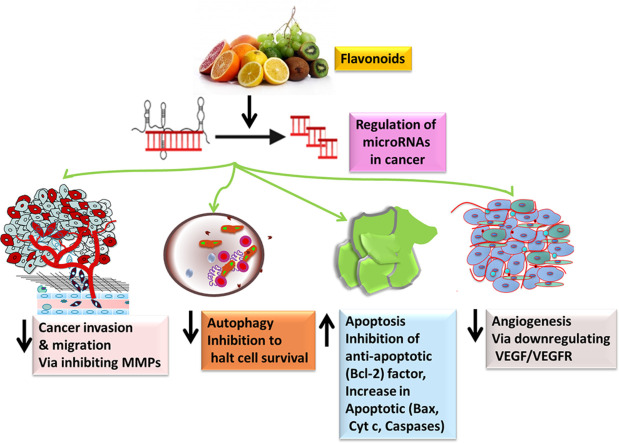
Highlights
-
•
microRNAs play critical role in the development of cancer.
-
•
microRNAs can regulate various hallmarks of cancer.
-
•
Natural flavonoids can modulate microRNAs to exhibit their anticancer actions.
Keywords: MicroRNAs, Flavonoids, Cancer, Anti-proliferation, Apoptosis, Synergism
Abstract
Cancer prevalence and its rate of incidence are constantly rising since the past few decades. Owing to the toxicity of present-day antineoplastic drugs, it is imperative to explore safer and more effective molecules to combat and/or prevent this dreaded disease. Flavonoids, a class of polyphenols, have exhibited multifaceted implications against several diseases including cancer, without showing significant toxicity towards the normal cells. Shredded pieces of evidence suggest that flavonoids can enhance drug sensitivity and suppress proliferation, metastasis, and angiogenesis of cancer cells by modulating several oncogenic or oncosuppressor microRNAs (miRNAs, miRs). They play pivotal roles in regulation of various biological and pathological processes, including various cancers. In the present review, the structure, chemistry and miR targeting efficacy of quercetin, luteolin, silibinin, genistein, epigallocatechin gallate, and cyanidin against several cancer types are comprehensively discussed. miRs are considered as next-generation medicine of recent times, and their targeting by naturally occurring flavonoids in cancer cells could be deemed as a signature step. We anticipate that our compilations related to miRNA-mediated regulation of cancer cells by flavonoids might catapult the clinical investigations and affirmation in the future.
Graphical abstract
Introduction
Cancer represents a group of malignant disorders developing in different tissues and organs, being characterized by uncontrolled growth and spreading towards distant sites [1,2]. This heterogeneous disease is a global leading cause of death [3], [4], [5], [6]. Regrettably, the incidence rate of malignant neoplasms is steadily rising all over the world, with a 47% increase expected to occur from 2020 to 2040, meaning 28.4 million new cancer cases in 2040 [7]. It is clear that new efficient treatment modalities are highly needed for fighting against this devastating disease.
Many anticancer drugs currently used in clinical settings are originally derived from different natural sources [4,8]. Vinca alkaloids vincristine and vinblastine from Catharanthus roseus (L.) G.Don and taxane paclitaxel from Taxus brevifolia Nutt. are just some examples of plant-derived anticancer agents that are clinically approved and widely exploited [9]. During the last few decades, interest of researchers around the globe in bioactive phytochemicals has rapidly increased, focusing especially on polyphenolic compounds. Flavonoids are a large group of natural polyphenols, occurring abundantly in fruits, vegetables, nuts, seeds, grains, tea and medicinal herbs [10]. These plant secondary metabolites exhibit a variety of different bioactivities, including antiinflammatory, immunomodulatory, antiproliferative, anti-migratory, anti-invasive, antimetastatic and anti-angiogenic effects in diverse malignant tissues [11], [12], [13], [14], [15]. In doing so, flavonoids modulate multiple molecular targets and intervene in different intracellular pathways [16], [17], [18].
Recent research investigations suggest that dysregulation of microRNAs (miRNAs) is a signature step in the anticancer activity of flavonoids. Deregulation of these small non-coding nucleotide sequences has been demonstrated to be associated with the initiation, promotion and progression of malignant cells, affecting different carcinogenesis-related processes such as epithelial-to-mesenchymal transition, autophagy and angiogenesis [19]. Therefore, modulatory action of flavonoids on the expression of miRNAs might be important in the suppression of cancer development, growth and spread, possibly representing a new promising mode to combat such a dreaded malignancy [19]. In this review article we provide an up-to-date insight into the anticancer effects of structurally different flavonoids (flavonol quercetin, flavonolignan silibinin, isoflavone genistein, flavanol epigallocatechin gallate, flavone luteolin and anthocyanidin cyanidin) which are mediated by the modulation of miRNAs. Additionally, we also discuss the role of miRNAs involved in synergistic and/or additive effects of flavonoids with traditional chemotherapeutic drugs. The uniqueness of this review is compilation of anti-cancer potential of flavonoids from chemistry, miRNa modulation, and clinical investigations to synergistic perspectives at a single platform. We believe that the preclinical information summarized in this comprehensive review article will accelerate the progress towards initiating the clinical investigation into flavonoids as potential anticancer drugs.
Flavonoids: understanding about the structure and chemistry
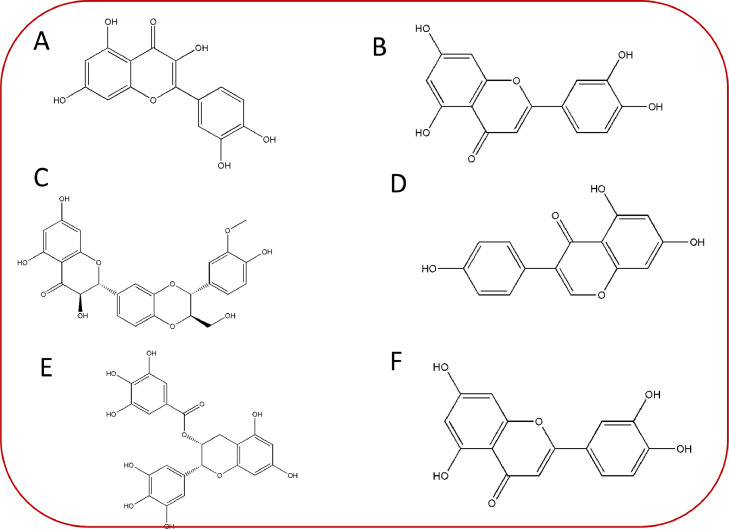
It is a well-known fact that the chemical structure of flavonoids depends on 15-carbon skeleton which comprises of 2 benzene rings i.e., “ring A” and “ring B”, respectively [20]. These rings are further linked by heterocyclic pyrane “ring C”. Interestingly, flavonoids with “ring B” attached to “position 3” of the “ring C” are known as “isoflavones” (Fig. 1). However, those phytocompounds where “ring B” is attached to 4th position with reference to “ring C” are known as” neoflavonoids”. Moreover, linkage of “ring B” at “position 2” leads to the categorization of flavonoids into numerous classes including flavones (e.g., luteolin, apigenin), flavonols (e.g., quercetin, myricetin, kaempferol etc.), flavanones (hesperetin, naringenin), and flavan-3-ols (catechin, epicatechin etc.) [21]. Generally, different classes of flavonoids vary at the oxidation levels and the pattern associated with substitution of “ring C”. In contrast to this, the diversity of individual compounds/members within a particular class rely upon the pattern of substitution at rings A and B, respectively [22,23]. Considering the above facts and the potential of flavonoids to play a critical role in therapeutic issues (Tables 1 and 2) possessing antioxidant, gene regulation, and enzyme inhibition, we discuss important members of flavonoids in the further subsections.
Fig. 1.
Chemical structures of some commonly occurring derivatives of natural flavonoid's subclasses (A) Quercetin (B) Luteolin (C) Silibinin (D) Genistein (E) Epigallocatechin gallate (F) Cyanidin.
Table 1.
A birdeye view of various anti-proliferatory activities of flavonoids in cancer.
| S. No | Flavonoids | Type of cancer | Cell model | Mechanistic insight | Refs. |
|---|---|---|---|---|---|
| 1 | Quercetin | Prostate cancer | LNCaP, DU-145, and PC-3 | ↑ROS, ↓ Akt, ↓NF-κB apoptosis | [154] |
| 2 | Quercetin | Breast cancer | MCF-7 | ↓Bcl-2, ↓ RIPK1, ↓ RIPK3 | [155] |
| 3 | Quercetin | Lung cancer | A549, and H1299 | ↑ SIRT1/AMPK | [156] |
| 4 | Quercetin | Bladder cancer | T24 | ↑Apoptosis, ↓Cellular elasticity | [157] |
| 5 | Quercetin | Hepatocellular carcinoma Breast cancerColorectal cancer cells | HepG2, and Hep3B MDA-MB-231 HCT116 | ↑Lysosome, transcription factor EB, ROS | [158] |
| 6 | Luteolin | Glioblastoma | A172, and U-373MG | ↑PARP, ↑autophagy | [159] |
| 7 | Luteolin | Gliomablastoma | U251MG, and U87MG | ↑ROS/ER, ↑caspase-3/Bax/Bcl-2, ↓ p-PERK/p-eIF2α/ATF4/CHOP | [160] |
| 8 | Luteolin | Colon Cancer | HCT116 | ↑p53, ↑autophagy | [161] |
| 9 | Luteolin | Breast Cancer | MCF7-TamR | ↓PI3K/AKT/mTOR | [162] |
| 10 | Luteolin | Hepatocellular cancer | Hep3B | ↑p21, ↑ER stress | [163] |
| 11 | Silibinin | Pancreatic cancer | SW1990 | ↑G1 arrest, ↑apoptosis and ↑JNK/SAPK | [164] |
| 12 | Silibinin | Breast cancer | MCF-7 | ↓ERα, ↑ERβ, ↑apoptosis | [165] |
| 13 | Encapsulated silibinin | Pancreatic cancer | MIA PaCa-2 | ↓miR-155, miR-222 and miR-21, AKT3, MASPINE, and SERPINEA12, | [166] |
| 14 | Silibinin | Renal cell carcinoma | 769-P, 786-O, ACHN, and OS-RC-2 | ↓mTOR-GLI1-BCL2 | [167] |
| 15 | Silibinin | Epidermal cancer | A431 | ↑NO, ↑eNOS phosphorylation | [168] |
| 16 | Genistein | Colon cancer | LoVo, and HT-29 | ↓NF-κB, ↓Bcl-2, ↑Bax | [169] |
| 17 | Genistein | Ovarian cancer | SK-OV-3 | ↑G1 or G2/M, ↑ LDH | [170] |
| 18 | Genistein | Hepatocellular carcinoma | PLC/PRF5, and HepG2 | ↑ apoptosis, ↑ DNMT1, ↑DNMT3a and ↑DNMT3b | [171] |
| 19 | Genistein | Cervical cancer | HeLa, CaSki, and C33A | ↑Caspase-3, -8, and -9, ↑PARP, ↑Bax, bcl-2 | [172] |
| 20 | Genistein | Lung adenocarcinoma | A549 | ↓Bcl-2 and ↑Bax, ↑apoptosis | [173] |
| 21 | Epigallocatechin-3-gallate | Myeloid leukaemia | NB4 | ↓peptidyl-prolyl isomerase NIMA-interacting 1 (PIN1), ↓ cyclin D1, ↓ NF-κB, c-MYC, and AKT | [174] |
| 22 | Epigallocatechin-3-gallate | Bladder cancer | 5,637, and T24 | ↑caspase9, ↑caspase3 and ↑BAX | [175] |
| 23 | Epigallocatechin-3-gallate | Esophageal cancer | Eca109, and Ec9706 | ↓Bcl-2, ↑Bax, ↑caspase-3 | [176] |
| 24 | Epigallocatechin-3-gallate | Pancreatic cancer | PANC-1, and BxPC-3 | ↓PI3K/Akt/mTOR | [177] |
| 25 | Epigallocatechin-3-gallate | Melanoma cells | A375 | ↓Bcl-2, ↑Caspase-3, ↓PI3K/AKT/mTOR | [178] |
| 26 | Cyanidin chloride | Colorectal cancer | HCT116, HT29, and SW620 | ↓NF-κB, ↑Nrf2, | [179] |
| 27 | Cyanidin 3-O-Glucoside | Osteosarcoma | Saso-2, MG-63, and G-292 | ↑P21, ↑PPARγ, ↑Bax | [180] |
| 28 | Cyanidin-3-O-β-glucopyranoside | Prostatic cancer | DU145, and LnCap | ↑ROS, ↑tumor suppressor P75NGFR, DNA fragmentation | [181] |
| 29 | Cyanidin-3-O-glucoside and cisplatin | Cervical cancer | HeLa | ↓PI3K/AKT/mTOR, cyclin D1 and Bcl-2 | [182] |
| 30 | Cyanidin-3-glucoside | Breast cancer | MDA-MB-453 | ↑caspase-3, ↑DNA fragmentation, ↓Bcl-2, ↑ Bax | [95] |
Table 2.
