Abstract
Infections with Streptococcus pneumoniae remain a significant cause of morbidity and mortality. To gain insight into structure-function relationships for human antibodies to pneumococcal capsular polysaccharide (PPS), we studied the response of transgenic mice reconstituted with human immunoglobulin loci, XenoMouse, to PPS antigens in a pneumococcal vaccine. Enzyme-linked immunosorbent assays of sera from mice vaccinated with a 23-valent pneumococcal vaccine revealed that they produced serotype-specific human antibodies, with the greatest response being to the PPS of serotype 3 (PPS 3). Molecular sequence analysis of three monoclonal antibodies (MAbs) to PPS 3 generated from lymphoid cells from mice vaccinated with a 23-valent pneumococcal vaccine or a PPS 3-bovine serum albumin conjugate revealed that they all used heavy-chain immunoglobulin genes from the VH3 family, two expressed light chain genes from the human Vκ1 family, and one expressed a mouse λ light chain. The protective efficacy of the two MAbs was examined in mice. A 10-μg dose of both, and a 1-μg dose of one, significantly prolonged survival from a lethal serotype 3 infection in CBA/N mice. Our data show that XenoMouse mice produced protective, serotype-specific human antibodies to PPS 3, and they lend support to the proposal that these animals represent a useful model to study the human antibody response to PPS antigens.
Available pneumococcal vaccines consist of the purified capsular polysaccharides (PPS) of the most common serotypes of Streptococcus pneumoniae that cause disease in adults and children. Though immunogenic in normal individuals, PPS-based vaccines are poorly immunogenic in adult patients who are at increased risk for pneumococcal infections (reviewed in reference 33). Our group and others have shown that human antibodies to PPS are derived from restricted B-cell subsets which express VH3 gene family segments (3, 27, 40). However, these studies were based largely on studies of polyclonal serum antibodies. Characterization of the molecular genetic structure of antibodies to the capsular polysaccharide of Haemophilus influenzae type b (4, 28) has provided insight into possible mechanisms of disease susceptibility and vaccine failure in patients with decreased expression of the genetic elements used in the response (16). This is relevant to S. pneumoniae, because overall (8) and serotype-specific VH3 expression is decreased in human immunodeficiency virus-infected individuals (3), a group with reduced responses to pneumococcal vaccines (reviewed in reference 33) and increased susceptibility to pneumococcal infection (23).
To date, the genetic makeup of a limited number of human monoclonal antibodies (MAbs) to a limited number of PPS serotypes generated by Epstein-Barr virus transformation of lymphocytes from vaccinated recipients has been described, namely, two MAbs to PPS 3 derived from different individuals (39), one to PPS 8 (46) and one to PPS 6B (40). Epstein-Barr virus transformation is difficult and unpredictable. In addition, all approaches to producing MAbs from vaccinated humans are hampered because the immune response cannot be manipulated experimentally. The development of a transgenic mouse strain reconstituted with human immunoglobulin loci, XenoMouse, has made it possible to generate fully human antibodies in mice (29). XenoMouse animals were created by introducing yeast artificial chromosomes (YACs) containing 66 human heavy-chain and 32 κ light-chain immunoglobulin genes into mice whose endogenous heavy-chain and κ loci were functionally inactivated by targeted deletion (29). The mice express human μ, δ, γ2, and κ chains and mouse λ chains, with a human κ-to-mouse λ ratio of 75:1 (29). XenoMouse animals were previously shown to produce human antibodies to protein antigens (19, 29), but their ability to respond to T-independent antigens such as polysaccharides was unknown. This study was undertaken to investigate whether XenoMouse animals represent a useful model to investigate the human antibody repertoire to PPS.
MATERIALS AND METHODS
S. pneumoniae and PPS.
S. pneumoniae serotype 3, strain 10813 (American Type Culture Collection [ATCC], Manassas, Va.), was used for mouse protection experiments. Organisms were prepared for animal inoculation as described previously (1) and stored at −80°C until use. A 23-valent pneumococcal vaccine (Pneumovax 23; Merck & Co., Inc., West Point, Pa.) was used for vaccinations and to coat enzyme-linked immunosorbent assay (ELISA) plates. Purified PPS from S. pneumoniae serotypes 3, 4, 6B, 8, and 19F (ATCC) were used for ELISAs. A PPS 3-bovine serum albumin (BSA) conjugate was produced with the purified PPS from serotype 3 (ATCC) and BSA (Sigma Chemical Co., St. Louis, Mo.) as described elsewhere (26).
XenoMouse animals and vaccination protocols.
Two genetically distinct groups of XenoMouse animals were used: Xm2a-3, reconstituted with one double YAC containing both heavy- and light-chain genes; and Xm2a-5, reconstituted with two YACs, one with heavy-chain and the other with light-chain genes (22). All mice were bred and maintained at Abgenix (Fremont, Calif.). Overall, 40 XenoMouse animals (37 female and 3 male) were vaccinated with 11.5 μg of a 23-valent pneumococcal vaccine (consisting of 0.5 μg of each of the 23 PPS antigens in the vaccine) either without (group A [5 animals]) or with (groups B [5 animals] and C and D [15 animals each]) 25 μg of the adjuvant monophosphoryl lipid A (Sigma) as described elsewhere (18). The vaccinations were administered either intraperitoneally (groups A, B, and C) or subcutaneously at the base of the tail (group D). In addition, a group of 10 Xm2a-3 mice was vaccinated at the base of the tail with 10 μg of the PPS 3-BSA conjugate (see above) without adjuvant on days 1, 15, and 18. The PPS 3-BSA conjugate was used because the Pneumovax-vaccinated mice had the greatest serum antibody responses to PPS 3 (see below).
Serologic studies of antibody responses.
