Abstract
Melioidosis is a complex tropical disease linked with many complications. It is increasingly diagnosed in India. The clinical mimicry of this disease with several other common causes of pneumonia has kept the clinicians in ignorance. Usually, the diagnosis and appropriate management get delayed. The organism closely resembles the common contaminant Pseudomonas and is easily misidentified in microbiology laboratories. The diagnosis is often missed because of poor diagnostic sensitivity of blood culture, the gold standard of the diagnosis. All this contributes to increased morbidity and mortality. The rampant use of high-end broad-spectrum antibiotics like ceftazidime and meropenem at suboptimal dose and duration suppresses the diagnosis without eradicating the disease, leaving the chance of recurrence from its latency even after years. As an infectious disease, the cure and prevention depend on early diagnosis and treatment. An awareness of its peculiar presentations and history can differentiate clinically and suspect the condition much easily from other mimickers of tuberculosis to sepsis. Ultimately, the prevention of melioidosis remains the critical strategy. Increasing the number of cases and intricated management of this fatal but potentially curable disease had prompted us to take up the mission of preventing the disease by spreading knowledge and awareness.
Keywords: Boots, Burkholderia pseudomallei, gloves, melioidosis, prevention, soil
Introduction
Melioidosis is a serious human disease caused by a saprophytic gram-negative bacillus of soil and water origin called Burkholderia pseudomallei. It is a disease of hot and humid regions within tropical countries and is endemic in several regions where health systems are developed unequally. The bacteria can survive long in extreme environments. This disease typically manifests with acute melioidosis as pneumonia, sepsis, and multiorgan abscesses, with a variable high mortality rate. The projected mortality from the disease is similar to that of measles.
Global Burden
The estimated global burden of melioidosis is over 46 lakh disability-adjusted life-years (DALYs) and is higher than other tropical diseases like intestinal nematode infections, leptospirosis schistosomiasis and dengue.[1] The global burden of the disease is estimated to be having 1.65 lakh human melioidosis cases annually worldwide, out of which nearly 89,000 people die annually. The actual mortality rates vary between 9 and 70% across the regions.[2] Melioidosis is grossly underreported in at least 45 countries, including Bangladesh and Sri Lanka, where it is endemic. Nearly 99% of the DALY’s burden was due to deaths from the disease. Around 1,65,000 melioidosis cases and 89,000 deaths worldwide as of 2015 have been estimated. These data exclude the critical data of melioidosis, which is “severely underreported in 45 countries it is to be endemic”.[3] Global warming along with soil changes, hot and humid weather, and unrestricted urbanisation may predispose to the additional problem and spread of melioidosis.[4]
As per the Centres for Disease Control and Prevention (CDC) of the USA, B. pseudomallei and B. mallei (which were being used for bioterrorism in World War I) have been classified as the second-highest priority (category-B) agents. Melioidosis is much commoner than ever believed to be diagnosed in tropical countries like India. Like any other infectious disease, infection prevention remains the essential strategy.
Current Gaps in Diagnosis and Prevention
The true epidemiology of melioidosis, particularly in developing tropical countries, is unknown. Many factors are contributing to the current gaps in preventing this disease. In many regions, lack of awareness among clinicians misses the clinically suspected cases. Second, melioidosis being a great mimicker, the clinical presentation occurs with varied manifestations leading to a delay in the diagnosis. Melioidosis necessitates a laboratory confirmation of the condition as the clinical manifestations are inconsistent and nonspecific. But the laboratory diagnostic systems are also less accessible in rural areas of poorly developed countries, where most cases have been estimated to be present. Inadequate laboratory infrastructure and diagnostic capacity in many tropical countries are other contributing factors for the underdiagnosis of the cases.[1,3] Therefore, it is largely misdiagnosed and underdiagnosed across the tropics. Even when diagnosed, it is too late or reported after the death of the patient in many cases. The varied and shared clinical manifestations confound and mimic other diseases, indicating that it is not easy to describe this clinical condition in standard terms. Probably, the lack of awareness, understanding and familiarity with this disease were the reasons for rare reporting.
Clinical presentation
The Burkholderia pseudomallei infection can present as acute (up to 2 months of symptoms) in some cases or chronic or latent with dormant bacilli. This disease frequently presents as a sub-clinical disease in immunocompetent individuals who cleared the infection due to immunity. The patients who, after acquiring the infection, progress to clinical manifestations are suspected of having melioidosis. The microbiologic confirmation is essential to establish the treatment. These patients are targeted for management and proper drug therapy.
