Abstract
Photosynthesis is a key process in plants that can be strongly affected by the actions of environmental stressors. The stressor-induced photosynthetic responses are based on numerous and interacted processes that can restrict their experimental investigation. The development of mathematical models of photosynthetic processes is an important way of investigating these responses. Our work was devoted to the development of a two-dimensional model of photosynthesis in plant leaves that was based on the Farquhar–von Caemmerer–Berry model of CO2 assimilation and descriptions of other processes including the stomatal and transmembrane CO2 fluxes, lateral CO2 and HCO3− fluxes, transmembrane and lateral transport of H+ and K+, interaction of these ions with buffers in the apoplast and cytoplasm, light-dependent regulation of H+-ATPase in the plasma membrane, etc. Verification of the model showed that the simulated light dependences of the CO2 assimilation rate were similar to the experimental ones and dependences of the CO2 assimilation rate of an average leaf CO2 conductance were also similar to the experimental dependences. An analysis of the model showed that a spatial heterogeneity of the CO2 assimilation rate on a leaf surface should be stimulated under an increase in light intensity and a decrease in the stomatal CO2 conductance or quantity of the open stomata; this prediction was supported by the experimental verification. Results of the work can be the basis of the development of new methods of the remote sensing of the influence of abiotic stressors (at least, excess light and drought) on plants.
Keywords: CO2 assimilation, excess light, spatial heterogeneity, leaf CO2 conductance, two-dimensional photosynthetic model, drought
1. Introduction
Photosynthesis is a key process in the life of green plants and the basis of their productivity. It is a complex process [1,2] that can be strongly affected by numerous abiotic stressors, including excess light [3,4,5] and fluctuations in light intensity [6,7,8,9], drought [10,11,12], decrease [13] and increase [14,15,16] in temperatures, and others.
Changes in the photosynthetic processes induced by the action of stressors include both the damage of photosynthetic machinery and numerous protective responses. The stressor-induced damages include photodamage under excess light [3,4,5], increase in proton leakage across the thylakoid membrane under heating [14], damage of photosynthetic complexes through the stimulation of the production of reactive oxygen species induced by the decrease in photosynthetic dark reactions under the action of various stressors [17], and others. The protective responses include the induction of a non-photochemical quenching [3,4,18,19], stimulation of a cyclic electron flow around photosystem I [7,19,20], translocation of Ferredoxin-NADP+ Reductase [21,22], activation of photorespiration [23], changes in the positions of chloroplasts [24,25,26], and others. These processes can strongly interact, e.g., the stimulation of the cyclic electron flow increases the acidification of the lumen in chloroplasts and can increase an energy-dependent component of the non-photochemical quenching caused by this acidification [19,27,28] through the interacted protonation of PsbS proteins [3,4,29] and the synthesis of zeaxanthin and antheraxanthin from violaxanthin in the xanthophyll cycle [30].
The complexity of the photosynthetic stress responses is a reason for the active development of mathematical models of photosynthetic processes [31], because these models can be effective tools for the prediction of changes in photosynthesis under the action of adverse factors. There are models simulating processes on different levels of the organization of photosynthesis [31]: models of the ways of energy utilization in the reaction centers of photosystem II [32,33,34], models focusing on the description of photosynthetic light reactions and their regulation by stressors [5,35,36,37,38,39,40], models focusing on the description of photosynthetic dark reactions and CO2 fluxes [41,42,43,44], complex models of plant productivity [45,46], and global photosynthetic models [47,48].
The photosynthetic model by Farquhar, von Caemmerer, and Berry (FvCB model) [42,49,50,51] is a widely-used model of C3 photosynthesis that can describe the photosynthetic processes in mesophyll cells, leaves, plant canopies, and ecosystems [31]. This model is based on a stationary description of a photosynthetic CO2 assimilation rate (Ahv) that is dependent on the slowest process of three processes that can limit the dark reactions of photosynthesis [50]: CO2 fixation by Rubisco, linear electron flow (LEF) in the electron transport chain of thylakoids, and triose flux from the stroma of chloroplasts. Particularly, the FvCB model can be used for the description of the heterogeneity of the photosynthetic processes in the leaves and canopies of plants [52,53,54,55,56]; analysis of this heterogeneity has great importance for revealing new factors that can regulate photosynthetic processes (e.g., the influence of changes in the intensity and spectrum of light caused by an increase in the distance from the leaf surface during photosynthetic processes or the influence of 3-D microstructures of leaf tissues and chloroplast movements on photosynthesis).
However, the simulation of photosynthetic processes in the scale of a leaf surface that can also be based on the FvCB model is weakly developed. A model of photosynthetic processes in the scale of a leaf surface is a potential tool for the theoretical investigation of the spatial heterogeneity of photosynthetic parameters on this surface, including revealing possible modifications of the heterogeneity under the action of stressors. There are several reasons supporting the importance of the development of the leaf photosynthesis model and its theoretical analysis.
First, revealing stressor-induced changes in the photosynthetic heterogeneity can provide an additional indicator of the action of adverse factors on plants. It can be used for the development of new methods for remote sensing plant stress changes. Particularly, these methods can be based on the measurements of the spatial heterogeneity of the distribution of a photochemical reflectance index (PRI), which is calculated based on reflectance at 531 and 570 nm [57,58,59,60] and is strongly related to photosynthetic parameters (the non-photochemical quenching of fluorescence, effective quantum yield of photosystem II, light-use efficiency, and photosynthetic CO2 assimilation rate) [61,62,63,64,65,66,67].
Second, the development of the leaf photosynthesis model and revealing stressor-induced changes in the spatial photosynthetic heterogeneity can be an important step for further investigation into new mechanisms influencing plant tolerance to stressors. Particularly, it was theoretically shown that the spatial heterogeneity in the physiological parameters of two-dimensional models of living cells can modify their responses to the actions of external factors through a diversity-induced resonance [68,69,70], e.g., this effect was shown for excitable plant cells under cooling [70,71]. It cannot be excluded that the spatial heterogeneity in photosynthetic processes can also influence the plant response to stressors. Potentially, the leaf photosynthesis model can also be used as an analysis tool for this influence.
Thus, there were three main purposes of our work: (i) The development and verification of the two-dimensional model of C3 photosynthesis in the plant leaf, which was based on the FvCB model. (ii) The model-based analysis of the induction of the spatial heterogeneity of the CO2 assimilation rate under excess light conditions and a decrease in leaf CO2 conductance (gS) (this gS decrease imitated the action of a short-term drought). (iii) Additional experimental verification of the results of this analysis.
2. Description of the Two-Dimensional Model of C3 Photosynthesis in Plant Leaves
The two-dimensional model of C3 photosynthesis in the plant leaf was based on the round system of elements (Figure 1a). Each element included descriptions of the photosynthetic cell and the apoplast; some elements (central elements in 3 × 3 elements squares or in 5 × 5 elements squares) additionally included stomata. Figure 1b shows the main processes considered in the model. Equations and parameters of the two-dimensional model of C3 photosynthesis in the plant leaf are described in File S1 “Equations and parameters of the two-dimensional photosynthetic model” in detail.
Figure 1.
A general scheme of the developed two-dimensional model (a) and main processes described by the model on the cell level (b). Model elements (squares) include both mesophyll cells and stomata or only mesophyll cells (without stomata). Small arrows in the general scheme show transport of carbon dioxide, H+, and K+ between apoplastic volumes of neighboring cells and across the plasma membrane. PAR is the photosynthetic active radiation. pHap, pHcyt, and pHstr are pH in the apoplast, cytoplasm, and stroma of chloroplasts, respectively. Bcyt− and BcytH are the free and proton-bound cytoplasmic buffers. Bap−, BapH, and BapK are the free, proton-bound, and potassium-bound apoplastic buffers. Em is the difference of electrical potentials across the plasma membrane. FvCB model is the Farquhar–von Caemmerer–Berry model. The main systems of ion transport at rest, including H+-ATP-ases, H+/K+-antiporters, inwardly rectifying K+ channels, and outwardly rectifying K+ channels, are described in the two-dimensional photosynthetic model.
Briefly, the simplified FvCB model, which described only two limiting stages (the CO2 fixation by Rubisco and the linear electron flow in the electron transport chain of thylakoids in accordance with [51]), was used as the basis for the simulation of the photosynthetic CO2 assimilation in mesophyll cells (in accordance with standard Equation (1) [50,51]):
| (1) |
where Wc and Wj are carboxylation rates at the Rubisco-limited CO2 assimilation and electron transport-limited CO2 assimilation conditions, respectively (both values were calculated based on standard Equations (S2) and (S3) in accordance with [50]), [CO2]str is the concentration of CO2 in the stroma of chloroplasts, Γ* is the photosynthetic CO2 compensation point in the absence of mitochondrial respiration. It should be noted that Equation (1) was used for the estimation of the measured photosynthetic CO2 assimilation and for comparison with the experimental results. The photosynthetic consumption of CO2 in the stroma was described as min(Wc, Wj); i.e., the correction relating to photorespiration was not used in this case. Photorespiration was separately described as the CO2 source in the cytoplasm in accordance with Equation (2) based on Equation (1):
| (2) |
A dark respiration was described as another CO2 source in the cytoplasm. In accordance with von Caemmerer et al. [1], it was assumed that the rate of the dark respiration (Rd) was constant.
Carbon fluxes between cells and compartments were described based on Fick’s law [72,73,74]. CO2 fluxes across the stomata (jS), plasma membrane (jPM), and envelopes of chloroplasts (jChl), which depended on the CO2 conductance [74,75], were analyzed in the model (Equations (3)–(5)):
| (3) |
| (4) |
| (5) |
where [CO2]out, [CO2]ap, and [CO2]cyt are concentrations of CO2 in the air, apoplast and cytoplasm, respectively; gS0, gPM, and gChl are CO2 conductance for the stomata, plasma membrane, and chloroplast envelopes (jChl), respectively.
