Abstract
Japanese quails in wild life live in small groups with females being even solitary during the laying period. Although it is a poultry species widely used for egg production, information regarding laying behavior motivations or influencing variables is scarce. Our study focuses on evaluating along 7 d the quail laying behavior in a novel environmental set up. This set up allows the female to choose between remaining separated from a conspecific in one side of the apparatus or to voluntarily enter their space (box-mate side) and interact with it. We evaluated whether the female insemination status prior to enter the environmental set up, and the presence of a female or a male partner in the box-mate side can influence their laying and social behavior. Thus, 4 experimental groups were established. Females spent a higher (P < 0.05) percentage of time in the box-mate side than in their separated sector in all groups. In 3 of the 4 experimental groups (non-inseminated females interacting with a female or a male box-mate, and inseminated females interacting with a male box-mate) females also laid a greater percentage (≥65%, P < 0.05, in all cases) of eggs in the box-mate sector than in their separated sector. However, the group of inseminated females that interacted with a female box-mate shifted their egg distribution and laid equally between both sides of the apparatus. Aggressive social interactions were reduced (P < 0.05) throughout the testing days but this was depending upon the female insemination status and the sex of their box-mate. Findings suggest that females can change their laying side choice when they are inseminated but depending on the sex identity of their box-mate partners. Thus, providing quail female breeders with the option of laying their eggs in separated enclosures from conspecifics could be key to favor their well-being.
Key words: Japanese quail, egg laying behavior, behavioral need
INTRODUCTION
In nature, the nest sites are chosen by birds to avoid exposure to predation and other environmental adversities while nesting. For example, most species have evolved the strategy of building special structures in “hidden” environments (Mainwaring et al., 2014). Thus, the selection of nesting sites appears governed by the need for adequate support for the nests and the need for birds´ protection. Therefore, biotic and abiotic influences in nest site selection are complex and often confounded, since the attainment of one of them often make the other also present (Martin, 2001); one of the most common examples in birds is the non-random distribution of nests in dense vegetation.
Egg-laying behavior is also influenced by intraspecific social relationships. Because nesting sites can be considered a limited resource, new competitive interactions can arise between females (Rosvall, 2011). Indeed, several studies have suggested that female aggressive behavior may provide a competitive advantage when breeding or mating opportunities are scarce. For example, increased aggression has been observed in front and inside nests when multiple hens are motivated to use that area (Meijsser and Hughes, 1989; Appleby and Hughes, 1991). Competition among females has been linked to benefits for the winner female, and is related on one hand with the availability of resources and partners, and, on the other, with the direct or indirect benefits provided by those possible partners in regards to their offspring success (Rosvall, 2011). Additionally, the type of interaction that is established between females and males (usually sexual or parental interactions) is also a remarkable aspect to consider when evaluating egg-laying behavior. There are studies reporting that male mating related behaviors and/or the changes induced by the female insemination can trigger hormonal changes (i.e., estradiol, corticosterone and progesterone) that help explain the significant variability in both their maternal physiology and reproductive investment (Correa et al., 2011; Rutkowska et al., 2011). Because changes in circulating androgens have positive competition, nest acquisition and parental behaviors (Langmore et al., 2002; Cain and Ketterson, 2013), it is also possible that female social behavior will be modified not only in the presence of a female but also a male.
Because of the intense selective breeding aimed to improve egg production, nowadays these poultry species appear adapted to lay eggs under conditions that a priori would not be found in wildlife. For example, laying the eggs in extremely small environments shared with several conspecifics and even without any material to build a nest structure. Thus, the chosen nesting sites during intense farming could be the preferred site, but it could also be the less avoided one, or the only site available to complete a physiological process and/or fulfil their behavioral needs. It is also used to be thought that hens raised under commercial conditions (protected from predators, extreme weather conditions and hunger) do not have the need to perform behaviors that promote offspring survival. However, a large body of evidence has shown the importance of females being able to perform those behaviors for their well-being (Keeling, 2004). Nevertheless, it is clear that egg laying behavior under captive breeding is not fully understood. Specifically, in quails, no information is available regarding whether the female insemination status or the presence of a male or another female in the close environment can affect differentially the laying behavior motivations or the laying site selection. Understanding the influence of those factors could be highly relevant to improve both their management and welfare.
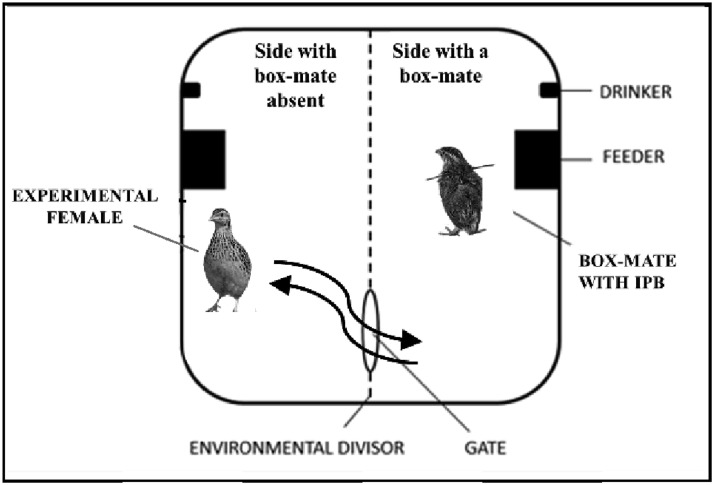
As a first approach to the aforementioned topic, this study focuses on evaluating the laying behavior of female quail in a novel environmental set up using individual physical barrier devices (IPB) (Pellegrini et al., 2015). This device allows an experimental bird to choose between remaining partially separated from a conspecific or to enter their “space” with the possibility of also engaging in physical interactions in that “shared” environment. Specifically, the test apparatus is divided in 2 sectors by a wire mesh that holds an open gate in it (Figure 1). One sector (box-mate sector) holds an unknown conspecific (either a male or a female) that is fitted with an IPB that precludes them from escaping from that area by crossing through the open gate. The other sector initially holds an experimental female (separated sector) that is able to freely ambulate in the apparatus and get access (through the open gate) to the box-mate sector. The present experimental setup enabled us to address 4 main questions: (1) were the Japanese quails able to differentially use the 2 environmental sides (shared with the box-mate or separated from it)? (2) Did the quails show preferences for laying their eggs in one of the 2 environments? (3) Did the female insemination status have an influence on the selection of the laying site and/or their social behavior? And, (4) was the selection of the laying site differentially influenced by the presence of a male or a female box-mate?
