Abstract
Central nervous system (CNS) injuries, including stroke, traumatic brain injury, and spinal cord injury, are leading causes of long-term disability. It is estimated that more than half of the survivors of severe unilateral injury are unable to use the denervated limb. Previous studies have focused on neuroprotective interventions in the affected hemisphere to limit brain lesions and neurorepair measures to promote recovery. However, the ability to increase plasticity in the injured brain is restricted and difficult to improve. Therefore, over several decades, researchers have been prompted to enhance the compensation by the unaffected hemisphere. Animal experiments have revealed that regrowth of ipsilateral descending fibers from the unaffected hemisphere to denervated motor neurons plays a significant role in the restoration of motor function. In addition, several clinical treatments have been designed to restore ipsilateral motor control, including brain stimulation, nerve transfer surgery, and brain–computer interface systems. Here, we comprehensively review the neural mechanisms as well as translational applications of ipsilateral motor control upon rehabilitation after CNS injuries.
Keywords: Stroke, Traumatic brain injury, Spinal cord injury, Brain–computer interface system, Neuroplasticity, Ipsilateral motor control, Axon regrowth
Introduction
In adult mammals, most corticospinal (CS) neurons (CSNs) in the cerebral cortex project directly to the opposite side of the spinal cord and control contralateral limb movements [1–3]. Therefore, the extremities on the side contralateral to the lesion often display motor deficits following injuries to the cortical descending pathways, such as stroke or traumatic brain injury [3–5]. Among central nervous system (CNS) injuries, stroke is the main worldwide cause of death and disability [6–10]. During the acute phase of injury, the majority of patients experience some degree of spontaneous recovery, thereby the impaired function is partially restored with time [11–13]. After entering the chronic phase of recovery (> 6 months), it is estimated that 55%–75% of stroke survivors exhibit permanent functional impairment of the paralyzed extremities [14, 15].
In recent decades, many efforts have been devoted to rescuing abnormal descending connections in the acute phase; for example, pharmacological interventions, stem cells, behavioral therapies, and non-invasive brain stimulation (NIBS) [11, 16–23]. Although such early interventions can improve parts of motor function, most patients still have difficulty in flexibly using the affected arm and/or hand for completing skilled movements, such as grasping and manipulating objects [24]. Notably, skilled movements of the upper extremities serve as the main predictor of whether a patient will resume a usual professional and personal life after brain injury [11, 24]. Recent studies have revealed that the unaffected hemisphere can compensate for the function of the damaged hemisphere and control the movements of the ipsilateral, paralyzed limb [19, 25–30]. The ipsilateral CS tract (CST) from the unaffected hemisphere to the paralyzed hand is one of the most common routes through which skilled motor function is restored following CNS injuries [30–33]. However, the compensatory ability to regain ipsilateral motor control gradually diminishes with age and is largely determined by the location and extent of the lesion [34–36]. Emerging technologies have focused on using the unaffected hemisphere to control the paralyzed upper extremity, such as novel NIBS strategies, brain–computer interface (BCI)-based rehabilitation systems, and a unique nerve transfer surgery named contralateral cervical seventh nerve transfer (CC7), which can effectively enhance skilled motor performance [27, 37–42].
In this review, we first describe the various effects of CNS injury on the cortical descending pathway and summarize the neural mechanisms of plasticity-dependent motor recovery. We next introduce interventions aimed at protecting or repairing the damaged brain in the early stage and highlight the limitations of their clinical application. Then, we shift our focus from the ipsilesional hemisphere to the contralesional hemisphere and summarize the research advances related to using the unaffected hemisphere to control the paralyzed upper extremity in both animal research and clinical application. Furthermore, we propose a sensorimotor integration-based treatment concept, the individualized, combined application of multiple methods to manipulate sensory input and motor output, which may contribute to driving neuroplasticity and accelerate the recovery of skilled motor function of the paralyzed hand.
CNS Injury and Plasticity-Dependent Spontaneous Recovery
CNS injuries associated with motor dysfunction results from the cortical denervation due to the destruction of contralesional CST axons [3, 5], also known as the pyramidal tract. The cortical neurons that constitute the CST are also known as upper motor neurons, and postsynaptic neurons of CST in the spinal cord are referred to as lower motor neurons, which are connected to skeletal muscle through neuromuscular junctions and control muscle contraction [1–3, 43]. Anatomically, most CST axons cross the midline in medulla oblongata, forming the pyramidal decussation. During typical development, the CST axons of motor cortices initially project bilaterally to the spinal cord. Continued development is characterized by the progressive weakening of uncrossed CST axons and the strengthening of crossed CST axons through synaptic competition driven by cortical activity [44–46]. Consequently, the uncrossed CST axons target motor neurons innervating the proximal and axial muscles, while the crossed CST axons target motor neurons in the distal muscles and are mainly involved in skilled movements. Most of the CST axons originate from pyramidal neurons located in the deep cortical layers (layers V and VI) of the primary motor and sensory cortex (M1 and S1, respectively). In mammalian species, the terminal distribution of the CST shows marked similarities, but the cortical-motor connections differ among species. In felines and rodents, motor commands conveyed by the CST are eventually transmitted to forelimb motoneurons relayed via segmental interneurons and propriospinal neurons [47]. In contrast, direct cortical-motor connections in the distal muscles are a specific feature of primates, thus enabling more advanced hand function and manual dexterity [3, 48–50].
Depending on their location, CNS injuries are divided into upper and lower motor neuron lesions [51–54]. An upper motor neuron lesion refers to an injury/lesion that occurs between the brain and the spinal cord, i.e., proximal to the ventral horn, including cortical damage, internal capsular infarction, brainstem injury, and spinal cord injury (SCI). An injury that occurs in the ventral horn of the spinal cord is considered to be a lower motor neuron lesion. In cortical damage caused by traumatic brain injury and stroke, the CSNs are greatly affected and exhibit degeneration or death, resulting in termination of the transmission of cortical commands [34]. In addition, internal capsular infarction and brainstem injury are primarily found in subcortical regions, known to be vital structures containing CS fibers carrying motor commands from some cortices to lower motor neurons. There are two main categories of SCI: complete and incomplete. Incomplete SCI is commonly caused by compression or damage to the spinal cord, which decreases motor signal transmission. However, complete SCI is the most severe and results from serious trauma to the spinal cord, bilaterally eliminating signal transmission between the spinal cord below the injury site and the brain. Therefore, these types of CNS injury disrupt CS fibers, causing motor dysfunction and paralysis of the limb.
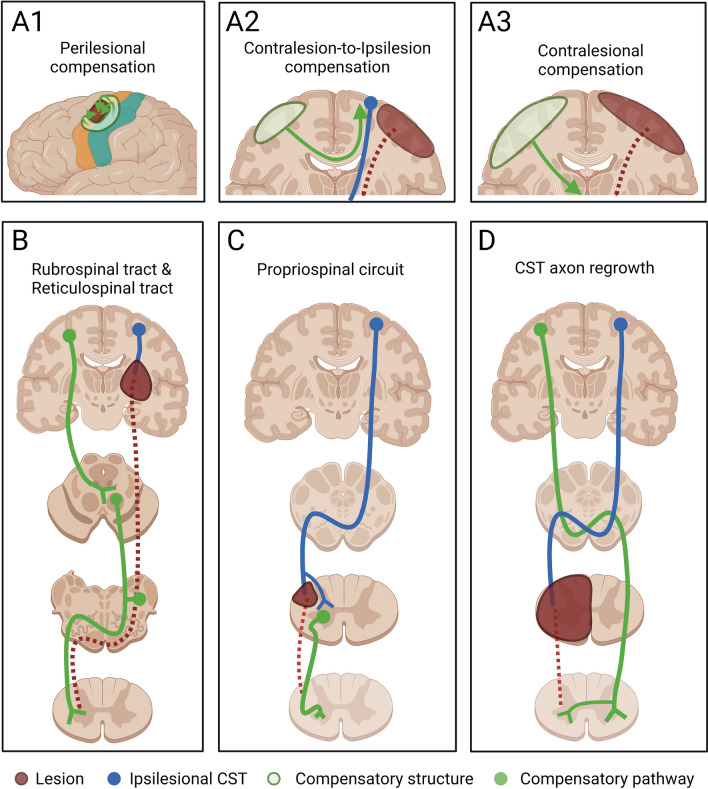
In general, CNS injury often results in a permanent loss of motor function, causing mature neurons to typically fail to regenerate. Nevertheless, an accelerating amount of research has revealed that the brain exhibits substantial neuroplasticity during the acute stage after damage, and this can compensate for the damage through reorganization and the creation of new connections among undamaged neurons [55–57]. Injuries occurring in different regions, such as the cerebral cortex, internal capsule, brainstem, and spinal cord, with different lesion extent are compensated for by different alternative pathways [58, 59]. In the event of motor cortical lesions, the perilesional area compensates for the functional loss [55, 60, 61] (Fig. 1A1). In contrast, the contralesional hemisphere exhibits a pronounced compensatory effect for lesions involving sensorimotor cortical areas [25, 59, 62] (Fig. 1A2, A3). The more serous the injury, the greater the activation of the ipsilateral hemisphere that is used during paretic limb movement [24, 63, 64]. Sprouting of the cortico-rubro-spinal pathway contributes to recovery in internal capsule injury [65–67] (Fig. 1B). Likewise, the rubrospinal and reticulospinal tracts play an important part in functional recovery from pyramidal lesions [66, 68, 69]. After a dorsolateral funiculus (DLF) lesion at the C4/C5 segments, an indirect pathway composed of the propriospinal tract contributes to recovery [70] (Fig. 1C). In the event of the hemisection of the lower cervical cord, the CST axons from the ipsilateral cortex descend to the contralesional DLF, and sprout across the midline below the lesion site (Fig. 1D).
Fig. 1.
Compensatory pathways during the acute stage after different CNS injuries. A1 After a local motor cortical lesion, significant reorganization occurs in the perilesional areas to compensate for the injured region. A2 The contralesional cortex controls the ipsilesional cortex by functional cortico-cortical connections to compensate for the lesion in a moderate cortical lesion. A3 A severe cortical lesion affects interhemispheric inhibition, while the cortex on the injured side completely loses its function, and the contralesional cortex directly controls the paretic side. B The cortico-rubro-spinal pathway is enhanced after damage to the internal capsule or brainstem pyramid. The reticulospinal tracts can be another compensatory pathway after pyramidal lesions. C After a DLF lesion, the spared propriospinal tract connecting different segments of the spinal cord plays an important role in functional recovery. D CST axons from the ipsilesional cortex sprout and cross below the lesion after hemisection of the spinal cord.
Notably, spontaneous recovery is generally incomplete. The degree of spontaneous recovery varies among individuals, and spontaneous recovery usually plateaus 6 months after CNS injury [11, 24]. The majority of patients still have difficulty in flexibly using the affected arm and/or hand for performing motor skills, such as grasping and manipulating objects. Therefore, in recent decades, various restorative therapies have been developed or are under development and aim to potentiate the remaining function of the damaged brain or enhance the compensatory capacity of the undamaged brain by fostering neuroplastic changes.
Neuroprotection, Chemical Neuromodulation, and Neurorepair Strategies in the Damaged CNS
In patients, neuroprotective agents or endovascular therapy should be administered strictly within the neuroprotection time window, which is 4–6 h after CNS injury [71, 72]. Clinical treatments administered beyond this brief window have limited neuroprotective effects [73, 74]. In the damaged brain, the neuroinflammatory cascade amplifies the primary injury [75, 76]. These secondary biochemical changes lead to tissue damage with associated cell death [77]. The time over these effects occur has been assumed to be hours–weeks, allowing a potential window for interventions.
