Abstract
Campylobacteriosis is a gastroenteritis caused by pathogenic Campylobacter species and an important topic in public health. Here we review the global epidemiology of campylobacteriosis in the last eight years between 2014-2021, providing comprehensive and updated information on the reported incidence and outbreaks of Campylobacter infections. The government public health website of each of the 195 countries and publications from 2014 to September 2022 in public databases were searched. The reported incidence of campylobacteriosis in pre-COVID-19 years was compared to that during the COVID-19 pandemic in countries where data were available. Czech Republic had the highest reported incidence of campylobacteriosis worldwide (215 per 100,000 in 2019), followed by Australia (146.8 per 100,000 in 2016) and New Zealand (126.1 per 100,000 in 2019). Campylobacter was one of the most common human enteric pathogens in both developed and developing countries. About 90% of cases of campylobacteriosis were caused by Campylobacter jejuni, whereas less than 10% of cases were caused by Campylobacter coli. Other Campylobacter species were also isolated. The reported incidence and case numbers of campylobacteriosis in developed nations have remained steadily high prior to the COVID-19 pandemic, whilst some countries reported an increasing trend such as France and Japan. While outbreaks were more frequently reported in some countries, Campylobacter infections were mainly sporadic cases in most of the developed countries. Campylobacter infection was more common in summer in some but not all countries. Campylobacter infection was more common in males than females. The COVID-19 pandemic has reduced the reported incidence of campylobacteriosis in most countries where 2020 epidemiology data were available. In conclusion, Campylobacter infection remains a global health concern. Increased research and improved strategies are needed for prevention and reduction of Campylobacter infection.
Keywords: campylobacteriosis, Campylobacter, Campylobacter jejuni, epidemiology, diarrhea, gastroenteritis, transmission
1 Introduction
Campylobacteriosis is a gastroenteritis caused by pathogenic Campylobacter species, which are gram-negative bacteria with a curved or spiral shape. Bacterial cells of most Campylobacter species are motile with a flagellum present at one or both ends of the bacteria, allowing them to have a corkscrew-like motion during movement (Lastovica et al., 2014). Cases of campylobacteriosis in humans are mainly caused by Campylobacter jejuni and most commonly present as gastroenteritis. Individuals suffering from Campylobacter infection often have diarrhea, fever, and abdominal pain; sometimes nausea and vomiting are also present. Illness usually resolves within two to five days but may last up to several weeks. It is usually self-limiting, with 5-10% requiring hospitalisation and a fatality rate of five in 10,000 (Galanis, 2007). In a very small number of cases (one in every 1,000), C. jejuni also causes Guillain-Barré syndrome, an autoimmune neurological disease triggered by the molecular mimicry between C. jejuni outer membrane lipooligosaccharides and human peripheral nerve gangliosides (Li et al., 2020a; Centers for Disease Control and Prevention, 2022). Campylobacteriosis is most often caused by consumption of contaminated poultry; ruminants such as cattle, sheep and goats can also be a source for human Campylobacter infection (Chlebicz and Śliżewska, 2018). Several Campylobacter species use humans as their natural host and are referred to as human hosted Campylobacter species (Liu et al., 2018). Translocation of some of the human hosted Campylobacter species such as Campylobacter concisus may cause chronic inflammatory diseases of the gastrointestinal tract (Liu et al., 2018).
Campylobacter is a common gastroenteric bacterial pathogen in humans. Given this, the global epidemiology of campylobacteriosis is an important topic in public health. Here we review the global epidemiology of campylobacteriosis in the last eight years between 2014-2021, providing comprehensive and updated information on the reported incidence and outbreaks of Campylobacter infections. The reported incidence of campylobacteriosis in pre-COVID-19 years was compared to that during the COVID-19 pandemic within individual countries where data were available.
2 Data collection
The government public health website of each of the 195 countries was searched. Data that were available in English were directly used in this review. In countries where data were presented in non-English language, the reported campylobacteriosis incidence and case numbers were obtained from English publications that were based on their government data.
Furthermore, publications on human Campylobacter infection using data collected from 2014 to September 2022 in PubMed and Web of Science were searched. Keyword combinations of country or continent name with Campylobacter, campylobacteriosis, diarrhea, enteritis, or gastroenteritis were used. The search yielded 4,766 publications. Examination of these 4,766 publications revealed that 66 publications contained data of Campylobacter outbreaks and sporadic cases between 2014 and 2021, which were included in this review. Other relevant publications were included in the discussion.
3 Epidemiology
3.1 Reported incidence and outbreaks of campylobacteriosis in countries with national surveillance data
Campylobacteriosis is a notifiable disease in countries with surveillance programs available ( Table 1 ). Most of the countries with surveillance programs provided data of both incidence and case numbers, while some only provided case numbers. The reported incidence and case numbers of campylobacteriosis in countries with surveillance programs are in Table 1 , arranged alphabetically based on continents and countries within each continent.
Table 1.
Incidence and national case numbers of campylobacteriosis between 2014-2021 in countries with national surveillance.
Age group and season with highest incidence rate. Gender: male to female ratio or incidence rate for male versus female are noted. M: male. F: female. *Disruption of seasonal pattern due to outbreak. &Notifiable in NSW since 2017. ^Only subset of cases has species confirmed. #Case numbers were from outbreaks only. NSW: New South Wales. -Information not available.
3.1.1 Asia
National surveillance data of campylobacteriosis were available in three Asian countries namely Japan, Korea and Singapore.
3.1.1.1 Japan
National data of campylobacteriosis in Japan were available for years 2014 to 2018 from the Food Poisoning Statistics, Ministry of Health, Labour and Welfare of Japan. The data were interpreted in English in related publications (Vetchapitak and Misawa, 2019; Yoshikura, 2020). The national case numbers of foodborne infection reported each year varied from 1,893 to 3,272 ( Table 1 ) (Vetchapitak and Misawa, 2019).
Campylobacteriosis in Japan was mainly acquired through outbreaks occurring in restaurants (Yoshikura, 2020). For example, in 2018, there were 278 outbreaks totalling to 1,830 cases of Campylobacter infection in Japan, of which 262 (94.2%) outbreaks were due to dining in restaurants (Yoshikura, 2020). Similar infection patterns were also seen in other years. Foodborne outbreaks caused by Campylobacters in Japan increased from 212 in year 2014 to 278 in year 2018 (Yoshikura, 2020). About 15-28% of Campylobacter infection outbreaks were related to the consumption of chicken meat (Yoshikura, 2020). For example, in 2016, a Campylobacter foodborne outbreak happened in Fukuoka city in which 266 individuals suffered from gastroenteritis due to the consumption of sushi topped with undercooked chicken meat (Asakura et al., 2017).