Similar HCO3− fluxes were assumed to be absent, because charged HCO3− should weakly diffuse across biological membranes [75].
The lateral fluxes of both neutral CO2 and charged HCO3− in the apoplast were considered in the model [73] and were described by Equations (S12) and (S13). In accordance with our previous work [70], it was assumed that each cell had its section of apoplast. The lateral fluxes were described between nearest sections (four lateral fluxes for each apoplast section, Figure 1a).
The ratios between the concentrations of CO2 and HCO3− were dependent on pH in the apoplast, cytoplasm, and stroma of chloroplasts [75]. It was assumed that the transitions between CO2 and HCO3− were fast; this meant that the stationary distribution between these molecules could be used. Equation (6) described the portion of CO2 in the total content of CO2 and HCO3−:
| (6) |
where pK is the negative logarithm of the equilibrium constant in the reaction of the transition between CO2 and HCO3−.
The stromal pH was assumed to be constant; the pH in the apoplast and cytoplasm was described based on our early model of ion transport and electrogenesis in plant cells [76].
The description of H+ and K+ fluxes across the plasma membrane was based on our previous model [70,76], which was simplified. Only H+-ATPase, inwardly and outwardly rectifying K+ channels, and K+/H+-antiporters were described, because the interaction of these systems could support stationary H+ concentrations in the cytoplasm and the apoplast: the H+-ATPase provided the primary transport of H+ across the plasma membrane; the K+ channels provided the K+ transport, which electrically compensated the charge transfer related to the proton transport through H+-ATPase; the K+/H+-antiporter prevented the non-physiological increase in cytoplasmic pH and K+ concentration and the decrease in apoplastic pH and K+ concentration.
The buffer properties of the cytoplasm (for H+) (Equation (S37)) and the apoplast (for K+ and H+) (Equations (S38) and (S39)) were described in accordance with Sukhova et al. [70]. H+-ATPase was described based on the “two-state model” [77] (Equation (S18)); a regulation of its activity by blue light and ATP concentration in the cytoplasm [78] was included in the model using the Hill Equation (Equations (S19) and (S20)). We used a stationary description of this ATP concentration (Equation (S40)), which was based on the ATP synthesis dependent on the dark respiration (constant) and the CO2 assimilation rate (the FvCB model) and the ATP hydrolysis with the assumed velocity constant.
K+ fluxes through inwardly and outwardly rectifying K+ channels were described based on the Goldman–Hodgkin–Katz Equation [76,79] (Equations (S21) and (S22)); the regulation of activities of these channels by the electrical potential across the plasma membrane of mesophyll cells was described based on the stationary solution of the Equation for the open probability for these channels [70] (Equations (S23) and (S24)).
H+ and K+ fluxes through the K+/H+-antiporter were described in accordance with our previous works [70,76] (Equation (S25)); this description was based on the simple Equation of the chemical kinetics. The K+/H+-antiporter was described as the electroneutral transporter because the transport of charges was compensated in this system.
The lateral fluxes of H+ and K+ were described based on Fick’s law in accordance with Sukhov et al. [80], (Equations (S31) and (S32)). The electrical potential across the plasma membrane was described as the stationary value in accordance with Sukhov et al. [80], (Equation (S26)); it was assumed that the electrical conductance between cells was zero.
The developed model included numerous parameters that made it difficult for the direct experimental parameterization of a specific plant object. Considering this point, we mainly used standard parameters from the FvCB model [50] and from our previous model of ion transport and electrogenesis in plant cells [70] (Table S1 in File S1); other data from the literature were also used for the parameterization. As a result, this model could rather show the qualitive properties of forming spatial heterogeneity in the photosynthetic parameters in the leaf surface. In contrast, this model (with the current parameters) was not optimal for the quantitative predictions of the specific plant object. It should be additionally noted that using standard parameters, which provided good descriptions of investigated processes in earlier works, minimized the probability of qualitive errors in the results of the simulation. In contrast, the broad experimental search of parameters in specific species of plants could, potentially, induce these errors (strong experimental errors in the estimation of even one of numerous parameters can disrupt the results of a simulation).
The developed model was numerically analyzed using the forward Euler method. The special computer program (Microsoft Visual C++ 2019, Microsoft Corporation, Redmond, WA, USA) was developed for the numerical analysis of the model. Equation (1) was used for the calculation of the Ahv simulated by the developed model.
The action of excess light and drought on the spatial heterogeneity was analyzed in our work. The excess light action was provided by using the high values of the Photosynthetic Active Radiation (PAR) in Equation (S5). It was assumed that the drought action on plants was mainly related to the stomatal closure. At the model analysis, this action was imitated by using the decreased stomatal CO2 conductance (the decreased parameter gS0 in Equation (3), the quantity of open stomata per leaf area was not changed) or the decreased quantity of open stomata per leaf area (from one stomata per 3 × 3 elements square to one stomata per 5 × 5 elements square, the stomatal CO2 conductance was not changed). The average leaf CO2 conductance (gS) was decreased from 0.064 mol m−2s−1 to 0.023 mol m−2s−1 in both cases of the model analysis.
3. Results
3.1. Verification of the Developed Model on the Basis of Light Curves of Simulated and Experimental Photosynthetic CO2 Assimilation Rate
The first question of the current analysis was: could the developed model simulate the experimental light curve of the photosynthetic CO2 assimilation rate? We used the average photosynthetic CO2 assimilation rates in pea plant leaves under the actinic light with different intensities and these assimilation rates at different average leaf CO2 conductance for this verification. Experimental and simulated results were compared in a quality manner by using the standard parameters of the FvCB model [50], which were not adapted for pea plants. The details of the experimental procedures are described in Section 5 “Materials and Methods”.
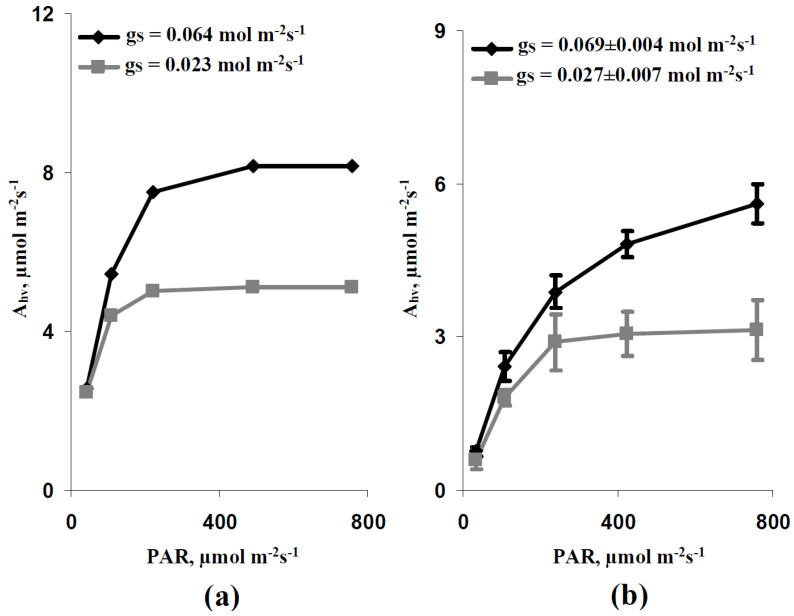
It is shown (Figure 2a) that the simulated dependence of average Ahv on the intensity of the actinic light at the basic gS (0.064 mol m−2s−1) included two parts: the increase in the CO2 assimilation rate with increasing intensity of illumination (low and moderate light intensities) and the light saturation of this assimilation rate (high light intensities). This effect was also observed at the decreased average gS (0.023 mol m−2s−1), which was imitated by using the decreased stomatal CO2 conductance; however, the maximal Ahv at gS = 0.064 mol m−2s−1 was higher than one at gS = 0.023 mol m−2s−1. Additionally, the minimal light intensity for the Ahv saturation at gS = 0.064 mol m−2s−1 was higher than one at gS = 0.023 mol m−2s−1.
Figure 2.
Simulated (a) and experimental (b) dependences of the average photosynthetic CO2 assimilation rate (Ahv) on the intensity of the photosynthetic active radiation (PAR) at the varied average leaf CO2 conductance (gS). Simulated dependences were calculated at average gS = 0.064 mol m−2s−1 (the basic gS) and gS = 0.023 mol m−2s−1 (the decreased gS). Each stomata in the model was located in the center of square (3 × 3 elements); the average gS was calculated as the CO2 conductance in the element with stomata divided by 9. In order to obtain experimental dependences, all experimental records in this series were ranged and divided into two groups with the low (gS < 0.04 mol m−2s−1, n = 5) and high (gS > 0.04 mol m−2s−1, n = 9) CO2 conductance (see Section 5.1). A combination of Dual-PAM-300 and GFS-3000 was used in the experimental measurements of pea seedlings.
Experimental plants were ranged in accordance with their gS and were divided into two groups with high and low CO2 conductance (average gS in leaves was 0.069 ± 0.004 and 0.027 ± 0.007 mol m−2s−1, respectively, see Section 5.1). It is shown (Figure 2b) that experimental Ahv dependences on light intensity were similar to simulated ones: (i) there were stages of increase in the photosynthetic CO2 assimilation rate and stage of Ahv light saturation, (ii) the maximal Ahv was increased with increasing gS, and (iii) the minimal light intensity for the Ahv saturation was increased with increasing stomatal CO2 conductance. It should be additionally noted that the values of the maximal Ahv differed in the experimental and the simulated results. This moderate quantitative difference could be caused by the used standard values of the model parameters, which were not adapted for pea seedlings (see Section 2).
