Abstract
Urotensin-II is a polypeptide ligand with neurohormone-like activity. It mediates downstream signaling pathways through G-protein-coupled receptor 14 (GPR14) also known as urotensin receptor (UTR). Urotensin-II is the most potent endogenous vasoconstrictor in mammals, promoting cardiovascular remodelling, cardiac fibrosis, and cardiomyocyte hypertrophy. It is also involved in other physiological and pathological activities, including neurosecretory effects, insulin resistance, atherosclerosis, kidney disease, and carcinogenic effects. Moreover, it is a notable player in the process of inflammatory injury, which leads to the development of inflammatory diseases. Urotensin-II/UTR expression stimulates the accumulation of monocytes and macrophages, which promote the adhesion molecules expression, chemokines activation and release of inflammatory cytokines at inflammatory injury sites. Therefore, urotensin-II turns out to be an important therapeutic target for the treatment options and management of associated diseases. The main downstream signaling pathways mediated through this urotensin-II /UTR system are RhoA/ROCK, MAPKs and PI3K/AKT. Due to the importance of urotensin-II systems in biomedicine, we consolidated a network map of urotensin-II /UTR signaling. The described signaling map comprises 33 activation/inhibition events, 31 catalysis events, 15 molecular associations, 40 gene regulation events, 60 types of protein expression, and 11 protein translocation events. The urotensin-II signaling pathway map is made freely accessible through the WikiPathways Database (https://www.wikipathways.org/index.php/Pathway:WP5158). The availability of comprehensive urotensin-II signaling in the public resource will help understand the regulation and function of this pathway in normal and pathological conditions. We believe this resource will provide a platform to the scientific community in facilitating the identification of novel therapeutic drug targets for diseases associated with urotensin-II signaling.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-022-00672-4.
Keywords: Intercellular signaling, Ligand-receptor interactions, Omics integration, Pathways, Signaling network
Introduction
Urotensin-II is a neurohormone-like polypeptide molecule (Sun and Liu 2019). It was first isolated in 1980 by D Pearson et al. from the urophysis of the goby fish (Gillichthys mirabilis) (Pearson et al. 1980). Urotensin-II is the most potent vasoconstrictor cyclic peptide, with similar domain sequences to somatostatin (Ames et al. 1999). The urotensin-II system is involved in multiple biological systems, including cardiovascular, nervous, endocrine, and renal (Douglas et al. 2004; Vaudry et al. 2010, 2015). Urotensin-II is encoded by a gene UTS2, which is located on chromosome 1p36 (Tostivint et al. 2006). In humans, the alternative splicing of the UTS2 gene yielding 124 (isoform b, NP_006777) and 139 (isoform a, NP_068835.1) amino acid variants of prepro-urotensin-II. The proteolytic cleavage of preproprotein urotensin-II by a specific urotensin converting enzyme (UCE) results in a mature 11- amino acid sequence peptide urotensin-II. Urotensin-II selectively binds to G-protein -coupled orphan receptor 14 (GPR14), known as the urotensin-II receptor encoded by the UTS2R gene, located on human chromosome 17q25.3 (Muravenko et al. 2000).
In physiological conditions, the vascular effect of urotensin-II is a balance between vasoconstriction and vasodilation, resulting in the release of nitric oxide, prostaglandins, and endothelium-derived hyperpolarizing factors from endothelial cells. In addition, urotensin-II plays a role in the immune response, which leads to activation of cytokine expression and the promotion of immune cell infiltration (Castel et al. 2017). The altered expression of urotensin-II and urotensin receptor (UTR) in plasma, urine and cerebrospinal fluid can be seen in vascular inflammatory diseases, including essential hypertension, chronic heart failure, atherosclerosis, renal failure, renal dysfunction, portal hypertension/cirrhosis, and diabetes mellitus (Tsoukas et al. 2011). It suggests that the urotensin-II/UTR system may not contribute to the regulation of organ function in healthy states but also plays a significant role in inflammatory diseases (Ong et al. 2005; Tsoukas et al. 2011).
Urotensin-II mediated activation of UTR increases intracellular Ca2+ that causes vasoconstriction (Gibson et al. 1988; Ames et al. 1999; Filipeanu et al. 2002; Song et al. 2006). In addition, the influx of extracellular calcium through L-type calcium channels is also responsible for vasoconstriction (Zhang et al. 2015). Urotensin-II is a well-known potent vasoconstrictor, which turns out to be an important molecule for treatment options and management of the diseases (Douglas and Ohlstein 2000; Zhu et al. 2006). Many peptidic and nonpeptidic urotensin-II antagonists have been developed to reveal the role of urotensinergic system. Urantide is a UTR competitive antagonist that fastidiously blocks the urotensin-II induced effects in the rat aorta and human monocyte-derived macrophages (Patacchini et al. 2003; Watanabe et al. 2005). Another UTR competitive antagonist, BIM-23127 inhibited calcium mobilization in human embryonic kidney cells expressing UTR and inhibited urotensin -II induced hypertrophy in cultured H9c2 cardiomyocytes (Herold et al. 2003; Johns et al. 2004). The treatment of a non-peptide UTR antagonist, SB-611812, improved cardiac function and reduced cardiac remodelling after congenital heart failure (Watanabe et al. 2005; Bousette et al. 2006). Another non-peptide UTR antagonist, Palosuran inhibited urotensin-II-induced inflammation through MAPK phosphorylation in CHO cell transfected with hUT, suggesting an anti-inflammatory role (Clozel et al. 2004). Palosuran has the ability to improve renal dysfunction and injury-induced ischemia, revealing its role in treating diseases in humans (Clozel et al. 2004). A non-peptide UTR antagonist, Piperazino-isoindolinone strongly and specifically bind to human UTR and suggested that this may provide interesting research opportunity to treat diseases caused by the altered expression of urotensin-II (Lawson et al. 2009).
