Abstract
Background
Risk factors for intervention in terminal ileal (TI) stricturing Crohn's disease (CD) are poorly defined. Novel and rigorous definitions for TI strictures recently became available.
Objective
We aimed to describe the rates of symptoms or need for endoscopic balloon dilation (EBD) or surgery as well as risk factors of progression in a well‐defined stricturing CD cohort.
Methods
Consecutive adult patients with non‐penetrating stricturing TI CD, as defined by centrally‐read magnetic resonance enterography CONSTRICT criteria, were separated into a derivation and validation cohort. Clinical and imaging characteristics were collected following prespecified scoring conventions. Primary outcome was a composite endpoint of EBD or surgery (“intervention”). Multivariable analysis was performed.
Results
Eighty‐six patients (48.8% female, median age 36 years) met selection criteria, 17.4% had prior EBD, 59.3% previously received biologics and 58.1% of strictures were anastomotic. Median follow‐up was 63.4 [95% CI: 57, 68.9] months. In the derivation cohort, at 12 and 48 months, 26% and 45% of patients had intervention, respectively. Multivariable analysis showed obstructive symptoms (Hazard ratio [HR] 1.444; 95% CI 1.126–1.852), stricture duration (HR 0.974; 95% CI, 0.954–0.995) and length (HR 1.039; 95% CI, 1.011–1.069) predicted intervention. The concordance index for split‐sample validation was 0.74 and 0.67, respectively. Biologics were not associated with intervention. An online risk calculator was constructed.
Conclusion
In patients with TI stricturing CD, 26% and 45% required intervention at 1 and 4 years. Obstructive symptoms, stricture duration and length were independent and validated predictors of the need for intervention. These findings are important for clinical practice and aid in the design of future trials for CD strictures.
Keywords: Crohn's disease, dilation, intervention, stricture, surgery
Key summary.
Summarise the established knowledge on this subject
A significant number of patients with Crohn's disease develop stricturing complications.
Risk factors for progression to intervention are poorly defined, especially in purely stricturing CD without internal penetration.
No information is available about progression rate and risk factors in patients using the novel stricture CONSTRICT criteria.
What are the significant and/or new findings of this study?
Twenty six percent of patients with stricturing disease undergo balloon dilation or surgery within 1 year and 45% within 4 years.
The number of obstructive symptoms, stricture duration and length predict subsequent intervention.
INTRODUCTION
Stricturing disease is a significant clinical problem with more than half of Crohn's disease (CD) patients developing clinically‐apparent bowel obstruction in their lifetime. 1 Current long‐term therapy is interventional only, such as endoscopic balloon dilation (EBD) or surgery. 2 Anti‐inflammatory therapies can provide short‐term relief, but obstruction recurs in most patients. 3 Despite frequent occurrence of CD strictures there is limited information about progression rate and risk factors for progression, which would guide clinical decision‐making between medical therapy, dilation and surgery. A major limitation of existing studies 4 , 5 , 6 , 7 , 8 , 9 is the absence of a commonly accepted definition for stricturing disease, leading to heterogenous patient populations. 10 Many studies incorporated patients with internal penetrating disease, 4 , 5 , 9 , 11 confounding results as penetrating complications are themselves an indication for surgery. 12 No central reading based on scoring conventions has been performed and stricture location was heterogenous with studies including upper gastrointestinal, mid‐small bowel and colonic strictures in addition to terminal ileal (TI) strictures. 4 , 5 , 9 , 11 , 13 , 14 Different imaging modalities, such as CT, MRI, CT enterography, magnetic resonance enterography (MRE) and intestinal ultrasound have been combined into single studies with their reliability and comparison between them being unknown.
Surprisingly, the progression rate and risk factors for progression of non‐penetrating TI stricturing CD, the most common stricture location, remains an unmet need. A global expert panel through the Stenosis Therapy and Anti‐fibrosis Research Consortium is working on developing endpoints and validated clinical, radiologic and histopathologic scoring systems, 15 and established definitions for TI stricturing disease, the so‐called CONSTRICT criteria. 16 MRE was determined the most appropriate imaging modality to assess strictures in CD. 16 , 17 Patients with TI stricturing CD have been deemed the most appropriate population for inclusion in clinical trials for fibrostenosing CD. 16 Determination of progression rate to intervention is critical for patient counseling and decision‐making as well as for feasibility assessments and power calculations in potential anti‐fibrotic therapeutic trials.
To fill this knowledge gap, we investigated progression rate to and risk factors for intervention of non‐penetrating stricturing TI CD in a well‐defined population using accepted objective pre‐specified stricture criteria and central reading.
METHODS
Study population
We performed a single center retrospective cohort study of patients with TI stricturing CD. All consecutive patients with an established CD diagnosis who underwent MRE at the Cleveland Clinic between September 2013 and April 2016 were identified through a prospectively maintained IRB‐approved radiology database. This timeframe was chosen to include MRE performed with state‐of‐the‐art protocols, 17 while allowing adequate follow‐up to meet the study objective. To ensure study rigor, MREs were centrally read by an expert radiologist with experience in inflammatory bowel disease (IBD) imaging (M.B.) blinded to the outcome parameters. Magnetic resonance enterography scoring conventions for each item were developed prior to central reading (Supplementary Table S1, Supplementary Appendix).
We included: (1) adult patients with a confirmed diagnosis of CD for at least 3 months (2) the presence of no more than two TI naïve or anastomotic small bowel strictures on MRE as defined by the CONSTRICT criteria (see ‘Definitions’) (3) MRE performed according to commonly accepted technical parameters 17 with imaging quality being deemed sufficient by the central reader radiologist. We excluded: (1) internal penetrating disease including fistula, abscess or inflammatory mass at baseline (a blind‐ending sinus was not excluded); (2) prior strictureplasty at the stricture site; (3) multifocal strictures (>2 TI strictures at the time of baseline MRE [where two strictures within 3 cm are considered the same stricture; a long segment with multiple areas of narrowing or multiple strictures with inflammation between them is counted as one stricture]); (4) stricture located >15 cm proximal to the ileocecal valve or ileocolic anastomosis; (5) ileal pouch anal anastomosis or Kock pouch; (6) proximal diverting loop ileostomy; (7) stricture associated with an ileostomy; (8) gastrointestinal malignancy; (9) stricture due to other pathologies; (10) concurrent colonic stricture. The selection criteria are consistent with a CD population with TI strictures in reach of ileocolonoscopy without associated complications. Symptoms were recorded at baseline, but were not an inclusion criterion. This population was chosen as it represents the most commonly encountered patient group and was determined to be the population to be included in the initial trials for fibrostenosing CD. 16
The dataset was chronologically separated into two subsets for split sample validation on predicting intervention: a derivation subset with the first 58 patients and a validation subset with the subsequent 28 patients. The patients were ordered chronologically based on MRE date.
Data collection and definitions
The prospectively‐maintained radiology database was linked with a prospectively‐maintained electronic medical record for all patients. Patient demographics, medical and surgical CD history, endoscopic and laboratory data, as well as multiple radiologic characteristics were documented. Specific collected data points can be found in Supplementary Table S1, Supplementary Appendix. Items were identified in a systematic review 18 and are consistent with the stricture criteria developed by the CONSTRICT group. 16
Obstructive symptoms 16 were collected and an obstructive symptom index was devised ranging from 7 (maximal) to 0 (minimal) (Supplementary Table S2). If no obstructive symptoms were recorded in the chart, patient files were assessed for documentation of any CD‐related symptoms to ensure that the lack of obstructive symptoms was not due to lack of recording of symptoms in general. Strictures on MRE were defined according to the CONSTRICT criteria 16 : (1) luminal diameter reduction of at least 50% relative to normal adjacent bowel loop; (2) 25% bowel wall increase relative to adjacent nonaffected bowel; and (3) >3 cm pre‐stricture dilation. Anastomotic strictures were defined the same as naïve strictures. Baseline MRE was defined as the first MRE that met inclusion criteria.
