Abstract
Bacteria are being actively investigated as vaccine carriers for inducing or boosting protective immune responses. In this study, human monocyte-derived dendritic cells (DCs) and normal B cells were compared for their capacity to present the C fragment of tetanus toxin (TTFC), expressed on the surface of recombinant Streptococcus gordonii, to specific CD4+ T lymphocytes. DCs were more efficient than B cells at presenting soluble TTFC and remarkably more capable of presenting bacterium-associated TTFC both in terms of the amount of antigen required to obtain a given T-cell response and on a per-cell basis. This difference was associated with a much lower capacity of B cells to endocytose soluble TTFC and phagocytose recombinant S. gordonii. In addition, S. gordonii induced the phenotypic maturation of DCs but not of B cells. The results thus indicate that DCs but not B cells play a crucial role in the amplification of class II-restricted immune responses induced by immunization with recombinant gram-positive bacteria.
Recent efforts in developing more efficacious vaccine strategies have pointed to the use of bacteria as vectors of heterologous antigens. Bacteria can be easily manipulated at the genetic level and can be engineered to express the gene product in different forms (11, 21, 26). Alternatively, attenuated bacteria can serve as carriers for delivering antigen-encoding DNA to antigen-presenting cells (APCs) (6, 17, 30). These approaches have been used for inducing protective immune responses against viral and tumor antigens in mouse models (6, 17, 26). In particular, recombinant strains of Streptococcus gordonii, which is a commensal bacterium of the human oral cavity, induce both local and systemic antibody responses, as well as a T-cell response, to viral antigens in both mice and macaques (7, 15, 16). However, the APCs and the mechanisms involved in these bacterium-based immunostimulating systems have been only marginally investigated.
Dendritic cells (DCs) and B lymphocytes show important differences in their APC functions (1, 19). They differ in the capacity to endocytose antigens, to cluster with T cells, to provide proper costimulation, and to secrete regulatory cytokines (2, 5, 9, 13, 28). A number of studies have indeed demonstrated that DCs are much more potent APCs than are antigen-specific and non-antigen-specific B lymphocytes in the activation of both naive and memory T cells (2, 4, 9, 14, 20, 27). Most experiments, however, has been performed using soluble antigens given in the form of native proteins or immunogenic peptides, and very few studies have compared the capacity of DCs and B cells to present particulate or bacterium-associated antigens (8, 23, 29).
Mouse DCs present a major histocompatibility complex (MHC) class I-restricted heterologous antigen, expressed on the surface of recombinant S. gordonii, to T lymphocytes at high efficiency, and S. gordonii induces neobiosynthesis and membrane stabilization of MHC class I and class II molecules (25). Moreover, human DCs fed with recombinant bacteria stimulated the specific CD4+-T-cell response with much higher efficiency than did DCs pulsed with soluble antigen. Finally, S. gordonii provided a potent stimulus for inducing DC maturation and release of chemokines active on T cells (3). In the present study, DCs and B cells were compared for their capacity to present the C fragment of tetanus toxin (TTFC) expressed on recombinant S. gordonii to CD4+ T lymphocytes.
MATERIALS AND METHODS
Recombinant bacteria.
Recombinant S. gordonii expressing TTFC (strain GP1253) was prepared using the host-vector system GP1221-pSMB55, as described in detail previously (3, 21). Surface expression of TTFC on GP1253 was achieved using the M6 protein as a fusion partner and monitored by flow cytometric analysis on whole cells and Western blotting of cell fractions, using M6- and TTFC-specific rabbit polyclonal antibodies (Abs). The concentration of TTFC for each bacterium was calculated by densitometric analysis of dot blots of purified TTFC (Calbiochem, San Diego, Calif.) and the envelope fraction of bacterial lysate and estimated to be approximately 1,000 molecules/cell, corresponding to 10−7 ng of TTFC/CFU (3). S. gordonii strains GP1221 and GP1253 were grown at 37°C in tryptic soy broth without dextrose (Difco, Detroit, Mich.) and harvested by centrifugation at the end of the exponential phase of growth. Bacterial cells were then washed and resuspended at 1:500 of the original culture volume in fresh medium containing 10% glycerol. Aliquots were stored at −80°C until use.
