Abstract
Hypertension is the most important risk factor for the development of terminal cardiovascular diseases, such as heart failure, chronic kidney disease, and atherosclerosis. Lifestyle interventions to lower blood pressure are generally desirable prior to initiating pharmaceutical drug treatments, which may have undesirable side effects. Ketogenic interventions are popular but the scientific literature supporting their efficacy is specific to certain interventions and outcomes in animal models and patient populations. For example, although caloric restriction has its own inherent difficulties (e.g. it requires high levels of motivation and adherence is difficult), it has unequivocally been associated with lowering blood pressure in hypertensive patients. On the other hand, the antihypertensive efficacy of ketogenic diets is inconclusive, and this is surprising, given that these diets have been largely helpful in mitigating metabolic syndrome and promoting longevity. It is possible that side effects associated with ketogenic diets (e.g. dyslipidemia) aggravate the hypertensive phenotype. However, given the recent data from our group, and others, reporting that the most abundant ketone body, β-hydroxybutyrate, can have positive effects on endothelial and vascular health, there is hope that ketone bodies can be harnessed as a therapeutic strategy to combat hypertension. Therefore, we conclude this review with a summary of the type and efficacy of ketone supplements. We propose that ketone supplements warrant investigation as low-dose antihypertensive therapy that decreases total peripheral resistance with minimal adverse side effects.
Keywords: 1,3-butanediol; caloric restriction; high blood pressure; ketogenesis; ketogenic diets; ketone supplements
Graphical Abstract:
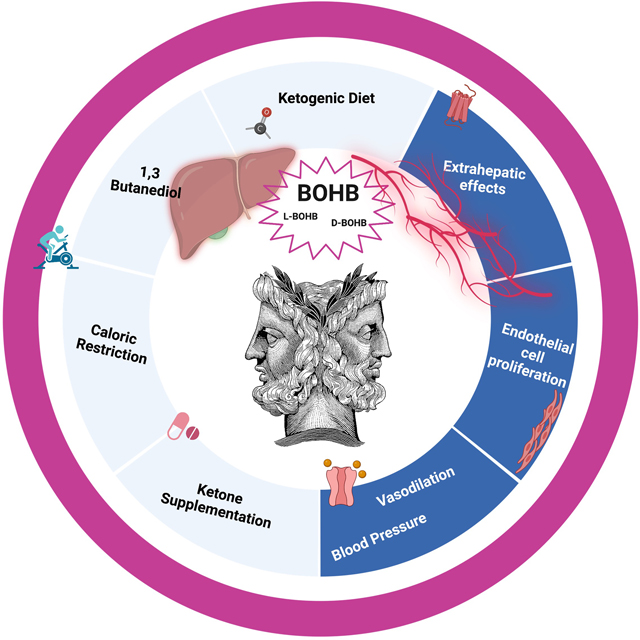
INTRODUCTION
According to the updated Guidelines for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults by the American College of Cardiology and American Heart Association (2017), 130/80 mmHg is considered high blood pressure, rather than 140/90 mmHg, as previously accepted [1,2]. As a result, approximately half of the United States adult population meets this criterion for hypertension [3]. These guidelines recognize that blood pressures previously classified as ‘prehypertensive’ correlate with pathophysiological changes in the cardiovascular system and increase a patient’s risk for other terminal cardiovascular diseases and even death.
Lifestyle interventions to lower blood pressure are generally desirable before initiating pharmaceutical drug treatments, which may have undesirable side effects and can lower one’s quality of life. Ketogenic interventions, such as extreme caloric restriction and diets constituted of high-fat and low-carbohydrate consumption, are popular. However, long-term adherence to, and sustainability of, these interventions is difficult [4], and the scientific support surrounding ketogenic diets is questionable, particularly in hypertension literature. In the present review, we will describe the biosynthetic pathway of ketone bodies and their signaling capabilities beyond their classical function as an energy source. Subsequently, we summarize the literature supporting and opposing ketogenic interventions in hypertension. Finally, we will propose that ketone supplements could be a titrated strategy to harness the positive effects of ketone bodies, while avoiding the difficulty of adherence to ketogenic inventions and some of the negative effects that have been associated with ketogenic diets (e.g. dyslipidemia).
KETONE BODIES AND KETOGENESIS
Ketone bodies are derived from small lipid molecules and predominantly generated in the liver from fatty acid oxidation-derived acetyl-coenzyme A (CoA) (Fig. 1). In the absence of carbohydrates, ketone bodies become a primary energy source and they are produced through an intricate web of vital metabolic pathways, such as the tricarboxylic acid cycle (Krebs cycle), gluconeogenesis, and de novo lipogenesis biosynthesis of sterols [5]. Ketone bodies, including acetone, acetoacetate (AcAc), and the most abundant circulating ketone body β-hydroxybutyrate (βOHB) are predominantly biosynthesized in the liver to be transported to the peripheral tissues for conversion into energy [6] (Fig. 1 and Table 1). Although the liver is the primary organ that produces ketone bodies, the liver does not use ketone bodies as it lacks the necessary enzyme ketoacyl-CoA transferase (SCOT)1 [5].
