Abstract
Despite its prevalence in the management of peripheral tumors, compared to surgery and radiation therapy, chemotherapy is still a suboptimal intervention in fighting against brain cancer and cancer brain metastases. This discrepancy is mainly derived from the complicatedly physiological characteristic of intracranial tumors, including the presence of blood-brain barrier (BBB) and limited enhanced permeability and retention (EPR) effect attributed to blood-brain tumor barrier (BBTB), which largely lead to insufficient therapeutics penetrating to tumor lesions to produce pharmacological effects. Therefore, dependable methodologies that can boost the efficacy of chemotherapy for brain tumors are urgently needed. Recently, nanomedicines have shown great therapeutic potential in brain tumors by employing various transcellular strategies, paracellular strategies, and their hybrids, such as adsorptive-mediated transcytosis, receptor-mediated transcytosis, BBB disruption technology, and so on. It is compulsory to comprehensively summarize these practices to shed light on future directions in developing therapeutic regimens for brain tumors. In this review, the biological and pathological characteristics of brain tumors, including BBB and BBTB, are illustrated. After that, the emerging delivery strategies for brain tumor management are summarized into different classifications and supported with detailed examples. Finally, the potential challenges and prospects for developing and clinical application of brain tumor-oriented nanomedicine are discussed.
Keywords: nanoparticle, blood-brain barrier, transport, transcytosis, brain tumor
1. Introduction
Brain tumor, referring to both a primary brain tumor and a metastatic brain tumor, remains one of the most lethal cancers, with median survival between 4 and 15 months after diagnosis [1, 2]. Glioblastoma multiforme (GBM), a grade IV astrocytoma classified by WHO, is the most common and aggressive primary brain tumor with a 5-year survival rate of less than 5% and accounts for around 80 % of new diagnosed malignant central nervous system (CNS) tumors [3, 4]. A metastatic brain tumor is a devastating complication of periphery tumors with a high propensity to metastasize to the brain, such as lung cancer (50%), breast cancer (15%), and melanoma (6–11%) [5, 6]. Despite tremendous efforts devoted in the past decades, at present, the standard care for newly diagnosed GBM is surgery, followed by adjuvant radiation therapy and adjuvant chemotherapy with alkylating agent temozolomide (TMZ) [1]. Similar to primary brain tumors, although surgery and radiation therapy are typically adopted as first-line treatments for metastatic brain tumors, there are no specific chemotherapeutics proven effective for brain metastasis therapy, including TMZ [7]. The biggest challenge for surgery is that, due to heterogeneous and invasive growth, it is hard to remove the tumor tissue completely without compromising healthy neurological tissue due to the lack of finely defined glioma boundary. Thus, the residue of neoplastic tissue would lead to rapid glioma recurrence and poor prognosis [8]. Radiation therapy, especially whole-brain radiation therapy (WBRT), is restrained by significant side effects, such as cognitive impairment [9, 10]. Meanwhile, the improved overall survival is conservative and heterogeneous [11].
Compared to significant progress in managing peripheral tumors, chemotherapy is still a suboptimal intervention, which serves as an adjuvant role in a state-of-art regimen for brain tumor treatment. Although most tumoricidal agents for peripheral tumors exhibit comparable pharmacological effects to brain tumor cells in vitro, they barely yield a plausible anti-tumor outcome in preclinical brain tumor models and clinical trials. This discrepancy mainly derives from the insufficient accumulation of therapeutic agents in the brain tumor to produce a pharmacological effect, attributing to the presence of brain and tumor-related physioanatomical barriers, such as blood-brain barrier (BBB) and blood-brain tumor barrier (BBTB) to form a pharmacological sanctuary. The intrinsic characteristic of brain tumors, especially metastatic brain tumors, including invasive and aggressive growth, substantial heterogeneity, and readily acquired drug resistance, further reinforce their refractory [12, 13]. During drug research and development for brain tumors, it is of equal importance to develop some novel and practicable approaches, including biological, chemical, and physical strategies to improve drug delivery to intracranial tumors, rather than merely devoted to the discovery and development of the first in class drugs, considering the vast cost in labor, finance, and time. Thus, it is crucial to have a complete picture of the biological and pathological characteristics of intracranial tumors, including BBB and BBTB. Hereby, we review and discuss recent emerging strategies for overcoming the BBB and increasing theranostic agents penetrating BBB (Figure 1), including detailed examples of different categories. Finally, we also summarize the potential challenges and prospects for the design and clinic application of nanomedicine for brain tumors.
Figure 1.
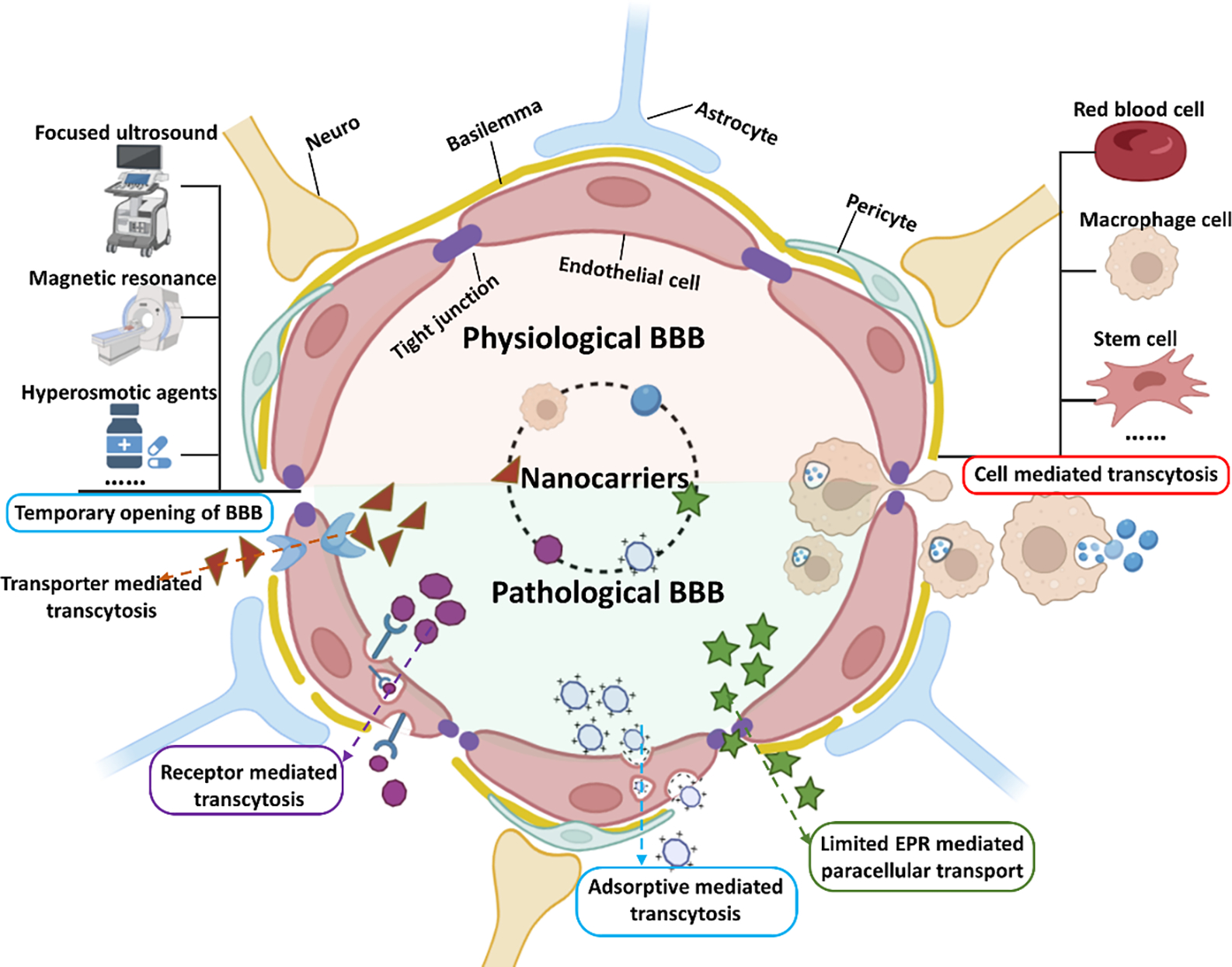
The structure of BBB and recent emerging strategies for drugs and theranostic agents crossing BBB for treating brain cancer and cancer brain metastases.
2. The physiological barriers of brain tumor: BBB and BBTB
Despite marvelous efforts that have been devoted to the development of the diagnosis and therapeutic avenues, the theranostic of brain tumors remains a significant challenge. This obstacle mainly comes from the presence of the blood-brain barrier (BBB), restricting the access of diagnostic and/or therapeutic agents to brain tumors. BBB is a distinct physiological structure of microvessels in the brain related to the peripheral ones, which exerts critical functions on precisely regulating the agents’ transport between the luminol blood and brain tissue and maintaining the dynamic balance of the brain microenvironment in the CNS. The BBB (Figure 1) is composed of endothelial cells (ECs), pericytes (PCs), basement membrane, and end-feet of astrocytes. ECs are held together interactionally through intermolecular tight junctions (TJs) through a number of transmembrane proteins (such as occludin, claudins, and junction adhesion molecule (JAM) proteins), making up the substantial wall of brain capillaries. A discontinuous layer of PCs surrounds the abluminal surface of ECs. The ECs and PCs are further covered by a stroma-like basement membrane, which is tightly ensheathed by astrocytes’ end feet. On the one hand, this nest-like structure of BBB serves as a physical barrier that strictly controls the passage of specific nutrients, such as amino acids, glucose, and fatty acids, from the bloodstream to the CNS via passive diffusion, as well as restricts the passage of potentially harmful substances such as pathogens and neurotoxic compounds. On the other hand, ECs express a bulk of active efflux proteins such as P-glycoprotein and multidrug resistance-associated proteins (MRPs) on the luminal side of the ECs, which have a broad spectrum of substrates to efflux them back to circulation and minimize the substance transportation across the BBB. As a result, a substantially higher electrical resistance was observed between the BBB compared to that of peripheral ones, which would restrict polar and ionic substances transporting to the brain liberally. All these barriers preclude the access of most large molecule drugs and more than 98% of small molecular agents to the brain tissue [14].
Like most peripheral tumors, the original primary brain tumor may derive from the excess “embryonic rests” activation in a particular microenvironment and the imbalance between oncogenes and tumor suppressors [15]. At the early stage of the development of a primary brain tumor, the surrounding nutrients and oxygen can orchestrate inceptive brain cancer cells to grow and develop until the tumor size expands to 2–3 mm (in diameter). After that, the tumor will progress to angiogenesis-dependent nutrient supply mode due to the explosive mass expansion, which leads to the loss of balance between pro- and anti-angiogenic factors [16], resulting in neovascularization. The angiogenetic vessels possess an intact BBB at the initial stage or on the invasive margin. Following the primary tumor’s growth, the neovascularization and intratumor vasculature would deteriorate to form a compromised BBB, whose structure and function display a vast difference from the BBB, named blood-brain tumor barrier (BBTB). There are three populations of micro-vessels in a brain tumor, including continuous and non-fenestrated capillaries, continuous and fenestrated capillaries, and discontinuous (with or without fenestrations) capillaries, based on their orientation and identification of structure and organization [17]. The first group is unique to brain tumors, while the other two groups are similar to vessels found in peripheric tumors. Although no apparent difference in the component, BBTB holds differentiated permeability compared to BBB, deriving from functional compromise of intratumor vasculature, including disrupted endothelial lining, disrupted TJs, and snatchy basement membrane, and so on [18]. However, the leakage structure and the penetrating efficiency of BBTB for macromolecule and nanoparticles are highly heterogenous, not limited to different grades of brain tumor but also different regions within a particular brain tumor. This heterogeneity is prominent among different grades of brain tumors. For example, contrast-enhanced MRI exhibited limited ability in delineating the margin of most of low-grade gliomas (grade II) and more than 40% of high-grade gliomas (grade III), even sometimes failed to accurately classify malignant gliomas due to the heterogeneous BBTB induced limited and un-uniform distribution of paramagnetic contrast agent in the tumor [19]. Moreover, the permeability of capillaries progressively increases from minor to moderate, along with the progression of gliomas [17]. The intertumoral difference in permeability is attributed to the heterogeneous distribution of variable microvessel and spatial variables, such as relatively intact and continuous lipid layer at the margin of brain tumor and enlarged lumen inside the tumor [20]. Even so, BBTB still holds some original characteristics of BBB and its permeability for achieving an ideal leakage for theranostics agents is still significantly different from that of peripheral tumor.
In contrast to the internal germination pattern of primary brain tumor, brain metastasis formation is mainly due to hematogenous dissemination from a peripheral tumor into the brain, followed by the progress of a series of the metastatic cascade, including escape and intravasation into the bloodstream from the primary tumor site, navigation and arrest in the cerebral microcapillaries, and extravasation and proliferation in the brain [21]. It was found that this sowing is always of co-blossom of seed-like cancer cells with their soil-stromal components, such as tumor-associated fibroblast, which can create a modest circumstance for tumor cells colonization and proliferation at the metastatic foci in the early stage [22]. Generally, after the spread of tumor cells to the brain, these progenitor like-tumor cells mainly display a co-opted pattern for proliferation and growth, along with the BBB basement membrane depending on the existing blood vessel at the early stage, and the BBB integrity is maintained until the size of the tumor expands to 3 mm [21]. At the following advanced stage, the co-opting metastatic tumor will evolve to angiogenesis-dependent growth as the aforementioned primary brain tumor and compromise the integrity of BBB. Moreover, due to the original malignant and aggressive nature of metastatic cells, cancer brain metastasis always exhibits multi-nodules and invasive progression, heterogeneity, and poor prognosis [23].
3. Strategies for enhanced BBB penetration
Brain is the most complex system in the body. The accurate controlling passage of various endogenous and exogenous agents from the vasculature into the CNS, and vice versa, is critical for maintaining the homeostasis of the CNS. Due to the presence of a unique physiologic barrier, BBB, endogenous molecules penetrating across the BBB are precisely controlled by diffusion and transcytosis, while most external agents (95%) are blocked by this physical and physiological barrier. Therefore, how to make theranostic agents overcome the BBB barrier and be delivered to the brain without disturbing the homeostasis of the CNS is highly desired. Recently, nanoparticle-based drug delivery systems have been widely investigated to provide therapeutic agents into the brain for the treatment of CNS diseases, including brain tumors, due to their distinctive physiochemical properties, such as high drug loading, prolonged blood circulation time, enhanced stability, facile surface modification for active targeting and controlled drug release. Some nanoparticles based strategies for the diagnosis and treatment of brain tumors have been advanced to different clinical phases (Table 1). The blooming development of nanoparticles would lead to new opportunities for managing brain tumors in both diagnosis and therapy.
Table1.
Summary of clinical trials of nanoparticles based strategies for the diagnosis and treatment of brain tumors
| Name | Vehicle | Cargo | Brain tumor | Objective | Status of Clinical trial | Delivery target and ligand |
|---|---|---|---|---|---|---|
| Caelyx [24, 25] (liposomal doxorubicin) | Liposome | Doxorubicin | Recurrent malignant glioma | Chemotherapy | Marketed (off-label employment) | Passive targeting (EPR) |
| Ferumoxytol [26] | Ultra-small superparamagnetic iron oxide | / | Recurrent and progressed malignant glioma | MRI imaging | Phase I | EPR |
| Nab paclitaxel (Abraxane) [27] | Albumin nanoparticles | Paclitaxel | Breast cancer, brain metastasis of breast cancer | Chemotherapy | Marketed (off-label employment) | EPR |
| AGuIX [28] | Gadolinium-based nanoparticle | / | Multiple brain metastases | Radiosensitizer Imaging enhance | Phase II | EPR |
| MTX110 [29] | Gold nanoparticle | Panobinostat | Diffuse Intrinsic Pontine Glioma | Antineoplastic activity | Phase II | Interventional (Convection-Enhanced Delivery) |
| CPT-11 [30] | Liposome | Irinotecan | Recurrent High-Grade Gliomas | Chemotherapy | Phase I | EPR |
| SGT-53 [31] | Cationic liposome | p53 cDNA | Recurrent Glioblastoma | Gene therapy | Phase II | Transferrin receptor; anti-transferrin receptor single chain antibody fragment |
| EGFR(V)-EDV-Dox [32] | Nanocells | Doxorubicin | Recurrent Glioblastoma Multiforme | Chemotherapy | Phase I | Epidermal growth factor receptor (EGFR); bispecific antibodies |
| 2B3-101 [33] | Liposome | Doxorubicin | Breast Cancer and Leptomeningeal Metastases | Chemotherapy | Phase II | GSH transport; Glutathione |
3.1. Passive targeting
Enhanced permeability and retention (EPR) effect is the theoretical basis of nanoparticles based passive targeting therapy in most tumors, especial peripheral ones, in which nanoparticles of the size range of 1 to 200 nm utilize the unique structure of tumors, including impaired lymphatic drainage system, hyper-vasculature, and defective vascular architecture to realize nanoparticles entry and accumulation within the tumor mass [34]. The EPR effect is extensively size dependent, referring to the size of the particles and pore dimensions of the vasculature within the tumor, whereas it exhibits little impact on the distribution of small molecular weight compounds such as chemotherapeutic agents, which mainly enter tumor mass by free diffusion [35]. However, the EPR effect displays a significant heterogeneity. Different types of tumors may have different pore dimensions. A given tumor could also have a vast difference in pore size at different locations and in various stages. Attributing to the existence of BBB, the EPR effect in brain tumors may be somewhat different from that of periphery tumors, which is ambiguous and controversial. Brain tumors are divided into four levels based mainly on the histologic progress by World Health Organization standard (WHO) [36], in which tumor microvessel population is an important parameter [17]. As aforementioned, continuous endothelial cells and inter-endothelial gaps are the primary aspects of the microvessels population. It is easy to comprehend that EPR-based passive targeting to access the intratumoral space is associated with the grade of brain tumor. For instance, glioblastoma (WHO grade is IV) has a tortuous and disorganized vessel wall, and its vessel pore size is as large as 550 nm in some regions [37], which gives the reasonability for passive targeting. However, this kind of gap-dependent permeability merely exists in WHO-defined grade II astrocytomas and oligodendrogliomas vessels [19], which leads to low permeability of contrast agents for CT and MRI and following faint contrast signals in the tumor. Similar to most peripheral tumors, only a small fraction of administered nanoparticles will be located in brain tumors via the EPR effect. Most nanoparticles will be susceptible to opsonized by the reticuloendothelial system (RES, such as liver and spleen). This tumor aggravative progress and grade-dependent permeability in brain tumors are heterogenous and may yield enormous challenges for EPR base nanomedicine, which could be an excellent opportunity for personalized medicine.
3.2. Nanoparticle-BBB interaction mediated BBB penetration
Although the inherent features of BBB and BBTB prevent macromolecule and nanoparticles from shuttling freely between the peripheric circulation system and brain tumor, the large blood-brain interface of BBB (around 20 m2) and the exuberant blood [38], some inevitable, widespread and/or special interactions between circulatory nanoparticles with BBB, including biological interfacial interaction (such as receptor-mediated transcytosis and transporter-mediated transcytosis) and physicochemical interaction (such as absorption-mediated transcytosis and BBB opening) and can be fully utilized to realize nanoparticle delivery to brain tumors. Here we mainly review and discuss the recent progress in developing nanotechnology-based treatments for brain tumors.`
3.2.1. Receptor-mediated transcytosis (RMT)
RMT is considered as the most promising method and is widely employed for realizing intracerebral delivery of nanoparticles with the characterization of high specificity, selectivity, and affinity, especially for brain tumors [39]. Most endogenous macromolecules are transported into brain parenchyma via RMT, usually involving the following three essential steps, including (1) interactive binding of a ligand to its cognate receptor on the luminal membrane of capillary endothelial cells mediated the formation of corpuscles at the luminal wall; (2) intracellular trafficking from luminal side to abluminal side; (3) exocytosis of the endocytic agent from abluminal side to brain parenchyma [40]. RMT-based transport is usually energy-dependent and with relatively high efficiency. To take advantage of this strategy, the surface of nanoparticles can be decorated with specific ligands to improve the intracerebral transport of loaded therapeutic agents. Theoretically, to achieve an optimal delivery efficiency of therapeutic to a brain tumor, the target receptor should be highly expressed in the endothelial cells of the brain tumor, whereas minimally expressed in other vascular endothelial cells to minimize the off-target effect induced safety concern. However, to date, nearly no receptor can meet this criterion. Meanwhile, an endogenous ligand may exert a competitive bind to the receptor, which will compromise the target delivery efficiency of RMT. Over the past decades, most receptors served as targets for CNS-associated diseases, including brain tumors, are upregulated on the endothelial cells of the brain capillary. Recent advancements in biomedical sciences and nanotechnology provide a better resource for the design of nanoparticle-based RMT. The following section will summarize several typical receptors utilized in nanoparticle-based drug delivery for brain tumors.
