Keywords: mitochondria, mitochondrial protein import, mitochondrial quality control, secondary mitochondrial disease
Abstract
Mitochondrial diseases are a broad, genetically heterogeneous class of metabolic disorders characterized by deficits in oxidative phosphorylation (OXPHOS). Primary mitochondrial disease (PMD) defines pathologies resulting from mutation of mitochondrial DNA (mtDNA) or nuclear genes affecting either mtDNA expression or the biogenesis and function of the respiratory chain. Secondary mitochondrial disease (SMD) arises due to mutation of nuclear-encoded genes independent of, or indirectly influencing OXPHOS assembly and operation. Despite instances of novel SMD increasing year-on-year, PMD is much more widely discussed in the literature. Indeed, since the implementation of next generation sequencing (NGS) techniques in 2010, many novel mitochondrial disease genes have been identified, approximately half of which are linked to SMD. This review will consolidate existing knowledge of SMDs and outline discrete categories within which to better understand the diversity of SMD phenotypes. By providing context to the biochemical and molecular pathways perturbed in SMD, we hope to further demonstrate the intricacies of SMD pathologies outside of their indirect contribution to mitochondrial energy generation.
1. Introduction
Modern-day mitochondria arose from an ancient symbiotic union between a primitive eukaryotic precursor cell and an α-proteobacterium [1]. Accordingly, mitochondria possess a double membrane architecture and are divided into four sub-compartments: the outer membrane (OM), intermembrane space (IMS), inner membrane (IM) and the matrix. Across billions of years of coevolution, endosymbiotic gene transfer has significantly depleted the size of the existing mitochondrial genome, and only a fraction of mitochondrial proteins are believed to have proteobacterial origin [2]. An extensive amount of proteomic rewiring has occurred to accommodate the energetic and metabolic requirements of modern eukaryotic organisms. As it exists today, the human mitochondrial genome (mtDNA) encodes only 13 proteins, 22 transfer RNAs and 2 mitochondrial ribosomal RNAs [3]. All mtDNA-encoded proteins are core components of oxidative phosphorylation (OXPHOS) Complexes I, III, IV or V. Complexes I, III and IV work to establish an electrochemical gradient across the IM, which fuels ATP generation through the F1F0 ATP synthase (Complex V), drives import and sorting of nuclear-encoded mitochondrial proteins, and powers metabolite exchange across inner membrane-embedded carrier proteins [3].
In addition to having a pivotal role in energy generation, mitochondria are critical in numerous cellular processes, including amino acid metabolism, iron-sulfur cluster biogenesis, intrinsic cell death and intraorganellar signalling [4]. Consequently, mitochondrial dysfunction often propagates and/or underlies many disease states, including cancer, neurodegenerative disease and diabetes [5–9], as well as the process of ageing [10,11]. Mitochondrial dysfunction also results in mitochondrial diseases, a group of heterogenous, often rare metabolic disorders characterized by defective cellular energy generation. Of the estimated 1136 total mtDNA and nuclear DNA (nDNA) encoded mitochondrial genes [12], over 330 have been linked to mitochondrial disease onset [13–16]. Mitochondrial diseases can be categorized into two broad categories: primary mitochondrial disease (PMD) and secondary mitochondrial disease (SMD). PMDs arise from inherited variants in mtDNA genes or nuclear genes that are directly linked to OXPHOS function [13]. Currently, pathogenic variants linked to PMD have been identified in 36 of the 37 mtDNA genes and over 150 nDNA-encoded genes [14,17]. Some of these genes encode OXPHOS subunits themselves, while others have supportive roles as assembly factors, cofactors or electron carriers [13]. In addition, genes responsible for mtDNA maintenance, mtRNA expression/translation and biogenesis of the mitoribosome can also be classified as PMDs [13]. SMD defines disease states in which the causative mutation indirectly impairs OXPHOS function via other crucial mitochondrial pathways [13,17]. This can include perturbations in protein biogenesis pathways, mitochondrial morphology, lipid biogenesis and the TCA cycle, among others [13]. Although individual mitochondrial diseases are rare, mitochondrial diseases overall are prevalent and represent the most common class of inborn errors of metabolism, with an estimated minimum birth prevalence of 1/5000 [18,19]. Despite marked improvements in the diagnosis of mitochondrial disease, development of effective treatments has lagged significantly. This in part is due to challenges imposed by the diversity of the observed symptoms, but also a lack of information on the molecular mechanisms underscoring individual disease states.
PMDs have been extensively reviewed in the literature [13,14,20–24]. In this review, we will explore the biology underscoring mitochondrial dysfunction in SMD. We will emphasize the growing significance of SMD in diagnostic settings and how expanding our knowledge of fundamental mitochondrial biology is crucial to driving understanding of disease pathomechanisms.
2. Secondary mitochondrial disease and associated genes
Prior to the advent of next-generation sequencing (NGS) technologies in 2010, identification of novel disease-causing mutations was reliant upon candidate gene sequencing and linkage studies [13]. These techniques enabled the successful identification of 32 mtDNA genes and 94 nuclear-encoded genes associated with mitochondrial disease over 22 years, the majority associated with PMD [13]. Since the implementation of NGS, over 150 additional genes linked to mitochondrial disease have been identified, greatly exceeding the output of earlier techniques in roughly half the time [14,24,25]. The proportion of genes associated with SMD has steadily increased since the introduction of NGS techniques [13], demonstrating the diversity of mitochondrial functions critical to cellular homeostasis and viability. Such functions include metabolic regulation, mitochondrial homeostasis, protein quality control and maturation, and broader mitochondrial morphology, among others. The list of SMD genes in figure 1 indirectly impact OXPHOS and probably involve additional cellular/mitochondrial functions. These genes have been compiled with some stringency from available literature [13,20], and include several additional recently described novel disease genes. It is important to highlight that numerous additional disease genes with links to mitochondrial dysfunction have been flagged [20] but are not included here, as their impact on OXPHOS or role in mitochondrial function is less clear.
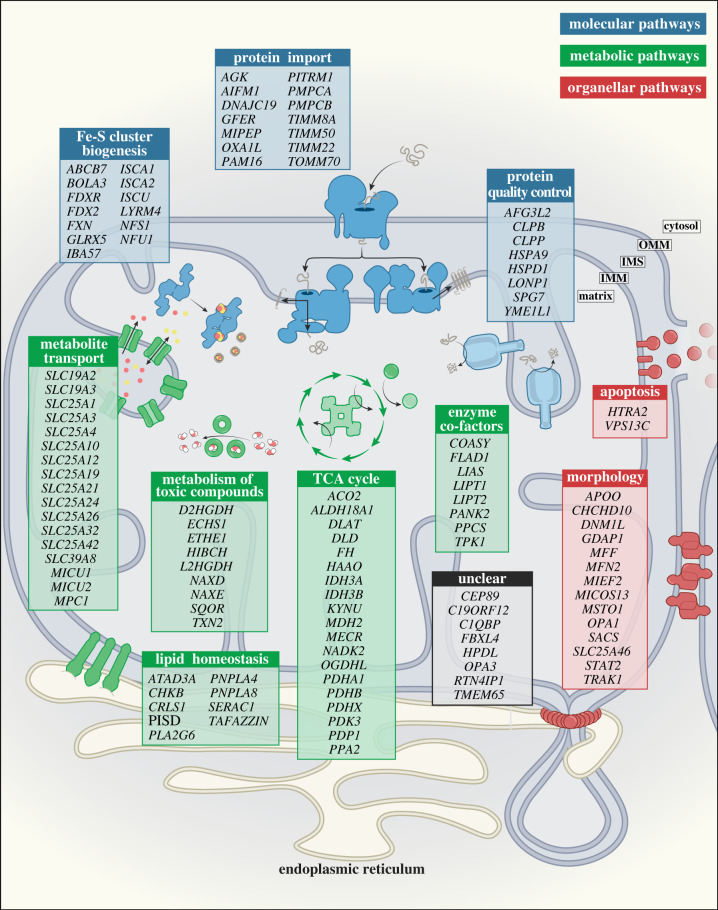
Figure 1.
Categorical organization of mitochondrial genes associated with secondary mitochondrial disease (SMD). Genes with deleterious mutations impacting mitochondrial functions secondary to OXPHOS have been broadly classified into three main categories: (1) Molecular pathways related to protein biogenesis, including protein import, protein quality control and Fe-S cluster biogenesis (indicated in blue); (2) Metabolic pathways involving metabolite transport, metabolism of toxic compounds, enzymatic cofactors, TCA cycle metabolism and lipid homeostasis (indicated in green); and (3) Organellar pathways linked to mitochondrial health, including mitochondrial morphology and apoptosis (indicated in red). Genes linked to SMD with currently unclear functions are listed in the Unclear category (indicated in grey). OMM, outer mitochondrial membrane; IMS, intermembrane space; IMM, inner mitochondrial membrane.
This is an expansive topic with many diseases, hence for the purpose of this review, we will broadly classify mitochondrial functions linked to SMD into three (overlapping) categories shown in figure 1.
-
1.
Molecular pathways related to protein biogenesis, including protein import/processing, protein quality control and Fe-S cluster biogenesis.
-
2.
Metabolic pathways including metabolite transport, metabolism of toxic compounds (ROS), enzyme cofactors, TCA cycle and lipid homeostasis.
-
3.
Organellar pathways linked to mitochondrial health, including mitochondrial dynamics and cell death.
A subset of genes with currently unclear biological functions is included in figure 1. For these genes, although a pathogenic correlation is recognized, appropriate classification as SMD or PMD cannot yet be assigned. Conversely, while some genes are clearly imperative to mitochondrial metabolism and health and have been flagged as mitochondrial disease genes in other reviews [15,20], they have been excluded here on the basis of a strict requirement for demonstrable OXPHOS defect. It is beyond the scope of this review to cover each gene linked to SMD. Our descriptions below will cover key pathways and genes to provide an overview of the myriad of ways in which dysfunctional mitochondria can intersect and impinge on OXPHOS function, and lead to diverse disease pathologies.
3. Molecular pathways related to protein biogenesis
Mitochondrial protein biogenesis refers to the processes that permit inter- or intra-organellar protein synthesis (mitochondrial or cytosolic ribosomes, respectively), followed by the coordinated action of translocases, proteases, chaperones and assembly factors to mediate the correct compartmentalization of newly synthesized proteins. Due to the dual organellar location of genes encoding subunits comprising OXPHOS complexes, protein biogenesis pathways are essential in the establishment of a functional OXPHOS system. Perturbations to systems involved in mtDNA transcription, translation and maintenance contribute to the onset of PMD, as their loss solely impedes OXPHOS biogenesis. Conversely, mitochondrial import, protein homeostasis and other post-translational actions support broader mitochondrial function, loss of which results in SMD. Accordingly, the list of SMDs linked to protein biogenesis dysfunction has steadily grown over the last decade (figure 1).
3.1. Protein import/processing
The unique double membrane architecture of mitochondria necessitates the presence of targeting signals, delivering nuclear-encoded precursors to one of five major translocases/import pathways [26]. Figure 2a illustrates these translocases and highlights subunits with a known connection to SMD. Most SMDs in this category are associated with the two Translocases of the Inner Mitochondrial Membrane, the TIMM22 and TIMM23 complexes, which are responsible for the delivery of the majority of the matrix and inner membrane proteome [27]. Pathogenic variants within the translocase of the outer mitochondrial membrane (TOMM) complex have only recently been reported in the receptor protein TOMM70 [28,29]. Three patients have been identified so far, presenting with dystonia, hyper-reflexia, ataxia, lactic acidosis, anaemia and mild developmental delay [28,29].
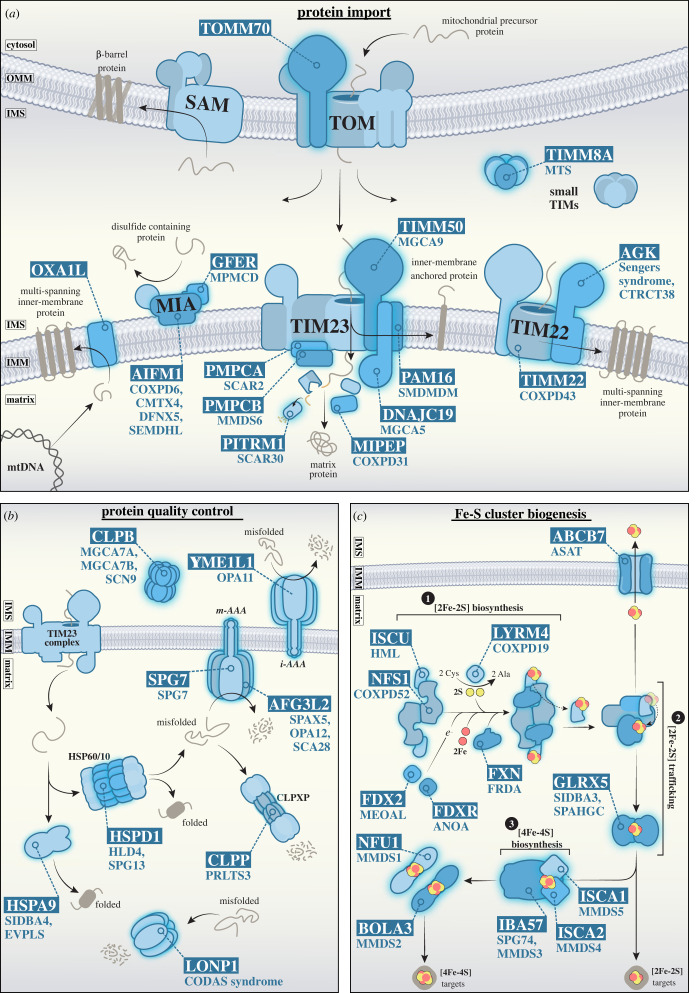
Figure 2.
Overview of protein biogenesis pathways linked to secondary mitochondrial disease. (a) Schematic depicting mitochondrial import pathways and genes associated with SMD. The majority of nuclear-encoded mitochondrial precursor proteins are targeted to mitochondria and imported via the translocase of the outer mitochondrial membrane (TOMM) complex. From here, precursor import pathways diverge to one of four key routes: (1) β-barrel insertion and assembly into the outer membrane via the sorting and assembly machinery (SAM); (2) cysteine-rich precursors of the intermembrane space are oxidized by CHCHD4 (MIA40) at the mitochondrial intermembrane space and assembly complex (MIA); (3) the N-terminal pre-sequence pathway via the translocase of the inner mitochondrial membrane 23 (TIMM23) complex, for import into the mitochondrial matrix, or lateral insertion of proteins into the inner membrane; and (4) the carrier pathway, where proteins with multi-spanning transmembrane domains are chaperoned by members of the small TIM family and delivered to the TIMM22 complex for lateral insertion into the inner membrane. The OXA1L insertase is responsible for the biogenesis of a number of inner membrane proteins, including components of the respiratory chain encoded by the mitochondrial genome. (b) Protein quality control systems within the mitochondrial intermembrane space and matrix. Within the IMS, the i-AAA protease (YME1L1 hexamer) and CLPB disaggregase clear misfolded and aggregated proteins, respectively. In the matrix, the HSP60/10 chaperone complex (HSPD1/HSPE1, respectively) facilitates protein folding while the m-AAA (AFG3L2/SPG7 hetero-hexamer or AFG3L2 homo-hexamer), CLPXP (CLPP and CLPX oligomer), and LONP1 proteases cooperate to degrade misfolded protein precursors. (c) Fe-S cluster biogenesis occurs through three major steps 1) [2Fe-2S] biosynthesis: NFS1, in complex with LYRM4, catalyses the release of sulfane (-SSH) from cysteine. FXN likely chaperones imported iron to the ISCU scaffold. FDX2 and FDXR reduce sulfane to sulfide and finalize [2Fe-2S] assembly at ISCU. 2) [2Fe-2S] trafficking: chaperone HSPA9 reacts with and detaches [2Fe-2S] from the ISCU. GLRX5 binds to and transfers the mature [2Fe-2S] cluster to apoproteins or shuttles the cluster for export. 3) [4Fe-4S] biosynthesis: IBA57, ISCA1 and ISCA2 facilitate the maturation of [4Fe-4S] clusters and can either deliver them to apoproteins directly or pass clusters on to other proteins (such as NFU1 or BOLA3) to target more specific downstream [4Fe-4S]-containing proteins. Gene names are boxed, and associated diseases are listed below or indicated here: (a) AGK (Sengers syndrome (MIM #212350) and CTRCT38 (MIM #614691)); AIFM1 (COXPD6 (MIM #300816), CMTX4 (MIM #310490), DFNX5 (MIM #300614) and SEMDHL (MIM #300232)); DNACJ19 (MGCA5 (MIM #610198)); GFER (MPMCD (MIM #613076)); MIPEP (COXPD31 (MIM #617228)); OXA1L (-); PAM16 (SMDMDM (MIM #613320)); PITRM1 (SCAR30 (MIM #619405)); PMPCA (SCAR2 (MIM #213200)); PMPCB (MMDS6 (MIM #617954)); TIMM22 (COXPD43 (MIM #618851)); TIMM50 (MGCA9 (MIM #617698)); TIMM8A (MTS (MIM #304700)); TOMM70 (-). (b) AFG3L2 (SPAX5 (MIM #614487)), OPA12 (MIM #618977) and SCA28 (MIM #610246)); CLPB (MGCA7A (MIM #619835), MGCA7B (MIM #616271) and SCN9 (MIM #619813)); CLPP (PRLTS3 (MIM #614129)); HSPA9 (SIDBA4 (MIM #182170) and EVPLS (MIM #616854)); HSPD1 (HLD4 (MIM #612233) and SPG13 (MIM #605280)); LONP1 (CODAS syndrome (MIM #600373)); SPG7 (SPG7 (MIM #607259)); YME1L1 (OPA11 (MIM #617302)). (c) ABCB7 (ASAT (MIM #301310)); BOLA3 (MMDS2 (MIM #614299)); FDX2 (MEOAL (MIM #251900)); FDXR (ANOA (MIM #617717)); FXN (FRDA (MIM #229300)); GLRX5 (SIDBA3 (MIM #616860) and SPAHGC (MIM #616859)); IBA57 (SPG74 (MIM# 616451) and MMDS3 (MIM #615330)); ISCA1 (MMDS5 (MIM #617613)); ISCA2 (MMDS4 (MIM #616370)); ISCU (HML (MIM #255125)); LYRM4 (COXPD19 (MIM #615595)), NFS1 (COXPD52 (MIM #619386)); NFU1 (MIM #MMDS1 (605711)).
