Abstract
Purpose:
To assess the safety and efficacy of extracorporeal carbon dioxide removal (ECCO2R) versus standard care in patients with acute hypoxaemic respiratory failure (AHRF).
Methods:
MEDLINE, Embase and clinical trial registries were searched from 1994 to 31 December 2021. We included randomised controlled trials (RCTs) and observational studies. Pairs of reviewers independently extracted data and assessed the risk of bias. The primary outcome was mortality. Secondary outcomes included ventilator-free days, length of stay, safety and adverse events and physiological changes. As a primary analysis, we performed a meta-analysis of mortality until day 30 using a Bayesian random effects model. We then performed a trial sequential analysis of RCTs.
Results:
21 studies met inclusion criteria: three RCTs, enrolling 531 patients, and 18 observational studies. In a pooled analysis of RCTs, the posterior probability of increased mortality with the use of ECCO2R was 73% (relative risk 1.19, 95% credible interval 0.70–2.29). There was substantial heterogeneity in the reporting of safety and adverse events. However, the incidence of extra and intracranial haemorrhage was higher (relative risk 3.00, 95% credible interval 0.41–20.51) among those randomised to ECCO2R. Current trials have accumulated 80.8% of the diversity-adjusted required information size and the lack of effect reaches futility for a 10% absolute risk reduction in mortality.
Conclusions:
The use of ECCO2R in patients with AHRF is not associated with improvements in clinical outcomes. Furthermore, it is likely that further trials of ECCO2R aiming to achieve an absolute risk reduction in mortality of ≥10% are futile.
Short abstract
ECCO2R for AHRF is associated with increased risk of intracranial haemorrhage, fewer ventilator-free days and increased mortality. Trials aiming to show ARR ≥10% are probably futile. Use is not recommended except in clinical trials or highly selected cases. https://bit.ly/3eyYRPO
Introduction
Acute hypoxaemic respiratory failure (AHRF) is a common indication for intensive care unit (ICU) admission and frequently requires the use of invasive mechanical ventilation [1]. Despite best practice, including the use of lung-protective ventilation strategies, AHRF remains associated with substantial morbidity and mortality [2]. Acute respiratory distress syndrome (ARDS) is a leading cause of AHRF. It is characterised by the acute onset of bilateral pulmonary infiltrates that are noncardiogenic in origin. A diagnosis of ARDS is associated with a mortality of ∼35–45% and in those who survive long-term functional recovery is often limited [3]. While it is known that mechanical ventilation with limited tidal volumes (6–8 mL·kg−1 predicted body weight) is associated with reduced mortality compared to higher tidal volumes [4], it is uncertain whether additional benefit can be accrued by further tidal volume reduction.
The implementation of lower tidal volume ventilation can be limited by hypercapnia. Extracorporeal carbon dioxide removal (ECCO2R) may be used to mitigate this, allowing a reduction in tidal volumes by ex vivo decarboxylation. The technique has been investigated since the 1970s [5]; however, recent technological advances have improved the efficiency of gas exchange and potentially reduced the adverse effects associated with prolonged extracorporeal circulation. As a result, ECCO2R has been used in the management of patients mechanically ventilated for AHRF [6]. The rate of clinical adoption has not been matched by an expansion in the quality of the evidence base, which is characterised by numerous small technical studies evaluating efficacy [7]. A large randomised controlled trial has been completed recently [8]; however, it was terminated early due to futility. Given the uncertainty surrounding the role of ECCO2R in AHRF, we conducted a systematic review and meta-analysis of the available data to assess the safety and efficacy of ECCO2R for AHRF.
Methods
The systematic review and meta-analysis protocol was registered on the International Prospective Register of Systematic Reviews (registration number CRD42021235939). The review is reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [9].
Search strategy and selection criteria
In conjunction with a medical information specialist, we designed and ran a search of the MEDLINE and Embase databases from inception to 31 December 2021, including in-process and ahead-of-print articles. There were no language restrictions. We subsequently filtered these results to include studies reported after 1 January 1994, to exclude historic technologies. A summary of clinical studies published between 1946 and 31 December 1993 is provided in supplementary file S1. In addition, we queried the International Clinical Trials Registry Platform, ClinicalTrials.gov and the Chinese Clinical Trial Registry for unpublished studies. The reference lists of included articles were screened by hand to identify relevant studies not found by our search. The full search strategy is provided in supplementary file S2.
We included studies of ECCO2R which recruited patients with AHRF of any cause. Veno-venous and veno-arterial ECCO2R were eligible. Similarly, we permitted the inclusion of studies in which co-interventions were trialled in either the ECCO2R or control group. We included randomised controlled trials of ECCO2R, matched cohort studies and observational studies with a clearly stated hypothesis and that included >10 patients. We excluded studies primarily recruiting children (aged <18 years), studies of chronic respiratory failure, studies of hypercapnic respiratory failure, studies of extracorporeal membrane oxygenation (author defined, where oxygenation by device was described, and/or where extracorporeal flows exceeded 1.5 L·min−1) and observational studies lacking a clear hypothesis and/or including ≤10 participants.
Outcomes
The selection of outcome measures was informed by the core outcome set for research evaluating patients on extracorporeal membrane oxygenation [10]. The primary outcome measure was mortality. We chose to analyse mortality up to day 30, as this was the most likely outcome to be reported in common. We also specified mortality at the longest available follow-up. If mortality up to day 30 was not available, we chose to extrapolate based on available data (survival curves or Consolidated Standards of Reporting Trials diagrams) or to substitute the latest available time point. Secondary outcomes included ventilator-free days, rates of intracranial haemorrhage and major bleeding, complications associated with the ECCO2R circuit or cannulation, physiological changes (quantification of carbon dioxide (CO2) removal or change in tidal volume, minute volume, pH and plateau airway pressure) at the latest time until day 3 and life impact measures (i.e. health-related quality of life, neurological recovery, disability, activities of daily living, return to work).
