Abstract
The resurgence of interest in using psychedelic drugs, including lysergic acid diethylamide (LSD), in psychiatry has drawn attention to the medically unsupervised practice of ‘microdosing’. Thousands of users claim that very low doses of LSD, taken at 3–4-day intervals, improve mood and cognitive function., However, few controlled studies have described the effects of the drug when taken in this way. Here, in a double-blind controlled study, we studied the effects of four repeated doses of LSD tartrate (13 or 26 μg) or placebo, administered to healthy adults at 3–4 day intervals, on mood, cognitive performance and responses to emotional tasks. Participants were randomly assigned to one of three drug conditions: placebo (N = 18), 13 μg LSD (N = 19), or 26 μg LSD (N = 19). They attended four 5-hour drug-administration sessions separated by 3–4 days, followed by a drug-free follow-up session 3–4 days after the last session. LSD (26 μg) produced modest subjective effects including increased ratings of ‘feeling a drug effect’ and both stimulant-like and LSD-like effects, but the drug did not improve mood or affect performance on psychomotor or most emotional tasks. No residual effects were detected on mood or task performance on the drug-free follow-up session. We conclude that within the context of a controlled setting and a limited number of administrations, repeated low doses of LSD are safe, but produce negligible changes in mood or cognition in healthy volunteers.
Keywords: behavior, cognition, LSD, microdosing, mood, psychopharmacology
1 |. INTRODUCTION
The practice of ‘microdosing’ of lysergic acid diethylamide (LSD) has received a great deal of media attention in recent years. Thousands of people report that ingesting very low doses of LSD once every 3 or 4 days produces a wide range of beneficial mood and cognitive effects.1–5 They report benefits including improved mental function (e.g. relief of negative moods and depression), increased positive mood, energy level, work effectiveness and ‘healthy habits’, as well as relief from medical conditions such as migraines, pre-menstrual discomfort, traumatic brain injury and shingles. The drug is taken in doses of 10–20 μg, or about one-tenth of the dose that produces psychedelic experiences. Until now, the drug is being used without medical supervision, and there have been few controlled studies to determine its effects under these conditions. What are the direct effects of the drug, do these effects change with repeated dosing, and are there lasting psychological benefits?
There are good reasons to expect that a serotonergic drug like LSD might improve mood.
The serotonergic system is critically involved in the neurobiology of depression, and 5HT2A signalling in particular may underlie the effectiveness of selective serotonin reuptake inhibitor (SSRI) antide-pressants.6 LSD acts as a direct agonist on serotonin receptors, whereas SSRI’s block reuptake of serotonin and often take weeks to be clinically effective. LSD also acts on other neurotransmitter systems, including notably the dopamine system,7 which has itself been the target of antidepressant drugs such as bupropion.8 At higher doses, however, the altered states of consciousness induced by LSD appear to depend on its effects on 5HT2A.9–11 Interestingly, there is some evidence for antidepressant effects of repeated low doses of LSD and other psychedelic drugs from animal models. Small, repeatedly administered doses of the psychedelic drug N,N-dimethyltryptamine (DMT) enhance fear extinction learning and time to immobility on the forced swim test in rodents, another metric of possible antidepressant effects.12 In an animal model of antidepressant effects (olfactory bulbectomy), repeated small doses of LSD and other psychedelic drugs improved deficits in active avoidance learning, a defining feature of other antidepressant drugs.13 The authors suggested that repeated activation of 5HT2A receptors led to a rebalancing of 5HT1A/2A receptors and a resulting downregulation of 5HT2A receptors that has been linked to effectiveness of antidepressants. LSD has a long history of use in psychotherapy, which has recently been revisited in clinical research studies. In the 1950s and 1960s, over 1000 studies were published supporting therapeutic effects of LSD in combination with psychotherapy.14–16 Although the findings were promising, many of these early studies lacked adequate control groups and did not isolate drug effects from effects of the psychotherapy itself. More recently, several controlled clinical studies report therapeutic effects of moderate to high doses of LSD (200–800 μg) or psilocybin in the treatment of depression, end-of-life anxiety in terminally ill patients and addictive disorders.17–19 These high-dose clinical studies are promising, suggesting that psychedelic drugs have the potential to yield lasting changes in mood and behaviour.20
Several studies have documented subjective and physiological effects of single low doses of LSD,21–23 but relatively few studies have examined effects of repeated low doses of LSD, the pattern known as microdosing. One study24,25 examined the effects of six repeated doses, taken every 4 days, of 5, 10 and 20 μg LSD or placebo in 48 healthy older adults (mean age 63). The drug was well tolerated and produced modest effects on a measure of time perception: Subjects over-reproduced temporal intervals of 2000 ms and longer, especially in the 10 μg condition. However, the drug did not significantly alter mood, or impair cognition, balance or proprioception. Another recent study26 used an innovative design in which experienced users of microdoses of LSD or psilocybin ingested drug or placebo under double-blind conditions in their home environments. Subjects (N = 191) were instructed on blinding their preferred psychedelic drug and dose (obtained from their own sources) and a placebo using online instructions for use during a 4-week dosing period. All subjects, regardless of drug condition, reported improvements in well-being and cognition across the 4 weeks of treatment. This suggested the drug had little effect on these measures. Subjects did report acute subjective effects from the drug (compared with placebo), including increased energy, mood and creativity and post-acute decreases in anxiety. However, when the authors removed the data from subjects who correctly identified the drug as active (thereby breaking the double blind), these drug effects were no longer significant. Even though there were no lasting improvements in mood or cognition, the authors reasoned that any apparent benefits from the drug could have been due to expectancy or placebo effects. The Szigeti study raises interesting questions about whether detectable subjective effects truly nullify any observed therapeutic benefits because the blind is broken or whether therapeutic benefits can occur at doses that are detectable by the users. This presents a challenge for psychiatric research, because it is possible that some beneficial effects occur at doses that produce detectable acute effects. Indeed, individuals with a low threshold for detecting the drug’s effects may be especially sensitive to its antidepressant effects.
In the present study, we administered repeated doses of LSD (13 or 26 μg LSD tartrate, which is equivalent to a dose of 10 or 20 μg of LSD base) or placebo to healthy volunteers under controlled and fully blinded conditions. The subjects were not experienced with micro-dosing and were informed that they might receive any of several drug types during the study (e.g. stimulant, sedative and hallucinogen). Participants attended four 5-h laboratory sessions in which they received LSD or placebo, once session every 3–4 days, followed by one drug-free session 3–4 days later. We assessed mood and performance on cognitive and emotional tasks during the drug administration sessions and at follow-up. We hypothesized that repeated doses of drug, compared with placebo, would improve mood and cognitive performance and that these effects would persist to the follow-up session.
2 |. MATERIALS AND METHODS
2.1 |. Design
Participants (N = 56) were healthy adults aged 18–35 who reported having used a psychedelic drug or MDMA at least once in their life-time. After screening, they were randomly assigned to one of three conditions to receive placebo, LSD (13 μg) or LSD (26 μg) during four 5-h laboratory sessions, conducted at 3- to 4-day intervals (Figure 1). After ingesting their dose, subjects completed mood questionnaires every hour (for detailed descriptions of all subjective/self-report measures, see Supporting Information), and cardiovascular measures were obtained. On Sessions 1 and 4, and at the follow-up session, subjects also completed cognitive and behavioural tasks (for detailed descriptions of all tasks, see Supporting Information). Primary outcome measures were ratings of mood and performance on cognitive tasks during drug sessions and at the drug-free follow-up.
FIGURE 1.
