Abstract
Introduction:
The present in vitro study evaluated the cytotoxicity and bioactivity of commonly-used calcium silicate-based cements in a culture of stem cells from the apical papilla (SCAPs).
Materials and Methods:
NeoMTA Plus (Avalon Biomed), BiodentineTM (Septodont) and MTA HP Repair (Angelus) cements were evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and sulphorhodamine-B (SRB) viability assays. Cells were seeded (1*104 cells mL-1) in 96-well plates and exposed to 1:4 diluted extract in 24 h and 72 h. For the analysis of bioactivity, alkaline phosphatase (ALP) enzyme activity and Alizarin Red S (AZR) were assessed after 24 h of cell culture in 12-well plates (1*104 cells mL-1), where cells were exposed to 1:4 diluted extract on days 1 and 7. Minimum Essential Eagle’s Medium alpha modification was used as control. ANOVA and Tukey’s post hoc test were used to compare the different cements at each experimental time point.
Results:
No significant differences were found between the cements and the control specimens on MTT at 24 h and 72 h (P>0.05); however, the calcium silicate-based cement materials showed higher cell viability compared to the control group (P<0.05). In the 24-h SRB, NeoMTA Plus showed lower cell viability than BiodentineTM and MTA HP Repair (P<0.05), with all groups similar to the control group (P>0.05). Compared to 24-h results, only NeoMTA Plus presented increased cell viability at 72 h (P<0.05). ALP activity was similar across the materials at 1 day (P>0.05). ALP activity was higher for BiodentineTM when compared to NeoMTA Plus (P<0.05), nevertheless, it was similar to MTA HP Repair and control groups (P>0.05) at 7 days. At 1- and 7-day periods of AZR assay, BiodentineTM presented higher levels of mineralized nodule formation (P<0.05).
Conclusion:
All evaluated calcium silicate-based cements demonstrated cell viability and bioactivity, suggesting that these (bio)materials may be indicated for use in regenerative dentine-pulp complex procedures.
Key Words: Bioactivity, Biomaterials, Calcium Silicate, Cytotoxicity, Endodontics
Introduction
Bioceramic materials have become consolidated as the (bio)materials of choice for the treatment of immature necrotic teeth, perforation repair and root-end filling; as they are able to stimulate the osteogenic process through their bioactive potential [1, 2]. These materials react with physiological solutions to produce calcium silicate hydrate as well as calcium hydroxide, and form a layer of hydroxyapatite on the cement surface [3].
To improve the physicochemical and biological properties of calcium silicate-based cements, and especially to minimize the disadvantages of mineral trioxide aggregate (MTA) [4], different compounds have been combined and new materials have been proposed. BiodentineTM (Septodont, Saint-Maur-de-Fosses, France) has been reported to present superior physicochemical and biological characteristics, due to its modified composition, e.g., smaller particle size, use of zirconium oxide as radiopacifier, purity of tricalcium silicate, absence of dicalcium silicate, and the addition of calcium chloride/a water-soluble polymer, when compared to MTA [5-9]. MTA Repair HP (Angelus Industria de Produtos Odontológicos S/A, Londrina, PR, Brazil), differently from its predecessor MTA White (Angelus Industria de Produtos Odontológicos S/A, Londrina, PR, Brazil), uses calcium tungstate rather than bismuth oxide as radiopacifier. Furthermore, the addition of a polymer to the liquid component has improved material handling. Additionally, MTA Repair HP has demonstrated extended alkalinizing activity and calcium release, favouring calcium phosphate nucleation [10]. NeoMTA Plus (Avalon Biomed Inc., Bradenton, FL, USA) includes tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium sulphate and tantalum oxide instead of zirconium oxide as radiopacifier, causing no discolouration [11]. However, it consists of a water-based gel with thickening agents and polymers in its liquid. Seemingly, NeoMTA Plus has been shown to have high bioactivity and low cytotoxicity on human dental pulp stem cells (DPSCs) as well as osteoblast-like cells (SAOS-2) [6, 12-14].
Studies have been developed to elucidate the cytotoxic and bioactive potentials of (bio) materials on tissues; and the first tests recommended are those involving cell cultures [2]. This in vitro tests are the first step to evaluate and classify the bioactive potential of the materials before using them in vivo. These tests are reproducible, relevant and suitable for the assessment of basic biological properties.
