Abstract
Phase angle is a composite measure that combines two raw bioelectrical impedance analysis measures: resistance and reactance. Phase angle has been considered an indicator of cellular health, integrity, and hydration. As inflammation and oxidative stress can damage cellular structures, phase angle has potential utility in early detecting inflammatory and oxidative status. Herein, we aimed to critically review the current understanding on the determinants of phase angle and its relationship with markers of inflammation and oxidative stress. We also discussed the potential role of phase angle in detecting chronic inflammation and related adverse outcomes. Several factors have been identified as predictors of phase angle, including age, sex, extracellular to intracellular water ratio, and fat-free mass. In addition to these factors, body mass index (BMI) also seems to influence phase angle. Available data also show that lower phase angle values are correlated (negligible to high correlation coefficients) with higher c-reactive protein, tumour necrosis factor-α, interleukin-6, and interleukin-10 in studies involving the general and aging populations, as well as patients with chronic conditions. Although fewer studies have evaluated the relationship between phase angle and markers of oxidative stress, available data also suggest that phase angle has potential to be used as an indicator (for screening) of oxidative damage. Future studies including diverse populations and bioelectrical impedance devices are required to confirm the validity and accuracy of phase angle as a marker of inflammation and oxidative stress for clinical use.
Keywords: Bioelectrical impedance analysis, Phase angle, Chronic diseases, Inflammation, Oxidative stress
Introduction
Inflammation is a critical component of the body’s response to damage, injury, and infection to restore homeostasis [1]. The immune system triggers inflammatory responses as a defense mechanism, usually temporary in acute conditions [2, 3]. Conversely, chronic inflammation is an uncontrolled, prolonged process associated with abnormal proinflammatory cytokine production and its consequential tissue damage and physiological imbalances; this low-grade inflammatory response is often subclinical [4].
Chronic inflammation can contribute to metabolic abnormalities, changes in body composition, and the onset of many chronic diseases, such as sarcopenia, diabetes, metabolic syndrome, cardiovascular disease, neurodegeneration, and cancer [5–8]. Obesity can compound the inflammatory response through an increased production of tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), prompting a proinflammatory milieu and oxidative stress [9]. This is particularly important in view of the obesity epidemic worldwide [10]. In more severe cases, chronic inflammation can also result in organ dysfunction and death [11–13].
Given the negative effects of inflammation on health, assessing and monitoring inflammatory status can provide valuable information in clinical practice. In such settings, inflammation is often evaluated by measuring blood biomarkers, such as cellular factors (e.g., lymphocytes), C-reactive protein (CRP), TNF-α, interleukins, and negative acute-phase proteins (e.g., albumin, and transferrin) among others [14, 15]. However, these markers require invasive blood tests and subsequent costly laboratory analyses, delaying the assessment of inflammatory status. Early assessment of inflammation is critical to initiate personalized treatment, evaluate patient prognosis, and screen for diseases [15–17].
Phase angle (PhA) may be a low-cost, real-time alternative approach to measuring blood inflammatory biomarkers. During inflammation and oxidative stress, reactive oxygen species disrupt cell membranes and fluid balance between intracellular and extracellular spaces; this fluid imbalance influences the capacitive effect of membranes and consequently, PhA [18]. PhA is therefore considered a marker of cell membrane integrity and a potential surrogate—yet reliable method—to screen for inflammatory and oxidative abnormalities [18]. Notably, several studies have shown the relationship between PhA and medical issues, prognoses, and mortality [19, 20]. Here, we summarized the literature, outlining the determinants of PhA as well as the relationships between PhA, inflammation, and oxidative damage. We further discuss the utility of PhA in clinical practice and future research on this important topic.
Phase angle and its determinants
PhA is obtained as a ratio between the raw measures resistance (R) and reactance (Xc) from bioelectrical impedance analysis (BIA) at 50 kHz frequency, according to the following formula [21]:
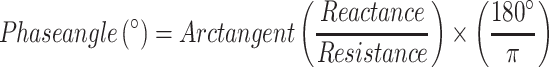 |
PhA results from the relationship between the body’s resistive behaviour, the opposition offered by the body fluids and electrolytes (i.e., intracellular and extracellular resistance) and cell membrane-specific capacitance / Xc (i.e., membrane’s ability to sustain the electrical potential) to the BIA’s electric current [22, 23]. In other words, the alternating current passing through the body’s tissues is associated with changes in R and Xc, which are the foundation of the PhA concept [24]. Therefore, PhA depends on many parameters, including cellular content and fluids as well as cell membranes’ integrity and permeability [24]. As a result, PhA is regarded as a marker of cell size, hydration, integrity, and death [25]. Decreased cellular structure and increased cell death are associated with lower PhA values, whereas improved overall cell function and health are associated with higher PhA values [26, 27].
Several factors can determine PhA, and the extent of their influence vary with age and health status. For instance, a large cohort study showed that sex and age were the main PhA determinants in heathy adults (n = 214,732), but age and body mass index (BMI) predicted PhA to a greater extent in healthy children and adolescents (n = 15,605) [28]. Although BMI was weakly correlated with PhA in healthy adults, PhA seems to decrease after a BMI of 40 kg/m2 [28]. This inverse association between PhA and BMI > 40 kg/m2 has been confirmed by others [29, 30] and might be explained by factors such as increased tissue hydration [22] and inflammation [28]. At very high BMI values (i.e., ≥ 40 kg/m2), individuals present with fluid overload that is characterized by altered extracellular water (ECW) and intracellular water (ICW) ratio (ECW/ICW) [24, 31], which is negatively related to PhA [24]. As obesity is associated with a chronic proinflammatory status, individuals with higher BMI values may also present with cellular membrane damage, further contributing to fluid imbalance and a lower PhA [28].
In addition to the ECW/ICW ratio, fat-free mass is another strong predictor of PhA. In a study with healthy individuals (N = 1,442), fat-free mass was one of the most critical determinants of PhA, explaining the association between PhA, sex, and age [24]. Variability in PhA has also been explored using a machine learning approach, with ICW and sex having the highest effect on PhA differences, followed by total body water, basal metabolic rate, and age [32]. PhA has also been linked to other health markers, such as frailty, muscle mass quantity and composition, physical function, muscular strength, and cardiorespiratory fitness levels [33–36]. In healthy older adults, Matias et al. reported that PhA predicted body strength and agility independently of sex, age, and skeletal muscle mass [37].
Other factors can impact PhA in the presence of diseases, with patients generally presenting with lower PhA values than healthy individuals [38]. PhA can be affected by specific elements of the disease, infection, inflammation, or oxidative stress [22, 39]. In this sense, PhA has been proposed as an independent predictor of disease severity in clinical conditions, such as malnutrition [40], increased nutritional risk [41], and sarcopenia [42]. These conditions all have inflammation in common [43], which affects tissue electrical properties, body cell mass, cell membrane integrity, and ultimately PhA [44]. Nevertheless, a systematic review of 33 articles could not conclusively indicate that PhA can independently predict malnutrition in clinical populations, as assessed by Subjective Global Assessment [45]. This result could have been impacted by the selected studies failing to account for confounding variables and/or to select proper PhA cut-offs [45]. Hydration is another potential confounder; thus, it is recommended to interpret PhA along with the RXc graph from the bioimpedance vector analysis in clinical populations [44]. Conditions in which malnutrition is prevalent and PhA is considered a prognostic tool include patients with human immunodeficiency virus [46], kidney chronic disease [47, 48], breast [49], colorectal [50], head and neck [51], hepatic cancers and other liver diseases [52], and COVID-19 [53, 54]. Higher ECW/total body water ratio played a significant role in mediating the relationship between PhA and mortality in patients with cancer cachexia even at a low BMI range [55]. Finally, PhA can also be affected by lifestyle factors. For example, smoking negatively affects PhA, primarily due to oxidative stress and inflammatory pathways [56]. Research has additionally shown a positive impact of physical activity intervention programs and antioxidants supplementation (e.g., zinc) on PhA values [57–59].
The biological and medical implications of PhA can be better appreciated in light of technological advancements, such as the field of Proteomics. Proteomics is a cutting-edge application of technologies for identifying and quantifying the total amount of proteins in a cell, tissue, or organism [60]. Huemer et al., 2022 employed proteomics to investigate PhA’s biomedical factors by identifying protein markers and its related biological processes [61]. The authors found that N-terminal prohormone brain natriuretic peptide (a marker of heart failure) was a significant PhA marker. They also indicated that the biological process related to PhA’s protein profile was involved in the protein profile control of cell quantity and growth, supporting the consensus that PhA reflects body cell mass from a biomedical perspective [61].
Phase angle, inflammation and cellular damage
Overview
Inflammation and oxidative stress are intimately related and can be triggered by many factors, including pathogens, diseases, environmental factors (e.g., chemical substances, radiation and pollution), lifestyle factors (e.g., smoking and alcohol consumption), and obesity [62, 63]. Both are also associated with cellular damage and are involved in the onset of several chronic disease through mobilization of immune cells and proinflammatory cytokine production [64].
Chronic inflammation, in a vicious cycle, can promote reactive oxidative species production, which increases inflammatory response and cellular injury [12]. As such, an inflammatory and oxidative environment are associated with constant cell damage. These injuries can cause alterations in diverse cellular structures and organelles (e.g., membranes, mitochondria, and nucleus) by oxidation of various components, such as lipids, protein, and deoxyribonucleic acid, which prompts apoptosis [65]. Consequently, this prolonged process can affect cell structure and integrity, cellular water content distribution, and cellular function, potentially causing tissue damage or illness. For this reason, inflammation is considered as an important etiologic factor of cancer, diabetes, cardiovascular disease, neurodegeneration, aging, and sarcopenia [3, 8, 66].