TIMM22 functions to insert multi-spanning inner membrane proteins, which include mitochondrial solute carriers (SLC25 family), the largest family of carrier proteins responsible for the transport of nucleotides, amino acids and inorganic compounds across the inner membrane [30–32]. The best characterized member of this family is the ADP/ATP translocase (ANT1-4 or SLC25A4-6 and SLC25A31 in humans) [33,34]. Two subunits of the TIMM22 complex are currently connected to mitochondrial disease: the pore subunit TIMM22, and the receptor subunit AGK (acylglycerol kinase) [35,36]. TIMM22 mutation is rare, with only a single patient reported, carrying p.(Tyr25Ter) and p.(Val33Leu) pathogenic variants [35]. The patient presented with autosomal recessive combined OXPHOS deficiency 43 (COXPD34, MIM #617872), characterized by intrauterine growth retardation, hypotonia, feeding difficulties, gastroesophageal reflux, delayed myelination of white matter and increased plasma lactate and creatine levels [35]. Activities of OXPHOS Complexes I, III and IV were reduced, likely due to impaired carrier protein import and metabolite imbalance [35]. The mild respiratory deficit observed in this lone COXPD34 patient may be partially explained by perturbed import of Complex I accessory subunits with multiple transmembrane domains, such as NDUFA11, NDUFC2 [37,38] and SFXN4, a novel Complex I assembly factor [39] and newly identified TIMM22 substrate [40]. AGK mutations cause Sengers syndrome (MIM #212350), a rare, autosomal recessive mitochondrial disorder associated with congenital cataracts, hypertrophic cardiomyopathy, lactic acidosis and skeletal myopathy [41,42] Loss of AGK perturbs TIMM22 complex stability, disrupting carrier protein import and dampening the rate of mitochondrial respiration and metabolic flux through the TCA cycle [43,44]. One-carbon metabolism is altered in Sengers syndrome patient cells [43,45], owing to the perturbed biogenesis of the novel TIMM22 complex substrates, SFXN1, SFXN2 and SFXN3 [40,46]. It is believed that defects in lipid metabolism and/or mitochondrial carrier import due to the absence of functional AGK contribute to Sengers syndrome pathogenesis.
The TIMM23 complex facilitates the import of N-terminal presequence-containing precursors into the matrix and inner membrane [26,47]. The TIMM23 core complex (TIMM23, TIMM50, TIMM17A/B and TIMM44) can associate with either the matrix-localized presequence associated motor (PAM) to drive protein import into the matrix in an ATP-dependent manner (TIMM23MOTOR) [47], or ROMO1 and TIMM21 to mediate lateral insertion of precursors into the inner membrane (TIMM23SORT) [47]. No pathological variants of TIMM23 and TIMM17A/B have yet been described, but this is not surprising as mouse models carrying heterozygous Timm23 mutations present with neurological phenotypes and a reduced lifespan, and complete deletion of the gene is embryonically lethal [48]. The PAM module comprises the ATPase HSPA9 and associated regulatory co-chaperone DNACJ19, a modulator of HSPA9 ATP hydrolysis activity and precursor shuttling [47,49]. Additional co-chaperones include GRPEL1, PAM16 (MAGMAS), DNAJC19 and DNAJC15 [47]. Dnajc15-null mice have no obvious phenotype [50], but mutations in DNAJC19 are associated with dilated cardiomyopathy with ataxia syndrome (DMCA, or MGCA5 (3-methylglutaconic aciduria type V), MIM #610198) [51]. DMCA patients present with severe, early onset dilated cardiomyopathy (DCM), growth failure, mild cerebellar ataxia and notably, exacerbated urinary excretion of 3-methyglutaconic acid (3-MGA) [51]. While mild elevation of 3-MGA (20–40 mmol mol−1 creatinine) in urine is frequently observed among disorders impacting OXPHOS [52], extremely elevated levels of 3-MGA (40-greater than 1000 mmol mol−1 creatinine) are consistent with a limited set of disorders entitled ‘3-methyglutaconic acidurias' (3-MGA-urias) [53]. DMCA itself is classified as a ‘secondary 3-MGA-uria’, as this defect arises via an unknown mechanism that is unrelated to the primary metabolic disorder 3-methylglutaconyl-CoA hydratase (AUH) deficiency (MGCA1, MIM #250950) [53].
TIMM50 is a core subunit of the TIMM23 translocase, receiving presequence-containing precursors from the outer membrane TOM complex and directing their passage through the TIMM23 channel [54]. Patients presenting with pathogenic mutations in TIMM50 are rare, but common symptoms include severe intellectual disabilities, epileptic spasms, microcephaly, moderate elevation of plasma lactate levels, variable mitochondrial Complex V deficiency and 3-MGA-uria (MGCA9, MIM #617698) [55–57]. Isolated Complex V deficiency is a confounding phenotype, considering that efficient function of the TIMM23 complex is necessary for import of a range of precursors, including multiple OXPHOS components [58]. Further detailed study of TIMM50 function and other members of the human mitochondrial import machinery will provide crucial insight into diseases of protein import, alongside the prevalence of secondary 3-MGA-uria across mitochondrial disorders. While not a constitutive member of the TIMM23 complex itself, the OXA1L general insertase facilitates the integration of multiple inner membrane resident and OXPHOS components from the matrix into the inner membrane and has recently been linked to a severe form of combined respiratory chain deficiency with mitochondrial encephalopathy [59].
TIMM8A, together with TIMM8B and TIMM13, are intermembrane space chaperones with a broadly appreciated role in mitochondrial import and biogenesis, receiving nascent precursor proteins from the TOMM complex and shuttling them either to the inner membrane TIMM22 translocase or the outer membrane via the dedicated sorting and assembly machinery (SAM) β-barrel insertase [60–62]. Loss of function mutations in TIMM8A manifest in the X-linked autosomal recessive neurodegenerative disorder Mohr-Tranebjærg syndrome (MTS, MIM #304700) [63,64], characterized by early onset progressive sensorineural deafness, progressive dystonia, cortical blindness and dysphagia. The mode of MTS pathogenesis was initially believed to be a defect in TIMM23 complex assembly, due to impaired import of TIMM23 protein via TIMM8A [60,61]. However, an alternative role for TIMM8A in Complex IV biogenesis within the neuronal SH-SY5Y cell type has recently been reported [65]. The neuronal SH-SY5Y TIMM8A-deficient model also demonstrates increased apoptotic sensitivity due to elevated levels of reactive oxygen species, and a concurrent defect in oxidative phosphorylation [65]. This novel, cell-type specific role for the TIMM8A chaperone challenges current understanding of MTS pathomechanisms and necessitates some leniency in primary and secondary disease classifications as our understanding of mitochondrial biology expands.
Further reading on mitochondrial protein import/processing can be found in [58,62,66,67].
3.2. Protein quality control
The dynamic nature of mitochondria and their heightened susceptibility to oxidative damage via OXPHOS [68,69] creates a volatile environment for protein folding and maturation. Intraorganellar chaperones and proteases provide a first line of defence in protein quality control (QC), surveying the protein folding environment and mediating the removal of damaged or potentially toxic compounds [8]. Key members of this pathway are highlighted in figure 2b and include: CLPB, an intermembrane space disaggregase [70]; the matrix-localized, ATP-dependant proteases LONP1 and CLPP (together with CLPX), which mediate the proteolytic degradation of misfolded protein substrates [69]; chaperones of the Hsp60 family [71]; and ATP-dependent inner membrane localized proteases, i-AAA and m-AAA, with proteolytic domains in the intermembrane space and matrix, respectively. Effective mitochondrial proteostasis is therefore largely dependent on the action of AAA + domain-containing proteins (ATPases associated with diverse cellular activities) [72]. Failure of these QC systems can result in extensive oxidative protein damage, aggregate accumulation and mitochondrial network fragmentation [8], and can manifest in a range of novel mitochondrial pathologies as outlined herein.
The m-AAA protease exists either as an AFG3L2 homo-hexamer, or as a hetero-hexamer in conjunction with paraplegin, encoded by the SPG7 gene [73]. m-AAA activity is critical in the biogenesis of OXPHOS complexes [74] and mitochondrial ribosomes [75], and for OMA1 maturation, a regulator of mitochondrial dynamics [76]. Both AFG3L2 and paraplegin contain AAA-ATPase domains and zinc-dependent metalloprotease domains [77]. Loss of function mutations in SPG7 contribute to hereditary spastic paraplegia type 7 (SPG7, MIM #607259), characterized by adult-onset progressive weakness and spasticity of extremities due to degeneration of corticospinal axons [78,79]. The mode of inheritance of SPG7 is complex—while autosomal dominant cases of SPG7 have been described [80,81], autosomal recessive inheritance is also widely reported [82]. The recent identification of a deep intronic variant alongside a previously identified missense mutation within SPG7 [83] has raised conjecture over the true inheritance pattern of SPG7. Digenic inheritance is another possibility, and it is recommended that patients carrying heterozygous mutations in SPG7 should also be screened for pathogenic variants in known paraplegin interactor AFG3L2 as well as genetically associated variants in CACNA1A and MORC2 [84].
Missense mutations of AFG3L2 can occur in either domain, severely impeding the proteolytic capacity of m-AAA, leading to Complex IV deficiency and impaired cellular respiration [85]. Heterozygous mutations of AFG3L2 contribute to autosomal dominant hereditary spinocerebellar ataxia type 28 (SCA28, MIM #610246) [85] and optic atrophy-12 (OPA12, MIM #604581) [86]. Homozygous mutations of AFG3L2 are also implicated in autosomal recessive spastic ataxia-5 (SPAX5, MIM #614487) [87]. AFG3L2 mutations associated with SCA28 and SPAX5 frequently occur in the metalloprotease domain, whereas mutations linked to OPA12 exclusively affect the AAA-domain [86]. SCA28 is characterized by late-onset cerebellar ataxia, dysarthria and ptosis [85,87]. Selective upregulation of AFG3L2 in Purkinje cells of SCA28 patients, along with an exclusive neuronal biochemical phenotype, suggests that AFG3L2 might have a specific, neuro-protective role within the human cerebellum, aligning with the proposed model of cerebellar degeneration within SCA28 patients [85]. The SPAX5 phenotype shares cerebellar ataxia and ptosis as similarities with SCA28 [85,87], along with additional traits such as early onset spasticity, oculomotor apraxia, dystonia and progressive myoclonic epilepsy [87]. While OPA12 patients predominantly present with optic atrophy, a small subset can experience additional neurologic involvement, including mild intellectual disabilities, ataxia and dystonia [86].
YME1L1 (i-AAA) has its proteolytic domain orientated towards the intermembrane space. Both i-AAA and m-AAA regulate mitochondrial cristae morphology by way of OPA1 processing in conjunction with OMA1 [76,88]. OPA1 is linked to mitochondrial morphology and will be described further in §5.1 ('Mitochondrial morphology'). Patients carrying mutations in YME1L1 present with optic atrophy-11 (OPA11, MIM #617302), characterized by intellectual disabilities, muscular degradation and optic nerve atrophy, associated with abnormal OPA1 processing and mitochondrial fragmentation [88].
Under intense cellular stressors, nascent proteins can quickly misfold and accumulate as toxic aggregates, overwhelming these conventional proteases [89]. Human CLPB is a mitochondrial AAA + domain-containing protein with demonstrable disaggregase activity and intermembrane space localization [70,90]. In the presence of substrate, CLPB oligomerizes into a large (approx. 800 kDa) dodecameric species, comprising two CLPB hexamers interacting via highly versatile ankyrin repeat (ANK) domains [91]. Autosomal recessive mutations within CLPB cause 3-methylglutaconic aciduria, type 7B (MGCA7B, MIM #616271) [92], though autosomal dominant, de novo missense mutations have also been described (MGCA7A, MIM #619835) [93]. Both forms display similar phenotypic outcomes, including progressive encephalopathy, impaired psychomotor development, intellectual disabilities, bilateral cataracts, congenital neutropenia and are specifically characterized via the 3-MGA-uria biomarker [92,93]. More recently, heterozygous missense mutations within the nucleotide-binding domain (NBD) of CLPB have been reported to contribute to autosomal dominant severe congenital neutropenia 9 (SCN9 MIM #619813) [94]. These patients predominantly present with early onset neutropenia and recurrent infections and can occasionally develop cataracts or minimal neurologic involvement. However, SCN9 is distinct from MGCA7A/B in that these SCN9 patients do not have 3-MGA-uria [94]. It is not yet known why certain mutations throughout the ANK or NBD of CLPB give rise to such clinical heterogeneity.
Further reading on mitochondrial quality control can be found in [8,68,69]
3.3. Fe-S cluster biogenesis
Mitochondria are thought to be essential for the generation of all Fe-S-containing proteins in the cell [95]. Fe-S clusters are highly versatile, facilitating electron transfer, catalysis, signalling and protein–protein interactions [96]. Suspected to have supported the earliest metabolic reactions giving rise to complex life [97], these ancient, critical cofactors have a diverse functional repertoire within the cell: coordinating DNA repair, the citric acid cycle, and comprising components of OXPHOS subunits [98,99]. Together with haem synthesis, mitochondrial Fe-S biogenesis also helps regulate total cellular levels of iron and sulfide, preventing the accumulation of cytotoxic Fe-S cluster constituents [100]. Hence, it is not surprising that perturbation to Fe-S cluster biogenesis is implicated in SMD [99,101].
Figure 2c illustrates the Fe-S cluster machinery in mitochondria and components of the process that have been linked to SMD. Mutations of core proteins in step one of Fe-S biogenesis (LYRM4, FXN, ISCU and HSPA9) are typically more common and have broader biochemical consequence, as these factors are essential in the generation of all cellular Fe-S clusters [98,102]. Mutation of proteins involved in step three (NFU1, BOLA3, IBA57, ISCA2 and ISCA1) disrupt [2Fe-2S] trafficking and [4Fe-4S] biogenesis and shuttling, perturbing synthesis of downstream apoproteins with key mitochondrial functions, such as lipoic acid synthase (LIAS; see §4.4, 'Enzyme cofactors') [98,103]. This can result in the onset of multiple mitochondrial dysfunction syndrome types 1–5, respectively (MMDS1-5, MIM #605711, MIM #614299, MIM #615330, MIM #616370 and MIM #617613), a class of severe yet heterogeneous neurodegenerative disorders sharing symptoms of early onset leukoencephalopathy and elevated levels of glycine, lactate and pyruvate within the blood, urine or cerebrospinal fluid [103].