Sera were separated by centrifugation from blood obtained from the retro-orbital sinus plexus of the mice and stored at −20°C until analyzed. The sera were adsorbed with purified pneumococcal cell wall polysaccharide (CWPS; Statens Seruminstitut, Copenhagen, Denmark), and an antigen capture ELISA was used to detect antibodies to PPS as described elsewhere (3). Briefly, polystyrene ELISA plates (Corning Glass Works, Corning, N.Y.) were coated with either PVX (10 μg/ml) or PPS 3, 4, 6B, 8, or 19F (10 μg/ml), blocked with PBS–1% BSA, washed, and incubated at 37°C for 1 h with a 1:50 dilution of the serum samples. After washing, the plates were incubated at 37°C for 1 h with alkaline phosphatase (AP)-conjugated goat antibodies to human immunoglobulin G (IgG), IgM, and kappa light chains and to mouse lambda light chains (Fisher Biotech, Fisher Scientific, Pittsburgh, Pa.) and were then washed. Antibody binding was detected with p-nitrophenyl phosphate substrate (Sigma) in diethanolamine buffer (pH 9.8). Optical densities (ODs) were measured at 405 nm with an MRX microplate reader (Dynatech Laboratories, Chantilly, Va.). Serum from a human PVX recipient which was taken on day 28 postvaccination used at a 1:50 dilution was the positive control on each plate (3). Antibody levels were defined as the average OD of duplicate wells of the samples minus two times the background. The background for each plate was defined as the OD of the detection antibody alone.
Generation and molecular characterization of hybridomas.
Hybridomas were generated by fusion of splenic and/or lymph node cells from XenoMouse animals with the nonsecreting myeloma cell line NSO-bcl 2 as described elsewhere (29). The cells used for the fusions were from mice vaccinated with the PPS 3-BSA on days 1, 15, and 18 before fusion on day 21 or from mice that had received PVX vaccinations on days 1, 26, 40, 56, 82, and 87 before fusion on day 90. The latter vaccination protocol had been used previously for immunization of XenoMouse animals with other antigens. Supernatants from hypoxanthine-aminopterin-thymidine-selected hybridomas were screened for the production of human antibodies to PPS by ELISA as described above. Nucleic acid sequencing of hybridoma heavy (VH) and light (VL) chains was performed on PCR-amplified VH and VL cDNAs as described elsewhere (29, 34). PCR products corresponding to the human IgM VH and Vκ were isolated from Tris-acetate-EDTA agarose gels by centrifugation through Spin X columns (Fisher Biotech), and both strands of the gel-purified product were directly sequenced as described previously (19). Sequence analysis was performed using the Vbase database for sequence alignment and identification (I. M. Tomlinson et al., MRC Centre for Protein Engineering) (21). D regions were assigned according to Corbett et al. (12).
Specificity studies of the MAbs.
The serotype specificity of the MAbs was determined by ELISA using plates coated with PPS 3, 4, 6B, 8 and 19F as described above. The PPS 3 binding of the MAbs was examined by inhibition ELISA after adsorption of the MAbs with 50 μg of CWPS per ml. PPS 3-coated ELISA plates were incubated with the MAbs (5 μg/ml) and concentrations of PPS 3 from 0.01 to 100 μg/ml at 37°C for 1 h. The plates were then washed and incubated with AP-conjugated goat antibody to human IgM and developed as described above. Cross-reactivity of the MAbs with tetanus toxoid (TT; Connaught, Inc., Swiftwater, Pa.), human IgG Fc (Calbiochem, San Francisco, Calif.), human DNA (Sigma), and CWPS was examined by ELISA as described elsewhere (34). Briefly, serial dilutions of the MAbs starting at a concentration of 5 μg/ml (except for MAb 1.4, which was available only at a concentration of 1 μg/ml) were incubated with antigen-coated plates. A human MAb with reactivity with DNA, TT, and IgG Fc (provided by Anne Davidson, Albert Einstein College of Medicine) was used as a positive control. The human IgM MAb to PPS 8, D11, was used as a negative control (46). The MAbs were purified by column chromatography from ascites (MAbs 1.2, 1.10, and 1.1) or hybridoma supernatants (MAb 1.4).
MAb-mediated complement activation.
ELISAs were performed as described elsewhere (46) to determine if the MAbs to PPS 3 could deposit complement component 3 (C3) on PPS 3. ELISA plates coated with 10 μg of PPS 3 per ml were incubated at 37°C for 1 h with solutions containing a final MAb concentration of 1 or 10 μg/ml and 10% of each complement source. Factor B-deficient human serum (Calbiochem), which has an inactive alternative complement pathway, was used to evaluate the influence of the classical pathway as described elsewhere (46, 47). Normal human serum, adsorbed with PPS 3, before and after chelation with 10 mM EGTA (Sigma) and 10 mM MgCl2, was used to examine the influence of the alternative pathway as described elsewhere (17). A myeloma IgM MAb (Calbiochem) was used as a negative control (46, 47). After incubation, the plates were washed, incubated at 37°C for 1 h with goat anti-human C3 (Sigma), washed, incubated at 37°C for 1 h with AP-conjugated rabbit antibody to goat IgG (Sigma), and the developed and read as described above. C3 deposition was characterized by OD after subtraction of the background due to the complement sources alone. The C3 deposition experiment was performed twice.
Mouse protection experiments.