3.2.1.1. Transferrin receptor mediated transcytosis
Transferrin receptor (TfR) has been extensively investigated for RMT for brain delivery due to its over-expression in the endothelial cells of brain capillaries [40]. In the brain, TfRs are mainly responsible for the transport of iron ions into the brain parenchyma in the complex form of transferrin-Fe (Tf-Fe) to maintain the homeostasis of iron, which is vital for metabolism, neural signal transduction, and brain function [41]. Transferrin, TfR targeted peptides, anti-TfRs antibodies, and its fragments (such as Fab, sFabs, and scFv) consist of the targeting ligands of TfRs. Moreover, as in most peripheral tumors, due to the proliferation of cancer cells induced exponential demand for iron and iron metabolism, the expression of TfR can rise to 100 folds compared to normal cells [42]. Therefore, those ligand molecules can be engineered onto the surface of nanoparticles via physical, chemical, and biological manners to initiate the RMT [43], realizing a TfR receptor mediated sequential dual-targeted delivery [43]. For example, Dixit et al. prepared transferrin-modified zoledronic acid (ZOL) loaded liposomes (NPs-ZOL-Tf), and evaluated the in vitro and in vivo anti-brain tumor efficiency [44]. It was demonstrated that NPs-ZOL-Tf exhibited a higher in vitro anti-proliferative activity than free ZOL and non-targeted NPs-ZOL, which was attributed to an enhanced receptor-mediated intracellular internalization. Moreover, this TfR-mediated superiority was further validated in intratumor localization and antitumor efficiency in orthotropic and heterotopic brain tumor models [44]. Adopting a similar strategy, Cui et al. developed a dual-targeting PLGA nanoparticles (MNP/T7-PLGA NPs), which combined with magnetic guidance and peptide T7 mediated active targeting, for co-delivery of paclitaxel and curcumin for brain tumor therapy [45]. T7 (sequence HAIYPRH), a human TfR binding peptide, was modified on the nanoparticles surface to mediate the active targeted delivery of NPs through RMT. Since T7 and transferrin bind to distinct sites on TfR, there is no competitive binding between them. The integration of magnetic iron oxide into the delivery system endowed the nanoparticles magnetic-guided targeting ability by favoring particles-endothelial cells and receptor-ligand (TfR-T7) interaction and attenuating sheering stress applied on the BBB, thus achieving an improved active targeting effect [45]. The following Table 2 give a brief summarization of systems utilizing TfR mediated RMT.
Table 2.
Nanocarriers utilize Transferrin receptor mediated transcytosis.
| Ligands | Nanoparticles | Cargoes | Cell lines | application |
|---|---|---|---|---|
| T7 peptide (HAIYPRH) | Liposomes | Anti-EGFR siRNA | U87 | Decrease EGFR, anti-glioma[46] |
| Transferrin, cell penetrating peptide PFVYLI (PFV) | Liposomes | doxorubicin (Dox) and Erlotinib (Erlo) | U87, brain endothelial (bEnd.3) | Anti- glioblastoma tumor [47] |
| T7 peptide | PLGA nanoparticles, magnetic nanoparticles | Paclitaxel, curcumin | U87, bEnd.3 | Anti- glioblastoma tumor [45] |
| Transferrin peptide (Tfpep) | gold nanoparticles | photodynamic pro-drug, Pc 4 | LN229, U87 | Fluorescence imaging [48] |
| Transferrin | Carbon dots | Dox | SJGBM2, CHLA266 | No in vivo data [49] |
| Transferrin, transferrin receptor monoclonal antibodies (OX26 or R17217) | Human serum albumin | Loperamide | No data | Anti-nociceptive effects [50] |
| Y-shaped ligands (with Tat peptide and transferrin) | iron oxide (Fe3O4) particles | Chlorin e6 | U87-MG | Photodynamic glioblastoma tumor therapy [51] |
| Transferrin | magnetic silica PLGA nanoparticles | Dox, paclitaxel (PTX) | U87-MG | Anti-brain glioma [52] |
| EGF peptide (YHWYGYTPQNVI), Transferrin peptide (HAIYPRH) | gold nanoparticles | photosensitizer phthalocyanine 4 (Pc 4) | LN229 | Photodynamic glioblastoma tumor therapy [53] |
| Transferrin | carbon dots | Epirubicin, temozolomide | SJGBM2, CHLA266, CHLA200 U87 | Anti- glioblastoma tumor [54] |
| D-T7 peptide | PEGylated bilirubin nanoparticles (BRNPs) | PTX, cediranib | bEnd.3, C6 cells | Antiangiogenesis and Chemotherapy of Glioma [55] |
Although TfR has been extensively utilized as a targeting receptor, it is worth noting that TfR-based RMT is still full of debate. One scenario is that the unique receptor-ligand interaction between TfR and TfR’s ligands decorated on the surface of nanocarriers may be severely compromised by the competed binding of TfR with endogenous ligand, which will reduce the chance of nanocarrier crossing the BBB. In another case, the efficiency of TfR-mediated transcytosis is relatively low. Some early research in cultured cells and in situ brain perfusion models found that nearly 90 percent of transferrin was recycled back to the luminal side after being endocytosed by brain endothelial cells, and only 10 percent of transferrin was perfused into the brain [56, 57]. More recent follow-up studies revealed that the affinity and the valency of TfR antibody and the density of the ligand decorated on the nanoparticles could have a significant influence on the intracellular TfR trafficking and transport of the nanoparticles [58–60]. Therefore, the microstructure and function of the Tf-TfR complex and its efficiency in TfR-mediated transcytosis should be further explored and improved.
3.2.1.2. Low-density lipoprotein receptor-mediated transcytosis
Low-density lipoprotein receptors related protein (LRP, such as LRP1, LRP2, and LRP8) is another common family of receptors, which have been widely reported to mediate the transport of various nanocarriers across BBB through RMT. Among them, LRP1 and its targeting ligands are extensively investigated and employed for the diagnosis and therapy of CNS diseases, including brain tumors. LRP1, a membrane of the LDL receptor family, is involved in numerous essential processes in the brain under both physiological and pathological conditions [61]. LRP1 is usually expressed at a high level at BBB, participates in the transport of amyloid-beta peptide in the CNS together with its ligands, such as alpha-2-macroglulin and apoliliprotein [61], controls the degradation of extracellular matrix by regulating the level of matrix-degradation proteases, such as matrix metalloproteinases (MMPs) and plasminogen activators (PAs) [62]. Angiopep 2, a 19-amnino acid length peptide derived from aprotinin, a natural ligand of LRP1, is widely used to mediate brain-targeted drug delivery. Sun et al. reported that the modification of Angiopep on the cationic liposome could realize effective codelivery of genetic agent and paclitaxel to brain tumors [63]. After treatment with these liposomes, the median survival time of brain tumor-bearing mice was significantly longer than their non-targeted counterpart (69.5 days vs 35.5 days), even substantially longer than the standard clinical administration of temozolomide (47 days). Thus, this Angiopep-functionalized liposome holds great promise for clinical translation. Apart from the application on the surface modification for nanoplatforms, Angiopep 2 also serves as the key guiding agent for some Engineered Peptide Compound (EPiC) [64], including ANG1005, a novel paclitaxel derivative, consisting of three molecules paclitaxel and one 19-amino-acid peptide Angiopep-2 via cleavable ester bond [65]. It was founded that reducing the activity of LRP1 on U87 glioblastoma cells through gene silencing or corresponding competitors could reduce the cellular uptake of ANG1005, and on the other hand, increase the internalization of ANG1005 into U87 cells under the microenvironment of hypoxic and acidic condition mimicking aggressive tumor [65]. Moreover, in vivo imaging and immunolocalization studies demonstrated that ANG1005 and Cy5.5-Angiopep-2 boosted accumulation in the intracranial Mu87MG.EGFRvII glioblastoma tumor model compared to surrounding or contralateral tissue, mainly attributing to the over-expression of LRP-1 and LRP-1 RMT [65].
Tween 80 is a surfactant agent that can increase nanoparticle’s brain delivery by coating the surface of nanoparticles and is considered the “gold standard” coating for brain delivery [66, 67]. Gulyaev et al. found that 1% Tween 80 coating on the surface of doxorubicin-loaded poly(butyl cyanoacrylate) nanoparticles achieved 60-fold brain concertation of doxorubicin as compared to bare nanoparticles and free drug in mice [68]. Wang et al. also verified that 1% Tween 80 coating on the gemcitabine-loaded Polybutylcyanoacrylate (PBCA) nanoparticles could significantly inhibit the growth of C6 glioma cells in vitro and extend the survival time of corresponding brain tumor model mice in vivo, thus realizing a preferable antitumor efficiency [69]. Although the exact mechanism of Tween 80 assisting nanoparticles in crossing the BBB is unclear, it is speculated that the interaction between brush like Tween 80 and LDL receptor is the driving force to initiate the RMT [70]. Another possible explanation is that the Tween 80 may increase the adsorption of plasma-derived apolipoproteins E and B to the surface of nanoparticles [71], which have been confirmed to interact with LDL receptors and trigger receptor-mediated endocytosis [72–74].
3.2.1.3. Insulin receptor-mediated transcytosis
Similarly, insulin receptor expressed on the endothelial cells of brain capillary has also been employed for RMT-based drug delivery to the brain [75, 76]. It was founded that a strong binding force between insulin and insulin-like growth factor I (IGF-I) in the brain tumor, which provides evidence that insulin receptor could be a promising candidate for insulin as a ligand for brain tumor target drug delivery [77]. The 83–14 murine mAb is an insulin peptidomimetic mAb, which has a high affinity with the exofacial epitope of the insulin receptor. Moreover, 83–14 murine mAb could initiate an RMT through the BBB in Rhesus monkeys in vivo [78], holding a great promise as a vector or ligand for delivering drugs to brain tumors. Because of this property, Dieu et al. grafted 83–14 murine mAb on the surface of polymersomes and realized enhanced cellular binding and uptake by the endothelial cells of brain capillary [79]. Employing a similar strategy by modifying 83–14 mAb on the surface of nanoparticles, Kuo et al. achieved the transport of solid lipid nanoparticles carrying chemotherapeutic carmustine across the BBB and brain-targeting delivery with the help of insulin receptor [80]. It was worth noting that the role of insulin receptors in mediating the transport of insulin across BBB in vivo was receptor type dependent and sometimes controversial. For instance, researchers revealed that the transport of insulin across the BBB was independent of insulin receptors, especially for signaling-related insulin receptors [81]. Another looming concern is that potential hypoglycemia may be present when insulin is acted as a targeting ligand, which should be monitored carefully, or prophylactic measures should be taken first, such as co-administration of dextrose [82]. Thus, as an alternative to insulin, peptidomimetic antibodies, insulin-derived peptides and its derivatives, which have similar binding ability to the exofacial epitope of the insulin receptor, could be a great choice.
3.2.1.4. Nicotinic acetylcholine receptor-mediated transcytosis
Nicotinic acetylcholine receptors (nAChRs), a ligand-gated ion channel widely expressed on the brain capillary endothelial cell, were recently widely investigated as a targeted receptor to facilitate BBB crossing and intracranial transporting of nanocarriers [83, 84]. One of the prominent features of nAChRs is their super susceptibility to inhibition, such as peptide neurotoxins and neurotropic viral proteins, which endowed their ability to mediate various agents crossing into the brain. So far, some synthetic and nature-derived peptides have been explored as nAChRs targeting ligand to facilitate therapeutic agent delivery to the brain, such as RVG-9R [85, 86], a 29-amino-acid peptide derived from rabies virus glycoprotein (RVG), CDX [83], a 16-residue peptide derived from the loop II region of the snake neurotoxin candoxin, and KC2S [87], a synthetic peptide similar to the loop 2 segments of three-finger snake neurotoxins. More recently, adopting similar tactics, Lu’s group utilized 2-methacryloyloxyethyl phosphorylcholine (MPC) as a dual targeting ligand to deliver protein therapeutics for the treatment of central nervous system diseases, such as primary brain tumor, metastatic brain tumor and neural degeneration [84, 88–90]. Wherein, MPC, a molecule containing a choline and an acetylcholine analogues, interacts with nAChRs and choline transporter (ChT) via hydrogen bonding and electrostatic interaction, respectively [84], to initiate RMT and realize brain drug delivery. In these systems, MPC monomer and crosslinker were polymerized around a therapeutical protein to yield a nanocapsule shell, which can assist the protein in penetrating the BBB via RMT. One of the representative examples was the systemic delivery of monoclonal antibodies to the CNS for the treatment of brain tumors (Figure 2) [84]. The MPC monomer and matrix metalloproteinase-2 (MMP-2) cleavable peptide crosslinker were assembled around the nimotuzumab (Nimo) or trastuzumab (Tras) to form nanocapsules of antibodies. By virtual of the choline transporters or acetylcholine receptors expressed on the luminal side of BBB, MPC decoration enables the nanocapsules to penetrate through the BBB effectively via RMT. Subsequently, MMP-2, highly expressed in the brain tumor environment, cleaved the peptide linker, broke the shell, and released the encapsulated monoclonal antibodies to realize their pharmacological function [84]. In vivo assay on an orthotopic U87-EGFRwt glioma model demonstrated the effective and safe delivery of the therapeutic protein for brain tumor treatment. It was more interesting to note that excepting for brain targeting capacity, the MPC shell can also prevent the core protein from being phagocytosed by macrophages, which is a key mechanism of nanoparticles clearance during in vivo application, and extend the circulation time of encapsuled protein [91]. Moreover, the polymer shell could prevent the protein from being identified by immune cells, therefore reducing the immunogenicity derived from the therapeutic proteins [91].
Figure 2.
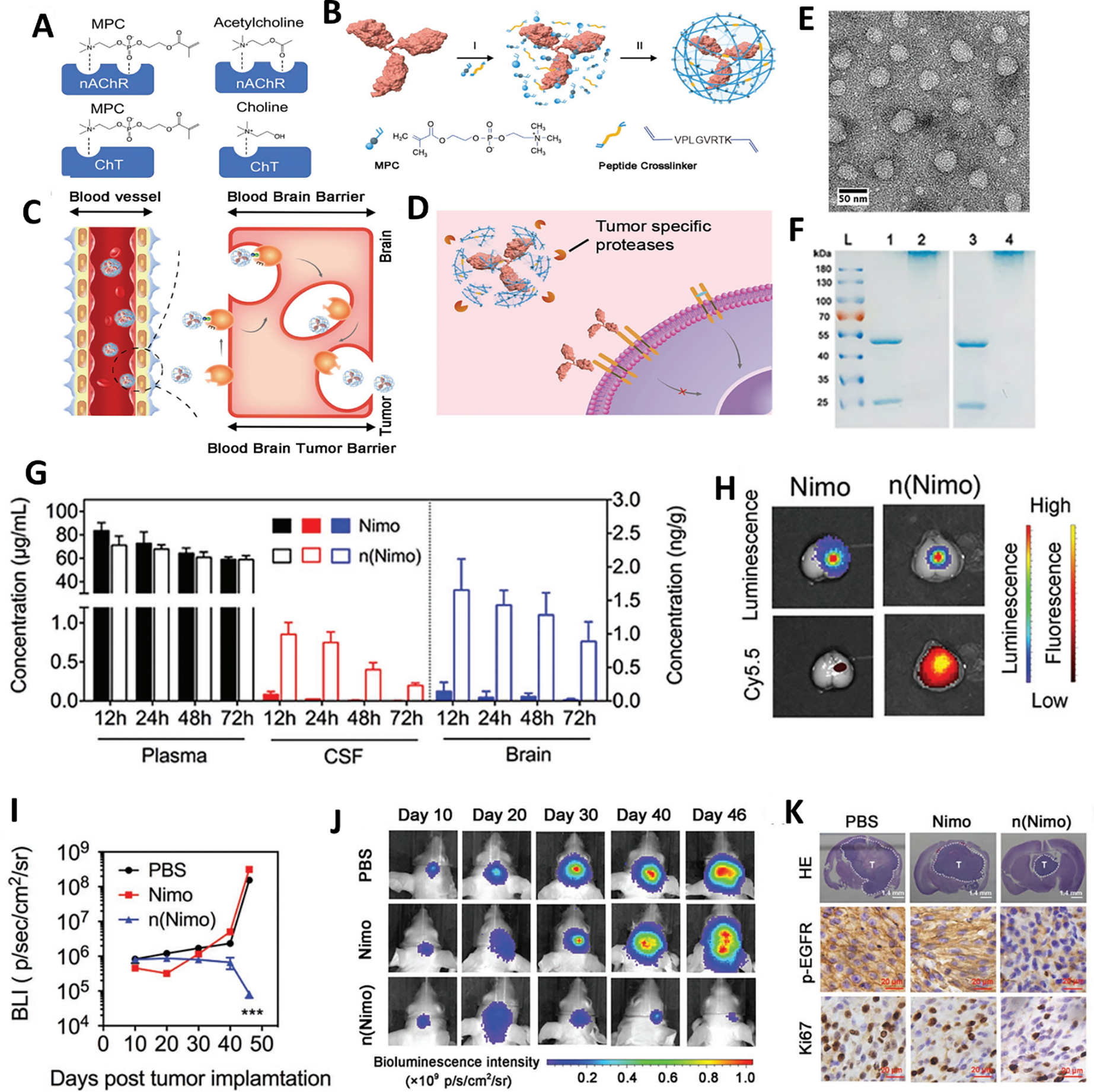
Nicotinic acetylcholine receptor (nAChR) mediated BBB penetration of nimotuzumab for brain tumor therapy. (A) Schematic illustrating the interaction between nAChRs and MPC. (B) The synthetic schemes of nanocapsules that contain acetylcholine and choline analogs. (C) nAChRs mediated BBB penetration of n(Nimo). (D) Nimotuzumab releases from n(Nimo) with the help of MMP-2. (E) The representative TEM image of n(Nimo). (F) SDS-PAGE of (L) ladder, (1) nimotuzumab, (2) n(Nimo), (3) trastuzumab, and (4) n(Tras). (G) The concentration of free nimotuzumab and n(Nimo) in the plasma, CSF, and brain. (H) Representative ex vivo fluorescence images of cy5.5 labeled nimotuzumab and n(Nimo) distribution in brain tumor-bearing brain. Bioluminescence intensity (I) and representative bioluminescence image (J) of the glioma-bearing mice at different times after receiving different treatments. (K) Representative images of the HE-stained brain, immunohistochemistry staining of p-EGFR expression and Ki67 in different treatment groups at 46 days. Adapted with permission from ref. [84].
3.2.2. Transporter-mediated transcytosis (TMT)
TMT, also known as carrier-mediated transcytosis, is a vital strategy for transporting low molecular weight nutrients from the bloodstream into the brain. Many transporters have been discovered on the BBB [38,39]. Among them, glucose transporter and glutathione transporter are the two most explored transporters for facilitating nanoparticles crossing the BBB in brain tumor treatment.
3.2.2.1. Glucose transporter-mediated transcytosis
Glucose transporter (such as GLUT1), which is found highly expressed on BBB cells and various tumors, including brain tumors, are mainly responsible for the transport of glucose from the blood to the brain and tumor, the primary energy source for their high metabolism. Firstly, abundant blood flow and hypermetabolic characterization of the brain is the prerequisite for its acting as the central role of CNS, which may partly account for the high expression of GLUT1 on endothelial cells for high and uninterrupted energy requirement. Secondly, the characterization of rapid differentiation, renewal, and metabolism of brain tumors further reinforces the expression of GLUT1 on BBB, as well as the brain tumor itself. Moreover, it seems that the BBB penetration is closely associated with the GLUT1, whose building up glycosylation is a meaningful methodology for enhancing biodistribution to the brain [92, 93]. All of them render GLUT1 an excellent target for brain-targeted drug delivery research. It was revealed that glycemia control boosts the BBB crossing and brain accumulation of glucosylated nanocarrier via GLUT1 (Figure 2) [93]. The surface of self-assembled supramolecular nanocarriers is featured with properly configured glucose, which can regulate the nanocarriers’ distribution in the brain by mimicking the glucose transporting BBB through the GLUT1, especially in the circumvent of an external trigger of a glycaemic increase after a fasting state. It was founded that 25% Glu(6/m), the optimal density of glucose on the surface of nanocarriers had the highest facilitating accumulation rate to 6% dose/g-brain [93], which was significantly higher than a previous report [94]. By fully utilizing the over-expression of GLUT1 on both BBB and glioma cells, Jiang et al. employed glucose as a ligand to develop a 2-deoxy-D-glucose modified poly(ethylene glycol)-co-poly(trimethylene carbonate) nanosystem (DGlu-NP) to realize a dual-targeting strategy, enhanced BBB penetration via GLUT-mediated transcytosis and improved drug accumulation in the intracranial tumor via GLUT-mediated endocytosis [95]. All in vitro and in vivo assays validated the feasibility and potential of GLUT-1 as a targeting receptor for enhanced drug delivery to the brain tissue, especially for brain tumors. It is worth mentioning that due to the abundant expression of GLUT-1 (approximately 100 fold higher than transferrin receptor ) and its facilitative function [76], the possible toxicity, especially neurotoxicity, should be considered when adopting this strategy. Both acute toxicity and long-term toxicity should be monitored and evaluated.in case a GLUT-mediated transcytosis is employed.
