Abstract
Racial and racialized economic residential segregation has been empirically associated with outcomes across multiple health conditions but not yet explored in relation to out-of-hospital cardiac arrest (OHCA). We sought to examine if measures of racial and economic residential segregation are associated with differences in survival to discharge after OHCA for Black and White Medicare beneficiaries. Utilizing age-eligible Medicare fee-for-service claims data from 2013 to 2015, we identified OHCA claims and determined survival to discharge. The primary predictor, residential segregation, was calculated using the index of concentration at the extremes (ICE) for the beneficiary residential ZIP code. Multilevel modified Poisson regression models were used to determine the association of OHCA outcomes and ZIP code level ICE measures. In total, 194,263 OHCA cases were identified among beneficiaries residing in 75% of US ZIP codes. Black beneficiaries exhibited 12.1% survival to discharge, compared with 12.5% of White beneficiaries. In fully adjusted models of the three ICE measures accounting for differences in treating hospital characteristics, there was as high as a 28% (RR 1.28, CI 1.23–1.26) higher relative likelihood of survival to discharge in the most segregated White ZIP codes (Q5) as compared to the most segregated Black ZIP codes (Q1). Racial residential segregation is independently associated with disparities in OHCA outcomes; among Medicare beneficiaries who generated a claim after suffering an OHCA, ICE measures of racial segregation are associated with a lower likelihood of survival to discharge for those living in the most segregated Black and lower income quintiles compared to higher quintiles.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11524-022-00691-x.
Keywords: Out-of-hospital cardiac arrest, Health disparities, Racial residential segregation
Introduction
An estimated 350,000 cases of out-of-hospital cardiac arrest (OHCA) occur each year in the USA [1] with variability in survival across regions of the country [2–4]. Racial disparities in incidence and survival after OHCA have been demonstrated: cardiac arrest incidence is higher for Black populations when compared to White populations [5, 6], and survival rates are worse in Black and Hispanic patients when compared to White patients [7, 8]. However, inconsistency across studies persists given known regional differences in outcomes across the USA and the usage of different data sources [9, 10].
The mechanisms that underlie racial disparities in survival after OHCA have been explored at the individual level, identifying biophysical factors and pre-hospital conditions such as arrest rhythm, rates of bystander CPR, and EMS response times that may account for some differences in patient outcomes by racial and ethnic group. The larger impact of the built and social environment in which OHCA occur and racial disparities arise has been less explored. Socioeconomic status (SES) and educational attainment of a neighborhood have been shown to be significantly associated with rates of bystander CPR and intact neurological survival [11, 12]. Prior studies have also found that neighborhood racial composition and income may be significant factors in the prediction of survival to discharge and survival with good neurological status once patients arrive at a healthcare facility [13, 14].
Racial residential segregation, or the geographical separation of populations by race and ethnicity, is a product of long-standing structural and institutional racism in the USA, which has resulted in deep population-based inequality in living environments and social conditions that can affect health and health outcomes. Environments characterized by high levels of isolation and segregation of Black populations, in particular, have been associated with limited employment opportunities, poor housing conditions, and a paucity of high-quality and accessible healthcare which, in turn, is linked to a myriad of poor health outcomes [15, 16]. Research has demonstrated that racial disparities in hypertension diagnosis, cardiovascular disease risk, pre-term birth, and infant mortality may be accounted for by the extent of racial segregation in a specific geography and the historical policies through which racial segregation was structurally established and reinforced (i.e., Redlining) [17–19]. The association of OHCA outcomes and specific measures of racial and economic residential segregation have not been deeply examined to our knowledge. Specifically, we sought to examine if racial and economic residential segregation is associated with differences in survival to discharge after OHCA for Medicare beneficiaries when accounting for patient demographics and comorbidities, ZIP code level residence and socioeconomic status, and treating hospital characteristics.
Methods
Data Sources and Study Population
We conducted a retrospective observational cohort study, utilizing age-eligible (≥ 65 years old) Medicare fee-for-service claims data from the Medicare Provider Analysis and Review (MedPAR) and Outpatient Research Identifiable Files (RIF) for January 2013–December 2015. Individual patient demographics including race and ethnicity, sex, and age were abstracted from the Medicare Beneficiary Summary file. The Vital Status File was used to identify survival past discharge based on validated dates of death for beneficiaries. The analysis focused on Black and White beneficiaries due to the small sample size and distribution across a large number of ZIP codes for all other Medicare designated racial and ethnic groups in the OHCA cohort (Hispanic, Asian/Native Hawaiian, or Pacific Islander, and Alaska Native or American Indian). Claims were excluded if the patient age was < 65 years.
We identified individuals with emergency department (ED)–based claims (including those later admitted to the hospital) by ICD-9-CM codes 427.5, 427.4, 427.41, and 427.42 and mapped ICD-10-CM codes I46, I49.0, I49.01, and I49.02 (International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification) as the primary or admitting diagnosis, based on prior methods for identifying OHCA patients [20–23]. The primary outcome, survival to discharge, was identified from the patient discharge status at the end of the hospital stay. Beneficiaries undergoing interhospital transfer were excluded from these analyses.
Comorbidities were identified from secondary diagnoses flagged as present on admission in the MedPAR or outpatient RIF and adjusted for using the AHRQ Elixhauser Comorbidity index [24, 25]. US Census Bureau American Community Survey (ACS) 5-year estimates for each year of data were used to calculate ZIP code-level racial composition and income and mapped to the beneficiary based on the ZIP code of residence listed on the OHCA claim. Treating hospital characteristics including number of hospital beds (< 100, ≥ 100–399, ≥ 400), hospital teaching status (major academic, minor academic, non-teaching) availability of medical and cardiac intensive care beds (yes/no), and cardiac catheterization availability (yes/no) were identified from the American Hospital Association Survey dataset to account for differences in treatment at the hospital level.