Although urotensin-II mediated UTR activation plays a role in many physiological and pathophysiological conditions, the information pertaining to urotensin-II mediated UTR signaling network is dispersed throughout the literature. In this study, we compiled the research articles containing molecular information about urotensin-II mediated signaling mechanisms and built a detailed pathway map of urotensin-II mediated UTR signaling. We developed a resource of signaling events mediated by UTR similar to the previously published pathways related to cardiac vascular diseases, including endothelin, apelin and elabela (Dagamajalu et al. 2021, 2022a, b). The urotensin-II mediated UTR signaling pathway described in this study comprises 129 proteins undergoing six different molecular reactions stimulated by urotensin-II. These reactions were gathered together through a manual annotation of the scientific literature and depicted as a single pathway map. The urotensin-II mediated UTR signaling pathway map is available through the WikiPathways Database (https://www.wikipathways.org/index.php/Pathway:WP5158).
Methodology
We carried out an extensive literature search in PubMed for the downstream effects of urotensin-II/UTR. The scientific articles were fetched from PubMed using the following search terms (“UTS2” OR “PRO1068” OR “U-II” OR “UCN2” OR “UII” OR “urotensin 2” OR “UTS2R” OR “GPR14” OR “UR-2-R” OR “UTR2” OR “urotensin 2 receptor” OR “urotensin-2 receptor” OR “UR-II-R” OR “UT receptor”) AND (“pathway” OR “signalling” OR “signalling”). Except reviews and short communications, only original research studies were considered for the annotation. The abstract of each of the research articles was manually screened for urotensin-II/UTR mediated signaling reactions that categorized into the following five different groups: (1) molecular association (protein–protein interactions), (2) post-translational modification (PTM), (3) translocation/transport of proteins between subcellular compartments, (4) activation/inhibition, and finally, (5) gene regulation at the mRNA and protein level (both up-and down-regulation). We manually curated the signaling reactions based on the previously published NetPath annotation criteria (Kandasamy et al. 2010). Each signaling event described in urotensin-II mediated UTR pathway was hyperlinked to the PubMed entry of the corresponding articles.
An instructive pathway map was generated using PathVisio, a pathway drawing tool (van Iersel et al. 2008). The map provides a pictorial summary of all the reactions annotated, including downstream effectors at both the transcriptional and translational levels. Annotated pathway reactions were exported to the WiKiPathways with the ID: WP5158.
Results and discussion
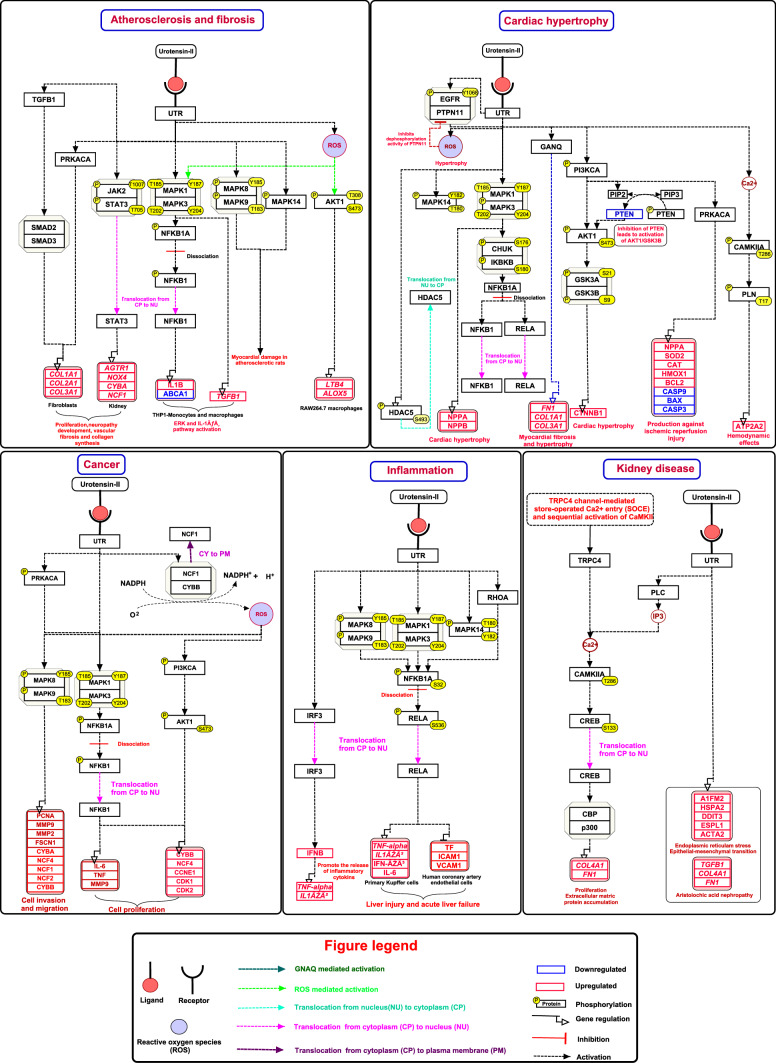
The PubMed search retrieved 68 articles, which were relevant to urotensin-II mediated UTR signaling molecular events were used for annotation. The manual annotation yielded a total of 15 molecular associations, 31 post-translational modifications (PTMs), 33 activation/inhibition reactions, 11 translocation events, 60 protein regulation and 40 gene regulation events were induced by rotensin-II mediated UTR signaling. Of the 40 gene regulation events, 38 genes were found to be up-regulated and 2 genes were downregulated. While in the case of 60 induced protein expression events, 8 proteins were downregulated and 52 were up-regulated. These reactions were incorporated into a corresponding map of the urotensin-II mediated UTR signaling pathway (Fig. 1). The pathway datasheet explains the molecular events involved in urotensin-II ligand-mediated UTR signaling (Supplementary data S1). The consolidated pathway map of the UTR-mediated signaling pathway was submitted to the WikiPathways.
