Abstract
Background
Many COVID-19 patients are left with symptoms several months after resolution of the acute illness; this syndrome is known as post-acute sequalae of COVID-19 (PASC). We aimed to determine the prevalence of objective hemodynamic cardiovascular autonomic abnormalities (CAA), explore sex differences, and assess the prevalence of CAA among hospitalized vs nonhospitalized patients with PASC.
Methods
Patients with PASC (n = 70; female [F] = 56; 42 years of age; 95% confidence interval [CI], 40-48) completed standard autonomic tests, including an active stand test 399 days (338, 455) after their COVID-19 infection. Clinical autonomic abnormalities were evaluated.
Results
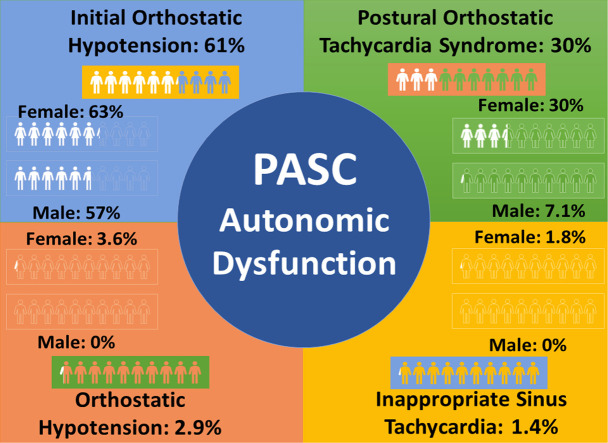
Most patients with PASC met the criteria for at least 1 CAA (51; 73%; F = 43). The postural orthostatic tachycardia syndrome hemodynamic (POTSHR) criterion of a heart rate increase of > 30 beats per minute within 5 to 10 minutes of standing was seen in 21 patients (30%; F = 20; P = 0.037 [by sex]). The initial orthostatic hypotension hemodynamic (IOH40) criterion of a transient systolic blood pressure change of > 40 mm Hg in the first 15 seconds of standing was seen in 43 (61%) patients and equally among female and male patients (63% vs 57%; P = 0.7). Only 9 (13%) patients were hospitalized; hospitalized vs nonhospitalized patients had similar frequencies of abnormalities (67% vs 74%; P = 0.7).
Conclusions
Patients with PASC have evidence of CAA, most commonly IOH40, which will be missed unless an active stand test is used. Female patients have increased frequency of POTSHR, but IOH40 is equally prevalent between sexes. Finally, even nonhospitalized “mild” infections can result in long-term CAAs.
Graphical abstract
Résumé
Contexte
De nombreux patients ayant contracté la COVID-19 présentent toujours des symptômes plusieurs mois après la phase aiguë de la maladie; on parle alors du syndrome post-COVID-19. L’étude visait à établir objectivement la prévalence des anomalies hémodynamiques de la fonction autonome cardiovasculaire (AHFAC), notamment chez les patients hospitalisés et non hospitalisés présentant le syndrome post-COVID-19, et à déterminer s’il y a des différences entre les sexes.
Méthodologie
Des patients atteints du syndrome post-COVID-19 (n = 70; femmes [F] = 56; âge moyen = 42 ans; intervalle de confiance [IC] à 95 % : 40 à 48) se sont soumis à des tests standard de la fonction autonome, notamment à un test d’orthostatisme, 399 jours (338, 455) après l’infection par le virus de la COVID-19, qui visaient à évaluer les anomalies cliniques.
Résultats
La plupart des patients présentant le syndrome post-COVID-19 satisfaisaient aux critères définissant au moins une AHFAC (51; 73 %; F = 43). Un syndrome de tachycardie orthostatique posturale (STOPFC), soit une augmentation de la fréquence cardiaque (FC) de plus de 30 battements par minute après une période de 5 à 10 minutes en position debout, a été observé chez 21 patients (30 %; F = 20; p = 0,037 [par sexe]). Une hypotension orthostatique initiale (HOI40), soit une variation transitoire de la pression artérielle systolique supérieure à 40 mmHg dans les 15 secondes suivant la mise en position debout, a été observée chez 43 (61 %) patients, aussi bien des hommes que des femmes (63 % c. 57 %; p = 0,7). Seulement neuf (13 %) patients avaient été hospitalisés, et les anomalies étaient aussi fréquentes chez les patients hospitalisés que chez les patients non hospitalisés (67 % c. 74 %; p = 0,7).
Conclusions
Les patients présentant le syndrome post-COVID-19 montraient des signes d’AHFAC, le plus souvent une HOI40, qui risquent de passer inaperçus si un test d’orthostatisme n’est pas réalisé. Les femmes sont plus sujettes au STOPFC, mais l’HOI40 est tout aussi prévalente chez les hommes que chez les femmes. Enfin, même une infection « légère » n’ayant pas nécessité d’hospitalisation peut entraîner des AHFAC à long terme.
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has affected the health and economies of Canada and the world. Worldwide, there have been over 560 million cases and 6 million deaths attributed to COVID-19 (SARS-CoV-2 disease).1 Although most patients recover, many are left with residual and sometimes disabling symptoms several months after resolution of the acute illness.
These ongoing symptoms have been termed post-acute sequelae of COVID-19 syndrome (PASC), defined as symptoms that develop during or after an infection consistent with COVID-19, continue for >12 weeks, and are not explained by an alternative diagnosis.2 Common symptoms include exercise intolerance, dyspnea, fatigue, lightheadedness, and tachycardia- palpitation,2 , 3 which are common in cardiovascular autonomic abnormalities (CAA).
