Abstract
Peptides are potential therapeutic alternatives against global diseases, such as antimicrobial-resistant infections and cancer. Venoms are a rich source of bioactive peptides that have evolved over time to act on specific targets of the prey. Peptides are one of the main components responsible for the biological activity and toxicity of venoms. South American organisms such as scorpions, snakes, and spiders are important producers of a myriad of peptides with different biological activities. In this review, we report the main venom-derived peptide families produced from South American organisms and their corresponding activities and biological targets.
Keywords: antimicrobial peptides, venom, cancer, South America, neurotoxins
1. Introduction—Drug Discovery in Venoms
Venoms are evolved complex mixtures of toxins that take part in the defensive and predation processes of the producer organism [1,2]. These toxins typically display toxicity against neuronal, red blood, and other mammalian cells, but also therapeutic biological activities such as antimicrobial, antitumoral, or analgesic properties [3]. Indeed, venoms can contain up to 500 biologically active compounds and are considered a rich source of potential drugs with novel molecular scaffolds [3,4,5]. Although it is estimated that up to 50 million different natural products exist in venoms, only 0.01% of such molecules have been identified and characterized [3]. The main reasons for this limitation are the challenging isolation and purification processes involved, and the low amount of these molecules produced by the venomous animals [5]. Recent advances in bioanalytical technologies, genomics, transcriptomics, and computational biology have enabled obtaining new insights into the structure and function of the venom content [5,6,7,8,9,10,11,12]. Such studies revealed, for example, that venoms are mainly composed of a heterogenous mixture of inorganic salts, low-molecular-weight organic molecules, peptides (2–10 KDa), and enzymes (>10 KDa), even though the pharmacology and complexity of their content differ substantially between venomous animals [2,4]. In recent years, there has been an increase in the number of reports describing therapeutic peptides isolated from venoms with potent activity in preclinical animal models [13]. Multiple venomous organisms of interest, such as insects, arthropods, and reptiles, can be found in different parts of South America. In this review, we highlight peptides with interesting biological features isolated from venoms of organisms originally from South America.
2. Venom-Derived Peptides
Available treatments for drug-resistant infections and cancer have side effects and have lost effectiveness due to the emergence of evolved resistant bacteria and tumors [14,15]. In addition, the drug development pipeline in these areas, particularly antimicrobial-resistant (AMR) infections, has not yielded truly novel structural candidates in decades [16]. AMR infections are responsible for 700,000 deaths annually [17], and cancer is the second leading cause of death in our society, reaching a ratio of 1 out of 6 deaths and with an estimated 27.5 million cases and 16.3 million deaths by 2040 [18]. Venom-derived peptides have been exploited as novel potential medicines for the treatment of AMR infections and cancer. This class of peptides comprises short biopolymers formed by amino acid residues that naturally evolved over time to reach specific targets in the prey, such as G protein-coupled receptors, ion channels, or enzymes, without suffering proteolytic cleavage [4,5]. Venom-derived peptides are also known for displaying therapeutic properties, including bacteria and cancer-targeting capabilities [19,20]. Their structural characteristics allow them to interact selectively with cancer and bacterial cell membranes through electrostatic interactions followed by hydrophobic interactions with the lipid bilayer [21]. The activity displayed by these compounds correlates with their physicochemical properties, including net charge, hydrophobicity, and solvent accessibility, which in turn dictate their mechanisms of action, selectivity, and specificity towards their targets [12]. Even though many venoms contain antimicrobial peptides (AMPs), other families of biologically active peptides can be also found in venoms [22], such as ICK peptides and α- and β-toxins.
2.1. Snake Venoms
Snake venoms are known for their toxicity, causing over 80,000 human deaths annually [23,24]. Components from these venoms have evolved to interact with specific mammalian proteins [3], and with the muscles, brain, nervous and cardiovascular systems of their prey, exhibiting high cyto-, neuro-, and hemotoxicity [25]. Snake bites often lead to punctured wounds that rarely get infected, despite the presence of microbes in the oral cavity of the snake [26,27]. This characteristic extended throughout different snake families (e.g., vip rippers and rattlesnakes), and has been at least partially explained by the presence of AMPs [26]. AMPs are commonly produced as part of host defense mechanisms, are also referred to as endogenous host defense peptides (HDPs), and can be found in the venom of multiple organisms [28]. These peptides are generally short cationic peptides containing fewer than 100 amino acid residues and hydrophobic and amphipathic properties [21].
Snake venom AMPs (SV-AMPs) mainly belong to the defensin-like and cathelicidin peptide families (Figure 1). The best example organism is the rattlesnake Crotalus durissus terrificus, a producer of both peptide families. Defensins are endogenous cationic peptides that contain from six to eight conserved cysteine residues that stabilize the β-sheet structure of the peptide by forming three disulfide bridges [25,29]. There are three types of defensins (α, β, and θ), which differ in the distance between cysteine residues within the sequence [29,30]. Specifically, β-defensins are interesting antimicrobial peptides composed of 18–45 amino acid residues [31]. These peptides take part in the defense barrier of vertebrates and display broad-spectrum activities against bacteria, fungi, and viruses [32]. Crotamine, from the rattlesnake Crotalus durissus terrificus, is a 42-amino acid residue β-defensin-like myoneurotoxin described as an antimicrobial and cell-penetrating peptide (CPP) (Figure 1 and Table 1). Even though this peptide presented cytotoxicity in vivo (2.5 mg Kg−1), leading to limb paralysis and necrosis, and in vitro toxicity against muscle cells (10 μg mL−1) [25,32], it also displayed significant antimicrobial and anticancer activities. Crotamine was one of the first AMPs that was also described as a CPP with selective antifungal activity [25,33]. The CPP properties of crotamine have been explored to selectively target cancer cells by interacting with the mitochondria and lysosomes without any observed side effect or alteration to normal cells [25,33,34].
Figure 1.
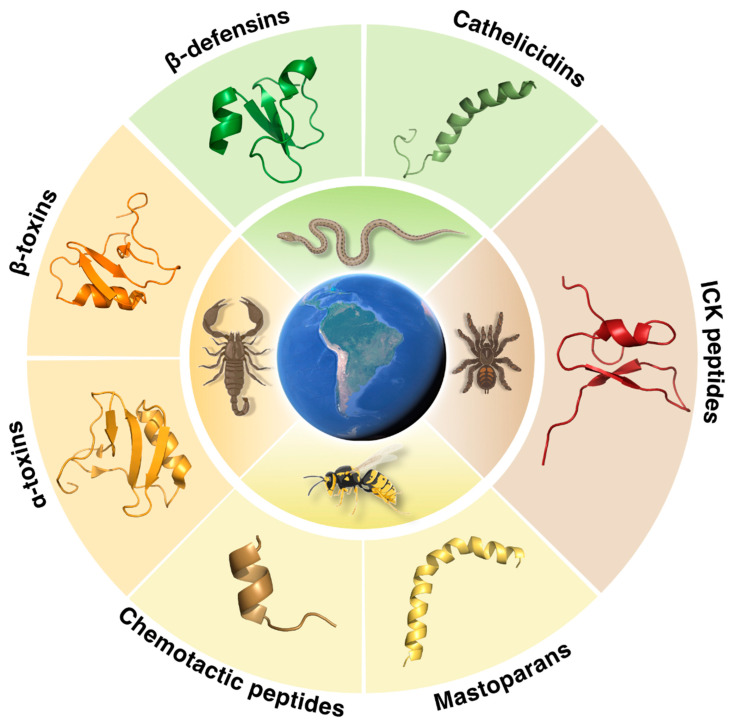
Families of venom-derived peptides from South American organisms. Schematic representation of the venom producers from South America and main peptide families found in their venoms, such as β-defensins (e.g., crotamine), cathelicidins (e.g., crotalicidine), ICK peptides (e.g., psalmotoxin 1), α-toxins (e.g., Ts1), β-toxins (e.g., Ts3), mastoparans, and chemotactic peptides (e.g., polybia-CP). This figure was created with BioRender.com.
Table 1.
