Abstract
Background
The use of antimicrobial-impregnated peripherally inserted central catheters (PICCs) has been introduced in the last few years to neonatal units aiming to reduce central line-associated bloodstream infection (CLABSI).
Methods
This retrospective observational study aimed to compare the CLABSI rates and other catheter-related parameters including the insertion success rates and catheter-related complications in the antimicrobial-impregnated and conventional (ordinary) PICCs in NICU between 2017 and 2020.
Results
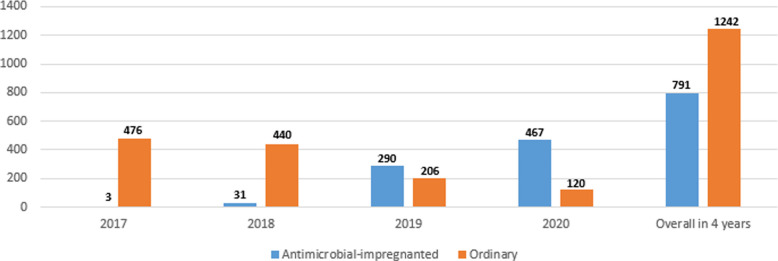
Our dedicated PICC team including physicians and nurses inserted 1,242 conventional (PremiCath and NutriLine) and 791 antimicrobial-impregnated PICCs (PremiStar) over the study period from 2017 to 2020. Of those 1,242 conventional PICCs, 1,171 (94.3%) were 1 Fr single lumen and only 71 (5.7%) were 2 Fr double lumen. The mean ± SD [median (IQR)] for the birth weight in all babies who had a PICC line was 1,343.3 ± 686.75 [1,200 (900, 1,500)] g, while the mean ± SD for the gestational age was 29.6 ± 4.03 [29 (27, 31)] weeks. The mean ± SD [median (IQR)] age at the time of insertion for all catheters was 9.3 ± 21.47 [2 (1, 9)] days, while the mean ± SD [median (IQR)] dwell time was 15.7 ± 14.03 [12 (8, 17)] days. The overall success rate of the PICC insertion is 1,815/2,033 (89.3%), while the first attempt success rate is 1,290/2,033 (63.5%). The mean ± SD [median (IQR)] gestational age, birth weight, age at catheter insertion, and catheter dwell time were 28.8 ± 3.24 [29, (26, 31)] weeks, 1,192.1 ± 410.3 [1,150, (900, 1,450)] g, 6.3 ± 10.85 [2, (1, 8)] days, and 17.73 ± 17.532 [13, (9, 18)] days in the antimicrobial-impregnated catheter compared with 30.1 ± 4.39 [29, (27, 32)] weeks (P < 0.001), 1,439.5 ± 800.8 [1,240, (920, 1,520)] g (P < 0.001), 11.1 ± 25.9 [1, (1, 9)] days (P < 0.001), and 14.30 ± 10.964 [12, (8, 17)] days (P < 0.001), respectively, in the conventional PICCs. The use of the antimicrobial-impregnated catheter was not associated with any significant reduction in the CLABSI rate (per 1,000 days dwell time), either the overall [P = 0.11, risk ratio (RR) (95% CI): 0.60 (0.32, 1.13)] or the yearly CLABSI rates.
Conclusions
The use of miconazole and rifampicin-impregnated PICCs did not reduce the CLABSI rate in neonates compared with conventional PICCs. However, it has a higher overall rate of elective removal after completion of therapy and less extravasation/infiltration, occlusion, and phlebitis compared with the conventional PICCs. Further large RCTs are recommended to enrich the current paucity of evidence and to reduce the risk of bias. Neonatal PICCs impregnation by other antimicrobials is a recommendation for vascular access device manufacturers.
Keywords: vascular access, vascular access devices, peripherally inserted central catheter, antimicrobial-impregnated central venous catheters, central line-associated bloodstream infection, newborn, neonatal intensive care unit, neonate
Introduction
Since its first discovery in 1973, peripherally inserted central catheters (PICCs) have been progressively used in neonatal intensive care units (NICUs) (1). PICCs offer long-term venous access for neonates and are indicated for total parental nutrition (TPN), long-term IV medications and antibiotic therapy, and vesicant drug therapy (2, 3).
Central line-associated bloodstream infection (CLABSI) is the most common and serious complication related to PICC insertion in neonates. The causative organisms usually adhere and stick to the catheter and secrete a biologically coated film that protects them from systemically administered antimicrobials, allowing for prolonged microbial colonization (4–6). The organisms most frequently isolated from preterm babies in hospital-acquired infections are coagulase-negative Staphylococci, and other gram-positive cocci (Staphylococcus aureus and Enterococci), gram-negative bacilli (E. coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae), and fungi (Candida pathogens) (7). CLABSI is associated with death, serious morbidities especially in very preterm babies, increased healthcare costs, prolonged length of hospital stay, and subsequent long-term adverse neurodevelopmental outcomes and lung diseases (8, 9).
The use of antimicrobial-impregnated central venous catheters (CVCs) has been recently recommended for patients at high risk of infection in addition to all other preventive measures (10, 11). The antimicrobial-impregnated catheter PremiStar; Vygon, Swindon, UK is miconazole and rifampicin-impregnated PICC, designed and manufactured aiming to reduce the incidence of CLABSI in newborns, by the combined synergistic effect of the two medications (12). Extruded polyurethane tubes are placed into a solution made of two anti-infective substances. By thermal activation, the combination of rifampicin and miconazole is chemically incorporated into the structure of the catheter. Miconazole is a systemic antifungal medication proven to be effective against systemic fungal infections. Rifampicin is a systemic antibacterial medication that has been investigated and tested in rifampicin and minocycline CVCs impregnation in children and adults (13–16). PremiStar was introduced to our neonatal unit in August 2016.
There is a limited number of studies comparing antimicrobial-impregnated vs. conventional PICCs in neonates that necessitated further research studies to compare both types of catheters (17). This retrospective observational study aimed to compare the CLABSI rates and other catheter-related parameters including the insertion success rates and catheter-related complications in the antimicrobial-impregnated vs. conventional PICCs in NICU from 2017 to 2020. The main objective is to assess the ability of the antimicrobial-impregnated PICCs in reducing the CLABSI rate in the NICU compared with the conventional PICCs using retrospectively collected data.
Materials and methods
This is a retrospective observational study conducted in the NICU, Women's Wellness and Research Center (WWRC) at Hamad Medical Corporation (HMC), Doha, Qatar. The NICU in WWRC is a tertiary-level neonatal unit with 112 cots. The hospital has more than 18,000 deliveries per year and the NICU has more than 3,000 admissions per year.
