Summary
Obesity and diabetes are independent risk factors for death during sepsis. S100A8, an alarmin, is related to inflammation, obesity, and diabetes. Here, we examine the role of S100A8 in sepsis of obesity and diabetes models. Injection of S100A8 prolongs the survival of septic mice induced by lethal endotoxemia, Escherichia coli injection, or cecal ligation and puncture. S100A8 decrease the LPS-induced expression of proinflammatory cytokines in peritoneal macrophages by inhibiting TLR4-mediated signals in an autocrine manner. db/db, ob/ob, and western diet-fed mice demonstrate reduced upregulation of S100A8 induced by LPS treatment in both serum and peritoneal cells. These mice also show shorter survival after LPS injection, and S100A8 supplementation prolonged the survival. While myelomonocytic cells-specific S100A8-deficient mice (Lyz2cre:S100A8floxed/floxed) exhibit shorter survival after LPS treatment, S100A8 supplementation prolonged the survival. Thus, myelomonocytic cell-derived S100A8 is crucial for protection from sepsis, and S100A8 supplementation improves sepsis, particularly in mice with obesity and diabetes.
Subject areas: physiology, pathophysiology, immunology
Graphical abstract
Highlights
-
•
Obese and diabetic mice demonstrate impaired induction of S100A8 during endotoxemia
-
•
S100A8 suppresses TLR4-mediated inflammatory cytokine production
-
•
S100A8 from myelomonocytic cells is critical for survival during sepsis
-
•
S100A8 supplementation rescues mice with obesity and diabetes from septic death
Physiology; Pathophysiology; Immunology.
Introduction
Patients with obesity or type 2 diabetes often have serious infections caused by bacteria.1,2 Monocytes from people with obesity exhibit activated nuclear factor κβ (NF-κβ) and increased mRNA expression of tumor necrosis factor-a (Tnfa) and interleukin (Il)-6.3,4 Hyperglycemia impairs neutrophil and monocyte function and increases inflammatory cytokine levels.5 Pathogen-associated molecular patterns (PAMPs), which are substances derived from pathogenic microorganisms, have been assumed to play important roles in the inflammatory response and sepsis development.6 In addition to PAMPs, Alarmins (also referred to as damage-associated molecular patterns (DAMPs)), which are host-derived endogenous substances in infection and trauma, are now considered to be crucial regulators in severe sepsis.7,8 S100 calcium-binding protein A8 (S100A8), an alarmin, is an inflammation-related protein predominantly secreted by activated neutrophils and macrophages during acute inflammation.9,10,11,12 S100A8 is known to be a ligand of Toll-like receptor (TLR) 4 and receptor for advanced glycation end products (RAGE) that induces inflammation10,13 and forms a homodimer or a heterodimer with S100A9.14 The serum S100A8 level is elevated in patients with obesity or type 2 diabetes, in addition to those with sepsis, cancer, or autoimmune diseases.15,16,17,18,19 Circulating S100A8/A9 levels are also increased in hospitalized patients with septic shock, and sepsis survivors show lower serum S100A8/A9 levels than nonsurvivors.20,21 The serum S100A8 level has been reported to be an indicator of damaged cells and a predictor of mortality in patients with septic shock.20,21 We previously showed that the chronic stimulation of islets with glucose increased the expression of S100A8 and that islet-derived S100A8 triggered inflammation through mutual interactions with surrounding macrophages.22,23,24 Thus, S100A8 has been thought to be a mediator of inflammation in people with obesity or diabetes.
On the other hand, whether S100A8 has a proinflammatory or anti-inflammatory role in sepsis is controversial. S100A8 has been reported to attenuate the production of proinflammatory mediators such as cytokines, chemokines, reactive oxygen species (ROS), and nitric oxide (NO).25 S100A8 decreases mast cell degranulation and IL-4, IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF) secretion in response to immunoglobulin E (IgE) cross-linking by inhibiting intracellular ROS production in vitro.26 Prestimulation of mouse and human monocytes with S100A8 attenuates the production of IL-6 and TNF-α in response to lipopolysaccharide (LPS) and bacteria via downregulation of phosphorylated p38 mitogen-activated protein kinases (MAPKs) and protects the host from sepsis-induced death.27 Furthermore, the noncovalent high-affinity binding of S100A8/A9 with proinflammatory IL-1β, IL-6, and TNF-α suggests the ability of S100A8/A9 to capture cytokines.28 S100A8 promotes anti-inflammatory IL-10 expression in airway epithelial cells, causes impaired LPS-induced neutrophil infiltration, and reduces inflammatory cytokine induction.29,30 S100A8 negatively regulates leukocyte adhesion and migration by reducing p38 MAPK phosphorylation.31 In neonatal sepsis, S100A8/A9 is an essential modulator of neonatal immunity to prevent sepsis.32,33 In addition, high concentrations of S100A8/A9 protein complexes have antibacterial effects due to their metal ion chelating ability.34,35 Thus, it remains controversial whether S100A8 has a harmful or protective effect on survival in sepsis.
In this study, we investigated the role of serum S100A8 in mouse models of sepsis using conditional knockout (KO) mice and mouse models of obesity and diabetes.
Results
S100A8 induced inflammatory cytokines in peritoneal macrophages but did not result in a septic response
To assess the effects of S100A8 on the expression of inflammatory cytokines, peritoneal macrophages isolated from wild-type (WT) mice were treated with PBS, recombinant S100A8, or recombinant S100A8/S100A9 in vitro (Figure S1A). The mRNA expression of Tnfa was significantly increased with S100A8 but not with S100A8/S100A9, while the mRNA expression of Il-1b, Il-6, C-X-C motif chemokine ligand 10 (Cxcl10), Mip-1a, and Mcp1 was not changed (Figure S1B). We next examined the effects of S100A8 on cytokine production in WT mice in vivo (Figure S1C). Intraperitoneal injection of LPS upregulated the mRNA expression of Tnfa, Il-1b, Il-6, Cxcl10, Mip-1a, Mcp1, S100a8, and S100a9 in peritoneal cells (Figure S1D). S100A8 injection also increased Tnfa, Il-1b, Il-6, Mip-1a, Mcp1, S100a8, and S100a9 expression, whereas the expression of Tnfa, Il-6, Cxcl10, and Mip-1a induced by S100A8 injection was lower than that induced by LPS injection (Figure S1D). We also investigated the effects of S100A8 supplementation on the liver. S100A8 and S100A8/S100A9 supplementation did not change the mRNA expression of Tnfa, Il-1b, Il-6, Cxcl10, Mip-1a, Mcp1, S100a8 and S100a9 in Hepa1-6 mouse hepatoma cells compared to PBS treatment (Figure S2A). Intraperitoneal injection of LPS significantly upregulated the mRNA expression of Tnfa, Il-1b, Il-6, Mip-1a, S100a8 and S100a9 and tended to increase Cxcl10 and Mcp1 expression in the liver compared to PBS treatment (Figure S2B). However, S100A8 did not change the expression of those cytokines in the liver compared to PBS (Figure S2B). There were no changes in the serum AST, ALT, total cholesterol, or triglyceride levels among the PBS, LPS, and S100A8 injection groups (Figure S2C). Since intraperitoneal injection of S100A8 induced inflammatory cytokines in peritoneal cells, similar to LPS, we investigated the survival rate after the intraperitoneal injection of PBS, S100A8, or LPS. All mice died within 48 h after LPS injection, while S100A8 did not reduce survival through 72 h, similar to PBS (Figures S3A and S3B). Although injection with LPS decreased body temperature, PBS or S100A8 injection did not alter it (Figure S3C). Blood glucose levels were reduced by LPS but not by PBS or S100A8 (Figure S3D). We then examined the serum S100A8 levels after LPS injection. Mice showed a time-dependent increase in circulating S100A8 levels after LPS injection (Figure S3E). These results in turn suggest that the increase in S100A8 is an adaptive response triggered by endotoxemia.
