Abstract
The Candida albicans gene HWP1 encodes a surface protein that is required for normal hyphal development in vitro. We used mutants lacking one or both alleles of HWP1 to investigate the role of this gene in virulence. Mice infected intravenously with the homozygous hwp1 null mutant, CAL3, survived a median of >14 days, whereas mice infected with a control strain containing two functional alleles of HWP1 survived only 3.5 days. After 1 day of infection, all strains produced similar levels of infection in the kidneys, spleen, and blood. However, after 2 and 3 days, there was a significant decrease in the number of organisms in the kidneys of the mice infected with CAL3. This finding suggests that the hwp1 homozygous null mutant is normal in its ability to initiate infection but deficient in its capacity to maintain infection. CAL3 also germinated minimally in the kidneys. The ability of the heterozygous null mutant to germinate and cause mortality in mice was intermediate to CAL3, suggesting a gene dosage effect. To investigate potential mechanisms for the diminished virulence of CAL3, we examined its interactions with endothelial cells and neutrophils in vitro. CAL3 caused less endothelial cell injury than the heterozygous hwp1 mutant. We conclude that the HWP1 gene product is important for both in vivo hyphal development and pathogenicity of C. albicans. Also, the ability to form filaments may be critical for candidal virulence by enabling the fungus to induce cellular injury and maintain a deep-seated infection.
Candida albicans is an opportunistic fungal pathogen that causes hematogenously disseminated infections in certain compromised hosts (4, 22). Vascular endothelium may play a critical role during the initiation of hematogenous infection, because blood-borne organisms likely adhere to and penetrate the endothelial cell lining of the blood vessel to gain access to the tissue parenchyma. Furthermore, endothelial cells have the ability to express proinflammatory mediators that may recruit leukocytes to sites of vascular infection (5).
C. albicans can grow in either the yeast or hyphal form, and the ability to germinate and form hyphae is thought to be essential for the virulence of this organism in vivo (2, 19). We have shown previously that germinated organisms interact with host cells, such as vascular endothelium, differently than do blastospores. For example, germinated organisms are significantly more adherent to endothelial cells than are blastospores (23). Also, germination is required for C. albicans to damage endothelial cells and stimulate them to express leukocyte adhesion molecules in vitro (5, 6).
We have been investigating the function of HWP1, a gene that is expressed by hyphae but not blastospores of C. albicans (24). Immunological evidence indicates that this gene encodes a surface protein (26). Deletion of both alleles of HWP1 results in a conditional defect in hyphal formation in vitro (24). Homozygous hwp1 null mutants exhibit almost no germination on all solid media lacking serum. On agar containing serum, the mutants germinate but produce fewer and shorter filaments than do wild-type strains. However, the hwp1 null mutants germinate normally in liquid culture. To evaluate the role of HWP1 in the pathogenicity of C. albicans, we investigated the virulence and morphology of hwp1 null mutants during hematogenous candidal infection in mice. In addition, to investigate potential mechanisms for the diminished virulence of the hwp1 null mutants, we examined the interactions of these organisms with human endothelial cells and neutrophils in vitro.
MATERIALS AND METHODS
Organisms.
Heterozygous and homozygous hwp1 null mutants were constructed from C. albicans CAI4 by using the hisG URA3 hisG disruption cassette method as described elsewhere (7, 24). Briefly, CAL1 (Δhwp1::hisG URA3 hisG/HWP1 Δura3::imm434/Δura3::imm434) was generated from strain CAI4 (Δura3::imm434/Δura3::imm434). In CAL1, a 434-bp BglII-BamHI fragment in the 5′ coding region of one allele of HWP1 replaced with the HisG URA3 HisG disruption construct. The homozygous hwp1 null mutant, CAL3 (Δhwp1::hisG/Δhwp1::hisG URA3 hisG Δura3::imm434/Δura3::imm434) was generated from CAL1 by using the same disruption construct. CAL5, the HWP1 revertant (Δhwp1::hisG/Δhwp1::hisG HWP1 URA3 Δura3::imm434/Δura3::imm434) was constructed from CAL3. The genotypes of all mutants were confirmed by Southern blotting.