In 2017, the PICC insertion team was developed and introduced to our NICU. Since then, the members are expanding and currently, the team includes 15 neonatologists, 1 neonatal nurse practitioner, and 7 NICU nurses. The central line simulation course is an accredited course that was designed by mobile pediatric simulation (MPS) and the neonatal simulation team based on the educational needs assessment. The aim was to train the team members and enhance their cognitive, technical and behavioral skills related to central line insertion. The participants were trained to insert PICC by MicroFlash insertion, the split steel needle insertion and the modified Seldinger technique (MST) (18–20). Since its launch, the PICC team has been working in collaboration with the neonatal specialized nursing (NSN) team. NSN members are highly trained NICU competent in IV line insertion. The NSN team's roles are the determination of the insertion eligibility criteria, VAD selection, line maintenance ensuring closed system care, blood samples collection, and insertion data entry in the specially designed online data collection system. In our day-to-day practice, there is no difference in the line maintenance quality, personnel, and frequency of all types of CVCs (21, 22).
In our NICU, we insert PICC for very low birth weight (VLBW) infants who are less than 1.5 kg, if intravenous fluids are needed for more than 5 days, if intravenous medications are needed for more than 7 days, the osmolality of the intravenous fluids exceeds 700 mOsmol/L and if the patient requires more than 3 peripheral intravenous catheters (PIVCs) insertions in 24 h. This is according to the locally developed protocol based on international guidelines (23).
Two types of conventional (ordinary) PICCs are available in our NICU (NutriLine 2 Fr; Vygon and PremiCath 1 Fr; Vygon). The PremiStar 1 Fr; Vygon, Swindon, UK is the only antimicrobial-impregnated PICC available in our unit. Based on our vascular access device (VAD) selection criteria, we insert PremiStar for extremely preterm infants less than 28 weeks gestation babies or in babies with suspected early or late-onset neonatal sepsis in the presence of risk factors. In big babies with older gestational ages or sick babies when a double lumen catheter is needed, NutriLine 2 Fr; 30 cm length; Vygon, was the right choice. For the rest of the babies who required PICC insertion, PremiCath 1 Fr; Vygon was used. The most frequently used veins for PICC insertions in the lower extremities were the great saphenous vein, the small saphenous vein, and the posterior tibial vein. The most frequently used veins for PICC insertions in the upper extremities were the antecubital vein, the cephalic vein, the basilic vein, and the ulnar vein. For all catheters, either split steel needle, MicroFlash over-the-needle technique, or MST was followed for the catheter insertion. A central venous catheter was considered to be successfully inserted when its tip was located either in the superior vena cava (SVC) or inferior vena cava (IVC) just outside the right atrium. As per our evidence-based guidelines for the insertion and management of PICC in neonates, two pricks are allowed for each PICC team member. After a total of three failed insertion attempts, the procedure was stopped. Exceptions are very limited with chronic patients who have difficult vascular access. Preventive removal refers to the unnecessary premature catheter withdrawal before completion of the IV therapy (21, 22, 24, 25).
The line maintenance and care bundles for preventing CLABSI in our unit include a fully sterile insertion bundle, a daily VAD assessment bundle, a bundle for change of the line dressing, and a catheter access bundle. The core bundle elements include but are not limited to applying maximal personal protective equipment (PPE), proper hand hygiene techniques, optimal catheter site, and device selection, a daily central line needs assessment, using a specialized central line trolley named Mayo Stand, performing the procedure time out, timing the duration of the procedure, using a central line insertion sterile closed kit, cleaning the line hub using chlorhexidine, applying a transparent semipermeable line dressing, using a needleless connector, using an alcohol-impregnated line port protector, applying two-person technique in each step all the time, using cyanoacrylate glue for catheter fixation and sealing of the insertion site, using diluted lipid emulsion solution as a lubricant to facilitate the guidewire removal, using the vein viewer imaging system for vascular assessment, using the microsite and the MST for the catheter insertion, early PICC insertion-early PICC removal approach, use of antimicrobial-impregnated catheters, speak-up campaigns to pick up any infection control breaches, and meticulously keeping a closed IV tubing system all the time. We have locally developed a mnemonic for neonatal vascular access pre-briefing, the “5Rights for Vascular Access” that is, choosing the Right vascular access device, for the Right patient, administering the Right therapy, in the Right vein, for the Right duration (24–28).
The Centers for Disease Control and Prevention (CDC) defines CLABSI as a laboratory-confirmed bloodstream infection not related to an infection at any other site, which develops within 48 h of central line insertion. The CLABSI rate is defined as the number of CLABSI infections per 1,000 central line days (29–31). These CDC definitions for CLABSI and CLABSI rate were followed in our unit.
An electronic web-based data registry was designed to collect the study variables and catheter-related parameters in both groups.
Statistical analysis
The primary analysis in this observational research study was to evaluate and compare the CLABSI rates and other catheter-related parameters including the insertion success rates and catheter-related complications between the antimicrobial-impregnated and conventional PICCs in NICU. Descriptive statistics were used to summarize the sample characteristics using mean and standard deviation (SD) for normally distributed data and median and interquartile range (IQR) for skewed data. Qualitative data were summarized using frequencies and percentages. Associations between two or more categorical variables were compared using Chi-square (χ2) test or Fisher Exact test as applicable. Quantitative data between the two independent groups (e.g., antimicrobial-impregnated and conventional catheters; CLABSI vs. non-CLABSI, etc.) were analyzed using a t-test or nonparametric Mann–Whitney U-test as appropriate.
Logistic regression analysis was used to examine and evaluate the potential confounders and covariates (as shown in Tables 4, 5) associated with CLABSI. For multivariate logistic regression models, predictor variables were included in consideration with both statistical and clinical significance and relevance. The results of logistic regression analysis were presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs). Subsequently, we used the receiver operating characteristic curve (ROC) to evaluate the discriminative ability (predictive accuracy of the developed logistic regression model) of potentially significant predictors associated with CLABSI. The linear relationship between two continuous variables was assessed using Pearson's or Spearman's correlation coefficients. Two-tailed P-values <0.05 were considered statistically significant. It was written as numbers and if it is very low, i.e., less than 0.001, it was put as <0.001. All statistical analyses were performed using statistical packages SPSS version 28.0 (Armonk, NY: IBM Corp) and Epi-info (Centers for Disease Control and Prevention, Atlanta, GA) software.
Table 4.
CLABSI rates (per 1,000 days dwell time) between the two types of catheters across the 4 years.
| Year | CLABSI rate in antimicrobial-impregnated catheters | CLABSI rate in conventional catheters | Risk ratio (RR) (95% CI) | P-value |
|---|---|---|---|---|
| 2017 | 0 | 2.44 | NA | 0.843 |
| 2018 | 2.11 | 2.05 | 1.03 (0.13, 7.91) | 0.977 |
| 2019 | 1.52 | 0.46 | 3.30 (0.42, 25.77) | 0.227 |
| 2020 | 0.54 | 0 | NA | 0.593 |
| Overall in the 4 years | 1.11 | 1.86 | 0.60 (0.32, 1.13) | 0.11 |
CLABSI, central line-associated bloodstream infection; CI, confidence interval; NA, not applicable.
CLABSI rate is the number of CLABSI cases per 1,000 days dwell time.
Table 5.
