Abstract
Depression is one of the most poorly understood diseases due to its elusive pathogenesis. There is an urgency to identify molecular and biological mechanisms underlying depression and the gut microbiome is a novel area of interest. Here we investigate the relation of fecal microbiome diversity and composition with depressive symptoms in 1,054 participants from the Rotterdam Study cohort and validate these findings in the Amsterdam HELIUS cohort in 1,539 subjects. We identify association of thirteen microbial taxa, including genera Eggerthella, Subdoligranulum, Coprococcus, Sellimonas, Lachnoclostridium, Hungatella, Ruminococcaceae (UCG002, UCG003 and UCG005), LachnospiraceaeUCG001, Eubacterium ventriosum and Ruminococcusgauvreauiigroup, and family Ruminococcaceae with depressive symptoms. These bacteria are known to be involved in the synthesis of glutamate, butyrate, serotonin and gamma amino butyric acid (GABA), which are key neurotransmitters for depression. Our study suggests that the gut microbiome composition may play a key role in depression.
Subject terms: Microbiology, Microbiome
Here, the authors analyze the relation of fecal microbiota diversity and composition with depressive symptoms in 1,054 participants from the Rotterdam Study cohort and in 1,539 subjects of the Amsterdam HELIUS cohort, finding associations with bacteria known to be involved in the synthesis of key neurotransmitters for depression.
Introduction
Depression is one of the most common mental disorders experienced worldwide with an average lifetime prevalence of 11–15%1. The prevalence has doubled and, in some countries, even tripled during the COVID-19 pandemic2. Yet, depression is also one of the most common and poorly understood diseases courtesy of its elusive pathogenesis. Treatment options are sub-optimal with most antidepressants performing only marginally better than placebo3,4 with additional costs of having side effects ranging from minor cognitive complaints to even suicide5. The low to moderate heritability6 and the small effects of genetic variants (odds ratio <1.05) identified in large genome-wide association studies (GWAS) of depression7 entails the need to go beyond genetics in the search of molecular biomarkers of depression.
Evidence is accumulating that gut microbiota may influence brain activity and behavior via neural and humoral pathways8,9 and may have translational applications in the treatment of neuropsychiatric disorders10–12. Several animal studies suggest that gut microbiota might have impact on the neurobiological features of depression13–21. Fecal microbiota transplantation of either stressed or obese animals to control animals showed significant alteration of anxiety22. Kelly et al.23 showed that transferring gut microbiota of depressed human patients to germfree rats induces behavioral and physiological features characteristic of depression in the recipient animals suggesting that gut microbiota may be involved in causal pathways leading to depression. Another study showed that pre- and probiotic consumption positively affects mood and anxiety in humans24. There have been very few studies systematically exploring the association between gut microbiome and depression in humans25. Further, the existing studies are based on very small samples (<60 cases), lacking statistical power to detect robust and reproducible associations. The most recent study including 121 cases reported depletion of butyrate producing bacteria (Coprococcus and Dialister) in individuals with depression26. However, these studies did not adjust for confounders including life style factors and medication use25, which are known to modify the gut microbiome27. A parallel study investigated the association of the gut microbiome with depressive symptoms in the multiethnic HELIUS cohort comprising of six different ethnic groups. This study has identified genera/species belonging to the families Christensencellaceae, Lachnospiraceae, and Ruminococcaceae consistently associated with depressive symptoms across ethnicities, taking a wide range of confounders into account [NCOMMS-21-20669B]. Thus far, most consistent associations have been reported for genera Eggerthella, Coproccocus, Subdoligranulum, Mitsuokella, Paraprevotella, Sutterella and family Prevotellaceae28. However, the results of existing studies are conflicting with little overlap asking for larger and more carefully designed studies28.
Here, we study the effect of gut microbiome diversity and composition on depression scores in 1,133 individuals from the Rotterdam Study while controlling for lifestyle factors and medication use. The analyses were replicated in the native Dutch participants of the HELIUS cohort (N = 1,539). Finally, we performed Mendelian Randomization (MR) to elucidate causal relationships between the identified microbiota and major depression.
Results
Microbiome association analysis reveals association of thirteen taxa with depressive symptoms
The cohort characteristics are provided in Table 1. After exclusion of individuals using antidepressants and non-European subjects, 1,054 samples from RS and 1,539 samples from the HELIUS-study were included in the analyses (Table 1). The resulting microbiome data consisted of 17 phyla (for both cohorts), 33 classes for RS and 36 classes for HELIUS, 59 orders in RS and 61 orders for HELIUS, 116 families for RS and 108 families for HELIUS, and 439 genera for RS and 418 genera for HELIUS. In both cohorts, microbiome was dominated by phyla Firmicutes (77% in RS and 70% in HELIUS), Bacteroidetes (13% in RS and 21% in HELIUS), Actinobacteria (0.42% in RS and 0.42% in HELIUS) and Proteobacteria (0.48% in RS and 0.22% in HELIUS).
Table 1.
Descriptive statistics of the study populations
| RS | HELIUS | |
|---|---|---|
| Age: mean(±SD; range) | 56 (±5.9; 45–87) | 51 (±12.8; 19–71) |
| Sex (female%) | 56% | 49% |
| BMI: mean(±SD; range) | 27 (±4.4; 16–51) | 26 (±4.4; 16–53) |
| Smoking (current, ever, never) | (137, 533, 384) | (305, 664, 568) |
| Antidepressants (Yes) | 79 | 66 |
| Depression Score mean(±SD; range) | 4.7 (±6.2; 0–49) | 3 (±3.6; 0–24) |
Descriptive statistics of the Rotterdam Study (N = 1054) and HELIUS (N = 1539) cohorts.
Alpha diversity was negatively associated with depressive symptoms in both RS (Shannon index; beta = −1.57, p value = 1.5 × 10−03) and HELIUS cohorts (Shannon index; beta = −0.64, p value = 2.84 × 10−02). Beta diversity showed significant association with depressive symptoms in RS (Permanova; R2 = 0.003, p value = 0.001) but not in the HELIUS cohort (R2 = 0.0005, p value = 0.51).