Measuring Racial Residential Segregation
To measure racial residential segregation, the primary predictor of interest, the validated Index of Concentration at the Extremes (ICE) measure, was used [26, 27]. First developed by Massey [28], ICE is a measure of spatial social polarization and accounts for the extreme ends of deprived and privileged groups simultaneously. We followed methods previously used by Krieger and colleagues demonstrating that a combined racial/ethnic and economic ICE measure, or racialized economic segregation, can identify more extreme gradients of risk in health outcomes [18, 29]. Using the ICE measure also avoids the issues of collinearity when accounting for race and income disparities simultaneously in research addressing health disparities [26–28]. Other approaches to measure residential segregation, such as percentage composition of racial and ethnic groups per geographic unit of analysis or the dissimilarity index (DI), have demonstrated that they can underestimate associations with health outcomes [26].
The ICE measure is calculated as the difference between the count of privileged population and disadvantaged population, divided by the total population (Supplemental Fig. 1). The index is the scaled difference between the population counts of the more privileged group and the less privileged group. We calculated three measures for ICE to evaluate racial residential segregation: race alone, income alone, and race and income combined [27] (derived from US Census ACS 5-year estimates). Beneficiary residence was determined from the primary claim present at index admission. To align with available geographic data within the Medicare claims, estimates were calculated at the ZIP code level [30]. Black and White race were utilized for racial ICE calculations as these two groups represent the extreme ends of racial privilege and deprivation for the USA [26] and were also the most representative among the Medicare datasets. For the ICE measures of income, the lowest income group was defined as < $25,000 and ≥ $100,000 for the highest income group, representative of the 20th and 80th percentiles of income for the USA [18]. ZIP code level ICE scores were then binned into quintiles, with Q5 representing the more economically advantaged ZIP codes and Q1 representing the less economically advantaged ZIP codes. For ICE measures of race and income combined, we examined high-income White and low-income Black based on the original design of this measure and prior work in this area [18, 27, 29, 31]. This represents the extreme ends of racialized economic segregation in the USA. ICE measures for other racial and ethnic groups were not explored given the low number of claims in the data. Values for ICE measures are continuous and range from − 1 (most deprived) to 1 (most privileged).
Statistical Analysis
Descriptive statistics were calculated using means with standard deviation or medians with interquartile ranges, as appropriate for continuous covariates of interest, and frequencies with proportions for nominal variables. We constructed multilevel modified Poisson regression models with a random effect for treating hospital. We also included an offset to model rates of survival to discharge. The models were used to estimate the rate ratios and 95% confidence intervals between beneficiary level survival to discharge and (1) ICE race, (2) ICE income, and (3) ICE racialized income (income + race). Three models were built to examine the association between ICE measures and rates of survival to discharge: model 1, unadjusted models including each covariate independently (with hospital random effects term); model 2, a beneficiary-level model with each ICE measure, demographics, and the comorbidity index plus the hospital random effects term; and model 3, a fully adjusted model including each ICE measure, all covariates (beneficiary and hospital), and the hospital random-effects term. Covariates included in the final models included beneficiary demographics (sex, age, race), Elixhauser comorbidity index, and treating hospital characteristics. We also performed several secondary and sensitivity analyses to assess the validity of our results. To examine the role of individual level race in our models, we ran a separate secondary analysis utilizing an interaction term between race and each ICE measures in the three full models (model 3). We also sought to determine if those with residential ZIP codes associated with institutionalized care facilities versus community residence would change outcomes. To perform this, we identified those beneficiaries with an open skilled nursing facility (SNF) claim at the time of the index OCHA or within 1 day of admission and excluded them from the models. All analyses and graphics were conducted using standard statistical software (R version 4.04; R Core Team, Vienna, Austria). This study was completed in accordance with the STROBE guidelines [32]. The study was reviewed and approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai.
Results
We identified 194,263 OHCA beneficiaries with a mean age of 78 years (SD 8.6 years) and with an overall age distribution of 65–74 years: 37.6%; 75–84 years: 35.7%; > 85 years: 26.8% that was 44.7% female (see Table 1). Black beneficiaries made up 16.5% of the cohort (n = 32,029) and 77.2% were White (n = 149,993). Comorbidity burden was similar between both Black and White OHCA beneficiaries. Over half of beneficiaries (54%, n = 104,921) were treated in non-academic hospitals and a significant proportion were treated in hospitals with medical ICU capabilities (79.3%, n = 154,028). Overall, 23,568 (12.1%) of beneficiaries survived to hospital discharge. Unadjusted rates of survival were similar in both Black and White beneficiaries (12.1% and 12.5%, respectively) (Table 1).
Table 1.