We also confirmed that only a single copy of the HWP1 URA3 construct had been reintroduced into the HWP1 locus in CAL5. Southern blotting of DNA from CAL5 that was digested with EcoRI yielded three bands of 4.2, 4.9, and 7.4 kb (24). If the strain had contained a tandem duplication of the integrative sequence, then an additional 6.6-kb band would have been present. This band was not present in Southern blots prepared from any of the independently constructed HWP1 revertants that were used in these studies.
In the animal studies, the control strain was CAI12 (Δura3::imm434/URA3), a revertant of CAI4 in which the wild-type URA3 locus was reconstructed. All of these strains had similar growth rates and similar levels of activity of orotidylate monophosphate decarboxylase (the enzyme encoded by URA3) (reference 24 and unpublished data).
For each experiment, the organisms were grown on a rotating drum at 25°C in yeast nitrogen base broth (Difco, Detroit, Mich.) supplemented with 0.5% glucose (5). The organisms were harvested by centrifugation and washed twice in Dulbecco's phosphate-buffered saline (PBS). Next, they were counted in a hemacytometer and adjusted to the desired concentration in either PBS or RPMI 1640 medium.
Measurement of extracellular proteinase and phospholipase activity.
The extracellular proteinase activity of the different strains was measured by using bovine hemoglobin as the substrate by the method of Hube et al. (10). The extracellular phospholipase activity of the various mutants was determined by growing them on egg yolk agar and measuring the size of the zone of precipitation according to our previously described method (13).
Mouse studies.
Nine- to eleven-week-old male BALB/c mice (20 to 25 g) were used in this study. They were given food and water ad libitum. Each mouse was inoculated with 106 organisms in 200 μl of PBS via the tail vein. Survival was monitored twice daily, and moribund mice were euthanized by cervical dislocation. To quantify the number of organisms in the tissues, mice were sacrificed after 1, 2, and 3 days of infection by cervical dislocation. Next, 200 μl of blood was obtained by cardiac puncture and plated on Sabouraud dextrose agar. The kidneys and spleen were removed aseptically, weighed, and homogenized in sterile saline, and then serial dilutions were inoculated in Sabouraud dextrose agar. Colonies were counted after incubation at 37°C for 24 h.
Histology.
The kidneys and spleens from the mice were fixed in 10% buffered formalin and then embedded in paraffin. Thin sections were prepared and stained with hematoxylin and eosin, as well as Gomori methenamine silver. They were examined by light microscopy. Tissue sections from two mice infected with each mutant were studied.
Endothelial cells.
Endothelial cells were harvested from human umbilical veins by the method of Jaffe et al. (14). The cells were grown in M-199 medium supplemented with 10% fetal bovine serum, 10% defined bovine calf serum, and 2 mM l-glutamine with penicillin and streptomycin (5). In all experiments, the cells were grown in multiwell tissue culture plates coated with gelatin and incubated at 37°C in 5% CO2.
Endothelial cell adherence.
The adherence of C. albicans to endothelial cells was determined by our standard method (9). Briefly, 102 blastospores were added to each well of endothelial cells in six-well tissue culture plates and then incubated for 90 min. Virtually all of the organisms germinated during this time. Next, the nonadherent organisms were removed by rinsing in a standardized manner, and the wells were overlaid with Sabouraud dextrose agar. The number of adherent organisms was determined by colony counting. Adherence was expressed as a percentage of the original inoculum, which was confirmed by quantitative culture in Sabouraud dextrose agar. Each experiment was performed in triplicate on three separate occasions.
Endothelial cell phagocytosis of C. albicans.
The number of organisms phagocytized by the endothelial cells was measured by a modification of our previously described method (12). Endothelial cells were grown to confluency on 12-mm-diameter glass coverslips coated with human fibronectin. Each coverslip was incubated with 105 organisms for 2 h. Next, the media were aspirated and the cells were fixed with 3% paraformaldehyde in PBS for 30 min. The coverslips were rinsed with 1% bovine serum albumin in PBS (PBS-BSA) and then incubated for 1 h with Texas red-conjugated antiserum directed against C. albicans (Biodesign International, Kennebunk Port, Maine) to label the nonphagocytized organisms. After extensive rinsing in PBS-BSA, the endothelial cell membranes were made permeable by exposing the cells to 0.1% Triton X-100 in PBS. The coverslips were rinsed three times with PBS-BSA and then incubated with 1% Uvitex (a generous gift from Jay Isharani, Novartis, Greensboro, N.C.) in PBS for 30 min (17). This fluorescent chitin stain labeled both the phagocytized and nonphagocytized organisms. Finally, the coverslips were rinsed in PBS-BSA and mounted inverted on microscope slides. The coverslips were examined with a Zeiss Axiovert 10 microscope equipped for epifluorescence. The number of phagocytized organisms was calculated by subtracting the number of nonphagocytized organisms from the total organisms. At least 100 organisms per coverslip were counted, and each experiment was performed in triplicate on three different days.
