Abstract
Background
Different forms of vaccines have been developed to prevent the SARS‐CoV‐2 virus and subsequent COVID‐19 disease. Several are in widespread use globally.
Objectives
To assess the efficacy and safety of COVID‐19 vaccines (as a full primary vaccination series or a booster dose) against SARS‐CoV‐2.
Search methods
We searched the Cochrane COVID‐19 Study Register and the COVID‐19 L·OVE platform (last search date 5 November 2021). We also searched the WHO International Clinical Trials Registry Platform, regulatory agency websites, and Retraction Watch.
Selection criteria
We included randomized controlled trials (RCTs) comparing COVID‐19 vaccines to placebo, no vaccine, other active vaccines, or other vaccine schedules.
Data collection and analysis
We used standard Cochrane methods. We used GRADE to assess the certainty of evidence for all except immunogenicity outcomes.
We synthesized data for each vaccine separately and presented summary effect estimates with 95% confidence intervals (CIs).
Main results
We included and analyzed 41 RCTs assessing 12 different vaccines, including homologous and heterologous vaccine schedules and the effect of booster doses. Thirty‐two RCTs were multicentre and five were multinational. The sample sizes of RCTs were 60 to 44,325 participants. Participants were aged: 18 years or older in 36 RCTs; 12 years or older in one RCT; 12 to 17 years in two RCTs; and three to 17 years in two RCTs. Twenty‐nine RCTs provided results for individuals aged over 60 years, and three RCTs included immunocompromized patients. No trials included pregnant women. Sixteen RCTs had two‐month follow‐up or less, 20 RCTs had two to six months, and five RCTs had greater than six to 12 months or less. Eighteen reports were based on preplanned interim analyses.
Overall risk of bias was low for all outcomes in eight RCTs, while 33 had concerns for at least one outcome.
We identified 343 registered RCTs with results not yet available.
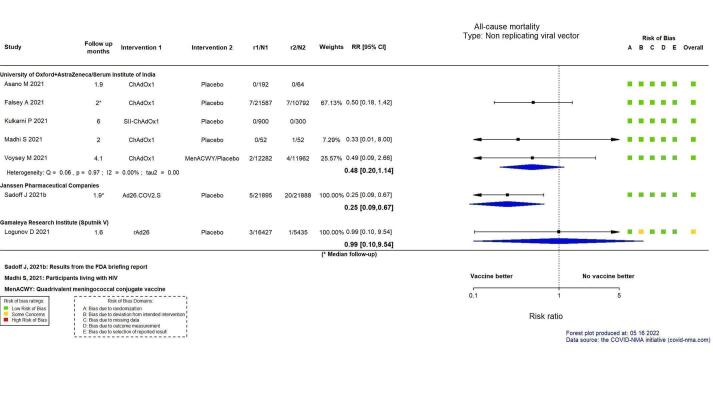
This abstract reports results for the critical outcomes of confirmed symptomatic COVID‐19, severe and critical COVID‐19, and serious adverse events only for the 10 WHO‐approved vaccines. For remaining outcomes and vaccines, see main text. The evidence for mortality was generally sparse and of low or very low certainty for all WHO‐approved vaccines, except AD26.COV2.S (Janssen), which probably reduces the risk of all‐cause mortality (risk ratio (RR) 0.25, 95% CI 0.09 to 0.67; 1 RCT, 43,783 participants; high‐certainty evidence).
Confirmed symptomatic COVID‐19
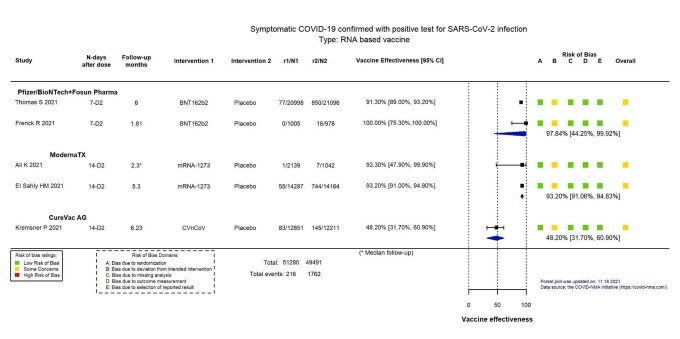
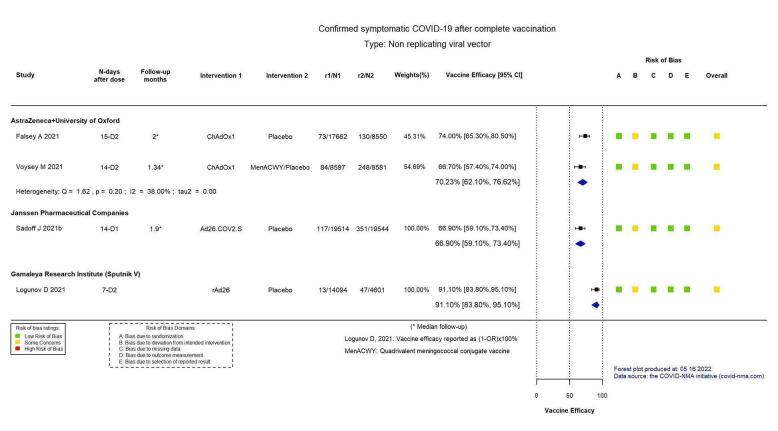
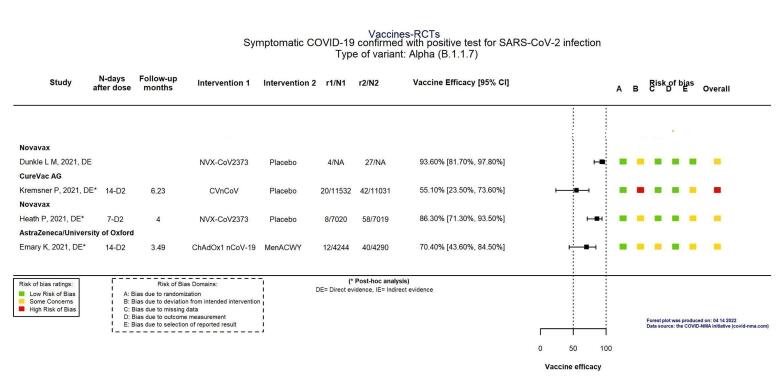
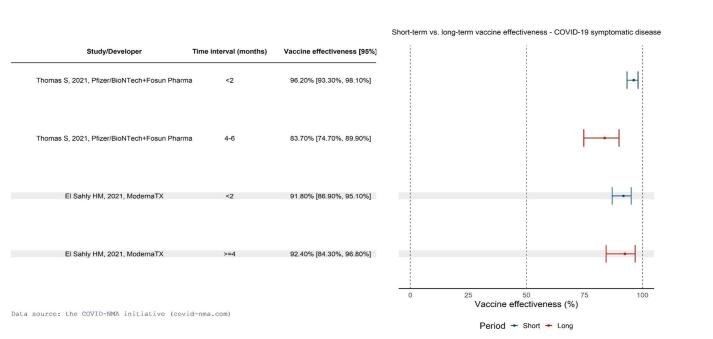
High‐certainty evidence found that BNT162b2 (BioNtech/Fosun Pharma/Pfizer), mRNA‐1273 (ModernaTx), ChAdOx1 (Oxford/AstraZeneca), Ad26.COV2.S, BBIBP‐CorV (Sinopharm‐Beijing), and BBV152 (Bharat Biotect) reduce the incidence of symptomatic COVID‐19 compared to placebo (vaccine efficacy (VE): BNT162b2: 97.84%, 95% CI 44.25% to 99.92%; 2 RCTs, 44,077 participants; mRNA‐1273: 93.20%, 95% CI 91.06% to 94.83%; 2 RCTs, 31,632 participants; ChAdOx1: 70.23%, 95% CI 62.10% to 76.62%; 2 RCTs, 43,390 participants; Ad26.COV2.S: 66.90%, 95% CI 59.10% to 73.40%; 1 RCT, 39,058 participants; BBIBP‐CorV: 78.10%, 95% CI 64.80% to 86.30%; 1 RCT, 25,463 participants; BBV152: 77.80%, 95% CI 65.20% to 86.40%; 1 RCT, 16,973 participants).
Moderate‐certainty evidence found that NVX‐CoV2373 (Novavax) probably reduces the incidence of symptomatic COVID‐19 compared to placebo (VE 82.91%, 95% CI 50.49% to 94.10%; 3 RCTs, 42,175 participants).
There is low‐certainty evidence for CoronaVac (Sinovac) for this outcome (VE 69.81%, 95% CI 12.27% to 89.61%; 2 RCTs, 19,852 participants).
Severe or critical COVID‐19
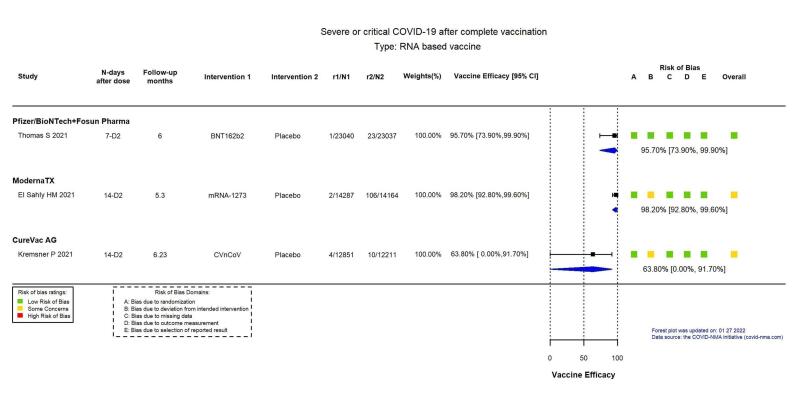
High‐certainty evidence found that BNT162b2, mRNA‐1273, Ad26.COV2.S, and BBV152 result in a large reduction in incidence of severe or critical disease due to COVID‐19 compared to placebo (VE: BNT162b2: 95.70%, 95% CI 73.90% to 99.90%; 1 RCT, 46,077 participants; mRNA‐1273: 98.20%, 95% CI 92.80% to 99.60%; 1 RCT, 28,451 participants; AD26.COV2.S: 76.30%, 95% CI 57.90% to 87.50%; 1 RCT, 39,058 participants; BBV152: 93.40%, 95% CI 57.10% to 99.80%; 1 RCT, 16,976 participants).
Moderate‐certainty evidence found that NVX‐CoV2373 probably reduces the incidence of severe or critical COVID‐19 (VE 100.00%, 95% CI 86.99% to 100.00%; 1 RCT, 25,452 participants).
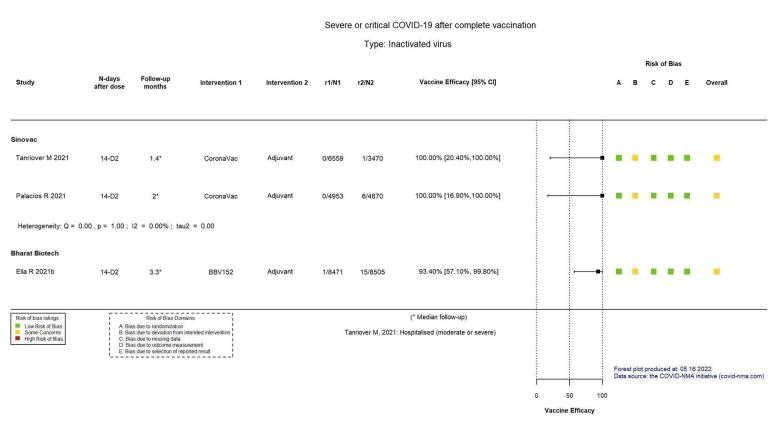
Two trials reported high efficacy of CoronaVac for severe or critical disease with wide CIs, but these results could not be pooled.
Serious adverse events (SAEs)
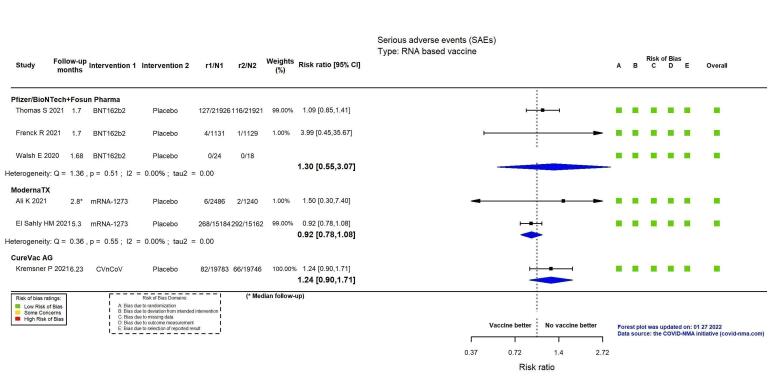
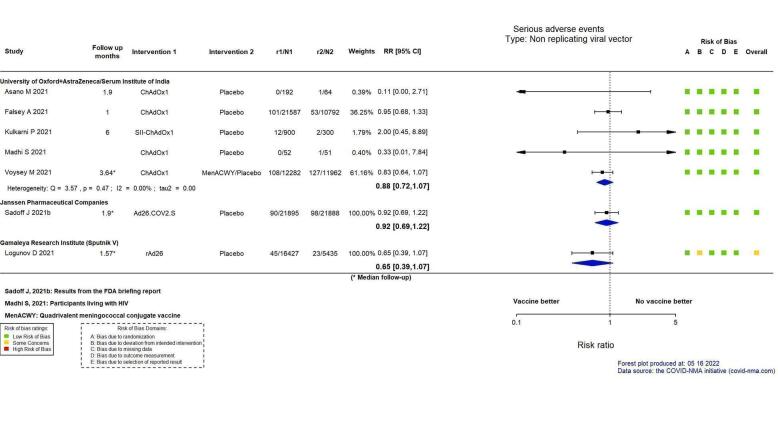
mRNA‐1273, ChAdOx1 (Oxford‐AstraZeneca)/SII‐ChAdOx1 (Serum Institute of India), Ad26.COV2.S, and BBV152 probably result in little or no difference in SAEs compared to placebo (RR: mRNA‐1273: 0.92, 95% CI 0.78 to 1.08; 2 RCTs, 34,072 participants; ChAdOx1/SII‐ChAdOx1: 0.88, 95% CI 0.72 to 1.07; 7 RCTs, 58,182 participants; Ad26.COV2.S: 0.92, 95% CI 0.69 to 1.22; 1 RCT, 43,783 participants); BBV152: 0.65, 95% CI 0.43 to 0.97; 1 RCT, 25,928 participants). In each of these, the likely absolute difference in effects was fewer than 5/1000 participants.
Evidence for SAEs is uncertain for BNT162b2, CoronaVac, BBIBP‐CorV, and NVX‐CoV2373 compared to placebo (RR: BNT162b2: 1.30, 95% CI 0.55 to 3.07; 2 RCTs, 46,107 participants; CoronaVac: 0.97, 95% CI 0.62 to 1.51; 4 RCTs, 23,139 participants; BBIBP‐CorV: 0.76, 95% CI 0.54 to 1.06; 1 RCT, 26,924 participants; NVX‐CoV2373: 0.92, 95% CI 0.74 to 1.14; 4 RCTs, 38,802 participants).
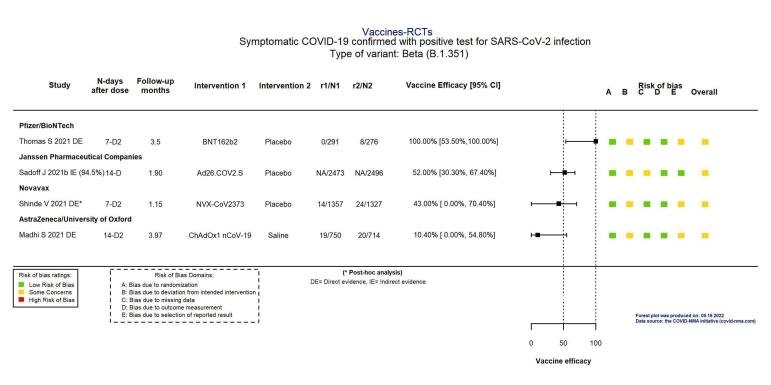
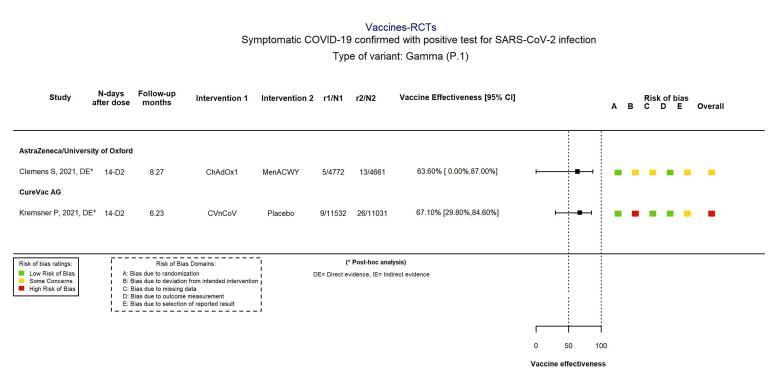
For the evaluation of heterologous schedules, booster doses, and efficacy against variants of concern, see main text of review.
Authors' conclusions
Compared to placebo, most vaccines reduce, or likely reduce, the proportion of participants with confirmed symptomatic COVID‐19, and for some, there is high‐certainty evidence that they reduce severe or critical disease. There is probably little or no difference between most vaccines and placebo for serious adverse events. Over 300 registered RCTs are evaluating the efficacy of COVID‐19 vaccines, and this review is updated regularly on the COVID‐NMA platform (covid-nma.com).
Implications for practice
Due to the trial exclusions, these results cannot be generalized to pregnant women, individuals with a history of SARS‐CoV‐2 infection, or immunocompromized people. Most trials had a short follow‐up and were conducted before the emergence of variants of concern.
Implications for research
Future research should evaluate the long‐term effect of vaccines, compare different vaccines and vaccine schedules, assess vaccine efficacy and safety in specific populations, and include outcomes such as preventing long COVID‐19. Ongoing evaluation of vaccine efficacy and effectiveness against emerging variants of concern is also vital.
Keywords: Adolescent, Aged, Humans, Middle Aged, 2019-nCoV Vaccine mRNA-1273, COVID-19, COVID-19/prevention & control, SARS-CoV-2
Plain language summary
What are the benefits and risks of vaccines for preventing COVID‐19?
Key messages
– Most vaccines reduce, or probably reduce, the number of people who get COVID‐19 disease and severe COVID‐19 disease.
– Many vaccines likely increase number of people experiencing events such as fever or headache compared to placebo (sham vaccine that contains no medicine but looks identical to the vaccine being tested). This is expected because these events are mainly due to the body's response to the vaccine; they are usually mild and short‐term.
– Many vaccines have little or no difference in the incidence of serious adverse events compared to placebo.
– There is insufficient evidence to determine whether there was a difference between the vaccine and placebo in terms of death because the numbers of deaths were low in the trials.
– Most trials assessed vaccine efficacy over a short time, and did not evaluate efficacy to the COVID variants of concern.
What is SARS‐CoV‐2 and COVID‐19?
SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2) is the virus that causes COVID‐19 disease. Not everyone infected with SARS‐CoV‐2 will develop symptoms of COVID‐19. Symptoms can be mild (e.g. fever and headaches) to life‐threatening (e.g. difficulty breathing), or death.
How do vaccines prevent COVID‐19?
While vaccines work slightly differently, they all prepare the body's immune system to prevent people from getting infected with SARS‐CoV‐2 or, if they do get infected, to prevent severe disease.
What did we want to find out?
We wanted to find out how well each vaccine works in reducing SARS‐CoV‐2 infection, COVID‐19 disease with symptoms, severe COVID‐19 disease, and total number of deaths (including any death, not only those related to COVID‐19).
We wanted to find out about serious adverse events that might require hospitalization, be life‐threatening, or both; systemic reactogenicity events (immediate short‐term reactions to vaccines mainly due to immunological responses; e.g. fever, headache, body aches, fatigue); and any adverse events (which include non‐serious adverse events).
What did we do?
We searched for studies that examined any COVID‐19 vaccine compared to placebo, no vaccine, or another COVID‐19 vaccine.
We selected only randomized trials (a study design that provides the most robust evidence because they evaluate interventions under ideal conditions among participants assigned by chance to one of two or more groups). We compared and summarized the results of the studies, and rated our confidence in the evidence based on factors such as how the study was conducted.
What did we find?
We found 41 worldwide studies involving 433,838 people assessing 12 different vaccines. Thirty‐five studies included only healthy people who had never had COVID‐19. Thirty‐six studies included only adults, two only adolescents, two children and adolescents, and one included adolescents and adults. Three studied people with weakened immune systems, and none studied pregnant women.
Most cases assessed results less than six months after the primary vaccination. Most received co‐funding from academic institutions and pharmaceutical companies. Most studies compared a COVID‐19 vaccine with placebo. Five evaluated the addition of a 'mix and match' booster dose.
Main results
We report below results for three main outcomes and for 10 World Health Organization (WHO)‐approved vaccines (for the remaining outcomes and vaccines, see main text). There is insufficient evidence regarding deaths between vaccines and placebo (mainly because the number of deaths was low), except for the Janssen vaccine, which probably reduces the risk of all‐cause deaths.
People with symptoms
The Pfizer, Moderna, AstraZeneca, Sinopharm‐Beijing, and Bharat vaccines produce a large reduction in the number of people with symptomatic COVID‐19.
The Janssen vaccine reduces the number of people with symptomatic COVID‐19.
The Novavax vaccine probably has a large reduction in the number of people with symptomatic COVID‐19.
There is insufficient evidence to determine whether CoronaVac vaccine affects the number of people with symptomatic COVID‐19 because results differed between the two studies (one involved only healthcare workers with a higher risk of exposure).
Severe disease
The Pfizer, Moderna, Janssen, and Bharat vaccines produce a large reduction in the number of people with severe disease.
There is insufficient evidence about CoronaVac vaccine on severe disease because results differed between the two studies (one involved only healthcare workers with a higher risk of exposure).
Serious adverse events
For the Pfizer, CoronaVac, Sinopharm‐Beijing, and Novavax vaccines, there is insufficient evidence to determine whether there was a difference between the vaccine and placebo mainly because the number of serious adverse events was low.
Moderna, AstraZeneca, Janssen, and Bharat vaccines probably result in no or little difference in the number of serious adverse events.
What are the limitations of the evidence?
Most studies assessed the vaccine for a short time after injection, and it is unclear if and how vaccine protection wanes over time. Due to the exclusion criteria of COVID‐19 vaccine trials, results cannot be generalized to pregnant women, people with a history of SARS‐CoV‐2 infection, or people with weakened immune systems. More research is needed comparing vaccines and vaccine schedules, and effectiveness and safety in specific populations and outcomes (e.g. preventing long COVID‐19). Further, most studies were conducted before the emergence of variants of concerns.
How up to date is this evidence?
The evidence is up to date to November 2021. This is a living systematic review. Our results are available and updated bi‐weekly on the COVID‐NMA platform at covid‐nma.com.
Summary of findings
Summary of findings 1. BNT162b2 – Pfizer/BioNTech + Fosun Pharma compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with BNT162b2 | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19b | 3923 per 100,000 | 85 per 100,000 (3 to 2187) |
VE 97.84 (44.25 to 99.92) |
44,077 (2 RCTs)c | ⊕⊕⊕⊕ Highd | — |
| Severe or critical COVID‐19e | 100 per 100,000 | 4 per 100,000 (0 to 26) | VE 95.70 (73.90 to 99.90) | 46,077 (1 RCT)f | ⊕⊕⊕⊕ High | — |
| All‐cause mortalityg | 64 per 100,000 | 68 per 100,000 (33 to 142) | RR 1.07 (0.52 to 2.22) | 43,847 (1 RCT)f | ⊕⊕⊖⊖ Lowh | 2 additional studies (Frenck 2021 (adolescents aged 12–15 years); Walsh 2020 (adults aged 18–85 years)) reported this outcome in 2302 participants (1131 versus 1129 participants and 24 versus 18 participants in the BNT162b2 versus placebo groups, respectively). There were no events in either group and the trials did not contribute to the effect estimate. |
| Systemic reactogenicity events | Outcome not yet measured or reported | |||||
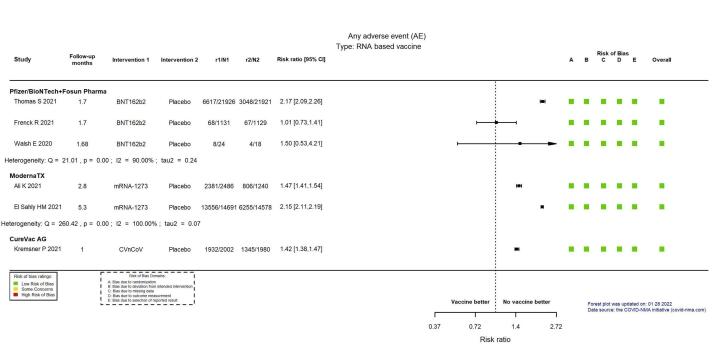
| Any adverse eventi | Outcome not pooled due to considerable heterogeneity (I² = 90%) between included studies: Thomas 2021 (≥ 16 years): RR 2.17, 95% CI 2.09 to 2.26; n = 43,847; Frenck 2021 (12–15 years): RR 1.01, 95% CI 0.73 to 1.41; n = 2260; Walsh 2020 (≥ 18 years): RR 1.50, 95% CI 0.53 to 4.21; n = 42 | 46,149 (3 RCTs)j | ⊕⊕⊖⊖ Lowk | — | ||
| Serious adverse eventsi | 508 per 100,000 | 660 per 100,000 (279 to 1558) | RR 1.30 (0.55 to 3.07) | 46,107 (2 RCTs)c | ⊕⊕⊖⊖ Lowl,m | 1 additional trial (Walsh 2020 (adults aged 18–85 years)) reported this outcome in 42 participants (24 BNT162b2 versus 18 placebo). There were no events in either group and the trial did not contribute to the effect estimate. |
| Local reactogenicity events | Outcome not yet measured or reported | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 3 May 2022 bFollow‐up: from 7 days following the second dose to 1.81 months and six months. cBioNTech/Fosun Pharma/Pfizer: Thomas 2021 (adolescents and adults aged from 16 years); Frenck 2021 (adolescents aged 12–15 years) dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eFollow‐up: from seven days following the second dose to six months. fBioNTech/Fosun Pharma/Pfizer: Thomas 2021 (adolescents and adults aged from 16 years) gFollow‐up: six months hImprecision: downgraded two levels due to small number of events observed and a wide CIs that encompasses a potential benefit and a potential harm with the intervention. iFollow‐up: 1.7 months jBioNTech/Fosun Pharma/Pfizer: Thomas 2021 (adolescents and adults aged from 16 years); Frenck 2021 (adolescents aged 12–15 years); Walsh 2020 (adults aged 18–85 years) kInconsistency: downgraded two levels (I² = 90%) lInconsistency: downgraded one level (I² = 76%) mImprecision: downgraded one level due to wide CIs consistent with the possibility of benefit and the possibility of harm. This outcome was not downgraded an additional level for imprecision because it was downgraded one level for inconsistency, which is related to and would have contributed to the severity of the imprecision.
Summary of findings 2. mRNA‐1273 – ModernaTX compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with mRNA‐1273 | |||||
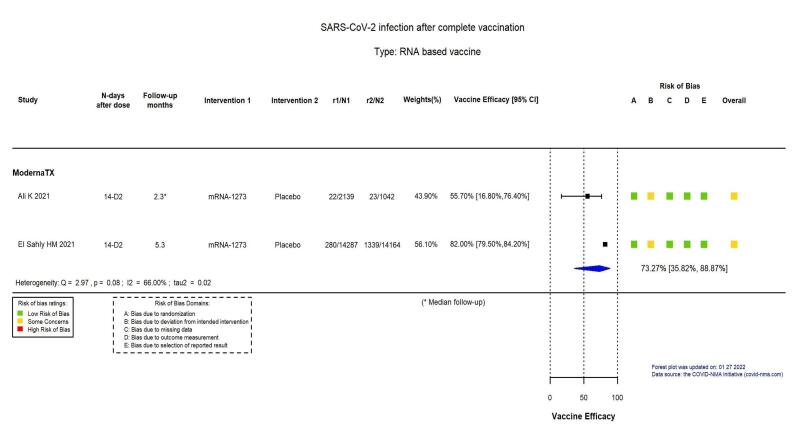
| Confirmed SARS‐CoV‐2 infectionb | 8957 per 100,000 | 2394 per 100,000 (997 to 5749) | VE 73.27 (35.82 to 88.87) | 31,632 (2 RCTs)c | ⨁⨁⨁◯ Moderated,e | Substantial heterogeneity (I² = 66%) between included studies: Ali 2021 (adolescents aged 12–17 years, median 2.3 months' follow‐up): VE 55.7% (95% CI 16.8 to 76.4), n = 3181; El Sahly 2021 (adults aged 18–95 years, 5.3 months' follow‐up): VE 82% (95% CI 79.5 to 84.2), n = 28,451 |
| Confirmed symptomatic COVID‐19 b | 4939 per 100,000 | 336 per 100,000 (255 to 442) |
VE 93.20 (91.06 to 94.83) |
31,632 (2 RCTs)c | ⨁⨁⨁⨁ Highd | — |
| Severe or critical COVID‐19f | 748 per 100,000 | 13 per 100,000 (3 to 54) |
VE 98.20 (92.80 to 99.60) |
28,451 (1 RCT)g | ⨁⨁⨁⨁ Highd | — |
| All‐cause mortalityf | 106 per 100,000 | 112 per 100,000 (57 to 222) |
RR 1.06 (0.54 to 2.10) | 30,346 (1 RCT)g | ⨁⨁◯◯ Lowh | 1 additional trial: (Ali 2021 (adolescents aged 12–17 years)) reported on this outcome in 3726 participants (2486 mRNA‐1273 and 1240 placebo). There were no events in either group and the trial did not contribute to the pooled effect estimate |
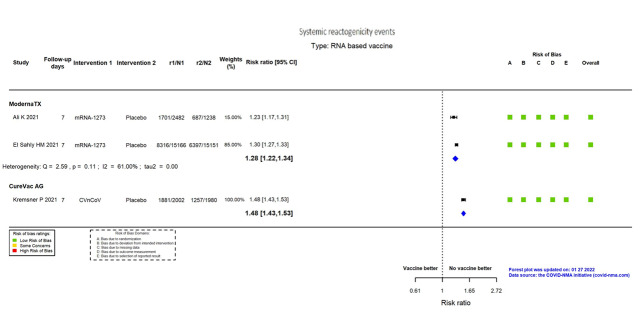
| Systemic reactogenicity eventsi | 432 per 1000 | 553 per 1000 (527 to 579) | RR 1.28 (1.22 to 1.34) | 34,037 (2 RCTs)c | ⨁⨁⨁⨁ Highj | — |
| Any adverse eventk | Outcome not pooled due to considerable heterogeneity (I² = 100%) between included studies: Ali 2021 (all solicited adverse events, adolescents aged 12–17 years, median 2.8 months' follow‐up): RR 1.47 (95% CI 1.41 to 1.54), n = 3726; El Sahly 2021 (all solicited adverse events, adults aged 18–95 years, 5.3 months' follow‐up): RR 2.15 (95% CI 2.11 to 2.19), n = 29,269 | — | 32,995 (2 RCTs)c | ⨁⨁◯◯ Lowl | — | |
| Serious adverse eventsl | 1792 per 100,000 | 1649 per 100,000 (1398 to 1936) | RR 0.92 (0.78 to 1.08) | 34,072 (2 RCTs)c | ⨁⨁⨁◯ Moderatem | — |
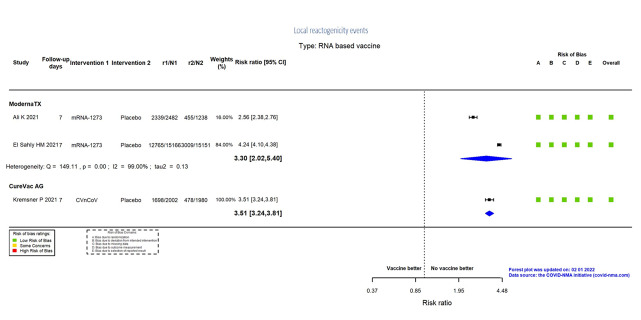
| Local reactogenicity eventsi | 211 per 1000 | 697 per 1000 (427 to 1000) | RR 3.30 (2.02 to 5.40) | 34,037 (2 RCTs)c | ⨁⨁⨁⨁ Highn | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a. Last updated: 01 March 2023
b. Follow‐up: from 14 days after dose 2 to 2.3 months (median) and 5.3 months
c. Moderna TX: Ali 2021 (adolescents aged 12–17 years); El Sahly 2021 (adults aged 18–95 years)
d. Despite some concerns with deviations from intervention, not downgraded for risk of bias
e. Inconsistency: downgraded one level: I² = 66.37%
f. Follow‐up: 5.3 months
g. Moderna TX: El Sahly 2021 (adults aged 18–95 years)
h. Imprecision downgraded two levels due to small number of events observed and wide CIs that encompass a potential benefit and a potential harm with the intervention
i. Follow‐up: seven days
j. Despite inconsistency (I² = 61%) not downgraded for inconsistency, as the same direction of effect in both effect estimates
k. Follow‐up: 2.8 months (median) and 5.3 months
l. Inconsistency: downgraded two levels (I² = 100%)
m. Imprecision: downgraded one level due to wide CIs that encompass a potential benefit and a potential harm with the intervention.
n. Despite inconsistency (I² = 99%), not downgraded for inconsistency, as the same direction of effect in both effect estimates
Summary of findings 3. CVnCoV – CureVac AG compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with CVnCOV | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19b | 1187 per 100,000 | 615 per 100,000 (464 to 811) |
VE 48.20 (31.70 to 60.90) |
25,062 (1 RCT)c | ⊕⊕⊕⊖ Moderated,e | — |
| Severe or critical COVID‐19f | 82 per 100,000 | 30 per 100,000 (7 to 82) |
VE 63.80 (0.00 to 91.70) |
25,062 (1 RCT)c | ⊕⊖⊖⊖ Very lowd,e,g | — |
| All‐cause mortalityh | 30 per 100,000 | 40 per 100,000 (14 to 116) | RR 1.33 (0.46 to 3.83) | 39,529 (1 RCT)c | ⊕⊖⊖⊖ Very lowe,g | — |
| Systemic reactogenicity eventsi | 635 per 1000 | 940 per 1000 (908 to 971) | RR 1.48 (1.43 to 1.53) | 3982 (1 RCT)c | ⊕⊕⊕⊕ High | — |
| Any adverse eventj | 679 per 1000 | 965 per 1000 (937 to 999) | RR 1.42 (1.38 to 1.47) | 3982 (1 RCT)c | ⊕⊕⊕⊖ Moderatee | — |
| Serious adverse eventsk | 334 per 100,000 | 414 per 100,000 (301 to 572) | RR 1.24 (0.90 to 1.71) | 39,529 (1 RCT)c | ⊕⊕⊖⊖ Lowe,l | — |
| Local reactogenicity eventsi | 241 per 1000 | 847 per 1000 (782 to 920) | RR 3.51 (3.24 to 3.81) | 3982 (1 RCT)c | ⊕⊕⊕⊕ High | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 10 May 2022 bFollow‐up: from 14 days following the second dose to 6.23 months cCureVac AG: Kremsner 2021 (adults aged 18–98 years) dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eIndirectness: downgraded one level as data are from interim analyses of the trial and from the available information it is unclear whether these were preplanned. fFollow‐up: from seven days following the second dose to six months gImprecision: downgraded two levels due to small number of events observed and wide CIs that encompass a potential benefit and a potential harm with the intervention. hFollow‐up: 6.23 months iFollow‐up: seven days jFollow‐up: one month kFollow‐up: 1.7 months lImprecision: downgraded one level due to wide CIs consistent with the possibility of benefit and the possibility of harm.
Summary of findings 4. ChAdOx1 – AstraZeneca + University of Oxford compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with ChAdOx1 | |||||
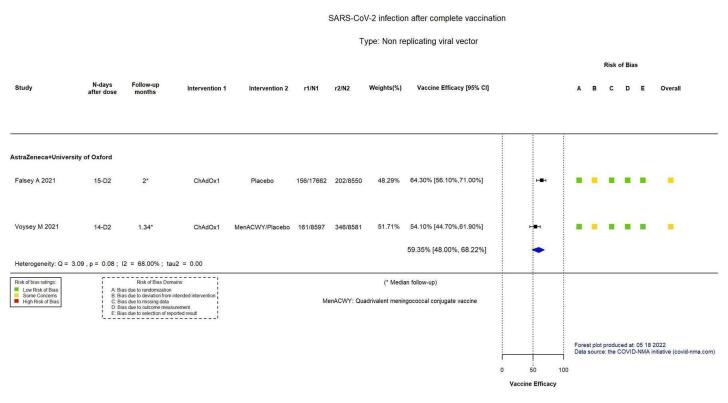
| Confirmed SARS‐CoV‐2 infectionb | 3199 per 100,000 | 1300 per 100,000 (1017 to 1663) |
VE 59.35 (48.00 to 68.22) |
43,390 (5 RCTs)c | ⊕⊕⊕⊖ Moderated,e | Substantial heterogeneity (I² = 68%) between included studies: Falsey 2021 (VE 64.35%, 95% CI 56.10% to 71.00%; n = 26,212); Voysey 2021a (VE 54.10%, 95% CI 44.70% to 61.90%; n = 17,178) |
| Confirmed symptomatic COVID‐19b | 2207 per 100,000 | 657 per 100,000 (516 to 836) |
VE 70.23 (62.10 to 76.62) |
43,390 (5 RCTs)c | ⊕⊕⊕⊕ Highd | — |
| Severe or critical COVID‐19 | Outcome not yet measured or reported | |||||
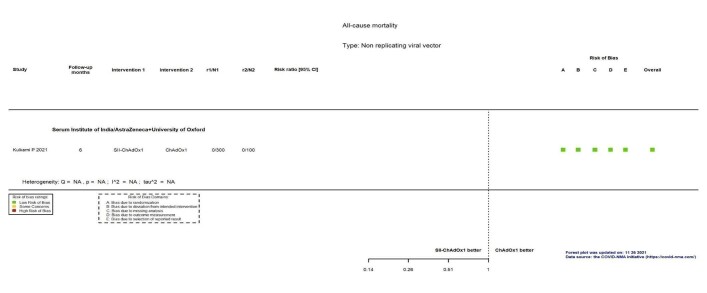
| All‐cause mortalityf | 52 per 100,000 | 25 per 100,000 (10 to 59) | RR 0.48 (0.20 to 1.14) | 56,727 (5 RCTs)g | ⊕⊕⊖⊖ Lowh | 2 additional trials (Asano 2022; Kulkarni 2021) reported this outcome in 1392 participants (192 ChAdOx1 versus 64 placebo and 900 SII‐ChAdOx1 versus 300 placebo, respectively). There were no events in either group in either trial and they did not contribute to the pooled effect estimate. |
| Systemic reactogenicity eventsi | 141 per 1000 | 553 per 1000 (297 to 1000) | RR 3.93 (2.11 to 7.29) | 256 (1 RCT)j | ⊕⊕⊕⊖ Moderatek | — |
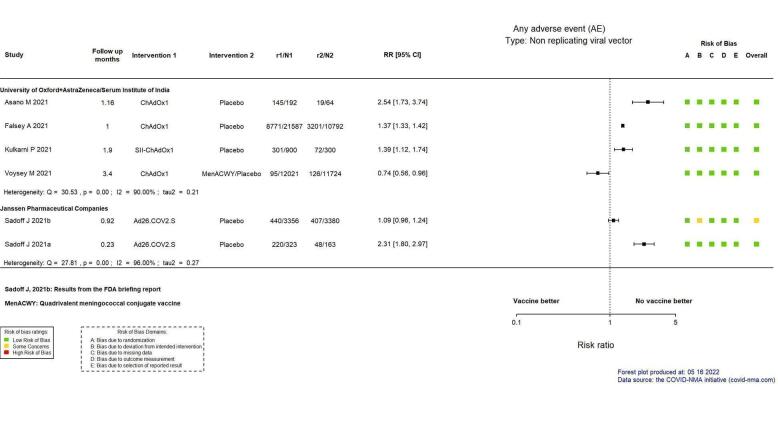
| Any adverse eventl | Outcome not pooled due to considerable heterogeneity (I² = 90%) between included studies: Asano 2022 (RR 2.54, 95% CI 1.73 to 3.74; n = 256); Falsey 2021 (RR 1.37, 95% CI 1.33 to 1.42; n = 32,379); Kulkarni 2021 (RR 1.39, 95% CI 1.12 to 1.74; n = 1200); Voysey 2021a (RR 0.74, 95% CI 0.56 to 0.96; n = 23,745) | — | 57,580 (7 RCTs)m | ⊕⊕⊖⊖ Lown | — | |
| Serious adverse eventso | 794 per 100,000 | 699 per 100,000 (572 to 850) | RR 0.88 (0.72 to 1.07) | 58,182 (7 RCTs)p | ⊕⊕⊕⊖ Moderateq | — |
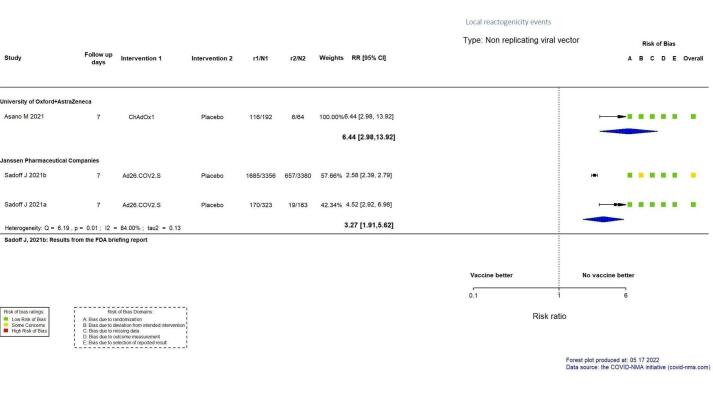
| Local reactogenicity eventsi | 94 per 1000 | 604 per 1000 (279 to 1000) | RR 6.44 (2.98 to 13.92) | 256 (1 RCT)j | ⊕⊕⊕⊖ Moderatek,r | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 4 May 2022 bFollow‐up: from 14 days after second dose up to 1.34 months (median) and 2 months (median) cFalsey 2021; Voysey 2021a (data from four pooled RCTs) dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eInconsistency: downgraded one level (I² = 68%). fFollow‐up: 2 months, 4.2 months and 2 months (median) gFalsey 2021; Voysey 2021a (data from four pooled RCTs); Madhi 2021a (participants with HIV, trial already counted in Voysey 2021a) hImprecision: downgraded two levels due to small number of events observed and wide CIs that encompass a potential benefit and a potential harm with the intervention. iFollow‐up: seven days jAsano 2022 kImprecision: downgraded one level due to low number of participants/few events observed. lFollow‐up: 1 month, 1.16 months, 1.9 months, and 3.4 months mAsano 2022; Falsey 2021; Kulkarni 2021; Voysey 2021a (data from four pooled RCTs) nInconsistency: downgraded two levels (I² = 90%). oFollow‐up: 1 month, 1.9 months, 6 months, and 3.64 months (median) pAsano 2022; Falsey 2021; Kulkarni 2021; Voysey 2021a (data from four pooled RCTs). Madhi 2021a (participants with HIV, trial already counted in Voysey 2021a) qImprecision: downgraded one level due to wide CIs consistent with the possibility of benefit and the possibility of no effect. rDespite some concerns with selection of reported results, not downgraded for risk of bias.
Summary of findings 5. SII‐ChAdOx1 – Serum Institute of India/AstraZeneca + University of Oxford compared to ChAdOx1 – University of Oxford for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ChAdOx1 | Risk with SII‐ChAdOx1 | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19 | Outcome not yet measured or reported | |||||
| Severe or critical COVID‐19 | Outcome not yet measured or reported | |||||
| All‐cause mortality | — | — | — | — | — | 1 study reported this outcome in 400 participants (Kulkarni 2021). There were no events in either group and no effect estimate could be calculated. |
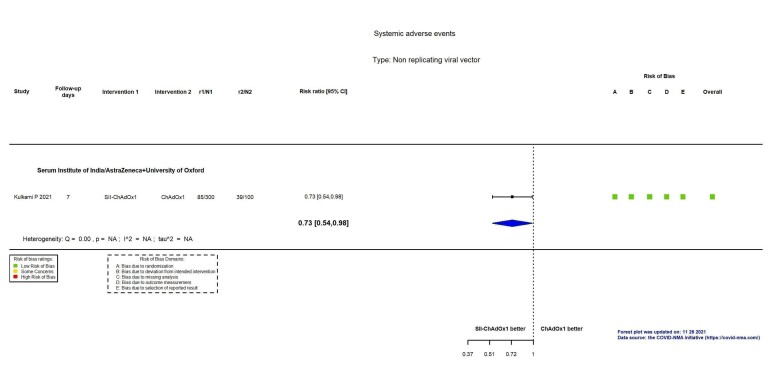
| Systemic reactogenicity eventsb | 390 per 1000 | 285 per 1000 (211 to 382) | RR 0.73 (0.54 to 0.98) | 400 (1 RCT)c | ⊕⊕⊕⊖ Moderated | — |
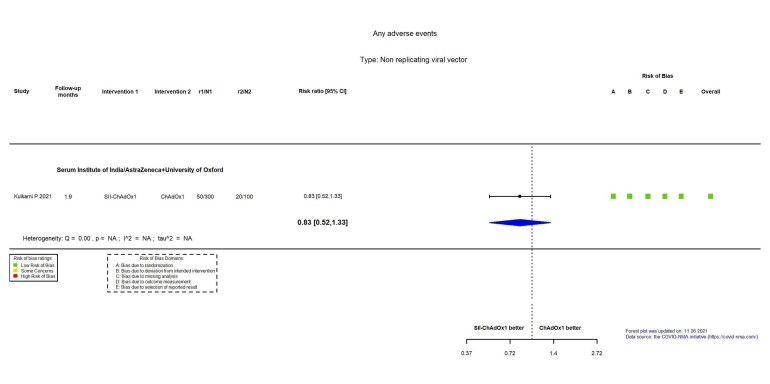
| Any adverse evente | 200 per 1000 | 166 per 1000 (104 to 266) | RR 0.83 (0.52 to 1.33) | 400 (1 RCT)c | ⊕⊕⊖⊖ Lowf | — |
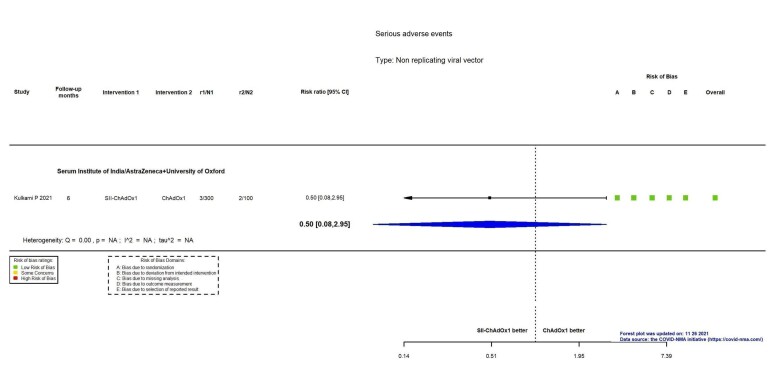
| Serious adverse eventsg | 2000 per 100,000 | 1000 per 100,000 (160 to 5900) | RR 0.50 (0.08 to 2.95) | 400 (1 RCT)c | ⊕⊕⊖⊖ Lowf | — |
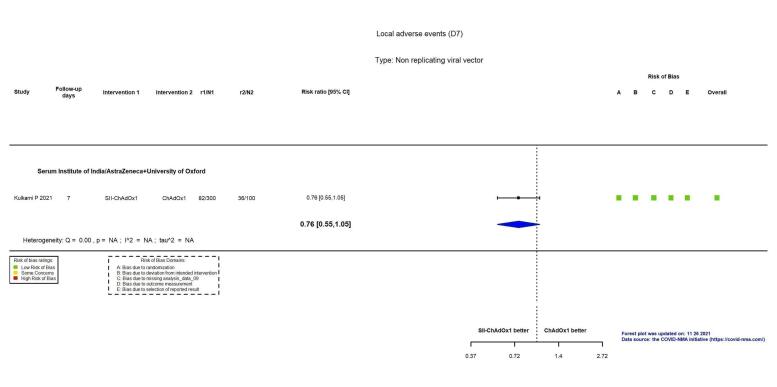
| Local reactogenicity eventsb | 360 per 1000 | 274 per 1000 (198 to 378) | RR 0.76 (0.55 to 1.05) | 400 (1 RCT)c | ⊕⊕⊖⊖ Lowh | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 10 May 2022 bFollow‐up: seven days cKulkarni 2021 dImprecision: downgraded one level due to low number of events/participants. eFollow‐up: 1.9 months fImprecision: downgraded two levels due to wide CIs consistent with the possibility of benefit and the possibility of harm and low number of events/participants. gFollow‐up: six months hImprecision: downgraded two levels due to wide CIs consistent with the possibility of no effect and the possibility of benefit and low number of events/participants.
Summary of findings 6. AD26.COV2.S – Janssen Pharmaceutical Companies compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with AD26.COV2.S | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19b | 1796 per 100,000 | 594 per 100,000 (478 to 735) |
VE 66.90 (59.10 to 73.40) |
39,058 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| Severe or critical COVID‐19b | 409 per 100,000 | 97 per 100,000 (51 to 172) |
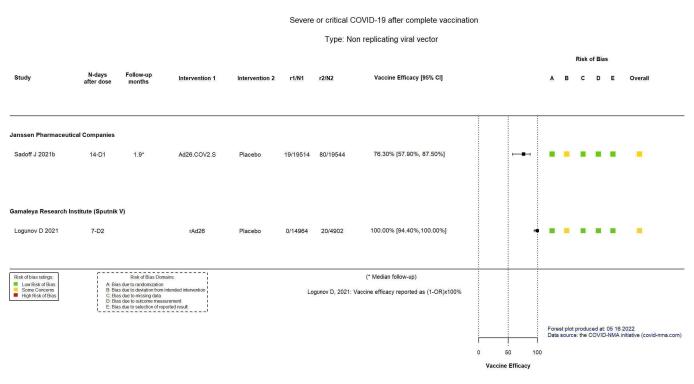
VE 76.30 (57.90 to 87.50) |
39,058 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| All‐cause mortalityb | 91 per 100,000 | 23 per 100,000 (8 to 61) | RR 0.25 (0.09 to 0.67) | 43,783 (1 RCT)c | ⊕⊕⊕⊕ High | — |
| Serious adverse eventsb | 448 per 100,000 | 412 per 100,000 (309 to 546) | RR 0.92 (0.69 to 1.22) | 43,783 (1 RCT)c | ⊕⊕⊕⊖ Moderatej | — |
| Systemic reactogenicity eventse | 34,575 per 100,000 | 63,273 per 100,000 (44,602 to 89,896) | RR 1.83 (1.29 to 2.60) | 7222 (2 RCTs)f | ⊕⊕⊕⊕ Highd,g | — |
| Any adverse eventh | Outcome not pooled due to considerable heterogeneity (I² = 96%) between included studies: Sadoff 2021a (RR 1.09, 95% CI 0.96 to 1.24; n = 6736); Sadoff 2021b (RR 2.31, 95% CI 1.80 to 2.97; n = 486) | — | 7222 (2 RCTs)f | ⊕⊕⊖⊖ Lowd,i | — | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 4 May 2022 bFollow‐up: 1.9 months (median) cSadoff 2021b dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eFollow‐up: seven days and 14 days fSadoff 2021a; Sadoff 2021b gDespite I² = 83%, not downgraded for inconsistency, as the same direction of effect in both effect estimates. hFollow‐up: 0.23 months and 0.92 months iInconsistency: downgraded two levels (I² = 96%). jImprecision: downgraded one level due to wide CIs consistent with the possibility of no effect and the possibility of benefit. kFollow‐up: seven days lDespite I² = 84%, not downgraded for inconsistency, as the same direction of effect in both effect estimates.
Summary of findings 7. Gam‐COVID‐VAC – Sputnik V compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Gam‐COVID‐VAC | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19b | 1022 per 100,000 | 92 per 100,000 (51 to 167) |
VE 91.10 (83.80 to 95.10) |
18,695 (1 RCT)c | ⊕⊕⊕⊖ Moderated,e | — |
| Severe or critical COVID‐19b | 408 per 100,000 | 0 per 100,000 (0 to 23) |
VE 100.00 (94.40 to 100.00) |
19,866 (1 RCT)c | ⊕⊕⊕⊖ Moderated,e | — |
| All‐cause mortalityf | 18 per 100,000 | 18 per 100,000 (2 to 176) | RR 0.99 (0.10 to 9.54) | 21,862 (1 RCT)c | ⊕⊖⊖⊖ Very lowd,e,g | — |
| Systemic reactogenicity events | Outcome not yet measured or reported | |||||
| Any adverse event | Outcome not yet measured or reported | |||||
| Serious adverse eventsf | 423 per 100,000 | 275 per 100,000 (165 to 453) | RR 0.65 (0.39 to 1.07) | 21,862 (1 RCT)c | ⊕⊕⊖⊖ Lowd,e,h | — |
| Local reactogenicity events | Outcome not yet measured or reported | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 27 May 2022 bFollow‐up: from seven days after second dose cLogunov 2021 dIndirectness: downgraded one level as data are from interim analyses of the trial and from the available information it is unclear whether these were preplanned. eConcern regarding the internal validity of the trial. fFollow‐up: 1.6 months (median) gImprecision: downgraded two levels due to wide CIs consistent with the possibility of benefit and the possibility of harm and few events. hImprecision: downgraded one level due to wide CIs consistent with the possibility of no effect and the possibility of benefit.
Summary of findings 8. CoronaVac – Sinovac compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with CoronaVac | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19b | 2398 per 100,000 | 724 per 100,000 (249 to 2104) |
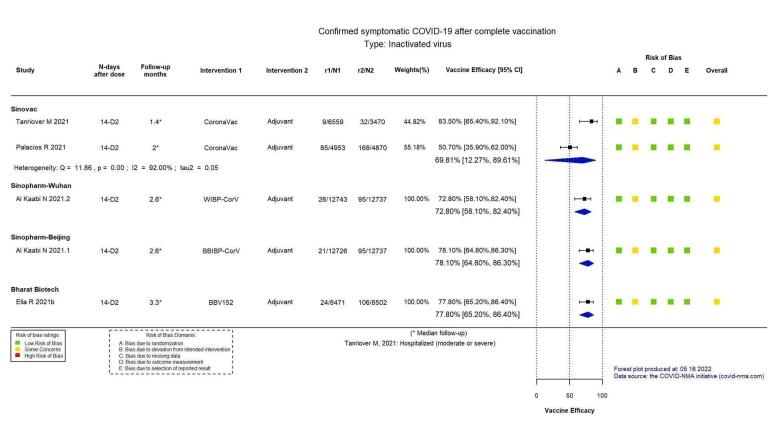
VE 69.81 (12.27 to 89.61) |
19,852 (2 RCTs)c | ⊕⊕⊖⊖ Lowd,e,f | Considerable heterogeneity (I² = 92%) between included studies: Tanriover 2021 (VE 83.50%, 95% CI 65.40% to 92.10%; n = 10,029); Palacios 2020 (VE 50.70%, 95% CI 35.90 to 62.00%; n = 9823) |
| Severe or critical COVID‐19b | 2 studies report on severe or critical disease due to COVID‐19: Tanriover 2021, with 0/6559 events in the CoronaVac group versus 1/3470 events in the placebo group and a VE of 100%, 95% CI (20.40% to 100.00%); and Palacios 2020, with 0/4953 events in the CoronaVac group and 6/4870 events in the placebo group and a VE of 100%, 95% CI (16.90% to 100.00%). (Note: estimates could not be pooled due to asymmetry in the CIs) | — | 19,852 (2 RCTs)c | ⊕⊕⊖⊖ Lowd,g | — | |
| All‐cause mortalityh | 20 per 100,000 | 10 per 100,000 (1 to 113) | RR 0.50 (0.05 to 5.52) | 22,610 (2 RCTs)c | ⊕⊕⊖⊖ Lowi | — |
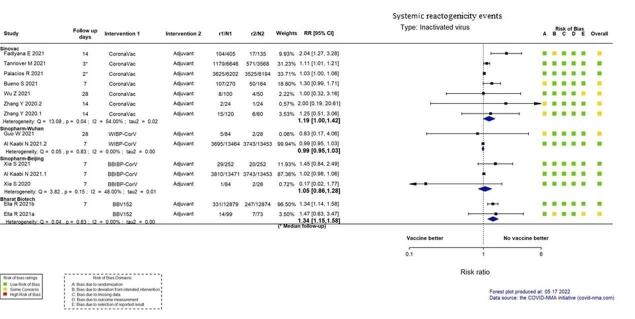
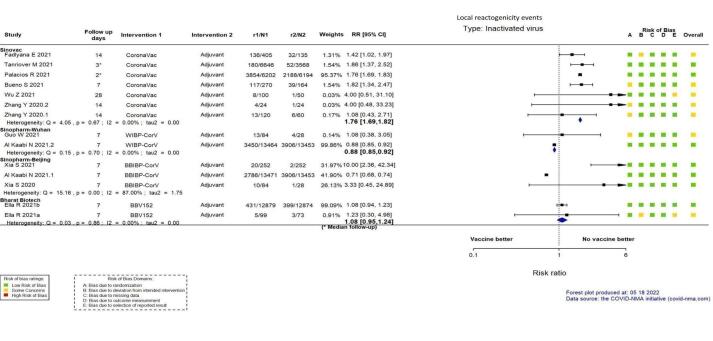
| Systemic reactogenicity eventsj | 409 per 1000 | 487 per 1000 (409 to 581) | RR 1.19 (1.00 to 1.42) | 23,966 (6 RCTs)k | ⊕⊕⊖⊖ Lowl,m,n | — |
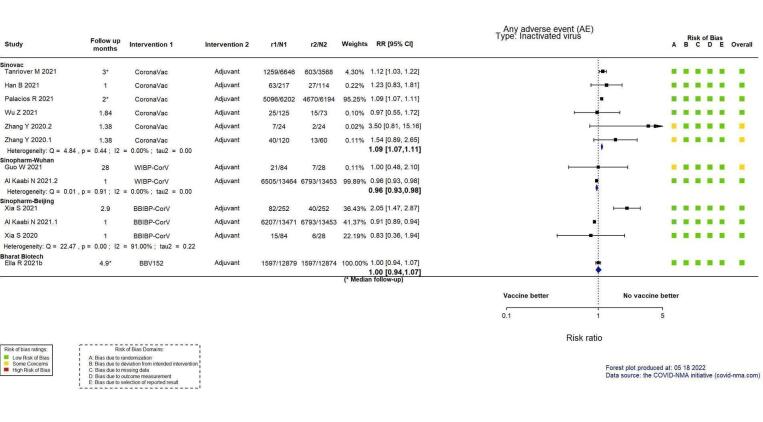
| Any adverse evento | 531 per 1000 | 579 per 1000 (568 to 590) | RR 1.09 (1.07 to 1.11) | 23,367 (6 RCTs)p | ⊕⊕⊕⊕ Highq | — |
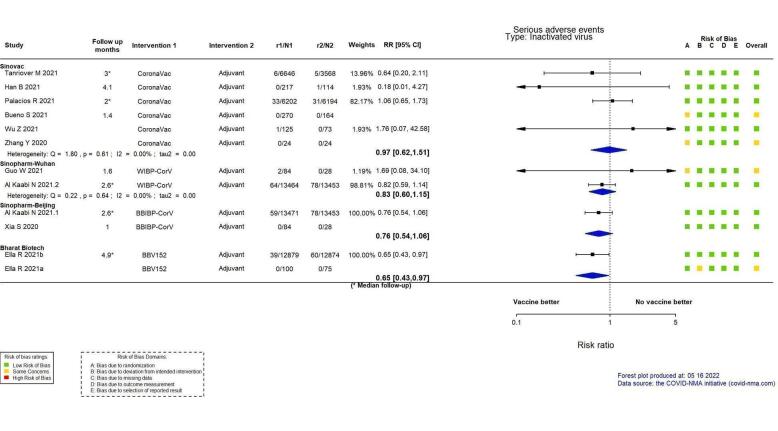
| Serious adverse eventsr | 372 per 100,000 | 361 per 100,000 (231 to 562) | RR 0.97 (0.62 to 1.51) | 23,139 (4 RCTs)s | ⊕⊕⊖⊖ Lowi,q | 2 additional trials (Bueno 2021; Zhang 2021) reported this outcome in 482 participants (270 versus 164 and 24 versus 24 respectively, receiving CoronaVac versus placebo). There were no events in either group and the trials did not contribute to the pooled effect estimate. |
| Local reactogenicity eventsj | 227 per 1000 | 400 per 1000 (384 to 414) | RR 1.76 (1.69 to 1.82) | 23,962 (6 RCTs)k | ⊕⊕⊕⊕ Highl | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 4 May 2022 bFollow‐up: from 14 days after the second dose up to two months (median) cPalacios 2020; Tanriover 2021 dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eInconsistency: downgraded one level (I² = 92%). fImprecision: downgraded one level due to wide CIs consistent with the possibility of benefit and the possibility of harm. gImprecision: downgraded two levels due to low number of events and wide CIs. hFollow‐up: 1.4 and 2 months (median) iImprecision: downgraded two levels due to wide CIs consistent with the possibility of benefit and the possibility of harm and few events. jFollow‐up: 7–28 days kBueno 2021; Fadlyana 2021; Palacios 2020; Tanriover 2021; Wu 2021a; Zhang 2021 lDespite some concerns with adequate randomisation, deviation from intended intervention, missing data, and selection of reported results not downgraded for risk of bias. mInconsistency: downgraded one level (I² = 55%). nImprecision: downgraded one level due to wide CIs consistent with the possibility of no effect and the possibility of harm. oFollow‐up: one to three months (median) pBueno 2021; Han 2021; Palacios 2020; Tanriover 2021; Wu 2021a; Zhang 2021 qDespite some concerns with adequate randomisation, not downgraded for risk of bias. rFollow‐up: 4.1 months, 2 months (median), 3 months (median) sHan 2021; Palacios 2020; Tanriover 2021; Wu 2021a
Summary of findings 9. WIBP‐CorV – Sinopharm‐Wuhan compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with WIBP‐CorV | |||||
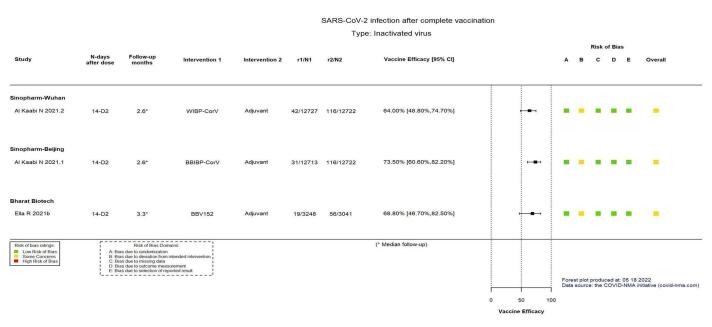
| Confirmed SARS‐CoV‐2 infectionb | 912 per 100,000 | 328 per 100,000 (231 to 467) |
VE 64.00 (48.80 to 74.70) |
25,449 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| Confirmed symptomatic COVID‐19b | 746 per 100,000 | 203 per 100,000 (131 to 313) |
VE 72.80 (58.10 to 82.40) |
25,480 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| Severe or critical COVID‐19 | Outcome not yet measured or reported | |||||
| All‐cause mortality | — | — | — | — | — | 1 trial reported on this outcome in 26,917 participants (13,464 WIBP‐CorV versus 13,453 placebo) (Al Kaabi 2021). There were no events in either group and no effect estimate could be calculated for this outcome. |
| Systemic reactogenicity eventse | 278 per 1000 | 275 per 1000 (264 to 286) | RR 0.99 (0.95 to 1.03) | 27,029 (2 RCTs)f | ⊕⊕⊕⊕ Highg | — |
| Any adverse eventh | 504 per 1000 | 484 per 1000 (469 to 494) | RR 0.96 (0.93 to 0.98) | 27,029 (2 RCTs)f | ⊕⊕⊕⊕ High | — |
| Serious adverse eventsi | 579 per 100,000 | 480 per 100,000 (347 to 665) | RR 0.83 (0.60 to 1.15) | 27,029 (2 RCTs)f | ⊕⊕⊖⊖ Lowg,j | — |
| Local reactogenicity eventsk | 290 per 1000 | 255 per 1000 (247 to 267) | RR 0.88 (0.85 to 0.92) | 27,029 (2 RCTs)f | ⊕⊕⊕⊕ Highg | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 4 May 2022 bFollow‐up: from 2 weeks after the second dose up to 2.6 months (median) cAl Kaabi 2021 dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eFollow‐up: seven days and 28 days fAl Kaabi 2021; Guo 2021 gDespite some concerns with adequate randomisation, not downgraded for risk of bias. hFollow‐up: one month iFollow‐up: 1.6 and 2.6 months (median) jImprecision: downgraded two levels due to wide CIs consistent with the possibility of no effect and the possibility of benefit and few events. kFollow‐up: seven days
Summary of findings 10. BBIBP‐CorV – Sinopharm‐Beijing compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with BBIBP‐CorV | |||||
| Confirmed SARS‐CoV‐2 infectionb | 912 per 100,000 | 242 per 100,000 (162 to 359) |
VE 73.50 (60.60 to 82.20) |
25,435 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| Confirmed symptomatic COVID‐19b | 746 per 100,000 | 163 per 100,000 (102 to 263) |
VE 78.10 (64.80 to 86.30) |
25,463 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| Severe or critical COVID‐19 | Outcome not yet measured or reported | |||||
| All‐cause mortality | — | — | — | — | — | 1 study reported this outcome in 26,924 participants (13,471 BBIBP‐CorV versus 13,453 placebo) (Al Kaabi 2021). There were no events in either group and no effect estimate could be calculated for this outcome. |
| Systemic reactogenicity eventse | 274 per 1000 | 288 per 1000 (236 to 351) | RR 1.05 (0.86 to 1.28) | 27,540 (3 RCTs)f | ⊕⊕⊕⊖ Moderateg | — |
| Any adverse eventh | 3 studies (n = 27,540) reported any adverse event with 1 month or 2.9 months' follow‐up. 2 of the studies reported an effect estimate in favour of BBIBP‐CorV: 1 with RR 0.91, 95% CI 0.89 to 0.94; n = 26,924; and 1 with CIs crossing the line of no effect (RR 0.83, 95% CI 0.36 to 1.95; n = 112). 1 study reported an effect estimate in favour of placebo with CIs not crossing the line of null effect (RR 2.05, 95% CI 1.47 to 2.87; n = 504) | — | 26,924 (3 RCTs)f | ⊕⊕⊖⊖ Lowi,j | — | |
| Serious adverse eventsk | 580 per 100,000 | 441 per 100,000 (313 to 615) | RR 0.76 (0.54 to 1.06) | 26,924 (1 RCT)c | ⊕⊕⊖⊖ Lowl | 1 additional study reported this outcome in 112 participants (84 BBIBP‐CorV versus 28 placebo) (Xia 2020). There were no events in either group and the trial did not contribute to the effect estimate. |
| Local reactogenicity eventse | 3 studies (n = 27,540) reported local adverse events with 7 days' follow‐up. 1 study reported an effect estimate in favour of BBIBP‐CorV: RR 0.71, 95% CI 0.68 to 0.74; n = 26,924. 2 studies reported an effect estimate in favour of placebo with CIs not crossing the line of null effect (RR 10.00, 95% CI 2.36 to 42.34; n = 504 and RR 3.33, 95% CI 0.45 to 24.89; n = 112). | — | 26,924 (3 RCTs)f | ⊕⊕⊖⊖ Lowi,j | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 4 May 2022 bFollow‐up: from 2 weeks after second dose up to 2.6 months (median) cAl Kaabi 2021 dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eFollow‐up: seven days fAl Kaabi 2021; Xia 2021 (children); Xia 2020 gImprecision: downgraded one level due to wide CIs consistent with the possibility of no effect and the possibility of harm. hFollow‐up: one month and 2.9 months iInconsistency: downgraded one level as studies are not pooled, effect estimates and direction of effect inconsistent between included studies. jImprecision: downgraded one level due to wide CIs consistent with the possibility of benefit and the possibility of harm. kFollow‐up: 2.6 months (median) lImprecision: downgraded two levels due to wide CIs consistent with the possibility of no effect and the possibility of benefit and few events.
