Abstract
Circadian rhythms drive our daily behaviors to coincide with the earth’s rotation on an approximate 24-hour cycle. The circadian clock mechanism present in nearly every cell is responsible for our circadian rhythms and is comprised of a transcriptional – translational feedback loop in mammals. The central clock resides in the hypothalamus responding to external light cues, whereas peripheral clocks, receive signals from the central clock and are also sensitive to cues from feeding and activity. Of the peripheral clocks, the skeletal muscle clock is particularly sensitive to exercise which has shown to be an important time cue with the ability to influence and adjust the muscle clock phase in response to exercise timing. Since the skeletal muscle clock is also involved in the expression of tissue specific gene expression – including glucoregulatory genes – this might suggest a role for exercise timing as a therapeutic strategy in metabolic diseases, like type 2 diabetes. Notably, type 2 diabetics have accompanied disruptions in their skeletal muscle clock mechanism which may also be related to the increased risk of type 2 diabetes seen among shift workers. Therefore, the direct influence of exercise on the skeletal muscle clock might support the use of exercise timing to provide disease-mitigating effects. Here we highlight the potential use of time-of-day exercise as a chronotherapeutic to improve the metabolic profile of type 2 diabetes and support long-term glycemic control, potentially working through the skeletal muscle clock and circadian physiology.
Keywords: Circadian Rhythm, Skeletal Muscle, Exercise, Muscle Clock, Metabolism
Introduction
Circadian rhythms which influence our overall physiology and health, have evolved to coincide with the earth’s rotation on an approximate 24-hour cycle (Takahashi et al., 2008; Richards and Gumz, 2013; Gerhart-Hines and Lazar, 2015; Pilorz et al., 2018). The circadian clock mechanism that is responsible for our circadian rhythms is present in nearly every cell, whereupon its disruption can lead to deleterious consequences. Evidence of clock disruption is commonly seen among shift workers, who display metabolic disruptions similar to prediabetes, likely contributing to high rates of developing type 2 diabetes in shift workers (Wang et al., 2011; Gan et al., 2015; Manodpitipong et al., 2017). Additionally, type 2 diabetics also show signs indicative of clock disruption, further highlighting the connection between clock function and metabolic diseases (Hansen et al., 2016; Gabriel et al., 2021). However, in the last 10 years, research has demonstrated that clocks in peripheral tissues can respond to non-photic cues such as exercise and feeding even though the central clock in the brain stays locked in phase with the light-dark cycle. This raises the possibility of new health related interventions, such as scheduled exercise, as a therapeutic strategy to support clock and systems health.
Exercise has long been recognized as a treatment strategy to combat metabolic diseases (Thyfault and Bergouignan, 2020) and has recently been shown to directly influence the skeletal muscle clock. Specifically, exercise can serve as a time-cue for the muscle clock, with the phase of the muscle clock sensitive to the timing of exercise (Wolff and Esser, 2012; Kemler et al., 2020; Adamovich et al., 2021). Since the muscle clock is a significant contributor to muscle-specific gene expression – many of which are metabolism related – suggests that exercise timing may provide a pretext for reinforcing good metabolic health. Therefore, exercise timing could be used as a promising preventative or therapeutic avenue for metabolic diseases, such as type 2 diabetes.
In this review we start by highlighting the fundamental muscle clock mechanism and its interactions with exercise, with a particular focus of the role of exercise as a time-cue. We next present the close association between disrupted circadian rhythms (i.e., misalignment) and metabolic health. Lastly, we propose a paradigm through which consistent time-of-day exercise could be tested as a therapeutic strategy to improve glucose handling and long-term glycemic control, thus serving as a promising chronotherapy.
The Muscle Clock Mechanism
The circadian clock mechanism is found in virtually all cells throughout the body and is defined as a transcriptional-translational feedback loop (TTFL). The positive arm of the feedback loop consists of basic helix-loop-helix (bHLH)-PAS family of transcription factors, circadian locomotor output cycles kaput (CLOCK) (Gekakis, 1998) and Brain and Muscle ARNT-like 1 (BMAL1) (Bunger et al., 2000), that heterodimerize and bind to E box elements (Hardin, 2004) within the promoters of the negative arm genes, Period (Per 1/2/3) and Cryptochrome (Cry 1/2) genes, facilitating their transcription. Once translated, PERS and CRYS dimerize, translocate back to the nucleus and inhibit the transcriptional activity of the CLOCK: BMAL1 complex facilitating the negative limb of the feedback loop (Kume et al., 1999; Eide et al., 2002). Other regulatory proteins including the retinoic acid-related orphan nuclear receptors, REV-ERBs (Nr1d1, Nr1d2) and ROR, which compete for binding to retinoic acid-related orphan receptor response elements (ROREs) within the BMAL1 promotor either inhibiting or activating BMAL1 transcription, respectively (Preitner et al., 2002; Akashi and Takumi, 2005). This fundamental mechanism cycles within approximately 24-hours and is required for circadian rhythms (Figure 1).
Figure 1. Basic mechanism of the transcriptional – translational feedback loop of the molecular clock.
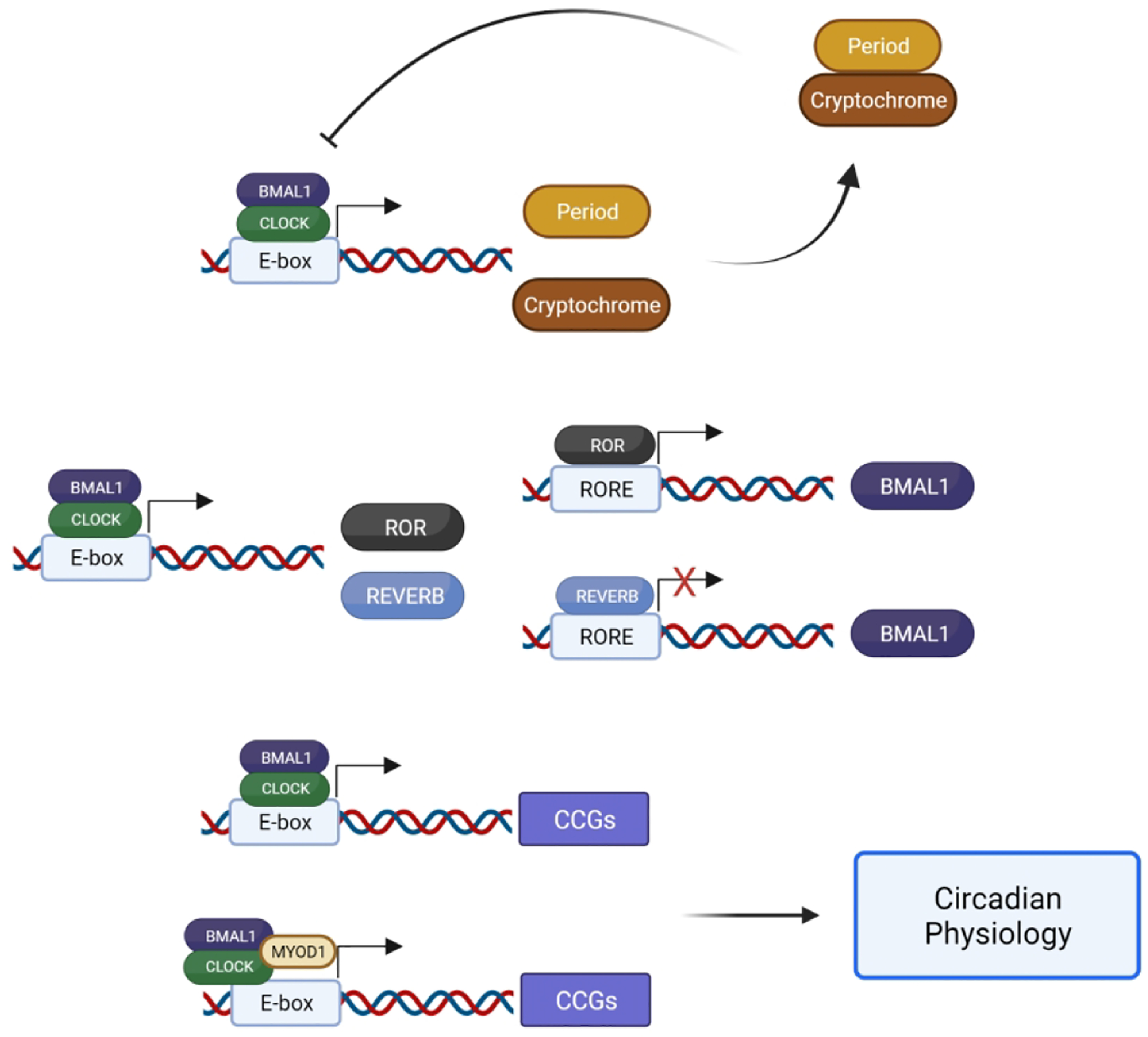
The constituents of the positive arm (BMAL1 and CLOCK) bind to E-box elements, facilitating the transcription of repressive genes of the negative arm (Per and Cry), which repress their own transcription by inhibiting the BMAL1: CLOCK heterodimer. BMAL1 and CLOCK also facilitate the transcription of Ror and Reverb genes that promote or inhibit the expression of Bmal1, respectively. Additionally, the BMAL1: CLOCK heterodimer binds to E-box elements of genes, often accompanied by tissues specific transcription factors like MYOD1 in skeletal muscle, facilitating the expression of clock-controlled genes (CCGs) and contributing to circadian physiology.