S100A8 alleviated lipopolysaccharide-induced inflammation and improved survival in sepsis models
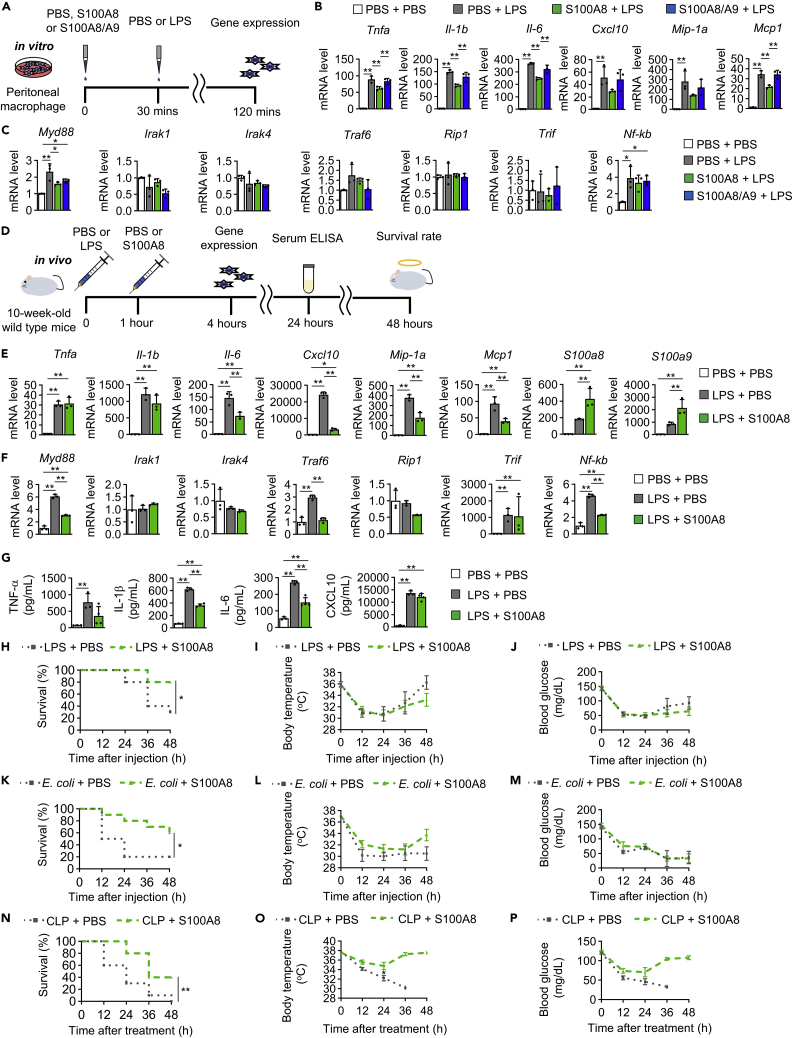
Here, we explored the protective effects of S100A8 on LPS-induced endotoxic shock. Isolated peritoneal macrophages were pretreated with S100A8 or S100A8/A9 for 30 min before LPS stimulation (Figure 1A). Pretreatment with S100A8 but not S100A8/S100A9 significantly attenuated the LPS-induced increases in the expression of Tnfa, Il-1b, Il-6 and Mcp1 and tended to reduce the expression of Cxcl10 and Mip-1a (Figure 1B). In contrast, those reductions were not observed when S100A8 was treated after LPS stimulation (Figure S4A). TLR4 signaling is transduced through either myeloid differentiation primary response gene 88 (MyD88) or toll-Il-1 receptor domain-containing adaptor inducing IFN-β (TRIF) pathways, with the latter known as the Myd88-independent pathway.36 Pretreatment with S100A8 also significantly suppressed the LPS-induced increase in the TLR4 signaling pathway-related gene expression of Myd88, but there were no changes in Il-1 receptor-associated kinase 1 (Irak1), Irak4, Tnf receptor-associated factors 6 (Traf6), receptor-interacting protein-1 (Rip1), Trif, or Nf-kb expression (Figure 1C). Those suppressions also disappeared when S100A8 was added after LPS (Figure S4B). Then, the impact of an additional injection of S100A8 1 h after LPS stimulation was evaluated (Figure 1D). Supplementation via S100A8 injection into the peritoneal cavity significantly reduced the elevated expression of Il-6, Cxcl10, Mip-1a, and Mcp1 in peritoneal cells after the LPS challenge at 4 h but did not change the expression of Tnfa or Il-1b (Figure 1E). The mRNA expression of S100a8 and S100a9 in peritoneal cells was further increased by injecting S100A8 in addition to LPS (Figure 1E). Intraperitoneal injection of S100A8 significantly attenuated the LPS-induced increases in the expression of Myd88, Traf6 and Nf-kb, whereas there were no changes in Irak1, Irak4, Rip1, or Trif expression (Figure 1F). Supplementation by S100A8 injection into the peritoneal cavity after the LPS challenge also significantly increased the expression of Il-1b, Il-6, Cxcl10, Mcp1 and S100a8 in the liver but did not change Tnfa, Mip-1a, or S100a9 expression (Figure S5A). Intraperitoneal injection of S100A8 after LPS treatment significantly increased the expression of Myd88 and Nf-kb in the liver, while Irak1, Irak4, Traf6, Rip1 and Trif expression showed no significant changes (Figure S5B). There were no differences in serum AST, ALT, total cholesterol, or triglyceride levels between PBS injection and S100A8 injection at 4 h (Figure S5C) and 24 h (Figure S5D) after the intraperitoneal injection of LPS. We next assessed the effects of S100A8 in mice injected with a synthetic TLR4 agonist CRX-527. S100A8 also prevented the elevation of Tnfa, Cxcl10, Mcp1, and Myd88 expression in peritoneal cells induced by CRX-527, suggesting the attenuation of TLR4 signaling (Figures S6A and S6B). Intraperitoneal injection of S100A8 after LPS administration decreased serum IL-1β and IL-6 levels at 24 h but did not change serum TNF-α and CXCL10 levels (Figure 1G). S100A8 supplementation after intraperitoneal LPS injection significantly improved the survival rate from 30% to 80% at 48 h (Figure 1H). S100A8 sustained survival of LPS-injected mice at 72 h (60 vs. 20%) (Figure S7A). S100A8 administration after LPS injection did not rescue the decreased body temperature or reduced blood glucose levels (Figures 1I and 1J). Furthermore, we also examined the protective effects of S100A8 in practical models of sepsis, namely, intraperitoneal injection of Escherichia coli and CLP models.37 Notably, S100A8 supplementation could improve survival in the contexts of sepsis induced by either E. coli injection or CLP (Figures 1K, 1N, S7B, and S7C). Supplementation with S100A8 tended to restore body temperature and blood glucose levels after CLP but not after E. coli injection (Figures 1L, 1M, 1O, and 1P). These results suggested potential therapeutic applications for S100A8 to reduce inflammation and lethality in the context of septic shock.
Figure 1.
S100A8 alleviated LPS-induced inflammation and improved the survival rate in LPS-induced lethal endotoxemia
Wild-type mice at 10 weeks of age were used for in vitro and in vivo experiments.
(A) Experimental outline of an in vitro study. Peritoneal macrophages from mice were pretreated with PBS, S100A8 (0.3 μg/mL), or S100A8/A9 (0.65 μg/mL) for 30 min before LPS (2 μg/mL) stimulation for 2 h and analyzed by qPCR.
(B) mRNA expression of the indicated inflammatory cytokine genes in peritoneal macrophages (n = 3).
(C) mRNA expression of the indicated TLR4 signaling pathway-related genes in peritoneal macrophages (n = 3).
(D) Experimental outline of an in vivo study. Peritoneal cells from mice injected with either PBS or S100A8 (0.1 μg/gBW) 1 h after LPS (12.5 μg/gBW) stimulation were collected at 4 h and analyzed by qPCR. Serum collected from mice at 24 h after the first injection was analyzed by ELISA. The survival rates, body temperature, and blood glucose levels of mice were monitored for 48 h.
(E) mRNA expression of the indicated inflammatory cytokine genes in peritoneal cells (n = 3).
(F) mRNA expression of the indicated TLR4 signaling pathway-related genes in peritoneal cells (n = 3).
(G) Serum TNF-a, IL-1b, IL-6, and CXCL10 levels (n = 3).
(H) The survival rates, (I) body temperature, and (J) blood glucose levels of mice were measured at 12, 24, 36, and 48 h after the first injection (n = 10).
(K) The survival rates, (L) body temperature, and (M) blood glucose levels of mice injected with either PBS or S100A8 (0.1 μg/gBW) at 1 h after E. coli DH5α (2.0∗109 CFU per mouse) injection were measured at 12, 24, 36, and 48 h (n = 10).
(N) The survival rates, (O) body temperature, and (P) blood glucose levels of mice injected with either PBS or S100A8 (0.1 μg/gBW) twice (immediately and at 24 h) after cecal ligation and puncture (CLP) treatment were measured at 12, 24, 36, and 48 h (n = 10).
All data from three or four independent experiments are represented as the mean ± SEM ∗p < 0.05, ∗∗p < 0.01.