Summary of findings 11. BBV152 – Bharat Biotech compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with BBV152 | |||||
| Confirmed SARS‐CoV‐2 infectionb | 1841 per 100,000 | 575 per 100,000 (322 to 982) |
VE 68.80 (46.70 to 82.50) |
6289 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| Confirmed symptomatic COVID‐19b | 1247 per 100,000 | 277 per 100,000 (170 to 434) |
VE 77.80 (65.20 to 86.40) |
16,973 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| Severe or critical COVID‐19b | 176 per 100,000 | 12 per 100,000 (0 to 76) |
VE 93.40 (57.10 to 99.80 |
16,976 (1 RCT)c | ⊕⊕⊕⊕ Highd | — |
| All‐cause mortalitye | 78 per 100,000 | 39 per 100,000 (13 to 113) | RR 0.50 (0.17 to 1.46) | 25,753 (1 RCT)c | ⊕⊕⊖⊖ Lowf | — |
| Systemic reactogenicity eventsg | 20 per 1000 | 26 per 1000 (23 to 31) | RR 1.34 (1.15 to 1.58) | 25,925 (2 RCTs)h | ⊕⊕⊕⊕ Highd | — |
| Any adverse eventi | 124 per 1000 | 124 per 1000 (117 to 133) | RR 1.00 (0.94 to 1.07) | 25,753 (1 RCT)j | ⊕⊕⊕⊕ High | — |
| Serious adverse eventsi | 463 per 100,000 | 301 per 100,000 (199 to 449) | RR 0.65 (0.43 to 0.97) | 25,928 (1 RCT)j | ⊕⊕⊕⊕ Highd | 1 additional trial reported this outcome in 175 participants (100 BBV152 versus 75 placebo) (Ella 2021a). There were no events in either group and the trial did not contribute to the pooled effect estimate. |
| Local reactogenicity eventsg | 31 per 1000 | 34 per 1000 (30 to 39) | RR 1.08 (0.95 to 1.24) | 25,750 (2 RCTs)h | ⊕⊕⊕⊕ Highd | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 4 May 2022 bFollow‐up: from two weeks after second dose to 3.3 months (median) cElla 2021a dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eFollow‐up: 3.3 months (median) fImprecision: downgraded two levels due to wide CIs consistent with the possibility of benefit and the possibility of harm and low number of events. gFollow‐up: seven days hElla 2021a; Ella 2021b iFollow‐up: 4.9 months (median) jElla 2021b
Summary of findings 12. NVX‐CoV2373 – Novavax compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with NVX‐CoV2373 | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
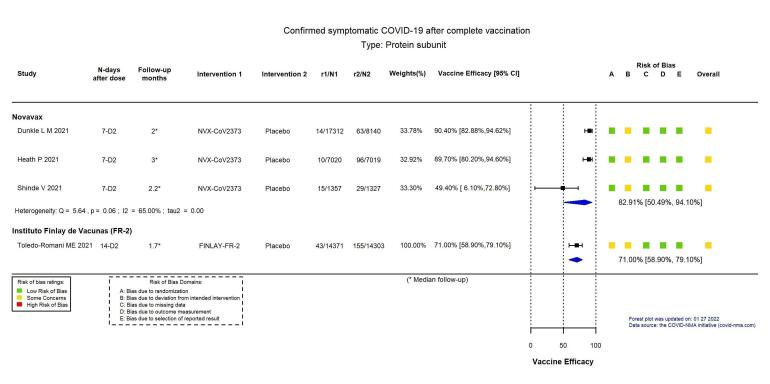
| Confirmed symptomatic COVID‐19b | 1140 per 100,000 | 195 per 100,000 (67 to 564) |
VE 82.91 (50.49 to 94.10) |
42,175 (3 RCTs)c | ⊕⊕⊕⊖ Moderated,e | Substantial heterogeneity (I² = 65%) between included studies: Dunkle 2021 (VE 90.40%, 95% CI 82.88 to 94.62%; n = 25,452); Heath 2021 (VE 89.70%, 95% CI 80.20% to 94.60%; n = 14,039); Shinde 2021 (VE 49.40%, 95% CI 6.10% to 72.80%; n = 2684) |
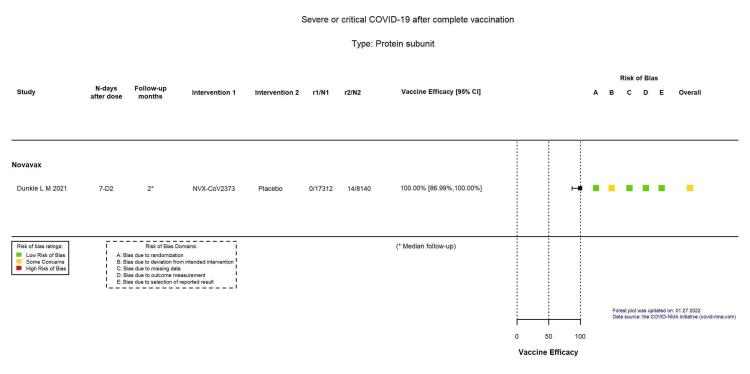
| Severe or critical COVID‐19 | 172 per 100,000 | 0 per 100,000 (0 to 22) | VE 100.00 (86.99 to 100.00) | 25,452 (1 RCT)f | ⊕⊕⊕⊖ Moderated,g | — |
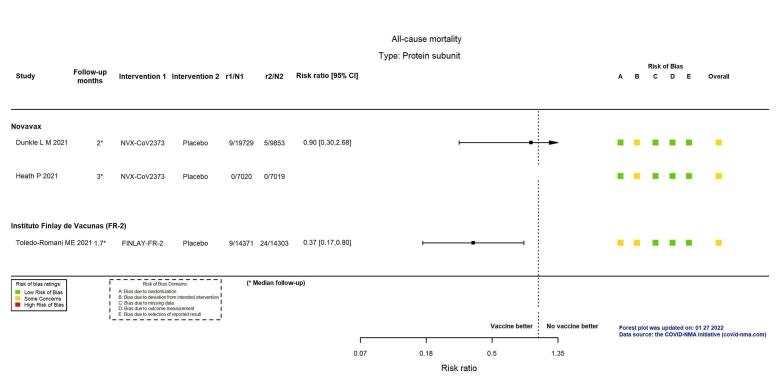
| All‐cause mortalityh | 51 per 100,000 | 46 per 100,000 (15 to 136) | RR 0.90 (0.30 to 2.68) | 29,582 (1 RCT)f | ⊕⊕⊖⊖ Lowd,i | 1 additional study reported on this outcome in 14,039 participants (7020 NVX‐CoV2373 versus 7019 placebo) (Heath 2021). There were no events in either group and the trial did not contribute to the pooled effect estimate. |
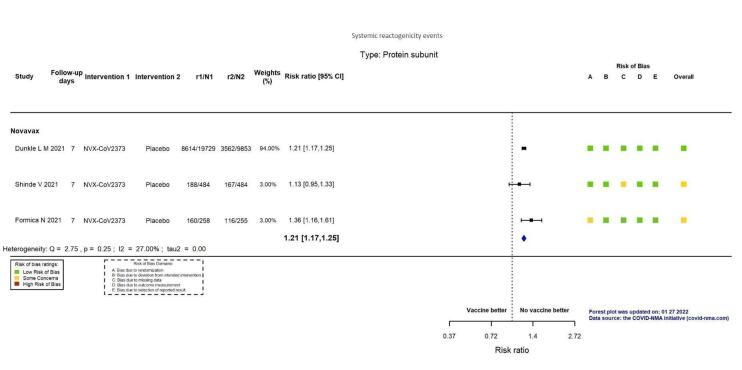
| Systemic reactogenicity eventsj | 363 per 1000 | 439 per 1000 (425 to 454) | RR 1.21 (1.17 to 1.25) | 31,063 (3 RCTs)k | ⊕⊕⊕⊕ Highl | — |
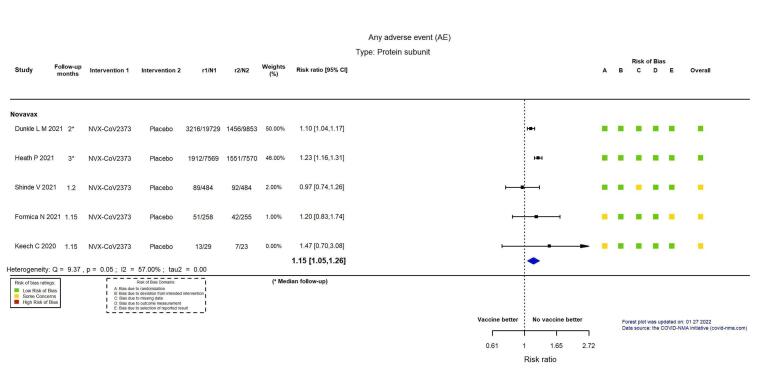
| Any adverse eventm | 173 per 1000 | 199 per 1000 (182 to 218) | RR 1.15 (1.05 to 1.26) | 46,231 (5 RCTs)n | ⊕⊕⊕⊖ Moderatel,o | Substantial heterogeneity (I² = 57%) between the 5 included studies. |
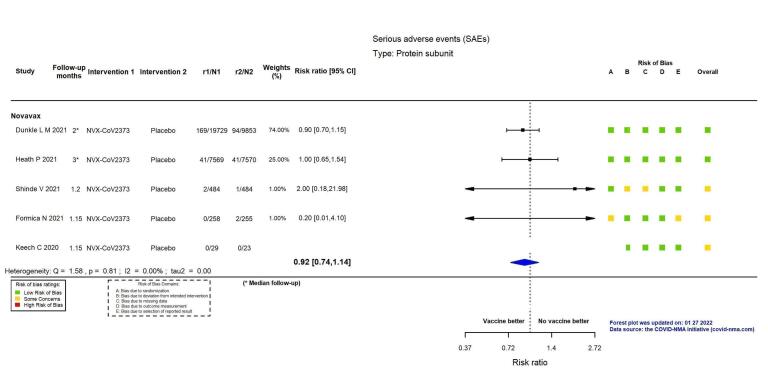
| Serious adverse eventsm | 777 per 100,000 | 715 per 100,000 (575 to 886) | RR 0.92 (0.74 to 1.14) | 38,802 (4 RCTs)p | ⊕⊕⊖⊖ Lowi,q | 1 additional trial reported on this outcome in 52 participants (29 NVX‐CoV2373 versus 23 placebo) (Keech 2020). There were no events in either group and the trial did not contribute to the pooled effect estimate. |
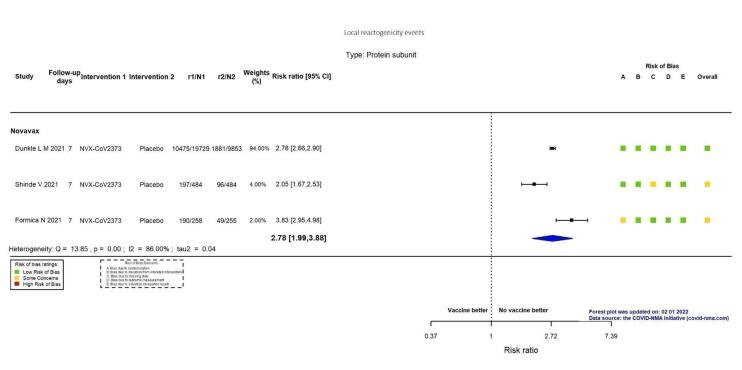
| Local reactogenicity eventsj | 191 per 1000 | 532 per 1000 (381 to 742) | RR 2.78 (1.99 to 3.88) | 31,063 (3 RCTs)k | ⊕⊕⊕⊕ Highl,r | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 2 June 2022 bFollow‐up: from seven days after second dose up to three months (median) cDunkle 2021; Heath 2021; Shinde 2021 dDespite some concerns with deviations from intervention, not downgraded for risk of bias. eInconsistency: downgraded one level (I² = 65%). fDunkle 2021 gIndirectness: downgraded one level as outcome in this trial included participants with moderate severity. hFollow‐up: two months (median) iImprecision: downgraded two levels due to wide CIs consistent with the possibility of benefit and the possibility of harm and few events. jFollow‐up: seven days kDunkle 2021; Frenck 2021; Shinde 2021 lDespite some concerns with adequate randomisation and missing data, not downgraded for risk of bias. mUnsolicited adverse events, follow‐up to three months (median) nDunkle 2021; Formica 2021; Heath 2021; Keech 2020; Shinde 2021 oInconsistency: downgraded one level (I² = 57%). pDunkle 2021; Formica 2021; Heath 2021; Shinde 2021 qDespite some concerns with adequate randomisation, deviation from intended intervention and missing data, not downgraded for risk of bias. rDespite I² = 86%, not downgraded for inconsistency, as the same direction of effect in both effect estimates.
Summary of findings 13. FINLAY‐FR‐2 – Instituto Finlay de Vacunas compared to placebo for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with FINLAY‐FR‐2 | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19b | 1084 per 100,000 | 314 per 100,000 (226 to 445) |
VE 71.00 (58.90 to 79.10) |
28,674 (1 RCT)c | ⊕⊕⊕⊖ Moderated | — |
| Severe or critical COVID‐19 | Outcome not yet measured or reported | |||||
| All‐cause mortalitye | 168 per 100,000 | 62 per 100,000 (29 to 134) | RR 0.37 (0.17 to 0.80) | 28,674 (1 RCT)c | ⊕⊕⊕⊖ Moderated | — |
| Systemic reactogenicity events | Outcome not yet measured or reported | |||||
| Any adverse event | Outcome not yet measured or reported | |||||
| Serious adverse events | Outcome not yet measured or reported | |||||
| Local reactogenicity events | Outcome not yet measured or reported | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 6 May 2022 bFollow‐up: from seven days after second dose up to three months (median) cToledo‐Romani 2021 dRisk of bias downgraded one level: some concerns regarding adequate randomisation and deviation from intended intervention. eFollow‐up: 1.7 months (median)
Summary of findings 14. Heterologous vaccination scheme compared to homologous vaccination scheme for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence (GRADE) | Comments | |
| Risk with homologous vaccination scheme | Risk with heterologous vaccination scheme | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19 | Outcome not yet measured or reported | |||||
| Severe or critical COVID‐19 | Outcome not yet measured or reported | |||||
| All‐cause mortality | Outcome not yet measured or reported | |||||
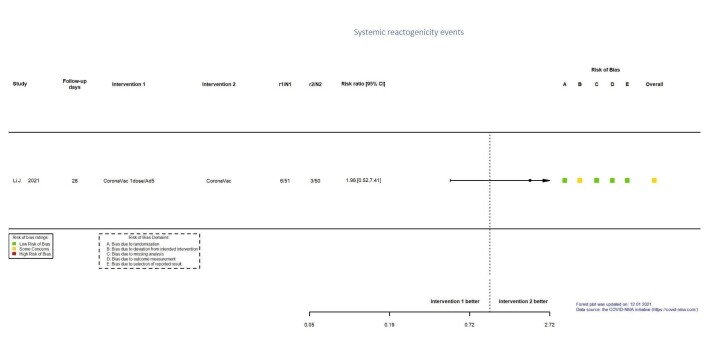
| Systemic reactogenicity eventsb | 60 per 1000 | 118 per 1000 (31 to 445) | RR 1.96 (0.52 to 7.41) | 101 (1 RCT)c | ⊕⊕⊖⊖ Lowd,e | — |
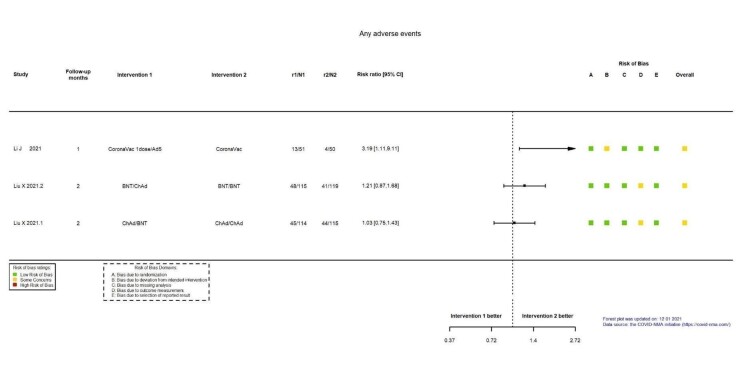
| Any adverse eventf | 3 studies (n = 564) that compared heterologous versus homologous vaccination schemes reported any adverse event with 1 or 2 months' follow‐up. 2 of the studies reported an effect estimate in favour of homologous scheme but with CIs crossing the line of no effect (RR 1.21, 95% CI 0.87 to 1.68; n = 234; and RR 1.03, 95% CI 0.75 to 1.43; n = 229). 1 study reported an effect estimate in favour of homologous scheme with CIs not crossing the line of null effect (RR 3.19, 95% CI 1.11 to 9.11; n = 101) | — | (3 RCTs)g | ⊕⊖⊖⊖ Very lowh,i,j | — | |
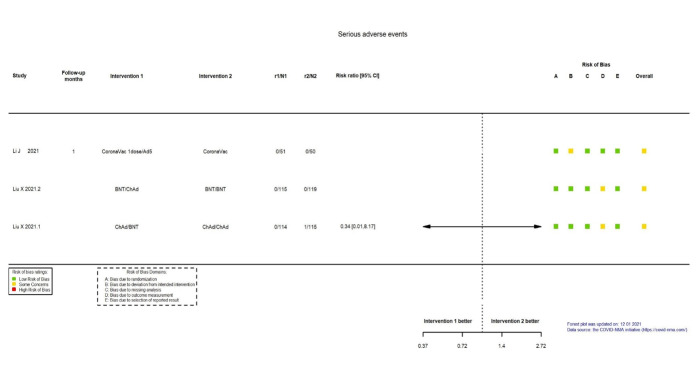
| Serious adverse eventsk | 1 study (Liu 2021: ChAdOx1/BNT162b2 versus ChAdOx1/ChAdOx1) that compared heterologous versus homologous vaccination schemes reported no serious adverse events in the heterologous scheme (0/114) versus 1 serious adverse event (1/115) in the homologous scheme (RR 0.34, 95% CI 0.01 to 8.17). 2 more studies reported the outcome, with 0 events in both groups: Li 2021a: CoronaVac/Ad5 versus CoronaVac/CoronaVac in n = 51 versus n = 50 and Liu 2021: BNT162b2/ChAdOx1 versus BNT162b2/BNT162b2 in n = 115 versus n = 119 respectively, in heterologous versus homologous scheme | — | 229 (1 RCT)l | ⊕⊖⊖⊖ Very lowh,m | — | |
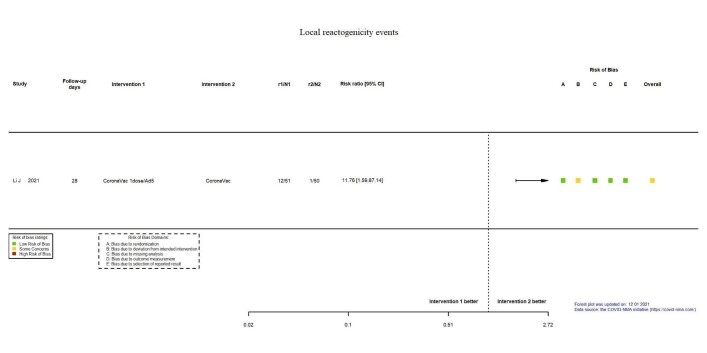
| Local reactogenicity eventsb | 20 per 1000 | 235 per 1000 (32 to 1000) | RR 11.76 (1.59 to 87.14) | 101 (1 RCT)c | ⊕⊕⊖⊖ Lowd,n | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 4 May 2022 bFollow‐up: 28 days cLi 2021a: CoronaVac/Ad5 versus CoronaVac/CoronaVac dDespite some concerns with deviation from intended intervention, not downgraded for risk of bias. eImprecision: downgraded two levels due to wide CIs consistent with the possibility of benefit for heterologous and benefit for homologous vaccination scheme and the low number of events/participants. fFollow‐up: one and two months gLi 2021a: CoronaVac/Ad5 versus CoronaVac/CoronaVac; Liu 2021: BNT162b2/ChAdOx1 versus BNT162b2/BNT162b2; Liu 2021: ChAdOx1/BNT162b2 versus ChAdOx1/ChAdOx1 hRisk of bias downgraded one level: some concerns regarding outcome measurement. iInconsistency: downgraded one level as studies are not pooled, effect estimates and direction of effect inconsistent between included studies. jImprecision: downgraded one level due to wide CIs consistent with the possibility of no effect and benefit for homologous vaccination scheme and the low number of events/participants. kFollow‐up: one month lLiu 2021: ChAdOx1/BNT162b2 versus ChAdOx1/ChAdOx1 mImprecision: downgraded two levels due to wide CIs consistent with the possibility of benefit for the heterologous and benefit for homologous vaccination scheme and the low number of events/participants. nImprecision: downgraded two levels due to very few events or participants (or both).
Summary of findings 15. Booster compared to placebo/no booster for vaccination against COVID‐19a.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no booster | Risk with booster | |||||
| Confirmed SARS‐CoV‐2 infection | Outcome not yet measured or reported | |||||
| Confirmed symptomatic COVID‐19 | Outcome not yet measured or reported | |||||
| Severe or critical COVID‐19 | Outcome not yet measured or reported | |||||
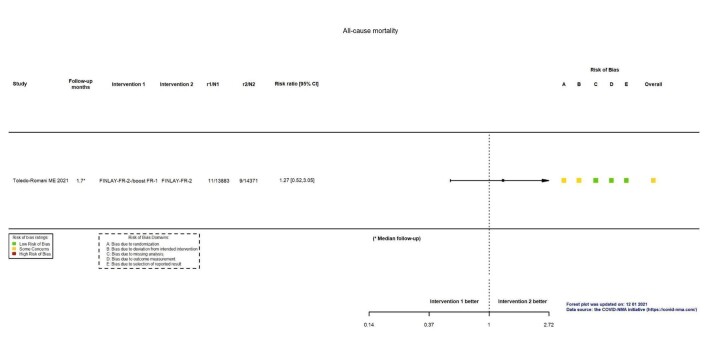
| All‐cause mortalityb | 63 per 100,000 | 80 per 100,000 (33 to 191) | RR 1.27 (0.52 to 3.05) | 28,254 (1 RCT)c | ⊕⊖⊖⊖ Very lowd,e | — |
| Systemic reactogenicity eventsf | 102 per 1000 | 183 per 1000 (72 to 464) | RR 1.80 (0.71 to 4.56) | 119 (1 RCT)g | ⊕⊕⊖⊖ Lowd | — |
| Any adverse event | Outcome not yet measured or reported | |||||
| Serious adverse events | Outcome not yet measured or reported | |||||
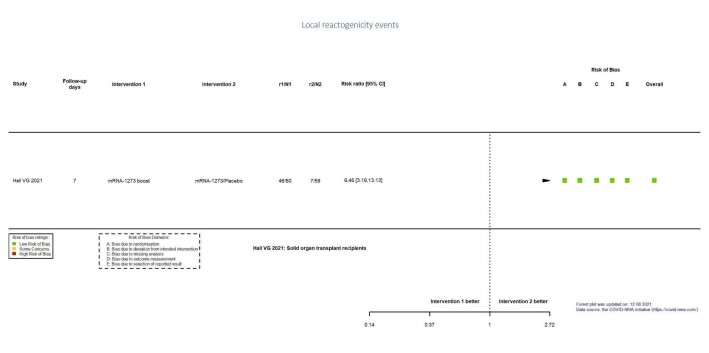
| Local reactogenicity eventsf | 119 per 1000 | 766 per 1000 (377 to 1000) | RR 6.46 (3.18 to 13.13) | 119 (1 RCT)g | ⊕⊕⊕⊖ Moderateh | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). COVID‐19: coronavirus disease 2019 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLast updated: 4 May 2022 bFollow‐up: 1.7 months (median) cToledo‐Romani 2021: FINLAY‐FR‐2/booster FR‐1 versus FINLAY‐FR‐2 dImprecision: downgraded two levels due to wide CIs consistent with the possibility of benefit and the possibility of harm and few events. eRisk of bias downgraded one level: some concerns regarding adequate randomization and deviation from intended intervention. fFollow‐up: seven days gHall 2021: mRNA‐1273 booster versus placebo (solid organ transplant recipients). hImprecision: downgraded one level due to low number of participants.
Background
Description of the condition
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) outbreak began in Wuhan, Hubei Province, China. SARS‐CoV‐2 began to spread worldwide, and on 11 March 2020, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID‐19) a pandemic (WHO 2020a).
In many countries, the number of cases increased exponentially during the first and subsequent waves (Worldometer 2022). The clinical spectrum of COVID‐19 ranges from mild to critical, and approximately 15% to 30% of patients infected with the wild‐type variant of SARS‐CoV‐2 experienced acute respiratory distress syndrome (Attaway 2021). Persons with underlying conditions and weakened immune systems were at higher risk of becoming severely sick (Formica 2021).
Further, genetic variants of SARS‐CoV‐2 have been emerging and circulating at a global level: B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta) variants, and more recently B.1.1.529 (Omicron) (WHO 2022a). Consequently, the WHO has developed a definition of variants of concern for molecular surveillance (WHO 2022a).
Intensive research and development of vaccines is currently underway to curtail the pandemic and prevent disease outbreaks that could overwhelm health systems worldwide (van Riel 2020; WHO 2022b).
Description of the intervention
Vaccines exploit the ability of the immune system to respond to and remember encounters with pathogenic antigens. COVID‐19 vaccine development, aimed at conferring protection against infection, or symptomatic disease, or both, has been accelerated due to priority funding over other diseases.
Different vaccine platform technologies (i.e. technologies that have in common the use of a ‘backbone’ carrier or vector) are being, and have been tested: live attenuated virus vaccines or inactivated virus vaccines (either inactivated whole or altered pathogens); protein‐based vaccines (protein subunits or virus‐like particles); viral vector vaccines (non‐replicating viral vector, replicating viral vector); and nucleic acid‐based vaccines (DNA‐ and RNA‐based vaccines)(Abbasi 2020).
Vaccines may be categorized as either live or non‐live (CDC 2021), distinguishing those vaccines that contain an attenuated (live) form of the pathogen from those that harbour the killed (inactivated, non‐live) version of the pathogen. Non‐live vaccines predominantly induce humoral immunity, whereas live vaccines create a robust cellular and humoral response. The present review includes 12 vaccines within four different non‐live vaccine platform technologies.
-
Inactivated virus vaccines
CoronaVac
WIBP‐CorV
BBIBP‐CorV
BBV152
-
Protein subunit vaccines
NVX‐CoV2373
FINLAY‐FR‐2
-
Viral vector (non‐replicating) vaccines
ChAdOx1
Ad26.COV2.S
Gam‐COVID‐Vac
-
Nucleic acid‐based (RNA) vaccines
BNT162b2
mRNA‐1273
CVnCoV
How the intervention might work
Vaccines aim to generate an immune response that prevents SARS‐CoV‐2 infection or reduces the risk of severe disease or death.
Live attenuated virus vaccines
Live attenuated virus vaccines use a weakened form of the virus and are developed so that in an immunocompetent host, they replicate sufficiently to generate a robust immune response (Pollard 2021). Live attenuated vaccines may potentially replicate in an uncontrolled manner in immunosuppressed individuals, thus rendering them less suitable for use within this population (Rubin 2013).
Inactivated virus vaccines
In contrast, inactivated vaccines contain either inactivated whole or altered pathogens, thus precluding their replication; however, inactivated vaccines do not always induce as strong or long‐lasting an immune response as live attenuated vaccines.
Inactivated virus technologies present multiple viral proteins for immune recognition. They have a stable expression of conformation‐dependent antigenic epitopes (Roper 2009). Pitfalls include their potential to alter viral epitopes, which may adversely affect immunogenicity if the native structure of the viral antigen is not maintained (DeZure 2016). As a result, the administration of multiple doses, booster injections, or adjuvant addition is often needed to elicit protective humoral immune responses(Pollard 2021).
Protein subunit vaccines are composed of fragments of the virus. Akin to inactivated whole‐cell vaccines, protein subunit vaccines do not harbour live components of the pathogen. They are distinguished from inactivated whole‐cell vaccines by containing only the necessary antigenic parts of the pathogen for mounting a protective immune response. As the subunit vaccine only relies on the antigen of interest made using recombinant technology, it is considered a more reliable and safer technique than inactivated vaccines(Dong 2020). Nevertheless, this advantage may be offset by its inability to display the virus's full antigenic complexity. This may cause an unbalanced immune response and lower its protective effect (Enjuanes 2016). Consequently, adjuvants may be required to boost immune responses and increase immunogenicity.
Several other platforms have developed over the past few decades. These include virus‐like particles, viral vectors, nucleic acid‐based RNA and DNA vaccines (Pollard 2021), all of which have been employed in COVID‐19 vaccine development.
Virus‐like particle (VLP) vaccines contain virus‐like particles which closely resemble viruses, but are non‐infectious as they contain no viral genetic material (Oxford Vaccine Group 2020). This platform has been used against hepatitis B and human papillomavirus (HPV), and constitutes another protein‐based vaccine composed of proteins from the viral capsid (Fuenmayor 2017). VLP vaccines consist of self‐assembled viral structural proteins that mimic the conformation of native SARS‐CoV virions (Mortola 2004), making them immunogenic and inducing highly neutralizing‐antibody titres. In light of their non‐replicating and non‐infectious constructs, VLPs may have an enhanced safety profile.
Unlike previous vaccines, viral vectors and nucleic acid‐based RNA and DNA vaccines do not contain antigens, but rather nucleic acid sequences (RNA or DNA) that code for the proteins of interest inside the organism (Pollard 2021).
Viral vector vaccines
They differ from most conventional vaccines because they do not contain antigens (Gavi 2020). They are generally constructed from a carrier virus, such as an adeno‐ or pox‐virus, and are engineered to carry the key target for COVID‐19 vaccines (Dong 2020). Whilst vector vaccines confer the key advantage of including the innate immune responses required for eliciting adaptive immune responses, a potential disadvantage is that the host may already possess immunity against the vector due to prior exposure, thus reducing its effect (Pollard 2021). However, this disadvantage does not exist for all vectors. If the anti‐vector response is likely to interfere with the efficacy induced by adenovirus vectors widely used for SARS‐CoV‐2 vaccines, this is not the case with Pox virus vectors (Dong 2020).
Nucleic acid‐based vaccine – mRNA vaccine
Whilst mRNA vaccines are considered a new type of vaccine (CDC 2021), this platform has garnered interest among researchers for decades. The mechanism of action of mRNA vaccines is to instruct cells how to make a protein that may trigger an immune response (CDC 2021). mRNA translation occurs in the host cell's cytosol, circumventing the risk of integration into the host genome (CDC 2021). Like viral vectors, mRNA vaccines induce dendritic cell sensing – mRNA can stimulate TLR7, thus avoiding the use of adjuvants. Like viral vectors, attenuated vaccines and DNA vaccines, these vaccines can induce a CD8 T cell response. Finally, RNAs rapidly destroy mRNAs in the extracellular medium; these vaccines must be encapsulated.
Nucleic acid‐based vaccine – DNA vaccine
DNA vaccine candidates function by injecting a plasmid containing the DNA sequence encoding a SARS‐CoV‐2 antigen which will stimulate the immune response. Due to the biocompatibility of plasmid DNA, their cost‐efficient production and long shelf life, DNA vaccine‐based immunotherapeutic strategies have been developed for treatment of infections (Hobernik 2018). However, their disadvantage is that the DNA molecules must cross the nuclear membrane to be transcribed, and they generally have low immunogenicity (Dong 2020).
These vaccines are used systemically (usually intramuscular injection), but mucosal SARS‐CoV‐2 vaccines are under development. This type of vaccine is predicted to have a better efficacy against infection. Apart from COVID‐19, only one vaccine used via the nasal route has been approved to date: an attenuated vaccine against the influenza virus.
Why it is important to do this review
Given the importance to global health and the increasing number of vaccine candidates now being tested in phase 2 and phase 3 trials, there is a need to produce and maintain a living synthesis of the efficacy and safety of COVID‐19 vaccines.
This review is part of a larger project: the COVID‐NMA initiative (Boutron 2020a). The COVID‐NMA initiative provides decision‐makers with a complete, high‐quality, and up‐to‐date mapping and synthesis of evidence on interventions for preventing and treating COVID‐19. We developed a master protocol on the effect of all interventions for preventing and treating COVID‐19 (Boutron 2020b), followed by specific protocols for more specific questions. Our results are made available and updated bi‐weekly on the COVID‐NMA platform at covid-nma.com.
We followed the PRISMA guidelines (Page 2021). The protocol is available at doi.org/10.5281/zenodo.6458272 and registered on PROSPERO (www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021271897). It was peer‐reviewed and processed by Cochrane's Central Editorial Service.
This review will be updated as soon as new evidence changes the conclusions or certainty of the evidence of the review, or at least twice a year if no substantial changes occur.
Objectives
To assess the efficacy and safety of COVID‐19 vaccines (as a full primary vaccination series or as a booster dose) against SARS‐CoV‐2.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel individually or cluster‐randomized controlled trials (RCTs) evaluating COVID‐19 vaccines in humans with no restrictions on language. Single‐arm studies, non‐randomized studies, and modelling studies of interventions for COVID‐19 were not eligible to be included in the review.
Types of participants
We included individuals with no restriction on age and comorbidities, irrespective of their serological status at baseline.
Types of interventions
Eligible interventions included any COVID‐19 vaccines, particularly:
live attenuated virus vaccine;
inactivated virus vaccine;
protein subunit vaccine;
virus‐like particle (VLP) vaccine;
non‐replicating viral vector (e.g. recombinant adenovirus) vaccine;
replicating viral vector vaccine;
RNA‐based vaccine.
DNA‐based vaccine;
Other vaccine types for COVID‐19, if any.
In the analysis, we included only results for vaccine candidates with a selected dose evaluated in phases 2‐3 or phase 3 trials and their corresponding early phases.
Comparators included placebo (placebo could consist of saline placebo, injecting only the vaccine adjuvant or injecting a vaccine protecting against other diseases, such as meningococcal conjugate vaccine), no vaccine, or another COVID‐19 vaccine.
Types of outcome measures
Our outcomes were identified with content experts, considering the outcomes most frequently evaluated in the registered RCTs, and after consulting the main outcomes recommended by the US Food and Drug Administration (FDA) guidance for developing a vaccine (FDA 2020a).
Efficacy outcomes
Incidence of confirmed SARS‐CoV‐2 infection after complete vaccination (all doses of the primary vaccination schedule)*
Incidence of confirmed symptomatic COVID‐19 after complete vaccination
Severe or critical COVID‐19 after complete vaccination, as reported by authors (a table summarising the definitions used in each study can be found in Appendix 1)
All‐cause mortality
*confirmed by reverse transcription polymerase chain reaction (RT‐PCR), nucleic acid amplification testing (NAAT), or any other validated test.
Safety outcomes
Incidence of systemic reactogenicity events (i.e. the immediate short‐term reactions of a system to vaccines mainly due to immunological responses, such as fever) reported at day 14 after first dose.
When the number of participants with at least one systemic reactogenicity event is not reported, we used proxy measures as follows.
For adults: the number of participants with malaise as first choice, headache as second choice, and fever 37.5 °C or greater as third choice;
For children: irritability as first choice, decreased activity/weakness as second choice, and fever 37.5 °C or greater as third choice.
Incidence of any adverse event (including non‐serious adverse events). We considered any adverse event reported by authors, prioritizing 'solicited' adverse events. However, when these were not available, we collected 'unsolicited' adverse events.
Incidence of any serious adverse events (SAEs) as reported by authors (a table reporting the definitions used in each study can be found in Appendix 1).
Immunogenicity outcomes
Geometric mean titre (GMT) of a specific antibody against SARS‐CoV‐2 (two weeks after the first dose or nearest follow‐up, as mentioned in the manuscript)
GMT of a neutralizing antibody against SARS‐CoV‐2 (two weeks after second dose or nearest follow‐up, as mentioned in the manuscript)
Cellular immune responses (i.e. interferon gamma (IFN‐γ) enzyme‐linked immunospot (ELISpot)) (any time point reported by authors)
Specific safety outcomes
Incidence of local reactogenicity events (i.e. the immediate local short‐term reactions of a system to vaccines mainly due to immunological responses, such as pain and swelling) reported at day seven after first dose.
When the number of participants with at least one local adverse event is not reported, we used as a proxy measure pain as the first choice, local swelling/induration as the second choice, and erythema (redness) as the third choice.
Incidence of specific safety outcomes
Cardioembolic events (i.e. pulmonary embolism, stroke, venous thrombosis, cavernous sinus thrombosis, pericarditis, myocardial infarction)
Haematological events (i.e. thrombocytopenia, haemorrhage, neutropenia, anaemia, lymphadenopathy)
Neurological events (i.e. nervous system diseases)
Vaccine‐enhanced disease
Note: as the start of follow‐up (T0) varies (e.g. follow‐up starts "14 days after the last dose" or "21 days after the first dose"), we systematically recorded the T0 considered in the study report. For safety outcomes, we considered T0 = time the first dose is injected when the comparison is vaccine versus placebo/no vaccine; T0 = time after the second dose when the comparison focuses on heterologous vaccination; and T0 = time after the booster or placebo when the comparison assessed the booster dose. We systematically recorded the follow‐up duration for the outcomes considered. When the same outcome was recorded at several time points, we recorded the latest.
For specific antibodies against SARS‐CoV‐2, we considered T0 = 2 weeks after the first dose where available, or the nearest time point.
For neutralizing antibodies against SARS‐CoV‐2, we considered T0 = 2 weeks after the second dose where available, or the nearest time point.
Search methods for identification of studies
We used the search strategies defined in the protocol of the larger COVID‐NMA initiative (covid-nma.com) (Boutron 2020b), and outlined in Appendix 2 to identify randomized trials evaluating vaccines for COVID‐19. The search methods and strategies to identify records for this review are being revised approximately yearly, to ensure that they reflect any terminology changes in the topic area, or in the databases.
Electronic searches
The Epistemonikos L·OVE COVID‐19 platform was searched regularly from 4 September 2020 until 5 November 2021 (Epistemonikos) (app.iloveevidence.com/covid19). This platform is a digital repository built by systematic searches in multiple databases, trial registries and preprint servers. Complete data sources and search methods are available at: app.iloveevidence.com/covid19/methods.
The Cochrane COVID‐19 Study Register has been searched on a regular basis (covid-19.cochrane.org/; last searched 5 November 2021). The Cochrane COVID‐19 Study Register is a specialized register built within the Cochrane Register of Studies (CRS) and is maintained by Cochrane Information Specialists. The register contains study reports from several sources, including:
daily searches of PubMed;
daily searches of ClinicalTrials.gov;
weekly searches of Embase.com;
weekly searches of the WHO International Clinical Trials Registry Platform (ICTRP);
weekly searches of medRxiv;
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL).
Complete data sources and search methods for the register are available at: community.cochrane.org/about-covid-19-study-register.
We also searched the Retraction Watch Database for retracted studies (retractionwatch.com/retracted-coronavirus-covid-19-papers/; last searched 5 November 2021).
We also systematically searched for updates or publications of preprints using a preprint tracker, developed in collaboration with a research team from the French National Centre for Scientific Research (CNRS) (Cabanac 2021).
Searching other resources
We searched the following trial registries for unpublished and ongoing studies.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/), to identify ongoing and completed clinical trials on COVID‐19 (last searched 3 November 2021). We used the List by Health Topic: 2019‐nCoV / COVID‐19 filter to retrieve all studies identified.
European Medicines Agency (EMA) clinical data website (clinicaldata.ema.europa.eu/web/cdp/home) to identify trials submitted to the EMA and also for the clinical study report (CSR) of eligible studies (last searched 5 November 2021).
FDA website (www.fda.gov) to identify FDA approval trials (last searched 5 November 2021).
Data collection and analysis
We search, screen and extract data weekly. The analysis is updated online every 2 weeks (covid-nma.com). The next update will be conducted soon after the publication of this review.
Selection of studies
We searched and screened the citations retrieved and used a spreadsheet to document search dates and citations identified. We identified duplicates in Rayyan (Ouzzani 2016), and then in a spreadsheet to enhance sensitivity. Two review authors (CR, HB) independently screened records and abstracts; a third review author (RA) resolved any disagreements.
We did not check the references of included reports as the living search process identifies COVID‐19 trial records prospectively from the point of trial registration.
Whenever both preprints and subsequent peer‐reviewed publications were available, we favoured the latter as they are the latest documents of trial findings (Boutron 2020b).
We retrieved CSRs for four vaccines (BNT162b2 – BioNtech/Fosun Pharma/Pfizer; mRNA‐123 – ModernaTX; ChAdOx1 – Astra Zeneca+University of Oxford; and AD26.COV2.S – Janssen Pharmaceutical Companies) from the EMA website (www.ema.europa.eu/en). For three vaccines (BNT162b2, mRNA‐123 – ModernaTX and Ad26.COV2.S), we found minor discrepancies when compared to the data reported in the peer‐reviewed publication. Discrepancies were due to different cut‐off dates and follow‐up lengths. We were unable to compare data between the CSR and the peer‐reviewed publication for one vaccine (ChAdOx1) since the publication reports pooled results for four trials (COV001, COV002, COV003, and COV005) and the CSR contains data for only two of them (COV002 and COV003).
Data extraction and management
All data were extracted in duplicate. Two review authors (HB, BB) independently read each preprint, publication, protocol, or other study reports, evaluated the completeness of the data, and assessed the risk of bias. Based on a pilot data extraction form, we designed, evaluated and modified a specific structured data extraction form whenever needed to ensure consistency in the extraction of information. The form was implemented on the COVID‐NMA platform on the extraction module explicitly developed for this purpose (covid-nma.com). All discrepancies automatically identified by the platform data extraction module were discussed by the two review authors to reach a consensus.
Information extracted included study characteristics (such as first author, publication year and journal), number of participants randomized, patient characteristics (age, sex, pre‐existing neutralizing or specific antibodies or participants seropositive, comorbidities), intervention details (type of vaccines, dosing, schedule and route of administration), outcome measures, and risk of bias assessment.
For dichotomous outcomes, we extracted the number of events and number of total participants in each study arm.
For efficacy outcomes, we extracted vaccine efficacy as reported by the authors and 95% confidence interval (CI) for each outcome, when available. Vaccine efficacy measures the percentage reduction in incidence of cases among vaccinated persons compared to unvaccinated persons. It is usually calculated as the incidence rate among unvaccinated – incidence rate among vaccinated / the incidence rate among unvaccinated.
For immunogenicity outcomes, we recorded GMTs and 95% CIs for specific and neutralizing antibodies in the control and intervention. We extracted results related to cellular response as reported by authors.
For safety outcomes, we extracted the data as analyzed by the authors.
We extracted the data as analyzed by the trial authors.
To explore vaccine efficacy on variants of concern, such as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529), we also took into account that:
vaccine efficacy on variants of concern is determined by sequencing all available cases where available;
-
study authors extrapolated vaccine efficacy on variants of concern
considering the prevalent variant during the study period
from other sources: the information was extrapolated from data on the prevalence of the variant in the population during the study period. This information was obtained from outbreak.info or other sources.
This was done only for critical outcomes of efficacy.
Assessment of risk of bias in included studies
We assessed each study with the Cochrane RoB 2 tool for randomized controlled trials (Sterne 2019). We assessed risk of bias for the critical outcomes of the review. We recorded judgements for each domain using the online data extraction tool we developed. Risk of bias was assessed independently, in duplicate with consensus by researchers with epidemiological training (currently 4 people) or Cochrane Response members (the number of people involved varies). All have been previously trained in clinical epidemiology and systematic reviews. All have participated in a training programme where they had to read the training material and perform data extraction and RoB assessments with a team of experienced researchers. The data quality was assessed by the Cochrane Bias Methods Group, who checked a random sample of 10% of the extracted reports.
The Cochrane RoB 2 tool is structured into five domains: 1) risk of bias arising from the randomisation process; 2) risk of bias due to deviations from intended interventions; 3) risk of bias due to missing outcome data; 4) risk of bias in the measurement of the outcome; and 5) risk of bias in the selection of the reported result. Within each domain, a series of 'signalling questions' elicit information relevant to the risk of bias assessment. The response options to the signalling questions are: "yes"; "probably yes"; "probably no"; "no"; and "no information." A risk of bias judgement for each domain is generated by an algorithm, based on answers to the signalling questions. Judgement can be 'low', ‘some concerns’ or 'high' risk of bias. Overall, risk of bias will be considered as "low risk of bias" if all domains are at 'low risk;' "some concerns" if at least one domain is of ‘some concern’ and no domains are 'high risk of bias;' and "high risk of bias" if there is at least one domain assessed as 'high risk,' or several domains with 'some concerns.' In the context of this review, we are interested in quantifying the effect of assignment to the interventions at baseline, regardless of whether the interventions are received as intended (i.e. the intention‐to‐treat effect).
For cluster‐randomized trials, if any, we planned to rely on the extension of the RoB tool 2 for cluster‐randomized trials. Particularly, we planned to add the domain 1b: risk of bias arising from the timing of identification or recruitment of participants in a cluster‐randomized trial. There were no cluster‐RCTs reported by the date of the last search.
While we relied on the signalling questions to assess each domain and justify our assessment, we did not record the answers of systematic reviewers or how consensus was obtained for the signalling questions; this was done only at the domain level.
The risk of bias assessment was considered part of an evaluation of the certainty of the evidence and sensitivity analysis.
Measures of treatment effect
For dichotomous outcomes, we used vaccine efficacy and risk ratio accompanied by the 95% CI as a measure of effect. For outcomes measured with GMTs, we calculated the geometric mean ratios (GMRs) by taking the anti‐log of the mean difference of the log transformed data between arms.
To date, all trials reported vaccine efficacy. In the future if we identify trials reporting only rate ratio, we will calculate vaccine efficacy using the formula rate ratio = 1 − VE/100.
Unit of analysis issues
We analyzed separately different comparisons from multiple‐arm trials for all pairwise meta‐analyses.
Dealing with missing data
For missing outcome data, we extracted the number of participants who dropped out before the completion of the study, and how the study authors handled missing data. We assessed the appropriateness of any imputation methods used to account for early dropouts in our risk of bias assessments. We conducted sensitivity analysis to assess the potential impact of missing outcome data on the results.
Assessment of heterogeneity
We first generated descriptive statistics for study and population characteristics, and we examined the distribution of important clinical and methodological variables (such as age, immunocompromized status, location etc.). We have considered the variability in point estimates and the overlap in CIs in addition to the I2 statistic to assess the level of statistical heterogeneity (Riley 2011).
Assessment of reporting biases
We assessed the selective non‐reporting or under‐reporting of results in the trials identified according to the framework proposed in Chapter 13 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Assessment of risk of bias due to missing results in the included studies
We checked whether the results of all our critical and important outcomes were reported as prespecified in the first version of the trial registry. When more than one version was available and the outcomes were modified, we checked the date of the modification using “history of changes.” Of note, some platforms do not provide information about previous versions of the registers. In these cases, we could not know whether we were assessing the original. When registration was not prospective, we also checked the protocol or statistical analysis plan if available.
We used a matrix indicating the availability of study results as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021; Kirkham 2018).
We evaluated whether results were unavailable because of the results' P value, magnitude, or direction. We considered the risk of bias due to missing results if an outcome specified in the registry was missing from the main report.
Due to the small number of trials, we could not assess the potential for reporting bias across studies graphically or statistically.
Data synthesis
We analyzed each type of vaccine separately. We combined trials with comparators as placebo or adjuvant or other control together under the same comparison at the specific vaccine level. We included all eligible RCTs in the primary analysis, whatever the risk of bias assessment. We included early‐phase trials in the analysis only when the selected dose was clearly defined and efficacy outcomes (usually assessed in Phase 3 trials) were available.
We performed a pairwise meta‐analysis and presented summary effect estimates with 95% CIs for each direct comparison, with at least two studies providing data. We used the random‐effects model to incorporate the anticipated clinical and methodological heterogeneity across studies. We presented trials reporting zero events in both arms in the forest plot but did not incorporate these in the analysis.
In the presence of excessive heterogeneity across studies (i.e. diverse forest plots or Tau2 > 75% quartile of empirical distributions, or both) (Turner 2012), we did not synthesize the trial data quantitatively but qualitatively unless we could set up homogeneous subsets of the available trials.
All analyses were undertaken with the statistical software environment R (version 4.0.3) using the packages metafor and meta (Balduzzi 2019; Viechtbauer 2010).
We initially planned to conduct a network meta‐analysis (NMA); however, the network of vaccines appeared very sparse, included mainly comparisons of vaccines against placebo, and only one or two studies informed most of the available comparisons (Figure 1). A network of such structure does not allow proper evaluation of the synthesis assumptions. Additionally, the NMA estimates from this network would not be substantially more precise (and could even be less precise for some comparisons) than the direct ones. We will reassess the feasibility of conducting an NMA regularly as part of the living systematic review process (details of the NMA methods considered for future update versions are available in Appendix 3).
1.
Network graph. The size of the nodes is proportional to the number of participants randomized and the thickness of the lines to the number of studies in each comparison.
Subgroup analysis and investigation of heterogeneity
We had planned to perform subgroup analyses for critical outcomes only (Boutron 2020b). For future updates, we will pursue our prespecified subgroup analyses to explore whether the following population characteristics explain sources of heterogeneity.
-
Age:
children or adolescents (aged less than 18 years);
adults (aged 18 to 59 years);
older adults (aged greater than 60 years).
-
Specific populations:
immunocompromized people;
pregnant women.
It should be noted that, as the evidence base on COVID‐19/SARS‐CoV‐2 and its variants continues to evolve, we will reassess the feasibility of performing these subgroup analyses in future updates of the review when we could also evaluate the impact of the different SARS‐CoV‐2 variants in a meta‐regression model.
For the current review, we assessed the level of heterogeneity by visual inspection of forest plots, the I2 statistic, the between‐study variance (Tau2), and prediction intervals.
Sensitivity analysis
We performed sensitivity analyses for critical outcomes only. We performed sensitivity analyses by excluding RCTs reported in preprint only and early‐phase trials (1 and 2). We also ran the analyses using the number of participants randomized instead of those analyzed for safety outcomes to assess the potential impact of missing outcome data on the results. For efficacy outcomes, it was not possible to calculate the effect estimate (vaccine efficacy) using the number of participants randomized. We did not perform the planned sensitivity analysis that excluded RCTs with an overall high risk of bias since no RCTs were considered at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
To evaluate the certainty of the evidence in the results of the pairwise comparisons for all outcomes except immunogenetic outcomes, overall certainty of the evidence for each outcome was assessed by one review author (KP) and cross‐checked by another review author (AJ) using the GRADE approach (Schünemann 2021). We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence. The assessment of imprecision was based on a non‐contextualized approach i.e. rating the certainty that there is any effect (Hultcrantz 2017; Zeng 2021a), with the null effect as the threshold for the critical outcomes of mortality and SAEs (Guyatt 2011). In the description of the results for each outcome, we use different thresholds for the size of the effects.
For outcomes reported as vaccine efficacy, we used a threshold of 30%, based on the WHO guidance document which indicated that the primary efficacy endpoint estimate for a placebo‐controlled trial should be at least 50%, with a statistical success criterion that the lower bound of the confidence interval be more than 30% (WHO 2020b; WHO 2020c). For additional adverse event outcomes (i.e. any adverse event, systemic reactogenicity events, and local reactogenicity events), we considered the thresholds for an effect to be RRs of 0.75 and 1.25 for downgrading imprecision.
For all‐cause mortality and SAEs, we considered the effect was "large" when the absolute difference was greater than 5%; there was a "slight" effect when the absolute difference was from 1% to 5%, and there was "little or no effect" when the absolute difference was less than 1%.
For vaccine efficacy outcomes, when the effect estimate was 70% or greater we considered the vaccine to have a "large effect" (WHO 2020b; WHO 2020c).
For any adverse event, systemic reactogenicity events, and local reactogenicity events, we considered the effect as a "large effect" when the absolute difference was greater than 25%; a "slight effect" when the absolute difference was from 10% to 25%, and "little or no effect" when the absolute difference was less than 10%.
We prepared summary of findings tables to present estimated relative and absolute risks for critical and important outcomes, except for immunogenicity outcomes. We calculated absolute effects with GRADEpro GDT using the pooled baseline risks from the control groups of the included studies. Absolute effects are presented per 1000 for the outcomes 'any adverse event,' 'systemic adverse events,' and 'local adverse events,' and in remaining outcomes with low baseline risk (control group event rates less than 1%) per 100,000. We did not report absolute effect for results with low or very low certainty. For outcomes where vaccine efficacy is presented as the effect measure in the summary of findings tables, we used the corresponding RR to calculate the absolute effect. The rationale for using a footnote for the length of follow‐up was to add the specific time per individual study for each outcome.
Results
Description of studies
The full description of included studies is available at zenodo.org/record/6963352#.YuvhdhzP3RY. Characteristics of excluded studies and unpublished registered studies are summarized in the Characteristics of excluded studies section and in Appendix 4, respectively.
Results of the search
The results of the searches are detailed in Figure 2. On 5 November 2021, after excluding duplicates, we screened 48,047 records: 701 were eligible for full‐text screening; we included 111 reports of 76 studies evaluating vaccine candidates against SARS‐CoV‐2. Thirty early‐phase randomized trials (36 reports) are pending due to uncertainty regarding concentration of the vaccine candidate to be selected for the phase 3 trial or lack of results on efficacy for the selected dose reported in a phase 3 trial (Appendix 5). In seven reports of trials already included in the analysis and in five other reports of trials not included in the analysis, we did not find any outcomes of interest or we were unable to extract the data (i.e. results reported only as figures or in graphs) (Appendix 6). Overall, we included 41 studies in the analyses. These studies assessed four different types of vaccine platforms: RNA‐based vaccines (six studies), non‐replicating viral vector vaccines (10 studies), inactivated virus vaccines (13 studies), and protein subunit vaccines (six studies). They also assessed heterologous vaccine schedules and the effect of booster doses (six studies). Of note, we did not identify any trials reporting the efficacy outcome of interest for the vaccine Ad5‐vectored (non‐replicant viral vector) (Zhu 2021a); however, its efficacy as part of a heterologous scheme is assessed in a trial included in the analysis (Li 2021a).
2.
PRISMA flow diagram of included randomized controlled trials (RCTs) (last search date 5 November 2021). COVID‐NMA is a living systematic review of all trials assessing treatment and preventive interventions for COVID‐19 (Boutron 2020a). This review is a subreview of the COVID‐NMA.
FDA: Food and Drug Administration; ICTRP: World Health Organization (WHO) International Clinical Trials Registry Platform.
Included studies
Source of the data
We identified 41 trials overall. There were 37 primary analyses (Ali 2021; Al Kaabi 2021; Asano 2022; Bonelli 2021; Bueno 2021; Dunkle 2021; Ella 2021a; Ella 2021b; El Sahly 2021; Fadlyana 2021; Falsey 2021; Formica 2021; Frenck 2021; Guo 2021; Hall 2021; Han 2021; Heath 2021; Keech 2020; Kremsner 2021; Kulkarni 2021; Li 2021a; Liu 2021; Logunov 2021; Mok 2021; Palacios 2020; Sablerolles 2021; Sadoff 2021a; Sadoff 2021b; Shinde 2021; Tanriover 2021; Thomas 2021; Toledo‐Romani 2021; Walsh 2020; Wu 2021a; Xia 2020; Xia 2021; Zhang 2021), and Voysey 2021a, which was a combined analysis of four trials ((COV001 (NCT04324606), COV002 (NCT04400838), COV003 (ISRCTN89951424), COV005 (NCT04444674)).
We also identified four articles reporting secondary analyses of the four trials included in Voysey 2021a. Emary 2021 reported results by variants for COV002 (NCT04400838); Clemens 2021 reported results by variants for COV003 (ISRCTN89951424); Madhi 2021b reported results by variants for COV005 (NCT04444674); and Madhi 2021a reported results for participants with HIV included in COV005 (NCT04444674).
The 41 included trials were reported in 63 reports (34 peer‐reviewed publications, 22 reports of preprints, four clinical study reports, and three FDA briefings). Of the 34 peer‐reviewed publications, 17 were published with earlier versions (Appendix 7). Data were initially extracted from these reports and then updated with subsequent publications. Only the latest versions of the reports are referenced. Most of the trials included were performed and results were retrieved before the detection of variants of concern. Overall, 10 trials reported results for a specific SARS‐CoV‐2 variant of concern; four trials presented results on the Alpha variant (B.1.1.7) (Dunkle 2021; Emary 2021; Heath 2021; Kremsner 2021), four on Beta variant (B.1.351) (Madhi 2021b; Sadoff 2021b; Shinde 2021; Thomas 2021), two on Gamma variant (P.1) (Clemens 2021; Kremsner 2021), and one on Delta (B.1.617.2) (Ella 2021b).
Study design
All trials used a parallel‐group individually randomized design. Twenty‐six of the RCTs included in the analysis had two arms (63.4%) and 15 (36.6%) were multiple‐arm trials. There were 13 early‐phase trials: three phase 1 (Ella 2021a; Keech 2020; Walsh 2020), seven phase 1‐2 (Asano 2022; Guo 2021; Han 2021; Sadoff 2021a; Wu 2021a; Xia 2021; Zhang 2021), and three phase 2 (Formica 2021; Liu 2021; Xia 2020). In 40 trials (97.5%) the outcome assessor was blinded. All trials evaluating BNT162b2 (three), mRNA‐1273 (two), CVnCoV RNA (one), Ad26.COV2.S (two), and NVX‐CoV2373 (five) used placebo (normal saline) in the control arm. All trials assessing Gam‐COVID‐Vac (one), CoronaVac (six), WIBP‐CorV (two), BBIBP‐CorV (three), BBV152 (two), and FINLAY‐FR‐2 (one) used adjuvant in the control arm. In the case of ChAdOx1/SII‐ChAdOx1, three trials used placebo (normal saline) in the control arm (Asano 2022; Falsey 2021; Madhi 2021b), three used a non‐COVID‐19 vaccine (MenACWY) (COV001, COV002 and COV003 included in Voysey 2021a), and one used adjuvant (Kulkarni 2021). Two trials assessing heterologous vaccine schedules used regular homologous vaccine schedules as control (Li 2021a; Liu 2021), and four trials compared the effect of different vaccine booster schedules (Bonelli 2021; Li 2021a; Mok 2021; Sablerolles 2021).
Recruitment was completed for 33 trials (80.4%), ongoing for seven trials that reported results of prespecified interim analyses (Frenck 2021; Sadoff 2021a; Sadoff 2021b; Voysey 2021a), and one trial was terminated due to an emergency use authorization for the vaccine candidate (Tanriover 2021). The mean sample size was 10,581 participants with median of 504 (interquartile range (IQR) 180 to 21,977; range: minimum 42 to maximum 44,325).
Study registration
All trial registration records were available; three trials were registered retrospectively (Asano 2022; Shinde 2021; Tanriover 2021).
Settings
Overall, 32 RCTs were multicentre and nine were single‐centre trials (Bonelli 2021; Fadlyana 2021; Hall 2021; Han 2021; Li 2021a; Wu 2021a; Xia 2020; Xia 2021; Zhang 2021). The trials took place in Asia (14 trials, 34.1%), Europe (eight trials, 19.5%), North America (seven trials, 17.0%), worldwide (five trials, 12.1%), South America (four trials, 9.7%), Africa (two trials, 4.8%), and Oceania (one trial, 2.4%).
Characteristics of participants
There were 433,838 participants randomized; 250,200 (57.7%) were assigned to the intervention and 183,638 (42.3%) to the control arm. The number of participants analyzed varied by outcome, from 408 to 418,803 participants. The age range was between three and 100 years; 26 trials included participants 18 years of age or older, seven trials included adults between 18 and 65 years of age, two trials included participants 50 years or older (Liu 2021; Wu 2021a), two trials included participants 12 years old or older (Thomas 2021; Walsh 2020), two trials included only adolescents 12 to 17 years old (Ali 2021; Frenck 2021). Two trials included children and adolescents three to 17 years of age (Han 2021; Xia 2021). Overall, 54.0% of participants were male and the mean age ranged between 14 years (minimum) to 61 years (maximum).
Most trials (n = 35, 85.3%) enrolled healthy or clinically stable participants with no history of SARS‐CoV‐2 infection or COVID‐19 diagnosis, four trials enrolled healthcare workers or individuals considered at substantial risk of exposure to and infection with SARS‐CoV‐2 (Bueno 2021; Dunkle 2021; Palacios 2020; Sablerolles 2021), and two trials included immunocompromized participants in trials assessing booster dose; transplant recipients (Hall 2021) and adults under current rituximab therapy (Bonelli 2021). Thirty‐seven of 41 trials reported that pregnancy was an exclusion criterion. No trials reported data on vaccine efficacy and safety in pregnant women.
Details of the intervention
The included trials reported on four types of vaccine platforms and 12 vaccine candidates: three RNA‐based vaccines (BNT162b2, mRNA‐1273 and CVnCoV), three non‐replicating viral vector vaccines (Ad26.COV2.S, ChAdOx1/SII‐ChAdOx1 and Gam‐COVID‐Vac), four inactivated virus vaccines (CoronaVac, WIBP‐CorV, BBIBP‐CorV and BBV152), and two protein subunit vaccines (NVX‐CoV2373 and FINLAY‐FR‐2). As SII‐ChAdOx1, manufactured in India at Serum Institute of India, is the equivalent of ChAdOx1, we pooled the results for both vaccines in the analysis.
All COVID‐19 vaccine candidates are to be administered through an intramuscular injection. Most of the vaccine candidates full vaccination schedules relied on two doses with a between‐dose time interval varying from 14 to 28 days; however, four trials reported a time interval between doses of less than six weeks to 12 weeks or greater for ChAdOx1 (Voysey 2021a), and one trial one to three months for heterologous compared to a homologous scheme (CoronaVac/Ad5 versus CoronaVac/CoronaVac) (Li 2021a). One vaccine candidate had a two‐dose schedule in adults and three‐dose schedule in children and adolescents (BBIBP‐CorV); one vaccine candidate necessitates a single dose (Ad26.COV2.S), and six studies evaluated the effect of a homologous compared to a heterologous booster dose; the time intervals between complete vaccination and boosters are 28 days (Toledo‐Romani 2021), one month (Bonelli 2021), two months (Hall 2021), three months (Sablerolles 2021), four months (mean) (Mok 2021), and three to six months (Li 2021a). There were no studies on variant‐adapted booster doses.
Outcome measurement
There was some heterogeneity in the way outcomes were assessed.
The definition of 'severe or critical disease' was most often based on the WHO clinical progression scale (Marshall 2020). 'Serious adverse events' were assessed using different grading scales such as ICH and EU Guidelines on Pharmacovigilance for Medicinal Products for Human Use (Sadoff 2021b), Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events (Kulkarni 2021), and toxicity grading scales adapted from Food and Drug Administration (FDA) grading guidance (Asano 2022). The list of definitions used for both outcomes is in Appendix 1.
Funding and conflict of interest
Trials received mixed (private and public) funding (20 trials, 48.78%), public/non‐profit funding (14 trials, 34.14%), and private funding (seven trials, 17.07%). Overall, 37 trials declared competing interests and four trials declared no competing interests (Fadlyana 2021; Mok 2021; Sablerolles 2021; Tanriover 2021).
Excluded studies
We excluded 590 reports; 579 were RCTs evaluating other interventions and were consequently included in the COVID‐NMA platform (covid-nma.com); 11 reports evaluated vaccines but were not eligible for the review (Baden 2021; Barrett 2021; Ewer 2021; Flaxman 2021; Hsieh 2021; Irfan 2021; Lazarus 2021; Patamatamkul 2021; Ward 2021a; Wu 2021b; Zdanowski 2021). Reasons for exclusion included: not randomized (five reports), secondary analysis (two reports), intervention not relevant to the review (two reports), exploratory analysis (one report), and comment (one report). See Characteristics of excluded studies table.
Ongoing studies
We identified 343 trials from registries; 10 were completed, two were terminated, 172 were not recruiting, 155 were ongoing and four were cancelled (Appendix 4).
