Abstract
Immunization of cattle with native MSP1 induces protection against Anaplasma marginale. The native immunogen is composed of a single MSP1a protein and multiple, undefined MSP1b polypeptides. In addition to the originally sequenced gene, designated msp1β(F1), we identified three complete msp1β genes in the Florida strain: msp1β(F2), msp1β(F3), and msp1β(F4). Each of these polymorphic genes encodes a structurally unique MSP1b protein, and unique transcripts can be identified during acute A. marginale rickettsemia. The structural polymorphism is clustered in discrete variable regions, and each MSP1b protein results from a unique mosaic of five variable regions. Although each of the MSP1b proteins in the Florida strain contains epitopes recognized by serum antibody induced by protective immunization with the native MSP1 complex, the variable regions also include epitopes expressed by some but not all of the MSP1b proteins. These data support testing recombinant vaccines composed of the multiple antigenically and structurally unique MSP1b proteins combined with MSP1a in order to mimic the efficacy of native MSP1 immunization.
Anaplasma marginale is a tick-transmitted rickettsial pathogen of cattle, classified within erhrlichial genogroup II, that causes significant morbidity and mortality in tropical and subtropical regions worldwide (15, 31). Following transmission, A. marginale invades and replicates in mature erythrocytes. Sequential rounds of invasion and replication result in a high level of rickettsemia (≥109/ml of blood) and consequent severe anemia, weight loss, abortion, and death (15). Individuals that survive acute rickettsemia remain persistently infected and serve as reservoirs for tick transmission to susceptible cattle (9).
A. marginale infection occurs in temperate and subtropical climates but is most prevalent in tropical regions (15). A recent study of cattle in northern Veracruz State in Mexico identified 69% of cattle as being rickettsemic (7), and similar infection rates of 73 and 78% have been reported for cattle in St. Lucia (13) and El Salvador (25), respectively. This high prevalence is associated with significant rates of transmission; 26% of total cattle deaths in Mexico during 1995 were due to the movement of susceptible cattle into high-prevalence areas and subsequent A. marginale transmission (12). Consequently, there is an acute need for a safe and effective vaccine.
Immunization with A. marginale outer membranes induces protection against challenge, and this immunity correlates with the titer of antibody to the major surface proteins (MSPs) (24, 29). Antibody specific for MSP1 blocks the binding of A. marginale to erythrocytes (16, 17) and opsonizes live organisms for macrophage phagocytosis (6). Immunization of cattle with native purified MSP1, a heteromeric complex of MSP1a and MSP1b (MSP1a/b) (5, 30), confers protection against acute rickettsemia and disease (20, 21). As a result MSP1 has been investigated for recombinant vaccine development. However, unlike the results obtained using the native MSP1a/b complex, immunization with recombinant MSP1a, MSP1b, or the combination of these two proteins has not induced significant protection (23). MSP1a is encoded by a single gene copy and is invariant within a strain (4). In contrast, MSP1b is proposed to be encoded by a multigene family, since four partially homologous msp1β copies have been detected in the Florida strain using restriction enzyme and Southern blot analysis (5). The recombinant MSP1b used in the unsuccessful immunization trials was expressed from the only msp1β gene copy sequenced to date (5), now designated msp1β(F1) to indicate its derivation from the Florida strain. Whether additional complete msp1β copies are present in the genome and are expressed by the organism or, alternatively, are pseudogenes has not been previously addressed. If these other gene copies encode unique MSP1b proteins that are expressed by A. marginale, this may explain why immunization with a single copy of MSP1b combined with MSP1a fails to induce the same level of protection as that afforded by the native MSP1a/b complex. In this paper, we report the cloning and sequencing of these additional gene copies, analysis of their polymorphic regions, expression of the unique transcripts during acute A. marginale rickettsemia, and the binding of antibody from protectively immunized cattle to each expressed MSP1b variant.
MATERIALS AND METHODS
Cloning and sequencing of genomic copies of msp1β.
Genomic DNA was extracted from stabilates of the Florida and Havana, Cuba, strains of A. marginale by using a Puregene (Gentra) DNA extraction kit. Gene copies of msp1β were amplified using forward and reverse primers derived from the 5′ and 3′ ends, respectively, of the previously cloned Florida strain msp1β(F1) copy (5). The sequence of the forward primer was 5′-ATGACAGAAGACGACAAGCAACAACA, and that of the reverse primer was 5′-TTACCTAGACCAACCAGAAGACTG. Amplification using Pwo DNA polymerase (Boehringer Mannheim), ligation of the 2.2-kb amplicons into pCR-Blunt (Invitrogen), and transformation of Escherichia coli One Shot were done as previously described (10). The presence of inserts in plasmids from transformed colonies was confirmed by restriction digestion using EcoRI or by PCR using primers derived from the flanking regions. Insert DNA was sequenced in both directions by using an ABI PRISM (Applied Biosystems) automated sequencer. Sequence analysis was performed on a VAX11/785 computer, using the Genetics Computer Group package from the University of Wisconsin, Madison, according to the instructions in the manual.
