Introduction
Small fiber neuropathies are a common cause of peripheral chronic pain, where mechanical, chemical, and/or metabolic damage results in the degeneration and aberrant firing of peripheral neurons. Therapeutic options available to patients suffering from pain in small fiber neuropathies include lifestyle intervention and minimally effective pharmacological interventions, and even fewer therapies address the underlying damage sustained by the peripheral nervous system. The most common etiology for small fiber neuropathy is diabetes [7], and diabetic peripheral neuropathy (DPN) commonly affects the distal limbs in a stocking-glove distribution. DPN involves aberrant metabolic processes that produce toxic metabolites, such as methylglyoxal (MGO) [8; 22; 26], causing pain and damage to sensory neurons [3; 6; 8; 16; 18; 22]. Importantly, MGO has been implicated in small fiber neuropathies of different etiologies as well, including chemotherapy-induced neuropathy [42] and radiculopathy following lumbar disc herniation [27].
MGO is a toxic reactive dicarbonyl that is produced primarily as a byproduct of glycolysis by decomposition of dihydroxy-acetate-phosphate, as well as through other pathways, such as condensation of acetate [39]. MGO is normally scavenged and detoxified through the endogenous glyoxalase enzyme system [5; 38; 39]. In this system, MGO reacts with glutathione to produce hemithioacetal, which is enzymatically converted by glyoxalase I (GLO1) into S-D-lactoylglutathione and subsequently D-lactate by glyoxalase II. Under periods of intense MGO production, such as diabetes, glyoxalase activity can be reduced [30] and is not sufficient to scavenge MGO. This is thought to lead to accumulation of MGO and advanced glycation endproducts (AGEs) [8; 42]. MGO and AGE accumulation results in the activation of pro-nociceptive signaling cascades, including activation of transient receptor potential channel ankyrin-1 (TRPA1)[3; 18; 22], increased voltage-gated sodium channel NaV1.8 open probability [8], activation of the integrated stress response [6], and activation of the receptor for AGEs (RAGE) [42]. MGO scavengers, such as aminoguanidine, have shown promise in preclinical models of diabetic complications like neuropathy and nephropathy, but have failed in clinical studies due to toxicity and safety concerns [40].
There is a growing body of clinical and preclinical evidence supporting the use of a ketogenic diet in treating chronic pain conditions [12-14; 17; 31; 32]. We and others have demonstrated success in using a ketogenic diet in reversing small fiber neuropathies where MGO accumulation has been implicated, including diabetic peripheral neuropathy [12; 13; 17] and chemotherapy-induced neuropathy [45]. Moreover, Salomón, et. al. (2017) demonstrated MGO can be scavenged in in blood by the ketone body acetoacetate [33].
Here, tested whether a ketogenic diet and ketone bodies could improve MGO-evoked nociception in mice. As an experimental model, we directly injected mice with MGO or MGO coincubated with various ketone bodies. Our results suggest that ketone bodies elevated by consuming a ketogenic diet 1) improve MGO-evoked nociception, 2) reduce spinal activation in response to MGO, and 3) contribute to MGO scavenging. These findings identify potential mechanisms by which a ketogenic diet improves DPN and supports the view that a ketogenic diet may be a powerful intervention for MGO-scavenging in diabetic complications and pathologies associated with MGO.
Materials and Methods
Animals and Diet
All animal work was performed following review and approval by the Institutional Animal Care and Use Committee of Kansas University Medical Center. Eight-week-old C57/Bl6 mice #027 were purchased from Charles River Laboratories (Wilmington, MA) and maintained on a 12:12 light: dark cycle in the animal research facility at Kansas University Medical Center. Mice were given ad libitum access to water and either a control rodent chow (TD.8604; Envigo, Madison, WI; 14% fat, 32% protein, and 54% carbohydrate by kcal) or a ketogenic diet (TD.96355; Envigo, 90.5% fat, 9.2% protein, and 0.3% carbohydrate by kcal). Animals fed a ketogenic diet were given fresh diet every 3–4 days.
Methylglyoxal Injection
For systemic methylglyoxal (MGO) administration, MGO (Sigma; 40% by weight in water) was diluted in sterile saline to a working concentration of 28.8 ng/μl (pH 7.0). Mice received a single intraperitoneal (I.P.) injection of either sterile saline or 720 ng methylglyoxal in saline [6].
For peripheral MGO administration, MGO was diluted in sterile saline to a working concentration of 1.5 μg/μl (pH 7.0). In in vitro MGO-scavenging experiments, equimolar concentrations of acetoacetate, β-hydroxybutyrate, or aminoguanidine HCl (Sigma; St. Louis) were incubated with MGO for 18 hours at 4°C. Mice then received 30 μg of the resultant mixture by 20 μl intraplantar injection [18].
Blood Measurements
Blood ketones were measured using a hand-held blood monitor and β-hydroxybutyrate blood ketone strips (β-Ketone blood test strips, Abbott Laboratories, Chicago, IL; Precision Xtra, Abbott Laboratories). Saline- and MGO-injected animals were not fasted prior to blood ketone measures.
For measurement of plasma MG-H1, 60 μl tail blood was collected and allowed to coagulate on ice. Samples were then centrifuged for 30 minutes at 4 °C and 3000 rpm, and serum was drawn off. Serum was then depleted of endogenous IgG by overnight incubation with Protein A-conjugated Sepharose beads (Abcam) at 4 °C on an orbital shaker and plasma MGO was determined by ELISA (Abcam) the next day.
Glyoxalase 1 Activity Assay
Glyoxalase 1 (GLO1) activity was measured in several relevant peripheral tissues using a microassay as previously described [5]. Briefly, protein was extracted from the lumbar spinal cord and footpad by sonication in EDTA-free RIPA buffer. 2 μg protein per sample were loaded in triplicate to a 96-well round-bottomed plate. 2 mM MGO and 2 mM reduced glutathione (Sigma) were allowed to react in 10 mM sodium phosphate buffer (pH 6.6) for 10 minutes at 37°C, forming hemithioacetal. The resultant mixture was loaded on a 96-well plate and absorbance changes were collected at 240 nm every minute for 5 minutes, corresponding to the conversion from hemithioacetal to S-D-lactoylglutathione. From this, units of GLO1 activity as units/μg protein were calculated, where one unit equaled the amount of GLO1 activity to catalyze 1 μmol S-D-lactoyalglutathion/minute.
Sensory Behavioral Testing
Sensory behavioral testing was performed at baseline and on days 1, 3, 6, and 13 for animals receiving I.P. MGO injection. Prior to collection of baseline data, mice were acclimated to testing areas for 30 minutes and either the mesh table for 30 minutes on at least two occasions separated by 24 hours. Prior to collection of all sensory behavioral data, mice were again acclimated to the testing area and mesh table for 30 minutes each. Selected Von Frey microfilaments were applied to the plantar surface of the hindpaw following the “up-down” method for one second [11]. Animals were observed for either a negative or a positive response, and mechanical withdrawal threshold was calculated following five positive responses.
Sensory behavior in animals receiving intraplantar injections was visually assessed by observation of spontaneous nocifensive behavior (e.g., licking, biting, lifting, and shaking the injected paw). Mice were acclimated to a clear plastic cage without bedding for 5 minutes prior to injection. Following intraplantar injection, mice were returned to the cage. A blinded investigator then observed the mouse for 5 minutes following injection and recorded both the total number of nocifensive events displayed and the total time spent engaged in nocifensive behavior.