Gastroenteritis caused by Campylobacter species in Japan was found to be most prevalent between May and July. Males were found to have a higher incidence rate reported than females, peaking in individuals aged 10 to 20 years (Vetchapitak and Misawa, 2019).
Campylobacter was the leading bacterial enteric pathogen causing foodborne diseases in Japan, causing far more infections than other foodborne bacterial pathogens such as Salmonella and Vibrio parahaemolyticus(Vetchapitak and Misawa, 2019). For example, in 2018, 1,830 cases of foodborne infection were due to Campylobacter, while 619 cases were due to Salmonella and 218 cases due to V. parahaemolyticus(Yoshikura, 2020). A similar pattern was seen in other years.
C. jejuni was the main causative species in Campylobacter caused gastroenteritis in Japan (Vetchapitak and Misawa, 2019). However, other Campylobacter species causing human infections were also reported. A study by Hatanaka et al. examined the presence of Campylobacter in 586 stool samples collected from paediatric patients with diarrhea between 2013 and 2015. They found that 48.3% (283/586) of samples were positive for Campylobacter genus specific polymerase chain reaction (PCR), among which 51.9% (147/283) were surprisingly positive for C. ureolytius, indicating in addition to C. jejuni, emerging Campylobacter species also contribute to Campylobacter induced gastroenteritis in this area (Hatanaka et al., 2017). C. ureolyticus was previously detected in patients with inflammatory bowel disease (Zhang et al., 2009).
3.1.1.2 Korea
National data in Korea were available for years 2014-2019 from the Korea epidemiological investigation of infectious diseases annual reports (Korea Disease Control and Prevention Agency, 2015; Korea Disease Control and Prevention Agency, 2016; Korea Disease Control and Prevention Agency, 2017; Korea Disease Control and Prevention Agency, 2018; Korea Disease Control and Prevention Agency, 2019; Korea Disease Control and Prevention Agency, 2021). The annual case numbers of Campylobacter infection outbreaks in Korea varied greatly. Campylobacter infection cases were the lowest in 2017 (103 cases) and highest in 2016 (902 cases) ( Table 1 ).
Outbreaks of food and waterborne diseases caused by Campylobacter were found to peak during late spring and summer from May to August which contributed to 40-80% of all outbreaks ( Table 1 ) (Korea Disease Control and Prevention Agency, 2015; Korea Disease Control and Prevention Agency, 2016; Korea Disease Control and Prevention Agency, 2017; Korea Disease Control and Prevention Agency, 2018; Korea Disease Control and Prevention Agency, 2019; Korea Disease Control and Prevention Agency, 2021). The common places of outbreaks included workplaces, militaries, schools, and restaurants (Korea Disease Control and Prevention Agency, 2015; Korea Disease Control and Prevention Agency, 2016; Korea Disease Control and Prevention Agency, 2017; Korea Disease Control and Prevention Agency, 2018; Korea Disease Control and Prevention Agency, 2019; Korea Disease Control and Prevention Agency, 2021). For example, in 2019, 12 outbreaks were reported, among which four originated from workplaces, three from school, three from restaurants and two from militaries (Korea Disease Control and Prevention Agency, 2021).
C. jejuni was usually the third most common enteric bacterial pathogen in Korea. In 2019, the case numbers of Campylobacter, Salmonella and pathogenic E. coli infections were 270, 606 and 361, respectively (Korea Disease Control and Prevention Agency, 2021). An exception was the year 2016, where 18 outbreaks caused by Campylobacter were noted, which contributed to the highest yearly Campylobacter cases (902 cases) and made Campylobacter the leading bacterial enteric pathogen in that year ( Table 1 ) (Korea Disease Control and Prevention Agency, 2017).
3.1.1.3 Singapore
The latest data from the Communicable Diseases Surveillance in Singapore for foodborne disease was reported in 2018, in which the reported incidence of Campylobacter caused gastroenteritis was 7.6 in 100,000 population, similar to that in 2017 and 2016 which were 8.8 and 7.6 in 100,000 population, respectively ( Table 1 ) (Ministry of Health Singapore, 2015; Ministry of Health Singapore, 2016; Ministry of Health Singapore, 2017). The yearly cases between 2014-2018 were approximately 430, among which 80-85% were caused by C. jejuni, 4-7% by C. coli and 10-13% by other unspecified Campylobacter species. Children less than four years of age were found to have the highest reported incidence of Campylobacter gastroenteritis, being 71.7 in 100,000 population in 2018. There was no obvious gender pattern with the male to female ratio being 1.5:1 in 2018, 1.2:1 in 2017 and 1:1.4 in 2015. Specific information on Campylobacter infection outbreaks was not available, but 44.5% (192/431) of foodborne infection notifications were classified as outbreaks in 2016, 93.9% (214/228) in 2015, and 94.4% (284/301) in 2014, indicating outbreaks are the main form of foodborne diseases in Singapore (Ministry of Health Singapore, 2015; Ministry of Health Singapore, 2016; Ministry of Health Singapore, 2017).
Campylobacter was the second most common foodborne bacterial pathogen in Singapore following Salmonella. In 2018, the reported incidence of salmonellosis was 28.7 per 100,000 population. The reported incidence of foodborne diseases such as typhoid and paratyphoid fever were 0.8 and 0.3, respectively. There were only two imported cases of cholera; therefore the reported incidence of cholera was zero in 2018 (Ministry of Health Singapore, 2017).
A recent study examining the aetiology of acute gastroenteritis using stool specimens collected between February and October 2016 reported that Campylobacter(9.4%, 28/299) was the second leading bacterial pathogen detected, following Salmonella(19.1%, 57/299) (Koo et al., 2022). Chau et al. examined the presence of enteric pathogens in faecal samples collected from adult patients with acute diarrhea between October 2013 and January 2014 using multiplex PCR (Chau et al., 2015). They reported that the most frequently detected microorganisms were norovirus genogroup II (11%, 11/100), Aeromonas(9%, 9/100) and Campylobacter(5%, 5/100) (Chau et al., 2015).
3.1.2 Europe
Campylobacteriosis in Europe is monitored by the European Centre for Disease Prevention and Control. According to the European Union One Health 2020 Zoonoses Report, campylobacteriosis was the leading cause of human zoonoses, consisting of 120,946 cases, representing more than 60% of all reported zoonotic disease cases (European Food Safety Authority, 2021a). Other major zoonoses included salmonellosis (52,702 cases), yersiniosis (5,668) and Shiga toxin-producing E. coli(STEC) infections (4,446 cases) (European Food Safety Authority, 2021b). Subsets of the confirmed cases of campylobacteriosis had available information on Campylobacter species. In 2020, 64.7% of confirmed cases had available information on Campylobacter species, among which 88.1% were caused by C. jejuni, 10.6% caused by C. coli, and the remaining caused by C. fetus(0.16%), C. upsaliensis(0.11%), C. lari(0.09%), and other Campylobacter species (0.94%) ( Table 1 ) (European Food Safety Authority, 2021b). This Campylobacter species distribution pattern was consistent with previous years (European Food Safety Authority, 2019a; European Food Safety Authority, 2021a).