Detection of specific msp1β transcripts.
Calf 97B37 was inoculated with the Florida strain of A. marginale and developed acute rickettsemia characterized by >109 organisms per ml of blood (69% infected erythrocytes). Total RNA was extracted from whole blood obtained at the peak level of acute rickettsemia using TRIzol (BRL) and then reverse transcribed with random hexamers, as previously described in detail (8, 11). To identify specific msp1β gene copy transcripts, the cDNA was amplified using msp1β-specific primer sets and then sequenced. The primer sets used to amplify msp1β(F2) cDNA were as follows: forward primer, 5′-CGGGATCCGAAGACCATCGTCAGCG; reverse primer, 5′-CGGGATCCGTACTGCTGCAAGTAAG. The primer sets for amplification of msp1β(F3) cDNA were as follows: forward primer, 5′-GCCCAGAAACGATATATGC; reverse primer, 5′-GGGATCCGTTACCTAGACCAACCAGA. Amplification using Pwo polymerase, ligation, transformation, sequencing, and sequence analysis were done as described above.
Expression of variant MSP1b proteins.
The proteins encoded by the polymorphic copies of msp1β in the Florida strain were expressed as His-tagged fusion proteins. Full-length msp1β(F2), msp1β(F3), and msp1β(F4) were subcloned from plasmids containing the individual gene copies into pET19b (Novagen). The primers used for subcloning were identical to those used in the initial cloning from genomic DNA (sequences provided above), with the exception that BamHI sites were incorporated into each primer. Following PCR amplification, the individual amplicons were digested with BamHI and ligated in-frame into BamHI-digested and dephosphorylated pET19b. Competent E. coli XL-1 Blue cells were transformed with the ligated vector. Plasmids with inserts in the correct orientation were selected following analysis by restriction enzyme digestion and confirmation by sequencing the vector-insert junction. These plasmids were designated pET(F2), pET(F3), and pET(F4) and were then used to transform competent E. coli BL21(DE3) cells. The expressed MSP1bF2, -F3, and -F4 His-tagged fusion proteins were purified on Ni2+-charged columns under denaturing conditions as recommended by the manufacturer (Novagen), but the procedure was modified by adding imidazole in the wash buffer (0.5 M NaCl, 20 mM Tris [pH 7.9], 80 mM imidazole) to minimize nonspecific binding of proteins to the column (11). Eluted protein fractions were dialyzed against phosphate-buffered saline (PBS) for 48 h at 4°C. Protein fractions were then separated by electrophoresis on a sodium dodecyl sulfate (SDS)-containing polyacrylamide gel, and the purity of the eluted proteins was examined by silver staining (27).
Reactivity of anti-native MSP1a/b complex antibody with variant MSP1b proteins.
Native MSP1a/b complex was purified from the Florida strain of A. marginale by affinity chromatography using monoclonal antibody ANAF15D2, as previously described in detail (20, 21). Prior to immunization, age-matched male holstein calves were shown to be seronegative for A. marginale and to be negative when Giemsa-stained blood smears were examined microscopically (20). Five calves were immunized subcutaneously four times, at 3-week intervals, with 500 μg of native MSP1a/b in 6 mg of saponin. One of the immunized calves died from an unrelated cause prior to challenge. Five control calves were immunized on the identical schedule but with saponin alone. Prior to challenge, titers of antibody against native MSP1a/b in serum were determined using a previously described enzyme-linked immunosorbent assay (ELISA) (21). All nine calves were challenged by intravenous inoculation of 105 erythrocytes infected with live A. marginale Florida strain. Development of acute rickettsemia was monitored by daily microscopic examination of blood smears and quantified by determining the percentage of infected erythrocytes.
Serum collected after MSP1a/b immunization but prior to challenge was tested for ability to bind each of the His-tagged MSP1b fusion proteins using immunoblots. Briefly, 3.5 μg of each fusion protein was electrophoresed in a 7.5 to 17.5% polyacrylamide gel containing SDS, transferred to nitrocellulose, and then incubated with a 1:2,000 dilution of serum from immunized calf 541 (10). Bound antibody was detected using a 1:20,000 dilution of peroxidase-conjugated protein G followed by enhanced chemiluminescence. A His-tagged Babesia bigemina RAP-1 fusion protein, expressed using the same plasmid vector and E. coli host strain and purified using identical conditions, was used as a negative antigen control. Preimmunization serum from calf 541 and postimmunization serum from calf 535, inoculated only with saponin, were used as negative antibody controls.
Detection of MSP1b variant-specific B-cell epitopes.
