Abstract
This review highlights the relationship between the metabolism of reactive oxygen species (ROS), reactive nitrogen species (RNS), and H2S-reactive sulfur species (RSS). These three metabolic pathways, collectively termed reactive oxygen, nitrogen, and sulfur species (RONSS), constitute a conglomerate of reactions that function as an energy dissipation mechanism, in addition to allowing environmental signals to be transduced into cellular information. This information, in the form of proteins with posttranslational modifications or signaling metabolites derived from RONSS, serves as an inducer of many processes for redoxtasis and metabolic adjustment to the changing environmental conditions to which plants are subjected. Although it is thought that the role of reactive chemical species was originally energy dissipation, during evolution they seem to form a cluster of RONSS that, in addition to dissipating excess excitation potential or reducing potential, also fulfils essential signaling functions that play a vital role in the stress acclimation of plants. Signaling occurs by synthesizing many biomolecules that modify the activity of transcription factors and through modifications in thiol groups of enzymes. The result is a series of adjustments in plants’ gene expression, biochemistry, and physiology. Therefore, we present an overview of the synthesis and functions of the RONSS, considering the importance and implications in agronomic management, particularly on the biostimulation of crops.
Keywords: biostimulants, redox homeostasis, plant stress, tolerance inductors, elemental sulfur, sulfur nanoparticles, nitric oxide, ROS, RNS, RSS
1. RONSS Integration as a Metabolic Cluster
Plant metabolism consists of a conglomerate of chemical reactions in which free energy is dissipated from physical sources such as radiation or chemical sources that store energy in chemical bonds or chemical potentials. What is obtained in organisms is metabolic energy, biomolecules, and information to maintain cellular, tissue, and organ structures in a dynamic steady state.
Cellular metabolism processes are believed to be descendants of ancient abiotic processes that dissipate free energy from physical and chemical sources, which occurred before the emergence of organized cell life [1,2,3]. Such abiotic processes are supposed to have arisen spontaneously as one of several physicochemical mechanisms through which the primordial Earth system dissipated free energy from the Sun or the stores of substances in the Earth’s crust [4,5]. It is thought that many of these processes occurred through reactions that involved the transfer of electrons [6], which could partly explain the preponderance of redox processes in the metabolism of modern organisms [7].
The goal of the above processes was to maximize entropy generation from free energy [4,8]. One way to maximize the entropy produced is to carry out cooperative work between different molecular species, which implies the collective organization of diverse functions in conglomerates or clusters [1,4]. It can be assumed that molecular conglomerates functioned as collaborative energy-channeling mechanisms. Different molecular complexes probably organized themselves to transfer energy from one molecular species to another, making the process of energy dissipation (or entropy generation) more effective than the result of the individual functions [9]. The chemical conglomerates or clusters were dedicated to dissipating free energy in the form of reduction potential to produce reactive chemical species such as reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS), collectively termed reactive oxygen, nitrogen, and sulfur species (RONSS).
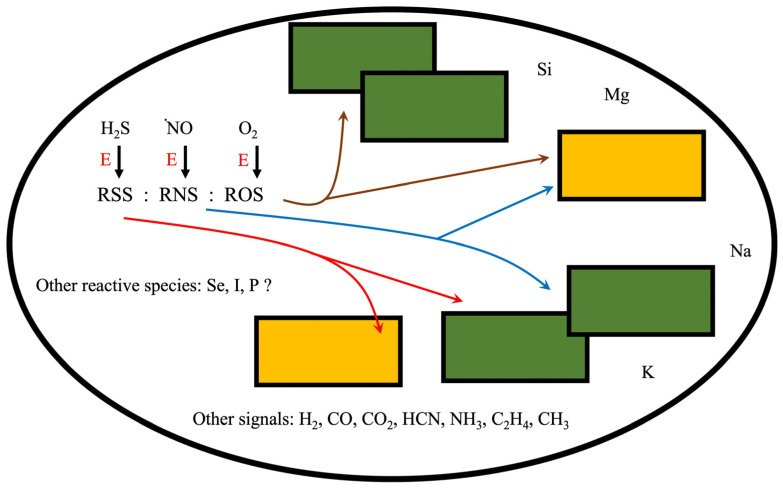
The cooperative work of the different components in different compartments required the creation of networks for the transmission of endogenous information that improved the ability to adjust to the conditions of the environment [1,4,10]. These information networks, which could include organic and inorganic soluble and volatile compounds (Figure 1), were possibly the ancestors of cell signaling processes. In Figure 1, RONSS resulting from the energy dissipation by H2S, ·NO, and inorganic and organic compounds (and lately O2) became part of the information system that monitored the energy state or redoxtasis of the different processes, regulating the joint action of the different components. In particular, the RSSH derived from the interaction of H2S with thiols could be, due to their amphiphilic nature, chemical agents that increased the system’s flexibility in terms of the degrees of freedom available for the flow of electrons. Some elements such as K, Mg, Fe, Na, and Si possibly formed activation or protection systems for various components of the system. It is possible that some abundant elements, such as Fe and other heavy metals, have not been used in more significant volumes due to their ability to trigger oxidative reactions, which could cause system instability due to RONSS saturation. Therefore, Fe in biological organisms functions as a trace element.
Figure 1.
Schematic representation of a prebiotic supramolecular complex that processes energy and matter [1]. The different components represented by the colored rectangles carried out specialized functions and interacted with each other coordinating through energy signals (redoxtasis) and chemical signals created by metabolites. It is possible that the RSS:RNS ratio, and later the RSS:RNS:ROS ratio, modified the redox homeostasis of the prebiotic system, modified internal signals, and caused changes in the nucleic acids, proteins, peptides, and other organic molecules of the prebiotic supramolecular complexes [12,13]. E: energy; RSS: reactive sulfur species; RNS: reactive nitrogen species; ROS: reactive oxygen species.
Compartmentalization gave rise to more sophisticated systems for copying structures, the precursors of reproduction systems, probably based on the ability to store information on functional patterns through the emergence of Hopfield-like attractor dynamics [11]. Such compartmentalization may have given rise to the first cellular organisms with different metabolic abilities, according to the energy and matter use niche in which they evolved [1].
As a consequence of the above, the metabolic processes of living organisms not only function as a mechanism to maintain the structure and functions of organisms but, as a consequence of their intrinsic dissipative nature, they still operate cooperatively to maximize the generation of entropy [8,14]. Metabolism is the set of biochemical processes that, in addition to processing matter and information, allows the acquisition, transformation, and dissipation of free energy available in the environment. Metabolism comprises a set of supramolecular conglomerates or clusters that work cooperatively, giving rise to the different phenomena that allow cellular life. The metabolic pathways that produce reactive species of certain elements, such as S (RSS), N (RNS), and O (ROS), can be an example of the above since they are linked to energy metabolism, functioning as dissipative processes of the reduction potential in excess [15,16] and can, to a certain extent, be visualized as a cluster of processes with diverse functions: the primary being energy dissipation, followed by information transfer or signaling. The dissipative processes possibly did not initially have a goal of regulation or control of the redoxtasis but were spontaneous processes for energy dissipation. Their use as regulatory or signaling agents may be a later adaptation [17].
Other inorganic reactive species, e.g., I, Se, and P reactive species, and RONSS-derived reactive species such as lipid hydroperoxides (LOOH), carbonyl species (RCS), and malondialdehyde (MDA), have similar signaling functions [18,19,20,21,22,23]. However, they may operate at smaller concentrations than S, N, and O reactive species.
Perhaps initially with a preponderance of the RSS (H2S) and RNS (·NO) during the long Archean anoxygenic phase of planetary evolution, to later incorporate ROS [24,25], when O2 increased its concentration during the Proterozoic phase of Earth’s evolution [6,26,27]. However, if O2 or oxygen compounds such as H2O2 were present as traces before the complete oxygenic phase ([atmospheric O2] > 2%) [28], they could be sources of ROS. In the latter case, the joint evolution of the RONSS could have started before the concentration of O2 rose substantially.
The final integration and cooperation of RONSS may result, through the self-organization and creation of novelties that characterize complex systems [29], in the obtention of cooperative systems to transform free energy into information [30,31]. The information accumulated in the dynamic structures and the complexes of structures coordinated through signaling allowed the synchronization of the activities of the metabolism: first, coordinated abiotic processes, and later cellular metabolism [6,7,32,33].
Considering the abovementioned assumptions and that the different metabolic pathways for the energy dissipation and matter transformation may have formed cooperative clusters during the prebiotic era, it is to be expected that RONSS constitutes in modern organisms a system tightly coupled and coordinated with the rest of the cellular processes (Figure 2) [7,27,34]. The impact and biological functions of reactive species on plants have been extensively described in the scientific literature for ROS, RNS, and RSS individually [12,22,33,35,36,37,38,39,40,41,42,43,44]. It has been determined to a much lesser extent for the ROS–RNS, ROS–RSS, and RNS–RSS pairs [45,46,47,48,49,50,51,52,53,54,55,56] and to a lesser extent for the RONSS cluster [13,34,57,58,59,59,60,61].
Figure 2.
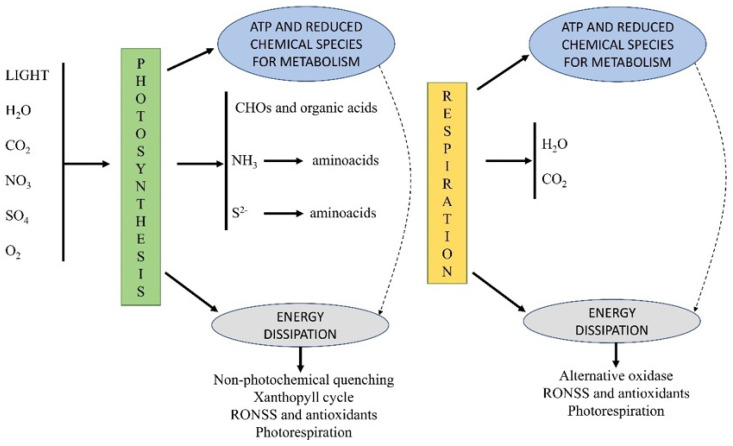
Model of different energy capture and dissipation processes. Both photosynthesis and respiration, as well as the metabolism coupled with these activities, constitute dissipative mechanisms. Photosynthesis and respiration are further associated with other photochemical and biochemical energy dissipation pathways, including the production of RONSS. During the abiotic evolutionary process and later during the early biotic evolution, the production of RONSS went from being only a mechanism for the dissipation of free energy, with the consequent generation of entropy, also constituting a mechanism for regulation and transfer of information on redox and energy status between the different components of the system.
The issue of the agricultural application of the RONSS constitutes, in addition to a fertile field for scientific research, a potential seedbed for the development of innovations in the field of biostimulants for crop production [34,57,60,61]. This manuscript aims to present a brief view of the metabolism of RONSS and their use as plant biostimulants. Different literature sources are presented, which comprise the application of at least two of the various reactive species in priming, signaling, and adaptive processes.
2. RONSS in Plant Metabolism
The sources of RONSS for plants are O2·− from atmospheric O2; ·NO generated mainly from nitrogenous compounds (NO3−, NO2−, and amino acids) that the plant takes as nutrients and to a much lesser extent from traces of ·NO present in the atmosphere; and the H2S produced as part of the assimilation of the sulfur compounds that the plant takes as nutrients and to a much lesser extent the traces of H2S and other compounds such as dimethyl sulfide (DMS) and carbonyl sulfide (COS) present in the atmosphere.
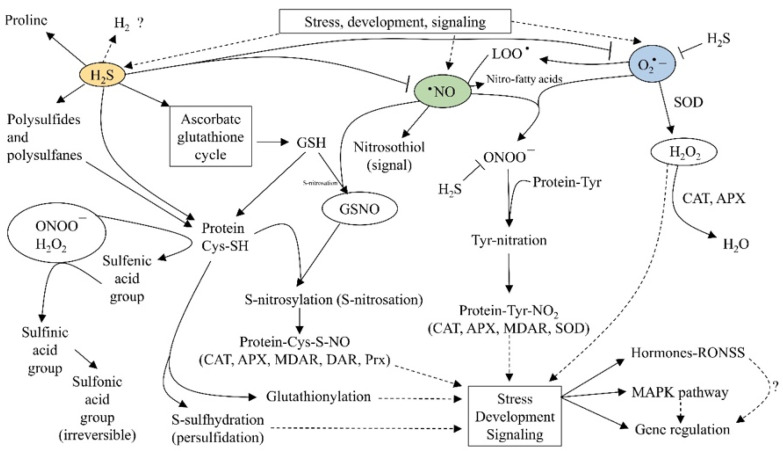
Figure 3, Figure 4 and Figure 5 present the transformations in plant cells to obtain the different reactive species, ROS, RNS, and RSS.
Figure 3.
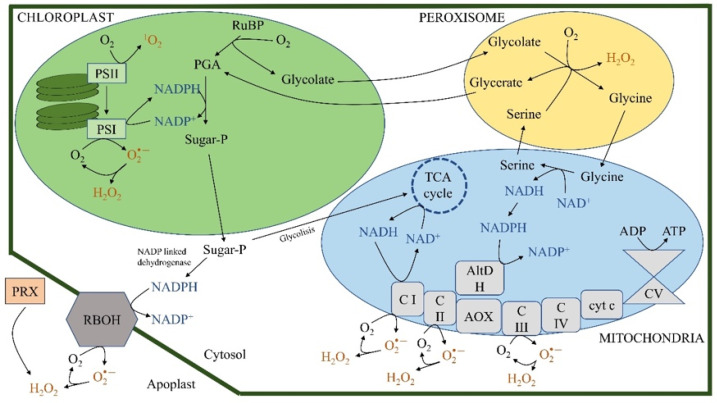
Most important sources of ROS in plant cells. Singlet oxygen (1O2) may originate from excited triplet chlorophylls (Chl) that activate ground-state O2 in the photosystem II (PSII) reaction center. In PSI, superoxide and hydrogen peroxide can be produced by reducing O2. In the mitochondrial electron transport chain, complexes CI, CII, and CIII are ROS-generating systems. AltDH, alternative dehydrogenase; AOX, alternative oxidase; cyt. c, cytochrome c; CI-V, mitochondrial complex I–V; PGA, phosphoglycerate; PS, photosystem; PRX, peroxidase; RBOH, respiratory burst oxidase homologs; RuBP, ribulose 1,5-bisphosphate; Sugar-P, sugar–phosphate; TCA, tricarboxylic acid. Modified from [62].
Figure 4.
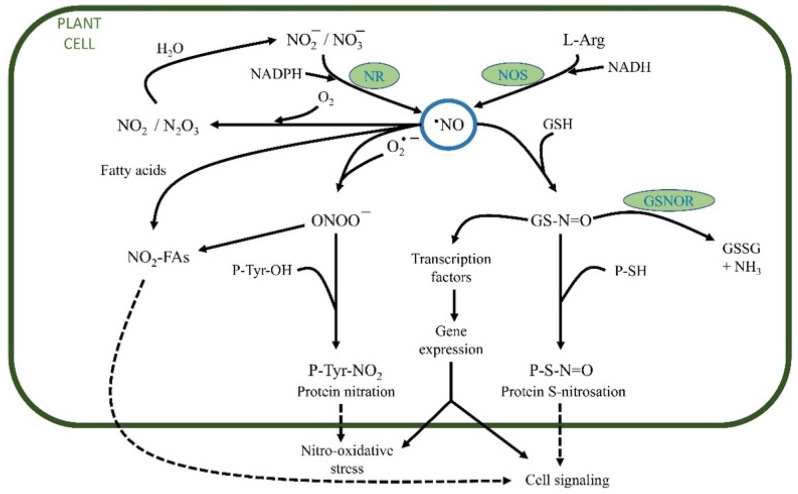
Metabolism of ·NO in plant cells. ·NO can be produced by nitrate reductase (NR), L-Arg NO synthase (NOS), or other reductive processes. ·NO can react by S-nitrosation with glutathione (GSH) to form S-nitrosoglutathione (GS-N=O). GS-N=O can be converted by S-nitrosoglutathione reductase (GSNOR) into oxidized glutathione (GSSG) and NH3. As part of the signaling process, the protein sulfhydryl groups can react with GS-N=O and other S-nitrosothiols to produce S-nitrosated proteins (P-S-N=O). Peroxynitrite (ONOO−) is an oxidant obtained by interacting ·NO with O2·−. The NOOO− can mediate the nitration of proteins (P-Tyr-NO2) and fatty acids (NO2-FAs). ·NO in the presence of O2 is transformed into N2O3 and NO2, which are subsequently transformed into NO2− and NO3− in aqueous media. Modified from [40].