3.3.2.2. Glutathione transporter-mediated transcytosis
Glutathione (GSH) is an endogenous antioxidant that can minimize oxidative stress within the brain and protect neurons from oxidative stress-induced damage. It was revealed that GSH concentration within the brain is much higher than in the blood and other tissues. Recent studies found that GSH transporters (MRP1) are highly expressed on the endothelia of the BBB and are vital for GSH homeostasis in the brain [96]. Thus, several GSH-conjugated nanoparticles have been developed to boost the delivery of paclitaxel and Doxil to the brain through a glutathione transporter-mediated transcytosis route [97, 98]. Tellingen et al. utilized GSH-conjugated PEGylated liposomes, named G-Technology™, to fabricate (GSH-Doxil) for the treatment of intracranial xenograft brain tumors [97]. After three consecutive weekly treatments of GSH-Doxil at the 5 mg/kg of doxorubicin equivalents dose, two animals showed complete regression, which was not observed in the Doxil treatment group. In addition, the GSH-Doxil treatment was well tolerated.
3.3.2.3. Other transporter-mediated transcytosis
Apart from the aforementioned two prevalent and widely-adopted transporters as the target for transcytosis, some other transporters, such as monocarboxylate transporters (MCTs) and L-type amino transporters (LATs) expressed on the luminal membrane of brain endothelial cells also drawn some attention in drug delivery. MCTs are mainly responsible for the transport of endogenous short chain monocarboxylate solutes, such as lactate, pyruvate, and acetoacetic acid, which could serve as an alternative energy source and play an important role in energy metabolism [99]. They are also responsible for some exogenous acid drugs’ intra-brain transport, such as salicylic acid, valproic acid, benzoic acid, etc [100]. Therefore, some drug delivery systems have leveraged the specific substrates of MCTa as the target ligand for high efficient brain delivery of therapeutic agents. For example, β-Hydroxybutyric acid (HBA), as a novel ligand of MCT1, was grafted on the surface of docetaxel, carmustine and temozolomide loaded solid lipid nanoparticles (HD-SLNs) to improve their delivery to brain [101, 102]. However, due to the universal expression of MCTs almost in all tissues, including kidney, liver, intestine, heart, muscle, etc., the strategy of targeting MCTs may encounter fluctuated disposition of delivery system in different organs and compromise the brain targeting efficiency. Yet it is worth noting that the different isoform of MCTs has a inequable distribution in the different subcellular regions of the brain and types of intracerebral cell population [100, 103], which provides a meaningful guideline for the design of MCTs targeting drug delivery system for tumor located in a different region of the brain.
LATs mainly assist the internalization of neutral amino acids into cells and are commonly upregulated in most tumors [104], including brain tumors, and serve as a great potential molecular target for brain tumor treatment [105, 106]. It was also found that LATs are highly expressed on the luminal and abluminal membranes of capillary endothelial cells of BBB [107]. All of this makes LATs great potential targets for brain tumor dual drug targeted delivery by rational design of the drugs/delivery system mimicking the structure of LATs substrates [108]. One prominent representative methodology is coupling neutral amino acids or analogs to a therapeutic agent or on the surface of a drug delivery system to produce targeted pro-drugs and targeted delivery systems. For example, Parul Kharya et al. grafted L-phenylalanine (PA), a most common BBB penetrating neutral amino acid, on the surface of solid lipid nanoparticles for targeted delivery of DOX to brain tumors [109]. However, one of the critical aspects that should be carefully considered is avoiding the loss of affinity of modified amino acids to LATs after their modification to a drug delivery system. It was revealed that the presence of a free amino group and a free carboxyl group on the α-carbon atom of the amino acid is critical for the specific interaction between amino acid and LATs [110]. So, the biggest challenge and verification is how to maintain the conformational consistency of amino acids. For example, Li et al. induced γ-carboxyl of glutamate to the surface of liposomes through the side chain linkage as the targeted ligand to retain the intact structure of a free amino group and a free carboxyl group on the α-carbon atom to preserve the active targeting capacity [111].
3.3.3. Adsorptive mediated transcytosis (AMT)
Adsorptive mediated transcytosis (AMT) is another widely explored approach for delivering drugs across the BBB. AMT is triggered by the electrostatic interaction between positively charged agents and negatively charged luminal membranes of brain endothelial cells. Similar to the RMT, the tour of agents from capillary to brain parenchyma also go through a three-step procedure: positively charged agents firstly interact with negatively charged molecules or region on the luminal surface of endothelial cells via electrostatic interaction, thus triggering the formation of transcytotic vesicles; The transcytotic vesicles navigate in the cytoplasm from luminal side to the abluminal membrane of BBB cells; At last, transcytotic vesicles fuse with the membrane and release the carried agent into the brain [40]. Cationic bovine serum albumin (CBSA) and cell-penetration peptides are two kinds of the most frequently used moieties conjugated on NP to trigger NPs across the BBB through AMT [112]. For instance, Lu et al. conjugated CBSA to pegylated PLA nanoparticles to yield CBSA-NP for brain-targeted drug delivery [113]. In an in vitro co-culture BBB model, it was found that the modification of CBSA on the surface of nanoparticles could more effectively facilitate the NP transport across the BBB compared to the parental bovine serum albumin (BSA) modified NP (7.76 times higher). Moreover, the incorporation of CBAS had no impact on BBB’s permeability and integrity, ensuring the carrier system’s safety [113]. Through encapsulating cytotoxic plasmid pORF-hTRAIL, CBSA-NP-hTRAIL was employed to realize malignant gliomas gene therapy. It was demonstrated that at 30 min after administration, there were some distributions of CBSA-NP-hTRAIL in the brain and brain tumor, main colocalized with the glycoproteins. Subsequently, hTRAIL mRNA and hTRAIL protein were detected in normal brain and tumors at 24 and 48 hour later [114]. Furthermore, the repeated administration of CBSA-NP-hTRAIL in vivo realized the prospective apoptosis of C6 glioma and significantly delayed tumor growth [114, 115].
Cell-penetrating peptides (CPPs), a type of short positively charged peptide heterogeneous in size and sequence, show outstanding ability in assisting various therapeutic agents, such as proteins, oligonucleotide, antibodies, imaging agents, and drug-loaded nanoparticles, crossing the lipophilic barrier of cellular membranes, including BBB [116]. The first discovered and mostly investigated CPPs is the transactivating transcription factor TAT (AYGRKKRRQRRR), a polypeptide motif derived from the surface protein of human immunodeficiency virus-1 (HIV-1) [117]. By integrating TAT into the Angiopep-2 decorated docetaxel-loaded nanoparticles with a rational ratio, Zhong et al. developed co-functional tandem nanomicelles, termed ANG20/TAT10-Ms (Figure 4) [118]. In vivo imaging found that ANG20/TAT10-Ms had a significant higher glioma accumulation compared to the parent nanoparticles (including only Angiopep-2 decorated nanomicelles and no ligand modified ones) in an orthotopic U87MG glioma-bearing mouse model. Furthermore, DTX-loaded ANG20/TAT10-Ms exhibited the strongest glioma inhibitory effect and extend the survival time of U87MG glioma-bearing mice [118]. It was of convince that ANG20/TAT10-Ms not only hold a high glioma cell selectivity via Angiopep-2 peptide mediated targeting recognition but also displayed a markedly enhanced BBB permeation with the help of TAT. Although many potential pathways for CPPs mediated brain tumor-targeted delivery have been proposed, the cellular membrane transport mechanism of CPPs is still unclear. Earlier research found that around 0.126 % of intravenously injected TAT peptide could cross the BBB and accumulate in the CNS, probably through a nonsaturable mechanism with a unidirectional influx rate of about 0.490 ul/g/min [117]. More overly, it was speculated the CPPs either directly initiate the BBB penetration though RMT as a result of electrostatic interaction between its positive charge and negatively charged luminal surface of BBB or interacted with some particular receptors/proteins to trigger endocytosis/transcytosis [40]. It is worth noticing that although the travel route of AMT is similar to RMT, there is a significant difference between these two pathways. The prominent feature of AMT is its high binding capacity to BBB cells and associated with poor tissue selectivity due to non-special binging. It was striking that the binding potential of AMT between cargoes and endothelial cells via positive and negative electrostatic interaction could be a thousand times higher than that of RMT, which is the main reason for the high transcytosis efficacy of AMT [119]. Likewise, due to the non-special interaction between opposite charges, limited tissue selectivity, and high background noise, system toxicity is the primary shortcoming that should be taken into consideration when employing this approach.
Figure 4.
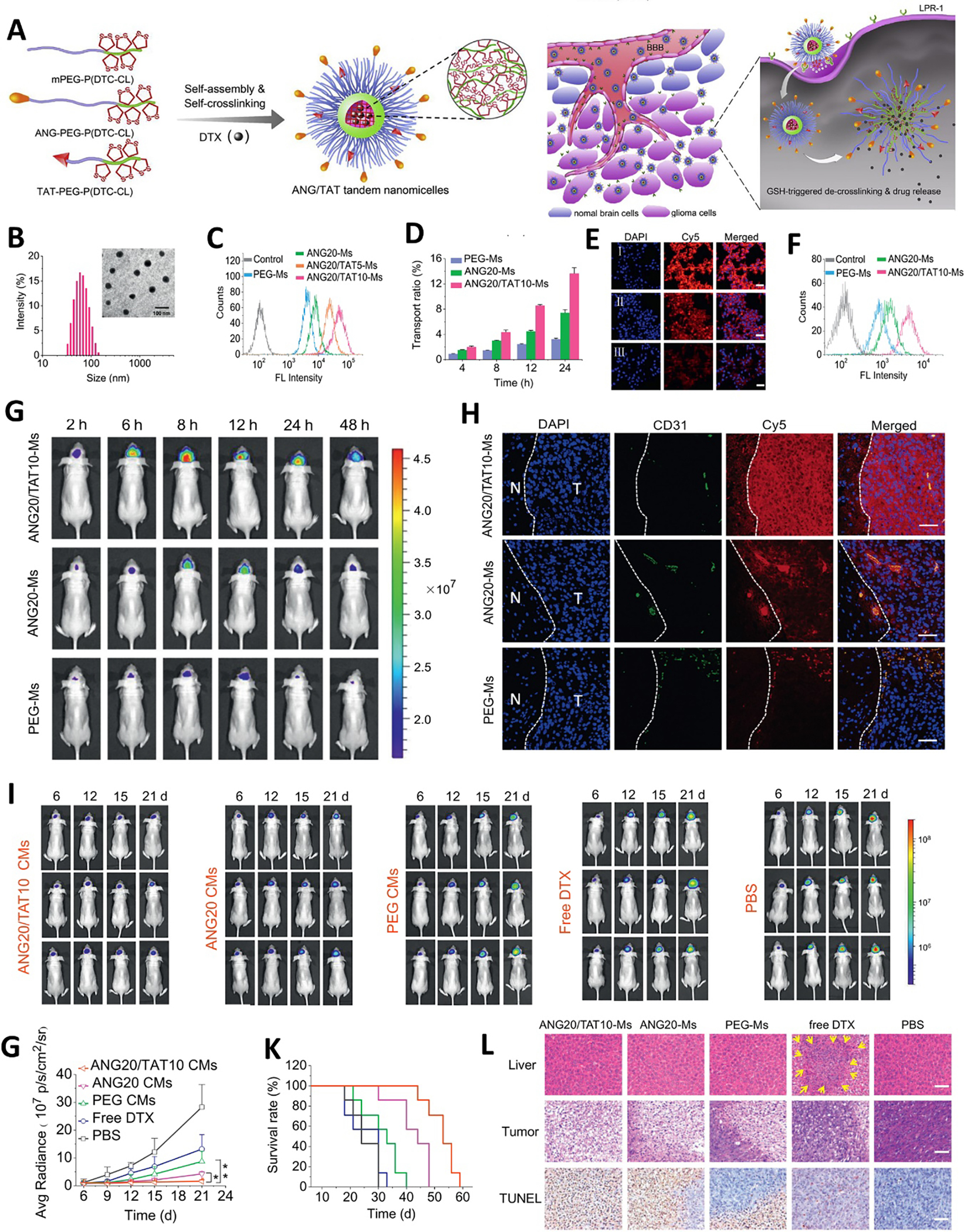
Cell-penetrating peptides TAT mediated BBB penetration of nanomedicine for brain tumor therapy. (A) Schematic illustrating the preparation of ANG/TAG tandem nanomicelles (ANG/TAT-Ms) and their in vivo anti-glioma pathway. (B) The size distribution of ANG/TAT-Ms by DLS and TEM. (C) Cellular uptake of different nanoparticles in U87MG after 4 h incubation by FCS. (D) Cumulative transport of different particles across in vitro BBB model. Cellular uptake of different nanoparticles after transporting the in vitro BBB model by U87MG by CLSM (E) and FCS (F). (G) In vivo distribution of different nanoparticles in orthotopic U87MG glioma bearing mice. (H) The penetration behavior of different nanoparticles in orthotopic U87MG glioma observed by CLSM. In vivo anti-orthotopic U87MG glioma evaluation of different treatments monitored by bioluminescence imaging (I) and bioluminescence intensity (G). (K) Kaplan-Meier survival curves of mice in different treatment groups. (L) H&E staining of liver and tumor and TUNEL assay of tumor in different treatment groups. Adapted with permission from ref. [118].
3.4. The temporary BBB opening and disrupting facilitated penetration
Another straightforward, effective, and extensively explored strategy for brain drug delivery is temporary opening and disruption of BBB by using biochemical reagents or physical approaches. Different from interaction-mediated transport, of which there is nearly no influence on the integrity of BBB, the disruption of BBB by physicochemical method is usually referred to as compromising the integrity of BBB to a certain degree temporarily and reversibly. Subsequently, therapeutic agents transport into the brain parenchyma by diffusion. The commonly adopted disruption methods include hyperosmotic agents induced osmotic disruption, ultrasound disruption, magnetic disruption, and their orthogonal combination.
3.4.1. Chemical agents mediated disruption
Common hyperosmotic agents, mannitol, glycerol, and arabinose, may induce a high osmotic pressure and result in the BBB opening temporarily. The primary mechanism of hyperosmotic agents induced the BBB opening is that the osmotic pressure difference leads to the shrinking of vascular endothelial cells and disruption of the tight junction, which results in the broadening of the paracellular space within BBB and subsequent transportation of drugs into the brain parenchyma by diffusion. Moreover, this kind of disruption-induced transportation efficiency can be controlled by regulating the type of hyperosmotic agent, its concentration, injection speed, and retention time [120, 121]. Riina et al. reported that there was no dosed-limiting toxicity from a single dose of super-selective intraarterial cerebral infusion (SIACI) of bevacizumab up to 15 mg/kg after osmotic BBB disruption with mannitol in patients with recurrent malignant glioma [121], demonstrating the safety and toleration of SIACI of mannitol followed by bevacizumab. One month later, magnetic resonance imaging furtherly showed the efficiency of SIACI treatment with bevacizumab for recurrent malignant glioma, referring to the reduction in the tumor area, volume, perfusion and T2-weighted/FLAIR signal, no matter whether the patients previously received the intravenous bevacizumab exposure or not [121]. It was noted that osmotic-induced BBB disruption might also allow some other macromolecules, toxic and harmful agents, to enter the CNS, which may result in neuropathological changes and dysfunction. Moreover, this osmotic BBB disruption is an invasive technique and requires the collaboration of highly trained neurosurgeons to achieve a plausible therapeutic benefit.
Apart from hyperosmotic agents induced BBB temporary shrinking, biological agents-mediated integrity alternation of BBB/BBTB is another alternative for BBB disruption. These chemical agents are mainly comprised of vasoactive compounds [122], such as histamine and bradykinin, which could selectively target B2 receptor expressed on the endothelium, followed by triggering transiently intracellular Ca2+ increase and sequentially leading to tight junctions (TJs) disruption and increased the drug permeability [123, 124]. It was revealed that some chemical agents, such as alkyl glycerols (AGs) [125], exhibited bioactivity similar to vasoactive compounds and could disrupt the BBB. However, it was independent of the alteration of TJs integrity, suggesting the complexity of chemical meditated BBB disruption and their promising research potential [122]. Zhou et al. developed a panel of nano-drug delivery systems by leveraging BBB modulating molecules to increase the accumulation of pharmaceutical agents in brain tumors [126–128]. One representative work utilizes an autocatalytic approach for improving the transport of nanoparticles into brain tumor for a theranostic purpose [126]. The autocatalytic nanoparticles (ABTT NPs) were composed of a biodegradable poly(amine-co-ester) terpolymer, a 36-amino acid peptide (chlorotoxin, CTX), a BBB disruption agent lexiscan, and a chemotherapeutic agent paclitaxel (PTX). Firstly, a small portion of ABTT NPs entered the brain tumor microenvironment through a traditional mechanism, such as active targeting mediated transcytosis (RMT). After penetrating the BBB, ABTT NPs could locally release the loaded BBB modulators lexiscan, which in turn transiently enhanced the BBB permeability to allow more ABTT NPs accumulation in the tumor microenvironment. This cascade amplification triggered by autocatalysis driving positive feedback loop would make nanoparticles readily cross the BBB and preferentially accumulate in the brain tumor [126]. Impressively, it was founded that the accumulation of ABTT NPs in the brain tumor region was 4.3 and 94.0 folds higher than that in the liver and in the non-tumor regions of brain, respectively [126]. Adopting a similar strategy, more recently, they developed an autocatalytic nano-delivery system, LANPs, integrated with breast cancer brain metastasis targeting, tumor microenvironment responsive size shrinking, and BBB disrupting ability [128]. The enhanced brain penetration of LANPs mainly derived from the encapsulated lexiscan, an adenosine receptor agonist, could pharmacologically modulate the permeability of the BBB [129]. It was founded that the enhancement of BBB permeability was dose-dependent, and the discrete TJs would rapidly recover flawlessly [129]. Considering the FDA’s approvement and practice in the clinical application of lexiscan and combining with the superiority property of nanomedicine, LANPs hold great potential of translating into clinical application for the treatment of brain tumors, including metastatic brain tumors [128, 129]. Similarly, minoxidil sulfate (MS) could serve as a BBB modulator by upregulating the expression of caveolin-1 on endothelial cells and downregulating the expression of tight junction proteins to ameliorate BBTB permeability, following boosting the transport of nanoparticles across the BBB and entering brain tumor nidus through transcellular and paracellular pathways [130].
3.4.2. Ultrasound-mediated disruption
Apart from its clinical application in imaging modality, recently, ultrasound, especially focused ultrasound (FUS), is also widely explored as a physical tool to reversibly disrupt BBB and shows great potential for enhancing the bioavailability of therapeutics for the treatment of CNS diseases, such as brain tumor [131]. Mechanically, by combined with microbubbles (MB), FUS can concentrate acoustic energy to trigger the cavitation activity and produce shear stress in endothelial cells, induce transient and reversible opening of TJs, and/or activate the signaling pathway, which in turn leads to the disruption of BBB [132]. As a non-invasive and readily repeatable therapeutic modality, FUS has been widely used to improve various chemotherapeutics delivery to brain tumors, including doxorubicin [133], temozolomide [134], and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) [135]. Due to the advantage of focused and defined BBB disruption by FUS, the combination of nanoparticles-based drug delivery system (nanomedicine) coupled with MB-facilitated FUS has attracted tremendous attention in brain tumor-targeted therapy and achieved superior pharmacokinetics (PK) properties in contrast to its free drug [136]. An early study investigated the marriage between FUS and liposomal doxorubicin for enhanced brain targeted drug delivery and antitumor effect in glioblastoma multiforme (Figure 5) [137]. The study tested a human atherosclerotic plaque-specific peptide-1 (AP-1)-conjugated liposomes containing doxorubicin (AP-1 Lipo-Dox) for the targeted drug delivery to the tumor highly expressing interleukin-4 receptors (IL-4R). AP-1 Lipo-Dox was administered intravenously in the experimental brain tumor model, followed by pulsed FUS. It was founded that, compared to control mice treated with AP-1 Lipo-Dox or unconjugated Lipo-Dox, the mice that received liposome coupled with FUS showed an enhanced accumulation of doxorubicin in the tumor region. Moreover, AP-1 Lipo-Dox coupled with FUS increased the doxorubicin ratio of the tumor-to-normal brain by two-fold compared to that in the control group [137]. Consequently, the combination treatment yielded an enhanced antitumor efficiency and reduced side effects on normal brain tissue. Based on a similar approach, Price et al. utilized magnetic resonance image (MRI)–guided focused ultrasound (FUS) together with circulating microbubbles to realize brain tumor gene transfection with the help of systemically administrated “brain-penetrating” nanoparticle (BPN) gene vectors [138]. Besides enhancing nanoparticle accumulation in brain tumors via transient and reversible BBB disruption, FUS could also serve as an external stimulus to control drug release from nanoparticle/MBs. Multifunctional MBs not only acted as an ultrasound contrast agent to facilitate the FUS-induced BBB disruption but also served as drug-carrying vehicles that were responsive to ultrasound for drug release [139]. In this case, the microbubble (BCNU-MBs) had a super high 1,3-bis(2-chloroethyl)-1- nitrosourea (BCNU) loading capability (68.01 % loading efficiency) and free of premature release. Upon FUS, the intravenous administrated BCNU-MBs readily facilitated local BBB disruption, permeated into the brain tumor, and released BCNU at the targeted site [139]. Attributed to the high BBB penetration and tumor-specific release effect of BCNU-MBs, the progression of glioblastoma multiforme was significantly suppressed (39.6%), and the median survival was extended to 32.5 days [139]. It is of worthy noting that the potential adverse effects from BBB disruption induced by FUS, such as hemorrhage, brain damage, obnubilation, and brain inflammation, should be closely monitored. To balance between achieving the maximum efficiency for brain tumor and minimizing the potential side effects of the treatment, the parameters of FUS, such as frequency and power, should be carefully optimized [67, 140].