In Europe, children under five years of age contributed to approximately 13% of all confirmed cases. There was a higher rate in males than females (1.2:1) (European Centre for Disease Prevention and Control, 2018b; European Centre for Disease Prevention and Control, 2018a; European Centre for Disease Prevention and Control, 2019). A clear seasonal distribution of campylobacteriosis in Europe was seen with most cases being reported during summer months ( Table 1 ) (European Centre for Disease Prevention and Control, 2018b; European Centre for Disease Prevention and Control, 2018a; European Centre for Disease Prevention and Control, 2019).
In addition to the summer peak, a smaller but distinct winter peak was also observed in European countries including Austria, Belgium, Finland, Germany, Luxembourg, the Netherlands, Switzerland and Sweden (European Food Safety Authority, 2021a). Risk factors contributing to the winter peak included consumption of meat fondue or table-top grilling during the festive season as well as increased travel during Christmas and New Year (Bless et al., 2017). However this winter peak was not observed in Denmark, France, Ireland, Italy, Norway and the United Kingdom (Bless et al., 2017).
Sporadic cases were the main form of campylobacteriosis in Europe. In 2020, among the 120,946 cases reported, 1,319 cases were caused by 317 foodborne outbreaks, contributing to only 1% of total number of cases. Similar proportions were also observed in previous years. Among ready-to-eat food, main categories with occurrence of Campylobacter included fruit, vegetables and juices (36.6%), milk and milk products (24.2%), meat and meat products (12.9%) and salads (10.2%). Among non-ready-to-eat food, meat and meat products (79.7%) were the main contributor, followed by milk and milk products (5.4%), and fruit, vegetables and juices (3.3%).
3.1.2.1 Austria
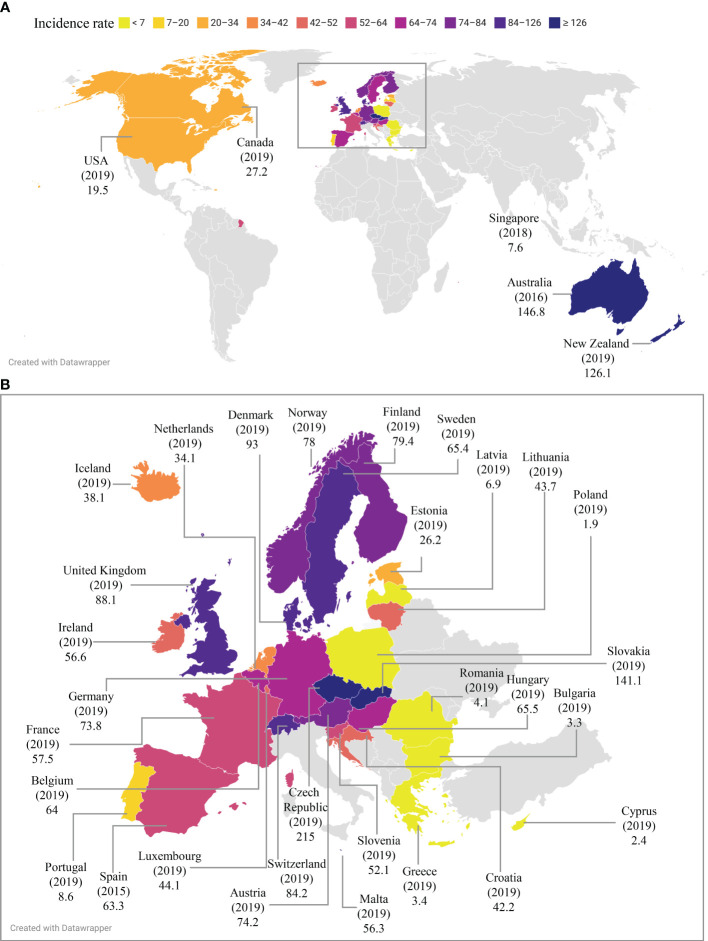
The reported incidence of campylobacteriosis in Austria was 76.6 per 100,000 in year 2014, followed by an increasing trend from 2015 to 2018 with the reported incidence being 72.9 to 90.7 per 100,000. In 2019, the reported incidence was 74.2 per 100,000, which further decreased to 60.7 per 100,000 in 2020 ( Table 1 ). Campylobacter was the leading bacterial enteric pathogen in Austria. The reported incidence of other bacterial pathogens in 2020 including Salmonella, STEC, Yersinia, and Listeria monocytogenes were 9.2, 3.2, 1.4 and 0.46 per 100,000, respectively (European Food Safety Authority, 2021b).
3.1.2.2 Belgium
The highest reported incidence of campylobacteriosis in Belgium was in year 2016, being 88.9 per 100,000 population ( Table 1 ). The reported incidence then gradually decreased and reached 48.6 per 100,000 in 2020 (European Food Safety Authority, 2021b). Campylobacter was the leading bacterial enteric pathogen in Belgium. In 2020, the reported incidence of other bacterial pathogens causing gastroenteritis including Salmonella, Yersinia, STEC, and L. monocytogenes were 13.8, 2.3, 0.73, and 0.59 per 100,000, respectively.
3.1.2.3 Bulgaria
In Bulgaria, the reported incidence of Campylobacter infection during 2014 and 2020 varied between 1.8 and 3.3 per 100,000 population, and the number of annual cases ranged between 144 and 229 ( Table 1 ) (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021a). Campylobacter is not the leading zoonotic pathogen in Bulgaria. In 2020, Salmonella was the leading bacterial pathogen with the reported incidence being 2.7 per 100,000 population, followed by Campylobacter(1.8), Trichinella(0.19), L. monocytogenes and Yersinia(0.06). A recent study examining the bacterial etiological agents causing acute diarrhea in Bulgaria between 2014 and 2018 also showed that Salmonella was the leading bacterial pathogen, causing 44% of cases examined, followed by Shigella(26%), STEC (20%), and Campylobacter(8%) (Pavlova et al., 2019).