FIGURE 1.
The biochemistry of ketogenesis and ketone utilization. Although ketone bodies are predominantly produced in the liver, their biosynthesis can be divided into hepatic and extrahepatic metabolism. Fatty acids are brought into the mitochondria via carnitine CPT-1 and broken down into acetyl CoA via beta-oxidation. Two acetyl-CoA molecules are converted into acetoacetyl-CoA via the enzyme ACAT. Afterward, acetoacetyl-CoA is converted to HMG-CoA via the enzyme HMG-CoA synthase 2. HMG-CoA lyase then converts HMG-CoA to AcAc. The AcAc can be converted to either acetone through nonenzymatic decarboxylation or to βOHB via BDH. Once they reach extrahepatic tissues, βOHB is converted to AcAc via the enzyme BDH, and AcAc is converted back to acetoacetyl-CoA via the enzyme SCOT1, and acetoacetyl-CoA is converted into two acetyl-CoA molecules via ACAT. Acetyl-CoA goes through the citric acid cycle, and after oxidative phosphorylation, produces ATP. AcAc, acetoacetate; ACAT, acetyl-coenzyme A acetyltransferases; βOHB, β-hydroxybutyrate; BDH, βOHB-dehydrogenase; CPT-1, carnitine palmitoyltransferase; HMGCL, HMG-CoA lyase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; HMGCS2, 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase 2; NADH, nicotinamide adenine dinucleotide; SCOT1, succinyl-CoA:3-ketoacid CoA transferase 1. Figure created by Biorender.com under license.
TABLE 1.
Names and structures of commonly referred ketone body molecules
| Molecule Name | Molecular Line Structure | Molecular 3D Structure |
|---|---|---|
|
| ||
| Acetone |
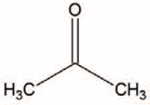
|

|
| Acetoacetate |

|

|
| β-hydroxybutyrate |
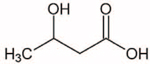
|
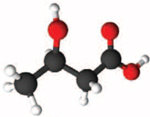
|
| 1,3-Butanediol |

|

|
| βOHB Salt* |

|

|
| βOHB monoester (D-β-hydroxybutyl D-β-hydroxybutyrate) |
|

|
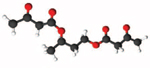
| Acetoacetate Diester (D,L- Butanediol Diacetoacetate) |
|
|
| Bis-hexanoyl- 1,3-butanediol |
|
|
Ketone bodies are water-soluble lipid molecules of two R-groups attached to a carbonyl group (C = O) that contribute to extrahepatic transport. In physiological conditions, serum levels of βOHB are in the low micromolar range but begin to rise to a few hundred micromoles after 12–16 h of fasting, reaching 1–2 mmol/l after 2 days of fasting [7,8], 6–8 mmol/l with prolonged starvation [9], and 1–2 mmol/l after 90 min of intense exercise [10].
Hepatic ketogenesis proceeds through mitochondrial β-oxidation of free fatty acids, which generates acetyl-CoA. Fatty acids are brought into the mitochondria via carnitine palmitoyl transferase (CPT-1) and subsequently broken into two molecules of acetyl-CoA via beta-oxidation. The acetyl-CoA molecules are converted into acetoacetyl-CoA via thiolase, a ubiquitous enzyme family that has important roles in many vital biochemical pathways, including β-oxidation. Thiolases, including acetyl-CoA acetyltransferases (ACAT), convert two molecules of acetyl-CoA into acetoacetyl-CoA by the mevalonate pathway. Next, two hormone- and nutrient-responsive pathways are responsible for converting and regulating transcription of the rate-limiting enzyme, mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2), which condenses acetyl-CoA with CoA-SH to generates the 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). The HMG-CoA lyase then cleaves acetyl-CoA from HMG-CoA, resulting in the formation of AcAc. AcAc can then either be converted to acetone through nonenzymatic decarboxylation, or to βOHB via βOHB-dehydrogenase (BDH) (Fig. 1).
Acetone cannot be further metabolized to produce ATP and is generally believed to be excreted through the respiratory system. However, both AcAc and βOHB are exported into the blood for energy in extrahepatic tissues. The oxidation of AcAc produces 23 mol of ATP and oxidation βOHB produces 26 mol of ATP. βOHB is transported to the blood by the monocarboxylate transporter SLC16A6, and into the extrahepatic target, across the plasmatic membrane by specific monocarboxylate transporters [6].