Protection studies were performed with MAbs 1.10 and 1.2. The design of the experiments was based on our group's and others' studies of S. pneumoniae infections in mice (9, 10). Female CBA/N mice (6 to 8 weeks of age) were obtained from the National Cancer Institute, Bethesda, Md., and maintained in the Albert Einstein College of Medicine animal facility prior to use. Mouse inoculations were performed as follows: groups of 10 mice each received 0.5 ml (1 or 10 μg in sterile saline) of MAb 1.2, MAb 1.10, the human myeloma IgM control (Calbiochem) used as an isotype control as described elsewhere (46), or PBS intraperitoneally; 1 h later, 0.2 ml (300 CFU in tryptic soy broth) of S. pneumoniae (ATCC strain 10813) was given via intravenous inoculation into the lateral tail vein. The number of CFU injected was confirmed by plating each inoculum that was injected into the mice. The inoculum was based on the known susceptibility of CBA/N mice to infection with strain 10813 as described previously (9, 10) and was >100 times greater than the 90% lethal dose for CBA/N mice determined in our laboratory (data not shown). Mice were observed daily. The number of surviving mice in each group was compared with the Kaplan-Meier log-rank survival test using the statistical software package SPSS (SPSS, Inc., Chicago, Ill.). The protection experiments were performed two times.
RESULTS
Antibody responses to pneumococcal vaccination.
The XenoMouse animals in all four vaccination groups produced human IgM and IgG to the PPS antigens in the vaccine. The magnitude of the IgM response for each group was greater than the IgG response, and there was no significant difference in the response of the four groups. The binding of preimmune sera to PVX and individual PPS antigens was equal to or less than background (not shown). Five mice were found to have mouse λ antibodies to the PPS in the vaccine 1 of 5 from group A, 1 of 5 from group B, 2 of 15 from group C, and 1 of 15 from group D. IgM and IgG reactive with PPS 3, 4, 6B, 8, and 19F is shown for a subset of 19 mice with the highest levels of antibodies to the PPS in the whole vaccine (Fig. 1). Overall, 17 of 19 mice had levels of IgM to PPS 3 that were greater than or equal to the level of a human vaccinee. Vaccination of Xm2a-3 XenoMouse animals with the PPS 3-BSA conjugate elicited IgM to PPS 3 in 10 of 10 Xm2a-3 mice, but only 3 mice produced IgG to PPS 3 (Fig. 1). The PPS 3 binding of sera from the PPS 3-BSA-vaccinated mice was inhibited by soluble PPS 3 (not shown).
FIG. 1.
Serotype-specific responses to the PPS 3, 4, 6B, 8, and 19F in Pneumovax-vaccinated mice. The PPS 3 responses of the PPS 3-BSA-vaccinated mice are shown in the top right panel. The y axes represent the ODs of serum samples from the individual mice depicted on the x axes. 007 and 009 series mice are from groups A and B; all other mice are from groups C and D. The y-axis scales differ for each serotype. For groups A and B, samples tested were from day 47 after vaccinations on days 1, 26, and 40. For groups C and D, samples tested were from day 21 after vaccinations on days 1 and 14. The sera from the PPS 3-BSA-vaccinated mice were from day 18 after vaccinations on days 1 and 15. The sera were used at a dilution of 1:50. The ODs shown represent the values after subtracting two times the background. A day 28 postvaccination serum from a human vaccinee was used as a positive control on each plate at a dilution of 1:50. It had the following serotype-specific ODs: PPS 3, 0.83 (IgM) and 2.15 (IgG; PPS 4, 0.90 and 2.11; PPS 6B, 0.58 and 1.89; PPS 8, 1.42 and 2.09; PPS 19, 1.16 and 2.15. Black bars represent IgM, and cross-hatched bars represent IgG.
Specificity of MAbs.
MAbs 1.10, 1.2, and 1.4 reacted with PPS 3, without significant binding (less than or equal to background) to the PPS of serotypes 4, 6B, 8, 14, and 19F. Fifty percent of the binding of MAbs 1.10 and 1.2 (each at 5 μg/ml) to PPS 3 was inhibited by soluble PPS 3 at 0.8 and 2 to 7 μg/ml, respectively; 46% of the binding of a culture supernatant of MAb 1.4 (0.45 μg/ml) was inhibited by soluble PPS 3 at 1 μg/ml (not shown). Soluble PPS 3 did not inhibit the PPS 3 binding of MAbs 1.1 and 2.1 (data not shown), which was interpreted to indicate that their PPS 3 binding was nonspecific. The MAbs did not bind to CWPS, DNA, TT, or human IgG Fc (not shown). Hence, the MAbs were not polyreactive and had different avidity for PPS 3.
MAb-mediated complement activation.
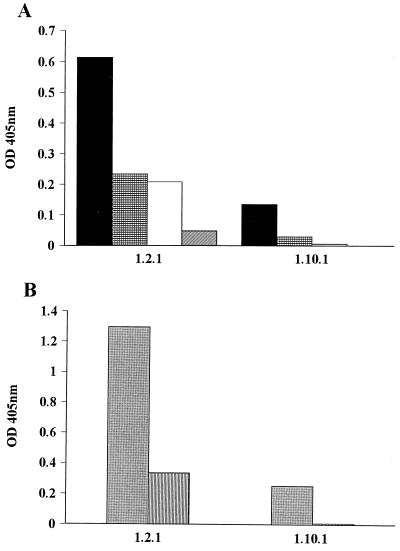
Since antibody is required for most pneumococcal serotypes, including serotype 3, to active complement (43), the ability of the PPS 3-specific MAbs 1.2 and 1.10 to deposit C3 on solid-phase PPS 3 was studied. More studies with MAb 1.4 were precluded by the loss of the cell line. In the presence of EGTA-treated and untreated human serum, the magnitude of C3 deposition on solid-phase PPS 3 was greater with MAb 1.2 than MAb 1.10 for both MAb concentrations (Fig. 2A). Similarly, in the presence of factor B-deficient serum, the magnitude of C3 deposition was greatest for MAb 1.2, and there was greater deposition at the higher concentration (Fig. 2B). These results demonstrate that MAb-mediated C3 deposition on PPS 3 was greater via the classical than the alternative complement pathway and that MAb 1.2 mediated more complement deposition. The experiments were repeated two times with similar results.