Although studies have reported CAA in patients with PASC, including reports of new-onset postural orthostatic tachycardia syndrome (POTS),4, 5, 6, 7, 8, 9, 10 inappropriate sinus tachycardia (IST),9 , 11 and orthostatic hypotension (OH),12 these studies have been retrospective,7 had small patient sample sizes,5, 6, 7 or did not perform autonomic evaluations using standard tests of autonomic function.13 Further, most studies used a tilt table test8 , 14 and not an active stand test,15 which, in conjunction with beat-to-beat hemodynamic monitoring, is required to diagnose initial orthostatic hypotension (IOH).15 , 16 As such, at present, we do not know the prevalence of objective CAA in patients with PASC based on standard tests of autonomic function.17
We determined the frequency of objective CAA in a cohort of patients with PASC and examined whether there was a sex dimorphism in the frequency of these abnormalities and whether they were more frequent among patients hospitalized for their COVID-19 infections.
Methods
Participants
Patients with PASC between 18 and 80 years of age were recruited from the Calgary Autonomic Investigation and Management Clinic, the Alberta Health Services Calgary COVID Clinics, and through local advertising. All patients met the consensus statement criteria for PASC,9 including a positive SARS-CoV-2 polymerase chain reaction test and symptoms consistent with COVID-19 lasting > 12 weeks following a COVID-19 infection.18 In our cohort, 80% of patients were not vaccinated before their initial COVID-19 infection. Participants were excluded if they were unable to provide informed consent or could not safely withdraw from medications affecting heart rate and blood pressure. The Conjoint Health Research Ethics Board at the University of Calgary (REB21-1188) provided ethical oversight and approval for this study. All participants provided written informed consent before study participation. Participants also completed the Composite Autonomic Symptom Score (COMPASS-31) questionnaire and a general autonomic symptoms questionnaire via research electronic data capture (REDCap).19
Instrumentation
Studies were conducted in a postvoid and postabsorptive state at least 2 hours after a meal. Continuous heart rate (HR) and blood pressure (BP) were recorded using a 5-lead electrocardiogram (ECG) (IVY Biomedical Model 450C, Branford, CT) and noninvasive beat-to-beat finger cuff (BMEYE, Amsterdam, The Netherlands). Brachial BP measurements were obtained before, during, and after the tests to verify finger cuff BP recordings. Advanced hemodynamics, including stroke volume (SV), cardiac output (CO), and systemic vascular resistance (SVR), as well as indices corrected for body surface area (BSA) (ie, SV index [SVI], cardiac index [CI], and SVR index [SVRI]) were calculated using waveform based Modelflow software (BMEYE, Amsterdam, The Netherlands). All analogue signals were sampled at 500 Hz (WinDaq, DATAQ Corporation, San Diego, CA) and stored digitally for off-line analysis using custom software written in MATLAB r2021b (December 2021, Mathworks, Natick, MA).
Assessment of hemodynamic criteria for cardiovascular autonomic abnormalities
Following a 10-minute baseline in the supine position, participants completed an active stand test,15 in which they were instructed to stand as quickly as possible and remain standing for 10 minutes.
Hemodynamic criteria for cardiovascular autonomic disorders were determined using HR and BP changes during the active stand test. The hemodynamic criterion for OH was defined as a systolic BP (SBP) drop of ≥ 20 mm Hg within 3 minutes of standing (OH20).20, 21, 22 The hemodynamic criterion for POTS was defined as a HR increase ≥ 30 beats per minute (bpm) within 10 minutes of standing in the absence of OH (POTSHR).21 , 23 IOH was defined as a transient SBP drop of ≥ 40 mm Hg within 15 seconds of standing, with recovery within 45 seconds (IOH40).24 IST was assessed as a resting supine HR >100 bpm (IST100).25
Autonomic function testing
Participants completed the following standard tests of autonomic function: quantitative sudomotor axon reflex testing (QSART), deep breathing, and Valsalva manoeuvre. QSART (QSWEAT, WR Medical Electronics Co., Stillwater, MN) was used to evaluate postganglionic peripheral sympathetic nerve integrity. Axon terminals were transdermally iontophoresed at a constant current (2 mA) for 5 minutes with 10% acetylcholine at 4 standard sites (left forearm, left proximal leg, left distal leg, and left foot),26 followed by a 5-minute poststimulation period. Total sweat volumes were calculated as the area under the curve over 10 minutes.
Cardiovagal function was assessed using deep breathing and the Valsalva manoeuvre. During deep breathing,17 participants were instructed to breathe at a rate of 6 breaths per minute for 90 seconds. The peak-to-trough difference over 5 consecutive breaths was averaged to provide an average HR response to deep breathing (ΔHRDB). For the Valsalva manoeuvre,17 participants were instructed to blow into a tube with an air leak (to ensure an open glottis) and to maintain an expiratory pressure of 40 mmHg for 15 seconds. A Valsalva ratio was calculated by dividing the maximum HR obtained during the manoeuvre by the minimum HR in the 30s immediately following release.
The Composite Autonomic Severity Score (CASS) was calculated for each participant to quantify the severity and distribution of autonomic dysfunction normalized for age and sex.17 This 10-point scale evaluates autonomic dysfunction across 3 functional domains: sudomotor, adrenergic, and cardiovagal.17
Autonomic symptom assessment
Before their visit, all participants completed the COMPASS-31 Autonomic Symptom Score questionnaire.27 The COMPASS-31, composed of 31 self-reported questions with a total score of 100, quantifies symptoms associated with autonomic dysfunction in the following domains: orthostatic intolerance (maximum score of 40), vasomotor (maximum score of 5), secretomotor (maximum score of 15), gastrointestinal (maximum score of 25), bladder (maximum score of 10), and pupillomotor (maximum score of 5). Participants also completed a questionnaire pertaining to common clinical symptoms in PASC9 (Supplemental Table S1).
Active stand symptom assessment
Participants were asked to report symptoms they experienced during the active stand test. Specifically, participants were asked to report if they experienced symptoms within the first 60 seconds of the active stand. At the end of each stand, orthostatic symptoms were also assessed using the Vanderbilt Orthostatic Symptoms Score (VOSS).28, 29, 30 In brief, the VOSS assesses 9 orthostatic symptoms, with scores ranging from 0 (no symptoms) to 10 (worst symptoms), with higher scores indicating worse orthostatic intolerance.