Once in extrahepatic organs, βOHB is oxidized to AcAc by catalysis via BDH. Succinyl-CoA then donates its CoA to AcAc, through the action of SCOT1, forming acetoacetyl-CoA. Finally, acetyl-CoA acetyltransferase1 (ACAT1) cleaves acetoacetyl-CoA into two acetyl-CoAs, which can then be used as substrate to supply the tricarboxylic acid cycle for ATP synthesis. Interestingly, SCOT1 is expressed in embryonic livers but not adult livers. This has been suggested as the major reason for the absence of ketone body consumption in adult livers [11].
KETONE BODIES AS SIGNALING METABOLITES
In addition to the ability to provide energy, ketone bodies can promote different signaling responses [12]. Accordingly, several studies have demonstrated ketone’s ability to bind to different receptors and even modify ion channel function. The most well characterized ketone body in this regard is βOHB. βOHB can activate Gpr109a (also known as hydroxycarboxylic acid receptor 2, niacin receptor 1, or PUMA-G) a high-affinity Gi/o-GPCR [13]. βOHB is also generally recognized as an antagonist of the Gpr41 (also known as free fatty acid receptor 3), another Gi/o-GPCR [14]. However, there are conflicting reports on whether βOHB functions as an antagonist or agonist for Gpr41 [15]. More recently, AcAc has also been revealed as an endogenous agonist for Gpr43 (also known as free fatty acid receptor 2) [16]. Thus, ketone bodies can act as both agonist and antagonist of different G-protein coupled receptors (GPCRs), and their pharmacological role may be tissue-dependent.
In addition to its ability to bind receptors, βOHB causes voltage-gated M-type potassium channel (KCNQ2/3) activation [17]. Potassium ion (K+) efflux is extremely important for maintaining cell membrane potential, and disturbances of K+ homeostasis may lead to hyperexcitability of the cells, which is related to pathological conditions. Interestingly, βOHB increases the KCNQ2/3 current, which causes hyperpolarization of the membrane potential and prevented hyperexcitability in neurons [17]. This ability of βOHB to alter membrane potential and cell excitation has been studied in conditions, such as epilepsy and alcohol withdrawal crises, and most often involves the participation of K+ channels [18]. The protective role of βOHB and K+ has also been evaluated in other tissues, such as heart, lymphatic vessels, resistance arteries, liver, and kidney [19–23]. Overall, these studies support the concept that ketone bodies, mainly βOHB and AcAc, are not only energy sources but also have important signaling properties via GPCRs and ion channels.
KETOGENESIS AND KETOGENIC INTERVENTIONS IN HYPERTENSION
Interventions that promote ketogenesis, such as caloric restriction, have long been associated with increased longevity and therapeutic potential [24]. However, whether ketone bodies are responsible for these positive effects are less clear [25]. Additionally, the efficacy of ketogenic diets is conflicting. For example, a ketogenic diet can increase life span in mice, even compared with mice that consume a relatively low carbohydrate diet [26]. Furthermore, it has been reported that ketogenic diets are beneficial for weight loss, improving insulin sensitivity, and the management of diabetes [27,28]. However, the scientific support for ketogenic diets as an antihypertensive therapy is debatable [29–31]. Therefore, whether ketogenic diets are helpful or harmful may be specific to the underlying disease condition.
In the context of hypertension, it was recently observed that βOHB biosynthesis is reduced after a 24-h fast in rats with salt-sensitive hypertension, as well as normotensive rats chronically fed a high-salt diet [22,32]. Although reconstitution of βOHB bioavailability with the secondary alcohol, 1,3-butanediol, was associated with reduced SBP and DBP, lowered kidney damage, and the amelioration of vascular dysfunction, it is not clear whether this is because of βOHB, or direct protective actions of 1,3-butanediol per se (e.g. vasodilation) [33,34] Also, it should be noted that 1,3-butanediol (at a concentration that is sufficient to raise circulating βOHB) was associated with a host of undesirable side effects including, stunted growth and cachexia, hepatotoxicity, metabolic acidosis, dehydration, and hepatic sinusoidal dilation [22,35]. These negative phenotypes are corroborated by rodent studies using ketogenic chow diets that have observed exacerbated blood pressure, endothelial dysfunction, renal damage, and cardiac hypertrophy in spontaneously hypertensive rats [29–31]. In humans, while the data are not so damaging, there is a lack of conclusive evidence on the potential beneficial effect of ketogenic diets on blood pressure [25,36]. These studies are in direct contrast with an abundance of literature demonstrating that caloric restriction is an effective antihypertensive therapy [37]. It is possible that side effects associated with ketogenic diets (e.g. nonalcoholic fatty liver, insulin resistance, hyperglycemia, and/or dyslipidemia) may aggravate other underlying conditions in hypertensive patients and animals that negate the positive effects of ketogenesis on blood pressure [36].
