Abstract
Aims
Pulmonary vein isolation (PVI) either by balloon devices or radiofrequency forms the cornerstone of invasive atrial fibrillation (AF) treatment. Although equally effective cryoballoon (CB)-based PVI offers shorter procedure duration and a better safety profile. Beside the worldwide established Arctic Front Advance system, a novel CB device, POLARx, was recently introduced. This CB incorporates unique features, which may translate into improved efficacy and safety. However, multicentre assessment of periprocedural efficacy and safety is lacking up to date.
Methods and results
A total of 317 patients with paroxysmal or persistent AF were included and underwent POLARx CB-based PVI in 6 centres from Germany and Italy. Acute efficacy and safety were assessed in this prospective multicenter observational study. In 317 patients [mean age: 64 ± 12 years, 209 of 317 (66%) paroxysmal AF], a total of 1256 pulmonary veins (PVs) were identified and 1252 (99,7%) PVs were successfully isolated utilizing mainly the short tip POLARx CB (82%). The mean minimal CB temperature was −57.9 ± 7°C. Real-time PVI was registered in 72% of PVs. The rate of serious adverse events was 6.0% which was significantly reduced after a learning curve of 25 cases (9.3% vs. 3.0%, P = 0.018). The rate of recurrence-free survival after mean follow-up of 226 ± 115 days including a 90-day blanking period was 86.1%.
Conclusion
In this large multicentre assessment, the novel POLARx CB shows a promising efficacy and safety profile after a short learning curve.
Keywords: Atrial fibrillation, Catheter ablation, Cryoballoon, Pulmonary vein isolation
What's new?
The novel POLARx cryoballon incorporates unique features which may translate into improved efficacy and safety. Here, we provide a multicentre assessment of periprocedural efficacy and safety.
With 99.7% of PVs, the POLARx provides a high rate of acute efficacy.
The rate of periprocedural complications was comparable with data of the current cryoballoon system.
In this large multicentre assessment, the novel POLARx shows a promising efficacy and safety profile.
Introduction
Pulmonary vein isolation (PVI) either by catheter ablation forms the cornerstone of invasive atrial fibrillation (AF) treatment and has demonstrated high procedural success and encouraging long-term outcomes.1 Although equally effective cryoballoon (CB)-based PVI offers shorter procedure duration, as well as lower complication rates compared with radiofrequency (RF) point-by-point based PVI.2,3 Beside the worldwide established Arctic Front Advance (AFA) cryoablation system (Medtronic, INC, Minneapolis, USA), a novel CB ablation system, POLARx (Boston Scientific, St. Paul, USA) was recently introduced.4 This novel CB incorporates several modifications which possibly translate into improved efficacy and safety as well as to further simplify CB-based PVI procedures.
One of the most important differences between the two CB systems is the constant pressure inside the POLARx during positioning and freezing, while with the AFA, the pressure differs between the positioning and freezing status if the CB which might lead to pop-out phenomenon as well as insufficient ablation through slightly positioning changing after starting the freezing process.5 The first reported analysis comparing the POLARx with the AFA showed similar efficacy and safety for both systems.5–9 However, the reported patient numbers are relatively low and lacks in multicenter assessments and large patient numbers. With every novel ablation system beside efficacy, safety is the key factor for success or failure. We therefore aimed to pool periprocedural data from different centers to improve data quality and quantity of this novel ablation system with the focus on acute efficacy and safety.
Methods
Study population
Between August and October 2021, a total of 317 patients with paroxysmal or persistent AF, treated with the POLARx CB (Figure 1) for PVI, were included in this multicentre observational study. Participating centres from Germany and Italy were University Heart Center Lübeck; University Medical Center Ulm; Department of Cardiology, University of Essen; University Heart Center of Hamburg Eppendorf, Herz- und Diabeteszentrum NRW, Bad Oeynhausen, Germany; and IRCCS Centro Cardiologico Monzino, Milan, Italy. The data acquision of 5 of 6 centers was prospective, whereas one centre provided retrospectively acquired data.
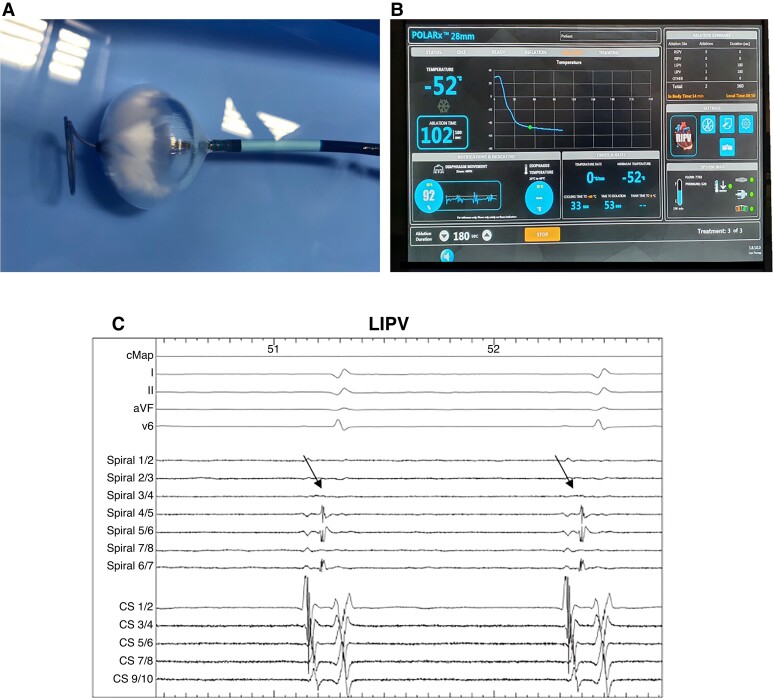
Figure 1.
