Abstract
The cytokine response to the agent of human granulocytic ehrlichiosis (HGE) was assessed in a murine infection model and the role of gamma interferon (IFN-γ), a cytokine that is crucial for host defenses against intracellular pathogens, was investigated by using IFN-γ-deficient mice. The agent of HGE (aoHGE) is an obligate intracellular bacterium that survives within neutrophils: morulae (vacuoles containing HGE organisms) are evident in polymorphonuclear leukocytes of experimentally infected immunocompetent mice for 1 to 2 weeks. We now show that IFN-γ levels increase during early infection of C3H/HeN or C57BL/6 mice with HGE bacteria. Moreover, in response to aoHGE extracts or concanavalin A, splenocytes from ehrlichia-infected mice produced more IFN-γ and less interleukin-4 than controls, suggesting that aoHGE partially skewed the immune response towards a Th1 phenotype. Absolute concentration of morulae containing neutrophils in blood was 122 ± 22 cells/μl on day 8. The bacterial DNA burden was also highest on day 8 and then declined after IFN-γ levels peaked. In contrast, IFN-γ-deficient mice had a markedly elevated HGE bacteria burden with morulae concentration of 282 ± 48 cells/μl on day 5 (P = 0.004) and 242 ± 63 cells/μl on day 8 (P = 0.005). Rickettsemia resolved in immunocompetent and IFN-γ deficient mice after 2 weeks, while both the immunocompetent and the IFN-γ-deficient mice had increased serum antibodies against aoHGE antigens at this time point. These data demonstrate that the HGE agent elicits a prominent IFN-γ response in mice and that IFN-γ is important in controlling the degree of rickettsemia during the early phase of infection, while IFN-γ independent mechanisms play a role at later time points.
Human granulocytic ehrlichiosis (HGE) is a tick-borne disease caused by an obligate intracellular gram-negative organism that persists in granulocytes (14). HGE has recently been described in the United States and Europe and is found in areas where Borrelia burgdorferi and Babesia microti, pathogens transmitted by Ixodes scapularis or Ixodes ricinus ticks, are prevalent (3, 8, 9, 15, 16, 30, 31, 33, 34, 43). Fever, malaise, headache, myalgia, and leukopenia are common during infection. The illness is usually mild and limited, but severe symptoms, such as carditis and demyelinating polyneuropathy occasionally occur, and fatalities have been documented (6, 7, 19, 24). Immunocompromised patients may be at increased risk of developing disease (2, 4). Most patients develop early humoral responses toward products of the HGE-44 gene family and other antigens during infection (3, 5, 23).
Laboratory mice can be infected with the agent of HGE (aoHGE) and serve as a model for studying granulocytic ehrlichiosis (20, 28, 40). aoHGE-infected C3H mice developed a transient bloodstream infection, thereby somewhat resembling human disease (20, 40). In contrast, aoHGE infection within polymorphonuclear leukocytes (PMNs) persisted in SCID mice (20). The kinetics of rickettsemia vary somewhat among the studies: the peak rickettsemia generally occurs between days 6 to 10, and the bacteremia can persist up to day 39 (20, 42). Differences in the onset and the duration of rickettsemia may be due to variations in the mode of inoculation (tick versus syringe challenge), the inoculum size, and the virulence of the aoHGE isolate used (20, 40, 42). Immunocompetent mice also developed antibodies to several aoHGE antigens, including HGE-44, providing another similarity with human infection (20, 40). In addition, C3H mice actively immunized with aoHGE lysates or passively immunized with anti-aoHGE sera were protected against aoHGE challenge (40). The protective anti-aoHGE sera contained high-titer antibodies to the HGE-44 protein, and a monoclonal antibody to a member of the HGE-44 family has also been shown to afford partial protection to infection, suggesting that this antigen is a target for host defenses (28, 40). These results demonstrated that mice can be used to study granulocytic ehrlichiosis and that the humoral response to aoHGE was important in immunity to infection (40).
Cellular immune responses have a prominent role in the control and outcome of many infections, particularly those caused by intracellular pathogens. The HGE agent is unusual, however, because this bacterium persists within neutrophils—a cell that only lives for several days. The nature of the T-cell response and the role of cytokines in the evolution of aoHGE infection are not known. T-helper (Th) 1 responses are commonly associated with gamma interferon (IFN-γ) and interleukin 12 (IL-12), while IL-4, IL-5, and IL-10 are related to Th2 responses (1). In general, Th1 responses are important in the eradication of intracellular organisms, while Th2 responses facilitate the clearance of extracellular pathogens. This paradigm is not without exception, and the progression and resolution of infection is often complex and multifactorial (12, 18, 22, 37). We therefore now investigated the role of the cytokine response to aoHGE during granulocytic ehrlichiosis in mice.
MATERIALS AND METHODS
Bacterial strains and maintenance of aoHGE infection.
The NCH-1 isolate of aoHGE, which was recovered from a patient with granulocytic ehrlichiosis and has been shown to be infectious in mice, was used throughout these studies (41). aoHGE was initially cultured in HL-60 cells (240-CCL; American Type Culture Collection, Manassas, Va.) grown in Iscove's modified Dulbecco (Gibco BRL/Life Technologies, Grand Island, N.Y.) medium supplemented with 20% fetal calf serum at 37°C with 5% CO2 as described earlier (23). Inbred SCID mice were used to further cultivate aoHGE for subsequent inoculation into inbred immunocompetent C3H/HeN (C3H) or C57BL/6 (B6) mice. The use of blood from syngenic SCID mice infected with aoHGE prevents a potential inflammatory response to HL-60 cells during the murine studies. C3H-scid and B6-scid mice were obtained from the Frederick Cancer Research Center (Frederick, Md.) and challenged with aoHGE by the intraperitoneal (i.p.) injection of 100 μl of aoHGE-infected HL-60 cells.