Endothelial cell damage.
The amount of endothelial injury caused by C. albicans was quantified by chromium release assay as described earlier (6). The inoculum was 106 organisms per well of a 24-well tissue culture plate, and the incubation period was 3 h. All experiments were performed in triplicate and repeated three times.
Germ tube elongation.
The extent of germ tube elongation by the different mutants on endothelial cells was measured by using a micrometer as described previously (11). Blastospores (5 × 104 organisms per well) in RPMI 1640 medium were added to endothelial cells in 24-well tissue culture plates. After incubation for 3 h, the organisms were fixed in 2% glutaraldehyde, and the lengths of 100 germ tubes of each mutant were measured.
Leukocyte adhesion molecule expression by endothelial cells.
Endothelial cells in 96-well plates were infected with 2 × 104 blastospores in RPMI 1640 medium containing 1% fetal bovine serum. Control wells containing the same medium, but no organisms were processed in parallel. After incubating the plates for 8 h, the relative amounts of E-selectin and intracellular adhesion molecule 1 (ICAM-1) expressed by the endothelial cells was determined by whole-cell enzyme-linked immunosorbent assay by the method of Noel et al. (21). The results were corrected for nonspecific binding of the antibodies, which was determined by incubating the wells with the secondary antibody in the absence of the primary antibody. In preliminary experiments, we determined that none of the primary antibodies bound to C. albicans germinated on bare plastic. These experiments were performed in triplicate on three different days.
Neutrophil-mediated growth inhibition of C. albicans.
The ability of neutrophils to inhibit the growth of C. albicans on an endothelial cell monolayer was determined by a modification of the method of Meshulam et al. (20). Neutrophils were isolated from heparinized human blood by dextran sedimentation, followed by centrifugation through Ficoll-Hypaque. After removing contaminating erythrocytes by hypotonic lysis, the neutrophils were washed, counted with a hemacytometer, and suspended in RPMI 1640 medium containing 10% pooled human serum (Sigma Chemical Co., St. Louis, Mo.). These leukocytes were added to endothelial cells in a 48-well tissue culture plate that had been infected for 3 h with 2.5 × 104 C. albicans blastospores per well. The ratio of organisms to neutrophils was 1 to 4. After a 1-h incubation, the neutrophils and endothelial cells were lysed with distilled water, and the number of metabolically active organisms was determined spectrophotometrically by the reduction of the water-soluble tetrazolium salt, 2,3-bis(2-methoxy-4-nitro-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (20). The number of organisms inhibited by the neutrophils was calculated from a standard curve, which was constructed by incubating known numbers of organisms with endothelial cells in the absence of neutrophils. These experiments were repeated twice in triplicate.
Data analysis.
Differences in survival of the mice infected with the various strains were analyzed using the Wilcoxon rank sum test. In all other experiments, differences were compared using analysis of variance. When multiple comparisons were performed, the Bonferroni correction was used. P values of ≤0.05 were considered significant.
RESULTS
Virulence of C. albicans strains in mice.