Figure 1.
Representative diagram of the experimental box, divided into 2 environments by a wire divisor with a circular gate. Box-mate animals with IPB cannot cross through while experimental females (without IPB) can freely ambulate from one to the other side of the box. Abbreviation: IPB, individual physical barrier.
MATERIALS AND METHODS
Animals and Breeding Conditions
This study was conducted with 148 Japanese quail (Coturnix japonica) taken from a larger population of animals obtained from 3 incubation series (3 batches). Ninety birds were used during behavioral testing (Testing apparatus and behavioral records Section) and the rest were used during initial visits to obtain inseminated vs. non-inseminated females (Experimental female treatments Section). At hatch, chicks were leg-banded (Red bird products, Inc. #35YN) to maintain individual identification and housed in mixed-sex groups of about 30 birds, in white wooden rearing boxes (90 × 90 × 60 cm, length × width × high, respectively). Each box had a feeder covering the entire front of the box and 16 automatic nipple drinkers. A wire-mesh floor (1 cm grid) was raised 5 cm to allow the passage of excreta. On the floor, pieces of 2 sheet corrugated fibreboard (linerboard plus corrugated board facing upwards) were also provided to facilitate walking and scratching behaviors. A lid was placed on top to prevent the birds from escaping. Brooding temperature was 37.5°C during the first week of life, with a weekly decline of 3.0°C until room temperature (24 ± 2°C) was achieved. Starter feed (Marcelo E. Hoffman e Hijos S.A., Entre Ríos, Argentina) and water were provided ad libitum. Quails were subjected to a daily cycle of 14 h light (250–300 lux): 10 h dark during the whole study.
When quails reached 28 d of age, they were sexed by plumage coloration and leg bands were replaced (Red bird products, Inc. #FCN1, Mount Aukum, CA). Depending on their role in the experiment and to avoid isolation, all birds were housed in pairs of either 2 females (Experimental and Female Box-mates groups) or 1 male and 1 female (Male-Box-mates). At this time, birds were switched to a laying feed, with plain water provision ad libitum. Laying feed (Marcelo E. Hoffman e Hijos S.A., Entre Ríos, Argentina) was provided to meet the minimum nutritional requirement of the quails. Environment temperature was kept at 24 ± 2°C. Maintenance and feeding chores were done every day at the same time (09:00 h).
Experimental Female Treatments
At 11 wk of age, when all females had peak egg-laying, half of the experimental females (n = 18; 6 per batch) received short (2 h) visits on alternate days from a male (different males on each visit) during weeks 11 and 12, and then received same scheduled visits from a female (also a different female on each visit) during weeks 13 and 14. The other half were submitted to the same procedure but receiving the visits of females in the first 2 wk and males in the last 2 wk. At the end of the 4 wk, all experimental females had experienced social interactions with both females and males. During male to female visits, we observed their behavior and ensured that all the experimental females were involved in copulation sequences with males (sexually experienced). The experimental quails receiving visits from males during weeks 13 and 14 were considered “inseminated” and able to lay fertilized eggs, while the females receiving female visits on those weeks were considered “non-inseminated” and therefore, laying non-fertilized eggs (Sittmann and Abplanalp, 1965; Birkhead and Fletcher, 1994). After experiment was finished birds remained in the quail facility as breeders.
Box-Mate Animals
At 13 wk of age, birds participating as box-mates (see below) were randomly assigned to one experimental female and provided with an IPB device that remained fitted on their back for at least 7 d prior testing to facilitate habituation. A full IPB description and its usefulness for social behavioral studies were published by Pellegrini et al. (2015). Being fitted with the IPB allows us to ensure that those birds were restricted to only one area of their testing boxes. It is important to recall that box-mate females were housed with a female partner and therefore at the time of the experiment they were all non-inseminated (laying infertile eggs).
Testing Apparatus and Behavioral Records
A white wooden box, 40 cm × 45 cm × 40 cm (width × length × height, respectively) was used (Figure 1) as test box. The box was divided into 2 compartments by a wire partition (environmental divisor) with a circular hole as a gate through which the animals with an IPB fitted could not cross (box-mate animals), while animals with no IPB fitted (experimental females) could move freely throughout the 2 environmental compartments. During the week before the testing, the experimental females were placed for about 2 h in the test apparatus to ensure all females were able to explore both environments by crossing through the gate.
The test-box contained one side where the experimental female would enter and “share” with the box-mate and the opposite side where it could remain separated. The test began when the box-mate is placed on one side and the experimental female is placed on the other side of the box (Figure 1). The box activity was video recorded continuously during 7 d (day and night) using cameras placed 2 m above the boxes and fitted with infrared vision for night recording. Eggs laid during this experimental week were not removed.
A total of 36 experimental females (12 per batch) were evaluated along the 7 d starting when they were 15 wk of age. Six inseminated and 6 non-inseminated females per batch were simultaneously tested (half of them with a male box-mate and the other half with a female box-mate). Thus, 4 experimental groups with 9 replicates each were defined: Inseminated females with a male box-mate; Inseminated females with a female box-mate; non-inseminated females with a male box-mate and non-inseminated females with a female box-mate.
