Abstract
SARS-CoV-2 is a highly infectious virus and etiologic agent of COVID-19, which is spread by respiratory droplets, aerosols, and contaminated surfaces. Copper is a known antiviral agent, and has resulted in successful reduction of pathogens and infections by 83–99.9% when coated on surfaces in intensive care units. Additionally, copper has been shown to inactivate pathogens such as Coronavirus 226E, a close relative of SARS-CoV-2. Here, we examine the ability of two copper blends with differing compositions to inactivate SARS-CoV-2 virus at different time points. Copper Blend 2 (75.07% pure copper) was found to significantly reduce (over 50%) the viability of SARS-CoV-2 at 5 min of contact, with at least 98% reduction in recovered virus at 20 min (vs. plastic control). However, Copper Blend 1 (48.26% pure copper), was not found to significantly reduce viability of SARS-CoV-2 at any time point when compared to plastic. This may indicate that there is an important percentage of copper content in materials that is needed to effectively inactivate SARS-CoV-2. Overall, this study shows that over the course of 20 min, coatings made of copper materials can significantly reduce the recovery of infectious SARS-CoV-2 compared to uncoated controls, indicating the effective use of copper for viral inactivation on surfaces. Furthermore, it may suggest higher copper content has stronger antiviral properties. This could have important implications when short turnaround times are needed for cleaning and disinfecting rooms or equipment, especially in strained healthcare settings which are struggling to keep up with demand.
Keywords: SARS-CoV-2, COVID-19, Copper, Copper blend, Viral inactivation, Antiviral agent
Introduction
SARS-Coronavirus-2 (SARS-CoV-2), the etiologic agent of COVID-19, is primarily spread by respiratory droplets, but also through contaminated surfaces and aerosols (Govind et al. 2021; Jin et al. 2020; Kraay et al. 2020; Marquès and Domingo 2021; Ren et al. 2020; van Doremalen et al. 2020; Ye et al. 2020; Zuo et al. 2020). To prevent spread of the virus, emphasis was initially put on basic public health practices, including isolating, hand washing, and mask wearing (CDC, 2021, 2020; Gostin et al. 2020; Johns Hopkins University, 2021). These mitigation efforts were largely aimed at stopping the spread of droplet-associated SARS-CoV-2 viral particles both in the context of human-to-human transmission, as well as surface contamination. Even with these efforts, SARS-CoV-2 spread across the globe, indicating a need for additional research on transmission prevention.
Recent research suggests that respiratory droplets from breathing, speaking, coughing, and sneezing can be carried distances greater than 2 m (Bahl et al. 2020). These droplets often land on high-touch surfaces (e.g., doorknobs, elevator buttons, cell phones), which allows infectious virus to be mechanically transmitted to the mouth, nasal mucosa, or conjunctiva of others. Droplet spread and pathogen survival depend on factors including surface porosity, temperature, ventilation, and relative humidity, but some droplet-associated viruses remain infectious on common surfaces for days or weeks (Aboubakr et al. 2021; Bueckert et al. 2020). Infectious pathogens have been found to survive and remain infectious for extended periods of time on high-touch surfaces, including those in healthcare settings (e.g., bed rails, IV poles) (CDC, 2021; Mantlo et al. 2020; Otter et al. 2013; Zerbib et al. 2020). Specifically, SARS-CoV-2 has been found to be viable on non-porous surfaces for days to weeks (Biryukov et al. 2020; Chin et al. 2020; Liu et al. 2021; Riddell et al. 2020; van Doremalen et al. 2020). Additionally, SARS-CoV-2 was found to have substantial transfer from non-porous solids to artificial skin through light touch when the droplet was wet (13–16% virus transfer) as well as after it had evaporated (3–9% virus transfer) (Behzadinasab et al. 2021a). Consequently, disinfection of high-touch surfaces, particularly during a pandemic and in healthcare settings, provides an opportunity to decrease the spread of deadly pathogens (Bahl et al. 2020; Otter et al. 2016).