Numerous experimental studies have suggested that excitation (glutamate and excitotoxicity), radicals (oxidative stress and free radicals), and cellular suicide (activation of apoptotic-like pathways) are the main reasons for the continuous cell death after CNS injuries [78–80]. Recent animal studies have overwhelmingly established that neuroprotective interventions can relieve secondary tissue damage and improve motor function after stroke [16, 81, 82]. These interventions include the pharmacological blockade of neurotransmitter receptors to inhibit cell death pathways [83] and inducing hypothermia [84] or hyperoxygenation [85, 86]. Besides, these restorative processes mainly depend on the severity of CNS injury and are appropriate for treating mild to moderate injuries but are largely ineffective in severe injuries. However, a number of clinical trials have shown that drugs modulating one or more of these mechanisms do not significantly improve the outcome for stroke patients [16].
In recent decades, it has gradually been recognized that neurovascular units (NVUs) play a major part in neuroprotection. The NVU is defined as a complex functional and anatomical structure including neurons, astrocytes, endothelial cells of the blood–brain barrier (BBB), myocytes, pericytes, and extracellular matrix components [87]. The modulation of interactions among different components of the NVU is essential for rescuing the damaged brain at an early stage. For example, enhancing microglial phagocytosis can reduce the brain damage caused by an excessive inflammatory response in brain tissue, avoiding a large degree of neuronal apoptosis and maintaining a number of neurons as much as possible [88]. In addition to affecting microglia, CNS injury can also continuously induce astrocyte reactivation and regeneration to reduce neurotoxic events and encourage scar tissue formation to avoid further injury [89–91]. Moreover, blood vessels, as multi-branched structures, continuously provide relevant nutritional materials to promote the recovery of injured brain tissue [92]. Furthermore, related studies have also suggested that pericytes contribute to maintaining the stability of the NVU and rescuing the normal BBB structure [93, 94]. Overall, neuroprotection in the damaged brain can accelerate functional and structural recovery in multiple animal models by stimulating endogenous neurorestorative events, such as neurogenesis, gliogenesis, angiogenesis, anti-inflammation, and synaptic plasticity.
Although many neurons around the lesion die after CNS injury, some survive but become dormant. These neurons may still have the ability to be excited in response to inputs from spared circuits and pharmacological neuromodulation. Neurotransmitter agonists, such as serotonin, dopamine, and norepinephrine temporarily restore locomotion when applied to paralyzed SCI animals [95]. Furthermore, systemic administration of CLP290, an agonist of the neuron-specific K+/Cl− co-transporter KCC2, enhances locomotion in paralyzed SCI mice [96]. CLP290 downregulates the excitability of spinal inhibitory interneurons enabling increased activity in propriospinal circuits to ameliorate the locomotor dysfunction. These drug treatments targeting propriospinal interneurons enhance their activity to promote locomotion by facilitating the transmission of motor signals through propriospinal circuits [96], and by raising the excitability of downstream locomotor networks [97]. But so far, these pharmacological neuromodulations have not been tested in patients.
In addition, neurorepair strategies, such as the transplantation of neural stem/progenitor cells (NSPCs), can also promote neuroprotection by repopulating areas of damaged cells and tissues [98, 99]. NSPCs are multipotent progenitors and can form neurospheres to differentiate into a wide range of cell types, such as neurons, glial cells, and precursor cells. NSPCs tend to differentiate into oligodendrocytes when transplanted into host parenchyma, while they are more to differentiate into astrocytes when transplanted into a lesion site [100]. Following transplantation into the damaged brain, they can induce regeneration of the axons of damaged neurons to bridge the break caused by injury and thereby recover the regular function of nerve cells and restore brain area function [99, 101]. NSPCs transplanted into sites of SCI enable the regeneration of CS axons by maintaining CST neurons in an embryonic growth state [102], and the regenerating axons can form synaptic projections to appropriate NSPCs-derived neurons [103]. These grafted neurons, functioning as interneuronal relays, extend abundant axons for long distances to the host spinal neurons below an injury [104, 105], and these neurons can respond to cortical stimulation or sensory stimulation below the lesion in vivo [106]. Recent studies have also reported that NSPCs derived from human pluripotent stem cells (hPSCs) can differentiate into distinct spinal cord neurons, enable robust CS regeneration, integrate various intraspinal and supraspinal systems, and improve functional outcomes after injuries [107–109]. Enhancing activity within the transplanted hPSCs-NSPCs or in combination with rehabilitation training can promote the efficiency and efficacy of cell transplantation therapy for SCI [110, 111]. Although animal studies have revealed that NSPC transplantation is a feasible and promising means of compensating for lost functions, there are still some limitations and potential side-effects in their clinical application, including a large-scale bottleneck in stem cell production and potential allogeneic rejection of the cells [112].
In summary, neuroprotection, neuromodulation, and neurorepair can not only effectively rescue the damaged CST but also induce neuroplasticity to restore the motor function of a paralyzed limb. However, these successes are limited to animal studies, and translation of these interventions from animal studies into clinical practice has yield disappointing results. Clinical failure may be due to insufficient efficacy of current treatments and ignorance of the heterogeneity among patient populations [16]. Particularly, it is still difficult to achieve persistent recovery of motor function by repairing the damaged brain when injury-related recovery enters the chronic stage.
The Cortical Physiology of Ipsilateral Motor Control
Is it possible to use the intact hemisphere to control the paralyzed limb on the same side? In fact, as early as 1874, Brown-Sequard declared that it was quite possible and that one hemisphere was sufficient to act on both sides of the body [113]. Anatomical and physiological studies have established that almost 15% of CST fibers directly project to the ipsilateral spinal cord without crossing the medulla [1–3, 114]. Notably, the location, proportion, and distribution of the uncrossed CST axons differ by species and stages of development. Continued development is characterized by the gradual weakening of uncrossed CST axons and the strengthening of crossed CST axons through synaptic competition, driven by cortical activity [114]. With the exception of the uncrossed CST axons described above, the unilateral motor cortex can control ipsilateral movements by the corpus callosum, as well as neural networks interconnecting brainstem and spinal cords [115, 116]. Consequently, this indicates the potential involvement of the contralesional hemisphere in the restoration of motor function after unilateral CNS injury.
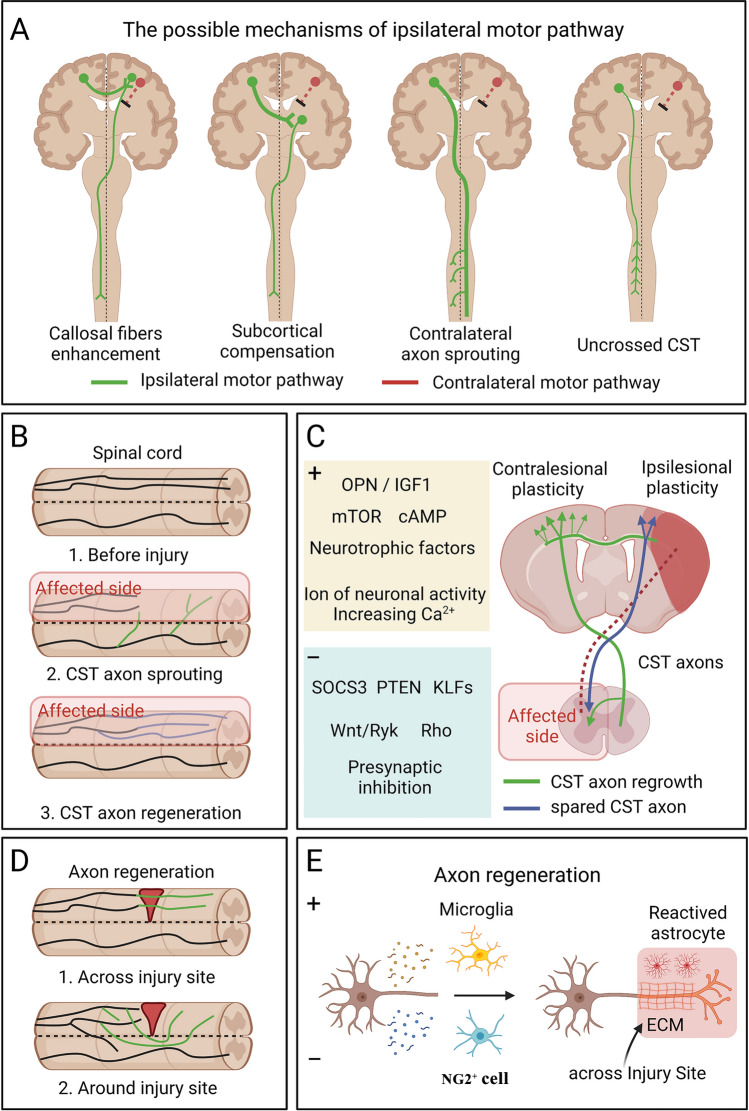
It is well known that the juvenile brain has more powerful neural plasticity than the adult brain, thus allowing better functional recovery after CNS injury [117, 118]. Patients who undergo brain injury at a young age often exhibit substantial improvement in motor function [119]. Animal studies have revealed that the ipsilateral motor pathways from the contralesional sensorimotor cortex to the opposite side of the spinal cord play a vital role in motor recovery following a sizable cortical lesion at a young age [30, 118, 120]. The possible mechanisms involved in ipsilateral motor control are as follows: (1) enhancing the neuronal activity of the ipsilateral cortex via the corpus callosum and the more substantial contralateral CST [121]; (2) regenerating new synaptic connections of the contralateral CST in subcortical areas such as the brainstem, leading to the reorganization of descending motor pathways [122–124]; (3) promoting axon sprouting of the CST in the contralesional hemisphere across the midline and growing into the denervated area of the spinal cord [2, 33]; and (4) enhancing the efficacy, increasing the regeneration, or decreasing the degeneration of the uncrossed CST axons of the ipsilateral spinal cord [31, 125] (Fig. 2A). Certainly, multiple mechanisms must be involved in the reorganization of ipsilateral motor pathways after injury, and these mechanisms need to be further elucidated.
Fig. 2.
The potential circuit basis and molecular mechanism for implementation of ipsilateral motor control and the cellular mechanism of axon regeneration. A Schematic organization of ipsilateral motor pathways. The mechanism of ipsilateral motor control may include the following possibilities: 1. The contralesional cortex controls the remaining CST in the impaired hemisphere by callosal fiber enhancement; 2. Synaptic connections between the contralesional cortex and the ipsilesional subcortical areas are increased; 3. The crossed CST originating from the contralesional cortex sprouts across the midline to the damage-denervated side of the spinal cord; 4. The number and activity of uncrossed CST fibers from the contralesional cortex to the affected spinal cord are increased. B Cartoon showing the difference between CST axonal sprouting and regeneration in the spinal cord. Axon growth including axon sprouting and axon regeneration. The sprouting refers to axon regrowth from intact neurons on the unaffected side, and regenerating axons arise from the cut ends of the transected axon of injured neurons. C Factors that enhance axon growth are shown in the yellow box, while factors that inhibit axon growth are shown in the blue box. Note that both CST regrowth (green) and spared CST axons (blue) aim to re-innervate the spinal cord of the affected side. D, There are two forms of axon regeneration in spinal cord injury, one in which regenerating axons cross through the glial scars, and one in which regenerating axons bypass the injury site. E Cellular mechanisms that facilitate the passage of regenerating axons across the astroglial scar. The neonatal microglia secrete fibronectin and their binding proteins to establish a bridge to the extracellular matrix and express various peptidase inhibitors to promote CST regrowth to pass through the injury site. Nerve/glial antigen 2 positive (NG2+) cells increase scar formation by secreting pro-inflammatory factors that impede the passage of regenerating axons through glial scars.
