Abstract
The tumor microbiome is increasingly implicated in cancer progression and resistance to chemotherapy. In pancreatic ductal adenocarcinoma (PDAC), high intratumoral loads of Fusobacterium nucleatum correlate with shorter survival in patients. Here, we investigated the potential mechanisms underlying this association. We found that F. nucleatum infection induced both normal pancreatic epithelial cells and PDAC cells to secrete increased amounts of the cytokines GM-CSF, CXCL1, IL-8, and MIP-3α. These cytokines increased proliferation, migration, and invasive cell motility in both infected and noninfected PDAC cells but not in non-cancerous pancreatic epithelial cells, suggesting autocrine and paracrine signaling to PDAC cells. Notably, this phenomenon occurred in response to Fusobacterium infection regardless of the strain and in the absence of immune and other stromal cells. Blocking GM-CSF signaling markedly limited proliferative gains after infection. Thus, F. nucleatum infection in the pancreas elicits cytokine secretion from both normal and cancerous cells that promotes phenotypes in PDAC cells associated with tumor progression. The findings support the importance of exploring host-microbe interactions in pancreatic cancer to guide future therapeutic interventions.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy that has a median survival of fewer than 6 months and a 5-year survival rate of 3-7%1. Factors that promote disease progression from early, preinvasive precursor lesions to a malignant and invasive adenocarcinoma include oncogenic mutations2-4 and a dense, complex tumor microenvironment (TME). Characterized by extensive stromal remodeling and desmoplasia, the PDAC TME has limited vascularization and poor immune infiltration which makes it highly intractable to chemotherapy5,6. Furthermore, its TME is enriched with immunosuppressive cells such as T regulatory (Treg) cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs)7,8. This immune suppression is further compounded by tumor-specific microbial species residing within the PDAC TME that are implicated in worse outcomes for patients 9-11.
The systematic characterization of intratumoral bacteria in multiple cancer types details a diverse bacterial ecosystem within tumors that is not found in adjacent healthy tissue12. These observations have elicited several fundamental questions on the precise role that microbes play within the TME to impact tumorigenesis, cancer progression, and therapy response13-15. Little is known about how these bacteria reach the tumor, the survival strategies that they employ within the TME, the host tumor cells’ response to the infection, and whether elimination of the bacteria could augment cancer therapy. Previous studies have revealed positive associations of certain microbes with the development of PDAC. These include Fusobacterium nucleatum16, Porphyromonas gingivalis, Neisseria elongata, Streptococcus mitis11,17, and Helicobacter pylori18. Moreover, the PDAC tumor microbiome has been shown to influence immune response and can impact chemotherapy19. For example, Gammaproteobacteria in PDAC tissue regulates drug resistance to a common chemotherapeutic agent, gemcitabine20. However, there is still limited insight into the individual roles these bacteria play in worsening cancer prognosis. Our work aims to shed light on the role of a specific microbe, F. nucleatum, and its impact within the PDAC TME.
F. nucleatum is a gram-negative, anaerobic, rod-shaped bacterium usually found within the oral cavity. It is a commensal microorganism that plays a supportive role as a bridge-species within biofilms connecting primary and secondary microbial colonizers21. However, in certain disease conditions, it can act as an opportunistic pathogen. In addition to participating in inflammatory diseases within the oral cavity, such as in gingival and periodontal disease, it is a risk-factor in preterm birth and intrauterine infections22. There is also associative evidence of its role in appendicitis23, urinary tract infection, endocarditis24, and other respiratory tract infections25. Moreover, F. nucleatum is increasingly recognized as an oncomicrobe due to its disproportionate presence in several cancers and association with a worse prognosis. These cancers include colorectal cancer26,27, esophageal cancer28, pancreatic cancer16,29, and Lauren’s diffuse type gastric cancer30.
Much of the current insight into the mechanisms of F. nucleatum infection and effects come from studies in colorectal cancer (CRC). F. nucleatum uses its surface adhesin, Fap2, to initially bind to host Gal/Gal-NAc31 sugar residues found abundantly in CRC, which then drives entry into host cells and intracellular colonization. In fact, Gal/Gal-NAc is highly expressed in a wide variety of tumors and may act as a key factor in bacterial homing to these sites32-34. In addition, the adhesin FadA has been shown to bind to E-cadherin, which activates β-catenin and stimulates downstream signaling that promotes carcinogenesis35. However, it is unclear how F. nucleatum adapts to the TME and exacerbates the aggressiveness of both early and late-stage cancers36. Moreover, it has been shown that F. nucleatum may travel within the primary tumor cells to distant metastatic sites37 and could contribute to pre-metastatic niche formation. We have previously shown that F. nucleatum infects human CRC cells in a Fap2-driven mechanism and leads to the secretion of potent cytokines, IL-8 and CXCL1, that induced cell migration 38, suggesting a way in which F. nucleatum may facilitate the metastatic spread of CRC.
Emerging evidence has tied F. nucleatum to increased mortality from pancreatic cancer. Mitsuhashi et al. initially uncovered the association of Fusobacterium species in PDAC16. They tested cancer tissue specimens from 283 patients with PDAC and identified an 8.8% detection rate of Fusobacterium and observed highly significant mortality in the Fusobacterium positive group. In a separate study, Kohi et al. observed higher enrichment of Fusobacterium in duodenal fluid microbiomes from patients with PDAC when compared to normal pancreas controls and was more abundant in patients with shorter survival due to PDAC39. Fusobacteria were also detected in 18.84% of pancreatic cyst fluid samples collected in a study with 69 patients40. Taxonomically, F. nucleatum is further classified into several subspecies which include nucleatum, animalis, polymorphum, and vincentii, which may variably impact their pathogenesis. For example, F. nucleatum subsp. animalis is the most prevalent in human CRC41. In pancreatic cancer, F. nucleatum subsp. animalis was found to co-occur with 10 other bacterial species in cyst fluid from intraductal papillary mucinous neoplasms (IPMNs) with high-grade dysplasia (HGD)29. Furthermore, Chung et al. compared oral and pancreatic tissues from 52 subjects and identified a consistent presence of F. nucleatum subsp. vincentii in pancreatic tumors42. The same subspecies vincentii was identified in a study by del Castillo et al. but its relative proportion among patient samples was dependent on the origin of the samples43. Alkharaan et al. identified circulating IgG reactivity to F. nucleatum and high salivary IgA reactivity to F. nucleatum and Fap2 of F. nucleatum in patients with HGD44. Further corroboration comes from the study by Nejman et al. which identified F. nucleatum in pancreatic cancer with a prevalence of 15-20% from the 67 tumor samples that were tested12. In contrast, other independent studies that have delineated the composition of the PDAC tumor microbiome have revealed discordant results with respect to Fusobacterial abundance among patient samples43. These studies show that Fusobacterial abundance was low or absent in PDAC tumors 9,10,19,20 indicating high variability in Fusobacterial co-occurrence and colonization in tumors. Together, these observations suggest a disproportionate presence of F. nucleatum within the PDAC TME, warranting further research into its function in pancreatic cancer pathogenesis.
A distinguishing feature of PDAC is that tumor cells form only a small fraction of the TME. In fact, studies have shown how PDAC progression and resistance to therapy is considerably driven by its dense stroma, which can constitute up to 80% of the tumor45. It consists of multiple non-oncogenic cell types including cancer associated fibroblasts, pancreatic stellate cells, immune cells, and endothelial cells that shape the behavior of PDAC cells, by remodeling the extracellular matrix, promoting epithelial-mesenchymal transition (EMT), and fostering aberrant cell signaling46,47. The dense fibrosis and limited vascularization hence formed creates a stiff and hypoxic TME that could provide a favorable niche for anaerobic organisms such as F. nucleatum.
Our previous findings from F. nucleatum infection of CRC cells showed that bacterial infection can exert local effects on cancer cells without the involvement of immune cells38. We hypothesized that F. nucleatum may play an analogous role in PDAC to impact tumor progression. Here, using healthy and cancerous pancreatic cell lines and several subspecies of F. nucleatum, we investigated the potential role of F. nucleatum in pancreatic cancer and identified key secretory factors from host cells that could contribute to worse outcomes in patients harboring this bacterium in their tumors.
