Abstract
Critically ill patients undergo early impairment of their gut microbiota (GM) due to routine antibiotic therapies and other environmental factors leading to intestinal dysbiosis. The GM establishes connections with the rest of the human body along several axes representing critical inter-organ crosstalks that, once disrupted, play a major role in the pathophysiology of numerous diseases and their complications. Key players in this communication are GM metabolites such as short-chain fatty acids and bile acids, neurotransmitters, hormones, interleukins, and toxins. Intensivists juggle at the crossroad of multiple connections between the intestine and the rest of the body. Harnessing the GM in ICU could improve the management of several challenges, such as infections, traumatic brain injury, heart failure, kidney injury, and liver dysfunction. The study of molecular pathways affected by the GM in different clinical conditions is still at an early stage, and evidence in critically ill patients is lacking. This review aims to describe dysbiosis in critical illness and provide intensivists with a perspective on the potential as adjuvant strategies (e.g., nutrition, probiotics, prebiotics and synbiotics supplementation, adsorbent charcoal, beta-lactamase, and fecal microbiota transplantation) to modulate the GM in ICU patients and attempt to restore eubiosis.
Keywords: Microbiota, Microbiome, Intensive care, Dysbiosis, Probiotics, Prebiotics, Synbiotics, Fecal microbiota transplantation, Critical illness, Multidrug-resistant bacteria
Introduction
The total number of human cells is approximately 3.0·1013while the number of microorganisms inhabiting humans is approximately 3.8·1013 [1]. Collectively, they constitute the human microbiota, representing an organ itself [2].
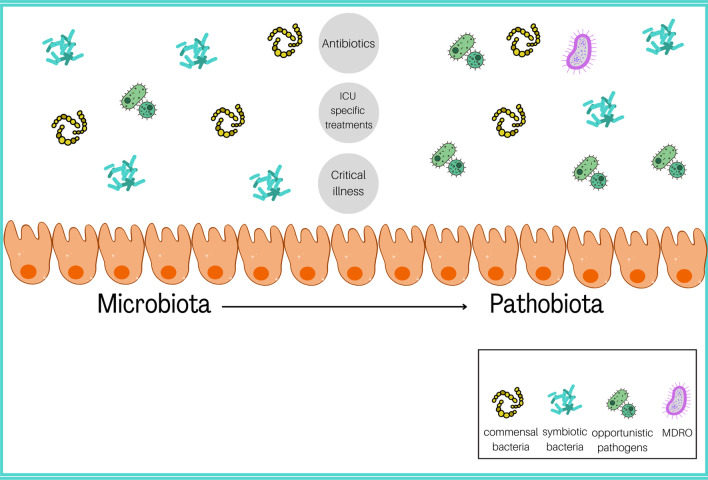
Most of the microbiota colonizes the gut establishing a symbiosis with their host [3]. The gut microbiota (GM) of a healthy subject harbors all three main life domains: bacteria, archaea, and eukarya. The bacterial domain is the most represented [4]. There are six known bacterial phyla [5]. Firmicutes and Bacteroidetes are the most abundant, followed by Actinobacteria and Proteobacteria [6, 7]. The GM composition varies among individuals, changing throughout life due to intrinsic factors like age and genetics and extrinsic modifiable factors like diet [8], environment, and drug use [9]. In healthy individuals, the GM has prerogative functions, including enterocyte renewal modulation, metabolic and antimicrobic actions, and systemic activities such as improvement of glucose sensitivity [10], reduction of systemic inflammation, and even longevity [11]. Illness and drugs can disrupt the GM balance. In the intensive care unit (ICU), patients are subjected to antibiotics, gastrointestinal transit changes, nutritional changes, and sepsis [4], collectively leading to a GM imbalance, namely dysbiosis, whose most common symptom is diarrhea [12]. Ninety percent of the intestinal microflora is lost within 6 h of ICU admission [13]. ICU patients have lower bacterial diversity and variability, and opportunistic pathogens are enriched over symbiotics [14–16]. Most opportunistic pathogens are Gram-negative aerobic proteobacteria such as Enterobacteriaceae and Gram-positive bacteria such as Staphylococcus spp. and Enterococcus spp. [14]. This imbalance can lead to Candida albicans overgrowth in critically ill patients [2, 14, 15] while beneficial species such as Ruminococcus spp., Pseudobutyrivibrio spp., and Faecalibacterium prausnitzii become less represented [17, 18]. For these reasons, the GM in critically ill patients is defined as "pathobiota" (Fig. 1) [19]. The GM is also the main reservoir for "multidrug-resistant" bacteria (MDROs): Initially, these bacteria are counteracted by the resident beneficial bacteria, but then, as antibiotics eradicate them, MDROs take over and may promote infections during hospitalization [reviewed in [20]].
Fig. 1.
P1:The shift from microbiota to pathobiota in the ICU is driven by antibiotics and ICU-specific treatments like artificial feeding, mechanical ventilation, proton pump inhibitors, vasopressors, and opioids
In critically ill patients, dysbiosis could be considered an organ dysfunction [21]. This review aims to describe this impairment and provide intensivists with a perspective on the currently available strategies to modulate the GM in ICU patients.
The gut microbiota and the host
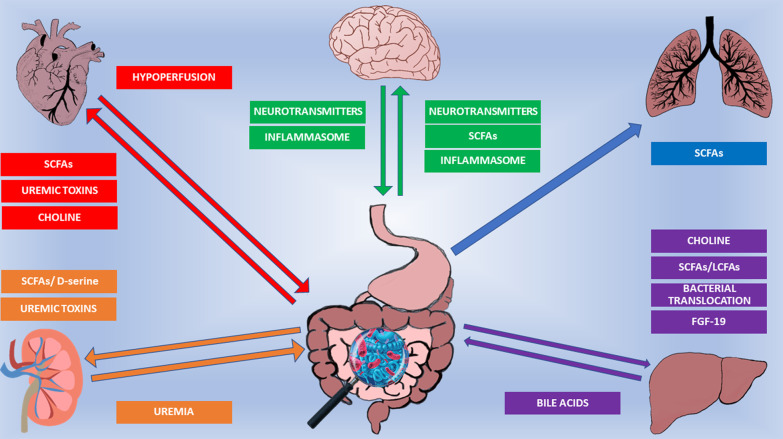
The GM interacts with its host (Fig. 2), and scientists are just beginning to characterize GM composition and function in health and disease. This paragraph describes the GM functions and how it interacts with the body, focusing on critically ill patients.
Fig. 2.
The GM interacts with its host along several axes. The GM is at the crossroad of multiple arrows representing molecular pathways involved in axes’communication. Further details in the main text. SCFAs: Short-Chain Fatty Acids. LCFAs: long chain fatty acids. FGF-19: Fibroblast growth factor 19
The gut–brain axis
The blood–brain barrier (BBB) has always been considered molecule impermeable. However, immune cells, neurotransmitters, and some gut bacterial metabolites (SCFAs, vitamins, bile acids) can pass through it [reviewed in [22]]. These molecules affect memory, learning, behavior, and locomotion. Unbalanced molecular communication contributes to neurodegenerative and neuropsychiatric diseases [23], traumatic brain injury (TBI) [24], and sepsis-correlated brain impairment [25, 26]. The gut–brain axis (GBA) bidirectionally connects the central nervous system with the enteric one. It goes beyond a mere anatomical network and includes immune, endocrine, metabolic, and humoral communication routes. The GBA was first described in the early 2000s when antibiotic-treated germ-free (GF) or specific pathogen-free mice developed neurological problems [27]. The GBA links brain’s emotional and cognitive centers with the gut [28]. Lactobacillus, Bifidobacteria, Enterococcus, and Streptococcus produce acetylcholine,γ-aminobutyric acid (GABA), and serotonin [29]. Serotonin impacts brain functions, heart, bowel motility, bladder control, and platelet aggregation. Serotonin-based treatments in psychiatry and neurology regulate sleep, mood, and behavior [30]. Ninety-five percent of serotonin is produced from tryptophan in the gut by microbes [31, 32]. The GM also produces SCFAs butyrate, acetate, and propionate by fiber fermentation [22]. SCFAs contribute to maintaining the gut and BBB physiology. GF mice have increased gut and BBB permeability, and supplementation with the SCFAs-producing Clostridium tyrobutyricum restores both gut and brain homeostasis [33]. SCFAs influence the production of glutamate, glutamine, GABA, and neurotrophic factors. Propionate and butyrate modulate the expression of serotonin- and catecholamine-synthesizing enzymes and regulate intracellular potassium levels [reviewed in [34]].
Dysbiosis in the GBA is linked to neuroinflammation through the creation of the inflammasome, a biological complex of innate immune system multiprotein oligomers that activates inflammatory responses. Activated by pathogens or stress signals, the inflammasome assembles, producing pro-inflammatory cytokines [e.g.,interleukins (IL)-1 and IL-18] implicated in neuroimmunomodulation, neuroinflammation, neurodegeneration, and pyroptosis [35]. GM and inflammasome are strongly connected. Indeed, the inflammasome binds pathogen-associated and/or danger-associated molecular patterns, specific molecular motifs carried by gut microorganisms. Thus, a perturbation of the GM composition could overstimulate the inflammasome and compromise the GBA homeostasis, promoting neuroinflammation as seen in multiple sclerosis [36], Alzheimer's disease [37], Parkinson's disease [38], neuropsychiatric disorders [39], and sepsis [25, 26]. While no human studies are available, a recent preclinical study has shown how the GM plays a part in sepsis-associated encephalopathy by improving neurological outcomes when indole-3-propionic acid, a microbial neuroprotective metabolite, is produced, leading to the inhibition of NLRP3 inflammasome activation and IL-1β secretion in the microglia [26]. Similar preclinical results have been found in model of sepsis-induced cognitive decline [25], emphasizing the link between GM dysbiosis and the brain.