RNA‐based vaccine
We identified 73 unpublished trials; 34 were not recruiting (67,412 participants planned) and 39 were ongoing (192 participants planned).
Non‐replicating viral vector
We identified 73 unpublished trials; there was one completed trial without results available (27 participants planned), 39 not recruiting (60,018 participants planned), 32 ongoing (157,387 participants planned), and one cancelled (1210 participants planned).
Replicating viral vector
We identified 10 unpublished trials; one completed trial without results available (90 participants planned), four not recruiting (40,950 participants planned), three ongoing (6434 participants planned), and two terminated (495 participants planned).
Inactivated virus
We identified 61 unpublished trials; four completed trials without results available (19,512 participants planned), 25 not recruiting (146,312 participants planned), and 32 ongoing (122,182 participants planned).
Protein subunit
We identified 91 unpublished trials; two completed trials without results available (173 participants planned), 56 not recruiting (605,200 participants planned), 31 ongoing (260,273 participants planned), and two terminated (no participants).
Live attenuated virus
We identified two studies not recruiting (163 participants planned).
DNA‐based vaccine
We identified 18 unpublished trials; two completed trials without results available (30 participants planned), nine not recruiting (16,238 participants planned), and seven ongoing (997 participants planned).
Virus‐like particles
We identified 12 unpublished trials; two not recruiting (1818 participants planned), nine ongoing (2546 participants planned), and one terminated (997 participants planned).
Any SARS‐CoV‐2 vaccine
We identified three trials; two recruiting (2300 participants planned) and one not recruiting (1314 participants planned).
Risk of bias in included studies
For the overall risk of bias across trials, we judged 34 trials to have 'some concerns' for at least one outcome; eight trials were at low risk of bias for all outcomes (Asano 2022; Hall 2021; Han 2021; Kulkarni 2021; Sadoff 2021a; Walsh 2020; Xia 2020; Xia 2021). Further details of these assessments are available in the risk of bias assessment tables (Appendix 8).
Risk of bias arising from the randomisation process
We judged the risk of bias due to randomization to be appropriate and adequately done in 32 trials. In other trials, the allocation concealment was either unclear (Bueno 2021; Guo 2021; Zhang 2021), or not reported (Bonelli 2021; Formica 2021; Keech 2020; Mok 2021; Sablerolles 2021); we downgraded Toledo‐Romani 2021 due to imbalances in baseline characteristics.
Risk of bias due to deviations from intended interventions
Thirty‐four trials were blinded for participants, outcome assessors or healthcare providers, or both. Participants were blinded in six trials (Liu 2021; Sablerolles 2021; Voysey 2021a (which reported pooled results for four trials)), and blinding was unclear in one (Mok 2021). Nevertheless, no deviations from the intended intervention occurred due to awareness of the intervention received, and we did not downgrade the trials for this reason.
For efficacy outcomes, we judged the risk of bias due to deviation from intended interventions to be low in 13 trials and have 'some concerns' in 28 trials, mainly because analyses used to estimate the effect of assignment to intervention was inappropriate as most analyses were per protocol for efficacy outcomes. Participants were excluded for positive or unknown baseline SARS‐CoV‐2 status, not receiving a scheduled injection, not receiving the correct injection or major protocol deviation. We considered there would be no substantial impact of failure to analyse participants according to their randomized assignment due to the relatively small number of exclusions or a balanced number of exclusions between arms. In contrast, safety outcomes mainly were analyzed using intention‐to‐treat analysis. We considered this method appropriate to estimate the effect of assignment to intervention.
Risk of bias due to missing outcome data
We judged the risk of bias due to missing outcome data as low for all outcomes for 33 trials. There were no missing data or any missing outcome data were reasonably well‐balanced across intervention groups, with similar reasons for missing data across the groups. Additionally, when missingness was related to deviations from the protocol, it was accounted for in the assessment of bias due to deviations from intended interventions and we did not downgrade the trial due to missing outcome data. For eight trials (Bonelli 2021; Bueno 2021; Ella 2021b; Frenck 2021; Hall 2021; Liu 2021; Sablerolles 2021; Shinde 2021), we judged the risk of bias as having 'some concerns' since trialists failed to report data for all or nearly all participants for at least one outcome, and missingness could depend on the true value of the outcome, for instance, unbalanced loss to follow‐up due to adverse events or deceased participants not included in the analysis.
Risk of bias in the measurement of the outcome
We judged the risk of bias as low for all outcomes in 38 trials. We judged three trials as having 'some concerns' due to unclear or not blinded assessment of the safety outcomes whose evaluation can be influenced by knowledge of the intervention assignment (Bonelli 2021; Liu 2021; Mok 2021).
Risk of bias in the selection of the reported results
Thirty‐three trials had prospective registrations or protocols (or both) available with no discrepancies between prespecified and reported outcomes; we judged these trials to be at low risk of bias. Six trials had risk of bias concerns due to reported outcomes that were not prespecified or had discrepancies in time points (Bonelli 2021; Ella 2021a; Fadlyana 2021; Formica 2021; Mok 2021; Wu 2021a).
Bias due to missing results in the synthesis
We present matrices indicating the availability of trial results for critical and important review outcomes in Appendix 9. There was evidence of bias due to missing results in four trials: El Sahly 2021, Dunkle 2021, Sadoff 2021b, and Shinde 2021 planned to assess 'GMTs of neutralizing and specific antibodies' but did not report on them. Toledo‐Romani 2021 reported 'total adverse events', but only reported on 'local and systemic reactogenicity events', in addition to outcomes 'confirmed SARS‐CoV‐2 infection after complete vaccination' and 'GMTs of neutralizing and specific antibodies', which were not reported as well. Kulkarni 2021 did not report on the preplanned analysis for 'GMTs of neutralizing and specific antibodies' as well as 'systemic and local reactogenicity events' when compared to placebo. Tanriover 2021 planned to assess 'GMTs of neutralizing and specific antibodies' and 'confirmed SARS‐CoV‐2 infection after complete vaccination' but did not report on them. Zhang 2021 in phase 2 did not report on the results of 'serious adverse events'. Clemens 2021 did not report on the prespecified outcomes 'systemic and serious adverse events'. Liu 2021 did not report on the prespecified 'local and systemic reactogenicity events'. Hall 2021 and Kremsner 2021 did not report on the prespecified outcome 'confirmed SARS‐CoV‐2 infection after complete vaccination'. Finally, Voysey 2021a reporting on results of four trials did not report on results of 'local adverse events'. Ten registered trials are completed but not yet published (19,832 participants planned); the dates of completion range between 15 January 2021 and 13 October 2021. Publication delay since study completion ranged between 23 days and 295 days.
Overview of the risk of bias assessments by outcome
The outcome 'SARS‐CoV‐2 infection after complete vaccination' was reported in seven trials; in all trials we assessed the overall risk of bias to have 'some concerns'.
The outcome 'confirmed symptomatic COVID‐19 after complete vaccination' was reported in 18 trials; in all trials we assessed the overall risk of bias to have 'some concerns'.
The outcome 'severe or critical COVID‐19 after complete vaccination' was reported in 11 trials. In one of them, we assessed the overall risk of bias for this outcome to be 'low' (Thomas 2021). In 10 trials, we assessed the overall risk of bias for this outcome to have 'some concerns'.
The outcome 'all‐cause mortality' was reported in 22 trials. In 17 trials, we assessed the overall risk of bias for this outcome to be 'low'. In five trials, we assessed the overall risk of bias for this outcome to have 'some concerns'.
The outcome 'serious adverse events' was reported in 32 trials. In 21 of them, we assessed the overall risk of bias for this outcome to be low. In 11 trials, we assessed the overall risk of bias for this outcome to have 'some concerns'.
The outcome 'any adverse event' was reported in 35 trials. In 24 of them, we assessed the overall risk of bias for this outcome to be 'low'. In 11 trials, we assessed the overall risk of bias for this outcome to have 'some concerns'.
The outcome 'systemic adverse events' was reported in 31 trials. In 15 of them, we assessed the overall risk of bias for this outcome to be 'low'. In 16 trials, we assessed the overall risk of bias for this outcome to have 'some concerns'.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15
We report the network structure, irrespective of the outcomes in Figure 1 and the certainty of evidence for all critical outcomes in the summary of findings tables.
RNA‐based vaccines
BNT162b2 – BioNtech/Fosun Pharma/Pfizer versus placebo (normal saline)
See Table 1 and table of results in Appendix 10.
We identified and included three trials in the analysis assessing BNT162b2. The outcomes 'confirmed SARS‐CoV‐2 infection after complete vaccination', 'systemic reactogenicity events', 'GMT of specific antibodies against SARS‐CoV‐2' and 'cellular immune response' were not reported for this comparison.
Critical outcomes
Confirmed symptomatic COVID‐19 after complete vaccination
Two trials reported this outcome (Frenck 2021; Thomas 2021). BNT162b2 results in a large reduction in the incidence of symptomatic COVID‐19 after complete vaccination compared to placebo measured at 1.8 months' and six months' follow‐up (vaccine efficacy (VE) 97.84%, 95% confidence interval (CI) 44.25% to 99.92%; I² = 66%; 2 RCTs, 44,077 participants; high‐certainty evidence; Figure 3).
3.
Analysis 1.1.2: RNA‐based vaccine. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
Ali 2021 included only participants 3 to 17 years of age. Frenck 2021 included only participants 12 to 15 years of age.
Severe or critical COVID‐19 after complete vaccination
One trial reported severe or critical COVID‐19 (Thomas 2021). BNT162b2 results in a large reduction in the incidence of severe or critical disease due to COVID‐19 compared to placebo measured at six months' follow‐up (VE 95.70%, 95% CI 73.90% to 99.90%; 1 RCT, 46,077 participants; high‐certainty evidence; Figure 4).
4.
Analysis 1.1.3: RNA‐based vaccine. Outcome: severe or critical COVID‐19 after complete vaccination.
*Thomas 2021 reports pooled results including adults' participants from Thomas 2021 and adolescent participants from Frenck 2021.
All‐cause mortality
Two trials reported the outcome in 2302 participants at 1.7 months and 4.7 months' follow‐up (Frenck 2021; Walsh 2020); there were no events and the trials did not contribute to the effect estimate. Only one study contributed to the analysis (Thomas 2021), with a follow‐up of six months. The evidence is uncertain for an effect of BNT162b2 on all‐cause mortality compared to placebo due to very serious imprecision (risk ratio (RR) 1.07, 95% CI 0.52 to 2.22; 1 RCT, 43,847 participants; low‐certainty evidence; Figure 5).
5.
Analysis 1.1.4: RNA‐based vaccine. Outcome: all‐cause mortality.
Ali 2021 included only participants 3 to 17 years of age. Frenck 2021 included only participants 12 to 15 years of age.
Serious adverse events
One trial reported the outcome in 42 participants at 1.7 months' follow‐up (Walsh 2020); there were no events and the trial did not contribute to the effect estimate. Two trials contributed to the analysis at 1.7 months' follow‐up (Frenck 2021; Thomas 2021). The evidence is uncertain for an effect of BNT162b2 on SAEs compared to placebo due to serious inconsistency and serious imprecision (RR 1.30, 95% CI 0.55 to 3.07; I² = 76%; 2 RCTs, 46,107 participants; low‐certainty evidence; Figure 6).
6.
Analysis 1.1.5: RNA‐based vaccine. Outcome: serious adverse events (SAEs).
Ali 2021 included only participants 3 to 17 years of age. Frenck 2021 included only participants 12 to 15 years of age.
Any adverse event
Three RCTs reported the outcome at 1.7 months' follow‐up (Frenck 2021; Thomas 2021; Walsh 2020). We decided not to pool the results due to considerable heterogeneity (I² = 90%) probably caused by studies assessing participants in different age groups; Thomas 2021 included adults while Frenck 2021 included adolescents.
One trial reported results for 43,847 participants 16 years and older (Thomas 2021), the RR for any adverse event was 2.17 (95% CI 2.09 to 2.26). Another trial reported results for 2260 participants between 12 and 15 years of age (Frenck 2021); the RR for any adverse event was 1.01 (95% CI 0.73 to 1.41). A third trial reported results for 42 participants 18 years or older (Walsh 2020); the RR for any adverse event in the study was 1.50 (95% CI 0.53 to 4.21) (Figure 7).
7.
Analysis 1.1.7: RNA‐based vaccine. Outcome: any adverse event (AE).
Ali 2021 included only participants 3 to 17 years of age. Frenck 2021 included only participants 12 to 15 years of age.
Important outcomes
GMTs of a neutralizing antibody against SARS‐COV‐2
Two trials reported GMTs of neutralizing antibodies against SARS‐COV‐2 (Frenck 2021; Walsh 2020). Results are detailed in Appendix 11.
Incidence of specific safety outcomes
Specific safety outcomes were not consistently reported throughout the included trials. Thomas 2021 reported the number of participants with stroke and myocardial infarction, Frenck 2021 reported the number of participants with cavernous sinus thrombosis, venous thrombosis and lymphadenopathy, and Walsh 2020 did not report any specific safety outcome of interest. These outcomes are summarized in detail in Appendix 12.
Vaccine‐enhanced disease
This outcome was reported in a single trial which reported no vaccine‐enhanced disease effect (Thomas 2021).
mRNA‐1273 – ModernaTX versus placebo (normal saline)
See Table 2 and table of results in Appendix 13.
We identified and included two trials in the analysis assessing mRNA‐1273. The outcomes 'GMT of specific antibodies against SARS‐CoV‐2', 'GMT of neutralizing antibodies against SARS‐CoV‐2' and 'cellular immune response' were not reported for this comparison.
Critical outcomes
Confirmed SARS‐CoV‐2 infection after complete vaccination
Two trials reported this outcome (Ali 2021; El Sahly 2021). mRNA‐1273 probably results in a large reduction in the incidence of SARS‐CoV‐2 infection compared to placebo at 2.3 months (median) and 5.3 months' follow‐up (VE 73.27%, 95% CI 35.82% to 88.87%; I² = 66%; 2 RCTs, 31,632 participants; moderate‐certainty evidence; Figure 8).
8.
Analysis 1.1.1: RNA‐based vaccine. Outcome: confirmed SARS‐CoV‐2 infection after complete vaccination.
Ali 2021 included only participants 3 to 17 years of age.
Confirmed symptomatic COVID‐19 after complete vaccination
Two trials reported on this outcome (Ali 2021; El Sahly 2021). mRNA‐1273 results in a large reduction in the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to placebo at 2.3 months (median) and 5.3 months' follow‐up (VE 93.20%, 95% CI 91.06% to 94.83%; I² = 0%; 2 RCTs, 31,632 participants; high‐certainty evidence; Figure 3).
Severe or critical COVID‐19 after complete vaccination
The outcome was reported in one trial (El Sahly 2021). mRNA‐1273 results in a large reduction of the incidence of severe or critical disease due to COVID‐19 compared to placebo at 5.3 months' follow‐up (VE 98.20%, 95% CI 92.80% to 99.60%; 1 RCT, 28,451 participants; high‐certainty evidence; Figure 4).
All‐cause mortality
One study reported the outcome in 3726 participants at 2.3 months (median) follow‐up (Ali 2021); there were no events and the trial did not contribute to the effect estimate. One trial contributed to the analysis with follow‐up of 5.3 months (El Sahly 2021). The evidence is uncertain for an effect of mRNA‐1273 on all‐cause mortality compared to placebo due to very serious imprecision (RR 1.06, 95% CI 0.54 to 2.10; 1 RCT, 30,346 participants; low‐certainty evidence; Figure 5).
Serious adverse events
Two trials reported SAEs (Ali 2021; El Sahly 2021). mRNA‐1273 probably results in no or little difference in the incidence of SAEs compared to placebo at 2.8 months (median) and 5.3 months' follow‐up (RR 0.92, 95% CI 0.78 to 1.08; I² = 0%; 2 RCTs, 34,072 participants; absolute effect: 143 fewer per 100,000 (from 394 fewer to 143 more); moderate‐certainty evidence; Figure 6).
Systemic reactogenicity events
Two trials reported the outcome (Ali 2021; El Sahly 2021). mRNA‐1273 results in a slight increase in the occurrence of any systemic reactogenicity event compared to placebo (RR 1.28, 95% CI 1.22 to 1.34; I² = 61%; 2 RCTs, 34,037 participants; absolute effect: 121 more with systemic reactogenicity events per 1000 (from 95 fewer to 147 more); high‐certainty evidence; Figure 9).
9.
Analysis 1.1.6: RNA‐based vaccine. Outcome: systemic reactogenicity events.
Ali 2021 included only participants 3 to 17 years of age.
Any adverse event
Two RCTs reported the outcome at 2.8 months (median) and 5.3 months' follow‐up (Ali 2021; El Sahly 2021). We decided not to pool the results due to considerable heterogeneity (I² = 100%) probably caused by studies assessing participants in different age groups; Ali 2021 included participants aged three years to 17 years while El Sahly 2021 included adults. One trial reported results for 3726 participants between 12 and 17 years of age (Ali 2021); the risk for any adverse event in the study was 1.47 (95% CI 1.41 to 1.54), the other study reported results for 29,269 participants 18 years and older (El Sahly 2021), the risk for any adverse event in this study was 2.15 (95% CI 2.11 to 2.19) (Figure 7).
Important outcomes
Local reactogenicity events
Two trials reported this outcome (Ali 2021; El Sahly 2021). mRNA‐1273 results in a large increase of local reactogenicity events compared to placebo (RR 3.30, 95% CI 2.02 to 5.40; I² = 99%; 2 RCTs, 34,037 participants; absolute effect: 486 more with local reactogenicity events per 1000 (from 216 more to 930 more); high‐certainty evidence; Figure 10).
10.
Analysis 1.1.8: RNA‐based vaccine. Outcome: local reactogenicity events.
Ali 2021 included only participants 3 to 17 years of age.
Incidence of specific safety outcomes
Specific safety outcomes were not consistently reported throughout the included trials. One trial reported number of participants with pulmonary embolism, pericarditis, venous thrombosis, myocardial infarction, thrombocytopaenia, anaemia and nervous system diseases (El Sahly 2021); the other trial reported number of participants with pericarditis myocardial infarction and lymphadenopathy (Ali 2021). Outcomes were summarized in detail in Appendix 12.
Vaccine‐enhanced disease
One trial reported no vaccine‐enhanced disease effect (El Sahly 2021).
CVnCoV – CureVac AG versus placebo (normal saline)
See Table 3 and table of results in Appendix 14.
We identified and included in the analysis one trial assessing CVnCoV. The outcomes 'SARS‐CoV‐2 infection after complete vaccination', 'GMT of specific antibodies against SARS‐CoV‐2', 'GMT of neutralizing antibodies against SARS‐CoV‐2', 'cellular immune response', 'incidence of specific safety outcomes' and 'vaccine‐enhanced disease' were not reported for this comparison.
Critical outcomes
Confirmed symptomatic COVID‐19 after complete vaccination
One trial reported this outcome at 6.2 months' follow‐up (Kremsner 2021). CVnCoV probably results in a small reduction of confirmed symptomatic COVID‐19 after complete vaccination compared to placebo (VE 48.20%, 95% CI 31.70% to 60.90%; 1 RCT, 25,062 participants; moderate‐certainty evidence; Figure 3).
Severe or critical COVID‐19 after complete vaccination
One trial reported the outcome at six months' follow‐up (Kremsner 2021). The evidence is very uncertain for an effect of CVnCoV in reducing severe or critical COVID‐19 compared to placebo due to serious indirectness and very serious imprecision (VE 63.80%, 95% CI 0.00% to 91.70%; 1 RCT, 25,062 participants; very low‐certainty evidence; Figure 4).
All‐cause mortality
One trial reported this outcome at six months' follow‐up (Kremsner 2021). The evidence is very uncertain for an effect of CVnCoV on all‐cause mortality compared to placebo due to serious indirectness and very serious imprecision (RR 1.33, 95% CI 0.46 to 3.83; 1 RCT, 39,529 participants; very low‐certainty evidence; Figure 5).
Serious adverse events
One trial reported this outcome (Kremsner 2021). The evidence is very uncertain for an effect of CVnCoV on SAEs compared to placebo at 1.7 months' follow‐up (RR 1.24, 95% CI 0.90 to 1.71; 1 RCT, 39,529 participants; low‐certainty evidence; Figure 6).
Systemic reactogenicity events
One trial reported this outcome (Kremsner 2021). CVnCoV results in a large increase in the incidence of systemic reactogenicity events compared to placebo at 6.2 months' follow‐up (RR 1.48, 95% CI 1.43 to 1.53; 1 RCT, 3982 participants; absolute effect: 305 more with systemic reactogenicity events per 1000 (from 273 more to 336 more); high‐certainty evidence; Figure 9).
Any adverse event
One trial reported this outcome (Kremsner 2021). CVnCoV probably results in a large increase in the incidence of any adverse event compared to placebo at one‐month follow‐up (RR 1.42, 95% CI 1.38 to 1.47; 1 RCT, 3982 participants; absolute effect: 285 more with any adverse event per 1000 (from 258 more to 319 more); moderate‐certainty evidence; Figure 7).
Important outcomes
Local reactogenicity events
One trial reported this outcome (Kremsner 2021). CVnCoV results in a large increase in the incidence of local reactogenicity events compared to placebo (RR 3.51, 95% CI 3.24 to 3.81; 1 RCT, 3982 participants; absolute effect: 606 more with local reactogenicity events per 1000 (from 541 more to 678 more); high‐certainty evidence; Figure 10).
Non‐replicant viral vector vaccines
ChAdOx1/SII‐ChAdOx1 – AstraZeneca+University of Oxford/Serum Institute of India versus placebo (normal saline/adjuvant/MenACWY)
See Table 4 and table of results in Appendix 15.
We identified and included in the analysis seven trials assessing ChAdOx1 – AstraZeneca/University of Oxford and one trial assessing SII‐ChAdOx1, the equivalent of ChAdOx1 manufactured in India at Serum Institute of India (Kulkarni 2021). The latter did not report efficacy outcomes.
The outcomes 'severe or critical COVID‐19 after complete vaccination', 'GMT of neutralizing antibodies against SARS‐CoV‐2' and 'cellular immune response' were not reported for this comparison.
Critical outcomes
Confirmed SARS‐CoV‐2 infection after complete vaccination
This outcome was reported in five RCTs (Falsey 2021; Voysey 2021a (which reported pooled results for four trials)). ChAdOx1 probably reduces SARS‐CoV‐2 infection compared to placebo and MenACWY vaccine at 1.3 months (median) and two months (median) follow‐up (VE 59.35%, 95% CI 48.00% to 68.22%; I² = 68%; 5 RCTs, 43,390 participants; moderate‐certainty evidence; Figure 11).
11.
Analysis 2.1.1: Non‐replicating viral vector vaccine. Outcome: confirmed SARS‐CoV‐2 infection after complete vaccination.
Voysey 2021a: data pooled from four trials.
Confirmed symptomatic COVID‐19 after complete vaccination
Five RCTs reported this outcome (Falsey 2021; Voysey 2021a) (Voysey 2021a (which reported pooled results for four trials)). ChAdOx1 results in a large reduction of the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to placebo and MenACWY vaccine at 1.3 months (median) and two months (median) follow‐up (VE 70.23%, 95% CI 62.10% to 76.62%; I² = 38%; 5 RCTs, 43,390 participants; high‐certainty evidence; Figure 12).
12.
Analysis 2.1.2: non‐replicating viral vector vaccine. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
Voysey 2021a: data pooled from four trials.
All‐cause mortality
Two trials reported this outcome in 1456 participants at 2‐month follow‐up (Asano 2022; Kulkarni 2021); there were no events and the trials did not contribute to the effect estimate. Five trials contributed to the analysis with follow‐up from 2.0 months to 4.2 months (Falsey 2021; Madhi 2021a (which reported on HIV‐positive participants who were not included in this pooled analysis); Voysey 2021a (which reported pooled results for four trials)). The evidence is uncertain for an effect of ChAdOx1 on all‐cause mortality compared to placebo and MenACWY vaccine due to very serious imprecision (RR 0.48, 95% CI 0.20 to 1.14; I² = 0%; 5 RCTs, 56,727 participants; low‐certainty evidence; Figure 13).
13.
Analysis 2.1.4: non‐replicating viral vector vaccine. Outcome: all‐cause mortality.
In Kulkarni 2021, the control arm received adjuvant. Voysey 2021a: data pooled from four trials.
Serious adverse events
Seven trials reported this outcome (Asano 2022; Falsey 2021; Kulkarni 2021; Madhi 2021a (which reported on HIV‐positive participants who were not included in this pooled analysis); Voysey 2021a (which reported pooled results for four trials)). ChAdOx1 probably results in no or little increase in the incidence of SAEs compared to placebo and at one month' to 6 months' follow‐up (RR 0.88, 95% CI 0.72 to 1.07; I² = 6%; 7 RCTs, 58,182 participants; absolute effect: 1 fewer with SAEs per 1000 (from 2 fewer to 1 more); moderate‐certainty evidence; Figure 14).
14.
Analysis 2.1.5: non‐replicating viral vector vaccine. Outcome: serious adverse events (SAEs).
In Kulkarni 2021, the control arm received adjuvant. Voysey 2021a: data pooled from four trials.
Systemic reactogenicity events
This outcome was reported in one trial (Asano 2022). ChAdOx1 probably results in a large increase of systemic reactogenicity events compared to placebo (RR 3.93, 95% CI 2.11 to 7.29; 1 RCT, 256 participants; absolute effect: 412 more with systemic reactogenicity events per 1000 (from 156 more to 885 more); moderate‐certainty evidence; Figure 15).
15.
Analysis 2.1.6: non‐replicating viral vector vaccine. Outcome: systemic reactogenicity events.
Any adverse event
Seven trials reported this outcome (Asano 2022; Falsey 2021; Kulkarni 2021; Voysey 2021a (which reported pooled results for four trials). Due to considerable heterogeneity, we decided not to pool the results (I² = 90%). Asano 2022 reported results for 256 participants at 1.2 months' follow‐up; the risk of any adverse event in the study was 2.54 (95% CI 1.73 to 3.74). Falsey 2021 reported results for 32,379 participants at one‐month follow‐up; the risk for any adverse event was 1.37 (95% CI 1.33 to 1.42). Kulkarni 2021 reported results for 1200 participants at 1.9 months' follow‐up; the risk for any adverse event was 1.39 (95% CI 1.12 to 1.74). Lastly, a report pooling four trials presented results for 23,745 participants, the risk for any adverse event was 0.74 (95% CI 0.56 to 0.96) at 3.4 months' follow‐up (Voysey 2021a). Of note, participants in the control arm received different interventions across studies; three trials used normal saline as placebo (Asano 2022; COV005 included in Voysey 2021a; Falsey 2021) and three used MenACWY vaccine (COV001, COV002, COV003 included in Voysey 2021a) and one trial used adjuvant (Kulkarni 2021) (Figure 16).
16.
Analysis 2.1.7: non‐replicating viral vector vaccine. Outcome: any adverse event (AE).
In Kulkarni 2021, the control arm received adjuvant. Voysey 2021a merged results from four different trials where three used quadrivalent meningococcal conjugate vaccine as placebo and one trial used normal saline.
Important outcomes
GMTs of a specific antibody against SARS‐COV‐2
Voysey 2021a reported GMTs of specific antibodies against SARS‐COV‐2. Results are detailed in Appendix 16.
Local reactogenicity events
The outcome was reported in one trial (Asano 2022). ChAdOx1 probably results in a large increase in the number of local reactogenicity events compared to placebo (RR 6.44, 95% CI 2.98 to 13.92; 1 RCT, 256 participants; absolute effect: 510 more with local reactogenicity events per 1000 (from 186 more to 1000 more); moderate‐certainty evidence; Figure 17).
17.
Analysis 2.1.8: non‐replicating viral vector vaccine. Outcome: local reactogenicity events.
Incidence of specific safety outcomes
Specific safety outcomes were not consistently reported throughout the included trials. Madhi 2021a reported number of participants with subsequent nervous system diseases, Falsey 2021 reported number of participants with stroke, cavernous sinus thrombosis, venous thrombosis and nervous system disorders, Voysey 2021a presented results for the number of participants with pulmonary embolism, pericarditis, venous thrombosis, myocardial infarction, anaemia and nervous system diseases, and Asano 2022 and Kulkarni 2021 did not report any specific safety outcome of interest. Outcomes are summarized in detail in Appendix 12.
Vaccine‐enhanced disease
Falsey 2021 reported no vaccine‐enhanced disease effect.
ChAdOx1 – AstraZeneca+University of Oxford versus SII‐ChAdOx1 – Serum Institute of India
See Table 5 and table of results in Appendix 17.
Kulkarni 2021 reported results on ChAdOx1 compared to SII‐ChAdOx1 (the equivalent of ChAdOx1 manufactured in India at Serum Institute of India).
Critical outcomes
All‐cause mortality
Kulkarni 2021 reported this outcome at six months' follow‐up. The trial including 400 participants reported zero events for both groups for this outcome (Figure 18).
18.
Analysis 2.2.1: serum Institute of India/Astra Zeneca+University of Oxford – SII‐ChAdOx1 versus University of Oxford – ChAdOx1. Outcome: all‐cause mortality.
Serious adverse events
Kulkarni 2021 reported this outcome at six months' follow‐up. The evidence is uncertain for an effect of SII‐ChAdOx1 on the incidence of SAEs compared to ChAdOx1 due to very serious imprecision (RR 0.50, 95% CI 0.08 to 2.95; 1 RCT, 400 participants; low‐certainty evidence; Figure 19).
19.
Analysis 2.2.2: SII‐ChAdOx1 versus ChAdOx1. Outcome: serious adverse events (SAEs).
Systemic reactogenicity events
Kulkarni 2021 reported this outcome. SII‐ChAdOx1 probably results in a slight decrease in the number of systemic reactogenicity events compared to ChAdOx1 (RR 0.73, 95% CI 0.54 to 0.98; 1 RCT, 400 participants; absolute effect: 105 fewer with systemic reactogenicity events per 1000 (from 179 fewer to 8 fewer); moderate‐certainty evidence; Figure 20).
20.
Analysis 2.2.3: SII‐ChAdOx1 versus ChAdOx1. Outcome: systemic reactogenicity events.
Any adverse event
Kulkarni 2021 reported this outcome at 1.9 months' follow‐up. The evidence is uncertain for an effect of SII‐ChAdOx1 on the incidence of any adverse event compared to ChAdOx1 due to very serious imprecision (RR 0.83, 95% CI 0.52 to 1.33; 1 RCT, 400 participants; low‐certainty evidence; Figure 21).
21.
Analysis 2.2.4: SII‐ChAdOx1 versus ChAdOx1. Outcome: any adverse event (AE).
Important outcomes
Immunogenicity outcomes
Kulkarni 2021 reported that SII‐ChAdOx1 elicited slightly higher levels of specific antibodies against SARS‐COV‐2 (GMR 1.52, 95% CI 1.03 to 2.26) compared to ChAdOx1 (Appendix 16). Results for neutralizing antibodies against SARS‐COV‐2 were not conclusive because of imprecision (GMR 1.23, 95% CI 0.92 to 1.63).
Local reactogenicity events
Kulkarni 2021 reported this outcome. The evidence is uncertain for an effect of SII‐ChAdOx1 on the incidence of local reactogenicity events compared to ChAdOx1 (RR 0.76, 95% CI 0.55 to 1.05; 1 RCT, 400 participants; low‐certainty evidence; Figure 22).
22.
Analysis 2.2.5: SII‐ChAdOx1 versus ChAdOx1. Outcome: local reactogenicity events.
Ad26.COV2.S – Janssen Pharmaceutical Companies versus placebo (normal saline)
See Table 6 and table of results in Appendix 18.
We identified and included in the analysis two trials assessing Ad26.COV2.S. The outcomes 'SARS‐CoV‐2 infection after complete vaccination', 'GMT of specific antibodies against SARS‐CoV‐2', and 'cellular immune response' were not reported for this comparison.
Critical outcomes
Confirmed symptomatic COVID‐19 after complete vaccination
This outcome was reported in Sadoff 2021b. Ad26.COV2.S reduces the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to placebo at 1.9 months (median) follow‐up (VE 66.90%, 95% CI 59.10% to 73.40%; 1 RCT, 39,058 participants; high‐certainty evidence; Figure 12).
Severe or critical COVID‐19 after complete vaccination
This outcome was reported in Sadoff 2021b. Ad26.COV2.S results in a large reduction of severe or critical COVID‐19 compared to placebo at 1.9 months (median) follow‐up (VE 76.30%, 95% CI 57.90% to 87.50%; 1 RCT, 39,058 participants; high‐certainty evidence; Figure 23).
23.
Analysis 2.1.3: non‐replicating viral vector vaccine. Outcome: severe or critical COVID‐19 after complete vaccination.
All‐cause mortality
This outcome was reported in Sadoff 2021b. Ad26.COV2.S probably results in a reduction in all‐cause mortality compared to placebo at 1.9 months (median) follow‐up (RR 0.25, 95% CI 0.09 to 0.67; 1 RCT, 43,783 participants; absolute effect: 69 fewer per 100,000 (from 83 fewer to 30 fewer); high‐certainty evidence; Figure 13).
Serious adverse events
This outcome was reported in Sadoff 2021b. Ad26.COV2.S probably results in little or no difference in the incidence of SAEs at 1.9 months (median) follow‐up (RR 0.92, 95% CI 0.69 to 1.22; 1 RCT, 43,783 participants; absolute effect: 36 fewer per 100,000 (from 139 fewer to 99 more); moderate‐certainty evidence; Figure 14).
Systemic reactogenicity events
Two trials reported this outcome (Sadoff 2021a; Sadoff 2021b). Ad26.COV2.S results in a large increase in systemic reactogenicity events compared to placebo (RR 1.83, 95% CI 1.29 to 2.60; I² = 83%; 2 RCTs, 7222 participants; absolute effect: 28,697 more per 100,000 (from 10,027 more to 55,320 more); high‐certainty evidence; Figure 15).
Any adverse event
The outcome was reported in two trials (Sadoff 2021a; Sadoff 2021b). We decided not to pool the results due to considerable heterogeneity (I² = 96%). Sadoff 2021b reported results for 6736 participants at one‐month follow‐up; the risk for any adverse event was 1.09 (95% CI 0.96 to 1.24). Sadoff 2021a reported results for 486 participants; the risk for adverse events was 2.31 (95% CI 1.80 to 2.97; Figure 16).
Important outcomes
Immunogenicity outcomes
Sadoff 2021a reported GMTs of neutralizing antibodies against SARS‐COV‐2. Results are detailed in Appendix 11.
Local reactogenicity events
Two trials reported this outcome (Sadoff 2021a; Sadoff 2021b). Ad26.COV2.S results in a large increase in local reactogenicity events compared to placebo (RR 3.27, 95% CI 1.91 to 5.62; I² = 84%; 2 RCTs, 7222 participants; absolute effect: 433 more with local reactogenicity events per 1000 (from 174 more to 881 more); high‐certainty evidence; Figure 17).
Incidence of specific safety outcomes
Specific safety outcomes were not consistently reported throughout the included trials: Sadoff 2021b reported the number of participants with pulmonary embolism, cavernous sinus thrombosis, pericarditis and venous thrombosis; Sadoff 2021a did not report any specific safety outcomes of interest. Outcomes are summarized in detail in Appendix 12.
Vaccine‐enhanced disease
Sadoff 2021b reported no vaccine‐enhanced disease effect.
Gam‐COVID‐Vac – Gamaleya Research Institute (Sputnik V) versus placebo (adjuvant)
See Table 7 and table of results in Appendix 19.
We identified and included one trial in the analysis assessing Gam‐COVID‐Vac (Logunov 2021).
The outcomes 'SARS‐CoV‐2 infection after complete vaccination', 'incidence of any adverse event', 'systemic reactogenicity events' and 'vaccine‐enhanced disease' were not reported for this comparison.
Some important concerns were raised concerning Logunov 2021: lack of clarity in the definition of the primary outcome; addition of interim analyses; change in outcomes; inadequate reporting with inconsistencies in numbers; and excess of homogeneity of vaccine efficacy across age groups (Bucci 2021). The authors responded to some of these concerns and the manuscript was corrected (Logunov 2021). Nevertheless, uncertainty persists related to the prespecification of the interim analysis and excess of homogeneity of vaccine efficacy across age groups. Consequently, we decided to downgrade the certainty of evidence for these reasons.
Critical outcomes
Confirmed symptomatic COVID‐19 after complete vaccination
This outcome was reported in Logunov 2021. Gam‐COVID‐Vac probably results in a large reduction in the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to placebo (follow‐up time not reported) (VE 91.10%, 95% CI 83.80% to 95.10%; 1 RCT, 18,695 participants; moderate‐certainty evidence). Of note, vaccine efficacy for this outcome was calculated using RR (Figure 12).
Severe or critical COVID‐19 after complete vaccination
This outcome was reported in Logunov 2021. Gam‐COVID‐Vac probably results in a large reduction in the incidence of severe or critical COVID‐19 compared to placebo (follow‐up time not reported) (VE 100.00%, 95% CI 94.40% to 100.00%; 1 RCT, 19,866 participants; moderate‐certainty evidence; Figure 23).
All‐cause mortality
Logunov 2021 reported this outcome at 1.6 months' follow‐up. The evidence is very uncertain for an effect of Gam‐COVID‐Vac in all‐cause mortality compared to placebo due to serious imprecision (RR 0.99, 95% CI 0.10 to 9.54; 1 RCT, 21,862 participants; very low‐certainty evidence; Figure 13).
Serious adverse events
Logunov 2021 reported this outcome. The evidence is uncertain for an effect of Gam‐COVID‐Vac in the incidence of SAEs compared to placebo at 1.6 months' follow‐up (RR 0.65, 95% CI 0.39 to 1.07; 1 RCT, 21,862 participants; low‐certainty evidence; Figure 14).
Important outcomes
Immunogenicity outcomes
Logunov 2021 reported GMTs of neutralizing and specific antibodies against SARS‐CoV‐2, and cellular immune response. Results are detailed in Appendix 11, Appendix 16, and Appendix 20, respectively.
Incidence of specific safety outcomes
Logunov 2021 reported number of participants with cavernous sinus thrombosis, venous thrombosis, myocardial infarction, lymphadenopathy and nervous system diseases. Details are in Appendix 12.
Inactivated virus vaccines
CoronaVac – Sinovac versus placebo (adjuvant)
See Table 8 and table of results in Appendix 21.
We identified and included in the analysis seven trials assessing CoronaVac – Sinovac. The outcome 'SARS‐CoV‐2 infection after complete vaccination' was not reported for this comparison.
Critical outcomes
Confirmed symptomatic COVID‐19 after complete vaccination
This outcome was reported in two trials at 1.4 months (median) to 2 months (median) follow‐up (Palacios 2020; Tanriover 2021). The evidence is uncertain for an effect of CoronaVac on the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to adjuvant due to serious inconsistency and imprecision (VE 69.81%, 95% CI 12.27% to 89.61%; I² = 92%; 2 RCTs, 19,852 participants; low‐certainty evidence). There was considerable heterogeneity between included studies which could be due to participant’s different level of exposure to the virus across studies (all participants included in Palacios 2020 were healthcare workers compared to a third in Tanriover 2021) (Figure 24).
24.
Analysis 3.1.2: inactivated virus vaccine. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
Al Kaabi 2021.1 and Al Kaabi N 2021.2 refers to two different comparisons from the same report (Al Kaabi 2021).
Severe or critical COVID‐19 after complete vaccination
Two trials reported this outcome (Palacios 2020; Tanriover 2021). We did not conduct a meta‐analysis for this outcome since the typical normality assumption of the meta‐analysis model would be invalid due to the skewness of the data. This can be seen in the forest plots where the CI is not symmetric around the point estimate. Tanriover 2021, with 0/6559 events in the CoronaVac group versus 1/3470 events in the control group reported a vaccine efficacy of 100.00%, 95% CI 20.40% to 100.00% at 1.4 months (median) follow‐up; and Palacios 2020, with 0/4953 events in the CoronaVac group and 6/4870 events in the control group reported a vaccine efficacy of 100.00%, 95% CI 16.90% to 100.00% at two months (median) follow‐up (Figure 25).
25.
Analysis 3.1.3: inactivated virus vaccine. Outcome: severe or critical COVID‐19 after complete vaccination.
All‐cause mortality
This outcome was reported in two trials at 1.4 months (median) to two months (median) follow‐up (Palacios 2020; Tanriover 2021). The evidence is uncertain for an effect of CoronaVac on all‐cause mortality compared to adjuvant due to very serious imprecision (RR 0.50, 95% CI 0.05 to 5.52; 2 RCTs, 22,610 participants; I² = 32%; low‐certainty evidence; Figure 26).
26.
Analysis 3.1.4: inactivated virus vaccine. Outcome: all‐cause mortality.
Al Kaabi 2021.1 and Al Kaabi N 2021.2 refers to two different comparisons from the same report (Al Kaabi 2021).
Serious adverse events
Two trials reported this outcome in 482 participants at 1.4 months' follow‐up (Bueno 2021; Zhang 2021); there were no events and the trials did not contribute to the effect estimate. Four RCTs contributed to the analysis with follow‐up of two months (median) to four months (Han 2021; Palacios 2020; Tanriover 2021; Wu 2021a). The evidence is uncertain for an effect of CoronaVac on SAEs compared to adjuvant due to very serious imprecision (RR 0.97, 95% CI 0.62 to 1.51; 4 RCTs, 23,139 participants; I² = 0%; low‐certainty evidence; Figure 27).
27.
Analysis 3.1.5: inactivated virus vaccine. Outcome: serious adverse events (SAEs).
Han 2021 included only participants 3 to 17 years of age. Wu 2021a included only participants 60 years of age and older.
Wu 2021a reports data for phase 1 and 2. Al Kaabi 2021.1 and Al Kaabi N 2021.2 refer to two different comparisons from the same report (Al Kaabi 2021).
Systemic reactogenicity events
Six trials reported this outcome (Bueno 2021; Fadlyana 2021; Palacios 2020; Tanriover 2021; Wu 2021a; Zhang 2021). The evidence is uncertain for an effect of CoronaVac on systemic reactogenicity events compared to adjuvant due to serious inconsistency and imprecision (RR 1.19, 95% CI 1.00 to 1.42; 6 RCTs, 23,966 participants; I² = 55%; low‐certainty evidence; Figure 28).
28.
Analysis 3.1.6: inactivated virus vaccine. Outcome: systemic reactogenicity events.
Xia S 2021 included only participants 3 to 17 years of age (Xia 2021). Wu Z 2021 included only participants 60 years of age and older (Wu 2021a).
Wu Z 2021 reports data for phase 2 (Wu 2021a). Al Kaabi 2021.1 and Al Kaabi N 2021.2 refer to two different comparisons from the same report (Al Kaabi 2021). Zhang 2020.1 and Zhang 2020.2 refers to two different comparisons from the same report (Zhang 2021).
Any adverse event
This outcome was reported in five trials at one month' to three months' (median) follow‐up (Han 2021; Palacios 2020; Tanriover 2021; Wu 2021a; Zhang 2021). CoronaVac results in a slight difference in the incidence of any adverse event compared to adjuvant (RR 1.09, 95% CI 1.07 to 1.11; 6 RCTs, 23,367 participants; absolute effect: 48 more with any adverse event per 1000 (from 37 more to 58 more); high‐certainty evidence; Figure 29).
29.
Analysis 3.1.7: inactivated virus vaccine. Outcome: any adverse event (AE).
Han B 2021 and Xia 2021 included only participants 3 to 17 years of age (Han 2021; Xia 2021). Wu Z 2021 included only participants 60 years of age and older (Wu 2021a).
Wu Z 2021 reports data for phase 1 and 2 (Wu 2021a), Al Kaabi 2021.1 and Al Kaabi N 2021.2 refer to two different comparisons from the same report (Al Kaabi 2021). Zhang 2020.1 and Zhang 2020.2 refers to two different comparisons from the same report (Zhang 2021).
Important outcomes
Immunogenicity outcomes
Five trials reported GMTs of neutralizing and specific antibodies against SARS‐COV‐2 (Bueno 2021; Fadlyana 2021; Han 2021; Wu 2021a; Zhang 2021), and one trial reported results for cellular immune response (Zhang 2021). Results are detailed in Appendix 11, Appendix 16, and Appendix 20.
Local reactogenicity events
Six trials reported this outcome (Bueno 2021; Fadlyana 2021; Palacios 2020; Tanriover 2021; Wu 2021a; Zhang 2021) CoronaVac results in a slight increase in the occurrence of local reactogenicity events compared to adjuvant (RR 1.76, 95% CI 1.69 to 1.82; 6 RCTs, 23,962 participants; I² = 0%; absolute effect: 173 more per 1000 (from 157 more to 187 more); high‐certainty evidence; Figure 30).
30.
Analysis 3.1.8: inactivated virus vaccine. Outcome: local reactogenicity events.
Xia S 2021 included only participants 3 to 17 years of age (Xia 2021). Wu Z 2021 included only participants 60 years of age and older (Wu 2021a).
Wu Z 2021 reports data for phase 2 (Wu 2021a). Al Kaabi 2021.1 and Al Kaabi N 2021.2 refer to two different comparisons from the same report (Al Kaabi 2021). Zhang 2020.1 and Zhang 2020.2 refers to two different comparisons from the same report (Zhang 2021).
Incidence of specific safety outcomes
Specific safety outcomes were not consistently reported throughout the included trials: Tanriover 2021 reported number of participants with myocardial infarction and nervous system diseases; Fadlyana 2021 reported the number of participants with venous thrombosis and nervous system diseases; and five trials reported no specific safety outcome of interest (Bueno 2021; Han 2021; Palacios 2020; Wu 2021a; Zhang 2021). Outcomes of interest are summarized in Appendix 12.
Vaccine‐enhanced disease
Palacios 2020 reported no vaccine‐enhanced disease effect.
WIBP‐CorV – Sinopharm‐Wuhan versus placebo (adjuvant)
See Table 9 and table of results in Appendix 22.
We identified and included two trials in the analysis assessing WIBP‐CorV. The outcomes 'severe or critical COVID‐19 after complete vaccination', 'cellular immune response' and 'incidence of specific safety outcomes' were not reported for this comparison.
Critical outcomes
Confirmed SARS‐CoV‐2 infection after complete vaccination
This outcome was reported in Al Kaabi 2021. WIBP‐CorV results in a reduction in the incidence of confirmed SARS‐CoV‐2 infection compared to adjuvant at 2.6 months (median) follow‐up (VE 64.00%, 95% CI 48.80% to 74.70%; 1 RCT, 25,449 participants; high‐certainty evidence; Figure 31).
31.
Analysis 3.1.1: inactivated virus vaccine. Outcome: confirmed SARS‐CoV‐2 infection after complete vaccination.
Al Kaabi 2021.1 and Al Kaabi N 2021.2 refer to two different comparisons from the same report (Al Kaabi 2021).
Confirmed symptomatic COVID‐19 after complete vaccination
This outcome was reported in Al Kaabi 2021. WIBP‐CorV results in a large reduction in the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to adjuvant at 2.6 months (median) follow‐up (VE 72.80%, 95% CI 58.10% to 82.40%; 1 RCT, 25,480 participants; high‐certainty evidence; Figure 24).
All‐cause mortality
This outcome was assessed in one trial (26,917 participants) at 2.6 months (median) follow‐up (Al Kaabi 2021). There were zero events in both groups, therefore no effect estimate could be calculated for this outcome (Figure 26).
Serious adverse events
Two trials assessed this outcome (Guo 2021; Al Kaabi 2021). The evidence is uncertain for an effect of WIBP‐CorV on SAEs compared to adjuvant at 1.6 months (median) and 2.6 months (median) follow‐up due to serious imprecision (RR 0.83, 95% CI 0.60 to 1.15; I² = 0%; 2 RCTs, 27,029 participants; low‐certainty evidence; Figure 27).
Systemic reactogenicity events
Two trials reported this outcome (Guo 2021; Al Kaabi 2021). WIBP‐CorV results in no or little difference in the occurrence of systemic reactogenicity events compared to adjuvant (RR 0.99, 95% CI 0.95 to 1.03; I² = 0%; 2 RCTs, 27,029 participants; absolute effect: 3 fewer with systemic reactogenicity events per 1000 (from 14 fewer to 8 more); high‐certainty evidence; Figure 28).
Any adverse event
Two trials assessed the outcome (Guo 2021; Al Kaabi 2021). WIBP‐CorV results in little difference in the incidence of any adverse event compared to adjuvant at one‐month follow‐up (RR 0.96, 95% CI 0.93 to 0.98; I² = 0%; 2 RCTs, 27,029 participants; absolute effect: 20 fewer with any adverse event per 1000 (from 35 fewer to 10 fewer); high‐certainty evidence; Figure 29).
Important outcomes
Immunogenicity outcomes
Two trials reported GMTs of neutralizing and specific antibodies against SARS‐COV‐2 (Guo 2021; Al Kaabi 2021). Results are reported in Appendix 11 and Appendix 16.
Local reactogenicity events
Two trials reported this outcome (Guo 2021; Al Kaabi 2021). WIBP‐CorV results in little difference in the occurrence of local reactogenicity events compared to adjuvant (RR 0.88, 95% CI 0.85 to 0.92; I² = 0%; 2 RCTs, 27,029 participants; absolute effect: 35 fewer with local reactogenicity events per 1000 (from 44 fewer to 23 fewer); high‐certainty evidence; Figure 30).
Vaccine‐enhanced disease
One trial reported no vaccine‐enhanced disease effect (Al Kaabi 2021).
BBIBP‐CorV – Sinopharm‐Beijing versus placebo (adjuvant)
See Table 10 and table of results in Appendix 23.
We identified and included in the analysis three trials assessing BBIBP‐CorV. The outcomes 'severe or critical COVID‐19 after complete vaccination', 'cellular immune response' and 'incidence of specific safety outcomes' were not reported for this comparison.
Critical outcomes
Confirmed SARS‐CoV‐2 infection after complete vaccination
This outcome was reported in one trial (Al Kaabi 2021). BBIBP‐CorV results in a large reduction in SARS‐CoV‐2 infection compared to adjuvant (VE 73.50%, 95% CI 60.60% to 82.20%; 1 RCT, 25,435 participants; high‐certainty evidence; Figure 31).
Confirmed symptomatic COVID‐19 after complete vaccination
This outcome was reported in one trial (Al Kaabi 2021). BBIBP‐CorV results in a large reduction in the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to placebo (adjuvant) (VE 78.10%, 95% CI 64.80% to 86.30%; 1 RCT, 25,463 participants; high‐certainty evidence; Figure 24).
All‐cause mortality
This outcome was assessed in one trial (26,924 participants) (Al Kaabi 2021). There were zero events in both groups, therefore no effect estimate could be calculated for this outcome (Figure 26).
Serious adverse events
One study assessed this outcome in 112 participants (Xia 2020). There were zero events in both groups and the trial did not contribute to the analysis. One trial contributed to the analysis (Al Kaabi 2021). The evidence is uncertain for an effect of BBIBP‐CorV on SAEs compared to adjuvant at 2.6 months (median) follow‐up (RR 0.76, 95% CI 0.54 to 1.06; 1 RCT, 26,924 participants; low‐certainty evidence; Figure 27).
Systemic reactogenicity events
This outcome was reported in three trials (Al Kaabi 2021; Xia 2020; Xia 2021). BBIBP‐CorV probably results in no or little difference in the occurrence of systemic reactogenicity events compared to adjuvant (RR 1.05, 95% CI 0.86 to 1.28; 3 RCTs, 27,540 participants; absolute effect: 14 more per 1000 (from 38 fewer to 77 more); moderate‐certainty evidence; Figure 28).
Any adverse event
This outcome was reported in three trials (Al Kaabi 2021; Xia 2020; Xia 2021). We decided not to pool the results due to considerable heterogeneity (I² = 90%) probably caused by studies assessing participants in different age groups; reported data for participants aged three years to 17 years old. Xia 2021 reported results for 504 participants at 2.9 months' follow‐up; the risk of any adverse event in the study was 2.05 (95% CI 1.47 to 2.87). Al Kaabi 2021 reported results for 26,941 participants at one‐month follow‐up; the risk for any adverse event was 0.91 (95% CI 0.89 to 0.94). Xia 2020 reported results for 112 participants at one‐month follow‐up; the risk for any adverse event was 0.83 (95% CI 0.36 to 1.94; Figure 29).
Important outcomes
Immunogenicity outcomes
Three trials reported GMTs of neutralizing and specific antibodies against SARS‐COV‐2 (Al Kaabi 2021; Xia 2020; Xia 2021). Results are reported in Appendix 11 and Appendix 16.
Local reactogenicity events
This outcome was reported in three trials (Al Kaabi 2021; Xia 2020; Xia 2021). We decided not to pool the results due to considerable heterogeneity (I² = 90%) probably caused by studies assessing participants in different age groups. Xia 2021 reported results for 504 participants at seven days' follow‐up; the risk of local reactogenicity events in the study was 10.00 (95% CI 2.36 to 42.34). Al Kaabi 2021 reported results for 26,924 participants at seven days' follow‐up; the risk for local reactogenicity events was 0.71 (95% CI 0.68 to 0.74). Xia 2020 reported results for 112 participants at seven days' follow‐up; the risk for local reactogenicity events was 3.33 (95% CI 0.45 to 24.89; Figure 30).
Vaccine‐enhanced disease
One trial reported no vaccine‐enhanced disease effect (Al Kaabi 2021).
BBV152 – Bharat Biotech versus placebo (adjuvant)
See Table 11 and table of results in Appendix 24.
We identified and included two trials in the analysis assessing BBV152. The outcome 'vaccine‐enhanced disease' was not reported for this comparison.
Critical outcomes
Confirmed SARS‐CoV‐2 infection after complete vaccination
One trial reported this outcome (Ella 2021b). BBV152 results in a reduction in the incidence of SARS‐CoV‐2 infections compared to adjuvant at 3.3 months (median) follow‐up (VE 68.80%, 95% CI 46.70% to 82.50%; 1 RCT, 6289 participants; high‐certainty evidence; Figure 31).
Confirmed symptomatic COVID‐19 after complete vaccination
This outcome was reported in one trial (Ella 2021b). BBV152 results in a large reduction in the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to adjuvant at 3.3 months (median) follow‐up (VE 77.80%, 95% CI 65.20% to 86.40%; 1 RCT, 16,973 participants; high‐certainty evidence; Figure 24).
Severe or critical COVID‐19 after complete vaccination
This outcome was reported in one trial at 3.3 months (median) follow‐up (Ella 2021b). BBV152 results in a large reduction of severe or critical COVID‐19 after complete vaccination compared to adjuvant due to very serious imprecision (VE 93.40%, 95% CI 57.10% to 99.80%; 1 RCT, 16,976 participants; high‐certainty evidence; Figure 25).
All‐cause mortality
One trial reported this outcome at 3.3 months (median) follow‐up (Ella 2021b). The evidence is uncertain for an effect of BBV152 on all‐cause mortality compared to adjuvant due to very serious imprecision (RR 0.50, 95% CI 0.17 to 1.46; 1 RCT, 25,753 participants; low‐certainty evidence; Figure 26).
Serious adverse events
This outcome was reported in two trials (Ella 2021a; Ella 2021b); Ella 2021b contributed to the analysis. BBV152 results in little or no difference in the incidence of SAEs compared to adjuvant at 4.9 months (median) follow‐up (RR 0.65, 95% CI 0.43 to 0.97; 1 RCT, 25,928 participants; absolute effect: 162 fewer per 100,000 (from 264 fewer to 14 fewer); high‐certainty evidence; Figure 27).
Systemic reactogenicity events
This outcome was reported in two trials (Ella 2021a; Ella 2021b). BBV152 results in little increase in the occurrence of systemic reactogenicity events compared to adjuvant (RR 1.34, 95% CI 1.15 to 1.58; I² = 0%; 2 RCTs, 25,925 participants; absolute effect: 7 more with systemic reactogenicity events per 1000 (from 3 more to 11 more); high‐certainty evidence; Figure 28).
Any adverse event
This outcome was reported in Ella 2021b. BBV152 results in no or little difference in the occurrence of any adverse event compared to adjuvant at 4.9 months (median) follow‐up (RR 1.00, 95% CI 0.94 to 1.07; 1 RCT, 25,753 participants; absolute effect: 0 fewer with any adverse event per 1000 (from 7 fewer to 9 more); high‐certainty evidence; Figure 29).
Important outcomes
Immunogenicity outcomes
Two trials reported GMTs of neutralizing and specific antibodies against SARS‐COV‐2 (Ella 2021a; Ella 2021b), and Ella 2021a reported results for cellular immune response. Results are detailed in Appendix 11, Appendix 16 and Appendix 20.
Local reactogenicity events
This outcome was reported in two trials (Ella 2021b; Ella 2021a). BBV152 results in no or little difference in the occurrence of local reactogenicity events compared to adjuvant (RR 1.08, 95% CI 0.95 to 1.24; I² = 0%; 2 RCTs, 25,750 participants; absolute effect: 2 more with local reactogenicity events per 1000 (from 2 fewer to 7 more); high‐certainty evidence; Figure 30).
Incidence of specific safety outcomes
Specific safety outcomes were not consistently reported throughout the included trials and are summarized in detail in Appendix 12, rather than pooled in a meta‐analysis.
Protein subunit vaccines
NVX‐CoV2373 – Novavax versus placebo (normal saline)
See Table 12 and table of results in Appendix 25.
We identified and included five trials in the analysis assessing NVX‐CoV2373. The outcomes 'SARS‐CoV‐2 infection after complete vaccination', 'severe or critical COVID‐19 after complete vaccination' and 'cellular immune response' were not reported for this comparison.
Low‐certainty evidence for the efficacy outcomes might be explained by the inclusion of results from a trial conducted in South Africa during a period of high prevalence of the Beta variant (Shinde 2021). Vaccine efficacy against this variant was considerably lower than the efficacy reported in the primary analysis or against the Alpha variant.
Critical outcomes
Confirmed symptomatic COVID‐19 after complete vaccination
This outcome was reported in three trials at two months (median) and three months (median) follow‐up (Dunkle 2021; Heath 2021; Shinde 2021). NVX‐CoV2373 probably results in a large reduction of the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to placebo (VE 82.91%, 95% CI 50.49% to 94.10%; I² = 65%; 3 RCTs, 42,175 participants; moderate‐certainty evidence; Figure 32).
32.
Analysis 4.1.1: protein subunit vaccine. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
Severe or critical COVID‐19 after complete vaccination
This outcome was reported in one trial at two months (median) follow‐up (Dunkle 2021). NVX‐CoV2373 results in a large reduction of severe or critical COVID‐19 after complete vaccination compared to adjuvant due to very serious imprecision (VE 100.00%, 95% CI 86.99% to 100.00%; 1 RCT, 25,452 participants; moderate‐certainty evidence; Figure 33).
33.
Analysis 4.1.2: protein subunit vaccine. Outcome: severe or critical COVID‐19 after complete vaccination.
All‐cause mortality
One trial reported this outcome in 14,039 participants at three months (median) follow‐up (Heath 2021); there were no events and the trial did not contribute to the effect estimate. Dunkle 2021 contributed to the analysis with follow‐up of two months (median); the evidence is uncertain for an effect of NVX‐CoV2373 on all‐cause mortality compared to placebo due to very serious imprecision (RR 0.90, 95% CI 0.30 to 2.68; 1 RCT, 29,582 participants; low‐certainty evidence; Figure 34).
34.
Analysis 4.1.3: protein subunit vaccine. Outcome: all‐cause mortality.
Serious adverse events
One trial reported the outcome in 52 participants at 1.15 months' follow‐up (Keech 2020); there were no events and the trial did not contribute to the effect estimate. Four trials contributed to the analysis with follow‐up of 1.15 months, two months (median), and three months (Dunkle 2021; Formica 2021; Heath 2021; Shinde 2021). The evidence is uncertain for an effect of NVX‐CoV2373 on SAEs compared to placebo due to very serious imprecision (RR 0.92, 95% CI 0.74 to 1.14, I² = 0%; 4 RCTs, 38,802 participants; low‐certainty evidence; Figure 35).
35.
Analysis 4.1.4: protein subunit vaccine. Outcome: serious adverse events (SAEs).
Systemic reactogenicity events
This outcome was reported in three trials (Dunkle 2021; Formica 2021; Shinde 2021). NVX‐CoV2373 increases slightly the occurrence of systemic reactogenicity events compared to placebo (RR 1.21, 95% CI 1.17 to 1.25, I² = 27%, 3 RCTs, 31,063 participants; absolute effect 76 more per 1000 (from 62 more to 91 more); high‐certainty evidence; Figure 36).
36.
Analysis 4.1.5: protein subunit vaccine. Outcome: systemic reactogenicity events.
Any adverse event
This outcome was reported in five trials (Dunkle 2021; Formica 2021; Heath 2021; Keech 2020; Shinde 2021). NVX‐CoV2373 probably results in little increase in the incidence of any adverse event compared to placebo at 1.15 months (median) to three months (median) follow‐up (RR 1.15, 95% CI 1.05 to 1.26; I² = 57%; 5 RCTs, 46,231 participants; absolute effect: 26 more with any adverse event per 1000 (from 9 more to 45 more); moderate‐certainty evidence; Figure 37).
37.
Analysis 4.1.6: protein subunit vaccine. Outcome: any adverse event (AE).
Important outcomes
Immunogenetic outcomes
Two trials reported GMTs of specific antibodies against SARS‐COV‐2 (Formica 2021; Keech 2020), and Keech 2020 reported GMTs of neutralizing antibodies against SARS‐COV‐2. Results are detailed in Appendix 16 and Appendix 11.
Local reactogenicity events
Three trials reported the outcome (Dunkle 2021; Formica 2021; Shinde 2021). NVX‐CoV2373 results in a large increase in local reactogenicity events compared to placebo (RR 2.78, 95% CI 1.99 to 3.88; I² = 86%; 3 RCTs, 31,063 participants; absolute effect: 340 more with local reactogenicity events per 1000 (from 189 more to 551 more); high‐certainty evidence; Figure 38).
38.
Analysis 4.1.7 Protein subunit vaccine. Outcome: local reactogenicity events
Incidence of specific safety outcomes
Specific safety outcomes were not consistently reported throughout the included trials: Formica 2021 reported number of participants with venous thrombosis, lymphadenopathy and nervous system diseases; Shinde 2021 reported number of participants with anaemia and nervous system diseases; Heath 2021 reported number of participants with myocardial infarction, thrombocytopaenia and nervous system diseases; and Dunkle 2021 reported on the number of events for pulmonary embolism, stroke, venous thrombosis, thrombocytopenia, haemorrhage, neutropenia, anaemia, lymphadenopathy and nervous system diseases. Outcomes are summarized in detail in Appendix 12.
Vaccine‐enhanced disease
One report mentioned this outcome without presenting results (Keech 2020), and two trials reported no vaccine‐enhanced disease effect (Dunkle 2021; Heath 2021).
FINLAY‐FR‐2 – Instituto Finlay de Vacunas versus placebo (adjuvant)
See Table 13 and table of results in Appendix 26.
We identified and included in the analysis one trial assessing FINLAY‐FR‐2. The outcomes 'SARS‐CoV‐2 infection after complete vaccination', 'severe or critical COVID‐19 after complete vaccination', 'systemic reactogenicity events', 'incidence of any adverse event', 'incidence of serious adverse events', 'GMT of specific antibodies against SARS‐CoV‐2', 'GMT of neutralizing antibodies against SARS‐CoV‐2', 'cellular immune response', 'incidence of specific safety outcomes' and 'vaccine‐enhanced disease' were not reported for this comparison.
Critical outcomes
Confirmed symptomatic COVID‐19 after complete vaccination
We found one trial reporting this outcome (Toledo‐Romani 2021). FINLAY‐FR‐2 probably results in a large reduction in the incidence of confirmed symptomatic COVID‐19 after complete vaccination compared to adjuvant (VE 71.00%, 95% CI 58.90% to 79.10%; 1 RCT, 28,674 participants; moderate‐certainty evidence; Figure 32).
All‐cause mortality
This outcome was reported in one trial (Toledo‐Romani 2021). FINLAY‐FR‐2 probably results in a reduction of all‐cause mortality compared to adjuvant due to serious risk of bias and imprecision (RR 0.37, 95% CI 0.17 to 0.80; 1 RCT, 28,674 participants; absolute effect: 106 fewer per 100,000 (from 139 fewer to 34 fewer) moderate‐certainty evidence; Figure 34).
Primary series heterologous vaccination scheme versus homologous vaccination scheme
See Table 14 and table of results in Appendix 27.
Two publications reported results for three different comparisons involving an RNA‐based vaccine (BNT162b2 – BioNtech/Fosun Pharma/Pfizer), non‐replicating viral vector vaccine (ChAdOx1 – AstraZeneca/University of Oxford), and inactivated virus vaccine (CoronaVac – Sinovac). More specifically the following schemes were compared (vaccine first dose/vaccine second dose): BNT162b2/ChAdOx1 versus BNT162b2/BNT162b2 (Liu 2021), and ChAdOx1/BNT162b2 versus ChAdOx1/ChAdOx1 (Liu 2021), and CoronaVac/Ad5 versus CoronaVac/CoronaVac (Li 2021a).
The outcomes 'SARS‐CoV‐2 infection after complete vaccination', 'symptomatic COVID‐19 after complete vaccination', 'severe or critical COVID‐19', 'all‐cause mortality', 'systemic reactogenicity events' and 'vaccine‐enhanced disease' were not reported for these comparisons.
Critical outcomes
Serious adverse events
One trial reported this outcome in 101 participants at one‐month follow‐up for the comparison CoronaVac/Ad5 versus CoronaVac homologous (Li 2021a), and reported zero events in both groups. Liu 2021 reported the outcome in 234 participants for the comparison BNT162b2/ChAdOx1 versus BNT162b2 homologous and also reported zero events in both groups. The same trial reported the outcome for the comparison ChAdOx1/BNT162b2 versus ChAdOx1 homologous. The evidence is uncertain for an effect of ChAdOx1/BNT162b2 on SAEs compared to ChAdOx1/ChAdOx1 due to serious risk of bias, inconsistency and imprecision (RR 0.34, 95% CI 0.01 to 8.17; 1 RCT, 229 participants; very low‐certainty evidence; Figure 39).
39.
Analysis 5.1.1: heterologous vaccination scheme versus homologous vaccination scheme. Outcome: serious adverse events (SAEs).
Liu X 2021.1 and Liu X 2021.2 are different comparisons for the same report (Liu 2021).