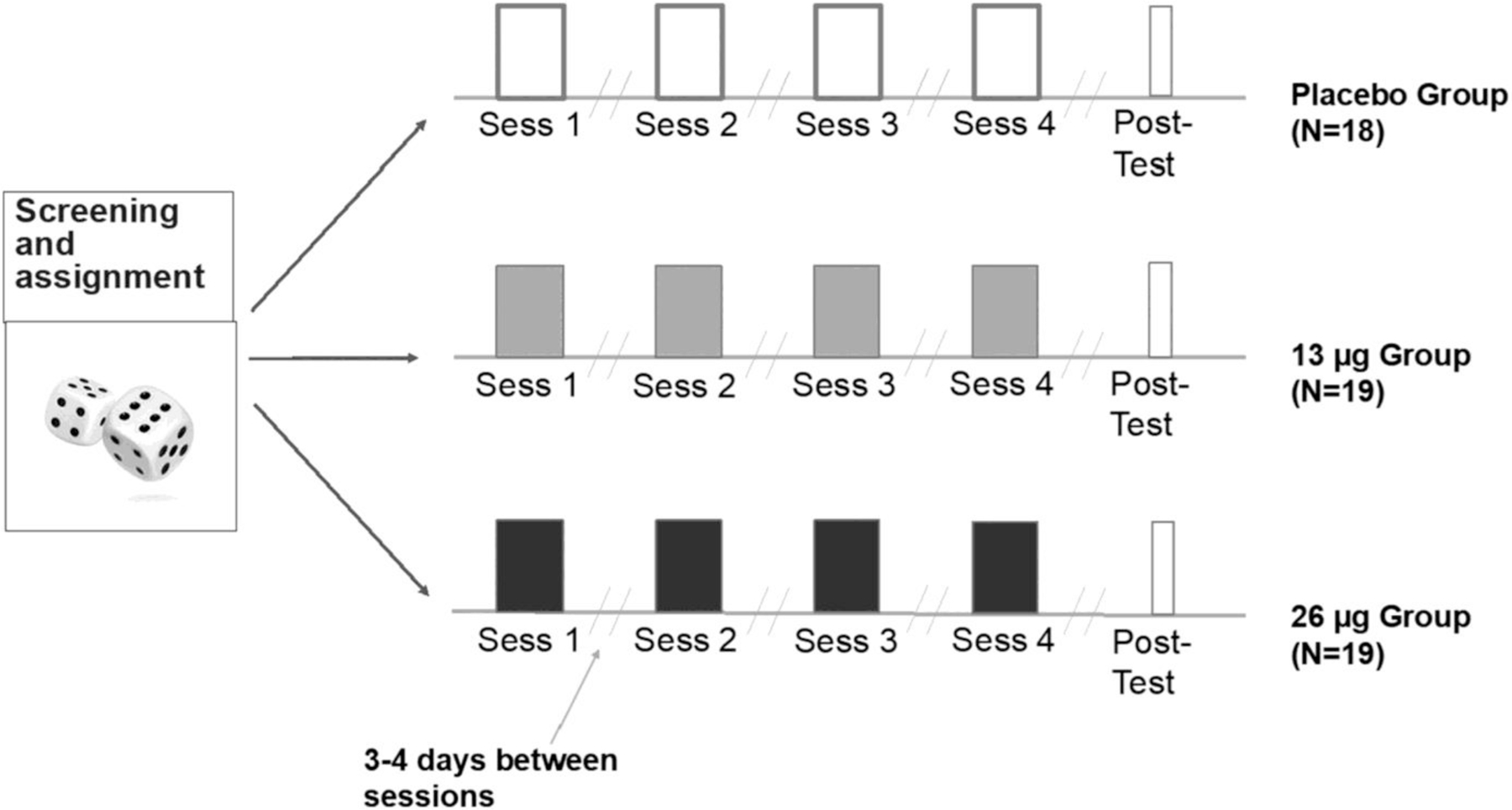
Diagram of the study design
2.2 |. Subjects
Participants were recruited by flyers and social media ads. Subjects provided informed consent before beginning the study, and the study was approved by the University of Chicago Institutional Review Board. Initial eligibility criteria were age 18–35, fluent in English, minimum high school education, BMI 19–30, not taking medications and some lifetime experience with a psychedelic drug (e.g. psilocybin, LSD, mescaline, dimethyltryptamine or MDMA). At an in-person interview, candidates underwent further screening including a semi-structured psychiatric interview conducted by a clinical psychologist, a physical examination and electrocardiogram. They also completed the Depression, Anxiety and Stress Scale (DASS-21)27 and a detailed drug use history questionnaire. Exclusion criteria were a medical condition contraindicating study participation (e.g. liver or kidney disease), current or past substance use disorder, an unwillingness to use a psychedelic drug, serious psychiatric conditions including current suicidal ideation, psychosis or current panic disorder and pregnancy or planned pregnancy in women.
2.3 |. Procedure
2.3.1 |. Orientation
Participants attended an orientation session to explain procedures and obtain informed consent, and subjects practised tasks and questionnaires. They were instructed to abstain from illicit drugs and medications for 48 h before each session, from cannabis 7 days and from alcohol for 24 h before each session. Adherence to these instructions was verified by urine and breathalyser screens at the beginning of each session. Participants were permitted to consume their normal amounts of caffeine and nicotine before the sessions.
Subjects were instructed to have a normal night’s sleep and fast for 12 h before the session. A granola bar was provided at arrival, and lunch was provided 120 min after drug administration. Subjects were told that on any session, they might receive a placebo, stimulant (e.g. methylphenidate), sedative/tranquilizer (e.g. Valium) or a ‘hallucinogenic’ drug (e.g. LSD).
2.3.2 |. Drug administration session
The drug administration sessions were conducted from 9 AM to 2 PM at 3- to 4-day intervals. Participants remained in a comfortable room with movies and reading materials, and they were allowed to relax during times when no activities were scheduled. Upon arrival, they provided urine and breath samples to confirm compliance to drug abstinence instructions (Instant Drug Test Cup; CLIAwaived, San Diego, CA) (Alco-Sensor III; Intoximeters, St. Louis, MO), and women were tested for pregnancy. After compliance was confirmed, subjects completed baseline measures of subjective mood, blood pressure and heart rate. Then at 9:30 AM, they ingested a sublingual dose of placebo (water), 13 μg LSD or 26 μg LSD (see below) under double-blind conditions. The subject held the solution under the tongue without swallowing for 60 s under observation by a research assistant. At regular intervals during the session, subjects completed subjective effect measures, and heart rate and blood pressure were monitored (Table 1). Subjects were given a standardized lunch 120 min after drug administration. On Sessions 1 and 4, they completed cognitive and emotion tasks (for detailed descriptions of all tasks, see Supporting Information) at 150 min after drug administration, coinciding with the expected peak effect of the drug. They completed the tasks in a counterbalanced order. Subjects completed two additional questionnaires at the end of session, including the 5D-ASC questionnaire28 and questions about the drug effect (feel drug, like drug and what they thought they received).
TABLE 1.
Summary of measures
| Sess 1 pre |
Sess 1 post |
Sess 2 pre |
Sess 2 post |
Sess 3 pre |
Sess 3 post |
Sess 4 pre |
Sess 4 post |
Sess 5 |
|
|---|---|---|---|---|---|---|---|---|---|
| Depression, Anxiety and Stress Scale | x | x | |||||||
| Positive and Negative Affect Scale | x | x | x | x | |||||
| Addiction Research Center Inventory, Drug Effects Questionnaire, Profile of Mood States | x | D | x | D | x | D | x | D | |
| Heart rate, blood pressure | x | D | x | D | x | D | x | D | |
|
Cognitive tasks: Digital Symbol Substitution Test, N-back Emotion tasks: Cyberball, Emotional Images |
D | D | x | ||||||
| Task, Emotional Faces Task | |||||||||
| 5D-ASC and End-of-Session Questionnaire | D | D | D | D |
Notes: X measures in drug-free state. D measures of direct drug effects.
2.3.2 |. Follow-up session (Session 5)
Three to four days after the fourth session, subjects attended a 1-h follow-up session to assess their post-drug mood and behavioural responses. During this session, they first provided urine and breathalyser samples to confirm that they were drug-free. They completed the DASS-21 to assess their mood and completed the same cognitive and emotion tasks administered during Sessions 1 and 4.
2.3.4 |. Drug and doses
The drug was obtained from Organix Inc and placed in solution with tartaric acid by the University of Chicago Investigational Pharmacy Service. It was administered sublingually in a volume of 0.2 ml, and placebo consisted of 0.2 ml distilled water. The 13 and 26 μg doses of LSD were selected because they produced marginally detectable subjective effects without any hallucinatory or perceptual effects.21 The expected time to onset was 30 min, and peak plasma concentrations were expected to occur at 1.5–3 h.29,30
2.4 |. Physiological measures
Heart rate and blood pressure were obtained with a monitor (Omron BP791IT, Omron Healthcare, Lake Forest, IL, USA) before drug administration and every hour during the sessions.
2.5 |. Data analysis
2.5.1 |. All analyses were conducted using IBM SPSS, Version 25.0 (Armonk, NY)
For direct effects of drug on Sessions 1–4, subjective and physiological responses to the drug were assessed using three-way mixed-model analysis of variance (ANOVA). Drug (LSD 13, LSD 26, placebo) was the between-group factor. Session day (Days 1–4) and time (within sessions) were within-subject factors. After determining that the time course of effects were similar across outcome measures, we reduced the data in all figures (with the exception of DEQ data in Figure 3), for clarity, by calculating change scores. For the DEQ, which was not completed pre-drug, we used peak scores, and on other measures, we calculated peak change from pre-drug values for each subject. We confirmed that participants in the three groups did not differ at baseline. Behavioural tasks completed on Sessions 1 and 4 were compared using two-way ANOVA (drug, day). Post hoc comparisons were performed using t-tests. Analyses were not corrected for multiple comparisons.