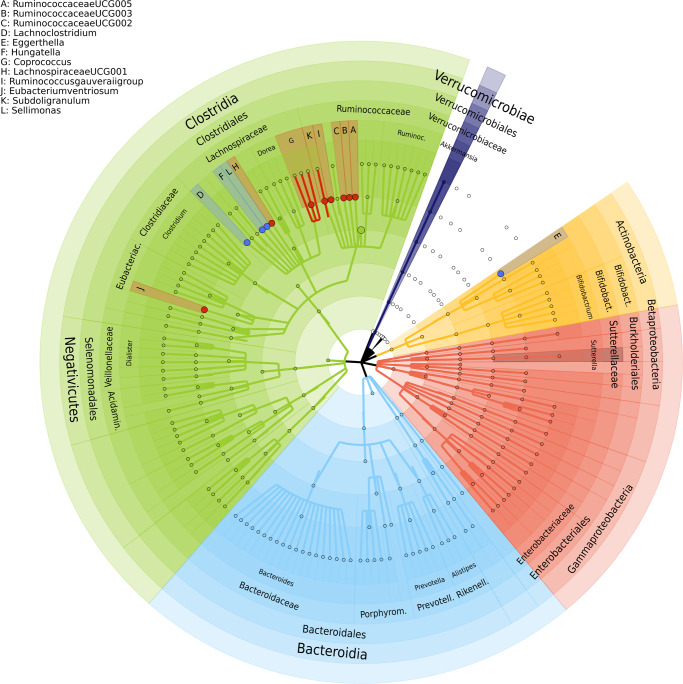
At taxonomic level, 24 genera, three microbial families, one class, two orders and a phylum were significantly (false discovery rate (FDR) < 5%) associated with depressive symptoms in the Rotterdam Study (Table 2, Source Data). We replicated these results in the HELIUS cohort for 12 genera, which were associated with depressive symptoms scores in the same direction (Table 2, Fig. 1). These include Sellimonas, Eggerthella, Ruminococcaceae (UCG002, UCG003, UCG005), Coprococcus3, Lachnoclostridium, Hungatella, LachnospiraceaeUCG001, Ruminococcusgauvreauiigroup, Eubacterium ventriosum and Subdoligranulum. Of the three microbial families significantly associated with depressive symptoms in RS, only family Ruminococcaceae was significantly associated with depressive symptoms in the HELIUS cohort. The direction of association was consistent for all associated taxa across both cohorts and the meta-analysis of results from both cohorts improved association p-values (Table 2). Of the 12 significantly associated genera 10 belong to the families Ruminococcaceae and Lachnospiraceae. All significantly associated genera belonging to the family Ruminococcaceae were depleted in those with higher depressive symptoms (Fig. 1). While most of the significantly associated genera within family Lachnospiraceae were increased in those reporting higher depressive symptoms (Fig. 1).
Table 2.
Microbiota significantly associated with depressive symptoms
| Taxon | Rotterdam Study (N = 1054) | HELIUS (N = 1539) | Meta-analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | Se | p | fdr | Beta | Se | P-value | N | Zscore | P-value | |
| family.Christensenellaceae.id.1866 | −0.59 | 0.12 | 4.56E–07 | 8.04E–05 | −0.10 | 0.06 | 1.32E–01 | 2593 | −1.88 | 5.96E–02 |
| genus.ChristensenellaceaeR7group.id.11283 | −0.55 | 0.11 | 3.31E–07 | 8.04E–05 | −0.10 | 0.06 | 1.30E–01 | 2593 | −4.42 | 9.80E–06 |
| genus.RuminococcaceaeUCG005.id.11363 | −0.59 | 0.13 | 1.36E–05 | 1.57E–03 | −0.12 | 0.07 | 7.62E–02 | 2593 | −4.14 | 3.48E–05 |
| genus.RuminococcaceaeUCG010.id.11367 | −0.50 | 0.12 | 1.77E–05 | 1.57E–03 | −0.07 | 0.06 | 2.08E–01 | 2593 | −3.71 | 2.11E–04 |
| family.Ruminococcaceae.id.2050 | −1.59 | 0.38 | 2.51E–05 | 1.77E–03 | −0.52 | 0.24 | 3.11E–02 | 2593 | −4.35 | 1.38E–05 |
| genus.Coprococcus2.id.11302 | −0.29 | 0.08 | 2.74E–04 | 1.03E–02 | −0.02 | 0.04 | 5.79E–01 | 2593 | −2.75 | 6.02E–03 |
| genus.FamilyXIIIAD3011group.id.11293 | −0.74 | 0.20 | 2.56E–04 | 1.03E–02 | −0.06 | 0.10 | 5.78E–01 | 2593 | −2.76 | 5.78E–03 |
| genus.Lachnoclostridium.id.11308 | 0.57 | 0.16 | 3.11E–04 | 1.03E–02 | 0.27 | 0.11 | 1.80E–02 | 2593 | 4.12 | 3.76E–05 |
| genus.RuminococcaceaeUCG002.id.11360 | −0.42 | 0.12 | 3.21E–04 | 1.03E–02 | −0.16 | 0.07 | 2.33E–02 | 2593 | −4.04 | 5.30E–05 |
| genus.RuminococcaceaeUCG003.id.11361 | −0.51 | 0.15 | 4.60E–04 | 1.33E–02 | −0.25 | 0.08 | 3.10E–03 | 2593 | −4.51 | 6.42E–06 |
| class.Mollicutes.id.3920 | −0.39 | 0.12 | 8.63E–04 | 1.38E–02 | −0.01 | 0.05 | 8.04E–01 | 2593 | −2.32 | 2.06E–02 |
| genus.Eggerthella.id.819 | 0.65 | 0.19 | 6.28E–04 | 1.38E–02 | 0.31 | 0.12 | 1.18E–02 | 2593 | 4.12 | 3.80E–05 |
| genus.Hungatella.id.11306 | 0.77 | 0.22 | 6.73E–04 | 1.38E–02 | 0.34 | 0.14 | 1.34E–02 | 2593 | 4.07 | 4.65E–05 |
| genus.Ruminiclostridium6.id.11356 | −0.36 | 0.11 | 6.23E–04 | 1.38E–02 | −0.01 | 0.05 | 9.06E–01 | 2593 | −2.27 | 2.31E–02 |
| genus.RuminococcaceaeUCG014.id.11371 | −0.29 | 0.08 | 5.70E–04 | 1.38E–02 | −0.05 | 0.05 | 3.16E–01 | 2593 | −2.97 | 2.99E–03 |
| order.MollicutesRF9.id.11579 | −0.40 | 0.12 | 8.28E–04 | 1.38E–02 | 0.00 | 0.05 | 9.55E–01 | 2593 | −2.18 | 2.96E–02 |
| phylum.Tenericutes.id.3919 | −0.39 | 0.12 | 8.63E–04 | 1.38E–02 | −0.01 | 0.05 | 8.04E–01 | 2593 | −2.32 | 2.06E–02 |
| genus.Coprococcus3.id.11303 | −0.43 | 0.13 | 9.09E–04 | 1.39E–02 | −0.29 | 0.09 | 1.20E–03 | 2593 | −4.61 | 4.04E–06 |
| genus.LachnospiraceaeUCG001.id.11321 | −0.52 | 0.16 | 1.01E–03 | 1.48E–02 | −0.16 | 0.07 | 2.34E–02 | 2593 | −3.84 | 1.21E–04 |
| family.Micrococcaceae.id.636 | 1.53 | 0.47 | 1.26E–03 | 1.71E–02 | −0.14 | 0.16 | 3.75E–01 | 2593 | 2.92 | 3.55E–03 |
| genus..Ruminococcusgauvreauiigroup.id.11342 | −0.34 | 0.11 | 1.54E–03 | 2.01E–02 | −0.13 | 0.07 | 4.90E–02 | 2593 | −3.54 | 4.06E–04 |
| genus..Eubacteriumventriosumgroup.id.11341 | −0.49 | 0.16 | 2.09E–03 | 2.47E–02 | −0.17 | 0.08 | 4.20E–02 | 2593 | −3.53 | 4.19E–04 |