Mice were immunized subcutaneously with 10 μg of purified recombinant His-tagged MSP1bF2 or -F3 emulsified in Freund's complete adjuvant. Three booster immunizations using 10 μg of each recombinant protein in Freund's incomplete adjuvant were given at approximately 3-week intervals. To detect variant-specific antibody, serum from a mouse immunized with MSP1bF2 was adsorbed against MSP1bF3. For adsorption, 5 μg of MSP1bF3 was electrophoresed on an SDS-containing polyacrylamide gel, transferred to nitrocellulose, and incubated for 1 h at room temperature with anti-MSP1bF2 serum diluted 1:80,000 in TNT (0.01 M Tris, 0.067 M NaCl, 0.05% Tween 20 [pH 8.0]) containing 3% bovine serum albumin. Adsorption was repeated until there was no reactivity with MSP1bF3, and the adsorbed serum was then retested for remaining antibody specific for MSP1bF2 using immunoblots. Detection used a 1:5,000 dilution of goat anti-murine immunoglobulin and enhanced chemiluminescence, as described elsewhere (11). This procedure was repeated using MSP1bF3 as the immunogen, adsorbing anti-MSP1bF3 serum against MSP1bF2, and then testing the adsorbed serum for specific binding to MSP1bF3.
A second approach used immunization of mice with recombinant polypeptides representing variable region 4 (VR4) of either MSP1bF2 or MSP1bF3 and testing whether the induced antibody bound only to those full-length MSP1b proteins containing the homologous VR4. The VR4s were amplified from the individual full-length clones of both msp1β(F2) and msp1β(F3) using conserved primers that flanked the variable region. These primers, which included BamHI restriction sites (forward, 5′-CGGGATCCGAAGACCATCGTCAGCG; reverse, 5′-CGGGATCCGTACTGCTGCAAGTAAG), directed amplification of the region encoding amino acids 502 to 629 in MSP1bF2 and 495 to 607 in MSP1bF3. The amplicons were digested with BamHI and ligated in-frame into pET19b. Transformation, confirmation of the correct open reading frame by sequencing, purification of the His-tagged MSP1bF2 VR4 and MSP1bF3 VR4 on Ni2+-charged columns, and immunization of mice were carried out as described for the full-length fusion proteins described above. The anti-MSP1bF2 VR4 and anti-MSP1bF3 VR4 sera were tested at a 1:2,000 dilution for binding to the full-length recombinant MSP1bF2, -F3, and -F4 using immunoblots as described above. B. bigemina RAP-1 was used as a negative antigen control, and normal mouse serum was used as a negative antibody control.
Nucleotide sequence accession numbers.
The nucleotide sequences have been deposited in GenBank with the following accession numbers: AF110808 for msp1β(F2), AF110809 for msp1β(F3), AF110810 for msp1β(F4), AF112479 for msp1β(C1), and AF112480 for msp1β(C2).
RESULTS
Polymorphism in genomic copies of msp1β.
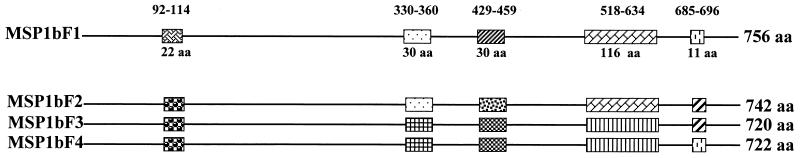
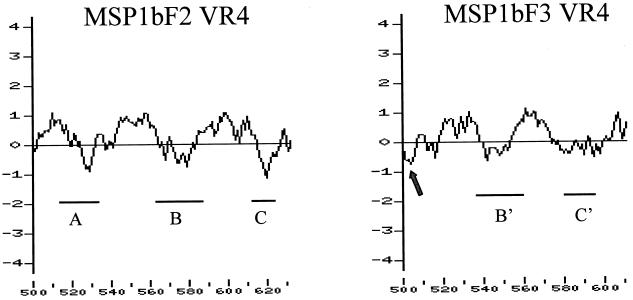
Three unique genomic copies of msp1β were newly identified in the Florida strain: msp1β(F2), msp1β(F3), and msp1β(F4). Each msp1β gene has a unique sequence compared to that of the originally described gene, designated msp1β(F1), and varies in the size of the open reading frame, ranging from 2,160 to 2,226 bp. This size polymorphism reflects the occurrence of nucleotide deletions and insertions in individual members of this gene family. The start of the open reading frame is identical in all copies and is the same as that reported in the original msp1β(F1) gene copy (5). Sequence alignment of the encoded MSP1b proteins from the Florida strain revealed five major regions of sequence polymorphism separated by large blocks of conserved amino acid sequence (Fig. 1 and 2). The variable regions, characterized by amino acid insertions, deletions, and substitutions, are as follows: VR1, amino acids 92 to 114 (numbering is based on MSP1bF1); VR2, 330 to 360; VR3, 429 to 459; VR4, 518 to 634; and VR5, 686 to 696 (Fig. 1 and 2). Although individual variable regions are shared among two or more of the Florida strain variants (for example, VR1 is identical in MSP1bF2, -F3, and -F4, while VR3 is identical in MSP1bF1, -F3, and -F4), each MSP1b protein is composed of a unique set of the five variable regions. Consequently, the identity between the encoded MSP1b proteins ranges from 79%, between MSP1bF2 and -F4, to 96%, between MSP1bF3 and -F4. The variation between the closely related MSP1bF3 and -F4 is limited to VR5 and the combination of substitutions and a short deletion at the extreme carboxyl ends of the proteins (Fig. 1 and 2). Analysis of hydrophilicity using a sliding window length of 17 amino acids (14) revealed that VR1, VR3, and VR5 are located in amphipathic domains, while VR2 is within the most hydrophilic domain of the full-length protein (data not shown). In contrast, VR4 is composed of alternating hydrophilic and hydrophobic domains (Fig. 3). Interestingly, comparison of the two forms of VR4, represented by MSP1bF2 and -F3, revealed that the domains that are most hydrophilic, and thus most likely to be surface exposed, include the variable oligopeptides, while the hydrophobic domains are conserved (Fig. 3).