Friedreich ataxia (FRDA, MIM #229300) is considered the most common Fe-S disorder, with a prevalence of 1 : 20 000–1 : 50 000 [98]. It is an autosomal recessive disorder, caused by mutation of frataxin (FXN) [104], a matrix protein associated with the inner membrane [105]. Fxn knockout in mice is embryonic lethal [106], and the yeast FXN homologue, YFH1, modulates mitochondrial iron efflux [107]. Frataxin contains a conserved iron binding site [108], and is suspected to act as an allosteric activator together with NFS1, LYRM4 and ISCU [109], shuttling iron to the site of [2Fe-2S] synthesis. In FRDA patients, Fe-S assembly is stalled and mitochondria become overloaded with iron [105]. Respiratory chain malfunction in FRDA patients leads to an accumulation of H2O2, which oxidizes ferrous iron to yield hydroxyl free radicals (•OH) through the Fenton reaction [110]. FRDA patients become hypersensitive to oxidative stress, resulting in mtDNA damage and the onset of FRDA pathologies such as ataxia, diabetes mellitus, visual loss, deafness and cardiomyopathy [111]. A number of treatment options are being explored for FRDA patients, with some attempting to restore basal levels of frataxin by blocking its ubiquitination and degradation [112], while others are aimed at negating the secondary effects of frataxin deficiency, such as iron chelators and antioxidants [113].
Further reading on mitochondrial Fe-S cluster biogenesis can be found in [98,101–103].
4. Molecular and organellar pathways linked to metabolism
Bidirectional metabolite exchange between mitochondria and the cytosol facilitates energy generation, nucleotide biosynthesis, calcium storage and lipid homeostasis, among other events. Aberrant activity of mitochondrial proteins either directly or indirectly involved in supporting mitochondrial and cellular metabolism constitute a significant proportion of all SMD's, each with equitably heterogeneous pathologies.
4.1. TCA cycle and metabolism
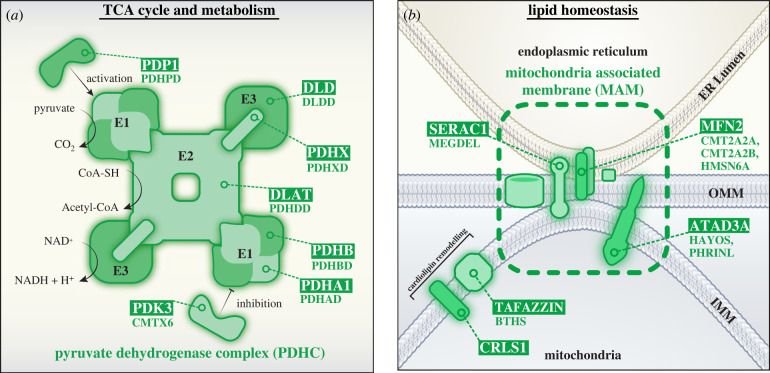
The product of glycolysis, pyruvate, can be metabolized in a non-oxidative (anaerobic) or oxidative-dependent (aerobic) manner. Under anaerobic conditions, pyruvate can be converted to lactate to generate two ATP for one NADH2, a comparatively inefficient pathway against that of aerobic metabolism [114]. In an oxygen-rich environment, pyruvate feeds into the TCA cycle and in a reaction mediated by the pyruvate dehydrogenase complex (PDHC), two pyruvate molecules are converted into two acetyl-CoA (that enter the TCA cycle), and two NADH2 (that are consumed in OXPHOS) [114]. Hence, PDHC operates as a vital link between glycolysis and aerobic respiration. Figure 3a depicts the human PDHC, a multienzyme complex that comprises a large dihydrolipoyl transacetylase (DLAT) (E2) core, anchored to dihydrolipoamide dehydrogenase (DLD) (E3) units via pyruvate dehydrogenase protein component X (PDHX) [115,116]. Associated are multiple pyruvate dehydrogenase α/β heterotetrametric (α2β2) subunits (PDH-α/PDH-β) which constitute the E1 component and catalyse the rate-limiting conversion of pyruvate into acetyl-CoA. Transiently associated with the complex are PDHC regulatory subunits pyruvate dehydrogenase kinase (PDK) and pyruvate dehydrogenase phosphatase (PDP) [115,116]. PDK inactivates the complex via phosphorylation of PDH-α, which can be reversed by PDP activity. Of the four PDK isoforms (PDK1-4), PDK3 has the greatest binding affinity, and hence activity [117]. Mg2+ binding is essential for the activity of both PDP1 and PDP2 isoforms, though PDP1 is functional at much lower Mg2+ concentrations [118]. As each subunit of the PDHC is crucial for structural integrity or overall catalytic activity, mutation of any PDHC component can significantly alter glucose metabolism, oxidative phosphorylation efficiency and cellular viability. Described broadly as ‘PDHC deficiency’, such mutations result in a variety of heterogeneous phenotypes, which include developmental delay, neurological degeneration, peripheral neuropathy, seizures, ataxia and fatal infantile lactic acidosis [119,120]. Most instances of PDHC deficiency arise due to mutation of the X-linked PDHA1 gene (encoding PDH-α), resulting in pyruvate dehydrogenase E1-alpha deficiency (PDHAD, MIM #312170) [119], though disease causing mutations in PDHB, DLAT, DLD, PDHX, PDP1 and PDK3 have been described at a considerably lower frequency [118,120–124].
Figure 3.
TCA and lipid metabolic pathways compromised in secondary mitochondrial disease. (a) At the core of the PDHC complex is dihydrolipoyl transacetylase (DLAT; E2). The E2 core is anchored to dihydrolipoamide dehydrogenase (DLD; E3) via PDHX. Pyruvate dehydrogenase a/β heterotetrametric subunits (PDHA1/PDHB; E1), associate with E2. The PDHC catalyses the production of acetyl-CoA from pyruvate in a three-step process: (1) E1, in conjunction with thiamine pyrophosphate (TPP) cofactor, catalyses the decarboxylation of pyruvate, releasing CO2 and forming a hydroxyethyl-TPP intermediate. (2) E2 transfers the hydroxyethyl group from TPP to an oxidized lipoamide cofactor, releasing an acetyl group which is then transferred to coenzyme A (CoA-SH) to form acetyl-CoA and a reduced dihydrolipoamide-E2 core. (3) E3 then oxidizes the lipoyl group of dihydrolipoamide-E2 to form lipoamide-E2 and NADH. Activity of the PDHC can be tightly modulated by associated kinases and phosphatases; phosphorylation of E1 by pyruvate dehydrogenase kinase 3 (PDK3) inactivates PDC, and dephosphorylation of E1 by pyruvate dehydrogenase phosphatase 1 (PDP1) reactivates PDC. (b)Mitochondria-associated membranes (MAMs) are direct points of contact between mitochondria and endoplasmic reticulum (ER). MAMs support phospholipid exchange between the ER and mitochondria, with links to SERAC1 in this process. In addition, MAMs serve as complex signalling platforms, as selective enrichment of proteins at these intraorganellar contact points enables robust coordination of intracellular events, such as apoptosis, autophagy and calcium homeostasis. ER-mitochondrial contacts also regulate mitochondrial dynamics, recruiting proteins such as MFF and MFN2 to coordinate mitochondrial fission and fusion, respectively. Proteins such as TAFAZZIN and CRLS1 coordinate cardiolipin remodelling at the inner membrane to preserve correct lipid composition. ATAD3A is proposed to tether mitochondrial membranes at MAM sites and is therefore broadly implicated in the retention of mitochondrial network structure and cholesterol homeostasis, in addition to mtDNA nucleoid regulation. Gene names are boxed, and associated diseases are listed below or indicated here: (a) DLAT (PDHDD (MIM #245348)); DLD (DLDD (MIM #246900)); PDHA1 (PDHAD (MIM #312170)); PDHB (PDHBD (MIM #614111)); PDHX (PDHXD (MIM #245349)); PDK3 (CMTX6 (MIM #300905)); PDP1 (PDHPD (MIM #608782)). (b) ATAD3A (HAYOS (MIM #617183) and PHRINL (MIM #618810)); CRLS1 (-); MFN2 (CMT2A2A (MIM #609260), CMTA2A2B (MIM #617087) and HMSN6A (MIM #601152)); SERAC1 (MEGDEL (MIM #614739)); TAFAZZIN (BTHS (MIM #302060)).
Further reading concerning mitochondrial TCA cycle metabolism can be found in [125].
4.2. Metabolite transport
While the outer membrane is permeable to smaller solutes, the mitochondrial inner membrane is largely impermeable, and the passage of hydrophilic metabolites, nucleotides and other compounds requires mitochondrial carrier proteins (described in §3.1, 'Protein import/processing') [126]. The electrochemical gradient, established by the mitochondrial respiratory chain, provides the driving force required by carrier proteins to mediate the directional transport of substrates either with or against the concentration gradient [126]. In humans, over 65 carrier proteins [127] are responsible for the maintenance of sufficient metabolite flux across the inner membrane to fuel metabolism. Of these 65 carriers, 15 are linked to mitochondrial disease to date (figure 1, metabolite transport) [13]. Pathogenic mutations across the majority of these carriers typically impact OXPHOS and energy generation among tissues where the respective carrier protein or isoform is expressed.
The phosphate carrier (PiC or SLC25A3) transports inorganic phosphate across the inner membrane into the matrix and is an essential component of OXPHOS and ATP generation [128,129]. In humans, mutually exclusive alternative splicing of exon 3 of SLC25A3 results in two tissue-specific isoforms: PiC-A and PiC-B [129,130]. PiC-A containing exon 3A is predominantly expressed in heart and skeletal muscle, whereas PiC-B containing exon 3B is ubiquitously expressed [129]. Pathogenic mutations in SLC25A3 are rare, and 6 of the 7 described patients had homozygous mutations impacting exon 3A directly [129,131,132]. Patients with mitochondrial phosphate carrier deficiency (MPCD, MIM #610773) consistently displayed prenatal or neonatal hypertrophic cardiomyopathy, skeletal myopathy and elevated lactate levels [129,132]. One patient reported to carry compound heterozygous variants impacting exons 4 and 6 did not exhibit clinical myopathy or lactic acidosis [131]. This discrepancy is likely a consequence of the tissue specific expression of PiC-A, and the importance of exon 3A specifically in the phosphate shuttling mechanism.
Mitochondria are intimately involved in cellular calcium flux, a key signalling pathway, facilitating dynamic calcium storage, calcium signalling propagation throughout cells, and influencing total cellular calcium uptake [133]. Mitochondrial calcium uptake is an electrogenic process mediated via the mitochondrial calcium uniporter (MCU), a pore-forming transmembrane protein positioned in the inner membrane [134,135], though not a formal carrier member or known substrate of TIMM22 [136,137]. Associated with the MCU are two calcium-binding heterodimers, MICU1 and MICU2, which are suggested to serve as positive and negative regulators of calcium flux through the MCU, respectively [134,138,139]. At low [Ca2+], the strong inhibitory effect of MICU2 keeps MCU closed. With increasing [Ca2+], MICU1-2 heterodimers undergo a conformational change which dampens MICU2 inhibition and promotes MICU1-mediated enhancement of Ca2+ flux through MCU [139]. While no pathogenic variants in MCU itself have been described, impaired mitochondrial calcium signalling has devastating effects on patients with MICU mutations. Individuals carrying MICU1 mutations present with proximal myopathy, axonal peripheral neuropathy, varied involuntary movement and severe learning difficulties (myopathy with extrapyramidal signs, MPXPS #615673) [140]. Interestingly, mutations in MICU2 manifest as acute encephalopathy and associated cognitive impairment, but myopathy is absent – though these mutations are less frequently observed than those in MICU1 [141].
Further reading concerning mitochondrial carrier proteins and calcium homeostasis can be found in [31,126,142,143].
4.3. Metabolism of toxic compounds
As integral metabolic hubs of the cell, mitochondria are uniquely vulnerable to toxic by-product and metabolite accumulation. Consequently, an array of specialized protein systems exists to prevent deleterious accumulation of harmful compounds in the organelle. For example, sulfur dioxygenase protein ETHE1 mediates the catabolic conversion of hydrogen sulfide to sulfite [144]. Mutation of ETHE1 prevents efficient clearance of hydrogen sulfide from tissues, indirectly impairing mitochondrial respiration and manifesting as ethylmalonic encephalopathy (EE, MIM #602473) [145,146]. Another example is the toxic metabolite NAD(P)HX, which can accumulate via spontaneous hydration of NAD(P)H under stress conditions, or enzymatic action of glyceraldehyde 3-phoshate (GAPDH) [147]. Damaged NAD(P)HX cannot act as an election carrier and will strongly inhibit cellular dehydrogenases and OXPHOS efficiency [147]. The dehydratase, NAXD, can only convert S-NAD(P)HX back to usable NAD(P)H, and so R-NAD(P)HX must first be converted into S-epimers by a dedicated epimerase, NAXE [148]. Loss of function mutations in NAXE disturb S-NAD(P)HX conversion, resulting in irreparable metabolite accumulation and the development of an early onset progressive neurometabolic encephalopathy, with brain edema and leukoencephalopathy (PEBEL1, MIM #617186) [149]. Pathogenic variants in NAXD have only recently been described [150,151], and phenotypic outcomes are similar to NAXE mutations (PEBEL2, MIM #618321).
Almost all intracellular reactive oxygen species (ROS) are derived from hazardous superoxides generated as by-products of oxygen consumption at the mitochondrial respiratory chain [152]. At low concentrations, ROS is an important signalling molecule and can stimulate transcriptional upregulation of scavenger enzymes such as glutathione peroxidase and thioredoxin [153]. Under conditions of stress, excessive ROS production by the respiratory chain can cause extensive oxidative damage if left unchecked [152]. Mutations in genes linked to both PMD and SMD can contribute to exacerbated ROS production and consequent oxidative stress via a direct or indirect impact on OXPHOS integrity. In mitochondria, either spontaneously or with the help of the manganese superoxide dismutase (MnSOD), superoxides are converted into H2O2, which can passively diffuse into other cellular compartments [154]. Fittingly, H2O2 production in mitochondria is tightly regulated by detoxifying agents, including the glutathione and thioredoxin enzymatic systems [152,155]. Within the mitochondrial matrix, these systems function in parallel to detoxify H2O2 into water, retaining a reducing environment and protecting against cellular degeneration and apoptotic induction.
The mitochondrial thioredoxin system is composed of nuclear-encoded peroxiredoxin 3 & 5 (PRDX3 & 5), thioredoxin 2 (TXN2) and thioredoxin reductase 2 (TXNRD2). PRDX3/5 uses TXN2 as an electron donor in the reduction of H2O2 into water. Oxidized TXN2 is then reduced by TXNRD2 in the presence of NADPH, resetting the system [155–157]. Together, these proteins operate to efficiently eliminate H2O2 from the mitochondrial environment and maintain organelle health. Mitochondrial TXN2 is ubiquitously expressed, yet highest levels of expression are observed in the brain [158]. Interestingly, while Txn2 deficiency is embryonic-lethal in mice [156], human patients with loss-of-function mutations in TXN2 survive to term and beyond [155], which may be explained by partial redundancy between the glutathione and thioredoxin detoxifying systems. Despite this, TXN2-deficiency manifests as an infantile-onset neurodegenerative disorder, characterized by cerebellar and optic atrophy, epilepsy, dystonia and peripheral neuropathy (COXPD29, MIM #616811) [155]. Such severe phenotypic expression in the absence of TXN2 clearly demonstrates the importance of efficient ROS clearance from mitochondria, specifically in the development and preservation of mature neuronal networks.
Further reading concerning mitochondrial ROS can be found in [152,159,160].
4.4. Enzyme cofactors
Perturbation to enzyme cofactor synthesis can also contribute to the onset of SMD. FLAD1 encodes FAD synthase, an enzyme responsible for flavin mononucleotide (FMN) adenylation, generating flavin adenine dinucleotide (FAD), a critical cofactor to many mitochondrial dehydrogenases, such as the α-ketoglutarate (α-KGDH) and pyruvate (PDHC) dehydrogenase complexes [161]. FLAD1 mutation results in an autosomal recessive lipid storage myopathy characterized by extreme heterogeneity in severity (LSMFLAD, MIM #255100) [161,162]. Lipoic acid is another essential cofactor of α-KGDH and PDHC, in addition to the branched chain keto acid dehydrogenase (BCKDH) and the glycine cleavage system (GCS) [163]. Loss of lipoic acid synthase (LIAS) contributes to PDHC lipoic acid synthetase deficiency (PDHLD, MIM #614462), characterized by lactic acidosis, hyperglycaemia, delayed psychomotor development and seizures [163,164]. Similarly, loss of the lipoyl-transferases LIPT1 and LIPT2 impairs the attachment of lipoic acid to downstream dehydrogenases, resulting in equitable pathologic outcomes to PDHLD in lipoyl-transferase 1 and 2 deficiency (LIPT1D, MIM #616299) (LIPT2D, MIM #617668) [165]. Thiamine pyrophosphokinase 1 (TPK1) catalyses the conversion of thiamine to thiamine pyrophosphate (TPP), which is also an essential cofactor to α-KGDH, PDHC and BCKDH complexes [166,167]. Loss of functional TPK1 results in thiamine metabolism dysfunction syndrome 5 (THMD5, MIM #614458) an autosomal recessive episodic encephalopathy, which usually spares cognitive function [166,167].
