Abstract
Secondary bacterial infections and bacterial coinfections are an important complication of coronavirus disease 2019 (COVID-19), leading to antibiotic overuse and increased rates of antimicrobial resistance (AMR) during the COVID-19 pandemic. In this literature review, we summarize the reported rates of secondary bacterial infections and bacterial coinfections in patients with COVID-19, the impact on patient outcomes, the antibiotic treatment approaches employed, and the resistance patterns observed. The reported data suggest that although the incidence of secondary bacterial infections or bacterial coinfections is relatively low, they are associated with worse outcomes such as prolonged hospitalization, intensive care unit admission, mechanical ventilator use, and increased mortality. Interestingly, antibiotic prescription rates are typically higher than secondary bacterial and bacterial coinfection rates, and reports of AMR are common. These findings highlight the need for an improved understanding of secondary bacterial and bacterial coinfection in patients with COVID-19, as well as improved treatment options, to mitigate inappropriate antibiotic prescribing and AMR.
Viral pandemics have historically been associated with secondary bacterial infections, and coronavirus disease 2019 (COVID-19) has been no exception. Subsequent bacterial infections, particularly lower respiratory tract infections, which are the leading cause of infectious disease mortality worldwide, 1,2 have been associated with increased mortality both during the 1918 Spanish influenza pandemic and during seasonal influenza outbreaks. 3,4 However, differentiating viral versus bacterial infection is a challenge for clinicians, which has led to inappropriate or prolonged use of antibiotics in patients with COVID-19. As previously described, the overuse of antibiotics increases the risk of antimicrobial resistance (AMR) 5–7 which can cause severe infections and complications, such as disruption of the gut microbiota leading to outbreaks of Clostridium difficile infection. 8,9
In this review, we examined the prevalence of secondary bacterial infections and bacterial coinfections in patients with COVID-19 and the use of antibiotics associated with these infections. A literature search of PubMed and Embase was conducted to identify relevant studies published up to June 2, 2021 (Supplementary Table 1). The main types of bacterial infections studied were (1) coinfections or community-acquired (CA) infections prior to or within the first 3 d of hospitalization, (2) secondary or hospital-acquired (HA) infections on or after day 4 of hospitalization, according to the National Healthcare Safety Network definition, 10 and (3) both CA and HA infections.
We also reviewed the impact of secondary bacterial infections and bacterial coinfections on clinical outcomes (eg, length of hospitalization, intensive care unit [ICU] admission and mortality), the etiology of these bacterial infections, the antibiotic treatment approaches, and discuss the development of AMR.
Prevalence of secondary bacterial infections and bacterial coinfections in patients with COVID-19
Most studies have reported an estimated rate of secondary bacterial infections and bacterial coinfections <20% (Tables 1–3). 11–16 However, CA bacterial infection has been less commonly reported, with rates ranging between 1% and 7.5% (Table 1). 13,14,17,18 The rates of HA bacterial infections were variable and ranged from 9.3% to 32% for overall secondary bacterial infections (Table 3). 19,20 Although the heterogeneity of the methodologies and populations (eg, moderate-to-severe COVID-19 cases, outpatients vs inpatients) make it difficult to compare rates of bacterial infections, in general, HA infection rates tended to be higher than CA infection rates in studies that recorded data on both (Table 2). 15,16,21,22
Table 1.
Summary of Included Studies Involving Patients With COVID-19 and Community-Acquired (CA) Bacterial Coinfections
| Study | Study Type/Date | Total COVID-19 Patients, No. | Coinfection Type a /Acquisition Setting | Time of Infection Diagnosis a | Rate of Coinfection b |
Mortality Outcomes in COVID-19 Patients With Coinfection c | Other Outcomes Reported in COVID-19 Patients With or Confection |
|---|---|---|---|---|---|---|---|
| Observational studies, United States | |||||||
| Vaughn et al, 2020 14 | Retrospective cohort, 8 hospitals, USA (March–June 2020) |
1,705 | CA bacterial infection/outpatient | ≤3 d of hospitalization | 59 (3.5%) of 1,705 CA bacterial infections; bacterial respiratory infection, 29 (1.7%) of 1,705; BSI, 31 (1.8%) of 1,705 | Mortality rate, 28 (47.5%) of 71 in patients with CA bacterial infections vs 297 (18.0%) of 1,634 w/o confirmed infection (P < .001) |
Hospital LOS |
| Karaba et al, 2020 13 | Retrospective cohort, 5 hospitals, USA (March–May 2020) |
1,016 | CABP/outpatient | ≤48 h of admission | Proven/probable CABP, 12 (1.2%) of 1,016; nonrespiratory coinfection, 42 (4%) of 1,016 | NR | Hospital LOS, ICU admission rate |
| Observational studies: Europe | |||||||
| Thelen et al, 2021 17 | Retrospective cohort, 2 hospitals, Netherlands (February–June 2020) | 678 | CA bacterial infection/outpatient | ≤48 h of COVID-19 test | Bacteremia, 7 (1.0%) of 678 | 30-d all-cause mortality rate in patients with BSI, 26.2% | NR |
| Observational studies: Asia | |||||||
| Wang et al, 2020 18 | Retrospective cohort, 1 hospital, China (January 2020) | 67 (69 enrolled but 2 lost) | CABP/outpatient | NR | Bacterial respiratory infections: 5 (7.5%) of 67 | Mortality rate, 5 (7.5%) of 67 | NR d |
| Hoshyama et al, 2020 63 | Retrospective cohort, 1 hospital, Japan (February–July 2020) | 7 | CA bacterial infection/outpatient | On admission | Bacterial respiratory, 4 (57.1%) of 7 | Mortality rate, 0%; all 7 patients were discharged | NR |
Note. CABP, community-acquired bacterial pneumonia; ICU, intensive care unit; LOS, length of stay; NR, not reported; w/o, without.
Based on published information, including clinical details, or on the time of infection diagnosis: outpatient/≤3 d of hospitalization = community acquired infection, unless otherwise stated in the source.
Rates were reported per total number of patients with COVID-19.
Data for hospital LOS, ICU admission rates in patients with COVID-19 who had coinfections.
Outcomes were reported in total patient population.
Table 3.
