Abstract
Cancer immunotherapies have changed the landscape of cancer treatment during the past few decades. Among them, immune checkpoint inhibitors (ICIs), which target PD-1, PD-L1 and CTLA-4, are increasingly used for certain cancers; however, this increased use has resulted in an increased reports of immune-related adverse events (irAEs). These irAEs are unique and are different to those of traditional cancer therapies, and typically have a delayed onset and prolonged duration. IrAEs can involve any organ or system. These effects are frequently low-grade and are treatable and reversible; however, some adverse effects can be severe and lead to permanent disorders. Management is based primarily on corticosteroids and other immunomodulatory agents, which should be prescribed carefully to reduce the potential for short-term and long-term complications. Thoughtful management of irAEs is important in optimizing quality of life and long-term outcomes.
ToC blurb
Checkpoint inhibitors are increasingly being used in clinical practice; however, these therapies can be associated with adverse events that can affect almost any organ system. This Primer by Ramos-Casals and colleagues summarizes the epidemiology, mechanisms, diagnosis and treatment of these adverse events.
Introduction
Cancer immunotherapies are broadly defined as therapies that directly or indirectly target any component of the immune system that is involved in the anticancer immune response, including the stimulation, enhancement, suppression or desensitization of the immune system. These therapies comprise different approaches that include the use of specific drugs (monoclonal antibodies, small proteins or fusion proteins) that target proteins on the surface of cancer cells or immune cells, and other therapies, such as cytokines, oncolytic virus therapies, cancer vaccines or cell-based therapies (such as adoptive T cell transfer and chimeric antigen receptor T cell therapies).1
One class of these therapies — immune-checkpoint inhibitors (ICIs) — serve to induce an antitumour immune response by blocking immune checkpoints. Normally, immune checkpoints, key examples of which include CTLA-4 and PD-1 pathways, downregulate T cell responses and act to protect the body from possibly damaging immune responses, such as autoimmune disease (Fig. 1). However, tumours can hijack this system to evade the immune system, through the activation of immune checkpoints and inhibition of the T cell response. Thus, interfering with these immune checkpoint pathways can induce an antitumour immune response and convey therapeutic benefits in patients with cancer.
Figure 1. Mechanism of immune checkpoints and ICIs.
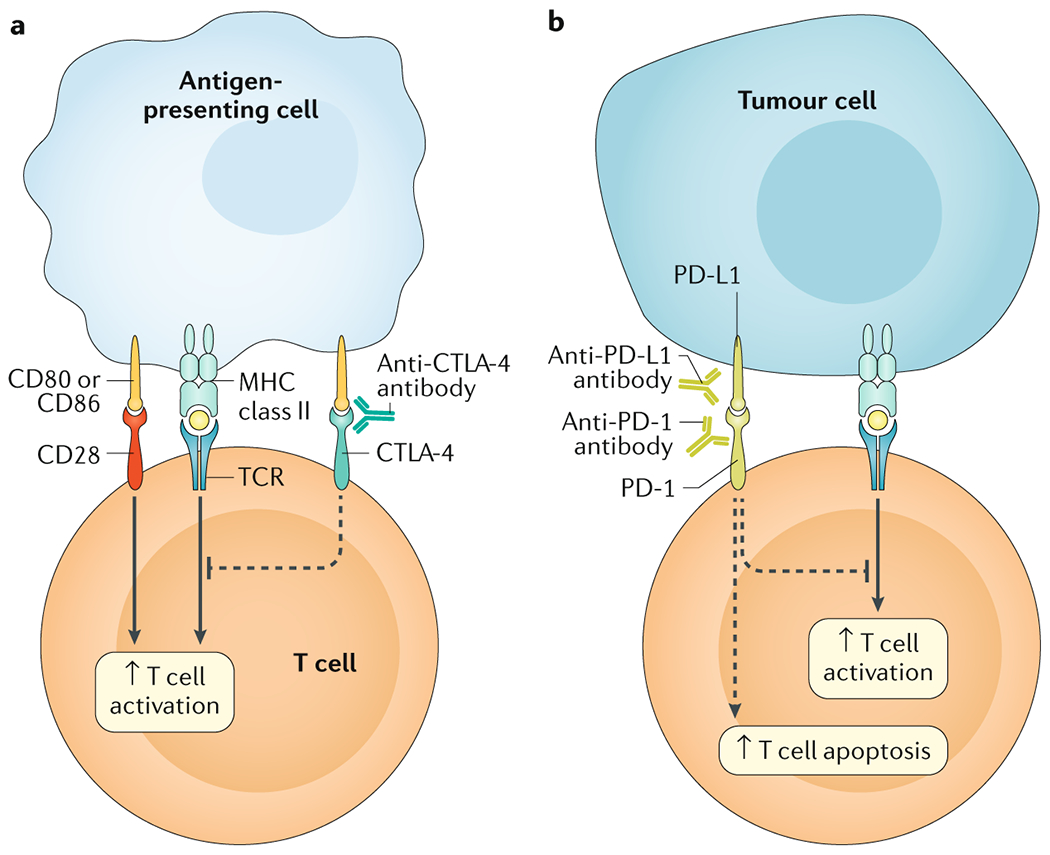
The main immunotherapy approaches that are approved for clinical use in cancer are the immune checkpoint inhibitors (ICIs). These therapies are monoclonal antibodies that target the receptors CTLA-4 and PD-1 and the PD-1 ligand, PD-L1, which are involved in the regulation of T cell activation. A| T cell activation requires two signals: first, antigen recognition by the T cell receptor (TCR) following antigen presentation by major histocompatibility complex (MHC) class II molecules on the surface of antigen-presenting cells and, second, signal modulation by CD80 or CD86 binding to the CD28 receptor. CTLA-4 is located on the T-cell surface and competes with the CD28 receptor to bind CD80 or CD86, thereby blocking T cell activation. CTLA-4 inhibitors block CTLA-4–CD80 or CTLA-4–CD86 binding to facilitate T cell activation (dashed line). B| PD-1 is a surface receptor that is expressed by T-cells and promotes apoptosis of antigen-specific T-cells and reduces apoptosis of regulatory T-cells 252,253 through its interaction with its ligand PD-L1, which is expressed by tumour cells and myeloid cells. This interaction is useful in preventing autoimmunity in physiological conditions, but cancer cells exploit this process to escape from immune system activity upregulating PD-L1 expression.254,255 PD-1 and PD-L1 inhibitors block the PD-1–PD-L1 interaction, facilitating T cell activation and survival (dashed lines).
Several ICIs are approved for the treatment of various cancer types (Box 1). These drugs are all monoclonal antibodies that target either CTLA-4 signalling or PD-1 signalling (by targeting PD-1 or the PD-1 ligand, PD-L1), and have a universal effect on immune responses that is not dependent on individual cancer-specific antigens.2 The use of ICIs for cancer therapy is increasing; however, a key challenge that has emerged with the progressive implementation of ICIs in clinical practice is their uncontrolled collateral effects on the immune system that can lead to so-called immune-related adverse events (irAEs).3 ICIs have a different spectrum of toxicities than standard chemotherapy or other biological agents, and most toxicities result from excessive immunity against normal organs 3 (Box 2).
Box 1. Approved ICIs according to cancer type.
Anti-CTLA-4 antibodies
Anti-PD-1 antibodies
- Nivolumab
- Bladder cancer
- Colorectal cancer
- Head and neck cancer
- Hepatocellular carcinoma
- Hodgkin lymphoma
- Melanoma
- Non-small cell lung cancer
- Renal cell carcinoma
- Pembrolizumab
- Bladder cancer
- Cervical cancer
- Gastroesophageal junction cancers
- Head and neck cancer
- Hepatocellular carcinoma
- Hodgkin lymphoma
- Merkel cell carcinoma
- Metastatic solid tumours classified as MSI-H or dMMR
- Non-small cell lung cancer
- Primary mediastinal large B-cell lymphoma
- Stomach cancer
- Cemiplimab
- Cutaneous squamous cell carcinoma
Anti-PD-L1 antibodies
- Atezolizumab
- Bladder cancer
- Breast cancer
- Non-small cell lung cancer
- Avelumab
- Bladder cancer
- Merkel cell carcinoma
- Durvalumab
- Bladder cancer
- Non-small cell lung cancer
aIn combination with nivolumab. ICIs, immune checkpoint inhibitors.
Box 2. Organ-based classification of irAEs in patients with cancer treated with ICIs.
Cardiac
- Myocarditisa
- Autoimmune myocarditis
- Myocardial fibrosis
- Pericarditis
- Autoimmune pericarditis
- Pericardial effusion
- Pericardial tamponade
Dermatological
Alopecia areata/universalis
Dermatitis herpetiforme
Erythema multiforme
Granuloma annulare
Lichen planopilaris/planus/lichenoid dermatitis
Panniculitis/Erythema nodosum
Pemphigoid/Pemphigus
Psoriasis
Pyoderma gangrenosum
Sweet syndrome
Vitiligoa
Endocrine
- Adrenitisa
- Adrenal insufficiency
- Cortisol deficiency
- Hypercortisolism
- Hypoadrenalism
- Isolated ACTH deficiency
Autoimmune diabetes mellitus
Hyperparathyroidism
Hypogonadism
- Hypophysitisa
- Autoimmune hypophysitis
- Hypopituitarism
- Panhypopituitarism
- Thyroiditisa
- Autoimmune thyroiditis
- Hyperthyroidism
- Hypothyroidism
- Graves disease
- Thyrotoxicosis
Gastrointestinal
Haematological
Aplastic anemia/pure red cell aplasia
Autoimmune hemolytic anemia
Autoimmune neutropenia
Hemophagocytic lymphohistiocytosis
Immune thrombocytopenic purpura
Muscular
Neurological
Aseptic meningitis
Encephalitis
- Cranial nerve involvement
- Bilateral hearing loss
- Facial palsy
- Oculomotor paresis
- Motor neuropathy
- Acute generalized motor neuropathy
- Multifocal motor block neuropathy
Myasthenia gravis
- Neuromyelitis optica spectrum disorders
- Optic neuritis
- Transverse myelitis
- Polyneuropathiesa
- Axonal sensory motor polyneuropathy
- Multiplex mononeuritis
- Peripheral sensory neuropathy
- Polyradiculopathies
- Chronic inflammatory demyelinating polyneuropathy
- Guillain-Barré syndrome
Ocular
Conjunctivitis
Episcleritis/scleritis
Orbital inflammation
- Uveitisa
- Anterior uveitis
- Chrorioretinopathy
- Iridocyclitis/iritis
- Panuveitis
- Posterior uveitis
Vogt-Koyanagi-Harada syndrome
Pulmonary
- Interstitial lung diseasea
- Alveolitis
- Organizative pneumonitis
- Pneumonitis
- Pulmonary fibrosis
- Pulmonary hemorrhage
Renal
Acute tubulointerstitial nephritis/renal tubular acidosis
Glomerulonephritis
Skeletal
Systemic
Antiphospholipid syndrome
- Lupus
- Lupus nephropathy
- Subacute cutaneous lupus erythematosus
- Systemic lupus erythematosus
- Sarcoidosis
- Cutaneous sarcoidosis
- Pulmonary sarcoidosis
- Renal sarcoidosis
Sicca syndromea/Sjogren syndrome
Systemic sclerosis
- Vasculitisa
- Cerebral vasculitis
- Cryoglobulinemia
- Cutaneous vasculitis
- Eosinophilic granulomatosis with polyangiitis
- Giant cell arteritis
- Pulmonary vasculitis
- Schonlein-Henoch purpura
aMore than 100 cases reported4. ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events.
Owing to the increased use of ICIs for cancer treatment, the cumulated annual number of irAEs is increasing exponentially, with nearly 13,000 cases reported up to 2018(Ref.4). More than two thirds of reported cases of cancer immunotherapy-related irAEs are related to ICIs, and only 3 drugs (that is, ipilimumab, nivolumab and pembrolizumab) are responsible for almost 60% of reported cases4.
This Primer provides an update on ICI-associated irAEs (subsequently referred to as irAEs) from a multidisciplinary perspective. Owing to their increased prevalence and potentially severe nature, this Primer focuses on the adverse effects of ICIs only, rather than other types of cancer immunotherapy.
Epidemiology
Frequency of irAEs
Adverse events in clinical trials are reported and graded using the Common Terminology Criteria for Adverse Events (CTCAE) from the National Cancer Institute. CTCAE ranges from grade 1 to grade 5, which refers to mild, moderate, severe, life-threatening or death, in ascending order.5 The general safety of ICIs has been estimated in a meta-analysis of 36 phase II/III trials, showing a pooled incidence ranging between 54% and 76% for all adverse events6.
IrAEs can occur in any organ system, with the median onset usually within 2–16 weeks from the commencement of therapy, depending on the organ system involved7. However, onset of irAEs has been described within a few days of ICI initiation, and ≥1 year after completion of therapy.8,9 The risk of first-onset irAEs is 3-fold higher during the first 4 weeks of treatment than between 4 weeks and the end of treatment.10 As the T cell autoreactive clones that underlie these irAEs are presumably present long after the cessation of treatment, it is possible that irAEs may even present many years after treatment. Early toxicity (that is, between 1 and 12 weeks after treatment initiation) is most commonly dermatological effects for both CTLA-4 and PD-1 inhibitors.11 Among individual ICIs, data from one meta-analysis showed that the most-common irAEs for ipilimumab are dermatological, gastrointestinal and renal toxicities, for pembrolizumab arthralgia, pneumonitis and hepatic toxicities, for nivolumab endocrine toxicities, and for atezolizumab hypothyroidism6.
Pharmacological class-specific irAEs
Consistent with the distinct functions of immune checkpoints, the types of irAEs related to single drug therapy targeting the CTLA-4 or PD-1 pathways differ12. Typically, PD-1 and PD-L1 inhibitors are better tolerated than CTLA-4 inhibitors13; indeed, grade 3 and 4 irAEs were more common with CTLA-4 inhibitors than PD-1 inhibitors in one systematic review14. In this review, grade 3 or 4 irAEs comprised 31% of all irAEs for CTLA-4 inhibitors and 10% for PD-1 inhibitors); of note, colitis (OR 8.7, 95% CI 5.8–12.9), hypophysitis (OR 6.5, 95% CI 3.0–14.3) and rash (OR 2.0, 95% CI 1.8–2.3) were more common with CTLA-4 inhibitors, whereas pneumonitis (OR 6.4, 95% CI 3.2–12.7), hypothyroidism (OR 4.3, 95% CI 2.9–6.3), arthralgia (OR 3.5, 95% CI 2.6–4.8) and vitiligo (OR 3.5, 95% CI 2.3–5.3) were more common with PD-1 inhibitors14. The precise biological explanations for the differences in irAE localization and severity with different ICIs are not entirely known. Theoretically, CTLA-4 blockade might induce a greater magnitude of T-cell proliferation or reduced regulatory T cell (Treg)-mediated immunosuppression, and PD-1 blockade might activate a smaller number of T-cell clones15,16 (although most circulating T-cells do not express PD-1, they can be induced to do so upon stimulation during TCR-dependent signalling)15 (see Mechanisms/pathophysiology, below).