Results
Fusobacterium binds and invades BxPC3 and Panc1 cells through a Fap2-driven mechanism
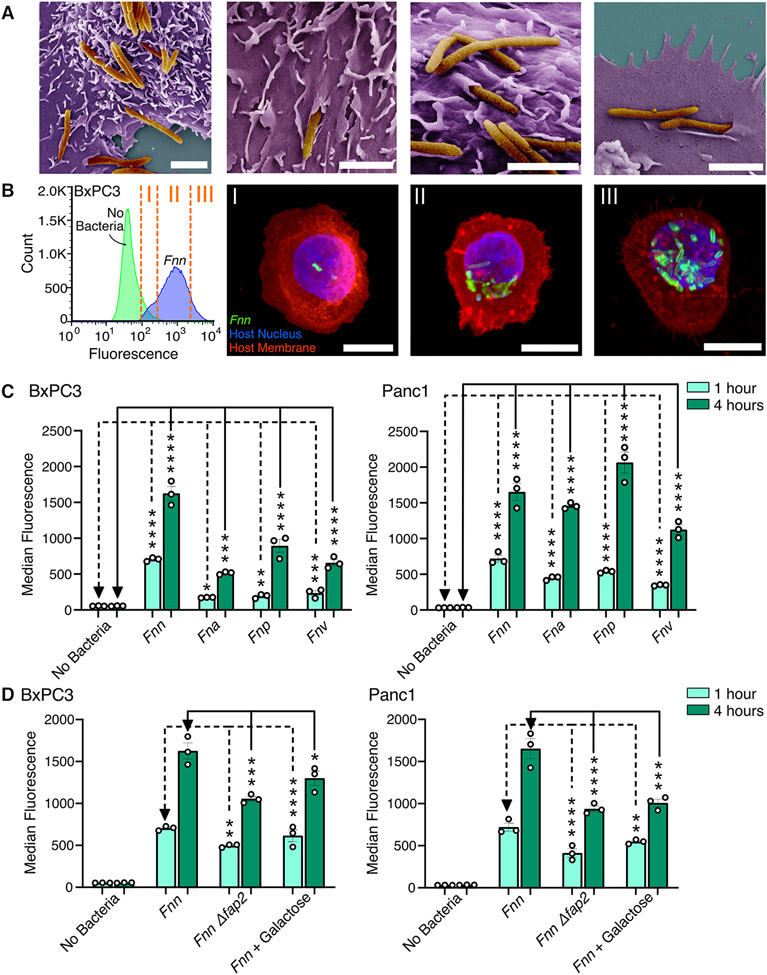
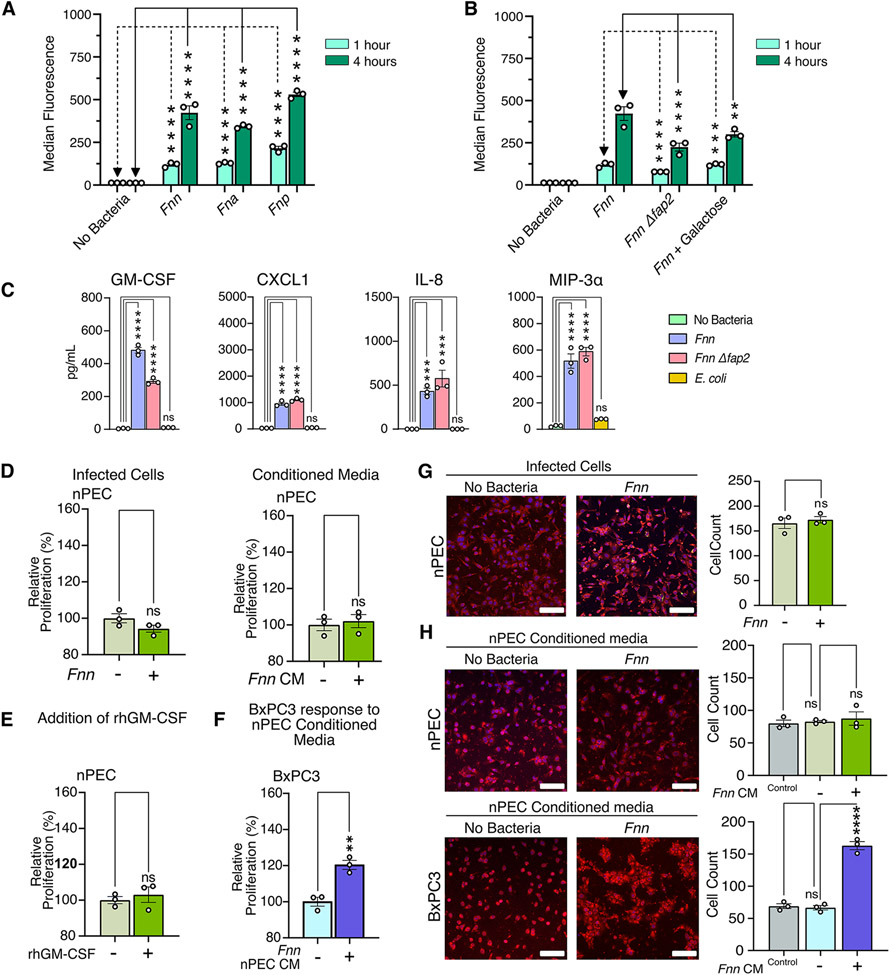
For this study, we used four pancreatic cancer cell lines (BxPC3, Panc1, HPAC, and Capan1), chosen because they are extensively characterized48 and are representative of different sexes (male and female patient-derived), metastatic propensity, and differentiation status among PDACs, which could impact their response to F. nucleatum infection. We first confirmed that F. nucleatum can bind and invade pancreatic cancer cells. Scanning electron microscopy (SEM) imaging enabled direct visualization of the various modes of binding and invasion of F. nucleatum subsp. nucleatum ATCC 23726 (hereafter referred to as Fnn) into BxPC3 cells (Fig. 1A). We further confirmed the intracellular localization of Fnn within BxPC3 cells through confocal imaging and flow cytometry (Fig. 1B). We sorted the cells based on the intensity of fluorescence observed and imaged them in high resolution z-stacks to visualize the number of intracellular bacteria within each cell. We then demonstrated that other subspecies of F. nucleatum, including F. nucleatum subsp. animalis (Fna), F. nucleatum subsp. vincentii (Fnv), and F. nucleatum subsp. polymorphum (Fnp) are also highly invasive (Fig. 1C). Though subspecies level differences exist between these clinically relevant strains, we observed that all the tested strains are invasive. Thus, additional experiments were focused on Fnn infection as it is well characterized in the literature and is genetically tractable compared to the other strains. Based on prior research highlighting the importance of Fap2 in F. nucleatum binding, we hypothesized an analogous interaction with pancreatic cancer cells. Using flow cytometry, we identified that the Fap2 surface adhesin deletion mutant (Fnn Δfap2), previously created and used in our work38,49, infects both BxPC3 and Panc1 cells at a significantly decreased rate compared to the wild-type Fnn (Fig. 1D). Furthermore, we demonstrate that the addition of galactose, a sugar that binds to Fap2, significantly inhibits binding and invasion of Fnn in both BxPC3 and Panc1 cells, underscoring the importance—but not exclusivity—of Fap2 binding in Fnn infection of pancreatic cancer cells.
Figure 1. Fusobacterium nucleatum binds to and invades pancreatic cell lines.
(A) Scanning electron microscopy images of F. nucleatum subsp. nucleatum 23726 (Fnn) invading BxPC3 pancreatic cancer cells. Pseudocolored with orange for bacteria and purple for host cells. Scale bar: 2μm. (B) Flow cytometry analysis of BxPC3 cells cultured without bacteria or with Fnn at 50:1 MOI. Cells sorted based on fluorescence intensity correspond to intracellular bacterial loads (I: low, II: medium, III: high). These cells were plated and imaged on a confocal microscope to identify relative levels of infection. Host cell nuclei were stained with DAPI (blue), Fnn membranes were stained with FM 1-43 FX (green), and host cell membranes were stained with MemBrite 568 (red). Scale bar: 10 μm. (C) Flow cytometry analysis of infection of BxCP3 and Panc1 cells cultured with Fnn, Fna, Fnp, and Fnv at 50:1 MOI after 1 and 4 hours. (D) Flow cytometry analysis of infection of BxpC3 and Panc1 cells cultured with Fnn, Fnn Δfap2, or Fnn in the presence of Fap2-binding inhibitor galactose, at 50:1 MOI for 1 and 4 hours. Data in (B to D) are means ± SEM from N = 3 independent experiments per condition; comparisons by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test (C) or Šídák's multiple comparisons test (D): * P≤0.05, ** P≤0.01, *** P≤0.001, and **** P≤0.0001; ns, not significant.
Host pancreatic cancer cells secrete specific cytokines upon Fusobacterium infection
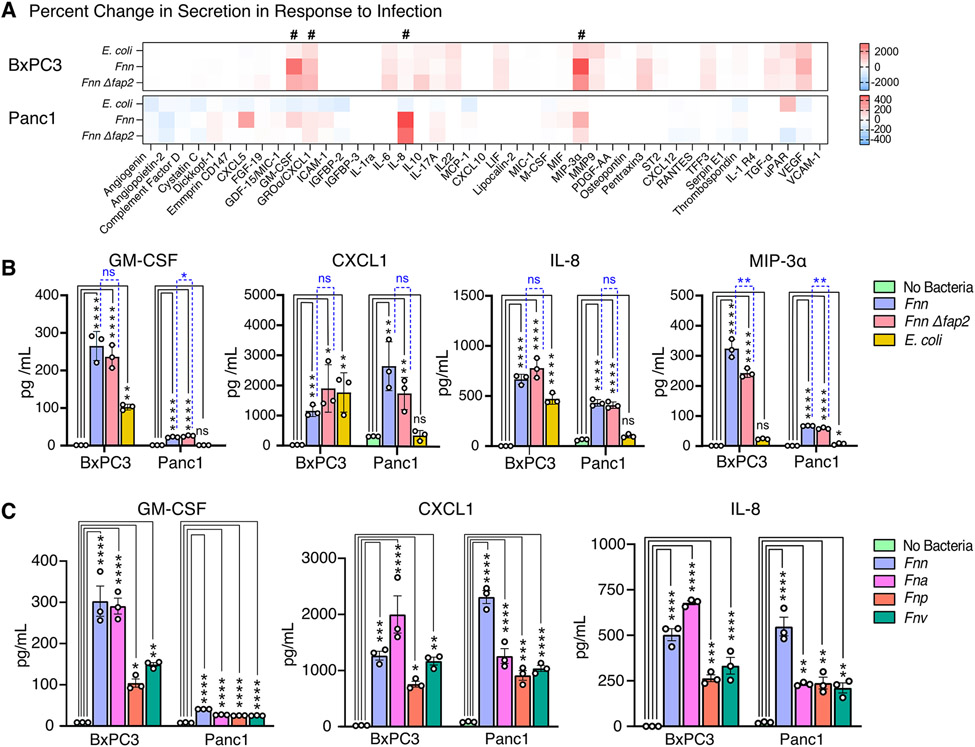
Once we confirmed host cell binding to Fnn and subsequent intracellular invasion into host cells, we then analyzed the host cell response to infection. Because of our prior work in Fnn-infected CRC cells38, we focused our investigation here to cytokine secretion. Western blot arrays were used to identify infection-induced cytokine secretions from the pancreatic cancer cell lines tested in four conditions: without bacteria, infection with E. coli, infection with Fnn, and infection with Fnn Δfap2. Heat maps were constructed based on the raw intensities (fig. S1, A to C) and the percent fold change in protein secretion compared to the No Bacteria condition was determined (Fig. 2A). Whereas pancreatic cancer cell lines are highly secretory without infection, we identified Fnn infection-specific increases in secretion for GM-CSF, CXCL1, IL-8, and MIP-3α. There was minimal or no change in secretion of these cytokines in response to infection by E. coli. ELISAs were performed to confirm and quantify the secretion of these cytokines, and significant increased secretion was noted for the four cytokines from Fnn-infected BxPC3 and Panc1 cells relative to the condition lacking bacteria (Fig. 2B, and fig. S2, A to D). There was a significant increase in IL-8 and CXCL1 secretion from BxPC3 cells upon infection with E. coli, but there was not from Panc1 cells, and there was a significant increase in the secretion of GM-CSF from BxPC3 cells upon infection with E. coli relative to the no-bacteria control, but this was significantly lower than that elicited by infection with Fnn and Fnn Δfap2. Notably, there was no significant difference in the response to measured cytokine values between infection with Fnn and Fnn Δfap2 for GM-CSF, CXCL1, and IL-8, but was significantly lower in Fnn Δfap2 for MIP-3α. To confirm that other F. nucleatum subspecies elicit the same secretory phenotype upon infection of host cancer cells, we measured cytokine levels elicited from the infection of BxPC3 and Panc1 cells with Fna, Fnp, and Fnv, and observed significantly increased secretion of all four cytokines for these additional bacterial subspecies (Fig. 2C). These findings suggest that a cytokine response is induced by Fusobacteria species broadly in PDAC cells, though the strength and specificity of this response may vary with the tumor cell background.