Figure 5.
A simplified model of the interactive action of ROS, RNS, and RSS (RONSS) on plant responses during development events or stress-inducing environmental challenges.
2.1. Reactive Oxygen Species
Figure 3 illustrates the processes associated with ROS synthesis in plant cells. ROS are the result of a sequential series of one-electron reductions of dioxygen:
| O2 ← e− → O2·− ← e− → O22− ← e− → O23− → O− + e− → O2− | (1) |
| ↓ + 2H+ ↓ + 2H+ |
| H2O H2O |
With the contribution of H+, the ROS are transformed as follows:
| O2− + H+ → HO2· (perhydroxyl radical) | (2) |
| O22− + 2H+ → H2O2 | (3) |
| O− + H+ → OH· (hydroxyl radical) | (4) |
| O2− + 2H+ → H2O | (5) |
The above processes allow the dissipation of the excess reducing potential that occurs; for example, in chloroplasts under conditions of high irradiance, or low or high temperature, in mitochondria when low temperatures occur, and in general under any situation that causes an imbalance between the production and the metabolic use of the reducing potential.
The presence of unpaired electrons in O2, the high electronegativity (only less than that of F), and the various oxidation states of oxygen (Table 1) explain its ability to accept electrons successively, forming different ROS. S and N are also highly electronegative elements (with S > N), partly explaining their ability to form reactive species.
Table 1.
Representative oxygen compounds and their oxidation state. ROS are in bold letters.
| Oxidation State | Representative Compound and Formula |
|---|---|
| +2 | OF2 |
| +1 | O2F2 |
| 0 | O2 |
| −1/2 | All superoxides, O2−, O2−, HO2 |
| −1 | All the peroxides, H2O2, HO2−, HO |
| −2 | All the oxides, H2O, CO2 |
In addition to their energy dissipation role, ROS act as signaling agents in practically all metabolic and plant development processes [6,63,64]. Interaction with ROS produces peroxidative changes in membranes, protein cysteine and transcription factors, nucleic acids and histones, and low molecular weight metabolites. The above changes modify the functionality of biomolecules, allowing cellular behavior adjustments in response to changes in the redox balance. An example is ROS peroxidation of protein cysteines to sulfenic acid (RSOH). This is a class of oxidative posttranslational modification (oxPTM) of proteins that modifies the redox properties and the capacity for interaction in the cellular environment of the modified protein [6,65]:
| RSH + H2O2 → RSOH + H2O | (6) |
This kind of modification can, for example, change the ability of transcription factors or histones to interact with DNA or the stability or capacity of an enzyme to bind to its substrate. The ROS oxPTM of proteins occurs, for example, in Calvin cycle enzymes, sulfur and starch metabolism, and the proteins hormone-responsive associated with adaptation to stressful environments [66].
The above oxidation of thiols can be reversed using the reducing potential of NADPH:
| RSOH + NADPH → RSH + H2O + NADP+ | (7) |
acting effectively as a redox switch to move from one protein signaling state to a different one. Most likely, during the prebiotic stage of evolution, this reversible mode of chemical reaction was greatly favored by the ability to dissipate large amounts of free energy using a relatively modest investment in molecular infrastructure.
The epigenomic, proteomic, and metabolomic modifications mentioned above change plants´ phenotype and developmental events, leading to substantial changes in chemical composition, morphology, life cycle, and in general adaptive capacity in a dynamic environment [34,67]. On the other hand, the products of the oxidation of the fatty acids of the membranes, of the thiol groups of the proteins, and several metabolites also constitute a signaling mechanism (through the sensing of the reduction:oxidation balances, e.g., NADPH:NADP+, ascorbate:dehydroascorbate, and GSH:GSSH), functioning as a system for perceiving the internal energy states of the system [67,68].
2.2. Reactive Nitrogen Species
Nitric oxide (·NO) can be considered the primary RNS (Table 2). It is an ancient signaling molecule present in prokaryotes and eukaryotes, including animals and plants [69]. According to Astier et al. [70], although ·NO is a common chemical theme in the signaling of all living organisms, the way of using the signal to obtain cellular responses (the ·NO signaling enzymes) seems to have diverged among the different lineages of eukaryotes, and it is different between plants and animals. Those mentioned above may be part of the explanation for the different responses of animals and plants when exposed to RNS. For example, ·NO2 is toxic and is an allergenic agent for animals, but in plants, it is used as a signaling agent [71]. S-nitrosothiols occur as signaling agents in both animals and plants [70]. On the other hand, peroxynitrites are characterized as signaling agents capable of inducing more significant toxicity in animals than in plants. In fact, in mammals can cause guanine nitration leading to mutations and cancer due to guanine mispairs. Meanwhile, in plants, peroxynitrites can be inactivated in the presence of CO2, producing CO3− and ·NO2. The toxicity of ·NO is mainly due to the formation of NO-derived oxidants characterized by greater reactivity than ·NO [72].
Table 2.
Representative nitrogen compounds and their oxidation state. RNS are in bold letters.
| Oxidation State | Representative Compound and Formula |
|---|---|
| +5 | HNO3, NO3−, ONOO− (peroxynitrite) |
| +4 | ·NO2 (nitrogen dioxide), N2O4 |
| +3 | HNO2, NO2−, S-nitrosothiols (RS-N=O), NO+ |
| +2 | ·NO (nitric oxide) |
| +1 | N2O (nitrous oxide), NO− |
| 0 | N2 |
| −1 | NH2OH (hydroxylamine) |
| −2 | N2H4 (hydrazine) |
| −3 | NH3, NH4+ |
In animals, ·NO is primarily synthesized by nitric oxide synthase [73], while in plants, ·NO is endogenously produced by different enzyme systems. Among them are the oxidative pathway of the L-Arg NO synthase analogs, by reductive mechanisms such as nitrate reductase (NR) that produces NO2 that is reduced to ·NO by the NR itself, or by the plasma membrane-bound NO-forming nitrite reductase (NOFNiR). The mitochondrial complexes, mainly III and IV, as well as complexes I, II, alternative dehydrogenase, and cytochrome c, also generate ·NO reductively from NO2− [74]. Alternative oxidase (AOX) also produces ·NO under anoxic or hypoxic conditions in the mitochondria [75], although under normoxia, AOX removes excess ·NO. Under anoxic conditions and in N2 fixation nodules, nonsymbiotic hemoglobins collaborate with mitochondria creating a Phytogb1-NO cycle of ·NO → NO3− → ·NO that generates anoxic ATP and allows the control of NADPH levels. In addition to NR and NOFNiR, some molybdoenzymes, such as xanthine oxidases, aldehyde oxidases, and sulfite oxidases, seem to possess NO2− reductive capacity [22,74,76].
As in the case of ROS, the presence of RNS is associated with dissipation processes of free energy/reducing potential. The preceding is because the main RNS depend on their synthesis on the interaction of ·NO with the other reactive species that dissipate free energy, ROS, and RSS; secondly, the synthesis of ·NO is privileged when a plentiful supply of reducing potential (electron pressure) occurs. Electron pressure is substantial, for example, under high irradiance or stress conditions that disturb the flow of electrons in transport chains such as low temperature, water deficit, or salinity. The nonenzymatic reduction of NO2− to ·NO in the presence of high nitrate concentrations in a highly reducing condition or low pH can indeed occur [71,76].
·NO generates other SNRs such as peroxynitrite (ONOO−), a reaction product between O2− and ·NO [77]. This reaction allows the dissipation of the stored reducing potential resulting from the reduction of NO3− to ·NO:
| O2− + ·NO → ONOO− | (8) |
| ONOO− + H+ → ONOOH → HO· + ·NO2 → NO3− + H+ | (9) |
S-Nitrosothiols are another class of RNS resulting from the reaction of ·NO with thiol groups, as occurs, for example, when reacting with specific protein sulfhydryl groups to mediate signaling by the S-nitrosated proteins or with H2S or glutathione (GSH), to form S-nitrosoglutathione (GS-N=O) [77].
| ·NO + H2S → HS-N=O + H+ | (10) |
| ·NO + GSH → GS-N=O + H+ | (11) |
These latter reactions also allow the reduction potential to dissipate. As previously stated, the recovery of the reduced state of thiols requires the consumption of reducing potential (NADH, NADPH, GSH) and the action of the enzyme S-nitrosoglutathione reductase (GSNOR), which catalyzes the irreversible GS-N=O conversion to oxidized glutathione (GSSH) [22].
In addition to their energy dissipation role, RNS are signaling molecules in practically all metabolic and plant development processes (Figure 4). The main mechanisms by which RNS modify cell behavior are through S-nitrosation, nitration, and metal nitrosylation [20,40,77].
S-Nitrosation consists of the formation of S-N=O due to the covalent attachment between ·NO and the thiol (–SH) of cysteine (Cys). This reversible posttranslational modification (PTM) of proteins is one of the most important mechanisms for NO signaling. The S-N=O group additionally functions as a donor and reservoir of ·NO. Proteins modified by S-nitrosation change their functionality, inducing rapid and reversible cellular proteome changes [40].
Nitration is the addition, mediated by ONOO−, of a nitro group (–NO2) into proteins, fatty acids, or nucleic acids. In proteins, the most-studied nitration type results in a nitro-tyrosine formation. However, it also occurs in other amino acids such as cysteine, tryptophan, and methionine. Nitration of amino acids can lead to gain or loss of protein function or even absence of an effect. The most common result is the loss of function [40].
Metal nitrosylation occurs when ·NO interacts with the transition metals present in proteins. Little information is available on the plants in this process [40].
Similar to ROS, RNS (·NO, ONOO−, ·NO2) react with fatty acids or LOO· (lipid peroxy radicals), forming reactive lipid species called nitro-fatty acids (NO2-FAs). NO2-FAs constitute signaling molecules and modulate gene expression during stress events and developmental processes [22,78].
2.3. Reactive Sulfur Species
The synthesis of H2S and other RSS is coupled with the metabolism of S that allows for obtaining the S2− and S− necessary for cellular functions. At the same time, it is a dissipative process that consumes reducing potential, transforming oxidized sulfur species such as S0, SO42−, SO32−, and S2O32− into species with a very high reducing potential, such as H2S with −0.23 V and glutathione (GSSG/GSH) with −0.24 V [79,80]. The reverse oxidative process, from H2S to S0 through sequential one-electron oxidations, is the source of RSS (Table 3) such as thiyl radical (HS·), hydrogen persulfide (H2S2), persulfide ‘supersulfide’ radical (HS2·−), and elemental sulfur (S0) [6].
| S0 ← e− → HS2− ← e− → H2S2 ← e−→ HS· ← e− → H2S | (12) |
Table 3.
Representative sulfur compounds and their oxidation state. RSS are in bold letters. Modified from [79].
| Oxidation State | Representative Compound and Formula |
|---|---|
| +6 | Sulfate, SO42− |
| +6 and −2 | Thiosulfate, S2O32− |
| +5 and −2 | Polythionates (-O3S-Sn-SO3-)2− Dithionate, S2O62− Trithionate, S3O62− Tetrathionate, S4O62− |
| +4 | Sulfur dioxide, SO2 Sulfite, SO32− Disulfite, S2O52− Sulfonic acid (RSO3H) from ROS-mediated protein sulfonylation. Sulfone, OS(S) the oxidation product of sulfoxides. |
| +3 | Dithionite, S2O42− Disulfide-S-dioxide (thiosulfonate) RS(O2)SR |
| +2 | Carbonyl Sulfide (COS), OCS. Sulfinic acid (RSO2H) from ROS-mediated protein sulfinylation. |
| +1 | Disulfide-S-monoxide (thiosulfinate) RS(O)SR |
| 0 | S0 (sulfane sulfur), elemental sulfur, mainly S8 (cycloocta-S). Sulfoxide (R-S(-O)-R such as the dimethyl sulfoxide (DMSO). Oxidized derivatives of sulfide and sulfenic acid (RSOH) from ROS-mediated protein sulfenylation. Near the six electrons, valence S0 never exists by itself. Sulfane sulfur (S0, S-S, or S2) is labile. There are a variety of compounds such as S8, thiosulfate, polysulfanes, and polysulfides, that contain S0 |
| −1 |
Disulfide (RSSR) is a persulfide −S-S− found in the linkages between two cysteine residues in proteins. RSSH denotes persulfides (also called hydrosulfides or hydropersulfides) obtained by the action of H2S on cysteine residues (R-SH). Thioethers and thiols can be oxidized to disulfides. Persulfides such as CysSSH, GSSH, and protein-SSH act as signaling compounds in organisms. Major products of the decomposition of persulfides are polysulfanes Disulfide-S-monoxide (thiosulfinate) RS(O)SR Disulfide-S-dioxide (thiosulfonate) RS(O2)SR Thiyl-radical HS· or RS· |
| −2 |
Sulfide, S2− and organic polysulfides, S22−, S32−, S52− Disulfides (R-S-S-R) Carbon disulfide (CS2) FeS2 NaHS and Na2S are sources of S2− and of its conjugated acids SH− and H2S. Organic and inorganic polysulfides (with Sn > 2) contain S0 atoms, which allows a diversity of oxidation states. |
| −2 | Hydrogen sulfide (H2S), disulfane or hydrogen persulfide (H2S2), H2S3, other inorganic polysulfides (H2Sx) x ≥ 1, and polysulfanes (RSSnH, RSSnSR, n > 2). Polysulfanes contain S0 atoms, which allows a diversity of oxidation states. |
| −2 | Thioethers (C-S-C) such as dimethyl sulfide (DMS), CH3-S-CH3 and dimethyl disulfide (DMDS), CH3-S-S-CH3. |
| −2 |
Thiols (R-SH) such as glutathione (GSH) and methyl mercaptan, CH3-SH. Thiols are derived from the sulfhydryl group -SH of cysteine that enables multiple oxidation states (−2 to +6). Thiolates are anionic derivatives of thiols in which a metal or other cation replaces H. |
The reducing potential of H2S can also be used to reduce disulfides, such as glutathione disulfide (GSSG) and certain protein-based disulfides (PrSSG, PrSSPr). The persulfuration and polysulfuration of protein thiols to obtain persulfides R-S-SH are of great importance in cell signaling [20,81], as well as S2− found in biomolecules and H2S, which can be partially oxidized to obtain polysulfides (H2Sx y S22−, S32−, S52−) that are RSS involved in cell signaling. It appears that H2S exerts signaling actions indirectly via H2S-derived polysulfides, such as the persulfides RS-SH obtained by the action of H2S on thiols and cysteine residues (R-SH), and higher-order polysulfur compounds, i.e., RSxH, RSxR, with R = glutathione or protein and x ≥ 3. This mechanism can be considered a reversible switch with value to dissipate reducing potential, to signal the redox state of the system, to protect protein thiols from oxidation by ROS (e.g., carbonylation) and to regulate the function of proteins in different metabolic pathways [82]:
| RSH (thiol) ← e− → R-S-SH ← e− → R-Sx-SH | (13) |
RS-SH contains bound (or sulfane) sulfur, the reactive form of sulfur with a formal oxidation number of −1, but with the capacity of -S-S- to adopt different oxidation states (0 to −2), allowing greater diversity and flexibility of posttranslational modification states in proteins [80,83].