Pantothenate kinase (PANK), phosphopantothenoylcystine synthetase (PPCS) and CoA synthase (COASY) are three enzymes involved in coenzyme A (CoA) cofactor synthesis [168]. Coenzyme A is an essential cofactor which participates in a diverse range of cellular processes, including the citric acid cycle, fatty acid metabolism and amino acid synthesis, among others [168,169]. PANK catalyses the first committed step in the biosynthesis of CoA, converting pantothenate (Vitamin B5) to 4’-phosphopantothenate [168,170], which is an essential prosthetic group across many biosynthetic reactions [171]. This is a key rate limiting step in CoA biosynthesis, and positions PANK as a critical regulator of intracellular CoA concentration [171]. PPCS catalyses the second step in CoA synthesis, converting 4′-phosphopantothenate to phosphopantothenoylcystine [172]. COASY is a bifunctional enzyme that synthesizes the final two steps of CoA biosynthesis; converting 4′-phosphopantetheine into dephospho-CoA, and then into CoA [173]. These final two steps are mediated by the phosphoribosyl pyrophosphate amidotransferase (PPAT) and dephospho-CoA kinase (DPCK) domains of COASY respectively [174], and mutation of either domain is highly pathogenic [175]. Loss of function mutations in either PANK or COASY gives rise to disorders which fall into a group categorized broadly as neurodegeneration with brain iron accumulation (NBIA) [176]. Diseases classified under NBIA share common phenotypes, such as progressive degradation of the nervous system and substantial iron accumulation within the brain [177,178]. Broader symptoms of these disorders include hypo-and/or hyperkinetic movement disorder, coupled with any of central/peripheral nervous system, cognitive and neuropsychiatric abnormalities [178]. By contrast, PPCS deficiency manifests as dilated cardiomyopathy of variable severity (CMD2C, MIM #618189), with no NBIA-related phenotypes [172]. It is not yet understood how disruption to sequential steps in CoA-synthesis can lead to such variable pathologic outcomes.
Of the four PANK isoforms in humans, only one, PANK2, localizes to mitochondria [170]. Mutation of PANK2 leads to the onset of pantothenate kinase-associated neurodegeneration (PKAN, MIM #234200). In early onset PKAN, disease progression is rapid, with symptoms of dystonia, spasticity, intellectual disability, high globus pallidus iron content, optic atrophy and pigmentary retinopathy [176,179]. Late-onset PKAN progresses more slowly, with significantly different symptoms including obsessive-compulsive behaviour, schizophrenia and depression [176]. CoA deficiency resulting in oxidative stress may partly explain the highly specific phenotypes of early onset PKAN, as in other mitochondrial diseases. Mutation of COASY (encoding CoA synthase) contributes to the onset of COASY protein-associated neurodegeneration (CoPAN, MIM #615643), a rare autosomal recessive NBIA [180]. CoA synthase is localized to the mitochondrial matrix [180] and CoPAN causing mutations have been identified in both the ubiquitously expressed COASY alpha isoform and the brain specific beta isoform [174]. The COASY protein is critical to two CoA biosynthesis pathways: de novo CoA biosynthesis from pantothenate and CoA generation from externally acquired 4′-phosphopantetheine [181]. Given that functional COASY is absent in CoPAN, both of these CoA synthesis pathways fail, resulting in non-viability. Most CoPAN patients die within a few weeks of birth [175], and it is assumed that maternal CoA supports the foetus through gestation via an unidentified cell membrane CoA transporter [174]. External CoA can effectively reverse a coasy-null phenotype in zebrafish [182], but more work is needed to develop feasible treatment and accessible delivery options for CoPAN patients.
Further reading concerning NBIA and mitochondrial enzyme cofactors can be found in [183,184]
4.5. Lipid modification and homeostasis
Mitochondria are key sites for lipid homeostasis and defects in these pathways are linked to SMD (figure 3b). The mitochondrial inner membrane has a unique membrane lipid composition with cardiolipin (CL) and phosphatidylethanolamine (PE) compromising approximately 50% of total inner membrane phospholipid mass [185]. The cone-shaped topology of CL and PE is essential in the formation of curved membranes and supporting architecture of cristae [185], which are the predominant site of OXPHOS organization and operation [186]. CL also directly interacts with OXPHOS components and is known to be required for Complex III and IV stability, as well as promoting the formation of III2 + IV1-2 OXPHOS supercomplexes [187,188]. De novo synthesis of CL occurs at the inner membrane [189], and nascent CL is further matured in remodelling events via acyltransferases such as tafazzin [190]. Pathogenic mutation of TAFAZZIN results in Barth syndrome (BTHS, MIM #302060), an X-linked autosomal recessive disorder characterized by cardiomyopathy, skeletal myopathy, growth retardation, neutropenia and 3-MGA-uria [191,192]. In BTHS, lack of functional TAFAZZIN results in the accumulation of immature CL remodelling intermediates, compromising supercomplex stability, impeding OXPHOS efficiency and increasing ROS generation [193,194]. Pathogenic variants in CRLS1 were also recently shown to cause a defect in cardiolipin synthesis with altered acyl-chain composition, resulting in multisystem disease [195]. Therefore, the maintenance of proper inner membrane lipid composition is intimately linked to optimal OXPHOS functionality.
The endoplasmic reticulum (ER) is the major site of phospholipid, triacylglycerol and sterol biosynthesis within the cell [196]. Organelles source lipids from the ER through vesicle exchange, carrier proteins or in the case of mitochondria, via specific contact sites termed mitochondria-associated membranes (MAMs) [196]. MAMs also serve as intracellular signalling platforms, recruiting a specialized and specific proteome to facilitate their operation [197]. The mammalian ER–mitochondrion interface is markedly enriched at MAMs with proteins such as MFN2, FIS1, PINK1 and VDAC1, illustrating the necessity of ER–mitochondrial contacts in regulating mitochondrial dynamics, apoptosis, autophagy and calcium homeostasis, respectively [197,198]. Defects in MAM components have been attributed to a wide variety of neurodegenerative and metabolic diseases. SERAC1 has previously been linked to phospholipid exchange between ER and mitochondrial membranes, reportedly supporting mitochondrial function and cholesterol trafficking [199,200]. More recently, SERAC1 has been implicated in one-carbon metabolism, cooperating with the inner membrane transporter SFXN1 to mediate serine transport into mitochondria [201]. Loss of function mutations in SERAC1 contribute to 3-MGA-uria with psychomotor regression, encephalopathy, deafness and hepatopathy (MEGDEL, MIM #614739) [199,200].
Another SMD disease gene with links to mitochondrial lipid and membrane homeostasis is ATAD3A, encoding a eukaryotic, ubiquitously expressed AAA-ATPase domain containing protein of the ATAD3 family. Contrary to most species, primates contain three ATAD3 paralogues positioned in tandem (ATAD3A, ATAD3B and ATAD3C), which share extensive homology making them prone to frequent non-allelic homologous recombination (NAHR) events [202–205]. ATAD3C is likely to be non-functional, and ATAD3B is expressed at relatively low levels, except in embryonic cells, and the brain, heart and pituitary gland of adults [202,206]. While the precise molecular function is unknown, ATAD3 is positioned within and proposed to tether the mitochondrial inner membrane to the outer membrane at MAM sites [207,208]. Because of this positioning, ATAD3A has been suggested as a critical regulator of mitochondrial dynamics and inner membrane structure, cholesterol channelling, and mtDNA-containing nucleoids [209,210]. Pathogenic ATAD3 variants display an array of recessive and dominant inheritance patterns, both inherited and de novo, along with recurrent deletions and duplications arising from NAHR; hence, they are among the most common causes of SMD in children [204]. The resulting phenotypes range from milder neurodevelopmental disorders (Harel-Yoon syndrome, MIM #617183) to severe neonatal lethal presentations linked to either biallelic deletions (MIM #618810) or de novo duplications (MIM #618815), typically featuring pontocerebellar hypoplasia or cardiomyopathy, respectively. Intriguingly, patients with ATAD3A duplications show severe Complex I deficiency in heart and variable OXPHOS changes in other tissues, although it is unclear if this is a primary or secondary consequence of ATAD3 dysfunction [204]. Likewise, the tissue specificity and genotype-phenotype links to ATAD3 variants are not well understood.
Further reading concerning mitochondrial lipid modification/homeostasis can be found in [196,211,212].
5. Organellar pathways linked to mitochondrial health
As discussed in §3.2 ('Protein quality control'), the mitochondrion tempers volatile insults to proteostasis via the recruitment of designated chaperones and proteases. Mitochondria have multiple levels of protein quality control beyond these molecular systems to maintain homeostasis. If part of a mitochondrial network becomes irreparably damaged it can be cleared on a macro scale via coordinated fission and mitophagy events. Targeted elimination of terminally damaged mitochondrial units allows the cell to evade apoptotic cell death, which is also coordinated within mitochondria. As mitochondrial dynamics, mitophagy and coordination of intrinsic cell death are integral to correct cellular functionality, a number of SMD's linked to greater mitochondrial homeostasis have been extensively documented.
5.1. Mitochondrial morphology
Mitochondrial morphology is mediated by a balance of opposing fission and fusion events at both the inner- and outer-mitochondrial membranes [213]. Cellular bioenergetics is dependent on the modular nature of mitochondrial units, with fusion events enabling the exchange of contents, membrane potential and mtDNA [214]. Conversely, fission events are imperative during mitosis, ensuring equal distribution of mitochondria among both daughter cells [215]. Mitochondrial fission is also intimately linked to mitophagy, enabling the selective clearance of damaged mitochondria, an extreme form of quality control secondary to apoptosis [216]. Both fission and fusion are facilitated by dynamin family GTPases, including DRP1, MFN2 and OPA1. The mitochondrial contact site and cristae organizing system (MICOS) is a large IM structure that is crucial for the formation of cristae junctions and maintenance of cristae morphology [217]. Perturbation to these delicate systems severely impedes the functional capacity of mitochondria, and significantly reduces the viability of the affected organism figure 4.
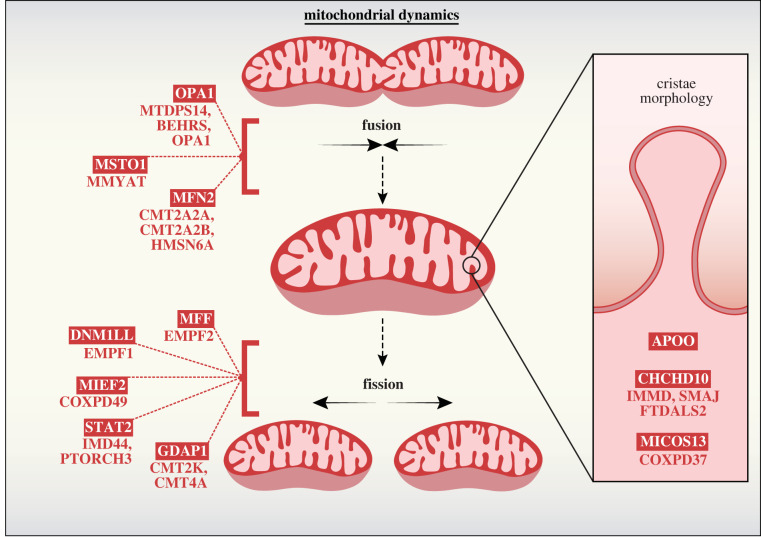
Figure 4.
Regulators of mitochondrial fission and fusion and secondary mitochondrial disease. Mitochondrial fusion events occur by successive fusion of outer and inner membranes. Mitochondrial fusion GTPases MFN1 and MFN2 mediate OM fusion in conjunction with MSTO1, a cytosolic accessory protein recruited to the OM via an unknown mechanism. Mitochondrial IM fusion is coordinated by balanced processing of the OPA1 GTPase from long-form (L-OPA1) into short-form (S-OPA1). L-OPA1 can form oligomers and promote fusion upon GTP hydrolysis. Excessive L-OPA1 processing into S-OPA1 can disrupt L-OPA1 fusion events and tip the balance towards mitochondrial network fission. Mitochondrial fission is an essential component in cellular proliferation and is also used to clear terminally damaged or toxic nodes from the network via mitophagy. Fission factors such as MFF, MIEF1 and MIEF2 recruit cytosolic GTPase DNM1L to the OM, where it assembles in a spiral formation to restrict mitochondria and sever the double membrane upon GTP hydrolysis. DNM1L GTPase activity is dynamically controlled via a number of post translational modifications, including phosphorylation as mediated by kinases such as STAT2. GDAP1 is another fission factor localized to the OM. While loss of GDAP1 prevents efficient mitochondrial fission, the exact role of GDAP1 in cooperation with other OM fission mediators is yet to be uncovered. Within the mitochondrial contact site and cristae organizing system (MICOS) only two components have been connected to secondary mitochondrial disease: CHCHD10 and MICOS13. MICOS13 is an IM scaffolding protein required for the integration of other MICOS members into the mature complex. CHCHD10 is an IMS protein peripherally associated with the MICOS and is believed to maintain complex stability. Gene names are boxed, and associated diseases are listed below or indicated here: CHCHD10 (IMMD (MIM #616209), SMAJ (MIM #615048) and FTDALS2 (MIM #615911)); DNM1L (EMPF1 (MIM #614388)); GDAP1 (CMT2K (MIM #607831) and CMT4A (MIM #214400)); MFF (EMPF2 (MIM #617086)); MFN2 (CMT2A2A (MIM #609260), CMT2A2B (MIM #617087) and HMSN6A (MIM #601152)); MICOS13 (COXPD37 (MIM #618329)); MIEF2 (COXPD49 (MIM #619024)); MSTO1 (MMYAT (MIM #617675)); OPA1 (MTDPS14 (MIM #616896), BEHRS (MIM #210000) and OPA1 (MIM #165500)); STAT2 (IMD44 (MIM #616636) and PTORCH3 (MIM #618886)).
DRP1 exists in both cytosolic and mitochondrial pools, with the latter forming punctate clusters along mitochondrial tubules and mediating mitochondrial scission [218]. ER-mitochondria contacts facilitate tubule constriction, and act as a platform onto which DRP1-receptor proteins such as FIS1 and MFF can be recruited [219]. DRP1 activity can be actively regulated by phosphorylation, linking mitochondrial fission to a range of diverse cellular events, such as calcium storage regulation and mitosis [220,221]. Knockout of Drp1 is embryonic lethal in mice, and targeted ablation in mouse brain results in significant developmental defects [222]. In humans, DRP1 mutation is typically lethal in the first few weeks of life, where patients present with neonatal encephalopathy, microcephaly, demyelination of brain matter, optic atrophy, epilepsy and global developmental delay (EMPF1, MIM #614388) [223,224]. In some cases, disease onset can be delayed into early childhood, but the course of disease remains severe [225].
The mitofusins, MFN1 and MFN2, are mediators of outer membrane fusion [226]. In the absence of either homologue, the mitochondrial network becomes extensively fragmented and mitochondrial fusion is significantly reduced, though not completely abolished [227]. Adjacent mitochondria require the homotypic or heterotypic interaction between MFN1 and/or MFN2 on opposing membranes to facilitate fusion events [228]. MFN2 mutation is widely recognized as the most prevalent cause of Charcot-Marie-Tooth disease 2 (CMT2) with autosomal dominant inheritance, accounting for roughly 20% of diagnosed patients [229]. Clinical symptoms (CMT2A2A, MIM #609260) typically present in infancy or early childhood and consist of muscle atrophy, sensory loss, atypical gait and eventual immobility [230]. Most CMT neuropathies demonstrate evidence of neuronal demyelination, though a smaller population, including CMT2A are classified as primary axonal disorders [229].