| genus.Sellimonas.id.14369 | 0.57 | 0.18 | 2.10E–03 | 2.47E–02 | 0.54 | 0.13 | 3.55E–05 | 2593 | 5.15 | 2.65E–07 |
| genus.Rothia.id.646 | 1.47 | 0.48 | 2.21E–03 | 2.52E–02 | 0.32 | 0.23 | 1.66E–01 | 2593 | 3.02 | 2.55E–03 |
| order.Micrococcales.id.510 | 1.41 | 0.47 | 2.58E–03 | 2.84E–02 | −0.21 | 0.15 | 1.68E–01 | 2593 | 2.96 | 3.09E–03 |
| genus.LachnospiraceaeNK4A136group.id.11319 | −0.41 | 0.14 | 2.80E–03 | 2.99E–02 | −0.08 | 0.08 | 3.48E–01 | 2593 | −2.63 | 8.56E–03 |
| genus.Subdoligranulum.id.2070 | −0.44 | 0.15 | 3.36E–03 | 3.48E–02 | −0.28 | 0.09 | 1.66E–03 | 2593 | −4.29 | 1.77E–05 |
| genus.RuminococcaceaeNK4A214group.id.11358 | −0.34 | 0.12 | 4.66E–03 | 4.70E–02 | −0.04 | 0.06 | 5.73E–01 | 2593 | −2.24 | 2.52E–02 |
| genus.PrevotellaceaeUCG001.id.11186 | −0.49 | 0.17 | 4.80E–03 | 4.71E–02 | −0.02 | 0.08 | 8.38E–01 | 2593 | −1.96 | 5.06E–02 |
Significantly associated taxa with depressive symptom levels in both Rotterdam Study (N = 1,054) and HELIUS (N = 1,539) cohorts. Linear regression models were used adjusting for several life-style confounders (such as smoking and alcohol use) and medical confounders (such as PPI, metformin). The taxa at genus level are in italic face and taxa that remain significant in both discovery and replication cohort and after the meta-analysis are highlighted in bold face. The taxa Data for the regression analysis of the Rotterdam Study are provided as a Source Data file.
Fig. 1. The taxonomic tree showing the 13 genera associated with depressive symptoms.
Red dots depict negatively associated genera with depressive symptoms and blue ones depict positively associated genera with depressive symptoms. The outer most layer depicts the phylum level followed by class, order, family and genus levels.
Random forest analysis with RS as the training cohort and HELIUS as the testing cohort revealed RuminococcaceaeUCG005 as the most important genus in predicting depressive symptoms (Source Data), showing the highest percentage increase in mean squared error (%incMSE) in out of bag analysis. Other important predictors of depressive symptoms include ChristensenellaceaeR7group, Lachnoclostridium, Eggerthella, Sellimonas, and Hungatella, which overlap with the findings of the linear regression analysis in this study (Source Data). Further, important predictors identified by random forest analysis include Roseburia, Streptococcus, Bacteroides, Anaerotruncus, Dorea, Blautia, Veillonella, Desulfovibrio, Anaerostipes and Bifidobacterium, which replicate associations reported earlier28.
Mendelian Randomization (MR) analysis identifies a causal link between major depression and Eggerthella
Results of MR analysis are provided in the Source Data. With major depression as the exposure, Eggerthella, showed significant MR results under the IVW method (effect = 0.237, p value = 0.027) (Source Data). Tests for heterogeneity and horizontal pleiotropy were negative for Eggerthella (Supplementary Data 1, Supplementary Data 2). Further the effect estimates for Eggerthella was also consistent with the findings of this study, i.e., increase in the abundance of Eggerthella in those with higher depressive symptoms. Interestingly, the Steiger test for directionality suggests that Eggerthella is more likely to be causally associated with MDD (Supplementary Data 3). With microbiome as exposure, significant MR was observed for genus Sellimonas under the IVW method (effect = −0.046, p-value = 5.5*10−04) but effect estimate was inconsistent with the findings of our study (Supplementary Data 3).
Among the 87 depression-associated SNPs7 significant association was observed for one SNP rs17641524 with the genus Acidaminococcus after correction for multiple testing (Supplementary Data 4). No significant association was observed for the MDD GRS (Supplementary Data 5).
Discussion
In this large study of 2593 individuals profiled for depressive symptoms and fecal microbiome, we identified 12 genera and 1 microbial family associated with depressive symptoms. These include genera Sellimonas, Eggerthella, Ruminococcaceae (UCG002, UCG003, UCG005), Lachnoclostridium, Hungatella, Coprococcus, LachnospiraceaeUCG001, Ruminococcusgauvreauiigroup, Eubacterium ventriosum, Subdoligranulum and family Ruminococcaceae. Sellimonas, Eggerthella, Lachnoclostridium and Hungatella were more abundant in individuals with higher depressive symptoms. All other taxa were depleted in depression. Alpha diversity was significantly associated with depressive symptoms in both discovery and replication cohorts.
