Abstract
Background
There is growing interest in a “treatable traits” approach to pulmonary rehabilitation in chronic airways disease. The frequency with which pulmonary rehabilitation programmes address treatable traits is unknown.
Methods
Randomised controlled trials of pulmonary rehabilitation compared to usual care in patients with stable chronic airways disease were included. The components of pulmonary rehabilitation delivered were extracted and mapped to treatable traits in pulmonary, extrapulmonary and behavioural/lifestyle domains. Meta-analysis was used to evaluate the impact of addressing >1 treatable trait on exercise capacity and health-related quality of life (HRQoL).
Results
116 trials were included (6893 participants). Almost all pulmonary rehabilitation programmes addressed deconditioning (97% of trials). The most commonly addressed extrapulmonary traits were nutritional status (obesity and cachexia, 18% each) and mood disturbance (anxiety and depression, 10% each). Behavioural/lifestyle traits most frequently addressed were nonadherence (46%), poor inhalation technique (24%) and poor family/social support (19%). Exercise capacity and HRQoL outcomes did not differ between studies that addressed deconditioning alone and those that targeted additional traits, but heterogeneity was high.
Conclusion
Aside from deconditioning, treatable traits are infrequently addressed in existing trials of pulmonary rehabilitation. The potential of the treatable traits approach to improve pulmonary rehabilitation outcomes remains to be explored.
Short abstract
Deconditioning is commonly targeted in pulmonary rehabilitation, but other treatable traits are infrequently addressed. The potential of the treatable traits approach to improve pulmonary rehabilitation outcomes remains to be explored. https://bit.ly/3xVYeGe
Background
Pulmonary rehabilitation is a highly effective intervention to enhance exercise capacity, improve symptoms and reduce hospitalisations in people with chronic airways disease [1–4]. Pulmonary rehabilitation is defined as the delivery of “patient tailored therapies … designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviours” [5]. This definition is not restricted to a particular patient group, and is consistent with a personalised, treatable traits approach to patient management [6]. The treatable traits approach is a precision medicine model that does not apply diagnostic labels such as asthma or chronic obstructive pulmonary disease (COPD). Rather, it involves identifying the treatable traits in an individual based on established biomarkers, and targeting these traits with treatments of known efficacy [6]. There is growing interest in a treatable traits approach to pulmonary rehabilitation, comprising a thorough assessment to identify relevant treatable traits and an individually tailored rehabilitation programme [7].
Treatable traits have been classified into three domains: pulmonary, extrapulmonary and behavioural/lifestyle traits [6]. Although some pulmonary treatable traits may be infrequently addressed in pulmonary rehabilitation (e.g. emphysema, eosinophilic airway inflammation, pulmonary hypertension), interventions that target extrapulmonary and behavioural/lifestyle traits are perceived to be key components of a comprehensive pulmonary rehabilitation model [5]. This includes the extrapulmonary trait of deconditioning, addressed through the provision of an individualised exercise training programme, an essential component of all pulmonary rehabilitation programmes [5]. However, the frequency with which pulmonary rehabilitation programmes assess and specifically address treatable traits, and any gaps in the treatable traits approach in its application within pulmonary rehabilitation, have not been established. The aim of this systematic review was to understand the frequency with which treatable traits are addressed in pulmonary rehabilitation clinical trials, in order to inform future model development.
Methods
The protocol was registered prospectively on 4 March 2021 on Open Science Framework (https://osf.io/9rq3f).
Participants
We included studies of patients with chronic airways disease of any severity. As we expected that most studies used a diagnosis-based approach, for the purposes of this review we examined studies that included patients with COPD, asthma and bronchiectasis. We excluded studies of patients with fibrotic lung disease or pulmonary hypertension as, to date, the treatable traits approach has not been applied to these groups.
