Abstract
Cognitive impairment represents a leading residual symptom of COVID-19 infection, which lasts for months after the virus clearance. Up-to-date scientific reports documented a wide spectrum of brain changes in COVID-19 survivors following the illness's resolution, mainly related to neurological and neuropsychiatric consequences. Preliminary insights suggest abnormal brain metabolism, microstructure, and functionality as neural under-layer of post-acute cognitive dysfunction. While previous works focused on brain correlates of impaired cognition as objectively assessed, herein we investigated long-term neural correlates of subjective cognitive decline in a sample of 58 COVID-19 survivors with a multimodal imaging approach.
Diffusion Tensor Imaging (DTI) analyses revealed widespread white matter disruption in the sub-group of cognitive complainers compared to the non-complainer one, as indexed by increased axial, radial, and mean diffusivity in several commissural, projection and associative fibres. Likewise, the Multivoxel Pattern Connectivity analysis (MVPA) revealed highly discriminant patterns of functional connectivity in resting-state among the two groups in the right frontal pole and in the middle temporal gyrus, suggestive of inefficient dynamic modulation of frontal brain activity and possible metacognitive dysfunction at rest.
Beyond COVID-19 actual pathophysiological brain processes, our findings point toward brain connectome disruption conceivably translating into clinical post-COVID cognitive symptomatology. Our results could pave the way for a potential brain signature of cognitive complaints experienced by COVID-19 survivors, possibly leading to identify early therapeutic targets and thus mitigating its detrimental long-term impact on quality of life in the post-COVID-19 stages.
Keywords: COVID-19, Magnetic resonance imaging, Subjective cognitive dysfunction, Diffusion tensor imaging, Resting-state, Functional connectivity
1. Introduction
As the world moves into the third year of COVID-19 pandemic, much effort is expended in further defining mental health clinical patterns exhibited by COVID-19 survivors, their endurance, and their putative pathophysiological mechanisms. Scientific and clinical data are evolving on the residual symptoms of the disease persisting in the long run, possibly linked to the COVID-19 multiple organ involvement (Jiang et al., 2020, Nalbandian et al., 2021). Although the direct neurotropism of the SARS-CoV-2 has not been confirmed to date (Spudich and Nath, 2022), there are heeds regarding COVID-19-induced brain demyelinating lesions (Zanin et al., 2020), limbic and subcortical hypo metabolism in extensive areas, along with abnormal functional intra- and inter-network connectivity in survivors experiencing neuropsychiatric sequelae (Zanin et al., 2020, Voruz et al., 2022, Zhang et al., 2022). According to the most favoured hypothesis, several factors entailing dysregulated peripheral immune system activation and prompted neuroinflammation, coagulopathy, and endothelial dysfunction may critically affect brain's morphology and functionality via indirect pathways (O'Shea et al., 2021, Tang et al., 2021, Tang et al., 2022). A key concern is the long-lasting cognitive consequences of the infection (Mazza et al., 2020, Mazza et al., 2021, Poletti et al., 2021), representing a critical and debilitating feature of the so-called Post-acute COVID-19 syndrome (PACS) (Nalbandian et al., 2021, Hampshire et al., 2021). Most strikingly, consistent evidence highlights an impressive rate of COVID-19 patients who still exhibit impaired cognitive performance six months after the virus clearance, showing a constant impaired cognitive profile over time (Poletti et al., 2021). Beyond cognitive deficits as evaluated through standardized batteries, recent investigations pinpoint a high prevalence of clinically relevant subjective cognitive failure among COVID-19 survivors in the months following the infection (Miskowiak et al., 2021). Notably, cognitive decline as reported by COVID-19 patients does not seem to be affected by the severity of acute pneumonia (Andersson et al., 2022); rather, existing data showed that subjective cognitive deficits in COVID-19 survivors (Ferrando et al., 2022) are associated with anxiety and depressive symptomatology and lower neuropsychological performance (Gouraud et al., 2021). These symptoms persist even several months after the acute infection, suggesting that the perception of cognitive complaints is a sign of long-term actual cognitive deficits. In keeping with this, it has been documented that most of COVID-19 patients exhibit intercorrelated objective and subjective cognitive impairments, altogether worsening quality of life (Miskowiak et al., 2021).
Given the well-documented altered brain patterns underlying neuropsychological deficits, it is crucial to further explore potential neural endophenotypes of cognitive impairments experienced by COVID-19 patients. Despite being also a hallmark of PACS, both when objectively measured or subjectively reported by the patients (Ceban et al., 2022, Schou et al., 2021), studies exploring brain correlates of post-COVID cognitive impairment are sparse.
Novel insights provide evidence of altered brain metabolism, microstructure, and functionality in COVID-19 survivors exhibiting cognitive deficiency, possibly underpinning acute and post-acute cognitive dysfunction in patients recovering from the illness (Voruz et al., 2022, Silva et al., 2021, Voruz et al., 2022, Yesilkaya et al., 2021). However, available investigations focused on standardized measures of cognitive functioning, and most of them, with cross-sectional design, cannot effectively disentangle between deficits acquired after COVID-19 and pre-infection cognitive status (Hampshire et al., 2021, Silva et al., 2021).
In our study we are now focusing on investigating long-term neural correlates of subjective cognitive decline in a sample of COVID-19 survivors studied with a multimodal brain imaging approach. Subjective perception of cognitive impairments may identify an early and possibly more sensitive sign of emerging deficits, although subjective difficulties could also reflect levels of depressive symptoms (Ott et al., 2016, Petersen et al., 2019). Specifically, we compared patients with and without subjective cognitive complaints on Diffusion Tensor Imaging (DTI) measures and Resting-State functional connectivity (rs-FC).
2. Material and methods
2.1. Participants and data collection
58 COVID-19 survivors were enrolled starting from January 2021 until January 2022 in the context of an ongoing prospective cohort study taking place at San Raffaele Hospital in Milan (De Lorenzo et al., 2020).
To keep a naturalistic study design, we enrolled inpatients aged between 18 and 70, who were hospitalized at San Raffaele Hospital for a SARS-CoV-2 infection.