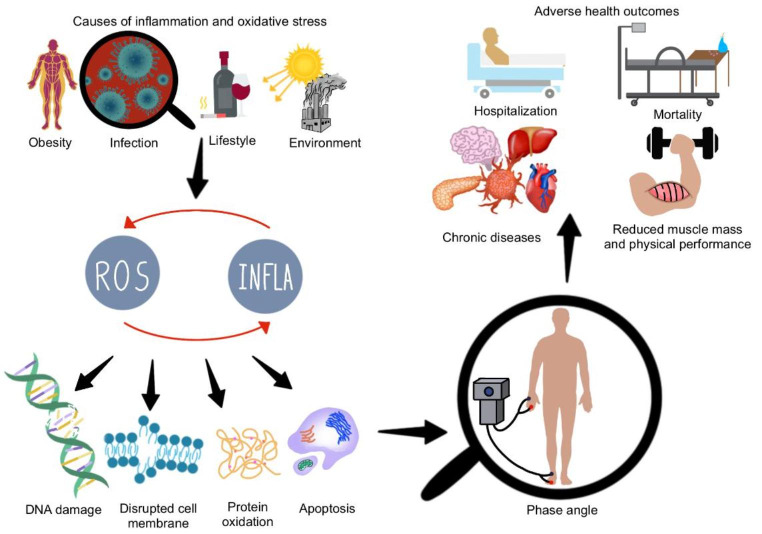
PhA may indirectly reflect inflammation as it measures cellular integrity and health and is associated with body cell mass and hydration [67, 68]. Lower PhA values are associated with a lower body cell mass and imbalances in cellular water (i.e., expansion of absolute ECW or increased ECW/ICW). This occurs in disease states [3, 69], and might be explained by the more oxidative environment of the extracellular fluid, versus that of the intracellular space [70]. During inflammation, Xc decreases in response to the lower capacitance of damaged cell membranes, and this occurs simultaneously with a reduction in R because of increases in ECW (measured at 50 kHz). As PhA represents the ratio between Xc and R, and the values of the former measure decrease to a greater extent than those of the latter, inflammation causes a decrease in PhA. Lower PhA also implies lower overall health, which might explain why low PhA has been linked to different diseases, muscle wasting [42], low physical performance [34], hospitalization [71], and mortality [53]. In contrast to clinical populations, healthy adults (i.e., athletes) showed positive associations between PhA and ICW but negative association with ECW/ICW ratio in both cross-sectional and longitudinal analyses [72, 73]. Thus, it is reasonable to expect that PhA can also reflect inflammation. Figure 1 provides an overview of the interconnection among inflammation, oxidative stress, low PhA, and adverse health effects.
Fig. 1.
The relation between inflammation, oxidative stress, cellular damage, low phase angle, and poor outcomes. Pathogens, environmental factors, high fat mass and lifestyle factors, such as alcohol consumption or smoking, can trigger an inflammatory and oxidative stress responses, and both can promote damage in several cellular structures, prompting cell death. This proinflammatory signaling, when chronically maintained, is related to several diseases, such as cardiovascular, metabolic, neurodegenerative disease, and cancer. Prolonged proinflammatory signaling can also lead to muscle wasting, lower physical function, sarcopenia, and increased length of hospitalization and mortality. Additionally, the cellular damage promoted by the inflammatory response can contribute to a low phase angle. For this reason, phase angle has been associated with several chronic diseases and mortality, including inflammation and oxidative stress. Note that the figure depicts phase angle being measured by a tetrapolar bioelectrical impedance analysis (BIA) device; although other BIA devices can also be used, PhA is always obtained at a frequency of 50 kHz. Images retrieved from smart.servier.com
Literature search
We conducted a non-systematic literature search in PubMed (from database inception to July 2022) to identify studies addressing the associations between PhA, inflammation, and oxidative stress. A combination of the following medical subject headings and keywords was used in the title/abstract field: “inflammation,” “inflammatory,” “low-grade inflammation,” “chronic inflammation,” “inflammatory markers,” “meta-inflammation,” “oxidative stress,” “reactive oxygen species (ROS),” “oxygen metabolites,” “free radicals,” “redox imbalance,” “phase angle,” and “bioelectrical impedance phase angle.” Articles published in English or Portuguese were selected for critical synthesis, and only significant associations are reported.
Main findings of selected studies
Phase angle and inflammation
Seventeen of 60 search results were identified as relevant studies describing the associations between PhA and inflammation (Table 1). These studies were published between 2003 and 2022 and included healthy adults and clinical populations. Overall, PhA was associated with several direct inflammatory markers, such as CRP, TNF-α, IL-6, interleukin 1-β (IL-1β), interleukin-10 (IL-10), and indirect markers (i.e., malnutrition-inflammation score). Studies included patients with: chronic kidney disease [74–79], hospitalized [39], primary care [80], psoriasis [81], Prader–Willi Syndrome [82], COVID-19 [54]; additionally, studies also included older women [83, 84], women with obesity [85], general population [86, 87], and professional soccer players [88].
Table 1.
Summary of the main results of the relationship between phase angle, inflammatory and oxidative stress markers
| Authors/ year Country |
Population | Study design | Device type, model (manufacturer, country) | BIA protocol | Marker | PhA / R / Xc | Association coefficient | p-value | Main conclusion | Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation | ||||||||||
|
Johansen et al., 2003 [74] US |
- N = 54 (18 ♀) - Patients undergoing HD treatment - Age: 51.5 ± 17 y |
Longitudinal | SF-BIA, model NR (RJL Systems®, USA) | NR | IL 1-β | 5.3 ± 1.5° / NR / NR | NR | 0.004 | Negative association with IL1-β | Age, sex, race, diabetes status, and dialysis vintage |
|
Demirci et al., 2010 [75] Turkey |
- N = 95 (53 ♀) - Patients undergoing PD treatment - Age: 50 ± 13 y - BMI: 26.0 ± 3.9 kg/m2 - Diabetes: 10.5% |
Case-control |
MF-BIA, QuadScan 4000 (Bodystat®, Isle of Man) |
- Empty peritoneal cavity - ≥ 15 min in supine position prior to test. |
CRP, albumin | Men: 5.8 ± 1.4° / NR / NR; women: 5.7 ± 1.0° / NR / NR |
CRP: r = − 0.330 Albumin: r = 0.440 |
All p < 0.01 | Negative association with CRP and positive association with albumin | NR |
|
Stobäus., 2012 [39] Germany |
- N = 777 (410 ♀) - Hospitalized patients - Age: 53.6 ± 16.7 - OW/OB: 37.8% - Malnutrition by SGA: 54.8% - Malignant tumours: 34% |
Cross-sectional | MF-BIA, Nutrigard M (Data Input®, Germany) | - As described in Kyle et al. [97] | CRP | 4.91 ± 1.17° / NR / NR |
Unadjusted analysis: R = − 0.248 Adjusted analysis: β = −0.003 |
All p < 0.0001 | Negative association with CRP | Regression analysis was adjusted for age, sex, BMI, nutritional status, disease, and inflammation |
|
Beberashvili et al., 2014 [76] Israel |
- N = 91 (34 ♀) - Patients undergoing HD treatment (all clinically stable) - Age: 64.0 ± 11.5 y |
Cohort study |
MF-BIA, Nutrigard M (Data Input®, Germany) |
- As described in Kyle et al. [97] | IL-6 | 4.7 ± 1.3° / NR / NR | Correlations between changes in IL-6 and changes in PhA: r = − 0.32 | 0.005 | PhA changes over time were associated with lower IL-6 levels | Partial correlation adjusted for age, sex, diabetes, dialysis vintage, and cardiovascular disease history |
|
Beberashvili et al., 2014 [79] Israel |
- N = 250 (82 ♀) - Patients undergoing HD treatment - Age: 68.7 ± 13.6 y |
Cohort study (baseline assessment) |
MF-BIA, Nutrigard M (Data Input®, Germany) |
- As described in Kyle et al. [97] | Albumin, IL-6, MIS | 4.7 ± 1.3° / NR / NR | NR | All p < 0.001 | Patients in the lowest PhA tertile (≤ 4.0°) had the highest IL-6 concentrations and MIS values but the lowest albumin concentrations | NR |
|
Tsigos et al., 2015 [80] Italy |
- Outpatients (routine check-up) - Group A: N = 10,416 (1,606 ♀); (−)OW/OB/MUS; Age: 35.7 ± 11.0; BMI: 24.3 ± 2.5 kg/m2 - Group B: N = 58,710 (53,129 ♀); (−)OW/OB (+)MUS; Age: 41.2 ± 12.5; BMI: 23.3 ± 2.6 kg/m2 - Group C: N = 30,445 (15,312 ♀); (+)OW/OB (−)MUS; Age: 43.1 ± 12.7; BMI: 31.6 ± 5.0 kg/m2 |
Case-control |
MF-BIA, BIA-ACC® (Biotekna srl., Italy) |
- Supine position - Standard placement of electrodes |
CRP | Group A: 4.89 ± 1.82° / NR / NR, group B: 3.26 ± 1.38° / NR / NR, group C: 3.69 ± 1.51° / NR / NR | R2 = 0.759 (negative slope) | NR | Negatively correlation with CRP | NR |
|
Barrea el al., 2016 [81] Italy |
- N = 180 (52 ♀) - Patients with psoriasis - Age: median (range), 50 (21 − 65) y - BMI: 30.2 ± 6.1 kg/m2 - OW/OB: 77.8% - Diabetes: 22.8% |
Case-control | SF-BIA, BIA 101 (Akern Bioresearch, Italy) |
- Supine position - Limbs slightly apart the body - Fasting and not exercising for 6 h prior test - No alcohol intake for 24 h prior test - Without shoes and socks - Contact areas scrubbed with alcohol |
CRP | 5.2 ± 1.0° 504.5 ± 85.8 Ω / 45.4 ± 9.2 Ω | r = − 0.283 | < 0.001 | Negatively correlation with CRP | BMI |
|
Sarmento-Dias et al., 2017 [77] Portugal |
- Patients undergoing PD treatment - Cross-sectional: N = 61 (27 ♀); age = 48 ± 13 y; residual renal function: 95% - Longitudinal: N = 33 (15 ♀); age: 47.8 ± 12 y |
Cross-sectional; longitudinal analysis of a subgroup of patients | MF-BIA, InBody S10 (Biospace, Korea) | NR | CRP, albumin |
NR for cross-sectional analysis Longitudinal: PhA at baseline: 5.6 ± 1.4° PhA at follow-up: 6.0 ± 1.1° |
Cross-sectional: CRP: β = −0.419 Albumin: β = 0.302 Longitudinal: CRP: r = − 0.426 (correlation between % change of both variables) |
Cross-sectional: CRP: p = 0.003 Albumin: p = 0.047 Longitudinal: CRP: p = 0.021 |
Cross-sectional: Negative association with CRP and positive with albumin Longitudinal: PhA change negatively associated with changes in CRP levels |
Cross-sectional: Multivariable analysis including age, ultrafiltration volume, serum urea, residual renal function, fat-free mass, Charlson comorbidity index Longitudinal: NR |
|
Moreto et al., 2017 [86] Brazil |
- N = 417 (341 ♀) - General population - Age: 53.9 ± 9.4 y - OW/OB: 72% - Metabolic syndrome: 52% |
Cross-sectional | SF-BIA, Biodynamics 450, (Biodynamics® Corp., USA) |
- No vigorous exercises for 24 h prior test - No alcohol/ caffeinated drinks for 72 h prior test - Emptied bladder - Not during menstruation (women) |
CRP | NR / NR / NR | OR = 1.62 for CRP ≥ 3.0 mg/L | < 0.05 | Higher CRP increased the odds of low PhA | Age, sex, BMI, and muscle mass index |