Brief list of undergoing / completed clinical trials related to natural flavonoids as a preventive and/or therapeutic intervention as assessed from clinicaltrials.gov. Untimely terminated and unsuccessful trials were excluded for drafting the table.
| Compound | Intervention / Treatment | Cancer type | Clinical trial stage | Identifier |
|---|---|---|---|---|
| Quercetin | Combination of quercetin and genistein | Prostate cancer | Intended to start with unknown phase | NCT01538316 |
| Quercetin | Combination of quercetin and EGCG | Prostate cancer | Phase I completed | NCT01912820 |
| Quercetin | Combination of radiotherapy, IAluril and IAluril soft gels (combination of curcumin, quercetin, hyaluronic acid, chondroitin sulfate) | Prostate cancer | Phase II completed | NCT03493997 |
| Quercetin | Combination of Sunitinib and quercetin | Renal cell carcinoma & kidney cancer | Phase II; active and recruiting | NCT02446795 |
| Quercetin | Quercetin or quercetin-encapsulated nanoparticle | Oral cancer | Phase II; not yet recruiting | NCT05456022 |
| Quercetin | Alone | Squamous cell carcinoma | Phase II; active with unknown recruitment | NCT03476330 |
| Quercetin | Combination of quercetin, EGCG, ellagic acid, allicin, selenium and omega-3 fatty acids | Follicular Lymphoma | Phase II; unknown recruitment | NCT00455416 |
| Luteolin | Luteolin or nanoparticle encapsulated-luteolin | Tongue neoplasms | Early phase I; not yet recruiting | NCT03288298 |
| Silibinin | Alone | Prostate cancer | Phase II completed | NCT00487721 |
| Silibinin | Alone | Advanced hepatocellularcarcinoma | Phase I completed | NCT01129570 |
| Silibinin | Combination of Erlotinib and Silybin-phytosome | Non-small-cell-lung carcinoma | Phase II completed | NCT02146118 |
| EGCG | Alone | Colorectal cancer | Early phase I; recruitment in process | NCT02891538 |
| EGCG | Combination of EGCG with other polyphenols | Basal cell carcinoma | Phase III completed | NCT02029352 |
| EGCG | Polyphenon E (EGCG as main component) | Prostate carcinoma | Phase II completed | NCT00676780 |
| EGCG | Combination of Erlotinib and Polyphenon E | Head & Neck carcinoma | Phase I completed | NCT01116336 |
| EGCG | Alone | Small cell lung carcinoma | Undergoing Phase I with expanded access status | NCT01317953 |
| Genistein | Combination of Interleukin-2 and Genistein | Metastatic melanoma or kidney cancer | Early phase I; recruitment completed | NCT 00276835 |
| Genistein | Alone | Colorectal cancer | Phase II completed | NCT01985763 |
| Genistein | Genistein combined polysaccharide | Prostate cancer | Phase III completed | NCT00584532 |
| Genistein | Combination of Cholecalciferol and Genistein | Prostate cancer | Phase II completed | NCT01325311 |
| Genistein | Combination of Gemcitabine and Genistein | Breast cancer | Phase II completed | NCT00244933 |
| Genistein | Alone | Breast cancer | Phase II completed | NCT00290758 |
| Genistein | Alone | Bladder cancer | Phase II completed | NCT00118040 |
| Genistein | Combination of Decitabine and Genistein | Non-small cell lung cancer | Phase II completed | NCT01628471 |
| Genistein | Combination of Erlotinib, Gemcitabine and Genistein | Metastatic pancreatic cancer | Phase II completed | NCT00376948 |
| Genistein | Combination of Decitabine and Genistein | Childhood cancer | Phase II completed | NCT02499861 |
| C3G | Combination of Mirtoselect (C3G) and Meriva (curcumin) | Colorectal Adenoma | Phase II; active but not recruiting | NCT01948661 |
Quercetin
Among the array of bioflavonoids, quercetin is one of the key dietary phytoconstituents which is ubiquitously found in plants including vegetables, fruits, leaves, seeds etc. Its name is derived from “quercetum” which represents “oak forest”. Chemically, it is known as 2-(3,4-dihydroxyphenyl)-3,5,7- trihydroxy-4H-chromen-4-one [24,25]. It possesses a bitter flavor and is utilized as an ingredient in many dietary supplements, foods and beverages. The biosynthesis of quercetin involves the conversion of phenylalanine to 4-coumaroyl-CoA via phenylpropanoid pathway in the presence of enzyme phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA ligase, respectively [26]. Once 4-coumaroyl-CoA is formed it is further combined to malonyl-CoA (3 molecules) under the enzymatic action of 7,2’-dihydroxy-4’-methoxyisoflavanol synthase which leads to the tetrahydroxychalcone generation. Furthermore, the action of chalcone isomerase converts tetrahydroxychalcone to naringenin and this compound is again converted to eriodictyol with the help of enzyme flavonoid 3′-hydroxylase. Further, eriodictyol is converted into dihydroquercetin which in turn forms quercetin via enzymatic action of flavanol synthase [27].
In the recent years, quercetin has gained significant attention from scientists due to its antioxidant and therapeutic abilities, which include anticancer, anti-inflammatory, and anti-obesity activities [28], [29], [30]. Moreover, it has also shown its potential against many ailments related to gout, pancreas, coronary artery, asthma and Alzheimer like diseases [31]. Interestingly, quercetin has been reported to reduce the risk of cataract owing to diabetic complication and dermatological issues [32].
Luteolin
Luteolin, also known as 3′,4′,5,7-tetrahydroxyflavone, is another widely occurring natural phytoconstituent belonging to the class “flavones” of flavonoids [33]. It possesses a C6-C3-C6 structure along with three rings having a carbon double bond at 2-3 position. Luteolin is known to have hydroxyl groups at third, fifth, and seventh carbon positions, respectively [12]. Moreover, the bioactivities of luteolin are observed to be dependent upon hydroxyl moieties and a double bond at positions “second” and “third” as described above. Additionally, luteolin is reported to be associated with glycosylation in plants which in turn gets hydrolyzed further to free luteolin [12].
Luteolin is a nontoxic as well as heat stable phytocompound. Several reports have pointed toward its anticarcinogenic, anti-inflammatory, and antioxidant properties [34,35]. It has also shown the capacity to protect against cardiovascular diseases and diabetes via stimulating the immune response. It also acts as an agent to scavenge nitrogen and oxygen species which can harm cellular integrity directly or indirectly [36]. Moreover, luteolin can behave as antiestrogenic compound which leads to a reduction of cell proliferation, therefore establishing it as a potent antiproliferative agent [37].
Silibinin
One of the important flavonoids in plants is silibinin whose name is based upon the source plant i.e., Silybum marianum (L.) Gaertn. (milk thistle). Generally, milk thistle possesses a variety of phytocompounds that together form a mixture called “flavonolignan” [38]. However, the first identified constituent in this complex is sylibin also known as silibinin which itself is a mixture comprising of two diastereomers, i.e., silybin A and silybin B. Silibinin is a semipurified commercially available fraction of silymarin [39,40]. Interestingly, the process of biosynthesis of silibinin involves the coupling of two important components viz., taxifolin and coniferyl alcohol. It has been known to exhibit numerous pharmacological activities, predominantly related to the fatty liver including non-alcoholic fatty liver and steatohepatitis and alcoholic liver cirrhosis [38,41,42].
Genistein
Genistein belongs to the subgroup of isoflavones. It is a phytoestrogen which is mainly derived from leguminaceae plants viz., Vicia faba L., Glycine max (L.) Merr., Pueraria lobata (Wild.), Lupinus albus L., etc. Chemically, genistein is known as 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one with a similar structure to that of mammalian estrogens [43]. It comprises 15 carbons possessing two aromatic rings, i.e., “ring A” and “ring B” which are further associated with “ring C” which is a carbon pyran ring. The carbon skeleton/structure of this phytoconstituent consists of a double bond between second and third positions, in addition to the oxo group at the fourth position of ring C. It also shows the presence of three hydroxyl groups at fifth and seventh positions of ring A and at the fourth position of ring B.
Recent research studies have established the therapeutic potency of genistein in providing protection against cardiovascular diseases, osteoporosis, postmenopausal problems and cancer [44], [45], [46]. It has also shown an important role in inflammatory conditions as an anti-inflammatory agent [43]. Current findings associated with genistein have suggested its role in neurodegenerative disorders through autophagy-dependent action [47,48].
Epigallocatechin gallate
Epigallocatechin gallate (EGCG), alternatively known as epigallocatechin-3-gallate belongs to the class catechin of polyphenols, which is an ester of epigallocatechin and gallic acid. It is observed that the cancer chemopreventive potential of Camellia sinensis (L.) Kuntze (tea) is regulated by catechins [49]. Among the plethora of catechins, EGCG itself is the key phytoconstituent of green tea. Interestingly, the overall content of EGCG is found to be maximum in the dried leaves of green tea followed by white tea and least in black tea, respectively [50]. In the recent years, many researchers have established numerous beneficial effects of consuming tea that can be attributed to the presence of EGCG. It has shown beneficial action against many diseases including inflammation, cardiovascular, rheumatoid arthritis and cancer [51,52].
Cyanidin
One of the important subgroups of flavonoids is anthocyanins which are commonly found in plants. These phytocompounds are widely distributed in fruits and vegetables viz., blueberries, blackberries, raspberries, grapes, cranberries, plums, eggplant, spinach and red cabbage [53]. The basic structure of anthocyanin possesses C15 skeleton of “anthocyanidins” which consists of “chromane ring” having a second aromatic ring B at “position 2” which are glycosylated or acylated at particular hydroxylated position, respectively. Overall, there are 600 natural cyanins and mostly they are either 3,5-diglycosides or 3-glycosides. Interestingly, among these cyanins four phytocompounds viz., cyanidin 3-, cyanidin 3-glucoside, cyanidin 3-xylosylrutinoside and cyanidin 3-rutinoside have been regularly reported and identified in black raspberries [54]. Since cyanidin is chief pigment in berries and other fruits, consequently it has been reported to play medicinal action in complications related to diabetes, asthma, atherosclerosis and cancer via triggering anti-inflammatory response through hydroxylation [55,56].
Overview of miRNAs and their role in cancer development and progression
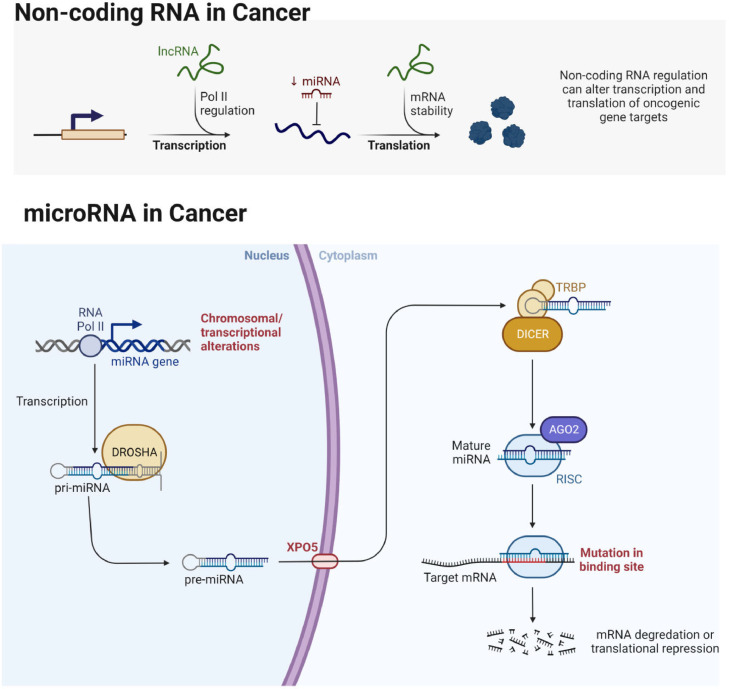
Most organisms express small non-coding RNAs called microRNAs (miRNAs), which have 21–23 nucleotides [57], [58], [59]. In addition, miRNAs account for 1-5% of the total human genome (∼28,000 miRNAs), which play an essential role in regulating gene expression post-translationally [60]. Depending on the functions of the cell, miRNAs act as a tumor suppressors or oncogenes in various cancer types such as lung [58,59], breast [61], ovarian [61], prostate [57], gastric [62], colorectal [63], brain cancers [64]. Upregulation of miRNAs may result in the repression of tumor suppressor gene expression. At the same time, downregulation of miRNAs leads to an increased expression of oncogenes, inducing cell differentiation, proliferation, and apoptosis (Fig. 2). In addition phytochemicals including flavonoids can regulate the expression and mode of action of miRNAs via modulating receptors and/or intracellular targets in cancer [65], [66], [67], [68], [69], [70], [71].
Fig. 2.
Biogenesis of miRNA and its role in cancer development and progression.
Regulation of miRNAs in different cancer types by flavonoids
Nowadays, flavonoids as plant-based naturally-derived secondary metabolites (for example, quercetin, silibinin, genistein, epigallocatechin gallate, luteolin, cyanidin) play a significant role in the anticancer drug discovery field. They are a broad category of compounds with anti-inflammatory, antioxidant, anticarcinogenic, and antimutagenic effects. These compounds are primarily present in vegetables, seeds, fruits, cereals, tea, and some alcoholic beverages, such as wine [72], [73], [74], [75], [76], [77]. These flavonoids possess low toxicity, regulate growth, proliferation, differentiation, inflammation, angiogenesis, invasion, and metastasis of different cancer types. More recently they have been shown to impact the expression of various miRNAs.
Many studies have shown that natural compounds also used in traditional medicine contribute to epigenetic regulation, including DNA methylation, histone modification, and the activity of microRNAs due to the presence of numerous bioactive compounds [75]. Numerous phytochemicals from the group of flavonoids, polyphenols, and alkaloids are known for their ability to fight cancer [78]. Although natural substances derived from plants have various biological effects, epigenetic modification gathers attention as powerful cancer prevention and therapy method [79]. By altering the expression of methyltransferases and histone deacetylases, miRNA can control the epigenetic system [80].