Although the reported incidence of campylobacteriosis remained low in Bulgaria, a research study performed between February 2014 and January 2015 examining patients with diarrheal syndrome reported high detection rate of Campylobacter from their stool samples, in which 43% (40/93) of the samples examined were PCR positive for Campylobacter(Pavlova et al., 2016). Furthermore, a recent study examining the presence of Campylobacter in children with acute diarrhea under five years of age reported a high infection rate, raising concerns regarding Campylobacter infection in paediatric patients. Campylobacter was found in 37.8% (139/368) of faecal samples examined, among which 33.2% were C. jejuni and 4.6% were C. coli(Pavlova et al., 2020). Boys were found to have a significant higher positive rate than girls (64 versus 36%, respectively). Furthermore, children between three and five years of age were found to have a significantly higher infection rate than other age groups, accounting for half of the positive cases. Authors suggested that this might be due to a more varied diet in older children (Pavlova et al., 2020).
3.1.2.4 Croatia
The reported incidence of Campylobacter infection remained relative steady in Croatia, ranging from 38.8 per 100, 000 in 2016 to 42.2 in 2019. In 2020, the reported incidence dropped to 26 per 100,000, however still remained the leading bacterial enteric pathogen ( Table 1 ) (European Food Safety Authority, 2021b). The reported incidence of other enteric agents including Salmonella, Yersinia, STEC, and L. monocytogenes in the year 2020 were 19.4, 0.27, 0.20 and 0.12, respectively (European Food Safety Authority, 2021b).
3.1.2.5 Cyprus
Cyprus has a low reported incidence of campylobacteriosis, ranging from 4.7 per 100,000 in 2014 to 2.4 in 2019, further dropping to 2.0 in 2020 ( Table 1 ). Salmonella was the leading bacterial enteric pathogen in Cyprus with a reported incidence of 7.9 in 2020, followed by Campylobacter(2.0) and L. monocytogenes(0.23). No cases of STEC and Yersinia infection were reported in 2020.
3.1.2.6 Czech Republic
Czech Republic has the highest reported incidence of campylobacteriosis among all European countries. The reported incidence increased from 197.4 per 100,000 in 2014 to 230.0 in 2017, then dropped to 215.0 in 2019 before further decreasing to 163.8 in 2020 ( Table 1 ) (European Food Safety Authority, 2021b). Campylobacter was the leading bacterial enteric pathogen in Czech Republic. Additionally, its reported incidence of Salmonella infection was also the highest in Europe, being 98.4 in 2020. The reported incidence rates of L. monocytogenes, Yersinia and STEC infections in Czech Republic were 0.15, 4.1 and 0.3, respectively in 2020 (European Food Safety Authority, 2021b).
3.1.2.7 Denmark
There has been a continuous increase in Campylobacter infection in Denmark since 2012 (Statens Serum Institut, 2019). The reported incidence of campylobacteriosis in 2014 was 67.0 per 100,000 population, which increased to 82 in 2016, and to 93 in 2019 ( Table 1 ). A rapid decrease in Campylobacter infection was noted in 2020, with a reported incidence of 64.3 and the case numbers of 3,742 (National Food Institute, 2021).
The majority of the Campylobacter infections in Denmark were sporadic, in which only 5% of the cases in 2020 were due to outbreaks. In May 2020, a large outbreak consisting of 161 cases was reported on Bornholm, most likely due to consumption of a particular brand of pasteurised milk. Another two smaller outbreaks involving 20 and 18 cases due to the consumption of Danish-produced chicken and unknown causes were also reported (National Food Institute, 2021). In 2019, a total of 5,389 cases were notified, which was the highest number ever recorded (Statens Serum Institut, 2019; Joensen et al., 2021). Among those cases, 701 human clinical isolates were analysed, of which 93% (652/701) were C. jejuni and 7% were C. coli(Joensen et al., 2021). Furthermore, through whole genome sequencing and cluster analysis, it was found that one third of all clinical isolates (31%, 219/701) matched isolates from chicken meat, suggesting chicken could be a major source of foodborne outbreaks (Joensen et al., 2021).
There has been a consistent gender predominance of Campylobacter infection in Denmark, where males were more often infected than females. Elders more than 85 years of age and young adults between 20 and 29 were shown to have a high reported incidence among all age groups. Between 2016 and 2019, the highest reported incidence was found in Bornholm where more than 200 cases per 100,000 inhabitants were reported (Statens Serum Institut, 2018; Statens Serum Institut, 2019).
A prospective study conducted in 2016 among Danish subjects aged 1-30 years old identified several determinants of sporadic Campylobacter infections. Such risk factors included bathing in fresh water, contact with beach sand, owning a pet dog with diarrhea, eating minced beef or chicken, as well as foreign travel to Asia, Africa or Turkey and eating street food (Kuhn et al., 2018).
Campylobacter was the leading bacterial enteric pathogen in Denmark, followed by Salmonella, STEC, Yersinia and L. monocytogenes. The reported incidence rates of Salmonella, STEC, Yersinia and L. monocytogenes infections in 2020 were 10.5, 7.6, 7.1 and 0.76, respectively (European Food Safety Authority, 2021b).
3.1.2.8 Estonia
The reported incidence of campylobacteriosis in Estonia remained steady during the past years, ranging from 21.7 to 31.2 per 100,000 population during 2014 to 2019. In 2020, the reported incidence dropped slightly to 19.9 ( Table 1 ) (European Food Safety Authority, 2021b). Campylobacter was the leading bacterial enteric pathogen in Estonia, followed by Salmonella(6.8), L. monocytogenes(3.3), STEC (0.75), and Yersinia(0.23) (European Food Safety Authority, 2021b).
3.1.2.9 Finland
Finland had a decreasing trend of campylobacteriosis during the period between 2014 and 2019, as the reported incidence decreased from 89.7 per 100,000 population in 2014 to 79.4 in 2019, then rapidly dropped to 37.5 in 2020 ( Table 1 ) (European Food Safety Authority, 2021b). Campylobacter outbreaks were rarely reported in Finland. In 2019, two outbreaks consisting of six cases were reported, broiler meat and mixed food were found to be the source of contamination; whereas in 2018, three outbreaks were reported consisting of a total number of 19 cases; mixed food were found to be the vehicle of transmission (European Food Safety Authority, 2019b; European Food Safety Authority, 2021c).
In 2019, Finland reported the highest proportion of travel associated cases (49.2%) among all European countries, whereas in other European countries, the proportion of travel associated cases accounted to less than 26% (European Food Safety Authority, 2021b).
Campylobacter was the leading bacterial enteric pathogen in Finland, followed by Salmonella(reported incidence of 9.3 per 100,000 in 2020), Yersinia(7.0), STEC (3.2), and L. monocytogenes(1.7) (European Food Safety Authority, 2021b).
3.1.2.10 France
A continuously increasing trend of campylobacteriosis was observed in France from 2014 to 2020, with the reported incidence increasing from 45.2 per 100,000 in 2014 to 57.5 in 2019 and 58.8 in 2020 ( Table 1 ) (European Food Safety Authority, 2021b). Along with Luxembourg, France was one of the two European countries that observed an increase in the number of cases in 2020 as compared to 2019 (European Food Safety Authority, 2021b). Campylobacter was the leading bacterial enteric pathogen in France, followed by STEC (reported incidence of 21.9 in 2020), Salmonella(9.3), and L. monocytogenes(0.5) (European Food Safety Authority, 2021b).