Structural features and biological activities of venom-derived peptides from South American organisms. Extended overview of the number of amino acids (referred to as AA), sequence, peptide family, structure, biological activity, and producer organism of the venom-derived peptides. n.e. = not elucidated.
| Peptide | AA | Sequence | Peptide Family | Structure | Activity | Organism Producer | Reference |
|---|---|---|---|---|---|---|---|
| Snake Venom-Derived Peptides | |||||||
| Crotamine | 42 | YKQCHKKGGHCFPKEKICLPPSSDFGKMDCRWRWKCCKKGSG | β-defensin-like | β1αβ2β3 N-terminal 𝛼-helix, two stranded antiparallel β-sheets, and two β-turns. |
Antifungal (MIC = 12.5–50.0 μg mL−1) Anticancer activity (5 μg mL−1) |
Crotalus durissus terrificus | [25,34] |
| Crotalicidine | 34 | KRFKKFFKKVKKSVKKRLKKIFKKPMVIGVTIPF | Cathelicidin | α-helix at the N-terminal and random coil conformation at the C-terminal of the peptide. | Antibacterial (MIC < 10 μmol L−1), Anticancer (IC50 < 5 μmol L−1), Immunomodulatory activities |
Crotalus durissus terrificus | [27,35] |
| Spider Venom-Derived Peptides | |||||||
| PnTx1 | 78 | AELTSCFPVGHECDGDASNCNCCGDDVYCGCGWGRWNCKCKVADQSYSYGICKDKVNCPNRHLWPAKVCKKCRRNCGG | ICK peptide | Disulfide bridge pattern | LD50 = 5.5 pmol g−1 (mice) Target: Nav channels, antagonist |
Phoneutria nigriventer | [36,37] |
| PnTx2-1 | 53 | ATCAGQDKPCKETCDCCGERGECVCALSYEGKYRCICRQGNFLIAWHKLASCK | ICK peptide | n.e. | Lethal in mice model at 0.02 pmol g−1 |
Phoneutria nigriventer | [36,37] |
| PnTx2-5 | 48 | ATCAGQDQTCKVTCDCCGERGECVCGGPCICRQGNFLIAWYKLASCKK | ICK peptide | n.e. | Lethal in mice model at 2.4 pmol g−1 |
Phoneutria nigriventer | [36,37] |
| PnTx2-6 | 48 | ATCAGQDQPCKETCDCCGERGECVCGGPCICRQGYFWIAWYKLANCKK | ICK peptide | n.e. | Lethal in mice model at 7.5 pmol g−1 |
Phoneutria nigriventer | [36,37] |
| PnTx2-9 | 32 | SFCIPFKPCKSDENCCKKFKCKTTGIVKLCRW | ICK peptide | n.e. | - | Phoneutria nigriventer | [36,37] |
| PnTx3-1 | 41 | AECAAVYERCGKGYKRCCEERPCKCNIVMDNCTCKKFISEL | ICK peptide | n.e. | Paralysis in mice at 0.07 pmol g−1 Target: agonist of K+ channels |
Phoneutria nigriventer | [36,37] |
| PnTx3-2 | 46 | ACAGLYKKCGKGASPCCEDRPCKCDLAMGNCICKKKFIEFFGGGK | ICK peptide | n.e. | Antagonist of L-type CaV channels. Paralysis in mice 0.08 pmol g−1 | Phoneutria nigriventer | [36,37] |
| PnTx3-3 | 34 | GCANAYKSCNGPHTCCWGYNGYLLACICSGXNWK | ICK peptide | n.e. | Lethal to mice at 0.07 pmol g−1 Antagonist of L-, P/Q- and R-type Cav channels |
Phoneutria nigriventer | [36,37] |
| PnTx3-4 | 77 | SCINVGDFCDGKKDDCQCCRDNAFCSCSVIFGYKTNCRCEVGTTATSYGICMAKHKCGRQTTCTKPCLSKRCKKNHG | ICK peptide | n.e. | Lethal to mice at 5 μg/mouse Target: antagonist of N-, P/Q- and R-type Cav channels |
Phoneutria nigriventer | [36,37] |
| PnTx3-5 | 45 | GCIGRNESCKFDRHGCCWPWSCSCWNKEGQPESDVWCECSLKIGK | ICK peptide | n.e. | Paralysis in mice 0.07 pmol g−1 Target: L-type Cav channels |
Phoneutria nigriventer | [36,37] |
| PnTx3-6 | 54 | ACIPRGEICTDDCECCGCDNQCYCPPGSSLGIFKCSCAHANKYFCNRKKEKCKK | ICK peptide | n.e. | Paralysis in mice 0.05 pmol g−1 Target: N-, P/Q- and L-type Cav channels |
Phoneutria nigriventer | [36,37] |
| PnTx4-3 | 48 | CGDINAACKEDCDCCGYTTACDCYWSSSCKCREAAIVIYTAPKKKLTC | ICK peptide | n.e. | Non-toxic to mice (288.5 pmol g−1) LD50 = 192.3 pmol g−1 (house fly) |
Phoneutria nigriventer | [36,37] |
| PnTx4 (5-5) | 47 | CADINGACKSDCDCCGDSVTCDCYWSDSCKCRESNFKIGMAIRKKF-C | ICK peptide | n.e. | Non-toxic to mice (290 pmol g−1) Target: NMDAR (antagonist), NaV channels (agonist) |
Phoneutria nigriventer | [36,37] |
| PnTx4 (6-1) | 48 | CGDINAACKEDCDCCGYTTACDCYWSKSCKCREAAIVIYTAPKKKLTC | ICK peptide | n.e. | LD50=9.3 ng/house fly Non-toxic to mice (286.2 pmol g−1) ED50= 36.3 pmol g−1 (house fly) Target: agonist NaV channels |
Phoneutria nigriventer | [36,37] |
| Psalmotoxin 1 (PcTX1) | 40 | EDCIPKWKGCVNRHGDCCEGLECWKRRRSFEVCVPKTPKT | ICK peptide | Three antiparallel ®-sheet structure with three disulfide bridges tightly folded into the “knottin” fold pattern | Antinociceptive effects IC50 = 36 pmol L−1 in glioma cells. |
Psalmopoeus cambridgei | [38,39] |
| U1-SCRTX-Lg1a | 16 | VGTDFSGNDDISDVQK | Anionic antimicrobial peptide (AAMP) | Random coil conformation with a <-helix structure between the ISDV residues | Active against Gram-negative bacteria (MIC 1.5–4.6 μmol L−1) | Loxosceles gaucho | [40] |
| Scorpion Venom-Derived Peptides | |||||||
| Ts1 | 61 | KEGYLMDHEGCKLSCFIRPSGYCGRECGIKKGSSGYCAWPACYCYGLPNWVKVWDRATNKC | ®-like neurotoxin | Three antiparallel β-strands and a α-helix bonded by disulfide bridges | Toxic against mammals and insects Intravenous LD50 = 76 ± 9 μg kg−1 Target: Na+ channels |
Tityus serrulatus | [41] |
| Ts2 | 62 | KEGYAMDHEGCKFSCFIRPAGFCDGYCKTHLKASSGYCAWPACYCYGVPDHIKVWDYATNKC | ®-like neurotoxin | three β-strands and one α-helix, and is arranged in a triangular shape forming a cysteine-stabilized α-helix/ β-sheet (CSab) motif. three β-strands and one α-helix, and is arranged in a triangular shape forming a cysteine-stabilized α-helix/ β-sheet (CSab) motif. three β-strands and one α-helix, and is arranged in a triangular shape forming a cysteine-stabilized α-helix/ β-sheet (CSab) motif three β-strands and one α-helix, and is arranged in a triangular shape forming a cysteine-stabilized α-helix/β-sheet (CSab) motif Cysteine-stabilized α-helix/β-sheet (CSαβ) motif composed of three β-strands and one α-helix arranged in a triangular shape |
Induction of inflammation and production of cytokines Inhibition of the rapid inactivation of some NaV channels |
Tityus serrulatus | [41,42,43] |
| Ts3 | 62 | KKDGYPVEYDNCAYICWNYDNAYCDKLCKDKKADSGYCYWVHILCYCYGLPDSEPTKTNGKC | α-neurotoxin | α-helix and three-stranded antiparallel β-sheet | Inhibition of the inactivation of NaV channels Muscle relaxation |
Tityus serrulatus | [41,44] |
| Ts5 | 64 | KKDGYPVEGDNCAFACFGYDNAYCDKLCKDKKADDGYCVWSPDCYCYGLPEHILKEPTKTSGRC | α-neurotoxin | Core composed of three β-strands and one α-helix | LD50 = 94 ± 7 μg kg−1 in mice Causes hypertension Target: Na+ channels |
Tityus serrulatus | [41,45] |
| Ts6 | 40 | WCSTCLDLACGASRECYDPCFKAFGRAHGKCMNNKCRCYT | α-KTx (Potassium channel toxin) | α-helix and triple-stranded β-sheet stabilized by four disulfide bridges | Induction of inflammation and production of cytokines Blockage of KV channels |
Tityus serrulatus | [41,43] |
| Ts7 | 37 | VFINAKCRGSPECLPKCKEAIGKAAGKCMNGKCKCYP | α-KTx (Potassium channel toxin) | n.e. | Blockage of K+ current Blockage of 86Rb efflux |
Tityus serrulatus | [41] |
| Ts8 | 60 | KLVALIPNDQLRSILKAVVHKVAKTQFGCPAYEGYCNDHCNDIERKDGECHGFKCKCAKD | ®-KTx (Potassium channel toxin) | n.e. | Blockage of voltage-gated non-inactivating K+ channels from rat brain synaptosomes at IC50: 30 nmol L−1 |
Tityus serrulatus | [41] |
| Ts9 | 35 | VVIGQRCYRSPDCYSACKKLVGKATGKCTNGRCDC | κ-KTx (Kappa potassium channel toxin) | Core composed of a short <-helix and a three-stranded antiparallel ®-sheet | Ligand for small-conductance apamin-sensitive calcium-activated potassium channel | Tityus serrulatus | [41] |
| Wasp Venom-Derived Peptides | |||||||
| Polybia-MPI | 14 | IDWKKLLDAAKQIL | Mastoparan | 71.43% α-helix | Antifungal activity (ED50 = 8–16 μmol L−1 Antimicrobial activity against Gram-positive and Gram-negative bacteria (MIC = 4–15 μg mL−1 |
Polybia paulista | [46,47] |
| Polybia-MPII | 14 | INWLKLGKMVIDAL | Mastoparan | α-helix | Antimicrobial activity against Gram-positive bacteria (MIC = 2–5 μmol L−1 Antifungal activity (ED50 = 111–12.9 μmol L−1 Hemolytic properties (ED50 of 5 × 10 -5 mol L−1 |