FIGURE 3.
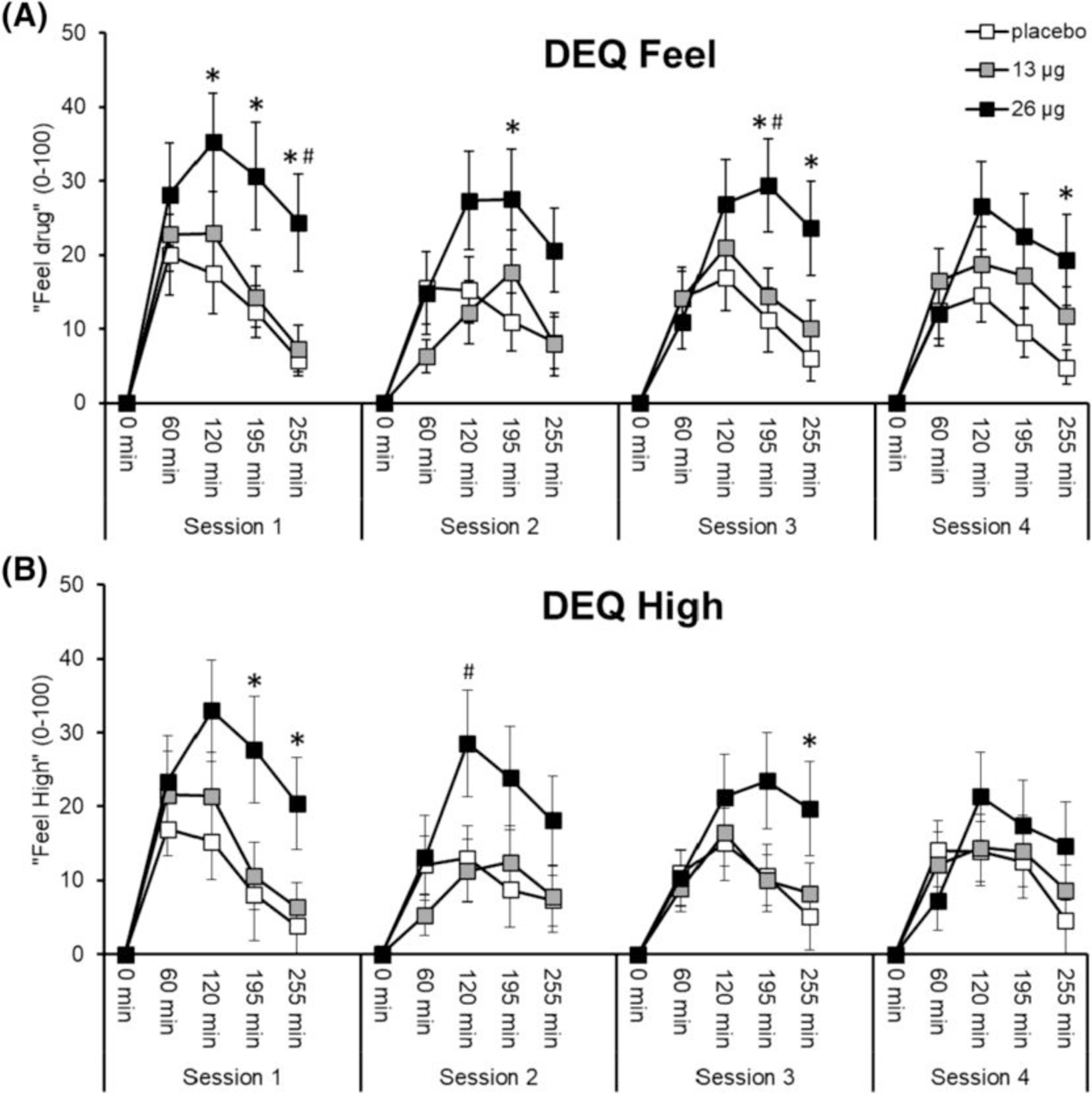
Effects of LSD on ratings of ‘feel drug’ (A) and ‘feel high’ (B). Points depict mean ratings ± SEM during Sessions 1–4. Significance between 26 μg and placebo, *p < 0.05. Significance between 26 and 13 μg, #p < 0.05
2.5.2 |. Lasting effects of drug
PANAS scores at the beginning of Sessions 1–4 were compared across the three groups using mixed-model two-way ANOVA with session day as a within-subject factor and drug as a between-subject factor. Subjective and behavioural task data from Session 5 were analysed using one-way ANOVA with drug condition as a between subject factor. DASS scores (Depression, Anxiety, Stress) and total were compared using mixed-model two-way ANOVA across the three drug treatment groups (between-subject factor), at screening, before the first drug session and at follow-up (within-subject factor).
3 |. RESULTS
3.1 |. Demographics
The groups were matched on most variables, including sex, drug use and DASS scores. Most of the participants were Caucasian in their mid-20s with at least some college education, who reported light to moderate drug and alcohol use (Table 2). The groups did not differ on demographic characteristics or drug use history.
TABLE 2.
Demographic characteristics and drug use history of participants in the three groups
| Participant demographics | Placebo n or mean ± SD (range) |
13 μg n or mean ± SD (range) |
26 μg n or mean ± SD (range) |
|---|---|---|---|
| N (male/female) | 18 (11/7) | 19 (13/6) | 19 (13/6) |
| Race/ethnicity | |||
| Caucasian | 12 | 11 | 13 |
| African American | 0 | 2 | 1 |
| Asian | 1 | 2 | 2 |
| Other/more than one race | 5 | 4 | 3 |
| Hispanic | 4 | 2 | 0 |
| Age, years | 24.4 ± 4 (19–33) | 26.6 ± 4 (21–34) | 25.9 ± 5 (19–35) |
| BMI | 24.6 ± 4 (17.7–31.7) | 24.2 ± 24 (20.8–28.6) | 22.4 ± 4 (18.2–29.7) |
| Education in years | 15 ± 1 (14–16) | 15.8 ± 2 (14–20) | 15.9 ± 2 (14–18) |
| Drug use in past month | |||
| Caffeine, servings/day | 1.4 ± 1 (0–4) | 1.2 ± 0.7 (0–3) | 1.6 ± 0.9 (0–3.5) |
| Tobacco, uses per week | 0.03 ± 0.1 (0–0.3) | 1.5 ± 4 (0–12) | 0.01 ± 0.03 (0–0.2) |
| Alcohol, drinks/week | 2.5 ± 2 (0–7) | 1.9 ± 2 (0–5) | 2± 1 (0–5) |
| Alcohol, drinking days/week | 2.6 ± 1 (0–5) | 2.3 ± 2 (0–5) | 2.09 ± .8 (0–3.5) |
| Cannabis, times/month | 7.6 ± 13 (0–50) | 5.6 ± 8 (0–25) | 5.3 ± 9 (0–30) |
| Lifetime drug use | |||
| Cannabis | |||
| 1–10, 11–50, 51–100, >100 times | 1, 6, 1, 9 | 2, 2, 4, 11 | 3, 6, 3, 7 |
| Tranquilizer | |||
| Never used, 1–10 times | 14, 4 | 16, 3 | 17, 2 |
| Stimulant | |||
| Never used | 9 | 9 | 10 |
| 1–10, 11–50, >50 times | 5, 4, 0 | 6, 2, 2 | 5, 3, 1 |
| Opiate | |||
| Ever used | 2 | 1 | 5 |
| Psychedelics | |||
| Ever used | 17 | 17 | 16 |
| Times useda | 9.7 ± 19 (1–90) | 9.6 ± 13 (1–50) | 13.5 ± 24 (1–100) |
| MDMA | |||
| Ever used | 10 | 12 | 11 |
| Times useda | 16.8 ± 35 (1–113) | 6.9 ± 9 (1–34) | 6.6 ± 14 (1–50) |
Mean for users only.
3.2 |. DASS27
Subjects’ DASS scores were within the range of a ‘normal’ sample.27 The groups did not differ on DASS scores either at screening (one-way ANOVA, drug, F2,53 = 0.277, p = 0.759) or before the first session (one-way ANOVA, drug, F2,53 = 1.67, p = 0.198). Scores in all three groups declined after the four sessions, regardless of what drug they received (Table 3 and Figure S1).