Figure 5.
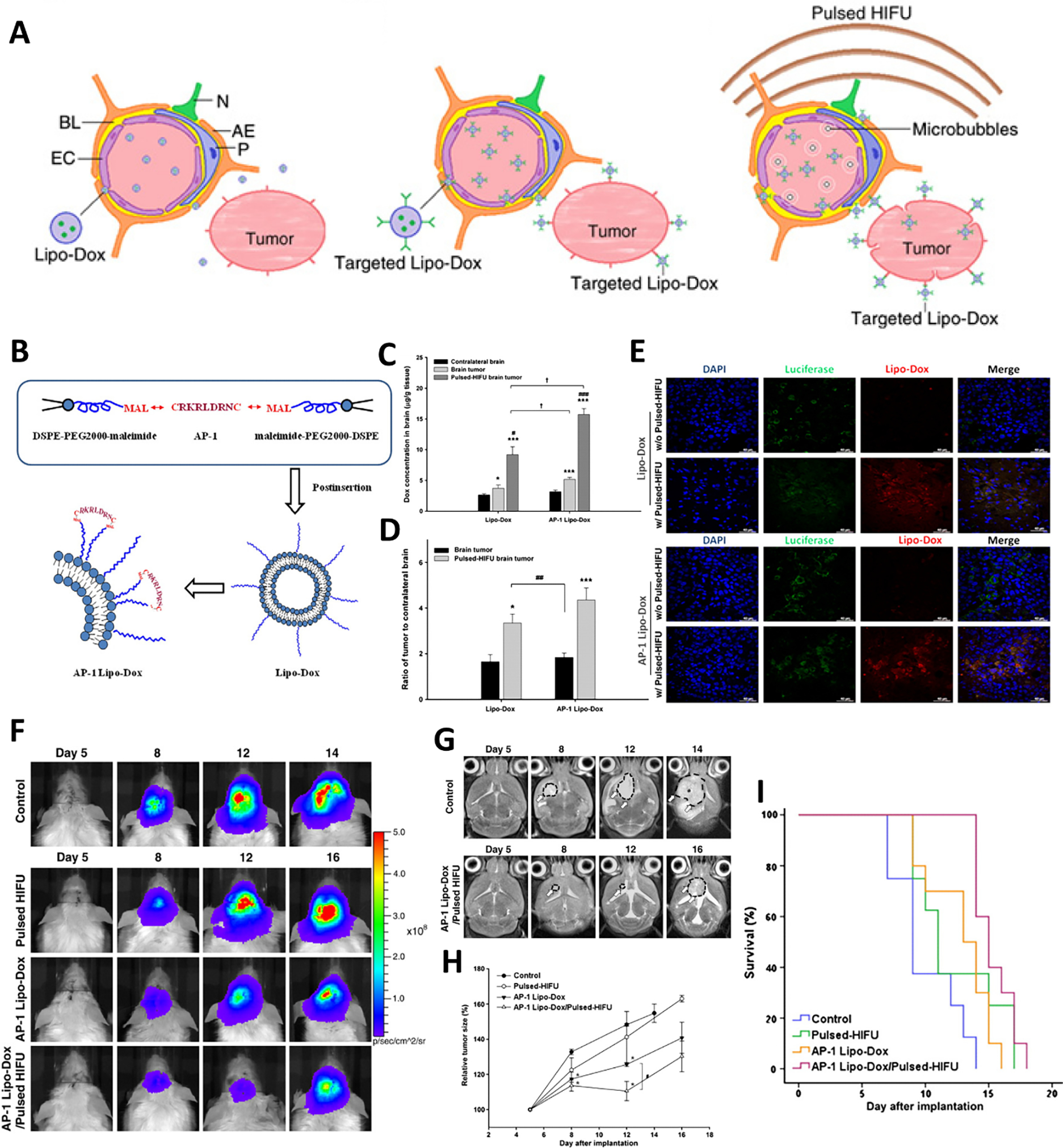
Focused ultrasound and interleukin-4 receptor mediated BBB penetration of liposomes for glioblastoma multiforme therapy. (A) Schematic illustration of receptor and FUS enhanced BBB penetration of DOX loaded liposome and superior anti-brain tumor. (B) Schematic illustrating the preparation of DOX loaded AP-1 conjugated liposomes. Measurements of Lipo-Dox and AP-1 Lipo-Dox in the brain tumor (C) and contralateral normal brain regions (D) with or without sonication. (E) Immunocytochemistry staining of brain tumor to evaluate the distribution of Lipo-Dox and AP-1 Lipo-Dox in glioma xenografts with/without FUS. (F) Bioluminescence imaging of glioma xenografts in different treatment groups to evaluate the anti-tumor efficiency. (G) Representative T2-weighted magnetic resonance imaging of glioma xenografts in the brain in different treatment groups. (H) Relative tumor growth trend of GMB 8401 glioma xenografts in different treatment groups (relative to day 5). (I) Kaplan–Meier survival plot of GBM-bearing mice in different treatment groups. Adapted with permission from ref. [137].
3.4.3. Magnetic resonance-mediated disruption
Apart from severing as an imaging modality for brain tumors, magnetic resonance can also be used to increase the BBB permeability locally [115]. When magnetic nanoparticles are exposed to a gradient/alternating magnetic field, local hyperthermia will be generated from the magnetic nanoparticles as a local heat source through a mechanism named Néel relaxation [141], to induce a substantial but reversible opening of the BBB, which is sensitivity to physiologically relevant temperature change (38–39 °C) [142, 143]. Martel et al. validated the concept of brain-targeted drug delivery by remotely controlling the permeability of BBB through magnetic hyperthermia [143]. In the study, poly (maleic acid-co-olefin)-coated Fe3O4 (PMO-MNPs) was injected via a microcatheter, which was inserted into the External Carotid Artery (ECA) and advanced to the Internal Carotid Artery (ICA) using a cannulation technique. Subsequently, the brain was exposed to a low radio frequency (RF) field for 30 min. Evans blue (EB) staining revealed that EB has distributed into the brain parenchyma in the magnetic hyperthermia treated groups, indicating the BBB opening by magnetic heating. Immunohistochemistry analysis of CD68 showed no other trace of CD68 was found in the parenchyma of the magnetic heating treated brain, indicating no apparent immune response to magnetic heating in the brain tissue. Moreover, the amount of fluorescent units counted after 2 h of recovery from magnetic heating was substantially lower than hyperthermia and normothermia. In addition, transmission electron microscopy (TEM) imaging did not reveal abnormal brain structure after 2 hours of recovery. Taken together, the author concluded that magnetic hyperthermia could transiently induce BBB opening, and the opening could be recovered within 2 hours [143]. Noticeably, although EB had a substantive extravascular infiltration into brain parenchyma after magnetic hyperthermia, there was no significant appearance of PMO-MNPs (5–20 nm) in the brain parenchyma, suggesting the disruption of BBB by magnetic hyperthermia might have a limitation of size cut-off. However, in another research, Jin et al. found that MNPs (~100 nm) could permeate the BBB and accumulate in the perivascular zone of the brain parenchyma through a transcellular trafficking mechanism when subjected to an external magnetic field without apparent toxicity [144]. Although the paradoxicality in permeability of MNPs into the brain, all these results proved the practicality, relative safety, and recovery of magnetic hyperthermia-mediated BBB opening. Therefore, further investigation about the size limitation of magnetic hyperthermia-induced BBB opening should be systematically performed. And the parameter of magnetic resonance, such as the strength and frequency of alternating magnetic field (AMF), the size and concentration of MNPs, and the time and region of AMF application, should be optimized to maximize the benefits of magnetic hyperthermia-induced disruption of BBB and minimize the risk of systemic toxicities [145].
3.4.4. Multimodality-mediated disruption
Integrating different BBB disruption modalities for brain tumor treatment could be a promising strategy, which not only can accurately change the permeability of BBB for theranostics agents across the brain but also can real-time display their penetration efficiency and monitor the treatment outcome. Among them, the integration of MRI with FUS is one of the most commonly explored combinations. Usually, FUS with microbubble is performed to induce the increase of BBB permeability, of which enhanced BBB permeability would be facilely characterized by MRI whenever the MRI contract agents transport across the disrupted BBB and retained in brain tumor, which is similar to FUS induced accumulation of chemotherapy agents or nanoparticles [133]. More significantly, MRI contrast agents can be coupled with the FUS microbubble to serve as an integrated and multifunctional contrast agent for theranostic purposes. Lammers et al. embedded ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles into the shell of poly(butyl cyanoacrylate)-based microbubbles (MB) to yield USPIO-MB, which was used to mediate and monitor BBB permeation (Figure 6) [146]. Upon encountering transcranial ultrasound pulse, USPIO-MB induced vessel permeability through acoustic force. Simultaneously, the structure of the microbubbles was destroyed to release the trapped USPIO. Thus, the available and small size of USPIO (around 5.5 nm) would easily cross the disrupted BBB and accumulate in the parenchyma, which could be evidence of BBB opening and be verified by MRI [146]. [146]. Moreover, by leveraging the high spatial resolution, MRI can also be employed as an invasive method to guide FUS to targeted open the BBB at a specific localization, named MR-guided focused ultrasound (MRgFUS) [147]. Attributing to the fine spatial control over the treatment field and real-time image guidance of transcranial non-invasive MRgFUS, a low-intensity ultrasound could meet the requirement of temporary disruption of the BBB, the region of BBB disruption could also be customized with a special shape and location in the intracranial vault [147], emphasizing its safety and feasibility. Using an MRI-based transport analysis, it was further revealed that apart from BBB/BBTB opening, FUS could also significantly augment brain tumor interstitial flow velocity and change “per voxel” flow direction, which might also play a critical role in enhancing nanoparticles transporting through BBB [138].
Figure 6.
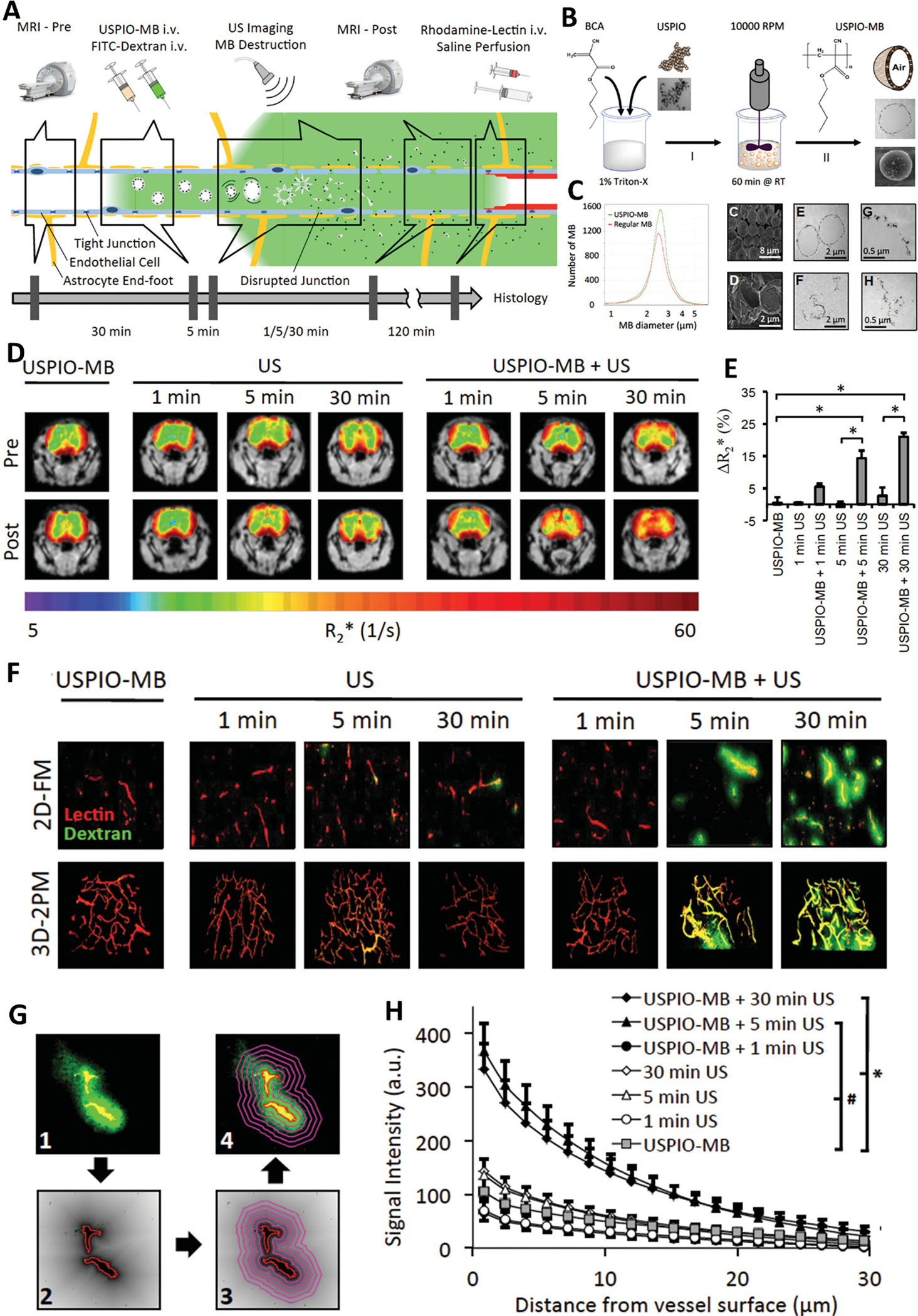
Theranostic USPIO-MB for mediating and monitoring BBB permeation. (A) Schematic illustration of FUS-mediated disruption of BBB and MRI-based monitoring of BBB permeation and drug delivery across BBB by using USPIO-MB. (B) Schematic illustrating the preparation of USPIO-MB. (C) Characterization of USPIO-MB with DLS, TEM, and SEM. (D) MRI imaging of USPIO deposition across the BBB upon USPIO-MB destruction. (E) Quantification of R2* values in D. (F) 2D fluorescence (2D-FM) and 3D two-photon microscopy (3D-2PM) imaging of FITC-dextran (green) and Rhodamine-lectin-stained blood vessels (red) to evaluate the BBB permeation. (G) Schematic illustrating the protocol of quantifying the extravasation and penetration of FITC-dextran. (H) Signal intensity of extravasated FITC-dextran as a function of distance to vessel surface. Adapted with permission from ref. [146].
Photoacoustic (PA) imaging is a nonionizing imaging technique with a superior imaging capability for deep tissue with high resolution by integrating optical excitation with ultrasound detection. By taking advantage of deep tissue imaging, PA holds great potential for imaging theranostic agents penetrating BBB and entering brain parenchyma by overcoming the skull-led light and ultrasound attenuation [148]. Zheng et al. prepared a multifunctional theranostic nanosystem, named as DOX-HCu, by integrating ultrasmall Cu2–xSe and doxorubicin (DOX)-loaded organic-inorganic hybrid hollow mesoporous organosilica nanoparticles (HMONs) [149]. Upon FUS, DOX-HCu could be accurately delivered into glioblastoma multiforme (GBM) and penetrated the tumor tissue to execute its antitumor mission. More importantly, this process could be visualized through PA imaging [149], realizing real-time triggered drug delivery and monitoring of antitumor outcome.
Another promising strategy for enhancing theranostic agents penetrating BBB and delivery to brain tumors is the joint of BBB disruption and receptor-mediated active targeting. The transient and reversible opening of BBB would pave the way for theranostic agents to enter the brain parenchyma. Whereas receptor-mediated active targeting would guide the theranostic agents, especially nanoparticles, to precisely navigate to brain tumor tissue, thus alleviating non-specific distribution-induced background signal enhancement and side effects. For instance, the combination of FUS and interleukin-4 receptor-targeted liposomal doxorubicin realized enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme [137]. In the presence of MB, pulsed focused ultrasound waves could transcranially disrupt the BBB, creating the condition for the leakage of pre-injected human atherosclerotic plaque-specific peptide-1 (AP-1)-conjugated liposomes containing doxorubicin (AP-1 Lipo-Dox) into brain tumor, similar as EPR effect. Subsequently, AP-1 Lipo-Dox was internalized by glioblastoma multiforme (GBM) 8401, which highly expressed IL-4 receptors, through receptor-mediated endocytosis [137]. Compared to the control tumor, pulsed FUS could increase liposome distribution in the brain tumor, including AP-1 Lipo-Dox and control Lipo-Dox. In addition, the tumor-to-normal brain doxorubicin of pulsed FUS treated tumor was increased twofold compared to that of control tumors, indicating FUS mediated the disruption of BBB. Moreover, the tumor-to-normal brain ratio is the highest in the mice treated with AP-1 Lipo-Dox followed by pulsed FUS. Consequently, combining sonication with AP-1 Lipo-Dox significantly inhibited GBM growth and increased median survival [137].
3.5. Living cell and its membrane-mediated BBB crossing
Recently, cells with intrinsic tropism towards tumors, such as mesenchymal stem cells (MSCs), neural stem cells (NSCs), macrophages, and neutrophils (NEs) have been explored as “Trojan Horse” for the delivery of cargo across the BBB through cell-mediated transportation [150]. The tumor tropism nature of these cells is mainly attributed to their innate bio-interfacing property or genetic modification. The expression of some receptors or markers on the membrane, such as CXCR4, CX3CR1, and L-selectin, could perceive tumor cells and orchestrate the tumor microenvironment (TME). The TME is featured of chemoattractant gradients of various tumor tissue derived chemokines, such as stromal cell-derived factor-1 (SDF-1), colony-stimulating factor 1 (CSF-1), tumor necrosis factor-alpha (TNF-α), hypoxia-inducible factor-1α (HIF-1α), and interleukin 8 (IL-8) and so on [150]. Although the exact mechanisms of these carrier cells trafficking to the brain tumor is fully understood, and different kind of cells might have different trafficking mechanisms, they may still share several general stages [150–152], (1) capture: the navigating carrier cells in circulation system are recruited to tumor region by chemokines (inflammatory factor) as paracrine by tumor tissue; (2) contact: the carrier cells are tethered to the endothelial surface through the specific interaction between receptor and ligand expressed on the membrane of carrier cells and inflamed endothelium, respectively; (3) rolling, the carrier cells roll along the endothelium and eventually adhere them totally along with elevated interaction; (4) extravasation: under the attraction of chemokine or cytokine gradient, the adhered carrier cells adaptively emigrate to the tumor perivascular region and penetrate tumor mass (Figure 7). Because of this intrinsic homing property, many cargos, such as therapeutic agents loaded nanoparticles, liposomes, immunomodulators, imaging contrast agents, and oncolytic viruses, could be efficiently delivered to brain tumors [150]. Generally, Trojan horse and backpacker are the two main approaches that are used by carrier cells to deliver specific payloads to brain tumors [153]. The former indicates that the cargos are internalized into the carrier cells first and protected inside the cell carriers before they are delivered to the destination, followed by being released from the carriers to excrete their function [153, 154]. The backpacker means that the payloads are attached to the surface of carrier cells through a covalent bond, electrostatic interaction, and/or biological interaction [155]. Similar to a trojan horse, a package like cargo also should be unloaded from the backpacker-like carrier cells to perform their task. Moreover, cell carriers can be genetically modified to express specific therapeutic agents and directly target, regulate, and kill cancer cells. These secreted therapeutic agents could be cytotoxic proteins, such as TRAIL [156], immunomodulatory-associated cytokines, human interleukin-2 (IL-2) [157], and specific enzymes, such as rabbit CE [158].
Figure 7.