In summary, βOHB does appear to have therapeutic potential as an antihypertensive therapy because of its vasodilatory properties [22] and its propensity to promote endothelial cell proliferation [23]. However, the literature supporting the efficacy of ketogenic diets is not conclusive and ketogenic diets have even produced deleterious side effects in some instances. Therefore, therapies that harness the vasodilatory effects of βOHB should be the focus of ketogenic interventions in the hypertension field.
KETONE SUPPLEMENTS
As noted above, the ketone bodies, βOHB and AcAc are produced endogenously primarily by the liver under conditions of extreme caloric restriction, very-low carbohydrate intake (i.e. ketogenic diets), and prolonged glycogen-depleting exercise [7,38]. Although there are obvious drawbacks of living under a state of starvation, many individuals are also unable, or unwilling, to consume very low carbohydrate diets [39] or routinely engage in prolonged exercise [40]. Historically, outside of the research setting, the only way to achieve ketosis was through these arduous methods. Within the research setting, until recently, most studies seeking to examine the direct effects of ketosis on metabolic control in human and animal models used ketone infusion methods [41–44]. Thus, the hopes of extending the potential benefits of ketosis to a wider range of applications in the research, clinical, and athletics settings has spurred the development of ingestible ketone supplements in the form of ketone precursors (e.g. R-1,3-butanediol), ketone salts (e.g. R-3-hydroxybutyric acid sodium salt), and ketone esters (e.g. ketone monoester drinks) [45]. An overview of these supplements and their application to vascular and cardiometabolic health is presented in this section. The structures of the endogenous ketone bodies and the exogenous ketone supplements are presented in Table 1.
Types of ketone supplements
The various formulations of ketone supplements have distinct ketogenic properties pertaining to the magnitude and duration of ketosis they induce [46–48]. Importantly, most commercially available oral ketone supplements are a racemic mixture (i.e. equal amounts of left-handed and right-handed enantiomers of a chiral molecule) of d-βOHB and l-βOHB enantiomers of βOHB (e.g. many ketone salts) or their respective precursors (e.g. 1,3-butanediol). Exceptions include the R-1,3-Butanediol d-βOHB monoester and some other specific 1,3-butanediol and ketone salt products. Presumably the distinct effects that ketone supplements have on the magnitude and duration of ketosis lead to unique metabolic and signaling effects. The variety of ketone supplements available and their unique signaling effects necessitate the need for continued research to promote a clearer understanding of the utility for the individual ketone supplements regarding their therapeutic potentials [45,49].
Medium chain triglycerides
Many consider medium chain triglycerides (MCTs) to be a ketogenic aid. MCTs are composed of fatty acids that are 6–12 carbons in length. Compared with long-chain fatty acids, which are absorbed via the lymphatic system, the 6–12 carbon fatty acids can be absorbed via hepatic portal circulation and enter the hepatic mitochondria without enzymatic conversions via CPT-1. Thus, the MCT-derived fatty acids are rapidly metabolized to acetyl-CoA, and subsequently to ketones. Therefore, MCTs are considered ketogenic fats as they result in ketogenesis without requiring carbohydrate restriction [45]. However, original studies in humans have demonstrated that MCTs have limited efficacy in substantially elevating βOHB in healthy adults [50].
1,3-Butanediol
1,3-Butanediol is a widely available nontoxic secondary alcohol [35,45]. Importantly, 1,3-butanediol is Food and Drug Administration (FDA)-approved and has been studied as a ketone-producing compound for nearly 50 years. Following ingestion, 1,3-butanediol is passively absorbed in the gut and increases blood 1,3-butanediol, and subsequently undergoes hepatic conversion to the isotopic enantiomer of βOHB [51]. Thus, the nonracemic form of 1,3-butanediol is metabolized into the d-isoform of βOHB. 1,3-Butanediol is commonly used as a backbone in the synthesis of the commonly used d-1,3-butanediol d-βOHB ketone ester (Table 1) [45] and the novel bis-hexanoyl-d-1,3-butanediol ketogenic ester (Table 1) [52].
Ketone salts
βOHB and AcAc in their free acid forms are not stable, and thus, ineffective at producing sustained ketosis [45]. Therefore, the ketone bodies need to be buffered with sodium, potassium, or other electrolytes to enhance the efficacy of ketone delivery. There are some combination ketone salts containing multiple electrolytes to prevent overload of any single ion (e.g. concerns of ingesting excess sodium). In theory, balanced mineral formulations of ketone salts may be useful in attenuating symptoms of mineral depletion that occur early in response to ketogenic diets. However, at large doses, excessive salt intake from ketone salt supplements can lead to gastric hyperosmolarity along with potential gastrointestinal distress [45]. Commonly available ketone salts are also typically racemic and less effective than ketone esters at raising the d-isoform of βOHB [46].