TBI is another condition compromising the GBA. Preliminary evidence on non-ICU patients shows that primary brain damage compromises the vagal nuclei and tractus solitarius nucleus [40], possibly leading to dysautonomia in the gastrointestinal tract and leaky-gut occurrence, which in turn affects BBB's permeability, reduces intestinal motility and leads to immune system dysregulation, as shown in a rat study [41].
The gut–heart axis
The gut–heart axis is bidirectional. Beneficial microbial metabolites like SCFAs and uremic toxins (UT) wire this connection [42, 43]. Dysbiosis and, consequently, downregulated production of SCFAs concomitant to a high level of UT negatively affect the gut–heart axis. In this context, lower SCFAs and higher UT production have been observed. This is accompanied by increased absorption of lipopolysaccharide and endotoxin due to epithelial dysfunction, which ultimately triggers the systemic inflammatory response. This sequence of events facilitates the development of atherosclerosis and heart failure (HF) (reviewed in [44]). Conversely, it has been shown that HF causes dysbiosis, which promotes intestinal barrier damage, impairs nutrient absorption, and primes a vicious cycle leading to harmful microbial product translocation into systemic circulation, further aggravating HF [45–47]. Indoxyl sulfate (IS) and p-cresyl sulfate (PCS), UT derived from tryptophan and tyrosine fermentation, play a key role in this context. They promote fibrosis in klotho deficient and wild-type mice, negatively affecting heart and kidney function [48, 49]. Moreover, GF mice have less angiotensin II-induced hypertension and cardiac fibrosis than conventionally raised mice [50]. Observational data in patients with HF show decreased intestinal blood flow [46] and edema in the terminal ileum and colon [45], which lead to a change in the GM composition, with enrichment of Campylobacter, Shigella, and Salmonella [47]. Several HF therapeutic strategies targeting the GM, such as probiotic supplementation and diets, are being preclinically and clinically tested. In a rat ischemia–reperfusion model, probiotics administration reduces myocardial infarct size and remodeling [51].In a pilot human trial, Saccharomyces boulardii supplementation in HF patients improved left ventricular fraction and decreased left atrium diameter[52]. However, data are controversial so far, possibly because not all subjects included in these studies present with different grades of dysbiosis. The gutHeart trial studying GM manipulation to treat HF did not confirm the previous findings, possibly due to the lack of substantial dysbiosis in the enrolled patients[53].
A high-fiber diet prevents hypertension and cardiac hypertrophy in hypertensive mice by reshaping the intestinal microbial community GM and increasing the abundance of acetate-producing bacteria [54]. Similarly, a plant-based diet rich in complex carbohydrates and fibers while low in fat reduces HF events in both men [55] and women [56], improving arterial compliance, exercise endurance, and quality of life [57]. Choline is another soluble nutrient linked to HF. HF is worse in wild-type mice fed choline-supplemented diets [58]. Certain gut bacteria, such as Enterobacteriaceae[59], can metabolize choline to trimethylamine (TMA). TMA should remain in the intestine; however, in a condition of leaky-gut secondary to other pathological conditions, TMA can translocate to the liver, where it is converted to trimethylamineNoxide (TMAO), causing liver [60, 61] and heart damage [58]. Dysbiosis also promotes atherosclerosis. In fact, GF ApoE-deficient mice fed a low-cholesterol diet have more atherosclerosis plaques than conventionally raised mice [62]. However, when administered with Akkermansia muciniphila, which prevents endotoxemia-induced inflammation, they present with smaller atherosclerotic plaques [63].
As the emerging data show the contribution of the GM in the onset or progression of heart diseases and preclinical studies start to lay the foundation of interventions, data on the potential of these strategies in critically ill patients are lacking.
The gut–lung axis
Evidence suggests that the lung and gut also communicate with each other, and complex pathways between their cognate microbiota strengthen the gut–lung axis (GLA). Neonatal gut dysbiosis with reduced Bifidobacteria, Akkermansia, and Faecalibacteria may cause CD4+ T cell dysfunction associated with childhood atopy and asthma [64]. IBD patients carry a higher risk of pulmonary diseases. In fact, IBD patients commonly have small and large airway dysfunction, obstructive and interstitial pulmonary diseases [65, 66] and, even when asymptomatic, may display early respiratory symptoms that could lead to bronchiectasis, mosaic perfusion, and air trapping, indicating obliterative bronchiolitis and centrilobular nodules and bronchial linear opacities bronchiolitis or bronchiectasis with mucoid secretion [67, 68]. Early after colectomy, several IBD patients developed respiratory symptoms. No bacterial pathogens were identified in these patients' sputum cultures, and corticosteroid therapy was required, suggesting a pulmonary impairment due to inflammation and not infection [69].
SCFAs may act as anti-inflammatory molecules and barrier keepers in the respiratory tract [70]. Mice administered with high-fiber diets display high intestinal SCFAs levels [71]. No SCFAs were found in lung tissue, but their levels promoted dendritic cell hematopoiesis and activated T helper 2 effector cells in the airways, establishing a de facto gut–bone marrow-lung axis protecting mice from allergic lung inflammation [71]. Wild-type mice depleted of GM with antibiotics, subsequently intranasally infected with pneumococcal pneumonia and then subjected to fecal microbiotal transplantation have an enhanced primary alveolar macrophage function thanks to a GM beneficial reshape [72].
The GLA could be a route for gut-to-lung bacterial migration. In a recent case–control study, Klebsiella and Enterococcus physically translocated to the bloodstream and pulmonary system, causing sepsis with disruption of GM diversity and enrichment of antibiotic-resistant bacteria, which led to secondary bloodstream and abdominal infections [73]. Acute respiratory distress syndrome(ARDS) could also be linked to GM dysbiosis as ARDS patients present enrichment of Enterobacteriaceae in the gut and lung while displaying lower bacterial diversity [74].
The gut–kidney axis
A dysbalanced GM has also been associated with kidney diseases, including acute kidney injury (AKI) [reviewed in [75]] and chronic kidney disease (CKD) [76]. UT, SCFAs, and D-serine establish the so-called gut–kidney axis. Abnormal levels of IS, PCS, and TMA contribute to the onset of renal tubular cell dysfunction, pruritus, fatigue, neurological damage, coagulation and endothelial dysfunction, mineral bone disorder, cardiovascular impairment, and insulin resistance, especially in patients affected by chronic kidney disease (CKD) [77]. Renal failure leads to higher urea concentration in both blood and intestine. This cause an overgrowth of intestinal bacteria owning urease activity, converting urea to ammonia [78], leading to dysbiosis. Patients with CKD and end-stage renal disease (ESRD) subjected to hemodialysis display enrichment of the genus Faecalibacterium and the families Bifidobacteriaceae and Prevotellaceae. Conversely, in ESRD patients subjected to peritoneal dialysis, the Escherichiagenusand Enterobacteriaceae-Enterococcaceae families are predominant [79].UT also accumulate in AKI, but the underlying cause of this association remains uncovered [80].
Kidneys also express SCFAs receptors[81]. The olfactory receptor 78 (Olfr78) is a renal SCFA receptor, which promotes renin secretion. In experimental murine AKI models, butyrate decreases the production of reactive oxygen species and several pro-inflammatory cytokines [74] and exerts direct epigenetic activities by inhibiting histone deacetylases involved in the progression of glomerulopathies [82]. Renal Toll-like receptor-4mRNA levels are lower in mice supplemented with SCFAs, and inflammation is tampered [83]. Furthermore, Bifidobacterium bifidum BGN4 supplementation in mice previously subjected to bilateral renal ischemia–reperfusion injury attenuates AKI severity by modulating the inflammatory-immune response [84].
Free D-aminoacids are produced by bacteria in mice [85] and play a role in the physiology of several organs, including the kidneys. In fact, AKI-associated dysbiosis in mice leads to a lower D-serine associated with kidney injury aggravation [85]. Oral administration of D-serine alleviated renal damage pointing to D-serine’s renoprotective properties [85]. In the same study, authors have also shown a direct correlation between impaired kidney function in AKI patients and D-serine levels [83]. Despite the lack of mechanism of this association, these data suggest that D-serine levels could be a biomarker for AKI patients.
The gut–liver axis
The portal vein, biliary tree, and systemic circulation connect the gut and liver. The liver interacts with the intestine by producing bile acids (BAs)[86], while the intestine interacts with the liver by metabolizing BAs, aminoacids, and exogenous components like alcohol and choline [87]. Dietary molecules, xenobiotics, free fatty acids, choline, and ethanol metabolites in the bloodstream reinforce this crosstalk [88]. Several signaling cues go through GM metabolism. In fact, gut dysbiosis contributes to several gut–liver conditions. Enterococcus faecalis contributes to alcoholic liver disease (ALD) [89], Klebsiella pneumonia to non-alcoholic steatohepatitis [90], and Enterococcus gallinarum to autoimmune hepatitis [91] pathogenesis.