Nociception-Evoked Activation of the Spinal Dorsal Horn Cells
To assess the response of spinal dorsal horn cells to peripheral noxious stimulation, the lumbar enlargement of the spinal cord was dissected 10 minutes following intraplantar injection and post-fixed in 4% paraformaldehyde overnight. Spinal cords were then cryopreserved in 30% sucrose, frozen in Optimal Cutting Temperature Compound (Sekura Tissue-Tek) and sectioned by cryostat. Thirty-micron coronal sections of the spinal cord were blocked for two hours in Superblock (ThermoFisher; Grand Island, NY), 1.5% Normal Donkey Serum, 0.5% Porcine Gelatin, and 0.5% Triton X-100 (Sigma) at room temperature. Slides were then incubated overnight at 4°C with rabbit α-phospho-ERK 42/44 (1:500; Cell Signaling Technologies). Slides were next incubated with AlexaFluor-555 tagged donkey-α-rabbit secondary antibody (1:1000, Molecular Probes) for one hour and imaged with a Nikon Eclipse 90i microscope at 20x. A blinded investigator counted the number of cells in the dorsal horn grey matter that were 5–15 μm diameter, roughly spherical, and showed obvious increase in p-ERK staining compared to background. This process was repeated across at least five independent sections for each animal, and the average count for each mouse was used for statistical analyses.
Statistics and Data Analysis
All statistical analyses were performed using R version 3.6.2 and packages “Rmisc”, “car”, and “ggplot2”. All analyses for which data were collected over time were performed using a three-way repeated measure analysis of variance (ANOVA). Correlations between MGO and β-hydroxybutyrate (β-HB) were assessed by Spearman’s Rank Correlation. All other analyses were performed using a three-way ANOVA or Student’s t-test, as appropriate. Data were analyzed posthoc by pairwise t-test or Tukey’s Honest Significant Difference (HSD), as indicated. Assumptions of normal distribution and homogeneity of variance were confirmed by Shapiro-Wilks and Levene tests, respectively. Biological sex was considered in all analyses to account for potential sexual dimorphism. All data are presented as mean +/− standard error of the mean, and an α-level of 0.05 was considered statistically significant.
Results
Consumption of a Ketogenic Diet Prevents Methylglyoxal-Evoked Mechanical Allodynia
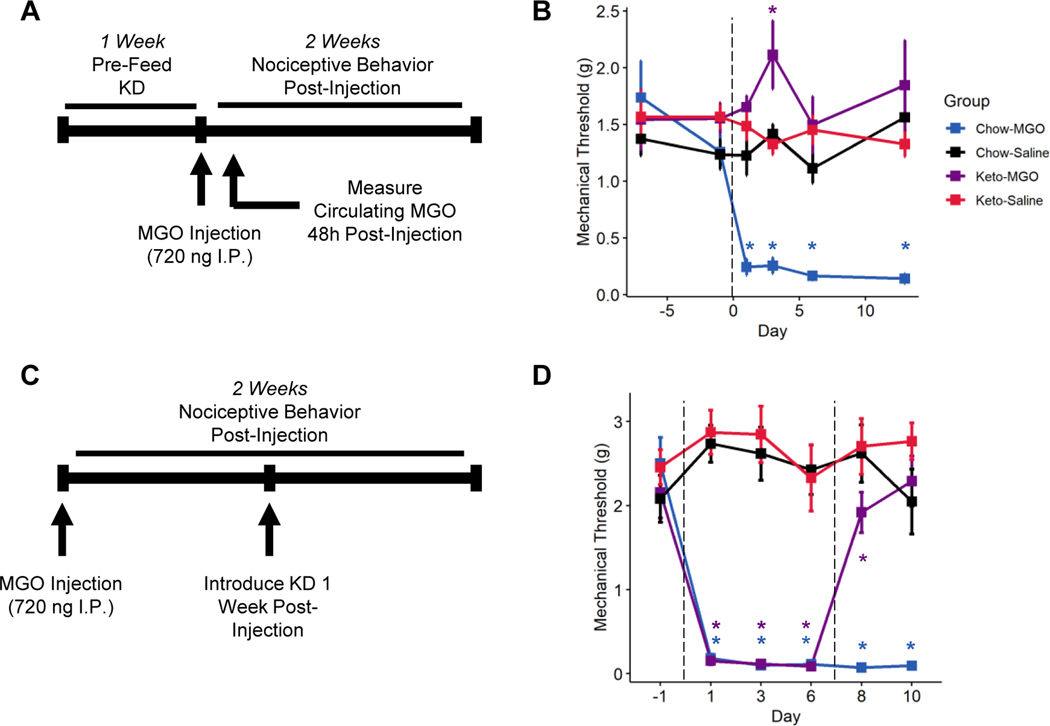
Mice were randomized and placed into four groups: mice fed chow before receiving an intraperitoneal (I.P.) saline injection (Chow-Ctrl); mice fed a ketogenic diet before I.P. saline injection (Keto-Ctrl); mice fed chow before a single high-dose (720 ng) I.P. MGO injection (Chow-MGO); and mice fed a ketogenic diet before I.P. MGO injection (Keto-MGO). We then injected mice with MGO and measured mechanical allodynia of the hindpaw for two weeks (Figure 1A). Within 24 hours of MGO injection, Chow-fed, MGO-injected mice displayed significant mechanical allodynia, which persisted at least 13 days (Figure 1B, 3-way repeated measures ANOVA, MGO: p < 0.00726, diet: p < 9.48e−12, MGO-diet interaction: p < 2.19e−5). No mechanical allodynia was detected in ketogenic diet-fed mice injected with MGO, revealing that consumption of a ketogenic diet for one week provides significant protection from MGO-induced pain. We detected no statistically significant sex differences in response to either MGO-injection or response to a ketogenic diet (Figure 1B, 3-way repeated measures ANOVA, sex: p = 0.57, MGO-sex interaction: p = 0.067, diet-sex interaction: p = 0.55, MGO-diet-sex interaction: p = 0.50).
Figure 1. A ketogenic diet prevents methylglyoxal-evoked mechanical allodynia.
(A) Experimental design for assessing effects of a ketogenic diet on methylglyoxal (MGO) evoked nociception. Mice were provided chow or a ketogenic diet for one week before receiving a 25 μl intraperitoneal injection of 720 ng MGO. Sensory behavioral testing with Von Frey filaments was conducted for two weeks following injection prior to sacrifice. (B) Chow-fed mice receiving an injection of MGO quickly developed mechanical allodynia that persisted through the course of the experiment. Mice fed a ketogenic diet, however, never developed allodynia following MGO injection (n=8). Mixed-models ANOVA with repeated measures and Tukey’s post hoc test; *p < 0.05 compared to chow-fed saline-injected mice.
A Ketogenic Diet Rescues Methylglyoxal-Evoked Mechanical Allodynia
We previously reported that a ketogenic diet could reverse mechanical allodynia in mice with longstanding DPN [17]. It is possible, however, neuropathy improvement by a ketogenic diet is specific to DPN. Thus, to improve the translatability of our findings, we tested whether consumption of a ketogenic diet could reverse established MGO-evoked mechanical allodynia. Mice were randomized at baseline to receive either MGO or vehicle I.P. injections (720 ng) followed by intervention with a ketogenic diet one week following injection. We assessed mechanical thresholds of the mouse hindpaw for two weeks (Figure 1C). As seen previously, I.P. MGO injection caused robust, lasting mechanical allodynia within 24 hours (Figure 1D, 3-way repeated measures ANOVA, MGO: p < 2e−16, diet: p < 0.00223, MGO-diet interaction: p < 0.166). However, within 24 hours of intervention with a ketogenic diet, MGO-injected mice exhibit a rapid improvement of mechanical sensation toward baseline, and the interaction between dietary intervention and MGO-injection was significant over time (3-way repeated measure ANOVA, MGO-diet-time interaction: p < 0.0039), revealing that intervention with a ketogenic diet is sufficient to reverse established MGO-evoked nociception. Again, we detected no sex differences in response to MGO-injection or rescue by a ketogenic diet (Figure 1D, 3-way ANOVA, sex: p = 0.55, MGO-sex interaction: p = 0.19, diet-sex interaction: p = 0.24, MGO-diet-sex interaction: 0.95), nor did the response to any of these factors vary by sex with respect to time.