Melioidosis usually presents as an acute or fulminant case and less commonly as a chronic disease. It can present with a wide variety of infections like a localised infection to sepsis, pneumonia, meningitis, abscesses of different organs (liver, spleen, kidneys, prostate, brain), septic arthritis, osteomyelitis and pyrexia of unknown origin (PUO) [Figure 1]. Patients with fever, cough, weight loss and lung infiltration are often misdiagnosed as tuberculosis and wrongly treated. Therefore, the diagnosis is delayed, and timely appropriate treatment of melioidosis is not always practicable. The presence of broad-based signs and symptoms can share with other diseases without a clue, resembling other conditions, often delaying melioidosis diagnosis and management. Melioidosis is called by the nickname ‘the great mimicker’.
Figure 1.
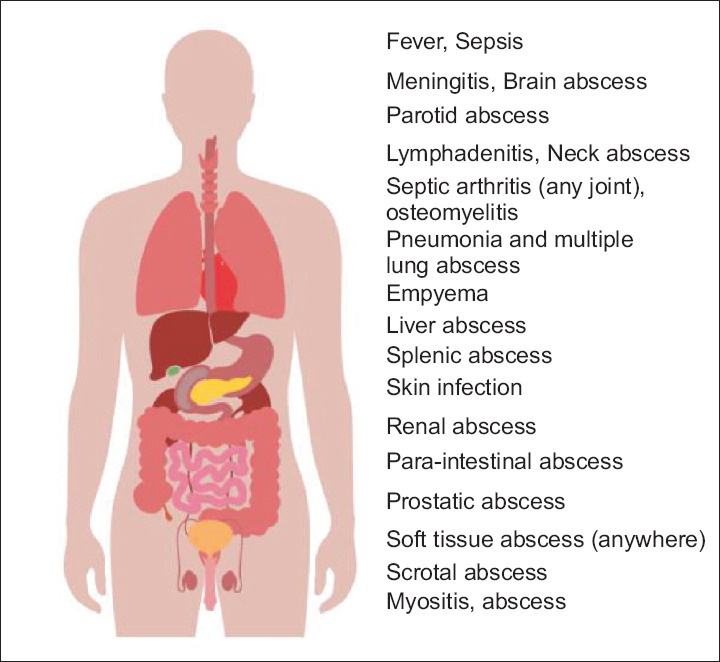
Spectrum of clinical manifestation of melioidosis
Transmission and prevention
As an infectious disease, the cure and prevention depend on early diagnosis and treatment. Awareness of its peculiar presentations and history can differentiate clinically and suspect the condition much easily from other mimickers of tuberculosis to sepsis. The common saying for infectious diseases is that “prevention is better than cure”; therefore, preventive actions are needed to strengthen them. It rarely spreads from person to person. So, isolating and treating the disease (one of the World Health Organisation [WHO] approaches) as in TB or corona virus disease (COVID-19) may not help in preventing melioidosis. Evidence-based guidelines and interventions targeting the risk factors, both behavioural and occupational, are also critical. Melioidosis mainly affects poverty-stricken rural segments of people in low and middle-income countries; over 99% of the deaths due to melioidosis happen in low-income countries.[3] The public health strategies are discussed here.
Prevention of transmission of melioidosis through soil and water
The infection is caused after exposure to B. pseudomallei in a susceptible person through contact of cracked or damaged unprotected skin, less commonly by aerosol inhalation and less commonly via B. pseudomallei-contaminated water.[5] Important modes of transmission and their preventions are discussed in Figure 2.
Figure 2.
Modes of transmission and prevention of melioidosis
The organism is commonly found in the soil region near plant roots, where the nutrient exchange is feasible, and surface groundwater of tropical and sub-tropical areas.[3,6] These highly pathogenic bacteria generally remain in the soil of tropical countries like some areas of Southeast Asia and Northern Australia.[7,8,9]
It is essential to increase the use of protective water-resistant ‘knee-high boots’ in the paddy field and gloves when working outdoors for protection from rain, loose soil and mud, where the bacteria survive. Precautions should be more during the rainy season and for farmers working in watery fields, like paddy fields.[10] In a country like northern Australia, basic public health instructions are usually given periodically to the high-risk occupational groups and the general population to protect themselves from contact directly with soil and water, particularly at the beginning of the rainy season.[10]
A study on the barriers for prevention revealed that 97% of the rice-farming rural population did not know melioidosis.[5] It has been established that providing information alone is unlikely to change their routine habits and accept the recommendations. Barriers to the change are the inherent tradition of continuing old habits, time and economic constraints for not wearing protective footwear due to discomfort.[5] In Thailand, to prevent the transmission of the organisms, all residents, rice-cultivating farmers and companions are recommended in a study to wear protective equipment, such as knee-length boots and gloves, at the time of direct contact with soil or water.