Several cell types reside in the periodontium around a perforated root, root-end or immature permanent tooth, including cementoblasts, endothelial cells, fibroblasts, osteoblasts and stem cells [15]. Dental papilla at the apex contains stem cells (stem cells from apical papilla, “SCAPs”) that have been described to be more robust stem cells than DPSCs [16, 17]. SCAPs may survive infection, allow root maturation and possibly odontoblast growth. The use of mesenchymal stem cells (e.g. SCAPs) in regenerative procedures to treat immature necrotic teeth has shown high success rates [18]. Therefore, SCAPs have been identified as the most important cells for the continued root development process due to the survival and maintenance of their stemness [16-18]. Regenerative treatment relies on the survival of stem cells from Hertwig’s epithelial root sheath and SCAPs, along with growth factors released from dentinal walls, for continued root development and bone regeneration [19].
Recently, a systematic review observed positive cytocompatibility and bioactivity results for BiodentineTM, MTA and iRoot BP Plus when cultured with stem cells from human exfoliated deciduous teeth (SHEDs)[20]. In a study using human DPSCs, MTA-HP, ACTIVA and iRoot-BP-Plus have promoted proliferation, mineralization and attachment [21]. Moreover, the biological effects of Bio-C Repair, BiodentineTM and ProRoot MTA on human DPSCs were evaluated, with excellent results [4]. In another investigation, NeoMTA Plus has been compared with MTA-Angelus and MTA Repair HP; with all three materials showing biological effects on human DPSCs in terms of cell proliferation, morphology, migration and attachment [6]. Additionally, NeoMTA Plus have been evaluated and compared with BiodentineTM and MTA HP Repair on human DPSCs, with good responses [14].
Since calcium silicate-based materials should present cytocompatibility and bioactivity, the objective of the current study was to assess the biological response(s) of SCAPs to three different commonly-used calcium silicate-based cements; NeoMTA Plus, BiodentineTM and MTA Repair HP.
Materials and Methods
This study was approved by the research ethics committee of the Federal University, Rio Grande do Sul (protocol no. 38542614.6.0000.5347). All experiments were performed considering the guidelines of ISO 10993-5 directive [22].
SCAPs were collected from patients through the apical opening of third molars with incomplete root formation and stored in a culture plate (35 mm*10 mm; TPP-Techno Plastic Products, Zollstrasse, Trasadingen, Switzerland) containing minimum essential eagle’s medium alpha modification (α-MEM) (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% of fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), and 1% of penicillin and streptomycin (PenStrep; Gibco). Tissues were sectioned in fragments of approximately 1 mm*3 mm and maintained in an incubator at 37°C, 100% humidity and 5% CO2. The culture medium was changed 24 h after the collection and then, every 48 h to obtain the explants of SCAPs. When the cells reached 80% of confluence, they were washed with phosphate buffer solution (PBS) 1X (Sigma-Aldrich, St Louis, MO, USA) and separated using 0.25% trypsin-EDTA solution (Sigma-Aldrich, St Louis, MO, USA), re-suspended in the culture medium and transferred to a Falcon tube (TPP-Techno Plastic Products), forming a suspension of cells. To confirm the potential of mesenchymal cells, SCAPs were subjected to flow cytometric analysis using specific molecular markers. Cells were incubated at 4 °C during 30 min with the following antibodies: STRO1, CD146, CD45, CD14 and CD105, diluted to 1:10. Subsequently, SCAPs were washed with PBS, fixed with a mixed solution (1% paraformaldehyde, 0.1% sodium azide, and 0.5% FBS) and subjected to analysis.
Successive passages were performed and the experiments were conducted at the fourth cell passage. After trypsinization, for viability and bioactivity assays, cells were collected and centrifugated at 800 rpm for 5 min, and pellets were dispersed in the culture medium. Cell suspension was counted under a microscope with a Neubauer counting chamber and cells (1*104) were seeded with culture medium in an oven at 37° C, 95% humidity and 5% CO2 until confluence was established.
Material preparation
NeoMTA Plus (Avalon Biomed, Bradenton, FL, USA), BiodentineTM (Septodont, Saint-Maur-des-Fosses, France) and MTA Repair HP (Angelus, Londrina, Brazil) were handled on sterilized glass slides (Table 1). Following preparation, the cements were placed in two 24-well plates. The surface-to-volume ratio used during preparation of the extract was approximately 250 mm2/mL.
Table 1.