At the inner mitochondrial membrane, fusion and cristae morphogenesis are regulated by OPA1 processing. Populations of long form (L-OPA1) and short form (S-OPA1) tip the balance toward mitochondrial fusion and fission, respectively. Regulatory processing of L-OPA1 by inner membrane proteases OMA1 and YME1L1 results in an equilibrium between L-OPA1 and S-OPA1, maintaining a healthy mitochondrial network [231]. This balance is acutely sensitive to cellular stressors, such as changes in inner membrane polarity. For example, deliberate dissipation of the membrane potential upon addition of protonophores [232] can trigger OMA1-dependent OPA1 processing and subsequent mitochondrial network fragmentation. In humans, heterozygous mutation of OPA1 accounts for approximately 60% of all autosomal dominant optic atrophy (DOA) cases, which have an estimated minimum prevalence of 1 in 25 000 [233]. OPA1-mutant DOA (OPA1, MIM #165500) is primarily characterized by early onset retinal ganglion cell degeneration and up to 20% of patients will also present with additional symptoms (DOA+), including deafness, ataxia, peripheral neuropathy and a worsened visual prognosis in comparison to pure DOA [234]. The existence of differing pathologies may partly be explained by genotype-phenotype correlation, in which OPA1 GTPase domain mutations are most frequently associated with DOA+, while mutation of the dynamin domain is more strongly associated with a pure DOA presentation [234].
Further reading concerning mitochondrial morphology can be found in [215–217,235,236].
5.2. Apoptosis
Mitochondria mediate intrinsic cell death that can be inhibited or exacerbated by specific Bcl-2 family proteins, such as BAK and BAX, which oligomerize to form pores in the mitochondrial outer membrane and allow the efflux of pro-apoptotic cytochrome c into the cytosol [237]. SMAC and HTRA2 proteases can also be released from the intermembrane space, interacting with and inhibiting the inhibitor of apoptosis (IAP), XIAP [238]. HTRA2 may also have anti-apoptotic capacity, in conjunction with HAX1, mediating BAX inhibition following it's activation by the inner membrane rhomboid protease, PARL [239]. Htra2 knock-out mice die approximately one month after birth, and display neurological and behavioural abnormalities, lack of coordination, decreased mobility, tremor, selective loss of striatal neurons, as well as decreased heart and spleen mass, and abnormal mitochondrial morphology [240]. Patients lacking functional HTRA2 present with more severe phenotypes and are born with extensive encephalopathy, acquire no developmental milestones and die soon after birth (MGCA8, MIM #617248) [241]. In addition, patients may exhibit 3-MGA-uria, and/or neutropenia, which were suggested to be a consequence of abnormal cristae architecture within cultured patient muscle cells [241]. Further, patient cells devoid of HTRA2 are more susceptible to apoptotic induction [241], which implies an anti-apoptotic role for HTRA2 under normal physiological conditions. Clarifying the breadth of HTRA2 functionality outside of apoptosis will be imperative in understanding the phenotypic basis of patient conditions.
Apoptosis inducing factor (AIF, AIFM1) is an apoptogenic, mitochondrial intermembrane space protein with dual functionality. Under physiological conditions, AIFM1 functions as an integral component of respiratory chain complex biogenesis, tethered to the inner membrane and operating upstream of the MIA machinery, mediating CHCHD4 (human Mia40) import (figure 2a) [242]. During apoptotic induction, the mitochondrial outer membrane is permeabilized, membrane bound AIFM1 is cleaved and soluble AIFM1 is released from the intermembrane space into the cytosol. Here, AIFM1 promotes apoptosis by interacting with EIF3G (subunit of eIF3) and inhibiting de novo protein synthesis, or via caspase-7 activation and subsequent degradation of EIF3G [243]. AIFM1 mutation can contribute to several primary X-linked pathologies, two of which include: (i) combined oxidative phosphorylation deficiency 6 (COXPD6, MIM #300816) [244] and (ii) Cowchock syndrome (CMTX4, MIM #310490) [245]. COXPD6 is a neurodegenerative disease characterized by OXPHOS-related encephalopathy, psychomotor delay, hypotonia, muscle atrophy and early death. Causative mutations in AIFM1 in COXPD6 impede CHCHD4 interaction, resulting in severely reduced OXPHOS complex activities and enhanced nuclear DNA binding in the soluble form [244,246]. Conversely, CMTX4 is a less-severe neuromuscular disorder characterized by progressive axonal neuropathy, distal sensory impairment, cognitive impairment and deafness. As AIFM1 is an FAD-dependent flavoprotein, treatment with riboflavin has been shown to ameliorate some COXPD6 patient symptoms and return OXPHOS complex activity to basal levels in patient fibroblasts [244]. Ultimately, further research will be required to fully understand the basal function of human AIFM1, and to comprehend the apparent genotype–phenotype association between these aberrant mutations.
Further reading on intrinsic apoptosis can be found in [238,247]
6. Conclusion
Over the past decade, NGS has facilitated the identification of putative disease-causing mutations in hundreds of nuclear-encoded mitochondrial genes. This technology, in conjunction with clinical examination, diagnostic pathways and accredited disease scoring systems, is enabling the rapid delivery of accurate prognoses and earlier application of effective treatment plans [14,17]. However, existing mitochondrial disease therapies are largely supportive and preventive approaches [248], with treatments typically focused on countering disease-specific symptoms and enhancing mitochondrial function. Examples of such therapies include regular exercise regimes prescribed to patients with hypotonia and motor delays, ubiquinone (Coenzyme Q10), thiamine (vitamin B1) and riboflavin (vitamin B2) supplementation to enhance OXPHOS functionality, and antioxidant administration to dampen excessive ROS generation, among others [249].
Genetic therapies are being developed, in particular for PMD, including mutant mtDNA elimination via mitoTALENs [250] or zinc finger nucleases [251], that specifically target heteroplasmic mitochondrial diseases, stabilizing wild-type mtDNA levels and thereby reversing pathogenic phenotypes. As the proportion of pathogenic gene variants associated with SMD increases, it is evident that mitochondrial dysfunction can be attributed to a number of defective mitochondrial processes beyond OXPHOS. Given the clinical heterogeneity of mitochondrial diseases, a universal treatment scheme is highly unlikely. The advancement of effective treatment strategies against both PMD and SMD requires a deep understanding of mechanisms underscoring mitochondrial dysfunction in individual diseases. This personalized approach to mitochondrial disease can then drive targeted therapeutic intervention.
Contributor Information
Ann E. Frazier, Email: ann.frazier@mcri.edu.au.
Diana Stojanovski, Email: d.stojanovski@unimelb.edu.au.
Data accessibility
This article has no additional data.
Authors' contributions
M.J.B.: conceptualization, writing—original draft; J.J.C.: conceptualization, writing—review and editing; D.R.T.: writing—review and editing; A.E.F.: supervision, writing—review and editing; D.S.: supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Gabaldón T, Huynen MA. 2004. Shaping the mitochondrial proteome. Biochim. Biophys. Acta (BBA) - Bioenerg. 1659, 212-220. ( 10.1016/j.bbabio.2004.07.011) [DOI] [PubMed] [Google Scholar]
- 2.Gabaldón T, Huynen MA. 2003. Reconstruction of the proto-mitochondrial metabolism. Science 301, 609. ( 10.1126/science.1085463) [DOI] [PubMed] [Google Scholar]
- 3.Friedman JR, Nunnari J. 2014. Mitochondrial form and function. Nature 505, 335-343. ( 10.1038/nature12985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfanner N, Warscheid B, Wiedemann N. 2019. Mitochondrial proteins: from biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 20, 267-284. ( 10.1038/s41580-018-0092-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burté F, Carelli V, Chinnery PF, Yu-Wai-Man P. 2015. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 11, 11-24. ( 10.1038/nrneurol.2014.228) [DOI] [PubMed] [Google Scholar]
- 6.Vyas S, Zaganjor E, Haigis MC. 2016. Mitochondria and cancer. Cell 166, 555-566. ( 10.1016/j.cell.2016.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam MT. 2017. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 39, 73-82. ( 10.1080/01616412.2016.1251711) [DOI] [PubMed] [Google Scholar]
- 8.Tatsuta T, Langer T. 2008. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 27, 306-314. ( 10.1038/sj.emboj.7601972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorzano A, Liesa M, Palacín M. 2009. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int. J. Biochem. Cell Biol. 41, 1846-1854. ( 10.1016/j.biocel.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 10.Baker BM, Haynes CM. 2011. Mitochondrial protein quality control during biogenesis and aging. Trends Biochem. Sci. 36, 254-261. ( 10.1016/j.tibs.2011.01.004) [DOI] [PubMed] [Google Scholar]
- 11.Moehle EA, Shen K, Dillin A. 2019. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J. Biol. Chem. 294, 5396-5407. ( 10.1074/jbc.TM117.000893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rath S, et al. 2021. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49(D1), D1541-D1547. ( 10.1093/nar/gkaa1011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazier AE, Thorburn DR, Compton AG. 2019. Mitochondrial energy generation disorders: genes, mechanisms, and clues to pathology. J. Biol. Chem. 294, 5386-5395. ( 10.1074/jbc.R117.809194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson K, et al. 2020. Recent advances in understanding the molecular genetic basis of mitochondrial disease. J. Inherit. Metab. Dis. 43, 36-50. ( 10.1002/jimd.12104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenton SL, Prokisch H. 2020. Genetics of mitochondrial diseases: identifying mutations to help diagnosis. EBioMed. 56, 102784. ( 10.1016/j.ebiom.2020.102784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gusic M, Prokisch H. 2021. Genetic basis of mitochondrial diseases. FEBS Lett. 595, 1132-1158. ( 10.1002/1873-3468.14068) [DOI] [PubMed] [Google Scholar]
- 17.Niyazov DM, Kahler SG, Frye RE. 2016. Primary mitochondrial disease and secondary mitochondrial dysfunction: importance of distinction for diagnosis and treatment. Mol. Syndromol. 7, 122-137. ( 10.1159/000446586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorburn DR. 2004. Mitochondrial disorders: prevalence, myths and advances. J. Inherit. Metab. Dis. 27, 349-362. ( 10.1023/B:BOLI.0000031098.41409.55) [DOI] [PubMed] [Google Scholar]
- 19.Gorman GS, et al. 2015. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753-759. ( 10.1002/ana.24362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlieben LD, Prokisch H. 2020. The Dimensions of primary mitochondrial disorders. Front. Cell Dev. Biol. 8, 600079. ( 10.3389/fcell.2020.600079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alston CL, Stenton SL, Hudson G, Prokisch H, Taylor RW. 2021. The genetics of mitochondrial disease: dissecting mitochondrial pathology using multi-omic pipelines. J. Pathol. 254, 430-442. ( 10.1002/path.5641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schapira AHV. 2006. Mitochondrial disease. Lancet 368, 70-82. ( 10.1016/S0140-6736(06)68970-8) [DOI] [PubMed] [Google Scholar]
- 23.Rahman S. 2020. Mitochondrial disease in children. J. Intern. Med. 287, 609-633. ( 10.1111/joim.13054) [DOI] [PubMed] [Google Scholar]
- 24.Craven L, Alston CL, Taylor RW, Turnbull DM. 2017. Recent advances in mitochondrial disease. Ann. Rev. Genom. Hum. Genet. 18, 257-275. ( 10.1146/annurev-genom-091416-035426) [DOI] [PubMed] [Google Scholar]
- 25.Schon KR, Ratnaike T, van den Ameele J, Horvath R, Chinnery PF. 2020. Mitochondrial diseases: a diagnostic revolution. Trends Genet. 36, 702-717. ( 10.1016/j.tig.2020.06.009) [DOI] [PubMed] [Google Scholar]
- 26.Wiedemann N, Pfanner N. 2017. Mitochondrial machineries for protein import and assembly. Annu. Rev. Biochem. 86, 685-714. ( 10.1146/annurev-biochem-060815-014352) [DOI] [PubMed] [Google Scholar]
- 27.Palmer CS, Anderson AJ, Stojanovski D. 2021. Mitochondrial protein import dysfunction: mitochondrial disease, neurodegenerative disease and cancer. FEBS Lett. 595, 1107-1131. ( 10.1002/1873-3468.14022) [DOI] [PubMed] [Google Scholar]
- 28.Dutta D, et al. 2020. De novo mutations in TOMM70, a receptor of the mitochondrial import translocase, cause neurological impairment. Hum. Mol. Genet. 29, 1568-1579. ( 10.1093/hmg/ddaa081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei X, et al. 2020. Mutations in TOMM70 lead to multi-OXPHOS deficiencies and cause severe anemia, lactic acidosis, and developmental delay. J. Hum. Genet. 65, 231-240. ( 10.1038/s10038-019-0714-1) [DOI] [PubMed] [Google Scholar]
- 30.Palmieri F. 2004. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflügers Archiv. 447, 689-709. ( 10.1007/s00424-003-1099-7) [DOI] [PubMed] [Google Scholar]
- 31.Ferramosca A, Zara V. 2013. Biogenesis of mitochondrial carrier proteins: molecular mechanisms of import into mitochondria. Biochim. Biophys. Acta (BBA) - Mol. Cell Res., 1833, 494-502. ( 10.1016/j.bbamcr.2012.11.014) [DOI] [PubMed] [Google Scholar]
- 32.Horten P, Colina-Tenorio L, Rampelt H. 2020. Biogenesis of mitochondrial metabolite carriers. Biomolecules 10, 1008. ( 10.3390/biom10071008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunji ER, et al. 2016. The transport mechanism of the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta. 1863, 2379-2393. ( 10.1016/j.bbamcr.2016.03.015) [DOI] [PubMed] [Google Scholar]