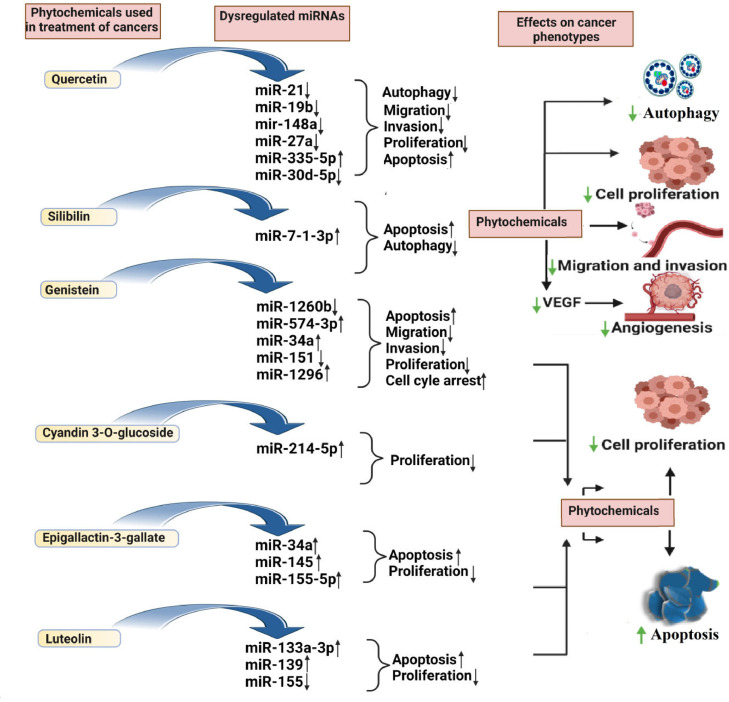
According to the current studies, natural substances could directly or indirectly affect miRNAs and restrict cancer development ((Fig. 3)). They control post-transcriptional gene expression by either silencing or degrading the target mRNA, which regulates cell physiological processes like cell death and proliferation [81]. Using dietary polyphenol supplementation, miRNA and mRNA levels in the livers of apolipoprotein E-deficient mice was assessed by researchers [82]. Study revealed that out of 119 miRNAs, 92 showed up-regulation and 27 showed down-regulations. For example, mmu-miR-374* is up-regulated after mutagenesis in apoE mice by a factor of 2.96 comparing to the wild-type mice, however when supplemented by polyphenols, this miRNA is downregulated in apoE by an average factor of −2.44. The same observation of opposite miRNA expression is also reported for the miRNA that are downregulated in the liver after mutagenesis and for which an increase in expression was observed after polyphenol supplementation. Dysregulation of miRNAs has been implicated in many cancers [57]. Mechanistically, flavonoids (quercetin, silibilin, genistein, cyanidine-3-O-glucoside, epigallactin-3-gallate, Luteolin) suppresses or activates miRNAs expression and modulating the regulation of apoptosis [Caspase-3/9, B-cell lymphoma 2 (Bcl 2), sirtuin 1 (Sirt1)/p53, ATP binding cassette subfamily E member 1 (ABCE1), multidrug resistance (MDR1), retinoid X receptor alpha (RxRα) forkhead Box O3 (FOXO3) phosphatase and tensin homolog (PTEN)], cell proliferation (p21, and p27), migration and invasion [Smad-2/4, Slug, Sox-4, cyclin G2 (CCNG2), PTEN, Sprouty 2, zinc finger E-box binding homeobox 1 (ZEB-1), E-Cadherin, phosphoinositide 3-kinase (PI3K)/ protein kinase B (Akt)]. Interestingly, these flavonoids trigger the binding domain of apoptosis, cell proliferation and migration, and invasion-related markers, resulting in a conformational change in their structure and hence modulating miRNA expression by affecting various signaling pathways such as NOTCH, PI3K/Akt, etc., [19]. In the subsequent sections, we discuss the role of different flavonoids (quercetin, silibinin, genistein, epigallocatechin gallate, luteolin, and cyanidin) in miRNA expression regulation in various human cancers.
Fig. 3.
The effect of phytochemicals in regulating miRNAs results in inhibition of proliferation, autophagy, and metastasis while promoting apoptosis of cancer cells.
Brain tumors
Glioma is considered to be a very prominent cancer and not easily detectable in its early stages. Despite the availability of various techniques and therapeutic advancements in cancer treatment, still, the cure of glioma is difficult and patients generally survive up to a few months in advance stages. For a decade, it has been observed that the survival rate of glioma patients is not improving and extensive clinical research needs to be conducted. Several case reports suggested that only maximum 5% of patients are found to survive more than 3 years. The higher mobility rate of glioma is known to impart strong proliferation and infiltration features. Therefore, further investigations are necessary to analyze molecular bases of glioma therapeutic resistance. Shreds of evidences suggested that several signaling cascades are found to be activated in glioma to inhibit apoptosis and autophagy [83]. In addition, the expression of various chaperones is upregulated to prolong glioma survival. Therefore, it is highly essential to investigate novel molecular targets to inhibit cell proliferation and initiate natural cell death of glioma. A synergistic study using silibinin and luteolin in Glioblastoma Multiforme cells (U87MG, T98G) upregulated Caspases-3, -8, -9 via miR-7-1-3p [84]. Genistein has been found to upregulate miR-218 in combination with Propofol to increase apoptotic cell death in Glioblastoma Multiforme (U251, 9L) via activating caspases [85]. In another study using derivatives of cyanidin, proliferation of temozolomide-resistant Glioblastoma Multiforme cells (LN-18, Temozolomide resistant-LN-18) were inhibited via upregulated miR-214-5p and downregulated β-catenin, O-6-methylguanine-DNA methyltransferase [86].
Breast cancer
Breast cancer is the most common type of tumor and the leading cause of cancer-related deaths among women all over the world [87]. The American Cancer Society (ACS) estimates that every year, breast cancer accounts for 2-3% of all fatalities [7]. The advancement in cancer treatment options has increased patients' life expectancy [88,89]. However, there is still room to identify novel targets and their underlying mechanisms. miRNAs are emerging targets with gene regulatory roles and are crucial for cell proliferation and their differentiation processes, as well as for the development of breast cancer [61]. Several flavonoids have been identified for their role in targeting miRNAs regulating breast cancer progression [60,77]. Numerous studies have demonstrated that flavonoid quercetin (3,3`,4`,5,7-pentahydroxyflavone) can cause cytotoxic effects, such as reducing cell growth and induction of apoptosis in breast cancer cells [90], [91], [92]. Quercetin is commonly distributed in the human diet because it is simple to extract, isolate, and detect. It is plentiful in various plants, including fruits, vegetables, nuts, seeds, tea, olive oil, and red wine [92].
In this regard, feng Tao et al. found that quercetin reduced cell proliferation in a dose- and time-dependent manner in human breast cancer cell lines MCF-7 and MDA-MB-231 [93]. Quercetin administration increases miR-146a expression and inhibits tumor growth in an in vivo orthotopic xenograft nude mouse model [93] suggesting overexpression of miR-146a caused by quercetin treatment improved the suppression of cell growth and proliferation [93]. Mechanistically, quercetin treatment activates mitochondrial-dependent pathways, including caspase-3, promotes apoptosis, and prevents invasion by down-regulating the expression of the EGFR [93].
Furthermore, a quercetin derivative, methoxylated quercetin glycoside (MQG), showed remarkable anticancer properties for triple-negative breast cancer (TNBC) [94]. Mechanistically, MQG restricted TNBC progression by suppressing the MALAT-1/miR-155/miR-146a axis, altering the NO biosynthesis route, immunological ligands for natural killer cells, and cytotoxic T-lymphocytes, and ultimately reducing the immune-suppressive character of the tumor microenvironment of TNBC cells [94].
Another most prevalent flavonoid found in fresh fruits and vegetables, cyanidin-3-glucoside (C3G), shows outstanding antioxidant characteristics. C3G accelerates cell death by altering the apoptotic proteins, including cleaved caspase-3 and DNA fragmentation [95]. In addition, C3G reduced the expression of miR-138 in TNBC, which elevated Sirt1 protein via mRNA translation suppression and consequentially modulated epithelial marker E-cadherin, mesenchymal marker N-cadherin, vimentin, and EMT-associated transcription factors Snail1/Snail2, reversing breast cancer EMT [96]. This suggests that the aggressiveness of TNBC could be reduced by C3G by causing the mesenchymal to epithelial transition (MET).
Next, one more important isoflavonoid found in soybeans called genistein is also found to inhibit protein tyrosine kinase phosphorylation, induce apoptosis, and promote cell differentiation in breast cancer [97]. A recent study discovered that genistein treatment alters miRNAs' expression in the MCF-7 breast cancer cell line. Genistein was administered to the cells for three days at an IC50 dosage. As a result, miR-23b expression was elevated in the genistein-treated group. Previous studies have validated the tumor suppressor role of miR-23b, which suppresses P21-activated kinase 2 (PAK2) gene expression in MCF-7 and MDA-MB-231 cell lines [98]. De La Parra et al. showed that genistein could prevent breast cancer by blocking miR-155 [99]. Genistein-induced miR-155 downregulation has been shown to prevent tumor growth and cancer cell proliferation while causing cell-cycle arrest through the overexpression of CK1. CK1 then breaks down β-catenin and inhibits the production of the β-catenin target, cyclin D1, which in turn prevents tumor development [99].
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is another common flavonoid naturally occurring as a glycosylated form found in fruits, vegetables, and medicinal herbs [99]. Luteolin's anticancer activity is related to inducing apoptosis, which inhibits cancer cell growth and lowers metastasis and angiogenesis by involving protein kinases, redox regulation, and DNA damage [100]. The first study on luteolin to find its role in breast cancer suggested that it activates ERK and p38, which help in the nuclear translocation of apoptosis-inducing factor (AIF), ultimately leading to caspase-independent apoptotic cell death in human breast cancer cell lines [101]. In another study, luteolin was found to suppress breast cancer progression by inhibiting the expression of Notch-1, Hey1, Hes-1, Hey2, and cyclin D1 genes of Notch signaling pathway [102]. Furthermore, luteolin treatment, along with Notch-1 silencing, also suppressed angiogenesis and metastasis in breast cancer by downregulating VEGF, MMP-2, and MMP-9 expressions. Interestingly, they also found that luteolin treatment regulates certain miRNAs, ultimately leading to inhibition of breast cancer progression. Upregulated miR-139-5p, miR-246, miR-34a, miR-181a, miR-224, and downregulated miR-155 could inhibit breast cancer progression in luteolin-dependent as well as luteolin-independent manner [102]. Gao et al. revealed that the anticancer and anti-EMT agent luteolin is effective against breast cancer cells [103]. The anti-tumor effects of luteolin have been brought about by luteolin's ability to promote miR-203 expression and decrease Ras/Raf/MEK/ERK signaling [103]. This study also found that luteolin caused apoptosis while suppressing the viability of MCF-7 and MDA-MB-453 cells [103]. Given that the equilibrium between Bax and Bcl-2 was skewed and caspase-3 was cleaved, the apoptotic death caused by luteolin administration may have occurred via a mitochondria-dependent mechanism [103].
Zhang et al. investigated how the anti-TNBC mechanism of luteolin was affected by the TINCR- miR-761 molecular module. According to their findings, luteolin may inhibit tumor cell proliferation and cause apoptosis in TNBC by down-regulating TINCR and miR-761 expression levels in a dose-dependent way. However, this protective effect was somewhat reduced when TINCR and miR-761 were abnormally upregulated. Additionally, the effect of miR-761 on the anti-TNBC activity of luteolin was largely eliminated with the use of si-TINCR [104]. Magura et al. studied luteolin cytotoxicity against the breast cancer cell line (MCF-7) in a dose- and time-dependent manner [105]. It exerts an anticancer effect in MCF-7 cells by attaching to multi-mature miRNAs (miR-16, miR-21, and miR-34a) and modifying the levels of their cytoplasmic expression. Bcl-2 is known to be regulated by the miRNAs miR-16, miR-21, and miR-34a [105]. Interestingly, during treatment with 100 μg/mL of luteolin for 48 h, miR-16 and miR-34a were upregulated, and miR-21 and Bcl-2 were downregulated [105]. Thus, treatment with luteolin increases the expression of miR-16, miR-21, and miR-34a while decreasing the production of miR-21, which inhibits the expression of Bcl-2 and causes apoptosis in breast cancer [105].
Lung cancer
Not only is lung cancer the most prevalent cancer type diagnosed globally, but it also accounts for the majority of cancer deaths, with around 1.6 million new cases and 1.4 million fatalities each year [106], [107], [108]. The two morphological subtypes of this disease are non-small cell lung cancer (NSCLC) and small cell lung cancer. The majority of lung cancer patients are diagnosed at an advanced stage, which is nearly incurable [109]. Less than 15% of patients receiving chemotherapeutic medicines reach five years of survival as their ability to prolong life has been severely restricted by the emergence of multidrug resistance [110]. Thus, innovative therapeutic agents and therapeutic targets may offer more clinical advantages and signal improved outcomes in the treatment of lung cancer.
In a significant population-based case-control study, Lam et al. found that increased consumption of foods rich in quercetin was linked to a decreased risk of lung cancer. Their research revealed that eating foods high in quercetin is connected to specific miR expression, as per the histological analysis [111]. In conclusion, diets rich in quercetin affected the expression of several miRNAs in the tumor tissues of former smokers with adenocarcinoma but not those of squamous cell carcinoma and current smokers [111]. Kang et al. showed that Notch-1 overexpression is responsible for radioresistance in NSCLC cells. Their findings revealed that rhamnetin (a quercetin derivative) administration with radiation has a lot of potential to be a successful anti-lung cancer therapy approach [112]. Mechanistically, it increased p53 acetylation, which upregulates miR-34a expression, producing radiosensitization effects [112]. Thus, targeting Notch-1 with rhamnetin in combination with radiotherapy could overcome radioresistance and ultimately increase the efficacy of radiation therapy for treating NSCLC [112].
Next, Sonoki et al. proved that quercetin reduces claudin-2 production by increasing its mRNA instability in A549 cells. Additionally, their findings suggest that quercetin reduces claudin-2 expression through increased miR-16 expression. So, quercetin partially inhibits carcinogenesis in human lung cancer via increasing miR-16 and decreasing claudin-2 expression. Consequently, a diet rich in quercetin influences the expression of miRNA and is linked to a lower risk of lung cancer [113].
Li et al. demonstrated that hyperoside (a quercetin derivative) could inhibit the proliferation of A549 cells by causing apoptosis and G1/S phase arrest. Mechanistically, hyperoside caused apoptosis by activating caspase-3 under the control of Bcl-2/Bax, whereas in combination with let-7a-5p the antiproliferative response was synergistic. A bioinformatics investigation revealed that let-7a-5p possesses binding sites on the 3′UTR of cyclin D1 (CCND1), indicating that let-7a-5p may target CCND1. In addition, let-7a-5p suppressed cellular growth by causing cell cycle arrest at the G1 phase in A549 cells by controlling CCND1 gene expression [114].