Materials, composition and proportions employed
| Material | Composition | Proportion |
|---|---|---|
| NeoMTA Plus | Powder: Tricalcium silicate, dicalcium silicate, tantalum oxide, tricalcium aluminate, and calcium sulfate Liquid: Water-based gel with thickening agents and water-soluble polymers |
1 g/10 drops |
| Biodentine TM | Powder: Tricalcium silicate, calcium carbonate, and zirconium oxide Liquid: Carboxylate-, calcium chloride-, water-based water-soluble polymer |
Single-dose capsule, mixed in an amalgamator for 30 seconds |
| MTA HP Repair | Powder: Tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium oxide, calcium tungstate Liquid: water and plasticizer |
0.8 g/320 μL |
The plates were immediately filled with α-MEM culture medium supplemented with 1% PenStrep and maintained for 24 h in an incubator at 37°C with 95% humidity and 5% CO2, so as to obtain the extract solutions of materials. After collection, the extracts were diluted to 1:4 for different assays. Every experiment was performed in triplicate (n=9 specimens per group).
Viability assays
Viability was determined using 3-(4, 5-dimethyl-thiazolyl)- 2,5-diphenyl-tetrazolium bromide (MTT) and sulphorhodamine-B (SRB) assays. Cells were placed (1×104 cells per well) in 96-well plates, and exposed to the diluted extracts 24 h later. The α-MEM culture medium was used as control.
After 24 h and 72 h of exposure, 10 µL of the solution containing 5 mg/mL of MTT salt (Sigma-Aldrich) was added to each well and maintained in an incubator for 3 h at 37°C, 100% humidity and 5% CO2. Then, the contents of wells were removed and MTT-formazan crystals were solubilized using acidified isopropanol at 0.04 N HCl (Sigma-Aldrich). Optical density was measured using an automatic microplate reader (EL×800; Bio-Tek Instruments, Winooski, VT, USA) at 570 nm wavelength.
For the SRB assay, cells were fixed with 100 µL trichloroacetic acid 10% (Sigma-Aldrich) and stained with 100 µL of 0.4% SRB dye (Sigma-Aldrich) for 1 h. The resulting colorimetric product was dissolved in 100 µL of Trizma-base solution 10 mmol/L (Sigma-Aldrich). Optical density was measured at 560 nm using an automatic microplate reader (ELx800). Results were expressed in percentage of viability in relation to the control group (cells exposed to α-MEM culture medium).
Bioactivity assays
For bioactivity assays, cells exposed to α-MEM culture medium were considered as controls. The alkaline phosphatase enzyme (ALP) activity assay was used to assess the enzyme activity, by means of a commercially available kit (Labtest, Lagoa Santa, MG, Brazil). Additionally, an osteogenic culture medium was used; α-MEM supplemented with 10% FBS, 1% PenStrep, 0.05 µM L-ascorbic acid (Sigma-Aldrich) and 10 mM ß-glycerol phosphate (Sigma-Aldrich).
After 24 h of culture in 12-well plates (1×104 cells per well), cells were exposed to the extracts diluted to 1:4 for days 1 and 7, with the medium changed every 3 days. At each experimental time point, the culture medium containing the material extracts was removed from the wells and the cells were washed with 500 µL of PBS 1X. Then, 500 µL of sodium dodecyl sulfate solution 1% (SDS, Sigma-Aldrich) was added to each well. Samples were allowed to rest for 30 min at room temperature and the protocol was followed/conducted according to the manufacturer’s instructions (Labtest). Samples were transferred to a 12-well plate for the measurement of absorbance using a spectrophotometer at 590 nm wavelength (ELx800; Bio-Tek Instruments, Vermont, USA). Data were expressed as unit/liter (U/L), using to the formula below:
The alizarin red dye (AZR assay) was used to identify calcium deposits. After 24 h of culture in 12-well plates (1×104, cells per well), cells were exposed to the materials diluted to 1:4 for 1 and 7 days with the medium changed every 3 days. At each experimental time point, extracts were removed from the wells and the cells were washed with 500 µL of PBS 1X and then fixed with 500 µL of formaldehyde solution 10% (Sigma-Aldrich) at room temperature for 15 min. Subsequently, the wells were washed with 500 µL of distilled water and then, 500 µL of 2%, AZR solution with a pH of 4.2 (Sigma-Aldrich) was added to each well and stored at room temperature for 20 min. Next, the dye was aspirated and the wells were washed 4 times with distilled water (500 µL). Once dry, the plates were photographed (Canon EOS-1D; Canon Inc., Tokyo, Japan). Digital images were processed and the AZR-coloured areas were measured using the ImageJ 1.45 software (National Institutes of Health, NIH, Bethesda, MD, USA). Results were expressed in percentage values, according to the stained area in each well.