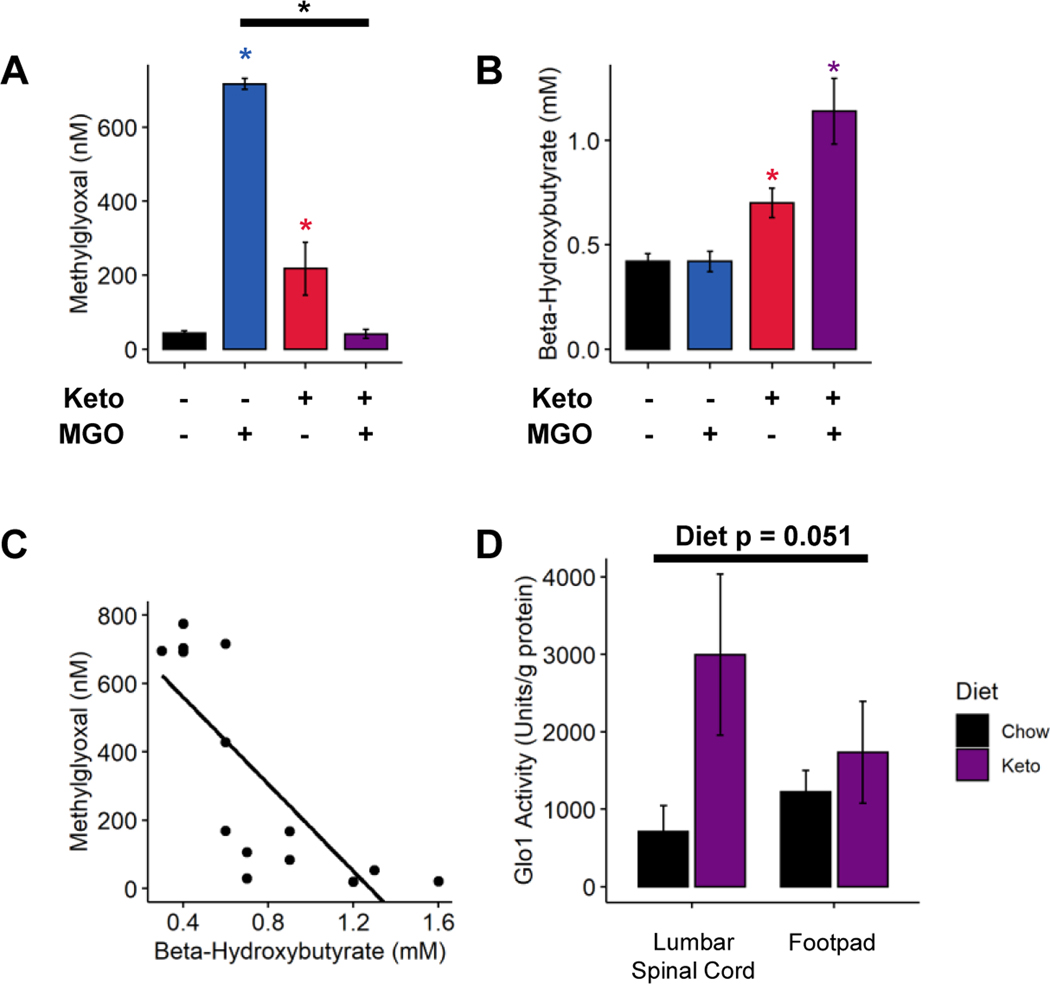
A Ketogenic Diet Prevents Plasma Accumulation of Methylglyoxal
In 2017, Salomón et. al. demonstrated non-enzymatic clearance of MGO from blood by ketone bodies [33]. We therefore reasoned that a ketogenic diet might lower plasma MGO concentrations. To test this, blood was collected from the four groups of mice described above. At 48 hours post-injection, Chow-MGO mice displayed significantly elevated plasma concentration of MG-H1, a protein modification formed by the reaction of methylglyoxal and arginine (Figure 2A; 2-way ANOVA, injection: p < 5.94e−7, diet: p < 2.67e−7, injection-diet interaction: p < 8.28e−10). Keto-MGO mice, however, had a similar concentration of plasma MG-H1 as Chow-Ctrl (Tukey’s HSD, adjusted p < 1.0). Surprisingly, Keto-Ctrl mice also exhibited elevated plasma MG-H1 (Tukey’s HSD, Keto-Ctrl and Chow-Ctrl adjusted p < 0.0082), though MG-H1 concentration was still significantly higher in Chow-MGO mice (Tukey’s HSD, adjusted p < 0.0075).
Figure 2. A ketogenic diet contributes to methylglyoxal scavenging.
(A) Quantification of blood MG-H1 levels 48 hours post-injection in chow- and ketogenic diet-fed mice (n=4–5). Chow-fed mice exhibit significantly elevated circulating MGO 48 hours post-injection, whereas ketogenic diet-fed mice show no such increase. (B) Quantification of blood β-hydroxybutyrate (β-HB) levels in chow- and ketogenic diet-fed mice 48 hours after MGO injection (n=4–5). Chow-fed mice show normal blood ketone levels, while mice fed a ketogenic diet exhibit elevated β-HB, indicative of nutritional ketosis. (C) Negative correlation between circulating MGO and β-HB 48 hours after MGO injection in mice fed a chow or ketogenic diet (n=14; p < 7.28e−5, ρ = −0.86). (D) Quantification of Glyoxalase 1 enzyme activity from various tissues in chow and ketogenic diet-fed mice. Both spinal cord and footpad skin from ketogenic diet fed mice show a strong trend toward increased glyoxalase 1 activity compared to those from chow fed mice (n=4). (A-B and D) Two-way ANOVA and Tukey’s post hoc test; * p < 0.05 compared to chow-fed saline-injected, *p < 0.05 between two conditions. (C) Spearman’s Rank Correlation.
We next reasoned that if ketone bodies were directly scavenging MGO, an inverse relationship may exist between blood concentrations of ketone bodies and MG-H1. Keto-Ctrl and Keto-MGO mice exhibited elevated blood β-HB concentrations as expected (Figure 2B; 2-way ANOVA, MGO: p < 0.0242, diet: p < 6.74e−5, injection-diet interaction: p < 0.0340). There was, however, a slight but significant decrease in β-HB concentration in Keto-Ctrl mice compared to Keto-MGO mice (Tukey’s HSD, adjusted p < 0.027). We then compared β-HB concentrations to plasma MG-H1 levels across all groups and identified a slight inverse correlation (Spearman’s Rank Correlation, p = 0.1006, rho = −0.39). As the Chow-Ctrl group exhibited low concentrations of both β-HB and MG-H1, there was concern that this could mask potential correlations. Upon removal of the Chow-Ctrl group, we noted a strong inverse correlation between blood concentrations of β-HB and MG-H1 (Figure 2C; Spearman’s Rank Correlation, p < 7.28e−5, ρ = −0.86).