Video Analysis
To register behaviors we used the ANY-maze tracking software interface that allows manual registration of behaviors by using the computer keyboard while the video recording is played (ANY-maze, 2018). Twenty-four hours of video recording were analyzed on days 1, 4, and 7 of the study, aiming to cover since the beginning of the social interactions until interactions to establish dominance should be significantly reduced (Vaisanen et al., 2005).
Egg-Related Variables and General Behaviors
In the case of the experimental females housed with a female box-mate, the eggs from each female were identified according to their eggshell stain pattern. We registered:
-
•
N° of total eggs
-
•
N° of broken eggs
-
•
Percentage of eggs laid in each box side
-
•
Percentage of time spent in each box side
-
•
Number of times the experimental female entered the box-mate side
-
•
N° of aggressive behaviors (pecks and chases) the experimental female performed (given aggressions) towards the box-mate (either a male or a female) or were received from the box-mate.
-
•
Number of mating attempts: sum of grabs and mounts received by the female when interacting with a male.
-
•
Number of effective matings: number of cloacal contacts observed when interacting with a male.
-
•
For a more detailed description of the behavioral variables, please see Pellegrini et al. (2019).
Statistical Analysis
Differences in total number of eggs, number of broken eggs, number of visits from experimental females to the box-mate side, and total aggressive behaviors, matings attempts and effective matings were determined using Generalized Linear Mixed Model. A Poisson error distribution with a log-link function was used on the mentioned variables. A 2 × 2 experimental design was used with experimental female status (inseminated or non-inseminated) and box-mate sex (male or female) included as main (fixed) effects. For aggressive and mating related behavioral variables, testing day (1, 4, or 7, repeated factor) was also included in the analysis as a main effect. Testing box and batch were included as random effects. Differences in percentage of eggs laid in each side of the box and percentage of time in each box side were analyzed using General Linear Models. The experimental female condition and box-mate sex were included as main (fixed) effects. The box side and days were included in the model as non-independent factors. Testing box was also included as a random effect. All models were fitted using the nlme, glm, and glmer R library through a user-friendly interface implemented in InfoStat software (InfoStat. et al., 2016). Whenever significant main effects were detected, Fisher post-hoc test (alpha = 0.05) was used to compare the means.
A proportion test was used to determine whether a bird has preferences for laying eggs in one of the 2 sides of the box (separated or “shared” with a box-mate). A P < 0.05 was considered indicative of significant differences.
RESULTS
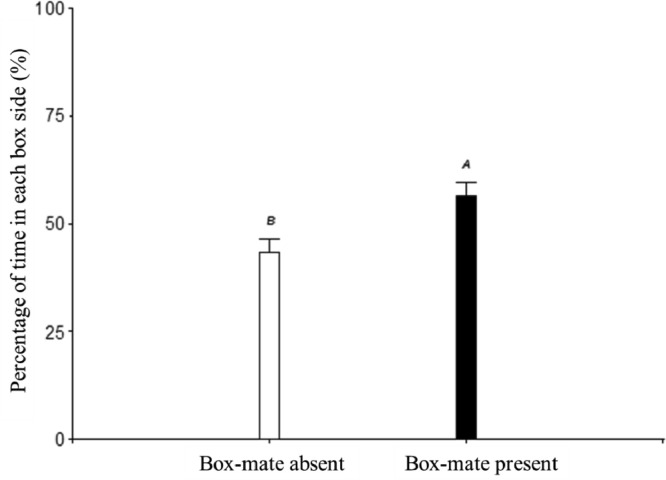
Figure 2 shows the percentage of time spent by experimental females on each side of the box. A main effect of the box side (either with or without a box-mate) was observed in the percentage of time spent by the females in the apparatus (P = 0.01). No effect of the box-mate sex or interaction between the female insemination status and the testing day (P ≥ 0.16, in all cases). Females spent more time in the side containing a box-mate than in the separated side.
Figure 2.
Percentage of time spent by experimental females in the side without a box mate (box-mate absent) or in the side “shared” with the box-mate (either a male or a female; Box-mate present), during the 3 d of registration. A-BDifferent letters indicate differences between groups (P ≤ 0.05).
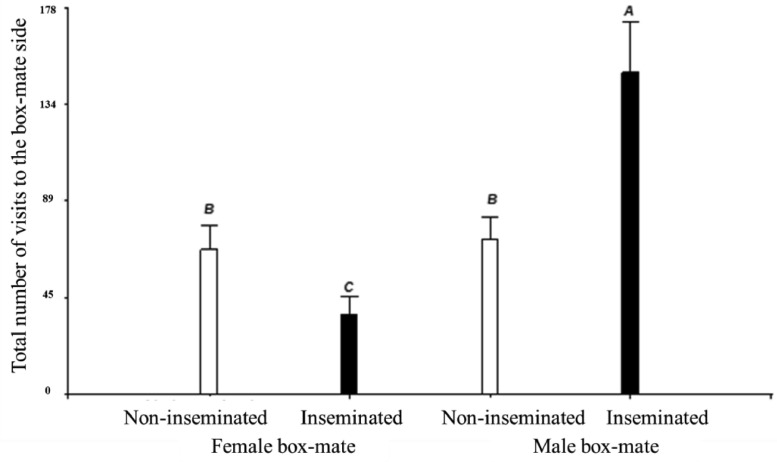
The number of times the experimental female visited the box-mate side is shown in Figure 3. A significant interaction (P = 0.0003) between the effects of the experimental female condition and the box-mate sex was found. The inseminated females tested with a male box-mate visited more frequently the male side than those females that were tested with a female box-mate. On the other hand, the females that were non-inseminated visited a similar amount of time the box-mate side regardless of whether they hold a male or a female box-mate.
Figure 3.
Number of visits made by the experimental females to the box-mate side of the apparatus along the 3 d of behavior registrations. A-CDifferent letters indicate differences between groups (P ≤ 0.05). White bar: female non-inseminated. Black bar: inseminated female.