Statistical analysis
All the data were assessed for normality of distribution using the Kolmogorov-Smirnov test. Analysis of variance (ANOVA) and Tukey’s post hoc test were used to compare the different cements at each experimental time point. Analyses were performed using GraphPad Prism 5.0 software. Significance was set at 5%.
Results
Cell characterization
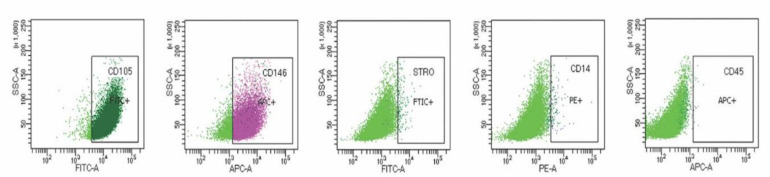
The SCAPs showed typical levels of surface markers for mesenchymal cells (Figure 1). The cultures expressed positivity for mesenchymal cell markers (CD105, CD146), whereas hematopoietic cell markers (STRO-1, CD45, CD14) were absent or minimally present. The percentages of cells with positive results were CD105 98%, CD146 86.6%, STRO-1 0.8%, CD14 0.6% and CD45 0.1%.
Figure 1.
Flow cytometric analysis of mesenchymal cell surface markers expressed in the human stem cells of apical papilla. Positive markers: CD105, CD146, STRO-1; negative markers: CD14, CD45. Fluorescent conjugated isotype control antibodies: FITC-A, APC-A, PE-A
Cell viability
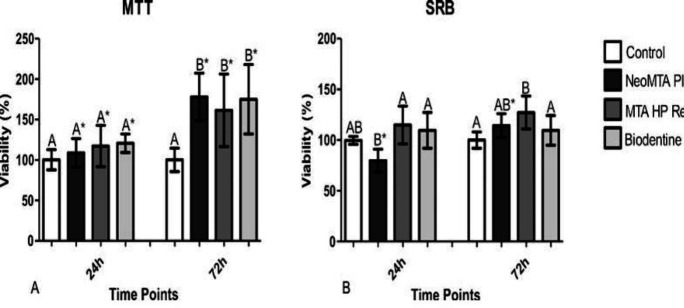
In MTT assay, no differences were observed amongst the cements and the control group (P>0.05) at 24 h. All cements showed cell viability above 70%. At 72 h, repair materials presented higher cell viability when compared to the control group (P<0.05), without statistical difference amongst the cements investigated (P>0.05) (Figure 2A).
Figure 2.
Cytocompatibility of repair materials evaluated using MTT and SRB assays Different letters indicate significant differences among repair materials at the same time point (P≤0.05; analysis of variance). Asterisks indicate significant differences obtained with the same repair material at different time points (P≤0.05; t test). [A-MTT=3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; B-SRB=sulphorhodamine-B assay]
In the 24-h SRB assay, NeoMTA Plus presented lower cell viability results than BiodentineTM and MTA Repair HP (P<0.05), and all were similar to the control group (P>0.05). At 72 h, an increase in cell viability was observed for NeoMTA Plus; however at a non-significant difference in relation to BiodentineTM and MTA Repair HP (P>0.05) (Figure 2B).
Bioactivity
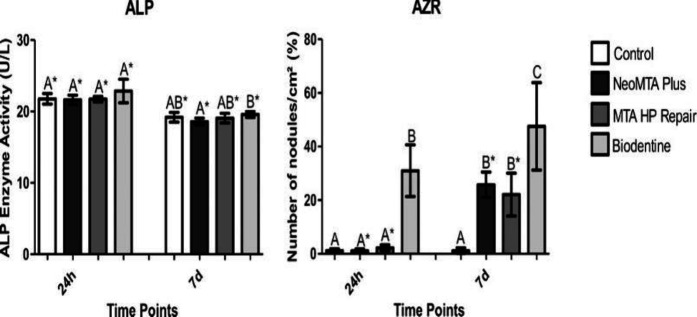
ALP enzyme activity assessment did not show statistically significant differences across the cements on day 1 (P>0.05). However, at 7 days, BiodentineTM presented higher ALP activity results compared to NeoMTA Plus (P<0.05) but similar outcomes to MTA Repair HP and the control group (P>0.05) (Figure 3A).
Figure 3.