Ketogenic Diet Increases Glyoxalase Activity
Experiments next tested whether increased MGO scavenging in mice fed a ketogenic diet could be explained by increased glyoxalase activity. As GLO1 is the rate-limiting enzyme of the glyoxalase system [38], we assayed GLO1 activity from protein harvested from the plantar footpads and spinal cords of mice fed a ketogenic diet for one week. Our results revealed a positive, albeit non-statistically significant, trend toward increased GLO1 activity in tissues from ketogenic diet fed mice (Figure 2D; 2-way ANOVA, diet: p < 0.051, tissue: p < 0.62, diet-tissue interaction: p <0.17), which appeared to be primarily driven by an increase in GLO1 activity in the spinal cord.
Ketone Bodies Detoxify Methylglyoxal and Prevent Methylglyoxal Evoked Nociception
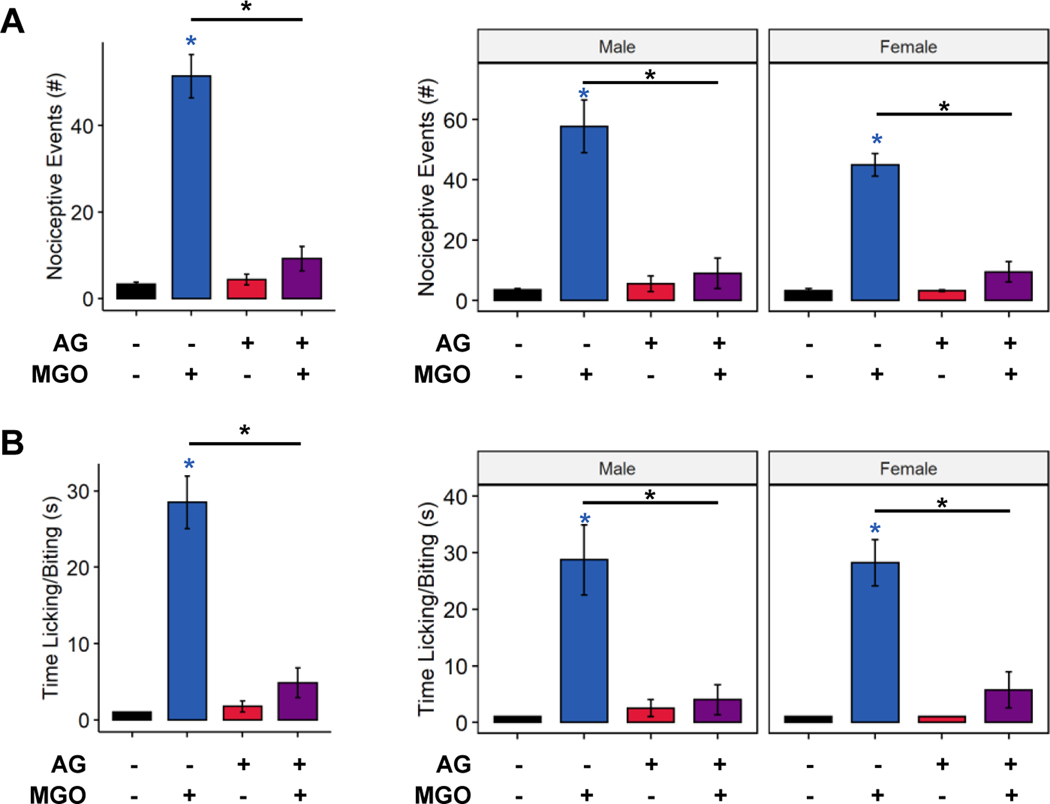
In addition to increased GLO1 activity in ketogenic diet-fed mice, it is possible that ketone bodies directly scavenge and detoxify MGO [33]. This is supported by the strong negative correlation between β-HB and methylglyoxylated-protein levels (Figure 2C). To separate and identify possible effects of ketone bodies on scavenging MGO from increased GLO1 activity in mice fed a ketogenic diet, we developed a behavioral assay for MGO detoxification. MGO was allowed to react in vitro with an MGO scavenger or ketone bodies prior to intraplantar injection, followed by subsequent assessment of nociceptive behavior and spinal cord activation. We validated this assay by first incubating MGO with the known scavenger aminoguanidine [40]. As expected, control mice receiving intraplantar injections of only MGO elicited a sharp nocifensive response, whereas mice injected with MGO preincubated with aminoguanidine showed a significantly diminished behavioral response (Figure 3A, 3-way ANOVA, MGO: p < 3.08e−9, scavenger: p < 2.63e−7, MGO-scavenger interaction: p < 1.19e−7; Figure 3B, 3-way ANOVA, MGO: p < 2.54e−7, scavenger: p < 1.99e−5, MGO-scavenger interaction: p < 8.36e−6). We did not detect any statistically significant sex differences in the effect of intraplantar MGO injection or prevention by aminoguanidine (3-way ANOVA, number of nociceptive events, sex: p = 0.0.217, sex-scavenger interaction: p = 0.343; 3-way ANOVA, time engaged in nocifensive behavior, sex: p = 0.977, sex-scavenger interaction: p = 0.932).
Figure 3. Validation of a behavioral assay for methylglyoxal detoxification.
(A-B) Quantification of the number of nociceptive events engaged in nociceptive behavior following intraplantar injection of MGO with, and without, aminoguanidine (n=8). Mice receiving intraplantar MGO injections exhibit increased number (A) and duration (B) of nocifensive behaviors, which is abrogated by pre-incubating MGO with aminoguanidine. (A-B) Three-way ANOVA and Tukey’s post hoc test; * p < 0.05 compared to chow-fed saline-injected, *p < 0.05 between two conditions.
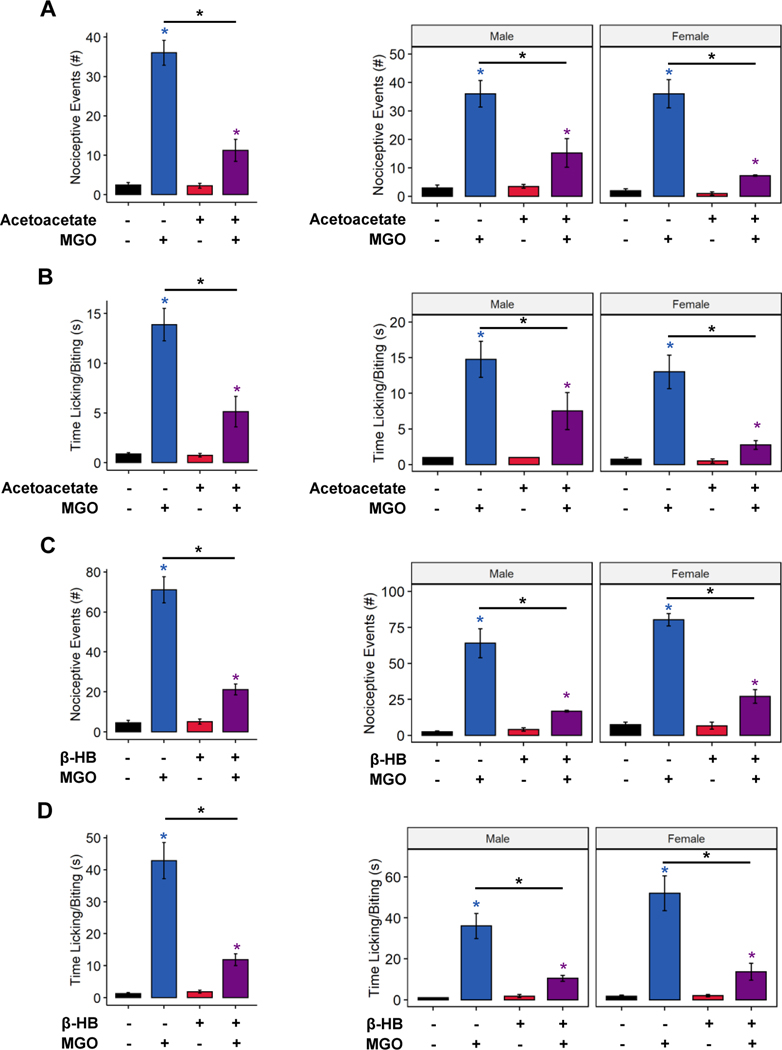
To date, the only ketone known reported to scavenge MGO is acetoacetate [34]. Here, we tested whether MGO scavenging by acetoacetate could prevent MGO-evoked nociception in via preincubation of MGO with acetoacetate. Mice receiving an intraplantar injection of MGO exhibited a significant increase in nocifensive behavior (licking, lifting, biting, shaking) compared to control-injected mice (Figure 4A, 3-way ANOVA, MGO: p < 5.71e−10, ketone: p < 5.09e−6, MGO-ketone interaction: p < 6.80e−6; Figure 4B, 3-way ANOVA, MGO: p < 3.66e−8, ketone: p < 0.000462, MGO-ketone interaction: p < 0.000616). No statistically significant sex differences were detected. In comparison, mice receiving injections of MGO preincubated with acetoacetate, however, exhibited significantly reduced nocifensive behavior (MGO compared to acetoacetate + MGO, Tukey’s Honest Significant Difference, Figure 4A p < 1.0e−7, Figure 4B p < 0.0000455). In addition, mice injected with a premixture of β-HB and MGO exhibited similarly reduced nocifensive responses compared to those receiving an injection of MGO alone (Figure 4C, 3-way ANOVA, MGO: p < 7.65e−11, ketone: p < 3.72e−7, MGO-ketone interaction: p < 2.64e−7; Figure 4D, 3-way ANOVA, MGO: p < 6.52e−9, ketone: p < 1.57e−5, MGO-ketone interaction: 9.81e−6). We did observe a slight sex difference in number of nocifensive events (3-way ANOVA, sex: p < 0.0197) and a trend toward increased time engaged in nocifensive behaviors in males (3-way ANOVA, sex: p = 0.0798); however, these differences did not appear to interact with either MGO-injection or presence of β-HB. Together, these data indicate both ketone bodies may act as scavengers of MGO and reduce MGO-evoked nociception.