Clinical and socio-demographic information - including age, sex, date of COVID symptoms’ onset, length of hospitalization, setting of care, presence of depressive symptomatology, presence of intellectual disability or organic illness - were gathered in the context of an unstructured clinical interview conducted by well-trained psychologists. Inclusion criteria were: I) diagnosis of COVID-19 infection as suggested from clinical and radiological findings obtained at the Emergency department and confirmed via reverse transcriptase polymerase chain reaction assays on the nasopharyngeal, throat, or lower respiratory tract swab; II) aged between 18 and 70. Exclusion criteria were: intellectual disabilities, history of drug or alcohol use disorder within the last six months, major neurological disorders, and pregnancy. After a complete description of the study procedure, written informed consent was obtained.
Cognitive complaints were assessed in the context of the clinical interview; they were considered present if participants answered ‘yes’ to at least one of the following clinical questions: ‘Did you develop regular cognitive difficulties in any of the following domains after the illness’ resolution?’ i) forgetfulness in activities of daily living; ii) difficulty with paying attention to external stimuli or maintaining concentration on a task. On the basis of the answer, we divided patients as cognitive complainer and non-complainer. A similar approach was previously validated in general population in a wide-cohort study and proven to be advantageous to investigate subjective cognitive complaints in COVID-19 survivors (Gouraud et al., 2021, Goldberg et al., 2017).
2.2. DTI images preprocessing and statistical analyses
All imaging was performed on a 3.0 T scanner (Ingenia CX, Philips, The Netherlands) with spin-echo echo-planar imaging (EPI) and the following parameters: TR/TE = 5900/78 ms, FoV (mm) 240 (ap), 129 (fh), 232 (rl); acquisition matrix 2.14 × 2.73 × 2.30; 56 contiguous, 2.3 mm thick axial slices reconstructed with in-plane pixel size 1.88 × 1.88 × 2.30 mm; SENSE acceleration factor = 2; 1 b0 and 40 non-collinear directions of the diffusion gradients; b value = 1000 s/mm2. Fat saturation was performed to avoid chemical shift artifacts.
Image analyses and tensor calculations were done using the “Oxford Center for Functional Magnetic Resonance Imaging of the Brain Software Library” (FSL 6.0; www.fmrib.ox.ac.uk/fsl/index.html) (Woolrich et al., 2009). Each DTI volume was affine registered to the T2-weighted b=0 volume using FLIRT (FMRIB's Linear Image Registration Tool) (Jenkinson and Smith, 2001). Correction for susceptibility-induced off-resonance field, eddy current-induced distortions, and subject movements was performed (Andersson et al., 2016) . Least-square fits were performed to estimate the fractional anisotropy (FA), eigenvector, and eigenvalue maps. Mean diffusivity (MD) was defined as the mean of all three eigenvalues (λ 1 + λ 2 + λ 3)/3, axial diffusivity (AD) as the principal diffusion eigenvalue (λ 1), and radial diffusivity (RD) as the mean of the second and third eigenvalues (λ 2 + λ 3)/2. Next, all individuals’ volumes were skeletonized and transformed into a common space as used in Tract-Based Spatial Statistics (Smith et al., 2006). Briefly, all volumes were nonlinearly warped to the FMRIB58_FA template supplied with FSL (http://www.fmrib.ox.ac.uk/fsl/tbss/FMRIB58_FA.html) and normalized to the Montreal Neurological Institute (MNI) space. Next, a mean FA volume of all subjects was generated and thinned to create a mean FA skeleton representing the centres of all common tracts. Individual FA values were warped onto this mean skeleton mask. The resulting tract invariant skeletons for each participant were fed into voxel-wise permutation-based cross-subject statistics. Similar warping and analyses were used on MD, AD, and RD data.
Voxel-wise DTI analyses were performed using nonparametric permutation-based testing (Nichols and Holmes, 2002) as implemented in Randomise in FSL. Within the GLM framework, a Two-Sample unpaired T-Test was performed between cognitive and non-cognitive complainers on FA, MD, AD, and RD across the WM skeleton; we entered age, sex, time from COVID-19 onset to MRI scan, and BMI, thus correcting the results for covariates known to affect the brain integrity. Furthermore, given its close association with subjective cognitive deficits, the presence of depressive symptomatology was also considered as a nuisance covariate in the analyses (Benedetti et al., 2021, Douaud et al., 2022, Stanek et al., 2011). Threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009) was used to avoid defining arbitrary cluster forming thresholds and smoothing levels. Voxel-wise levels of significance, corrected for multiple comparisons, were then calculated with standard permutation testing by building up the null distribution (across permutation of the input data) of the maximum (across voxels) TFCE scores, and then using the 95th percentile of the null distribution to threshold signals at corrected p < 0.05. The data were tested against an empirical null distribution generated by 5000 permutations for each contrast, thus providing statistical maps fully corrected for multiple comparisons across space. Corrected p < 0.05 in a minimum cluster size of k = 100 was considered significant.
2.3. fMRI data preprocessing and statistical analyses
Scanning sessions for resting-state fMRI images comprised 200 sequential T2*-weighted volumes (interleaved ascending transverse slices covering whole brain, tilted 30° downward with respect to bicommissural line to reduce susceptibility artifacts in orbitofrontal region), acquired using an EPI pulse sequence (TR = 2000 ms; TE = 30 ms; flip angle= 85°; field of view = 192 mm; number of slices = 38; slice thickness = 3.7 mm; matrix size = 64 × 62 reconstructed up to 96 × 96 pixels). Six dummy scans before fMRI acquisition allowed obtaining longitudinal magnetization equilibrium. Total time acquisition was 6 min and 56 s.T2*-weighted images were preprocessed using CONN toolbox (www.nitrc.org/projects/conn), running within Statistical Parametric Mapping (SPM 12). The standard preprocessing pipeline was implemented, which included the following procedures: i) realignment to a reference image in order to minimize variance due to head movements and unwarping; ii) slice timing correction was applied to mitigate temporal misalignment between different slices of the functional data; iii) detection of potential outlier scans (subject motion above 0.9mm and 0.02 rad or spikes in global signal intensity above 5 SD) by means of Artifact Detection Tool (ART, www.nitrc.org/projects/artifact_detect) – a threshold of 20% scans flagged as outliers was set to determine subject exclusion; iv) normalization to a standard MNI space and segmentation of the brain into GM, WM and CSF tissue classes; v) application of spatial smoothing using an 8-mm full-width at half-maximum isotropic Gaussian kernel to enhance the signal-to-noise ratio; vi) removal of confounding effects via an anatomical component-based noise correction procedure (aCompCor), which involves WM, CSF, pshysiological noise source reduction (e.g. six standard motion parameters and ART-based “scrubbed” signal artifacts) with relative derivatives, all taken as covariates in first-level analyses; vii) application of linear detrending to remove linear drift artifacts and high-frequency noise.