(A) POLARx cryoablation system: POLARx balloon with POLARMAP spiral mapping catheter inside saline 30 s after initializing the freezing process. Please note the increasing ice formation on the distal hemisphere of the balloon. (B) Periprocedural scene during PVI of the RIPV on the SMART-FREEZE console. Please note the information of the novel diaphragm movement sensor in the left lower corner. (C) Periprocedural recordings with ECG lead I, II, aVF and V6, CS catheter and POLARMAP catheter (spiral) during ablation at the LIPV. Arrows mark the PV spike. CS = coronary sinus catheter; PV = pulmonary vein; LIPV = left inferior PV; RIPV = right inferior PV.
Exclusion criteria were permanent AF, previous left atrial (LA) surgical or catheter-based ablation, presence of LA thrombus on transesophageal echocardiography prior to the procedure, LA diameter >60 mm, uncontrolled heart failure (NYHA Class IV), moderate or severe valvular disease, and acute coronary syndrome and/or percutaneous coronary intervention (PCI) within 1 month before the procedure. The study complies with the Declaration of Helsinki and was approved by the local institutional ethics committees. All patients gave written informed consent and all patient information was anonymized. The multicentre study was approved by the local ethical review board of the University of Luebeck, Germany (AZ 15-347). The data acquisition was based on the prospective Luebeck ablation registry (Ethical Review Board number: WF-028/15). Each participating centre was responsible for its ethics approval by the local ethics committee. The primary endpoint was acute success for PVI. Secondary endpoints were periprocedural characteristics including complications.
Aim of the study
The aim of the study was to assess the incidence of periprocedural complications, such as bleeding events (defined as bleedings acquiring medical action), pericardial effusion and/or pericardial tamponade, cerebral stroke, ST-elevation myocardial infarction, phrenic nerve injury (PNI) or air embolism. Furthermore, this study aimed to analyse procedural efficacy and periprocedural data as indicated by acute PVI, time to isolation (TTI), lowest CB temperature during cryoenergy application, procedure duration, as well as fluoroscopy time. Periprocedural complications were defined according to latest guidelines. Only adverse events adjudicated as possible, probable, or definitely related to the ablation procedure were mentioned as safety events. An adverse event was considered serious if it resulted in permanent injury or death, required an intervention for treatment, or required hospitalization for more than 24 h.10
Intraprocedural management
The detailed intraprocedural management has been described in previous studies.6,9,11 In brief, the procedure was performed under deep sedation using midazolam, sufentanyl, and propofol. Two right femoral vein punctures were performed, and two 8F short sheath were inserted. Prior to transseptal puncture, one diagnostic catheter was introduced via the right femoral vein and positioned within the coronary sinus. Single transseptal puncture was performed under fluoroscopic guidance using a modified Brockenbrough technique and an 8.5F transseptal sheath (SL1, St. Jude Medical, Inc., St. Paul, MN, USA; or TSX transseptal delivery system and TSX transseptal needle, Boston scientific). Selective PV angiography was performed to identify the pulmonary vein (PV) ostia utilizing a 7F multipurpose catheter or was performed directly via the transseptal sheath. The transseptal sheath was exchanged over a guidewire for the 15.9 F POLAR-SHEATH (Boston Scientific). The sheath was continuously flushed with heparinized saline (20 mL/h). After transseptal puncture heparin boluses were administered targeting an activated clotting time of >300 s.
POLARx-based pulmonary vein isolation
Subsequently the 28 mm POLARx CB [POLARx short tip (ST) or long tip (LT), Boston Scientific] was advanced to the LA via the steerable sheath with a 20 mm spiral mapping catheter (10-polar, PolarMap, Boston Scientific) as a guidewire. The CB was inflated proximal to the PV ostium, advanced, and pushed to the PV ostium aiming at complete sealing of the PV without advancing the balloon into the PV. The PVs were treated following a clockwise sequence [left superior pulmonary vein (LSPV), left inferior pulmonary vein (LIPV), right inferior pulmonary vein (RIPV), right superior pulmonary vein (RSPV)]. A gentle pull-down manoeuver was performed for LIPV and RIPV after 60–70 s of freezing time. A TTI-based ablation protocol was utilized.
The standard freeze-cycle duration was 180 s. If the TTI could be visualized and was measured <60 s, the freeze-cycle duration was 180 s, and no further bonus-freeze application was performed. If no PVI was achieved after 60 s–90 s, the freeze-cycle was terminated. If TTI was measured ≥60 s, the freeze-cycle duration was 180 s and a bonus-freeze application of 180 s was performed. The procedural endpoint was disappearance of PV recordings verified via the circular mapping catheter after the freeze cycle (entrance block). No additional pacing of adenosine testing has been performed. The occlusion of the PV ostium was verified by contrast dye injections. Balloon temperature < −70°C during ablation led to immediate interruption of cryoenergy application.
A temperature probe (CIRCA S-CATH, Circa Scientific, Englewood, CO, USA or SensiTherm; St Jude Medical, St. Paul, MN, USA) was advanced into the oesophagus in 5 of 6 centres. A luminal oesophageal temperature below 15–20°C was used as a cut-off to trigger termination of the freeze-cycle.12,13
During energy delivery along the septal PVs, continuous phrenic nerve (PN) pacing was performed using a diagnostic catheter introduced into the superior vena cava. Pacing was set at maximum output and pulse width (12 mA, 2.9 ms) and a cycle length of 700–1000 ms. PN capture was monitored by tactile feedback of diaphragmatic contraction and assessment of the right diaphragmatic compound motor action potential (CMAP). Energy delivery was interrupted immediately if weakening or loss of diaphragmatic contraction was noted or a decrease of the CMAP amplitude of ≥30% was seen.