Summary of Included Studies Involving Patients With COVID-19 and Hospital-Acquired (HA) Secondary Bacterial Infections
| Study | Study Type/Date | Total COVID-19 Patients, No. | Secondary Infection Type a /Acquisition Setting | Time of Infection Diagnosis a | Rate of Secondary Bacterial Infection b | Mortality Outcomes in COVID-19 Patients With Secondary Bacterial Infection c | Other Outcomes Reported in COVID-19 Patients With Secondary Bacterial Infection d |
|---|---|---|---|---|---|---|---|
| Observational studies, South America | |||||||
| Martinez-Guerra et al, 2021 12 | Prospective cohort, 1 hospital, Mexico (March–June 2020) | 794 | HA bacterial infection/inpatient | Median hospital stay at diagnosis, 9 d | Overall infection, 74 (11.3%) of 656; VAP/HAP, 56 (50.9%) of 110 episodes; BSI, 6 (29.1%) of 110 episodes |
Mortality rate, 30 (40.5%) of 74 vs 33% w/o infection (P < .05) | Hospital LOS |
| Silva et al, 2021 11 | Retrospective cohort, 1 hospital, Brazil (May–November 2020) |
212 | HA bacterial and fungal infection/inpatient | NR | Bacterial, 34 (16%) of 212 | Increased risk of death with bacterial infection | NR |
| Observational studies, United States | |||||||
| Nori et al, 2020 28 | Retrospective cohort, 1 hospital, USA (March–April 2020) |
4,267 | HA bacterial or fungal infection, inpatient | Time to culture positivity, 6–7 d | Bacterial/fungal, 152 (3.6%) of 4,267 | Mortality rate, 68 (57%) of 152 | Hospital LOS, ICU admission |
| Gomez-Simmonds et al, 2021 45 | Retrospective cohort, 1 hospital, USA (March–April 2020) |
3,152; 13 with CPE infection | HA CPE infection/inpatient | NR | CPE, 13 (0.4%) of 3,152; respiratory, 11 (0.3%) of 3,152; bacteremia, 7 (0.2%) of 3,152 | Mortality rate, 5 (38.5%) of 13 | ICU admission rate and MV rate |
| Adelman et al, 2021 37 | Retrospective cohort, 4 hospitals, USA (February–May 2020) |
774 | HA bacterial pneumonia and BSIs/inpatient | NR | BSI, 36 (4.7%) of 774; respiratory, 65 (27.3%) of 238 intubated patients; VAP, 2% |
Mortality rate in patients with BSI, 50%; m ortality rate in intubated patients, 41.5% |
NR |
| Chong et al, 2021 40 | Retrospective cohort, 1 hospital, USA (March–June 2020) |
244 | HA pulmonary bacterial infection/inpatient | ≥48 h after admission | Pulmonary, 13 (5%) of 244 | No difference in mortality vs w/o infection | hospital LOS, ICU admission rate, MV rate |
| Obata et al, 2020 48 | Retrospective cohort, 1 hospital, USA (March–May 2020) |
226: 57 received steroid; 169 w/o steroids (n=169) | HA bacterial infection/inpatient | NR | Bacterial infection in steroid group, 14 (24.6%) of 57 vs 19 (11.2%) of 169 w/o steroids | NR d | NR d |
| Kimmig et al, 2020 47 | Retrospective cohort, 1 ICU, USA (March–April 2020) | 111: 48 treated with tocilizumab; 63 w/o tocilizumab | HA bacterial infection/inpatient | NR | Patients treated with tocilizumab: bacterial, 24 (50%) of 48, VAP/HAP, 18 (37.5%) of 48. Patients w/o tocilizumab: bacterial, 18 (28.6%) of 63, VAP/HAP, 11 (17.5%) of 63 | NR d | NR d |
| Observational studies, Europe | |||||||
| Ripa et al, 2020 19 | Cohort study, 1 hospital, Italy (February–April 2020) | 731 | HA bacterial infection/inpatient | ≥48 h after admission | Overall infection, 68 (9.3%) of 731g; possible LRTI 22 (3.0%) of 731; BSI, 58 (7.9%) of 731 | Mortality rate, 30 (44.1%) of 68 vs 24.7% w/o HA infection (P < .001) |
ICU admission rate |
| Posteraro et al, 2021 35 | Retrospective cohort, 1 hospital, Italy (March–May 2020) |
293; 46 with BSI | HA BSI/inpatient | >48 h after admission or after discharge from previous hospital | BSI, 46 (15.7%) of 293g | Mortality rate, 20 (43.5%) of 46 vs 52 (24.2%) of 215 w/o positive blood cultures (P = .008) | NR |
| Guisado-Gil et al, 2020 50 | Retrospective cohort, 1 hospital, Spain (March–May 2020) |
282 | HA candidemia and MDR BSIs/inpatient | HA, >48 h after admission | Incidence candidemia and bacterial BSI per 1,000 OBD: Q1 2020, 0.37 cases; Q2 2020, 0.24 cases | Mortality rate, 17.6% at day 14 and 26.5% at day 30 in patients with MDR BSIs | NR |
| Magnasco et al, 2020 51 | Retrospective cohort, 2 ICUs, Italy (February– May 2020) | 118 | HA drug-resistant infectione/inpatient | HA, 10–30 d after ICU admission | Drug resistant, 14 (11.9%) of 118; CRPA, 12 (10.2%) of 118; CR-Kp, 2 (1.6%) of 118f | Patients with CRPA: crude mortality rate, 5 (41.7%) of 12. Patients with CR-Kp: observed mortality rate, 1 (50%) of 2 |
ICU LOS |
| Pink et al, 2021 20 | Retrospective cohort, 1 hospital, Germany (March–October 2020) | 99 | HA bacterial infection/inpatient | NR | Bacterial, 32% | NR d | NR d |
| Bogossian et al, 2020 42 | Retrospective case control study, 1 ICU, Belgium (March–April 2020) | 72 | HA MDR bacterial infection/inpatient | NR | MDR bacterial, 24 (33%) of 72 | ICU mortality rate, 6 (25%) of 24; hospital mortality rate, 6 (25%) of 23 |
Hospital LOS, ICU LOS, MV rate, MV duration |
| García-Meniño et al, 2021 44 | Case series, 1 ICU, Spain (February 2020) |
62 | HA CP-Kp infection/inpatient | 4–15 d after ICU admission | CP-Kp, 7 (11.3%) of 62 | Mortality rate, 1 (14.3%) of 7 | MV rate |
| Buehler et al, 2021 30 | Prospective cohort study, 1 ICU, Switzerland (April–June 2020) | 45 | HA pulmonary infection/inpatient | Day 10 after ICU admission (mean) | Bacterial/fungal, 19 (42.2%) of 45 | NR | ICU LOS, MV duration |
| Montrucchio et al, 2020 65 | Cohort study, 1 ICU, Italy (March–May 2020) | 35 | HA CP-Kp infection/inpatient | 6–22 d after ICU admission | CP-Kp, 7 (20%) of 35 | 28-d mortality rate, 2 (28.6%) of 7 | ICU LOS, MV duration |
| Observational studies, Asia | |||||||
| Lee et al, 2021 38 | Retrospective cohort, 1 hospital Korea (February– July 2020) | 140 | HA infection/inpatient | 5.8 ± 6.7 d after admission | Overall secondary infection, 31 (22.1%) of 140; secondary bacterial infection, 30 (21.4%) of 140 | Mortality rate, 6.5% vs 0% w/o HA infection (P = .048) | MV rate |
| Yang et al, 2020 29 | Retrospective cohort, 1 ICU, China (December 2020–January 2021) | 52 | HA bacterial and fungal infection/inpatient | NR | Bacterial/fungal, 7 (13.5%) of 52 | NR d | NR d |
| Fu et al, 2020 43 | Retrospective cohort, 1 ICU, China (February–April 2020) | 36 | HA bacterial infection/inpatient | Average time from ICU admission, 11 d | Bacterial, 5 (13.9%) of 36 | Mortality rate, 1 (20%) of 5 | MV rate |
Note. BSI, bloodstream infections; CPE, carbapenemase-producing Enterobacterales; CR-Kp, carbapenem-resistant Klebsiella pneumoniae; CRPA, carbapenem-resistant Pseudomonas aeruginosa; ED, emergency department; EU, European Union; HA, hospital-acquired; HAP, hospital-acquired pneumonia; HFNT, high flow nasal therapy; ICU, intensive care unit; LOS, length of stay; MDR, multidrug resistant; MV, mechanical ventilation; NR, not reported; OBD, occupied bed d; OR, odds ratio; patients, patients; VAP, ventilator-associated pneumonia; w/o, without.