BAs aid in the absorption of dietary fats and fat-soluble vitamins in the small intestine [92, 93] and act as signaling molecules by binding to the farnesoid X receptor (FXR) and G proteincoupled bile acid receptor 1 [94]. This triggers molecular events impacting their own hepatic synthesis(via the action of the FXR-FGF19 duo) and glucose and lipid homeostasis [94]. As BAs and GM communicate with each other, disrupting this delicate balance can lead to intestinal barrier impairment, inflammation, and even contribute to cancer onset and development. A damaged intestinal barrier allows bacteria to enter the portal vein and reach the liver, exacerbating liver [95] and intestinal diseases [96]. Akkermansia muciniphila, a gram-negative anaerobe colonizing intestinal mucus, is decreased in liver damage [97]. In this respect, bile duct ligated mice in which BA-FXR physiology is restored via the administration of a semisynthetic BA and FXR ligand, display a restored gut barrier integrity and Akkermansia muciniphila enrichment [96]. Loss of barrier caused by pathogenic bacteria disrupting epithelial integrity can lead to sepsis by triggering systemic inflammation (reviewed in [98]), and, in turn, sepsis increases the risk of liver damage [99]. Intriguingly, these studies suggest that sepsis-induced liver damage could potentially be prevented by harnessing the GM in ICU septic patients.
SCFAs and LCFAs are gut–liver messengers. Studies in rodents have shown that reducing SCFAs increases intestinal permeability [100] while reducing LCFAs decreases luminal Lactobacilli [101]. Supplementation with Lactobacillus spp. probiotics in animal models improves intestinal barrier integrity, inhibits colonization of infectious bacteria like Clostridium difficile and Clostridium perfringens, Campylobacter jejuni, Salmonella Enteritidis, Escherichia coli, Staphylococcus aureus, and Yersinia, and eases intestinal inflammation [102, 103].
Choline is metabolized into lecithin, contributing to VLDL hepatic excretion. Low choline may cause hepatic steatosis [104–106]. Lecithin levels depend on the host's choline-to-lecithin conversion. Any impairment in enterocyte metabolism could harm the liver by reducing lecithin supply. Personalized nutrition could slow the choline-TMAO pathway to prevent liver damage [60, 61, 107, 108].
Acute (caused by toxins or infections without underlying liver disease) or chronic (caused by pre-existing liver disease) liver dysfunction is common in ICU [reviewed in [109]]. No study has explored preventing or treating liver dysfunction in the ICU using GM modulators. Previous research on the adjuvant benefits of probiotics/prebiotics/symbiotics, fecal microbiota transplantation (FMT), and bacteriophages in non-alcoholic fatty liver disease [110, 111] and ALD [89, 112] could lay the groundwork for their future employment in other clinical contexts.
Harnessing the microbiota in critically ill patients
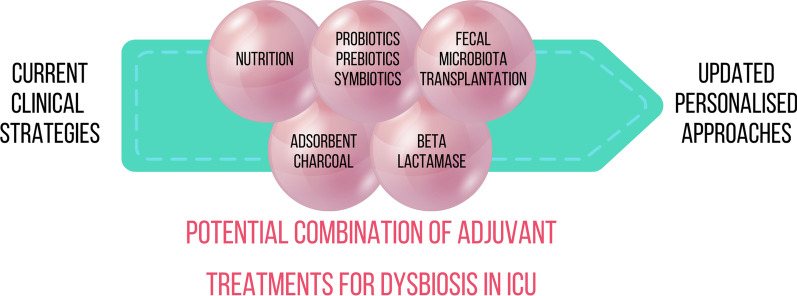
Despite the emerging data, there is a lack of human studies, especially on critically ill patients, and the available data should be expanded and validated in clinical trials. Moreover, a further effort to address biomarker discovery should be made in this field, as indicated by the potential shown by TMAO and D-serine. GM manipulation could be used as an adjuvant strategy in some comorbidities rising in critically ill patients. An overview of the current preclinical and clinical trials with grade of evidence with potential application to the ICU is provided in Table 1. Moreover, we propose a potential strategy (Fig. 3), where nutrition is the first and easiest step in managing dysbiosis in ICU patients, followed by probiotics, prebiotics, synbiotics, beta-lactamase, absorbent charcoal administration, and, finally, FMT. These treatments require validation with a high level of evidence before they can be routinely applied in ICU. They must be used with extreme caution aiming for a tailored approach to the patient with ideal efficacy and safety.
Table 1.
Overview of the current preclinical and clinical trials with grade of evidence with potential application to the ICU
| Interventions | Potential ICU applications | Grade of evidence |
|---|---|---|
| Nutritional approach (EN) |
Promote GM eubiosis Reduce mortality/morbidity Reduce infectious complications |
Very weak [113] |
| Adsorbent Charcoal | Prevention of RCDI |
Very weak Pre clinical studies [114] One phase 2 trial completed[115] |
| Probiotics Prebiotics Synbiotics | Reduce infection rate, notably VAP |
RCTs in ICU with low quality of evidence |
| Coadjuvant in sepsis |
Preclinical studies |
|
| Coadjuvant in SARS-CoV-2 infection |
Weak |
|
| Coadjuvant in TBI |
Very weak [127] Preclinical studies |
|
| Prevention of RCDI | Very weak [128] | |
| Beta-Lactamase | Prevention of RCDI |
Very weak Phase 2 trials completed [129] One phase 1b/2a trial ongoing [130] |
| FMT | Treatment of RCDI |
One RCT [133] |
| Coadjuvant in TBI |
Very weak [138] Pre clinical studies |
|
| Coadjuvant in sepsis |
Case reports and pre clinical studies |
Evidence reported in ICU or critically ill settings have been specified
ICU intensive care unit, VAP ventilator associated pneumonia, TBI traumatic brain injury, RCDI recurrent clostridioides difficile infection, FMT fecal microbiota transplantation
Fig. 3.
Potential personalized approach for treating dysbiosis in ICU patients
Nutrition
Different dietary patterns can modulate the GM in different ways. Quantity, quality, fiber content, and feeding patterns [141, 142] affect GM abundance and diversity [143]. ICU patients may undergo fasting or limited nutrition [144], resulting in dysbiosis [145]and compromised bacterial metabolite levels [4]. Nutrition in critical illness is a complex topic, and evidence is limited. Nevertheless, it has been observed that enteral nutrition (EN) benefits the GM more than parenteral nutrition (PN). PN raises Bacteroidetes levels and intestinal permeability in wild-type mice [146], while these effects are absent with EN [146]. GM is affected by the macronutrient-to-fiber ratio in EN formulas. A high protein and animal fat load will enrich GM Bacteroides, while carbohydrate-rich diets increase Prevotella strains [147]. Low fiber intake impacts gut epithelium integrity, mucus layer thickness, and the enrichment of pathogenic strains [148]. EN should be preferred over PN to preserve intestinal barrier integrity, prevent villi atrophy, and promote eubiosis, ultimately leading to lower mortality and morbidity [149] and fewer infectious complications [reviewed in [113]]. Anyhow, caution should be taken in formula selection. It has been observed that mice fed dietary emulsifiers show an imbalanced GM linked to colitis and metabolic syndrome [150]. Since EN formulas include preservatives and emulsifiers, like soy lecithin and glycerol derivates [151], they could potentially harm ICU patients.
Probiotics, prebiotics, synbiotics
Probiotics
Probiotics are live microorganisms that provide a health benefit to the host when supplemented in sufficient quantities [152]. Probiotics promote eubiosis, reduce gut cell apoptosis, and support the immune system [153–155].
A meta-analysis analyzing several trials in about 2.900 critically ill patients on probiotic use in the ICU reported that strains such as Saccharomyces boulardii, Lactobacillus spp., and Bifobacterium spp. are associated with a reduction in infections, particularly in patients with ventilator-associated pneumonia (VAP) and treated with antibiotics, but not to increased survival [118]. Conversely, a more recent study did not confirm the beneficial role of Lactobacillus rhamnosus GG in reducing VAP incidence in ICU patients [156]. Another meta-analysis, which analyzed 4893 patients, has shown that probiotics reduce VAP, ICU length of stay, and duration of antibiotic therapy; however, the high variability in treatments and type of patients prevents the introduction of the use of probiotics as VAP prophylaxis [116]. Other positive effects of probiotics in specific patient subgroups, such as polytrauma patients, include improvement in clinical conditions, less use of vasopressors, reduction in the "sequential organ failure assessment" (SOFA) score, and a shorter ICU stay [157]. In particular, four probiotic preparations in polytrauma patients under mechanical ventilation lowered the incidence of VAP from 23.8% in the placebo group to 11.9% in the interventional group [157].