Types of studies
We included clinical trials of pulmonary rehabilitation compared to usual care in patients with stable chronic airways disease, identified from published systematic reviews [1, 4, 8]. Systematic reviews were identified from Medline (Ovid) searches using keywords for diagnosis (COPD, asthma or bronchiectasis) and rehabilitation or exercise training, limited to “review articles” or “topic reviews (Cochrane)”. We selected Cochrane reviews when these were available because of their robust methodological approach. Where a Cochrane review was not available, we selected the most recently published review where the methodology (inclusion and exclusion criteria, definition of pulmonary rehabilitation) aligned with our registered protocol. Where the search strategy had been executed more than 12 months ago, we repeated the search using the original strategy, to identify new clinical trials.
Types of interventions
For the purposes of this review, we identified pulmonary rehabilitation as “any inpatient, outpatient, community-based or home-based rehabilitation programme of at least 4 weeks’ duration that included exercise therapy with or without any form of education and/or psychological support” [1]. Usual care was defined as conventional care, which could include verbal advice or medical review.
Outcomes
The primary outcome of interest was the delivery of an intervention component to address treatable traits. For the purposes of this review, treatable traits and their treatments were defined according to the original list of traits proposed by Agusti et al. [6], with minor adaptations to operationalise the identification of treatments and to ensure treatments delivered in pulmonary rehabilitation were included (table S1). For example, airway clearance techniques were included for treatment of chronic sputum production, and smoking cessation was included only under treatment of the trait of smoking (not under chronic sputum production) to aid operationalisation.
For each treatable trait, the intervention component was coded as definitely delivered, probably delivered or not delivered. A treatment for a trait was considered to be “definitely delivered” if it was specifically mentioned in the text and was congruent with our definition (table S1). In cases where the study methods indicated it was likely that the trait had been treated, but the specific treatment was not named in the text, we classified it as “probably delivered”. An example is where studies indicated that respiratory medications were optimised according to best practice guidelines, but specific medications (e.g. bronchodilators) were not mentioned. Evidence to support the delivery of the treatment was extracted from each paper as a direct quote.
Where there were sufficient data available, we compared effects across programmes that addressed different combinations of treatable traits (deconditioning only or deconditioning plus other treatable traits) for the outcomes of exercise capacity and health-related quality of life (HRQoL). Consistent with the treatable traits approach, which does not employ diagnostic labels [6], data for the three diagnostic groups were pooled, and we did not perform subgroup analyses for COPD, asthma and bronchiectasis.
Identification of systematic reviews and conducting of updated searches
Relevant systematic reviews [1, 4, 8] were identified through a Medline search by one author (A.H.). Cochrane systematic reviews were used where available. All included studies from the original systematic reviews were obtained as full text and included in this review. Updated searches for each of the reviews were performed in May 2021 to identify any new studies, using the original search strategy (table S2). Two reviewers (A.H. and B.W.) screened titles and abstracts from the updated search independently. Those identified as “include” or “unsure” were obtained as full text. Full-text review was conducted by two reviewers independently (A.H. and B.W.) to determine eligibility for inclusion. A record of reasons for exclusion was kept for full-text reviews.
Assessment of risk of bias
Risk of bias was assessed by two reviewers independently (B.W., A.J., M.H. and A.L.). For individual studies identified from the original systematic reviews [1, 4, 8], we retrieved the risk of bias assessments that had been made in the reviews. In order to support this decision, methodological quality of the systematic reviews was assessed using the AMSTAR-II checklist [9]. For individual studies in the updated search, risk of bias was assessed using the Cochrane risk of bias tool [10], consistent with the approach used in the original systematic reviews [1, 4, 8].
Data extraction
Two reviewers extracted data from the original papers independently (B.W., A.J., M.H. and A.L.) on a custom-designed data collection form. The data extracted were study design, inclusion and exclusion criteria for participants, demographic characteristics including age, gender, lung function, number of participants, setting and characteristics of the rehabilitation programme (duration, inpatient/outpatient, etc.), components of the rehabilitation programme, treatable traits addressed, characteristics of usual care, outcomes assessed, outcome data for exercise capacity and HRQoL, and adverse events.