Pseudopolybia vespiceps Testacea, Polybia paulista | [46] |
| Polybia-CP | 12 | ILGTILGLLKSL | Chemotactic peptide | 50% random coil 50% ambiguous conformations |
Antimicrobial activity against Gram-positive bacteria (MIC = 15 μg mL−1 Mast cell degranulation (10−5 mol L−1) Low hemolytic activity |
Polybia paulista | [47] |
Cathelicidins (CATHs) are likely the larger family of SV-AMPs and SV-CPPs peptides worldwide. Indeed, 25 SV-CATHs have been identified to date [27]. CATHs are released upon proteolytic cleavage of a precursor that encodes the bioactive peptide in a hypervariable C-domain, hence it is possible to find different types of peptides inside this family. Notwithstanding, the majority of CATHs are short and linear peptides of 25–35 amino acid residues with an amphipathic α-helical structure [27,48,49,50]. In addition to their known antimicrobial and anticancer activities, CATHs also present immunomodulatory properties by acting on biological processes such as wound healing, angiogenesis, or the inhibition of apoptosis, among others [48,51]. South American pit vipers produce cathelicidin-related antimicrobial peptides (CRAMPs) named vipericidins. This family of peptides possesses an anionic moiety followed by a Lys-/Arg-rich and amphipathic domain [35]. Vipericidins are usually active at low micromolar concentrations (0.05–3.8 μmol L−1) against AMR infections caused by Gram-negative bacteria. In contrast, vipericidins are only active against Gram-positive bacteria at concentrations ranging from 16 to 128 μmol L−1. Toxicity studies showed that these peptides led to 50% hemolysis at millimolar concentrations, similar to the human defensin LL-37, allowing a selectivity window of 2-log for targeting bacteria versus eukaryotic cells [35,50]. Crotalus durissus terrificus venom also contains crotalicidin, a 34 amino acid residues vipericidin that, in hydrophobic environment, structures into an α-helix at the N-terminal while keeping random coil conformation at the C-terminal of the peptide (Figure 1, Table 1) [27]. Crotalicidin showed broad antibacterial activity against Gram-negative and Gram-positive bacteria (MIC < 10 μmol L−1), potent anticancer activity against leukemia cells (IC50 < 5 μmol L−1), antiparasitic activity and immunomodulatory activities by promoting autoinflammation with low toxicity levels (10% hemolysis at 25 μmol L−1) [27,35]. These activities are derived from the ability of this peptide to interact and disrupt the targeted bacterial membranes and to display membranolytic effects while interfering with intracellular pathways in cancer cells [27].
2.2. Spider Venoms
Spiders produce venoms, which are lethal complex cocktails of toxins (neurotoxins, enzymes, proteins, biologically active peptides, nucleotides, salts, amino acids, and neurotransmitters) that allow them to overcome preys that can be considerably larger [52]. Some of these venoms are so potent that even small amounts can be lethal to humans. Up to 1000 peptides (2–10 kDa) can be found in a crude venom sample, making them the main component of spider venoms [53]. It is estimated that there are approximately 12 million peptides from spider venoms, most of them still uncharacterized. As for snakes, peptides are typically produced to clean the biological conducts where the venom is transported from the gland to the tip of the spider fangs to block potential infections [54]. Peptides bearing an inhibitory cysteine knot (ICK) in their structure, known as ICK peptides or knottins, are the most abundant class of peptides in spider venoms and are the ones responsible for the neurotoxicity displayed by these venoms (Figure 1) [52,55]. These peptides are small (commonly from 3 to 10 KDa) and present from 6 to 14 cysteine residues that create a knot-like structure composed of antiparallel β-sheets with a ring disposition, made by two disulfide bonds, which are crossed by a third disulfide bond [52,55]. This motif contributes to the rigidity and stability of the peptides against thermal and enzymatic degradation and helps maintain their active conformation so they can interact with their target [52]. Ion channels (e.g., voltage-dependent sodium (Nav), potassium (Kv), and calcium (Cav) channels) and neuroreceptors are the main targets of ICK peptides, leading to not only antimicrobial activity but also antiarrhythmic, analgesic, antiparasitic, cytolytic, hemolytic, and enzyme inhibitory activities [56].
Phoneutria nigriventer is one of the most important sources of ICK peptides from South America [36]. This spider, also known as “armed” spider, is an extremely aggressive and dangerous spider that can be found throughout South America [57]. The bite from P. nigriventer causes severe and irradiating pain along with toxic symptoms such as convulsions, arrhythmias, spastic paralysis, or priapism [58]. These symptoms are generated by the production of neurotoxic ICK peptides that cross the blood–brain barrier and interact with ionic channels and different receptors from the nervous system [37,57,58]. The knottins display a wide range of biological activities caused by their different mechanisms of action, such as inactivation of Na+ channels or blockage of voltage-gated calcium channels (VGCCs) [58]. To date, 41 neurotoxins from P. nigriventer, divided into 5 fractions (PhTx-1 to 5), have been identified (Table 1) [37]. Intracerebral administration of the different fractions (PhTx-1 to 5) in vivo showed toxic symptomatology in all cases, including excitation, spastic paralysis, progressive flaccid paralysis, hyperactivity, and smoothing of the muscles [37]. All the bioactive peptides found in these fractions are cysteine-rich peptides with lengths that vary from 34 to 78 amino acid residues [37]. Further biological studies on each of the peptides found in the venom of P. nigriventer showed their therapeutic potential primarily due to their ability to interact with ion channels and opioid receptors [37]. Most of these peptides show promising antinociceptive effects in different pain models (e.g., chronic pain, cancer-related pain, postoperative pain syndrome, or treatment-related pain) [37]. Indeed, they exhibited similar or more favorable antinociceptive effects than opioids, specifically morphine, and the well-known toxin ω-conotoxin MVIIA. These peptides generated no side effects and were able to revert tolerance to opioids in mice [37]. However, in addition to their antinociceptive properties, these peptides showed neuroprotective properties, insecticidal activity, and potential in the treatment of erectile dysfunction treatment, among others [37].
Another important ICK venom-derived peptide to mention is Psalmotoxin 1, also known as PcTx1, isolated from Psalmopoeus cambridgei, a tarantula originally from the West Indies (Figure 1, Table 1). PcTx1 is composed of 40 amino acid residues and was the first compound to be described as an inhibitor of the acid-sensing ion channels (AISCs) that are extensively expressed throughout the nervous system [38,39]. Study of the therapeutic role of PcTx1 in different in vivo pain models, such as acute and neuropathic pain, showed similar or even better antinociceptive effects than morphine and reverted thermal hyperalgesia and tactile allodynia without side effects. This peptide also showed inhibition of the AISCs expressed in glioblastoma multiforme (GBM) (IC50 of 36 pmol L−1), while not affecting normal cells, thus constituting a promising candidate for the treatment of cancer [39].