Based on published information, including clinical details, or on the time of infection diagnosis: ≥4 d of hospitalization = hospital-acquired infection, unless otherwise stated in the source.
Rates were reported per total number of patients with COVID-19.
Data for hospital/ICU LOS, ICU admission rates, MV rates, MV duration in patients with COVID-19 who secondary bacterial infections.
Outcomes were reported in total patient population.
Table 2.
Summary of Included Studies Involving Patients With COVID-19 and Community-Acquired (CA) Bacterial Coinfections or Hospital-Acquired (HA) Secondary Bacterial Infections
| Study | Study Type/Date | Total COVID-19 Patients, No. | Coinfection or Secondary Infection Type a /Acquisition Setting | Time of Infection Diagnosis a | Rate of Secondary Bacterial Infection or Coinfection b | Mortality Outcomes in COVID-19 Patients With Secondary Bacterial Infection or Coinfection c | Other Outcomes Reported in COVID-19 Patients With Secondary Bacterial Infection or Coinfection c |
|---|---|---|---|---|---|---|---|
| Observational studies, United States | |||||||
| Singh et al, 2021 33 | Analysis of respiratory samples, 1 laboratory, USA (March–August 2020) | 4,259 | CA and HA respiratory bacterial infections/outpatient and inpatient | NR | Bacterial, 33.2% | NR | NR |
| Kubin et al, 2021 21 | Retrospective cohort, 1 hospital, USA (March–May 2020) |
3,028 | CA and HA bacterial/fungal infection/outpatient and inpatient | ≤72 h of hospitalization or ≤5 d prior to admission from outpatient/ED visit (CA infection); after hospital day 3 (HA infection) | Overall bacterial/fungal infection, 516 (17.0%) of 3,028; CA infection, 183 (6.0%) of 3,028; HA infection, 350 (11.6%) of 3,028 |
Mortality rate, 168 (33%) of 516 | Hospital LOS, ICU admission rate, MV rate |
| Cusumano et al, 2020 25 | Retrospective case series, 2 hospitals, USA (March–May 2020) |
2,679; 42 with S. aureus bacteremia | CA and HA Staphylococcus aureus bacteremia/outpatient and inpatient | On admission; ≥4 d after admission (HA bacteremia) |
S. aureus bacteremia, 42 (1.6%) of 2,679; HA-bacteremia, 28 (66.7%) of 42 |
14-d mortality rate, 23 (54.8%) of 42; 30-d mortality rate, 28 (66.7%) of 42 | Hospital LOS |
| Observational studies, Europe | |||||||
| Amin-Chowdhury et al, 2020 46 | Prospective national cohort study, England (February–June 2020) | 160,886 | Bacterial coinfection with IPD/outpatient and inpatient | Coinfection, ≤2 d of positive COVID test | Coinfection, 40 (0.025%) of 160,886 | Mortality rate (<28 d), 25 (63.2%) of 40 | NR |
| Russell et al, 2021 41 | Prospective cohort, 260 hospitals, UK (February–June 2020) | 48,902 | CA and HA-acquired bacterial infection/outpatient and inpatient | ≤2 d of admission (coinfection) and ≥3 d (HA infection) | Respiratory or BSI, 1,107 (2.3%) of 48,902g; unrelated infections, 1,002 (2.0%) of 48,902g; 70.6% of infections were HA |
No association between respiratory infection or BSI and mortality in ICU patients | NR |
| Calderón-Parra et al, 2021 53 | Retrospective cohort SEMI-COVID-19 registry, 150 hospitals, Spain (March–June 2020) | 13,932 | CA and HA bacterial infection/outpatient and inpatient | NR | NR | NR d | NR d |
| Sharov et al, 2020 26 | Two sampling sets, Russia (March–May 2020) |
Set 1: 147 of 3,382 patients with COVID-19-related pneumonia; Set 2: 1,204 patients with pneumonia and COVID-19 | CA and HA bacterial pneumonia/outpatient and inpatient | At admission, day 4, and day 10 of hospitalization or with clinical deterioration | Set 1: bacterial pneumonia, 61 (41.5%) of 147. Set 2: 433 (36.0%) of 1,204; HA, 239 (55.2%) of 433 | Set 1: 91.7% of lethal COVID-19 cases associated with secondary bacterial pneumonia. Set 2: patients with diagnosed bacterial pneumonia, 57 (17.7%) of 322 |
NR |
| Garcia-Vidal et al, 2021 15 | Retrospective cohort, 1 hospital, Spain (February–April 2020) |
989 | CA bacterial infection/outpatient | ≤24 h of admission | CA bacterial infections, 25 (2.5%) of 989; bacterial pneumonia, 21 (2.1%) of 989 | Mortality rate, 5 (16.1%) of 31 patients with CA coinfections | Hospital LOS, ICU admission rate, ICU LOS |
| HA bacterial infection/inpatient | ≥48 h after admission; mean time to diagnosis, 10.6 d | HA bacterial infections, 38 (3.8%) of 989 | Mortality rate, 8 (18.6%) of 43 patients with HA superinfections | Hospital LOS, ICU admission rate, ICU LOS | |||
| Hughes et al, 2021 23 | Retrospective cohort, 2 hospitals, UK (February–April 2020) |
836 | CA bacterial infection/outpatient | <5 d from admission (CA infection) | 27 (3.2%) of 836 early bacterial infections (0–5 d after admission); bacterial CA pathogens, 14 (35.9%) of 39 among 112 respiratory samples taken |
Relative risk of death of patients with true pathogens in blood against baseline of admitted patients, 1.51 (P = .3543) | |
| HA bacterial infection/inpatient | >5 d from admission (HA infection) | 51 (6.1%) of 836 bacterial infections throughout admission; bacterial HA pathogens, 25 (64.1%) of 39 among 112 respiratory samples taken |
|||||
| Rouze et al, 2021 39 | Retrospective cohort, 36 ICUs, EU (March–May 2020) | 568 | CA and HA pneumonia infection/outpatient and inpatient | ≤48 h intubation (n=359) | Overall pneumonia, 55 (9.7%) of 568; pneumonia with <48 h hospital stay, 29 (8.1%) of 359 | 28-d mortality rate, 24 (43.6%) of 55; increased adjusted HR for 28-d mortality, 1.57 (95% CI, 1.01–2.44; P =.043) | ICU LOS, ICU LOS, MV duration, ICU mortality |
| Baskaran et al, 2021 36 | Retrospective cohort, 7 ICUs, England, (February–May) | 254 | CA and HA infection/outpatient and inpatient | <48 h (CA infection), >48 h (HA infection) | Overall bacterial infection, 83 (32.7%) of 254g; bacterial CA, 14 (5.5%) of 254; bacterial/fungal HA, 77 (30.3%) of 254 | Mortality rate, 8 of 43 (18.6%); P = .047 vs patients w/o HA infection |
ICU LOS |
| Foschi et al, 2021 32 | Retrospective cohort, Italy, ICUs, (March–December 2020) | 178 critically ill | CA and HA respiratory bacterial infection, mostly HA/outpatient and inpatient | NR | Respiratory bacterial infections, 79 (34.3%) of 230 samples among 178 patients | NR | NR |