Data synthesis
The primary outcome was the number and proportion of studies that addressed each of the treatable traits. Where sufficient data were available from trials that were clinically homogeneous (e.g. addressed the same combination of treatable traits, either definitely or probably delivered) we performed meta-analysis to derive pooled effects for the outcomes of exercise capacity (6-min walk distance (6MWD)) and HRQoL (St George's respiratory questionnaire total score), for comparison across models. Meta-analysis was performed using Review Manager version 5.4.1 and used a random effects model. The I2 statistic was used to measure heterogeneity among studies in each subgroup. Funnel plots were used to assess reporting bias.
Results
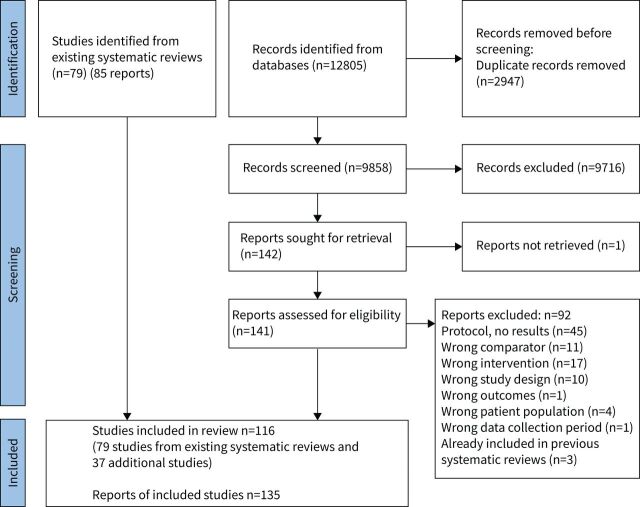
We included 79 studies from previous systematic reviews, including 65 studies from the COPD review (70 reports), nine from the asthma review, one of which was also in the COPD review [11] (10 reports), and six from the bronchiectasis review. An additional 37 studies were identified in the updated searches (49 reports) including 32 from the COPD search (43 reports), five from the asthma search (six reports) and none from the bronchiectasis search. A total of 116 studies (135 reports) were included in this review (figure 1). There were 6893 participants, with 3821 randomised to pulmonary rehabilitation. Settings for pulmonary rehabilitation included outpatient (n=69, 60%), inpatient (n=4, 3%) and home (n=23, 20%), a combination of inpatient and home (n=2, 2%), and a combination of outpatient and home (n=5, 4%), with no setting reported in 13 studies (11%). Across studies, average age ranged from 22 to 78 years (mean 63 (sd 10) years) and average forced expiratory volume in 1 s ranged from 26 to 90% predicted (mean 53 (sd 16) % pred). Characteristics of the included studies are shown in table S3.
FIGURE 1.
PRISMA flow diagram for study selection.
The systematic reviews used to identify studies [1, 4, 8] scored well in almost all domains of the AMSTAR-2 checklist, except for those related to reporting source of funding for included studies and investigation of publication bias (table S2). Risk of bias for individual studies in the updated search is provided in table S3. Similar to the individual studies included in the systematic reviews [1, 4, 8], risk of bias was generally low for sequence generation and allocation concealment, but high for performance and detection bias due to lack of blinding.
Delivery of interventions to address treatable traits varied across domains (tables 1–3, table S3). Pulmonary treatable traits were rarely addressed, with individual pulmonary treatable traits not addressed in 78–100% of studies. Airway smooth muscle contraction was the most frequently addressed pulmonary treatable trait (definitely delivered 7%, probably delivered 15%), usually through interventions to optimise bronchodilator therapy, followed by chronic sputum production (definitely delivered 11%, probably delivered 6%), predominantly in studies where participants were trained in use of airway clearance techniques. The extrapulmonary treatable trait of deconditioning was addressed in 97% of included studies through exercise training (table 2). Other extrapulmonary treatable traits were infrequently addressed, including cachexia (definitely delivered 9%, probably delivered 9%), obesity (definitely delivered 7%, probably delivered 11%), depression (definitely delivered 1%, probably delivered 9%) and anxiety (definitely delivered 2%, probably delivered 8%). There was marked variation across behavioural and lifestyle treatable traits (table 3), with nonadherence to treatment addressed most frequently (definitely delivered 23%, probably delivered 23%) followed by poor inhalation technique (definitely delivered 6%, probably delivered 18%), poor family and social support (definitely delivered 5%, probably delivered 14%), and smoking (definitely delivered 6%, probably delivered 10%).