APCs.
DCs were prepared from peripheral blood monocytes of healthy individuals by the method described by Sallusto and Lanzavecchia (27). In brief, peripheral blood mononuclear cells (PBMC) isolated by standard density gradient centrifugation were further separated on multistep Percoll gradients (Pharmacia, Uppsala, Sweden). Cells from the low-density fraction were recovered and cultured in RPMI 1640 (Life Technologies, Gaithersburg, Md.) plus 10% fetal bovine serum (FBS) (HyClone Laboratories, Logan, Utah), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 2 mM l-glutamine, 25 mM HEPES, 100 U of penicillin per ml, 100 μg of streptomycin per ml (all from Life Technologies), and 0.05 mM 2-mercaptoethanol (Merck, Darmstadt, Germany) (complete medium) at 37°C under 5% CO2, in the presence of 200 ng of recombinant human granulocyte-macrophage colony-stimulating factor (Mielogen; Schering-Plough, Milan, Italy) per ml and 200 U of recombinant human interleukin-4 (Genzyme, Cambridge, Mass.) per ml. The medium was changed after 3 days, and on day 6 of culture, the cells were collected and depleted of CD2+ and CD19+ cells by using immunomagnetics beads (Dynal, Oslo, Norway). This procedure gave >97% pure CD1a+ CD14− cell preparations. B cells were separated from PBMC by incubation with anti-CD19-conjugated magnetic beads followed by the DETACHaBEAD Ab (Dynal). Thereafter, a second incubation with beads conjugated to anti-CD2+ and anti-CD14+ monoclonal Abs (MAbs) was performed. The resulting cell population was ≥95% pure CD19+. Autologous B-cell lines were generated by standard procedures by incubating PBMC with supernatant from the Epstein-Barr virus (EBV)-producing marmoset line B95/8 (Rockville, Md.) in the presence of 2 μg of cyclosporin A per ml for 7 to 15 days.
T cells.
CD4+-T-cell clones specific for TTFC (CP7 and ALS4) were prepared by limiting dilution of TTFC-specific CD4+-T-cell lines generated from PBMC from two distinct healthy volunteers. Briefly, the nonadherent fraction was further separated in CD4+ or CD8+ cells by using immunomagnetic beads (Dynal). CD4+ cells were cocultured with irradiated adherent monocytes in the presence of 1 μg of soluble TTFC per ml. After 5 days, cell lines were cloned by limiting dilution at 1 cell/well in the presence of irradiated 105 feeder cells/well and 1% phytohemagglutinin. The T-cell clones were CD4+ CD8− TCR-α/β+ TCR-γ/δ− CD28+ and secreted high levels of gamma interferon but no interleukin-4 following activation with 1 μg of immobilized anti-CD3 (OKT3, immunoglobulin G1 [IgG1]; Immunotech, Marseille, France) per ml and soluble 1 μg of anti-CD28 (L293, IgG1; Becton Dickinson, San Jose, Calif.) per ml or in an antigen-specific stimulation assay. TTFC-specific CD4+-T-cell clones were strictly MHC class II dependent, as determined by inhibition studies with anti-HLA-DR MAb (L243, IgG2a; Becton Dickinson).
Antigen presentation assay.
Autologous DCs, B cells, or EBV-B cells were incubated at 37°C with S. gordonii GP1253, control strain GP1221, or soluble TTFC in complete medium at the indicated concentrations. After 18 h, APCs were washed, examined for cell viability by the trypan blue exclusion test, and cocultured with T cells (2 × 104 to 3 × 104 cells/well) in triplicate wells. Cocultures were pulsed with 1 μCi of [3H]thymidine per well on day 2 or 3. Radioactivity was measured in a beta counter (Topcount; Packard Instruments, Groningen, The Netherlands). Results are given as mean counts per minute (cpm) ± standard deviation (SD) of triplicate cultures.
Endocytosis and phagocytosis assays.