Apart from the aforementioned safety manoeuvers for PNI prevention, the novel DMS (diaphragm movement sensor) was utilized to monitor PN function. The DMS sensor is based on an accelerometer technology and is placed on a disposable electrode below the right-sided costal cartilage. Baseline DMS is automatically assessed when general PN pacing is started. The DMS cut-off was set at 60% of diaphragm movement. The freeze cycle was terminated by double stop if the cut-off was reached and no PN capture was detected immediately. The double stop was conducted via the orange foot pedal. In case of persistent PNI, no further cryoenergy was delivered to the septal PVs.14,15 The pop-out phenomenon was defined by the observation of a balloon dislodgement from the PV ostium after initializing the freezing process. This was evaluated by a second injection of contrast medium and fluoroscopy 5–10 s after initializing the freezing process.
Post-procedural care
A figure-of-eight suture and/or a pressure bandage were used to prevent femoral bleeding. The pressure bandage was removed after 4–6 h and the figure-of-eight suture was removed on the next day. Following ablation, all patients underwent transthoracic echocardiography immediately, after 2 h, and at Day 1 to rule out a pericardial effusion. Low-molecular-weight heparin was administered in patients on vitamin K antagonists and an INR <2.0 until a therapeutic INR of 2–3 was achieved. New oral anticoagulants were re-initiated 6 h post-ablation. Anticoagulation was continued for at least 3 months and continued thereafter based on the individual CHA2DS2-VASc score. Previously ineffective antiarrhythmic drugs were discontinued after 3 months post-ablation. All patients were treated with proton-pump inhibitors for 6 weeks.
Clinical follow-up
Following a blanking period of 3 months, patients completed outpatient clinic visits at 3, 6, and 12 months including ECGs and 24 h Holter ECGs. In addition, regular telephonic interviews were performed. Recurrence was defined as any episode of documented AF/atrial tachycardia recurrence lasting longer than 30 s after a 90-day blanking period. Only patients with at least 3 months follow-up were evaluated in this analysis.
Statistical analysis
To take account for learning curve effects, the 1st 25 patients of each centre were denoted as T1 and the patients from the 26th case are denoted as T2. All analyses were performed using STATA software, version 14.0 (STATA Corp, Lake Drive Way, Texas, USA). Distributions of continuous variables were tested for normality using the Shapiro–Wilk test. Continuous variables are expressed as mean ± standard deviation (SD) for normally distributed, or as median [inter-quartile range (IQR)], for non-normally distributed otherwise. Categorical variables are reported as counts (percentage). Comparisons of continuous variables were performed using the Student’s t-test for two groups or ANOVAs in case of multiple groups, or the corresponding non-parametric test, if not normally distributed. Comparisons of categorical variables were performed using χ2 or Fisher’s exact test, as appropriate.
Recurrence-free survival was estimated with the Kaplan–Meier method. All P-values reported are two-sided, and a P-value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 317 patients underwent POLARx-based PVI. Patient baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics
| Variable | POLARx |
|---|---|
| Patients, n | 317 |
| Age (years) | 64 ± 12 |
| Body mass index | 29 ± 6 |
| Female gender, (%) | 136 (43) |
| Duration of Afib, months | 28 ± 36 |
| Paroxysmal AF, n (%) | 209 (66) |
| LA diameter, mm | 36 ± 8 |
| Congestive heart failure (%) | 53 (17) |
| Arterial hypertension, n (%) | 207 (65) |
| Diabetes mellitus type II, n (%) | 39 (12) |
| Coronary artery disease, n (%) | 13 (22) |
| Previous stroke/TIA, n (%) | 18 (6) |
| CHA2DS2-VASc score | 2.0 ± 1.6 |
| HASBLED score | 1.6 ± 1.4 |
Values expressed as n (%) or median (range). AF = atrial fibrillation, LA = left atrium.
Acute ablation results
In 317 patients, a total of 1256 PVs were identified and targeted for ablation [305 RSPVs, 305 RIPVs, 317 LSPVs, 317 LIPVs, and 12 left common pulmonary veins (LCPVs)]. A total of 1252 PVs (99.7%) were successfully isolated using the POLARx. The POLARx ST was utilized in 260 (82%) of cases, whereas the POLARx LT was utilized in 57 (18%) cases. Periprocedural characteristics are reported in Table 2. The mean minimal CB temperature was −57.9 ± 7.2°C. Real-time PVI was visualized in 71.9% of PVs. The mean procedures time utilizing the POLARx was 9241 min.
Table 2.
Procedural details
| Variable | POLARx |
|---|---|
| Number of patients, n | 317 |
| Number of PVs, n | 1256 |
| Short tip POLARx CB | 260 (82) |
| Long tip POLARx CB | 57 (18) |
| Total number of isolated PVs, (%) | 1252 (99.7) |
| Total CB cycles until PVI | 1.2 ± 0.5 |
| Total CB cycles | 1.3 ± 0.6 |
| FAAVI, n (%) | 146 (46) |
| Minimal CB temp. (C°) | −57.9 ± 7.2 |
| Minimal oesophageal temp., (C°) | 31.6 ± 6.2 |
| Time to PVI, s | 46.1 ± 28.5 |
| Rate of TTI recordings, (%) | 761/1059 (71.9) |
| Duration of total freezing time, s | 234.9 ± 116.4 |
| Total procedure time, min. | 92 ± 41 |
| Total procedure time, min. (T1: 1–25) | 97.1 ± 46 |
| Total procedure time, min. (T2: >25) | 87.8 ± 35 |
| Total flouroscopy time, min. | 15 ± 10 |
| Total fluoroscopy time, min. (T1: 1–25) | 16.4 ± 11 |
| Total fluoroscopy time, min. (T2: >25) | 13.9 ± 9 |
| Total amount of contrast, mL | 83 ± 67 |
Values expressed as n (%), mean ± standard deviation or median (range) as appropriate. PV(s) = Pulmonary vein(s), PVI = pulmonary vein isolation, CB = cryoballoon, temp. = temperature, TTI = time to isolation, FAAVI = first attempt all veins isolated.