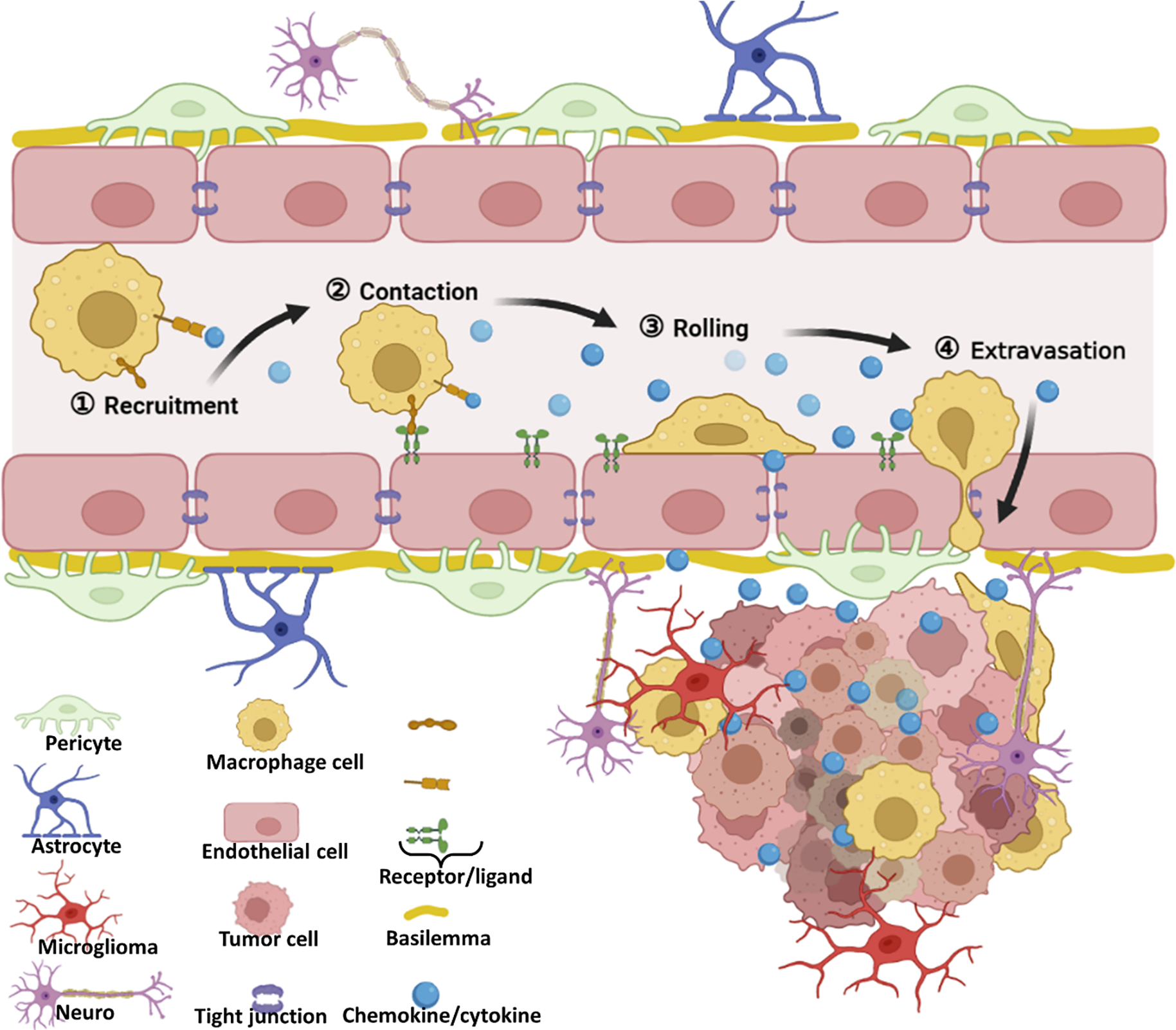
The potential pathway of living cell-mediated BBB crossing.
Despite living cell-based carriers holding promising potential in brain tumor application, several concerns should be well taken into consideration. The first and foremost is that the loading of theranostic cargos should have negligible influence on the cell viability and their tropism toward brain tumor, which means that the ratio between the number of carrier cells and the cargos concentration, or loading content, is critical for desired delivery efficiency, and should be optimized [159], especially for cytotoxic drugs. Another important aspect is the loaded cargos need to be released from cell carriers in a controlled manner. The desired drug release profile of a cell carrier system is a limited cargo premature release in the circulation system and effective drug unload in the destination, which would achieve a preferable theranostic effect [160]. Moreover, much attention should be paid to the final fate of carrier cells in the targeted destination and throughout the organism after their delivery, especially for the potential adverse effects [150].
As the extension of living cell-mediated BBB crossing, recently, cell membrane cloaked nanoparticles have drawn mounting attention in the diagnosis and therapy of brain tumors [150]. As an aforementioned statement, contact interaction between carrier cells with tropism toward brain tumor cells and tumor surrounding environment is the prerequisite for BBB penetration into the brain. This interaction is mainly modulated by carrier cell-surface receptors and adhesions molecules on the endothelial cells, involving multiple cell-surface and secreted proteins. It does not depend exclusively on the expression of a single protein or ‘receptor’ [158]. Therefore, it was postulated that the membrane-associated biological functions, such as tumor tropism, would be preserved after they are isolated and followed by cloaking onto nanoparticles if their membrane proteins are retained. In 2011, for the first time, Zhang et al. designed this strategy and put it into practice by coating plasma membrane derived from red blood cell (RBC) onto the surface of PLGA nanoparticles [161], creating the precedent of biological membrane mimetic nanoparticles. By integrating membranes from different types of cells, core nanoparticles, and loaded agents according to different objectives, this biomimetic strategy would create a variety of new cell membrane-camouflaged nanocarriers with different functions. This coating generally confers the biomimetic nanocarriers with the capacity to evade confiscation by the RES, prolong the circulation time in the body, cross biological barriers, and actively target disease tissue [162–165]. Inspired by this biomimetic concept, some research works have adopted this strategy for the treatment of brain-related diseases, including glioblastoma multiforme [166, 167], ischemic stock [168, 169], Parkinson’s disease [170], and brain metastases tumor [166]. As to brain tumors, the first research work that adopted this biomimetic strategy was carried out in 2017. Thereof, apart from the coating of RBC membrane on the surface of DOX-loaded PLGA nanoparticles (RBCNP), a CDX peptide, which had a high binding affinity with nicotinic acetylcholine receptors (nAChRs) highly expressed on the surface of brain tumor ECs, was grafted on the surface of RBCNP to increase the brain tumor targeting ability to yield DCDX-RBCNPs [167]. By integrating the active targeting effect of CDX peptide and the merits of RBCs membrane coating, such as prolonged blood circulation, good biocompatibility, and low immunogenicity, DOX-loaded DCDX-RBCNPs exhibited superior therapeutic efficacy with limited toxicity to normal cells in comparison to non-targeting counterpart [167]. Different from RBCs membrane coated nanocarriers integrated with targeted ligand, Liu et al. directly employed brain metastatic tumor cell membranes to camouflage nanoparticles [166]. By inheriting the intrinsic brain-homing property of the brain metastatic tumor cell, the coating of brain metastatic tumor cell membranes would bestow the nanoparticles with BBB penetrating capacity, as well as target brain tumor by a homotypic binding mechanism [171, 172], which offers a new perspective for brain tumor diagnosis and therapy. Recently, our group employed a similar Trojan horse strategy to combat the early-stage brain metastasis of breast cancer (BMBC) with the cell membrane of BMBC cells (Figure 8) [173]. Compared to other brain tumors, the BBB of early-stage BMBC is intact and has not yet deteriorated to form BBTB. Therefore, minimal drugs, including nanomedicines, can penetrate the BBB and reach metastasis lesions, which partially leads to the exclusion of chemotherapy from the standard care for BMBC [174]. In our study, we collected and purified brain-homing breast cancer cells (MDA-MB-231/Br) after several rounds of intracardiac injection and isolation from the brain. It was founded that after camouflaging the cell membrane of MDA-MB-231/Br onto the surface of DOX loaded poly (D, L-lactic-co-glycolic acid) (PLGA) nanoparticles (DOX-PLGA@CM), the DOX-PLGA@CM was endowed the magical ability to penetrate the BBB of normal mice and homing to early-stage of BMBC behind an intact BBB. In vivo study found that DOX-PLGA@CM could significantly slow down the progression of BMBC, reduce tumor burden, and extend the survival time for the mice with BMBC [173]. This study offered a paradigm of integrating drug-loaded nanoparticles and the cell membrane of brain homing cancer cells, which opened a new window for the treatment of BMBC and other brain metastatic cancers.
Figure 8.
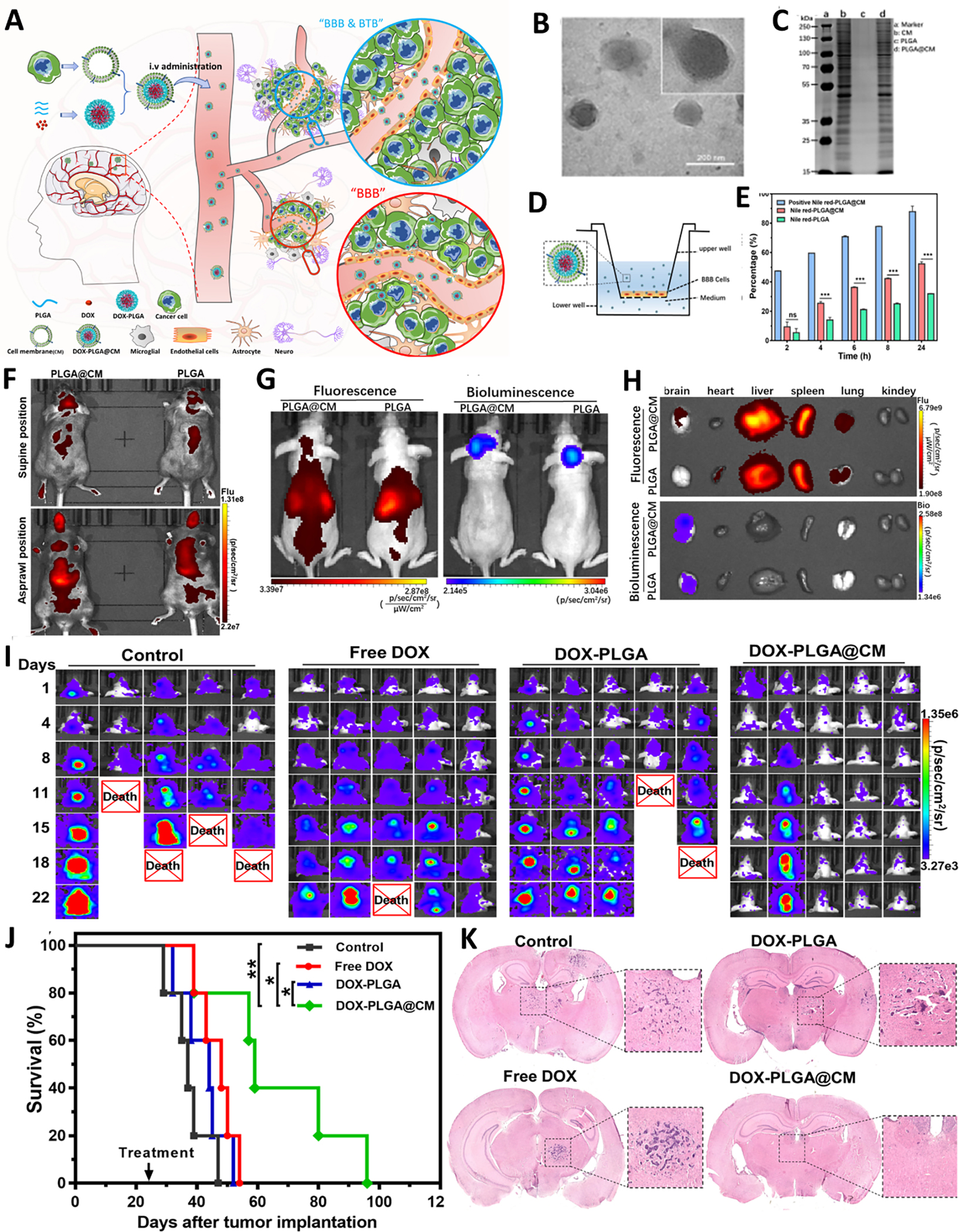
Brain metastatic tumor cell membrane mediated nanoparticles BBB crossing for the treatment of brain metastasis of breast cancer. (A) The schematic illustration for the fabrication of DOX@PLGA@CM and its BBB penetration. B The TEM of DOX@PLGA@CM. (C) The protein analysis of CM on the surface of PLGA@CM by SDS-PAGE. (D) The scheme of BBB crossing of PLGA@CM in vitro BBB model. (E) Quantitative analysis of transcytosis efficiency of PLGA@CM in vitro BBB model. (F) In vivo fluorescence imaging indicating the distribution of DIR labeled PLGA@CM in normal C57BL/6J mice. (G) In vivo fluorescence imaging indicating the distribution of DIR labeled PLGA@CM in BMBC model. (H) Ex vivo fluorescence imaging of DIR labeled PLGA@CM in different organs of BMBC model mice. (I) In vivo bioluminescence imaging of BMBC in different groups at the indicated time. (J) Kaplan–Meier survival curves of BMBC bearing mice in different treatment groups. n=5. (K) Representative whole brain H&E staining of BMBC bearing mice in different treatment groups. Adapted with permission from ref. BMBC [173].
4. The influence of nanoparticles fabrication parameters on the BBB penetration
In addition to the aforementioned pathological and physiological characteristics of brain tumors, as well as the interaction between BBB and nanoparticles, the parameters of nanoparticles also influence their crossing the BBB and entering brain tumors, including surface modification, size, morphology, and even the loaded cargo. To maximize BBB penetrating efficacy and subsequent therapeutic effect of nanoparticles on brain tumors, those parameters should be optimized during the design and construction of nanoparticles. The following sections briefly summarize the influence of nanoparticles parameters on the BBB transcytosis behavior.
4.1. Surface functionalization
Since both RMT and AMT involve the interaction between nanoparticles and BBB, the primary aim of the surface functionality of nanoparticles is to increase their interaction with BBB to further facilitate them across the BBB into brain parenchyma. These functionalizations include specifical ligand decoration to trigger RMT, surface chemistry modification to improve pharmacokinetics [175, 176], and surface charge optimization to facilitate AMT, etc.
As for ligand decoration, except for the choice of ligands as discussed above, another important aspect is the ligand density decorated on the surface of the nanoparticles [177]. Unsurprisingly, it is easy to understand that a low density of ligand decoration would not be sufficient to trigger a strong multivalent binding for RMT. In contrast, a too high density of ligand decoration might trap nanoparticles on the luminal side of BBB due to too strong binding and preventing the release of nanoparticles inside the brain [178]. For instance, by tuning the transferrin (Tf) density on gold nanoparticles to optimize their binding to transferrin receptor on brain endothelial cells, Wiley et al. found that 30 Tf and 20 Tf molecules per nanoparticles decoration were the optimized densities for gold nanoparticles with a size of 45 nm and 80 nm, respectively, to realize their best traversing into the brain parenchyma [178]. It is noted that there are no universal principles to define the best number or density of ligands for nanoparticle surface decoration, the optimal ligand density for a brain-targeted nanoparticle is ligand and nanoparticle specific. Another scenario is decorating multiple types of targeting ligands on the surface of nanoparticles, which would make the nanoparticles interact with different receptors simultaneously, increasing BBB penetrating efficacy and brain tissue selectivity [118, 179, 180]. However, the use of a combination of different ligands onto the nanoparticles is an artistic and specialized skill, referring to the choice of ligands, the ratio optimization of different ligands, and the ligand specific grafting technology.
Pegylation is one of the most common surface chemistry functionalizations for nanoparticles [181]. The coating of polyethylene glycol (PEG) on the surface of nanoparticles can prevent them from aggregation, opsonization and phagocytosis, and improve the systemic circulation time by forming a hydrophilic layer on the surface, which subsequently increases the interacting opportunity between nanoparticles and brain vascular endothelial cells [182]. Moreover, the grafted PEG can also serve as a linker between ligand molecules and nanoparticles. However, many parameters of PEG, such as its molecular weight, content, density, and conformation, influence the function and should be optimized during the fabrication of brain tumor-targeted nanoparticles [181]. In addition, some potential drawbacks of Pegylation, such as shielding the exposure of targeting ligands, interfering with the interaction between nanoparticle and targeted cells, and possible activation of anti-PEG immune response, should be thoroughly considered. Except for PEG, others polymers, such as hyperbranched glycerol (HPG) [183], polysaccharide [184], vitamin E TPGS [185], polyvinyl alcohol (PVA) [186] and polyethylene oxide (PEO) [187], etc., are also used as alternative surface chemistry functionalization approaches to improve nanoparticles in vivo pharmacokinetics and brain tumor targeting.
The surface charge of nanoparticles also has a significant influence on their brain tumor targeting capacity and pharmacokinetic properties [188]. It is well known that nanoparticles with positive charges could readily interact with negatively charged cell membranes through electrostatic interaction, followed by triggering AMT [188, 189]. However, highly positively and negatively charged nanoparticles could alter and disrupt the integrity and permeability of BBB [190], facilitate the formation of protein coronas that would shield and compromise the functional modification of the surface [191], and be cleared from blood circulation system [192]. Another main aim of surface functionality, including the Pegylation mentioned above, is to optimize the surface zeta potentials of nanoparticles to increase their stability and limit their interaction with RES.
4.2. Size
Size is a fundamental feature of nanoparticles. Due to the existence of the inter-endothelial gap (IEG) of BBTB, the size of the nanoparticles is one of the most vital parameters affecting nanoparticles penetrating across the BBB, especially for paracellular leakage pathways [23, 130]. Along with the aggravation of brain tumors, IEG gradually appears and increases, reaching 550 nm in some regions of glioblastoma [37]. Moreover, the IEG is usually of full heterogeneity, driven by different pore sizes of IEG in various types of brain tumors, different IEG in different grades of brain tumors, and the differentiated distribution of IEG within other regions of the same brain tumor. All these heterogeneities of IEG lead to the heterogeneous distribution of theranostics agents in tumors [23, 193]. Sarin et al. used different size Gd labeled polyamidoamine dendrimers to systematically investigate the physiologic upper limit of pore size of rodent malignant gliomas through dynamic contrast-enhanced MRI [194]. It was revealed that along with the increase of particles size from generation 5 (G5) to generation 8 (G8) (approximately 2 nm increase), there was a significant decrease in nanoparticles penetrating across the BBTB to accumulation in the orthotopic RG-2 glioma tumor, and nearly no extravasation of G8 dendrimers across the BBTB. Therefore, the upper limit of pore size within the BBTB of orthotopic RG-2 rat gliomas was approximately 12 nm. Meanwhile, it was founded that the fibrous glycocalyx coated on the surface of the pore in the BBTB was the major obstacle to the transvascular extravasation of particles across the BBTB of brain tumors [194]. It was noted that although G1-G4 dendrimers with smaller sizes could traverse the BBTB, their signal-to-background ratios were weak and attenuated rapidly due to their blood half-time derived from the fast excretion via renal filtration [195]. These investigations indicated that when employing paracellular leakage pathways, the size of nanoparticles should be optimized with the consideration of balancing between their extravasation across the BBTB and their filtration through the kidney. Different from the influence of nanoparticles size on IEG depending on paracellular extravasation, the influence of nanoparticle size on the transcytosis penetration is moderated. The nanoparticles systemically administrated usually have a diameter between 30–200 nm, which can realize the coordination of “Trojan horse” delivery and long half-life [177].
4.3. Shape
Currently, most nanoparticles developed for brain-targeted delivery are spherical. However, several recent studies showed that nanoparticles’ morphology, including nanosphere, nanostar, nanorod, and nanocage, would greatly influence their BBB passage by affecting nanoparticle-cell interaction [196, 197], which is a critical step for transcytosis. A previous study found that particles with a high aspect ratio showed a higher cellular uptake by tumor cells due to their high surface area, which facilitates the multivalent interaction between nanoparticles and cell membranes [198]. Specific to transcytosis of endothelium cells, Poornima et al. systemically probed the influence of ligand-displaying nanoparticles’ shape (nanorod vs nanosphere) on their specificity to lung and brain endothelial [199]. It was revealed that, in the microfluidic system that mimics the vasculature in vitro, nanorod displayed a higher selectivity and efficacy than that of nanosphere and exhibited high specific and lower nonspecific accumulation in the targeted region. In vivo study in mice further confirmed that nanorods showed a seven-fold enhanced vascular accumulation compared to that of nanosphere [199]. A similar phenomenon was also observed on gold nanorods (AuNRs) [200]. Lee et al. developed an interesting gold nanorod, RVG-PEG-AuRNs@SiO2, by mimicking the rabies virus in terms of size, surface glycoprotein property, and especially the shape with an aspect ratio of 2.34, which was very close to that of a live rabies virus (2.4) [200]. It found that the rod shape of AuNRs played a crucial role in increasing RVG29 mediated cellular uptake, BBB active targeting, and BBB penetration. Ruling out the influence of RVG29 ligand, AuNRs were easily entering N2a cells in vitro, and this merit was more prominent in the orthotopic glioma tumor model. It was emphasized that although the RVG29 ligand decorated on the surface of AuNRs could trigger the receptor (nicotinic acetylcholine receptor, AchR) mediated active targeting, the elongated shape of AuNR would increase the chance of interaction between the ligand on the nanoparticle surface and its receptor on the cell membrane due to a greater surface area [176, 199]. In addition, the shape of the nanoparticles may also have a significant influence on their pharmacokinetics in vivo [175, 201]. These results suggested that the shape of nanoparticles should also be carefully considered when utilizing nanoparticles to realize the diagnosis and therapy of brain tumors.