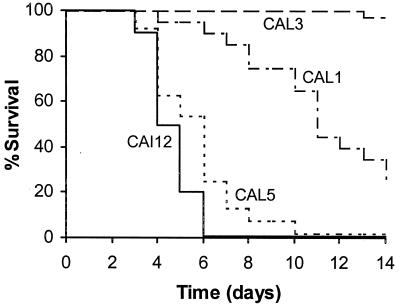
The hwp1 null mutants exhibited defects in hyphal formation in vitro (24). Hyphal formation has been implicated in the virulence of C. albicans (19). Therefore, the contribution of HWP1 to the virulence of C. albicans in the murine model of hematogenously disseminated candidiasis was determined. Mice infected with the control strain, CAI12, survived a median of 3.5 days (Fig. 1). CAL1, the heterozygous hwp1 null mutant, was significantly less virulent than was CAI12, since mice infected with CAL1 had a median survival of 10 days (P < 0.001 compared to CAI12). The virulence of the homozygous hwp1 null mutant, CAL3, was even less than that of CAL1. The median survival of mice infected with CAL3 was greater than 14 days (P < 0.001 compared to CAI12 and CAL1). Similar results were obtained with a second homozygous hwp1 null mutant that was constructed independently of CAL3 (data not shown). These results suggested that the HWP1 gene product is important for the virulence of C. albicans. Also, the relationship between the number of functional alleles of HWP1 and in vivo virulence suggested a gene dosage effect in this animal model of infection.
FIG. 1.
Survival of mice following intravenous challenge with 106 blastospores of CAI12 (n = 10), CAL1 (n = 20), CAL3 (n = 33), and CAL5 (n = 24).
When HWP1 was reintroduced into CAL3 to form CAL5, the virulence of the organisms was restored. Mice infected with CAL5 had a median survival of 5 days, which was similar to that of mice infected with CAI12 (P = 0.14) but significantly shorter than that of mice infected with CAL1 (P < 0.001) (Fig. 1). A second HWP1 revertant was also tested in the mouse model of infection, and its virulence was similar to that of CAL5 (data not shown).
Quantitative culture results.
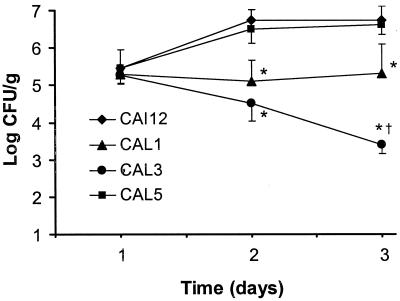
All strains tested produced a similar level of infection in the kidneys after 1 day of infection (Fig. 2). However, after 2 and 3 days of infection, the kidneys of mice infected with either the heterozygous or the homozygous hwp1 null mutants (CAL1 and CAL3, respectively) contained significantly fewer organisms than did those of mice infected with either CAI12 or the HWP1 revertant, CAL5. In addition, after 3 days of infection, there were significantly fewer organisms in the kidneys of mice infected with CAL3 compared with those infected with CAL1. Finally, the number of organisms in the kidneys of mice infected with CAL3 decreased progressively after the first day of infection. In contrast, the number of organisms in the kidneys of the mice infected with the other strains either remained constant or increased during the course of the study.
FIG. 2.
Colony counts of the kidneys of mice infected with different strains of C. albicans. Seven mice were infected with each strain. Results are the mean ± the standard deviation. ∗, P ≤ 0.001 compared to CAI12; †, P < 0.001 compared to CAL3.
As stated above, the mice infected with the hwp1 homozygote, CAL3 survived for at least 14 days. At the end of one of these experiments, some of the mice were sacrificed, and their kidneys were cultured to determine if the infection had been cleared completely. All kidneys tested contained at least 105 CFU/g of tissue. Therefore, the null mutant had not been cleared completely from the kidneys during the first few days of the infection, and the remaining organisms had apparently multiplied.
Although there were significant differences at 48 and 72 h in organism burden in the kidneys of mice infected with the different strains of C. albicans, there was no significant difference in the number of organisms in the spleens and blood of these mice (data not shown). Thus, the HWP1 gene product did not appear to be essential for the initiation or maintenance of infection in these anatomic sites.
Histopathologic examination of the kidneys.