Outcome measures
Four outcomes were pre‐defined: 19 , 20 , 21 (1) surgery (and time to surgery) during follow‐up defined as a surgical procedure performed at the stricture site; (2) EBD (and time to EBD); (3) time to intervention (composite endpoint of surgery or EBD; primary endpoint); (4) the presence of obstructive symptoms at baseline and throughout follow‐up. Exploratory outcome variables included the development of penetrating complications during follow‐up, time to biologic therapy (among those who were not on biologic therapy at baseline) and time to symptom occurrence for those asymptomatic at baseline. Follow‐up for each patient started at the time of the baseline MRE and was censored at the time of surgery, when follow up was lost or at 48 months, whatever came first.
Statistical analysis and Ethical considerations followed standard approaches and can be found in Supplementary Methods.
RESULTS
Patient demographics and disease characteristics
A total of 1101 MREs were reviewed for this study, of which 527 (47.8%) did not meet CONSTRICT criteria, 295 (26.8%) were excluded as they occurred in pediatric patients, 59 were excluded due to internal penetrating disease, 28 due to multifocal or proximal strictures, and 21 were excluded as they were associated with a pouch. Eighty six consecutive scans from 86 individual patients were eligible (Supplementary Table S3; Table 1). Median follow‐up from baseline MRE was 63.4 (95% CI: 57, 68.9) months. Except for age at baseline, there was no significant difference between the derivation and validation cohort in terms of demographic features.
TABLE 1.
Patient characteristics at baseline
| Characteristics | [ALL] | Derivation | Validation | p‐value | N |
|---|---|---|---|---|---|
| N = 86 | N = 58 | N = 28 | |||
| Age at CD diagnosis | 21.3 [16.8; 28.0] | 20.6 [15.9; 26.3] | 24.5 [17.9; 37.1] | 0.07 | 85 |
| Age at baseline | 36.7 [26.5; 56.1] | 35.0 [24.4; 48.5] | 47.2 [31.6; 61.7] | 0.04 | 86 |
| Gender | 0.70 | 86 | |||
| Female | 42 (48.8%) | 27 (46.6%) | 15 (53.6%) | ||
| Race | 0.55 | 86 | |||
| Black or African American | 2 (2.33%) | 1 (1.72%) | 1 (3.57%) | ||
| White | 84 (97.7%) | 57 (98.3%) | 27 (96.4%) | ||
| Smoking | 0.81 | 86 | |||
| Never | 52 (60.5%) | 36 (62.1%) | 16 (57.1%) | ||
| Active | 13 (15.1%) | 9 (15.5%) | 4 (14.3%) | ||
| Past | 21 (24.4%) | 13 (22.4%) | 8 (28.6%) | ||
| BMI (kg/m2) | 25.5 [21.9; 27.9] | 24.9 [20.8; 27.9] | 25.9 [22.9; 27.9] | 0.54 | 86 |
| Family history of IBD | 0.50 | 86 | |||
| Yes | 32 (37.2%) | 22 (37.9%) | 10 (35.7%) | ||
| Unknown | 3 (3.49%) | 1 (1.72%) | 2 (7.14%) | ||
| Time since diagnosis (months) | 154 [65.5; 327] | 155 [69.0; 297] | 144 [68.3; 344] | 0.84 | 85 |
| Time from diagnosis of stricture to baseline MRE (months) | 6.31 [0.00; 35.7] | 14.4 [0.00; 36.9] | 1.40 [0.00; 34.8] | 0.34 | 86 |
| Disease location (non‐exclusive) | |||||
| Esophagus | 2 (2.33%) | 2 (3.45%) | 0 (0.00%) | >0.99 | 86 |
| Stomach | 3 (3.49%) | 1 (1.72%) | 2 (7.14%) | 0.25 | 86 |
| Duodenum | 8 (9.30%) | 5 (8.62%) | 3 (10.7%) | 0.71 | 86 |
| Jejunum | 7 (8.14%) | 6 (10.3%) | 1 (3.57%) | 0.42 | 86 |
| Ileum | 85 (98.8%) | 57 (98.3%) | 28 (100%) | >0.99 | 86 |
| Colon | 41 (47.7%) | 29 (50.0%) | 12 (42.9%) | 0.69 | 86 |
| Montreal classification: | 0.96 | 84 | |||
| B2 | 34 (40.5%) | 22 (38.6%) | 12 (44.4%) | ||
| B2p | 23 (27.4%) | 16 (28.1%) | 7 (25.9%) | ||
| B3 | 18 (21.4%) | 13 (22.8%) | 5 (18.5%) | ||
| B3p | 9 (10.7%) | 6 (10.5%) | 3 (11.1%) | ||
| Prior ileal resection | 52 (60.5%) | 32 (55.2%) | 20 (71.4%) | 0.23 | 86 |
| Number of prior resections | 1.50 [1.00; 2.00] | 2.00 [1.00; 2.00] | 1.00 [1.00; 2.00] | 0.53 | 52 |
| Anastomotic stricture | 50 (58.1%) | 32 (55.2%) | 18 (64.3%) | 0.57 | 86 |
| Presence of sinus at baseline | 6 (6.98%) | 3 (5.17%) | 3 (10.7%) | 0.39 | 86 |
| Prior EBD of current stricture | 15 (17.4%) | 13 (22.4%) | 2 (7.14%) | 0.13 | 86 |
| Time from last EBD to baseline (months) | 9.17 [6.49; 35.2] | 9.17 [5.55; 36.3] | 20.7 [14.1; 27.4] | >0.99 | 15 |
| Duration of obstructive symptoms (months) | 10.6 [3.45; 21.9] | 12.6 [3.30; 23.8] | 6.59 [5.36; 15.9] | 0.61 | 41 |
| Medications at baseline | |||||
| Five‐Aminosalicylic acid | 19 (22.1%) | 13 (22.4%) | 6 (21.4%) | >0.99 | 86 |
| Systemic corticosteroids | 21 (24.4%) | 14 (24.1%) | 7 (25.0%) | >0.99 | 86 |
| Anti‐metabolites | 9 (10.5%) | 8 (13.8%) | 1 (3.57%) | 0.26 | 86 |
| Methotrexate | 3 (3.49%) | 2 (3.45%) | 1 (3.57%) | >0.99 | 86 |
| Biologics | 51 (59.3%) | 35 (60.3%) | 16 (57.1%) | 0.96 | 86 |
| Albumin g/dl | 4.20 [3.90; 4.50] | 4.20 [3.90; 4.50] | 4.20 [3.88; 4.50] | 0.79 | 69 |
| Hemoglobin g/dl | 13.1 [11.7; 14.1] | 13.1 [11.4; 14.1] | 13.1 [12.0; 14.1] | 0.69 | 74 |
| Fecal calprotectin (mg/kg) | 318 [187; 374] | 309 [201; 395] | 318 [200; 322] | 0.61 | 9 |
| C‐reactive protein (mg/dl) | 0.45 [0.20; 0.92] | 0.45 [0.20; 1.18] | 0.40 [0.15; 0.65] | 0.63 | 28 |
Note: Bold values denote statistical significance at the p < 0.05 level.
Abbreviations: BMI, body mass index; CD, Crohn's disease; EBD, endoscopic balloon dilation; IBD, inflammatory bowel disease; MRE, magnetic resonance enterography.