Figure 4. Pre-incubation with ketone bodies prevents methylglyoxal-evoked nociception.
(A-D) The number of nociceptive events and time engaged in nociceptive behavior following intraplantar injection of MGO with, and without, ketone bodies (n=7–8). MGO injection increased the number of nocifensive events (A and C), and time engaged in nocifensive behavior (B and D), which was largely prevented by pre-incubation of MGO with acetoacetate (A-B) or β-HB (C-D). (A-D) Three-way ANOVA and Tukey’s post hoc test; * p < 0.05 compared to chow-fed saline-injected, *p < 0.05 between two conditions.
β-Hydroxybutyrate Does Not Detoxify Methylglyoxal Adducts
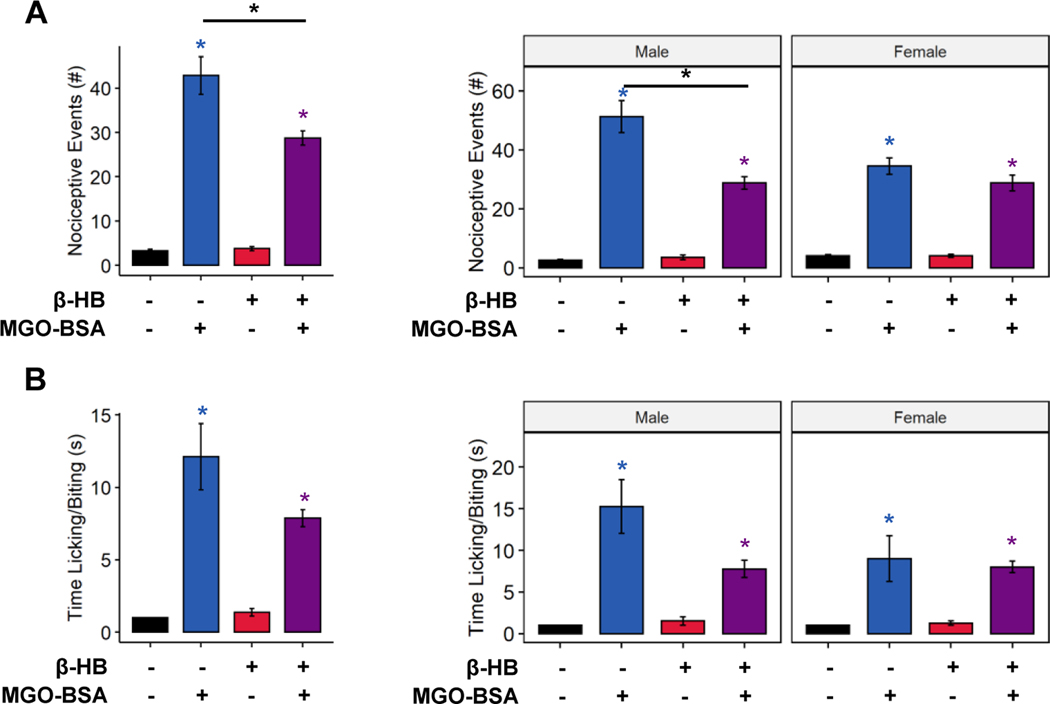
In addition to direct nociceptive and pathological signaling, MGO reacts with amino acids to form noxious and toxic peptide adducts. Formation of these adducts, such as the hydroimidazolones MG-H1 and MG-H2, can be irreversible [1; 2], precluding the possibility of their detoxification. We tested whether ketone bodies prevented nociception evoked by MGO-peptide adducts by using MGO-modified bovine serum albumin (MGO-BSA) in the MGO-detoxifying assay. MGO-BSA evoked strong nociceptive behaviors following intraplantar injection (Figure 5A, 3-way ANOVA, MGO-BSA: p < 1.45−15, ketone: p < 0.00078, MGO-BSA-ketone interaction: p < 0.00038; Figure 5B, 3-way ANOVA, MGO-BSA: p < 3.70e−8, ketone: p < 0.094, MGO-BSA-ketone interaction: p < 0.0483). Coincubation with β-HB caused a slight but statistically significant reduction in number of nociceptive events compared to MGO-BSA injection alone (Figure 5A, Tukey’s HSD, p < 0.0000472; Figure 5B, Tukey’s HSD, p = 0.056), yet the number of nociceptive events and time engaged in nociceptive behavior was still significantly greater than those of animals injected with bovine serum albumin alone (Figure 5A, Tukey’s HSD, p < 1.0e−7; Figure 5B, Tukey’s HSD, p < 0.0011). Of note, female mice exhibited a greater number of nocifensive events than male mice in response to MGO-BSA (3-way ANOVA, sex: p < 0.048, sex-MGO-BSA interaction: p < 0.014, sex-ketone interaction: p < 0.036, sex-MGO-BSA-ketone interaction: p < 0.0195; Tukey’s HSD, p < 0.0018), though coincubation with β-HB eliminated this effect (Tukey’s HSD, p = 1.0). These data suggest that ketone bodies only modify MGO-evoked nociception prior to protein modification by MGO and MGO-mediated advanced glycation end-product generation.
Figure 5. Pre-incubation with β-HB does not prevent nociception evoked by methylglyoxal-protein adducts.
Nociceptive events (A) and time engaged in nocifensive behavior (B) were increased by MGO conjugated to bovine serum albumin (MGO-BSA). Pre-incubation with β-HB caused a slight, but statistically significant, improvement in number of MGO-BSA-evoked nociceptive events (A) but did not affect time engaged in nocifensive behaviors (B) (n=8). (A-B) Three-way ANOVA and Tukey’s post hoc test; * p < 0.05 compared to chow-fed saline-injected, *p < 0.05 between two conditions.