A- ALP activity in repair materials at 1 and 7 days. B- Percentage of mineralized nodule formation assessed using AZR after exposure to diluted repair materials at different time points (1 and 7 days). Different letters indicate significant differences among repair materials at the same time point (P≤0.05; analysis of variance). Asterisks indicate significant differences obtained with the same repair material at different time points (P≤0.05; t test). (ALP=alkaline phosphatase enzyme. AZR=alizarin red)
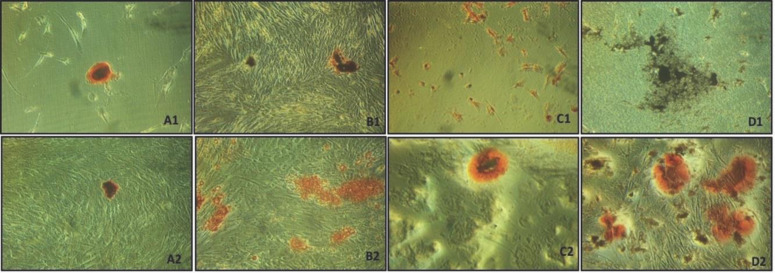
In the AZR assay, on day 1, the control, NeoMTA Plus and MTA Repair HP groups showed lower levels of mineralized nodule formation when compared with BiodentineTM (P<0.05). At 7 days, an increase in mineralised nodules was observed for NeoMTA Plus and MTA Repair HP (P<0.05). Biodentine presented more mineralized nodules when compared to the other groups at 7 days (P<0.05) (Figures 3B and 4).
Figure 4.
Photographs of AZR-coloured mineralized nodules in the SCAP after exposure to repair materials at different time points (1 and 7 days); A1) Control group, 1 day; A2) Control group, 7 days. B1) NeoMTA Plus, 1 day; B2) NeoMTA Plus, 7 days. C1) MTA HP Repair, 1 day; C2) MTA HP Repair, 7 days. D1) BiodentineTM, 1 day; D2) BiodentineTM, 7 days [AZR = alizarin red]
Discussion
When severe root canal infection occurs, cellular components within the root canal system are damaged. In these situations, calcium silicate-based cements may interact with at least three kinds of stem cells; SCAPs, periodontal ligament stem cells and alveolar bone marrow mesenchymal stem cells [16, 23]. SCAPs are the source of primary odontoblasts for the formation of root dentine. Calcium silicate-based cements can promote odonto/osteoblastic SCAP differentiation; enhancing dentinogenesis in vivo and creating a potent inductive microenvironment necessary for pulp regeneration [16, 19].
One of the components of calcium silicate-based cements with known deleterious physicochemical effects is the radiopacifier bismuth oxide, which has been replaced with alternative compounds in more recent formulations [24, 25]. NeoMTA Plus uses tantalum oxide, a compound considered to be biocompatible for not interfering with bone formation after injection into a distal femoral condyle model in rabbits along with a cement used in dental implants [26]. BiodentineTM contains zirconium oxide as radiopacifier with the main advantages of improving biological response and mechanical properties [5]. In addition, BiodentineTM has shown low cytotoxicity when tested on fibroblasts in an experimental sealer. 24 MTA Repair HP uses calcium tungstate as radiopacifier and has shown good biological results in terms of cell proliferation, morphology, migration and fixation in human DPSCs [6, 14].
When evaluating the potential toxicity of materials, different cell parameters have to be analyzed. The MTT assay mainly relies on the enzymatic conversion of tetrazolium dye into formazan crystals, which occurs in numerous organelles including the mitochondria and endoplasmic reticulum [27-29]. However, many endogenous and exogenous compounds can catalyze this dye [30]. The SRB assay measures total cellular protein content and does not rely on cell functionality [31].
Fresh cements were used to prepare the extracts aiming to simulate the clinical condition of the initial contact between the cement(s) and the tissue. All the cements investigated in the current study presented cell viability results above 70% in the two tests (MTT and SRB) and can therefore be considered cytocompatible according to ISO 10993-5 directive [22]. This suggests that the compounds present in the tested repair materials with the aim of improving their physicochemical properties did not interfere with their cytocompatibility properties in SCAPs.
In the present study, NeoMTA Plus, BiodentineTM and MTA HP Repair did not show significant differences in the MTT assay after 24 and 72 h. These results are in line with previous investigations that compared the DPSCs cell viability of MTA HP Repair, NeoMTA Plus, BiodentineTM and MTA Angelus within the same culture times [6, 14]. In addition, some studies have reported the cell viability of BiodentineTM in SCAPs [1, 32], SHEDs [5], and human DPSCs [4, 21] at 24 h.