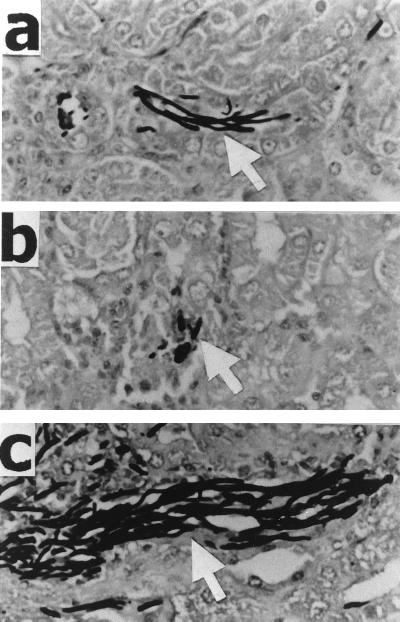
Because deletion of one or both copies of HWP1 reduces the ability of C. albicans to germinate on solid media in vitro (24), we investigated the germination of the different hwp1 mutants in vivo after 48 h of infection. The majority of the organisms were in the hyphal phase in the kidneys of the mice infected with the hwp1 heterozygote, CAL1 (Fig. 3). In the kidneys of the mice infected with the hwp1 homozygote, CAL3, the fungi appeared to be a mixture of ovoid and elongated cells. The longest hyphae were observed in the kidneys of the mice infected with the HWP1 revertant, CAL5. Thus, the extent of hyphal formation of HWP1 mutants in the kidneys of mice paralleled their virulence.
FIG. 3.
Micrographs of organisms in the kidneys of mice at 48 h postinfection. Mice were infected with 106 blastospores of CAL1 (a), CAL3 (b), and CAL5 (c). Sections of the kidney were stained with silver (magnification, ×224). Arrows indicate C. albicans.
In kidney sections stained with hematoxylin and eosin, a significant inflammatory infiltrate was visible around the organisms in the mice infected with CAL1 and CAL5. However, there were very few inflammatory cells surrounding the fungi in the mice infected with CAL3 (data not shown). Whether this paucity of inflammatory cells was due to the low number of organisms present in the tissue or a diminished host response elicited by this null mutant could not be ascertained.
After 48 h of infection, virtually no organisms were visible in the spleens of the mice infected with any of the different mutants. Thus, it was not possible to compare differences in hyphal length or inflammatory response in this organ.
Interactions of the hwp1 mutants with endothelial cells and neutrophils in vitro.
To investigate potential mechanisms for the diminished virulence of the hwp1 mutants, we examined the interactions of the different strains with endothelial cells and neutrophils in vitro. We found that deleting both copies of hwp1 had a significant effect on only a few of these interactions. The homozygous hwp1 null mutant, CAL3, produced shorter germ tubes and caused somewhat less endothelial cell injury than did either CAL1 or CAL5 (Table 1). Although CAL3 produced shorter hyphae on endothelial cells than did the other strains, the percentage of the homozygous hwp1 mutants that germinated on these cells was similar to that of the other strains (data not shown). In addition, CAL3 was phagocytized by endothelial cells only slightly less efficiently than were the two other strains. Furthermore, although lytic enzymes released by the fungi likely contribute to endothelial cell injury (12), the extracellular proteinase and phospholipase activities of all strains studied were similar (data not shown).
TABLE 1.
Interactions of HWP1 mutant strains with endothelial cells and neutrophils in vitroa
| Strain | Germ tube length (μm) | % Endothelial cell adherence (% of CAL1) | % Phagocytosis by endothelial cells | Endothelial cell injury (% specific 51Cr release) | % Neutrophil-mediated growth inhibition | % Induction of d:
|
|
|---|---|---|---|---|---|---|---|
| E-selectin expression | ICAM-1 expression | ||||||
| CAL1 | 26 ± 5 | 100 ± 30 | 67 ± 5 | 57 ± 3 | 54 ± 6 | 163 ± 11 | 250 ± 30 |
| CAL3 | 10 ± 4bc | 124 ± 32 | 59 ± 7b | 40 ± 4bc | 61 ± 9 | 166 ± 24 | 247 ± 28 |
| CAL5 | 16 ± 5b | 164 ± 12 | 64 ± 7 | 50 ± 5 | 56 ± 7 | 168 ± 23 | 275 ± 42 |
All values are mean ± the standard deviation.
P < 0.05 compared to CAL1.
P < 0.05 compared to CAL5.
% Increase compared to uninfected endothelial cells.
Interestingly, the HWP1 revertant, CAL5, interacted with endothelial cells in a markedly different manner than did the hwp1 heterozygote, CAL1. CAL5 was 64% more adherent to endothelial cells than was CAL1 (Table 1). When grown on endothelial cells, the average germ tube length of CAL5 was 38% shorter than that of CAL1 (Table 1). Corresponding to its reduced germ tube length, CAL5 caused slightly less injury to endothelial cells than did CAL1.
