Abstract
Background
Enhanced Recovery After Surgery (ERAS) has been widely applied in liver surgery since the publication of the first ERAS guidelines in 2016. The aim of the present article was to update the ERAS guidelines in liver surgery using a modified Delphi method based on a systematic review of the literature.
Methods
A systematic literature review was performed using MEDLINE/PubMed, Embase, and the Cochrane Library. A modified Delphi method including 15 international experts was used. Consensus was judged to be reached when >80% of the experts agreed on the recommended items. Recommendations were based on the Grading of Recommendations, Assessment, Development and Evaluations system.
Results
A total of 7541 manuscripts were screened, and 240 articles were finally included. Twenty-five recommendation items were elaborated. All of them obtained consensus (>80% agreement) after 3 Delphi rounds. Nine items (36%) had a high level of evidence and 16 (64%) a strong recommendation grade. Compared to the first ERAS guidelines published, 3 novel items were introduced: prehabilitation in high-risk patients, preoperative biliary drainage in cholestatic liver, and preoperative smoking and alcohol cessation at least 4 weeks before hepatectomy.
Conclusions
These guidelines based on the best available evidence allow standardization of the perioperative management of patients undergoing liver surgery. Specific studies on hepatectomy in cirrhotic patients following an ERAS program are still needed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00268-022-06732-5.
Introduction
Enhanced Recovery After Surgery (ERAS) is a multimodal and perioperative management pathway. ERAS offers to reduce the response to surgical stress and has been shown to decrease postoperative complications and length of stay (LoS) after several types of surgery [1, 2].
The first ERAS guidelines for liver surgery were published in 2016 [3]. Since then, several publications have shown that implementation of ERAS in liver surgery improves postoperative outcomes [4, 5]. Three recent meta-analyses showed that ERAS in liver surgery decreased postoperative complications, LoS, and costs [6–8]. In the first ERAS guidelines for liver surgery, 7 out of 23 recommendation items were based on non-liver surgery studies. The selected items described in the current updated recommendations are all issued from studies specifically on liver surgery.
The aim of the present systematic review was to update the guidelines for ERAS in liver surgery by reviewing the current literature and using a modified Delphi method to obtain an expert consensus.
Material and methods
Creation of these guidelines followed the recommendations of the ERAS Society [9].
Literature search and data selection
A systematic review was performed by searching MEDLINE/PubMed, Embase, and the Cochrane Library. For each specific ERAS item, a search query was created by two professional librarians. The specific queries for each item can be found in Supplementary files.
Inclusion and exclusion criteria
Studies included in the previous guidelines and new studies published between January 1, 2010, and May 31, 2020, were considered. Eligible articles were meta-analyses, systematic reviews, reviews, expert consensus, randomized controlled trials (RCT), or prospective studies. Retrospective studies were considered only if better data were not available.
Quality assessment and grade of recommendation
Levels of evidence of the recommendations were based on the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system, where levels were graded as low, moderate, or high [10]. The GRADE system includes RCT and observational studies. RCT were primarily defined as high level of evidence. Risk of bias, inconsistency, indirectness of evidence (different populations of interest or different outcomes), imprecision, and publication bias were then assessed, and, if present, level of evidence was downgraded by one or two steps. Observational studies were primarily defined as low level of evidence. The level of evidence was potentially upgraded by one or two levels based on the large magnitude of effect, the dose–response gradient, and the effect of residual confounding. The grades of recommendation, also based on the GRADE system, were defined as weak or strong [10]. Definition of the grade of recommendation considered the quality of evidence, the uncertainty of values and preferences, the balance between desirable and undesirable effect of alternative strategy of management, and the costs of intervention [10].
Analyzed items
The items were chosen at the beginning of the study by the experts and were based on the previous guidelines and on the current available literature. Twenty-five recommendation items were elaborated.
Modified Delphi method
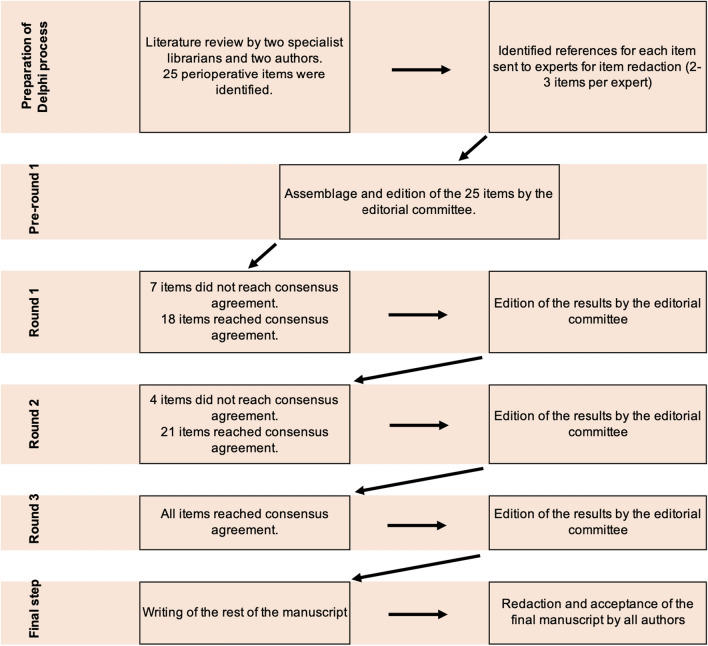
An expert committee was chosen by the group responsible for the guidelines (Lausanne University Hospital group). The experts represent an international panel from Asia, America, and Europe. Twelve experts were asked by email whether they wanted to take part in the elaboration of these guidelines. All of them agreed. Each expert was then assigned 2–3 recommendation items to elaborate. After each expert wrote their individual parts, all items were put together by the editorial team. The Delphi rounds then started. For each round, the manuscript was sent individually to each expert to avoid influence of the results by reading other expert comments. Each expert was asked to comment and edit the manuscript using the Word Track Changes system. The editorial team edited the manuscript before every new round. Levels of evidence and grades of recommendation were modified for the next round only if the majority of experts agreed. A total of 3 web-based rounds was performed to reach the highest level of consensus. Consensus was determined to be reached if >80% agreement (i.e., 12/15 experts) was obtained for an item.
Results
The PRISMA flowchart of the study is summarized in Fig. 1, and the process of the modified Delphi method is depicted in Fig. 2. Table 1 shows the percentage of agreement for each round for items that did not reach consensus after round 1, and Table 2 summarizes the recommendations.
Fig. 1.
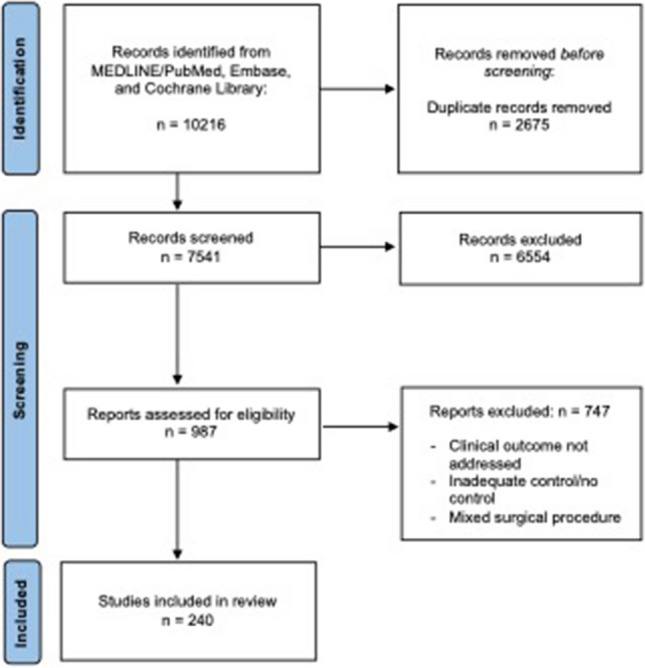
PRISMA flowchart of the systematic review of the literature
Fig. 2.
Process of the modified Delphi method for the development of the present consensus ERAS guidelines for perioperative care for liver surgery
Table 1.
Percentage of agreement (regarding summary, level of evidence, and grade of recommendation) after the different Delphi rounds for items that did not reach consensus (< 80% agreement) after the first round
| Round 1 (%) | Round 2 (%) | Round 3 (%) | |
|---|---|---|---|
| Preoperative biliary drainage | 67 | 86 | 93 |
| Anti-thrombotic prophylaxis | 73 | 93 | 100 |
| Preoperative steroids administration | 60 | 73 | 93 |
| Prophylactic abdominal drainage | 60 | 73 | 100 |
| Delayed gastric emptying | 73 | 100 | 100 |
| Early and scheduled mobilization | 73 | 75 | 100 |
| Fluid management | 73 | 75 | 100 |
Table 2.
Summary of ERAS recommendations for liver surgery for each item, including the levels of evidence and the grades of recommendation
| ERAS item | Summary | Evidence level | Grade of recommendation |
|---|---|---|---|
| 1. Preoperative counseling | Patients should receive preoperative information and counseling regarding the upcoming liver surgery. Brochures and multimedia supports might help to improve the verbal counseling. | Low | Weak |
| 2. Prehabilitation | Prehabilitation should be performed in high-risk patients (elderly, malnourished or overweight patients, smokers, or patients with psychological disorder) prior to liver surgery. Prehabilitation should be commenced 4–6 weeks before the operation depending upon the urgency of surgery. The content (physical exercises, dietary interventions, or anxiety reduction exercises) and duration of the prehabilitation program for liver surgery are not clearly established. | Moderate | Weak |
| 3. Preoperative biliary drainage | Biliary drainage in cholestatic liver (>50 mmol/l) is recommended. For perihilar cholangiocarcinoma, percutaneous biliary drainage should be preferred to endoscopic biliary drainage. Surgery should ideally not be performed until bilirubin level drops below 50 mmol/l. | Moderate | Strong |
| 4. Preoperative smoking and alcohol cessation | Preoperative smoking cessation should be counseled at least 4 weeks prior to hepatectomy. Alcohol cessation is recommended for heavy drinkers (> 24 g/day for women and >36 g/day for men) 4–8 weeks before surgery. | High | Strong |
| 5. Preoperative nutrition | A nutritional assessment is necessary prior to all hepatic surgery. Malnourished patients (i.e., weight loss >10% or >5% over 3 months and reduced body mass index or a low fat-free mass index) should be optimized with enteral supplementation at least 7–14 days prior to surgery. | High | Strong |
| 6. Perioperative oral immunonutrition | Due to the lack of evidence, the use of immunonutrition in hepatic surgery is not recommended yet. | Low | Weak |
| 7. Preoperative fasting and preoperative carbohydrate load | Preoperative fasting of 2 h for liquids and 6 h for solids before anesthesia is safe and can be recommended. | Moderate | Strong |
| Carbohydrate loading is recommended the evening before liver surgery and 2–4 h before induction of anesthesia. Preoperative carbohydrate loading is safe and improves perioperative insulin resistance, but it is not clear if it is associated with a reduction of length of stay in liver surgery. | Low | Weak | |
| 8. Pre-anesthetic medication | Long-acting anxiolytic drugs should be avoided, particularly in the elderly. Preoperative gabapentinoids and nonsteroidal anti-inflammatory drugs are not recommended. Preoperative acetaminophen should be dose-adjusted according to extent of resection. Preoperative hyoscine patches can be used in patients with high risk for postoperative nausea and vomiting but should be avoided in the elderly. | Moderate | Strong |
| 9. Anti-thrombotic prophylaxis | Low molecular weight heparin or unfragmented heparin reduces the risk of thromboembolic events and should be routinely started postoperatively unless exceptional circumstances make this unsafe. Intermittent pneumatic compression devices should be used to further reduce this risk. | Moderate | Strong |
| 10. Preoperative steroids administration | Steroid administration (methylprednisolone at a dose of 500 mg) is recommended. No recommendation can be formulated on diabetic patients undergoing liver surgery. | Moderate | Weak |
| 11. Antimicrobial prophylaxis and skin preparation | Antibiotic prophylaxis (such as cefazolin) within 60 min before surgical incision is recommended, with no benefit extending it into the postoperative period. In case of complex liver surgery with biliary reconstruction, a targeted antibiotic pre-emptive regimen based on preoperative bile culture may be recommended but its duration is unknown. | Moderate | Weak |
| Skin preparation with chlorhexidine-alcoholic solution is associated with a lower rate of surgical site infections, compared to povidone-iodine solution. | Moderate | Strong | |
| 12. Minimally invasive surgery | In trained teams and when clinically appropriate, laparoscopic liver resection is recommended since it reduces postoperative length of stay and complication rates. | Moderate | Strong |
| 13. Epidural, postoperative intravenous, and postoperative per oral analgesia | For open liver surgery, thoracic epidural analgesia can provide excellent analgesia but has significant disadvantages. In fact, optimal postoperative management is key to avoid hypotension and mobility issues which can be detrimental to rapid recovery. Multimodal analgesia (including potential use of intrathecal opiates) is recommended. | High | Strong |
| Regarding laparoscopic surgery, there is no need for regional anesthesia techniques, as multimodal analgesia combined with judicious intravenous opiates provides functional analgesia. | Low | Weak | |
| 14. Wound catheter and transversus abdominis plane (TAP) block | Continuous local anesthetic wound infiltration provides lower complication rates and overall equivalent analgesia to thoracic epidural analgesia. Local anesthetic transversus abdominis plane blockade as a supplement to standard analgesia improves pain control and reduces opiate usage. | High | Strong |
| 15. Prophylactic nasogastric intubation | Prophylactic nasogastric intubation does not offer postoperative benefits and may in fact increase hospital length of stay. Routine use of prophylactic nasogastric intubation is not recommended. | High | Strong |
| 16. Prophylactic abdominal drainage | The routine use of abdominal drain placement is not indicated for hepatectomy without biliary reconstruction. No recommendation can be made for hepatectomy with biliary reconstruction. | High | Strong |
| 17. Preventing intraoperative hypothermia | Perioperative normothermia using multimodal temperature management (including circulating water garments or forced warm air) should be maintained during open and minimally invasive liver surgery. | Moderate | Strong |
| 18. Postoperative artificial nutrition and early oral intake | Early oral intake with normal diet should be implemented after hepatectomy. Individualized need for artificial nutrition should be assessed for malnourished patients, patients with complications causing several days of fasting, and patients with liver cirrhosis. If artificial nutrition is considered, enteral administration should be preferred. | High | Strong |
| 19. Postoperative glycemic control | Insulin therapy for maintenance of normoglycemia (<8.3 mmol/l) is recommended. | High | Strong |
| 20. Prevention of delayed gastric emptying (DGE) | Use of an omental flap to cover the cut surface of the liver might reduce the risk of delayed gastric emptying after left-sided liver resection. | Low | Weak |
| 21. Stimulation of bowel movement | Postoperative laxatives, gum chewing, herbal medicine, or decoction after hepatectomy might reduce the time to first flatus or stool but do not impact the morbidity rate. Current data do not permit the recommendation of the routine use of postoperative laxatives, gum chewing, herbal medicine, or decoction to stimulate bowel movement after liver surgery. | Moderate | Weak |
| 22. Early and scheduled mobilization | Early mobilization (out of bed) after liver surgery should be established from the operative day until hospital discharge. No recommendation can be made regarding the optimal duration of mobilization. | Moderate | Strong |
| 23. Postoperative nausea and vomiting (PONV) prophylaxis | A multimodal approach to postoperative nausea and vomiting should be used. Patients should receive postoperative nausea and vomiting prophylaxis with at least 2 antiemetic drugs such as dexamethasone and ondansetron. | High | Strong |
| 24. Fluid management | Low central venous pressure (below 5 cm H2O) with close monitoring is recommended during hepatic transection. As maintenance fluid balanced crystalloid should be preferred over 0.9% saline or colloids. Goal-directed fluid therapy optimizes cardiac output and end-organ perfusion. This may be particularly beneficial after the intraoperative liver resection during a low central venous pressure state to restore tissue perfusion. Patients who have comorbidities and reduced cardiac function may benefit most. | High | Strong |
| 25. Monitoring/Audit | Substantial literature exists supporting that audit and feedback improve outcomes in health care and surgery. Regular audit and feedback should be implemented and performed in liver surgery to monitor and improve postoperative outcomes and compliance to the ERAS program. | Moderate | Strong |
Preoperative counseling
No RCT assessing preoperative counseling specifically in liver surgery exist. A recent RCT (PEDUCAT trial) compared a 1-h preoperative counseling and information seminar associated with brochure to brochure only with standard management (control group) in major abdominal surgery, including 25 liver resections [11]. No difference in complications or mortality was observed between both groups except for hospital falls, which were more frequent in the control group. Moreover, no difference in patient satisfaction was found. The preoperative information seminar was nevertheless beneficial for the training of patients and nursing staff. In one study, a mobile application for hepatopancreatobiliary (HPB) surgery within an ERAS pathway containing preoperative information was developed and was found to be feasible [12]. A systematic review on preoperative information before elective surgery did not show any differences in terms of perioperative anxiety and postoperative outcomes between specific formats and timings [13].