Data extraction
Two authors (J.E. Millar and A.J. Boyle) independently reviewed titles and abstracts of all citations and assessed eligibility. Full-text articles were retrieved and reassessed. Disagreements were resolved by a third author (D.F. McAuley). Data from included studies were independently extracted using a pre-piloted data collection proforma and using the relevant Cochrane risk of bias (RoB) summary tools, by four authors (J.E. Millar, A.J. Boyle, C.E. Adams and A.W. Glass). Details for each study were cross-checked by another member of the team. Anomalies were resolved by a consensus of the group. Data for study details, patient characteristics, technical aspects of ECCO2R, physiological variables, complications and outcomes were extracted. Where data were presented in a figure, but were not available in the text of a manuscript, digital retrieval was attempted using the metaDigitize package [11] in R (v4.1.1; the R Foundation for Statistical Computing, Vienna, Austria). Similarly, where appropriate summary statistics were not presented but raw data were available, summaries were produced using the skimr package in R [12].
Risk-of-bias assessment
We assessed the risk of bias for randomised controlled trials using the Cochrane RoB tool (v2.0) in the following domains: randomisation, deviations from the intended intervention, missing outcome data, measurement of the outcome and selection of the reported result [13]. Each domain was rated as being of “low”, “some concerns” or “high” risk of bias. For observational studies, we used the Cochrane Risk of Bias in Non-Randomized Studies – of Interventions (ROBINS-I) tool [14]. Domains were rated as containing no information or being at “low”, “moderate”, “serious” or “critical” risk of bias. Studies were assessed in seven domains: confounding, selection of participants, classification of interventions, deviations from the intended intervention, missing data, measurement of outcomes and selection of the reported result. Risk-of-bias assessments were performed independently by two authors, and the overall risk of bias was determined by the highest risk assigned to any one domain.
Data analysis
We anticipated high levels of clinical heterogeneity arising from the differences in the clinical use of ECCO2R (i.e. carbon dioxide removal rates, veno-arterial/veno-venous access, different ventilatory strategies) and so opted to perform meta-analysis using a Bayesian random-effect model implemented in the Bayesmeta package [15] in R. As the belief that the technology may be beneficial has been largely expressed through observational research, we planned to perform a Bayesian meta-analysis using the observational studies, with noninformative priors, and to use the results from this to inform the prior distribution for pooling of the randomised controlled trials. However, only a single controlled trial reported a mortality end-point. Therefore, for the primary mortality end-point we adopted this approach, but performed a sensitivity analysis using a noninformative prior. For secondary, outcomes we used noninformative priors. Posterior relative effects are presented as mean relative risk or mean difference (MD), alongside the 95% credible interval (CrI) using Markov chain Monte Carlo simulations. Estimates are presented in forest plots, with the individual study estimates and shrinkage estimates. Shrinkage estimates consider interstudy heterogeneity and therefore appear smaller than the original estimate. Interstudy statistical heterogeneity was measured using the I2 value, where we defined 0–40% as not being important, 40–60% as moderate heterogeneity and 60–100% as considerable heterogeneity. Publication bias was studied by visual inspection of funnel plots. As a further sensitivity analysis, we calculated the posterior probability across varying degrees of optimism in the effect size. We calculated the priors which represented no effect (absolute risk reduction (ARR) 0%), some conservative belief that the intervention may work (ARR 5%) and an optimistic belief (ARR 10%).
To see if the number of patients included in the trials had achieved the minimum information size for the treatment effect estimated in our models, we performed a cumulative frequentist meta-analysis with trial sequential analysis (TSA). The minimum information size calculation was based on an a priori ARR in mortality of 10% (α=5%, power 80%). This is in line with the ARR of 9% on which the REST trial was powered [8]. The minimum information size was then updated to adjust for diversity to give the required information size that was used in the TSA. Bounds were constructed using O'Brien–Fleming α- and β-spending functions. We also conducted a TSA based on a more conservative ARR of 5% (α=5%, power 80%). All analyses were conducted in R v4.1.1.
Certainty of evidence
Finally, using the framework proposed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) group [16], we rated the overall quality of the evidence based on the risk of bias, inconsistency, indirectness, imprecision and publication bias.
Results
Our search identified 603 unique citations and a flowchart of exclusions is shown in supplementary figure S1. 33 of these studies were published prior to 1994 and were excluded (supplementary file S1). 54 articles were retrieved for full-text review, 21 of which were finally included in the systematic review and meta-analysis. These included three randomised controlled trials [8, 17, 18], totalling 531 patients, and 18 observational studies [19–36], ranging from non-randomised controlled designs to retrospective cohort studies. Most studies were published in the past decade (n=15, 71%) [8, 18, 24–36] and evaluated veno-venous ECCO2R (n=12, 57%) [8, 17, 19, 21, 27, 29–33, 35, 36]. Studies were largely undertaken in Europe or North America. The average (mean or median) age ranged from 35 to 68 years. The average arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FIO2) ratio at inclusion ranged from 58 to 211 mmHg.
Characteristics of randomised controlled trials
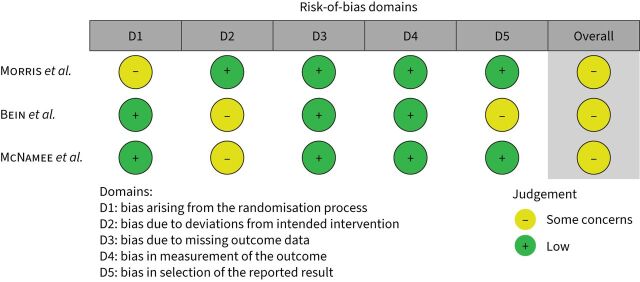
The baseline characteristics of three randomised controlled trials (RCTs) are shown in table 1. All were conducted in Europe [8, 18] or the United States [17] between 1994 and 2021. McNamee et al. [8] was the only RCT to recruit patients with AHRF, as opposed to patients meeting a definition of ARDS (table 1). All were designed as parallel-group, allocation-concealed, nonblinded RCTs. All were terminated early due to futility. Overall, 531 patients were randomised, and 244 patients received ECCO2R, from a total planned sample of 1300 patients. The ratios of patients screened to patients randomised were 6:1 (Morris et al. [17]), 4:1 (Bein et al. [18]) and 17:1 (McNamee et al. [8]). Two studies used veno-venous configurations [8, 17] and one a pumpless arterio-venous configuration [18]. Technical details of ECCO2R devices, management strategies and anticoagulation protocols employed in RCTs are summarised in supplementary table 1. The average durations of ECCO2R were 9±2 days (Morris et al. [17]), 7±4 days (Bein et al. [18]) and 4±2 days (McNamee et al. [8]). A variety of mortality and non-mortality-based outcome measures were reported; however, few were reported consistently in all trials (tables 1 and 2). No RCT was judged to be of “low” risk of bias (figure 1). A detailed description of the rationale for RoB assessments is provided in supplementary table S2.