Unfortunately, such compensatory ability gradually diminishes as the brain matures, mainly because the ipsilateral motor pathway, which perform as compensation for the early stage of development, becomes sparse with maturity [3, 115, 116]. Recent studies have established that the ipsilateral CST is also necessary for behavioral recovery after CNS injury in adult rodents [19, 126], monkeys [127], and humans [128, 129]. However, the regenerative characteristics of the ipsilateral CST are significantly different at different developmental stages. As the ipsilateral CST is not fully pruned after CNS injury in childhood, the axons of CSNs in the uninjured motor cortex project to both halves of the spinal cord to control both limbs [33, 130]. Nevertheless, in adulthood, the decline in cortical neural activity on the injured side promotes axon sprouting, but this compensatory effect cannot flexibly control the impaired limbs by the weakened ipsilateral CST. To achieve ipsilateral motor control after CNS injury, the underlying mechanism is related to the selective activation of CSNs and cortical layer V neurons, promoting neuronal plasticity, and ameliorating injury-induced sensory-motor deficits. Therefore, new therapies are urgently needed to stimulate the compensatory ability within the contralesional cortex to recruit the spared circuit in adult patients with CNS injury.
Cellular and Molecular Interventions for Promoting Axon Regrowth
By definition, axon growth includes both regeneration and sprouting, with the difference between the two being whether the neuron was damaged in the first place (Fig. 2B) [131, 132]. Regeneration refers to axon growth of damaged neurons, whereas sprouting refers to axon growth of undamaged neurons. It is now widely recognized that both the intracellular and extracellular environments can influence axon growth [133, 134]. Myelin-associated inhibitors (MAIs), such as Nogo and myelin associated glycoprotein, are an important class of neuron-extrinsic mechanisms that inhibit axon growth. Interestingly, inhibition of MAIs alone increases compensatory axon sprouting, but has poor effect on the axon regeneration of injured neurons [135–137]. Notably, research progress in the mechanisms underlying the change of axon growth capacity in the development period contributes to understanding the neuron-intrinsic mechanisms underlying axon regeneration [34, 133]. During development, the axon growth competence of neurons diminishes after synapse-formation is complete and functional neuronal circuits are established. After CNS injury, the cellular processes in the affected neurons are altered, and the neuron transitions from an active, electrically-transmitting state to an electrically-silent, growth-competent state similar to that during development, regaining the intrinsic capacity for axon growth [133, 138]. Next, we summarize the optimal interventions related to axon regrowth from cellular and molecular perspectives.
Recent studies have shown that multiple steps of signal initiation and transduction, including receptor availability [139] and signal propagation [140, 141], or silencing inhibitory pathways [142, 143], can be targeted to enhance axon regeneration of the mature neuron (Fig. 2C). For example, a combination of osteopontin/insulin-like growth factor 1 (IGF1) induces robust sprouting of CST axons and associated behavioral recovery after injuries [144]. In addition, IGF1-dependent axon regeneration is down-regulated by presynaptic neurons [139]. Interestingly, in the case of a lack of growth factors, direct molecular intervention targeting adult neurons promotes robust CST axon sprouting and restores motor function after injury, including enhancers such as WNT/RYK signaling [145], ras homolog gene family member A [146], cyclic adenosine monophosphate [147], and mammalian target of rapamycin [148], and repressors such as phosphatase and tensin homolog [149–151], Kruppel-like transcription factors [152], and suppressor of cytokine signaling 3 [149]. Similarly, some ions involved in neuronal activity play an important part in promoting axon regeneration. An increase of the Ca2+ concentration in damaged neurons within minutes after the lesion promotes the initiation of axon regeneration by promoting neuronal growth cone assembly and other stages of axon regeneration [133, 153, 154]. But the continuous Ca2+ influx through voltage-gated Ca2+ channels after injury suppresses the axon regeneration, and the ablation of Cacna1c (Calcium Channel Subunit Alpha1c, encoding the Cav1.2 channel) [155] or Cacna2d1 (encoding subunits necessary for the Cav2 channel) [156] efficiently promote axon regeneration in dorsal roots ganglion ( DRG) neurons after peripheral axotomy or SCI.
In addition, extensive scar tissue is formed after CNS injury, and newly-formed axons need to reinnervate and redistribute axon terminals through scars (Fig. 2D). The CNS scar is a compartmentalized structure and consist of two major parts: the reactive astrocytes constituting the outer glial scar [157] and the fibrotic scar at the core of the lesion with pericyte derived fibroblasts [158] and inflammatory cells. Recently, different types of loss-of-function study have revealed that scar formation and fibrosis in the CNS involves complex interactions among multiple types of CNS glia and non-neural stromal cells. A partial reduction of Type-A pericytes reduces the fibrosis, enhances the tissue repair and promotes locomotor recovery, whereas a more complete prevention of the scarring produced by Type-A pericytes appears to be detrimental and worsens the outcome [159]. Preventing astrocytic scar formation or specifically ablating chronic astrocyte scars does not lead to axonal regrowth after SCI [160], while pharmacological blocking of the interaction between reactive astrocytes and type I collagen prevents glial scar formation, allowing for a more loosely-arranged glial architecture and promoting axonal regrowth [161]. Besides, astrocytes activated by injury synthesize and secrete various neurotrophic or axon-growth-supporting factors for axon regeneration, including brain-derived neurotrophic factor [162], ciliary neurotrophic factor [163, 164], and chondroitin sulfate proteoglycan 5 (also known as neuroglycan C) [160], which contribute to the composition of the injury microenvironment. Depleting microglia specifically results in an increase in systemic immune cell infiltration and neuronal cell death, disruption of glial scar formation, and worsened behavioral outcomes after SCI [165–167]. Moreover, the latest research has revealed that a subgroup of microglia secrete fibronectin and its binding proteins to establish a bridge to the extracellular matrix and express various peptidase inhibitors to promote scarless healing of spinal cord lesions [168], thus allowing descending axons to pass through the injury site in neonatal mice. Such new insights from cell type-specific loss-of-function studies and next-generation sequencing results have yielded a more complex portrait of the molecular mechanisms governing the glial and neuronal responses to injury, and provide possible combinatorial therapeutic approaches to scar modulation to restore function after severe CNS injury [169].
Notably, robust axon regrowth and the establishment of appropriate neural connectivity does not mean restoration of neural function [170]. Combined rehabilitation training at an adequate time encourages CST regrowth and helps to form, select, and stabilize new functional neural circuitry [19]. Overall, sequentially combining sensory-motor rehabilitation with molecular therapies may act synergistically to enhance cortical plasticity in the intact brain and thereby promote long-term recovery after CNS injury [117]. However, these successes are currently restricted to animal studies and are challenging to translate immediately into clinical applications.
Clinical Interventions for Enhancing Compensatory Capacity
Current treatments to improve long-term outcomes in CNS injury patients include rehabilitative training, electrical stimulation, and pharmacological interventions [11, 18]. However, these treatments have had only limited success, and it is difficult to achieve the recovery of motor skill at the chronic stage. In recent years, several advanced neuromodulation treatment strategies have been developed to improve motor performance in people with functional impairments [117]. We next summarize the neurotechnologies designed for applying appropriate stimulation to the nervous system to help enhance neuroplasticity in the intact hemisphere, thus facilitating functional recovery of the paralyzed arm/hand.
Electrical Stimulation to Drives Neuroplasticity for Functional Recovery
Electrical stimulation can be delivered by non-invasive surface electrodes or invasive implanted electrodes to activate or inhibit the CNS. This stimulation can enhance the neural plasticity of specific brain regions, modify local neural circuits in combination with appropriate neurological rehabilitation training, and correct maladaptive neural reorganization caused by neurological injury [11, 18, 171].
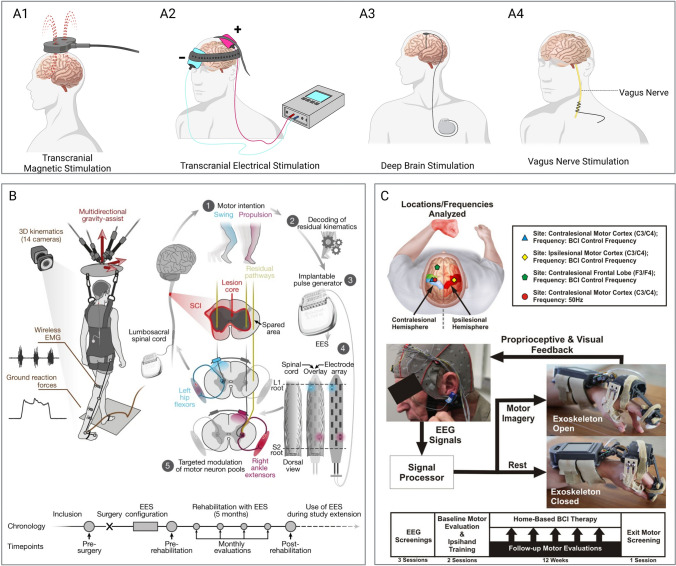
Noninvasive brain stimulation (NIBS), including transcranial magnetic stimulation (TMS) and transcranial electrical stimulation, is available to activate or inhibit the CNS by electrodes placed on the epicranium (Fig. 3A1 and A2) [7]. Based on the finding of interhemispheric imbalance, NIBS was first used to suppress the contralesional hemisphere and attenuate transcallosal inhibition to activate the ipsilesional primary motor cortex. However, an increasing number of negative results have appeared, and no additional beneficial effects of the re-activation of the ipsilesional hemisphere have been reported [172]. A plausible explanation is that suppression of the contralesional motor cortex reduces the excitability of the ipsilateral motor pathways that are also important for movement of the paralyzed limb. Several neuroimaging results have demonstrated that the contralesional hemisphere also participates in functional restoration by involving the ipsilateral CST pathway and the cortico-reticulo-propriospinal pathway [40, 65, 173, 174]. We then conclude that NIBS is not the “one size fits all” solution for all patients but that it can be tailored to individuals according to the extent and location of damage, the degree of functional deficiency, or the stage of the recovery process [11]. Moreover, NIBS should not only focus on interventions in the primary motor cortex. In addition, it is notable that the premotor cortex, supplementary motor areas, and the cerebellum, as well as other areas connected to the primary and secondary motor areas and form a complex network, are also involved in the processes of restoration of motor function after brain damage [39, 175]. Therefore, patient-specific characteristics might be considered for determining the best neuromodulation target and more focal stimulation of specific cortical targets. The combination of EEG and TMS has recently offered a new direction named “closed-loop NIBS”, which provides a novel and effective strategy of neuromodulation to restore the impaired motor function [176]. These closed-loop strategy-based therapeutic approaches can successfully restore injured motor function in patients after SCI. Even though various animal experiments and clinical trials have established that NIBS can treat the dysfunction caused by CNS injury to a certain extent, the results are not satisfactory [18]. In future, the underlying concepts of NIBS need to be deeply elucidated, and NIBS-based treatments, such as personalized NIBS, multi-site NIBS, and closed-loop NIBS need to be further developed.
Fig. 3.