Factors associated with CLABSI: univariate logistic regression analysis.
| Variables | CLABSI (Yes), n (%) N = 43 | Unadjusted odds ratio (95% CI) | P-value |
|---|---|---|---|
| Catheter type | |||
| Antimicrobial-impregnated | 14 (1.8%) | 1.0 (reference) | |
| Conventional | 29 (2.3%) | 1.33 (0.69, 2.53) | 0.390 |
| Gestational age at birth | |||
| <28 weeks | 33 (5.0%) | 1.0 (reference) | |
| 28 to <32 weeks | 6 (0.7%) | 0.13 (0.05, 0.31) | <0.001 |
| 32 to 36 weeks | 2 (0.6%) | 0.12 (0.03, 0.51) | 0.004 |
| >36 weeks | 2 (1.1%) | 0.21 (0.05, 0.90) | 0.036 |
| Birth weight (g) | |||
| <1,000 g | 30 (4.5%) | 1.0 (reference) | |
| 1,000 to <1,500 g | 9 (1.0%) | 0.22 (0.11, 0.47) | <0.001 |
| 1,500 to 2,500 g | 2 (0.6%) | 0.13 (0.03, 0.53) | 0.005 |
| >2,500 g | 2 (1.2%) | 0.25 (0.06, 1.06) | 0.060 |
| Age at insertion (days) | |||
| ≤5 days | 19 (1.4%) | 1.0 (reference) | |
| 5–10 days | 16 (3.9%) | 2.83 (1.44, 5.55) | 0.003 |
| >10 days | 8 (2.8%) | 1.97 (0.85, 4.53) | 0.113 |
| Dwell time (days) | |||
| ≤14 days | 23 (2.0%) | 1.0 (reference) | |
| >14 days | 20 (3.2%) | 1.64 (0.89, 3.01) | 0.112 |
| Gender | |||
| Male | 24 (2.2%) | 1.0 (reference) | |
| Female | 19 (2.0%) | 0.94 (0.51, 1.73) | 0.851 |
| Reason for insertion | |||
| Long-term IV fluid therapy | 41 (2.3%) | 1.0 (reference) | |
| Long-term IV medication therapy | 2 (3.7%) | 1.66 (0.39, 7.07) | 0.490 |
| Number of catheter lumens | |||
| Single lumen antimicrobial-impregnated | 14 (1.8%) | 1.0 (reference) | |
| Single lumen conventional | 29 (2.5%) | 1.41 (0.74, 2.68) | 0.297 |
| Insertion technique | |||
| MST | 10 (0.9%) | 1.0 (reference) | |
| MicroFlash | 10 (2.8%) | 3.16 (1.30, 7.66) | 0.011 |
| Split steel needle | 7 (6.5%) | 7.65 (2.85, 20.54) | <0.001 |
| No. of attempts/pricks | |||
| 1 | 26 (2.0%) | 1.0 (reference) | |
| 2 | 14 (3.2%) | 1.60 (0.83, 3.09) | 0.161 |
| 3 | 3 (1.2%) | 0.58 (0.18, 1.94) | 0.380 |
| Site of insertion | |||
| Lower extremities | 28 (1.8%) | 1.0 (reference) | |
| Upper extremities | 15 (3.0%) | 1.69 (0.90, 3.26) | 0.104 |
| Side of the body | |||
| Right | 24 (1.8%) | 1.0 (reference) | |
| Left | 19 (2.7%) | 1.50 (0.81, 2.75) | 0.196 |
| Glue applied | |||
| No | 30 (3.2%) | 1.0 (reference) | |
| Yes | 13 (1.5%) | 0.47 (0.24, 0.90) | 0.024 |
| Type of preflush | |||
| Normal saline | 39 (2.5%) | 1.0 (reference) | |
| Lipid emulsion | 4 (0.8%) | 0.33 (0.12, 0.92) | 0.035 |
CI, confidence interval; CLABSI, central line-associated bloodstream infection; MST, modified Seldinger technique.
Outcome variable: the non-CLABSI group was considered the reference group.
All the percentage values were computed using nonmissing values.
Results
Our dedicated PICC team including physicians and nurses inserted 1,242 conventional (PremiCath and NutriLine) and 791 antimicrobial-impregnated PICCs (PremiStar) over the study period from 2017 to 2020. Of those 1,242 conventional PICCs, 1,171 (94.3%) were 1 Fr single lumen and only 71 (5.7%) were 2 Fr double lumen. Table 1 shows the mean ± SD [median (IQR)] for the birth weight, gestational age, age at the time of insertion, and catheter dwell time for the whole study population.
Table 1.
Demographics of the study sample.
| Variables | Mean ± SD [median (IQR)] |
|---|---|
| Gestational age at birth (weeks) | 29.6 ± 4.03 [29 (27, 31)] |
| <28 weeks | 655 (32.2%) |
| 28 to <32 weeks | 889 (43.7%) |
| 32 to 36 weeks | 311 (15.3%) |
| >36 weeks | 178 (8.8%) |
| Age at insertion (days) | 9.3 ± 21.47 [2 (1, 9)] |
| Birth weight (g) | 1,343.3 ± 686.75 [1,200 (900, 1,500)] |
| <1,000 g | 663 (32.6%) |
| 1,000 to <1,500 g | 860 (42.3%) |
| 1,500 to 2,500 g | 340 (16.7%) |
| >2,500 g | 170 (8.4%) |
| Dwell time (days) | 15.7 ± 14.03 [12 (8, 17)] |
Median and interquartile ranges (IQRs) were used for skewed data.
All the percentage values were computed using nonmissing values.
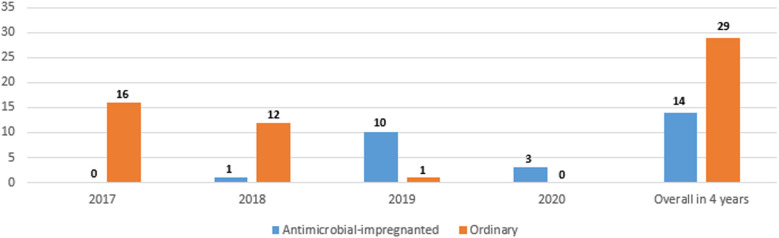
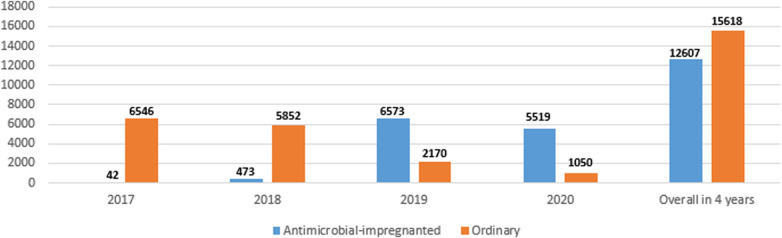
Figure 1 shows the numbers of the two types of catheters across the 4 years showing a gradual increase in the use of antimicrobial-impregnated catheters. The main indication for PICC line insertion in our study was the need for long-term IV fluid therapy 1,815/2,033 (89.3%) and the most commonly used catheter was the single lumen conventional PICC 1,171/2,033 (57.6%) followed by the single lumen antimicrobial-impregnated PICC 791/2,033 (38.9%). The overall success rate of the PICC insertion is 1,815/2,033 (89.3%), while the first attempt success rate is 1,290/2,033 (63.5%). The main reason for the central venous catheter removal in our study was the elective removal after completion of therapy 1,449/2,033 (79.8%). We had 43/2,033 (2.1%) CLABSI cases in our study sample (Table 2). Figure 2 shows the number of CLABSI cases between the two types of catheters across the 4 years and Figure 3 shows the dwell time in days between the two types of catheters across the 4 years.