Whole-brain functional connectivity (FC) patterns were investigated through multivariate pattern connectivity analyses (MVPA), which allow computing the pairwise connectivity patterns between each voxel and all the other voxels in the brain by reducing the dimensionality of these multi-voxel patterns with principal component analysis (PCA) (Whitfield-Gabrieli et al., 2016). By estimating multivariate connectivity maps for each subject, MVPA provides a more sensitive and analytical approach to the study of the functional organization of the brain than traditional univariate analyses (Haxby, 2012). As a consequence, MVPA allows to decode perceptual stimuli and mental states from brain activation patterns, with evidence of efficacy in the prediction of diagnostic groups (Sundermann et al., 2014) and antidepressant treatment response (Wang et al., 2019). First-level MVPA was performed using 64 dimensions. MVPA-derived maps were then entered in the second-level analyses exploring differences in connectivity patterns between cognitive complainers and non-complainers. Analyses were designed in the context of GLM: the group was considered as a categorical predictor, whereas age, sex, time elapsed from COVID diagnosis to the MRI scan, BMI, and presence of depressive symptoms at the time of recovery as nuisance covariates. The resulting regions of significance indicate clusters of voxels that consistently share similar between-subject variance of their spatial connectivity associated with the presence or otherwise of subjective cognitive complaints. Considering the sample size, 4 components were kept for each voxel. Analyses were thresholded at peak level (p < 0.001, uncorrected; cluster level: p < 0.05 FWE-corrected). To further investigate the direction of the effects, the identified areas were used as seeds for the following seed-to-voxel analyses, aiming at determining whether their functional connectivity with the whole brain raise or decrease according to the group of belonging.
3. Results
Clinical and demographic characteristics of the sample can be seen in Table 1 .
Table 1.
clinical and demographic characteristics of the sample.
| Whole Sample (n=58) | Non-cognitive complainers (n=29) | Cognitive complainers (n=29) | t-test or chi-squared significance (p) | |
|---|---|---|---|---|
| Age | 52,34 ± 11,73 | 54,41 ± 9,93 | 50,27 ± 13,13 | 0,181 |
| Sex | M=41, F=17 | M=23, F=6 | M=18, F=11 | 0,149 |
| BMI | 26,99 ± 4,86 | 28,04 ± 5,35 | 25,93 ± 4,15 | 0,100 |
| Onset – MRI | 173,12 ± 174,03 | 179,14 ± 173,52 | 167,10 ± 177,41 | 0,795 |
| Days of Hospitalization | 13,79 ± 13,83 | 16,20 ± 15,10 | 11,37 ± 12,23 | 0,186 |
| ICU admission | 9 (15,51%) | 5 (17,24%) | 4 (13,79%) | 0,716 |
| Presence of Depressive Symptomatology | 30 (51,72%) | 10 (34,48%) | 20 (68,96%) | 0,009* |
p < 0.05
Twenty-nine participants (50%) presented with cognitive complaints one month after the virus clearance, and in the vast majority of them (twenty-four out of twenty-nine) they were still present at the time of MRI scan. All patients were hospitalized due to COVID-19 infection, and 9 patients required treatment in the ICU: no effect of ICU admission on the development of subjective cognitive impairment was found. No significant differences emerged for age, sex, and other variables, including day of hospitalization. However, 51,72% of the sample reported the presence of depressive symptomatology after discharge, with much higher rates in cognitive complainers (68,96%) than in non-complainers (34,48%).
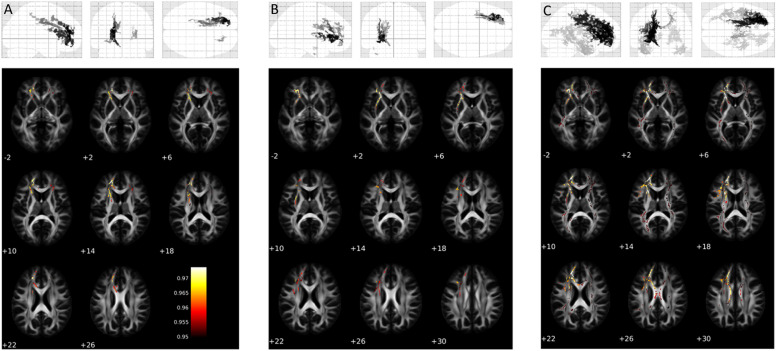
Cognitive complaints associated with differences in WM microstructure, as revealed by significant differences in DTI measures between cognitive and non-cognitive complainers (Supplementary Table 1). COVID-19 survivors with subjective cognitive impairments showed greater MD in widespread portions of the WM skeleton, affecting bilaterally the inferior fronto-occipital fasciculus, uncinate fasciculus, corona radiata as well as several sections of corpus callosum. The increase in MD associated both, with increased RD in several WM tracts located in the left hemisphere (corona radiata, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus and uncinate fasciculus), and with higher AD in some inter-hemispheric associative tracts (Fig. 1 ). Differences in FA were not significant at pFWE<0.05 but showed a marginal trend of lower values in cognitive complainers (pFWE= 0.09).