Ketone Body Preincubation of MGO Prevents Methylglyoxal-Evoked ERK Phosphorylation in the Spinal Cord
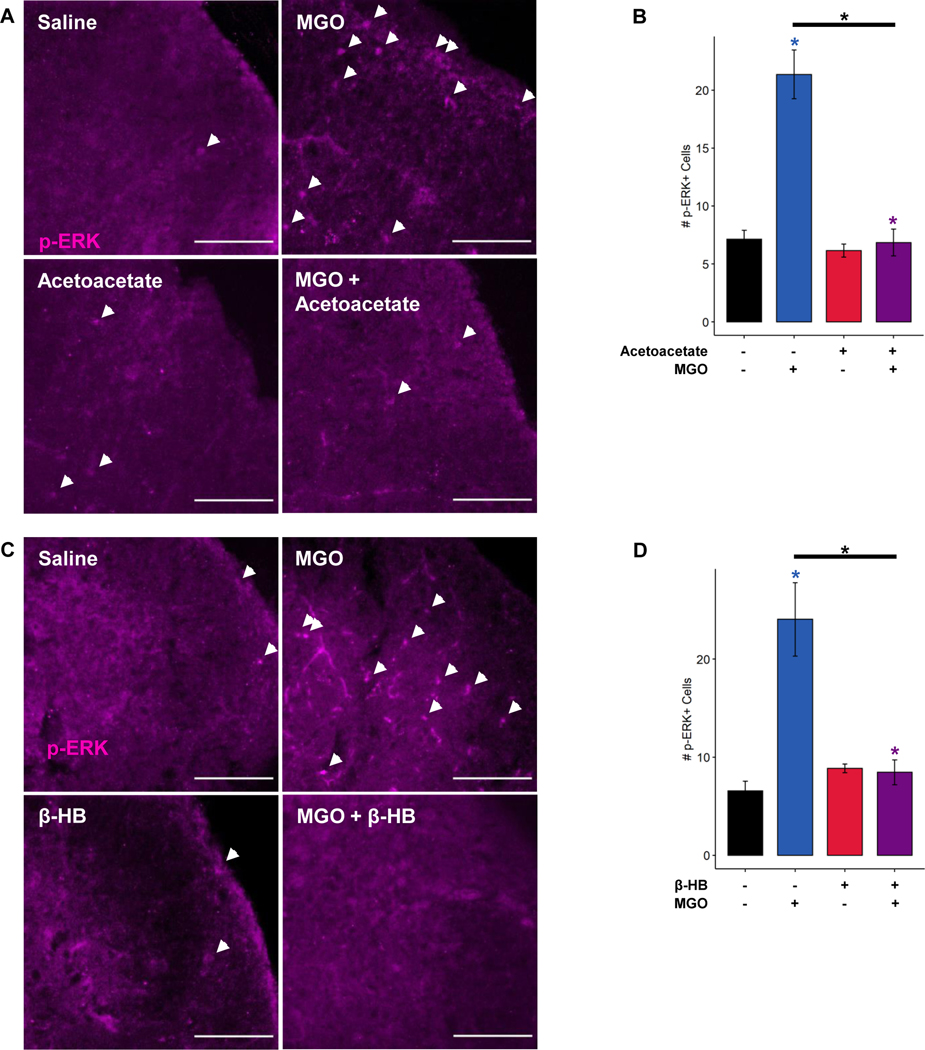
To measure whether acetoacetate or β-HB affect activation of spinal neurons during MGO-evoked nociception, we quantified the number of phospho-ERK (p-ERK)-positive cells in the spinal dorsal horn following intraplantar MGO injection. MGO injection significantly increased the number of p-ERK+ cells ipsilateral to the injection site compared to control injected mice (Figure 6), indicating spinal activation in response to a peripheral noxious stimulus. Consistent with behavioral data, MGO-evoked increases in p-ERK activation were significantly reduced by coincubation of MGO with acetoacetate prior to injection (Figure 6A-B; 3-way ANOVA, MGO: p < 0.00206, ketone: p < 0.00124, MGO-ketone interaction: p < 0.00397; Tukey’s HSD, MGO and MGO-Acetoacetate adjusted p-value: 0.00123). In addition, the number of spinal p-ERK+ cells was similarly reduced following MGO coincubation with β-HB (Figure 6C-D; 3-way ANOVA, MGO: p < 0.00724, ketone: p < 0.02391, MGO-ketone interaction: p < 0.00538; Tukey’s HSD, MGO and MGO-β-HB adjusted p-value: 0.00744). We detected no sex differences in p-ERK positivity in the spinal dorsal horn following injection of MGO or either ketone body.
Figure 6. Ketone bodies prevent methylglyoxal-evoked early activation in spinal dorsal horn.
Representative images (A) and quantification (B) of phospho-ERK (p-ERK)-positive cells (white arrows) in the dorsal horn of the spinal cord from mice receiving footpad injections of MGO with and without acetoacetate. MGO injection increased the number of p-ERK-positive cells 10 minutes after injection, which was prevented by coincubation of MGO with acetoacetate. (C-D) Representative images and quantification of p-ERK-positive cells (white arrows) in the dorsal horn of the spinal cord following intraplantar MGO injection with and without β-HB. MGO again increased p-ERK positivity in the dorsal horn, which was abrogated by coincubation with β-HB. Scale bar depicts 100 μm. (B and D) Three-way ANOVA and Tukey’s post hoc test; *p < 0.05 compared to chow-fed saline-injected, *p < 0.05 between two conditions.
Discussion
Detoxification of MGO has long been a clinical target for treating pathological conditions associated with diabetes, including DPN. Aminoguanidine is a small molecule scavenger of MGO that showed modest promise in ameliorating symptoms of diabetic neuropathy in preclinical models [25; 44]. However, aminoguanidine failed in a clinical trial for diabetic nephropathy due to poor tolerability [40]; leaving the niche for a well-tolerated MGO-targeting therapy unfilled in diabetic complications. A ketogenic diet is well-tolerated in patients with diabetes [10; 15; 41; 43] as well as preclinical models of diabetes [13; 17; 28]. Moreover, ketogenic diets are showing efficacy in an ever-increasing number of preclinical models of chronic pain related to MGO [13; 17; 45].
In this study, we demonstrated that a ketogenic diet prevents and reverses MGO-evoked nociception and activation in the spinal dorsal horn. Consistent with prior literature [6], a single I.P injection of MGO induced mechanical allodynia lasting at least two weeks. We report that this MGO-induced mechanical allodynia was absent in mice fed a ketogenic diet (Figure 1B). Importantly, intervention with a ketogenic diet reversed established MGO-induced mechanical allodynia (Figure 1D), lending to the translatability of this model. Chow-fed mice that were injected with MGO had significantly higher circulating methylglyoxylated protein levels compared to mice receiving saline injection. This was not observed in mice fed a ketogenic diet (Figure 2A), consistent with the idea that a ketogenic diet contributes to MGO scavenging and detoxification in vivo. We also observed increased circulating MGO in ketogenic diet-fed, saline-injected mice. We attribute this to an alternate MGO synthesis pathway, in which acetoacetate decomposes to acetone, which then condensates to form MGO [24]. It is also worthwhile to note that the levels of MGO observed in this group were well below those reported in painful conditions [8; 27] and following MGO-injection in our experiments.
