Alzheimer’s disease (AD) is the main neurodegenerative disease leading to dementia and cognitive impairment in the elderly. Considering AD to be an epidemic, an increase from the current 50 million to more than 150 million patients is expected by the year 2050. AD is characterized by a slow, progressive and asymptomatic onset; making it difficult to decipher the precise etiology. It is well established that AD presents two characteristic features, extracellular β-amyloid plaques and intracellular tau tangles, that eventually lead to the impairment of cognitive functions. Unfortunately, AD symptomatology shares many similarities with other dementias once is present, which makes it difficult an accurate premortem diagnosis.
Although AD is mainly considered an aging-related condition that affects cognitive function, several cardio- and cerebrovascular comorbidities such as hypertension or diabetes are also risk factors for cognitive impairment. Accordingly, brain vascular-associated alterations underlie many pathophysiological mechanisms of AD. We have recently reviewed the latest evidence supporting the detrimental role of vascular and angiogenic alterations during AD (Custodia et al., 2022). Remarkably, cerebral blood-brain barrier (BBB) leakage and microbleeds are associated with cognitive decline in patients with mild cognitive impairment (MCI) and early AD. Accordingly, the two-hit vascular hypothesis points at initial damage in cerebral vasculature (hit one) that eventually induces the accumulation of β-amyloid (Aβ) in the brain (hit two; Zlokovic, 2005).
CD34+ bone marrow-derived progenitor cells (BMPCs) define a group of stem and progenitor cell populations released by the bone marrow that covers different subpopulations of cells from the hematopoietic linage, including endothelial progenitor cells (EPCs). EPCs exhibit characteristics of both endothelial and stem cells, and, accordingly, proangiogenic early EPCs expressing both CD34 and CD133 (a progenitor surface marker) can be distinguished from late EPCs additionally expressing KDR and/or CD146 (endothelial markers), which participate in the process of angiogenesis and vasculogenesis (Figure 1). Therefore, EPCs participate in angiogenesis and the maintenance of the endothelium by acting as a cellular reservoir for the replacement of dysfunctional endothelial cells, or by the secretion of angiogenic growth factors.
Figure 1.
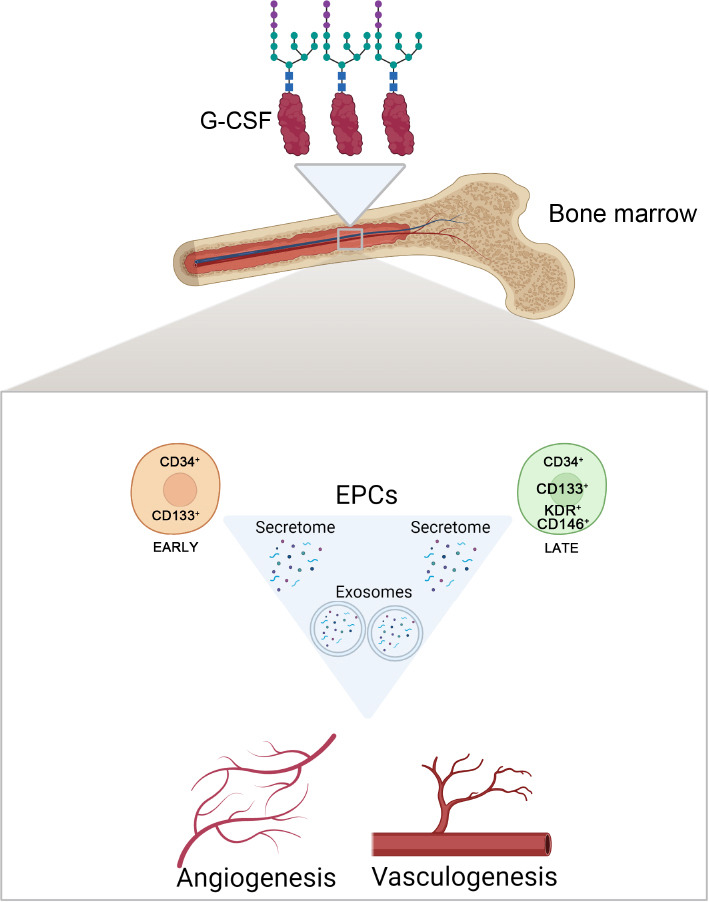
Beneficial roles of CD34+ BMPCs following CNS injury.