TABLE 1.
Pulmonary treatable traits addressed in pulmonary rehabilitation trials
| Trait | Definitely delivered (%) | Probably delivered (%) | Not delivered (%) |
| Airway smooth muscle contraction | 7 | 15 | 78 |
| Eosinophilic airway inflammation | 2 | 4 | 94 |
| Chronic sputum production | 11 | 6 | 83 |
| Bacterial colonisation | 0 | 6 | 94 |
| Bronchiectasis | 2 | 2 | 96 |
| Cough reflex hypersensitivity | 0 | 0 | 100 |
| Chronic respiratory failure | 1 | 2 | 97 |
| Pulmonary hypertension | 0 | 0 | 100 |
| Emphysema | 0 | 0 | 100 |
TABLE 3.
Behavioural/lifestyle treatable traits addressed in pulmonary rehabilitation trials
| Trait | Definitely delivered (%) | Probably delivered (%) | Not delivered (%) |
| Poor inhalation technique | 6 | 18 | 76 |
| Nonadherence to treatment | 23 | 23 | 54 |
| Smoking | 6 | 10 | 84 |
| Exposure to sensitising agents | 1 | 3 | 96 |
| Side effects of treatment | 3 | 8 | 89 |
| Polypharmacy | 1 | 2 | 97 |
| Poor family and social support | 5 | 14 | 81 |
TABLE 2.
Extrapulmonary treatable traits addressed in pulmonary rehabilitation trials
| Trait | Definitely delivered (%) | Probably delivered (%) | Not delivered (%) |
| Rhinosinusitis | 0 | 1 | 99 |
| Deconditioning | 97 | 1 | 2 |
| Cachexia | 9 | 9 | 82 |
| Obesity | 7 | 11 | 82 |
| Cardiovascular disease | 0 | 2 | 98 |
| Vocal cord dysfunction | 0 | 0 | 100 |
| Depression | 1 | 9 | 90 |
| Anxiety | 2 | 8 | 90 |
| Systemic inflammation | 1 | 1 | 98 |
One quarter of studies (n=29) addressed the trait of deconditioning only. The most common combinations of treatable traits addressed in pulmonary rehabilitation programmes were deconditioning plus airway smooth muscle contraction (n=26, 22%, commonly optimisation of inhalation technique), deconditioning plus nutritional status (n=24, 21%, addressing cachexia and/or obesity) and deconditioning and mood (n=15, 13%, addressing anxiety and/or depression). A total of 63 studies (54%) addressed deconditioning as well as at least one lifestyle/behavioural treatable trait. This included nonadherence to treatment (n=52), but this was frequently limited to encouragement with independent (home) exercise, rather than a broader approach to nonadherence. The other behavioural trait commonly addressed was assessment of inhalation technique (n=28 studies, overlapping with those addressing airway smooth muscle contraction). Subgroup analyses for common combinations of treatable traits are shown in table 4 and figures S1 and S2. Clinically important effects of pulmonary rehabilitation on exercise capacity and HRQoL were seen across all subgroups, with no evidence of larger effects in studies that addressed a greater number of treatable traits; however, statistical heterogeneity was moderate to high (I2 range 41–93%, table 4). There was asymmetry in the funnel plot for the 6MWD (figure S3), particularly for studies that addressed only the trait of deconditioning, but this was not evident for the outcome of St George's respiratory questionnaire (figure S4).
TABLE 4.