Spiders from the genus Loxosceles cause the necrotic and systemic disease known as loxoscelism. Although some of the Loxosceles genus members, such as Loxosceles intermedia, produce ICK peptides, others such as Loxosceles gaucho present biologically active peptides that do not belong to the family of ICK peptides. The study of the composition of L. gaucho venom showed the presence of U1-SCRTX-Lg1a, a hydrophobic anionic antimicrobial peptide (AAMP) composed of 16 amino acid residues (Table 1). Contrary to broad-spectrum AMPs, this peptide was selective against Gram-negative bacteria (MIC 1.5–4.6 μmol L−1), displaying even increased activity compared to the potent peptide gomesin isolated from the spider Acanthoscurria gomesiana (MIC < 6.5 μmol L−1). U1-SCRTX-Lg1a was not hemolytic (approximately 0.15% hemolysis at 137 μM) or cytotoxic against HeLa cells at any of the concentrations tested (i.e., 0.88–112.74 μmol L−1) [40]. These data indicate that the AMP U1-SCRTX-Lg1a, considered an important component of the innate immunity of the spider L. gaucho, interacts with an intramolecular target after translocating into the bacterial cell. The peptide acts through non-membranolytic mechanisms by creating cationic salt bridges between the negatively charged components of the bacterial cell membrane and metallic chelates in its aspartic and glutamic acids [40].
2.3. Scorpion Venoms
Scorpion venoms are rich arsenals of biological compounds that comprise mainly small neurotoxic peptides along with minor components such as sugars, salts, the neurotransmitter serotonin, and protease inhibitors [41,59,60]. Scorpion venoms display toxicity against both vertebrate and invertebrate organisms [59]. The neurotoxicity displayed is related to the peptide content present in their venom and, more specifically, due to the production of α- and β-toxins (Figure 1). Both families of peptides are composed of 58–76 amino acid residues, and their structure is stabilized by disulfide bridges [59]. α- and β-toxins target and interact with the NaV channels present in the membrane of excitable and non-excitable cells, affecting their ion flux [41,59,60]. Specifically, α-toxins extend the depolarization time of NaV channels leading to extended potential action in nerves and muscles [59,60] whereas β-toxins change the NaV channel activation threshold leading to hyperpolarized membrane voltages [41]. Scorpions from the genus Tityus are important producers of these toxins and, in Brazil alone, 22 different scorpions from this genus have been identified [59].
Tityus serrulatus has been rated as the most dangerous scorpion in Brazil [41]. Its envenomation can induce hyperglycemia, glycogenolysis, leukocytes, or even pulmonary edema, which can lead to death [41]. Tityus serrulatus venom (Tsv) has been extensively studied over the years due to its rich peptide content, namely small neurotoxins bearing disulfide bonds able to interact with ionic channels, and linear peptides with specific activity on receptors. Insights into its peptide content led to the characterization of 14 peptides belonging to different families such as α- and β-toxins or K+ channel neurotoxins (Table 1) [41]. One of the most important toxins found in this venom, Ts1, is a β-like neurotoxin with high affinity for mammalian NaV channels, which showed competition with AahIT, the most potent excitatory insect-selective toxin (Figure 1, Table 1) [60]. Structurally, Ts1 is composed of three antiparallel β-strands and an α-helix bonded by disulfide bridges with a significantly different orientation compared to that of α-toxins [41]. On the other hand, Ts3 is the only α-neurotoxin found in this venom (Figure 1, Table 1). This peptide, composed of 62 amino acid residues, inhibits the inactivation of NaV channels, favors the release of neurotransmitters, and depolarizes nitrergic fibers to induce smooth muscle relaxation [41].
2.4. Wasp Venoms
Peptides comprise up to 70% of dried wasp venoms, while the rest of the content contains other biologically active compounds such as biogenic amines, polyamine toxins, enzymes, and allergens [46,61]. Mastoparans and chemotactic peptides are the main peptides produced in wasp venoms, although it is also possible to find neurotoxic peptides and kinins (Figure 1) [61]. Mastoparans are short cationic peptides with an amidated C-terminal leucyl residue, two to four lysine residues, and no cysteine residues. This family of peptides is involved in the inflammation, hemolysis, and cell membrane lysis that occur upon envenomation. In water, mastoparans present a random coiled structure, and upon contact with the target membrane, their structure adopts an amphiphilic α-helix conformation in which the hydrophobic and hydrophilic residues are distributed in opposite faces of the helix (Figure 1) [46,61,62]. This structural transition leads to the creation of pores and destabilization of bacterial membranes through different membrane-related mechanisms of action, such as the carpet model and the toroidal pore formation [47,61]. Their ability to translocate or permeabilize membranes, also including that of the mitochondria, make these peptides versatile therapeutic alternatives for the treatment of bacterial, fungal, viral infections, and even cancer [46]. However, mastoparans also target erythrocytes and mast cells, showing unwanted hemolytic and mast cell degranulation activities [61]. The latter activity can be partially explained by the ability of mastoparans to mimic G protein-coupled receptors (GPCRs) allowing the activation of these proteins and leading to the production of pores in lipidic membranes [46,61,62]. For instance, Polybia-MPII, a mastoparan peptide isolated from Pseudopolybia vespiceps Testacea, which is an important source of biologically active peptides [46,63], presents potent antimicrobial activity against Gram-positive (MIC = 2-5 μmol L−1) and modest activity against Gram-negative (MIC = 5-38 μmol L−1) bacteria. However, this peptide is hemolytic at an ED50 of 5 × 10 −5 mol L−1 (Table 1) [47,64].
Chemotactic peptides are composed of 12 or 13 amino acid residues, are mainly hydrophobic, and possess a single basic residue. These peptides typically cause hemolysis and reduce mast cell degranulation [47]. Polybia-CP, for example, is a chemotactic peptide composed of 12 amino acid residues isolated from the venom of the social wasp Polybia paulista (Figure 1, Table 1). This peptide reduced mast cell degranulation (10−5 mol L−1) and exhibited low hemolytic activity [47] and antichagasic activity [63]. In a comprehensive study, the structure of polybia-CP was elucidated, and amino acid substitutions throughout the peptide using alanine residues revealed insights into the specific role of each of these residues on biological function (Figure 1) [65]. The hydrophobic face of the peptide’s helical structure was crucial for its activity, while substitutions made within its hydrophilic face did not affect the helicity nor the antimicrobial activity of the peptide. Further studies with the same peptide highlighted the importance of the position of the positively charged residues within the hydrophilic face of the peptide, which led to increased helical content and antimicrobial activity. This structure-function-guided design approach led to an optimized hit, [Lys]7-Polybia-CP, with improved structural features, such as net positive charge and helicity, in addition to lower toxicity and increased antimicrobial in vivo activity at concentrations as low as 4 μmol L−1 [65]. The synthetic peptide [Lys]7-Polybia-CP was also used in conjunction with micro- and nanomotors systems for the autonomous treatment of infections in an animal model [66]. These studies underscore the role of venom-derived peptides as scaffolds for novel antimicrobials.
2.5. Venom-Derived Peptides in the Clinic
From 2015 to 2020 the U.S. Food Drug Administration (FDA) approved 273 new drugs. Out of those, 21 were peptides or peptide-derived drugs described as medicines or drug delivery systems [67,68,69,70]. The sales of peptide drugs exceeded US$ 70 billion in 2019 with 10 non-insulin peptide drugs in the top 200 drug sales, representing a substantial part of the pharmaceutical market [71].