Statistical analyses
Continuous data are presented as median (95% CI). Categorical data are presented as number (%). A Fisher’s exact test was used to compare categorical variables, whereas continuous variables were compared using Mann-Whitney U tests. A 2-tailed P value of < 0.05 was deemed statistically significant.
Subgroup analyses were conducted to evaluate sex (male vs female) differences and hospitalization (hospitalized vs nonhospitalized) differences. Statistical analyses were performed using SPSS statistical software for Windows version 28 (SPSS, Inc., IBM, Armonk, NY). Figures were created using GraphPad Prism (version 8.0.0 for Windows, GraphPad Software, San Diego, CA, www.graphpad.com).
Results
Demographics
Patients with PASC (n = 70; 42 years [40,48]) were evaluated for CAA a median of 399 days (338,455) following an acute infection with COVID-19 (Table 1 ).
Table 1.
Participant characteristics, hemodynamics, and symptoms in patients with PASC with and without hemodynamic autonomic abnormalities
| Characteristic | Patients with PASC: overall (n = 70) | Patients with PASC with CAA (n = 51) | Patients with PASC without CAA (n = 19) | P value |
|---|---|---|---|---|
| Age, years | 42 (40, 48) | 44 (40, 49) | 41 (36, 49) | 0.409 |
| Female | 56 (80%) | 43 (84%) | 13 (68%) | 0.139 |
| Height, cm | 168 (165, 170) | 167 (165, 170) | 170 (161, 173) | 0.979 |
| Weight, kg | 77 (71, 88) | 78 (71, 90) | 73 (60, 88) | 0.222 |
| BMI, kg/m2 | 27.2 (24.7, 28.7) | 27.8 (25.2, 31.5) | 26.1 (22.7, 28.5) | 0.237 |
| BSA, m2 | 1.92 (1.82, 2.02) | 1.92 (1.83, 2.07) | 1.85 (1.69, 2.08) | 0.202 |
| Race | 0.727 | |||
| Caucasians | 64 (91.4%) | 46 (90.2%) | 18 (94.7%) | |
| South Asians | 2 (2.9%) | 2 (3.8%) | 0 (0%) | |
| Indigenous Canadians | 1 (1.4%) | 1 (2.0%) | 0 (0%) | |
| West Asians | 1 (1.4%) | 1 (2.0%) | 0 (0%) | |
| ≥1 race | 2 (2.9%) | 1 (2.0%) | 1 (5.3%) | |
| Duration since initial COVID infection, days | 399 (338, 455) | 418 (341, 456) | 344 (319, 477) | 0.989 |
| Hospitalized with COVID-19 infection | 9 (13%) | 6 (12%) | 3 (16%) | 0.655 |
| Autonomic function testing | ||||
| Sympathetic nerve integrity (total sweat volumes) | ||||
| Forearm, μL | 0.51 (0.39, 0.74) | 0.48 (0.35, 0.64) | 0.86 (0.39, 1.47) | 0.093 |
| Proximal leg, μL | 0.39 (0.32, 0.66) | 0.38 (0.30, 0.54) | 0.70 (0.12, 1.23) | 0.276 |
| Distal leg, μL | 0.49 (0.40, 0.67) | 0.46 (0.36, 0.54) | 0.74 (0.20, 0.167) | 0.063 |
| Foot, μL | 0.45 (0.30, 0.55) | 0.44 (0.29, 0.55) | 0.48 (0.22, 0.81) | 0.663 |
| Cardiovagal function | ||||
| ΔHRDB (bpm) | 15 (12, 18) | 15 (12, 18) | 15 (10, 20) | 0.769 |
| VR | 1.75 (1.67, 1.91) | 1.74 (1.64, 1.92) | 1.82 (1.55, 2.11) | 0.840 |
| CASS | ||||
| Overall CASS | 1 (1, 2) | 1 (1, 2) | 1 (0, 2) | 0.163 |
| Sudomotor | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | 0.701 |
| Cardiovagal | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | 0.702 |
| Adrenergic | 1 (1, 1) | 1 (1, 1) | 0 (0, 1) | 0.06 |
| Autonomic symptom assessment | ||||
| COMPASS-31 domains | ||||
| Orthostatic intolerance | 20 (16, 20) | 20 (16, 24) | 16 (12, 20) | 0.038 |
| Vasomotor | 0 (0, 0) | 0 (0, 0) | 0 (0, 0.33) | 0.578 |
| Secretomotor | 6.43 (4.29, 6.43) | 6.43 (4.29, 6.43) | 6.43 (4.29, 8.57) | 0.757 |
| Gastrointestinal | 7.14 (6.25, 8.92) | 8.04 (6.25, 9.82) | 7.14 (4.46, 10.7) | 0.736 |
| Bladder | 1.11 (0, 1.11) | 1.11 (0, 1.11) | 1.11 (0, 2.22) | 0.693 |
| Pupillomotor | 2.33 (2.0, 2.67) | 2.33 (2.0, 2.67) | 2.33 (1.67, 3.0) | 0.873 |
| Total | 36 (31, 40) | 37 (31, 40) | 32 (23, 40) | 0.288 |
| PASC symptoms | ||||
| Lightheadedness | 55 (79%) | 40 (78%) | 15 (79%) | 0.963 |
| Shortness of breath | 51 (74%) | 37 (74%) | 14 (74%) | 0.979 |
| Palpitations | 49 (70%) | 37 (73%) | 12 (63%) | 0.446 |
| Fatigue | 64 (91%) | 48 (94%) | 16 (84%) | 0.188 |
| Headache | 41 (59%) | 30 (60%) | 11 (58%) | 0.874 |
| Loss or change in taste | 21 (31%) | 16 (33%) | 5 (26%) | 0.612 |
| Constipation | 20 (29%) | 16 (32%) | 4 (21%) | 0.371 |
| Problems with sleeping | 51 (75%) | 38 (76%) | 13 (72%) | 0.751 |
Values are expressed as Median (95% CI). P values were generated from Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables.