Three more meta-analyses analyzing the use of probiotics in critically ill patients to prevent VAP or mortality and ICU-acquired infections were recently published [117, 158, 159]. Consistently, authors concluded that probiotics administration is safe and beneficial and leads to a decrease in the incidence of ICU infections, notably VAP, whose prevention was the most effective in trauma patients. Conversely, the largest network meta-analysis, including 8339 patients from 31 RCTs, advocates that the safety of probiotics should be further studied, especially in critically ill patients, as in some cases, high dosages have been linked to an increase in infection complications such as sepsis, pneumonia, abscesses, and endocarditis due to bacteremia and fungemia [117].
Other potential indications for probiotics in ICU include sepsis, TBI, and, most recently, SARS-CoV-2 infection. Since 2007, the gut has been conceptualized as a "motor" that, when impaired, could drive systemic inflammation and multiple organ failure [160]. Experimental evidence point to Faecalibacterium prausnitzii as a probiotic strain potentially blunting systemic inflammation during sepsis as it produces an anti-inflammatory peptide that can counteract chemically induced colitis in mice [119]. Moreover, daily oral intake of Lactobacillus rhamnosus GG and Bifidobacterium longum has been shown to reduce mortality and improve intestinal epithelial homeostasis in a murine model of septic peritonitis [120]. Probiotics could also be a valid asset in TBI, as shown in mice subjected to traumatic spinal cord injury and supplemented with VSL#3, a mixture of eight bacterial strains [161]. This intervention supported mice's immune response in the gut and better locomotor recovery, improving post-injury outcomes [127].
The SARS-CoV-2 pandemic has impacted every field of medicine, including intensive care, where each virus variant has differently affected critically ill patients [162]. The role of probiotics in infected patients in intensive wards has not been systematically explored. However, their supplementation could influence the host's immune response [163]. Clinical trials studying the effect of probiotic supplementation in SARS-CoV-2 patients are ongoing or have recently been completed [121–126]. Emerging evidence points to the modulation of the immune function and reduction of secondary infections achieved by probiotics supplementation [164].
Next-generation probiotics are in development. SER-109 is obtained by transplanting fecal microbiota with alcohol-triggered massive sporulation. This new probiotic reduces the risk of recurrent Clostridium Difficile infection (RCDI) in patients treated with antibiotics per guidelines [128].
Prebiotics and synbiotics
Prebiotics are specific nutrients for intestinal bacteria, while synbiotics combine prebiotics and probiotics. They can both be administered to modulate the GM [2]. A meta-analysis has shown that there is no difference in the incidence of infections in ICU patients between using probiotics alone or synbiotics [118]. Conversely, in VAP, synbiotics supplementation seems to produce a more significant benefit, like reducing infection rates, than probiotic supplementation alone [116, 117].
Despite the emerging evidence on harnessing the GM through the use of nutrition, pro-, pre-, and symbiotics, the fine mechanisms describing the published observation are still lacking, and this complicates the translation from bench to bedside. Moreover, caution should be taken. Studies on safety are imperative as these strategies do not come without pitfalls, as demonstrated by a recent retrospective study showing a correlation between probiotic administration and an increase in probiotic-associated central line infections leading to increased mortality [165].
Beta-lactamase
GM changes in ICU patients are mostly due to broad-spectrum antibiotics, but not all induce dysbiosis [2]. Antibiotic stewardship includes switching from broad-spectrum molecules to a narrower spectrum and shortening antibiotic therapy whenever possible [166]. The use of beta-lactamase in dogs treated with ampicillin lowered its jejunal concentration and prevented it from reaching the colon [167]. This approach could lessen antibiotics' negative effects on the colonic microbial community. Ribaxamase (formerly SYN-004), an orally administered beta-lactamase with IV penicillins and cephalosporin, has shown promising results in preventing dysbiosis in hospitalized patients with lower respiratory tract infections treated with ceftriaxone [129]. A phase-2b study has shown a RCDI risk reduction in the interventional group [129]. This reduction occurred with macrolide plus ceftriaxone or ceftriaxone alone [129]. Ribaxamase is also effective when administered with beta-lactamase inhibitors like tazobactam and sulbactam [168]. Moreover, a phase-1b/2a clinical trial study to assess the safety, tolerability, and efficacy of orally administered SYN-004 in adult patients undergoing allogeneic hematopoietic cell transplantation is also ongoing [130].
Adsorbent charcoal
The use of adsorbent charcoal is an additional approach to prevent antibiotic-induced dysbiosis. Dav-132 is a charcoal-based adsorbent that is actively studied [169]. It was developed for oncological patients for whom therapy is mandatory to prevent infections. Dav-132 is designed to become active in the ileum, cecum and colon before antibiotics could start impairing the GM [169, 170]. A phase-2 trial has confirmed that Dav-132 can be safely used in patients as it is well tolerated and promotes GM diversity by improving Clostridioides difficile colonization resistance [115]. Notably, a study on hamsters with moxifloxacin-induced Clostridioides difficile infection showed that a modified version of Dav-132 suitable for mice, namely Dav-131, could reduce mortality in a dose-dependent manner by lowering the fecal-free moxifloxacin concentration [114]. Therefore, despite lack of studies in critical illness, Dav-132 could potentially be an additional tool, together with probiotics administration, to reduce RCDI incidence and progression risks in ICU patients.
Fecal microbiota transplantation
FMT is a medical technique in which stools from a "healthy" donor are delivered to a dysbiotic patient to restore eubiosis [2]. An FMT sample contains all microorganisms that naturally harbor in the gut and all their associated metabolites. For this reason, this treatment may restore dysbiosis better than others [171, 172]. However, this procedure does not come without risks. In fact, undesired and/or undetected pathogens could also be delivered from the donor to the recipient subject, sometimes even with fatal complications. In a case report, two patients developed drug-resistant E. coli bacteremia following FMT, causing one death [173]. Therefore, extreme caution should be taken, especially in critically ill patients with a higher risk of infection. In 2017, a panel of experts from the European Consensus Conference drafted FMT recommendations [132]. To date, the main indication for FMT is recurrent RCDI infection, where efficacy is superior to antibiotics [132, 174].
However, its application is currently being trialed in other conditions and could potentially be studied in critically ill patients [175], particularly to eradicate intestinal MDRO burden [176] and manage ICU common illnesses such as TBI [138], sepsis and multi-organ failure (MOF) [177].
A group of patients affected by RCDI with dysbiosis and severe gastrointestinal symptoms improved significantly after FMT, possibly via increased resistance MDROs’ gut colonization [176]. Intriguingly, FMT for sepsis has also been successfully used in patients with septic shock of undetermined cause with profuse diarrhea or diarrhea associated with antibiotic administration [139, 140]. FMT has been shown to promote eubiosis in a mouse model in which sepsis was induced post-administration of stool collected from a septic patient [177].
FMT has also been studied in TBI, which causes autonomic dysregulation, impaired BBB integrity, intestinal mucosal impairment, and brain immunity dysregulation. A recent study evaluated the effect of FMT following TBI in rats with a controlled cortical impact model, showing that this strategy could effectively restore eubiosis and resolve neurological deficits [138].
The emerging literature represents a great starting point for the exploitation of FMT in different ICU conditions and complications. Although European guidelines to regulate the use of FMT are in place, targeted RCTs should be initiated to explore the safety and efficacy of this procedure in the ICU without putting patients at risk of post-antibiotic discontinuation, a key step in FMT protocols. Rehorová et al. proposed an experimental standardized operating procedure for FMT in critically ill patients using a two-month-quarantined frozen multi-donor transplant administered by enema (seven donors, 50 ml from each donor to make a final graft of 350 ml). FMT should be done 48 h after the last antibiotic to maximize engraftment. Due to safety concerns, new studies should only include hemodynamically stable patients without perforated viscous or immunoparalysis [12].
Conclusions
Recent evidence has shown that critically ill patients display a changed GM, known as pathobiota. The pathobiota composition is one of the leading causes of clinical complications. Metagenomic and meta-metabolomic studies are growing and dissecting mechanisms leading to dysbiosis in different ICU conditions. Harnessing the GM in ICU patients is intriguing, and this review summarizes the currently available results and outlines potential strategies for critically ill patients. Targeting intestinal bacteria has the potential to preserve or restore barrier integrity, which would then prime a beneficial cascade on the organ dysfunctions arising in ICU patients. New studies designed for critically ill patients should reinforce the current evidence, such as preventing VAP by probiotics, treating RCDI and MDRO colonization by FMT, preventing and resolving dysbiosis by personalized nutrition and antibiotic “damage control” tools, and have the potential to uncover critical biomarkers, stratify patients according to infection risk, immune response and inflammation status, and identify combination therapy/adjuvant responders vs. non-responders. Intensivists juggle the multiple connections between the intestine and the rest of the body. Unraveling this communication could transform them into modern "enterointensivists" at the front line of critical care management, placing the intestine at the center of critically ill patients as a "jack of all trades" card.
Acknowledgements
In memory of Nicola Brienza, esteemed professor, researcher, doctor, mentor, friend and father, who had understood the central role of the gut in ICU.