Event rates of and factors associated with EBD, surgery and symptom occurrence in the derivation cohort
EBD during follow‐up
In the derivation cohort, median follow‐up was 55.3 (95% CI: 44.8, 59.6) months, during which 8%, 13% and 23% of patients required EBD at 12, 24 and 48 months, respectively (Figure 1a, Table 2). The only factor associated with EBD on univariate analysis was the presence of an anastomotic stricture (HR 8.10 [1.02–64.16]; p = 0.047) (Supplementary Table S4).
FIGURE 1.

(a) Time from baseline magnetic resonance enterography (MRE) to endoscopic balloon dilation (EBD). (b) Time from baseline MRE to surgery. (c) Time from baseline MRE in asymptomatic patients to occurrence of obstructive symptoms. (d) Time from baseline MRE to intervention
TABLE 2.
Cumulative rates of events during follow‐up
| Months | N | EBD rate (95% CI) | N | Surgery rate (95% CI) | N | Intervention rate (95% CI) | N | Obstructive rate (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Entire cohort | ||||||||
| 0 | 86 | 0 (0,0) | 86 | 0 (0,0) | 86 | 0 (0,0) | 45 | 0 (0,0) |
| 6 | 69 | 0.04 (0.01,0.1) | 71 | 0.17 (0.11,0.27) | 69 | 0.2 (0.13,0.3) | 40 | 0.09 (0.03,0.22) |
| 12 | 60 | 0.09 (0.05,0.19) | 65 | 0.23 (0.16,0.34) | 60 | 0.29 (0.2,0.4) | 35 | 0.2 (0.11,0.35) |
| 18 | 54 | 0.13 (0.07,0.23) | 61 | 0.26 (0.18,0.36) | 54 | 0.34 (0.25,0.45) | 31 | 0.27 (0.16,0.43) |
| 24 | 52 | 0.13 (0.07,0.23) | 57 | 0.29 (0.21,0.4) | 52 | 0.35 (0.26,0.46) | 28 | 0.32 (0.2,0.48) |
| 36 | 44 | 0.21 (0.13,0.34) | 53 | 0.31 (0.22,0.42) | 44 | 0.42 (0.33,0.54) | 24 | 0.39 (0.27,0.55) |
| 48 | 32 | 0.27 (0.17,0.4) | 40 | 0.36 (0.27,0.47) | 32 | 0.50 (0.39,0.61) | 18 | 0.47 (0.33,0.63) |
| Derivation cohort | ||||||||
| 0 | 58 | 0 (0, 0) | 58 | 0 (0, 0) | 58 | 0 (0, 0) | 35 | 0 (0, 0) |
| 6 | 48 | 0.02 (0, 0.12) | 49 | 0.16 (0.08, 0.28) | 48 | 0.17 (0.1, 0.3) | 32 | 0.06 (0.01, 0.21) |
| 12 | 42 | 0.08 (0.03, 0.2) | 45 | 0.21 (0.12, 0.34) | 42 | 0.26 (0.16, 0.39) | 27 | 0.21 (0.1, 0.38) |
| 18 | 36 | 0.13 (0.06, 0.27) | 41 | 0.24 (0.15, 0.38) | 36 | 0.33 (0.23, 0.47) | 23 | 0.29 (0.17, 0.48) |
| 24 | 34 | 0.13 (0.06, 0.27) | 39 | 0.26 (0.17, 0.4) | 34 | 0.35 (0.24, 0.49) | 20 | 0.36 (0.22, 0.54) |
| 36 | 31 | 0.18 (0.09, 0.33) | 37 | 0.26 (0.17, 0.4) | 31 | 0.39 (0.28, 0.53) | 17 | 0.42 (0.27, 0.61) |
| 48 | 26 | 0.23 (0.13, 0.4) | 31 | 0.32 (0.22, 0.47) | 26 | 0.45 (0.33, 0.59) | 14 | 0.49 (0.33, 0.67) |
| Validation cohort | ||||||||
| 0 | 28 | 0 (0, 0) | 28 | 0 (0, 0) | 28 | 0 (0, 0) | 10 | 0 (0, 0) |
| 6 | 21 | 0.07 (0.02, 0.26) | 22 | 0.21 (0.1, 0.42) | 21 | 0.25 (0.13, 0.45) | 8 | 0.2 (0.05, 0.59) |
| 12 | 18 | 0.12 (0.04, 0.33) | 20 | 0.29 (0.15, 0.49) | 18 | 0.36 (0.21, 0.56) | 8 | 0.2 (0.05, 0.59) |
| 18 | 18 | 0.12 (0.04, 0.33) | 20 | 0.29 (0.15, 0.49) | 18 | 0.36 (0.21, 0.56) | 8 | 0.2 (0.05, 0.59) |
| 24 | 18 | 0.12 (0.04, 0.33) | 18 | 0.36 (0.21, 0.56) | 18 | 0.36 (0.21, 0.56) | 8 | 0.2 (0.05, 0.59) |
| 36 | 13 | 0.28 (0.14, 0.53) | 16 | 0.39 (0.24, 0.6) | 13 | 0.51 (0.34, 0.7) | 7 | 0.3 (0.11, 0.67) |
| 48 | 6 | 0.34 (0.17, 0.59) | 9 | 0.44 (0.28, 0.64) | 6 | 0.59 (0.41, 0.78) | 4 | 0.4 (0.17, 0.75) |
Abbreviations: CI, Confidence interval; EBD, Endoscopic balloon dilation.
Surgery during follow‐up
Median follow‐up was 63.4 (95% CI: 57, 68.9) months, during which 21%, 26% and 32% of patients required surgery at 12, 24 and 48 months, respectively (Figure 1b, Table 2). During follow‐up, 37 patients (42.5%) required bowel resection, while two patients underwent strictureplasty. Only one patient (2.8%) was found to have dysplasia (local and non‐invasive cancer). Univariate factors associated with surgery were nausea/vomiting (HR 2.62 [1.03–6.67]; p = 0.04), the obstructive index (HR 1.41 [1.07–1.87]; p = 0.02), past smoking history (HR 3.75 [1.31–10.77]; p = 0.01), stricture length (HR 1.04 [1.01–1.07]; p = 0.003), restricted diffusion (stratified pattern) on MRE (HR 10.62 [1.24–91.13]; p = 0.03) (Supplementary Table S5).
Occurrence of symptoms during follow‐up
At baseline, 59.3% of patients had obstructive symptoms. Symptomatic patients at baseline had a median symptom duration of 12.6 (IQR, 3.30–23.8) months prior to MRE. Among asymptomatic patients, 21%, 36% and 49% patients developed symptoms at 12, 24 and 48 months, respectively (Figure 1c).
Event rates of and factors associated with intervention in the derivation cohort
The primary endpoint of intervention was defined as the composite need for EBD or surgery during follow‐up (whichever came first). Intervention rates at 12, 24 and 48 months were 26%, 35% and 45%, respectively (Figure 1d, Table 2). By univariate analysis, factors associated with intervention were nausea/vomiting (HR 2.43 [1.09–5.44]; p = 0.03), the obstructive index (HR 1.40 [1.10–1.79]; p = 0.01), body mass index (HR 1.06 [1–1.12]; p = 0.049), stricture duration (HR 0.98 [0.96–0.99]; p = 0.04), stricture length (HR 1.03 [1.002–1.05]; p = 0.03), restricted diffusion (stratified pattern) on MRE (HR 8.59 [1.03–71.46]; p = 0.047), and fibrofatty proliferation on MRE (HR 2.36 [1.02–5.47]; p = 0.046) (Table 3).
TABLE 3.