3.1.2.11 Germany
There has been a decreasing trend of the reported incidence of campylobacteriosis in Germany from 2014 to 2020, from 87.4 per 100,000 population in 2014 to 73.8 in 2019, which further dropped to 55.8 in 2020 ( Table 1 ) (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021a). Campylobacter was the leading bacterial enteric pathogen in Germany, in which its reported incidence was far higher than other pathogens such as Salmonella(reported incidence of 10.4 in 2020), Yersinia(2.2), STEC (1.7) and L. monocytogenes(0.65) (European Food Safety Authority, 2021b).
3.1.2.12 Greece
Greece is one of the countries with a low reported incidence of campylobacteriosis in Europe. The reported incidence rates in 2018, 2019 and 2020 were 3.3. 3.4 and 2.0 per 100,000 population, respectively, with case numbers being 357, 366 and 218, respectively ( Table 1 ) (European Food Safety Authority, 2021b; European Food Safety Authority, 2021a). Salmonella infection in Greece was more common than that of Campylobacter, with the reported incidence being 3.6 in 2020, and 6.0 in 2018 and 2017. The reported incidence of infections caused by other enteric bacterial pathogens such as L. monocytogenes and STEC remained low, which were 0.2 and 0.05 in past years, respectively (European Food Safety Authority, 2021b).
A large waterborne outbreak was notified in Northern Greece in 2019 in which a total of 638 cases of gastroenteritis were recorded (Tzani et al., 2020). Eleven of these cases were examined for the presence of enteric pathogens and eight of them (8/11) were PCR positive for C. jejuni. Additionally, other pathogens including norovirus, E. coli O15 and EPEC were also identified.
3.1.2.13 Hungary
There has been a decrease in the reported incidence of campylobacteriosis in Hungary during 2014-2019, from 85.5 per 100,000 population in 2014 to 65.5 in 2019, which further dropped to 45.7 in 2020 ( Table 1 ) (European Food Safety Authority, 2021b). Salmonella was the second common enteric pathogen causing zoonotic gastroenteritis, with the reported incidence being 30.3, consisting of 2,964 cases in 2020. The reported incidence rates of other bacterial enteric pathogens including L. monocytogenes, Yersinia, and STEC were much lower, with the reported incidence in 2020 being 0.33, 0.26 and 0.08, respectively (European Food Safety Authority, 2021b).
3.1.2.14 Iceland
The rate of Campylobacter infection in Iceland has slightly decreased since 2014, with the reported incidence being 43.6 per 100,000 in 2014, 38.1 in 2019, further decreasing to 26.1 in 2020. The case numbers were 142 in 2014, 136 in 2019 and 95 in 2020, respectively ( Table 1 ) (European Food Safety Authority, 2021b). Campylobacter was the leading bacterial enteric pathogen in Iceland. The reported incidence rates of gastroenteritis caused by other zoonotic enteric bacterial pathogens including Salmonella, L. monocytogenes, STEC and Yersinia were much lower, being 8.8, 1.1, 1.1 and 0.26 per 100,000 population in 2020, respectively (European Food Safety Authority, 2021b).
3.1.2.15 Ireland
The rate of campylobacteriosis remained stable in Ireland for the course between 2014 and 2017, with the reported incidence varying between 52.8 and 58.5 per 100,000 population, with case numbers between 2,451 and 2,786 ( Table 1 ) (European Food Safety Authority, 2021b). The reported incidence increased to 63.6 per 100,000 population in 2018 (case number 3,030), but reduced to 56.6 in 2019 (case number 2,777) and further dropped to 48.7 in 2020 (case number 2,419) (European Food Safety Authority, 2021b). During 2015 to 2019 where species information was available for subsets of Campylobacter isolates, 86-93% were C. jejuni, 6-12% were C. coli, whilst the remaining were other Campylobacter species (Health Protection Surveillance Centre, 2018; Health Protection Surveillance Centre, 2019; Brehony et al., 2021; Health Protection Surveillance Centre, 2021).
Most of the Campylobacter infections in Ireland were sporadic, with only six outbreaks notified in 2017 and five outbreaks in 2018, consisting of 28 and 19 cases, respectively (Health Protection Surveillance Centre, 2018; Health Protection Surveillance Centre, 2019). Known routes of transmission contributing to such outbreaks included foodborne, waterborne, person-to-person or animal contact (Health Protection Surveillance Centre, 2018; Health Protection Surveillance Centre, 2019). In Ireland, the age group of 0-4 years was consistently found to have the highest reported incidence rate (Health Protection Surveillance Centre, 2018; Health Protection Surveillance Centre, 2019; Health Protection Surveillance Centre, 2021). There was also a well-documented seasonal distribution with an early summer peak observed every year.
Campylobacteriosis was the most common form of bacterial gastroenteritis in Ireland (Health Protection Surveillance Centre, 2019). The reported incidence rates of other bacterial pathogens including STEC, Salmonella, and L. monocytogenes were much lower, being 14.8, 4.3 and 0.12 in 2020, respectively (European Food Safety Authority, 2021b).
3.1.2.16 Italy
According to the European Union One Health 2020 Zoonoses Report, the information of reported incidence was not available due to the lack of information on population coverage. The number of notified Campylobacter cases in Italy between 2014 and 2020 varied between 1,060 and 1,633 ( Table 1 ) (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021b; European Food Safety Authority, 2021a).
A recent study by Stanyevic et al. examined the evolving epidemiology on acute gastroenteritis in hospitalised children in Italy. Among the 74 stool samples from 2019 subjected for examination of aetiological agents, 8.1% (6/74) was positive for C. jejuni, similar to that from 2012 (12.5%, 8/64) (Stanyevic et al., 2021). Other less prevalent enteric bacterial pathogens reported in 2019 included non-Typhi Salmonella(4/74), Yersinia enterocolitica(1/74) and E. coli(1/74). Adenovirus (9/74), rotavirus (4/74), and norovirus (4/74) were the leading enteric viral pathogens causing paediatric gastroenteritis (Stanyevic et al., 2021). A similar detection rate of Campylobacter was reported by another study performed between 2018 and 2020 in which among stool samples from 2,066 children with severe acute gastroenteritis, 9.21% were positive for Campylobacter, whereas enteropathogenic E. coli(EPEC) (19.14%), Clostridioides difficile(14.42%), norovirus (10.36%) and enterovirus (9.44%) were detected more frequently (De Conto et al., 2022). In 2018, Sorgentone et al. reported a large foodborne outbreak in kindergarteners and primary schools in Pescara, Italy (Sorgentone et al., 2021). In this study, a total of 224 human stools were collected; 40.6% (91/224) were positive for Campylobacter using real-time polymerase chain reaction (rt-PCR), and 62 C. jejuni strains were isolated from 60 patients. An investigation of possible causes suggested a failure in the pasteurisation process of the milk used for cheese.