Few data are available about the potential differences in irAEs owing to the specific pharmaco-immunological composition of ICIs. However, some studies have demonstrated that antigenic effects of immunotherapies can result in the development of neutralizing antibodies and may depend on the degree of ‘humanization’ of the therapy17. Indeed, the formation of antibodies against biological agents is quite common, although these antibodies seem to have primarily neutralizing effects decreasing therapeutic efficacy and causing allergic or hypersensitivity reactions18. The immunogenicity of ICIs has been assessed in a few studies. In 6 clinical studies with nivolumab, anti-drug antibodies were found at least once in 12.7% of patients and persistently (detected in at least 2 consecutive samples, separated at least 16 weeks apart) in only 0.3% of patients without any clinical consequence (lack of association with hypersensitivity, infusion reactions, or loss of efficacy)19, where as the maximum anti-drug antibody-positive rates were 54.1% for atezolizumab, 5.9% for durvalumab, 2.9% for avelumab and 2.1% for pembrolizumab20. Although the clinical implications of anti-ICI antibodies remain to be elucidated, these effects seem to primarily affect treatment efficacy rather than cause adverse events 20.
Combination strategies
Increasing use of combination strategies (combining immunotherapies with traditional treatments, such as chemotherapy, or the combining two types of immunotherapy) might improve the efficacy of cancer immunotherapy but could also amplify irAEs.2
With respect to the development of unexpected toxicities, combining chemotherapies with PD-1 inhibitors is not characterized by additional irAEs, and reported adverse effects are consistent with those of each agent.21,22 Similarly, combining CTLA-4 inhibitors with PD-1 or PD-L1 inhibitors did not lead to unexpected new irAEs 15,16. However, the frequency of adverse events with combinatorial therapy is higher than with monotherapy.8 Indeed, in one study, the overall prevalence of irAEs (>90%) and severity (with grade ≥3 adverse events representing ~60% of the total irAEs) with combining CTLA-4 inhibitors with PD-1 or PD-L1 inhibitors were higher than that of monotherapy.16
In addition, the phenotype of the organ-specific irAEs can be modified with combination therapies. One study of 30 patients with clinically-confirmed arthritis demonstrated that individuals treated with combination ICI therapy were more likely to present with knee arthritis, to have higher levels of C-reactive protein, to have a prior irAE, and to have a reactive arthritis-like phenotype, compared with those treated with ICI monotherapy, who were more likely to have initial small joint involvement and arthritis as their only irAE23. Moreover, combination therapy was associated with greater risk and earlier onset of irAEs, with an up to 5-fold shorter median time to onset than monotherapy (32 for combination therapy versus 146 days for monotherapy).10
Tumour-specific patterns of irAEs
Overall, the types of irAEs do not seem to be specific to the type of cancer 12. Indeed, although cross-study comparisons should be interpreted with caution, some data advise that the frequency of specific irAEs varies between individuals with different cancers who receive the same ICI, suggesting that different organ-specific immune microenvironments could drive specific irAE patterns in some types of cancer. For example, comparison of irAEs owing to PD-1 inhibitors in melanoma, non-small-cell lung cancer and renal cell carcinoma , showed that patients with melanoma had a higher frequency of dermatological (especially vitiligo) and gastrointestinal irAEs and a lower frequency of pneumonitis than patients with other cancers.14
Risk factors
The frequency of irAEs varies widely according to the ICI used and the organ-specific damage triggered, suggesting that there is a specific population of individuals who are prone to developing irAEs, maybe involving an unknown genetic background.11 In addition, there are substantial individual variations in risks of irAEs, as some patients do not develop adverse events after months of therapy whereas others have life-threatening irAEs after a single infusion.
One explanation for the difference in risk of irAEs could be that some individuals have a predisposition to autoimmunity. To this end, some studies have reported that <10% of individuals who developed rheumatic irAEs had a family history of autoimmune disorders, and ~25% of individuals had a personal history of autoimmune disorders.24–27 Furthermore, a number of CTLA4 and PDCD1 (encoding PD-1) polymorphisms have been associated with several autoimmune disorders.28,29 Genetic variations could have a role in risk of irAEs, although no clear evidence of strong genetic associations have been demonstrated. Several studies have reported some personal risk factors linked to the development of irAEs. A history of autoimmune disease, use of CTLA-4 inhibitors and poor kidney function of grade ≥3 have been associated with a higher risk of developing irAEs.30 In addition, one study has linked an elevated BMI with an increased risk of irAEs in patients treated with pembrolizumab31, but this association was not confirmed in patients treated with atezolizumab32 or ipilimumab33. By comparison, female sex and corticosteroid use were identified as protective markers against development of irAEs in one study of 78 patients treated with ipilimumab, nivolumab or pembrolizumab30. All these data require replication before firm conclusions regarding these associations can be drawn. Age has not been associated with a differentiated toxicity profile in patients treated with ICI monotherapy 34,35 and tolerance in elderly patients seems similar to younger people34. Whether other epidemiological features, such as patient’s ethnicity, are associated with risk of irAEs is unknown.
Mechanisms/Pathophysiology
CTLA-4 and PD-1 or PD-L1 inhibitors result in non-specific upregulation of immune pathways. Although there are commonalities between the immune toxicity profiles of these therapies, there are important differences in the frequency and clinical presentation of specific irAEs and the most frequently affected organs14. These phenotypic variations suggest that the mechanisms of irAEs differ between ICIs, although the precise mechanisms remain to be elucidated. Both CTLA-4 and PD-1 inhibition increases T cell activation and proliferation, abrogate Treg functions, and possibly boosts humoral autoimmunity36 (Fig. 2), although it is likely that specific pathways are more prominent in one therapy type versus the other, resulting in differences in irAE phenotypes.
Figure 2. Mechanism of irAE.

The mechanisms of immune-related adverse events (irAE) owing to immune checkpoint inhibitors (ICIs) depend on the type of ICI therapy used (anti-PD-1 or anti-PD-L1 inhibitors versus anti-CTLA-4 inhibitors). CTLA-4 inhibitors can induce several cellular alterations, such as T cell activation and proliferation, impaired regulatory T cell (Treg) survival and increased levels of TH17 cells, in addition to the induction of cross-reactivity between antitumour T cells and antigens on healthy cells and autoantibody production. PD-1 and PD-L1 inhibitors lead to a reduction in Treg survival and Treg inhibitory function and increase cytokine production. TCR, T cell receptor.
T-cell activation
Studies in animal models that lack immune checkpoints or in patients with genetic disorders that affect immune checkpoints can be used to study the immunological consequence of immune checkpoint inhibition, and can shed some light on the mechanisms underlying irAEs. CTLA4-knockout mice develop profound and rapidly fatal T cell lymphoproliferation, hypergammaglobulinaemia and T cell-mediated autoimmunity.37,38 Similarly, CTLA4fl/fl mice with acquired CTLA-4 deficiency during adulthood develop multi-organ autoimmune disease, although this disease is less severe than that observed in congenital deficiency.37 In humans, two genetic diseases, CHAI (CTLA-4 haploinsufficiency with autoimmune infiltration) and LATAIE (LRBA deficiency with autoantibodies, regulatory Treg cell defects, autoimmune infiltration, and enteropathy) which cause functional abnormalities in CTLA-4 pathways have been described, and result in widespread multi-organ lymphocytic infiltration, Treg defects and autoantibody production39. Although the disease phenotypes in animal models and individuals with CTLA-4-related genetic disorders are substantially more severe than irAEs in patients who receive CTLA-4 inhibitors, they all have similar types of functional immune abnormalities.
Normally, Tregs express CTLA-4 and downregulate immune responses by inhibiting effector T cell proliferation and cytokine release, to maintain self-tolerance. In mice, administration of anti-CTLA-4 antibodies impairs Treg function and survival via antibody-dependent cell-mediated cytotoxicity, therefore, increasing the ratio of effector T cells to Tregs in the tumour microenvironment40, possibly enhancing antitumour responses and contributing to the therapeutic benefit observed with CTLA-4 inhibitors40. In accordance with these animal data , numbers of circulating Tregs are decreased in patients receiving ipilimumab with no significant changes in the relative frequencies of naïve, central memory and effector memory cells41, although not all studies confirmed this reduction42. An imbalance in Tregs and Th17 cells could contribute to irAEs associated with ICIs43. Enhanced Th17 responses have prominent roles in the pathogenesis of many autoimmune diseases including rheumatoid arthritis, psoriatic arthritis, inflammatory bowel disease and many others44 owing to the production of pro-inflammatory cytokines such as IL-17A, IL-21, and IL-22 by these cells. Indeed, CTLA-4 inhibitors increase numbers of circulating Th17 cells in patients with melanoma, especially in those who developed irAEs 45, and increased IL-17 levels have been associated with severe irAE, particularly colitis, in patients receiving ipilimumab, suggesting that the effect of ICIs on Th17 cells contributes to the manifestations of irAEs 46.
PD-1 inhibition also enhances T cell activation, but results in different phenotypes of irAE than CTLA-4 inhibition. PD-1 is expressed on T cells, whereas its ligands PD-L1 and PD-L2 are present on antigen presenting cells, tumour cells and various normal tissues, and normally act to downregulate T cell activation (Fig. 1).47 Both PD-1 and PD-L1 are expressed by Tregs, and this pathway appears to be involved in the differentiation of Th1 cells into Tregs.48,49 Mice deficient in PD-1 or PD-L1 develop various autoimmune manifestations depending on their genetic backgrounds, which, in some cases is mediated by autoantibodies, for example anti-troponin I antibodies in PD-1 deficient mice who develop cardiomyopathy.50–52 PD-1 and PD-L1 inhibition with monoclonal antibodies leads to a decrease in the number of circulating Tregs that was associated with a more favourable progression-free survival in patients treated for melanoma 53.
In addition to these cellular changes, both CTLA-4 and PD-1/PD-L1 inhibition result in increased cytokine production. Indeed, blocking CTLA-4 with monoclonal antibodies in humans results in enhanced CD4+ and CD8+ T cell activation, with subsequent release of cytokines such as TNF, IFNγ and IL-215,54, which can lead to further T cell proliferation and activation. As discussed above, IL-17 might mediate irAEs given its marked pro-inflammatory functions, and the empirical evidence showing increased circulating levels in individuals with some irAEs, such as colitis46. The role of other pro-inflammatory cytokines is less evident, although there have been reports of increased IL-1Ra, CXCL10 and TNF levels in patients with irAEs.55 Anti-TNF agents have been successfully used to treat different irAEs in patients receiving ICIs, suggesting a role for this cytokine in the development of these adverse events 56. However, the precise roles of these cytokines in the development of irAEs is unknown and requires further study.
Cross-reactivity tumoural antigenicity
Cross-reactivity between antitumour T cells and similar antigens on healthy cells might underlie the development of some irAEs57, such as vitiligo in patients with melanoma treated with ICIs.58 Data from the ICIR-BIOGEAS Registry revealed that among 368 reported cases of vitiligo, 96% of cases were in patients with melanoma, which is suggestive of cross reactivity between T cells against tumour antigen and melanocytes.11 In addition, cross-reactivity has been suggested for ICI-related myocarditis 59 owing to low selectivity among the tumour-reactive T-cell population, and, therefore, cross-reactivity with normal tissues.8 In support of this mechanism, post-mortem tissue from two patients who received combination immunotherapy (CTLA-4 and PD-1 inhibitors) with fatal myocarditis had robust T-cell infiltration and clonal expansion with shared T-cell receptors in both myocardium and the tumour60. In one of the patients, a 10-fold increased expression of PD-L1 was demonstrated in affected cardiac tissue compared with non-diseased muscle tissue.60
B-cell mediated autoantibody production
Owing to increased T cell activation with ICIs, augmented T cell–B cell interactions can result in autoantibody production. Indeed, interactions between follicular T cells and B cells in germinal centres is vital for humoural immunity and abnormal interactions have been associated with autoimmunity61. The production of autoantibodies in mouse models of anti-CTLA-4-induced irAE is common.62 Indeed, wild-type mice administered with repeated injections of anti-CTLA-4 antibodies develop anti-pituitary antibodies and hypophysitis (inflammation of the pituitary gland) is an irAE commonly observed with ipilimumab but not PD-1 and PD-L1 inhibitors.62 Further data supporting a potential role for B cells in immunotoxicity is from recent data showing that patients treated with ICI had B cell changes after a single dose, including reduced numbers of circulating B cells and increased numbers of CD21lo B cells and plasmablasts. These early changes were strong predictors of subsequent irAE.63
The detection of autoantibodies during an adverse event would support an immune-mediated aetiology and could assist in guiding specific therapeutic intervention. To this end, several autoantibodies have been identified in some patients with specific irAEs, although their presence is not universal across patients. For example, autoantibodies to thyrotropin, FSH and corticotropin-secreting cells have been identified in patients with melanoma who received ipilimumab and developed hypophysitis62. Other examples of patients with irAEs and circulating autoantibodies against specific tissues after CTLA-4 or PD-1/PD-L1 inhibition64 include anti-thyroid antibodies in patients developing thyroiditis, anti-BP180 antibodies in pemphigoid, and rheumatoid factor and anti-CCP in patients developing arthritis 62,65–69. In addition, positive autoantibodies to diabetic autoantigens including GAD65, IA-2, ICA-512, ZnT8 and insulin have been found in some individuals with PD-1 inhibitor-associated type 1 diabetes mellitus
Despite the discovery of autoantibodies in patients who have developed autoimmune diseases after receiving ICIs, their frequency is significantly lower than that reported in patients with the same autoimmune disease who did not receive ICIs. This has been reported for type 1 diabetes mellitus26, Sjögren syndrome70,71, rheumatoid arthritis72 or myasthenia gravis73,74. The predominant lack of serum autoantibodies may suggest a unique mechanism23, although whether this finding reflects an underlying mechanism or the inability to detect as-yet unidentified autoantibodies or a very low level of the specific autoantibody involved is not well known.8,23
Direct effect of monoclonal antibody
As ICIs are monoclonal antibodies against molecules that are expressed by both immune cells and other tissues, it is likely that some irAEs could be caused by complement-mediated direct injury from these therapies. For instance, CTLA-4 is strongly expressed in the anterior pituitary62, and hypophysitis is primarily seen with ipilimumab, not with PD-1 or PD-L1 inhibitors. In addition, myocardial PD-L1 is mainly localized on endothelium and is critical for control of immune-mediated cardiac injury75, and a 10-fold increased expression of PD-L1 was demonstrated in the affected cardiac tissue in an individual with fatal myocarditis who received ICI combination therapy, compared with non-diseased muscle tissue.60
Diagnosis, screening and prevention
Organ-specific irAEs
We summarize the incidence, typical presentation and recommended clinical workup for the most frequent irAEs (a more detailed list is included in Box 1).
Cardiac.
Cardiac toxicity owing to ICIs is rare (occurring in <1% of patients76 but can be fulminant and potentially fatal. Several cardiac pathologies have been reported associated with ICIs, of which myocarditis is one of the most common. Risk of myocarditis is higher with combination therapy than monotherapy.60,77 The onset for myocarditis is a relatively early event, with most cases developing within ~4 weeks of a single dose of ICI.77 Other cardiac irAEs include myocardial fibrosis, pericarditis, cardiomyopathy with Takotsubo-like syndrome, acute heart failure and cardiac arrythymia.78,79 Clinical presentation of evolving cardiac irAEs can include dyspnoea (difficulty breathing), palpitations or symptoms of congestive heart failure (such as fluid retention and oedema), depending on the type of cardiac dysfunction, although preserved ventricular function assessed by echocardiography does not rule out possibility of cardiac arrhythmia.77 Investigation of suspected cardiac toxicity should include electrocardiography and assessment of cardiac serum biomarkers including creatinine kinase and troponin levels. Useful imaging assessments include echocardiography to assess left ventricular ejection fraction and cardiac MRI with gadolinium enhancement to assess inflammation secondary to myocarditis.5 Cardiac biopsy is the gold standard for diagnosing of myocarditis, and characteristic pathological findings consistent with immune cell infiltration of the myocardium support this diagnosis. The involvement of a cardiologist and close monitoring are necessary if myocarditis is suspected.