Fig. 1.
Differences in DTI indexes between the cognitive-complainer group and the non-complainer counterpart. A) Axial Diffusivity; B) Radial Diffusivity; C) Mean Diffusivity. Only the voxels surviving the statistical threshold of corrected p < 0.05 at TFCE are shown.
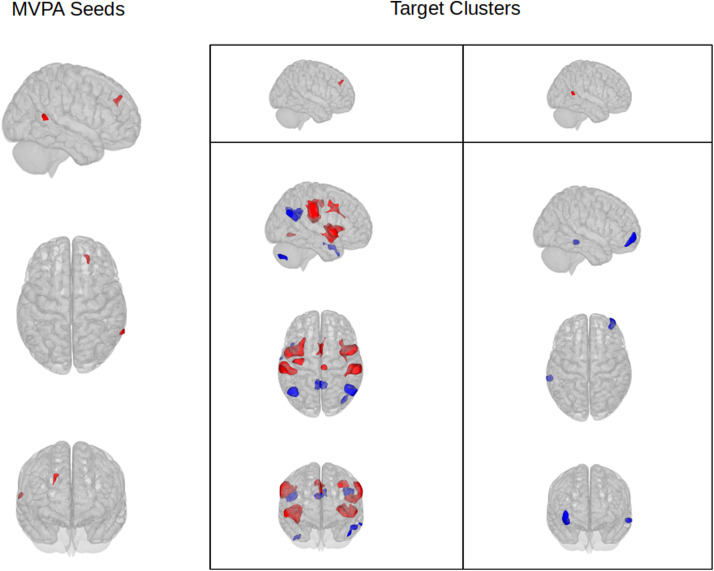
MVPA analysis revealed 2 significant clusters of different rs-FC between the two groups: the first cluster was located in the right frontal pole (peak MNI +20 +38 +32, Cluster Size 120, pFWE=0.0016) and the second in the middle temporal gyrus (peak MNI +68 -54 +06, Cluster Size 92, pFWE=0.0088) (Fig. 2 , Supplementary Table 2).
Fig. 2.
Differences in resting-state functional connectivity between the cognitive-complainers and the non-complainer group. First column: seeds of altered connectivity identified in the MVPA analysis. Second and third column: target clusters resulting from post-hoc seed to voxel analysis. Red: areas of increased rs-FC in cognitive complainers; Blue: areas of reduced rs-FC in cognitive complainers.
Post-hoc seed-to-voxel analysis using the identified clusters as seeds highlighted several brain regions of increased or decreased FC between the two groups; concerning the first seed (right frontal pole), increased FC in subjects complaining deficits was found with eight clusters located in the bilateral Insular Cortex, bilateral Supramarginal and Opercular cortex, Anterior Cingulate gyrus, bilateral Precentral Gyrus and inferior Lateral Occipital cortex; reduced FC was instead found with five clusters located in bilateral superior Occipital cortex, posterior Cingulate gyrus, left middle temporal gyrus (MTG) and right Cerebellum. All the areas with increased FC were part of the Salience, Sensori-Motor or Dorsal Attention networks (except for the small cluster in the inferior LOC belonging to the Visual network); among clusters with decreased FC, the three with the highest statistical significance were found to be part of the Default Mode Network (DMN); no Network was identified for the left MTG cluster, while the last cluster belonged to cerebellar networks.
Concerning the second seed, in subjects reporting cognitive deficits it exhibited lower FC with two clusters located in the right Frontal Pole and in the left posterior MTG. No cluster of increased FC was identified. The Frontal Pole cluster was identified as part of the frontoparietal network, while no cluster was identified for the temporal cluster.
4. Discussion
This is the first study investigating MRI brain correlates of subjective cognitive deficits in COVID-19 survivors. The main finding of the present study is that WM microstructure and resting-state FC differ in patients reporting subjective cognitive impairment. When compared to non-cognitive complainers, patients with newly onset cognitive decline after COVID-19 exhibited far-reaching alterations of WM microstructure and FC. Specifically, patients experiencing cognitive impairment at one month follow-up showed increased widespread MD in several bilateral WM tracts; increased levels of RD and AD were also identified, while FA exhibited a trend towards statistical significance with lower values in subjects reporting cognitive deficits.
Higher rates of depressive symptomatology were reported in the complainer group, thus making it challenging to completely ascribe the connectivity abnormalities we found to the subjective cognitive status; however, the presence of depression was taken into account in all our analyses.
Alterations of DTI indexes have been widely described in patients exhibiting or reporting cognitive impairments; Increased MD and decreased FA are common findings in patients suffering from Alzheimer Disease and Mild Cognitive Impairment (Chandra et al., 2019); higher values of MD and lower FA were also found in preclinical subjects with subjective cognitive decline compared to healthy controls, in anterior and posterior WM tracts also identified in our analysis (Corona Radiata, superior and inferior longitudinal fasciculum, Corpus Callosum) (Brueggen et al., 2019). DTI alterations have been reported also in non-age –related forms of cognitive decline, such as post trauma (Oehr and Anderson, 2017) or iatrogenic post radio or chemotherapy ones (Durán-Gómez et al., 2022, Yahya and Manan, 2021).
As for COVID-19, abnormalities in brain microstructure and functionality linked to severity of neuropsychiatric sequelae are starting to be reported; a previous study by our group showed that indexes of post-COVID depressive and post-traumatic symptomatology significantly associates with disruption in WM integrity and abnormal functional connectivity at rest (Benedetti et al., 2021). Furthermore, alterations of WM microstructure were found to persist up to 1 year after COVID-19 recovery (Huang et al., 2022). In a large longitudinal study performed on 785 subjects from the UK Biobank, greater reductions in Cortical Thickness and increases in DTI Mean Diffusivity in parahippocampal, orbitofrontal anterior cingulate, and insular cortex were observed in subjects that contracted COVID-19 compared to controls. The same study also reported a greater decline of objectively measured cognitive abilities in post-COVID patients, revealing a close relationship between impaired cognitive performance and reduced cerebellar volume (Douaud et al., 2022).