TABLE 3.
DASS scores for the three groups at screening, on Day 1 before drug administration and 3–4 days after the final drug administration session
| Placebo Mean ± SD |
13 μg Mean ± SD |
26 μg Mean ± SD |
|
|---|---|---|---|
| Depression score | |||
| Screen | 6.2 (5.9) | 6.1 (6.7) | 7.5 (5.5) |
| Session 1 | 3.6 (3.9) | 3.7 (3.9) | 4.9 (3.9) |
| Session 5 | 2.9 (3.0) | 2.7 (2.9) | 4.5 (3.7) |
| Anxiety score | |||
| Screen | 3.1 (3.6) | 2.1 (2.2) | 2.2 (2.5) |
| Session 1 | 1.6 (2.3) | 1.7 (2.4) | 2.1 (2.3) |
| Session 5 | 2.4 (2.9) | 0.89 (1.1) | 1.4 (1.6) |
| Stress score | |||
| Screen | 5.4 (4.3) | 4.9 (3.7) | 6.3 (4.6) |
| Session 1 | 3.8 (2.5) | 4.1 (3.5) | 6.3 (3.7) |
| Session 5 | 3.9 (3.1) | 3.6 (3.2) | 5.3 (3.4) |
| DASS total score | |||
| Screen | 14.8 (12.6) | 13.2 (11.4) | 15.9 (10.5) |
| Session 1 | 9.1 (7.7) | 9.5 (8.6) | 13.3 (8.6) |
| Session 5 | 9.2 (8.3) | 7.2 (6.0) | 11.2 (8.1) |
Notes: Two-way ANOVA for depression score: Drug, ns; Session, F2,106 = 20.47, p = 0.001; Drug × session, ns. Two-way ANOVA for anxiety score: Drug, ns; Session, F2,106 = 4.42, p = 0.014; Drug × session, ns. Two-way ANOVA for stress score: Drug, ns; Session, F2,106 = 4.82, p = 0.010; Drug × session, ns. Two-way ANOVA for total DASS score: Drug, ns; Session, F2,106 = 15.1, p = 0.001; Drug × session, ns.
3.3 |. Positive and Negative Affect Scale31
Positive and negative mood ratings on the Positive and Negative Affect Scale (PANAS) did not differ across groups, either before the first session (positive mood: one-way ANOVA, drug, F2,52 = 0.778, p = 0.465; negative mood: one-way ANOVA, drug, F2,52 = 1.101, p = 0.340) or before sessions 2–4 (i.e. 3 days after first, second and third doses) (Table S1).
3.4 |. Direct effects of the drug32
3.4.1 |. Addiction Research Center Inventory30
LSD, primarily 26 μg, increased scores on several Addiction Research Center Inventory (ARCI) subscales relative to placebo, including the A scale (main effect of drug, F2,53 = 6.40, p = 0.003, ƞp2 = 0.195; 26 μg vs. placebo, p < 0.01; 26 μg vs. 13 μg, p < 0.01), the MBG scale (drug × session F6,159 = 2.56, p = 0.022, ƞp2 = 0.088; 26 μg vs. placebo, p < 0.001 at session 1 and p < 0.05 at session 3; 13 μg vs. placebo, ƞp2 = 0.088 at session 1) and the LSD scale (main effect of drug, F2,53 = 3.67, p = 0.032, ƞp2 = 0.121; 26 μg vs. placebo, p < 0.05) (Figure 2). No drug effects were observed on PCAG or BG subscales (Figure S2).
FIGURE 2.
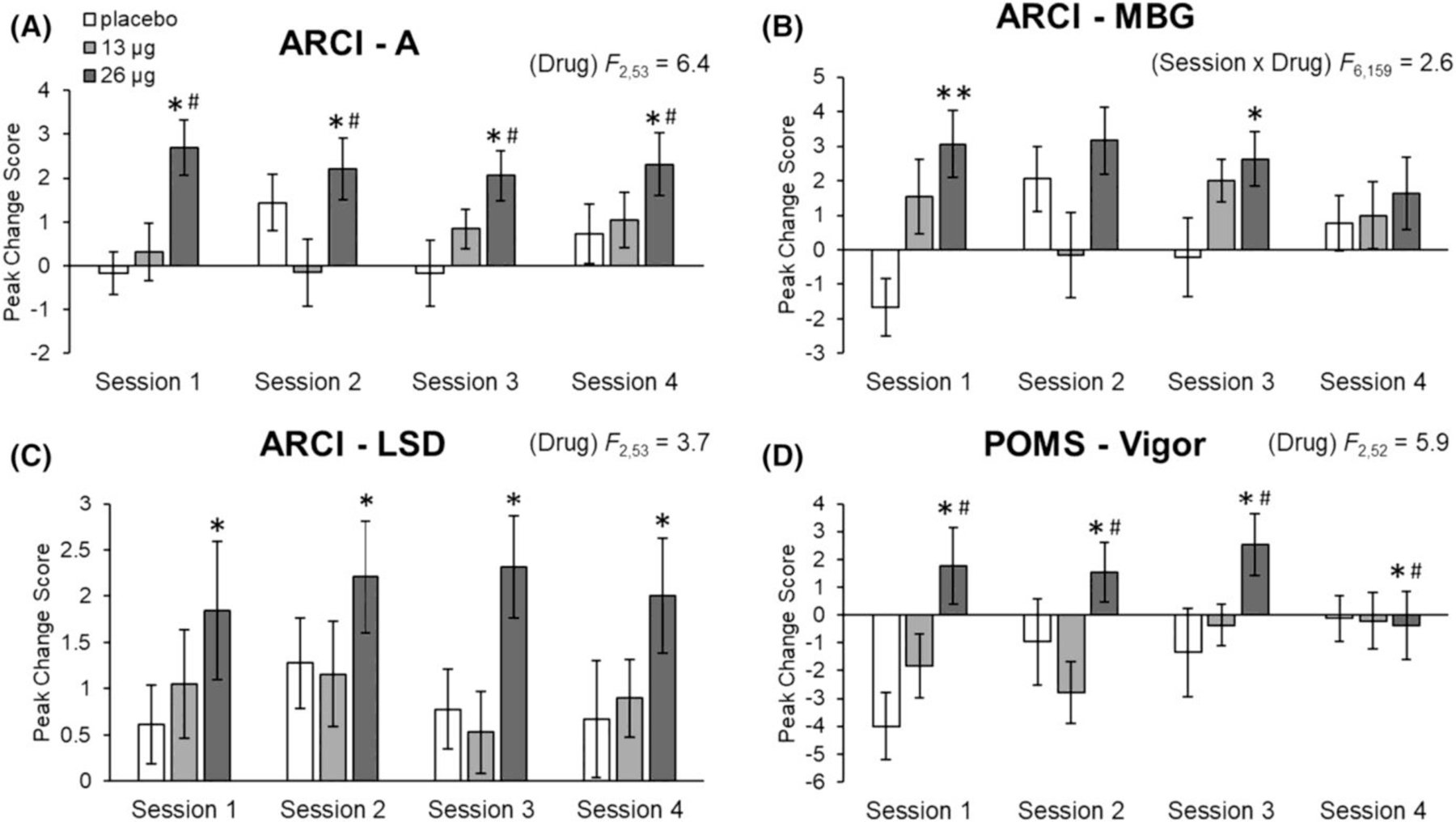
Effects of LSD on the Addiction Research Inventory (ARCI) amphetamine scale (A), MBG euphoria scale (B), LSD scale (C) and the Profile of Mood States (POMS) Vigor scale (D). Bars depict peak change scores ± SEM for Sessions 1–4. Significance between 26 μg and placebo, *p < 0.05, **p < 0.001. Significance between 26 and 13 μg, #p < 0.05
3.4.2 |. Profile of Mood State33
The high dose of LSD significantly increased peak change scores for the Vigor subscale, on the Profile of Mood States (POMS) (Figure 2; main effect of drug, F2,52 = 5.86, p = 0.005, ƞp2 = 0.184; 26 μg vs. placebo, p < 0.05; 26 μg vs. 13 μg, p < 0.05). No main effect of drug or drug interactions was observed on other subscales of the POMS including Anger, Anxiety, Depression, Confusion, Elation, Fatigue or Friendliness (Figure S4).