Fig. 1.
Schematic representation of urotensin-II-mediated signaling pathway. Schematic representation of urotensin-II mediated signaling reactions. The signaling pathway map represents molecules involved in ligand-receptor interactions and UTR activated downstream molecular events including molecular association, enzyme catalysis, translocation, and gene regulation events. In addition, information regarding the post-translational modification site and the residue is also shown in the pathway
Summary of the urotensin pathway map
Urotensin-II binds to a G protein-coupled receptor called GPR14 or UTR signals through intracellular biochemical cascades, inducing many physiological and pathophysiological events (Carlson and Childress 1975; Saetrum Opgaard et al. 2000; Brule et al. 2014; Zhang et al. 2015). The expression of urotensin-II has been elevated in cardiovascular diseases associated with cardiovascular remodeling, including atherosclerosis, fibrosis, systemic, pulmonary hypertension, and congestive heart failure (Djordjevic and Gorlach 2007). Urotensin-II induces ROS production via NADPH oxidases, leading to JNK pathways and insulin resistance in HepG2 cells (Li et al. 2016). The NOX4-mediated ROS production by urotensin-II phosphorylates JNK and 14-3-3 and activates FoxO3a. This pathway leads to the up-regulation of MMP2 and plays an important role in vascular remodeling by enhanced proliferation of smooth muscle cells (SMCs) in human pulmonary artery SMCs (Diebold et al. 2011). Chen et al., 2014, have reported that the protective role of urotensin-II against doxorubicin-induced apoptosis in human umbilical vein endothelial cells (HUVEC) via the upregulation of ATF3, downregulation of p53, via AKT1 (Ser 473) and MAPK1/3 Tyr 204/Thr 202) phosphorylation (Chen et al. 2014; Staff 2015). Li et al. reported that urotensin-II induces collagen synthesis and mediates the proliferation of pulmonary artery smooth muscle cells (PASMCs) by activating MAPK1/3, MAP2K7 and EGR-1 expression. This represents the role of urotensin-II in the pathophysiology of pulmonary hypertension (Li et al. 2015).
Cardiac hypertrophy
Urotensin-II is a small peptide extensively expressed in the cardiovascular system, which induces cardiomyocyte hypertrophy. In cultured rat cardiac myocytes, urotensin-II strongly up-regulated NPPA and NPPB through activation of MAPK1/3 signaling mechanism (Zou et al. 2001). The urotensin-II stimulates UTR and induces the activation of MAPK1/3 and MAPK14 via the tyrosine phosphorylation (Y1068) of epidermal growth factor receptors (EGFRs), which lead to cardiomyocyte hypertrophy in rat cardiomyocytes (Liu et al. 2009). Another study in rat cardiomyocytes reported that urotensin-II-induced cardiac hypertrophy by ROS generation via NADPH oxidase, which inhibits SHP-2 and facilitates the phosphorylation of EGFR (Tyr 1068) and MAPKs (Liu et al. 2009; Shi et al. 2016). Park et al. reported that the urotensin-II induces the activation and phosphorylation of NF-kB and HDAC5 (Ser 493) via MAPK1/3 and GSK3a and GSK3β independent pathways, leading to hypertrophy in rat H9c2 cells (Park et al. 2016). CaMKII/PLN/SERCA2a, signaling pathway contributed in urotensin-II induced cardiomyocyte hypertrophy reported in rat cardiac myocytes. In neonatal rat cardiomyocytes, urotensin-II induces cardiomyocyte hypertrophy via Akt/GSK-3β pathway by inhibiting PTEN and ERK pathways (Chao et al. 2014). The urotensin-II-induced adult cardiomyocyte hypertrophy reported in adult cardiomyocytes involves the Akt/GSK-3β signaling pathways and is accompanied by the stabilization of the beta-catenin (Gruson et al. 2010). Tzanidis et al. reported that urotensin-II stimulation in neonatal cardiac fibroblasts increased mRNA level transcripts for FN1, COL1A1, and COL3A1 through Galpha(q)- and Ras-dependent pathways are implicated in myocardial fibrosis and pathological cardiac hypertrophy (Tzanidis et al. 2003).
Inflammation
Urotensin-II expression is closely related to tissue damage caused by immune inflammation by promoting the production and release of proinflammatory cytokines. A study in primary Kupffer cells (KCs) shows that urotensin-II/UTR system induces the upregulation of TNF and IL1β and RELA via the MAPK pathway, which promotes inflammatory liver injury (Liu et al. 2015a). Another study reported that urotensin-II/UTR system plays a key role in the pathogenesis of acute liver failure (ALF) by promoting the release of inflammatory cytokines including TNF-a, IL-1β and IFN-γ through NF-κB mediated signaling pathway (Liang et al. 2014). In the study in ALF mice, urotensin-II/UTR system induces the secretion of IFN-β via IRF3 signaling and IL-6 and IFN-γ via MAPK/NF-κB signaling, which mediates inflammatory liver injury in ALF (Liu et al. 2016). Furthermore, urotensin-II induces Rho-A activation and cellular adhesion molecules such as ICAM1, VCAM1 and tissue factors expression via NF-κB signaling pathway, which response to athero-thrombosis in human coronary artery endothelial cells (Cirillo et al. 2008). Urotensin-II/UTR system also induces neuropathic pain, a devastating disease related to excessive inflammation in the spinal dorsal horn by protein upregulation of IL1β, IL-6, TNF-alpha via JNK/NF-kB signaling pathway (Liao et al. 2017).