In addition to the cell timing, it is now clear that the core clock factors contribute to transcriptional regulation of a large number of genes that are important for daily cell physiology. Work by Zhang et al., described circadian output genes in over 12 mouse organs and found that the number of oscillating mRNAs varies across tissues ranging from ~1000 to 3000 mRNAs (Zhang et al., 2014). While the core clock mechanism is common to all tissues, they found that the circadian output genes were largely unique to each tissue and reflected the cell and organ specific functions. Data supporting a significant role for CLOCK and BMAL1 in genome wide gene expression was first highlighted by Koike et al., when they performed a comprehensive study looking at temporal patterns of core clock factor binding across liver chromatin, demonstrating that CLOCK and BMAL1 target binding at 5000–6000 sites in a time of day pattern in the liver (Koike et al., 2012). Likewise, clock output genes have been identified in mouse and human skeletal muscle (Miller et al., 2007; Dyar et al., 2018). Skeletal muscle exhibits a sleuth of clock-controlled genes that are reflective of the myogenic cell specificity, partially through interactions with the muscle transcription factor Myogenic cell differentiation factor 1 (MYOD1) and the CLOCK: BMAL1 heterodimer (Hodge et al., 2019). Most recently, BMAL1:CLOCK binding to over 5000 sites within chromatin was reported in mouse skeletal muscle (Gabriel et al., 2021). These studies demonstrate the clock mechanism in skeletal muscle contributes to a fundamental daily myogenic specific transcriptional program.
As prior investigations highlighted transcriptomic differences in clock outputs across a variety of tissues, additional studies using mouse genetic approaches have sought to understand the physiological influence of the skeletal muscle clock. Both skeletal muscle specific and inducible skeletal muscle specific mouse models of Bmal1 ablation have been performed. The combination of these studies has consistently found that loss of Bmal1 function in skeletal muscle is sufficient to induce alterations in the ability of muscle to uptake and utilize glucose coupled with an inability to respond to insulin.
For the purpose of this review, we will focus on the role of the skeletal muscle clock, which has emerged as a key regulator in metabolism. As skeletal muscle is the primary site of glucose uptake, storage, and utilization, the disruption of the muscle clock has implications in metabolic disease. Disruption of the skeletal muscle clock using Clock mutant mice and the muscle-specific Bmal1−/− model have demonstrated widespread transcriptional disruption and loss of rhythmicity, particularly of genes related to glucose and lipid metabolism (Miller et al., 2007; Dyar et al., 2014; Hodge et al., 2015; Schroder et al., 2015; Dyar et al., 2018). Specifically, muscle-specific Bmal1−/− mice display genes related to carbohydrate metabolism are downregulated whereas genes associated with lipid metabolism are upregulated (Hodge et al., 2015; Harfmann et al., 2016). As a result, muscle-specific Bmal1−/− mice exhibit higher levels of insulin while maintaining a normal fasting blood glucose and glucose tolerance tests (GTT) confirm that muscle-specific Bmal1−/− mice are glucose intolerant (Harfmann et al., 2016). Consistent with the GTT results, muscles in the muscle specific Bmal1−/− mice have lower mRNA and protein levels of Glut4 (Slc2a4) as well as reduced glucose uptake stimulated through insulin-dependent or independent mechanisms (Dyar et al., 2014; Harfmann et al., 2016). Comparatively, myotubes generated from human muscle primary myoblasts treated with small-interfering RNA complementary to Clock also exhibited reduced expression of Glut4 (Perrin et al., 2015; Perrin et al., 2018), further demonstrating the close association of the muscle clock and glucose uptake. Aside from glucose uptake, rate limiting enzymes associated with glycolysis including hexokinase 2 (hk2), phosphofructokinase (pfk1) show reduced transcript levels and enzymatic activity (Harfmann et al., 2016). Others using the same muscle-specific Bmal1−/− mice also showed an upregulation of pyruvate dehydrogenase 4 kinase (pdk4) and a downregulation of pyruvate dehydrogenase phosphatase 1 (Pdp1) which are involved in the reversible phosphorylation of the PDH-E1α subunit of PDH, necessary for catalyzing pyruvate to acetyl-CoA for oxidative metabolism (Dyar et al., 2014). Thus, as glycolytic pathways become obstructed due to altered enzyme expression and activity brought on by a disrupted muscle clock, shifts substrate metabolism away from carbohydrates and toward lipid and potential protein sources. This also means that the ability of muscle to serve as a glucose sink in the body is diminished, challenging other organs in the system to manage circulating glucose levels with feeding (Table 1).
Table 1.
Summary of Metabolic Outcomes of Global and Muscle-specific clock models
| Model | Metabolic Outcome |
|---|---|
| Clock Mutant (Clock Δ19) | Hyperglycemic, Hypoinsulinemic |
| Muscle-specific BMAL1 KO (MS-BMAL1) | Reduced Glucose Uptake, Increased Insulin Resistance |
| Inducible Muscle BMAL1 KO (iMS-BMAL1) | Reduced Glucose Uptake, Increased Insulin Resistance |
The resultant metabolic impact from the loss of Bmal1 function in skeletal muscle demonstrates the role of the skeletal muscle clock in metabolic physiology. Importantly, the transcriptional regulation of clock-controlled genes related to muscle metabolism indicate that changes related to the skeletal muscle clock may also influence the clock output and resulting physiology. Thus, means of influencing the skeletal muscle clock may provide an avenue of affecting whole-body physiology.
Exercise and the Muscle Clock
It is now recognized that human exercise performance displays a diurnal pattern, as peak levels of performance most frequently occur in the late afternoon/evening compared to the morning hours. Such aspects of performance are not specific to exercise modality as measures of endurance capacity, power output, force production, and maximal uptake of VO2 have been shown to be elevated in the evening (Souissi et al., 2007; Chtourou et al., 2011; van Moorsel et al., 2016; Sedliak et al., 2018; Knaier et al., 2021). The observation that performance displays a diurnal pattern has led to the suggestion of a relationship between exercise and the circadian clock, especially in skeletal muscle.