Summary and recommendation: Patients should receive preoperative information and counseling regarding the upcoming liver surgery. Brochures and multimedia supports might help to improve the verbal counseling.
Evidence level: Low.
Grade of recommendation: Weak.
Prehabilitation
Two recent systematic reviews (including 419 and 1377 patients) focusing on prehabilitation for liver surgery have been published [14, 15]. Both found no difference in terms of postoperative complications and LoS. Only a trend toward less postoperative complications and shorter LoS was found in the pooled analysis of the systematic review by Dewulf et al. [15]. Both reviews underlined that several included studies were underpowered and that standardized outcome measures should be defined in future analyses. A narrative review on prehabilitation for patients with steatosis suggested that the 4–6-week period before the operation could be used for prehabilitation including dietary intervention to decrease intrahepatic fat and improve postoperative outcomes [16]. Another further narrative review on older patients recommended a focus on high-risk patients who could benefit from prehabilitation [17]. Aging is often associated with sarcopenia and malnutrition, rendering these patients more at risk of a complication [17]. Frail patients might therefore benefit the most from prehabilitation [17]. In their review, Walcott-Sapp and Billingsley [18] recommended that candidates for major liver resection should have their nutritional and functional status evaluated preoperatively and improved if necessary. Two RCT on prehabilitation in liver surgery have been performed [19, 20]. One study, including a total of 51 patients, found that postoperative complications were similar but that serum insulin levels were decreased and anaerobic threshold increased in the prehabilitation group [19]. The other RCT of 35 patients found an improvement in cardiopulmonary testing and quality of life in patients who had prehabilitation [20]. Two other studies specific to liver surgery (one prospective study and one propensity score matching study) found improved outcomes in the prehabilitation group (decreased complications and LoS) [21, 22]. Nine systematic reviews and meta-analyses on major abdominal surgery found globally that postoperative complications were reduced in patients having prehabilitation but heterogeneity of the included studies was high and quality of the evidence low [23–31].
Summary and recommendation: Prehabilitation should be performed in high-risk patients (elderly, malnourished or overweight patients, smokers, or patients with psychological disorder) prior to liver surgery. Prehabilitation should be commenced 4–6 weeks before the operation depending upon the urgency of surgery. The content (physical exercises, dietary interventions, or anxiety reduction exercises) and duration of the prehabilitation program for liver surgery are not clearly established.
Evidence level: Moderate.
Grade of recommendation: Weak.
Preoperative biliary drainage (PBD)
The results of 2 meta-analyses showed that the mortality rate was similar between patients with or without PBD for perihilar cholangiocarcinoma, but that PBD increased the incidence of complications such as pancreatitis, cholangitis, and surgical site infection (SSI) [32, 33]. In the meta-analysis by Moole et al. [34], PBD was associated with fewer overall major adverse events than surgery itself, especially in patients undergoing endoscopic PBD. Moreover, PBD has been proven beneficial in the presence of cholangitis, severe malnutrition, and coagulation abnormalities [35]. Most reports have described PBD on the future liver remnant side. Prolonged preoperative jaundice was associated with increased postoperative morbidity and mortality after hepatic resection because of severe cholestatic liver dysfunction [36]. Regarding hilar cholangiocarcinoma, an expert consensus statement (American Hepato-Pancreato-Biliary Association-sponsored consensus meeting) recommended PBD in patients with cholangitis, hyperbilirubinemia-induced malnutrition, hepatic insufficiency, or renal insufficiency and in patients undergoing preoperative chemotherapy or portal vein embolization [37].
Endoscopic biliary drainage (EBD) and percutaneous biliary drainage (PTBD) for hilar tumors are the 2 main strategies of PBD. According to different meta-analyses, PTBD is associated with a lower rate of complications such as pancreatitis and cholangitis than EBD, and PTBD has a higher therapeutic success rate than EBD [38–40]. Conversely, a meta-analysis by Wang et al. [41] showed that the incidence of seeding metastasis was significantly higher in the PTBD than EBD group, and EBD was superior to PTBD in terms of overall survival in patients with resectable perihilar cholangiocarcinoma.
Neither the timing of surgery nor the duration of PBD has been defined. Most institutions define these parameters based on the serum bilirubin concentration, which also shows variance. Some centers recommended PBD duration until the bilirubin level is <2 to 3 mg/dl (about 30–50 mmol/l) [37]. Only one study has assessed the optimal interval between PBD and liver resection [42]. Son et al. [42] classified patients into either a long-term (≥2 weeks) or a short-term (<2 weeks) group. They showed that PBD <2 weeks before surgery was associated with significantly fewer PBD-related complications after resection.
Summary and recommendation: Biliary drainage in cholestatic liver (>50 mmol/l) is recommended. For perihilar cholangiocarcinoma, percutaneous biliary drainage should be preferred to endoscopic biliary drainage. Surgery should ideally not be performed until bilirubin level drops below 50 mmol/l.
Evidence level: Moderate.
Grade of recommendation: Strong.
Preoperative smoking and alcohol cessation
Smoking is a risk factor for overall complications, SSI, pulmonary complications, neurological complications, and admission to the intensive care unit after surgery [43]. Lv et al. [44] showed in a retrospective study of 425 patients that smoking was an independent risk factor for liver-related and infectious complications after hepatectomy in patients with hepatocellular carcinoma (HCC). No prospective study specific to smoking cessation and liver surgery has been published yet. Two older RCT found a benefit of preoperative smoking cessation in terms of complications before various types of operations [45, 46]. A systematic review of 25 articles published in 2012 confirmed that smoking cessation at least 4 weeks before surgery reduced the risk of respiratory and wound-associated complications [47]. Conversely, smoking cessation less than 4 weeks prior surgery did not improve postoperative outcomes. A Cochrane systematic review published in 2014 including 13 RCT found that an intensive intervention for smoking cessation prior to surgery reduced postoperative complications compared to no intervention (risk ratio 0.42) [48]. Two retrospective studies also suggested that smoking was a risk factor for higher recurrence and liver-specific mortality after hepatectomy for HCC [49, 50].
In a systematic review with meta-analysis including 55 studies, alcohol was found to be an independent risk factor for overall, infectious, and respiratory complications after surgery [51]. Nevertheless, low-to-moderate alcohol consumption was not associated with postoperative morbidity but data remained scarce [51]. In liver surgery, alcoholic hepatitis is a risk factor for postoperative complications [52]. Alcohol consumption should therefore be reduced and ideally stopped in the perioperative period.
Summary and recommendation: Preoperative smoking cessation should be counseled at least 4 weeks prior to hepatectomy. Alcohol cessation is recommended for heavy drinkers (>24 g/day for women or >36 g/day for men) 4–8 weeks before surgery.
Evidence level: Smoking: high, alcohol: high.
Grade of recommendation: Smoking: strong, alcohol: strong.
Preoperative nutrition
Prior to hepatic surgery, a nutritional risk screening is required to determine patients at higher risk of postoperative complications. Numerous nutritional screening tools have been validated [53]. Multiple meta-analyses have prognosticated nutritional scoring systems utilizing a combination of serum albumin and lymphocyte count (prognostic nutritional index) [54, 55] and, in addition, serum cholesterol (controlling nutritional status score) [56] in patients undergoing hepatectomy for HCC.
Delaying surgery to optimize preoperative malnutrition (body mass index, BMI <18.5 kg/m2) and disease-related malnutrition (weight loss >10% or >5% over 3 months and reduced BMI or a low fat-free mass index) is necessary [57]. A number of adverse outcomes have been associated with poor perioperative nutrition, including septic complications [58, 59]. Perioperative enteral nutritional therapy should be utilized for a period of 7–14 days in such patients, preferably in an outpatient setting [57]. Parenteral nutrition should only be considered in patients where requirements cannot be met by enteral nutrition alone [57]. A single RCT has demonstrated that intraoperative blood loss can be minimized in obese patients undergoing hepatectomy by introducing a low-fat, low-calorie diet one week prior to surgery [60].
Summary and recommendation: A nutritional assessment is necessary prior to all hepatic surgery. Malnourished patients (i.e., weight loss >10% or >5% over 3 months and reduced body mass index or a low fat-free mass index) should be optimized with enteral supplementation at least 7–14 days prior to surgery.
Evidence level: High.
Grade of recommendation: Strong.
Perioperative oral immunonutrition
Immunomodulation through the use of branched-chain amino acids (BCAA), L-arginine, omega-3 fatty acids, and nucleotides has been reported to control inflammation, prevent immunosuppression, and thus reduce postoperative sepsis [59, 61].
The reduction of inflammation and improvement in hepatic function as a result of immunonutrition may improve outcomes. Omega-3 fatty acid administration has been associated with a reduction in infections and improved liver functions in patients undergoing hepatic resection [62]. Yang et al. [63] suggested that omega-3 fatty acid administration in rats undergoing large hepatic resection reduced hepatic fibrosis and improved hepatic regeneration. Akbari et al. [64] failed to demonstrate this benefit in rats, although the hepatic resections were significantly smaller. Beppu et al. [65] concluded that BCAA supplementation in humans was beneficial in hepatic regeneration after portal vein embolization.
A few systematic reviews suggested promising results. Decreased postoperative complications, improved nutritional state, and shortened hospitalization have all been demonstrated [59, 66]. Numerous biochemical parameters (alanine aminotransferase, aspartate aminotransferase, white cell count and pre-albumin) have been used and have demonstrated a benefit of omega-3 fatty acids in hepatic surgery [67]. None of these beneficial aspects have shown any improvement in postoperative mortality [59, 66–68]. McKay et al. [59] noted that the majority of RCT in their systematic review were of poor quality. More recent RCT failed to demonstrate a benefit for immunonutrition in elective hepatic surgery [69, 70].
Ichikawa et al. [71] demonstrated an oncological benefit for the use of BCAA. Tumor recurrence (at 30 months) as well as alpha-fetoprotein (at 36 months) was significantly reduced as a result of BCAA use; however, overall mortality was unaffected.
The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines currently do not support the use of glutamine, arginine, and omega-3 fatty acids in well-nourished patients; however, supplementation may be indicated in malnourished patients or patients that are unable to be fed enterally [57].
Summary and recommendation: Due to the lack of evidence, the use of immunonutrition in hepatic surgery is not recommended yet.
Evidence level: Low.
Grade of recommendation: Weak.
Preoperative fasting and preoperative carbohydrate load
The ESPEN and the American Society of Anesthesiologists guidelines currently recommend fasting for solids for 6 h before anesthesia and for liquids no more than 2 h before anesthesia [57, 72].
The purpose of giving carbohydrate drinks the evening before and 2–4 h before surgery is to ensure hydration and to reduce insulin resistance [73, 74]. Preoperative carbohydrate drinks have been associated with reduced anxiety, postoperative nausea and vomiting, postoperative insulin resistance, and length of hospitalization [57]. Preoperative carbohydrate loading reduces the resistance to insulin after liver surgery [75–77]. Moreover, Kobayashi et al. [78] found that giving late-evening carbohydrate load and an amino acid snack preoperatively improved the nutritional status of patients with perturbation of liver function. A Cochrane meta-analysis found that carbohydrate loading before elective surgery (18 studies for abdominal surgery) allowed a small reduction of LoS [79]. No difference in terms of complications was found. In a network meta-analysis published in 2017, carbohydrate loading before surgery was associated with a reduction of LoS in patients who were fasting, but did not show any benefit compared to water or placebo [80]. A 2010 RCT on major abdominal surgery (including liver surgery) found that preoperative carbohydrate loading did not improve postoperative outcomes [81]. However, patients with carbohydrate loading who underwent open surgery without epidural analgesia had a trend toward a shorter median LoS (7 vs. 9 days, p = 0.054) [81]. A recent systematic review of RCT in general surgery concluded that carbohydrate loading up to 2 h prior to surgery was safe and could decrease insulin resistance [82]. Some data support the deleterious effect of insulin resistance on liver regeneration [83]. Type 1 diabetes or active gastroesophageal reflux is relative contraindication to carbohydrate loading in the 2–4 h period before surgery although type 2 diabetes can receive it [84].
Summary and recommendation: Preoperative fasting of 2 h for liquids and 6 h for solids before anesthesia is safe and can be recommended. Carbohydrate loading is recommended the evening before liver surgery and 2–4 h before induction of anesthesia. Preoperative carbohydrate loading is safe and improves perioperative insulin resistance, but it is not clear if it is associated with a reduction of length of stay in liver surgery.
Evidence level: Preoperative fasting: moderate, carbohydrate loading: low.
Grade of recommendation: Preoperative fasting: strong, carbohydrate loading: weak.
Pre-anesthetic medication
Pre-anesthetic medication has traditionally been given to allay anxiety, but long-acting agents impair psychomotor recovery after general anesthesia. A Cochrane review on anxiolytic premedication for outpatient surgery showed that patients who received oral anxiolytics had psychomotor function impairment 4 h after surgery, reducing their ability to ambulate, eat, and drink [85]. In selected cases, short-acting anxiolytics (such as 1–2 mg midazolam) can be given to ease regional anesthesia before general anesthesia induction. The American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older patient populations (aged 65 years and older) strongly advise against using benzodiazepines as they may cause cognitive impairment and increase the risk of delirium and falls in the elderly [86].
More recently, preoperative medication was more commonly used as perioperative multimodal analgesic adjuncts. In liver surgery, the use of nonsteroidal anti-inflammatory drugs (NSAIDS) preoperatively is not recommended because of the risk of acute kidney injury. A meta-analysis of 281 trials (n = 24,682 participants) examining the use of gabapentinoids in major surgery showed that although there was a mild analgesic effect, there were significant problems with blurred vision and dizziness [87]. These appeared to be dose-related but could occur with normal dosing in the elderly, so their use in liver surgery was not recommended. Acetaminophen has to be dose-adjusted if significant liver parenchyma is removed. Scopolamine patches are effective in patients with high risk of postoperative nausea and vomiting (PONV) but should be avoided in the elderly due to central effects [88].