Dermatological.
A wide range of dermatological manifestations of varying severity can occur associated with ICIs including vitiligo, lichenoid dermatitis, psoriasis, bullous pemphigoid, granulomatous diseases, drug rash with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome and Sweet syndrome.80–83 In most cases, dermatological toxicities occur early, and have been observed 2–3 weeks after ipilimumab treatment initiation and ~5 weeks after initiation of PD-1 inhibitors.84,85 Although common with both CTLA-4 inhibitors and PD-1 inhibitors, pruritis, rash and vitiligo are usually low grade (mild and localized or widespread and intermittent) (Fig. 3).86 Vitiligo-like depigmentation (VLD) is a characteristic cutaneous alteration predominantly described in patients with melanoma who receive ICIs, it has been reported in some untreated individuals and isolated cases in patients with non-cutaneous cancer.87 Severe dermatological irAEs (that is, ≥grade 3 according to CTCAE) occur in only ~2–10% of patients receiving ICIs.83,88 Expert input from dermatologists should be sought for progressive or high grade skin conditions, such Stevens-Johnson Syndrome, toxic epidermal necrolysis and DRESS syndrome 89. Diagnostic work-up typically includes physical examination to assess the dermatological manifestations, and skin biopsies to histologically assess aetiology according to the dermatologist’ clinical diagnosis.90
Figure 3. Common radiological and/or photographical appearance of irAEs.
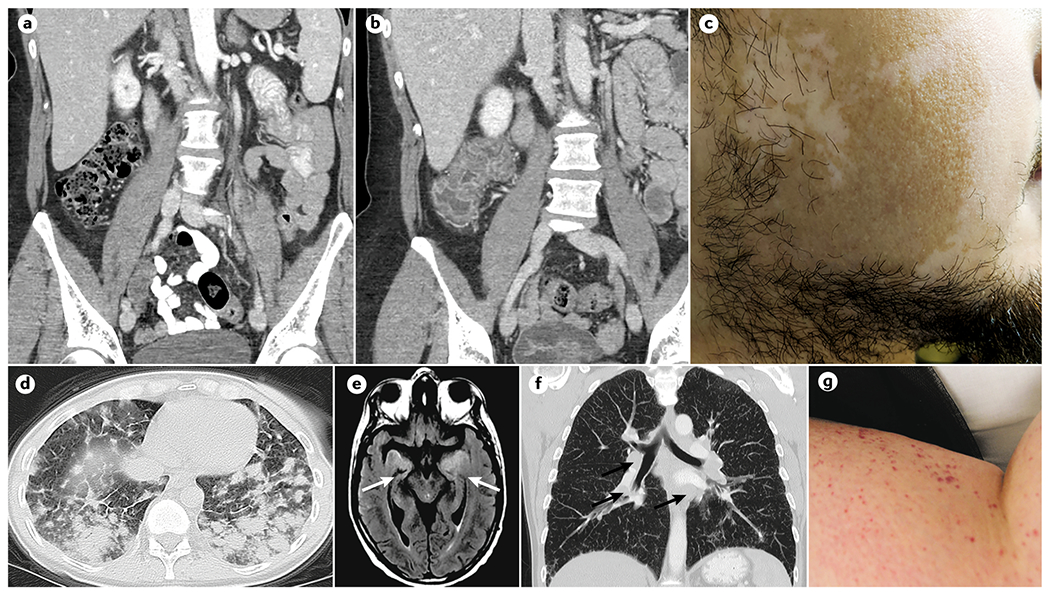
A| CT image of immune checkpoint inhibitor (ICI)-associated before the onset of immune related colitis. B| CT image of ICI-associated colitis after colitis onset. C| Vitiligo. D| High-resolution pulmonary CT showing interstitial lung disease of ICI-associated pneumonitis. E| MRI with T2 flair of ICI-associated encephalitis; an abnormal signal can be observed bilaterally in the insula and medial temporal lobes. The patient presented with new-onset confusion and weakness whilst using anti PD-1 therapy. F| Pulmonary CT showing multiple hilar adenopathy in a patient with sarcoidosis after ipilimumab treatment. Biopsy of an adenopathy demonstrated non-caseating granulomas. G| Cutaneous purpura in a patient treated with nivolumab.
Endocrine.
The more commonly occurring endocrinopathies include hypophysitis, thyroid dysfunction and, less frequently, type 1 diabetes mellitus.91 Endocrinopathies are observed in up to 10% of patients who are treated with CTLA-4 inhibitors92,93 and in 4–14% of those treated with anti-PD-1 inhibitors94.
Hypophysitis has been reported in 3.3% of patients who receive ICIs in a meta-analysis of 61 clinical trials, with a higher rate in those who received CTLA-4 inhibitors (4.5%) and combination therapy (7.7%), whereas the rate in patients who received PD-1 or PD-L1 inhibitors was very low (in <1% of individuals)95,96. Symptoms of pituitary dysfunction can be nonspecific, including fatigue, headache or weakness with additional symptoms of headache or visual changes in those with hypophysitis causing pituitary enlargement.97 Polyuria and polydipsia representing signs of diabetes insipidus and posterior pituitary hormone deficiency are more rarely reported than anterior pituitary hormone deficiencies5,98. Hypophysitis can lead to secondary adrenocorticotropic hormone (ACTH) deficiency with secondary adrenal insufficiency, hypogonadotropic hypogonadism and secondary hypothyroidism owing to thyroid-stimulating hormone (TSH) deficiency. Symptoms indicative of hypophysitis should prompt an evaluation of cortisol, follicle-stimulating hormone (FSH), luteinizing hormone (LH), TSH and free thyroxine (T4) levels, in addition to testosterone levels in men and oestrogen levels in premenopausal women. MRI of the pituitary gland should be carried out in patients presenting with neurological deficits to rule out tumoural involvement.5 Although auto-antibodies against thyrotropin, FSH and corticotropin-secreting cells and expression of CTLA-4 on pituitary endocrine cells have been described in individuals with ICI-associated hypophysitis, testing for these markers is not part of routine workup.62
Thyroid dysfunction is one of the most common ICI-related endocrinopathies, and occurs slightly more frequently with PD-1 inhibitors than with CTLA-4 inhibitors.99,100 Primary hypothyroidism of any grade occurs in 8–14% of patients treated with pembrolizumab,96,101,102 whereas thyroiditis or hypothyroidism have been reported in 6% of patients with melanoma treated with ipilimumab and in 10–22% of patients treated with ipilimumab plus nivolumab.92,96,103 ICI-associated thyroid dysfunction is typically mild (CTCAE grade 1–2) and mainly consists of hypothyroidism (which manifests with symptoms such as fatigue, increased sensitivity to cold, constipation or weight gain) and, less rarely, of hyperthyroidism (symptoms of which include weight loss, increased appetite, palpitations, irritability, tachycardia or arrhythmia). Hyperthyroidism can resolve to normal thyroid function over time although it can develop to hypothyroidism.91,104 Investigation of thyroid dysfunction should distinguish between primary hypothyroidism (in which TSH level is high with low T4 levels) and secondary hypothyroidism (in which TSH and T4 levels are low and can be representative of pituitary dysfunction or hypophysitis).99 Guidelines from the American Society of Clinical Oncology recommend monitoring thyroid function before ICI initiation and every 4–6 weeks during therapy, repeating testing annually or as indicated by symptoms90.
Primary adrenal insufficiency is a less frequent irAE than secondary adrenal insufficiency related to hypophysitis with a reported frequency between 0.6 and 2.6%94,95. In severe cases, primary and secondary adrenal insufficiency can lead to adrenal crisis (a life threatening adrenal insufficiency caused by a lack of ACTH production in the pituitary gland in secondary adrenal insufficiency, or by lack of cortisol production in primary adrenal insufficiency), symptoms of which include hypotension, electrolyte imbalances (particularly hyponatraemia (low serum sodium levels) and dehydration, and requires immediate treatment. Low cortisol levels can indicate primary or secondary adrenal insufficiency, which can be further differentiated with dynamic ACTH deficiency testing under specialist guidance. A lack of ACTH stimulation during dynamic ACTH testing indicates secondary adrenal insufficiency, although both primary and secondary deficiency likely require lifelong steroid replacement 94.
The ICI treatment emergent diagnosis of type 1 diabetes mellitus has been reported in ≤1% of patients treated with atezolizumab or pembrolizumab in phase III trials.101,105,106 Type 1 diabetes mellitus associated with CTLA-4 inhibitors has not yet been reported with ipilimumab monotherapy.91 Although rare, presentations with this irAE can be emergent, with 59% of individuals with ICI-associated type 1 diabetes mellitus presenting with ketoacidosis and 42% presenting with pancreatitis in a retrospective case series of patients receiving PD-1 or PD-L1 inhibitors.26 Fasting glucose is the preferred diagnostic test for suspected new onset hyperglycaemia and testing for autoantibodies (against GAD65, IA-2, ICA-512, ZnT8 and insulin) may be considered as part of a more specific work-up, although patients are not always positive for these antibodies.91
Gastrointestinal.
Diarrhoea is one of the most common adverse events, and has an incidence of ~35% for CTLA-4 inhibitors, ~20% for PD-1 inhibitors and >40% for combinatorial therapy107. By contrast, colitis (evidence of inflammation of the colon) is reported in 12%, 1% and 14%, respectively103. Typical diagnostic workup for colitis can include assessments to exclude infectious causes and CT to evaluate the extent and severity of colitis and rule out bowel perforation (Fig. 3). Endoscopy is not routine for mild cases of colitis as correlation between the grade of diarrhoea and endoscopic features of colitis severity is poor108 but can be helpful when diagnosis is elusive, in those with severe, refractory or recurrent colitis, to guide biopsy for the exclusion of cytomegalovirus-associated colitis or examine high-risk features that can guide escalation of therapy (ulceration or extensive colitis).107,109 On colonoscopy, CTLA-4 inhibitor-related colitis can show mucosal erythema and ulcerations similar to those in Crohn’s disease, although findings can be variable.110 Colonic biopsies from individuals with ICI-associated colitis have shown both neutrophilic and lymphocytic inflammation, with a significant histological overlap with other etiologies of colitis making the differential diagnosis difficult111. Fatal intestinal perforation in individuals with severe colitis has been reported in up to 1% of patients who received ipilimumab, which was more commonly related to higher dose ipilimumab or ipilimumab combinatorial therapy with radiotherapy or other treatments.112–114
ICI-related hepatitis has a prevalence of 1–6% in anti-PD-1 or anti-PD-L1 trials, 1–25% in CTLA-4 inhibitor trials and 17–22% in combination anti-PD-1 and CTLA-4 inhibitor trials.5,13,105,106,112,115–117 The most common presentation of hepatitis is an asymptomatic rise in transaminase levels, which is observed more frequently than increased bilirubin levels (which tends to only occur in very severe or chronic cases). Hepatitis screening via monitoring transaminase and bilirubin levels before initiating ICI and before every dose are necessary. Individuals who develop liver enzyme abnormalities should undergo additional testing to rule out viral aetiology or disease-related hepatic dysfunction. In addition, a liver biopsy may be considered for those with higher grade hepatitis (defined as transaminases >5 times the upper limit of normal) to help expedite the identification of aetiology. For patients treated with ipilimumab, liver biopsies demonstrate changes overlapped with acute hepatitis similar to findings in drug-induced liver injury, acute viral hepatitis or autoimmune hepatitis.118
Haematological.
Haematological irAEs are rare (account for ~3–4% of the total irAEs) but can be potentially fatal.119 The most common clinical presentations are neutropenia, autoimmune haemolytic anaemia, immune thrombocytopenia and aplastic anaemia.119 Most haematological irAEs are asymptomatic and diagnosis is based on full blood count. However, severe cytopenias can present with fatigue and jaundice (haemolytic anaemia), purpura, bruising and/or bleeding from mucosal surfaces (thrombocytopenia), or fever and recurrent infections (neutropenia).
Neurological.
Neurological irAEs are rare but can cause substantial morbidity if not recognized and treated early.120 When all neurological irAEs are pooled, an incidence of 3.8% for CTLA-4 inhibitors, 6% for PD-1 inhibitors and 12% with combination therapy has been reported in one review of 59 clinical trials.121 Several neurological irAEs have been reported including peripheral neuropathies (reported in 1.3% of individuals with ICIs), myasthenia gravis (1.2%), myelitis (0.8%), meningitis (0.4%), encephalitis (0.3%) or Guillain-Barré syndrome (<0.1%).121,122
Several peripheral neuropathies have been associated with ICIs, including non-length-dependent polyradiculoneuropathies, small-fiber/autonomic neuropathy, mononeuritis multiplex, sensory neuronopathy and length-dependent sensorimotor axonal polyneuropathy123. Individuals with suspected neuropathies should undergo evaluation for alternative causes, as it could be caused by other medications, infectious disease and metabolic, endocrine or vascular disorders. Nerve conduction studies can be useful in this regard.5
Symptoms of myasthenia gravis include muscle weakness, commonly affecting the face. One review of 23 cases73 of myasthenia gravis reported an average onset of symptoms within 6 weeks of initiating therapy and two-thirds of cases presenting with severe symptoms, with bulbar symptoms and myasthenic crisis (severe muscle weakness requiring respiratory ventilation) being observed more frequently in ICI-associated myasthenia gravis than in idiopathic cases73.
Patients presenting with headache, neck stiffness and photophobia (extreme sensitivity to light) whilst using ICIs should raise suspicion for aseptic meningitis, which can be clinically distinguished from encephalitis owing to preserved mental status with aseptic meningitis (if mental status changes and seizures are present, encephalitis should be suspected). Investigations should include brain MRI with and without contrast in individuals with suspected meningitis or encephalitis, in addition to lumbar puncture to identify infectious causes (particularly viral aetiology) and electroencephalography to assess for subclinical seizures (Fig. 3).
Ocular.
Ocular irAEs encompasses inflammation of the eye, which can include uveitis, episcleritis, conjunctivitis and orbital myopathy, with an incidence of <1%.124 Onset of uveitis associated with CTLA-4 inhibitors is usually CTCAE grades 1 or 2.125 Symptoms of inflammation in the eye can include photophobia, pain in the orbital region, dryness and blurry vision.126 A change in vision should prompt vision testing under the guidance of an ophthalmologist to assess visual acuity, pupil reactivity and fundus changes to diagnose uveitis89.
Pulmonary.