TLR4 signaling-induced S100A8 and S100A8 suppressed TLR4-mediated inflammation in peritoneal macrophages
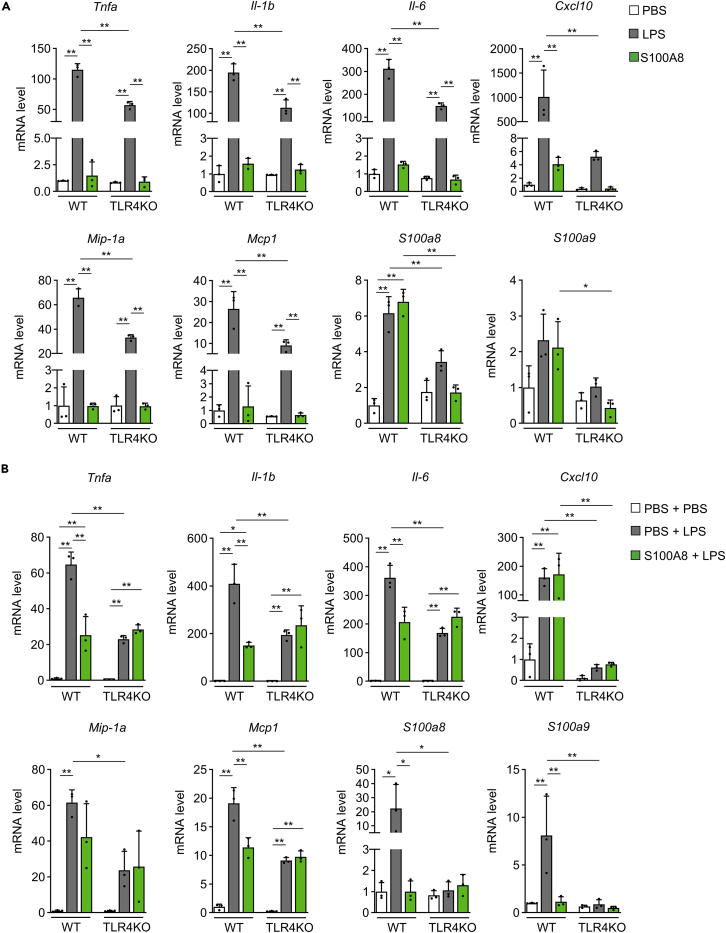
To investigate the mechanism of the S100A8-mediated suppression of LPS-induced inflammation, we performed in vitro experiments with peritoneal macrophages from TLR4 knockout (TLR4KO) mice. LPS stimulation significantly increased the gene expression of Tnfa, Il-1b, Il-6, Cxcl10, Mip-1a, Mcp1, and S100a8 in peritoneal macrophages from WT mice and tended to upregulate the expression of S100a9 (Figure 2A). LPS also significantly upregulated the expression of Tnfa, Il-1b, Il-6, Mip-1a, and Mcp1 in peritoneal macrophages from TLR4KO mice, but the Tnfa, Il-1b, Il-6, Cxcl10, Mip-1a, and Mcp1 levels were lower than those in peritoneal macrophages from WT mice (Figure 2A). Notably, LPS-induced S100a8 expression was completely blunted in TLR4KO mice (Figure 2A). Although S100A8 treatment increased the gene expression of S100a8 in peritoneal macrophages from WT mice, the expression of Tnfa, Il-1b, Il-6, Cxcl10, Mip-1a, Mcp1, and S100a9 was not altered by S100A8 treatment (Figure 2A). The increase in S100A8 expression observed after treatment with S100A8 was also diminished in TLR4KO macrophages (Figure 2A). Thus, TLR4 is required for the production of S100a8 induced by LPS or S100A8 in peritoneal macrophages. Pretreatment with S100A8 before LPS stimulation significantly suppressed the increases in the gene expression of Tnfa, Il-1b, Il-6, Mcp1, S100a8, and S100a9 but not that of Cxcl10 and Mip-1a in peritoneal macrophages from WT mice (Figure 2B). The Tnfa, Il-1b, Il-6, Cxcl10, Mip-1a, Mcp1, S100a8, and S100a9 expression induced by PBS plus LPS stimulation was reduced in peritoneal macrophages from TLR4KO mice (Figure 2B). Pretreatment with S100A8 failed to reduce the gene expression of Tnfa, Il-1b, Il-6, Cxcl10, Mcp1, Mip-1a, S100a8, and S100a9 in TLR4KO peritoneal macrophages (Figure 2B). These results suggested that S100A8 prevented the LPS-induced upregulation of inflammatory cytokine expression through the modulation of TLR4-mediated signaling.
Figure 2.
S100A8 attenuated LPS-induced inflammation via TLR4
TLR4 knockout (TLR4KO) mice or wild-type (WT) mice at 12 weeks of age were used for experiments.
(A) Peritoneal macrophages from the indicated mice were incubated with PBS, LPS (2 μg/mL), or S100A8 (0.3 μg/mL) for 2 h and then analyzed by qPCR. mRNA expression of the indicated inflammatory cytokine and S100 genes in peritoneal macrophages (n = 3).
(B) Peritoneal macrophages from the indicated mice were pretreated with PBS or S100A8 (0.3 μg/mL) for 30 min before LPS (2 μg/mL) stimulation for 2 h and then analyzed by qPCR. mRNA expression of the indicated inflammatory cytokine and S100 genes in peritoneal macrophages (n = 3).
All data from three independent experiments are represented as the mean ± SEM ∗p < 0.05, ∗∗p < 0.01.
Attenuated increases in S100A8 levels during septic shock in the circulation and peritoneal cells of mice with obesity and diabetes
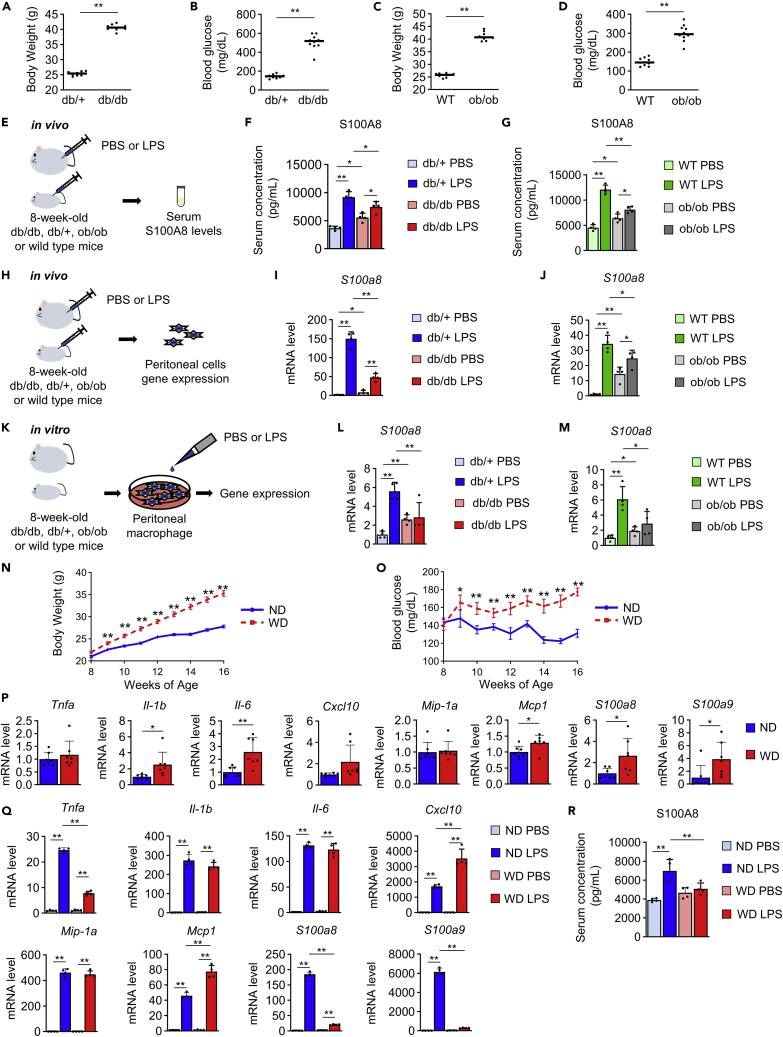
First, we investigated whether S100A8 levels were altered in the circulation or peritoneal cells during the lethal endotoxemia in mouse models of obesity and diabetes by injecting LPS intraperitoneally into ob/ob or db/db mice, respectively. The BW and blood glucose levels of both db/db mice and ob/ob mice were significantly higher than those of control lean mice (Figures 3A–3D). The serum S100A8 levels in db/db or ob/ob mice after intraperitoneal LPS or PBS injection were evaluated (Figure 3E). As expected, the serum S100A8 levels in both db/db mice and ob/ob mice were significantly higher than those in controls under nonseptic conditions (PBS treatment) (Figures 3F and 3G). Although all mice showed increased serum S100A8 levels after LPS-induced endotoxemia, the increment and concentration of S100A8 in the serum were significantly reduced in db/db and ob/ob mice after LPS administration (Figures 3F and 3G). Then, we examined the expression of the S100a8 gene in peritoneal cells from the above-described mice (Figure 3H). Peritoneal cells from db/db or ob/ob mice showed significantly higher expression of S100a8 than those from control mice without endotoxemia (Figures 3I and 3J). In contrast, the expression of S100a8 in peritoneal cells was significantly lower in db/db and ob/ob mice during endotoxemia by LPS administration (Figures 3I and 3J). In db/db mice, the response of peritoneal cells to the intraperitoneal injection of S100A8 was also downregulated (Figures S8A and S8B). We next investigated the expression of S100a8 in isolated peritoneal macrophages from db/db or ob/ob mice in the presence or absence of LPS in vitro (Figure 3K). Peritoneal macrophages from db/db or ob/ob mice had increased S100a8 expression compared to the respective controls in the absence of LPS (Figures 3L and 3M). However, peritoneal macrophages from both db/db mice and ob/ob mice showed marked attenuation of S100a8 induction after LPS administration compared with those from lean and nondiabetic controls (Figures 3L and 3M). These results suggested that obesity and diabetes cause insufficient S100A8 production in peritoneal macrophages during endotoxemia. Patients with obesity show relatively high sepsis mortality and increased serum S100A8 levels.38 Mice with obesity-fed Western diet (WD) demonstrated increases in both BW (Figure 3N) and blood glucose levels (Figure 3O) compared with normal diet (ND)-fed mice. The expression of Il-1b, Il-6, Mcp1, S100a8, and S100a9 in peritoneal cells but not that of Tnfa, Cxcl10, and Mip-1a was significantly elevated in mice fed a WD for 8 weeks compared with ND-fed mice (Figure 3P). The induction of Tnfa, S100a8, and S100a9 was significantly blunted in peritoneal cells after the intraperitoneal injection of LPS in WD-fed mice compared to ND-fed mice (Figure 3Q). In contrast, WD-fed mice demonstrated enhanced LPS-mediated upregulation of Cxcl10 and Mcp1 expression in peritoneal cells (Figure 3Q). The increases in Il-1b, Il-6, and Mip-1a expression induced by LPS were similar between the groups (Figure 3Q). WD-fed mice failed to increase the circulating S100A8 level in the serum after LPS injection (Figure 3R).
Figure 3.
Peritoneal macrophages and peritoneal cells from mice with obesity and diabetes exhibited insufficient production of S100A8 after LPS treatment
db/db and db/+ mice or ob/ob and wild-type (WT) mice at 8 weeks of age were used for experiments (A–M).
(A) Body weight of db/db and db/+ mice (n = 10).
(B) Casual blood glucose levels of db/db and db/+ mice (n = 10).
(C) Body weight of ob/ob and WT mice (n = 10).
(D) Casual blood glucose levels of ob/ob and WT mice (n = 10).