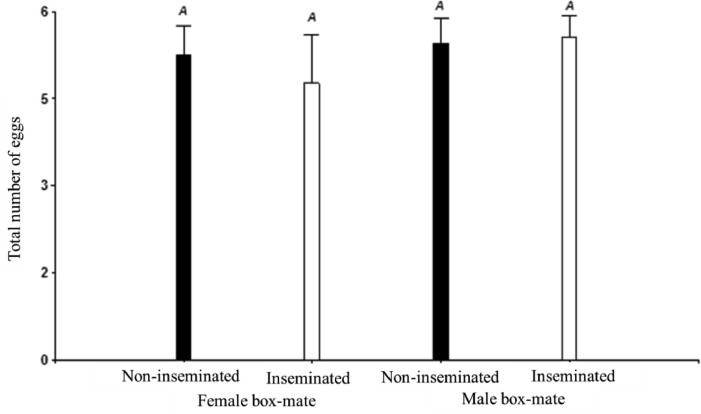
The total number of eggs laid by the experimental females is shown in Figure 4. No differences were detected between treatments (P = 0.99) and no differences were found on the number of broken eggs (∼1) that were found at the end of the 7 d of testing (P = 0.72).
Figure 4.
Number of eggs laid in each experimental treatment. Black bars: experimental females non-inseminated; White bars: experimental females inseminated.
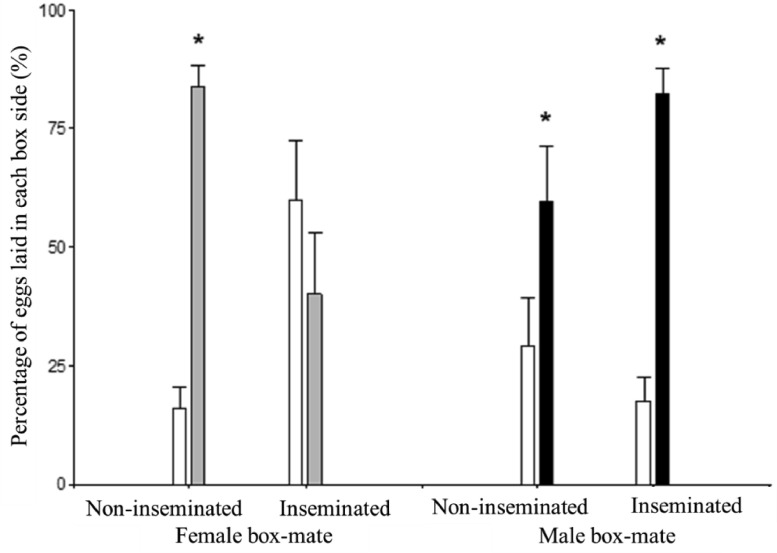
The percentage of eggs laid in each side of the box is shown in Figure 5. A main interaction between the experimental female condition and the box-mate sex was found (P < 0.0001). Post-hoc showed that in 3 of the 4 experimental groups (non-inseminated females that interacted with a female or a male box-mate, and the inseminated females that interacted with a male box-mate) the females laid a greater (P < 0.05) percentage of their eggs in the box-mate sector of the apparatus than in the side where the box-mate was absent. However, the group of females that entered the trial being inseminated, when interacted with a female box-mate distributed their eggs similarly between the box-mate sector “shared” with the female and the side where the female box-mate was absent.
Figure 5.
Percentage of eggs laid in each experimental box side by inseminated and non-inseminated females that were housed during 7 d either with a female or a male box-mate. The asterisks indicate differences (P ≤ 0.05) between the percentages of eggs laid in one side of the box related to the other side of the same box. White bar: box side where the box-mate was absent. Gray bar: box side “shared” with female box-mate. Black bar: box side “shared” with male box-mate.
The proportion of females within each experimental condition laying their eggs on one or the other side of the experimental apparatus was also evaluated. A higher proportion of non-inseminated females (tested either with a female or a male box-mate), and inseminated females but tested with a male box-mate laid the majority of their eggs within the box-mate side (P = 0.0001, P = 0.0092, and P = 0.0001, respectively). On the other hand, 5 out of 9 inseminated females that were tested with a female box-mate showed a the opposite behavior laying the majority of their eggs within the side of the box where they could remain separated from their female box-mate counterpart.
The aggressions given and received by experimental females are shown in Figure 6. A significant interaction was detected between treatments (P < 0.0001). Post-hoc showed that on the first day of testing, non-inseminated females that were tested with a female box-mate behaved more aggressively than their box-mate counterparts. However, on days 4 and 7 of the study, the aggressive level between the experimental female and the female box-mate was balanced. On the other hand, when the inseminated females were tested with a female box-mate (non-inseminated), they were similarly aggressive on the first day of testing. Then, on days 4 and 7, the inseminated females remained performing a high level of aggressions while their female box-mates practically stopped performing aggressive behaviors. Interestingly, non-inseminated females tested with a male box-mate, showed on day 1 a similar aggressiveness than their male box-mate. However, on days 4 and 7, while the aggressiveness of the male remained similarly high, the aggressions performed by the experimental females markedly decreased compared to the first day. Finally, when inseminated females were tested with a male box-mate, initially they were significantly more aggressive than their male box-mate and then, on days 4 and 7 of testing, they drastically decreased their aggressive behaviors while their male box-mate remained showing similar levels of aggressions than on the first day of testing.
Figure 6.
Number of aggressive behaviors (pecks and chases) the experimental female performed (given aggressions) toward the box-mate (either a male or a female) or were received from the box-mate. Behaviors were recorded on days 1, 4, and 7 of the testing. A-LDifferent letters indicate differences between groups (P ≤ 0.05).