Quercetin was also found to suppress Bcl-2 and Chk1 expression while inducing p53 and Bax expression in NSCLC cells. The expression of miR-16-5p was also upregulated by quercetin, and the effects of quercetin were proportional to its dose and duration of therapy [115]. Furthermore, miR-16-5p inhibitors remarkably reversed the inhibitory effect of quercetin on NSCLC cell survival and partially diminished quercetin's contribution to the promotion of NSCLC radiosensitivity [115]. In light of this, it was determined that quercetin affects the expression of miR-16-5p to reduce vitality and increase the radiosensitivity of NSCLC cells. Additionally, quercetin increased the expression of miR-16-5p, and its effects were related to both its concentration and the length of the therapy [115]. Quercetin's inhibition of NSCLC cell survival was also drastically reversed by miR-16-5p inhibitors, and its role in boosting NSCLC radiosensitivity was diminished. Thus, it was found that quercetin regulates the expression of miR-16-5p to decrease NSCLC cell survival and increase radiosensitivity [115]. Furthermore, miR-16-5p was discovered to significantly lower WEE1 expression in NSCLC cells. The dual luciferase reporter assay showed that miR-16-5p had no effect on mutant WEE1 but drastically reduced the luciferase activity of wild-type WEE1, indicating WEE1 as a miR-16-5p target gene in NSCLC cells [115]. Additionally, quercetin administration significantly decreased WEE1 expression. Therefore, quercetin affects the miR-16-5p/WEE1 axis in NSCLC [115].
Quercetin lowered SNHG7 and increased miR-34a-5p levels in NSCLC cells. Target-binding sequences between SNHG7 and miR-34a-5p were discovered using the RNA-binding protein immunoprecipitation assay, luciferase reporter gene assay, and transfection assays. Overexpression of SNHG7 or the miR-34a-5p inhibitor increased the development and spread of tumor cells and promoted NSCLC cell proliferation. Co-transfection of an inhibitor of miR-34a-5p or an SNHG7 mimic blocked the therapeutic effects of quercetin on NSCLC cells. NSCLC cells' survival, proliferation, migration, and invasion were decreased by quercetin, while their apoptosis was boosted [116].
Many cellular regulatory pathways in lung cancer have been reported to be affected by epigallocatechin-3-gallate (EGCG). EGCG specifically upregulated the expression of miR-210, a major miRNA regulated by HIF-1, in both human and mouse lung cancer cells [117]. Furthermore, they discovered that overexpression of miR-210 reduces cell proliferation rate, anchorage-independent growth, and sensitivity to EGCG. EGCG regulates miR-210 in a mechanism that involves the hypoxia-response element in the miR-210 promoter. In addition, it was discovered that the overexpression of miR-210 following EGCG therapy was associated with the stabilized HIF-1 in lung cancer cell lines [117]. The stabilization of HA-tagged HIF-1 explained the EGCG-induced stabilization of HIF-1, implying that EGCG targets the oxygen-dependent degradation (ODD) domain. Thus, EGCG anticancer activity involves an increase in miR-210 in lung cancer cell lines, which is mediated by HIF-1 stabilization [117].
Another study suggests that epigallocatechin-3-gallate (EGCG) increased the efficacy of cisplatin in NSCLC. The tumor size in the cisplatin and EGCG group was considerably lower than in the cisplatin-only group in an in vivo model. In contrast, EGCG reduced the effectiveness of cisplatin in NCI-H460 cells, a different kind of NSCLC cell. Following EGCG treatment, hsa-miR-98-5p and hsa-miR-125a-3p expression levels in these two cell lines were found to differ. After transfection with the hsa-miR-98-5p inhibitor, both the A549 and NCI-H460 cells' survival fraction after receiving cisplatin was decreased [118]. Hsa-miR-98-5p was silenced, which resulted in an increase in p53, a crucial regulator of cisplatin-induced apoptosis. These results suggested that EGCG increased the effectiveness of cisplatin by decreasing the expression of hsa-miR-98-5p, which was followed by an elevation in p53 [118]. The traditional MAPK pathway and hsa-miR-125a-3p have a close interaction, according to a bioinformatics study. These results imply that the clinical cisplatin therapy of NSCLC may consider targeting hsa-miR-98-5p. Certain forms of NSCLC may respond favorably to the therapeutic combination of EGCG and cisplatin [118].
According to Jiang et al. miR-485 is crucial in controlling NSCLC chemoresistance compared to A549 cells. MiR-485 was shown to be downregulated in A549/Cisplatin-resistant cells. MiR-485 inhibitors improved the stemness of A549/cisplatin-resistant cells, whereas miR-485 overexpression decreased CSC-like characteristics. Additionally, EGCG promoted cell death and reduced the growth of A549/Cisplatin-resistant cells. EGCG increased the expression of miR-485 and suppressed the CSC-like characteristics induced by miR-485 [119].
Jiang et al. also demonstrated that EGCG, a potent anticancer compound derived from green tea, can boost hsa-miR-4855p expression. Mir-485-5p mimics significantly inhibited NSCLC cell growth and induced apoptosis. Inhibiting hsa-mir-485-5p significantly enriched CSC-like traits [120]. MiR-485 was found to be crucial in controlling NSCLC chemoresistance. Interestingly, miR-485 was downregulated in A549/Cisplatin-resistant cells compared to A549 cells [119]. A549/Cisplatin-resistant cells had more stemness when miR-485 was inhibited than when it was overexpressed, which decreased CSC-like characteristics. Furthermore, A549/Cisplatin-resistant cell proliferation was reduced by EGCG, which also caused cell death [119].
Bhardwaj et al. used NGS to study the effect of EGCG in regulating miRNA expression in NSCLC and validated the functional role of hsa-miR-548o-5p, hsa-miR-21-5p, hsa-miR-212-5p, and hsa-miR-181c in EGCG treated cell line A549. The MAPK pathway was found to be the most effective pathway by which EGCG modulated microRNAs according to KEGG and PANTHER pathway analyses [121].
A study demonstrated the capacity of the genistein-miRNA-29b-loaded hybrid nanoparticles (GMLHN) to attack numerous targets, resulting in an improved anticancer effect, making GMLHN a promising therapeutic option for NSCLC. Additionally, GMLHN over individual miRNA-29b and genistein-loaded nanoparticles showed enhanced apoptosis [122]. Moreover, genistein limits the growth of NSCLC cells by controlling the circ 0031250/miR-873-5p/FOXM1 axis. This indicates that the presence of genistein in soy foods may make them useful in the diet for treating NSCLC [123].
Another study showed that luteolin inhibits the formation of the tumor by causing apoptosis and decreasing the proliferation of NSCLC cells both in vivo and in vitro. MiR-34a-5p greatly adds to the anticancer effects produced by luteolin by preferentially targeting MDM4 and increasing the activation of the p53 pathway. Furthermore, cells were treated with luteolin and transfected after NSCLC-vascular endothelial cells (VECs). As a result, luteolin decreased the viability, migration, angiogenesis, and invasion of NSCLC-VECs while increasing the expression of miR-133a-3p. A miR-133a-3p inhibitor overrode this effect by promoting vitality, migration, angiogenesis, and invasion. VEGF, MMP-2, and MMP-9, as well as components relevant to the PI3K/Akt and MAPK signaling pathways, were all suppressed by luteolin, while the inhibitor of miR-133a-3p reversed this effect on NSCLC-VECs. MiR-133a-3p was inhibited by luteolin, which reduced the amount of purine-rich element binding protein B (PURB). ShPURB increased the level of miR-133a-3p in NSCLC-VECs while counteracting the effects of miR-133a-3p inhibitors that boosted migration and invasion in NSCLC [124].
Ovarian cancer
In addition to breast and lung cancer, ovarian cancer is also known as the "silent killer" because of its poor diagnosis at early stages. It is one of the most highly fatal gynecological malignancies [125]. Recent studies identified the significant contribution of flavonoids in regulating the expression of underpinnings molecules, such as dysregulated miRNA, which is responsible for the alteration of the cascade of downstream molecules. For example, quercetin, a dietary flavonoid, induces the apoptosis of human ovarian cancer cell lines SKOV-3 and A2780 via regulating miR-145 expression. Zhou et al. demonstrated that quercetin treatment of 25, 50, and 100 μM/ml stimulates the expression of miR-145 in SKOV-3 and A2780 cells and upregulates caspase-3 levels regulating cell growth and apoptosis [126].
Another dietary flavonoid, genistein, regulates miRNA expression in ovarian cancer. According to Xu et al. ovarian cancer cells treated with genistein had their proliferation and migration inhibited. Sprouty2, a suspected miR-27a target gene, was expressed more when genistein decreased miR-27a expression. This suggests that genistein acts as a non-toxic inactivator of miR-27a and inhibits the proliferation and migration of ovarian cancer cells [127].
Gastric cancer
Gastric cancer is found to be 3rd most common cause of death among cancer patients worldwide. Surprisingly, it has been observed that more than 50 % of GC cases are observed in Eastern Asian population [128]. Reports suggested that 20% of patients survive up to 5 years after detection and the majority of patients eventually die of cancer metastasis or poor diagnosis [129]. Presently, investigations are going on to explore alternative treatment methods including combinatorial therapeutics with novel RNA-based mechanistic insight. In a study, miR-143 pretreated cells of gastric cancer (AGS, MNK28) were found to be sensitized toward quercetin. Treatment leads to decrease in cell viability, and can increase apoptotic cell death via caspase activation [130]. Another study, using gastric cancer cells (AGS, SGC7901) revealed the modulation of LINC00511/miR-29b/KDM2A axis and apoptosis induction by epigallocatechin-3-gallate treatment [131].
Prostate cancer
Prostate cancer is among the leading cause of cancer-associated mortalities in old age men. Currently, androgen deprivation is considered as a treatment therapy for newly diagnosed patients. However, prostate cancer may convert into castration-resistant even after androgen depletion therapy [132]. Prostate cancer in advanced stages shows limited response, therefore novel therapeutic methods such as miRNAs could be beneficial to inhibit tumor progressions. Chen et al., in their study found that quercetin exhibits its anticancer effect in prostate cancer (C4-2-IRR, 22Rv1) via modulating circNHS / miR-512-5p / XRCC5 signaling [133]. Similarly, genistein mediated downregulation of miR-1260b in prostate cancer (PC3, DU145) leading to a decrease in cell viability by inducing apoptosis, cell cycle arrest, and inhibiting tumor mobility [134]. In another study, genistein upregulated miR-574-3p, mir-34a, and downregulated miR-151 in prostate cancer (PC3, DU145) resulting in apoptotic cell death [135,136]. Majid et al. [137] reported that genistein mediated upregulation of miR-1296 and cell cycle arrest via inhibiting CDK2, CDC7, CDT1 in prostate cancer (PC3, LNCaP). Downregulation of miR-221 and miR-222 in prostate cancer (PC3) by genistein resulted in upregulation of ARH1 that is essential for maintaining ADP-ribosylation and cancer suppression [138]. Epigallocatechin-3-gallate downregulated androgen receptor and the proliferation rate of prostate cancer (LNCaP, 22Rv1) in mice models via upregulation of miR-330 and downregulation of miR-21 [139].
Colorectal cancer
Colorectal cancer (CRC) is found to be 2nd most common cause of death worldwide. The diagnosis and mortality rates of CRC are increasing rapidly among low- and middle-income countries. Statistical analysis revealed that incidences of CRC will rise to 60 % more and might be responsible for the death of 1.1 million cancer patients by 2030. Hence, more investigations are needed to lower down adverse effects of CRC and to improve early diagnosis and treatment aspects [140]. Novel therapeutic strategies using miRNA may not only provide an alternative route of treatment but may also reduce toxicity and adverse effects. Quercetin-mediated differential modulation of 83 miRNAs (19 downregulated and 64 upregulated) was found to suppress the growth of colorectal cancer (HCT116) proliferation via apoptosis induction [130]. In a study using colorectal cancer cell line model, a combination of quercetin and resveratrol resulted in suppression of miR-27a along with decreased cell proliferation and increase in caspase-3, PARP cleavage [141]. Genistein-mediated downregulation of miR-95 was found to induce apoptosis via inhibiting Bcl-2, ERK1, SGK1 in colorectal cancer (SW620) [142]. Upregulation of miR-34a, miR-145, miR-200c was observed in colorectal cancer with 5-fluorouracil-resistant cells (HCT116, SW480) with epigallocatechin-3-gallate treatment. Mechanistic insight revealed induction of apoptosis and cell cycle arrest along with the downregulation of cMYC, NOTCH1, OCT4, and Nanog [143].
Intervention of microRNAs in the synergistic effect of flavonoids and antineoplastic agents
As described in the previous section, miRNAs are known to play crucial role in cancer dissemination and are thus rendered as a promising target for the prevention and/or treatment of various malignancies [144]. The potential of flavonoids to alter the levels of miRNAs through a variety of pathways, including transcription, epigenetic, and miRNA processing, modulating tumor suppressor and oncogenic miRNAs, is remarkable. Furthermore, as shown in Table 3, flavonoids can increase the sensitivity of chemotherapeutic agents and drug molecules, suggesting a promising new anticancer medication for malignancies [60]. Many previous studies showed that flavonoids like EGCG, quercetin etc. show a strong antineoplastic role when combined with several anticancer agents by regulating different types of miRNAs [145]. Efficacy of cisplatin has been shown to be enhanced by EGCG with a mechanism involving downregulation of hsa-miR-98-5p and upregulation of p53 in non-small-cell lung cancer (NSCLC) cells [118]. Another study showed that EGCG is an effective adjuvant therapy in combination with cisplatin for lung cancer treatment as it targets miR-98-5p which in turn reduces CTRI-induced chemoresistance [146]. Furthermore, according to La et al. and colleagues, EGCG inhibits the GRP78/NF-B/miR-155-5p/MDR1 pathway, increasing the susceptibility of colorectal cancer cells to 5-fluorouracil [147]. In a mouse model of castration-resistance prostate cancer (CRPC), the potential enhancement of the therapeutic efficacy of docetaxel using a combination of green tea and quercetin was examined. The treatment combination was found to increase docetaxel's effectiveness by twofold, and significant increase in the tumor-suppressor genes miR-15a and miR-330 in cancerous tissue [148]. According to Zhang et al. quercetin at a concentration of 5 μM increases sensitivity of cisplatin by modifying the miR-217/KRAS axis in osteosarcoma cells. The oncogene KRAS was downregulated both at the mRNA and protein levels, whilst miR-217 expression was upregulated. MiR-217 knockdown reversed the effects of quercetin on the induction of cisplatin sensitivity [149]. Recent studies have demonstrated that a curcumin derivative (difluorinated curcumin) suppressed colorectal cancer cells resistance to oxaliplatin and 5-fluorouracil by downregulating miR-21. Authors showed that PTEN, a tumor suppressor gene, is downregulated by miR-21. Resistance to standard treatments and resurgence of cancer after initial treatment is caused by decreased PTEN function [150]. Cyanidin-3-O-glucoside (C3G) also showed tumor-suppressive role in TMZ-resistant LN-18/TR glioma cells by upregulating miR-215-5p and enhancing the cytotoxic effect of temozolomide (TMZ) [86]. Chakrabarti and Ray in 2016 found that silibinin (50 µM) and luteolin (20 µM) synergistically increased apoptosis, inhibition of invasion and migration in two human glioblastoma cell lines (U87MG and T98G) by upregulating miR-7-1-3p [84]. Zaman et al demonstrated that genistein synergistically acts with 5-aza-2′-deoxycytidine, Trichostatin A in PC-3 cells, and induces apoptosis/ cell cycle arrest via up regulating miR-145 [138]. In addition, cancer is also characterized by tumor escape from immune eradication. The most effective immunotherapeutic treatment for cancer to date is therapeutic targeting with checkpoint blockage [151,152]. The combinatorial method, however, appears to be a superior option to enhance the treatment and inhibit immunosuppressive signals in the tumour microenvironment. For instance, using HCC, single immunotherapeutic drug is linked to therapeutic failure, whereas plant-based antiangiogenic flavonoids and immunotherapy combination found to be more effective [153].