Further supporting the notion of brain involvement in COVID-19 survivors, a higher prevalence and volume of White Matter Hyperintensities (WMH) seem to be a common finding among subjects who recover from the illness with residual cognitive impairments or depressive symptomatology (Andriuta et al., 2022, Cecchetti et al., 2022, Hellgren et al., 2021, Poletti et al., 2022). Finally, a diffusion microstructure imaging study comparing post-COVID subjects exhibiting neurological symptoms or cognitive impairment and healthy controls, found evidence of redistribution of water molecules with widespread decreasing intra-axonal and extra-axonal volume and increasing free water/CSF fractions in COVID-19 survivors; the magnitude of V-CSF-increase was found to correlate with the degree of cognitive impairment, and speculated by the authors to be reflective of vasogenic edema (Rau et al., 2022).
Our study also identified an increase of both AD and RD and a trend towards reduced FA values in subjects reporting deficits: higher values of RD and lower of FA could also be reflective of areas of WM integrity disruption (Winklewski et al., 2018). AD on the other hand reflects the diffusion coefficient along the principal eigenvector (λ 1), and has been proposed as a marker of axonal integrity (Winklewski et al., 2018); however when its increase is not associated with a corresponding increase of FA values, but is associated with higher MD, it is usually thought to be reflective of increased extracellular space and higher water diffusion (Alves et al., 2015), and has been repeatedly reported in patients with cognitive impairment and Alzheimer's disease (Mayo et al., 2019, O'Dwyer et al., 2011).
With regards to resting-state fMRI results, MVPA analyses exposed highly discriminant FC patterns among the two groups, with the complainer cohort showing abnormally high connectivity at rest of the frontal pole with networks critically involved in cognitive-demanding tasks, as well as lower functional pairing with the DMN. Reproducible negative correlations between DMN and the salience and dorsal attentive networks at rest have been widely reported in the literature (Zhou et al., 2018). Specifically, the DMN shows coordinated temporal activity at rest, whereas deactivates during attentional-demanding tasks. Conversely, the dorsal attention network increases its activity during goal-directed tasks, exerting an inhibitory control over the DMN (Di and Biswal, 2014, Smallwood et al., 2021). Notably, the switching processes from the DMN to the attentive networks are promoted by connections between the anterior prefrontal cortex and the insula within the salience network, suggesting that the anterior frontal lobe is fundamental for the transition between resting and cognitively-demanding states (Peng et al., 2018). Increased FC within the dorsal attentive network during resting-state has been previously associated with low cognitive performance in healthy elderly subjects (Charroud et al., 2016, Sala-Llonch et al., 2012). Similar results were found in HIV patients, where over activations in task-related regions are linked with poor cognitive performance at complex behavioural tasks (Hakkers et al., 2017). These results could be explained according to the brain reserve theory, which states that higher activations in task-positive networks are needed to counterweight inefficient cognitive functioning in low-performing subjects (Stern, 2009). Notably, we found that, albeit not involved in performing a cognitive-demanding task, subjective cognitive complainers exhibit increased connectivity of the frontal pole with the salience and dorsal attentive networks, which might indicate an inefficient dynamic modulation of frontal brain activity in these subjects that occurs even during rest.
Together with patterns of increased FC, negative correlations between the right frontal pole and regions encompassing the DMN, such as the superior occipital cortex and posterior cingulate gyrus, were observed in subjective cognitive complainers. Previous studies demonstrated that reduced functional coupling between frontal and posterior cingulate regions is predictive of poor performance during attentional and working memory tasks, both in healthy and pathological aging (Sala-Llonch et al., 2012, Damoiseaux et al., 2008, Greicius et al., 2004, Sambataro et al., 2010). In line with these results, we observed that COVID-19 survivors reporting subjective cognitive impairments exhibit reduced FC between the frontal pole and the DMN during rest, possibly reflecting altered attentional and memory processes which are independent from performance at cognitive tasks.
Finally, we found that subjective cognitive impairments are associated with reduced rs-FC of the right middle temporal gyrus with the right frontal pole and left middle temporal gyrus. Increased activations in the frontal pole, along with medial temporal regions and posterior cingulate cortices, have been associated with metacognitive processes, which represent the ability to monitor and reflect on one's cognition and experience (Chua et al., 2006, Moritz et al., 2006, Yokoyama et al., 2010). Previous evidence suggests that subjective cognitive complainers could be less confident in their cognitive performance compared to non-complainers, reflecting impairments in metacognition and memory self-efficacy (Ponds and Jolles, 1996, Reid and MacLullich, 2006). In our sample, the negative functional coupling between the middle temporal gyrus and frontal pole in subjective cognitive complainers may indicate that an underlying prefrontal hypo-connectivity might promote metacognitive dysfunction, representing a potential neural correlate of long-term cognitive impairments in these subjects.
It should be considered, though, that much higher rates of depressive symptomatology were found in the cognitive complainer group compared to the non-complainer counterpart. Besides being recognized as a marker of cognitive decline (Burmester et al., 2016), subjective cognitive complaints are a common feature of the depressive syndrome (Serra-Blasco et al., 2019, Srisurapanont et al., 2017). Extensive literature supports the notion of a close interplay between depressive symptoms and subjective cognitive failure, further corroborated by the treatment-induced parallel improvement of both affective and cognitive symptomatology in psychiatric populations (Allott et al., 2020). At the same time, since they have been both reported to be highly prevalent in post COVID-19 patients, we could speculate low mood and cognitive impairment to be closely related manifestations of the neuropsychiatric symptomatology of COVID-19 infection (Almeria et al., 2020). In any case, in a bid to investigate specifically the brain correlates of subjective cognitive impairment, we entered the presence of depressive symptomatology as a covariate in all our analyses.