4.4. Cargo
Apart from being used for tumor diagnosis, imaging, and therapeutic agents, many BBB regulator agents can also be loaded into the nanoparticles as an auxiliary approach to promoting the nanoparticle penetrating the BBB to brain tumor through pharmacological and physical modulation strategies with or without external stimulus [136, 202]. In the strict sense, the cargo loaded in the nanoparticles is not a parameter of the nanoparticles. Whereas, because of its flexibility and accessibility, the loaded BBB regulator could be an auxiliary parameter of the delivery system. These disrupting cargos are divided into two categories, chemical disruption agents and physical disruption agents[136]. Universal chemical disrupting agents include osmotic agents [203], vasodilator [130, 166], and efflux pump inhibitors [136], etc.. One prominent feature of these disrupting cargos is their enhanced and selected BBB disruption and reduced systemic side effects by leveraging the nanoparticles. For example, lexiscan, an adenosine receptor agonist with a definite capacity for modulating the permeability of BBB, was loaded into active breast cancer brain metastases (BCBMs) and shrinkable nanoparticles (LANPs) [128]. LANPs were decorated with AMD3100, a ligand that can actively recognize and bind the overexpressed CXCR4 on tumor cells and could respond to the tumor microenvironment (enriched neutrophil elastase) to shrink their size. These two prominent characterizations of LANPs made lexiscan efficiently open the BBB without remarkable side effects, cooperatively inhibiting BCBMs growth and prolonging the survival of BCBMs bearing mice [128]. A similar strategy was also applied to minoxidil, which could boost transcytosis and down-regulated the tight junction proteins, to endow the nanoparticles (M@HNPs) with enhanced transcellular and paracellular BBB surmounting capacity [130]. As to physical disruption agents, most of the nanoparticles themselves play the role of BBB disruption and vehicle simultaneously, represented as magnetic iron oxide nanoparticles (IONP). IONP can not only act as the classic magnetic imaging contrast for T2 MR imaging, but also leverage the low radiofrequency magnetic field to produce hyperthermia, which had been verified to transiently increase BBB permeability and drug penetration to brain tumor [67]. Another representative agent is microbubble, one of widely adopted focused ultrasound (FUS) contrasts. The microbubble can not only execute cavitation activity mediated mechanical stress to increase the transcytosis activity and induce BBB temporary open [204, 205] but also can serve as the reliable vehicle of ultrasmall nanoparticles [206] and small molecular drug [207] to realize the FUS augmented brain tumor imaging and treatment.
Besides promoting nanoparticles penetrating the BBB through tuning the permeability of BBB, some loaded cargoes (or detachable blocks) could change the properties of the nanocarriers themselves, such as the charge, size, shape, and surface geomorphology, by leveraging the pathophysiological features of the brain and/or external stimulus, to facilitate the nanocarriers penetrate brain tumors [208, 209]. Therefore, the investigations involving potentially multifunctioal cargoes, adaptive nanocarriers and their rational mechanism would pioneer a new methodology of cargo-driving nanocarriers penetrating the brain and flourishing drug delivery systems-assisted theranostic for brain tumor.
5. The potential challenges and future directions
Unlike peripheral tumors, the major challenge for the diagnosis and therapy of brain tumors is the presence of the substantive physiological barrier, such as BBB, which severely prevents the access of diagnostic and therapeutic agents to brain tumors. Although various BBB crossing/disturbing strategies with different advantages have been explored, they also encounter some inevitable limitations (Table 3). Nevertheless the BBB would deteriorate to yield BBTB as the progress of brain tumor, in which the integrity of the physiological barrier is partially broken, the resulted fenestration remains an obstacle for the permeation of most agents, especially for nanoparticles. Therefore, nanoparticle-mediated drug delivery systems represent a promising approach for improving brain tumor chemotherapy. On the one hand, compared with free drugs, NPs are endowed with unique pharmaceutical properties, such as high drug loading, controlled drug release features, and prolonged half-life. On the other hand, attributed to the exclusive recognition between ligand decorated on the surface of NPs and the corresponding receptor expressed on the surface of brain endothelial cells, an improved cargo delivery to the brain would be realized by RMT [188]. Even so, due to the competitive binding with a high concentration of endogenous ligands [189] and reduced targeting efficacy induced by the rapid formation of plasma protein corona on the nanoparticle surface in the circulation system [210], the accumulation of most NPs in the brain via RMT is still lower than 1% [211], which may not be adequate to yield a desired therapeutic response. The multiple targeting and/or multistage targeting by multi-ligands mediated active targeting may not only enhance BBB penetration of nanoparticles but also distinguish the normal brain tissue and brain tumor, thus achieving enhanced antitumor efficiency and reducing potential side effects.
Table 3.
Pros and cons of different BBB crossing/disturbing strategies
| Strategies | Methodology | Pros | Cons |
|---|---|---|---|
| Passive targeting | / | Simple structure of nanoparticle; High specificity (lesion dependent distribution); safety | Heterogeneous distribution; low distribution; brain tumor type and stage dependent |
| Biological interfacial interaction | Receptor/Transporter-mediated transcytosis | Receptor/transporter mediated high efficiency and relative high distribution; Active targeting and multiple targeting mediated highly specific distribution in brain tumor | Relative complicated and high-cost nanoparticles; Off-target distribution deriving from non-exclusive distribution of receptor/transporter; Compromised target ability due to the competition from endogenous ligand and the formation of protein corona |
| Physicochemical interaction | Adsorptive mediated transcytosis | High transcytosis efficacy; High distribution in brain tumor; | No-specific high distribution in brain; limited tissue selectivity; Potential system toxicity |
| Temporary BBB opening and disrupting | Highly efficient, enables focus on targeted region; temporary and reversible BBB opening; most method approved in the clinic; | Potential side effects, such as increased intracranial pressure, chronic inflammation; Non-specific opening of the BBB; | |
| Living cell | / | High specificity; High efficiency; High carry capacity; Decreased drug immunogenicity | Complicated operation; High cost; Heterogeneous outcome; Potential system toxicity; Poor spatial and temporal control of cargoes release |
Temporary disruption and opening of BBB by chemical and physical methods is a promising method to enhance therapeutic drugs across the BBB for enhanced diagnosis and therapy of brain tumors. On the one hand, the opening of BBB may be well controlled not only by manipulating the mechanical parameters, such as ultrasound intensity and frequency, magnetic field intensity, and intervention time but also by choosing the optimal time and location to implement the intervention and real-time monitoring the outcome by integrating multimodality imaging into the system [146]. On the other hand, the combination of temporary disruption with other brain active targeting strategies, such as FUS plus RMT, would realize an enhanced targeted drug delivery and achieve boosted anti-cancer efficacy [137]. It is also noted that further study should be performed to uncover the mechanism of BBB opening and recovery, which might be critical for their clinic application [147]. Meanwhile, it is vital to monitor the potential adverse effects associated with the opening of BBB [212].
Although possessing promising potential in treating and detecting brain tumors, living cells and their derivatives-based delivery systems are still in their infancy. There are several concerns that must be taken into careful consideration before their translation to clinic practice. First, the mechanism behind the brain tumor tropism and BBB penetration of living cells should be extensively explored to balance the potential theranostic benefits and side effects associated with the treatment. Second, how to maintain and strengthen the inherent characteristics of the cells during external expansion and cargo loading to achieve a reliable strategy should be investigated. Thus, developing a standard operating protocol for utilizing living cells is well desired. Moreover, living cells-based diagnosis and therapy may bring broad application prospects to individual precision medicine, but how to reduce the time and financial cost of developing a versatile and reliable platform should be considered for general applications in CNS disease therapy.
Figure 3.
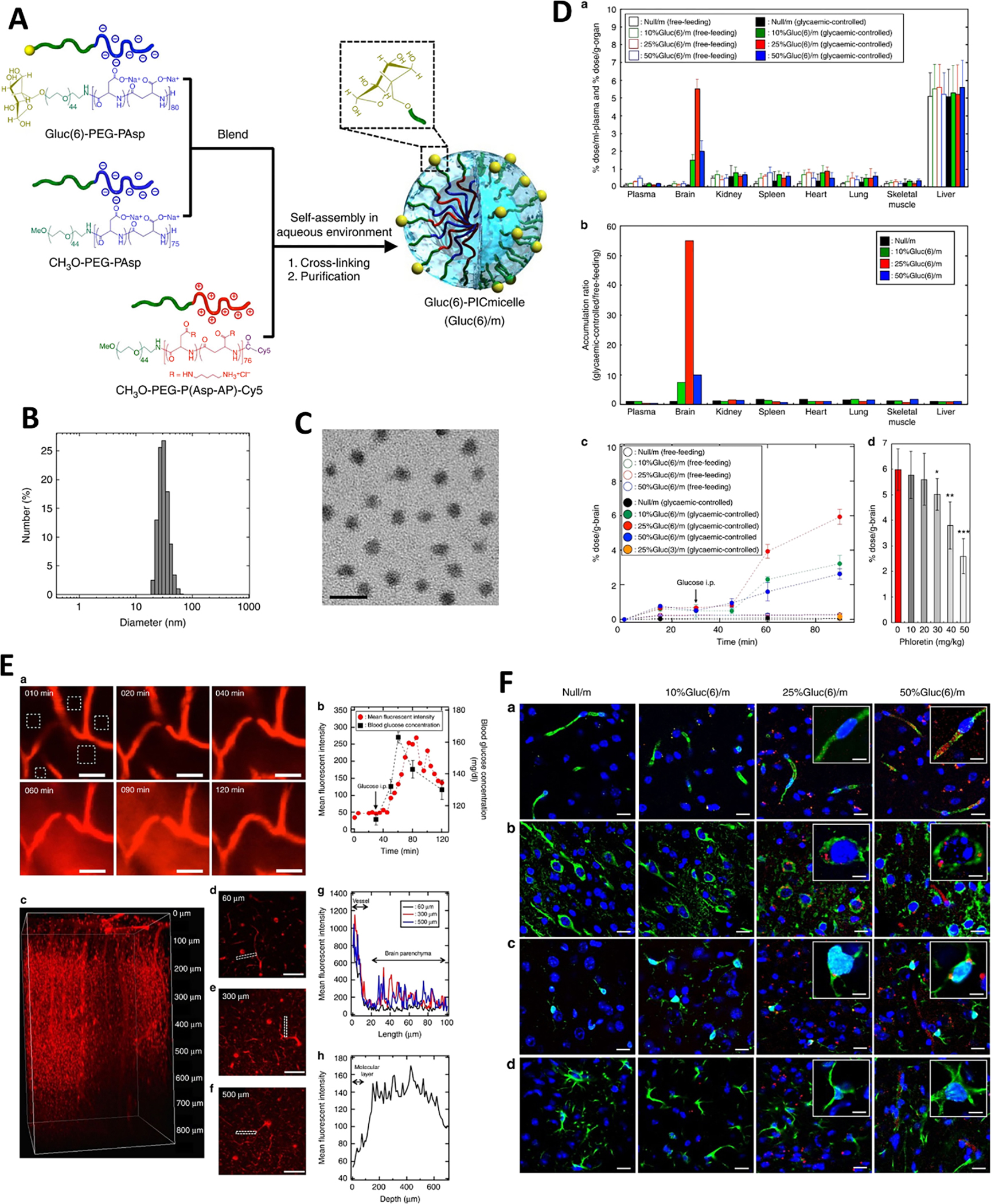
Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain through glucose transporter-1 (GLUT1). (A) Scheme of Gluc(6)/m preparation. (B) DLS size distribution of 25% Gluc(6)/m. (C) TEM image of 25% Gluc(6)/m, scale bar=50 nm. (D) Da). Biodistribution of different micelles in different organs under different feeding conditions at 48 h after injection. Db) Relative accumulation ratio of glycaemic-controlled micelle/free-feeding micelle in various organs calculated from the value from Da. Dc) Time course of different micelles accumulation in brain under different feeding conditions. Dd) In vivo inhibition study of the brain accumulation of glycaemic-controlled/free-feeding by phloretin. The data are expressed as the mean ± SEM, n=5, *p<0.05. E Real-time observation of the 25%Gluc(6)/m crossing the BBB. (E) Ea) Representative images of 25%Gluc(6)/m distribution in mouse cerebrum at different time by using IVRT-CLSM. Scale bar is 50 μm. Eb) Time course of blood glucose concentration and the mean fluorescent intensities of the 25%Gluc(6)/m in the region of interest (ROI) in (Ea). Ec) Images of Gluc(6)/m in the cerebrum observed using intravital multiphoton microscopy 48 h after administration. Cross-sections at depths of 60 μm (Ed), 300 μm (Ee) and 500 μm (Ef) are also shown. Scale bar is 100 μm. Eg) Distribution profiles of Gluc(6)/m from blood vessels to the brain parenchyma in the selected region in (Ed), (Ee) and (Ef). Eh) Depth profiles of the mean fluorescent intensities from 0 to 700 μm in the brain parenchymal region. (F) Immunohistochemical analysis of the mouse brains Cerebral sections at 48 h after administration of different polymeric micelles. BCECs (Fa), neurons (Fb), microglia (Fc) and astrocytes (Fd) (green) are stained by corresponding antibodies. Nuclei (blue) are stained with DAPI. Scale bar is 20 μm (10 μm in insets). Adapted with permission from ref. [93].
Acknowledgments
The authors want to thank the National Institutes of Health (1R01CA263747-01A1, 1R01AG054839-01A1, 1R41CA254500-01A1, and 1R21CA252360-01) for financial support of the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma, New England journal of medicine, 352 (2005) 987–996. [DOI] [PubMed] [Google Scholar]
- [2].Maher EA, Mietz J, Arteaga CL, DePinho RA, Mohla S, Brain metastasis: opportunities in basic and translational research, AACR, 2009. [DOI] [PubMed] [Google Scholar]
- [3].Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J, CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011, Neuro-oncology, 16 (2014) iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL, Epidemiologic and molecular prognostic review of glioblastoma, Cancer Epidemiology and Prevention Biomarkers, 23 (2014) 1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nayak L, Lee EQ, Wen PY, Epidemiology of brain metastases, Current oncology reports, 14 (2012) 48–54. [DOI] [PubMed] [Google Scholar]
- [6].Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS, Brain metastases, Nature Reviews Disease Primers, 5 (2019) 1–26. [DOI] [PubMed] [Google Scholar]
- [7].Parrish K, Sarkaria JN, Elmquist WF, Improving drug delivery to primary and metastatic brain tumors: strategies to overcome the blood–brain barrier, Clinical Pharmacology & Therapeutics, 97 (2015) 336–346. [DOI] [PubMed] [Google Scholar]
- [8].Minniti G, Traish D, Ashley S, Gonsalves A, Brada M, Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years, The Journal of clinical endocrinology & metabolism, 90 (2005) 800–804. [DOI] [PubMed] [Google Scholar]
- [9].Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE, Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications, Journal of vascular Research, 50 (2013) 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jacob J, Durand T, Feuvret L, Mazeron J-J, Delattre J-Y, Hoang-Xuan K, Psimaras D, Douzane H, Ribeiro M, Capelle L, Cognitive impairment and morphological changes after radiation therapy in brain tumors: a review, Radiotherapy and Oncology, 128 (2018) 221–228. [DOI] [PubMed] [Google Scholar]
- [11].Mehta MP, Rodrigus P, Terhaard C, Rao A, Suh J, Roa W, Souhami L, Bezjak A, Leibenhaut M, Komaki R, Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases, Journal of Clinical Oncology, 21 (2003) 2529–2536. [DOI] [PubMed] [Google Scholar]
- [12].DeAngelis LM, Chemotherapy for brain tumors—a new beginning, Mass Medical Soc, 2005, pp. 1036–1038. [DOI] [PubMed] [Google Scholar]
- [13].Toms SA, Lin W-C, Weil RJ, Johnson MD, Jansen ED, Mahadevan-Jansen A, Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity, Operative Neurosurgery, 57 (2005) ONS-382–ONS-391. [DOI] [PubMed] [Google Scholar]
- [14].Pardridge WM, Crossing the blood–brain barrier: are we getting it right?, Drug discovery today, 6 (2001) 1–2. [DOI] [PubMed] [Google Scholar]
- [15].Rather LJ, The genesis of cancer: A study in the history of ideas, (1978).