BMI, body mass index; BSA, body surface area; CAA, cardiovascular autonomic abnormalities; CASS, Composite Autonomic Severity Score; CI, confidence interval; COMPASS-31, Composite Autonomic Symptom Score; ΔHRDB, delta heart rate in deep breathing; PASC, post-acute sequelae of COVID-19; VR, Valsalva ratio.
Sex-based analysis
Fifty-six female patients (80%; 42 years [40,47]), and 14 male patients (20%; 50 years [31,63]) were evaluated a median of 427 days (357,461) and 324 days (285,475) after a COVID-19 infection (Table 2 ).
Table 2.
Participant characteristics, hemodynamics, and symptoms in male vs female patients with PASC
| Characteristic | Female patients with PASC (n = 56) | Male patients with PASC (n = 14) | P value |
|---|---|---|---|
| Age, years | 42 (40, 47) | 50 (31, 63) | 0.577 |
| Height, cm | 167 (163, 168) | 179 (172, 183) | < 0.001 |
| Weight, kg | 72 (67, 83) | 89 (78, 109) | 0.015 |
| BMI, kg/m2 | 26.3 (23.7, 29.1) | 27.9 (26.1, 33.6) | 0.322 |
| BSA, m2 | 1.84 (1.77, 1.92) | 2.1 (2.0, 2.33) | 0.004 |
| Race | 0.060 | ||
| Caucasians | 52 (92.8%) | 12 (85.8%) | |
| South Asians | 2 (3.6%) | 0 (0%) | |
| West Asians | 0 (0%) | 1 (7.1%) | |
| Indigenous Canadian | 0 (0%) | 1 (7.1%) | |
| ≥ 1 race | 2 (3.6%) | 0 (0%) | |
| Duration since initial COVID infection, days | 427 (357, 461) | 324 (285, 475) | 0.083 |
| Hospitalized with COVID-19 infection | 6 (10.7%) | 3 (4.3%) | 0.284 |
| Autonomic function testing | |||
| Sympathetic nerve integrity (total sweat volumes) | |||
| Forearm, μL | 0.47 (0.37, 0.59) | 1.08 (0.28, 1.84) | 0.01 |
| Proximal leg, μL | 0.38 (0.27, 0.55) | 0.72 (0.10, 1.41) | 0.093 |
| Distal leg, μL | 0.48 (0.38, 0.57) | 0.75 (0, 1.77) | 0.277 |
| Foot, μL | 0.39 (0.28, 0.50) | 1.18 (0, 2.04) | 0.028 |
| Cardiovagal function | |||
| ΔHRDB (bpm) | 15 (12, 18) | 14 (11, 24) | 0.489 |
| VR | 1.82 (1.67, 1.94) | 1.73 (1.38, 2.11) | 0.146 |
| CASS | |||
| Overall CASS | 1 (1, 2) | 2 (1, 3) | 0.468 |
| Sudomotor | 0 (0, 0) | 0 (0, 2) | 0.245 |
| Cardiovagal | 0 (0, 0) | 0 (0, 1) | 0.972 |
| Adrenergic | 1 (1, 1) | 1 (0, 1) | 0.960 |
| Autonomic symptom assessment | |||
| COMPASS-31 domains | |||
| Orthostatic intolerance | 20 (16, 24) | 16 (0, 20) | 0.163 |
| Vasomotor | 0 (0, 0) | 0 (0, 0) | 0.172 |
| Secretomotor | 6.43 (4.29, 6.43) | 5.36 (2.14, 6.43) | 0.468 |
| Gastrointestinal | 8.04 (7.14, 9.82) | 4.91 (3.57, 10.7) | 0.032 |
| Bladder | 1.11 (0, 1.11) | 0 (0, 2.22) | 0.742 |
| Pupillomotor | 2.33 (2.0, 2.67) | 1.83 (0.67, 2.67) | 0.065 |
| Total | 37 (32, 40) | 28 (12, 40) | 0.083 |
| PASC symptoms | |||
| Lightheadedness | 45 (80%) | 10 (71%) | 0.466 |
| Shortness of breath | 40 (73%) | 11 (79%) | 0.657 |
| Palpitations | 43 (77%) | 6 (43%) | 0.013 |
| Fatigue | 53 (95%) | 11 (79%) | 0.055 |
| Headache | 37 (67%) | 4 (29%) | 0.008 |
| Loss or change in taste | 20 (37%) | 1 (7.1%) | 0.031 |
| Constipation | 19 (35%) | 1 (7.1%) | 0.044 |
| Problems with sleeping | 43 (78%) | 8 (62%) | 0.213 |
Values are expressed as median (95% CI). P values were generated from Mann-Whitney U test for continuous variables and
Fisher's exact test for categorical variables.
BMI, body mass index; BSA, body surface area; CASS, Composite Autonomic Severity Score; COMPASS-31, Composite Autonomic Symptom Score; CI, confidence interval; ΔHRDB, delta heart rate in deep breathing; PASC, post-acute sequelae of COVID-19; VR, Valsalva ratio.
Hospitalization-based analysis
A minority of patients were hospitalized during their initial COVID-19 infections (13%; Supplemental Table S2). Hospitalized patients trended older (53 years [41,59] vs 41 years [39,46]; P = 0.051). Hospitalization frequency did not differ between sexes (P = 0.3).