Risk factors associated with the outcome of intervention on univariate analysis
| Derivation cohort | |||||
|---|---|---|---|---|---|
| Intervention status | |||||
| Risk factor | No intervention (N = 33) | Intervention (N = 25) | HR | 95% CI | p‐value |
| BMI (kg/m2) | 22.0 [19.9; 27.4] | 26.2 [24.3; 27.9] | 1.06 | 1–1.12 | 0.049 |
| Smoking status | |||||
| Never | 23 (69.7%) | 13 (52.0%) | Reference | ||
| Active | 5 (15.2%) | 4 (16.0%) | 1.37 | 0.45–4.19 | 0.59 |
| Past | 5 (15.2%) | 8 (32.0%) | 2.32 | 0.96–5.64 | 0.06 |
| Duration of stricture (Months) | 25.9 [0.00; 41.2] | 0.56 [0.00; 22.9] | 0.98 | 0.96–0.999 | 0.04 |
| Presence of nausea/vomiting | |||||
| No | 28 (84.8%) | 15 (60.0%) | Reference | ||
| Yes | 5 (15.2%) | 10 (40.0%) | 2.43 | 1.09–5.44 | 0.03 |
| Obstructive symptom index | 0.00 [0.00; 0.00] | 2.00 [0.00; 3.00] | 1.40 | 1.10–1.79 | 0.01 |
| Duration of obstructive symptoms (Months) | 19.9 [5.82; 30.1] | 10.6 [3.10; 23.8] | 0.98 | 0.95–1.02 | 0.37 |
| Biologic therapy before baseline | |||||
| No | 14 (42.4%) | 9 (36.0%) | Reference | ||
| Yes | 19 (57.6%) | 16 (64.0%) | 1.280 | 0.57–2.90 | 0.56 |
| Fecal calprotectin (mg/kg) | 244 [187; 374] | 624 [624; 624] | NA | NA | NA |
| C‐reactive protein (mg/dl) | 0.60 [0.30; 1.75] | 0.40 [0.20; 0.55] | 0.63 | 0.28–1.40 | 0.26 |
| Anastomotic stricture | |||||
| Naïve | 16 (48.5%) | 10 (40.0%) | Reference | ||
| Anastomotic | 17 (51.5%) | 15 (60.0%) | 1.30 | 0.58–2.89 | 0.53 |
| Presence of sacculations | |||||
| No | 18 (56.2%) | 13 (54.2%) | Reference | ||
| Yes | 14 (43.8%) | 11 (45.8%) | 1.05 | 0.47–2.35 | 0.91 |
| Narrowest luminal diameter within stricture (mm) | 18.5 [16.8; 21.0] | 18.0 [17.0; 22.0] | 1.04 | 0.94–1.16 | 0.42 |
| Length of stricture (cm) | 7.00 [4.35; 21.0] | 17.0 [10.0; 28.0] | 1.03 | 1.002–1.05 | 0.03 |
| Maximal diameter of proximal small bowel dilation (mm) | 35.0 [33.0; 41.0] | 36.0 [33.0; 44.0] | 1.04 | 0.995–1.08 | 0.09 |
| Perienteric fat stranding | |||||
| Absent | 26 (83.9%) | 17 (68.0%) | Reference | ||
| Present | 5 (16.1%) | 8 (32.0%) | 1.52 | 0.65–3.52 | 0.34 |
| Presence of sinus: | |||||
| Absent | 32 (97.0%) | 23 (92.0%) | Reference | ||
| Present | 1 (3.03%) | 2 (8.00%) | 2.19 | 0.51–9.31 | 0.29 |
| Maximal wall thickness of stricture | 9.00 [8.00; 10.0] | 11.0 [8.00; 12.0] | 1.12 | 0.99–1.28 | 0.08 |
| Ulceration: | |||||
| Absent | 15 (45.5%) | 10 (40.0%) | Reference | ||
| Present | 18 (54.5%) | 15 (60.0%) | 1.05 | 0.47–2.34 | 0.90 |
| Pattern of enhancement portal or enteric phase | |||||
| Stratified | 9 (27.3%) | 12 (48.0%) | Reference | ||
| Homogenous | 6 (18.2%) | 1 (4.00%) | 0.20 | 0.03–1.53 | 0.12 |
| Luminal (inner wall) only | 18 (54.5%) | 12 (48.0%) | 0.69 | 0.31–1.54 | 0.36 |
| Pattern of enhancement delayed | |||||
| Delayed GD not available | 1 (3.12%) | 2 (8.00%) | Reference | ||
| Stratified | 3 (9.38%) | 7 (28.0%) | 2.14 | 0.44–10.37 | 0.35 |
| Homogenous | 16 (50.0%) | 12 (48.0%) | 0.85 | 0.19–3.82 | 0.84 |
| Luminal (inner wall) only | 12 (37.5%) | 4 (16.0%) | 0.43 | 0.08–2.37 | 0.33 |
| Intensity of delayed enhancement | |||||
| Delayed GD not available | 1 (3.03%) | 2 (8.00%) | Reference | ||
| Greater than portal | 12 (36.4%) | 13 (52.0%) | 1.11 | 0.25–4.93 | 0.89 |
| Equal to portal | 17 (51.5%) | 10 (40.0%) | 0.72 | 0.16–3.30 | 0.67 |
| Less than portal | 3 (9.09%) | 0 (0.00%) | NA | NA | NA |
| Restricted diffusion | |||||
| DWI not available | 29 (87.9%) | 23 (92.0%) | Reference | ||
| Absent | 1 (3.03%) | 0 (0.00%) | NA | NA | NA |
| Present homogenous | 1 (3.03%) | 0 (0.00%) | NA | NA | NA |
| Present stratified | 0 (0.00%) | 1 (4.00%) | 8.59 | 1.03–71.46 | 0.047 |
| Present luminal | 2 (6.06%) | 1 (4.00%) | 0.82 | 0.11–6.09 | 0.85 |
| Intramural fat | |||||
| Absent | 5 (15.2%) | 1 (4.00%) | Reference | ||
| Present | 28 (84.8%) | 24 (96.0%) | 2.66 | 0.36–19.72 | 0.34 |
| Whiskering | |||||
| Absent | 14 (46.7%) | 8 (33.3%) | Reference | ||
| Present | 16 (53.3%) | 16 (66.7%) | 1.43 | 0.61–3.34 | 0.41 |
| Perienteric edema or fluid | |||||
| Absent | 27 (84.4%) | 20 (83.3%) | Reference | ||
| Present | 5 (15.6%) | 4 (16.7%) | 0.95 | 0.33–2.78 | 0.92 |
| Vasa recta distension | |||||
| Absent | 27 (81.8%) | 19 (76.0%) | Reference | ||
| Present | 6 (18.2%) | 6 (24.0%) | 1.23 | 0.49–3.07 | 0.67 |
| Fibrofatty proliferation | |||||
| Absent | 20 (60.6%) | 8 (32.0%) | Reference | ||
| Present | 13 (39.4%) | 17 (68.0%) | 2.36 | 1.02–5.47 | 0.046 |
| VAS increasing inflammation | 15.0 [10.0; 15.0] | 15.0 [10.0; 20.0] | 1.03 | 0.98–1.10 | 0.27 |
| VAS non‐inflammation damage | 20.0 [10.0; 30.0] | 20.0 [15.0; 30.0] | 1.02 | 0.99–1.05 | 0.26 |
| VAS global | 25.0 [20.0; 35.0] | 27.5 [20.0; 32.5] | 1.02 | 0.99–1.05 | 0.24 |
| Validation cohort | |||
|---|---|---|---|
| Intervention status | N | ||
| Risk factor | No intervention (N = 45) | Intervention (N = 41) | |
| BMI (kg/m2) | 25.8 [22.1; 27.9] | 25.9 [23.2; 27.7] | 28 |
| Smoking status | 28 | ||
| Never | 7 (58.3%) | 9 (56.2%) | |
| Active | 2 (16.7%) | 2 (12.5%) | |
| Past | 3 (25.0%) | 5 (31.2%) | |
| Duration of stricture (Months) | 0.00 [0.00; 1.41] | 16.5 [0.00; 42.7] | 28 |
| Presence of nausea/vomiting | 28 | ||