Numerous studies have demonstrated the antibacterial and antiviral activity of copper against a wide range of pathogens including E. coli, Influenza A, Norovirus, SARS-CoV-1, herpes simplex, Junin, HIV-1, poliovirus, monkeypox, and Marburg and Ebola viruses (Champagne et al. 2019; Cortes and Zuñiga 2020; Govind et al. 2021; Grass et al. 2011; Han et al. 2005; Imai et al. 2012; Mantlo et al. 2020; Manuel et al. 2015; Michels et al. 2015; Montero et al. 2019; Noyce et al. 2007; Rakowska et al. 2021; Rosenberg et al. 2018; Różańska et al. 2017; Warnes et al. 2012; Wilks et al. 2005). In addition, laboratory results have led to the use of copper materials in clinical trials in healthcare facilities and community centers (Casey et al. 2010; Colin et al. 2018; Hinsa-Leasure et al. 2016; Ibrahim et al. 2018; Poggio et al. 2020; Zerbib et al. 2020; Schmidt et al. 2012), with hospital intensive care units containing copper-coated appliances reporting 83–99.9% reduction in the burden of pathogens and infections (Montero et al. 2019; Salgado et al. 2013). The exact mechanism behind the ability of copper to inactivate or kill pathogens is believed to differ between pathogen types (Festa and Thiele 2011; Manuel et al. 2015; Rosenberg et al. 2018; Warnes et al. 2012, 2015). For viruses, the proposed mechanism is that copper ions disrupt viral envelopes, prevent cellular respiration, produce free radicals, and destroy the DNA/RNA of microbes when in contact with copper surfaces (Rakowska et al. 2021).
Copper inactivation of Coronavirus 226E, another common respiratory virus and close relative of SARS-CoV-2, has been successful, with inactivation observed in 40 min or less (Warnes et al. 2015). Recent studies by Behzadinasab et al. (2020), Hutasoit et al. (2020), and Mantlo et al. (2020) examined Cu2O/PU film, 3D-printed copper-coated surfaces, and cold-spray copper coating, respectively, for SARS-CoV-2 inactivation capabilities. Behzadinasab et al. (2020) reported 99.99% inactivation after 1-h incubation while Hutasoit et al. (2020) and Mantlo et al. (2020) found 96% and 99% inactivation after 2 h. Another study by Behzadinasab et al. (2021b) studied two transparent surface coatings, PDA/Cu and PDA/Cu2O, which also found 99.98% and 99.88% inactivation of SARS-CoV-2 after 1-h incubation, respectively. However, viability of the novel SARS-CoV-2 virus immediately after contact with copper materials has not been adequately tested. It may be that, coating surfaces with copper infused materials can more rapidly inactivate SARS-CoV-2 than we currently understand. Accordingly, in this study, we evaluated the inactivation of SARS-CoV-2 virus upon contact with two copper blend coatings from 1 to 20 min.
Materials and methods
Viral strains and cell lines
Vero E6 (ATCC® CRL-1586™) cells were cultured in 1× Dulbecco’s Modification of Eagle’s Medium with 4.5 g/l glucose supplemented with 1% l-glutamine (DMEM; Corning, Corning, NY) and 10% (v/v) fetal bovine serum (FBS; Corning, Corning, NY). Vero E6 cells were grown in T175 flasks and passaged when the cells were 90% confluent. 1.2 × 106 cells were plated in 6- well plates overnight before use.
The SARS-CoV-2 Chile strain was obtained from BEI (NR-52439). One hundred μl was added to a confluent T75 flask of Vero E6 cells in serum-free media (SFM) with antibiotics. Forty-eight hours later, 100 μl of supernatant was added to a second flask with uninfected cells. If cytopathic effects were observed after 48 h, the supernatant was removed and the flask containing the remainder of the cells were placed in a freezer for 5 min. The flask was then thawed, and the cells were collected using 5 ml of new media. The cell lysate was mixed with supernatant and centrifuged at 500×g for 5 min before aliquoting.