Infection of C3H, C3H-scid, IFN-γ-deficient (IFN-γ−/−), and B6 mice with aoHGE and collection of specimens.
Six-week-old, female C3H mice were obtained from the Frederick Cancer Research Center laboratories. Twenty-eight C3H mice were inoculated with aoHGE by i.p. injection of 100 μl of aoHGE-containing blood from donor C3H-scid mice. As a control, seven C3H mice were injected with blood from uninfected C3H-scid mice in an identical fashion. Groups of four infected mice and one control mouse were sacrificed on days 2, 5, 8, 15, 21, 30, and 45. Experiments were repeated five times. Spleen, anticoagulant-treated peripheral blood, and sera were collected from each mouse upon necropsy. Splenic RNA was extracted, and splenocytes were used for in vitro stimulation experiments. Twenty-eight 6-week-old male IFN-γ−/− and twenty-eight age-matched B6 mice from the Jackson Laboratories (Bar Harbor, Maine) were inoculated by using blood from donor B6-scid mice and then sacrificed in a similar manner as the C3H mice. Bacterial infection was assessed by determining the percentage of morulae containing cells among 200 granulocytes examined in each peripheral blood smear, followed by the multiplication of morulae percentage by absolute neutrophil counts. Slides were stained with Diff-Quick (Baxter Healthcare Corp., Miami, Fla.) and examined for morulae by light microscopy. The inoculum donor mouse blood morula-containing cell concentrations were 168 ± 47 (mean ± the standard deviation [SD]) in any of the five sets of experiments for C3H mice and 158 ± 57 (mean ± SD) for two sets of experiments in B6 mice. All mice were maintained in barrier-filtered cages, received a standard laboratory diet, and were given water ad libitum.
PCR amplification of aoHGE DNA.
Prior to extraction of blood DNA with the QIAamp Tissue Kit (Qiagen, Inc. Valencia, Calif.) 100 μl of anticoagulated peripheral blood was incubated with 900 μl of erythrocyte lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 1 mM EDTA) at room temperature for 10 min. Pelleted cells were suspended in 200 μl of phosphate-buffered saline (PBS), pH 7.2, and DNA was extracted with the QIAamp Tissue Kit according to the manufacturer's instructions.
A semiquantitative competitive PCR was employed to determine the aoHGE blood DNA concentrations in C3H mice (21). A 549-bp competitor DNA fragment carrying an unrelated internal 504-bp B. burgdorferi OspA insert with external aoHGE primers was a kind gift of A. de Silva. An increasing known amount of competitor DNA was mixed with a series of tubes containing constant amounts of target mouse blood DNA in a 50-μl PCR reaction mixture with specific primers for aoHGE 16S ribosomal DNA (rDNA): bp 497 to 521 (5′-TGT AGG CGG CGG TTC GGT AAG TTA AAG-3′) and bp 747 to 727 (5′-GCA CTC ATC GTT TAC AGC GTG-3′) (32). PCR was performed by using the following intervals: 1 min at 94°C, 1 min at 54°C, and 2 min at 72°C. Amplified products were resolved in a 2% agarose gel and stained with ethidium bromide. Since both the competitor and the target aoHGE DNA competed for the same primers, the DNA amount in the two samples were considered equal when the PCR band intensities of these two DNA molecules were equivalent. Experiments were repeated twice for each mouse sample, and the mean value of four mice in each group was recorded.
The differences in aoHGE DNA content of blood from different days of infection was also assessed by comparison of the intensity of the DNA band from different samples. For this purpose pooled DNA samples from each group were initially subjected to PCR amplification by using primers specific for the housekeeping gene, hypoxanthine-guanine phosphoribosyltransferase (HPRT) (control) for the purpose of equalizing the amount of DNA in different samples. Normalized DNA samples were amplified with the above-described 16S aoHGE rDNA primers by using the above-described PCR intervals. Appearances of PCR band densities from different days were compared to assess the differences. Experiments were repeated at least twice.
Measurement of cytokine mRNA levels using reverse transcriptase PCR.
Murine total RNA was extracted by the guanidine isothiocyanate-phenol-chloroform single step method using the Stratagene (La Jolla, Calif.) RNA isolation kit. Five micrograms of RNA was reverse transcribed by using random primers to obtain cDNA with a Stratagene kit in a 50-μl reaction mixture. The cDNAs from individual mice at each time point were pooled. HPRT primers were used to assess and normalize the cDNA concentrations among samples from the seven time points. An equal amount of DNA was added to PCR reaction mixtures to amplify IFN-γ, IL-4, IL-12, and IL-10 cDNA by using specific primers (35). PCR experiments were repeated three times to validate the result.
Secretion of cytokines from cultured splenocytes.
Single cell suspensions of splenocytes from aoHGE-infected and uninfected (control) mice were prepared on days 2, 5, 8, and 15 of infection following lysis of erythrocytes with lysis buffer. Cells prepared from each mouse were suspended in 1 ml of Click's medium containing 10% fetal calf serum, 100 U of penicillin per ml, 100 U of streptomycin per ml, and 2 mM l-glutamine and incubated in 24-well tissue culture plates at a density of 2 × 106 cells/ml. Five micrograms of concanavalin A (ConA) and 100, 25, and 5 μg of sonication supernatants per ml from aoHGE-containing HL-60 cells or uninfected HL-60 cells (see below) were added to the wells as stimulants. After 24 or 48 h of incubation at 37°C in 5% CO2, culture supernatants were collected and kept at −70°C until testing in cytokine enzyme-linked immunosorbent assays (ELISAs).