|
Tomeleri et al., 2018 [84] Brazil |
- N = 46 ♀ - Older women - Age, Int: 71.0 ± 5.4 y; Ctr: 68.8 ± 4.6 y - BMI, Int: 26.8 ± 4.3 kg/m2; Ctr: 26.9 ± 4.1 kg/m2 |
RCT | MF-BIA, Hydra ECF/ICF 4200 (Xitron Technologies, USA) |
- Supine position for 5 min prior test - Euhydration by post-voiding first-morning body weights and urine color |
IL-10, TNF-α and CRP | Int: 5.4 ± 0.6° / 560.3 ± 56.1 Ω/ 53.3 ± 7.9 Ω; Ctr: 5.6 ± 0.5° / 579.8 ± 71.5 Ω / 57.0 ± 9.8 Ω |
Unadjusted analysis: TNF-α (r = − 0.71); IL-10 (r = 0.46); CRP (r = − 0.65) (correlation between % change of both variables) |
< 0.01 | Negative association with TNF-α and CRP, and positive association with IL-10 | NR |
|
Tomeleri et al., 2018 [83] Brazil |
- N = 155 ♀ - Older women - Age: 67.7 ± 5.7 y - BMI: 27.0 ± 4.4 kg/m2 - 75% of women had up to two diseases |
Cross-sectional | MF-BIA, Hydra ECF/ICF 4200 (Xitron Technologies, USA) |
- Supine position - Voided bladder 30 min prior test - Fasting for 4 h prior test - No strenuous exercise for 24 h - No alcohol/ caffeinated drinks for 48 h prior test - Euhydration confirmed by post-voiding first-morning body weights and urine color - Metals removed - Exam table isolated from electrical conductors - BIA calibrated |
IL-6, TNF-α and CRP | 5.7 ± 0.6° / NR / NR |
Unadjusted analysis: TNF-α (r = −0.63); IL-6 (r = − 0.55); CRP (r = − 0.33) Adjusted analysis: TNF-α (β = −0.84); IL-6 (β = −0.97); CRP (β = −0.58) |
< 0.05 | Negative association with TNF-α, CRP and IL-6 | Age, trunk fat mas, appendicular lean soft tissue, and comorbidities |
|
Wang et al., 2019 [78] China |
- N = 144 (50 ♀) - Patients with CKD - Age, median (IQR): 53 (38 − 63) y - BMI, median (IQR): 24.8 (22.6 − 27.4) kg/m2 - CVD: 22.2% - Diabetes: 33.3% - Hypertension: 75.7% |
Cross-sectional |
BIS-BIA, BCM (Fresenius Medical Care, Germany) |
- Electrodes attached to hand and foot (nondominant side) - Supine position for ≥ 5 min prior test |
MIS | 5.38 ± 1.25° / NR / NR |
Unadjusted analysis: r = − 0.475 Adjusted analysis: β = −0.842 |
< 0.005 | Negative association with MIS. Association remained significant in multivariable analysis. | Age, sex, diabetes, handgrip strength, BMI, eGFR, overhydration, albumin, IL 6 |
|
Barrea et al., 2020 [82] Italy |
- N = 15 (9 ♀) - Adult patients with Prader–Willi Syndrome - Age: 28.0 ± 6.8 y - BMI: 43.8 ± 10.7 kg/m2 |
Cross-sectional | SF-BIA, BIA 101 (Akern Bioresearch, Italy) | - As described in Kyle et al. [97] | CRP | 4.5 ± 0.8° / 445.6 ± 63.7 Ω / 35.1 ± 9.4 Ω | r = − 0.69 | 0.01 | Negative association with CRP | Sex, BMI, and waist circumference |
|
Barrea et al., 2021 [87] Italy |
- N = 1855 (1175 ♀) - General population - Age, ♂: 34.8 ± 11.3 y; ♀: 34.4 ± 11.2 y - BMI, ♂: 33.8 ± 8.1; ♀: 35.6 ± 8.6 - OW/OB: 84.9% |
Cross-sectional | SF-BIA, BIA 101 (Akern Bioresearch, Italy) | - As described in Kyle et al. [97] | CRP |
♂: 6.1 ± 0.8° / 471.5 ± 90.2 Ω / 49.9 ± 10.3 Ω; ♀: 5.6 ± 0.7° / 481.2 ± 85.7 Ω / 46.5 ± 9.4 Ω |
Unadjusted analysis: r = − 0.55 Adjusted analysis: β = −0.35 |
< 0.0001 | Negative association with CRP | Age, sex, physical activity, BMI, waist circumference, and PREDIMED score |
|
Cornejo-Pareja et al., 2021 [54] Spain |
- N = 127 (52 ♀) - Patients with COVID-19 - Age, median (IQR): 69 (59 − 80) y - BMI, median (IQR): 28.2 (25.7 − 31.8) kg/m2 - OW/OB: 60.6% - Diabetes: 29.1% - Dyslipidemia: 40.9% - Hypertension: 59.1% - CVD: 20.5% - Lung disease: 16.5% - Chronic renal failure: 14.2% - ICU admission: 18.1% |
Cohort study |
SF-BIA, BIA 101 Whole Body BIVA (Akern Bioresearch, Italy) |
- Standard protocol - Daily check for accuracy of BIA device |
CRP, Albumin | Median (IQR): 4.4 (3.2 − 5.4)° / R/height: 302.5 (272.2 − 366.3) Ω / Xc/height: 24.7 (16.3 − 31.1) Ω | NR | < 0.001 | Patients in the lowest standardized PhA quartile had increased inflammation (high CRP, low albumin) | NR |
|
Moya-Amaya et al., 2021 [88] Spain |
- N = 18 (0 ♀) - Professional soccer players |
Clinical trial | MF-BIA, MC-780 MA (Tanita Corp., Japan) |
- Fasting for 8 h - Morning of the competitive match and 36 h after the match - No moderate/ intense exercise 24 h prior test - Voided bladder - Metals removed |
CRP | 7.69 ± 0.38° / NR / NR | r = 0.554 | 0.017 | Positive association with CRP | NR |
|
Barrea et al., 2022 [85] Italy |
- N = 260 ♀ - Women with OW/OB - Age: 37.6 ± 14.1 y - BMI: 35.7 ± 5.4 kg/m2 - OW/OB: 100% |
Pilot clinical study | SF-BIA, BIA 101 (Akern Bioresearch, Italy) | - As described in Kyle et al. [97] | CRP | At baseline: 5.5 ± 0.8° / 478.0 ± 73.1 Ω / 46.3 ± 9.4 Ω | Correlation between changes in PhA and changes in CRP: r = − 0.16 | 0.024 | Negative association with CRP | Age, physical activity, BMI and waist circumference |
| Oxidative stress | ||||||||||
|
Zouridakis et al., 2016 [89] Greece |
- N = 30 (14 ♀) - Patients with CKD - Age: 64 ± 14 y |
Case-control | SF-BIA, BIA 101 using the Bodygram software (Akern Bioresearch, Italy) | NR | TAC | At baseline: 5.9 ± 0.9° / 500.5 ± 102.9 Ω / 50.8 ± 9.9 Ω | r = 0.606 | < 0.001 | Positive correlation with TAC | NR |
|
Tomeleri et al., 2018 [84] Brazil |
- N = 46 ♀ - Older women - Age: 70.6 ± 5.1 y - BMI: 26.9 ± 4.2 kg/m2 |
RCT | MF-BIA, Hydra ECF/ICF Model 4200 (Xitron Technologies, USA) |
- Supine position for 5 min - Euhydration confirmed by post-voiding first-morning body weights and urine color |
FOX, AOCC, and CAT | Int: 5.4 ± 0.6° / 560.3 ± 56.1 Ω/ 53.3 ± 7.9 Ω; Ctr: 5.6 ± 0.5° / 579.8 ± 71.5 Ω / 57.0 ± 9.8 Ω |
Unadjusted analysis: Correlation between changes in PhA and changes in oxidative stress markers: FOX (r = − 0.43); AOPP (r = − 0.55); CAT (r = 0.73) Adjusted analysis: AOPP (β = −1.84); CAT (β = 2.03) |
< 0.01 | Negative correlation with AOPP and positive correlation with CAT | Skeletal muscle and body fat |
|
Tomeleri et al., 2018 [83] Brazil |
- N = 155 ♀ - Older women - Age: 67.7 ± 5.7 y - BMI: 27.0 ± 4.4 kg/m2 - 75% of women had up to two diseases |
Cross-sectional | MF-BIA, Hydra ECF/ICF 4200 (Xitron Technologies, USA) |
- Supine position - Voided bladder 30 min prior test - Fasting for 4 h prior test - No strenuous exercise for 24 h - No alcohol/ caffeinated drinks for 48 h prior test - Euhydration confirmed by post-voiding first-morning body weights and urine color - Metals removed - Exam table isolated from electrical conductors - BIA calibrated |
CAT, SOD and TRAP | 5.7 ± 0.6° / NR / NR | Partial correlations: CAT (r = 0.48); SOD (r = 0.31) and TRAP (r = 0.30) | < 0.01 | Positive correlation with CAT, SOD and TRAP | Age, trunk fat mass, appendicular lean soft tissue, and comorbidities |
|
Venancio et al., 2021 [90] Brazil |
- N = 39 (32 ♀) - Patients undergoing bariatric surgery - Age range: 18 − 60 y - BMI: ≥35 kg/m2 - Diabetes: 41% - Hypertension: 61.5% - Dyslipidemia: 40.9% |
Cohort study | SF-BIA, Biodynamics 450, (Biodynamics® Corp., USA) | - As described for Kyle et al. [97] | AOPP |
Median at baseline: RYGB = 7.0 (6.6–7.5)° / 410.6 (339.2–455.9) Ω / 49.9 (42.9–57.8) Ω; SG = 6.9 (6.6–7.2)° / 465.8 (456.0–517.2) Ω / 55.6 (51.7–61.0) Ω |
Correlations between changes in PhA and AOPP: r = − 0.549 | 0.041 | Negative correlation with AOPP | NR |
Age, body mass index, and phase angle values are mean ± SD, unless otherwise specified. Abbreviations: (−), without; (+), with; ♀, female; ♂, male; AOPP, advanced oxidation protein products; BIA, bioelectrical impedance analysis; BMI, body mass index; CAT, catalase; CKD, chronic kidney disease; CRP, C-reactive protein; Ctr, control group; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FOX, ferrous oxidation-xylenol orange; HD, hemodialysis; ICU, intensive care unit; IL-10, Interleukin 10; IL-6, Interleukin-6; IL 1-β, interleukin 1-β; Int, intervention group; IQR, interquartile range; MF-BIA, multi-frequency bioelectrical impedance analysis; MIS, malnutrition inflammation score; MUS: medically unexplained symptoms; N, sample size; NR, not reported; OB, obesity; OR, odd ratio; OW, overweight; PD, peritoneal dialysis; PhA, phase angle; R, resistance; RCT, randomized controlled trial; RYGB, roux-en-y gastric bypass; SF-BIA: single-frequency bioelectrical impedance analysis; SG, sleeve gastrectomy; SGA, Subjective Global Assessment; SOD, superoxide dismutase; SOFA, Sequential Organ Failure Assessment; TAC, total antioxidant capacity; TNF-α, tumor necrosis factor‐α; TRAP, radical-trapping antioxidant potential; Xc, reactance
PhA was negatively associated with CRP in 12 of 13 studies. Strength of these correlations varied among the studies, with coefficients ranging from r = − 0.69 to − 0.16, except for one study reporting a positive correlation (r = 0.55) [88]; higher CRP also increased the odds of low PhA. The most extensive study conducted to date found a high negative correlation between PhA and CRP in 99,571 individuals of varied BMI visiting medical centres for routine check-up with or without medically unexplained symptoms [80]. The linear regression model showed that CRP explained 75.9% of PhA variability [80]. Tomeleri et al. also explored the association of PhA with CRP in two studies in older women. One of these studies was an RCT (N = 46), and percent changes in CRP showed moderate negative correlations with percent changes in PhA [84]. The other study had a cross-sectional design (N = 155) and also showed negative correlations between PhA and CRP; this association was maintained in multivariate analysis controlling for age, trunk fat mass, appendicular lean soft tissue, and comorbidities [83].