The environmental and demographic factors of the coastal areas of India and other tropical countries are very favourable due to their increased rainfall and temperature, loose soil, paddy cultivation, percentage of the population with diabetes and construction boom.[11] The transmission of melioidosis can be by air, which moves from the contaminated soil to aerosols, and then, to humans in this endemic area.[12] The climatic predictors of melioidosis are mentioned in Table 1.
Table 1.
| Climatic Predictor of Melioidosis |
| Humidity |
| Rainfall |
| Wind speed |
| Temperature |
| Anthrosol (modified as organic waste by human activities) and acrisol (clay-rich associated with humid, tropical climates) |
Prevention of water contamination
In Australia, surveys of groundwater accessed by drilling (bore water) confirmed about 33% of the cases with B. pseudomallei contamination. A successive study demonstrated the efficacy of ultraviolet (UV) irradiation to disinfect untreated bore water supplies.[13,14] Additionally, chlorination has been an effective way of disinfection of potable water and is efficiently used to control melioidosis.[15,16] Large-scale chlorination of water has been shown to protect B. pseudomallei by chlorination of water settings in Australia.[15] In low-income and middle-income countries, where UV light treatment of water is not feasible, people must use traditionally boiled water before consumption.[13]
Risk Factors and their prevention
There are several underlying risk factors for the development of melioidosis [Table 2]. Among the risk factors, diabetes (leading risk factors, particularly when uncontrolled), alcohol (heavy), chronic lung disease, chronic renal disease and smoking are common. The patient with poorly controlled diabetes has defects in intracellular bactericidal activity, neutrophil adhesion and chemotaxis. Patients with chronic renal failure usually have defective granulocyte, and the T-cell function has a lower immunity, which predisposes to melioidosis with or without other infections.[17] The multimodal approach for primary prevention and glycaemic treatment of type 2 diabetes would possibly reduce the overall problem of melioidosis more effectively than any of the above five strategies for neglected tropical diseases (NTD) prevention. Heavy alcoholics are also prone to getting the infection of Burkholderia. pseudomallei.[18] Cardiac failure, a significant smoking history, chronic respiratory disease like COPD also predispose to pulmonary melioidosis due to impaired pulmonary defence mechanisms.[19] We need to understand each component of the risk factors well. The primary and primordial prevention and modification of the risk factors are essential to prevent the emergence of favourable conditions for the growth of Burkholderia pseudomallei. There is a need to reassure people not to go out during storms, restrict alcohol consumption and wear masks when not working in public health water pipes. All the common risk factors for melioidosis are listed in Table 2.
Table 2.
| Risk factors for melioidosis |
| Diabetes (if uncontrolled, up to 12 times) |
| Heavy alcohol use |
| Chronic pulmonary disease |
| Chronic renal disease |
| Chronic liver disease |
| Smoking |
| Prolonged glucocorticoid therapy |
| Cancer patients on chemotherapy |
| Occupational exposures |
| Chronic heart diseases |
| Immunosuppression |
| Thalassemia |
| Systemic lupus erythematosus (SLE) |
| Exposure to soil (rainy season) |
| Male sex (more environmental exposure) |
Other modalities
Intensified case finding and case management could reduce the spread of infections The early treatment decreased the case fatality rate of melioidosis to less than 15% as reported by Currie et al.[21] from Australia. Several chemicals have been used to reduce the bacterial content in the soil. Chitosan, a natural and nontoxic biopolymer, a linear polysaccharide (d-glucosamine) can break the cell membrane of the bacteria, including Burkholderia, and cause cell death by releasing intracellular content.[25] It has demonstrated the potential for control in soil but needs further research in real-life scenarios. Calcium oxide has shown significant and more extended bactericidal effects by in vitro study for decreasing the risk of infection from contaminated paddy field soil.[25] This type of preventive soil management with calcium oxide in a Thai zoo has shown possible benefits for environmental control.[26] However, the feasibility and potential effects on crop ecology are unknown and need to be explored.[25]
Public Health Awareness Campaign
Public awareness and knowledge on melioidosis, in particular, in developing and tropical countries, are grossly inadequate. Public awareness campaigns through media and preventive approaches like protective footwear and water chlorination, particularly for the high-risk public, are much needed.