3.1.2.17 Latvia
Although Latvia was one of the European countries with a low reported incidence of Campylobacter infection, there has been an increasing trend over the period between 2014 and 2020 ( Table 1 ). The reported incidence in 2014 and 2015 was 1.8 and 3.7 per 100,000 population, respectively, whereas in 2019 and 2020 was 6.9 and 5.5, respectively. The case numbers increased from 37 in 2014 to 133 in 2019 and 104 in 2020 (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021b; European Food Safety Authority, 2021a). A study examining the presence of Campylobacter species in sporadic gastroenteritis cases during 2015 and 2016 reported low isolation rates of Campylobacter species, in which C. jejuni and C. coli were isolated from 5% (23/434) and 0.2% (1/434) of human stool samples, respectively ( Table 2 ) (Meistere et al., 2019).
Table 2.
Campylobacter infection in countries without national surveillance program.
| Country | Year | Region | Campylobacter | C. jejuni | C. coli | Other Campylobacter species | Peak age(range) | Gender | Season | Method | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | |||||||||||
| Egypt | 2019 | Beni-Suef | 48% (25) | Isolation & PCR | (Zeinhom et al., 2021) | ||||||
| 2018 | Cairo, Giza, Fayoum and Qalyubia | 38.09% (105) | 35.2% (105) | 2.9% (105) | PCR | (Barakat et al., 2020) | |||||
| 2017-2018 | Sharkia | 30% (100) | Isolation & PCR | (Ammar et al., 2020) | |||||||
| 2017-2018 | Ismailia | 31.2% (80) | (Abdelmageed et al., 2021) | ||||||||
| 2015-2018 | Zagazig | 4.1% (270) | Isolation & PCR | (Abd El-Hamid et al., 2019) | |||||||
| 2015-2016 | Cairo, Giza, Fayoum, Qalubia & Minya | 17.33% (75) | PCR | (Ghoneim et al., 2020) | |||||||
| 2013-2014 | Fayuom, Cairo, Qaluobia, Bin-suef and Assuit | 21.5% (93) | Isolation & PCR | (Elgabry et al., 2016) | |||||||
| 2013-2014 | Mansoura | 7.84% (102) | (18-50y) | PCR | (Ramadan et al., 2015) | ||||||
| 2013-2014 | Zagazig | 4.1% (246) | Isolation & rtPCR | (Ahmed et al., 2015) | |||||||
| Ethiopia | 2019 | Arba Minch | 4.4% (180) | (≥15y) | Isolation | (Ayele et al., 2020)# | |||||
| Ghana | 2015-2016 | Accra | 50.7% (140) | 50.7% (140) | 31-50y (20-80y) |
Female | Isolation & PCR | (Forson et al., 2020)# | |||
| Madagascar | 2011-2014 | Moramanga and Antananarivo | 1% (199) | (<5y) | Isolation | (Randremanana et al., 2016)^ | |||||
| Malawi | 2012-2015 | Blantyre | 16.5% (684) | (<5y) | rtPCR | (Iturriza-Gómara et al., 2019)^ | |||||
| South Sudan | 2017 | United Kingdom military personnel in South Sudan | 0.8% (127) | (18->45y) | PCR | (Biswas et al., 2019)& | |||||
| Asia | |||||||||||
| Bangladesh | 2019-2020 | Mymensingh | 31.5% (330) | 21.8% (330) | 9.6% (330) | 0-5y (0->60y) |
Female | Isolation & PCR | (Rahman et al., 2021) | ||
| 2019-2020 | Dhaka | 3.6% (2135) | (≥5y) | Isolation | (Garbern et al., 2021) | ||||||
| China | 2019 | Beijing | 85.7% (12/14) | rtPCR | (Li et al., 2020b)* | ||||||
| 2017-2019 | Wenzhou | 10.5% (850) | 9.3% (850) | 1.2% (850) | (6m-91y) | Isolation | (Zhang et al., 2020a) | ||||
| 2009-2018 | 31 provinces | 0.87% (90391) | 0.15% (89049) | 1-17y (<5->60y) |
Female | Spring | Isolation | (Wang et al., 2021) | |||
| 2017-2018 | Beijing | 7.8% (2945) | 6–17y (<5->65y) |
Male | Autumn | Isolation | (Zhang et al., 2020b) | ||||
| 2018 | Beijing | 90.0% (11) | (15-40y) | rtPCR | (Qu et al., 2019)* | ||||||
| 2018 | Hangzhou | 55.6% (27) | Isolation | (Yu et al., 2020)* | |||||||
| 2016-2017 | Beijing | 7% (370) | 6.5% (370) | 0.5% (370) | 76–87y (15-87y) |
Male | Winter | Isolation | (Li et al., 2018) | ||
| 2012-2016 | Shanghai | 1.2% (8797) | 1.1% (8797) | 0.08% (8797) | (18->67) | Isolation | (Gong et al., 2018) | ||||
| 2014-2015 | Shenzhen | 4.9% (412) | 5-9y (1m-78y) |
Isolation | (Shen et al., 2016) | ||||||
| 2014-2015 | Wuhan | 2.9% (381) | 2-5y (<5y) |
Isolation | (Zhu et al., 2016)^ | ||||||
| 2011-2014 | Henan | 0.13% (755) | (<5y) | Isolation | (Wang et al., 2015)^ | ||||||
| 2010-2014 | Southeast | 0.0003% (3175) | (<5->65y) | Isolation | (Chen et al., 2019) | ||||||
| 2009-2014 | Mainland | 0.5% (5967) | (>65y) | Isolation | (Zhang et al., 2017) | ||||||
| 2009-2014 | Zhejiang | 0.4% (2318) | <1y (<5y) |
Summer | Isolation | (Zheng et al., 2016)^ | |||||
| India | 2019-2020 | Vellore | 12% (400) | 4.3% (400) | 2.5% (400) | 31-40y (0-80y) |
Isolation & rtPCR | (Lakshmi Ss et al., 2022) | |||
| 2016-2017 | Odisha | 16.77% (310) | 2-5y (0->5y) |
Female | PCR | (Mohakud et al., 2019)^ | |||||
| 2014-2016 | Northeast | 10.1% (407) | 8.1% (407) | <2y (<5y) |
Male | Summer | PCR | (Borkakoty et al., 2020)^ | |||
| Iran | 2017 | Mazandaran | 27% (74) | Isolation & PCR | (Divsalar et al., 2019) | ||||||
| 2016 | East Azerbaijan | 35.4% (223) | 27.8% (223) | 10.8% (223) | 18-30y (18-70y) |
Male | Isolation & PCR | (Ranjbar et al., 2017) | |||
| 2015 | Central Iran | 33% (230) | 1-3y (<1-10y) |
Male | PCR | (Abbasi et al., 2019)^ | |||||
| 2014-2015 | Semnan | 8.6% (419) | 6-12y (<2->6y) |
Female | Isolation | (Mazaheri et al., 2016)^ | |||||