- [16].Ahir BK, Engelhard HH, Lakka SS, Tumor development and angiogenesis in adult brain tumor: Glioblastoma, Molecular neurobiology, 57 (2020) 2461–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Groothuis DR, The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery, Neuro-oncology, 2 (2000) 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gao X, Li C, Nanoprobes visualizing gliomas by crossing the blood brain tumor barrier, Small, 10 (2014) 426–440. [DOI] [PubMed] [Google Scholar]
- [19].Ewelt C, Floeth FW, Felsberg J, Steiger HJ, Sabel M, Langen K-J, Stoffels G, Stummer W, Finding the anaplastic focus in diffuse gliomas: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence, Clinical neurology and neurosurgery, 113 (2011) 541–547. [DOI] [PubMed] [Google Scholar]
- [20].Roose T, Netti PA, Munn LL, Boucher Y, Jain RK, Solid stress generated by spheroid growth estimated using a linear poroelasticity model☆, Microvascular research, 66 (2003) 204–212. [DOI] [PubMed] [Google Scholar]
- [21].Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK, The biology of brain metastases—translation to new therapies, Nature reviews Clinical oncology, 8 (2011) 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK, Malignant cells facilitate lung metastasis by bringing their own soil, Proceedings of the National Academy of Sciences, 107 (2010) 21677–21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer, Clinical cancer research, 16 (2010) 5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fabel K, Dietrich J, Hau P, Wismeth C, Winner B, Przywara S, Steinbrecher A, Ullrich W, Bogdahn U, Long - term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin, Cancer: Interdisciplinary International Journal of the American Cancer Society, 92 (2001) 1936–1942. [DOI] [PubMed] [Google Scholar]
- [25].Hau P, Fabel K, Baumgart U, Rümmele P, Grauer O, Bock A, Dietmaier C, Dietmaier W, Dietrich J, Dudel C, Pegylated liposomal doxorubicin - efficacy in patients with recurrent high - grade glioma, Cancer: Interdisciplinary International Journal of the American Cancer Society, 100 (2004) 1199–1207. [DOI] [PubMed] [Google Scholar]
- [26].Barajas RF Jr, Hamilton BE, Schwartz D, McConnell HL, Pettersson DR, Horvath A, Szidonya L, Varallyay CG, Firkins J, Jaboin JJ, Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI define glioblastoma pseudoprogression, Neuro-oncology, 21 (2019) 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ricciardi GRR, Russo A, Franchina T, Ferraro G, Adamo V, Efficacy of nab-paclitaxel plus trastuzumab in a long-surviving heavily pretreated HER2-positive breast cancer patient with brain metastases, OncoTargets and therapy, 8 (2015) 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Verry C, Sancey L, Dufort S, Le Duc G, Mendoza C, Lux F, Grand S, Arnaud J, Quesada JL, Villa J, Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol, BMJ open, 9 (2019) e023591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zacharoulis S, Szalontay L, CreveCoeur T, Neira J, Higgins D, Englander Z, Spinazzi E, Sethi C, Canoll P, Garvin J, DDEL-07. A Phase I study examining the feasibility of intermittent convection-enhanced delivery (CED) of MTX110 for the treatment of children with newly diagnosed diffuse midline gliomas (DMGs), Neuro-Oncology, 24 (2022) i35. [Google Scholar]
- [30].Clarke JL, Molinaro AM, Cabrera JR, DeSilva AA, Rabbitt JE, Prey J, Drummond DC, Kim J, Noble C, Fitzgerald JB, A phase 1 trial of intravenous liposomal irinotecan in patients with recurrent high-grade glioma, Cancer chemotherapy and pharmacology, 79 (2017) 603–610. [DOI] [PubMed] [Google Scholar]
- [31].Leung CP, Barve MA, Wu M-S, Pirollo KF, Strauss JF, Liao W-C, Yang S-H, Nunan RA, Adams J, Harford JB, A phase II trial combining tumor-targeting TP53 gene therapy with gemcitabine/nab-paclitaxel as a second-line treatment for metastatic pancreatic cancer, Wolters Kluwer Health, 2021. [Google Scholar]
- [32].Sagnella SM, Yang L, Stubbs GE, Boslem E, Martino-Echarri E, Smolarczyk K, Pattison SL, Vanegas N, Clair ES, Clarke S, Cyto-immuno-therapy for cancer: a pathway elicited by tumor-targeted, cytotoxic drug-packaged bacterially derived nanocells, Cancer Cell, 37 (2020) 354–370. e357. [DOI] [PubMed] [Google Scholar]
- [33].Brandsma D, Kerklaan BM, Diéras V, Altintas S, Anders C, Ballester MA, Gelderblom H, Soetekouw P, Gladdines W, Lonnqvist F, Phase 1/2a study of glutathione pegylated liposomal doxorubicin (2b3–101) in patients with brain metastases (BM) from solid tumors or recurrent high grade gliomas (HGG), Annals of Oncology, 25 (2014) iv157. [Google Scholar]
- [34].Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC, Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology, AACR, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bhojani MS, Van Dort M, Rehemtulla A, Ross BD, Targeted imaging and therapy of brain cancer using theranostic nanoparticles, Molecular pharmaceutics, 7 (2010) 1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW, The 2016 World Health Organization classification of tumors of the central nervous system: a summary, Acta neuropathologica, 131 (2016) 803–820. [DOI] [PubMed] [Google Scholar]
- [37].Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK, Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment, Proceedings of the National Academy of Sciences, 95 (1998) 4607–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pardridge WM, Molecular biology of the blood-brain barrier, Mol Biotechnol, 30 (2005) 57–70. [DOI] [PubMed] [Google Scholar]
- [39].Xie J, Shen Z, Anraku Y, Kataoka K, Chen X, Nanomaterial-based blood-brain-barrier (BBB) crossing strategies, Biomaterials, 224 (2019) 119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Terstappen GC, Meyer AH, Bell RD, Zhang W, Strategies for delivering therapeutics across the blood–brain barrier, Nature Reviews Drug Discovery, 20 (2021) 362–383. [DOI] [PubMed] [Google Scholar]
- [41].Leitner DF, Connor JR, Functional roles of transferrin in the brain, Biochimica et Biophysica Acta (BBA)-General Subjects, 1820 (2012) 393–402. [DOI] [PubMed] [Google Scholar]
- [42].Danhier F, Feron O, Préat V, To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery, Journal of controlled release, 148 (2010) 135–146. [DOI] [PubMed] [Google Scholar]
- [43].Choudhury H, Pandey M, Chin PX, Phang YL, Cheah JY, Ooi SC, Mak K-K, Pichika MR, Kesharwani P, Hussain Z, Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: a review of recent advancements and emerging trends, Drug delivery and translational research, 8 (2018) 1545–1563. [DOI] [PubMed] [Google Scholar]
- [44].Porru M, Zappavigna S, Salzano G, Luce A, Stoppacciaro A, Balestrieri ML, Artuso S, Lusa S, De Rosa G, Leonetti C, Medical treatment of orthotopic glioblastoma with transferrin-conjugated nanoparticles encapsulating zoledronic acid, Oncotarget, 5 (2014) 10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cui Y, Zhang M, Zeng F, Jin H, Xu Q, Huang Y, Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy, ACS applied materials & interfaces, 8 (2016) 32159–32169. [DOI] [PubMed] [Google Scholar]
- [46].Wei L, Guo X-Y, Yang T, Yu M-Z, Chen D-W, Wang J-C, Brain tumor-targeted therapy by systemic delivery of siRNA with Transferrin receptor-mediated core-shell nanoparticles, International journal of pharmaceutics, 510 (2016) 394–405. [DOI] [PubMed] [Google Scholar]
- [47].Lakkadwala S, Singh J, Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model, Colloids and Surfaces B: Biointerfaces, 173 (2019) 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dixit S, Novak T, Miller K, Zhu Y, Kenney ME, Broome A-M, Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors, Nanoscale, 7 (2015) 1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li S, Amat D, Peng Z, Vanni S, Raskin S, De Angulo G, Othman AM, Graham RM, Leblanc RM, Transferrin conjugated nontoxic carbon dots for doxorubicin delivery to target pediatric brain tumor cells, Nanoscale, 8 (2016) 16662–16669. [DOI] [PubMed] [Google Scholar]
- [50].Ulbrich K, Hekmatara T, Herbert E, Kreuter J, Transferrin-and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB), European Journal of Pharmaceutics and Biopharmaceutics, 71 (2009) 251–256. [DOI] [PubMed] [Google Scholar]
- [51].Noh G, Youn YS, Lee ES, Preparation of iron oxide nanoparticles functionalized with Y-shaped ligands for brain tumor targeting, Journal of Materials Chemistry B, 4 (2016) 6074–6080. [DOI] [PubMed] [Google Scholar]
- [52].Cui Y, Xu Q, Chow PK-H, Wang D, Wang C-H, Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment, Biomaterials, 34 (2013) 8511–8520. [DOI] [PubMed] [Google Scholar]
- [53].Dixit S, Miller K, Zhu Y, McKinnon E, Novak T, Kenney ME, Broome A-M, Dual receptor-targeted theranostic nanoparticles for localized delivery and activation of photodynamic therapy drug in glioblastomas, Molecular pharmaceutics, 12 (2015) 3250–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hettiarachchi SD, Graham RM, Mintz KJ, Zhou Y, Vanni S, Peng Z, Leblanc RM, Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors, Nanoscale, 11 (2019) 6192–6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yu M, Su D, Yang Y, Qin L, Hu C, Liu R, Zhou Y, Yang C, Yang X, Wang G, D-T7 peptide-modified PEGylated bilirubin nanoparticles loaded with cediranib and paclitaxel for antiangiogenesis and chemotherapy of glioma, ACS applied materials & interfaces, 11 (2018) 176–186. [DOI] [PubMed] [Google Scholar]
- [56].Roberts RL, Fine RE, Sandra A, Receptor-mediated endocytosis of transferrin at the blood-brain barrier, Journal of cell science, 104 (1993) 521–532. [DOI] [PubMed] [Google Scholar]
- [57].Sheff DR, Daro EA, Hull M, Mellman I, The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions, The Journal of cell biology, 145 (1999) 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle, Neuron, 81 (2014) 49–60. [DOI] [PubMed] [Google Scholar]
- [59].Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, Atwal J, Elliott JM, Prabhu S, Watts RJ, Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target, Science translational medicine, 3 (2011) 84ra44–84ra44. [DOI] [PubMed] [Google Scholar]
- [60].Bien-Ly N, Yu YJ, Bumbaca D, Elstrott J, Boswell CA, Zhang Y, Luk W, Lu Y, Dennis MS, Weimer RM, Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants, Journal of Experimental Medicine, 211 (2014) 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhao Y, Li D, Zhao J, Song J, Zhao Y, The role of the low-density lipoprotein receptor–related protein 1 (LRP-1) in regulating blood-brain barrier integrity, Reviews in the neurosciences, 27 (2016) 623–634. [DOI] [PubMed] [Google Scholar]
- [62].Etique N, Verzeaux L, Dedieu S, Emonard H, LRP-1: a checkpoint for the extracellular matrix proteolysis, BioMed research international, 2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sun X, Pang Z, Ye H, Qiu B, Guo L, Li J, Ren J, Qian Y, Zhang Q, Chen J, Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome, Biomaterials, 33 (2012) 916–924. [DOI] [PubMed] [Google Scholar]
- [64].Gabathuler R, An engineered peptide compound platform technology incorporating angiopep for crossing the BBB, Drug Delivery to the Central Nervous System, Springer; 2010, pp. 249–260. [Google Scholar]
- [65].Bertrand Y, Currie J, Poirier J, Demeule M, Abulrob A, Fatehi D, Stanimirovic D, Sartelet H, Castaigne J, Béliveau R, Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1, British journal of cancer, 105 (2011) 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kreuter J, Ramge P, Petrov V, Hamm S, Gelperina SE, Engelhardt B, Alyautdin R, Von Briesen H, Begley DJ, Direct evidence that polysorbate-80-coated poly (butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles, Pharmaceutical research, 20 (2003) 409–416. [DOI] [PubMed] [Google Scholar]
- [67].Sun C, Ding Y, Zhou L, Shi D, Sun L, Webster TJ, Shen Y, Noninvasive nanoparticle strategies for brain tumor targeting, Nanomedicine: Nanotechnology, Biology and Medicine, 13 (2017) 2605–2621. [DOI] [PubMed] [Google Scholar]
- [68].Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J, Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles, Pharmaceutical research, 16 (1999) 1564–1569. [DOI] [PubMed] [Google Scholar]
- [69].Wang C-X, Huang L-S, Hou L-B, Jiang L, Yan Z-T, Wang Y-L, Chen Z-L, Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model, Brain research, 1261 (2009) 91–99. [DOI] [PubMed] [Google Scholar]
- [70].Georgieva JV, Hoekstra D, Zuhorn IS, Smuggling drugs into the brain: an overview of ligands targeting transcytosis for drug delivery across the blood–brain barrier, Pharmaceutics, 6 (2014) 557–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Das D, Lin S, Double-coated poly (butylcynanoacrylate) nanoparticulate delivery systems for brain targeting of dalargin via oral administration, Journal of pharmaceutical sciences, 94 (2005) 1343–1353. [DOI] [PubMed] [Google Scholar]
- [72].Li Z, Zhu J, Wang Y, Zhou M, Li D, Zheng S, Yin L, Luo C, Zhang H, Zhong L, In situ apolipoprotein E-enriched corona guides dihydroartemisinin-decorating nanoparticles towards LDLr-mediated tumor-homing chemotherapy, Asian journal of pharmaceutical sciences, 15 (2020) 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pedersen JC, Berg K, Interaction between low density lipoprotein receptor (LDLR) and apolipoprotein E (apoE) alleles contributes to normal variation in lipid level, Clinical genetics, 35 (1989) 331–337. [DOI] [PubMed] [Google Scholar]
- [74].Ulbrich K, Knobloch T, Kreuter J, Targeting the insulin receptor: nanoparticles for drug delivery across the blood–brain barrier (BBB), Journal of drug targeting, 19 (2011) 125–132. [DOI] [PubMed] [Google Scholar]
- [75].Pardridge WM, Eisenberg J, Yang J, Human blood—brain barrier insulin receptor, Journal of neurochemistry, 44 (1985) 1771–1778. [DOI] [PubMed] [Google Scholar]
- [76].Pardridge WM, Kang Y-S, Buciak JL, Yang J, Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood–brain barrier in vivo in the primate, Pharmaceutical research, 12 (1995) 807–816. [DOI] [PubMed] [Google Scholar]
- [77].Glick RP, Gettleman R, Patel K, Lakshman R, Tsibris JC, Insulin and insulin-like growth factor I in brain tumors: binding and in vitro effects, Neurosurgery, 24 (1989) 791–797. [DOI] [PubMed] [Google Scholar]
- [78].Coloma MJ, Lee HJ, Kurihara A, Landaw EM, Boado RJ, Morrison SL, Pardridge WM, Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor, Pharmaceutical research, 17 (2000) 266–274. [DOI] [PubMed] [Google Scholar]
- [79].Dieu L-H, Wu D, Palivan CG, Balasubramanian V, Huwyler J, Polymersomes conjugated to 83–14 monoclonal antibodies: in vitro targeting of brain capillary endothelial cells, European journal of pharmaceutics and biopharmaceutics, 88 (2014) 316–324. [DOI] [PubMed] [Google Scholar]
- [80].Kuo Y-C, Shih-Huang C-Y, Solid lipid nanoparticles carrying chemotherapeutic drug across the blood–brain barrier through insulin receptor-mediated pathway, Journal of drug targeting, 21 (2013) 730–738. [DOI] [PubMed] [Google Scholar]
- [81].Rhea EM, Rask-Madsen C, Banks WA, Insulin transport across the blood–brain barrier can occur independently of the insulin receptor, The Journal of physiology, 596 (2018) 4753–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Giugliani R, Giugliani L, de Oliveira Poswar F, Donis KC, Dalla Corte A, Schmidt M, Boado RJ, Nestrasil I, Nguyen C, Chen S, Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): an open label phase 1–2 trial, Orphanet journal of rare diseases, 13 (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhan C, Li B, Hu L, Wei X, Feng L, Fu W, Lu W, Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand, Angewandte Chemie, 123 (2011) 5596–5599. [DOI] [PubMed] [Google Scholar]
- [84].Han L, Liu C, Qi H, Zhou J, Wen J, Wu D, Xu D, Qin M, Ren J, Wang Q, Systemic delivery of monoclonal antibodies to the central nervous system for brain tumor therapy, Advanced Materials, 31 (2019) 1805697. [DOI] [PubMed] [Google Scholar]
- [85].Kumar P, Wu H, McBride JL, Jung K-E, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N, Transvascular delivery of small interfering RNA to the central nervous system, Nature, 448 (2007) 39–43. [DOI] [PubMed] [Google Scholar]
- [86].Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, Lou J, Jiang C, Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles, Biomaterials, 30 (2009) 4195–4202. [DOI] [PubMed] [Google Scholar]
- [87].Zhan C, Yan Z, Xie C, Lu W, Loop 2 of Ophiophagus hannah toxin b binds with neuronal nicotinic acetylcholine receptors and enhances intracranial drug delivery, Molecular pharmaceutics, 7 (2010) 1940–1947. [DOI] [PubMed] [Google Scholar]
- [88].Wen J, Wu D, Qin M, Liu C, Wang L, Xu D, Vinters HV, Liu Y, Kranz E, Guan X, Sustained delivery and molecular targeting of a therapeutic monoclonal antibody to metastases in the central nervous system of mice, Nature biomedical engineering, 3 (2019) 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wu D, Qin M, Xu D, Wang L, Liu C, Ren J, Zhou G, Chen C, Yang F, Li Y, A bioinspired platform for effective delivery of protein therapeutics to the central nervous system, Advanced Materials, 31 (2019) 1807557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Xu D, Wu D, Qin M, Nih LR, Liu C, Cao Z, Ren J, Chen X, He Z, Yu W, Efficient delivery of nerve growth factors to the central nervous system for neural regeneration, Advanced Materials, 31 (2019) 1900727. [DOI] [PubMed] [Google Scholar]
- [91].Liang S, Liu Y, Jin X, Liu G, Wen J, Zhang L, Li J, Yuan X, Chen IS, Chen W, Phosphorylcholine polymer nanocapsules prolong the circulation time and reduce the immunogenicity of therapeutic proteins, Nano Research, 9 (2016) 1022–1031. [Google Scholar]
- [92].Egleton R, Mitchell S, Huber J, Palian M, Polt R, Davis T, Improved blood-brain barrier penetration and enhanced analgesia of an opioid peptide by glycosylation, Journal of Pharmacology and Experimental Therapeutics, 299 (2001) 967–972. [PubMed] [Google Scholar]
- [93].Anraku Y, Kuwahara H, Fukusato Y, Mizoguchi A, Ishii T, Nitta K, Matsumoto Y, Toh K, Miyata K, Uchida S, Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain, Nature communications, 8 (2017) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Qin Y, Fan W, Chen H, Yao N, Tang W, Tang J, Yuan W, Kuai R, Zhang Z, Wu Y, In vitro and in vivo investigation of glucose-mediated brain-targeting liposomes, Journal of drug targeting, 18 (2010) 536–549. [DOI] [PubMed] [Google Scholar]
- [95].Jiang X, Xin H, Ren Q, Gu J, Zhu L, Du F, Feng C, Xie Y, Sha X, Fang X, Nanoparticles of 2-deoxy-D-glucose functionalized poly (ethylene glycol)-co-poly (trimethylene carbonate) for dual-targeted drug delivery in glioma treatment, Biomaterials, 35 (2014) 518–529. [DOI] [PubMed] [Google Scholar]
- [96].Okamura T, Okada M, Kikuchi T, Wakizaka H, Zhang MR, Mechanisms of glutathione-conjugate efflux from the brain into blood: Involvement of multiple transporters in the course, J Cereb Blood Flow Metab, 40 (2020) 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].van Tellingen O, Brandsma D, Boogerd W, Appeldoorn C, Manca F, Rip J, Dorland R, van Kregten J, Gaillard P, Gsh-Conjugation Improves Efficacy of Doxil against Intracranial Xenografts, Neuro-Oncology, 12 (2010) 34–34. [Google Scholar]
- [98].Nosrati H, Tarantash M, Bochani S, Charmi J, Bagheri Z, Fridoni M, Abdollahifar MA, Davaran S, Danafar H, Manjili HK, Glutathione (GSH) Peptide Conjugated Magnetic Nanoparticles As Blood-Brain Barrier Shuttle for MRI-Monitored Brain Delivery of Paclitaxel, Acs Biomater Sci Eng, 5 (2019) 1677–1685. [Google Scholar]
- [99].Li G, Shao K, Umeshappa CS, Recent progress in blood-brain barrier transportation research, Brain targeted drug delivery system, (2019) 33–51. [Google Scholar]
- [100].Vijay N, Morris ME, Role of monocarboxylate transporters in drug delivery to the brain, Current pharmaceutical design, 20 (2014) 1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Venishetty VK, Samala R, Komuravelli R, Kuncha M, Sistla R, Diwan PV, β-Hydroxybutyric acid grafted solid lipid nanoparticles: A novel strategy to improve drug delivery to brain, Nanomedicine: Nanotechnology, Biology and Medicine, 9 (2013) 388–397. [DOI] [PubMed] [Google Scholar]
- [102].Ak G, Ünal A, Karakayalı T, Özel B, Günel NS, Şanlıer ŞH, Brain-targeted, drug-loaded solid lipid nanoparticles against glioblastoma cells in culture, Colloids and Surfaces B: Biointerfaces, 206 (2021) 111946. [DOI] [PubMed] [Google Scholar]
- [103].Simpson IA, Carruthers A, Vannucci SJ, Supply and demand in cerebral energy metabolism: the role of nutrient transporters, Journal of Cerebral Blood Flow & Metabolism, 27 (2007) 1766–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hayashi K, Anzai N, Novel therapeutic approaches targeting L-type amino acid transporters for cancer treatment, World Journal of Gastrointestinal Oncology, 9 (2017) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nawashiro H, Otani N, Uozumi Y, Ooigawa H, Toyooka T, Suzuki T, Katoh H, Tsuzuki N, Ohnuki A, Shima K, High expression of L-type amino acid transporter 1 in infiltrating glioma cells, Brain tumor pathology, 22 (2005) 89–91. [DOI] [PubMed] [Google Scholar]
- [106].Nawashiro H, Otani N, Shinomiya N, Fukui S, Ooigawa H, Shima K, Matsuo H, Kanai Y, Endou H, L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors, International journal of cancer, 119 (2006) 484–492. [DOI] [PubMed] [Google Scholar]
- [107].Kageyama T, Nakamura M, Matsuo A, Yamasaki Y, Takakura Y, Hashida M, Kanai Y, Naito M, Tsuruo T, Minato N, The 4F2hc/LAT1 complex transports L-DOPA across the blood–brain barrier, Brain research, 879 (2000) 115–121. [DOI] [PubMed] [Google Scholar]
- [108].Puris E, Gynther M, Auriola S, Huttunen KM, L-Type amino acid transporter 1 as a target for drug delivery, Pharmaceutical Research, 37 (2020) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kharya P, Jain A, Gulbake A, Shilpi S, Jain A, Hurkat P, Majumdar S, Jain SK, Phenylalanine-coupled solid lipid nanoparticles for brain tumor targeting, Journal of nanoparticle research, 15 (2013) 1–12. [Google Scholar]
- [110].Rautio J, Gynther M, Laine K, LAT1-mediated prodrug uptake: a way to breach the blood–brain barrier?, Therapeutic delivery, 4 (2013) 281–284. [DOI] [PubMed] [Google Scholar]