Exercise and Circadian Behaviors
More recent studies of exercise have shown that it can function as a potent, non-photic time cue (i.e., zeitgeber, or time giver) capable of influencing circadian rhythms. Youngstedt et al. showed that 3 days of 1-hour of scheduled moderate exercise was sufficient to shift the onset of urinary 6-sulphatoxymelatonin (aMT6s), a metabolite of melatonin used in characterizing human circadian rhythms (Youngstedt et al., 2019). Interestingly, early morning exercise (0700) induced an advance in circadian rhythm, as indicated by aMT6s onset; whereas evening exercisers (1900) were accompanied with a phase delay. Melatonin secretion originates from the pineal gland, downstream of the SCN, so these results suggest that time of exercise does impact the SCN in humans and may serve to coincide with anticipated activity times (Youngstedt et al., 2019).
Furthermore, young sedentary adults exercised for 5 consecutive days during either morning or evening hours. The morning exercise group exhibited a phase advance in dim light melatonin onset (DLMO) whereas there was no phase shift was observed in the afternoon exercise group (Thomas et al., 2020). Thus, these recent studies indicate that exercise does function as a non-photic time cue and can influence circadian rhythms in humans.
Earlier studies in mice have corroborated the influences of exercise on circadian rhythms. Mice exposed to consistently timed exercise have shown to align rhythmic activity and drinking behaviors with exercise timing after several days (Edgar and Dement, 1991; Marchant and Mistlberger, 1996). Hughes et al. showed that activity patterns in free running mice, showed entrainment to scheduled daily exercise up to 70 days (Hughes et al., 2021), indicating exercise does serve as a key time-cue for activity behaviors. Furthermore, Sasaki et al. utilized treadmill running on mice during the inactive phase which resulted in activity patterns shifting to correspond with exercise timing, where upon removal of the exercise stimulus led to a gradual re-shift in activity patterns, regressing to the innate rhythm prior to the exercise intervention (Sasaki et al., 2016). Prior behavioral studies have shown that non-photic time cues have an impact on activity and drinking behaviors (Dallmann and Mrosovsky, 2006; Dallmann et al., 2007), consistent with an impact of activity timing on the central clock. Indeed, running wheel access in mice while on an inverted L:D cycle show greater rates of entrainment, indicating that physical activity feeds back onto the central clock (Castillo et al., 2011; Hughes and Piggins, 2012). These studies support a model in which time of exercise has direct effects on the central clock and this change will regulate the changes in the peripheral clocks through neural and humoral pathways.
Exercise directly influences the Muscle Clock
In this section, we review the recent studies that have analyzed muscle clock function following scheduled exercise and we raise the potential that exercise can directly modify the muscle clock independent or concomitant with cues from the SCN. The availability of the Period 2::Luciferase (Per2::Luc) reporter mouse model allows for investigators to monitor clock function through tracking PER2 protein levels using real time bioluminescence recording over days and this data provide a sensitive output of core clock function in tissues of interest (Yoo et al., 2004). Wolff and Esser performed a training study that included both treadmill running and voluntary wheel running during the light phase to test the role of scheduled exercise on Per2::Luc rhythms. The ability to compare results from voluntary wheel running to treadmill running was a unique aspect of this design and controlled for the potential for stress, as a result of forced treadmill exposure, to be a modifying factor of peripheral clocks. Following 4 weeks of training, the tissues were collected 24 hours after the last exercise bout and they found that lung and different skeletal muscles displayed significant ~2–4 hour changes in Per2::Luc rhythms demonstrating a significant phase shift compared to non-exercised mice. Importantly, they also found that there was no change in Per2::Luc rhythms in the SCN with exercise (Wolff and Esser, 2012). However, it is possible the absence of a phase shift in the SCN was due to re-setting by light during the L:D cycle as phase shifts in the SCN can respond to activity rather than light (Castillo et al., 2011).
More recently, a shift in muscle clock phase has been shown in response to a single bout of running. In this study, Per2::Luc mice underwent a 60 minutes of moderate treadmill exercise at times corresponding with the middle of the resting, end of rest, and middle of active phases. Exercise at the midpoint of the active phase did not induce a significant shift as normal activity is typical at this time. However, exercise during the middle of resting phase produced an advance in Per2::Luc rhythms and exercise at the end of the rest phase induced a delay in Per2::LUC rhythms. The observation that exercise can produce either a phase advance or delay to the muscle clock is consistent with exercise being a bonafide time cue for the muscle clock (Kemler et al., 2020). While the acute response of the muscle clocks to exercise suggests a direct effect, these results cannot discriminate between the SCN regulating the muscle clocks vs. direct effects of exercise on muscle clocks.
Evidence for direct effects of exercise or contractions on muscle clocks comes from two different studies. Small et al., used electrical stimulation of muscle ex vivo and showed this was sufficient to induce Per2 gene expression and implicated calcium as the intracellular signaling pathway linking contractions to clock gene expression (Small et al., 2020). Another study used a muscle cell line transfected with the Bmal1-luciferase reporter vector and tested time of day stimulations. In this study, bioluminescence recording demonstrated that a 60-minute bout of contractions of muscle cells in vitro was sufficient to induce a sustained advance or delay in circadian clock phase. In addition, they found a similar phase advance and phase delay were seen in vitro when compared to the in vivo treadmill running (Kemler et al., 2020). These ex vivo and in vitro studies demonstrate that the circadian clock in mouse skeletal muscle does receive direct time setting information from contractions/exercise. We note that these findings are also consistent with the changes in clock genes following an acute bout of resistance exercise in human subjects (Zambon et al., 2003) Thus, we propose that there is a mechanism whereby exercise can directly modulate the clock mechanism in muscle. We caution, however, that there is still much research to be done to determine how exercise information from the SCN is integrated into the muscle clock exercise response in vivo.
Beyond acute exercise or contractions, consistent exercise timing has also demonstrated the ability to induce a muscle clock phase shift over the course of several weeks. Recently, Adamovich et al. restricted wheel running activity (i.e., exercise) in Per2::Luc mice to 6 hours either during the early active phase or late active phase for 2 weeks. A phase shift was only observed in the late exercise group, inducing a phase delay in the gastrocnemius muscle; whereas the early exercise group displayed no phase shift (Adamovich et al., 2021). Additionally, our lab has demonstrated in mice that underwent 6 weeks of treadmill running at 70% max performance at either the early active or late active was accompanied by a phase advance or delay, respectively. While phase shifts in both groups were responsive to the respective time of exercise, both early and late exercise groups achieved similar exercise capacities after the 6-week exercise period (Hesketh biorxiv 2022). Thus, in addition to acute exercise or muscle contractions, repetitive exercise bouts at a consistent time of day shifts and maintains the muscle clock phase toward regular exercise timing (Figure 2).
Figure 2. The exercise-induced phase shift of the skeletal muscle clock.

Exercise can induce a phase shift in the skeletal muscle clock in response to the timing of exercise shown here as a representative effect of either Early Active (AM) or Late Active (PM) phase exercise (Ex; dashed vertical lines). Shifts in the muscle clock phase (dashed curve) are identified through Period 2 (Per2) of the muscle clock’s negative arm, from its rhythm prior to exercise (solid curve). Arrows indicate the direction of the phase shift.
Other considerations involved with the effect of exercise on the skeletal muscle clock is whether forced or voluntary exercise results in similar modifications to the muscle clock phase. Importantly, the exercise field has recognized that the use of electrical shocks during forced exercise can have detrimental effects on exercise performance (Poole et al., 2020; Casanova-Vallve et al., 2022) while also inducing stress-related sympathetic and glucocorticoid responses that can influence peripheral tissue phase (Sasaki et al., 2016). Additionally, timing of tissue collection is critically important for observing phase shifts, as modulations to circadian phase are not detectable until several hours from the introduction of the time-cue (Yannielli et al., 2002). We note that our previous study included groups with either forced treadmill exercise or voluntary wheel running to study time of exercise on muscle clocks. The tissues were removed from the exercise mice 24 hours after the final exercise bout and we found comparable shifts in peripheral tissue phases, indicating that both forced exercise and voluntary exercise can modulate muscle clocks in a similar manner (Wolff and Esser, 2012).