Endocytosis was quantitated by incubating DCs or B cells with fluorescein isothiocyanate (FITC)-conjugated TTFC (Calbiochem) at 1 mg/ml. For detection of phagocytosis, bacteria were labeled using the PKH-26 kit (Sigma Chemical Co., St. Louis, Mo.) as specified by the manufacturer. The cells were incubated with fluorochrome-conjugated TTFC or bacteria at 37 or 4°C, and at selected time points, uptake was stopped by adding cold phosphate-buffered saline containing 2% FBS and 0.01% NaN3. The cells were then washed four times and finally analyzed by flow cytometry using a FACScan and Cellquest analysis software (Becton Dickinson, Mountain View, Calif.). Surface binding values obtained by incubating cells at 4°C were subtracted from the values measured at 37°C.
Immunophenotype of DCs and B cells.
After 18 to 48 h of incubation with S. gordonii strain GP1253, strain GP1221 or medium alone, DCs or B cells were washed and then stained in phosphate-buffered saline containing 2% FBS and 0.01% NaN3. The following FITC-conjugated MAbs were used: anti-HLA-DR (L243, IgG2a), anti-CD14 (MφP9, IgG2b), anti-CD19 (4G7, IgG1), and anti-CD25 (2A3, IgG1) from Becton-Dickinson; anti-CD1a (HI149, IgG1) and anti-CD86 (FUN-1, IgG1) from Pharmingen, San Diego, Calif.; anti-CD40 (BB20, IgG1) from Ylem, Avezzano, Italy; and anti-CD54 (84H10, IgG1) and anti-CD80 (MAB104, IgG1) from Immunotech. Pure anti-CD83 (HB15, IgG2b) and anti-MHC class I (W6/32, IgG1) were from Immunotech and Dako (Glostrup, Denmark), respectively. FITC-conjugated anti-mouse Ig F(ab′)2 came from Southern Biotechnology, Birmingham, Ala. In control samples, the MAb was replaced by matched isotype control mouse Ig (Becton Dickinson). Cells were analyzed with a FACScan.
Statistical analysis.
Wilcoxon's signed rank test was used (SigmaStat; Jandel, San Rafael, Calif.) to compare differences in T-cell proliferation, endocytosis, and phagocytosis. P ≤ 0.05 were considered significant.
RESULTS
DCs are much more efficient than B cells at presenting a recombinant class II-restricted antigen expressed on gram-positive bacteria.
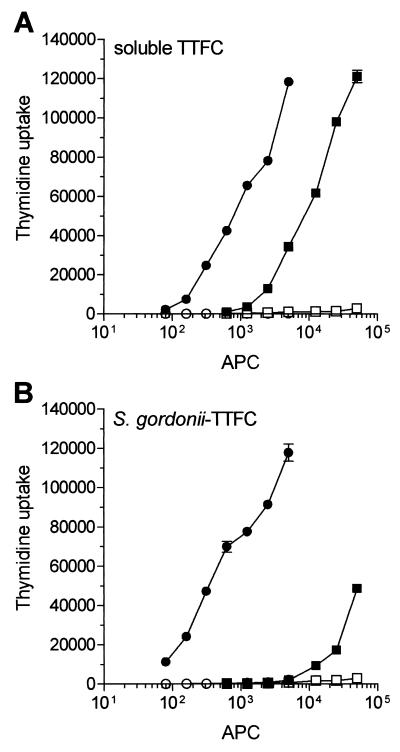
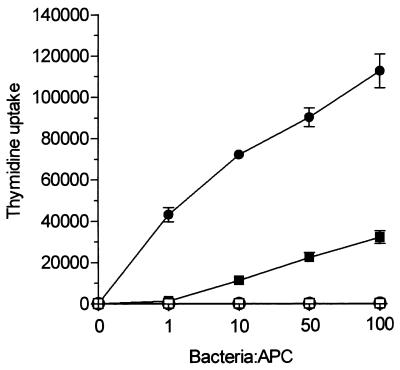
S. gordonii, a gram-positive coccus and a normal commensal of the human oral cavity, has been used as the carrier for mucosal delivery of heterologous vaccine antigens in both mice and nonhuman primates (7, 15, 16). In this study, we used a recombinant strain of S. gordonii (GP1253) expressing TTFC fused with the M6 protein on the cell surface and the parental strain GP1221 as the control (3, 21). Two types of APCs, monocyte-derived DCs (≥95% CD1a+ CD14− CD19−) and normal B cells (≥95% CD19+ CD14− CD1a−), were prepared at high purity and compared for their capacity to present recombinant TTFC expressed on S. gordonii. As responder T cells, two TTFC-specific, HLA-DR-restricted Th1 clones (CP7 and ALS4) were used. DCs were more efficient than nonspecific B cells at presenting soluble TTFC (Fig. 1A), as shown previously (27). Additionally, the DCs were considerably more potent than the B cells at presenting bacterium-associated TTFC (Fig. 1B). In fact, to obtain a comparable T-cell response, 10- and 150-fold fewer APCs were required using soluble antigen and GP1253, respectively. Figure 1 also shows that DCs incubated with 1 μg of soluble TTFC per ml (Fig. 1A) induced T-cell