Clinical advances for enhancing neuroplasticity to promote motor recovery. Schematic of brain stimulation systems for patients: A1 The noninvasive brain stimulation method TMS controls cortical activity via magnetic signals. A2 tES modulate the activation of cortex by low-intensity current. A3 In invasive brain stimulation systems, such as DBS, electrodes are implanted in a deep brain region and a generator is implanted in the upper chest. A4 The VNS system requires the implantation of electrodes into the vagus nerve in the left neck. B Targeted neurotechnologies for EES during overground walking in patients with SCI [187]. Upper: During training, the wireless communication environment ensures that the EES on the spinal cord can be independently adjusted in real time. An auxiliary device applies multidirectional forces to the trunk against gravity and a real-time processing system records the full-body movements, ground reaction forces, and the electrical activity of leg muscles. A 16-channels electrode paddle array with pulse generator is implanted in the lumbosacral dorsal roots that connect to specific motor neuron pools innervating different leg muscles. EES sequences under voluntary intention induce different lower extremity movements, such as hip flexors and ankle extensors. Lower: The study timeline of this system. C The mechanism and study methodology of IpsiHand [37]. Left: The EEG electrode placement strategy of IpsiHand. The recording electrodes are placed in the bilateral motor cortex (blue triangle in the contralesional motor cortex and yellow diamond in the ipsilesional motor cortex), a spatial control electrode (green pentagon) in the contralesional frontal lobe, and a spectral control electrode in the contralesional motor cortex (red dot). Right: The images above show how IpsiHand works. The exoskeleton is attached to a patient’s impaired forearm, palm, and intermediate phalanges of the index and middle finger. A microprocessor in the forearm controls the exoskeleton by an assembly that processes EEG signals. Based on the decoded EEG signals, a linear actuator drives fine hand movements in a 3-finger pinch grip.
In deep brain stimulation (DBS), the most invasive therapy, electrodes are implanted in brain regions, such as deep nuclei and white matter tracts, and a generator is implanted in the upper chest (Fig. 3A3). The stimulation pattern of DBS is often continuous, and its intensity and frequency can be customized according to individual characteristics. Originally used to treat Parkinson’s disease, DBS can relieve symptoms such tremors, stiffness, and involuntary movements. Recent studies have made a breakthrough in the application of DBS to treat severe traumatic brain injury (STBI) [177, 178]. Direct activation of central thalamic neurons through DBS in patients with STBI tends to normalize cortico-striato-pallido-thalamocortical function. For example, a clinical trial demonstrated that DBS of the bilateral central thalamus alleviated the arousal disorders and increased functional limb control in a 38-years-old patient who remained in a minimally conscious state for 6 years following STBI [179]. Therefore, DBS provides a feasible solution to activate dormant networks or reorganize aberrant or desynchronized connections across brain regions to accelerate functional recovery.
Vagus nerve stimulation (VNS) consists of electrodes connected to the left vagal nerve and a pulse generator implanted under the skin (Fig. 3A4). Previously, VNS was applied in patients with pharmaco-resistant epilepsy, and several studies demonstrated that it triggers neural plasticity to reorganize the motor response area by activating ascending cholinergic pathways [180]. Recently, various animal studies and clinical trials have confirmed that VNS combined with rehabilitative therapies is a promising treatment option for hemiplegic patients [181, 182]. VNS paired with rehabilitative training in adult rats with stroke improves forelimb motor performance in an untrained task, and the treatment effects can persist for several months, even after stopping the treatment [182]. Anatomically, VNS can enhance plasticity in ipsilateral CST regeneration to enhance synaptic connectivity to the muscles of the paralyzed forelimb. Hence, VNS acts synergistically with rehabilitation exercise to restore impaired motor function by enhancing plasticity in the descending motor pathway.
Recent clinical studies of spinal cord epidural electrical stimulation (EES) have demonstrated that delivery of current to the dorsal spinal cord can restore several voluntary movements below the level of a SCI (Fig. 3B) [183–185]. Further investigations have suggested that EES and task-specific training can reconstruct independent stepping in completely paralyzed patients [186–188], revealing the reactivation of previously dormant spared spinal circuits and the enhancement of use-stimulation dependent plasticity [183, 184]. On the other hand, EES can recruit proprioceptive input from afferent dorsal roots, which has been suggested to be central to the ability of EES to engage motor neurons at specific spinal cord segments [187]. Spatiotemporal EES applied to specific dorsal roots allows for selectively stimulation to be timed to coincide with the desired movement [187, 189].
In sum, electrical stimulation therapies are promising; however, based on the individual characteristics of patients evaluated by electrophysiology and imaging, current neuromodulation techniques need to be combined with traditional rehabilitation and necessary pharmacological treatment, ultimately leading to the formation of a complete rehabilitation treatment program for patients with motor disorders after CNS injury.
BCI-assisted Movement Control
BCI systems record brain signals, analyze them, and translate them into specific commands that are relayed to output equipment that achieves the desired actions [190]. BCI systems record and decode specific signals related to motion execution, and provide appropriate feedback to the CNS for enhancing neural plasticity. To date, BCI systems have been used as promising treatments to restore motor function in patients following chronic CNS injury. Studies have demonstrated that BCI-controlled electrical stimulators targeted to muscles or peripheral nerves and BCI-driven neuroprostheses can effectively improve motor performance in stroke survivors. But these BCI systems for stroke patients have only focused on residual signals from the perilesional cortex [191, 192]. However, in patients with moderate-to-severe cortical damage, it is difficult to modulate perilesional cortical activity, and new therapies should be directed to focus on the non-lesioned, or ipsilateral, cortical hemisphere.
It is universally acknowledged that unilateral limb movements derive primarily from the cortical hemisphere contralateral to the limb, but the ipsilateral motor cortex is also involved in the control of these movement [116]. This ipsilateral motor activation can be used to decode specific motor intentions [193, 194]. Recently, the FDA approved a BCI system, “IpsiHand”, from the company Neurolutions, as a novel and potentially powerful tool that processes movement-related EEG signals from the contralesional hemisphere to control the exoskeleton of the paralyzed hand, which may lead to functional improvements in patients with chronic stroke (Fig. 3C). This contralesional BCI was designed and configured for stroke rehabilitation in the home environment [37, 38]. Despite much progress, the development of this BCI-driven powered exoskeleton system for stroke patients to restore dexterous movements and perform daily living activities remains an ambitious project. The current design mainly has the following deficiencies: (1) This product cannot separate the signals of both upper extremities so that the contralesional hemisphere cannot spontaneously control both the healthy and the paralyzed hands independently. (2) IpsiHand has a flexible exoskeleton only for the index finger with a single degree of freedom and cannot achieve motion assistance for multiple joints of the hand. (3) IpsiHand does not induce neuroplasticity in the contralesional cortex, and the restored functions cannot be maintained without the assistance of the BCI device. Therefore, compared with traditional rehabilitation, the IpsiHand system-assisted rehabilitation improves the Fugl-Meyer (FM) score by only 0.79 points, far from reaching the minimum difference of clinical significance (FM score 5.25 points).
While various interventions targeting the CNS can effectively increase neuroplasticity, peripheral sensory-motor training may play an important part in remodeling brain function. Neuromuscular electrical stimulation (NMES) provides proprioceptive feedback through the residual sensory afferent pathway to reactivate spared cortical circuits and achieve motor recovery [195, 196]. Recent studies have established that an NMES-based BCI approach may achieve functional recovery in patients with moderate and severe chronic stroke, and such recovery can continue to be maintained 6–12 months after completing treatment [197]. In addition to proprioception, the sense of touch is also essential for motor control. A closed-loop BCI uses residual touch signaling from the patient’s own hand to restore the ability to detect object touch and improve motor functions after SCI [198]. Notably, it is difficult to use a prosthesis or perform any simple task without being able to sense the interacting object. In patients with complete SCI, the brain loses all neural connections below the injury site and disrupts sensory-motor control of the extremities. Recent studies have reported a novel bidirectional BCI that records neural activity from the motor cortex and generates sensations by intracortical microsimulation of the sensory cortex [192, 199]. As a result, bidirectional BCI can enable a tetraplegia patient to substantially restore motor function with a robotic limb [192]. Therefore, we believe that bidirectional closed-loop BCI integrating sensory feedback and motor control is a promising strategy for recovering skilled motor function after CNS injury.
Crossing Nerve Transfer Surgery to Reconstruct Ipsilaterally-based Sensory-motor Function
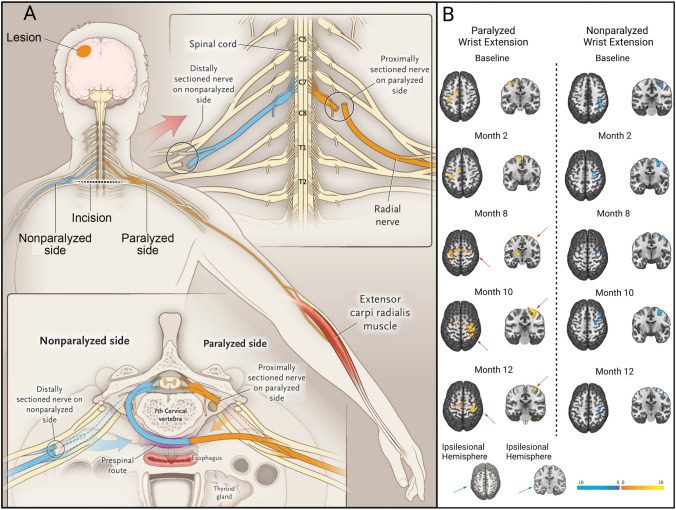
The brachial plexus (BP) is a network of five nerves from distinct spinal cord segments (C5, C6, C7, C8, and T1) containing ~ 80,000 nerve fibers and innervating the upper limb [200]. With the widespread use of nerve transfer, we have gained more knowledge of the internal anatomy of individual nerve fibers within the major peripheral nerves. Within the BP, the C7 nerve accounts for ~ 20% and contains both sensory and motor fibers. The unique feature of the C7 nerve is that the its innervation largely overlaps with that of the other four nerves that give rise to the BP. Resection of the C7 nerve usually results in transient weakness and numbness in the upper extremity [201, 202]. Therefore, the healthy side of the C7 nerve in humans can be used as a donor in crossing nerve transfer surgery. Crossing nerve transfer surgery has been widely performed to repair avulsion of the BP by reconnecting the injured nerve ends to the C7 nerve on the healthy side [203, 204]. Recently, we confirmed the possibility of the creative use of crossing nerve transfer as a new peripheral nerve strategy for CNS injury. A crossing nerve transfer surgery, named contralateral cervical seventh nerve transfer (CC7), was applied to achieve significant functional recovery of the paralyzed arm by transferring the C7 nerve from the non-paralyzed side to the paralyzed side in a patient after brain injury (Fig. 4A) [41]. A unique feature of this operation is that the sensory and motor signals of the paralyzed upper extremity communicate with the contralesional hemisphere through the displaced “left-right crossover” nerve. Patients who underwent this surgery showed significant improvements, especially in skilled movements of the paralyzed hand, as measured by Fugl–Meyer assessment; and this recovery led to improved self-care in daily life. In addition, TMS and functional magnetic resonance imaging (fMRI) showed the establishment of physiological connectivity between the contralesional cortex and the paralyzed arm (Fig. 4B). Notably, the functional recovery resulting from CC7 surgery is not limited to function innervated by the C7 nerve itself. Therefore, there are sufficient reasons to believe that CC7 surgery can stimulate neuroplasticity to accelerate motor recovery from CNS injury by modulating peripheral sensorimotor interactions.
Fig. 4.