In addition to reporting a moderated negative correlation between CRP and PhA in both males and females, another article also proposed PhA threshold values to predict high high-sensitivity CRP levels (hs-CRP, above sex-specific, data-driven median values) in 1855 healthy individuals, with most having overweight or obesity (84.9%) [87]. However, their proposed ROC derived thresholds to predict the highest hs-CRP levels (5.5° for males and 5.4° for females) [87] were notably higher than mean values for older adults, as they are associated with the sex and age distribution of the population. This would overestimate the prevalence of high hs-CRP levels. As such, the use of PhA cut-offs values in this context are discouraged. Other studies confirmed negligible to moderate negative associations between PhA and CRP in adults, mostly with excess body weight [85, 86]. These negative associations of similar strength between PhA and CRP were also found in clinical populations, including peritoneal dialysis [75, 77], hospitalized patients (54.8% with malnutrition, and 34% with cancer) [39], psoriasis [81], Prader-Willi Syndrome [82], and COVID-19 [54].
Unexpected results (i.e., positive correlation) between PhA and CRP were observed in one study in athletes with very high (highest among included studies) PhA values (7.69 ± 0.38°) [88]. Although these athletes (healthy soccer players) experienced post exercise-induced muscle damage as an acute recovery response, they were not actually injured. The study also had a small sample size (N = 18) and differences between pre- and post-matches were not significant, yet mean PhA values decreased and CRP increased [88].
Other inflammatory markers were also consistently associated with PhA values. Four of 5 studies showed that higher interleukin concentrations (IL 1-β and IL-6) were associated with lower PhA in patients undergoing maintenance hemodialysis [74, 76, 79] and older women [83]. In contrast, changes in PhA positively correlated with changes in IL-10 concentration (r = 0.46) in older women, mostly with a high BMI and comorbidities [84]. These studies in older women also reported moderate negative correlations (r = − 0.71 to − 0.63) between TNF-α and PhA [83, 84]. Albumin was another inflammatory marker explored, and 4 of 4 studies found a low positive association between PhA and albumin levels in patients undergoing peritoneal dialysis [75, 77], maintenance hemodialysis [79], and COVID-19 [54] treatment. Furthermore, higher malnutrition inflammation score was associated with lower PhA in patients with chronic kidney disease [78] and undergoing hemodialysis treatment [79].
Phase angle and oxidative stress
Four of 16 search results were identified as relevant studies describing the associations between PhA and markers of oxidative stress (Table 1). Our group published a review on the topic in 2021 [18], and the current updated search revelated that no new studies have been conducted since then. Overall, PhA was significantly associated with markers of oxidative stress, highlighting the link between inflammation and oxidative stress with PhA [18]. For instance, Zouridakis et al. were the first to observe a moderate positive correlation between PhA and total antioxidant capacity in 30 patients with chronic kidney disease [89]. Tomeleri et al. also found that PhA positively correlated with antioxidants, such as catalase (low to high correlations), superoxide dismutase (low correlation), and total radical trapping antioxidant (low correlation) in older women, mostly with high a BMI and comorbidities [83, 84]. Conversely, PhA was negatively correlated with ferric-xylenol orange (low correlation) and advanced oxidation protein products (moderate correlation) [83, 84]. The most recent study also showed a moderate negative correlation between PhA and oxidative protein damage (caused by advanced oxidation protein products) in adults undergoing bariatric surgery [90].
Additional points of included studies
As expected, PhA varied according to the population and disease studied as well as the BIA device used to acquire the bioimpedance data, with mean or median values ranging from 3.26° to 7.69° (Table 1). The lowest mean PhA value was found in outpatients of normal weight with medically unexplained symptoms [80]. Although the study by Cornejo-Pareja et al. did not reported the lowest PhA value among the select studies, it showed that patients with COVID-19 in the intensive care unit (ICU) or hospital ward also had very low PhA values (IQR : 3.2 − 5.4°) [54], 16 of which died. In fact, non-survivors of COVID-19 had lower PhA values than survivors (median difference = − 1.7°, p < 0.001) and PhA predicted mortality in adjusted models for several confounders (Hazard ratio = 3.912, 95% confidence interval = 1.322 − 11.572). As mentioned above, the study conducted in a cohort of professional soccer players reported the highest PhA value (7.69 ± 0.38°) [88].
Some studies (7 of 16) failed to adjust for confounding variables, and there was no standardization of which variables to include when adjustments were considered (Table 1). When conducted, most adjustments included age and/or sex as confounding variables. Additional variables were BMI, handgrip strength, physical activity status, diabetes and overall comorbidities, waist circumference, albumin concentration or body composition parameters (i.e., ECW, muscle mass index, body fat, trunk fat and appendicular lean soft tissue), among others. Although some confounding variables are specific to the study population, others determine PhA, such as age, sex, and body composition parameters, as previously addressed. For instance, in a population of patients with chronic kidney disease, Wang et al. adjusted their analyses for estimated glomerular filtration rate and overhydration, in addition to age, sex, and BMI, handgrip strength and inflammatory markers [78].
As expected, we also observed the use of diverse BIA devices, which ranged from single frequency, dual-frequency, multi-frequency, and spectroscopy, and from different brands (Table 1). This is important given the potential variation in PhA values among devices. For instance, while comparing several BIA devices, Genton et al. found different relationships between PhA and mortality, highlighting the need to standardize device selection [91]. Inconsistencies were also observed regarding the protocol applied for BIA assessment (Table 1). Most studies followed a standardized protocol for BIA assessment (i.e., electrodes and participant position). Only a few described pre-study visit procedures, such as instructions for not performing vigorous physical activity, fasting, avoiding alcohol and/or caffeinated drinks before testing, and previous emptying of the bladder. These are certainly important for reliability [92]. Furthermore, seven studies reported followed The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines for BIA analysis [25]. Only one study considered participants’ menstrual cycle, one reported previous BIA calibration, and none reported smoking status (Table 1).
Utility of phase angle in interventional studies
In the light of current literature, PhA appears to be a promising inflammatory marker. As such, it may be a relevant clinical outcome and a helpful tool to assess and monitor the effectiveness of interventions to improve inflammation status. Although only a few studies have explored the role of different interventions on PhA and inflammation status, results are promising. Nevertheless, these findings should be interpreted considering the technical variability of the PhA measurement, which is device- and procedure-specific. An overview of interventional studies conducted, and their respective findings are shown in Table 2.
Table 2.