Prevalence of hemodynamic criteria for cardiovascular autonomic disorders
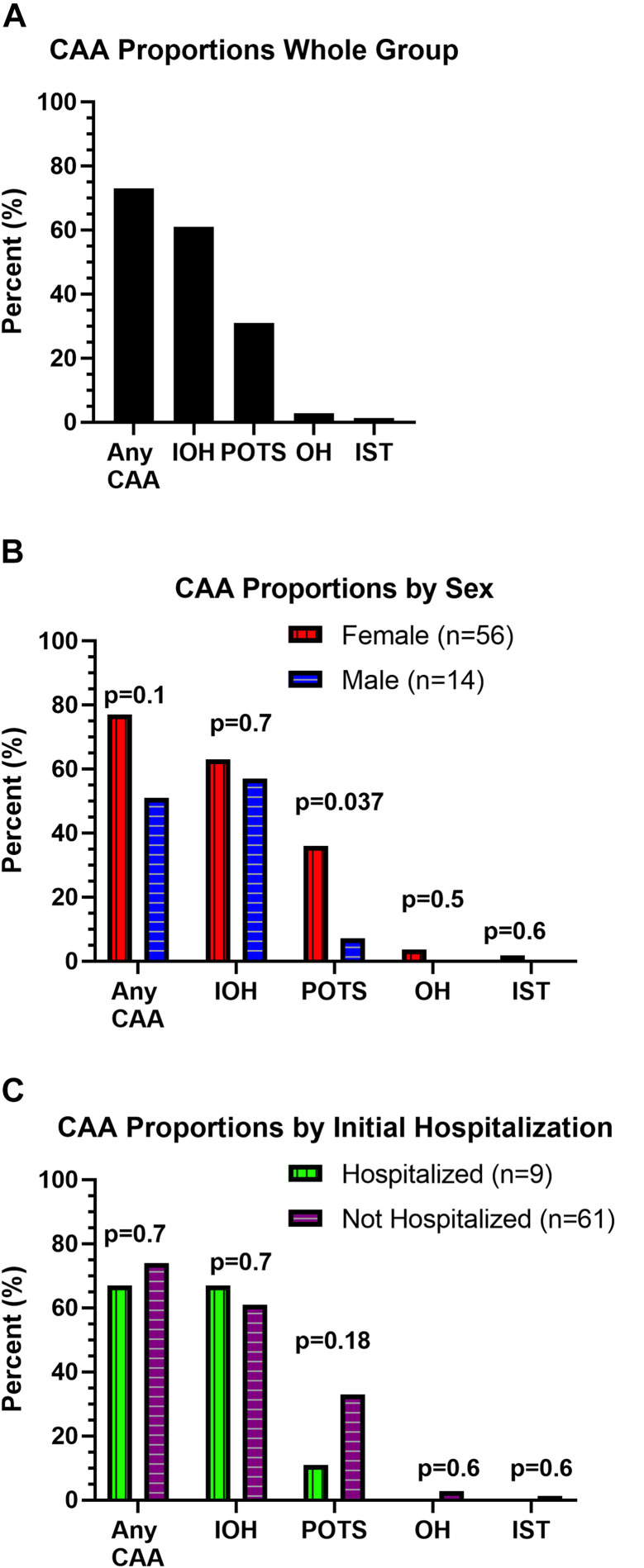
Most patients with PASC (73%) met the criteria for at least 1 CAA (Fig. 1 ). Among the CAA group, 16 (31%) patients met the hemodynamic criteria for > 1 abnormality. The POTSHR criterion was met in 21 (30%) patients with PASC (40 years [35,45]) with a median ΔHR of +41 bpm (+34, +59) from supine to standing. IST100 was seen in 1 (1.4%; 18-year-old) patient (supine HR: 136 bpm). Sustained OH20 was evident in 2 (2.9%) patients with PASC (ΔSBP: –21 mm Hg [–21, –35]) with an average age of 52 years [46,58]. In contrast, the IOH40 criterion was evident in 43 (61%) patients (45 years [39,51]) with PASC with a median ΔSBP of –52 mm Hg (–61, –46) in the first 15 seconds of an active stand.
Figure 1.
Proportions of cardiovascular autonomic abnormalities (CAA) in patients with post-acute sequelae of COVID-19 (PASC), including sex-based and hospitalization status-based differences. (A) CAAs were evaluated using hemodynamic criteria from an active stand test, which assessed hemodynamic criteria for postural orthostatic tachycardia syndrome (POTS), inappropriate sinus tachycardia (IST), orthostatic hypotension (OH), and initial orthostatic hypotension (IOH). (B) Proportions of patients with PASC meeting hemodynamic criteria for a CAA, broken down by patient sex. (C) Proportions of patients with PASC meeting hemodynamic criteria for a CAA, broken down by patient hospitalization status during initial COVID-19 infection.
Sex-based analysis
The POTSHR criterion was evident in 36% female vs 7.1% male patients (P = 0.037; Fig.1). In contrast, IOH40 criterion was equally prevalent in female and male patients (63% vs 57%; P = 0.7). The OH20 (P = 0.5) and IST100 criterion (P = 0.6) were only met in female patients. Overall, the increased estimates of female patients vs male patients meeting any CAA criteria were not significant (77% vs 51%; P = 0.14).
Hospitalization-based analysis
The overall prevalence of CAA was not different (67% vs 74%; P = 0.7). IOH40 was similar between the groups (67% vs 61%; P = 0.7). There was not a significant difference in the prevalence of patients with POTSHR who were hospitalized (11% vs 33%; P = 0.18). The OH20 and IST100 patients were not hospitalized (Fig. 1).
Autonomic function testing
Postganglionic sympathetic nerve integrity
Reduced nerve integrity was evident in 15 patients with CAA and in 4 patients without (P = 0.5). There was a trend for sympathetic nerve function to be attenuated in the forearm and distal leg for patients with CAA (Table 1). Between sexes, reduced nerve integrity was present in 13 female patients and 6 male patients (P = 0.14). Sympathetic nerve function was reduced in female patients at the forearm and foot (Table 2). Abnormal nerve function was observed in both hospitalized and nonhospitalized patients (P = 0.2). The forearm had worse sympathetic nerve integrity in nonhospitalized patients (Supplemental Table S2).
Cardiovagal
HR responses to deep breathing and Valsalva manoeuvre were not different between patient groups (Tables 1 and 2; Supplemental Table S2).