The ablation data per individual PV is summarized in Table 3. No pop-out phenomenon after initializing the freezing process has been reported by the operators. For the comparison of the mean procedure time of the 1st 25 cases vs. the later cases from the 26th patient, a trend towards short duration but no statistical difference has been observed (T1: 97.1 ± 46 min vs. T2: 87.8 ± 35.4 min, 0.635). Similar findings have been detected for fluoroscopy time (T1: 16.4 ± 11 min vs. T2: 13.9 ± 8.6 min, P = 0.406).
Table 3.
Procedural details—individual pulmonary veins
| Variable | POLARx |
|---|---|
| LSPV: | 305 |
| Total cycles until PVI | 1.2 ± 0.5 |
| Total cycles | 1.4 ± 0.6 |
| FAVI, (%) | 228 (75) |
| Bonus freeze cycles | 19 (6) |
| Minimal temp., (C°) | −59.2 ± 5.6 |
| Minimal oesophageal temp., (C°) | 31.9 ± 5 |
| Time to PVI, s | 48.1 ± 25.1 |
| Rate of TTI recordings, (%) | 201/258 (77.9) |
| Duration of total freezing time, s | 251 ± 115 |
| LIPV: | 305 |
| Total cycles until PVI | 1.1 ± 0.2 |
| Total cycles | 1.2 ± 0.5 |
| FAVI, (%) | 263 (86) |
| Bonus freeze cycles | 16 (5) |
| Minimal temp., (C°) | −56.0 ± 5.2 |
| Minimal oesophageal temp., (C°) | 28.5 ± 8.2 |
| Time to PVI, s | 43.5 ± 29.2 |
| Rate of TTI recordings, (%) | 181/258 (70.2) |
| Duration of total freezing time, s | 217 ± 100 |
| LCPV: | 12 |
| Total cycles until PVI | 1.6 ± 0.9 |
| Total cycles | 2.3 ± 1.1 |
| FAVI, (%) | 6 (50) |
| Bonus freeze cycles | 2 (17) |
| Minimal temp., (C°) | −63.9 ± 8.1 |
| Minimal oesophageal temp., (C°) | 33.5 ± 1.2 |
| Time to PVI, s | 46.4 ± 13.6 |
| Rate of TTI recordings, (%) | 5/9 (55.6) |
| Duration of total freezing time, s | 413 ± 148 |
| RSPV: | 317 |
| Total cycles until PVI | 1.2 ± 0.5 |
| Total cycles | 1.4 ± 0.8 |
| FAVI, (%) | 239 (75) |
| Bonus freeze cycles | 18 (7) |
| Minimal temp., (C°) | −58.3 ± 9.5 |
| Minimal oesophageal temp., (C°) | 33.7 ± 3.6 |
| Time to PVI, s | 43.7 ± 29.4 |
| Rate of TTI recordings, (%) | 193/277 (69.7) |
| Duration of total freezing time, s | 240 ± 135 |
| RIPV: | 317 |
| Total cycles until PVI | 1.1 ± 0.4 |
| Total cycles | 1.3 ± 0.6 |
| FAVI, (%) | 249 (79) |
| Bonus freeze cycles | 16 (5) |
| Minimal temp., (C°) | −57.5 ± 7.0 |
| Minimal oesophageal temp., (C°) | 31.9 ± 6.0 |
| Time to PVI, s | 50.0 ± 30.0 |
| Rate of TTI recordings, (%) | 181/277 (65.3) |
| Duration of total freezing time, s | 226 ± 105 |
Values expressed as n (%), mean ± standard deviation or median (range) as appropriate. PV(s) = Pulmonary vein(s), PVI = pulmonary vein isolation, RSPV = right superior pulmonary vein, RIPV = right inferior pulmonary vein, LCPV = left common pulmonary vein, LSPV = left superior pulmonary vein LIPV left inferior pulmonary vein, temp. temperature, TTI = time to isolation, FAVI = first attempt vein isolated.
Peri- and post-procedural complications
Data on periprocedural complications are summarized in Table 4. The rate of serious adverse events was 6.0%. One patient (1/317, 0.3%) experienced a cardiac tamponade and was successfully treated by pericardiocentesis. A total of 4 of 317 patients (1.3%) experienced pericardial effusion without necessity of pericardiocentesis and conservative treatment. Three of those patients were discharged from the clinic without a prolonged hospital stay and were therefore not denoted as serious adverse event. One patient experienced a prolonged hospitalization with multiple echocardiographic controls and was discharged after 3 days and followed-up in the outpatient’s department after one week. A PNI occurred in 13 of 317 (4.1%) patients and was persistent until discharge in 10 of 317 (3.2%) patients and until 6 months of follow-up in 7 of 317 (2.2%) patients. The median time to PNI during the freeze cycle was 121 s (IQR: 81, 144), and the median temperature at PNI was −55°C (IQR −59, −53). A total of 12 of 13 PVs (92.3%) were isolated at the time of PNI. Concerning the PNI during PVI, the DMS warning was available in 6 (46%) of 13 patients. The median minimal DMS was 27% (IQR 21, 34).