Several pathophysiological mechanisms have been suggested for the development of post-COVID cognitive impairment: a direct neurotropism of the virus is supposed to play a secondary role, while a higher impact is attributed to neuroinflammation and abnormal immune response, endothelial and blood–brain barrier dysfunction and oxidative stress (Stefanou et al., 2022). Therefore, we can hypothesize the alterations of structural and functional connectivity we detected to be reflective of these pathophysiological brain processes, and to translate clinically to the post-COVID cognitive symptomatology. An alternative explanation is to imagine the brain connectome disruption to precede the infection and to confer a vulnerability towards the development of subjective cognitive deficits. Further studies with a longitudinal design will have to address this issue.
Strengths of the present study include a focused research question, state of the art MRI methods, and a real-world experimental setting, but our results must be viewed in light of some limitations. Patients were studied at variable intervals from symptoms’ onset. In any case, time from COVID-19 infection to MRI scan has been considered as nuisance covariate in all the analyses. Patients enrolled in this study were evaluated in a single clinical outpatient service, therefore it cannot be ruled out stratification issues. The drug treatments administered during the course of the illness could have influenced the clinical and biological picture.
The cross-sectional design of the current study limits the possibility to establish a clear causal link between the infection and cognitive deficits. However, subjective cognitive decline, even though difficult to disentangle from depressive symptomatology, may better mirror the dynamic changes of cognitive status before and after the illness than objective cognitive measures. Furthermore, subjective cognitive complaints have been previously found to be reflective of objective cognitive status in COVID-19 patients (Miskowiak et al., 2021). The onset of cognitive complaints could also be influenced by the severity of the infection and particularly by hypoxia; we checked the effect of setting of care (i.e., standard hospitalization or ICU) on the development of cognitive complaints, but this represents only a rough estimation of illness severity. The lack of a healthy control group prevents us from drawing definite conclusions about the pattern of brain alterations underlying subjective cognitive decline. Lastly, as already mentioned in the methods section, the assessment of cognitive status was not performed through a structured questionnaire. Nevertheless, the procedure we employed was adapted from previously validated protocols (Gouraud et al., 2021, Goldberg et al., 2017).
Considering widespread concern about cognitive complaints in the post-COVID stages, the above findings have high clinical relevance in driving early identification of brain patterns strictly associated with the experience of cognitive failure in everyday activities. Although further research is needed to replicate our results, hopefully a solid brain signature may lead to prompt interventions targeting cognitive dysfunction, allowing to early tackle neuropsychiatric sequelae possibly arising afterwards.
5. Contributors
FB, MP (Marco Paolini) and MP (Mariagrazia Palladini) designed the study and wrote the protocol. Authors FB, MP (Marco Paolini), MP (Mariagrazia Palladini), and FC managed the literature searches and analyses. Authors MP (Marco Paolini), MP (Mariagrazia Palladini) and FC undertook the statistical analysis, and authors MP (Marco Paolini), MP (Mariagrazia Palladini), MGM, and FC wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Declaration of Competing Interest
All the authors declare that they have no conflicts of interest.
Acknowledgments
Funding source
None
Acknowledgements
Funding: MP salary: Italian Ministry of University, XXXVII PhD cycle, FSE REACT-EU 2021 PON projects, Action IV.5. We also thank Miss Valentina Bettonagli, who kindly provided the data necessary for our analysis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.euroneuro.2022.12.002.
Appendix. Supplementary materials
References
- Allott K., Gao C., Hetrick S.E., Filia K.M., Menssink J.M., Fisher C., et al. Subjective cognitive functioning in relation to changes in levels of depression and anxiety in youth over 3 months of treatment. BJPsych Open. 2020;6(5) doi: 10.1192/bjo.2020.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain, Behav. Immunity Health. 2020;9 doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves G.S., Oertel Knöchel V., Knöchel C., Carvalho A.F., Pantel J., Engelhardt E., et al. Integrating retrogenesis theory to Alzheimer's disease pathology: insight from DTI-TBSS investigation of the white matter microstructural integrity. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/291658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Graham M.S., Zsoldos E., Sotiropoulos S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556–572. doi: 10.1016/j.neuroimage.2016.06.058. [DOI] [PubMed] [Google Scholar]
- Andersson S., Hestad K., Johannessen D., Okkenhaug I., Gramstad A., Andreassen O., et al. Subjective cognitive complaints and neuropsychological performance at six months post COVID-19. J. Psychosom. Res. 2022;157 [Google Scholar]
- Andriuta D., Si-Ahmed C., Roussel M., Constans J.-M., Makki M., Aarabi A., et al. Clinical and imaging determinants of neurocognitive disorders in post-acute COVID-19 patients with cognitive complaints. J. Alzheimers Dis. 2022;(Preprint):1–12. doi: 10.3233/JAD-215506. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Palladini M., Paolini M., Melloni E., Vai B., De Lorenzo R., et al. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: a multimodal magnetic resonance imaging study. Brain, Behavior Immunity-Health. 2021;18 doi: 10.1016/j.bbih.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggen K., Dyrba M., Cardenas-Blanco A., Schneider A., Fliessbach K., Buerger K., et al. Structural integrity in subjective cognitive decline, mild cognitive impairment and Alzheimer's disease based on multicenter diffusion tensor imaging. J. Neurol. 2019;266(10):2465–2474. doi: 10.1007/s00415-019-09429-3. [DOI] [PubMed] [Google Scholar]