| No | 10 (83.3%) | 4 (25.0%) | |
| Yes | 2 (16.7%) | 12 (75.0%) | |
| Obstructive symptom index | 0.00 [0.00; 2.00] | 2.00 [2.00; 3.00] | 28 |
| Duration of obstructive symptoms (Months) | 5.26 [3.09; 5.65] | 14.4 [5.98; 16.8] | 18 |
| Biologic therapy before baseline | 28 | ||
| No | 4 (33.3%) | 8 (50.0%) | |
| Yes | 8 (66.7%) | 8 (50.0%) | |
| Fecal calprotectin (mg/kg) | 322 [320; 323] | 83.0 [83.0; 83.0] | 3 |
| C‐reactive protein (mg/dl) | 0.50 [0.30; 0.70] | 0.10 [0.10; 0.10] | 6 |
| Anastomotic stricture | 28 | ||
| Naïve | 5 (41.7%) | 5 (31.2%) | |
| Anastomotic | 7 (58.3%) | 11 (68.8%) | |
| Presence of sacculations | 28 | ||
| No | 9 (75.0%) | 12 (75.0%) | |
| Yes | 3 (25.0%) | 4 (25.0%) | |
| Narrowest luminal diameter within stricture (mm) | 17.0 [16.0; 20.0] | 20.0 [18.0; 21.0] | 27 |
| Length of stricture (cm) | 8.20 [4.75; 24.2] | 9.75 [4.90; 16.5] | 28 |
| Maximal diameter of proximal small bowel dilation (mm) | 35.0 [32.0; 36.5] | 34.5 [32.5; 45.0] | 28 |
| Perienteric fat stranding | 28 | ||
| Absent | 10 (83.3%) | 13 (81.2%) | |
| Present | 2 (16.7%) | 3 (18.8%) | |
| Presence of sinus | 28 | ||
| Absent | 10 (83.3%) | 15 (93.8%) | |
| Present | 2 (16.7%) | 1 (6.25%) | |
| Maximal wall thickness of stricture | 10.0 [7.00; 11.2] | 9.50 [8.00; 11.0] | 28 |
| Ulceration | 28 | ||
| Absent | 5 (41.7%) | 8 (50.0%) | |
| Present | 7 (58.3%) | 8 (50.0%) | |
| Pattern of enhancement portal or enteric phase | 28 | ||
| Stratified | 4 (33.3%) | 6 (37.5%) | |
| Homogenous | 4 (33.3%) | 3 (18.8%) | |
| Luminal (inner wall) only | 4 (33.3%) | 7 (43.8%) | |
| Pattern of enhancement delayed | 28 | ||
| Delayed GD not available | 1 (8.33%) | 0 (0.00%) | |
| Stratified | 1 (8.33%) | 6 (37.5%) | |
| Homogenous | 8 (66.7%) | 10 (62.5%) | |
| Luminal (inner wall) only | 2 (16.7%) | 0 (0.00%) | |
| Intensity of delayed enhancement | 28 | ||
| Delayed GD not available | 1 (8.33%) | 0 (0.00%) | |
| Greater than portal | 6 (50.0%) | 6 (37.5%) | |
| Equal to portal | 4 (33.3%) | 10 (62.5%) | |
| Less than portal | 1 (8.33%) | 0 (0.00%) | |
| Restricted diffusion | 28 | ||
| DWI not available | 2 (16.7%) | 0 (0.00%) | |
| Absent | 2 (16.7%) | 4 (25.0%) | |
| Present homogenous | 3 (25.0%) | 4 (25.0%) | |
| Present stratified | 2 (16.7%) | 3 (18.8%) | |
| Present luminal | 3 (25.0%) | 5 (31.2%) | |
| Intramural fat | 28 | ||
| Absent | 0 (0.00%) | 2 (12.5%) | |
| Present | 12 (100%) | 14 (87.5%) | |
| Whiskering | 27 | ||
| Absent | 6 (54.5%) | 8 (50.0%) | |
| Present | 5 (45.5%) | 8 (50.0%) | |
| Perienteric edema or fluid | 27 | ||
| Absent | 11 (91.7%) | 13 (86.7%) | |
| Present | 1 (8.33%) | 2 (13.3%) | |
| Vasa recta distension: | 28 | ||
| Absent | 10 (83.3%) | 14 (87.5%) | |
| Present | 2 (16.7%) | 2 (12.5%) | |
| Fibrofatty proliferation | 28 | ||
| Absent | 6 (50.0%) | 7 (43.8%) | |
| Present | 6 (50.0%) | 9 (56.2%) | |
| VAS increasing inflammation | 15.0 [10.0; 21.2] | 17.5 [10.0; 25.0] | 28 |
| VAS non‐inflammation damage | 20.0 [15.0; 25.0] | 27.5 [15.0; 35.0] | 28 |
| VAS global | 25.0 [23.8; 36.2] | 32.5 [23.8; 40.0] | 28 |
Note: Statistics presented as Median [P25, P75] and N (column %). Bold values denote statistical significance at the p 〈 0.05 level.
Abbreviations: BMI, body mass index; CI, confidence interval; DWI, diffusion‐weighted imaging; EBD, endoscopic balloon dilation; GD, gadolinium; HR, hazard ratio; NA, not applicable; VAS, visual analog scale.
On multivariable analysis, predictors of intervention in the derivation cohort were duration of stricture (HR 0.97 [0.95–0.995]; p = 0.016 for longer stricture duration), stricture length (HR 1.04 [1.01–1.07]; p = 0.007) and the obstructive symptom index (HR 1.44 [1.13–1.85]; p = 0.004) (Table 4). After adjusting for other predictors, with every unit increase in the obstructive index reflecting one additional obstructive symptom, the risk of intervention increased by 44.4%.
TABLE 4.
Multivariable model predicting intervention with split sample validation
| Risk factor | HR | 95% CI | p‐value |
|---|---|---|---|
| Obstructive index | 1.44 | 1.13–1.85 | 0.004 |
| Length of stricture | 1.04 | 1.01–1.07 | 0.007 |
| Duration of stricture | 0.97 | 0.95–0.995 | 0.016 |
| Biologics before baseline | 2.12 | 0.88–5.08 | 0.09 |
| Maximal wall thickness of stricture | 1.12 | 0.98–1.28 | 0.09 |
Note: Bold values denote statistical significance at the p < 0.05 level.
Validation of risk factors for intervention in the validation cohort
Including the factors stricture duration, length and obstructive index, the concordance index of the model for the derivation dataset was 0.74 (Figure 2a). In the validation cohort the model could predict intervention with a concordance index of 0.67 (Figure 2b), indicating a good model fit. A nomogram describing the relationship between predictors and the predicted intervention probability at one and 4 years in the dataset was developed (Figure 3, see Supplemental Figure S1 for an example explaining the use of the nomogram). In addition, the model was used to create an online risk calculator for predicting intervention, accessible at https://riskcalc.org/CrohnsDiseaseSmallBowelStricture.
FIGURE 2.