Figure 1.
Numbers of the two types of catheters across the 4 years.
Table 2.
Clinical variables of the study sample.
| Variables | (n = 2,033) (%) |
|---|---|
| Gender | |
| Male | 1,106 (54.4%) |
| Female | 927 (45.6%) |
| Reason for insertion | |
| Long-term IV fluid therapy | 1,815 (89.3%) |
| Long-term IV medication therapy | 54 (2.7%) |
| Hypoglycemia | 58 (2.9%) |
| Difficult IV insertion | 106 (5.2%) |
| Catheter type | |
| Antimicrobial-impregnated | 791 (38.9%) |
| Conventional | 1,242 (61.1%) |
| Number of catheter lumens | |
| Single lumen antimicrobial-impregnated | 791 (38.9%) |
| Single lumen conventional | 1,171 (57.6%) |
| Double lumen conventional | 71 (3.5%) |
| Insertion technique | |
| MST | 1,103 (70.4%) |
| MicroFlash | 356 (22.7%) |
| Split steel needle | 107 (6.8%) |
| No. of attempts/pricks | |
| 1 | 1,290 (63.5%) |
| 2 | 439 (21.6%) |
| 3 | 253 (12.4%) |
| ≥4 | 51 (2.5%) |
| Site of insertion | |
| Lower extremities | 1,539 (75.7%) |
| Upper extremities | 493 (24.3%) |
| Side of the body | |
| Right | 1,325 (65.2%) |
| Left | 708 (34.8%) |
| Successful insertion | |
| No | 218 (10.7%) |
| Yes | 1,815 (89.3%) |
| Glue applied | |
| No | 951 (52.3%) |
| Yes | 866 (47.7%) |
| Type of preflush | |
| Normal saline | 1,554 (76.5%) |
| Lipid emulsion | 478 (23.5%) |
| Resistance during the guidewire removal | |
| No | 602 (85.3%) |
| Yes | 104 (14.7%) |
| Reason for removal | |
| Elective removal | 1,449 (79.8%) |
| Accidental removal | 8 (0.4%) |
| Broken catheter | 14 (0.8%) |
| Leaking | 28 (1.5%) |
| Extravasation/infiltration | 43 (2.4%) |
| Malposition | 22 (1.2%) |
| Occlusion | 42 (2.3%) |
| Phlebitis | 55 (3.0%) |
| CLABSI | 43 (2.4%) |
| Preventive | 78 (4.3%) |
| Death | 34 (1.9%) |
| CLABSI | |
| No | 1,990 (97.9%) |
| Yes | 43 (2.1%) |
CLABSI, central line-associated bloodstream infection; MST, modified Seldinger technique.
All the percentage values were computed using nonmissing values.
Figure 2.
Number of CLABSI cases between the two types of catheters across the 4 years.
Figure 3.
Dwell time in days between the two types of catheters across the 4 years.
Table 3 shows the mean ± SD [median (IQR)] gestational age, birth weight, age at catheter insertion, and catheter dwell time in the antimicrobial-impregnated catheter compared with the conventional PICCs.
Table 3.
Association between catheter types and other parameters.
| Parameters | Antimicrobial-impregnated catheters, n (%) (N = 791) | Conventional catheters, n (%) (N = 1,242) | P-value |
|---|---|---|---|
| Gestational age at birth (weeks) | 28.8 ± 3.24 (median 29, IQR 26, 31) | 30.1 ± 4.39 (median 29, IQR 27, 32) | <0.001 |
| <28 weeks | 315 (39.8%) | 340 (27.4%) | <0.001 |
| 28 to <32 weeks | 342 (43.2%) | 547 (44.0%) | |
| 32 to 36 weeks | 120 (15.2%) | 191 (15.4%) | |
| >36 weeks | 14 (1.8%) | 164 (13.2%) | |
| Age at insertion (days) | 6.3 ± 10.85 (median 2, IQR 1, 8) | 11.1 ± 25.9 (median 1, IQR 1, 9) | <0.001 |
| Birth weight (g) | 1,192.1 ± 410.3 (median 1,150, IQR 900, 1,450) | 1,439.5 ± 800.8 (median 1,240, IQR 920, 1,520) | <0.001 |
| <1,000 g | 282 (35.7%) | 381 (30.7%) | <0.001 |
| 1,000 to <1,500 g | 339 (42.9%) | 521 (41.9%) | |
| 1,500 to 2,500 g | 158 (20.0%) | 182 (14.7%) | |
| >2,500 g | 12 (1.5%) | 158 (12.7%) | |
| Dwell time (days) | 17.73 ± 17.532 (median 13, IQR 9, 18) | 14.30 ± 10.964 (median 12, IQR 8, 17) | <0.001 |
| Gender | |||
| Male | 438 (55.4%) | 668 (53.8%) | 0.483 |
| Female | 353 (44.6%) | 574 (46.2%) | |
| Reason for insertion | |||
| Long-term IV fluid therapy | 715 (90.4%) | 1,100 (88.6%) | 0.027 |
| Long-term IV medication therapy | 27 (3.4%) | 27 (2.2%) | |
| Hypoglycemia | 15 (1.9%) | 43 (3.5%) | |
| Difficult IV insertion | 34 (4.3%) | 72 (5.8%) | |
| Number of catheter lumens | |||
| Single lumen | 791 (100.0%) | 1,171 (94.3%) | <0.001 |
| Double lumen | 0 (0.0%) | 71 (5.7%) | |
| Insertion technique | |||
| MST | 694 (88.1%) | 409 (52.6%) | <0.001 |
| MicroFlash | 53 (6.7%) | 303 (38.9%) | |
| Split steel needle | 41 (5.2%) | 66 (8.5%) | |
| No. of attempts/pricks | |||
| 1 | 523 (66.1%) | 767 (61.8%) | 0.021 |
| 2 | 174 (22.0%) | 265 (21.3%) | |
| 3 | 78 (9.9%) | 175 (14.1%) | |
| ≥4 | 16 (2.0%) | 35 (2.8%) | |
| Site of insertion | |||
| Lower extremities | 679 (85.8%) | 860 (69.3%) | <0.001 |
| Upper extremities | 112 (14.2%) | 381 (30.7%) | |
| Side of the body | |||
| Right | 566 (71.6%) | 759 (61.1%) | <0.001 |
| Left | 225 (28.4%) | 483 (38.9%) | |
| Successful insertion | |||
| No | 72 (9.1%) | 146 (11.8%) | 0.059 |
| Yes | 719 (90.9%) | 1,096 (88.2%) | |
| Glue applied | |||
| No | 84 (11.7%) | 867 (79.0%) | <0.001 |
| Yes | 635 (88.3%) | 231 (21.0%) | |
| Type of preflush | |||
| Normal saline | 370 (46.8%) | 1,184 (95.4%) | <0.001 |
| Lipid emulsion | 421 (53.2%) | 57 (4.6%) | |
| Resistance during the guidewire removal | |||
| No | 487 (86.7%) | 115 (79.9%) | 0.040 |
| Yes | 75 (13.3%) | 29 (20.1%) | |
| Reason for removal | |||
| Elective removal | 637 (88.6%) | 812 (74.0%) | <0.001 |
| Accidental removal | 2 (0.3%) | 6 (0.5%) | 0.398 |
| Broken catheter | 6 (0.8%) | 8 (0.7%) | 0.802 |