3.4.3 |. Drug Effects Questionnaire34
The high microdose of LSD significantly increased ‘feel drug’ ratings (drug × timepoint, F8,212 = 3.33, p = 0.001, ƞp2 = 0.112; 26 μg vs. placebo, p < 0.05, at 120, 195 and 255 min on session 1, 195 min on Session 2, 195 and 255 min on Session 3 and 255 min on session 4; 26 μg vs. 13 μg, p < 0.05, at 255 min on Session 1 and 195 min on Session 3). 26 μg also increased ‘feel high’ ratings (drug × timepoint, F8,212 = 2.50, p = 0.013, ƞp2 = 0.086; 26 μg vs. placebo, p < 0.05, at 195 and 255 min on Session 1 and 255 min on Session 3; 26 μg vs. 13 μg, p < 0.05, at 120 min on Session 2) (Figure 3). Neither dose of LSD significantly changed ratings of ‘like drug’, ‘dislike drug’ or ‘want more’ (Figure S3).
3.4.4 |. 5 Dimensions of Altered States of Consciousness28,35
This assessed altered states of consciousness in five domains and is sensitive to LSD administration.30,36 LSD dose-dependently increased scores on several subscales of the 5 Dimensions of Altered States of Consciousness (5D-ASC), including experience of unity (main effect of drug, F2,50 = 3.30, p = 0.045, ƞp2 = 0.117; post hoc analysis did not report a significant effect), blissful state (drug, F2,50 = 5.54, p = 0.007, ƞp2 = 0.181; 26 μg vs. placebo, p < 0.05; 26 μg vs. 13 μg, p < 0.05), insightfulness (drug, F2,50 = 3.83, p = 0.028, ƞp2 = 0.133; 26 μg vs. placebo, p < 0.05) and complex imagery (drug, F2,50 = 3.88, p = 0.027, ƞp2 = 0.134; post hoc analysis did not report a significant effect). No significant drug effects between LSD and placebo groups were observed on the following subscales: spiritual experience, elementary imagery, audio-visual synesthesiae, changed meaning of percepts, disembodiment, impaired control and cognition and anxiety (Figure 4 and Table S3). The effects of the drug were most pronounced during the first session and declined during Sessions 2–4, although this change did not reach statistical significance.
FIGURE 4.
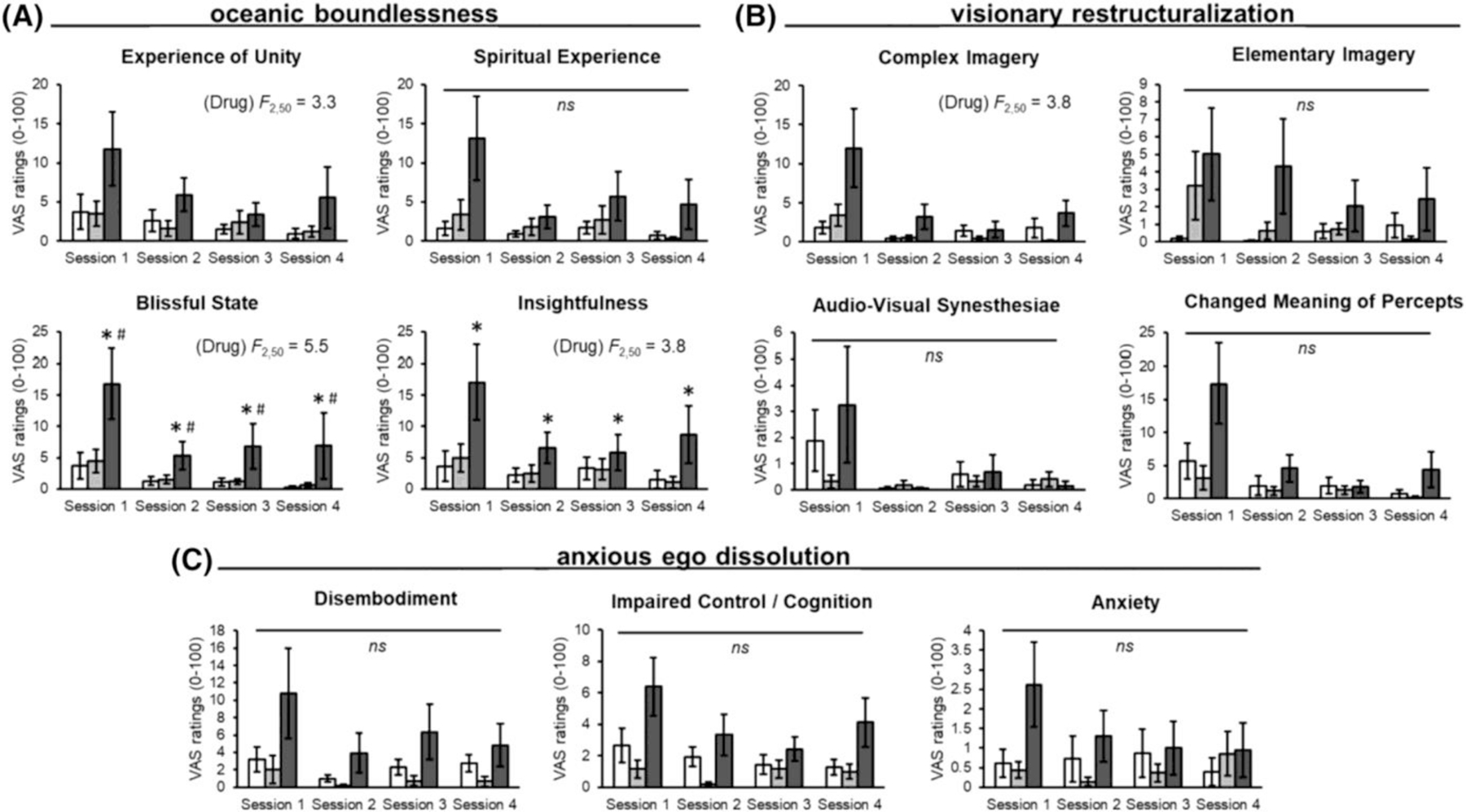
Effects of LSD on the 5 Dimensions of Altered States of Consciousness domains of (A) oceanic boundlessness, (B) visionary restructuralization and (C) anxious ego dissolution. Bars (placebo in white, 13 μg in light grey and 26 μg in dark gray) depict mean VAS ratings ± SEM for Sessions 1–4. Significance between 26 μg and placebo, *p < 0.05. Significance between 26 and 13 μg, #p < 0.05. No significance between 26 μg versus placebo and 13 μg versus placebo, ns
3.4.5 |. End-of-Session Questionnaire
The number of subjects who identified the drug as stimulant, sedative, placebo or hallucinogen is shown in Figure 5. We compared the likelihood of correctly identifying the three substances administered (placebo, 13 μg LSD and 26 μg LSD), across the three groups and across the four sessions. We used GEE logistic regression and found that only the placebo and low-dose groups differed significantly (z = −2.44, p = 0.0147). The group receiving placebo was almost five times more likely to identify their substance as placebo than the low-dose LSD group was to identify their substance as LSD.
FIGURE 5.