Other public health management approaches, such as IEC (information, education, communication) activities in the community to reduce exposure or contamination should be appropriate against melioidosis. There can be health education on the menace and prevention of ailments in school, social media and the environment.
The WHO follows five strategies for the prevention and control of NTDs: (1) preventive chemotherapy, (2) intensified case management, (3) control of disease vectors, (4) provision of clean water and sanitation and (5) veterinary public health measures. Therefore, a step-by-step approach at multiple levels, like community and government, is necessary to improve long-term infection prevention. First, the WHO needs to include this disease as NTD, which has long been due. There is an urgent need to create awareness among the clinician microbiologists, capacity-building of the microbiology laboratories and create a national melioidosis registry and networking to report, manage and prevent melioidosis.[27]
Prevention of Infection in Health Care Settings
Theoretically, there is a risk for transmission of B. pseudomallei from the laboratory. But the risk is usually low, and there are occasionally documented cases.[28,29,30] The laboratory staff must undergo desirable training and practice on handling this organism under a biosafety level 3 (BSL-3) condition.[30] But BSL-3 facilities in developing countries have been a constraint even for highly transmissible severe acute respiratory syndrome coronavirus (SARS- CoV2). In addition, the staff is required to use universal precautions and must wear suitable personal protective equipment, which includes at least standard gowns, gloves and a respiratory mask when handlings suspected material or during sample centrifugation.[30]
Post-Exposure Prophylaxis
Practically, there is no evidence of infection during simple laboratory work such as identification and susceptibility testing. The high-risk events include the contact of the organism to penetrating or skin injuries, to mouth or eyes through contaminated materials, and exposure to the contaminated aerosols while outside of a biosafety cabinet.[31] After an episode of exposure to B. pseudomallei in the laboratory, there should be a risk assessment and post-exposure prophylaxis (PEP). The PEP consists of oral treatment of trimethoprim-sulfamethoxazole for 21 days. If the patient is intolerant, doxycycline or co-amoxiclav may be given for 21 days.[31] PEP is generally recommended for high-risk events, including exposure to open skin injuries, piercing injuries and direct exposure of eyes and mouth to B. pseudomallei -contaminated tissues, materials and aerosols. The potential benefit of PEP must be weighed against the adverse effect of trimethoprim-sulfamethoxazole, particularly for persons with low-risk exposures. However, starting PEP should be based on known risk factors like uncontrolled diabetes for naturally progressing to melioidosis. Individuals with definite risk factors and exposure should receive PEP, whereas routine monitoring should be sufficient in the absence of known risk factors.[31] This is as per the CDC workshop based on the consensus statements of a large working group on the management of melioidosis.[32] It is also essential to follow the country-specific guidelines changed from time to time.
B. pseudomallei is inherently resistant to several antibiotics, and an extended duration of appropriate antibiotics, often using multiple drugs, is typically required.[33] In addition, many cases of melioidosis are very much progressed when diagnosed and treated, so mortality is high.[7]
Development of Vaccines
A suitable vaccine for the vulnerable population should be valuable for preventing melioidosis. Numerous vaccine candidates are at the early stages of development; only one vaccine is at the forefront to lunch its human trial (phase 1 clinical trial). If at all an effective vaccine is available, it needs to be utilised on priority for high-risk individuals, in particular, in endemic regions.[34] Only a single study on the vaccination has been published so far from north-eastern Thailand to find the possible cost-effectiveness.[35] Some of the vaccine candidates have demonstrated promising results in the animal study, but only one clinical trial (phase-1) so far has been conducted.[34,35,36] Till then, prevention remains the key strategy.
Melioidosis Surveillance
Disease notification compels to document the substantial burden and brings opportunities to alleviate public health risks [Table 3]. Although medically interesting melioidosis cases are increasingly being reported at individual levels in various journals, no surveillance systems exist to record the incidence of the cases. Many patients might be dying unreasonably due to a lack of diagnosis. If there is a diagnosis, it is too late to receive appropriate treatment in some cases. The countries need to confirm and report the actual number of cases projected but these remain unrecognised in regions where the melioidosis is yet to be documented as endemic.
Table 3.