| Leaking | 7 (1%) | 21 (1.9%) | 0.112 |
| Extravasation/infiltration | 3 (0.4%) | 40 (3.6%) | <0.001 |
| Malposition | 7 (1%) | 15 (1.4%) | 0.453 |
| Occlusion | 10 (1.4%) | 32 (2.9%) | 0.034 |
| Phlebitis | 6 (0.8%) | 49 (4.5%) | <0.001 |
| CLABSI | 14 (1.9%) | 29 (2.6%) | 0.340 |
| Preventive | 21 (2.9%) | 57 (5.2%) | 0.019 |
| Death | 6 (0.8%) | 28 (2.6%) | 0.008 |
| CLABSI | |||
| No | 777 (98.2%) | 1,213 (97.7%) | 0.388 |
| Yes | 14 (1.8%) | 29 (2.3%) | |
| Group of the CLABSI causative organism | |||
| Gram-positive organism | 4 (28.6%) | 8 (27.6%) | 0.946 |
| Gram-negative organism | 10 (71.4%) | 21 (72.4%) | |
| The subgroup of the CLABSI causative organisms | |||
| Gram-positive cocci | 4 (28.6%) | 7 (24.1%) | 0.757 |
| Gram-positive bacilli | 0 (0%) | 1 (3.4%) | |
| Gram-negative bacilli | 10 (71.4%) | 21 (72.4%) | |
CLABSI, central line-associated bloodstream infection; MST, modified Seldinger technique; IQR, interquartile range.
Yates corrected Chi-square test was applied in case of small cell frequencies (50% or more cells have expected frequencies <5), whereas the quantitative outcome measures were compared using a t-test or nonparametric Mann–Whitney U-test as appropriate.
All the percentage values were computed using nonmissing values.
The majority of our PICCs were inserted in the lower extremities 1,539/2,033 (75.7%). The veins used for central line insertion in our study population are great saphenous 1,536 (75.6%), antecubital 364 (17.9%), cephalic 61 (3.0%), basilic 41 (2.0%), brachial 18 (0.9%), axillary 5 (0.2%), metacarpal 2 (0.1%), popliteal 2 (0.1%), temporal 2 (0.1%), and femoral 1 (0%) veins.
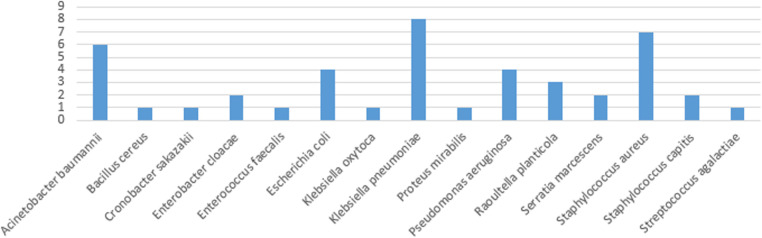
Of the 43 organisms causing CLABSI, 31 (72%) were gram-negative while the remaining 12 (28%) were gram-positive organisms. Of those gram-positive organisms, 1 (8.3%) was gram-positive bacilli, 9 (75%) were gram-positive cocci in clusters, and 2 (16.7%) were gram-positive cocci in pairs/chains. All the gram-negative organisms were gram-negative bacilli.
The use of the antimicrobial-impregnated catheter was not associated with any significant reduction in the CLABSI rate (per 1,000 days dwell time), either the overall [P = 0.11, risk ratio (RR) (95% CI): 0.60 (0.32, 1.13)] or the yearly CLABSI rates (Table 4). The CLABSI-causative organisms in both types of catheters in the 4 years are shown in Figure 4.
Figure 4.
CLABSI-causative organisms in both types of catheters across the 4 years.
Univariate and multivariate logistic regression analysis was performed to test for the potential factors, predictors and possible association with the dichotomous outcome variable (CLABSI and non-CLABSI). Results of the univariate analysis indicated that gestational age at birth, birth weight, age at insertion, insertion techniques, cyanoacrylate glue application, and type of preflush had a significant association with CLABSI. The mean gestational age at birth for those who developed CLABSI was 26.58 ± 3.83 vs. 29.64 ± 4.01 weeks for those who did not, unadjusted odds ratio (95% CI) 0.75 (0.67, 0.85), P < 0.001. The mean birth weight for those who had CLABSI was 1,014.05 ± 743.06 vs. 1,350.36 ± 683.93 g for those who did not, unadjusted odds ratio (95% CI) 0.98 (0.98, 0.99), P = 0.001. The mean age at the central line insertion for those who developed CLABSI was 11.56 ± 18.64 vs. 9.23 ± 21.53 days for those who did not, unadjusted odds ratio (95% CI) 1.01 (0.99, 1.02), P = 0.084. The mean dwells time for those who had CLABSI was 20.49 ± 16.64 vs. 15.54 ± 13.94 days for those who did not, unadjusted odds ratio (95% CI) of 1.02 (1.01, 1.03), P = 0.036. The insertion technique most associated with CLABSI was the use of the split needle 7 (6.5%), unadjusted odds ratio (95% CI) 7.65 (2.85, 20.54), P < 0.001 compared with the MicroFlash 10 (2.8%), unadjusted odds ratio (95% CI) 3.16 (1.30, 7.65), P = 0.011 and MST which had the least CLABSI 10 (0.9%) (Table 5).
The multivariate logistic regression analysis showed that gestational age at birth (adjusted OR 0.19, 95% CI, 0.07, 0.51, P < 0.001, when compared groups gestational age 28 to <32 weeks with reference group <28 weeks), number of attempts/pricks (adjusted OR 2.68, 95% CI, 1.19, 6.01, P = 0.017, when compared the number of attempts/pricks 2 with 1), and insertion techniques (adjusted OR 3.39, 95% CI, 1.37, 8.37, P = 0.008, when compared MicroFlash with reference group MST and adjusted OR 5.34, 95% CI, 1.91, 14.89, P = 0.001, when compared split steel needle with reference group MST) were associated with significantly more CLABSI after adjusting for other predictors and factors (Table 6). None of the clinical variables or catheter-related parameters was significantly associated with any gram stain type of the CLABSI-causative organisms in both groups. The discriminative ability of the significant predictors (observed in multivariate analysis) in predicting CLABSI was found to be good with an area under the ROC curve value of 0.871 (95% CI, 0.82, 0.92), which indicates that this developed regression model demonstrated an excellent fit (Figure 5).