Atherosclerosis and fibrosis
Zhao et al (2020) reported that foam cell formation in the arterial wall plays a key role in the development of atherosclerosis. The increased levels of urotensin-II expression are associated with atherosclerotic myocardial injury in rats by activating MAPKs signaling pathways such as MAPK1/3, MAPK14 and MAPK8/9 (Zhao et al. 2020). Urotensin-II activates MAPK1/3 signaling through NF-kB mediated pathway and suppresses the ATP-binding cassette transporter A1 (ABCA1) protein and gene expression in THP-1 macrophages. This study suggests that urotensin-II may reduce cholesterol efflux to promote macrophage foam cell formation to promote the progression of atherosclerosis (Wang et al. 2014). In THP-1 monocytes, MAPK1/3 induces activation of the IL-1ß pathway, which in turn downregulates expression of ABCA1, which speeds up foam cell formation leads to atherosclerotic lesions (Kim et al. 2017a). Kim et al. (2017a, b) reported that UTR activation by urotensin-II induces ROS generation and MAPK1/3 activation in vascular smooth muscle cells, which are associated with atherosclerosis and restenosis (Kim et al. 2017b). urotensin-II stimulates UTR and induces the overexpression of 5-lipoxygenase (ALOX5) protein and release of leukotriene B4 (LTB4) via ROS/AKT pathways, which initiates macrophage activation and promotes atherosclerosis in mouse RAW264.7 macrophages (Lu et al. 2019). The urotensin-II /UTR system phosphorylates JAK2 (Tyr 1007)/STAT3 (Tyr 705) molecules and activation of MAPKs signaling pathways, p-MAPK1/3 translocated into the nucleus, which induces the atherosclerosis-related kidney injury by overexpression of Agtr1α, Nox4, Cyba and Ncf1 genes in an atherosclerotic rat model (Wang et al. 2020).
Urotensin-II is mainly involved in the occurrence and progression of cardiac fibrosis by stimulating collagen synthesis (Tran et al. 2010; Liu et al. 2015b). Similarly, urotensin-II promotes collagen synthesis via MAPK1/3-dependent and independent TGF-β1 pathway in neonatal cardiac fibroblasts (Zou et al. 2001). Urotensin-II induces enhanced expression of Collagen I (COL1A1) and Collagen III (COL3A1) via the cAMP-PKA signaling pathway, which promotes myocardial fibrosis in the pressure-overload rat model (Liu et al. 2015b). In vascular smooth muscle cells (VSMCs), urotensin-II/UTR stimulates TGF-β1/Smad 2/3 pathway activation, which induces the upregulation of COL1A1 production and vascular fibrosis in VSMCs (Zhao et al. 2013).
Cancer
Recent studies have demonstrated that urotensin-II/UTR system is involved in the pathogenesis of many malignant tumors (Federico et al. 2017). The study by Yu et al. has reported that urotensin-II/UTR system enhanced the proliferation of human hepatoma cells via PKC, MAPK1/3 and MAPK14 pathways (Yu et al. 2014). The study by Yu et al. (2015) has reported that the urotensin-II/UTR system increases ROS production through NADPH oxidase, translocation of NCF1 and NCF2 from the cytoplasm to membrane and upregulated the expression of CYBB, NCF4, CCNE1 and CDK2 via PI3K/AKT and MAPK1/3 pathways. This accelerates the G1/S transition, leading to cell proliferation in human hepatocellular carcinoma (Yu et al. 2015). In another study in hepatocellular carcinoma, urotensin-II/UTR induced ROS generation through NADPH oxidase, activated MAPK8/9 pathways, and increased expression of MMP2, CYBA, NCF4, NCF1, NCF2, CYBB, PCNA, FSCN1, which are involved in migration and invasion (Li et al. 2017). Urotensin-II induced proliferation and migration via MAPK and FAK pathways in TSC2-deficient human angiomyolipoma cells excised from a patient with Lymphangioleiomyomatosis (LAM) (Goldberg et al. 2016). In lung adenocarcinoma tumor-bearing nude mice model, urotensin-II forms an inflammatory microenvironment by increased expression of IL-6, TNF-α and MMP-9 via NF-kB pathway leading to enhanced proliferation (Zhou et al. 2012).
Kidney diseases
The increased levels of urotensin-II and UTR are already reported in kidney specimens of diabetic nephropathy (Langham et al. 2004). In diabetic conditions, UTR activation by urotensin-II induces the elevation of intracellular calcium via the activation of TRPC4 channels and successive activation of CaMKII/CREB signaling pathways by phosphorylation, which contribute to proliferation and extracellular matrix protein accumulation in glomerular mesangial cells (GMCs), which cause diabetic nephropathy (Soni and Adebiyi 2017). Chen et al. (2017) has reported that urotensin-II increased the TGF-β1 expression and secretion during aristolochic acid-induced nephropathy (Chen et al. 2017). In rat proximal tubular epithelial cells, the advanced glycation end product (AGE) stimulates the increased expression of urotensin-II involved in the upregulation of TGFB1, FN1 and COL4A1, which promotes tubulointerstitial nephropathy in diabetes (Tian et al. 2016). In the diabetic nephropathy model, urotensin-II induces the upregulation of AIFM2, HSPA5, DDIT3, ESPL1, ACTA2, which induces ER stress and EMT in human renal proximal tubular epithelial cell line HK-2 cells (Pang et al. 2016).
Conclusions
Urotensin-II mediated signaling is involved in several pathophysiological roles in pro-fibrotic activities, neurosecretory effects, atherosclerosis, kidney disease, cardiomyocyte hypertrophy, carcinogenic effects and inflammatory diseases. Therefore, the availability of comprehensive urotensin-II signaling in the public resource will help understand the regulation and function of this pathway in normal and pathological conditions. Furthermore, we anticipate that this resource about urotensin-II and its signaling system will help and provide more insights to understand the role of urotensin-II signaling in disease progression and proteins with undefined functionality in the pathway, which provides a new hypothesis that will assist them to design better near future therapeutics.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (XLSM 81 kb) Supplementary Data S1: A compendium of urotensin-II mediated signaling events
Acknowledgements
We thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka, for the support to the Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017). RDAB is a recipient of the Senior Research Fellowship from the Indian Council of Medical Research (ICMR), Government of India. AP is a recipient of the Junior Research Fellowship from the University Grants Commission (UGC), Government of India.