Exercise Timing and Muscle Clock Output
Recent work has described differences among the skeletal muscle transcriptional and metabolomic profiles accompanying time-of-day exercise. Importantly, genes related to glycolytic pathways and mitochondrial respiration have been shown to be under clock control (Zambon et al., 2003; Schmitt et al., 2018; Sato et al., 2019), indicating a possible interaction with time-of-day exercise. A recent study by Ezagouri et al. aimed to dissect the transcriptional and metabolomic changes related to time-of-day and exercise and noted that several of the clock, as well as core metabolism genes, including Per1/2, Bmal1, Gck, PPARα, and Klf10, are influenced by both time-of-day and exercise, indicating an overlap of controlled expression (Ezagouri et al., 2019). Additionally, exercise timing influences the skeletal muscle metabolome of mice exposed to treadmill exercise in either the early rest or early active phase. Whereas exercise during the early rest phase primarily induced the upregulation of amino acid related metabolites, exercise in the early active phase substantially increased the overall number of metabolites of amino acids, lipids, and carbohydrate metabolism, which coupled with an activation of the glycolytic pathway in skeletal muscle (Sato et al., 2019). Together, time-of-day exercise has a substantial effect of the transcriptional and metabolic response in skeletal muscle.
As skeletal muscle transcriptional and metabolomic profiles are affected by time-of-day exercise, additional work has provided insight into the potential feedback between metabolic associated processes and their influence on the muscle clock. Metabolic pathways associated with mitochondrial bioenergetics, including the regulation of nicotinamide adenosine dinucleotide (NAD+) by phosphoribosyltransferase (NAMPT), have been implemented as having an influence on the muscle clock. NAD+ can further influence mitochondrial respiration through NAD-dependent deacetylases Sirtuins (SIRTs) (Peek et al., 2013) which belong to the class III histone deacetylation proteins (HDACs) involved in chromatin remodeling (Asher et al., 2008; Nakahata et al., 2008), providing a possible connection between metabolism and transcriptional regulation. Other pathways, including AMP-activated protein kinase (AMPK), which is highly responsive to exercise (Wang et al., 2018) has also shown to influence the regulation of circadian genes (Lassiter et al., 2018). Additionally, transcription factors like hypoxia-inducible factor 1α (HIF1α), when expressed under hypoxic conditions (1% O2) in vitro, can interact with BMAL1, inducing Per2 expression to a similar extent to the CLOCK: BMAL1 heterodimer (Peek et al., 2017). Acetylation of histone lysine residues have been identified in whole skeletal muscle and myotubes by CREB-p300 which are thought to be coactivators under HIF1α control, further suggesting remodeling of chromatin structure and transcriptional influence (Dengler et al., 2014; Sato et al., 2019). Thus, the transcriptional and metabolic changes as well as associated signaling pathways in response to time-of-day exercise could provide a connection between the intramyocellular milieu and the respective response of clock genes. However, whether these metabolic mechanism(s) contribute to a muscle phase shift or if the metabolic profile is significantly different after a muscle phase shift has yet to be revealed.
We are only beginning to understand the multiple connections between exercise and its effects on circadian rhythms and the muscle clock. Nonetheless, the demonstration that time-of-day exercise can directly modify the muscle clock points to time-of-day exercise as a possible therapeutic strategy to help with various diseases, including those accompanied with clock disruptions.
Circadian Misalignment and Metabolic Disruption: A Potential Target for Exercise Timing?
Circadian misalignment is defined by the body clocks throughout the system being out of synchrony. Such misalignment can be attributed to a shifted light/dark cycle with our behaviors and/or the misalignment between or within tissue clocks. This most commonly occurs with shift work or travel across time zones (i.e., jet lag) but can also occur as a result from altered times of light exposure (i.e., light at night), feeding (i.e., night feeding) and activity occurring during the overnight hours (Vetter, 2020). These behaviors, either independently or in combination induce circadian misalignment, which has been shown to have deleterious consequences on metabolic health and contribute to disease development.
Although the underlying mechanisms remain elusive, circadian misalignment is closely associated with the development of type 2 diabetes. Individuals involved in shift work are particularly vulnerable as they are continuously exposed to circadian misalignment with behaviors occurring outside of the normal wake/sleep hours. Prior observational and epidemiological studies have indicated that shift working individuals are more likely to develop type 2 diabetes than those working traditional daytime shift hours (Wang et al., 2011; Gan et al., 2015). Of shift working individuals, odds ratios appear to show men to be disproportionately affected (1.37) compared to women (1.09) (Gan et al., 2015). Moreover, night shift workers, with diagnosed diabetes display higher HbA1c levels, a measure of long-term glycemic control, compared to their diabetic day shift counterparts (Anothaisintawee et al., 2017; Manodpitipong et al., 2017). Such findings support an association between shift workers and type 2 diabetes, indicative of an underlying effect from circadian misalignment and metabolic disruption.
As epidemiological studies have suggested an association between circadian misalignment and type 2 diabetes, prior laboratory-controlled studies have showed that brief periods of induced circadian misalignment with healthy participants results in altered glycemic control that is reminiscent of either pre- or fully-developed diabetes (Scheer et al., 2009). Ten healthy adults exposed to a 28-hour day resulting in circadian misalignment, showed elevated blood glucose and insulin across 24-hours by approximately 6% and 22%, respectively. Importantly, the postprandial glucose response to standardized meals were higher in misaligned subjects compared to normal alignment, suggestive of insulin insensitivity as the primary contributor to the overall increases in glucose and insulin across the 24-hour period (Scheer et al., 2009). Others show similar increases in postprandial blood glucose after misalignment further demonstrating a reduced ability to appropriately respond to glucose excursions (Morris et al., 2015; Morris et al., 2016; Qian et al., 2018). During an entire 24-hour period of misalignment, Morris et al. found overall higher blood glucose levels compared to control subjects while secreted insulin responses remained unchanged in response to regular meals (Morris et al., 2016). This study also utilized subjects who were also shift workers, indicating that prior long-term shift work provides no protective means to induced misalignment. Additionally, others have also incorporated sleep loss as another modifier of circadian misalignment that results in insulin resistance and poorer glycemic control (Leproult et al., 2014; Eckel et al., 2015; Simon et al., 2019). Leproult et al found that circadian misalignment resulted in a 47% reduction in insulin sensitivity when accompanied with sleep loss compared to 34% with sleep loss alone (Leproult et al., 2014). Moreover, insulin sensitivity was shown to be reduced as much as 39% after a 5-hour sleep schedule coupled with elevated melatonin and increased wakefulness (Eckel et al., 2015). As there appears to be an inverse relationship between insulin sensitivity and melatonin levels (Rubio-Sastre et al., 2014), shift work which is often accompanied by reduced sleep and increased wakefulness during a period of higher melatonin levels (Birkeland, 1982; Lane et al., 2016), supports the association of circadian misalignment and the development of type 2 diabetes. Thus, misalignment of behavioral and circadian rhythmicity induces a clinically significant disruption of glycemic control and could be a contributing factor to type 2 diabetes.
Studies have sought to investigate the underpinnings of circadian misalignment and glycemic control. Wefers et al. demonstrated in human muscle biopsy samples, insulin sensitivity was primarily disrupted at the level of skeletal muscle, as hepatic glucose production (a measure of liver gluconeogenesis and glycogenolysis) remained unchanged (Wefers et al., 2018). As skeletal muscle accounts for approximately 80–85% of glucose storage (Carnagarin et al., 2015), disruptions in the storage and/or utilization of glucose at the site of skeletal muscle has implications in diabetes. Inasmuch, circadian misalignment has shown to reduce non-oxidative glucose storage (i.e. glycogen synthesis) (Morris et al., 2015; Wefers et al., 2018; Harmsen et al., 2021). Furthermore, the Bmal1−/− mouse model also displays reduced glucose uptake and utilization, similar to misaligned humans, indicating the need for appropriate muscle clock function (Dyar et al., 2014; Harfmann et al., 2016). Isolated human primary muscle cells from diabetic donors have previously showed lower amplitude of REVERB, a key repressor of BMAL1 expression compared to their healthy counterparts, further suggesting a connection between type 2 diabetes and muscle clock function (Hansen et al., 2016). Thus, elevated blood glucose as a result of lower glucose uptake and utilization highlights circadian misalignment and its effects on metabolic disruption and development of disease, with a pivotal role of skeletal muscle.