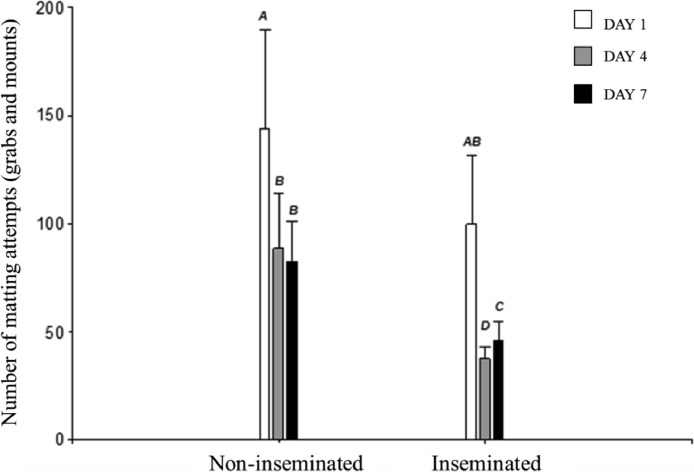
Regarding sexual behaviors, an interaction between the female insemination status when entering the test box and the testing days was found on the number of mating attempts (P < 0.0001) (Figure 7). Both, the inseminated and the non-inseminated females received a higher number of grabs and mounts on day 1 than on days 4 and 7 of testing. However, it should also be noted that beside all females (inseminated and non-inseminated) received a lower number of mating attempts on days 4 and 7, the non-inseminated females remained receiving a similar number of mating attempts that those received by the inseminated females on the first day of testing (P < 0.05, in all cases).
Figure 7.
Number of mating attempts (grabs and mounts) received by the experimental females on days 1, 4, and 7 of testing. The groups that do not share letters differ significantly at P ≤ 0.05.
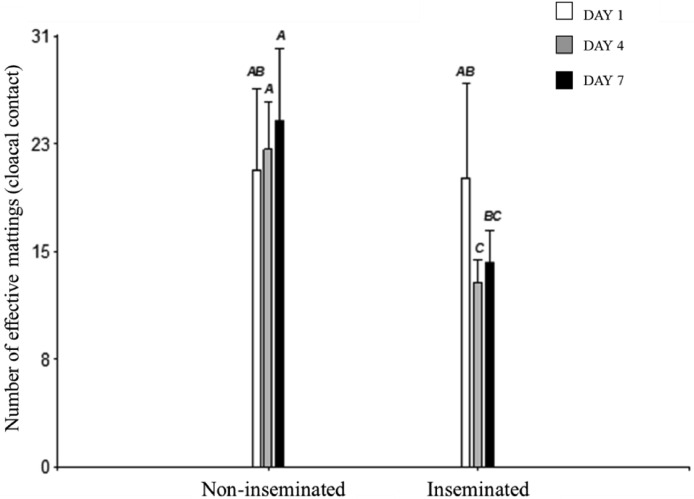
The number of effective mating (cloacal contacts performed) was evaluated. An interaction between the female inseminated condition when entering the box and the days of testing was found (P = 0.0013) (Figure 8). While the non-inseminated females received a similarly higher number of cloacal contacts throughout the study (days 1, 4, and 7), the inseminated females received a lower number of cloacal contacts on day 4 of study and remained similar on day 7.
Figure 8.
Number of effective matings (cloacal contacts) observed between experimental females and their male box-mate on days 1, 4, and 7 of testing. The groups that do not share letters differ significantly at P ≤ 0.05.
DISCUSSION
This study evaluated whether the female insemination status and the sex of the box-mate can influence the female egg laying behavior and their social aggressive interactions with conspecifics. First, it was observed that females spent more time in the apparatus side containing a box-mate than in the side where they remained separated, regardless of whether they were inseminated or not inseminated, or whether the box-mate was a female or a male. The longer time spent in the box-mate side of the apparatus is consistent with the social characteristic of poultry species. For example, both domestic chicks and Japanese quail, usually approached conspecifics more readily than an empty environment or one containing members of different avian or mammalian species (Mills et al., 1995; Jones and Mills, 1999). Furthermore, social proximity and social reinstatement behaviors are also frequently observed at the beginning of the trials when fearful reactions due to stressful stimulations (i.e., exposure to novel environments and/or capture by the experimenter) are expected to be exacerbated (Marin et al., 2001; Guzman and Marin, 2008). Nevertheless, when the number of visits to the box-mate side was evaluated, the groups behaved differently depending on their particular experimental situation. While the inseminated females visited more frequently the box mate side of the apparatus containing a male partner than when it contained a female one, the non-inseminated females visited a similar number of times the box-mate side regardless of whether they held a male or a female box-mate partner. Thus, it appears that besides the social need to be in close proximity to a conspecific, other influential variables such as the pair sex-combination (either female-male or female-female) or the physiological status of the experimental female (either inseminated or non-inseminated) were interactively influencing their behavior. The wide range of visits observed between groups in combination with their reproductive status and the aggressive social interactions observed (see below), also suggest that experimental females not only are able to recognize the open gate as a passage from one side of the apparatus to the other, but they are also able to regulate the time spent in each visit. For example, in the group where the highest incidence of aggressive behavior was observed, a higher number of visits was also recorded (i.e., shorter times spent per visit).
All groups of experimental females laid equal number of eggs suggesting that the treatments studied did not affect the egg formation process. Nevertheless, when the laying side choice was evaluated, all experimental groups but the inseminated females that were tested with a female box-mate behaved similarly. Consistently with a higher percentage of time spent in the box-mate side of the apparatus, the non-inseminated females that interacted with a female or a male box-mate, and the inseminated females that interacted with a male box-mate, laid also a greater percentage (∼75%) of their eggs within the box-mate side of the apparatus than in the separated sector. However, on average, the group of inseminated females that were tested with a female box-mate showed a shift towards distributing their eggs similarly between the 2 sides of the apparatus. Furthermore, when the chosen site was evaluated for each of those particular inseminated females, it was observed a different trend with 5 out of 9 cases laying the majority of their eggs in the area where their female box-mate did not have access (separated side of the apparatus). Findings are closely related to what has already been observed in other species in which different females within the same population can evidence different laying strategies (Duncan et al., 1978). Clearly, in our study, and depending on the experimental conditions, some females selected only one box side of the apparatus and others distributed their eggs in both sides. Considering the observed differences between our 4 experimental groups, the results suggest that the female laying strategy is influenced by their internal reproductive-physiological condition (inseminated or non-inseminated) and the social context in which they find themselves (in close proximity with a male or a female box-mate).