FIG. 1.
Map of conserved and variable regions encoded by full-length msp1β genes in the Florida strain of A. marginale. Line, conserved regions; boxes, major variable regions. The position and length of each major variable region is indicated, respectively, above and below the map of MSP1bF1. Variable regions shared among MSP1b proteins are indicated by the same pattern within the boxes. The number of amino acids in each protein is given at the right.
FIG. 2.
Amino acid sequence alignment of the MSP1b proteins encoded by the Florida strain (designated MSP1bF1 through -F4) and the Havana, Cuba, strain (designated MSP1bC1 and -C2). Areas of amino acid substitutions, insertions, and deletions are indicated by a white background, and conserved substitutions have a shaded background. Identical amino acids are represented by white letters on a black background. Dots indicate deletions.
FIG. 3.
Hydrophobicity/hydrophilicity profile and sequence variation in VR4 encoded by msp1β(F2) or msp1β(F3). The profile was calculated using the Kyte-Doolittle method (14) over a sliding window of 17 amino acids for the full-length MSP1bF2 and -F3. Relatively hydrophobic and hydrophilic domains are, respectively, above and below the x axis. The VR4 of MSP1bF2 (left panel) is shown with the positions of the three stretches of variant-specific oligopeptides indicated by lines labeled A, B, and C. The VR4 of MSP1bF3 (right panel) is shown with the positions of its variant-specific oligopeptides indicated by B′ and C′. There is no counterpart of the A stretch in MSP1bF3, because there is a deletion relative to MSP1bF2. The position of the deletion is indicated by the arrow.
Only two unique msp1β copies, designated msp1β(C1) and msp1β(C2), were identified in the Havana, Cuba, strain. Sequencing of additional independent clones revealed only the identical msp1β(C1) and msp1β(C2) sequences. The proteins encoded by msp1β(C1) and msp1β(C2) are 82% identical, with the polymorphism occurring in VR1, VR2, VR4, and VR5 (Fig. 2). The VR3s in the two copies of MSP1b in the Havana strain are very similar, with differences limited to amino acid substitutions at two positions (amino acids 436 and 437 in MSP1bC1 [Fig. 2]). None of the genomic or predicted protein sequences are identical between the Havana and Florida strains. Overall, the highest identity in MSP1b protein sequences between strains is found for MSP1bC1 and -F4 and for MSP1bC2 and -F2 (both 90%). The only variable region that is shared between strains is VR2, which is identical among MSP1bC2, MSP1bF1, and MSP1bF2. The VR1, VR3, VR4, and VR5 regions in both MSP1bC1 and MSP1bC2 are unique compared to those of each of the four Florida strain MSP1b variants due to individual substitutions and relatively small insertions or deletions encoding as many as 7 amino acids (Fig. 2). Notably, the region from the start of VR5 to the carboxyl end in MSP1bC1 and -C2 are mosaics of the two different VR5 sequences identified in the Florida strain. For example, 13 of the 17 variable amino acids in the MSP1bC2 sequence spanning amino acids 668 to 726 are identical to those contained in the VR5 shared by Florida strain MSP1bF2 and -F3, 3 are identical to those of the VR5 in MSP1bF1 and -F4, and 1 substitution is unique to MSP1bC2 (Fig. 2). In contrast, in the same region in MSP1bC1, represented by amino acids 649 to 707, 9 of the 22 variable amino acids are identical to those of the VR5 in MSP1bF1 and -F4, 6 are identical to those of the VR5 in MSP1bF2 and -F3, and 7 are unique (Fig. 2).
Polymorphic msp1β genes are transcribed during acute rickettsemia.