Crossing nerve transfer surgery to improve motor function by enhancing neuroplasticity in the contralesional hemisphere in patients with unilateral arm paralysis [41]. A Procedure of contralateral C7 nerve transfer surgery. After harvesting the bilateral C7 nerves in adequate sites, the C7 nerve on the non-paralyzed side (blue) is drawn behind the trachea and esophagus via a pre-spinal route to the paralyzed side (yellow) and coapted directly to the C7 nerve on the paralyzed side. B Functional MRI assessment in patients with CC7 surgery. The changes in brain activation on fMRI are evaluated during the 12 months after surgery. Left: Brain activation (yellow) during active extension of the paralyzed wrist. Before surgery, activation was only evident in the ipsilesional hemisphere. At month 8, activation began to appear in both hemispheres. Contralesional activation was enhanced and extended to a larger area than ipsilesional activation at 10 months after surgery, and it was weaker and covered a smaller region at month 12 than at month 10. Right: Brain activation (blue) during active extension of the non-paralyzed wrist. Brain activation associated with movements of the non-paralyzed wrist was stable in the contralesional hemisphere before and after surgery.
Future Prospects
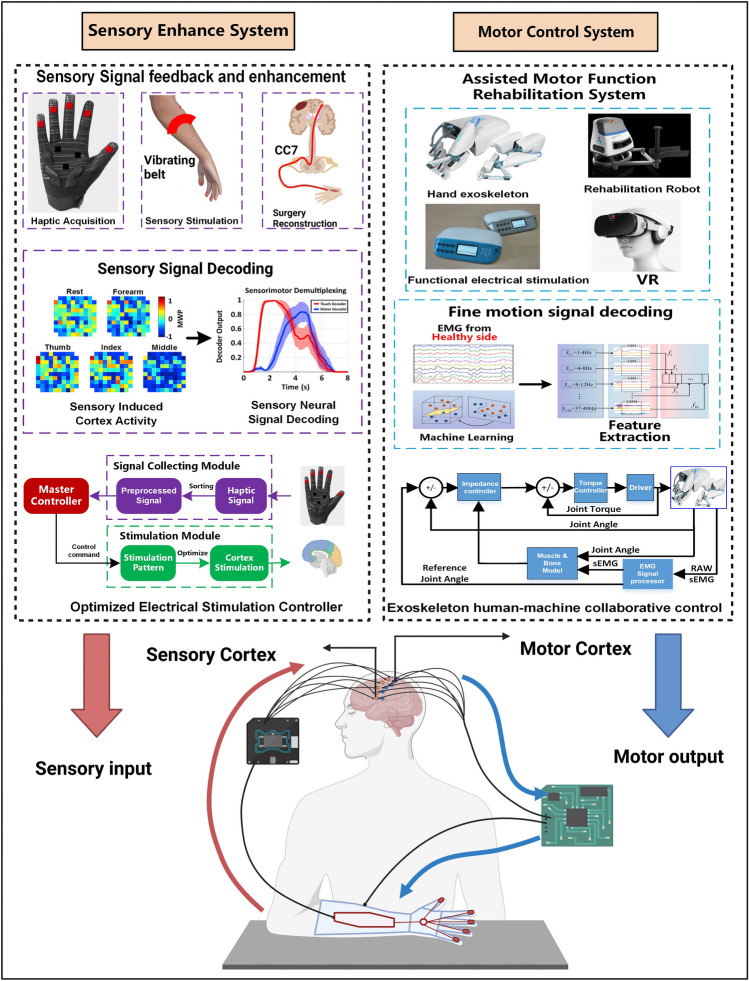
To date, many emerging neurotechnologies, such as NIBS, BCI, and crossing nerve transfer surgery, have supported the direct modulation of cortical plasticity in the intact brain to improve the fine motor function of the paralyzed limb in adult patients after CNS injury. However, a novel technology is still needed to specifically focus on the coordinated combination of various methodologies to maximize their strengths. Sensory input is quite important for motor function [205]. Sensory signals affect motor functions by transmitting environmental information and intrinsic physiological status as well as by guiding the initiation of the motor system [206, 207]. Nevertheless, the modulation of sensorimotor integration can reactivate dormant plasticity in the adult neocortex. Hence, the next step needs an in-depth exploration of the underlying mechanisms of how sensorimotor integration contributes to skilled hand movements in both normal humans and patients.
In this review, we propose that those new therapeutic designs should focus on targeting sensorimotor integration and combining innovative neurotechnologies to drive neuroplasticity in the adult brain to achieve ipsilateral motor control and thus accelerate the recovery of skilled motor performance (Fig. 5). To date, a growing number of amputees are using neuroprosthetics that restore some somatosensation [192, 208–210]. The greatest advantage of crossing nerve transfer is that the sensory inputs from the paralyzed hand can be physiologically transmitted to the ipsilateral (contralesional) brain by avoiding the injured side. Future studies should pay more attention to bidirectional BCI systems, in which sensory enhancement is achieved through peripheral stimulation, surgical reconstruction, or patient-specific cortical stimulation and decoding the signals related to motion intention during motor preparation to control a robotic prosthesis (Fig. 4). We believe that the bidirectional BCI system can be used to maximally enhance intrinsic neuroplasticity while functionally bridging undamaged ipsilateral motor pathways and thus contribute to promoting recovery after brain injury.
Fig. 5.
A new rehabilitation strategy that integrates multiple technologies and integrates sensorimotor information to achieve ipsilateral motor control. In patients with unilateral CNS injury, sensory information from the paralyzed limb is increased by using sensors, electrical stimulation, and surgery, and specific sensory information is decoded. The decoded information is then directly transmitted to specific regions of the contralesional hemisphere by electrical stimulation. At the same time, the motor commands from the contralesional cortex are extracted, and the motor information is decoded according to the data set during training, and then the exoskeleton is directly controlled to drive the affected limb and complete the corresponding action.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81902296, 82071406, 82021002, 92168105), Shanghai Municipal Science and Technology Major Project (2018SHZDZX05), and Shanghai Natural Science Foundation [20XD1420700, 22ZR1479000].
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Zhengrun Gao and Zhen Pang contributed equally to this work.
References
- 1.Nudo RJ, Masterton RB. Descending pathways to the spinal cord, III: Sites of origin of the corticospinal tract. J Comp Neurol. 1990;296:559–583. doi: 10.1002/cne.902960405. [DOI] [PubMed] [Google Scholar]
- 2.Li HL, Asante CO. Developmental plasticity of descending motor pathways. J Neurophysiol. 2011;105:1963–1965. doi: 10.1152/jn.01104.2010. [DOI] [PubMed] [Google Scholar]
- 3.Welniarz Q, Dusart I, Roze E. The corticospinal tract: Evolution, development, and human disorders. Dev Neurobiol. 2017;77:810–829. doi: 10.1002/dneu.22455. [DOI] [PubMed] [Google Scholar]
- 4.Wang AC, Ibrahim GM, Poliakov AV, Wang PI, Fallah A, Mathern GW, et al. Corticospinal tract atrophy and motor fMRI predict motor preservation after functional cerebral hemispherectomy. J Neurosurg Pediatr. 2018;21:81–89. doi: 10.3171/2017.7.PEDS17137. [DOI] [PubMed] [Google Scholar]
- 5.Lin DJ, Cloutier AM, Erler KS, Cassidy JM, Snider SB, Ranford J, et al. Corticospinal tract injury estimated from acute stroke imaging predicts upper extremity motor recovery after stroke. Stroke. 2019;50:3569–3577. doi: 10.1161/STROKEAHA.119.025898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the American Heart Association. Stroke. 2010;41:2402–2448. doi: 10.1161/STR.0b013e3181e7512b. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham DA, Machado A, Janini D, Varnerin N, Bonnett C, Yue G, et al. Assessment of inter-hemispheric imbalance using imaging and noninvasive brain stimulation in patients with chronic stroke. Arch Phys Med Rehabil. 2015;96:S94–S103. doi: 10.1016/j.apmr.2014.07.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayis S, Wellwood I, Rudd AG, McKevitt C, Parkin D, Wolfe CD. Variations in Health-Related Quality of Life (HRQoL) and survival 1 year after stroke: Five European population-based registers. BMJ Open. 2015;5:e007101. doi: 10.1136/bmjopen-2014-007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding QQ, Liu SW, Yao YD, Liu HN, Cai T, Han LY. Global, regional, and national burden of ischemic stroke, 1990–2019. Neurology. 2022;98:e279–e290. doi: 10.1212/WNL.0000000000013115. [DOI] [PubMed] [Google Scholar]
- 10.Collaborators GBD2S Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micera S, Caleo M, Chisari C, Hummel FC, Pedrocchi A. Advanced neurotechnologies for the restoration of motor function. Neuron. 2020;105:604–620. doi: 10.1016/j.neuron.2020.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, et al. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci. 2016;10:442. doi: 10.3389/fnhum.2016.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair. 2006;20:444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa H, Ueno M, Itokazu T, Yamashita T. Bilateral movement training promotes axonal remodeling of the corticospinal tract and recovery of motor function following traumatic brain injury in mice. Cell Death Dis. 2013;4:e534. doi: 10.1038/cddis.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevignard MP, Lind K. Long-term outcome of abusive head trauma. Pediatr Radiol. 2014;44(Suppl 4):S548–S558. doi: 10.1007/s00247-014-3169-8. [DOI] [PubMed] [Google Scholar]
- 16.Stocchetti N, Taccone FS, Citerio G, Pepe PE, le Roux PD, Oddo M, et al. Neuroprotection in acute brain injury: An up-to-date review. Crit Care. 2015;19:186. doi: 10.1186/s13054-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janowitz T, Menon DK. Exploring new routes for neuroprotective drug development in traumatic brain injury. Sci Transl Med. 2010;2:27rv1. doi: 10.1126/scitranslmed.3000330. [DOI] [PubMed] [Google Scholar]
- 18.Won SM, Song EM, Reeder JT, Rogers JA. Emerging modalities and implantable technologies for neuromodulation. Cell. 2020;181:115–135. doi: 10.1016/j.cell.2020.02.054. [DOI] [PubMed] [Google Scholar]
- 19.Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schröter A, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–1255. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- 20.Friel KM, Williams PT, Serradj N, Chakrabarty S, Martin JH. Activity-based therapies for repair of the corticospinal system injured during development. Front Neurol. 2014;5:229. doi: 10.3389/fneur.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang LY, Xiong XX, Zhang LY, Shen J. Neurovascular Unit: A critical role in ischemic stroke. CNS Neurosci Ther. 2021;27:7–16. doi: 10.1111/cns.13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dąbrowski J, Czajka A, Zielińska-Turek J, Jaroszyński J, Furtak-Niczyporuk M, Mela A, et al. Brain functional reserve in the context of neuroplasticity after stroke. Neural Plast. 2019;2019:9708905. doi: 10.1155/2019/9708905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joy MT, Carmichael ST. Encouraging an excitable brain state: Mechanisms of brain repair in stroke. Nat Rev Neurosci. 2021;22:38–53. doi: 10.1038/s41583-020-00396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat Rev Neurol. 2014;10:597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- 25.Buetefisch CM. Role of the contralesional hemisphere in post-stroke recovery of upper extremity motor function. Front Neurol. 2015;6:214. doi: 10.3389/fneur.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruitt DT, Danaphongse TT, Schmid AN, Morrison RA, Kilgard MP, Rennaker RL, 2nd, et al. Traumatic brain injury occludes training-dependent cortical reorganization in the contralesional hemisphere. J Neurotrauma. 2017;34:2495–2503. doi: 10.1089/neu.2016.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradnam LV, Stinear CM, Byblow WD. Ipsilateral motor pathways after stroke: Implications for non-invasive brain stimulation. Front Hum Neurosci. 2013;7:184. doi: 10.3389/fnhum.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain. 2013;136:1288–1303. doi: 10.1093/brain/aws283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sebastianelli L, Versace V, Taylor A, Brigo F, Nothdurfter W, Saltuari L, et al. Functional reorganization after hemispherectomy in humans and animal models: What can we learn about the brain's resilience to extensive unilateral lesions? Brain Res Bull. 2017;131:156–167. doi: 10.1016/j.brainresbull.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Umeda T, Takahashi M, Isa K, Isa T. Formation of descending pathways mediating cortical command to forelimb motoneurons in neonatally hemidecorticated rats. J Neurophysiol. 2010;104:1707–1716. doi: 10.1152/jn.00968.2009. [DOI] [PubMed] [Google Scholar]