Cultivation and harvesting of aoHGE from HL-60 cells.
One liter of aoHGE-infected HL-60 cells was used to isolate the bacteria. As a control, uninfected HL-60 cells were processed in an identical fashion. To harvest extracellular aoHGE, cells were centrifuged at 300 × g for 10 min, and the supernatants were collected. Pellets were resuspended in PBS-glucose (0.02%) and sonicated (Branson Sonifier, Danbury, Conn.) four times for 5 s each time to disrupt HL-60 cells and release the aoHGE. Unbroken HL-60 cells were pelleted by centrifugation at 300 × g for 10 min. Released aoHGE was collected after centrifugation of the supernatant at 3,000 × g for 15 min at 4°C. These aoHGE and extracellular aoHGE collected from the culture supernatant were pooled and treated by sonication for 3 min on ice. Unbroken cells were pelleted by centrifugation at 1,200 × g for 10 min. Released aoHGE were collected by centrifugation at 15,000 × g at 4°C and kept at −20°C until use in in vitro splenocyte assays.
Determination of cytokine levels in sera and culture supernatants.
Sera from animals in each group were pooled, and then the IFN-γ levels were assessed in sandwich ELISAs. In vitro-stimulated splenocyte culture supernatants from individual mice were also assayed for IFN-γ and IL-4 levels. A total of 100 μl of murine IFN-γ or IL-4 (Pharmingen, San Diego, Calif.) antibodies, suspended at a concentration of 200 ng/well in 0.1 mM NaHCO2− (pH 9.6) were coated onto 96-well plates (Nalge Nunc International) and incubated overnight at 4°C. Nonspecific binding sites in each well were blocked by incubating with 200 μl of 1.5% bovine serum albumin in PBS (blocking buffer). Serially diluted culture supernatants or sera were applied in wells coated with the cytokine antibodies and incubated at room temperature for 4 h. A total of 100 μl of purified cytokines (Pharmingen), diluted in blocking buffer containing 0.05% Tween 20, was also added at twofold serial dilutions to develop a standard curve. Wells were washed with 0.05% Tween 20 in PBS. Then, 100 μl of biotinylated antibodies (Pharmingen), diluted 1:4,000 in blocking buffer containing 0.05% Tween 20, was added to the wells and incubated for 45 min at room temperature. Bound biotin was detected with peroxidase-conjugated streptavidin (Bio-Rad Laboratories, Hercules, Calif.) used at a 1:500 dilution. A chromogenic reaction of peroxidase was developed with 3,3′,5,5′-tetramethylbenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, Md.), and the absorbancy was read at 450 nm following the addition of stop solution (Kirkegaard and Perry).
Serum anti-aoHGE ELISA.
Serum immunoglobulin G (IgG) antibodies to aoHGE antigens in aoHGE-infected C3H, IFN-γ−/−, and B6 mice were measured in ELISAs. Wells were coated with 100 ng of aoHGE antigen per well as described above. After the blocking step, serially diluted sera from the designated days of infection were added to wells. A hyperimmune mouse serum containing aoHGE antibodies was also added to each plate in serial dilutions to correct plate-to-plate variations. Sera pooled from uninfected mice C3H, IFN-γ−/−, and B6 mice were used as a negative control. Plates with added sera were incubated at room temperature for 4 h. Wells were washed with washing buffer, and goat anti-mouse IgG antibodies (Sigma) diluted 1:4,000 in 0.05% Tween 20 containing blocking buffer were added. After 1-h incubation and washing steps, development of chromogenic reaction and the reading of plates were done as described above.
Statistics.
Differences between experimental groups were analyzed by the Student's t test. The means ± the SD were calculated, and a P value of <0.05 was considered significant.
RESULTS
Progression of granulocytic ehrlichiosis in C3H mice.
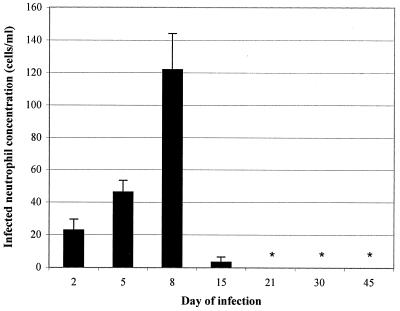
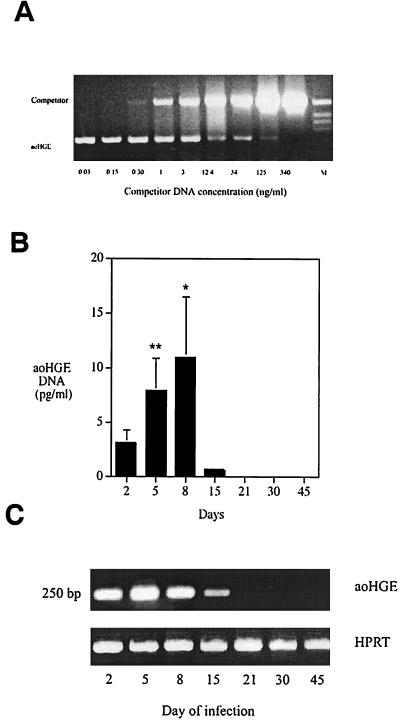
The course of aoHGE infection in C3H mice was examined over a period of 45 days so that the progression of ehrlichiosis could then be correlated with the cytokine responses. Infected neutrophil concentration and bacteria DNA burden were assessed as indices of infection. Morulae were evident 2 days after challenge, and the concentration of morulae containing neutrophils increased until day 8, when 122 ± 22 cells/μl of the neutrophils had detectable morulae (Fig. 1). At later time points morulae were not evident, except in one animal on day 15. The aoHGE burden in C3H mice was also evaluated by a semiquantitative competitive PCR (Fig. 2A). aoHGE DNA was readily present in blood on day 2, and the mean value of 4 mice was 3.05 ± 1.16 pg/ml (Fig. 2B). DNA concentrations measured on days 5 and 8 were 7.81 ± 3.0 and 11.0 ± 5.4 pg/ml, respectively. The level of aoHGE DNA had the lowest values on day 15 (0.51 ± 0.13 pg/ml) and was not detectable from days 21 to 45. In another approach, pooled DNA samples from each group were amplified with 16S rDNA aoHGE primers after normalizing the total DNA for the housekeeping gene HPRT in each sample (Fig. 2C). Comparison of DNA band intensity appearances of each group revealed that the rickettsemia peaked on days 5 to 8, followed by a significant decrease on day 15. aoHGE DNA was not detected after day 15. The infection kinetics were similar with both methods. Therefore, in subsequent studies, the intensity of the amplified products were compared since this method is considerably less laborious than semiquantitative competitive PCR when handling big sample sizes.