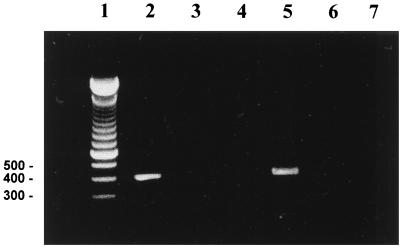
The msp1β(F1) copy had previously been shown to be transcribed in acute rickettsemia (5). To determine if multiple polymorphic msp1β transcripts could be expressed, copy-specific primer sets were used to amplify cDNA derived by reverse transcription of blood obtained from calf 97B37 during acute Florida strain rickettsemia. Transcripts from msp1β(F2) and msp1β(F3) were targeted because these differ in the largest variable region, VR4, and could be amplified using primers specific for the individual gene. In contrast, msp1β(F4) is 96% identical to msp1β(F3) and has no sequences that are not also present in msp1β(F3) or msp1β(F1). The MSP1bF2 primers were selected from the sequences flanking the msp1β(F2) VR4 region and were expected to amplify a fragment from nucleotide 1503 to 1884. The resulting amplicon had the predicted size of 381 bp (Fig. 4). The cDNA sequence was 100% identical with the MSP1bF2 genomic sequence. Sequences of four additional, independently derived cDNA clones were also identical to the MSP1bF2 genomic sequence (data not shown). Similarly, MSP1bF3 primers were selected to amplify across part of msp1β(F3) VR4 and all of VR5, from nucleotides 1723 to 2160. The resulting amplicon had the predicted size of 437 bp (Fig. 4). Ten independently derived cDNA clones were randomly selected and sequenced. Multiple alignment of all MSP1bF3 cDNA clones showed no changes among them and 100% identity with the MSP1bF3 genomic copy of msp1β.
FIG. 4.
Reverse transcriptase PCR (RT-PCR) of total RNA obtained during acute A. marginale rickettsemia and amplified using primers specific for msp1β(F2) or msp1β(F3). Primers specific for msp1β(F2) were used in lanes 2 to 4, and msp1β(F3) primers were used in lanes 5 to 7. Products from RT-PCR (lanes 2 and 5), PCR without reverse transcriptase as a control for amplification of contaminating DNA (lanes 3 and 6), and RT-PCR without RNA as a template control (lanes 4 and 7) were detected using ethidium bromide staining and agarose gel electrophoresis. Lane 1, molecular size markers. Sizes (in base pairs) are shown on the left.
MSP1b variants contain epitopes bound by antibodies induced by protective MSP1a/b immunization.
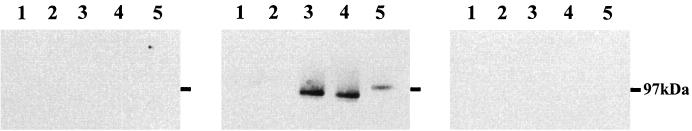
The calves immunized with the native Florida strain MSP1a/b all developed high titers of antibody to the immunogen in serum (ELISA titer range, 103 to 105), while all control calves, inoculated with adjuvant alone on the identical schedule, had no detectable antibody at the lowest dilution tested, 1:100. Upon challenge with the Florida strain, the MSP1a/b-immunized group developed a mean peak rickettsemia of 1.3 ± 2.4% infected erythrocytes. Two of the immunized calves, calves 541 and 537, did not develop any microscopically detectable rickettsemia during the 75-day postchallenge observation period. In contrast, all control calves developed rickettsemia, with a group mean of 4.7 ± 2.5% infected erythrocytes. The rickettsemia in the MSP1a/b-immunized calves was significantly lower (P ≤ 0.05) compared to that in the control group using the one-tailed t test for comparison of means with unequal variances (28). Whether each of the Florida strain MSP1b variants contained epitopes bound by serum antibody induced by native MSP1a/b immunization was tested using serum from calf 541 in Western blots. This calf had the highest ELISA titer, 105, to the native MSP1a/b. The His-tagged recombinant MSP1bF2, -F3, and -F4 proteins were individually isolated using Ni2+-charged affinity columns, and the purity was analyzed by silver staining of polyacrylamide gels (Fig. 5). Serum from calf 541 bound each of the MSP1b variants (Fig. 6, center panel). In contrast, there was no binding of either preimmunization serum from calf 541 (Fig. 6, right panel) or serum from calf 535, immunized with adjuvant alone (Fig. 6, left panel).
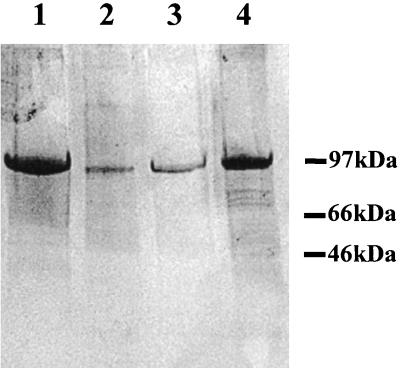
FIG. 5.
Purification of His-tagged MSP1b fusion proteins. MSP1bF2 (lane 1), MSP1bF3 (lanes 2 and 3), and MSP1bF4 (lane 4) were electrophoresed on SDS-containing polyacrylamide gels and detected using silver staining (27). Lanes 2 and 3 represent different batches of purified MSP1bF3. Positions of molecular size markers are indicated in the right margin.