As noted above, biologically active peptides from venomous animals from South America display relevant and diverse activities and constitute promising clinical candidates. To date, some peptides from the cornucopia of venoms have been studied extensively based on their structure, activities, and toxicity. Captopril is a pioneering and successful example of the transition from venom to drug. This blockbuster angiotensin-converting enzyme (ACE) inhibitor was approved in 1981, under the name Capoten® (Bristol-Myers Squibb, BMS, New York, NY, USA), for the treatment of cardiovascular diseases [72,73]. Captopril was designed based on bradykinin-potentiating peptides (BPP). Pro-rich peptides were found in the venom of the Brazilian pit viper Bothrops jararaca [2,72,73,74]. Just four years after its approval, Enalapril, another peptide produced by the same snake, was also approved under the name Vasotec® (Merck, Darmstadt, Germany) for the treatment of hypertension and cardiac failure with higher therapeutic potency than Capoten® but limited oral availability (Table 2) [72,75,76]. Batroxobin, found in the Brazilian lancehead snake Bothrops moojeni venom cocktail [72], is a thrombin-like serine protease that displays a high defibrinogenating effect with anti-inflammatory effects [72,77,78]. This activity allows the use of this peptide as a drug against thrombotic diseases (such as deep vein thrombosis, myocardial infarction, pulmonary embolism, and acute ischemic stroke), and as a diagnostic tool (Reptilase®) for the quantification of fibrinogen levels and blood coagulation capabilities (Table 2) [72,79]. This peptide has not yet been approved for clinical use in the USA by the FDA in the USA; however, it is commercialized in its original form under diverse brand names in other countries (Table 2), and it is currently in clinical phase IV for its use against cerebral venous sinus thrombosis [72]. The attractive activities displayed by venom-derived peptides enable their translation into preclinical development. Currently, ICK peptides from tarantulas are catching the attention of pharmaceutical companies for their ability to block NaV channels and treat pain. For instance, the peptide PcTx-1 mentioned above in the spider venom section is currently undergoing preclinical trials, not only to assess its activity but also for its valuable role in furthering our understanding of ASICS heteromeric channels (Table 2) [72,80,81]. Recently, the ICK peptide protocin-II (ProTX-II) from the Peruvian tarantula Thrixopelma pruriens was optimized by Janssen via directed evolution achieving the novel peptide JNJ63955918, which exhibited improved selectivity for NaV1.7 channels along with in vivo tolerability (Table 2) [2,81,82]. The company Amgen has also explored this family of peptides and described and characterized the peptide GpTx-1, widely produced among South American tarantulas such as Grammostila porter, Grammostila rosea, and Paraphysa scrofa (Table 2). GpTx-1 is a peptide with high selectivity for the NaV1.7 channel (IC50 = 10 nM) that demonstrated to be an excellent template for the engineering of new bioactive peptides, such as the analog [Ala5, Phe6, Leu26, Arg28]GpTx-1 that demonstrated exceptional selectivity towards NaV1.7 (IC50 = 1.6 nM) and >1000 fold selectivity for NaV1.4 and NaV1.5 (Table 2) [2,81,83,84,85]. Of note, peptides can be administered within a mixture of active compounds to enhance the activity of a lead compound. This is the case with AMPs and CPPs, which primarily target the cell membrane, forming pores through diverse mechanisms of action that lead to cellular apoptosis. This characteristic not only allows their use as a single treatment but also the exploration of synergistic activities with different approved drugs. These peptides can act as adjuvants for compounds that have their target within the intracellular environment, allowing them to reach their active site efficiently, and increasing the effectiveness of the treatment by attacking the infection or illness through different mechanisms of action [86,87]. HYL, an α-helical antimicrobial peptide secreted within the venom of the solitary bee Hylaeus signatus, is an example of this synergistic activity. This peptide of poor antimicrobial activity was optimized following a structure−activity study that led to the generation of 25 new peptides (from HYL −1 to HYL-25) with optimized antimicrobial activities [88]. Combination of HYL and most of these analogs with rifampicin resulted in synergistic interactions (FIC ≤ 0.5) against Pseudomona aeruginosa [88]. This synergy was also observed when HYL-1, HYL-18, and HYL-25 were combined with tetracycline against the same bacterium [88]. Synergistic studies were also carried out against the Gram-positive bacterium Staphylococcus aureus by combination of the different HYL analogs with amoxicillin. Ten different analogs demonstrated FIC ≤ 0.5 against this bacterium [88].
Table 2.
Clinical or preclinical status of venom-derived peptides from South America. Extended overview of the producer organism, description of the peptide, main biological target, target indication, company and brand, and clinical trial stage of the venom-derived peptides.
| Peptide | Producer Organism | Description | Target | Target Indication |
Company (Brand) |
Clinical Trial | Reference |
|---|---|---|---|---|---|---|---|
| Captopril | Bothrops jararaca | Synthetic peptide based on bradykinin-potentiating peptides (BPP) | Angiotensin-converting enzyme (ACE) | Hypertension, cardiac failure |
Bristol-Myers Squibb. (Capoten®) |
Completed | [2,72,73,74,89] |
| Enalapril | Bothrops jararaca | Synthetic peptide based on bradykinin-potentiating peptides (BPP) | Angiotensin-converting enzyme (ACE) | hypertension and cardiac failure | Merck (Vasotec®) |
Completed | [72,75,76,89] |
| Batroxobin | Brazilian lancehead snake (Bothrops moojeni) |
Peptide isolated from venom | Cleavage of the Aα chain of fibrinogen at the [Ala]16-[Gly]17 bond | Defibrinogenating effect Anticoagulation therapy (thrombotic diseases) Diagnosis of fibrinogen levels and blood coagulation capabilities |
Tobishi Pharmaceutical (Batroxobin, Reptilase, Defibrase) DSM Nutritional Products Ltd/Branch Pentapharm (Defibrase) Hanlim (Botropase) Juggat Pharma (Botropase, Botroclot) Drugs.com, (Botroclot) Plateltex S.R.O. (Plateltex-Act®) Vivostat A/S (Vivostat System). |
Phase IV (Combination with anticoagulation in cerebral venous sinus thrombosis, NCT04269954) Completed in different countries |
[72,77,78,79,90] |
| Psalmotoxin 1 (PcTx-1) | Psalmopoeus cambridgei | Peptide isolated from venom | Inhibitor of AISCs | Pain treatment | - | Preclinical | [72,80,81] |
| JNJ63955918 | Thrixopelma pruriens | Synthetic peptide based on the natural peptide ProTX-II | NaV1.7 channels | Pain treatment | Janssen | Preclinical | [2,81,82] |
| GpTx-1 | Grammostila porter, Grammostila rosea, and Paraphysa scrofa | Peptide isolated from the venom | NaV1.7 channels | Pain treatment | Amgen | Preclinical | [2,81,83,84,85] |
| [Ala5, Phe6, Leu26, Arg28]GpTx-1 | Grammostila porter, Grammostila rosea, and Paraphysa scrofa | Synthetic peptide based on the natural peptide GpTx-1 | NaV1.7, NaV1.5 and NaV1.4 channels | Pain treatment | Amgen | Preclinical | [2,81,83,84,85] |
3. Conclusions
Venom-derived peptides have demonstrated promising biological activities and potential as scaffolds for the generation of potent antimicrobials and anticancer agents through rational design. South American venomous organisms are important sources of biologically active peptides with antinociceptive, anticancer, and antibacterial properties. Recent advances in chemical and computational methods promise to accelerate the screening, testing, and lead identification of biologically active compounds.
Acknowledgments
Cesar de la Fuente-Nunez holds a Presidential Professorship at the University of Pennsylvania and acknowledges funding from the Procter & Gamble Company, United Therapeutics, a BBRF Young Investigator Grant, the Nemirovsky Prize, Penn Health-Tech Accelerator Award, and the Dean’s Innovation Fund from the Perelman School of Medicine at the University of Pennsylvania. Research reported in this publication was supported by the Langer Prize (AIChE Foundation), the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM138201 and the Defense Threat Reduction Agency (DTRA; HDTRA11810041 and HDTRA1-21-1-0014). The figure shown in the manuscript was prepared in BioRender.com. We thank Xunta de Galicia for a pre-doctoral fellowship 2019 co-funded with the social European funding (FSE) of the European Union (ED481A-2019/081).