- [111].Li L, Di X, Zhang S, Kan Q, Liu H, Lu T, Wang Y, Fu Q, Sun J, He Z, Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting, Colloids and Surfaces B: Biointerfaces, 141 (2016) 260–267. [DOI] [PubMed] [Google Scholar]
- [112].Wei X, Chen X, Ying M, Lu W, Brain tumor-targeted drug delivery strategies, Acta pharmaceutica sinica B, 4 (2014) 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lu W, Tan Y-Z, Hu K-L, Jiang X-G, Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood–brain barrier, International journal of pharmaceutics, 295 (2005) 247–260. [DOI] [PubMed] [Google Scholar]
- [114].Lu W, Sun Q, Wan J, She Z, Jiang X-G, Cationic albumin–conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration, Cancer research, 66 (2006) 11878–11887. [DOI] [PubMed] [Google Scholar]
- [115].Munyendo WL, Lv H, Benza-Ingoula H, Baraza LD, Zhou J, Cell penetrating peptides in the delivery of biopharmaceuticals, Biomolecules, 2 (2012) 187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gupta B, Levchenko TS, Torchilin VP, Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides, Advanced drug delivery reviews, 57 (2005) 637–651. [DOI] [PubMed] [Google Scholar]
- [117].Banks WA, Robinson SM, Nath A, Permeability of the blood–brain barrier to HIV-1 Tat, Experimental neurology, 193 (2005) 218–227. [DOI] [PubMed] [Google Scholar]
- [118].Zhu Y, Jiang Y, Meng F, Deng C, Cheng R, Zhang J, Feijen J, Zhong Z, Highly efficacious and specific anti-glioma chemotherapy by tandem nanomicelles co-functionalized with brain tumor-targeting and cell-penetrating peptides, Journal of controlled release, 278 (2018) 1–8. [DOI] [PubMed] [Google Scholar]
- [119].Bickel U, Yoshikawa T, Pardridge WM, Delivery of peptides and proteins through the blood–brain barrier, Advanced drug delivery reviews, 46 (2001) 247–279. [DOI] [PubMed] [Google Scholar]
- [120].Foley CP, Rubin DG, Santillan A, Sondhi D, Dyke JP, Gobin YP, Crystal RG, Ballon DJ, Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption, Journal of controlled release, 196 (2014) 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Boockvar JA, Tsiouris AJ, Hofstetter CP, Kovanlikaya I, Fralin S, Kesavabhotla K, Seedial SM, Pannullo SC, Schwartz TH, Stieg P, Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma, Journal of neurosurgery, 114 (2011) 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang D, Wang C, Wang L, Chen Y, A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment, Drug delivery, 26 (2019) 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Oberoi RK, Parrish KE, Sio TT, Mittapalli RK, Elmquist WF, Sarkaria JN, Strategies to improve delivery of anticancer drugs across the blood–brain barrier to treat glioblastoma, Neuro-oncology, 18 (2015) 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Miranda A, Blanco-Prieto MJ, Sousa J, Pais A, Vitorino C, Breaching barriers in glioblastoma. Part II: Targeted drug delivery and lipid nanoparticles, International journal of pharmaceutics, 531 (2017) 389–410. [DOI] [PubMed] [Google Scholar]
- [125].Hülper P, Veszelka S, Walter F, Wolburg H, Fallier-Becker P, Piontek J, Blasig I, Lakomek M, Kugler W, Deli M, Acute effects of short-chain alkylglycerols on blood-brain barrier properties of cultured brain endothelial cells, British journal of pharmacology, 169 (2013) 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Han L, Kong DK, Zheng M.-q., Murikinati S, Ma C, Yuan P, Li L, Tian D, Cai Q, Ye C, Increased nanoparticle delivery to brain tumors by autocatalytic priming for improved treatment and imaging, ACS nano, 10 (2016) 4209–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li S, Su W, Wu H, Yuan T, Yuan C, Liu J, Deng G, Gao X, Chen Z, Bao Y, Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids, Nature biomedical engineering, 4 (2020) 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhang S, Deng G, Liu F, Peng B, Bao Y, Du F, Chen AT, Liu J, Chen Z, Ma J, Autocatalytic Delivery of Brain Tumor–Targeting, Size-Shrinkable Nanoparticles for Treatment of Breast Cancer Brain Metastases, Advanced functional materials, 30 (2020) 1910651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Carman AJ, Mills JH, Krenz A, Kim D-G, Bynoe MS, Adenosine receptor signaling modulates permeability of the blood–brain barrier, Journal of Neuroscience, 31 (2011) 13272–13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Miao T, Ju X, Zhu Q, Wang Y, Guo Q, Sun T, Lu C, Han L, Nanoparticles surmounting blood–brain tumor barrier through both transcellular and paracellular pathways to target brain metastases, Advanced Functional Materials, 29 (2019) 1900259. [Google Scholar]
- [131].Deprez J, Lajoinie G, Engelen Y, De Smedt S, Lentacker I, Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery, Advanced drug delivery reviews, 172 (2021) 9–36. [DOI] [PubMed] [Google Scholar]
- [132].O’Reilly MA, Huang Y, Hynynen K, The impact of standing wave effects on transcranial focused ultrasound disruption of the blood–brain barrier in a rat model, Physics in Medicine & Biology, 55 (2010) 5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Park J, Aryal M, Vykhodtseva N, Zhang Y-Z, McDannold N, Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption, Journal of Controlled Release, 250 (2017) 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wei K-C, Chu P-C, Wang H-YJ, Huang C-Y, Chen P-Y, Tsai H-C, Lu Y-J, Lee P-Y, Tseng I-C, Feng L-Y, Focused ultrasound-induced blood–brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study, PloS one, 8 (2013) e58995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Liu H-L, Hua M-Y, Chen P-Y, Chu P-C, Pan C-H, Yang H-W, Huang C-Y, Wang J-J, Yen T-C, Wei K-C, Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment, Radiology, 255 (2010) 415–425. [DOI] [PubMed] [Google Scholar]
- [136].Mangraviti A, Gullotti D, Tyler B, Brem H, Nanobiotechnology-based delivery strategies: New frontiers in brain tumor targeted therapies, Journal of Controlled Release, 240 (2016) 443–453. [DOI] [PubMed] [Google Scholar]
- [137].Yang F-Y, Wong T-T, Teng M-C, Liu R-S, Lu M, Liang H-F, Wei M-C, Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme, Journal of controlled release, 160 (2012) 652–658. [DOI] [PubMed] [Google Scholar]
- [138].Curley CT, Mead BP, Negron K, Kim N, Garrison WJ, Miller GW, Kingsmore KM, Thim EA, Song J, Munson JM, Augmentation of brain tumor interstitial flow via focused ultrasound promotes brain-penetrating nanoparticle dispersion and transfection, Science advances, 6 (2020) eaay1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ting C-Y, Fan C-H, Liu H-L, Huang C-Y, Hsieh H-Y, Yen T-C, Wei K-C, Yeh C-K, Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment, Biomaterials, 33 (2012) 704–712. [DOI] [PubMed] [Google Scholar]
- [140].Liu H-L, Fan C-H, Ting C-Y, Yeh C-K, Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview, Theranostics, 4 (2014) 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ng EYK, Kumar SD, Physical mechanism and modeling of heat generation and transfer in magnetic fluid hyperthermia through Néelian and Brownian relaxation: a review, Biomedical engineering online, 16 (2017) 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tabatabaei SN, Girouard H, Carret A-S, Martel S, Remote control of the permeability of the blood–brain barrier by magnetic heating of nanoparticles: a proof of concept for brain drug delivery, Journal of Controlled Release, 206 (2015) 49–57. [DOI] [PubMed] [Google Scholar]
- [143].Kiyatkin EA, Sharma HS, Permeability of the blood–brain barrier depends on brain temperature, Neuroscience, 161 (2009) 926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kong SD, Lee J, Ramachandran S, Eliceiri BP, Shubayev VI, Lal R, Jin S, Magnetic targeting of nanoparticles across the intact blood–brain barrier, Journal of controlled release, 164 (2012) 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Mahmoudi K, Hadjipanayis CG, The application of magnetic nanoparticles for the treatment of brain tumors, Frontiers in chemistry, 2 (2014) 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Lammers T, Koczera P, Fokong S, Gremse F, Ehling J, Vogt M, Pich A, Storm G, Van Zandvoort M, Kiessling F, Theranostic USPIO-loaded microbubbles for mediating and monitoring blood-brain barrier permeation, Advanced functional materials, 25 (2015) 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study, Scientific reports, 9 (2019) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Liu X, Duan Y, Liu B, Nanoparticles as contrast agents for photoacoustic brain imaging, Aggregate, 2 (2021) 4–19. [Google Scholar]
- [149].Wu M, Chen W, Chen Y, Zhang H, Liu C, Deng Z, Sheng Z, Chen J, Liu X, Yan F, Focused Ultrasound-Augmented Delivery of Biodegradable Multifunctional Nanoplatforms for Imaging-Guided Brain Tumor Treatment, Advanced Science, 5 (2018) 1700474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Mendanha D, de Castro JV, Ferreira H, Neves N, Biomimetic and cell-based nanocarriers–New strategies for brain tumor targeting, Journal of Controlled Release, 337 (2021) 482–493. [DOI] [PubMed] [Google Scholar]
- [151].Yu H, Yang Z, Li F, Xu L, Sun Y, Cell-mediated targeting drugs delivery systems, Drug Delivery, 27 (2020) 1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lee H-W, Choi H-J, Ha S-J, Lee K-T, Kwon Y-G, Recruitment of monocytes/macrophages in different tumor microenvironments, Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1835 (2013) 170–179. [DOI] [PubMed] [Google Scholar]
- [153].Pardridge WM, Drug transport across the blood–brain barrier, Journal of cerebral blood flow & metabolism, 32 (2012) 1959–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Choi M-R, Bardhan R, Stanton-Maxey KJ, Badve S, Nakshatri H, Stantz KM, Cao N, Halas NJ, Clare SE, Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse, Cancer nanotechnology, 3 (2012) 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Huang B, Abraham WD, Zheng Y, López SCB, Luo SS, Irvine DJ, Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells, Science translational medicine, 7 (2015) 291ra294–291ra294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Bexell D, Scheding S, Bengzon J, Toward brain tumor gene therapy using multipotent mesenchymal stromal cell vectors, Molecular Therapy, 18 (2010) 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H, Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model, Gene therapy, 11 (2004) 1155–1164. [DOI] [PubMed] [Google Scholar]
- [158].Aboody K, Najbauer J, Danks M, Stem and progenitor cell-mediated tumor selective gene therapy, Gene therapy, 15 (2008) 739–752. [DOI] [PubMed] [Google Scholar]
- [159].Zhang X, Yao S, Liu C, Jiang Y, Tumor tropic delivery of doxorubicin-polymer conjugates using mesenchymal stem cells for glioma therapy, Biomaterials, 39 (2015) 269–281. [DOI] [PubMed] [Google Scholar]
- [160].Batrakova EV, Gendelman HE, Kabanov AV, Cell-mediated drug delivery, Expert opinion on drug delivery, 8 (2011) 415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L, Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform, Proceedings of the National Academy of Sciences, 108 (2011) 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L, Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery, Nano letters, 14 (2014) 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Liu H.j., Wang J, Wang M, Wang Y, Shi S, Hu X, Zhang Q, Fan D, Xu P, Biomimetic Nanomedicine Coupled with Neoadjuvant Chemotherapy to Suppress Breast Cancer Metastasis via Tumor Microenvironment Remodeling, Advanced Functional Materials, (2021) 2100262. [Google Scholar]
- [164].Dong X, Mu LL, Liu XL, Zhu H, Yang SC, Lai X, Liu HJ, Feng HY, Lu Q, Zhou BBS, Biomimetic, Hypoxia-Responsive Nanoparticles Overcome Residual Chemoresistant Leukemic Cells with Co-Targeting of Therapy-Induced Bone Marrow Niches, Advanced Functional Materials, 30 (2020) 2000309. [Google Scholar]
- [165].Liu XL, Dong X, Yang SC, Lai X, Liu HJ, Gao Y, Feng HY, Zhu MH, Yuan Y, Lu Q, Biomimetic liposomal nanoplatinum for targeted cancer chemophototherapy, Advanced Science, 8 (2021) 2003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Wang C, Wu B, Wu Y, Song X, Zhang S, Liu Z, Camouflaging nanoparticles with brain metastatic tumor cell membranes: a new strategy to traverse blood–brain barrier for imaging and therapy of brain tumors, Advanced Functional Materials, 30 (2020) 1909369. [Google Scholar]
- [167].Chai Z, Hu X, Wei X, Zhan C, Lu L, Jiang K, Su B, Ruan H, Ran D, Fang RH, A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery, Journal of Controlled Release, 264 (2017) 102–111. [DOI] [PubMed] [Google Scholar]
- [168].Ma J, Zhang S, Liu J, Liu F, Du F, Li M, Chen AT, Bao Y, Suh HW, Avery J, Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells, Small, 15 (2019) 1902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Feng L, Dou C, Xia Y, Li B, Zhao M, Yu P, Zheng Y, El-Toni AM, Atta NF, Galal A, Neutrophil-like Cell-Membrane-Coated Nanozyme Therapy for Ischemic Brain Damage and Long-Term Neurological Functional Recovery, ACS nano, 15 (2021) 2263–2280. [DOI] [PubMed] [Google Scholar]
- [170].Liu H, Han Y, Wang T, Zhang H, Xu Q, Yuan J, Li Z, Targeting Microglia for Therapy of Parkinson’s Disease by Using Biomimetic Ultrasmall Nanoparticles, Journal of the American Chemical Society, (2020). [DOI] [PubMed] [Google Scholar]
- [171].Harris JC, Scully MA, Day ES, Cancer cell membrane-coated nanoparticles for cancer management, Cancers, 11 (2019) 1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Fang RH, Kroll AV, Gao W, Zhang L, Cell membrane coating nanotechnology, Advanced Materials, 30 (2018) 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Liu HJ, Wang MM, Shi SS, Hu XX, Xu PS, A Therapeutic Sheep in Metastatic Wolf’s Clothing: Trojan Horse Approach for Cancer Brain Metastases Treatment, Nano-Micro Lett, 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK, The biology of brain metastases-translation to new therapies, Nat Rev Clin Oncol, 8 (2011) 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ernsting MJ, Murakami M, Roy A, Li S-D, Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles, Journal of controlled release, 172 (2013) 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Salatin S, Maleki Dizaj S, Yari Khosroushahi A, Effect of the surface modification, size, and shape on cellular uptake of nanoparticles, Cell biology international, 39 (2015) 881–890. [DOI] [PubMed] [Google Scholar]
- [177].Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X, Emerging blood–brain-barrier-crossing nanotechnology for brain cancer theranostics, Chemical Society Reviews, 48 (2019) 2967–3014. [DOI] [PubMed] [Google Scholar]
- [178].Wiley DT, Webster P, Gale A, Davis ME, Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor, Proceedings of the National Academy of Sciences, 110 (2013) 8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].He H, Li Y, Jia X-R, Du J, Ying X, Lu W-L, Lou J-N, Wei Y, PEGylated Poly (amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors, Biomaterials, 32 (2011) 478–487. [DOI] [PubMed] [Google Scholar]
- [180].Wu H, Lu H, Xiao W, Yang J, Du H, Shen Y, Qu H, Jia B, Manna SK, Ramachandran M, Sequential targeting in crosslinking nanotheranostics for tackling the multibarriers of brain tumors, Advanced Materials, 32 (2020) 1903759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, PEGylation as a strategy for improving nanoparticle-based drug and gene delivery, Advanced drug delivery reviews, 99 (2016) 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Gajbhiye K, Pawar A, Mahadik K, Gajbhiye V, PEGylated nanocarriers: A promising tool for targeted delivery to the brain, Colloids and Surfaces B: Biointerfaces, 187 (2020) 110770. [DOI] [PubMed] [Google Scholar]
- [183].Deng Y, Saucier-Sawyer JK, Hoimes CJ, Zhang J, Seo Y-E, Andrejecsk JW, Saltzman WM, The effect of hyperbranched polyglycerol coatings on drug delivery using degradable polymer nanoparticles, Biomaterials, 35 (2014) 6595–6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lemarchand C, Gref R, Couvreur P, Polysaccharide-decorated nanoparticles, European Journal of Pharmaceutics and Biopharmaceutics, 58 (2004) 327–341. [DOI] [PubMed] [Google Scholar]
- [185].Sonali P. Agrawal, Singh RP, Rajesh CV, Singh S, Vijayakumar MR, Pandey BL, Muthu MS, Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: preparation, characterization and brain distribution in rats, Drug delivery, 23 (2016) 1788–1798. [DOI] [PubMed] [Google Scholar]
- [186].Fang JH, Lai YH, Chiu TL, Chen YY, Hu SH, Chen SY, Magnetic Core–Shell Nanocapsules with Dual - Targeting Capabilities and Co - Delivery of Multiple Drugs to Treat Brain Gliomas, Advanced healthcare materials, 3 (2014) 1250–1260. [DOI] [PubMed] [Google Scholar]
- [187].Lavasanifar A, Samuel J, Kwon GS, Poly (ethylene oxide)-block-poly (L-amino acid) micelles for drug delivery, Advanced drug delivery reviews, 54 (2002) 169–190. [DOI] [PubMed] [Google Scholar]
- [188].Wohlfart S, Gelperina S, Kreuter J, Transport of drugs across the blood–brain barrier by nanoparticles, Journal of controlled release, 161 (2012) 264–273. [DOI] [PubMed] [Google Scholar]
- [189].Tsou YH, Zhang XQ, Zhu H, Syed S, Xu X, Drug delivery to the brain across the blood–brain barrier using nanomaterials, Small, 13 (2017) 1701921. [DOI] [PubMed] [Google Scholar]
- [190].Lockman PR, Koziara JM, Mumper RJ, Allen DD, Nanoparticle surface charges alter blood–brain barrier integrity and permeability, Journal of drug targeting, 12 (2004) 635–641. [DOI] [PubMed] [Google Scholar]
- [191].Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA, Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts, Proceedings of the National Academy of Sciences, 105 (2008) 14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS, The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles, Biomaterials, 32 (2011) 3435–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zhan C, Lu W, The blood-brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery, Current pharmaceutical biotechnology, 13 (2012) 2380–2387. [DOI] [PubMed] [Google Scholar]
- [194].Sarin H, Kanevsky AS, Wu H, Sousa AA, Wilson CM, Aronova MA, Griffiths GL, Leapman RD, Vo HQ, Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors, Journal of translational medicine, 7 (2009) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Sarin H, Kanevsky AS, Wu H, Brimacombe KR, Fung SH, Sousa AA, Auh S, Wilson CM, Sharma K, Aronova MA, Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells, Journal of translational medicine, 6 (2008) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Meola A, Rao J, Chaudhary N, Sharma M, Chang SD, Gold nanoparticles for brain tumor imaging: a systematic review, Frontiers in neurology, 9 (2018) 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Verma A, Stellacci F, Effect of surface properties on nanoparticle–cell interactions, small, 6 (2010) 12–21. [DOI] [PubMed] [Google Scholar]
- [198].Wilhelm C, Billotey C, Roger J, Pons J, Bacri J-C, Gazeau F, Intracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coating, Biomaterials, 24 (2003) 1001–1011. [DOI] [PubMed] [Google Scholar]
- [199].Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S, Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium, Proceedings of the National Academy of Sciences, 110 (2013) 10753–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Lee C, Hwang HS, Lee S, Kim B, Kim JO, Oh KT, Lee ES, Choi HG, Youn YS, Rabies virus-inspired silica-coated gold nanorods as a photothermal therapeutic platform for treating brain tumors, Advanced Materials, 29 (2017) 1605563. [DOI] [PubMed] [Google Scholar]
- [201].Hoshyar N, Gray S, Han H, Bao G, The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction, Nanomedicine, 11 (2016) 673–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Ojha T, Pathak V, Shi Y, Hennink WE, Moonen CT, Storm G, Kiessling F, Lammers T, Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors, Advanced drug delivery reviews, 119 (2017) 44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Bozzuto G, Molinari A, Liposomes as nanomedical devices, International journal of nanomedicine, 10 (2015) 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K, Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles, Ultrasound Med Biol, 30 (2004) 979–989. [DOI] [PubMed] [Google Scholar]
- [205].Shang X, Wang P, Liu Y, Zhang Z, Xue Y, Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction, J Mol Neurosci, 43 (2011) 364–369. [DOI] [PubMed] [Google Scholar]
- [206].Lammers T, Koczera P, Fokong S, Gremse F, Ehling J, Vogt M, Pich A, Storm G, van Zandvoort M, Kiessling F, Theranostic USPIO-Loaded Microbubbles for Mediating and Monitoring Blood-Brain Barrier Permeation, Adv Funct Mater, 25 (2015) 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Wu M, Chen W, Chen Y, Zhang H, Liu C, Deng Z, Sheng Z, Chen J, Liu X, Yan F, Zheng H, Focused Ultrasound-Augmented Delivery of Biodegradable Multifunctional Nanoplatforms for Imaging-Guided Brain Tumor Treatment, Adv Sci (Weinh), 5 (2018) 1700474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Tian Y, Mi G, Chen Q, Chaurasiya B, Li Y, Shi D, Zhang Y, Webster TJ, Sun C, Shen Y, Acid-Induced Activated Cell-Penetrating Peptide-Modified Cholesterol-Conjugated Polyoxyethylene Sorbitol Oleate Mixed Micelles for pH-Triggered Drug Release and Efficient Brain Tumor Targeting Based on a Charge Reversal Mechanism, ACS applied materials & interfaces, 10 (2018) 43411–43428. [DOI] [PubMed] [Google Scholar]
- [209].Wu H, Lu HW, Xiao WW, Yang JF, Du HX, Shen YB, Qu HJ, Jia B, Manna SK, Ramachandran M, Xue XD, Ma Z, Xu XB, Wang ZL, He YX, Lam KS, Zawadzki RJ, Li YP, Lin TY, Sequential Targeting in Crosslinking Nanotheranostics for Tackling the Multibarriers of Brain Tumors, Advanced Materials, 32 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Åberg C, Mahon E, Dawson KA, Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface, Nature nanotechnology, 8 (2013) 137–143. [DOI] [PubMed] [Google Scholar]
- [211].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WC, Analysis of nanoparticle delivery to tumours, Nature reviews materials, 1 (2016) 1–12. [Google Scholar]
- [212].Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, Asquier N, Law-Ye B, Leclercq D, Bissery A, Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma, Clinical Cancer Research, 25 (2019) 3793–3801. [DOI] [PubMed] [Google Scholar]