Abbreviations
- ALD
Alcoholic liver disease
- AKI
Acute kidney injury
- ARDS
Acute respiratory distress syndrome
- BAs
Bile acids
- BBB
Blood–brain barrier
- CKD
Chronic kidney disease
- COVID-19
The coronavirus disease 2019
- EN
Enteral nutrition
- ESRD
End-stage renal disease
- FGF-19
Fibroblast growth factor 19
- FMT
Fecal microbiota transplantation
- FXR
Farnesoid X receptor
- GBA
Gut–brain axis
- GM
Gut microbiota
- GF
Germ-free
- HF
Heart failure
- IBD
Intestinal bowel disease
- ICU
Intensive care unit
- IS
Indoxyl sulfate
- LCFAs
Long-chain fatty acids
- MDROs
Multidrug resistant bacteria
- PCS
P-cresyl sulfate
- PN
Parenteral nutrition
- RCDI
Recurrent clostridioides difficile infection
- SCFAs
Short-chain fatty acids
- SOFAscore
Sequential organ failure assessment score
- TBI
Traumatic brain injury
- TMA
Trimethylamine
- TMAO
TrimethylamineNoxide
- UT
Uremic toxins
- VAP
Ventilator-associated pneumonia
Author contributions
A.C. and N.B. wrote the first draft of the manuscript which was critically revised by R.M.G, A.M., F.I. and F.P.; A.C. and R.M.G. prepared the figures. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Passed away on September 29th 2022. Rest in peace Professor
Contributor Information
Alberto Corriero, Email: alberto.corriero@gmail.com.
Raffaella Maria Gadaleta, Email: raffaella.gadaleta@uniba.it.
Filomena Puntillo, Email: filomena.puntillo@uniba.it.
Francesco Inchingolo, Email: francesco.inchingolo@uniba.it.
Antonio Moschetta, Email: antonio.moschetta@uniba.it.
Nicola Brienza, Email: nicola.brienza@uniba.it.
References
- 1.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szychowiak P, Villageois-Tran K, Patrier J, Timsit JF, Ruppé É. The role of the microbiota in the management of intensive care patients. Ann Intensive Care. 2022;12(1):3. doi: 10.1186/s13613-021-00976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70(Suppl 1):S38–44. doi: 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff NS, Hugenholtz F, Wiersinga WJ. The emerging role of the microbiota in the ICU. Crit Care. 2018;22(1):78. doi: 10.1186/s13054-018-1999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, et al. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11(10):2393. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38(5):996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inchingolo AD, Malcangi G, Inchingolo AM, Piras F, Settanni V, Garofoli G, et al. Benefits and implications of resveratrol supplementation on microbiota modulations: a systematic review of the literature. Int J Mol Sci. 2022;23(7):4027. doi: 10.3390/ijms23074027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stedtfeld RD, Chai B, Crawford RB, Stedtfeld TM, Williams MR, Xiangwen S, et al. Modulatory Influence of Segmented Filamentous Bacteria on Transcriptomic Response of Gnotobiotic Mice Exposed to TCDD. Front Microbiol [Internet]. 2017 [cited 2022 Oct 24];8. Available from: https://www.frontiersin.org/articles//10.3389/fmicb.2017.01708 [DOI] [PMC free article] [PubMed]
- 10.Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Krämer M, et al. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020;32(3):379–390.e3. doi: 10.1016/j.cmet.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Yang Y, Su J, Zheng X, Wang C, Chen S, et al. Age-related compositional changes and correlations of gut microbiome, serum metabolome, and immune factor in rats. GeroScience. 2021;43(2):709–725. doi: 10.1007/s11357-020-00188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Řehořová V, Cibulková I, Soukupová H, Duška F. Multi-donor fecal microbial transplantation for critically Ill patients: rationale and standard operating procedure. Future Pharmacol. 2022;2(1):55–63. doi: 10.3390/futurepharmacol2010005. [DOI] [Google Scholar]
- 13.Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci. 2011;56(8):2361–2365. doi: 10.1007/s10620-011-1649-3. [DOI] [PubMed] [Google Scholar]
- 14.Lankelma JM, van Vught LA, Belzer C, Schultz MJ, van der Poll T, de Vos WM, et al. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: a pilot study. Intensive Care Med. 2017;43(1):59–68. doi: 10.1007/s00134-016-4613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald D, Ackermann G, Khailova L, Baird C, Heyland D, Kozar R, et al. Extreme dysbiosis of the microbiome in critical illness. mSphere. 2016;1(4):0e00199–16. doi: 10.1128/mSphere.00199-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wischmeyer PE, McDonald D, Knight R. Role of the microbiome, probiotics, and ‘dysbiosis therapy’ in critical illness. Curr Opin Crit Care. 2016;22(4):347–353. doi: 10.1097/MCC.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio. 2014;5(5):e01361–e11314. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krezalek MA, DeFazio J, Zaborina O, Zaborin A, Alverdy JC. The shift of an intestinal “microbiome” to a “pathobiome” governs the course and outcome of sepsis following surgical injury. Shock Augusta Ga. 2016;45(5):475–482. doi: 10.1097/SHK.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13(11):790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4(1):59–72. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutsch A, Kantsjö JB, Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front Immunol [Internet]. 2020 [cited 2022 Jul 12];11. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2020.604179 [DOI] [PMC free article] [PubMed]
- 23.Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res. 2015;77(6):823–828. doi: 10.1038/pr.2015.51. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara M, Bertozzi G, Zanza C, Longhitano Y, Piccolella F, Lauritano CE, et al. Traumatic Brain Injury and Gut Brain Axis: The Disruption of an Alliance. Rev Recent Clin Trials. 2022 Jun 22; [DOI] [PubMed]
- 25.Giridharan VV, Generoso JS, Lence L, Candiotto G, Streck E, Petronilho F, et al. A crosstalk between gut and brain in sepsis-induced cognitive decline. J Neuroinflammation. 2022;19(1):114. doi: 10.1186/s12974-022-02472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang H, Wang Y, Deng J, Zhang H, Wu Q, He L, et al. Sepsis-induced gut dysbiosis mediates the susceptibility to sepsis-associated encephalopathy in mice. mSystems. 2022;7(3):e01399–e1421. doi: 10.1128/msystems.01399-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furness JB. Enteric nervous system. Scholarpedia. 2007;2(10):4064. doi: 10.4249/scholarpedia.4064. [DOI] [Google Scholar]
- 29.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erspamer V, Testini A. Observations on the release and turnover rate of 5-hydroxytryptamine in the gastrointestinal tract. J Pharm Pharmacol. 1959;11(1):618–623. doi: 10.1111/j.2042-7158.1959.tb12603.x. [DOI] [PubMed] [Google Scholar]
- 32.Bertaccini G. Tissue 5-hydroxytryptamine and urinary 5-hydroxyindoleacetic acid after partial or total removal of the gastro-intestinal tract in the rat. J Physiol. 1960;153(2):239–249. doi: 10.1113/jphysiol.1960.sp006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med [Internet]. 2014 Nov 19 [cited 2022 Jul 14];6(263). Available from: https://www.science.org/doi/10.1126/scitranslmed.3009759 [DOI] [PMC free article] [PubMed]
- 34.Oleskin AV, Shenderov BA. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb Ecol Health Dis. 2016;27:30971. doi: 10.3402/mehd.v27.30971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21(6):797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govindarajan V, de Rivero Vaccari JP, Keane RW. Role of inflammasomes in multiple sclerosis and their potential as therapeutic targets. J Neuroinflammation. 2020;17(1):260. doi: 10.1186/s12974-020-01944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai H, Zhang Q. Activation of NLRP3 Inflammasome and Onset of Alzheimer’s Disease. Front Immunol [Internet]. 2021 [cited 2022 Sep 13];12. Available from:https://www.frontiersin.org/articles/10.3389/fimmu.2021.701282 [DOI] [PMC free article] [PubMed]
- 38.Yan YQ, Fang Y, Zheng R, Pu JL, Zhang BR. NLRP3 inflammasomes in Parkinson’s disease and their Regulation by Parkin. Neuroscience. 2020;15(446):323–334. doi: 10.1016/j.neuroscience.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Shen Y, Qian L, Luo H, Li X, Ruan Y, Fan R, et al. The significance of NLRP inflammasome in neuropsychiatric disorders. Brain Sci. 2022;12(8):1057. doi: 10.3390/brainsci12081057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baguley IJ, Heriseanu RE, Nott MT, Chapman J, Sandanam J. Dysautonomia after severe traumatic brain injury: evidence of persisting overresponsiveness to afferent stimuli. Am J Phys Med Rehabil. 2009;88(8):615–622. doi: 10.1097/PHM.0b013e3181aeab96. [DOI] [PubMed] [Google Scholar]
- 41.Hang CH, Shi JX, Li JS, Wu W, Li WQ, Yin HX. Levels of vasoactive intestinal peptide, cholecystokinin and calcitonin gene-related peptide in plasma and jejunum of rats following traumatic brain injury and underlying significance in gastrointestinal dysfunction. World J Gastroenterol. 2004;10(6):875–880. doi: 10.3748/wjg.v10.i6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devlin AS, Marcobal A, Dodd D, Nayfach S, Plummer N, Meyer T, et al. Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe. 2016;20(6):709–715. doi: 10.1016/j.chom.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66(1):343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamo T, Akazawa H, Suzuki JI, Komuro I. Novel Concept of a heart-gut axis in the pathophysiology of heart failure. Korean Circ J. 2017;47(5):663–669. doi: 10.4070/kcj.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(16):1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64(11):1092–1102. doi: 10.1016/j.jacc.2014.06.1179. [DOI] [PubMed] [Google Scholar]