In addition to the reductions in glucose uptake and metabolism, circadian misalignment is also associated with an enhanced preference toward lipid metabolism. Misaligned humans have shown increases in lipid metabolism after simulated shiftwork, indicating an associated altered metabolic profile (Wefers et al., 2018). Others also highlight increases in lipid utilization up to 18% whereas lipid oxidation increased as high as 50% in the days following the onset of laboratory induced misalignment (McHill et al., 2014; Morris et al., 2015). As the associated between circadian misalignment leads to an altered metabolic preference toward lipids, may lead to the accumulation of lipid metabolism by-products implicated in the development of type 2 diabetes and skeletal muscle insulin resistance (Bandet et al., 2019). Indeed, altered lipid species have been found within human skeletal muscle after circadian misalignment (Harmsen et al., 2021). Furthermore, the skeletal muscle Bmal1−/− model also shows a shift in substrate preference favoring lipid metabolism, extending the connection between the muscle clock and unbalanced substrate utilization (Harfmann et al., 2016). Therefore, whole body and skeletal muscle metabolic milieus favoring lipid metabolism with reductions in glucose uptake and utilization in response to circadian misalignment, indicates a need of synchrony among the body clocks, and particularly skeletal muscle, for appropriate metabolic control.
Circadian misalignment is a prime example in which clock disruption can result in metabolic disease, with a particular effect on skeletal muscle. As the muscle clock strongly influences metabolism, coupled with muscle’s sensitivity to exercise timing, suggests that exercise timing could play a role as a treatment strategy for metabolic disease or a means of long-term glycemic control.
Time-of-Day Exercise
As metabolic health is influenced by the skeletal muscle clock, treatment options operating through the skeletal muscle clock could be a promising avenue of therapeutic investigation. While exercise is well accepted to have a beneficial role in the treatment of metabolic disease, the direct influence of consistent time-of-day exercise on the skeletal muscle clock could be a suitable countermeasure to metabolic diseases, like type 2 diabetes.
Here we have described the importance of the skeletal muscle clock in metabolic health with a particular focus in type 2 diabetes. Importantly, skeletal muscle is the primary site of glucose handling and metabolism (Carnagarin et al., 2015) which is strongly influenced by the skeletal muscle clock (Dyar et al., 2014; Harfmann et al., 2016). Exercise, which has time setting capabilities, can directly affect and modulate the skeletal muscle clock and thus influence the metabolic profile. Whereas disruptions in the skeletal muscle clock are associated with metabolic disease as seen in type 2 diabetic muscle (Hansen et al., 2016; Gabriel et al., 2021), exercise timing may be a noteworthy therapeutic strategy. Therefore, we propose a paradigm in which consistent time-of-day exercise facilitates changes in skeletal muscle physiology through directly modulating the skeletal muscle clock and in turn the expression of skeletal muscle CCGs, reinforcing whole-body metabolic health (Figure 3). The underlying mechanism(s) through which consistent time-of-day exercise improves metabolic health are not fully investigated, however could stem from synchrony within muscle or between skeletal muscle and other organs by the direct effect of exercise on the skeletal muscle clock. Nonetheless, such a paradigm and its mechanism(s) has yet to be described, however the expression of glucoregulatory genes by the skeletal muscle clock (Dyar et al., 2014; Harfmann et al., 2016) stemming from consistent time-of-day exercise, could benefit individuals with metabolic diseases, like type 2 diabetes.
Figure 3. Paradigm of consistent time-of-day exercise improving metabolic health through modulating the skeletal muscle clock.
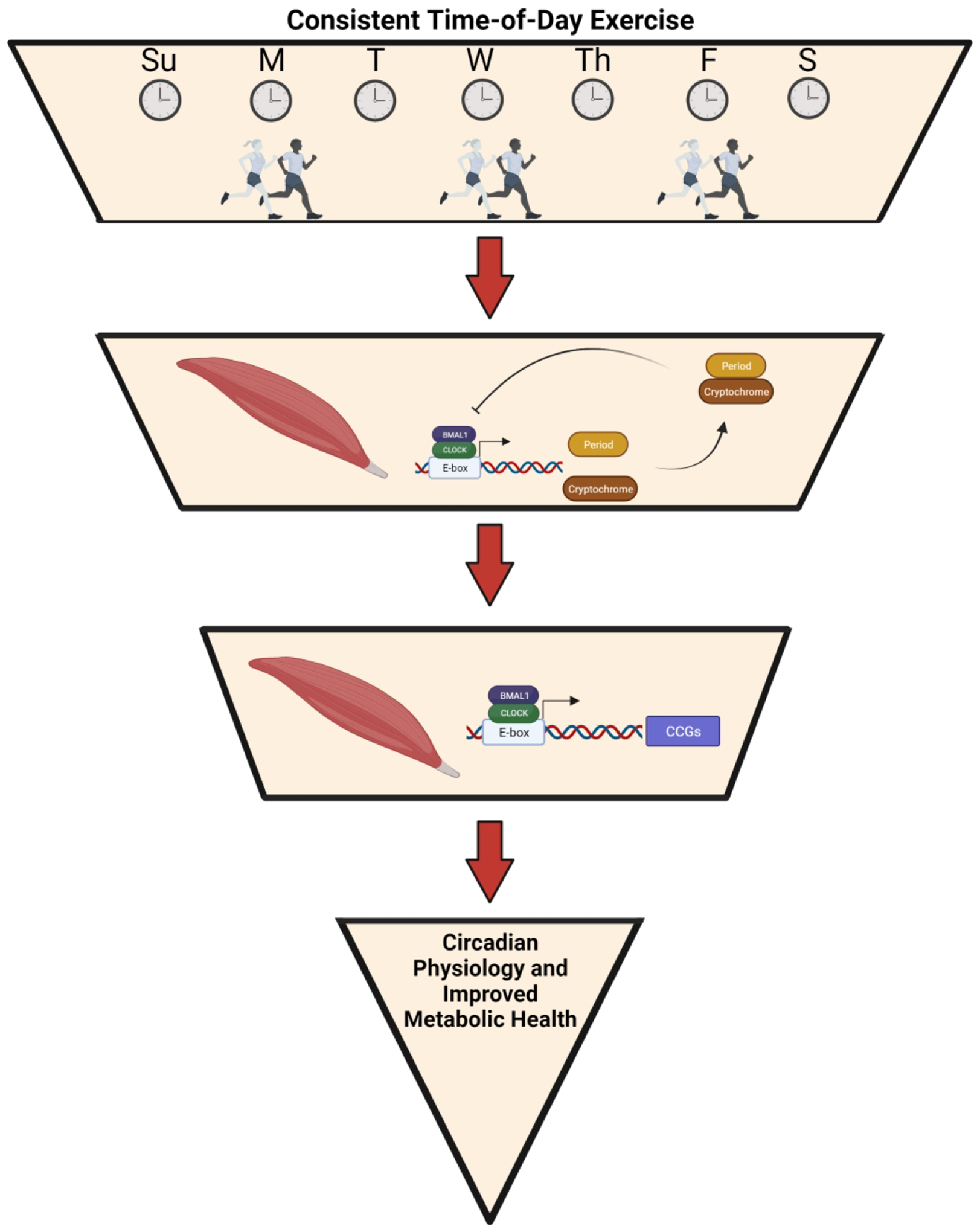
Proposed paradigm of consistent time-of-day exercise directly influencing the skeletal muscle clock. In turn, the muscle clock then modulates the transcriptional control of muscle clock-controlled genes involved with muscle physiology, leading to improved metabolic health.