Ketone monoesters
Ketone monoester supplementation provides a well tolerated method to rapidly inducing ketosis, irrespective of diet composition [52]. Indeed, many of the studies using ketone monoesters have been able to achieve βOHB blood concentration of greater than 3 mmol/l [45]. Importantly, raising βOHB to this high of a concentration is comparable to extreme fasted or carbohydrate restricted states while also remaining under the clinically dangerous threshold for developing ketoacidosis [45]. Once ingested, nonracemic ketone monoester drinks are metabolized into the d-isoform of βOHB, which is the isoform produced by endogenous ketogenesis [45].
Exogenous ketones and vascular health in humans
Although there is exciting preclinical data demonstrating that ketogenesis can improve vascular function [22], there is very limited data on ketone supplements and vascular health in humans. For example, 6 weeks of ketone salt supplementation modestly reduced blood pressure in young adults [53]. Another study demonstrated that 2 weeks of ketone ester supplementation improves peripheral vascular function in adults with obesity, assessed via brachial artery flow-mediated dilation [54]. In the same cohort, ketone ester supplementation improved cerebrovascular function [55]. Therefore, there is a knowledge gap in the literature pertaining to how ketone supplements affect arterial stiffness, peripheral and central vascular function, and blood pressure in human participants, particularly in at-risk populations, such as patients with hypertension and older adults. Additionally, there has been excitement surrounding ketones and kidney health [56]. Nonetheless, there is also a need for additional human data regarding ketone supplements and renal blood flow and kidney health in the context of hypertension.
Exogenous ketones and cardiometabolic health in humans
Although there are limited data regarding exogenous ketone supplements and vascular health, there has been greater interest pertaining to exogenous ketones and metabolic health, and it is well established that metabolic derangement (e.g. insulin resistance, impaired glucose tolerance, hyperlipidemia) contributes to cardiovascular dysfunction [57], and hypertension [58]. For example, recent studies demonstrate that a ketone monoester drink effectively improves glucose clearance in healthy young adults [59], and in participants with obesity [60]. Regarding longer term studies, two 1-month studies in which healthy participants consumed ketone esters daily found that despite regular elevations in βOHB, the ketone esters had no effect on body weight or body composition, fasting blood glucose, cholesterol, or triglyceride concentrations [52]. In a similar study design, patients with type 2 diabetes experienced modest improvements in markers of glycemic control, including mean blood glucose and HbA1c [61].
One of the mechanisms through which ketones could improve glycemic control includes promoting skeletal muscle health. Skeletal muscle is a major tissue for mediating whole body glucose homeostasis as it accounts for the majority of insulin-stimulated glucose disposal [61]. Therefore, interventions, such as resistance training and therapies that aid in preventing loss of muscle mass could have important implications for preserving cardiometabolic health. To that end, multiple human studies have demonstrated that βOHB may support retention of muscle mass. Specific examples include that βOHB infusion augments muscle protein synthesis in part by reducing leucine oxidation [44], and also attenuates muscle protein catabolism during stimulated acute inflammation with lipopolysaccharide [43], which is well known to elicit catabolic cascades [62].
In addition to skeletal muscle health, energy balance and appetite also play important roles in cardiometabolic health and may be influenced by ketones [63,64]. For example, ingestion of a ketone monoester acutely reduces ghrelin (a gut hormone that stimulates the desire to eat), self-reported hunger, and enhances satiation [65]. Despite these promising findings acutely, longer term studies of ketone supplementation have reported no change in bodyweight [52], and therefore, the limited data available suggest that long-term ketone supplementation may not have an appreciable effect on caloric intake.
In summary, the data on ketone supplementation and cardiometabolic health suggest that ketone supplementation may have utility in improving glycemic control in patients with metabolic derangement but to a lesser extent in healthy adults. Additional studies with oral ketone supplements are also needed to determine whether exogenous ketones can help attenuate muscle wasting over time and/or augment muscle strength and hypertrophy in response to resistance training in at-risk populations. There is also enthusiasm for the use of ketones during exercise beyond the potential anticatabolic effects discussed, thus far.
Ketone supplementation in the context of exercise
Ketone bodies are oxidized as a fuel source during exercise and are markedly elevated during the postexercise recovery period [10,66]. The ability to metabolize ketones varies considerably across muscle types (e.g. type I vs. type II fibers) [67], and ketone body utilization is higher in exercise-trained skeletal muscle, suggesting that regular exercise permits more efficient use of ketones as substrate [68]. Collectively, these observations contributed to enthusiasm surrounding research into the use of exogenous ketone supplements as ergogenic aids. Regarding cardiovascular health, if ketone supplements were to promote greater exercise tolerance (i.e. greater ability to tolerate higher exercise volume or intensity), this could have important implications for beneficial training adaptations in at-risk populations. However, multiple studies have reported that oral 1,3-butanediol [47,48], ketone salts [69], or ketone esters [70,71] do not improve endurance exercise performance. Nonetheless, these studies were primarily conducted in healthy, young and/or middle-aged male individuals, and additional data are needed in female and less fit populations, such as those with cardiovascular risk factors and comorbidities.