The intestinal bacterial strains Eggerthella, Subdoligranulum, Coprococcus and Ruminococcaceae have been reported to be associated with major depression in earlier studies. Eggerthella has been consistently found to be increased in depression and anxiety cases in 8 studies25,26,28, which is in line with the findings of our study. MR analysis suggests a causal link between MDD and Eggerthella, which requires further investigation. Also in line with our findings Subdoligranulum and Coprococcus were consistently found to be depleted in individuals with generalized anxiety disorder and depression in several studies28. In a recent study Coproccocus was depleted in rats that exhibited depressed behavior upon fecal transplantation from depressed human subjects29, suggesting that Coproccocus may have a causal impact on depression. Both Subdoligranulum and Coprococcus are involved in the production of butyrate26 and Subdoligranulum was found to be increased in omega 3 rich diet30. A previous meta-analysis shows that omega 3 fatty acids, more specifically eicosapentaenoic acid (EPA) supplementation are beneficial for depression31. Ruminococcaceae at genus and family levels have been found to be depleted in cases of both uni- and bipolar depression25,26,28,32–34. A similar pattern is observed in the study by Bosch et al. [NCOMMS-21-20669B] with several genera belonging to the family Ruminococcaceae depleted in those reporting higher depressive symptoms, which is again consistent with the results of our study.
Other findings of this study that have previously not been reported include association with genera Sellimonas, Lachnoclostridium, Hungatella, Eubacterium ventriosum, LachnospiraceaeUCG001, and Ruminococcusgauvreauiigroup. Sellimonas and Hungatella were positively associated with depressive symptoms. Sellimonas is the most significant finding of this study. It belongs to the family Lachnospiraceae and phylum Firmicutes. Species belonging to Sellimonas have been reported to be increased in inflammatory diseases including ankylosing spondylitis, atherosclerosis and liver cirrhosis35. Further, increased abundance of Sellimonas have been observed after dysbiosis36. Lachnoclostridium belongs to the family Lachnospiraceae. Higher levels of Lachnoclostridium were associated with increased depressive symptoms in our study and also consistent with the findings of the Bosch et al. study [NCOMMS-21-20669B]. Lachnoclostridium has previously found to be depleted in other psychiatric disorders including schizophrenia37 and autism38 and in patients with gastrointestinal tract neoplasms39. Hungatella belongs to the family Clostridiaceae and phylum Firmicutes. It has previously been associated with paleolithic diet and is known to produce the precursor molecule for trimethylamine-N-oxide (TMAO)40. TMAO has been implicated in cardio-vascular and neurological diseases including depression41,42. Eubacterium ventriosum belongs to the family Eubacteriaceae and has been found to be significantly depleted after traumatic brain injury in mice43. Major depression is a frequent complication of traumatic brain injury44. In our study we also observed depletion of Eubacterium ventriosum with the increase in depressive symptoms, which fits well with association with traumatic brain injury. In human studies Eubacterium ventriosum was found to be slightly more abundant in obese individuals45,46. Obesity is one of the most prevalent somatic comorbidities of major depressive disorder47,48 and is partly attributed to a side effect of selective serotonin reuptake inhibitors (SSRI). However, in our study we excluded those using antidepressants and adjusted for BMI in the linear regression analysis thus our finding is independent of the association with body weight. LachnospiraceaeUCG001, at species level, was found to be associated with anhedonia in mice49. Ruminococcusgauvreauii belongs to the family Ruminococcaceae and at species level was found to be increased in atherosclerotic conditions35. Interestingly depression is known to be causally associated with atherosclerosis50. It may be worth to investigate the genera Sellimonas and Ruminococcusgauvreauii as potential mediators in the relationship between depression and atherosclerotic conditions.
Most identified microbiota in our study show potential involvement in the synthesis of glutamate and butyrate (see Supplementary Data of Valles-Colomer et al. 2019)26. Eggerthella is further involved in the synthesis of serotonin and gamma aminobutyric acid (GABA). Glutamate is widely distributed in the brain and a major excitatory synaptic neurotransmitter51. It is known to be involved in regulating neuroplasticity, learning and memory52. Glutamate levels in plasma, serum, cerebrospinal fluid and brain tissue have been associated with mood and psychotic disorders and suicide53–58. With increasing evidence of its role in the etiology of depressive disorders, glutamate is rapidly becoming the novel therapeutic target for depressive disorders. Ketamine, for instance, has been shown to increase glutamate signaling in rodents and humans59,60 and has shown to reduce depressive symptoms rapidly61. Glutamate plays a role as a neurotransmitter in the enteric nervous system, which sustains the reciprocal influence between the gastrointestinal tract and the central nervous system8,62. Butyrate on the hand is a short chain fatty acid and modulates biological responses of host gastrointestinal health by acting as a histone deacetylase inhibitor and binding to specific G protein-coupled receptors (GPCRs)63. Butyrate can affect the gut-brain axis by enhancing the cholinergic neurons via epigenetic mechanisms64 and can cross the blood brain barrier and activate the vagus nerve and hypothalamus65,66. Sodium butyrate has shown anti-depressant effects in animal models of depression and mania67,68. Serotonin and GABA are both important neurotransmitters relevant to depression. Evidence suggests that serotonin may be the key neurotransmitter to the gut-brain axis17. Enteric nervous system accounts for >90% of the body’s serotonin production where it is produced by enterochromaffin cells and in the neurons of the enteric nervous system69. The neuronal production of serotonin is most critical for the development and motility of the enteric nervous system, affecting neurogenesis and guiding development of neurons expressing dopamine and GABA69–71. Although serotonin produced by the gut cannot cross the blood-brain barrier72, it can affect the blood-brain barrier permeability, which can lead to inflammation of the brain73. Further, vagus nerve stimulation by the gut microbiota can alter concentration of serotonin, GABA and glutamate within the brain in animals and humans42,74 and germ-free male mice exhibit anxiety-like behaviors and altered serotonin abundance in the brain14. GABA is the main inhibitory neurotransmitter of the central nervous system that counterbalances the action of glutamate75. Low levels of GABA are linked to depression and mood disorders75. Animal studies show that gut microbiota can alter GABA activity in the brain through the vagus nerve76. While each of the metabolites mentioned above are highly relevant for depression, most are known to be unable to cross the blood-brain barrier. However, an increasing number of animal studies show that the peripheral production of neurotransmitters by the gut microbiome can alter brain chemistry and therefore influence mood and behavior42.