Pneumonitis (focal or diffuse inflammation of the lung parenchyma) is a relatively rare irAE that is potentially life threatening117,127. Pneumonitis has an incidence of ~1 and 3% in individuals who received PD-1 or PD-L1 inhibitor monotherapy for melanoma, non-small-cell lung cancer and renal cell carcinoma128, with the highest incidence of all-grade and high-grade pneumonitis in those with non-small-cell lung cancer (3.1%)129. One study reported a significantly higher frequency of all-grade interstitial lung disease (3.6% vs 1.3%) and high-grade interstitial lung disease (1.1% vs 0.4%) in patients treated with PD-1 inhibitors than those who received PD-L1 inhibitors130. However, some studies have reported a higher incidence, such as one study of durvalumab treatment after chemotherapy and radiotherapy in individuals with stage 3 non-small-cell lung cancer, in which incidence was 34%, supporting the hypothesis that radiotherapy could be a risk factor for pneumonitis.131
Patients with pneumonitis present most commonly with dyspnoea (shortness of breath) and cough but can also present with fever or chest pain102, or as asymptomatic radiological findings in some individuals. The median time to onset of treatment-related pneumonitis for nivolumab was 15.1 weeks.132 Suspicion of pneumonitis should be investigated by checking oxygen saturation on room air and while ambulatory, ruling out infectious aetiology, and CT to exclude alternative causes and evaluate the extent of inflammation. CT findings can be variable and include the following patterns: cryptogenic organizing pneumonia like, ground glass opacities, interstitial with interlobular septal thickening, hypersensitivity with a bronchiolitis like appearance and tree-in-bud (Fig. 3).102 Bronchoscopy can also be helpful to establish diagnosis, particularly if alternative aetiologies are under consideration.5
Renal.
An overall incidence of 2.2% was reported for acute kidney injury (0.6% for grade III/IV renal events) in one systematic review of randomized controlled trials including 3,695 patients treated with ICIs133, although more recent studies have suggested a higher frequency134. Increased serum creatinine levels are an almost universal feature of ICI-induced renal toxicity and most renal irAEs occurred 6-12 weeks following the start of ICI treatment134. An e valuation of potential alternative nephrotoxic therapies or contrast agents is suggested in individuals with newly-increased creatinine levels. In addition, investigation of urinary protein is recommended, followed by an autoimmune screen, including ANA, ANCA, rheumatoid factor, anti-dsDNA antibodies and serum complement levels, which might offer insight into the underlying pathological process.5 Clinical findings and laboratory tests are suboptimal in diagnosing the underlying renal lesion, making kidney biopsy necessary in the majority of cases to definitely diagnose what type of renal damage is and potentially guide therapy135. Pathological features have been described as ranging from acute tubulointerstitial nephritis to granulomatous features and evidence of thrombotic microangiopathy.
Systemic and rheumatic.
Systemic and rheumatic irAEs have mainly been reported in retrospective studies and case reports, and can be clustered into articular (with a prevalence of 36% in the ICIR-BIOGEAS Registry), muscular (prevalence of 34%), granulomatous (pre valence of 6%), vasculitic (prevalence of 12%) and systemic (prevalence of 12%) irAEs 136.
The most frequent articular irAEs are inflammatory arthralgias, arthritis (which manifest as joint inflammation and pain) and polymyalgia rheumatica (which manifests as stiffness and pain in the shoulders and hips). The frequency of arthralgias in randomized controlled trials of ICIs is 8% and are classified as mild (CTCAE grades 1 or 2 in >95% of cases, whereas arthritis has been reported in 1% of individuals4 . The key features of articular irAEs include a median onset time of 70 days after ICI commencement, the predominance of seronegative arthritis (80% of individuals) and among individuals with available information about the distribution of the arthritis, ~60% were classified as polyarthritis23,137–140. Well-characterized inflammatory arthritis such as rheumatoid arthritis or psoriatic arthritis have been reported in ~30 individuals, with isolated reported cases of other forms (spondyloarthropathy, reactive arthritis-like, stenosing tenosynovitis, Jaccoud arthropathy, RS3PE syndrome, entesitis or osteonecrosis).4 Patients receiving ICIs should be asked about joint symptoms, and referred as soon as possible to a rheumatologist if arthritis is suspected for diagnostic work up, which includes ultrasound/CT/MRI studies and analysis of serum inflammatory parameters and autoantibodies.
Muscular features can include myalgias and myositis, typical symptoms of which include varying degrees of muscle weakness and pain. The frequency of myalgias is 4% in randomized controlled trials, and they are often classified as mild (CTCAE grades 1 and 2), whereas myositis has been reported in 0.6% of individuals receiving ICIs.4 Myositis is predominantly reported without a detailed clinical, immunological and histopathological characterization, with well-characterized inflammatory myopathies (dermatomyositis and polymyositis) being reported in ~20 cases, of which most cases were positive for myositis-related autoantibodies. Two specific studies141,142 have characterized ICI-associated inflammatory myopathies, and have reported a mean time of onset of 25 days after ICI initiation and an association with other muscular irAEs (16–40% of people with inflammatory myopathies also had myocarditis or myasthenia gravis)and hepatitis (found in 8–10% of individuals with inflammatory myopathies). In addition, ICI-associated inflammatory myopathies are associated with a high mortality rate (21%), which was higher in patients who also developed myocarditis (52%).141 Patients with a suspected muscular irAE should be investigated for raised serum muscular enzymes and myositis-related autoantibodies (Jo-1, PL-7/12, EJ, OJ, Mi-2, SRP, TIF, MDA5, PM-Scl, Ku, RNP and Ro) , and muscular involvement should be objectively assessed using electrodiagnostic and imaging studies and, whenever possible, with histopathology.
Granulomatous disorders, predominantly sarcoidosis (Fig. 3), are also an increasingly recognized irAE in patients treated with ICIs143,4. A review of 23 cases reported that sarcoidosis developed between 3 and 36 weeks after treatment initiation, with the lymph nodes, lungs and skin bring the primary affected organs, although other systemic manifestations have also been reported.144 A thoracic CT is mandatory in patients with suspected sarcoidosis. Histopathological confirmation of non-caseating granulomas in the absence of cancer progression and other causes (especially infectious) of granulomatosis is highly recommended for diagnosis.
Almost 200 cases of vasculitis associated with cancer immunotherapies have been reported, 64% of which are related to ICIs, mainly presenting with cutaneous purpura and less frequently as neuropathy or visceral vasculitis.4 Based on the size of the vessel affected, giant cell arteritis is the most frequently reported systemic vasculitis (of which 28 cases have been reported145), with isolated reported cases of Schonlein-Henoch purpura, cryoglobulinemic vasculitis and eosinophilic granulomatosis with polyangiitis.4,146 Patients with suspected vasculitis should be investigated for the presence anti-ANCA antibodies and cryoglobulins, and vascular involvement should be assessed using imaging and, whenever possible, with histopathological study.
Sicca syndrome has been reported in 5% of patients receiving ICI monotherapy and 10% receiving combination therapy in randomized controlled trials4. 22 cases of Sjögren syndrome triggered by ICIs have been reported, predominantly related to the use of PD-1 inhibitors71,147. Patients presenting with sicca symptoms after being treated with ICIs should be investigated for the presence of abnormal function of lachrymal and parotid glands, positive autoantibodies (mainly for anti-Ro antibodies) and focal lymphocytic sialadenitis via histopathological analysis of minor salivary glands. In addition, isolated cases of systemic sclerosis, lupus and antiphospholipid syndrome have been reported in individuals receiving ICIs.24,138,148,149
Screening and prevention
Safety and efficacy in patients with pre-existing autoimmune diseases.
As ICIs can induce irAEs, and given that patients with pre-existing autoimmune diseases were excluded from clinical trials of ICIs, the safety and efficacy of these drugs in this population is a key issue before extending treatment recommendations to include these patients.
Several studies have suggested an enhanced risk of irAEs in patients with pre-existing autoimmune diseases. A systematic review that included 123 patients with pre-existing autoimmune disease and treated for cancer by ICIs150 demonstrated autoimmune disease exacerbation in 50% of individuals, de novo irAEs in 34% and no autoimmune symptoms in only 16%. Interestingly, no difference was observed in patients with active versus those with inactive pre-existing autoimmune disease, and patients receiving treatment for their autoimmune disease at initiation of cancer immunotherapy had a little fewer adverse events (59%) than those who did not receive treatment at initiation of immunotherapy (83%). Further evidence supporting an increased risk of irAEs in those with autoimmune disease is based on data from the systematic REISAMIC registry151, the prevalence of irAEs was higher in patients with pre-existing autoimmune diseases (44%) compared with patients without autoimmune diseases (29%), of which 55% of irAEs in patients with previous autoimmune disease were related to the same autoimmune disease and 45% were new-onset irAEs. In this study, ICIs were stopped only in five of 20 patients with irAE and the overall survival of the patients with previous autoimmune diseases was the same as in the patients without. In many patients, irAEs were manageable with corticosteroids (only 16% required other immunosuppressive therapies) and discontinuation of ICIs was required in 17% of patients in the systematic literature review150 and in 11% of individuals in the Gustave Roussy experience151. Thus, even if the risk of irAEs is higher in patients with pre-existing autoimmune disorders, there is no reason to exclude these patients from cancer immunotherapy 152.
The rates of reactivation or flare of the pre-existing autoimmune diseases after initiating ICIs are summarized in Fig. 4 (Supplementary Figure 1 and Supplementary Table 1)150,151,153–159.
Figure 4.
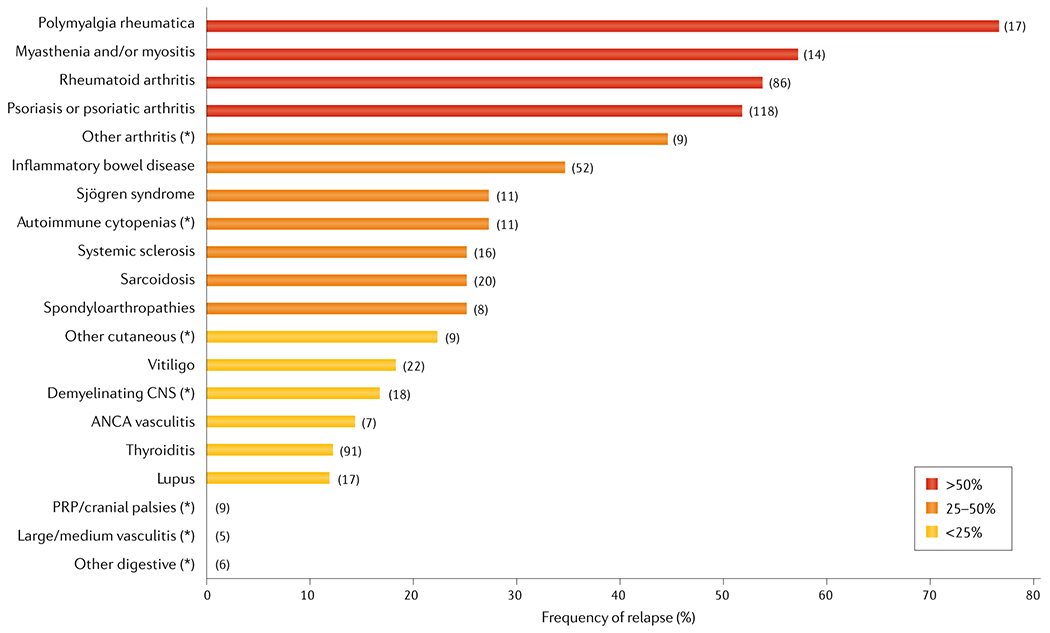
Rate of reactivation/flare of pre-existing autoimmune diseases after ICI therapy. We searched MEDLINE for articles published until January 1st 2020 using the terms “pre-existing (preexisting)” and “autoimmune” in combination with “checkpoint”, ”CTLA-4”, ”PD-1” and “PD-L1”, with no search restrictions. Study designs were considered in the following order (listed from highest to lowest evidence quality): systematic reviews, controlled trials, prospective cohort studies, case-control studies, retrospective studies and case series. The following relevant information was defined as selection criteria: a well-defined “population at risk” (patients diagnosed with an autoimmune disease before the initiation of the ICI therapy), available information to calculate the rate of relapse (patients who relapsed/total number of patients exposed to the drug, to be calculated for every different type of underlying autoimmune disease), and whether the relapse was linked to the underlying autoimmune disease or not. Duplicate publications, case reports, experimental studies and articles including incomplete/irrelevant information were excluded. We also manually searched the reference list of relevant articles retrieved and the EMBASE database. The following Figure shows a flow diagram of our search results. The available information about the use of immune checkpoint inhibitors was extracted from nine studies including patients with cancer and pre-existing autoimmune diseases150,151,153–159. Rates of reactivation/flare were defined as “number of patients who relapsed/total number of patients exposed to the drug”, to be calculated for each underlying autoimmune disease. Rates in individual diseases or grouped diseases with at least 5 treated individuals are represented in this Figure. For more detailed information about the methodology and rates for each individual autoimmune disease, see the Supplementary Material.
Autoimmune screening before starting therapy.
One of the most common factors that predisposes to autoimmune diseases is the presence of autoantibodies without clinical signs160. Some patients with irAEs might have pre-existing subclinical autoimmune conditions that manifest clinically as a full autoimmune disease after ICI therapy. There is no argument for recommending a universal screening for auto-antibodies before initiating ICI as even if an individual screens-positive, it will not be a contraindication for treating them with ICIs for cancer therapy. However, in patients with a personal or familial history of autoimmune diseases, or in those who presented signs or symptoms suggesting an underlying autoimmune disease, screening for autoantibodies may be considered before starting ICI, since these patients have an enhanced risk of developing a full autoimmune disease after treatment and therefore, should be followed up more closely.
Potential markers predicting occurrence of irAEs.
Some biomarkers that are present before starting ICI therapy are associated with a high risk of occurrence of irAEs. Several studies have linked the presence of pre-therapeutic serum autoantibodies with an increased risk of some irAEs8, such as myositis in patients with anti-mAChR antibodies161, thyroiditis in patients with anti-thyroid antibodies162, dermatological irAEs in those with anti-BP180 antibodies163, colitis in patients with antinuclear antibodies164, hypophysitis in those with anti-GNAL antibodies or anti-ITM2B antibodies and pneumonitis in those with anti-CD74 antibodies165. B cell changes detected after a median of 3 weeks of starting ICI therapy (reduced number of total B cells, increased percentage of CD21low B cells and plasmablasts and greater clonality in CD21low B cells) has also been suggested to help identify patients at increased risk of irAEs.63 Serum cytokine levels might also provide predictive value and mechanistic insight for patient susceptibility to ICI-induced irAEs; for example, pre-existing, circulating IL-17 levels may help to predict which patients with ipilimumab-treated melanoma could develop severe diarrhoea and colitis, since patients who developed grade 3 CTCAE colitis had higher mean IL-17 serum levels than those who presented with grades 0-2166 All these data have to be confirmed in larger studies before recommending its use in clinical practice.
Management
Therapeutic management
Treatment of irAEs depends on the organ system affected and the grade of toxicity according to the CTCAE classification, although it should be emphasized that common CTCAE grading might not be useful to grade the severity of some complex irAEs (such as systemic and rheumatic irAEs). Therapeutic algorithms have been developed by several organizations to help simplify and effectively diagnose and manage these adverse effects (such as ASCO, NCCN, ESMO, SITC and EULAR).88–90,152,167 To summarize, patients with CTCAE grade 1 irAEs typically do not require interventional treatment, and in most cases ICIs can be continued or temporarily halted with close monitoring. Patients with grade 2 adverse effects should stop ICIs until adverse effects abate, although glucocorticoids can be considered in some individuals, depending on the severity of the organ-specific damage or if irAEs persist after ICI therapy is stopped, where patients presenting with grade 3 or 4 irAEs should initially receive steroids In general, grade 1 irAEs can be treated and monitored by the patient’s oncologist (particular for non-bullous dermatitis, colitis, hepatitis, ocular, renal, musculoskeletal and haematological irAEs), whereas patients with grade >2 irAEs or those with symptomatic endocrine irAEs such as diabetes mellitus or thyroid disease should be referred to a specialist. For some organ-specific irAEs (pancreatitis, hypophysitis, pneumonitis, neurological, rheumatic and systemic autoimmune diseases), referral to a specialist should be strongly considered regardless the degree of CTCAE severity.