(E) Experimental protocol for an in vivo study. Mice were injected with PBS or LPS (12.5 μg/gBW), and serum was collected at 4 h after injection.
(F) Serum S100A8 levels in db/db and db/+ mice (n = 4).
(G) Serum S100A8 levels in ob/ob and WT mice (n = 4).
(H) Experimental protocol for an in vivo study. Mice were injected with PBS or LPS (12.5 μg/gBW), and peritoneal cells were analyzed by qPCR at 4 h after injection.
(I) mRNA expression of S100a8 in peritoneal cells from db/db or db/+ mice (n = 4).
(J) mRNA expression of S100a8 in peritoneal cells from ob/ob or WT mice (n = 4).
(K) Experimental outline of an in vitro study. Peritoneal macrophages were incubated with PBS or LPS (2 μg/mL) for 2 h and analyzed by qPCR.
(L) mRNA expression of S100a8 in peritoneal macrophages from db/db or db/+ mice (n = 4).
(M) mRNA expression of S100a8 in peritoneal macrophages from ob/ob or WT mice (n = 4).
C57BL/6J mice fed a Western diet (WD) or normal diet (ND) from 8 to 16 weeks of age were used for experiments (N-R).
(N) Body weight and (O) casual blood glucose levels (n = 20-30).
(P) mRNA expression of the indicated inflammatory cytokine and S100 genes in peritoneal cells (n = 7).
(Q) mRNA expression of the indicated inflammatory cytokine and S100 genes in peritoneal cells at 4 h after PBS or LPS injection (12.5 μg/gBW) (n = 4).
(R) Serum S100A8 levels at 4 h after PBS or LPS (12.5 μg/gBW) injection (n = 4).
All data from three independent experiments are represented as the mean ± SEM ∗p < 0.05, ∗∗p < 0.01.
S100A8 supplementation improves lethal endotoxemia in mouse models of diabetes and obesity
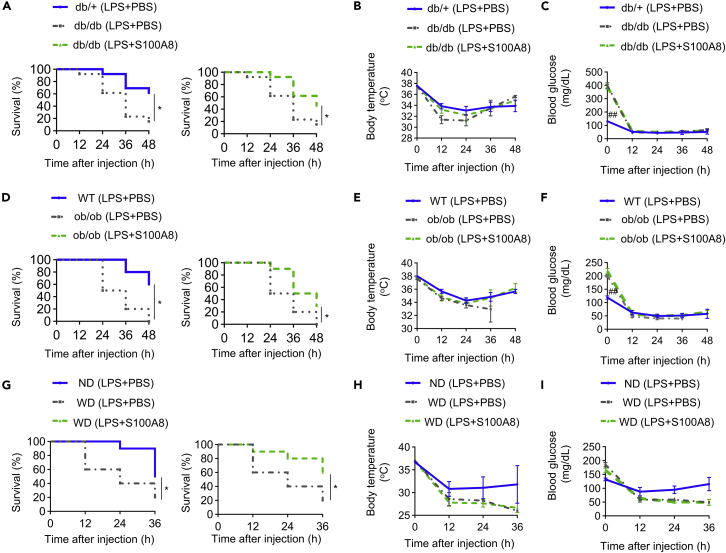
db/db mice had poorer survival rate than db/+ mice, the lean control mice, in LPS treatments (Figure 4A left), and injecting of S100A8 after LPS treatment improved db/db mice from death in LPS-induced endotoxemia (Figures 4A right and S9A). On the other hand, S100A8 did not modify either body temperature or blood glucose levels in db/db mice after LPS treatment (Figures 4B and 4C). ob/ob mice also had lower survival rates than wild-type mice during LPS-induced endotoxemia (Figure 4D left), and S100A8 injection improved survival rates of ob/ob mice after LPS administration (Figure 4D right and S9B). S100A8 injection also changed neither body temperature nor blood glucose levels in ob/ob mice after LPS treatment (Figures 4E and 4F). In LPS-induced endotoxemia, WD-fed mice with obesity showed a lower survival rate than ND-fed mice (Figure 4G left). Supplementation with S100A8 after LPS injection rescued WD-fed mice from death (Figures 4G right and S9C). However, S100A8 did not reverse the decreases in either body temperature or blood glucose levels in WD-fed mice after LPS-induced endotoxemia (Figures 4H and 4I). Thus, the increased mortality by endotoxemia in mice with obesity and diabetes can be remedied by the addition of exogenous S100A8.
Figure 4.
S100A8 supplementation improves survival rates in mice with obesity and diabetes during endotoxemia
db/db and db/+ mice at 8 weeks of age were used for experiments (A–C).
(A) The survival rates, (B) body temperature, and (C) blood glucose levels of mice injected with either PBS or S100A8 (0.1 μg/gBW) 1 h after LPS (12.5 μg/gBW) stimulation were measured at 12, 24, 36, and 48 h (n = 13).
ob/ob and wild-type (WT) mice at 8 weeks of age were used for experiments (D–F).
(D) The survival rates, (E) body temperature, and (F) blood glucose levels of mice injected with either PBS or S100A8 (0.1 μg/gBW) 1 h after LPS (12.5 μg/gBW) stimulation were measured at 12, 24, 36, and 48 h (n = 10).
C57BL/6J mice fed a Western diet (WD) or normal diet (ND) from 8 to 16 weeks of age were used for experiments (G–I).
(G) The survival rates, (H) body temperature, and (I) blood glucose levels of mice injected with either PBS or S100A8 (0.1 μg/gBW) 1 h after LPS (12.5 μg/gBW) stimulation were measured at 12, 24, and 36 h (n = 10).
All data from three independent experiments are represented as the mean ± SEM ∗p < 0.05, ∗∗p < 0.01.
Myelomonocytic cell-specific S100A8 knockout mice are vulnerable to lipopolysaccharide-induced endotoxemia
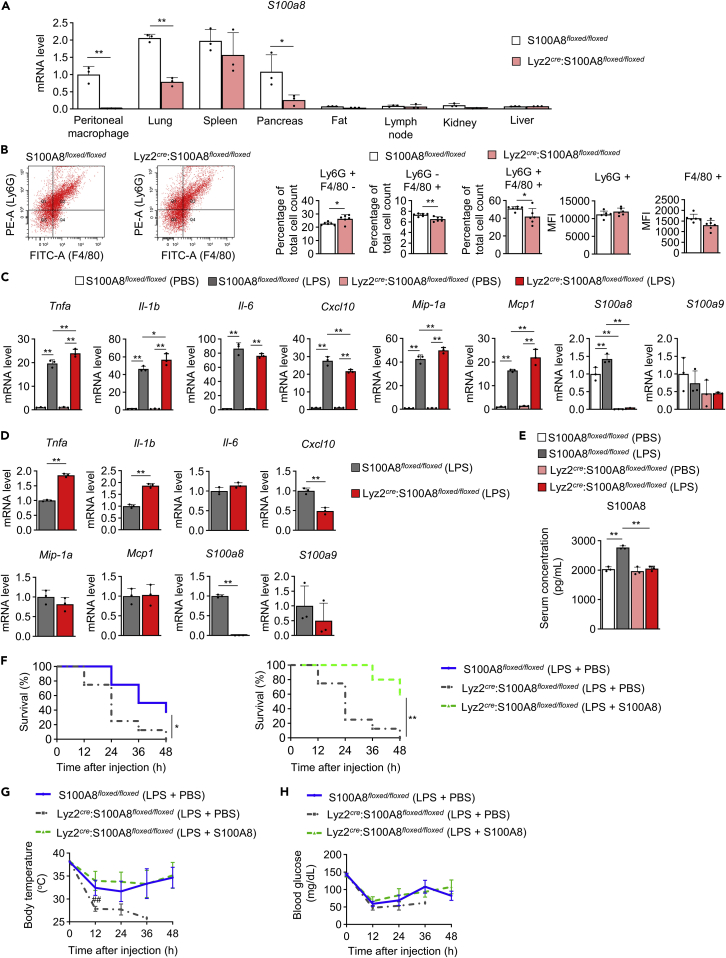
Because S100A8 production was attenuated in peritoneal macrophages of mice with obesity and diabetes, we generated myelomonocytic cell-specific S100A8 KO (Lyz2cre:S100A8floxed/floxed) mice by crossing S100A8-floxed (S100A8floxed/floxed) mice (Figure S10) with Lyz2-Cre mice. S100a8 mRNA expression was significantly reduced in peritoneal macrophages, the lungs, and the pancreas but not in the spleen, fat, lymph nodes, kidneys, or liver in Lyz2cre:S100A8floxed/floxed mice compared to S100A8floxed/floxed mice (Figure 5A). The proportion of Ly6G+F4/80- cells was increased in the peritoneal cells of Lyz2cre:S100A8floxed/floxed mice compared to those of S100A8floxed/floxed mice, while the proportions of Ly6G−F4/80+ cells and Ly6G+F4/80+ cells were decreased (Figure 5B). However, there was no significant difference in the mean fluorescence intensity (MFI) of Ly6G or F4/80 between S100A8floxed/floxed and Lyz2cre:S100A8floxed/floxed peritoneal cells (Figure 5B). The expression of Tnfa, Il-1b, Mip-1a, and Mcp1 was significantly upregulated, while Cxcl10 and S100a8 expression was significantly downregulated in peritoneal macrophages from Lyz2cre:S100A8floxed/floxed mice compared to those from S100A8floxed/floxed mice after stimulation with LPS in vitro (Figure 5C). The expression of Il-6 and S100a9 was not different between Lyz2cre:S100A8floxed/floxed and S100A8floxed/floxed macrophages. Furthermore, the intraperitoneal injection of LPS produced higher Tnfa and Il-1b expression and lower Cxcl10 and S100a8 expression in Lyz2cre:S100A8floxed/floxed peritoneal cells than in S100A8floxed/floxed peritoneal cells, while the expression of Il-6, Mip-1a, Mcp1 and S100a9 was not altered (Figure 5D). Il-6, Mip-1a, Mcp1, and S100a9 expression in peritoneal cells was not different between the genotypes after LPS injection (Figure 5D). Then, the effects of S100A8 supplementation in addition to LPS treatment were evaluated in the liver of Lyz2cre:S100A8floxed/floxed mice. There were no differences in serum AST, ALT, total cholesterol, or triglyceride levels between Lyz2cre:S100A8floxed/floxed and S100A8floxed/floxed mice after LPS + S100A8 injection (Figure S11). The circulating S100A8 level in the serum was increased by LPS treatment in S100A8floxed/floxed mice, but this increase was blunted in Lyz2cre:S100A8floxed/floxed mice (Figure 5E). S100A8 deficiency in myelomonocytic cells was associated with increased mortality after LPS treatment through 48 h (Figure 5F left). Supplementation with recombinant S100A8 after the intraperitoneal injection of LPS improved the survival of Lyz2cre:S100A8floxed/floxed mice at 48 h (Figures 5F right and S12). S100A8 injection recovered the decrease in body temperature caused by LPS-induced lethal endotoxemia in Lyz2cre:S100A8floxed/floxed mice (Figure 5G). Blood glucose levels showed no significant differences among all groups (Figure 5H).