As stated above, mice infected with CAL3 had fewer organisms in their kidneys after 2 and 3 days of infection (Fig. 2). Also, fewer inflammatory cells were visible around these mutants at this time point. Therefore, it was of interest to examine the susceptibility of the hwp1 mutants to neutrophil-mediated growth inhibition and their ability to stimulate endothelial cells to express leukocyte adhesion molecules in vitro. We found that all three mutants were inhibited equally by the neutrophils and all of them induced endothelial cells to express similar amounts of E-selectin and ICAM-1 (Table 1).
DISCUSSION
In C. albicans, the formation of hyphae or pseudohyphae is triggered by a diverse set of environmental signals, and different genes appear to regulate this morphologic transformation. The majority of investigations on the regulation of filamentation in C. albicans have focused on genes that regulate this process in vitro. However, it is possible that different genes are important in regulating filamentation in vivo. Therefore, investigating the role of HWP1 in candidal germination in the murine model of hematogenous infection complemented our in vitro studies. We found that hwp1 mutants exhibited marked defects in germination in the murine kidney following intravenous infection. This reduction in germination was accompanied by a significant decrease in virulence. The role of the HWP1 gene product in the formation of filaments in vivo is consistent with our findings that hwp1 null mutants are defective in filamentation on solid media in vitro (24).
When tested in vitro, the hwp1 mutants germinated normally in liquid media containing 10% serum but exhibited reduced hyphal development when grown on serum-containing agar (24). In the murine kidney, the organisms were almost certainly exposed to serum constituents. The finding that the null mutants germinated very poorly in vivo suggests that the signal transduction pathway(s) that induces germination on solid media may also regulate germination in the murine kidney.
To date, at least two distinct signal transduction pathways that induce the yeast to hyphal transformation in C. albicans have been identified. One pathway contains the transcription factor encoded by EFG1, and the other is the mitogen-activated protein (MAP) kinase pathway (1, 15, 16, 18, 19, 27). HWP1 expression is dependent on EFG1 in vitro, because there is no detectable HWP1 expression in a homozygous efg1 null mutant (24). In vivo, HWP1 may be regulated by factors other than Efg1p because the hwp1 mutants had markedly reduced virulence, whereas the virulence of efg1 mutants appears to be diminished to a lesser extent (19). However, because the virulence of the different mutants of C. albicans was assessed in different strains of mice, this conclusion requires additional experimental confirmation.
HWP1 also appears to be regulated independently of the MAP kinase pathway in vitro and in vivo. We have found that, in vitro, HWP1 is expressed at normal levels in a homozygous cph1 mutant, which lacks the terminal transcription factor of the MAP kinase pathway (24). In the murine model, the hwp1 null mutants also had a different phenotype from any of the MAP kinase mutants. The hwp1 mutants exhibited deficient germination in the murine kidney. In contrast, although some of the MAP kinase mutants have reduced virulence in vivo (1), in the one study where histopathology was performed, no reduction in filamentation was seen in the tissues (16). Thus, the reduction in virulence of the MAP kinase mutants may be caused by factor(s) other than decreased filamentation.
The C. albicans gene, INT1, resembles HWP1 in that its product appears to be a surface protein that plays a role in hyphal formation (8). In vitro, int1 null mutants germinate normally in liquid media but have reduced germination on solid media. Like the hwp1 mutants, int1 mutants are less virulent in the murine model of hematogenously disseminated candidiasis. However, it is unknown whether the reduced virulence of the int1 mutants is due to impaired hyphal formation because the ability of these mutants to germinate in vivo has not been reported. Furthermore, the signal transduction pathways that regulate int1 expression are currently unknown.
When testing the virulence of the hwp1 mutants, we saw evidence of a gene dosage effect. The virulence of the heterozygous mutant, CAL1 was intermediate to that of the wild-type strain and the homozygous null mutant, CAL3. This gene dosage effect was also evident in the ability of the organisms to form hyphae in vivo and in vitro (24).