Summary and recommendation: Long-acting anxiolytic drugs should be avoided, particularly in the elderly. Preoperative gabapentinoids and nonsteroidal anti-inflammatory drugs are not recommended. Preoperative acetaminophen should be dose-adjusted according to extent of resection. Preoperative hyoscine patches can be used in patients with high risk for postoperative nausea and vomiting but should be avoided in the elderly.
Evidence level: Moderate.
Grade of recommendation: Strong.
Anti-thrombotic prophylaxis
Liver surgery is an independent risk factor for postoperative thromboembolic events, and this risk is directly proportional to the magnitude of hepatectomy [89]. In addition, this risk extends beyond hospital discharge. Since the last published ERAS liver guidelines, one prospective study on the use of enoxaparin treatment in patients after curative HPB surgery for malignancies was published [90]. In this prospective multicenter study including 74 hepatectomies and 35 pancreaticoduodenectomies, subcutaneous injection of enoxaparin was initiated 48–72 h after surgery and repeated for 8 days. Neither major bleeding (primary endpoint) nor symptomatic venous thromboembolism (VTE) was observed. In a recent meta-analysis of 5 retrospective studies including 2256 patients who received chemical thromboprophylaxis, 1412 with mechanical prophylaxis only, the use of chemical thromboprophylaxis reduced the VTE incidence (2.6% vs. 4.6%) following liver surgery without any apparent risk of bleeding [91]. However, chemical thromboprophylaxis dosing was varied and was initiated at different times of the perioperative pathway. The results of the updated Cochrane review (7 RCT, 1,728 participants) on the use of prolonged thromboprophylaxis with low molecular weight heparin (LMWH) in abdominal and pelvic surgery favor the use of prolonged LMWH ≥14 days after surgery [92]. The overall incidence of VTE was reduced from 13.2% in the control group (i.e., only hospital thromboprophylaxis) to 5.3% in the study group. This reduction was also observed when analyzing the rate of deep venous thrombosis and symptomatic VTE. There is no clear evidence to start chemical thromboprophylaxis the day before liver surgery, although this issue has never been addressed in liver surgery studies. There are no reports suggesting that omission of the preoperative dose places patients at higher risk for VTE after hepatectomy. Although no specific studies for liver surgery were found, the use of intermittent pneumatic compression devices applied prior to induction of anesthesia combined with chemical thromboprophylaxis is supported in the literature [93–95]. A meta-analysis of 16,164 patients from 70 studies found almost a 50% risk reduction of VTE when combining intermittent pneumatic compression and chemical thromboprophylaxis compared to intermittent pneumatic compression alone (RR 0.54, 95% CI 0.32–0.91, p = 0.02) [93].
Summary and recommendation: Low molecular weight heparin or unfragmented heparin reduces the risk of thromboembolic events and should be routinely started postoperatively unless exceptional circumstances make this unsafe. Intermittent pneumatic compression devices should be used to further reduce this risk.
Evidence level: Use of low molecular weight heparin or unfragmented heparin: moderate, use of intermittent pneumatic compression devices: moderate.
Grade of recommendation: Use of low molecular weight heparin or unfragmented heparin: strong, use of intermittent pneumatic compression devices: strong.
Preoperative steroids administration
According to a recently published meta-analysis including 6 RCT (n = 411 patients) focusing on liver surgery, the preoperative administration of steroids compared to placebo was not associated with a significant difference in the incidence of postoperative complications or LoS [96]. Of note, most of the studies included in the previous meta-analysis had relatively small sample sizes (n = 20–200, range of patients included) and lacked long-term follow-up data [96]. A supplementary double-blind RCT on 124 patients with laparoscopic liver surgery, which was not included in the previous meta-analysis, found similar results [97]. The doses used ranged from 500 to 30 mg/kg [97]. Both the above-cited studies reported that short-term administration of steroids before the operation was associated with reduction of surgical stress following liver resection, measured by IL-6 and C-reactive protein (both significantly reduced on postoperative day 1 in the steroids group compared to control) [96, 97]. Moreover, a recent RCT (n = 151 patients) comparing the preoperative use of 500 mg of methylprednisolone versus placebo showed that the methylprednisolone group had fewer postoperative complications (31% vs. 47%, p = 0.042), and in particular a lower rate of organ space SSI (7% vs. 18%, p = 0.036) [98].
Although preoperative administration of steroids in liver resection may promote the recovery of liver function, its systematic use in diabetic patients remains unresolved.
Summary and recommendation: Steroid administration (methylprednisolone at a dose of 500 mg) is recommended. No recommendation can be formulated on diabetic patients undergoing liver surgery.
Evidence level: Moderate.
Grade of recommendation: Weak.
Antimicrobial prophylaxis and skin preparation
SSI and wound complications after liver surgery are associated with increased mortality, morbidity, hospital stay, and costs [99–102]. Despite recommendations to administer antibiotics in liver surgery before skin incision, hard data are lacking [103]. The highest level of evidence is offered by a recent network meta-analysis on 5 RCT accumulating 701 patients, from 1998 to 2016, comparing 4 antibiotic prophylaxis strategies (preoperative or postoperative short-duration, postoperative long-duration or no antibiotic prophylaxis) and their combination [104]. Surprisingly, the lowest rate of SSI was demonstrated for patients who received no antibiotic prophylaxis. However, this observation should be mitigated by the fact that the “no antibiotic prophylaxis” strategy was reported in only one of the RCT, a single-center study with moderate risk of bias, enrolling 120 patients for open hepatectomy without bile duct resection [105].
If a prophylactic antibiotic regimen is delivered, its duration should not exceed 24 h: This statement is based on the results of 2 Japanese trials with 670 patients undergoing open liver surgery without biliary reconstruction [106, 107]. The authors observed no difference in the incidence of SSI with a 1-day versus 3-day antibiotic regimen.
In case of complex surgery requiring biliary reconstruction, the frequency of SSI seems to be decreased with a targeted regimen (based on preoperative bile culture) compared to standard antibiotic treatment [108]. In this particular case, and based on the findings from an open-label single-center RCT of 86 patients, 2-day administration of antimicrobial prophylaxis offers similar results to a 4-day regimen, with respect to the absence of infectious complications or sepsis [109]. In this context, it has not been evaluated if preoperative or 1-day antibioprophylaxis is equivalent to 2-day antimicrobial prophylaxis. Moreover, preoperative biliary stenting might also be associated with SSI occurrence, but no robust data exist on the role of antibiotic prophylaxis in these patients [110, 111].
Regarding skin preparation, 2 robust trials comparing 2 different strategies were published during the last decade [112, 113]. A double-blind, single-center RCT including 100 patients undergoing liver surgery assessed the efficacy of a pre-disinfection process with chlorhexidine gluconate (CHG) vs. saline, before the application of povidone–alcohol solution (in both groups) [112]. No differences were observed in terms of postoperative SSI. One supplementary double-blind trial compared the efficacy of CHG–alcohol solution versus povidone-iodine for the prevention of SSI, on a heterogeneous target population (upper and lower gastrointestinal, biliary, thoracic and urogynecology or hepatobiliary and gastroesophageal surgeries) [113]. This large (n = 897), multicenter, international, and high-quality trial [113] reported a decrease in the rates of SSI infection in the CHG–alcohol solution group.
Summary and recommendation: Antibiotic prophylaxis (such as cefazolin) within 60 min before surgical incision is recommended, with no benefit extending it into the postoperative period. In case of complex liver surgery with biliary reconstruction, a targeted antibiotic pre-emptive regimen based on preoperative bile culture may be recommended, but its duration is unknown. Skin preparation with chlorhexidine-alcoholic solution is associated with a lower rate of surgical site infections, compared to povidone-iodine solution.
Evidence level: Antibiotics: moderate, skin preparation: moderate.
Grade of recommendation: Antibiotics: weak, skin preparation: strong.
Minimally invasive approach
Two international consensus conferences (Louisville and Morioka) [114, 115] followed by one European consensus guideline (Southampton) [116] emphasized the benefits of laparoscopic liver resection for both benign and malignant tumors, including primary and metastatic diseases.
Regarding minor liver resections, 2 RCT focusing on colorectal liver metastases have demonstrated the short-term benefits of laparoscopy compared to open surgery, especially leading to a lower rate of morbidity, shorter hospital stay, lower postoperative morphine consumption, and better quality of life [117, 118].
Major hepatectomies performed by laparoscopy have not been assessed by RCT. However, several meta-analyses and propensity score studies showed potential short-term advantages in trained teams [119–122]. Even complex procedures such as staged hepatectomies for liver metastases have been reported and showed not only short-term advantage but also shorter delay between the postoperative course and chemotherapy restart [123]. Recently, a European experience from 9 tertiary referral centers reported lower bleeding, shorter hospital stay, and lower postoperative morbidity for both left and right hemihepatectomies [124]. These results need to be confirmed in future RCT.
Finally, in the setting of a living donor, the laparoscopic approach has not been evaluated in a controlled trial given the small number of performed procedures that would lead to insufficient patient recruitment. However, international registries and propensity score studies demonstrated postoperative advantages of laparoscopy, especially for left sectionectomy. For this indication, the risk of laparoscopic approach has been shown to be lower than donor nephrectomy. In expert centers, consensus conferences have claimed the benefit of laparoscopy for pediatric living liver donor transplantation, and a recent international registry showed the benefit of laparoscopy in major hepatectomies [115, 125–127].
Currently, data on robotic minimally invasive liver resection from RCT are lacking.
Summary and recommendation: In trained teams and when clinically appropriate, laparoscopic liver resection is recommended since it reduces postoperative length of stay and complication rates.
Evidence level: Moderate.
Grade of recommendation: Strong.
Epidural, postoperative intravenous, and postoperative per oral analgesia
The advantage of thoracic epidural analgesia (TEA) is that it can modify the stress response as measured by biomarkers, which may have the potential to improve downstream oncological outcomes as shown in a RCT of 62 patients [128]. The sympathectomy from TEA can induce hypotension due to vasodilation, which can complicate fluid therapy, necessitate the need for low-dose vasopressors, and compound the risk of acute kidney injury [129]. In liver surgery, the postoperative prolongation of prothrombin time can make timing of removal of the epidural catheter problematic [130]. A RCT of 140 patients comparing TEA to intravenous patient-controlled analgesia (PCA) in hepatopancreatobiliary surgery found that TEA had better pain control and less use of opioids with similar LoS and complications in both groups [131]. A Cochrane analysis of 32 studies (1716 patients) of TEA vs. PCA opiate in open surgery showed a slightly better pain reduction with TEA but increased risk of technical failure, more frequent episodes of hypotension, and more pruritus [132]. Evidence level was graded as moderate.
Intrathecal opiates have been used to reduce opiate requirement postoperatively when combined with a multimodal analgesic regimen and avoid the need for continuous infusions [133, 134]. A recent review of 11 studies confirmed this technique to have similar results to TEA but with a lower likelihood of postoperative hypotension and a reduced LoS [134]. In a 2017 RCT (56 patients), addition of a selective COX-2 inhibitor (parecoxib, unauthorized in certain countries) to PCA for patients undergoing open liver resection was found to decrease postoperative pain compared to PCA alone [135]. For open living donor hepatectomy, the use of ketorolac infusion in addition to intravenous fentanyl PCA improved postoperative analgesia and decreased the dose of used fentanyl as shown in a RCT of 60 patients [136]. Therapeutic acetaminophen has been shown to be safe after major hepatectomy if liver function was preserved [137]. However, it is prudent to reduce the dose to 2 g per day if significant liver parenchyma is resected. Postoperatively, NSAIDS should be used only if renal function is normal.
The analgesic requirements after laparoscopic surgery combined with earlier gut function enable analgesia to be achieved by oral route soon after surgery. This and the smaller incisions (the main one being to deliver the specimen) reduced the need for regional analgesic techniques. In laparoscopic liver surgery, a RCT of 124 patients showed that an intravenous infusion pump of parecoxib provided superior analgesia and fewer adverse outcomes compared to an intravenous infusion pump of fentanyl [138].
Summary and recommendation: For open liver surgery, thoracic epidural analgesia can provide excellent analgesia but has significant disadvantages. In fact, optimal postoperative management is key to avoid hypotension and mobility issues which can be detrimental to rapid recovery. Multimodal analgesia (including potential use of intrathecal opiates) is recommended. Regarding laparoscopic surgery, there is no need for regional anesthesia techniques, as multimodal analgesia combined with judicious intravenous opiates provide functional analgesia.
Evidence level: Open (multimodal analgesia): high, laparoscopy (multimodal analgesia): low.
Grade of recommendation: Open (multimodal analgesia): strong, laparoscopy (multimodal analgesia): weak.
Wound catheter and transversus abdominis plane (TAP) block
Continuous wound infiltration (CWI) of local anesthetic using wound catheters has been compared with TEA in a number of RCT in patients undergoing liver surgery. The Liver 1 trial (RCT, n = 65) compared CWI to TEA and showed similar static pain scores, better dynamic and early pain scores with TEA and shorter LoS with CWI [139]. No difference was found in terms of mobilization and overall complication rate. A similar RCT of 83 patients showed equivalent pain scores and reduced treatment failure for CWI but did not show reduced LoS [140]. The Liver 2 trial (RCT, n = 93) combined CWI with TAP blocks and compared it with TEA [141]. This trial showed equivalent static and dynamic pain scores in both arms both early and later after surgery and earlier discharge from hospital in the CWI and TAP block group. A further multicentric RCT of 105 patients containing patients undergoing liver resection and other HPB procedures showed non-inferiority in analgesia between CWI and TEA [142]. Other RCT (sample size range: 40–153) using ropivacaine as a continuous infusion in CWI showed reduction in opiate requirements and/or reduced LoS in patients receiving CWI [143–149]. One RCT of 99 patients allocated patients after liver resection who received patient-controlled opiate analgesia to either ropivacaine CWI infusion or placebo and showed no difference in pain scores or LoS between the groups [150]. Two RCT have compared TAP blocks to TEA in abdominal surgery; the first (n = 62) showed equivalent pain scores but higher opiate requirements in the TAP group [151]. The second RCT (n = 65) in major general cancer resections showed lower opiate requirements in the TAP group and less frequent postoperative hypotension [152]. A further 3 RCT of TAP blocks showed reduced opiate requirements in patients undergoing liver surgery in different contexts [153–155]. Two RCT in patients with cirrhosis undergoing liver surgery also showed benefit of TAP blocks in reducing opiate requirements of patients [156, 157]. A recent RCT of 63 patients showed benefit from regional blockade using quadratus lumborum blocks in patients undergoing liver resection [158]. There is one RCT reported which looked at the use of sponges placed over port sites from minimally invasive liver surgery soaked with ropivacaine vs. placebo which showed benefit in pain scores from the ropivacaine group [159].
Pooled data in 2 meta-analyses of patients undergoing major abdominal open surgery (inclusion of 16 RCT) [160] and specifically liver resections (inclusion of 3 RCT) [161] compared outcomes of patients receiving CWI vs. TEA, and both showed lower complication rates and faster recovery after surgery with CWI but slightly better pain control with TEA at certain time points following surgery.
Summary and recommendation: Continuous local anesthetic wound infiltration provides lower complication rates and overall equivalent analgesia to thoracic epidural analgesia. Local anesthetic transversus abdominis plane blockade as a supplement to standard analgesia improves pain control and reduces opiate usage.
Evidence level: High.
Grade of recommendation: Strong.