CONCLUSION AND FUTURE PERSPECTIVES
Evolutionarily, ketone bodies are an important energy source during nutrient scarcity. The most well characterized ketone body is βOHB because of its abundant bioavailability. In addition to serving as an energy source, βOHB has been revealed to be an important signaling molecule in a range of extrahepatic tissues by acting on different receptors and channels, including Gpr109a, Gpr41, and potassium channels. Although βOHB biosynthesis is diminished in animal models of hypertension [22,32], βOHB does have potential as a novel antihypertensive therapy because of its ability to promote endothelial-dependent vasodilation [22] and proliferation [23]. However, the evidence for the blood pressure-lowering effects of ketogenic diets is not conclusive and may actually be contraindicated in hypertensive patients because of adverse side effects (e.g. dyslipidemia). Therefore, we suggest the utilization of ketone supplements as a novel means to tightly raise the concentration of ketone bodies such that the endothelial effects are promoted and the negative effects of high concentrations of ketones on systemic and liver physiology are subdued (Fig, 2). Nonetheless, the type of ketone supplement, dosage, and route of administration all need to be optimized for translational application.
FIGURE 2.
Low-dose ketone supplementation could be a novel antihypertensive therapy by promoting endothelium-dependent vasodilation and proliferation. Exogenous supplementation is a means of bypassing endogenous ketogenesis, as interventions such as caloric restriction, ketogenic diets, and 1,3-butanediol all have concerns regarding their long-term utilization and/or efficacy at lowering blood pressure. Figure created by Biorender.com under license.
ACKNOWLEDGEMENTS
Sources of funding: this work was supported by grants from the National Institutes of Health (R00GM118885, R01HL149762, K01HL147998, R00HL151889, and P20GM103641-Pilot Project).
Abbreviations:
- βOHB
β-hydroxybutyrate
- AcAc
acetoacetate
- ACAT
acetyl-CoA acetyltransferases
- ACAT1
acetyl-CoA acetyltransferase 1
- BDH
βOHB-dehydrogenase
- CoA
coenzyme A
- CPT-1
carnitine palmitoyl transferase
- GPCR
G-protein coupled receptor
- HMGCL
HMG-CoA lyase
- HMG-CoA
3-hydroxy-3-methylglutaryl-CoA
- HMGCS2
3-hydroxy-3-methylglutaryl-CoA synthase 2
- MCTs
medium chain triglycerides
- NADH
nicotinamide adenine dinucleotide
- OXCT1
3-oxoacid CoA-transferase 1
- SCOT1
succinyl-CoA:3-ketoacid CoA transferase 1
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71:2199–2269.29146533 [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics—2022 update: a report from the American Heart Association. Circulation 2022; 145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 4.Phelan JP, Rose MR. Why dietary restriction substantially increases longevity in animal models but won’t in humans. Ageing Res Rev 2005; 4:339–350. [DOI] [PubMed] [Google Scholar]
- 5.Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol 2013; 304:H1060–H1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2014; 25:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 1980; 60:143–187. [DOI] [PubMed] [Google Scholar]
- 8.Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr 2006; 26:1–22. [DOI] [PubMed] [Google Scholar]
- 9.Cahill GF, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, et al. Hormone-fuel interrelationships during fasting. J Clin Invest 1966; 45:1751–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol 2017; 595:2857–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids 2004; 70:243–251. [DOI] [PubMed] [Google Scholar]
- 12.Puchalska P, Crawford PA. Metabolic and signaling roles of ketone bodies in health and disease. Annu Rev Nutr 2021; 41:49–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 2005; 280:26649–26652. [DOI] [PubMed] [Google Scholar]
- 14.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A 2011; 108:8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Won YJ, Lu VB, Puhl HL, Ikeda SR. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J Neurosci 2013; 33:19314–19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto J, Ohue-Kitano R, Mukouyama H, Nishida A, Watanabe K, Igarashi M, et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc Natl Acad Sci U S A 2019; 116:23813–23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manville RW, Papanikolaou M, Abbott GW. M-channel activation contributes to the anticonvulsant action of the ketone body. J Pharmacol Exp Ther 2020; 372:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J Neurosci 2007; 27:3618–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, et al. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 2016; 7:66444–66454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Caballero M, Zecchin A, Souffreau J, Truong AK, Teuwen LA, Vermaelen W, et al. Role and therapeutic potential of dietary ketone bodies in lymph vessel growth. Nat Metab 2019; 1:666–675. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy CG, Chakraborty S, Singh G, Yeoh BS, Schreckenberger ZJ, Singh A, et al. Ketone body β-hydroxybutyrate is an autophagy-dependent vasodilator. JCI Insight 2021; 6:e149037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weis EM, Puchalska P, Nelson AB, Taylor J, Moll I, Hasan SS, et al. Ketone body oxidation increases cardiac endothelial cell proliferation. EMBO Mol Med 2022; 14:e14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, Anson RM, et al. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab 2019; 29:221.e3–228.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Raimondo D, Buscemi S, Musiari G, Rizzo G, Pirera E, Corleo D, et al. Ketogenic diet, physical activity, and hypertension-a narrative review. Nutrients 2021; 13:2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 2017; 26:539.e5–546,e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 2015; 31:1–13. [DOI] [PubMed] [Google Scholar]
- 28.Kossoff EH, Hartman AL. Ketogenic diets: new advances for metabolism-based therapies. Curr Opin Neurol 2012; 25:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia P, Huang B, You Y, Su H, Gao L. Ketogenic diet aggravates kidney dysfunction by exacerbating metabolic disorders and inhibiting autophagy in spontaneously hypertensive rats. Biochem Biophys Res Commun 2021; 573:13–18. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Wang X, Jia P, You Y, Cheng Y, Deng H, et al. Ketogenic diet aggravates hypertension via NF-(B-mediated endothelial dysfunction in spontaneously hypertensive rats. Life Sci 2020; 258:118124. [DOI] [PubMed] [Google Scholar]
- 31.You Y, Guo Y, Jia P, Zhuang B, Cheng Y, Deng H, et al. Ketogenic diet aggravates cardiac remodeling in adult spontaneously hypertensive rats. Nutr Metab (Lond) 2020; 17:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakraborty S, Galla S, Cheng X, Yeo JY, Mell B, Singh V, et al. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep 2018; 25:677.e4–689.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy CG, Waigi EW, Yeoh BS, Mell B, Vijay-Kumar M, Wenceslau CF, Joe B. Low-dose 1,3-butanediol reverses age-associated vascular dysfunction independent of ketone body β-hydroxybutyrate. Am J Physiol Heart Circ Physiol 2022; 322:H466–H473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilon D, Brodeur J, Plaa GL. 1,3-Butanediol-induced increases in ketone bodies and potentiation of CCl4 hepatotoxicity. Toxicology 1986; 40:165–180. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy CG, Waigi EW, Singh G, Castaneda TR, Mell B, Chakraborty S, et al. Physiologic, metabolic, and toxicologic profile of 1,3-butanediol. J Pharmacol Exp Ther 2021; 379:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosinski C, Jornayvaz FR. Effects of ketogenic diets on cardiovascular risk factors: evidence from animal and human studies. Nutrients 2017; 9:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Daniele N, Marrone G, Di Lauro M, Di Daniele F, Palazzetti D, Guerriero C, et al. Effects of caloric restriction diet on arterial hypertension and endothelial dysfunction. Nutrients 2021; 13:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, et al. Medical aspects of ketone body metabolism. Clin Invest Med 1995; 18:193–216. [PubMed] [Google Scholar]
- 39.Alhassan S, Kim S, Bersamin A, King AC, Gardner CD. Dietary adherence and weight loss success among overweight women: results from the A TO Z weight loss study. Int J Obes (Lond) 2008; 32:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennie JA, De Cocker K, Teychenne MJ, Brown WJ, Biddle SJH. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 U.S. adults. Int J Behav Nutr Phys Act 2019; 16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balasse E, Ooms HA. Changes in the concentrations of glucose, free fatty acids, insulin and ketone bodies in the blood during sodium beta-hydroxybutyrate infusions in man. Diabetologia 1968; 4:133–135. [DOI] [PubMed] [Google Scholar]
- 42.Miles JM, Haymond MW, Gerich JE. Suppression of glucose production and stimulation of insulin secretion by physiological concentrations of ketone bodies in man. J Clin Endocrinol Metab 1981; 52:34–37. [DOI] [PubMed] [Google Scholar]
- 43.Thomsen HH, Rittig N, Johannsen M, Møller AB, Jørgensen JO, Jessen N, et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr 2018; 108:857–867. [DOI] [PubMed] [Google Scholar]
- 44.Nair KS, Welle SL, Halliday D, Campbell RG. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J Clin Invest 1988; 82:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poff AM, Koutnik AP, Egan B. Nutritional ketosis with ketogenic diets or exogenous ketones: features, convergence, and divergence. Curr Sports Med Rep 2020; 19:251–259. [DOI] [PubMed] [Google Scholar]