Surfaces tested
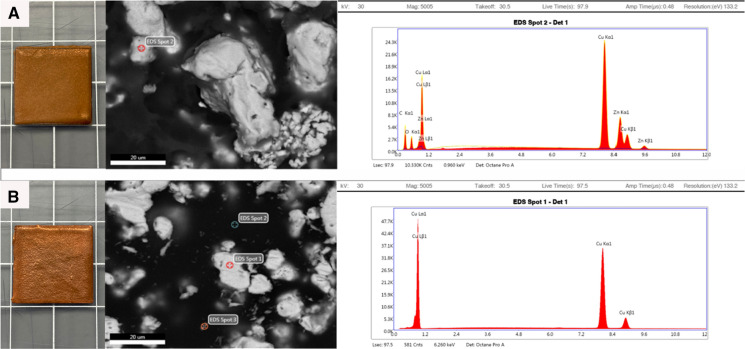
Two distinct copper blends were formulated by Alloi, with Copper Blend 1 containing 48.26% pure copper and Copper Blend 2 containing 75.07% pure copper. The composition of each blend’s inputs is characterized in Table 1. To create these materials, copper and zinc were ordered from a third-party and then mixed with a non-metal binder (styrene resin sold under COR75-AQ-010L [INTERPLASTICS Unsaturated Polyester Resin product code: SIL94BA-990]) and catalyst (Methyl ethyl ketone peroxide [MEKP]). Both blends consist of metallic components as well as the binder, to make the material as a whole not conductive, and the catalyst, to harden the binder. All materials were measured using a scale with an uncertainty of ± 0.01 g. The two copper blends were then used to completely cover pieces of 2 cm × 2 cm polycarbonate plastic sheets with 0.1 mm thickness using cold-spray technology. These materials were then brought to the George Washington University Nanofabrication and Imaging Center and imaged using the GEI Teneo LV scanning electron microscope (SEM) with an EDAX Octane Pro detector for elemental analysis. SEM was done at 5000 × and energy-dispersive x-ray spectroscopy (EDS) was performed at 30 kV for 100 s showing Copper Blend 1 to be mainly copper (Cu) 41.61% and zinc (Zn) 14.59%, and Copper Blend 2 to be predominately Cu 100%. Further analysis of these coated materials by SEM and energy-dispersive X-ray spectroscopy (EDS) is shown in Fig. 1 and Table 2. The copper blend-coated samples arrived in sealed bags and were tested as is. The uncoated plastic samples arrived with a thin sheet of plastic adhesive covering one side. The plastic adhesive was removed with 70% ethanol and allowed to dry before experimentation. The components of the copper blends were unknown to the researchers at the time of experimentation; however, composition was revealed to the researchers after analysis of results.
Table 1.
Composition of the inputs for Copper Blend 1 and 2 by weight and percent of total weight
| Material | Copper Blend 1 | Copper Blend 2 | ||
|---|---|---|---|---|
| Weight (g) | Percent of total weight | Weight (g) | Percent of total weight | |
| Binder (styrene resin [COR75-AQ-010L]) | 45.0 | 24.13% | 45.0 | 24.13% |
| Catalyst (methyl ethyl ketone peroxide [MEKP]) | 1.5 | 0.80% | 1.5 | 0.80% |
| Pure copper (without brass) | 90.0 | 48.26% | 140.0 | 75.07% |
| Brass (70% copper/30% zinc) | 50.0 | 26.81% | 0.0 | 0.00% |
| Total weight | 186.5 | – | 186.5 | – |
| Zinca | 15.0 | 8.04% | 0.0 | 0.00% |
| Total copper (pure copper + brass)a | 125.0 | 67.02% | 140.0 | 75.07% |
aZinc weight and percent of total weight were calculated based on brass content. Total copper weight and percent of total weight were calculated based on brass and pure copper content
Fig. 1.
Photographs of the appearance of: A) Copper Blend 1 from left to right: tested material in a 2 × 2 cm square, scanning electron microscopy (SEM) done at 5000x, energy-dispersive X-ray spectroscopy (EDS) at 30 kV for 100 s showing composition of Copper Blend 1 Spot 2 to be predominately copper (Cu) 41.61% and zinc (Zn) 14.59%; B) Copper Blend 2 from left to right: tested material in a 2 × 2 cm square, SEM done at 5000x, EDS at 30 kV for 100 s showing composition of Copper Blend 2 Spot 1 to be predominately Cu 100% (background analysis of Copper Blend 2 Spot 2 and Spot 3 were predominantly carbon, 57.64% and 43.18%, respectively)
Table 2.