Aim of melioidosis surveillance
| Aim of melioidosis surveillance |
| To estimate the burden of the disease |
| To detect disease outbreaks |
| Characterise persons with greater risk for diseases |
Conclusion
The reported cases of melioidosis are just the tip of the iceberg. The disease is much more common than realised, and there has been gross underreporting across the tropics.[4] The health and economic impacts of melioidosis in tropical countries need increased attention. Our approach should be mutual and multimodal, even to restrict the emergence of the risk factors. It is time for the world authority to consider melioidosis as a neglected tropical disease. The primary public health preventive measures, as recommended by the WHO, are accurate case detection, improvement in patient management, and provision of good hygiene, sanitation, and safe drinking water.[37]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Birnie E, Virk HS, Savelkoel J, Spijker R, Bertherat E, Dance DAB, et al. Global burden of melioidosis in 2015: A systematic review and data synthesis. Lancet Infect Dis. 2019;19:892–902. doi: 10.1016/S1473-3099(19)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gassiep I, Armstrong M, Norton R. Human melioidosis. Clin Microbiol Rev. 2020;33:e00006–19. doi: 10.1128/CMR.00006-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Golding N, Dance DAB, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 4.Chai LYA, Fisher D. Earth, wind, rain, and melioidosis. Lancet Planet Health. 2018;2:e329–30. doi: 10.1016/S2542-5196(18)30165-7. [DOI] [PubMed] [Google Scholar]
- 5.Suntornsut P, Wongsuwan N, Malasit M, Kitphati R, Michie S, Peacock SJ, et al. Barriers and recommended interventions to prevent melioidosis in Northeast Thailand: A focus group study using the behaviour change wheel. PLoS Negl Trop Dis. 2016;10:e0004823. doi: 10.1371/journal.pntd.0004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaestli M, Schmid M, Mayo M, Rothballer M, Harrington G, Richardson L, et al. Out of the ground: Aerial and exotic habitats of the melioidosis bacterium Burkholderia pseudomallei in grasses in Australia. Environ Microbiol. 2012;14:2058–70. doi: 10.1111/j.1462-2920.2011.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–44. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 8.Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, et al. Melioidosis. Nat Rev Dis Primers. 2018;4:17107. doi: 10.1038/nrdp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldhous P. Tropical medicine: Melioidosis? Never heard of it. Nature. 2005;434:692–3. doi: 10.1038/434692a. [DOI] [PubMed] [Google Scholar]
- 10.Hinjoy S, Hantrakun V, Kongyu S, Kaewrakmuk J, Wangrangsimakul T, Jitsuronk S, et al. Melioidosis in Thailand: Present and future. Trop Med Infect Dis. 2018;3:38. doi: 10.3390/tropicalmed3020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behera B, Mohanty S, Mahapatra A, Hallur VK, Mishra B, Dey A, et al. Melioidosis in Odisha: A clinico-microbiological and epidemiological description of culture-confirmed cases over a 2-year period. Indian J Med Microbiol. 2019;37:430–2. doi: 10.4103/ijmm.IJMM_19_367. [DOI] [PubMed] [Google Scholar]
- 12.Chen PS, Chen YS, Lin HH, Liu PJ, Ni WF, Hsueh PT, et al. Airborne transmission of melioidosis to humans from environmental aerosols contaminated with B. pseudomallei. PLoS Negl Trop Dis. 2015;9:e0003834. doi: 10.1371/journal.pntd.0003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McRobb E, Kaestli M, Mayo M, Price EP, Sarovich DS, Godoy D, et al. Melioidosis from contaminated bore water and successful UV sterilisation. Am J Trop Med Hyg. 2013;89:367–8. doi: 10.4269/ajtmh.13-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayo M, Kaesti M, Harrington G, Cheng AC, Ward L, Karp D, et al. Burkholderia pseudomallei in unchlorinated domestic bore water, Tropical Northern Australia. Emerg Infect Dis. 2011;17:1283–5. doi: 10.3201/eid1707.100614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard K, Inglis TJ. Disinfection of Burkholderia pseudomallei in potable water. Water Res. 2005;39:1085–92. doi: 10.1016/j.watres.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O'Reilly L, et al. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis. 2000;6:56–9. doi: 10.3201/eid0601.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon R, Baby P, Kumar VA, Surendran S, Pradeep M, Rajendran A, et al. Risk factors for mortality in melioidosis: A single-centre, 10-year retrospective cohort study. ScientificWorldJournal. 