The interaction between thiols and ROS was mentioned previously. The interaction between H2S and RNS, e.g., ·NO, also generates several classes of H2Sx, which seems to establish a direct chemical link between the two reactive molecules [20]. Similarly, GS-N=O when reacting with H2S produces ·NO and a series of RNSS, e.g., SSNO−, HSNO, and HNO [84]. Polysulfides can also be obtained by a reductive route using GSH and other RSS (sulfenic acid and thiosulfinates R-S(O)-S-R) and organic polysulfanes (RSSnSR, n > 2) as precursors [20]. Thiosulfinates are highly reactive toward the thiol groups of GSH and proteins; they are disulfide-S-monoxides found naturally in Allium spp. and Petiveria spp. Among the thiosulfinates, allicin is one of the most-studied compounds used as a biostimulant, microbicide, and medicine [85]. Organic polysulfanes and those contained in elemental sulfur (S8) and sulfur nanoparticles constitute another group of thiol-reactive compounds with great potential for agricultural use as biostimulants and microbicides [79,86,87,88]. On the other hand, organic polysulfanes (diallyl and dipropyl polysulfanes) subject to reduction can generate RSS- (reduced organic persulfides), which when reacting with GS-N=O produce RSS and ·NO [89].
Similar to ROS and RNS, RSS are important in cell signaling. Indeed, Olson (2020) [6] notes that RSS has much greater importance than it has been given. The author mentions that RSS includes a more significant amount of reactive chemical species, in addition to the fact that once sulfur is oxidized from its −2 state (H2S and S2−) to −1, it can be utilized again to reductively regenerate H2S from a diversity of organic and inorganic persulfides (RSSH) and polysulfides (H2Sx), e.g., H2S3, H2S4, CysSSH, CysSSSH, GSH(Sx)H, GSH(Sx)GSH. Therefore, it is highly likely that a significant source and sink for RSS, compared with ROS and RNS, exists in the cells. Additionally, RSS signaling flexibility is increased by modifying the number of S atoms in persulfides and polysulfides. The higher the number of S atoms, the greater promoted the anionic forms (RSS-) with a nucleophilic character in the terminal S and electrophilic in the nonterminal S, contrary to what occurs with the protonated forms (RSSH) with an electrophilic character in both S atoms [82].
Redox signaling in proteins occurs mainly through redox-sensitive cysteine residues. The -SH group of cysteine has multiple oxidation states (from −2 to +6) that allow a great diversity of modifications when reacting with ROS, RNS, and RSS (Figure 5). The mechanism by which RSS works is called persulfidation:
| RSH + H2S2 → RSSH + H2S | (14) |
Persulfidation is an oxidation that can be reversed through thiol exchange:
| R1SSH + R2SH → R1SH + R2SSH | (15) |
using antioxidant pathways such as peroxiredoxin (Prx), thioredoxin/thioredoxin reductase (Trx/TrxR), or glutaredoxin (Grx). R1 and R2 can be H or small thiols such as cysteine (CysSH) or glutathione (GSH) [6].
2.4. Reactive Oxygen, Nitrogen, Sulfur Species (RONSS)
Although the reducing capacity of H2S could directly counteract the oxidizing capacity of · ·NO and O2− (Figure 5), the direct antioxidant action of H2S under physiological conditions does not seem particularly important. This is derived from the volatility and reactivity of H2S, making it a short-lived chemical species in cells, with HS− and other RSS being more abundant. Therefore, the antioxidant action of H2S is indirect through the abovementioned interactions between RSS, RNS, and ROS [39,80].
Crosstalk has been shown to occur between RSS, RNS, and ROS; these interactions have been studied mainly in signaling molecules ·NO, O2−, and H2S [60]. For example, H2O2 10 mM induces the synthesis of ·NO in leaf epidermal preparations of Phaseolus aureus [90], and during the induction of thermotolerance by applying H2O2 in corn seedlings, it was shown that H2O2 causes an increase in the synthesis of ·NO, which, in turn, causes that of H2S [91]. With the stimulation of heat shock (45 °C for 30 min), A. thaliana plants sprayed with H2O2 (20–200 μM) increased ·NO; ·NO, in turn, stimulated the activity of catalase, ascorbate peroxidase, and glutathione reductase that eliminated excess H2O2, reducing the risk of oxidative damage [92]; ·NO also favors the expression of the mitochondrial alternative oxidase under salt stress [93].
Similarly, the increase in endogenous H2S by the application of NaHS increased the activity and gene expression associated with catalase, superoxide dismutase, and peroxidase, reducing the oxidative damage induced by osmotic stress with 0.3 M mannitol [94], Cd toxicity [95], or Cr stress [96]. An analogous impact of the H2S donor GYY4137 by reducing ·NO accumulation on stomata has been described [97]. Similarly, in tomato plants subjected to salinity, ·NO functioned as an inducer of H2S synthesis, but not vice versa [98]. Otherwise, a study with barley seedlings subjected to salinity determined that the biostimulant impact of H2S depends on the endogenous synthesis of ·NO [99]. However, the effects of H2S on ROS metabolism do not always occur through the promotion of antioxidant enzyme activity, as was demonstrated in peroxisomes, in which H2S is associated with catalase inhibition [100].
Subsequently, hormones such as auxin [101], melatonin [102], and salicylic acid [103] can function as downstream signaling in the biostimulation process and improve stress tolerance. It has also been found that the reverse is true and that applying gibberellic acid induces the endogenous synthesis of H2S, reducing oxidative damage by boron toxicity [104]. ·NO has also been associated with plant responses to nanomaterials (NMs), either in the induction of tolerance to stress by NMs or in the plant response to stress caused by NMs [105].
RONSS crosstalk also occurs with other gasotransmitters. For example, it was reported that the favorable impact of H2 on cut flowers seems to be mediated by H2S, which decreases the expression of genes associated with senescence [106]. Similarly, the CO-dependent root architecture and the organogenesis of adventitious roots induced by CH4 depends on the induction of the synthesis of ·NO and H2S [107,108]; the greater tolerance to stress caused by CH4 relies on the synthesis of ·NO [109]. The crosstalk between RSS, RNS, and ROS and their subsequent impact on signaling molecules and growth regulators promote cell redoxtasis and could cause different molar ratios between the reactive species depending on the environmental factors and the cellular development context.
RONSS crosstalk also occurs with Se. Se is an element located in the same group as S, and like the latter, it also fulfills functions associated with redox homeostasis. Selenium is an essential element in mammals and macroalgae, with a broad spectrum of functions. One of the most studied functions is participating in antioxidant selenoproteins, which protect against oxidative stress and neutralize ROS and RNS. Selenoproteins contain selenocysteine and selenomethionine, and to date, the best-identified are those of the glutathione peroxidase (GPx), iodothyronine deiodinase, thioredoxin reductase, and selenophosphate synthetase families, which contribute to the maintenance of redoxtasis [110]. Furthermore, it has been established that the application of Se at low concentrations promotes stress tolerance, growth, and nutraceutical value [111] due to its impact on antioxidant enzymatic activity and the synthesis of redox-active metabolites.
It has been shown that the activity of glutathione peroxidase, ascorbate peroxidase, superoxide dismutase, dehydroascorbate reductase, and monodehydroascorbate reductase is increased [112,113]. These antioxidant enzymes directly impact ROS, and their effect on RNS and RSS is indirect, considering what has been exposed about the association between reactive species (Figure 5). Se has also been related to the increase in the activity of other non-catalytic proteins, such as thioredoxin (TrxR) and protein P [114]. The impact of Se on antioxidant metabolites is associated with sulfur metabolism since both elements share uptake and assimilation pathways; the effects on the concentration of GSH and GSSH have been described in Allium [115] and Prunus domestica [116]. In species that can reach high concentrations of S, such as broccoli, the accumulation of glucosinolates is increased [117].
Additionally, it has been shown that the presence of selenium increases the activity of phenylalanine ammonium lyase (PAL) and the accumulation of phenolic compounds, which due to their reducing capacity can modify the balance of RONSS [118]. The direct action of Se on redox homeostasis has also been proposed through the induction of antioxidant activity by spontaneous reduction of O2− by GPx or by promoting the synthesis of ascorbic acid [119]. Another form of the direct action of Se is as a pro-oxidant, causing moderate oxidative stress with the formation of ROS that triggers the synthesis of enzymatic and non-enzymatic antioxidants [120].
Despite the close physiological and nutritional relationship between S and Se [121], the interaction between these elements in their impact on redoxtasis is poorly understood [122].
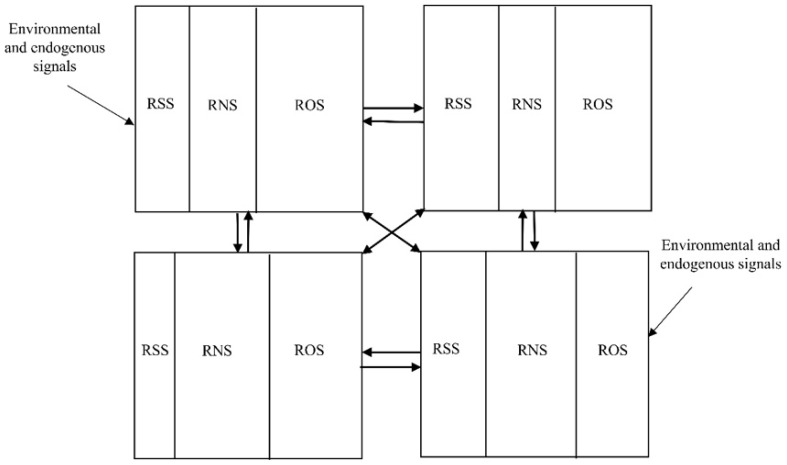
The adjustments in the molar balances between the different RSS, RNS, and ROS (Figure 6), as a result of various environmental stimuli and different physiological conditions, give rise, on the one hand, to the diversity of ratios between reactive species, metabolites, and enzymatic activities that define the cellular redoxtasis [123] and, on the other hand, to the presence of multiple proteomic [124] and metabolomic landscapes. The proteomic differences between individuals at different stages of development and/or in different environments or growth conditions are a consequence of the interaction of RSS, RNS, and ROS with cysteine residues or other amino acids such as tyrosine, which can be subjected to peroxidation, carbonylation, nitrosation, glutathionylation, persulfidation, sulfenylation, and sulfonylation [66,125].
Figure 6.
The dynamic balance in the relative amount of RSS, RNS, and ROS molecules is represented. In addition to the modifications of the RSS, RNS, and ROS species profiles, each change in the balance between the relative quantities (redoxtasis) would imply adjustments in the phenotype (transcriptome, proteome, metabolome, etc.) of the plant. The changes would be responses to external environmental signals, such as temperature and irradiance, and endogenous signals from the organism itself.
3. RONSS as Biostimulants
From the point of view of biostimulation or priming with RONSS, the application of ROS, RNS, or RSS, or the use in pairs ROS–RNS, ROS–RSS, RNS–RSS constitutes a relevant and dynamic topic in plant science [50,57,60,61] (Table 4). In the same way, it is known that the mechanism of action of seed magnetopriming and some biostimulants, such as melatonin, salicylic acid, and silicon, includes the action of RONSS as signaling agents [102,103,126,127,128]. Although many examples are known where the application of RONSS induces favorable responses to stress, an increase in productivity or yield, or an improvement in nutritional composition in plants, there are still many gaps in knowledge about the molecular mechanisms involved in cellular responses [34,60,61]. The explanation of the above gaps lies in the great complexity of the interactions of the RONSS with the different cellular components [57,60].
Table 4.
Some examples of studies where the favorable impact of applying at least two of the reactive species: ROS, RNS, and RSS or their precursors in plants has been demonstrated.
| Impact on the Plant | Reactive Species | Plant Species | Reference |
|---|---|---|---|
| Decreased absorption and/or toxicity of heavy metals | H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO |
Medicago sativa
Sesamum indicum Triticum aestivum Triticum aestivum |
[129] [130] [131] [51] |
| Increase in the concentration of essential elements | H2S, ·NO H2S, ·NO |
Triticum aestivum
Sesamum indicum |
[131] [130] |
| Increase in Relative Growth Rate (RGR) and/or biomass | H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2O2, ·NO H2O2, ·NO H2O2, ·NO |
Cynodon dactylon
Medicago sativa Sesamum indicum Solanum lycopersicum Triticum aestivum Triticum aestivum Ocimum basilicum Oriza sativa Triticum aestivum |
[129] [132] [130] [133] [51] [131] [134] [135] [136] |
| Improved crop yield and/or quality | H2O2, ·NO | Ocimum basilicum | [134] |
| Increase in Relative Water Content (RWC) | H2S, ·NO H2O2, ·NO |
Triticum aestivum
Fragaria × ananassa |
[51] [137] |
| Increment in stomatal conductance (gs) | H2S, ·NO H2S, ·NO |
Medicago sativa
Triticum aestivum |
[50] [51] |
| Increase in the quantum efficiency of PSII (Fv/Fm) | H2S, ·NO H2S, ·NO H2O2, ·NO H2O2, ·NO |
Medicago sativa
Triticum aestivum Citrus aurantium Fragaria × ananassa |
[50] [51] [125] [137] |
| Increase in CO2 assimilation (A) | H2O2, ·NO | Citrus aurantium | [125] |
| Increment in the concentration of photosynthetic pigments | H2S, ·NO H2S, ·NO H2O2, ·NO H2O2, ·NO H2O2, ·NO |
Sesamum indicum
Triticum aestivum Citrus aurantium Fragaria × ananassa Ocimum basilicum |
[130] [131] [125] [137] [134] |
| Increased activity of antioxidant enzymes (e.g., SOD and CAT) and the ascorbate–glutathione (AsA–GSH) cycle | H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2O2, ·NO |
Cynodon dactylon
Medicago sativa Medicago sativa Medicago sativa Solanum lycopersicum Triticum aestivum Triticum aestivum Ocimum basilicum |
[132] [138] [129] [50] [133] [131] [51] [134] |
| Proteome reprogramming through reversible or irreversible posttranslational modifications (PTM) and changes in gene expression | H2S, ·NO H2S, ·NO H2O2, ·NO |
Citrus aurantium
Citrus aurantium Citrus aurantium |
[139] [140] [124] |
| Mitigation of the relative electrolyte leakage under stress | H2S, ·NO H2O2, ·NO H2O2, ·NO H2O2, ·NO |
Cynodon dactylon
Citrus aurantium Citrus aurantium Fragaria × ananassa |
[132] [124] [125] [137] |
| Mitigation of lipid peroxidation under stress | H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2O2, ·NO |
Cynodon dactylon
Medicago sativa Medicago sativa Solanum lycopersicum Triticum aestivum Fragaria × ananassa |
[132] [50] [129] [133] [131] [137] |
| Increased accumulation of proline and other osmolytes | H2S, ·NO H2O2, ·NO |
Medicago sativa
Triticum aestivum |
[50] [136] |
Table 4 shows that coincidences occur in the proposed functions or impact on plants for the different reactive species. For example, the mitigation of electrolyte leakage and the decrease in lipid peroxidation can be achieved with the combination of ROS–RNS and RSS–RNS. Therefore, as confirmed by the studies cited in Table 4, the RONSS seems to function non-independently through crosstalk between the different signaling pathways [13,34,57,108], as depicted in Figure 5 and Figure 6. The mechanism that enables the RONSS to exert their effects in a coordinated way, as explained in the first section, is thought to have been the result of prebiotic evolution that had the goal of developing processes coordinated to obtain the maximum capacity for free energy processing and entropy production [8]. The biochemical descendants of that primordial processes are still active in cells. Through billions of years of biological evolution, natural selection adjusted and adapted them to permit the maximum capacity of the cells and multicellular organisms to process free energy and transform it into entropy [10].