| Søgaard et al, 2020 16 | Retrospective cohort, 1 hospital, Switzerland (February–May 2020) | 162 | CA and HA respiratory tract infection/outpatient and inpatient | ≤48 h of admission (CA infection) | Bacterial CA pneumonia and bacteremia, 1 (0.6%) of 162; bacterial HA infection, 23 (13.6%) of 162 | NR | NR |
| Observational studies, Asia | |||||||
| Tan et al, 2020 59 | Antibiotic use point prevalence survey, 2 hospitals, Singapore (April 2020) | 577 (554 confirmed, 23 suspected) | CA and HA infection/outpatient and inpatient | NR | NR | NR d | NR d |
| Chen et al, 2021 27 | Retrospective cohort, 1 hospital, China (January–March 2020) | 408 | CA and HA infection/outpatient and inpatient | <48 h (CA infection); >48 h (HA infection) | Bacterial/viral CA, 33 (8.1%) of 408; bacterial/fungal HA, 21 (5.1%) of 408 | NR d | NR d |
| Nasir et al, 2021 24 | Retrospective case-control study, 1 hospital, Pakistan, (February–June 2020) | 100: 50 cases with and 50 controls w/o bacterial infection | CA and HA bacterial infection/outpatient and inpatient | NR | 28% CA bacterial infections and 72% HA bacterial infections. Most common infection: HA pneumonia, 28 (56%) of 50; CA pneumonia, 8 (16%) of 50 |
Mortality rate, 21 (42%) of 50, vs 18% w/o bacterial infection (P < .05) |
Hospital LOS, ICU admission rate, MV rate |
| Meta-analyses and reviews | |||||||
| Langford et al, 2021 52 | Systematic review/meta-analysis, 154 studies (December 2019–May 2020) | 35,263 | Coinfection and secondary bacterial infection/outpatient and inpatient | NR | Bacterial coinfection, 8.6% from 31 studies pooled | NR | NR |
| Lansbury et al, 2020 34 | Systematic review/meta-analysis, 30 studies (January–April 2020) | 3,834 | Coinfection and secondary infection/outpatient and inpatient | NR | Bacterial, 7% for hospitalized patients; 14% for ICU patients | Crude pooled OR for death patients with vs w/o coinfection, 5.82 (95% CI, 3.4–9.9) | NR |
| Vazzana et al, 2021 64 | Systematic review/meta-analysis, 355 studies (December 2019–April 2020) |
3,492 | CA and HA bacterial infection/outpatient and inpatient | NR | Secondary bacterial infections, 4.8%–19.5% from 8 studies pooled | Risk of severe course and/or fatal outcomes was significantly increased in patients with evidence of bacterial infection (OR, 20.8; 95% CI, 11.6–37.4) | NR |
| Langford et al, 2020 22 | Systematic review/meta-analysis, 24 studies (December 2019–March 2020) | 3,338 | Coinfection and secondary bacterial infection/outpatient and inpatient | On presentation (coinfection); emerging during illness or hospital stay (secondary infection) | Overall bacterial, 6.9%; CA, 3.5%; HA, 14.3% | NR | NR |
| Rawson et al, 2020 31 | Systematic review, 9 studies (January–April 2020) | 806 (infection rates); 2010 (antimicrobial prescribing) | CA and HA bacterial and fungal infection/outpatient and inpatient | NR | Bacterial/fungal infection, 62 (8%) of 806 | NR | NR |
Note. BSI, bloodstream infections; CA, community acquired; CABP, community-acquired bacterial pneumonia; CPE, carbapenemase-producing Enterobacterales; CRKp, carbapenem-resistant Klebsiella pneumoniae; CRPA, carbapenem-resistant Pseudomonas aeruginosa; ED, emergency department; EU, European Union; HA, hospital-acquired; HAP, hospital-acquired pneumonia; ICU, intensive care unit; IPD, invasive pneumonococcal disease; LOS, length of stay; MDR, multidrug resistant; MV, mechanical ventilation; NR, not reported; OBD, occupied bed days; OR, odds ratio; patients, patients; VAP, ventilator-associated pneumonia; w/o, without.
Based on published information, including clinical details, or on the time of infection diagnosis: outpatient/≤3 d of hospitalization = community acquired infection; ≥4 d of hospitalization = hospital-acquired infection, unless otherwise stated in the source.
Rates were reported per total number of patients with COVID-19.
Data for hospital LOS, ICU admission rates in patients with COVID-19 who secondary bacterial infections or coinfections.
Outcomes were reported in total patient population.
Respiratory tract infections and bloodstream infections were the most common bacterial HA infections observed. 14,23 Specifically, in a case–control study of 50 COVID-19 patients with bacterial infections, 56% had HA bacterial pneumonia versus 16% with CA pneumonia. 24 The higher rates of HA infections may be linked to ICU admission, ventilator-associated infections, and prolonged hospital stay. 16 Indeed, a single-center study of hospitalized patients with COVID-19 in the United States reported that ICU stay and mechanical ventilation were independent predictors of HA infection in patients hospitalized with COVID-19. 21 In several studies the rates of HA and/or ventilator-associated pneumonia (VAP) infection were >50% (Table 3). 12,24–26
Although we specifically looked at bacterial infections, a few studies reported rates both for bacterial and fungal infections together. 21,27–30 Rates reported in hospitalized patients with COVID-19 varied from 3.6% up to 42.2% (Tables 2–3). 28,30 In a meta-analysis including 9 studies, 8% of patients with COVID-19 experienced bacterial or fungal coinfections during hospital admission. 31
Etiology of bacterial infections
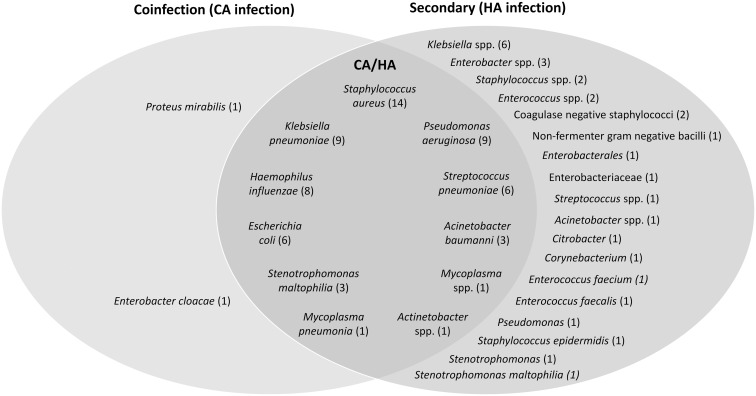
Common microorganisms causing secondary bacterial infections and/or bacterial coinfections in patients with COVID-19 are shown in Figure 1. No clear pattern of preponderant pathogens was observed; however, the most frequently reported pathogens associated with both CA and HA infections were Escherichia coli, Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Haemophilus influenzae, Acinetobacter baumannii, Mycoplasma spp, M. pneumoniae, Stenotrophomonas maltophilia, and Acinetobacter spp (Fig. 1). 22,24,26,27,32–34 Notably, only 2 additional microorganisms were observed to only cause CA infection: Enterobacter cloacae (2 cases among 5 patients with bacterial respiratory infections) 18 and Proteus mirabilis (18 (8%) of 221 cultures from 183 patients with COVID-19 and CA infections). 21 Each was reported in 1 study. A wide range of other pathogens were reported to only cause HA infection across a range of studies (Fig. 1). 11,12,15,28,30,35–38
Fig. 1.