TABLE 1.
Baseline characteristics of included randomised controlled trials
| Morris et al., 1994 [ 17 ] | Bein et al., 2013 [ 18 ] | McNamee et al., 2021 [ 8 ] | |
| Planned | 60 | 120 | 1120 |
| Randomised | 40 | 79 | 412 |
| Randomised to ECCO2R | 21 | 40 | 202 |
| Received ECCO2R | 19+ | 40 | 185§ |
| Inclusion criteria | Severe ARDS and met ECMO criteriaƒ; ≤21 days of mechanical ventilation; aged ≥12 and ≤65 years | Moderate–severe ARDS# (PaO2/FIO2 <200 mmHg for ≥2 h); <7 days of mechanical ventilation; plateau airway pressure >25 cmH2O; absence of haemodynamic instability | Moderate–severe AHRF (PaO2/FIO2 <150 mmHg); within 48 h of onset; ≤7 days of mechanical ventilation; potentially reversible |
| Age, years | |||
| ECCO2R | 33±3 | 50±12 | 60 (51–69) |
| Standard care | 38±3 | 49±17 | 62 (50–70) |
| Sex, female/male | |||
| ECCO2R | 13/8 | 2/38 | 64/138 |
| Standard care | 10/9 | 9/30 | 79/131 |
| PaO2/FIO2 ratio, mmHg | |||
| ECCO2R | 63±4 | 152±37 | 118 (96–134) |
| Standard care | 64±4 | 168±37 | 116 (94–133) |
| APACHE II score, 0–71 | |||
| ECCO2R | 18±1 | NR | 19 (15–23) |
| Standard care | 17±1 | NR | 20 (16–23) |
| Aetiology of ARDS/AHRF, % total patients randomised#,¶ | |||
| Pneumonia | 60 | 57 | 80 |
| Sepsis | 8 | 48 | |
| Aspiration | 9 | 9 | |
| Trauma | 8 | 24 | 2 |
| Inhalation | 1 | 2 | |
| Emboli | 5 | ||
| Other | 27 | 1 | 11 |
| Mode of ECCO2R | Veno-venous | Arterio-venous | Veno-venous |
| Mechanical ventilation protocol in ECCO2R arm | Variable## LFPPV Minimum VT 4 mL·kg−1 (>250 mL) |
VT 3 mL⋅kg−1 PBW PEEP by ARDSNet “high PEEP/FIO2” table fR 10–25 I:E ratio 1:1 |
Incremental reduction of VT to ≤3 mL·kg−1 PBW PEEP by ARDSNet protocol |
| Mechanical ventilation protocol in control arm | PC-IRV Minimum FIO2 and PEEP (maximum 35 cmH2O) to maintain PaO2 55–60 mmHg (60–65 mmHg if no evidence of barotrauma) |
VT 6 mL⋅kg−1 PBW PEEP set by ARDSNet “high PEEP/FIO2” table fR 10–25 I:E ratio 1:1 |
Recommendation to use 6 mL⋅kg−1 PBW and set PEEP using the ARDSNet protocol |
| Crossover | No | No | No |
| Primary outcome | 30-day survival | 28-day VFDs | 90-day mortality |
| Secondary outcomes | ICU LOS | Hospital mortality | VT at days 2 and 3 |
| Hospital LOS | Organ-failure-free days | VFDs at day 28 | |
| Daily and total costs | Requirement for RRT | Duration of IMV in survivors | |
| Ventilatory and physiological data | Transfusion requirements | Need for ECMO up to day 7 | |
| Major complications | Use of adjunctive therapies | 28-day mortality | |
| Sedative/analgaesic requirements | Adverse event rate | ||
| Ventilatory and physiological data | |||
| Complications or adverse reactions | |||
Data are presented as n, mean±sd or median (interquartile range), unless otherwise stated. ECCO2R: extracorporeal carbon dioxide removal; PaO2: arterial oxygen tension; FIO2: inspiratory oxygen fraction; APACHE: Acute Physiology and Chronic Health Evaluation; ARDS: acute respiratory distress syndrome; AHRF: acute hypoxaemic respiratory failure; LOS: length of stay; RRT: renal replacement therapy; VFDs: ventilator-free days; IMV: invasive mechanical ventilation; ECMO: extracorporeal membrane oxygenation; NR: not reported; LFPPV: low-frequency positive pressure ventilation; VT: tidal volume; PBW: predicted body weight; PEEP: positive end-expiratory pressure; fR: respiratory frequency; I:E: inspiratory to expiratory time ratio; PC-IRV: pressure controlled inverse ratio ventilation; ICU: intensive care unit. #: American–European Consensus Conference (AECC) definition of ARDS; ¶: McNamee et al. [8] report aetiology only for the subgroup of patients meeting the Berlin definition of ARDS (n=248). Here, patients may have had more than one aetiology and thus the reported percentages do not sum to 100; +: one patient died before initiation of ECCO2R and one patient improved with mechanical ventilation alone; §: eight patients improved prior to ECCO2R, six had a technical issue with ECCO2R, two patients deteriorated and one patient withdrew consent; ƒ: defined as per Fowler et al. [37] and Suchyta et al. [38]. The trial was conducted prior to publication of the AECC definition of ARDS; ##: in the first 10 patients a peak airway pressure limit of 35 cmH2O was used. Thereafter, the study switched to a VT target.
TABLE 2.