- 31.Alawieh A, Tomlinson S, Adkins D, Kautz S, Feng WW. Preclinical and clinical evidence on ipsilateral corticospinal projections: Implication for motor recovery. Transl Stroke Res. 2017;8:529–540. doi: 10.1007/s12975-017-0551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosazza C, Deleo F, D'Incerti L, Antelmi L, Tringali G, Didato G, et al. Tracking the re-organization of motor functions after disconnective surgery: A longitudinal fMRI and DTI study. Front Neurol. 2018;9:400. doi: 10.3389/fneur.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen TC, Lall S, Pagnotta C, Markward J, Gupta D, Ratnadurai-Giridharan S, et al. Plasticity in one hemisphere, control from two: Adaptation in descending motor pathways after unilateral corticospinal injury in neonatal rats. Front Neural Circuits. 2018;12:28. doi: 10.3389/fncir.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen MF, Zheng BH. Axon plasticity in the mammalian central nervous system after injury. Trends Neurosci. 2014;37:583–593. doi: 10.1016/j.tins.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krucoff MO, Miller JP, Saxena T, Bellamkonda R, Rahimpour S, Harward SC, et al. Toward functional restoration of the central nervous system: A review of translational neuroscience principles. Neurosurgery. 2019;84:30–40. doi: 10.1093/neuros/nyy128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmichael ST, Kathirvelu B, Schweppe CA, Nie EH. Molecular, cellular and functional events in axonal sprouting after stroke. Exp Neurol. 2017;287:384–394. doi: 10.1016/j.expneurol.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bundy DT, Souders L, Baranyai K, Leonard L, Schalk G, Coker R, et al. Contralesional brain-computer interface control of a powered exoskeleton for motor recovery in chronic stroke survivors. Stroke. 2017;48:1908–1915. doi: 10.1161/STROKEAHA.116.016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisneski KJ, Anderson N, Schalk G, Smyth M, Moran D, Leuthardt EC. Unique cortical physiology associated with ipsilateral hand movements and neuroprosthetic implications. Stroke. 2008;39:3351–3359. doi: 10.1161/STROKEAHA.108.518175. [DOI] [PubMed] [Google Scholar]
- 39.Latchoumane CV, Barany DA, Karumbaiah L, Singh T. Neurostimulation and reach-to-grasp function recovery following acquired brain injury: Insight from pre-clinical rodent models and human applications. Front Neurol. 2020;11:835. doi: 10.3389/fneur.2020.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zareen N, Shinozaki M, Ryan D, Alexander H, Amer A, Truong DQ, et al. Motor cortex and spinal cord neuromodulation promote corticospinal tract axonal outgrowth and motor recovery after cervical contusion spinal cord injury. Exp Neurol. 2017;297:179–189. doi: 10.1016/j.expneurol.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng MX, Hua XY, Feng JT, Li T, Lu YC, Shen YD, et al. Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. N Engl J Med. 2018;378:22–34. doi: 10.1056/NEJMoa1615208. [DOI] [PubMed] [Google Scholar]
- 42.Hua XY, Qiu YQ, Wang M, Zheng MX, Li T, Shen YD, et al. Enhancement of contralesional motor control promotes locomotor recovery after unilateral brain lesion. Sci Rep. 2016;6:18784. doi: 10.1038/srep18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olivares-Moreno R, Rodriguez-Moreno P, Lopez-Virgen V, Macías M, Altamira-Camacho M, Rojas-Piloni G. Corticospinal vs rubrospinal revisited: An evolutionary perspective for sensorimotor integration. Front Neurosci. 2021;15:686481. doi: 10.3389/fnins.2021.686481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon AM, Bleyenheuft Y, Steenbergen B. Pathophysiology of impaired hand function in children with unilateral cerebral palsy. Dev Med Child Neurol. 2013;55:32–37. doi: 10.1111/dmcn.12304. [DOI] [PubMed] [Google Scholar]
- 45.Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123(Pt 1):51–64. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- 46.Martin GF, Megirian D, Conner JB. The origin, course and termination of the corticospinal tracts of the Tasmanian potoroo (Potorous apicalis) J Anat. 1972;111:263–281. [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XH, Liu YY, Li XJ, Zhang ZC, Yang HF, Zhang Y, et al. Deconstruction of corticospinal circuits for goal-directed motor skills. Cell. 2017;171:440–455.e14. doi: 10.1016/j.cell.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemon R. Recent advances in our understanding of the primate corticospinal system. F1000Res 2019, 8: F1000FacultyRev–F1000Faculty274. [DOI] [PMC free article] [PubMed]
- 49.Griffin DM, Hoffman DS, Strick PL. Corticomotoneuronal cells are “functionally tuned”. Science. 2015;350:667–670. doi: 10.1126/science.aaa8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu ZR, Kalambogias J, Yoshioka S, Han WQ, Li Z, Kawasawa YI, et al. Control of species-dependent cortico-motoneuronal connections underlying manual dexterity. Science. 2017;357:400–404. doi: 10.1126/science.aan3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isa T, Mitsuhashi M, Yamaguchi R. Alternative routes for recovery of hand functions after corticospinal tract injury in Primates and rodents. Curr Opin Neurol. 2019;32:836–843. doi: 10.1097/WCO.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 52.Javed K, Daly DT. Neuroanatomy, Lower Motor Neuron Lesion. StatPearls 2022. [PubMed]
- 53.Lohia A, McKenzie J. Neuroanatomy, pyramidal tract lesions. StatPearls 2021 [PubMed]
- 54.Emos MC, Agarwal S. Neuroanatomy, upper motor neuron lesion. StatPearls 2021. [PubMed]
- 55.Harrison TC, Silasi G, Boyd JD, Murphy TH. Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke. 2013;44:2300–2306. doi: 10.1161/STROKEAHA.113.001272. [DOI] [PubMed] [Google Scholar]
- 56.Murata Y, Higo N, Hayashi T, Nishimura Y, Sugiyama Y, Oishi T, et al. Temporal plasticity involved in recovery from manual dexterity deficit after motor cortex lesion in macaque monkeys. J Neurosci. 2015;35:84–95. doi: 10.1523/JNEUROSCI.1737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cirillo C, Brihmat N, Castel-Lacanal E, le Friec A, Barbieux-Guillot M, Raposo N, et al. Post-stroke remodeling processes in animal models and humans. J Cereb Blood Flow Metab. 2020;40:3–22. doi: 10.1177/0271678X19882788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Touvykine B, Mansoori BK, Jean-Charles L, Deffeyes J, Quessy S, Dancause N. The effect of lesion size on the organization of the ipsilesional and contralesional motor cortex. Neurorehabil Neural Repair. 2016;30:280–292. doi: 10.1177/1545968315585356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones TA. Motor compensation and its effects on neural reorganization after stroke. Nat Rev Neurosci. 2017;18:267–280. doi: 10.1038/nrn.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castro-Alamancos MA, Borrel J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 1995;68:793–805. doi: 10.1016/0306-4522(95)00178-L. [DOI] [PubMed] [Google Scholar]
- 61.Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 62.Takatsuru Y, Fukumoto D, Yoshitomo M, Nemoto T, Tsukada H, Nabekura J. Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. J Neurosci. 2009;29:10081–10086. doi: 10.1523/JNEUROSCI.1638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrington RM, Chan E, Rounds AK, Wutzke CJ, Dromerick AW, Turkeltaub PE, et al. Roles of lesioned and nonlesioned hemispheres in reaching performance poststroke. Neurorehabil Neural Repair. 2020;34:61–71. doi: 10.1177/1545968319876253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 65.Rüber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012;79:515–522. doi: 10.1212/WNL.0b013e31826356e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takenobu Y, Hayashi T, Moriwaki H, Nagatsuka K, Naritomi H, Fukuyama H. Motor recovery and microstructural change in rubro-spinal tract in subcortical stroke. Neuroimage Clin. 2013;4:201–208. doi: 10.1016/j.nicl.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishida A, Kobayashi K, Ueda Y, Shimizu T, Tajiri N, Isa T, et al. Dynamic interaction between cortico-brainstem pathways during training-induced recovery in stroke model rats. J Neurosci. 2019;39:7306–7320. doi: 10.1523/JNEUROSCI.0649-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H, Lee H, Jung KI, Ohn SH, Yoo WK. Changes in diffusion metrics of the red nucleus in chronic stroke patients with severe corticospinal tract injury: A preliminary study. Ann Rehabil Med. 2018;42:396–405. doi: 10.5535/arm.2018.42.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, et al. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- 71.Yang JP, Liu HJ, Yang H, Feng PY. Therapeutic time window for the neuroprotective effects of NGF when administered after focal cerebral ischemia. Neurol Sci. 2011;32:433–441. doi: 10.1007/s10072-011-0512-9. [DOI] [PubMed] [Google Scholar]
- 72.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young B, Runge JW, Waxman KS, Harrington T, Wilberger J, Muizelaar JP, et al. Effects of pegorgotein on neurologic outcome of patients with severe head injury. A multicenter, randomized controlled trial. JAMA. 1996;276:538–543. doi: 10.1001/jama.1996.03540070034027. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura K, Shichita T. Cellular and molecular mechanisms of sterile inflammation in ischaemic stroke. J Biochem. 2019;165:459–464. doi: 10.1093/jb/mvz017. [DOI] [PubMed] [Google Scholar]
- 75.Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation. 2017;14:99. doi: 10.1186/s12974-017-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi YH, Laaker C, Hsu M, Cismaru P, Sandor M, Fabry Z. Molecular mechanisms of neuroimmune crosstalk in the pathogenesis of stroke. Int J Mol Sci. 2021;22:9486. doi: 10.3390/ijms22179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: How brain cells Die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 79.Yan WJ, Sun W, Fan JH, Wang HQ, Han S, Li JF, et al. Sirt1-ROS-TRAF6 signaling-induced pyroptosis contributes to early injury in ischemic mice. Neurosci Bull. 2020;36:845–859. doi: 10.1007/s12264-020-00489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu X, Cui WX, Guo W, Liu HX, Luo JN, Zhao L, et al. Acrolein aggravates secondary brain injury after intracerebral hemorrhage through Drp1-mediated mitochondrial oxidative damage in mice. Neurosci Bull. 2020;36:1158–1170. doi: 10.1007/s12264-020-00505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie Q, Gu YX, Hua Y, Liu WQ, Keep RF, Xi GH. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke. 2014;45:290–292. doi: 10.1161/STROKEAHA.113.003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gu Y, Xi G, Liu W, Keep RF, Hua Y. Estrogen reduces iron-mediated brain edema and neuronal death. Acta Neurochir Suppl. 2010;106:159–162. doi: 10.1007/978-3-211-98811-4_29. [DOI] [PubMed] [Google Scholar]
- 83.Degterev A, Huang ZH, Boyce M, Li YQ, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 84.Sun HW, Tang Y, Guan XQ, Li LF, Wang DS. Effects of selective hypothermia on blood-brain barrier integrity and tight junction protein expression levels after intracerebral hemorrhage in rats. Biol Chem. 2013;394:1317–1324. doi: 10.1515/hsz-2013-0142. [DOI] [PubMed] [Google Scholar]
- 85.Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 86.Poli S, Veltkamp R. Oxygen therapy in acute ischemic stroke - experimental efficacy and molecular mechanisms. Curr Mol Med. 2009;9:227–241. doi: 10.2174/156652409787581619. [DOI] [PubMed] [Google Scholar]
- 87.Lo EH, Rosenberg GA. The neurovascular unit in health and disease: Introduction. Stroke. 2009;40:S2–S3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loane DJ, Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol. 2016;275(Pt 3):316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol. 2016;275(Pt 3):305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol. 2016;173:692–702. doi: 10.1111/bph.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zamanian JL, Xu LJ, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monson KL, Converse MI, Manley GT. Cerebral blood vessel damage in traumatic brain injury. Clin Biomech (Bristol, Avon) 2019;64:98–113. doi: 10.1016/j.clinbiomech.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Cai W, Liu H, Zhao JY, Chen LY, Chen J, Lu ZQ, et al. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res. 2017;8:107–121. doi: 10.1007/s12975-016-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Omote Y, Deguchi K, Kono S, Liu N, Liu WT, Kurata T, et al. Neurovascular protection of cilostazol in stroke-prone spontaneous hypertensive rats associated with angiogenesis and pericyte proliferation. J Neurosci Res. 2014;92:369–374. doi: 10.1002/jnr.23327. [DOI] [PubMed] [Google Scholar]