proliferation similar to that obtained with DCs treated with 5 ng of bacterium-associated TTFC per ml (Fig. 1B), confirming that recombinant bacteria represent a very effective means of delivering antigens to APCs (3). A marked difference in the APC efficiency between DCs and B cells was also measured when a constant number of DCs (1,000 cells/well) and B cells (30,000 cells/well) was tested with increasing doses of antigen (Fig. 2). In particular, DCs could still activate T cells when pulsed with 1 bacterium/cell whereas B cells required at least 100 bacteria/cell to give an even smaller T-cell response. These differences in APC function could not be attributed to loss of cell viability in the B-cell preparations, since both DCs and B cells were >90% viable, as shown by trypan blue staining, after 18 to 48 h of incubation with either soluble TTFC or recombinant bacteria (data not shown).
FIG. 1.
TTFC expressed on recombinant gram-positive bacteria is presented much more efficiently by DCs than by B cells to specific CD4+ T cells. Graded numbers of autologous DCs (solid circles) or fresh B cells (solid squares) were treated with 1 μg of soluble TTFC per ml or (A) with S. gordonii GP1253 at a bacterium-to-APC ratio of 50 (corresponding to 5 ng/ml of TTFC) (B) and then cocultured for 3 days with specific CD4+ T cells (clone CP7; 30,000 cells/well). Open symbols represent T-cell proliferation in the absence of antigen (A) or in the presence of APCs pulsed with S. gordonii control strain GP1221 (B). Results are expressed as mean cpm and SD of triplicate cultures. Differences in T-cell proliferation induced by DC and B cells incubated with GP1253 were statistically significant (P ≤ 0.001) at each APC dose. The results of one representative experiment out of four performed are shown.
FIG. 2.
DCs are superior to B cells at presenting different doses of recombinant antigen. A fixed number of DCs (solid circles) (1,000 cells/well) or fresh B cells (solid squares) (30,000 cells/well) was incubated with increasing doses of S. gordonii GP1253 and then cocultured for 3 days with specific CD4+ T cells (clone CP7; 30,000 cells/well). Open symbols represent T-cell proliferation in the presence of APCs treated with control strain GP1221. Results are expressed as mean cpm and SD of triplicate cultures. Similar results were observed in three independent experiments.
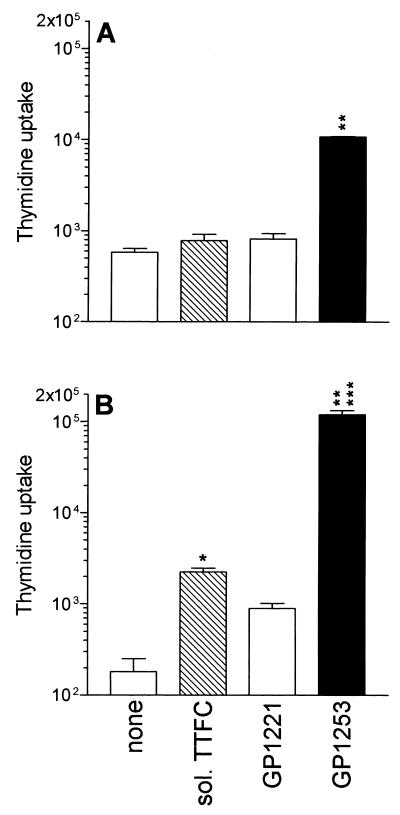
Next, we compared the capacity of DCs and B cells to present equivalent amounts of antigen in the soluble or bacterium-associated form. Figure 3A shows that B cells pulsed with 5 ng of soluble TTFC per ml were not capable of inducing T-cell proliferation whereas B cells incubated with recombinant bacteria at a bacterium-to-B-cell ratio of 50:1 (corresponding to 5 ng of TTFC per ml) induced a significant T-cell response. In contrast to B cells, DCs pulsed with 5 ng of soluble TTFC per ml could induce a significant T-lymphocyte proliferation; however, the proliferation was much greater when DCs were fed with a corresponding dose of bacterium-associated TTFC. These results suggested that recombinant antigen expressed on bacteria is presented more efficiently than soluble antigen by both B cells and DCs. Results similar to those obtained with normal B cells were observed using autologous EBV-B-cell lines (data not shown).