Summary of findings and certainty of evidence for randomised controlled trials
| Participants/studies [references] | Pooled event rate (%) or pooled mean±sd # | Mean posterior relative effect¶ (95% CrI) | Posterior probability of harm from ECCO2R + | Certainty of the evidence (GRADE) | ||
| ECCO2R | Standard care | |||||
| Primary outcome | ||||||
| Mortality up to day 30 (or latest)§ | 524/3 [8, 17, 18] | 97/261 (37.2) | 91/263 (34.6) | Relative risk 1.19 (0.70–2.29) | 0.73 | ⊕⊕◯◯ Low## |
| Secondary outcomes | ||||||
| 90-day mortality | 405/1 [8] | 83/200 (41.5) | 81/205 (39.5) | |||
| Hospital mortalityƒ | 119/2 [18] | 21/61 (34.4) | 17/58 (29.3) | ⊕◯◯◯ Very low¶¶ | ||
| 28-day ventilator-free days (days) | 484/2 [8, 18] | 7.6±8.7 | 9.2±9.2 | MD −1.4 (−3.6–0.9) | 0.90 | ⊕⊕⊕◯ Moderate++ |
| ICU length of stay (days) | 531/3 [8, 17, 18] | 17.4±16.6 | 15.2±11.5 | MD 0.9 (−1.3–3.1) | 0.79 | ⊕⊕◯◯ Low§§ |
| Hospital length of stay (days) | 513/3 [8, 17, 18] | 24.1±25.8 | 22.1±19.2 | MD 0.8 (−2.2–3.9) | 0.70 | ⊕⊕◯◯ Low§§ |
| Haemorrhage | 452/2 [8, 17] | 38/223 (17) | 3/229 (1.3) | ⊕⊕◯◯ Lowƒƒ,###,¶¶¶ | ||
| Intracranial haemorrhage | 452/2 [8, 17] | 11/223 (4.9) | 3/229 (1.3) | Relative risk 3.00 (0.41–20.51) | 0.88 | ⊕⊕◯◯ Low### |
Data are presented as n/N, n/N (%) or mean±sd, unless otherwise stated. ECCO2R: extracorporeal carbon dioxide removal; 95% CrI: 95% credible interval; GRADE: Grading of Recommendations, Assessment, Development, and Evaluations; ICU: intensive care unit; MD: mean difference. #: calculated as per the Cochrane Handbook [39]; ¶: relative risk for binary outcomes or mean difference for continuous outcomes; +: set for relative risk >1 or MD <0 days; §: Morris et al. [17] reported 30-day mortality, Bein et al. [18] reported hospital mortality and McNamee et al. [8] reported 28-day mortality; ƒ: all models failed to converge, probably due to low sample sizes and imprecision; ##: downgraded one point due to indirectness (given the differing outcome measures) and one point for imprecision (as the number of participants did not reach the optimal information size); ¶¶: downgraded one point for risk of bias and two points for serious imprecision (as the number of participants did not reach the optimal information size); ++: downgraded one point for imprecision (as the number of participants did not reach the optimal information size); §§: downgraded one point for imprecision (as the number of participants did not reach the optimal information size) and one point for indirectness (given the time elapsed between studies and differences in practice relating to ICU and hospital admission and discharge); ƒƒ: no study offered a consistent definition of major haemorrhage. Haemorrhage was not reported as an adverse event by Bein et al. [18]. Bleeding adverse events reported by McNamee et al. [8] are included. These were defined as bleeding events (excluding intracranial bleeding) that were possibly, probably or definitely related to the intervention; ###: downgraded two points for serious imprecision (as the number of participants did not reach the optimal information size and the event rate is low); ¶¶¶: could not be pooled due to the absence of events in the Morris et al. [17] standard care arm.
FIGURE 1.
Risk-of-bias assessment for randomised controlled trials. Performed using the Cochrane risk-of-bias tool v 2.0. A detailed rationale for each assessment is provided in supplementary table S2.
Characteristics of observational studies
The baseline characteristics of 17 observational studies, reported between 1997 and 2021, are shown in supplementary table S3. In total, eight studies were conducted in Germany [20, 23–25, 28, 32–34], three in France [19, 27, 35], two in Italy [21, 30], two were international [29, 31], one was conducted in South Korea [26] and one in China [36]. There was an almost even split between veno-venous (n=10, 56%) [19, 21, 27, 29–33, 35, 36] and arterio-venous (n=8, 44%) [20, 22–26, 28, 34] configurations. Study designs included two nonrandomised controlled trials [19, 21], two matched cohort studies [24, 30], one quasi-experimental study [35] and 13 prospective or retrospective cohort studies [20, 22, 23, 25–29, 31–34, 36]. Overall, 571 patients received ECCO2R, from a total population of 826. Most studies specified the inclusion of patients meeting a definition of ARDS (n=12, 67%) [19–23, 25, 29–31, 34–36]; the remainder included patients matching disparate definitions consistent with AHRF (n=6, 33%) [24, 26–28, 32, 33]. Five studies included continuous renal replacement therapy as a co-intervention [27, 30, 32, 33, 36]; one included aspirin [24]; and one included high-frequency oscillatory ventilation [23]. Like RCTs of ECCO2R, selected outcomes were heterogeneous and inconsistently reported (supplementary table S4). Two studies, with appropriate control groups, were assessed using ROBINS-I (supplementary figure S2) [19, 21]. They were rated as being of either “serious” or “critical” risk. A detailed description of the rationale for RoB assessments is provided in supplementary table S5.