Author Contributions
Writing—original draft preparation, L.A. and M.D.T.T.; writing—review and editing, C.d.l.F.-N.; supervision, C.d.l.F.-N.; funding acquisition, C.d.l.F.-N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
National Institute of General Medical Sciences of the National Institutes of Health (R35GM138201); Defense Threat Reduction Agency (DTRA; HDTRA11810041 and HDTRA1-21-1-0014).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lewis R.J., Garcia M.L. Therapeutic Potential of Venom Peptides. Nat. Rev. Drug Discov. 2003;2:790–802. doi: 10.1038/nrd1197. [DOI] [PubMed] [Google Scholar]
- 2.Pennington M.W., Czerwinski A., Norton R.S. Peptide Therapeutics from Venom: Current Status and Potential. Bioorg. Med. Chem. 2018;26:2738–2758. doi: 10.1016/j.bmc.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 3.El-Aziz T.M.A., Soares A.G., Stockand J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins. 2019;11:564. doi: 10.3390/toxins11100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King G.F. Venoms as a Platform for Human Drugs: Translating Toxins into Therapeutics. Expert Opin. Biol. Ther. 2011;11:1469–1484. doi: 10.1517/14712598.2011.621940. [DOI] [PubMed] [Google Scholar]
- 5.Prashanth J.R., Hasaballah N., Vetter I. Pharmacological Screening Technologies for Venom Peptide Discovery. Neuropharmacology. 2017;127:4–19. doi: 10.1016/j.neuropharm.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Torres M.D.T., de la Fuente-Nunez C. Toward Computer-Made Artificial Antibiotics. Curr. Opin. Microbiol. 2019;51:30–38. doi: 10.1016/j.mib.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Porto W.F., Irazazabal L., Alves E.S.F., Ribeiro S.M., Matos C.O., Pires Á.S., Fensterseifer I.C.M., Miranda V.J., Haney E.F., Humblot V., et al. In Silico Optimization of a Guava Antimicrobial Peptide Enables Combinatorial Exploration for Peptide Design. Nat. Commun. 2018;9:1490. doi: 10.1038/s41467-018-03746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pane K., Cafaro V., Avitabile A., Torres M.D.T., Vollaro A., De Gregorio E., Catania M.R., Di Maro A., Bosso A., Gallo G., et al. Identification of Novel Cryptic Multifunctional Antimicrobial Peptides from the Human Stomach Enabled by a Computational-Experimental Platform. ACS Synth. Biol. 2018;7:2105–2115. doi: 10.1021/acssynbio.8b00084. [DOI] [PubMed] [Google Scholar]
- 9.Torres M.D.T., Sothiselvam S., Lu T.K., de la Fuente-Nunez C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019;431:3547–3567. doi: 10.1016/j.jmb.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Torres M.D.T., Cao J., Franco O.L., Lu T.K., de la Fuente-Nunez C. Synthetic Biology and Computer-Based Frameworks for Antimicrobial Peptide Discovery. ACS Nano. 2021;15:2143–2164. doi: 10.1021/acsnano.0c09509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer N., Maasch J.R.M.A., Torres M.D.T., de la Fuente-Nunez C. Molecular Dynamics for Antimicrobial Peptide Discovery. Infect. Immun. 2021;89:e00703-20. doi: 10.1128/IAI.00703-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Der Torossian Torres M., De La Fuente-Nunez C. Reprogramming Biological Peptides to Combat Infectious Diseases. Chem. Commun. 2019;55:15020–15032. doi: 10.1039/C9CC07898C. [DOI] [PubMed] [Google Scholar]
- 13.Silveira G.G.O.S., Torres M.D.T., Ribeiro C.F.A., Meneguetti B.T., Carvalho C.M.E., de la Fuente-Nunez C., Franco O.L., Cardoso M.H. Antibiofilm Peptides: Relevant Preclinical Animal Infection Models and Translational Potential. ACS Pharmacol. Transl. Sci. 2021;4:55–73. doi: 10.1021/acsptsci.0c00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberio M., Joanitti G., Fontes W., Castro M.S. Anticancer Peptides and Proteins: A Panoramic View. Protein Pept. Lett. 2012;20:380–391. doi: 10.2174/092986613805290435. [DOI] [PubMed] [Google Scholar]
- 15.Pfalzgraff A., Brandenburg K., Weindl G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018;9:281. doi: 10.3389/fphar.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Fuente-Nunez C., Torres M.D., Mojica F.J., Lu T.K. Next-Generation Precision Antimicrobials: Towards Personalized Treatment of Infectious Diseases. Curr. Opin. Microbiol. 2017;37:95–102. doi: 10.1016/j.mib.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Commission . A European One Health Action Plan against Antimicrobial Resistance. European Commission; Brussels, Belgium: 2017. [Google Scholar]
- 18.World Health Organization . Global Cancer Facts & Figures. 4th ed. American Cancer Society; Atlanta, GA, USA: 2018. [Google Scholar]
- 19.Akef H.M. Anticancer and Antimicrobial Activities of Scorpion Venoms and Their Peptides. Toxin Rev. 2019;38:41–53. doi: 10.1080/15569543.2017.1414847. [DOI] [Google Scholar]
- 20.Torres M.D.T., Silva A.F., Andrade G.P., Pedron C.N., Cerchiaro G., Ribeiro A.O., Oliveira V.X., de la Fuente-Nunez C. The Wasp Venom Antimicrobial Peptide Polybia-CP and Its Synthetic Derivatives Display Antiplasmodial and Anticancer Properties. Bioeng. Transl. Med. 2020;5:e10167. doi: 10.1002/btm2.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koehbach J., Craik D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019;40:517–528. doi: 10.1016/j.tips.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Perumal Samy R., Stiles B.G., Franco O.L., Sethi G., Lim L.H.K. Animal Venoms as Antimicrobial Agents. Biochem. Pharmacol. 2017;134:127–138. doi: 10.1016/j.bcp.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Gutiérrez J.M., Calvete J.J., Habib A.G., Harrison R.A., Williams D.J., Warrell D.A. Snakebite Envenoming. Nat. Rev. Dis. Prim. 2017;3:17063. doi: 10.1038/nrdp.2017.63. [DOI] [PubMed] [Google Scholar]
- 24.Ferraz C.R., Arrahman A., Xie C., Casewell N.R., Lewis R.J., Kool J., Cardoso F.C. Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 2019;7:218. doi: 10.3389/fevo.2019.00218. [DOI] [Google Scholar]
- 25.Kerkis I., Hayashi M.A.F., Prieto Da Silva A.R.B., Pereira A., De Sá Júnior P.L., Zaharenko A.J., Rádis-Baptista G., Kerkis A., Yamane T. State of the Art in the Studies on Crotamine, a Cell Penetrating Peptide from South American Rattlesnake. Biomed. Res. Int. 2014;2014:675985. doi: 10.1155/2014/675985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lima D.C., Alvarez Abreu P., de Freitas C.C., Santos D.O., Borges R.O., Dos Santos T.C., Mendes Cabral L., Rodrigues C.R., Castro H.C. Snake Venom: Any Clue for Antibiotics and CAM? Evid. Based Complement. Alternat. Med. 2005;2:39–47. doi: 10.1093/ecam/neh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Peinado C., Defaus S., Andreu D. Hitchhiking with Nature: Snake Venom Peptides to Fight Cancer and Superbugs. Toxins. 2020;12:255. doi: 10.3390/toxins12040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercer D.K., Torres M.D.T., Duay S.S., Lovie E., Simpson L., von Köckritz-Blickwede M., de la Fuente-Nunez C., O’Neil D.A., Angeles-Boza A.M. Antimicrobial Susceptibility Testing of Antimicrobial Peptides to Better Predict Efficacy. Front. Cell. Infect. Microbiol. 2020;10:326. doi: 10.3389/fcimb.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehrer R.I., Bevins C.L., Ganz T. Defensins and Other Antimicrobial Peptides and Proteins. Mucosal Immunol. 2005:95–110. doi: 10.1016/B978-012491543-5/50010-3. [DOI] [Google Scholar]
- 30.Ganz T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 31.Meade K.G., O’Farrelly C. β-Defensins: Farming the Microbiome for Homeostasis and Health. Front. Immunol. 2019;9:3072. doi: 10.3389/fimmu.2018.03072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerkis I., Silva F.D.S., Pereira A., Kerkis A., Rádis-Baptista G. Biological Versatility of Crotamine a Cationic Peptide from the Venom of a South American Rattlesnake. Expert Opin. Investig. Drugs. 2010;19:1515–1525. doi: 10.1517/13543784.2010.534457. [DOI] [PubMed] [Google Scholar]
- 33.Yamane E.S., Bizerra F.C., Oliveira E.B., Moreira J.T., Rajabi M., Nunes G.L.C., de Souza A.O., da Silva I.D.C.G., Yamane T., Karpel R.L., et al. Unraveling the Antifungal Activity of a South American Rattlesnake Toxin Crotamine. Biochimie. 2013;95:231–240. doi: 10.1016/j.biochi.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Pereira A., Kerkis A., Hayashi M.A.F., Pereira A.S.P., Silva F.S., Oliveira E.B., Prieto da Silva A.R.B., Yamane T., Rádis-Baptista G., Kerkis I. Crotamine Toxicity and Efficacy in Mouse Models of Melanoma. Expert Opin. Investig. Drugs. 2011;20:1189–1200. doi: 10.1517/13543784.2011.602064. [DOI] [PubMed] [Google Scholar]
- 35.Falcao C.B., De La Torre B.G., Pérez-Peinado C., Barron A.E., Andreu D., Rádis-Baptista G. Vipericidins: A Novel Family of Cathelicidin-Related Peptides from the Venom Gland of South American Pit Vipers. Amino Acids. 2014;46:2561–2571. doi: 10.1007/s00726-014-1801-4. [DOI] [PubMed] [Google Scholar]