Figure 2: Fusobacterium infection elicits secretion of specific cytokines from host cells.
(A) Heat maps depicting the percent change in cytokine secretion from BxPC3 and Panc1 cells in response to E. coli, Fnn, or Fnn Δfap2 infection in comparison to non-infected cells using the Human XL Cytokine Array. # denotes increases in secretion of GM-CSF, CXCL1, IL-8, and MIP-3α. (B) Quantitative analysis of select cytokine secretion from BxPC3 and Panc1 cells upon infection with E. coli, Fnn, or Fnn Δfap2, using ELISA. (C) Quantitative analysis of select cytokine secretion from BxPC3 and Panc1 cells upon infection with other F. nucleatum strains: animalis (Fna), polymorphum (Fnp), and vincentii (Fnv). Data in (B and C) are means ± SEM from N = 3 independent experiments per condition; comparisons by ordinary one-way ANOVA followed by Tukey’s multiple comparisons test (B) or Dunnett’s multiple comparisons test (C): * P≤0.05, ** P≤0.01, *** P≤0.001, and **** P≤0.0001; ns, not significant.
GM-CSF increases the proliferation of pancreatic cancer cells
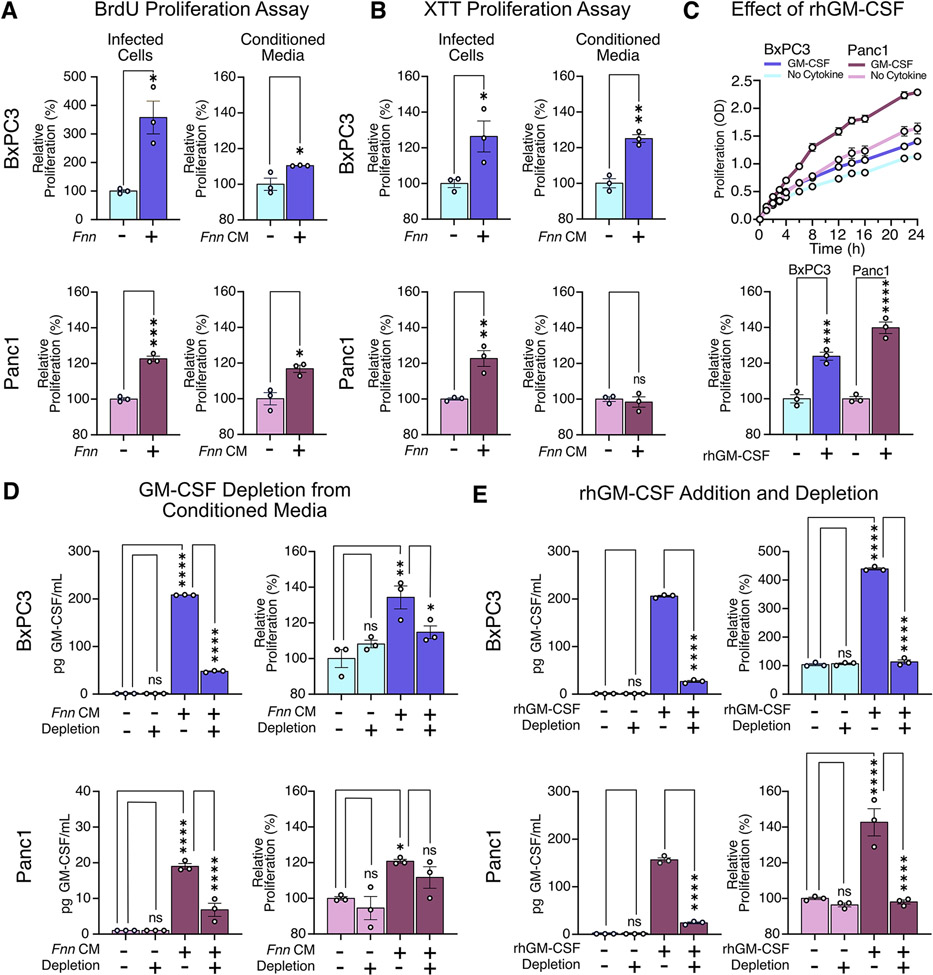
We hypothesized that the four cytokines we identified from the cytokine arrays could be involved in impacting the proliferation of pancreatic cells, which is a common feature in aggressive cancers. We first observed their individual impact on the proliferation of BxPC3 cells using an XTT assay (fig. S3). From this preliminary assay, we discovered that GM-CSF at a concentration of 200 pg/mL increased the proliferation of BxPC3 cells, whereas the other cytokines—IL-8, CXCL1, and MIP-3a—did not. In both BxPC3 and Panc1 cells, we observed increased proliferation of cells when infected with Fnn. Furthermore, conditioned media obtained from infected cells after a 4-hour infection was sufficient to induce the proliferation of uninfected cells. This was confirmed by three independent assays to quantify proliferation: BrdU (Fig. 3A), XTT (Fig. 3B) and Ki67 (fig. S4). We additionally confirmed that the measured proliferative increase was not due to bacterial secretions (fig. S5, A and B). The addition of recombinant human GM-CSF (rhGM-CSF) at 200 pg/mL was sufficient to increase the proliferation of BxPC3 and Panc1 cells (Fig. 3C). To confirm that GM-CSF was primarily responsible for the proliferative effect, we depleted GM-CSF from the conditioned media using biotinylated anti-GM-CSF antibodies to capture free GM-CSF and used streptavidin-coated magnetic particles to capture and spin down the cytokines and observed a concomitant decrease in proliferation using an XTT assay (Fig. 3D). Because Panc1 cells showed lower but measurable secretion of GM-CSF, we supplemented 200 pg/mL rhGM-CSF into the culture medium on these cells, and then depleted it from the medium and measured proliferation; the results confirmed that GM-CSF directly contributed to the increase in cell proliferation of this pancreatic cancer cell line (Fig. 3E). This last assay also suggests that exogenous GM-CSF within the TME may directly impact the proliferation of pancreatic cancer cells.
Figure 3: GM-CSF induces pancreatic cell proliferation.
(A and B) Results of BrdU and XTT assays to assess proliferation of BxPC3 and Panc1 cells upon Fnn infection and when cultured in conditioned medium from infected cells from the same line (Fnn CM). (C) Effect of recombinant human GM-CSF (rhGM-CSF, 200 pg/mL) on the proliferation of BxPC3 and Panc1 cells, measured using the XTT assay. (D) Effect of GM-CSF depletion from conditioned media obtained from Fnn infected cells on the proliferation of BxPC3 and Panc1 cells, measured using the XTT assay. (E) Proliferation of BxPC3 and Panc1 cells upon GM-CSF depletion from the regular culture medium, upon supplementation of the regular culture medium with rhGM-CSF, and upon depletion of the supplemented rhGM-CSF, measured using the XTT assay. Data in (A to E) are means ± SEM from N = 3 independent experiments per condition; comparisons by unpaired t-test (A, B) or ordinary one-way ANOVA followed by Šídák's multiple comparisons test (C, D, E): * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001; ns, not significant.