Prophylactic nasogastric intubation
Historically, nasogastric tubes (NGTs) were routinely placed after major abdominal surgery due to concerns for postoperative abdominal distention, nausea, and vomiting. The benefits of this approach, however, were questionable. Two recent studies focusing on hepatectomy patients have added to the body of literature that speaks against routine prophylactic nasogastric intubation [162, 163]. The first is a RCT that showed no difference in the rates of overall morbidity, pulmonary complications, postoperative vomiting, time to oral intake, and hospital LoS between patients randomized to a NGT group vs. a no-NGT group [162]. The second is a systematic review and meta-analysis with over 1300 hepatectomy patients from 7 RCT and likewise demonstrated no benefits to NGT with regard to return of bowel function [163]. In fact, NGT was associated with greater LoS and delay to starting diet. Taken together in the context of the existing literature, these studies suggest that routine prophylactic NGT should be discouraged.
Summary and recommendation: Prophylactic nasogastric intubation does not offer postoperative benefits and may in fact increase hospital length of stay. Routine use of prophylactic nasogastric intubation is not recommended.
Evidence level: High.
Grade of recommendation: Strong.
Prophylactic abdominal drainage
The existing literature on the topic of prophylactic abdominal drainage after liver surgery has been inconclusive at best to this point, with contradictory results. More recently, several studies have emerged that speak against this practice [164–168]. A RCT found no improvement in postoperative morbidity with abdominal drainage after hepatectomy; instead, the drain was associated with some inherent complications such as bleeding and infection, especially among patients with chronic liver diseases [164]. Likewise, an analysis of 199 patients undergoing liver surgery found that intraoperative placement of perihepatic drainage failed to decrease rates of perioperative complications [165]. In addition, it should be noted that selection bias likely tainted the outcomes of this study, as drains were more likely to be placed for more complex tumors and resections. More recently, a review by Messager et al. [166] on the topic of prophylactic intra-abdominal drainage in elective major gastrointestinal surgery concluded that, based on high-quality evidence, no argument could be made for routine abdominal drainage following hepatic resection without biliary anastomosis. Finally, a systematic review and meta-analysis reviewed results from 6 RCT including 665 patients; its main finding was that routine use of abdominal drains after elective uncomplicated liver surgery was not associated with a reduction in postoperative complications [167]. Rather than routine abdominal drainage, clinicians may wish to consider other maneuvers to reduce the rate of post-hepatectomy complications such as bile leaks and organ space infections. One such practice is the systematic use of an intraoperative air leak test after major hepatectomy [169, 170].
Summary and recommendation: The routine use of abdominal drain placement is not indicated for hepatectomy without biliary reconstruction. No recommendation can be made for hepatectomy with biliary reconstruction.
Evidence level: High.
Grade of recommendation: Strong.
Preventing intraoperative hypothermia
Maintaining body temperature >36 °C has been recommended to reduce both cardiac and non-cardiac complications [171–176]. One meta-analysis [172] of 67 trials has shown that mild hypothermia was associated with increased SSI and blood loss. There is a lack of studies relating specifically to liver surgery. One meta-analysis of 23 trials comparing warming systems suggested that circulating water-based systems offer better warming than forced air systems [177] although a recent retrospective study (n = 50) has used a multimodal approach to temperature management during surgery with good temperature control [178]. A systematic review of 22 studies has suggested heating insufflated gases in laparoscopic abdominal surgery to maintain body temperature but patient outcomes were not improved [179]. Liver cooling techniques have been used to minimize ischemia reperfusion injury to the liver, but temperature changes in the whole body in such procedures have not been reported [180].
Summary and recommendation: Perioperative normothermia using multimodal temperature management (including circulating water garments or forced warm air) should be maintained during open and minimally invasive liver surgery.
Evidence level: Moderate.
Grade of recommendation: Strong.
Postoperative artificial nutrition and early oral intake
Early oral diet has been shown to be safe and to decrease the time to first bowel movement in abdominal and liver surgery [181]. The ESPEN guidelines on clinical nutrition in surgery published in 2017 recommend oral nutrition postoperatively [57]. Moreover, full nutritional assessment preoperatively is required before liver surgery to identify malnutrition and to better tailor the nutritional management [182, 183]. Nutrient deficiency should be corrected before, during, and after liver surgery according to identified deficits.
Oral nutrition was found to be better than total parenteral nutrition (TPN) after hepatectomy in a study of 32 patients [184]. Ishikawa et al. [185] randomly assigned 24 patients to usual oral diet vs. oral and parenteral diet (preoperatively and during 7 postoperative days). No difference in terms of complications was found.
A review based on 6 articles recently showed that artificial (i.e., enteral and parenteral) nutrition in liver surgery is poorly defined and that it should not be routinely given [186]. Patients with malnutrition or complications inducing fasting or appetite loss might benefit from artificial nutrition. If artificial nutrition support is considered, Gao et al. [187] in a meta-analysis (9 studies) showed that enteral nutrition (EN) induced better outcomes than TPN. In a systematic review and meta-analysis including 18 RCT with 2540 patients, Zhao et al. [188] assessed TPN vs. EN in major abdominal surgery for cancer. The authors found better outcomes with EN (decreased LoS and first flatus time, increased serum albumin level).
A more general review on patients with liver disease by Sun et al. [189] recommended that if a patient has a need for nutritional support, EN should first be considered. EN can then be complemented with parenteral nutrition when EN cannot bring all energy needs (60%). Nutritional support should be individualized based on patient need, disease characteristics, liver tolerance, and integrity of the gastrointestinal tract.
Summary and recommendation: Early oral intake with normal diet should be implemented after hepatectomy. Individualized need for artificial nutrition should be assessed for malnourished patients, patients with complications causing several days of fasting, and patients with liver cirrhosis. If artificial nutrition is considered, enteral administration should be preferred.
Evidence level: High.
Grade of recommendation: Strong.
Postoperative glycemic control
Perioperative hyperglycemia is frequently observed after major surgery [190, 191]. The cause of perioperative hyperglycemia is a transitory resistance to insulin leading to a decreased peripheral uptake of glucose [192]. Surgical stress produces a raise in blood sugar, which modifies hepatic metabolism regulation and immune function, impacting the recovery after surgery. In patients undergoing colorectal and pancreatic surgery, hyperglycemia occurring in the initial postoperative days is related to postoperative complications [193, 194]. Sensitivity to insulin after surgery is significantly decreased when insulin is not used intraoperatively to treat hyperglycemia [195]. Moreover, the glucose concentration rapidly changes during liver resection using the Pringle maneuver, because hypoxia induces glycogen breakdown within hepatocytes [196]. One RCT including 88 patients undergoing hepatectomy compared the use of an artificial pancreas (closed-loop glycemic control system) to the usual sliding-scale method for insulin therapy and showed that SSI and total hospital costs were decreased in patients who were treated with closed-loop glycemic control system [197]. It has also been shown that supplementation with carbohydrates and branched-chain amino acid-enriched nutrients before liver surgery lowers the resistance to insulin [77]. Additionally, elevated serum lactate after hepatectomy that has been shown to be associated with postoperative complications is linked to insulin resistance and/or ischemia–reperfusion injury [198]. In some RCT, high-dose insulin therapy or intensive insulin therapy reduced postoperative liver dysfunction, infections, and complications and improved the liver glycogen content in patients undergoing HPB surgery [199–201]. One RCT showed that perioperative glucose and insulin administration more effectively resulted in normoglycemia than did the standard insulin therapy for patients undergoing liver resection [202]. A systematic review of intensive insulin therapy showed that high blood sugar was not predictive of SSI among diabetic patients and recommended a target blood glucose level of <150 mg/dl (<8.3 mmol/l) in patients without diabetes undergoing gastroenterological surgery [203]. A conventional protocol is indicated for patients admitted to the general ward (not the intensive care unit) to avoid the risk of hypoglycemia [203]. According to one recent RCT, preoperative administration of liraglutide stabilized the perioperative plasma glucose level and reduced the perioperative insulin requirement without increasing the risk of hypoglycemia [204].
Summary and recommendation: Insulin therapy for maintenance of normoglycemia (<8.3 mmol/l) is recommended.
Evidence level: High.
Grade of recommendation: Strong.
Prevention of delayed gastric emptying (DGE)
Left hepatic resection might induce a higher DGE risk (15–20%) because of disturbance of regular gastrointestinal motion at the contact plane between the stomach and the surface of the cut liver [205, 206]. One RCT (n = 40) found no difference of DGE incidence if an omental flap to cover the liver cut surface after left-sided liver resection was used [205], whereas another RCT (n = 49) found a reduced incidence of DGE when using an omental flap [206]. In addition, one cross-sectional study (n = 42, 15 patients in the fixation group) showed that fixation of the round ligament reduced the incidence of DGE [207].
Summary and recommendation: Use of an omental flap to cover the cut surface of the liver might reduce the risk of delayed gastric emptying after left-sided liver resection.
Evidence level: Low.
Grade of recommendation: Weak.
Stimulation of bowel movement
Shimada et al. [208] found in a multicenter RCT that daikenchuto (TU-100, traditional herbal medicine) significantly decreased the median time to first bowel movement by 5 h in 231 patients who underwent hepatectomy for cancer. Although significant, this 5-h difference is probably not clinically relevant as complication rates were similar in both groups. Another RCT assessing the effect of daikenchuto after hepatectomy found that the daikenchuto group had shorter time to bowel movement and oral intake, but complications were similar [209]. You et al. [210] performed a 3-arm RCT to assess ileus rates in patients with HCC undergoing liver resection. Simo decoction (traditional Chinese herbal medicine) with acupuncture was compared to gum chewing and no specific postoperative intervention (control group). Both interventions were found to diminish the time to first stool compared to the control group, whereas only the group with simo decoction and acupuncture had a shorter length of hospital stay. In a RCT of 68 patients undergoing liver surgery, the group with laxatives had reduced time to passage of stool but similar secondary outcomes, such as DGE, LoS, or time to functional recovery [211]. Jang et al. [212] showed in a prospective case–control study including 42 patients that gum chewing permitted to decrease the time to first flatus and the xerostomia rate, but did not have an effect on LoS or analgesic use.
Summary and recommendation: Postoperative laxatives, gum chewing, herbal medicine, or decoction after hepatectomy might reduce the time to first flatus or stool but do not impact the morbidity rate. Current data do not permit the recommendation of the routine use of postoperative laxatives, gum chewing, herbal medicine, or decoction to stimulate bowel movement after liver surgery.
Evidence level: Moderate.
Grade of recommendation: Weak.
Early and scheduled mobilization
Bed rest is associated with multiple established deleterious effects including muscle atrophy, thromboembolic disease, and insulin resistance [213–215]. A RCT involving 120 patients undergoing liver resection showed a significantly faster postoperative gastrointestinal function and shorter length of hospital stay after performing early activity (from postoperative day 1) [216]. An early postoperative mobilization program based on supervised exercises improved functional capacity in patients undergoing major elective abdominal oncologic surgery [217].
So far, however, no consensus has been defined regarding the type, frequency, and intensity of physical therapy in liver surgery [218].
Summary and recommendation: Early mobilization (out of bed) after liver surgery should be established from the operative day until hospital discharge. No recommendation can be made regarding the optimal duration of mobilization.
Evidence level: Moderate.
Grade of recommendation: Strong.
Postoperative nausea and vomiting (PONV) prophylaxis
PONV occurs frequently after major surgery (25–30%). The multimodal approach and opioid reduction provided by ERAS enable the majority of patients to eat early after hepatectomy [219]. Known risk factors, such as previous PONV, female gender, younger age, non-smoker, and use of volatile anesthetic agents and opioids, should be evaluated before the operation [220]. The 5-HT3 antagonists are the primary treatment because of their safe side effect profile. Low-dose dexamethasone is a good additive preventative agent and facilitates hepatic regeneration [221]. Of note, there is no supplementary advantage of using higher doses [221]. However, dexamethasone should be used with caution in diabetics as it can transiently worsen glycemic control [222]. Antihistamines, butyrophenones, and phenothiazines can also be used as second-line therapy [88]. The international consensus group on PONV recommends using 2 antiemetic drugs to decrease PONV and to improve efficacy [88]. Table 3 summarizes potential antiemetic drugs with doses and timing of use.
Table 3.
Antiemetic drugs for postoperative nausea and vomiting (PONV) prophylaxis with doses and timing of use
| Medication | Doses | Timing of use |
|---|---|---|
| Ondansetron | 4 mg IV | End of surgery |
| Dexamethasone | 4–8 mg IV | At induction |
| Droperidol | 0.625 mg IV | End of surgery |
| Metoclopramide | 10 mg IV/PO | Postoperatively |
| Scopolamine | 1 mg transdermal patch | Before surgery |
This table is based on the recommendations from the international consensus group on PONV
IV: intravenous, PO: per os
Summary and recommendation: A multimodal approach to postoperative nausea and vomiting should be used. Patients should receive postoperative nausea and vomiting prophylaxis with at least 2 antiemetic drugs such as dexamethasone and ondansetron.
Evidence level: High.
Grade of recommendation: Strong.
Fluid management
Blood loss and transfusion rates remain central risk factors leading to higher morbidity and mortality after liver resections [223–225]. A Cochrane review showed that a lower central venous pressure (CVP) decreased blood loss, but without significant difference in red blood cell transfusion requirements, intraoperative morbidity, or long-term survival benefits [226]. Another systematic review and meta-analysis also confirmed that low CVP was associated with less blood loss [227].
Regarding fluid management for major hepatic surgery, there is currently no protocol available providing the optimum amount of fluid to be given to patients. The current concept must focus on the maintenance of central euvolemia, thereby preventing any excess of salt or water. Goal-directed fluid therapy (GDFT), targeting adequate cardiac output and end-organ perfusion, has attracted much attention. A meta-analysis of 32 RCT including about 3000 patients testing the impact of GDFT during major surgery, i.e., not only liver surgery, demonstrated significant benefits in reducing morbidity and mortality [228]. A RCT published in 2015 found that stroke volume variation (SVV)-guided GDFT compared to standard fluid resuscitation decreased the intraoperative infused fluid volume without decreasing postoperative complications [229]. On multivariable analysis, higher intraoperative fluid volume was an independent risk factor for 30-day morbidity. Recently, Weinberg et al. [230] showed in an RCT that a restrictive GDFT did not decrease LoS and fluid-related complications compared to conventional care within an ERAS pathway for major liver resection. Of note, only 24 patients were included in each arm.
Excessive administration of crystalloids should be avoided as much as blood loss during liver surgery. To guide fluid management during surgery, the measurement of SVV has been proposed to replace CVP monitoring [231]. A randomized prospective trial comparing SVV monitoring versus CVP recording in 90 patients undergoing laparoscopic liver surgery showed a reduced conversion rate as well as reduced blood loss in favor of the SVV approach [232]. The choice for intravenous fluid therapy in liver surgery is still under debate. A systematic review covering 43 RCT compared 18 fluid types (9 crystalloids and 9 colloids) in major abdominal surgery concluded that the best approach was balanced crystalloids (e.g., Ringer’s lactate) as maintenance fluid and colloids as volume expander (e.g., human albumin) [233]. Concerning the postoperative period, a recent retrospective study showed that a weight gain ≥3.5 kg on postoperative day 2 was an independent risk factor for major complication after liver surgery [234]. This suggests that postoperative weight fluctuation should be carefully monitored and potentially minimized.
Summary and recommendation: Low central venous pressure (below 5 cm H2O) with close monitoring is recommended during hepatic transection. As maintenance fluid balanced crystalloid should be preferred over 0.9% saline or colloids. Goal-directed fluid therapy optimizes cardiac output and end-organ perfusion. This may be particularly beneficial after the intraoperative liver resection during a low central venous pressure state to restore tissue perfusion. Patients who have comorbidities and reduced cardiac function may benefit most.