Systemic reactogenicity events
There was one comparison with results for this outcome (Li 2021a). The evidence is uncertain for an effect of CoronaVac/Ad5 on the incidence of systemic reactogenicity events compared to CoronaVac/CoronaVac due to very serious imprecision (RR 1.96, 95% CI 0.52 to 7.41; 1 RCT, 101 participants; low‐certainty evidence; Figure 40).
40.
Analysis 5.1.2: heterologous vaccination scheme versus homologous vaccination scheme. Outcome: systemic reactogenicity events.
Any adverse event
Two trials reported any adverse event on three different comparisons (Li 2021a; Liu 2021). CoronaVac/Ad5 versus CoronaVac homologous at 1‐month follow‐up (RR 3.19, 95% CI 1.11 to 9.11), BNT162b2/ChAdOx1 versus BNT162b2 homologous at two months' follow‐up (RR 1.21, 95% CI 0.87 to 1.68) and ChAdOx1/BNT162b2 versus ChAdOx1 homologous at 2 months' follow‐up (RR 1.03, 95% CI 0.75 to 1.43). The evidence is very uncertain about the effect of heterologous schemes on the incidence of any adverse event compared to homologous schemes due to serious risk of bias, inconsistency and imprecision (Figure 41).
41.
Analysis 5.1.3: heterologous vaccination scheme versus homologous vaccination scheme. Outcome: any adverse event (AE).
Liu 2021 included only participants 50 years of age or older.
Liu X 2021.1 and Liu X 2021.2 are different comparisons for the same report (Liu 2021).
Important outcomes
Immunogenicity outcomes
Li 2021a reported that the heterologous schedule CoronaVac/Ad5 elicited higher levels of specific antibodies against SARS‐COV‐2 (GMR 6.11, 95% CI 3.90 to 9.57) and neutralizing antibodies against SARS‐COV‐2 (GMR 4.25, 95% CI 2.63 to 6.86) compared to the homologous schedule CoronaVac/CoronaVac (Appendix 16 and Appendix 11).
Liu 2021 reported this outcome for two different comparisons. The outcome was measured using IFN‐γ ELISpot 28 days after the administration of the second dose.
The heterologous schedule ChAdOx1/BNT162b2 elicited a larger immune cellular response compared to the homologous schedule ChAdOx1/ChAdOx1 (GMR of number of spot‐forming cells (SFCs) per million peripheral blood mononuclear cell (PBMC)) 3.9 (95% CI 2.9 to 5.3)).
The GMR of SFCs per million PBMCs was 1.2 (95% CI 0.87 to 1.7) for the comparison of the heterologous schedule BNT162b2/ChAdOx to the homologous schedule BNT162b2/BNT162b2 (Appendix 20).
Local reactogenicity events
One trial reported this outcome (Li 2021a). The heterologous schedule (CoronaVac/Ad5) probably results in a large increase in the number of local reactogenicity events compared to the homologous schedule (CoronaVac/CoronaVac) (RR 11.76, 95% CI 1.59 to 87.14; 1 RCT, 101 participants; absolute effect: 215 more with local reactogenicity events per 1000 (from 12 more to 1000 more); low‐certainty evidence; Figure 42).
42.
Analysis 5.1.4: heterologous vaccination scheme versus homologous vaccination scheme. Outcome: local reactogenicity events.
Incidence of specific safety outcomes
Specific safety outcomes were not consistently reported throughout the included trials. Two trials reported on the number of participants with venous thrombosis (Li 2021a; Liu 2021). Outcomes are summarized in detail in Appendix 12.
Boosters
Homologous or heterologous booster versus placebo/no booster
See Table 15 and table of results in Appendix 28.
We identified and included two trials in the analysis (Hall 2021; Toledo‐Romani 2021). Hall 2021 included only kidney transplant recipient participants; in our judgement results from this trial are not generally applicable.
mRNA‐1273 booster versus placebo (normal saline)
Hall 2021 compared a booster dose of mRNA‐1273 to placebo after complete vaccination of mRNA‐1273 in kidney transplant recipients. They reported three outcomes of interest.
Systemic reactogenicity events
Follow‐up was seven days, starting after injection of the booster dose. There were 11 systemic reactogenicity events in the intervention arm (60 participants) compared to six in the control arm (59 participants). We assessed the overall risk of bias for the outcome to be low.
The evidence is uncertain for an effect of mRNA‐1273 booster on the incidence of systemic reactogenicity events compared to placebo due to serious imprecision (RR 1.80, 95% CI 0.71 to 4.56; 1 RCT, 119 participants; low‐certainty evidence; Figure 43).
43.
Analysis 6.1.2: booster versus placebo/no booster. Outcome: systemic reactogenicity events.
Immunogenicity outcomes
One trial reported results for cellular immune response (Hall 2021). The outcome was measured using intracellular cytokine staining 28 days after the administration of the booster or placebo. The median CD4+ T cells per million was higher in the booster arm than in the placebo arm (432 versus 67 cells per 100 CD4+ T cells; 95% CI for the between‐group difference, 46 to 986; Appendix 20).
Local reactogenicity events
The follow‐up period was seven days starting after the injection of the booster dose. There were 46 local reactogenicity events in the intervention arm (N = 60) compared to seven in the control arm (N = 59). We assessed the overall risk of bias for the outcome to be low. A‐1273 booster probably results in a large increase in the number of local reactogenicity events compared to placebo (RR 6.46, 95% CI 3.18 to 13.13; 1 RCT, 119 participants; absolute effect: 648 more local adverse event per 1000 (from 259 more to 1000 more); moderate‐certainty evidence; Figure 44).
44.
Analysis 6.1.3: booster versus placebo/no booster. Outcome: local reactogenicity events.
FINLAY‐FR‐1 booster versus no booster dose
All‐cause mortality
Toledo‐Romani 2021 compared a booster dose of FINLAY‐FR‐1 to no booster dose after complete vaccination of FINLAY‐FR‐2 in adults; only all‐cause mortality with a median follow‐up of 1.7 months was reported.
There were 11 deaths in the intervention arm (of 13,883 participants) compared to nine in the control arm (of 14,371 participants). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information about allocation concealment and the use of per‐protocol analysis.
The evidence is very uncertain about the effect of the booster dose of FR‐1 compared to adjuvant due to serious risk of bias and very serious imprecision (RR 1.27, 95% CI 0.52 to 3.05; 1 RCT, 28,254 participants; very low‐certainty evidence; Figure 45).
45.
Analysis 6.1.1: booster versus placebo/no booster. Outcome: all‐cause mortality.
Homologous booster versus heterologous booster
We identified four trials for this comparison (Bonelli 2021; Li 2021a; Mok 2021; Sablerolles 2021). Of note, in all trials specific safety outcomes were not consistently reported; these are summarized in Appendix 9.
BNT162b2 or mRNA‐1273 with homologous booster versus heterologous ChAdOx1 booster
One trial compared a homologous booster dose of BNT162b2 or mRNA‐1273 to a booster dose of ChAdOx1 in immunocompromized adults under current rituximab therapy (Bonelli 2021). They only reported on two outcomes of interest.
Immunogenicity outcomes
Bonelli 2021 reported results for cellular immune response. The outcome was measured using IFN‐γ ELISpot seven days after the administration of the booster dose. The median interquartile range (IQR) number of SFCs per million PBMCs was 459 (133 to 722) in the heterologous booster arm versus 305 (717 to 416) in the homologous booster arm (Appendix 20).
Local reactogenicity events
Follow‐up was two days starting after the injection of the booster dose. There were fewer local reactogenicity events in the ChAdOx1 heterologous booster arm (8/27) compared to the homologous booster arm (16/28) (RR 0.52, 95% CI 0.27 to 1.01). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information about allocation concealment, missingness of outcome data, unclear blinding which could have influenced the measurement of the outcome, and no information on whether the outcome was analyzed as prespecified (Figure 46).
46.
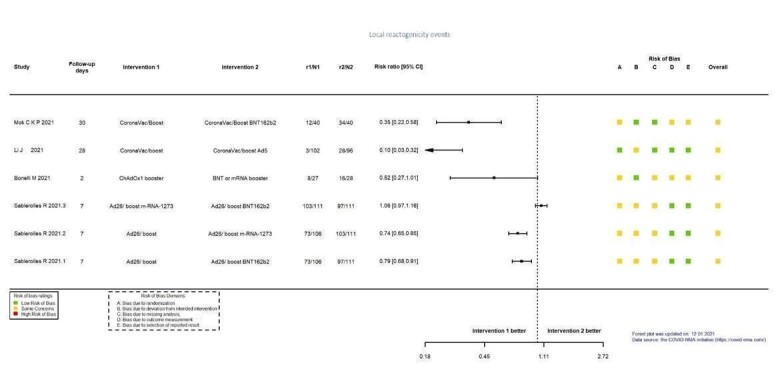
Analysis 6.2.4: homologous booster versus heterologous booster. Outcome: local reactogenicity events.
Bonelli 2021 included only participants under current Rituximab therapy.
Incidence of specific safety outcomes
Bonelli 2021 reported on the number of participants with thrombocytopaenia and nervous system diseases; details are in Appendix 12.
Ad26.COV2.S with homologous booster versus heterologous mRNA‐1273 booster
One trial compared a homologous booster dose of Ad26.COV2.S to a booster dose of mRNA‐1273 in healthcare workers (Sablerolles 2021). They only reported on three outcomes of interest.
Systemic reactogenicity events
Follow‐up was seven days starting after the injection of the booster dose. There were fewer systemic reactogenicity events in the homologous booster arm (62/106) compared to the mRNA‐1273 booster arm (84/111) (RR 0.77, 95% CI 0.64 to 0.94). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information on allocation concealment, use of per‐protocol analysis and missing outcome data (Figure 47).
47.
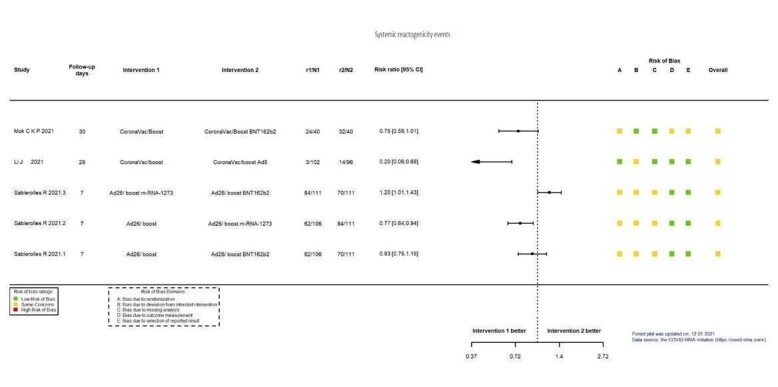
Analysis 6.2.2: homologous booster versus heterologous booster. Outcome: systemic reactogenicity events.
Immunogenicity outcomes
Sablerolles 2021 reported results for cellular immune response. The proportion of responders was measured using IFN‐y release assay (cut‐off is 0.15 IU/mL) 28 days after the administration of the booster dose. The proportion of responders was lower in the homologous booster arm (32/44; 72.7%) than in the heterologous booster arm (44/48; 91.7%) (RR 0.79, 95% CI 0.64 to 0.96; Appendix 20).
Local reactogenicity events
Follow‐up was seven days starting after the injection of the booster dose. There were fewer local reactogenicity events in the homologous booster arm (73/106) compared to the mRNA‐1273 booster arm (103/111) (RR 0.74, 95% CI 0.65 to 0.85). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information on allocation concealment, use of per‐protocol analysis and missing outcome data (Figure 46).
Ad26.COV2.S with homologous booster versus heterologous BNT162b2 booster
Sablerolles 2021 assessed complete vaccination of Ad26.COV2.S with a homologous booster dose of Ad26.COV2.S versus a heterologous booster dose of BNT162b2 in healthcare workers. They reported on three outcomes of interest.
Systemic reactogenicity events
Follow‐up was seven days starting after the injection of the booster dose. There were 62/106 systemic reactogenicity events in the homologous booster arm compared to 70/111 in the BNT162b2 booster arm (RR 0.93, 95% CI 0.75 to 1.15). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information on allocation concealment, use of per‐protocol analysis and missing outcome data (Figure 47).
Immunogenicity outcomes
Sablerolles 2021 reported results for cellular immune response. The proportion of responders was measured using IFN‐y release assay (cut‐off is 0.15 IU/mL) 28 days after the administration of the booster dose. The response rate was lower in the homologous booster arm (32/44; 72.7%) than in the heterologous booster arm (43/47; 91.5%) (RR 0.79, 95% CI 0.65 to 0.97; Appendix 20).
Local reactogenicity events
Follow‐up was seven days starting after the injection of the booster dose. There were fewer local reactogenicity events in the homologous booster arm (73/106) compared to the BNT162b2 booster arm (97/111) (RR 0.79, 95% CI 0.66 to 0.91). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information on allocation concealment, use of per‐protocol analysis and missing outcome data (Figure 46).
CoronaVac with homologous booster versus heterologous Ad5 booster
One trial compared a complete vaccination of CoronaVac with a homologous booster dose of CoronaVac to a heterologous booster dose of Ad5 in healthy adults (Li 2021a). They reported five outcomes of interest.
Serious adverse events
Zero SAEs were reported in both groups (Figure 48).
48.
Analysis 6.2.1: homologous booster versus heterologous booster. Outcome: serious adverse events.
Systemic reactogenicity events
Follow‐up was one month starting after the injection of the booster dose. There were fewer systemic reactogenicity events in the homologous booster arm (3/102) compared to the Ad5 booster arm (14/96) (RR 0.20, 95% CI 0.06 to 0.68). We assessed the overall risk of bias for the outcome to have some concerns due to the use of per‐protocol analysis (Figure 47).
Any adverse event
Follow‐up was one month starting after the injection of the booster dose. There were fewer adverse events in the homologous booster arm (5/102) compared to the Ad5 booster arm (34/96) (RR 0.14, 95% CI 0.06 to 0.34). We assessed the overall risk of bias for the outcome to have some concerns due to the use of per‐protocol analysis (Figure 49).
49.
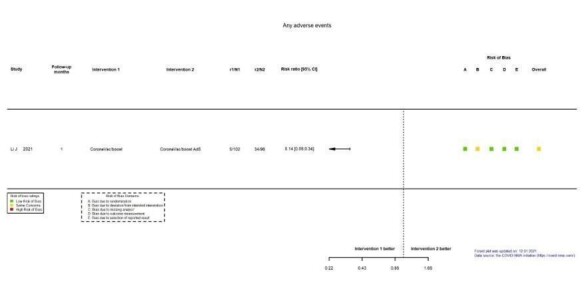
Analysis 6.2.3: homologous booster versus heterologous booster. Outcome: any adverse event.
Immunogenicity outcomes
Li 2021a reported that the heterologous booster CoronaVac/Ad5 elicited higher levels of specific antibodies against SARS‐COV‐2 (GMR 8.37, 95% CI 6.52 to 10.75) and neutralizing antibodies against SARS‐COV‐2 (GMR 5.87, 95% CI 4.64 to 7.43) compared to the homologous booster CoronaVac/CoronaVac (Appendix 16; Appendix 11).
Local reactogenicity events
Follow‐up was one month starting after the injection of the booster dose. There were fewer local reactogenicity events in the homologous booster arm (3/102) compared to the Ad5 booster arm (28/96) (RR 0.10, 95% CI 0.03 to 0.32). We assessed the overall risk of bias for the outcome to have some concerns due to the use of per‐protocol analysis (Figure 46).
CoronaVac with a homologous booster versus heterologous BNT162b2 booster
One trial compared complete vaccination of CoronaVac with a homologous booster dose of CoronaVac to a heterologous booster dose of BNT162b2 in adults with low‐immune response against SARS‐CoV‐2 after complete vaccination of CoronaVac (Mok 2021). They reported two outcomes of interest.
Systemic reactogenicity events
Follow‐up was one month starting after the injection of the booster dose. There were fewer systemic reactogenicity events in the homologous booster arm (24/40) compared to the BNT162b2 booster arm (32/40) (RR 0.75, 95% CI 0.56 to 1.01). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information on allocation concealment, unclear blinding which could have influenced the measurement of the outcome, and the outcome not being prespecified (Figure 47).
Local reactogenicity events
Follow‐up was one month starting after the injection of the booster dose. There were fewer local reactogenicity events in the homologous booster arm (12/40) compared to the BNT162b2 booster arm (34/40) (RR 0.35, 95% CI 0.22 to 0.58). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information on allocation concealment, unclear blinding which could have influenced the measurement of the outcome, and the outcome not being prespecified (Figure 46).
Heterologous booster versus heterologous booster
Ad26.COV2.S with mRNA‐1273 booster versus Ad26.COV2.S with BNT162b2 booster
One trial compared mRNA‐1273 booster to BNT162b2 booster in healthcare workers vaccinated with Ad26.COV2.S (Sablerolles 2021). They reported on three outcomes of interest.
Systemic reactogenicity events
Follow‐up was seven days starting after the injection of the booster dose. There were more systemic reactogenicity events in the mRNA‐1273 booster arm (84/111) compared to the BNT162b2 booster arm (70/111) (RR 1.20, 95% CI 1.01 to 1.43). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information on allocation concealment, use of per‐protocol analysis, and missing outcome data (Figure 47).
Immunogenicity outcomes
Sablerolles 2021 reported results for cellular immune response. The proportion of responders was measured using IFN‐y release assay (cut‐off is 0.15 IU/mL) 28 days after the administration of the booster dose. The number of responders was similar in the mRNA‐1273 booster arm (44/48; 91.7%) compared to the BNT162b2 booster arm (43/47; 91.5%) (RR 1.00, 95% CI 0.88 to 1.13; Appendix 20).
Local reactogenicity events
Follow‐up was seven days starting after the injection of the booster dose. There were 103/111 participants with local reactogenicity events in the mRNA‐1273 booster arm compared to 97/111 in the BNT162b2 booster arm (RR 1.06, 95% CI 0.97 to 1.16). We assessed the overall risk of bias for the outcome to have some concerns due to lack of information on allocation concealment, use of per‐protocol analysis, and missing outcome data (Figure 46).
Effects of the intervention on variants of concern
Given that the prevalence of more than one variant in the same population changes and shifts over time, it is to be expected that most of the trials, which collect data over several months, reflect the heterogeneity of COVID‐19 variants in their sample. However, among our included studies, 10 did report vaccine efficacy on confirmed symptomatic COVID‐19 after complete vaccination against four variants of concern: Alpha (Dunkle 2021; Emary 2021; Heath 2021; Kremsner 2021), Beta (Madhi 2021b; Sadoff 2021b; Shinde 2021; Thomas 2021), Gamma (Clemens 2021; Kremsner 2021), and Delta (Ella 2021b). No study had yet reported data regarding the Omicron variant at the time of the data cut‐off (5 November 2021).
We considered the direct evidence when study reports provided evidence on a sequenced sample. When sequencing was not performed, we extrapolated the exposure to variants from the prevalence in the study setting.
Alpha variant (B.1.1.7)
Vaccine efficacy against the Alpha variant was reported in three trials, assessing three different vaccines. All cases of the Alpha variant were detected with genome sequencing. Of note, Emary 2021 includes only participants of the COV002 trial (Voysey 2021a).
Reported vaccine efficacy on confirmed symptomatic COVID‐19 after complete vaccination was 55.10%, 95% CI 23.50% to 73.60% for CVnCoV (Kremsner 2021); 70.40%, 95% CI 43.60% to 84.50% for ChAdOx1 (Emary 2021); and for NVX‐CoV2373 was 86.30%, 95% CI 71.30% to 93.50% (Heath 2021) and 93.60%, 95% CI 81.70% to 97.80% (Dunkle 2021) (Figure 50).
50.
Analysis 7.1.1: variant‐Alpha. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
Beta variant (B.1.351)
Vaccine efficacy against the Beta variant was reported in four trials, assessing four different vaccines. Results from three trials are based only on genetically sequenced cases (direct evidence) (Madhi 2021b; Shinde 2021; Thomas 2021). In contrast, results in Sadoff 2021b include all cases identified and the prevalence of the Beta variant among participants (94.5%), obtained by sequencing a sample of RT‐PCR positive cases, was extrapolated to the results (indirect evidence). Of note, Madhi 2021b includes only participants of the COV005 trial (Voysey 2021a).
Reported vaccine efficacy on confirmed symptomatic COVID‐19 after complete vaccination was 100.00%, 95% CI 53.50% to 100.00% for BNT16b2 (Thomas 2021); 10.40%, 95% CI 0.00% to 54.80% for ChAdOx1 (Madhi 2021b); 52.00%, 95% CI 30.30% to 67.40% for Ad26.COV2.S (Sadoff 2021b), and 43.00%, 95% CI 0.00% to 70.40% for NVX‐CoV2373 (Shinde 2021) (Figure 51).
51.
Analysis 7.2.1: variant‐Beta. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
Gamma variant (P.1)
Vaccine efficacy against the Gamma variant was reported in two trials, assessing two different vaccines. All cases of the Gamma variant were detected with genome sequencing. Reported vaccine efficacy on confirmed symptomatic COVID‐19 after complete vaccination was 67.10%, 95% CI 29.80% to 84.60% for CVnCoV (Kremsner 2021), and 63.60%, 95% CI 0.00% to 87.00% for ChAdOx1 (Clemens 2021) (Figure 52).
52.
Analysis 7.3.1: variant‐Gamma. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
Delta (B.1.617.2)
Vaccine efficacy against the Delta variant was reported in one trial. All cases of the Delta variant were detected with genome sequencing.
Reported vaccine efficacy on confirmed symptomatic COVID‐19 after complete vaccination was 65.20%, 95% CI 33.10% to 83.00% for BBV152 (Ella 2021b) (Figure 53).
53.
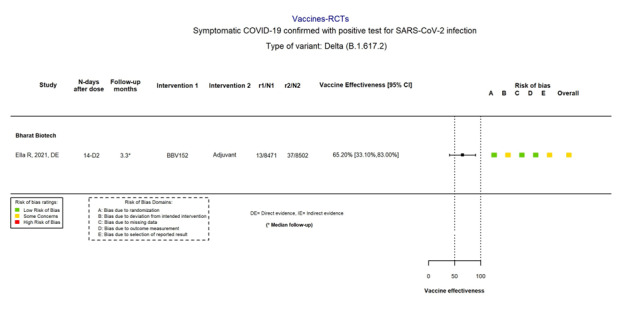
Analysis 7.4.1: variant‐Delta. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
Assessment of vaccine efficacy over time
Out of the 41 included trials, only two studies reported on the change of vaccine efficacy over time for the outcome 'incidence of confirmed symptomatic COVID‐19 after complete vaccination' for comparisons BNT162b2 versus placebo (BioNtech/Fosun Pharma/Pfizer) and mRNA‐1273 versus placebo (ModernaTX) (El Sahly 2021; Thomas 2021).
For the comparison BNT162b2 versus placebo, vaccine efficacy seems to decrease slightly over time. However, the effect remains large: VE 96.20%, 95% CI 93.30% to 98.10% after a median follow‐up less than 2 months and VE 83.70%, 95% CI 74.70% to 89.90% after a median follow‐up of 4 months to 6 months (Figure 54).
54.
Analysis 8.1: follow‐up. RNA‐based vaccine. Outcome: confirmed symptomatic COVID‐19 after complete vaccination.
When comparing mRNA‐1273 with placebo, vaccine efficacy was consistent over time (VE 91.80%, 95% CI 86.90% to 95.10%; median follow‐up less than two months and VE 92.40%, 84.30% to 96.80%; median follow‐up four months or greater) (Figure 54).
Exploration of heterogeneity
Subgroup analysis
We had planned to perform subgroup analysis for different age groups and immunocompromized patients; however due to the low number of studies we could not undertake formal subgroup analyses for each comparison.
Sensitivity analysis
Overall, all results for all outcomes were consistent in every sensitivity analysis as compared with the primary analysis. Small differences were mostly observed due to the increase of uncertainty in the summary estimate when excluding some trials.
RNA‐based vaccines
Overall, results were consistent in all the analyses (Table 16).
1. Sensitivity analysis: RNA‐based vaccines.
| Developer – comparison | Analysesa | Outcomes | ||||||
| SARS‐CoV‐2 infection | Symptomatic COVID‐19 | Severe COVID‐19 | All‐cause mortality | SAEs | Systemic reactogenicity events | AEs | ||
|
VE (95% CI) No. of trials (No. of participants) |
RR (95% CI) No. of trials (No. of participants) |
|||||||
| BNT162b2 – Pfizer/BioNTech+Fosun Pharma versus placebo | Main analysis | — | 97.84% (44.25% to 99.92%) 2 RCTs (44,077) |
95.70% (73.90% to 99.90%) 1 RCT (46,077) |
1.07 (0.52 to 2.22) 1 RCT (43,846) |
1.30 (0.55 to 3.07) 2 RCTs (46,107) |
— | 1.52 (0.88 to 2.63) 3 RCTs (46,419) |
| Sensitivity 1 | — | — | — | 1.07 (0.52 to 2.22) 1 RCT (44,165) |
1.30 (0.55 to 3.05) 2 RCTs (46,429) |
— | 1.52 (0.88 to 2.63) 3 RCTs (46,471) |
|
| Sensitivity 2 | — | — | — | — | — | — | — | |
| Sensitivity 3 | — | — | — | — |
— |
— | — | |
| mRNA‐1273 – ModernaTX versus placebo | Main analysis | 73.27% (35.82% to 88.87%) 2 RCTs (31,632) |
93.20% (91.06% to 94.83%) 2 RCTs (31,632) |
98.20% (92.80% to 99.60%) 1 RCT (28,451) |
1.06 (0.54 to 2.10) 1 RCT (30,346) |
0.92 (0.78 to 1.08) 2 RCTs (34,072) |
1.28 (1.22 to 1.34) 2 RCTs (34,037) |
1.19 (0.79 to 1.80) 2 RCTs (34,072) |
| Sensitivity 1 | — | — | — | 1.06 (0.54 to 2.10) 1 RCT (30,415) |
0.92 (0.78 to 1.09) 2 RCTs (34,147) |
1.28 (1.22 to 1.34) 2 RCTs (34,147) |
1.20 (0.79 to 1.80) 2 RCTs (34,147) |
|
| Sensitivity 2 | — | — | — | — | — | — | — |
|
| Sensitivity 3 | — | — | — | — |
— |
— | — | |
| CVnCoV – CureVac AG versus placebo | Main analysis | — | 48.20% (31.70% to 60.90%) 1 RCT (25,062) |
63.80% (0.00% to 91.70%) 1 RCT (25,062) |
1.33 (0.46 to 3.83) 1 RCT (39,529) |
1.24 (0.90 to 1.71) 1 RCT (39,529) |
1.48 (1.43 to 1.53) 1 RCT (3982) |
1.42 (1.38 to 1.47) 1 RCT (3982) |
| Sensitivity 1 | — | — | — | — | — | 1.49 (1.39 to 1.60) 1 RCT (39,529) |
1.43 (1.34 to 1.53) 1 RCT (39,529) |
|
| Sensitivity 2 | — | — | — | — | — | — | — | |
| Sensitivity 3 | — | — | — | — | — | — | — | |
| AE: adverse event; CI: confidence interval; COVID‐19: coronavirus disease 2019; RCT: randomized controlled trial; RR: risk ratio; SAE: serious adverse event; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||||
aSensitivity 1: participants randomized; Sensitivity 2: early‐phase studies excluded; Sensitivity 3: only published studies.
Non‐replicating viral vector vaccines
Overall, results were consistent in all the analyses (Table 17). An important but not statistically significant reduction in the RR for adverse event was observed, though, when excluding the early‐phase trial.
2. Sensitivity analysis: non‐replicating viral vector vaccine .
| Developer – comparison | Analysesa | Outcomes | ||||||
| SARS‐CoV‐2 infection | Symptomatic COVID‐19 | Severe COVID‐19 | All‐cause mortality | SAEs | Systemic reactogenicity events | AEs | ||
|
VE (95% CI) No. of trials (No. of participants) |
RR (95% CI) No. of trials (No. of participants) |
|||||||
| ChAdOx1 – AstraZeneca + University of Oxford versus placebo | Main analysis | 59.35% (48.00% to 68.22%) 5 RCTs (43,390) |
70.23% (62.10% to 76.62%) 5 RCTs (43,390) |
— | 0.48 (0.20 to 1.14) 5 RCTs (56,726) |
0.88 (0.72 to 1.07) 7 RCTs (58,182) |
3.93 (2.11 to 7.29) 1 RCT (256) |
Not pooled |
| Sensitivity 1 | — | — | — | 0.50 (0.20 to 1.21) 5 RCTs (56,873) |
0.86 (0.70 to 1.06) 7 RCTs (58,329) |
— | — | |
| Sensitivity 2 | — | — | — | 0.48 (0.20 to 1.14) 5 RCTs (56,623) |
0.88 (0.72 to 1.08) 6 RCTs (57,823) |
— | — | |
| Sensitivity 3 | — | — | — | 0.50 (0.20 to 1.21) 5 RCTs (56,623) |
0.86 (0.70 to 1.05) 6 RCTs (56,879) |
— | — | |
| Ad26.COV2.S – Janssen Pharmaceutical Companies versus placebo | Main analysis | — | 66.90% (59.10% to 73.40%) 1 RCT (39,058) |
76.30% (57.90% to 87.50%) 1 RCT (39,058) |
0.25 (0.09 to 0.67) 1 RCT (43,783) |
0.92 (0.69 to 1.22) 1 RCT (43,783) |
1.83 (1.29 to 2.60) 2 RCTs (7222) |
1.57 (0.75 to 3.29) 2 RCTs (7222) |
| Sensitivity 1 | — | — | — | 0.25 (0.09 to 0.67) 1 RCT (44,325) |
0.92 (0.69 to 1.22) 1 RCT (44,325) |
1.83 (1.27 to 2.63) 2 RCTs (44,813) |
1.57 (0.74 to 3.32) 2 RCTs (44,813) |
|
| Sensitivity 2 | — | — | — | — | — | — | 1.09 (0.96 to 1.24) 1 RCT (6736) |
|
| Sensitivity 3 | — | — | — | — | — | — | — | |
| Gam‐COVID‐Vac – Gamaleya Research Institute (Sputnik V) Gam‐COVID‐Vac versus placebo | Main analysis | — | 91.10% (83.80% to 95.10%) 1 RCT (18,695) |
100.00% (94.40% to 100.00%) 1 RCT (19,866) |
0.99 (0.10 to 9.54) 1 RCT (21,862) |
0.65 (0.39 to 1.07) 1 RCT (21,862) |
— | — |
| Sensitivity 1 | — | — | — | 1.00 (0.10 to 9.57) 1 RCT (21,977) |
0.65 (0.39 to 1.07) 1 RCT (21,977) |
— | — | |
| Sensitivity 2 | — | — | — | — | — | — | — | |
| Sensitivity 3 | — | — | — | — | — | — | — | |
| AE: adverse event; CI: confidence interval; COVID‐19: coronavirus disease 2019; RCT: randomized controlled trial; RR: risk ratio; SAE: serious adverse event; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||||
aSensitivity 1: participants randomized; Sensitivity 2: early‐phase studies excluded; Sensitivity 3: only published studies.
Inactivated virus vaccines
Results were consistent, with the exception of an increase in vaccine efficacy against confirmed symptomatic COVID‐19 after complete vaccination for CoronaVac compared to placebo when excluding results reported as preprints (VE 83.5%, 95% CI 65.4% to 92.1%) (Tanriover 2021) (Table 18). Using the participants randomized instead of those analyzed seemed to increase the heterogeneity, whereas excluding early‐phase trials slightly decreased the heterogeneity and increased the precision of the summary estimate.
3. Sensitivity analysis: inactivated virus vaccine.
| Developer – comparison | Analysesa | Outcomes | ||||||
| SARS‐CoV‐2 infection | Symptomatic COVID‐19 | Severe COVID‐19 | All‐cause mortality | SAEs | Systemic reactogenicity events | AEs | ||
|
VE (95% CI) No. of trials (No. of participants) |
RR (95% CI) No. of trials (No. of participants) |
|||||||
| CoronaVac – Sinovac versus placebo | Main analysis | — | 69.81% (12.27% to 89.61%) 2 RCTs (19,852) |
— | 0.50 (0.05 to 5.52) 1 RCT (12,396) |
0.97 (0.62 to 1.51) 4 RCTs (23,139) |
0.95 (0.55 to 1.62) 7 RCTs (23,956) |
1.09 (1.07 to 1.11) 6 RCTs (23,367) |
| Sensitivity 1 | — | — | — | 0.50 (0.05 to 5.52) 1 RCT (12,408) |
0.99 (0.64 to 1.51) 4 RCTs (23,157) |
1.56 (0.91 to 2.69) 7 RCTs (25,106) |
1.09 (1.07 to 1.11) 6 RCTs (23,385) |
|
| Sensitivity 2 | — | — | — | — | 0.99 (0.63 to 1.55) 2 RCTs (22,610) |
1.21 (0.98 to 1.49) 4 RCTs (23,584) |
1.09 (1.07 to 1.11) 2 RCTs (22,610) |
|
| Sensitivity 3 | — | 83.50% (65.40% to 92.10%) 1 RCT (10,029) |
— | — | 0.73 (0.24 to 2.21) 4 RCTs (10,894) |
0.94 (0.49 to 1.81) 6 RCTs (11,617) |
1.13 (1.04 to 1.23) 4 RCTs (10,640) |
|
| WIBP‐CorV – Sinopharm‐Wuhan versus placebo | Main analysis | 64.00% (48.80% to 74.70%) 1 RCT (25,449) |
72.80% (58.10% to 82.40%) 1 RCT (25,480) |
— | — | 0.83 (0.60 to 1.15) 2 RCTs (27,029) |
0.99 (0.95 to 1.03) 2 RCTs (27,029) |
0.96 (0.93 to 0.98) 2 RCTs (27,029) |
| Sensitivity 1 | — | — | — | — | 0.83 (0.60 to 1.15) 2 RCTs (27,053) |
0.99 (0.95 to 1.03) 2 RCTs (27,053) |
0.96 (0.93 to 0.98) 2 RCTs (27,053) |
|
| Sensitivity 2 | — | — | — | — | 0.82 (0.59 to 1.14) 1 RCT (26,917) |
0.99 (0.95 to 1.03) 1 RCT (26,917) |
0.96 (0.93 to 0.98) 1 RCT (26,917) |
|
| Sensitivity 3 | — | — | — | — | — | — | — | |
| BBIBP‐CorV – Sinopharm‐Beijing versus placebo | Main analysis | 73.50% (60.60% to 82.20%) 1 RCT (25,463) |
78.10% (64.80% to 86.30%) 1 RCT (25,463) |
— | — | 0.76 (0.54 to 1.06) 1 RCT (26,924) |
1.05 (0.86 to 1.28) 3 RCTs (27,540) |
Not pooled |
| Sensitivity 1 | — | — | — | — | — |
1.05 (0.86 to 1.28) 3 RCTs (27,557) |
Not pooled | |
| Sensitivity 2 | — | — | — | — | — |
1.02 (0.98 to 1.06) 1 RCT (26,924) |
||
| Sensitivity 3 | — | — | — | — | — | — | — | |
| BBV152 – Bharat Biotech versus placebo | Main analysis | 68.80% (46.70% to 82.50%) 1 RCT (6289) |
77.80% (65.20% to 86.40%) 1 RCT (16,973) |
99.70% (96.79% to 99.79%) 1 RCT (16,976) |
0.50 (0.17 to 1.46) 1 RCT (25,753) |
0.65 (0.43 to 0.97) 1 RCT (25,753) |
1.34 (1.15 to 1.58) 2 RCTs (25,925) |
1.00 (0.94 to 1.07) 1 RCT (25,753) |
| Sensitivity 1 | — | — | — | 0.50 (0.17 to 1.46) 1 RCT (25,778) |
0.65 (0.43 to 0.97) 2 RCTs (25,953) |
1.35 (1.15 to 1.58) 2 RCTs (25,953) |
1.00 (0.94 to 1.07) 1 RCT (25,778) |
|
| Sensitivity 2 | — | — | — | — | 0.65 (0.43 to 0.97) 1 RCT (25,753) |
1.34 (1.14 to 1.58) 1 RCT (25,753) |
— | |
| Sensitivity 3 | — | — | — | — | — | 1.47 (0.63 to 3.47) 1 RCT (172) |
— | |
| AE: adverse event; CI: confidence interval; COVID‐19: coronavirus disease 2019; RCT: randomized controlled trial; RR: risk ratio; SAE: serious adverse event; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||||
aSensitivity 1: participants randomized; Sensitivity 2: early‐phase studies excluded; Sensitivity 3: only published studies.
Protein subunit vaccines
Overall, results were consistent in all the analyses (Table 19).
4. Sensitivity analysis: protein subunit vaccine.
| Developer‐comparison | Analysesa | Outcomes | ||||||
| SARS‐CoV‐2 infection | Symptomatic COVID‐19 | Severe COVID‐19 | All‐cause mortality | SAEs |
Systemic reactogenicity events |
AEs | ||
|
VE (95% CI) No. of trials (No. of participants) |
RR (95% CI) No. of trials (No. of participants) |
|||||||
| NVX‐CoV2373 – Novavax versus placebo | Main analysis | — | 82.91% (50.49% to 94.10%) 3 RCTs (42,175) |
100.00% (86.99% to 100.00%) 1 RCT (25,452) |
0.90 (0.30 to 2.68) 1 RCT (29,582) |
0.92 (0.74 to 1.14) 4 RCTs (46,202) |
1.21 (1.17 to 1.25) 3 RCTs (31,063) |
1.15 (1.05 to 1.26) 5 RCTs (46,231) |
| Sensitivity 1 | — | — | — | — |
0.92 (0.74 to 1.14) 4 RCTs (50,111) |
1.21 (1.17 to 1.26) 3 RCTs (34,870) |
1.16 (1.05 to 1.27) 5 RCTs (50,111) |
|
| Sensitivity 2 | — | — | — | — | 0.93 (0.75 to 1.15) 3 RCTs (45,689) |
1.20 (1.17 to 1.24) 2 RCTs (30,550) |
1.14 (1.02 to 1.27) 3 RCTs (45,689) |
|
| Sensitivity 3 | — | 77.10% (0.00% to 95.19%) 2 RCTs (16,723) |
— | — |
0.99 (0.65 to 1.51) 3 RCTs (16,620) |
1.24 (1.03 to 1.49) 2 RCTs (1481) |
1.18 (1.03 to 1.35) 4 RCTs (16,672) |
|
| FINLAY‐FR‐2 – Instituto Finlay de Vacunas versus placebo | Main analysis | — | 71.00% (58.90% to 79.10%) 1 RCT (28,674) |
— | 0.37 (0.17 to 0.80) 1 RCT (28,674) |
— | — | — |
| Sensitivity 1 | — | — | — | — |
— | — | — | |
| Sensitivity 2 | — | — | — | — |
— | — | — | |
| Sensitivity 3 | — | — | — | — |
— |
— | — | |
| AE: adverse event; CI: confidence interval; COVID‐19: coronavirus disease 2019; RCT: randomized controlled trial; RR: risk ratio; SAE: serious adverse event; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VE: vaccine efficacy. | ||||||||
aSensitivity 1: participants randomized; Sensitivity 2: early‐phase studies excluded; Sensitivity 3: only published studies.
Discussion
Summary of main results
We identified and included 41 RCTs evaluating four different vaccine platforms and 12 vaccine candidates published in 65 reports in the analysis. Six RCTs reported results for three RNA‐based vaccines (BNT162b2 from BioNtech/Fosun Pharma/Pfizer; mRNA‐1273 from ModernaTX; CVnCoV by CureVac AG), and 10 RCTs evaluated three non‐replicating viral vector vaccines (ChAdOx1 by AstraZeneca/University of Oxford and SII‐ChAdOx1; Ad26.COV2.S by Janssen Pharmaceutical Companies; Gam‐COVID‐Vac by Gamaleya Research Institute), 13 RCTs evaluated four inactivated virus vaccines (CoronaVac by Sinovac; WIBP‐CorV by Sinopharm‐Wuhan; BBIBP‐CorV by Sinopharm‐Beijing; BBV152 by Bharat Biotech), and 6 RCTs evaluated two protein subunit vaccines (NVX‐CoV2373 by Novavax; FINLAY‐FR‐2 by Instituto Finlay de Vacunas).
Our review also retrieved two trials comparing heterologous vaccination schemes with homologous vaccination schemes, two trials comparing booster versus placebo/no booster, and four trials comparing homologous and heterologous booster doses. Only 10 studies reported results on vaccine efficacy of six different vaccine candidates against any specific variant, which limits our ability to make any variant‐specific claims.
Efficacy outcomes for vaccines versus placebo
There is moderate‐ to high‐certainty evidence that several vaccine candidates are effective in preventing SARS‐CoV‐2 infection (i.e. mRNA‐1273, ChAdOx1, WIBP‐CorV, BBIBP‐CorV, BBV152); symptomatic COVID‐19 (i.e. BNT162b2, mRNA‐1273, CVnCoV, ChAdOx1, Ad26.COV2.S, Gam‐COVID‐Vac, WIBP‐CorV, BBIBP‐CorV, BBV152, NVX‐CoV2373, FINLAY‐FR‐2), and severe or critical disease compared to placebo (i.e. BNT162b2, mRNA‐1273, Ad26.COV2.S, Gam‐COVID‐Vac, BBV152, NVX‐CoV2373).
There is moderate‐certainty evidence that Ad26.COV2.S and FINLAY‐FR‐2 result in a decrease in all‐cause mortality compared to placebo. Evidence was uncertain and very uncertain for death for all other vaccines because of the low number of events.
Safety outcomes for vaccines versus placebo
Overall, we identified an increase in local reactogenicity events such as pain, redness, swelling, and systemic reactogenicities such as tiredness, headache, muscle pain, chills, fever, and nausea. There is moderate‐ to high‐certainty evidence that most vaccine candidates have an increased risk of systemic reactogenicity events (e.g. fever) compared to placebo (mRNA‐1273, CVnCoV, ChAdOx1, Ad26.COV2.S, WIBP‐CorV, BBIBP‐CorV, BBV152, NVX‐CoV2373). These events were expected.
We did not find evidence of an increase in SAEs. There is moderate‐ to high‐certainty evidence that there is probably little or no difference between mRNA‐1273, ChAdOx1, Ad26.COV2.S and BBV152, and placebo in terms of SAEs. Evidence was uncertain and very uncertain for SAEs for other vaccines because of the low number of events.
We also extracted some specific adverse events, that is, cardioembolic events (pulmonary embolism, stroke, cavernous sinus thrombosis, pericarditis, venous thrombosis, myocardial infarction); haematological events (thrombocytopaenia, haemorrhage, neutropenia, anaemia, lymphadenopathy); and neurological events. The reporting of these events was very inconsistent and the number of events reported was very low.
The outcome 'any adverse event' was reported inconsistently. Some considered only the non‐SAE including local and systemic reactogenicity events. Some also considered SAEs, and frequently it was unclear how these events were classified. Overall, we found moderate‐ to high‐certainty evidence that vaccine increases any adverse event for three vaccines (i.e. CVnCoV, NVX‐CoV2373, CoronaVac) and that vaccine results in no increase in any adverse event for two vaccines (i.e. WIBP‐CorV, BBV152). Evidence was uncertain for other vaccines.
As trials' follow‐up was short and the incidence of SAEs was very low, vaccine safety surveillance systems have been put in place to detect rare adverse events and concerns have been raised related to the occurrence of vaccine‐induced immune thrombocytopaenia and thrombosis (Makris 2021; Ostrowski 2021; Rizk 2021; Sharifian‐Dorche 2021).
Other evidence
We found little evidence regarding the differences between heterologous and homologous vaccination schemes, and the effect of booster vaccines (homologous or heterologous). Outcomes considered were mainly immunogenicity outcomes.
In the two studies (assessing mRNA‐1273 and BNT162b2) for which we have data at different time points, vaccine efficacy at short term was consistent with longer‐term results.
Effects of the interventions on specific subpopulations
Given the sparsity of data, we were unable to explore heterogeneity in the results by conducting subgroup analyses, and therefore decided to present results separately for specific subpopulations. We identified only four clinical trials including children and adolescents, and assessed BNT162b2, mRNA‐1273, CoronaVac and BBIBP‐CorV (Ali 2021; Frenck 2021; Han 2021; Xia 2020). We found more studies focused on, or reporting subgroup data for elderly participants, with single studies reporting different outcomes in elderly participants. However, data were still sparse and should be interpreted with caution. Finally, only three studies reported data for immunocompromized participants, each assessing a different vaccine candidate (ChAdOx1, NVX‐CoV2373, and mRNA‐1273 booster versus placebo). No studies were conducted on pregnant women, and pregnant women were very rarely included in trials although it has been reported that they are at greater risk of severe COVID‐19 disease (Qiao 2020).
Impact of the results on future research
The high efficacy of several vaccine candidates, their marketing authorization and the rapid roll‐out population‐wide, raise the question of the feasibility and ethics of placebo RCTs assessing a new vaccine candidate.
For the ongoing placebo trials, the question is whether participants randomized to the placebo group should be unblinded and offered vaccine. Some argue the need to pursue follow‐up to obtain strong data on long‐term efficacy and safety (WHO Ad Hoc Expert Group 2021); others argue that given the clear evidence of a benefit for important outcomes, it would be unethical not to provide a vaccine to all participants (Dal‐Ré 2021a; Dal‐Ré 2021b).
Assessing vaccine efficacy and safety in randomized trials is also difficult considering the rapid evolution of the disease and the emergence of new variants that could impact vaccine efficacy. Large population‐based observational data provide useful complementary information, although they need to be interpreted carefully because of the risk of bias.
Future research questions should focus on the efficacy and safety of vaccines on specific populations, such as pregnant women, immunosuppressed patients and other vulnerable populations, on variants of concerns, and on how we can overcome the waning of vaccine efficacy over time.
An increasing number of trials consider only immunogenetic outcomes to allow a smaller sample size to generate a more rapid answer. However, there is considerable heterogeneity in assessing these outcomes and a consensus is needed on a core outcome set to enable effective comparison and synthesis of studies. Further, their results must be interpreted with caution.
Overall completeness and applicability of evidence
The evidence identified is incomplete. We identified 344 registered RCTs from registries evaluating the efficacy of COVID‐19, of which 10 were completed but not published (non‐replicating viral vector, replicating viral vector, inactivated virus, protein subunit and DNA‐based platforms). The planned sample size of the completed trials for non‐replicating viral vector vaccines is 27 participants, 90 participants for replicating viral vector vaccines, 19,512 for inactivated virus vaccines, 173 for protein subunit vaccines, and 30 for DNA‐based vaccines, yielding a total planned sample size of 19,832.
The applicability of the results should be interpreted with caution. The trials spanned all geographical regions: seven trials were conducted in North America, 14 in Asia, four in South America, eight in Europe, two in Africa, and one in Oceania. Notwithstanding the worldwide geographical representation of trials, it is noteworthy that the representation is skewed. Inactivated vaccine and protein subunit vaccine trials were mostly limited to India, Cuba, and China. Furthermore, trials for mRNA‐1273 were only conducted in the USA.
Our review also highlights the lack of evidence from RCTs regarding the efficacy of vaccines against specific variants. This is not surprising, given the relatively short period between the dominance of one variant and the next. Future studies might report more consistently on the specific variant predominating in their sample or report results stratified by variant, which would allow for more specific meta‐analyses in the future. It is likely that data on efficacy by variant will mainly come from large population‐based observational studies. The COVID‐NMA initiative identified observational studies evaluating vaccine efficacy on the Delta variant, and provides some results on the platform (covid-nma.com). Given that Omicron has replaced all other variants in most countries, data may not be applicable to the current situation.
We found high‐ or moderate‐certainty evidence for many of the main efficacy results of our review. However, the impact of effect modifiers, such as age or immunocompromized status, could not be explored adequately through subgroup analyses nor by meta‐regression. Specific trials including these specific populations should be conducted. Vaccine efficacy on these subgroups could also be explored through large observational studies using routinely collected data.
Certainty of the evidence
Overall, evidence of the critical outcomes exhibited a certainty of evidence ranging from very low certainty to high certainty. The evidence for outcomes of efficacy against SARS‐CoV‐2 infection, symptomatic COVID‐19, and severe or critical COVID‐19 was most often of moderate or high certainty. In contrast, we frequently downgraded safety outcomes and all‐cause mortality.
The reason for which we downgraded certainty of evidence most often, throughout the results for all vaccine types, was imprecision, referring to wide CIs in our results. This was often the result of a low number of events, and less often due to inconsistencies between the included studies or risk of bias. This explains why so few of the results related to mortality or severe adverse events, which are more rare events, achieved levels of moderate‐ or high‐certainty evidence. We expect higher levels of certainty to be reached as more studies are published, and the body of evidence grows.
In one trial (Logunov 2021), we downgraded the certainty of evidence due to concerns about the trustfulness of the analyses (Bucci 2021). The authors responded to some of these concerns, and the manuscript was corrected (Logunov 2021). Nevertheless, uncertainty persists particularly related to the prespecification of the interim analysis and the excess of homogeneity of vaccine efficacy across age groups.
Potential biases in the review process
We followed the guidance of the Cochrane Handbook for Systematic Reviews of Interventions in order to minimize several potential biases in the review process (Higgins 2021). First, the search strategy was peer reviewed. We initially performed a thorough search in several electronic databases and then considered only high‐quality sources, particularly the L·OVE platform and the Cochrane COVID‐19 Study Register. Second, all data were extracted in duplicate with consensus. Third, to increase our review's informative value, we track all registered trials in a living mapping. Finally, the review is updated continually; each week, we search for new trials and collect data, and bi‐weekly we update the syntheses. All updates of this review are available on the COVID‐NMA platform (covid-nma.com).
Another consideration for this rapidly evolving field is the availability of preprint articles that have not yet undergone peer review. In this review, we also included preprints. However, we are aware of these publications' potentially differing quality and that results could change once the peer‐reviewed journal publications are available (Oikonomidi 2020). To overcome this issue, we developed a preprint tracker to keep us informed of updates, so we can update data collection and data analysis when a preprint is modified or published (Cabanac 2021). We also conducted sensitivity analyses excluding preprints, and found consistent results.
Agreements and disagreements with other studies or reviews
We identified seven systematic reviews reporting on the efficacy of vaccines against COVID‐19 and whose search strategy was run in the second half of 2021 or later. One included only RCTs (Rotshild 2021), three only observational studies (Harder 2021; Kow 2022; Liu 2021), and three a hybrid of RCTs and observational studies (Hayawi 2021; Higdon 2021; Zeng 2021b). We identified one systematic review focused on children and adolescents (Lv 2021). Overall, all the trials included in these reviews were identified in our search and our results are consistent.
There are other living systematic reviews of vaccines for COVID‐19, such as Castagneto Gissey 2021, which includes only RCTs; Harder 2021, which includes, but is not limited to RCTs (the second interim results were published in October 2021). Finally, the Living Vaccine Project, a living systematic review with network meta‐analysis that includes only RCTs recently published their results (Korang 2022). All studies included in their review were included in our review (either the same publication or another with more up‐to‐date data). For the most part, their results are consistent with ours. Concurrently, there are over a dozen protocols of systematic reviews assessing the safety or efficacy of vaccines registered in PROSPERO and listed as ongoing.
Authors' conclusions
Implications for practice.
Several COVID‐19 vaccines are highly effective or probably highly effective in preventing SARS‐CoV‐2 infection, symptomatic COVID‐19 and severe or critical COVID‐19.
There is moderate‐ to high‐certainty evidence that most vaccine candidates increased the risk of systemic reactogenicity events (e.g. fever). Evidence related to any adverse event was mainly uncertain.
There is moderate‐ to high‐certainty evidence that there is probably no difference between mRNA‐1273, CVnCoV, ChAdOx1, Ad26.COV2.S, Gam‐COVID‐Vac, WIBP‐CorV and BBIBP‐CorV and placebo in terms of serious adverse events. Evidence was uncertain and very uncertain for serious adverse events for other vaccines and for all‐cause mortality for most vaccines, mainly because of the low number of events.
In addition, as most RCTs only followed up participants for 2 months after full vaccination, all reports are related to short‐term impacts of the vaccine.
Results cannot easily be generalized to pregnant women and immunocompromized individuals; more evidence is needed to elucidate the degree of additional protection conferred by COVID‐19 vaccines in these populations.
Finally, the advent of variants of concern has highlighted the need for further research on each of the vaccine’s capacity to limit infection, disease, and death in regard to specific variants of concern.
Implications for research.
Three hundred and forty‐four RCTs are currently registered, of which 10 are completed. The findings from these trials will contribute to the body of evidence on efficacy and safety outcomes. The findings of this review will be updated as soon as new data are available on the COVID‐NMA platform.
Since the efficacy of vaccines is well established at this point, the ethics of RCT designs using a placebo as the comparison group should be questioned, and active comparators should be considered.
With the notable impact of variants of concern on vaccine efficacy, it is crucial that variant type is assessed in clinical trials and reported for future meta‐analyses to assess vaccine efficacy on considerably different variants.
As a non‐negligible global population has been infected by SARS‐CoV‐2, robust evidence‐based vaccination schemes are also required.
Finally, considering the rapidly changing situation (in terms of variants, policies, etc.) and the increasing and important heterogeneity in the population in terms of combinations of vaccines received, history of SARS‐CoV‐2 infection (and by which variant), type of booster vaccine received, and predominant variants at the time of data collection, RCTs might become increasingly difficult to conduct in such a rapidly‐changing context and large population‐based observational studies could provide relevant information.
What's new
| Date | Event | Description |
|---|---|---|
| 13 March 2023 | Amended | Minor edits made to correct error in the number of events for the outcome 'All‐cause mortality' for the Moderna TX‐mRNA‐1273 vaccine. We previously entered 16 deaths in the intervention arm and 17 deaths in the control arm. The correct data should be 17 deaths in the intervention arm and 16 deaths in the control arm (RR 1.06, 95% CI 0.54 to 2.10). Corresponding edits have been made throughout the review. |
History
Review first published: Issue 12, 2022
| Date | Event | Description |
|---|---|---|
| 3 January 2023 | Amended | URL correction in the abstract. |
| 12 December 2022 | Amended | 'Acknowledgements' updated. |
Acknowledgements
We particularly thank Elise Diard for her help on the website and extraction tool development.
Solaf Alawadhi1,2, Camila Ávila3,, Camilla Hansen Neistgaard4, Fulvia Baldassarre5, Rita Banzi6, Julien Barnier7, Julia Baudry8, Guillaume Cabanac9, Sarah Charpy2, David Chavalarias10, Sarah Cohen‐Boulakia11, Elise Cogo12, Françoise Conil13, Emmanuel Coquery13, Elise Diard14, Bastien Doreau15, Mishelle Engleton2, Laura Esmail2, Gilles Feron16, Leopold Fezeu8, Mathilde Fouet17, Joly Ghanawi18, Robin Featherstone19, François Grolleau1, Candyce Hamel12, Vernon Hedge20, Harald Herkner20, Mona Hersi12, Philipp Kapp14, Ameer Hohlfeld21, Chantal Julia8, Joey Kwong12, Ruben Martinez15, Pauline Martinot2, Dimitris Mavridis22, Brice Meyer15, Nadia Meziou1 , Nathan Pace20, Matthew Page23, Jennifer Petkovic12, Elizabeth Pienaar21, Fiona Quirke24, Pierre Ripoll13, Philippe Rivière13, Jelena Savovic25, Yanina Sguassero12, Marialena Trivella20, Janne Vendt20, Romain Vuillemot13, Stephanie Weibel20
Université de Paris, France.
Centre of Research in Epidemiology and StatisticS (CRESS UMR1153), Methods team, France.
Epistemonikos Foundation, Chile.
Open Patient data Explorative Network (OPEN), Odense University Hospital, Odense, Denmark.
McMaster University, Canada.
Center for Health Regulatory Policies, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Italy.
Centre Max Weber, CNRS, France.
Centre of Research in Epidemiology and StatisticS (CRESS UMR1153), Eren team, France.
Université Toulouse 3 – Paul Sabatier ‐ Institut de Recherche en Informatique de Toulouse – IRIT UMR 5505, France.
Institut des Systèmes Complexes de Paris IDF (ISC‐PIF), CNRS, France.
Laboratoire de recherche en Informatique (LRI), CNRS, Université Paris‐Saclay, France.
Cochrane Response, Cochrane, London, UK.
Laboratoire d’InfoRmatique en Image et Systèmes d’information (LIRIS), CNRS, Université Claude Bernard Lyon 1, France.
Cochrane France.
Laboratoire d'Informatique, de Modélisation et d'Optimisation des Systèmes (LIMOS), CNRS, Université Clermont Auvergne.
Centre for Evidence‐Based Medicine Odense (CEBMO) and Cochrane Denmark, University of Southern Denmark, Denmark.
Service de Neurochirurgie, Hôpital d'Instruction des Armées Percy (HIA), France.
The Collaborative Approach to Meta‐Analysis and Review of Animal Data from Experimental Studies (CAMARADES), Centre for Clinical Brain Sciences, University of Edinburgh, Scotland.
Cochrane Editorial and Methods Department, Cochrane Central.
Cochrane Emergency and Critical Care
Cochrane South Africa.
Department of Primary Education, University of Ioannina, Greece.
Research Methodology Division, School of Public Health and Preventive Medicine, Monash University, Australia.
Evidence Synthesis Ireland, Cochrane Ireland and HRB‐Trials Methodology Research Network, National University of Ireland, Galway, Ireland
Population Health Sciences, Bristol Medical School, University of Bristol, UK; NIHR CLAHRC West, University Hospitals Bristol and Weston NHS Foundation Trust, UK.
Cochrane Emergency and Critical Care Group supported the authors in the development of this review. The following people conducted the editorial process for this review.
Sign‐off Editor (final editorial decision): Harald Herkner, Medical University of Vienna, Austria, Co‐ordinating Editor of the Cochrane Emergency and Critical Care Group.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service.
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service.
Copy Editor (copy‐editing and production): Clare Dooley, c/o Cochrane Production Service.
Proofreader: Anne Lawson, Central Production Service, Cochrane.