Table 3.
Synergistic action of flavonoids with various drug molecules.
| Flavonoid | miRNA and its expression | Combination drug molecule | Physiological effect | Model system (cell lines) | Refs. |
|---|---|---|---|---|---|
| Quercetin | Suppresses miR-21 | Hyperoside | Apoptosis ↑, Caspase-3↑, Migration ↓, Invasion ↓, ROS↓ | Prostate cancer (PC3) | [183] |
| Quercetin | Suppresses miR-21, miR-19b, miR-148a | Arctigenin | Proliferation↓, Migration ↓, AR signaling↓, PI3K/AKT signaling↓ | Prostate cancer (LAPC4, LNCaP) | [184] |
| Quercetin | Suppresses miR-27a | Resveratrol | Cell proliferation↓, Apoptosis ↑, Caspase-3, PARP cleavage ↑ | Colorectal cancer (HT29) | [141] |
| Quercetin | Suppresses mir15a and mir330 | Docetaxel | Tumor growth↓, Ki67↓, cleaved caspase 7↑ | Prostate cancer (PC3) | [148] |
| Quercetin | Upregulates miR-217 | Cisplatin | KRAS↓ | Osteosarcoma (143B) | [149] |
| Difluorinated curcumin | Downregulates miR-21 | 5-Flurouracil + Oxaliplatin | PTEN↓, Akt phosphorylation↓ | colon cancer HCT116 cells SCID mice xenografts |
[150] |
| Silibinin | Upregulates miR-7-1-3p | Luteolin | Apoptosis ↑, Autophagy ↓, Caspase-3, -8, -9↑ | Glioblastoma Multiforme (U87MG, T98G) | [84] |
| Genistein | Upregulates miR-145 | 5-aza-2′-deoxycytidine, Trichostatin A | Apoptosis ↑, Cell cycle arrest in G2/M phase↑ | Prostate cancer (PC3) | [138] |
| Genistein | Upregulates miR-218 | Propofol | Apoptosis↑, Proliferation↓, Caspase-3 ↑ | Glioblastoma Multiforme (U251, 9L) | [85] |
| Epigallocatechin-3-gallate | Upregulates miR-155-5p | 5-FluoroUracil | Apoptosis ↑, MDR1↓, GRP78 ↓, NF-κB ↓, Proliferation ↓, tumor growth in mice ↓ | Colorectal cancer (HCT116, DLD1) | [147] |
| Epigallocatechin-3-gallate | Downregulates hsa-miR-98-5p | Cisplatin | Apoptosis↑, p53↑, MAPK↓ | Non-small cell lung cancer (A549) | [118] |
| Epigallocatechin-3-gallate | NEAT1/hsa-mir-98-5p crosstalk | Cisplatin | CTR1↑, Cell growth↓, | Non-small cell lung cancer (A549, H460 and H1299) Nude mouse xenograft | [146] |
| Cyanidin-3-O-glucoside (C3G) | Upregulates miR-214-5p | Temozolomide (TMZ) | β-catenin↑, MGMT↑ | Glioma TMZ-resistant LN-18/TR | [86] |
Conclusion and further perspectives
The data presented in this review article clearly show the strong potential of structurally different flavonoids to modulate the expression of miRNAs in many different cancer types, affecting the development, growth and spread of malignant tumors. Despite this preclinical evidence, further investigations are definitely required to establish the clinical usefulness of these effects and validate flavonoids as a miRNAs-targeting cancer treatment. Modulation of the expression of cancer-related miRNAs might be an important mechanism among the multitargeted bioactivities of flavonoids, intensifying their complex anticancer action and providing an additional weapon to the current anticancer arsenal. Since bioavailability of flavonoids is known to be low representing the major challenge of their in vivo application, the future studies should focus also on the possibilities of chemical modification and targeted delivery of these promising natural molecules to gain the maximal benefit of their anticancer potential.
Authorship contribution statement
HST and VKG: Initial draft and writng. SB: contributed in chemistry section. VU, US, and AJ: Contributed in miRNA Section. KS and VY: contributed in introduction and table section. JML, KD, and TB: Edited the draft. GS: Conceptualization, Final proof reading and editing.
Declaration of Competing Interest
There is no conflict of interest to declare.
Acknowledgment
Authors would like to acknowledge BioRender software to draw some of the figures.
Contributor Information
Tapan Behl, Email: tapanbehl31@gmail.com.
Gautam Sethi, Email: phcgs@nus.edu.sg.
References
- 1.Gurunathan S., Kang M.H., Qasim M., Kim J.H. Nanoparticle-mediated combination therapy: two-in-one approach for cancer. Int. J. Mol. Sci. 2018;19:3264. doi: 10.3390/ijms19103264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashyap D., Garg V.K., Goel N. 1st ed. Elsevier Inc.; 2021. Intrinsic and Extrinsic Pathways of Apoptosis: Role in Cancer Development and Prognosis. [DOI] [PubMed] [Google Scholar]
- 3.Sharma D., Goel N., Garg V.K. Algorithms Intell. Syst. Springer; Singapore: 2022. Predicting survivability in oral cancer patients; pp. 153–162. [DOI] [Google Scholar]
- 4.Kashyap D., Tuli H.S., Garg V.K., Goel N., Bishayee A. Oncogenic and tumor-suppressive roles of MicroRNAs with special reference to apoptosis: molecular mechanisms and therapeutic potential. Mol. Diagn. Ther. 2018;22:179–201. doi: 10.1007/s40291-018-0316-1. [DOI] [PubMed] [Google Scholar]
- 5.Xia X., Li R., Zhou P., Xing Z., Lu C., Long Z., Wang F., Wang R. Decreased NSG3 enhances PD-L1 expression by Erk1/2 pathway to promote pancreatic cancer progress. Am. J. Cancer Res. 2021;11:916–929. https://pubmed.ncbi.nlm.nih.gov/33791163/ accessed September 29, 2022. [PMC free article] [PubMed] [Google Scholar]
- 6.Huang R., Chen Z., He L., He N., Xi Z., Li Z., Deng Y., Zeng X. Mass spectrometry-assisted gel-based proteomics in cancer biomarker discovery: approaches and application. Theranostics. 2017;7:3559–3572. doi: 10.7150/THNO.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021:caac.21660. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap D., Garg V.K., Sandberg E.N., Goel N., Bishayee A. Oncogenic and tumor suppressive components of the cell cycle in breast cancer progression and prognosis. Pharmaceutics. 2021;13:1–28. doi: 10.3390/pharmaceutics13040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichota A., Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018;19:3533. doi: 10.3390/IJMS19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014;8:122–146. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Z.P., Peng Z.Y., Peng M.J., Yan W.B., Ouyang Y.Z., Zhu H.L. Flavonoids health benefits and their molecular mechanism. Mini Rev. Med. Chem. 2011;11:169–177. doi: 10.2174/138955711794519546. [DOI] [PubMed] [Google Scholar]
- 12.Weng C.J., Yen G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012;31:323–351. doi: 10.1007/S10555-012-9347-Y. [DOI] [PubMed] [Google Scholar]
- 13.Tuli H.S., Mistry H., Kaur G., Aggarwal D., Garg V.K., Mittal S., Yerer M.B., Sak K., Khan M.A. Gallic acid: a dietary polyphenol that exhibits anti-neoplastic activities by modulating multiple oncogenic targets. Anti Cancer Agents Med. Chem. 2021;22:499–514. doi: 10.2174/1871520621666211119085834. [DOI] [PubMed] [Google Scholar]
- 14.Tuli H.S., Sak K., Iqubal A., Garg V.K., Varol M., Sharma U., Chauhan A., Yerer M.B., Dhama K., Jain M., Jain A. STAT signaling as a target for intervention: from cancer inflammation and angiogenesis to non-coding RNAs modulation. Mol. Biol. Rep. 2022;49 doi: 10.1007/s11033-022-07399-w. [DOI] [PubMed] [Google Scholar]
- 15.Kashyap D., Garg V.K., Tuli H.S., Yerer M.B., Sak K., Sharma A.K., Kumar M., Aggarwal V., Sandhu S.S. Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules. 2019;9 doi: 10.3390/biom9050174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namdeo A.G., Boddu S.H.S., Amawi H., Ashby C.R., Tukaramrao D.B., Trivedi P., Babu R.J., Tiwari A.K. Flavonoids as multi-target compounds: a special emphasis on their potential as chemo-adjuvants in cancer therapy. Curr. Pharm. Des. 2020;26:1712–1728. doi: 10.2174/1381612826666200128095248. [DOI] [PubMed] [Google Scholar]
- 17.Tuli H.S., Sak K., Gupta D.S., Kaur G., Aggarwal D., Parashar N.C., Choudhary R., Yerer M.B., Kaur J., Kumar M., Garg V.K., Sethi G. Anti-inflammatory and anticancer properties of birch bark-derived betulin: recent developments. Plants. 2021;10 doi: 10.3390/plants10122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q., You L., Nepovimova E., Heger Z., Wu W., Kuca K., Adam V. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022;15:77. doi: 10.1186/S13045-022-01292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S., Raza W., Parveen S., Meena A., Luqman S. Flavonoid display ability to target microRNAs in cancer pathogenesis. Biochem. Pharmacol. 2021;189 doi: 10.1016/J.BCP.2021.114409. [DOI] [PubMed] [Google Scholar]
- 20.Brodowska K.M. Natural flavonoids: classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017;7:108–123. doi: 10.5281/ZENODO.545778. [DOI] [Google Scholar]
- 21.Sharma A., Singh Tuli H., Sharma A.K. Current Aspects of Flavonoids: Their Role in Cancer Treatment. Springer; Singapore: 2019. Chemistry and synthetic overview of flavonoids; pp. 23–38. [DOI] [Google Scholar]
- 22.Ignat I., Volf I., Popa V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/J.FOODCHEM.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magar R.T., Sohng J.K. A review on structure, modifications and structure-activity relation of quercetin and its derivatives. J. Microbiol. Biotechnol. 2020;30:11–20. doi: 10.4014/JMB.1907.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miltonprabu S. Nonvitamin and Nonmineral Nutritional Supplements. Academic Press; 2018. Quercetin: a flavonol with versatile therapeutic applications and its interactions with other drugs; pp. 75–83. [DOI] [Google Scholar]
- 26.Lesjak M., Beara I., Simin N., Pintać D., Majkić T., Bekvalac K., Orčić D., Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018;40:68–75. doi: 10.1016/j.jff.2017.10.047. [DOI] [Google Scholar]
- 27.Tuli H.S. Springer; Singapore: 2019. Current Aspects of Flavonoids: Their Role in Cancer Treatment. [Google Scholar]
- 28.Devappa R.K., Rakshit S.K., Dekker R.F.H. Forest biorefinery: potential of poplar phytochemicals as value-added co-products. Biotechnol. Adv. 2015;33:681–716. doi: 10.1016/J.BIOTECHADV.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Ezzati M., Yousefi B., Velaei K., Safa A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020;248 doi: 10.1016/J.LFS.2020.117463. [DOI] [PubMed] [Google Scholar]
- 30.Khazdair M.R., Anaeigoudari A., Agbor G.A. Anti-viral and anti-inflammatory effects of kaempferol and quercetin and COVID-2019: a scoping review. Asian Pac. J. Trop. Biomed. 2021;11:327–334. doi: 10.4103/2221-1691.319567. [DOI] [Google Scholar]
- 31.Ulusoy H.G., Sanlier N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020;60:3290–3303. doi: 10.1080/10408398.2019.1683810. [DOI] [PubMed] [Google Scholar]
- 32.Mishra N., Rizvi S.I. Quercetin modulates Na+/K+ atpase and sodium hydrogen exchanger in type 2 diabetic erythrocytes. Cell. Mol. Biol. 2012;58:148–152. doi: 10.1170/T934. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y., Shi R., Wang X., Shen H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aziz N., Kim M.Y., Cho J.Y. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018;225:342–358. doi: 10.1016/J.JEP.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Choi J.S., Islam M.N., Ali M.Y., Kim Y.M., Park H.J., Sohn H.S., Jung H.A. The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer's disease, anti-diabetic, and anti-inflammatory activities. Arch. Pharm. Res. 2014;37:1354–1363. doi: 10.1007/s12272-014-0351-3. [DOI] [PubMed] [Google Scholar]
- 36.Tuorkey M.J. Molecular targets of luteolin in cancer. Eur. J. Cancer Prev. 2016;25:65–76. doi: 10.1097/CEJ.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bharti S., Misro M.M., Rai U. Quercetin supplementation restores testicular function and augments germ cell survival in the estrogenized rats. Mol. Cell. Endocrinol. 2014;383:10–20. doi: 10.1016/j.mce.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Porwal O., Mohammed Ameen M.S., Anwer E.T., Uthirapathy S., Ahamad J., Tahsin A. Silybum marianum (Milk Thistle): review on Its chemistry, morphology, ethno medical uses, phytochemistry and pharmacological activities. J. Drug Deliv. Ther. 2019;9:199–206. doi: 10.22270/jddt.v9i5.3666. [DOI] [Google Scholar]
- 39.Samu Z., Nyiredy S., Baitz-Gacs E., Varga Z., Kurtan T., Dinya Z., Antus S. Structure elucidation and antioxidant activity of (-)-isosilandrin isolated from silybum marianum L. Chem. Biodivers. 2004;1:1668–1677. doi: 10.1002/cbdv.200490125. [DOI] [PubMed] [Google Scholar]
- 40.Ung L., Le Q.U., Lay H.L., Wu M.C., Kumar Joshi R., Ming-Chang Wu C. Phytoconstituents and pharmacological activities of Silybum marianum (Milk Thistle): a critical review. Am. J. Essent. Oils Nat. Prod. 2018;6:41–47. https://www.researchgate.net/publication/331438694 accessed September 23, 2022. [Google Scholar]