Medicare beneficiary OHCA cohort characteristics 2013–2015
| Variable | Overall n (%) | Black n (%) | White n (%) | Othera n (%) | Unknown n (%) |
|---|---|---|---|---|---|
| Overall OHCA cohort beneficiary level | 194,263 | 32,029 (16.5) | 149,993 (77.2) | 11,247 (5.8) | 994 (0.51) |
| Sex | |||||
| Female | 86,870 (44.7) | 17,316 (54.1) | 64,073 (42.7) | 5194 (46.2) | 287 (28.9) |
| Male | 107,393 (55.3) | 14,713 (45.9) | 85,920 (57.3) | 6053 (53.8) | 707 (71.1) |
| Age group category | |||||
| 65–74 y | 72,961 (37.6) | 13,619 (42.5) | 54,835 (36.6) | 3776 (33.6) | 731 (73.5) |
| 75–84 y | 69,267 (35.7) | 10,915 (34.1) | 54,398 (36.3) | 3809 (33.9) | 145 (14.6) |
| 85 + y | 52,035 (26.8) | 7495 (23.4) | 40,760 (27.2) | 3662 (32.6) | 118 (11.9) |
| AHRQ comorbidity index | |||||
| < 0 | 27,291 (14.0) | 4671 (14.6) | 20,833 (13.9) | 1641 (14.6) | 146 (14.7) |
| 0 | 28,430 (14.6) | 3482 (10.9) | 23,405 (15.6) | 1396 (12.4) | 147 (14.8) |
| 1–4 | 12,959 (6.7) | 1691 (5.3) | 10,550 (7.0) | 664 (5.9) | 54 (5.4) |
| > 5 | 80,251 (41.3) | 14,715 (45.9) | 59,701 (39.8) | 5389 (47.9) | 446 (44.9) |
| NA | 45,332 (23.3) | 7470 (23.3) | 35,504 (23.7) | 2157 (19.2) | 201 (20.2) |
| Treating hospital characteristics (beneficiary level) | |||||
| Hospital no. of beds | |||||
| < 100 | 29,240 (15.1) | 3075 (9.6) | 25,012 (16.7) | 1017 (9.0) | 136 (13.7) |
| 100–399 | 107,933 (55.6) | 16,405 (51.2) | 84,335 (56.2) | 6640 (59.0) | 533 (53.6) |
| > 400 | 55,099 (28.4) | 12,249 (38.2) | 39,176 (26.1) | 3364 (29.9) | 310 (31.2) |
| NA | 1991 (1.0) | 300 (0.9) | 1450 (1.0) | 226 (2.0) | 15 (1.5) |
| Hospital teaching status | |||||
| Major teaching | 24,527 (12.6) | 6720 (21.0) | 16,027 (10.7) | 1625 (14.4) | 155 (15.6) |
| Minor teaching | 64,815 (33.4) | 11,004 (34.4) | 49,226 (32.8) | 4252 (37.8) | 333 (33.5) |
| Non-teaching | 104,921 (54.0) | 14,305 (44.7) | 84,740 (56.5) | 5370 (47.7) | 506 (50.9) |
| Medical intensive care unit (MICU) beds (Y or N) | |||||
| MICU beds Y | 154,028 (79.3) | 25,965 (81.1) | 118,690 (79.1) | 8584 (76.3) | 789 (79.4) |
| MICU beds N | 11,457 (5.9) | 1239 (3.9) | 9680 (6.5) | 485 (4.3) | 53 (5.3) |
| NA | 28,778 (14.8) | 4825 (15.1) | 21,623 (14.4) | 2178 (19.4) | 152 (15.3) |
| Cardiac intensive care beds (CIC) (Y or N) | |||||
| CIC beds Y | 88,212 (45.4) | 16,293 (50.9) | 66,243 (44.2) | 5209 (46.3) | 467 (47.0) |
| CIC beds N | 77,273 (39.8) | 10,911 (34.1) | 62,127 (41.4) | 3860 (34.3) | 375 (37.7) |
| NA | 28,778 (14.8) | 4825 (15.1) | 21,623 (14.4) | 2178 (19.4) | 152 (15.3) |
| Cardiac catheterization capability (Y or N) | |||||
| Cardiac catheterization Y | 126,189 (65.0) | 21,667 (67.6) | 96,489 (64.3) | 7363 (65.5) | 670 (67.4) |
| Cardiac catheterization N | 68,074 (35.0) | 10,362 (32.4) | 53,504 (35.7) | 3884 (34.5) | 324 (32.6) |
| Outcomes | |||||
| Expired | 170,695 (87.9) | 28,016 (87.5) | 131,909 (87.9) | 9980 (88.7) | 790 (79.5) |
| Survival to discharge | 23,568 (12.1) | 4013 (12.5) | 18,084 (12.1) | 1267 (11.3) | 204 (20.5) |
aOther: Hispanic; Asian/Native Hawaiian, or Pacific Islander; American Indian or Alaska Native
For ICE measure calculations, a total of 24,685 of 33,120 US ZIP codes were included in the final analytic dataset. For included ZIP codes, the median population was 6910 persons (IQR 2174–2645 persons). Median racial composition for Black beneficiaries by ZIP code was 1.9% (IQR 0.3–9.6%) and for White beneficiaries by ZIP code was 89.8% (IQR 74.7–96.4%). Overall distribution of beneficiaries by urban/rural ZIP code included 50% (n = 97,193) large metro, 31.2% small/mid-metro (n = 60,678), and 18.4 non-metro (n = 35,386) using the 2013 National Center for Health Statistics (NCHS) Urban–Rural Classification system. Median ICE distributions at the beneficiary level for each ICE measure are seen in Supplementary Table 2. Median ICE distributions for ICE race ranged from − 0.19 [IQR − 0.63, 0.09] for Q1 to 0.96 [IQR 0.94, 0.98] for Q5, ICE income − 0.32 [IQR − 0.39, − 0.28] for Q1 to 0.30 [IQR 0.22, 0.40] for Q5, and ICE race + income − 0.16 [IQR − 0.31, − 0.07] for Q1 to 0.39 [IQR 0.33, 0.47] for Q5. Median ICE HHI by ICE measure and quintile ranged from $36,180 to $50,200 for race measure Q1–Q5, $31,920 to $85,250 for income measure, and $33,192 to $83,958 for race + income measure (Supplementary Table 3).