Main results of the interventional studies included in the review
| Authors/ year Country |
Population Study design |
Device | BIA protocol | Intervention | Control | Intervention effects on PhA | Intervention effects on inflammation | Conclusion |
|---|---|---|---|---|---|---|---|---|
|
Roberts et al., 2017 [93] UK |
- N = 14 (6 ♀) - Physically active individuals - Age: 31 ± 6 y Crossover RCT |
SF-BIA, Impedimed DF50 (Impedimed, USA) Reliability NR |
- No strenuous exercise ~ 48 h prior test - No caffeinated products ~ 48 h prior test - ~3–4 h fasting prior test - Supine |
- PROHIGH: 2.9 g. kg− 1.d− 1 of PRO for 10 days - Resistance exercise on days 8–10 (T1-T3) |
- PROMOD: 1.8 g. kg− 1.d− 1 of PRO for 10 days - Resistance exercise on days 8–10 (T1-T3) |
- No difference in PhA change from baseline to follow-up between groups - PROHIGH had greater PhA at T3 than PROMOD (MD = + 0.18°, p = 0.012) |
- No difference in TNF-α change from baseline to follow-up between groups - No difference in TNF-α between groups at follow-up |
PhA values were maintained at T3 for the group with higher protein intake. The exercise protocol and diet did not influence TNF-α values |
|
Tomeleri et al., 2018 [84] Brazil |
- N = 46 ♀ - Older women - Age: 70.6 ± 5.1 y BMI: 26.9 ± 4.2 kg/m2 RCT |
MF-BIA, Hydra ECF/ICF 4200 (Xitron Technologies, USA) Reliability NR |
- Supine position for 5 min - Euhydration confirmed by post-voiding first-morning body weights and urine color - Same technician performing pre-/post-tests |
− 12 weeks of resistance training under professional supervision performed 3 times per week (45–50 min per session)(TG) | - No physical exercise of any type performed for 12 weeks (CG) |
- TG had a greater PhA than CG at follow-up (MD = + 0.4°, p < 0.05) - TG had a greater PhA at follow-up than at baseline (MD = + 0.4°, p < 0.05) - CG had a decrease in PhA from baseline to follow-up (MD = − 0.2°, p < 0.05) |
- TG had a lower TNF- α (MD = − 0.3, p < 0.05), IL-6 (MD = − 1.2, p < 0.05) and CRP (MD = − 2.4, p < 0.05) and a greater IL-10 (MD = + 10.3, p < 0.05) than CG at follow-up - TG had a decrease in TNF-α (MD = − 0.5, p < 0.05), IL-6 (MD = − 0.5, p < 0.05), and CRP (MD = − 0.7, p < 0.05) and an increase in IL-10 (MD = + 0.5, p < 0.05) from baseline to follow-up |
12 weeks of resistance training improved PhA and inflammation |
|
Di Renzo et al., 2019 [94] Italy |
- N = 44 (10 ♀) - Head and neck squamous carcinoma (stage III) - Age: 65.48 ± 12.66 y Clinical trial |
SF-BIA, BIA 101 S (Akern/RJL Systems, Italy) Reliability NR |
NR |
- Enteral standard nutrition for days 0–3 (ESN); 1500 kcal/d, 43% CHO, 27% PRO, 30% fat, 14 g fibre - Enteral immunonutrition for days 4–8 (EIN); 1500 kcal/d, 53% CHO, 22% PRO, 25% fat, < 3 g fibre, 9.15 MCT) |
- Oral standard diet (OD) for 8 days; 2448.45 kcal/d, 50% CHO, 17% PRO, 33% fat, 32–35 g fibre |
- No difference in PhA change between OD and ESN at day 3 - EIN had a greater PhA than OD at day 8 (MD = + 0.75, p = 0.045) |
- No difference in leukocyte, neutrophils, lymphocytes, albumin, transferrin, and CRP change between OD and ESN at day 3 and between OD from days 0–8 - EIN trended towards a lower transferrin (MD = − 30.25, p = 0.050) and CRP (MD = − 6.64, p = 0.066) than OD at day 8, but no difference in leukocyte, neutrophils, lymphocytes and albumin change - EIN had a higher transferrin than ESN (MD = + 39.25, p < 0.05), but no difference in leukocyte, neutrophils, lymphocytes, albumin, transferrin, and CRP - ESN had higher lymphocytes at day 3 than day 0 (MD = + 0.33, p < 0.05), but no difference in leukocyte, neutrophils, albumin, transferrin, and CRP |
Immunonutrition treatment improves PhA and inflammation more than an oral diet and standard enteral nutrition |
|
Moya-Amaya et al., 2021 [88] Spain |
- N = 18 (0 ♀) - Professional soccer players Clinical trial |
MF-BIA, MC-780 MA (Tanita Corp., Japan) Reliability NR |
- Overnight fast of ≥ 8 h - Tests performed between 8am and 10am - No moderate or intense exercise 24 h prior test - Voided bladder - Metals removed - Standing erect - Same technician performing pre-/post-tests - Performed according to manufacturer guidelines |
- Players (except goalkeepers) who played > 45 min were evaluated in the morning prior to the start of and 36 h after a competitive match during the first half of the season | NA | - No difference in PhA change from prior to and 36 h after a match | - No difference in CRP and IL-6 changes from prior to and 36 h after a match | 36 h of intense exercise did not affect PhA nor CRP |
|
Barrea et al., 2022 [85] Italy |
- N = 260 ♀ - Women with OW/OB - Age: 37.6 ± 14.1 y - BMI: 35.7 ± 5.4 kg/m2 - OW/OB: 100% Pilot clinical trial |
SF-BIA, BIA 101 (Akern Bioresearch, Italy) Reliability for intraday (R < 2%; Xc < 2.5%) and interday (R < 3.3%, Xc < 2.8%) tests assessed using same observer. Coefficient of variation of repeated measurements at 50 kHz assessed in 10 females using same observer: R = 1.4% and Xc = 1.3%. |
- Same device and technician performing pre-/post-tests - Supine - Limbs slightly apart - No food, drink, or exercise 6 h prior to test - No alcohol 24 h prior to test - Voided bladder |
- Very low-calorie ketogenic diet for 31 d using a commercial weight-loss program, replacement meals with a biological value of 110, a multivitamin, and saline supplements − 13% CHO (< 30 g/d), 43% PRO (1.2–1.5 g/kg IBW), 44% fat (including 10 g/d of EVOO) − 30 min of moderate physical activity 3 times per week |
NA | - Participants had a greater PhA at follow-up than at baseline (MD = + 0.5°, p < 0.001) | - Participants had a lower hs-CRP at follow-up than at baseline (MD = − 1.7, p < 0.001) | 31 days of a low-calorie ketogenic diet promoted a significant reduction in patient inflammation and improved PhA |
Abbreviations: ♀, female; BIA, bioelectrical impedance analysis; BMI, body mass index; CHO, carbohydrates; CG, control group; CRP, C-reactive protein; EIN, enteral immunonutrition; ESN, enteral standard nutrition; EVOO, extra virgin olive oil; hs-CRP, high-sensitivity CRP; IBW, ideal body weight; IL-10, interleukin-10; IL-6, interleukin-6; MCT, medium chain triglycerides; MD, mean/median difference; MF-BIA, multi-frequency BIA; N, sample size; NR, not reported; OD, oral diet; PhA, phase angle; PRO, protein; R, resistance; RCT, randomized controlled trial; SF-BIA, single-frequency BIA; TG, training group; TNF-α, tumor necrosis factor-α; Xc, reactance;
Robert and colleagues conducted the first study evaluating the role of a 10-day high protein diet to support improved markers of muscle damage (i.e., PhA and TNF-α) after extenuating exercise in 14 resistance trained adults [93]. Although PhA was higher in the group receiving 2.9 g∙kg− 1∙d− 1 versus 1.8 g∙kg− 1∙d− 1 at the end of the intervention (mean difference = + 0.18°, p = 0.012), no intervention effect was observed on both PhA and inflammation levels.
Di Renzo et al. explored the effects of immunonutrition compared to a control standard oral diet on PhA and inflammatory response in patients with advanced stage head and neck cancer [94]. At the end of treatment (day 8), participants who received immunonutrition had a greater PhA (median difference = +0.75°, p = 0.045) and higher pre-albumin levels (median difference = 3.35 mg/L, p = 0.048) than controls. From days 4 to 8, the immunonutrition group increased PhA (Δ = +0.3°, p = 0.045), pre-albumin (Δ = +4.5 mg/L, p < 0.05), and transferrin (Δ = +39.3 mg/dL, p < 0.05); no significant changes from baseline or day 3 were observed in the standard oral diet group for these inflammatory markers. Although the intervention had no effect on CRP levels, the authors highlighted the potential use of PhA to monitor nutritional and biochemical status [94].
Using exploratory dietary approaches, Barrea et al. conducted a pilot clinical trial to evaluate the effect of a very low-calorie ketogenic diet in improving PhA and inflammation status in 260 women with overweight or obesity [85]. The diet (< 800 kcal/ day; 1.2–1.5 g∙kg− 1 of ideal body weight∙d− 1 g; 44% lipids, including 10 g/d extra virgin olive oil) was followed for 31 days [85]. The authors reported an improvement in PhA from 5.5 ± 0.8° to 6.0 ± 0.8° and in hs-CRP from 3.5 ± 4.1 to 1.8 ± 2.5 mg/L (p < 0.001) after the intervention (Table 2). This was the only study that reported reliability measures, with a coefficient of variation for repeated measures at 50 kHZ of 1.3% for Xc and 1.4% for R. Although it is unclear how the variations in individual PhA components could have reflected the coefficient of variation for PhA values, it is possible that small changes in PhA could be attributed to measurement errors and not to the true intervention effect per se.
Tomeleri et al. explored the effects of a 12-week resistance training program in a sample of 46 older women [84]. They reported a significant improvement in several inflammatory markers (CRP, IL-6, TNF-α, and IL-10) and in PhA values [84]. Conversely, Moya-Amaya et al. described no effects on PhA and CRP levels after 36 h of a competitive soccer math [88]. Despite expected, increased muscle damage was not observed in the athletes [95]. No changes were observed, although this could be explained by a return to baseline levels after 36 h, likely associated to athletes’ greater muscle mass and strength [37].
Other applications / segmental analysis
An alternative approach of BIA use to detect cellular abnormalities, injury, and therefore inflammation, is its segmental application, which can be potentially used to evaluate cellular health at a localized level. According to Lukaski & Moore., localized BIA is a sensitive technique to determine alterations in bioelectrical components (R, Xc, and PhA), which can be used to assess changes in soft-tissue hydration, cell membrane integrity, and structure in a specific body area [96]. Localized BIA has been used to evaluate specific body segments such as arms, trunk, and legs and has been suggested over whole-body analyzes in the presence of specific conditions, such as cardiac insufficiency, liver or kidney failure, neuromuscular diseases, or limb amputation [97].
To the best of our knowledge, no studies have been conducted to evaluate the presence of localized inflammation using the segmental approach. However, insights related directly or indirectly to inflammation have been explored including muscle lesions, wound healing, and cancer diagnoses or its severity. Lukaski & Moore examined the application of localized single-frequency BIA(Quantum IV; RJL Systems, Clinton Township, MI) for patients with lower leg wounds by placing four electrodes around the wound, and showed PhA as a healing indicator [96]. Additionally, PhA assessed by localized BIA has the potential to detect neuromuscular disease, changes in skeletal muscle, injury and lesions [98–102]. In patients with neuromuscular disease, Rutkove et al. reported very low segmental PhA values (using several electrodes placed in line over the thigh for tetrapolar measures) among those with advanced disease state and values close to the healthy range (control subjects) during illness remission [102]. Freeborn & Fu demonstrated that localized PhA, and its components (R and Xc), reduced with increased exercise induced fatigue, using four electrodes placed on the lateral side of the biceps and a multifrequency BIA (E4990A Impedance Analyzer; Keysight Technologies, Santa Rosa, CA) [100]. Although muscle swelling was not evaluated in this study, the authors speculated that it might have caused R values to drop. Future investigations into the connection between muscle swelling and PhA should also assess potential confounders, such as changes in blood flow, interstitial fluid, and skin blood flow and temperature. Using four electrode placed on the calf and proximal hamstring muscles and a single-frequency BIA (BIA-101; AKERN-Srl, Florence, Italy), localized PhA measures agreed with the diagnosis of muscle injury severity assessed by magnetic resonance imaging [98].