Declarations
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D. A. B. Rex, Email: rexprem@yenepoya.edu.in
G. P. Suchitha, Email: suchithagp1@gmail.com
Akhina Palollathil, Email: akhinap@yenepoya.edu.in.
Anagha Kanichery, Email: anaghak0092@gmail.com.
T. S. Keshava Prasad, Email: keshav@yenepoya.edu.in.
Shobha Dagamajalu, Email: shobha_d@yenepoya.edu.in.
References
- Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW, Ao Z, Disa J, Holmes SD, Stadel JM, Martin JD, Liu WS, Glover GI, Wilson S, McNulty DE, Ellis CE, Elshourbagy NA, Shabon U, Trill JJ, Hay DW, Ohlstein EH, Bergsma DJ, Douglas SA. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401(6750):282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- Bousette N, Hu F, Ohlstein EH, Dhanak D, Douglas SA, Giaid A. Urotensin-II blockade with SB-611812 attenuates cardiac dysfunction in a rat model of coronary artery ligation. J Mol Cell Cardiol. 2006;41(2):285–295. doi: 10.1016/j.yjmcc.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Brule C, Perzo N, Joubert JE, Sainsily X, Leduc R, Castel H, Prezeau L. Biased signaling regulates the pleiotropic effects of the urotensin II receptor to modulate its cellular behaviors. FASEB J. 2014;28(12):5148–5162. doi: 10.1096/fj.14-249771. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Childress DS. The lift lock: a device to increase the lifting ability of dual-control prostheses. Bull Prosthet Res. 1975;1975(01/01):158–168. [PubMed] [Google Scholar]
- Castel H, Desrues L, Joubert JE, Tonon MC, Prezeau L, Chabbert M, Morin F, Gandolfo P. The G protein-coupled receptor UT of the neuropeptide urotensin II displays structural and functional chemokine features. Front Endocrinol (Lausanne) 2017;8:76. doi: 10.3389/fendo.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HH, Sung LC, Chen CH, Liu JC, Chen JJ, Cheng TH. Lycopene inhibits urotensin-II-induced cardiomyocyte hypertrophy in neonatal rat cardiomyocytes. Evid Based Complement Alternat Med. 2014;2014:724670. doi: 10.1155/2014/724670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Tsai YT, Lee CY, Lee CH, Chen CY, Liu CM, Chen JJ, Loh SH, Tsai CS. Urotensin II inhibits doxorubicin-induced human umbilical vein endothelial cell death by modulating ATF expression and via the ERK and Akt pathway. PLoS ONE. 2014;9(9):e106812. doi: 10.1371/journal.pone.0106812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang Y, Wan Y. Urotensin II enhances transforming growth factorbeta1 expression and secretion in the kidney during aristolochic acid nephropathy. Mol Med Rep. 2017;16(5):6904–6909. doi: 10.3892/mmr.2017.7424. [DOI] [PubMed] [Google Scholar]
- Cirillo P, De Rosa S, Pacileo M, Gargiulo A, Angri V, Fiorentino I, Prevete N, Petrillo G, De Palma R, Leonardi A, De Paulis A, Chiariello M. Human urotensin II induces tissue factor and cellular adhesion molecules expression in human coronary endothelial cells: an emerging role for urotensin II in cardiovascular disease. J Thromb Haemost. 2008;6(5):726–736. doi: 10.1111/j.1538-7836.2008.02923.x. [DOI] [PubMed] [Google Scholar]
- Clozel M, Binkert C, Birker-Robaczewska M, Boukhadra C, Ding SS, Fischli W, Hess P, Mathys B, Morrison K, Muller C, Muller C, Nayler O, Qiu C, Rey M, Scherz MW, Velker J, Weller T, Xi JF, Ziltener P. Pharmacology of the urotensin-II receptor antagonist palosuran (ACT-058362; 1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulfate salt): first demonstration of a pathophysiological role of the urotensin system. J Pharmacol Exp Ther. 2004;311(1):204–212. doi: 10.1124/jpet.104.068320. [DOI] [PubMed] [Google Scholar]
- Dagamajalu S, Rex DAB, Gopalakrishnan L, Karthikkeyan G, Gurtoo S, Modi PK, Mohanty V, Mujeeburahiman M, Soman S, Raju R, Tiwari V, Prasad TSK. A network map of endothelin mediated signaling pathway. J Cell Commun Signal. 2021;15(2):277–282. doi: 10.1007/s12079-020-00581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagamajalu S, Rex DAB, Philem PD, Rainey JK, Keshava Prasad TS. A network map of apelin-mediated signaling. J Cell Commun Signal. 2022;16(1):137–143. doi: 10.1007/s12079-021-00614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagamajalu S, Rex DAB, Suchitha GP, Rai AB, Rainey JK, Prasad TSK. The network map of Elabela signaling pathway in physiological and pathological conditions. J Cell Commun Signal. 2022;16(1):145–154. doi: 10.1007/s12079-021-00640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold I, Petry A, Burger M, Hess J, Gorlach A. NOX4 mediates activation of FoxO3a and matrix metalloproteinase-2 expression by urotensin-II. Mol Biol Cell. 2011;22(22):4424–4434. doi: 10.1091/mbc.e10-12-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic T, Gorlach A. Urotensin-II in the lung: a matter for vascular remodelling and pulmonary hypertension? Thromb Haemost. 2007;98(5):952–962. doi: 10.1160/TH07-04-0294. [DOI] [PubMed] [Google Scholar]