FIG. 2.
MAb-mediated C3 deposition on PPS 3 via activation of the alternative or classical complement pathway. The y axes depict the OD representing C3 deposited on solid-phase PPS 3 in the presence of the MAbs depicted on the x axis, along with the influence of the alternative complement pathway activation in the presence of human sera before and after chelation with EGTA and MgCl2 (A) and the influence of the classical pathway in the presence of factor B-deficient human serum (B). Each bar represents the average of duplicate wells. For the control IgM in both experiments, C3 binding was detected at ODs of ≤0.1 after subtraction of the background; hence no bars are depicted. Symbols in panel A represent the MAbs at 10 μg/ml in the presence of normal human serum (solid black bars) and in the presence of serum chelated with EGTA and MgCl2 (hatched black bars) and at 1 μg/ml in the presence of normal human serum (white bars) and in the presence of chelated serum (hatched white bars). In panel B, gray bars represent MAbs at 10 μg/ml, while hatched gray bars represent mAbs at 1 μg/ml; all are in the presence of factor B-deficient serum. The experiments were performed twice, and the results of one experiment are shown.
Genetic makeup of XenoMouse-derived MAbs.
The immunoglobulin gene segments used by the three PPS 3-specific MAbs are shown in Table 1. MAb 1.10 was a chimeric antibody that expressed a human μ and mouse λ transcript, but it had a human κ transcript with an 18-bp deletion in its Jκ2 sequence which prevented its expression. All the other MAbs expressed both human μ and κ transcripts without deletions. Each MAb had a different heavy-chain gene element, each from the VH3 family: MAb 1.10 used the DP-38/9.1/V3-15 gene, MAb 1.2 used the DP 77/V3-21 gene, and MAb 1.4 used the DP 50/V3-33 gene (Table 1). Each MAb had a unique VH CDR 3. Table 2 shows the CDR 3 sequences of the PPS 3-specific XenoMouse-derived MAbs compared to the human MAbs to PPS 3 reported by Shaw et al. (39) and the nonspecific MAbs produced from the vaccinated XenoMouse mice. The D regions of MAbs 1.10, 1.2, and 1.4 use reading frame 1, and those of the nonspecific MAbs use reading frame 2. All of the D regions encode a number of polar and hydrophilic amino acids: glutamine (Q), glycine (G), serine (S), asparagine (N), tyrosine (Y), and threonine (T). The PPS 3-specific XenoMouse-derived MAbs all used JH4 gene segments, whereas the nonspecific MAbs use different JH genes. All PPS 3-specific MAbs had germ line VH and Vκ sequences, but the nonspecific MAb 1.1 had 3 silent base changes in codons 69, 88, and 93 of the VH. Taken together, the sequence information revealed that all of the MAbs used genes from the VH3 family, but the junctional diversity of the MAbs indicated that each was the product of a distinct recombination event.
TABLE 1.
Heavy- and light-chain variable gene use of PPS 3-specific MAbs
| MAba | Specificityb | Heavy-chain gene segments
|
Light-chain gene segments
|
|||||
|---|---|---|---|---|---|---|---|---|
| VH | DH | CDR 3 lengthc | JH | VL | CDR 3 lengthc | JL | ||
| 1.10 | PPS 3 | VH3/V3-15 | D6-19 | 10 | JH4b | Mouse λd | Mouse λ | Mouse λ |
| 1.2 | PPS 3 | VH3/V3-21 | D5-5/5-18 | 12 | JH4b | Vκ1/L5 | 9 | Jκ1 |
| 1.4 | PPS 3 | VH3/V3-33 | D1-26 | 9 | JH4b | Vκ1/A30 | 9 | Jκ1 |
| 1.1 | Nonspecific | VH3/V3-15 | D1-1rc | 15 | JH6 | Vκ1/A30 | 9 | Jκ3 |
| 2.1 | Nonspecific | VH3/V3-15 | D1-7 | 8 | JH3 | Vκ1/A30 | 9 | Jκ3 |
GenBank accession numbers of MAbs (VH/VL): 1.10, AF209733/AF209734; 1.2, AF209735/AF209736; 1.4, AF209737/AF209738; 1.1, AF209741/AF209742; 2.1, AF209739/AF209740.
PPS 3 specificity for the XenoMouse-derived MAbs was determined as indicated in the text by inhibition ELISA. MAbs 1.1 and 2.1 were not inhibited by PPS 3 and were designated nonspecific.
Number of amino acids in the VH (amino acids 95 to 101) or Vκ (amino acids 89 to 96) CDR 3.
MAb 1.10 expressed a mouse λ which was not sequenced, though it contained a transcript for Vκ1/A17-Jκ2 which had an 18-bp deletion in Jκ (see text).
TABLE 2.
Heavy- and light-chain CDR3 sequences of PPS 3-specific and nonspecific MAbs
| Cell line | Specificitya | VH CDR 3b | Vκ CDR 3 |
|---|---|---|---|
| 1.10 | PPS 3 | K YSSGWLPFAY | Nonec |
| 1.2 | PPS 3 | R DHSYGWGKSFDY | QQANSFPRT |
| 1.4 | PPS 3 | R GVGATLFDY | LQHNSYPPT |
| 1.1 | Nonspecific | D YQLSRYYYYYYGMNVW | LQHNSYPFT |
| 2.1 | Nonspecific | T GTTIAFDI | LQHNSYPFT |
| HU AB 14-3d | PPS 3 | R SNYFDS | QQYYSTPLT |
| HU AB 2-3d | PPS 3, polyreactive | G HQPLTTVTTPHWFDP | LLSYSGARV |
PPS 3 specificity for the XenoMouse-derived MAbs was determined as indicated in the text by inhibition ELISA.