Common etiologies of bacterial coinfections and/or secondary bacterial infections in patients with COVID-19. The most frequently reported bacterial microorganisms from 22 studies (up to 5 of the most common bacterial microorganisms) were included for each type of infection from each study: 6 studies for CA infection, 17,18,21,27,36,63 12 studies for HA infection, 11,12,15,20,27,28,30,35–38,40 and 6 studies for both CA and HA 22,24,26,32–34 (studies could report >1 type of infection). The number of studies that reported each organism are shown in parenthesis. Note. CA, community-acquired; COVID-19, coronavirus disease 2019; HA, hospital-acquired.
In a retrospective study of 254 hospitalized patients with COVID-19, the proportion of pathogens detected increased with the duration of ICU stay, consisting mainly of gram-negative bacteria, particularly K. pneumoniae and E. coli. 36 In contrast, S. aureus and S. pneumoniae were the pathogens most commonly detected within 48 hours of hospital admission. 36 Similarly, a retrospective study of 3,028 hospitalized COVID-19 patients showed that the proportion of gram-negative bacteria causing HA infections increased with longer hospital stay, whereas staphylococci were more commonly isolated within the first 14 days of hospitalization. 21 Beyond day 14 of hospitalization, Enterobacterales and Pseudomonas spp predominated. 21 Overall, this finding reflects an increased acquisition of pathogens and wider range of organisms with length of hospitalization.
Impact of secondary bacterial infections and bacterial coinfections on outcomes in patients with COVID-19
Mortality rates reported in patients with COVID-19 who had bacterial coinfections and/or secondary bacterial infections ranged between 6.5% and 66.7%; however, observation periods and population differed, which may account for the wide variation in rates (Tables 1–3).
In several studies, mortality rates were significantly higher in COVID-19 patients with bacterial coinfections or secondary bacterial infections compared with those without. 12,14,15,19,24,35,37–39 Notably, in one study, high 14-day mortality rates (54.8%) and 30-day mortality rates (66.7%) were reported among 42 hospitalized patients with COVID-19 and S. aureus bacteremia. 25 In a second study of 1,705 patients with COVID-19, mortality rates were significantly higher in patients with CA bacterial infections compared to those without (47.5% vs 18.0%; P < .001). 14 However, in other studies, no difference in mortality rates between patients with and without bacterial coinfection or secondary bacterial infection was reported, with an overall mortality rate at 28 days of 31.5% in patients with COVID-19. 40,41 These discrepancies may be due to low sample size in some studies, leading to inadequate power to detect a mortality difference. 40 In other studies, most deaths occurred early during hospitalization; therefore, less time was available to collect microbiological samples. 41 This finding suggests that the rate of bacterial infections in patients with COVID-19 might be underestimated.
Along with increased mortality, other noteworthy trends among COVID-19 patients with bacterial coinfection or secondary bacterial infection included prolonged length of hospital stay, 14,15,21,24,40 more frequent ICU admission, 13,15,19,21,24,40 and use of invasive mechanical ventilation. 21,24,28,38,40,42–45 In a study of 100 COVID-19 patients, patients severely or critically ill at the time of admission were 4.4 times more likely to develop a bacterial infection, 24 and those with bacterial infections were more likely to be admitted to the ICU compared with patients without bacterial infections (56% vs 18%; P < .001). 24 Similarly, patients with CA bacterial pneumonia (CABP) were more likely to be admitted to the ICU compared with patients without coinfections (33% vs 16%; P < .01). 13 Interestingly, in a single-center retrospective study of 989 patients, hospital length of stay was only significantly increased in patients with HA bacterial infections, and not in those with CA bacterial infections. 15 Furthermore, other studies have also reported that patients with bacterial coinfections or secondary bacterial infections were older in age 13–15,17,21,33,36,38,46,47 and were immunocompromised. 15,21,48 These populations typically at greater risk of developing severe COVID-19 and frequently have chronic underlying conditions and comorbidities, such as diabetes, kidney disease, or cancer. 14,15,27
Nasir et al 24 reported that a larger proportion of patients with COVID-19 and bacterial infections received treatment with systemic steroids compared with patients without bacterial infections (92% vs 62% respectively; P = .001) and that treatment with steroids was a significant risk factor for bacterial infections. 24 In a study of 226 hospitalized COVID-19 patients, treatment with steroids increased the risk of bacterial infections but steroid use did not affect the mortality rate (Table 3). 48 In another study of 111 hospitalized COVID-19 patients, tocilizumab use was associated with patients with high risk of developing bacterial or fungal infections (Table 3). 47 Although mortality in the group of patients who received tocilizumab was higher than those not receiving treatment (39.6% vs 17.4% respectively; P = .016), 47 this may be due to the fact that patients in the tocilizumab group were sicker and tocilizumab use predisposes to secondary bacterial infections.
Antibiotic treatment approaches in patients with COVID-19 and secondary bacterial infections or bacterial coinfections
Considerable heterogeneity in reported treatment rates and antibiotic treatment approaches was reported across the studies included in this review, perhaps in part due to the variability in study locations and differing local and national guidelines to antibiotic treatment (Fig. 2 and Fig. 3). National US guidelines (from the National Institutes of Health), updated in April 2021, recommend empiric antibiotics if secondary bacterial pneumonia or sepsis is suspected in patients with COVID-19 but to re-evaluate patients daily and de-escalate or stop antibiotic treatment if there is no evidence of bacterial infection. 49
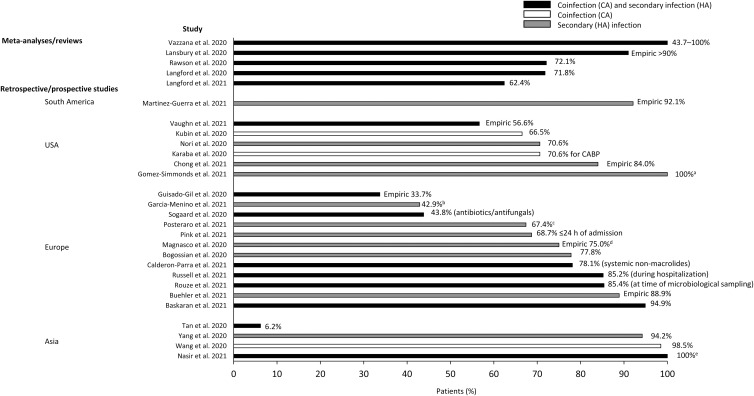
Fig. 2.