At 72 h, viability levels were observed to be higher in all cements tested, with statistically significant differences in relation to the control group. This finding can be explained by the high release of calcium ions increasing the medium pH [12, 30]. In addition, calcium release and medium alkalization promote antibacterial action and endodontic/periodontal regeneration [12, 25]. Furthermore, calcium ion release has been shown to be adequate for the survival of osteoblasts [33]. The increase of viability levels over time in MTT and SRB assays corroborates findings from others studies that assessed the cytotoxicity of calcium silicate-based material using different culture times, from 1 to 7 days [1, 4-6, 14, 21].
The ALP enzyme is a marker of osteoblast maturation [34]. The cements evaluated showed similar ALP activity when compared with the control group after 1 day. Despite the decrease in ALP activity over time, at 7 days, BiodentineTM showed higher enzyme expression than NeoMTA Plus, indicating that BiodentineTM has a higher potential of mineralization. The ALP enzyme releases free phosphate ions that react with calcium ions, forming precipitated calcium phosphates, which is the molecular unit of hydroxyapatite [34]. Therefore, when tricalcium silicate-based cements come into contact with tissue fluids, calcium hydroxide can be formed, which is an important component of mineralized tissue [35]. In AZR assay, the materials evaluated showed the formation of mineralized nodules in both periods assessed, in line with previous reports [21, 36].
Corroborating the greater potential of mineralization demonstrated in the ALP activity assay, BiodentineTM presented a higher number of nodules when compared to MTA Repair HP and NeoMTA Plus. The same correlation was previously observed between ALP activity and the number of AZR-stained nodules in a study assessing the bioactivity of BiodentineTM and MTA when in contact with osteogenic cells [36]. According to the results presented, all tested (bio)materials demonstrated adequate biological behavior; nevertheless, BiodentineTM presented greater bioactivity in both assays suggesting that this material may achieve superior hard tissue formation in a clinical situation when compared to other tested cements. However, it should be noted that in vitro studies are only the first step to evaluate biological properties of (bio)materials, and that they should be complemented by in vivo studies, to confirm/improve the knowledge first obtained.
Conclusions
Despite the limitations of the methods used in this study, MTA Repair HP, BiodentineTM, and NeoMTA Plus demonstrated bioactivity and to maintain cell viability. BiodentineTM presented greater bioactivity in both performed assays suggesting that BiodentineTM may be indicated for use in regenerative dentine-pulp complex procedures. More studies are nevertheless needed to confirm the suitability of these materials.
Acknowledgements
This study was supported by the Center for Basic Research in Dentistry (Núcleo de Pesquisa Básica em Odontologia, NPBO), Federal University of Rio Grande do Sul (UFRGS) and Brazilian Lutheran University (ULBRA). The funding sources did not have any role in (i) the study design, (ii) the collection, analysis or interpretation of data, (iii) the writing of the report or (iv) the decision to submit the article for publication.
Conflict of Interest:
‘None declared’.
References
- 1.Saberi E, Farhad-Mollashahi N, Sargolzaei Aval F, Saberi M. Proliferation, odontogenic/osteogenic differentiation, and cytokine production by human stem cells of the apical papilla induced by biomaterials: a comparative study. Clin Cosmet Investig Dent. 2019;11:181–93. doi: 10.2147/CCIDE.S211893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa F, Sousa Gomes P, Fernandes MH. Osteogenic and Angiogenic Response to Calcium Silicate-based Endodontic Sealers. J Endod. 2016;42(1):113–9. doi: 10.1016/j.joen.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. 2013;29(5):580–93. doi: 10.1016/j.dental.