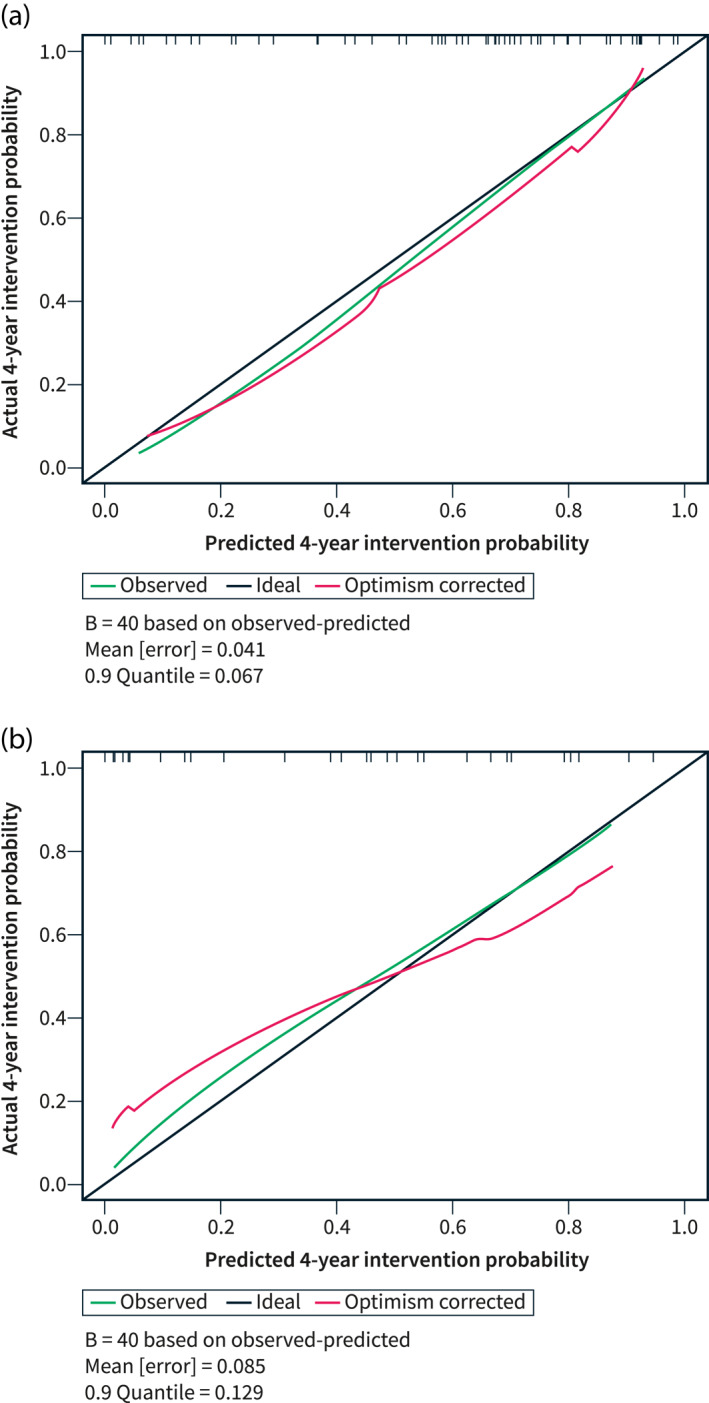
Calibration plot of the multivariable model. (a) Derivation dataset. (b) Validation dataset
FIGURE 3.
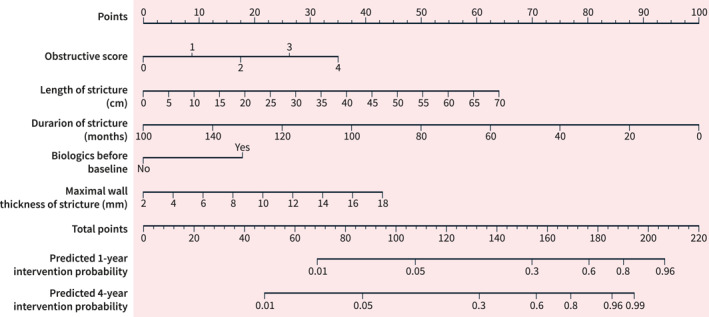
Nomogram depicting intervention probability
A subgroup analysis was performed on patients with naïve strictures in the validation cohort. Although this was limited by the small size (10 out of 28 patients had naïve strictures), the concordance index was 0.88, which indicates a good prediction. The model is therefore still valid among patients without anastomotic strictures.
Penetrating complications and impact of biologics during follow‐up
Six patients (6.90%) developed penetrating complications during follow‐up. None of them had a sinus on baseline imaging. Importantly, there was no association between biologic use during follow‐up and outcomes, including the occurrence of symptoms, EBD, surgery and the composite outcome of intervention (Supplementary Table S6).
DISCUSSION
In patients with stricturing TI CD as defined by the CONSTRICT criteria 16 and treated according to contemporary standard of care, obstructive symptoms, shorter stricture duration and increased stricture length were found to be associated with intervention (EBD or surgery) in a derivation and validation design.
A major limitation of the current literature is the lack of stricture definitions. A systematic review assessing cross‐sectional imaging for stricturing CD found significant heterogeneity in the definitions used among studies. 18 A consensus‐based set of diagnostic criteria has therefore been recommended by the CONSTRICT group in order to standardize stricture definitions. Our study is the first to describe the rate of progression and risk factors of stricturing CD using the new criteria. Other limitations in the available literature include the inclusion of mixed populations, the lack of validation of predictors, inclusion of patients with pre‐existing internal penetrating disease, the presence of multifocal strictures (including colonic strictures) and the lack of central reading. 4 , 14 , 22 , 23 The importance of central reading has previously been demonstrated for endoscopy and histopathology. 24 Our study addresses these gaps by including a well‐defined population from a prospectively‐maintained registry using a consensual stricture definition, with central reading and validation of results. Of note, included patients had to have no more than 2 small bowel strictures. This criterion was selected in order to represent the majority of the stricture patient population, as well as to reflect the patient population selected in current or future clinical trials (NCT05013385) and thereby allowing development of a prediction model that can be used for power calculations in these settings.
Information on the progression rate and risk factors of intervention of stricturing disease is limited. Our findings are in line with a prospective cohort study 4 which assessed outcomes of adalimumab therapy in stricturing small bowel CD. Increased stricture length, obstructive symptoms as well as “recent” onset of obstructive symptoms were associated with failure of medical therapy, among others. In that study however, heterogenous stricture definitions were used, only adalimumab was allowed as a therapy, specified outcomes were different and images were not centrally evaluated. Increased stricture length was also associated with surgery in other studies. 14 , 22 Importantly, stricture length has recently been found to be measurable in a highly reliable manner, making this risk factor for intervention broadly applicable. 25
In our study, the number of obstructive symptoms was associated with the need for intervention. Using a simple obstructive symptom index, we demonstrate that a higher score is linked with a higher likelihood to require intervention. This can be a useful tool that is simple to use in practice to help identify patients at higher risk of requiring intervention despite standard medical therapy and help in decision‐making in clinical practice until a validated patient‐reported outcome instrument is available. An important finding was the high rate of patients without obstructive symptoms. It is critical to highlight that most patients reported symptoms consistent with IBD but that up to 40% had no obstructive symptoms at baseline (as defined by the CONSTRICT panel and used in prior studies 16 ). This indicates, comparable to luminal CD, a disconnect between symptoms and objective markers of disease. Among asymptomatic patients, less than half developed symptoms at 4 years. This finding also challenges the Montreal classification as the latter generally relies on symptoms triggering imaging to correctly classify patients. Since it does not require imaging, it may in fact need to be revisited given the high rate of “silent” strictures.