- 36.Diniz M.R.V., Paiva A.L.B., Guerra-Duarte C., Nishiyama M.Y., Jr., Mudadu M.A., de Oliveira U., Borges M.H., Yates J.R., Junqueira-de-Azevedo I.d.L. An Overview of Phoneutria Nigriventer Spider Venom Using Combined Transcriptomic and Proteomic Approaches. PLoS ONE. 2018;13:e0200628. doi: 10.1371/journal.pone.0200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peigneur S., de Lima M.E., Tytgat J. Phoneutria Nigriventer Venom: A Pharmacological Treasure. Toxicon. 2018;151:96–110. doi: 10.1016/j.toxicon.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Escoubas P., De Weille J.R., Lecoq A., Diochot S., Waldmann R., Champigny G., Moinier D., Ménez A., Lazdunski M. Isolation of a Tarantula Toxin Specific for a Class of Proton-Gated Na+ Channels. J. Biol. Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- 39.Bubien J.K., Ji H.-L., Gillespie G.Y., Fuller C.M., Markert J.M., Mapstone T.B., Benos D.J. Cation Selectivity and Inhibition of Malignant Glioma Na+ Channels by Psalmotoxin 1. Am. J. Physiol. Cell Physiol. 2004;287:C1282–C1291. doi: 10.1152/ajpcell.00077.2004. [DOI] [PubMed] [Google Scholar]
- 40.Segura-Ramírez P.J., Silva Júnior P.I. Loxosceles Gaucho Spider Venom: An Untapped Source of Antimicrobial Agents. Toxins. 2018;10:522. doi: 10.3390/toxins10120522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cologna C., Marcussi S., Giglio J., Soares A., Arantes E. Tityus Serrulatus Scorpion Venom and Toxins: An Overview. Protein Pept. Lett. 2009;16:920–932. doi: 10.2174/092986609788923329. [DOI] [PubMed] [Google Scholar]
- 42.Cologna C.T., Peigneur S., Rustiguel J.K., Nonato M.C., Tytgat J., Arantes E.C. Investigation of the Relationship between the Structure and Function of Ts2, a Neurotoxin from Tityus Serrulatus Venom. FEBS J. 2012;279:1495–1504. doi: 10.1111/j.1742-4658.2012.08545.x. [DOI] [PubMed] [Google Scholar]
- 43.Zoccal K.F., da Silva Bitencourt C., Sorgi C.A., de Castro Figueiredo Bordon K., Sampaio S.V., Arantes E.C., Faccioli L.H. Ts6 and Ts2 from Tityus Serrulatus Venom Induce Inflammation by Mechanisms Dependent on Lipid Mediators and Cytokine Production. Toxicon. 2013;61:1–10. doi: 10.1016/j.toxicon.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Dang B., Kubota T., Mandal K., Correa A.M., Bezanilla F., Kent S.B.H. Elucidation of the Covalent and Tertiary Structures of Biologically Active Ts3 Toxin. Angew. Chem. Int. Ed. Engl. 2016;55:8639–8642. doi: 10.1002/anie.201603420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arantes E.C., Riccioppo Neto F., Sampaio S.V., Vieira C.A., Giglio J. Isolation and Characterization of TsTX-V, a New Neurotoxin from Tityus Serrulatus Scorpion Venom Which Delays the Inactivation of NA+ Channels. Biochim. Biophys. Acta (BBA) Gen. Subj. 1994;1199:69–75. doi: 10.1016/0304-4165(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 46.Silva J.C., Neto L.M., Neves R.C., Gonçalves J.C., Trentini M.M., Mucury-Filho R., Smidt K.S., Fensterseifer I.C., Silva O.N., Lima L.D., et al. Evaluation of the Antimicrobial Activity of the Mastoparan Polybia-MPII Isolated from Venom of the Social Wasp Pseudopolybia Vespiceps Testacea (Vespidae, Hymenoptera) Int. J. Antimicrob. Agents. 2017;49:167–175. doi: 10.1016/j.ijantimicag.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Souza B.M., Mendes M.A., Santos L.D., Marques M.R., César L.M.M., Almeida R.N.A., Pagnocca F.C., Konno K., Palma M.S. Structural and Functional Characterization of Two Novel Peptide Toxins Isolated from the Venom of the Social Wasp Polybia Paulista. Peptides. 2005;26:2157–2164. doi: 10.1016/j.peptides.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 48.Gao W., Xing L., Qu P., Tan T., Yang N., Li D., Chen H., Feng X. Identification of a Novel Cathelicidin Antimicrobial Peptide from Ducks and Determination of Its Functional Activity and Antibacterial Mechanism. Sci. Rep. 2015;5:17260. doi: 10.1038/srep17260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanetti M., Gennaro R., Romeo D. Cathelicidins: A Novel Protein Family with a Common Proregion and a Variable C-Terminal Antimicrobial Domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-O. [DOI] [PubMed] [Google Scholar]
- 50.De Barros E., Gonçalves R.M., Cardoso M.H., Santos N.C., Franco O.L., Cândido E.S. Snake Venom Cathelicidins as Natural Antimicrobial Peptides. Front. Pharmacol. 2019;10:1415. doi: 10.3389/fphar.2019.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanetti M. The Role of Cathelicidins in the Innate Host Defenses of Mammals. Curr. Issues Mol. Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- 52.Estrada-Gomez S., Cardoso F.C., Vargas-Muñoz L.J., Quintana-Castillo J.C., Arenas Gómez C.M., Pineda S.S., Saldarriaga-Cordoba M.M. Venomic, Transcriptomic, and Bioactivity Analyses of Pamphobeteus Verdolaga Venom Reveal Complex Disulfide-Rich Peptides That Modulate Calcium Channels. Toxins. 2019;11:496. doi: 10.3390/toxins11090496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Escoubas P., Sollod B., King G.F. Venom Landscapes: Mining the Complexity of Spider Venoms via a Combined CDNA and Mass Spectrometric Approach. Toxicon. 2006;47:650–663. doi: 10.1016/j.toxicon.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Garcia F., Villegas E., Espino-Solis G.P., Rodriguez A., Paniagua-Solis J.F., Sandoval-Lopez G., Possani L.D., Corzo G. Antimicrobial Peptides from Arachnid Venoms and Their Microbicidal Activity in the Presence of Commercial Antibiotics. J. Antibiot. 2013;66:3–10. doi: 10.1038/ja.2012.87. [DOI] [PubMed] [Google Scholar]
- 55.Langenegger N., Nentwig W., Kuhn-Nentwig L. Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses. Toxins. 2019;11:611. doi: 10.3390/toxins11100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saez N.J., Senff S., Jensen J.E., Er S.Y., Herzig V., Rash L.D., King G.F. Spider-Venom Peptides as Therapeutics. Toxins. 2010;2:2851–2871. doi: 10.3390/toxins2122851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lauria P.S.S., Villarreal C.F., Casais-e-Silva L.L. Pain Modulatory Properties of Phoneutria Nigriventer Crude Venom and Derived Peptides: A Double-Edged Sword. Toxicon. 2020;185:120–128. doi: 10.1016/j.toxicon.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Gomez M.V., Kalapothakis E., Guatimosim C., Prado M.A.M. Phoneutria Nigriventer Venom: A Cocktail of Toxins That Affect Ion Channels. Cell. Mol. Neurobiol. 2002;22:579–588. doi: 10.1023/A:1021836403433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furtado A., Silva A., Silva-Júnior A., Fernandes-Pedrosa M. Biology, Venom Composition, and Scorpionism Induced by Brazilian Scorpion Tityus Stigmurus (Thorell, 1876) (Scorpiones: Buthidae): A Mini-Review. Toxicon. 2020;185:36–45. doi: 10.1016/j.toxicon.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Martin-Eauclaire M.-F., Bougis P.E., de Lima M.E. Ts1 from the Brazilian Scorpion Tityus Serrulatus: A Half-Century of Studies on a Multifunctional Beta like-Toxin. Toxicon. 2018;152:106–120. doi: 10.1016/j.toxicon.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 61.Lee S.H., Baek J.H., Yoon K.A. Differential Properties of Venom Peptides and Proteins in Solitary vs. Social Hunting Wasps. Toxins. 2016;8:32. doi: 10.3390/toxins8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Konno K., Kazuma K., Rangel M., Stolarz-de-Oliveira J., Fontana R., Kawano M., Fuchino H., Hide I., Yasuhara T., Nakata Y. New Mastoparan Peptides in the Venom of the Solitary Eumenine Wasp Eumenes Micado. Toxins. 2019;11:155. doi: 10.3390/toxins11030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freire K.A., Torres M.D.T., Lima D.B., Monteiro M.L., Bezerra de Menezes R.R.P.P., Martins A.M.C., Oliveira V.X., Jr. Wasp Venom Peptide as a New Antichagasic Agent. Toxicon. 2020;181:71–78. doi: 10.1016/j.toxicon.2020.04.099. [DOI] [PubMed] [Google Scholar]
- 64.de Souza B.M., da Silva A.V.R., Resende V.M.F., Arcuri H.A., dos Santos Cabrera M.P., Ruggiero Neto J., Palma M.S. Characterization of Two Novel Polyfunctional Mastoparan Peptides from the Venom of the Social Wasp Polybia Paulista. Peptides. 2009;30:1387–1395. doi: 10.1016/j.peptides.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 65.Torres M.D.T., Pedron C.N., Higashikuni Y., Kramer R.M., Cardoso M.H., Oshiro K.G.N., Franco O.L., Silva Junior P.I., Silva F.D., Oliveira Junior V.X., et al. Structure-Function-Guided Exploration of the Antimicrobial Peptide Polybia-CP Identifies Activity Determinants and Generates Synthetic Therapeutic Candidates. Commun. Biol. 2018;1:221. doi: 10.1038/s42003-018-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arqué X., Torres M.D.T., Patiño T., Boaro A., Sánchez S., de la Fuente-Nunez C. Autonomous Treatment of Bacterial Infections in Vivo Using Antimicrobial Micro- and Nanomotors. ACS Nano. 2022;16:7547–7558. doi: 10.1021/acsnano.1c11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de la Torre B.G., Albericio F. Peptide Therapeutics 2.0. Molecules. 2020;25:2293. doi: 10.3390/molecules25102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de la Torre B.G., Albericio F. The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules. 2020;25:745. doi: 10.3390/molecules25030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al Musaimi O., Al Shaer D., Albericio F., de la Torre B.G. 2020 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals. 2021;14:145. doi: 10.3390/ph14020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.FDA New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products. [(accessed on 1 October 2022)]; Available online: https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products.