- 95.Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen B, Li Y, Yu B, Zhang ZC, Brommer B, Williams PR, et al. Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell. 2018;174:1599. doi: 10.1016/j.cell.2018.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brommer B, He M, Zhang ZC, Yang ZY, Page JC, Su JF, et al. Improving hindlimb locomotor function by Non-invasive AAV-mediated manipulations of propriospinal neurons in mice with complete spinal cord injury. Nat Commun. 2021;12:781. doi: 10.1038/s41467-021-20980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat Neurosci. 2012;15:1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- 99.Fischer I, Dulin JN, Lane MA. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat Rev Neurosci. 2020;21:366–383. doi: 10.1038/s41583-020-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piltti KM, Salazar DL, Uchida N, Cummings BJ, Anderson AJ. Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem Cells Transl Med. 2013;2:204–216. doi: 10.5966/sctm.2012-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okada S, Ishii K, Yamane J, Iwanami A, Ikegami T, Katoh H, et al. In vivo imaging of engrafted neural stem cells: Its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 2005;19:1839–1841. doi: 10.1096/fj.05-4082fje. [DOI] [PubMed] [Google Scholar]
- 102.Poplawski GHD, Kawaguchi R, van Niekerk E, Lu P, Mehta N, Canete P, et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020;581:77–82. doi: 10.1038/s41586-020-2200-5. [DOI] [PubMed] [Google Scholar]
- 103.Dulin JN, Adler AF, Kumamaru H, Poplawski GHD, Lee-Kubli C, Strobl H, et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat Commun. 2018;9:84. doi: 10.1038/s41467-017-02613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu P, Wang YZ, Graham L, McHale K, Gao MY, Wu D, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu P, Gomes-Leal W, Anil S, Dobkins G, Huie JR, Ferguson AR, et al. Origins of neural progenitor cell-derived axons projecting caudally after spinal cord injury. Stem Cell Reports. 2019;13:105–114. doi: 10.1016/j.stemcr.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ceto S, Sekiguchi KJ, Takashima Y, Nimmerjahn A, Tuszynski MH. Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after spinal cord injury. Cell Stem Cell. 2020;27:430–440.e5. doi: 10.1016/j.stem.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950.e6. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 108.Bacigaluppi M, Russo GL, Peruzzotti-Jametti L, Rossi S, Sandrone S, Butti E, et al. Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter GLT-1 in astrocytes. J Neurosci. 2016;36:10529–10544. doi: 10.1523/JNEUROSCI.1643-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumamaru H, Kadoya K, Adler AF, Takashima Y, Graham L, Coppola G, et al. Generation and post-injury integration of human spinal cord neural stem cells. Nat Methods. 2018;15:723–731. doi: 10.1038/s41592-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 110.Tashiro S, Nishimura S, Iwai H, Sugai K, Zhang L, Shinozaki M, et al. Functional recovery from neural stem/progenitor cell transplantation combined with treadmill training in mice with chronic spinal cord injury. Sci Rep. 2016;6:30898. doi: 10.1038/srep30898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kawai M, Imaizumi K, Ishikawa M, Shibata S, Shinozaki M, Shibata T, et al. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 2021;37:110019. doi: 10.1016/j.celrep.2021.110019. [DOI] [PubMed] [Google Scholar]
- 112.Tsintou M, Dalamagkas K, Makris N. Taking central nervous system regenerative therapies to the clinic: Curing rodents versus nonhuman Primates versus humans. Neural Regen Res. 2020;15:425–437. doi: 10.4103/1673-5374.266048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brown-Sequard The brain power of man. Am J Dent Sci. 1874;8:82–87. [PMC free article] [PubMed] [Google Scholar]
- 114.Eyre JA. Development and plasticity of the corticospinal system in man. Neural Plast. 2003;10:93–106. doi: 10.1155/NP.2003.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bundy DT, Leuthardt EC. The cortical physiology of ipsilateral limb movements. Trends Neurosci. 2019;42:825–839. doi: 10.1016/j.tins.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hübener M, Bonhoeffer T. Neuronal plasticity: Beyond the critical period. Cell. 2014;159:727–737. doi: 10.1016/j.cell.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 118.Umeda T, Funakoshi K. Reorganization of motor circuits after neonatal hemidecortication. Neurosci Res. 2014;78:30–37. doi: 10.1016/j.neures.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 119.McGovern RA, Moosa ANV, Jehi L, Busch R, Ferguson L, Gupta A, et al. Hemispherectomy in adults and adolescents: Seizure and functional outcomes in 47 patients. Epilepsia. 2019;60:2416–2427. doi: 10.1111/epi.16378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gennaro M, Mattiello A, Mazziotti R, Antonelli C, Gherardini L, Guzzetta A, et al. Focal stroke in the developing rat motor cortex induces age- and experience-dependent maladaptive plasticity of corticospinal system. Front Neural Circuits. 2017;11:47. doi: 10.3389/fncir.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Juenger H, Koerte IK, Muehlmann M, Mayinger M, Mall V, Krägeloh-Mann I, et al. Microstructure of transcallosal motor fibers reflects type of cortical (re-) organization in congenital hemiparesis. Eur J Paediatr Neurol. 2014;18:691–697. doi: 10.1016/j.ejpn.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 122.Williams PTJA, Jiang YQ, Martin JH. Motor system plasticity after unilateral injury in the developing brain. Dev Med Child Neurol. 2017;59:1224–1229. doi: 10.1111/dmcn.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fink KL, Cafferty WBJ. Reorganization of intact descending motor circuits to replace lost connections after injury. Neurotherapeutics. 2016;13:370–381. doi: 10.1007/s13311-016-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Z'Graggen WJ, Metz GA, Kartje GL, Thallmair M, Schwab ME. Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: A TMS and fMRI study. Brain. 2002;125:2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- 126.Carmel JB, Kimura H, Martin JH. Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control. J Neurosci. 2014;34:462–466. doi: 10.1523/JNEUROSCI.3315-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sawada M, Kato K, Kunieda T, Mikuni N, Miyamoto S, Onoe H, et al. Function of the nucleus accumbens in motor control during recovery after spinal cord injury. Science. 2015;350:98–101. doi: 10.1126/science.aab3825. [DOI] [PubMed] [Google Scholar]
- 128.Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.STR.31.3.656. [DOI] [PubMed] [Google Scholar]
- 129.Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31:1136–1149. doi: 10.1016/j.neubiorev.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 131.He ZG. Intrinsic control of axon regeneration. J Biomed Res. 2010;24:2–5. doi: 10.1016/S1674-8301(10)60002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19:323–337. doi: 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quraishe S, Forbes LH, Andrews MR. The extracellular environment of the CNS: Influence on plasticity, sprouting, and axonal regeneration after spinal cord injury. Neural Plast. 2018;2018:2952386. doi: 10.1155/2018/2952386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee JK, Chan AF, Luu SM, Zhu YH, Ho C, Tessier-Lavigne M, et al. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci. 2009;29:8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ueno R, Takase H, Suenaga J, Kishimoto M, Kurihara Y, Takei K, et al. Axonal regeneration and functional recovery driven by endogenous Nogo receptor antagonist LOTUS in a rat model of unilateral pyramidotomy. Exp Neurol. 2020;323:113068. doi: 10.1016/j.expneurol.2019.113068. [DOI] [PubMed] [Google Scholar]
- 138.Tedeschi A, Bradke F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr Opin Neurobiol. 2017;42:118–127. doi: 10.1016/j.conb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 139.Zhang YL, Williams PR, Jacobi A, Wang C, Goel A, Hirano AA, et al. Elevating growth factor responsiveness and axon regeneration by modulating presynaptic inputs. Neuron. 2019;103:39–51.e5. doi: 10.1016/j.neuron.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nawabi H, Belin S, Cartoni R, Williams PR, Wang C, Latremolière A, et al. Doublecortin-like kinases promote neuronal survival and induce growth cone reformation via distinct mechanisms. Neuron. 2015;88:704–719. doi: 10.1016/j.neuron.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rishal I, Fainzilber M. Axon-soma communication in neuronal injury. Nat Rev Neurosci. 2014;15:32–42. doi: 10.1038/nrn3609. [DOI] [PubMed] [Google Scholar]
- 142.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li F, Lo TY, Miles L, Wang Q, Noristani HN, Li D, et al. The Atr-Chek1 pathway inhibits axon regeneration in response to Piezo-dependent mechanosensation. Nat Commun. 2021;12:3845. doi: 10.1038/s41467-021-24131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu YY, Wang XH, Li WL, Zhang Q, Li Y, Zhang ZC, et al. A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron. 2017;95:817–833.e4. doi: 10.1016/j.neuron.2017.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hollis ER, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, et al. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci. 2016;19:697–705. doi: 10.1038/nn.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stern S, Hilton BJ, Burnside ER, Dupraz S, Handley EE, Gonyer JM, et al. RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron. 2021;109:3436–3455.e9. doi: 10.1016/j.neuron.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 147.Caracciolo L, Marosi M, Mazzitelli J, Latifi S, Sano Y, Galvan L, et al. CREB controls cortical circuit plasticity and functional recovery after stroke. Nat Commun. 2018;9:2250. doi: 10.1038/s41467-018-04445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lim JHA, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, et al. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci. 2016;19:1073–1084. doi: 10.1038/nn.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jin D, Liu YY, Sun F, Wang XH, Liu XF, He ZG. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat Commun. 2015;6:8074. doi: 10.1038/ncomms9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zukor K, Belin S, Wang C, Keelan N, Wang XH, He ZG. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci. 2013;33:15350–15361. doi: 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blackmore MG, Wang ZM, Lerch JK, Motti D, Zhang YP, Shields CB, et al. Krüppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci USA. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kulbatski I, Cook DJ, Tator CH. Calcium entry through L-type calcium channels is essential for neurite regeneration in cultured sympathetic neurons. J Neurotrauma. 2004;21:357–374. doi: 10.1089/089771504322972130. [DOI] [PubMed] [Google Scholar]