Composition of Copper Blend 1 and 2 products by percent weight using energy-dispersive x-ray spectroscopy (EDS)
| Copper Blend 1 (spot 2)a | Copper Blend 2 (spot 1)a | |||
|---|---|---|---|---|
| Element | Weight % | Error % | Weight % | Error % |
| Copper | 41.61 | 1.34 | 100 | 0.99 |
| Zinc | 14.59 | 1.45 | – | – |
| Carbon | 33.98 | 8.96 | – | – |
| Oxygen | 9.82 | 9.90 | – | – |
aEDS spot locations are displayed in Fig. 1 above
Infectivity assay for SARS-CoV-2
Viral stock solution was thawed and placed in ten 1 μl droplets on two different copper blend-coated surfaces to simulate respiratory droplet contact (Lindsley et al. 2013; Warnes et al. 2015). The distinct 1 μl droplets were used instead of one 10 μl droplet to allow for more direct contact with the copper blend surfaces due to the surface tension of the solution (Mantlo et al. 2020). The virus was removed from the test surfaces by washing with 490 μl EMEM supplemented with 1% penicillin, streptomycin, amphotericin B and non-essential nucleic acids after incubation at room temperature for various time points: 1 min, 5 min, 10 min, and 20 min. The virus and media mixture was assayed for infectious virus survival using a plaque assay, quantified as plaque forming units (PFU). Serial dilutions of the virus mixture were prepared in infection medium before 200 μl aliquots were plated onto confluent monolayers of Vero E6 cells in Corning® 6-well plates. After 1 h, a 1:1 agar and EMEM overlay was added and plates were incubated at 37 °C and 5% CO2 for 72 h. Following incubation, the plates were fixed with 10% (w/v) formaldehyde, stained with 0.5% Crystal Violet and allowed to dry before plaques were counted.
Statistical analyses
All statistical analyses were performed in R Studio (version 1.3.1093) with base R (version 3.6.3) and significance was assessed at the α = 0.05 level. We first wanted to compare whether the recovery of viable SARS-CoV-2 was differentially affected by incubation on three different materials (Copper Blend 1, Copper Blend 2, and plastic) at four different time points. Median rank titers (Log PFU/ml) across the three materials were compared using the Kruskal–Wallis non-parametric analysis of variance (kruskal.test) after determination that data were not distributed normally (shapiro.test, p < 0.05). When warranted, post-hoc tests for pairwise differences were performed using the Dunn Test (dunnTest, package FSA). We next wanted to compare the performance of each blend compared to the baseline material of plastic. We took the difference of recovered viral titer (log PFU/mL) for each trial-blend combination and calculated the percent reduction from the corresponding trial’s plastic control at each time point. We then used a t-test to determine whether these calculated differences were significantly greater from select null values; namely, 50%, 75%, and 98%. A rejection of the null in this case would indicate that an blend had at least or greater reduction than the null value tested.
Results
Comparison of recovered titer from each type of material
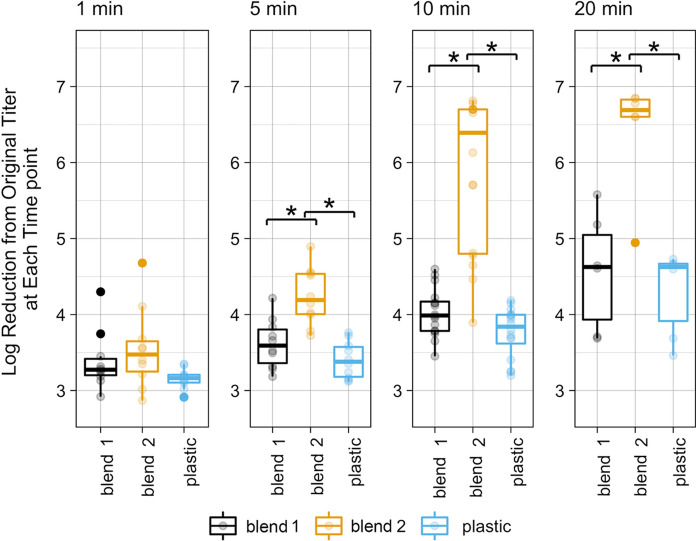
At 1-min exposure time, there was no significant difference in the recovered viral titer (PFUs/mL) among the three materials. At 5, 10, and 20 min contact with Copper Blend 2, there were significantly lower recovered titers of SARS-CoV-2 than both Copper Blend 1 and plastic. The viral titer recovered from Copper Blend 1 was not significantly different from the plastic control at any time point measured (Fig. 2).
Fig. 2.