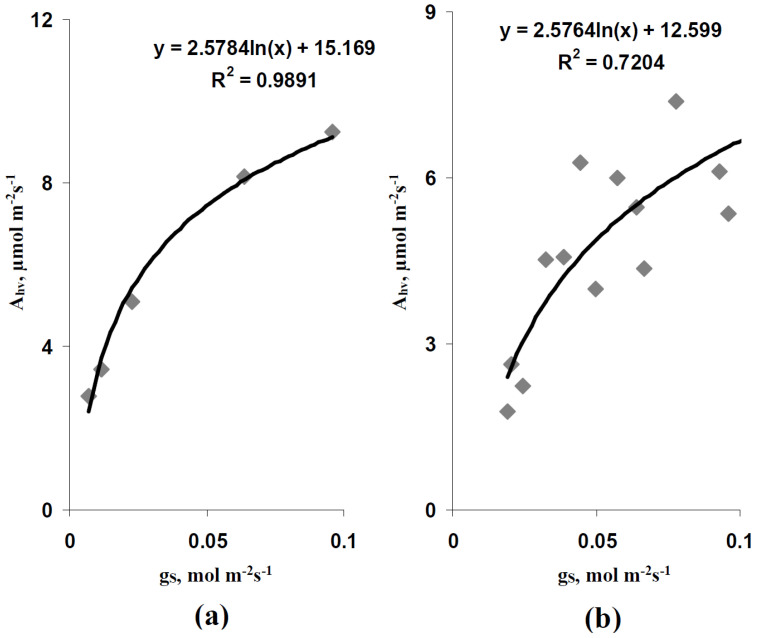
Simulated (Figure 3a) and experimental (Figure 3b) dependences of Ahv on gS at the high light intensity (758 µmol m−2s−1) were analyzed in the next stage of our work. It is shown that both dependences were qualitatively similar and could be described by logarithmic Equations with similar coefficients. Quantitative differences between dependences were probably caused by the absence of adaptation of parameters for pea plants.
Figure 3.
Simulated (a) and experimental (b) scatter plots between the average photosynthetic CO2 assimilation rate (Ahv) and the average leaf CO2 conductance (gS) under high intensity of the photosynthetic active radiation (758 µmol m−2s−1). Simulated Ahv were calculated at the average gS equaling 0.007, 0.012, 0.023, 0.064, and 0.096 mol m−2s−1. Each stomata in the model was located in the center of square (3 × 3 elements); the average gS was calculated as the CO2 conductance in the element with stomata divided by 9. Pea seedlings were experimentally investigated; all gS and Ahv (under the 758 µmol m−2s−1 PAR intensity) were used (n = 14). R2 is the determination coefficient.
Thus, these results showed that the developed model based on the two-dimensional system of photosynthetic cells could qualitatively describe important characteristics of Ahv, including the shape of the light dependence of the photosynthetic CO2 assimilation rate and changes in this shape and maximal Ahv during changes in the stomatal CO2 conductance. As a result, the developed model could be used for further analysis in our current work.
3.2. Analysis of Simulated and Experimental Spatial Heterogeneities in the Photosynthetic CO2 Assimilation Rate under Various Light Intensity and Stomatal CO2 Conductance
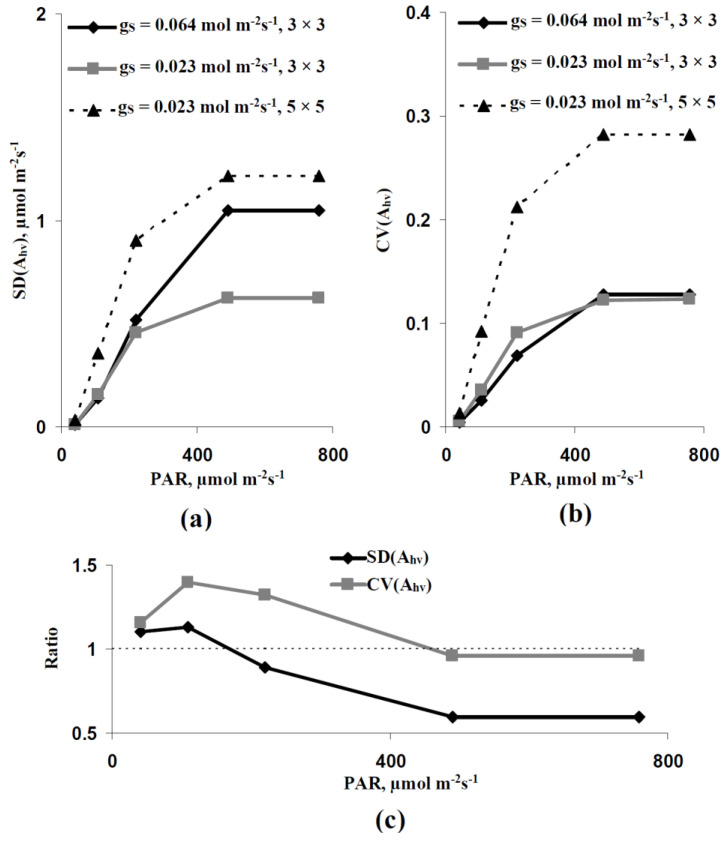
The spatial heterogeneity of Ahv simulated by the developed model was analyzed in the next stage of investigation. First, the standard deviation of Ahv (SD(Ahv)), which was calculated based on the values of Ahv in all elements of the two-dimensional model of the leaf, was analyzed. It is shown (Figure 4a) that SD(Ahv) was increased with the increase in light intensity at all variants of the average leaf CO2 conductance. A decrease in the average gS (from 0.064 to 0.023 mol m−2s−1) caused by the decrease in the stomatal CO2 conductance strongly decreased SD(Ahv). In contrast, the similar decrease in the average gS caused by the decrease in the quantity of stomata per area unit weakly influenced SD(Ahv).
Figure 4.
Dependences of parameters of the simulated spatial heterogeneity of the photosynthetic CO2 assimilation rate (Ahv) on the intensity of the photosynthetic active radiation (PAR). (a) Dependence of the standard deviation of Ahv (SD(Ahv)) on the PAR intensity. There were three variants of parameters. (i) The average gS of the leaf was 0.064 mol m−2s−1, each stomata was located in the center of the 3 × 3 elements square. This variant was assumed as the control. (ii) The average gS of the leaf was decreased to 0.023 mol m−2s−1. The CO2 conductance in individual stomata was decreased; each stomata was located in the center of the 3 × 3 elements square. (iii) The average gS of the leaf was decreased to 0.023 mol m−2s−1. The CO2 conductance in individual stomata was not changed; each stomata was located in the center of the 5 × 5 elements square. (b) Dependence of the coefficient of variation of Ahv (CV(Ahv)) on the PAR intensity. (c) Dependence of the ratio of the SD(Ahv) at gS = 0.023 mol m−2s−1 (3 × 3 elements) to the SD(Ahv) at gS = 0.064 mol m−2s−1 (3 × 3 elements) on the PAR intensity and the analogical dependence for CV(Ahv).
However, SD(Ahv) should be strongly related to the absolute value of Ahv; thus, all revealed changes could be related to changes in this value. We analyzed the coefficient of variation (CV(Ahv)) to eliminate the influence of the absolute value of Ahv on the estimation of the spatial heterogeneity, because the variation coefficient was calculated as the standard deviation divided by the average value. It is shown (Figure 4b) that CV(Ahv) was also strongly increased with increasing light intensity in all analyzed variants. The decrease in the average gS caused by the decrease in the quantity of stomata per area unit strongly increased CV(Ahv). The decrease in the average gS caused by the decrease in the stomatal CO2 conductance weakly influenced CV(Ahv); however, CV(Ahv) in this variant was higher than CV(Ahv) at the control average gS (0.064 mol m−2s−1) under low and moderate light intensities.
We analyzed a ratio between SD(Ahv) at the control average gS and at the decreased average gS, which was caused by the decrease in the stomatal CO2 conductance (with no change in the quantity of stomata), and the analogical ratio between CV(Ahv) to additionally estimate the last effect. It is shown (Figure 4c) that these ratios were increased under the low light intensity and the ratio of CV(Ahv) was also increased under the moderate light intensity.