FIG. 6.
Binding of serum antibody induced by protective MSP1a/b immunization to Florida strain MSP1b variants. Purified MSP1bF2 (lanes 5), MSP1bF3 (lanes 4), MSP1bF4 (lanes 3), and, as a negative antigen control, B. bigemina RAP-1 (lanes 1) were electrophoresed on SDS-containing polyacrylamide gels and transferred to nitrocellulose. Lane 2 in each gel was unloaded. Membranes were reacted with a 1:2,000 dilution of serum from calf 541 prior to MSP1a/b immunization (right panel), calf 541 after MSP1a/b immunization (center panel), or calf 535, immunized with saponin alone (left panel). The position of the 97-kDa molecular size marker is indicated by a dash to the right of each panel.
Detection of MSP1b variant-specific B-cell epitopes.
MSP1bF2 and MSP1bF3 differ markedly in VR2, VR3, and VR4 (Fig. 1 and 2). To detect MSP1bF2-specific epitopes, serum from a mouse immunized with purified recombinant MSP1bF2 was adsorbed with MSP1bF3. The preadsorption sera bound recombinant MSP1bF2 and MSP1bF3; however, complete adsorption of the MSP1bF2 antiserum with MSP1bF3 also ablated detectable binding to MSP1bF2 (data not shown). A similar result was obtained using anti-MSP1bF3 serum adsorbed with MSP1bF2 and then retested for binding to MSP1bF3 (data not shown).
A second approach for detection of MSP1b variant-specific epitopes, using immunization of mice with recombinant VR4 derived from either MSP1bF2 or MSP1bF3, was then tested. None of the three mice immunized with the MSP1bF2 VR4 developed specific antibody that bound the full-length homologous MSP1bF2 (data not shown). In contrast, two of the three mice immunized with the MSP1bF3 VR4 developed specific antibody. This serum antibody bound full-length MSP1bF3 and -F4, which contain the identical VR4 (Fig. 1 and 2), but not MSP1bF2, which contains a different VR4, or the negative control, His-tagged recombinant B. bigemina protein (Fig. 7). Normal mouse serum did not react with any of the MSP1b variants or the His-tagged B. bigemina protein (Fig. 7).
FIG. 7.
Anti-VR4 serum antibody binds only MSP1b proteins bearing the homologous VR4. Purified MSP1bF2 (lanes 4), MSP1bF3 (lanes 3), and MSP1bF4 (lanes 2), and, as a negative antigen control, B. bigemina RAP-1 (lane 1) were electrophoresed on SDS-containing polyacrylamide gels and then transferred to nitrocellulose. Membranes were reacted with a 1:1,000 dilution of serum from a mouse immunized with the VR4 region of MSP1bF3 (left panel) or normal mouse serum (right panel). Positions of molecular size markers are given in the right margins.
DISCUSSION
We have identified three complete msp1β genes in the Florida strain, in addition to the originally sequenced msp1β(F1) (5), and shown that each of these encodes a unique MSP1b protein. Both msp1β(F2) and msp1β(F3) were shown to be transcribed in acute A. marginale rickettsemia (Fig. 4), and msp1β(F1) specific transcripts have been previously identified in the acute stage (5). Thus, the msp1β genes appear to constitute a true multigene family as opposed to a single msp1β(F1) gene accompanied by related pseudogenes. Like the well-characterized A. marginale msp2 and msp3 gene families (3, 10, 22, 26), the msp1β polymorphism is clustered in discrete regions interspersed among highly conserved sequences. However unlike MSP2 and MSP3, identical variable regions are shared by more than one of the encoded MSP1b proteins (Fig. 1 and 2). As a result, each MSP1b variant may be seen as a unique mosaic of the five variable regions. The functional significance of the individual variable regions and how the presence of different combinations of variable regions may affect protein conformation and subsequent function are intriguing questions raised by the pattern of MSP1b polymorphism. Interestingly, VR2 and the polymorphic oligopeptides of VR4 are highly hydrophilic and are predicted to be surface exposed. This likely surface exposure, in combination with the previous identification of MSP1b as an adhesin (16, 17), suggests testing hypotheses regarding the role of these variable regions in binding to different erythrocyte receptors, as shown for erythrocyte-binding proteins encoded by multigene families in Plasmodium spp. (1, 2).