Figure 5.
Myelomonocytic cell-specific S100A8 knockout mice were vulnerable to lethal endotoxemia, and exogenous S100A8 supplementation it was protective
Myelomonocytic cell-specific S100A8 knockout (Lyz2cre:S100A8floxed/floxed) mice or S100A8floxed/floxed mice at 10 weeks of age were used for experiments.
(A) The mRNA expression of S100a8 in peritoneal macrophages and the lungs, spleen, pancreas, fat, inguinal lymph nodes, kidneys, and liver (n = 3).
(B) The percentage of Ly6G or F4/80 positive cells in peritoneal cells and the mean fluorescence intensity (MFI) of PE (Ly6G) and FITC (F4/80) staining were analyzed by flow cytometry with anti-Ly6G-PE and anti-F4/80-Alexa488 antibodies (n = 6).
(C) mRNA expression of the indicated inflammatory cytokine and S100 genes in peritoneal macrophages incubated with PBS or LPS (2 μg/mL) for 2 h (n = 3).
(D) mRNA expression of the indicated inflammatory cytokine and S100 genes in peritoneal cells at 4 h after LPS (2 μg/mL) injection (n = 3).
(E) Serum S100A8 levels at 4 h after PBS or LPS (12.5 μg/gBW) injection (n = 3).
(F) The survival rates, (G) body temperature, and (H) blood glucose levels of mice injected with either PBS or S100A8 (0.1 μg/gBW) at 1 h after LPS (12.5 μg/gBW) stimulation were measured at 12, 24, 36, and 48 h (n = 5–8).
All data from three or four independent experiments are represented as the mean ± SEM ∗p < 0.05, ∗∗p < 0.01; ##p < 0.01 vs. Lyz2cre:S100A8floxed/floxed (LPS + S100A8).
Discussion
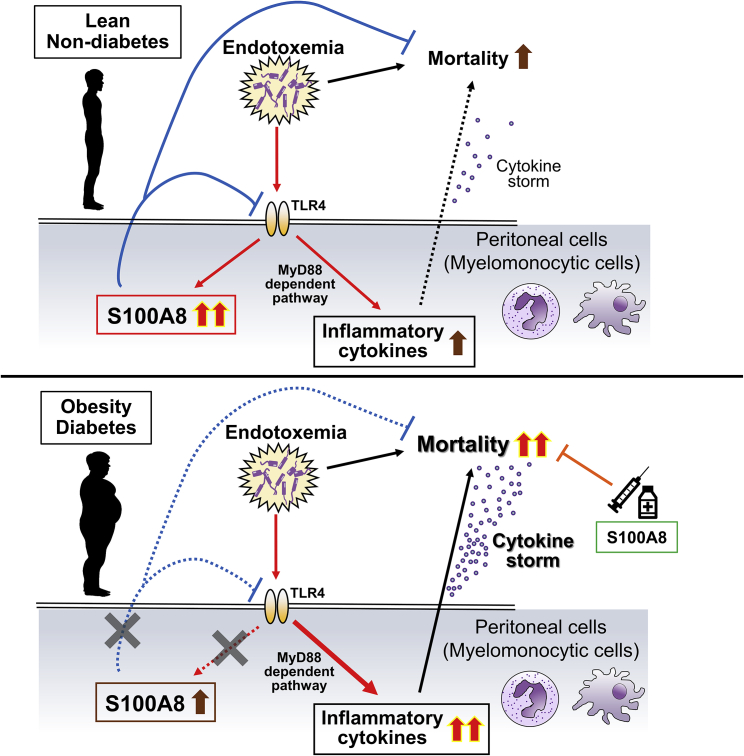
In this study, we reported impaired S100A8 production in myelomonocytic cells during lethal endotoxemia associated with obesity and diabetes and the protective effects of S100A8 on early mortality during endotoxic shock (Figure 6). S100A8 prevented the LPS-induced upregulation of inflammatory cytokines in peritoneal cells both in vitro and in vivo, possibly through attenuating the TLR4-MyD88 pathway. Severe septic shock is caused by an autoactivation loop in the cytokine production cascade: cytokine storm.39 S100A8 may improve cytokine storm by suppressing key pathogenic cytokines. Here, we evaluated LPS-induced lethal endotoxemia and multiple septic models including E. coli injection and CLP. The results for conditional KO mice and models of obesity and diabetes also supported the therapeutic potential of S100A8 in preventing death from endotoxic shock in subjects with obesity.
Figure 6.
Schematic diagram showing the role of S100A8 in the prevention of cytokine storm during endotoxemia in lean subjects, and patients with obesity and diabetes
In lean subjects, adaptive induction of S100A8 in myelomonocytic cells through TLR4-mediated signaling during endotoxemia attenuates the excessive production of inflammatory cytokines and prevents lethal endotoxemia. In people with obesity and diabetes, the insufficient induction of S100A8 by endotoxemia, possibly due to chronic low-grade inflammation, fails to suppress the chain reaction of inflammatory cytokine production and results in cytokine storm and increased mortality. Exogenous supplementation with S100A8 may be able to prevent death in patients with obesity and diabetes during sepsis.
Although the S100A8 homodimer reduced LPS-induced cytokine production, the S100A8/A9 heterodimer did not alter this cytokine production. In the serum, both the S100A8 homodimer and the S100A8/A9 heterodimer are considered ligands for TLR4 and RAGE.10,13 Previous reports have indicated that the S100A8 homodimer shows a more potent immunomodulatory effect in neonatal sepsis than does the S100A8/A9 heterodimeric complex.32,33 S100A8 attenuates the production of IL-6 and TNF-α in mouse monocytes and human monocytes in response to LPS or bacteria via downregulation of phosphorylated p38 MAPK.27 It has been reported that S100A8 binds with several kinds of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, to form S100A8-inflammatory cytokine complexes in vivo in acute inflammation.40 Thus, S100A8 may compete with LPS for receptor binding or lead to the attenuation of inflammatory responses independent of TLR4 or RAGE. In this study, the protective effects of S100A8 against inflammatory responses were assumed to be dependent on TLR4 because S100A8 was unable to prevent LPS-induced inflammatory cytokine production in the absence of TLR4 in peritoneal macrophages.
CXCL10 expression showed different trends from other inflammatory cytokine expressions after S100A8 treatment. Both S100A8 and CXCL10 are known as markers for psoriasis.41 Therefore, it may be possible that S100A8 has a different effect on CXCL10 compared with other cytokines. Since each cytokine shows divergent time-dependent expression pattern in peritoneal macrophages after LPS stimulation, time-course analysis might be required. Macrophages cultured with a large amount of LPS spontaneously internalize LPS. The LPS-induced production of inflammatory cytokines in TLR4KO peritoneal macrophages may occur through noncanonical inflammasome-mediated caspase-11 activation by intracellular LPS independent of TLR4.42 Furthermore, our results also demonstrated that TLR4 activation by LPS induced S100a8 expression in peritoneal macrophages. Thus, signaling via S100A8 may constitute an autoregulatory negative feedback loop of the TLR4 pathway to prevent excess cytokine storm.
S100A8 supplementation after LPS injection improved inflammation in peritoneal cells, and S100A8 administration alone did not induce inflammation in the liver. However, supplementation with S100A8 after the LPS challenge did not reduce the production of inflammatory cytokines in the liver via the activation of the TLR4-MyD88 pathway. A previous study reported that hepatocyte-specific TLR4 knockout mice exhibited a decrease in LPS clearance in the liver and elevated serum IL-6 level after the intravenous injection of LPS, while myeloid cell-specific TLR4 knockout mice demonstrated reduced serum IL-6 level.43 The liver eliminates pathogens, pathogen-associated molecular patterns, debris, cytokines, and other inflammatory metabolites during sepsis.44 The effects of S100A8 on the clearance of LPS in the liver has not yet been worked out in detail. It also remains unclear why S100A8 showed different effects between peritoneal cells and the liver in terms of the inflammatory response to sepsis. It might be due to the differences in RAGE-mediated effects of S100A8 on each organ, non-circulating direct exposure of LPS to the liver through peritoneal fluid, or the influence of Kupffer cells or liver sinusoidal endothelial cells in addition to hepatocytes.