The VH (amino acids 95 to 101) and Vκ (amino acids 89 to 96) CDR 3 regions are shown preceded by amino acid 94, which is shown in boldface letters.
MAb 1.10 did not express a human Vκ (see text).
EBV-transformed cell line secreting IgM antibodies to PPS 3 generated by Shaw et al. (39).
Mouse protection experiments.
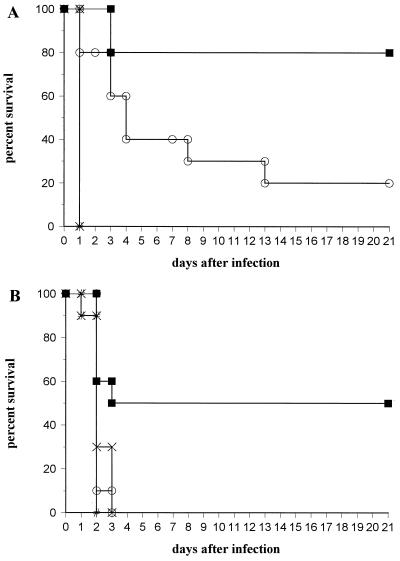
Treatment with MAb 1.2 prolonged the survival of mice infected with serotype 3 S. pneumoniae at both 1- and 10-μg doses, whereas MAb 1.10 prolonged survival at the 10-μg dose only (Fig. 3). At the 10-μg dose, mean survival was 1 day for the PBS group, 1 day for the control IgM group, 7.8 ± 2.3 days for the MAb 1.10 group, and 17.2 ± 2.4 days for the MAb 1.2 group. At this dose, the groups receiving both MAbs lived significantly longer than the IgM and untreated control groups (P ≤ 0.0004, Kaplan-Meier log-rank survival test), and survival of the group receiving MAb 1.2 was greater than that of the group receiving MAb 1.10 (P = 0.016). For the 1-μg dose, mean survival was 1.9 ± 0.1 days for the PBS group, 2.2 ± 0.2 days for the control IgM group, 2.1 ± 0.1 days for the MAb 1.10 group, and 8.1 ± 1.9 days for the MAb 1.2 group. At this dose, survival was significantly greater for the group receiving MAb 1.2 than the other groups (P ≤ 0.017). All mice surviving to day 21 postinfection were alive 3 months postinfection. Protection experiments were performed two times with similar results.
FIG. 3.
Survival of CBA/N mice after infection with serotype 3 S. pneumoniae. (A) Survival of mice treated with MAb doses of 10 μg; (B) survival of mice treated with mAb doses of 1 μg. The y axes show percentages of mice surviving the number of days after infection depicted on the x axes. Mice were infected as described in the text after the administration of IgM (depicted by ×), PBS (#), MAb 1.2 (■), or mAb 1.10 (○). The experiments were performed twice, and the results of one experiment are shown. In panel A, IgM (x) and PBS (#) had identical survival.
DISCUSSION
Our data show that XenoMouse animals vaccinated with a pneumococcal vaccine produced fully human antibodies to PPS antigens in the vaccine and that MAbs to PPS 3 generated from lymphocytes of PPS-vaccinated animals were protective in mice against serotype 3 infection. XenoMouse mice are transgenic mice reconstituted with human immunoglobulin loci and inactivated endogenous heavy and kappa light-chain loci. The lambda loci of the animals are intact. Previous work with these mice has shown that the size of their immunoglobulin loci, which contain 66 VH and 32 Vκ genes, promotes both B-cell development (20) and human antigen-specific antibody responses (29). Whereas the latter had been shown only for protein antigens, this report is the first to demonstrate that the XenoMouse repertoire is sufficient to generate an antipolysaccharide response. More work is needed to establish how closely the anti-PPS response of XenoMouse mice resembles that of humans, since the repertoire of XenoMouse mice does not contain a human λ locus and the λ light chain contributes to the human antibody response to PPS (27, 32). However, our finding that like antibodies to PPS from humans (3, 25, 27, 40), XenoMouse-derived MAbs to PPS 3 use VH3 genes suggests that the anti-PPS response in these animals is restricted to VH3 as it is in humans.
Our data show that the antibody repertoire of XenoMouse animals is sufficient to generate fully human antibodies to PPS antigens. Similar to humans, the mice responded differently to different serogroups, with the greatest response to PPS 3. This is interesting in light of data showing that PPS 3 was immunogenic in infants, whereas serogroups 6 and 19 were not (7). Although our findings suggest a possible parallel with the responses of young children to PPS serotypes (15), our studies were not designed to compare the immunogenicities of different PPSs in the mice or to directly compare the responses of the mice and humans. PPS is a weak immunogen which elicits a T-independent type 2 response (30), though it has been noted that the adult response to some serotypes resembles a recall response (27). Since environmental, cross-reactive antigens and/or exposure to pneumococcal serotypes are proposed to play a role in establishing the antibody repertoire to PPS (27, 36), more work is necessary to determine whether and how the preimmune repertoire influences the responses of XenoMouse mice to certain PPSs, e.g., 6B and 19F. At present, the serotype-to-serotype and mouse-to-mouse variability observed in the response to the PPS serotypes examined in this study is not understood. A factor which could contribute is that the sera were evaluated after multiple vaccinations. It has been reported that antibody levels to PPS antigens can be reduced in the presence of high concentrations of polysaccharide (6) and the amount of vaccine administered and its clearance may have varied for different PPSs among the mice.