CASS
Hospitalized patients had higher cardiovagal scores (Supplemental Table S2; P = 0.022).
Autonomic symptom assessment
All patients reported at least 1 symptom on the COMPASS-31, with an overall median score of 36 of 100 (31,40). Patients with CAA had higher COMPASS-31 scores in the orthostatic intolerance domain vs patients without CAA (P = 0.038; Table 1). Among all patients, female patients had higher gastrointestinal scores compared with male patients (P = 0.032; Table 2) on the COMPASS-31. On the PASC-symptom questionnaire, more female patients than male patients reported loss or change in taste (P = 0.031), constipation (P = 0.044), palpitations (P = 0.013), and headache (P = 0.008) and a trend toward more fatigue (P = 0.055) (Table 2). Supplemental Table S1 provides an assessment of all symptoms in the general PASC symptom questionnaire.
Active stand symptom assessment
In our cohort, 71% of patients reported symptoms in the first 60 seconds of standing, and 100% of patients reported a score of ≥ 1 on the VOSS. Among patients with symptom assessments, 74% of patients with IOH reported symptoms within the first 60 seconds of standing, and 84% reported symptoms of new onset orthostatic intolerance (OI), including lightheadedness or palpitations. In addition, 100% of patients meeting POTS criteria reported scores of ≥ 1 on the VOSS, and 91% reported new-onset OI symptoms of lightheadedness or palpitations following their COVID-19 infections in the PASC questionnaire. Finally, 50% of patients meeting the OH criterion reported symptoms while standing, and the single patient with IST (100%) did not complete a symptom assessment during the active stand. Notably, with the IOH subgroup, the change in BP in the first 60 seconds of stand was negatively correlated with higher OI scores (r s = –0.328; P = 0.006). Similarly, patients with higher orthostatic tachycardia also reported higher OI symptoms (r s = 0.284; P = 0.017).
Discussion
In this study we report 1 of the largest PASC cohorts that have undergone autonomic testing. Here, we report that many patients with PASC have objective evidence of CAA; the most common abnormality is IOH, followed by POTS; both male and female patients with PASC meet the IOH criterion with similar frequency, but POTS skewed heavily toward female patients, and hospitalized patients with PASC did not have increased rates of CAA compared with nonhospitalized patients, suggesting even mild, nonhospitalized COVID-19 infections can result in long-term CAA.
Prevalence of cardiovascular autonomic abnormalities
Within our cohort, IOH was the most prevalent hemodynamic abnormality (61%). Previous studies have observed IOH ranging from 15% to 30% in the general population,31 suggesting that IOH may be more prevalent among patients with PASC. IOH requires an active stand with beat-to-beat hemodynamics for accurate detection.15 , 24 , 32 Few studies have used these methods, suggesting that underdiagnosis of IOH is likely in patients with PASC. This is important, as there are targeted nonpharmacologic treatments that can help patients with IOH.14 , 24 , 33 The POTS hemodynamic criterion was met in 30% of patients with PASC, which is consistent with previous reports14 , 34 but higher than the prevalence seen in the general population (0.2%),25 and specifically within females aged 20 to 40 years. For example, in a Croatian cohort, the prevalence of POTS in female patients 20 to 40 years of age ranged from 3% to 21%.35 Finally, IST and OH were relatively uncommon in our PASC cohort.
In addition to abnormal orthostatic hemodynamics, patients with CAA also had higher OI scores, and these scores were related to orthostatic hemodynamic abnormalities. For example, patients with IOH and larger BP drops and patients with POTS and higher HRs both reported higher OI scores. These findings further emphasize the need to incorporate an active stand and beat-to-beat hemodynamics when evaluating patients presenting with chronic PASC symptoms, especially symptoms of OI (eg. lightheadedness or palpitations).
Sex-based differences
There was a trend toward increased frequency of a CAA among female patients compared with male patients. These findings are consistent with many disorders of the autonomic nervous system, including POTS23, which predominantly affects premenopausal Caucasian women (5-fold female predominance).36 It is important, however, to acknowledge that our PASC cohort was predominantly composed of Caucasian women. It is hard to know if this represents the broader PASC population or if this reflects our sampling and recruitment. Despite a strong overall female predominance, we found that patients meeting the cardiovascular criteria for IOH were equally prevalent among male and female patients. IOH can be a common cause of syncope in persons with unexplained syncope,33 , 37 suggesting that men and women are at equal risk of experiencing syncopal episodes related to PASC.
There were several symptoms that were more prevalent among women, including reports of headache, loss or change in taste, gastrointestinal (eg, constipation), fatigue, and palpitations. Many of these symptoms are reported among patients with POTS, which was also female dominated in our PASC cohort. Palpitations is a common cardiac symptoms of POTS38; however, what might be less appreciated is that many patients with POTS also experience several noncardiac symptoms, including significant debilitating fatigue,38, 39, 40 which has important implications in all facets of life, including increased absences from school, work, and loss of productivity.41 Other noncardiac symptoms reported in patients with POTS include headache and gastrointestinal disorders,38 which are also consistent with our findings. Conversely, shortness of breath, lightheadedness, and sleep difficulties were not different between female and male patients within our cohort. Lightheadedness is commonly reported in patients with autonomic disorders.24 , 38 Given that IOH was equally prevalent among male and female patients and was frequently reported within the first 60 seconds of standing, it is not surprising that lightheadedness was not different between sexes.
Hospitalization status
Contrary to our hypothesis, patients with PASC hospitalized because of their COVID-19 infections did not have increased rates of CAA compared with nonhospitalized patients. Similarly, reported symptoms did not differ between groups. These findings suggest that even “mild” COVID-19 infections may result in CAA with significant symptomatology.