Table 4.
Periprocedural complications
| All | T1 (1–25) | T2 (>25) | P | |
|---|---|---|---|---|
| Patients | 317 | 150 | 167 | |
| Serious adverse events | 19 (6.0) | 14 (9.3) | 5 (3.0) | 0.018 |
| Minor complications | 12 (3.8) | 8 (6.5) | 4 (2.4) | 0.171 |
| Death from any cause, n (%) | 0 | 0 | 0 | 1.000 |
| Pericardial tamponade with intervention, n (%) | 1 (0.3) | 1 (0.7) | 0 | 0.473 |
| Pericardial effusion without intervention, n (%) | 3 (0.9) | 2 (1.3) | 1 (0.6) | 0.500 |
| Pericardial effusion without intervention and prolonged hospitalization, n (%) | 1 (0.3) | 1 (0.7) | 0 | 0.473 |
| Atrioesophageal fistula, n (%) | 0 | 0 | 0 | 1.000 |
| Phrenic nerve injury, n (%) | 13 (4.1) | 10 (6.7) | 3 (1.8) | 0.029 |
| Phrenic nerve recovered until end of procedure, n (%) | 3 (0.9) | 2 (1.3) | 1 (0.6) | 0.500 |
| Phrenic nerve injury at RSPV, (%) | 9 (69.2) | 5 (3.3) | 4 (2.4) | 0.616 |
| Phrenic nerve injury at RIPV, (%) | 4 (30.8) | 2 (1.3) | 2 (1.2) | 0.914 |
| Phrenic nerve injury persistent until discharge, n (%) | 10 (3.2) | 8 (6.5) | 2 (1.2) | 0.035 |
| Phrenic nerve injury persistent at 6 months follow-up, n (%) | 7 (2.2) | 6 (4) | 1 (0.6) | 0.040 |
| Time to phrenic nerve injury (s) | 121 (81, 144) | |||
| Temperature at phrenic nerve injury (°C) | −55 (−59, −53) | |||
| PV isolated at time of phrenic nerve injury, n (%) | 12 (92.3) | |||
| Immediate stop at phrenic nerve injury, (%) | 10 (77) | |||
| Double stop at phrenic nerve injury, n (%) | 8 (62) | |||
| DMS warning at phrenic nerve injury, (%) | 6 (46) | |||
| Minimal DMS at phrenic nerve injury, (%) | 27 (21, 34) | |||
| Stroke, n (%) | 2 (1.7) | 1 (0.7) | 1 (0.6) | 0.939 |
| Transient ischaemic attack, n (%) | 1 (0.3) | 1 (0.7) | 0 | 0.473 |
| Severe bleeding of the puncture site, n (%) | 0 | 0 | 0 | 1.000 |
| Minor bleeding of the puncture site, n (%) | 2 (1.7) | 1 (0.7) | 1 (0.6) | 0.939 |
| Groin aneurysm with conservative treatment, n (%) | 1 (0.3) | 0 | 1 (0.6) | 0.999 |
| Arterial-venous fistula with conservative treatment, n (%) | 2 (1.7) | 2 (1.3) | 0 | 0.223 |
| Transient air embolism, n (%) | 5 (1.6) | 3 (2) | 2 (1.2) | 0.567 |
| ST-elevation during air embolism, n (%) | 5 (1.6) | 3 (2) | 2 (1.2) | 0.567 |
Values expressed as n (%), mean ± standard deviation or median (range) as appropriate. PV(s) = Pulmonary vein(s), PVI = pulmonary vein isolation, RSPV = right superior pulmonary vein, RIPV = right inferior pulmonary vein, DMS = diaphragm movement sensor. Only events adjudicated as possible, probable, or definitely related to the ablation procedure were mentioned. Bold numbers are denoted as serious adverse events. An adverse event was considered serious if it resulted in permanent injury or death, required an intervention for treatment, or required hospitalization for more than 24 h.
Stroke and transient ischaemic attack were reported in 3 of 317 (0.9%) patients. In one patient, the neurological impairment was completely regredient during the hospitalization. Two other patients showed prolonged neurological impairment.
An air embolism was observed in 5 of 317 (1.6%) patients. It was detected by a significant ST-elevation in the 12-lead ECG. In all cases, an immediate coronary angiography was performed and intracoronary air-bubbles were detected within the right coronary artery (4/5, 80%) or the left descending coronary artery (1/5, 20%). In 4 of 5 cases, the ST-elevation disappeared during the coronary angiography, while in one patient a significant stenosis of the left descending artery was detected and the patient was treated by PCI. No durable impairment of left ventricular systolic function was detected in patients with periprocedural coronary air embolism. In 4 of 5 (80%) cases, the air embolism occurred directly after PV angiography and in patients where the angiography was performed utilizing a 7F multipurpose catheter together with the POLAR-SHEATH. In all cases, the procedure was finished after regression of air embolism. No known atrioesophageal fistula, periprocedural death or PV stenosis were observed. For the comparison of periprocedural complications for T1 and T2 significant differences were found for serious adverse events (T1: 9.3%, T2: 3.0%. P = 0.018). For minor complications no differences have been detected, P = 0.171.