Reports from several groups show that VH3 genes are used in the antibody responses to PPS (3, 27, 39, 40, 46). Our finding that the PPS 3-specific XenoMouse-derived MAbs expressed VH3 genes lends further support to the concept of restriction in the human anti-PPS response. A mechanism to explain this phenomenon has not been described. VH3 is the largest and most prevalent human VH gene family subgroup, though its representation in the expressed antibody repertoire has been attributed to antigen selection in addition to the size of the subgroup (14). In this regard, it is noteworthy that common structural characteristics have been found among antibodies to defined types of antigens, e.g., polysaccharides (44), suggesting the possibility that only certain immunoglobulin receptors can bind to polysaccharides. Consistent with the latter, VH3 immunoglobulin receptors have unique structural characteristics which place them in a separate clan (clan III) and distinguish them from the immunoglobulin receptors derived from other VH subgroups (24). Interestingly, human antibodies to other capsular polysaccharides, namely, H. influenzae and Cryptococcus neoformans, also use VH3 genes (3, 4, 34).
VH3 gene use alone appears to be insufficient to confer PPS specificity. DP 38/V3-15 was used in both the PPS3-specific MAb 1.10 and human MAbs to PPS 8 and 6B (40, 46) as well as in the nonspecific XenoMouse MAbs 1.1 and 2.1 and Fabs from a human combinatorial library that cross-react with PVX, phosphorycholine, and DNA (25). The fact that the XenoMouse-derived MAbs were germ line encoded indicates that the diversity generated by recombinational and combinatorial mechanisms was sufficient to produce antibodies specific to PPS 3. A major influence on antibody specificity is thought to be the structure of antigen-antibody contact regions, particularly the CDR 3 which is created by a unique V-DJ joining event in each B cell (37). The PPS 3-specific MAbs 1.10, 1.2, and 1.4 had distinct CDR 3 regions and different avidity for PPS 3. However, each MAb had a positively charged residue at VH position 94, as did the protective MAb described by Shaw et al. (39), whereas the nonspecific MAbs did not (Table 2). Such residues have also been found in antibodies to DNA (25), suggesting that charge interactions may play a role in binding to negatively charged molecules. The frequency of CDR 3-encoded aromatic residues in the PPS 3-specific XenoMouse-derived MAbs is consistent with the concept that aromatic amino acids may enhance binding to capsular polysaccharides (42, 45). However, a fuller understanding of the contribution of charge, polarity, and aromaticity to PPS 3-antibody interactions must await formal structure-function analysis and/or crystallographic studies.
Four of the XenoMouse-derived MAbs, including two that were specific for PPS 3 (MAbs 1.2 and 1.4), used Vκ1 genes. While it has been suggested that certain cationic Vκ1 light chains might preferentially bind to negatively charged molecules, e.g., DNA and capsular polysaccharides (38, 41), this concept requires further investigation. The use of proximal Vκ genes is consistent with previous studies of the antibody repertoires of XenoMouse mice (29) and humans (13). The mice were reconstituted with 32 Jκ-proximal κ genes, which represent about 95% of the expressed κ genes in the human antibody repertoire (13). Thus, the PPS-elicited light-chain antibody repertoire in the mice resembled the naturally occurring human antibody repertoire. Consistent with other studies (25, 27) but in contrast to antibodies to H. influenzae (5, 16), we did not find a predilection for expression of certain light chains in the anti-PPS 3 response. Interestingly, a human MAb to PPS 3 reported by the Shaw group (HU AB 14-3 [39]) also used a Vκ CDR 3 motif QQ--S-P-T motif. Although the significance of this motif for PPS 3 binding is uncertain, its existence in both XenoMouse- and human-derived MAbs that were protective against serotype 3 is intriguing. Lambda genes are used in antibodies to PPS 6B (27, 40) and the polyreactive MAb to PPS 3 reported by Shaw et al. (39). The significance of λ use by MAb 1.10 is uncertain since this MAb had an unexpressed Vκ2/A27 transcript, and the XenoMouse animals used in our studies lacked a human λ locus. Hence, the importance of the human λ repertoire in the anti-PPS response cannot be examined in this model.
The XenoMouse-derived MAbs 1.10 and 1.2 were protective in mice. This is a significant finding, since some human antibodies enhanced pneumococcal infection in mice (2, 35). MAb 1.2 was more effective than MAb 1.10 in that it mediated survival of a greater proportion of animals at a 10-fold-lower dose. Since complement opsonins enhance antibody-mediated opsonization of S. pneumoniae (11) and are required for IgM-mediated protection against the pneumococcus (46), the greater complement deposition on PPS 3 mediated by MAb 1.2 may be a correlate of its superior protective efficacy. However, other mechanisms such as phagocytosis of antigen-antibody aggregates may also play a role in IgM-mediated protection (31). The use of different genetic elements and the unique CDR 3 sequences of MAbs 1.10 and 1.2 raise the possibility that they had differences in fine specificity which translated into differences in biological activity. Alternatively, MAb 1.10 may have had a reduced ability to activate complement and/or different pharmacokinetics because it expressed a mouse λ. Thus, further studies are needed to fully understand the observed differences in the protective efficacy of MAb 1.10 as compared to MAb 1.2.
In summary, our data demonstrate that XenoMouse mice produced protective antibodies to PPS 3 that use VH3 genes. The germ line configuration of the MAbs indicates that the antibody repertoire of these animals is sufficient to generate an anti-PPS 3 response which reflects the VH usage of the native human repertoire. The study of human antibodies to PPS in the XenoMouse model has the potential enlarge the basic science knowledge base regarding the molecular characteristics of protective antibodies to S. pneumoniae. Such information is likely to provide insight into possible mechanisms of vaccine failure and facilitate the development of new approaches to treating and preventing pneumococcal infections.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI 35370 to L.P. and NIH Microbial Pathogenesis Training Grant 1 P32 AI 07506 to N.D.R.