Here, we follow a pharmacologically-guided schedule about the overall therapeutic approach for irAEs; for more detailed organ-by-organ management guidelines, the reader is directed to guidelines. 88–90,152,167
Glucocorticoids.
Glucocorticoids are the mainstay of treatment for irAEs except for endocrine irAEs (Fig. 5). Prednisone is usually the preferred corticosteroid and the dose varies depending on the grade and clinical severity88–90,167 and for most irAEs, is tapered slowly over 4–6 weeks if clinical improvement is confirmed within days of steroid initiation. In those with grade 3 or 4 irAEs, methylprednisolone pulses can be started, and if clinical improvement is confirmed after 48–72 hours, then tapered slowly over 4–6 weeks. As a general rule, glucocorticoids should be used at the minimum dose and length of time necessary to control active systemic disease, and in case of anticipating a prolonged use, the need for introducing steroid-sparing strategies or an early initiation of anti-TNF and other monoclonal antibodies. Although endocrine irAEs are common they rarely require treatment with steroids , steroids can provide symptom relief in patients presenting with pituitary or thyroid gland acute inflammation.168 The prophylactic use of glucocorticoids is not recommended for the prevention of irAEs as some study showed that did not prevent the development of diarrhoea or colitis in patients receiving ipilimumab monotherapy169.
Figure 5.
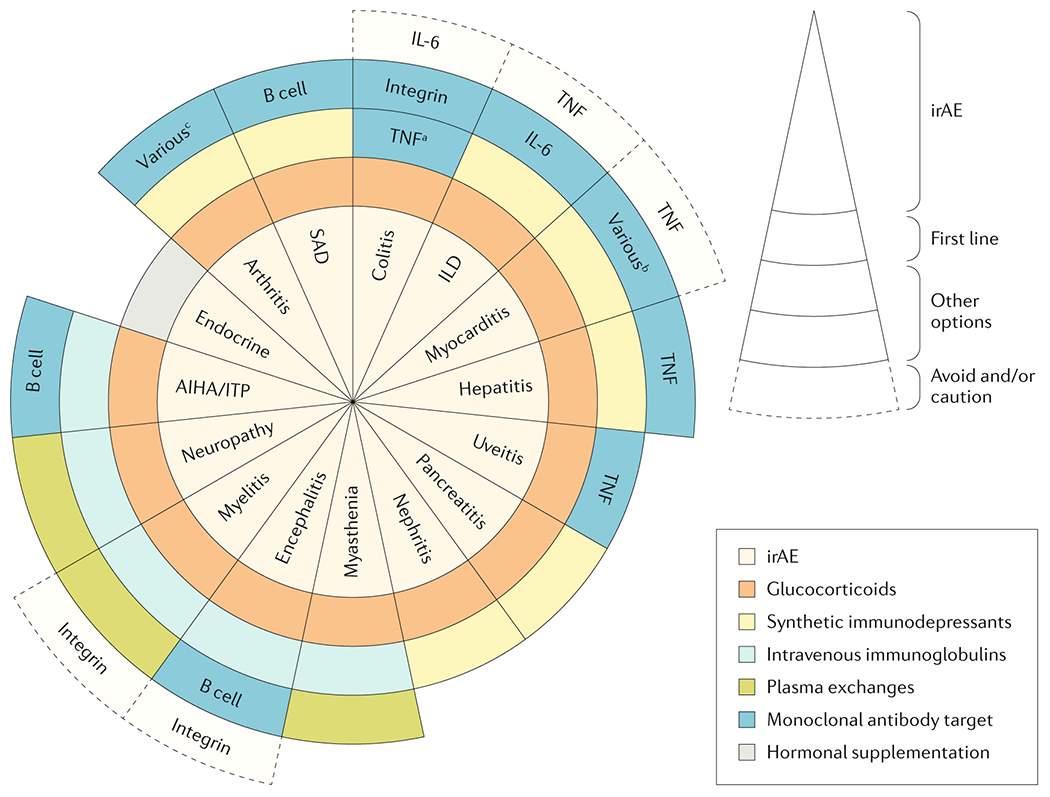
Suggested therapeutic algorithm for the organ-by-organ management of irAEs. When a systemic therapy is considered in patients presenting with immune-related adverse events owing to immune checkpoint inhibitors (irAEs), the first-line treatment are glucocorticoids with the exception of adverse effects that affect the endocrine system. Other therapies to be considered in severe/refractory cases depend on the affected organ system but can include synthetic immunosuppressants, intravenous immunoglobulin (IVIG), plasma exchange and monoclonal antibodies. These therapeutic suggestions are based on recommendations included in official guidelines, data from some retrospective studies, isolated published cases and personal experience of the authors. SAD: systemic autoimmune diseases; ILD: interstitial lung disease. aAvoid etanercept owing to the risk of autoimmune inflammatory colitis; b consider abatacept or alemtuzumab; c consider infliximab or tocilizumab.
Hormonal replacement.
Typically, patients with symptomatic thyroid dysfunction, hypopituitarism, hypoadrenalism, or type I diabetes mellitus are given replacement hormones or insulin. These irAEs rarely recover fully, so patient’s often require permanent treatment94,170 especially for the corticotrope axis dysfunction. In addition, holding ICI is not required for endocrinopathies unless the patient is symptomatic or unstable. ICI treatment can resume once replacement therapy or treatment is started for the endocrine irAE.89
Non-steroidal immunosuppressive agents.
If irAEs do not markedly improve within 48–72 hours of steroid treatment, or cannot be tapered without a flare of the symptoms, synthetic immunosuppressive agents should be added as glucocorticoid-sparing agents, with no evidence supporting the choice of one drug over another. For example, mycophenolate-containing immunosuppressants can be used for management of steroid-refractory irAEs particularly for immune-related hepatitis, nephritis, pancreatitis and uveitis, while patients with steroid-refractory pneumonitis can be treated with either mycophenolate or cyclophosphamide,89 and those with arthritis can receive hydroxychloroquine or methotrexate171. Other immunosuppressive therapies have been less frequently used for corticosteroid-refractory irAEs including tacrolimus, cyclosporine and sulfasalazine.89 Use of these drugs should be considered only for refractory irAEs in addition consultation with appropriate disease specific specialists.
Intravenous immunoglobulin and plasma exchange.
Intravenous immunoglobulin (IVIG) is used as a second-line therapy for neurological and haematological irAEs.172 IrAEs that are caused directly by autoantibodies, such as some haematological or neuromuscular irAEs can also be treated by plasma exchange (Fig. 5)173, which can remove the pathogenetic autoantibody from the circulation and is particularly effective in severe cases of myasthenia gravis or Guillain-Barré syndrome.
Monoclonal antibodies.
Non-controlled descriptive studies have suggested the use of infliximab (a TNF inhibitor174) for severe, refractory immune-related colitis or inflammatory arthritis. In most cases, only a single dose of infliximab is required to improve these irAEs; however, a second dose 2 weeks later is required in some patients. One study has demonstrated that the frontline addition of infliximab to glucocorticoids for grade 3 and 4 colitis was associated with a significantly shorter time to symptom resolution, compared with patients who received glucocorticoids alone.175 In a mouse model of colon cancer, anti-TNF monoclonal antibodies prevented the occurrence of colitis concomitantly administered with combined CTLA-4 and PD-1 inhibitors and increased survival, providing clinically feasible strategies to dissociate efficacy and toxicity in human trials176. Prior to administering TNF inhibitors, tests to identify infectious disease, such as a tuberculosis spot test, should be performed as TNF inhibitors can increase the risk of reactivation of certain infections.89,174
Vedolizumab (a monoclonal antibody against the integrin α4β7 that inhibits the migration of T cells into inflamed gastrointestinal mucosa can be used instead of infliximab for immune-related colitis.177 The theoretical advantage of using vedolizumab is that the immunosuppression would be limited to the gastrointestinal tract and, therefore, spares the systemic immune suppression. In a retrospective study of patients refractory to steroids (n=19) and infliximab (n=9) who received vedolizumab, 86% achieved a sustained clinical remission and 54% an endoscopic remission178.
Tocilizumab (an anti-IL-6 antibody) has been suggested for the management of some steroid-refractory irAEs.179 One study in people with nivolumab-associated grade 3–4 irAEs (n=34; predominantly pneumonitis, serum sickness and systemic inflammatory response syndrome or cerebritis) reported a clinical improvement in 80% of patients who received tocilizumab , which, in most cases, required only 1–2 doses to cause clinical improvement.180 Another study has reported the effective use of tocilizumab in three cases of severe polyarthritis.56
Other monoclonal antibodies have also shown some promise for the treatment of some steroid-refractory irAEs. Rituximab has shown efficacy for treatment of glucocorticoid-refractory cases of severe encephalitis,181 autoimmune cytopenias182 or severe bullous skin disease.183 In addition, two cases of successful response to abatacept184 or alemtuzumab185 have been reported in patients with steroid-refractory autoimmune myocarditis.
Despite the benefits of monoclonal antibodies for treatment of steroid-refractor irAEs, they are associated with specific adverse effects that may preclude their use for some irAEs (Fig. 5). For example, anti-TNF antibodies should be used with caution to treat pneumonitis because can risk exacerbating interstitial lung disease 186, as well as etanercept and tocilizumab to treat colitis due to their association with increased risk of inflammatory bowel disease 187 and increased risk of perforation in patients with Crohn’s disease 188,189, respectively. Moreover, some cases of progressive multifocal leukoencephalopathy during therapy with natalizumab (anti-α4-integrin monoclonal antibody) — although not with vedolizumab 190 — have been reported and the use of anti-integrin monoclonal antibodies for neurological irAEs should be proposed with caution.
Follow-up and monitoring
The therapies used for the management of irAEs can be associated with adverse effects, of which, some can be severe. Whether treatment of irAEs affects the treatment of the cancer (Box 3) and when to reintroduce ICIs to the patient are important questions.
Box 3. Treatment of irAEs without affecting cancer treatment.
Whether the use of steroids and/or immunosuppressants to treat immune related adverse events (irAEs) could reduce efficacy of cancer immunotherapy has been evaluated in several studies. In this regard, results from retrospective studies in melanoma were reassuring; steroid use was not associated with a loss of efficacy of CTLA-4 inhibitors and PD-1 or PD-L1 inhibitors256,257; however, prospective studies are needed to determine the effects of steroid use on the outcomes of other cancers. In lung cancer, the use of ≥10 mg of prednisone equivalent upfront has been associated with poorer outcome, but the irAEs were not the only indications for steroid use (common indications were dyspnoea, fatigue and brain metastases).258 Two different situations could be considered based on these results; if steroids are used before commencing ICIs for cancer-related indications, a dose >10mg/day may be deleterious258, whereas no deleterious effect has been demonstrated so far for the use of steroids for irAE treatment. Interestingly, progression-free survival was longer in patients with urothelial cancers who developed irAE than those who did not, and this benefit was not altered by steroid use259.
Despite those trials showing no effect on cancer outcomes with irAE treatment, other studies have suggested that caution is needed. For example, glucocorticoids have been suggested to promote breast cancer metastasis260 through activation of the glucocorticoid receptor. In addition, another study has suggested that clinically-relevant doses of infliximab only had a minor influence on the activity of tumour-specific T-cells in vitro, whereas even low doses of corticosteroids markedly impaired the antitumour activity of tumour-specific T cells.261
Adverse effects and infections.
Most irAEs resolve after a median of 4–8 weeks after the ICI is stopped, or with the addition of glucocorticoids in severe cases.115,191 Close monitoring is mandatory to detect a relapse of the irAE or a complication of immunosuppressive therapies. Some adverse effects can be easily identified, such as worsening of pre-existent diabetes mellitus, hypertension and mood disorders, whereas others, such as infections, are more challenging to detect. Although ICIs do not seem to directly increase risk of infection,190 the use of immunosuppressive therapies and biological agents to treat irAEs may increase the risk192 . A retrospective study in 740 patients with melanoma who received CTLA-4 and/or PD-1 inhibitors identified severe infections (defined as infection requiring hospitalization or parenteral antimicrobials) in 54 (7%) patients (steroids were used in 46% and infliximab in 16% of these patients). In this study, opportunistic infections were reported but most infections were bacterial pneumonia or septicaemia, and the main factors significantly associated with severe infection were the use of corticosteroids or infliximab192. Owing to the overlap with intestinal, pulmonary and hepatic irAEs, some opportunistic infections should be always ruled out in patients in whom irAEs did not improve or worsen despite treatment with immunosuppressive agents. Infections to be ruled out include cytomegalovirus193 and Clostridium difficile194 in refractory colitis, pneumocystis pneumonia, pulmonary aspergillosis195 and tuberculosis196 in pneumonitis, and viral infections or reactivations (hepatitis B virus and cytomegalovirus) in hepatitis197–199. Thus, a history of previous infections and risk factors for viral infections such as HIV or viral hepatitis should be evaluated before individuals with cancer are given ICIs 196,200 although a chronic viral hepatitis infection with negative viremia are not contraindication for an ICI prescription as shown in liver carcinoma studies with PD-1 inhibitors201.
Rechallenge.
In the ASCO and ESMO guidelines, permanent discontinuation of the ICI is recommended for all grade 4 irAEs, and the ASCO guidelines recommend permanently stopping ICIs for those with grade 3 myocarditis, pneumonitis, nephritis, hepatitis and severe neurological toxicities. In the remaining cases, the oncologist has to determine if a patient will benefit from the reintroduction of ICI therapy (rechallenge) once the irAE has resolved. No randomized phase III trials evaluating ICIs rechallenge after resolution of grade ≥2 irAEs have been published, and the main evidence available comes from studies with more than ten patients rechallenged 202–205. These studies showed that 33–50% of the patients who had an irAE during treatment with a PD-1 or PD-L1 inhibitors had a recurrent or new-onset irAE after reintroducing PD-1 or PD-L1 inhibitors, in contrast to 18–21% of those who had an irAE during combination treatment (with CTLA-4 and PD-1 inhibitors) after reintroducing only PD-1 inhibitors. Three deaths were reported in patients rechallenged due to Steven-Johnson syndrome, colitis and hepatic failure, and pneumonitis. In all these studies, patients with myocarditis or severe neurological irAEs were not rechallenged, therefore, data from these conditions are lacking.
The decision to rechallenge should be based on the potential risk/benefit ratio for each patient. When to re-initiate ICIs should be undertaken by a multidisciplinary committee involving organ specialists in each cancer centre to provide recommendations with a personalized care/patient centred approach. No risk factors associated with irAE relapse after rechallenge have been clearly identified, except a shorter time to the initial irAE, and the increased risk of relapse after resumption of CTLA-4 inhibitors than for PD-1 or PD-L1 inhibitors205.