- 47.Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4(3):220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol JASN. 2015;26(10):2434–2446. doi: 10.1681/ASN.2014060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, et al. Gut microbiota promote angiotensin Ii–induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5(9):e003698. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7(3):491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 52.Costanza AC, Moscavitch SD, Faria Neto HCC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015;20(179):348–350. doi: 10.1016/j.ijcard.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 53.Awoyemi A, Mayerhofer C, Felix AS, Hov JR, Moscavitch SD, Lappegård KT, et al. Rifaximin or Saccharomyces boulardii in heart failure with reduced ejection fraction: Results from the randomized GutHeart trial. eBioMedicine [Internet]. 2021 Aug 1 [cited 2022 Oct 23];70. Available from: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00304-2/fulltext [DOI] [PMC free article] [PubMed]
- 54.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 55.Levitan EB, Wolk A, Mittleman MA. Relation of consistency with the dietary approaches to stop hypertension diet and incidence of heart failure in men aged 45 to 79 years. Am J Cardiol. 2009;104(10):1416–1420. doi: 10.1016/j.amjcard.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch Intern Med. 2009;169(9):851–857. doi: 10.1001/archinternmed.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rifai L, Pisano C, Hayden J, Sulo S, Silver MA. Impact of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. Proc Bayl Univ Med Cent. 2015;28(2):151–156. doi: 10.1080/08998280.2015.11929216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9(1):e002314. doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dyer FE, Wood AJ. Action of enterobacteriaceae on choline and related compounds. J Fish Res Board Can. 1947;7(1):17–21. doi: 10.1139/f47-002. [DOI] [Google Scholar]
- 60.Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. doi: 10.1038/srep19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140(3):976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stepankova R, Tonar Z, Bartova J, Nedorost L, Rossman P, Poledne R, et al. Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J Atheroscler Thromb. 2010;17(8):796–804. doi: 10.5551/jat.3285. [DOI] [PubMed] [Google Scholar]
- 63.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila Protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe −/− Mice. Circulation. 2016;133(24):2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 64.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yazar A, Atis S, Konca K, Pata C, Akbay E, Calikoglu M, et al. Respiratory symptoms and pulmonary functional changes in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96(5):1511–1516. doi: 10.1111/j.1572-0241.2001.03748.x. [DOI] [PubMed] [Google Scholar]
- 66.Kraft SC, Earle RH, Roesler M, Esterly JR. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch Intern Med. 1976;136(4):454–459. doi: 10.1001/archinte.1976.03630040056012. [DOI] [PubMed] [Google Scholar]
- 67.Ward H, Fisher KL, Waghray R, Wright JL, Card SE, Cockcroft DW. Constrictive bronchiolitis and ulcerative colitis. Can Respir J. 1999;6(2):197–200. doi: 10.1155/1999/392575. [DOI] [PubMed] [Google Scholar]
- 68.Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9(2):104–115. doi: 10.1097/00054725-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Eaton TE, Lambie N, Wells AU. Bronchiectasis following colectomy for Crohn’s disease. Thorax. 1998;53(6):529–531. doi: 10.1136/thx.53.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 72.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJTH, de Boer JD, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mu S, Xiang H, Wang Y, Wei W, Long X, Han Y, et al. The pathogens of secondary infection in septic patients share a similar genotype to those that predominate in the gut. Crit Care Lond Engl. 2022;26(1):68. doi: 10.1186/s13054-022-03943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kyo M, Nishioka K, Nakaya T, Kida Y, Tanabe Y, Ohshimo S, et al. Unique patterns of lower respiratory tract microbiota are associated with inflammation and hospital mortality in acute respiratory distress syndrome. Respir Res. 2019;20(1):246. doi: 10.1186/s12931-019-1203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noel S, Martina-Lingua MN, Bandapalle S, Pluznick J, Hamad ARA, Peterson DA, et al. Intestinal microbiota-kidney cross talk in acute kidney injury and chronic kidney disease. Nephron Clin Pract. 2014;127(1–4):139–143. doi: 10.1159/000363209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borges NA, Stenvinkel P, Bergman P, Qureshi AR, Lindholm B, Moraes C, et al. Effects of probiotic supplementation on trimethylamine-N-oxide plasma levels in hemodialysis patients: a pilot study. Probiotics Antimicrob Proteins. 2019;11(2):648–654. doi: 10.1007/s12602-018-9411-1. [DOI] [PubMed] [Google Scholar]
- 77.Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE. 2016;11(1):e0141738. doi: 10.1371/journal.pone.0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39(3):230–237. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu J, Zhong X, Yan J, Zhou D, Qin D, Xiao X, et al. High-throughput sequencing analysis of intestinal flora changes in ESRD and CKD patients. BMC Nephrol. 2020;21(1):12. doi: 10.1186/s12882-019-1668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W, Hao G, Pan Y, Ma S, Yang T, Shi P, et al. Serum indoxyl sulfate is associated with mortality in hospital-acquired acute kidney injury: a prospective cohort study. BMC Nephrol. 2019;20(1):57. doi: 10.1186/s12882-019-1238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci. 2013;110(11):4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Felizardo RJF, de Almeida DC, Pereira RL, Watanabe IKM, Doimo NTS, Ribeiro WR, et al. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33(11):11894–11908. doi: 10.1096/fj.201901080R. [DOI] [PubMed] [Google Scholar]
- 83.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJF, de Almeida DC, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol JASN. 2015;26(8):1877–1888. doi: 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang J, Ji GE, Park MS, Seong YJ, Go YS, Lee HY, et al. Probiotics partially attenuate the severity of acute kidney injury through an immunomodulatory effect. Kidney Res Clin Pract. 2021;40(4):620–633. doi: 10.23876/j.krcp.20.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakade Y, Iwata Y, Furuichi K, Mita M, Hamase K, Konno R, et al. Gut microbiota–derived D-serine protects against acute kidney injury. JCI Insight. 2018;3(20):e97957. doi: 10.1172/jci.insight.97957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, et al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J. 2013;27(9):3583–3593. doi: 10.1096/fj.13-231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen P, Miyamoto Y, Mazagova M, Lee KC, Eckmann L, Schnabl B. Microbiota protects mice against acute alcohol-induced liver injury. Alcohol Clin Exp Res. 2015;39(12):2313–2323. doi: 10.1111/acer.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie G, Zhong W, Zheng X, Li Q, Qiu Y, Li H, et al. Chronic Ethanol consumption alters mammalian gastrointestinal content metabolites. J Proteome Res. 2013;12(7):3297–3306. doi: 10.1021/pr400362z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575(7783):505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019;30(4):675–688.e7. doi: 10.1016/j.cmet.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 91.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–1161. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishibashi S, Schwarz M, Frykman PK, Herz J, Russell DW. Disruption of cholesterol 7alpha-hydroxylase gene in mice. I. Postnatal lethality reversed by bile acid and vitamin supplementation. J Biol Chem. 1996;271(30):18017–18023. doi: 10.1074/jbc.271.30.18017. [DOI] [PubMed] [Google Scholar]
- 93.Cheng JB, Jacquemin E, Gerhardt M, Nazer H, Cresteil D, Heubi JE, et al. Molecular genetics of 3beta-hydroxy-Delta5-C27-steroid oxidoreductase deficiency in 16 patients with loss of bile acid synthesis and liver disease. J Clin Endocrinol Metab. 2003;88(4):1833–1841. doi: 10.1210/jc.2002-021580. [DOI] [PubMed] [Google Scholar]
- 94.Gadaleta RM, Cariello M, Crudele L, Moschetta A. Bile salt hydrolase-competent probiotics in the management of IBD: unlocking the ‘Bile Acid Code’. Nutrients. 2022;14(15):3212. doi: 10.3390/nu14153212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filliol A, Piquet-Pellorce C, Raguénès-Nicol C, Dion S, Farooq M, Lucas-Clerc C, et al. RIPK1 protects hepatocytes from Kupffer cells-mediated TNF-induced apoptosis in mouse models of PAMP-induced hepatitis. J Hepatol. 2017;66(6):1205–1213. doi: 10.1016/j.jhep.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Marzano M, Fosso B, Colliva C, Notario E, Passeri D, Intranuovo M, et al. Farnesoid X receptor activation by the novel agonist TC-100 (3α, 7α, 11β-Trihydroxy-6α-ethyl-5β-cholan-24-oic Acid) preserves the intestinal barrier integrity and promotes intestinal microbial reshaping in a mouse model of obstructed bile acid flow. Biomed Pharmacother Biomed Pharmacother. 2022;153:113380. doi: 10.1016/j.biopha.2022.113380. [DOI] [PubMed] [Google Scholar]