The purpose of maximum entropy requires that organisms have a process for obtaining information that allows them to adjust to environmental changes, which is achieved by determining the energy condition through the evaluation of the redox status of the system [141], which can be equivalent to the variations in the molar ratios of the different reactive species (Figure 6). Information on redox status causes changes in gene expression and phenotype adjustments and proteomic and metabolomic responses that modulate the metabolism according to the organism’s needs in a particular environment. The RONSS are relevant messengers of the above metabolic adjustments [34].
The number of known chemical agents involved in cell signaling and biostimulation will likely grow as new information about other signaling molecules that work in coordination with RONSS is acquired. H2 and CO can be examples [108,142]. RONSS work in coordination with many other biomolecules, forming an intricate network of cellular information about energy status and responses to environmental stimuli [143,144]. The preceding points to the joint use of RONSS with biostimulants such as silicon, selenium, or iodine, plant and seaweed extracts, chitosan and other biopolymers, humic substances, and metabolites such as melatonin and salicylic acid [50,102,145,146,147,148,149].
As mentioned in Table 4, the application of RONSS for signaling and as a biostimulant has been evaluated in several plants with economic purposes, such as Triticum aestivum, Solanum tuberosum, Citrus aurantium, among others, which have shown promissory results. In this regard, early studies with exogenous application of sodium hydrosulphide (SHS) as a donor of H2S on T. aestivum seedlings under Cu stress showed an improvement in the activity of glutathione reductase, dehydroascorbate reductase, L-galactono-1,4-lactone dehydrogenase and gamma-glutamyleysteine synthetase. Moreover, the levels of ascorbic acid, glutathione, and total ascorbate increased, alleviating the damage produced by Cu [150]. Reduced damage of plasma membrane integrity in T. aestivum seeds exposed to Cu, promotion of amylase and esterase activities and lower levels of malondialdehyde, and H2O2 in germinating seeds treated with H2S donors have also been reported [151]. Tolerance against Cd stress in T. aestivum through the application of NO and H2S using sodium nitroprusside (SNP) and SHS as donors, respectively, showed an increase in dry matter, chlorophyll a and b, and Fv/Fm ratio between 39.1–47.8, 61.5–92.3, and 27.2–29.1, respectively, related to the control [152]. Under cobalt (Co) stress, T. aestivum exposed to Co concentrations of 150–300 µM and treated with NO and H2S donors showed an increase of glutathione (GSH), superoxide dismutase (SOD), peroxidase (POX), monodehydroascorbate reductase (MDHAR), APX, glutathione reductase (GR), dehydroascorbate reductase (DHAR), ascorbate (tAsA), and counteracted the negative effect caused by Co on growth, water relations, redox, and antioxidant capacity in chloroplasts [51]. The addition of SNP (100 µM) as a donor of NO in T. aestivum has also been demonstrated to counteract the negatives effects of 400 µM Fe, enhanced seed germination, decreasing Fe accumulation, and proline and malondialdehyde (MDA) content [153]. Under water deficit conditions, RONSS application has also demonstrated that T. aestivum seeds can mitigate the damage produced by water scarcity. The seeds soaked with SNP (0.1 mM) or H2O2 (1 mM) or a combination of both improved Ψw, Ψs, Ψp, photosynthetic pigment content, osmolytes accumulation (GB and Pro), TSP, and the antioxidative defense mechanism. Moreover, it also reduced MDA accumulation [154].
Other species with commercial importance, such as Citrus aurantium or Solanum lycopersicum have also been evaluated. In this regard, adverse effects caused by salinity stress (120 mM NaCl) on S. lycopersicum (47% of decrease in dry leaf mass and root length) were alleviated by exogenous application of SNP (100 µM) enhanced the leaf dry mass (30%) and root length (23%) compared with the non-treated plants [155]. NO has been associated with root development in S. lycopersicum growing under elevated CO2 concentration, especially in lateral roots, and increasing nitric oxide synthase activity [156]. SNP applied as NO donor at 100 µM in S. lycopersicum showed a good capacity to immobilize As in the root but also its translocation in the shoots by upregulation of γ-glutamylcysteine synthetase (GSH1), glutathione synthetase (GSH2), phytochelatin synthase (PCS), metallothionein (MT), and ABC transporter (ABC1). Interestingly, the authors reported that the plants subjected to As stress (10 mg/L) and treated with SNP were able to restore the growth retardation through modulating the chlorophyll and proline metabolism, with an increase of stomatal conductance and NO accumulation [157]. Studies carried out with Citrus aurantium have also demonstrated how nitrosative and oxidative signals play an important role in regulating cellular adjustments to environmental conditions. In this regard, plants subjected to salinity stress (150 mM NaCl) and pre-treated with H2O2 (10 mM for 8 h) and SNP (100 µM for 48 h) showed a strong reduction of phenotypical and physiological effects, as well as a higher net photosynthetic rate compared with the non-treated plants that showed clear foliar injury (necrosis) and low net photosynthetic rates [140]. Moreover, these same authors reported that proteomics analysis reveals quantitative variations in 85 leaf proteins in plants subjected to salinity. Many of these were not present in H2O2 or SNP pre-treated plants. Histochemical and fluorescent probes in C. aurantium plants pre-treated with H2O2 and SNP showed ROS movement by vascular tissues over long distances and NO signaling pathways [125].
In other species, such as Solanum tuberosum, the use of NO donors (SNP, S-nitroso-N-acetylpenicillamine or a mixture of ascorbic acid and NaNO2) demonstrated that NO could protect plants from methylviologen damage produced by herbicides [158], but could also stimulate phytoalexin accumulation, which can be used as a mechanism of induction of defense against pathogens in plants [159] or to participate in the wound–healing response of potato leaves by the induction of cell wall glucan callose production [160]. On the other hand, since H2O2 is relatively stable compared to other ROS molecules such as NO, a recent study demonstrated that foliar spraying of H2O2 at 1% consecutively (7 days) on S. tuberosum caused an increase in the photosynthetic apparatus and antioxidant capacity [161].
Strawberries are a highly demanded fruit consumed globally, known for their biological properties such as antioxidant, antimicrobial, or anti-inflammatory capacity [162]. In early studies developed with Fragaria × ananassa it was demonstrated that fumigation for 5 h with NO at 200 µL/L NO atmospheres and maintained at 18 °C in air delayed the onset of ethylene production and reduced the respiration, maintaining the fruit’s quality and prolonging its shelf life [163]. Similar results were obtained fumigating F. × ananassa with NO (between 1.0 to 4000 µL L−1) immediately after harvest and held at 5 °C and 20 °C in air containing 0.1 µL L−1 [164]. At both temperatures, the postharvest life of F. × ananassa was extended, but the optimal NO concentration was 5–10 µL L−1, causing > 50% extension in shelf life. The application of sodium hydrosulfide (NaHS) as a donor of H2S on F. × ananassa under iron deficiency has also been evaluated [165]. Leaf interveinal chlorosis caused by iron deficiency was overcome by foliar application of NaHS. Moreover, applying H2S donors enhanced chlorophyll contents and iron accumulation in young leaves. However, the H2S enhanced not only iron deficiency but also the assimilation of other micronutrients such as Zn, Ca, and Mg [166]. Iron deficiency in F. × ananassa concomitant with salinity stress (50 mM NaCl) has also been overcome by the exogenous application of NO through SNP as a donor. SNP applied at 0.1 mM showed that plants under iron deficiency and salinity reduced the exacerbated electrolyte leakage, malondialdehyde levels, and H2O2 levels caused by the stress [165]. In recent work, [167] determined that applying SNP as NO donor at 100 µM alleviated heat injury in F. × ananassa plants. NO controlled the overaccumulation of H2O2, reduced lipid peroxidation, and improved the relative water content and a higher expression of heat shock transcription factor genes involved in thermotolerance. According to the information shown above, NO or H2S are gaseous signaling molecules with an important role in response to diverse biotic and abiotic stresses in plants, regulating normal plant growth and development. This evidence suggests that RONSS are a potential tool for use in the biostimulation of crops.
The RONSS studies for their potential as signaling molecules or biostimulants have also been evaluated in medicinal plants. Although they have been less studied, medicinal plants have also been used as a model in some assays. In this regard, Catharanthus roseus, an endemic medicinal plant from Madagascar, was used as a model to evaluate its tolerance to metal stress in the presence of NO [168]. The plants were exposed to 30 mg kg−1 of Cu (CuCl2·2H2O) alone or mixed with SNP as a donor of NO in concentrations of 0–400 µM. The results showed that the damages produced by Cu in C. roseus (Cu+2 accumulation, decrease in NO production, disruption in mineral equilibrium, and high ROS production) were alleviated by SNP presence and in a more significant proportion by 50 µM of SNP. Moreover, the treatment with SNP and Cu + SNP significantly prevented or restored the Cu-induced depression of iron in the root. In addition, interestingly, the authors found that the application of SNP caused an increase in leaf vincristine and vinblastine, two potential anticancer compounds [169], which have been previously reported in C. roseus [170].
Artemisia annua is an important vegetal source against malaria [171]. Adverse effects caused by Cu+2 (20 to 40 mg kg−1) on A. annua can be alleviated by exogenous application of H2S (200 µM), restoring physiological and biochemical parameters, reducing lipid peroxidation and enhancing the antioxidant activity of plants [172]. Additionally, H2S application increased the photosynthetic efficiency and trichome density and the production of artemisinin content [171], a well-known compound used against malaria, but also with anti-inflammatory, antioxidant, and antimicrobial effects [173].
H2S has also been effectively used in Carthamus tinctorius, an Asteraceae with essential medicinal properties and a source of food-grade color in the food industry [174]. The exogenous application of H2S (1 mM) on C. tinctorius plants subjected to drought demonstrated that the harmful effects caused by the water scarcity were countered, increasing the accumulation of secondary metabolites and antioxidant capacity [175]. Exogenous application of SNP as a NO donor on Gingko biloba at different concentrations (50, 100, 250, and 500 μM) demonstrated that the high concentrations (500 μM) favored the increase of phenolic compounds, glycosides, tannins, and saponins. Moreover, a significant increase in an oxidative burst of O2− was also detected, enhanced phenylalanine ammonia-lyase (PAL) activities and antioxidant defense enzymes such as superoxide dismutase and ascorbate peroxidase [176]. Similar results were obtained in G. biloba by applying 250 μM L−1 of SNP under drought stress. The authors reported that after the treatment with SNP, remarkably soluble sugar, proline, flavonoid, and ginkgolide content was obtained in G. biloba leaves, as well as increased PAL activity, demonstrating the capacity of NO to alleviate the adverse effects caused by drought stress [177].
Another medicinal plant is Silybum marianum, which is used to treat liver and biliary disorders. S. marianum contains silymarin, a mixture of flavonoid complexes with a protective component against drugs, including chemotherapy [178]. Field assays with two genotypes of S. marianum demonstrated that applying the SNP (100 µM) as a NO donor compensates for 40% of the adverse effects caused for drought stress, and all yield components responded significantly to treatment with SNP [179]. Applying 100 µM SNP also decreased malondialdehyde content and H2O2 in S. marianum plants submitted to water deficit and prevented a silymarin yield reduction but increased taxifolin production, silychristin, silybin, and isosilybin B [180], compounds that have been associated with the treatment of diseases due to pharmacological properties as hepatoprotective drugs [181,182]. Under drought stress applying 100 µM SNP on S. marianum, the leaf photosynthesis rate increased between 80 and 100% compared with the non-treated plants [179].
Ginsenosides are compounds associated with rhizomes and roots of Panax ginseng. It has a therapeutic potential as an adjuvant in treating diabetes mellitus [183]. In this regard, using SNP as a NO donor, together with methyl jasmonate and applied in adventitious roots of P. ginseng, has shown that a high concentration of ginsenoside was obtained with 200 µM SNP. Additionally, the application of 200 µM SNP and 100 µM methyl jasmonate caused a high induction of ginsenoside biosynthesis-related genes and detected a high sensitivity of the superoxide dismutase 1 gene [184]. In another interesting work, [185] reported stimulatory responses in Origanum majorana German type under drought stress and treated with SNP at 30 and 60 µM. Its application enhanced the growth and yield of essential oil, improved water use efficiency, and caused an upregulation in the antioxidant system. Interestingly, the use of SNP also caused a significant increase in the production of phytopharmaceuticals (total soluble phenol, anthocyanin, flavonoids, and ascorbic acid) in the herbal extract. As mentioned above, most studies have been performed under drought conditions. However, using NO has also caused stimulatory effects in medicinal plants under salt stress. In this regard, [186] developed a study to evaluate the use of NO and spermidine, a known polyamine protector of plants [187], as pretreatment of Matricaria recuita plants. The results showed increased growth parameters, significant malondialdehyde and H2O2 content reduction, and increased ascorbate peroxidase activity.
Finally, it is essential to mention that medicinal plant extract’s biological efficacy in preventing oxidative damage is well documented [188,189,190]. However, their capacity as free radical scavenging or as biostimulant agents favoring the RONSS formation or the increase of antioxidant enzymes has been focused mainly on treating human inflammation or wounds [189,191]. On the other hand, we cannot ignore that plant-derived extracts can act as biostimulants in sustainable agriculture. The systematic application of plant-based products has been shown to promote plant growth and improve damage caused by environmental stresses, which has been associated with the presence of polysaccharides, polyphenols, vitamins, phytohormones, etc. [192,193]. In this regard, recent excellent reviews have focused on the role of moringa leaf as a plant biostimulant to improve the quality of agricultural products [194,195]. Hydrolysate-based biostimulants from Medicago sativa containing triacontanol and indole-3-acetic acid have been reported to stimulate the growth of Zea mays under salinity stress [196]. Since this review was focused only on RONSS species and their use as signaling molecules or biostimulant agents, this aspect will not be addressed in detail, but for more information, see [197] and [193].
NO is a labile molecule and challenging to apply in an exogenous way due to its gaseous nature and short in vivo half-life (between 1 and 5 s). NO has been successfully applied in maize to alleviate the damage produced by saline stress [198]. The authors used chitosan nanoparticles containing the NO donor S-nitroso-mercaptosuccinic acid as a carrier. As a result, a sustained NO release was reported, and amelioration of the harmful effects of salinity on the photosystem II activity, chlorophyll content, and growth of maize plants was observed [198]. In this same way, NO release from chitosan nanoparticles containing S-nitrosoglutathione (GSNO) as an NO donor was demonstrated to attenuate the effects of water deficit on sugarcane plants [199]. Furthermore, encapsulating GSNO into chitosan nanoparticles was shown to cause higher photosynthetic rates under water deficit, and increased the root/shoot ratio.
From a practical point of view, it can be thought that considering the great availability in the atmosphere and the ease of absorption of O2 by plants through stomata and lenticels, the presence of ROS in plant cells will always be ensured at the necessary quantities. The above considers the many mechanisms and environmental factors associated with ROS synthesis (Figure 3). However, despite the potential abundance of ROS in plant cells, different studies show that priming with ROS yields favorable results in different plant species [53,57,136].