FIG. 1.
The concentration of morula-containing neutrophils in the bloodstream of C3H mice up to 45 days after challenge. Results represent the mean ± the SD of four mice from each time point. This set of animals were infected with 100 μl of SCID mouse blood containing 195 cells of aoHGE infected neutrophils per μl. ∗, Not detected.
FIG. 2.
PCR analysis to determine the blood aoHGE DNA levels. (A) Representative semiquantitative competitive PCR analysis. Mouse blood DNA and a competitor DNA with primers specific for aoHGE 16S rDNA was added to the PCR reaction mixture to allow competition for the primers. Reaction tubes containing decreasing amounts of competitor DNA facilitated comparison of sample DNA and the competitor DNA. When the ratio of these two DNA bands was 1:1, they were considered equivalent, and the sample DNA concentration was equal to the competitor concentration. (B) DNA levels at different time points after infection with semiquantitative competitive PCR. Starting from day 2, aoHGE DNA concentrations increased until day 8. On day 15, aoHGE DNA levels were significantly decreased. ∗, difference in values of day 8 and day 5 are statistically insignificant (P = 0.14); ∗∗, difference in values of day 5 and day 2 are statistically significant (P = 0.001). (C) PCR analysis of blood DNA samples pooled among groups from different time points that were simultaneously amplified following an initial DNA normalization step with HPRT-based PCR.
Cytokine responses in C3H mice during aoHGE infection.
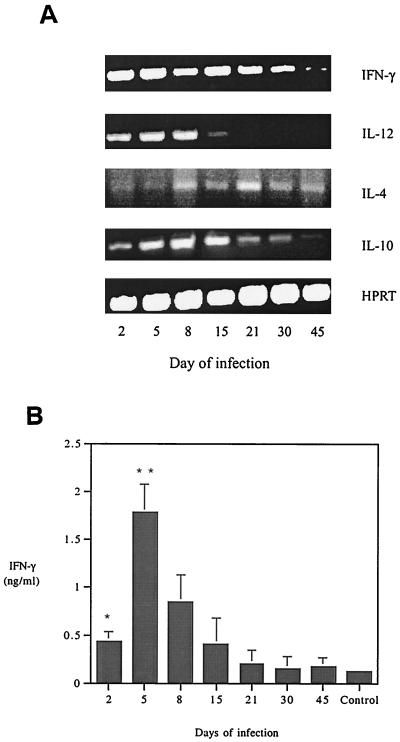
Comparative cytokine mRNA levels were determined in splenocytes of C3H mice during aoHGE infection, and culture supernatant cytokine levels were measured in in vitro stimulation assays by using splenocytes from infected C3H mice. Sera pooled among each group were also screened for IFN-γ levels. IFN-γ and IL-12 levels, which generally reflect Th1 responses, and IL-4 and IL-10, which are associated with Th2 responses were assessed in vivo, and IFN-γ and IL-4 levels were measured in vitro. In the splenocytes of C3H mice, IFN-γ mRNA was readily detected on days 2 through 30 (Fig. 3A). The PCR band intensity of IFN-γ mRNA showed a decrease toward day 45. IL-12 mRNA was evident through day 8. IL-4 mRNA PCR bands were faintly detected on day 5 and persisted through day 45, and IL-10 was detected at most intervals.
FIG. 3.
(A) Splenic cytokine mRNA levels in C3H mice during infection with aoHGE. cDNA levels of each sample were normalized by using HPRT primers. (B) Serum IFN-γ concentrations measured in sandwich ELISAs. Sera of animals from each time point were pooled and applied to wells as undiluted. Means of triplicate samples are recorded. ∗, P = 0.02 compared to uninfected mice; ∗∗, P = 0.04 compared to day 2 values.
Pooled sera from groups of four mice were subjected to ELISA test to measure the serum IFN-γ levels. IFN-γ levels were significantly higher (P = 0.02) than control mice on day 2 of infection (Fig. 3B) and peaked on day 8, followed by a gradual decrease on the remaining days. Serum IFN-γ levels reflected the mRNA levels with the exception of day 2. The PCR band intensities were similar on days 2 and 5, while day 2 serum levels were significantly less than those of day 5 (P = 0.04). This variance during the early stage of infection could be due to temporal differences between mRNA transcription and protein expression.