Table 6.
Predictors associated with CLABSI: multivariate logistic regression analysis.
| Predictors | Adjusted odds ratio (OR) | 95% CI for adjusted OR | P-value |
|---|---|---|---|
| Gestational age at birth | |||
| <28 weeks | 1.0 (reference) | ||
| 28 to <32 weeks | 0.19 | 0.07, 0.51 | <0.001 |
| 32 to 36 weeks | 0.23 | 0.05, 1.01 | 0.052 |
| >36 weeks | – | – | – |
| No. of attempts/pricks | |||
| 1 | 1.0 (reference) | ||
| 2 | 2.68 | 1.19, 6.01 | 0.017 |
| 3 | 0.35 | 0.04, 2.78 | 0.321 |
| Insertion technique | |||
| MST | 1.0 (reference) | ||
| MicroFlash | 3.39 | 1.37, 8.37 | 0.008 |
| Split steel needle | 5.34 | 1.91, 14.89 | 0.001 |
CI, confidence interval; CLABSI, central line-associated bloodstream infection; MST, modified Seldinger technique.
Outcome variable: the non-CLABSI group was considered the reference group.
All the percentage values were computed using nonmissing values.
Figure 5.
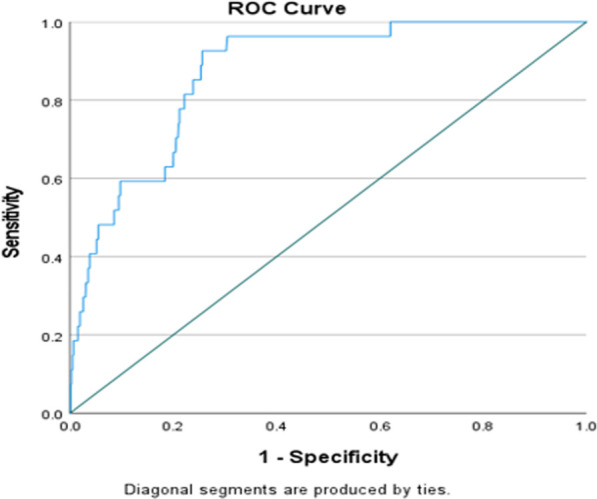
Area under the curve (AUC) value: 0.871 (95% CI, 0.82, 0.92).
Discussion
The neonatal antimicrobial-impregnated catheter was introduced to the market in December 2012 after getting the appropriate approvals and certification under the Conformité Européenne process (certificate number Z/12/02895) to reduce the rate and severity of CLABSI in NICUs. It has been used in many countries including Germany, Italy, and the UK. Despite the overall gradual decrease in the CLABSI rate in our NICU from 12.18 per 1,000 days dwell time in 2010 to 2.14 per 1,000 days dwell time in 2019, this improvement remained unsteady and showed a lack of sustainability (21, 22, 24). In 2016, the antimicrobial-impregnated PICC was introduced to the unit to help in reducing the CLABSI rate aiming for Zero-CLABSI.
From our study, no statistically significant differences or reductions in CLABSI rates were observed in neonates with the miconazole and rifampicin-impregnated catheters compared with those with the conventional PICCs. This applies to both the yearly and the overall CLABSI rates. In 2018, 2019, and 2020, the CLABSI rates were even higher in the antimicrobial-impregnated catheters compared with the conventional catheters; however, this difference was not statistically significant. This is related to the number of catheters in each category per year as displayed in Figure 1. The PREVAIL trial in neonates reported similar findings to ours with no evidence to support the use of the miconazole and rifampicin-impregnated catheters over the conventional catheter (12). The CATCH trial in children also reported no significant difference or superiority of the antimicrobial-impregnated catheters over the conventional ones (13). A meta-analysis to investigate the efficacy of the antimicrobial-impregnated catheters for the prevention of CLABSI in the neonatal and pediatric populations had also similar findings to ours and the PREVAIL trial (14). Furthermore, the antimicrobial-impregnated catheter; PremiStar is more expensive (QAR 720) compared with the conventional ones; PremiCath (QAR 305) and NutriLine (QAR 335). A randomized controlled trial was conducted in the UK to investigate the cost-effectiveness of different strategies to prevent late-onset sepsis in preterms and concluded that the antimicrobial-impregnated catheters are not likely to be cost-effective (32, 33). On the other hand, in a small trial available in the Cochrane database involving 98 patients, the authors found that silver zeolite-impregnated umbilical venous catheters (UVCs) significantly reduced the incidence of catheter-related bloodstream infections in preterms (34). A systematic review and network meta-analysis of the adult population demonstrated that antimicrobial-impregnated CVCs significantly reduced the CLABSI rate compared with standard catheters (15).
The early insertion, use of glue, MST insertion technique, single lumen catheters, a small number of attempts, and the use of lipid emulsion to facilitate the guidewire removal are all significantly higher in the miconazole and rifampicin-impregnated catheter and are all supposed to reduce the CLABSI rate (25, 35, 36). On the other hand, the birth weight and the gestational age were significantly lower in the miconazole and rifampicin-impregnated catheter group together with the longer dwell time compared with the conventional PICC. These factors are known to contribute to a higher CLABSI rate (37, 38). The multivariate logistic regression analysis of our results showed that the gestational age, the number of attempts, and the insertion technique are the predictors associated with CLABSI. However, the net result from our study is that the use of the miconazole and rifampicin-impregnated catheter did not improve the CLABSI rate compared with the conventional PICCs.
Results from our study show that the use of antimicrobial-impregnated catheters is not without benefits. Compared with conventional catheters, it has an overall elective removal rate of 637/791 (88.6%) compared with 812/1,242 (74.0%) in conventional catheters (P < 0.001). Also, the rate of extravasation/infiltration, occlusion, phlebitis, and preventive removals were all less in the antimicrobial-impregnated catheters (0.4%, 1.4%, 0.8%, and 2.9%) compared with the conventional ones (3.6% P < 0.001, 2.9% P = 0.034, 4.5% P = 0.001, and 5.2% P = 0.019), respectively. This can not be explained by the smaller caliber in the antimicrobial-impregnated catheters as most conventional PICC 1,171/1,242 (94.3%) has the same caliber. Preventive removals are related to the PICC team members who removed the line prematurely without a clear indication but extravasation/infiltration, occlusion, and phlebitis are related to the catheter itself. These complications have considerable clinical and healthcare cost impacts on neonates in NICU.