Cancer cells exhibit increased migration in response to Fnn induced host cell secretion
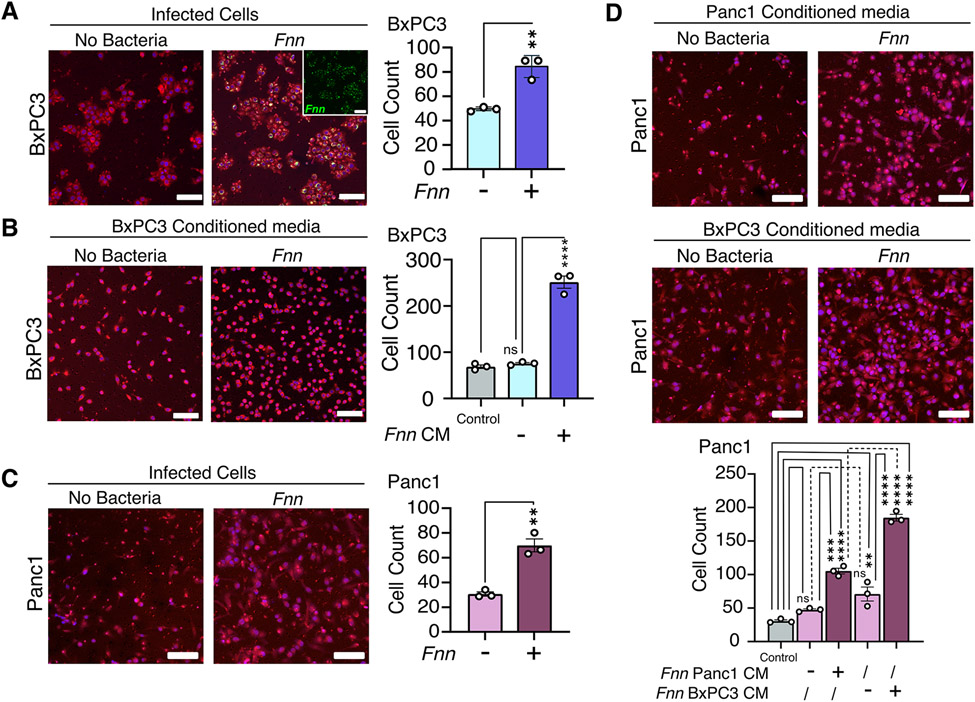
Several studies have implicated the four upregulated cytokines we identified in cell migration 38,57, 58,64. Therefore, we hypothesized that the Fnn induced host secretion would impact pancreatic cancer cell migration. We determined the migration of Fnn infected and uninfected host cells in an in vitro transwell co-culture model. Host cells were infected with Fnn at 50:1 MOI for 4 hours after which they were collected and added to the top chamber of a transwell with a membrane that consisted of 8μm pores. Fnn infected BxPC3 cells trans-migrated across the membrane in a transwell assay at a significantly increased rate compared to uninfected cells over 16 hours (Fig. 4A). To study the influence of secreted factors on migration, we collected conditioned media from the 4-hour infection of host cells with Fnn at 50:1 MOI, filtered and concentrated it 16x, and then added the concentrated media to the outer (lower) chamber of the transwell. We observed significantly greater migration of uninfected BxPC3 cells from the top of the membrane to the bottom in response to concentrated conditioned media obtained from infected cells than in response to concentrated conditioned media obtained from uninfected cells over 16 hours (Fig. 4B). The short time period of 16 hours and using 0.5% serum supplemented media restricted the contribution of cellular proliferation in this assay. Conditioned media obtained from BxPC3 cells infected with the other F. nucleatum strains (Fna, Fnp, and Fnv), also induced increased migration of uninfected BxPC3 cells (fig. S6). These observations were reproduced in Panc1 cells. Infected Panc1 cells showed significantly increased migration at 12 hours (Fig. 4C). Because Panc1 cells are highly migratory, a shorter duration of 12 hours was used to dissect the differences in their response to conditioned media. Similar to BxPC3 cells, Panc1 cells show significantly increased migration in response to concentrated conditioned media obtained from infected Panc1 cells. To test whether migration correlated to the extent of cytokine secretion, and to demonstrate paracrine crosstalk, we exposed Panc1 cells to concentrated conditioned media obtained from infected BxPC3 cells and observed significantly increased migration of Panc1 cells (Fig. 4D). Notably, this increase was significantly higher than the increase in migration observed in response to Panc1-conditioned media. Together, these findings suggest that F. nucleatum infection confers increased cell motility to pancreatic cancer cells through autocrine and paracrine crosstalk.
Figure 4: Increased cell migration observed for both F. nucleatum-infected cells and non-infected cells in response to conditioned media obtained from F. nucleatum infected cells.
(A) Representative images and results from Transwell assays to measure BxPC3 cell migration over 16 hours in response to Fnn infection. Staining by Cell Tracker Red, DAPI (blue), and FM 1-43X (inset; Fnn, green). (B) Transwell assays assessing BxPC3 cell migration in response to conditioned, concentrated media obtained from Fnn-infected BxPC3 cells, stained as described in (A). Control: 0.5% FBS. (C) Transwell assays assessing the migration of Panc1 cells upon infection with Fnn. (D) Transwells assays assessing migration in Panc1 cells cultured with conditioned, concentrated medium from Fnn-infected Panc1 or BxPC3 cells. Data in (A to D) are means ± SEM from N = 3 independent experiments; comparisons by unpaired t-tests (A and C) or ordinary one-way ANOVA followed by Tukey's multiple comparisons test (B) or Šídák's multiple comparisons test (D): * P≤0.05, ** P≤0.01, *** P≤0.001, and **** P≤0.0001; ns, not significant. Scale bars = 100μm.
Normal pancreatic epithelial cells secrete cytokines upon F. nucleatum invasion that stimulate proliferation and migration of cancer cells
We next studied the infection of a normal pancreatic epithelial cell line (hereafter called nPEC) and compared its response with that of pancreatic cancer cell lines. Flow cytometry analysis of nPECs upon infection with Fnn, Fna, and Fnp indicate that these tested strains were all invasive (Fig. 5A), though reduced in comparison to invasion of the pancreatic cancer cells (Fig. 1C). Furthermore, we show that infection with Fnn Δfap2 is significantly lower than the infection with Fnn and the addition of galactose (an inhibitor of Fap2 binding) significantly decreases infection of nPECs by Fnn (Fig. 5B). Next, ELISA of conditioned media from nPECs confirmed a significant secretion of GM-CSF, CXCL1, IL-8, and MIP-3α in response to Fnn and Fnn Δfap2 infection, which was absent upon infection with E. coli (Fig. 5C). There was also reduced secretion of GM-CSF upon infection with Fnn Δfap2 when compared to infection with Fnn. Proliferation assays were subsequently performed and in contrast to the cancer cell lines, nPECs did not increase proliferation upon infection or in the presence of conditioned media obtained from infected nPECs (Fig. 5D). Similarly, nPECs did not proliferate in the presence of 200pg/mL recombinant hGM-CSF (Fig. 5E). However, when conditioned media obtained from infected nPECs was added to BxPC3 cells, it stimulated significant proliferation of BxPC3 cells (Fig. 5F). Next, we studied nPEC migratory response in a transwell assay and observed that infection of nPECs did not impact migration in comparison to uninfected nPECs (Fig. 5G). Furthermore, we observed that concentrated conditioned media from infected nPEC cells did not significantly impact nPEC migration; however, it did significantly increase the cell migration of BxPC3 cells (Fig. 5H). Together, these findings suggest that noncancerous pancreatic cells, too, can be infected by F. nucleatum, which elicits cytokine secretory response that can impact the proliferation and migration of cancer cells in a paracrine manner.
Figure 5: Normal pancreatic cells secrete cytokines upon Fusobacterium infection.
(A) Flow cytometry analysis of nPEC infection with Fnn, Fna, and Fnp and Fnn Δfap2 at 1 and 4 hours to assess bacterial invasion. (B) Flow cytometry analysis of nPEC infection with Fnn, Fnn Δfap2, and Fnn + Fap2-binding inhibitor galactose at 1 and 4 hours to assess bacterial invasion. (C) Secretion of GM-CSF, CXCL1, IL-8, and MIP-3α from nPECs upon infection with Fnn, Fnn Δfap2, or E. coli compared with each from uninfected nPECs, measured using ELISA. (D) XTT proliferation assays assessing proliferation of nPECs upon infection or culture in conditioned media from infected nPECs. (E) Proliferation of nPECs in response to rhGM-CSF, measured using the XTT assay. (F) Proliferation of BxPC3 cells cultured in conditioned media from infected nPECs, measured using the XTT assay. (G and H) Transwell migration assays to assess cell migration of infected nPECs (G) and of nPECs and BxPC3 cells cultured in conditioned, concentrated media from infected nPECs (H), stained with Cell Tracker Red. Scale bar: 100 μm. Data in (A to H) are means ± SEM from N = 3 independent experiments; comparisons by unpaired t-test (D, E, F, and G), and ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test (A and C), Tukey’s multiple comparisons test (H), or Šídák's multiple comparisons test (B): * P≤0.05, ** P≤0.01, *** P≤0.001, and **** P≤0.0001; ns, not significant.
Cells from BxPC3 spheroids proliferate and migrate in a 3D environment in response to F. nucleatum infection
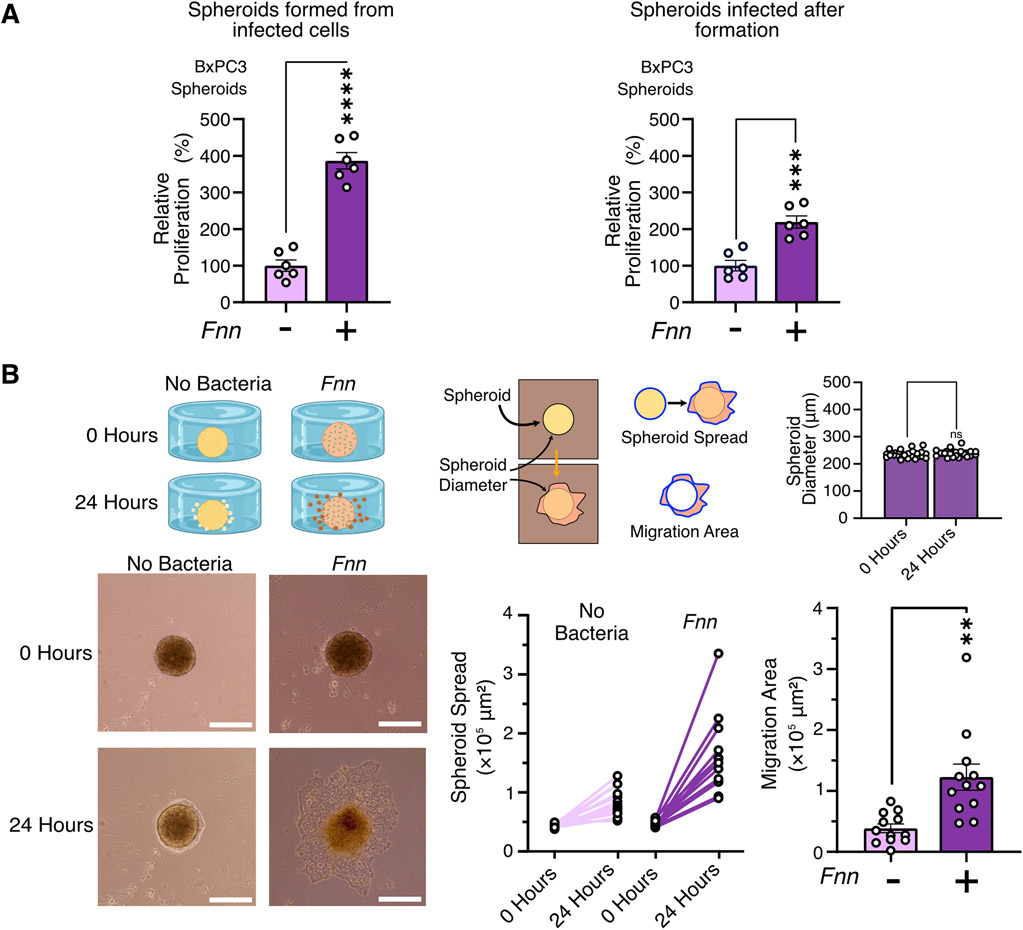
To extend the physiological relevance of our observations from 2D co-cultures, we next utilized a 3D spheroid model of pancreatic cancer to study its response to Fnn infection. Spheroids containing ~5000 cells were formed from either uninfected or infected BxPC3 cells using a multi-spheroid well plate and proliferation quantified using the XTT assay. Spheroids formed from cells initially infected with Fnn at 50:1 MOI for 4 hours as well as spheroids infected after formation with Fnn at 50:1 MOI for 4 hours both showed a significant increase in proliferating cells compared to uninfected spheroids (Fig. 6A). Next, to assess the migration of cells from the spheroid, a custom designed 3D printed chamber was used to form 5000 cell BxPC3 spheroids in hanging drops. Once formed, the spheroids were infected with Fnn at 50:1 MOI for 4 hours, and subsequently embedded in 2.5mg/mL collagen I (fig. S7). The spheroids were monitored for 24 hours, and the extent of spheroid spread and migration area within the collagen gel were quantified (Fig. 6B). Fnn infected spheroids showed significantly greater area of migration in comparison to uninfected spheroids. These findings reveal that proliferative and migratory phenotypes induced in pancreatic cancer cells by F. nucleatum infection can be reproduced in a more physiologically relevant 3D co-culture model.