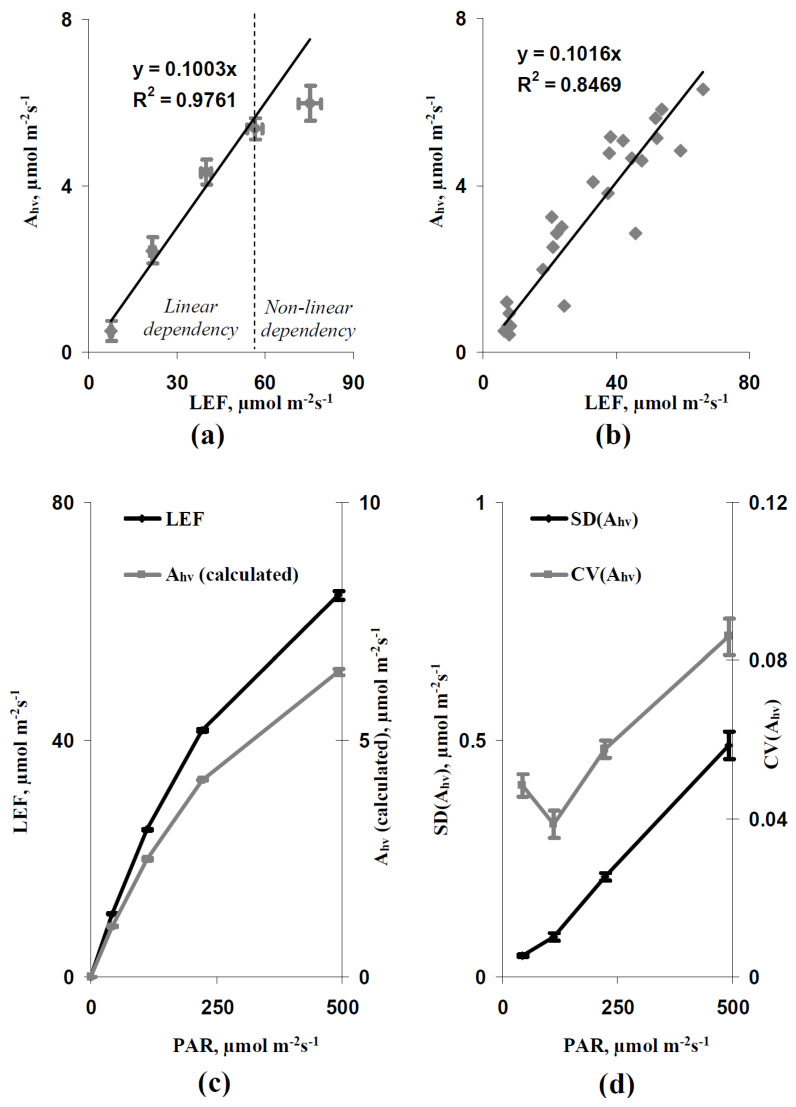
Thus, the results of the simulation show that the increase in light intensity and the decrease in leaf CO2 conductance could increase the spatial heterogeneity of the photosynthetic CO2 assimilation rate. After that, we experimentally analyzed this heterogeneity to check the revealed results. The direct experimental analysis of Ahv was not possible. However, the FvCB model [42,49,50,51] predicted that the linear relation between Ahv and LEF could be probable at the limitation of photosynthesis by the linear electron flow. Figure 5a shows that the average Ahv and LEF were strongly linearly related with increasing LEF (with increasing intensity of the actinic light) to about 60 µmol m−2s−1; this linear relation was disrupted at higher values of LEF (75 µmol m−2s−1 LEF at the 758 µmol m−2s−1 light intensity). Analysis of individual Ahv and LEF (excluding LEF at 758 µmol m−2s−1 light intensity) showed a similar linear relation at LEF equaling 6.5–66.2 µmol m−2s−1 (Figure 5b). Thus, linear regression Ahv = 0.1 LEF was used for the calculation of Ahv based on the measured LEF at LEF ≤ 66 µmol m−2s−1.
Figure 5.
The dependence of average photosynthetic CO2 assimilation rate (Ahv) on the average linear electron flow (LEF) at 34, 108, 239, 425, and 758 µmol m−2s−1 intensities of actinic light (n = 5–7) and the linear calibration Equation (a), the dependence of individual Ahv on individual LEF at 34, 108, 239, and 425 µmol m−2s−1 light intensities (n = 25) and the linear calibration Equation (b), dependences of LEF and Ahv (calculated) on the PAR intensity (n = 6) (c), and dependences of parameters of the spatial heterogeneity of Ahv (calculated) (SD(Ahv) and CV(Ahv)) on the PAR intensity (n = 6) (d). R2 is the determination coefficient. Ahv (calculated) was calculated based on LEF and the calibration Equation. A combination of Dual-PAM-300 and GFS-3000 was used for development of the calibration Equation. IMAGING-PAM M-Series MINI Version was used for analysis of the spatial heterogeneity of Ahv. Pea seedlings were used in all variants of experiments.
It is shown that the increase in light intensity increased the linear electron flow and calculated Ahv (Figure 5c). The experimental SD(Ahv) and CV(Ahv), which showed the spatial heterogeneity of the photosynthetic CO2 assimilation rate in leaves, were also increased with increasing light intensity (Figure 5d). This result was in good accordance with the results of the simulation and supported the induction of the photosynthetic spatial heterogeneity under excess light conditions.
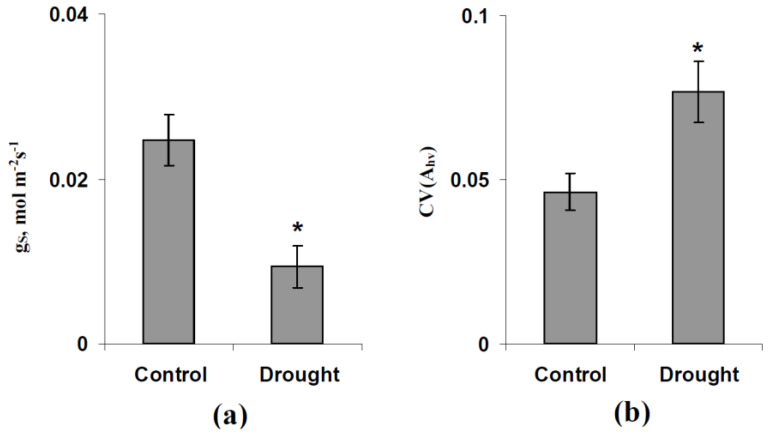
Finally, we experimentally checked the increase in CV(Ahv) at the decreased average gS that was predicted by the developed model. It is shown that the short-term drought (1 day) decreased the gS in pea leaves (Figure 6a), which was probably related to the stomata closing. CV(Ahv), calculated based on the variation coefficient of LEF, was significantly increased during the short-term drought (Figure 6b). This result experimentally supported the increase in photosynthetic spatial heterogeneity due to the stomata closing.
Figure 6.
Influence of the short-term drought (1 day) on the leaf CO2 conductance (gS) (a) and the coefficient of variation of Ahv (CV(Ahv)) showing the relative spatial heterogeneity of this parameter in the leaf (b) (n = 6). GFS-3000 was used for the gS measurement (averaged in the investigated area of the leaf) and IMAGING-PAM M-Series MINI Version was used for the analysis of the spatial heterogeneity of Ahv (based on the spatial heterogeneity of LEF and the calibration Equation). The moderate light intensity (249 µmol m−2s−1) was used in this experiment. Pea seedlings were irrigated in the control and were not irrigated under drought conditions. *, difference with the control was significant.
4. Discussion
Photosynthesis is a complex process [1,2] that can be strongly affected by numerous abiotic stressors [3,4,15,16]. The simulation of photosynthetic processes is an effective prediction tool of photosynthetic changes under the action of stressors [31]. There are photosynthetic models focusing on descriptions of the primary light absorption [32,33,34], photosynthetic light reactions [5,35,36,37,38,39,40], photosynthetic dark reactions, and CO2 fluxes [41,42,43,44], etc. However, mathematical models of photosynthetic processes in the scale of the leaf surface, which can be used for revealing the spatial heterogeneity of the distribution of photosynthetic parameters on this surface, are weakly developed. Our current work—devoted to the solution of this problem—shows two important results.
First, the developed two-dimensional model of C3 photosynthesis in the leaf, which is based on the FvCB model [42,49,50,51], descriptions of stomatal and transmembrane fluxes of CO2 and lateral fluxes of CO2 and HCO3− [73,74,75], and the simplified model of the H+ and K+ transport [70,76,77,80] can qualitatively simulate the experimental results, including the shape of dependence of the average Ahv in the leaf on the light intensity and the influence of the average gS on the photosynthetic CO2 assimilation rate (see Figure 2 and Figure 3). It is important that this accordance between the experimental and the simulated results does not require additional adaptation of parameters of the photosynthetic description in the developed model because standard parameters of the FvCB model [50] are used (Table S1 in File S1). This result verifies the efficiency of the developed model for the simulation of the average Ahv. Furthermore, considering that this model can also describe the spatial heterogeneity of the Ahv distribution on the leaf surface, it is a potential tool for the investigation of the influence of stressors on this heterogeneity.
Second, the developed model predicts the increase in the Ahv spatial heterogeneity on the leaf surface with increasing light intensity (Figure 4). This effect is related to the stomatal CO2 conductance and the quantity of open stomata supporting the CO2 flux into the leaf, because the decrease in this conductance or the quantity of open stomata per leaf area increases the simulated photosynthetic spatial heterogeneity (especially at the weak and moderate light intensities). The results of analysis of the developed model are in good accordance with works showing the relations of the spatial heterogeneity and the dynamics of the stomata opening to the distribution of photosynthetic parameters in leaves [81,82,83]. Additionally, there are works [83,84,85,86] showing an increase in the spatial heterogeneity of photosynthetic parameters under drought conditions. The participation of the stomata closing due to this effect is a discussion question [85,86]; however, considering the influence of drought on stomata [87,88], this participation cannot be excluded.
Our experimental results support the prediction of the developed model: an increase in light intensity increases the variation coefficient of the photosynthetic CO2 assimilation rate in pea leaves (Figure 5d) and a decrease in leaf CO2 conductance, induced by the short-term drought, also increases this coefficient (Figure 6). These results, which are in good accordance with the noted experimental works by other authors showing the positive drought influence on the photosynthetic spatial heterogeneity in leaves [83,84,85,86] additionally verify the developed model.