CD34+ BMPCs and the EPCs subtypes, early and late, can promote both angiogenesis and vasculogenesis following CNS injury by specializing in endothelial cells, and/or indirectly by secreting free and exosome-enveloped growth factors. G-CSF is a glycoprotein that acts in the bone marrow to mobilize both EPCs and CD34+ BMPCs after damage. BMPCs: Bone marrow progenitor cells; CNS: central nervous system; EPCs: endothelial progenitor cells; G-CSF: granulocyte colony-stimulating factor. Created with BioRender.com.
Given that both dysfunctional angiogenesis and compromised BBB integrity seem critical in the onset and/or progression of AD, CD34+ progenitor cells, primarily EPCs, appear as potential targets for the early diagnosis and/or treatment of the disease. In this way, early and late EPCs would work synergistically: early EPCs reach the site of injury due to the high concentration of angiogenic factors and inflammatory cytokines, from which they paracrinally release different factors promoting angiogenesis and recruiting late EPCs, which either restore the endothelium or form new vessels guided by the early EPCs. Here, we discuss recent work and ongoing human clinical trials testing the feasibility of CD34+ BMPCs and EPCs as early biomarkers of AD and pharmacological targets for future treatments.
Association of circulating levels of CD34+ BMPCs and cognitive decline in healthy and MCI subjects: Several cross-sectional studies have shown that the number of circulating CD34+ BMPCs decreases with age, and this may impact cognition. In this regard, a longitudinal study regarding cognition and CD34+ BMPCs levels reported that older healthy subjects had lower levels of CD34+ BMPCs than younger counterparts at baseline measurements (Hajjar et al., 2016). Moreover, this investigation revealed that subjects with higher baseline levels of several subgroups of CD34+ BMPCs such as early and late EPCs, and CD34+/KDR+ cells, among others, had better executive-derived and working memory scores over 4 years of follow-up (Hajjar et al., 2016). Recently, a large transverse study has shown the association between CD34+ BMPCs and different memory-related tests in cognitively normal subjects with coronary artery disease (Moazzami et al., 2020). Notably, circulating numbers of late EPCs were positively correlated with a better performance in tasks assessing visual, logical, and verbal immediate/delayed memory. Therefore, the amount of circulating CD34+ BMPCs subtypes appears to be negatively correlated to the cognitive decline of both healthy subjects and patients with vascular-associated conditions. Although more longitudinal clinical studies are needed to fully confirm the harmful effect of low levels of CD34+ BMPCs on the cognitive state, and other factors may be also taking part in this cognitive decline, it is still plausible that larger amounts of circulating endothelial progenitors exert a protective effect, probably by the maintenance of vascular endothelium integrity.
MCI often precedes clinical symptoms of AD, and MCI patients show an increased risk of developing dementia in the future. Thus, it is very interesting to study CD34+ BMPCs/EPCs levels in patients with MCI in order to test whether such levels can be used as potential non-invasive diagnostic biomarkers to detect cognitive decline or its progression from MCI to dementia.
Some studies have observed a decrease in CD34+ BMPCs and EPCs populations from MCI patients (Nation et al., 2018; Callahan et al., 2020). In this sense, MCI patients with lower levels of circulating CD34+ BMPCs and both subtypes of EPCs exhibited worse scores in memory tests and reduced cortical thickness compared to control subjects (Nation et al., 2018). Considering the angiogenesis ratio (pro-angiogenic/non-angiogenic BMPCs, including early and late EPCs), Callahan et al. (2020) showed a positive association between angiogenesis ratio and white matter hyperintensities, but not with global cerebral blood flow, hippocampal volume, or accumulation of tau and Aβ. By contrast, measurements in an older cohort of MCI patients did not show significant changes in CD34+, early EPCs, and late EPCs circulating levels compared to control subjects (Breining et al., 2016). This discrepancy may highlight that aging decreases CD34+ BMPCs to such a reduced level that is no longer different in controls compared to MCI.