Subgroup analyses for studies addressing common combinations of treatable traits
| Subgroup | Number of studies | Number of participants | Mean difference rehabilitation – control (95% confidence interval) | I2 (%) |
| 6-min walk distance (m) | ||||
| Deconditioning only | 18 | 847 | 50 (39–62) | 50 |
| Deconditioning and airway smooth muscle contraction | 13 | 766 | 48 (25–72) | 81 |
| Deconditioning and mood | 7 | 684 | 37 (11–64) | 76 |
| Deconditioning and nutrition | 15 | 1243 | 45 (29–62) | 79 |
| Deconditioning and at least one behavioural/lifestyle trait | 28 | 2041 | 40 (26–53) | 80 |
| Health-related quality of life – St George's respiratory questionnaire total score (points) | ||||
| Deconditioning only | 8 | 328 | −12.2 (−21.4– −3.0) | 93 |
| Deconditioning and airway smooth muscle contraction | 8 | 583 | −9.7 (−13.3– −6.1) | 41 |
| Deconditioning and mood | 4 | 626 | −13.9 (−21.9– −6.0) | 90 |
| Deconditioning and nutrition | 15 | 1349 | −8.44 (−12.0– −4.9) | 89 |
| Deconditioning and at least one behavioural/lifestyle trait | 21 | 1761 | −8.25 (−11.3– −5.2) | 82 |
Discussion
This systematic review examined whether the current model of pulmonary rehabilitation, as described in 116 randomised controlled trials including 6893 participants, addressed treatable traits proposed to be important in patients with chronic airways disease [6]. As expected, almost all studies (97%) addressed deconditioning through the provision of exercise training. Extrapulmonary treatable traits were addressed less commonly than expected, including nutritional status (obesity and cachexia, 18% of studies each) and mood disturbance (anxiety and depression, 10% each). The behavioural and lifestyle traits most frequently addressed were nonadherence, primarily to encourage independent exercise (46%), poor inhalation technique (24%), and poor family and social support (19%). There was no evidence that addressing specific combinations of treatable traits in pulmonary rehabilitation resulted in better outcomes; however, there was considerable variation in outcomes across studies.
Pulmonary rehabilitation is considered a highly effective treatment for people with chronic airways disease and is recommended in clinical practice guidelines around the world. A key feature of pulmonary rehabilitation is a comprehensive assessment to identify an individual's needs and goals, and many suggested assessment items address the treatable traits examined in this study [6, 12]. It was therefore surprising that, aside from deconditioning, treatable traits appeared to be infrequently addressed in the clinical trials that form the evidence base on which modern pulmonary rehabilitation practice is based. It is possible that this reflects the limited description of programme components in the methods sections of included studies, rather than the absence of this individualised approach. A more comprehensive and systematic reporting of intervention components would be helpful in future pulmonary rehabilitation trials [13]. However, it is also possible that many pulmonary rehabilitation programmes do not directly address many of the proposed treatable traits. Although an approach consistent with treatable traits is certainly applied in some centres [7], substantial variation in the content and organisational structure of pulmonary rehabilitation programmes has been well documented [14]. Pulmonary rehabilitation programmes frequently focus on nonpharmacological treatment strategies, such that some pulmonary treatable traits (e.g emphysema, eosinophilic airway inflammation, pulmonary hypertension) might be better addressed in other clinical contexts. However, the strong emphasis of pulmonary rehabilitation on addressing the extrapulmonary features of chronic airways disease, and facilitating health behaviour change, makes it surprising that traits in these domains appeared to be infrequently addressed.
The most relevant treatable traits to apply in the pulmonary rehabilitation context have not yet been established. The approach to treatable traits was primarily developed in patients with asthma [6], and whilst a “label-free”, diagnosis-agnostic approach has been proposed, it is unclear whether the traits applied in this review are optimal for patients with other respiratory conditions. A recent study has proposed a slightly different list of treatable traits in patients with COPD, including shortness of breath, anaemia, disability and arthritis [15]. Importantly, the traits that predicted decline in lung function were not the same as those predicting decline in HRQoL, indicating that the most important traits vary according to the outcome under consideration [15]. In pulmonary rehabilitation, the important outcomes include exercise capacity, HRQoL and future hospitalisations, so the traits addressed should be relevant to these outcomes. In COPD, nine treatable traits which point towards nonpharmacological interventions (such as pulmonary rehabilitation) have been identified, with an outcome of health status [16]. Whilst some of these proposed traits are similar to previous approaches (e.g. depression, nutritional status) others are new (frequent exacerbations, severe fatigue, low physical activity, patient activation for self-management). If pulmonary rehabilitation programmes are to adopt a treatable traits approach, there is a need for consensus on the most relevant traits to address in the pulmonary rehabilitation context.