At 5, 10, and 20 min, contact with Copper Blend 2 produced significantly (*) lower titers (Log PFU/ml) than exposure to either Copper Blend 1 or plastic (p < 0.05). Presented are data points and boxplot data summaries
Percent reduction of recovered titer of Copper Blend 1 or Copper Blend 2 compared to the plastic control
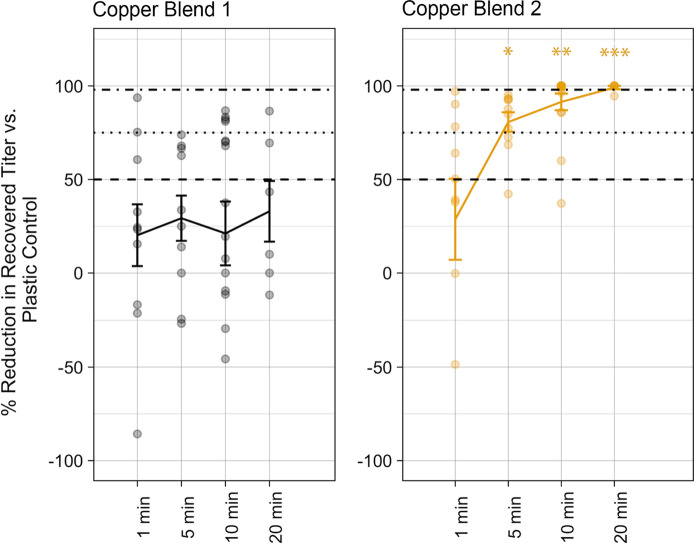
When the recovered viral titer of each copper blend was compared to that of the plastic control, Copper Blend 1 did not result in a SARS-CoV-2 titer reduction that was significantly greater than 50% at any timepoint. However, for Copper Blend 2, the percent reduction in titer compared to the plastic control was significantly higher. Reduction was significantly greater than a maximum of 50% at time point 5 min, 75% at 10 min, and 98% at 20 min (Fig. 3).
Fig. 3.
For each copper blend material, points represent percent reduction from matching plastic control. Lines represent the mean percent reduction with ± standard error. *Indicates significantly greater reduction than 50%, **Indicates significantly greater reduction than 75%, ***Indicates significantly greater reduction than 98%
Discussion
Basic infection prevention and control practices are important to prevent the rapid spread of novel viruses, such as SARS-CoV-2. Part of this strategy includes timely and consistent disinfection of surfaces, especially in high-trafficked spaces where respiratory droplets may contain viruses capable of surviving for extended periods of time on commonly used surfaces. Reducing environmental contamination is particularly important during pandemics and in health care settings. Previous studies found copper is an effective option to combat fomite transmission both in laboratory and in clinical settings (Behzadinasab et al. 2020; Hutasoit et al. 2020; Mantlo et al. 2020; Warnes et al. 2015). This current study sought to build on the growing body of knowledge regarding the uses of copper and copper materials as deterrents of surface-mediated transmission of viruses.
Our results indicate that contact with a high copper-content material inactivated SARS-CoV-2 as compared to a plastic control in a time-dependent manner. There was no significant difference in recovered titer between Copper Blend 1, Copper Blend 2, and the plastic control after 1 min of contact, suggesting a minimum time of contact is needed for efficacy. Copper Blend 2 begins to significantly reduce the viability of SARS-CoV-2 at 5 min of contact with at least 98% reduction in recovered virus at 20 min of contact.
Copper Blend 2 is 75.07% pure copper composition by weight compared to Copper Blend 1, which has a pure copper composition of 48.26%. The difference in recovered virus between the two suggests that higher copper content may have stronger antiviral properties. It is important to note that Copper Blend 1 also contained brass, which is a copper alloy with 70% copper/30% zinc, meaning Copper Blend 1 has a total copper content of 67.02%. Our results are similar to a study that demonstrated copper was efficacious at inactivating a human Alphacoronavirus, HuCoV-229E (Warnes et al. 2015). Pure copper and 90% copper materials were able to inactivate HuCoV-229E in 20 min (Warnes et al. 2015). Interestingly, inactivation efficiency began to decrease when the pure copper content was reduced to 85%. As SARS-CoV-2 is more stable on surfaces than HuCoV-229E, determining the efficacy of a specific material’s copper content is critical to evaluate further (Rabenau et al. 2005; van Doremalen et al. 2020).