Evidence level: High.
Grade of recommendation: Strong.
Monitoring/audit
Monitoring the outcomes after implementation of ERAS allows performing a precise audit. Outcome monitoring therefore represents the first step to establish an audit of quality. A recent study reported the successful implementation of a nationwide audit for liver surgery in the Netherlands [235]. This audit on postoperative outcomes after liver surgery was intended to evaluate the quality of centers performing these operations and to reach or maintain the best surgical quality. Otherwise, no study specifically designed for liver surgery has been published yet. A Cochrane systematic review on the effects of audit and feedback analyzed 140 studies [236]. It was found that audit and feedback generally induce improvements. The audit was more efficient when the baseline performance was low. The structure of the audit or feedback played a role. It was, for example, of interest to identify specific targets and put in place a plan of action. Another review by Ivers et al. [237] revealed that the body of evidence showing that audit improves outcomes was substantial, but that progress and evolution in this field were not present in recent literature. To a larger scale such as healthcare system, Grimshaw et al. [238] recommended the implementation of laboratories to better understand the science behind audit and feedback and to improve audit and feedback and their impact. One article highlighted the importance of undertaking actions over just measurement [239]. Recently, the Clinical Performance Feedback Intervention Theory issued from a systematic review and meta-synthesis postulated that an effective feedback was a cyclical process and that every missing links stopping the “cycle” cause effect loss of the feedback [240]. This theory includes recommendations for optimally designing or implementing an audit intervention. Practical suggestions on how to effectively display or deliver feedback have also been published [241].
Summary and recommendation: Substantial literature exists supporting that audit and feedback improve outcomes in health care and surgery. Regular audit and feedback should be implemented and performed in liver surgery to monitor and improve postoperative outcomes and compliance to the ERAS program.
Evidence level: Moderate.
Grade of recommendation: Strong.
Discussion
This systematic review and modified Delphi consensus elaborated 25 recommendations based on the best available evidence published until mid-2020. Nine items had a high level of evidence: preoperative smoking and alcohol cessation, preoperative nutrition, wound catheter and TAP block, prophylactic nasogastric intubation, prophylactic abdominal drainage, postoperative artificial nutrition and early oral intake, postoperative glycemic control, PONV prophylaxis, and fluid management.
Regarding differences with 2016 recommendations, more evidence regarding use of steroids before hepatectomy has been published since. It is now routinely recommended in non-diabetic patients. Routine drainage after hepatectomy without biliary reconstruction is not recommended in the present guidelines, whereas in 2016 no conclusive evidence and no recommendation for or against the use of drain were given. In addition, 3 novel items were introduced: prehabilitation, preoperative biliary drainage, and preoperative smoking and alcohol cessation. The novelties of these guidelines are the addition of the 3 novel items mentioned hereabove and the reassessment of the previously published items based on the most recent literature data.
It is not clear if patients with cirrhosis undergoing liver surgery should be managed differently within an ERAS program. Preliminary data showed that ERAS was safe in these patients [242, 243]. Further robust data are needed, and it remains unclear if ERAS pathways should be adapted in cirrhotic patients undergoing liver surgery.
It is important to mention that the compliance (adherence) to all ERAS items is paramount. It has been clearly shown that higher compliance to the ERAS pathway allows to have better postoperative outcomes compared to lower compliance [244, 245].
In conclusion, these guidelines for perioperative care after liver surgery were developed based on the best available evidence and recommend management for 25 perioperative items.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Cécile Jaques and Alexia Trombert, Biomedical Information Specialists (Medical Library, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland), for support with the systematic literature search.
Funding
Open access funding provided by University of Lausanne.
Declarations
Conflict of interest
Prof. Nicolas Demartines is implementation chair of the ERAS Society. The other authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coolsen MME, van Dam RM, van der Wilt AA, et al. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg. 2013;37:1909–1918. doi: 10.1007/s00268-013-2044-3. [DOI] [PubMed] [Google Scholar]
- 2.Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531–1541. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 3.Melloul E, Hübner M, Scott M, et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg. 2016;40:2425–2440. doi: 10.1007/s00268-016-3700-1. [DOI] [PubMed] [Google Scholar]
- 4.Liang X, Ying H, Wang H, et al. Enhanced recovery program versus traditional care in laparoscopic hepatectomy. Medicine (Baltimore) 2016;95:e2835. doi: 10.1097/MD.0000000000002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang X, Ying H, Wang H, et al. Enhanced recovery care versus traditional care after laparoscopic liver resections: a randomized controlled trial. Surg Endosc. 2018;32:2746–2757. doi: 10.1007/s00464-017-5973-3. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Chen J, Liu Z, et al. Enhanced recovery program versus traditional care after hepatectomy: a meta-analysis. Medicine (Baltimore) 2017;96:e8052. doi: 10.1097/MD.0000000000008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Qin H, Wu Y, Xiang B. Enhanced recovery after surgery program reduces length of hospital stay and complications in liver resection: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e7628. doi: 10.1097/MD.0000000000007628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noba L, Rodgers S, Chandler C, et al. Enhanced recovery after surgery (ERAS) reduces hospital costs and improve clinical outcomes in liver surgery: a systematic review and meta-analysis. J Gastrointest Surg. 2020;24:918–932. doi: 10.1007/s11605-019-04499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brindle M, Nelson G, Lobo DN, et al. Recommendations from the ERAS® Society for standards for the development of enhanced recovery after surgery guidelines. BJS Open. 2020;4:157–163. doi: 10.1002/bjs5.50238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Klaiber U, Stephan-Paulsen LM, Bruckner T, et al. Impact of preoperative patient education on the prevention of postoperative complications after major visceral surgery: the cluster randomized controlled PEDUCAT trial. Trials. 2018;19:288. doi: 10.1186/s13063-018-2676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickens R, Cochran A, Tezber K, et al. Using a mobile application for real-time collection of patient-reported outcomes in hepatopancreatobiliary surgery within an ERAS® pathway. Am Surg. 2019;85:909–917. doi: 10.1177/000313481908500847. [DOI] [PubMed] [Google Scholar]
- 13.Hounsome J, Lee A, Greenhalgh J, et al. A systematic review of information format and timing before scheduled adult surgery for peri-operative anxiety. Anaesthesia. 2017;72:1265–1272. doi: 10.1111/anae.14018. [DOI] [PubMed] [Google Scholar]
- 14.Dagorno C, Sommacale D, Laurent A, et al (2021) Prehabilitation in hepato-pancreato-biliary surgery: a systematic review and meta-analysis. A necessary step forward evidence-based sample size calculation for future trials. J Visc Surg S1878–7886(21)00111–9 [DOI] [PubMed]
- 15.Dewulf M, Verrips M, Coolsen MME, et al. The effect of prehabilitation on postoperative complications and postoperative hospital stay in hepatopancreatobiliary surgery a systematic review. HPB (Oxford) 2021;23:1299–1310. doi: 10.1016/j.hpb.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Doherty DT, Coe PO, Rimmer L, et al. Hepatic steatosis in patients undergoing resection of colorectal liver metastases: a target for prehabilitation? A narrative review. Surg Oncol. 2019;30:147–158. doi: 10.1016/j.suronc.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Bongers BC, Dejong CHC, den Dulk M. Enhanced recovery after surgery programmes in older patients undergoing hepatopancreatobiliary surgery: What benefits might prehabilitation have? Eur J Surg Oncol. 2020;47:551–559. doi: 10.1016/j.ejso.2020.03.211. [DOI] [PubMed] [Google Scholar]
- 18.Walcott-Sapp S, Billingsley KG. Preoperative optimization for major hepatic resection. Langenbecks Arch Surg. 2018;403:23–35. doi: 10.1007/s00423-017-1638-x. [DOI] [PubMed] [Google Scholar]
- 19.Kaibori M, Ishizaki M, Matsui K, et al. Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg. 2013;206:202–209. doi: 10.1016/j.amjsurg.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Dunne DFJ, Jack S, Jones RP, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016;103:504–512. doi: 10.1002/bjs.10096. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima H, Yokoyama Y, Inoue T, et al. Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato-pancreato-biliary surgeries for malignancy. Ann Surg Oncol. 2019;26:264–272. doi: 10.1245/s10434-018-6943-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Shelat VG, Chow JJL, et al. Prehabilitation program improves outcomes of patients undergoing elective liver resection. J Surg Res. 2020;251:119–125. doi: 10.1016/j.jss.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Thillainadesan J, Yumol MF, Hilmer S, et al. Interventions to improve clinical outcomes in older adults admitted to a surgical service: a systematic review and meta-analysis. J Am Med Dir Assoc. 2020;21:1833–1843. doi: 10.1016/j.jamda.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg. 2017;39:156–162. doi: 10.1016/j.ijsu.2017.01.111. [DOI] [PubMed] [Google Scholar]
- 25.Lau CSM, Chamberlain RS. Prehabilitation programs improve exercise capacity before and after surgery in gastrointestinal cancer surgery patients: a meta-analysis. J Gastrointest Surg. 2019;24:2829–2837. doi: 10.1007/s11605-019-04436-1. [DOI] [PubMed] [Google Scholar]
- 26.Kamarajah SK, Bundred J, Weblin J, Tan BHL. Critical appraisal on the impact of preoperative rehabilitation and outcomes after major abdominal and cardiothoracic surgery: a systematic review and meta-analysis. Surgery. 2020;167:540–549. doi: 10.1016/j.surg.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 27.Heger P, Probst P, Wiskemann J, et al. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366) J Gastrointest Surg. 2020;24:1375–1385. doi: 10.1007/s11605-019-04287-w. [DOI] [PubMed] [Google Scholar]
- 28.Thomas G, Tahir MR, Bongers BC, et al. Prehabilitation before major intra-abdominal cancer surgery: a systematic review of randomised controlled trials. Eur J Anaesthesiol. 2019;36:933–945. doi: 10.1097/EJA.0000000000001030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes MJ, Hackney RJ, Lamb PJ, et al. Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg. 2019;43:1661–1668. doi: 10.1007/s00268-019-04950-y. [DOI] [PubMed] [Google Scholar]
- 30.Luther A, Gabriel J, Watson RP, Francis NK. The impact of total body prehabilitation on post-operative outcomes after major abdominal surgery: a systematic review. World J Surg. 2018;42:2781–2791. doi: 10.1007/s00268-018-4569-y. [DOI] [PubMed] [Google Scholar]
- 31.Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160:1189–1201. doi: 10.1016/j.surg.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Celotti A, Solaini L, Montori G, et al. Preoperative biliary drainage in hilar cholangiocarcinoma: systematic review and meta-analysis. Eur J Surg Oncol. 2017;43:1628–1635. doi: 10.1016/j.ejso.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Mehrabi A, Khajeh E, Ghamarnejad O, et al. Meta-analysis of the efficacy of preoperative biliary drainage in patients undergoing liver resection for perihilar cholangiocarcinoma. Eur J Radiol. 2020;125:108897. doi: 10.1016/j.ejrad.2020.108897. [DOI] [PubMed] [Google Scholar]
- 34.Moole H, Bechtold M, Puli SR. Efficacy of preoperative biliary drainage in malignant obstructive jaundice: a meta-analysis and systematic review. World J Surg Oncol. 2016;14:182. doi: 10.1186/s12957-016-0933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aly EA, Johnson CD. Preoperative biliary drainage before resection in obstructive jaundice. Dig Surg. 2001;18:84–89. doi: 10.1159/000050105. [DOI] [PubMed] [Google Scholar]
- 36.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–394. doi: 10.1097/00000658-199604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691–699. doi: 10.1111/hpb.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Mahjoub A, Menahem B, Fohlen A, et al. Preoperative biliary drainage in patients with resectable perihilar cholangiocarcinoma: is percutaneous transhepatic biliary drainage safer and more effective than endoscopic biliary drainage? A meta-analysis. J Vasc Interv Radiol. 2017;28:576–582. doi: 10.1016/j.jvir.2016.12.1218. [DOI] [PubMed] [Google Scholar]
- 39.Hameed A, Pang T, Chiou J, et al. Percutaneous vs. endoscopic pre-operative biliary drainage in hilar cholangiocarcinoma—a systematic review and meta-analysis. HPB (Oxford) 2016;18:400–410. doi: 10.1016/j.hpb.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Z, Yang Y, Meng W, Li X. Best option for preoperative biliary drainage in Klatskin tumor: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e8372. doi: 10.1097/MD.0000000000008372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Lin N, Xin F, et al. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J Surg Oncol. 2019;17:116. doi: 10.1186/s12957-019-1656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Son JH, Kim J, Lee SH, et al. The optimal duration of preoperative biliary drainage for periampullary tumors that cause severe obstructive jaundice. Am J Surg. 2013;206:40–46. doi: 10.1016/j.amjsurg.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 43.Grønkjær M, Eliasen M, Skov-Ettrup LS, et al. Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann Surg. 2014;259:52–71. doi: 10.1097/SLA.0b013e3182911913. [DOI] [PubMed] [Google Scholar]
- 44.Lv Y, Liu C, Wei T, et al. Cigarette smoking increases risk of early morbidity after hepatic resection in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2015;41:513–519. doi: 10.1016/j.ejso.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Lindström D, Sadr Azodi O, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg. 2008;248:739–745. doi: 10.1097/SLA.0b013e3181889d0d. [DOI] [PubMed] [Google Scholar]
- 46.Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359:114–117. doi: 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
- 47.Wong J, Lam DP, Abrishami A, et al. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth. 2012;59:268–279. doi: 10.1007/s12630-011-9652-x. [DOI] [PubMed] [Google Scholar]
- 48.Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. 2014;2014:CD002294. doi: 10.1002/14651858.CD002294.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kai K, Koga H, Aishima S, et al. Impact of smoking habit on surgical outcomes in non-B non-C patients with curative resection for hepatocellular carcinoma. World J Gastroenterol. 2017;23:1397–1405. doi: 10.3748/wjg.v23.i8.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X-F, Wei T, Liu X-M, et al. Impact of cigarette smoking on outcome of hepatocellular carcinoma after surgery in patients with hepatitis B. PLOS ONE. 2014;9:e85077. doi: 10.1371/journal.pone.0085077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eliasen M, Grønkjær M, Skov-Ettrup LS, et al. Preoperative alcohol consumption and postoperative complications: a systematic review and meta-analysis. Ann Surg. 2013;258:930–942. doi: 10.1097/SLA.0b013e3182988d59. [DOI] [PubMed] [Google Scholar]