Mouse models of obesity and diabetes, including db/db, ob/ob, and WD-fed mice, showed insufficient elevations in the S100A8 levels in the circulation and peritoneal cells during lethal endotoxemia. Plasma-free fatty acid (FFA) levels are increased in obesity, and FFAs have an effect on macrophages that regulates the inflammatory response, possibly through TLR2 and TLR4.45,46,47 Persistent chronic inflammation is a fundamental pathology of various diseases, such as obesity and diabetes.48 Chronic or persistent low-grade stimulation of innate immune receptors causes desensitization of TLR signaling to prevent excessive inflammation.49 Obesity-induced chronic inflammation attenuates responses to LPS in immune cells due to impaired p38 MAPK signaling.50 Although obesity-induced increases in serum S100A8 levels have been reported,51 it remains unclear whether the responsiveness of S100A8 induction is altered. Obesity-related chronic low-grade inflammation may impair S100A8 elevation after LPS administration due to TLR desensitization. The reduced responsiveness to S100A8 in db/db mice also could be explained by chronic elevation of basal S100A8 level, which results in TLR desensitization. It has been reported that mice fed a WD have increased disease severity and higher sepsis mortality than mice fed a standard fiber-rich diet.52 Because sepsis-prone mice with obesity and diabetes showed impaired elevations in S100A8 levels during endotoxemia, adaptive S100A8 induction probably plays an important role in the prevention of lethal cytokine storm.
Considering the embryonic lethality of systemically knocking out S100A8 in mice, S100A9 KO mice have often been used as an alternative for analysis of S100A8 deletion because these mice do not express the mature S100A8 protein due to its high metabolic turnover in the absence of S100A9.53,54,55 Since Lyz2cre:S100A8floxed/floxed peritoneal cells showed no changes in the fluorescence intensities of Ly6G and F4/80 staining compared to peritoneal cells from S100A8floxed/floxed mice, the deletion of S100A8 in myelomonocytic cells did not affect the populations of neutrophils and monocytes. Since serum S100A8 levels in Lyz2cre:S100A8floxed/floxed mice were similar to those in control mice, the serum S100A8 under non-septic conditions might be predominantly derived from other cells including adipocytes. The significant decreases in the expression of S100a8 in the lungs and pancreas of Lyz2cre:S100A8floxed/floxed mice might reflect abundant resident macrophages in these tissues. Our conditional KO mouse model indicated the pathological significance of S100A8 in each tissue.
It has been reported that the serum S100A8 levels in patients with sepsis are positively correlated with disease severity.56 Based on our results, S100A8 may be adaptively elevated in severe sepsis because of its protective function, rather than acting to aggravate the infection. Although serum S100A8 levels in individuals with obesity are higher than those in lean individuals,38 whether the sepsis-induced elevation in S100A8 is altered in patients with obesity is still unknown. Further clinical studies on the association between S100A8 and sepsis in individuals with obesity or diabetes need to be conducted.
In summary, S100A8 in peritoneal cells reduces sepsis-related inflammation and improves the lethality of sepsis, and S100A8 supplementation may be a potential treatment for sepsis, especially in patients with obesity and diabetes.
Limitations of the study
There are limitations to this study. The LPS used in this experiment was purified by phenol extraction from E. coli 0111: B4 with a protein content of 1.72%. Therefore, the purity of LPS seems to be comparable to other reagents for use in other reports. However, it is possible that non-specific effects of LPS, including lipoproteins that activate TLR2, were observed in the analysis of TLR4KO mice. It was not clear which type of myelomonocytic cell contributes to the production of S100A8 during endotoxemia. In addition, which organ was involved in the S100A8-mediated regulation of endotoxemia remains unclear. Although we demonstrated the protective effects of S100A8 on endotoxemia or sepsis models until 72 h, one has to also consider long-term survival rate or combination therapy with antibacterial agents for the clinical application. The CFU assay of peritoneal lavage fluid after S100A8 administration in sepsis models might reveal the direct effects of S100A8 on pathogens.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE Rat Anti-Mouse Ly-6G and Ly-6C Clone RB6-8C5 | Beckton Dickinson | Cat# 561084; RRID: AB_394644 |
| Ms F4/80-Like Rcptr Alexa 488 6F12 | Beckton Dickinson | Cat# 564227; RRID: AB_2738682 |
| Chemicals, peptides, and recombinant proteins | ||
| RNeasy Mini Kit | QIAGEN | Cat# 74106 |
| High-Capacity cDNA Reverse Transcription Kit | Thermo Fisher Scientific | Cat# 4368813 |
| THUNDERBIRD SYBR qPCR Master Mix | TOYOBO | Cat# QPS-101 |
| LPS | SantaCruz | Cat# sc3535a |
| Recombinant Mouse S100A8 Protein,CF | R&D | Cat# 9877-S8-050 |
| Recombinant Mouse S100A8/S100A9 Heterodimer Protein,CF | R&D | Cat# 8916-S8-050 |
| MF diet | Oriental Yeast | Cat# orien-mf500 |
| Western diet | Research Diets, Inc | Cat# D12079B |
| CRX-527 | Invivogen | Cat# tlrl-crx527 |
| Critical commercial assays | ||
| TNF alpha Mouse Uncoated ELISA Kit with Plates | eBioscience | Cat# 88-7324-22 |
| IL-6 Mouse Uncoated ELISA Kit with Plates | eBioscience | Cat# 88-7064-22 |
| IL-1 beta Mouse Uncoated ELISA Kit with Plates | eBioscience | Cat# 88-7013-22 |
| Mouse S100A8 ELISA Kit | Abcam | Car# ab213886 |
| TG assay kit | FUJIFILM Wako Pure Chemical Corporation | Cat# 467-08994 |
| Aspartate aminotransferase assay kit | FUJIFILM Wako Pure Chemical Corporation | Cat# 631-22751 |
| Alanine aminotransferase assay kit | FUJIFILM Wako Pure Chemical Corporation | Cat# 638-22761 |
| Cholesterol assay kit | FUJIFILM Wako Pure Chemical Corporation | Cat# 635-50981 |
| Glutest Neo Super | Sanwa Chemical Co. | N/A |
| Experimental models: Cell lines | ||
| Hepa 1-6 | RIKEN Bioresource Center | RCB1638 |
| Experimental models: Organisms/strains | ||
| Mouse: WT C57BL6/J | Jackson Laboratory | JAX. 000664 |
| Mouse: BKS.Cg-m+/+Leprdb/Jcl (Db/m) | Jackson Laboratory | JAX. 000662 |
| Mouse: BKS.Cg- + Leprdb/+Leprdb/Jcl (Db/db) | Jackson Laboratory | JAX. 000642 |
| Mouse: B6.V-Lepob/J | Jackson Laboratory | JAX. 000632 |
| Mouse: B6(Cg)-Tlr4tm1.2Karp/J | Jackson Laboratory | JAX. 029015 |
| Mouse: B6.129P2-Lyz2tm1(cre)Ifo/J | Jackson Laboratory | JAX. 004781 |
| Oligonucleotides | ||
| Primers for qPCR, see Table S1 | This paper | N/A |
| Software and algorithms | ||
| Prism 8 software (GraphPad Software) | https://www.graphpad.com/scientific-software/prism/ | N/A |
| JMP 16 | https://www.jmp.com/ja_jp/home.html | N/A |
| Other | ||
| 7900 Real-Time PCR System | Applied Biosystems | N/A |
| FACS Canto II | BD biosciences | N/A |
| Rectal thermometer | Natsume Seisakusho Co. | KN-91-AD-1687-M |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directly to and will be fulfilled by Jun Shirakawa (jshira@gunma-u.ac.jp).
Materials availability
Mouse lines generated in this study (S100A8-floxed mice) are available from the lead contact upon request.
Experimental model and subject details
Animals and animal care
Animals were housed in a room maintained at a constant temperature (25°C) under a 12-hour light (07:00)/12-hour dark (19:00) cycle, and the animals were provided free access to food and water. C57BL/6J mice were purchased from CLEA Japan. All mice used in this study were male and on the C57BL/6J background. TLR4 KO, db/db, db/+, and ob/ob mice were purchased from Oriental Bio Service. To generate S100A8-floxed mice, a 516-bp region containing exons 2 and 3 of the mouse S100a8 gene was replaced with a loxP-flanked neomycin resistance (neo) gene cassette. Targeted embryonic stem cell clones were injected into 8-cell-stage ICR embryos to generate chimeric mice. The neo cassette was removed by crossing mice heterozygous for the targeted conditional KO allele with Flp-deleter mice. S100A8floxed/floxed mice were then crossed with mice expressing the Cre recombinase gene under the control of the lysozyme M gene regulatory region (Lyz2cre, The Jaxon laboratory, JAX 004781) to generate the KO allele (Figure S10). S100A8floxed /floxed mice were studied as controls. The primers for genotyping the conditional KO mice were P1 (5′-CTTTCCCATGACTGCTCCTC-3′) and P2 (5′-CCACCTACCCCAAGACAGAA-3′), which yielded a 350-bp product for the wild-type (WT) allele and a 431-bp product for the floxed allele. MF diet (Carbohydrate 61.4%, Protein 25.8%, Fat 12.8%; Oriental Yeast) was used as the standard diet. The Western Diet, D12079B (Carbohydrate 43.0%, Protein 17.0%, Fat 41.0%; Research Diets, Inc), was given to mice used to model diet-induced obesity.