- 97.Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease | Gut [Internet]. [cited 2022 Aug 28]. Available from: https://gut.bmj.com/content/67/5/891 [DOI] [PubMed]
- 98.Kang H, Thomas RM. Bacteria and sepsis: microbiome to the rescue? J Clin Med. 2021;10(16):3578. doi: 10.3390/jcm10163578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brienza N, Dalfino L, Cinnella G, Diele C, Bruno F, Fiore T. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med. 2006;32(2):267–274. doi: 10.1007/s00134-005-0023-3. [DOI] [PubMed] [Google Scholar]
- 100.Cresci GA, Glueck B, McMullen MR, Xin W, Allende D, Nagy LE. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J Gastroenterol Hepatol. 2017;32(9):1587–1597. doi: 10.1111/jgh.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen P, Torralba M, Tan J, Embree M, Zengler K, Stärkel P, et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148(1):203–214.e16. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martín R, Chamignon C, Mhedbi-Hajri N, Chain F, Derrien M, Escribano-Vázquez U, et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep. 2019;1(9):5398. doi: 10.1038/s41598-019-41738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahl D, Liu H, Schreiber O, Roos S, Phillipson M, Holm L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. 2016;217(4):300–310. doi: 10.1111/apha.12695. [DOI] [PubMed] [Google Scholar]
- 104.Buchman AL, Moukarzel A, Jenden DJ, Roch M, Rice K, Ament ME. Low plasma free choline is prevalent in patients receiving long term parenteral nutrition and is associated with hepatic aminotransferase abnormalities. Clin Nutr Edinb Scotl. 1993;12(1):33–37. doi: 10.1016/0261-5614(93)90143-R. [DOI] [PubMed] [Google Scholar]
- 105.Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatol Baltim Md. 1995;22(5):1399–1403. [PubMed] [Google Scholar]
- 106.Marcolin E, Forgiarini LF, Tieppo J, Dias AS, de Freitas LAR, Marroni NP. Methionine- and choline-deficient diet induces hepatic changes characteristic of non-alcoholic steatohepatitis. Arq Gastroenterol. 2011;48(1):72–79. doi: 10.1590/S0004-28032011000100015. [DOI] [PubMed] [Google Scholar]
- 107.Flores-Guerrero JL, Post A, van Dijk PR, Connelly MA, Garcia E, Navis G, et al. Circulating trimethylamine-N-oxide is associated with all-cause mortality in subjects with nonalcoholic fatty liver disease. Liver Int Off J Int Assoc Study Liver. 2021;41(10):2371–2382. doi: 10.1111/liv.14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, et al. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol Nutr Food Res. 2019;63(17):e1900257. doi: 10.1002/mnfr.201900257. [DOI] [PubMed] [Google Scholar]
- 109.Paugam-Burtz C, Levesque E, Louvet A, Thabut D, Amathieu R, Bureau C, et al. Management of liver failure in general intensive care unit. Anaesth Crit Care Pain Med. 2020;39(1):143–161. doi: 10.1016/j.accpm.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 110.Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr. 2017;64(3):413–417. doi: 10.1097/MPG.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 111.Xue L, Deng Z, Luo W, He X, Chen Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front Cell Infect Microbiol [Internet]. 2022 [cited 2022 Oct 25];12. Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2022.759306 [DOI] [PMC free article] [PubMed]
- 112.Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study - PubMed [Internet]. [cited 2022 Aug 28]. Available from: https://pubmed.ncbi.nlm.nih.gov/26302024/
- 113.Elke G, van Zanten ARH, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20(1):117. doi: 10.1186/s13054-016-1298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burdet C, Sayah-Jeanne S, Nguyen TT, Miossec C, Saint-Lu N, Pulse M, et al. Protection of hamsters from mortality by reducing fecal moxifloxacin concentration with DAV131A in a model of moxifloxacin-induced clostridium difficile colitis. Antimicrob Agents Chemother. 2017;61(10):e00543–e617. doi: 10.1128/AAC.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vehreschild MJGT, Ducher A, Louie T, Cornely OA, Feger C, Dane A, et al. An open randomized multicentre Phase 2 trial to assess the safety of DAV132 and its efficacy to protect gut microbiota diversity in hospitalized patients treated with fluoroquinolones. J Antimicrob Chemother. 2022;77(4):1155–1165. doi: 10.1093/jac/dkab474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheema HA, Shahid A, Ayyan M, Mustafa B, Zahid A, Fatima M, et al. Probiotics for the prevention of ventilator-associated pneumonia: an updated systematic review and meta-analysis of randomised controlled trials. Nutrients. 2022;14(8):1600. doi: 10.3390/nu14081600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li C, Lu F, Chen J, Ma J, Xu N. Probiotic supplementation prevents the development of ventilator-associated pneumonia for mechanically ventilated ICU patients: a systematic review and network meta-analysis of randomized controlled trials. Front Nutr [Internet]. 2022 [cited 2022 Jul 29];9. Available from: https://www.frontiersin.org/articles/10.3389/fnut.2022.919156 [DOI] [PMC free article] [PubMed]
- 118.Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care Lond Engl. 2016;19(19):262. doi: 10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Quévrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65(3):415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khailova L, Frank DN, Dominguez JA, Wischmeyer PE. Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis. Anesthesiology. 2013;119(1):166–177. doi: 10.1097/ALN.0b013e318291c2fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biosearch SA. Multicentric Study to Assess the Effect of Consumption of Lactobacillus Coryniformis K8 on Healthcare Personnel Exposed to COVID-19 [Internet]. clinicaltrials.gov; 2020 Apr [cited 2022 Aug 28]. Report No.: NCT04366180. Available from: https://clinicaltrials.gov/ct2/show/NCT04366180
- 122.Örebro University, Sweden. Exploratory Study on the Effects of Probiotic Supplementation on SARS-CoV-2 Antibody Response in Healthy Adults [Internet]. clinicaltrials.gov; 2021 Oct [cited 2022 Aug 28]. Report No.: NCT04734886. Available from: https://clinicaltrials.gov/ct2/show/NCT04734886
- 123.Duke University. A Randomized Trial of the Effect of Lactobacillus on the Microbiome of Household Contacts Exposed to COVID-19 [Internet]. clinicaltrials.gov; 2022 Feb [cited 2022 Aug 28]. Report No.: NCT04399252. Available from: https://clinicaltrials.gov/ct2/show/NCT04399252
- 124.I.M. Sechenov First Moscow State Medical University. Efficacy of Probiotics (Lactobacillus Rhamnosus, Bifidobacterium Bifidum, Bifidobacterium Longum Subsp. Infantis and Bifidobacterium Longum) in the Treatment of Hospitalised Patients With Novel Coronavirus Infection [Internet]. clinicaltrials.gov; 2022 Jan [cited 2022 Aug 28]. Report No.: NCT04854941. Available from: https://clinicaltrials.gov/ct2/show/NCT04854941
- 125.Centre hospitalier de l’Université de Montréal (CHUM). Randomised Single Blinded Clinical Study of Efficacy of Intranasal Probiotic Treatment to Reduce Severity of Symptoms in COVID19 Infection [Internet]. clinicaltrials.gov; 2021 May [cited 2022 Aug 28]. Report No.: NCT04458519. Available from: https://clinicaltrials.gov/ct2/show/NCT04458519
- 126.AB Biotics, SA. Efficacy and Safety of Lactobacillus Plantarum and Pediococcus Acidilactici as Co-adjuvant Therapy for Reducing the Risk of Severe Disease in Adults With SARS-CoV-2 and Its Modulation of the Fecal Microbiota: A Randomized Clinical Trial [Internet]. clinicaltrials.gov; 2021 May [cited 2022 Aug 28]. Report No.: NCT04517422. Available from: https://clinicaltrials.gov/ct2/show/NCT04517422
- 127.Kigerl KA, Hall JCE, Wang L, Mo X, Yu Z, Popovich PG. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213(12):2603–2620. doi: 10.1084/jem.20151345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, et al. SER-109, an oral microbiome therapy for recurrent clostridioides difficile infection. N Engl J Med. 2022;386(3):220–229. doi: 10.1056/NEJMoa2106516. [DOI] [PubMed] [Google Scholar]
- 129.Kokai-Kun J, Connelly S. Ribaxamase, an orally administered β-lactamase, protects the gut microbiome in patients treated with ceftriaxone. J Transl Sci [Internet]. 2020 [cited 2022 Aug 30];6(3). Available from: https://www.oatext.com/ribaxamase-an-orally-administered-v-lactamase-protects-the-gut-microbiome-in-patients-treated-with-ceftriaxone.php#gsc.tab=0
- 130.Synthetic Biologics Inc. Phase 1b/2a Evaluation of the Safety and Tolerability of SYN-004 in Adult Allogeneic Hematopoietic Cell Transplantation (Allo-HCT) Recipients [Internet]. clinicaltrials.gov; 2022 Apr [cited 2022 Oct 24]. Report No.: NCT04692181. Available from: https://clinicaltrials.gov/ct2/show/NCT04692181
- 131.Quraishi MN, Widlak M, Bhala N, Moore D, Price M, Sharma N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46(5):479–493. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 132.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ianiro G, Masucci L, Quaranta G, Simonelli C, Lopetuso LR, Sanguinetti M, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection—single versus multiple infusions. Aliment Pharmacol Ther. 2018;48(2):152–159. doi: 10.1111/apt.14816. [DOI] [PubMed] [Google Scholar]