| 2013-2014 | Hamadan | 10% (120) | (<10y) | Isolation & PCR | (Rastyani et al., 2015)^ | ||||||
| 2012-2014 | Tehran | 3.6% (980) | 3.4% (980) | 0.2% (980) | Summer | Isolation & PCR | (Shams et al., 2017)^ | ||||
| Iraq | 2017 | Thi-Qar | 10.9% (155) | (<5y) | PCR | (Harb et al., 2019)^ | |||||
| Lebanon | 2018 | South | 12% (291) | >5y (1m-12y) |
Male | Isolation | (Ghssein et al., 2021)^ | ||||
| 2016-2017 | 8 districts | 21.5% (1000) | 17.9% (1000) | 3% (1000) | <12 (<12->65) |
Male | Summer | Isolation & PCR | (Ibrahim et al., 2019) | ||
| Nepal | 2017-2018 | Kathmandu | 56.8% (303) | <6m (<5y) |
Isolation | (Bhattarai et al., 2020)^ | |||||
| 2011-2014 | International travelers to Nepal | 16% (480) | 2.7% (480) |
C. concisus
31.3% (83); C. ureolyticus 7.2% (83) |
PCR | (Serichantalergs et al., 2017)& | |||||
| 2012-2014 | International travelers to Nepal | 20% (433) | Isolation & rtPCR | (Murphy et al., 2019)& | |||||||
| Pakistan | 2014-2015 | Rawalpindi, Islamabad, Lahore, Peshawar, Khairpur, Mardan and Nowshera | 54.6% (150) | Isolation & PCR | (Noreen et al., 2020)^ | ||||||
| 2014 | Rawalpindi & Islamabad | 52% (500) | 48.2% (500) | 6-11m | Male | Summer | rtPCR | (Sadiq et al., 2019a)^ | |||
| Thailand | 2016-2018 | Nationwide | 10.8% (370) | <5y | Isolation & rtPCR | (Okada et al., 2020) | |||||
| 2013-2017 | United States military personnel in Thailand | 43.8% (248) | 25% (48) | 18.8% (48) | Isolation, rtPCR & ELISA | (Lurchachaiwong et al., 2020)& | |||||
| 2012-2014 | International travelers to Thailand | 31.2% (154) | rtPCR | (Lertsethtakarn et al., 2018)& | |||||||
| 2011-2014 | International travelers to Thailand | 24.9% (173) | 5.2% (173) |
C. concisus
11.5% (26); C. ureolyticus 7.7% (26) |
PCR | (Serichantalergs et al., 2017)& | |||||
| United Arab Emirates | 2017-2019 | Al Ain | 1.9% (203) | (<5y) | rtPCR | (Alsuwaidi et al., 2021)^ | |||||
| Oceania | |||||||||||
| Papua New Guinea | 2013-2014 | Hela, Eastern Highlands, Madang and Central Provinces | 33.1% (118) | (1m-69y) | rtPCR | (Abdad et al., 2020) | |||||
| Europe | |||||||||||
| North Macedonia | 2016-2017 | Skopje | 2.5% (3820) | 2.2% (3820) | 0.3% (3820) | <15y (<15->50y) |
Isolation | (Trajkovska-Dokic et al., 2019) | |||
| South America | |||||||||||
| Colombia | 2013-2014 | Bucaramanga | 3.5% (431) | (<5y) | Isolation | (Farfán-García et al., 2020)^ | |||||
Gender and season with highest isolation or detection rate were noted. Age group with highest isolation or detection rate (range of ages included in the study). M: month. Y: year. Incidence: expressed as percentage (total number of cases). *Outbreak. ^Pediatric studies. &Traveler’s diarrhea. #HIV patients with diarrhea. If multiple methods were used for detection, the higher detection rate is noted. Studies of sample size less than 10 were excluded. EIA: enzyme immunochromatographic assay. PCR: polymerase chain reaction. rtPCR: real-time polymerase chain reaction.
Campylobacter was the second leading bacterial enteric pathogen in Latvia, following Salmonella(European Food Safety Authority, 2021b). The reported incidence of salmonellosis in 2020 was 15.5, consisting of 296 cases. Other less prevalent enteric bacterial pathogens such as Yersinia and STEC had reported incidence rates of 4.6 and 0.1, respectively in 2020 (European Food Safety Authority, 2021b).
3.1.2.18 Lithuania
The rate of campylobacteriosis in Lithuania remained mostly stable during 2014 to 2020, with the reported incidence being 40.2 per 100,000 individuals in 2014 and 42.3 in 2020 ( Table 1 ). A decrease in reported incidence was observed in years between 2017 and 2018, with the reported incidence being 34.8 and 32.7, respectively (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021b; European Food Safety Authority, 2021a). Infections caused by other enteric pathogens such as Salmonella and Yersinia had a much lower reported incidence as compared to Campylobacter, namely 17.8 and 4.9, respectively in 2020 (European Food Safety Authority, 2021b).
3.1.2.19 Luxembourg
Between 2014 and 2020, the reported incidence of campylobacteriosis in Luxembourg greatly fluctuated from 45.1 per 100,000 individuals in 2015, to more than double (103.8) in 2017 and 2018 ( Table 1 ) (European Centre for Disease Prevention and Control, 2019). There was a sharp decrease in the reported incidence (44.1) in 2019 which was due to a surveillance artefact caused by a change in diagnostic methods in private laboratories, resulting in reduced numbers of isolates sent to the national reference laboratory (European Food Safety Authority, 2021a). An electronic laboratory notification system was established in March 2020, and the reported incidence rate in 2020 (116.4) increased back to the level that was comparable to that in 2018 and 2017 (European Food Safety Authority, 2021b).
Campylobacter was the leading enteric pathogen in Luxembourg. The reported incidence rates of enteric infections caused by Salmonella, Yersinia, and L. monocytogenes were much lower, being 14.9, 4.2 and 0.64, respectively, in 2020 (European Food Safety Authority, 2021b).