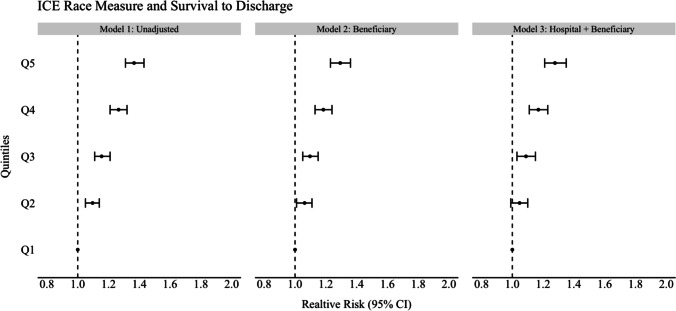
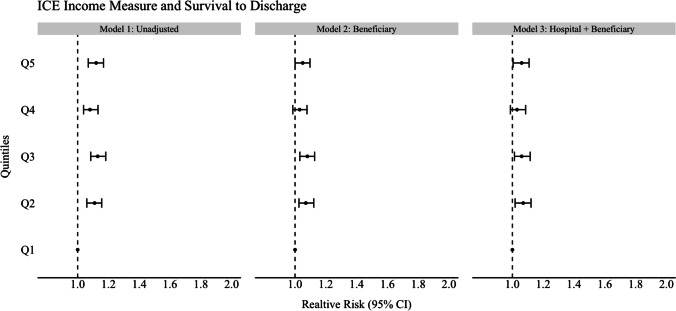
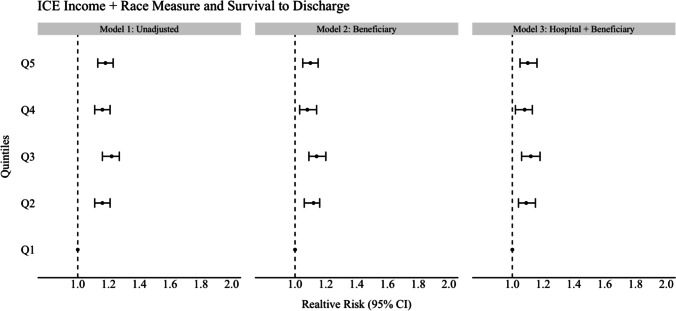
In unadjusted analyses for the ICE race model, beneficiaries residing in the most segregated White ZIP codes (Q5) were 37% more likely to survive to discharge compared to beneficiaries residing in the most segregated Black ZIP codes (Q1, ref) (RR 1.37, CI 1.31–1.43). This increased likelihood of survival persisted and was significant across all other quintiles for the ICE race measure (model 1; see Figs. 1, 2, and 3 and Tables 2, 3, and 4). For the unadjusted ICE income model, this effect was also seen for beneficiaries residing in higher income ZIP codes (Q5) compared to lower income ZIP codes (Q1, ref) with at least 8% (RR 1.08, CI 1.04–1.13) higher likelihood of survival to discharge (Q2) and as much as 13% (Q3) (RR 1.13, 1.08–1.18) compared to reference (Q1). In the unadjusted racialized income ICE model (income + race), beneficiaries residing in the most segregated and higher income White communities (Q5) were 18% more likely to survive to discharge (RR 1.18, CI 1.13–1.23) and as high as 22% (Q3) (RR 1.22, CI 1.16–1.27) compared to reference (Q1). This was present and significant across all quintiles.
Fig. 1.
Forest plot of association of ICE measure and survival to discharge for ICE race measure
Fig. 2.
Forest plot of association of ice measure and survival to discharge for ICE income measure
Fig. 3.
Forest plot of association of ICE measure and survival to discharge for ICE income + race measure
Table 2.
Unadjusted and multilevel models with random effects for relative risks and 95% CIs for association of ICE race measure and survival to discharge among Medicare beneficiaries
| Variable | ICE race model 1 unadjusted | ICE race model 2 (beneficiary) | ICE race model 3 (beneficiary + hospital) | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| ICE race Q1 (most segregated Black) | Ref | Ref | Ref | Ref | Ref | Ref |
| ICE race Q2 | 1.10 | 1.05–1.14 | 1.06 | 1.01–1.11 | 1.05 | 0.99–1.1 |
| ICE race Q3 | 1.15 | 1.11–1.21 | 1.10 | 1.05–1.15 | 1.09 | 1.03–1.15 |
| ICE race Q4 | 1.27 | 1.21–1.32 | 1.18 | 1.13–1.24 | 1.17 | 1.11–1.23 |
| ICE race Q5 (most segregated White) | 1.37 | 1.31–1.43 | 1.29 | 1.23–1.36 | 1.28 | 1.21–1.35 |
| Sex: female | – | – | Ref | Ref | Ref | Ref |
| Sex: male | – | – | 1.07 | 1.04–1.10 | 1.06 | 1.03–1.10 |
| Individual White race | – | – | Ref | Ref | Ref | Ref |
| Individual Black race | – | – | 0.89 | 0.85–0.93 | 0.90 | 0.86–0.94 |
| Age category 65–74 y | – | Ref | Ref | Ref | Ref | |
| Age category 75–84 y | – | – | 0.90 | 0.88–0.93 | 0.90 | 0.88–0.93 |
| Age category 85 + y | – | – | 0.85 | 0.82–0.89 | 0.85 | 0.82–0.89 |
| Elixhauser AHRQ Comorbidity Index < 0 | – | – | Ref | Ref | Ref | Ref |
| Elixhauser AHRQ Index = 0 | – | – | 0.72 | 0.67–0.78 | 0.72 | 0.67–0.78 |
| Elixhauser AHRQ Index = 1–4 | – | – | 0.80 | 0.75–0.86 | 0.79 | 0.74–0.86 |
| Elixhauser AHRQ Index ≥ 5 | – | – | 0.62 | 0.60–0.65 | 0.63 | 0.60–0.66 |
| Hospital size: no. of beds < 100 | – | – | – | – | Ref | Ref |
| Hospital size: no. of beds > 100–399 | – | – | – | – | 0.89 | 0.82–0.96 |
| Hospital size: no. of beds ≥ 400 | – | – | – | – | 0.87 | 0.79–0.94 |
| Hospital teaching status: major academic teaching | – | – | – | – | Ref | Ref |
| Hospital teaching status: minor academic teaching | – | – | – | – | 1.04 | 0.99–1.10 |
| Hospital teaching status: non-teaching | – | – | – | – | 1.11 | 1.04–1.17 |
| Hospital medical intensive care unit beds (no) | – | – | – | – | Ref | Ref |
| Hospital medical intensive care unit beds (yes) | – | – | – | – | 0.94 | 0.86–1.02 |
| Hospital cardiac intensive care beds (CIC) (no) | – | – | – | – | Ref | Ref |
| Hospital cardiac intensive care beds (CIC) (yes/no) | – | – | – | – | 0.99 | 0.95–1.03 |
| Hospital cardiac catheterization availability (no) | – | – | – | – | Ref | Ref |
| Hospital cardiac catheterization availability (yes) | – | – | – | – | 1.13 | 1.07–1.20 |
Ref = Reference Category
Table 3.