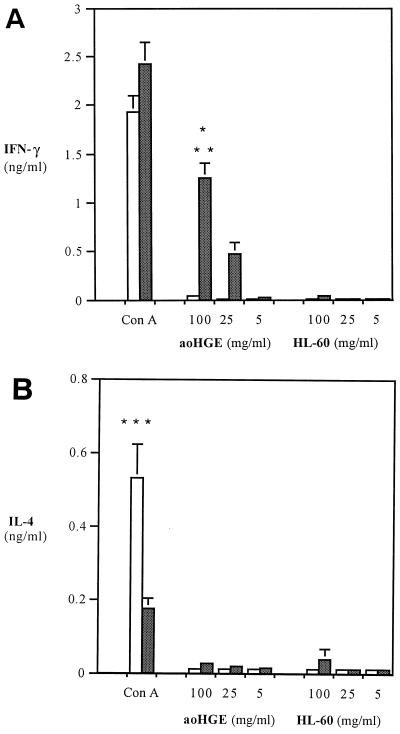
Stimulation assays with single-cell splenocyte suspensions at 2, 5, 8, and 15 days revealed increased IFN-γ levels. The highest level of IFN-γ was detected on day 8 after aoHGE challenge (Fig. 4A). Splenocytes from infected C3H mice produced 1.36 ± 0.14 ng of IFN-γ per ml when incubated with 100 μg of aoHGE extract per ml, while cells from control (uninfected) mice did not produce IFN-γ (P = 0.002). The increase in IFN-γ production was dose dependent. Cells from infected animals also had significantly higher IFN-γ levels (P = 0.002) when incubated with aoHGE extract than with HL-60 extract (control), demonstrating that the increased secretion of IFN-γ was not due to components of HL-60 cells that could have been retained during aoHGE purification. ConA also induced higher IFN-γ levels in aoHGE-infected splenocytes than in uninfected splenocytes (P < 0.002) and, conversely, higher levels of IL-4 secretion (P < 0.01) in splenocytes from uninfected mice (controls) than from splenocytes from aoHGE-infected mice (Fig. 4B). These responses to ConA suggest that aoHGE infection skewed the immune response toward a Th1 phenotype. aoHGE and HL-60 cell extracts did not stimulate detectable IL-4 in infected or uninfected mouse splenocyte cultures (Fig. 4B).
FIG. 4.
Levels of IFN-γ (A) and IL-4 (B) in stimulation assays with splenocytes from aoHGE-infected C3H mice (8 days). Splenocytes were incubated with 5 μg of ConA per ml and 100, 25, or 5 μg of sonication supernatants of aoHGE per ml containing HL-60 cells or HL-60 cells alone. IFN-γ levels were measured at 48 h, and IL-4 levels were measured at 24 h. All values are the means ± the SD of four mice. ∗, P < 0.002 compared to uninfected mice; ∗∗, P = 0.001 compared to HL-60 extract stimulated splenocytes; ∗∗∗, P < 0.01 compared to infected mice. □, Uninfected mice; ■, infected mice.
aoHGE infection and cytokine profile in IFN-γ−/− and B6 mice.
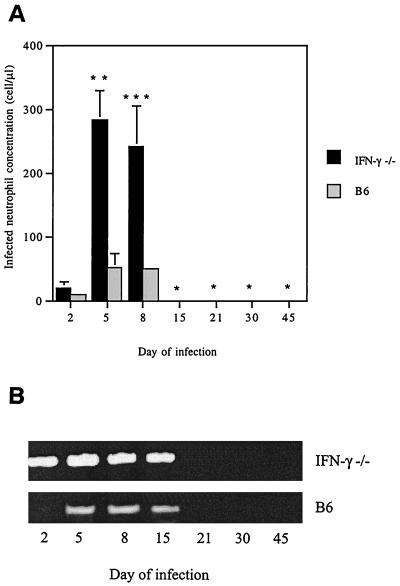
These data imply that IFN-γ is a prominent cytokine during murine infection with aoHGE and that bacterial levels decrease after IFN-γ become evident. IFN-γ−/− mice were then used to further explore the importance of IFN-γ in the course of aoHGE infection. Groups of IFN-γ−/− and the wild-type B6 (control) mice were assessed for ehrlichiosis on days 2, 5, 8, 15, 21, 30, and 45. The concentration of morulae containing neutrophils was markedly higher in the IFN-γ−/− mice than in control animals (51 ± 21 cells/μl) on days 2, 5, and 8, reaching levels of 282 ± 48 cells/μl (P = 0.004) (Fig. 5A). In addition, PCR revealed more aoHGE DNA in IFN-γ−/− than in control animals (Fig. 5B). Morulae were not detectable after day 8 and Ehrlichia DNA was not evident after day 15 in both IFN-γ−/− and B6 mice.
FIG. 5.
aoHGE infection in IFN-γ−/− and B/6 mice up to 45 days after challenge with ehrlichiae. (A) Morulae containing neutrophil concentrations in the bloodstream. Means ± the SD are derived from three mice examined at each time point. This set of animals were infected with 100 μl of SCID mice blood containing 198.4 cells/μl of aoHGE infected neutrophils. ∗, Not detected; ∗∗, P = 0.004 compared to B6 mice; ∗∗∗, P = 0.005 compared to B6 mice. (B) Blood aoHGE DNA levels, amplified by using 16S DNA primers. DNA levels of each sample were normalized by using HPRT (not shown).
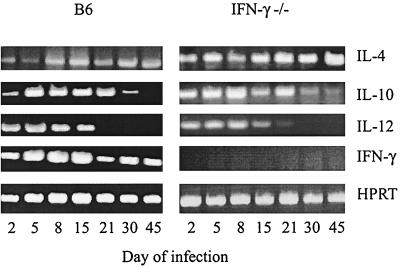
aoHGE-infected B6 mice had persistently detectable IFN-γ mRNA, which were most prominent on days 5, 8, and 15 (Fig. 6) and, as expected, IFN-γ−/− mice did not produce any IFN-γ. IL-4 mRNA PCR band intensities were greater in the IFN-γ−/− mice than in the wild-type B6 mice (Fig. 6). There was no significant difference in IL-10 and IL-12 mRNA PCR band intensities between the IFN-γ−/− and the control mice.