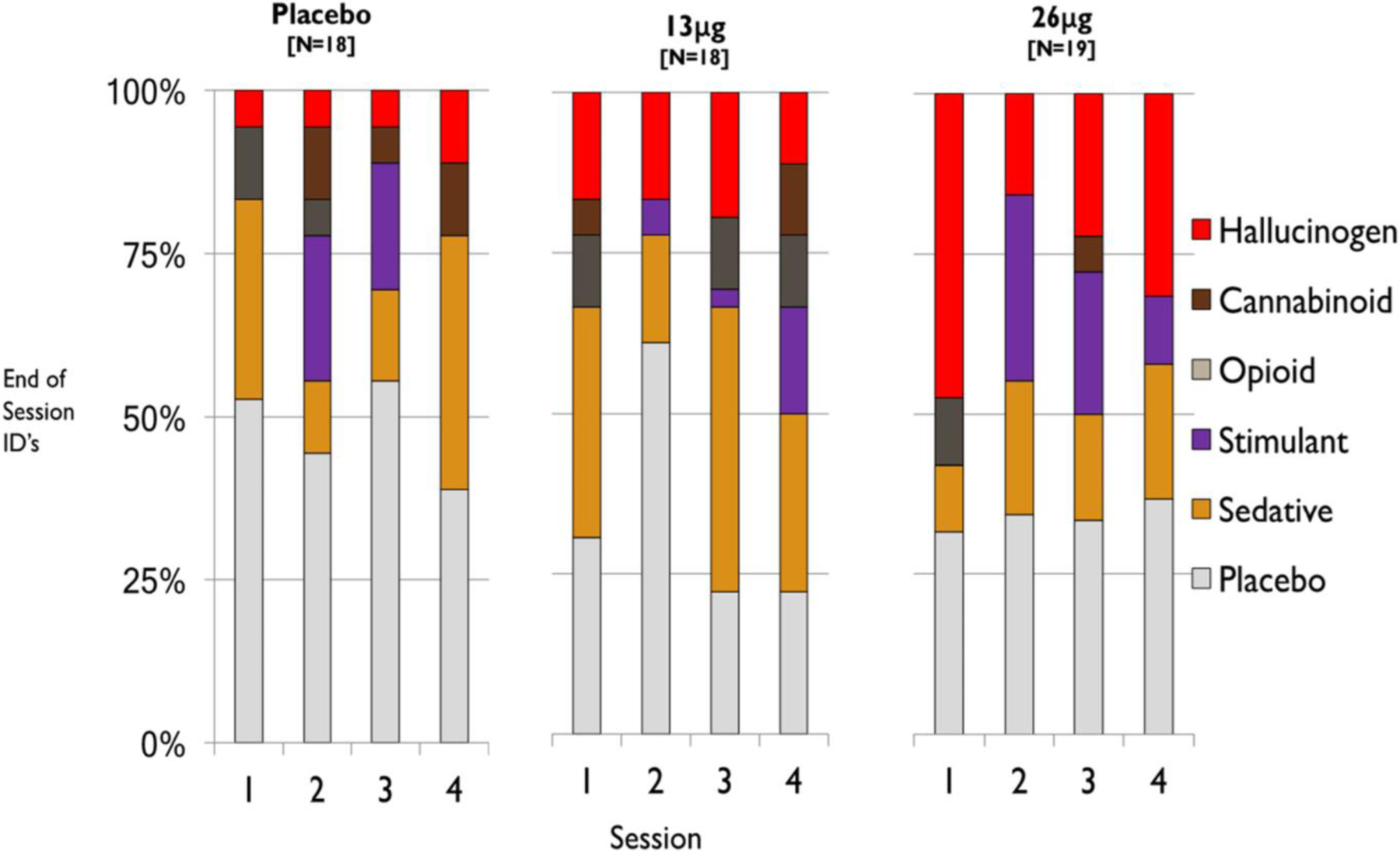
Responses to the End-of-Session Questionnaire item ‘Which category do you think the drug came from?’ for Sessions 1–4
3.5 |. Cardiovascular measures
The drug did not significantly alter heart rate or blood pressure during any of the sessions (Figure 6).
FIGURE 6.
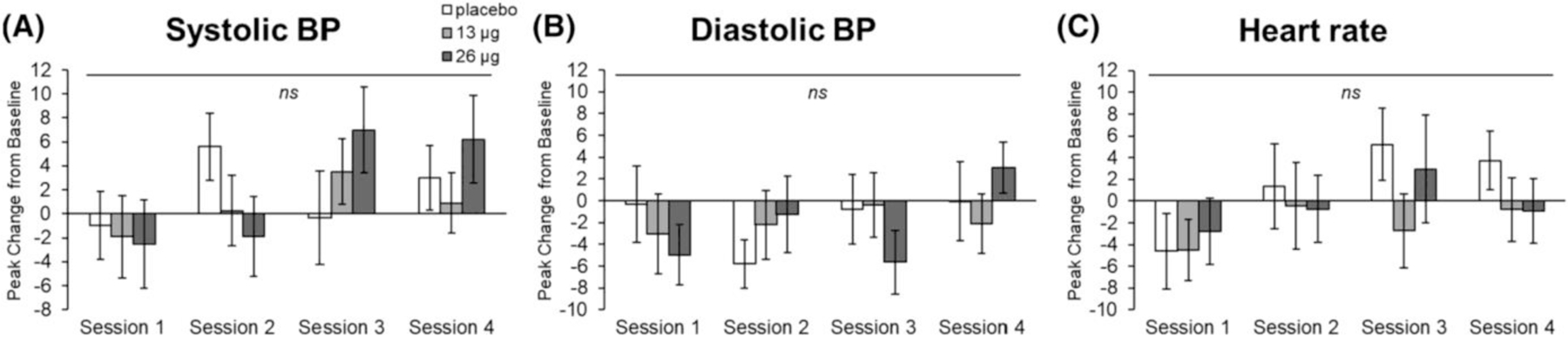
Effects of LSD on systolic blood pressure (A), diastolic blood pressure (B) and heart rate (C). Bars depict peak change from baseline ± SEM for Sessions 1–4. No significance between 26 μg versus placebo and 13 μg versus placebo, ns
3.6 |. Tasks: Session 1–4
3.6.1 |. Emotional faces task
The drug (26 μg) decreased false alarm rates on fear faces only, during the first and last days of drug administration (main effect of drug F2,52 = 3.26, p = 0.046, ƞp2 = 0.111; 26 μg vs. placebo, p < 0.050; Figure 7). The drug did not change hit rates for any emotion compared with placebo (Figure S7).
FIGURE 7.
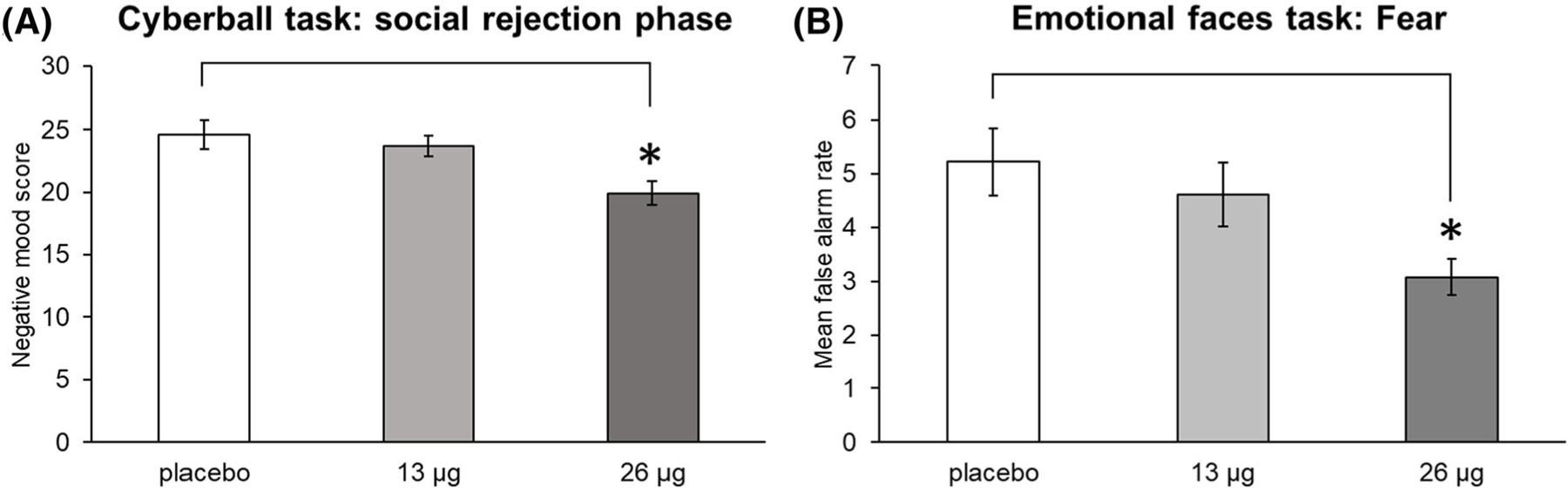
Effects of LSD during Sessions 1 and 4 on the cyberball task during the social rejection phase (A) and the emotional faces task—fear emotion (B). Bars depict mean negative mood scores and mean false alarm rates, collapsed across both sessions ± SEM. Significance between 26 μg and placebo, *p < 0.05
3.6.2 |. Emotional images task
Neither dose of LSD significantly altered either positive ratings of positive images or negative ratings of negative images during Sessions 1 and 4, when compared with placebo (Figure S5).
3.6.3 |. Cognitive performance
On the n-back task, neither dose of LSD significantly affected performance on two- or three-back trials during Sessions 1 and 4 (Figure S6).
Performance on the DSST improved across the two sessions (main effect of session, F1,53 = 45.3, p = 0.001, ƞp2 = 0.461). There was a non-significant trend for a session × drug interaction in the direction of improved DSST performance after the drug (F2,53 = 3.02, p = 0.057, ƞp2 = 0.102) (Figure S6).