Unadjusted and multilevel models with random effects for relative risks and 95% CIs for association of ICE income measure and survival to discharge among Medicare beneficiaries
| ICE income model 1 unadjusted | ICE income model 2 (beneficiary) | ICE income model 3 (beneficiary + hospital) | ||||
|---|---|---|---|---|---|---|
| Variable | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| ICE income Q1 (low income) | Ref | Ref | Ref | Ref | Ref | Ref |
| ICE income Q2 | 1.11 | 1.05–1.16 | 1.07 | 1.03–1.12 | 1.07 | 1.02–1.12 |
| ICE income Q3 | 1.13 | 1.08–1.18 | 1.08 | 1.03–1.13 | 1.06 | 1.01–1.12 |
| ICE income Q4 | 1.08 | 1.04–1.13 | 1.03 | 0.99–1.08 | 1.03 | 0.99–1.09 |
| ICE income Q5 (high income) | 1.12 | 1.07–1.17 | 1.05 | 1.0–1.10 | 1.06 | 1.0–1.11 |
| Sex: female | – | – | Ref | Ref | Ref | Ref |
| Sex: male | – | – | 1.07 | 1.04–1.10 | 1.07 | 1.04–1.10 |
| Individual White race | – | – | Ref | Ref | Ref | Ref |
| Individual Black race | – | – | 0.82 | 0.79–0.85 | 0.84 | 0.80–0.87 |
| Age category 65–74 y | – | – | Ref | Ref | Ref | Ref |
| Age category 75–84 y | – | – | 0.90 | 0.88–0.93 | 0.90 | 0.88–0.93 |
| Age category 85 + y | – | – | 0.85 | 0.82–0.88 | 0.85 | 0.82–0.89 |
| Elixhauser AHRQ Comorbidity Index < 0 | – | – | Ref | Ref | Ref | Ref |
| Elixhauser AHRQ Index = 0 | – | – | 0.72 | 0.67–0.78 | 0.72 | 0.67–0.78 |
| Elixhauser AHRQ Index = 1–4 | – | – | 0.80 | 0.74–0.86 | 0.79 | 0.73–0.85 |
| Elixhauser AHRQ Index ≥ 5 | – | – | 0.62 | 0.59–0.65 | 0.63 | 0.60–0.66 |
| Hospital size: no. of beds < 100 | – | – | – | – | Ref | Ref |
| Hospital size: no. of beds > 100–399 | – | – | – | – | 0.86 | 0.80–0.93 |
| Hospital size: no. of beds ≥ 400 | – | – | – | – | 0.83 | 0.76–0.91 |
| Hospital teaching status: major academic teaching | – | – | – | – | Ref | Ref |
| Hospital teaching status: minor academic teaching | – | – | – | – | 1.05 | 1.0–1.11 |
| Hospital teaching status: non-teaching | – | – | – | – | 1.11 | 1.05–1.18 |
| Hospital medical intensive care unit beds (no) | – | – | – | – | Ref | Ref |
| Hospital medical intensive care unit beds (yes) | – | – | – | – | 0.92 | 0.85–1.01 |
| Hospital cardiac intensive care beds (CIC) (no) | – | – | – | – | Ref | Ref |
| Hospital cardiac intensive care beds (CIC) (yes/no) | – | – | – | – | 0.99 | 0.95–1.03 |
| Hospital cardiac catheterization availability (no) | – | – | – | – | Ref | Ref |
| Hospital cardiac catheterization availability (yes) | – | – | – | – | 1.14 | 1.07–1.20 |
Ref = Reference Category
Table 4.
Unadjusted and multilevel models with random effects for relative risks and 95% CIs for association of ICE income + race measure and survival to discharge among Medicare beneficiaries
| Variable | ICE income + race model 1 unadjusted | ICE income + race model 2 (beneficiary) | ICE income + race model 3 (beneficiary + hospital) | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| ICE income + race Q1 (low-income Black) | Ref | Ref | Ref | Ref | Ref | Ref |
| ICE income + race Q2 | 1.16 | 1.11–1.21 | 1.12 | 1.06–1.16 | 1.09 | 1.04–1.15 |
| ICE income + race Q3 | 1.22 | 1.16–1.27 | 1.14 | 1.09–1.20 | 1.12 | 1.06–1.18 |
| ICE income + race Q4 | 1.16 | 1.11–1.21 | 1.08 | 1.03–1.14 | 1.08 | 1.02–1.13 |
| ICE income + race Q5 (high-income White) | 1.18 | 1.13–1.23 | 1.10 | 1.05–1.15 | 1.10 | 1.05–1.16 |
| Sex: female | – | – | Ref | Ref | Ref | Ref |
| Sex: male | – | – | 1.07 | 1.04–1.10 | 1.07 | 1.04–1.10 |
| Individual White race | – | – | Ref | Ref | Ref | Ref |
| Individual Black race | – | – | 0.85 | 0.82–0.89 | 0.86 | 0.82–0.90 |
| Age category 65–74 y | – | – | Ref | Ref | Ref | Ref |
| Age category 75–84 y | – | – | 0.90 | 0.88–0.93 | 0.90 | 0.88 |
| Age category 85 + y | – | – | 0.85 | 0.82–0.88 | 0.85 | 0.82 |
| Elixhauser AHRQ Comorbidity Index < 0 | – | – | Ref | Ref | Ref | Ref |
| Elixhauser AHRQ Index = 0 | – | – | 0.72 | 0.67–0.78 | 0.72 | 0.67–0.78 |
| Elixhauser AHRQ Index = 1–4 | – | – | 0.80 | 0.74–0.86 | 0.79 | 0.73–0.85 |
| Elixhauser AHRQ Index ≥ 5 | – | – | 0.62 | 0.59–0.64 | 0.63 | 0.60–0.66 |
| Hospital size: no. of beds < 100 | – | – | – | – | Ref | Ref |
| Hospital size: no. of beds > 100–399 | – | – | – | – | 0.86 | 0.80–0.93 |
| Hospital size: no. of beds ≥ 400 | – | – | – | – | 0.84 | 0.77–0.92 |
| Hospital teaching status: major academic teaching | – | – | – | – | Ref | Ref |
| Hospital teaching status: minor academic teaching | – | – | – | – | 1.05 | 1.0–1.11 |
| Hospital teaching status: non-teaching | – | – | – | – | 1.11 | 1.04–1.18 |
| Hospital medical intensive care unit beds (no) | – | – | – | – | Ref | Ref |
| Hospital medical intensive care unit beds (yes) | – | – | – | – | 0.92 | 0.85–1.01 |
| Hospital cardiac intensive care beds (CIC) (no) | – | – | – | – | Ref | Ref |
| Hospital cardiac intensive care beds (CIC) (yes/no) | – | – | – | – | 0.99 | 0.95–1.03 |
| Hospital cardiac catheterization availability (no) | – | – | – | – | Ref | Ref |
| Hospital cardiac catheterization availability (yes) | – | – | – | – | 1.13 | 1.07–1.20 |
Ref = Reference Category
After adjusting for beneficiary demographics and individual comorbidities (model 2, see Figs. 1–3 and Tables 2–4), the relative likelihood of survival to discharge for Medicare beneficiaries was higher in ICE quintiles Q2–Q5 as compared to reference. This was significant across all ICE race and income + race quintiles but not significant across all ICE income quintiles. The relative likelihood of survival was most pronounced in the ICE race measure in Q5 (model 2) with a 29% relative likelihood of survival to discharge (RR 1.29 CI 1.23–1.36) when compared to Q1. After additional adjustment for treating hospital characteristics (model 3), the relative likelihood of survival to discharge was only slightly attenuated (RR 1.28, CI 1.23–1.26). This remained significant across all quintiles for the ICE income + race measure only when compared to reference (Q5 1.10, CI 1.05–1.16). In this fully adjusted model, beneficiaries who identified as Black were at least 10% (RR 0.90, CI 0.86–0.94) less likely to survive to discharge compared to White beneficiaries in the ICE race model and this was significant in the two other ICE measures (ICE income, RR 0.84. CI 0.80–0.87), (ICE race + income, RR 0.86, CI 0.82–0.90). Among treating hospital level characteristics in the three fully adjusted ICE models (model 3), there was a consistent and statistically significant decreased relative likelihood of survival to discharge in academic hospitals with greater numbers of hospital beds and an increased relative likelihood of survival to discharge among hospitals with cardiac catheterization capability (RR 1.13, CI 1.07–1.20) compared to those without.