FIG. 3.
Antigen expressed on recombinant bacteria is presented better than soluble antigen by both DCs and B cells. B cells (20,000 cells/well) (A) or DCs (5,000 cells/well) (B) were treated with soluble TTFC (5 ng/ml), S. gordonii strain GP1253 (at a bacterium-to-APC ratio of 50:1, corresponding to 5 ng of TTFC per ml), or strain GP1221 (at a bacterium-to-APC ratio of 50:1) and then cocultured for 3 days with specific CD4+ T cells (clone ALS4; 30,000 cells/well). Results are expressed as mean cpm and SD of triplicate cultures. Comparable results were obtained in three independent experiments. ∗, P < 0.003 compared to DCs not treated with antigen; ∗∗, P < 0.0002 compared to B cells or DCs incubated with control bacteria; ∗∗∗, P < 0.0001 compared to B cells treated with recombinant bacteria.
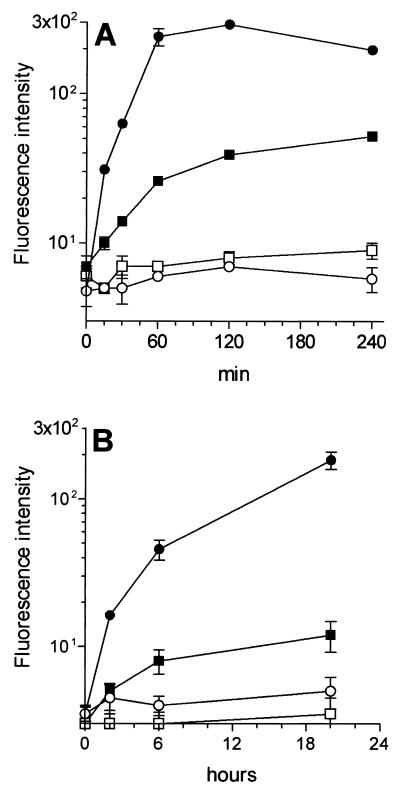
B cells are less endocytic and phagocytic than DCs and are not sensitive to gram-positive bacteria in terms of membrane activation.