Shorter duration of obstructive symptoms as a risk factor for intervention is interesting. This may be related to the clinically observed phenomenon of accommodation of symptoms with longer stricture duration or patients adapting by dietary or lifestyle changes or may be due to separation of ‘acute’ and more severe obstructions from ‘chronic’ but less severe presentations.
Of note, although anastomotic strictures were more likely to be shorter, they were not associated with the outcome of intervention, suggesting a similar approach to managing anastomotic and naïve strictures can be considered. An interesting observation was the low rate of fistulizing complications. This is critical information regarding the progression of stricturing disease. It may represent a true low rate of fistulizing complications in our patient population with purely stricturing disease as MRE was required for inclusion and hence no ‘silent’ internal penetrating disease was present. Development of fistulizing disease in the setting of a stricture may take several years and follow‐up may not have been long enough to detect the development of penetrating complications.
Importantly, biologic use was not associated with any of the outcomes in the present study. In CREOLE, patients were treated with adalimumab but the study was observational and there was no control arm in order to evaluate of the true impact of biologics. 4 In a systematic review assessing biologics in stricturing CD, 10 28.3% of patients required surgery at 23 months, comparable to our findings, where 29% at 24 months required surgery. Given the observational nature of available data, no firm conclusion regarding the impact of biologics in terms of delaying or preventing intervention can be drawn. This raises the question of whether biologic therapy changes the natural history of stricturing CD. Our results suggest that they may not. This may be explained by the fact that once fibrosis is established, it may be too late for biologics to make an impact. In addition, biologics do not appear to change the progression to stricturing or complicated CD, 26 , 27 even when introduced early in a pediatric prospective cohort. 28 Further prospective studies are needed to clarify the role of biologics in this setting. 3 , 29 In concordance with this, factors associated with active inflammation such as MRE features of inflammation and C‐reactive protein were not associated with any of the outcomes. This suggests that inflammation (or treatment of inflammation with biologics as discussed above) may not impact outcomes once strictures are established.
This study also highlights the pressing need for antifibrotic development and the establishment of adequate endpoints in stricturing CD to support it. Until recently no pathway to design clinical trials existed and respective endpoints were missing. This is being addressed by a global consortium 15 , 16 and in fact the population examined in this manuscript largely represents a population that was included in the first global clinical trial testing antifibrotics in stricturing CD (NCT05013385). The information provided herein supports power calculations for this scenario as patients treated with standard of care medical therapy would comprise the placebo arm in this setting.
The limitations of our study include the observational nature of the study and the limited number of patients (albeit comparable to other published studies of this kind) 5 , 7 , 8 , 9 , 11 , 22 as well as non‐protocolized medical treatments. However, these patients were a well‐defined group treated according to currently accepted standards of care in a quaternary care IBD center and outcomes seen here likely reflect outcomes that can be expected in “real‐world” practice. This manuscript noted a disconnect between symptoms and the presence of strictures. Although this may be due to variations in symptom reporting, a standardized electronic template for symptom evaluation was used in most cases, which would make this possibility unlikely. Another explanation would be that patients may adapt their diet to avoid symptoms. Dietary restrictions are taken into account in the obstructive symptom index, but may not be assessed systematically or in‐depth during clinic visits. Finally, another more likely explanation may be that obstructive symptoms develop later in the disease course and that when they do occur, this is associated with a need for intervention and portends poor response to medical therapy. Nevertheless, this is an important consideration when planning for clinical trials in stricturing disease in the future and does highlight the need for further work to better define PROs in stricturing CD, which is currently ongoing. 16
In conclusion, the present study shows that in a cohort of patients with TI stricturing CD defined by the CONSTRICT criteria and treated according to current standard of care, obstructive symptoms, duration and length of stricture were independent and validated predictors of the need for intervention. Future studies are needed to establish appropriate endpoints in stricturing CD and explore treatment targets. This should pave the way for clinical trials to evaluate the role of antifibrotics in this setting, which are eagerly awaited.
AUTHOR CONTRIBUTIONS
All authors had substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work, drafting the work or revising it critically for important intellectual content and provided final approval of the version to be published. All authors provided agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
Sara El Ouali has received lecture fees from Janssen and Abbvie. Mark E. Baker receives support to the institution for salary, software and hardware from Siemens Healthineers, Pfizer Inc and the Helmsley Charitable Trust. Ruishen Lyu has no conflict of interest. Joel G. Fletcher receives research support to the institution: Siemens Healthineers, Takeda, Medtronics, Helmsley Foundation; consulting (funds to institution) Pfizer, Takeda, Glaxo Smith Kline, Boehringer Ingelheim. David H. Bruining receives research support: Takeda and Medtronics and consulting: Medtronics. Stefan D. Holubar receives the following consultant fees: Shionogi, Takeda, Guidepoint. Benjamin Click serves on the speaker's bureau for Takeda and as consultant to TARGET‐RWE. Taha Qazi has no conflict of interest. Benjamin L. Cohen receives the following financial support: Advisory Boards and Consultant for Abbvie, Celgene‐Bristol Myers Squibb, Pfizer, Sublimity Therapeutics, TARGET RWE; CME Companies: Cornerstones, Vindico; Speaking: Abbvie. Florian Rieder is on the advisory board or consultant for Agomab, Allergan, AbbVie, Boehringer‐Ingelheim, Celgene, CDISC, Cowen, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Janssen, Koutif, Metacrine, Morphic, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Takeda, Techlab, Theravance, Thetis, UCB.
ETHICS STATEMENT
The Cleveland Clinic Institutional Review Board approved the study (CCF 20–146). The need for informed consent was waived as there was no direct contact with patients for the purpose of the study.
WRITING ASSISTANCE
None.
Supporting information
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Figure S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
ACKNOWLEDGMENTS
This study was supported in part by the Helmsley Charitable Trust through the Stenosis Therapy and Anti‐Fibrotic Research (STAR) Consortium, National Institutes of Health (K08DK110415 & R01DK123233) to F.R. and Pfizer Inc. through a sponsored research agreement.