The freeze cycle was prematurely terminated due to achievement of the oesophageal temperature cut-off as followed: LSPV: n = 3 (0.9%), median time to termination: 170 s (IQR: 165, 170); LIPV: n = 5 (1.6%), median time to termination 120 s (IQR: 120, 150); RIPV: n = 4 (1.3%), median time to termination 126 s (IQR: 109, 140); RSPV: n = 1 (0.3%) time to termination: 127 s. In all cases, the individual PV was already isolated when the freeze cycle was terminated.
Follow-up and clinical success
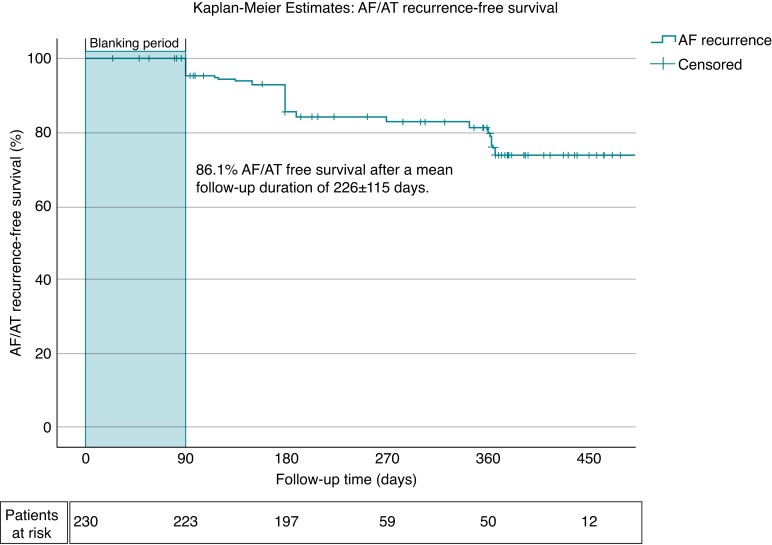
In a total of 230 of 317 patients (72.6%), at least 3 months follow-up was available. The rate of AF-/AT-free survival after mean follow-up duration of 226 ± 115 days and a 90-day blanking period was 86.1% (198/230 patients, Figure 2). The mean time to recurrence was 177 ± 85 days.
Figure 2.
Kaplan–Meier estimates of AF/AT recurrence-free survival: The Kaplan–Meier estimates demonstrate the relative proportion of patients in stable sinus rhythm following index PVI using the POLARx cryoballoon. AF = atrial fibrillation, AT = atrial tachycardia.
Discussion
The current ANTARCTICA study set out to assess the procedural efficacy, mid-term outcome, safety and characteristics of the novel POLARx CB for PVI. The study offers the up-to-date largest patient number treated by this ablation system in a multicentre international study. The major findings are (i) with 99.7% of PVs, the POLARx provides a high rate of acute efficacy, (ii) the rate of real-time PV recordings was 71.9%, (iii) the procedural duration as well as fluoroscopy time were 92 ± 41 min and 15 ± 10 min, (iv) with 6.0% of overall periprocedural complication rate, the POLARx ablation system provides an acceptable safety profile. (v) After a learning curve of 25 cases, the rate of severe adverse events significantly decreased (3%) with comparable findings to recent observations utilizing the AFA-CB2 system.2
Since the Fire-and-Ice trial showed non-inferiority of CB-based PVI compared with RF, the number of performed CB-based PVI has significantly increased.2 Owing to the fact that balloon-based PVI provides shorter learning curves, shorter procedures times combined with an favourable efficacy and safety profile, various catheter ablation systems utilizing different energy sources, as well as catheter designs and ablation techniques are currently under investigation. However, randomized controlled data are only available for the AFA-CB2 and the laserballoon (Heartlight, Cardiofocus).2,16,17 Although the AFA-CB2 and its recent update AFA-Pro (CB4) provides excellent clinical outcome and performance, the POLARx system offers some unique tools which possibly improve patient safety, efficacy, and the operators convenience.6,9 The ICE-AGE-X as well as some other studies demonstrates that the POLARx ablation system provides equal acute success and equal rate of periprocedural complications compared to the AFA-CB4.6,9,18 The aim of the ANTARCTICA study was to pool multicentre data to improve data quality and quantity with the focus on acute efficacy and safety of the novel POLARx CB.
Efficacy
With 99.7% the acute efficacy of the POLARx was promising and is in line with recent studies and findings of the AFA.6,12 Recent studies focusing on the POLARx found that the minimal CB nadir temperature was significantly lower compared with the AFA-CB (e.g. ICE-AGE-X: −57 ± 7°C vs −50 ± 6°C, P = 0.0046, Moser et al: −60 [−65, −55]°C vs. −48 [−54, −45] °C, P < 0.0019, Yap et al.: −55°C vs. −47°C, P < 0.0015). The reason for this observation is still unknown. Since the location of the thermocouple on the CB shaft is similar between both CB systems, a reason for this effect may be a different CB material or the fact that the POLARx is more compliant compared with the AFA. This is possibly leading (i) to a more efficient PV occlusion with reduced cryoenergy conduction by reduced blood flow around the balloon and (ii) to a more distal CB position inside the PVs. However, the suggested minimal CB temperature threshold is −70°C which is 10°C lower compared with the AFA.
As mentioned previously, one of the most important differences of the two CB systems is the different balloon pressure during positioning and freezing. Since the pressure of the POLARx stays at the same level after initializing the refrigerant injection CB dislodgement from the PV ostium (pop-out phenomenon) might be reduced. Remarkably, pop-out phenomenon was not observed in single procedure of 317 cases, strongly suggesting that the intended concept of a more compliant balloon translates into a procedural benefit in daily practice compared to the established CB ablation system (CB2/CB4). Although a trend towards shorter procedure and fluoroscopy duration have been found after a learning curve of 25 cases no statistical differences were detected.