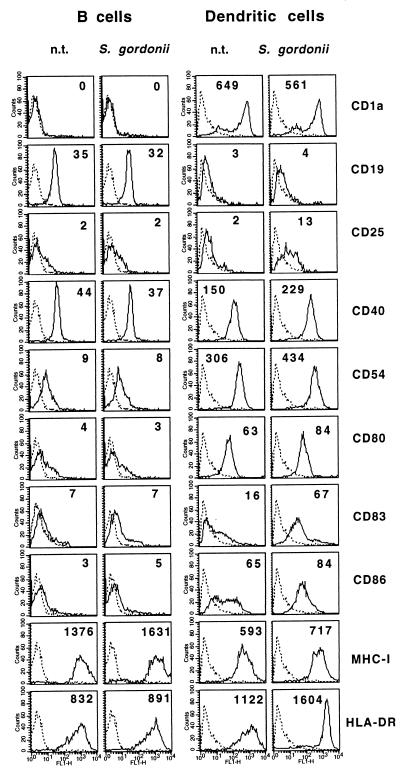
To identify the reasons why DCs were more efficient than B cells in presenting TTFC, they were compared for the capacity to take up both soluble antigen and recombinant bacteria. Cells were incubated at 37°C with FITC-conjugated TTFC or with PKH-26-labeled bacteria and, at selected time points, analyzed by flow cytometry. As shown in Fig. 4, B cells were much less capable than DCs at internalizing TTFC or S. gordonii, although B cells did uptake up a small amount of TTFC or a few bacteria, as confirmed by transmission electron microscopy (results not shown). These results are in agreement with those of previous studies performed with mouse or human B-cell lines (13, 28). Next, we wanted to see whether DCs and B cells were equally sensitive to S. gordonii in terms of cell activation, and so we examined changes in the expression of cell membrane molecules following incubation with bacteria. As shown in Fig. 5, resting B cells (>95% CD19+) showed a weaker expression of CD40, CD54, CD80, and CD86 and MHC class II than did immature DCs. In contrast to DCs, B cells that were incubated for 18 to 48 h with S. gordonii did not show significant changes in membrane expression of CD25, CD40, CD54, CD80, or CD86, although they displayed a modest but consistent induction of MHC molecules. Moreover, no B-cell proliferative response was observed after treatment with S. gordonii. Upregulation of selected activation markers (CD25, CD40, CD54, and HLA-DR) and proliferation were instead induced when B cells were treated with pokeweed mitogen or lipopolysaccharide (data not shown). Finally, S. gordonii promoted high tumor necrosis factor alpha release from DCs but not from B cells (data not shown). These results indicated that S. gordonii is a potent inducer of DC but not B-cell maturation.
FIG. 4.
B cells display poor endocytic and phagocytic capacities compared to DCs. DCs (solid circle) or B cells (solid square) were incubated with 1 mg of FITC-conjugated TTFC per ml (A) or with PKH-26-labeled S. gordonii (bacterium-to-cell ratio, 50:1) (B) at 37°C. At the indicated time points, uptake was stopped and cells were analyzed by flow cytometry. Data are expressed as mean fluorescence intensity and SD of data collected from four distinct experiments. The values measured at 37°C were adjusted by subtraction of those measured at 4°C (open symbols). Differences in both endocytosis and phagocytosis between DC and B cells were statistically significant (P < 0.002) at each time points after time zero.
FIG. 5.
S. gordonii induces membrane maturation of DCs but not of B cells. APCs were incubated with S. gordonii at bacterium-to-APC ratio of 50:1 or left untreated (n.t.). After 48 h, the cells were stained for the indicated membrane markers and then analyzed by flow cytometry. The numbers indicate the mean fluorescence intensity adjusted by subtraction of the fluorescence by control matched-isotype Ab (dotted lines). Similar results were measured in seven independent experiments.
DISCUSSION
In this study, we have analyzed the ability of DCs and B lymphocytes to present an MHC class II-restricted antigen expressed on recombinant S. gordonii to specific Th1 cells. Purified populations of DCs and B cells were used to avoid the influence of contaminating cells. The results indicated that DCs were markedly more efficient than non-antigen-specific B cells at phagocytosing bacteria and presenting bacterium-associated TTFC to CD4+ T lymphocytes. DCs also displayed a higher capacity to endocytose soluble TTFC and present soluble antigen to T cells. However, differences between DCs and B cells in both antigen uptake and T-cell activation were more prominent when particulate antigen was used rather than soluble antigen. At any rate, bacterium-associated antigen was also presented with higher efficiency than soluble antigen by B cells, indicating that phagocytosis represents a very effective mechanism for providing antigens to the MHC class II processing pathway in different APC types (8, 12, 31, 32). Moreover, S. gordonii enhanced the expression of presenting and costimulatory molecules in DCs but not in B cells, suggesting that recombinant gram-positive bacteria, as well as providing an effective antigen delivery system, can act as strong adjuvants by stimulating primarily DC maturation (3). Another important difference between DCs and B cells is that, in contrast to DCs, B cells have minimal or no ability to produce IL-12 in response to stimulation with gram-positive bacteria or upon CD40/CD40L-dependent interaction with T cells (1, 3, 10) and may therefore favor the differentiation of Th2 rather than Th1 lymphocytes.