Figure 6: F. nucleatum infection induces proliferation of BxPC3 spheroids and migration of BxPC3 cells from spheroids.
(A) XTT proliferation assays measure proliferation of BxPC3 spheroids (5000 cells) when infected with F. nucleatum at 50:1 MOI both before spheroid formation and after spheroid formation. Data are representative of means ± SEM from N = 6 independent experiments per condition. (B) Representative images of the migration of BxPC3 cells from 5000 cell spheroids in the custom designed chamber. Spheroid diameters were quantified at the start and at the end of the experiment to confirm no expansion and contraction of the spheroids during the duration of the experiment. Migration over 24 hours is quantified by measuring extent of spheroid spread and overall area of migration after normalization to spheroid sizes. Data are representative of means ± SE from N=4 with 3 biological replicates per condition; comparisons by unpaired t-test: * P≤0.05, ** P≤0.01, *** P≤0.001, and **** P≤0.0001; ns, not significant.
Discussion
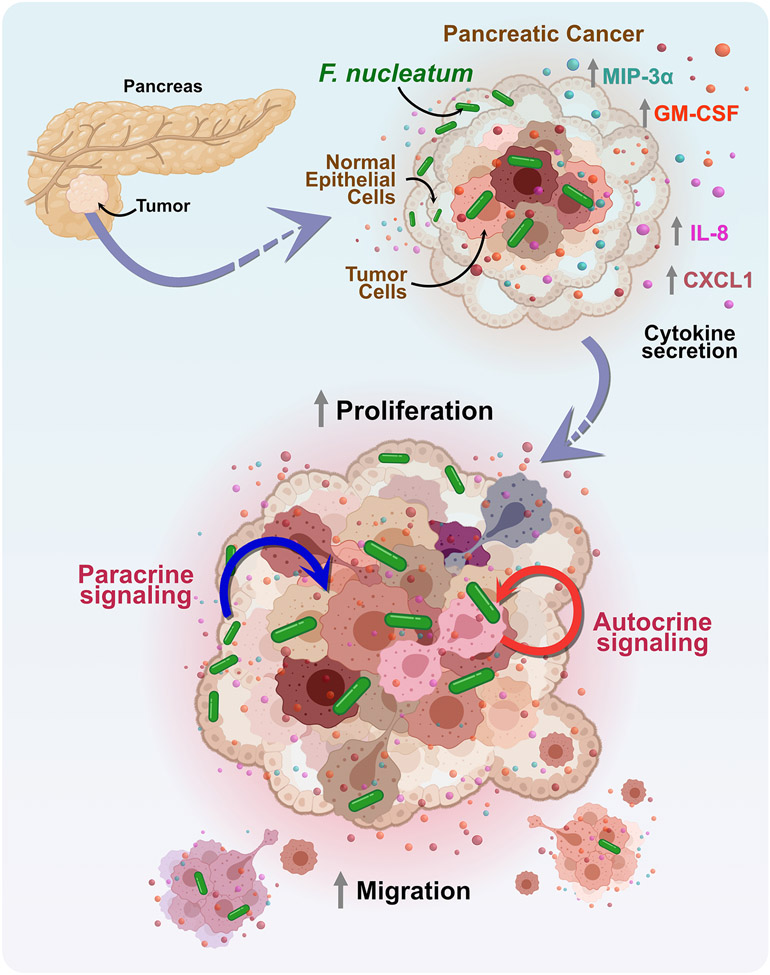
We sought in this study to leverage in vitro co-culture models to begin to define and quantify the impact of localized and intracellular F. nucleatum in the PDAC microenvironment (findings summarized in Fig. 7). Using flow cytometry, SEM, and confocal imaging, we first confirmed intracellular invasion and localization of F. nucleatum in pancreatic cell lines. F. nucleatum is well-known to bind and invade a variety of epithelial and immune cell types 21,50. Among the host of binding adhesins expressed on F. nucleatum, the role of Fap2 has received significant attention due to its defined functions in adhesion, cell-cell aggregation, biofilm formation, and bacterial invasion. In CRC, it has been shown that Fap2 binding to human inhibitory receptor TIGIT on natural killer (NK) cells and the tumor itself can protect tumors from being targeted by NK cells and also inhibits T cell function 51,52. F. nucleatum binding to tumor cells is also mediated by Fap2, evidenced in CRC31 and breast cancer33. Its target is Gal-GalNAc (D-galactose-β(1-3)-N-acetyl-D-galactosamine) that is expressed at high levels in adenocarcinomas, including pancreatic cancer32,34. Furthermore, it was identified that the Fap2 lectin domain can be inhibited by galactose and galactose derivative molecules 38,53,54. Our results reveal significantly decreased infection of BxPC3 and Panc1 cells by the Fap2 deficient F. nucleatum in comparison to the wild-type, indicating that Fap2 binding plays a critical role in the intracellular invasion of the bacterium in pancreatic cancer as well. We observed significantly decreased invasion of F. nucleatum in the presence of galactose, which further supports our conclusions. This difference is sustained upon binding and invasion of normal pancreatic cells by the fap2 deletion mutant, indicating that healthy pancreatic cell invasion could at least partially be driven by lower levels of Gal-GalNAc. Additionally, we acknowledge that adhesins other than Fap2 could contribute to binding and invasion of cells through undiscovered protein-protein interactions.
Figure 7: A model of pancreatic cancer response to F. nucleatum infection induced cytokine signaling.
Model of the mechanism, depicting that F. nucleatum binds to pancreatic cancer cells in a Fap2-driven mechanism and induces the specific secretion of cytokines, GM-CSF, CXCL1, IL-8, and MIP-3α. These cytokines play a role to enhance proliferation and migration of pancreatic cancer cells through paracrine and autocrine signaling to adversely impact cancer progression.
The next focus of our study was the pancreatic cancer cells' response to F. nucleatum infection, focusing on host cytokine secretions55,56. Our findings from the cytokine arrays revealed a trend that F. nucleatum specifically induced the secretion of GM-CSF, CXCL1, IL-8, and MIP-3α by BxPC3, Panc1, and HPAC cells, and increased IL-6, GM-CSF, and MIP-3α secretion in addition to the constitutive secretion of IL-8 and CXCL1 by Capan1 cells. Notably, IL-8 is known to regulate multiple aspects of tumor progression57, CXCL1 has been implicated in chemoresistance and metastasis58 as well as pre-metastatic niche formation in CRC59, and MIP-3α (or CCL20) has multifaceted roles within the TME60. MIP-3α production has also been observed in F. nucleatum infection of human oral epithelial cells61. Intriguingly, GM-CSF is known to exhibit both stimulatory and suppressive effects on tumor progression62,63 and has been shown to play a role in metastasis to the liver64. Previous studies have also revealed roles for these cytokines in pancreatic cancer 65,70,71. GM-CSF, which is upregulated by oncogenic mutations in the protein KRAS, seen in many patient tumors (including two of the four cell lines—Panc1 and Capan1—used here), can promote the development of pancreatic neoplasia66 and the evasion of antitumor immunity in PDAC67,68. These multiple roles have been tied to poor patient outcomes69.
Our new findings reveal an additional role for GM-CSF in pancreatic cancer. We isolated conditioned media from the F. nucleatum infected cancer cells and found that it was sufficient to increase proliferation of uninfected pancreatic cancer cells, and this effect was partially negated when we depleted GM-CSF from the media. We also demonstrated that the addition of exogenous recombinant GM-CSF increased cell proliferation and its depletion negated the observed effect. Together, these observations define an independent role of GM-CSF in BxPC3 and Panc1 cells, to directly contribute to cancer progression by impacting proliferation. We additionally observed increased cell migration of infected cells compared to uninfected cells, and hypothesized that secreted cytokines could account for this altered phenotype. We demonstrated that conditioned and concentrated media obtained from F. nucleatum infected cells was sufficient to significantly increase the cellular migration of uninfected cells. Together, these observations indicate that infected cancer cells can influence both infected as well as uninfected cancer cells within the PDAC TME through both autocrine and paracrine signaling (Fig. 7), an important potential mechanism whereby the impact of relatively low numbers of bacteria in vivo72 may be amplified locally through host cell crosstalk. Furthermore, we show that the observations of increased proliferation and migration are sustained in 3D spheroid models.