- 52.Bennett K, Enki DG, Thursz M, et al. Systematic review with meta-analysis: high mortality in patients with non-severe alcoholic hepatitis. Aliment Pharmacol Ther. 2019;50:249–257. doi: 10.1111/apt.15376. [DOI] [PubMed] [Google Scholar]
- 53.Bo Y, Yao M, Zhang L, et al. Preoperative Nutritional Risk Index to predict postoperative survival time in primary liver cancer patients. Asia Pac J Clin Nutr. 2015;24:591–597. doi: 10.6133/apjcn.2015.24.4.26. [DOI] [PubMed] [Google Scholar]
- 54.Fan X, Chen G, Li Y, et al. The preoperative prognostic nutritional index in hepatocellular carcinoma after curative hepatectomy: a retrospective cohort study and meta-analysis. J Invest Surg. 2021;34:826–833. doi: 10.1080/08941939.2019.1698679. [DOI] [PubMed] [Google Scholar]
- 55.Man Z, Pang Q, Zhou L, et al. Prognostic significance of preoperative prognostic nutritional index in hepatocellular carcinoma: a meta-analysis. HPB (Oxford) 2018;20:888–895. doi: 10.1016/j.hpb.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Takagi K, Domagala P, Polak WG, et al. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:211. doi: 10.1186/s12876-019-1126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–650. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Masuda T, Shirabe K, Yoshiya S, et al. Nutrition support and infections associated with hepatic resection and liver transplantation in patients with chronic liver disease. JPEN J Parenter Enteral Nutr. 2013;37:318–326. doi: 10.1177/0148607112456041. [DOI] [PubMed] [Google Scholar]
- 59.McKay BP, Larder AL, Lam V. Pre-operative vs. peri-operative nutrition supplementation in hepatic resection for cancer: a systematic review. Nutr Cancer. 2019;71:179–198. doi: 10.1080/01635581.2018.1560479. [DOI] [PubMed] [Google Scholar]
- 60.Barth R, Mills J, Suriawinata A, et al. Short-term preoperative diet decreases bleeding after partial hepatectomy: results from a multi-institutional randomized controlled trial. Ann Surg. 2019;269:48–52. doi: 10.1097/SLA.0000000000002709. [DOI] [PubMed] [Google Scholar]
- 61.Mikagi K, Kawahara R, Kinoshita H, Aoyagi S. Effect of preoperative immunonutrition in patients undergoing hepatectomy; a randomized controlled trial. Kurume Med J. 2011;58:1–8. doi: 10.2739/kurumemedj.58.1. [DOI] [PubMed] [Google Scholar]
- 62.Wu Z, Qin J, Pu L. Omega-3 fatty acid improves the clinical outcome of hepatectomized patients with hepatitis B virus (HBV)-associated hepatocellular carcinoma. J Biomed Res. 2012;26:395–399. doi: 10.7555/JBR.26.20120058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y, Shao C, Zhang W, et al. Omega-3 polyunsaturated fatty acids prevent progression of liver fibrosis and promote liver regeneration after partial hepatectomy in cirrhotic rats. Eur Rev Med Pharmacol Sci. 2019;23:10151–10160. doi: 10.26355/eurrev_201911_19585. [DOI] [PubMed] [Google Scholar]
- 64.Akbari M, Celik SU, Kocaay AF, et al. Omega-3 fatty acid supplementation does not influence liver regeneration in rats after partial hepatectomy. Clin Exp Hepatol. 2018;4:253–259. doi: 10.5114/ceh.2018.80127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beppu T, Nitta H, Hayashi H, et al. Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: a randomized controlled trial. J Gastroenterol. 2015;50:1197–1205. doi: 10.1007/s00535-015-1067-y. [DOI] [PubMed] [Google Scholar]
- 66.Zhang C, Chen B, Jiao A, et al. The benefit of immunonutrition in patients undergoing hepatectomy: a systematic review and meta-analysis. Oncotarget. 2017;8:86843–86852. doi: 10.18632/oncotarget.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Lin J, Li F, et al. Effect of omega-3 polyunsaturated fatty acids on liver function and inflammatory reaction in patients undergoing hepatectomy: a systematic review and meta-analysis of randomized control trials. Expert Rev. 2019;13:375–384. doi: 10.1080/17474124.2019.1578648. [DOI] [PubMed] [Google Scholar]
- 68.Meng J, Zhong J, Zhang H, et al. Pre-, peri-, and postoperative oral administration of branched-chain amino acids for primary liver cancer patients for hepatic resection: a systematic review. Nutr Cancer. 2014;66:517–522. doi: 10.1080/01635581.2013.780628. [DOI] [PubMed] [Google Scholar]
- 69.Russell K, Zhang HG, Gillanders LK, et al. Preoperative immunonutrition in patients undergoing liver resection: a prospective randomized trial. World J Hepatol. 2019;11:305–317. doi: 10.4254/wjh.v11.i3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linecker M, Botea F, Aristotele Raptis D, et al. Perioperative omega-3 fatty acids fail to confer protection in liver surgery: results of a multicentric, double-blind, randomized controlled trial. J Hepatol. 2019;15:15. doi: 10.1016/j.jhep.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Ichikawa K, Okabayashi T, Maeda H, et al. Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today. 2013;43:720–726. doi: 10.1007/s00595-012-0288-4. [DOI] [PubMed] [Google Scholar]
- 72.American Society of Anesthesiologists Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures an updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126:376–393. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 73.Scott MJ, Fawcett WJ. Oral carbohydrate preload drink for major surgery—the first steps from famine to feast. Anaesthesia. 2014;69:1308–1313. doi: 10.1111/anae.12921. [DOI] [PubMed] [Google Scholar]
- 74.Svanfeldt M, Thorell A, Hausel J, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342–1350. doi: 10.1002/bjs.5919. [DOI] [PubMed] [Google Scholar]
- 75.Gjessing PF, Constantin-Teodosiu D, Hagve M, et al. Preoperative carbohydrate supplementation attenuates post-surgery insulin resistance via reduced inflammatory inhibition of the insulin-mediated restraint on muscle pyruvate dehydrogenase kinase 4 expression. Clin Nutr. 2015;34:1177–1183. doi: 10.1016/j.clnu.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Gjessing PF, Hagve M, Fuskevåg O-M, et al. Single-dose carbohydrate treatment in the immediate preoperative phase diminishes development of postoperative peripheral insulin resistance. Clin Nutr. 2015;34:156–164. doi: 10.1016/j.clnu.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 77.Okabayashi T, Nishimori I, Yamashita K, et al. Preoperative oral supplementation with carbohydrate and branched-chain amino acid-enriched nutrient improves insulin resistance in patients undergoing a hepatectomy: a randomized clinical trial using an artificial pancreas. Amino Acids. 2010;38:901–907. doi: 10.1007/s00726-009-0297-9. [DOI] [PubMed] [Google Scholar]
- 78.Kobayashi K, Kaneko J, Yamaguchi T, et al. Late-evening carbohydrate and branched-chain amino acid snacks improve the nutritional status of patients undergoing hepatectomy based on bioelectrical impedance analysis of body composition. Gastrointest Tumors. 2019;6:81–91. doi: 10.1159/000501452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith MD, McCall J, Plank L, et al (2014) Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev CD009161 [DOI] [PMC free article] [PubMed]
- 80.Amer MA, Smith MD, Herbison GP, et al. Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg. 2017;104:187–197. doi: 10.1002/bjs.10408. [DOI] [PubMed] [Google Scholar]
- 81.Mathur S, Plank LD, McCall JL, et al. Randomized controlled trial of preoperative oral carbohydrate treatment in major abdominal surgery. Br J Surg. 2010;97:485–494. doi: 10.1002/bjs.7026. [DOI] [PubMed] [Google Scholar]
- 82.Noba L, Wakefield A. Are carbohydrate drinks more effective than preoperative fasting: a systematic review of randomised controlled trials. J Clin Nurs. 2019;28:3096–3116. doi: 10.1111/jocn.14919. [DOI] [PubMed] [Google Scholar]
- 83.Beyer TA, Werner S. The cytoprotective Nrf2 transcription factor controls insulin receptor signaling in the regenerating liver. Cell Cycle. 2008;7:874–878. doi: 10.4161/cc.7.7.5617. [DOI] [PubMed] [Google Scholar]
- 84.Gustafsson UO, Nygren J, Thorell A, et al. Pre-operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand. 2008;52:946–951. doi: 10.1111/j.1399-6576.2008.01599.x. [DOI] [PubMed] [Google Scholar]
- 85.Walker KJ, Smith AF (2009) Premedication for anxiety in adult day surgery. Cochrane Database Syst Rev CD002192 [DOI] [PMC free article] [PubMed]
- 86.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel (2019) American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 67:674–694 [DOI] [PubMed]
- 87.Verret M, Lauzier F, Zarychanski R, et al. Perioperative use of gabapentinoids for the management of postoperative acute pain: a systematic review and meta-analysis. Anesthesiology. 2020;133:265–279. doi: 10.1097/ALN.0000000000003428. [DOI] [PubMed] [Google Scholar]
- 88.Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131:411–448. doi: 10.1213/ANE.0000000000004833. [DOI] [PubMed] [Google Scholar]
- 89.Melloul E, Dondéro F, Vilgrain V, et al. Pulmonary embolism after elective liver resection: a prospective analysis of risk factors. J Hepatol. 2012;57:1268–1275. doi: 10.1016/j.jhep.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Eguchi H, Kawamoto K, Tsujie M, et al. A prospective, multi-center phase i study of postoperative enoxaparin treatment in patients undergoing curative hepatobiliary-pancreatic surgery for malignancies. Dig Surg. 2020;37:81–86. doi: 10.1159/000497451. [DOI] [PubMed] [Google Scholar]
- 91.Baltatzis M, Low R, Stathakis P, et al. Efficacy and safety of pharmacological venous thromboembolism prophylaxis following liver resection: a systematic review and meta-analysis. HPB (Oxford) 2017;19:289–296. doi: 10.1016/j.hpb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Felder S, Rasmussen MS, King R, et al. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2019;8:CD004318. doi: 10.1002/14651858.CD004318.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128:1003–1020. doi: 10.1161/CIRCULATIONAHA.113.002690. [DOI] [PubMed] [Google Scholar]
- 94.Morris RJ, Woodcock JP. Intermittent pneumatic compression or graduated compression stockings for deep vein thrombosis prophylaxis? A systematic review of direct clinical comparisons. Ann Surg. 2010;251:393–396. doi: 10.1097/SLA.0b013e3181b5d61c. [DOI] [PubMed] [Google Scholar]
- 95.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S–e277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang L, Zhang Z, Kong J, Wang W. Systematic review and meta-analysis of the benefit and safety of preoperative administration of steroid in patients undergoing liver resection. Front Pharmacol. 2019;10:1442. doi: 10.3389/fphar.2019.01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasegawa Y, Nitta H, Takahara T, et al. Glucocorticoid use and ischemia-reperfusion injury in laparoscopic liver resection: Randomized controlled trial. Ann Gastroenterol Surg. 2019;4:76–83. doi: 10.1002/ags3.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bressan AK, Isherwood S, Bathe OF, et al. Preoperative single-dose methylprednisolone prevents surgical site infections after major liver resection: a randomized controlled trial. Ann Surg. 2022;275:281–287. doi: 10.1097/SLA.0000000000004720. [DOI] [PubMed] [Google Scholar]
- 99.Brustia R, Fleres F, Tamby E, et al. Postoperative collections after liver surgery: Risk factors and impact on long-term outcomes. J Visc Surg. 2020;157:199–209. doi: 10.1016/j.jviscsurg.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 100.Margonis GA, Sasaki K, Andreatos N, et al. Prognostic impact of complications after resection of early stage hepatocellular carcinoma. J Surg Oncol. 2017;115:791–804. doi: 10.1002/jso.24576. [DOI] [PubMed] [Google Scholar]
- 101.Matsuda A, Matsumoto S, Seya T, et al. Does postoperative complication have a negative impact on long-term outcomes following hepatic resection for colorectal liver metastasis? A meta-analysis. Ann Surg Oncol. 2013;20:2485–2492. doi: 10.1245/s10434-013-2972-z. [DOI] [PubMed] [Google Scholar]
- 102.Moreno Elola-Olaso A, Davenport DL, Hundley JC, et al. Predictors of surgical site infection after liver resection: a multicentre analysis using National Surgical Quality Improvement Program data. HPB (Oxford) 2012;14:136–141. doi: 10.1111/j.1477-2574.2011.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bratzler DW, Houck PM, Workgroup SIPGW. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395–404. doi: 10.1016/j.amjsurg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 104.Guo T, Ding R, Yang J, et al. Evaluation of different antibiotic prophylaxis strategies for hepatectomy: a network meta-analysis. Medicine (Baltimore) 2019;98:e16241. doi: 10.1097/MD.0000000000016241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou YM, Chen ZY, Li XD, et al. Preoperative antibiotic prophylaxis does not reduce the risk of postoperative infectious complications in patients undergoing elective hepatectomy. Dig Dis Sci. 2015;61:1707–13. doi: 10.1007/s10620-015-4008-y. [DOI] [PubMed] [Google Scholar]
- 106.Hirokawa F, Hayashi M, Miyamoto Y, et al. Evaluation of postoperative antibiotic prophylaxis after liver resection: a randomized controlled trial. Am J Surg. 2013;206:8–15. doi: 10.1016/j.amjsurg.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 107.Takayama T, Aramaki O, Shibata T, et al. Antimicrobial prophylaxis for 1 day versus 3 days in liver cancer surgery: a randomized controlled non-inferiority trial. Surg Today. 2019;49:859–869. doi: 10.1007/s00595-019-01813-w. [DOI] [PubMed] [Google Scholar]
- 108.Okamura K, Tanaka K, Miura T, et al. Randomized controlled trial of perioperative antimicrobial therapy based on the results of preoperative bile cultures in patients undergoing biliary reconstruction. J Hepatobiliary Pancreat Sci. 2017;24:382–393. doi: 10.1002/jhbp.453. [DOI] [PubMed] [Google Scholar]
- 109.Sugawara G, Yokoyama Y, Ebata T, et al. Duration of antimicrobial prophylaxis in patients undergoing major hepatectomy with extrahepatic bile duct resection: a randomized controlled trial. Ann Surg. 2018;267:142–148. doi: 10.1097/SLA.0000000000002049. [DOI] [PubMed] [Google Scholar]
- 110.Ramanathan R, Borrebach J, Tohme S, Tsung A. Preoperative biliary drainage is associated with increased complications after liver resection for proximal cholangiocarcinoma. J Gastrointest Surg. 2018;22:1950–1957. doi: 10.1007/s11605-018-3861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takahashi Y, Takesue Y, Fujiwara M, et al. Risk factors for surgical site infection after major hepatobiliary and pancreatic surgery. J Infect Chemother. 2018;24:739–743. doi: 10.1016/j.jiac.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Hsieh CS, Cheng HC, Lin JS, et al. Effect of 4% chlorhexidine gluconate predisinfection skin scrub prior to hepatectomy: a double-blinded, randomized control study. Int Surg. 2014;99:787–794. doi: 10.9738/INTSURG-D-13-00179.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Darouiche RO, Wall MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 114.Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/SLA.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 115.Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–629. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 116.Hilal MA, Aldrighetti L, Dagher I, et al. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg. 2018;268:11–18. doi: 10.1097/SLA.0000000000002524. [DOI] [PubMed] [Google Scholar]