Peer‐reviewers (provided comments and recommended an editorial decision): Ariel Izcovich, Internal Medicine Department, Hospital Alemán de Buenos Aires, Argentina (clinical/content review); Romina Brignardello‐Petersen, Department of Health Research Methods, Evidence, and Impact, McMaster University (clinical/content review); Ana Katherine Gonçalves, Obstetric and Gynecology Department, Federal University of Rio Grande Do Norte, Brazil (clinical/content review); Stella O'Brien (consumer review); Robert Walton, Cochrane UK (summary versions review); Rachel Richardson, Cochrane Evidence Production and Methods Directorate (methods review); Kerry Dwan, Cochrane Methods Support Unit (statistical review); Robin Featherstone, Cochrane Central Editorial Service (search review). Two additional peer reviewers provided clinical/content peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. List of definitions used for outcomes 'serious adverse events' and 'severe or critical disease'
| Definition: serious adverse events (SAEs) | Definition: severe or critical disease | |
| RNA‐based | ||
| BNT162b2 – BioNTech/Fosun Pharma/Pfizer | ||
| Walsh 2020 | An SAE is defined as any untoward medical occurrence that, at any dose: results in death; is life‐threatening; requires inpatient hospitalisation or prolongation of existing hospitalisation; results in persistent disability/incapacity; is a congenital anomaly/birth defect; other situations. Medical or scientific judgement should be exercised in deciding whether SAE reporting is appropriate in other situations, such as important medical events that may not be immediately life‐threatening or result in death or hospitalisation, but may jeopardize the participant or may require medical or surgical intervention to prevent 1 of the other outcomes listed in the above definition. | NR |
| Frenck 2021 | An SAE is defined as any untoward medical occurrence that, at any dose: results in death; is life‐threatening; requires inpatient hospitalisation or prolongation of existing hospitalisation; results in persistent disability/incapacity; is a congenital anomaly/birth defect. |
Diagnosis of severe COVID‐19 included confirmed COVID‐19 and the presence of any of the following: (1) clinical signs at rest indicative of severe systemic illness (e.g. respiratory rate ≥ 30 breaths/min, heart rate ≥ 125 beats/min, SpO2 ≤ 93% on room air at sea level, or PaO2/FiO2 < 300 mmHg; (2) respiratory failure (i.e. needing high‐flow oxygen, non‐invasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation); (3) evidence of shock (i.e. systemic blood pressure < 90 mmHg, diastolic blood pressure < 60 mmHg, or requiring vasopressors); (4) significant acute renal, hepatic, or neurological dysfunction; (5) intensive care unit admission; or (6) death. |
| Thomas 2021 | An SAE is defined as any untoward medical occurrence that, at any dose: results in death; is life‐threatening; requires inpatient hospitalisation or prolongation of existing hospitalisation; results in persistent disability/incapacity; is a congenital anomaly/birth defect. |
Confirmed severe COVID‐19 required confirmation of COVID‐19 and the presence of ≥ 1 of the following: clinical signs at rest indicative of severe systemic illness (respiratory rate ≥ 30 breaths/min, heart rate ≥ 125 beats/min, SpO2 ≤ 93% on room air at sea level, or PaO2/FiO2 < 300 mmHg); respiratory failure (defined as needing high‐flow oxygen, non‐invasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation); evidence of shock (systolic blood pressure < 90 mmHg, diastolic blood pressure < 60 mmHg, or requiring vasopressors); significant acute renal, hepatic, or neurological dysfunction; intensive care unit admission; death; or a combination of these. |
| mRNA‐1273 – ModernaTX | ||
| Ali 2021 | An SAE results in any of the following outcomes: death; is life‐threatening; requires inpatient hospitalisation or prolongation of existing hospitalisation; persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions; is a congenital anomaly or birth defect; is a medically important event. | NR |
| El Sahly 2021 | An adverse event (including an adverse reaction) is considered an SAE if, in the view of either the investigator or sponsor, it results in any of the following outcomes: death; is life‐threatening; inpatient hospitalisation or prolongation of existing hospitalisation; persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions; congenital anomaly or birth defect; medically important event. | Confirmed severe COVID‐19 requires any of the following criteria had to be met: clinical signs of severe systemic illness; respiratory rate ≥ 30 breaths/min; heart rate ≥ 125 beats/min; SpO2 ≤ 93% on room air at sea level or PaO2/FIO2 < 300 mmHg, or respiratory failure or acute respiratory distress syndrome (defined as needing high‐flow oxygen, non‐invasive or mechanical ventilation, or extracorporeal membrane oxygenation); evidence of shock (systolic blood pressure < 90 mmHg, diastolic blood pressure < 60 mmHg or requiring vasopressors) or significant acute renal, hepatic or neurological dysfunction or admission to an intensive care unit or death. |
| CVnCoV – CureVac AG | ||
| Kremsner 2021 | NR | Severe COVID‐19 was defined by clinical signs at rest that are indicative of severe systemic illness (respiratory rate ≥ 30 breaths/min, heart rate ≥ 125 beats/min, altitude‐adjusted SpO2 ≤ 93% or PaO2/FIO2 < 300 mmHg), respiratory failure, evidence of shock, significant renal, hepatic, or neurological dysfunction, admission to an intensive care unit, or death. |
| Non‐replicating viral vector | ||
| ChAdOx1/SII‐ChAdOx1 nCoV‐19 – AstraZeneca + University of Oxford | ||
| Asano 2022 | Severity of safety endpoints was assessed according to toxicity grading scales adapted from Food and Drug Administration (FDA) grading guidance |
NR |
| Emary 2021 | NR | NR |
| Falsey 2021 | An adverse event that fulfils ≥ 1 of the following criteria: results in death; is immediately life‐threatening; requires in‐participant hospitalisation or prolongation of existing hospitalisation; results in persistent or significant disability or incapacity; is a congenital abnormality or birth defect; is an important medical event that may jeopardize the participant or may require medical treatment to prevent 1 of the outcomes listed above. |
Laboratory‐confirmed COVID‐19 (SARS‐CoV‐2 RT‐PCR‐positive symptomatic illness) plus any of the following: clinical signs at rest indicative of severe systemic illness (respiratory rate ≥ 30 breaths/min, heart rate ≥ 125 beats/min, oxygen saturation ≤ 93% on room air at sea level, or PaO2/FIO2 < 300 mmHg); respiratory failure (defined as needing high‐flow oxygen, non‐invasive ventilation, mechanical ventilation or extracorporeal membrane oxygenation); evidence of shock (systolic blood pressure < 90 mmHg, diastolic blood pressure < 60 mmHg, or requiring vasopressors); significant acute renal, hepatic, or neurological dysfunction; admission to an intensive care unit; death. |
| Kulkarni 2021 | All adverse events were graded for severity using the Division of AIDS (DAIDS) table for Grading the Severity of Adult and Pediatric Adverse Events (corrected version 2.1, July 2017) from the US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases. | Severe cases as per the WHO clinical progression scale |
| Madhi 2021b | NR | As defined by WHO ordinal scale |
| Voysey 2021a | NR | Severe COVID‐19 (WHO clinical progression score ≥ 6) |
| Gam‐COVID‐Vac (Sputnik V) – Gamaleya Research Institute | ||
| Logunov 2021 | SAEs were diagnosed on the basis of the event requiring hospital admission. |
Moderate or severe COVID‐19: fever > 38.5 °C; respiratory rate > 22 breaths/min; shortness of breath during physical exertion; pneumonia (confirmed by computed tomography of the lungs); oxygen saturation level < 95%. |
| Ad26.COV2.S – Janssen Pharmaceutical Companies | ||
| Sadoff 2021a | NR | NR |
| Sadoff 2021b | An SAE based on ICH and EU guidelines on pharmacovigilance for medicinal products for human use is any untoward medical occurrence that at any dose: results in death; is life‐threatening (the participant was at risk of death at the time of the event; it does not refer to an event that hypothetically might have caused death if it were more severe); requires inpatient hospitalisation or prolongation of existing hospitalisation; results in persistent or significant disability/incapacity; is a congenital anomaly/birth defect; is a suspected transmission of any infectious agent via a medicinal product; is medically important. | A SARS‐CoV‐2 positive RT‐PCR or molecular test result. Respiratory rate ≥ 30 breaths/min; heart rate ≥ 125 beats/min; oxygen saturation (SpO2) ≤ 93% on room air at sea level, or PaO2/FiO2 < 300 mmHg; respiratory failure; evidence of shock; significant acute renal, hepatic, or neurological dysfunction; admission to the ICU; death |
| Inactivated virus | ||
| BBV152 – Bharat Biotech | ||
| Ella 2021a | NR | NR |
| Ella 2021b | NR | NR |
| CoronaVac – Sinovac | ||
| Zhang 2021 | NR | NR |
| Bueno 2021 | Any untoward medical occurrence that: results in death; is life‐threatening (i.e. the subject was, in the opinion of the investigator, at immediate risk of death from the event as it occurred; it does not refer to an event which hypothetically might have caused death if it were more severe); requires or prolongs subject’s hospitalisation; results in persistent or significant disability/incapacity (i.e. the event causes a substantial disruption of a personal ability to conduct normal life functions); results in a congenital anomaly/birth defect; requires intervention to prevent permanent impairment or damage; is an important and significant medical event that may not be immediately life‐threatening or resulting in death or hospitalisation but, based upon appropriate medical judgement, may jeopardize the subject or may require intervention to prevent 1 of the other outcomes listed above. | NR |
| Han 2021 | NR | NR |
| Fadlyana 2021 | NR | Severe or critical COVID‐19 confirmed by RT‐PCR |
| Palacios 2020 | Any adverse event that results in any of the following outcomes: death; threat to life; there is a risk of death at the time of the event; hospitalisation or extension of hospitalisation; significant or persistent disability; congenital anomaly; any suspicion of transmission of an infectious agent by means of a medication; clinically significant event; any event resulting from the use of drugs that require medical intervention, in order to avoid death, risk to life, significant disability or hospitalisation. | Score ≥ 6 on WHO 10‐point clinical progression scale (hospitalized with severe COVID‐19 through to death) |
| Tanriover 2021 | An SAE is an adverse event that results in any of the following outcomes, whether or not considered related to the study intervention: death; life‐threatening event (i.e. the volunteer was, in the view of the investigator, at immediate risk of death from the event that occurred); persistent or significant disability or incapacity (i.e. substantial disruption of one’s ability to carry out normal life functions); hospitalisation or prolongation of existing hospitalisation, regardless of length of stay, even if it is a precautionary measure for continued observation (hospitalisation (including inpatient or outpatient hospitalisation for an elective procedure) for a pre‐existing condition that has not worsened unexpectedly does not constitute an SAE); an important medical event (that may not cause death, be life‐threatening, or require hospitalisation) that may, based upon appropriate medical judgement, jeopardise the volunteer, require medical or surgical intervention to prevent 1 of the outcomes listed above, or a combination of these. Examples of such medical events include allergic reaction requiring intensive treatment in an emergency room or clinic, blood dyscrasias, or convulsions that do not result in inpatient hospitalisation. | WHO clinical progression scale ≥ 6: hospitalized, needing oxygen by non‐invasive or high‐flow ventilation or worse |
| Wu 2021a | Events during the clinical trial that need hospitalisation treatment, prolong hospitalisation time, disability, affect working ability, endanger life or death, cause congenital malformation, etc. | NR |
| WIBP‐CorV – Sinopharm Wuhan | ||
| Al Kaabi 2021 | NR | Confirmed COVID‐19 case, meeting any 1 of the following criteria: respiratory distress (respiratory rate ≥ 30 breaths/min); O2 saturation ≤ 93% at rest; PaO2/FiO2 < 300 mmHg (1 mmHg = 0.133 kPa); clinical symptoms progressively worsened, and chest imaging showed > 50% obvious lesion progression within 24–48 hours. |
| Guo 2021 | NR | |
| Protein subunit | ||
| NVX‐CoV2373 – Novavax | ||
| Dunkle 2021 | NR | Severe refers to ≥ 1 of the following: tachypnoea ≥ 30 breaths/min at rest; resting heart rate ≥ 125 beats/min; SpO2 ≤ 93% on room air or PaO2/FiO2 < 300 mmHg; high‐flow O2 therapy or non‐invasive ventilation/non‐invasive positive pressure ventilation (e.g. continuous positive airway pressure or bilevel positive airway pressure; mechanical ventilation or extracorporeal membrane oxygenation; ≥ 1 major organ system dysfunction or failure to be defined by diagnostic testing/clinical syndrome/interventions, including any of the following – acute respiratory failure, including acute respiratory distress syndrome, acute renal failure, acute hepatic failure, acute right or left heart failure, septic or cardiogenic shock (with shock defined as systolic blood pressure < 90 mmHg or diastolic blood pressure < 60 mmHg), acute stroke (ischaemic or haemorrhagic), acute thrombotic event; acute myocardial infarction, deep vein thrombosis, pulmonary embolism, requirement for: vasopressors, systemic corticosteroids, or haemodialysis; admission to an intensive care unit; death. |
| Formica 2021 | NR | NR |
| Heath 2021 | An SAE is defined as any event that results in death, is immediately life‐threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect. |
Tachypnoea ≥ 30 breaths/min at rest; resting heart rate ≥ 125 beats/min; SpO2 ≤ 93% on room air or PaO2/FiO2 < 300 mmHg; high‐flow O2 therapy or non‐invasive ventilation/non‐invasive positive pressure ventilation (e.g. continuous positive airway pressure or bilevel positive airway pressure; mechanical ventilation or extracorporeal membrane oxygenation; ≥ 1 major organ system dysfunction or failure to be defined by diagnostic testing/clinical syndrome/interventions, including any of the following – acute respiratory failure, including acute respiratory distress syndrome, acute renal failure, acute hepatic failure, acute right or left heart failure, septic or cardiogenic shock (with shock defined as systolic blood pressure < 90 mmHg or diastolic blood pressure < 60 mmHg), acute stroke (ischaemic or haemorrhagic), acute thrombotic event; acute myocardial infarction, deep vein thrombosis, pulmonary embolism, requirement for: vasopressors, systemic corticosteroids, or haemodialysis; admission to an intensive care unit; death. |
| Shinde 2021 | NR | Severe refers to ≥ 1 of the following: tachypnoea ≥ 30 breaths/min at rest; resting heart rate ≥ 125 beats/min; SpO2 ≤ 93% on room air or PaO2/FiO2 < 300 mmHg; high‐flow O2 therapy or non‐invasive ventilation/non‐invasive positive pressure ventilation (e.g. continuous positive airway pressure or bilevel positive airway pressure; mechanical ventilation or extracorporeal membrane oxygenation; ≥ 1 major organ system dysfunction or failure to be defined by diagnostic testing/clinical syndrome/interventions, including any of the following – acute respiratory failure, including acute respiratory distress syndrome, acute renal failure, acute hepatic failure, acute right or left heart failure, septic or cardiogenic shock (with shock defined as systolic blood pressure < 90 mmHg or diastolic blood pressure < 60 mmHg), acute stroke (ischaemic or haemorrhagic), acute thrombotic event; acute myocardial infarction, deep vein thrombosis, pulmonary embolism, requirement for: vasopressors, systemic corticosteroids, or haemodialysis; admission to an intensive care unit; death. |
| FINLAY‐FR‐2 – Instituto Finlay de Vacunas | ||
| Toledo‐Romani 2021 | NR | Severe systemic confirmed COVID‐19 disease (serious or critical), defined by 1 of the following criteria: polypnoea; x‐ray infiltration/condensation, pulmonary echography; oxygen saturation ≤ 90% or assisted mechanical ventilation (serious disease), acute respiratory distress syndrome or evidence of septic shock (critical disease). |
| Heterologous vaccination | ||
| Liu 2021 | Any untoward medical occurrence that: results in death; is life‐threatening; requires inpatient hospitalisation or prolongation of existing hospitalisation; results in persistent or significant disability/incapacity; consists of a congenital anomaly or birth defect. | NR |
Appendix 2. Search strategies
Cochrane COVID‐19 Study Register
| Source | Search strategy (last search date 5 November 2021) |
| PubMed | (2019 nCoV[tiab] OR 2019nCoV[tiab] OR corona virus[tiab] OR corona viruses[tiab] OR coronavirus[tiab] OR coronaviruses[tiab] OR COVID[tiab] OR COVID19[tiab] OR nCov 2019[tiab] OR SARS‐CoV2[tiab] OR SARS CoV‐2[tiab] OR SARSCoV2[tiab] OR SARSCoV‐2[tiab] OR "COVID‐19"[Mesh] OR "COVID‐19 Testing"[Mesh] OR "COVID‐19 Vaccines"[Mesh] OR "Coronavirus"[Mesh:NoExp] OR "Receptors, Coronavirus"[Mesh] OR "SARS‐CoV‐2"[Mesh] OR "Spike Glycoprotein, Coronavirus"[Mesh]) NOT ("animals"[mh] NOT "humans"[mh]) NOT (editorial[pt] OR newspaper article[pt]) |
| Embase | ((('anti‐SARS‐CoV‐2 agent'/exp OR 'coronaviridae'/de OR 'coronavirinae'/de OR 'coronaviridae infection'/de OR 'coronavirus disease 2019'/exp OR 'coronavirus infection'/de OR 'COVID‐19 testing'/exp OR 'sars coronavirus 2 test kit'/exp OR 'sars‐related coronavirus'/de OR 'severe acute respiratory syndrome coronavirus 2'/exp OR '2019 ncov':ti,ab,kw OR 2019ncov:ti,ab,kw OR (((corona* OR corono*) NEAR/1 (virus* OR viral* OR virinae*)):ti,ab,kw) OR coronavir*:ti,ab,kw OR coronovir*:ti,ab,kw OR covid:ti,ab,kw OR covid19:ti,ab,kw OR hcov*:ti,ab,kw OR 'ncov 2019':ti,ab,kw OR 'sars cov2':ti,ab,kw OR 'sars cov 2':ti,ab,kw OR sarscov2:ti,ab,kw OR 'sarscov 2':ti,ab,kw) NOT (('animal experiment'/de OR 'animal'/exp) NOT ('human'/exp OR 'human experiment'/de))) NOT 'editorial'/it) NOT ([medline]/lim OR [pubmed‐not‐medline]/lim) AND [1‐12‐2019]/sd |
| CENTRAL | 1 ("2019 nCoV" OR 2019nCoV OR "corona virus*" OR coronavirus* OR COVID OR COVID19 OR "nCov 2019" OR "SARS‐CoV2" OR "SARS CoV‐2" OR SARSCoV2 OR "SARSCoV‐2"):TI,AB AND CENTRAL:TARGET 2 Coronavirus:MH AND CENTRAL:TARGET 3 Coronavirus:EH AND CENTRAL:TARGET 4 #1 OR #2 OR #3 5 2019 TO 2021:YR AND CENTRAL:TARGET 6 #5 AND #4 7 INSEGMENT 8 #6 NOT #7 |
| ClinicalTrials.gov | COVID‐19 OR 2019‐nCoV OR SARS‐CoV‐2 OR coronavirus |
| WHO ICTRP | COVID OR 2019‐nCoV OR SARS‐CoV‐2 OR coronavirus OR corona virus |
| medRxiv | All new medRxiv records are imported each week into the Cochrane Register of Studies. Records captured by this strategy are then evaluated: ("2019 nCoV" OR 2019nCoV OR "corona virus*" OR coronavirus* OR COVID OR COVID19 OR "nCov 2019" OR "SARS‐CoV2" OR "SARS CoV‐2" OR SARSCoV2 OR "SARSCoV‐2"):TI,AB |
Epistemonikos L·OVE COVID‐19 platform
| Search strategy |
| coronavir* OR coronovirus* OR betacoronavir* OR "beta‐coronavirus" OR "beta‐coronaviruses" OR "corona virus" OR "virus corona" OR "corono virus" OR "virus corono" OR hcov* OR covid* OR "2019‐ncov" OR cv19* OR "cv‐19" OR "cv 19" OR "n‐cov" OR ncov* OR (wuhan* AND (virus OR viruses OR viral)) OR "2019‐ncov‐related" OR "cv‐19‐related" OR "n‐cov‐related" OR sars* OR sari OR "severe acute respiratory syndrome" OR antisars* OR "anti‐sars‐cov‐2" OR "anti‐sars‐cov2" OR "anti‐sarscov‐2" OR "anti‐sarscov‐2" OR "post‐COVID‐19" OR "Not‐of‐COVID‐19" OR "corona patients" OR "article‐covid‐19" OR "post‐covid‐19" OR "post‐covid" OR "with‐covid‐19" OR "pre‐covid" OR "pre‐covid‐19" OR "with‐covid" OR "anti‐covid‐19" OR "n‐covid" OR "no‐covid" |
For the Epistemonikos L*OVE COVID‐19 platform we:
select type of question “Prevention or treatment”
select intervention “Public health”, “Vaccination” and “SARS‐CoV‐2 vaccines”
select “Primary studies”
filter results by “RCT”
export the results in a.ris file
upload the results into Rayyan ®
export results in an excel file
eliminate duplicates
cross‐check with the latest extraction to eliminate duplicates and obtain only new articles (L*OVE platform does not filter results by day)
For the Cochrane COVID‐19 Study Register we:
select new studies “Last week”
select update new references “Last week”
select results available “Report results”
select study characteristics, study type “Interventional”
select study characteristics, study aim “Treatment and management”
select study characteristics, intervention assignment “Randomized”
For the Retraction Watch Website:
click in « Retracted coronavirus (COVID‐19) papers »
check the list of news Retracted papers
For the ICTRP:
The records are automatically extracted in the platform https://ctr-dwh.limos.fr/
For the EMA Website we:
select « Vaccines » in Covid‐19 pandemic
select « name of vaccine » in Authorized for use in the European Union
search « Assessment report »
export the results in a PDF file
For the FDA Website we:
click in « FDA Covid‐19 Response »
select « name of vaccine » in COVID‐19 Vaccines
search reports of interest
export the results in a PDF file
in the home page, search in search Search Toolbar « Briefing Document » for each FDA‐approved vaccine
Appendix 3. Additional methods for future network meta‐analysis (NMA) updates
Below are additional methods to consider if a NMA and subgroup analyses are to be conducted in future updates.
Unit of analysis issues
If we perform a NMA, we will properly account for the correlation of effect sizes coming from multiple‐arm trials.
If we identify any eligible cluster‐randomized trials, we will extract results that properly account for the cluster design (such as based on a multiple‐level model or on generalized estimating equations). If such an analysis is not reported, we will contact study authors to try to obtain the parameters required to be able to calculate an estimate of the intraclass correlation coefficient for the meta‐analyses to adjust for the design effect. Should these not be obtained, the trial will still be included, although it will be mentioned as a limitation of the analysis.
Assessment of transitivity
If a certain number of studies are available (e.g. at least 3 studies for 30% of the available direct comparisons), we will opt for conducting a NMA. Prior to this analysis, we will assess whether the assumption that the anchor treatments are transitive to allow valid indirect inference is likely to be plausible. Specifically, we will evaluate the similarity of the distribution of the potential effect modifiers (variants of the virus, baseline risk such as rate of transmission of COVID‐19 at the time the trials were conducted, immune status) across the available comparisons. Throughout the living review, we will be consulting content experts and update, if necessary, the list of potential effect modifiers. We will use boxplots to depict the distributions of these variables across comparisons. In terms of node (i.e. vaccine) definition, we do not expect substantial heterogeneity that could threaten the transitivity assumption.
Assessment of reporting biases
We will use funnel plots (in the presence of at least 10 studies per meta‐analysis) and statistical tests (such as the Egger’s test) (Egger 1997) to assess the potential for small‐study effects. If asymmetry is found, we will explore possible reasons for the apparent association between study size and study effect. If publication bias is suspected, we will apply selection models that make assumptions about the probability of publication based on the study results (Mavridis 2014). If NMA is deemed feasible, we will also draw comparison‐adjusted funnel plots; these are modified funnel plots appropriate for putting together all studies from a NMA, irrespective of the comparison they evaluate(Chaimani 2013). This will be done only for critical outcomes.
If there are no major concerns about transitivity (see above), we will also perform a random‐effects NMA for each outcome. The analysis will be performed at the vaccine level (not the type of vaccine), hence we will not combine different vaccines. We will assume a common heterogeneity parameter for each network. We will present the results in terms of effect sizes and 95% CIs in league tables and will use colours to represent the certainty of the evidence for every comparison. We will assess the impact of heterogeneity on the results by using prediction intervals. To rank the interventions, in the absence of excessive uncertainty in the relative effects, we will use the surface under the cumulative ranking curve (SUCRA) (Salanti 2011). This will be done for critical outcomes. We will run analyses and produce graphical displays using R (netmeta package)(Rucker 2013) and Stata network (White 2008), and network graphs packages (Chaimani 2015). If important concerns about transitivity are detected, we will only perform pairwise meta‐analyses.
Assessment of incoherence
We will evaluate the assumption coherence, which refers to the agreement between direct and indirect evidence, using local and global tests. Local approaches assess coherence in parts of the network, while global approaches assess coherence in the entire network jointly. Specifically, we will use the side‐splitting method (Dias 2010) and the design‐by‐treatment interaction model (Higgins 2021). We will consider P values < 0.10 as suspicious for incoherence. Tests for incoherence are known to have low power and may not be able to detect incoherence even when present, so we will interpret the results of the tests with caution.
Subgroup analysis and investigation of heterogeneity/incoherence
In the NMA, we will conduct the same subgroup analysis already prespecified for the for pairwise comparisons.
Sensitivity analysis
We will perform sensitivity analyses by excluding RCTs with an overall high risk of bias, RCTs reported in preprint only, and early‐phase trials. For the NMA, we will also perform a sensitivity analysis assuming that the effects of the vaccines of the same type (e.g. RNA‐based vaccine) are related, although not identical.
Summary of findings and assessment of the certainty of the evidence of the review findings
We will prepare separate summary of findings tables of the NMA for each critical outcome. These tables will report the different comparisons included in the network, relative and absolute effect estimates, and the certainty of the evidence (Chaimani 2022; Yepes‐Nuñez 2019). We will calculate absolute effects using the baseline risks in the control groups of the included studies. Two review authors will independently rate the evidence's overall certainty for each outcome using the CINeMA tool and all decisions to downgrade or update the certainty of evidence will be made explicit.
To evaluate the certainty of the evidence in the NMA for the critical outcomes, we will use the CINeMA tool that considers the following domains: within‐study‐bias, across‐studies bias, indirectness, imprecision, heterogeneity and incoherence (Nikolakopoulou 2020). For within‐study bias and indirectness, CINeMA calculates the contribution of each study in the estimation and combines these contributions with the study‐specific evaluations (low, moderate, high) to rate the relative effect for each comparison in the network. The domains of imprecision, heterogeneity and incoherence use a prespecified important size of effect to specify the margin of equivalence between two interventions. This will be defined by consulting the content experts.
Appendix 4. Characteristics of unpublished registered studies
| Characteristics of unpublished registered studies: RNA‐based vaccine (73 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions |
Estimated sample size |
Phase |
| ChiCTR2000034112 | 24 June 2020 | Not recruiting | Parallel | ARCoV | 168 | Phase 1 |
| ChiCTR2100041855 | 8 January 2021 | Not recruiting | Parallel | ARCoV | 420 | Phase 2 |
| NCT04847102 | 15 April 2021 | Not recruiting | Cross‐over | ARCoV | 28,000 | Phase 3 |
| NCT04668339 | 16 December | Not recruiting | Parallel | ARCT‐021 | 600 | Phase 2 |
| ChiCTR2000040044 | 19 November 2020 | Not recruiting | Parallel | BNT162b2 | 960 | Phase 2 |
| NCT04588480 | 19 October 2020 | Not recruiting | Parallel | BNT162b2 | 160 | Phase 1/Phase 2 |
| NCT04649021 | 2 December 2020 | Not recruiting | Parallel | BNT162b2 | 950 | Phase 2 |
| NCT04816669 | 25 March 2021 | Not recruiting | Parallel | BNT162b2 | 610 | Phase 3 |
| NCT04955626 | 9 July 2021 | Not recruiting | Parallel | BNT162b2 | 10,000 | Phase 3 |
| NCT04961229 | 14 July 2021 | Not recruiting | Parallel | BNT162b2 | 450 | Phase 4 |
| NCT04969250 | 20 July 2021 | Not recruiting | Factorial | BNT162b2 | 640 | Phase 4 |
| NCT05057169 | 27 September 2021 | Not recruiting | Parallel | BNT162b2 | 400 | Phase 4 |
| NCT05029245 | 31 August 2021 | Not recruiting | Parallel | BNT162b2 | 1000 | Phase 3 |
| NCT05081271 | 18 October 2021 | Not recruiting | Parallel | BNT162b2 | 60 | Not reported |
| NCT05077254 | 14 October 2021 | Not recruiting | Parallel | BNT162b2 | 400 | Phase 2 |
| TCTR20210923012 | 23 September 2021 | Not recruiting | Parallel | BNT162b2 + CoronaVac | 80 | Phase 2 |
| ACTRN12621001465842 | 26 October 2021 | Not recruiting | Parallel | BNT162b2 + inulin | 120 | Not reported |
| ACTRN12621001412820 | 20 October 2021 | Not recruiting | Parallel | BNT162b2 + sirolimus | 120 | Not reported |
| NCT04566276 | 28 September 2020 | Not recruiting | Sequential assignment | ChulaCov19 mRNA vaccine | 96 | Phase 1/Phase 2 |
| NCT04674189 | 19 December 2020 | Not recruiting | Parallel | CVnCoV | 2520 | Phase 3 |
| NCT04848467 | 19 April 2021 | Not recruiting | Parallel | CVnCoV + influenza vaccine | 1000 | Phase 3 |
| NCT04821674 | 29 March 2021 | Not recruiting | Parallel | DS‐5670a | 152 | Phase 1/Phase 2 |
| NCT04844268 | 14 April 2021 | Not recruiting | Parallel | HDT 301 vaccine | 78 | Phase 1 |
| ChiCTR2100049349 | 31 July 2021 | Not recruiting | Parallel | LVRNA009 | 144 | Phase 1 |
| ChiCTR2100049104 | 21 July 2021 | Not recruiting | Parallel | mRNA vaccine | 2000 | Phase 3 |
| ChiCTR2100049521 | 2 August 2021 | Not recruiting | Parallel | mRNA vaccine | 320 | Phase 1/Phase 2 |
| NCT04677660 | 21 December 2020 | Not recruiting | Parallel | mRNA‐1273 | 200 | Phase 1/Phase 2 |
| NCT04805125 | 18 March 2021 | Not recruiting | Parallel | mRNA‐1273 | 380 | Phase 3 |
| PACTR202105817814362 | 20 May 2021 | Not recruiting | Cross‐over | mRNA‐1273 | 14,000 | Phase 3 |
| NCT05000216 | 11 August 2021 | Not recruiting | Parallel | mRNA‐1273 | 600 | Phase 2 |
| NCT04978038 | 27 July 2021 | Not recruiting | Parallel | mRNA‐1273 | 414 | Phase 4 |
| NCT04785144 | 5 March 2021 | Not recruiting | Parallel | mRNA‐1273.351 | 210 | Phase 1 |
| NCT05069636 | 6 October 2021 | Not recruiting | Parallel | mRNA‐1273 + osteopathic manipulative medicine | 100 | Not reported |
| NCT04765436 | 21 February 2021 | Not recruiting | Parallel | PTX‐COVID19‐B | 60 | Phase 1 |
| EUCTR2021‐005043‐71‐NL | 9 October 2021 | Ongoing | Parallel | BNT162b2 | 400 | Phase 2 |
| ChiCTR2000039212 | 22 October 2020 | Ongoing | Parallel | ARCoV | 120 | Phase 1 |
| ISRCTN15779782 | 8 October 2021 | Ongoing | Adaptive | ARCT‐021 | 100,000 | Phase 3 |
| NCT05012943 | 19 August 2021 | Ongoing | Parallel | ARCT‐154 | 21,000 | Phase 2/Phase 3 |
| NCT05037097 | 8 September 2021 | Ongoing | Parallel | ARCT‐165 | 72 | Phase 1/Phase 2 |
| ACTRN12621000661875 | 1 June 2021 | Ongoing | Parallel | BNT162b2 | 100 | Phase 4 |
| NCT04713553 | 19 January 2021 | Ongoing | Parallel | BNT162b2 | 1530 | Phase 3 |
| NCT04754594 | 15 February 2021 | Ongoing | Parallel | BNT162b2 | 4000 | Phase 3 |
| NCT04907331 | 28 May 2021 | Ongoing | Parallel | BNT162b2 | 3000 | Phase 2 |
| NCT04949490 | 2 July 2021 | Ongoing | Sequential assignment | BNT162b2 | 549 | Phase 2 |
| EUCTR2021‐003331‐28‐ES | 21 June 2021 | Ongoing | Parallel | BNT162b2 | 776 | Phase 4 |
| EUCTR2020‐005442‐42‐PL | 11 August 2021 | Ongoing | Parallel | BNT162b2 | 4644 | Phase 1/Phase 2/Phase 3 |
| NCT05022329 | 26 August 2021 | Ongoing | Parallel | BNT162b2 | 300 | Phase 2/Phase 3 |
| NCT05047640 | 17 September 2021 | Ongoing | Parallel | BNT162b2 | 200 | Phase 3 |
| ISRCTN12348322 | 16 September 2021 | Ongoing | Parallel | BNT162b2 | 360 | Phase 2 |
| TCTR20210917004 | 17 September 2021 | Ongoing | Parallel | BNT162b2 | 120 | Phase 2 |
| NCT04977479 | 27 July 2021 | Ongoing | Cross‐over | BNT162b2 | 100 | Phase 2 |
| EUCTR2021‐004526‐29‐DE | 6 September 2021 | Ongoing | Adaptive | BNT162b2 | 85 | Phase 2 |
| EUCTR2021‐001993‐52‐BE | 5 May 2021 | Ongoing | Parallel | BNT162b2 | 840 | Phase 4 |
| NCT04887948 | 14 May 2021 | Ongoing | Parallel | BNT162b2 + pneumococcal vaccine | 600 | Phase 3 |
| NCT05060991 | 29 September 2021 | Ongoing | Parallel | BNT162b2 + reduction in antimetabolite immunosuppression | 50 | Phase 4 |
| ChiCTR2100045984 | 1 May 2021 | Ongoing | Parallel | COVID‐19 mRNA vaccine (nucleoside‐modified) | 240 | Phase 1 |
| NCT05028361 | 31 August 2021 | Ongoing | Parallel | COVID‐19 mRNA vaccine (nucleoside‐modified) + influenza vaccine | 450 | Phase 4 |
| NCT04863131 | 28 April 2021 | Ongoing | Parallel | EXG‐5003 | 60 | Phase 1/Phase 2 |
| CTRI/2021/04/032688 | 28 April 2021 | Ongoing | Parallel | HGCO19 | 620 | Phase 1/Phase 2 |
| ISRCTN17072692 | 4 June 2020 | Ongoing | Parallel | LNP‐nCoVsaRNA | 320 | Phase 1 |
| ISRCTN27841311 | 26 March 2021 | Ongoing | Parallel | mRNA‐1273 | 1050 | Phase 2 |
| NCT04761822 | 21 February 2021 | Ongoing | Parallel | mRNA‐1273 | 3400 | Phase 2 |
| NCT04796896 | 15 March 2021 | Ongoing | Parallel | mRNA‐1273 | 7050 | Phase 2/Phase 3 |
| NCT04811664 | 23 March 2021 | Ongoing | Cross‐over | mRNA‐1273 | 37,500 | Phase 3 |
| NCT04894435 | 20 May 2021 | Ongoing | Parallel | mRNA‐1273 | 1300 | Phase 1/Phase 2 |
| EUCTR2021‐004558‐44‐NL | 13 September 2021 | Ongoing | Parallel | mRNA‐1273 | 460 | Phase 4 |
| NCT04900467 | 25 May 2021 | Ongoing | Parallel | mRNA‐1273 | 400 | Not reported |
| NCT04852978 | 21 April 2021 | Ongoing | Parallel | mRNA‐1273 + casirivimab + imdevimab | 180 | Phase 2 |
| NCT04969276 | 20 July 2021 | Ongoing | Parallel | mRNA‐1273 + quadrivalent influenza vaccine | 300 | Phase 2 |
| NCT04813796 | 24 March 2021 | Ongoing | Parallel | mRNA‐1283 | 125 | Phase 1 |
| NCT04798027 | 15 March 2021 | Ongoing | Parallel | MRT5500 | 333 | Phase 1/Phase 2 |
| NCT05079633 | 15 October 2021 | Ongoing | Parallel | MVC‐COV1901 + mRNA‐1273 | 220 | Phase 4 |
| JPRN‐jRCT2071210067 | 28 September 2021 | Ongoing | Parallel | VLPCOV‐01 | 45 | Phase 1 |
| Characteristics of unpublished registered studies: non‐replicating viral vector (73 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions | Estimated sample size | Phase |
| NCT04690387 | 30 December 2020 | Completed | Adaptive | AV‐COVID‐19 | 27 | Phase 1 |
| ChiCTR2000031781 | 10 April 2020 | Not recruiting | Parallel | Recombinant novel coronavirus (2019‐ncov) vaccine (adenovirus vector) | 500 | Phase 2 |
| CTRI/2021/02/031295 | 15 February 2021 | Not recruiting | Parallel | BBV154 | 175 | Phase 1 |
| CTRI/2021/05/033665 | 18 May 2021 | Not recruiting | Parallel | COVID‐Vac Combined Vector Vaccine | 228 | Phase 3 |
| NCT04398147 | 21 May 2020 | Not recruiting | Adaptive | Ad5‐nCoV | 696 | Phase 1/Phase 2 |
| NCT04509947 | 12 August 2020 | Not recruiting | Parallel | Ad26.COV2.S | 250 | Phase 1 |
| NCT04540419 | 7 September 2020 | Not recruiting | Parallel | Ad5‐nCoV | 500 | Phase 3 |
| NCT04564716 | 25 September 2020 | Not recruiting | Parallel | Gam‐COVID‐Vac | 100 | Phase 3 |
| NCT04614948 | 4 November 2020 | Not recruiting | Parallel | Ad26.COV2.S | 30,000 | Phase 3 |
| NCT04640233 | 23 November 2020 | Not recruiting | Adaptive | Gam‐COVID‐Vac | 1600 | Phase 2/Phase 3 |
| NCT04642339 | 24 November 2020 | Not recruiting | Parallel | Gam‐COVID‐Vac | 2000 | Phase 3 |
| NCT04656613 | 7 December 2020 | Not recruiting | Parallel | Gam‐COVID‐Vac | 1000 | Phase 3 |
| NCT04679909 | 22 December 2020 | Not recruiting | Parallel | AdCOVID | 180 | Phase 1 |
| NCT04751682 | 12 February 2021 | Not recruiting | Parallel | BBV154 | 175 | Phase 1 |
| NCT04760730 | 18 February 2021 | Not recruiting | Parallel | ChAdOx1 nCoV‐19 + rAd26‐S | 100 | Phase 1/Phase 2 |
| NCT04791423 | 10 March 2021 | Not recruiting | Parallel | GRAd‐COV2 | 10,300 | Phase 2/Phase 3 |
| NCT04840992 | 12 April 2021 | Not recruiting | Parallel | Ad5‐nCoV | 840 | Phase 1/Phase 2 |
| NCT04843722 | 13 April 2021 | Not recruiting | Sequential assignment | hAd5‐S‐Fusion/N‐ETSD vaccine | 540 | Phase 1/Phase 2 |
| NCT04845191 | 14 April 2021 | Not recruiting | Sequential assignment | hAd5‐S‐Fusion/N‐ETSD vaccine | 540 | Phase 1/Phase 2 |
| NCT04894305 | 20 May 2021 | Not recruiting | Parallel | Ad26.COV2.S | 380 | Phase 1 |
| NCT04895449 | 20 May 2021 | Not recruiting | Parallel | MVA‐SARS‐2‐S | 240 | Phase 1/Phase 2 |
| NCT04977024 | 26 July 2021 | Not recruiting | Parallel | COH04S1 | 240 | Phase 2 |
| PACTR202104601572565 | 12 April 2021 | Not recruiting | Parallel | Sputnik light vaccine | 2200 | Phase 3 |
| NCT05011526 | 18 August 2021 | Not recruiting | Parallel | ChAdOx1 nCoV‐19 | 1020 | Phase 3 |
| NCT05027672 | 30 August 2021 | Not recruiting | Parallel | Gam‐COVID‐Vac | 348 | Phase 2 |
| NCT05030974 | 1 September 2021 | Not recruiting | Parallel | Ad26.COV2.S vaccine | 460 | Phase 4 |
| NCT04998240 | 10 August 2021 | Not recruiting | Parallel | BBIBP‐CorV + ChAdOx1 nCoV‐19 | 360 | Phase 2 |
| TCTR20210717002 | 17 July 2021 | Not recruiting | Parallel | ChAdOx1 nCoV‐19 + CoronaVac | 165 | Phase 4 |
| TCTR20210903006 | 3 September 2021 | Not recruiting | Sequential assignment | ChAdOx1 nCoV‐19 + CoronaVac | 80 | Phase 1/Phase 2 |
| TCTR20210904004 | 4 September 2021 | Not recruiting | Sequential assignment | ChAdOx1 nCoV‐19 + inactivated COVID‐19 vaccine | 40 | Phase 1/Phase 2 |
| NCT05091307 | 25 October 2021 | Not recruiting | Parallel | Ad26.COV2.S + influenza vaccine | 1680 | Phase 3 |
| NCT04833101 | 6 April 2021 | Not recruiting | Parallel | Ad5‐nCoV + ZF2001 | 120 | Phase 4 |
| NCT05048940 | 17 September 2021 | Not recruiting | Parallel | Ad26.COV2.S | 386 | Phase 3 |
| NCT05049226 | 20 September 2021 | Not recruiting | Parallel | ChAdOx1 nCoV‐19 | 1320 | Phase 2 |
| ChiCTR2100049530 | 2 August 2021 | Not recruiting | Parallel | ChAdTS‐S | 360 | Phase 2 |
| TCTR20210907003 | 7 September 2021 | Not recruiting | Sequential assignment | ChAdOx1 nCoV‐19 | 60 | Phase 1/Phase 2 |
| TCTR20211004005 | 4 October 2021 | Not recruiting | Parallel | ChAdOx1 nCoV‐19 | 300 | Phase 2 |
| NCT04730895 | 29 January 2021 | Not recruiting | Parallel | ChAdOx1 nCoV‐19 | 360 | Phase 1/Phase 2 |
| NCT05094609 | 26 October 2021 | Not recruiting | Parallel | Ad5‐triCoV/Mac | 30 | Phase 1 |
| NCT05007496 | 16 August 2021 | Not recruiting | Parallel | AV‐COVID‐19 | 145 | Phase 2 |
| EUCTR2020‐005226‐28‐IT | 23 November 2020 | Ongoing | Parallel | ChAdOx1 nCoV‐19 | 40,000 | Phase 3 |
| ChiCTR2100044249 | 12 March 2021 | Ongoing | Adaptive | Ad5‐nCoV | 40,000 | Phase 3 |
| EUCTR2020‐002584‐63‐DE | 12 August 2020 | Ongoing | Parallel | Ad26.COV2.S | 225 | Phase 2 |
| EUCTR2020‐005801‐14‐PL | 30 December 2020 | Ongoing | Parallel | Ad26.COV2.S | 570 | Phase 3 |
| EUCTR2021‐002693‐10‐AT | 19 May 2021 | Ongoing | Parallel | ChAdOx1 nCoV‐19 | 150 | Phase 2 |
| ISRCTN73765130 | 13 May 2021 | Ongoing | Adaptive | ChAdOx1 nCoV‐19 | 2886 | Phase 2 |
| NCT04526990 | 26 August 2020 | Ongoing | Adaptive | Ad5‐nCoV | 40,000 | Phase 3 |
| NCT04536051 | 2 September 2020 | Ongoing | Sequential assignment | ChAdOx1 nCoV‐19 | 10,300 | Phase 3 |
| NCT04639466 | 20 November 2020 | Ongoing | Parallel | COH04S1 | 129 | Phase 1 |
| NCT04666012 | 14 December 2020 | Ongoing | Sequential assignment | AdCLD‐CoV19 | 150 | Phase 1/Phase 2 |
| NCT04684446 | 24 December 2020 | Ongoing | Parallel | ChAdOx1 nCoV‐19 + rAd26‐S | 100 | Phase 1/Phase 2 |
| NCT04741061 | 5 February 2021 | Ongoing | Parallel | Sputnik light vaccine | 6000 | Phase 3 |
| NCT04776317 | 1 March 2021 | Ongoing | Parallel | ChAdV68‐S‐TCE | 140 | Phase 1 |
| NCT04816019 | 25 March 2021 | Ongoing | Sequential assignment | ChAdOx1 nCoV‐19 | 54 | Phase 1 |
| NCT04830800 | 5 April 2021 | Ongoing | Parallel | COVIVAC | 420 | Phase 1/Phase 2 |
| NCT04916886 | 8 June 2021 | Ongoing | Parallel | Ad5‐nCoV | 2016 | Not reported |
| NCT04952727 | 7 July 2021 | Ongoing | Parallel | Ad5‐nCoV | 300 | Phase 4 |
| NCT04954092 | 8 July 2021 | Ongoing | Sequential assignment | Gam‐COVID‐Vac M | 350 | Phase 2/Phase 3 |
| NCT04962906 | 15 July 2021 | Ongoing | Parallel | Gam‐COVID‐Vac + ChAdOx1 nCov‐19 | 150 | Phase 2 |
| NCT04973449 | 22 July 2021 | Ongoing | Parallel | ChAdOx1 nCov‐19 | 2475 | Phase 2/Phase 3 |
| PACTR202006922165132 | 22 June 2020 | Ongoing | Parallel | ChAdOx1 nCoV‐19 | 2000 | Phase 1/Phase 2 |
| NCT04983537 | 30 July 2021 | Ongoing | Parallel | Gam‐COVID‐Vac | 120 | Phase 2 |
| NCT04988048 | 3 August 2021 | Ongoing | Parallel | Gam‐COVID‐Vac + ChAdOx1 nCov‐19 | 1760 | Phase 2 |
| NCT05007951 | 17 August 2021 | Ongoing | Parallel | ChAdOx1 nCov‐19 | 3990 | Phase 3 |
| NCT05005156 | 13 August 2021 | Ongoing | Parallel | Ad5‐nCoV | 876 | Phase 2 |
| EUCTR2019‐003102‐26‐IT | 7 June 2021 | Ongoing | Parallel | ChAdOx1 nCov‐19 | 33 | Phase 1/Phase 2 |
| NCT05054621 | 23 September 2021 | Ongoing | Parallel | ChAdOx1 nCoV‐19 + MVC‐COV1901 | 110 | Phase 2 |
| EUCTR2021‐001978‐37‐ES | 6 May 2021 | Ongoing | Adaptive | ChAdOx1 nCoV‐19 + BNT162b2 | 600 | Phase 2 |
| NCT05037188 | 8 September 2021 | Ongoing | Sequential assignment | BCD‐250 | 160 | Phase 1/Phase 2 |
| NCT05067933 | 5 October 2021 | Ongoing | Sequential assignment | VXA‐CoV2‐1.1‐S | 896 | Phase 2 |
| TCTR20210722003 | 22 July 2021 | Ongoing | Parallel | ChAdOx1 nCov‐19 | 400 | Phase 2 |
| NCT04685603 | 25 December 2020 | Ongoing | Adaptive | AV‐COVID‐19 | 27 | Phase 1 |
| NCT04535453 | 2 September 2020 | Cancelled | Parallel | Ad26.COV2.S | 1210 | Phase 2 |
| Characteristics of unpublished registered studies: replicating viral vector (10 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions | Estimated sample size | Phase |
| NCT04497298 | 4 August 2020 | Completed | Parallel | TMV‐083/V‐591 | 90 | Phase 1 |
| ChiCTR2000037782 | 1 September 2020 | Not recruiting | Parallel | DelNS1‐2019‐nCoV‐RBD‐OPT1 | 60 | Phase 1 |
| ChiCTR2100048316 | 5 July 2021 | Not recruiting | Parallel | DelNS1‐2019‐nCoV‐RBD‐OPT1 | 400 | Not reported |
| NCT04990466 | 4 August 2021 | Not recruiting | Parallel | rVSV‐SARS‐CoV‐2‐S vaccine | 550 | Phase 2/Phase 3 |
| ChiCTR2100051391 | 22 September 2021 | Not recruiting | Parallel | DelNS1‐2019‐nCoV‐RBD‐OPT1 | 40,000 | Phase 3 |
| ChiCTR2000039715 | 6 November 2020 | Ongoing | Parallel | DelNS1‐2019‐nCoV‐RBD‐OPT1 | 720 | Phase 2 |
| NCT04608305 | 29 October 2020 | Ongoing | Sequential assignment | rVSV‐SARS‐CoV‐2‐S vaccine | 1040 | Phase 1/Phase 2 |
| NCT04993209 | 6 August 2021 | Ongoing | Adaptive | NDV‐HXP‐S | 5394 | Phase 1/Phase 2 |
| NCT04498247 | 4 August 2020 | Terminated | Sequential assignment | V591‐001 | 263 | Phase 1/Phase 2 |
| NCT04569786 | 30 September 2020 | Terminated | Sequential assignment | V590‐001 | 232 | Phase 1 |
| Characteristics of unpublished registered studies: inactivated virus vaccine (61 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions | Estimated sample size | Phase |
| ChiCTR2000034780 | 8 July 2020 | Completed | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 15,000 | Phase 3 |
| ChiCTR2100041704 | 1 January 2021 | Completed | Parallel | SARS‐CoV‐2 vaccine | 360 | Not reported |
| NCT04691908 | 31 December 2020 | Completed | Parallel | QazCovid‐in | 3000 | Phase 3 |
| NCT04790851 | 10 March 2021 | Completed | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) + IIV4 + Inactivated SARS‐CoV‐2 vaccine (vero cell) + pneumococcal vaccine | 1152 | Phase 4 |
| ChiCTR2100046174 | 8 May 2021 | Not recruiting | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 1152 | Phase 4 |
| ChiCTR2000040146 | 22 November 2020 | Not recruiting | Parallel | INO‐4800 + CoronaVac | 640 | Phase 2 |
| ChiCTR2100046227 | 11 May 2021 | Not recruiting | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 1404 | Phase 4 |
| JPRN‐jRCT2071200106 | 3 March 2021 | Not recruiting | Parallel | KD‐414 | 210 | Phase 1/Phase 2 |
| NCT04560881 | 23 September 2020 | Not recruiting | Parallel | BBIBP‐CorV | 3000 | Phase 3 |
| NCT04612972 | 3 November 2020 | Not recruiting | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 12,000 | Phase 3 |
| NCT04747821 | 10 February 2021 | Not recruiting | Parallel | CoronaVac | 27,711 | Phase 4 |
| NCT04852705 | 21 April 2021 | Not recruiting | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 28,000 | Phase 3 |
| NCT04884685 | 13 May 2021 | Not recruiting | Parallel | CoronaVac | 500 | Phase 2 |
| NCT04894227 | 20 May 2021 | Not recruiting | Parallel | CoronaVac | 1080 | Phase 4 |
| NCT04917523 | 8 June 2021 | Not recruiting | Parallel | BBIBP‐CorV | 1800 | Phase 3 |
| NCT04953325 | 7 July 2021 | Not recruiting | Parallel | CoronaVac | 270 | Phase 4 |
| NCT04956224 | 9 July 2021 | Not recruiting | Parallel | VLA2001 | 750 | Phase 3 |
| PER‐051‐20 | 18 August 2020 | Not recruiting | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 12,000 | Phase 3 |
| NCT04984408 | 30 July 2021 | Not recruiting | Parallel | BBIBP‐CorV | 8825 | Phase 3 |
| NCT04992182 | 5 August 2021 | Not recruiting | Parallel | CoronaVac | 534 | Phase 2 |
| NCT05003466 | 12 August 2021 | Not recruiting | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 480 | Phase 2 |
| NCT05003479 | 12 August 2021 | Not recruiting | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 84 | Phase 1 |
| IRCT20210206050259N3 | 29 August 2021 | Not recruiting | Parallel | Inactivated SARS‐CoV‐2 vaccine FAKHRAVAC (MIVAC) | 41,128 | Phase 3 |
| NCT05035238 | 3 September 2021 | Not recruiting | Parallel | Turkovac | 200 | Phase 2 |
| CTRI/2021/08/035648 | 13 August 2021 | Not recruiting | Parallel | Covaxin | 1100 | Phase 4 |
| NCT05046548 | 16 September 2021 | Not recruiting | Parallel | Kovivac | 400 | Phase 1/Phase 2 |
| NCT05079217 | 15 October 2021 | Not recruiting | Parallel | CoronaVac | 1200 | Phase 4 |
| NCT04993365 | 6 August 2021 | Not recruiting | Parallel | CoronaVac + influenza vaccine + pneumococcal vaccine | 440 | Phase 4 |
| NCT05079152 | 15 October 2021 | Not recruiting | Parallel | BBIBP‐CorV + influenza vaccine + pneumococcal vaccine | 1404 | Phase 4 |
| IRCT20201202049567N1 | 15 December 2020 | Ongoing | Parallel | SARS‐CoV‐2 vaccine | 56 | Phase 1 |
| IRCT20201202049567N2 | 13 March 2021 | Ongoing | Parallel | Antigen protein | 32 | Phase 1 |
| IRCT20201202049567N3 | 13 March 2021 | Ongoing | Parallel | Antigen protein | 20,000 | Phase 2/Phase 3 |
| IRCT20210206050259N1 | 8 March 2021 | Ongoing | Factorial | Inactivated SARS‐CoV‐2 vaccine FAKHRAVAC (MIVAC) | 135 | Phase 1 |
| IRCT20210206050259N2 | 8 June 2021 | Ongoing | Parallel | Inactivated SARS‐CoV‐2 vaccine FAKHRAVAC (MIVAC) | 500 | Phase 2 |
| ChiCTR2000039000 | 13 October 2020 | Ongoing | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 600 | Phase 3 |
| ChiCTR2100043907 | 5 March 2021 | Ongoing | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 16 | Phase 4 |
| ChiCTR2100045109 | 7 April 2021 | Ongoing | Parallel | Inactivated COVID‐19 vaccine | 472 | Not reported |
| ChiCTR2100047917 | 27 June 2021 | Ongoing | Sequential assignment | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 20 | Phase 1 |
| CTRI/2020/07/026300 | 26 August 2020 | Ongoing | Parallel | Covaxin | 1125 | Phase 1/Phase 2 |
| CTRI/2020/09/027674 | 8 September 2020 | Ongoing | Adaptive | Covaxin | 124 | Phase 1/Phase 2 |
| NCT04470609 | 14 July 2020 | Ongoing | Parallel | SARS‐CoV‐2 vaccine | 471 | Phase 1/Phase 2 |
| NCT04617483 | 5 November 2020 | Ongoing | Parallel | SARS‐CoV‐2 vaccine (inactivated) | 1040 | Phase 3 |
| NCT04659239 | 9 December 2020 | Ongoing | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 34,020 | Phase 3 |
| NCT04691947 | 31 December 2020 | Ongoing | Parallel | ERUCOV‐VAC | 44 | Phase 1 |
| NCT04824391 | 1 April 2021 | Ongoing | Parallel | ERUCOV‐VAC | 250 | Phase 2 |
| NCT04838080 | 8 April 2021 | Ongoing | Parallel | Inactivated COVID‐19 vaccine | 38 | Phase 1 |
| NCT04863638 | 28 April 2021 | Ongoing | Parallel | BBIBP‐CorV | 4400 | Phase 4 |
| NCT04864561 | 29 April 2021 | Ongoing | Parallel | VLA2001 | 4000 | Phase 3 |
| NCT04866069 | 29 April 2021 | Ongoing | Parallel | SARS‐CoV‐2 vaccine | 50 | Phase 1 |
| NCT04942405 | 28 June 2021 | Ongoing | Parallel | CoronaVac | 40,800 | Phase 3 |
| NCT04962308 | 14 July 2021 | Ongoing | Parallel | CoronaVac | 1400 | Phase 4 |
| CTRI/2021/04/032942 | 19 April 2021 | Ongoing | Parallel | Covaxin | 190 | Phase 2 |
| NCT04979949 | 28 July 2021 | Ongoing | Parallel | CoronaVac | 111 | Phase 2 |
| NCT04992260 | 5 August 2021 | Ongoing | Parallel | CoronaVac | 7000 | Phase 3 |
| NCT05033847 | 3 September 2021 | Ongoing | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 1800 | Phase 3 |
| CTRI/2021/08/035993 | 27 August 2021 | Ongoing | Parallel | Covaxin | 608 | Phase 2/Phase 3 |
| NCT05043259 | 14 September 2021 | Ongoing | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 420 | Phase 1/Phase 2 |
| ChiCTR2100050589 | 31 August 2021 | Ongoing | Sequential assignment | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 500 | Phase 4 |
| TCTR20210731003 | 31 July 2021 | Ongoing | Parallel | BBIBP‐CorV | 960 | Phase 2 |
| ChiCTR2100051645 | 29 September 2021 | Ongoing | Parallel | Inactivated SARS‐CoV‐2 vaccine (vero cell) | 600 | Phase 2 |
| NCT05095298 | 27 October 2021 | Ongoing | Parallel | SARS‐CoV‐2 vaccine (inactivated) | 400 | Phase 4 |
| Characteristics of unpublished registered studies: protein subunit (91 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions | Estimated sample size | Phase |
| NCT04453852 | 1 July 2020 | Completed | Parallel | COVAX‐19 | 40 | Phase 1 |
| IRCT20201214049709N1 | 21 January 2021 | Completed | Parallel | RAZI‐COV PARS | 133 | Phase 1 |
| RPCEC00000345 | 26 November 2020 | Not recruiting | Parallel | CIGB‐669 (RBD/AgnHB) | 88 | Phase 1/Phase 2 |
| RPCEC00000381 | 1 July 2021 | Not recruiting | Parallel | CIGB‐66 (RBD/aluminium hydroxide) | 592 | Phase 1/Phase 2 |
| RPCEC00000382 | 9 July 2021 | Not recruiting | Parallel | CIGB‐669 (RBD/HBcAg) | 120 | Phase 1/Phase 2 |
| NCT05084989 | 20 October 2021 | Not recruiting | Cross‐over | Recov – recombinant 2‐component COVID‐19 vaccine (cho cell) | 20,301 | Phase 2/Phase 3 |
| RPCEC00000346 | 26 November 2020 | Not recruiting | Factorial | CIGB‐66 (RBD/aluminium hydroxide) | 132 | Phase 1/Phase 2 |
| PACTR202107562417077 | 23 July 2021 | Not recruiting | Factorial | Recombinant SARS‐CoV‐2 fusion protein vaccine (v‐01) | 22,500 | Phase 3 |
| PACTR202108616900606 | 6 August 2021 | Not recruiting | Factorial | CpG 1018/alum adjuvant + scb‐2019 | 600 | Phase 3 |
| ACTRN12620001308987 | 4 December 2020 | Not recruiting | Parallel | RBD + alum adjuvant | 255 | Phase 1/Phase 2 |
| ACTRN12621000882820 | 8 July 2021 | Not recruiting | Parallel | IVX‐411 | 84 | Phase 2 |
| ChiCTR2000035691 | 16 August 2020 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 50 | Phase 1 |
| ChiCTR2000037518 | 28 August 2020 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 168 | Phase 1 |
| ChiCTR2000040153 | 22 November 2020 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 29,000 | Phase 3 |
| ChiCTR2100048439 | 7 July 2021 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO cell) | 75 | Phase 1 |
| CTRI/2020/11/029032 | 10 November 2020 | Not recruiting | Parallel | BECOV2 | 360 | Phase 1/Phase 2 |
| CTRI/2021/02/031554 | 25 February 2021 | Not recruiting | Parallel | SARS‐CoV‐2 rS/Matrix M1‐adjuvant | 1600 | Phase 2/Phase 3 |
| JPRN‐jRCT2051200092 | 9 December 2020 | Not recruiting | Parallel | S‐268019 | 300 | Phase 1/Phase 2 |
| NCT04473690 | 16 July 2020 | Not recruiting | Parallel | KBP‐COVID‐19 | 180 | Phase 1/Phase 2 |
| NCT04672395 | 17 December 2020 | Not recruiting | Parallel | SCB‐2019 + CpG 1018/Alum‐adjuvant | 22,000 | Phase 2/Phase 3 |
| NCT04683224 | 24 December 2020 | Not recruiting | Parallel | UB‐612 | 7320 | Phase 2/Phase 3 |
| NCT04712110 | 15 January 2021 | Not recruiting | Parallel | TAK‐019 | 200 | Phase 1/Phase 2 |
| NCT04742738 | 8 February 2021 | Not recruiting | Parallel | GBP510 + aluminium hydroxide adjuvant | 260 | Phase 1/Phase 2 |
| NCT04750343 | 11 February 2021 | Not recruiting | Parallel | GBP510 + AS03 adjuvant | 320 | Phase 1/Phase 2 |
| NCT04760743 | 18 February 2021 | Not recruiting | Parallel | NBP2001 | 50 | Phase 1 |
| NCT04780035 | 3 March 2021 | Not recruiting | Parallel | EpiVacCorona | 3000 | Phase 3 |
| NCT04784767 | 5 March 2021 | Not recruiting | Parallel | SpFN_1B‐06‐PL + ALFQ (QS21 adjuvant) | 72 | Phase 1 |
| NCT04887207 | 14 May 2021 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 40,000 | Phase 3 |
| NCT04930003 | 18 June 2021 | Not recruiting | Parallel | QazCoVac‐P | 244 | Phase 1/Phase 2 |
| NCT04944368 | 29 June 2021 | Not recruiting | Parallel | SARS‐CoV‐2 recombinant spike protein + Advax‐SM adjuvant | 400 | Phase 2 |
| NCT04950751 | 6 July 2021 | Not recruiting | Parallel | SCB‐2020S | 150 | Phase 2 |
| NCT04951388 | 6 July 2021 | Not recruiting | Parallel | COV1901 | 385 | Phase 2 |
| NCT04954131 | 8 July 2021 | Not recruiting | Parallel | SCB‐2019 | 800 | Phase 2 |
| PACTR202011523101903 | 2 November 2020 | Not recruiting | Parallel | SARS‐CoV‐2 recombinant protein vaccine + AS03 adjuvant | 34,520 | Not reported |
| PACTR202103845381761 | 3 March 2021 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 40,000 | Phase 3 |
| RPCEC00000347 | 17 December 2020 | Not recruiting | Parallel | FINLAY‐FR‐2 anti‐SARS‐CoV‐2 vaccine | 910 | Phase 2 |
| RPCEC00000359 | 18 March 2021 | Not recruiting | Parallel | CIGB‐66 (RBD/aluminium hydroxide) | 48,000 | Phase 3 |
| RPCEC00000366 | 9 April 2021 | Not recruiting | Parallel | FINLAY‐FR‐1A anti‐SARS‐CoV‐2 Vaccine | 450 | Phase 2 |
| NCT05007509 | 16 August 2021 | Not recruiting | Parallel | Hipra | 30 | Phase 1/Phase 2 |
| NCT05005559 | 13 August 2021 | Not recruiting | Parallel | SARS‐CoV‐2 recombinant spike p + Advax‐cpg adjuvant | 16,876 | Phase 3 |
| NCT05012787 | 19 August 2021 | Not recruiting | Parallel | SCB‐2019 + CpG 1018 adjuvant | 300 | Phase 3 |
| NCT05013983 | 20 August 2021 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 600 | Phase 1/Phase 2 |
| NCT05016934 | 23 August 2021 | Not recruiting | Parallel | Versamune‐CoV‐2FC | 360 | Phase 1/Phase 2 |
| JPRN‐jRCT2051210057 | 29 July 2021 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 240 | Phase 1/Phase 2 |
| NCT05029856 | 1 September 2021 | Not recruiting | Parallel | Monovalent B.1.351 vaccine + Matrix‐M1 Adjuvant | 240 | Phase 1/Phase 2 |
| CTRI/2021/08/036074 | 31 August 2021 | Not recruiting | Parallel | Corbevax | 2140 | Phase 3 |
| NCT05043285 | 14 September 2021 | Not recruiting | Parallel | SCTV01C | 8420 | Phase 2/Phase 3 |
| NCT05043311 | 14 September 2021 | Not recruiting | Parallel | SCTV01C | 12,420 | Phase 2/Phase 3 |
| NCT05067894 | 5 October 2021 | Not recruiting | Parallel | SARS‐CoV‐2 recombinant protein vaccine | 780 | Phase 1/Phase 2 |
| JPRN‐jRCT2031210269 | 23 August 2021 | Not recruiting | Parallel | S‐268019 | 60 | Phase 1/Phase 2 |
| RPCEC00000385 | 23 July 2021 | Not recruiting | Parallel | Finlay‐fr‐1a anti‐SARS‐CoV‐2 vaccine + FINLAY‐Fr‐1 anti‐SARS‐CoV‐2 vaccine | 1166 | Phase 2 |
| NCT05096832 | 27 October 2021 | Not recruiting | Parallel | Recombinant SARS‐CoV‐2 fusion protein vaccine (v‐01) | 10,722 | Phase 3 |
| NCT04961541 | 14 July 2021 | Not recruiting | Parallel | ICC vaccine | 720 | Phase 1/Phase 2 |
| NCT05087368 | 21 October 2021 | Not recruiting | Parallel | Alum adjuvant + SCB‐2019 | 520 | Phase 2 |
| NCT04522089 | 21 August 2020 | Not recruiting | Sequential assignment | AdimrSC‐2f | 70 | Phase 1 |
| NCT04550351 | 16 September 2020 | Not recruiting | Sequential assignment | Recombinant SARS‐CoV‐2 vaccine (CHO cell) | 50 | Phase 1/Phase 2 |
| RPCEC00000360 | 19 March 2021 | Not recruiting | Single‐group assignment | FINLAY‐FR‐2 anti‐SARS‐CoV‐2 vaccine + FINLAY‐FR‐1A anti‐SARS‐CoV‐2 vaccine | 150,000 | Not reported |
| RPCEC00000363 | 27 March 2021 | Not recruiting | Single group assignment | CIGB‐66 (RBD/aluminium hydroxide) | 124,000 | Not reported |
| IRCT20150303021315N23 | 24 May 2021 | Ongoing | Parallel | SARS‐CoV‐2 spike (S) protein subunit vaccine + Advax‐CpG adjuvant | 400 | Phase 2 |
| IRCT20201214049709N2 | 13 April 2021 | Ongoing | Parallel | RAZI‐COV PARS | 500 | Phase 2 |
| IRCT20150303021315N24 | 3 August 2021 | Ongoing | Parallel | SARS‐CoV‐2 recombinant spike protein + Advax‐SM adjuvant | 16,876 | Phase 3 |
| IRCT20210303050558N1 | 24 April 2021 | Ongoing | Parallel | FINLAY‐FR‐2 anti‐SARS‐CoV‐2 vaccine | 24,000 | Phase 3 |
| ACTRN12621000738820 | 11 June 2021 | Ongoing | Parallel | IVX‐411 | 84 | Phase 1 |
| ChiCTR2000039994 | 17 November 2020 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 960 | Phase 2 |
| ChiCTR2100042374 | 21 January 2021 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 4800 | Phase 2 |
| EUCTR2020‐004272‐17‐DE | 6 January 2021 | Ongoing | Parallel | SCB‐2019 | 800 | Phase 2/Phase 3 |
| IRCT20210620051639N1 | 25 June 2021 | Ongoing | Parallel | Noora | 70 | Phase 1 |
| NCT04636333 | 19 November 2020 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 216 | Phase 1 |
| NCT04646590 | 30 November 2020 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 29,000 | Phase 3 |
| NCT04773067 | 26 February 2021 | Ongoing | Parallel | UB‐612 | 3850 | Phase 2 |
| NCT04783311 | 5 March 2021 | Ongoing | Parallel | EuCorVac‐19 | 280 | Phase 1/Phase 2 |
| NCT04813562 | 24 March 2021 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 480 | Phase 2 |
| NCT04818801 | 26 March 2021 | Ongoing | Parallel | ReCOV – recombinant 2‐component COVID‐19 vaccine (CHO cell) | 160 | Phase 1 |
| NCT04822025 | 30 March 2021 | Ongoing | Parallel | MVC‐COV1901 | 400 | Phase 2 |
| NCT04869592 | 3 May 2021 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 3580 | Phase 1/Phase 2 |
| NCT04885361 | 13 May 2021 | Ongoing | Parallel | CoVepiT (OSE13E) | 48 | Phase 1 |
| NCT04904471 | 27 May 2021 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 40,000 | Phase 3 |
| NCT04904549 | 27 May 2021 | Ongoing | Parallel | SARS‐CoV‐2 adjuvanted recombinant protein vaccine (monovalent) | 37,430 | Phase 3 |
| NCT04922788 | 11 June 2021 | Ongoing | Parallel | Nanocovax | 13,000 | Phase 3 |
| NCT04982068 | 29 July 2021 | Ongoing | Parallel | 202‐CoV | 144 | Phase 1 |
| NCT04990544 | 4 August 2021 | Ongoing | Parallel | 202‐CoV | 1056 | Phase 2 |
| IRCT20201214049709N3 | 29 August 2021 | Ongoing | Parallel | RAZI‐COV PARS | 41,128 | Phase 3 |
| NCT05069129 | 6 October 2021 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 1848 | Phase 1/Phase 2 |
| NCT05091411 | 25 October 2021 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 1680 | Phase 3 |
| NCT05096845 | 27 October 2021 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 fusion protein vaccine (v‐01) | 22,500 | Phase 3 |
| NCT05097053 | 27 October 2021 | Ongoing | Parallel | Mvc‐cov1901 | 200 | Phase 4 |
| ChiCTR2100050849 | 5 September 2021 | Ongoing | Parallel | Recombinant SARS‐CoV‐2 vaccine (CHO Cell) | 14,600 | Phase 3 |
| NCT04702178 | 8 January 2021 | Ongoing | Sequential assignment | COVAC‐2 | 108 | Phase 1/Phase 2 |
| NCT04961359 | 14 July 2021 | Ongoing | Sequential assignment | Recombinant SARS‐CoV‐2 vaccine (CHO cell) | 75 | Phase 1 |
| NCT04718467 | 22 January 2021 | Cancelled | Parallel | Recombinant SARS‐CoV‐2 vaccine (Sf9 Cell) | 0 | Phase 2 |
| NCT04806529 | 19 March 2021 | Cancelled | Parallel | Adjuvanted SARS‐CoV‐2 subunit vaccine (aCoV2) | 0 | Phase 2/Phase 3 |
| Characteristics of unpublished registered studies: live attenuated virus (2 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions | Estimated sample size | Phase |
| NCT04619628 | 6 November 2020 | Not recruiting | Parallel | COVI‐VAC | 48 | Phase 1 |
| NCT04809389 | 22 March 2021 | Not recruiting | Parallel | DelNS1‐nCoV‐RBD LAIV | 115 | Phase 1 |
| Characteristics of unpublished registered studies: DNA‐based vaccine (18 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions | Estimated sample size | Phase |
| ChiCTR2000038152 | 11 September 2020 | Completed | Parallel | INO‐4800 + electroporation | 45 | Phase 1 |
| NCT04527081 | 26 August 2020 | Completed | Parallel | AG0302‐COVID19 | 30 | Phase 1/Phase 2 |
| CTRI/2020/07/026352 | 4 July 2020 | Not recruiting | Adaptive | nCov vaccine | 1048 | Phase 1/Phase 2 |
| CTRI/2021/03/032051 | 16 March 2021 | Not recruiting | Parallel | ZyCov‐D | 150 | Phase 1/Phase 2 |
| NCT04655625 | 7 July 2020 | Not recruiting | Parallel | AG0302‐COVID19 | 500 | Phase 2/Phase 3 |
| NCT04742842 | 8 February 2021 | Not recruiting | Sequential assignment | COVIGEN | 150 | Phase 1 |
| NCT04993586 | 6 August 2021 | Not recruiting | Parallel | AG0302‐COVID19 | 80 | Phase 1/Phase 2 |
| JPRN‐jRCT2051210052 | 16 July 2021 | Not recruiting | Parallel | AG0302‐COVID19 | 400 | Phase 1/Phase 2 |
| NCT05067946 | 5 October 2021 | Not recruiting | Parallel | Gx‐19n | 14,000 | Phase 2/Phase 3 |
| NCT05085639 | 20 October 2021 | Not recruiting | Parallel | GLS‐5130 | 30 | Phase 1 |
| NCT05102643 | 1 November 2021 | Not recruiting | Sequential assignment | SARS‐CoV‐2 DNA vaccine + electroporation | 30 | Phase 1 |
| CTRI/2021/01/030416 | 12 January 2021 | Ongoing | Parallel | ZyCov‐D | 28,216 | Phase 3 |
| NCT04445389 | 24 June 2020 | Ongoing | Parallel | GX‐19 | 210 | Phase 1/Phase 2 |
| NCT04447781 | 25 June 2020 | Ongoing | Sequential assignment | INO‐4800 + electroporation | 160 | Phase 1/Phase 2 |
| NCT04591184 | 19 October 2020 | Ongoing | Parallel | Covigenix VAX‐001 | 72 | Phase 1/Phase 2 |
| NCT04673149 | 17 December 2020 | Ongoing | Parallel | GLS‐5310 | 345 | Phase 1/Phase 2 |
| NCT05047445 | 17 September 2021 | Ongoing | Parallel | Covidity | 40 | Phase 1 |
| NCT04715997 | 20 January 2021 | Ongoing | Sequential assignment | GX‐19N | 170 | Phase 1/Phase 2 |
| Characteristics of unpublished registered studies: virus‐like particle (12 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions | Estimated sample size | Phase |
| NCT04662697 | 10 December 2020 | Not recruiting | Parallel | Coronavirus‐like particle COVID‐19 + adjuvant | 918 | Phase 2 |
| NCT05040789 | 10 September 2021 | Not recruiting | Parallel | SARS‐CoV‐2 VLP vaccine | 900 | Phase 3 |
| JPRN‐jRCT2051210093 | 28 September 2021 | Ongoing | Parallel | Adjuvant + coronavirus‐like particle COVID‐19 | 145 | Phase 1/Phase 2 |
| NCT05065619 | 4 October 2021 | Ongoing | Parallel | Coronavirus‐like particle COVID‐19 | 145 | Phase 1/Phase 2 |
| ACTRN12620000817943 | 14 August 2020 | Ongoing | Parallel | RBD SARS‐CoV‐2 HBsAg VLP vaccine | 280 | Phase 1/Phase 2 |
| IRCT20210620051639N2 | 11 October 2021 | Ongoing | Parallel | RBD SARS‐CoV‐2 HBsAg VLP vaccine | 300 | Phase 2 |
| NCT04935528 | 23 June 2021 | Ongoing | Single‐group assignment | SARS‐COV‐2 vaccine | 430 | Not reported |
| NCT04844346 | 14 April 2021 | Ongoing | Parallel | SARS‐CoV‐2 vaccine + plant stanol esters | 100 | Not reported |
| NCT04818281 | 26 March 2021 | Ongoing | Parallel | SARS‐CoV‐2 VLP vaccine | 36 | Phase 1 |
| NCT04962893 | 15 July 2021 | Ongoing | Parallel | SARS‐CoV‐2 VLP vaccine‐Wuhan | 330 | Phase 2 |
| NCT04773665 | 26 February 2021 | Ongoing | Sequential assignment | VBI‐2902a | 780 | Phase 1 |
| NCT04854876 | 22 April 2021 | Cancelled | Parallel | SARS‐CoV‐2 vaccine + 5‐ALA/SFC | 200 | Not reported |
| Characteristics of unpublished registered studies: any COVID‐19 vaccine (3 studies) | ||||||
| Registration number | Registration date | Status | Design | Interventions | Estimated sample size | Phase |
| ChiCTR2100049467 | 2 August 2021 | Not recruiting | Parallel | COVID‐19 vaccine | 1314 | Phase 3 |
| ChiCTR2100051297 | 18 September 2021 | Ongoing | Single‐group assignment | COVID‐19 vaccine | 1500 | Phase 0 |
| ISRCTN15279830 | 14 October 2021 | Ongoing | Parallel | COVID‐19 vaccine | 800 | Phase 2 |
Appendix 5. Baseline characteristics of early‐phase studies not included in the analysis
| Type of vaccine | Reference ID | Register | Phase | Vaccine | Comparator | Sample size | Participants/centre/location |
| RNA‐based vaccine | Roozen 2021 | NL9275 | 1‐2 | mRNA‐1273 20 μg ID | mRNA‐1273 20 μg IM | 30 | Healthy adults/single centre/the Netherlands |
| Low 2021 | NCT04480957 | 1‐2 | ARCT‐021 (1 μg; 5 μg; 7.5 μg; 10 μg) |
Placebo | 106 | Healthy adults/single centre/Singapore | |
| Li 2021b | ChiCTR2000034825 NCT04523571 |
1 | BNT162b1 10 μg | BNT162b1 30 μg | 144 | Healthy young adults/single centre/China | |
| Mulligan 2020 | NCT04368728 | 1‐2 | BNT162b1 (10 μg; 30 μg; 100 μg) | Placebo | 45 | Healthy adults/2 centres/USA | |
| Non‐replicating viral vector | Ramasamy 2020 | NCT04400838; ISRCTN, 15281137 | 2/3 | ChAdOx1 (2.2 × 1010 vp; 1 or 2 doses) | MenACWY | 300 | Healthy adults/2 centres/UK |
| Inactivated virus | Wu 2021c | NCT04552366 | 1 | Ad5‐nCoV 0.2 mL neb 2D | Ad5‐nCoV (0.1 mL neb 2D; 0.5 mL IM + 0.2 mL neb; 0.5 mL IM; 1.0 mL IM) | 130 | Adults/single centre/China |
| Zhu 2020 | NCT04341389 | 2 | Ad5‐vectored (1 × 1011 vp; 5 × 1010 vp) | Placebo | 508 | Healthy young adults/single centre/China | |
| Zhu 2022 | NCT04566770 | 2 | Ad5‐vectored (3 × 1010 vp) | Placebo | 400 | Healthy children and adolescents/single centre/China | |
| Che 2021 | NCT04412538 | 2 | KMS‐1 (100 EU; 150 EU) D0/14; D0/28 KMS‐1 |
Placebo | 750 | Healthy adults/2 centres/China | |
| Lazarus 2021 | NCT04671017, ISRCTN 82411169 | 1‐2 | VL A2001 3 AU |
VL A2001 35 AU; 7 AU | 153 | Healthy adults/4 centres/UK | |
| Pan 2021a | NCT04758273 | 1 | KCONVAC 5 μg | KCONVAC 10 μg | 60 | Healthy adults/single centre/China | |
| Pan 2021a | NCT04756323 | 2 | KCONVAC (5 μg; 10 μg) (D0/14; D0/28) |
Placebo | 500 | Healthy adults/single centre/China | |
| Pitisuttithum 2021 | NCT04764422 | 1 | NDV‐HXP‐S (1 μg; 1 μg + CpG1018; 3 μg; 3 μg + CpG1018; 10 μg) | Placebo | 210 | Healthy adults/single centre/Thailand | |
| Pu 2021 | NCT04412538 | 1 | KMS‐1 100 EU D0/14; D0/28 | Placebo | 192 | Healthy adults/single centre/China | |
| Zakarya 2021 | NCT04530357 | 1 | QazCovid‐in | Placebo | 44 | Healthy adults/single centre/Kazakhstan | |
| Protein subunit | Chappell 2021 | NCT04495933 | 1 | SARS‐CoV‐2 Sclamp (5 μg; 15 μg; 45 μg) |
Placebo | 120 | Healthy adults/single centre/Australia |
| Goepfert 2021 | NCT04537208 | 1‐2 | CoV2 preS dTM LD + AFO3 CoV2 preS dTM LD + ASO3 CoV2 preS dTM HD + AFO3 CoV2 preS dTM HD + ASO3 CoV2 preS dTM HD |
Placebo | 271 | Healthy adults/10 centres/USA | |
| Hsieh 2021 | NCT04695652 | 2 | MVC‐COV1901 | Placebo | 3854 | Healthy adults/11 centres/Taiwan | |
| Zhang 2021b | ChiCTR2100045108 | 1 | V‐01 (10 μg; 25 μg; 50 μg) | Placebo | 180 | Healthy adults/single centre/China | |
| Meng 2021b | NCT04530656 | 1 | Sf9 cells vaccine (low dose in 2 doses; high dose in 2 or 3 doses) | Placebo | 168 | Healthy adults/single centre/China | |
| Meng 2021b | NCT04640402 | 2 | Sf9 cells vaccine (low dose or high dose in 2 or 3 doses) | Placebo | 960 | Healthy adults/single centre/China | |
| Nguyen 2021 | NCT04683484 | 2 | Nanocovax (25 μg; 50 μg; 75 μg) |
Placebo | 560 | Healthy adults/2 centres/Vietnam | |
| Nguyen 2021 | NCT04683484 | 1 | Nanocovax (25 μg; 50 μg) |
Nanocovax 75 μg | 60 | Healthy adults/2 centres/Vietnam | |
| Richmond 2021 | NCT04405908 | 1 | SCB‐2019 (3 μg; 3 μg + AS03; 3 μg + CpG/Alum; 9 μg; 9 μg + AS03 9 μg + CpG/Alum; 30 μg; 30 μg + AS03; 30 μg + CpG/Alu) |
Placebo | 151 | Healthy adults/single centre/Australia | |
| Ryzhikov 2021 | NCT04527575 | 2 | EpiVacCorona | Placebo | 86 | Healthy adults/single centre/Russia | |
| Shu 2021 | ChiCTR2100045107 | 2 | V‐01 (10 μg; 25 μg; 50 μg) | Placebo | 880 | Healthy adults/single centre/China | |
| Sridhar 2021 | NCT04762680 | 2 | CoV2 preS dTM (15 µg; 10 µg) | CoV2 preS dTM 5 µg | 722 | Adults with and without prior SARS‐CoV‐2 infection and risk factors for severe disease/20 centres/USA and Honduras | |
| Yang 2021 | NCT04466085 | 2 | ZF2001 (25 μg 2 doses; 50 μg 2 doses; 25 μg 3 doses; 50 μg 3 doses) | Placebo | 900 | Healthy adults/single centre/China | |
|
Yang 2021 |
NCT04445194 | 2 | ZF2001 (25 μg 3 doses; 50 μg 3 dose) | Placebo | 50 | Healthy adults/single centre/China | |
| DNA‐based vaccine | Mammen 2021 | NCT04642638 | INO‐4800 (1 mg; 2 mg) D0/28 |
Placebo | 201 | Healthy adults/19 centres/USA | |
| Virus‐like particle (VLP) | Gobeil 2021 | NCT04636697 | CoVLP 3.75 μg + AS03 | Placebo | 753 | Healthy adults/multiple centres/Canada and USA | |
| Ward 2021b | NCT04450004 | CoVLP (3.75 μg with CpG1018, AS03 or without adjuvant; 7.5 μg with CpG1018, AS03 or without adjuvant; 15 μg with CpG1018, AS03 or without adjuvant) | Placebo | 180 | Canada |
Appendix 6. Baseline characteristics of studies with no outcomes of interest or not extractable
| Reports of trials not included in the analysis (5 studies) | |||||||
| Type of vaccine | Reference | Register | Phase | Vaccine | Comparator | Sample size | Population/centre/location |
| RNA‐based vaccine | Chu 2021 | NCT04405076 | 2 | mRNA‐1273 (50 μg; 100 μg) | Placebo | 600 | Healthy adults/8 centres/USA |
| Inactivated virus | Feng 2021 | ChiCTR2100041705; ChiCTR2100041706 | * | BBIBP‐CorV D0/14; D0/21 | BBIBP‐CorV D0/28 | 809 | Healthy adults /single centre/China |
| Protein subunit | Pérez‐Rodríguez 2021 | RPCEC00000338‐En | 1 | FINLAY‐FR‐1A (25 μg; 50 μg) | FINLAY‐FR‐1 | 60 | Healthy adults/single centre/Cuba |
| Heterologous scheme | Borobia 2021 | NCT04860739; EudraCT2021‐001978 | 2 | BNT162b2 after 1 dose ChAdOx1‐S – 1 IM dose 30 μg/0.3 mL BNT162b2 8‐12 weeks after 1 dose ChAdOx1‐S | No second vaccine dose | 676 | Adults/multicentre/Spain |
| Non‐replicating viral vector/inactivated virus | Angkasekwinai 2022 | TCTR20210720002 | CoronaVac 3 μg | ChAdOx1 (5 × 1010 vp) | 360 | Healthcare workers/single centre/Thailand | |
| Reports of trials already included in the analysis (7 studies) | |||||||
| RNA‐based vaccine | Pajon 2021 | NCT04470427 | 3 | mRNA‐1273 | Placebo | 791 | Healthy adults/99 centres/USA |
| Non‐replicating viral vector | Voysey 2021b | NCT04324606; ISRCTN89951424; NCT04400838; NCT04444674 | 1/2/3 | ChAdOx1 (5 × 1010 vp or 2.2 × 1010 vp) | Placebo/MenACWY | 17, 177 | Adults/multicentre/Brazil, South Africa and UK |
| Stephenson 2021 | NCT04436276 | 1 | Ad26.COV2.S | Placebo | 10 | Healthy adults/single centre/USA | |
| Inactivated virus | Pan 2021b | NCT04352608 | 2 | CoronaVac (3 doses, 4 different schedules, 3 μg and 6 μg) | Placebo | 600 | Healthy adults/single centre/China |
|
Ella 2021a |
NCT04471519 | 2 | 6 μg BBV152 + Algel‐IMDG | 3 μg BBV152 + Algel‐IMDG | 380 | Healthy adults/9 centres/India | |
| Li 2021c | NCT04383574 | 1/2 | CoronaVac (3 doses) | Placebo | 350 | Healthy adults aged ≥ 60 years/single centre/China | |
| Ella 2020b | NCT04471519 | 2 | 6 μg BBV152 + Algel‐IMDG | 3 μg BBV152 + Algel‐IMDG | 380 | Healthy adults/9 centres/India | |
Appendix 7. List of previous publications later updated
| Reference/study ID | Registry | |
| RNA‐based vaccine | FDA 2020b | NCT04470427 |
| Baden 2021 | NCT04470427 | |
| Walsh 2021 | NCT04368728 | |
| Thomas 2021 | NCT04368728 | |
| FDA 2020c | NCT04368728 | |
| Polack 2020 | NCT04368728 | |
| Non‐replicating viral vector | Madhi 2021 | NCT04444674 |
| Folegatti 2020 | NCT04324606 | |
| FDA 2021 | NCT04505722 | |
| Sadoff 2020c | NCT04436276 | |
| Inactivated virus | Bueno 2021 | NCT04651790 |
| Xia 2020 | ChiCTR2000031809 | |
| Formica 2021 | NCT04368988 | |
| Protein subunit | Shinde 2021 | NCT04533399 |
| Heath 2021 | NCT04583995 | |
| Heterologous schedule | Liu 2021 | ISRCTN69254139 |
Appendix 8. Risk of bias assessments
RNA‐based vaccines
BNT162b2 – BioNTech/Fosun Pharma/Pfizer versus placebo
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Frenck 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
| Thomas 2021 | Low | Some concernsb | Low | Low | Low | Some concerns |
aFrenck 2021, RoB 2. Deviations from intervention: Quote: "observer‐blinded" (report) “Masking: Triple (Participant, Care Provider, Investigator)” (registry) Comment: blinded study (participants, personnel, investigators). Per‐protocol analysis as planned in the trial protocol) was performed on the outcomes: 'confirmed symptomatic COVID'. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. Risk assessed as some concerns for this outcome.