- 41.Shaker E., Mahmoud H., Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem. Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Zhu S.Y., Jiang N., Yang J., Tu J., Zhou Y., Xiao X., Dong Y. Silybum marianum oil attenuates hepatic steatosis and oxidative stress in high fat diet-fed mice. Biomed. Pharmacother. 2018;100:191–197. doi: 10.1016/j.biopha.2018.01.144. [DOI] [PubMed] [Google Scholar]
- 43.Dixon R.A., Ferreira D. Genistein. Phytochemistry. 2002;60:205–211. doi: 10.1016/S0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- 44.Thangavel P., Puga-Olguín A., Rodríguez-Landa J.F., Zepeda R.C. Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules. 2019;24:3892. doi: 10.3390/molecules24213892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marini H., Minutoli L., Polito F., Bitto A., Altavilla D., Atteritano M., Gaudio A., Mazzaferro S., Frisina A., Frisina N., Lubrano C., Bonaiuto M., D'Anna R., Cannata M.L., Corrado F., Adamo E.B., Wilson S., Squadrito F. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann. Intern. Med. 2007;146:839–847. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- 46.Chen X., Wu Y., Gu J., Liang P., Shen M., Xi J., Qin J. Anti-invasive effect and pharmacological mechanism of genistein against colorectal cancer. Biofactors. 2020;46:620–628. doi: 10.1002/biof.1627. [DOI] [PubMed] [Google Scholar]
- 47.Nazari-Khanamiri F., Ghasemnejad-Berenji M. Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: an overview. J. Food Biochem. 2021;45:e13972. doi: 10.1111/jfbc.13972. [DOI] [PubMed] [Google Scholar]
- 48.Pierzynowska K., Cyske Z., Gaffke L., Rintz E., Mantej J., Podlacha M., Wiśniewska K., Ĺťabińska M., Sochocka M., Lorenc P., Bielańska P., Giecewicz I., Węgrzyn G. Potencjał autofagii indukowanej przez genisteinę w leczeniu chorób neurodegeneracyjnych. Postepy Biochem. 2021;67:117–129. doi: 10.18388/pb.2021_380. [DOI] [PubMed] [Google Scholar]
- 49.Chu C., Deng J., Man Y., Qu Y. Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed Res. Int. 2017;2017 doi: 10.1155/2017/5615647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin P., Li M., Xu G., Zhang K., Zheng L., Zhao J. Role of (-)-epigallocatechin-3-gallate in the osteogenic differentiation of human bone marrow mesenchymal stem cells: an enhancer or an inducer? Exp. Ther. Med. 2015;10:828–834. doi: 10.3892/etm.2015.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecumberri E., Dupertuis Y.M., Miralbell R., Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013;32:894–903. doi: 10.1016/J.CLNU.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Chourasia M., Koppula P.R., Battu A., Ouseph M.M., Singh A.K. EGCG, a green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules. 2021;26 doi: 10.3390/molecules26051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jo Y.H., Park H.C., Choi S., Kim S., Bao C., Kim H.W., Choi H.K., Lee H.J., Auh J.H. Metabolomic analysis reveals cyanidins in black raspberry as candidates for suppression of lipopolysaccharide-induced inflammation in murine macrophages. J. Agric. Food Chem. 2015;63:5449–5458. doi: 10.1021/acs.jafc.5b00560. [DOI] [PubMed] [Google Scholar]
- 54.Bendokas V., Šarkinas A., Jasinauskienë D., Anisimovienë N., Morkûnaitë-Haimi Š., Stanys V., Šikšnianas T. Antimicrobial activity of berries extracts of four Ribes species, their phenolic content and anthocyanin composition. Folia Hortic. 2018;30:249–257. doi: 10.2478/fhort-2018-0021. [DOI] [Google Scholar]
- 55.Lyu S.Y., Shin A.H., Hahn D.R., Park W.B. Antioxidant activity of cyanidins isolated from ogapy (acanthopanax divaricatus var. albeofructus) fruits in U937 macrophages. Food Sci. Biotechnol. 2012;21:1445–1450. doi: 10.1007/s10068-012-0190-2. [DOI] [Google Scholar]
- 56.Bahrani H., Thoms K., Båga M., Larsen J., Graf R., Laroche A., Sammynaiken R., Chibbar R.N. Preferential accumulation of glycosylated cyanidins in winter-hardy rye (Secale cereale L.) genotypes during cold acclimation. Environ. Exp. Bot. 2019;164:203–212. doi: 10.1016/j.envexpbot.2019.05.006. [DOI] [Google Scholar]
- 57.Barwal T.S., Singh N., Sharma U., Bazala S., Rani M., Behera A., Kumawat R.K., Kumar P., Uttam V., Khandelwal A., Barwal J., Jain M., Jain A. miR-590–5p: a double-edged sword in the oncogenesis process. Cancer Treat. Res. Commun. 2022;32 doi: 10.1016/j.ctarc.2022.100593. [DOI] [PubMed] [Google Scholar]
- 58.Khandelwal A., Sharma U., Barwal T.S., Seam R.K., Gupta M., Rana M.K., Vasquez K.M., Jain A. Circulating miR-320a acts as a tumor suppressor and prognostic factor in non-small cell lung cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.645475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khandelwal A., Seam R.K., Gupta M., Rana M.K., Prakash H., Vasquez K.M., Jain A. Circulating microRNA-590-5p functions as a liquid biopsy marker in non-small cell lung cancer. Cancer Sci. 2020;111:826–839. doi: 10.1111/cas.14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adinew G.M., Taka E., Mendonca P., Messeha S.S., Soliman K.F.A. The anticancer effects of flavonoids through miRNAs modulations in triple-negative breast cancer. Nutrients. 2021;13:1212. doi: 10.3390/nu13041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barwal T.S., Sharma U., Bazala S., Singh I., Jain M., Prakash H., Shekhar S., Sandberg E.N., Bishayee A., Jain A. Micrornas and long noncoding rnas as novel therapeutic targets in estrogen receptor-positive breast and ovarian cancers. Int. J. Mol. Sci. 2021;22:4072. doi: 10.3390/ijms22084072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J., Zhang M., Hu X., She J., Sun R., Qin S., Li D. miRNA-194 predicts favorable prognosis in gastric cancer and inhibits gastric cancer cell growth by targeting CCND1. FEBS Open Bio. 2021;11:1814–1826. doi: 10.1002/2211-5463.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu J., Xu Y., Liu S., Qiao L., Sun J., Zhao Q. MicroRNAs associated with colon cancer: new potential prognostic markers and targets for therapy. Front. Bioeng. Biotechnol. 2020;8:176. doi: 10.3389/fbioe.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tantawy M., Elzayat M.G., Yehia D., Taha H. Identification of microRNA signature in different pediatric brain tumors. Genet. Mol. Biol. 2018;41:27–34. doi: 10.1590/1678-4685-gmb-2016-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yonekura-Sakakibara K., Higashi Y., Nakabayashi R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019;10:943. doi: 10.3389/fpls.2019.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wen W., Alseekh S., Fernie A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020;55:100–108. doi: 10.1016/j.pbi.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Ngwa W., Kumar R., Thompson D., Lyerly W., Moore R., Reid T.E., Lowe H., Toyang N. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules. 2020;25:2702. doi: 10.3390/molecules25112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang B., Liu H., Yang J., Gupta V.K., Jiang Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018;79:116–124. doi: 10.1016/J.TIFS.2018.07.006. [DOI] [Google Scholar]
- 69.Khan A., Ikram M., Hahm J.R., Kim M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: special focus on neurological disorders. Antioxidants. 2020;9:1–15. doi: 10.3390/ANTIOX9070609. (Basel, Switzerland) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang C.H., Sun T.L., Xiang D.X., Wei S.S., Li W.Q. Anticancer activity and mechanism of xanthohumol: a prenylated flavonoid from hops (Humulus lupulus L.) Front. Pharmacol. 2018;9:530. doi: 10.3389/FPHAR.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pasam V.R., Kiran S., Rohini P., Bhagyasree P. Flavonoid : a review on naringenin. J. Pharmacogn. Phytochem. 2017;6:2778–2783. https://www.phytojournal.com/archives/2017.v6.i5.2042/flavonoid-a-review-on-naringenin (accessed September 23, 2022) [Google Scholar]
- 72.Khan M.F., Khan M.A., Khan Z.A., Ahamad T., Ansari W.A. Identification of dietary molecules as therapeutic agents to combat COVID-19 using molecular docking studies. Res. Sq. 2020:1–17. doi: 10.21203/rs.3.rs-19560/v1. [DOI] [Google Scholar]
- 73.Wang W., Xie Y., Malhotra A. Potential of curcumin and quercetin in modulation of premature mitochondrial senescence and related changes during lung carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2021;40:53–60. doi: 10.1615/JEnvironPatholToxicolOncol.2021039371. [DOI] [PubMed] [Google Scholar]
- 74.Varghese E., Liskova A., Kubatka P., Samuel S.M., Büsselberg D. Anti-angiogenic effects of phytochemicals on miRNA regulating breast cancer progression. Biomolecules. 2020;10:191. doi: 10.3390/BIOM10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gianfredi V., Nucci D., Vannini S., Villarini M., Moretti M. In vitro biological effects of sulforaphane (SFN), epigallocatechin-3-gallate (EGCG), and curcumin on breast cancer cells: a systematic review of the literature. Nutr. Cancer. 2017;69:969–978. doi: 10.1080/01635581.2017.1359322. [DOI] [PubMed] [Google Scholar]
- 76.Alqarni A.A., Alamoudi A.A., Allam R.M., Ajabnoor G.M., Harakeh S.M., Al-Abd A.M. The influence of antioxidant dietary-derived polyphenolic combination on breast cancer: molecular study. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112835. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed F., Ijaz B., Ahmad Z., Farooq N., Sarwar M.B., Husnain T. Modification of miRNA expression through plant extracts and compounds against breast cancer: mechanism and translational significance. Phytomedicine. 2020;68 doi: 10.1016/j.phymed.2020.153168. [DOI] [PubMed] [Google Scholar]
- 78.Samec M., Liskova A., Kubatka P., Uramova S., Zubor P., Samuel S.M., Zulli A., Pec M., Bielik T., Biringer K., Kudela E., Benacka J., Adamek M., Rodrigo L., Ciccocioppo R., Kwon T.K., Baranenko D., Kruzliak P., Büsselberg D. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019;145:1665–1679. doi: 10.1007/s00432-019-02940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saleh H.A., Yousef M.H., Abdelnaser A. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.606069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laengsri V., Kerdpin U., Plabplueng C., Treeratanapiboon L., Nuchnoi P. Cervical cancer markers: epigenetics and microRNAs. Lab. Med. 2018;49:97–111. doi: 10.1093/LABMED/LMX080. [DOI] [PubMed] [Google Scholar]
- 81.Ling H., Fabbri M., Calin G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milenkovic D., Deval C., Gouranton E., Landrier J.F., Scalbert A., Morand C., Mazur A. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: a new mechanism of the action of polyphenols. PLoS One. 2012;7:e29837. doi: 10.1371/journal.pone.0029837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakada M., Kita D., Furuta T., Watanabe T., Hayashi Y., Hamada J.I. Glioma Cell Biol. Springer-Verlag Wien; 2012. Signaling cascades driving the malignant phenotype of glioma cells; pp. 47–75. [DOI] [Google Scholar]
- 84.Chakrabarti M., Ray S.K. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis. 2016;21:312–328. doi: 10.1007/s10495-015-1198-x. [DOI] [PubMed] [Google Scholar]
- 85.Zheng Y., Liu H., Liang Y. Genistein exerts potent antitumour effects alongside anaesthetic, propofol, by suppressing cell proliferation and nuclear factor-κB-mediated signalling and through upregulating microRNA-218 expression in an intracranial rat brain tumour model. J. Pharm. Pharmacol. 2017;69:1565–1577. doi: 10.1111/jphp.12781. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Y., Chen L., Ding D., Li Z., Cheng L., You Q., Zhang S. Cyanidin-3-O-glucoside inhibits the β-catenin/MGMT pathway by upregulating miR-214-5p to reverse chemotherapy resistance in glioma cells. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-11757-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kashyap D., Pal D., Sharma R., Garg V.K., Goel N., Koundal D., Zaguia A., Koundal S., Belay A. Global increase in breast cancer incidence: risk factors and preventive measures. Biomed Res. Int. 2022;2022 doi: 10.1155/2022/9605439. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Ren B., Kwah M.X.Y., Liu C., Ma Z., Shanmugam M.K., Ding L., Xiang X., Ho P.C.L., Wang L., Ong P.S., Goh B.C. Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett. 2021;515:63–72. doi: 10.1016/j.canlet.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Ma Z., Xiang X., Li S., Xie P., Gong Q., Goh B.C., Wang L. Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Semin. Cancer Biol. 2022;80:379–390. doi: 10.1016/J.SEMCANCER.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 90.Chien S.Y., Wu Y.C., Chung J.G., Yang J.S., Lu H.F., Tsou M.F., Wood W., Kuo S.J., Chen D.R. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 2009;28:493–503. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 91.Chou C.C., Yang J.S., Lu H.F., Ip S.W., Lo C., Wu C.C., Lin J.P., Tang N.Y., Chung J.G., Chou M.J., Teng Y.H., Chen D.R. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2010;33:1181–1191. doi: 10.1007/s12272-010-0808-y. [DOI] [PubMed] [Google Scholar]