3.6.4 |. Simulated social rejection
On the cyberball task, the high dose of LSD decreased negative mood ratings during the social rejection phase on Sessions 1 and 4 (main effect of drug, F2,53 = 3.65, p = 0.033, ƞp2 = 0.121; 26 μg vs. placebo, p < 0.05) (Figure 7). This effect did not differ significantly across Sessions 1 and 4. The drug did not significantly alter negative mood during the social acceptance phase (Figure S5).
3.7 |. Tasks: Follow-up session (Session 5)
3.7.1 |. Emotional faces task
On the follow-up session (Session 5), the LSD- and placebo-treated groups did not differ on hit rates or false alarms in identifying facial expressions associated with anger, disgust, fear, happiness, sadness or surprise (Figure S8).
3.7.2 |. Emotional images task
The LSD groups (13 or 26 μg) did not differ from the placebo group in ratings of positive or negative images (Figure S8).
3.7.3 |. Cognitive performance
The LSD (13 or 26 μg) groups did not differ from placebo on the n-back or DSST tasks on session 5 (Figure S9). Interestingly, when subjects were asked to rate (on a 7-point scale) how well they thought they performed on the task, subjects in the high-microdose LSD group self-reported performing significantly above average relative to other participants (one-way ANOVA: drug, F2,49 = 3.86, p = 0.028, 26 μg vs. placebo, p < 0.050) and significantly better compared with the first time they completed the task (one-way ANOVA: drug, F2,49 = 4.77, p = 0.013, 26 μg vs. placebo, p < 0.050).
3.7.4 |. Simulated social rejection
The LSD (13 or 26 μg) groups did not differ from placebo on ratings of negative mood on the cyberball task, during either social acceptance or rejection phase (Figure S10).
4 |. DISCUSSION
During the four drug administration sessions, LSD (26 μg) produced modest, dose-related increases in stimulant-like (ARCI A and POMS Vigor) and LSD-like effects (ARCI) and ratings of ‘feeling a drug effect’ during the sessions. These effects appeared to be stronger on the earlier sessions. The drug had no effect on most cognitive or emotional tasks or on cardiovascular measures, except for a small decrease in false alarm rates for recognizing fearful emotions, a decrease in feelings of rejection on the social rejection task and a non-significant trend for improved performance on the DSST. Most subjects did not correctly identify the drug as a hallucinogen/psychedelic at either dose. There were no lasting effects of the drug on mood or cognitive or emotional performance on the follow-up session.
26 μg of LSD produced mainly stimulant-like subjective and behavioural effects, including increased amphetamine-like effects (ARCI A scale) and increased ratings of ‘Vigor’. Stimulant-like effects have been reported in previous studies with LSD, at a range of doses.11,21,22 Hutton et al reported that 20 μg of LSD hydrate increased ratings of arousal and decreased lapses in attention, and Bershad et al reported that 26 μg LSD tartrate increased ratings of vigour. Holze et al reported dose-related increases in stimulant-like effects from 25 to 200 μg. In addition to its effects on serotonin, LSD is also known to act on dopaminergic and other neurotransmitter receptor systems,37,38 and the increase in stimulant-like effect may be related to the effects of LSD on the dopaminergic system.39 Though the receptor activity profile of very low doses is not fully understood, it is possible that the stimulant-like effects observed here are related to actions on dopaminergic receptors.
An important challenge in this study is the low magnitude of drug effect combined with a high level of variability in responses to the drug, both within and between subjects. By design, the study examined doses near the threshold of detectability. This, combined with variability in response to the drug, makes the findings difficult to interpret. Variability is evident on the subjects’ ratings on the Feeling of Unity scale of the 5D-ASC, one of the most sensitive measures of drug effect in this study. Scores on this scale for the highest dose of LSD ranged from 0 to 72 (out of 100) on the first session and from 0 to 75 on the fourth session. On the end-of-session drug identification questionnaire, less than half the subjects correctly identified LSD at the higher dose, and this level of accuracy declined on the subsequent sessions. No associations between body weight and ratings of ‘feeling a drug effect’ were found (not presented), indicating differences in body weight do not account for the variance. Variability in response to LSD may be due in part to variation in pharmacokinetic factors. Indeed, Holze et al reported substantial variability in peak plasma levels after a dose of 20 μg LSD base, ranging from about 250 to 900 pg/mL. This variability could be due to differences in absorption or hepatic metabolism, as CYP2D6 poor metabolizers have been shown to have higher plasma concentrations of LSD relative to extensive metabolizers.40,41 Similarly, these subjects’ rating of feeling ‘under the influence’ ranged from less than 1 to over 7 on a scale of 0–10. Even greater variability in plasma levels may have occurred in the present study, which used a different form of LSD (tartrate). The variability in plasma concentrations reinforces the importance of obtaining plasma levels in each subject in future studies.
Effects of 26 μg of LSD were detected on two measures of emotional response: a small decrease in false alarm rates in recognizing fearful emotions and a decrease in feelings of rejection on the social rejection task. Reduced erroneous observations of fearful faces may indicate a reduced bias towards negative emotions with low doses of LSD. Alterations in fear processing have also previously been observed at higher doses (100 and 200 μg), where LSD impaired fear recognition while detection of other emotions including happiness and anger were not affected.42 The difference in doses across studies may account for differences in fear-related emotional processing, although further work is needed. In this study, we also found that 26 μg of LSD reduced feelings of rejection during a task that emulates social exclusion. A previous study found similar effects with a moderately high dose of the 5HT2A/1A receptor agonist psilocybin (0.215 mg/kg), and these effects were associated with reduced activation of the dorsal anterior cingulate cortex and middle frontal gyrus, regions involved in social pain processing.43 Our results suggest that 5HT2A/1A stimulation with low doses of LSD may be sufficient to elicit a similar effect. Of note, the effects of 26 μg LSD on emotional processing were not observed 3–4 days after the last dose, suggesting the effects are not enduring. It is important to note that most of the effects of LSD were only observed in the 26 μg group, a dose that was also most detectable with regard to subjective drug experience. Whether a perceivable change in drug experience is necessary to have a significant effect on various emotional and cognitive outcome measures remains unknown and should be further explored in future studies.
In the present study, we examined effects on various measures following four repeated low doses of LSD, which were administered every 3–4 days. Prior to this study, it was unknown whether repeated dosing would result in either sensitization or tolerance to any of the drug’s effects. Here, we provide novel evidence showing a modest decline in subjective and altered consciousness effects across the four sessions, particularly with 26 μg of LSD. Such effects appeared to be more pronounced on Session 1 relative to subsequent sessions, suggestive of tolerance. The decline in response is biologically plausible, given that LSD has prolonged agonist activity on the 5-HT2A receptor,44 which may lead to desensitization of the function of the metabotropic receptor.45 It remains to be determined if there are emotional or cognitive phenomena that emerge as a consequence of 5-HT2A receptor desensitization and/or repeated dosing. We did not find evidence that new mood-enhancing effects emerged with repeated dose, but it is possible that such effects were present but not measured.
This study had limitations, and many questions remain. First, the participants in the study did not report high levels of emotional distress before enrolling in the study, and it is possible that the beneficial effects of low doses of LSD manifest themselves in more symptomatic individuals. We note that self-reported anxiety and depression ratings, as measured by the DASS, declined substantially from the initial screening to the first study session and then to the follow-up up session, regardless of what drug the participants received. Thus, symptoms may decline simply with time or with contact with clinical research staff, reinforcing the importance of a placebo control condition. Nevertheless, future studies with more symptomatic participants are needed. A second limitation is that the drug was administered only four times and behaviour was measured while the drug active at the receptor. It is possible that some effects appear only after extended use, over periods longer than 2 weeks, between acute doses, or that the therapeutic effects are delayed, as they are with SSRIs. This remains to be studied. Another possible limitation or explanation of the discrepancy between anecdotal reports and relatively modest effects we describe here is that the tasks and questionnaires used in this study are relatively simple standard measures that may not be sensitive to the specific effects of psychedelic drugs. These drugs seem to affect the way people perceive meaning in their lives, and although we did administer the 5D-ASC, which is meant to capture some of these effects, it is possible that the effects described by microdosers in the community are not fully captured by our measures. An important limitation of the study was the lack of pharmacokinetic data, or plasma levels for each of the subjects. As noted above, previous studies report significant variability in plasma levels in LSD levels,30,46 which may have contributed to the variability in this study.