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Ghilotti J, Sanz JL, López-García S, Guerrero-Gironés J, Pecci-Lloret MP, Lozano A, Llena C, Rodríguez-Lozano FJ, Forner L, Spagnuolo G. Comparative Surface Morphology, Chemical Composition, and Cytocompatibility of Bio-C Repair, Biodentine, and ProRoot MTA on hDPCs. Materials (Basel) 2020;13 doi: 10.3390/ma13092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araújo LB, Cosme-Silva L, Fernandes AP, Oliveira TM, Cavalcanti BDN, Gomes Filho JE, Sakai VT. Effects of mineral trioxide aggregate, BiodentineTM and calcium hydroxide on viability, proliferation, migration and differentiation of stem cells from human exfoliated deciduous teeth. J Appl Oral Sci. 2018;26:e20160629. doi: 10.1590/1678-7757-2016-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomás-Catalá CJ, Collado-González M, García-Bernal D, Oñate-Sánchez RE, Forner L, Llena C, Lozano A, Castelo-Baz P, Moraleda JM, Rodríguez-Lozano FJ. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int Endod J. 2017;50 Suppl 2:e63–e72. doi: 10.1111/iej.12859. [DOI] [PubMed] [Google Scholar]
- 7.Olcay K, Taşli PN, Güven EP, Ülker GMY, Öğüt EE, Çiftçioğlu E, Kiratli B, Şahin F. Effect of a novel bioceramic root canal sealer on the angiogenesis-enhancing potential of assorted human odontogenic stem cells compared with principal tricalcium silicate-based cements. J Appl Oral Sci. 2020;28:e20190215. doi: 10.1590/1678-7757-2019-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AA, Takimoto K, Wealleans J, Diogenes A. Effect of 3 Bioceramic Materials on Stem Cells of the Apical Papilla Proliferation and Differentiation Using a Dentin Disk Model. J Endod. 2018;44(4):599–603. doi: 10.1016/j.joen.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Sultana N, Singh M, Nawal RR, Chaudhry S, Yadav S, Mohanty S, Talwar S. Evaluation of Biocompatibility and Osteogenic Potential of Tricalcium Silicate-based Cements Using Human Bone Marrow-derived Mesenchymal Stem Cells. J Endod. 2018;44(3):446–51. doi: 10.1016/j.joen.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Guimarães BM, Prati C, Duarte MAH, Bramante CM, Gandolfi MG. Physicochemical properties of calcium silicate-based formulations MTA Repair HP and MTA Vitalcem. J Appl Oral Sci. 2018;26:e2017115. doi: 10.1590/1678-7757-2017-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagogeni I, Metlerska J, Lipski M, Falgowski T, Maciej G, Nowicka A. Materials used in regenerative endodontic procedures and their impact on tooth discoloration. J Oral Sci. 2019;61(3):379–85. doi: 10.2334/josnusd.18-0467. [DOI] [PubMed] [Google Scholar]
- 12.Siboni F, Taddei P, Prati C, Gandolfi MG. Properties of NeoMTA Plus and MTA Plus cements for endodontics. Int Endod J. 2017;50 Suppl 2:e83–e94. doi: 10.1111/iej.12787. [DOI] [PubMed] [Google Scholar]
- 13.Zordan-Bronzel CL, Tanomaru-Filho M, Rodrigues EM, Chávez-Andrade GM, Faria G, Guerreiro-Tanomaru JM. Cytocompatibility, bioactive potential and antimicrobial activity of an experimental calcium silicate-based endodontic sealer. Int Endod J. 2019;52(7):979–86. doi: 10.1111/iej.13086. [DOI] [PubMed] [Google Scholar]
- 14.Tomás-Catalá CJ, Collado-González M, García-Bernal D, Oñate-Sánchez RE, Forner L, Llena C, Lozano A, Moraleda JM, Rodríguez-Lozano FJ. Biocompatibility of New Pulp-capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J Endod. 2018;44(1):126–32. doi: 10.1016/j.joen.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Lin NH, Gronthos S, Bartold PM. Stem cells and periodontal regeneration. Aust Dent J. 2008;53(2):108–21. doi: 10.1111/j.1834-7819.2008.00019.x. [DOI] [PubMed] [Google Scholar]
- 16.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34(2):166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1(1) doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XM, Liu Y, Yu S, Jiang LM, Song B, Chen X. Potential immunomodulatory effects of stem cells from the apical papilla on Treg conversion in tissue regeneration for regenerative endodontic treatment. Int Endod J. 2019;52(12):1758–67. doi: 10.1111/iej.13197. [DOI] [PubMed] [Google Scholar]