2021;2021:8154810. doi: 10.1155/2021/8154810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sridharan S, Princess IB, Ramakrishnan N. Melioidosis in critical care: A review. Indian J Crit Care Med. 2021;25(Suppl 2):S161–5. doi: 10.5005/jp-journals-10071-23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meumann EM, Cheng AC, Ward L, Currie BJ. Clinical features and epidemiology of melioidosis pneumonia: Results from a 21-year study and review of the literature. Clin Infect Dis. 2012;54:362–9. doi: 10.1093/cid/cir808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010;82:1113–7. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Currie BJ, Jacups SP, Cheng AC, Fisher DA, Anstey NM, Huffam SE, et al. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop Med Int Health. 2004;9:1167–74. doi: 10.1111/j.1365-3156.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 23.Fong SM, Wong KJ, Fukushima M, Yeo TW. Thalassemia major is a major risk factor for pediatric melioidosis in Kota Kinabalu, Sabah, Malaysia. Clin Infect Dis. 2015;60:1802–7. doi: 10.1093/cid/civ189. [DOI] [PubMed] [Google Scholar]
- 24.Kronsteiner B, Chaichana P, Sumonwiriya M, Jenjaroen K, Chowdhury FR, Chumseng S, et al. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol. 2019;49:1092–106. doi: 10.1002/eji.201848037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamjumphol W, Chareonsudjai P, Chareonsudjai S. Antibacterial activity of chitosan against Burkholderia pseudomallei. Microbiologyopen. 2018;7:e00534. doi: 10.1002/mbo3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommanustweechai A, Kasantikul T, Somsa W, Wongratanacheewin S, Sermswan RW, Kongmakee P, et al. Environmental management procedures following fatal melioidosis in a captive chimpanzee (Pan troglodytes) J Zoo Wildl Med. 2013;44:475–9. doi: 10.1638/2012-0025R5.1. [DOI] [PubMed] [Google Scholar]
- 27.Mohapatra PR, Behera B, Mohanty S, Bhuniya S, Mishra B. Melioidosis. Lancet Infect Dis. 2019;19:1056–7. doi: 10.1016/S1473-3099(19)30480-3. [DOI] [PubMed] [Google Scholar]
- 28.Green RN, Tuffnell PG. Laboratory acquired melioidosis. Am J Med. 1968;44:599–605. doi: 10.1016/0002-9343(68)90060-0. [DOI] [PubMed] [Google Scholar]
- 29.Schlech WF, 3rd, Turchik JB, Westlake RE, Jr, Klein GC, Band JD, Weaver RE. Laboratory-acquired infection with Pseudomonas pseudomallei (melioidosis) N Engl J Med. 1981;305:1133–5. doi: 10.1056/NEJM198111053051907. [DOI] [PubMed] [Google Scholar]
- 30.Peacock SJ, Schweizer HP, Dance DA, Smith TL, Gee JE, Wuthiekanun V, et al. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg Infect Dis. 2008;14:e2. doi: 10.3201/eid1407.071501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipsitz R, Garges S, Aurigemma R, Baccam P, Blaney DD, Cheng AC, et al. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei Infection, 2010. Emerg Infect Dis. 2012;18:e2. doi: 10.3201/eid1812.120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dance DA, Limmathurotsakul D, Currie BJ. Burkholderia pseudomallei: Challenges for the clinical microbiology laboratory-A response from the front line. J Clin Microbiol. 2017;55:980–2. doi: 10.1128/JCM.02378-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wuthiekanun V, Peacock SJ. Management of melioidosis. Expert Rev Anti Infect Ther. 2006;4:445–55. doi: 10.1586/14787210.4.3.445. [DOI] [PubMed] [Google Scholar]
- 34.Luangasanatip N, Flasche S, Dance DAB, Limmathurotsakul D, Currie BJ, Mukhopadhyay C, et al. The global impact and cost-effectiveness of a melioidosis vaccine. BMC Med. 2019;17:129. doi: 10.1186/s12916-019-1358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peacock SJ, Limmathurotsakul D, Lubell Y, Koh GC, White LJ, Day NP, et al. Melioidosis vaccines: A systematic review and appraisal of the potential to exploit biodefense vaccines for public health purposes. PLoS Negl Trop Dis. 2012;6:e1488. doi: 10.1371/journal.pntd.0001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Titball RW, Burtnick MN, Bancroft GJ, Brett P. Burkholderia pseudomallei and Burkholderia mallei vaccines: Are we close to clinical trials? Vaccine. 2017;35:5981–9. doi: 10.1016/j.vaccine.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Savelkoel J, Dance DAB, Currie BJ, Limmathurotsakul D, Wiersinga WJ. A call to action: Time to recognise melioidosis as a neglected tropical disease. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00394-7. S1473-3099(21)00394-7. doi: 10.1016/S1473-3099(21)00394-7. [DOI] [PubMed] [Google Scholar]