One mechanism by which this may occur is through upregulation of glyoxalase activity, specifically GLO1, the rate-limiting step of this enzyme system [38]. Mutations in GLO1 and reductions in its activity have been linked to increased susceptibility to diabetic peripheral neuropathy [19; 36], and we have previously reported that substrains of mice with GLO1 gene duplications are protected from mechanical allodynia and diabetic peripheral neuropathy [23]. We assayed the activity of GLO1 and observed a non-statistically significant trend toward increased GLO1 activity in the spinal cords and footpads of mice fed a ketogenic diet for one week (Figure 2D). This result suggests that a ketogenic diet may, in part, reduce MGO-evoked pain and nociception by increasing systemic glyoxalase activity, though this is likely not the only mechanism by which a ketogenic diet results in improved allodynia. This result has clear implications for the efficacy of a ketogenic diet in preventing and reversing mechanical allodynia in models of diabetic peripheral neuropathy [17] and neuropathy induced by metabolic syndrome [13], where MGO may play an important role.
Another mechanism by which a ketogenic diet may detoxify MGO is by direct interaction and reaction between MGO and ketone bodies [33]. In the 1930s, Henze and colleagues discovered the reaction between MGO and acetoacetate to form 3-hydroxy-hexane-2,5-dione, or Henze’s ketol, initially hypothesizing a role in converting fatty acids to carbohydrates [20; 21; 37]. Salomón and colleagues demonstrated a nonenzymatic formation on Henze’s ketol in vitro and in the blood of diabetics with elevated ketones due to an insulin fast [33], however, the biological significance of this reaction has not yet been identified. We therefore hypothesized that elevated circulating ketones resulting from a ketogenic diet could directly detoxify MGO, rendering it non-noxious. To assess this possibility, we developed a novel behavioral assay for MGO toxicity and nociception (Figure 3). Using this paradigm, we demonstrate that coincubation with either acetoacetate or β-HB was sufficient to reduce MGO-evoked nociception (Figure 4) and spinal biomarkers of nociception (Figure 6). Importantly, β-HB was unable to detoxify MGO following conjugation to BSA (Figure 5), consistent with a model in which ketones are only able to detoxify MGO before it has formed protein adducts.
Despite strong evidence for MGO detoxification by a ketogenic diet and ketone bodies, it remains possible that these interventions modify other aspects of MGO signaling. Griggs and colleagues have demonstrated a TRPA1-adenylyl cyclase 1 signaling circuit that contributes to MGO-evoked nociception and nociceptive biomarker elevations in the spinal dorsal horn [18]. As nutritional ketosis is often described as mimicking starvation or a fasted condition, it is plausible that a ketogenic diet or ketone bodies modify adenylyl cyclase activity or available cyclic AMP levels. It is also possible that ketone bodies modify TRPA1 signaling. We have previously reported reduced hydrogen peroxide production in the sciatic nerves of ketogenic diet-fed mice [12], which may lead to reduced TRPA1 sensitization or activation [4; 35]. Additionally, MGO-BSA conjugates are traditionally considered ligands for RAGE [42]. Thus, the ability for β-HB to prevent MGO-evoked but not MGO-BSA-evoked nociception may be consistent with modification of TRPA1 activation as well as MGO detoxification. It is, however, unlikely that the antinociceptive effects of a ketogenic diet are solely mediated through regulation of TRPA1. Barragán-Iglesias et. al. (2019) reported an incomplete rescue of MGO-evoked mechanical allodynia following treatment with the TRPA1 inhibitor A967079 [6]. Here, we report a full rescue with a ketogenic diet (Figure 1D), suggesting intervention with a ketogenic diet likley rescues MGO-evoked pain-like behaviors through multiple mechanisms that could include MGO scavenging, improved mitochondrial function, and reduced inflammation[9; 29; 34].
Generation, accumulation, and pathological signaling of MGO lays at the crux of many debilitating pain conditions, including DPN [8; 22], chemotherapy-induced neuropathy [42], and radiculopathy resulting from lumbar disc herniation [27]. Here we demonstrate that a ketogenic diet prevents and rescues nociception and activation of nociceptive circuits evoked by MGO. Further, our findings identify MGO scavenging and detoxification as a potential mechanism by which a ketogenic diet impacts pain. Overall, these findings are consistent with emerging evidence that suggest a ketogenic diet may benefit MGO-driven painful pathologies [13; 17; 31; 32; 45]. These results suggest a broader application of ketogenic diets as therapeutic interventions in other MGO-related pathologies and chronic pain conditions.
Acknowledgments
This work was supported by NIH grants RO1 NS043314 (DEW), the Kansas Institutional Development Award (IDeA) P20 GM103418, Kansas University Training Program in Neurological and Rehabilitation Sciences (NIH T32 award) supported by NIH Award Number T32HD057850, and core support from the Kansas IDDRC P30 HD00228. Thanks to Dr. Bret Freudenthal and Ben Ryan for their help in purification of acetoacetate.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
Bibliography
- [1].Ahmed N, Argirov OK, Minhas HS, Cordeiro CAA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J 2002;364(Pt 1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahmed N, Thornalley PJ. Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and intrinsic fluorescence. Biochem J 2002;364(Pt 1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andersson DA, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, Fleming T, Bevan S. Methylglyoxal evokes pain by stimulating TRPA1. PloS one 2013;8(10):e77986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. Journal of Neuroscience 2008;28(10):2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arai M, Nihonmatsu-Kikuchi N, Itokawa M, Rabbani N, Thornalley PJ. Measurement of glyoxalase activities: Portland Press Ltd, 2014. [DOI] [PubMed]
- [6].Barragán-Iglesias P, Kuhn J, Vidal-Cantú GC, Salinas-Abarca AB, Granados-Soto V, Dussor GO, Campbell ZT, Price TJ. Activation of the integrated stress response in nociceptors drives methylglyoxal-induced pain. Pain 2019;160(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bednarik J, Vlckova‐Moravcova E, Bursova S, Belobradkova J, Dusek L, Sommer C. Etiology of small‐fiber neuropathy. Journal of the Peripheral Nervous System 2009;14(3):177–183. [DOI] [PubMed] [Google Scholar]
- [8].Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F. Methylglyoxal modification of Na v 1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nature medicine 2012;18(6):926–933. [DOI] [PubMed] [Google Scholar]
- [9].Brings S, Fleming T, De Buhr S, Beijer B, Lindner T, Wischnjow A, Kender Z, Peters V, Kopf S, Haberkorn U, Mier W, Nawroth PP. A scavenger peptide prevents methylglyoxal induced pain in mice. Biochim Biophys Acta Mol Basis Dis 2017;1863(3):654–662. [DOI] [PubMed] [Google Scholar]
- [10].Chandrasekaran P, Rani PK. Reversal of diabetic tractional retinal detachment attributed to keto diet. BMJ Case Reports 2020;13(10):e235873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chaplan SR, Bach F, Pogrel J, Chung J, Yaksh T. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [12].Cooper MA, McCoin C, Pei D, Thyfault JP, Koestler D, Wright DE. Reduced mitochondrial reactive oxygen species production in peripheral nerves of mice fed a ketogenic diet. Experimental physiology 2018;103(9):1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cooper MA, Menta BW, Perez-Sanchez C, Jack MM, Khan ZW, Ryals JM, Winter M, Wright DE. A ketogenic diet reduces metabolic syndrome-induced allodynia and promotes peripheral nerve growth in mice. Experimental neurology 2018;306:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Di Lorenzo C, Coppola G, Bracaglia M, Di Lenola D, Sirianni G, Rossi P, Di Lorenzo G, Parisi V, Serrao M, Cervenka MC. A ketogenic diet normalizes interictal cortical but not subcortical responsivity in migraineurs. BMC neurology 2019;19(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dressler AR E; Trimmel-Schwahofer P; Klbermaxz K; Prayer D; Kaspiran G; Rami B; Schober E; Feucht M. Type 1 diabetes and epilepsy: efficacy and safety of the ketogenic diet. Epilepsia 2010;51(6):1806–1809. [DOI] [PubMed] [Google Scholar]
- [16].Düll MM, Riegel K, Tappenbeck J, Ries V, Strupf M, Fleming T, Sauer SK, Namer B. Methylglyoxal causes pain and hyperalgesia in human through C-fiber activation. Pain 2019;160(11):2497–2507. [DOI] [PubMed] [Google Scholar]
- [17].Enders J, Swanson MT, Ryals J, Wright D. A ketogenic diet reduces mechanical allodynia and improves epidermal innervation in diabetic mice. Pain 2021. [DOI] [PMC free article] [PubMed]
- [18].Griggs RB, Laird DE, Donahue RR, Fu W, Taylor BK. Methylglyoxal requires AC1 and TRPA1 to produce pain and spinal neuron activation. Frontiers in neuroscience 2017;11:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Groener J, Reismann P, Fleming T, Kalscheuer H, Lehnhoff D, Hamann A, Roser P, Bierhaus A, Nawroth PP, Rudofsky G. C332C genotype of glyoxalase 1 and its association with late diabetic complications. Experimental and Clinical Endocrinology & Diabetes 2013;121(07):436–439. [DOI] [PubMed] [Google Scholar]
- [20].Henze M. Die Umwandlung der Acetessigsäure durch Methylglyoxal. I. Mitteilung. 1930.