To date, msp1β genes have been sequenced only from the Florida and Havana strains examined here. Consequently, the ability to compare polymorphism among strains and to identify strain-specific regions is very limited. Nonetheless, the MSP1b structure of conserved regions and defined polymorphic regions appears to be maintained. The two variants, MSP1bC1 and -C2, identified in the Havana strain differed from each other in each of the five variable regions. This is in contrast to the Florida strain, in which none of the four variants differed from each other in all the variable regions. Thus, although only two variants were identified in the Havana strain, the overall degree of variation is quite similar to that represented by the four Florida strain MSP1b variants. Interestingly, the composition of VR5 and its flanking regions in both MSP1bC1 and -C2 is itself a mosaic of the two different VR5 sequences contained within the Florida strain. Although MSP1bC1 and -C2 have different sequences in this region, most of the individual VR5 substitutions in MSP1bC1 and -C2 match either those in the Florida strain MSP1bF1 and -F4 or those in MSP1bF2 and -F3. This occurrence of apparently limited allowable amino acid substitutions suggests functional constraints on the MSP1b sequence, even within the variable regions.
The polymorphism encoded in the variant MSP1b proteins provides the structural basis for dissecting the difference in induction of protective immunity between native MSP1a/b, which induces protection, and the mixture of recombinant MSP1a and the single recombinant MSP1bF1, which does not (20, 21, 23). In fact the first evidence for expression of more than a single MSP1b was the presence of multiple polypeptides, differing in apparent molecular size by 1 to 3 kDa, in the purified native Florida strain MSP1a/b (19). That observed variation in size is consistent with the differences in the predicted molecular sizes of the four variant Florida strain MSP1b proteins reported here. To determine if antibody from protectively immunized calves recognized each of the MSP1b variant proteins, we essentially replicated the initial experiments showing that purified native MSP1a/b induced protective immunity (20, 21). In the present experiment, the peak rickettsemia upon challenge in the MSP1a/b-immunized calves was significantly lower than that in adjuvant control calves and was consistent with that previously reported for native MSP1a/b immunization (20, 21). Serum antibody from calf 541, immunized with native MSP1a/b complex, bound all four Florida strain MSP1b copies (Fig. 6). This may reflect induction of antibodies to epitopes shared among all four MSP1b variants or, alternatively, antibodies to the variable regions expressed by each MSP1b variant. We were unable to detect MSP1b variant-specific antibody from calf 541 by adsorbing antibody against one variant and then retesting reactivity with a different variant (data not shown). However, this failure to detect variant-specific antibody may reflect the technical limitations of adsorption and detection using immunoblots that do not maintain the native conformation (18). We were similarly unable to detect variant-specific antibody using the same technique with serum antibodies induced by immunization of mice with purified recombinant MSP1bF2 and -F3, despite the extensive polymorphism occurring in VR2, VR3, and VR4 between the two variants (Fig. 1 and 2). In contrast, immunization of mice with the VR4 region from MSP1bF3 induced antibody that bound only those full-length MSP1b variants, MSP1bF3 and -F4, that contained the identical VR4 region. Interestingly, the variant-specific differences in VR4 occur within the hydrophilic domains that are predicted to be surface exposed. The importance of surface-reactive antibody in immunity to A. marginale (24) suggests that the VR4 structural and antigenic polymorphism may be relevant to MSP1a/b vaccine development.
While these data clearly establish that variant B-cell epitopes can be expressed by the unique MSP1b proteins, they leave several key questions unanswered regarding the role of MSP1b variant epitopes in protective immunity. First, what is the full spectrum of epitope variation encoded by the different variable regions? Second, are these epitopes recognized by cattle protectively immunized with native MSP1a/b? Third, is antibody binding to these variant epitopes required for induction of protective immunity? Fourth, does the variation in MSP1b observed between strains arise as a result of immune selection, and, if so, how rapidly is this variation generated? Using the information reported in the present article, these questions can now be addressed by immunization with unique combinations of MSP1a and MSP1b variants. These proposed experiments could provide substantial progress toward developing an effective recombinant vaccine against bovine anaplasmosis.
ACKNOWLEDGMENTS
We thank Siomara Martinez and Teresita Blandino for providing the DNA from the Havana, Cuba, strain and Beverly Hunter and Carla Robertson for technical assistance.
This work was supported by NIH R01 AI44005, USDA BARD US-2799-96C, and CONACyT grants 8.35 and E120.2714.