An unexpected finding was that the HWP1 revertant, CAL5, was as virulent as the parent strain. Phenotypic differences between CAL1 and CAL5 were also observed in our in vitro studies (Table 1) (24). These phenotypic differences were not likely due to extraneous mutations because the differences were reproducible in independently constructed revertants. The results of the Southern blotting precluded the possibility that any of the revertants contained more than one copy of HWP1 (24). However, it is possible that the fragment of HWP1 that was used to construct the revertant did not contain the full-length promoter, and the presence of this incomplete promoter resulted in higher-level expression of the reintroduced allele. Alternatively, the structure of the reverted locus in CAL5 may have been altered in such a way that the expression of HWP1 was increased. Nevertheless, the finding that reintroduction of HWP1 into the homozygous null mutant reversed its filamentation and virulence defects demonstrates that the deletion of HWP1 was directly responsible for these defects.
When investigating the number of organisms in the kidneys, we found that significant differences among the various hwp1 mutants were observed only after 2 days of infection but not at the earlier time point (Fig. 2). These results may indicate that germination or another process associated the HWP1 gene product is required for the organism to persist in the kidney and avoid clearance by host defense mechanisms. They also suggest that initiation of a renal infection can occur independently of the HWP1 gene product.
Recently, Staab et al. (25) reported that the Hwp1 protein may act as an adhesin by serving as a substrate for host cell transglutaminases. As with our findings, these authors determined that hwp1 null mutants of C. albicans are less lethal in the murine model of intravenous infection. Thus, it is possible that the reduction in virulence of the hwp1 null mutants may be due to a decrease in the ability of these mutants to adhere to host cells, as well as a reduction in their capacity to form hyphae.
Although the different mutants produced significantly different organism burdens in the kidneys, all mutants tested achieved similar levels of infection in the spleen and blood. This finding suggests that candidal factors other than the HWP1 gene product are required for the maintenance of infection in these anatomic sites. Evidence of niche-specific virulence factors of C. albicans has been reported previously with the PHR1 and PHR2 gene products (3).
To develop in vitro correlates of the in vivo pathogenicity of the different mutants, we examined their interactions with endothelial cells and neutrophils. These in vitro studies demonstrated some differences among CAL1, CAL3, and CAL5 (Table 1). For example, CAL3 produced the shortest germ tubes and caused the least amount of endothelial cell injury. We have found previously that a sap2 mutant of C. albicans that is deficient in secreted aspartyl proteinase 2 causes less injury to endothelial cells in vitro (12). This mutant also has attenuated virulence in two different animal models (10). Therefore, it is possible that endothelial cell injury in vitro may be useful as a surrogate marker for virulence in animal models of hematogenously disseminated infection.
We also observed that when the mutants were grown on endothelial cells, the average germ tube length of CAL5 was significantly shorter than that of CAL1. This result is the opposite of what was observed in vivo. The germ tube elongation experiments were performed in RPMI 1640 medium. We repeated them in M199 medium containing 20% bovine serum to determine if a richer medium might give results more similar to those obtained in vivo. However, even in this medium, CAL1 still produced longer germ tubes than did CAL5 (data not shown). These findings indicate that the in vitro conditions do not always elicit the same candidal responses as were induced in the murine model. This difference between the in vitro and in vivo responses may also explain why there was no detectable difference in the susceptibility of the different mutants to neutrophil-mediated inhibition in vitro, even though the homozygous hwp1 null mutant appeared to be cleared more rapidly from the kidneys in vivo.
In conclusion, our results indicate that the HWP1 gene product is important for both the germination and pathogenicity of C. albicans in vivo. These findings provide strong evidence for the importance of filamentation in candidal virulence and suggest that the yeast-to-hypha transition may enable the fungus to avoid host clearance mechanisms in some organs. Therapeutic strategies that inhibit this morphological change may be useful adjuncts for treating serious infections caused by C. albicans.
ACKNOWLEDGMENTS
We thank the nurses at Harbor-UCLA Medical Center for collecting umbilical cords, as well as Alison Orozco and Michael Mador for help in preparing endothelial cells. We also thank Takashi Fukuoka for his assistance with the animal studies and Ashraf Ibrahim and Paul Belanger for helping with the in vitro studies. The Olympus IMT-2 phase-contrast microscope used in these studies was graciously donated by Toyota U.S.A.