- Douglas SA, Ohlstein EH. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc Med. 2000;10(6):229–237. doi: 10.1016/S1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- Douglas SA, Dhanak D, Johns DG. From ‘gills to pills’: urotensin-II as a regulator of mammalian cardiorenal function. Trends Pharmacol Sci. 2004;25(2):76–85. doi: 10.1016/j.tips.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Federico A, Zappavigna S, Dallio M, Misso G, Merlino F, Loguercio C, Novellino E, Grieco P, Caraglia M. Urotensin-II receptor: a double identity receptor involved in vasoconstriction and in the development of digestive tract cancers and other tumors. Curr Cancer Drug Targets. 2017;17(2):109–121. doi: 10.2174/1568009616666160621101248. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Brailoiu E, Le Dun S, Dun NJ. Urotensin-II regulates intracellular calcium in dissociated rat spinal cord neurons. J Neurochem. 2002;83(4):879–884. doi: 10.1046/j.1471-4159.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- Gibson A, Conyers S, Bern HA. The influence of urotensin II on calcium flux in rat aorta. J Pharm Pharmacol. 1988;40(12):893–895. doi: 10.1111/j.2042-7158.1988.tb06298.x. [DOI] [PubMed] [Google Scholar]
- Goldberg AA, Joung KB, Mansuri A, Kang Y, Echavarria R, Nikolajev L, Sun Y, Yu JJ, Laporte SA, Schwertani A, Kristof AS. Oncogenic effects of urotensin-II in cells lacking tuberous sclerosis complex-2. Oncotarget. 2016;7(38):61152–61165. doi: 10.18632/oncotarget.10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruson D, Ginion A, Decroly N, Lause P, Vanoverschelde JL, Ketelslegers JM, Bertrand L, Thissen JP. Urotensin II induction of adult cardiomyocytes hypertrophy involves the Akt/GSK-3beta signaling pathway. Peptides. 2010;31(7):1326–1333. doi: 10.1016/j.peptides.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Herold CL, Behm DJ, Buckley PT, Foley JJ, Wixted WE, Sarau HM, Douglas SA. The neuromedin B receptor antagonist, BIM-23127, is a potent antagonist at human and rat urotensin-II receptors. Br J Pharmacol. 2003;139(2):203–207. doi: 10.1038/sj.bjp.0705251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Ao Z, Naselsky D, Herold CL, Maniscalco K, Sarov-Blat L, Steplewski K, Aiyar N, Douglas SA. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol. 2004;370(4):238–250. doi: 10.1007/s00210-004-0980-z. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, Tattikota SG, Mohan S, Padhukasahasram H, Subbannayya Y, Goel R, Jacob HK, Zhong J, Sekhar R, Nanjappa V, Balakrishnan L, Subbaiah R, Ramachandra YL, Rahiman BA, Prasad TS, Lin JX, Houtman JC, Desiderio S, Renauld JC, Constantinescu SN, Ohara O, Hirano T, Kubo M, Singh S, Khatri P, Draghici S, Bader GD, Sander C, Leonard WJ, Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11(1):R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Ilyosbek S, Lee BH, Yi KY, Jung YS. A novel urotensin II receptor antagonist, KR-36676, prevents ABCA1 repression via ERK/IL-1beta pathway. Eur J Pharmacol. 2017;803:174–178. doi: 10.1016/j.ejphar.2017.03.056. [DOI] [PubMed] [Google Scholar]
- Kim TH, Lee DG, Kim YA, Lee BH, Yi KY, Jung YS. A novel urotensin II receptor antagonist, KR-36996 inhibits smooth muscle proliferation through ERK/ROS pathway. Biomol Ther (Seoul) 2017;25(3):308–314. doi: 10.4062/biomolther.2016.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langham RG, Kelly DJ, Gow RM, Zhang Y, Dowling JK, Thomson NM, Gilbert RE. Increased expression of urotensin II and urotensin II receptor in human diabetic nephropathy. Am J Kidney Dis. 2004;44(5):826–831. doi: 10.1016/S0272-6386(04)01130-8. [DOI] [PubMed] [Google Scholar]
- Lawson EC, Luci DK, Ghosh S, Kinney WA, Reynolds CH, Qi J, Smith CE, Wang Y, Minor LK, Haertlein BJ, Parry TJ, Damiano BP, Maryanoff BE. Nonpeptide urotensin-II receptor antagonists: a new ligand class based on piperazino-phthalimide and piperazino-isoindolinone subunits. J Med Chem. 2009;52(23):7432–7445. doi: 10.1021/jm900683d. [DOI] [PubMed] [Google Scholar]
- Li W, Cai Z, Liu M, Zhao C, Li D, Lv C, Wang Y, Xu T. Urotensin II contributes to collagen synthesis and up-regulates Egr-1 expression in cultured pulmonary arterial smooth muscle cells through the ERK1/2 pathway. Biochem Biophys Res Commun. 2015;467(4):1076–1082. doi: 10.1016/j.bbrc.2015.09.148. [DOI] [PubMed] [Google Scholar]
- Li YY, Shi ZM, Yu XY, Feng P, Wang XJ. Urotensin II-induced insulin resistance is mediated by NADPH oxidase-derived reactive oxygen species in HepG2 cells. World J Gastroenterol. 2016;22(25):5769–5779. doi: 10.3748/wjg.v22.i25.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Shi ZM, Yu XT, Feng P, Wang XJ. The effects of urotensin II on migration and invasion are mediated by NADPH oxidase-derived reactive oxygen species through the c-Jun N-terminal kinase pathway in human hepatoma cells. Peptides. 2017;88:106–114. doi: 10.1016/j.peptides.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Liang DY, Liu LM, Ye CG, Zhao L, Yu FP, Gao DY, Wang YY, Yang ZW, Wang YY. Inhibition of UII/UTR system relieves acute inflammation of liver through preventing activation of NF-kappaB pathway in ALF mice. PLoS ONE. 2014;8(6):e64895. doi: 10.1371/journal.pone.0064895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao TL, Chen YM, Hsieh CW, Chen HH, Lee HC, Hung WT, Tang KT, Chen DY. Upregulation of circulating microRNA-134 in adult-onset Still's disease and its use as potential biomarker. Sci Rep. 2017;7(1):4214. doi: 10.1038/s41598-017-04086-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Chen CH, Chen JJ, Cheng TH. Urotensin II induces rat cardiomyocyte hypertrophy via the transient oxidization of Src homology 2-containing tyrosine phosphatase and transactivation of epidermal growth factor receptor. Mol Pharmacol. 2009;76(6):1186–1195. doi: 10.1124/mol.109.058297. [DOI] [PubMed] [Google Scholar]