A potential mechanism of the revealed light-induced increase in the Ahv spatial heterogeneity can be related to the heterogeneity of the stromal CO2 concentration in the different cells. In accordance with the FvCB model [42,49,50,51], this concentration can strongly influence Ahv in cells. On the other hand, CO2 is propagated from stomata through lateral diffusion [89,90] and is consumed by photosynthetic processes, which can be dependent on the light intensity. It means that an increase in this intensity and the stimulation of photosynthesis should increase the variability of the CO2 concentration in different cells; i.e., the light intensity should influence the spatial heterogeneity of the stromal CO2 concentration. The additional model analysis of the variation coefficient of this concentration shows that this coefficient is strongly increased by changes in the light intensity from 42 µmol m−2s−1 to 221 µmol m−2s−1 (from 0.013 to 0.100, respectively); thus, this mechanism can participate in an increase in the Ahv spatial heterogeneity under the excess light.
A decrease in the quantity of open stomata per leaf area should stimulate this effect by increasing the distance of the CO2 diffusion. This supposition is supported by an increase in the variation coefficient of the simulated stromal CO2 concentration from 0.100 to 0.180 by decreasing this quantity from one stomata per 9 cells to one stomata per 25 cells under the 221 µmol m−2s−1 light intensity. In contrast, a decrease in the stomatal CO2 conductance (without changes in the open stomata quantity) weakly influences this coefficient (data not shown). The last result shows that there are additional induction mechanisms of the Ahv spatial heterogeneity in the leaf. It cannot be excluded that these additional mechanisms also participate in influencing the light intensity on the Ahv heterogeneity.
The revealed stimulation of the Ahv spatial heterogeneity under excess light conditions and/or under the decreased leaf CO2 conductance (imitation of the drought) can potentially modify the non-photochemical quenching of the chlorophyll fluorescence, including photodamage, state-transition in the light-harvesting complex, and energy-dependent quenching [3,4,18,19], because low Ahv in some parts of a leaf can strongly limit photosynthetic light reactions and can contribute to the induction of these processes. It means that this spatial heterogeneity can potentially modify the plant tolerance to the actions of the excess light. Particularly, cells with low CO2 concentration in the stroma and weak activity of the photosynthetic CO2 assimilation should have a low threshold for both photodamage and induction of protective changes in the photosynthetic machinery. It can be expected that these cells can influence damage and tolerance of whole leaves under the action of stressors (e.g., through the production and propagation of reactive oxygen species [71]); however, this supposition requires further development of the model (e.g., a description of the damage of photosynthetic machinery in the model can be included in the model) and the model-based investigations.
Additionally, the increased Ahv spatial heterogeneity and related changes in photosynthetic light reactions can be used for the development of methods of remote sensing plant stress changes under excess light or drought conditions. Particularly, it can be expected that these stressors should increase the heterogeneity of the spatial distribution of PRI because this reflectance index is strongly related to photosynthetic parameters [61,62,64,66,67]. Potentially, this effect can be used for the development of methods of remote sensing the actions of excess light and drought on plants (based on the measurements of the spatial heterogeneity of PRI); however, this possible stimulation of PRI under the action of stressors requires future model-based and experimental investigations.
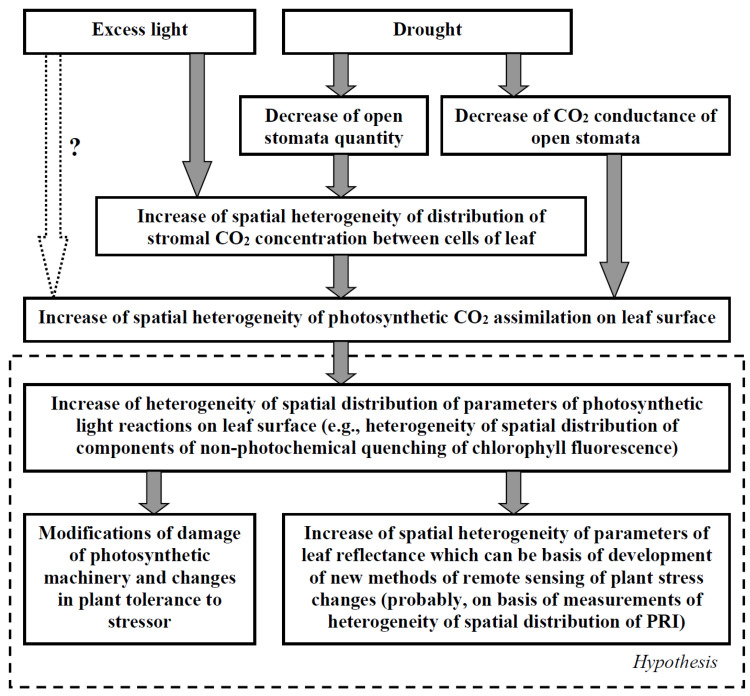
Figure 7 summarizes the results of our work and their potential importance for understanding the ways of plant damage and tolerance under the action of stressors and the development of methods for plant remote sensing. It should be additionally noted that the developed model can be used for future analysis of the influence of the stochastic spatial heterogeneity of its parameters on photosynthetic processes; e.g., the influence of the stochastic heterogeneity of the activity of H+-ATPases in the plasma membrane [31], which is related to the CO2 flux into mesophyll cells [71], or the influence of the stochastic heterogeneity of the CO2 conductance of individual stomata can be investigated. It is known that the stochastic spatial heterogeneity of biological objects (including plants) can influence their systemic parameters (e.g., through “diversity-induced resonance” or similar effects, [31,68,69]); thus, the analysis of this problem based on the developed model can be an important task.
Figure 7.
A scheme of potential ways the excess light and drought influencing the heterogeneity of the spatial distribution of photosynthetic parameters and the hypothetical importance of this heterogeneity for the plant tolerance and remote sensing of plant stress changes. The scheme is based on analysis of the developed model and experimental results (see Section 4 for details).
Other interesting perspectives of the model development can be: description of stomata regulation mechanisms by light intensity and drought (and potential interactions between these mechanisms), description of the light damage to photosynthetic machinery (and relation of this damage with stomata opening, the plasma membrane and chloroplast envelope CO2 conductance, and activity of the CO2 carboxylation), and description of the influence of photosynthetic processes to leaf reflectance (this description can be important for the development of methods of remote sensing). Finally, the parameterization of the model for specific plant species (e.g., plant species that are widely used in agriculture) can be an additional important task for the future development of the model.
5. Materials and Methods
5.1. Experimental Procedure of Verification of Two-Dimensional Model of the C3 Photosynthesis in Plant Leaves
We did not parameterize the two-dimensional model of C3 photosynthesis in leaves for the specific plant, because using the standard parameters from earlier models, which were included in the current model, simplified parameterization and minimized potential errors in parameter values that were probable at the broad experimental search and could disrupt the model analysis.
Therefore, we could not expect a quantitative accordance between the simulated and the experimental photosynthetic parameters at verification. As a result, we analyzed the qualitive accordance between the results of the simulation and the results of the experimental investigation of the pea plant. Pea plants were selected based on our numerous early works, which investigated photosynthesis and its regulation in this plant object (e.g., [5,66,67,91]).
Thus, 2–3-week-old pea seedlings (Pisum sativum L., cultivar “Albumen”) were used for verification of the two-dimensional model of C3 photosynthesis in plant leaves. The plants were cultivated in a sand substrate in a Binder KBW 240, with irrigation by the 50% Hoagland–Arnon medium (about 50 mL) performed every two days. Luminescent lamps FSL YZ18RR (Foshan Electrical And Lighting Co., Ltd., Foshan, China) were used for illumination (about 100 µmol m−2s−1). The weak water deficit (the short-term drought) was induced by an absence of irrigation of the experimental seedlings for 1 day.
A combination of a PAM-fluorometer Dual-PAM-100 and an infrared gas analyzer GFS-3000 (Heinz Walz GmbH, Effeltrich, Germany) was used for the investigation of the average photosynthetic parameters in the second mature leaves of the pea plant. Ahv was measured as the difference between the CO2 assimilation rate after 10 min under the actinic blue light (Dual-PAM-100 was used as the source of this light) and this assimilation rate under dark conditions. The current CO2 assimilation rate was measured by the gas analyzer GFS-3000. The leaf CO2 conductance was calculated based on the leaf water conductance, which was measured by GFS-3000, in accordance with Cabrera et al. [92]. The GFS-3000 was also used for supporting the 360 ppm concentration of CO2 and the 70% relative air humidity in the measuring cuvette.
A photosynthetic linear electron flow (LEF) was calculated based on the effective quantum yield of the photosystem II (ΦPSII), the intensity of the actinic light (PAR), the fraction of absorbed light distributed to the photosystem II (dII = 0.42), and the fraction of PAR absorbed by the leaves (p = 0.88) in accordance with Equation (7) [91]:
| (7) |
ΦPSII was estimated after 10 min under the actinic light. This parameter was automatically calculated by the Dual-PAM-100 software based on the current levels of fluorescence (F) and the maximal fluorescence level after the preliminary illumination (), which were measured before initiation and before termination of the saturation pulse (300 ms, red light, 10,000 µmol m−2s−1), respectively, in accordance with the standard procedure of measurement by the PAM fluorometer. Equation (8) was used for the ΦPSII calculation [93]:
| (8) |
The blue light from the standard source of Dual-PAM-100 was used as the actinic light; its intensity was varied.
There were two variants of experiments combining the Dual-PAM-100 and the GFS-3000. First, we preliminary experimentally estimated the basic gS that was used for the calculation of the stomatal CO2 conductance in the model (gS0 = gS·9 because one stomata per nine elements was used as the control variant in the model, Table S1 in File S1). Experiments were performed for 1 day; light curves were not analyzed. It was shown that gS = 0.064 ± 0.04 mol m−2s−1 (n = 6). As a result, gS = 0.064 mol m−2s−1 (and gS0 = 0.576 mol m−2s−1) was used as the basic leaf CO2 conductance. In the model, the decreased gS was provided by the decreased gS0 or the decreased quantity of stomata per leaf area (from one stomata per 3 × 3 elements square to one stomata per 5 × 5 elements square, see Section 2); both decreased gS should be the same when compared. Thus, the decreased gS was calculated as the multiplication between the basic gS and 9/25 (the decreased gS0 was similarly calculated, Table S1 in File S1).