Whether the inseminated females make their laying side selection based on a true site preference or just because they are avoiding a close proximity with their female box-mate conspecific still remains unknown. Although the bibliography informs that some species lay in gregarious form and in close proximity between conspecifics (e.g., penguins, cormorants and chickens in captivity conditions) (Clark and Robertson, 1979; McNeil and Léger, 1987), in most species it is observed that females choose to isolate themselves to lay their eggs and incubate them (Duncan et al., 1978; Guyomarc'h and Saint-Jalme, 1986). As stated above, the laying behavior of quail are still not fully understood, in wildlife it has been observed that female quail can be solitary when in their laying period (Stanford, 1957). However in captive conditions, females were also observed to lay in the presence of conspecifics (Orcutt and Orcutt, 1976). Habituation to a certain situation, learning, lived experiences, and domestication are strongly influential processes on the behavior of animals (Gerken and Petersen, 1992; Jones, 1995; Jones, 1996). Consequently, the laying strategy observed in our experimental groups could be related to both, the particular conditions in which the birds were raised and the conditions where most of the domestication/selection process has been occurred during many generations (i.e., housed in cages in small groups). It is important to recall that our experimental females were raised since hatch in groups, and prior to puberty they were re-housed in pairs. Because they initiated (and continued) their egg laying within a reduced enclosure, it is conceivable that at the time of this study in our new experimental set up, females were already habituated (or learned) to lay their eggs in close proximity with a cage-mate. Interestingly, while most females appeared to repeat that initial pattern of laying the majority of their eggs in close proximity with conspecifics (“shared” side of the apparatus), the group of inseminated females entering the apparatus with a female cage-mate showed a shift towards laying their eggs in the separated side of the box. The observed behavior in this particular group combination is consistent with Rosvall (2011) proposal which postulates that females compete either for the territory itself or for a nesting site at the time of laying (Slagsvold, 1993; Sandell and Smith, 1997). In fact, when considering the aggressive behaviors observed among these females, both females started the trial interacting similarly aggressive. However, along the trial days, the inseminated female managed to position itself as dominant (on days 4 and 7 of the study, they practically did not receive any aggressions from the female box-mate). Nevertheless, it is important to recall that female box-mates were all non-inseminated and therefore, although laying eggs, they were probably less motivated to sustain a defensive/aggressive behavior. The result of such competition would give the inseminated female an advantage (Chek and Robertson, 1991) since it would reduce the aggressiveness of the female box-mate. On the other hand, because inseminated females were laying eggs not only in the separated side of the apparatus but also in the “shared” side with another female, it is possible that a parasitizing phenomenon could also underlie the observed behavior. Intraspecific nest parasitism has already been reported for this species and other members of the order Galliformes (Yom-Tov, 2001; Andersson, 2017). According to Pilz et al. (2005), parasitism would present several advantages for the experimental female, especially if, as in our case, the nest resource and available space are critical. However, to be able to define whether parasitism was a true strategy used by some of the inseminated females, further studies should be carried out to complement the current experimental conditions and to include assessing of breeding success.
Focusing on the behavioral results, as mentioned, the inseminated females appeared positioned as dominants throughout the testing days. However, when the experimental females were non-inseminated and interacted with another female that situation was not observed and both females evidenced similar levels of aggression among them throughout the whole study suggesting that a hierarchy were not yet established. Regarding the aggressive behaviors observed in the interactions with male box-mates, males were always constant in the number of aggressions shown towards the females, being them inseminated or non-inseminated. The levels of male aggressions are consistent with a coercive copulations strategy that is perceived from a human observer (Clutton-Brock and Parker, 1995). Regarding the aggressive behavior shown by the females towards the males, the inseminated females showed a higher number of aggressive events towards the males than their non-inseminated counterparts, although both groups of females (inseminated or not) also drastically reduced the number of aggressions toward the last days of the study. This reduction on the female aggressions performed is consistent with their establishment in a lower hierarchy position respecting to their male counterpart. That process of hierarchies and/or dominances establishment has already been reported when new social groups, and particularly small groups, are initially formed (François et al., 2000; Beacham, 2003; Marin et al., 2014).
The female behavior on the first day of testing deserves additional considerations. First, the inseminated females were not only more aggressive than the non-inseminated females but they were even more aggressive than the males with whom they interacted. This findings are consistent with a reduced female receptivity towards males after being inseminated (Persaud and Galef Jr, 2004), as well as with exacerbated behaviors related to maintaining control of the resources and/or safeguarding their clutch. Reduction in aggressions in the following days is also consistent with a sustained attack by the male and the establishment of a male dominance (Zayan, 1987; Bradshaw, 1992). Interestingly, in spite of the aggressions received by the females when interacting with both a male and a female box-mate, the time spent within the box-mate side was always greater than the time they spent in the box side where they could be separated (and therefore, without direct social interactions). This also suggests that the tendency to approach, stay and interact physically with other congeners was greater than the tendency to remain separated (that could have otherwise been exacerbated by the aggressions received by females). It is important to note that the increased time in the box-mate side was also observed even on days 4 and 7 of the test, and both, when the levels of aggressions received by the females were similar to the ones they performed (group of non-inseminated females with another female), when the levels of aggressions received by females were lowered (group of inseminated females with another female), and when the levels of aggressions received were higher than the aggressions they performed (both groups of females paired with a male).