- [21].Henze M, Müller R. Die Umwandlung der Acetessigsäure durch Methylglyoxal. II. Mitteilung. 1930.
- [22].Huang Q, Chen Y, Gong N, Wang Y-X. Methylglyoxal mediates streptozotocin-induced diabetic neuropathic pain via activation of the peripheral TRPA1 and Nav1. 8 channels. Metabolism 2016;65(4):463–474. [DOI] [PubMed] [Google Scholar]
- [23].Jack MM, Ryals JM, Wright DE. Protection from diabetes-induced peripheral sensory neuropathy — A role for elevated glyoxalase I? Experimental Neurology 2012;234(1):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kalapos MP. On the mammalian acetone metabolism: from chemistry to clinical implications. Biochimica et Biophysica Acta (BBA) - General Subjects 2003;1621(2):122–139. [DOI] [PubMed] [Google Scholar]
- [25].Kihara M, Schmelzer JD, Poduslo JF, Curran GL, Nickander KK, Low PA. Aminoguanidine effects on nerve blood flow, vascular permeability, electrophysiology, and oxygen free radicals. Proceedings of the National Academy of Sciences 1991;88(14):6107–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kikuchi S, Shinpo K, Moriwaka F, Makita Z, Miyata T, Tashiro K. Neurotoxicity of methylglyoxal and 3‐deoxyglucosone on cultured cortical neurons: synergism between glycation and oxidative stress, possibly involved in neurodegenerative diseases. Journal of neuroscience research 1999;57(2):280–289. [DOI] [PubMed] [Google Scholar]
- [27].Liu C-C, Zhang X-S, Ruan Y-T, Huang Z-X, Zhang S-B, Liu M, Luo H-J, Wu S-L, Ma C. Accumulation of methylglyoxal increases the advanced glycation end-product levels in DRG and contributes to lumbar disk herniation-induced persistent pain. Journal of Neurophysiology 2017;118(2):1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of Diabetic Nephropathy by a Ketogenic Diet. PloS one 2011;6(4):e18604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Puchalska P, Crawford PA. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab 2017;25(2):262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reichert O, Fleming T, Neufang G, Schmelz M, Genth H, Kaever V, Wenck H, Stäb F, Terstegen L, Kolbe L. Impaired glyoxalase activity is associated with reduced expression of neurotrophic factors and pro‐inflammatory processes in diabetic skin cells. Experimental dermatology 2017;26(1):44–50. [DOI] [PubMed] [Google Scholar]
- [31].Ruskin DN, Kawamura M Jr, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PloS one 2009;4(12):e8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ruskin DN, Sturdevant IC, Wyss LS, Masino SA. Ketogenic diet effects on inflammatory allodynia and ongoing pain in rodents. Scientific reports 2021;11(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Salomón T, Sibbersen C, Hansen J, Britz D, Svart MV, Voss TS, Møller N, Gregersen N, Jørgensen KA, Palmfeldt J, Poulsen TB, Johannsen M. Ketone Body Acetoacetate Buffers Methylglyoxal via a Non-enzymatic Conversion during Diabetic and Dietary Ketosis. Cell Chemical Biology 2017;24(8):935–943.e937. [DOI] [PubMed] [Google Scholar]
- [34].Salomón T, Sibbersen C, Hansen J, Britz D, Svart MV, Voss TS, Møller N, Gregersen N, Jørgensen KA, Palmfeldt J, Poulsen TB, Johannsen M. Ketone Body Acetoacetate Buffers Methylglyoxal via a Non-enzymatic Conversion during Diabetic and Dietary Ketosis. Cell Chem Biol 2017;24(8):935–943.e937. [DOI] [PubMed] [Google Scholar]
- [35].Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. European Journal of Neuroscience 2008;27(5):1131–1142. [DOI] [PubMed] [Google Scholar]
- [36].Skapare E, Konrade I, Liepinsh E, Strele I, Makrecka M, Bierhaus A, Lejnieks A, Pirags V, Dambrova M. Association of reduced glyoxalase 1 activity and painful peripheral diabetic neuropathy in type 1 and 2 diabetes mellitus patients. J Diabetes Complications 2013;27(3):262–267. [DOI] [PubMed] [Google Scholar]
- [37].Stöhr R, Henze M. Die Umwandlung der Acetessigsäure durch Methylglyoxal. III. Mitteilung. Das Ketol (C6H10O3) als Glykogenbildner. 1932.
- [38].Thornalley P. Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochemical Society Transactions 2003;31(6):1343–1348. [DOI] [PubMed] [Google Scholar]
- [39].Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification-A role in pathogenesis and antiproliferative chemotherapy. General Pharmacology: The Vascular System 1996;27(4):565–573. [DOI] [PubMed] [Google Scholar]
- [40].Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Archives of Biochemistry and Biophysics 2003;419(1):31–40. [DOI] [PubMed] [Google Scholar]
- [41].Tóth CC Type 1 diabetes mellitus successfully managed with the paleolithic ketogenic diet. Int J Case Rep Images 2014;5(10):699–703. [Google Scholar]
- [42].Wei J-Y, Liu C-C, Ouyang H-D, Ma C, Xie M-X, Liu M, Lei W-L, Ding H-H, Wu S-L, Xin W-J. Activation of RAGE/STAT3 pathway by methylglyoxal contributes to spinal central sensitization and persistent pain induced by bortezomib. Experimental neurology 2017;296:74–82. [DOI] [PubMed] [Google Scholar]
- [43].Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutrition & Metabolism 2008;5(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yagihashi S, Kamijo M, Baba M, Yagihashi N, Nagai K. Effect of aminoguanidine on functional and structural abnormalities in peripheral nerve of STZ-induced diabetic rats. Diabetes 1992;41(1):47–52. [DOI] [PubMed] [Google Scholar]
- [45].Zhong S, Zhou Z, Lin X, Liu F, Liu C, Liu Z, Deng W, Zhang X, Chang H, Zhao C. Ketogenic diet prevents paclitaxel-induced neuropathic nociception through activation of PPARγ signalling pathway and inhibition of neuroinflammation in rat dorsal root ganglion. European Journal of Neuroscience 2021;54(4):5341–5356. [DOI] [PubMed] [Google Scholar]