In summary, it seems that the reduction in CD34+ BMPCs is directly related to vascular dysfunction, increasing brain white matter microlesions and impairing cognition in MCI patients.
Association of circulating levels of CD34+ BMPCs and AD: Several studies have been performed in order to determine the relationship between CD34+ BMPCs/EPCs circulating levels and the progression of AD (Maler et al., 2006; Lee et al., 2009; Stellos et al., 2010; Bigalke et al., 2011; Kong et al., 2011; Breining et al., 2016; Callahan et al., 2020; Haiyuan et al., 2020). In this way, AD patients in the early symptomatic phase already showed lower levels of CD34+ and CD34+/KDR+ cells compared to their control counterparts (Maler et al., 2006; Haiyuan et al., 2020). Notably, CD34+ BMPCs counts have negatively correlated with levels of Aβ1–42 in cerebrospinal fluid and the Aβ ratio 42/40, two well-known biomarkers for AD, as well as with age, only in the early AD group (Maler et al., 2006). Furthermore, the homing capacity of EPCs from early AD patients was already impaired (Haiyuan et al., 2020). Overall, it is becoming clear that dysfunctional CD34+ BMPCs are related to a reduced ability to repair brain endothelial cells, which appears to mediate neurotoxicity by affecting the BBB permeability.
On the other hand, different outcomes were described in studies assessing the number of progenitor cells during AD progression. Specifically, lower counts of CD34+ BMPCs and EPCs have been observed in moderate and severe AD patients compared to both early AD stage (Haiyuan et al., 2020) and control subjects (Lee et al., 2009; Kong et al., 2011; Haiyuan et al., 2020). Such studies also revealed that homing and adhesion features of EPCs from AD patients were impaired (Haiyuan et al., 2020), as well as EPCs levels were inversely correlated with the mini-mental state exam (MMSE) score (Lee et al., 2009; Stellos et al., 2010; Kong et al., 2011). Furthermore, moderate to severe AD patients displayed a reduced flow velocity of the middle cerebral artery (Kong et al., 2011). In contrast, other studies reported higher levels of CD34+ BMPCs and EPCs compared to controls (Stellos et al., 2010; Bigalke et al., 2011), or even no changes (Breining et al., 2016). Intriguingly, the work from Stellos and colleagues reported an increase in both CD34+ BMPCs and early EPCs counting when comparing moderate to severe AD patients versus control subjects; however, within the AD group, there was an inverse correlation between CD34+ BMPCs and early EPCs counting and the MMSE score. Although these results seem contrary to each other, it is noteworthy that most AD patients from this study (Stellos et al., 2010) were treated with cholinesterase inhibitor; a drug involved in EPCs proliferation. Therefore, this fact may bias the results and it could explain why cell counting in the AD group was higher than in controls, but they were inversely correlated with MMSE scores. The other study that showed increased levels of CD34+ BMPCs/EPCs (Bigalke et al., 2011) only measured the numbers of CD34+ BMPCs in early to moderate AD compared to controls, with no information regarding cholinesterase inhibitor treatment.
In summary, most of the studies in later AD stages support the studies performed on MCI and early AD stages. Therefore, AD-mediated loss of CD34+ BMPCs/EPCs, as well as loss of EPCs-intrinsic features, are likely present in AD patients and may constitute novel diagnostic and therapeutic targets.
Potential therapy with granulocyte colony-stimulating factor (G-CSF) in AD: The G-CSF is a glycoprotein secreted by endothelial and immune cells that acts as a hematopoietic growth factor (Figure 1). Among other beneficial mechanisms following vascular injury, the G-CSF can promote angiogenesis by mobilizing EPCs (Figure 1). Therefore, G-CSF may be a potential target to enhance vascular repair in AD patients. Indeed, it has been recently shown that a G-CSF treatment improved memory as well as reduced blood levels of amyloid and tau in mild to moderate patients of AD (Potter et al., 2021). Based on these achievements, it is currently conducting a phase2b clinical trial in order to evaluate the long-term treatment of G-CSF in AD patients (NCT04902703; ClinicalTrials.gov).