FIG. 6.
Spleen cytokine mRNA levels of aoHGE-infected IFN-γ−/− and B6 mice after various intervals after aoHGE challenge. cDNA levels of each sample were normalized by using HPRT primers.
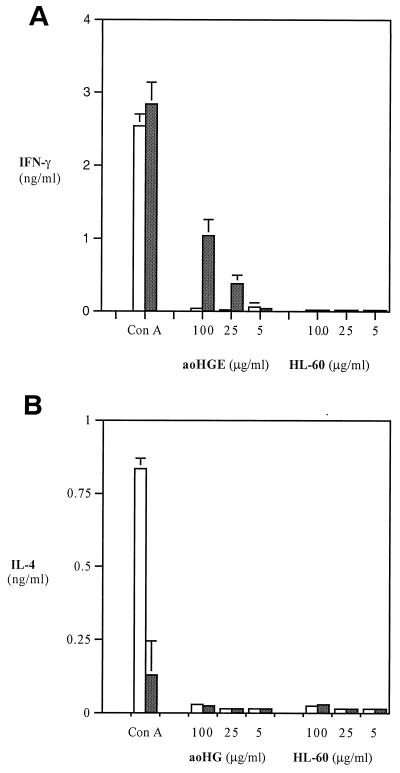
Splenocytes from infected B6 mice produced 1.0 ± 0.1 ng of IFN-γ per ml when incubated with 100 μg of aoHGE extract per ml, whereas uninfected mice did not produce IFN-γ (Fig. 7A). IL-4 was not detected in infected mice stimulated with aoHGE extract. In contrast, ConA induced much stronger IL-4 responses in splenocytes from uninfected mice (Fig. 7B). IFN-γ levels were slightly higher when splenocytes from infected, rather that uninfected, mice were stimulated with ConA.
FIG. 7.
Levels of IFN-γ (A) and IL-4 (B) in stimulation assays with splenocytes from aoHGE-infected B6 mice (8 days). Splenocytes were incubated with 5 μg of ConA per ml and 100 μg of sonication supernatants of aoHGE per ml containing HL-60 cells or HL-60 cells alone. IFN-γ levels were measured at 48 h, and IL-4 levels were measured at 24 h. All values are the means ± the SD of four mice. □, Uninfected mice; ■, infected mice.
Antibody response to aoHGE in C3H, IFN-γ−/−, and B6 mice.
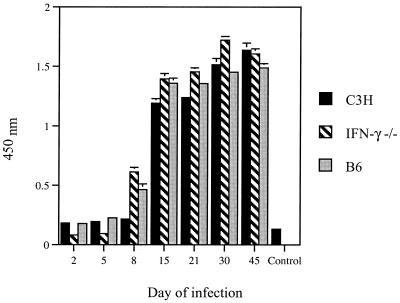
Serum IgG antibodies to aoHGE antigens manifested a significant increase in IFN-γ−/− (P = 0.01), and B6 (P = 0.02) mice on day 8 compared to uninfected mice, while C3H mice had increased antibody levels (P = 0.001) only on day 15 (Fig. 8). On day 15 all three mice had comparable antibody levels to aoHGE. The increase in antibodies to aoHGE continued in all mice until day 30 and then stayed at the same level on day 45.
FIG. 8.
Development of anti-aoHGE IgG antibodies in the sera of C3H, IFN-γ−/−, and B6 mice. Sera were pooled among four animals from each time point. All values are the means ± the SD of three experiments.
DISCUSSION
These studies investigated the role of IFN-γ in the development of murine aoHGE infection. It was first demonstrated that IFN-γ is a dominant cytokine during the early phase of granulocytic ehrlichiosis in immunocompetent C3H and B6 mice. In addition, morulae were prominent on day 8 and then cleared from the bloodstream when IFN-γ were at their highest level. The aoHGE bacterial burden, as assessed by PCR, followed a similar course. These results suggest that IFN-γ may facilitate bacterial clearance. To explore this hypothesis in greater detail, we assessed the course of infection in IFN-γ−/− mice. The IFN-γ−/− mice had markedly increased concentrations of infected neutrophils at day 5 (282 ± 48 cells/μl) and elevated levels of aoHGE DNA, further suggesting that this cytokine plays a role in controlling the degree of rickettsemia during the early phase of infection. Although ehrlichiosis was more prominent at 8 days in the IFN-γ−/− mice than in the control mice, both groups of animals cleared aoHGE from the bloodstream at 2 to 3 weeks. This suggests that IFN-γ-independent mechanisms such as humoral immunity and cellular responses may play an important role in the control of the pathogen at these later time points. Indeed, immunocompetent and IFN-γ−/− mice had IgG antibodies to aoHGE that were readily detectable after 8 to 15 days of infection.
For several years, mice have been used as an experimental model for aoHGE infection (20, 21, 40, 42). The kinetics of infection, including the peak of aoHGE infection and the duration of rickettsemia, varies somewhat among the studies, although all of the studies suggest a peak infection during the first several weeks with subsequent bacterial clearance. Differences in the results may be attributed to the dosage of inoculum, the mode of inoculum (tick or syringe challenge), or the virulence of the inoculated bacteria. The course of rickettsemia is shorter in the current study (15 days) than in our earlier report (30 days) (21). This difference may be attributed to the smaller inoculum size used in this study compared to the earlier study (ca. 7% versus 17 to 20% percentage of morulae).