Two studies explored the effectiveness of localized BIA and its PhA as a screening and prognostic tool for patients with prostate cancer [103, 104]. Tyagi et al. demonstrated that low PhA allowed discriminating prostate cancer patients using BIA electrodes on the right upper and right lower limb and a multifrequency BIA (QuadScan 4000 (Bodystat®; Isle of Man) [103]. PhA was lower in patients with prostate cancer (compared to matched controls) and worse comparing more advanced versus earlier disease stages [103]. In the other study, four electrodes were placed around the prostate area and a single frequency BIA (BIA 101 ASE®; Akern Srl, Italy) was used to measure PhA values in individuals with prostate cancer, benign prostatic disease, and healthy volunteers. Although Xc and R values differed between studied groups, PhA had a good sensitivity (0.925) but a poor specificity (0.200) to diagnose patients with prostate cancer [104]. Although none of these studies have evaluated the presence of inflammation, it is well known that the inflammatory state is associated with cancer, muscle damage and wound healing [3, 105, 106]. In that sense, it is expected that segmental PhA could also reflect inflammatory status, assessing localized inflammation. Further research is needed to confirm the role of segmental PhA to screen for inflammation in patients with cancer.
Localized PhA seems to be a potential technique to evaluate the health condition of a specific body part, also reflecting the impact of changes in tissue physiology on changes in tissue’s electrical properties. However, it is still unclear whether localized PhA is superior to whole-body PhA. Obayashi et al. compared segmental and whole-body PhA in adolescent athletes using a multifrequency BIA (Body S10 Body Water Analyzer; InBody Co., Seoul, Korea) with eight electrodes placed on five body segments (right and left arms, trunk, and right and left legs), and found that localized PhA was related to the performance of corresponding parts of the body (except trunk, which was excluded from the analysis), suggesting its use in association with whole-body PhA to assess local physical fitness levels [107]. Conversely, Jiang et al. concluded that PhA in the trunk estimated with a segmental BIA (InBody S10, InBody, Seoul, Korea) was underestimated compared to measures obtained by a tetrapolar, single-frequency BIA (Quantum IV, RJL Systems, Clinton Township, MI) in older adults [108].
Strengths and limitations of current literature
There is consistent evidence suggesting PhA can be used as a screening tool to detect inflammation, and several factors support this association. Studies were conducted in people with various medical conditions (chronic kidney disease, COVID-19, hospitalized and intensive care unit patients, obesity, psoriasis, general population, older people, and athletes), and across the globe (United States, Germany, Brazil, Israel, Italy, Portugal, Spain, Turkey, China, and Greece) (Table 1). The association between PhA and inflammation was explored using different inflammatory markers (CRP, TNF-α, IL-6, IL-1β and IL-10), presented in various designs (cross-section, cohort, case-control and clinical trials), and direction of associations were consistent among studies despite variations in the strength of associations. Furthermore, PhA may also provide supplemental clinical information that could be helpful in health care settings as PhA can be a prognostic marker for a variety of poor outcomes [37, 49, 109, 110].
Unfortunately, results hereby presented are not generalizable. The clinical significance of PhA as a predictor of inflammation and oxidative stress in a more diverse population, particularly with less severe conditions, has yet to be explored. We have also noted the need for standardized procedures during BIA assessment. Although some argue that PhA cut-offs to identify inflammation are needed, these cut-offs would have lower accuracy when used in populations that differ in age, sex, BMI, body composition, and ethnicity. Future research should therefore explore the utility of PhA in evaluating inflammatory changes and in determining the efficacy of nutritional and exercise interventions to improve inflammation and oxidative stress, as results are still scarce. Moreover, future research should assess the biological importance of localized PhA measurement and whether it is superior to the whole-body technique.
Conclusion
Inflammation and oxidative stress are intimately related and can cause tissue damage and lead to several chronic diseases. PhA is a feasible tool for assessing cellular health and integrity and could be a potential marker of inflammation and oxidative stress. Lower PhA was consistently associated with higher levels of CRP, interleukins, and TNF-α as well as lower albumin levels, despite heterogeneity in populations, study designs, and BIA devices. PhA is an affordable assessment, reliable, and non-invasive, which might be an efficient measure to be used in clinical settings to detect inflammation, where faster and simpler alternatives are urgently needed. Future studies should explore the relationship between PhA, inflammation, and oxidative stress using single and other multifrequency devices and standardized BIA protocols. Studies should also include large populations of varied age and health status.
Acknowledgements
The authors acknowledge Leticia Ramos da Silva and Lucas Yoshio Matsuo Hashimoto for their assistance with the graphical illustration, and Anne Caretero for her assistance updating Tables 1 and 2.
Abbreviations
- BIA
Bioelectrical impedance analysis
- BMI
Body mass index
- CRP
C-reactive protein
- ECW
Extracellular water
- hs-CRP
High-sensitivity c-reactive protein
- ICW
Intracellular water
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- IL-1β
Interleukin 1-β
- PhA
Phase angle
- R
Resistance
- TNF-α
Tumour necrosis factor-α
- Xc
Reactance
Declarations
Competing interests
CEO has received honoraria from Abbott Nutrition. MCG has received paid consultancy from Abbott Nutrition, Nutricia, and Nestlé Brazil. CMP has previously received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, Nestlé Health Science, AMRA medical, and Pfizer. BRS, JMFS, MSM, and AAJ have nothing to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000. 2013;63:149–64. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–18. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004;18:385–405. doi: 10.1016/j.bpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Hong Y, Huang H. Triptolide attenuates inflammatory response in Membranous Glomerulo-Nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press Res. 2016;41:901–10. doi: 10.1159/000452591. [DOI] [PubMed] [Google Scholar]
- 6.Scarpellini E, Tack J. Obesity and metabolic syndrome: an inflammatory condition. Dig Dis. 2012;30:148–53. doi: 10.1159/000336664. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17:324–8. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 8.Zacarías-Flores M, Sánchez-Rodríguez MA, García-Anaya OD, Correa-Muñoz E, Mendoza-Núñez VM. Relationship between oxidative stress and muscle mass loss in early postmenopause: an exploratory study. Endocrinol Diabetes y Nutr (English ed) 2018;65:328–34. doi: 10.1016/j.endien.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–63. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO). Obesity and overweight: Key facts [Internet]. World Heal. Organ. 2021 [cited 2022 Sep 27]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 11.Proctor MJ, McMillan DC, Horgan PG, Fletcher CD, Talwar D, Morrison DS. Systemic inflammation predicts all-cause mortality: a glasgow inflammation outcome study. PLoS ONE. 2015;10:1–12. doi: 10.1371/journal.pone.0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett JM, Reeves G, Billman GE, Sturmberg JP. Inflammation-nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Front Med. 2018;5:1–30. doi: 10.3389/fmed.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med Nature Publishing Group. 2019;25:1822–32. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CH, Abrams ND, Carrick DM, Chander P, Dwyer J, Hamlet MRJ, et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat Immunol. 2017;18:1175–80. doi: 10.1038/ni.3828. [DOI] [PubMed] [Google Scholar]
- 15.Ansar W, Ghosh S. Inflammation and inflammatory diseases, markers, and mediators: role of CRP in some inflammatory diseases. Biol C React Protein Heal Dis. 2016;24:67–107. doi: 10.1007/978-81-322-2680-2_4. [DOI] [Google Scholar]
- 16.Merker M, Felder M, Gueissaz L, Bolliger R, Tribolet P, Kägi-Braun N, et al. Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: a secondary analysis of a randomized clinical trial. JAMA Netw Open Netw open. 2020;3:e200663. doi: 10.1001/jamanetworkopen.2020.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson J, Salisbury C, Banks J, Whiting P, Hamilton W. Predictive value of inflammatory markers for cancer diagnosis in primary care: a prospective cohort study using electronic health records. Br J Cancer. 2019;120:1045–51. doi: 10.1038/s41416-019-0458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva BR, Gonzalez MC, Cereda E, Prado CM. Exploring the potential role of phase angle as a marker of oxidative stress: a narrative review. Nutrition. 2021;93:111493. doi: 10.1016/j.nut.2021.111493. [DOI] [PubMed] [Google Scholar]
- 19.Stapel SN, Looijaard WGPM, Dekker IM, Girbes ARJ, Weijs PJM, Oudemans-Van Straaten HM. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur J Clin Nutr. 2018;72:1019–25. doi: 10.1038/s41430-018-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman K, Stobäus N, Zocher D, Bosy-Westphal A, Szramek A, Scheufele R, et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr. 2010;92:612–9. doi: 10.3945/ajcn.2010.29215. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine (US) Committee on Military Nutrition Research. Emerging technologies for nutrition research: Potential for assessing military performance capability. Carlson-Newberry S, Costello R, editors. Emerg. Technol. Nutr. Res. Washington (DC): National Academies Press (US); 1997. [PubMed]