The POLARMAP catheter provides a high rate of online visualization of PV signals (71%) by mainly using the ST POLARx. Recent observation of real-time isolations utilizing the AF-CB4 showed 69%–84.8% which are similar to observed rate for the POLARx found in our analysis.12,13 With recurrence-free survival of 86.1% after a mean of >6 months short-term follow-up seems to be promising.
Safety
With a serious adverse event rate of 6.0% the POLARx showed an acceptable rate of periprocedural complications. After a learning curve of 25 cases, a significant decrease of the rate of serious adverse events have been detected which was comparable with the findings of multicentre trials such as the FIRE and ICE trial2 and the CIRCA DOSE trial.10 Characteristic complications of CB-based PVI such as PNI could be significantly reduced by utilizing recent strategies such as CMAP, PN pacing, and tactile feedback.14,15,19,20 The new DMS sensor offers an additional tool to further improve safety of CB-based procedures. Nevertheless, the rate of periprocedural PNI was 4.1% which is similar to the finding of the recently published multicentre multinational YETI registry (4.2%).14 Almost 50% of PNI recovered within 6 months of follow-up.14 However, the DMS sensor was only warning the operator in 46% of PNI cases and did not lead to a relevant reduction of PNI compared with the finding of the YETI registry where no DMS sensor was available. This observation might be part of a learning curve effect, yet the DMS sensors efficacy and benefit needs to be evaluated on further studies.
A relatively high rate of transient air embolism was observed, mainly related to the PV angiography. The fact that the PV angiography was performed with a combination of a 7F multipurpose catheter together with the 15.9F steerable sheath in 80% of those patients might explain this observation. Therefore, the authors suggest to avoid this mismatched combination and either perform the PV angiography directly via the 8.5F transseptal sheath or via a 7F multipurpose catheter in combination with the 8.5F transseptal sheath. Although some data have been published for the comparison of POLARx and AF-CB, no randomized data are available to date. Currently, there is one ongoing randomized controlled trial comparing POLARx and AF-CB4 for the treatment of paroxysmal AF (COMPARE-CRYO, ClinicalTrials.gov Identifier: NCT04704986). The primary endpoint is time to recurrence of AF or AT within 3–12 months, secondary endpoints are periprocedural complications, procedure duration and further periprocedural characteristics.
Although CB-based PVI is increasingly performed worldwide, recent findings of the newly developed pulsed field ablation (PFA) energy source are very promising, and PFA will be a potentially strong competitor for single-shot PVI procedures. Yet, no randomized data on head-to-head comparison to RF or CB is available yet.
Limitations
Only patients with POLARx-based PVI have been included and no comparison to a control group was conducted. Yet, consecutive patients were evaluated in this multicentre study, which represents the largest analysis on the POLARx CB up to date. The data was acquired prospectively in 5 of 6 centres, whereas one centre provided retrospective data. This fact is limiting the data quality. Owing to the recent launch of the POLARx CB, only acute efficacy, mid-term follow-up, and safety data are provided, whereas long-term clinical outcome will need future assessment. No independent data and safety monitoring board was installed to monitor the study. Therefore, a monitoring bias cannot be excluded. The rate of available follow-up was relatively low, which is limiting the findings of mid-term follow-up. The patient’s outcome was relatively good despite the fact that the rate of PAF patients was only 66%. This observation may be linked to the relatively short mid-term follow-up period. Larger studies and randomized studies are needed to compare the safety and efficacy of the POLARx CB on a longer follow-up period.
Conclusions
To the best of our knowledge, this is the first study reporting on the acute efficacy, mid-term outcome and safety of POLARx-based PVI in a multicentre study. Even experienced CB users may observe significantly more complications during the initial 25 cases. After passing the learning curve, the POLARx CB showed a promising acute efficacy and safety profile.
Contributor Information
Christian-H Heeger, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein (UKSH), Ratzeburger Allee 160, D-23538 Lübeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Alexander Pott, Internal Medicine II, Department of Cardiology, Ulm, Germany.
Christian Sohns, Herz und Diabeteszentrum, Klinik für Rhythmologie, Herz- und Diabeteszentrum NRW, Bad Oeynhausen, Germany.
Lisa Riesinger, Universitätsklinikum Essen, Westdeutsches Herz- und Gefäßzentrum Essen, Germany.
Philipp Sommer, Herz und Diabeteszentrum, Klinik für Rhythmologie, Herz- und Diabeteszentrum NRW, Bad Oeynhausen, Germany.
Alessio Gasperetti, Centro Cardiologico Monzino, Heart Rhythm Center at IRCCS Centro Cardiologico Monzino, Milan, Italy.
Claudio Tondo, Centro Cardiologico Monzino, Heart Rhythm Center at IRCCS Centro Cardiologico Monzino, Milan, Italy.
Gaetano Fassini, Centro Cardiologico Monzino, Heart Rhythm Center at IRCCS Centro Cardiologico Monzino, Milan, Italy.
Fabian Moser, University heart center of Hamburg Eppendorf, Germany.
Philipp Lucas, Herz und Diabeteszentrum, Klinik für Rhythmologie, Herz- und Diabeteszentrum NRW, Bad Oeynhausen, Germany.
Karolina Weinmann, Internal Medicine II, Department of Cardiology, Ulm, Germany.
Jan-Eric Bohnen, Universitätsklinikum Essen, Westdeutsches Herz- und Gefäßzentrum Essen, Germany.
Tillman Dahme, Internal Medicine II, Department of Cardiology, Ulm, Germany.
Andreas Rillig, University heart center of Hamburg Eppendorf, Germany.