In the secondary analysis testing an interaction term of individual level Black race and all ICE measures in the full model (model 3), we found that there were no notable changes to our outcomes. In the sensitivity analysis including only a cohort of community-dwelling individuals and excluding those with residential ZIP codes associated with institutionalized care facilities, our outcomes also did not change across all three ICE measures and models.
Discussion
In this study of more than 190,000 Medicare beneficiaries who generated a claim after suffering an OHCA, we demonstrated that ICE measures of racial segregation are associated with disparities and adverse outcomes for those living in the most segregated Black and lower income communities. We found in fully adjusted models accounting for differences in patient and treating hospital characteristics that there was as high as a 28% (ICE race model 3) and as high as 29% in beneficiary level models (ICE race model 2) higher likelihood of survival to discharge in the most segregated White ZIP codes (Q5) as compared to the most segregated Black ZIP codes (Q1). We also found a linear trend for the ICE race model with decreasing relative likelihood of survival from Q5 to Q2. This linear trend was not seen across the ICE income and ICE income + race quintiles. While this study was not designed to ascertain a causal pathway between racial residential segregation and OHCA, it does suggest that residential segregation in the USA may have implications for short-term outcomes in emergency care conditions such as OHCA.
Racial residential segregation is associated with health disparities and worse outcomes for Black individuals with several conditions including hypertension and end-stage renal disease and is associated with increased risk for infant mortality and pre-term birth [17, 33, 34]. One previous study illustrated that across 265 US metropolitan areas, there is a significant association between living in a the most segregated areas and higher risk of cardiac and stroke mortality when compared to less segregated areas across multiple racial and ethnic groups [20]. However, the impact of segregation on mortality was more pronounced for Black residents when compared to White residents and in older adult residents when compared to younger adult residents. Prior studies have also shown that racial disparities exist in OHCA survival [5, 7, 8] although the role of residential segregation has not been closely examined. Previous research to evaluate race or ethnicity in outcomes for OHCA have utilized racial composition or other proxy measures of segregation [13]. Starks et al. demonstrated that individuals residing in areas with higher percentages of Black residents were less likely to less likely to undergo bystander CPR and less likely to survive to discharge compared with patients in White neighborhoods [14]. Another recent study examined the association between neighborhood ethnicity and found that in neighborhoods with a higher percentage of residents of Hispanic ethnicity, the likelihood of both bystander CPR and survival were lower compared with neighborhoods where a lower percentage of residents were of Hispanic ethnicity [35]. Our findings indicate that residential segregation may contribute to racial disparities in survival after OHCA by concentrating social and economic disadvantage and perpetuating environments that could impact pre-arrest or peri-arrest conditions. The decreased likelihood of survival between the most segregated Black communities and the most segregated White communities suggests that spatial living environments are potential drivers of differences seen in outcomes; however, this study was not designed to establish causation.
Findings from our study demonstrate that measures of racial segregation are associated with disparities in outcomes after OHCA across the USA for Medicare beneficiaries. There are some noted differences in our results from other studies employing the ICE measures. In our work, we did not find as strong a gradient of risk using the ICE income and income + race measure as seen in previous studies [17, 18, 27, 29] which may be unique to the Medicare population and the OHCA cohort. As an emergency care sensitive condition (ECSC) [36], OHCA differs from other chronic health conditions that have been studied in the context of ICE measures. OHCA is a time-sensitive condition, with high morbidity and mortality and requiring complex linkage across systems of care which may lead to differences in its association with ICE measures of income. This may also be related to outcomes that are unique to the Medicare population as related to income: ubiquitous insurance status or different distributions in HHI not seen among younger populations who suffer an OHCA. The distributions of income among a younger cohort could potentially be associated with greater financial instability leading to different outcomes. In other work incorporating ICE measures of income and racialized income, the studied population included younger individuals and utilized smaller geographic units of analysis than ZIP code for our study. Prior research has demonstrated that ZIP code level socioeconomic measures may not be as sensitive at identifying associations with health outcomes [37], which may also explain the differences in our results. However, our findings overall suggest that among Medicare beneficiaries, race may be a more significant driver of OHCA disparities than income in the context of segregation. More study is needed to better understand the mechanisms leading to different outcomes among a highly insured population such as Medicare beneficiaries and this intersection with race and income. We also noted decreased relative likelihood of survival to discharge in larger academic medical centers with medical and cardiac ICUs which may reflect EMS treatment and referral patterns for higher acuity, elderly OHCA beneficiaries.