In the current study, we aimed to identify gut microbiota associated with depressive symptoms in the general population. The strengths of our study include a large sample, controlling for most known confounders including comorbid conditions, performing analysis in individuals free of anti-depressive medication and finally the use of quantitative depression scales. A large study consisting of 252,503 individuals from 68 countries showed that subthreshold depressive disorders produce significant decrements in health and do not qualitatively differ from full-blown episodes of depression77. Use of rating scales is thus more powerful in omics association studies78. There may have been a loss of statistical power as the depression assessment scales were different in the discovery and replication cohorts. Further, despite the use of the largest GWAS for both microbiome and depression, the MR analysis lacked power. There are 87 SNPs identified for depression, however, their effect on depression is small (individual odds ratio <1.05, combined odds ratio <2.0), which makes unlikely that the individual genetic variants show association with microbiome. For microbiome, there were no SNPs significantly associated at the genome-wide level. Therefore, we had to lower the threshold to 10−05 to identify at least more than one independent instrument for the identified microbiota. This limits the value of the MR. Another limitation of this study is using different methods for stool sampling and sequencing variable regions. These factors might influence the microbial profiles substantially. For example, reads generated by the V4 primer pair showed a higher alpha diversity of the gut microbial community than V1-V2 and V3-V479. In addition, a recent study showed significant differences in bacterial composition that result from collecting stool samples using different stool collection methods compared to immediate freezing80. This may have a negative impact on statistical power. However, despite the differences there is a significant overlap and consistency in effect estimates between the discovery and the replication cohorts. The overlapping results of this study are, therefore, of greater importance, as they are consistent despite methodological differences. It is interesting to note that despite the fact that we replicate most of our findings in the European participants of the HELIUS cohort, there’s only partial overlap with the findings of the study by Bosch et al. [NCOMMS-21-20669B]. However, the lack of significant ethnic differences in that study suggests that non-replication between cohorts (as inferred by p-value testing) likely is in the realm of normal sample variation and coupled to small effect sizes, i.e., may (at least partially) reflect Type 2 statistical error. Another difference is the classification method used for taxonomic identification of bacteria. Bosch et al. uses Amplicon Sequence Variants in 3,211 participants from 6 ethnic groups [NCOMMS-21-20669B], while this study uses closed reference OTU clustering with the same SILVA database in only European participants. Nevertheless, irrespective of the above methodological differences reproduced associations are observed for Lachnoclostridium, Coproccocus and Ruminoccocaceae [NCOMMS-21-20669B] suggesting robust association of depressive symptoms with these taxa.
To summarize, we have identified several bacteria at genera level that might influence depression in humans. We confirm the association of Eggerthella, Coprococcus, Subdoligranulum and family Ruminococcaceae and identify bacteria including Sellimonas, Lachnoclostridium, Hungatella, Ruminococcus, Subdoligranulum, LachnospiraceaeUCG001, Eubacterium ventriosum and Ruminococcusgauvreauiigroup. These bacteria are involved in the synthesis of glutamate, butyrate, serotonin and GABA, which are the key neurotransmitters relevant for depression.
Methods
Study population
The discovery cohort includes 1054 participants from the Rotterdam Study who were not using anti-depressants at the time of assessment. The Rotterdam Study is a population-based cohort study from the well-defined Ommoord district within Rotterdam, The Netherlands. It is designed to investigate occurrence and determinants of diseases in the elderly81. Initially, the RS included 7,983 participants in 1990 who underwent an at-home interview, extensive physical examination at baseline and during follow-up examinations that occur every 3–4 years (RS-I). The RS was extended with two more cohorts in 2000 (RS-II) and 2005 (RS-III) and contains a total of 14,926 participants. In this study we used the data of individuals from the second follow up of the third Rotterdam Study cohort (RS-III-2) as these individuals were profiled for the gut microbiome. The Rotterdam Study (RS-III-2) consists of individuals of European background. The RS is approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The RS was entered into the Netherlands National Trial Register (NTR; www.trialregister.nl) and into the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/network/primary/en/) under shared catalog number NTR6831. All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians. Participants were not compensated for their participation.
The replication cohort included 1539 participants from the Healthy Life in an Urban Setting (HELIUS) cohort. The HELIUS cohort is a multiethnic cohort consisting of individuals of Dutch, Surinamese, Ghanaian, Turkish and Moroccan origin from Amsterdam. People in the age range of 18–70 years were randomly sampled, stratified by ethnic origin through the municipality register of Amsterdam. This register contains data on country of birth of citizens and of their parents, thus allowing for sampling based on the widely accepted Dutch standard indicator for ethnic origin82. The Dutch sample includes people who were born in the Netherlands and whose parents were born in the Netherlands. The current study used data from Dutch samples only. The Medical Ethics Committee of the Amsterdam UMC, location AMC approved the study protocols. Written informed consent was obtained from all participants, who were not compensated for their participation.
Fecal sample collection and microbiome profiling
Detailed description on how the gut microbiome composition was generated at RS-III-2 (2012-2013) and in the HELIUS cohort are described elsewhere83,84. Briefly, in RS, participants were instructed to collect a stool sample at their home in sterile tubes and to send the sample by regular mail to the research location of Erasmus Medical Center (EMC), Rotterdam, the Netherlands. Upon arrival at Erasmus MC, samples were checked and stored at −20 °C. Samples, which were underway for more than 3 days, were excluded84. Subsequently, an automated stool DNA isolation kit (Diasorin, Saluggia, Italy) was used to isolate bacterial DNA from approximately 300 mg stool aliquot using a bead-beating step. The V3 and V4 hypervariable regions of the bacterial 16 S rRNA gene were amplified and sequenced on an Illumina MiSeq platform with the V3 kit (2 × 300 bp paired-end reads; Illumina).
Participants from the HELIUS-study were given a stool collection tube and requested to collect a stool sample and bring their samples to the research location within 6 h after collection and if not possible kept in their freezer overnight and bring it to the research location the next morning. At the research location, the samples were temporarily stored at −20 °C until daily transportation to the Amsterdam Medical Center (AMC), Amsterdam, the Netherlands, where the samples were checked and stored at −80 °C. Total genomic DNA was extracted from a 150 mg aliquot using a repeated bead beating method85. Briefly, fecal samples were bead beated twice and after each bead-beating cycle, samples were heated at 95 °C for 5 min. Supernatants from two extractions were pooled and DNA was purified using the QIAamp DNA Mini kit (QIAGEN Benelux B.V., Venlo, The Netherlands) on the QIAcube (QIAGEN) instrument using the procedure for human DNA analysis.
The composition of fecal microbiota was determined by sequencing the V4 region of the 16 S rRNA gene on a MiSeq system (Illumina) with 515 F and 806 R primers designed for dual indexing (42) and the V2 kit (2 × 250 bp paired-end reads; Illumina). Raw sequencing data from both cohorts were run through the same microbiome-profiling pipeline to harmonize the microbiome data.