Karl-Heinz Kuck, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein (UKSH), Ratzeburger Allee 160, D-23538 Lübeck, Germany; LANS Cardio, Stephansplatz 5, 20354, Hamburg, Germany.
Reza Wakili, Universitätsklinikum Essen, Westdeutsches Herz- und Gefäßzentrum Essen, Germany.
Andreas Metzner, University heart center of Hamburg Eppendorf, Germany.
Roland R Tilz, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein (UKSH), Ratzeburger Allee 160, D-23538 Lübeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Data availability
Sharing of data is not possible due to ethical aspects.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KRet al. . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann E, Straube F, Wegscheider K, Kuniss M, Andresen D, Wu LQet al. . Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace 2019;21:1313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su WW. A second cryoballoon system—New and improved? J Cardiovasc Electr 2021;32:931–2. [DOI] [PubMed] [Google Scholar]
- 5. Yap S, Anic A, Breskovic T, Haas A, Bhagwandien RE, Jurisic Zet al. . Comparison of procedural efficacy and biophysical parameters between two competing cryoballoon technologies for pulmonary vein isolation: Insights from an initial multicenter experience. J Cardiovasc Electr 2021;32:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tilz RR, Meyer-Saraei R, Eitel C, Fink T, Sciacca V, Lopez LDet al. . Novel cryoballoon ablation system for single shot pulmonary vein isolation - The prospective ICE-AGE-X study. Circ J 2021;85:1296–304. [DOI] [PubMed] [Google Scholar]
- 7. Kochi AN, Moltrasio M, Tundo F, Riva S, Ascione C, Dessanai MAet al. . Cryoballoon atrial fibrillation ablation: single-center safety and efficacy data using a novel cryoballoon technology compared to a historical balloon platform. J Cardiovasc Electr 2021;32:588–94. [DOI] [PubMed] [Google Scholar]
- 8. Creta A, Kanthasamy V, Schilling RJ, Rosengarten J, Khan F, Honarbakhsh Set al. . First experience of POLARxTM versus Arctic Front AdvanceTM: an early technology comparison. J Cardiovasc Electr 2021;32:925–30. [DOI] [PubMed] [Google Scholar]
- 9. Moser F, Rottner L, Moser J, Schleberger R, Lemoine M, Münkler Pet al. . The established and the challenger: a direct comparison of current cryoballoon technologies for pulmonary vein isolation. J Cardiovasc Electr 2022;33:48–54. [DOI] [PubMed] [Google Scholar]
- 10. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle Let al. . Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring. Circulation 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 11. Pott A, Messemer M, Petscher K, Iturbe-Orbe M, Bothner C, Rottbauer Wet al. . Clinical outcome of 2nd generation cryoballoon pulmonary vein isolation in patients over 75 years of age. J Cardiol 2017;69:24–9. [DOI] [PubMed] [Google Scholar]
- 12. Heeger C-H, Popescu SS, Saraei R, Kirstein B, Hatahet S, Samara Oet al. . Individualized or fixed approach to pulmonary vein isolation utilizing the fourth-generation cryoballoon in patients with paroxysmal atrial fibrillation: the randomized INDI-FREEZE trial. Europace 2022;24:921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Straube F, Dorwarth U, Pongratz J, Bruck B, Wankerl M, Hartl Set al. . The fourth cryoballoon generation with a shorter tip to facilitate real-time pulmonary vein potential recording: feasibility and safety results. J Cardiovasc Electrophysiol 2019;30:918–25. [DOI] [PubMed] [Google Scholar]
- 14. Heeger C-H, Sohns C, Pott A, Metzner A, Inaba O, Straube Fet al. . Phrenic nerve injury during cryoballoon-based pulmonary vein isolation: results of the worldwide YETI registry. Circulation Arrhythmia Electrophysiol 2021:CIRCEP121010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyazaki S, Kajiyama T, Watanabe T, Hada M, Yamao K, Kusa Set al. . Characteristics of phrenic nerve injury during pulmonary vein isolation using a 28-mm second-generation cryoballoon and short freeze strategy. J Am Heart Assoc 2018;7:e008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt B, Neuzil P, Luik A, Asensi JO, Schrickel JW, Deneke Tet al. . Laser balloon or wide-area circumferential irrigated radiofrequency ablation for persistent atrial fibrillation. Circulation Arrhythmia Electrophysiol 2018;10:e005767. [DOI] [PubMed] [Google Scholar]
- 17. Dukkipati SR, Cuoco F, Kutinsky I, Aryana A, Bahnson TD, Lakkireddy Det al. . Pulmonary vein isolation using the visually guided laser balloon: a prospective, multicenter, and randomized comparison to standard radiofrequency ablation. J Am Coll Cardiol 2015;66:1350–60. [DOI] [PubMed] [Google Scholar]
- 18. Guckel D, Lucas P, Isgandarova K, Hamriti ME, Bergau L, Fink Tet al. . News from the cold chamber: clinical experiences of POLARx versus Arctic Front Advance for single-shot pulmonary vein isolation. J Cardiovasc Dev Dis 2022;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franceschi F, Koutbi L, Gitenay E, Hourdain J, Maille B, Trevisan Let al. . Electromyographic monitoring for prevention of phrenic nerve palsy in second-generation cryoballoon procedures. Circ ArrhythmElectrophysiol 2015;8:303–7. [DOI] [PubMed] [Google Scholar]
- 20. Metzner A, Rausch P, Lemes C, Reissmann B, Bardyszewski A, Tilz Ret al. . The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol 2014;25:466–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sharing of data is not possible due to ethical aspects.