REFERENCES
- 1.Aaberge I S, Eng J, Lermark G, Lovik M. Virulence of Streptococcus pneumococcus in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb Pathog. 1995;18:141–152. doi: 10.1016/s0882-4010(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 2.Aaberge I S, Hvalbye B, Lovik M. Enhancement of Streptococcus pneumoniae serotype 6B infection in mice after passive immunization with human serum. Microb Pathog. 1996;21:125–137. doi: 10.1006/mpat.1996.0048. [DOI] [PubMed] [Google Scholar]
- 3.Abadi J, Friedman J, Jefferis R, Mageed R A, Pirofski L. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J Infect Dis. 1998;178:707–716. doi: 10.1086/515369. [DOI] [PubMed] [Google Scholar]
- 4.Adderson E E, Azmi F H, Wilson P M, Shackelford P G, Carroll W L. The human VH3b gene subfamily is highly polymorphic. J Immunol. 1993;151:800–809. [PubMed] [Google Scholar]
- 5.Adderson E E, Shackelford P G, Insel R A, Quinn A, Wilson P M, Carroll W L. Immunoglobulin light chain variable region gene sequences for human antibodies to Haemophilus influenzae type b capsular polysaccharide are dominated by a limited number of V kappa and V lambda segments and VJ combinations. J Clin Investig. 1992;89:729–738. doi: 10.1172/JCI115649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker P J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect Immun. 1990;58:3465–3468. doi: 10.1128/iai.58.11.3465-3468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett D J, Lee C G, Ammann A J, Ayoub E M. IgG and IgM pneumococcal polysaccharide antibody responses in infants. Pediatr Res. 1984;18:1067–1071. doi: 10.1203/00006450-198411000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Berberian L, Goodglick L, Kipps T J, Braun J. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science. 1993;261:1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- 9.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briles D E, Forman C, Crain M. Mouse antibody to phosphorylcholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:1957–1962. doi: 10.1128/iai.60.5.1957-1962.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown E J, Joiner K A, Cole R M, Berger M. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect Immun. 1983;39:403–409. doi: 10.1128/iai.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett S J, Tomlinson I M, Sonnhammer E L L, Buck D, Winter G. Sequence of the human immunoglobulin diversity (D) segment locus: a systematic analysis provides no evidence for the use of DIR segments, inverted D segments, “minor” D segments or D-D recombination. J Mol Biol. 1997;270:587–597. doi: 10.1006/jmbi.1997.1141. [DOI] [PubMed] [Google Scholar]
- 13.Cox J P, Tomlinson I M, Winter G. A directory of human germ-line Vk segments reveals a strong bias is their usage. Eur J Immunol. 1994;24:827–836. doi: 10.1002/eji.1830240409. [DOI] [PubMed] [Google Scholar]
- 14.Dorner T, Brezinschek H P, Brezinschek R I, Foster S J, Domiati-Saad R, Lipsky P E. Analysis of the frequency and pattern of somatic mutations within nonproductively rearranged human variable heavy chain genes. J Immunol. 1997;158:2779–2789. [PubMed] [Google Scholar]
- 15.Douglas R M, Paton J C, Duncan S J, Hansman D J. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 16.Feeney A J, Atkinson M J, Cowan M J, Escuro G, Lugo G. A defective VK A2 allele is Navajos which may play a role in increased susceptibility to Haemophilus influenzae type b disease. J Clin Investig. 1996;97:2277–2282. doi: 10.1172/JCI118669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsgren A, Quie P G. Opsonic activity in human serum chelated with ethylene glycoltetra-acetic acid. Immunology. 1974;26:1251–1256. [PMC free article] [PubMed] [Google Scholar]
- 18.Garg M, Subbarao B. Immune responses of systemic and mucosal lymphoid organs to Pnu-Imune vaccine as a function of age and the efficacy of monophosphoryl lipid A as an adjuvant. Infect Immun. 1992;60:2329–2336. doi: 10.1128/iai.60.6.2329-2336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green L L, Hardy M C, Maynard-Currie C E, Tsuda H, Louie D M, Mendez M J, Abderrahim H, Noguchi M, Smith D H, Zeng Y, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Gen. 1994;7:13–21. doi: 10.1038/ng0594-13. [DOI] [PubMed] [Google Scholar]
- 20.Green L L, Jakobovits A. Regulation of B cell development by variable gene complexity in mice reconstituted with human immunoglobulin yeast artificial chromosomes. J Exp Med. 1998;188:483–495. doi: 10.1084/jem.188.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidicelli V, Chaume D, Bodmer J, Muller W, Busin C, Mash S, Bontrop R, Marc L, Malik A, LeFranc M P. The International Immunogenetics database. Nucleic Acids Res. 1997;25:206–211. doi: 10.1093/nar/25.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakobovits A. Production of fully human antibodies by transgenic mice. Curr Opin Biotechnol. 1995;6:561–566. doi: 10.1016/0958-1669(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 23.Janoff E N, Breiman R F, Daley C L, Hopewell P C. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–324. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- 24.Kirkham P M, Mortari R F, Newton J A, Schroeder H W. Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 1992;11:603–609. doi: 10.1002/j.1460-2075.1992.tb05092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowal C, Weinstein A, Diamond B. Molecular mimicry between bacterial and self antigen in a patient with systemic lupus erythematosus. Eur J Immunol. 1999;29:1901–1911. doi: 10.1002/(SICI)1521-4141(199906)29:06<1901::AID-IMMU1901>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Lees A, Nelson B L, Mond J J. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine. 1996;14:190–198. doi: 10.1016/0264-410x(95)00195-7. [DOI] [PubMed] [Google Scholar]