In conclusion, the present study demonstrated the feasibility of studying low doses of a psychedelic drug under placebo-controlled conditions but provided little support for beneficial effects of the drug on mood, emotional function or cognition. Despite our current findings, the anecdotal reports of beneficial effects of the drug remain compelling, suggesting that future studies may detect improvements in mood or performance under other conditions (i.e. greater number of repeated doses or when examined in clinically depressed populations).
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Institute on Drug Abuse (NIDA) DA02812; a pilot award from the Institute of Translational Medicine at the University of Chicago (UL1 RR024999); and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) through Grant Number 5UL1TR002389–05 that funds the Institute for Translational Medicine. HM was supported by NIH/National Institute of General Medical Sciences (NIGMS) T32GM007019. The Medical Scientist Training Program training grant is acknowledged as T32GM007281. AB was supported by the University of Chicago Medical Scientist Training Program.
Funding information
National Institute on Drug Abuse, Grant/Award Number: DA02812; University of Chicago Medical Scientist Training program; National Institute of Health/National Institute of General Medical Sciences, Grant/Award Number: T32GM007019; Institute of Translational Medicine at the University of Chicago, Grant/Award Number: UL1RR024999
Footnotes
CONFLICT OF INTERESTS
HdW has served as scientific advisor to Schedule I Therapeutics, Gilgamesh Pharmaceuticals and Awakn Life Sciences, and she serves on the Board of Directors of PharmAla. None of these are directly related to this study. The other authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in PsychArchives at http://doi.org/10.23668/psycharchives.5153, reference number 5153.
REFERENCES
- 1.Fadiman J The Psychedelic Explorer’s Guide: Safe, Therapeutic, and Sacred Journeys Park Street Press; 2011. [DOI] [PubMed] [Google Scholar]
- 2.Waldman A A Really Good Day: How Microdosing Made a Mega Difference in My Mood, My Marriage, and My Life Knopf; 2017. [Google Scholar]
- 3.Fadiman J, Korb S. Might microdosing psychedelics be safe and beneficial? An initial exploration. J Psychoactive Drugs 2019;51(2):118–122. [DOI] [PubMed] [Google Scholar]
- 4.Kuypers KP, Ng L, Erritzoe D, et al. Microdosing psychedelics: more questions than answers? An overview and suggestions for future research. J Psychopharmacol 2019;33(9):1039–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polito V, Stevenson RJ. A systematic study of microdosing psychedelics. PloS One 2019;14(2):e0211023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci 2004;29(4):252–265. [PMC free article] [PubMed] [Google Scholar]
- 7.Minuzzi L, Nomikos GG, Wade MR, Jensen SB, Olsen AK, Cumming P. Interaction between LSD and dopamine D2/3 binding sites in pig brain. Synapse 2005;56(4):198–204. [DOI] [PubMed] [Google Scholar]
- 8.Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 1995;56(9):395–401. [PubMed] [Google Scholar]
- 9.Preller KH, Herdener M, Pokorny T, et al. The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Current Biology: CB 2017;27(3):451–457. [DOI] [PubMed] [Google Scholar]
- 10.Preller KH, Burt JB, Ji JL, et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife 2018;7:e35082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holze F, Vizeli P, Ley L, et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology 2021b;46(3): 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron LP, Benson CJ, DeFelice BC, Fiehn O, Olson DE. Chronic, intermittent microdoses of the psychedelic N,N-dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chem Nerosci 2019;10(7):3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchborn T, Schroder H, Hollt V, Grecksch G. Repeated lysergic acid diethylamide in an animal model of depression: normalisation of learning behaviour and hippocampal serotonin 5-HT2 signalling. J Psychopharmacol 2014;28(6):545–552. [DOI] [PubMed] [Google Scholar]
- 14.Savage C Lysergic acid diethylamide; a clinical-psychological study. Am J Psychiatry 1952;108(12):896–900. [DOI] [PubMed] [Google Scholar]
- 15.Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A. The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther 2008;14(4):295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 2010;11(9):642–651. [DOI] [PubMed] [Google Scholar]
- 17.Krebs TS, Johansen PO. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol 2012;26(7):994–1002. [DOI] [PubMed] [Google Scholar]
- 18.Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 2014;202(7):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis AK, Barrett FS, May DG, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiat 2021;78(5):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med 2021;384(15): 1402–1411. [DOI] [PubMed] [Google Scholar]
- 21.Bershad AK, Schepers ST, Bremmer MP, Lee R, de Wit H. Acute subjective and behavioral effects of microdoses of lysergic acid diethylamide in healthy human volunteers. Biol Psychiatry 2019; 86(10):792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutten NRPW, Mason NL, Dolder PC, et al. Low doses of LSD acutely increase BDNF blood plasma levels in healthy volunteers. ACS Pharmacol Transl Sci 2020a;4(2):461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutten N, Mason NL, Dolder PC, et al. Mood and cognition after administration of low LSD doses in healthy volunteers: a placebo controlled dose-effect finding study. Eur Neuropsychopharmacol 2020b; 41:81–91. [DOI] [PubMed] [Google Scholar]
- 24.Yanakieva S, Polychroni N, Family N, Williams L, Luke DP, Terhune DB. The effects of microdose LSD on time perception: a randomised, double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2019;236(4):1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl) 2020;237(3):841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szigeti B, Kartner L, Blemings A, et al. Self-blinding citizen science to explore psychedelic microdosing. Elife 2021;10:e62878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety & Stress Scales 2nded. Psychology Foundation; 1995. [Google Scholar]
- 28.Dittrich A The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 1998; 31(Suppl 2):80–84. [DOI] [PubMed] [Google Scholar]
- 29.Dolder PC, Schmid Y, Haschke M, Rentsch KM, Liechti ME. Pharmacokinetics and concentration-effect relationship of oral LSD in humans. Int J Neuropsychopharmacol 2015;19(1):pyv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holze F, Liechti ME, Hutten N, et al. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide microdoses in healthy participants. Clin Pharmacol Ther 2021a;109(3):658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 32.Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol TherClin Pharmacol Ther 1971;12(2part1):245–258. [DOI] [PubMed] [Google Scholar]
- 33.McNair D, Lorr M, Droppleman L. POMS, Profile of Mood States Educational and Industrial Testing Services; 1971. [Google Scholar]
- 34.Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict 1991; 86(12):1563–1570. [DOI] [PubMed] [Google Scholar]
- 35.Studerus E, Gamma A, Vollenweider FX. Psychometric evaluation of the altered states of consciousness rating scale (OAV). PloS One 2010;5(8):e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry 2015;78(8):544–553. [DOI] [PubMed] [Google Scholar]
- 37.Giacomelli S, Palmery M, Romanelli L, Cheng CY, Silvestrini B. Lysergic acid diethylamide (LSD) is a partial agonist of D2 dopaminergic receptors and it potentiates dopamine-mediated prolactin secretion in lactotrophs in vitro. Life Sci 1998;63(3):215–222. [DOI] [PubMed] [Google Scholar]
- 38.Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 2002;26(5):634–642. [DOI] [PubMed] [Google Scholar]
- 39.Kelly PH, Iversen LL. LSD as an agonist at mesolimbic dopamine receptors. Psychopharmacologia 1975;45(2):221–224. [DOI] [PubMed] [Google Scholar]
- 40.Luethi D, Hoener MC, Krähenbühl S, Liechti ME, Duthaler U. Cytochrome P450 enzymes contribute to the metabolism of LSD to nor-LSD and 2-oxo-3-hydroxy-LSD: Implications for clinical LSD use. Biochem Pharmacol 2019;164:129–138. [DOI] [PubMed] [Google Scholar]
- 41.Vizeli P, Straumann I, Holze F, Schmid Y, Dolder PC, Liechti ME. Genetic influence of CYP2D6 on pharmacokinetics and acute subjective effects of LSD in a pooled analysis. Sci Rep 2021;11(1):10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolder PC, Schmid Y, Müller F, Borgwardt S, Liechti ME. LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology 2016;41(11):2638–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preller KH, Pokorny T, Hock A, et al. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci US A 2016;113(18):5119–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wacker D, Wang S, McCorvy JD, et al. Crystal structure of an LSD-bound human serotonin receptor. Cell 2017;168(3):377–389.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamauchi M, Miyara T, Matsushima T, Imanishi T. Desensitization of 5-HT2A receptor function by chronic administration of selective serotonin reuptake inhibitors. Brain Res 2006;1067(1):164–169. [DOI] [PubMed] [Google Scholar]
- 46.Dolder PC, Schmid Y, Steuer AE, et al. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin Pharmacokinet 2017;56(10):1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in PsychArchives at http://doi.org/10.23668/psycharchives.5153, reference number 5153.