Proportion of patients with COVID-19 receiving antibiotics: (a) in patients with COVID-19 and carbapenemase-producing Enterobacterales; (b) in patients with carbapenem-resistant Klebsiella pneumoniae; (c) in patients with COVID-19 and bloodstream infection; (d) in patients with carbapenem-resistant Pseudomonas aeruginosa for suspected bacterial superinfection; and (e) in patients with COVID-19 and bacterial infection. Note. CA, community-acquired; COVID-19, coronavirus disease 2019; HA, hospital-acquired.
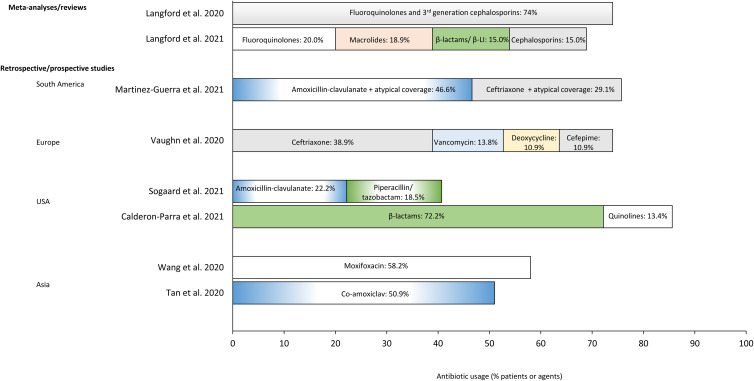
Fig. 3.
Most frequently used antibiotics in patients with COVID-19.a (a) Studies expressed data as percentage of patients receiving antibiotic treatments, except the study by Langford et al, 52 in which data were presented as percentage of prescriptions of an antibiotic class per total number of antibiotic prescriptions. Data are provided only for antibiotic classes that were used in >10% of patients or > 10% of prescriptions. Note. β-LI, β-lactamase inhibitors; COVID-19, coronavirus disease 2019.
Despite the low rates of secondary bacterial infections observed, most studies reported the use of empiric antibiotic treatment, with 33.7% to > 90% of COVID-19 patients treated (Tables 2 and 3). 12,30,34,40,50,51 However, data are limited and information was not available on the duration of treatment. Although many patients did not have a confirmed bacterial infection at the start of treatment, data were not available on patients who stopped or altered treatment once microbial testing to confirm bacterial infection was performed. The variation in the range of patients receiving antibiotics could be explained by the differences in geographic location, the diversity of the populations treated, the time when studies were done, and so on.
These findings suggest that antibiotic utilization was high in patients who did not have bacterial infection. In a study of 48 COVID-19 patients, no significant difference was reported in the use of empiric antimicrobial therapy in critically ill patients either with bacterial superinfection (88%) or without bacterial superinfection (94.7%). 30 Notably, all studies that included data on both infection rates and antibiotic use reported mismatch between use of antibiotics versus confirmed secondary or coinfection, regardless of whether infection was CA or HA (see Tables 1–3 and Fig. 2). 12,14,21 In a meta-analysis of patients with COVID-19, the prevalence of antibiotic prescribing was 62.4%, whereas the estimated rate of bacterial coinfection was 8.6%. 52 In a systematic review reporting bacterial and fungal coinfections in 806 patients with COVID-19, 72.1% received antimicrobial therapy despite only 8% of patients having bacterial or fungal coinfections during their hospitalization. 31
A recent meta-analysis of antibiotic prescribing in 30,623 patients with COVID-19 reported considerable heterogeneity across regions with a prevalence of 63.1% (95% confidence interval [CI], 41.7%–80.4%) in Europe, 64.8% (95% CI, 54.0%–74.2%) in the United States, 76.2% (95% CI, 66.8%–82.3%) in China, 86.0% (95% CI, 77.4%–91.7%) in the Middle East, and 87.5% (95% CI, 47.8%–98.2%) in East and Southeast Asia (excluding China). 52 Only 5 (3.2%) of 154 studies included in this meta-analysis provided data on duration of antibiotic treatment. 52 Antibiotic stewardship strategies were reported in 3 studies (1.9%), including recommendations to avoid antibiotics in patients without suspected coinfection (n = 2) or to de-escalate antibiotics when additional data became available (n = 1). 52 In a retrospective study of 13,932 hospitalized patients with COVID-19 who were prescribed antibiotics in 150 hospitals in Spain from March 1 to June 23, 2020, antibiotics were prescribed for respiratory bacterial coinfections and/or secondary infections in 10.9% of patients with COVID-19 and 43.8% of total antibiotic prescriptions were considered inappropriate. 53 Interestingly, younger age and fewer comorbidities were independently associated with inappropriate antibiotic prescribing. 53 Notably, a lower percentage of inappropriate antibiotic prescribing was observed in patients hospitalized after March 2020 in this study, which suggests increased awareness of the problem among healthcare professionals and a better understanding of the disease.
The types of antibiotics prescribed differed across the studies we reviewed, although most were broad-spectrum agents, including fluoroquinolones, β-lactam and β-lactamase inhibitors, cephalosporins, macrolides, and penicillin-like agents (Fig. 3). This pattern of antibiotic prescribing likely reflects the empirical use of these agents, which tends to provide coverage of multiple organisms while awaiting culture results or confirmation of coinfection or secondary infection.
The potential overuse or misuse of antibiotics in the context of the COVID-19 pandemic could contribute to increased AMR. 54 AMR has been widely reported, including infections with multidrug-resistant (MDR) organisms, 15,16,19,28,30,35,37,42 and methicillin-resistant Staphylococcus aureus. 25,32,34–36,40,48 In a study of 989 COVID-19 patients, MDR gram-negative bacteria were isolated in 7 of 43 patients with HA infections: 3 had MDR P. aeruginosa infection, 2 had extended-spectrum β-lactamase E. coli, and 2 extended-spectrum β-lactamase K. pneumoniae. 15 Søgaard et al 16 only reported 1 MDR pathogen (Acinetobacter baumannii, Oxa-23) isolated in a case transferred from a hospital abroad. Buehler et al 30 reported that MDR bacteria (Pseudomonas aeruginosa, Enterobacter cloacae, and Burkholderia cepacia) were detected in 22.2% of all hospitalized COVID-19 patients.
Increased AMR leads to high exposure to antibiotic treatments, which can have detrimental consequences and can facilitate subsequent infections during ICU stay, particularly by gram-positive pathogens such as enterococci. 55 In a US cohort study of hospitalized patients with sepsis, inadequate broad-spectrum empiric antibiotic treatment was associated with ICU hospitalization and increased mortality. 56 Interestingly, inadequate antibiotic therapy was 4 times more likely in patients with resistant pathogens (eg, methicillin-resistant Staphylococcus aureus) than with nonresistant pathogens (P < .001), older patients, and patients with comorbidities. 56 Thus, improved treatment strategies (antimicrobial stewardship) and treatment options with newer antibiotics that have lower resistance rates are needed.