- 34.Ruprecht JJ, et al. 2019. The molecular mechanism of transport by the mitochondrial ADP/ATP carrier. Cell 176, 435-447. ( 10.1016/j.cell.2018.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacheu-Grau D, et al. 2018. Mutations of the mitochondrial carrier translocase channel subunit TIM22 cause early-onset mitochondrial myopathy. Hum. Mol. Genet. 27, 4135-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haghighi A, et al. 2014. Sengers syndrome: six novel AGK mutations in seven new families and review of the phenotypic and mutational spectrum of 29 patients. Orphanet J. Rare Dis. 9, 119. ( 10.1186/s13023-014-0119-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Caballero L, Guerrero-Castillo S, Nijtmans L. 2016. Unraveling the complexity of mitochondrial complex I assembly: a dynamic process. Biochim. Biophys. Acta. 1857, 980-990. ( 10.1016/j.bbabio.2016.03.031) [DOI] [PubMed] [Google Scholar]
- 38.Andrews B, Carroll J, Ding S, Fearnley IM, Walker JE. 2013. Assembly factors for the membrane arm of human complex I. Proc. Natl Acad. Sci. USA 110, 18 934-18 939. ( 10.1073/pnas.1319247110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson TD, et al. 2022. Sideroflexin 4 is a complex I assembly factor that interacts with the MCIA complex and is required for the assembly of the ND2 module. Proc. Natl Acad. Sci. USA 119, e2115566119. ( 10.1073/pnas.2115566119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson TD, et al. 2021. The TIM22 complex mediates the import of sideroflexins and is required for efficient mitochondrial one-carbon metabolism. Mol Biol Cell. 32, 475-491. ( 10.1091/mbc.E20-06-0390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvo SE, et al. 2012. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 4, 118ra10. ( 10.1126/scitranslmed.3003310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johannes AM, et al. 2012. Lack of the mitochondrial protein acylglycerol kinase causes Sengers syndrome. Am. J. Hum. Genet. 90, 314-320. ( 10.1016/j.ajhg.2011.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang Y, et al. 2017. Sengers syndrome-associated mitochondrial acylglycerol kinase is a subunit of the human TIM22 protein import complex. Mol. Cell. 67, 457-470. ( 10.1016/j.molcel.2017.06.014) [DOI] [PubMed] [Google Scholar]
- 44.Vukotic M, Nolte H, König T, Saita S, Ananjew M, Krüger M, Tatsuta T, Langer T. 2017. Acylglycerol kinase mutated in Sengers syndrome is a subunit of the TIM22 protein translocase in mitochondria. Mol. Cell. 67, 471-483. ( 10.1016/j.molcel.2017.06.013) [DOI] [PubMed] [Google Scholar]
- 45.Barbosa-Gouveia S, et al. 2021. Characterization of a novel splicing variant in acylglycerol kinase (AGK) associated with fatal Sengers Syndrome. Int. J. Mol. Sci. 22, 13484. ( 10.3390/ijms222413484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acoba MG, et al. 2021. The mitochondrial carrier SFXN1 is critical for complex III integrity and cellular metabolism. Cell Rep. 34, 108869. ( 10.1016/j.celrep.2021.108869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang Y, Fielden LF, Stojanovski D. 2018. Mitochondrial protein transport in health and disease. Semin. Cell Dev. Biol. 76, 142-153. ( 10.1016/j.semcdb.2017.07.028) [DOI] [PubMed] [Google Scholar]
- 48.Ahting U, et al. 2009. Neurological phenotype and reduced lifespan in heterozygous Tim23 knockout mice, the first mouse model of defective mitochondrial import. Biochim. Biophys. Acta 1787, 371-376. ( 10.1016/j.bbabio.2008.12.001) [DOI] [PubMed] [Google Scholar]
- 49.Sinha D, Srivastava S, Krishna L, D'Silva P. 2014. Unraveling the intricate organization of mammalian mitochondrial presequence translocases: existence of multiple translocases for maintenance of mitochondrial function. Mol. Cell Biol. 34, 1757-1775. ( 10.1128/MCB.01527-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatle KM, et al. 2013. MCJ/DnaJC15, an endogenous mitochondrial repressor of the respiratory chain that controls metabolic alterations. Mol. Cell Biol. 33, 2302-2314. ( 10.1128/MCB.00189-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davey KM, et al. 2006. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J. Med. Genet. 43, 385-393. ( 10.1136/jmg.2005.036657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wortmann SB, et al. 2013. 3-Methylglutaconic aciduria–lessons from 50 genes and 977 patients. J. Inherit. Metab. Dis. 36, 913-921. ( 10.1007/s10545-012-9579-6) [DOI] [PubMed] [Google Scholar]
- 53.Wortmann SB, Duran M, Anikster Y, Barth PG, Sperl W, Zschocke J, Morava E, Wevers RA. 2013. Inborn errors of metabolism with 3-methylglutaconic aciduria as discriminative feature: proper classification and nomenclature. J. Inherit. Metab. Dis. 36, 923-928. ( 10.1007/s10545-012-9580-0) [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto H, Esaki M, Kanamori T, Tamura Y, Nishikawa S-I, Endo T. 2002. Tim50 is a subunit of the TIM23 complex that links protein translocation across the outer and inner mitochondrial membranes. Cell 111, 519-528. ( 10.1016/S0092-8674(02)01053-X) [DOI] [PubMed] [Google Scholar]
- 55.Mir A, Hadab S, Sammak M, Alhazmi R, Housawi Y, Bashir S. 2020. Complete resolution of epileptic spasms with vigabatrin in a patient with 3-methylglutaconic aciduria caused by TIMM50 gene mutation. Clin. Genet. 98, 102-103. ( 10.1111/cge.13763) [DOI] [PubMed] [Google Scholar]
- 56.Shahrour MA et al. 2017. Mitochondrial epileptic encephalopathy, 3-methylglutaconic aciduria and variable complex V deficiency associated with TIMM50 mutations. Clin. Genet. 91, 690-696. ( 10.1111/cge.12855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyes A, Melchionda L, Burlina A, Robinson AJ, Ghezzi D, Zeviani M. 2018. Mutations in TIMM50 compromise cell survival in OxPhos-dependent metabolic conditions. EMBO Mol. Med. 10, e8698. ( 10.15252/emmm.201708698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt O, Pfanner N, Meisinger C. 2010. Mitochondrial protein import: from proteomics to functional mechanisms. Nat. Rev. Mol. Cell Biol. 11, 655-667. ( 10.1038/nrm2959) [DOI] [PubMed] [Google Scholar]
- 59.Thompson K, et al. 2018. OXA1L mutations cause mitochondrial encephalopathy and a combined oxidative phosphorylation defect. EMBO Mol. Med. 10, e9060. ( 10.15252/emmm.201809060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paschen SA, Rothbauer U, Káldi K, Bauer MF, Neupert W, Brunner M. 2000. The role of the TIM8–13 complex in the import of Tim23 into mitochondria. EMBO J. 19, 6392-6400. ( 10.1093/emboj/19.23.6392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothbauer U, Hofmann S, Mühlenbein N, Paschen SA, Gerbitz KD, Neupert W, Brunner M, Bauer MF. 2001. Role of the deafness dystonia peptide 1 (DDP1) in import of human Tim23 into the inner membrane of mitochondria. J. Biol. Chem. 276, 37 327-37 334. ( 10.1074/jbc.M105313200) [DOI] [PubMed] [Google Scholar]
- 62.Dudek J, Rehling P, van der Laan M. 2013. Mitochondrial protein import: common principles and physiological networks. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1833, 274-285. ( 10.1016/j.bbamcr.2012.05.028) [DOI] [PubMed] [Google Scholar]
- 63.Tranebjaerg L, Hamel BC, Gabreels FJ, Renier WO, Van Ghelue M. 2000. A de novo missense mutation in a critical domain of the X-linked DDP gene causes the typical deafness-dystonia-optic atrophy syndrome. Eur. J. Hum. Genet. 8, 464-467. ( 10.1038/sj.ejhg.5200483) [DOI] [PubMed] [Google Scholar]
- 64.Ujike H, Tanabe Y, Takehisa Y, Hayabara T, Kuroda S. 2001. A family with X-linked dystonia-deafness syndrome with a novel mutation of the DDP gene. Arch. Neurol. 58, 1004-1007. ( 10.1001/archneur.58.6.1004) [DOI] [PubMed] [Google Scholar]
- 65.Kang Y, et al. 2019. Function of hTim8a in complex IV assembly in neuronal cells provides insight into pathomechanism underlying Mohr-Tranebjærg syndrome. Elife 8, e56968. ( 10.7554/eLife.48828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner K, Mick DU, Rehling P. 2009. Protein transport machineries for precursor translocation across the inner mitochondrial membrane. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1793, 52-59. ( 10.1016/j.bbamcr.2008.05.026) [DOI] [PubMed] [Google Scholar]
- 67.Callegari S, Cruz-Zaragoza LD, Rehling P. 2020. From TOM to the TIM23 complex – handing over of a precursor. Biol. Chem. 401, 709-721. ( 10.1515/hsz-2020-0101) [DOI] [PubMed] [Google Scholar]
- 68.Fischer F, Hamann A, Osiewacz HD. 2012. Mitochondrial quality control: an integrated network of pathways. Trends Biochem. Sci. 37, 284-292. ( 10.1016/j.tibs.2012.02.004) [DOI] [PubMed] [Google Scholar]
- 69.Koppen M, Langer T. 2007. Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit. Rev. Biochem. Mol. Biol. 42, 221-242. ( 10.1080/10409230701380452) [DOI] [PubMed] [Google Scholar]
- 70.Cupo RR, Shorter J. 2020. Skd3 (human ClpB) is a potent mitochondrial protein disaggregase that is inactivated by 3-methylglutaconic aciduria-linked mutations. Elife 9, e55279. ( 10.7554/eLife.55279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magen D, et al. 2008. Mitochondrial Hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am. J. Hum. Genet. 83, 30-42. ( 10.1016/j.ajhg.2008.05.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glynn SE. 2017. Multifunctional mitochondrial AAA proteases. Front. Mol. Biosci. 4, 34. ( 10.3389/fmolb.2017.00034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koppen M, Metodiev MD, Casari G, Rugarli EI, Langer T. 2007. Variable and tissue-specific subunit composition of mitochondrial m-AAA protease complexes linked to hereditary spastic paraplegia. Mol. Cell Biol. 27, 758-767. ( 10.1128/MCB.01470-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arlt H, Steglich G, Perryman R, Guiard B, Neupert W, Langer T. 1998. The formation of respiratory chain complexes in mitochondria is under the proteolytic control of the m-AAA protease. EMBO J. 17, 4837-4847. ( 10.1093/emboj/17.16.4837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nolden M, Ehses S, Koppen M, Bernacchia A, Rugarli EI, Langer T. 2005. The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria. Cell 123, 277-289. ( 10.1016/j.cell.2005.08.003) [DOI] [PubMed] [Google Scholar]
- 76.Consolato F, Maltecca F, Tulli S, Sambri I. 2018. Casari G. m-AAA and i-AAA complexes coordinate to regulate OMA1, the stress-activated supervisor of mitochondrial dynamics. J. Cell Sci. 131, jcs213546. ( 10.1242/jcs.213546) [DOI] [PubMed] [Google Scholar]
- 77.Puchades C, Ding B, Song A, Wiseman RL, Lander GC, Glynn SE. 2019. Unique structural features of the mitochondrial AAA+ protease AFG3L2 reveal the molecular basis for activity in health and disease. Mol. Cell. 75, 1073-1085. ( 10.1016/j.molcel.2019.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atorino L, Silvestri L, Koppen M, Cassina L, Ballabio A, Marconi R, Langer T, Casari G. 2003. Loss of m-AAA protease in mitochondria causes complex I deficiency and increased sensitivity to oxidative stress in hereditary spastic paraplegia. J. Cell Biol. 163, 777-787. ( 10.1083/jcb.200304112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casari G, et al. 1998. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell 93, 973-983. ( 10.1016/S0092-8674(00)81203-9) [DOI] [PubMed] [Google Scholar]
- 80.Arnoldi A, et al. 2008. A clinical, genetic, and biochemical characterization of SPG7 mutations in a large cohort of patients with hereditary spastic paraplegia. Hum. Mutat. 29, 522-531. ( 10.1002/humu.20682) [DOI] [PubMed] [Google Scholar]
- 81.Sánchez-Ferrero E, et al. 2013. SPG7 mutational screening in spastic paraplegia patients supports a dominant effect for some mutations and a pathogenic role for p.A510V. Clin. Genet. 83, 257-262. ( 10.1111/j.1399-0004.2012.01896.x) [DOI] [PubMed] [Google Scholar]
- 82.Klebe S, et al. 2012. Spastic paraplegia gene 7 in patients with spasticity and/or optic neuropathy. Brain 135(Pt 10), 2980-2993. ( 10.1093/brain/aws240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verdura E, et al. 2020. A deep intronic splice variant advises reexamination of presumably dominant SPG7 Cases. Ann. Clin. Transl. Neurol. 7, 105-111. ( 10.1002/acn3.50967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Estiar MA, et al. 2021. Evidence for non-mendelian inheritance in spastic paraplegia 7. Mov. Disord. 36, 1664-1675. ( 10.1002/mds.28528) [DOI] [PubMed] [Google Scholar]
- 85.Di Bella D, et al. 2010. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat. Genet. 42, 313-321. ( 10.1038/ng.544) [DOI] [PubMed] [Google Scholar]
- 86.Caporali L, et al. 2020. ATPase domain AFG3L2 mutations alter OPA1 processing and cause optic neuropathy. Ann. Neurol. 88, 18-32. ( 10.1002/ana.25723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pierson TM, et al. 2011. Whole-exome sequencing identifies homozygous AFG3L2 mutations in a spastic ataxia-neuropathy syndrome linked to mitochondrial m-AAA proteases. PLOS Genet. 7, e1002325. ( 10.1371/journal.pgen.1002325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartmann B, et al. 2016. Homozygous YME1L1 mutation causes mitochondriopathy with optic atrophy and mitochondrial network fragmentation. Elife 5, e16078. ( 10.7554/eLife.16078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voos W. 2013. Chaperone–protease networks in mitochondrial protein homeostasis. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1833, 388-399. ( 10.1016/j.bbamcr.2012.06.005) [DOI] [PubMed] [Google Scholar]
- 90.Thevarajan I, Zolkiewski M, Zolkiewska A. 2020. Human CLPB forms ATP-dependent complexes in the mitochondrial intermembrane space. Int. J. Biochem. Cell Biol. 127, 105841. ( 10.1016/j.biocel.2020.105841) [DOI] [PubMed] [Google Scholar]
- 91.Cupo RR, et al. 2022. Unique structural features govern the activity of a human mitochondrial AAA+ disaggregase, Skd3. bioRxiv. 2022:2022.02.17.480866.