El Ouali S, Baker ME, Lyu R, Fletcher JG, Bruining DH, Holubar SD, et al. Validation of stricture length, duration and obstructive symptoms as predictors for intervention in ileal stricturing Crohn's disease. United European Gastroenterol J. 2022;10(9):958–72. 10.1002/ueg2.12314
Guarantor of the article: Florian Rieder
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):340–50.e6. 10.1053/j.gastro.2016.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El Ouali S, Click B, Holubar SD, Rieder F. Natural history, diagnosis and treatment approach to fibrostenosing Crohn's disease. United Eur Gastroenterol J. 2020;8(3):263–70. 10.1177/2050640620901960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sleiman J, El Ouali S, Qazi T, Cohen B, Steele SR, Baker ME, et al. Prevention and treatment of stricturing Crohn’s disease—perspectives and challenges. Expet Rev Gastroenterol Hepatol. 2021;15(4):401–11. 10.1080/17474124.2021.1854732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouhnik Y, Carbonnel F, Laharie D, Stefanescu C, Hebuterne X, Abitbol V, et al. Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67(1):53–60. 10.1136/gutjnl-2016-312581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allocca M, Bonifacio C, Fiorino G, Spinelli A, Furfaro F, Balzarini L, et al. Efficacy of tumor necrosis factor antagonists in stricturing Crohn's disease: a tertiary center real‐life experience. Dig Liver Dis. 2017;49(8):872–7. 10.1016/j.dld.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 6. Yaffe BH, Korelitz BI. Prognosis for nonoperative management of small‐bowel obstruction in Crohn's disease. J Clin Gastroenterol. 1983;5(3):211–5. 10.1097/00004836-198306000-00003 [DOI] [PubMed] [Google Scholar]
- 7. Amitai MM, Klang E, Levartovsky A, Rozendorn N, Soffer S, Taha GA, et al. Diffusion‐weighted magnetic resonance enterography for prediction of response to tumor necrosis factor inhibitors in stricturing Crohn's disease. Abdom Radiol (NY). 2018;43(12):3207–12. 10.1007/s00261-018-1626-9 [DOI] [PubMed] [Google Scholar]
- 8. Samimi R, Flasar MH, Kavic S, Tracy K, Cross RK. Outcome of medical treatment of stricturing and penetrating Crohn's disease: a retrospective study. Inflamm Bowel Dis. 2010;16(7):1187–94. 10.1002/ibd.21160 [DOI] [PubMed] [Google Scholar]
- 9. Campos C, Perrey A, Lambert C, Pereira B, Goutte M, Dubois A, et al. Medical therapies for stricturing Crohn's disease: efficacy and cross‐sectional imaging predictors of therapeutic failure. Dig Dis Sci. 2017;62(6):1628–36. 10.1007/s10620-017-4572-4 [DOI] [PubMed] [Google Scholar]
- 10. Lu C, Baraty B, Lee Robertson H, Filyk A, Shen H, Fung T, et al. Systematic review: medical therapy for fibrostenosing Crohn’s disease. Aliment Pharmacol Ther. 2020;51(12):1233–46. 10.1111/apt.15750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pelletier AL, Kalisazan B, Wienckiewicz J, Bouarioua N, Soule JC. Infliximab treatment for symptomatic Crohn's disease strictures. Aliment Pharmacol Ther. 2009;29(3):279–85. 10.1111/j.1365-2036.2008.03887.x [DOI] [PubMed] [Google Scholar]
- 12. Rieder F, Latella G, Magro F, Yuksel ES, Higgins PD, Di Sabatino A, et al. European Crohn's and colitis organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn's disease. J Crohns Colitis. 2016;10(8):873–85. 10.1093/ecco-jcc/jjw055 [DOI] [PubMed] [Google Scholar]
- 13. Nanda K, Courtney W, Keegan D, Byrne K, Nolan B, O'Donoghue D, et al. Prolonged avoidance of repeat surgery with endoscopic balloon dilatation of anastomotic strictures in Crohn's disease. J Crohn's Colitis. 2013;7(6):474–80. 10.1016/j.crohns.2012.07.019 [DOI] [PubMed] [Google Scholar]
- 14. Bamba S, Sakemi R, Fujii T, Takeda T, Fujioka S, Takenaka K, et al. A nationwide, multi‐center, retrospective study of symptomatic small bowel stricture in patients with Crohn's disease. J Gastroenterol 2020;55(6):615–26. 10.1007/s00535-020-01670-2 [DOI] [PubMed] [Google Scholar]
- 15. Gordon IO, Bettenworth D, Bokemeyer A, Srivastava A, Rosty C, de Hertogh G, et al. Histopathology scoring systems of stenosis associated with small bowel Crohn's disease: a systematic review. Gastroenterology. 2020;158(1):137–50.e1. 10.1053/j.gastro.2019.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rieder F, Bettenworth D, Ma C, Parker CE, Williamson LA, Nelson SA, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti‐fibrotic stricture therapies in Crohn's disease. Aliment Pharmacol Ther 2018;48(3):347–57. 10.1111/apt.14853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruining DH, Zimmermann EM, Loftus EV, Jr. , Sandborn WJ, Sauer CG, Strong SA, et al. Consensus recommendations for evaluation, interpretation, and utilization of Computed tomography and magnetic resonance enterography in patients with small bowel Crohn's disease. Gastroenterology. 2018;154(4):1172–94. 10.1053/j.gastro.2017.11.274 [DOI] [PubMed] [Google Scholar]
- 18. Bettenworth D, Bokemeyer A, Baker M, Mao R, Parker CE, Nguyen T, et al. Assessment of Crohn's disease‐associated small bowel strictures and fibrosis on cross‐sectional imaging: a systematic review. Gut. 2019;68(6):1115–26. 10.1136/gutjnl-2018-318081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bettenworth D, Mucke MM, Lopez R, Singh A, Zhu W, Guo F, et al. Efficacy of endoscopic dilation of gastroduodenal Crohn's disease strictures: a systematic review and meta‐analysis of individual patient data. Clin Gastroenterol Hepatol. 2019;17(12):2514–22e8. 10.1016/j.cgh.2018.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh A, Agrawal N, Kurada S, Lopez R, Kessler H, Philpott J, et al. Efficacy, safety, and long‐term outcome of serial endoscopic balloon dilation for upper gastrointestinal Crohn's disease‐associated strictures‐A cohort study. J Crohns Colitis. 2017;11(9):1044–51. 10.1093/ecco-jcc/jjx078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bettenworth D, Gustavsson A, Atreja A, Lopez R, Tysk C, van Assche G, et al. A pooled analysis of efficacy, safety, and long‐term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn's disease. Inflamm Bowel Dis. 2017;23(1):133–42. 10.1097/mib.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 22. Lowe SC, Ream J, Hudesman D, Malter L, Bosworth B, Xia Y, et al. A clinical and radiographic model to predict surgery for acute small bowel obstruction in Crohn’s disease. Abdom Radiol. 2020;45(9):2663–8. 10.1007/s00261-020-02514-6 [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez‐Lago I, Hoyo JD, Perez‐Girbes A, Garrido‐Marin A, Casanova MJ, Chaparro M, et al. Early treatment with anti‐tumor necrosis factor agents improves long‐term effectiveness in symptomatic stricturing Crohn's disease. Unit Eur Gastroenterol J. 2020;0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jairath V, Ordas I, Zou G, Panes J, Stoker J, Taylor SA, et al. Reliability of measuring ileo‐colonic disease activity in Crohn’s disease by magnetic resonance enterography. Inflamm Bowel Dis. 2018;24(2):440–9. 10.1093/ibd/izx040 [DOI] [PubMed] [Google Scholar]
- 25. Rieder F, Baker ME, Bruining DH, Fidler JL, Sheedy SP, Heiken J, et al. Mo1462: development of a magnetic resonance enterography index for assessing small bowel strictures in patients with Crohn's disease: validation of methods and item reliability. Gastroenterology. 2022;162(7):S‐774–S‐5. 10.1016/s0016-5085(22)61834-1 [DOI] [Google Scholar]
- 26. Jeuring SF, van den Heuvel TR, Liu LY, Zeegers MP, Hameeteman WH, Romberg‐Camps MJ, et al. Improvements in the long‐term outcome of Crohn's disease over the past two decades and the relation to changes in medical management: results from the population‐based IBDSL cohort. Am J Gastroenterol. 2017;112(2):325–36. 10.1038/ajg.2016.524 [DOI] [PubMed] [Google Scholar]
- 27. Lazarev M, Ullman T, Schraut WH, Kip KE, Saul M, Regueiro M. Small bowel resection rates in Crohn's disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis. 2010;16(5):830–5. 10.1002/ibd.21118 [DOI] [PubMed] [Google Scholar]
- 28. Kugathasan S, Denson LA, Walters TD, Kim MO, Marigorta UM, Schirmer M, et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet. 2017;389(10080):1710–8. 10.1016/s0140-6736(17)30317-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D’Amico F, Pugliese N, Peyrin‐Biroulet L, Danese S. Efficacy of anti‐TNFα drugs in patients with stricturing Crohn’s disease. Expet Rev Gastroenterol Hepatol. 2020;14(5):347–53. 10.1080/17474124.2020.1759417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Figure S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