It would be interesting to look at CD34+ BMPCs and EPCs levels from those clinical trials in order to elucidate whether such potential benefits promoted by G-CSF therapy are totally or partially mediated by increasing CD34+ BMPCs/EPCs mobilization.
Future challenges: The body of evidence supporting a vascular component underlying AD onset and/or progression is growing. However, further studies are mandatory to elucidate whether such vascular component triggers AD, is a consequence of AD, or both. Moreover, longitudinal studies are needed to confirm the relationship between CD34+ BMPCs/EPCs levels and AD progression. Given that vascular-related diseases may influence the amount of circulating progenitor cells, especially in AD patients, comorbidities present in those subjects deserve special attention when interpreting the results. Likewise, pharmacological treatments, such as a cholinesterase inhibitor, may bias the results from studies giving uncorrected information.
Despite the promising results in animal models of AD, the number of published results and clinical trials regarding the direct application of EPCs as a potential therapy in AD patients is absent. This is remarkable when there is compelling evidence that supports the role of endothelial dysfunction in the onset and progression of AD, and the potential of EPCs as a diagnostic biomarker and/or therapeutic target (Custodia et al., 2022). However, we were unable to find published data or ongoing clinical trials in humans using the application of EPCs to treat AD; as already seen in a stroke clinical trial (NCT01468064). Moreover, several recent studies have highlighted the beneficial role of EPCs secretome/exosomes by protecting and repairing the BBB following damage without using a cell-based therapy. So, clinical trials based on EPCs-derived secretome/exosomes might be a safer and more promising approach in AD research.
Finally, only the GCSF-based treatment is being tested in AD patients at later stages, with modest but promising results. Given that endothelium-related impairments are already seen in MCI patients, it would be really interesting to test this GCSF-based treatment in those subjects in order to increase the benefits and protect against the progression to AD.
This work was partially supported by grants from the Xunta de Galicia (IN607A2018/3 to TS, IN607D 2020/09 to TS, IN606A-2021/015 to AC; IN606B-2021/010 to DRS), and Science Ministry of Spain (RTI2018-102165-B-I00 to TS, RTC2019-007373-1 to TS). Furthermore, this work was also supported by grants from the INTERREG Atlantic Area (EAPA_791/2018_ NEUROATLANTIC project to TS), INTER-REG V A España Portugal (POCTEP) (0624_2IQBIONEURO_6_E to TS), and the European Regional Development Fund (ERDF). Moreover, DRS (CD21/00166) and TS (CPII17/00027) are recipients of research contracts from the Sara Borrell and Miguel Servet Programs, respectively, from the Instituto de Salud Carlos III.
Additional file: Open peer review reports 1 (96.1KB, pdf) and 2 (96KB, pdf) .
Footnotes
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewers: Yali Jia, Beijing Institute of Radiation Medicine, China; Rongcan Luo, Kunming Institute of Zoology Chinese Academy of Sciences, China.