While the influence of stromal cells such as tumor-associated fibroblasts and pancreatic stellate cells are well characterized in PDAC46,73-76, the role of normal epithelial cells have not received as much scrutiny. We show F. nucleatum infects normal pancreatic epithelial cells using a Fap2-dependent mechanism and this infection still results in the secretion of the same cytokines released by infected PDAC cells. These cytokines, which do not elicit a proliferative response from the normal cells, are able to impact tumor proliferation and migration. Cytokines such as IL-8 and CXCL1 are well known players in formation of pre-metastatic niches59,77 priming organs and tissues to attract circulating tumor cells (CTC), break dormancy, and establish micrometastatic sites. Thus, the crosstalk between normal and tumor cells through both paracrine and autocrine signaling could lead to the evolution tumor subpopulations with increased malignancy.
A planned limitation of this study is the lack of immune cells. However, we did this to show that autocrine and paracrine signaling from the tissue cells themselves is sufficient to exacerbate tumor growth. And although our work is restricted to the single bacterium F. nucleatum, we demonstrated the host invasion and similar phenotypic responses by additional subspecies of F. nucleatum identified clinically, including subsp. animalis and subsp. vincentii. Several other species of bacteria have been identified to co-occur within the PDAC tumor microbiome, with Fusobacterium found at varying abundance12,15,20,29,43. Thus, future studies should aim to understand if similar mechanisms are mediate an influence of other tumor-associated microbes in tumorigenesis and adverse patient outcomes. Our work reveals that the PDAC-resident bacterium F. nucleatum plays a role in directly enhancing tumor cell proliferation and migration, two key hallmarks of PDAC as well as other cancers (Fig. 7). The infection of normal cells surrounding the TME further extends the impact of F. nucleatum to surrounding tissues and organs, demonstrating that although F. nucleatum may not directly transform normal cells into cancer cells, it can still indirectly contribute to tumor progression through paracrine crosstalk. We believe that our data provides evidence that encourages future studies to investigate whether antibiotic-mediated therapy regimes in conjunction with chemotherapy or inhibition of cytokine and chemokine receptors may provide beneficial options for treating PDAC.
Materials & Methods
Bacteria cultures
Fusobacterium nucleatum subsp. nucleatum ATCC 23726 (referred to as Fnn) and its Fap2 surface adhesin deletion mutant, F. nucleatum Δfap2 (referred to as Fnn Δfap2), Fusobacterium nucleatum subsp. animalis (Fna), subsp. polymorphum (Fnp), and subsp. vincentii (Fnv) were grown as detailed previously38. Bacterial colonies are initiated on solid agar plates made with Columbia Broth (Gibco) substituted with hemin (5 μg/mL) and menadione (0.5 μg/mL) (CBHK) and grown in anaerobic conditions (90% N2, 5% H2, 5% CO2) at 37°C for two days. Single colonies were then retrieved and added to CBHK media to initiate the liquid culture and were grown for ~16 hours at 37°C anaerobically. The optical density (OD) at 600 nm was then measured (~0.6-0.8) to obtain bacteria at the mid-exponential growth phase of the culture. Bacterial counts were obtained based on standardized curves created for the spectrophotometer. All experiments were carried out with a 50:1 multiplicity of infection (MOI) which is the bacteria to host cell ratio.
To stain the bacteria, 1 mL of the bacterial cell suspension was collected and spun down at 1500g for 3 minutes. The pellet was resuspended in 100 μL CBHK media and then stained with FM 1-43FX lipophilic styryl dye (Invitrogen F35355) (5 μg/mL) for 5 minutes. This stains the outer membrane of the bacteria to emit green fluorescence upon excitation. The stained cells were subsequently spun down at 1500g for 3 minutes, washed with 500 μL media, and again resuspended in 1 mL of media and used for experiments.
Escherichia coli TOP10 was grown in the shaker incubator at 37°C in Luria Broth (LB) overnight. Bacterial culture was then accordingly diluted to OD600nm of 0.6-0.8 and used for experiments.
Cell cultures
Cancerous epithelial cells were purchased from ATCC and grown on tissue culture-treated plates and flasks in their respective cell culture media. BxPC3 (ATCC CRL-1687) cells were grown in RPMI-1640 supplemented with 10% FBS, and Panc1 (ATCC CRL-1469) cells were grown in DMEM supplemented with 10% FBS. Normal primary pancreatic epithelial cells were purchased from CellBiologics (H-6037), and grown in epithelial cell culture medium supplemented with 5% FBS (Cell Biologics H-6621). Additional cell lines HPAC (ATCC CRL-2119) and Capan1 (ATCC HTB-79) cells were grown in DMEM:F12 supplemented with 10% FBS and IMDM supplemented with 20% FBS, respectively. All cell culture media contained 1% penicillin and streptomycin, but infections were performed in serum-free and antibiotic-free media. Cells were grown at 37°C with 5% CO2 and were passaged every 2-3 days based on their confluency with gentle trypsinization (0.25% trypsin with EDTA) and reseeding. All cell lines were confirmed pathogen-free by the manufacturer and were not used beyond passage 12. For infection experiments, cells were grown to confluency in 6-, 12-, 24-, and 96-well plates.
Scanning electron microscopy
BxPC3 cells were seeded on coverslips and allowed to adhere overnight. The samples were then infected with Fnn for 2 hours at 50:1 MOI (multiplicity of infection). Preparation for scanning electron microscopy was performed as previously described78. In brief, samples were fixed for 24 h with 4% paraformaldehyde and 1% glutaraldehyde in PBS, post-fixed for 45 min with 1% osmium tetroxide in distilled water, and subsequently dehydrated in serial dilutions of ethanol of 25%, 50%, 70%, 95%, 100%, and 100% before critical point drying the samples. Samples were then sputter-coated with iridium fast coating (5 nm) on a Leica EM ACE600 and imaged on LEO (Zeiss) 1550 Field Emission SEM.
Flow cytometry
Fnn, Fna, Fnp, Fnv, and Fnn Δfap2 were obtained and first stained with FM 1-43FX as detailed above to facilitate green fluorescence detection. The epithelial cells were then infected in serum-free and antibiotic-free media with the stained bacteria at 50:1 MOI for 1 hour and 4 hours. Galactose at 10mM was added to the wells before infection with Fnn for the Fap2- inhibition experiment. Cells were then washed twice with PBS, trypsinized, and collected for flow cytometry and cell sorting. The population of cells containing a mix of non-infected and infected cells was then loaded onto an S3e flow cytometer (Bio-Rad). 50,000 cells were analyzed using single-cell gates to measure the median green fluorescence induced by intracellular bacteria. FlowJo10 was then used to determine the median fluorescence of the samples and the data was transferred to GraphPad Prism for statistical analysis.
To visualize intracellular bacterial counts based on fluorescence intensity, the cells were sorted based on gates created within the infected cell population for low, medium, and high fluorescence. The sorted cells were collected in 100% FBS, transferred to growth media, and then plated on coverslips for imaging. After adherence, the cells were stained with MemBrite 568/580 (Biotium BTM30095) and NucBlue (ThermoFisher Scientific R37605) before being fixed with 10% formalin. The cells were imaged on a Zeiss LSM 800 using a 63X oil objective and z-stacks were obtained to recreate the 3D structure of the infected cells on Zen Blue.
Cell proliferation assays
Proliferation assays were performed using XTT Assay Kit (ATCC 30-1011K) and BrdU Cell Proliferation Assay Kit (K306, BioVision) based on the protocols provided. Briefly, epithelial cells were seeded on 96 well plates at optimized cell numbers (10,000 cells/well) and allowed to adhere for 12 hours in their respective cell culture media. The media was then replaced with the treatment (conditioned media or cell culture media with added cytokines 200 pg/mL, each supplemented with 0.5% FBS) or the cells were directly infected on the 96-well plate at 50:1 MOI for 4 hours. The cancer cells were further incubated for 48 hours, and the primary normal cells were incubated for 24 hours at 37°C, which was optimized to account for their doubling time. The recombinant cytokines that were used were Recombinant Human GM-CSF Protein (R&D Systems, 215GM), Recombinant Human CCL20/MIP-3α Protein (R&D Systems, 360-MP), IL-8 Monocyte Recombinant Human Protein (ThermoFisher, PHC0884), and Recombinant Human CXCL1/GROα Protein (R&D Systems, 275-GR).
For the XTT assay, following the 24- to 48-hour incubation with conditioned/cytokine media, 50μL of activated XTT reagent is added to each well and the resulting color change was monitored for 2 to 24 hours by measuring the absorbance at 475 nm and 660 nm. For the BrdU assay, 10X BrdU solution is added to the treated cells to a final concentration of 1X and incubated for 4 hours. The medium was then removed and the cells were fixed with the Fixing/Denaturing solution for 30 minutes. Next, BrdU Detection Antibody was added and incubated for 1 hour with shaking, followed by washing thrice with Wash Buffer. Anti-mouse HRP-linked Antibody Solution was added to the wells and it was incubated for 1 hour and washed thrice again with Wash Buffer. Finally, TMB substrate was added to the wells and the color change was monitored for 5-20 minutes and the final absorbance was measured at 450 nm.
Ki-67 staining was also used as a marker for proliferation. BxPC3 cells were seeded at 750,000 cells per well in 6-well plates and allowed to adhere before being infected with Fnn at MOI of 50:1 for 4 hours in serum-free RPMI. Cells were then washed with PBS and media was replaced with RPMI supplemented with 2.5% FBS. Cells grew for 24 hours before being gently trypsinized. Following manufacturer’s instructions, cells were washed twice with PBS, then fixed with ice-cold 70% ethanol in a drop-wise fashion while vortexing before being stored overnight at −20°C. Prior to staining, cells were washed thrice with cell staining buffer (BioLegend, 420201) and then resuspended in 100μL of buffer containing 2.5μL of PE/Dazzle™ 594 anti-human Ki-67 Antibody (BioLegend, 350534). Cells were incubated at room temperature for 1 hour and then washed twice with the buffer. Stained cells were loaded into a S3e Cell Sorter (Bio-Rad) and 20,000 cells were analyzed through single-cell gates to measure fluorescence of the bound Ki-67 antibody. Two populations were identified as Ki-67+ or Ki-67− based on fluorescence and a bisecting gate was drawn between the two populations and analyzed in FlowJo10.