Exercise has previously demonstrated to have time setting capabilities as isolated skeletal muscle showed a skeletal muscle clock phase shift in response to muscle contractions (Kemler et al., 2020; Small et al., 2020), indicating a relationship between exercise timing and the muscle clock. Recent findings further indicate that consistent exercise timing can stimulate an exercise-induced muscle clock phase shift in vivo, shifting the muscle clock phase in response to exercise timing. Specifically, we and others show that consistent exercise timing can induce a phase shift while also achieving a similar level of fitness post-training (Adamovich et al., 2021). These data are evidence of consistent exercise timing modulating the skeletal muscle clock and thus, supports a paradigm in which exercise influences resultant circadian physiology.
Few studies have implemented time-of-day exercise as an intervention in type 2 diabetes. Sedentary overweight participants with or without diabetes were exposed to a multimodal (aerobic and resistance) exercise regimen for 12-weeks, which participants had to exercise during either morning (0800–1000) or evening (1700–1900) hours. All disease parameters including HbA1c, fasting glucose, HOMA-IR, and fasting insulin were lower after exercise training, however unaffected by time-of-day exercise (Teo et al., 2020). Furthermore, Chiang et al. monitored 20 type 2 diabetics engaging in either morning (0800–1000), afternoon (1400–1600), or evening (1800–2000) moderate-high intensity exercise for 12 weeks in a prospective longitudinal study which showed all participants improved baseline blood glucose after exercise training, regardless of exercise timing (Chiang et al., 2019). However, Mancilla et al. showed, using a hyperinsulinemic - euglycemic clamp in a retrospective study of 35 metabolically compromised males after a 12-week cycling and resistance training program, greater improvements in glucose disposal, carbohydrate oxidation, and fasting glucose after evening training (1500–1800) compared to the morning group (0800–1000). In addition to the improvements insulin-stimulated glucose handling, evening exercisers displayed larger reductions in fat mass and a greater increase in their maximal workload, highlighting improvements on body composition and exercise capacity, respectively (Mancilla et al., 2021). Importantly, only 12 of the 32 subjects were diabetic, which could limit interpretations. Nonetheless, improvements in disease parameters among both morning and evening exercisers in type 2 diabetics could potentially be due to modulations in the skeletal muscle clock adjusting to consistent exercise timing.
Although consistent time-of-day exercise over a few months appears to have a similar impact on type 2 diabetes, a recent study by Savijk et al. evaluated 20 participants with diagnosed type 2 diabetes before and after a morning and evening exercise bout of high intensity interval exercise 3 days/week for 2 weeks in a randomized cross-over approach (Savikj et al., 2019). Morning exercisers showed larger increases in blood glucose levels under continuous glucose monitoring compared to afternoon exercisers, which could be the result of high-intensity exercise induced hyperglycemia previously observed after morning exercise (Chiang et al., 2019). What is striking are the elevated blood glucose levels in morning exercisers on subsequent rest days by the second week; whereas afternoon exercisers achieving an overall lower blood glucose were largely unchanged. Thus, acute high intensity morning exercise appears to contribute to an unfavorable hyperglycemic effect. Type 2 diabetics are prone to elevated blood glucose upon waking known as the ‘dawn phenomenon’ (Roman et al., 2016; Zheng et al., 2020), however it is not clear whether the increases in blood glucose after morning exercise affected glucose production in the liver of this study, a known contributor to elevated morning glucose levels (Savikj et al., 2019). Although morning exercisers may elicit unfavorable effects from acute exercise, it is speculative to suggest that consistent morning or afternoon (i.e., early active or late active phase) exercise both would contribute to an improved metabolic profile, accounting for similar improvements in previous studies of time-of-day exercise in type 2 diabetics (Teo et al., 2020).
Additionally, consistent time-of-day exercise might also be a potential preventative measure, serving as a daily time cue modulating the skeletal muscle clock and associated glucoregulatory mechanisms, providing long-term glycemic control. Obese, prediabetic individuals showed that after 12-weeks of aerobic exercise intervention induced higher skeletal muscle PER2 protein and Bmal1 gene expression which positively correlated with glucose disposal rate (Erickson et al., 2020). Although time-of-day was not recorded in this study, the improved glycemic control associated with upregulated core clock genes may support the notion that consistent time-of-day exercise could act as a preventative measure for type 2 diabetes. Whether consistent time-of-day exercise ameliorates the development of type 2 diabetes in prediabetics requires longitudinal studies. Nonetheless, improvements in the metabolic profile in disease and non-disease states, may stem from resultant physiological changes accompanying modulations to the skeletal muscle clock as a result of consistent time-of-day exercise.
So far, the underlying mechanisms driving the modulation of the skeletal muscle clock from exercise are not yet established. However, what is clear is the emerging importance of the skeletal muscle clock and its involvement in metabolic health. We propose a paradigm in which consistent time-of-day exercise can directly adjust the skeletal muscle clock and with it, adjust our circadian physiology that contributes to a beneficial metabolic environment and good metabolic health. Thus, exercise timing coupled with its disease-mitigating benefits, could serve as an important chronotherapeutic in metabolic diseases.
Acknowledgements
Figures were created with BioRender.com
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health, [grant number U01AG055137; project number P0028939] and [grant number supplement U01AG055137; project number P0207911].
Footnotes
Declaration of Conflicting Interests
The authors declare that there are no conflicts of interest.
References
- Adamovich Y, Dandavate V, Ezagouri S, Manella G, Zwighaft Z, Sobel J, Kuperman Y, Golik M, Auerbach A, Itkin M, Malitsky S, and Asher G (2021) Clock proteins and training modify exercise capacity in a daytime-dependent manner. Proc. Natl. Acad. Sci 118:e2101115118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi M, and Takumi T (2005) The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat. Struct. Mol. Biol 12:441–448. [DOI] [PubMed] [Google Scholar]
- Anothaisintawee T, Lertrattananon D, Thamakaison S, Knutson KL, Thakkinstian A, and Reutrakul S (2017) Later chronotype is associated with higher hemoglobin A1c in prediabetes patients. Chronobiol. Int 34:393–402. [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, and Schibler U (2008) SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 134:317–328. [DOI] [PubMed] [Google Scholar]
- Bandet CL, Tan-Chen S, Bourron O, Stunff HL, and Hajduch E (2019) Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells. Int. J. Mol. Sci 20:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland AJ (1982) Plasma Melatonin Levels and Nocturnal Transitions between Sleep and Wakefulness. J Neuroendocrinol 34:126–131. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, and Bradfield CA (2000) Mop3 Is an Essential Component of the Master Circadian Pacemaker in Mammals. Cell 103:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnagarin R, Dharmarajan AM, and Dass CR (2015) Molecular aspects of glucose homeostasis in skeletal muscle – A focus on the molecular mechanisms of insulin resistance. Mol. Cell. Endocrinol 417:52–62. [DOI] [PubMed] [Google Scholar]
- Casanova-Vallve N, Duglan D, Vaughan ME, Pariollaud M, Handzlik MK, Fan W, Yu RT, Liddle C, Downes M, Delezie J, Mello R, Chan AB, Westermark PO, Metallo CM, Evans RM, and Lamia KA (2022) Daily running enhances molecular and physiological circadian rhythms in skeletal muscle. Mol Metab 61:101504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo C, Molyneux P, Carlson R, and Harrington ME (2011) Restricted wheel access following a light cycle inversion slows re-entrainment without internal desynchrony as measured in Per2Luc mice. Neuroscience 182:169–176. [DOI] [PubMed] [Google Scholar]