bThomas 2021, RoB 2. Deviations from intervention: Quote: "observer blinded" Comment: blinded study (participants and personnel/carers) Per‐protocol analysis was performed on the outcome: 'confirmed symptomatic COVID'. Reasons for exclusion: positive at baseline (689 versus 716) not received 2 vaccinations as randomized (326 versus 430) Reasons for exclusion in the 12–15‐year group not reported As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Thomas 2021 | Low | Low | Low | Low | Low | Low |
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Walsh 2020 | Low | Low | Low | Low | Low | Low |
| Frenck 2021 | Low | Low | Low | Low | Low | Low |
| Thomas 2021 | Low | Low | Low | Low | Low | Low |
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Walsh 2020 | Low | Low | Low | Low | Low | Low |
| Frenck 2021 | Low | Low | Low | Low | Low | Low |
| Thomas 2021 | Low | Low | Low | Low | Low | Low |
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Walsh 2020 | Low | Low | Low | Low | Low | Low |
| Frenck 2021 | Low | Low | Low | Low | Low | Low |
| Thomas 2021 | Low | Low | Low | Low | Low | Low |
mRNA‐1273 – ModernaTX versus placebo
SARS‐CoV‐2 infection after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ali 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
| El Sahly 2021 | Low | Some concernsb | Low | Low | Low | Some concerns |
aAli 2021, RoB 2. Deviations from intervention: Quote: "The investigators and trial staff, participants, site monitors, and sponsor personnel (or its designees) were unaware of the trial vaccine administered until unblinding of the trial data as specified in the protocol; however, pharmacists and vaccine administrators who were involved in injection preparation and administration and who had no other role in trial conduct were aware of these assignments." Comment: blinded study (participants and personnel/carers). Data for this outcome were analyzed using modified intention‐to‐treat or per protocol analysis. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment. Reasons for exclusion: mITT – did not receive at least one dose, had serological or virological evidence of previous SARS‐CoV‐2 infection before the first injection, received wrong injection; per protocol – did not receive planned injections of mRNA‐1273 or placebo, did not comply with the timing of the second injection, had immunological or virological evidence of previous COVID‐19 at baseline, and major protocol deviations. Risk assessed to have some concerns for this outcome.
bEl Sahly 2021, RoB 2. Deviations from intervention: Quote: "The investigator, study staff, study participants, site monitors, and Sponsor personnel (or its designees) will be blinded to the IP administered until study end." (protocol) "Masking: quadruple (participant, care provider, investigator, outcomes assessor." (registry) Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups (922 (6.1%) versus 1042 (6.9%)), with the majority of those excluded due to positive or unknown baseline SARS‐CoV‐2 status (434 versus 421). Other reasons: did not receive any injection (29 versus 40), received an incorrect injection (6 versus 7), discontinued without receiving second dose (334 versus 425), received dose 2 outside planned time frame (102 versus 119), other major protocol deviation (17 versus 30). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ali 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
| El Sahly 2021 | Low | Some concernsb | Low | Low | Low | Some concerns |
aAli 2021, RoB 2. Deviations from intervention: Quote: "The investigators and trial staff, participants, site monitors, and sponsor personnel (or its designees) were unaware of the trial vaccine administered until unblinding of the trial data as specified in the protocol; however, pharmacists and vaccine administrators who were involved in injection preparation and administration and who had no other role in trial conduct were aware of these assignments." Comment: blinded study (participants and personnel/carers). Data for this outcome were analyzed using modified intention‐to‐treat or per protocol analysis. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment. Reasons for exclusion: mITT – did not receive at least one dose, had serological or virological evidence of previous SARS‐CoV‐2 infection before the first injection, received wrong injection; per protocol – did not receive planned injections of mRNA‐1273 or placebo, did not comply with the timing of the second injection, had immunological or virological evidence of previous COVID‐19 at baseline, and major protocol deviations. Risk assessed to have some concerns for this outcome.
bEl Sahly 2021, RoB 2. Deviations from intervention: Quote: "The investigator, study staff, study participants, site monitors, and Sponsor personnel (or its designees) will be blinded to the IP administered until study end." (protocol) "Masking: quadruple (participant, care provider, investigator, outcomes assessor." (registry) Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups (922 (6.1%) versus 1042 (6.9%)), with the majority of those excluded due to positive or unknown baseline SARS‐CoV‐2 status (434 versus 421). Other reasons: did not receive any injection (29 versus 40), received an incorrect injection (6 versus 7), discontinued without receiving second dose (334 versus 425), received dose 2 outside planned time frame (102 versus 119), other major protocol deviation (17 versus 30). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| El Sahly 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aEl Sahly 2021, RoB 2. Deviations from intervention: Quote: "The investigator, study staff, study participants, site monitors, and Sponsor personnel (or its designees) will be blinded to the IP administered until study end." (protocol) "Masking: quadruple (participant, care provider, investigator, outcomes assessor." (registry) Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups (922 (6.1%) versus 1042 (6.9%)), with the majority of those excluded due to positive or unknown baseline SARS‐CoV‐2 status (434 versus 421). Other reasons: did not receive any injection (29 versus 40), received an incorrect injection (6 versus 7), discontinued without receiving second dose (334 versus 425), received dose 2 outside planned time frame (102 versus 119), other major protocol deviation (17 versus 30). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ali 2021 | Low | Low | Low | Low | Low | Low |
| El Sahly 2021 | Low | Low | Low | Low | Low | Low |
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ali 2021 | Low | Low | Low | Low | Low | Low |
| El Sahly 2021 | Low | Low | Low | Low | Low | Low |
| Hall 2021 | Low | Low | Low | Low | Low | Low |
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ali 2021 | Low | Low | Low | Low | Low | Low |
| El Sahly 2021 | Low | Low | Low | Low | Low | Low |
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ali 2021 | Low | Low | Low | Low | Low | Low |
| El Sahly 2021 | Low | Low | Low | Low | Low | Low |
CVnCoV – CureVac AG versus placebo
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kremsner 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aKremsner 2021, RoB 2. Deviations from intervention: Quote: "Due to the difference in appearance and presentation between the CVnCoV vaccine candidate and placebo, site personnel involved in preparing and administering the vaccine were not involved in the further conduct of the trial, and investigators, site personnel, and others directly involved in the conduct of the trial were blinded to participant treatment for the duration of the trial." Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the outcome: confirmed symptomatic COVID‐19. Analyses were carried out on participants who received both doses of CVnCoV or placebo according to their treatment allocation, who had not developed virologically confirmed COVID‐19 before day 43 (15 days after the second dose), and who were SARS‐CoV‐2 naïve at baseline and day 43. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to these being standard reasons from exclusion from per‐protocol analyses. Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kremsner 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aKremsner 2021, RoB 2. Deviations from intervention: Quote: "Due to the difference in appearance and presentation between the CVnCoV vaccine candidate and placebo, site personnel involved in preparing and administering the vaccine were not involved in the further conduct of the trial, and investigators, site personnel, and others directly involved in the conduct of the trial were blinded to participant treatment for the duration of the trial." Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the outcome: severe confirmed COVID‐19. Analyses were carried out on participants who received both doses of CVnCoV or placebo according to their treatment allocation, who had not developed virologically confirmed COVID‐19 before day 43 (15 days after the second dose), and who were SARS‐CoV‐2 naïve at baseline and day 43. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to these being standard reasons from exclusion from per‐protocol analyses. Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kremsner 2021 | Low | Low | Low | Low | Low | Low |
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kremsner 2021 | Low | Low | Low | Low | Low | Low |
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kremsner 2021 | Low | Low | Low | Low | Low | Low |
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kremsner 2021 | Low | Low | Low | Low | Low | Low |
Non‐replicating viral vector
ChAdOx1/SII‐ChAdOx1 nCoV‐19 – AstraZeneca + University of Oxford versus placebo/MenACWY
SARS‐CoV‐2 infection after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Falsey 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
| Voysey 2021a | Low | Some concernsb | Low | Low | Low | Some concerns |
aFalsey 2021, RoB 2. Deviations from intervention: Quote: "double‐blind" Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed for the outcome: confirmed COVID‐19. Reasons for exclusion: did not receive first dose: 52 (0.2%) versus 20 (0.2%), had a positive, missing, or indeterminate serostatus at baseline: 1046 (4.8%) versus 516 (4.8%); were followed for < 15 days after second dose: 2206 (10.2%) versus 920 (8.5%); had confirmed SARS‐CoV‐2 RT‐PCR–positive COVID‐19 infection < 15 days after second dose: 73 (0.3%) versus 69 (0.6%). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to similar levels of and reasons for exclusion in either arm. Risk assessed to have some concerns for this outcome.
bVoysey 2021a, RoB 2. Deviations from intervention: Quote: "three single‐blind randomized controlled trials in the UK (COV001/COV002), Brazil (COV003), and one double‐blind study in South Africa (COV005)" Comment: blinded studies (patients in 3 trials, patients and physicians in 1 trial). No participant cross‐over. Per‐protocol analysis (as planned in the trial protocol) was performed on the outcomes: confirmed COVID‐19. Reasons for exclusions: in non‐randomized open‐label group; in HIV cohorts; not enrolled in an efficacy cohort; not in SD/SD or LD/SD vaccine group; baseline seropositivity results unavailable; baseline seropositivity results positive; Vaccine administration errors; Less than 15 days of follow‐up accrued post second dose; PCR+ test < 14 days post‐second dose As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance in the number of exclusions. Risk assessed to have some concerns for this outcome.
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Falsey 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
| Voysey 2021a | Low | Some concernsb | Low | Low | Low | Some concerns |
aFalsey 2021, RoB 2. Deviations from intervention: Quotes: "double‐blind" Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed for the outcome: confirmed COVID‐19. Reasons for exclusion: did not receive first dose: 52 (0.2%) versus 20 (0.2%), had a positive, missing, or indeterminate serostatus at baseline: 1046 (4.8%) versus 516 (4.8%); were followed for < 15 days after second dose: 2206 (10.2%) versus 920 (8.5%); had confirmed SARS‐CoV‐2 RT‐PCR–positive COVID‐19 infection < 15 days after second dose: 73 (0.3%) versus 69 (0.6%). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to similar levels of and reasons for exclusion in either arm. Risk assessed to have some concerns for this outcome.
bVoysey 2021a, RoB 2. Deviations from intervention: Quote: "three single‐blind randomized controlled trials in the UK (COV001/COV002), Brazil (COV003), and one double‐blind study in South Africa (COV005)" Comment: blinded studies (patients in 3 trials, patients and physicians in 1 trial). No participant cross‐over. Per‐protocol analysis (as planned in the trial protocol) was performed on the outcomes: confirmed COVID‐19. Reasons for exclusions: in non‐randomized open‐label group; in HIV cohorts; not enrolled in an efficacy cohort; not in SD/SD or LD/SD vaccine group; baseline seropositivity results unavailable; baseline seropositivity results positive; Vaccine administration errors; Less than 15 days of follow‐up accrued post second dose; PCR+ test < 14 days post‐second dose As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance in the number of exclusions. Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kulkarni 2021 | Low | Low | Low | Low | Some concernsa | Some concerns |
aKulkarni 2021, RoB 5. Selection of the reported results: Comment: the trial registry was available (registered prospectively on 15 August 2020). No information on whether the result was selected from multiple outcome measurements or analyses of the data. Trial probably not analyzed as prespecified. Risk assessed as some concerns for this outcome. Outcome not prespecified.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Asano 2022 | Low | Low | Low | Low | Low | Low |
| Falsey 2021 | Low | Low | Low | Low | Low | Low |
| Kulkarni 2021 | Low | Low | Low | Low | Low | Low |
| Madhi 2021a | Low | Low | Low | Low | Low | Low |
| Voysey 2021a | Low | Low | Low | Low | Low | Low |
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Asano 2022 | Low | Low | Low | Low | Low | Low |
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Asano 2022 | Low | Low | Low | Low | Low | Low |
| Falsey 2021 | Low | Low | Low | Low | Low | Low |
| Kulkarni 2021 | Low | Low | Low | Low | Low | Low |
| Voysey 2021a | Low | Low | Low | Low | Low | Low |
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Asano 2022 | Low | Low | Low | Low | Low | Low |
| Falsey 2021 | Low | Low | Low | Low | Low | Low |
| Kulkarni 2021 | Low | Low | Low | Low | Low | Low |
| Madhi 2021a | Low | Low | Low | Low | Low | Low |
| Voysey 2021a | Low | Low | Low | Low | Low | Low |
Ad26.COV2.S – Janssen Pharmaceutical Companies versus placebo
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Sadoff 2021b | Low | Some concernsa | Low | Low | Low | Some concerns |
aSadoff 2021b, RoB 2. Deviations from intervention: Quote: "Quadruple (participant, care provider, investigator, outcomes assessor)." Comment: blinded study (participants and personnel/carers) Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusion: positive SARS‐CoV‐2 status at time of vaccination based on serology or PCR (or both); major protocol deviation evaluated to possibly impact efficacy (inclusion/exclusion criteria; received wrong treatment or incorrect dose; received a disallowed concomitant medication; other). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Sadoff 2021b | Low | Some concernsa | Low | Low | Low | Some concerns |
aSadoff 2021b, RoB 2. Deviations from intervention: Quote: "Quadruple (participant, care provider, investigator, outcomes assessor)." Comment: blinded study (participants and personnel/carers) Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusion: positive SARS‐CoV‐2 status at time of vaccination based on serology or PCR (or both); major protocol deviation evaluated to possibly impact efficacy (in/exclusion criteria; received wrong treatment or incorrect dose; received a disallowed concomitant medication; other) As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Sadoff 2021b | Low | Low | Low | Low | Low | Low |
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Sadoff 2021a | Low | Low | Low | Low | Low | Low |
| Sadoff 2021b | Low | Some concernsa | Low | Low | Low | Some concerns |
aSadoff 2021b, RoB 2. Deviations from intervention: Quote: "Quadruple (participant, care provider, investigator, outcomes assessor)." Comment: blinded study (participants and personnel/carers) Adverse events (solicited and unsolicited) were monitored in a safety subset of volunteers in centres (as planned in the trial protocol). Reasons: centres selected based on rapid start‐up capacity and projected incidence rates for COVID‐19 that would allow for rapid efficacy signal detection As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to:
the safety subset was prespecified and the researchers are transparent about any differences between the safety subset and the overall population;
furthermore, it was used as a way to gather detailed data on solicited local/systemic adverse events for the 7 days after each injection. All participants were trained in assessing and reporting events by study staff. All data was transferred automatically to the centres using e‐diaries. As a result, the participants were all at a subset of centres that had sufficient research capacity, which we considered a reasonable logistical decision. Risk assessed to have some concerns for this outcome.
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Sadoff 2021a | Low | Low | Low | Low | Low | Low |
| Sadoff 2021b | Low | Some concernsa | Low | Low | Low | Some concerns |
aSadoff 2021b, RoB 2. Deviations from intervention: Quote: "Quadruple (participant, care provider, investigator, outcomes assessor)." Comment: blinded study (participants and personnel/carers) Adverse events (solicited and unsolicited) were monitored in a safety subset of volunteers in centres (as planned in the trial protocol). Reasons: centres selected based on rapid start‐up capacity and projected incidence rates for COVID‐19 that would allow for rapid efficacy signal detection As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to:
the safety subset was prespecified and the researchers are transparent about any differences between the safety subset and the overall population;
furthermore, it was used as a way to gather detailed data on solicited local/systemic adverse events for the 7 days after each injection. All participants were trained in assessing and reporting events by study staff. All data was transferred automatically to the centres using e‐diaries. As a result, the participants were all at a subset of centres that had sufficient research capacity, which we considered a reasonable logistical decision. Risk assessed to have some concerns for this outcome.
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Sadoff 2021b | Low | Low | Low | Low | Low | Low |
Gam‐COVID‐Vac (Sputnik V) – Gamaleya Research Institute versus placebo
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Logunov 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aLogunov 2021, RoB 2. Deviations from intervention: Quote: "Investigators, participants, and all study staff were masked to group assignment." Comment: blinded study (participants, personnel/carers). Patients were excluded from analysis due to protocol violations such as vaccine administration error, not meeting eligibility criteria, receipt of other vaccines, error in date of second dose, skipped visits. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Logunov 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aLogunov 2021, RoB 2. Deviations from intervention: Quote: "Investigators, participants, and all study staff were masked to group assignment." Comment: blinded study (participants, personnel/carers). Patients were excluded from analysis due to protocol violations such as vaccine administration error, not meeting eligibility criteria, receipt of other vaccines, error in date of second dose, skipped visits. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Logunov 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aLogunov 2021, RoB 2. Deviations from intervention: Quote: "Investigators, participants, and all study staff were masked to group assignment." Comment: blinded study (participants, personnel/carers). Patients were excluded from analysis due to protocol violations such as vaccine administration error, not meeting eligibility criteria, receipt of other vaccines, error in date of second dose, skipped visits. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Logunov 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aLogunov 2021, RoB 2. Deviations from intervention: Quote: "Investigators, participants, and all study staff were masked to group assignment." Comment: blinded study (participants, personnel/carers). Patients were excluded from analysis due to protocol violations such as vaccine administration error, not meeting eligibility criteria, receipt of other vaccines, error in date of second dose, skipped visits. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment due to the small number. Risk assessed to have some concerns for this outcome.
Inactivated virus
CoronaVac – Sinovac versus adjuvant
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Palacios 2020 | Low | Some concernsa | Low | Low | Low | Some concerns |
| Tanriover 2021 | Low | Some concernsb | Low | Low | Low | Some concerns |
aPalacios 2020, RoB 2. Deviations from intervention: Quote: "Participants and all other study staff as well as monitors, lab technicians, and data management team remained unaware of the product allocation." Comment: blinded study (participants, personnel, investigators). Per‐protocol analysis (as planned in the trial protocol was performed on the outcome: confirmed symptomatic COVID‐19. 65 (1.0%) versus 74 (1.2%) participants were excluded due to protocol violations, reasons for exclusions: not eligible (0 versus 1), received 3rd dose or incorrect injection (11 versus 8), out of window for per‐protocol analysis (54 versus 65). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance of the exclusions between arms. Risk assessed to have some concerns for this outcome.
bTanriover 2021, RoB 2. Deviations from intervention: Quote: "Participants and practitioners were masked to the group allocation. The masking was removed in the event of a medical emergency requiring acute intervention, upon the responsible investigator's approval and the data and safety monitoring board's knowledge." "the placebo and study vaccine looked exactly the same, they were administered by staff masked to group allocation." Comment: blinded study (participants, staff, investigators). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). 69 (1%) versus 86 (2.4%) participants were excluded from the efficacy analysis post‐randomization because of protocol violations: positive for SARS‐CoV‐2 (60 (0.9%) versus 35 (1%)), unmasked before the second dose (due to emergency use authorization and commencement of community vaccination) (4 (0.06%) versus 45 (1.3%)), received incorrect injection (1 (0.02%) versus 4 (0.1%)), had protocol violations (2 (0.03%) versus 0), pregnant (2 (0.03%) versus 1 (0.03%)), withdrawn by study investigator (0 versus 1 (0.03%)). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), this method was considered inappropriate to estimate the effect of assignment to intervention. Although reasons for exclusions were not balanced between treatment groups, there was probably no substantial impact of failure to analyse participants according to their randomized groups since the imbalance was due to unmasking and subsequent vaccination after emergency use authorization. Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Palacios 2020 | Low | Some concernsa | Low | Low | Low | Some concerns |
| Tanriover 2021 | Low | Some concernsb | Low | Low | Low | Some concerns |
aPalacios 2020, RoB 2. Deviations from intervention: Quote: "Participants and all other study staff as well as monitors, lab technicians, and data management team remained unaware of the product allocation" Comment: blinded study (participants, personnel, investigators). Per‐protocol analysis (as planned in the trial protocol was performed on the outcome: confirmed symptomatic COVID‐19. 65 (1.0%) versus 74 (1.2%) participants were excluded due to protocol violations, reasons for exclusions: not eligible (0 versus 1), received 3rd dose or incorrect injection (11 versus 8), out of window for per‐protocol analysis (54 versus 65). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance of the exclusions between arms. Risk assessed to have some concerns for this outcome.
bTanriover 2021, RoB 2. Deviations from intervention: Quote: "Participants and practitioners were masked to the group allocation. The masking was removed in the event of a medical emergency requiring acute intervention, upon the responsible investigator's approval and the data and safety monitoring board's knowledge." "the placebo and study vaccine looked exactly the same, they were administered by staff masked to group allocation." Comment: blinded study (participants, staff, investigators). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). 69 (1%) versus 86 (2.4%) participants were excluded from the efficacy analysis post‐randomization because of protocol violations: positive for SARS‐CoV‐2 (60 (0.9%) versus 35 (1%)), unmasked before the second dose (due to emergency use authorization and commencement of community vaccination) (4 (0.06%) versus 45 (1.3%)), received incorrect injection (1 (0.02%) versus 4 (0.1%)), had protocol violations (2 (0.03%) versus 0), pregnant (2 (0.03%) versus 1 (0.03%)), withdrawn by study investigator (0 versus 1 (0.03%)). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), this method was considered inappropriate to estimate the effect of assignment to intervention. Although reasons for exclusions were not balanced between treatment groups, there was probably no substantial impact of failure to analyse participants according to their randomized groups since the imbalance was due to unmasking and subsequent vaccination after emergency use authorization. Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Palacios 2020 | Low | Low | Low | Low | Low | Low |
| Tanriover 2021 | Low | Low | Low | Low | Low | Low |
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Zhang 2021a | Some concernsb | Low | Low | Low | Low | Some concerns |
| Zhang 2021a | Some concernsc | Low | Low | Low | Low | Some concerns |
| Bueno 2021 | Some concernsd | Low | Low | Low | Low | Some concerns |
| Fadlyana 2021 | Low | Some concernse | Low | Low | Low | Some concerns |
| Palacios 2020 | Low | Low | Low | Low | Low | Low |
| Tanriover 2021 | Low | Low | Low | Low | Low | Low |
| Wu 2021a | Low | Low | Low | Low | Some concernsf | Some concerns |
aZhang 2021 reported two different comparisons/sets of participants.
bZhang 2021, RoB 1. Randomization: Quote: "no specific randomization was used when allocating participants to the vaccinations schedule cohorts." "The randomization codes for each vaccination schedule cohort were generated individually, using block randomization with a block size of six in phase 1 and a block size of five in phase 2, using SAS software (version 9.4). The randomization code was assigned to each participant in sequence in the order of enrolment, and then the participants received the investigational products labelled with the same code." Comment: no specific randomization between schedule cohorts. The allocation sequence between vaccine groups and placebo was generated adequately. Unclear allocation concealment. Risk assessed as some concerns
cZhang 2021, RoB 1. Randomization: Quote: "no specific randomization was used when allocating participants to the vaccinations schedule cohorts." "In phase 1, participants in blocks 1 and 2 in each schedule cohort were randomly assigned (2:1) to either CoronaVac or placebo." "The randomization codes for each vaccination schedule cohort were generated individually, using block randomization with a block size of six in phase 1 and a block size of five in phase 2, using SAS software (version 9.4). The randomization code was assigned to each participant in sequence in the order of enrolment, and then the participants received the investigational products labelled with the same code." Comment: no specific randomization between schedule cohorts or between low‐dose and high‐dose arms. The allocation sequence between vaccine groups and placebo was generated adequately. Unclear allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed as some concerns.
dBueno 2021, RoB 1. Randomization: Quote: "Volunteers were randomly assigned to immunization with CoronaVac or injection with placebo in a 1:1 ratio. A subgroup of volunteers was assigned to the immunogenicity arm and randomly received CoronaVac or placebo (3:1 ratio). Randomization was done using a sealed enveloped system integrated into the electronic Case Report Forms (eCRF) in the OpenClinica platform." Comment: authors report 1:1 allocation ratio for intervention/control group. However, in the flow chart and result tables there are 290 participants in the vaccine group and 164 in the control group. Comment: allocation sequence concealed. Allocation sequence unclear. Baseline characteristics not reported by arm. Risk assessed as some concerns.
eFadlyana 2021, RoB 2. Deviations from intervention: Quote: "Double‐blind." Comment: blinded study (participants and outcome assessors) Safety outcomes were monitored in a safety subset (first 540 participants randomized). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants all participants according to their randomized assignment Risk assessed to have some concerns for this outcome.
fWu 2021a, RoB 5. Selection of the reported results: Comment: the prospective registry was available (12 May 2020). The outcome: systemic adverse events was not prespecified. No information on whether the result was selected from multiple outcome measurements or analyses of the data. Trial not analyzed as prespecified. Risk assessed to have some concerns for this outcome.
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Zhang 2021 | Some concernsa | Low | Low | Low | Low | Some concerns |
| Zhang 2021 | Some concernsb | Low | Low | Low | Low | Some concerns |
| Han 2021 | Low | Low | Low | Low | Low | Low |
| Palacios 2020 | Low | Low | Low | Low | Low | Low |
| Tanriover 2021 | Low | Low | Low | Low | Low | Low |
| Wu 2021a | Low | Low | Low | Low | Low | Low |
aZhang 2021, RoB 1. Randomization: Quote: "no specific randomization was used when allocating participants to the vaccinations schedule cohorts." "The randomization codes for each vaccination schedule cohort were generated individually, using block randomization with a block size of six in phase 1 and a block size of five in phase 2, using SAS software (version 9.4). The randomization code was assigned to each participant in sequence in the order of enrolment, and then the participants received the investigational products labelled with the same code." Comment: no specific randomization between schedule cohorts. The allocation sequence between vaccine groups and placebo was generated adequately. Unclear allocation concealment. Risk assessed as some concerns
bZhang 2021, RoB 1. Randomization: Quote: "no specific randomization was used when allocating participants to the vaccinations schedule cohorts." "In phase 1, participants in blocks 1 and 2 in each schedule cohort were randomly assigned (2:1) to either CoronaVac or placebo." "The randomization codes for each vaccination schedule cohort were generated individually, using block randomization with a block size of six in phase 1 and a block size of five in phase 2, using SAS software (version 9.4). The randomization code was assigned to each participant in sequence in the order of enrolment, and then the participants received the investigational products labelled with the same code." Comment: no specific randomization between schedule cohorts or between low‐dose and high‐dose arms. The allocation sequence between vaccine groups and placebo was generated adequately. Unclear allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed as some concerns.
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Bueno 2021 | Some concernsa | Low | Low | Low | Low | Some concerns |
| Han 2021 | Low | Low | Low | Low | Low | Low |
| Palacios 2020 | Low | Low | Low | Low | Low | Low |
| Tanriover 2021 | Low | Low | Low | Low | Low | Low |
| Wu 2021a | Low | Low | Low | Low | Low | Low |
aBueno 2021, RoB 1. Randomization: Quote: "Volunteers were randomly assigned to immunization with CoronaVac or injection with placebo in a 1:1 ratio. A subgroup of volunteers was assigned to the immunogenicity arm and randomly received CoronaVac or placebo (3:1 ratio). Randomization was done using a sealed enveloped system integrated into the electronic Case Report Forms (eCRF) in the OpenClinica platform." Comment: authors report 1:1 allocation ratio for intervention/control group. However, in the flow chart and result tables there are 290 participants in the vaccine group and 164 in the control group. Comment: allocation sequence concealed. Allocation sequence unclear. Baseline characteristics not reported by arm. Risk assessed as some concerns.
WIBP‐CorV – Sinopharm – Wuhan versus adjuvant
SARS‐CoV‐2 infection after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aAl Kaabi 2021, RoB 2. Deviations from intervention: Quote: "The concealed random grouping allocation and blind codes were kept in signed and sealed envelopes and were blinded to the investigators, participants, and statisticians." "The vaccines and controls were approved by the National Institutes for Food and Drug Control of China, and were supplied in coded, identical‐appearing, single‐dose vials." (report) "Masking: quadruple (participant, care provider, investigator, outcomes assessor)." (registry) Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups: due to positive or unknown baseline SARS‐CoV‐2 RT‐PCR status (118 versus 134 versus 98), did not receive any injection (11 versus 5 versus 13), did not receive second dose (393 versus 379 versus 387). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment. Risk assessed to have some concerns for this outcome.
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aAl Kaabi 2021, RoB 2. Deviations from intervention: Quote: "The concealed random grouping allocation and blind codes were kept in signed and sealed envelopes and were blinded to the investigators, participants, and statisticians." "The vaccines and controls were approved by the National Institutes for Food and Drug Control of China, and were supplied in coded, identical‐appearing, single‐dose vials." (report) "Masking: quadruple (participant, care provider, investigator, outcomes assessor)." (registry) Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups: due to positive or unknown baseline SARS‐CoV‐2 RT‐PCR status (118 versus 134 versus 98), did not receive any injection (11 versus 5 versus 13), did not receive second dose (393 versus 379 versus 387). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment. Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aAl Kaabi 2021, RoB 2. Deviations from intervention: Quote: "The concealed random grouping allocation and blind codes were kept in signed and sealed envelopes and were blinded to the investigators, participants, and statisticians." "The vaccines and controls were approved by the National Institutes for Food and Drug Control of China, and were supplied in coded, identical‐appearing, single‐dose vials." (report) "Masking: quadruple (participant, care provider, investigator, outcomes assessor)." (registry) Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups: due to positive or unknown baseline SARS‐CoV‐2 RT‐PCR status (118 versus 134 versus 98), did not receive any injection (11 versus 5 versus 13), did not receive second dose (393 versus 379 versus 387). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment. Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Low | Low | Low | Low | Low |
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Low | Low | Low | Low | Low |
| Guo 2021 | Some concernsa | Low | Low | Low | Low | Some concerns |
aGuo 2021, RoB 1. Randomization: Quote: "Sequential computer‐generated randomization numbers were assigned to participants, and stratified block randomization by age and doses was adopted (block size 8)." Comment: allocation sequence random. Unclear allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed as some concerns
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Low | Low | Low | Low | Low |
| Guo 2021 | Some concernsa | Low | Low | Low | Low | Some concerns |
aGuo 2021, RoB 1. Randomization: Quote: "Sequential computer‐generated randomization numbers were assigned to participants, and stratified block randomization by age and doses was adopted (block size 8)." Comment: allocation sequence random. Unclear allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed as some concerns.
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Low | Low | Low | Low | Low |
| Guo 2021 | Some concernsa | Low | Low | Low | Low | Some concerns |
aGuo 2021, RoB 1. Randomization: Quote: "Sequential computer‐generated randomization numbers were assigned to participants, and stratified block randomization by age and doses was adopted (block size 8)." Comment: allocation sequence random. Unclear allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed as some concerns
BBIBP‐CorV – Sinopharm‐Beijing versus adjuvant
SARS‐CoV‐2 infection after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aAl Kaabi 2021, RoB 2. Deviations from intervention: Quote: "The concealed random grouping allocation and blind codes were kept in signed and sealed envelopes and were blinded to the investigators, participants, and statisticians." "The vaccines and controls were approved by the National Institutes for Food and Drug Control of China, and were supplied in coded, identical‐appearing, single‐dose vials." (report) "Masking: quadruple (participant, care provider, investigator, outcomes assessor)." (registry) Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups: due to positive or unknown baseline SARS‐CoV‐2 RT‐PCR status (118 versus 134 versus 98), did not receive any injection (11 versus 5 versus 13), did not receive second dose (393 versus 379 versus 387). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment. Risk assessed to have some concerns for this outcome.
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aAl Kaabi 2021, RoB 2. Deviations from intervention: Quote: "The concealed random grouping allocation and blind codes were kept in signed and sealed envelopes and were blinded to the investigators, participants, and statisticians." "The vaccines and controls were approved by the National Institutes for Food and Drug Control of China, and were supplied in coded, identical‐appearing, single‐dose vials." (report) "Masking: quadruple (participant, care provider, investigator, outcomes assessor)." (registry) Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups: due to positive or unknown baseline SARS‐CoV‐2 RT‐PCR status (118 versus 134 versus 98), did not receive any injection (11 versus 5 versus 13), did not receive second dose (393 versus 379 versus 387). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was no substantial impact of failure to analyse participants according to their randomized assignment. Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Low | Low | Low | Low | Low |
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Low | Low | Low | Low | Low |
| Xia 2020 | Low | Low | Low | Low | Low | Low |
| Xia 2021 | Low | Low | Low | Low | Low | Low |
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Low | Low | Low | Low | Low |
| Xia 2020 | Low | Low | Low | Low | Low | Low |
| Xia 2021 | Low | Low | Low | Low | Low | Low |
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Al Kaabi 2021 | Low | Low | Low | Low | Low | Low |
| Xia 2020 | Low | Low | Low | Low | Low | Low |
BBV152 – Bharat Biotech versus adjuvant
SARS‐CoV‐2 infection after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ella 2021b | Low | Some concernsa | Low | Low | Low | Some concerns |
aElla 2021b, RoB 2. Deviations from intervention: Quote: "Participants, investigators, study coordinators, study‐related personnel, and the sponsor were masked to the treatment group allocation, and masked study nurses at each site were responsible for vaccine preparation and administration." Comment: blinded study (participants, personnel, investigators). Per‐protocol analysis was performed on the outcome: Confirmed COVID (as planned in the trial protocol). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. Reasons for exclusions were balanced: did not received dose 1 (20 versus 25), did not received dose 2 (658 versus 676), positive for anti‐SARS‐CoV‐2 IgG (3932 versus 3886), positive for SARS‐CoV‐2 by PCR (108 versus 105). There was probably no substantial impact of failure to analyse participants according to their randomized assignment Risk assessed to have some concerns for this outcome.
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ella 2021b | Low | Some concernsa | Low | Low | Low | Some concerns |
aElla 2021b, RoB 2. Deviations from intervention: Quote: "Participants, investigators, study coordinators, study‐related personnel, and the sponsor were masked to the treatment group allocation, and masked study nurses at each site were responsible for vaccine preparation and administration." Comment: blinded study (participants, personnel, investigators). Per‐protocol analysis was performed on the outcomes: Confirmed symptomatic COVID (as planned in the trial protocol). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. Reasons for exclusions were balanced: did not received dose 1 (20 versus 25), did not received dose 2 (658 versus 676), positive for anti‐SARS‐CoV‐2 IgG (3932 versus 3886), positive for SARS‐CoV‐2 by PCR (108 versus 105). There was probably no substantial impact of failure to analyse participants according to their randomized assignment Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ella 2021b | Low | Some concernsa | Low | Low | Low | Some concerns |
aElla 2021b, RoB 2. Deviations from intervention: Quote: "Participants, investigators, study coordinators, study‐related personnel, and the sponsor were masked to the treatment group allocation, and masked study nurses at each site were responsible for vaccine preparation and administration." Comment: blinded study (participants, personnel, investigators). Per‐protocol analysis was performed on the outcomes: Confirmed severe COVID (as planned in the trial protocol). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. Reasons for exclusions were balanced: did not received dose 1 (20 versus 25), did not received dose 2 (658 versus 676), positive for anti‐SARS‐CoV‐2 IgG (3932 versus 3886), positive for SARS‐CoV‐2 by PCR (108 versus 105). There was probably no substantial impact of failure to analyse participants according to their randomized assignment Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ella 2021b | Low | Low | Low | Low | Low | Low |
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ella 2021b | Low | Low | Low | Low | Low | Low |
| Ella 2021a | Low | Some concernsa | Low | Low | Some concernsb | Some concerns |
aElla 2021a, RoB 2. Deviations from intervention: Quote: "The appearance, color, and viscosity were identical across all treatment and control formulations. Participants, investigators, study coordinators, study‐related personnel, and the sponsor were blinded to the treatment group allocation (excluding an unblinded CRO, who was tasked with the dispatch and labeling of vaccine vials and the generation of the master randomization code). Blinding was maintained using the randomization code." Comment: blinded study (patients, personnel, and investigators). No participant cross‐over. Per‐protocol analysis was performed on the outcomes. Reasons for exclusion: protocol deviation (1), positive for SARS‐CoV‐2 (1) As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to the small number of exclusions Risk assessed to have some concerns for this outcome.
bElla 2021a, RoB 5. Selection of the reported results: Comment: the prospective registry was available (July 15, 2020). Outcome not prespecified No information on whether the results were selected from multiple outcome measurements or analyses of the data. Trial not analyzed as prespecified. Risk assessed to have some concerns for this outcome.
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ella 2021b | Low | Low | Low | Low | Low | Low |
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Ella 2021b | Low | Low | Low | Low | Low | Low |
| Ella 2021a | Low | Some concernsa | Low | Low | Low | Some concerns |
aElla 2021a, RoB 2. Deviations from intervention: Quote: "The appearance, color, and viscosity were identical across all treatment and control formulations. Participants, investigators, study coordinators, study‐related personnel, and the sponsor were blinded to the treatment group allocation (excluding an unblinded CRO, who was tasked with the dispatch and labeling of vaccine vials and the generation of the master randomization code). Blinding was maintained using the randomization code." Comment: blinded study (patients, personnel, and investigators). No participant cross‐over. Per‐protocol analysis was performed on the outcomes. Reasons for exclusion: protocol deviation (1), positive for SARS‐CoV‐2 (1) As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to the small number of exclusions Risk assessed to have some concerns for this outcome.
Protein subunit
NVX‐CoV2373 – Novavax versus placebo
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Dunkle 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
| Heath 2021 | Low | Some concernsb | Low | Low | Low | Some concerns |
| Shinde 2021 | Low | Some concernsc | Low | Low | Low | Some concerns |
aDunkle 2021, RoB 2. Deviations from intervention: Quote: "Masking: quadruple (participant, care provider, investigator, outcomes assessor)." "Only unblinded site personnel managed study vaccine logistics/preparation and had no other role in trial conduct." "The trial is ongoing, and investigators and Novavax clinical team remain blinded to participant‐level treatment assignments." Comment: blinded study (participants and personnel/carers) Per‐protocol analysis was performed on the efficacy outcomes. Reasons for exclusion: were anti‐NP or PCR positive at baseline (vaccine 6.2%, placebo 6.8%), did not receive two Nv‐CXoV2373 doses or were dosed out of window (vaccine 3.2%, placebo 4.6%), had major protocol deviation, were unblinded, or had a censoring event (vaccine 3.3%, placebo 6.9%). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to relatively equal attrition in both arms. Risk assessed to have some concerns for this outcome.
bHeath 2021, RoB 2. Deviations from intervention: Quote: "This was an observer‐blinded study. Only unblinded site personnel managed study vaccine logistics and preparation and they were not involved in study‐related assessments or had participant contact for data collection following vaccine administration" (report) "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor" (NCT04583995 registry) "Double blind" (EudraCT 2020‐004123‐16 registry) Comment: blinded study (participants, personnel, investigators). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups (549 (7.2%) versus 551 (7.3%)), with the majority of those excluded due to seropositivity before 7 days after dose 2 (399 versus 402). Other reasons: received only one dose (102 versus 107); had major protocol deviation, missed dose, or censoring event (48 versus 42). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. Risk assessed to have some concerns for this outcome.
cShinde 2021, RoB 2. Deviations from intervention: Quote: "To maintain the blind, placebo vaccination via the intramuscular route was included, and unblinded site personnel managed vaccine logistics, preparation, and administration (if necessary) to maintain the blind from the remainder of the site personnel and participants." Comment: not fully blinded study (participants and some personnel were blinded). Two participants crossed over from placebo to vaccine group. This deviation was considered negligible among 2684 participants analyzed for efficacy outcomes. Per‐protocol analysis was performed on the efficacy outcomes evaluated in this cohort (as planned in the trial protocol). Reasons for exclusion: seropositivity at baseline (849 versus 873), SARS‐CoV‐2 positivity before day 28 (97 versus 78), did not receive both doses (24 versus 31), had important protocol deviations (4 versus 7), lost to follow‐up (6 versus 9), was withdrawn by physicians (1 versus 0), became pregnant (2 versus 3), withdrew with no reason reported (10 versus 15), had adverse event, not related to vaccine (1 versus 0). Risk assessed to have some concerns for this outcome.
Severe or critical COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Dunkle 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
aDunkle 2021, RoB 2. Deviations from intervention: Quote: "masking: quadruple (participant, care provider, investigator, outcomes assessor)." "Only unblinded site personnel managed study vaccine logistics/preparation and had no other role in trial conduct." "The trial is ongoing, and investigators and Novavax clinical team remain blinded to participant‐level treatment assignments." Comment: blinded study (participants and personnel/carers) Per‐protocol analysis was performed on the efficacy outcomes. Reasons for exclusion: Were Anti‐NP or PCR positive at baseline (vaccine 6.2%, placebo 6.8%), did not receive two Nv‐CXoV2373 doses or were dosed out of window (vaccine 3.2%, placebo 4.6%), had major protocol deviation, were unblinded, or had a censoring event (vaccine 3.3%, placebo 6.9%). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to relatively equal attrition in both arms. Risk assessed to have some concerns for this outcome.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Dunkle 2021 | Low | Some concernsa | Low | Low | Low | Some concerns |
| Heath 2021 | Low | Some concernsb | Low | Low | Low | Some concerns |
aDunkle 2021, RoB 2. Deviations from intervention: Quote: "Masking: quadruple (participant, care provider, investigator, outcomes assessor)." "Only unblinded site personnel managed study vaccine logistics/preparation and had no other role in trial conduct." "The trial is ongoing, and investigators and Novavax clinical team remain blinded to participant‐level treatment assignments." Comment: blinded study (participants and personnel/carers) Per‐protocol analysis was performed on the efficacy outcomes. Reasons for exclusion: were anti‐NP or PCR positive at baseline (vaccine 6.2%, placebo 6.8%), did not receive two Nv‐CXoV2373 doses or were dosed out of window (vaccine 3.2%, placebo 4.6%), had major protocol deviation, were unblinded, or had a censoring event (vaccine 3.3%, placebo 6.9%). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to relatively equal attrition in both arms. Risk assessed to have some concerns for this outcome.
bHeath 2021, RoB 2. Deviations from intervention: Quote: "This was an observer‐blinded study. Only unblinded site personnel managed study vaccine logistics and preparation and they were not involved in study‐related assessments or had participant contact for data collection following vaccine administration." (report) "Masking: quadruple (participant, care provider, investigator, outcomes assessor." (NCT04583995 registry) "Double blind" (EudraCT 2020‐004123‐16 registry) Comment: blinded study (participants, personnel, investigators). Per‐protocol analysis was performed on the efficacy outcomes (as planned in the trial protocol). Reasons for exclusions were balanced between treatment groups (549 (7.2%) versus 551 (7.3%)), with the majority of those excluded due to seropositivity before 7 days after dose 2 (399 versus 402). Other reasons: received only 1 dose (102 versus 107); had major protocol deviation, missed dose, or censoring event (48 versus 42). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. Risk assessed to have some concerns for this outcome.
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Dunkle 2021 | Low | Low | Low | Low | Low | Low |
| Formica 2021 | Some concernsa | Low | Low | Low | Low | Some concerns |
| Shinde 2021 | Low | Low | Some concernsb | Low | Low | Some concerns |
aFormica 2021, RoB 1. Randomization: Quote: "participants were randomly assigned in a blinded manner to one of five vaccine groups … according to pre‐generated randomization schedules with two‐factor, two‐level stratification employed." Comment: allocation sequence probably random No information on allocation concealment Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed to have some concerns
bShinde 2021, RoB 3. Missing outcome data: Comment: data from interim analysis 4406 participants randomized; 968 participants analyzed for safety. Data available for 22% of population for safety. For safety, only participants who were enrolled in the first stage were analyzed for the interim analysis. A large proportion (participants enrolled in the second stage of the trial) was missing, but it is unlikely that missingness depended on the true value of the outcome. Risk assessed to have some concerns for this outcome
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Keech 2020 | Some concernsa | Low | Low | Low | Low | Some concerns |
| Dunkle 2021 | Low | Low | Low | Low | Low | Low |
| Formica 2021 | Some concernsb | Low | Low | Low | Some concernsc | Some concerns |
| Heath 2021 | Low | Low | Low | Low | Low | Low |
| Shinde 2021 | Low | Low | Some concernsd | Low | Low | Some concerns |
aKeech 2020, RoB 1. Randomization: Quote: "As a safety measure, 6 participants were initially randomly assigned in a 1:1 ratio to the 5‐μg and 25‐μg rSARS‐CoV‐2 plus Matrix‐M1 groups (groups C and D), vaccinated in an open‐label manner, and observed for reactogenicity for 48 hours. Thereafter, the remaining 125 participants were randomly assigned, in a 1:1:1:1:1 ratio and in a blinded manner to one of five vaccine groups according to pregenerated randomization schedules, without stratification." Comment: allocation sequence probably random No information on allocation concealment Risk assessed to have some concerns
bFormica 2021, RoB 1. Randomization: Quote: "participants were randomly assigned in a blinded manner to one of five vaccine groups … according to pre‐generated randomization schedules with two‐factor, two‐level stratification employed." Comment: allocation sequence probably random No information on allocation concealment Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed to have some concerns
cFormica 2021, RoB 5. Selection of the reported results: Comment: the prospective trial registry was available (30 April). Different time point in the registry (prespecified at 28 days and reported at 35 days after first dose) No information on whether the result was selected from multiple outcome measurements or analyses of the data. Trial not analyzed as prespecified. Risk assessed to have some concerns for this outcome
dShinde 2021, RoB 3. Missing outcome data: Comment: data from interim analysis 4406 participants randomized; 968 participants analyzed for safety. For safety, only participants who were enrolled in the first stage were analyzed for the interim analysis. A large proportion (participants enrolled in the second stage of the trial) was missing, but it is unlikely that missingness depended on the true value of the outcome. Risk assessed to have some concerns for this outcome. Data available for 22% of population for safety.
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Keech 2020 | Some concernsa | Low | Low | Low | Low | Some concerns |
| Dunkle 2021 | Low | Low | Low | Low | Low | Low |
| Formica 2021 | Some concernsb | Low | Low | Low | Some concernsc | Some concerns |
| Heath 2021 | Low | Low | Low | Low | Low | Low |
| Shinde 2021 | Low | Some concernsd | Some concernse | Low | Low | Some concerns |
aKeech 2020, RoB 1. Randomization: Quote: "As a safety measure, 6 participants were initially randomly assigned in a 1:1 ratio to the 5‐μg and 25‐μg rSARS‐CoV‐2 plus Matrix‐M1 groups (groups C and D), vaccinated in an open‐label manner, and observed for reactogenicity for 48 hours. Thereafter, the remaining 125 participants were randomly assigned, in a 1:1:1:1:1 ratio and in a blinded manner to one of five vaccine groups according to pregenerated randomization schedules, without stratification". Comment: allocation sequence probably random No information on allocation concealment Risk assessed to have some concerns
bFormica 2021, RoB 1. Randomization: Quote: "participants were randomly assigned in a blinded manner to one of five vaccine groups … according to pre‐generated randomization schedules with two‐factor, two‐level stratification employed." Comment: allocation sequence probably random No information on allocation concealment Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed to have some concerns
cFormica 2021, RoB 5. Selection of the reported results: Comment: the prospective trial registry was available (April 30th). No information on whether the result was selected from multiple outcome measurements or analyses of the data. Trial not analyzed as prespecified. Risk assessed to have some concerns for this outcome. Outcome not prespecified
dShinde 2021, RoB 2. Deviations from intervention: Quote: "To maintain the blind, placebo vaccination via the intramuscular route was included, and unblinded site personnel managed vaccine logistics, preparation, and administration (if necessary) so as to maintain the blind from the remainder of the site personnel and participants." Comment: not fully blinded study (participants and some personnel were blinded). Two participants crossed over from placebo to vaccine group. This deviation was considered negligible among 968 participants analyzed for safety outcomes. The two participants randomized to the placebo group that crossed over were analyzed "as‐treated" in the intervention group. Nevertheless, due to the small proportion crossing over, we considered the safety analyses to be probably appropriate to estimate the effect of assignment to intervention. Risk assessed to have some concerns for this outcome.
eShinde 2021, RoB 3. Missing outcome data: Comment: data from interim analysis. 4406 participants randomized; 968 participants analyzed for safety. For safety, only participants who were enrolled in the first stage were analyzed for the interim analysis. A large proportion (participants enrolled in the second stage of the trial) was missing, but it is unlikely that missingness depended on the true value of the outcome. Risk assessed to have some concerns for this outcome. Data available for 22% of population for safety.
FINLAY‐FR‐2 – Instituto Finlay de Vacunas versus placebo
Confirmed symptomatic COVID‐19 after complete vaccination
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Toledo‐Romani 2021 | Some concernsa | Some concernsb | Low | Low | Low | Some concerns |
aToledo‐Romani 2021, RoB 1. Randomization: Quote: "Randomization into study arms (A and B) and placebo was done on day 0 at a 1:1:1 ratio using a site stratified random and previously defined risk strata (19–64 years without risk comorbidities, 19–64 years with risk comorbidities and ≥65 years)." Comment: allocation sequence random. No information on allocation concealment.
bToledo‐Romani 2021, RoB 2. Deviations from intervention: Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the outcomes. Reasons for exclusion: did not receive or discontinued the intervention. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance between groups. Imbalances in baseline characteristics appear to be compatible with chance.
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Toledo‐Romani 2021 | Some concernsa | Some concernsb | Low | Low | Low | Some concerns |
aToledo‐Romani 2021, RoB 1. Randomization: Quote: "Randomization into study arms (A and B) and placebo was done on day 0 at a 1:1:1 ratio using a site stratified random and previously defined risk strata (19–64 years without risk comorbidities, 19–64 years with risk comorbidities and ≥65 years)." Comment: allocation sequence random. No information on allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance.
bToledo‐Romani 2021, RoB 2. Deviations from intervention: Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the outcomes. Reasons for exclusion: did not receive or discontinued the intervention. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance between groups.
Heterologous vaccine
Comparison: heterologous vaccination scheme versus homologous vaccination scheme
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Li 2021a | Low | Some concernsa | Low | Low | Low | Some concerns |
aLi 2021a, RoB 2. Deviations from intervention: Quote "We masked participants, investigators, laboratory staff, and outcome assessors to the allocation of treatment groups … Designated unblinded personnel were responsible for the preparation and administration of the vaccination and were forbidden to reveal the identity of the study vaccines to the participants or other investigators" Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the outcomes. One participant randomized to the Convidecia boost group (additional arm in the study extracted separately) crossed over to the CoronaVac/Convidecia group because the participant had in fact only received one primary dose. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to the single participant that crossed over. Risk assessed to have some concerns for this outcome.
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Li 2021a | Low | Some concernsa | Low | Low | Low | Some concerns |
| Liu 2021 | Low | Low | Low | Some concernsb | Low | Some concerns |
| Liu 2021 | Low | Low | Low | Some concernsc | Low | Some concerns |
aLi 2021a, RoB 2. Deviations from intervention: Quote "We masked participants, investigators, laboratory staff, and outcome assessors to the allocation of treatment groups … Designated unblinded personnel were responsible for the preparation and administration of the vaccination and were forbidden to reveal the identity of the study vaccines to the participants or other investigators" Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the outcomes. One participant randomized to the Convidecia boost group (additional arm in the study extracted separately) crossed over to the CoronaVac/Convidecia group because the participant had in fact only received one primary dose. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to the single participant that crossed over. Risk assessed to have some concerns for this outcome.
bLiu 2021, RoB 4. Measurement of the outcome: Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Quote: "Laboratory staff will also be blinded to the vaccine schedule received." (protocol) "The clinical team assessing the safety endpoints were not blinded" (report) Comment: outcome assessment was unblinded for safety outcomes; Adverse events may contain both clinically and laboratory‐detected events, which can be influenced by knowledge of the intervention assignment but is not likely in the context of the pandemic. Risk assessed to have some concerns for this outcome.
cLiu 2021, RoB 4. Measurement of the outcome: Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Quote: "Laboratory staff will also be blinded to the vaccine schedule received." (protocol) "The clinical team assessing the safety endpoints were not blinded" (report) Comment: outcome assessment was unblinded for safety outcomes; Adverse events may contain both clinically and laboratory‐detected events, which can be influenced by knowledge of the intervention assignment but is not likely in the context of the pandemic. Risk assessed to have some concerns for this outcome.
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Li 2021a | Low | Some concernsa | Low | Low | Low | Some concerns |
| Liu 2021 | Low | Low | Low | Some concernsb | Low | Some concerns |
| Liu 2021 | Low | Low | Low | Some concernsc | Low | Some concerns |
aLi 2021a, RoB 2. Deviations from intervention: Quote "We masked participants, investigators, laboratory staff, and outcome assessors to the allocation of treatment groups … Designated unblinded personnel were responsible for the preparation and administration of the vaccination and were forbidden to reveal the identity of the study vaccines to the participants or other investigators" Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the outcomes. One participant randomized to the Convidecia boost group (additional arm in the study extracted separately) crossed over to the CoronaVac/Convidecia group because the participant had in fact only received one primary dose. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to the single participant that crossed over. Risk assessed to have some concerns for this outcome.
bLiu 2021, RoB 4. Measurement of the outcome: Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Quote: "Laboratory staff will also be blinded to the vaccine schedule received." (protocol) "The clinical team assessing the safety endpoints were not blinded" (report) Serious adverse events may contain both clinically and laboratory‐detected events, which can be influenced by knowledge of the intervention assignment but is not likely in the context of the pandemic. Risk assessed to have some concerns for this outcome. Outcome assessment was unblinded for safety outcomes
cLiu 2021, RoB 4. Measurement of the outcome: Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Quote: "Laboratory staff will also be blinded to the vaccine schedule received." (protocol) "The clinical team assessing the safety endpoints were not blinded" (report) Comment: outcome assessment was unblinded for safety outcomes; Serious adverse events may contain both clinically and laboratory‐detected events, which can be influenced by knowledge of the intervention assignment but is not likely in the context of the pandemic. Risk assessed to have some concerns for this outcome.