- 92.Wortmann SB, et al. 2015. CLPB mutations cause 3-methylglutaconic aciduria, progressive brain atrophy, intellectual disability, congenital neutropenia, cataracts, movement disorder. Am. J. Hum. Genet. 96, 245-257. ( 10.1016/j.ajhg.2014.12.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wortmann SB, et al. 2021. Neutropenia and intellectual disability are hallmarks of biallelic and de novo CLPB deficiency. Genet. Med. 23, 1705-1714. ( 10.1038/s41436-021-01194-x) [DOI] [PubMed] [Google Scholar]
- 94.Warren JT, et al. 2021. Heterozygous variants of CLPB are a cause of severe congenital neutropenia. Blood 139, 779-791. ( 10.1182/blood.2021010762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Braymer JJ, Lill R. 2017. Iron–sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 292, 12 754-12 763. ( 10.1074/jbc.R117.787101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beinert H. 2000. Iron-sulfur proteins: ancient structures, still full of surprises. J. Biol. Inorg. Chem. 5, 2-15. ( 10.1007/s007750050002) [DOI] [PubMed] [Google Scholar]
- 97.Daniel RM, Danson MJ. 1995. Did primitive microorganisms use nonhem iron proteins in place of NAD/P? J. Mol. Evol. 40, 559-563. ( 10.1007/BF00160501) [DOI] [Google Scholar]
- 98.Alfadhel M, Nashabat M, Abu Ali Q, Hundallah K. 2017. Mitochondrial iron-sulfur cluster biogenesis from molecular understanding to clinical disease. Neurosciences 22, 4-13. ( 10.17712/nsj.2017.1.20160542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rouault TA, Tong WH. 2008. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 24, 398-407. ( 10.1016/j.tig.2008.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lane DJ, Merlot AM, Huang ML, Bae DH, Jansson PJ, Sahni S, Kalinowski DS, Richardson DR. 2015. Cellular iron uptake, trafficking and metabolism: key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta 1853, 1130-1144. ( 10.1016/j.bbamcr.2015.01.021) [DOI] [PubMed] [Google Scholar]
- 101.Stehling O, Wilbrecht C, Lill R. 2014. Mitochondrial iron–sulfur protein biogenesis and human disease. Biochimie 100, 61-77. ( 10.1016/j.biochi.2014.01.010) [DOI] [PubMed] [Google Scholar]
- 102.Lill R, et al. 2012. The role of mitochondria in cellular iron–sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1823, 1491-1508. ( 10.1016/j.bbamcr.2012.05.009) [DOI] [PubMed] [Google Scholar]
- 103.Lebigot E, Schiff M, Golinelli-Cohen MP. 2021. A review of multiple mitochondrial dysfunction syndromes, syndromes associated with defective Fe-S protein maturation. Biomedicines 9, 989. ( 10.3390/biomedicines9080989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pandolfo M. 2008. Friedreich ataxia. Arch. Neurol. 65, 1296-1303. ( 10.1001/archneur.65.10.1296) [DOI] [PubMed] [Google Scholar]
- 105.Bürk K. 2017. Friedreich Ataxia: current status and future prospects. Cerebellum Ataxias 4, 4. ( 10.1186/s40673-017-0062-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cossée M, et al. 2000. Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum. Mol. Genet. 9, 1219-1226. ( 10.1093/hmg/9.8.1219) [DOI] [PubMed] [Google Scholar]
- 107.Radisky DC, Babcock MC, Kaplan J. 1999. The yeast frataxin homologue mediates mitochondrial iron efflux. evidence for a mitochondrial iron cycle. J. Biol. Chem. 274, 4497-4499. ( 10.1074/jbc.274.8.4497) [DOI] [PubMed] [Google Scholar]
- 108.Martelli A, Puccio H. 2014. Dysregulation of cellular iron metabolism in Friedreich ataxia: from primary iron-sulfur cluster deficit to mitochondrial iron accumulation. Front. Pharmacol. 5, 130. ( 10.3389/fphar.2014.00130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsai CL, Barondeau DP. 2010. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry 49, 9132-9139. ( 10.1021/bi1013062) [DOI] [PubMed] [Google Scholar]
- 110.Toyokuni S. 2002. Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep. 7, 189-197. ( 10.1179/135100002125000596) [DOI] [PubMed] [Google Scholar]
- 111.Heidari MM, Houshmand M, Hosseinkhani S, Nafissi S, Khatami M. 2009. Complex I and ATP content deficiency in lymphocytes from Friedreich's ataxia. Can. J. Neurol. Sci. 36, 26-31. ( 10.1017/S0317167100006260) [DOI] [PubMed] [Google Scholar]
- 112.Rufini A, et al. 2015. Highly specific ubiquitin-competing molecules effectively promote frataxin accumulation and partially rescue the aconitase defect in Friedreich ataxia cells. Neurobiol. Dis. 75, 91-99. ( 10.1016/j.nbd.2014.12.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tai G, Corben LA, Yiu EM, Milne SC, Delatycki MB. 2018. Progress in the treatment of Friedreich ataxia. Neurologia i Neurochirurgia Polska 52, 129-139. ( 10.1016/j.pjnns.2018.02.003) [DOI] [PubMed] [Google Scholar]
- 114.Dashty M. 2013. A quick look at biochemistry: carbohydrate metabolism. Clin. Biochem. 46, 1339-1352. ( 10.1016/j.clinbiochem.2013.04.027) [DOI] [PubMed] [Google Scholar]
- 115.Kato M, Wynn RM, Chuang JL, Tso S-C, Machius M, Li J, Chuang DT. 2008. Structural basis for inactivation of the human pyruvate dehydrogenase complex by phosphorylation: role of disordered phosphorylation loops. Structure 16, 1849-1859. ( 10.1016/j.str.2008.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patel MS, Korotchkina LG. 2006. Regulation of the pyruvate dehydrogenase complex. Biochem. Soc. Trans. 34(Pt 2), 217-222. ( 10.1042/BST0340217) [DOI] [PubMed] [Google Scholar]
- 117.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. 2008. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J. Biol. Chem. 283, 28 106-28 114. ( 10.1074/jbc.M803508200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maj MC, MacKay N, Levandovskiy V, Addis J, Baumgartner ER, Baumgartner MR, Robinson BH, Cameron JM. 2005. Pyruvate dehydrogenase phosphatase deficiency: identification of the first mutation in two brothers and restoration of activity by protein complementation. J. Clin. Endocrinol. Metab. 90, 4101-4107. ( 10.1210/jc.2005-0123) [DOI] [PubMed] [Google Scholar]
- 119.Cameron JM, Levandovskiy V, Mackay N, Tein I, Robinson BH. 2004. Deficiency of pyruvate dehydrogenase caused by novel and known mutations in the E1alpha subunit. Am. J. Med. Genet. A. 131, 59-66. ( 10.1002/ajmg.a.30287) [DOI] [PubMed] [Google Scholar]
- 120.Okajima K, Korotchkina LG, Prasad C, Rupar T, Phillips JA, Ficicioglu C, Hertecant J, Patel MS, Kerr DS. 2008. Mutations of the E1β subunit gene (PDHB) in four families with pyruvate dehydrogenase deficiency. Mol. Genet. Metabol. 93, 371-380. ( 10.1016/j.ymgme.2007.10.135) [DOI] [PubMed] [Google Scholar]
- 121.Head RA, Brown RM, Zolkipli Z, Shahdadpuri R, King MD, Clayton PT, Brown GK. 2005. Clinical and genetic spectrum of pyruvate dehydrogenase deficiency: dihydrolipoamide acetyltransferase (E2) deficiency. Ann. Neurol. 58, 234-241. ( 10.1002/ana.20550) [DOI] [PubMed] [Google Scholar]
- 122.Kennerson ML, et al. 2013. A new locus for X-linked dominant Charcot–Marie–Tooth disease (CMTX6) is caused by mutations in the pyruvate dehydrogenase kinase isoenzyme 3 (PDK3) gene. Hum. Mol. Genet. 22, 1404-1416. ( 10.1093/hmg/dds557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cameron JM, Levandovskiy V, Mackay N, Raiman J, Renaud DL, Clarke JT, Feigenbaum A, Elpeleg O, Robinson BH. 2006. Novel mutations in dihydrolipoamide dehydrogenase deficiency in two cousins with borderline-normal PDH complex activity. Am. J. Med. Genet. A. 140, 1542-1552. ( 10.1002/ajmg.a.31313) [DOI] [PubMed] [Google Scholar]
- 124.Dey R, Aral B, Abitbol M, Marsac C. 2002. Pyruvate dehydrogenase deficiency as a result of splice-site mutations in the PDX1 gene. Mol. Genet. Metab. 76, 344-347. ( 10.1016/S1096-7192(02)00104-X) [DOI] [PubMed] [Google Scholar]
- 125.Martínez-Reyes I, Chandel NS. 2020. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102. ( 10.1038/s41467-019-13668-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Palmieri F, Pierri CL. 2010. Mitochondrial metabolite transport. Essays Biochem. 47, 37-52. ( 10.1042/bse0470037) [DOI] [PubMed] [Google Scholar]
- 127.Wohlrab H. 2005. The human mitochondrial transport protein family: identification and protein regions significant for transport function and substrate specificity. Biochim. Biophys. Acta (BBA) - Bioenerg. 1709, 157-168. ( 10.1016/j.bbabio.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 128.Seifert EL, Ligeti E, Mayr JA, Sondheimer N, Hajnóczky G. 2015. The mitochondrial phosphate carrier: role in oxidative metabolism, calcium handling and mitochondrial disease. Biochem. Biophys. Res. Commun. 464, 369-375. ( 10.1016/j.bbrc.2015.06.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mayr JA, et al. 2007. Mitochondrial phosphate-carrier deficiency: a novel disorder of oxidative phosphorylation. Am. J. Hum. Genet. 80, 478-484. ( 10.1086/511788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dolce V, Iacobazzi V, Palmieri F, Walker JE. 1994. The sequences of human and bovine genes of the phosphate carrier from mitochondria contain evidence of alternatively spliced forms. J. Biol. Chem. 269, 10 451-10 460. ( 10.1016/S0021-9258(17)34081-4) [DOI] [PubMed] [Google Scholar]
- 131.Bhoj EJ, et al. 2015. Pathologic variants of the mitochondrial phosphate carrier SLC25A3: two new patients and expansion of the cardiomyopathy/skeletal myopathy phenotype with and without lactic acidosis. JIMD Rep. 19, 59-66. ( 10.1007/8904_2014_364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mayr JA, Zimmermann FA, Horváth R, Schneider HC, Schoser B, Holinski-Feder E, Czermin B, Freisinger P, Sperl W. 2011. Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children. Neuromuscul. Disord. 21, 803-808. ( 10.1016/j.nmd.2011.06.005) [DOI] [PubMed] [Google Scholar]
- 133.Duchen MR, Verkhratsky A, Muallem S. 2008. Mitochondria and calcium in health and disease. Cell Calcium 44, 1-5. ( 10.1016/j.ceca.2008.02.001) [DOI] [PubMed] [Google Scholar]
- 134.Kamer KJ, Mootha VK. 2014. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 15, 299-307. ( 10.1002/embr.201337946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kirichok Y, Krapivinsky G, Clapham DE. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360-364. ( 10.1038/nature02246) [DOI] [PubMed] [Google Scholar]
- 136.Taylor EB. 2017. Functional properties of the mitochondrial carrier system. Trends Cell Biol. 27, 633-644. ( 10.1016/j.tcb.2017.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Palmieri F, Monné M. 2016. Discoveries, metabolic roles and diseases of mitochondrial carriers: a review. Biochim. Biophys. Acta. 1863, 2362-2378. ( 10.1016/j.bbamcr.2016.03.007) [DOI] [PubMed] [Google Scholar]
- 138.Mallilankaraman K, et al. 2012. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell 151, 630-644. ( 10.1016/j.cell.2012.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patron M, et al. 2014. MICU1 and MICU2 finely tune the mitochondrial Ca2 + uniporter by exerting opposite effects on MCU activity. Mol. Cell 53, 726-737. ( 10.1016/j.molcel.2014.01.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Logan CV, et al. 2014. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat. Genet. 46, 188-193. ( 10.1038/ng.2851) [DOI] [PubMed] [Google Scholar]
- 141.Shamseldin HE, et al. 2017. A null mutation in MICU2 causes abnormal mitochondrial calcium homeostasis and a severe neurodevelopmental disorder. Brain 140, 2806-2813. ( 10.1093/brain/awx237) [DOI] [PubMed] [Google Scholar]
- 142.Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P. 2018. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 69, 62-72. ( 10.1016/j.ceca.2017.05.003) [DOI] [PubMed] [Google Scholar]
- 143.Palmieri F. 2008. Diseases caused by defects of mitochondrial carriers: a review. Biochim. Biophys. Acta 1777, 564-578. ( 10.1016/j.bbabio.2008.03.008) [DOI] [PubMed] [Google Scholar]
- 144.Tiranti V, et al. 2009. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 15, 200-205. ( 10.1038/nm.1907) [DOI] [PubMed] [Google Scholar]
- 145.Tiranti V, et al. 2004. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am. J. Hum. Genet. 74, 239-252. ( 10.1086/381653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Di Meo I, Lamperti C, Tiranti V. 2015. Mitochondrial diseases caused by toxic compound accumulation: from etiopathology to therapeutic approaches. EMBO Mol. Med. 7, 1257-1266. ( 10.15252/emmm.201505040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoshida A, Dave V. 1975. Inhibition of NADP-dependent dehydrogenases by modified products of NADPH. Arch. Biochem. Biophys. 169, 298-303. ( 10.1016/0003-9861(75)90344-6) [DOI] [PubMed] [Google Scholar]
- 148.Marbaix AY, Noël G, Detroux AM, Vertommen D, Van Schaftingen E, Linster CL. 2011. Extremely conserved ATP- or ADP-dependent enzymatic system for nicotinamide nucleotide repair. J. Biol. Chem. 286, 41 246-41 252. ( 10.1074/jbc.C111.310847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kremer LS, et al. 2016. NAXE mutations disrupt the cellular NAD(P)HX repair system and cause a lethal neurometabolic disorder of early childhood. Am. J. Hum. Genet. 99, 894-902. ( 10.1016/j.ajhg.2016.07.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van Bergen NJ, et al. 2019. NAD(P)HX dehydratase (NAXD) deficiency: a novel neurodegenerative disorder exacerbated by febrile illnesses. Brain 142, 50-58. ( 10.1093/brain/awy310) [DOI] [PubMed] [Google Scholar]
- 151.Borna NN, et al. 2020. NAD(P)HX dehydratase protein-truncating mutations are associated with neurodevelopmental disorder exacerbated by acute illness. Brain 143, e54. ( 10.1093/brain/awaa130) [DOI] [PubMed] [Google Scholar]
- 152.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. 2009. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 47, 333-343. ( 10.1016/j.freeradbiomed.2009.05.004) [DOI] [PubMed] [Google Scholar]
- 153.D'Autréaux B, Toledano MB. 2007. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813-824. ( 10.1038/nrm2256) [DOI] [PubMed] [Google Scholar]
- 154.Holley AK, Bakthavatchalu V, Velez-Roman JM, St. Clair DK. 2011. Manganese superoxide dismutase: guardian of the powerhouse. Int. J. Mol. Sci. 12, 7114-7162. ( 10.3390/ijms12107114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Holzerova E, et al. 2016. Human thioredoxin 2 deficiency impairs mitochondrial redox homeostasis and causes early-onset neurodegeneration. Brain 139(Pt 2), 346-354. ( 10.1093/brain/awv350) [DOI] [PubMed] [Google Scholar]
- 156.Nonn L, Williams RR, Erickson RP, Powis G. 2003. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 23, 916. ( 10.1128/MCB.23.3.916-922.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mahmood DF, Abderrazak A, El Hadri K, Simmet T, Rouis M. 2013. The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox Signal. 19, 1266-1303. ( 10.1089/ars.2012.4757) [DOI] [PubMed] [Google Scholar]
- 158.Rybnikova E, Damdimopoulos AE, Gustafsson J-Å, Spyrou G, Pelto-Huikko M. 2000. Expression of novel antioxidant thioredoxin-2 in the rat brain. Eur. J. Neurosci. 12, 1669-1678. ( 10.1046/j.1460-9568.2000.00059.x) [DOI] [PubMed] [Google Scholar]
- 159.Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. 2012. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 322, 254-262. ( 10.1016/j.jns.2012.05.030) [DOI] [PubMed] [Google Scholar]
- 160.Andreyev AY, Kushnareva YE, Murphy AN, Starkov AA. 2015. Mitochondrial ROS metabolism: 10 years later. Biochemistry 80, 517-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Auranen M, et al. 2017. Patient with multiple acyl-CoA dehydrogenation deficiency disease and FLAD1 mutations benefits from riboflavin therapy. Neuromuscul. Disord. 27, 581-584. ( 10.1016/j.nmd.2017.03.003) [DOI] [PubMed] [Google Scholar]
- 162.Olsen RKJ, et al. 2016. Riboflavin-responsive and -non-responsive mutations in FAD synthase cause multiple Acyl-CoA dehydrogenase and combined respiratory-chain deficiency. Am. J. Hum. Genet. 98, 1130-1145. ( 10.1016/j.ajhg.2016.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mayr JA, Zimmermann FA, Fauth C, Bergheim C, Meierhofer D, Radmayr D, Zschocke J, Koch J, Sperl W. 2011. Lipoic acid synthetase deficiency causes neonatal-onset epilepsy, defective mitochondrial energy metabolism, and glycine elevation. Am. J. Hum. Genet. 89, 792-797. ( 10.1016/j.ajhg.2011.11.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baker PR, et al. 2014. Variant non ketotic hyperglycinemia is caused by mutations in LIAS, BOLA3 and the novel gene GLRX5. Brain 137(Pt 2), 366-379. ( 10.1093/brain/awt328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Soreze Y, et al. 2013. Mutations in human lipoyltransferase gene LIPT1 cause a Leigh disease with secondary deficiency for pyruvate and alpha-ketoglutarate dehydrogenase. Orphanet. J. Rare. Dis. 8, 192. ( 10.1186/1750-1172-8-192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu L, Wu R, Ye Z, Gu R, Wang Y, Hou Y, Feng Z, Ma X. 2019. Identification of two novel TPK1 gene mutations in a Chinese patient with thiamine pyrophosphokinase deficiency undergoing whole exome sequencing. J. Pediatr. Endocrinol. Metab. 32, 295-300. ( 10.1515/jpem-2018-0363) [DOI] [PubMed] [Google Scholar]
- 167.Johannes AM, et al. 2011. Thiamine pyrophosphokinase deficiency in encephalopathic children with defects in the pyruvate oxidation pathway. Am. J. Hum. Genet. 89, 806-812. ( 10.1016/j.ajhg.2011.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Srinivasan B, Sibon OC. 2014. Coenzyme A, more than ‘just’ a metabolic cofactor. Biochem. Soc. Trans. 42, 1075-1079. ( 10.1042/BST20140125) [DOI] [PubMed] [Google Scholar]