Quality of life
Health-related QOL
Studies have addressed health-related quality of life (HRQOL) issues faced by people with irAEs, which have been shown to have a greater effect on older patients and potentially influence quality of life.206 Several studies have demonstrated that ICIs are well tolerated compared with other anticancer therapies207–209, and that even grade 3 and 4 irAEs does not translate into clinically meaningful differences in HRQOL210. By contrast, other studies have demonstrated significantly lower HRQOL scores than the general population211,212, increased psychological morbidity213, or a potential effect on cognitive function of survivors patients who received these therapies214. More people with melanoma and brain metastasis are surviving for longer owing to ICIs, and efforts to further comprehensively address psychosocial, neurocognitive, and HRQOL issues in this population are ongoing212.
Outcomes
Patients who developed irAEs often show a better therapeutic response to cancer compared with those who do not develop irAEs, suggesting a close link between autoimmunity and the antitumour effect elicited by ICIs215, 100. Indeed, a growing body of evidence suggests that patients who have irAEs have marked improvements in progression-free survival, overall survival and overall response rate than those who did not develop an irAE, with more consistent data in patients treated with PD-1 and PD-L1 inhibitors215 than in those treated with CTLA-4 inhibitors3. Of the different organ-specific irAEs that are associated with enhanced survival, the most consistent data are from dermatological irAEs, particularly rash and vitiligo216,217. However, many questions remain about what irAE-specific factors (such as affected organ, severity, timing of onset or therapeutic intervention) could have a prominent role in contributing to the increased survival 215.
Despite these apparent benefits in terms of cancer outcomes, the development of irAEs has been related to an irreversible organ damage and in some cases can be fatal. Irreversible organ damage is most frequently reported for irAEs of the endocrine system, which predominantly result in permanent damage or destruction of the endocrine organs and often require chronic therapy60. In addition, PD-1 inhibitors have been associated with a higher incidence of grade 3 or 4 pneumonitis (leading to irreversible fibrotic pulmonary damage)218, and severe colitis might require colectomy in some patients who have perforation219. Moreover, isolated cases of non-resolved xerostomia, alopecia or vitiligo with ICIs have been reported220.
The global rate of irAE-associated fatality is estimated as ~0.6% of treated with ICIs patients, with individual rates of 0.36% for patients who received PD-1 inhibitors, 0.38% for those who received PD-L1 inhibitors, 1.08% for those who received CTLA-4 inhibitors and 1.23% for individuals who received combined therapy.221,222 The reason for fatality differed widely between regimens; for example, 70% of CTLA-4-related deaths were due to colitis , whereas fatalities associated with PD-1 and PD-L1 inhibitors were often from pneumonitis (35%), hepatitis (22%) and neurotoxicity (15%).221 Among patients who received combination therapy (with PD-1 and CTLA-4 inhibitors) and died, 37% of deaths were caused by colitis and 25% by myocarditis.221 In addition, the time to fatal effects varied depending on the ICI used; fatalities occurred early after ICI initiation for combination therapy, with a median time from symptom onset to death of 14.5 in comparison with 32 days for monotherapy221. Myocarditis has the highest demonstrated mortality rate, reported in 48% of 351 individuals included in the two main studies223,224. The mortality rate of myositis is reported in 20% of 204 individuals,138,141,225,226 in whom the principal cause of death was associated myocarditis.141,226 A similar mortality rate has been reported for ICI-associated myasthenia gravis in 26% of individuals (n=35),73,74 whereas the rate is lower for colitis (6%).224 Other irAE-related deaths have been reported for interstitial lung disease,227–229 hepatitis,228 Guillain-Barré syndrome114 or autoimmune cytopenias.230
Outlook
International efforts are needed to develop evidence-based multidisciplinary management guidelines for irAEs, improve clinical characterization and diagnosis of irAEs, and anticipate that the certain rapid incorporation of novel therapeutic approaches could increase the diversity and severity of irAEs. The main aim of future research should be the development of more-effective therapeutic managements, which will require further clinical trials, and efforts to develop more precise mechanistic studies to learn more about pathophysiology.
Implications for treatment
The risk of irAEs with ICIs has several implications for cancer treatment, namely, whether the use of these therapies is appropriate and efficacious in individuals with chronic disorders. Data is now emerging from early phase II trials and case reports that suggest durable responses and a manageable safety profile of ICIs in patients with controlled hepatitis B or hepatitis C virus infection.231 With respect to the use of ICIs in patients with HIV, no unexpected irAEs have been observed.232 By contrast, data from case series suggest that there is a high risk of graft rejection with PD-1 and PD-L1 inhibitors in patients with solid organ transplantation and although responses to ICIs have been observed, the use of these therapies in this population should be weighed against the risk.233 Tolerance of ICIs in those ≥65 years seems similar to in younger individuals,34 although older patients should be closely monitored for cardiovascular disease.234 Compliance with vaccine recommendations is also important for protecting patients with cancer who are immunocompromised,235 and one study has demonstrated that influenza vaccination in patients with lung cancer receiving PD-1 inhibitors does not induce irAEs.236 With this complex clinical scenario, a multidisciplinary approach is mandatory237, with a central role for an oncologist,238 rheumatologist239 and internist,240 together with the support of the specialist of the affected organ/s.
Improving clinical characterization
The wide heterogeneity of both pharmacological and clinical scenarios of irAEs, together with the increasing identification of these events, suggest that the use of artificial intelligence (machine learning) and big data (incorporating large volumes of information into computers) will have a key role in optimizing clinical characterization.241,242 A key limitation for optimal clinical characterization of irAEs in randomized controlled trials is the use of CTCAE88 as these criteria can be difficult to apply and do not allow accurate reporting of the severity and effect of some irAEs, such as rheumatological or systemic irAEs.88 In addition, many randomized controlled trials only report adverse events of grade ≥3 and exclude those with lower severity27. One study has underscored critical challenges in assessing the occurrence, type, timing, and severity of irAEs, showing a poor inter-observer agreement for most organ-specific irAEs (except hypothyroidism).243
Some data from large databases such as the Vigibase145 or the ICIR-BIOGEAS Registry4 have begun to show a potential organ-driven clusterization of irAEs. Although >95% of irAEs are reported as single-organ autoimmune diseases, there is likely a publication bias, especially in cases reported in randomized controlled trials and pharmacovigilance studies in which irAEs are always counted as single-organ effects. However, a specific cluster of phenotypic associations among muscular irAEs (for myositis, myocarditis and myasthenia gravis) has been reported, particularly for the coexistence of myositis and myocarditis (which overlap in one third of reported cases),225 whereas rheumatic and systemic irAEs may be clustered into 5 differentiated clinical phenotypes.4 Future potential applications of artificial intelligence in cancer care and research must develop worldwide cancer networks and registries that investigate differential presentations and outcomes of irAEs according to individual, environmental or drug-related factors.241
Understanding environmental modulation
One rapidly expanding field is the effect that microbiota composition has on the efficacy and toxicity of ICIs.179 Understanding the effect of the microbiome on the immune response will be essentialin enabling the treatment of dysbiosis and to re-establish equilibrium of the protective gut microflora to limit and treat irAEs. The first irAEs in which a key effect of microbiota composition has been reported is for colitis, whereby patients with high levels of Bacteroidetes phylum have a decreased risk of colitis11, together with the use of adjunctive ‘oncomicrobiotics’ that have been proposed to indirectly promote beneficial immune responses through optimizing the gut microbiome.244 The first case series of successful treatment of ICI-associated colitis with fecal microbiota transplantation has been reported, with reconstitution of the gut microbiome and a relative increase in the proportion of Tregs within the colonic mucosa, lending further support to the hypothesis that alterations in the microbiota are involved in ICI-colitis.245 More than 200 fecal microbiota transplantation clinical trials are being carried out worldwide, but the knowledge of this microbial therapy is still limited, especially with respect to how the viabilities of transplanted microbiotas should be assessed.246 Determining the influence of non-gastrointestinal microbiota and whether irAEs are influenced by microbial composition and other environmental or geographical factors are critical.166
Future cancer immunotherapies
Newer-generation cancer immunotherapies and combination therapies will likely be associated with an enhanced prevalence of irAEs. 2 Combinatorial therapies pembrolizumab and avelumab with the multi-tyrosine kinase inhibitor axitinib has been approved in late 2019 for the treatment of advanced renal-cell carcinoma , with hypothyroidism (25-35%) and arthralgias (18-20%) being the most frequent reported irAEs247,248. In addition, the janus kinase inhibitor tofacitinib may enhance delivery of antibody-based therapeutics to tumour cells through modulation of inflammatory cells in a mouse model249 and could serve as a rationale for conducting trials in which short-term tofacitinib is administered with ICIs. In addition, other forms of immunotherapy, such as B cell targeting therapies, therapies targeting innate immunity including natural killer (NK) cell-targeting therapies are gaining interest.2 New bispecific antibodies, alternate antibody-based structures (which target multiple tumour and immune components simultaneously and could selectively enhance localized, tumour-targeted immune activity), and dual immunomodulators (targeting inhibitory PD-1 and LAG-3 or targeting stimulatory OX40 and inhibitory CTLA-4) are also in the horizon. As chimeric antigen receptor T-cell therapies become more widely used for cancer treatment, it is likely that these therapies will be tested in future trials in combination with ICIs. Chimeric antigen receptor-T-cell therapies have been shown to induce toxicities such as the cytokine-release syndrome (characterized by high fever, hypotension, and multiorgan toxicity) and CAR-T-cell-related encephalopathy syndrome (CRES, characterized by symptoms of confusion and delirium, seizures and cerebral oedema .250
As different routes of immunotherapy administration can influence their toxicity profile, with increasing interest in intratumoural therapies, it will be important to know whether more localized immunotherapies affect the frequency and severity of triggered irAEs. To minimize off-tissue effects, delivery systems have been designed for local and sustained release in vivo and many systems, including nanoparticles, scaffolds, hydrogels and cells, can be loaded with multiple therapeutic agents that are chosen on the basis of targets identified in patient biopsy samples, although whether these systems reduce the development of irAEs requires further study.251
Supplementary Material
Acknowledgements
This work was supported in part by the Memorial Sloan Kettering Cancer Center (MSKCC) NCI core grant P30 CA008748, and The University of Texas NCI core grant P30 CA016672. We thank Dr. Francesco Schettini from IDIBAPS/Hospital Clinic (Barcelona, Spain) for his assistance with the manuscript.
Competing interests
M.R.C. has received compensation for consulting services and/or speaking activities from BMS and Gilead. J.R.B. has received compensation for consulting services and/or speaking activities from Amgen, Astra Zeneca, Bristol-Myers Squibb, Genentech and Merck. M.K.C. declares intitutional research support and employment of a family member by Bristol-Myers Squibb and consulting, advisory, or speaking compensation from AstraZeneca/MedImmune, Incyte, Moderna and Merck. A.P. has received compensation for consulting services and/or speaking activities from Amgen, Bristol-Myers Squibb, Daiichi Sankyo, Novartis, Oncolytics Biotech, Pfizer, Puma and Roche, and received research support from Boehringer Ingelheim, Nanostring, Novartis and Roche. O.L. has received compensation for consulting services and/or speaking activities from AstraZeneca, Bristol-Myers Squibb France, Incyte, Janssen and MSD, and received research support from Gilead. X.M. has received compensation for consulting services and/or speaking activities from Bristol-Myers Squibb. M.E.S.A. has received funding for consulting services from AbbVie, Agile Pharmaceuticals, Amag Pharmaceuticals, Eli Lilly and Pfizer unrelated to this topic. All other authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Sanmamed MF & Chen L A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 175, 313–326 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoos A Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov 15, 235–247 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R & Hellmann MD Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med 378, 158–168 (2018). [DOI] [PubMed] [Google Scholar]; One of the first central reviews surveying the irAEs associated with ICIs and their management.
- 4.Ramos-Casals M et al. Immune-Related Adverse Events Induced By Cancer Immunotherapies. Big Data Analysis of 13,051 cases (ImmunoCancer International Registry). Ann. Rheum. Dis (2019). [Google Scholar]
- 5.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). (2019). [Google Scholar]
- 6.Xu C et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 363, k4226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; An elegant systematic review and network meta-analysis providing a complete toxicity profile, toxicity spectrum, and a safety ranking of the main ICIs drugs (nivolumab, pembrolizumab, ipilimumab, tremelimumab and atezolizumab).