Preparing conditioned media
The epithelial cells were grown to confluence in their respective media supplemented with 10% FBS and 1% penicillin-streptomycin in T-75 flasks or 12 well plates. One set of T-75 flasks/wells was then used for infection and another set was used as a control without infection. The flasks were washed with PBS twice and replaced with serum-free and PS-free media. For testing the impact of nPEC conditioned media on cancerous cells, serum-free and PS-free media of the respective cancer cell line was used. The flasks were then infected with Fnn, Fna, Fnp, or Fnv at 50:1 MOI for 4 hours. The media from both uninfected and infected cells was retrieved from the flasks and filtered through a 0.22 μm syringe filter (Millipore Sigma). For proliferation assays, conditioned media was used directly without concentration. For migration assays, the extracted media was concentrated to 16X, as determined from preliminary trials, using a 3000 MWCO Concentrator (Amplicon Millipore Sigma) by spinning the samples at 3000g for ~1.5 hours at 4°C. The samples were immediately used for the Transwell assays and ELISA.
Cell migration assays
Cell migration assays were performed on 8μm Transwells in 24-well plates (Corning CLS3422). Cells were first trypsinized (0.25% Trypsin in EDTA) and stained using CellTracker Red CMPTX (ThermoFisher C34552) for 45 minutes in serum-free media. Matrigel (Corning CB40234A) was diluted to a concentration of 250 μg/mL in serum-free media and 100μL was added to the upper chamber of each Transwell and incubated for 25 minutes at 37°C. The stained cells were resuspended to 2 million cells/mL in media containing 0.5% FBS and 100μL of the cell suspension was added on top of the Matrigel and incubated for 1 hour. The control and chemoattractant solutions were prepared in serum-free media or media with 0.5% FBS (100 ng/mL) and 600μL of the chemoattractant solution was added to the outer chamber of the Transwell. In the case of migration in response to conditioned media, 600 μL of concentrated conditioned media was added to the outer chamber of the Transwell. The Transwells were then incubated for the respective time points (12/16/24) hours at 37°C/5% CO2. The plate was collected and the cells in the upper chamber were removed using a cotton-tip applicator. The Transwells were transferred to a plate containing 10% formalin and fixed for 20 minutes. This was washed with PBS thrice and stained with DAPI (1:5000) (Sigma Aldrich D9542) in a permeabilization buffer (10 mL PBS + 0.5% Triton-X + 200 mg Bovine Serum Albumin) overnight at 4°C. Transwells were then washed thrice with PBS and stored at 4°C. The Transwells were imaged on a Zeiss LSM 800 confocal microscope using a 10X objective. The cells were counted using a custom-developed ImageJ protocol.
Cytokine arrays
Cytokine arrays were obtained using the Proteome Profiler Human XL Cytokine Array Kit (R&D Systems Inc. ARY022B) that screens for 105 cytokines. Briefly, cells were grown to confluence in 6 well plates. The cells were infected with Fnn, E. coli, and Fnn Δfap2 at 50:1 MOI for 4 hours at 37°C. Infection with E. coli TOP10 was used as a non-invasive infection control. The samples were first collected and filtered using a Spin-X column (Millipore Sigma) to remove bacteria within the sample. The membranes from the kit were first blocked using the Assay buffer for 1 hour and then incubated with the samples overnight at 4°C on a rocking platform shaker. Each membrane was then washed using the Wash buffer, and incubated with the Detection antibody cocktail for 1 hour. This was followed by washing thrice with Wash Buffer again and incubating with Streptavidin-HRP for 30 minutes. After a final washing procedure, Chemi-Reagent Mix was added to each membrane and it was covered for 1 minute. Finally, the membranes were transferred to an autoradiography film cassette and exposed to X-rays for 1-10 minutes.
Chemokine quantitation
ELISA was used to quantify cytokines IL-8, CXCL1, MIP-3α, and GM-CSF. The assays were performed using Duo Kits (R&D Systems DY208, DY275, DY360, and DY215, for the 4 cytokines respectively). Epithelial cells were grown to confluence on 12 well plates and infected with Fnn, E. coli, and Fnn Δfap2 at 50:1 MOI for 4 hours at 37°C. The ELISA plate was first coated with the capture antibody overnight and blocked the next day with the reagent diluent for 1 hour. Standards were prepared and conditioned media obtained from infected cells was filtered using a Spin-X column and then added to the wells, diluted, if necessary, with the reagent diluent. After a 2-hour incubation at room temperature, the plate was washed thrice with Wash buffer, and then incubated with the Detection Antibody for 2 hours at room temperature. The plate was again washed and incubated with Streptavidin-HRP for 30 minutes. Finally, the plate was washed and incubated with the Substrate solution for 20-30 minutes and the reaction was stopped by adding the Stop solution. The final absorbance was measured at 450 nm on a spectrophotometer.
Chemokine depletion assays
Conditioned and concentrated media was obtained as previously described. Human GM-CSF biotinylated antibody (R&D Systems, BAM215) was added to the medium to a final concentration of 90 ng/ml and incubated at room temperature with gentle shaking for 3 hours. Next, magnetic streptavidin particles (Sigma-Aldrich, 11641778001) were first washed twice with PBS and spun down at 1500 g and resuspended in the cell media, and 25 μL/mL was added to the sample media. This was incubated at room temperature for 1 hour. The samples were then spun down at 1500 g to pellet the heavier magnetic particles, and the supernatant was collected containing the conditioned medium with reduced cytokines. The medium was used for the proliferation assays and an ELISA was used to quantify and confirm the cytokine depletion.
3D spheroid proliferation assay
For the proliferation assay, 5000 cell BxPC3 spheroids were prepared in Corning Elplasia 24-well plates by seeding cells supplemented with 10% FBS and 5% methylcellulose. Methyl cellulose was prepared as described in79. Briefly, 10g of methyl cellulose (15cP, Sigma Aldrich M7027) is dissolved in 50mL of ultrapure water at 80°C and topped to 100mL with cold ultrapure water and stirred at 4°C. This 100mg/mL solution is then passed through a 0.45μm filter and stored at 4°C. The methylcellulose is then diluted in RPMI1640 supplemented with 10% FBS to form the spheroid formation medium which was again filtered through a 0.2 μm filter to ensure sterility. BxPC3 cells were collected from flasks after gentle trypsinization and resuspended in the spheroid formation medium before adding them to the plates. The plates were incubated at 37°C for 48 hours with media replaced every 24 hours. Once the spheroids had formed, the well was agitated by pipetting to release the spheroids from the wells to form a uniform suspension. The spheroids were collected in a microtube and the media was replaced with media containing 0.5% FBS. Infection with Fnn was performed (a) before spheroid formation for 4 hours at 50:1 MOI before infected cells were seeded in the plates or (b) after spheroid formation in the Elplasia plate at 50:1 MOI for 4 hours before collection. The spheroids in the microtube were uniformly resuspended with a large bore pipette and 50μL of the suspension was collected for each sample and replicates. The XTT assay was performed as detailed previously on these spheroids for 12 hours before the end points were measured.
3D spheroid migration assay
For the spheroid migration assay, 5000 cell BxPC3 spheroids were prepared in custom designed wells via the hanging drop method (fig. S7, A and B). The custom chamber was designed in Autodesk Inventor and 3D printed using PLA. It consists of a lower chamber to house the spheroid and a cap that functions as a cover as well as a reservoir for humidity control during the process of spheroid formation. Glass coverslips (#1.5) are attached to the chamber and the cap using PDMS (polydimethylsiloxane). PDMS was added to the chamber to a height of 7mm and 6mm diameter holes are punched out to form the wells on the coverslip. The chamber and cap were sterilized with 70% ethanol and UV-treated for 1 hour before use. Infected and uninfected cells extracted from T-75 flasks with gentle trypsinization were resuspended in spheroid formation medium at 1 million/mL concentration. 5μL droplets were then placed in each well of the chamber. The reservoir was filled with 2mL PBS to maintain humidity within the chamber and the chamber was inverted and placed on the cap. The chamber was incubated for 48 hours at 37°C. Once the spheroids formed, the chamber was removed from the reservoir and flipped, and the PBS in the reservoir was aspirated. The media surrounding the spheroid was carefully aspirated, and the 2.5mg/mL of collagen I solution was added to it to a height of 1 mm. The collagen was then crosslinked at 37°C for 20 minutes to embed the spheroid within the hydrogel. The hydrogels were prepared by mixing cold collagen stock (5 mg/mL) (50% of the final solution) with 10X DMEM (10% of final solution), 1X RPMI supplemented with 0.5% FBS (38% of the final solution), and 1N NaOH (2% of final solution). The embedded spheroids were imaged at 0 hours and 24 hours and the resulting migration spread was quantified using ImageJ.
Statistical analysis
Statistical analysis was performed on GraphPad Prism 9 using unpaired t-tests and ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test, Tukey's multiple comparisons test, or Šídák's multiple comparisons test based on the comparisons tested with P-value significance denoted by ns (not significant) for P>0.05, * for P≤0.05, ** for P≤0.01, *** for P≤0.001, and **** for P≤0.0001. All samples for analysis were collected in independent triplicates. Plots were created on GraphPad Prism 9, and figures were designed on Affinity Designer.
Supplementary Material
Acknowledgements:
We would like to acknowledge the Statistical Applications and Innovations Group (SAIG) at Virginia Tech for reviewing the statistical analysis presented. For SEM imaging experiments, we would like to acknowledge the Nanoscale Characterization and Fabrication Laboratory, which is supported by the Virginia Tech National Center for Earth and Environmental Nanotechnology Infrastructure (NanoEarth), a member of the National Nanotechnology Coordinated Infrastructure (NNCI), supported by NSF (ECCS 1542100 and ECCS 2025151). We would also like to acknowledge Biorender.com (Toronto) for figure editing tools.