On the other hand, unlike ROS, RNS and RSS are not obtained from a resource as abundant as O2. Instead, both RNS and RSS are synthesized from plant nutrients whose greater volume is assimilated by the root in the form of NO3−, NH4+, SO42+, and amino acids. In addition to being much smaller than those of O2 in volume, these nutrients require a previous absorption, transport, and assimilation process to produce the necessary RNS and RSS. The above implies the possibility that to obtain biostimulation with RONSS, only the exogenous application of RNS and RSS or the precursors of ·NO and H2S is necessary. It is even considered that the proper use of fertilizers with N and S can provide the amounts of RNS and RSS essential to achieving improvement in signaling and stress tolerance in plants or obtaining a more significant impact with the use of biostimulants, such as the use of elemental sulfur (S0) or organic fertilizers with S2− [79,200]. In the case of S, a regular supply of fertilizers is necessary, since repeated crop extractions and continuous land tillage that oxidizes soil organic matter cause a decrease in soil S stores [201].
A scheme similar to the one previously mentioned was presented in the study by [202], who used 100 μM ·NO (as donor sodium nitroprusside) in combination with split applications of N and S fertilizers (50 + 50 mg kg−1, two times) in plants of Brassica juncea. The results showed that the combination ·NO+N+S significantly promoted photosynthesis, stomatal performance, and growth in the absence of salt stress and meaningfully alleviated the impact of salt stress through increased proline, N- and S-use efficiency, and antioxidant system. Presumably, using ·NO in combination with the N and S fertilizer sources allowed an adequate balance of RNS and RSS.
4. Conclusions
RONSS exert their functions by interacting with many biomolecules forming a complex cellular information network that indicates the energy status of the system and regulates responses to environmental stimuli.
The use of RONSS as biostimulants in plants is feasible and practical, using techniques such as adequate fertilization with N and S and the use of tolerance-inducing biostimulants such as silicon, organic acids, or chitosan or with the application of precursors of RNS and RSS combined with direct application of ROS, e.g., H2O2. In this sense, applying exogenous NO incorporated in chitosan nanoparticles has proven to be a feasible alternative for alleviating the adverse effects in plants caused by abiotic stress. However, few works have been developed, and more in-depth studies are necessary.
The use of RONSS as biostimulants significantly modifies the phenotype and metabolic activity of plants since RONSS has impacts on and interactions with the main metabolic pathways such as photosynthesis, respiration, the flow of water, and nutrients, as well as with other signaling molecules, such as hormones.
Knowledge about the integration of interactive networks between ROS, RNS, and RSS and between RONSS and other signaling biomolecules is still incomplete. The enormous complexity of the processes, the mutual interactions between the system’s components, and the emergent properties that result from the system´s components´ interactions do not allow a simple approach to the functional scheme in which the RONSS are incorporated.
Author Contributions
Conceptualization (A.B.-M.); all authors performed the literature search and draft preparation. All authors critically revised the drafts and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
All authors agreed with the content, and all gave explicit consent to submit the manuscript.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Universidad de La Frontera DI22-1001.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ricard J. Systems Biology and the Origins of Life? Part I. Are Biochemical Networks Possible Ancestors of Living Systems? Reproduction, Identity and Sensitivity to Signals of Biochemical Networks. Comptes Rendus Biol. 2010;333:761–768. doi: 10.1016/j.crvi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Muchowska K.B., Varma S.J., Moran J. Nonenzymatic Metabolic Reactions and Life’s Origins. Chem. Rev. 2020;120:7708–7744. doi: 10.1021/acs.chemrev.0c00191. [DOI] [PubMed] [Google Scholar]
- 3.Becerra A. The Semi-Enzymatic Origin of Metabolic Pathways: Inferring a Very Early Stage of the Evolution of Life. J. Mol. Evol. 2021;89:183–188. doi: 10.1007/s00239-021-09994-0. [DOI] [PubMed] [Google Scholar]
- 4.Morowitz H.J., Deamer D.W., Smith T. Biogenesis as an Evolutionary Process. J. Mol. Evol. 1991;33:207–208. doi: 10.1007/BF02100670. [DOI] [PubMed] [Google Scholar]
- 5.Michaelian K. Non-Equilibrium Thermodynamic Foundations of the Origin of Life. Foundations. 2022;2:308–337. doi: 10.3390/foundations2010022. [DOI] [Google Scholar]
- 6.Olson K.R. Reactive Oxygen Species or Reactive Sulfur Species: Why We Should Consider the Latter. J. Exp. Biol. 2020;223:jeb196352. doi: 10.1242/jeb.196352. [DOI] [PubMed] [Google Scholar]
- 7.Jones D.P., Sies H. The Redox Code. Antioxid. Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotka A.J. Contribution to the Energetics of Evolution. Proc. Natl. Acad. Sci. USA. 1922;8:147–151. doi: 10.1073/pnas.8.6.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corning P.A. A Systems Theory of Biological Evolution. Biosystems. 2022;214:104630. doi: 10.1016/j.biosystems.2022.104630. [DOI] [PubMed] [Google Scholar]
- 10.Suki B. The Major Transitions of Life from a Network Perspective. Front. Physiol. 2012;3:1–13. doi: 10.3389/fphys.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De la Fuente I.M. Elements of the Cellular Metabolic Structure. Front. Mol. Biosci. 2015;2:16. doi: 10.3389/fmolb.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock J.T. Hydrogen Sulfide and Environmental Stresses. Environ. Exp. Bot. 2019;161:50–56. doi: 10.1016/j.envexpbot.2018.08.034. [DOI] [Google Scholar]
- 13.Hancock J.T., Whiteman M. Hydrogen Sulfide Signaling: Interactions with Nitric Oxide and Reactive Oxygen Species. Ann. N. Y. Acad. Sci. 2016;1365:5–14. doi: 10.1111/nyas.12733. [DOI] [PubMed] [Google Scholar]
- 14.Heylighen F., Beigi S., Busseniers E. The Role of Self-Maintaining Resilient Reaction Networks in the Origin and Evolution of Life. Biosystems. 2022;219:104720. doi: 10.1016/j.biosystems.2022.104720. [DOI] [PubMed] [Google Scholar]
- 15.De Bianchi S., Ballottari M., Dall’Osto L., Bassi R. Regulation of Plant Light Harvesting by Thermal Dissipation of Excess Energy. Biochem. Soc. Trans. 2010;38:651–660. doi: 10.1042/BST0380651. [DOI] [PubMed] [Google Scholar]
- 16.Haupt-Herting S., Fock H.P. Exchange of Oxygen and Its Role in Energy Dissipation during Drought Stress in Tomato Plants. Physiol. Plant. 2000;110:489–495. doi: 10.1111/j.1399-3054.2000.1100410.x. [DOI] [Google Scholar]
- 17.Baluška F., Reber A.S., Miller W.B. Cellular Sentience as the Primary Source of Biological Order and Evolution. Biosystems. 2022;218:104694. doi: 10.1016/j.biosystems.2022.104694. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan A.S., Oetjen H., Saiz-Lopez A., Lee J.D., McFiggans G.B., Plane J.M.C. Reactive Iodine Species in a Semi-Polluted Environment. Geophys. Res. Lett. 2009;36:L16803. doi: 10.1029/2009GL038018. [DOI] [Google Scholar]
- 19.Pasek M.A., Harnmeijer J.P., Buick R., Gull M., Atlas Z. Evidence for Reactive Reduced Phosphorus Species in the Early Archean Ocean. PNAS. 2013;110:10089–10094. doi: 10.1073/pnas.1303904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharma A., Grman M., Misak A., Domínguez-Álvarez E., Nasim M.J., Ondrias K., Chovanec M., Jacob C. Inorganic Polysulfides and Related Reactive Sulfur–Selenium Species from the Perspective of Chemistry. Molecules. 2019;24:1359. doi: 10.3390/molecules24071359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alché J.d.D. A Concise Appraisal of Lipid Oxidation and Lipoxidation in Higher Plants. Redox Biol. 2019;23:101136. doi: 10.1016/j.redox.2019.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolbert Z., Barroso J.B., Brouquisse R., Corpas F.J., Gupta K.J., Lindermayr C., Loake G.J., Palma J.M., Petřivalský M., Wendehenne D., et al. A Forty Year Journey: The Generation and Roles of NO in Plants. Nitric Oxide. 2019;93:53–70. doi: 10.1016/j.niox.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Tola A.J., Jaballi A., Missihoun T.D. Protein Carbonylation: Emerging Roles in Plant Redox Biology and Future Prospects. Plants. 2021;10:1451. doi: 10.3390/plants10071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson K.R., Straub K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology. 2015;31:60–72. doi: 10.1152/physiol.00024.2015. [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki H., Cohen M.F. Biological Consilience of Hydrogen Sulfide and Nitric Oxide in Plants: Gases of Primordial Earth Linking Plant, Microbial and Animal Physiologies. Nitric Oxide. 2016;55–56:91–100. doi: 10.1016/j.niox.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Gumsley A.P., Chamberlain K.R., Bleeker W., Söderlund U., de Kock M.O., Larsson E.R., Bekker A. Timing and Tempo of the Great Oxidation Event. PNAS. 2017;114:1811–1816. doi: 10.1073/pnas.1608824114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santolini J., Wootton S.A., Jackson A.A., Feelisch M. The Redox Architecture of Physiological Function. Curr. Opin. Physiol. 2019;9:34–47. doi: 10.1016/j.cophys.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen J.F., Thake B., Martin W.F. Nitrogenase Inhibition Limited Oxygenation of Earth’s Proterozoic Atmosphere. Trends Plant Sci. 2019;24:1022–1031. doi: 10.1016/j.tplants.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Jacob F. Evolution and Tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 30.Macklem P.T. Emergent Phenomena and the Secrets of Life. J. Appl. Physiol. 2008;104:1844–1846. doi: 10.1152/japplphysiol.00942.2007. [DOI] [PubMed] [Google Scholar]
- 31.Toyabe S., Sagawa T., Ueda M., Muneyuki E., Sano M. Experimental Demonstration of Information-to-Energy Conversion and Validation of the Generalized Jarzynski Equality. Nat. Phys. 2010;6:988–992. doi: 10.1038/nphys1821. [DOI] [Google Scholar]
- 32.Farooq M.A., Niazi A.K., Akhtar J., Saifullah, Farooq M., Souri Z., Karimi N., Rengel Z. Acquiring Control: The Evolution of ROS-Induced Oxidative Stress and Redox Signaling Pathways in Plant Stress Responses. Plant Physiol. Biochem. 2019;141:353–369. doi: 10.1016/j.plaphy.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 33.Noctor G., Reichheld J.-P., Foyer C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018;80:3–12. doi: 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Antoniou C., Savvides A., Christou A., Fotopoulos V. Unravelling Chemical Priming Machinery in Plants: The Role of Reactive Oxygen–Nitrogen–Sulfur Species in Abiotic Stress Tolerance Enhancement. Curr. Opin. Plant Biol. 2016;33:101–107. doi: 10.1016/j.pbi.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Corpas F.J., Carreras A., Valderrama R., Chaki M., Palma J.M. Reactive Nitrogen Species and Nitrosative Stress in Plants. Plant Stress. 2007;1:37–41. [Google Scholar]
- 36.Ahmad P., Sarwat M., Sharma S. Reactive Oxygen Species, Antioxidants and Signaling in Plants. J. Plant Biol. 2008;51:167–173. doi: 10.1007/BF03030694. [DOI] [Google Scholar]
- 37.Gruhlke M.C.H., Slusarenko A.J. The Biology of Reactive Sulfur Species (RSS) Plant Physiol. Biochem. 2012;59:98–107. doi: 10.1016/j.plaphy.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Corpas F.J., Gupta D.K., Palma J.M. Production Sites of Reactive Oxygen Species (ROS) in Organelles from Plant Cells. In: Gupta D.K., Palma J.M., Corpas F.J., editors. Reactive Oxygen Species and Oxidative Damage in Plants Under Stress. Springer International Publishing; Cham, Switzerland: 2015. pp. 1–22. [Google Scholar]
- 39.Giles G.I., Nasim M.J., Ali W., Jacob C. The Reactive Sulfur Species Concept: 15 Years On. Antioxidants. 2017;6:38. doi: 10.3390/antiox6020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corpas F.J., Palma J.M. Assessing Nitric Oxide (NO) in Higher Plants: An Outline. Nitrogen. 2020;1:12–20. doi: 10.3390/nitrogen1010003. [DOI] [Google Scholar]
- 41.González-Morales S., López-Sánchez R.C., Juárez-Maldonado A., Robledo-Olivo A., Benavides-Mendoza A. A Transcriptomic and Proteomic View of Hydrogen Sulfide Signaling in Plant Abiotic Stress. In: Khan M.N., Siddiqui M.H., Alamri S., Corpas F.J., editors. Hydrogen Sulfide and Plant Acclimation to Abiotic Stresses. Springer International Publishing; Cham, Switzerland: 2021. pp. 161–186. Plant in Challenging Environments. [Google Scholar]
- 42.Khanna K., Sharma N., Kour S., Ali M., Ohri P., Bhardwaj R. Hydrogen Sulfide: A Robust Combatant against Abiotic Stresses in Plants. Hydrogen. 2021;2:319–342. doi: 10.3390/hydrogen2030017. [DOI] [Google Scholar]
- 43.Mansoor S., Ali Wani O., Lone J.K., Manhas S., Kour N., Alam P., Ahmad A., Ahmad P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants. 2022;11:225. doi: 10.3390/antiox11020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittler R., Zandalinas S.I., Fichman Y., Van Breusegem F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022;23:663–679. doi: 10.1038/s41580-022-00499-2. [DOI] [PubMed] [Google Scholar]
- 45.Giles G.I., Tasker K.M., Jacob C. Hypothesis: The Role of Reactive Sulfur Species in Oxidative Stress. Free Radic. Biol. Med. 2001;31:1279–1283. doi: 10.1016/S0891-5849(01)00710-9. [DOI] [PubMed] [Google Scholar]
- 46.Del Río L.A. ROS and RNS in Plant Physiology: An Overview. J. Exp. Bot. 2015;66:2827–2837. doi: 10.1093/jxb/erv099. [DOI] [PubMed] [Google Scholar]