- 154.Chierzi S, Ratto GM, Verma P, Fawcett JW. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur J Neurosci. 2005;21:2051–2062. doi: 10.1111/j.1460-9568.2005.04066.x. [DOI] [PubMed] [Google Scholar]
- 155.Enes J, Langwieser N, Ruschel J, Carballosa-Gonzalez MM, Klug A, Traut MH, et al. Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr Biol. 2010;20:1154–1164. doi: 10.1016/j.cub.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 156.Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, et al. The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron. 2016;92:419–434. doi: 10.1016/j.neuron.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 157.Dorrier CE, Jones HE, Pintarić L, Siegenthaler JA, Daneman R. Emerging roles for CNS fibroblasts in health, injury and disease. Nat Rev Neurosci. 2022;23:23–34. doi: 10.1038/s41583-021-00525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 159.Dias DO, Kim H, Holl D, Werne Solnestam B, Lundeberg J, Carlén M, et al. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165.e22. doi: 10.1016/j.cell.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Anderson MA, Burda JE, Ren YL, Ao Y, O'Shea TM, Kawaguchi R, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med. 2017;23:818–828. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 162.Qian CH, Tan DD, Wang XH, Li LX, Wen JK, Pan MJ, et al. Peripheral nerve injury-induced astrocyte activation in spinal ventral horn contributes to nerve regeneration. Neural Plast. 2018;2018:8561704. doi: 10.1155/2018/8561704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Müller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- 164.Fischer D, Hauk TG, Müller A, Thanos S. Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci. 2008;37:471–479. doi: 10.1016/j.mcn.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 165.Bellver-Landete V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle ME, et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun. 2019;10:518. doi: 10.1038/s41467-019-08446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fu HT, Zhao YP, Hu D, Wang S, Yu TB, Zhang LC. Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death Dis. 2020;11:528. doi: 10.1038/s41419-020-2733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou X, Wahane S, Friedl MS, Kluge M, Friedel CC, Avrampou K, et al. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat Neurosci. 2020;23:337–350. doi: 10.1038/s41593-020-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Y, He XL, Kawaguchi R, Zhang Y, Wang Q, Monavarfeshani A, et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature. 2020;587:613–618. doi: 10.1038/s41586-020-2795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tran AP, Warren PM, Silver J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022;387:319–336. doi: 10.1007/s00441-021-03477-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bei FF, Lee HHC, Liu XF, Gunner G, Jin H, Ma L, et al. Restoration of visual function by enhancing conduction in regenerated axons. Cell. 2016;164:219–232. doi: 10.1016/j.cell.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Merzenich MM, van Vleet TM, Nahum M. Brain plasticity-based therapeutics. Front Hum Neurosci. 2014;8:385. doi: 10.3389/fnhum.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sankarasubramanian V, Machado AG, Conforto AB, Potter-Baker KA, Cunningham DA, Varnerin NM, et al. Inhibition versus facilitation of contralesional motor cortices in stroke: Deriving a model to tailor brain stimulation. Clin Neurophysiol. 2017;128:892–902. doi: 10.1016/j.clinph.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bachmann LC, Lindau NT, Felder P, Schwab ME. Sprouting of brainstem-spinal tracts in response to unilateral motor cortex stroke in mice. J Neurosci. 2014;34:3378–3389. doi: 10.1523/JNEUROSCI.4384-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karbasforoushan H, Cohen-Adad J, Dewald JPA. Brainstem and spinal cord MRI identifies altered sensorimotor pathways post-stroke. Nat Commun. 2019;10:3524. doi: 10.1038/s41467-019-11244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhong GL, Yang ZY, Jiang TZ. Precise modulation strategies for transcranial magnetic stimulation: Advances and future directions. Neurosci Bull. 2021;37:1718–1734. doi: 10.1007/s12264-021-00781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gordon PC, Zrenner C, Desideri D, Belardinelli P, Zrenner B, Brunoni AR, et al. Modulation of cortical responses by transcranial direct current stimulation of dorsolateral prefrontal cortex: A resting-state EEG and TMS-EEG study. Brain Stimul. 2018;11:1024–1032. doi: 10.1016/j.brs.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 177.Kundu B, Brock AA, Englot DJ, Butson CR, Rolston JD. Deep brain stimulation for the treatment of disorders of consciousness and cognition in traumatic brain injury patients: A review. Neurosurg Focus. 2018;45:E14. doi: 10.3171/2018.5.FOCUS18168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schiff ND. Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 180.Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, II, et al. Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul. 2016;9:174–181. doi: 10.1016/j.brs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, 2nd, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014;45:3097–3100. doi: 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Meyers EC, Kasliwal N, Solorzano BR, Lai E, Bendale G, Berry A, et al. Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat Commun. 2019;10:5782. doi: 10.1038/s41467-019-13695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen YS, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen YS, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379:1244–1250. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- 185.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24:1677–1682. doi: 10.1038/s41591-018-0175-7. [DOI] [PubMed] [Google Scholar]
- 187.Wagner FB, Mignardot JB, le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 188.Rowald A, Komi S, Demesmaeker R, Baaklini E, Hernandez-Charpak SD, Paoles E, et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat Med. 2022;28:260–271. doi: 10.1038/s41591-021-01663-5. [DOI] [PubMed] [Google Scholar]
- 189.Ji XR, Tang PF, Wang S, Zhang LC, Fu HT, Deng JH, et al. Epidural electrical stimulation effectively restores locomotion function in rats with complete spinal cord injury. Neural Regen Res. 2021;16:573. doi: 10.4103/1673-5374.290905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shih JJ, Krusienski DJ, Wolpaw JR. Brain-computer interfaces in medicine. Mayo Clin Proc. 2012;87:268–279. doi: 10.1016/j.mayocp.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mrachacz-Kersting N, Stevenson AJT, Jørgensen HRM, Severinsen KE, Aliakbaryhosseinabadi S, Jiang N, et al. Brain state-dependent stimulation boosts functional recovery following stroke. Ann Neurol. 2019;85:84–95. doi: 10.1002/ana.25375. [DOI] [PubMed] [Google Scholar]
- 192.Flesher SN, Downey JE, Weiss JM, Hughes CL, Herrera AJ, Tyler-Kabara EC, et al. A brain-computer interface that evokes tactile sensations improves robotic arm control. Science. 2021;372:831–836. doi: 10.1126/science.abd0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Berlot E, Prichard G, O'Reilly J, Ejaz N, Diedrichsen J. Ipsilateral finger representations in the sensorimotor cortex are driven by active movement processes, not passive sensory input. J Neurophysiol. 2019;121:418–426. doi: 10.1152/jn.00439.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Barany DA, Revill KP, Caliban A, Vernon I, Shukla A, Sathian K, et al. Primary motor cortical activity during unimanual movements with increasing demand on precision. J Neurophysiol. 2020;124:728–739. doi: 10.1152/jn.00546.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Crago PE. Neuromodulation by combined sensory and motor stimulation in the peripheral nerve: Tendon organ afferent activity. J Neural Eng. 2019;16:016015. doi: 10.1088/1741-2552/aaeaa9. [DOI] [PubMed] [Google Scholar]
- 196.Wilson RD, Page SJ, Delahanty M, Knutson JS, Gunzler DD, Sheffler LR, et al. Upper-limb recovery after stroke: A randomized controlled trial comparing EMG-triggered, cyclic, and sensory electrical stimulation. Neurorehabil Neural Repair. 2016;30:978–987. doi: 10.1177/1545968316650278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9:2421. doi: 10.1038/s41467-018-04673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ganzer PD, Colachis SC, 4th, Schwemmer MA, Friedenberg DA, Dunlap CF, Swiftney CE, et al. Restoring the sense of touch using a sensorimotor demultiplexing neural interface. Cell. 2020;181:763–773.e12. doi: 10.1016/j.cell.2020.03.054. [DOI] [PubMed] [Google Scholar]
- 199.Guggenmos DJ, Azin M, Barbay S, Mahnken JD, Dunham C, Mohseni P, et al. Restoration of function after brain damage using a neural prosthesis. Proc Natl Acad Sci USA. 2013;110:21177–21182. doi: 10.1073/pnas.1316885110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gesslbauer B, Hruby LA, Roche AD, Farina D, Blumer R, Aszmann OC. Axonal components of nerves innervating the human arm. Ann Neurol. 2017;82:396–408. doi: 10.1002/ana.25018. [DOI] [PubMed] [Google Scholar]
- 201.Gu YD, Zhang GM, Chen DS, Yan JG, Cheng XM, Chen L. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg Br. 1992;17:518–521. doi: 10.1016/S0266-7681(05)80235-9. [DOI] [PubMed] [Google Scholar]
- 202.Terzis JK, Kokkalis ZT. Selective contralateral c7 transfer in posttraumatic brachial plexus injuries: A report of 56 cases. Plast Reconstr Surg. 2009;123:927–938. doi: 10.1097/PRS.0b013e31819ba48a. [DOI] [PubMed] [Google Scholar]
- 203.Gu YD, Chen DS, Zhang GM, Cheng XM, Xu JG, Zhang LY, et al. Long-term functional results of contralateral C7 transfer. J Reconstr Microsurg. 1998;14:57–59. doi: 10.1055/s-2007-1006902. [DOI] [PubMed] [Google Scholar]
- 204.Gu YD, Xu JG, Chen L, Wang H, Hu SN. Long term outcome of contralateral C7 transfer: A report of 32 cases. Chin Med J (Engl) 2002;115:866–868. [PubMed] [Google Scholar]
- 205.Zandvliet SB, Kwakkel G, Nijland RHM, van Wegen EEH, Meskers CGM. Is recovery of somatosensory impairment conditional for upper-limb motor recovery early after stroke? Neurorehabil Neural Repair. 2020;34:403–416. doi: 10.1177/1545968320907075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chen XW, Liu FQ, Yan ZH, Cheng SH, Liu XC, Li H, et al. Therapeutic effects of sensory input training on motor function rehabilitation after stroke. Medicine (Baltimore) 2018;97:e13387. doi: 10.1097/MD.0000000000013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Edwards LL, King EM, Buetefisch CM, Borich MR. Putting the “sensory” into sensorimotor control: The role of sensorimotor integration in goal-directed hand movements after stroke. Front Integr Neurosci. 2019;13:16. doi: 10.3389/fnint.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Heming E, Sanden A, Kiss ZH. Designing a somatosensory neural prosthesis: Percepts evoked by different patterns of thalamic stimulation. J Neural Eng. 2010;7:064001. doi: 10.1088/1741-2560/7/6/064001. [DOI] [PubMed] [Google Scholar]
- 209.Tyler DJ. Neural interfaces for somatosensory feedback: Bringing life to a prosthesis. Curr Opin Neurol. 2015;28:574–581. doi: 10.1097/WCO.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pillette L, Lotte F, N'Kaoua B, Joseph PA, Jeunet C, Glize B. Why we should systematically assess, control and report somatosensory impairments in BCI-based motor rehabilitation after stroke studies. Neuroimage Clin. 2020;28:102417. doi: 10.1016/j.nicl.2020.102417. [DOI] [PMC free article] [PubMed] [Google Scholar]