- 46.Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, et al. On the metabolism of exogenous ketones in humans. Front Physiol 2017; 8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott BE, Laursen PB, James LJ, Boxer B, Chandler Z, Lam E, et al. The effect of 1,3-butanediol and carbohydrate supplementation on running performance. J Sci Med Sport 2019; 22:702–706. [DOI] [PubMed] [Google Scholar]
- 48.Shaw DM, Merien F, Braakhuis A, Plews D, Laursen P, Dulson DK. The effect of 1,3-butanediol on cycling time-trial performance. Int J Sport Nutr Exerc Metab 2019; 29:466–473. [DOI] [PubMed] [Google Scholar]
- 49.Cahill GF, Veech RL. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc 2003; 114:149–161. [PMC free article] [PubMed] [Google Scholar]
- 50.DC Harvey CJ, Schofield GM, Williden M, McQuillan JA. The effect of medium chain triglycerides on time to nutritional ketosis and symptoms of keto-induction in healthy adults: a randomised controlled clinical trial. J Nutr Metab 2018; 2018:2630565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tate RL, Mehlman MA, Tobin RB. Metabolic fate of 1,3-butanediol in the rat: conversion to -hydroxybutyrate. J Nutr 1971; 101:1719–1726. [DOI] [PubMed] [Google Scholar]
- 52.Chen O, Blonquist TM, Mah E, Sanoshy K, Beckman D, Nieman KM, et al. Tolerability and safety of a novel ketogenic ester, bis-hexanoyl (R)-1,3-butanediol: a randomized controlled trial in healthy adults. Nutrients 2021; 13:2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holland AM, Qaisi KS, Beasley KN, Bennett HR. Blood and cardiovascular health parameters after supplementing with ketone salts for six weeks. J Insulin Resist 2019; 4:a47. [Google Scholar]
- 54.Walsh JJ, Neudorf H, Little JP. 14-day ketone supplementation lowers glucose and improves vascular function in obesity: a randomized crossover trial. J Clin Endocrinol Metab 2021; 106:e1738–e1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh JJ, Caldwell HG, Neudorf H, Ainslie PN, Little JP. Short-term ketone monoester supplementation improves cerebral blood flow and cognition in obesity: a randomized cross-over trial. J Physiol 2021; 599:4763–4778. [DOI] [PubMed] [Google Scholar]
- 56.Pedro Rojas-Morales JP-C, Edilia T. Ketone bodies for kidney injury and disease. Adv Redox Res 2021; 2:100009. [Google Scholar]
- 57.Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res 2017; 183:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol 2014; 10:364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myette-Côté É, Neudorf H, Rafiei H, Clarke K, Little JP. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J Physiol 2018; 596:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myette-Côté É, Caldwell HG, Ainslie PN, Clarke K, Little JP. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. Am J Clin Nutr 2019; 110:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soto-Mota A, Norwitz NG, Evans R, Clarke K, Barber TM. Exogenous ketosis in patients with type 2 diabetes: safety, tolerability and effect on glycaemic control. Endocrinol Diabetes Metab 2021; 4:e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vesali RF, Cibicek N, Jakobsson T, Klaude M, Wernerman J, Rooyackers O. Protein metabolism in leg muscle following an endotoxin injection in healthy volunteers. Clin Sci (Lond) 2009; 118:421–427. [DOI] [PubMed] [Google Scholar]
- 63.Howell S, Kones R. Calories in, calories out’ and macronutrient intake: the hope, hype, and science of calories. Am J Physiol Endocrinol Metab 2017; 313:E608–E612. [DOI] [PubMed] [Google Scholar]
- 64.Deemer SE, Plaisance EP, Martins C. Impact of ketosis on appetite regulation-a review. Nutr Res 2020; 77:1–11. [DOI] [PubMed] [Google Scholar]
- 65.Vestergaard ET, Zubanovic NB, Rittig N, Møller N, Kuhre RE, Holst JJ, et al. Acute ketosis inhibits appetite and decreases plasma concentrations of acyl ghrelin in healthy young men. Diabetes Obes Metab 2021; 23:1834–1842. [DOI] [PubMed] [Google Scholar]
- 66.Féry F, Balasse EO. Ketone body turnover during and after exercise in overnight-fasted and starved humans. Am J Physiol 1983; 245:E318–E325. [DOI] [PubMed] [Google Scholar]
- 67.Winder WW, Baldwin KM, Holloszy JO. Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem 1974; 47:461–467. [DOI] [PubMed] [Google Scholar]
- 68.Winder WW, Baldwin KM, Holloszy JO. Exercise-induced increase in the capacity of rat skeletal muscle to oxidize ketones. Can J Physiol Pharmacol 1975; 53:86–91. [DOI] [PubMed] [Google Scholar]
- 69.O’Malley T, Myette-Cote E, Durrer C, Little JP. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab 2017; 42:1031–1035. [DOI] [PubMed] [Google Scholar]
- 70.Poffé C, Ramaekers M, Bogaerts S, Hespel P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J Appl Physiol (1985) 2020; 128:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sports Exerc 2019; 51:2506–2515. [DOI] [PubMed] [Google Scholar]