Second, we analyzed the experimental light curves, which were investigated for the long-time experimental series (about 2 weeks). In this case, the experimental gS was more varied than the gS in the first case (gS = 0.058 ± 0.11 mol m−2s−1, n = 14). This variability was used for the additional verification of the model; all experimental records in this series were ranged and divided into two groups with the low (gS < threshold value) and high (gS > threshold value) CO2 conductance. We found that using the 0.04 mol m−2s−1 threshold value provided an average gS which was similar to the leaf CO2 conductance in the model: 0.069 ± 0.004 mol m−2s−1 (n = 9) and 0.027 ± 0.007 mol m−2s−1 (n = 5). After that, we separately statistically analyzed the light dependences in these two groups (with the low and high CO2 conductance) to verify the developed model.
A system of PAM imaging IMAGING-PAM M-Series MINI Version (Heinz Walz GmbH, Effeltrich, Germany) was used for the measurements of the spatial distribution of photosynthetic parameters. The blue light from the standard source of this system was used as the actinic light; its intensity was varied. ΦPSII was estimated at the saturation pulse (in accordance with Equation (8)) after 10 min under the actinic light.
The analysis of the spatial distributions of LEF was based on the analysis of grayscale images of the spatial distribution of the quantum yield of photosystem II, which were created by software of the IMAGING-PAM M-Series MINI Version. These grayscale images were analyzed using ImageJ 1.46r. The analysis showed the average value and the standard deviation of ΦPSII in the standard round ROI in the center of the leaf. The coefficient of variation was calculated as the ratio of the standard deviation of the average value. The parameters of LEF (the average value, standard deviation, and coefficient of variation) were calculated based on Equation (7) as the simple proportion. These parameters were used for the estimation of the parameters of Ahv (the average value, standard deviation, and coefficient of variation) based on the calibration curve (see Section 3.2).
5.2. Statistics
Means and standard errors were used in the statistical analysis and Student’s t-test was used for the estimation of significance. The spatial heterogeneity was estimated based on the standard deviation of Ahv (SD(Ahv)) and the coefficient of variation of this photosynthetic parameter (CV(Ahv)). Numbers of repetitions were shown in figures.
6. Conclusions
The work was devoted to the development of a two-dimensional model of C3 photosynthesis in the plant leaf and further analysis of the induction of the spatial heterogeneity of the CO2 assimilation rate under the excess light and a decrease in the leaf CO2 conductance; this gS decrease imitated the action of a short-term drought. First, it was shown that the developed two-dimensional model of C3 photosynthesis in the leaf (based on the FvCB model, the descriptions of the fluxes of CO2 and HCO3−, and the simplified model of the H+ and K+ transport) qualitatively simulated the experimental results. Second, the analysis of the developed model showed that the increase in the light intensity and the decrease in the average leaf CO2 conductance should increase the spatial heterogeneity of the photosynthetic CO2 assimilation rate on the leaf surface. Experimental investigations supported these theoretical results. Thus, the developed model can be used as a tool for theoretical investigations of the influence of environmental factors on the spatial heterogeneity of the distribution of photosynthetic parameters in the leaf. Finally, there are some potential ways to further develop the model, including its parameterization for specific plant species, additional description of stomata regulation by light and drought, description of light damage to photosynthetic machinery, description of relations between photosynthesis and leaf reflectance, analysis of influence of stochastic heterogeneity in photosynthetic and stomata parameters, and others.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11233285/s1, File S1 “Equations and parameters of the two-dimensional photosynthetic model”, Refs [50,51,70,72,73,74,75,76,77,78,79,80,94,95,96,97,98] have mentioned in Supplementary Materials.
Author Contributions
Conceptualization, E.S. and V.S.; methodology, E.S., D.R. and V.S.; software, E.S.; formal analysis, E.S.; investigation, E.S., D.R. and E.G.; writing—original draft preparation, E.S. and V.S.; writing—review and editing, V.S.; supervision, V.S.; project administration, E.S.; funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
The investigation was funded by the Russian Foundation for Basic Research, project number 20-34-90086 Aspiranti.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allen J.F. Cyclic, pseudocyclic and noncyclic photophosphorylation: New links in the chain. Trends Plant Sci. 2003;8:15–19. doi: 10.1016/S1360-1385(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 2.Johnson M.P. Photosynthesis. Essays Biochem. 2016;60:255–273. doi: 10.1042/EBC20160016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruban A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015;66:7–23. doi: 10.1093/jxb/eru400. [DOI] [PubMed] [Google Scholar]
- 4.Ruban A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016;170:1903–1916. doi: 10.1104/pp.15.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukhova E., Khlopkov A., Vodeneev V., Sukhov V. Simulation of a nonphotochemical quenching in plant leaf under different light intensities. Biochim. Biophys. Acta Bioenerg. 2020;1861:148138. doi: 10.1016/j.bbabio.2019.148138. [DOI] [PubMed] [Google Scholar]
- 6.Tikkanen M., Grieco M., Nurmi M., Rantala M., Suorsa M., Aro E.-M. Regulation of the photosynthetic apparatus under fluctuating growth light. Phil. Trans. R. Soc. B. 2012;367:3486–3493. doi: 10.1098/rstb.2012.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang W., Hu H., Zhang S.B. Photorespiration plays an important role in the regulation of photosynthetic electron flow under fluctuating light in tobacco plants grown under full sunlight. Front. Plant Sci. 2015;6:621. doi: 10.3389/fpls.2015.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retkute R., Smith-Unna S.E., Smith R.W., Burgess A.J., Jensen O.E., Johnson G.N., Preston S.P., Murchie E.H. Exploiting heterogeneous environments: Does photosynthetic acclimation optimize carbon gain in fluctuating light? J. Exp. Bot. 2015;66:2437–2447. doi: 10.1093/jxb/erv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser E., Morales A., Harbinson J. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 2018;176:977–989. doi: 10.1104/pp.17.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flexas J., Medrano H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002;89:183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medrano H., Escalona J.M., Bota J., Gulías J., Flexas J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002;89:895–905. doi: 10.1093/aob/mcf079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zivcak M., Brestic M., Balatova Z., Drevenakova P., Olsovska K., Kalaji H.M., Yang X., Allakhverdiev S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013;117:529–546. doi: 10.1007/s11120-013-9885-3. [DOI] [PubMed] [Google Scholar]
- 13.Antolín M.C., Hekneby M., Sánchez-Díaz M. Contrasting responses of photosynthesis at low temperatures in different annual legume species. Photosynthetica. 2005;43:65–74. doi: 10.1007/s11099-005-5074-8. [DOI] [Google Scholar]
- 14.Bukhov N.G., Wiese C., Neimanis S., Heber U. Heat sensitivity of chloroplasts and leaves: Leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth. Res. 1999;59:81–93. doi: 10.1023/A:1006149317411. [DOI] [Google Scholar]
- 15.Allakhverdiev S.I., Kreslavski V.D., Klimov V.V., Los D.A., Carpentier R., Mohanty P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008;98:541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R., Sharkey T.D. Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth. Res. 2009;100:29–43. doi: 10.1007/s11120-009-9420-8. [DOI] [PubMed] [Google Scholar]
- 17.Fischer B.B., Hideg É., Krieger-Liszkay A. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid. Redox Signal. 2013;18:2145–2162. doi: 10.1089/ars.2012.5124. [DOI] [PubMed] [Google Scholar]
- 18.Müller P., Li X.P., Niyogi K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz J.A., Avenson T.J., Kanazawa A., Takizawa K., Edwards G.E., Kramer D.M. Plasticity in light reactions of photosynthesis for energy production and photoprotection. J. Exp. Bot. 2005;56:395–406. doi: 10.1093/jxb/eri022. [DOI] [PubMed] [Google Scholar]
- 20.Joliot P., Johnson G.N. Regulation of cyclic and linear electron flow in higher plants. Proc. Natl. Acad. Sci. USA. 2011;108:13317–13322. doi: 10.1073/pnas.1110189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alte F., Stengel A., Benz J.P., Petersen E., Soll J., Groll M., Bölter B. Ferredoxin: NADPH oxidoreductase is recruited to thylakoids by binding to a polyproline type II helix in a pH-dependent manner. Proc. Natl. Acad. Sci. USA. 2010;107:19260–19265. doi: 10.1073/pnas.1009124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benz J.P., Stengel A., Lintala M., Lee Y.H., Weber A., Philippar K., Gügel I.L., Kaieda S., Ikegami T., Mulo P., et al. Arabidopsis Tic62 and ferredoxin-NADP(H) oxidoreductase form light-regulated complexes that are integrated into the chloroplast redox poise. Plant Cell. 2010;21:3965–3983. doi: 10.1105/tpc.109.069815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozaki A., Takeba G. Photorespiration protects C3 plants from photooxidation. Nature. 1996;384:557–560. doi: 10.1038/384557a0. [DOI] [Google Scholar]