DCs have been repeatedly shown to be crucial for activation of naive T cells and successful immunization in mice, whereas B cells fail to show significant activity under normal circumstances (2, 4, 9, 14, 20). DCs and B cells perform distinct functions in the immune system (1, 19, 34). DCs activate T helper cells, which in turn induce B-cell growth and antibody production, and thus both DCs and B cells are very important for antibody responses. In addition, DCs activate and expand other effector T-cell populations, including cytotoxic T cells (1). On the other hand, B cells armed with specific Ig on the cell surface can effectively present antigen to T cells, albeit with a lower potency than that of DCs, and thus contribute to the magnitude and diversity of T-cell responses (33). DCs are strategically located in peripheral tissues for encounters with bacteria (1), display a broader array of innate recognition systems for bacteria than do B cells (18, 22, 24, 29), have a different sensitivity to bacterium-induced activation (10), and are the APCs most relevant for induction of primary immune responses (1). A major attribute of DCs is that they are mobile APCs, with the capacity to pick up incoming foreign antigens in the periphery and migrate to lymphoid tissue, thus rendering the antigens visible to the immune system (1, 34). In conclusion, the results of this study suggest that DCs, but not B cells, are the target APCs for the vaccination procedures which employ recombinant bacteria.
ACKNOWLEDGMENTS
This work was supported by the Associazione Italiana per la Ricerca sul Cancro, the European Community (BIOMED 2 contract BMH4 CT98-3713; BIOTECH contracts BIO2 CT94-3055 and BIO4 CT96-0542), the Istituto Superiore di Sanità (AIDS project, contracts 40A.0.83 and 40B/1.18), and the CNR (P. F. Biotecnologie, contract 97.01185.PF49).
REFERENCES
- 1.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Cassell D J, Schwartz R H. A quantitative analysis of antigen-presenting cell function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J Exp Med. 1994;180:1829–1840. doi: 10.1084/jem.180.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corinti S, Medaglini D, Cavani A, Rescigno M, Pozzi G, Ricciardi-Castagnoli P, Girolomoni G. Human dendritic cells present very efficiently a heterologous protein antigen expressed on the surface of recombinant Gram-positive bacteria to CD4+ T lymphocytes. J Immunol. 1999;163:3029–3036. [PubMed] [Google Scholar]
- 4.Croft M, Duncan D D, Swain S L. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992;176:1431–1437. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delon J, Bercovici N, Raposo G, Liblau R, Trautmann A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J Exp Med. 1998;188:1473–1484. doi: 10.1084/jem.188.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Simm A, Catic A, Kaufmann S H E, Hess J, Szalay A A, Goebel W. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 7.Di Fabio S, Medaglini D, Rush C M, Corrias F, Panzini G L, Pace M, Verani P, Pozzi G, Titti F. Vaginal immunization of cynomolgus monkeys with Streptococcus gordonii expressing HIV-1 and HPV-6 antigens. Vaccine. 1998;16:485–492. doi: 10.1016/s0264-410x(97)80002-3. [DOI] [PubMed] [Google Scholar]
- 8.Gengoux C, Leclerc C. In vivo induction of CD4+ T cell responses by antigens covalently linked to synthetic microspheres does not require adjuvant. Int Immunol. 1995;7:45–53. doi: 10.1093/intimm/7.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Guery J C, Ria F, Adorini L. Dendritic cells but not B cells present antigenic complexes to class II-restricted T cells after administration of proteins in adjuvant. J Exp Med. 1996;183:751–757. doi: 10.1084/jem.183.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guery J C, Ria F, Galbiati F, Adorini L. Normal B cells fail to secrete interleukin-12. Eur J Immunol. 1997;27:1632–1639. doi: 10.1002/eji.1830270707. [DOI] [PubMed] [Google Scholar]