- 117.Fretland AA, Dagenborg VJ, Bjornelv GMW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg. 2018;267:199–207. doi: 10.1097/SLA.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 118.Robles-Campos R, Lopez-Lopez V, Brusadin R, et al. Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc. 2019;33:3926–3936. doi: 10.1007/s00464-019-06679-0. [DOI] [PubMed] [Google Scholar]
- 119.Ciria R, Cherqui D, Geller DA, et al. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg. 2016;263:761–777. doi: 10.1097/SLA.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 120.Ciria R, Gomez-Luque I, Ocana S, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for hepatocellular carcinoma: updated results from the European Guidelines meeting on Laparoscopic Liver Surgery, Southampton, UK, 2017. Ann Surg Oncol. 2019;26:252–263. doi: 10.1245/s10434-018-6926-3. [DOI] [PubMed] [Google Scholar]
- 121.Ciria R, Ocana S, Gomez-Luque I, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc. 2020;34:349–360. doi: 10.1007/s00464-019-06774-2. [DOI] [PubMed] [Google Scholar]
- 122.Syn NL, Kabir T, Koh YX, et al. Survival advantage of laparoscopic versus open resection for colorectal liver metastases: a meta-analysis of individual patient data from randomized trials and propensity-score matched studies. Ann Surg. 2019;22:22. doi: 10.1097/SLA.0000000000003672. [DOI] [PubMed] [Google Scholar]
- 123.Okumura S, Goumard C, Gayet B, et al. Laparoscopic versus open two-stage hepatectomy for bilobar colorectal liver metastases: a bi-institutional, propensity score-matched study. Surgery. 2019;166:959–966. doi: 10.1016/j.surg.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 124.Cipriani F, Alzoubi M, Fuks D, et al. Pure laparoscopic versus open hemihepatectomy: a critical assessment and realistic expectations—a propensity score-based analysis of right and left hemihepatectomies from nine European tertiary referral centers. J Hepatobiliary Pancreat Sci. 2020;27:3–15. doi: 10.1002/jhbp.662. [DOI] [PubMed] [Google Scholar]
- 125.Soubrane O, de Rougemont O, Kim K-H, et al. Laparoscopic living donor left lateral sectionectomy: a new standard practice for donor hepatectomy. Ann Surg. 2015;262:757–761. doi: 10.1097/SLA.0000000000001485. [DOI] [PubMed] [Google Scholar]
- 126.Soubrane O, Eguchi S, Uemoto S, et al. Minimally invasive donor hepatectomy for adult living donor liver transplantation: an international, multi-institutional evaluation of safety, efficacy and early outcomes. Ann Surg. 2022;275:166–174. doi: 10.1097/SLA.0000000000003852. [DOI] [PubMed] [Google Scholar]
- 127.Scatton O, Katsanos G, Boillot O, et al. Pure laparoscopic left lateral sectionectomy in living donors: from innovation to development in France. Ann Surg. 2015;261:506–512. doi: 10.1097/SLA.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 128.Vicente D, Patino M, Marcus R, et al. Impact of epidural analgesia on the systemic biomarker response after hepatic resection. Oncotarget. 2019;10:584–594. doi: 10.18632/oncotarget.26549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kambakamba P, Slankamenac K, Tschuor C, et al. Epidural analgesia and perioperative kidney function after major liver resection. Br J Surg. 2015;102:805–812. doi: 10.1002/bjs.9810. [DOI] [PubMed] [Google Scholar]
- 130.Sakowska M, Docherty E, Linscott D, Connor S. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg. 2009;33:1802–1808. doi: 10.1007/s00268-009-0131-2. [DOI] [PubMed] [Google Scholar]
- 131.Aloia TA, Kim BJ, Segraves-Chun YS, et al. A randomized controlled trial of postoperative thoracic epidural analgesia versus intravenous patient-controlled analgesia after major hepatopancreatobiliary surgery. Ann Surg. 2017;266:545–554. doi: 10.1097/SLA.0000000000002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev. 2018;8:CD010434. doi: 10.1002/14651858.CD010434.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Niewinski G, Figiel W, Grat M, et al. A comparison of intrathecal and intravenous morphine for analgesia after hepatectomy: a randomized controlled trial. World J Surg. 2020;44:2340–2349. doi: 10.1007/s00268-020-05437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang JZJ, Weinberg L. A literature review of intrathecal morphine analgesia in patients undergoing major open hepato-pancreatic-biliary (Hpb) surgery. Anesthesiol Pain Med. 2019;9:e94441. doi: 10.5812/aapm.94441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen MT, Jin B, Du SD, et al. Role of a selective cyclooxygenase-2 inhibitor on pain and enhanced recovery after open hepatectomy: a randomized controlled trial. Transl Cancer Res. 2017;6:806–814. doi: 10.21037/tcr.2017.08.17. [DOI] [Google Scholar]
- 136.Yassen AM, Sayed GE. Low dose ketorolac infusion improves postoperative analgesia combined with patient controlled fentanyl analgesia after living donor hepatectomy—randomized controlled trial. Egypt J Anaesth. 2012;28:199–204. doi: 10.1016/j.egja.2012.02.010. [DOI] [Google Scholar]
- 137.Hughes MJ, Harrison EM, Jin Y, et al. Acetaminophen metabolism after liver resection: a prospective case-control study. Dig Liver Dis. 2015;47:1039–1046. doi: 10.1016/j.dld.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y, Song X, Sun D, et al. Evaluation of intravenous parecoxib infusion pump of patient-controlled analgesia compared to fentanyl for postoperative pain management in laparoscopic liver resection. Med Sci Monit. 2018;24:8224–8231. doi: 10.12659/MSM.913182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Revie EJ, McKeown DW, Wilson JA, et al. Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs. epidural analgesia following liver resection surgery. HPB (Oxford) 2012;14:611–618. doi: 10.1111/j.1477-2574.2012.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bell R, Ward D, Jeffery J, et al. A randomized controlled trial comparing epidural analgesia versus continuous local anesthetic infiltration via abdominal wound catheter in open liver resection. Ann Surg. 2019;269:413–419. doi: 10.1097/SLA.0000000000002988. [DOI] [PubMed] [Google Scholar]
- 141.Hughes MJ, Harrison EM, Peel NJ, et al. Randomized clinical trial of perioperative nerve block and continuous local anaesthetic infiltration via wound catheter versus epidural analgesia in open liver resection (LIVER 2 trial) Br J Surg. 2015;102:1619–1628. doi: 10.1002/bjs.9949. [DOI] [PubMed] [Google Scholar]
- 142.Mungroop TH, Veelo DP, Busch OR, et al. Continuous wound infiltration versus epidural analgesia after hepato-pancreato-biliary surgery (POP-UP): a randomised controlled, open-label, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:105–113. doi: 10.1016/S2468-1253(16)30012-7. [DOI] [PubMed] [Google Scholar]
- 143.Karanicolas PJ, Cleary S, McHardy P, et al. Medial open transversus abdominis plane (MOTAP) catheters reduce opioid requirements and improve pain control following open liver resection: a multicenter, blinded, randomized controlled trial. Ann Surg. 2018;268:233–240. doi: 10.1097/SLA.0000000000002657. [DOI] [PubMed] [Google Scholar]
- 144.Chan SK, Lai PB, Li PT, et al. The analgesic efficacy of continuous wound instillation with ropivacaine after open hepatic surgery. Anaesthesia. 2010;65:1180–1186. doi: 10.1111/j.1365-2044.2010.06530.x. [DOI] [PubMed] [Google Scholar]
- 145.Sun JX, Bai KY, Liu YF, et al. Effect of local wound infiltration with ropivacaine on postoperative pain relief and stress response reduction after open hepatectomy. World J Gastroenterol. 2017;23:6733–6740. doi: 10.3748/wjg.v23.i36.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee SH, Gwak MS, Choi SJ, et al. Prospective, randomized study of ropivacaine wound infusion versus intrathecal morphine with intravenous fentanyl for analgesia in living donors for liver transplantation. Liver Transpl. 2013;19:1036–1045. doi: 10.1002/lt.23691. [DOI] [PubMed] [Google Scholar]
- 147.Peres-Bachelot V, Blanc E, Oussaid N, et al. A 96-hour continuous wound infiltration with ropivacaine reduces analgesic consumption after liver resection: a randomized, double-blind, controlled trial. J Surg Oncol. 2019;119:47–55. doi: 10.1002/jso.25280. [DOI] [PubMed] [Google Scholar]
- 148.Yu H, Li ZY, Yu X. Efficacy of postoperative continuous wound infiltration with local anesthesia after open hepatectomy. Zhonghua Yi Xue Za Zhi. 2013;93:2723–2726. [PubMed] [Google Scholar]
- 149.Xin Y, Hong Y, Yong LZ. Efficacy of postoperative continuous wound infiltration with local anesthesia after open hepatectomy. Clin J Pain. 2014;30:571–576. doi: 10.1097/AJP.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dalmau A, Fustran N, Camprubi I, et al. Analgesia with continuous wound infusion of local anesthetic versus saline: double-blind randomized, controlled trial in hepatectomy. Am J Surg. 2018;215:138–143. doi: 10.1016/j.amjsurg.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 151.Niraj G, Kelkar A, Jeyapalan I, et al. Comparison of analgesic efficacy of subcostal transversus abdominis plane blocks with epidural analgesia following upper abdominal surgery. Anaesthesia. 2011;66:465–471. doi: 10.1111/j.1365-2044.2011.06700.x. [DOI] [PubMed] [Google Scholar]
- 152.Shaker TM, Carroll JT, Chung MH, et al. Efficacy and safety of transversus abdominis plane blocks versus thoracic epidural anesthesia in patients undergoing major abdominal oncologic resections: a prospective, randomized controlled trial. Am J Surg. 2018;215:498–501. doi: 10.1016/j.amjsurg.2017.10.055. [DOI] [PubMed] [Google Scholar]
- 153.Abdelsalam K, Mohamdin OW. Ultrasound-guided rectus sheath and transversus abdominis plane blocks for perioperative analgesia in upper abdominal surgery: a randomized controlled study. Saudi J Anaesth. 2016;10:25–28. doi: 10.4103/1658-354X.169470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Erdogan MA, Ozgul U, Ucar M, et al. Effect of transversus abdominis plane block in combination with general anesthesia on perioperative opioid consumption, hemodynamics, and recovery in living liver donors: the prospective, double-blinded, randomized study. Clin Transpl. 2017;31:e12931. doi: 10.1111/ctr.12931. [DOI] [PubMed] [Google Scholar]
- 155.Guo JG, Li HL, Pei QQ, Feng ZY. The analgesic efficacy of subcostal transversus abdominis plane block with Mercedes incision. BMC Anesthesiol. 2018;18:36. doi: 10.1186/s12871-018-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Serag Eldin M, Mahmoud F, El Hassan R, et al. Intravenous patient-controlled fentanyl with and without transversus abdominis plane block in cirrhotic patients post liver resection. Local Reg. 2014;7:27–37. doi: 10.2147/LRA.S60966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yassen K, Lotfy M, Miligi A, et al. Patient-controlled analgesia with and without transverse abdominis plane and rectus sheath space block in cirrhotic patients undergoing liver resection. J Anesthesiol Clin Pharmacol. 2019;35:58–64. doi: 10.4103/joacp.JOACP_36_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu Q, Li L, Yang Z, et al. Ultrasound guided continuous Quadratus Lumborum block hastened recovery in patients undergoing open liver resection: a randomized controlled, open-label trial. BMC Anesthesiol. 2019;19:23. doi: 10.1186/s12871-019-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang H, Du G, Liu YF, et al. Overlay of a sponge soaked with ropivacaine and multisite infiltration analgesia result in faster recovery after laparoscopic hepatectomy. World J Gastroenterol. 2019;25:5185–5196. doi: 10.3748/wjg.v25.i34.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li H, Chen R, Yang Z, et al. Comparison of the postoperative effect between epidural anesthesia and continuous wound infiltration on patients with open surgeries: a meta-analysis. J Clin Anesth. 2018;51:20–31. doi: 10.1016/j.jclinane.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 161.Gavriilidis P, Roberts KJ, Sutcliffe RP. Local anaesthetic infiltration via wound catheter versus epidural analgesia in open hepatectomy: a systematic review and meta-analysis of randomised controlled trials. HPB (Oxford) 2019;21:945–952. doi: 10.1016/j.hpb.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 162.Ichida H, Imamura H, Yoshimoto J, et al. Randomized controlled trial for evaluation of the routine use of nasogastric tube decompression after elective liver surgery. J Gastrointest Surg. 2016;20:1324–1330. doi: 10.1007/s11605-016-3116-0. [DOI] [PubMed] [Google Scholar]
- 163.Wen Z, Zhang X, Liu Y, et al. Is routine nasogastric decompression after hepatic surgery necessary? A systematic review and meta-analysis. Int J Nurs Stud. 2019;100:103406. doi: 10.1016/j.ijnurstu.2019.103406. [DOI] [PubMed] [Google Scholar]
- 164.Kim YI, Fujita S, Hwang VJ, Nagase Y. Comparison of abdominal drainage and no-drainage after elective hepatectomy: a randomized study. Hepatogastroenterology. 2014;61:707–711. [PubMed] [Google Scholar]
- 165.Butte JM, Grendar J, Bathe O, et al. The role of peri-hepatic drain placement in liver surgery: a prospective analysis. HPB (Oxford) 2014;16:936–942. doi: 10.1111/hpb.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Messager M, Sabbagh C, Denost Q, et al. Is there still a need for prophylactic intra-abdominal drainage in elective major gastro-intestinal surgery? J Visc Surg. 2015;152:305–313. doi: 10.1016/j.jviscsurg.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 167.Gavriilidis P, Hidalgo E, de’Angelis N, et al. Re-appraisal of prophylactic drainage in uncomplicated liver resections: a systematic review and meta-analysis. HPB (Oxford) 2017;19:16–20. doi: 10.1016/j.hpb.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 168.Petrowsky H, Demartines N, Rousson V, Clavien P-A. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240:1074–1084. doi: 10.1097/01.sla.0000146149.17411.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zimmitti G, Vauthey J-N, Shindoh J, et al. Systematic use of an intraoperative air leak test at the time of major liver resection reduces the rate of postoperative biliary complications. J Am Coll Surg. 2013;217:1028–1037. doi: 10.1016/j.jamcollsurg.2013.07.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tran Cao HS, Phuoc V, Ismael H, et al. Rate of organ space infection is reduced with the use of an air leak test during major hepatectomies. J Gastrointest Surg. 2017;21:85–93. doi: 10.1007/s11605-016-3209-9. [DOI] [PubMed] [Google Scholar]
- 171.Galvão CM, Liang Y, Clark AM. Effectiveness of cutaneous warming systems on temperature control: meta-analysis. J Adv Nurs. 2010;66:1196–1206. doi: 10.1111/j.1365-2648.2010.05312.x. [DOI] [PubMed] [Google Scholar]
- 172.Warttig S, Alderson P, Lewis SR, Smith AF. Intravenous nutrients for preventing inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev. 2016;11:CD009906. doi: 10.1002/14651858.CD009906.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Egan C, Bernstein E, Reddy D, et al. A randomized comparison of intraoperative PerfecTemp and forced-air warming during open abdominal surgery. Anesth Analg. 2011;113:1076–1081. doi: 10.1213/ANE.0b013e31822b896d. [DOI] [PubMed] [Google Scholar]
- 174.Campbell G, Alderson P, Smith AF, Warttig S (2015) Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev CD009891 [DOI] [PMC free article] [PubMed]
- 175.Alderson P, Campbell G, Smith AF, et al (2014) Thermal insulation for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev CD009908 [DOI] [PMC free article] [PubMed]
- 176.Madrid E, Urrútia G, Roqué i Figuls M, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev. 2016;4:CD009016. doi: 10.1002/14651858.CD009016.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moola S, Lockwood C. The effectiveness of strategies for the management and/or prevention of hypothermia within the adult perioperative environment: systematic review. JBI Libr Syst Rev. 2010;8:752–792. doi: 10.11124/jbisrir-2010-144. [DOI] [PubMed] [Google Scholar]