- 24.Davis P.A., Hangarter R.P. Chloroplast movement provides photoprotection to plants by redistributing PSII damage within leaves. Photosynth. Res. 2012;112:153–161. doi: 10.1007/s11120-012-9755-4. [DOI] [PubMed] [Google Scholar]
- 25.Wada M. Chloroplast movement. Plant Sci. 2013;210:177–182. doi: 10.1016/j.plantsci.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Ptushenko O.S., Ptushenko V.V., Solovchenko A.E. Spectrum of light as a determinant of plant functioning: A historical perspective. Life. 2020;10:25. doi: 10.3390/life10030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyake C., Yokota A. Cyclic flow of electrons within PSII in thylakoid membranes. Plant Cell Physiol. 2001;42:508–515. doi: 10.1093/pcp/pce063. [DOI] [PubMed] [Google Scholar]
- 28.Miyake C., Yonekura K., Kobayashi Y., Yokota A. Cyclic electron flow within PSII functions in intact chloroplasts from spinach leaves. Plant Cell Physiol. 2002;43:951–957. doi: 10.1093/pcp/pcf113. [DOI] [PubMed] [Google Scholar]
- 29.Jajoo A., Mekala N.R., Tongra T., Tiwari A., Grieco M., Tikkanen M., Aro E.M. Low pH-induced regulation of excitation energy between the two photosystems. FEBS Lett. 2014;588:970–974. doi: 10.1016/j.febslet.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 30.Demmig-Adams B., Adams W.W., III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. doi: 10.1016/S1360-1385(96)80019-7. [DOI] [Google Scholar]
- 31.Sukhova E.M., Vodeneev V.A., Sukhov V.S. Mathematical modeling of photosynthesis and analysis of plant productivity. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2021;15:52–72. doi: 10.1134/S1990747821010062. [DOI] [Google Scholar]
- 32.Bernhardt K., Trissl H.-W. Theories for kinetics and yields of fluorescence and photochemistry: How, if at all, can different models of antenna organization be distinguished experimentally? Biochim. Biophys. Acta Bioenerg. 1999;1409:125–142. doi: 10.1016/S0005-2728(98)00149-2. [DOI] [PubMed] [Google Scholar]
- 33.Vredenberg W.J. A three-state model for energy trapping and chlorophyll fluorescence in photosystem II incorporating radical pair recombination. Biophys. J. 2000;79:26–38. doi: 10.1016/S0006-3495(00)76271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulychev A.A., Vredenberg W.J. Modulation of photosystem II chlorophyll fluorescence by electrogenic events generated by photosystem I. Bioelectrochemistry. 2001;54:157–168. doi: 10.1016/S1567-5394(01)00124-4. [DOI] [PubMed] [Google Scholar]
- 35.Lazár D. Chlorophyll a fluorescence rise induced by high light illumination of dark-adapted plant tissue studied by means of a model of photosystem II and considering photosystem II heterogeneity. J. Theor. Biol. 2003;220:469–503. doi: 10.1006/jtbi.2003.3140. [DOI] [PubMed] [Google Scholar]
- 36.Porcar-Castell A., Bäck J., Juurola E., Hari P. Dynamics of the energy flow through photosystem II under changing light conditions: A model approach. Func. Plant Biol. 2006;33:229–239. doi: 10.1071/FP05133. [DOI] [PubMed] [Google Scholar]
- 37.Ebenhöh O., Houwaart T., Lokstein H., Schlede S., Tirok K. A minimal mathematical model of nonphotochemical quenching of chlorophyll fluorescence. Biosystems. 2011;103:196–204. doi: 10.1016/j.biosystems.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Tikhonov A.N., Vershubskii A.V. Computer modeling of electron and proton transport in chloroplasts. Biosystems. 2014;121:1–21. doi: 10.1016/j.biosystems.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Morales A., Yin X., Harbinson J., Driever S.M., Molenaar J., Kramer D.M., Struik P.C. In silico analysis of the regulation of the photosynthetic electron transport chain in C3 plants. Plant Physiol. 2018;176:1247–1261. doi: 10.1104/pp.17.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belyaeva N.E., Bulychev A.A., Riznichenko G.Y., Rubin A.B. Analyzing both the fast and the slow phases of chlorophyll a fluorescence and P700 absorbance changes in dark-adapted and preilluminated pea leaves using a thylakoid membrane model. Photosynth. Res. 2019;140:1–19. doi: 10.1007/s11120-019-00627-8. [DOI] [PubMed] [Google Scholar]
- 41.Laisk A., Eichelmann H., Oja V., Eatherall A., Walker D.A. A mathematical model of carbon metabolism in photosynthesis: Difficulties in explaining oscillations by fructose 2,6-bisphosphate regulation. Proc. R. Soc. Lond. B Biol. Sci. 1989;237:389–415. [Google Scholar]
- 42.Von Caemmerer S. Steady-state models of photosynthesis. Plant Cell Environ. 2013;36:1617–1630. doi: 10.1111/pce.12098. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X.-G., Wang Y., Ort D.R., Long S.P. E-photosynthesis: A comprehensive dynamic mechanistic model of C3 photosynthesis: From light capture to sucrose synthesis. Plant Cell Environ. 2013;36:1711–1727. doi: 10.1111/pce.12025. [DOI] [PubMed] [Google Scholar]
- 44.Berghuijs H.N., Yin X., Ho Q.T., Driever S.M., Retta M.A., Nicolaï B.M., Struik P.C. Mesophyll conductance and reaction-diffusion models for CO2 transport in C3 leaves; needs, opportunities and challenges. Plant Sci. 2016;252:62–75. doi: 10.1016/j.plantsci.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Wu A., Song Y., van Oosterom E.J., Hammer G.L. Connecting biochemical photosynthesis models with crop models to support crop improvement. Front. Plant Sci. 2016;7:1518. doi: 10.3389/fpls.2016.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin X., Struik P.C. Can increased leaf photosynthesis be converted into higher crop mass production? A simulation study for rice using the crop model GECROS. J. Exp. Bot. 2017;68:2345–2360. doi: 10.1093/jxb/erx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friend A.D., Geider R.J., Behrenfeld M.J., Still C.J. Photosynthesis in global-scale models. In: Laisk A., Nedbal L., Govindjee, editors. Photosynthesis in Silico. Advances in Photosynthesis and Respiration. Volume 29. Springer; Dordrecht, The Netherlands: 2009. pp. 465–497. [Google Scholar]
- 48.Pietsch S.A., Hasenauer H. Photosynthesis within large-scale ecosystem models. In: Laisk A., Nedbal L., Govindjee, editors. Photosynthesis in Silico. Advances in Photosynthesis and Respiration. Volume 29. Springer; Dordrecht, The Netherlands: 2009. pp. 441–464. [Google Scholar]
- 49.Farquhar G.D., von Caemmerer S., Berry J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 50.Von Caemmerer S., Farquhar G., Berry J. Biochemical model of C3 photosynthesis. In: Laisk A., Nedbal L., Govindjee, editors. Photosynthesis in Silico. Advances in Photosynthesis and Respiration. Volume 29. Springer; Dordrecht, The Netherlands: 2009. pp. 209–230. [Google Scholar]
- 51.Bernacchi C.J., Rosenthal D.M., Pimentel C., Long S.P., Farquhar G.D. Modeling the temperature dependence of C3. In: Laisk A., Nedbal L., Govindjee, editors. Photosynthesis in Silico. Advances in Photosynthesis and Respiration. Volume 29. Springer; Dordrecht, The Netherlands: 2009. pp. 231–246. [Google Scholar]
- 52.Niinemets Ü., Anten N.P.R. Packing the photosynthetic machinery: From leaf to canopy. In: Laisk A., Nedbal L., Govindjee, editors. Photosynthesis in Silico. Advances in Photosynthesis and Respiration. Volume 29. Springer; Dordrecht, The Netherlands: 2009. pp. 363–399. [Google Scholar]
- 53.Zhu X.G., Long S.P. Can increase in Rubisco specificity increase carbon gain by whole canopy? A modeling analysis. In: Laisk A., Nedbal L., Govindjee, editors. Photosynthesis in Silico. Advances in Photosynthesis and Respiration. Volume 29. Springer; Dordrecht, The Netherlands: 2009. pp. 401–416. [Google Scholar]
- 54.Song Q., Zhang G., Zhu X.-G. Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2—A theoretical study using a mechanistic model of canopy photosynthesis. Func. Plant Biol. 2013;40:109–124. doi: 10.1071/FP12056. [DOI] [PubMed] [Google Scholar]
- 55.Ho Q.T., Berghuijs H.N., Watté R., Verboven P., Herremans E., Yin X., Retta M.A., Aernouts B., Saeys W., Helfen L., et al. Three-dimensional microscale modelling of CO2 transport and light propagation in tomato leaves enlightens photosynthesis. Plant Cell Environ. 2016;39:50–61. doi: 10.1111/pce.12590. [DOI] [PubMed] [Google Scholar]
- 56.Wu A., Doherty A., Farquhar G.D., Hammer G.L. Simulating daily field crop canopy photosynthesis: An integrated software package. Funct. Plant Biol. 2018;45:362–377. doi: 10.1071/FP17225. [DOI] [PubMed] [Google Scholar]
- 57.Peñuelas J., Garbulsky M.F., Filella I. Photochemical reflectance index (PRI) and remote sensing of plant CO2 uptake. New Phytol. 2011;191:596–599. doi: 10.1111/j.1469-8137.2011.03791.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhang C., Filella I., Garbulsky M.F., Peñuelas J. Affecting factors and recent improvements of the photochemical reflectance index (PRI) for remotely sensing foliar, canopy and ecosystemic radiation-use efficiencies. Remote Sens. 2016;8:677. doi: 10.3390/rs8090677. [DOI] [Google Scholar]