- Chiang S-L, Heitkemper MM, Hung Y-J, Tzeng W-C, Lee M-S, and Lin C-H (2019) Effects of a 12-week moderate-intensity exercise training on blood glucose response in patients with type 2 diabetes: A prospective longitudinal study. Medicine 98:e16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtourou H, Zarrouk N, Chaouachi A, Dogui M, Behm DG, Chamari K, Hug F, and Souissi N (2011) Diurnal Variation in Wingate-Test Performance and Associated Electromyographic Parameters. Chronobiol. Int 28:706–713. [DOI] [PubMed] [Google Scholar]
- Dallmann R, Lemm G, and Mrosovsky N (2007) Toward easier methods of studying nonphotic behavioral entrainment in mice. J. Biol. Rhythms 22:458–461. [DOI] [PubMed] [Google Scholar]
- Dallmann R, and Mrosovsky N (2006) Scheduled wheel access during daytime: A method for studying conflicting zeitgebers. Physiol. Behav 88:459–465. [DOI] [PubMed] [Google Scholar]
- Dengler VL, Galbraith MD, and Espinosa JM (2014) Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol 49:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar KA, Ciciliot S, Wright LE, Biensø RS, Tagliazucchi GM, Patel VR, Forcato M, Paz MIP, Gudiksen A, Solagna F, Albiero M, Moretti I, Eckel-Mahan KL, Baldi P, Sassone-Corsi P, Rizzuto R, Bicciato S, Pilegaard H, Blaauw B, and Schiaffino S (2014) Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab 3:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar KA, Hubert MJ, Mir AA, Ciciliot S, Lutter D, Greulich F, Quagliarini F, Kleinert M, Fischer K, Eichmann TO, Wright LE, Peña Paz MI, Casarin A, Pertegato V, Romanello V, Albiero M, Mazzucco S, Rizzuto R, Salviati L, Biolo G, Blaauw B, Schiaffino S, and Uhlenhaut NH (2018) Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol 16:e2005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel Robert H, Depner Christopher M, Perreault L, Markwald Rachel R, Smith Mark R, McHill Andrew W, Higgins J, Melanson Edward L, and Wright Kenneth P (2015) Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr. Biol 25:3004–3010. [DOI] [PubMed] [Google Scholar]
- Edgar DM, and Dement WC (1991) Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am. J. Physiol. Regul. Integr. Comp. Physiol 261:R928–R933. [DOI] [PubMed] [Google Scholar]
- Eide EJ, Vielhaber EL, Hinz WA, and Virshup DM (2002) The Circadian Regulatory Proteins BMAL1 and Cryptochromes Are Substrates of Casein Kinase Iε. J. Biol. Chem 277:17248–17254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson ML, Zhang H, Mey JT, and Kirwan JP (2020) Exercise Training Impacts Skeletal Muscle Clock Machinery in Prediabetes. Med. Sci. Sports Exerc 52:2078–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezagouri S, Zwighaft Z, Sobel J, Baillieul S, Doutreleau S, Ladeuix B, Golik M, Verges S, and Asher G (2019) Physiological and Molecular Dissection of Daily Variance in Exercise Capacity. Cell Metab 30:78–91.e74. [DOI] [PubMed] [Google Scholar]
- Gabriel BM, Altıntaş A, Smith J, Sardon Puig L, Zhang X, Basse AL, Laker RC, Gao H, Liu Z, Dollet L, Treebak JT, Zorzano A, Huo Z, Ryden M, Lanner JT, Esser KA, Barrès R, Pillon NJ, Krook A, and Zierath JR (2021) Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle. Sci. Adv 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, Li L, Cao S, Dong X, Gong Y, Shi O, Deng J, Bi H, and Lu Z (2015) Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup. Environ. Med 72:72–78. [DOI] [PubMed] [Google Scholar]
- Gekakis N (1998) Role of the CLOCK Protein in the Mammalian Circadian Mechanism. Science 280:1564–1569. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, and Lazar MA (2015) Circadian Metabolism in the Light of Evolution. Endocr. Rev 36:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Timmers S, Moonen-Kornips E, Duez H, Staels B, Hesselink MKC, and Schrauwen P (2016) Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci. Rep 6:35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin PE (2004) Transcription Regulation within the Circadian Clock: The E-box and Beyond. J. Biol. Rhythms 19:348–360. [DOI] [PubMed] [Google Scholar]
- Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, and Esser KA (2016) Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen JF, Polanen N, Weeghel M, Wefers J, Hoeks J, Vaz FM, Pras‐Raves ML, Kampen AHC, Schaart G, Moorsel D, Hansen J, Hesselink MKC, Houtkooper RH, and Schrauwen P (2021) Circadian misalignment disturbs the skeletal muscle lipidome in healthy young men. FASEB J 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, Schroder EA, and Esser KA (2015) The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge BA, Zhang X, Gutierrez-Monreal MA, Cao Y, Hammers DW, Yao Z, Wolff CA, Du P, Kemler D, Judge AR, and Esser KA (2019) MYOD1 functions as a clock amplifier as well as a critical co-factor for downstream circadian gene expression in muscle. eLife 8:e43017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ATL, and Piggins HD (2012) Feedback actions of locomotor activity to the circadian clock. Prog. Brain Res 199:305–336. [DOI] [PubMed] [Google Scholar]
- Hughes ATL, Samuels RE, Baño-Otálora B, Belle MDC, Wegner S, Guilding C, Northeast RC, Loudon ASI, Gigg J, and Piggins HD (2021) Timed daily exercise remodels circadian rhythms in mice. Commun. Biol 4:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler D, Wolff CA, and Esser KA (2020) Time-of-day dependent effects of contractile activity on the phase of the skeletal muscle clock. J. Physiol 598:3631–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaier R, Qian J, Roth R, Infanger D, Notter T, Wang W, Cajochen C, and Scheer FAJL (2021) Diurnal Variation in Maximum Endurance and Maximum Strength Performance: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Yoo S-H, Huang H-C, Kumar V, Lee C, Kim T-K, and Takahashi JS (2012) Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science 338:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, and Reppert SM (1999) mCRY1 and mCRY2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop. Cell 98:193–205. [DOI] [PubMed] [Google Scholar]
- Lane JM, Chang A-M, Bjonnes AC, Aeschbach D, Anderson C, Cade BE, Cain SW, Czeisler CA, Gharib SA, Gooley JJ, Gottlieb DJ, Grant SFA, Klerman EB, Lauderdale DS, Lockley SW, Munch M, Patel S, Punjabi NM, Rajaratnam SMW, Rueger M, St. Hilaire MA, Santhi N, Scheuermaier K, Van Reen E, Zee PC, Shea SA, Duffy JF, Buxton OM, Redline S, Scheer FAJL, and Saxena R (2016) Impact of Common Diabetes Risk Variant in MTNR1B on Sleep, Circadian, and Melatonin Physiology. Diabetes 65:1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassiter DG, Sjögren RJO, Gabriel BM, Krook A, and Zierath JR (2018) AMPK activation negatively regulates GDAP1, which influences metabolic processes and circadian gene expression in skeletal muscle. Mol. Metab 16:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Holmbäck U, and Van Cauter E (2014) Circadian Misalignment Augments Markers of Insulin Resistance and Inflammation, Independently of Sleep Loss. Diabetes 63:1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla R, Brouwers B, Schrauwen‐Hinderling VB, Hesselink MKC, Hoeks J, and Schrauwen P (2021) Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol. Rep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manodpitipong A, Saetung S, Nimitphong H, Siwasaranond N, Wongphan T, Sornsiriwong C, Luckanajantachote P, Mangjit P, Keesukphan P, Crowley SJ, Hood MM, and Reutrakul S (2017) Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J. Sleep Res 26:764–772. [DOI] [PubMed] [Google Scholar]
- Marchant G, and Mistlberger E (1996) Entrainment and Phase Shifting of Circadian Rhythms in Mice by Forced Treadmill Running. Physiol. Behav 60:7. [DOI] [PubMed] [Google Scholar]
- McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, and Wright KP (2014) Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci 111:17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, and Takahashi JS (2007) Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl. Acad. Sci 104:3342–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Mistretta J, and Scheer FAJL (2016) Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J. Clin. Endocrinol. Metab 101:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, and Scheer FAJL (2015) Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci 112:E2225–E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, and Sassone-Corsi P (2008) The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Control. Cell 134:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, and Bass J (2013) Circadian Clock NAD+ Cycle Drives Mitochondrial Oxidative Metabolism in Mice. Science 342:1243417–1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, and Bass J (2017) Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab 25:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L, Loizides-Mangold U, Chanon S, Gobet C, Hulo N, Isenegger L, Weger BD, Migliavacca E, Charpagne A, Betts JA, Walhin J-P, Templeman I, Stokes K, Thompson D, Tsintzas K, Robert M, Howald C, Riezman H, Feige JN, Karagounis LG, Johnston JD, Dermitzakis ET, Gachon F, Lefai E, and Dibner C (2018) Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. eLife 7:e34114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L, Loizides-Mangold U, Skarupelova S, Pulimeno P, Chanon S, Robert M, Bouzakri K, Modoux C, Roux-Lombard P, Vidal H, Lefai E, and Dibner C (2015) Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol. Metab 4:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorz V, Helfrich-Förster C, and Oster H (2018) The role of the circadian clock system in physiology. Pflugers Arch 470:227–239. [DOI] [PubMed] [Google Scholar]
- Poole DC, Copp SW, Colburn TD, Craig JC, Allen DL, Sturek M, O’Leary DS, Zucker IH, and Musch TI (2020) Guidelines for animal exercise and training protocols for cardiovascular studies. Am. J. Physiol. Heart Circ. Physiol 318:H1100–H1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Zakany J, Duboule D, Albrecht U, and Schibler U (2002) The Orphan Nuclear Receptor REV-ERB␣ Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell 110:251–260. [DOI] [PubMed] [Google Scholar]
- Qian J, Dalla Man C, Morris CJ, Cobelli C, and Scheer FAJL (2018) Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes. Metab 20:2481–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, and Gumz ML (2013) Mechanism of the circadian clock in physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol 304:R1053–R1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Bala C, Craciun CI, Rusu A, and Craciun AE (2016) The correlation of dawn phenomenon with glycemic variability parameters in type 2 diabetes mellitus. Rev. Rom. Med. Lab 24:55–64. [Google Scholar]
- Rubio-Sastre P, Scheer FAJL, Gómez-Abellán P, Madrid JA, and Garaulet M (2014) Acute Melatonin Administration in Humans Impairs Glucose Tolerance in Both the Morning and Evening. Sleep 37:1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Hattori Y, Ikeda Y, Kamagata M, Iwami S, Yasuda S, Tahara Y, and Shibata S (2016) Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci. Rep 6:27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Basse AL, Schönke M, Chen S, Samad M, Altıntaş A, Laker RC, Dalbram E, Barrès R, Baldi P, Treebak JT, Zierath JR, and Sassone-Corsi P (2019) Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab 30:92–110.e114. [DOI] [PubMed] [Google Scholar]
- Savikj M, Gabriel BM, Alm PS, Smith J, Caidahl K, Björnholm M, Fritz T, Krook A, Zierath JR, and Wallberg-Henriksson H (2019) Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia 62:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FAJL, Hilton MF, Mantzoros CS, and Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci 106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt K, Grimm A, Dallmann R, Oettinghaus B, Restelli LM, Witzig M, Ishihara N, Mihara K, Ripperger JA, Albrecht U, Frank S, Brown SA, and Eckert A (2018) Circadian Control of DRP1 Activity Regulates Mitochondrial Dynamics and Bioenergetics. Cell Metab 27:657–666.e655. [DOI] [PubMed] [Google Scholar]
- Schroder EA, Harfmann BD, Zhang X, Srikuea R, England JH, Hodge BA, Wen Y, Riley LA, Yu Q, Christie A, Smith JD, Seward T, Wolf Horrell EM, Mula J, Peterson CA, Butterfield TA, and Esser KA (2015) Intrinsic muscle clock is necessary for musculoskeletal health: The molecular clock in skeletal muscle. J. Physiol 593:5387–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedliak M, Zeman M, Buzgó G, Cvecka J, Hamar D, Laczo E, Okuliarova M, Vanderka M, Kampmiller T, Häkkinen K, Ahtiainen JP, Hulmi JJ, Nilsen TS, Wiig H, and Raastad T (2018) Morphological, molecular and hormonal adaptations to early morning versus afternoon resistance training. Chronobiol. Int 35:450–464. [DOI] [PubMed] [Google Scholar]
- Simon SL, McWhirter L, Diniz Behn C, Bubar KM, Kaar JL, Pyle L, Rahat H, Garcia-Reyes Y, Carreau A-M, Wright KP, Nadeau KJ, and Cree-Green M (2019) Morning Circadian Misalignment Is Associated With Insulin Resistance in Girls With Obesity and Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab 104:3525–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small L, Altıntaş A, Laker RC, Ehrlich A, Pattamaprapanont P, Villarroel J, Pillon NJ, Zierath JR, and Barrès R (2020) Contraction influences Per2 gene expression in skeletal muscle through a calcium-dependent pathway. J. Physiol 598:5739–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souissi N, Bessot N, Chamari K, Gauthier A, Sesboüé B, and Davenne D (2007) Effect of Time of Day on Aerobic Contribution to the 30-s Wingate Test Performance. Chronobiol. Int 24:739–748. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong H-K, Ko CH, and McDearmon EL (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet 9:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo SYM, Kanaley JA, Guelfi KJ, Marston KJ, and Fairchild TJ (2020) The Effect of Exercise Timing on Glycemic Control: A Randomized Clinical Trial. Med. Sci. Sports Exerc 52:323–334. [DOI] [PubMed] [Google Scholar]
- Thomas JM, Kern PA, Bush HM, McQuerry KJ, Black WS, Clasey JL, and Pendergast JS (2020) Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 5:e134270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyfault JP, and Bergouignan A (2020) Exercise and metabolic health: beyond skeletal muscle. Diabetologia 63:1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Moorsel D, Hansen J, Havekes B, Scheer FAJL, Jörgensen JA, Hoeks J, Schrauwen-Hinderling VB, Duez H, Lefebvre P, Schaper NC, Hesselink MKC, Staels B, and Schrauwen P (2016) Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol. Metab 5:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter C (2020) Circadian disruption: What do we actually mean? Eur. J. Neurosci 51:531–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Arias EB, Pataky MW, Goodyear LJ, and Cartee GD (2018) Postexercise improvement in glucose uptake occurs concomitant with greater γ3-AMPK activation and AS160 phosphorylation in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab 315:E859–E871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Armstrong MEG, Cairns BJ, Key TJ, and Travis RC (2011) Shift work and chronic disease: the epidemiological evidence. Occup. Med 61:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers J, van Moorsel D, Hansen J, Connell NJ, Havekes B, Hoeks J, van Marken Lichtenbelt WD, Duez H, Phielix E, Kalsbeek A, Boekschoten MV, Hooiveld GJ, Hesselink MKC, Kersten S, Staels B, Scheer FAJL, and Schrauwen P (2018) Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci 115:7789–7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff G, and Esser KA (2012) Scheduled Exercise Phase Shifts the Circadian Clock in Skeletal Muscle. Med. Sci. Sports Exerc 44:1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannielli PC, McKinley Brewer J, and Harrington ME (2002) Is novel wheel inhibition of Per1 and Per2 expression linked to phase shift occurrence? Neuroscience 112:9. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, and Takahashi JS (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci 101:5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt SD, Elliott JA, and Kripke DF (2019) Human circadian phase–response curves for exercise. J. Physiol 597:2253–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon AC, McDearmon EL, Salomonis N, Vranizan KM, Johansen KL, Adey D, Takahashi JS, Schambelan M, and Conklin BR (2003) Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, and Hogenesch JB (2014) A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci 111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Qi Y, Bi L, Shi W, Zhang Y, Zhao D, Hu S, Li M, and Li Q (2020) Effects of Exercise on Blood Glucose and Glycemic Variability in Type 2 Diabetic Patients with Dawn Phenomenon. Biomed Res. Int 2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