- 178.Tandon M, Karna ST, Pandey CK, et al. Multimodal temperature management during donor hepatectomy under combined general anaesthesia and neuraxial analgesia: retrospective analysis. Indian J Anaesth. 2018;62:431–435. doi: 10.4103/ija.IJA_123_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Birch DW, Dang JT, Switzer NJ, et al. Heated insufflation with or without humidification for laparoscopic abdominal surgery. Cochrane Database Syst Rev. 2016;10:CD007821. doi: 10.1002/14651858.CD007821.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Olthof PB, Reiniers MJ, Dirkes MC, et al. Protective mechanisms of hypothermia in liver surgery and transplantation. Mol Med. 2016;21:833–846. doi: 10.2119/molmed.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg. 2008;247:721–729. doi: 10.1097/SLA.0b013e31815cca68. [DOI] [PubMed] [Google Scholar]
- 182.Ciuni R, Biondi A, Grosso G, et al. Nutritional aspects in patient undergoing liver resection. Updates Surg. 2011;63:249–252. doi: 10.1007/s13304-011-0121-4. [DOI] [PubMed] [Google Scholar]
- 183.Cornide-Petronio ME, Alvarez-Mercado AI, Jimenez-Castro MB, Peralta C. Current knowledge about the effect of nutritional status, supplemented nutrition diet, and gut microbiota on hepatic ischemia-reperfusion and regeneration in liver surgery. Nutrients. 2020;12:284. doi: 10.3390/nu12020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hotta T, Kobayashi Y, Taniguchi K, et al. Evaluation of postoperative nutritional state after hepatectomy for hepatocellular carcinoma. Hepatogastroenterology. 2003;50:1511–1516. [PubMed] [Google Scholar]
- 185.Ishikawa Y, Yoshida H, Mamada Y, et al. Prospective randomized controlled study of short-term perioperative oral nutrition with branched chain amino acids in patients undergoing liver surgery. Hepatogastroenterology. 2010;57:583–590. [PubMed] [Google Scholar]
- 186.Chiarla C, Giovannini I, Giuliante F, et al. Parenteral nutrition in liver resection. J Nutr Metab. 2012;2012:508103. doi: 10.1155/2012/508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gao LB, Tian H, Wang XG, et al. Early enteral and parenteral nutritional support after hepatectomy in patients with hepatic carcinoma: a systematic review and meta-analysis. Onco Targets Ther. 2015;8:623–631. doi: 10.2147/OTT.S73275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao XF, Wu N, Zhao GQ, et al. Enteral nutrition versus parenteral nutrition after major abdominal surgery in patients with gastrointestinal cancer: a systematic review and meta-analysis. J Investig Med. 2016;64:1061–1074. doi: 10.1136/jim-2016-000083. [DOI] [PubMed] [Google Scholar]
- 189.Sun Y, Yang Z, Tan H. Perioperative nutritional support and fluid therapy in patients with liver diseases. Hepatobiliary Surg. 2014;3:140–148. doi: 10.3978/j.issn.2304-3881.2014.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783–1788. doi: 10.2337/dc10-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.King JT, Goulet JL, Perkal MF, Rosenthal RA. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg. 2011;253:158–165. doi: 10.1097/SLA.0b013e3181f9bb3a. [DOI] [PubMed] [Google Scholar]
- 192.Lipshutz AKM, Gropper MA. Perioperative glycemic control: an evidence-based review. Anesthesiology. 2009;110:408–421. doi: 10.1097/ALN.0b013e3181948a80. [DOI] [PubMed] [Google Scholar]
- 193.Eshuis WJ, Hermanides J, van Dalen JW, et al. Early postoperative hyperglycemia is associated with postoperative complications after pancreatoduodenectomy. Ann Surg. 2011;253:739–744. doi: 10.1097/SLA.0b013e31820b4bfc. [DOI] [PubMed] [Google Scholar]
- 194.Jackson RS, Amdur RL, White JC, Macsata RA. Hyperglycemia is associated with increased risk of morbidity and mortality after colectomy for cancer. J Am Coll Surg. 2012;214:68–80. doi: 10.1016/j.jamcollsurg.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 195.Blixt C, Ahlstedt C, Ljungqvist O, et al. The effect of perioperative glucose control on postoperative insulin resistance. Clin Nutr. 2012;31:676–681. doi: 10.1016/j.clnu.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 196.Maeda H, Okabayashi T, Nishimori I, et al. Hyperglycemia during hepatic resection: continuous monitoring of blood glucose concentration. Am J Surg. 2010;199:8–13. doi: 10.1016/j.amjsurg.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 197.Okabayashi T, Nishimori I, Maeda H, et al. Effect of intensive insulin therapy using a closed-loop glycemic control system in hepatic resection patients: a prospective randomized clinical trial. Diabetes Care. 2009;32:1425–1427. doi: 10.2337/dc08-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vibert E, Boleslawski E, Cosse C, et al. Arterial lactate concentration at the end of an elective hepatectomy is an early predictor of the postoperative course and a potential surrogate of intraoperative events. Ann Surg. 2015;262:787–792. doi: 10.1097/SLA.0000000000001468. [DOI] [PubMed] [Google Scholar]
- 199.Fisette A, Hassanain M, Metrakos P, et al. High-dose insulin therapy reduces postoperative liver dysfunction and complications in liver resection patients through reduced apoptosis and altered inflammation. J Clin Endocrinol Metab. 2012;97:217–226. doi: 10.1210/jc.2011-1598. [DOI] [PubMed] [Google Scholar]
- 200.Okabayashi T, Shima Y, Sumiyoshi T, et al. Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care. 2014;37:1516–1524. doi: 10.2337/dc13-1771. [DOI] [PubMed] [Google Scholar]
- 201.Hassanain M, Metrakos P, Fisette A, et al. Randomized clinical trial of the impact of insulin therapy on liver function in patients undergoing major liver resection. Br J Surg. 2013;100:610–618. doi: 10.1002/bjs.9034. [DOI] [PubMed] [Google Scholar]
- 202.Sato H, Lattermann R, Carvalho G, et al. Perioperative glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing major liver resection. Anesth Analg. 2010;110:1711–1718. doi: 10.1213/ANE.0b013e3181d90087. [DOI] [PubMed] [Google Scholar]
- 203.Takesue Y, Tsuchida T. Strict glycemic control to prevent surgical site infections in gastroenterological surgery. Ann Gastroenterol Surg. 2017;1:52–59. doi: 10.1002/ags3.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Polderman JAW, van Steen SCJ, Thiel B, et al. Peri-operative management of patients with type-2 diabetes mellitus undergoing non-cardiac surgery using liraglutide, glucose–insulin–potassium infusion or intravenous insulin bolus regimens: a randomised controlled trial. Anaesthesia. 2018;73:332–339. doi: 10.1111/anae.14180. [DOI] [PubMed] [Google Scholar]
- 205.Igami T, Nishio H, Ebata T, et al. Using the greater omental flap to cover the cut surface of the liver for prevention of delayed gastric emptying after left-sided hepatobiliary resection: a prospective randomized controlled trial. J Hepatobiliary Pancreat Sci. 2011;18:176–183. doi: 10.1007/s00534-010-0323-z. [DOI] [PubMed] [Google Scholar]
- 206.Yoshida H, Mamada Y, Taniai N, et al. Fixation of the greater omentum for prevention of delayed gastric emptying after left-sided hepatectomy: a randomized controlled trial. Hepatogastroenterology. 2005;52:1334–1337. [PubMed] [Google Scholar]
- 207.Oida T, Mimatsu K, Kawasaki A, et al. Fixation of the round ligament to the peritoneum and wrapping of the cut surface of the liver for prevention of early delayed gastric emptying after hepatic lateral segmentectomy. Langenbecks Arch Surg. 2010;395:655–659. doi: 10.1007/s00423-008-0456-6. [DOI] [PubMed] [Google Scholar]
- 208.Shimada M, Morine Y, Nagano H, et al. Effect of TU-100, a traditional Japanese medicine, administered after hepatic resection in patients with liver cancer: a multi-center, phase III trial (JFMC40-1001) Int J Clin Oncol. 2015;20:95–104. doi: 10.1007/s10147-014-0678-2. [DOI] [PubMed] [Google Scholar]
- 209.Nishi M, Shimada M, Uchiyama H, et al. The beneficial effects of Kampo medicine Dai-ken-chu-to after hepatic resection: a prospective randomized control study. Hepatogastroenterology. 2012;59:2290–2294. doi: 10.5754/hge10115. [DOI] [PubMed] [Google Scholar]
- 210.You XM, Mo XS, Ma L, et al. Randomized clinical trial comparing efficacy of simo decoction and acupuncture or chewing gum alone on postoperative ileus in patients with hepatocellular carcinoma after hepatectomy. Medicine (Baltimore) 2015;94:e1968. doi: 10.1097/MD.0000000000001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hendry PO, van Dam RM, Bukkems SF, et al. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg. 2010;97:1198–1206. doi: 10.1002/bjs.7120. [DOI] [PubMed] [Google Scholar]
- 212.Jang SY, Ju EY, Kim DE, et al. First flatus time and xerostomia associated with gum-chewing after liver resection. J Clin Nurs. 2012;21:2188–2192. doi: 10.1111/j.1365-2702.2012.04132.x. [DOI] [PubMed] [Google Scholar]
- 213.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–641. doi: 10.1016/S0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 214.Convertino VA. Cardiovascular consequences of bed rest: effect on maximal oxygen uptake. Med Sci Sports Exerc. 1997;29:191–196. doi: 10.1097/00005768-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 215.Brower RG. Consequences of bed rest. Crit Care Med. 2009;37:S422–S428. doi: 10.1097/CCM.0b013e3181b6e30a. [DOI] [PubMed] [Google Scholar]
- 216.Ni CY, Wang ZH, Huang ZP, et al. Early enforced mobilization after liver resection: a prospective randomized controlled trial. Int J Surg. 2018;54:254–258. doi: 10.1016/j.ijsu.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 217.De Almeida EPM, De Almeida JP, Landoni G, et al. Early mobilization programme improves functional capacity after major abdominal cancer surgery: a randomized controlled trial. Br J Anaesth. 2017;119:900–907. doi: 10.1093/bja/aex250. [DOI] [PubMed] [Google Scholar]
- 218.Burgess LC, Immins T, Wainwright TW. What is the role of post-operative physiotherapy in general surgical Enhanced Recovery after Surgery pathways? Eur J Physiother. 2019;21:67–72. doi: 10.1080/21679169.2018.1468813. [DOI] [Google Scholar]
- 219.Jones C, Kelliher L, Dickinson M, et al. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg. 2013;100:1015–1024. doi: 10.1002/bjs.9165. [DOI] [PubMed] [Google Scholar]
- 220.Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742–753. doi: 10.1093/bja/aes276. [DOI] [PubMed] [Google Scholar]
- 221.Carlisle JB, Stevenson CA (2006) Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev CD004125 [DOI] [PMC free article] [PubMed]
- 222.DREAMS Trial Collaborators and West Midlands Research Collaborative Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS Trial) BMJ. 2017;357:j1455. doi: 10.1136/bmj.j1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/S1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 225.Page AJ, Kooby DA. Perioperative management of hepatic resection. J Gastrointest Oncol. 2012;3:19–27. doi: 10.3978/j.issn.2078-6891.2012.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gurusamy KS, Li J, Vaughan J, et al (2012) Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev CD007338 [DOI] [PMC free article] [PubMed]
- 227.Hughes MJ, Ventham NT, Harrison EM, Wigmore SJ. Central venous pressure and liver resection: a systematic review and meta-analysis. HPB (Oxford) 2015;17:863–871. doi: 10.1111/hpb.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cecconi M, Corredor C, Arulkumaran N, et al. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209. doi: 10.1186/cc11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Correa-Gallego C, Tan KS, Arslan-Carlon V, et al. Goal-directed fluid therapy using stroke volume variation for resuscitation after low central venous pressure-assisted liver resection: a randomized clinical trial. J Am Coll Surg. 2015;221:591–601. doi: 10.1016/j.jamcollsurg.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Weinberg L, Ianno D, Churilov L, et al. Goal directed fluid therapy for major liver resection: a multicentre randomized controlled trial. Ann Med Surg (Lond) 2019;45:45–53. doi: 10.1016/j.amsu.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dunki-Jacobs EM, Philips P, Scoggins CR, et al. Stroke volume variation in hepatic resection: a replacement for standard central venous pressure monitoring. Ann Surg Oncol. 2014;21:473–478. doi: 10.1245/s10434-013-3323-9. [DOI] [PubMed] [Google Scholar]
- 232.Ratti F, Cipriani F, Reineke R, et al. Intraoperative monitoring of stroke volume variation versus central venous pressure in laparoscopic liver surgery: a randomized prospective comparative trial. HPB (Oxford) 2016;18:136–144. doi: 10.1016/j.hpb.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Noonpradej S, Akaraborworn O. Intravenous fluid of choice in major abdominal surgery: a systematic review. Crit Care Res Pract. 2020;2020:2170828. doi: 10.1155/2020/2170828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Labgaa I, Joliat G-R, Grass F, et al. Impact of postoperative weight gain on complications after liver surgery. HPB (Oxford) 2020;22:744–749. doi: 10.1016/j.hpb.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 235.van der Werf LR, Kok NFM, Buis CI, et al. Implementation and first results of a mandatory, nationwide audit on liver surgery. HPB (Oxford) 2019;21:1400–1410. doi: 10.1016/j.hpb.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 236.Ivers N, Jamtvedt G, Flottorp S, et al (2012) Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev CD000259 [DOI] [PMC free article] [PubMed]
- 237.Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29:1534–1541. doi: 10.1007/s11606-014-2913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Grimshaw JM, Ivers N, Linklater S, et al. Reinvigorating stagnant science: implementation laboratories and a meta-laboratory to efficiently advance the science of audit and feedback. BMJ Qual Saf. 2019;28:416–423. doi: 10.1136/bmjqs-2018-008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Foy R, Skrypak M, Alderson S, et al. Revitalising audit and feedback to improve patient care. BMJ. 2020;368:m213. doi: 10.1136/bmj.m213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Brown B, Gude WT, Blakeman T, et al. Clinical performance feedback intervention theory (CP-FIT): a new theory for designing, implementing, and evaluating feedback in health care based on a systematic review and meta-synthesis of qualitative research. Implement Sci. 2019;14:40. doi: 10.1186/s13012-019-0883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Brehaut JC, Colquhoun HL, Eva KW, et al. Practice feedback interventions: 15 suggestions for optimizing effectiveness. Ann Intern Med. 2016;164:435–441. doi: 10.7326/M15-2248. [DOI] [PubMed] [Google Scholar]
- 242.Lunel T, Mohkam K, Merle P, et al. Impact of 2016 enhanced recovery after surgery (ERAS) recommendations on outcomes after hepatectomy in cirrhotic and non-cirrhotic patients. World J Surg. 2021;45:2964–2974. doi: 10.1007/s00268-021-06229-7. [DOI] [PubMed] [Google Scholar]
- 243.Zheng Y, Wang L, Wu F, et al. Enhanced recovery after surgery strategy for cirrhosis patients undergoing hepatectomy: experience in a single research center. Ann Surg Treat Res. 2020;98:224–234. doi: 10.4174/astr.2020.98.5.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Olson KA, Fleming RYD, Fox AW, et al. The enhanced recovery after surgery (ERAS) elements that most greatly impact length of stay and readmission. Am Surg. 2021;87:473–479. doi: 10.1177/0003134820951440. [DOI] [PubMed] [Google Scholar]
- 245.St-Amour P, St-Amour P, Joliat GR, et al. Impact of ERAS compliance on the delay between surgery and adjuvant chemotherapy in hepatobiliary and pancreatic malignancies. Langenbecks Arch Surg. 2020;405:959–966. doi: 10.1007/s00423-020-01981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.