The data obtained in this study also show that aoHGE infection skews the immune response toward a Th1 phenotype. Upon stimulation with aoHGE extract or ConA, splenocytes from aoHGE-infected C3H or B6 mice both showed a markedly elevated level of IFN-γ compared with splenocytes from uninfected mice (controls). Furthermore, the IL-4 levels were lower when infected, rather than uninfected, cells were stimulated with ConA. aoHGE antigens may therefore predominantly induce a Th1 response during the early stage of infection. Indeed, leishmaniae, which survive within macrophages, have been shown to express specific antigens which direct the immune response toward a Th1 or a Th2 response (26, 38, 39). Moreover, transgenic mice that are tolerant to LACK, a leishmania antigen that promotes Th2 responses, become resistant to infection (26). Identification of the aoHGE antigens that are involved in initiating a Th1 response may aid our understanding of the bacterial factors that influence the host response and the course of infection.
The IFN-γ response to aoHGE may lead to a number of effects that may affect infection. Natural killer (NK) cells and T lymphocytes are two known sources of IFN-γ (1, 36). Since IFN-γ was evident in aoHGE-infected SCID mice, it is likely that NK cells contribute to IFN-γ production. IFN-γ can induce nitric oxide synthesis by phagocytic cells, and this may facilitate pathogen clearance (10, 25, 27): increased mortality in mycobacterium-infected IFN-γ−/− mice has been associated with abolished nitric oxide activity and altered major histocompatibility complex (MHC) II expression (13). Furthermore, IFN-γ-induced MHC II expression of macrophages can be downregulated by Toxoplasma gondii (29). Cytokines such as IL-4, which elicit Th2 response, may alter some of the effects exerted by IFN-γ. Th2 responses can lead to downregulation of nitric oxide production by macrophages (11), and IL-10 blocks the IFN-γ-dependent microbicidal effect of macrophages on T. gondii and the extracellular killing of Schistosoma mansoni (17). Our study showed that murine aoHGE infection induces the production of IL-10 and, to a lesser degree, of IL-4 following the initial IFN-γ burst. The inhibitory activities of these two cytokines could contribute to the persistence of aoHGE.
The immunologic parameters that influence the progression of granulocytic ehrlichiosis are likely to be multifactorial. Most intracellular pathogens which are used to study cellular immune responses persist in macrophages (such as leishmaniae) or epithelial cells (such as listeriae). The HGE agent is unusual in that it persists with PMNs: a cell that only survives for several days. These studies show that IFN-γ plays a role in controlling the degree of early rickettsemia and aoHGE infection when antibodies are not yet readily available. The murine model of granulocytic ehrlichiosis will enable us to dissect the cytokine responses to aoHGE and the mechanisms by which these cytokines act to facilitate the clearance of this pathogen within neutrophils during different stages of infection.
ACKNOWLEDGMENTS
We thank Deborah Beck for technical assistance and Juan Anguita for critical reading of the manuscript.
This work was supported by NIH grant AI41440. M.A. was supported by the Brown-Coxe postdoctoral fellowship program at Yale, and E.F. is a recipient of a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund.
REFERENCES
- 1.Abbas A, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Adachi J A, Grimm E M, Johnson P, Uthman M, Kaplan B, Rakita R M. Human granulocytic ehrlichiosis in a renal transplant patient: case report and review of the literature. Transplantation. 1997;64:1139–1142. doi: 10.1097/00007890-199710270-00010. [DOI] [PubMed] [Google Scholar]
- 3.Aguero-Rosenfeld M E, Horowitz H W, Wormser G P, McKenna D F, Nowakowski J, Munoz J, Dumler J S. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996;125:904–908. doi: 10.7326/0003-4819-125-11-199612010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Anthony S J, Dumler J S, Hunter E. Human ehrlichiosis in a liver transplant recipient. Transplantation. 1995;60:879. [PubMed] [Google Scholar]
- 5.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 6.Bakken J S, Erlenmeyer S A, Kanoff R J, Silvesterini II T C, Goodwin D D, Dumler J S. Demyelinating polyneuropathy associated with human granulocytic ehrlichiosis. Clin Infect Dis. 1998;27:1323–1324. [PubMed] [Google Scholar]
- 7.Bakken J S, Dumler J S, Chen S M, Eckman M R, Van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 8.Bakken J S, Krueth J, Tilden R L, Dumler J S, Kristiansen B E. Serological evidence of human granulocytic ehrlichiosis in Norway. Eur J Clin Microbiol Infect Dis. 1996;15:829–832. doi: 10.1007/BF01701530. [DOI] [PubMed] [Google Scholar]
- 9.Bakken J S, Goellner P, van Etten M, Boyle D Z, Swonger O L, Mattson S, Krueth J K, Tilden R L, Asanovich K, Walls J, Dumler J S. Seroprevalance of human granulocytic ehrlichiosis among permanent residents of northwestern Wisconsin. Clin Infect Dis. 1998;27:1491–1496. doi: 10.1086/515048. [DOI] [PubMed] [Google Scholar]
- 10.Beckerman K P, Rogers H W, Corbett J A, Schreiber R D, McDaniel M L, Unanue E R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–895. [PubMed] [Google Scholar]
- 11.Bogdan C, Vodovotz Y, Paik J, Xie Q, Nathan C. Mechanisms of suppression of NOS expression by IL-4 in primary mouse macrophages. J Leukoc Biol. 1994;55:227–233. doi: 10.1002/jlb.55.2.227. [DOI] [PubMed] [Google Scholar]