- 22.Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis - clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31:854–61. doi: 10.1016/j.clnu.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Lukaski HC. Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur J Clin Nutr. 2013;67:2–9. doi: 10.1038/ejcn.2012.149. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. 2016;103:712–6. doi: 10.3945/ajcn.115.116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis - part I: review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Lim SK, Lim JY. Phase angle as a predictor of functional outcomes in patients undergoing in-hospital rehabilitation after hip fracture surgery. Arch Gerontol Geriatr. 2020;89:104060. doi: 10.1016/j.archger.2020.104060. [DOI] [PubMed] [Google Scholar]
- 27.Allison RD, Lewis AR, Liedtke R, Buchmeyer ND, Frank H. Early identification of hypovolemia using total body resistance measurements in long-term care facility residents. Gend Med. 2005;2:19–34. doi: 10.1016/S1550-8579(05)80006-3. [DOI] [PubMed] [Google Scholar]
- 28.Anja BW, Danielzik S, Dörhöfer RP, Later W, Wiese S, Müller MJ. Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. J Parenter Enter Nutr. 2006;30:309–16. doi: 10.1177/0148607106030004309. [DOI] [PubMed] [Google Scholar]
- 29.Di Vincenzo O, Marra M, Sacco AM, Pasanisi F, Scalfi L. Bioelectrical impedance (BIA)-derived phase angle in adults with obesity: a systematic review. Clin Nutr. 2021;40:5238–48. doi: 10.1016/j.clnu.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Brunani A, Perna S, Soranna D, Rondanelli M, Zambon A, Bertoli S, et al. Body composition assessment using bioelectrical impedance analysis (BIA) in a wide cohort of patients affected with mild to severe obesity. Clin Nutr. 2021;40:3973–81. doi: 10.1016/j.clnu.2021.04.033. [DOI] [PubMed] [Google Scholar]
- 31.Dittmar M. Reliability and variability of bioimpedance measures in normal adults: Effects of age, gender, and body mass. Am J Phys Anthropol. 2003;122:361–70. doi: 10.1002/ajpa.10301. [DOI] [PubMed] [Google Scholar]
- 32.Hosseini SAT, Rahimi F, Esmaeili M, Khalili M. Phase angle determinants in patients with cardiovascular disease using machine learning methods. Health Technol (Berl) 2022;12:83–8. doi: 10.1007/s12553-021-00622-x. [DOI] [Google Scholar]
- 33.Mundstock E, Amaral MA, Baptista RR, Sarria EE, dos Santos RRG, Filho AD, et al. Association between phase angle from bioelectrical impedance analysis and level of physical activity: systematic review and meta-analysis. Clin Nutr. 2019;38:1504–10. doi: 10.1016/j.clnu.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Genton L, Mareschal J, Norman K, Karsegard VL, Delsoglio M, Pichard C, et al. Association of phase angle and running performance. Clin Nutr ESPEN. 2020;37:65–8. doi: 10.1016/j.clnesp.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Mann S, Wade M, Fisher J, Giessing J, Gentil P, Steele J. Phase angle as an indicator of health and fitness in patients entering an exercise referral scheme. J Am Med Dir Assoc. 2018;19:809–10. doi: 10.1016/j.jamda.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Silva AA, de Melo GF, de Almeida Filho EJB, Silvino VO, de Albuquerque Neto SL, Ribeiro SLG, et al. Correlation between phase angle and muscle mass, muscle function, and health perception in community-dwelling older women. Sport Sci Health. 2022;1:1–9. [Google Scholar]
- 37.Matias CN, Nunes CL, Francisco S, Tomeleri CM, Cyrino ES, Sardinha LB, et al. Phase angle predicts physical function in older adults. Arch Gerontol Geriatr. 2020;90:104151. doi: 10.1016/j.archger.2020.104151. [DOI] [PubMed] [Google Scholar]
- 38.da Silva BR, Rufato S, Mialich MS, Cruz LP, Gozzo T, Jordao AA. An evaluation of metabolic, dietetic, and nutritional status reveals impaired nutritional outcomes in breast cancer patients. Nutr Cancer Nutr Cancer. 2022;74:3611–22. doi: 10.1080/01635581.2022.2093388. [DOI] [PubMed] [Google Scholar]
- 39.Stobäus N, Pirlich M, Valentini L, Schulzke JD, Norman K. Determinants of bioelectrical phase angle in disease. Br J Nutr. 2012;107:1217–20. doi: 10.1017/S0007114511004028. [DOI] [PubMed] [Google Scholar]
- 40.Player EL, Morris P, Thomas T, Chan WY, Vyas R, Dutton J, et al. Bioelectrical impedance analysis (BIA)-derived phase angle (PA) is a practical aid to nutritional assessment in hospital in-patients. Clin Nutr. 2019;38:1700–6. doi: 10.1016/j.clnu.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Ramos da Silva B, Mialich MS, Cruz LP, Rufato S, Gozzo T, Jordao AA. Performance of functionality measures and phase angle in women exposed to chemotherapy for early breast cancer. Clin Nutr ESPEN. 2021;42:105–16. doi: 10.1016/j.clnesp.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Margáin A, Xie JJ, Román-Calleja BM, Pauly M, White MG, Chapa-Ibargüengoitia M, et al. Phase angle from bioelectrical impedance for the assessment of sarcopenia in cirrhosis with or without ascites. Clin Gastroenterol Hepatol. 2021;19:1941–9.e2. doi: 10.1016/j.cgh.2020.08.066. [DOI] [PubMed] [Google Scholar]
- 43.Prado CM, Landi F, Chew ST, Atherton PJ, Molinger J, Ruck T, et al. Advances in muscle health and nutrition: a toolkit for healthcare professionals. Clin Nutr. 2022;41:2244–63. doi: 10.1016/j.clnu.2022.07.041. [DOI] [PubMed] [Google Scholar]
- 44.Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care. 2017;20:330–9. doi: 10.1097/MCO.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 45.Rinaldi S, Gilliland J, O’Connor C, Chesworth B, Madill J. Is phase angle an appropriate indicator of malnutrition in different disease states? A systematic review. Clin Nutr ESPEN. 2019;29:1–14. doi: 10.1016/j.clnesp.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Schwenk A, Beisenherz A, Romer K, Kremer G, Salzberger B, Elia M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am J Clin Nutr. 2000;72:496–501. doi: 10.1093/ajcn/72.2.496. [DOI] [PubMed] [Google Scholar]
- 47.Chertow GM, Jacobs DO, Lazarus JM, Lew NL, Lowrie EG. Phase angle predicts survival in hemodialysis patients. J Ren Nutr. 1997;7:204–7. doi: 10.1016/S1051-2276(97)90020-0. [DOI] [Google Scholar]
- 48.Saitoh M, Ogawa M, Kondo H, Suga K, Takahashi T, Itoh H, et al. Bioelectrical impedance analysis-derived phase angle as a determinant of protein-energy wasting and frailty in maintenance hemodialysis patients: retrospective cohort study. BMC Nephrol. 2020;21:438. doi: 10.1186/s12882-020-02102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta D, Lammersfeld CA, Vashi PG, King J, Dahlk SL, Grutsch JF. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer. 2008;8:1–7. doi: 10.1186/1471-2407-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta D, Lis CG, Dahlk SL, King J, Vashi PG, Grutsch JF, et al. The relationship between bioelectrical impedance phase angle and subjective global assessment in advanced colorectal cancer. Nutr J. 2008;7:1–6. doi: 10.1186/1475-2891-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Władysiuk MS, Mlak R, Morshed K, Surtel W, Brzozowska A, Małecka-Massalska T. Bioelectrical impedance phase angle as a prognostic indicator of survival in head-and-neck cancer. Curr Oncol. 2016;23:e481–7. doi: 10.3747/co.23.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pagano AP, Sicchieri JMF, Schiavoni IL, Barbeiro D, Manca CS, da Silva BR, et al. Phase angle as a severity indicator for liver diseases. Nutrition. 2020;70:110607. doi: 10.1016/j.nut.2019.110607. [DOI] [PubMed] [Google Scholar]
- 53.Osuna-Padilla IA, Rodríguez-Moguel NC, Rodríguez-Llamazares S, Aguilar-Vargas A, Casas-Aparicio GA, Ríos-Ayala MA, et al. Low phase angle is associated with 60-day mortality in critically ill patients with COVID-19. J Parenter Enter Nutr. 2022;46:828–35. doi: 10.1002/jpen.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornejo-Pareja I, Vegas-Aguilar IM, García-Almeida JM, Bellido-Guerrero D, Talluri A, Lukaski H, et al. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: a longitudinal cohort study. Clin Nutr. 2021;S0261-5614(21)00091 – 1. [DOI] [PMC free article] [PubMed]
- 55.Katsura N, Yamashita M, Ishihara T. Extracellular water to total body water ratio may mediate the association between phase angle and mortality in patients with cancer cachexia: a single-center, retrospective study. Clin Nutr ESPEN. 2021;46:193–9. doi: 10.1016/j.clnesp.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Portugal MRC, Brito FB, Curioni CC, Bezerra FF, Faerstein E, Koury JC. Smoking status affects bioimpedance-derived phase angle in men but not in women: the Pró-Saúde Study, Brazil. Nutrition. 2019;61:70–6. doi: 10.1016/j.nut.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 57.Toselli S, Badicu G, Bragonzoni L, Spiga F, Mazzuca P, Campa F. Comparison of the effect of different resistance training frequencies on phase angle and handgrip strength in obese women: a randomized controlled trial. Int J Environ Res Public Health. 2020;17:1163. doi: 10.3390/ijerph17041163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Souza MF, Tomeleri CM, Ribeiro AS, Schoenfeld BJ, Silva AM, Sardinha LB, et al. Effect of resistance training on phase angle in older women: a randomized controlled trial. Scand J Med Sci Sport. 2017;27:1308–16. doi: 10.1111/sms.12745. [DOI] [PubMed] [Google Scholar]
- 59.Vermeulen KM, Lopes MMGD, Alves CX, Brito NJN, Almeida MDG, Leite-Lais L, et al. Bioelectrical impedance vector analysis and phase angle on different oral zinc supplementation in eutrophic children: randomized triple-blind study. Nutrients. 2019;11:1215. doi: 10.3390/nu11061215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aslam B, Basit M, Nisar MA, Khurshid M, Rasool MH. Proteomics: Technologies and their applications. J Chromatogr Sci J Chromatogr Sci. 2017;55:182–96. doi: 10.1093/chromsci/bmw167. [DOI] [PubMed] [Google Scholar]
- 61.Huemer MT, Petrera A, Hauck SM, Drey M, Peters A, Thorand B. Proteomics of the phase angle: results from the population-based KORA S4 study. Clin Nutr Clin Nutr. 2022;41:1818–26. doi: 10.1016/j.clnu.2022.06.038. [DOI] [PubMed] [Google Scholar]
- 62.Görlach A, Dimova EY, Petry A, Martínez-Ruiz A, Hernansanz-Agustín P, Rolo AP, et al. Reactive oxygen species, nutrition, hypoxia and diseases: problems solved? Redox Biol. 6: Elsevier; 2015. pp. 372–85. [DOI] [PMC free article] [PubMed]
- 63.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González Á, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12:3117–32. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2013;18:1497–534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shankar K, Mehendale HM. Oxidative stress. Encycl Toxicol Third Ed. Third edit. Elsevier; 2014. pp. 735–7.