Materials used in central venous catheter impregnation in adults and pediatric populations available in the literature include antibiotics, such as vancomycin, teicoplanin, 5-fluorouracil, benzalkonium chloride, minocycline, minocycline/rifampicin, miconazole/rifampicin, and antiseptics; such as Oligon Vantex silver or silver, silver zeolite (AgION), chlorhexidine/silver sulfadiazine (15). To the best of our knowledge, the only type of neonatal antimicrobial-impregnated PICC is the one included in our study, which is impregnated with miconazole and rifampicin. These antimicrobials might not be fully and equally effective against the different CLABSI-causative organisms displayed in Figure 4. The emergence of resistant strains of the known CLABSI-causative organisms and the growth of new rare strains of bacteria should also be put into consideration. Those could be the reasons for our main study result stating the antimicrobial-impregnated catheters are not superior to the conventional ones in reducing the CLABSI rate. We encourage the manufacturers and vascular access industrial bodies to try the impregnation of neonatal catheters with other materials based on the types of neonatal CLABSI-causative organisms and their microbiome signatures (39).
The main limitation of our study is the retrospective and single-centered study design. However, the large sample size of 2,033 neonates increases its validity, power, and generalizability. Also, the two groups of neonates analyzed are quite different in terms of gestational age and weight. As many confounders might still exist, well-designed large-sample pragmatic randomized controlled trials (RCTs) are urgently indicated. Blinding both the caregivers and the study team is recommended to avoid introducing bias.
Reducing CLABSI is not dependent upon a single intervention. It is multifactorial and needs multidisciplinary team collaboration with continuous quality improvement projects. It remains a challenge to achieve and maintain zero-CLABSI in NICU.
Conclusions
The use of miconazole and rifampicin-impregnated PICCs did not reduce the CLABSI rate in neonates compared with conventional PICCs. However, it has a higher overall rate of elective removal after completion of therapy and less extravasation/infiltration, occlusion, and phlebitis compared with the conventional PICCs. Further large RCTs are recommended to enrich the current paucity of evidence and to reduce the risk of bias. Neonatal PICCs impregnation by other antimicrobials is a recommendation for VAD manufacturers.
Acknowledgments
Special thanks to the entire PICC, NSN, and infection control teams in WWRC who provide high-quality care to our newborns.
Funding
This work was funded and supported by the Medical Research Center (MRC), Hamad Medical Corporation, Doha, Qatar (Protocol number MRC-01-20-327).
Data availability statement
The datasets generated for this study are available on reasonable request to the corresponding author. Data requests should be made to Dr. Mohammad A. A. Bayoumi by email to moh.abdelwahab@hotmail.com.
Ethics statement
This research study was approved by the Institutional Review Board (IRB) of the Medical Research Center, Hamad Medical Corporation, Doha, Qatar (MRC-01-20-327). A waiver for the requirement of informed consent from the mothers whose records were analyzed was granted by the Chair of the Medical Research Center on the grounds of being a minimal-risk study. All methods were performed following the relevant guidelines and regulations. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
MAAB and MVR were the main investigators, conceptualized and designed the study, coordinated, and supervised data collection, drafted the initial manuscript, and reviewed and revised the subsequent manuscript versions. MVR, AlM, SD'S, FA, and MAAB collected, cleaned and anonymized the data. PC designed and performed the data analysis. MAAB, PC, and EE drafted the initial manuscript. AMK, AfS, SaA, RM, and SuS revised the manuscript. All authors reviewed and revised the manuscript, and approved the final submitted manuscript.
Conflict of interest
Author SS was employed by company VERITADYNE Strategic Consulting Pvt. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Barone G, Pittiruti M. Epicutaneo-caval catheters in neonates: new insights and new suggestions from the recent literature. J Vasc Access. (2020) 21(6):805–9. 10.1177/1129729819891546 [DOI] [PubMed] [Google Scholar]
- 2.McCay AS, Elliott EC, Walden M. Videos in clinical medicine. PICC placement in the neonate. N Engl J Med. (2014) 370(11):e17. 10.1056/NEJMvcm1101914 [DOI] [PubMed] [Google Scholar]
- 3.Yu X, Yue S, Wang M, Cao C, Liao Z, Ding Y, et al. Risk factors related to peripherally inserted central venous catheter nonselective removal in neonates. Biomed Res Int. (2018) 2018:3769376. 10.1155/2018/3769376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado JD, Suen VM, Figueiredo JF, Marchini JS. Biofilms, infection, and parenteral nutrition therapy. JPEN J Parenter Enteral Nutr. (2009) 33(4):397–403. 10.1177/0148607108327526 [DOI] [PubMed] [Google Scholar]
- 5.Sib E, Voigt AM, Wilbring G, Schreiber C, Faerber HA, Skutlarek D, et al. Antibiotic resistant bacteria and resistance genes in biofilms in clinical wastewater networks. Int J Hyg Environ Health. (2019) 222(4):655–62. 10.1016/j.ijheh.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Bertini G, Elia S, Ceciarini F, Dani C. Reduction of catheter-related bloodstream infections in preterm infants by the use of catheters with the AgION antimicrobial system. Early Hum Dev. (2013) 89(1):21–5. 10.1016/j.earlhumdev.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. (2002) 110(2 Pt 1):285–91. 10.1542/peds.110.2.285 [DOI] [PubMed] [Google Scholar]
- 8.Piening BC, Geffers C, Gastmeier P, Schwab F. Pathogen-specific mortality in very low birth weight infants with primary bloodstream infection. PLoS ONE. (2017) 12(6):e0180134. 10.1371/journal.pone.0180134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitha A, Foix-L’Helias L, Arnaud C, Marret S, Vieux R, Aujard Y, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. (2013) 132(2):e372–80. 10.1542/peds.2012-3979 [DOI] [PubMed] [Google Scholar]
- 10.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. (2011) 39(4 Suppl 1):S1–S34. 10.1016/j.ajic.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. (2014) 86(Suppl 1):S1–S70. 10.1016/S0195-6701(13)60012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert R, Brown M, Rainford N, Donohue C, Fraser C, Sinha A, et al. Antimicrobial-impregnated central venous catheters for prevention of neonatal bloodstream infection (PREVAIL): an open-label, parallel-group, pragmatic, randomised controlled trial. Lancet Child Adolesc Health. (2019) 3(6):381–90. 10.1016/S2352-4642(19)30114-2 [DOI] [PubMed] [Google Scholar]