On average, people with severe asthma and COPD present with 10 treatable traits [17, 18]. It is likely that many pulmonary rehabilitation programmes do not have the resources to comprehensively address all traits. It has been suggested that traits could be prioritised based on their clinical impact (severity, prevalence or impact on disease-important outcomes) and patient impact (traits rated as a high priority by patients). Whilst the clinical impact of treatable traits is becoming clearer [15], we do not yet know very much about which traits matter the most to patients. It may be important to identify traits that are nodal points in a disease network that may impact other traits, thus providing broader benefits [19]. An example might be targeting deconditioning with exercise training, which is also known to impact other important traits including anxiety and depression [20]. Similarly, targeting obesity with weight loss and resistance training improved depression and cardiovascular risk [21]. A deeper understanding of which treatable traits are most likely to enhance pulmonary rehabilitation outcomes may enhance translation of this approach into clinical practice.
At present, around half of the patients with COPD who undertake pulmonary rehabilitation do not achieve improvements exceeding the minimal important difference for the key outcomes of exercise capacity or HRQoL [22]. The treatable traits approach presents the possibility that outcomes for patients could be optimised by applying precision medicine [6]. This hypothesis should be tested in future clinical trials. Such trials would pose challenges related to both the intervention and the outcomes. A treatable traits approach to pulmonary rehabilitation meets the definition of a complex intervention, as demonstrated by the number of traits which would be targeted, a large number of intervention components, and significant tailoring of intervention components to individuals [23]. Guidance on developing and evaluating complex interventions is available and will be useful to inform the approach [23]. Both trait-specific and clinical outcomes will be required, all of which must be valid and responsive to the treatments used [6]. A comprehensive health economic analysis will also be needed, to ensure that the treatable traits approach provides value for patients and the healthcare system.
Strengths of this study include a systematic approach to identifying clinical trials of pulmonary rehabilitation across three disease groups and comprehensive data extraction from all studies to ensure that interventions targeting treatable traits were identified. Two reviewers independently screened studies for inclusion, assessed risk of bias and extracted data. Limitations include the reporting of intervention components in the included studies and an absence of registered trial protocols, particularly for older studies, such that some additional components may have been missed. We chose to use Cochrane reviews where available, due to their robust methodological approach including pre-registration of a detailed protocol, but it is possible that use of other systematic reviews might have identified a different group of clinical trials. We evaluated the interventions delivered in pulmonary rehabilitation against an accepted approach to treatable traits [6], but results may have differed if an alternative set of traits had been used. We chose to combine data across the subgroups of COPD, asthma and bronchiectasis, which is consistent with both the “label-free” treatable traits approach [6] and the individual tailoring of interventions in pulmonary rehabilitation [5], but it is possible that there may be differences in response across diagnostic groups. Most of the studies involved patients with COPD rather than other airways diseases, which may have influenced both the range of treatable traits that were addressed and the results of the meta-analysis. We restricted meta-analysis for the outcomes of 6MWD and St George's respiratory questionnaire total score, as insufficient data were available for other outcomes across diagnostic groups. Results may have differed if it had been possible to examine other outcomes, such as cardiopulmonary exercise test outcomes, which may be more commonly reported in asthma trials. Finally, we evaluated clinical trials of pulmonary rehabilitation, as this is the evidence base that underpins delivery of pulmonary rehabilitation, but this may not reflect the way that treatable traits are addressed in clinical practice.
In conclusion, this study has shown that the treatable trait of deconditioning is almost universally addressed in clinical trials of pulmonary rehabilitation, through provision of exercise training. However, other treatable traits were inconsistently addressed in clinical trials. The treatable traits approach has potential to optimise pulmonary rehabilitation outcomes, particularly for those who do not currently have a good response to this treatment. However, additional research will be required to understand the most relevant traits to be addressed in the pulmonary rehabilitation context, the optimal outcomes that should be assessed and the longer-term impact of this approach for patients and healthcare systems.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0042-2022.SUPPLEMENT (1.8MB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: A.E. Holland, B. Wageck, M. Hoffman, A.L. Lee and A.W. Jones have no conflicts of interest to declare.