- Burmester B., Leathem J., Merrick P. Subjective cognitive complaints and objective cognitive function in aging: a systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol. Rev. 2016;26(4):376–393. doi: 10.1007/s11065-016-9332-2. [DOI] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M., Lee Y., Gill H., Teopiz K.M., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchetti G., Agosta F., Canu E., Basaia S., Barbieri A., Cardamone R., et al. Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J. Neurol. 2022:1–13. doi: 10.1007/s00415-022-11047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A., Dervenoulas G., Politis M. Magnetic resonance imaging in Alzheimer's disease and mild cognitive impairment. J. Neurol. 2019;266(6):1293–1302. doi: 10.1007/s00415-018-9016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroud C., Le Bars E., Deverdun J., Steffener J., Molino F., Abdennour M., et al. Working memory performance is related to intrinsic resting state functional connectivity changes in community-dwelling elderly cohort. Neurobiol. Learn. Mem. 2016;132:57–66. doi: 10.1016/j.nlm.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Chua E.F., Schacter D.L., Rand-Giovannetti E., Sperling R.A. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29(4):1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Beckmann C., Arigita E.S., Barkhof F., Scheltens P., Stam C., et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb. Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X., Biswal B.B. Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ. 2014;2:e367. doi: 10.7717/peerj.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Gómez N., López-Jurado C.F., Nadal-Delgado M., Pérez-Civantos D., Guerrero-Martín J., Cáceres M.C. Chemotherapy-Related cognitive impairment in patients with breast cancer based on functional assessment and NIRS analysis. J. Clinic. Med. 2022;11(9):2363. doi: 10.3390/jcm11092363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando S.J., Dornbush R., Lynch S., Shahar S., Klepacz L., Karmen C.L., et al. Neuropsychological, medical, and psychiatric findings after recovery from acute COVID-19: a cross-sectional Study. J. Acad. Consultation-liaison Psychiatry. 2022 doi: 10.1016/j.jaclp.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M., Carton M., Descatha A., Leclerc A., Roquelaure Y., Santin G., et al. CONSTANCES: a general prospective population-based cohort for occupational and environmental epidemiology: cohort profile. Occup. Environ. Med. 2017;74(1):66–71. doi: 10.1136/oemed-2016-103678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouraud C., Bottemanne H., Lahlou-Laforêt K., Blanchard A., Günther S., Batti S.E., et al. Association between psychological distress, cognitive complaints, and neuropsychological status after a severe Covid-19 infection: a cross-sectional study. Front. Psychiatry. 2021:1530. doi: 10.3389/fpsyt.2021.725861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkers C., Arends J., Barth R., Du Plessis S., Hoepelman A., Vink M. Review of functional MRI in HIV: effects of aging and medication. J. Neurovirol. 2017;23(1):20–32. doi: 10.1007/s13365-016-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V. Multivariate pattern analysis of fMRI: the early beginnings. Neuroimage. 2012;62(2):852–855. doi: 10.1016/j.neuroimage.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren L., Thornberg U.B., Samuelsson K., Levi R., Divanoglou A., Blystad I. Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: an observational cohort study. BMJ Open. 2021;11(10) doi: 10.1136/bmjopen-2021-055164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Zhou Z., Yang D., Zhao W., Zeng M., Xie X., et al. Persistent white matter changes in recovered COVID-19 patients at the 1-year follow-up. Brain. 2022;145(5):1830–1838. doi: 10.1093/brain/awab435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jiang L., Tang K., Levin M., Irfan O., Morris S.K., Wilson K., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20(11):e276–ee88. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo C.D., Garcia-Barrera M.A., Mazerolle E.L., Ritchie L.J., Fisk J.D., Gawryluk J.R., et al. Relationship between DTI metrics and cognitive function in Alzheimer's disease. Front. Aging Neurosci. 2019;10:436. doi: 10.3389/fnagi.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K., Johnsen S., Sattler S., Nielsen S., Kunalan K., Rungby J., et al. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz S., Gläscher J., Sommer T., Büchel C., Braus D.F. Neural correlates of memory confidence. Neuroimage. 2006;33(4):1188–1193. doi: 10.1016/j.neuroimage.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dwyer L., Lamberton F., Bokde A.L., Ewers M., Faluyi Y.O., Tanner C., et al. Multiple indices of diffusion identifies white matter damage in mild cognitive impairment and Alzheimer's disease. PLoS One. 2011;6(6):e21745. doi: 10.1371/journal.pone.0021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea A., Parakh A., Hedgire S., Lee S.I. Multisystem assessment of the imaging manifestations of coagulopathy in hospitalized patients with coronavirus disease (COVID-19) Am. J. Roentgenol. 2021;216(4):1088–1098. doi: 10.2214/AJR.20.24132. [DOI] [PubMed] [Google Scholar]
- Oehr L., Anderson J. Diffusion-tensor imaging findings and cognitive function following hospitalized mixed-mechanism mild traumatic brain injury: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2017;98(11):2308–2319. doi: 10.1016/j.apmr.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Ott C.V., Bjertrup A.J., Jensen J.H., Ullum H., Sjælland R., Purdon S.E., et al. Screening for cognitive dysfunction in unipolar depression: validation and evaluation of objective and subjective tools. J. Affect. Disord. 2016;190:607–615. doi: 10.1016/j.jad.2015.10.059. [DOI] [PubMed] [Google Scholar]
- Peng K., Steele S.C., Becerra L., Borsook D. Brodmann area 10: collating, integrating and high level processing of nociception and pain. Prog. Neurobiol. 2018;161:1–22. doi: 10.1016/j.pneurobio.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J.Z., Porter R.J., Miskowiak K.W. Clinical characteristics associated with the discrepancy between subjective and objective cognitive impairment in depression. J. Affect. Disord. 2019;246:763–774. doi: 10.1016/j.jad.2018.12.105. [DOI] [PubMed] [Google Scholar]
- Poletti S., Palladini M., Mazza M.G., De Lorenzo R., Furlan R., Ciceri F., et al. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2021:1–10. doi: 10.1007/s00406-021-01346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti S., Paolini M., Mazza M.G., Palladini M., Furlan R., Querini P.R., et al. Lower levels of glutathione in the anterior cingulate cortex associate with depressive symptoms and white matter hyperintensities in COVID-19 survivors: Glutathione in COVID-19. Eur. Neuropsychopharmacol. 2022 doi: 10.1016/j.euroneuro.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponds R.W., Jolles J. Memory complaints in elderly people: The role of memory abilities, metamemory, depression, and personality. Educational Gerontol. 1996;22(4):341–357. [Google Scholar]