Mortality, ventilator-free days and length of stay
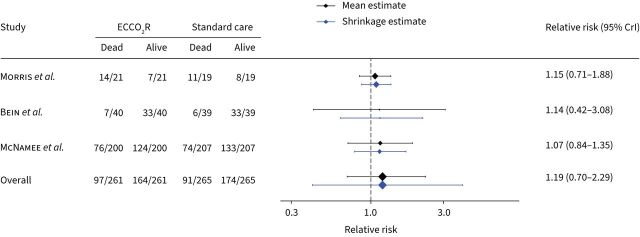
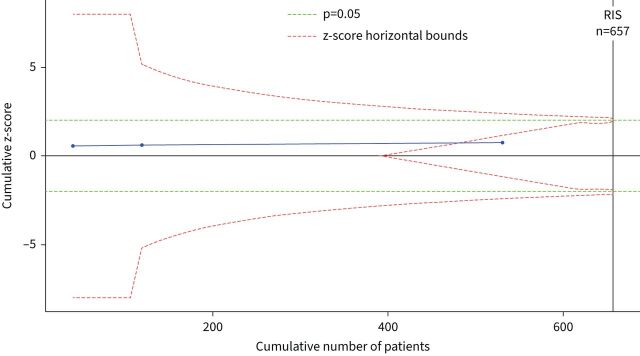
To estimate the effect of ECCO2R on mortality, we pooled the randomised trials together in a Bayesian meta-analysis model of mortality up to day 30 [8, 17] and hospital mortality [18]. We did not pool observational studies together as a secondary analysis, as only one study included a non-ECCO2R control group. However, we used the pooled effect size for mortality among observational studies (relative risk 1.43, 95% CrI 0.55–2.32) as a weakly informative prior for our primary analysis of RCTs. After combining studies, the use of ECCO2R was not associated with any difference in mortality (relative risk 1.19, 95% CrI 0.70–2.29) (table 2 and figure 2). There was moderate statistical heterogeneity present (I2=42%) and the prediction estimates were wide, indicating a considerable degree of uncertainty around the estimate. The effect of using observational data to inform the prior distribution, compared to a noninformative prior, was to increase our confidence that ECCO2R is associated with harm (supplementary table S6). For the weakly informative prior based on observational data, the posterior probabilities that ECCO2R resulted in an absolute risk reduction (ARR) >0%, >5% and >10% were 22.4%, 7.6% and 2.5%, respectively. For an uninformative prior, these were 33.9%, 18.3% and 11.2%. Visual inspection of the funnel plot including the RCTs identified no publication bias. In a sensitivity analysis of different prior degrees of reasonable clinical optimism, we did not observe large changes in the posterior probability that the intervention may have benefit (ARR 0% prior 33.1%, ARR 5% prior belief 34.2% and ARR 10% prior belief 35.6%). Frequentist cumulative meta-analysis with trial-sequential analysis showed that the RCTs to date had accrued 80.8% of the diversity adjusted required information size, but did demonstrate futility when considering an ARR ≥10% (figure 3). For an ARR ≥5%, futility bounds were not crossed, but the required information size was 2753 patients (supplementary figure S4). The certainty of evidence for mortality, using the GRADE approach, was low (table 2).
FIGURE 2.
Forest plot showing mortality up to day 30 (or latest available time point). ECCO2R: extracorporeal carbon dioxide removal; 95% CrI: 95% credible interval.
FIGURE 3.
Trial sequential analysis. The z-value is the test statistic where |z|=1.96 is equivalent to p=0.05 (green line). The z-score horizontal bounds are set with O'Brien–Fleming α-monitoring and β-futility boundaries (red lines). The required information size (RIS) is diversity adjusted and set to detect a 10% absolute difference in mortality (from 35% to 25%) at 80% power. Two-tailed α=0.05 and β=0.2.
Two studies reported 28 ventilator-free days (VFDs) (Bein et al. [18] as the primary outcome and McNamee et al. [8] as a secondary outcome). A meta-analysis of these studies showed that those randomised to ECCO2R had fewer VFDs (MD −1.4 days, 95% CrI −3.6–0.9 days) (table 2 and supplementary figure S3). The posterior probability of ECCO2R being associated with fewer VFDs was 90%. The certainty of evidence was moderate. All three RCTs reported ICU and hospital length of stay. For each, the mean relative effects were small and 95% credible intervals spanned a mean difference of 0 days (table 2 and supplementary figure S3).
For observational studies, 12 studies reported a mortality outcome (five hospital mortality [18, 19, 22, 24, 34], two 28-day mortality [29, 36], three both hospital and 28-day mortality [23, 28, 31] and two ICU mortality [25, 27]). Among studies including >50 patients, reported mortality ranged from 37.9% to 58.9% [20, 22, 28, 30, 31, 34].
Safety and adverse events
Safety and adverse event data were variably reported among RCTs and observational studies, which hampered our ability to conduct a meta-analysis. An overview is provided in supplementary table S7. No RCT offered a definition of major haemorrhage. Morris et al. [17] reported that non-central nervous system (CNS) haemorrhage occurred in 21 (100%) ECCO2R patients; sufficient to discontinue therapy in seven. There was no reported non-CNS haemorrhage among the control group. Likewise, they described daily packed red blood cell (PRBC) transfusion requirements as being significantly higher in the intervention (1.8±0.6 L·day−1) than the control group (0.2±0.0 L·day−1). Comparable data were not reported by Bein et al. [18], although the trial did report a higher PRBC transfusion requirement in patients receiving ECCO2R between randomisation and day 10 (3.7±2.4 units versus 1.5±1.3 units, p<0.05). McNamee et al. [8] reported non-CNS haemorrhage leading to a serious adverse event in six (3%) patients receiving ECCO2R versus one (0.5%) patient in the control group. In respect of intracerebral haemorrhage (ICH), Bein et al. [18] did not specifically report this complication. Morris et al. [17] reported one patient from each group as having suffered from ICH and McNamee et al. [8] reported 10 (5%) patients receiving ECCO2R as suffering an ICH versus two (1%) in the control group.
The lack of consistent definitions of haemorrhage hindered an effective meta-analysis. Pooling haemorrhage rates was not possible, due to zero events in the Morris et al. [17] control group. In the two trials which reported haemorrhage rates (Morris et al. [17] and McNamee et al. [8]), bleeding was much more frequent in the ECCO2R arms (table 2). Similarly, the relative risk of intracranial haemorrhage in those randomised to ECCO2R, as compared to standard care, was 3.00 (95% CrI 0.41–20.51). The Morris et al. [17] data must be interpreted cautiously, considering the age of the study and the technology employed at the time.
Physiological effects
A summary of the reported physiological effects of ECCO2R is presented in supplementary table S8. The consistency of reporting, the choice of variables to include, and the time points at which they were measured varied widely. McNamee et al. [8] reported a quantification of carbon dioxide removal, with a maximum average value of 85±35 mL·min−1 achieved on day 3. Of the RCTs, both McNamee et al. [8] and Bein et al. [18] achieved tidal volumes of ∼4 mL·kg−1 predicted body weight (PBW) by day 3. In the study by Morris et al. [17], a rapid reduction in tidal volume to 3±3 mL·kg−1 was reported within 3–6 h of commencing ECCO2R. Most studies describe a reduction in plateau (or peak) airway pressures, tidal volume, minute volume and arterial carbon dioxide tension over time.
Ongoing clinical trials
A search of trial registry databases found two planned or recruiting randomised controlled trials, seeking to enrol 244 participants (including SUPERNOVA (NCT04903262), n=230, and PROVE (NCT03525691), n=14) (supplementary table S9). Four ongoing observational studies and one registry study were also identified, with a planned combined sample size of 305 patients.