References
- 1.McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. doi: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; 12: CD005305. doi: 10.1002/14651858.CD005305.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowman L, Hill CJ, May A, et al. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2021; 2: CD006322. doi: 10.1002/14651858.CD006322.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AL, Gordon CS, Osadnik CR. Exercise training for bronchiectasis. Cochrane Database Syst Rev 2021; 4: CD013110. doi: 10.1002/14651858.CD013110.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 6.Agusti A, Bafadhel M, Beasley R, et al. Precision medicine in airway diseases: moving to clinical practice. Eur Respir J 2017; 50: 1701655. doi: 10.1183/13993003.01655-2017 [DOI] [PubMed] [Google Scholar]
- 7.Wouters EFM, Wouters B, Augustin IML, et al. Personalised pulmonary rehabilitation in COPD. Eur Respir Rev 2018; 27: 170125. doi: 10.1183/16000617.0125-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Z, Wang J, Xie Y, et al. Effects of exercise-based pulmonary rehabilitation on adults with asthma: a systematic review and meta-analysis. Respir Res 2021; 22: 33. doi: 10.1186/s12931-021-01627-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, John Wiley & Sons, 2019. [Google Scholar]
- 11.Cambach W, Chadwick-Straver RV, Wagenaar RC, et al. The effects of a community-based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. Eur Respir J 1997; 10: 104–113. doi: 10.1183/09031936.97.10010104 [DOI] [PubMed] [Google Scholar]
- 12.Holland AE, Cox NS, Houchen-Wolloff L, et al. Defining modern pulmonary rehabilitation. An official American Thoracic Society workshop report. Ann Am Thorac Soc 2021; 18: e12–e29. doi: 10.1513/AnnalsATS.202102-146ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 14.Spruit MA, Pitta F, Garvey C, et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J 2014; 43: 1326–1337. doi: 10.1183/09031936.00145613 [DOI] [PubMed] [Google Scholar]
- 15.Sarwar MR, McDonald VM, Abramson MJ, et al. Treatable traits in an English cohort: prevalence and predictors of future decline in lung function and quality of life in COPD. ERJ Open Res 2021; 7: 00934-2020. doi: 10.1183/23120541.00934-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van 't Hul AJ, Koolen EH, Antons JC, et al. Treatable traits qualifying for nonpharmacological interventions in COPD patients upon first referral to a pulmonologist: the COPD sTRAITosphere. ERJ Open Res 2020; 6: 00438-2020. doi: 10.1183/23120541.00438-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald VM, Clark VL, Cordova-Rivera L, et al. Targeting treatable traits in severe asthma: a randomised controlled trial. Eur Respir J 2020; 55: 1901509. doi: 10.1183/13993003.01509-2019 [DOI] [PubMed] [Google Scholar]
- 18.McDonald VM, Higgins I, Wood LG, et al. Multidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense? Thorax 2013; 68: 691–694. doi: 10.1136/thoraxjnl-2012-202646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agusti A, Sobradillo P, Celli B. Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am J Respir Crit Care Med 2011; 183: 1129–1137. doi: 10.1164/rccm.201009-1414PP [DOI] [PubMed] [Google Scholar]
- 20.Gordon CS, Waller JW, Cook RM, et al. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta-analysis. Chest 2019; 156: 80–91. doi: 10.1016/j.chest.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 21.McDonald VM, Gibson PG, Scott HA, et al. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology 2016; 21: 875–882. doi: 10.1111/resp.12746 [DOI] [PubMed] [Google Scholar]
- 22.Spruit MA, Augustin IM, Vanfleteren LE, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J 2015; 46: 1625–1635. doi: 10.1183/13993003.00350-2015 [DOI] [PubMed] [Google Scholar]
- 23.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021; 374: n2061. doi: 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0042-2022.SUPPLEMENT (1.8MB, pdf)