Antibiotic stewardship perspectives
The incidence of secondary bacterial infections and bacterial coinfections in patients with COVID-19 is relatively low, with lower rates of CA bacterial infections than HA infections. The incidence and variety of infecting pathogens increased with the length of hospitalization. Overall, the rates of secondary bacterial infections and bacterial coinfections in patients with COVID-19 were lower than rates of secondary bacterial infection and/or coinfection associated with other viral respiratory diseases such as influenza. 57 The relatively low incidence of bacterial coinfections and/or secondary infections reported during the COVID-19 pandemic could be a consequence of the implementation of national lockdowns and social distancing measures adopted by many countries during the pandemic, as was suggested in an international study demonstrating that COVID-19 lockdowns significantly reduced transmission of S. pneumoniae, H. influenzae, and N.meningitidis, leading to significant reductions in life-threatening invasive diseases worldwide. 58
Despite the relatively low rates of bacterial secondary infections and/or bacterial coinfections observed during the COVID-19 pandemic, high percentages of patients have been receiving antibiotic treatment. Empiric treatment was common, perhaps because COVID-19 patients are often hospitalized during the hyperinflammatory phase of the disease, making differentiation between viral and secondary bacterial infections challenging. 59 From a clinical perspective, the mismatch between antibiotic utilization and reported rates of bacterial infection is of particular concern because it may exacerbate the development of AMR and associated complications.
Increased empiric antibiotic prescribing may have been due to the diversion of stewardship efforts to pandemic responsibilities and away from core activities. 60 Investigation of the optimal antimicrobial stewardship program interventions into pandemic response efforts to limit antibiotic overuse is warranted. However, despite national guidelines aiming to rationalize antibiotic use and maintain safe medication use in the ICU, 49,61,62 the emergency caused by the COVID-19 pandemic probably made it difficult to apply these guidelines, with overwhelmed wards and ICUs and busy healthcare professionals. Moreover, the diagnosis of bacterial infections remains a challenge, and it is difficult to distinguish between severe viral pneumonia and bacterial infection. Microbiological investigations, which are not routinely performed in patients with COVID-19, take several days to result and do not differentiate bacterial colonization from infection. 14
Thus, the pandemic may have a lasting impact on AMR, and the long-term impact on antibiotic overuse during COVID-19 pandemic remains to be seen. The data reported here show multidrug-resistance pathogens and indicate that current empiric treatment strategies may not be effective. The development of newer antibiotics is urgently needed, particularly considering the increase in multidrug resistance for which there are no treatment options.
Although a strength of this review is the use of a comprehensive search strategy, several limitations must be considered. First, most of the included studies were small, retrospective, observational studies, with a large degree of heterogeneity between them in terms of patient populations, geographic locations, and treatment protocols. Many of the included studies lacked consistent bacteriological diagnostic and specific testing upon patient admission to hospital, which likely affected stratification of CA versus HA infections. Some studies did not give precise details regarding the timing of diagnosis, making the differentiation between CA and HA challenging. Finally, most studies included in this review were from Asia, Europe, and North America (United States), and regional differences in the patient populations, access to care, clinical practices among hospitals, and patient follow-up must be considered.
To conclude, recent data indicate that secondary bacterial infections and bacterial coinfections in patients with COVID-19 are associated with worse patient outcomes. Importantly, antibiotic utilization was consistently higher than bacterial infection rates, highlighting the need to improve appropriate treatment approaches to mitigate the complications of the misuse of antibiotics. Furthermore, due to the incidence of multidrug-resistant bacterial pathogens, new treatment and antibiotics that could overcome the problem of resistance are urgently needed. Implementing and following stewardship programs will be of crucial importance to prevent the development of resistance and to improve patient outcomes.
Acknowledgments
The authors thank Meridian HealthComms (Plumley, UK) for medical writing support, funded by Paratek Pharmaceuticals (King of Prussia, PA) in accordance with Good Publication Practice (GPP3).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/ash.2022.253.
click here to view supplementary material
Financial support
Support for medical writing by Meridian HealthComms was funded by Paratek Pharmaceuticals.
Conflicts of interest
E.M.G. is an employee of Dompé US (Boston, MA). All other authors declare no conflicts of interest related to this article.
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2019;7:69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008;198:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol 2017;8:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 6. Byarugaba DK. A view on antimicrobial resistance in developing countries and responsible risk factors. Int J Antimicrob Agents 2004;24:105–110. [DOI] [PubMed] [Google Scholar]
- 7. Strathdee SA, Davies SC, Marcelin JR. Confronting antimicrobial resistance beyond the COVID-19 pandemic and the 2020 US election. Lancet 2020;396:1050–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014;5:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imwattana K, Knight DR, Kullin B, et al. Antimicrobial resistance in Clostridium difficile ribotype 017. Expert Rev Anti Infect Ther 2020;18:17–25. [DOI] [PubMed] [Google Scholar]
- 10. National Healthcare Safety Network (NHSN). CDC/NHSN surveillance definitions for specific types of infections. Centers for Disease Control and Prevention website. https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. Accessed July 19, 2021.