Other studies have reported greater than 96% inactivation of SARS-CoV-2 after an hour of contact with different copper formulations (Behzadinasab et al. 2020; Hutasoit et al. 2020; Mantlo et al. 2020). Accordingly, our experimental limit of 20 min of contact between the viral inoculum and copper blends may offer a conservative estimate of the ultimate effectiveness of such materials. We cannot rule out the possibility that Copper Blend 1 may have more effectively inactivated SARS-CoV-2 or that Copper Blend 2 may have achieved even greater reduction at contact times exceeding 20 min. However, the effectiveness of Copper Blend 2 at such a short interval may prove beneficial during periods of high community transmission and healthcare stress, when shorter turnaround times for cleaning and disinfecting rooms or equipment is needed to keep pace with demand.
In conclusion, this study has found that over the course of 20 min, copper blend coatings can significantly reduce the recovery of infectious SARS-CoV-2 compared to uncoated controls. Our results indicate the continued use of copper as a viral inactivator for surfaces at-risk for contamination. Furthermore, it may be that there is an important percentage of copper content in materials that is needed for effectiveness against SARS-CoV-2.
Author contributions
AG performed the laboratory work and wrote the main manuscript text, KEK co-led the writing of the manuscript, RCC led the data analysis, CNM conceptualized the study and secured funding. All authors read and approved the final manuscript.
Funding
The research was funded by Teck Resources Limited. Copper blend materials provided by Isaac Lichter, Nick O'brien, and Andrew Medland at Alloi. Neither organization had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations
Conflict of interest
The authors have declared that no competing interests exist.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aboubakr HA, Sharafeldin TA, Goyal SM. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound Emerg Dis. 2021;68(2):296–312. doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR. Airborne or droplet precautions for health workers treating coronavirus disease 2019? J Infect Dis. 2020 doi: 10.1093/infdis/jiaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadinasab S, Chin A, Hosseini M, Poon L, Ducker WA. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl Mater Interfaces. 2020;12(31):34723–34727. doi: 10.1021/acsami.0c11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadinasab S, Chin AWH, Hosseini M, Poon LLM, Ducker WA. SARS-CoV-2 virus transfers to skin through contact with contaminated solids. Sci Rep. 2021 doi: 10.1038/s41598-021-00843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadinasab S, Williams MD, Hosseini M, Poon LLM, Chin AWH, Falkiinham JO, III, Ducker WA. Transparent and sprayable surface coatings that kill drug-resistant bacteria within minutes and inactivate SARS-CoV-2 virus. ACS Appl Mater Interfaces. 2021;13(46):54706–54714. doi: 10.1021/acsami.1c15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov J, Boydston JA, Dunning RA, Yeager JJ, Wood S, Reese AL, Ferris A, Miller D, Weaver W, Zeitouni NE, Phillips A, Freeburger D, Hooper I, Ratnesar-Shumate S, Yolitz J, Krause M, Williams G, Dawson DG, Herzog A, et al. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. Msphere. 2020;5(4):e00441–e00520. doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueckert M, Gupta R, Gupta A, Garg M, Mazumder A. Infectivity of SARS-CoV-2 and other coronaviruses on dry surfaces: potential for indirect transmission. Materials. 2020 doi: 10.3390/ma13225211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Shillam R, Christian P, Elliott TSJ. Role of copper in reducing hospital environment contamination. J Hosp Infect. 2010;74(1):72–77. doi: 10.1016/j.jhin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- CDC (2021) COVID-19 overview and infection prevention and control priorities in non-US healthcare settings. CDC. https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview/index.html. Accessed 13 Sept 2021
- CDC . CDC calls on Americans to wear masks to prevent covid-19 spread. Atlanta: CDC; 2020. [Google Scholar]
- Champagne V, Sundberg K, Helfritch D. Kinetically deposited copper antimicrobial surfaces. Coatings. 2019;9(4):257. doi: 10.3390/coatings9040257. [DOI] [Google Scholar]
- Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen H-L, Chan MCW, Peiris M, Poon LLM. Stability of SARS-CoV-2 in different environmental conditions. Lancet. 2020 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin M, Klingelschmitt F, Charpentier E, Josse J, Kanagaratnam L, De Champs C, Gangloff SC. Copper alloy touch surfaces in healthcare facilities: an effective solution to prevent bacterial spreading. Materials. 2018 doi: 10.3390/ma11122479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes AA, Zuñiga JM. The use of copper to help prevent transmission of SARS-coronavirus and influenza viruses. A general review. Diagnos Microbiol Infect Dis. 2020;98(4):115176. doi: 10.1016/j.diagmicrobio.2020.115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa RA, Thiele DJ. Copper: an essential metal in biology. Curr Biol. 2011;21(21):R877–R883. doi: 10.1016/j.cub.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostin L, Cohen I, Koplan J. Universal masking in the United States: the role of mandates. Health Educ CDC. 2020;324(9):837–838. doi: 10.1001/jama.2020.15271. [DOI] [PubMed] [Google Scholar]
- Govind V, Bharadwaj S, Sai Ganesh MR, Vishnu J, Shankar KV, Shankar B, Rajesh R. Antiviral properties of copper and its alloys to inactivate covid-19 virus: a review. Biometals. 2021;34(6):1217–1235. doi: 10.1007/s10534-021-00339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2011;77(5):1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Chen L, Duan S-M, Yang Q-X, Yang M, Gao C, Zhang B-Y, He H, Dong X-P. Efficient and quick inactivation of SARS coronavirus and other microbes exposed to the surfaces of some metal catalysts. Biomed Environ Sci BES. 2005;18(3):176–180. [PubMed] [Google Scholar]
- Hinsa-Leasure SM, Nartey Q, Vaverka J, Schmidt MG. Copper alloy surfaces sustain terminal cleaning levels in a rural hospital. Am J Infect Control. 2016;44(11):e195–e203. doi: 10.1016/j.ajic.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Hutasoit N, Kennedy B, Hamilton S, Luttick A, Rahman Rashid RA, Palanisamy S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf Lett. 2020;25:93–97. doi: 10.1016/j.mfglet.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim Z, Petrusan AJ, Hooke P, Hinsa-Leasure SM. Reduction of bacterial burden by copper alloys on high-touch athletic center surfaces. Am J Infect Control. 2018;46(2):197–201. doi: 10.1016/j.ajic.2017.08.028. [DOI] [PubMed] [Google Scholar]
- Imai K, Ogawa H, Bui VN, Inoue H, Fukuda J, Ohba M, Yamamoto Y, Nakamura K. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antiviral Res. 2012;93(2):225–233. doi: 10.1016/j.antiviral.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Jin T, Li J, Yang J, Li J, Hong F, Long H, Deng Q, Qin Y, Jiang J, Zhou X, Song Q, Pan C, Luo P. SARS-CoV-2 presented in the air of an intensive care unit (ICU) Sustain Cities Soc. 2020 doi: 10.1016/j.scs.2020.102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University (2021) COVID-19 map. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed 13 Sept 2021
- Kraay ANM, Hayashi MAL, Berendes DM, Sobolik JS, Leon JS, Lopman BA. Risk of fomite-mediated transmission of SARS-CoV-2 in child daycares, schools, and offices: a modeling study. MedRxiv. 2020 doi: 10.1101/2020.08.10.20171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley WG, Reynolds JS, Szalajda JV, Noti JD, Beezhold DH. A cough aerosol simulator for the study of disease transmission by human cough-generated aerosols. Aerosol Sci Technol. 2013;47(8):937–944. doi: 10.1080/02786826.2013.803019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li T, Deng Y, Liu S, Zhang D, Li H, Wang X, Jia L, Han J, Bei Z, Li L, Li J. Stability of SARS-CoV-2 on environmental surfaces and in human excreta. J Hosp Infect. 2021;107:105–107. doi: 10.1016/j.jhin.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantlo EK, Paessler S, Seregin A, Mitchell A. Luminore CopperTouch™ surface coating effectively inactivates SARS-CoV-2, Ebola, and Marburg viruses in vitro. MedRxiv. 2020 doi: 10.1101/2020.07.05.20146043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel CS, Moore MD, Jaykus LA. Destruction of the capsid and genome of GII.4 human norovirus occurs during exposure to metal alloys containing copper. Appl Environ Microbiol. 