This research was supported by Public Health Service grants RO1AI-19990, PO1AI-37194, R29AI-40636, and MO1RR-00425. W.A.F. was supported in addition by the Burroughs Wellcome Scholar Award in Molecular Pathogenic Mycology.
REFERENCES
- 1.Csank C, Schröppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas D Y, Whiteway M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 3.De Bernardus F, Mühlschlegel F A, Cassone A, Fonzi W A. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–3325. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards J E., Jr . Candida species. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2289–2306. [Google Scholar]
- 5.Filler S G, Pfunder A S, Spellberg B J, Spellberg J P, Edwards J E., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filler S G, Swerdloff J N, Hobbs C, Luckett P M. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–772. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale C A, Bendel C M, McClellan M, Hauser M, Becker J M, Berman J, Hostetter M K. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 9.Ghannoum M A, Filler S G, Ibrahim A S, Fu Y, Edwards J E., Jr Modulations of interactions of Candida albicans and endothelial cells by fluconazole and amphotericin B. Antimicrob Agents Chemother. 1992;36:2239–2244. doi: 10.1128/aac.36.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hube B, Sanglard D, Odds F C, Hess D, Monod M, Schafer W, Brown A J P, Gow N A R. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect Immun. 1997;65:3529–3538. doi: 10.1128/iai.65.9.3529-3538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim A S, Filler S G, Ghannoum M A, Edwards J E., Jr Interferon-gamma protects endothelial cells from damage by Candida albicans. J Infect Dis. 1993;167:1467–1470. doi: 10.1093/infdis/167.6.1467. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim A S, Filler S G, Sanglard D, Edwards J E, Jr, Hube B. Secreted aspartyl proteinases and interactions of Candida albicans with human endothelial cells. Infect Immun. 1998;66:3003–3005. doi: 10.1128/iai.66.6.3003-3005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim A S, Mirbod F, Filler S G, Banno Y, Cole G T, Kitajima Y, Edwards J E, Jr, Nozawa Y, Ghannoum M A. Evidence implicating phospholipase as a virulence factor in Candida albicans. Infect Immun. 1995;63:1993–1998. doi: 10.1128/iai.63.5.1993-1998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe E A, Nachman R L, Becker C G, Ninick C R. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köhler J R, Fink G R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leberer E, Harcus D, Broadbent I D, Clark K L, Dignard D, Ziegelbauer K, Schmidt A, Gow N A R, Brown A J P, Thomas D Y. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 1996;93:13217–13222. doi: 10.1073/pnas.93.23.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levitz S M, DiBenedetto D J, Diamond R D. A rapid fluorescent assay to distinguish attached from phagocytized yeast particles. J Immunol Methods. 1987;101:37–42. doi: 10.1016/0022-1759(87)90213-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Köhler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 19.Lo H-J, Köhler J, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 20.Meshulam T, Levitz S M, Christin K, Diamond R D. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanil ide (XTT) J Infect Dis. 1995;172:1153–1156. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 21.Noel R F, Jr, Sat T T, Mendez C, Johnson M C, Pohlman T H. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria requires soluble CD14. Infect Immun. 1995;63:4046–4053. doi: 10.1128/iai.63.10.4046-4053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller M A, Jones R N, Doern G V, Sader H S, Hollis R J, Messer S A. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. The SENTRY Participant Group. J Clin Microbiol. 1998;36:1886–1889. doi: 10.1128/jcm.36.7.1886-1889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotrosen D, Edwards J E, Jr, Gibson T R, Moore J D, Cohen A H, Green I. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis. 1985;152:1264–1273. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- 24.Sharkey L L, McNemar M D, Saporito-Irwin S M, Sypherd P S, Fonzi W A. HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1, and RBF1. J Bacteriol. 1999;181:5273–5279. doi: 10.1128/jb.181.17.5273-5279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staab J F, Bradway S D, Fidel P L, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 26.Staab J F, Ferrer C A, Sundstrom P. Developmental expression of a tandemly repeated, proline- and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J Biol Chem. 1996;271:6298–6305. doi: 10.1074/jbc.271.11.6298. [DOI] [PubMed] [Google Scholar]
- 27.Stoldt V R, Sonneborn A, Leuker C E, Ernst J F. Egf1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;6:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]