Reads were subsampled at 10,000 reads per sample. Taxonomy was assigned using the standard profiling pipeline developed by the MiBioGen consortium86. Briefly, we implemented the 16 S data processing pipeline, which comprised closed reference OTU clustering without a de-noising step based on the naive Bayesian classifier from the Ribosomal Database Project (version 2.12) and the most recent (version 128), full, SILVA database. We only analyzed taxonomical results at genus and higher taxonomic levels. Alpha diversity indices such as species richness, Shannon index and Inverse Simpson were calculated at the genus-level. We calculated Bray-Curtis distances based on absolute abundance of microbial communities at genus level to measure beta-diversity. For single taxon analyses, taxa that were present in less than 3% of the sample size (each cohort separately) and taxa with read counts less than 0.005% of the total number of reads were excluded. Taxa abundances (absolute counts) were then log transformed (to the absolute values 1 was added before log-transformation).
Depression assessment
In RS depressive symptoms were assessed using the 20-item version of the Center for Epidemiological Studies-Depression (CES-D) scale87. CES-D is a self-report measure of symptoms experienced during the prior week. It has been shown to be relatively stable over time and covers the major dimensions of depression including depressed mood, feelings of guilt and worthlessness, feelings of helplessness and hopelessness, psychomotor retardation, loss of appetite and sleep disturbance88. The total score ranges from 0 to 60, with higher scores indicating a greater burden of depressive symptoms. The CES-D detects current MDD cases with high sensitivity and specificity. We used the depression assessment from RS-III-2 (the same time as the collection of the feces).
For participants of the HELIUS cohort, depression was assessed using the Patient Health Questionnaire (PHQ-9) design89. PHQ-9 scores each of the DSM-IV criteria as “0” (not at all) to “3” (nearly every day). The total score ranges from 0 to 21, with higher scores indicating severity of depression. A PHQ-9 score of ≥10 has a sensitivity and specificity of 88% to detect major depression. Individuals with ethnic background other than Europeans and individuals using antidepressants were excluded.
Statistics and reproducibility
Overall, no statistical method was used to predetermine sample size. No data were excluded from the analysis and the experiments were not randomized. The investigations were not blinded to allocation during experiments and outcome assessment.
Microbiome association analysis
To test the association of depressive symptom scores with alpha diversity and individual taxa we used linear regression models using depression scores as the outcome and alpha diversity and taxa (log+1 transformed) as independent variables adjusting for several covariates including sex, age, alcohol use, body mass index (BMI), smoking, medication use (proton pump inhibitors (PPI), metformin, lipid-lowering and antibiotics) and technical covariates including time in mail and batch (in case of RS cohort). Association of the depression scores with microbiome beta-diversity was performed using permutation analysis of variance (PERMANOVA) in R-package “vegan” using the same model as described above.
Results from the discovery and replication cohorts were combined in a meta-analysis using METAL software90. Since the depressive symptoms assessment scales were different in the discovery and replication cohorts, we used sample-size weighted meta-analysis to combine the results. Adjustment for multiple testing was performed using false discovery rate (FDR) using Benjamini-Hochberg correction.
Further, we performed a random forest regression analysis using Breiman’s random forest algorithm91 for regression implemented in the “randomForest” library of the R software. Random forest is a tree-based machine learning algorithm that captures non-linear relationships and can deal with highly correlated input data by leveraging the power of multiple decision trees in order to control overfitting problem. In particular, each tree in the ensemble is built from a bootstrapped sample from the training sample and each node of the tree works on a random subset of the total feature. For this analysis RS stool microbiome profiles were used as predictors and depression scores as response for the training data set, while the HELIUS stool microbiome profiles and depression scores as predictors and response as the test data. Hyperparameters of the model including number of trees (ntree = 500) and number of variables randomly sampled as candidates at each split (mtry = 100) were tuned to give the best performance based on the increase in mean square error (%IncMSE) calculated from out-of-bag samples. In addition, we set the number of times the out of bag data is permuted per tree for assessing variable importance to 100 (nPerm = 100).
Mendelian randomization (MR) analysis
To ascertain causal links between the identified microbiota and major depressive disorder (MDD) we performed two-sample MR analysis using the results of the largest genome-wide association studies of both microbiome and major depression7,92. For major depression we used genome-wide significant single nucleotide polymorphisms (SNPs) as instruments7 (Source Data). For microbiome there were none to a very few SNPs that were genome-wide significantly associated with the identified microbiota, so we used SNPs with a p-value <10−05 as instruments (Source Data). MR analysis was performed using the “TwoSampleMR” library93 of the R software. Linkage disequilibrium pruning of the SNPs was performed using the ‘clump_data’ option with the clump r2 of 0.01 to identify independent instruments. MR report was generated using the ‘mr_report’ option. This method reports results from the weighted median, simple and weighted mode, Inverse variance weighted (IVW) and Egger methods. Variance explained (R2) per instrument for both the exposures and the outcomes were generated using the ‘add_rsq’ option.
We further examined the microbiome-wide association of each of the 87 SNPs associated with depression using the microbiome GWAS summary statistics from Kurilshikov et al.92 to identify the gut microbiota associated with these SNPs. Finally, we tested the association of the genetic risk score combining the summary level data of the 87 SNPs for each microbiota in an unweighted genetic risk score using inverse-weighted method in the ‘rmeta’ package of R software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The Rotterdam Study is a population-based cohort study from the well-defined Ommoord district within Rotterdam, The Netherlands. It is designed to investigate occurrence and determinants of diseases in the elderly. The RS cohort is approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The Rotterdam Study was entered into the Netherlands National Trial Register (NTR; www.trialregister.nl) and into the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/network/primary/en/) under shared catalog number NTR6831. We thank all participants and all others, who made this study possible.
The HELIUS study is conducted by the Amsterdam University Medical Centers, location AMC and the Public Health Service of Amsterdam. Both organisations provided core support for HELIUS. The HELIUS study is also funded by the Dutch Heart Foundation, the Netherlands Organization for Health Research and Development (ZonMw), the European Union (FP-7), and the European Fund for the Integration of non-EU immigrants (EIF). We are most grateful to the participants of the HELIUS study and the management team, research nurses, interviewers, research assistants and other staff who have taken part in gathering the data of this study. Grant numbers:
- ZonMW Memorabel: 733050814 (C.M.v.D.)