Our study importantly highlights that racial residential segregation, a product of long-standing systemic and institutional racism in the USA, is a significant factor in outcomes for emergency care sensitive conditions such as OHCA. This work identifies the need to continue to re-examine the mechanisms leading to disparities in OHCA, as well as other emergent cardiovascular conditions, and to understand more deeply how social and living spaces intersect with health. Future efforts building on our study should aim to include a more comprehensive exploration of the broader domains encompassing social determinants of health to better address the needs of patients more comprehensively in OHCA and across other ECSC.
Limitations
This study’s findings must be interpreted in the context of known limitations. First, utilization of Medicare administrative claims data loses some of the sensitivity and specificity for identifying OHCA incidence when compared with cardiac arrest registry data. Similarly, we may not have been able to account for all comorbidities given the reliance on ICD codes present in the claims data. To generate a claim for OHCA and be included in this analysis, a patient must survive at least to ED treatment, which can inflate the relative rates of OHCA survival to discharge compared to registry cohorts. This may reflect our crude survival to discharge of 12% or more for the cohort. This kind of inflation is likely more prevalent in rural areas where fewer cases are transported to the hospital due to unwitnessed arrests, and where EMS responses are associated with longer transport times [38]. With over 80% of our cohort residing in metro areas, we chose not to control for urbanicity of residence in our models. Second, the demographics of the age-eligible Medicare population is homogeneous. Approximately 77.1% of the overall Medicare population in 2013 was White, with 10.0% beneficiaries identified as Black [39]. This composition limits our ability to robustly apply our findings to other racial and ethnic groups. As the population ages, more nuanced examinations of the impact on other racial and ethnic groups can be evaluated. Despite these limitations and given the absence of a national registry for OHCA, the use of Medicare claims data allows for this important examination of the role of racial segregation across the USA using standardized data. The scale and geographic breadth of this study utilizing a large Medicare cohort provides important insight into OHCA outcomes at a population scale and has implications for examining health inequities and racial disparities across multiple health systems in the USA.
Conclusions
In this retrospective analysis of Medicare beneficiaries who generated a claim after suffering an OHCA, we demonstrated that ICE measures of racial segregation are associated with disparities and adverse outcomes for those living in the most segregated Black and lower income communities. This suggests that the role of spatial living environments and social structures may play a role in outcomes for emergency care conditions such as OHCA. More work is needed to identify the mechanisms through which racial residential segregation and associated place-based disinvestment can be addressed to improve health outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by a grant from the NIH-NHLBI (#7R01HL141841-03) (PI: Brendan Carr MD). E.E.A. was supported by NIH-NHLBI (5T32HL129974-04; PI: Lynne D. Richardson MD).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Albaeni A, Beydoun MA, Beydoun HA, Akinyele B, RaghavaKurup L, Chandra-Strobos N, et al. Regional variation in outcomes of hospitalized patients having out-of-hospital cardiac arrest. Am J Cardiol. 2017;120(3):421–427. doi: 10.1016/j.amjcard.2017.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girotra S, van Diepen S, Nallamothu BK, Carrel M, Vellano K, Anderson ML, et al. Regional variation in out-of-hospital cardiac arrest survival in the United States. Circulation. 2016;133(22):2159–2168. doi: 10.1161/CIRCULATIONAHA.115.018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinozaki K, Nonogi H, Nagao K, Becker LB. Strategies to improve cardiac arrest survival: a time to act. Acute Med Surg. 2016;3(2):61–64. doi: 10.1002/ams2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, et al. Racial differences in the incidence of cardiac arrest and subsequent survival The CPR Chicago Project. N Engl J Med. 1993;329(9):600–6. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 6.Reinier K, Nichols GA, Huertas-Vazquez A, Uy-Evanado A, Teodorescu C, Stecker EC, et al. Distinctive clinical profile of blacks versus whites presenting with sudden cardiac arrest. Circulation. 2015;132(5):380–387. doi: 10.1161/CIRCULATIONAHA.115.015673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowie MR, Fahrenbruch CE, Cobb LA, Hallstrom AP. Out-of-hospital cardiac arrest: racial differences in outcome in Seattle. Am J Public Health. 1993;83(7):955–959. doi: 10.2105/AJPH.83.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah KS, Shah AS, Bhopal R. Systematic review and meta-analysis of out-of-hospital cardiac arrest and race or ethnicity: black US populations fare worse. Eur J Prev Cardiol. 2014;21(5):619–638. doi: 10.1177/2047487312451815. [DOI] [PubMed] [Google Scholar]
- 9.Galea S, Blaney S, Nandi A, Silverman R, Vlahov D, Foltin G, et al. Explaining racial disparities in incidence of and survival from out-of-hospital cardiac arrest. Am J Epidemiol. 2007;166(5):534–543. doi: 10.1093/aje/kwm102. [DOI] [PubMed] [Google Scholar]