- 27.Lucas A H, Granoff D M, Mandrell R E, Connolly C C, Shah A S, Powers D C. Oligoclonality of serum immunoglobulin G antibody responses to Streptococcus pneumoniae capsular polysaccharide serotypes 6B, 14, and 23F. Infect Immun. 1997;65:5103–5109. doi: 10.1128/iai.65.12.5103-5109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas A H, Larrick J W, Reason D C. Variable region sequences of a protective human monoclonal antibody specific for the Haemophilus influenzae type b capsular polysaccharide. Infect Immun. 1994;62:3873–3880. doi: 10.1128/iai.62.9.3873-3880.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez M J, Green L L, Corvalan J R, Jia X C, Maynard-Currie C E, Yang X D, Gallo M L, Louie D M, Lee D V, Erickson K L, Luna J, Roy C M, Abderrahim H, Kirschenbaum F, Noguchi M, Smith D H, Fukushima A, Hales J F, Finer M H, Davis C G, Zsebo K M, Jakobovits A. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat Gen. 1997;15:146–156. doi: 10.1038/ng0297-146. [DOI] [PubMed] [Google Scholar]
- 30.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 31.Oshsenbein A F, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel R M. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 32.Park M K, Sun Y, Olander J V, Hoffmann J W, Nahm M H. The repertoire of human antibodies to the carbohydrate capsule of Streptococcus pneumoniae 6B. J Infect Dis. 1996;174:75–82. doi: 10.1093/infdis/174.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Pirofski L, Casadevall A. The use of licensed vaccines for active immunization of the immunocompromised host. Clin Microbiol Rev. 1998;11:1–26. doi: 10.1128/cmr.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pirofski L, Lui R, DeShaw M, Kressel A B, Zhong Z. Analysis of human monoclonal antibodies elicited by vaccination with a Cryptococcus neoformans glucuronoxylomannan capsular polysaccharide vaccine. Infect Immun. 1995;63:3005–3014. doi: 10.1128/iai.63.8.3005-3014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramisse F, Binder P, Szatanik M, Alonso J-M. Passive and active immunotherapy for experimental pneumococcal pneumonia by polyvalent human immunoglobulin or F(ab′)2 fragments administered intranasally. J Infect Dis. 1996;173:1123–1128. doi: 10.1093/infdis/173.5.1123. [DOI] [PubMed] [Google Scholar]
- 36.Robbins J B, Schneerson R, Glode M P, Vann W, Schiffer M, Liu T Y, Parke J C, Huntley C. Cross-reactive antigens and immunity to diseases caused by encapsulated bacteria. J Allergy Clin Immunol. 1975;56:141–151. doi: 10.1016/0091-6749(75)90119-0. [DOI] [PubMed] [Google Scholar]
- 37.Rock E P, Sibbald P R, Davis M M, Chien Y H. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott M G, Crimmins D L, McCourt D W, Chung G, Schable K F, Thiebe R, Quenzel E M, Zachau H G, Nahm M H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. IV. The less frequently expressed VL are heterogeneous. J Immunol. 1991;147:4007–4013. [PubMed] [Google Scholar]
- 39.Shaw D R, Kirkham P, Schroeder H W, Jr, Roben P, Silverman G J. Structure-function studies of human monoclonal antibodies to pneumococcus type 3 polysaccharide. Ann N Y Acad Sci. 1995;764:370–373. doi: 10.1111/j.1749-6632.1995.tb55849.x. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Park M K, Kim J, Nahm M H, Solomon A. Repertoire of human antibodies against the polysaccharide capsule of Streptococcus pneumoniae serotype 6B. Infect Immun. 1999;67:1172–1179. doi: 10.1128/iai.67.3.1172-1179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki N, Harada T, Mihara S, Sakane T. Characterization of a germline VK gene encoding cationic anti-DNA antibody and role of receptor editing for development of autoantibody in patients with systemic lupus erythematosus. J Clin Investig. 1996;98:1843–1850. doi: 10.1172/JCI118985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valadon P, Nussbaum G, Boyd L F, Margulies D H, Scharff M D. Peptide libraries define the fine specificity of antipolysaccharide antibodies to Cryptococcus neoformans. J Mol Biol. 1996;261:11–22. doi: 10.1006/jmbi.1996.0438. [DOI] [PubMed] [Google Scholar]
- 43.van Dam J E G, Fleer A, Snippe H. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Leeuwenhoek. 1990;58:1–47. doi: 10.1007/BF02388078. [DOI] [PubMed] [Google Scholar]
- 44.Vargas-Madrazo E, Lara-Ochoa F, Almagro J C. Canonical structure repertoire of the antigen-binding site of immunoglobulins suggests strong geometrical restrictions associated to the mechanism of immune recognition. J Mol Biol. 1995;254:497–504. doi: 10.1006/jmbi.1995.0633. [DOI] [PubMed] [Google Scholar]
- 45.Westerink M A, Giardina P C, Apicella M A, Kieber-Emmons T. Peptide mimicry of the meningococcal group C capsular polysaccharide. Proc Natl Acad Sci USA. 1995;92:4021–4025. doi: 10.1073/pnas.92.9.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong Z, Burns T, Chang Q, Carroll M, Pirofski L. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect Immun. 1999;67:4119–4127. doi: 10.1128/iai.67.8.4119-4127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong Z, Pirofski L. Antifungal activity of a human antiglucuronoxylomannan antibody. Clin Diagn Lab Immunol. 1998;5:58–64. doi: 10.1128/cdli.5.1.58-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]