- 47.Weidinger A., Kozlov A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules. 2015;5:472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turkan I. ROS and RNS: Key Signalling Molecules in Plants. J Exp Bot. 2018;69:3313–3315. doi: 10.1093/jxb/ery198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olson K.R. Hydrogen Sulfide, Reactive Sulfur Species and Coping with Reactive Oxygen Species. Free Radic. Biol. Med. 2019;140:74–83. doi: 10.1016/j.freeradbiomed.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Antoniou C., Xenofontos R., Chatzimichail G., Christou A., Kashfi K., Fotopoulos V. Exploring the Potential of Nitric Oxide and Hydrogen Sulfide (NOSH)-Releasing Synthetic Compounds as Novel Priming Agents against Drought Stress in Medicago Sativa Plants. Biomolecules. 2020;10:120. doi: 10.3390/biom10010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozfidan-Konakci C., Yildiztugay E., Elbasan F., Kucukoduk M., Turkan I. Hydrogen Sulfide (H2S) and Nitric Oxide (NO) Alleviate Cobalt Toxicity in Wheat (Trriticum aestivum L.) by Modulating Photosynthesis, Chloroplastic Redox and Antioxidant Capacity. J. Hazard. Mater. 2020;388:122061. doi: 10.1016/j.jhazmat.2020.122061. [DOI] [PubMed] [Google Scholar]
- 52.Palma J.M., Mateos R.M., López-Jaramillo J., Rodríguez-Ruiz M., González-Gordo S., Lechuga-Sancho A.M., Corpas F.J. Plant Catalases as NO and H2S Targets. Redox Biol. 2020;34:101525. doi: 10.1016/j.redox.2020.101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomar R.S., Kataria S., Jajoo A. Behind the Scene: Critical Role of Reactive Oxygen Species and Reactive Nitrogen Species in Salt Stress Tolerance. J. Agron. Crop. Sci. 2021;207:577–588. doi: 10.1111/jac.12490. [DOI] [Google Scholar]
- 54.Wani K.I., Naeem M., Castroverde C.D.M., Kalaji H.M., Albaqami M., Aftab T. Molecular Mechanisms of Nitric Oxide (NO) Signaling and Reactive Oxygen Species (ROS) Homeostasis during Abiotic Stresses in Plants. Int. J. Mol. Sci. 2021;22:9656. doi: 10.3390/ijms22179656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar S.P.J., Chintagunta A.D., Reddy Y.M., Rajjou L., Garlapati V.K., Agarwal D.K., Prasad S.R., Simal-Gandara J. Implications of Reactive Oxygen and Nitrogen Species in Seed Physiology for Sustainable Crop Productivity under Changing Climate Conditions. Curr. Plant Biol. 2021;26:100197. doi: 10.1016/j.cpb.2021.100197. [DOI] [Google Scholar]
- 56.Lushchak V.I., Lushchak O. Interplay between Reactive Oxygen and Nitrogen Species in Living Organisms. Chem. Biol. Interact. 2021;349:109680. doi: 10.1016/j.cbi.2021.109680. [DOI] [PubMed] [Google Scholar]
- 57.Savvides A., Ali S., Tester M., Fotopoulos V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016;21:329–340. doi: 10.1016/j.tplants.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Ashraf M.A., Rasheed R., Hussain I., Iqbal M., Riaz M., Arif M.S. Chemical Priming for Multiple Stress Tolerance. In: Hasanuzzaman M., Fotopoulos V., editors. Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants. Springer; Singapore: 2019. pp. 385–415. [Google Scholar]
- 59.Kaur P., Handa N., Verma V., Bakshi P., Kalia R., Sareen S., Nagpal A., Vig A.P., Mir B.A., Bhardwaj R. Reactive Oxygen, Nitrogen and Sulfur Species in Plants. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2019. Cross Talk Among Reactive Oxygen, Nitrogen and Sulfur During Abiotic Stress in Plants; pp. 857–871. [Google Scholar]
- 60.Zhou X., Joshi S., Patil S., Khare T., Kumar V. Reactive Oxygen, Nitrogen, Carbonyl and Sulfur Species and Their Roles in Plant Abiotic Stress Responses and Tolerance. J. Plant Growth Regul. 2021;41:119–142. doi: 10.1007/s00344-020-10294-y. [DOI] [Google Scholar]
- 61.Mangal V., Lal M.K., Tiwari R.K., Altaf M.A., Sood S., Kumar D., Bharadwaj V., Singh B., Singh R.K., Aftab T. Molecular Insights into the Role of Reactive Oxygen, Nitrogen and Sulphur Species in Conferring Salinity Stress Tolerance in Plants. J. Plant Growth Regul. 2022:1–21. doi: 10.1007/s00344-022-10591-8. [DOI] [Google Scholar]
- 62.Decros G., Baldet P., Beauvoit B., Stevens R., Flandin A., Colombié S., Gibon Y., Pétriacq P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant Sci. 2019;10:1091. doi: 10.3389/fpls.2019.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foyer C.H., Noctor G. Stress-Triggered Redox Signalling: What’s in PROSpect? Plant Cell Environ. 2016;39:951–964. doi: 10.1111/pce.12621. [DOI] [PubMed] [Google Scholar]
- 64.Foyer C.H., Hanke G. ROS Production and Signalling in Chloroplasts: Cornerstones and Evolving Concepts. Plant J. 2022;111:642–661. doi: 10.1111/tpj.15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.García-Caparrós P., De Filippis L., Gul A., Hasanuzzaman M., Ozturk M., Altay V., Lao M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021;87:421–466. doi: 10.1007/s12229-020-09231-1. [DOI] [Google Scholar]
- 66.Mukherjee S. Cysteine Modifications (OxPTM) and Protein Sulphenylation-Mediated Sulfenome Expression in Plants: Evolutionary Conserved Signaling Networks? Plant Signal. Behav. 2021;16:1831792. doi: 10.1080/15592324.2020.1831792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Considine M.J., María Sandalio L., Helen Foyer C. Unravelling How Plants Benefit from ROS and NO Reactions, While Resisting Oxidative Stress. Ann. Bot. 2015;116:469–473. doi: 10.1093/aob/mcv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Considine M.J., Foyer C.H. Oxygen and Reactive Oxygen Species-Dependent Regulation of Plant Growth and Development. Plant Physiol. 2021;186:79–92. doi: 10.1093/plphys/kiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Durner J., Gow A.J., Stamler J.S., Glazebrook J. Ancient Origins of Nitric Oxide Signaling in Biological Systems. Proc. Natl. Acad. Sci. USA. 1999;96:14206–14207. doi: 10.1073/pnas.96.25.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Astier J., Mounier A., Santolini J., Jeandroz S., Wendehenne D. The Evolution of Nitric Oxide Signalling Diverges between Animal and Green Lineages. J. Exp. Bot. 2019;70:4355–4364. doi: 10.1093/jxb/erz088. [DOI] [PubMed] [Google Scholar]
- 71.Mandal M., Sarkar M., Khan A., Biswas M., Masi A., Rakwal R., Agrawal G.K., Srivastava A., Sarkar A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants– Maintenance of Structural Individuality and Functional Blend. Adv. Redox Res. 2022;5:100039. doi: 10.1016/j.arres.2022.100039. [DOI] [Google Scholar]
- 72.Staszek P., Gniazdowska A. Peroxynitrite Induced Signaling Pathways in Plant Response to Non-Proteinogenic Amino Acids. Planta. 2020;252:5. doi: 10.1007/s00425-020-03411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murad F., Barber R. A Hypothesis about Cellular Signaling with Nitric Oxide in the Earliest Life Forms in Evolution. Free Radic. Biol. Med. 2009;47:1325–1327. doi: 10.1016/j.freeradbiomed.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Corpas F.J., González-Gordo S., Palma J.M. NO Source in Higher Plants: Present and Future of an Unresolved Question. Trends Plant Sci. 2022;27:116–119. doi: 10.1016/j.tplants.2021.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Gupta K.J., Stoimenova M., Kaiser W.M. In Higher Plants, Only Root Mitochondria, but Not Leaf Mitochondria Reduce Nitrite to NO, in Vitro and in Situ. J. Exp. Bot. 2005;56:2601–2609. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- 76.Astier J., Gross I., Durner J. Nitric Oxide Production in Plants: An Update. J. Exp. Bot. 2018;69:3401–3411. doi: 10.1093/jxb/erx420. [DOI] [PubMed] [Google Scholar]
- 77.Corpas F.J., González-Gordo S., Palma J.M. Protein Nitration: A Connecting Bridge between Nitric Oxide (NO) and Plant Stress. Plant Stress. 2021;2:100026. doi: 10.1016/j.stress.2021.100026. [DOI] [Google Scholar]
- 78.Di Fino L., Arruebarrena Di Palma A., Perk E.A., García-Mata C., Schopfer F.J., Laxalt A.M. Nitro-Fatty Acids: Electrophilic Signaling Molecules in Plant Physiology. Planta. 2021;254:120. doi: 10.1007/s00425-021-03777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuentes-Lara L.O., Medrano-Macías J., Pérez-Labrada F., Rivas-Martínez E.N., García-Enciso E.L., González-Morales S., Juárez-Maldonado A., Rincón-Sánchez F., Benavides-Mendoza A. From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants. Molecules. 2019;24:2282. doi: 10.3390/molecules24122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q., Lancaster J.R. Chemical Foundations of Hydrogen Sulfide Biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arif M.S., Yasmeen T., Abbas Z., Ali S., Rizwan M., Aljarba N.H., Alkahtani S., Abdel-Daim M.M. Role of Exogenous and Endogenous Hydrogen Sulfide (H2S) on Functional Traits of Plants Under Heavy Metal Stresses: A Recent Perspective. Front. Plant Sci. 2021;11:545453. doi: 10.3389/fpls.2020.545453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lau N., Pluth M.D. Reactive Sulfur Species (RSS): Persulfides, Polysulfides, Potential, and Problems. Curr. Opin. Chem. Biol. 2019;49:1–8. doi: 10.1016/j.cbpa.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 83.Koike S., Ogasawara Y. Sulfur Atom in Its Bound State Is a Unique Element Involved in Physiological Functions in Mammals. Molecules. 2016;21:1753. doi: 10.3390/molecules21121753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar M.R., Farmer P.J. Characterization of Polysulfides, Polysulfanes, and Other Unique Species in the Reaction between GSNO and H2S. Molecules. 2019;24:3090. doi: 10.3390/molecules24173090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leontiev R., Hohaus N., Jacob C., Gruhlke M.C.H., Slusarenko A.J. A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018;8:6763. doi: 10.1038/s41598-018-25154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao X., Wang C., Luo X., Yue L., White J.C., Elmer W., Dhankher O.P., Wang Z., Xing B. Elemental Sulfur Nanoparticles Enhance Disease Resistance in Tomatoes. ACS Nano. 2021;15:11817–11827. doi: 10.1021/acsnano.1c02917. [DOI] [PubMed] [Google Scholar]
- 87.Yuan H., Liu Q., Guo Z., Fu J., Sun Y., Gu C., Xing B., Dhankher O.P. Sulfur Nanoparticles Improved Plant Growth and Reduced Mercury Toxicity via Mitigating the Oxidative Stress in Brassica napus L. J. Clean. Prod. 2021;318:128589. doi: 10.1016/j.jclepro.2021.128589. [DOI] [Google Scholar]
- 88.Shankar S., Jaiswal L., Rhim J.-W. New Insight into Sulfur Nanoparticles: Synthesis and Applications. Crit. Rev. Environ. Sci. Technol. 2021;51:2329–2356. doi: 10.1080/10643389.2020.1780880. [DOI] [Google Scholar]
- 89.Grman M., Nasim M.J., Leontiev R., Misak A., Jakusova V., Ondrias K., Jacob C. Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red? Antioxidants. 2017;6:14. doi: 10.3390/antiox6010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lum H.K., Butt Y.K.C., Lo S.C.L. Hydrogen Peroxide Induces a Rapid Production of Nitric Oxide in Mung Bean (Phaseolus aureus) Nitric Oxide. 2002;6:205–213. doi: 10.1006/niox.2001.0395. [DOI] [PubMed] [Google Scholar]
- 91.Li Z.G., Luo L.J., Sun Y.F. Signal Crosstalk between Nitric Oxide and Hydrogen Sulfide May Be Involved in Hydrogen Peroxide-Induced Thermotolerance in Maize Seedlings. Russ. J. Plant Physiol. 2015;62:507–514. doi: 10.1134/S1021443715030127. [DOI] [Google Scholar]
- 92.Wu D., Chu H., Jia L., Chen K., Zhao L. A Feedback Inhibition between Nitric Oxide and Hydrogen Peroxide in the Heat Shock Pathway in Arabidopsis Seedlings. Plant Growth Regul. 2015;75:503–509. doi: 10.1007/s10725-014-0014-x. [DOI] [Google Scholar]
- 93.Jian W., Zhang D., Zhu F., Wang S., Pu X., Deng X.-G., Luo S.-S., Lin H. Alternative Oxidase Pathway Is Involved in the Exogenous SNP-Elevated Tolerance of Medicago Truncatula to Salt Stress. J. Plant Physiol. 2016;193:79–87. doi: 10.1016/j.jplph.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 94.Zhao M., Liu Q., Zhang Y., Yang N., Wu G., Li Q., Wang W. Alleviation of Osmotic Stress by H2S Is Related to Regulated PLDα1 and Suppressed ROS in Arabidopsis Thaliana. J. Plant Res. 2020;133:393–407. doi: 10.1007/s10265-020-01182-3. [DOI] [PubMed] [Google Scholar]
- 95.Zhang L., Pei Y., Wang H., Jin Z., Liu Z., Qiao Z., Fang H., Zhang Y. Hydrogen Sulfide Alleviates Cadmium-Induced Cell Death through Restraining ROS Accumulation in Roots of Brassica rapa L. Ssp. Pekinensis. Oxidative Med. Cell. Longev. 2015;2015:804603. doi: 10.1155/2015/804603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ahmad R., Ali S., Rizwan M., Dawood M., Farid M., Hussain A., Wijaya L., Alyemeni M.N., Ahmad P. Hydrogen Sulfide Alleviates Chromium Stress on Cauliflower by Restricting Its Uptake and Enhancing Antioxidative System. Physiol. Plant. 2020;168:289–300. doi: 10.1111/ppl.13001. [DOI] [PubMed] [Google Scholar]
- 97.Lisjak M., Srivastava N., Teklic T., Civale L., Lewandowski K., Wilson I., Wood M.E., Whiteman M., Hancock J.T. A Novel Hydrogen Sulfide Donor Causes Stomatal Opening and Reduces Nitric Oxide Accumulation. Plant Physiol. Biochem. 2010;48:931–935. doi: 10.1016/j.plaphy.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 98.Da-Silva C.J., Mollica D.C.F., Vicente M.H., Peres L.E.P., Modolo L.V. NO, Hydrogen Sulfide Does Not Come First during Tomato Response to High Salinity. Nitric Oxide. 2018;76:164–173. doi: 10.1016/j.niox.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 99.Chen J., Wang W.-H., Wu F.-H., He E.-M., Liu X., Shangguan Z.-P., Zheng H.-L. Hydrogen Sulfide Enhances Salt Tolerance through Nitric Oxide-Mediated Maintenance of Ion Homeostasis in Barley Seedling Roots. Sci. Rep. 2015;5:12516. doi: 10.1038/srep12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corpas F.J., Barroso J.B., González-Gordo S., Muñoz-Vargas M.A., Palma J.M. Hydrogen Sulfide: A Novel Component in Arabidopsis Peroxisomes Which Triggers Catalase Inhibition. J. Integr. Plant Biol. 2019;61:871–883. doi: 10.1111/jipb.12779. [DOI] [PubMed] [Google Scholar]