In conclusion, the findings suggest that the female laying strategy is influenced by both, their internal reproductive-physiological condition (inseminated or non-inseminated) and the social context in which they find themselves (in close proximity with a male or a female box-mate). It is conceivable that the physiological status induced by insemination modified the expression of a behavioral need (laying their eggs in a secluded environment) that becomes evident when females were paired with another female in a restricted environment (potential competitor for resources including a place for nesting). It is also possible that this need for finding a more isolated place to lay eggs (Duncan, 1998) has remained latent in the captive breeding conditions of forced gregariousness where they were bred since birth, and it was able to be expressed (at least partially) by using this novel environmental set up with the box-mate birds holding the IPB (i.e., allowing the experimental female to choose between remaining separated from box-mates in one side of the apparatus or to voluntarily enter their space). The possibility for animals to express their behavioral needs without major restrictions is a key aspect of improving animal welfare (Friend, 1989). Thus, providing quail female breeders with the option of laying their eggs in separated enclosures from conspecifics could be key to favor their well-being.
ACKNOWLEDGMENTS
The study complies with applicable Argentinean laws, with the local Argentinean Association for Science and Technology Laboratory Animals – (AACyTAL Bulletins number 15 and 16, 2001) and meets the Institutional Animal Care and Use Committee guidelines (Project approved; Acta N°5). The authors wish to thank Biol. Julia Ortiz and Pablo Prokopiuk for the animal care assistance and student Marcos Asis-Rodriguez for his assistance in analyzing video recordings.
This work was supported by grants from Secretaría de Ciencia y Técnica of Universidad Nacional de Córdoba (SECyT-UNC), Agencia Nacional de Promoción Científica y Tecnológica (FONCYT), Argentina. DAG and RHM are Career Members of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. SP and GO held PhD scholarships from the later institution.
DISCLOSURES
The authors have no conflicts of interest to declare.
REFERENCES
- Andersson M. Helping relatives survive and reproduce: inclusive fitness and reproductive value in brood parasitism. Am. Nat. 2017;189:138–152. doi: 10.1086/689991. [DOI] [PubMed] [Google Scholar]
- ANY-maze. 2018. Stoelting, Co., Wood Dale, IL.
- Appleby M.C., Hughes B.O. Welfare of laying hens in cages and alternative systems: environmental, physical and behavioural aspects. Worlds. Poult. Sci. J. 1991;47:109–128. [Google Scholar]
- Beacham J. Models of dominance hierarchy formation: effects of prior experience and intrinsic traits. Behaviour. 2003;140:1275–1303. [Google Scholar]
- Birkhead T.R., Fletcher F. Sperm storage and the release of sperm from the sperm storage tubules in Japanese quail Coturnix japonica. Ibis. 1994;136:101–105. [Google Scholar]
- Bradshaw R.H. Conspecific discrimination and social preference in the laying hen. Appl. Anim. Behav. Sci. 1992;33:69–75. [Google Scholar]
- Cain K.E., Ketterson E.D. Individual variation in testosterone and parental care in a female songbird; the dark-eyed junco (Junco hyemalis) Horm. Behav. 2013;64:685–692. doi: 10.1016/j.yhbeh.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K.L., Robertson R.J. Spatial and temporal multi-species nesting aggregations in birds as anti-parasite and anti-predator defenses. Behav. Ecol. Sociobiol. 1979;5:359–371. [Google Scholar]
- Clutton-Brock T.H., Parker G.A. Sexual coercion in animal societies. Anim. Behav. 1995;49:1345–1365. [Google Scholar]
- Correa S.M., Horan C.M., Johnson P.A., Adkins-Regan E. Copulatory behaviors and body condition predict post-mating female hormone concentrations, fertilization success, and primary sex ratios in Japanese quail. Horm. Behav. 2011;59:556–564. doi: 10.1016/j.yhbeh.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Chek A.A., Robertson R.J. Infanticide in female tree swallows: a role for sexual selection. Condor. 1991;93:454–457. [Google Scholar]
- Duncan I.J. Behavior and behavioral needs. Poult. Sci. 1998;77:1766–1772. doi: 10.1093/ps/77.12.1766. [DOI] [PubMed] [Google Scholar]
- Duncan I.J.H., Savory C.J., Wood-Gush D.G.M. Observations on the reproductive behaviour of domestic fowl in the wild. Appl. Anim. Ethol. 1978;4:29–42. [Google Scholar]
- Friend T. Recognizing behavioral needs. Appl. Anim. Behav. Sci. 1989;22:151–158. [Google Scholar]
- François N., Decros S., Picard M., Faure J.M., Mills A.D. Effect of group disruption on social behaviour in lines of Japanese quail (Coturnix japonica) selected for high or low levels of social reinstatement behaviour. Behav. Processes. 2000;48:171–181. doi: 10.1016/s0376-6357(99)00081-9. [DOI] [PubMed] [Google Scholar]
- Gerken M., Petersen J. Heritabilities for behavioral and production traits in Japanese quail (Coturnix coturnix japonica) bidirectionally selected for dustbathing activity. Poult. Sci. 1992;71:779–788. [Google Scholar]
- Guyomarc'h J.C., Saint-Jalme M. La reproduction chez la caille des blés (Coturnix C. coturnix). II: Croissance et développement sexuel des jeunes. Gibier Faune Sauvage. 1986;3:281–295. [Google Scholar]
- Guzman D.A., Marin R.H. Social reinstatement responses of meat-type chickens to familiar and unfamiliar conspecifics after exposure to an acute stressor. Appl. Anim. Behav. Sci. 2008;110:282–293. [Google Scholar]
- InfoStat. 2016. Di Rienzo, J. A., F. Casanoves, M. G. Balzarini, L. Gonzalez, M. Tablada, C. W. Robledo. InfoStat, versión 2016. ANY-maze. 2018. ANY-maze Video Tracking Software. National University of Córdoba, Córdoba, Argentina.