- 92.Duo J., Ying G.G., Wang G.W., Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012;5:1453–1456. doi: 10.3892/mmr.2012.845. [DOI] [PubMed] [Google Scholar]
- 93.feng Tao S., fei He H., Chen Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015;402:93–100. doi: 10.1007/s11010-014-2317-7. [DOI] [PubMed] [Google Scholar]
- 94.Abdel-Latif M., Riad A., Soliman R.A., Elkhouly A.M., Nafae H., Gad M.Z., Motaal A.A., Youness R.A. MALAT-1/p53/miR-155/miR-146a ceRNA circuit tuned by methoxylated quercitin glycoside alters immunogenic and oncogenic profiles of breast cancer. Mol. Cell. Biochem. 2022;477:1281–1293. doi: 10.1007/s11010-022-04378-4. [DOI] [PubMed] [Google Scholar]
- 95.Cho E., Chung E.Y., Jang H.Y., Hong O.Y., Chae H.S., Jeong Y.J., Kim S.Y., Kim B.S., Yoo D.J., Kim J.S., Park K.H. Anti-cancer effect of cyanidin-3-glucoside from mulberry via caspase-3 cleavage and DNA fragmentation in vitro and in vivo. Anti Cancer Agents Med. Chem. 2017;17:1519–1525. doi: 10.2174/1871520617666170327152026. [DOI] [PubMed] [Google Scholar]
- 96.Liang L., Liu X., He J., Shao Y., Liu J., Wang Z., Xia L., Han T., Wu P. Cyanidin-3-glucoside induces mesenchymal to epithelial transition via activating Sirt1 expression in triple negative breast cancer cells. Biochimie. 2019;162:107–115. doi: 10.1016/j.biochi.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 97.Orlando L., Schiavone P., Cinieri S. Genistein: the future of prevention and treatment of breast cancer? Cancer Biol. Ther. 2011;11:918–920. doi: 10.4161/CBT.11.10.15493. [DOI] [PubMed] [Google Scholar]
- 98.Pellegrino L., Stebbing J., Braga V.M., Frampton A.E., Jacob J., Buluwela L., Jiao L.R., Periyasamy M., Madsen C.D., Caley M.P., Ottaviani S., Roca-Alonso L., El-Bahrawy M., Coombes R.C., Krell J., Castellano L. miR-23b regulates cytoskeletal remodeling, motility and metastasis by directly targeting multiple transcripts. Nucl. Acids Res. 2013;41:5400–5412. doi: 10.1093/nar/gkt245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De La Parra C., Castillo-Pichardo L., Cruz-Collazo A., Cubano L., Redis R., Calin G.A., Dharmawardhane S. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr. Cancer. 2016;68:154–164. doi: 10.1080/01635581.2016.1115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galati G., Teng S., Moridani M.Y., Chan T.S., O'Brien P.J. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metabol. Drug Interact. 2000;17:311–349. doi: 10.1515/DMDI.2000.17.1-4.311. [DOI] [PubMed] [Google Scholar]
- 101.Kim M.J., Woo J.S., Kwon C.H., Kim J.H., Kim Y.K., Kim K.H. Luteolin induces apoptotic cell death through AIF nuclear translocation mediated by activation of ERK and p38 in human breast cancer cell lines. Cell Biol. Int. 2012;36:339–344. doi: 10.1042/cbi20110394. [DOI] [PubMed] [Google Scholar]
- 102.Sun D.W., Da Zhang H., Mao L., Mao C.F., Chen W., Cui M., Ma R., Cao H.X., Jing C.W., Wang Z., Wu J.Z., Tang J.H. Luteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing notch signaling and regulating MiRNAs. Cell. Physiol. Biochem. 2015;37:1693–1711. doi: 10.1159/000438535. [DOI] [PubMed] [Google Scholar]
- 103.Gao G., Ge R., Li Y., Liu S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019;47:3265–3271. doi: 10.1080/21691401.2019.1646749. [DOI] [PubMed] [Google Scholar]
- 104.Zhang M.L., Liu W.W., Li W.D. Imbalance of molecular module of tincr-mir-761 promotes the metastatic potential of early triple negative breast cancer and partially offsets the anti-tumor activity of luteolin. Cancer Manag. Res. 2021;13:1877–1886. doi: 10.2147/CMAR.S288271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Magura J., Moodley R., Mackraj I. The effect of hesperidin and luteolin isolated from eriocephalus africanus on apoptosis, cell cycle and miRNA expression in MCF-7. J. Biomol. Struct. Dyn. 2022;40:1791–1800. doi: 10.1080/07391102.2020.1833757. [DOI] [PubMed] [Google Scholar]
- 106.Barta J.A., Powell C.A., Wisnivesky J.P. Global epidemiology of lung cancer. Ann. Glob. Health. 2019;85:8. doi: 10.5334/AOGH.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cai W.Q., Zeng L.S., Wang L.F., Wang Y.Y., Cheng J.T., Zhang Y., Han Z.W., Zhou Y., Huang S.L., Wang X.W., Peng X.C., Xiang Y., Ma Z., Cui S.Z., Xin H.W. The latest battles between EGFR monoclonal antibodies and resistant tumor cells. Front. Oncol. 2020;10:1249. doi: 10.3389/fonc.2020.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu C., Xiang X., Han S., Lim H.Y., Li L., Zhang X., Ma Z., Yang L., Guo S., Soo R., Ren B., Wang L., Goh B.C. Blood-based liquid biopsy: insights into early detection and clinical management of lung cancer. Cancer Lett. 2022;524:91–102. doi: 10.1016/J.CANLET.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 109.Nasim F., Sabath B.F., Eapen G.A. Lung cancer. Med. Clin. N. Am. 2019;103:463–473. doi: 10.1016/J.MCNA.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 110.juan Liu W., Du Y., Wen R., Yang M., Xu J. Drug resistance to targeted therapeutic strategies in non-small cell lung cancer. Pharmacol. Ther. 2020;206 doi: 10.1016/j.pharmthera.2019.107438. [DOI] [PubMed] [Google Scholar]
- 111.Lam T.K., Shao S., Zhao Y., Marincola F., Pesatori A., Bertazzi P.A., Caporaso N.E., Wang E., Landi M.T. Influence of quercetin-rich food intake on microRNA expression in lung cancer tissues. Cancer Epidemiol. Biomark. Prev. 2012;21:2176–2184. doi: 10.1158/1055-9965.EPI-12-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kang J.H., Kim E.G., Kim W., Seong K.M., Youn H.S., Kim J.W., Kim J., Youn B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J. Biol. Chem. 2013;288:27343–27357. doi: 10.1074/jbc.M113.490482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sonoki H., Sato T., Endo S., Matsunaga T., Yamaguchi M., Yamazaki Y., Sugatani J., Ikari A. Quercetin decreases claudin-2 expression mediated by up-regulation of microRNA miR-16 in lung adenocarcinoma A549 cells. Nutrients. 2015;7:4578–4592. doi: 10.3390/nu7064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li J.P., Liao X.H., Xiang Y., Yao A., Song R.H., Zhang Z.J., Huang F., Dai Z.T., Zhang T.C. Hyperoside and let-7a-5p synergistically inhibits lung cancer cell proliferation via inducing G1/S phase arrest. Gene. 2018;679:232–240. doi: 10.1016/j.gene.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 115.Wang Q., Chen Y., Lu H., Wang H., Feng H., Xu J., Zhang B. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR-16-5p/WEE1 axis. IUBMB Life. 2020;72:1012–1022. doi: 10.1002/iub.2242. [DOI] [PubMed] [Google Scholar]
- 116.Chai R., Xu C., Lu L., Liu X., Ma Z. Quercetin inhibits proliferation of and induces apoptosis in non-small-cell lung carcinoma via the lncRNA SNHG7/miR-34a-5p pathway. Immunopharmacol. Immunotoxicol. 2021;43:693–703. doi: 10.1080/08923973.2021.1966032. [DOI] [PubMed] [Google Scholar]
- 117.Wang H., Bian S., Yang C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis. 2011;32:1881–1889. doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou D.H., Wang X., Feng Q. EGCG enhances the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC A549 cells. Nutr. Cancer. 2014;66:636–644. doi: 10.1080/01635581.2014.894101. [DOI] [PubMed] [Google Scholar]
- 119.Jiang P., Xu C., Chen L., Chen A., Wu X., Zhou M., ul Haq I., Mariyam Z., Feng Q. EGCG inhibits CSC-like properties through targeting miR-485/CD44 axis in A549-cisplatin resistant cells. Mol. Carcinog. 2018;57:1835–1844. doi: 10.1002/mc.22901. [DOI] [PubMed] [Google Scholar]
- 120.Jiang P., Xu C., Chen L., Chen A., Wu X., Zhou M., Haq I.U., Mariyam Z., Feng Q. Epigallocatechin-3-gallate inhibited cancer stem cell–like properties by targeting hsa-mir-485-5p/RXRα in lung cancer. J. Cell. Biochem. 2018;119:8623–8635. doi: 10.1002/jcb.27117. [DOI] [PubMed] [Google Scholar]
- 121.Bhardwaj V., Mandal A.K.A. Next-generation sequencing reveals the role of epigallocatechin-3-gallate in regulating putative novel and known microRNAs which target the MAPK pathway in non-small-cell lung cancer A549 cells. Molecules. 2019;24:368. doi: 10.3390/molecules24020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sacko K., Thangavel K., Shoyele S.A. Codelivery of genistein and miRNA-29b to a549 cells using aptamer-hybrid nanoparticle bioconjugates. Nanomaterials. 2019;9:1052. doi: 10.3390/nano9071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu Y., Xing Y., Zhang Q., Zhang Q., Huang S., Li X., Gao C. Soy isoflavone genistein inhibits hsa_circ_0031250/miR-873-5p/FOXM1 axis to suppress non-small-cell lung cancer progression. IUBMB Life. 2021;73:92–107. doi: 10.1002/iub.2404. [DOI] [PubMed] [Google Scholar]
- 124.Pan J., Cai X., Zheng X., Zhu X., Feng J., Wang X. Luteolin inhibits viability, migration, angiogenesis and invasion of non-small cell lung cancer vascular endothelial cells via miR-133a-3p/purine rich element binding protein B-mediated MAPK and PI3K/Akt signaling pathways. Tissue Cell. 2022;75 doi: 10.1016/j.tice.2022.101740. [DOI] [PubMed] [Google Scholar]
- 125.Barwal T.S., Sharma U., Rana M.K., Bazala S., Singh I., Murmu M., Kapoor H.S., Thakur S., Jain M., Jain A. A diagnostic and prognostic value of blood-based circulating long non-coding RNAs in thyroid, pancreatic and ovarian cancer. Crit. Rev. Oncol. Hematol. 2022;171 doi: 10.1016/j.critrevonc.2022.103598. [DOI] [PubMed] [Google Scholar]
- 126.Zhou J., Gong J., Ding C., Chen G. Quercetin induces the apoptosis of human ovarian carcinoma cells by upregulating the expression of microRNA-145. Mol. Med. Rep. 2015;12:3127–3131. doi: 10.3892/mmr.2015.3679. [DOI] [PubMed] [Google Scholar]
- 127.Xu L., Xiang J., Shen J., Zou X., Zhai S., Yin Y., Li P., Wang X., Sun Q. Oncogenic MicroRNA-27a is a target for genistein in ovarian cancer cells. Anti Cancer Agents Med. Chem. 2013;13:1126–1132. doi: 10.2174/18715206113139990006. [DOI] [PubMed] [Google Scholar]
- 128.Bishayee A., Haskell Y., Do C., Siveen K.S., Mohandas N., Sethi G., Stoner G.D. Potential benefits of edible berries in the management of aerodigestive and gastrointestinal tract cancers: preclinical and clinical evidence. Crit. Rev. Food Sci. Nutr. 2016;56:1753–1775. doi: 10.1080/10408398.2014.982243. [DOI] [PubMed] [Google Scholar]
- 129.Manu K.A., Shanmugam M.K., Li F., Chen L., Siveen K.S., Ahn K.S., Kumar A.P., Sethi G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014;92:267–276. doi: 10.1007/s00109-013-1095-0. [DOI] [PubMed] [Google Scholar]
- 130.Du F., Feng Y., Fang J., Yang M. MicroRNA-143 enhances chemosensitivity of Quercetin through autophagy inhibition via target GABARAPL1 in gastric cancer cells. Biomed. Pharmacother. 2015;74:169–177. doi: 10.1016/j.biopha.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 131.Zhao Y., Chen X., Jiang J., Wan X., Wang Y., Xu P. Epigallocatechin gallate reverses gastric cancer by regulating the long noncoding RNA LINC00511/miR-29b/KDM2A axis. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165856. [DOI] [PubMed] [Google Scholar]
- 132.Lee J.H., Kim C., Baek S.H., Ko J.H., Lee S.G., Yang W.M., Um J.Y., Sethi G., Ahn K.S. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget. 2017;8:17700–17711. doi: 10.18632/oncotarget.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen D., Chou F.J., Chen Y., Huang C.P., Tian H., Wang Y., Niu Y., You B., Yeh S., Xing N., Chang C. Targeting the radiation-induced ARv7-mediated circNHS/miR-512-5p/XRCC5 signaling with Quercetin increases prostate cancer radiosensitivity. J. Exp. Clin. Cancer Res. 2022;41:235. doi: 10.1186/s13046-022-02287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hirata H., Hinoda Y., Shahryari V., Deng G., Tanaka Y., Tabatabai Z.L., Dahiya R. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer. 2014;110:1645–1654. doi: 10.1038/bjc.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiyomaru T., Yamamura S., Fukuhara S., Hidaka H., Majid S., Saini S., Arora S., Deng G., Shahryari V., Chang I., Tanaka Y., Tabatabai Z.L., Enokida H., Seki N., Nakagawa M., Dahiya R. Genistein up-regulates tumor suppressor MicroRNA-574-3p in prostate cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chiyomaru T., Yamamura S., Fukuhara S., Yoshino H., Kinoshita T., Majid S., Saini S., Chang I., Tanaka Y., Enokida H., Seki N., Nakagawa M., Dahiya R. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Majid S., Dar A.A., Saini S., Chen Y., Shahryari V., Liu J., Zaman M.S., Hirata H., Yamamura S., Ueno K., Tanaka Y., Dahiya R. Regulation of minichromosome maintenance gene family by MicroRNA-1296 and genistein in prostate cancer. Cancer Res. 2010;70:2809–2818. doi: 10.1158/0008-5472.CAN-09-4176. [DOI] [PubMed] [Google Scholar]