Although we cannot infer causation from the current study, viral infections are commonly reported triggers of altered autonomic control and have been reported in various disorders of orthostatic intolerance,36 , 42 In addition, autoantibodies have been reported in patients with OH43 and POTS.44 , 45 Recently, Blitshteyn et al. reported that ∼20% of their PASC patient cohort had abnormal autoimmune or inflammatory biomarkers.7 These findings lead to the possibility that the observed CAA may result from an underlying autoimmune-inflammatory response. However, further studies including inclusion of a healthy control group are needed to explore whether these findings are related to PASC and to explore the relationship between autoimmune-inflammatory biomarkers and severity of autonomic dysfunction or symptoms.
Limitations
Our study highlighted the importance of testing autonomic function in patients with PASC, but there were some limitations. First, this was a descriptive study and lacked a proper control group to determine what is truly abnormal with PASC physiology. Our initial focus was to recruit and study patients with PASC, and there are plans to study a matched control group going forward. Despite this limitation, because of the societal impact of the COVID-19–related health crisis, we thought it was important to report these data quickly. Second, our cohort may not represent the full PASC patient population. We recruited through local advertising and “Long-COVID” clinics, but it is possible that some patients found our study based on knowledge of our interest and expertise in CAA. This could lead to an overestimation of the prevalence of CAA in the broader PASC population. Also, we acknowledge that the low number of male patients and hospitalized patients may have an impact on statistical power. Finally, we focussed on the hemodynamic criteria that could be identified with autonomic testing. Some of these disorders (such as POTS and IOH) also require a specific symptoms constellation during a clinical evaluation. Therefore, it is more correct for us to speak about the hemodynamics of these disorders being present, rather than the disorders being present. Finally, a 24-hour Holter monitor can also be used to diagnose IST. As a single-day study, we opted to assess IST as a resting supine HR >100 bpm.25 However, the use of a 24-hour Holter may provide additional insights that may not have been captured with the timeframe of our study, potentially resulting in underdiagnosis within our cohort.
Conclusions
Given the high prevalence of CAA among patients with PASC, these patients should have cardiovascular autonomic function testing, especially if they report symptoms of orthostatic intolerance. Ideally, this evaluation will include beat-to-beat BP and HR monitoring with an active stand test to evaluate for criteria consistent with IOH, in addition to POTS, OH, and IST. Whereas IOH was common in both sexes, POTS was seen primarily in female patients. Hemodynamic cardiovascular autonomic abnormalities were common even in patients who were not hospitalized with their COVID-19 infections, suggesting that even mild infections can result in CAAs.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgements
The authors would like to thank the patients who took the time to participate in our study.
Funding Sources
This work was supported by the Canadian Institutes of Health Research (CIHR, Ottawa, Ontario, Canada), grant G4A- 177741, Dysautonomia International Grant-in-Aid (2019), and the Vanderbilt Institute for Clinical and Translational Research (NIH UL1-TR000445).
Disclosures
Dr Raj has served as consultant to Lundbeck LLC, Theravance Biopharma, Amneal Pharma, Servier Affaires Medicales, Regeneron, and Argenx BV. Dr Morillo has served as consultant to Abbott, Medtronic, Novartis, and Boston Scientific and as Chair of the BETTY Trial DSMB, Drugs for Neglected Disease Advisory Board, and Global Chagas Platform Advisory Board.
Footnotes
See editorial by Ståhlberg and Fedorowski, pages 776-778 of this issue.
See page 774 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2022.12.002.
Supplementary Material
References
- 1.World Health Organization WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. https://covid19.who.int/ Available at:
- 2.National Institute for Health and Care Excellence COVID-19 rapid guideline: managing the long-term effects of COVID-19. 2020. https://www.nice.org.uk/guidance/ng188 Published 2020. [PubMed]
- 3.Paterson I., Ramanathan K., Aurora R., et al. Long COVID-19: a primer for cardiovascular health professionals, on behalf of the CCS rapid response team. Can J Cardiol. 2021;37:1260–1262. doi: 10.1016/j.cjca.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ståhlberg M., Reistam U., Fedorowski A., et al. Post-COVID-19 tachycardia syndrome: a distinct phenotype of post-acute COVID-19 syndrome. Am J Med. 2021;134:1451–1456. doi: 10.1016/j.amjmed.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson M., Ståhlberg M., Runold M., et al. Long-haul post–COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanjwal K., Jamal S., Kichloo A., Grubb B.P. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J Innov Card Rhythm Manag. 2020;11:4302–4304. doi: 10.19102/icrm.2020.111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blitshteyn S., Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69:205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamal S.M., Landers D.B., Hollenberg S.M., et al. Prospective evaluation of autonomic dysfunction in post-acute sequela of COVID-19. J Am Coll Cardiol. 2022;79:2325–2330. doi: 10.1016/j.jacc.2022.03.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozkurt B, Das SR, Addison D, et al. 2022 AHA / ACC key data elements and definitions for cardiovascular and noncardiovascular complications of COVID-19: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards. Circulation. 2022;80:388–465. doi: 10.1016/j.jacc.2022.03.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gall N., James S., Kavi L. Observational case series of postural tachycardia syndrome (PoTS) in post-COVID-19 patients. Br J Cardiol. 2022;29:3. doi: 10.5837/bjc.2022.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aranyó J., Bazan V., Lladós G., et al. Inappropriate sinus tachycardia in post-COVID-19 syndrome. Sci Rep. 2022;12:1–9. doi: 10.1038/s41598-021-03831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monaghan A., Jennings G., Xue F., Byrne L., Duggan E., Romero-Ortuno R. Orthostatic intolerance in adults reporting long COVID symptoms was not associated with postural Orthostatic tachycardia syndrome. Front Physiol. 2022;13:1–11. doi: 10.3389/fphys.2022.833650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladlow P., O’Sullivan O., Houston A., et al. Dysautonomia following COVID-19 is not associated with subjective limitations or symptoms but is associated with objective functional limitations. Heart Rhythm. 2022;19:613–620. doi: 10.1016/j.hrthm.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldokla A.M., Ali S.T. Autonomic function testing in long-COVID syndrome patients with orthostatic intolerance. Auton Neurosci. 2022;241 doi: 10.1016/j.autneu.2022.102997. [DOI] [PubMed] [Google Scholar]
- 15.Harms M.P.M., Finucane C., Pérez-Denia L., et al. Systemic and cerebral circulatory adjustment within the first 60 s after active standing: an integrative physiological view. Auton Neurosci Basic Clin. 2021;231 doi: 10.1016/j.autneu.2020.102756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finucane C., van Wijnen V.K., Fan C.W., et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res. 2019;29:427–441. doi: 10.1007/s10286-019-00606-y. [DOI] [PubMed] [Google Scholar]
- 17.Cheshire W.P., Freeman R., Gibbons C.H., et al. Electrodiagnostic assessment of the autonomic nervous system: a consensus statement endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. 2021;132:666–682. doi: 10.1016/j.clinph.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Coronavirus disease (COVID-19): Post COVID-19 condition. Published December 16, 2021. Available at: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition. Accessed May 31, 2022.