Boosters
Comparison: booster versus placebo/no booster
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Li 2021a | Low | Some concernsa | Low | Low | Low | Some concerns |
| Mok 2021 | Some concernsb | Low | Low | Some concernsc | Some concernsd | Some concerns |
| Sablerolles 2021 | Some concernse | Some concernsf | Some concernsg | Low | Low | Some concerns |
| Sablerolles 2021 | Some concernsh | Some concernsi | Some concernsj | Low | Low | Some concerns |
| Sablerolles 2021 | Some concernsk | Some concernsl | Some concernsm | Low | Low | Some concerns |
aLi 2021a, RoB 2. Deviations from intervention: Quote "We masked participants, investigators, laboratory staff, and outcome assessors to the allocation of treatment groups … Designated unblinded personnel were responsible for the preparation and administration of the vaccination and were forbidden to reveal the identity of the study vaccines to the participants or other investigators" Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed. Reasons for exclusion: three participants randomized to the Convidecia boost group crossed over to other groups. Two participants were wrongly administrated with a homogeneous boost dose of CoronaVac and were re‐classified into the CoronaVac boost group. One participant had in fact only received one primary dose and was re‐classified into the CoronaVac/Convidecia 2 dose group (additional arm in the study extracted separately). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to the small number of participants who crossed over. Risk assessed to have some concerns for this outcome.
bMok 2021, RoB 1. Randomization: Quote: "participants were randomized to receive either BNT162b2 (n = 40) or CoronaVac (n = 40) as the third dose." Comment: allocation sequence probably random. No information on allocation concealment.
cMok 2021, RoB 4. Measurement of the outcome: Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unclear blinding (outcome assessor). The authors reported on adverse events that may contain both clinically and laboratory‐detected events, which can be influenced by knowledge of the intervention assignment but is not likely in the context of the pandemic.
dMok 2021, RoB 5. Selection of the reported results: Comment: the protocol, statistical analysis plan, registry were available (revision dated August 17, 2021). Outcome not prespecified. No information on whether the result was selected from multiple outcome measurements or analyses of the data. Trial not analyzed as prespecified.
eSablerolles 2021, RoB 1. Randomization: Quote: "Participants were assigned to study groups in a 1:1:1:1 fashion; randomization was stratified by study site after obtaining written informed consent." Comment: allocation sequence probably random. No information on allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed as some concerns
fSablerolles 2021, RoB 2. Deviations from intervention: Quote: "single‐(participant)‐blinded Participants were unblinded for the booster vaccination by e‐mail eight days after injection, after completing the reactogenicity questionnaires." Comment: blinded study (participants). Deviations from intended intervention arising because of the study context: No participant cross‐over. Per‐protocol analysis was performed on the outcomes. Reasons for exclusion: baseline positive (2.6%, 1.7%, 0.9%, 1.7%), positive between baseline and follow‐up (1.8%, 0.9%, 0.0%, 0.0%). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance between groups. Risk assessed to have some concerns for this outcome.
gSablerolles 2021, RoB 3. Missing outcome data: Comment: 461 participants randomized; 433 participants analyzed for reactogenicity. No evidence that the result is not biased. Reasons (reactogenicity): baseline positive (2.6%, 1.7%, 0.9%, 1.7%), positive between baseline and follow‐up (1.8%, 0.9%, 0.0%, 0.0%), failed bleed at baseline or follow‐up (1.8%, 0.9%, 0.9%, 0.0%) or withdrew from the study (3.5%, 5.2%, 1.7%, 1.7%). Not likely that missingness depended on the true value of the outcome because there is no major imbalance between groups. Risk assessed to have some concerns for this outcome. Data not available for all or nearly all participants randomized. Missingness could depend on the true value of the outcome.
hSablerolles 2021, RoB 1. Randomization: Quote: "Participants were assigned to study groups in a 1:1:1:1 fashion; randomization was stratified by study site after obtaining written informed consent." Comment: allocation sequence probably random. No information on allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed as some concerns
iSablerolles 2021, RoB 2. Deviations from intervention: Quote: "single‐(participant)‐blinded Participants were unblinded for the booster vaccination by e‐mail eight days after injection, after completing the reactogenicity questionnaires." Comment: blinded study (participants). Deviations from intended intervention arising because of the study context: no participant cross‐over. Per‐protocol analysis was performed on the outcomes. Reasons for exclusion: baseline positive (2.6%, 1.7%, 0.9%, 1.7%), positive between baseline and follow‐up (1.8%, 0.9%, 0.0%, 0.0%). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance between groups. Risk assessed to have some concerns for this outcome.
jSablerolles 2021, RoB 3. Missing outcome data: Comment: 461 participants randomized; 433 participants analyzed for reactogenicity. Data not available for all or nearly all participants randomized. No evidence that the result is not biased. Reasons (reactogenicity): baseline positive (2.6%, 1.7%, 0.9%, 1.7%), positive between baseline and follow‐up (1.8%, 0.9%, 0.0%, 0.0%), failed bleed at baseline or follow‐up (1.8%, 0.9%, 0.9%, 0.0%) or withdrew from the study (3.5%, 5.2%, 1.7%, 1.7%). Missingness could depend on the true value of the outcome. Not likely that missingness depended on the true value of the outcome because there is no major imbalance between groups. Risk assessed to have some concerns for this outcome.
kSablerolles 2021, RoB 1. Randomization: Quote: "Participants were assigned to study groups in a 1:1:1:1 fashion; randomization was stratified by study site after obtaining written informed consent." Comment: allocation sequence probably random. No information on allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance. Risk assessed as some concerns
lSablerolles 2021, RoB 2. Deviations from intervention: Quote: "single‐(participant)‐blinded. Participants were unblinded for the booster vaccination by e‐mail eight days after injection, after completing the reactogenicity questionnaires." Comment: blinded study (participants) Deviations from intended intervention arising because of the study context: no participant cross‐over. Per‐protocol analysis was performed on the outcomes. Reasons for exclusion: baseline positive (2.6%, 1.7%, 0.9%, 1.7%), positive between baseline and follow‐up (1.8%, 0.9%, 0.0%, 0.0%). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance between groups. Risk assessed to have some concerns for this outcome.
mSablerolles 2021, RoB 3. Missing outcome data: Comment: 461 participants randomized; 433 participants analyzed for reactogenicity. Data not available for all or nearly all participants randomized. No evidence that the result is not biased. Reasons (reactogenicity): baseline positive (2.6%, 1.7%, 0.9%, 1.7%), positive between baseline and follow‐up (1.8%, 0.9%, 0.0%, 0.0%), failed bleed at baseline or follow‐up (1.8%, 0.9%, 0.9%, 0.0%) or withdrew from the study (3.5%, 5.2%, 1.7%, 1.7%). Missingness could depend on the true value of the outcome. Not likely that missingness depended on the true value of the outcome because there is no major imbalance between groups. Risk assessed to have some concerns for this outcome.
Any adverse event
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Li 2021a | Low | Some concernsa | Low | Low | Low | Some concerns |
aLi 2021a, RoB 2. Deviations from intervention: Quote "We masked participants, investigators, laboratory staff, and outcome assessors to the allocation of treatment groups … Designated unblinded personnel were responsible for the preparation and administration of the vaccination and were forbidden to reveal the identity of the study vaccines to the participants or other investigators" Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed. Reasons for exclusion: three participants randomized to the Convidecia boost group crossed over to other groups. Two participants were wrongly administrated with a homogeneous boost dose of CoronaVac and were reclassified into the CoronaVac boost group. One participant had in fact only received one primary dose and was re‐classified into the CoronaVac/Convidecia 2 dose group (additional arm in the study extracted separately). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to the small number of participants who crossed over. Risk assessed to have some concerns for this outcome.
Serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Li 2021a | Low | Some concernsa | Low | Low | Low | Some concerns |
aLi 2021a, RoB 2. Deviations from intervention: Quote "We masked participants, investigators, laboratory staff, and outcome assessors to the allocation of treatment groups … Designated unblinded personnel were responsible for the preparation and administration of the vaccination and were forbidden to reveal the identity of the study vaccines to the participants or other investigators" Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed. Reasons for exclusion: three participants randomized to the Convidecia boost group crossed over to other groups. Two participants were wrongly administrated with a homogeneous boost dose of CoronaVac and were reclassified into the CoronaVac boost group. One participant had in fact only received one primary dose and was re‐classified into the CoronaVac/Convidecia 2 dose group (additional arm in the study extracted separately). As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to the small number of participants who crossed over. Risk assessed to have some concerns for this outcome.
Comparison: booster versus booster
All‐cause mortality
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Toledo‐Romani 2021 | Some concernsa | Some concernsb | Low | Low | Low | Some concerns |
aToledo‐Romani 2021, RoB 1. Randomization: Quote: "Randomization into study arms (A and B) and placebo was done on day 0 at a 1:1:1 ratio using a site stratified random and previously defined risk strata (19–64 years without risk comorbidities, 19–64 years with risk comorbidities and ≥65 years)." Comment: allocation sequence random. No information on allocation concealment. Imbalances in baseline characteristics appear to be compatible with chance.
bToledo‐Romani 2021, RoB 2. Deviations from intervention: Comment: blinded study (participants and personnel/carers). Per‐protocol analysis was performed on the outcomes. Reasons for exclusion: did not receive or discontinued the intervention. As we are assessing the effect of assignment to intervention (intention‐to‐treat effect), we considered that the data were analyzed inappropriately. There was probably no substantial impact of failure to analyse participants according to their randomized assignment due to balance between groups.
Systemic reactogenicity events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Hall 2021a | Low | Low | Low | Low | Low | Low |
aTrial in immunocompromized participants.
Appendix 9. Matrix indicating availability of trial results for the critical and important outcomes of the review
Key to tables: ✓ A study result is available for inclusion in the synthesis. X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators. * No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results. ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study.
Abbreviations: AE: adverse event; GMR: geometric mean ratio; n: number of participants; SAE: serious adverse event.
RNA‐based vaccines
BNT162b2 – BioNTech/Fosun Pharma/Pfizer versus placebo
| Study ID | Study follow‐up (months) | BNT162b2 (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
|
Walsh 2020 (NCT04368728) |
1.68 | 24 | 18 | * | * | * | ✓ | ✓ | ✓ | ✓ |
| Frenck 2021 (NCT04368728) | 4.7 | 1134 | 1130 | * | ✓ |
✓ | ✓ | ✓ | ✓ | ✓ |
|
Thomas 2021 (NCT04368728) |
6 | 22,085 | 22,080 | * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study ID | Study follow‐up (months) | BNT162b2 (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
|
Walsh 2020 (NCT04368728) |
1.68 | 24 | 18 | * | ✓ | ✓ |
| Frenck 2021 (NCT04368728) | 4.7 | 1134 | 1130 | * | ✓ | ✓ |
| Thomas 2021 (NCT04368728) | 6 | 22,085 | 22,080 | * | * | ✓ |
mRNA‐1273 – ModernaTX versus placebo
| Study ID | Study follow‐up (months) | mRNA‐1273 (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
|
Ali 2021 (NCT04649151) |
2.8 | 2489 | 1243 | ✓ | ✓ | * | ✓ | ✓ | ✓ | ✓ |
| El Sahly 2021 (NCT04470427) | 5.3 | 15,209 | 15,206 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Results reported in Pajon 2021 are already reported in El Sahly 2021; consequently, Pajon 2021 is not included in the forest plots.
| Study ID | Study follow‐up (months) | mRNA‐1273 (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
|
Ali 2021 (NCT04649151) |
2.8 | 2489 | 1243 | * | * | ✓ |
| El Sahly 2021 (NCT04470427) | 5.3 | 15,209 | 15,206 | X | X | ✓ |
CVnCoV – CureVac AG versus placebo
| Study ID | Study follow‐up (months) | CVnCoV (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
| Kremsner 2021 (NCT04652102; EudraCT 2020‐003998‐22) | 6.23 | 19,783 | 19,746 | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study ID | Study follow‐up (months) | CVnCoV (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
| Kremsner 2021 (NCT04652102; EudraCT 2020‐003998‐22) | 6.23 | 19,783 | 19,746 | * | * | ✓ |
Non‐replicating viral vector
ChAdOx1/SII‐ChAdOx1 – AstraZeneca/University of Oxford versus placebo/MenACWY
| Study ID | Study follow‐up (months) | ChAdOx1 (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
| Asano 2022 (NCT04568031) | 1.9 | 192 | 64 | * | * | * | ✓ | ✓ | ✓ | ✓ |
| Falsey 2021 (NCT04516746) | 6.27 | 21,635 | 10,816 | ✓ | ✓ | ✓ | ✓ | * | ✓ | ✓ |
| Clemens 2021a (ISRCTN89951424) | 8.27 | 5207 | 5209 | * | ✓ | ✓ | ✓ | X | * | X |
|
Emary 2021 (NCT04400838) |
4.93 | 5600 | 5211 | * | ✓ | * | * | * | * | * |
|
Madhi 2021b (NCT04444674; PACTR202006922165132) |
2 | 52 | 52 | * | * | * | ✓ | * | * | ✓ |
|
Madhi 2021a (NCT04444674; PACTR202006922165132) |
6.73 | 1013 | 1013 | * | ✓ | * | * | * | * | * |
| Kulkarni 2021 (CTRI/2020/08/027170) | 6 | 900 | 300 | * | * | * | ✓ | X | ✓ | ✓ |
| Voysey 2021a (NCT04324606; ISRCTN89951424; NCT04400838; NCT04444674) | 3.94 | 12,408 | 12,014 | ✓ | ✓ | ✓ | ✓ | * | ✓ | ✓ |
| Voysey 2021ab (ISRCTN89951424; NCT04324606; NCT04400838; NCT04444674) | 3.94 | 12,048 | 12,014 | ✓ | ✓ | ✓ | ✓ | * | ✓ | ✓ |
aResults reported in Clemens 2021 are included in Voysey 2021a. Only results for "Confirmed SARS‐CoV‐2 infection after complete vaccination" against Gamma variant were extracted and analyzed. bResults reported in Voysey 2021b are already reported in Voysey 2021a, consequently Voysey 2021b is not included in the forest plots.
| Study ID | Study follow‐up (months) | ChAdOx1 (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
|
Asano 2022 (NCT04568031) |
1.9 | 192 | 64 | * | ✓ | ✓ |
|
Falsey 2021 (NCT04516746) |
6.27 | 21,635 | 10,816 | * | * | * |
| Clemens 2021 (ISRCTN89951424) | 8.27 | 5207 | 5209 | * | * | X |
|
Emary 2021 (NCT04400838) |
4.93 | 5600 | 5211 | * | * | * |
|
Madhi 2021b (NCT04444674; PACTR202006922165132) |
2 | 52 | 52 | * | * | * |
|
Madhi 2021a (NCT04444674; PACTR202006922165132) |
6.73 | 1013 | 1013 | * | * | * |
| Kulkarni 2021 (CTRI/2020/08/027170) | 6 | 900 | 300 | X | X | X |
| Voysey 2021a (NCT04324606; ISRCTN89951424; NCT04400838; NCT04444674) | 3.94 | 12,408 | 12,014 | ✓ | ✓ | X |
| Voysey 2021a (ISRCTN89951424; NCT04324606; NCT04400838; NCT04444674) | 3.94 | 12,048 | 12,014 | * | * | * |
ChAdOx1 – AstraZeneca/University of Oxford versus SII‐ChAdOx1
| Study ID | Study follow‐up (months) | ChAdOx1 (n) | SII‐ChAdOx (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | AE | ||||
| Kulkarni 2021 (CTRI/2020/08/027170) | 6 | 300 | 100 | * | * | * | ✓ | ✓ | ✓ | ✓ |
| Study ID | Study follow‐up (months) | ChAdOx1 (n) | SII‐ChAdOx (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
| Kulkarni 2021 (CTRI/2020/08/027170) | 6 | 300 | 100 | ✓ | ✓ | ✓ |
Ad26.COV2.S – Janssen Pharmaceutical Companies versus placebo
| Study ID | Study follow‐up (months) | Ad26.COV2.S (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
|
Sadoff 2021a (NCT04436276) |
2.33 | 324 | 164 | * | * | * | * | ✓ | ✓ | * |
|
Sadoff 2021b (NCT04505722) |
1.84 (median) | 22,174 | 22,151 | * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Stephenson 2021 reported on a subset of participants included in Sadoff 2021a. We could not retrieve data from Stephenson 2021 and it was not included in the analysis.
| Study ID | Study follow‐up (months) | Ad26.COV2.S (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
|
Sadoff 2021a (NCT04436276) |
2.33 | 324 | 164 | * | ✓ | * |
| Sadoff 2021b (NCT04505722) | 1.84 (median) | 22,174 | 22,151 | X | X | ✓ |
Gam‐COVID‐Vac (Sputnik V) – Gamaleya Research Institute versus placebo
| Critical outcomes | ||||||||||
| Study ID | Study follow‐up (months) | Gam‐COVID‐Vac (n) | Placebo (n) | Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE |
| Logunov 2021 (NCT04530396) | 2.56 | 16,501 | 5476 | * | ✓ | ✓ | ✓ | * | * | ✓ |
| Study ID | Study follow‐up (months) | Gam‐COVID‐Vac (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
| Logunov 2021 (NCT04530396) | 2.56 | 16,501 | 5476 | ✓ | ✓ | * |
Inactivated virus vaccine
CoronaVac – Sinovac versus adjuvant
| Study ID | Study follow‐up (months) | CoronaVac (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
|
Zhang 2021 (NCT04352608) Phase 2 |
1.41 | 120 | 60 | * | * | * | * | ✓ | ✓ | X |
|
Zhang 2021 (NCT04352608) Phase 1 |
1.41 | 24 | 24 | * | * | * | * | ✓ | ✓ | ✓ |
| Bueno 2021a (NCT04651790) | 1.4 | 270 | 164 | * | * | * | * | ✓ | * | ✓ |
|
Han 2021 (NCT04551547) |
4.1 | 219 | 114 | * | * | * | * | * | ✓ | ✓ |
| Palacios 2020 (NCT04456595) | 12 | 6201 | 6207 | * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tanriover 2021 (NCT04582344) | 6 | 6650 | 3568 | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
|
Wu 2021a (NCT04383574) |
1.84 | 124 | 74 | * | * | * | * | ✓ | ✓ | ✓ |
|
Li 2021aa (NCT04383574) |
10.46 | 100 | 50 | * | * | * | * | ✓ | ✓ | ✓ |
|
Pan 2021ab (NCT04352608) |
60 | 30 | * | * | * | * | ✓ | ✓ | ✓ | |
aResults reported in Li 2021a are already reported in Wu 2021a; consequently, Li 2021a is not included in the forest plots. bWe could not retrieve data from Pan 2021c; not included in the forest plots.
| Study ID | Study follow‐up (months) | CoronaVac (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
|
Zhang 2021 (NCT04352608) |
1.41 | 120 | 60 | ✓ | ✓ | ✓ |
|
Zhang 2021 (NCT04352608) |
1.41 | 24 | 24 | * | ✓ | ✓ |
|
Bueno 2021a (NCT04651790) |
1.4 | 270 | 164 | * | * | ✓ |
|
Han 2021 (NCT04551547) |
4.1 | 219 | 114 | * | ✓ | * |
| Palacios 2020 (NCT04456595) | 12 | 6201 | 6207 | * | * | ✓ |
| Tanriover 2021 (NCT04582344) | 6 | 6650 | 3568 | X | X | ✓ |
|
Wu 2021a (NCT04383574) |
1.84 | 100 | 74 | * | ✓ | ✓ |
|
Li 2021a (NCT04383574) |
10.46 | 100 | 50 | * | ✓ | ✓ |
|
Pan 2021a (NCT04352608) |
60 | 30 | * | ✓ | ✓ | |
WIBP‐CorV – Sinopharm‐Wuhan versus adjuvant
| Study ID | Study follow‐up (months) | WIV04 (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
|
Al Kaabi 2021 (NCT04510207; ChiCTR2000034780) |
5 | 13,470 | 13,471 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Guo 2021 (ChiCTR2000031809) | 4.77 | 168 | 168 | * | * | * | * | ✓ | ✓ | ✓ |
| Study ID | Study follow‐up (months) | WIV04 (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
| Al Kaabi 2021 (NCT04510207; ChiCTR2000034780) | 5 | 13,470 | 13,471 | * | ✓ | ✓ |
| Guo 2021 (ChiCTR2000031809) | 4.77 | 168 | 168 | * | ✓ | ✓ |
BBIBP‐CorV – Sinopharm‐ Beijing versus adjuvant
| Study ID | Study follow‐up (months) | BBIBP‐CorV (n) | Adjuvant (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
| Xia 2020 (ChiCTR2000032459) | 0.92 | 84 | 28 | * | * | * | * | ✓ | ✓ | ✓ |
| Al Kaabi 2021 (NCT04510207; ChiCTR2000034780) | 5 | 13,470 | 13,471 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Xia 2021 (ChiCTR2000032459) | 2.9 | 252 | 252 | * | * | * | * | ✓ | ✓ | * |
| Study ID | Study follow‐up (months) | BBIBP‐CorV (n) | Adjuvant (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
| Xia 2020 (ChiCTR2000032459) | 0.92 | 84 | 28 | * | ✓ | ✓ |
| Al Kaabi 2021(NCT04510207; ChiCTR2000034780) | 5 | 13,470 | 13,471 | * | ✓ | ✓ |
| Xia 2021 (ChiCTR2000032459) | 2.9 | 252 | 252 | * | ✓ | ✓ |
BBV152 – Bharat Biotech versus adjuvant
| Study ID | Study follow‐up (months) | BBV152 (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
| Ella 2021a (NCT04471519) | 6.38 | 100 | 75 | * | * | * | * | ✓ | * | ✓ |
| Ella 2021b (NCT04641481) | 12 | 12,889 | 12,889 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
|
Ella 2021aa (NCT04471519) |
3.87 | 190 | 190 | * | * | * | * | ✓ | * | ✓ |
aWe could not retrieve data from Ella 2021c and the trial is not included in the analysis.
| Study ID | Study follow‐up (months) | BBV152 (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
|
Ella 2021a (NCT04471519) |
6.38 | 100 | 75 | * | * | ✓ |
| Ella 2021b (NCT04641481) | 12 | 12,889 | 12,889 | * | * | ✓ |
|
Ella 2021a (NCT04471519) |
3.87 | 190 | 190 | * | * | ✓ |
Protein subunit
NVX‐CoV2373 – Novavax versus placebo
| Study ID | Study follow‐up (months) | NVX‐CoV2373 (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
| Keech 2020 (NCT04368988) | 1.15 | 29 | 25 | * | * | * | * | * | ✓ | ✓ |
| Dunkle 2021 (NCT04611802) | 2 | 19,965 | 9984 | * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Formica 2021 (NCT04368988) | 1.15 | 258 | 257 | * | * | * | * | ✓ | ✓ | ✓ |
| Heath 2021 (NCT04583995; EudraCT 2020‐004123‐16) | 13 | 7593 | 7594 | * | ✓ | ✓ | ✓ | * | ✓ | ✓ |
| Shinde 2021 (NCT04533399; PACTR202009726132275) | 1.15 | 2206 | 2200 | * | ✓ | ✓ | * | ✓ | ✓ | ✓ |
| Study ID | Study follow‐up (months) | NVX‐CoV2373 (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
| Keech 2020 (NCT04368988) | 1.15 | 29 | 25 | ✓ | ✓ | * |
| Dunkle 2021 (NCT04611802) | 2 | 19,965 | 9984 | X | X | ✓ |
| Formica 2021 (NCT04368988) | 1.15 | 258 | 257 | ✓ | * | ✓ |
| Heath 2021 (NCT04583995; EudraCT 2020‐004123‐16) | 13 | 7593 | 7594 | * | * | * |
| Shinde 2021 (NCT04533399; PACTR202009726132275) | 1.15 | 2206 | 2200 | X | X | ✓ |
FINLAY‐FR‐2 – FINLAY versus placebo
| Study ID | Study follow‐up (months) | FINLAY‐FR‐2 (n) | Placebo (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
| Toledo‐Romani 2021 (RPCEC00000354) | 5.2 | 14,679 | 14,675 | X | ✓ | ✓ | ✓ | * | X | * |
| Study ID | Study follow‐up (months) | FINLAY‐FR‐2 (n) | Placebo (n) | Important outcomes | ||
| GMT of specific antibody against 2019 novel coronavirus | GMT of neutralizing antibody against 2019 novel coronavirus | Local reactogenicity events | ||||
| Toledo‐Romani 2021 (RPCEC00000354) | 5.2 | 14,679 | 14,675 | X | X | * |
Heterologous vaccine
Comparison: CoronaVac/Ad5‐vectored versus homologous CoronaVac
| Study ID | Study follow‐up (months) | CoronaVac/ Ad5‐vectored (n) | CoronaVac (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
| Li 2021a (NCT04892459) | 1 | 50 | 50 | * | * | * | * | ✓ | ✓ | ✓ |
| Important outcomes | ||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | ||||||||
| ✓ | ✓ | ✓ | ||||||||
Comparison: ChAdOx1‐S/BNT162b2 versus ChAdOx1‐S
| Study ID | Study follow‐up (months) | ChAdOx1‐S/BNT162b2 (n) | ChAdOx1‐S (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
|
Liu 2021 (ISRCTN69254139; EudraCT 2020‐005085‐33) |
2 | 115 | 115 | * | * | * | * | X | ✓ | ✓ |
| Important outcomes | ||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | ||||||||
| * | * | X | ||||||||
Comparison: BNT162b2/ChAdOx1‐S versus BNT162b2
| Study ID | Study follow‐up (months) |
BNT162b2/ ChAdOx1‐S (n) |
BNT162b2 (n) | Critical outcomes | |||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | |||||
| Liu 2021 (ISRCTN69254139; EudraCT 2020‐005085‐33) | 2 | 114 | 119 | * | * | * | * | * | ✓ | ✓ | |
| Important outcomes | |||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | |||||||||
| ✓ | ✓ | * | |||||||||
Boosters
Comparison: BNT162b2 versus placebo
| Study ID | Study follow‐up (months) |
BNT162b2 (n) |
Placebo (n) | Critical outcomes | |||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | |||||
| Hall 2021 (NCT04885907)a | 1 | 60 | 60 | X | ✓ | * | * | ✓ | * | * | |
| Important outcomes | |||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | |||||||||
| * | * | ✓ | |||||||||
aTrial in immunocompromized participants.
Comparison: FINLAY‐FR‐2 (25 μg) + FR‐1 (50 μg) versus no booster
| Study ID | Study follow‐up (months) | FINLAY‐FR‐2 (25 μg) + FR‐1 (50 μg) (n) | Placebo (n) | Critical outcomes | |||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | |||||
| Toledo‐Romani 2021 (RPCEC00000354) | 5.2 | 14,679 | 14,675 | X | ✓ | ✓ | ✓ | * | X | * | |
| Important outcomes | |||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | |||||||||
| * | * | * | |||||||||
Booster versus booster
Comparison: BNT162b2 or mRNA‐1273/boost ChAdOx1 versus BNT162b2 or mRNA‐1273/boost BNT162b2 or mRNA‐1273
| Study ID | Study follow‐up (months) | BNT162b2 or mRNA‐1273/ Boost ChAdOx1 (n) | BNT162b2 or mRNA‐1273/ Boost BNT162b2 or mRNA‐1273 (n) | Critical outcomes | ||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||
| Bonelli 2021a (EudraCT 2021‐002348‐57) | 1 | 30 | 30 | * | * | * | * | * | * | * |
| Important outcomes | ||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | ||||||||
| * | * | ✓ | ||||||||
aTrial in immunocompromized participants.
Comparison: CoronaVac/boost Ad5‐vectored versus CoronaVac/boost
| Study ID | Study follow‐up (months) | CoronaVac/boost Ad5‐vectored (n) | CoronaVac/boost (n) | Critical outcomes | |||||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | |||||||
| Li 2021a (NCT04892459) | 1 | 100 | 100 | * | * | * | * | ✓ | ✓ | ✓ | |||
| Important outcomes | |||||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | |||||||||||
| ✓ | ✓ | ✓ | |||||||||||
Comparison: CoronaVac/boost BNT162b2 versus CoronaVac/boost
| Study ID | Study follow‐up (months) | CoronaVac/boost BNT162b2 (n) | CoronaVac/boost (n) | Critical outcomes | |||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | |||||
| Mok 2021 (NCT04611243) | 5.8 | 40 | 40 | * | * | * | * | ✓ | * | * | |
| Important outcomes | |||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | |||||||||
| * | * | ✓ | |||||||||
Comparison: Ad26/Boost mRNA‐1273 versus Ad26/boost
| Study ID | Study follow‐up (months | Ad26/Boost mRNA‐1273 (n) | Ad26/boost (n) | Critical outcomes | ||||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | ||||||
| Sablerolles 2021 (NCT04927936) | 1 | 106 | 111 | * | * | * | * | ✓ | * | * | ||
| Important outcomes | ||||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | ||||||||||
| * | * | ✓ | ||||||||||
Comparison: Ad26/Boost BNT162b2 versus Ad26/boost
| Study ID | Study follow‐up (months) | Ad26/Boost BNT162b2 (n) |
Ad26 /boost (n) |
Critical outcomes | |||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | |||||
| Sablerolles 2021 (NCT04927936) | 1 | 106 | 111 | * | * | * | * | ✓ | * | * | |
| Important outcomes | |||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | |||||||||
| * | * | ✓ | |||||||||
Comparison: Ad26/Boost BNT162b2 versus Ad26/Boost mRNA‐1273
| Study ID | Study follow‐up (months | Ad26/Boost mRNA‐1273 (n) |
Ad26/boost BNT162b2 (n) |
Critical outcomes | |||||||
| Confirmed SARS‐CoV‐2 infection after complete vaccination | Confirmed symptomatic COVID‐19 after complete vaccination | Severe or critical COVID‐19 | All‐cause mortality | Systemic reactogenicity events | Any AE | SAE | |||||
| Sablerolles 2021 (NCT04927936) | 1 | 111 | 111 | * | * | * | * | ✓ | * | * | |
| Important outcomes | |||||||||||
| GMT of specific antibody against SARS‐COV‐2 | GMT of neutralizing antibody against SARSCOV‐2 | Local reactogenicity events | |||||||||
| * | * | ✓ | |||||||||
Key: ✓ A study result is available for inclusion in the synthesis. X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators. * No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results. ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study.
Abbreviations: AE: adverse event; GMR: geometric mean ratio; n: number of participants; SAE: serious adverse event.
Appendix 10. BNT162b2 – BioNtech/Fosun Pharma/Pfizer versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | 2 | 44,077 | N/A | N/A | 97.84% (44.25% to 99.92%) |
| Severe or critical COVID‐19 after complete vaccination | 1 | 46,077 | N/A | N/A | 95.70% (73.90% to 99.90%) |
| All‐cause mortality | 1 | 43,847 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 (0.52 to 2.22) | N/A |
| Serious adverse events | 2 | 46,107 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 (0.55 to 3.07) | N/A |
| Systemic reactogenicity events | N/A | N/A | N/A | N/A | N/A |
| Any adverse event | 3 | 46,149 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 (0.88 to 2.63) | N/A |
| Local reactogenicity events | N/A | N/A | N/A | N/A | N/A |
| CI: confidence interval; N/A: not applicable. | |||||
Appendix 11. Neutralizing antibody geometric mean titre
RNA‐based vaccines
| Study | Intervention name | Results | Unit of analysis | Time point | Type of assay | Population | |
| GMT (95% CI) | GMR (95% CI) | ||||||
| COVID‐19 vaccine versus placebo | |||||||
| Frenck 2021 | BNT162b2 | 1283.00 (1139.60 to 1444.50) |
84.96 (58.90 to 122.55) | Not specified | 1 month after 2nd dose | SARS‐CoV‐2 50% neutralizing assay |
12–15 years |
| Placebo | 15.10 (10.70 to 21.40) | ||||||
| BNT162b2 | 730.80 (646.70 to 825.80) | 68.29 (56.55 to 82.48) | Not specified | 1 month after 2nd dose | SARS‐CoV‐2 50% neutralizing assay |
16–25 years | |
| Placebo | 10.70 (9.30 to 12.40) |
||||||
|
Walsh 2020 |
BNT162b2 | 163 (no CIs) | 16.30 | Not specified | 14 days after 2nd dose (time point not specified for placebo) | SARS‐CoV‐2 serum 50% neutralizing assay | 18–55 years |
| Placebo | |||||||
| BNT162b2 | 206 (no CIs) | 20.60 | Not specified | 14 days after 2nd dose (time point not specified for placebo) | SARS‐CoV‐2 serum 50% neutralizing assay | 65–85 years |
|
| Placebo | |||||||
CI: confidence interval; GMR: geometric mean ratio; GMT: geometric mean titre.
Non‐replicant viral vector vaccines
| Study | Intervention name | Results | Unit of analysis | Time point | Type of assay | Population | |
| GMT (95% CI) | GMR (95% CI) | ||||||
| COVID‐19 vaccine versus placebo | |||||||
|
Sadoff 2021a |
Ad26.COV2.S | 224 (158 to 318) | 3.86 (2.72 to 5.47) | Not reported | 29 days after vaccination | Wild‐type virus microneutralization assay using the Victoria/1/2020 SARSCoV‐2 strain | 18 and 55 years |
| Placebo | 58 (58 to 58) | ||||||
| Sadoff 2021a | Ad26.COV2.S | 212 (137 to 284) |
3.65 (2.53 to 5.26) | Not reported | 15 days after vaccination | Wild‐type virus microneutralization assay using the Victoria/1/2020 SARS‐CoV‐2 strain | ≥ 65 years |
| Placebo | 58 (58 to 58) | ||||||
|
Logunov 2021 |
Gam‐COVID‐Vac rAd26‐S | 44.50 (31.80 to 62.20) |
28.46 (17.71 to 45.75) | Not reported | 21 days after second dose | Microneutralization assay using SARS‐CoV‐2 (hCoV‐19/Russia/Moscow_PMVL‐1/2020) in a 96‐well plate and a 50% tissue culture infective dose (TCID50) of 100 | ≥ 18 years |
| Placebo | 1.60 (1.12 to 2.19) |
||||||
| COVID‐19 vaccine versus COVID‐19 vaccine | |||||||
| Kulkarni 2021 | SII‐ChAdOx1 | 69.90 (60.80 to 80.40) |
1.23 (0.92 to 1.63) | Not reported | 28 days after dose 2 |
Pseudo virus‐based microneutralization assay |
≥ 18 years |
| ChAdOx1 | 56.80 (44.40 to 72.50) | ||||||
CI: confidence interval; GMR: geometric mean ratio; GMT: geometric mean titre.
Inactivated virus vaccines
| Study | Intervention name | Results | Units of analysis | Timepoint | Type of assay | Population | |
| GMT (95% CI) | GMR (95% CI) | ||||||
| COVID‐19 vaccine versus adjuvant/placebo | |||||||
|
Bueno 2021 |
CoronaVac | 10.10 (7.28 to 14.01) |
1.84 (1.32 to 2.55) | Not reported | 2 weeks after 2nd dose | A SINOVAC standardized microtitre methodology, conventional virus neutralization | ≥ 18 years |
| Adjuvant |
5.48 (1.84 to 16.29) |
||||||
|
Zhang 2021 |
CoronaVac | 5.60 (3.60 to 8.70) | 2.80 (1.80 to 4.25) | Not reported | 2 weeks after 2nd dose | Microcytopathogenic effect assay | Phase 1: 18–59 years, healthy |
| Placebo | 2 (2 to 2) | ||||||
| Zhang 2021 | CoronaVac | 27.60 (22.70 to 33.50) | 11.90 (10.23 to 13.83) | Not reported | 2 weeks after 2nd dose | Microcytopathogenic effect assay | Phase 2: 18–59 years, healthy |
| Placebo | 2 (2 to 2) | ||||||
|
Han 2021 |
CoronaVac | 142.20 (124.70 to 162.10) | 67.71 (59.25 to 77.37) | Not reported | 28 days after 2nd dose | Microcytopathogenic effect assay | Phase 2: 3–17 years |
| Placebo | 2.10 (2 to 2.1) | ||||||
|
Wu 2021a |
CoronaVac | 42.20 (35.20 to 50.60) |
20.09 (16.73 to 24.13) | Not reported | 28 days after 2nd dose | Microcytopathogenic effect assay | Phase 2: ≥ 60 years |
| Adjuvant | 2.10 (2 to 2.10) | ||||||
| Fadlyana 2021 | CoronaVac | 15.76 (14.57 to 17.04) | 7.80 (7.20 to 8.45) | Not reported | 14 days after 2nd dose | Not clear | 18–59 years |
| Placebo | 2.02 (1.98 to 2.05) | ||||||
|
Al Kaabi 2021 |
WIBP‐CorV | 94.50 (89.70 to 99.50) | 35 (32.83 to 37.30) | Not reported | 14 days after 2nd dose | Not reported | ≥ 18 years |
| Placebo | 2.70 (2.60 to 2.80) | ||||||
|
Al Kaabi 2021 |
BBIBP‐CorV | 156 (149.60 to 162.70) | 57.77 (54.63 to 61.10) | Not reported | 14 days after 2nddose | Not reported | ≥ 18 years |
| Placebo | 2.70 (2.60 to 2.80) | ||||||
| Guo 2021 | WIBP‐CorV |
134 (104 to 174) |
26.80 (20.71 to 34.66) | Not reported | 28 days after whole course vaccination | Plaque reduction neutralization test (PRNT) |
18–59 years |
| Adjuvant |
5 (5 to 5) |
||||||
|
Xia 2020 |
BBIBP‐CorV |
218.90 (165.60 to 289.50) |
109.45 (82.77 to 144.73) | Not reported | 14 days after 1st inoculation | Not reported | Phase 2: ≥ 18 years |
| Placebo | 2 (2 to 2) |
||||||
| Xia 2021 | BBIBP‐CorV | 180.20 (163.60 to 198.40) |
90.10 (81.81 to 99.22) | Not reported | 28 days after 2nd inoculation | Not reported | 3–5 years |
| Adjuvant | 2 (2 to 2) |
||||||
| Xia 2021 | BBIBP‐CorV | 168.60 (151.90 to 187) | 84.30 (75.97 to 93.53) | Not reported | 28 days after 2nd inoculation | Not reported | 6–12 years |
| Adjuvant | 2 (2 to 2) |
||||||
|
Xia 2021 |
BBIBP‐CorV | 155.7 (137.7 to 176.5) | 77.87 (68.71 to 88.24) | Not reported | 28 days after 2nd inoculation | Not reported | 13–17 years |
| Adjuvant | 2 (2 to 2) |
||||||
| Ella 2021b | BBV152 | 125.60 (111.20 to 141.80) |
9.16 (2.28 to 36 to 78) | Not reported | 28 days after 2nd vaccination | MNT50 assay |
≥ 18 years |
| Adjuvant | 13.70 (10.70 to 170.40) |
||||||
| Ella 2021a | BBV152 | 66.40 (53.40 to 82.40) |
9.22 (7.25 to 11.80) | Not reported | Day 28 | MNT50 assay |
18–55 years |
| Adjuvant | 7.20 (6.40 to 8.10) |
||||||
CI: confidence interval; GMR: geometric mean ratio; GMT: geometric mean titre.
Protein subunit vaccines
| Study | Intervention name | Results | Unit of analysis | Time point | Type of assay | Population | ||
| GMT (95% CI) | GMR (95% CI) | |||||||
| COVID‐19 vaccine versus placebo | ||||||||
| Keech 2020 | NVX‐CoV2373 | 3906.30 (2555.90 to 5970) |
195.315 (127.79 to 298.50) | Not reported | Day 35 (14 days after 2nddose) | Wild‐type SARS‐CoV‐2 microneutralization |
18–59 years | |
| Placebo | 20 (20 to 20) |
|||||||
CI: confidence interval; GMR: geometric mean ratio; GMT: geometric mean titre.
Primary series heterologous vaccination scheme versus homologous vaccination scheme
| Study | Intervention name | Result | Unit of analysis | Time point | Type of assay | Population | |
| GMT (95% CI) | GMR (95% CI) | ||||||
| Heterologous schedule versus homologous schedule | |||||||
|
Li 2021a |
CoronaVac/Ad5 | 54.40 (37.90 to 78) |
4.25 (2.63 to 6.86) | Not reported | 14 days after 2nd dose | Cytopathic effect‐based microneutralization assay with a wild‐type SARS‐CoV‐2 virus strain | 18–59 years |
| CoronaVac | 12.80 (9.30 to 17.50) |
||||||
CI: confidence interval; GMR: geometric mean ratio; GMT: geometric mean titre.
Boosters
| Study | Intervention name | Estimate effect | Unit of analysis | Time point | Type of assay | Population | |
| GMT (95% CI) | GMR (95% CI) | ||||||
| Heterologous boost versus homologous boost | |||||||
|
Li 2021a |
CoronaVac/Ad5 boost | 197.40 (167.70 to 232.40) |
5.87 (4.64 to 7.43) | BAU/mL | 14 days after boost | ELISA RBD‐binding IgG | 18–59 years |
| CoronaVac/CoronaVac boost | 33.60 (28.30 to 39.80) |
||||||
BAU: binding antibody units; CI: confidence interval; ELISA: enzyme‐linked immunosorbent assay; GMR: geometric mean ratio; GMT: geometric mean titre; IgG: immunoglobulin G; RBD: receptor‐binding domain.
Appendix 12. Specific adverse events
Cardioembolic events
| Type of vaccine | Study ID |
Arms (number analyzed) |
Intervention |
Pulmonary embolism | Stroke | Cavernous sinus thrombosis | Pericarditis | Venous thrombosis | Myocardial infarction |
| RNA‐based vaccine | Thomas 2021 | Intervention (21,926) |
BNT162b2 |
NR | 0 | NR | NR | NR | 0 |
| Control (21,921) |
Placebo |
NR | 1 | NR | NR | NR | 2 | ||
|
Frenck 2021 |
Intervention (1131) |
BNT162b2 |
NR | NR | 0 | NR | 0 | NR | |
| Control (1129) |
Placebo |
NR | NR | 0 | NR | 0 | NR | ||
| Walsh 2020 | Intervention (24) |
BNT162b2 |
NR | NR | NR | NR | NR | NR | |
| Control (18) |
Placebo |
NR | NR | NR | NR | NR | NR | ||
|
El Sahly 2021 |
Intervention (15,166) |
mRNA‐1273 | 6 |
NR | NR | 2 | 47; 8 deep venous thrombosis |
7 | |
| Control (15,151) |
Placebo | 7 |
NR | NR | 2 | 43; 6 deep venous thrombosis |
9 | ||
|
Ali 2021 |
Intervention (2482) |
mRNA‐1273 | NR | NR | NR | 0 | NR | 0 | |
| Control (1238) |
Placebo | NR | NR | NR | 0 | NR | 0 | ||
| Kremsner 2021 | Intervention (2002) |
CVnCoV | NR | NR | NR | NR | NR | NR | |
| Control (1980) |
Placebo | NR | NR | NR | NR | NR | NR | ||
| Hall 2021 | Intervention (60) | mRNA‐1273 booster | NR | NR | NR | NR | NR | NR | |
| Control (59) |
mRNA‐1273/placebo | NR | NR | NR | NR | NR | NR | ||
| Non‐replicating viral vector | Madhi 2021b | Intervention (52) |
ChAdOx1 | NR | NR | NR | NR | NR | NR |
| Control (52) |
Placebo | NR | NR | NR | NR | NR | NR | ||
| Falsey 2021 | Intervention (21,587) |
ChAdOx1 | NR | 0 | 0 | NR | 0 | NR | |
| Control (10,792) |
Placebo | NR | 2 | 0 | NR | 0 | NR | ||
| Voysey 2021a | Intervention (12,021) |
ChAdOx1 | 0 | NR | NR | 1 | 0 deep venous thrombosis | 0 | |
| Control (11,724) |
Placebo | 1 | NR | NR | 2 | 0 deep venous thrombosis | 2 |
||
| Asano 2022 | Intervention (192) |
ChAdOx1 | NR | NR | NR | NR | NR | NR | |
| Control (64) |
Placebo | NR | NR | NR | NR | NR | NR | ||
| Kulkarni 2021 | Intervention (300) |
SII‐ChAdOx1 | NR | NR | NR | NR | NR | NR | |
| Control (100) |
ChAdOx1 | NR | NR | NR | NR | NR | NR | ||
|
Sadoff 2021a |
Intervention (323) |
Ad26.COV2.S | NR | NR | NR | NR | NR | NR | |
| Control (163) |
Placebo | NR | NR | NR | NR | NR | NR | ||
| Sadoff 2021b | Intervention (21,895) |
Ad26.COV2.S | 4 | NR | 1 | 1 | 6 deep venous thrombosis | NR | |
| Control (21,888) |
Placebo | 1 | NR | 0 | 0 | 2 deep venous thrombosis | NR | ||
| Logunov 2021 | Intervention (16,427) |
Gam‐COVID‐Vac | NR | NR | 0 | NR | 1 deep venous thrombosis | 2 | |
| Control (5435) |
Placebo | NR | NR | 1 | NR | 0 deep venous thrombosis | 1 | ||
| Inactivated virus | Ella 2021a | Intervention (99) |
BBV152 | NR | 2 | NR | NR | NR | NR |
| Control (73) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
|
Ella 2021b |
Intervention (12,879) |
BBV152 | NR | NR | NR | NR | NR | 0 | |
| Control (12,874) | Adjuvant | NR | NR | NR | NR | NR | 1 | ||
| Zhang 2021 | Intervention (24) |
CoronaVac | NR | NR | NR | NR | NR | NR | |
| Control (24) | Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Zhang 2021 | Intervention | CoronaVac | NR | NR | NR | NR | NR | NR | |
| Control | Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Bueno 2021 | Intervention (270) |
CoronaVac | NR | NR | NR | NR | NR | NR | |
| Control (164) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Han 2021 | Intervention (217) |
CoronaVac | NR | NR | NR | NR | NR | NR | |
| Control (114) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Palacios 2020 | Intervention (6202) | CoronaVac | NR | NR | NR | NR | NR | NR | |
| Control (6194) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Wu 2021a | Intervention (124) |
CoronaVac | NR | NR | NR | NR | NR | NR | |
| Control (74) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Al Kaabi 2021 | Intervention (13,464) |
WIV04 | NR | NR | NR | NR | NR | NR | |
| Intervention (13,471) |
HBO2 | NR | NR | NR | NR | NR | NR | ||
| Control (13,453) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
|
Tanriover 2021 |
Intervention (6646) |
CoronaVac | NR | NR | NR | NR | NR | 0 | |
| Control (3568) | Adjuvant | NR | NR | NR | NR | NR | 1 | ||
| Fadlyana 2021 | Intervention (405) | CoronaVac | NR | NR | NR | NR | 0 vascular disorders | NR |
|
| Control (135) |
Adjuvant | NR | NR | NR | NR | 1 vascular disorder | NR | ||
|
Xia 2020 Phase 1 and 2 |
Intervention (84) | WIBP‐CorV | NR | NR | NR | NR | NR | NR | |
| Control (28) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Xia 2021 | Intervention (252) | BBIBP‐CorV | NR | NR | NR | NR | NR | NR | |
| Control (252) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Guo 2021 | Intervention (84) |
WIBP‐CorV | NR | NR | NR | NR | NR | NR | |
| Control (28) |
Adjuvant | NR | NR | NR | NR | NR | NR | ||
| Protein subunit | Formica 2021 | Intervention (258) |
NVX‐CoV2373 | NR | NR | NR | NR | 2 vascular disorders | NR |
| Control (255) |
Placebo | NR | NR | NR | NR | 2 vascular disorders | NR | ||
| Keech 2020 | Intervention (29) |
NVX‐CoV2373 | NR | NR | NR | NR | NR | NR | |
| Control (23) |
Placebo | NR | NR | NR | NR | NR | NR | ||
| Shinde 2021 | Intervention (484) |
NVX‐CoV2373 | NR | NR | NR | NR | NR | NR | |
| Control (484) |
Placebo | NR | NR | NR | NR | NR | NR | ||
| Heath 2021 | Intervention (7569) |
NVX‐CoV2373 | NR | NR | NR | NR | NR | 1 myocarditis | |
| Control (7570) |
Placebo | NR | NR | NR | NR | NR | 0 myocarditis | ||
| Dunkle 2021 | Intervention (19,965) |
NVX‐CoV2373 | 3 | 2 | NR | NR | 2 deep venous thrombosis | NR | |
| Control (9984) |
Placebo | 2 | 0 | NR | NR | 0 deep venous thrombosis | NR | ||
| Toledo‐Romani 2021 | Intervention (14,675) |
FINLAY‐FR‐2 | NR | NR | NR | NR | NR | NR | |
| Intervention (14,679) |
FINLAY‐FR‐2/booster FR‐1 | NR | NR | NR | NR | NR | NR | ||
| Control (14,677) |
Placebo | NR | NR | NR | NR | NR | NR | ||
|
Homologous versus heterologous scheme |
Li 2021a | Intervention (51) |
CoronaVac/Ad5 | NR | NR | NR | NR | 0 | NR |
| Control (50) |
CoronaVac | NR | NR | NR | NR | 0 | NR | ||
| Liu 2021 | Intervention (115) |
ChAd/BNT | NR | NR | NR | NR | 1 deep venous thrombosis | NR | |
| Control (114) |
ChAd/ChAd | NR | NR | NR | NR | NR | NR | ||
| Intervention (119) |
BNT162b2/ChAdOx1 | NR | NR | NR | NR | NR | NR | ||
| Control (115) |
BNT162b2/BNT162b2 | NR | NR | NR | NR | NR | NR | ||
|
Homologous or heterologous booster versus heterologous booster |
Bonelli 2021 | Intervention (27) |
ChAdOx1 booster | NR | NR | NR | NR | NR | NR |
| Control (28) |
BNT162b2 or mRNA‐1273 booster | NR | NR | NR | NR | NR | NR | ||
| Sablerolles 2021 | Control (105) |
Ad26.COV2.S/no booster | NR | NR | NR | NR | NR | NR | |
| Intervention (106) |
Ad26/booster | NR | NR | NR | NR | NR | NR | ||
| Intervention (112) |
Ad26/booster mRNA‐1273 | NR | NR | NR | NR | NR | NR | ||
| Intervention (111) |
Ad26/booster BNT162b2 | NR | NR | NR | NR | NR | NR | ||
| Mok 2021 | Intervention (30) |
CoronaVac/booster | NR | NR | NR | NR | NR | NR | |
| Control (30) |
CoronaVac/booster BNT162b2 | NR | NR | NR | NR | NR | NR | ||
| Li 2021a | Intervention (96) |
CoronaVac/booster Ad5 | NR | NR | NR | NR | NR | NR | |
| Control (102) | CoronaVac/booster | NR | NR | NR | NR | NR | NR |
NR: not reported; RNA: ribonucleic acid.
Haematological events
| Type of vaccine | Study ID |
Arms (number analyzed) |
Intervention | Thrombocytopaenia | Haemorrhage | Neutropenia | Anaemia | Lymphadenopathy |
| RNA‐based vaccine | Thomas 2021 | Intervention (21,926) |
BNT162b2 |
NR | NR | NR | NR | NR |
| Control (21,921) |
Placebo |
NR | NR | NR | NR | NR | ||
|
Frenck 2021 |
Intervention (1131) |
BNT162b2 |
NR | NR | NR | NR | 10 | |
| Control (1129) |
Placebo |
NR | NR | NR | NR | 2 | ||
| Walsh 2020a | Intervention (24) |
BNT162b2 |
NR | NR | NR | NR | NR | |
| Control (18) |
Placebo |
NR | NR | NR | NR | NR | ||
|
El Sahly 2021 |
Intervention (15,166) |
mRNA‐1273 | 1 | NR | NR | 2 | NR | |
| Control (15,151) |
Placebo | 1 | NR | NR | 2 | NR | ||
|
Ali 2021 |
Intervention (2482) |
mRNA‐1273 | NR | NR | NR | NR | 108 | |
| Control (1238) |
Placebo | NR | NR | NR | NR | 5 | ||
| Kremsner 2021 | Intervention (2002) |
CVnCoV | NR | NR | NR | NR | NR | |
| Control (1980) |
Placebo | NR | NR | NR | NR | NR | ||
| Hall 2021 | Intervention (60) | mRNA‐1273 booster | NR | NR | NR | NR | NR | |
| Control (59) |
mRNA‐1273/placebo | NR | NR | NR | NR | NR | ||
| Non‐replicating viral vector | Madhi 2021b | Intervention (52) |
ChAdOx1 | NR | NR | NR | NR | NR |
| Control (52) |
Placebo | NR | NR | NR | NR | NR | ||
| Falsey 2021 | Intervention (21,587) |
ChAdOx1 | NR | NR | NR | NR | NR | |
| Control (10,792) |
Placebo | NR | NR | NR | NR | NR | ||
| Voysey 2021a | Intervention (12,021) |
ChAdOx1 | NR | NR | NR | 0 | NR | |
| Control (11,724) |
Placebo | NR | NR | NR | 1 | NR | ||
| Asano 2022 | Intervention (192) |
ChAdOx1 | NR | NR | NR | NR | NR | |
| Control (64) |
Placebo | NR | NR | NR | NR | NR | ||
| Kulkarni 2021 | Intervention (300) |
SII‐ChAdOx1 | NR | NR | NR | NR | NR | |
| Control (100) |
ChAdOx1 | NR | NR | NR | NR | NR | ||
| Sadoff 2021a | Intervention (323) |
Ad26.COV2.S | NR | NR | NR | NR | NR | |
| Control (163) |
Placebo | NR | NR | NR | NR | NR | ||
| Sadoff 2021b | Intervention (21,895) |
Ad26.COV2.S | NR | NR | NR | NR | NR | |
| Control (21,888) |
Placebo | NR | NR | NR | NR | NR | ||
| Logunov 2021 | Intervention (16,427) |
Gam‐COVID‐Vac | NR | NR | NR | NR | 6 | |
| Control (5435) |
Placebo | NR | NR | NR | NR | 1 | ||
| Inactivated virus | Ella 2021a | Intervention (99) |
BBV152 | NR | NR | NR | NR | NR |
| Control (73) |
Adjuvant | NR | NR | NR | NR | NR | ||
|
Ella 2021b |
Intervention (12,879) |
BBV152 | NR | 1 death due to cerebellar haemorrhage; 1 death due to haemorrhagic stroke | NR | NR | NR | |
| Control (12,874) | Adjuvant | NR | NR | NR | NR | NR | ||
| Zhang 2021 | Intervention (24) |
CoronaVac | NR | NR | NR | NR | NR | |
| Control (24) | Adjuvant | NR | NR | NR | NR | NR | ||
| Zhang 2021 | Intervention | CoronaVac | NR | NR | NR | NR | NR | |
| Control | Adjuvant | NR | NR | NR | NR | NR | ||
| Bueno 2021a | Intervention (270) |
CoronaVac | NR | NR | NR | NR | NR | |
| Control (164) |
Adjuvant | NR | NR | NR | NR | NR | ||
| Han 2021 | Intervention (217) |
CoronaVac | NR | NR | NR | NR | NR | |
| Control (114) |
Adjuvant | NR | NR | NR | NR | NR | ||
| Palacios 2020 | Intervention (6202) | CoronaVac | NR | NR | NR | NR | NR | |
| Control (6194) |
Adjuvant | NR | NR | NR | NR | NR | ||
| Wu 2021a | Intervention (124) |
CoronaVac | NR | NR | NR | NR | NR | |
| Control (74) |
Adjuvant | NR | NR | NR | NR | NR | ||
| Al Kaabi 2021 | Intervention (13,464) |
WIV04 | NR | NR | NR | NR | NR | |
| Intervention (13,471) |
HBO2 | NR | NR | NR | NR | NR | ||
| Control (13,453) |
Adjuvant | NR | NR | NR | NR | NR | ||
|
Tanriover 2021 |
Intervention (6646) |
CoronaVac | NR | NR | NR | NR | NR | |
| Control (3568) | Adjuvant | NR | NR | NR | NR | NR | ||
| Fadlyana 2021 | Intervention (405) |
CoronaVac | NR | NR | NR | NR | NR | |
| Control (135) |
Adjuvant | NR | NR | NR | NR | NR | ||
| Xia 2020 | Intervention (84) | WIBP‐CorV | NR | NR | NR | NR | NR | |
| Control (28) |
Adjuvant | NR | NR | NR | NR | NR | ||
| Xia 2021 | Intervention (252) | BBIBP‐CorV | NR | NR | NR | NR | NR | |
| Control (252) |
Adjuvant | NR | NR | NR | NR | NR | ||
| Guo 2021 | Intervention (84) |
WIBP‐CorV | NR | NR | NR | NR | NR | |
| Control (28) | Adjuvant | NR | NR | NR | NR | NR | ||
| Protein subunit |
Formica 2021 | Intervention (258) |
NVX‐CoV2373 | NR | NR | NR | NR | 3 |
| Control (255) |
Placebo | NR | NR | NR | NR | 1 | ||
| Keech 2020 | Intervention (29) |
NVX‐CoV2373 | NR | NR | NR | NR | NR | |
| Control (23) |
Placebo | NR | NR | NR | NR | NR | ||
| Shinde 2021 | Intervention (484) |
NVX‐CoV2373 | NR | NR | NR | 1 | NR | |
| Control (484) |
Placebo | NR | NR | NR | 0 | NR | ||
| Heath 2021 | Intervention (7569) |
NVX‐CoV2373 | 72 blood and lymphatic system disorders | NR | NR | NR | NR | |
| Control (7570) |
Placebo | 61 blood and lymphatic system disorders | NR | NR | NR | NR | ||
| Dunkle 2021 | Intervention (19,965) |
NVX‐CoV2373 | 1 | 2 | 1 | 3 | 53 | |
| Control (9984) |
Placebo | 0 | 1 | 0 | 0 | 13 | ||
| Toledo‐Romani 2021 | Intervention (14,675) |
FINLAY‐FR‐2 | NR | NR | NR | NR | NR | |
| Intervention (14,679) |
FINLAY‐FR‐2/boost FR‐1 | NR | NR | NR | NR | NR | ||
| Control (14,677) |
Placebo | NR | NR | NR | NR | NR | ||
|
Homologous versus heterologous scheme |
Li 2021a | Intervention (51) |
CoronaVac/Ad5 | NR | NR | NR | NR | NR |
| Control (50) |
CoronaVac | NR | NR | NR | NR | NR | ||
| Liu 2021 | Intervention (115) |
ChAdOx1/BNT162b2 | NR | NR | NR | NR | NR | |
| Control (114) |
ChAdOx1/ChAdOx1 | NR | NR | NR | NR | NR | ||
| Intervention (119) |
BNT162b2/ChAdOx1 | NR | NR | NR | NR | NR | ||
| Control (115) |
BNT162b2/BNT162b2 | NR | NR | NR | NR | NR | ||
|
Homologous or heterologous booster versus heterologous booster |
Bonelli 2021 | Intervention (27) |
ChAdOx1 booster | 0 | NR | NR | NR | NR |
| Control (28) |
BNT162b2 or mRNA‐1273 booster | 0 | NR | NR | NR | NR | ||
| Sablerolles 2021 | Control (105) |
Ad26.COV2.S/no boost | NR | NR | NR | NR | NR | |
| Intervention (106) |
Ad26/booster | NR | NR | NR | NR | NR | ||
| Intervention (112) |
Ad26/booster mRNA‐1273 | NR | NR | NR | NR | NR | ||
| Intervention (111) |
Ad26/booster BNT162b2 | NR | NR | NR | NR | NR | ||
| Mok 2021 | Intervention (30) |
CoronaVac/booster | NR | NR | NR | NR | NR | |
| Control (30) |
CoronaVac/booster BNT162b2 | NR | NR | NR | NR | NR | ||
| Li 2021a | Intervention (96) |
CoronaVac/booster Ad5 | NR | NR | NR | NR | NR | |
| Control (102) | CoronaVac/booster | NR | NR | NR | NR | NR |
NR: not reported; RNA: ribonucleic acid.
Neurological events
| Type of vaccine | Study ID |
Arms (number analyzed) |
Intervention | Nervous system diseases |
| RNA‐based vaccine | Thomas 2021 | Intervention (21,926) |
BNT162b2 |
NR |
| Control (21,921) |
Placebo |
NR | ||
|
Frenck 2021 |
Intervention (1131) |
BNT162b2 |
NR | |
| Control (1129) |
Placebo |
NR | ||
| Walsh 2020 | Intervention (24) |
BNT162b2 |
NR | |
| Control (18) |
Placebo |
NR | ||
|
El Sahly 2021 |
Intervention (15,166) |
mRNA‐1273 | 2 embolic stroke; 0 ischaemic stroke | |
| Control (15,151) |
Placebo | 0 embolic stroke; 1 ischaemic stroke | ||
|
Ali 2021 |
Intervention (2482) |
mRNA‐1273 | NR | |
| Control (1238) |
Placebo | NR | ||
| Kremsner 2021 | Intervention (2002) |
CVnCoV | NR | |
| Control (1980) |
Placebo | NR | ||
|
Hall 2021 |
Intervention (60) | mRNA‐1273 boost | NR | |
| Control (59) |
mRNA‐1273/placebo | NR | ||
| Non‐replicating viral vector | Madhi 2021b | Intervention (52) |
ChAdOx1 | 16 |
| Control (52) |
Placebo | 10 | ||
| Falsey 2021 | Intervention (21,587) |
ChAdOx1 | 34 paresthesia | |
| Control (10,792) |
Placebo | 16 paresthesia | ||
| Voysey 2021a | Intervention (12,021) |
ChAdOx1 | 1 ischaemic stroke | |
| Control (11,724) |
Placebo | 0 ischaemic stroke | ||
| Asano 2022 | Intervention (192) |
ChAdOx1 | NR | |
| Control (64) |
Placebo | NR | ||
| Kulkarni 2021 | Intervention (300) |
SII‐ChAdOx1 | NR | |
| Control (100) |
ChAdOx1 | NR | ||
| Sadoff 2021a | Intervention (323) |
Ad26.COV2.S | NR | |
| Control (163) |
Placebo | NR | ||
|
Sadoff 2021b |
Intervention (21,895) |
Ad26.COV2.S | NR | |
| Control (21,888) |
Placebo | NR | ||
| Logunov 2021 | Intervention (16,427) |
Gam‐COVID‐Vac | 0 haemorrhagic stroke; 1 paraesthesia | |
| Control (5435) |
Placebo | 1 haemorrhagic stroke; 1 paraesthesia | ||
| Inactivated virus | Ella 2021a | Intervention (99) |
BBV152 | NR |
| Control (73) |
Adjuvant | NR | ||
|
Ella 2021b |
Intervention (12,879) |
BBV152 | NR | |
| Control (12,874) | Adjuvant | NR | ||
| Zhang 2021 | Intervention (24) |
CoronaVac | NR | |
| Control (24) | Adjuvant | NR | ||
| Zhang 2021 | Intervention | CoronaVac | NR | |
| Control | Adjuvant | NR | ||
| Bueno 2021 | Intervention (270) |
CoronaVac | NR | |
| Control (164) |
Adjuvant | NR | ||
| Han 2021 | Intervention (217) |
CoronaVac | NR | |
| Control (114) |
Adjuvant | NR | ||
| Palacios 2020 | Intervention (6202) | CoronaVac | NR | |
| Control (6194) |
Adjuvant | NR | ||
| Wu 2021a | Intervention (124) |
CoronaVac | NR | |
| Control (74) |
Adjuvant | NR | ||
| Al Kaabi 2021 | Intervention (13,464) |
WIV04 Al Kaabi | NR | |
| Intervention (13,471) |
HBO2 | NR | ||
| Control (13,453) |
Adjuvant | NR | ||
| Tanriover 2021 |
Intervention (6646) | CoronaVac | NR | |
| Control (3568) | Adjuvant | 1 acute cerebellar infarction | ||
| Fadlyana 2021 | Intervention (405) | CoronaVac | 51 | |
| Control (135) |
Adjuvant | 20 | ||
| Xia 2020 | Intervention (84) | WIBP‐CorV | NR | |
| Control (28) |
Adjuvant | NR | ||
| Xia 2021 | Intervention (252) | BBIBP‐CorV | NR | |
| Control (252) |
Adjuvant | NR | ||
| Guo 2021 | Intervention (84) |
WIBP‐CorV | NR | |
| Control (28) |
Adjuvant | NR | ||
| Protein subunit | Formica 2021 | Intervention (258) |
NVX‐CoV2373 | 5 |
| Control (255) |
Placebo | 4 | ||
| Keech 2020 | Intervention (29) |
NVX‐CoV2373 | NR | |
| Control (23) |
Placebo | NR | ||
| Shinde 2021 | Intervention (484) |
NVX‐CoV2373 | 0 | |
| Control (484) |
Placebo | 1 | ||
| Heath 2021 | Intervention (7569) |
NVX‐CoV2373 | 32 | |
| Control (7570) |
Placebo | 31 | ||
| Dunkle 2021 | Intervention (19,965) |
NVX‐CoV2373 | 2 stroke | |
| Control (9984) |
Placebo | 0 stroke | ||
| Toledo‐Romani 2021 | Intervention (14,675) |
FINLAY‐FR‐2 | NR | |
| Intervention (14,679) |
FINLAY‐FR‐2/boostwe FR‐1 | NR | ||
| Control (14,677) |
Placebo | NR | ||
|
Homologous versus heterologous scheme |
Li 2021a | Intervention (51) |
CoronaVac/Ad5 | NR |
| Control (50) |
CoronaVac | NR | ||
| Liu 2021 |
Intervention (115) |
ChAdOx1/BNT162b2 | NR | |
| Control (114) |
ChAdOx1/ ChAdOx1NR |
NR | ||
| Intervention (119) |
BNT162b2/ChAdOx1 | NR | ||
| Control (115) |
BNT162b2/BNT162b2 | NR | ||
|
Homologous or heterologous booster versus heterologous booster |
Bonelli 2021 | Intervention (27) |
ChAdOx1 booster | 0 neurological complications |
| Control (28) |
BNT162b2 or mRNA‐1273 booster | 0 neurological complications | ||
| Sablerolles 2021 | Control (105) |
Ad26.COV2.S/no booster | NR | |
| Intervention (106) |
Ad26/booster | NR | ||
| Intervention (112) |
Ad26/booster mRNA‐1273 | NR | ||
| Intervention (111) |
Ad26/booster BNT162b2 | NR | ||
| Mok 2021 | Intervention (30) |
CoronaVac/booster | NR | |
| Control (30) |
CoronaVac/booster BNT162b2 | NR | ||
| Li 2021a | Intervention (96) |
CoronaVac/boost Ad5 | NR | |
| Control (102) | CoronaVac/booster | NR | ||
| NR: not reported; RNA: ribonucleic acid. | ||||
Appendix 13. mRNA‐1273 – ModernaTX versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | 2 | 31,632 | N/A | N/A | 73.27% (35.82% to 88.87%) |
| Confirmed symptomatic COVID‐19 after complete vaccination | 2 | 31,632 | N/A | N/A | 93.20% (91.06% to 94.83%) |
| Severe or critical COVID‐19 after complete vaccination | 1 | 28,451 | N/A | N/A | 98.20% (92.80% to 99.60%) |
| All‐cause mortality | 1 | 30,346 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 (0.54 to 2.10) | N/A |
| Serious adverse events | 2 | 34,072 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 (0.78 to 1.08) | N/A |
| Systemic reactogenicity events | 2 | 34,037 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 (1.22 to 1.34) | N/A |
| Any adverse event | 2 | 34,072 | N/A | Outcome not pooled due to considerable heterogeneity | N/A |
| Local reactogenicity events | 2 | 34,037 | Risk Ratio (M‐H, Random, 95% CI) | 3.30 (2.02 to 5.40) | N/A |
| CI: confidence interval; N/A: not applicable. | |||||
Appendix 14. CVnCoV – CureVac AG versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | 1 | 25,062 | N/A | N/A | 48.20% (31.70% to 60.90%) |
| Severe or critical COVID‐19 after complete vaccination | 1 | 25,062 | N/A | N/A | 63.80% (0.00% to 91.70%) |
| All‐cause mortality | 1 | 39,529 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 (0.46 to 3.83) | N/A |
| Serious adverse events | 1 | 39,529 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 (0.90 to 1.71) | N/A |
| Systemic reactogenicity events | 1 | 3982 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 (1.43 to 1.53) | N/A |
| Any adverse event | 1 | 3982 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 (1.38 to 1.47) | N/A |
| Local reactogenicity events | 1 | 3982 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 (3.24 to 3.81) | N/A |
| CI: confidence interval; N/A: not applicable | |||||
Appendix 15. ChAdOx1/SII‐ChAdOx1 – AstraZeneca + University of Oxford/Serum Institute of India versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | 5 | 43,390 | N/A | N/A | 59.35% (48.00% to 68.22%) |
| Confirmed symptomatic COVID‐19 after complete vaccination | 5 | 43,390 | N/A | N/A | 70.23% (62.10% to 76.62%) |
| Severe or critical COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| All‐cause mortality | 5 | 56,727 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 (0.20 to 1.14) | N/A |
| Serious adverse events | 7 | 58,182 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 (0.72 to 1.07) | N/A |
| Systemic reactogenicity events | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 3.93 (2.11 to 7.29) | N/A |
| Any adverse event | 7 | 57,580 | Not pooled | N/A | |
| Local reactogenicity events | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 6.44 (2.98 to 13.92) | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 16. Specific antibody geometric mean titre
Non‐replicant viral vector vaccines
| Study | Intervention name | Results | Unit of analysis | Time point | Type of assay | Population | ||
| GMT (95% CI) | GMR (95% CI) | |||||||
| COVID‐19 vaccine versus placebo/other no COVID‐19 vaccine | ||||||||
|
Voysey 2021a |
ChAdOx1 at < 6 weeks interval |
219.66 (197.53 to 244.27) |
296.83 (245.86 to 358.37) |
ELISA units | 28 days after second dose | Multiplexed immunoassay/RBD‐binding IgG | 18–55 years | |
| MenACWY vaccine/placebo | 74 (63 to 86) | |||||||
| Voysey 2021a | ChAdOx1 at < 6 weeks interval |
188.59 (169.00 to 210.46) |
471.47 (395.69 to 561.77) |
ELISA units | 28 days after second dose | Multiplexed immunoassay/RBD‐binding IgG | ≥ 56 years | |
| MenACWY vaccine/placebo | 40 (35 to 46) |
|||||||
| Logunov 2021 | Gam‐COVID‐Vac rAd26‐S | 8996 (7610 to 10 635) |
294.46 (188.27 to 460.56) | Not reported | 21 days after second dose |
ELISA RBD‐binding IgG |
≥ 18 | |
| Placebo | 30.55 (20.18 to 46.26) | |||||||
| COVID‐19 vaccine versus COVID‐19 vaccine | ||||||||
| Kulkarni 2021 | SII‐ChAdOx1 | 9636.70 (7983.70 to 11,631.90) |
1.52 (1.03 to 2.26) | Arbitrary units (AU)/mL |
28 days after dose 1 | ELISpot assay RBD‐binding IgG |
≥ 18 | |
| ChAdOx1 | 6311.20 (4470.10 to 8910.60) |
|||||||
CI: confidence interval; ELISA: enzyme‐linked immunosorbent assay; GMR: geometric mean rate; GMT: geometric mean titre; IgG: immunoglobulin G; RBD: receptor‐binding domain.