- 7.Weber JS et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet. Oncol 16, 375–384 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Yoest JM Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. ImmunoTargets Ther. 6, 73–82 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parakh S, Cebon J & Klein O Delayed Autoimmune Toxicity Occurring Several Months After Cessation of Anti-PD-1 Therapy. Oncologist 23, 849–851 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanjanapan Y et al. Delayed immune-related adverse events in assessment for dose-limiting toxicity in early phase immunotherapy trials. Eur. J. Cancer 107, 1–7 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Sandigursky S & Mor A Immune-Related Adverse Events in Cancer Patients Treated With Immune Checkpoint Inhibitors. Curr. Rheumatol. Rep 20, 65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauken KE, Dougan M, Rose NR, Lichtman AH & Sharpe AH Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 40, 511–523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med 373, 23–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoja L, Day D, Wei-Wu Chen T, Siu LL & Hansen AR Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 28, 2377–2385 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Seidel JA, Otsuka A & Kabashima K Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol 8, 86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolchok JD et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med 377, 1345–1356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu R-M et al. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci 27, 1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felis-Giemza A & Moots RJ Measurement of anti-drug antibodies to biologic drugs. Rheumatology (Oxford, England) 54, 1941–1943 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Agrawal S et al. Evaluation of Immunogenicity of Nivolumab Monotherapy and Its Clinical Relevance in Patients With Metastatic Solid Tumors. J. Clin. Pharmacol 57, 394–400 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Enrico D, Paci A, Chaput N, Karamouza E & Besse B Anti-drug Antibodies Against Immune Checkpoint Blockers: Impairment of drug efficacy or indication of immune activation? Clin. Cancer Res (2019). doi: 10.1158/1078-0432.CCR-19-2337 [DOI] [PubMed] [Google Scholar]
- 21.Langer CJ et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet. Oncol 17, 1497–1508 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid P et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med 379, 2108–2121 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Cappelli LC et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin. Arthritis Rheum 48, 553–557 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Burel S et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur. J. Cancer 82, 34–44 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Lidar M et al. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun. Rev 17, 284–289 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Stamatouli AM et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 67, 1471–1480 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappelli LC, Gutierrez AK, Bingham CO 3rd & Shah AA Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res. (Hoboken) 69, 1751–1763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizarro C et al. PD-L1 gene polymorphisms and low serum level of PD-L1 protein are associated to type 1 diabetes in Chile. Diabetes. Metab. Res. Rev 30, 761–766 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Vaidya B et al. An association between the CTLA4 exon 1 polymorphism and early rheumatoid arthritis with autoimmune endocrinopathies. Rheumatology (Oxford). 41, 180–183 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Kartolo A, Sattar J, Sahai V, Baetz T & Lakoff JM Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol 25, e403–e410 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eun Y et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci. Rep 9, 14039 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kichenadasse G et al. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. (2019). doi: 10.1001/jamaoncol.2019.5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richtig G et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLoS One 13, e0204729 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daste A et al. Immune checkpoint inhibitors and elderly people: A review. Eur. J. Cancer 82, 155–166 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Chiarion Sileni V et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J. Exp. Clin. Cancer Res 33, 30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sury K, Perazella MA & Shirali AC Cardiorenal complications of immune checkpoint inhibitors. Nat. Rev. Nephrol 14, 571–588 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Klocke K, Sakaguchi S, Holmdahl R & Wing K Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl. Acad. Sci. U. S. A 113, E2383–92 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wing K et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Lo B et al. CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood 128, 1037–1042 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selby MJ et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res 1, 32–42 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Pico de Coana Y et al. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol. Res 1, 158–162 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Sharma A et al. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3(+) Regulatory T Cells (Tregs) in Human Cancers. Clin. Cancer Res 25, 1233–1238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knochelmann HM et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol 15, 458–469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noack M & Miossec P Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev 13, 668–677 (2014). [DOI] [PubMed] [Google Scholar]
- 45.von Euw E et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J. Transl. Med 7, 35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarhini AA et al. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. cancer 3, 39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latchman YE et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. U. S. A 101, 10691–10696 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francisco LM et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med 206, 3015–3029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gianchecchi E & Fierabracci A Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front. Immunol 9, 2374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura H, Nose M, Hiai H, Minato N & Honjo T Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Okazaki T et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med 9, 1477–1483 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Okazaki T et al. Hydronephrosis associated with antiurothelial and antinuclear autoantibodies in BALB/c-Fcgr2b−/−Pdcd1−/− mice. J. Exp. Med 202, 1643–1648 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gambichler T et al. Decline of programmed death-1-positive circulating T regulatory cells predicts more favourable clinical outcome of patients with melanoma under immune checkpoint blockade. Br. J. Dermatol (2019). doi: 10.1111/bjd.18379 [DOI] [PubMed] [Google Scholar]
- 54.Laurent S et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-alpha production. J. Transl. Med 11, 108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murakami N, Borges TJ, Yamashita M & Riella LV Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin. Kidney J 9, 411–417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim ST et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann. Rheum. Dis 76, 2061–2064 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Michot JM et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Byrne EH & Fisher DE Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 123, 2143–2153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng F & Loscalzo J Autoimmune Cardiotoxicity of Cancer Immunotherapy. Trends Immunol. 38, 77–78 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Johnson DB et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med 375, 1749–1755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersone L et al. T Cell/B Cell Collaboration and Autoimmunity: An Intimate Relationship. Front. Immunol 9, 1941 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iwama S et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med 6, 230ra45 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Das R et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest 128, 715–720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Moel EC et al. Autoantibody Development under Treatment with Immune-Checkpoint Inhibitors. Cancer Immunol. Res 7, 6–11 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Delyon J, Mateus C & Lambert T Hemophilia A induced by ipilimumab. The New England journal of medicine 365, 1747–1748 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Min L, Vaidya A & Becker C Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur. J. Endocrinol 164, 303–307 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanameishi S et al. Idiopathic thrombocytopenic purpura induced by nivolumab in a metastatic melanoma patient with elevated PD-1 expression on B cells. Annals of oncology : official journal of the European Society for Medical Oncology 27, 546–547 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Kong Y-CM & Flynn JC Opportunistic Autoimmune Disorders Potentiated by Immune-Checkpoint Inhibitors Anti-CTLA-4 and Anti-PD-1. Front. Immunol 5, 206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sadik CD, Langan EA, Gratz V, Zillikens D & Terheyden P Checkpoint Inhibition May Trigger the Rare Variant of Anti-LAD-1 IgG-Positive, Anti-BP180 NC16A IgG-Negative Bullous Pemphigoid. Frontiers in immunology 10, 1934 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramos-Casals M et al. Sicca/Sjogren’s syndrome triggered by PD-1/PD-L1 checkpoint inhibitors. Data from the International ImmunoCancer Registry (ICIR). Clin. Exp. Rheumatol 37 Suppl 1, 114–122 (2019). [PubMed] [Google Scholar]
- 71.Warner BM et al. Sicca Syndrome Associated with Immune Checkpoint Inhibitor Therapy. Oncologist (2019). doi: 10.1634/theoncologist.2018-0823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cappelli LC et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann. Rheum. Dis 76, 43–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Makarious D, Horwood K & Coward JIG Myasthenia gravis: An emerging toxicity of immune checkpoint inhibitors. Eur. J. Cancer 82, 128–136 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Suzuki S et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 89, 1127–1134 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Grabie N et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 116, 2062–2071 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Zhang L et al. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr. Treat. Options Cardiovasc. Med 21, 32 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Mahmood SS et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol 71, 1755–1764 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heinzerling L et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. cancer 4, 50 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmer L et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur. J. Cancer 60, 210–225 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Belum VR et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur. J. Cancer 60, 12–25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voskens CJ et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 8, e53745 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldinger SM et al. Cytotoxic Cutaneous Adverse Drug Reactions during Anti - PD-1 Therapy. Clin. Cancer Res 22, 4023–4029 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Sibaud V Dermatologic Reactions to Immune Checkpoint Inhibitors : Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol 19, 345–361 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Weber JS, Kahler KC & Hauschild A Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol 30, 2691–2697 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N & Rosell R Clinical assessment of immune-related adverse events. Ther. Adv. Med. Oncol 10, 1758835918764628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minkis K, Garden BC, Wu S, Pulitzer MP & Lacouture ME The risk of rash associated with ipilimumab in patients with cancer: a systematic review of the literature and meta-analysis. J. Am. Acad. Dermatol 69, e121–8 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Liu X & Qin S Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges. Oncologist 24, S3–S10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puzanov I et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. cancer 5, 95 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson JA et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J. Natl. Compr. Canc. Netw 17, 255–289 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Brahmer JR et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol 36, 1714–1768 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; A practical, central position document convened by a multidisciplinary, multi-organizational panel of experts (from medical oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, hematology, emergency medicine, nursing, trialist, and advocacy) offering guidance on the recommended management of irAEs in patients treated with ICIs.
- 91.Girotra M et al. The Current Understanding of the Endocrine Effects From Immune Checkpoint Inhibitors and Recommendations for Management. JNCI cancer Spectr. 2, pky021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ryder M, Callahan M, Postow MA, Wolchok J & Fagin JA Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr. Relat. Cancer 21, 371–381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corsello SM et al. Endocrine side effects induced by immune checkpoint inhibitors. J. Clin. Endocrinol. Metab 98, 1361–1375 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Sznol M et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat. Rev 58, 70–76 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Lu J, Li L, Lan Y, Liang Y & Meng H Immune checkpoint inhibitor-associated pituitary-adrenal dysfunction: A systematic review and meta-analysis. Cancer Med. 8, 7503–7515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barroso-Sousa R et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 4, 173–182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; A systematic review and meta-analysis analyzing theincidence of all-grade hypothyroidism, hyperthyroidism, hypophysitis, primary adrenalinsu fficiency and insulin-deficient diabetes associated with ICIs.
- 97.Dillard T, Yedinak CG, Alumkal J & Fleseriu M Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 13, 29–38 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Faje AT et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J. Clin. Endocrinol. Metab 99, 4078–4085 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Byun DJ, Wolchok JD, Rosenberg LM & Girotra M Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol 13, 195–207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang L-S et al. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev 40, 17–65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eggermont AMM et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med 378, 1789–1801 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Naidoo J et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol 35, 709–717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hassel JC et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat. Rev 57, 36–49 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Delivanis DA et al. Pembrolizumab-induced thyroiditis. Comprehensive clinical review and insights into underlying involved mechanisms. J. Clin. Endocrinol. Metab (2017). doi: 10.1210/jc.2017-00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robert C et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med 372, 2521–2532 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Horn L et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med 379, 2220–2229 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Geukes Foppen MH et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO open 3, e000278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hughes MS et al. Colitis after checkpoint blockade: A retrospective cohort study of melanoma patients requiring admission for symptom control. Cancer Med. 8, 4986–4999 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abu-Sbeih H et al. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J. Immunother. cancer 6, 95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verschuren EC et al. Clinical, Endoscopic, and Histologic Characteristics of Ipilimumab-Associated Colitis. Clin. Gastroenterol. Hepatol 14, 836–842 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Karamchandani DM & Chetty R Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists’ perspective. J. Clin. Pathol 71, 665–671 (2018). [DOI] [PubMed] [Google Scholar]
- 112.Hodi FS et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kwon ED et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet. Oncol 15, 700–712 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eggermont AMM et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet. Oncol 16, 522–530 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Weber J et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med 377, 1824–1835 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Hellmann MD et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med 378, 2093–2104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Motzer RJ et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med 378, 1277–1290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kleiner DE & Berman D Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig. Dis. Sci 57, 2233–2240 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delanoy N et al. Haematologicalimmune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet. Haematol 6, e48–e57 (2019). [DOI] [PubMed] [Google Scholar]; A descriptive observational study describing patients presenting with haematological irAEs induced by PD-1 or PD-L1 inhibitors immunotherapy registered in three French pharmacovigilance databases (REISAMIC, ImmunoTOX and CeReCAI).
- 120.Perrinjaquet C, Desbaillets N & Hottinger AF Neurotoxicity associated with cancer immunotherapy: immune checkpoint inhibitors and chimeric antigen receptor T-cell therapy. Curr. Opin. Neurol 32, 500–510 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Cuzzubbo S et al. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur. J. Cancer 73, 1–8 (2017). [DOI] [PubMed] [Google Scholar]; A systematic search of literature including 59 clinical trials centered on analyse the incidence and characteristic of neurological irAEs.
- 122.Sato K, Mano T, Iwata A & Toda T Neurological and related adverse events in immune checkpoint inhibitors: a pharmacovigilance study from the Japanese Adverse Drug Event Report database. J. Neurooncol 145, 1–9 (2019). [DOI] [PubMed] [Google Scholar]
- 123.Dubey D et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology 93, e1093–e1103 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Bitton K et al. Prevalence and clinical patterns of ocular complications associated with anti-PD-1/PD-L1 anticancer immunotherapy. Am. J. Ophthalmol (2019). doi: 10.1016/j.ajo.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 125.Beck KE et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J. Clin. Oncol 24, 2283–2289 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Postow MA Managing immune checkpoint-blocking antibody side effects. Am. Soc. Clin. Oncol. Educ. book. Am. Soc. Clin. Oncol. Annu. Meet 76–83 (2015). doi: 10.14694/EdBook_AM.2015.35.76 [DOI] [PubMed] [Google Scholar]
- 127.Ferris RL et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med 375, 1856–1867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH & Hodi FS Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2, 1607–1616 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Ma K et al. The Relative Risk and Incidence of Immune Checkpoint Inhibitors Related Pneumonitis in Patients With Advanced Cancer: A Meta-Analysis. Front. Pharmacol 9, 1430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nishino M et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin. Cancer Res 22, 6051–6060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides a clear description of the main clinical characteristics, radiographic patterns, and treatment course of PD-1 inhibitor-related pneumonitis in patients with advanced cancer.
- 131.Antonia SJ et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med 377, 1919–1929 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Brahmer J et al. Nivolumab versus Docetaxelin Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med 373, 123–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cortazar FB et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 90, 638–647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wanchoo R et al. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am. J. Nephrol 45, 160–169 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Perazella MA & Shirali AC Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int. 97, 62–74 (2020). [DOI] [PubMed] [Google Scholar]
- 136.Ramos-Casals M et al. THU0649 PHENOTYPIC CLUSTERS OF RHEUMATIC/SYSTEMIC IMMUNE-RELATED ADVERSE EVENTS INDUCED BY CANCER IMMUNOTHERAPIES (IMMUNOCANCER INTERNATIONAL REGISTRY). Ann. Rheum. Dis 78, 620 LP–621 (2019). [Google Scholar]
- 137.Manolios N & Schrieber L Checkpoint inhibitors and arthritis. Annals of the rheumatic diseases 78, e58 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Richter MD et al. Rheumatic Syndromes Associated With Immune Checkpoint Inhibitors: A Single-Center Cohort of Sixty-One Patients. Arthritis Rheumatol. (Hoboken, N.J.) 71, 468–475 (2019). [DOI] [PubMed] [Google Scholar]
- 139.Kostine M et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann. Rheum. Dis 77, 393–398 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Buder-Bakhaya K et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol. Immunother 67, 175–182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anquetil C et al. Immune Checkpoint Inhibitor-Associated Myositis. Circulation 138, 743–745 (2018). [DOI] [PubMed] [Google Scholar]
- 142.Touat M et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 91, e985–e994 (2018). [DOI] [PubMed] [Google Scholar]
- 143.Cornejo CM, Haun P, English J 3rd & Rosenbach M Immune checkpoint inhibitors and the development of granulomatous reactions. J. Am. Acad. Dermatol (2018). doi: 10.1016/j.jaad.2018.07.051 [DOI] [PubMed] [Google Scholar]
- 144.Gkiozos I et al. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. J. Thorac. Oncol 13, 1076–1082 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Salem J-E et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet. Oncol 19, 1579–1589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Daxini A, Cronin K & Sreih AG Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin. Rheumatol 37, 2579–2584 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Ramos-Casals M, Maria A, Suárez-Almazor M & et al. Sicca/Sjögren Syndrome Triggered By PD-1/PD-L1 Checkpoint Inhibitors: Data from the International Immuno Cancer Registry (ICIR). Clin. Exp. Rheumatol. Supplement, (2019). [PubMed] [Google Scholar]
- 148.Hodi FS et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet. Oncol 19, 1480–1492 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Fadel F, El Karoui K & Knebelmann B Anti-CTLA4 antibody-induced lupus nephritis. The New England journal of medicine 361, 211–212 (2009). [DOI] [PubMed] [Google Scholar]
- 150.Abdel-Wahab N, Shah M, Lopez-Olivo MA & Suarez-Almazor ME Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann. Intern. Med 168, 121–130 (2018). [DOI] [PubMed] [Google Scholar]; A complete summary of the evidence on irAEs associated with ICIs in patients with cancer and preexisting autoimmune disease.