- 101.Zhang X.-W., Liu F.-J., Zhai J., Li F.-D., Bi H.-G., Ai X.-Z. Auxin Acts as a Downstream Signaling Molecule Involved in Hydrogen Sulfide-Induced Chilling Tolerance in Cucumber. Planta. 2020;251:69. doi: 10.1007/s00425-020-03362-w. [DOI] [PubMed] [Google Scholar]
- 102.Kaya C., Higgs D., Ashraf M., Alyemeni M.N., Ahmad P. Integrative Roles of Nitric Oxide and Hydrogen Sulfide in Melatonin-Induced Tolerance of Pepper (Capsicum annuum L.) Plants to Iron Deficiency and Salt Stress Alone or in Combination. Physiol. Plant. 2020;168:256–277. doi: 10.1111/ppl.12976. [DOI] [PubMed] [Google Scholar]
- 103.Kaya C., Ashraf M., Alyemeni M.N., Corpas F.J., Ahmad P. Salicylic Acid-Induced Nitric Oxide Enhances Arsenic Toxicity Tolerance in Maize Plants by Upregulating the Ascorbate-Glutathione Cycle and Glyoxalase System. J. Hazard. Mater. 2020;399:123020. doi: 10.1016/j.jhazmat.2020.123020. [DOI] [PubMed] [Google Scholar]
- 104.Kaya C., Sarıoğlu A., Ashraf M., Alyemeni M.N., Ahmad P. Gibberellic Acid-Induced Generation of Hydrogen Sulfide Alleviates Boron Toxicity in Tomato (Solanum lycopersicum L.) Plants. Plant Physiol. Biochem. 2020;153:53–63. doi: 10.1016/j.plaphy.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 105.Kolbert Z., Szőllősi R., Feigl G., Kónya Z., Rónavári A. Nitric Oxide Signalling in Plant Nanobiology: Current Status and Perspectives. J. Exp. Bot. 2021;72:928–940. doi: 10.1093/jxb/eraa470. [DOI] [PubMed] [Google Scholar]
- 106.Li L., Liu Y., Wang S., Zou J., Ding W., Shen W. Magnesium Hydride-Mediated Sustainable Hydrogen Supply Prolongs the Vase Life of Cut Carnation Flowers via Hydrogen Sulfide. Front. Plant Sci. 2020;11:595376. doi: 10.3389/fpls.2020.595376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kou N., Xiang Z., Cui W., Li L., Shen W. Hydrogen Sulfide Acts Downstream of Methane to Induce Cucumber Adventitious Root Development. J. Plant Physiol. 2018;228:113–120. doi: 10.1016/j.jplph.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 108.Mukherjee S., Corpas F.J. Crosstalk among Hydrogen Sulfide (H2S), Nitric Oxide (NO) and Carbon Monoxide (CO) in Root-System Development and Its Rhizosphere Interactions: A Gaseous Interactome. Plant Physiol. Biochem. 2020;155:800–814. doi: 10.1016/j.plaphy.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y., Su J., Cheng D., Wang R., Mei Y., Hu H., Shen W., Zhang Y. Nitric Oxide Contributes to Methane-Induced Osmotic Stress Tolerance in Mung Bean. BMC Plant Biol. 2018;18:207. doi: 10.1186/s12870-018-1426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pyrzynska K., Sentkowska A. Selenium in Plant Foods: Speciation Analysis, Bioavailability, and Factors Affecting Composition. Crit. Rev. Food Sci. Nutr. 2021;61:1340–1352. doi: 10.1080/10408398.2020.1758027. [DOI] [PubMed] [Google Scholar]
- 111.Morales-Espinoza M.C., Cadenas-Pliego G., Pérez-Alvarez M., Hernández-Fuentes A.D., Cabrera de la Fuente M., Benavides-Mendoza A., Valdés-Reyna J., Juárez-Maldonado A. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules. 2019;24:3030. doi: 10.3390/molecules24173030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schiavon M., Warzea L., Jiang Y., Hawkesfors M. Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects. Volume 114. Springer; Berlin/Heidelberg, Germany: 2017. Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers; p. 231. [Google Scholar]
- 113.Ghuge S.A., Kadam U.S., Hong J.C. Selenoprotein: Potential Player in Redox Regulation in Chlamydomonas Reinhardtii. Antioxidants. 2022;11:1630. doi: 10.3390/antiox11081630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.García Márquez V., Morelos Moreno Á., Benavides Mendoza A., Medrano Macías J. Ionic Selenium and Nanoselenium as Biofortifiers and Stimulators of Plant Metabolism. Agronomy. 2020;10:1399. doi: 10.3390/agronomy10091399. [DOI] [Google Scholar]
- 115.González-Morales S., Pérez-Labrada F., García-Enciso E.L., Leija-Martínez P., Medrano-Macías J., Dávila-Rangel I.E., Juárez-Maldonado A., Rivas-Martínez E.N., Benasvides-Mendoza A. Selenium and Sulfur to Produce Allium Functional Crops. Molecules. 2017;22:558. doi: 10.3390/molecules22040558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun X., Han G., Ye S., Luo Y., Zhou X. Effects of Selenium on Serotonin Synthesis and the Glutathione Redox Cycle in Plum Leaves. J. Soil Sci. Plant Nutr. 2020;20:2212–2221. doi: 10.1007/s42729-020-00288-w. [DOI] [Google Scholar]
- 117.Kopriva S., Suter M., von Ballmoos P., Hesse H., Krähenbühl U., Rennenberg H., Brunold C. Interaction of Sulfate Assimilation with Carbon and Nitrogen Metabolism in Lemna Minor. Plant Physiol. 2002;130:1406–1413. doi: 10.1104/pp.007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mimmo T., Tiziani R., Valentinuzzi F., Lucini L., Nicoletto C., Sambo P., Scampicchio M., Pii Y., Cesco S. Selenium Biofortification in Fragaria × Ananassa: Implications on Strawberry Fruits Quality, Content of Bioactive Health Beneficial Compounds and Metabolomic Profile. Front. Plant Sci. 2017;8:1887. doi: 10.3389/fpls.2017.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lanza M.G.D.B., dos Reis A.R. Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses against Abiotic Stresses. Plant Physiol. Biochem. 2021;164:27–43. doi: 10.1016/j.plaphy.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 120.Silva V.M., Rimoldi Tavanti R.F., Gratão P.L., Alcock T.D., dos Reis A.R. Selenate and Selenite Affect Photosynthetic Pigments and ROS Scavenging through Distinct Mechanisms in Cowpea (Vigna unguiculata (L.) Walp) Plants. Ecotoxicol. Environ. Saf. 2020;201:110777. doi: 10.1016/j.ecoenv.2020.110777. [DOI] [PubMed] [Google Scholar]
- 121.Yeasmin M., Lamb D., Choppala G., Rahman M.M. Selenium Accumulation and Speciation in Chickpea (Cicer arietinum) Impacted by S in Soils: Potential for Biofortification. ACS Agric. Sci. Technol. 2022;2:135–143. doi: 10.1021/acsagscitech.1c00237. [DOI] [Google Scholar]
- 122.Medrano-Macías J., Narvaéz-Ortiz W.A. Selenium and Nano-Selenium as a New Frontier of Plant Biostimulant. In: Hossain M.A., Ahammed G.J., Kolbert Z., El-Ramady H., Islam T., Schiavon M., editors. Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement. Springer International Publishing; Cham, Switzerland: 2022. pp. 41–54. Sustainable Plant Nutrition in a Changing World. [Google Scholar]
- 123.Solórzano E., Corpas F.J., González-Gordo S., Palma J.M. Reactive Oxygen Species (ROS) Metabolism and Nitric Oxide (NO) Content in Roots and Shoots of Rice (Oryza sativa L.) Plants under Arsenic-Induced Stress. Agronomy. 2020;10:1014. doi: 10.3390/agronomy10071014. [DOI] [Google Scholar]
- 124.Tanou G., Job C., Belghazi M., Molassiotis A., Diamantidis G., Job D. Proteomic Signatures Uncover Hydrogen Peroxide and Nitric Oxide Cross-Talk Signaling Network in Citrus Plants. J. Proteome Res. 2010;9:5994–6006. doi: 10.1021/pr100782h. [DOI] [PubMed] [Google Scholar]
- 125.Tanou G., Filippou P., Belghazi M., Job D., Diamantidis G., Fotopoulos V., Molassiotis A. Oxidative and Nitrosative-Based Signaling and Associated Post-Translational Modifications Orchestrate the Acclimation of Citrus Plants to Salinity Stress. Plant J. 2012;72:585–599. doi: 10.1111/j.1365-313X.2012.05100.x. [DOI] [PubMed] [Google Scholar]
- 126.Kataria S., Jain M., Tripathi D.K., Singh V.P. Involvement of Nitrate Reductase-Dependent Nitric Oxide Production in Magnetopriming-Induced Salt Tolerance in Soybean. Physiol. Plant. 2020;168:422–436. doi: 10.1111/ppl.13031. [DOI] [PubMed] [Google Scholar]
- 127.Kaur H., Bhatla S.C. Melatonin and Nitric Oxide Modulate Glutathione Content and Glutathione Reductase Activity in Sunflower Seedling Cotyledons Accompanying Salt Stress. Nitric Oxide. 2016;59:42–53. doi: 10.1016/j.niox.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 128.Kaya C., Ashraf M., Al-Huqail A.A., Alqahtani M.A., Ahmad P. Silicon Is Dependent on Hydrogen Sulphide to Improve Boron Toxicity Tolerance in Pepper Plants by Regulating the AsA-GSH Cycle and Glyoxalase System. Chemosphere. 2020;257:127241. doi: 10.1016/j.chemosphere.2020.127241. [DOI] [PubMed] [Google Scholar]
- 129.Li L., Wang Y., Shen W. Roles of Hydrogen Sulfide and Nitric Oxide in the Alleviation of Cadmium-Induced Oxidative Damage in Alfalfa Seedling Roots. Biometals. 2012;25:617–631. doi: 10.1007/s10534-012-9551-9. [DOI] [PubMed] [Google Scholar]
- 130.Amooaghaie R., Zangene-Madar F., Enteshari S. Role of Two-Sided Crosstalk between NO and H2S on Improvement of Mineral Homeostasis and Antioxidative Defense in Sesamum Indicum under Lead Stress. Ecotoxicol. Environ. Saf. 2017;139:210–218. doi: 10.1016/j.ecoenv.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 131.Kaya C., Ashraf M., Alyemeni M.N., Ahmad P. Responses of Nitric Oxide and Hydrogen Sulfide in Regulating Oxidative Defence System in Wheat Plants Grown under Cadmium Stress. Physiol. Plant. 2020;168:345–360. doi: 10.1111/ppl.13012. [DOI] [PubMed] [Google Scholar]
- 132.Shi H., Ye T., Chan Z. Nitric Oxide-Activated Hydrogen Sulfide Is Essential for Cadmium Stress Response in Bermudagrass (Cynodon dactylon (L). Pers.) Plant Physiol. Biochem. 2014;74:99–107. doi: 10.1016/j.plaphy.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 133.Liang Y., Zheng P., Li S., Li K., Xu H. Nitrate Reductase-Dependent NO Production Is Involved in H2S-Induced Nitrate Stress Tolerance in Tomato via Activation of Antioxidant Enzymes. Sci. Hortic. 2018;229:207–214. doi: 10.1016/j.scienta.2017.10.044. [DOI] [Google Scholar]
- 134.Gohari G., Alavi Z., Esfandiari E., Panahirad S., Hajihoseinlou S., Fotopoulos V. Interaction between Hydrogen Peroxide and Sodium Nitroprusside Following Chemical Priming of Ocimum basilicum L. against Salt Stress. Physiol. Plant. 2020;168:361–373. doi: 10.1111/ppl.13020. [DOI] [PubMed] [Google Scholar]
- 135.Uchida A., Jagendorf A.T., Hibino T., Takabe T., Takabe T. Effects of Hydrogen Peroxide and Nitric Oxide on Both Salt and Heat Stress Tolerance in Rice. Plant Sci. 2002;163:515–523. doi: 10.1016/S0168-9452(02)00159-0. [DOI] [Google Scholar]
- 136.Farooq M., Nawaz A., Chaudhary M.A.M., Rehman A. Foliage-Applied Sodium Nitroprusside and Hydrogen Peroxide Improves Resistance against Terminal Drought in Bread Wheat. J. Agron. Crop. Sci. 2017;203:473–482. doi: 10.1111/jac.12215. [DOI] [Google Scholar]
- 137.Christou A., Manganaris G.A., Fotopoulos V. Systemic Mitigation of Salt Stress by Hydrogen Peroxide and Sodium Nitroprusside in Strawberry Plants via Transcriptional Regulation of Enzymatic and Non-Enzymatic Antioxidants. Environ. Exp. Bot. 2014;107:46–54. doi: 10.1016/j.envexpbot.2014.05.009. [DOI] [Google Scholar]
- 138.Wang Y., Li L., Cui W., Xu S., Shen W., Wang R. Hydrogen Sulfide Enhances Alfalfa (Medicago sativa) Tolerance against Salinity during Seed Germination by Nitric Oxide Pathway. Plant Soil. 2012;351:107–119. doi: 10.1007/s11104-011-0936-2. [DOI] [Google Scholar]
- 139.Ziogas V., Tanou G., Belghazi M., Filippou P., Fotopoulos V., Grigorios D., Molassiotis A. Roles of Sodium Hydrosulfide and Sodium Nitroprusside as Priming Molecules during Drought Acclimation in Citrus Plants. Plant Mol. Biol. 2015;89:433–450. doi: 10.1007/s11103-015-0379-x. [DOI] [PubMed] [Google Scholar]
- 140.Tanou G., Job C., Rajjou L., Arc E., Belghazi M., Diamantidis G., Molassiotis A., Job D. Proteomics Reveals the Overlapping Roles of Hydrogen Peroxide and Nitric Oxide in the Acclimation of Citrus Plants to Salinity. Plant J. 2009;60:795–804. doi: 10.1111/j.1365-313X.2009.04000.x. [DOI] [PubMed] [Google Scholar]
- 141.Foyer C.H., Ruban A.V., Noctor G. Viewing Oxidative Stress through the Lens of Oxidative Signalling Rather than Damage. Biochem. J. 2017;474:877–883. doi: 10.1042/BCJ20160814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., Wink D.A., Fukuto J.M., Jackson A.A., van Goor H., et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Freschi L. Nitric Oxide and Phytohormone Interactions: Current Status and Perspectives. Front. Plant Sci. 2013;4:398. doi: 10.3389/fpls.2013.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He H., Garcia-Mata C., He L.-F. Interaction between Hydrogen Sulfide and Hormones in Plant Physiological Responses. Plant Growth Regul. 2019;87:175–186. doi: 10.1007/s10725-018-0454-9. [DOI] [Google Scholar]
- 145.Antoniou C., Chatzimichail G., Kashfi K., Fotopoulos V. P77: Exploring the Potential of NOSH-Aspirin as a Plant Priming Agent against Abiotic Stress Factors. Nitric Oxide. 2014;39:S39. doi: 10.1016/j.niox.2014.03.127. [DOI] [Google Scholar]
- 146.Shah Z.H., Rehman H.M., Akhtar T., Alsamadany H., Hamooh B.T., Mujtaba T., Daur I., Al Zahrani Y., Alzahrani H.A.S., Ali S., et al. Humic Substances: Determining Potential Molecular Regulatory Processes in Plants. Front. Plant Sci. 2018;9:263. doi: 10.3389/fpls.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rai K.K., Pandey N., Rai S.P. Salicylic Acid and Nitric Oxide Signaling in Plant Heat Stress. Physiol. Plant. 2020;168:241–255. doi: 10.1111/ppl.12958. [DOI] [PubMed] [Google Scholar]
- 148.Tripathi D.K., Vishwakarma K., Singh V.P., Prakash V., Sharma S., Muneer S., Nikolic M., Deshmukh R., Vaculík M., Corpas F.J. Silicon Crosstalk with Reactive Oxygen Species, Phytohormones and Other Signaling Molecules. J. Hazard. Mater. 2020;408:124820. doi: 10.1016/j.jhazmat.2020.124820. [DOI] [PubMed] [Google Scholar]
- 149.González-Morales S., Solís-Gaona S., Valdés-Caballero M.V., Juárez-Maldonado A., Loredo-Treviño A., Benavides-Mendoza A. Transcriptomics of Biostimulation of Plants under Abiotic Stress. Front. Genet. 2021;12:583888. doi: 10.3389/fgene.2021.583888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shan C., Dai H., Sun Y. Hydrogen Sulfide Protects Wheat Seedlings against Copper Stress by Regulating the Ascorbate and Glutathione Metabolism in Leaves. Aust. J. Crop. Sci. 2012;6:248–254. doi: 10.3316/informit.054397885307696. [DOI] [Google Scholar]