Funding:
This project was supported by the NIH R21 Exploratory/Developmental Research Grant 1R21CA238630-01A1 (Verbridge, Slade), NSF Career Award CBET-1652112 (Verbridge), College of Engineering, and the Center for Engineered Health of the Institute for Critical Technologies and Applied Sciences at Virginia Tech, and also by the Biostatistics Shared Resource at the Wake Forest Comprehensive Cancer Center.
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Data and Materials Availability: The cytokine array data have been deposited to OSF, at the following link: osf.io/gd7vb. All other data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and Notes
- 1.Adamska A, Domenichini A & Falasca M Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci 18, 1338 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buscail L, Bournet B & Cordelier P Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol 17, 153–168 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Singh K et al. Kras mutation rate precisely orchestrates ductal derived pancreatic intraepithelial neoplasia and pancreatic cancer. Lab. Invest 101, 177–192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters AM & Der CJ KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med 8, a031435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho WJ, Jaffee EM & Zheng L The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat. Rev. Clin. Oncol (2020) doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Liao Q & Zhao Y Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int. 17, 68 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du W, Pasca di Magliano M & Zhang Y Therapeutic Potential of Targeting Stromal Crosstalk-Mediated Immune Suppression in Pancreatic Cancer. Front. Oncol 11, 2606 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemp SB, di Magliano MP & Crawford HC Myeloid Cell Mediated Immune Suppression in Pancreatic Cancer. Cell. Mol. Gastroenterol. Hepatol 12, 1531–1542 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W et al. Tumor microbiome contributes to an aggressive phenotype in the basal-like subtype of pancreatic cancer. Commun. Biol 4, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pushalkar S et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 8, 403–416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei M-Y et al. The microbiota and microbiome in pancreatic cancer: more influential than expected. Mol. Cancer 18, 97 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nejman D et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 368, 973–980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udayasuryan B, Nguyen TTD, Slade DJ & Verbridge SS Harnessing Tissue Engineering Tools to Interrogate Host-Microbiota Crosstalk in Cancer. iScience 101878 (2020) doi: 10.1016/j.isci.2020.101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xavier JB et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 6, 192–204 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas RM & Jobin C Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol 17, 53–64 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Mitsuhashi K et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 6, 7209–7220 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell JJ et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61, 582–588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otieno S et al. Positive association between Helicobacter pylori infection and pancreatic cancer: A case-control study. J. Clin. Oncol 39, e16243–e16243 (2021). [Google Scholar]
- 19.Riquelme E et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 178, 795–806.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geller LT et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan CA & Garrett WS Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol 17, 156–166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han YW et al. Fusobacterium nucleatum Induces Premature and Term Stillbirths in Pregnant Mice: Implication of Oral Bacteria in Preterm Birth. Infect. Immun 72, 2272–2279 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swidsinski A et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 60, 34–40 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Shammas NW et al. Infective endocarditis due to Fusobacterium nucleatum: case report and review of the literature. Clin. Cardiol 16, 72–75 (1993). [DOI] [PubMed] [Google Scholar]
- 25.Li Q et al. Oral Pathogen Fusobacterium nucleatum Coaggregates With Pseudomonas aeruginosa to Modulate the Inflammatory Cytotoxicity of Pulmonary Epithelial Cells. Front. Cell. Infect. Microbiol 11, 643913 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostic AD et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mima K et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamura K et al. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin. Cancer Res 22, 5574–5581 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Gaiser RA et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 68, 2186–2194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boehm ET et al. Fusobacterium nucleatum is associated with worse prognosis in Lauren’s diffuse type gastric cancer patients. Sci. Rep 10, 16240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abed J et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 20, 215–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abed J et al. Tumor Targeting by Fusobacterium nucleatum: A Pilot Study and Future Perspectives. Front. Cell. Infect. Microbiol 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parhi L et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun 11, 3259 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shamsuddin AM, Tyner GT & Yang GY Common expression of the tumor marker D-galactose-beta-[1-->3]-N-acetyl-D-galactosamine by different adenocarcinomas: evidence of field effect phenomenon. Cancer Res. 55, 149–152 (1995). [PubMed] [Google Scholar]
- 35.Rubinstein MR et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvucci M et al. Patients with mesenchymal tumours and high Fusobacteriales prevalence have worse prognosis in colorectal cancer (CRC). Gut (2021) doi: 10.1136/gutjnl-2021-325193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullman S et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casasanta MA et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal 13, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohi S et al. Alterations in the Duodenal Fluid Microbiome of Patients With Pancreatic Cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc 20, e196–e227 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S et al. Pancreatic cyst fluid harbors a unique microbiome. Microbiome 5, 147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye X et al. Fusobacterium Nucleatum Subspecies Animalis Influences Proinflammatory Cytokine Expression and Monocyte Activation in Human Colorectal Tumors. Cancer Prev. Res. Phila. Pa 10, 398–409 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Chung M et al. Comparisons of oral, intestinal, and pancreatic bacterial microbiomes in patients with pancreatic cancer and other gastrointestinal diseases. J. Oral Microbiol 13, 1887680 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Castillo E et al. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol 28, 370–383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alkharaan H et al. Circulating and Salivary Antibodies to Fusobacterium nucleatum Are Associated With Cystic Pancreatic Neoplasm Malignancy. Front. Immunol 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy KJ, Chambers CR, Herrmann D, Timpson P & Pereira BA Dynamic Stromal Alterations Influence Tumor-Stroma Crosstalk to Promote Pancreatic Cancer and Treatment Resistance. Cancers 13, 3481 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulle A & Lim K-H Beyond just a tight fortress: contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct. Target. Ther 5, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas D & Radhakrishnan P Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 18, 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deer EL et al. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas 39, 425–435 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Umaña A et al. Utilizing Whole Fusobacterium Genomes To Identify, Correct, and Characterize Potential Virulence Protein Families. J. Bacteriol 201, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han YW et al. Interactions between Periodontal Bacteria and Human Oral Epithelial Cells: Fusobacterium nucleatum Adheres to and Invades Epithelial Cells. Infect. Immun 68, 3140–3146 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gur C et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gur C et al. Fusobacterium nucleatum supresses anti-tumor immunity by activating CEACAM1. Oncoimmunology 8, e1581531 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coppenhagen-Glazer S et al. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect. Immun 83, 1104–1113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park J, Shokeen B, Haake SK & Lux R Characterization of Fusobacterium nucleatum ATCC 23726 adhesins involved in strain-specific attachment to Porphyromonas gingivalis. Int. J. Oral Sci 8, 138–144 (2016). [Google Scholar]
- 55.Berraondo P et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dranoff G Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 4, 11–22 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Yuan A, Chen JJW, Yao P-L & Yang P-C The role of interleukin-8 in cancer cells and microenvironment interaction. Front. Biosci. J. Virtual Libr 10, 853–865 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Acharyya S et al. A CXCL1 Paracrine Network Links Cancer Chemoresistance and Metastasis. Cell 150, 165–178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D, Sun H, Wei J, Cen B & DuBois RN CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer. Cancer Res. 77, 3655–3665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korbecki J, Grochans S, Gutowska I, Barczak K & Baranowska-Bosiacka I CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci 21, E7619 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghosh SK, Gupta S, Jiang B & Weinberg A Fusobacterium nucleatum and human beta-defensins modulate the release of antimicrobial chemokine CCL20/macrophage inflammatory protein 3α. Infect. Immun 79, 4578–4587 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong I-S Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp. Mol. Med 48, e242–e242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhan Y, Lew AM & Chopin M The Pleiotropic Effects of the GM-CSF Rheostat on Myeloid Cell Differentiation and Function: More Than a Numbers Game. Front. Immunol 10, 2679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thorn M et al. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther. 23, 188–198 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Yako YY, Kruger D, Smith M & Brand M Cytokines as Biomarkers of Pancreatic Ductal Adenocarcinoma: A Systematic Review. PLOS ONE 11, e0154016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G & Bar-Sagi D Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21, 836–847 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayne LJ et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21, 822–835 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waghray M et al. GM-CSF Mediates Mesenchymal-Epithelial Cross-talk in Pancreatic Cancer. Cancer Discov. 6, 886–899 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeuchi S et al. Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer. Cancer Res. 75, 2629–2640 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Campbell AS, Albo D, Kimsey TF, White SL & Wang TN Macrophage inflammatory protein-3alpha promotes pancreatic cancer cell invasion. J. Surg. Res 123, 96–101 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Takamori H, Oades ZG, Hoch OC, Burger M & Schraufstatter IU Autocrine growth effect of IL-8 and GROalpha on a human pancreatic cancer cell line, Capan-1. Pancreas 21, 52–56 (2000). [DOI] [PubMed] [Google Scholar]
- 72.Sepich-Poore GD et al. The microbiome and human cancer. Science 371, eabc4552 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liot S et al. Stroma Involvement in Pancreatic Ductal Adenocarcinoma: An Overview Focusing on Extracellular Matrix Proteins. Front. Immunol 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang B et al. Stroma-Targeting Therapy in Pancreatic Cancer: One Coin With Two Sides? Front. Oncol 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Datta R et al. Interactions with stromal cells promote a more oxidized cancer cell redox state in pancreatic tumors. Sci. Adv 8, eabg6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sherman MH et al. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc. Natl. Acad. Sci. U. S. A 114, 1129–1134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li R, Wen A & Lin J Pro-Inflammatory Cytokines in the Formation of the Pre-Metastatic Niche. Cancers 12, 3752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shurer CR et al. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 177, 1757–1770.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maritan SM, Lian EY & Mulligan LM An Efficient and Flexible Cell Aggregation Method for 3D Spheroid Production. J. Vis. Exp. JoVE (2017) doi: 10.3791/55544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.