- 138.Zaman M.S., Chen Y., Deng G., Shahryari V., Suh S.O., Saini S., Majid S., Liu J., Khatri G., Tanaka Y., Dahiya R. The functional significance of microRNA-145 in prostate cancer. Br. J. Cancer. 2010;103:256–264. doi: 10.1038/sj.bjc.6605742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Siddiqui I.A., Asim M., Hafeez B.B., Adhami V.M., Tarapore R.S., Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25:1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jung Y.Y., Um J.Y., Chinnathambi A., Govindasamy C., Narula A.S., Namjoshi O.A., Blough B.E., Sethi G., Ahn K.S. Withanolide modulates the potential crosstalk between apoptosis and autophagy in different colorectal cancer cell lines. Eur. J. Pharmacol. 2022;928 doi: 10.1016/j.ejphar.2022.175113. [DOI] [PubMed] [Google Scholar]
- 141.Del Follo-Martinez A., Banerjee N., Li X., Safe S., Mertens-Talcott S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr. Cancer. 2013;65:494–504. doi: 10.1080/01635581.2012.725194. [DOI] [PubMed] [Google Scholar]
- 142.Chen X., Gu J., Wu Y., Liang P., Shen M., Xi J., Qin J. Clinical characteristics of colorectal cancer patients and anti-neoplasm activity of genistein. Biomed. Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109835. [DOI] [PubMed] [Google Scholar]
- 143.Toden S., Tran H.M., Tovar-Camargo O.A., Okugawa Y., Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. 2016;7:16158–16171. doi: 10.18632/oncotarget.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rana V., Parama D., Khatoon E., Girisa S., Sethi G., Kunnumakkara A.B. Reiterating the emergence of noncoding rnas as regulators of the critical hallmarks of gall bladder cancer. Biomolecules. 2021;11:1847. doi: 10.3390/biom11121847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yi J., Li S., Wang C., Cao N., Qu H., Cheng C., Wang Z., Wang L., Zhou L. Potential applications of polyphenols on main ncRNAs regulations as novel therapeutic strategy for cancer. Biomed. Pharmacother. 2019;113 doi: 10.1016/j.biopha.2019.108703. [DOI] [PubMed] [Google Scholar]
- 146.Jiang P., Wu X., Wang X., Huang W., Feng Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget. 2016;7:43337–43351. doi: 10.18632/oncotarget.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.La X., Zhang L., Li Z., Li H., Yang Y. (-)-Epigallocatechin gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway. J. Agric. Food Chem. 2019;67:2510–2518. doi: 10.1021/acs.jafc.8b06665. [DOI] [PubMed] [Google Scholar]
- 148.Wang P., Henning S.M., Magyar C.E., Elshimali Y., Heber D., Vadgama J.V. Green tea and quercetin sensitize PC-3 xenograft prostate tumors to docetaxel chemotherapy. J. Exp. Clin. Cancer Res. 2016;35 doi: 10.1186/s13046-016-0351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang X., Guo Q., Chen J., Chen Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS axis. Mol. Cells. 2015;38–38:638–642. doi: 10.14348/molcells.2015.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roy S., Yu Y., Padhye S.B., Sarkar F.H., Majumdar A.P.N. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grosser R., Cherkassky L., Chintala N., Adusumilli P.S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36:471–482. doi: 10.1016/J.CCELL.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liskova A., Samec M., Koklesova L., Brockmueller A., Zhai K., Abdellatif B., Siddiqui M., Biringer K., Kudela E., Pec M., Gadanec L.K., Šudomová M., Hassan S.T.S., Zulli A., Shakibaei M., Giordano F.A., Büsselberg D., Golubnitschaja O., Kubatka P. Flavonoids as an effective sensitizer for anti-cancer therapy: insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021;12:155–176. doi: 10.1007/s13167-021-00242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kumar A.R., Devan A.R., Nair B., Nath L.R. Anti-VEGF mediated immunomodulatory role of phytochemicals: scientific exposition for plausible HCC treatment. Curr. Drug Targets. 2021;22:1288–1316. doi: 10.2174/1389450122666210203194036. [DOI] [PubMed] [Google Scholar]
- 154.Ward A.B., Mir H., Kapur N., Gales D.N., Carriere P.P., Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018;16:108. doi: 10.1186/s12957-018-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khorsandi L., Orazizadeh M., Niazvand F., Abbaspour M.R., Mansouri E., Khodadadi A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratislava Med. J. 2017;118:123–128. doi: 10.4149/BLL_2017_025. [DOI] [PubMed] [Google Scholar]
- 156.Guo H., Ding H., Tang X., Liang M., Li S., Zhang J., Cao J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer. 2021;12:1415–1422. doi: 10.1111/1759-7714.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Adami B.S., Diz F.M., Oliveira Gonçalves G.P., Reghelin C.K., Scherer M., Dutra A.P., Papaléo R.M., de Oliveira J.R., Morrone F.B., Wieck A., Xavier L.L. Morphological and mechanical changes induced by quercetin in human T24 bladder cancer cells. Micron. 2021;151 doi: 10.1016/j.micron.2021.103152. [DOI] [PubMed] [Google Scholar]
- 158.Wang Z.X., Ma J., Li X.Y., Wu Y., Shi H., Chen Y., Lu G., Shen H.M., Lu G.D., Zhou J. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 2021;178:1133–1148. doi: 10.1111/bph.15350. [DOI] [PubMed] [Google Scholar]
- 159.Lee H.S., Park B.S., Kang H.M., Kim J.H., Shin S.H., Kim I.R. Role of luteolin-induced apoptosis and autophagy in human glioblastoma cell lines. Med. 2021;57:879. doi: 10.3390/medicina57090879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Q., Wang H., Jia Y., Pan H., Ding H. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother. Pharmacol. 2017;79:1031–1041. doi: 10.1007/s00280-017-3299-4. [DOI] [PubMed] [Google Scholar]
- 161.Yoo H.S., Won S.B., Kwon Y.H. Luteolin induces apoptosis and autophagy in HCT116 colon cancer cells via p53-dependent pathway. Nutr. Cancer. 2022;74:677–686. doi: 10.1080/01635581.2021.1903947. [DOI] [PubMed] [Google Scholar]
- 162.Wu H.T., Liu Y.E., Hsu K.W., Wang Y.F., Chan Y.C., Chen Y., Chen D.R. MLL3 induced by luteolin causes apoptosis in tamoxifen-resistant breast cancer cells through H3K4 monomethylation and suppression of the PI3K/AKT/mTOR pathway. Am. J. Chin. Med. 2020;48:1221–1241. doi: 10.1142/S0192415X20500603. [DOI] [PubMed] [Google Scholar]
- 163.Lee Y., Kwon Y.H. Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells. Biochem. Biophys. Res. Commun. 2019;517:617–622. doi: 10.1016/j.bbrc.2019.07.073. [DOI] [PubMed] [Google Scholar]
- 164.Zhang X., Liu J., Zhang P., Dai L., Wu Z., Wang L., Cao M., Jiang J. Silibinin induces G1 arrest, apoptosis and JNK/SAPK upregulation in SW1990 human pancreatic cancer cells. Oncol. Lett. 2018;15:9868–9876. doi: 10.3892/ol.2018.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu W., Ji Y., Sun Y., Si L., Fu J., Hayashi T., Onodera S., Ikejima T. Estrogen receptors participate in silibinin-caused nuclear translocation of apoptosis-inducing factor in human breast cancer MCF-7 cells. Arch. Biochem. Biophys. 2020;689 doi: 10.1016/j.abb.2020.108458. [DOI] [PubMed] [Google Scholar]
- 166.Tehrani F.K., Ranji N., Kouhkan F., Hosseinzadeh S. Apoptosis induction and proliferation inhibition by silibinin encapsulated in nanoparticles in MIA PaCa-2 cancer cells and deregulation of some miRNAs. Iran. J. Basic Med. Sci. 2020;23:469–482. doi: 10.22038/ijbms.2020.39427.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma Z., Liu W., Zeng J., Eng Zho U J., Guo P., Xie H., Yang Z., Zheng L., Xu S., Wang X., Chang L.S., He D., Li L. Silibinin induces apoptosis through inhibition of the mTOR-GLI1-BCL2 pathway in renal cell carcinoma. Oncol. Rep. 2015;34:2461–2468. doi: 10.3892/or.2015.4224. [DOI] [PubMed] [Google Scholar]
- 168.Yu Y., fang Li L., Tao J., mian Zhou X., Xu C. Silibinin induced apoptosis of human epidermal cancer A431 cells by promoting mitochondrial NOS. Free Radic. Res. 2019;53:714–726. doi: 10.1080/10715762.2019.1603376. [DOI] [PubMed] [Google Scholar]
- 169.Luo Y., xiang Wang S., quan Zhou Z., Wang Z., gao Zhang Y., Zhang Y., Zhao P. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-κB) pathway. Tumor Biol. 2014;35:11483–11488. doi: 10.1007/s13277-014-2487-7. [DOI] [PubMed] [Google Scholar]
- 170.Choi E.J., Kim T., Lee M.S. Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci. 2007;80:1403–1408. doi: 10.1016/j.lfs.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 171.Sanaei M., Kavoosi F., Roustazadeh A., Golestan F. Effect of genistein in comparison with trichostatin a on reactivation of dnmts genes in hepatocellular carcinoma. J. Clin. Transl. Hepatol. 2018;6:141–146. doi: 10.14218/JCTH.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim S.H., Kim S.H., Lee S.C., Song Y.S. Involvement of both extrinsic and intrinsic apoptotic pathways in apoptosis induced by genistein in human cervical cancer cells. Ann. N. Y. Acad. Sci. 2009:196–201. doi: 10.1111/j.1749-6632.2009.04902.x. Ann. N. Y. Acad. Sci. [DOI] [PubMed] [Google Scholar]
- 173.Zhang L., Ma X., Dong Y. Effect of genistein on apoptosis of lung adenocarcinoma A549 cells and expression of apoptosis factors. J. BUON. 2018;23:641–646. https://pubmed.ncbi.nlm.nih.gov/30003731/ accessed November 15, 2022. [PubMed] [Google Scholar]
- 174.Della Via F.I., Shiraishi R.N., Santos I., Ferro K.P., Salazar-Terreros M.J., Franchi Junior G.C., Rego E.M., Saad S.T.O., Torello C.O. (–)-Epigallocatechin-3-gallate induces apoptosis and differentiation in leukaemia by targeting reactive oxygen species and PIN1. Sci. Rep. 2021;11:9103. doi: 10.1038/s41598-021-88478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yin Z., Li J., Kang L., Liu X., Luo J., Zhang L., Li Y., Cai J. Epigallocatechin-3-gallate induces autophagy-related apoptosis associated with LC3B II and Beclin expression of bladder cancer cells. J. Food Biochem. 2021;45:e13758. doi: 10.1111/jfbc.13758. [DOI] [PubMed] [Google Scholar]
- 176.Liu L., Ju Y., Wang J., Zhou R. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol. Res. Pract. 2017;213:1242–1250. doi: 10.1016/j.prp.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 177.Liu S., Xu Z.L., Sun L., Liu Y., Li C.C., Li H.M., Zhang W., Li C.J., Qin W. (-)-Epigallocatechin-3-gallate induces apoptosis in human pancreatic cancer cells via PTEN. Mol. Med. Rep. 2016;14:599–605. doi: 10.3892/mmr.2016.5277. [DOI] [PubMed] [Google Scholar]
- 178.Du B.X., Lin P., Lin J. EGCG and ECG induce apoptosis and decrease autophagy via the AMPK/mTOR and PI3K/AKT/mTOR pathway in human melanoma cells. Chin. J. Nat. Med. 2022;20:290–300. doi: 10.1016/S1875-5364(22)60166-3. [DOI] [PubMed] [Google Scholar]
- 179.Lee D.Y., Yun S.M., Song M.Y., Jung K., Kim E.H. Cyanidin chloride induces apoptosis by inhibiting nf-κb signaling through activation of nrf2 in colorectal cancer cells. Antioxidants. 2020;9:285. doi: 10.3390/antiox9040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Atashi H.A., Arani H.Z., Shekarriz A., Nazari H., Zabolian A., Rakhshan R., Olya M. Cyanidin 3-O-glucoside induces the apoptosis in the osteosarcoma cells through upregulation of the PPARγ and P21: an in vitro study. Anti Cancer Agents Med. Chem. 2020;20:1087–1093. doi: 10.2174/1871520620666200408081111. [DOI] [PubMed] [Google Scholar]
- 181.Sorrenti V., Vanella L., Acquaviva R., Cardile V., Giofrè S., Di Giacomo C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015;47:1303–1310. doi: 10.3892/ijo.2015.3130. [DOI] [PubMed] [Google Scholar]
- 182.Li X., Zhao J., Yan T., Mu J., Lin Y., Chen J., Deng H., Meng X. Cyanidin-3-O-glucoside and cisplatin inhibit proliferation and downregulate the PI3K/AKT/mTOR pathway in cervical cancer cells. J. Food Sci. 2021;86:2700–2712. doi: 10.1111/1750-3841.15740. [DOI] [PubMed] [Google Scholar]
- 183.Yang F.Q., Liu M., Li W., Che J.P., Wang G.C., Zheng J.H. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA-21. Mol. Med. Rep. 2015;11:1085–1092. doi: 10.3892/mmr.2014.2813. [DOI] [PubMed] [Google Scholar]
- 184.Wang P., Phan T., Gordon D., Chung S., Henning S.M., Vadgama J.V. Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells. Mol. Nutr. Food Res. 2015;59:250–261. doi: 10.1002/mnfr.201400558. [DOI] [PMC free article] [PubMed] [Google Scholar]