- Liu LM, Liang DY, Ye CG, Tu WJ, Zhu T. The UII/UT system mediates upregulation of proinflammatory cytokines through p38 MAPK and NF-kappaB pathways in LPS-stimulated Kupffer cells. PLoS ONE. 2015;10(3):e0121383. doi: 10.1371/journal.pone.0121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Han Q, Liu Q, Liang G, Wang J, Liu C. An investigation into the expression and mechanism of action of urotensin II in chronic pressure-overloaded rat hearts. Mol Med Rep. 2015;12(5):6626–6634. doi: 10.3892/mmr.2015.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LM, Tu WJ, Zhu T, Wang XT, Tan ZL, Zhong H, Gao DY, Liang DY. IRF3 is an important molecule in the UII/UT system and mediates immune inflammatory injury in acute liver failure. Oncotarget. 2016;7(31):49027–49041. doi: 10.18632/oncotarget.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Peng F, Li J, Zhao J, Ye X, Li B, Ding W. Urotensin II promotes secretion of LTB4 through 5-lipoxygenase via the UT-ROS-Akt pathway in RAW264.7 macrophages. Arch Med Sci. 2019;15(4):1065–1072. doi: 10.5114/aoms.2019.85197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravenko OV, Gizatullin RZ, Al-Amin AN, Protopopov AI, Kashuba VI, Zelenin AV, Zabarovsky ER. Human ALY/BEF gene Map position 17q25.3. Chromosome Res. 2000;8(6):562. doi: 10.1023/A:1009236126053. [DOI] [PubMed] [Google Scholar]
- Ong KL, Lam KS, Cheung BM. Urotensin II: its function in health and its role in disease. Cardiovasc Drugs Ther. 2005;19(1):65–75. doi: 10.1007/s10557-005-6899-x. [DOI] [PubMed] [Google Scholar]
- Pang XX, Bai Q, Wu F, Chen GJ, Zhang AH, Tang CS. Urotensin II induces ER stress and EMT and increase extracellular matrix production in renal tubular epithelial cell in early diabetic mice. Kidney Blood Press Res. 2016;41(4):434–449. doi: 10.1159/000443445. [DOI] [PubMed] [Google Scholar]
- Park CH, Lee JH, Lee MY, Lee JH, Lee BH, Oh KS. A novel role of G protein-coupled receptor kinase 5 in urotensin II-stimulated cellular hypertrophy in H9c2UT cells. Mol Cell Biochem. 2016;422(1–2):151–160. doi: 10.1007/s11010-016-2814-y. [DOI] [PubMed] [Google Scholar]
- Patacchini R, Santicioli P, Giuliani S, Grieco P, Novellino E, Rovero P, Maggi CA. Urantide: an ultrapotent urotensin II antagonist peptide in the rat aorta. Br J Pharmacol. 2003;140(7):1155–1158. doi: 10.1038/sj.bjp.0705555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson D, Shively JE, Clark BR, Geschwind II, Barkley M, Nishioka RS, Bern HA. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA. 1980;77(8):5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetrum Opgaard O, Nothacker H, Ehlert FJ, Krause DN. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur J Pharmacol. 2000;406(2):265–271. doi: 10.1016/S0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- Shi H, Han Q, Xu J, Liu W, Chu T, Zhao L. Urotensin II induction of neonatal cardiomyocyte hypertrophy involves the CaMKII/PLN/SERCA 2a signaling pathway. Gene. 2016;583(1):8–14. doi: 10.1016/j.gene.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Song W, McDonald J, Camarda V, Calo G, Guerrini R, Marzola E, Thompson JP, Rowbotham DJ, Lambert DG. Cell and tissue responses of a range of urotensin II analogs at cloned and native urotensin II receptors. Evidence for coupling promiscuity. Naunyn Schmiedebergs Arch Pharmacol. 2006;373(2):148–157. doi: 10.1007/s00210-006-0057-2. [DOI] [PubMed] [Google Scholar]
- Soni H, Adebiyi A. Urotensin II-induced store-operated Ca(2+) entry contributes to glomerular mesangial cell proliferation and extracellular matrix protein production under high glucose conditions. Sci Rep. 2017;7(1):18049. doi: 10.1038/s41598-017-18143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff PO. Correction: urotensin II inhibits doxorubicin-induced human umbilical vein endothelial cell death by modulating ATF expression and via the ERK and Akt pathway. PLoS ONE. 2015;10(3):e0118879. doi: 10.1371/journal.pone.0118879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SL, Liu LM. Urotensin II: an inflammatory cytokine. J Endocrinol. 2019 doi: 10.1530/JOE-18-0505. [DOI] [PubMed] [Google Scholar]
- Tian L, Fu P, Zhou M, Gu Y, Li Y, Qi J. Role of urotensin II in advanced glycation end product-induced extracellular matrix synthesis in rat proximal tubular epithelial cells. Int J Mol Med. 2016;38(6):1831–1838. doi: 10.3892/ijmm.2016.2789. [DOI] [PubMed] [Google Scholar]