Discussion
This systematic review and Bayesian meta-analysis, including three randomised controlled trials and 18 observational studies, did not demonstrate a mortality benefit for ECCO2R in patients with AHRF. Most of the evidence for ECCO2R was comprised of observational studies of low quality and high risk of bias, which were often uncontrolled. When the available RCTs were pooled together, we found no evidence of benefit. A subsequent trial sequential analysis identified that current RCTs achieved 80.8% of the information size required to show benefit or harm and the cumulative z-score crossed into futility. Limited data were available for secondary outcomes, but these also suggested harms, particularly from haemorrhage. Therefore, based on an ARR of 10%, further trials of ECCO2R in this setting are futile and may lead to harm.
RCTs account for 39% of the patients included in the current review. All had a similar parallel-group design, although the included population, mechanical ventilation strategy and ECCO2R technique differed. Furthermore, the duration of ECCO2R ranged from 4 days to 9 days. Despite these differences, no study reported benefit with ECCO2R compared to standard care nor did any meta-analysis. It is important to note that each of these trials were terminated early due to futility, and therefore all are underpowered to detect a smaller difference between treatment arms. The result of our TSA, assuming an ARR ≥5%, suggests that the futility boundary may not have been crossed at this more conservative effect size. However, given the experience of the three RCTs to date and more broadly from trials of extracorporeal respiratory support, the required information size of 2753 patients seems infeasible.
While RCTs showed some consistency in design, there was considerable heterogeneity among observational studies. As described, only four out 18 studies had a non-ECCO2R comparator group. Similarly, mortality end-points were inconsistently reported, with in-hospital mortality being most frequently available and ranging from 6.7% to 75%.
Evaluating effectiveness by meta-analysis requires consistent outcome reporting, and future studies should consider the adoption of core outcome measures as defined previously [10, 40, 41]. This is particularly important for studies evaluating extracorporeal respiratory support, where clinical trials are frequently challenged by difficulties in recruitment, and no single study is likely to enrol a sufficient sample size. Of note, no study reported life impact outcomes; however, McNamee et al. [42] list several health-related quality-of-life and disability measures as secondary outcomes (at 6 months and/or 1 year) in their published trial protocol and intend to analyse and publish these in due course (unpublished data).
The assessment of safety and adverse events is an important part of the wider evaluation needed when considering the efficacy of a studied intervention. In this analysis, we pre-specified adverse events which have been commonly reported or are likely to be of concern to treating clinicians [43]. Furthermore, we adopted broad definitions across five domains to capture as much as possible. However, the consistency of reporting and the wide variety of definitions employed makes a definitive comparison difficult. This is compounded by advances in technology over time and by the inclusion of both arterio-venous and veno-venous ECCO2R configurations. Acknowledging these limitations, even in the most modern of included studies, ECCO2R is associated with an increased rate of major haemorrhage and intracranial haemorrhage. This is almost certainly related to the still-present requirement to provide systemic anticoagulation during ECCO2R and to the acquired coagulopathy which has been associated with extracorporeal respiratory support techniques [44, 45]. Future studies should endeavour to record bleeding complications using common definitions, such as those proposed in the International Extracorporeal Membrane Oxygenation Network core outcomes set [10]. Investigators should also be aware of mechanisms for the attribution of complications to ECCO2R. For example, it is recognised that occult intracranial haemorrhage may be present in critically ill patients at intensive care unit admission, which may subsequently be incorrectly linked to ECCO2R [46].
ECCO2R was originally developed to reduce the intensity of mechanical ventilation in patients with AHRF [5]. On this basis, it is reasonable to infer that the use of ECCO2R to reduce ventilator-induced lung injury, and ultimately improve outcomes, is unlikely to be successful unless there is in fact a reduction in the intensity of mechanical ventilation [47]. Of the three RCTs included in this study, two aimed to reduce tidal volume to 3 mL·kg−1 PBW (Bein et al. [18] and McNamee et al. [8]), and Morris et al. [17], evaluated a nonconventional lung protective ventilation strategy. In comparison, there were a wide range of ventilation strategies among the observational studies considered. These differences highlight the challenges in studying a complex intervention composed of a change to ventilation, the introduction of an extracorporeal circuit, and systemic anticoagulation. Characterisation of the physiological effect of the suite of interventions may be helpful in these circumstances; however, widespread heterogeneity in those measurements and their timing hinders comparison. In the absence of harmonised and robust biological and physiological endpoints, coupled with an incomplete understanding of the role of mechanical ventilation in the pathogenesis of AHRF, it is difficult to accurately quantify the net effect of ECCO2R on the intensity of mechanical ventilation. In future, advances in ECMO technology may reduce the relevance of ECCO2R where the aim is a reduction in the intensity of mechanical ventilation. However, ECCO2R may retain a role in patients with obstructive lung disease or chronic hypercapnia, where the mechanism differs.
This review has some limitations. First, the primary results are derived from three RCTs, one of which recruited more than twice as many patients as the remaining two combined. In addition, the inclusion criteria were broadest in the largest trial (McNamee et al. [8]). The combined effect is that the pooled estimates may not provide increased precision over evaluation of the studies individually, particularly when considering subgroups (e.g. those with ARDS) [48]. For example, the study by Bein et al. [18] reported a potential mortality benefit in a subgroup of patients with PaO2/FIO2 ratios <150 mmHg. This finding underpinned the subsequent trial by McNamee et al. [8], but due to limited numbers and the fact both trials were terminated prematurely, it may be that an alternative meta-analysis using individual patient data can reveal an important effect [49]. Second, ECCO2R has been studied in ARDS and AHRF since the 1970s [5]; in our study we limited the analysis to studies published in or after 1994. Therefore, it is possible that by excluding older studies an important effect was missed. However, prior to 1994 there was only one RCT, which did not have a non-ECCO2R arm [50]. Third, the evolution of ECCO2R technology, standards of intensive care and trial design during the time period between studies included in this analysis produces an array of challenges that hinder effective synthesis. These severely limit a proper comparison of the rates and types of complication associated with ECCO2R.