This study was conducted with the approval of the Animal Care Committee of Gunma University (21–035) and Yokohama City University (permit no. F-A-19–019). All the animal procedures were performed in accordance with the institutional animal care guidelines and the guidelines of the Animal Care Committee of Gunma University and Yokohama City University.
Method details
In vitro stimulation
Peritoneal cells, including macrophages, were harvested by peritoneal lavage with PBS, as previously described.57 Peritoneal macrophages (2.0∗105 cells) were stimulated with LPS (2 μg/mL) (Santa Cruz), S100A8 (0.3 μg/mL) (R&D), or S100A8/A9 (0.65 μg/mL) (R&D). Hepa1-6 murine hepatoma cells (5.0∗104 cells) (RIKEN Bioresource Center, RCB1638) were stimulated with S100A8 (0.3 μg/mL) (R&D) or S100A8/A9 (0.65 μg/mL) (R&D).
In vivo stimulation: lethal endotoxemia and experimental sepsis
Mice were injected intraperitoneally with PBS (100 μL), LPS (12.5 μg/g body weight (BW)) or S100A8 (0.1 μg/gBW). Peritoneal cells were harvested at 4 hours. Serum samples were collected after 24 hours. Peritoneal cells were harvested at 4 hours and survival rate was assessed at 48 or 72 hours after treatment were established. As practical models of sepsis, intraperitoneal injection of Escherichia coli (2.0∗109 CFU per mouse, DH5α) and cecal ligation and puncture (CLP) were performed. Mice underwent CLP with a 21-g needle to induce polymicrobial sepsis; the procedure was performed as previously described.58 Immediately and 24 hours after CLP, mice were fluid resuscitated with PBS (20 μL/gBW) or S100A8 (0.1 μg/gBW) injection. Core body temperature was monitored using a rectal thermometer (Natsume Seisakusho Co., KN-91-AD-1687-M).
Real-time PCR
Total RNA was isolated from each tissue using an RNase-free DNase and RNeasy Kit (Qiagen). cDNA was prepared using High-Capacity cDNA Reverse-Transcription Kits (Thermo Fisher Scientific). Quantitative PCR (qPCR) was performed by using Expression Assays (7900 Real-Time PCR System, Applied Biosystems) with THUNDERBIRD SYBR qPCR Master Mix (TOYOBO). Data were standardized according to the expression level of β-actin. The primer sequences are shown in Table S1.
Measurement of biochemical parameters
The serum S100A8 levels in mice were determined using a Mouse S100A8 ELISA Kit (Abcam, catalog no. ab213886). To measure the serum cytokine levels in mice, we used a TNF alpha Mouse Uncoated ELISA Kit (Thermo Fisher Scientific, catalog no. 88-7324-22), an IL-1 beta Mouse Uncoated ELISA Kit (Thermo Fisher Scientific, catalog no. 88-7013-22), and an IL-6 Mouse Uncoated ELISA Kit (Thermo Fisher Scientific, catalog no. 88-7064-22). Blood glucose levels were evaluated with Glutest Neo Super (Sanwa Chemical Co.). Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol, and triglyceride levels were assayed by enzymatic methods (Wako Pure Chemical Industries).
Flow cytometry
Peritoneal cells from myelomonocytic cell-specific S100A8 KO mice and control mice were stained with an Alexa Fluor 488-labeled anti-F4/80 (6F12) antibody (BD Biosciences) or a PE-labeled anti-Ly6G (RB6-8C5) antibody (BD Biosciences). Neutrophils and macrophages were identified as Ly6G-positive and F4/80-positive cells, respectively, by flow cytometry. Flow cytometry was performed using a FACSCanto II (BD Biosciences).
Quantification and statistical analysis
Statistical analysis
All experiments were independently repeated at least three times. All the data are expressed as the mean ± SEM. Differences between two groups were analyzed by Student’s t test. For comparisons among more than two groups, we used one-way ANOVA followed by Tukey’s HSD post hoc test. The log-rank test was used to evaluate mouse survival. Prism 8 (GraphPad 551 Software) and JMP 16 (SAS) were used for statistical analysis. Differences were considered significant if the p value was <0.05 (∗) or <0.01 (∗∗).
Acknowledgments
We thank Mitsuyo Kaji and Eri Sakamoto (Yokohama City University) for their technical assistance and Fuyumi Murai (Gunma University) and Misa Katayama (Yokohama City University) for their secretarial assistance. J.S. acknowledges support from a Grant-in-Aid for Scientific Research (C) 20K08866 from MEXT of Japan, the Japan IDDM network, Japan Diabetes Foundation, Astellas Foundation for Research on Metabolic Disorders, the Naito Foundation, Uehara Memorial Foundation, Taiju Life Social Welfare Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and A∗STAR - AMED JOINT CALL for the Strategic International Collaborative Research Program (SICORP).
Author contributions
J.S. designed the research. D.M., J.S., R.I., T.T., T.O., M.K., K.N., S.F., Y.I.. and Y.To. performed the experiments. J.S. contributed to the generation of S100A8-floxed mice. C.N. and A.S. contributed to cecal ligation and puncture experiments. J.S. and D.M. drafted the article. J.S. wrote, reviewed, and edited the article. D.M., J.S., R.I., T.T., T.O., M.K., K.N., S.F., Y.I., Y.To., and Y.Te. analyzed the data. D.M., R.I., T.T., T.O., M.K., C.N., K.N., S.F., A.S., Y.To., Y.Te., and J.S. revised the article. All authors approved the final version of the article. J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of interests
The authors declare that they have no conflicts of interest.
Published: December 22, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105662.
Supplemental information
Data and code availability
-
•
There are no new dataset in this paper.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Venmans L.M.A.J., Gorter K.J., Rutten G.E.H.M., Schellevis F.G., Hoepelman A.I.M., Hak E. A clinical prediction rule for urinary tract infections in patients with type 2 diabetes mellitus in primary care. Epidemiol. Infect. 2009;137:166–172. doi: 10.1017/s0950268808001015. [DOI] [PubMed] [Google Scholar]
- 2.Falagas M.E., Kompoti M. Obesity and infection. Lancet Infect. Dis. 2006;6:438–446. doi: 10.1016/s1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 3.Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghanim H., Aljada A., Hofmeyer D., Syed T., Mohanty P., Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. doi: 10.1161/01.Cir.0000142055.53122. [DOI] [PubMed] [Google Scholar]
- 5.Honiden S., Inzucchi S.E. Metabolic management during critical illness: glycemic control in the ICU. Semin. Respir. Crit. Care Med. 2015;36:859–869. doi: 10.1055/s-0035-1565253. [DOI] [PubMed] [Google Scholar]
- 6.Gentile L.F., Moldawer L.L. DAMPs, PAMPs, and the origins of SIRS in bacterial sepsis. Shock. 2013;39:113–114. doi: 10.1097/SHK.0b013e318277109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotze M.T., Tracey K.J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 9.Goyette J., Geczy C.L. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41:821–842. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 10.Foell D., Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50:3762–3771. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- 11.Roth J., Burwinkel F., van den Bos C., Goebeler M., Vollmer E., Sorg C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood. 1993;82:1875–1883. [PubMed] [Google Scholar]
- 12.Odink K., Cerletti N., Brüggen J., Clerc R.G., Tarcsay L., Zwadlo G., Gerhards G., Schlegel R., Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 13.Sekimoto R., Fukuda S., Maeda N., Tsushima Y., Matsuda K., Mori T., Nakatsuji H., Nishizawa H., Kishida K., Kikuta J., et al. Visualized macrophage dynamics and significance of S100A8 in obese fat. Proc. Natl. Acad. Sci. USA. 2015;112:E2058–E2066. doi: 10.1073/pnas.1409480112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korndörfer I.P., Brueckner F., Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J. Mol. Biol. 2007;370:887–898. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 15.Netea M.G., Balkwill F., Chonchol M., Cominelli F., Donath M.Y., Giamarellos-Bourboulis E.J., Golenbock D., Gresnigt M.S., Heneka M.T., Hoffman H.M., et al. A guiding map for inflammation. Nat. Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth J., Vogl T., Sorg C., Sunderkötter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 17.Bresnick A.R., Weber D.J., Zimmer D.B. S100 proteins in cancer. Nat. Rev. Cancer. 2015;15:96–109. doi: 10.1038/nrc3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouma G., Lam-Tse W.K., Wierenga-Wolf A.F., Drexhage H.A., Versnel M.A. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes. 2004;53:1979–1986. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- 19.Burkhardt K., Schwarz S., Pan C., Stelter F., Kotliar K., Von Eynatten M., Sollinger D., Lanzl I., Heemann U., Baumann M. Myeloid-related protein 8/14 complex describes microcirculatory alterations in patients with type 2 diabetes and nephropathy. Cardiovasc. Diabetol. 2009;8:10. doi: 10.1186/1475-2840-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofer S., Uhle F., Fleming T., Hell C., Schmoch T., Bruckner T., Weigand M.A., Brenner T. RAGE-mediated inflammation in patients with septic shock. J. Surg. Res. 2016;202:315–327. doi: 10.1016/j.jss.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Dubois C., Marcé D., Faivre V., Lukaszewicz A.C., Junot C., Fenaille F., Simon S., Becher F., Morel N., Payen D. High plasma level of S100A8/S100A9 and S100A12 at admission indicates a higher risk of death in septic shock patients. Sci. Rep. 2019;9:15660. doi: 10.1038/s41598-019-52184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirakawa J., Togashi Y., Sakamoto E., Kaji M., Tajima K., Orime K., Inoue H., Kubota N., Kadowaki T., Terauchi Y. Glucokinase activation ameliorates ER stress-induced apoptosis in pancreatic β-cells. Diabetes. 2013;62:3448–3458. doi: 10.2337/db13-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue H., Shirakawa J., Togashi Y., Tajima K., Okuyama T., Kyohara M., Tanaka Y., Orime K., Saisho Y., Yamada T., et al. Signaling between pancreatic β cells and macrophages via S100 calcium-binding protein A8 exacerbates β-cell apoptosis and islet inflammation. J. Biol. Chem. 2018;293:5934–5946. doi: 10.1074/jbc.M117.809228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirakawa J., Terauchi Y. Newer perspective on the coupling between glucose-mediated signaling and β-cell functionality. Endocr. J. 2020;67:1–8. doi: 10.1507/endocrj.EJ19-0335. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Song R., Wang Z., Jing Z., Wang S., Ma J. S100A8/A9 in inflammation. Front. Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J., Endoh I., Hsu K., Tedla N., Endoh Y., Geczy C.L. S100A8 modulates mast cell function and suppresses eosinophil migration in acute asthma. Antioxid. Redox Signal. 2011;14:1589–1600. doi: 10.1089/ars.2010.3583. [DOI] [PubMed] [Google Scholar]