2015;81(15):4940–4946. doi: 10.1128/AEM.00388-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquès M, Domingo JL. Contamination of inert surfaces by SARS-CoV-2: persistence, stability and infectivity. A Review. Environ Res. 2021;193:110559. doi: 10.1016/j.envres.2020.110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels HT, Keevil CW, Salgado CD, Schmidt MG. From Laboratory research to a clinical trial: copper alloy surfaces kill bacteria and reduce hospital-acquired infections. HERD Health Environ Res Des J. 2015;9(1):64–79. doi: 10.1177/1937586715592650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero DA, Arellano C, Pardo M, Vera R, Gálvez R, Cifuentes M, Berasain MA, Gómez M, Ramírez C, Vidal RM. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob Resist Infect Control. 2019;8(1):3. doi: 10.1186/s13756-018-0456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce JO, Michels H, Keevil CW. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol. 2007;73(8):2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter JA, Yezli S, Salkeld JAG, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control. 2013;41(5, Supplement):S6–S11. doi: 10.1016/j.ajic.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio C, Colombo M, Arciola CR, Greggi T, Scribante A, Dagna A. Copper-alloy surfaces and cleaning regimens against the spread of SARS-CoV-2 in dentistry and orthopedics. From fomites to anti-infective nanocoatings. Materials. 2020;13(15):3244. doi: 10.3390/ma13153244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194(1):1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowska P, Tiddia M, Farugui N, Bankier C, Pei Y, Pollard A, Zhang J, Gilmore I. Antiviral surfaces and coatings and their mechanisms of action. Commun Mater. 2021 doi: 10.1038/s43246-021-00153-y. [DOI] [Google Scholar]
- Ren S-Y, Wang W-B, Hao Y-G, Zhang H-R, Wang Z-C, Chen Y-L, Gao R-D. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases. 2020;8(8):1391–1399. doi: 10.12998/wjcc.v8.i8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S, Goldie S, Hill A, Eagles D, Drew TW. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol J. 2020;17(1):145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, Vija H, Kahru A, Keevil CW, Ivask A. Rapid in situ assessment of Cu-ion mediated effects and antibacterial efficacy of copper surfaces. Sci Rep. 2018;8(1):8172. doi: 10.1038/s41598-018-26391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Różańska A, Chmielarczyk A, Romaniszyn D, Sroka-Oleksiak A, Bulanda M, Walkowicz M, Osuch P, Knych T. Antimicrobial properties of selected copper alloys on staphylococcus aureus and Escherichia coli in different simulations of environmental conditions: with vs. without organic contamination. Int J Environ Res Public Health. 2017 doi: 10.3390/ijerph14070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, Sharpe PA, Michels HT, Schmidt MG. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol. 2013;34(5):479–486. doi: 10.1086/670207. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Attaway HH, Sharpe PA, John J, Jr, Sepkowitz K, Morgan A, Fairey S, Singh S, Steed L, Cantey JR, Freeman K, Michels HT, Salgado CD. Sustained reducation of microbial burden on common hospital surfaces through introduction of copper. J Clin Microbiol. 2012 doi: 10.1128/JCM.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes SL, Caves V, Keevil CW. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ Microbiol. 2012;14(7):1730–1743. doi: 10.1111/j.1462-2920.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- Warnes SL, Little ZR, Keevil CW. Human coronavirus 229E remains infectious on common touch surface materials. Mbio. 2015 doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks SA, Michels H, Keevil CW. The survival of Escherichia coli O157 on a range of metal surfaces. Int J Food Microbiol. 2005;105(3):445–454. doi: 10.1016/j.ijfoodmicro.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Ye G, Lin H, Chen S, Wang S, Zeng Z, Wang W, Zhang S, Rebmann T, Li Y, Pan Z, Yang Z, Wang Y, Wang F, Qian Z, Wang X. Environmental contamination of SARS-CoV-2 in healthcare premises. J Infect. 2020;81(2):e1–e5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbib S, Vallet L, Muggeo A, de Champs P, Lefebvre P, Jolly P, Kanagaratnam P. Copper for the prevention of outbreaks of health care-associated infections in a long-term care facility for older adults. ClinicalKey. 2020;21(1):68–71. doi: 10.1016/j.jamda.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Zuo YY, Uspal WE, Wei T. Airborne transmission of COVID-19: aerosol dispersion, lung deposition, and virus-receptor interactions. ACS Nano. 2020;14(12):16502–16524. doi: 10.1021/acsnano.0c08484. [DOI] [PubMed] [Google Scholar]