- 59.Sukhova E., Sukhov V. Connection of the Photochemical Reflectance Index (PRI) with the photosystem ii quantum yield and nonphotochemical quenching can be dependent on variations of photosynthetic parameters among investigated plants: A meta-analysis. Remote Sens. 2018;10:771. doi: 10.3390/rs10050771. [DOI] [Google Scholar]
- 60.Kior A., Sukhov V., Sukhova E. Application of reflectance indices for remote sensing of plants and revealing actions of stressors. Photonics. 2021;8:582. doi: 10.3390/photonics8120582. [DOI] [Google Scholar]
- 61.Gamon J.A., Peñuelas J., Field C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992;41:35–44. doi: 10.1016/0034-4257(92)90059-S. [DOI] [Google Scholar]
- 62.Evain S., Flexas J., Moya I. A new instrument for passive remote sensing: 2. Measurement of leaf and canopy reflectance changes at 531 nm and their relationship with photosynthesis and chlorophyll fluorescence. Remote Sens. Environ. 2004;91:175–185. doi: 10.1016/j.rse.2004.03.012. [DOI] [Google Scholar]
- 63.Kováč D., Veselovská P., Klem K., Večeřová K., Ač A., Peñuelas J., Urban O. Potential of photochemical reflectance index for indicating photochemistry and light use efficiency in leaves of European beech and Norway spruce trees. Remote Sens. 2018;10:1202. doi: 10.3390/rs10081202. [DOI] [Google Scholar]
- 64.Sukhova E., Sukhov V. Analysis of light-induced changes in the photochemical reflectance index (PRI) in leaves of pea, wheat, and pumpkin using pulses of green-yellow measuring light. Remote Sens. 2019;11:810. doi: 10.3390/rs11070810. [DOI] [Google Scholar]
- 65.Kohzuma K., Tamaki M., Hikosaka K. Corrected photochemical reflectance index (PRI) is an effective tool for detecting environmental stresses in agricultural crops under light conditions. J. Plant Res. 2021;134:683–694. doi: 10.1007/s10265-021-01316-1. [DOI] [PubMed] [Google Scholar]
- 66.Yudina L., Sukhova E., Gromova E., Nerush V., Vodeneev V., Sukhov V. A light-induced decrease in the photochemical reflectance index (PRI) can be used to estimate the energy-dependent component of non-photochemical quenching under heat stress and soil drought in pea, wheat, and pumpkin. Photosynth. Res. 2020;146:175–187. doi: 10.1007/s11120-020-00718-x. [DOI] [PubMed] [Google Scholar]
- 67.Sukhov V., Sukhova E., Khlopkov A., Yudina L., Ryabkova A., Telnykh A., Sergeeva E., Vodeneev V., Turchin I. Proximal imaging of changes in photochemical reflectance index in leaves based on using pulses of green-yellow light. Remote Sens. 2021;13:1762. doi: 10.3390/rs13091762. [DOI] [Google Scholar]
- 68.Tessone C.J., Mirasso C.R., Toral R., Gunton J.D. Diversity-induced resonance. Phys. Rev. Lett. 2006;97:194101. doi: 10.1103/PhysRevLett.97.194101. [DOI] [PubMed] [Google Scholar]
- 69.Liang X., Zhang X., Zhao L. Diversity-induced resonance for optimally suprathreshold signals. Chaos. 2020;30:103101. doi: 10.1063/5.0022065. [DOI] [PubMed] [Google Scholar]
- 70.Sukhova E., Ratnitsyna D., Sukhov V. Stochastic spatial heterogeneity in activities of H+-ATP-ases in electrically connected plant cells decreases threshold for cooling-induced electrical responses. Int. J. Mol. Sci. 2021;22:8254. doi: 10.3390/ijms22158254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sukhova E., Sukhov V. Electrical signals, plant tolerance to actions of stressors, and programmed cell death: Is interaction possible? Plants. 2021;10:1704. doi: 10.3390/plants10081704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winter H., Robinson D.G., Heldt H.W. Subcellular volumes and metabolite concentrations in spinach leaves. Planta. 1994;193:530–535. doi: 10.1007/BF02411558. [DOI] [Google Scholar]
- 73.Tholen D., Zhu X.-G. The mechanistic basis of internal conductance: A theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol. 2011;156:90–105. doi: 10.1104/pp.111.172346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evans J.R., Kaldenhoff R., Genty B., Terashima I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009;60:2235–2248. doi: 10.1093/jxb/erp117. [DOI] [PubMed] [Google Scholar]
- 75.Sukhova E.M., Sukhov V.S. Dependence of the CO2 uptake in a plant cell on the plasma membrane H+-ATPase activity: Theoretical analysis. Biochem. Mosc. Suppl. Ser. A. 2018;12:146–159. doi: 10.1134/S1990747818020149. [DOI] [Google Scholar]
- 76.Sukhov V., Vodeneev V. A mathematical model of action potential in cells of vascular plants. J. Membr. Biol. 2009;232:59–67. doi: 10.1007/s00232-009-9218-9. [DOI] [PubMed] [Google Scholar]
- 77.Sukhova E., Akinchits E., Sukhov V. Mathematical models of electrical activity in plants. J. Membr. Biol. 2017;250:407–423. doi: 10.1007/s00232-017-9969-7. [DOI] [PubMed] [Google Scholar]
- 78.Kinoshita T., Shimazaki K. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999;18:5548–5558. doi: 10.1093/emboj/18.20.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gradmann D. Impact of apoplast volume on ionic relations in plant cells. J. Membr. Biol. 2001;184:61–69. doi: 10.1007/s00232-001-0074-5. [DOI] [PubMed] [Google Scholar]
- 80.Sukhov V., Nerush V., Orlova L., Vodeneev V. Simulation of action potential propagation in plants. J. Theor. Biol. 2011;291:47–55. doi: 10.1016/j.jtbi.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 81.Cardon Z.G., Mott K.A., Berry J.A. Dynamics of patchy stomatal movements, and their contribution to steady-state and oscillating stomatal conductance calculated using gas-exchange techniques. Plant Cell Environ. 1994;17:995–1007. doi: 10.1111/j.1365-3040.1994.tb02033.x. [DOI] [Google Scholar]
- 82.Siebke K., Weis E. Assimilation images of leaves of Glechoma hederacea: Analysis of non-synchronous stomata related oscillations. Planta. 1995;196:155–165. doi: 10.1007/BF00193229. [DOI] [Google Scholar]
- 83.Schurr U., Walter A., Rascher U. Functional dynamics of plant growth and photosynthesis--from steady-state to dynamics--from homogeneity to heterogeneity. Plant Cell Environ. 2006;29:340–352. doi: 10.1111/j.1365-3040.2005.01490.x. [DOI] [PubMed] [Google Scholar]
- 84.Sharkey T.D., Seemann J.R. Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiol. 1989;89:1060–1065. doi: 10.1104/pp.89.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer S., Genty B. Heterogeneous inhibition of photosynthesis over the leaf surface of Rosa rubiginosa L. during water stress and abscisic acid treatment: Induction of a metabolic component by limitation of CO2 diffusion. Planta. 1999;210:126–131. doi: 10.1007/s004250050661. [DOI] [PubMed] [Google Scholar]
- 86.Osmond C.B., Kramer D., Lüttge U. Reversible, water stress-indiced non-uniform chlorophyll fluorescence quenching in wilting leaves of Potentilla reptans may not be due to patchy stomatal responses. Plant Biol. 1999;1:618–624. doi: 10.1111/j.1438-8677.1999.tb00272.x. [DOI] [Google Scholar]
- 87.Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Christmann A., Grill E., Huang J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013;16:293–300. doi: 10.1016/j.pbi.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Pieruschka R., Schurr U., Jahnke S. Lateral gas diffusion inside leaves. J. Exp. Bot. 2005;56:857–864. doi: 10.1093/jxb/eri072. [DOI] [PubMed] [Google Scholar]
- 90.Pieruschka R., Chavarría-Krauser A., Schurr U., Jahnke S. Photosynthesis in lightfleck areas of homobaric and heterobaric leaves. J. Exp. Bot. 2010;61:1031–1039. doi: 10.1093/jxb/erp368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sukhov V., Surova L., Sherstneva O., Katicheva L., Vodeneev V. Variation potential influence on photosynthetic cyclic electron flow in pea. Front. Plant Sci. 2015;5:766. doi: 10.3389/fpls.2014.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cabrera J.C.B., Hirl R.T., Schäufele R., Macdonald A., Schnyder H. Stomatal conductance limited the CO2 response of grassland in the last century. BMC Biol. 2021;19:50. doi: 10.1186/s12915-021-00988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maxwell K., Johnson G.N. Chlorophyll fluorescence–A practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 94.Flexas J., Barbour M.M., Brendel O., Cabrera H.M., Carriquí M., Díaz-Espejo A., Douthe C., Dreyer E., Ferrio J.P., Gago J., et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012;193:70–84. doi: 10.1016/j.plantsci.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 95.Day T.A., Vogelmann T.C. Alterations in photosynthesis and pigment distributions in pea leaves following UV-B exposure. Physiol. Plant. 1995;94:433–440. doi: 10.1111/j.1399-3054.1995.tb00950.x. [DOI] [Google Scholar]
- 96.Antal T.K., Kovalenko I.B., Rubin A.B., Tyystjärvi E. Photosynthesis-related quantities for education and modeling. Photosynth Res. 2013;117:1–30. doi: 10.1007/s11120-013-9945-8. [DOI] [PubMed] [Google Scholar]
- 97.Roeske C.A., Chollet R. Role of metabolites in the reversible light activation of pyruvate, orthophosphate dikinase in Zea mays mesophyll cells in Vivo. Plant Physiol. 1989;90:330–337. doi: 10.1104/pp.90.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Wu W.H. Plant sensing and signaling in response to K+-deficiency. Mol. Plant. 2010;3:280–287. doi: 10.1093/mp/ssq006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.