- Dutch Heart Foundation: 2010T084 (K.S)
- ZonMw: 200500003 (K.S)
- European Union (FP-7): 278901 (K.S)
- European Fund for the Integration of non-EU immigrants (EIF): 2013EIF013 (K.S)
- European Union (H2020) Research Innovation Action (RIA). Grant agreement ID: 848146 (JA Bosch)
Source data
Author contributions
Conception and design of the study: D.R., J.A.B., R.K., N.A. Collection of the data: D.R., A.G.U., C.M.v.D., R.K., N.A., M.A.I., J.A.B., A.H.Z., A.I.L., M.N., A.L. Analysis: D.R., N.A., R.K., C.M.v.D., J.A.B., M.N., A.L. Interpretation if the data: D.R., N.A., R.K., C.M.v.D., A.G.U, J.B.J.v.M., J.A.B., A.L., A.I.L., M.N., A.H.Z. Drafting of the paper: D.R., N.A., R.K., C.M.v.D. All authors read, revised, and approved the final draft.
Peer review
Peer review information
Nature Communications thanks Mary Kimmel, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
All relevant data supporting the key findings of this study are available within the article and its supplementary files. Full results of the genus-level linear models (Fig. 1, and Table 2), Random forest analyses and Mendelian randomization are supplied as Source Data. Individual-level data of Rotterdam Study and HELIUS Study are not publicly available due to privacy regulations (GDPR). Also, raw 16 S sequencing data from the Rotterdam Study is not publicly available as sharing of participant data, either pseudo-anonymized or anonymized, was not part of the informed consent. Raw 16 S sequencing data from HELIUS participants is available through European Genome-Phenome archive (EGAD00001004106). Rotterdam Study data are available upon request to the data manager Frank van Rooij (f.vanrooij@erasmusmc.nl) and subject to local rules and regulations. This includes submitting a proposal to the management team of RS, where upon approval, analysis needs to be done on a local server with protected access, complying with GDPR regulations. Source data are provided with this paper.
Code availability
The codes used for the analyses in this study are available at https://github.com/Djawad-Radj/Microbiome_Depression.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Robert Kraaij, Email: r.kraaij@erasmusmc.nl.
Najaf Amin, Email: najaf.amin@ndph.ox.ac.uk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-34502-3.
References
- 1.Bromet E, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salari N, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob. Health. 2020;16:57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur. Neuropsychopharmacol. 2009;19:34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Cipriani A, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morley JE. The effectiveness and harms of antidepressants. J. Am. Med. Dir. Assoc. 2017;18:279–281. doi: 10.1016/j.jamda.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 7.Howard DM, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019;22:343. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 9.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol. Motil. 2013;25:713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 10.Cryan JF, Dinan TG. Microbiota and neuroimmune signalling—Metchnikoff to microglia. Nat. Rev. Gastroenterol. Amp; Hepatol. 2015;12:494. doi: 10.1038/nrgastro.2015.127. [DOI] [PubMed] [Google Scholar]
- 11.Desbonnet L, et al. Microbiota is essential for social development in the mouse. Mol. Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey MT, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke G, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 15.El Aidy S, et al. The microbiota and the gut-brain axis: insights from the temporal and spatial mucosal alterations during colonisation of the germfree mouse intestine. Beneficial Microbes. 2012;3:251–259. doi: 10.3920/BM2012.0042. [DOI] [PubMed] [Google Scholar]
- 16.Möhle L, et al. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15:1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 17.O’Mahony SM, et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Ogbonnaya ES, et al. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry. 2015;78:e7–e9. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Park AJ, et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil.: Off. J. Eur. Gastrointest. Motil. Soc. 2013;25:733–e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudo N, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messaoudi M, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 23.Kelly JR, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Steenbergen L, et al. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, Behav., Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Cheung SG, et al. Systematic review of gut microbiota and major depression. Front. Psychiatry. 2019;10:34. doi: 10.3389/fpsyt.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valles-Colomer M, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 27.Fung TC, et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019;4:2064–2073. doi: 10.1038/s41564-019-0540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson CA, et al. The gut microbiota in anxiety and depression - A systematic review. Clin. Psychol. Rev. 2021;83:101943. doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- 29.Knudsen JK, et al. Faecal microbiota transplantation from patients with depression or healthy individuals into rats modulates mood-related behaviour. Sci. Rep. 2021;11:21869. doi: 10.1038/s41598-021-01248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noriega BS, et al. Understanding the impact of Omega-3 rich diet on the gut microbiota. Case Rep. Med. 2016;2016:3089303. doi: 10.1155/2016/3089303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y, et al. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry. 2019;9:190. doi: 10.1038/s41398-019-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu S, et al. Gut microbiota changes in patients with bipolar depression. Adv. Sci. 2019;6:1900752. doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, et al. The role of bacteria and its derived metabolites in chronic pain and depression: Recent findings and research progress. Int. J. Neuropsychopharmacol. 2020;23:26–41. doi: 10.1093/ijnp/pyz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu RT, et al. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun. 2020;88:308–324. doi: 10.1016/j.bbi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nayfach S, et al. New insights from uncultivated genomes of the global human gut microbiome. Nature. 2019;568:505–510. doi: 10.1038/s41586-019-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muñoz, M. et al. Comprehensive genome analyses of <em>Sellimonas intestinalis</em>, a potential biomarker of homeostasis gut recovery. bioRxiv, 2020: p. 2020.04.14.041921. [DOI] [PMC free article] [PubMed]
- 37.Zheng P, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019;5:eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma BJ, et al. Altered gut microbiota in chinese children with autism spectrum disorders. Front. Cellular Infect. Microbiol. 2019;9:40. doi: 10.3389/fcimb.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youssef O, et al. Stool microbiota composition differs in patients with stomach, colon, and rectal neoplasms. Digestive Dis. Sci. 2018;63:2950–2958. doi: 10.1007/s10620-018-5190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genoni A, et al. Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur. J. Nutr. 2020;59:1845–1858. doi: 10.1007/s00394-019-02036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janeiro MH, et al. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10:1398. doi: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caspani G, et al. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Micro. Cell. 2019;6:454–481. doi: 10.15698/mic2019.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treangen TJ, et al. Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front Immunol. 2018;9:2757. doi: 10.3389/fimmu.2018.02757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorge RE, et al. Major depression following traumatic brain injury. Arch. Gen. Psychiatry. 