- 19.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap):- a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedorowski A., Hamrefors V., Sutton R., et al. Do we need to evaluate diastolic blood pressure in patients with suspected orthostatic hypotension? Clin Auton Res. 2017;27:167–173. doi: 10.1007/s10286-017-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold A.C., Ng J., Lei L., Raj S.R. Autonomic dysfunction in cardiology: pathophysiology, investigation, and management. Can J Cardiol. 2017;33:1524–1534. doi: 10.1016/j.cjca.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman R., Wieling W., Axelrod F.B., et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Aut Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 23.Raj S.R., Guzman J.C., Harvey P., et al. Canadian Cardiovascular Society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020;36:357–372. doi: 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Sheikh N., Phillips A.A., Ranada S., et al. Mitigating initial orthostatic hypotension: mechanistic roles of muscle contraction versus sympathetic activation. Hypertension. 2022;79:638–647. doi: 10.1161/HYPERTENSIONAHA.121.18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheldon R.S., Grubb B.P., Olshansky B., et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novak P. Quantitative autonomic testing. J Vis Exp. 2011;53:1–22. doi: 10.3791/2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sletten D.M., Suarez G.A., Low P.A., Mandrekar J., Singer W. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87:1196–1201. doi: 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kpaeyeh A., Mar P., Raj V., et al. Hemodynamic profiles and tolerability of modafinil in the treatment of POTS: a randomized placebo-controlled trial. J Clin Psychopharmacol. 2014;34:738–741. doi: 10.1097/JCP.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raj S.R., Black B.K., Biaggioni I., Harris P.A., Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111:2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 30.Green E., Black B., Biaggioni I., et al. Melatonin reduces tachycardia in postural tachycardia syndrome (POTS): a randomized, crossover trial. Cardiovasc Ther. 2014;32:105–112. doi: 10.1111/1755-5922.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finucane C., O’Connell M.D.L., Fan C.W., et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from the Irish longitudinal study on ageing (TILDA) Circulation. 2014;130:1780–1789. doi: 10.1161/CIRCULATIONAHA.114.009831. [DOI] [PubMed] [Google Scholar]
- 32.Wieling W., Krediet C.T.P., Van Dijk N., Linzer M., Tschakovsky M.E. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci. 2007;112:157–165. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]
- 33.Sheikh N.A., Ranada S., Lloyd M., et al. Lower body muscle preactivation and tensing mitigate symptoms of initial orthostatic hypotension in young females. Heart Rhythm. 2022;19:604–610. doi: 10.1016/j.hrthm.2021.12.030. [DOI] [PubMed] [Google Scholar]
- 34.Shouman K., Vanichkachorn G., Cheshire W.P., et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31:385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamec I., Crnošija L., Ruška B., et al. The incidence of postural orthostatic tachycardia syndrome in the population of Zagreb, Croatia. Croat Med J. 2020;61:422–428. doi: 10.3325/cmj.2020.61.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw B.H., Stiles L.E., Bourne K., et al. The face of postural tachycardia syndrome: insights from a large cross-sectional online community-based survey. J Intern Med. 2019;286:438–448. doi: 10.1111/joim.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Twist D.J.L., Dinh T., Bouwmans E.M.E., Kroon A.A. Initial orthostatic hypotension among patients with unexplained syncope: an overlooked diagnosis? Int J Cardiol. 2018;271:269–273. doi: 10.1016/j.ijcard.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 38.Raj S.R. Postural tachycardia syndrome (POTS) Circulation. 2013;127:2336–2342. doi: 10.1161/CIRCULATIONAHA.112.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagai K., Song Y., Ling J.F., et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7:204–210. [PMC free article] [PubMed] [Google Scholar]
- 40.Wu R.E.Y., Khan F.M., Hockin B.C.D., Lobban T.C.A., Sanatani S., Claydon V.E. Faintly tired: a systematic review of fatigue in patients with orthostatic syncope. Clin Auton Res. 2022;32:185–203. doi: 10.1007/s10286-022-00868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricci J.A., Chee E., Lorandeau A.L., Berger J. Fatigue in the US workforce: prevalence and implications for lost productive work time. J Occup Env Med. 2007;49:1–10. doi: 10.1097/01.jom.0000249782.60321.2a. [DOI] [PubMed] [Google Scholar]
- 42.Carod-Artal F.J. Infectious diseases causing autonomic dysfunction. Clin Auton Res. 2018;28:67–81. doi: 10.1007/s10286-017-0452-4. [DOI] [PubMed] [Google Scholar]
- 43.Li H., Kem D.C., Reim S., et al. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension. 2012;59(2 suppl 1):402–408. doi: 10.1161/HYPERTENSIONAHA.111.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H., Yu X., Liles C., et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:1–10. doi: 10.1161/JAHA.113.000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fedorowski A., Li H., Yu X., et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. 2017;19:1211–1219. doi: 10.1093/europace/euw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.