- 151.Zhang H., Hu L.-Y., Hu K.-D., He Y.-D., Wang S.-H., Luo J.-P. Hydrogen Sulfide Promotes Wheat Seed Germination and Alleviates Oxidative Damage against Copper Stress. J. Integr. Plant Biol. 2008;50:1518–1529. doi: 10.1111/j.1744-7909.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 152.Khan M.N., Siddiqui Z.H., Naeem M., Abbas Z.K., Ansari M.W. Chapter 8—Nitric Oxide and Hydrogen Sulfide Interactions in Plants under Adverse Environmental Conditions. In: Aftab T., Naeem M., editors. Emerging Plant Growth Regulators in Agriculture. Academic Press; Cambridge, MA, USA: 2022. pp. 215–244. [Google Scholar]
- 153.Pandey N., Verma L. Nitric Oxide Alleviates Iron Toxicity by Reducing Oxidative Damage and Growth Inhibition in Wheat (Triticum aestivum L.) Seedlings. Int. J. Plant Environ. 2019;5:16–22. doi: 10.18811/ijpen.v5i01.3. [DOI] [Google Scholar]
- 154.Habib N., Ali Q., Ali S., Javed M.T., Zulqurnain Haider M., Perveen R., Shahid M.R., Rizwan M., Abdel-Daim M.M., Elkelish A., et al. Use of Nitric Oxide and Hydrogen Peroxide for Better Yield of Wheat (Triticum aestivum L.) under Water Deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants. 2020;9:285. doi: 10.3390/plants9020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manai J., Kalai T., Gouia H., Corpas F.J. Exogenous Nitric Oxide (NO) Ameliorates Salinity-Induced Oxidative Stress in Tomato (Solanum Lycopersicum) Plants. J. Soil Sci. Plant Nutr. 2014;14:433–446. doi: 10.4067/S0718-95162014005000034. [DOI] [Google Scholar]
- 156.Wang H., Xiao W., Niu Y., Jin C., Chai R., Tang C., Zhang Y. Nitric Oxide Enhances Development of Lateral Roots in Tomato (Solanum lycopersicum L.) under Elevated Carbon Dioxide. Planta. 2013;237:137–144. doi: 10.1007/s00425-012-1763-2. [DOI] [PubMed] [Google Scholar]
- 157.Ghorbani A., Pishkar L., Roodbari N., Pehlivan N., Wu C. Nitric Oxide Could Allay Arsenic Phytotoxicity in Tomato (Solanum lycopersicum L.) by Modulating Photosynthetic Pigments, Phytochelatin Metabolism, Molecular Redox Status and Arsenic Sequestration. Plant Physiol. Biochem. 2021;167:337–348. doi: 10.1016/j.plaphy.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 158.Beligni M.V., Lamattina L. Nitric Oxide Protects against Cellular Damage Produced by Methylviologen Herbicides in Potato Plants. Nitric Oxide. 1999;3:199–208. doi: 10.1006/niox.1999.0222. [DOI] [PubMed] [Google Scholar]
- 159.Noritake T., Kawakita K., Doke N. Nitric Oxide Induces Phytoalexin Accumulation in Potato Tuber Tissues. Plant Cell Physiol. 1996;37:113–116. doi: 10.1093/oxfordjournals.pcp.a028908. [DOI] [Google Scholar]
- 160.París R., Lamattina L., Casalongué C.A. Nitric Oxide Promotes the Wound-Healing Response of Potato Leaflets. Plant Physiol. Biochem. 2007;45:80–86. doi: 10.1016/j.plaphy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 161.Szpunar-Krok E., Jańczak-Pieniążek M., Skrobacz K., Bobrecka-Jamro D., Balawejder M. Response of Potato (Solanum tuberosum L.) Plants to Spraying by Hydrogen Peroxide. Sustainability. 2020;12:2469. doi: 10.3390/su12062469. [DOI] [Google Scholar]
- 162.Fierascu R.C., Temocico G., Fierascu I., Ortan A., Babeanu N.E. Fragaria Genus: Chemical Composition and Biological Activities. Molecules. 2020;25:498. doi: 10.3390/molecules25030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eum H.-L., Lee S.-K. The Responses of Yukbo Strawberry (Fragaria Ananassa Duch.) Fruit to Nitric Oxide. Food Sci. Biotechnol. 2007;16:123–126. [Google Scholar]
- 164.Wills R.B.H., Ku V.V.V., Leshem Y.Y. Fumigation with Nitric Oxide to Extend the Postharvest Life of Strawberries. Postharvest Biol. Technol. 2000;18:75–79. doi: 10.1016/S0925-5214(99)00061-7. [DOI] [Google Scholar]
- 165.Kaya C., Akram N.A., Ashraf M. Influence of Exogenously Applied Nitric Oxide on Strawberry (Fragaria × Ananassa) Plants Grown under Iron Deficiency and/or Saline Stress. Physiol. Plant. 2019;165:247–263. doi: 10.1111/ppl.12818. [DOI] [PubMed] [Google Scholar]
- 166.Kaya C., Ashraf M. The Mechanism of Hydrogen Sulfide Mitigation of Iron Deficiency-Induced Chlorosis in Strawberry (Fragaria × Ananassa) Plants. Protoplasma. 2019;256:371–382. doi: 10.1007/s00709-018-1298-x. [DOI] [PubMed] [Google Scholar]
- 167.Manafi H., Baninasab B., Gholami M., Talebi M. Nitric Oxide Induced Thermotolerance in Strawberry Plants by Activation of Antioxidant Systems and Transcriptional Regulation of Heat Shock Proteins. J. Hortic. Sci. Biotechnol. 2021;96:783–796. doi: 10.1080/14620316.2021.1927206. [DOI] [Google Scholar]
- 168.Liu S., Yang R., Pan Y., Ren B., Chen Q., Li X., Xiong X., Tao J., Cheng Q., Ma M. Beneficial Behavior of Nitric Oxide in Copper-Treated Medicinal Plants. J. Hazard. Mater. 2016;314:140–154. doi: 10.1016/j.jhazmat.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 169.Alam M.M., Naeem M., Khan M.M.M.A., Uddin M. Vincristine and Vinblastine Anticancer Catharanthus Alkaloids: Pharmacological Applications and Strategies for Yield Improvement. In: Naeem M., Aftab T., Khan M.M.A., editors. Catharanthus roseus: Current Research and Future Prospects. Springer International Publishing; Cham, Switzerland: 2017. pp. 277–307. [Google Scholar]
- 170.Gupta M.M., Singh D.V., Tripathi A.K., Pandey R., Verma R.K., Singh S., Shasany A.K., Khanuja S.P.S. Simultaneous Determination of Vincristine, Vinblastine, Catharanthine, and Vindoline in Leaves of Catharanthus Roseus by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2005;43:450–453. doi: 10.1093/chromsci/43.9.450. [DOI] [PubMed] [Google Scholar]
- 171.Weathers P.J., Towler M., Hassanali A., Lutgen P., Engeu P.O. Dried-Leaf Artemisia Annua: A Practical Malaria Therapeutic for Developing Countries? World J. Pharm. 2014;3:39–55. doi: 10.5497/wjp.v3.i4.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nomani L., Zehra A., Choudhary S., Wani K.I., Naeem M., Siddiqui M.H., Khan M.M.A., Aftab T. Exogenous Hydrogen Sulphide Alleviates Copper Stress Impacts in Artemisia annua L.: Growth, Antioxidant Metabolism, Glandular Trichome Development and Artemisinin Biosynthesis. Plant Biol. 2022;24:642–651. doi: 10.1111/plb.13242. [DOI] [PubMed] [Google Scholar]
- 173.Kim W.-S., Choi W.J., Lee S., Kim W.J., Lee D.C., Sohn U.D., Shin H.-S., Kim W. Anti-Inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharm. 2015;19:21–27. doi: 10.4196/kjpp.2015.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Adamska I., Biernacka P. Bioactive Substances in Safflower Flowers and Their Applicability in Medicine and Health-Promoting Foods. Int. J. Food Sci. 2021;2021:e6657639. doi: 10.1155/2021/6657639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Amir S.B., Rasheed R., Ashraf M.A., Hussain I., Iqbal M. Hydrogen Sulfide Mediates Defense Response in Safflower by Regulating Secondary Metabolism, Oxidative Defense, and Elemental Uptake under Drought. Physiol. Plant. 2021;172:795–808. doi: 10.1111/ppl.13267. [DOI] [PubMed] [Google Scholar]
- 176.El-Beltagi H.S., Ahmed O.K., Hegazy A.E. Molecular Role of Nitric Oxide in Secondary Products Production in Ginkgo biloba Cell Suspension Culture. Not. Bot. Horti Agrobot. Cluj-Napoca. 2015;43:12–18. doi: 10.15835/nbha4319660. [DOI] [Google Scholar]
- 177.Hao G.-P., Du X.-H., Shi R.-J. Exogenous nitric oxide accelerates soluble sugar, proline and secondary metabolite synthesis in Ginkgo biloba under drought stress. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2007;33:499–506. [PubMed] [Google Scholar]
- 178.Post-White J., Ladas E.J., Kelly K.M. Advances in the Use of Milk Thistle (Silybum marianum) Integr. Cancer. 2007;6:104–109. doi: 10.1177/1534735407301632. [DOI] [PubMed] [Google Scholar]
- 179.Zangani E., Zehtab-Salmasi S., Andalibi B., Zamani A.-A. Protective Effects of Nitric Oxide on Photosynthetic Stability and Performance of Silybum Marianum under Water Deficit Conditions. Agron. J. 2018;110:555–564. doi: 10.2134/agronj2017.07.0396. [DOI] [Google Scholar]
- 180.Zangani E., Zehtab-Salmasi S., Andalibi B., Zamani A.A., Hashemi M. Exogenous Nitric Oxide Improved Production and Content of Flavonolignans in Milk Thistle Seeds under Water Deficit System. Acta Physiol. Plant. 2021;43:87. doi: 10.1007/s11738-021-03257-7. [DOI] [Google Scholar]
- 181.Saller R., Meier R., Brignoli R. The Use of Silymarin in the Treatment of Liver Diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 182.Sunil C., Xu B. An Insight into the Health-Promoting Effects of Taxifolin (Dihydroquercetin) Phytochemistry. 2019;166:112066. doi: 10.1016/j.phytochem.2019.112066. [DOI] [PubMed] [Google Scholar]
- 183.Bai L., Gao J., Wei F., Zhao J., Wang D., Wei J. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front. Pharmacol. 2018;9:423. doi: 10.3389/fphar.2018.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rahimi S., Kim Y.-J., Devi B.S.R., Oh J.Y., Kim S.-Y., Kwon W.-S., Yang D.-C. Sodium Nitroprusside Enhances the Elicitation Power of Methyl Jasmonate for Ginsenoside Production in Panax Ginseng Roots. Res. Chem. Intermed. 2016;42:2937–2951. doi: 10.1007/s11164-015-2188-x. [DOI] [Google Scholar]
- 185.Farouk S., Al-Huqail A.A. Sodium Nitroprusside Application Regulates Antioxidant Capacity, Improves Phytopharmaceutical Production and Essential Oil Yield of Marjoram Herb under Drought. Ind. Crops Prod. 2020;158:113034. doi: 10.1016/j.indcrop.2020.113034. [DOI] [Google Scholar]
- 186.Nasrin F., Fatemeh N., Ramezan R. Comparison the Effects of Nitric Oxide and Spermidin Pretreatment on Alleviation of Salt Stress in Chamomile Plant (Matricaria recutita L.) J. Stress Physiol. Biochem. 2012;8:214–223. [Google Scholar]
- 187.Murkowski A. Heat Stress and Spermidine: Effect on Chlorophyll Fluorescence in Tomato Plants. Biol. Plant. 2001;44:53–57. doi: 10.1023/A:1017966203859. [DOI] [Google Scholar]
- 188.Hassan W., Noreen H., Rehman S., Gul S., Amjad Kamal M., Paul Kamdem J., Zaman B., da Rocha J. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr. Top. Med. Chem. 2017;17:1336–1370. doi: 10.2174/1568026617666170102125648. [DOI] [PubMed] [Google Scholar]
- 189.Banerjee J., Das A., Sinha M., Saha S. Biological Efficacy of Medicinal Plant Extracts in Preventing Oxidative Damage. Oxidative Med. Cell. Longev. 2018;2018:e7904349. doi: 10.1155/2018/7904349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vranješ M., Štajner D., Vranješ D., Blagojevic B., Pavlović K., Milanov D., Popović B.M. Medicinal Plants Extracts Impact on Oxidative Stress in Mice Brain Under the Physiological Conditions: The Effects of Corn Silk, Parsley, and Bearberry. Acta Chim. Slov. 2021;68:896–903. doi: 10.17344/acsi.2021.6885. [DOI] [PubMed] [Google Scholar]
- 191.Gu R., Wang Y., Wu S., Wang Y., Li P., Xu L., Zhou Y., Chen Z., Kennelly E.J., Long C. Three New Compounds with Nitric Oxide Inhibitory Activity from Tirpitzia Sinensis, an Ethnomedicinal Plant from Southwest China. BMC Chem. 2019;13:47. doi: 10.1186/s13065-019-0568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Singh A.L., Singh S., Kurella A., Verma A., Mahatama M.K., Venkatesh I. Chapter 12—Plant Bio-Stimulants, Their Functions and Use in Enhancing Stress Tolerance in Oilseeds. In: Singh H.B., Vaishnav A., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; Amsterdam, The Netherlands: 2022. pp. 239–259. [Google Scholar]
- 193.keya Tudu C., Dey A., Pandey D.K., Panwar J.S., Nandy S. Chapter 8—Role of Plant Derived Extracts as Biostimulants in Sustainable Agriculture: A Detailed Study on Research Advances, Bottlenecks and Future Prospects. In: Singh H.B., Vaishnav A., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; Amsterdam, The Netherlands: 2022. pp. 159–179. [Google Scholar]
- 194.Karthiga D., Chozhavendhan S., Gandhiraj V., Aniskumar M. The Effects of Moringa Oleifera Leaf Extract as an Organic Bio-Stimulant for the Growth of Various Plants: Review. Biocatal. Agric. Biotechnol. 2022;43:102446. doi: 10.1016/j.bcab.2022.102446. [DOI] [Google Scholar]
- 195.Yuniati N., Kusumiyati K., Mubarok S., Nurhadi B. The Role of Moringa Leaf Extract as a Plant Biostimulant in Improving the Quality of Agricultural Products. Plants. 2022;11:2186. doi: 10.3390/plants11172186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ertani A., Schiavon M., Muscolo A., Nardi S. Alfalfa Plant-Derived Biostimulant Stimulate Short-Term Growth of Salt Stressed Zea mays L. Plants. Plant Soil. 2013;364:145–158. doi: 10.1007/s11104-012-1335-z. [DOI] [Google Scholar]
- 197.Godlewska K., Ronga D., Michalak I. Plant Extracts—Importance in Sustainable Agriculture. Ital. J. Agron. 2021;16 doi: 10.4081/ija.2021.1851. [DOI] [Google Scholar]
- 198.Oliveira H.C., Gomes B.C.R., Pelegrino M.T., Seabra A.B. Nitric Oxide-Releasing Chitosan Nanoparticles Alleviate the Effects of Salt Stress in Maize Plants. Nitric Oxide. 2016;61:10–19. doi: 10.1016/j.niox.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 199.Silveira N.M., Seabra A.B., Marcos F.C.C., Pelegrino M.T., Machado E.C., Ribeiro R.V. Encapsulation of S-Nitrosoglutathione into Chitosan Nanoparticles Improves Drought Tolerance of Sugarcane Plants. Nitric Oxide. 2019;84:38–44. doi: 10.1016/j.niox.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 200.Hasanuzzaman M., Bhuyan M.H.M.B., Mahmud J.A., Nahar K., Mohsin S.M., Parvin K., Fujita M. Interaction of Sulfur with Phytohormones and Signaling Molecules in Conferring Abiotic Stress Tolerance to Plants. Plant Signal. Behav. 2018;13:e1477905. doi: 10.1080/15592324.2018.1477905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mikkelsen R., Norton R. Soil and Fertilizer Sulfur. Better Crops Plant Food. 2013;97:7–9. [Google Scholar]
- 202.Jahan B., AlAjmi M.F., Rehman M.T., Khan N.A. Treatment of Nitric Oxide Supplemented with Nitrogen and Sulfur Regulates Photosynthetic Performance and Stomatal Behavior in Mustard under Salt Stress. Physiol. Plant. 2020;168:490–510. doi: 10.1111/ppl.13056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.