Inactivated virus vaccines
| Study | Intervention name | Results |
Units of analysis |
Time point | Type of assay | Population | |
| GMT (95% CI) | GMR (95% CI) | ||||||
| COVID‐19 vaccine versus adjuvant/placebo | |||||||
|
Bueno 2021 |
CoronaVac | 10.10 (7.28 to 14.01) |
1.84 (1.32 to 2.55) | Not reported | 2 weeks after 2nd dose | A SINOVAC standardized microtitre methodology, conventional virus neutralization | ≥ 18 years |
| Adjuvant |
5.48 (1.84 to 16.29) |
||||||
|
Zhang 2021 |
CoronaVac | 5.60 (3.60 to 8.70) | 2.80 (1.80 to 4.25) | Not reported | 2 weeks after 2nd dose | Micro‐cytopathogenic effect assay | Phase 1: healthy and aged 18–59 years |
| Placebo | 2 (2 to 2) | ||||||
| Zhang 2021 | CoronaVac | 27.60 (22.70 to 33.5) | 11.90 (10.23 to 13.83) | Not reported | 2 weeks after 2nd dose | Micro‐cytopathogenic effect assay | Phase 2: healthy and aged 18–59 years |
| Placebo | 2 (2 to 2) | ||||||
|
Han 2021 |
CoronaVac | 142.20 (124.70 to 162.10) | 67.71 (59.25 to 77.37) | Not reported | 28 days after 2nd dose | Micro‐cytopathogenic effect assay | Phase 2: 3–17 years |
| Placebo | 2.10 (2 to 2.10) | ||||||
|
Wu 2021a |
CoronaVac | 42.20 (35.20 to 50.60) |
20.09 (16.73 to 24.13) | Not reported | 28 days after 2nd dose | Micro‐cytopathogenic effect assay | Phase 2: aged ≥ 60 years |
| Adjuvant | 2.10 (2 to 2.10) | ||||||
| Fadlyana 2021 | CoronaVac | 15.76 (14.57 to 17.04) | 7.80 (7.20 to 8.45) | Not reported | 14 days after 2nd dose | Not clear | 18–59 years |
| Placebo | 2.02 (1.98 to 2.05) | ||||||
|
Al Kaabi 2021 |
WIBP‐CorV | 94.50 (89.70 to 99.50) | 35 (32.83 to 37.30) | Not reported | 14 days after 2nd dose | Not reported | ≥ 18 years |
| Placebo | 2.70 (2.60 to 2.80) | ||||||
| Al Kaabi 2021 | BBIBP‐CorV | 156 (149.60 to 162.70) | 57.77 (54.63 to 61.10) | Not reported | 14 days after 2nd dose | Not reported | ≥ 80 years |
| Placebo | 2.70 (2.60 to 2.80) | ||||||
| Guo 2021 | WIBP‐CorV |
134 (104 to 174) |
26.80 (20.71 to 34.66) | Not reported | 28 days after whole course vaccination | Plaque reduction neutralization test (PRNT) |
18–59 years |
| Adjuvant |
5 (5 to 5) |
||||||
| Xia 2020 | BBIBP‐CorV |
218.90 (165.60 to 289.50) |
109.45 (82.77 to 144.73) | Not reported | 14 days after lst inoculation | Not reported | Phase 2 ≥ 18 years |
| Placebo | 2 (2 to 2) |
||||||
|
Xia 2021 |
BBIBP‐CorV | 180.20 (163.60 to 198.40) |
90.10 (81.81 to 99.22) | Not reported | 28 days after 2nd inoculation | Not reported | 3–5 years |
| Adjuvant | 20 (20 to 20) |
||||||
| BBIBP‐CorV | 168.60 (151.90 to 187) |
84.30 (75.97 to 93.53) | Not reported | 28 days after 2nd inoculation | Not reported | 6–12 years | |
| Adjuvant | 2 (2 to 2) |
||||||
| BBIBP‐CorV | 155.70 (137.70 to 176.50) |
77.87 (68.71 to 88.24) | Not reported | 28 days after 2nd inoculation | Not reported | 13–17 years | |
| Adjuvant | 2 (2 to 2) |
||||||
| Ella 2021b | BBV152 | 125.60 (111.20 to 141.80) |
9.16 (2.28 to 36.78) | Not reported | 28 days after 2nd vaccination | MNT50 assay |
≥ 18 years |
| Adjuvant | 13.70 (10.70 to 170.40) |
||||||
| Ella 2021a | BBV152 | 66.40 (53.40 to 82.40) |
9.22 (7.25 to 11.80) | Not reported | Day 28 | MNT50 assay |
18–55 years |
| Adjuvant | 7.20 (6.40 to 8.10) |
||||||
CI: confidence interval; GMR: geometric mean rate; GMT: geometric mean titre.
Protein subunit vaccines
| Study | Intervention name | Results | Unit of analysis | Time point | Type of assay | Population | ||
| GMT (95% CI) | GMR (95% CI) | |||||||
| COVID‐19 vaccine versus placebo | ||||||||
|
Keech 2020 |
NVX‐CoV2373 | 1984.20 (1405.80 to 2800.70) | 18.08 (12.18 to 26.85) | EU/mL | Day 21 after 1st dose | ELISA RBD‐binding IgG |
18–59 years | |
| Placebo | 109.70 (90.40 to 133.20) | |||||||
|
Formica 2021 |
NVX‐CoV2373 | 44,420.90 (37,929.10 to 52,023.80) | 352.26 (290 to 427.89) | EU/mL | Day 35 (14 days after the 2nd dose) |
ELISA | 18–84 years | |
| Placebo | 126.10 (114 to 139.40) | |||||||
CI: confidence interval; ELISA: enzyme‐linked immunosorbent assay; GMR: geometric mean rate; GMT: geometric mean titre; IgG: immunoglobulin G; RBD: receptor‐binding domain.
Primary series heterologous vaccination scheme versus homologous vaccination scheme
| Study | Intervention name | Results | Unit of analysis | Time point | Type of assay | Population | |
| GMT (95% CI) | GMR (95% CI) | ||||||
| Heterologous schedule versus homologous schedule | |||||||
|
Li 2021a |
CoronaVac/Ad5 | 941.80 (663.90 to 1336.10) | 6.11 (3.90 to 9.57) | Not reported | 14 days after 2nd dose | ELISA | 18–59 years |
| CoronaVac | 154.10 (116.30 to 204.30) | ||||||
CI: confidence interval; GMR: geometric mean rate; GMT: geometric mean titre.
Boosters
| Study | Intervention name | Results | Unit of analysis | Time point | Type of assay | Population | |
| GMT (95% CI) | GMR (95% CI) | ||||||
| Heterologous booster versus homologous booster | |||||||
|
Li 2021a |
CoronaVac/Ad5 booster | 3090.10 (2636.10 to 3622.30) | 8.37 (6.52 to 10.75) | Not reported | 14 days after boost | ELISA | 18–59 years |
| CoronaVac/CoronaVac boost | 369 (304.20 to 447.50) | ||||||
CI: confidence interval; GMR: geometric mean rate; GMT: geometric mean titre.
Appendix 17. ChAdOx1 – AstraZeneca + University of Oxford versus SII‐ChAdOx1 – Serum Institute of India
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Severe or critical COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| All‐cause mortality | N/A | N/A | N/A | N/A | N/A |
| Serious adverse events | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 (0.08 to 2.95) | N/A |
| Systemic reactogenicity events | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 (0.54 to 0.98) | N/A |
| Any adverse event | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 (0.52 to 1.33) | N/A |
| Local reactogenicity events | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 (0.55 to 1.05) | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 18. Ad26.COV2.S – Janssen Pharmaceutical Companies versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | 1 | 39,058 | N/A | N/A | 66.90% (59.10% to 73.40%) |
| Severe or critical COVID‐19 after complete vaccination | 1 | 39,058 | N/A | N/A | 76.30% (57.90% to 87.50%) |
| All‐cause mortality | 1 | 43,783 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 (0.09 to 0.67) | N/A |
| Serious adverse events | 1 | 43,783 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 (0.69 to 1.22) | N/A |
| Systemic reactogenicity events | 2 | 7222 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 (1.29 to 2.60) | N/A |
| Any adverse event | 2 | 7222 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 (0.75 to 3.29) | N/A |
| Local reactogenicity events | 2 | 7222 | Risk Ratio (M‐H, Random, 95% CI) | 3.27 (1.91 to 5.62) | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 19. Gam‐COVID‐Vac (Sputnik V) – Gamaleya Research Institute versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | 1 | 18,695 | N/A | N/A | 91.10% (83.80% to 95.10%) |
| Severe or critical COVID‐19 after complete vaccination | 1 | 19,866 | N/A | N/A | 100.00% (94.40% to 100.00%) |
| All‐cause mortality | 1 | 21,862 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 (0.10 to 9.54) | N/A |
| Serious adverse events | 1 | 21,862 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 (0.39 to 1.07) | N/A |
| Systemic reactogenicity events | N/A | N/A | N/A | N/A | N/A |
| Any adverse event | N/A | N/A | N/A | N/A | N/A |
| Local reactogenicity events | N/A | N/A | N/A | N/A | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 20. Cellular immune response
| Study | Intervention name | Estimate effect | Unit of analysis | Time point | Type of assay | Population | |
| Ella 2021a | Intervention: BBV152 | Median (IQR) | 55N (22 to 173.80) | Number of SFCs per million PBMCs | 28‐D1 | IFN‐γ ELISpot | 18–55 years |
| Control: placebo | Median (IQR) | 3 (1 to 23) | |||||
| Logunov 2021 | Intervention: Gam‐COVID‐Vac | Median (IQR) | 32.77 (13.94 to 50.76) | IFN‐γ concentration pg/mL | 28‐D1 | IFN‐γ measured by ELISA | ≥ 18 years |
| Control: placebo | Median (IQR) | 0.41 (0.11 to 0.85) | |||||
| Liu 2021 | Intervention: ChAdOx1/ BNT162b2 | Geometric mean ratio (95% CI) |
3.90 (95% CI 2.90 to 5.30) |
Number of spot‐forming cells (SFCs) per million PBMCs | 28‐D2 | IFN‐γ ELISpot | ≥ 50 years |
| Control: ChAdOx1/ChAdOx1 | |||||||
| Intervention: BNT162b2/ChAdOx1 | 1.20 (95% CI 0.87 to 1.70) | ||||||
| Control: BNT162b2/ BNT162b2 | |||||||
| Hall 2021 | Intervention: mRNA‐1273/mRNA‐1273 boost | Median | 432 versus 67; 95% CI for the between‐group difference, 46 to 986 | T‐cell counts – cells per million CD4+ T cells | 28‐D3 | Intracellular cytokine staining | Transplant recipients only |
| Control: mRNA‐1273/placebo boost | Median | ||||||
| Bonelli 2021 | Intervention: BNT162b2 or mRNA‐1273/ChAdOx1 boost | Median (IQR) | 459 (133 to 722) | Number of SFCs per million PBMCs | 7‐D3 | IFN‐γ ELISpot | People currently receiving rituximab |
| Control: BNT162b2 or mRNA‐1273/BNT162b2 or mRNA‐1273 boost | Median (IQR) | 305 (171 to 416) | |||||
| Zhang 2021 | Intervention: CoronaVac | Median (Min, Max) | 5.50 (0 to 35.70) | Number of SFCs per million PBMCs | 14‐D2 | IFN‐γ ELISpot | 18–59 years |
| Control: placebo | Median (Min, Max) | 0 (0 to 11.70) | |||||
| Sablerolles 2021 | Intervention: Ad26.COV2.S/mRNA‐1273 boost | Percentage of responders | 44/48 (91.66%) versus 32/44 (72.72%) (RR 0.79, 95% CI 0.64 to 0.96, P = 0.01726) | Number of responders (responder cut‐off is 0.15 IU/mL) | 28‐D2 | IFN‐y release assay | 18–65 years |
| Control: Ad26.COV2.S/Ad26.COV2.S boost | |||||||
| Intervention: Ad26.COV2.S/BNT162b2 boost | Percentage of responders |
43/47 (91.48%) versus 32/44 (72.72%) (RR 0.79, 95% CI 0.65 to 0.97, P = 0.01946) | |||||
| Control: Ad26.COV2.S/Ad26.COV2.S boost | |||||||
| Intervention: Ad26.COV2.S/BNT162b2 boost | Percentage of responders | 43/47 (91.48%) versus 44/48 (91.66%) (RR 1.00, 95% CI 0.88 to 1.13, P = 0.9753) | |||||
| Control: Ad26.COV2.S/mRNA‐1273 boost | |||||||
IFN: interferon; IQR: interquartile range; min: minimum; max: maximum; PBMC: peripheral blood mononuclear cell; RR: risk ratio; SFC: spot‐forming cell
Appendix 21. CoronaVac – Sinovac versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | 2 | 19,852 | N/A | N/A | 69.81% (12.27% to 89.61%) |
| Severe or critical COVID‐19 after complete vaccination | 2 | 19,852 | N/A | N/A | N/A |
| All‐cause mortality | 1 | 12,396 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 (0.05 to 5.52) | N/A |
| Serious adverse events | 4 | 23,139 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 (0.62 to 1.51) | N/A |
| Systemic reactogenicity events | 6 | 23,956 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 (0.55 to 1.62) | N/A |
| Any adverse event | 6 | 23,367 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 (1.07 to 1.11) | N/A |
| Local reactogenicity events | 6 | 23,962 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 (1.69 to 1.82) | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 22. WIBP‐CorV – Sinopharm‐Wuhan versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | 1 | 25,449 | N/A | N/A | 64.00% (48.80% to 74.70%) |
| Confirmed symptomatic COVID‐19 after complete vaccination | 1 | 25,480 | N/A | N/A | 72.80% (58.10% to 82.40%) |
| Severe or critical COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| All‐cause mortality | N/A | N/A | N/A | N/A | N/A |
| Serious adverse events | 2 | 27,029 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 (0.60 to 1.15) | N/A |
| Systemic reactogenicity events | 2 | 27,029 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 (0.95 to 1.03) | N/A |
| Any adverse event | 2 | 27,029 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 (0.93 to 0.98) | N/A |
| Local reactogenicity events | 2 | 27,029 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 (0.85 to 0.92) | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 23. BBIBP‐CorV – Sinopharm‐Beijing versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | 1 | 25,435 | N/A | N/A | 73.50% (60.60% to 82.20%) |
| Confirmed symptomatic COVID‐19 after complete vaccination | 1 | 25,463 | N/A | N/A | 78.10% (64.80% to 86.30%) |
| Severe or critical COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| All‐cause mortality | N/A | N/A | N/A | N/A | N/A |
| Serious adverse events | 1 | 26,924 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 (0.54 to 1.06) | N/A |
| Systemic reactogenicity events | 3 | 27,540 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 (0.86 to 1.28) | N/A |
| Any adverse event | 3 | 27,540 | – | Not pooled due to high heterogeneity | N/A |
| Local reactogenicity events | 3 | 27,540 | – | Not pooled due to high heterogeneity | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 24. BBV152 – Bharat Biotech versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | 1 | 6289 | N/A | N/A | 68.80% (46.70% to 82.50%) |
| Confirmed symptomatic COVID‐19 after complete vaccination | 1 | 16,973 | N/A | N/A | 77.80% (65.20% to 86.40%) |
| Severe or critical COVID‐19 after complete vaccination | 1 | 16,976 | N/A | N/A | 93.40% (57.10% to 99.80%) |
| All‐cause mortality | 1 | 25,753 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 (0.17 to 1.46) | N/A |
| Serious adverse events | 1 | 25,753 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 (0.43 to 0.97) | N/A |
| Systemic reactogenicity events | 2 | 25,925 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 (1.15 to 1.58) | N/A |
| Any adverse event | 1 | 25,753 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 (0.94 to 1.07) | N/A |
| Local reactogenicity events | 2 | 25,750 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 (0.95 to 1.24) | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 25. NVX‐CoV2373 – Novavax versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | 3 | 42,175 | N/A | N/A | 82.91% (50.49% to 94.10%) |
| Severe or critical COVID‐19 after complete vaccination | 1 | 25,452 | N/A | N/A | 100.00% (86.99% to 100.00%) |
| All‐cause mortality | 1 | 29,582 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 (0.30 to 2.68) | N/A |
| Serious adverse events | 4 | 46,202 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 (0.74 to 1.14) | N/A |
| Systemic reactogenicity events | 3 | 31,063 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 (1.17 to 1.25) | N/A |
| Any adverse event | 5 | 46,231 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 (1.05 to 1.26) | N/A |
| Local reactogenicity events | 3 | 31,063 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 (1.99 to 3.88) | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 26. FINLAY‐FR‐2 – Instituto Finlay de Vacunas versus placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | 1 | 28,674 | N/A | N/A | 71.00% (58.90% to 79.10%) |
| Severe or critical COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| All‐cause mortality | 1 | 28,674 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 (0.17 to 0.80) | N/A |
| Serious adverse events | N/A | N/A | N/A | N/A | N/A |
| Systemic reactogenicity events | N/A | N/A | N/A | N/A | N/A |
| Any adverse event | N/A | N/A | N/A | N/A | N/A |
| Local reactogenicity events | N/A | N/A | N/A | N/A | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 27. Heterologous vaccination scheme versus homologous vaccination scheme
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Severe or critical COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| All‐cause mortality | N/A | N/A | N/A | N/A | N/A |
| Serious adverse events | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 (0.01 to 8.17) | N/A |
| Systemic reactogenicity events | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 (0.52 to 7.41) | N/A |
| Any adverse event | 3 | N/A | Risk Ratio (M‐H, Random, 95% CI) Not pooled |
1.03 (0.75 to 1.43) 1.21 (0.87 to 1.68) 3.19 (1.11 to 9.11) |
N/A |
| Local reactogenicity events | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 11.76 (1.59 to 87.14) | N/A |
CI: confidence interval; N/A: not applicable.
Appendix 28. Booster versus placebo/no booster
| Outcome | No. of studies | No. of participants | Statistical method | Effect size | Vaccine efficacy (95% CI) |
| Confirmed SARS‐CoV‐2 infection after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Confirmed symptomatic COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| Severe or critical COVID‐19 after complete vaccination | N/A | N/A | N/A | N/A | N/A |
| All‐cause mortality | 1 | 28,254 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 (0.52 to 3.05) | N/A |
| Serious adverse events | N/A | N/A | N/A | N/A | N/A |
| Systemic reactogenicity events | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 (0.71 to 4.56) | N/A |
| Any adverse event | N/A | N/A | N/A | N/A | N/A |
| Local reactogenicity events | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 6.46 (3.18 to 13.13) | N/A |
CI: confidence interval; N/A: not applicable.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Al Kaabi 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Asano 2022.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Bonelli 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Bueno 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Clemens 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Dunkle 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Ella 2021a.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Ella 2021b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
El Sahly 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Emary 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Fadlyana 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Falsey 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Formica 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Frenck 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Guo 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Hall 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Han 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Heath 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Keech 2020.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Kremsner 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Kulkarni 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Li 2021a.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Liu 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Logunov 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Madhi 2021a.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Madhi 2021b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Mok 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Palacios 2020.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Sablerolles 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Sadoff 2021a.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Sadoff 2021b.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Shinde 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Tanriover 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Thomas 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Toledo‐Romani 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Voysey 2021a.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Walsh 2020.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Wu 2021a.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Xia 2020.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Xia 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Zhang 2021.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baden 2021 | Exploratory analysis |
| Barrett 2021 | Secondary analysis |
| Ewer 2021 | Secondary analysis |
| Flaxman 2021 | Not randomized |
| Hsieh 2021 | Not randomized |
| Irfan 2021 | Commentary |
| Lazarus 2021 | Intervention not relevant to review |
| Patamatamkul 2021 | Not randomized |
| Ward 2021a | Intervention not relevant to review |
| Wu 2021b | Not randomized |
| Zdanowski 2021 | Not randomized |
Differences between protocol and review
The protocol was developed early during the pandemic and is evolving.
We are no longer considering the following less relevant outcomes.
Incidence of confirmed symptomatic COVID‐19 after first dose (confirmed with positive test for SARS‐CoV‐2 infection by RT‐PCR or NAAT or any other validated test)
Incidence of participants with confirmed SARS‐CoV‐2 infection after first dose (confirmed by RT‐PCR or NAAT or any other validated test (symptomatic or asymptomatic))
Incidence of withdrawals due to adverse events
We clarified some outcomes. For the outcome 'specific adverse events' we collected data for 'nervous system diseases' (instead of stroke, headache, delirium, and paraesthesia) since we found this was reported more often. We did not collect data on 'bruising.'
As research and data on COVID‐19 vaccines evolved, we noticed that authors started using the term 'reactogenicity' to define the immediate, short‐in‐duration, and usually expected effects of the vaccine, and to differentiate them from any other medium‐term, long‐term, or unexpected adverse event (related or unrelated to the vaccine). Therefore, we adopted the term to describe local and systemic effects of the vaccine in the immediate days after the injection.
Post‐hoc analysis due to concern related to the waning of efficacy over time (Feikin 2022), we added a post‐hoc analysis of vaccine efficacy according to the delay since vaccination.
We initially planned to conduct an NMA; however, the network of vaccines appeared very sparse, included mainly comparisons of vaccines against placebo, and only one or two studies informed most of the available comparisons (Figure 1). A network of such structure does not allow proper evaluation of the synthesis assumptions. Additionally, the NMA estimates from this network would not be substantially more precise (and could even be less precise for some comparisons) than the direct ones. We decided not to perform a NMA and will revisit its feasibility throughout the living systematic review process.
We obtained clinical study reports (CSRs) after the corresponding publication was available and data were already extracted. When CSRs were available, we cross‐checked whether these provided data on the critical outcomes already extracted or critical outcome not available in the publication. In all cases, we did not obtain new data. The follow‐up of outcome assessment in the CSR was frequently lower than the one reported in the publication. We have not contacted study authors yet for missing results or to request additional information.
Contributions of authors
Conception and design of the review: CG, LG, AC, MM, PA, JDL, LA DD, JJM, GR, AH, GG, DT, PR, IB
Co‐ordination of the review: AC, LG, IB
Search and selection of studies for inclusion in the review: GF, CR, HB, RA
Collection of data for the review: BB, HB, KP, NH, EG, GV, CG, HB, MD, LG, SM
Assessment of the risk of bias in the included studies: BB, HB, KP, NH, EG, GV, CG, HB, MD, LG, IB
Analysis of data: AC, TE
Assessment of the certainty in the body of evidence: KP, HB, NH, GV
Interpretation of data: CG, LG, TE, AJ, SM, HB, BB, KP, GV, NH, HB, RA, SM, MM, DD, PM, JDL, LA, TK, GF, MD, CR, DT, JJM, GG, GR, AH, PR, AC, IB
Writing of and commenting on the review: CG, LG, AJ, AC, TE, BB, KP, NH, GF, CR, PK, HB, JDL, DD, JJM, GR, AH, GG, DT, PR, IB
Sources of support
Internal sources
Cochrane France, France
Center of Research in Epidemiology and Statistics (CRESS), France
Centre d’Epidémiologie Clinique (GHU Cochin, Hôtel Dieu), France
Université de Paris, France
National Institute of Health and Medical Research (Inserm), France
Assistance Publique Hôpitaux de Paris (APHP), France
Centre National de la Recherche Scientifique (CNRS), France
External sources
French Ministry of Health, France
French Ministry of Higher Education, Research and Innovation, France
Agence Nationale de la Recherche (ANR), France
World Health Organization (WHO), Switzerland
-
European Union’s Horizon 2020 Research and Innovation programme under the grant agreement N°101037867, Other
The research funding leading to these results was conducted as part of the VACCELERATE consortium. For further information please refer to https://www.vaccelerate.eu/.
Declarations of interest
Carolina Graña: none known.
Lina Ghosn: none known.
Theodoros Evrenoglou: none known.
Alexander Jarde: none known.
Silvia Minozzi: no relevant interests; Joint Co‐ordinating Editor and Method editor of the Drugs and Alcohol Group.
Hanna Bergman: Cochrane Response – consultant; WHO – grant/contract (Cochrane Response was commissioned by the WHO to perform review tasks that contribute to this publication).
Brian Buckley: none known.
Katrin Probyn: Cochrane Response – consultant; WHO – consultant (Cochrane Response was commissioned to perform review tasks that contribute to this publication).
Gemma Villanueva: Cochrane Response – employment (Cochrane Response has been commissioned by WHO to perform parts of this systematic review).
Nicholas Henschke: Cochrane Response – consultant; WHO – consultant (Cochrane Response was commissioned by the WHO to perform review tasks that contributed to this publication).
Hillary Bonnet: none known.
Rouba Assi: none known.
Sonia Menon: P95 – consultant.
Melanie Marti: no relevant interests; Medical Officer at WHO.
Declan Devane: Health Research Board (HRB) – grant/contract; registered nurse and registered midwife but no longer in clinical practice; Editor, Cochrane Pregnancy and Childbirth Group.
Patrick Mallon: AstraZeneca – Advisory Board; spoken of vaccine effectiveness to media (print, online, and live); works as a consultant in a hospital that provides vaccinations; employed by St Vincent's University Hospital.
Jean‐Daniel Lelievre: no relevant interests; published numerous interviews in the national press on the subject of COVID vaccination; Head of the Department of Infectious Diseases and Clinical Immunology CHU Henri Mondor APHP, Créteil; WHO (IVRI‐AC): expert Vaccelarate (European project on COVID19 Vaccine): head of WP; involved with COVICOMPARE P et M Studies (APHP, INSERM) (public fundings).
Lisa Askie: no relevant interests; Co‐convenor, Cochrane Prospective Meta‐analysis Methods Group.
Tamara Kredo: no relevant interests; Medical Officer in an Infectious Diseases Clinic at Tygerberg Hospital, Stellenbosch University.
Gabriel Ferrand: none known.
Mauricia Davidson: none known.
Carolina Riveros: no relevant interests; works as an epidemiologist.
David Tovey: no relevant interests; Emeritus Editor in Chief, Feedback Editors for 2 Cochrane review groups.
Joerg J Meerpohl: no relevant interests; member of the German Standing Vaccination Committee (STIKO).
Giacomo Grasselli: Pfizer – speaking engagement.
Gabriel Rada: none known.
Asbjørn Hróbjartsson: no relevant interests; Cochrane Methodology Review Group Editor.
Philippe Ravaud: no relevant interests; involved with Mariette CORIMUNO‐19 Collaborative 2021, the Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research, and AP‐HP Foundation.
Anna Chaimani: none known.
Isabelle Boutron: no relevant interests; member of Cochrane Editorial Board.
Edited (no change to conclusions)
References
References to studies included in this review
Ali 2021 {published data only}
- Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. New England Journal of Medicine 2021;385(24):2241-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al Kaabi 2021 {published data only}
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021;326(1):35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asano 2022 {published data only}
- Asano M, Okada H, Itoh Y, Hirata H, Ishikawa K, Yoshida E, et al. Immunogenicity and safety of AZD1222 (ChAdOx1 nCoV-19) against SARS-CoV-2 in Japan: a double-blind, randomized controlled phase 1/2 trial. International Journal of Infectious Diseases 2022;114:165-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonelli 2021 {published data only}
- Bonelli M, Mrak D, Tobudic S, Sieghart D, Koblischke M, Mandl P, et al. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomized controlled trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.09.05.21263125] [DOI] [PubMed]
Bueno 2021 {published data only}
- Bueno SM, Abarca K, González PA, Gálvez NM, Soto JA, Duarte LF, et al. Interim report: safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy Chilean adults in a phase 3 clinical trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.03.31.21254494] [DOI]
- Bueno SM, Abarca K, González PA, Gálvez NM, Soto JA, Duarte LF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in a subgroup of healthy adults in Chile. Clinical Infectious Diseases 2021 Sep 19 [Epub ahead of print]. [DOI: 10.1093/cid/ciab823] [DOI] [PMC free article] [PubMed]
Clemens 2021 {published data only}
- Clemens SA, Folegatti PM, Emary KR, Weckx LY, Ratcliff J, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 lineages circulating in Brazil. Nature Communications 2021;12(1):5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunkle 2021 {published data only}
- Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.10.05.21264567] [DOI] [PMC free article] [PubMed]
Ella 2021a {published data only}
- Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. A Phase 1: safety and immunogenicity trial of an inactivated SARS-CoV-2 vaccine-BBV152. medRxiv 2020 [Preprint]. [DOI: 10.1101/2020.12.11.20210419] [DOI] [PMC free article] [PubMed]
- Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomized, phase 1 trial. Lancet Infectious Diseases 2021;21(5):637-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ella 2021b {published data only}
- Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): a double-blind, randomized, controlled phase 3 trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.06.30.21259439] [DOI] [PMC free article] [PubMed]
El Sahly 2021 {published data only}
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England Journal of Medicine 2021;384(5):403-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. New England Journal of Medicine 2021;385(19):1774-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting; December 17, 2020; FDA Briefing Document: Moderna COVID-19 vaccine. www.fda.gov/media/144434/download (accessed prior to 1 November 2022).
Emary 2021 {published data only}
- Emary KR, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomized controlled trial. Lancet 2021;397(10282):1351-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fadlyana 2021 {published data only}
- Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine 2021;39(44):6520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falsey 2021 {published data only}
- Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. New England Journal of Medicine 2021;385(25):2348-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Formica 2021 {published data only}
- Formica N, Mallory R, Albert G, Robinson M, Plested JS, Cho I, et al. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: a phase 2 randomized placebo-controlled trial. PLOS Medicine 2021;18(10):e1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica N, Mallory R, Albert G, Robinson M, Plested JS, Cho I, et al. Evaluation of a SARS-CoV-2 vaccine NVX-CoV2373 in younger and older adults. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.02.26.21252482] [DOI] [PMC free article] [PubMed]
Frenck 2021 {published data only}
- Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. New England Journal of Medicine 2021;385(3):239-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2021 {published data only}
- Guo W, Duan K, Zhang Y, Yuan Z, Zhang YB, Wang Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 1/2 trial. eClinicalMedicine 2021;38:101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2021 {published data only}
- Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. New England Journal of Medicine 2021;385(13):1244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2021 {published data only}
- Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomized, controlled, phase 1/2 clinical trial. Lancet Infectious Diseases 2021;21(12):1645-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heath 2021 {published data only}
- Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. New England Journal of Medicine 2021;385(13):1172-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al . Efficacy of the NVX-CoV2373 Covid-19 vaccine against the B. 1.1.7 variant. medRxiv 2021 [Preprint].
Keech 2020 {published data only}
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. New England Journal of Medicine 2020;383:2320-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kremsner 2021 {published data only}
- Kremsner PG, Ahuad Guerrero RA, Arana-Arri E, Aroca Martinez GJ, Bonten M, Chandler R, et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate: results from Herald, a phase 2b/3, randomized, observer-blinded, placebo-controlled clinical trial in ten countries in Europe and Latin America. SSRN 2021 [Preprint]. [DOI: 10.2139/ssrn.3911826] [DOI] [PMC free article] [PubMed]
Kulkarni 2021 {published data only}
- Kulkarni PS, Padmapriyadarsini C, Vekemans J, Bavdekar A, Gupta M, Kulkarni P, et al. A phase 2/3, observer-blind, randomized, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCOV-19 (COVID-19 vaccine) in adults in India. eClinicalMedicine 2021;42:101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2021a {published data only}
- Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, et al. Heterologous prime-boost immunization with CoronaVac and Convidecia. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.09.03.21263062] [DOI]
Liu 2021 {published data only}
- Liu X, Shaw RH, Stuart AS, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomized, non-inferiority trial. Lancet 2021;398(10303):856-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shaw RH, Stuart AS, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity report from the Com-COV Study – a single-blind randomized non-inferiority trial comparing heterologous and homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine. SSRN 2021 [Preprint]. [DOI: 10.2139/ssrn.3874014] [DOI] [PMC free article] [PubMed]
Logunov 2021 {published data only}
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomized controlled phase 3 trial in Russia. Lancet 2021;397(10275):671-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhi 2021a {published data only}
- Madhi SA, Koen AL, Izu A, Fairlie L, Cutland CL, Baillie V, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomized, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021;8(9):e568-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhi 2021b {published data only}
- Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. New England Journal of Medicine 2021;384(20):1885-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mok 2021 {published data only}
- Mok C, Cheng S, Chen C, Yiu K, Chan TO, Lai KC, et al. A RCT of a third dose CoronaVac or BNT162b2 vaccine in adults with two doses of CoronaVac. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.11.02.21265843] [DOI]
Palacios 2020 {published data only}
- Palacios R, Patiño EG, Oliveira Piorelli R, Conde MT, Batista AP, Zeng G, et al. Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by Sinovac – PROFISCOV: a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sablerolles 2021 {published data only}
- Sablerolles RS, Rietdijk WJ, Goorhuis A, Postma DF, Visser LG, Geers D, et al. Immunogenicity and reactogenicity of booster vaccinations after Ad26.COV2.S priming. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.10.18.21264979] [DOI]
Sadoff 2021a {published data only}
- Food and Drug Administration. Vaccines and related biological products Advisory Committee Meeting; February 26, 2021; FDA briefing document: Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. www.fda.gov/media/146217/download (accessed prior to 1 November 2022).
- Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, Groot AM, et al. Interim results of a Phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. New England Journal of Medicine 2021;384(19):1824-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, Groot AM, et al. Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. medRxiv 2020 [Preprint]. [DOI: 10.1101/2020.09.23.20199604] [DOI]
Sadoff 2021b {published data only}
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. New England Journal of Medicine 2021;384(23):2187-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinde 2021 {published data only}
- Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. New England Journal of Medicine 2021;384(20):1899-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Preliminary efficacy of the NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.02.25.21252477] [DOI] [PMC free article] [PubMed]
Tanriover 2021 {published data only}
- Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomized, placebo-controlled, phase 3 trial in Turkey. Lancet 2021;398(10296):213-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2021 {published data only}
- Food and Drug Administration. Vaccines and related biological products Advisory Committee Meeting; December 10, 2020; FDA briefing document: Pfizer-BioNTech COVID-19 vaccine. www.fda.gov/media/144245/download (accessed prior to 1 November 2022).
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine 2020;383(27):2603-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. New England Journal of Medicine 2021;385(19):1761-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.07.28.21261159] [DOI] [PMC free article] [PubMed]
Toledo‐Romani 2021 {published data only}
- Toledo-Romani ME, Garcia-Carmenate M, Silva-Valenzuela C, Baldoquin-Rodriguez W, Martínez-Pérez M, Rodríguez-González M, et al. Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA-PLUS: double-blind, randomized, placebo-controlled phase 3 clinical trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.10.31.21265703] [DOI] [PMC free article] [PubMed]
Voysey 2021a {published data only}
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet 2020;396(10249):467-78. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397(10269):99-111. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2020 {published data only}
- Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv 2020 [Preprint]. [DOI: 10.1101/2020.08.17.20176651] [DOI]
- Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. New England Journal of Medicine 2020;383(25):2439-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2021a {published data only}
- Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomized, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infectious Diseases 2021;21(6):803-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2020 {published data only}
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020;324(10):951-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomized, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infectious Diseases 2020;21(1):39-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2021 {published data only}
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomized, double-blind, controlled, phase 1/2 trial. Lancet Infectious Diseases 2021;22(2):196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2021 {published data only}
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomized, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infectious Diseases 2021;21(2):181-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Baden 2021 {published data only}
- Baden LR, El Sahly HM, Essink B, Follmann D, Neuzil KM, August A, et al. Covid-19 in the phase 3 trial of mRNA-1273 during the Delta-variant surge. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.09.17.21263624] [DOI] [PMC free article] [PubMed]
Barrett 2021 {published data only}
- Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nature Medicine 2021;27(2):279-88. [DOI] [PubMed] [Google Scholar]
Ewer 2021 {published data only}
- Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nature Medicine 2021;27(2):270-8. [DOI] [PubMed] [Google Scholar]
Flaxman 2021 {published data only}
- Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomized controlled trials (COV001 and COV002). Lancet 2021;398(10304):981-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsieh 2021 {published data only}
- Hsieh SM, Liu WD, Huang YS, Lin YJ, Hsieh EF, Lian WC, et al. Safety and immunogenicity of a recombinant stabilized prefusion SARS-CoV-2 spike protein vaccine (MVC-COV1901) adjuvanted with CpG 1018 and aluminum hydroxide in healthy adults: a phase 1, dose-escalation study. eClinicalMedicine 2021;38:100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irfan 2021 {published data only}
- Irfan N, Chagla Z. In South Africa, a 2-dose Oxford/AZ vaccine did not prevent mild to moderate COVID-19 (cases mainly B.1.351 variant). Annals of Internal Medicine 2021;174(5):JC50. [DOI] [PubMed] [Google Scholar]
Lazarus 2021 {published data only}
- Lazarus R, Baos S, Cappel-Porter H, Carson-Stevens A, Clout M, Culliford L, et al. The safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults: a phase IV, multicentre randomized controlled trial with blinding (ComFluCOV). SSRN 2021 [Preprint]. [DOI: 10.2139/ssrn.3931758] [DOI] [PMC free article] [PubMed]
Patamatamkul 2021 {published data only}
- Patamatamkul S, Thammawat S, Buranrat B. Induction of robust neutralizing antibodies against the COVID-19 Delta variant with ChAdOx1 nCoV-19 or BNT162b2 as a booster following a primary vaccination series with CoronaVac. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.09.25.21264099] [DOI]
Ward 2021a {published data only}
- Ward BJ, Séguin A, Couillard J, Trépanier S, Landry N. Phase III: randomized observer-blind trial to evaluate lot-to-lot consistency of a new plant-derived quadrivalent virus like particle influenza vaccine in adults 18-49 years of age. Vaccine 2021;39(10):1528-33. [DOI] [PubMed] [Google Scholar]
Wu 2021b {published data only}
- Wu K, Choi A, Koch M, Ma LZ, Hill A, Nunna N, et al. Preliminary analysis of safety and immunogenicity of a SARS-CoV-2 variant vaccine booster. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.05.05.21256716] [DOI] [PMC free article] [PubMed]
Zdanowski 2021 {published data only}
- Zdanowski W, Waśniewski T. Evaluation of SARS-CoV-2 spike protein antibody titers in cord blood after COVID-19 vaccination during pregnancy in Polish healthcare workers: preliminary results. Vaccines 2021;9(6):675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abbasi 2020
- Abbasi J. COVID-19 and mRNA vaccines – first large test for a new approach. JAMA 2020;324(12):1125-7. [DOI] [PubMed] [Google Scholar]
Angkasekwinai 2022
- Angkasekwinai N, Sewatanon J, Niyomnaitham S, Phumiamorn S, Sukapirom K, Sapsutthipas S, et al. Comparison of safety and immunogenicity of CoronaVac and ChAdOx1 against the SARS-CoV-2 circulating variants of concern (Alpha, Delta, Beta) in Thai healthcare workers. Vaccine X 2022;10:100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Attaway 2021
- Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe Covid-19 pneumonia: pathogenesis and clinical management. BMJ 2021;372:n436. [DOI] [PubMed] [Google Scholar]
Baden 2021
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England Journal of Medicine 2021;384(5):403-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balduzzi 2019
- Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Mental Health 2019;22:153-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borobia 2021
- Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021;398(10295):121-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2020a
- Boutron I, Chaimani A, Meerpohl JJ, Hróbjartsson A, Devane D, Rada G, et al. The COVID-NMA Project: building an evidence ecosystem for the COVID-19 pandemic. Annals of Internal Medicine 2020;173(12):1015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2020b
- Boutron I, Chaimani A, Meerpohl JJ, Hróbjartsson A, Devane D, Rada G, et al. Interventions for preventing and treating COVID-19: protocol for a living mapping of research and a living systematic review. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013769. [DOI: 10.1002/14651858.CD013769] [DOI] [Google Scholar]
Bucci 2021
- Bucci EM, Berkhof J, Gillibert A, Gopalakrishna G, Calogero RA, Bouter LM, et al. Data discrepancies and substandard reporting of interim data of Sputnik V phase 3 trial. Lancet 2021;397(10288):1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bueno 2021
- Bueno SM, Abarca K, González PA, Gálvez NM, Soto JA, Duarte LF, et al. Interim report: safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy Chilean adults in a phase 3 clinical trial. medRxiv 2021 [Preprint].
Cabanac 2021
- Cabanac G, Oikonomidi T, Boutron I. Day-to-day discovery of preprint-publication links. Scientometrics 2021;126(6):5285-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castagneto Gissey 2021
- Castagneto Gissey L, Panunzi S, Maltese S, Russo MF, Angelini G, De Gaetano A, et al. Living systematic meta-analysis of COVID-19 vaccines and dose allocation strategies. SSRN 2021 [Preprint]. [DOI: 10.2139/ssrn.3827806] [DOI]
CDC 2021
- Centers for Disease Control and Prevention. Understanding mRNA COVID-19 vaccines. www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html (accessed prior to 1 November 2022).
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLOS One 2013;8 (10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2015
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the Network Graphs Package. Stata Journal 2015;15(4):905-50. [Google Scholar]
Chaimani 2022
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Chappell 2021
- Chappell KJ, Mordant FL, Li Z, Wijesundara DK, Ellenberg P, Lackenby JA, et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infectious Diseases 2021;21(10):1383-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Che 2021
- Che Y, Liu X, Pu Y, Zhou M, Zhao Z, Jiang R, et al. Randomized, double-blinded, placebo-controlled Phase 2 trial of an inactivated severe acute respiratory syndrome coronavirus 2 vaccine in healthy adults. Clinical Infectious Diseases 2021;73(11):e3949-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2021
- Chu L, McPhee R, Huang W, Bennett H, Pajon R, Nestorova B, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 2021;39(20):2791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dal‐Ré 2021a
- Dal-Ré R, Bekker LG, Gluud C, Holm S, Jha V, Poland GA, et al. Ongoing and future COVID-19 vaccine clinical trials: challenges and opportunities. Lancet Infectious Diseases 2021;21(11):e342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dal‐Ré 2021b
- Dal-Ré R, Orenstein W, Caplan AL. Being fair to participants in placebo-controlled COVID-19 vaccine trials. Nature Medicine 2021;27(6):938. [DOI] [PubMed] [Google Scholar]
DeZure 2016
- DeZure AD, Berkowitz NM, Graham BS, Ledgerwood JE. Whole-inactivated and virus-like particle vaccine strategies for chikungunya virus. Journal of Infectious Diseases 2016;214(Suppl 5):S497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. [DOI] [PubMed] [Google Scholar]
Dong 2020
- Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduction and Targeted Therapy 2020;5(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ella 2020b
- Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity clinical trial of an inactivated SARS-CoV-2 vaccine, BBV152 (a phase 2, double-blind, randomised controlled trial) and the persistence of immune responses from a phase 1 follow-up report. medRxiv 2020 [Preprint]. [DOI: 10.1101/2020.12.21.20248643] [DOI]
Ella 2021a
- Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomized, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomized phase 1 trial. Lancet Infectious Diseases 2021;21(7):950-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enjuanes 2016
- Enjuanes L, Zuñiga S, Castaño-Rodriguez C, Gutierrez-Alvarez J, Canton J, Sola I. Molecular basis of coronavirus virulence and vaccine development. Advances in Virus Research 2016;96:245-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epistemonikos
- Epistemonikos. Epistemonikos L·OVE COVID-19 platform. Available at app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d?utm=ile.
FDA 2020a
- Food and Drug Administration. Development and licensure of vaccines to prevent COVID-19. Guidance for industry; June 2020. www.fda.gov/media/139638/download (accessed prior to 1 November 2022).
FDA 2020b
- Food and Drug Administration. FDA Briefing Document Moderna COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee Meeting December 17, 2020. www.fda.gov/media/144434/download (accessed prior to 1 November 2022).
FDA 2020c
- Food and Drug Administration. FDA Briefing Document Pfizer-BioNTech COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee Meeting December 10, 2020. www.fda.gov/media/144245/download (accessed prior to 1 November 2022).
FDA 2021
- Food and Drug Administration. FDA Briefing Document Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee Meeting February 26, 2021. www.fda.gov/media/146217/download (accessed prior to 1 November 2022).
Feikin 2022
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022;399(10328):924-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2021
- Feng Y, Chen J, Yao T, Chang Y, Li X, Xing R, et al. Safety and Immunogenicity of Inactivated SARS-CoV-2 vaccine in high-risk occupational population: a randomized, parallel, controlled clinical trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.08.06.21261696] [DOI] [PMC free article] [PubMed]
Folegatti 2020
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396:467-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Formica 2021
- Formica N, Mallory R, Albert G, Robinson M, Plested JS, Cho I, et al, the 2019nCoV-101 Study Group. Evaluation of a SARS-CoV-2 vaccine NVX-CoV2373 in younger and older adults. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.02.26.21252482] [DOI] [PMC free article] [PubMed]
Fuenmayor 2017
- Fuenmayor J, Gòdia F, Cervera L. Production of virus-like particles for vaccines. New Biotechnology 2017;39(Pt B):174-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gavi 2020
- Gavi. What are viral vector-based vaccines and how could they be used against COVID-19? www.gavi.org/vaccineswork/what-are-viral-vector-based-vaccines-and-how-could-they-be-used-against-covid-19 (accessed prior to 1 November 2022).
Gobeil 2021
- Gobeil P, Pillet S, Séguin A, Boulay I, Mahmood A, Vinh DC, et al. Interim report of a Phase 2 randomized trial of a plant-produced virus-like particle vaccine for Covid-19 in healthy adults aged 18-64 and older adults aged 65 and older. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.05.14.21257248] [DOI]
Goepfert 2021
- Goepfert PA, Fu B, Chabanon AL, Bonaparte MI, Davis MG, Essink BJ, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. Lancet Infectious Diseases 2021;21(9):1257-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 6 December 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Harder 2021
- Harder T, Koch J, Vygen-Bonnet S, Kulper-Schiek W, Pilic A, Reda S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Eurosurveillance 2021;26(28):2100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayawi 2021
- Hayawi K, Shahriar S, Serhani MA, Alashwal H, Masud MM. Vaccine versus variants (3Vs): are the COVID-19 vaccines effective against the variants? A systematic review. Vaccines 2021;9(11):1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heath 2021
- Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Efficacy of the NVX-CoV2373 Covid-19 vaccine against the B.1.1.7 variant. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.05.13.21256639] [DOI]
Higdon 2021
- Higdon MM, Wahl B, Jones CB, Rosen JG, Truelove SA, Baidya A, et al. A systematic review of COVID-19 vaccine efficacy and effectiveness against SARS-CoV-2 infection and disease. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.09.17.21263549] [DOI]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hobernik 2018
- Hobernik D, Bros M. DNA vaccines – how far from clinical use? International Journal of Molecular Sciences 2018;19(11):3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hultcrantz 2017
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. Journal of Clinical Epidemiology 2017;87:4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2018
- Kirkham JJ, Altman DG, Chan AW, Gamble C, Dwan KM, Williamson PR. Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ 2018;362:k3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korang 2022
- Korang SK, Rohden E, Veroniki AA, Ong G, Ngalamika O, Siddiqui F, et al. Vaccines to prevent COVID-19: a living systematic review with trial sequential analysis and network meta-analysis of randomized clinical trials. PLOS One 2022;17(1):e0260733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kow 2022
- Kow CS, Ramachandram DS, Hasan SS. The effectiveness of mRNA-1273 vaccine against COVID-19 caused by Delta variant: a systematic review and meta-analysis. Journal of Medical Virology 2022;94(5):2269-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazarus 2021
- Lazarus R, Taucher C, Brown C, Čorbic I, Danon L, Dubischar K, et al. Immunogenicity and safety of inactivated whole virion Coronavirus vaccine with CpG (VLA2001) in healthy adults aged 18 to 55: a randomised phase 1 /2 clinical trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.08.13.21262021] [DOI]
Li 2021b
- Li J, Hui A, Zhang X, Yang Y, Tang R, Ye H, et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study. Nature Medicine 2021;27(6):1062-70. [DOI] [PubMed] [Google Scholar]
Li 2021c
- Li M, Yang J, Wang L, Wu Q, Wu Z, Zheng W, et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: a randomized, double-blind, placebo-controlled, phase 1/2 clinical trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.08.03.21261544] [DOI]
Liu 2021
- Liu X, Shaw RH, Stuart AS, Greenland M, Dinesh T, Provstgaard-Morys S, et al. Safety and immunogenicity report from the com-COV study – a single-blind randomised non-inferiority trial comparing heterologous and homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine. SSRN 2021 [Preprint]. [DOI] [PMC free article] [PubMed]
Low 2021
- Low JG, Alwis R, Chen S, Kalimuddin S, Leong YA, Mah TK, et al. A phase 1/2 randomized, double-blinded, placebo controlled ascending dose trial to assess the safety, tolerability and immunogenicity of ARCT-021 in healthy adults. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.07.01.21259831] [DOI]
Lv 2021
- Lv M, Luo X, Shen Q, Lei R, Liu X, Liu E, et al. Safety, immunogenicity, and efficacy of COVID-19 vaccines in children and adolescents: a systematic review. Vaccines 2021;9(10):1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhi 2021
- Madhi SA, Koen AL, Fairlie L, Cutland CL, Baillie V, Padayachee SD, et al. ChAdOx1 nCoV-19 (AZD1222) vaccine in people living with and without HIV. Research Square 2021 [Preprint]. [DOI: 10.21203/rs.3.rs-322470/v1] [DOI] [PMC free article] [PubMed]
Makris 2021
- Makris M, Pavord S, Lester W, Scully M, Hunt B. Vaccine-induced immune thrombocytopenia and thrombosis (VITT). Research and Practice in Thrombosis and Haemostasis 2021;5(5):e12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mammen 2021
- Mammen MP Jr, Tebas P, Agnes J, Giffear M, Kraynyak KA, Blackwood E, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.05.07.21256652] [DOI]
Marshall 2020
- Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mavridis 2014
- Mavridis D, Welton NJ, Sutton A, Salanti G. A selection model for accounting for publication bias in a full network meta-analysis. Statistics in Medicine 2014;33(30):5399-412. [DOI] [PubMed] [Google Scholar]
Meng 2021b
- Meng FY, Gao F, Jia SY, Wu XH, Li JX, Guo XL, et al. Safety and immunogenicity of a recombinant COVID-19 vaccine (Sf9 cells) in healthy population aged 18 years or older: two single-center, randomised, double-blind, placebo-controlled, phase 1 and phase 2 trials. Signal Transduction and Targeted Therapy 2021;6(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortola 2004
- Mortola E, Roy P. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Letters 2004;576(1-2):174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulligan 2020
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020;586(7830):589-93. [DOI] [PubMed] [Google Scholar]
Nguyen 2021
- Nguyen TP, Do Q, Phan LT, Dinh DV, Khong H, Hoang LV, et al. Safety and immunogenicity of Nanocovax, a SARS-CoV-2 recombinant spike protein vaccine. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.07.22.21260942] [DOI] [PMC free article] [PubMed]
Nikolakopoulou 2020
- Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLOS Medicine 2020;17 (4):e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oikonomidi 2020
- Oikonomidi T, Boutron I, Pierre O, Cabanac G, Ravaud P, COVID-19 NMA Consortium. Changes in evidence for studies assessing interventions for COVID-19 reported in preprints: meta-research study. BMC Medicine 2020;18(1):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostrowski 2021
- Ostrowski SR, Søgaard OS, Tolstrup M, Stærke NB, Lundgren J, Østergaard L, et al. Inflammation and platelet activation after COVID-19 vaccines – possible mechanisms behind vaccine-induced immune thrombocytopenia and thrombosis. Frontiers in Immunology 2021;12:779453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyanv – a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oxford Vaccine Group 2020
- Oxford Vaccine Group. Vaccine Knowledge Project: independent information about vaccines and infectious diseases. vk.ovg.ox.ac.uk/vk (accessed prior to 1 November 2022).
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pajon 2021
- Pajon R, Paila YD, Girard B, Dixon G, Kacena K, Baden LR, et al. Initial analysis of viral dynamics and circulating viral variants during the mRNA-1273 Phase 3 COVE trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.09.28.21264252] [DOI] [PMC free article] [PubMed]
Pan 2021a
- Pan HX, Liu JK, Huang BY, Li GF, Chang XY, Liu YF, et al. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine (KCONVAC) in healthy adults: two randomized, double-blind, and placebo-controlled Phase 1/2 clinical trials. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.04.07.21253850] [DOI]
Pan 2021b
- Pan HX, Wu QH, Zeng G, Yang J, Jiang DY, Deng XW, et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18–59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.07.23.21261026] [DOI]
Pérez‐Rodríguez 2021
- Pérez-Rodríguez S, la Caridad Rodríguez-González M, Ochoa-Azze R, Climent-Ruiz Y, Alberto González-Delgado C, Paredes-Moreno B, et al. A randomized, double-blind phase I clinical trial of two recombinant dimeric RBD COVID-19 vaccine candidates: safety, reactogenicity and immunogenicity. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.10.04.21264522] [DOI] [PMC free article] [PubMed]
Pitisuttithum 2021
- Pitisuttithum P, Luvira V, Lawpoolsri S, Muangnoicharoen S, Kamolratanakul S, Sivakorn C, et al. Safety and immunogenicity of an inactivated recombinant Newcastle disease virus vaccine expressing SARS-CoV-2 spike: interim results of a randomised, placebo-controlled, Phase 1/2 trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.09.17.21263758] [DOI] [PMC free article] [PubMed]
Polack 2020
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine 2020;383(27):2603-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollard 2021
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nature Reviews Immunology 2021;21(2):83-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pu 2021
- Pu J, Yu Q, Yin Z, Zhang Y, Li X, Yin Q, et al. The safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in Chinese adults aged 18–59 years: a phase I randomized, double-blinded, controlled trial. Vaccine 2021;39(20):2746-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiao 2020
- Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet 2020;395(10226):760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramasamy 2020
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomized, controlled, phase 2/3 trial. Lancet 2020;396(10267):1979-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richmond 2021
- Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomized, double-blind, placebo-controlled trial. Lancet 2021;397(10275):682-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Rizk 2021
- Rizk JG, Gupta A, Sardar P, Henry BM, Lewin JC, Lippi G, et al. Clinical characteristics and pharmacological management of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis: a review. JAMA Cardiology 2021;6(12):1451-60. [DOI] [PubMed] [Google Scholar]
Roozen 2021
- Roozen GV, Prins ML, Binnendijk R, den Hartog G, Kuiper VP, Prins C, et al. Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA-1273 SARS-CoV-2 vaccine in healthy adults as a dose sparing strategy. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.07.27.21261116] [DOI]
Roper 2009
- Roper RL, Rehm KE. SARS vaccines: where are we? Expert Review of Vaccines 2009;8(7):887-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotshild 2021
- Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Scientific Reports 2021;11(1):22777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubin 2013
- Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromized host. Clinical Infectious Diseases 2013;58(3):e44-e100. [DOI] [PubMed] [Google Scholar]
Rucker 2013
- Rucker G, Schwarzer G, Rücker G, Schwarzer G, Krahn U, König J. Network meta-analysis using frequentist methods – package netmeta. cran.r–project.org (accessed prior to 1 November 2022).
Ryzhikov 2021
- Ryzhikov AB, Ryzhikov ЕА, Bogryantseva MP, Usova SV, Danilenko ED, Nechaeva EA, et al. A single blind, placebo-controlled randomized study of the safety, reactogenicity and immunogenicity of the "EpiVacCorona" vaccine for the prevention of COVID-19. Russian Journal of Infection and Immunity 2021;11(2):283-96. [Google Scholar]
Sadoff 2020c
- Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, Groot AM, et al. Safety and immunogenicity of the Ad26. COV2. S COVID-19 vaccine candidate: interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. MedRxiv 2020 [Preprint].
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-7. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Sharifian‐Dorche 2021
- Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. Journal of the Neurological Sciences 2021;428:117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinde 2021
- Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al, for the 2019nCoV-501 Study Group. Preliminary efficacy of the NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. medRxiv 2021 [Preprint]. [DOI] [PMC free article] [PubMed]
Shu 2021
- Shu YJ, He JF, Pei RJ, He P, Huang ZH, Chen SM, et al. Immunogenicity and safety of a recombinant fusion protein vaccine (V-01) against coronavirus disease 2019 in healthy adults: a randomized, double-blind, placebo-controlled, phase II trial. Chinese Medical Journal 2021;134(16):1967-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sridhar 2021
- Sridhar S, Joaquin A, Bonaparte MI, Bueso A, Chabanon AL, Chen A, et al. Safety and immunogenicity of a SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant in healthy adults: interim findings from a phase 2, randomised, dose-finding, multi-centre study. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.10.08.21264302] [DOI] [PMC free article] [PubMed]
Stephenson 2021
- Stephenson KE, Le Gars M, Sadoff J, Groot AM, Heerwegh D, Truyers C, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 2021;325(15):1535-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Thomas 2021
- Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Six month safety and efficacy of the BNT162b2 Mrna Covid-19 vaccine. medRxiv 2021 [Preprint]. [DOI] [PMC free article] [PubMed]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Riel 2020
- Riel D, Wit E. Next-generation vaccine platforms for COVID-19. Nature Materials 2020;19(8):810-2. [DOI] [PubMed] [Google Scholar]
Viechtbauer 2010
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 2010;36(3):1-48. [Google Scholar]
Voysey 2021b
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomized trials. Lancet 2021;397(10277):881-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2021
- Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv 2021 [Preprint].
Ward 2021b
- Ward BJ, Gobeil P, Séguin A, Atkins J, Boulay I, Charbonneau PY, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nature Medicine 2021;27(6):1071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2008
- White IR, Higgins JP, Wood AM. Allowing for uncertainty due to missing data in meta-analysis – part 1: two-stage methods. Statistics in Medicine 2008;27(5):711-27. [DOI] [PubMed] [Google Scholar]
WHO 2020a
- World Health Organization. Coronavirus disease 2019 (COVID-19). Situation report – 51. apps.who.int/iris/handle/10665/331475 (accessed prior to 1 November 2022).
WHO 2020b
- World Health Organization. Considerations for evaluation of COVID19 vaccines. Points to consider for manufacturers of COVID-19 vaccines. Version 24 September 2020. www.who.int/docs/default-source/in-vitro-diagnostics/covid19/who-evaluation-covid-vaccine-w-lines.pdf?sfvrsn=701d3a65_2 (accessed prior to 1 November 2022).
WHO 2020c
- World Health Organization. WHO target product profiles for COVID-19 vaccines. Version 3 – 29 April 2020. www.who.int/docs/default-source/blue-print/who-target-product-profiles-for-covid-19-vaccines.pdf (accessed prior to 1 November 2022).
WHO 2022a
- World Health Organization. Tracking SARS-CoV-2 variants. www.who.int/activities/tracking-SARS-CoV-2-variants (accessed prior to 1 November 2022).
WHO 2022b
- World Health Organization. COVID-19 vaccine tracker and landscape. www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed prior to 1 November 2022).
WHO Ad Hoc Expert Group 2021
- WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation. Placebo-controlled trials of covid-19 vaccines – why we still need them. New England Journal of Medicine 2021;384:e2. [DOI: 10.1056/NEJMp2033538] [DOI] [PubMed] [Google Scholar]
Worldometer 2022
- Worldometer. COVID-19 coronavirus pandemic. www.worldometers.info/coronavirus/#countries (accessed prior to 1 November 2022).
Wu 2021c
- Wu S, Huang J, Zhang Z, Wu J, Zhang J, Hu H, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infectious Diseases 2021;21(12):1654-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2020
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020;324(10):951-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2021
- Yang S, Li Y, Dai L, Wang J, He P, Li C, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomized, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infectious Diseases 2021;21(8):1107-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yepes‐Nuñez 2019
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. [DOI] [PubMed] [Google Scholar]
Zakarya 2021
- Zakarya K, Kutumbetov L, Orynbayev M, Abduraimov Y, Sultankulova K, Kassenov M, et al. A single-centre, randomized, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. EClinicalMedicine 2021;39:101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2021a
- Zeng L, Brignardello-Petersen R, Hultcrantz M, Siemieniuk RA, Santesso N, Traversy G, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. Journal of Clinical Epidemiology 2021;137:163-75. [DOI] [PubMed] [Google Scholar]
Zeng 2021b
- Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.09.23.21264048] [DOI] [PMC free article] [PubMed]
Zhang 2021b
- Zhang J, Hu Z, He J, Liao Y, Li Y, Pei R, et al. Safety and immunogenicity of a recombinant interferon-armed RBD dimer vaccine (V-01) for COVID-19 in healthy adults: a randomized, double-blind, placebo-controlled, Phase I trial. Emerging Microbes & Infections 2021;10(1):1589-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2020
- Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet 2020;396(10249):479-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2021a
- Zhu F, Jin P, Zhu T, Wang W, Ye H, Pan H, et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: a randomized, double-blind, placebo-controlled, phase 2b trial. Clinical Infectious Diseases 2022;75(1):e783-91. [DOI: 10.1093/cid/ciab845] [DOI] [PMC free article] [PubMed]
Zhu 2022
- Zhu F, Jin P, Zhu T, Wang W, Ye H, Pan H, et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: a randomized, double-blind, placebo-controlled, phase 2b trial. Clinical Infectious Diseases 2022;75(1):e783-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Grana 2021
- Grana C, Ghosn L, Boutron I. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis. PROSPERO 2021 CRD42021271897. www.crd.york.ac.uk/prospero/display_record.php?RecordID=271897 (accessed prior to 1 November 2022).