- 71.Wang L., Wang N., Zhang W., Cheng X., Yan Z., Shao G., Wang X., Wang R., Fu C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022;7:48. doi: 10.1038/s41392-022-00904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bordon K.d.C.F., Cologna C.T., Fornari-Baldo E.C., Pinheiro-Júnior E.L., Cerni F.A., Amorim F.G., Anjolette F.A.P., Cordeiro F.A., Wiezel G.A., Cardoso I.A., et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020;11:1132. doi: 10.3389/fphar.2020.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferreira S.H. A Bradykinin-Potentiating Factor (BPF) Present in the Venom of Bothrops Jararaca. Br. J. Pharmacol. Chemother. 1965;24:163–169. doi: 10.1111/j.1476-5381.1965.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camargo A.C.M., Ianzer D., Guerreiro J.R., Serrano S.M.T. Bradykinin-Potentiating Peptides: Beyond Captopril. Toxicon. 2012;59:516–523. doi: 10.1016/j.toxicon.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Patchett A.A. The Chemistry of Enalapril. Br. J. Clin. Pharmacol. 1984;18:201S–207S. doi: 10.1111/j.1365-2125.1984.tb02599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biollaz J., Burnier M., Turini G.A., Brunner D.B., Porchet M., Gomez H.J., Jones K.H., Ferber F., Abrams W.B., Gavras H., et al. Three New Long-Acting Converting-Enzyme Inhibitors: Relationship between Plasma Converting-Enzyme Activity and Response to Angiotensin I. Clin. Pharmacol. Ther. 1981;29:665–670. doi: 10.1038/clpt.1981.92. [DOI] [PubMed] [Google Scholar]
- 77.Coulter-Parkhill A., McClean S., Gault V.A., Irwin N. Therapeutic Potential of Peptides Derived from Animal Venoms: Current Views and Emerging Drugs for Diabetes. Clin. Med. Insights Endocrinol. Diabetes. 2021;14 doi: 10.1177/11795514211006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stocker K., Barlow G.H. Methods in Enzymology. Volume 45. Academic Press; Cambridge, MA, USA: 1976. The Coagulant Enzyme from Bothrops Atrox Venom (Batroxobin) pp. 214–223. [DOI] [PubMed] [Google Scholar]
- 79.Funk C., Gmür J., Herold R., Straub P.W. Reptilase®-R—A New Reagent in Blood Coagulation. Br. J. Haematol. 1971;21:43–52. doi: 10.1111/j.1365-2141.1971.tb03415.x. [DOI] [PubMed] [Google Scholar]
- 80.Monge-Fuentes V., Arenas C., Galante P., Gonçalves J.C., Mortari M.R., Schwartz E.F. Arthropod Toxins and Their Antinociceptive Properties: From Venoms to Painkillers. Pharmacol. Ther. 2018;188:176–185. doi: 10.1016/j.pharmthera.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Smallwood T.B., Clark R.J. Advances in Venom Peptide Drug Discovery: Where Are We at and Where Are We Heading? Expert Opin. Drug Discov. 2021;16:1163–1173. doi: 10.1080/17460441.2021.1922386. [DOI] [PubMed] [Google Scholar]
- 82.Flinspach M., Xu Q., Piekarz A.D., Fellows R., Hagan R., Gibbs A., Liu Y., Neff R.A., Freedman J., Eckert W.A., et al. Insensitivity to Pain Induced by a Potent Selective Closed-State Nav1.7 Inhibitor. Sci. Rep. 2017;7:39662. doi: 10.1038/srep39662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray J.K., Long J., Zou A., Ligutti J., Andrews K.L., Poppe L., Biswas K., Moyer B.D., McDonough S.I., Miranda L.P. Single Residue Substitutions That Confer Voltage-Gated Sodium Ion Channel Subtype Selectivity in the NaV1.7 Inhibitory Peptide GpTx-1. J. Med. Chem. 2016;59:2704–2717. doi: 10.1021/acs.jmedchem.5b01947. [DOI] [PubMed] [Google Scholar]
- 84.Chen C., Hong M., Guo X., Wu F., Tian C., Wang Y., Xu Z. Facile Synthesis of Macrocyclic Peptide Toxins of GpTx-1 and Its Analogue. Org. Chem. Front. 2018;5:2143–2147. doi: 10.1039/C8QO00415C. [DOI] [Google Scholar]
- 85.Cherki R.S., Kolb E., Langut Y., Tsveyer L., Bajayo N., Meir A. Two Tarantula Venom Peptides as Potent and Differential NaV Channels Blockers. Toxicon. 2014;77:58–67. doi: 10.1016/j.toxicon.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 86.Lamiyan A.K., Dalal R., Kumar N.R. Venom Peptides in Association with Standard Drugs: A Novel Strategy for Combating Antibiotic Resistance – an Overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020;26:e20200001. doi: 10.1590/1678-9199-jvatitd-2020-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agbale C.M., Sarfo J.K., Galyuon I.K., Juliano S.A., Silva G.G.O., Buccini D.F., Cardoso M.H., Torres M.D.T., Angeles-Boza A.M., de la Fuente-Nunez C., et al. Antimicrobial and Antibiofilm Activities of Helical Antimicrobial Peptide Sequences Incorporating Metal-Binding Motifs. Biochemistry. 2019;58:3802–3812. doi: 10.1021/acs.biochem.9b00440. [DOI] [PubMed] [Google Scholar]
- 88.Nešuta O., Hexnerová R., Buděšínský M., Slaninová J., Bednárová L., Hadravová R., Straka J., Veverka V., Čeřovský V. Antimicrobial Peptide from the Wild Bee Hylaeus Signatus Venom and Its Analogues: Structure–Activity Study and Synergistic Effect with Antibiotics. J. Nat. Prod. 2016;79:1073–1083. doi: 10.1021/acs.jnatprod.5b01129. [DOI] [PubMed] [Google Scholar]
- 89.Drugs@FDA: FDA-Approved Drugs. [(accessed on 1 October 2022)]; Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process.
- 90.ClinicalTrials.Gov The Efficacy and Safety of Batroxobin Combined with Anticoagulation in Cerebral Venous Sinus Thrombosis—Full Text View. [(accessed on 1 October 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04269954?term=batroxobin&draw=3&rank=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.