REFERENCES
- 1.Adams J H, Hudson D E, Torii M, Ward G E, Wellems T E, Aikawa M, Miller L H. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell. 1990;63:141–153. doi: 10.1016/0092-8674(90)90295-p. [DOI] [PubMed] [Google Scholar]
- 2.Adams J H, Sim B K L, Dolan S A, Fang X, Kaslow D C, Miller L H. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1992;89:7085–7089. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alleman A R, Palmer G H, McGuire T C, McElwain T F, Perryman L E, Barbet A F. Anaplasma marginale major surface protein-3 (MSP-3) is encoded by a polymorphic multigene family. Infect Immun. 1997;65:156–163. doi: 10.1128/iai.65.1.156-163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allred D R, McGuire T C, Palmer G H, Leib S R, Harkins T M, McElwain T F, Barbet A F. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc Natl Acad Sci USA. 1990;87:3220–3224. doi: 10.1073/pnas.87.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbet A F, Allred D R. The msp1β multigene family of Anaplasma marginale: nucleotide sequence analysis of an expressed copy. Infect Immun. 1991;59:971–976. doi: 10.1128/iai.59.3.971-976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantor G H, Pontzer C H, Palmer G H. Opsonization of Anaplasma marginale mediated by bovine antibody against surface protein MSP-1. Vet Immunol Immunopathol. 1993;37:343–350. doi: 10.1016/0165-2427(93)90206-j. [DOI] [PubMed] [Google Scholar]
- 7.Cossio-Bayugar R, Rodriguez S D, Garcia-Ortiz M A, Garcia-Tapia D, Aboytes-Torres R. Bovine anaplasmosis prevalence in northern Veracruz state, Mexico. Prev Vet Med. 1997;32:165–170. doi: 10.1016/s0167-5877(97)00016-0. [DOI] [PubMed] [Google Scholar]
- 8.Eid G, French D M, Lundgren A M, Barbet A F, McElwain T F, Palmer G H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriks I S, Stiller D, Palmer G H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol. 1993;31:2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French D M, Brown W C, Palmer G H. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect Immun. 1999;67:5834–5840. doi: 10.1128/iai.67.11.5834-5840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French D M, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Ortiz M A, Angeles-Ojeda L E, Hernández-Salgado G, García-Tapia D, Aboytes-Torres R, Rodríguez-Camarillo S D. Caracterización de la virulencia de un aislado mexicano de Anaplasma marginale. Tec Pecu Mex. 1998;36:197–202. [Google Scholar]
- 13.Hugh-Jones M E, Scotland K, Applewhaite L M, Alexander F M. Seroprevalence of anaplasmosis and babesiosis in livestock on St. Lucia. 1983. Trop Anim Health Prod. 1988;20:137–139. doi: 10.1007/BF02240077. [DOI] [PubMed] [Google Scholar]
- 14.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 15.Losos G J. Anaplasmosis. In: Losos G J, editor. Infectious tropical diseases of domestic animals. Essex, United Kingdom: Longman House; 1986. pp. 743–795. [Google Scholar]
- 16.McGarey D J, Allred D R. Characterization of hemagglutinating components of the Anaplasma marginale initial body surface and identification of possible adhesins. Infect Immun. 1994;62:4587–4593. doi: 10.1128/iai.62.10.4587-4593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGarey D J, Barbet A F, Palmer G H, McGuire T C, Allred D R. Putative adhesins of Anaplasma marginale: major surface polypeptides (MSP) 1a and 1b. Infect Immun. 1994;62:4594–4601. doi: 10.1128/iai.62.10.4594-4601.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munodzana D, McElwain T F, Knowles D P, Palmer G H. Conformational dependence of Anaplasma marginale MSP5 surface-exposed B-cell epitopes. Infect Immun. 1998;66:2619–2624. doi: 10.1128/iai.66.6.2619-2624.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberle S M, Palmer G H, Barbet A F, McGuire T C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988;56:1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer G H, Barbet A F, Cantor G H, McGuire T C. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect Immun. 1989;57:3666–3669. doi: 10.1128/iai.57.11.3666-3669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer G H, Barbet A F, Davis W C, McGuire T C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986;231:1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- 22.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer G H, McElwain T F. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet Parasitol. 1995;57:233–253. doi: 10.1016/0304-4017(94)03123-e. [DOI] [PubMed] [Google Scholar]
- 24.Palmer G H, Rurangirwa F R, Kocan K M, Brown W C. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol Today. 1999;15:281–286. doi: 10.1016/s0169-4758(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 25.Payne R C, Scott J M. Anaplasmosis and babesiosis in El Salvador. Trop Anim Health Prod. 1982;14:75–80. doi: 10.1007/BF02282584. [DOI] [PubMed] [Google Scholar]
- 26.Rurangirwa F R, Stiller D, French D M, Palmer G H. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc Natl Acad Sci USA. 1999;96:3171–3176. doi: 10.1073/pnas.96.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasse J, Gallagher S R. Detection of proteins. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: Wiley Interscience; 1998. pp. 8.9.3–8.9.8. [Google Scholar]
- 28.Steele R G D, Torre J H. Comparisons involving two sample means. In: Steele R G D, Torre J H, editors. Principles and procedures of statistics: a biometrical approach. 2nd ed. New York, N.Y: McGraw-Hill Book Company; 1980. pp. 86–121. [Google Scholar]
- 29.Tebele N, McGuire T C, Palmer G H. Induction of protective immunity using Anaplasma marginale initial body membranes. Infect Immun. 1991;59:3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidotto M C, McGuire T C, McElwain T F, Palmer G H, Knowles D P. Intermolecular relationships of major surface proteins of Anaplasma marginale. Infect Immun. 1994;62:2940–2946. doi: 10.1128/iai.62.7.2940-2946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker D H, Dumler S J. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]