- Jones R.B. Ontogeny of response to humans in handled and non-handled female domestic chicks. Appl. Anim. Behav. Sci. 1995;42:261–269. [Google Scholar]
- Jones R.B. Fear and adaptability in poultry: insights, implications and imperatives. Worlds. Poult. Sci. J. 1996;52:131–174. [Google Scholar]
- Jones R.B., Mills A.D. Divergent selection for social reinstatement behaviour in Japanese Quail: effects on sociality and social discrimination. Poult. Avian Biol. Rev. 1999;10:213–223. [Google Scholar]
- Keeling L.J. In: Pages 203–213 in Welfare of the Laying Hen. Perry G.C., editor. CABI Publishing; Oxfordshire, MS: 2004. Nesting, perching and dustbathing. [Google Scholar]
- Langmore N.E., Cockrem J.F., Candy E.J. Competition for male reproductive investment elevates testosterone levels in female dunnocks, Prunella modularis. Proc. Biol. Sci. 2002;269:2473–2478. doi: 10.1098/rspb.2002.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring M.C., Hartley I.R., Lambrechts M.M., Deeming D.C. The design and function of birds' nests. Ecol. Evol. 2014;4:3909–3928. doi: 10.1002/ece3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin R.H., Freytes P., Guzman D., Jones R.B. Effects of an acute stressor on fear and on the social reinstatement responses of domestic chicks to cagemates and strangers. Appl. Anim. Behav. Sci. 2001;71:57–66. doi: 10.1016/s0168-1591(00)00167-2. [DOI] [PubMed] [Google Scholar]
- Marin R.H., Liste M.G., Campderrich I., Estevez I. The impact of phenotypic appearance on body weight and egg production in laying hens: a group-size- and experience-dependent phenomenon. Poult. Sci. 2014;93:1623–1635. doi: 10.3382/ps.2013-03705. [DOI] [PubMed] [Google Scholar]
- Martin T.E. Abiotic vs. biotic influences on habitat selection of coexisting species: climate change impacts? Ecology. 2001;82:175–188. [Google Scholar]
- McNeil R., Léger C. Nest-site quality and reproductive success of early- and late-nesting double-crested cormorants. Wilson Bull. 1987;99:262–267. [Google Scholar]
- Meijsser F.M., Hughes B.O. Comparative analysis of pre-laying behaviour in battery cages and in three alternative systems. Br. Poult. Sci. 1989;30:747–760. [Google Scholar]
- Mills A.D., Jones R.B., Faure J.M. Species specificity of social reinstatement in Japanese quail Coturnix japonica genetically selected for high or low levels of social reinstatement behaviour. Behav. Processes. 1995;34:13–22. doi: 10.1016/0376-6357(94)00044-h. [DOI] [PubMed] [Google Scholar]
- Orcutt F.S., Orcutt A.B. Nesting and parental behavior in domestic common quail. Auk. 1976;93:135–141. [Google Scholar]
- Pellegrini S., Condat L., Caliva J.M., Marin R.H., Guzman D.A. Can Japanese quail male aggressions toward a female cagemate predict aggressiveness toward unknown conspecifics? Livest. Sci. 2019;222:65–70. [Google Scholar]
- Pellegrini S., Marin R.H., Guzman D.A. An individually fitted physical barrier device as a tool to restrict the birds' spatial access: can their use alter behavioral responses? Poult. Sci. 2015;94:2315–2321. doi: 10.3382/ps/pev231. [DOI] [PubMed] [Google Scholar]
- Persaud K.N., Galef Jr B.G. Fertilized female quail avoid conspecific males: female tactics when potential benefits of new sexual encounters are reduced. Anim. Behav. 2004;68:1411–1416. [Google Scholar]
- Pilz K.M., Smith H.G., Andersson M. Brood parasitic European starlings do not lay high-quality eggs. Behav. Ecol. 2005;16:507–513. [Google Scholar]
- Rosvall K.A. Intrasexual competition in females: evidence for sexual selection? Behav. Ecol. 2011;22:1131–1140. doi: 10.1093/beheco/arr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowska J., Place N.J., Vincent S., Adkins-Regan E. Adrenocortical response to mating, social interaction and restraint in the female Japanese quail. Physiol. Behav. 2011;104:1037–1040. doi: 10.1016/j.physbeh.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Sandell M.I., Smith H.G. Female aggression in the European starling during the breeding season. Anim. Behav. 1997;53:13–23. [Google Scholar]
- Sittmann K., Abplanalp H. Duration and recovery of fertility in Japanese quail (Coturnix coturnix japonica) Br. Poult. Sci. 1965;6:245–250. doi: 10.1080/00071666508415580. [DOI] [PubMed] [Google Scholar]
- Slagsvold T. Female-female aggression and monogamy in great tits Parus major. Ornis Scand. 1993;24:155–158. [Google Scholar]
- Stanford J.A. Pages 316–359 in Proc. 22nd North American Wildlife Conference. 1957. A progress report of Coturnix quail investigations in Missouri. [Google Scholar]
- Vaisanen J., Hakansson J., Jensen P. Social interactions in Red Junglefowl (Gallus gallus) and White Leghorn layers in stable groups and after re-grouping. Br. Poult. Sci. 2005;46:156–168. doi: 10.1080/00071660500062638. [DOI] [PubMed] [Google Scholar]
- Yom-Tov Y. An updated list and some comments on the occurrence of intraspecific nest parasitism in birds. Ibis. 2001;143:133–143. [Google Scholar]
- Zayan R. In: Pages 321–438 in Cognitive Aspects of Social Behaviour in the Domestic Fowl. Zayan R., Duncan I.J.H., editors. Elsevier; Amsterdam, Netherlands: 1987. Recognition between individuals indicated by aggression and dominance in pairs of domestic fowl. [Google Scholar]