- Tostivint H, Joly L, Lihrmann I, Parmentier C, Lebon A, Morisson M, Calas A, Ekker M, Vaudry H. Comparative genomics provides evidence for close evolutionary relationships between the urotensin II and somatostatin gene families. Proc Natl Acad Sci USA. 2006;103(7):2237–2242. doi: 10.1073/pnas.0510700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Kompa AR, Kemp W, Phrommintikul A, Wang BH, Krum H. Chronic urotensin-II infusion induces diastolic dysfunction and enhances collagen production in rats. Am J Physiol Heart Circ Physiol. 2010;298(2):H608–H613. doi: 10.1152/ajpheart.00942.2009. [DOI] [PubMed] [Google Scholar]
- Tsoukas P, Kane E, Giaid A. Potential clinical implications of the urotensin II receptor antagonists. Front Pharmacol. 2011;2:38. doi: 10.3389/fphar.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanidis A, Hannan RD, Thomas WG, Onan D, Autelitano DJ, See F, Kelly DJ, Gilbert RE, Krum H. Direct actions of urotensin II on the heart: implications for cardiac fibrosis and hypertrophy. Circ Res. 2003;93(3):246–253. doi: 10.1161/01.RES.0000084382.64418.BC. [DOI] [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C. Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry H, Do Rego JC, Le Mevel JC, Chatenet D, Tostivint H, Fournier A, Tonon MC, Pelletier G, Conlon JM, Leprince J. Urotensin II, from fish to human. Ann N Y Acad Sci. 2010;1200:53–66. doi: 10.1111/j.1749-6632.2010.05514.x. [DOI] [PubMed] [Google Scholar]
- Vaudry H, Leprince J, Chatenet D, Fournier A, Lambert DG, Le Mevel JC, Ohlstein EH, Schwertani A, Tostivint H, Vaudry D. International union of basic and clinical pharmacology. XCII. Urotensin II, urotensin II-related peptide, and their receptor: from structure to function. Pharmacol Rev. 2015;67(1):214–258. doi: 10.1124/pr.114.009480. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu JF, Tang YY, Zhang M, Li Y, Chen K, Zeng MY, Yao F, Xie W, Zheng XL, Zeng GF, Tang CK. Urotensin II increases foam cell formation by repressing ABCA1 expression through the ERK/NF-kappaB pathway in THP-1 macrophages. Biochem Biophys Res Commun. 2014;452(4):998–1003. doi: 10.1016/j.bbrc.2014.09.030. [DOI] [PubMed] [Google Scholar]
- Wang T, Xie YQ, Miao GX, Cui HP, Liu K, Li Y, Li Y, Zhao J. Urotensin receptor antagonist urantide improves atherosclerosis-related kidney injury by inhibiting JAK2/STAT3 signaling pathway in rats. Life Sci. 2020;247:117421. doi: 10.1016/j.lfs.2020.117421. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Suguro T, Kanome T, Sakamoto Y, Kodate S, Hagiwara T, Hongo S, Hirano T, Adachi M, Miyazaki A. Human urotensin II accelerates foam cell formation in human monocyte-derived macrophages. Hypertension. 2005;46(4):738–744. doi: 10.1161/01.HYP.0000184226.99196.b5. [DOI] [PubMed] [Google Scholar]
- Yu Xiao-Tong, Wang Peng-Yan, Shi Zheng-Ming, Dong Kun, Feng Ping, Wang Hong-Xia, Wang Xue-Jiang. Up-Regulation of Urotensin II and Its Receptor Contributes to Human Hepatocellular Carcinoma Growth via Activation of the PKC, ERK1/2, and p38 MAPK Signaling Pathways. Molecules. 2014;19(12):20768–20779. doi: 10.3390/molecules191220768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang P, Shi Z, Dong K, Feng P, Wang H, Wang X. Urotensin-II-mediated reactive oxygen species generation via NADPH oxidase pathway contributes to hepatic oval cell proliferation. PLoS ONE. 2015;10(12):e0144433. doi: 10.1371/journal.pone.0144433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ying J, Jiang D, Chang Z, Li H, Zhang G, Gong S, Jiang X, Tao J. Urotensin-II receptor stimulation of cardiac L-type Ca2+ channels requires the betagamma subunits of Gi/o-protein and phosphatidylinositol 3-kinase-dependent protein kinase C beta1 isoform. J Biol Chem. 2015;290(13):8644–8655. doi: 10.1074/jbc.M114.615021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Ding W, Song N, Dong X, Di B, Peng F, Tang C. Urotensin II-induced collagen synthesis in cultured smooth muscle cells from rat aortic media and a possible involvement of transforming growth factor-beta1/Smad2/3 signaling pathway. Regul Pept. 2013;182:53–58. doi: 10.1016/j.regpep.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Zhao J, Miao G, Wang T, Li J, Xie L. Urantide attenuates myocardial damage in atherosclerotic rats by regulating the MAPK signalling pathway. Life Sci. 2020;262:118551. doi: 10.1016/j.lfs.2020.118551. [DOI] [PubMed] [Google Scholar]
- Zhou CH, Wan YY, Chu XH, Song Z, Xing SH, Wu YQ, Yin XX. Urotensin II contributes to the formation of lung adenocarcinoma inflammatory microenvironment through the NF-kappaB pathway in tumor-bearing nude mice. Oncol Lett. 2012;4(6):1259–1263. doi: 10.3892/ol.2012.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YC, Zhu YZ, Moore PK. The role of urotensin II in cardiovascular and renal physiology and diseases. Br J Pharmacol. 2006;148(7):884–901. doi: 10.1038/sj.bjp.0706800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Nagai R, Yamazaki T. Urotensin II induces hypertrophic responses in cultured cardiomyocytes from neonatal rats. FEBS Lett. 2001;508(1):57–60. doi: 10.1016/S0014-5793(01)03015-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (XLSM 81 kb) Supplementary Data S1: A compendium of urotensin-II mediated signaling events