- 27.Coveney A.P., Wang W., Kelly J., Liu J.H., Blankson S., Wu Q.D., Redmond H.P., Wang J.H. Myeloid-related protein 8 induces self-tolerance and cross-tolerance to bacterial infection via TLR4- and TLR2-mediated signal pathways. Sci. Rep. 2015;5:13694. doi: 10.1038/srep13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otsuka K., Terasaki F., Ikemoto M., Fujita S., Tsukada B., Katashima T., Kanzaki Y., Sohmiya K., Kono T., Toko H., et al. Suppression of inflammation in rat autoimmune myocarditis by S100A8/A9 through modulation of the proinflammatory cytokine network. Eur. J. Heart Fail. 2009;11:229–237. doi: 10.1093/eurjhf/hfn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiroshima Y., Hsu K., Tedla N., Chung Y.M., Chow S., Herbert C., Geczy C.L. S100A8 induces IL-10 and protects against acute lung injury. J. Immunol. 2014;192:2800–2811. doi: 10.4049/jimmunol.1302556. [DOI] [PubMed] [Google Scholar]
- 30.Başsorgun C.İ., Unal B., Erin N., Ozlük A., Uzun O.C., Elpek G.Ö. S100A8 and S100A9 positive cells in colorectal carcinoma: clinicopathological analysis. Gastroenterol. Res. Pract. 2014;2014:943175. doi: 10.1155/2014/943175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogl T., Ludwig S., Goebeler M., Strey A., Thorey I.S., Reichelt R., Foell D., Gerke V., Manitz M.P., Nacken W., et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104:4260–4268. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 32.Ulas T., Pirr S., Fehlhaber B., Bickes M.S., Loof T.G., Vogl T., Mellinger L., Heinemann A.S., Burgmann J., Schöning J., et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat. Immunol. 2017;18:622–632. doi: 10.1038/ni.3745. [DOI] [PubMed] [Google Scholar]
- 33.Heinemann A.S., Pirr S., Fehlhaber B., Mellinger L., Burgmann J., Busse M., Ginzel M., Friesenhagen J., von Köckritz-Blickwede M., Ulas T., et al. In neonates S100A8/S100A9 alarmins prevent the expansion of a specific inflammatory monocyte population promoting septic shock. Faseb j. 2017;31:1153–1164. doi: 10.1096/fj.201601083R. [DOI] [PubMed] [Google Scholar]
- 34.Achouiti A., Vogl T., Urban C.F., Röhm M., Hommes T.J., van Zoelen M.A.D., Florquin S., Roth J., van 't Veer C., de Vos A.F., van der Poll T. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog. 2012;8:e1002987. doi: 10.1371/journal.ppat.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbin B.D., Seeley E.H., Raab A., Feldmann J., Miller M.R., Torres V.J., Anderson K.L., Dattilo B.M., Dunman P.M., Gerads R., et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 36.Yang F.M., Zuo Y., Zhou W., Xia C., Hahm B., Sullivan M., Cheng J., Chang H.M., Yeh E.T. sNASP inhibits TLR signaling to regulate immune response in sepsis. J. Clin. Invest. 2018;128:2459–2472. doi: 10.1172/jci95720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toscano M.G., Ganea D., Gamero A.M. Cecal ligation puncture procedure. J Vis Exp. 2011;51:2860. doi: 10.3791/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riuzzi F., Chiappalupi S., Arcuri C., Giambanco I., Sorci G., Donato R. S100 proteins in obesity: liaisons dangereuses. Cell. Mol. Life Sci. 2020;77:129–147. doi: 10.1007/s00018-019-03257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishihara K., Namura T., Murayama H., Arai S., Totani M., Ikemoto M. [Possibility of formation of the S100A8/A9-proinflammatory cytokine complexes in vivo in acute inflammation and their functional roles] Rinsho Byori. 2009;57:324–331. [PubMed] [Google Scholar]
- 41.Pourani M.R., Abdollahimajd F., Zargari O., Shahidi Dadras M. Soluble biomarkers for diagnosis, monitoring, and therapeutic response assessment in psoriasis. J. Dermatolog. Treat. 2022;33:1967–1974. doi: 10.1080/09546634.2021.1966357. [DOI] [PubMed] [Google Scholar]
- 42.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., Miyake K., Zhang J., Lee W.P., Muszyński A., et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 43.Deng M., Scott M.J., Loughran P., Gibson G., Sodhi C., Watkins S., Hackam D., Billiar T.R. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J. Immunol. 2013;190:5152–5160. doi: 10.4049/jimmunol.1300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beyer D., Hoff J., Sommerfeld O., Zipprich A., Gaßler N., Press A.T. The liver in sepsis: molecular mechanism of liver failure and their potential for clinical translation. Mol. Med. 2022;28:84. doi: 10.1186/s10020-022-00510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/jci28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.Y., Zhao L., Youn H.S., Weatherill A.R., Tapping R., Feng L., Lee W.H., Fitzgerald K.A., Hwang D.H. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen M.T.A., Favelyukis S., Nguyen A.K., Reichart D., Scott P.A., Jenn A., Liu-Bryan R., Glass C.K., Neels J.G., Olefsky J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 48.Inoue S., Saito M., Kotani J. Immunosenescence in neurocritical care. J. Intensive Care. 2018;6:65. doi: 10.1186/s40560-018-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eppensteiner J., Kwun J., Scheuermann U., Barbas A., Limkakeng A.T., Kuchibhatla M., Elster E.A., Kirk A.D., Lee J. Damage- and pathogen-associated molecular patterns play differential roles in late mortality after critical illness. JCI Insight. 2019;4:127925. doi: 10.1172/jci.insight.127925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S., Lin G., You X., Lei L., Li Y., Lin M., Luo K., Yan F. Hyperlipidemia causes changes in inflammatory responses to periodontal pathogen challenge: implications in acute and chronic infections. Arch. Oral Biol. 2014;59:1075–1084. doi: 10.1016/j.archoralbio.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Yu S., Wu X., Shi Z., Huynh M., Jena P.K., Sheng L., Zhou Y., Han D., Wan Y.J.Y., Hwang S.T. Diet-induced obesity exacerbates imiquimod-mediated psoriasiform dermatitis in anti-PD-1 antibody-treated mice: implications for patients being treated with checkpoint inhibitors for cancer. J. Dermatol. Sci. 2020;97:194–200. doi: 10.1016/j.jdermsci.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Napier B.A., Andres-Terre M., Massis L.M., Hryckowian A.J., Higginbottom S.K., Cumnock K., Casey K.M., Haileselassie B., Lugo K.A., Schneider D.S., et al. Western diet regulates immune status and the response to LPS-driven sepsis independent of diet-associated microbiome. Proc. Natl. Acad. Sci. USA. 2019;116:3688–3694. doi: 10.1073/pnas.1814273116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantelmacher F.D., Zvibel I., Cohen K., Epshtein A., Pasmanik-Chor M., Vogl T., Kuperman Y., Weiss S., Drucker D.J., Varol C., Fishman S. GIP regulates inflammation and body weight by restraining myeloid-cell-derived S100A8/A9. Nat. Metab. 2019;1:58–69. doi: 10.1038/s42255-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 54.Manitz M.P., Horst B., Seeliger S., Strey A., Skryabin B.V., Gunzer M., Frings W., Schönlau F., Roth J., Sorg C., Nacken W. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol. Cell Biol. 2003;23:1034–1043. doi: 10.1128/mcb.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehrchen J.M., Sunderkötter C., Foell D., Vogl T., Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 56.Dubois C., Payen D., Simon S., Junot C., Fenaille F., Morel N., Becher F. Top-down and bottom-up proteomics of circulating S100A8/S100A9 in plasma of septic shock patients. J. Proteome Res. 2020;19:914–925. doi: 10.1021/acs.jproteome.9b00690. [DOI] [PubMed] [Google Scholar]
- 57.Cassado A.d.A., de Albuquerque J.A.T., Sardinha L.R., Buzzo C.d.L., Faustino L., Nascimento R., Ghosn E.E.B., Lima M.R.D., Alvarez J.M.M., Bortoluci K.R. Cellular renewal and improvement of local cell effector activity in peritoneal cavity in response to infectious stimuli. PLoS One. 2011;6:e22141. doi: 10.1371/journal.pone.0022141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan J., Nowell M., Chima R., Zingarelli B. Pioglitazone reduces inflammation through inhibition of NF-κB in polymicrobial sepsis. Innate Immun. 2014;20:519–528. doi: 10.1177/1753425913501565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
There are no new dataset in this paper.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.