- 151.Danlos F-X et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur. J. Cancer 91, 21–29 (2018). [DOI] [PubMed] [Google Scholar]
- 152.Kostine M et al. OP0165 EULAR RECOMMENDATIONS FOR THE DIAGNOSIS AND THE MANAGEMENT OF RHEUMATIC IMMUNE-RELATED ADVERSE EVENTS DUE TO CANCER IMMUNOTHERAPY. Ann. Rheum. Dis 78, 158 LP–158 (2019). [Google Scholar]
- 153.Kaur A et al. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: A single-center experience. Medicine (Baltimore). 98, e17348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kahler KC et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol. Immunother 67, 825–834 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tison A et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. (Hoboken, N.J.) 71, 2100–2111 (2019). [DOI] [PubMed] [Google Scholar]
- 156.Johnson DB et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2, 234–240 (2016). [DOI] [PubMed] [Google Scholar]
- 157.Leonardi GC et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients With Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J. Clin. Oncol 36, 1905–1912 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gutzmer R et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur. J. Cancer 75, 24–32 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Menzies AM et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 28, 368–376 (2017). [DOI] [PubMed] [Google Scholar]
- 160.Arbuckle MR et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med 349, 1526–1533 (2003). [DOI] [PubMed] [Google Scholar]
- 161.Mammen AL et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann. Rheum. Dis 78, 150–152 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kobayashi T et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J. Endocr. Soc 2, 241–251 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ali OH et al. BP180-specific IgG is associated with skin adverse events, therapy response and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J. Am. Acad. Dermatol (2019). doi: 10.1016/j.jaad.2019.08.045 [DOI] [PubMed] [Google Scholar]
- 164.Sakakida T et al. Safety and efficacy of PD-1/PD-L1 blockade in patients with preexisting antinuclear antibodies. Clin. Transl. Oncol (2019). doi: 10.1007/s12094-019-02214-8 [DOI] [PubMed] [Google Scholar]
- 165.Tahir SA et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc. Natl. Acad. Sci. U. S. A 116, 22246–22251 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Young A, Quandt Z & Bluestone JA The Balancing Act between Cancer Immunity and Autoimmunity in Response to Immunotherapy. Cancer Immunol. Res 6, 1445–1452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haanen JBAG et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 29, iv264–iv266 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Min L et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin. Cancer Res 21, 749–755 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weber J et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res 15, 5591–5598 (2009). [DOI] [PubMed] [Google Scholar]
- 170.Torino F, Corsello SM & Salvatori R Endocrinological side-effects of immune checkpoint inhibitors. Curr. Opin. Oncol 28, 278–287 (2016). [DOI] [PubMed] [Google Scholar]
- 171.Roberts J et al. Hydroxychloroquine is a safe and effective steroid-sparing agent for immune checkpoint inhibitor-induced inflammatory arthritis. Clin. Rheumatol (2019). doi: 10.1007/s10067-019-04451-2 [DOI] [PubMed] [Google Scholar]
- 172.Schwab I & Nimmerjahn F Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat. Rev. Immunol 13, 176–189 (2013). [DOI] [PubMed] [Google Scholar]
- 173.Touat M, Talmasov D, Ricard D & Psimaras D Neurological toxicities associated with immune-checkpoint inhibitors. Curr. Opin. Neurol 30, 659–668 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Jassen United States. Infliximab. (2019). [Google Scholar]
- 175.Johnson DH et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J. Immunother. cancer 6, 103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Perez-Ruiz E et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432 (2019). [DOI] [PubMed] [Google Scholar]
- 177.Bergqvist V et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol. Immunother 66, 581–592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J. Immunother. cancer 6, 142 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Siakavellas SI & Bamias G Checkpoint inhibitor colitis: a new model of inflammatory bowel disease? Curr. Opin. Gastroenterol 34, 377–383 (2018). [DOI] [PubMed] [Google Scholar]
- 180.Stroud CR et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pract 25, 551–557 (2019). [DOI] [PubMed] [Google Scholar]
- 181.Williams TJ et al. Association of Autoimmune Encephalitis With Combined Immune Checkpoint Inhibitor Treatment for Metastatic Cancer. JAMA Neurol. 73, 928–933 (2016). [DOI] [PubMed] [Google Scholar]
- 182.Khan U, Ali F, Khurram MS, Zaka A & Hadid T Immunotherapy-associated autoimmune hemolytic anemia. J. Immunother. cancer 5, 15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sowerby L, Dewan AK, Granter S, Gandhi L & LeBoeuf NR Rituximab Treatment of Nivolumab-Induced Bullous Pemphigoid. JAMA dermatology 153, 603–605 (2017). [DOI] [PubMed] [Google Scholar]
- 184.Salem J-E et al. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. The New England journal of medicine 380, 2377–2379 (2019). [DOI] [PubMed] [Google Scholar]
- 185.Esfahani K et al. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. The New England journal of medicine 380, 2375–2376 (2019). [DOI] [PubMed] [Google Scholar]
- 186.Akiyama M, Kaneko Y, Yamaoka K, Kondo H & Takeuchi T Association of disease activity with acute exacerbation of interstitial lung disease during tocilizumab treatment in patients with rheumatoid arthritis: a retrospective, case-control study. Rheumatol. Int 36, 881–889 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Korzenik J, Larsen MD, Nielsen J, Kjeldsen J & Norgard BM Increased risk of developing Crohn’s disease or ulcerative colitis in 17 018 patients while under treatment with anti-TNFalpha agents, particularly etanercept, for autoimmune diseases other than inflammatory bowel disease. Aliment. Pharmacol. Ther 50, 289–294 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Danese S & Fiorino G Anti-TNF Biosimilars in Inflammatory Bowel Disease: Searching the Proper Patient’s Profile. Curr. Med. Chem 26, 280–287 (2019). [DOI] [PubMed] [Google Scholar]
- 189.Strangfeld A et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann. Rheum. Dis 76, 504–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Redelman-Sidi G et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate rece. Clin. Microbiol. Infect 24 Suppl 2, S95–S107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andrews S & Holden R Characteristics and management of immunerelated adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma. Cancer Manag. Res 4, 299–307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Del Castillo M et al. The Spectrum of Serious Infections Among Patients Receiving Immune Checkpoint Blockade for the Treatment of Melanoma. Clin. Infect. Dis 63, 1490–1493 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Franklin C et al. Cytomegalovirus reactivation in patients with refractory checkpoint inhibitor-induced colitis. Eur. J. Cancer 86, 248–256 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Kuo JR et al. Severe Diarrhea in the Setting of Immune Checkpoint Inhibitors. Case reports in gastroenterology 12, 704–708 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schwarz M et al. Immunosuppression for Immune Checkpoint-related Toxicity Can Cause Pneumocystis Jirovecii Pneumonia (PJP) in Non-small-cell Lung Cancer (NSCLC): A Report of 2 Cases. Clin. Lung Cancer 20, e247–e250 (2019). [DOI] [PubMed] [Google Scholar]
- 196.Picchi H et al. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 24, 216–218 (2018). [DOI] [PubMed] [Google Scholar]
- 197.Koksal AS et al. HBV-related acute hepatitis due to immune checkpoint inhibitors in a patient with malignant melanoma. Annals of oncology : official journal of the European Society for Medical Oncology 28, 3103–3104 (2017). [DOI] [PubMed] [Google Scholar]
- 198.Pandey A, Ezemenari S, Liaukovich M, Richard I & Boris A A Rare Case of Pembrolizumab-Induced Reactivation of Hepatitis B. Case reports in oncological medicine 2018, 5985131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Uslu U et al. Autoimmune Colitis and Subsequent CMV-induced Hepatitis After Treatment With Ipilimumab. J. Immunother 38, 212–215 (2015). [DOI] [PubMed] [Google Scholar]
- 200.Johnson DB, Sullivan RJ & Menzies AM Immune checkpoint inhibitors in challenging populations. Cancer 123, 1904–1911 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.El-Khoueiry AB et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (London, England) 389, 2492–2502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pollack MH et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 29, 250–255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Santini FC et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol. Res 6, 1093–1099 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Simonaggio A et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. (2019). doi: 10.1001/jamaoncol.2019.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Abu-Sbeih H et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J. Clin. Oncol 37, 2738–2745 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.van Holstein Y et al. Efficacy and Adverse Events of Immunotherapy with Checkpoint Inhibitors in Older Patients with Cancer. Drugs Aging 36, 927–938 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hall ET et al. Patient-Reported Outcomes for Cancer Patients Receiving Checkpoint Inhibitors: Opportunities for Palliative Care-A Systematic Review. J. Pain Symptom Manage 58, 137–156.e1 (2019). [DOI] [PubMed] [Google Scholar]
- 208.Long GV et al. Effect of nivolumab on health-related quality of life in patients with treatment-naive advanced melanoma: results from the phase III CheckMate 066 study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 27, 1940–1946 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lebbe C et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial. J. Clin. Oncol 37, 867–875 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schadendorf D et al. Health-related quality of life results from the phase III CheckMate 067 study. Eur. J. Cancer 82, 80–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.O’Reilly A et al. An immunotherapy survivor population: health-related quality of life and toxicity in patients with metastatic melanoma treated with immune checkpoint inhibitors. Support. care cancer Off. J. Multinatl. Assoc. Support. Care Cancer (2019). doi: 10.1007/s00520-019-04818-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rogiers A et al. Long-Term Survival, Quality of Life, and Psychosocial Outcomes in Advanced Melanoma Patients Treated with Immune Checkpoint Inhibitors. J. Oncol 2019, 5269062 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lai-Kwon J et al. The survivorship experience for patients with metastatic melanoma on immune checkpoint and BRAF-MEK inhibitors. J. Cancer Surviv 13, 503–511 (2019). [DOI] [PubMed] [Google Scholar]
- 214.Joly F, Castel H, Tron L, Lange M & Vardy J Potential impact of immunotherapy agents on cognitive function in cancer patients. J. Natl. Cancer Inst (2019). doi: 10.1093/jnci/djz168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Das S & Johnson DB Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. cancer 7, 306 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Freeman-Keller M et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res 22, 886–894 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Teulings H-E et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J. Clin. Oncol 33, 773–781 (2015). [DOI] [PubMed] [Google Scholar]
- 218.Khunger M et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 152, 271–281 (2017). [DOI] [PubMed] [Google Scholar]
- 219.Hamamoto Y, Shin N, Hoshino T & Kanai T Management of challenging immune-related gastrointestinal adverse events associated with immune checkpoint inhibitors. Future Oncol. 14, 3187–3198 (2018). [DOI] [PubMed] [Google Scholar]
- 220.Hofmann L et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 60, 190–209 (2016). [DOI] [PubMed] [Google Scholar]
- 221.Wang DY et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 4, 1721–1728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the largest evaluation of fatal ICI-associated toxic effects performed after a retrospective evaluation of the WHO pharmacovigilance database “Vigilyze”.
- 222.De Velasco G et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res 5, 312–318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Al-Kindi SG & Oliveira GH Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet (London, England) 392, 382–383 (2018). [DOI] [PubMed] [Google Scholar]
- 224.Moslehi JJ, Salem J-E, Sosman JA, Lebrun-Vignes B & Johnson DB Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet (London, England) 391, 933 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Moreira A et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur. J. Cancer 106, 12–23 (2019). [DOI] [PubMed] [Google Scholar]
- 226.Liewluck T, Kao JC & Mauermann ML PD-1 Inhibitor-associated Myopathies: Emerging Immune-mediated Myopathies. J. Immunother 41, 208–211 (2018). [DOI] [PubMed] [Google Scholar]
- 227.Lisberg A et al. Treatment-Related Adverse Events Predict Improved Clinical Outcome in NSCLC Patients on KEYNOTE-001 at a Single Center. Cancer Immunol. Res (2018). doi: 10.1158/2326-6066.CIR-17-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Powles T et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. 3, e172411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sharma P et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet. Oncol 17, 1590–1598 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Davis EJ et al. Hematologic Complications of Immune Checkpoint Inhibitors. Oncologist 24, 584–588 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Davar D, Wilson M, Pruckner C & Kirkwood JM PD-1 Blockade in Advanced Melanoma in Patients with Hepatitis C and/or HIV. Case Rep. Oncol. Med 2015, 737389 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cook MR & Kim C Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients With HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. (2019). doi: 10.1001/jamaoncol.2018.6737 [DOI] [PubMed] [Google Scholar]
- 233.Tio M et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur. J. Cancer 104, 137–144 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Simons KH et al. T cell co-stimulation and co-inhibition in cardiovascular disease: a double-edged sword. Nat. Rev. Cardiol 16, 325–343 (2019). [DOI] [PubMed] [Google Scholar]
- 235.Ward EM, Flowers CR, Gansler T, Omer SB & Bednarczyk RA The importance of immunization in cancer prevention, treatment, and survivorship. CA. Cancer J. Clin 67, 398–410 (2017). [DOI] [PubMed] [Google Scholar]
- 236.Wijn DH et al. Influenza vaccination in patients with lung cancer receiving anti-programmed death receptor 1 immunotherapy does not induce immune-related adverse events. Eur. J. Cancer 104, 182–187 (2018). [DOI] [PubMed] [Google Scholar]
- 237.Naidoo J et al. A Multidisciplinary Toxicity Team for Cancer Immunotherapy-Related Adverse Events. J. Natl. Compr. Canc. Netw 17, 712–720 (2019). [DOI] [PubMed] [Google Scholar]
- 238.Scotte F, Ratta R & Beuzeboc P Side effects of immunotherapy: a constant challenge for oncologists. Curr. Opin. Oncol (2019). doi: 10.1097/CCO.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 239.Kostine M et al. Addressing immune-related adverse events of cancer immunotherapy: how prepared are rheumatologists? Annals of the rheumatic diseases 78, 860–862 (2019). [DOI] [PubMed] [Google Scholar]
- 240.Evens A et al. No Title. Am. J. Med (2019). doi: 10.1016/j.amjmed.2019.02.008 [DOI] [Google Scholar]
- 241.Kantarjian H & Yu PP Artificial intelligence, Big Data, and Cancer. JAMA Oncol. 1, 573–574 (2015). [DOI] [PubMed] [Google Scholar]
- 242.Mekki A et al. Machine learning defined diagnostic criteria for differentiating pituitary metastasis from autoimmune hypophysitis in patients undergoing immune checkpoint blockade therapy. Eur. J. Cancer 119, 44–56 (2019). [DOI] [PubMed] [Google Scholar]
- 243.Hsiehchen D, Watters MK, Lu R, Xie Y & Gerber DE Variation in the Assessment of Immune-Related Adverse Event Occurrence, Grade, and Timing in Patients Receiving Immune Checkpoint Inhibitors. JAMA Netw. open 2, e1911519 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pitt JM et al. Fine-Tuning Cancer Immunotherapy: Optimizing the Gut Microbiome. Cancer Res. 76, 4602–4607 (2016). [DOI] [PubMed] [Google Scholar]
- 245.Wang Y et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med 24, 1804–1808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang W et al. Assessing the viability of transplanted gut microbiota by sequential tagging with D-amino acid-based metabolic probes. Nat. Commun 10, 1317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rini BI et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med 380, 1116–1127 (2019). [DOI] [PubMed] [Google Scholar]
- 248.Motzer RJ et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med 380, 1103–1115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Simon N et al. Tofacitinib enhances delivery of antibody-based therapeutics to tumor cells through modulation of inflammatory cells. JCI insight 4, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Neelapu SS et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol 15, 47–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Riley RS, June CH, Langer R & Mitchell MJ Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov 18, 175–196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Francisco LM, Sage PT & Sharpe AH The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev 236, 219–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fife BT & Pauken KE The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci 1217, 45–59 (2011). [DOI] [PubMed] [Google Scholar]
- 254.Wang X, Teng F, Kong L & Yu J PD-L1 expression in human cancers and its association with clinical outcomes. Onco. Targets. Ther 9, 5023–5039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gandini S, Massi D & Mandala M PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol 100, 88–98 (2016). [DOI] [PubMed] [Google Scholar]
- 256.Weber JS et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J. Clin. Oncol 35, 785–792 (2017). [DOI] [PubMed] [Google Scholar]
- 257.Horvat TZ et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol 33, 3193–3198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Arbour KC et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J. Clin. Oncol 36, 2872–2878 (2018). [DOI] [PubMed] [Google Scholar]
- 259.Maher VE et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J. Clin. Oncol JCO1900318 (2019). doi: 10.1200/JCO.19.00318 [DOI] [PubMed] [Google Scholar]
- 260.Obradovic MMS et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019). [DOI] [PubMed] [Google Scholar]
- 261.Draghi A et al. Differential effects of corticosteroids and anti-TNF on tumor-specific immune responses: implications for the management of irAEs. Int. J. cancer (2018). doi: 10.1002/ijc.32080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