- 66.Sánchez-Castellano C, Martín-Aragón S, Bermejo-Bescós P, Vaquero-Pinto N, Miret-Corchado C, de MerelloMiguel A, et al. Biomarkers of sarcopenia in very old patients with hip fracture. J Cachexia Sarcopenia Muscle. 2020;11:478–86. doi: 10.1002/jcsm.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86:509–16. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 68.Fiaccadori E, Morabito S, Cabassi A, Regolisti G. Body cell mass evaluation in critically ill patients: killing two birds with one stone. Crit Care. 2014;18:1–2. doi: 10.1186/cc13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Low S, Pek S, Liu YL, Moh A, Ang K, Tang WE, et al. Higher extracellular water to total body water ratio was associated with chronic kidney disease progression in type 2 diabetes. J Diabetes Complications. 2021;35:107930. doi: 10.1016/j.jdiacomp.2021.107930. [DOI] [PubMed] [Google Scholar]
- 70.Palacio PL, Godoy JR, Aktas O, Hanschmann EM. Changing perspectives from oxidative stress to redox signaling—extracellular redox control in translational medicine. Antioxidants. 2022;11:1181. doi: 10.3390/antiox11061181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Del Giorno R, Quarenghi M, Stefanelli K, Rigamonti A, Stanglini C, De Vecchi V, et al. Phase angle is associated with length of hospital stay, readmissions, mortality, and falls in patients hospitalized in internal-medicine wards: a retrospective cohort study. Nutrition. 2021;85:111068. doi: 10.1016/j.nut.2020.111068. [DOI] [PubMed] [Google Scholar]
- 72.Marini E, Campa F, Buffa R, Stagi S, Matias CN, Toselli S, et al. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin Nutr. 2020;39:447–54. doi: 10.1016/j.clnu.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 73.Campa F, Matias CN, Marini E, Heymsfield SB, Toselli S, Sardinha LB, et al. Identifying athlete body fluid changes during a competitive season with bioelectrical impedance vector analysis. Int J Sports Physiol Perform. 2020;15:361–7. doi: 10.1123/ijspp.2019-0285. [DOI] [PubMed] [Google Scholar]
- 74.Johansen KL, Kaysen GA, Young BS, Hung AM, da Silva M, Chertow GM. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77:842–6. doi: 10.1093/ajcn/77.4.842. [DOI] [PubMed] [Google Scholar]
- 75.Demirci MS, Demirci C, Ozdogan O, Kircelli F, Akcicek F, Basci A, et al. Relations between malnutritioninflammationatherosclerosis and volume status. The usefulness of bioimpedance analysis in peritoneal dialysis patients. Nephrol Dial Transplant. 2011;26:1708–16. doi: 10.1093/ndt/gfq588. [DOI] [PubMed] [Google Scholar]
- 76.Beberashvili I, Azar A, Sinuani I, Kadoshi H, Shapiro G, Feldman L, et al. Longitudinal changes in bioimpedance phase angle reflect inverse changes in serum IL-6 levels in maintenance hemodialysis patients. Nutrition. 2014;30:297–304. doi: 10.1016/j.nut.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 77.Sarmento-Dias M, Santos-Araújo C, Poínhos R, Oliveira B, Sousa M, Simões-Silva L, et al. Phase angle predicts arterial stiffness and vascular calcification in peritoneal dialysis patients. Perit Dial Int. 2017;37:451–7. doi: 10.3747/pdi.2015.00276. [DOI] [PubMed] [Google Scholar]
- 78.Wang WL, Liang S, Zhu FL, Liu JQ, Chen XM, Cai GY. Association of the malnutrition-inflammation score with anthropometry and body composition measurements in patients with chronic kidney disease. Ann Palliat Med. 2019;8:596–603. doi: 10.21037/apm.2019.10.12. [DOI] [PubMed] [Google Scholar]
- 79.Beberashvili I, Azar A, Sinuani I, Shapiro G, Feldman L, Stav K, et al. Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur J Clin Nutr. 2014;68:683–9. doi: 10.1038/ejcn.2014.67. [DOI] [PubMed] [Google Scholar]
- 80.Tsigos C, Stefanaki C, Lambrou GI, Boschiero D, Chrousos GP. Stress and inflammatory biomarkers and symptoms are associated with bioimpedance measures. Eur J Clin Invest. 2015;45:126–34. doi: 10.1111/eci.12388. [DOI] [PubMed] [Google Scholar]
- 81.Barrea L, Macchia PE, Di Somma C, Napolitano M, Balato A, Falco A, et al. Bioelectrical phase angle and psoriasis: a novel association with psoriasis severity, quality of life and metabolic syndrome. J Transl Med. 2016;14:130. doi: 10.1186/s12967-016-0889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barrea L, Pugliese G, de Alteriis G, Colao A, Savastano S, Muscogiuri G. Phase angle: could be an easy tool to detect low-grade systemic inflammation in adults affected by Prader–Willi syndrome? Nutrients. 2020;12:1–16. doi: 10.3390/nu12072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomeleri CM, Cavaglieri CR, de Souza MF, Cavalcante EF, Antunes M, Nabbuco HCG, et al. Phase angle is related with inflammatory and oxidative stress biomarkers in older women. Exp Gerontol. 2018;102:12–8. doi: 10.1016/j.exger.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Tomeleri CM, Ribeiro AS, Cavaglieri CR, Deminice R, Schoenfeld BJ, Schiavoni D, et al. Correlations between resistance training-induced changes on phase angle and biochemical markers in older women. Scand J Med Sci Sport. 2018;28:2173–82. doi: 10.1111/sms.13232. [DOI] [PubMed] [Google Scholar]
- 85.Barrea L, Muscogiuri G, Aprano S, Vetrani C, de Alteriis G, Varcamonti L, et al. Phase angle as an easy diagnostic tool for the nutritionist in the evaluation of inflammatory changes during the active stage of a very low-calorie ketogenic diet. Int J Obes. 2022;46:1591–7. doi: 10.1038/s41366-022-01152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moreto F, de França NAG, Gondo FF, Callegari A, Corrente JE, Burini RC, et al. High C-reactive protein instead of metabolic syndrome is associated with lower bioimpedance phase angle in individuals clinically screened for a lifestyle modification program. Nutrire Nutrire. 2017;42:1–6. [Google Scholar]
- 87.Barrea L, Muscogiuri G, Pugliese G, Laudisio D, de Alteriis G, Graziadio C, et al. Phase angle as an easy diagnostic tool of meta-inflammation for the nutritionist. Nutrients. 2021;13:1446. doi: 10.3390/nu13051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moya-Amaya H, Molina-López A, Berralaguilar AJ, Rojano-Ortega D, La Rosa CJB, De, La Rosa FJB De. Bioelectrical phase angle, muscle damage markers and inflammatory response after a competitive match in professional soccer players. Polish J Sport Tour. 2021;28:8–13.
- 89.Zouridakis A, Simos YV, Verginadis II, Charalabopoulos K, Ragos V, Dounousi E, et al. Correlation of bioelectrical impedance analysis phase angle with changes in oxidative stress on end-stage renal disease patients, before, during, and after dialysis. Ren Fail. 2016;38:738–43. doi: 10.3109/0886022X.2016.1158042. [DOI] [PubMed] [Google Scholar]
- 90.Venâncio FA, Almeida LA, Zovico PV, Barauna VG, Miguel GPS, Pedrosa RG, et al. Roux-en-Y gastric bypass and sleeve gastrectomy differently affect oxidative damage markers and their correlations with body parameters. Obes Surg. 2021;31:1680–7. doi: 10.1007/s11695-020-05179-8. [DOI] [PubMed] [Google Scholar]
- 91.Genton L, Herrmann FR, Spörri A, Graf CE. Association of mortality and phase angle measured by different bioelectrical impedance analysis (BIA) devices. Clin Nutr. 2018;37:1066–9. doi: 10.1016/j.clnu.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 92.Mota JF, Gonzalez MC, Lukaski H, Oto GL, Trottier CF, Tibaes JRB, et al. The influence of coffee consumption on bioelectrical impedance parameters: a randomized, double-blind, cross-over trial. Eur J Clin Nutr. 2022;76:212–9. doi: 10.1038/s41430-021-00932-3. [DOI] [PubMed] [Google Scholar]
- 93.Roberts J, Zinchenko A, Suckling C, Smith L, Johnstone J, Henselmans M. The short-term effect of high versus moderate protein intake on recovery after strength training in resistance-trained individuals. J Int Soc Sports Nutr. 2017;14:1–11. doi: 10.1186/s12970-017-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Renzo L, Marchetti M, Cioccoloni G, Gratteri S, Capria G, Romano L, et al. Role of phase angle in the evaluation of effect of an immuno-enhanced formula in post-surgical cancer patients: a randomized clinical trial. Eur Rev Med Pharmacol Sci. 2019;23:1322–34. doi: 10.26355/eurrev_201902_17027. [DOI] [PubMed] [Google Scholar]
- 95.Delos D, Maak TG, Rodeo SA. Muscle injuries in athletes: enhancing recovery through scientific understanding and novel therapies. Sports Health. 2013;5:346–52. doi: 10.1177/1941738113480934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lukaski HC, Moore M. Bioelectrical impedance assessment of wound healing. J Diabetes Sci Technol. 2012;6:209–12. doi: 10.1177/193229681200600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis - part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–53. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 98.Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell-Ferrer J, Rodas G. Localized bioimpedance to assess muscle injury. Physiol Meas. 2013;34:237–45. doi: 10.1088/0967-3334/34/2/237. [DOI] [PubMed] [Google Scholar]
- 99.Francavilla VC, Bongiovanni T, Genovesi F, Minafra P, Francavilla G. Localized bioelectrical impedance analysis: how useful is it in the follow-up of muscle injury? A case report. Med dello Sport. 2015;68:323–4. [Google Scholar]
- 100.Freeborn TJ, Fu B. Time-course bicep tissue bio-impedance changes throughout a fatiguing exercise protocol. Med Eng Phys. 2019;69:109–15. doi: 10.1016/j.medengphy.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Aaron R, Shiffman CA. Using localized impedance measurements to study muscle changes in injury and disease. Ann N Y Acad Sci. 2000;904:171–80. doi: 10.1111/j.1749-6632.2000.tb06443.x. [DOI] [PubMed] [Google Scholar]
- 102.Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–7. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- 103.Tyagi R, Mishra S, Gaur N, Panwar A, Saini D, Singh K, et al. Bioelectric impedance phase angle in carcinoma prostate - a hospital-based study. Int J Med Sci Public Heal. 2016;5:1826. doi: 10.5455/ijmsph.2016.30122015335. [DOI] [Google Scholar]
- 104.Bartoletti R, Greco A, Di Vico T, Durante J, Ficarra V, Scilingo EP, et al. Bioelectric impedance analysis test improves the detection of prostate cancer in biopsy candidates: a multifeature decision support system. Front Oncol. 2021;11:555277. doi: 10.3389/fonc.2021.555277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol - Regul Integr Comp Physiol. 2005;288:R345-53. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 106.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Obayashi H, Ikuta Y, Fujishita H, Fukuhara K, Sakamitsu T, Ushio K, et al. The relevance of whole or segmental body bioelectrical impedance phase angle and physical performance in adolescent athletes. Physiol Meas. 2021;42. [DOI] [PubMed]
- 108.Jiang F, Tang S, Eom JJ, Song KH, Kim H, Chung S, et al. Accuracy of estimated bioimpedance parameters with octapolar segmental bioimpedance analysis. Sens Sens (Basel) 2022;22:2681. doi: 10.3390/s22072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shin J, Hwang JH, Han M, Cha RH, Kang SH, An WS, et al. Phase angle as a marker for muscle health and quality of life in patients with chronic kidney disease. Clin Nutr. 2022;41:1651–9. doi: 10.1016/j.clnu.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 110.Hirose S, Nakajima T, Nozawa N, Katayanagi S, Ishizaka H, Mizushima Y, et al. Phase angle as an indicator of sarcopenia, malnutrition, and cachexia in inpatients with cardiovascular diseases. J Clin Med. 2020;9:2554. doi: 10.3390/jcm9082554. [DOI] [PMC free article] [PubMed] [Google Scholar]