- 19.Hajizadeh N, Madani ZS, Zabihi E, Golpour M, Zahedpasha A, Mohammadnia M. Effect of MTA and CEM on Mineralization-Associated Gene Expression in Stem Cells Derived from Apical Papilla. Iran Endod J. 2018;13(1):94–101. doi: 10.22037/iej.v13i1.17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanz JL, Forner L, Llena C, Guerrero-Gironés J, Melo M, Rengo S, Spagnuolo G, Rodríguez-Lozano FJ. Cytocompatibility and Bioactive Properties of Hydraulic Calcium Silicate-Based Cements (HCSCs) on Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs): A Systematic Review of In Vitro Studies. J Clin Med. 2020;9:12. doi: 10.3390/jcm9123872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abou ElReash A, Hamama H, Grawish M, Saeed M, Zaen El-Din AM, Shahin MA, Zhenhuan W, Xiaoli X. A laboratory study to test the responses of human dental pulp stem cells to extracts from three dental pulp capping biomaterials. Int Endod J. 2021 doi: 10.1111/iej.13495. [DOI] [PubMed] [Google Scholar]
- 22.Tests for in vitro cytotoxicity EI. European Standard: Biological evaluation of medical devices-Part 5. 2009. pp. 10993–5. [Google Scholar]
- 23.Jung C, Kim S, Sun T, Cho YB, Song M. Pulp-dentin regeneration: current approaches and challenges. J Tissue Eng. 2019;10:2041731418819263. doi: 10.1177/2041731418819263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slompo C, Peres-Buzalaf C, Gasque KC, Damante CA, Ordinola-Zapata R, Duarte MA, de Oliveira RC. Experimental Calcium Silicate-Based Cement with and without Zirconium Oxide Modulates Fibroblasts Viability. Braz Dent J. 2015;26(6):587–91. doi: 10.1590/0103-6440201300316. [DOI] [PubMed] [Google Scholar]
- 25.Huck C, Barud HD, Basso FG, Costa CA, Hebling J, Garcia LD. Cytotoxicity of New Calcium Aluminate Cement (EndoBinder) Containing Different Radiopacifiers. Braz Dent J. 2017;28(1):57–64. doi: 10.1590/0103-6440201701023. [DOI] [PubMed] [Google Scholar]
- 26.Hoekstra JW, van den Beucken JJ, Leeuwenburgh SC, Bronkhorst EM, Meijer GJ, Jansen JA. Tantalum oxide and barium sulfate as radiopacifiers in injectable calcium phosphate-poly(lactic-co-glycolic acid) cements for monitoring in vivo degradation. J Biomed Mater Res A. 2014;102(1):141–9. doi: 10.1002/jbm.a.34677. [DOI] [PubMed] [Google Scholar]
- 27.Farhad Mollashahi N, Saberi E, Karkehabadi H. Evaluation of Cytotoxic Effects of Various Endodontic Irrigation Solutions on the Survival of Stem Cell of Human Apical Papilla. Iran Endod J. 2016;11(4):293–7. doi: 10.22037/iej.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinheiro LS, Iglesias JE, Boijink D, Mestieri LB, Poli Kopper PM, Figueiredo JAP, Grecca FS. Cell Viability and Tissue Reaction of NeoMTA Plus: An In Vitro and In Vivo Study. J Endod. 2018;44(7):1140–5. doi: 10.1016/j.joen.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Pintor AVB, Queiroz LD, Barcelos R, Primo LSG, Maia LC, Alves GG. MTT versus other cell viability assays to evaluate the biocompatibility of root canal filling materials: a systematic review. Int Endod J. 2020;53(10):1348–73. doi: 10.1111/iej.13353. [DOI] [PubMed] [Google Scholar]
- 30.van Tonder A, Joubert AM, Cromarty AD. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes. 2015;8 doi: 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva FSG, Starostina IG, Ivanova VV, Rizvanov AA, Oliveira PJ, Pereira SP. Determination of Metabolic Viability and Cell Mass Using a Tandem Resazurin/Sulforhodamine B Assay. Curr Protoc Toxicol. 2016;68:2.24.1–2. doi: 10.1002/cptx.1. [DOI] [PubMed] [Google Scholar]
- 32.Saberi EA, Karkehabadi H, Mollashahi NF. Cytotoxicity of Various Endodontic Materials on Stem Cells of Human Apical Papilla. Iran Endod J. 2016;11(1):17–22. doi: 10.7508/iej.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, Toyama Y, Taguchi T, Tanaka J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials. 2005;26(23):4847–55. doi: 10.1016/j.biomaterials.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Kuru L, Griffiths GS, Petrie A, Olsen I. Alkaline phosphatase activity is upregulated in regenerating human periodontal cells. J Periodontal Res. 1999;34(2):123–7. doi: 10.1111/j.1600-0765.1999.tb02231.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen I, Karabucak B, Wang C, Wang HG, Koyama E, Kohli MR, Nah HD, Kim S. Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J Endod. 2015;41(3):389–99. doi: 10.1016/j.joen.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues EM, Gomes-Cornélio AL, Soares-Costa A, Salles LP, Velayutham M, Rossa-Junior C, Guerreiro-Tanomaru JM, Tanomaru-Filho M. An assessment of the overexpression of BMP-2 in transfected human osteoblast cells stimulated by mineral trioxide aggregate and Biodentine. Int Endod J. 2017;50 Suppl 2:e9–e18. doi: 10.1111/iej.12745. [DOI] [PubMed] [Google Scholar]