In summary, this systematic review confirms that the use of ECCO2R in patients with AHRF is not associated with improvements in clinical outcomes. The relatively small number of patients studied and the heterogeneity between trials may have contributed to this finding. There remains an unmet need to better quantify lung injury and to identify patients who may benefit most from a reduction in the intensity of mechanical ventilation and current evidence cannot exclude the possibility of smaller beneficial effects or benefits to subgroups of patients. However, at present, ECCO2R should not be used outside of a clinical trial or in highly selected cases.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0030-2022.SUPPLEMENT (740.4KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: J.E. Millar, A.J. Boyle and D.F. McAuley conceived the review and designed and led its conduct. T.M. Drake led the statistical analysis. C.E. Adams and A.W. Glass contributed to data extraction and the interpretation of results. B. Blackwood and J.J. McNamee contributed to the protocol and to the interpretation of results. All the authors approved the final version of the manuscript and the decision to submit. D.F. McAuley is the guarantor of this work.
Conflict of interest: J.E. Millar is a co-author of the REST trial. Outside the submitted work, J.E. Millar reports personal speaking fees from Fisher & Paykel and Continulus. A.J. Boyle is a co-author of the REST trial. Outside the submitted work, T.M. Drake receives grant funding from Aligos. C.E. Adams, A.W. Glass and B. Blackwood have no related disclosures. J.J. McNamee reports a grant from the NIHR HTA Programme for the conduct of the REST trial. Outside the submitted work, J.J. McNamee reports personal speaking fees from Baxter. D.F. McAuley reports a grant from NIHR HTA Programme for the conduct of the REST trial. Outside the submitted work, D.F. McAuley reports personal fees from consultancy for GlaxoSmithKline, Boehringer Ingelheim, Bayer, Novartis, SOBI and Eli Lilly, and from sitting on DMECs for trials undertaken by Vir Biotechnology and Faron Pharmaceuticals. In addition, his institution has received funds from grants from several funders for studies in patients with ARDS and COVID-19. D.F. McAuley has a patent (US8962032) issued to his institution for a treatment for inflammatory disease. D.F. McAuley is the MRC/NIHR EME Programme Director. There was no funding associated with this review.
References
- 1.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 Countries. JAMA 2016; 315: 788–800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 2.Prescott HC, Sjoding MW, Langa KM, et al. Late mortality after acute hypoxic respiratory failure. Thorax 2017; 73: 618–625. doi: 10.1136/thoraxjnl-2017-210109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364: 1293–1304. doi: 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 4.Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308. doi: 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Kolobow T, Tomlinson T, et al. Low-frequency positive pressure ventilation with extracorporeal carbon dioxide removal (LFPPV-ECCO2R): an experimental study. Anesth Analg 1978; 57: 470–477. doi: 10.1213/00000539-197807000-00018 [DOI] [PubMed] [Google Scholar]
- 6.Boyle AJ, Sklar MC, McNamee JJ, et al. Extracorporeal carbon dioxide removal for lowering the risk of mechanical ventilation: research questions and clinical potential for the future. Lancet Respir Med 2018; 6: 874–884. doi: 10.1016/S2213-2600(18)30326-6 [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald M, Millar J, Blackwood B, et al. Extracorporeal carbon dioxide removal for patients with acute respiratory failure secondary to the acute respiratory distress syndrome: a systematic review. Crit Care 2014; 18: 222. doi: 10.1186/cc13875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamee JJ, Gillies MA, Barrett NA, et al. Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: the REST randomized clinical trial. JAMA 2021; 326: 1013–1023. doi: 10.1001/jama.2021.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodgson CL, Burrell AJC, Engeler DM, et al. Core outcome measures for research in critically ill patients receiving extracorporeal membrane oxygenation for acute respiratory or cardiac failure: an international, multidisciplinary, modified Delphi consensus study. Crit Care Med 2019; 47: 1557–1563. doi: 10.1097/CCM.0000000000003954 [DOI] [PubMed] [Google Scholar]
- 11.Pick JL, Nakagawa S, Noble DWA. Reproducible, flexible and high-throughput data extraction from primary literature: the metaDigitise R package. Methods Ecol Evol 2019: 10: 426–431. [Google Scholar]
- 12.Waring E, Quinn M, McNamara A, et al. skimr: Compact and Flexible Summaries of Data. 2021. https://cran.r-project.org/web/packages/skimr/index.html
- 13.Higgins JPT, Savović J, Page MJ, et al. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas JJC, Cumpston M, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2. Cochrane, 2021. doi: 10.1111/2041-210X.13118 [DOI] [Google Scholar]
- 14.Sterne JAC, Hernán MA, McAleenan A, et al. Chapter 25: assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, et al., , eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2. Cochrane, 2021. [Google Scholar]
- 15.Röver C. Bayesian random-effects meta-analysis using the bayesmeta R package. J Stat Software 2020; 93: 1–51. doi: 10.18637/jss.v093.i06 [DOI] [Google Scholar]
- 16.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490. doi: 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149: 295–305. doi: 10.1164/ajrccm.149.2.8306022 [DOI] [PubMed] [Google Scholar]
- 18.Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 2013; 39: 847–856. doi: 10.1007/s00134-012-2787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinard N, Beloucif S, Gatecel C, et al. Interest of a therapeutic optimization strategy in severe ARDS. Chest 1997; 111: 1000–1007. doi: 10.1378/chest.111.4.1000 [DOI] [PubMed] [Google Scholar]
- 20.Bein T, Weber F, Philipp A, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med 2006; 34: 1372–1377. doi: 10.1097/01.CCM.0000215111.85483.BD [DOI] [PubMed] [Google Scholar]