- 11. Silva DL, Lima CM, Magalhaes VCR, et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J Hosp Infect 2021;113:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinez-Guerra BA, Gonzalez-Lara MF, de-Leon-Cividanes NA, et al. Antimicrobial resistance patterns and antibiotic use during hospital conversion in the COVID-19 pandemic. Antibiotics (Basel) 2021;10:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karaba SM, Jones G, Helsel T, et al. Prevalence of coinfection at the time of hospital admission in COVID-19 patients, a multicenter study. Open Forum Infect Dis 2021;8:ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaughn VM, Gandhi T, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clin Infect Dis 2021;72:e533–e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, et al. Incidence of coinfections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 2021;27:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sogaard KK, Baettig V, Osthoff M, et al. Community-acquired and hospital-acquired respiratory tract infection and bloodstream infection in patients hospitalized with COVID-19 pneumonia. J Intensive Care 2021;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thelen JM, Buenen AGN, van Apeldoorn M, Wertheim HF, Hermans MHA, Wever PC. Community-acquired bacteraemia in COVID-19 in comparison to influenza A and influenza B: a retrospective cohort study. BMC Infect Dis 2021;21:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020;71:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ripa M, Galli L, Poli A, et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect 2021;27:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pink I, Raupach D, Fuge J, et al. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021;49:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kubin CJ, McConville TH, Dietz D, et al. Characterization of bacterial and fungal infections in hospitalized patients with coronavirus disease 2019 and factors associated with healthcare-associated infections. Open Forum Infect Dis 2021;8:ofab201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langford BJ, So M, Raybardhan S, et al. Bacterial coinfection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020;26:1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 2020;26:1395–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nasir N, Rehman F, Omair SF. Risk factors for bacterial infections in patients with moderate to severe COVID-19: a case–control study. J Med Virol 2021;93:4564–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cusumano JA, Dupper AC, Malik Y, et al. Staphylococcus aureus bacteremia in patients infected with COVID-19: a case series. Open Forum Infect Dis 2020;7:ofaa518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharov KS. SARS-CoV-2–related pneumonia cases in pneumonia picture in Russia in March–May 2020: secondary bacterial pneumonia and viral coinfections. J Glob Health 2020;10:020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen S, Zhu Q, Xiao Y, et al. Clinical and etiological analysis of coinfections and secondary infections in COVID-19 patients: an observational study. Clin Respir J 2021;15:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol 2021;42:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buehler PK, Zinkernagel AS, Hofmaenner DA, et al. Bacterial pulmonary superinfections are associated with longer duration of ventilation in critically ill COVID-19 patients. Cell Rep Med 2021;2:100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020;71:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foschi C, Zignoli A, Gaibani P, et al. Respiratory bacterial coinfections in intensive care unit-hospitalized COVID-19 patients: conventional culture vs BioFire FilmArray pneumonia plus panel. J Microbiol Methods 2021;186:106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh V, Upadhyay P, Reddy J, Granger J. SARS-CoV-2 respiratory coinfections: incidence of viral and bacterial copathogens. Int J Infect Dis 2021;105:617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lansbury L, Lim B, Baskaran V, Lim WS. Coinfections in people with COVID-19: a systematic review and meta-analysis. J Infec 2020;81:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Posteraro B, De Angelis G, Menchinelli G, et al. Risk factors for mortality in adult COVID-19 patients who develop bloodstream infections mostly caused by antimicrobial-resistant organisms: analysis at a large teaching hospital in Italy. J Clin Med 2021;10:1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baskaran V, Lawrence H, Lansbury LE, et al. Coinfection in critically ill patients with COVID-19: an observational cohort study from England. J Med Microbiol 2021;70:001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adelman MW, Bhamidipati DR, Hernandez-Romieu AC, et al. Secondary bacterial pneumonias and bloodstream infections in patients hospitalized with COVID-19. Ann Am Thorac Soc 2021;18:1584–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee SI, Koh JS, Kim YJ, et al. Secondary infection among hospitalized COVID-19 patients: a retrospective cohort study in a tertiary-care setting. Respirology 2021;26:277–278. [DOI] [PubMed] [Google Scholar]
- 39. Rouze A, Martin-Loeches I, Povoa P, et al. Early bacterial identification among intubated patients with COVID-19 or influenza pneumonia: a European multicenter comparative cohort study. Am J Respir Crit Care Med 2021;204:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chong WH, Chieng H, Tiwari A, et al. Incidence and risk factors for secondary pulmonary infections in patients hospitalized with coronavirus disease 2019 pneumonia. Am J Med Sci 2022;363:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Russell CD, Fairfield CJ, Drake TM, et al. Coinfections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe 2021;2:e354–e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bogossian EG, Taccone FS, Izzi A, et al. The acquisition of multidrug-resistant bacteria in patients admitted to COVID-19 intensive care units: a monocentric retrospective case control study. Microorganisms 2020;8:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fu Y, Yang Q, Xu M, et al. Secondary bacterial infections in critical ill patients with coronavirus disease 2019. Open Forum Infect Dis 2020;7:ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. García-Meniño I, Forcelledo L, Rosete Y, García-Prieto E, Escudero D, Fernández J. Spread of OXA-48–producing Klebsiella pneumoniae among COVID-19–infected patients: the storm after the storm. J Infect Public Health 2021;14:50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gomez-Simmonds A, Annavajhala MK, McConville TH, et al. Carbapenemase-producing Enterobacterales causing secondary infections during the COVID-19 crisis at a New York City hospital. J Antimicrob Chemother 2021;76:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective national cohort study, England. Clin Infect Dis 2021;72:e65–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kimmig LM, Wu D, Gold M, et al. IL-6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Front Med (Lausanne) 2020;7:583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Obata R, Maeda T, Do DR, Kuno T. Increased secondary infection in COVID-19 patients treated with steroids in New York City. Jpn J Infect Dis 2020;74:307–315. [DOI] [PubMed] [Google Scholar]
- 49. COVID-19 treatment guidelines. National Institutes of Health (NIH) website. https://www.covid19treatmentguidelines.nih.gov/search?q=antibiotic. Accessed July 20, 2021.
- 50. Guisado-Gil AB, Infante-Domínguez C, Peñalva G, et al. Impact of the COVID-19 pandemic on antimicrobial consumption and hospital-acquired candidemia and multidrug-resistant bloodstream infections. Antibiotics (Basel) 2020;9:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Magnasco L, Mikulska M, Giacobbe DR, et al. Spread of carbapenem-resistant gram-negatives and Candida auris during the COVID-19 pandemic in critically ill patients: one step back in antimicrobial stewardship? Microorganisms 2021;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect 2021;27:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Calderón-Parra J, Muiño-Miguez A, Bendala-Estrada AD, et al. Inappropriate antibiotic use in the COVID-19 era: factors associated with inappropriate prescribing and secondary complications. Analysis of the registry SEMI-COVID. PLoS One 2021;16:e0251340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rawson TM, Moore LSP, Castro-Sanchez E, et al. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother 2020;75:1681–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chakraborty R, Lam V, Kommineni S, et al. Ceftriaxone administration disrupatients intestinal homeostasis, mediating noninflammatory proliferation and dissemination of commensal enterococci. Infect Immun 2018;86:e00674–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open 2020;3:e202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses 2016;10:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health 2021;3:e360–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tan SH, Ng TM, Tay HL, et al. A point prevalence survey to assess antibiotic prescribing in patients hospitalized with confirmed and suspected coronavirus disease 2019 (COVID-19). J Glob Antimicrob Resist 2020;24:45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mazdeyasna H, Nori P, Patel P, et al. Antimicrobial stewardship at the core of COVID-19 response efforts: implications for sustaining and building programs. Curr Infect Dis Rep 2020;22(9):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. CoInfection and antimicrobial stewardship. The Infectious Diseases Society of America (IDSA) website. https://www.idsociety.org/covid-19-real-time-learning-network/disease-manifestations--complications/coinfection-and-Antimicrobial-Stewardship/. Accessed July 20, 2021.
- 62. Safe medication use in the ICU. Society of Critical Care Medicine website. https://www.sccm.org/Clinical-Resources/Guidelines/Guidelines/Safe-Medication-Use-in-the-ICU. Accessed August 10, 2021.
- 63. Hoshiyama T, Wada T, Nihonyanagi S, et al. Clinical and microbiological features of asymptomatic SARS-CoV-2 infection and mild COVID-19 in seven crewmembers of a cruise ship. Intern Med 2020;59:3135–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vazzana N, Dipaola F, Ognibene S. Procalcitonin and secondary bacterial infections in COVID-19: association with disease severity and outcomes. Acta Clin Belg 2020:1–5. [DOI] [PubMed] [Google Scholar]
- 65. Montrucchio G, Corcione S, Sales G, Curtoni A, De Rosa FG, Brazzi L. Carbapenem-resistant Klebsiella pneumoniae in ICU-admitted COVID-19 patients: keep an eye on the ball. J Glob Antimicrob Resist 2020;23:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/ash.2022.253.
click here to view supplementary material