- 134.Cheng YW, Phelps E, Nemes S, Rogers N, Sagi S, Bohm M, et al. Fecal microbiota transplant decreases mortality in patients with refractory severe or fulminant clostridioides difficile infection. Clin Gastroenterol Hepatol. 2020;18(10):2234–2243.e1. doi: 10.1016/j.cgh.2019.12.029. [DOI] [PubMed] [Google Scholar]
- 135.Cheng YW, Xu H, Phelps E, Rogers N, Sagi S, Bohm M, et al. Fecal microbiota transplant decreases mortality in patients with refractory severe and severe complicatedclostridium difficileinfection not eligible for colectomy: 2017 fellows-in-training award (Colon Category): 100. Off J Am Coll Gastroenterol ACG. 2017;112:S48. [Google Scholar]
- 136.Fischer M, Sipe B, Cheng YW, Phelps E, Rogers N, Sagi S, et al. Fecal microbiota transplant in severe and severe-complicated Clostridium difficile: a promising treatment approach. Gut Microbes. 2017;8(3):289–302. doi: 10.1080/19490976.2016.1273998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hocquart M, Lagier JC, Cassir N, Saidani N, Eldin C, Kerbaj J, et al. Early fecal microbiota transplantation improves survival in severe clostridium difficile infections. Clin Infect Dis. 2018;66(5):645–650. doi: 10.1093/cid/cix762. [DOI] [PubMed] [Google Scholar]
- 138.Du D, Tang W, Zhou C, Sun X, Wei Z, Zhong J, et al. Fecal microbiota transplantation is a promising method to restore gut microbiota dysbiosis and relieve neurological deficits after traumatic brain injury. Oxid Med Cell Longev. 2021;2021:5816837. doi: 10.1155/2021/5816837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Q, Wang C, Tang C, He Q, Zhao X, Li N, et al. Therapeutic modulation and reestablishment of the intestinal microbiota with fecal microbiota transplantation resolves sepsis and diarrhea in a patient. Am J Gastroenterol. 2014;109(11):1832–1834. doi: 10.1038/ajg.2014.299. [DOI] [PubMed] [Google Scholar]
- 140.Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care Lond Engl. 2016;20(1):332. doi: 10.1186/s13054-016-1491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaczmarek JL, Musaad SM, Holscher HD. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr. 2017;106(5):1220–1231. doi: 10.3945/ajcn.117.156380. [DOI] [PubMed] [Google Scholar]
- 142.Klingbeil E, de La Serre CB. Microbiota modulation by eating patterns and diet composition: impact on food intake. Am J Physiol Regul Integr Comp Physiol. 2018;315(6):R1254–R1260. doi: 10.1152/ajpregu.00037.2018. [DOI] [PubMed] [Google Scholar]
- 143.Oami T, Chihade DB, Coopersmith CM. The microbiome and nutrition in critical illness. Curr Opin Crit Care. 2019;25(2):145–149. doi: 10.1097/MCC.0000000000000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–1737. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 145.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4(8):1095–1119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wan X, Bi J, Gao X, Tian F, Wang X, Li N, et al. Partial enteral nutrition preserves elements of gut barrier function, including innate immunity, intestinal alkaline phosphatase (IAP) level, and intestinal microbiota in mice. Nutrients. 2015;7(8):6294–6312. doi: 10.3390/nu7085288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;13(361):k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 150.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moron R, Galvez J, Colmenero M, Anderson P, Cabeza J, Rodriguez-Cabezas ME. The importance of the microbiome in critically ill patients: role of nutrition. Nutrients. 2019;11(12):3002. doi: 10.3390/nu11123002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 153.Kabeerdoss J, Devi RS, Mary RR, Prabhavathi D, Vidya R, Mechenro J, et al. Effect of yoghurt containing Bifidobacterium lactis Bb12®on faecal excretion of secretory immunoglobulin A and human beta-defensin 2 in healthy adult volunteers. Nutr J. 2011;10(1):138. doi: 10.1186/1475-2891-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL#3 induce enterocyte β-defensin 2. Clin Exp Immunol. 2008;151(3):528–535. doi: 10.1111/j.1365-2249.2007.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Möndel M, Schroeder BO, Zimmermann K, Huber H, Nuding S, Beisner J, et al. Probiotic E. coli treatment mediates antimicrobial human β-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2009;2(2):166–172. doi: 10.1038/mi.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J, et al. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. JAMA. 2021;326(11):1024–1033. doi: 10.1001/jama.2021.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsilika M, Thoma G, Aidoni Z, Tsaousi G, Fotiadis K, Stavrou G, et al. A four-probiotic preparation for ventilator-associated pneumonia in multi-trauma patients: results of a randomized clinical trial. Int J Antimicrob Agents. 2022;59(1):106471. doi: 10.1016/j.ijantimicag.2021.106471. [DOI] [PubMed] [Google Scholar]
- 158.Lan SH, Hung SH, Chang SP, Lu LC, Lai CC, Lin WT. Pro-, pre- and synbiotics for the prevention of incidental ventilator-associated pneumonia among critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther. 2022;16:1–11. doi: 10.1080/14787210.2022.2089652. [DOI] [PubMed] [Google Scholar]
- 159.Alsuwaylihi AS, McCullough F. The safety and efficacy of probiotic supplementation for critically ill adult patients: a systematic review and meta-analysis. Nutr Rev. 2022 doi: 10.1093/nutrit/nuac059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clark JA, Coopersmith CM. Intestinal crosstalk—a new paradigm for understanding the gut as the “motor” of critical illness. Shock Augusta Ga. 2007;28(4):384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.George AK, Behera J, Homme RP, Tyagi N, Tyagi SC, Singh M. Rebuilding microbiome for mitigating traumatic brain injury: importance of restructuring the gut-microbiome-brain axis. Mol Neurobiol. 2021;58(8):3614–3627. doi: 10.1007/s12035-021-02357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Corriero A, Ribezzi M, Mele F, Angrisani C, Romaniello F, Daleno A, et al. COVID-19 variants in critically Ill patients: a comparison of the delta and omicron variant profiles. Infect Dis Rep. 2022;14(3):492–500. doi: 10.3390/idr14030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Batista KS, de Albuquerque JG, de Vasconcelos MHA, Bezerra MLR, da Silva Barbalho MB, Pinheiro RO, et al. Probiotics and prebiotics: potential prevention and therapeutic target for nutritional management of COVID-19? Nutr Res Rev. 2021;20:1–18. doi: 10.1017/S0954422421000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nguyen QV, Chong LC, Hor YY, Lew LC, Rather IA, Choi SB. Role of probiotics in the management of COVID-19: a computational perspective. Nutrients. 2022;14(2):274. doi: 10.3390/nu14020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mayer S, Bonhag CM, Jenkins P, Cornett BT, Scherbak D. Probiotic administration is associated with increased mortality in ICU patients with central venous catheters. Chest. 2022;162(4):A1148. doi: 10.1016/j.chest.2022.08.912. [DOI] [Google Scholar]
- 166.Luyt CE, Bréchot N, Trouillet JL, Chastre J. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18(5):480. doi: 10.1186/s13054-014-0480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harmoinen J, Vaali K, Koski P, Syrjänen K, Laitinen O, Lindevall K, et al. Enzymic degradation of a beta-lactam antibiotic, ampicillin, in the gut: a novel treatment modality. J Antimicrob Chemother. 2003;51(2):361–365. doi: 10.1093/jac/dkg095. [DOI] [PubMed] [Google Scholar]
- 168.Kaleko M, Bristol JA, Hubert S, Parsley T, Widmer G, Tzipori S, et al. Development of SYN-004, an oral beta-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe. 2016;41:58–67. doi: 10.1016/j.anaerobe.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 169.DAV132 Digestive Cancers [Internet]. Da Volterra. [cited 2022 Aug 30]. Available from: https://davolterra.com/dav132-digestive-cancers/
- 170.Blunting the Effects of Antibiotics on the Intestinal Microbiome [Internet]. [cited 2022 Aug 30]. Available from: https://www.jwatch.org/na46230/2018/03/06/blunting-effects-antibiotics-intestinal-microbiome
- 171.Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical application and potential of fecal microbiota transplantation. Annu Rev Med. 2019;27(70):335–351. doi: 10.1146/annurev-med-111717-122956. [DOI] [PubMed] [Google Scholar]
- 172.Limketkai BN, Hendler S, Ting PS, Parian AM. fecal microbiota transplantation for the critically Ill patient. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2019;34(1):73–79. doi: 10.1002/ncp.10228. [DOI] [PubMed] [Google Scholar]
- 173.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 174.Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile | NEJM [Internet]. [cited 2022 Jul 2]. Available from: https://www.nejm.org/doi/full/10.1056/nejmoa1205037 [DOI] [PubMed]
- 175.Keskey R, Cone JT, DeFazio JR, Alverdy JC. The use of fecal microbiota transplant in sepsis. Transl Res J Lab Clin Med. 2020;226:12–25. doi: 10.1016/j.trsl.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Millan B, Park H, Hotte N, Mathieu O, Burguiere P, Tompkins TA, et al. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent clostridium difficile infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;62(12):1479–1486. doi: 10.1093/cid/ciw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim SM, DeFazio JR, Hyoju SK, Sangani K, Keskey R, Krezalek MA, et al. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat Commun. 2020;11(1):2354. doi: 10.1038/s41467-020-15545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.