- 12.Brunet L R, Dunne D W, Pearce E J. Cytokine interaction and immune responses during Schistosoma mansoni infection. Parasitol Today. 1998;14:422–427. doi: 10.1016/s0169-4758(98)01317-9. [DOI] [PubMed] [Google Scholar]
- 13.Dalton D K, Pitts-Meek S, Keshav S, Figari S I, Bradley A, Steward T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 14.Dumler J S, Bakken J S. Human ehrlichiosis: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 15.Dumler J S, Bakken J S. Human granulocytic ehrlichiosis in Wisconsin and Minnesota: a frequent infection with the potential for persistence. J Infect Dis. 1996;173:1027–1030. doi: 10.1093/infdis/173.4.1027. [DOI] [PubMed] [Google Scholar]
- 16.Fingerle V, Goodman J L, Johnson R C, Kurtti T J, Munderloh U G, Wilske B. Human granulocytic ehrlichiosis in southern Germany: increased seroprevalence in high-risk groups. J Clin Microbiol. 1997;35:3244–3247. doi: 10.1128/jcm.35.12.3244-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazinelli R T, Oswald I P, James S L, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792. [PubMed] [Google Scholar]
- 18.Gazinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 19.Hardalo C J, Quagliarello V, Dumler J S. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1996;21:910–914. doi: 10.1093/clinids/21.4.910. [DOI] [PubMed] [Google Scholar]
- 20.Hodzic E, Ijdo J W, Feng S, Katavalos P, Sun W, Maretzki C H, Fish D, Fikrig E, Telford III S R, Barthold S W. Granulocytic ehrlichiosis in the laboratory mouse. J Infect Dis. 1998;177:737–745. doi: 10.1086/514236. [DOI] [PubMed] [Google Scholar]
- 21.Hodzic E, Fish D, Maretzki C M, De Silva A M, Feng S, Barthold S W. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol. 1998;36:3574–3578. doi: 10.1128/jcm.36.12.3574-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter C A, Ellis-Neyes L A, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo F G. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 23.Ijdo W J, Sun W, Zhang Y, Magnarelli L A, Fikrig E. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect Immun. 1998;66:3264–3269. doi: 10.1128/iai.66.7.3264-3269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahangir A, Kolbert C, Edwards W, Mitchell P, Dumler J S, Persing D H. Fatal pancarditis associated with human granulocytic ehrlichiosis in 44-year-old man. Clin Infect Dis. 1998;27:1424–1427. doi: 10.1086/515014. [DOI] [PubMed] [Google Scholar]
- 25.James S L, Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4808–4812. [PubMed] [Google Scholar]
- 26.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 27.Karupiah G, Xie Q W, Buller R M, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 28.Kim H Y, Rikihisa Y. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J Clin Microbiol. 1998;36:3278–3284. doi: 10.1128/jcm.36.11.3278-3284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luder C G, Lang T, Beuerle B, Gross U. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin Exp Immunol. 1998;112:308–316. doi: 10.1046/j.1365-2249.1998.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnarelli L A, Ijdo J W, Anderson J, Padula S J, Flavell R A, Fikrig E. Human exposure to a granulocytic Ehrlichia and other tick-borne agents in Connecticut. J Clin Microbiol. 1998;36:2823–2827. doi: 10.1128/jcm.36.10.2823-2827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadelman R B, Horowitz H W, Hsieh T C, Wu J M, Aguero-Rosenfeld M E, Schwartz I, Nowakowski J, Varde S, Wormser G P. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27–30. doi: 10.1056/NEJM199707033370105. [DOI] [PubMed] [Google Scholar]
- 32.Pancoli P, Kolbert C P, Mitchell P D, Reed K D, Jr, Dumler J S, Bakken J S, Telford S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 33.Petrovec M, Furlan S L, Zupanc T A, Strle F, Brouqui P, Roux V, Dumler J S. Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–1559. doi: 10.1128/jcm.35.6.1556-1559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pusterla N, Weber R, Wolfensberger C, Schar G, Zbinden R, Fierz W, Madigan J E, Dumler J S, Lutz H. Serological evidence of human granulocytic ehrlichiosis in Switzerland. Eur J Clin Microbiol Infect Dis. 1998;17:207–209. doi: 10.1007/BF01691120. [DOI] [PubMed] [Google Scholar]
- 35.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 36.Scharton T M, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 38.Skeiky Y A, Kennedy M, Kaufman D, Borges M M, Guderian J A, Scholler J K, Ovendale P J, Picha K S, Morrissey P J, Grabstein K H, Campos-Neto A, Reed S G. LeIF: a recombinant Leishmania protein that induces an IL-12-mediated Th1 cytokine profile. J Immunol. 1998;11:6171–6179. [PubMed] [Google Scholar]
- 39.Skeiky Y A, Guderian J A, Benson D R, Bacelar O, Carvalho E M, Kubin M, Badaro R, Trinchieri G, Reed S G. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun W, Ijdo J W, Telford III S R, Hodzic E, Zhang Y, Barthold S W, Fikrig E. Immunization against the agent of human granulocytic ehrlichiosis in a murine model. J Clin Investig. 1997;100:3014–3018. doi: 10.1172/JCI119855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telford I S R, Lepore T J, Snow P, Warner C K, Dawson J E. Human granulocytic ehrlichiosis in Massachusetts. N Engl J Med. 1995;334:209–215. doi: 10.7326/0003-4819-123-4-199508150-00006. [DOI] [PubMed] [Google Scholar]
- 42.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace B J, Brady G, Ackman D M, Wong S J, Jacquette G, Lloyd E E, Birkhead G S. Human granulocytic ehrlichiosis in New York. Arch Intern Med. 1998;158:769–773. doi: 10.1001/archinte.158.7.769. [DOI] [PubMed] [Google Scholar]