- 13.Harron K, Mok Q, Dwan K, Ridyard CH, Moitt T, Millar M, et al. CATheter infections in CHildren (CATCH): a randomised controlled trial and economic evaluation comparing impregnated and standard central venous catheters in children. Health Technol Assess. (2016) 20(18):vii–xxviii, 1–219. 10.3310/hta20180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai L, Yue X. Efficacy of antimicrobial-impregnated catheters for prevention of bloodstream infections in pediatric patients: a meta-analysis. Front Pediatr. (2021) 9:632308. 10.3389/fped.2021.632308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Tong H, Liu H, Wang Y, Wang R, Gao H, et al. Effectiveness of antimicrobial-coated central venous catheters for preventing catheter-related blood-stream infections with the implementation of bundles: a systematic review and network meta-analysis. Ann Intensive Care. (2018) 8(1):71. 10.1186/s13613-018-0416-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rump AF, Guttler K, Konig DP, Yucel N, Korenkov M, Schierholz JM. Pharmacokinetics of the antimicrobial agents rifampicin and miconazole released from a loaded central venous catheter. J Hosp Infect. (2003) 53(2):129–35. 10.1053/jhin.2002.1358 [DOI] [PubMed] [Google Scholar]
- 17.Sivanandan S. Do antimicrobial-impregnated central venous catheters prevent nosocomial bloodstream infection in neonates? Acta Paediatr. (2020) 109(9):1907–8. 10.1111/apa.15268 [DOI] [PubMed] [Google Scholar]
- 18.Uygun I, Okur MH, Otcu S, Ozturk H. Peripherally inserted central catheters in the neonatal period. Acta Cir Bras. (2011) 26(5):404–11. 10.1590/S0102-86502011000500014 [DOI] [PubMed] [Google Scholar]
- 19.Qin KR, Nataraja RM, Pacilli M. Long peripheral catheters: is it time to address the confusion? J Vasc Access. (2019) 20(5):457–60. 10.1177/1129729818819730 [DOI] [PubMed] [Google Scholar]
- 20.Bayoumi MAA, Elmalik EE, Ali H, D’Souza S, Furigay J, Romo A, et al. Neonatal simulation program: a 5 years educational journey from Qatar. Front Pediatr. (2022) 10:843147. 10.3389/fped.2022.843147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayoumi MAA, van Rens R, Chandra P, Shaltout D, Gad A, Elmalik EE, et al. Peripherally inserted central catheters versus non-tunnelled ultrasound-guided central venous catheters in newborns: a retrospective observational study. BMJ Open. (2022) 12(4):e058866. 10.1136/bmjopen-2021-058866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayoumi MAA, Van Rens MFP, Chandra P, Francia ALV, D’Souza S, George M, et al. Effect of implementing an epicutaneo-caval catheter team in neonatal intensive care unit. J Vasc Access. (2021) 22(2):243–53. 10.1177/1129729820928182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorski LA, Hadaway L, Hagle ME, Broadhurst D, Clare S, Kleidon T, et al. Infusion therapy standards of practice, 8th edition. J Infus Nurs. (2021) 44(1S Suppl 1):S1–S224. 10.1097/NAN.0000000000000396 [DOI] [PubMed] [Google Scholar]
- 24.van Rens M, Hugill K, Francia ALV, Mahmah MA, Al Shadad ABJ, Chiuco IC, et al. Closed intravenous systems for central vascular access: a difference maker for CLABSI rates in neonates? J Vasc Access. (2022):11297298221085480. 10.1177/11297298221085480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Rens M, Paramban R, Francia ALV, Chandra P, Mahmah MA, Thome UH, et al. Evaluation of a diluted lipid emulsion solution as a lubricant for improved peripherally inserted central catheter guidewire removal in a neonatal population. BMC Pediatr. (2022) 22(1):71. 10.1186/s12887-022-03119-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittiruti M, Annetta MG, Marche B, D’Andrea V, Scoppettuolo G. Ten years of clinical experience with cyanoacrylate glue for venous access in a 1300-bed university hospital. Br J Nurs. (2022) 31(8):S4–S13. 10.12968/bjon.2022.31.8.S4 [DOI] [PubMed] [Google Scholar]
- 27.Hussain AS, Ahmed AM, Arbab S, Ariff S, Ali R, Demas S, et al. CLABSI reduction using evidence based interventions and nurse empowerment: a quality improvement initiative from a tertiary care NICU in Pakistan. Arch Dis Child. (2021) 106(4):394–400. 10.1136/archdischild-2019-318779 [DOI] [PubMed] [Google Scholar]
- 28.Phipps K, Modic A, O’Riordan MA, Walsh M. A randomized trial of the vein viewer versus standard technique for placement of peripherally inserted central catheters (PICCs) in neonates. J Perinatol. (2012) 32(7):498–501. 10.1038/jp.2011.129 [DOI] [PubMed] [Google Scholar]
- 29.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. (2011) 52(9):e162–93. 10.1093/cid/cir257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain ASS, Ali SR, Ariff S, Arbab S, Demas S, Zeb J, et al. A protocol for quality improvement programme to reduce central line-associated bloodstream infections in NICU of low and middle income country. BMJ Paediatr Open. (2017) 1(1):e000008. 10.1136/bmjpo-2017-000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regunath YH, Pavan A, Hariharan R. Central line associated blood stream infections. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2022).
- 32.Grosso A, de Faria RIN, Bojke L, Donohue C, Fraser CI, Harron KL, Oddie SJ, et al. Correction: cost-effectiveness of strategies preventing late-onset infection in preterm infants. Arch Dis Child. (2021) 106(1):e1. 10.1136/archdischild-2019-317640corr1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grosso A, Neves de Faria RI, Bojke L, Donohue C, Fraser CI, Harron KL, et al. Cost-effectiveness of strategies preventing late-onset infection in preterm infants. Arch Dis Child. (2020) 105(5):452–7. 10.1136/archdischild-2019-317640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balain M, Oddie SJ, McGuire W. Antimicrobial-impregnated central venous catheters for prevention of catheter-related bloodstream infection in newborn infants. Cochrane Database Syst Rev. (2015) 2015(9):CD011078. 10.1002/14651858.CD011078.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rens M, Nimeri AMA, Spencer TR, Hugill K, Francia ALV, Olukade TO, et al. Cyanoacrylate securement in neonatal PICC use: a 4-year observational study. Adv Neonatal Care. (2022) 22(3):270–9. 10.1097/ANC.0000000000000963 [DOI] [PubMed] [Google Scholar]
- 36.Pearlman SA. Quality improvement to reduce neonatal CLABSI: the journey to zero. Am J Perinatol. (2020) 37(S 02):S14–7. 10.1055/s-0040-1713605 [DOI] [PubMed] [Google Scholar]
- 37.Bierlaire S, Danhaive O, Carkeek K, Piersigilli F. How to minimize central line-associated bloodstream infections in a neonatal intensive care unit: a quality improvement intervention based on a retrospective analysis and the adoption of an evidence-based bundle. Eur J Pediatr. (2021) 180(2):449–60. 10.1007/s00431-020-03844-9 [DOI] [PubMed] [Google Scholar]
- 38.Payne V, Hall M, Prieto J, Johnson M. Care bundles to reduce central line-associated bloodstream infections in the neonatal unit: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2018) 103(5):F422–9. 10.1136/archdischild-2017-313362 [DOI] [PubMed] [Google Scholar]
- 39.Pammi M, Thapa S, Balderas M, Runge JK, Venkatachalam A, Luna RA. Microbiome signatures in neonatal central line associated bloodstream infections. PLoS ONE. (2020) 15(1):e0227967. 10.1371/journal.pone.0227967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on reasonable request to the corresponding author. Data requests should be made to Dr. Mohammad A. A. Bayoumi by email to moh.abdelwahab@hotmail.com.