- 169.Leoni V, et al. 2012. Metabolic consequences of mitochondrial coenzyme A deficiency in patients with PANK2 mutations. Mol. Genet. Metab. 105, 463-471. ( 10.1016/j.ymgme.2011.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Johnson MA, et al. 2004. Mitochondrial localization of human PANK2 and hypotheses of secondary iron accumulation in pantothenate kinase-associated neurodegeneration. Ann. NY Acad. Sci. 1012, 282-298. ( 10.1196/annals.1306.023) [DOI] [PubMed] [Google Scholar]
- 171.Rock CO, Calder RB, Karim MA, Jackowski S. 2000. Pantothenate kinase regulation of the intracellular concentration of coenzyme A*. J. Biol. Chem. 275, 1377-1383. ( 10.1074/jbc.275.2.1377) [DOI] [PubMed] [Google Scholar]
- 172.Iuso A, et al. 2018. Mutations in PPCS, encoding phosphopantothenoylcysteine synthetase, cause autosomal-recessive dilated cardiomyopathy. Am. J. Hum. Genet. 102, 1018-1030. ( 10.1016/j.ajhg.2018.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leonardi R, Zhang YM, Rock CO, Jackowski S. 2005. Coenzyme A: back in action. Prog. Lipid Res. 44, 125-153. ( 10.1016/j.plipres.2005.04.001) [DOI] [PubMed] [Google Scholar]
- 174.van Dijk T, et al. 2018. Biallelic loss of function variants in COASY cause prenatal onset pontocerebellar hypoplasia, microcephaly, and arthrogryposis. Eur. J. Hum. Genet. 26, 1752-1758. ( 10.1038/s41431-018-0233-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Evers C, et al. 2017. Diagnosis of CoPAN by whole exome sequencing: waking up a sleeping tiger's eye. Am. J. Med. Genet. A 173, 1878-1886. ( 10.1002/ajmg.a.38252) [DOI] [PubMed] [Google Scholar]
- 176.Di Meo I, Tiranti V. 2018. Classification and molecular pathogenesis of NBIA syndromes. Eur. J. Paediatr. Neurol. 22, 272-284. ( 10.1016/j.ejpn.2018.01.008) [DOI] [PubMed] [Google Scholar]
- 177.Schneider SA. 2016. Neurodegenerations with brain iron accumulation. Park. Relat. Disord. 22, S21-SS5. ( 10.1016/j.parkreldis.2015.08.012) [DOI] [PubMed] [Google Scholar]
- 178.Annesi G, Gagliardi M, Iannello G, Quattrone A, Iannello G, Quattrone A. 2016. Mutational analysis of COASY in an Italian patient with NBIA. Park. Relat. Disord. 28, 150-151. ( 10.1016/j.parkreldis.2016.03.011) [DOI] [PubMed] [Google Scholar]
- 179.Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ. 2001. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat. Genet. 28, 345-349. ( 10.1038/ng572) [DOI] [PubMed] [Google Scholar]
- 180.Dusi S, et al. 2014. Exome sequence reveals mutations in CoA synthase as a cause of neurodegeneration with brain iron accumulation. Am. J. Hum. Genet. 94, 11-22. ( 10.1016/j.ajhg.2013.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sibon OC, Strauss E. 2016. Coenzyme A: to make it or uptake it? Nat. Rev. Mol. Cell Biol. 17, 605-606. ( 10.1038/nrm.2016.110) [DOI] [PubMed] [Google Scholar]
- 182.Khatri D, et al. 2016. Down-regulation of coasy, the gene associated with NBIA-VI, reduces Bmp signaling, perturbs dorso-ventral patterning and alters neuronal development in zebrafish. Sci. Rep. 6, 37660. ( 10.1038/srep37660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hinarejos I, Machuca-Arellano C, Sancho P, Espinós C. 2020. Mitochondrial dysfunction, oxidative stress and neuroinflammation in neurodegeneration with brain iron accumulation (NBIA). Antioxidants 9, 1020. ( 10.3390/antiox9101020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aoun M, Tiranti V. 2015. Mitochondria: a crossroads for lipid metabolism defect in neurodegeneration with brain iron accumulation diseases. Int. J. Biochem. Cell Biol. 63, 25-31. ( 10.1016/j.biocel.2015.01.018) [DOI] [PubMed] [Google Scholar]
- 185.Ikon N, Ryan RO. 2017. Cardiolipin and mitochondrial cristae organization. Biochim. Biophys. Acta Biomembr. 1859, 1156-1163. ( 10.1016/j.bbamem.2017.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gilkerson RW, Selker JML, Capaldi RA. 2003. The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett. 546, 355-358. ( 10.1016/S0014-5793(03)00633-1) [DOI] [PubMed] [Google Scholar]
- 187.Zhang M, Mileykovskaya E, Dowhan W. 2005. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 280, 29 403-29 408. ( 10.1074/jbc.M504955200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pfeiffer K, et al. 2003. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 278, 52 873-52 880. ( 10.1074/jbc.M308366200) [DOI] [PubMed] [Google Scholar]
- 189.Hatch GM. 1994. Cardiolipin biosynthesis in the isolated heart. Biochem J. 297(Pt 1), 201-208. ( 10.1042/bj2970201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mejia EM, Nguyen H, Hatch GM. 2014. Mammalian cardiolipin biosynthesis. Chem. Phys. Lipids 179, 11-16. ( 10.1016/j.chemphyslip.2013.10.001) [DOI] [PubMed] [Google Scholar]
- 191.Jefferies JL. 2013. Barth syndrome. Am. J. Med. Genet. C: Semin. Med. Genet. 163, 198-205. ( 10.1002/ajmg.c.31372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schlame M, Ren M. 2006. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 580, 5450-5455. ( 10.1016/j.febslet.2006.07.022) [DOI] [PubMed] [Google Scholar]
- 193.Dudek J, et al. 2013. Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome. Stem Cell Res. 11, 806-819. ( 10.1016/j.scr.2013.05.005) [DOI] [PubMed] [Google Scholar]
- 194.McKenzie M, Lazarou M, Thorburn DR, Ryan MT. 2006. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J. Mol. Biol. 361, 462-469. ( 10.1016/j.jmb.2006.06.057) [DOI] [PubMed] [Google Scholar]
- 195.Lee RG, et al. 2022. Deleterious variants in CRLS1 lead to cardiolipin deficiency and cause an autosomal recessive multi-system mitochondrial disease. Hum. Mol. Genet. 31, 3597-3612. ( 10.1093/hmg/ddac040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Flis VV, Daum G. 2013. Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harb. Perspect. Biol. 5, a013235. ( 10.1101/cshperspect.a013235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Raturi A, Simmen T. 2013. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim. Biophys. Acta 1833, 213-224. ( 10.1016/j.bbamcr.2012.04.013) [DOI] [PubMed] [Google Scholar]
- 198.Barazzuol L, Giamogante F, Calì T. 2021. Mitochondria Associated Membranes (MAMs): architecture and physiopathological role. Cell Calcium 94, 102343. ( 10.1016/j.ceca.2020.102343) [DOI] [PubMed] [Google Scholar]
- 199.Sarig O, et al. 2013. Infantile mitochondrial hepatopathy is a cardinal feature of MEGDEL syndrome (3-methylglutaconic aciduria type IV with sensorineural deafness, encephalopathy and Leigh-like syndrome) caused by novel mutations in SERAC1. Am. J. Med. Genet. A 161a, 2204-2215. ( 10.1002/ajmg.a.36059) [DOI] [PubMed] [Google Scholar]
- 200.Wortmann SB, et al. 2012. Mutations in the phospholipid remodeling gene SERAC1 impair mitochondrial function and intracellular cholesterol trafficking and cause dystonia and deafness. Nat. Genet. 44, 797-802. ( 10.1038/ng.2325) [DOI] [PubMed] [Google Scholar]
- 201.Fang H, et al. 2022. SERAC1 is a component of the mitochondrial serine transporter complex required for the maintenance of mitochondrial DNA. Sci. Transl. Med. 14, eabl6992. ( 10.1126/scitranslmed.abl6992) [DOI] [PubMed] [Google Scholar]
- 202.Harel T, et al. 2016. Recurrent de novo and biallelic variation of ATAD3A, encoding a mitochondrial membrane protein, results in distinct neurological syndromes. Am. J. Hum. Genet. 99, 831-845. ( 10.1016/j.ajhg.2016.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Desai R, et al. 2017. ATAD3 gene cluster deletions cause cerebellar dysfunction associated with altered mitochondrial DNA and cholesterol metabolism. Brain 140, 1595-1610. ( 10.1093/brain/awx094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Frazier AE, et al. 2021. Fatal perinatal mitochondrial cardiac failure caused by recurrent de novo duplications in the ATAD3 locus. Medicine 2, 49-73. ( 10.1016/j.medj.2020.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gunning AC, et al. 2020. Recurrent De Novo NAHR reciprocal duplications in the ATAD3 gene cluster cause a neurogenetic trait with perturbed cholesterol and mitochondrial metabolism. Am. J. Hum. Genet. 106, 272-279. ( 10.1016/j.ajhg.2020.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li S, Lamarche F, Charton R, Delphin C, Gires O, Hubstenberger A, Schlattner U, Rousseau D. 2014. Expression analysis of ATAD3 isoforms in rodent and human cell lines and tissues. Gene 535, 60-69. ( 10.1016/j.gene.2013.10.062) [DOI] [PubMed] [Google Scholar]
- 207.Gilquin B, et al. 2010. The AAA+ ATPase ATAD3A controls mitochondrial dynamics at the interface of the inner and outer membranes. Mol. Cell Biol. 30, 1984-1996. ( 10.1128/MCB.00007-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Baudier J. 2018. ATAD3 proteins: brokers of a mitochondria-endoplasmic reticulum connection in mammalian cells. Biol. Rev. Camb. Phil. Soc. 93, 827-844. ( 10.1111/brv.12373) [DOI] [PubMed] [Google Scholar]
- 209.Cooper HM, et al. 2017. ATPase-deficient mitochondrial inner membrane protein ATAD3A disturbs mitochondrial dynamics in dominant hereditary spastic paraplegia. Hum. Mol. Genet. 26, 1432-1443. ( 10.1093/hmg/ddx042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Arguello T, et al. 2021. ATAD3A has a scaffolding role regulating mitochondria inner membrane structure and protein assembly. Cell Rep. 37, 110139. ( 10.1016/j.celrep.2021.110139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Horvath SE, Daum G. 2013. Lipids of mitochondria. Prog. Lipid Res. 52, 590-614. ( 10.1016/j.plipres.2013.07.002) [DOI] [PubMed] [Google Scholar]
- 212.Cogliati S, Enriquez JA, Scorrano L. 2016. Mitochondrial cristae: where beauty meets functionality. Trends Biochem. Sci. 41, 261-273. ( 10.1016/j.tibs.2016.01.001) [DOI] [PubMed] [Google Scholar]
- 213.Elgass K, Pakay J, Ryan MT, Palmer CS. 2013. Recent advances into the understanding of mitochondrial fission. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1833, 150-161. ( 10.1016/j.bbamcr.2012.05.002) [DOI] [PubMed] [Google Scholar]
- 214.Chen H, Chan DC. 2004. Mitochondrial dynamics in mammals. In Current topics in developmental biology, vol. 59 (ed. Schatten GP), pp. 119-144. Cambridge, MA: Academic Press. [DOI] [PubMed] [Google Scholar]
- 215.Archer SL. 2013. Mitochondrial dynamics — mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 369, 2236-2251. ( 10.1056/NEJMra1215233) [DOI] [PubMed] [Google Scholar]
- 216.Youle RJ, van der Bliek AM. 2012. Mitochondrial fission, fusion, and stress. Science 337, 1062. ( 10.1126/science.1219855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kozjak-Pavlovic V. 2017. The MICOS complex of human mitochondria. Cell Tissue Res. 367, 83-93. ( 10.1007/s00441-016-2433-7) [DOI] [PubMed] [Google Scholar]
- 218.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. 2001. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245-2256. ( 10.1091/mbc.12.8.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. 2011. ER tubules mark sites of mitochondrial division. Science 334, 358-362. ( 10.1126/science.1207385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Han XJ, et al. 2008. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol. 182, 573-585. ( 10.1083/jcb.200802164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. 2007. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 282, 11 521-11 529. ( 10.1074/jbc.M607279200) [DOI] [PubMed] [Google Scholar]
- 222.Ishihara N, et al. 2009. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 11, 958-966. ( 10.1038/ncb1907) [DOI] [PubMed] [Google Scholar]
- 223.Vanstone JR, et al. 2016. DNM1L-related mitochondrial fission defect presenting as refractory epilepsy. Eur. J. Hum. Genet. 24, 1084-1088. ( 10.1038/ejhg.2015.243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. 2007. A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 356, 1736-1741. ( 10.1056/NEJMoa064436) [DOI] [PubMed] [Google Scholar]
- 225.Fahrner JA, Liu R, Perry MS, Klein J, Chan DC. 2016. A novel de novo dominant negative mutation in DNM1L impairs mitochondrial fission and presents as childhood epileptic encephalopathy. Am. J. Med. Genet. A 170, 2002-2011. ( 10.1002/ajmg.a.37721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schrepfer E, Scorrano L. 2016. Mitofusins, from mitochondria to metabolism. Mol. Cell 61, 683-694. ( 10.1016/j.molcel.2016.02.022) [DOI] [PubMed] [Google Scholar]
- 227.Chen H, Chomyn A, Chan DC. 2005. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26 185-26 192. ( 10.1074/jbc.M503062200) [DOI] [PubMed] [Google Scholar]
- 228.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. 2004. Structural basis of mitochondrial tethering by mitofusin complexes. Science 305, 858-862. ( 10.1126/science.1099793) [DOI] [PubMed] [Google Scholar]
- 229.Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME. 2011. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann. Neurol. 69, 22-33. ( 10.1002/ana.22166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cartoni R, Martinou J-C. 2009. Role of mitofusin 2 mutations in the physiopathology of Charcot–Marie–Tooth disease type 2A. Exp. Neurol. 218, 268-273. ( 10.1016/j.expneurol.2009.05.003) [DOI] [PubMed] [Google Scholar]
- 231.Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T. 2014. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 204, 919-929. ( 10.1083/jcb.201308006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Baker MJ, Lampe PA, Stojanovski D, Korwitz A, Anand R, Tatsuta T, Langer T. 2014. Stress-induced OMA1 activation and autocatalytic turnover regulate OPA1-dependent mitochondrial dynamics. EMBO J. 33, 578-593. ( 10.1002/embj.201386474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yu-Wai-Man P, et al. 2010. The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology 117, 1538-1546. ( 10.1016/j.ophtha.2009.12.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yu-Wai-Man P, et al. 2010. Multi-system neurological disease is common in patients with OPA1 mutations. Brain 133(Pt 3), 771-786. ( 10.1093/brain/awq007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hoppins S, Lackner L, Nunnari J. 2007. The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 76, 751-780. ( 10.1146/annurev.biochem.76.071905.090048) [DOI] [PubMed] [Google Scholar]
- 236.Westermann B. 2010. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 11, 872-884. ( 10.1038/nrm3013) [DOI] [PubMed] [Google Scholar]
- 237.Westphal D, Kluck RM, Dewson G. 2014. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 21, 196-205. ( 10.1038/cdd.2013.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang C, Youle RJ. 2009. The role of mitochondria in apoptosis. Ann. Rev. Genet. 43, 95-118. ( 10.1146/annurev-genet-102108-134850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chao JR, Parganas E, Boyd K, Hong CY, Opferman JT, Ihle JN. 2008. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature 452, 98-102. ( 10.1038/nature06604) [DOI] [PubMed] [Google Scholar]
- 240.Martins LM, et al. 2004. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol. Cell Biol. 24, 9848-9862. ( 10.1128/MCB.24.22.9848-9862.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mandel H, Saita S, Edvardson S, Jalas C, Shaag A, Goldsher D, Vlodavsky E, Langer T, Elpeleg O. 2016. Deficiency of HTRA2/Omi is associated with infantile neurodegeneration and 3-methylglutaconic aciduria. J. Med. Genet. 53, 690-696. ( 10.1136/jmedgenet-2016-103922) [DOI] [PubMed] [Google Scholar]
- 242.Hangen E, et al. 2015. Interaction between AIF and CHCHD4 regulates respiratory chain biogenesis. Mol. Cell 58, 1001-1014. ( 10.1016/j.molcel.2015.04.020) [DOI] [PubMed] [Google Scholar]
- 243.Kim JT, et al. 2006. Apoptosis-inducing factor (AIF) inhibits protein synthesis by interacting with the eukaryotic translation initiation factor 3 subunit p44 (eIF3g). FEBS Lett. 580, 6375-6383. ( 10.1016/j.febslet.2006.10.049) [DOI] [PubMed] [Google Scholar]
- 244.Ghezzi D, et al. 2010. Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am. J. Hum. Genet. 86, 639-649. ( 10.1016/j.ajhg.2010.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rinaldi C, et al. 2012. Cowchock syndrome is associated with a mutation in apoptosis-inducing factor. Am. J. Hum. Genet. 91, 1095-1102. ( 10.1016/j.ajhg.2012.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sevrioukova IF. 2016. Structure/function relations in AIFM1 variants associated with neurodegenerative disorders. J. Mol. Biol. 428, 3650-3665. ( 10.1016/j.jmb.2016.05.004) [DOI] [PubMed] [Google Scholar]
- 247.Abate M, et al. 2020. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 98, 139-153. ( 10.1016/j.semcdb.2019.05.022) [DOI] [PubMed] [Google Scholar]
- 248.Tinker RJ, Lim AZ, Stefanetti RJ, McFarland R. 2021. Current and emerging clinical treatment in mitochondrial disease. Mol. Diagn. Ther. 25, 181-206. ( 10.1007/s40291-020-00510-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.El-Hattab AW, Zarante AM, Almannai M, Scaglia F. 2017. Therapies for mitochondrial diseases and current clinical trials. Mol. Genet. Metab. 122, 1-9. ( 10.1016/j.ymgme.2017.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bacman SR, et al. 2018. MitoTALEN reduces mutant mtDNA load and restores tRNA(Ala) levels in a mouse model of heteroplasmic mtDNA mutation. Nat. Med. 24, 1696-1700. ( 10.1038/s41591-018-0166-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gammage PA, et al. 2018. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat. Med. 24, 1691-1695. ( 10.1038/s41591-018-0165-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.