- Rau A., Schroeter N., Blazhenets G., Dressing A., Walter L.I., Kellner E., et al. Widespread white matter oedema in subacute COVID-19 patients with neurological symptoms. Brain. 2022 doi: 10.1093/brain/awac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L.M., MacLullich A.M. Subjective memory complaints and cognitive impairment in older people. Dement. Geriatr. Cogn. Disord. 2006;22(5-6):471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R., Arenaza-Urquijo E.M., Valls-Pedret C., Vidal-Piñeiro D., Bargalló N., Junqué C., et al. Dynamic functional reorganizations and relationship with working memory performance in healthy aging. Front. Hum. Neurosci. 2012;6:152. doi: 10.3389/fnhum.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F., Murty V.P., Callicott J.H., Tan H-Y, Das S., Weinberger D.R., et al. Age-related alterations in default mode network: impact on working memory performance. Neurobiol. Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou T.M., Joca S., Wegener G., Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19–A systematic review. Brain Behav. Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Blasco M., Torres I.J., Vicent-Gil M., Goldberg X., Navarra-Ventura G., Aguilar E., et al. Discrepancy between objective and subjective cognition in major depressive disorder. Eur. Neuropsychopharmacol. 2019;29(1):46–56. doi: 10.1016/j.euroneuro.2018.11.1104. [DOI] [PubMed] [Google Scholar]
- Silva L.S., Joao R.B., Nogueira M.H., Aventurato I.K., de Campos B.M., de Brito M.R., et al. Functional and microstructural brain abnormalities, fatigue, and cognitive dysfunction after mild COVID-19. medRxiv. 2021 [Google Scholar]
- Smallwood J., Bernhardt B.C., Leech R., Bzdok D., Jefferies E., Margulies D.S. The default mode network in cognition: a topographical perspective. Nat. Rev. Neurosci. 2021;22(8):503–513. doi: 10.1038/s41583-021-00474-4. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Spudich S., Nath A. Nervous system consequences of COVID-19. Science. 2022;375(6578):267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M., Suttajit S., Eurviriyanukul K., Varnado P. Discrepancy between objective and subjective cognition in adults with major depressive disorder. Sci. Rep. 2017;7(1):1–7. doi: 10.1038/s41598-017-04353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek K.M., Grieve S.M., Brickman A.M., Korgaonkar M.S., Paul R.H., Cohen R.A., et al. Obesity is associated with reduced white matter integrity in otherwise healthy adults. obes. 2011;19(3):500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- Stefanou M-I, Palaiodimou L., Bakola E., Smyrnis N., Papadopoulou M., Paraskevas G.P., et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther. Adv. Chronic Dis. 2022;13 doi: 10.1177/20406223221076890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann B., Herr D., Schwindt W., Pfleiderer B. Multivariate classification of blood oxygen level–dependent fMRI data with diagnostic intention: a clinical perspective. Am. J. Neuroradiol. 2014;35(5):848–855. doi: 10.3174/ajnr.A3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.W., Helmeste D., Leonard B. Inflammatory neuropsychiatric disorders and COVID-19 neuroinflammation. Acta Neuropsychiatr. 2021;33(4):165–177. doi: 10.1017/neu.2021.13. [DOI] [PubMed] [Google Scholar]
- Tang S.W., Leonard B.E., Helmeste D.M. Long COVID, neuropsychiatric disorders, psychotropics, present and future. Acta Neuropsychiatr. 2022:1–18. doi: 10.1017/neu.2022.6. [DOI] [PubMed] [Google Scholar]
- Voruz P., Cionca A., Jacot de Alcântara I., Nuber-Champier A., Allali G., Benzakour L., et al. Functional connectivity underlying cognitive and psychiatric symptoms in post-COVID-19 syndrome: is anosognosia a key determinant? Brain Commun. 2022;4(2):fcac057. doi: 10.1093/braincomms/fcac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voruz, P., Cionca, A., Jacot, I., Nuber-Champier, A., Allali, G., Benzakour, L., et al. Brain functional connectivity alterations associated with neuropsychological post-COVID syndrome. 2022.
- Wang Y., Bernanke J., Peterson B.S., McGrath P., Stewart J., Chen Y., et al. The association between antidepressant treatment and brain connectivity in two double-blind, placebo-controlled clinical trials: a treatment mechanism study. Lancet Psychiatry. 2019;6(8):667–674. doi: 10.1016/S2215-0366(19)30179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ghosh S., Nieto-Castanon A., Saygin Z., Doehrmann O., Chai X., et al. Brain connectomics predict response to treatment in social anxiety disorder. Mol. Psychiatry. 2016;21(5):680–685. doi: 10.1038/mp.2015.109. [DOI] [PubMed] [Google Scholar]
- Winklewski P.J., Sabisz A., Naumczyk P., Jodzio K., Szurowska E., Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes—what do we know? Front. Neurol. 2018;9:92. doi: 10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1):S173–SS86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yahya N., Manan H.A. Diffusion tensor imaging indices to predict cognitive changes following adult radiotherapy. Eur. J. Cancer Care (Engl) 2021;30(1):e13329. doi: 10.1111/ecc.13329. [DOI] [PubMed] [Google Scholar]
- Yesilkaya U.H., Sen M., Balcioglu Y.H. COVID-19-related cognitive dysfunction may be associated with transient disruption in the DLPFC glutamatergic pathway. J. Clin. Neurosci. 2021;87:153–155. doi: 10.1016/j.jocn.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama O., Miura N., Watanabe J., Takemoto A., Uchida S., Sugiura M., et al. Right frontopolar cortex activity correlates with reliability of retrospective rating of confidence in short-term recognition memory performance. Neurosci. Res. 2010;68(3):199–206. doi: 10.1016/j.neures.2010.07.2041. [DOI] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. (Wien) 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chung TW-H, Wong FK-C, Hung IF-N, Mak HK-F. Changes in the intranetwork and internetwork connectivity of the default mode network and olfactory network in patients with COVID-19 and olfactory dysfunction. Brain Sci. 2022;12(4):511. doi: 10.3390/brainsci12040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Friston K.J., Zeidman P., Chen J., Li S., Razi A. The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb. Cortex. 2018;28(2):726–737. doi: 10.1093/cercor/bhx307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.