P-Reviewers: Jia Y, Luo R; C-Editors: Zhao M, Liu WJ, Wang Lu; T-Editor: Jia Y
References
- 1.Bigalke B, Schreitmüller B, Sopova K, Paul A, Stransky E, Gawaz M, Stellos K, Laske C. Adipocytokines and CD34 progenitor cells in Alzheimer's disease. PLoS One. 2011;6:e20286. doi: 10.1371/journal.pone.0020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breining A, Silvestre JS, Dieudonné B, Vilar J, Faucounau V, Verny M, Néri C, Boulanger CM, Boddaert J. Biomarkers of vascular dysfunction and cognitive decline in patients with Alzheimer's disease:no evidence for association in elderly subjects. Aging Clin Exp Res. 2016;28:1133–1141. doi: 10.1007/s40520-016-0535-4. [DOI] [PubMed] [Google Scholar]
- 3.Callahan CM, Apostolova LG, Gao S, Risacher SL, Case J, Saykin AJ, Lane KA, Swinford CG, Yoder MC. Novel markers of angiogenesis in the setting of cognitive impairment and dementia. J Alzheimers Dis. 2020;75:959–969. doi: 10.3233/JAD-191293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Custodia A, Ouro A, Romaus-Sanjurjo D, Pías-Peleteiro JM, de Vries HE, Castillo J, Sobrino T. Endothelial progenitor cells and vascular alterations in Alzheimer's disease. Front Aging Neurosci. 2022;13:811210. doi: 10.3389/fnagi.2021.811210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haiyuan L, Xue X, Min L, Lingyu W, Xianlin G, Hancong S, Qiulei C, Jia X. Study of quantity and function of endothelial progenitor cells in peripheral blood of patients with Alzheimer's disease. J New Med. 2020;51:590. [Google Scholar]
- 6.Hajjar I, Goldstein FC, Waller EK, Moss LD, Quyyumi A. Circulating progenitor cells is linked to cognitive decline in healthy adults. Am J Med Sci. 2016;351:147–152. doi: 10.1016/j.amjms.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong XD, Zhang Y, Liu L, Sun N, Zhang MY, Zhang JN. Endothelial progenitor cells with Alzheimer's disease. Chin Med J (Engl) 2011;124:901–906. [PubMed] [Google Scholar]
- 8.Lee ST, Chu K, Jung KH, Park HK, Kim DH, Bahn JJ, Kim JH, Oh MJ, Lee SK, Kim M, Roh JK. Reduced circulating angiogenic cells in Alzheimer disease. Neurology. 2009;72:1858–1863. doi: 10.1212/WNL.0b013e3181a711f4. [DOI] [PubMed] [Google Scholar]
- 9.Maler JM, Spitzer P, Lewczuk P, Kornhuber J, Herrmann M, Wiltfang J. Decreased circulating CD34+stem cells in early Alzheimer's disease:Evidence for a deficient hematopoietic brain support? Mol Psychiatry. 2006;11:1113–1115. doi: 10.1038/sj.mp.4001913. [DOI] [PubMed] [Google Scholar]
- 10.Moazzami K, Wittbrodt MT, Lima BB, Kim JH, Hammadah M, Ko YA, Obideen M, Abdelhadi N, Kaseer B, Gafeer MM, Nye JA, Shah AJ, Ward L, Raggi P, Waller EK, Bremner JD, Quyyumi AA, Vaccarino V. Circulating progenitor cells and cognitive impairment in men and women with coronary artery disease. J Alzheimers Dis. 2020;74:659–668. doi: 10.3233/JAD-191063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nation DA, Tan A, Dutt S, McIntosh EC, Yew B, Ho JK, Blanken AE, Jang JY, Rodgers KE, Gaubert A. Circulating progenitor cells correlate with memory, posterior cortical thickness, and hippocampal perfusion. J Alzheimers Dis. 2018;61:91–101. doi: 10.3233/JAD-170587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potter H, Woodcock JH, Boyd TD, Coughlan CM, O'Shaughnessy JR, Borges MT, Thaker AA, Raj BA, Adamszuk K, Scott D, Adame V, Anton P, Chial HJ, Gray H, Daniels J, Stocker ME, Sillau SH. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer's disease. Alzheimers Dement. 2021;7:e12158. doi: 10.1002/trc2.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stellos K, Panagiota V, Sachsenmaier S, Trunk T, Straten G, Leyhe T, Seizer P, Geisler T, Gawaz M, Laske C. Increased circulating progenitor cells in Alzheimer's disease patients with moderate to severe dementia:evidence for vascular repair and tissue regeneration?J Alzheimers Dis. 2010;19:591–600. doi: 10.3233/JAD-2010-1261. [DOI] [PubMed] [Google Scholar]
- 14.Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