- 21.Terragni PP, Del Sorbo L, Mascia L, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 2009; 111: 826–835. doi: 10.1097/ALN.0b013e3181b764d2 [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann M, Bein T, Arlt M, et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Crit Care 2009; 13: R10. doi: 10.1186/cc7703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubnow M, Luchner A, Philipp A, et al. Combination of high frequency oscillatory ventilation and interventional lung assist in severe acute respiratory distress syndrome. J Crit Care 2010; 25: 436–444. doi: 10.1016/j.jcrc.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 24.Bein T, Zimmermann M, Philipp A, et al. Addition of acetylsalicylic acid to heparin for anticoagulation management during pumpless extracorporeal lung assist. ASAIO J 2011; 57: 164–168. doi: 10.1097/MAT.0b013e318213f9e0 [DOI] [PubMed] [Google Scholar]
- 25.Nierhaus A, Frings DP, Braune S, et al. Interventional lung assist enables lung protective mechanical ventilation in acute respiratory distress syndrome. Minerva Anestesiol 2011; 77: 797–801. [PubMed] [Google Scholar]
- 26.Cho WH, Lee K, Huh JW, et al. Physiologic effect and safety of the pumpless extracorporeal interventional lung assist system in patients with acute respiratory failure – a pilot study. Artif Organs 2012; 36: 434–438. doi: 10.1111/j.1525-1594.2011.01359.x [DOI] [PubMed] [Google Scholar]
- 27.Quintard JM, Barbot O, Thevenot F, et al. Partial extracorporeal carbon dioxide removal using a standard continuous renal replacement therapy device: a preliminary study. ASAIO J 2014; 60: 564–569. doi: 10.1097/MAT.0000000000000114 [DOI] [PubMed] [Google Scholar]
- 28.Weingart C, Lubnow M, Philipp A, et al. Comparison of coagulation parameters, anticoagulation, and need for transfusion in patients on interventional lung assist or veno-venous extracorporeal membrane oxygenation. Artif Organs 2015; 39: 765–773. doi: 10.1111/aor.12464 [DOI] [PubMed] [Google Scholar]
- 29.Fanelli V, Ranieri MV, Mancebo J, et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care 2016; 20: 36. doi: 10.1186/s13054-016-1211-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fanelli V, Cantaluppi V, Alessandri F, et al. Extracorporeal CO2 removal may improve renal function of patients with acute respiratory distress syndrome and acute kidney injury: an open-label, interventional clinical trial. Am J Respir Crit Care Med 2018; 198: 687–690. doi: 10.1164/rccm.201712-2575LE [DOI] [PubMed] [Google Scholar]
- 31.Combes A, Fanelli V, Pham T, et al. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med 2019; 45: 592–600. doi: 10.1007/s00134-019-05567-4 [DOI] [PubMed] [Google Scholar]
- 32.Nentwich J, Wichmann D, Kluge S, et al. Low-flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: a pilot study. Ann Intensive Care 2019; 9: 3. doi: 10.1186/s13613-019-0480-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moerer O, Harnisch L-O, Barwing J, et al. Minimal-flow ECCO2R in patients needing CRRT does not facilitate lung-protective ventilation. J Artif Organs 2019; 22: 68–76. doi: 10.1007/s10047-018-1068-8 [DOI] [PubMed] [Google Scholar]
- 34.Petran J, Muelly T, Dembinski R, et al. Validation of RESP and PRESERVE score for ARDS patients with pumpless extracorporeal lung assist (pECLA). BMC Anesthesiol 2020; 20: 102. doi: 10.1186/s12871-020-01010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goursaud S, Valette X, Dupeyrat J, et al. Ultraprotective ventilation allowed by extracorporeal CO2 removal improves the right ventricular function in acute respiratory distress syndrome patients: a quasi-experimental pilot study. Ann Intensive Care 2021; 11: 3. doi: 10.1186/s13613-020-00784-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding X, Chen H, Zhao H, et al. ECCO2R in 12 COVID-19 ARDS patients with extremely low compliance and refractory hypercapnia. Front Med 2021; 8: 654658. doi: 10.3389/fmed.2021.654658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowler AA, Hamman RF, Good JT, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med 1983; 98: 593–597. 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 38.Suchyta MR, Clemmer TP, Orme JF Jr, et al. Increased survival of ARDS patients with severe hypoxemia (ECMO criteria). Chest 1991; 99: 951–955. 10.1378/chest.99.4.951. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, John Wiley & Sons, 2019. [Google Scholar]
- 40.Blackwood B, Ringrow S, Clarke M, et al. A core outcome set for critical care ventilation trials. Crit Care Med 2019; 47: 1324–1331. doi: 10.1097/CCM.0000000000003904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Needham DM, Sepulveda KA, Dinglas VD, et al. Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi consensus study. Am J Respir Crit Care Med 2017; 196: 1122–1130. doi: 10.1164/rccm.201702-0372OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNamee JJ, Gillies MA, Barrett NA, et al. Protective ventilation with veno-venous lung assist in respiratory failure: a protocol for a multicentre randomised controlled trial of extracorporeal carbon dioxide removal in patients with acute hypoxaemic respiratory failure. J Intensive Care Soc 2017; 18: 159–169. doi: 10.1177/1751143716681035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morelli A, Del Sorbo L, Pesenti A, et al. Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med 2017; 43: 519–530. doi: 10.1007/s00134-016-4673-0 [DOI] [PubMed] [Google Scholar]
- 44.Kalbhenn J, Neuffer N, Zieger B, et al. Is extracorporeal CO2 removal really “safe” and “less” invasive? Observation of blood injury and coagulation impairment during ECCO2R. ASAIO J 2017; 63: 666–671. doi: 10.1097/MAT.0000000000000544 [DOI] [PubMed] [Google Scholar]
- 45.Trudzinski F, Seiler F, Fähndrich S, et al. Acquired coagulation disorders during extracorporeal carbon dioxide removal. Eur Respir J 2016; 48: Suppl. 60, PA3574. [Google Scholar]
- 46.Oppenheim-Eden A, Glantz L, Eidelman LA, et al. Spontaneous intracerebral hemorrhage in critically ill patients: incidence over six years and associated factors. Intensive Care Med 1999; 25: 63–67. doi: 10.1007/s001340050788 [DOI] [PubMed] [Google Scholar]
- 47.Goligher EC, Combes A, Brodie D, et al. Determinants of the effect of extracorporeal carbon dioxide removal in the SUPERNOVA trial: implications for trial design. Intensive Care Med 2019; 45: 1219–1230. doi: 10.1007/s00134-019-05708-9 [DOI] [PubMed] [Google Scholar]
- 48.Turner RM, Bird SM, Higgins JP. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One 2013; 8: e59202. doi: 10.1371/journal.pone.0059202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340: c221. doi: 10.1136/bmj.c221 [DOI] [PubMed] [Google Scholar]
- 50.Knoch M, Köllen B, Dietrich G, et al. Progress in veno-venous long-term bypass techniques for the treatment of ARDS. Controlled clinical trial with the heparin-coated bypass circuit. Int J Artif Organs 1992; 15: 103–108. doi: 10.1177/039139889201500208 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0030-2022.SUPPLEMENT (740.4KB, pdf)