- 11.Guzmán C A, Saverino D, Madina E, Fenoglio D, Gerstel B, Merlo A, Li Pira G, Buffa F, Chakraborty T, Manca F. Attenuated Listeria monocytogenes carrier strains can deliver an HIV gp120 T helper epitope to MHC class II-restricted human CD4+ T cells. Eur J Immunol. 1998;28:1807–1814. doi: 10.1002/(SICI)1521-4141(199806)28:06<1807::AID-IMMU1807>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Inaba K, Turley S, Yamaide F, Yyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman R M. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz M B, Aßmann C U, Girolomoni G, Ricciardi-Castagnoli P. Different cytokines regulate antigen uptake and presentation of a precursor dendritic cell line. Eur J Immunol. 1996;26:586–594. doi: 10.1002/eji.1830260313. [DOI] [PubMed] [Google Scholar]
- 14.Masten B J, Lipscomb M F. Comparison of lung dendritic cells and B cells in stimulating naive antigen-specific T cells. J Immunol. 1999;162:1310–1317. [PubMed] [Google Scholar]
- 15.Medaglini D, Pozzi G, King T P, Fischetti V A. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc Natl Acad Sci USA. 1995;92:6868–6872. doi: 10.1073/pnas.92.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medaglini D, Rush C M, Sestini P, Pozzi G. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted disease: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine. 1997;15:1330–1337. doi: 10.1016/s0264-410x(97)00026-1. [DOI] [PubMed] [Google Scholar]
- 17.Medina E, Guzman C A, Staendner L H, Colombo M P, Paglia P. Salmonella vaccine carrier strains: effective delivery system to trigger anti-tumor immunity by oral route. Eur J Immunol. 1999;29:693–699. doi: 10.1002/(SICI)1521-4141(199902)29:02<693::AID-IMMU693>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 18.Medzhitov R, Janeway C A., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 19.Metlay J P, Puré E, Steinman R M. Control of the immune response at the level of antigen-presenting cells: a comparison of the function of dendritic cells and B lymphocytes. Adv Immunol. 1989;47:45–116. doi: 10.1016/s0065-2776(08)60662-8. [DOI] [PubMed] [Google Scholar]
- 20.Metlay J P, Puré E, Steinman R M. Distinct features of dendritic cells and anti-Ig activated B cells as stimulators of the primary mixed leukocyte reaction. J Exp Med. 1989;169:239–254. doi: 10.1084/jem.169.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oggioni M R, Pozzi G. A host-vector system for heterologous gene expression in Streptococcus gordonii. Gene. 1996;169:85–90. doi: 10.1016/0378-1119(95)00775-x. [DOI] [PubMed] [Google Scholar]
- 22.Okada N, Pentland A P, Falk P, Caparon M G. M protein and protein F act as important determinants of cell-specific tropism of Streptococcus pyogenes in skin tissue. J Clin Investig. 1994;94:965–977. doi: 10.1172/JCI117463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pancholi P, Steinman R M, Bhardwaj N. Dendritic cells efficiently immunoselect mycobacterial-reactive T cells in human blood, including clonable antigen-reactive precursors. Immunology. 1992;76:217–224. [PMC free article] [PubMed] [Google Scholar]
- 24.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–399. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 25.Rescigno M, Citterio S, Thèry C, Rittig M, Medaglini D, Pozzi G, Amigorena S, Ricciardi-Castagnoli P. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc Natl Acad Sci USA. 1998;95:5229–5234. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rüssmann H, Shams H, Poblete F, Fu Y, Galán J E, Donis R O. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281:565–568. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serre K, Machy P, Grivel J-C, Jolly G, Brun N, Barbet J, Leserman L. Efficient presentation of multivalent antigens targeted to various cell surface molecules of dendritic cells and surface Ig of antigen-specific B cells. J Immunol. 1998;161:6059–6067. [PubMed] [Google Scholar]
- 30.Sizemore D, Branstrom A, Sadoff J. Attenuated bacteria as DNA delivery vehicle for DNA-mediated immunization. Vaccine. 1997;15:804–807. doi: 10.1016/s0264-410x(96)00252-6. [DOI] [PubMed] [Google Scholar]
- 31.Svensson M, Stockinger B, Wick M J. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- 32.Vidard L, Kovacsovics-Bankowski M, Kraeft S-K, Chen L B, Benacerraf B, Rock K L. Analysis of MHC class II presentation of particulate antigens by B lymphocytes. J Immunol. 1996;156:2809–2818. [PubMed] [Google Scholar]
- 33.Watts C, Lanzavecchia A. Suppressive effect of antibody on processing of T cell epitopes. J Exp Med. 1993;178:1459–1463. doi: 10.1084/jem.178.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]