2004;61:42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- 45.Kasai C, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. doi: 10.1186/s12876-015-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tims S, et al. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013;7:707–717. doi: 10.1038/ismej.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Wit L, et al. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res. 2010;178:230–235. doi: 10.1016/j.psychres.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Luppino FS, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 49.Yang C, et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry. 2019;9:57. doi: 10.1038/s41398-019-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jee YH, et al. Cohort study on the effects of depression on atherosclerotic cardiovascular disease risk in Korea. BMJ Open. 2019;9:e026913. doi: 10.1136/bmjopen-2018-026913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72:1313–1333. doi: 10.2165/11633130-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 53.Frye MA, et al. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol. Psychiatry. 2007;61:162–166. doi: 10.1016/j.biopsych.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Holemans S, et al. NMDA glutamatergic receptors, labelled with [3H]MK-801, in brain samples from drug-free depressed suicides. Brain Res. 1993;616:138–143. doi: 10.1016/0006-8993(93)90202-X. [DOI] [PubMed] [Google Scholar]
- 55.Kim JS, et al. Increased serum glutamate in depressed patients. Arch. Psychiatr. Nervenkr (1970) 1982;232:299–304. doi: 10.1007/BF00345492. [DOI] [PubMed] [Google Scholar]
- 56.Levine J, et al. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol. Psychiatry. 2000;47:586–593. doi: 10.1016/S0006-3223(99)00284-X. [DOI] [PubMed] [Google Scholar]
- 57.Mitani H, et al. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 58.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-W. [DOI] [PubMed] [Google Scholar]
- 59.Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102:75–90. doi: 10.1016/j.neuron.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chowdhury GM, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol. Psychiatry. 2017;22:120–126. doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGirr A, et al. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: efficacy and tolerability. J. Psychiatr. Res. 2015;62:23–30. doi: 10.1016/j.jpsychires.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Mazzoli R, Pessione E. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front Microbiol. 2016;7:1934. doi: 10.3389/fmicb.2016.01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Clercq NC, et al. Gut microbiota in obesity and undernutrition. Adv. Nutr. 2016;7:1080–1089. doi: 10.3945/an.116.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soret R, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 65.Gagliano H, et al. High doses of the histone deacetylase inhibitor sodium butyrate trigger a stress-like response. Neuropharmacology. 2014;79:75–82. doi: 10.1016/j.neuropharm.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 66.Liu H, et al. Butyrate: a double-edged sword for health? Adv. Nutr. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valvassori SS, et al. Sodium butyrate, a histone deacetylase inhibitor, reverses behavioral and mitochondrial alterations in animal models of depression induced by early- or late-life stress. Curr. Neurovasc Res. 2015;12:312–320. doi: 10.2174/1567202612666150728121121. [DOI] [PubMed] [Google Scholar]
- 68.Resende WR, et al. Effects of sodium butyrate in animal models of mania and depression: implications as a new mood stabilizer. Behav. Pharm. 2013;24:569–79.. doi: 10.1097/FBP.0b013e32836546fc. [DOI] [PubMed] [Google Scholar]
- 69.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Z, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li ZS, et al. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J. Neurosci. 2004;24:1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawther BK, Kumar S, Krovvidi H. Blood-brain barrier. Continuing Educ. Anaesth. Crit. Care Pain. 2011;11:128–132. doi: 10.1093/bjaceaccp/mkr018. [DOI] [Google Scholar]
- 74.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat. Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lydiard RB. The role of GABA in anxiety disorders. J. Clin. Psychiatry. 2003;64:21–27. [PubMed] [Google Scholar]
- 76.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ayuso-Mateos JL, et al. From depressive symptoms to depressive disorders: the relevance of thresholds. Br. J. Psychiatry. 2010;196:365–371. doi: 10.1192/bjp.bp.109.071191. [DOI] [PubMed] [Google Scholar]
- 78.Story Jovanova O, et al. DNA methylation signatures of depressive symptoms in middle-aged and elderly persons: meta-analysis of multiethnic epigenome-wide studies. JAMA Psychiatry. 2018;75:949–959. doi: 10.1001/jamapsychiatry.2018.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen, Z. et al. Impact of preservation method and 16S rRNA hypervariable region on gut microbiota profiling. mSystems. 4. 10.1128/mSystems.00271-18 (2019). [DOI] [PMC free article] [PubMed]
- 80.Jones J, et al. Fecal sample collection methods and time of day impact microbiome composition and short chain fatty acid concentrations. Sci. Rep. 2021;11:13964. doi: 10.1038/s41598-021-93031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ikram MA, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur. J. Epidemiol. 2017;32:807–850. doi: 10.1007/s10654-017-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Snijder MB, et al. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, The Netherlands. BMJ Open. 2017;7:e017873. doi: 10.1136/bmjopen-2017-017873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deschasaux M, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018;24:1526–1531. doi: 10.1038/s41591-018-0160-1. [DOI] [PubMed] [Google Scholar]
- 84.Radjabzadeh D, et al. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 2020;10:1040. doi: 10.1038/s41598-020-57734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mobini R, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017;19:579–589. doi: 10.1111/dom.12861. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, et al. Meta-analysis of human genome-microbiome association studies: the MiBioGen consortium initiative. Microbiome. 2018;6:101. doi: 10.1186/s40168-018-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for epidemiologic studies depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging. 1997;12:277–287. doi: 10.1037/0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 88.Hek K, et al. A genome-wide association study of depressive symptoms. Biol. Psychiatry. 2013;73:667–678. doi: 10.1016/j.biopsych.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinforma. (Oxf., Engl.) 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Breiman L. Random forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 92.Kurilshikov, A. et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet53, 156–165 (2021) [DOI] [PMC free article] [PubMed]
- 93.Hemani G, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All relevant data supporting the key findings of this study are available within the article and its supplementary files. Full results of the genus-level linear models (Fig. 1, and Table 2), Random forest analyses and Mendelian randomization are supplied as Source Data. Individual-level data of Rotterdam Study and HELIUS Study are not publicly available due to privacy regulations (GDPR). Also, raw 16 S sequencing data from the Rotterdam Study is not publicly available as sharing of participant data, either pseudo-anonymized or anonymized, was not part of the informed consent. Raw 16 S sequencing data from HELIUS participants is available through European Genome-Phenome archive (EGAD00001004106). Rotterdam Study data are available upon request to the data manager Frank van Rooij (f.vanrooij@erasmusmc.nl) and subject to local rules and regulations. This includes submitting a proposal to the management team of RS, where upon approval, analysis needs to be done on a local server with protected access, complying with GDPR regulations. Source data are provided with this paper.
The codes used for the analyses in this study are available at https://github.com/Djawad-Radj/Microbiome_Depression.