- 10.Ghobrial J, Heckbert SR, Bartz TM, Lovasi G, Wallace E, Lemaitre RN, et al. Ethnic differences in sudden cardiac arrest resuscitation. Heart. 2016;102(17):1363–1370. doi: 10.1136/heartjnl-2015-308384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain RC, Barnetson C, Clegg GR, Halbesma N. Association of measures of socioeconomic position with survival following out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2020;157:49–59. doi: 10.1016/j.resuscitation.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 12.van Nieuwenhuizen BP, Oving I, Kunst AE, Daams J, Blom MT, Tan HL, et al. Socio-economic differences in incidence, bystander cardiopulmonary resuscitation and survival from out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2019;141:44–62. doi: 10.1016/j.resuscitation.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Chan PS, McNally B, Vellano K, Tang Y, Spertus JA. Association of neighborhood race and income with survival after out-of-hospital cardiac arrest. J Am Heart Assoc. 2020;9(4):e014178. doi: 10.1161/JAHA.119.014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starks MA, Schmicker RH, Peterson ED, May S, Buick JE, Kudenchuk PJ, et al. Association of neighborhood demographics with out-of-hospital cardiac arrest treatment and outcomes: where you live may matter. JAMA Cardiol. 2017;2(10):1110–1118. doi: 10.1001/jamacardio.2017.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 16.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1016/S0033-3549(04)50068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers BD, Baer RJ, McLemore MR, Jelliffe-Pawlowski LL. Using index of concentration at the extremes as indicators of structural racism to evaluate the association with preterm birth and infant mortality—California, 2011–2012. J Urban Health. 2019;96(2):159–170. doi: 10.1007/s11524-018-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman JM, Waterman PD, Coull BA, Krieger N. Spatial social polarisation: using the Index of Concentration at the Extremes jointly for income and race/ethnicity to analyse risk of hypertension. J Epidemiol Community Health. 2015;69(12):1199–1207. doi: 10.1136/jech-2015-205728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kershaw KN, Albrecht SS. Racial/ethnic residential segregation and cardiovascular disease risk. Curr Cardiovasc Risk Rep. 2015;9(3). [DOI] [PMC free article] [PubMed]
- 20.Shelton SK, Chukwulebe SB, Gaieski DF, Abella BS, Carr BG, Perman SM. Validation of an ICD code for accurately identifying emergency department patients who suffer an out-of-hospital cardiac arrest. Resuscitation. 2018;125:8–11. doi: 10.1016/j.resuscitation.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeZorzi C, Boyle B, Qazi A, Luthra K, Khera R, Chan PS, et al. Administrative billing codes for identifying patients with cardiac arrest. J Am Coll Cardiol. 2019;73(12):1598–1600. doi: 10.1016/j.jacc.2019.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennessy S, Leonard CE, Freeman CP, Deo R, Newcomb C, Kimmel SE, et al. Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol Drug Saf. 2010;19(6):555–562. doi: 10.1002/pds.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bruin ML, van Hemel NM, Leufkens HG, Hoes AW. Hospital discharge diagnoses of ventricular arrhythmias and cardiac arrest were useful for epidemiologic research. J Clin Epidemiol. 2005;58(12):1325–1329. doi: 10.1016/j.jclinepi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Gasparini A. Comorbidity: an R package for computing comorbidity scores. Journal of Open Source Software. 2018;3(23):648. doi: 10.21105/joss.00648. [DOI] [Google Scholar]
- 25.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698–705. doi: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 26.Krieger N, Feldman JM, Waterman PD, Chen JT, Coull BA, Hemenway D. Local residential segregation matters: stronger association of census tract compared to conventional city-level measures with fatal and non-fatal assaults (total and firearm related), using the Index of Concentration at the Extremes (ICE) for racial, economic, and racialized economic segregation, Massachusetts (US), 1995–2010. J Urban Health. 2017;94(2):244–258. doi: 10.1007/s11524-016-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger N, Waterman PD, Spasojevic J, Li W, Maduro G, Van Wye G. Public health monitoring of privilege and deprivation with the index of concentration at the extremes. Am J Public Health. 2016;106(2):256–263. doi: 10.2105/AJPH.2015.302955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massey DS. The age of extremes: concentrated affluence and poverty in the twenty-first century. Demography. 1996;33(4):395–412. doi: 10.2307/2061773. [DOI] [PubMed] [Google Scholar]
- 29.Krieger N, Waterman PD, Gryparis A, Coull BA. Black carbon exposure, socioeconomic and racial/ethnic spatial polarization, and the Index of Concentration at the Extremes (ICE) Health Place. 2015;34:215–228. doi: 10.1016/j.healthplace.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbott EEea. Abstract 134: Survival after out-of-hospital cardiac arrest: the role of urban-rural residence and demographic factors. Circulation. 2020;142(Suppl_4):A134-A. [Google Scholar]
- 31.Westrick AC, Bailey ZD, Schlumbrecht M, Hlaing WM, Kobetz EE, Feaster DJ, et al. Residential segregation and overall survival of women with epithelial ovarian cancer. Cancer. 2020;126(16):3698–3707. doi: 10.1002/cncr.32989. [DOI] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Kimmel PL, Fwu CW, Eggers PW. Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol. 2013;24(2):293–301. doi: 10.1681/ASN.2012070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White K, Borrell LN, Wong DW, Galea S, Ogedegbe G, Glymour MM. Racial/ethnic residential segregation and self-reported hypertension among US- and foreign-born blacks in New York City. Am J Hypertens. 2011;24(8):904–910. doi: 10.1038/ajh.2011.69. [DOI] [PubMed] [Google Scholar]
- 35.Blewer AL, Schmicker RH, Morrison LJ, Aufderheide TP, Daya M, Starks MA, et al. Variation in bystander cardiopulmonary resuscitation delivery and subsequent survival from out-of-hospital cardiac arrest based on neighborhood-level ethnic characteristics. Circulation. 2020;141(1):34–41. doi: 10.1161/CIRCULATIONAHA.119.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vashi AA, Urech T, Carr B, Greene L, Warsavage T, Jr, Hsia R, et al. Identification of emergency care-sensitive conditions and characteristics of emergency department utilization. JAMA Netw Open. 2019;2(8):e198642. doi: 10.1001/jamanetworkopen.2019.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger N, Waterman P, Chen JT, Soobader MJ, Subramanian SV, Carson R. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas—the Public Health Disparities Geocoding Project. Am J Public Health. 2002;92(7):1100–1102. doi: 10.2105/AJPH.92.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CC, Chen CW, Ho CK, Liu IC, Lin BC, Chan TC. Spatial variation and resuscitation process affecting survival after out-of-hospital cardiac arrests (OHCA) PLoS ONE. 2015;10(12):e0144882. doi: 10.1371/journal.pone.0144882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Kaiser Family Foundation State Health Facts. Data Source: 2008–2019 American Community Survey, 1-Year Estimates.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.