3.1.2.20 Malta
The trend in Campylobacter infection showed variations in Malta between 2014 and 2020, with the incidence varied between 40.4 and 70.0 per 100,000 individuals, and case numbers varied between 206 and 333 (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021b; European Food Safety Authority, 2021a). Campylobacter was the leading bacterial pathogen causing gastroenteritis in Malta, followed by Salmonella and L. monocytogenes, with the reported incidence rates being 34.2 and 0.97 in 2020, respectively (European Food Safety Authority, 2021b).
3.1.2.21 Netherlands
The reported incidence of campylobacteriosis in Netherlands was on a continuous decreasing trend, from 47.5 per 100,000 population in 2014, to 38.3 in 2016, further dropping to 34.1 in 2019 and 25.2 in 2020 ( Table 1 ) (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021b; European Food Safety Authority, 2021a). Other less prevalent bacterial pathogens including Salmonella, STEC, and L. monocytogenes had reported incidence rates of 6.2, 1.9 and 0.52 in 2020, respectively (European Food Safety Authority, 2021b).
3.1.2.22 Norway
There has been an increase in Campylobacter infections in Norway in the past years, from 44.9 per 100,000 population in 2015 to 73.9 in 2017 and 78.0 in 2019 ( Table 1 ). In 2019, Norway recorded a total of 4,154 cases, which was the highest level in the past decade (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021a). There was a sharp drop in reported Campylobacter case numbers (2,422 cases) and incidence (45.1) in 2020 (European Food Safety Authority, 2021b).
A large outbreak of gastroenteritis was notified in Askøy on 6th June 2019 (Hyllestad et al., 2020). C. jejuni isolates from 24 clinical samples and four water samples showed identical core genome multilocus sequence typing profiles, indicating the water supply as the source of the outbreak. Another study examining hospitalised individuals due to the same water outbreak showed that among 59 patients who had fecal Campylobacter tests taken, all tested positive for C. jejuni, either by bacterial cultivation or PCR (Mortensen et al., 2021). Contamination through cracks in the water reservoir most likely had occurred during heavy rainfall, indicating the importance of water safety planning and risk assessment (Hyllestad et al., 2020). A recent publication suggested the use of coagulation and UV radiation as treatments to improve water quality in areas where surface water is used as a source for drinking water production (Herrador et al., 2021).
Campylobacter was the leading bacterial enteric pathogen causing gastroenteritis in Norway, followed by Salmonella, STEC, Yersinia and L. monocytogenes, with the reported incidence being 8.2, 6.2, 1.5 and 0.69, respectively in 2020 (European Food Safety Authority, 2021b).
3.1.2.23 Poland
The reported incidence of campylobacteriosis in Poland was low, varying between 1.1 and 2.3 per 100,000 population, in the years between 2014 and 2020 ( Table 1 ) (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021b; European Food Safety Authority, 2021a). Despite the low reported incidence, Poland had a high hospitalisation rate with 76.6% of all reported cases in 2020 were hospitalised (European Food Safety Authority, 2021b). C. jejuni was the leading Campylobacter species causing campylobacteriosis in Poland. In 2015 and 2016 where species information was available for subsets of cases, C. jejuni accounted for 96% and 93% of cases examined, while C. coli accounted for 4% and 7%, respectively (Radziszewski et al., 2018). Males were more often infected than females. The age group of 0-4 years was consistently found to be most frequently infected, accounting for more than 70% of all reported cases.
Salmonellosis was more common than campylobacteriosis in Poland which had a reported incidence of 13.7 per 100,000 population in 2020. Other less common enteric bacterial pathogens such as Yersinia and STEC had a reported incidence of 0.23 and 0.01, respectively in 2020 (European Food Safety Authority, 2021b).
3.1.2.24 Portugal
Since 2015, the reported incidence of campylobacteriosis in Portugal showed an increasing trend, from 2.6 in 2015 to 5.8 per 100,000 population in 2017, and further increased to 8.6 in 2019 ( Table 1 ). However, in 2020, the reported incidence dropped slightly to 7.7. Much lower reported incidence rates of diarrheal disease caused by other bacterial pathogens including Salmonella, L. monocytogenes, Yersinia and STEC were reported in 2020, being 2.5, 0.46, 0.24 and 0.05 per 100, 000 population, respectively (European Food Safety Authority, 2021b).
3.1.2.25 Romania
The reported incidence of campylobacteriosis was low in Romania but showed an increasing trend, from 1.3 per 100,000 population in 2014, to 2.4 in 2017, and further increased to 4.1 in 2019 ( Table 1 ) (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021b; European Food Safety Authority, 2021a). There was a drop of Campylobacter infection in 2020 in which the reported incidence was 1.6 (European Food Safety Authority, 2021b).
Salmonella was the leading bacterial enteric pathogen causing gastroenteritis in Romania nationally, with the reported incidence varying between 5.9 and 7.6 in years between 2014 and 2019. In 2020, the reported incidence of salmonellosis dropped to 2.1. The reported incidence rates of gastroenteritis caused by other bacterial pathogens including STEC, Yersinia and L. monocytogenes were extremely low, being 0.07, 0.03 and 0.01 per 100,000 population in 2020, respectively (European Food Safety Authority, 2021b).
A study conducted between 2012 and 2016 showed that Campylobacter was the leading enteric pathogen causing acute bacterial gastroenteritis in children in north-eastern urban and rural regions of Romania (Chiriac et al., 2017). Among the 615 cases involved in the study, 69.6% of cases (428/615) were caused by Campylobacter, followed by Salmonella(67/615), E. coli(29/615), Shigella(12/615) and Y. enterocolitica(3/615) (Chiriac et al., 2017).
3.1.2.26 Slovakia
The rate of Campylobacter infection in Slovakia was reported to be high and on an upward trajectory. The reported incidence increased from 124.5 per 100,000 population in 2014, to 127.8 in 2017, and further rose to 141.1 in 2019 ( Table 1 ) (European Centre for Disease Prevention and Control, 2019; European Food Safety Authority, 2021a). There has been a rapid decrease of Campylobacter infection in 2020, with the reported incidence being 90.2 per 100,000 population (European Food Safety Authority, 2021b). In addition to campylobacteriosis, Slovakia also had a high reported incidence of salmonellosis, namely 62.1 per 100,000 in 2020. The reported incidence rates of gastroenteritis caused by Yersinia, L. monocytogenes and STEC were much lower, being 3.1, 0.13 and 0.02 in 2020, respectively (European Food Safety Authority, 2021b).
3.1.2.27 Slovenia
The highest reported incidence of campylobacteriosis in Slovenia was observed in 2016 (79.5 per 100,000 population). Since then, there has been a decreasing trend, the reported incidence dropped to 68.2 in 2017, 52.1 in 2019 and rapidly decreased to 38.7 in 2020 ( Table 1 ). Campylobacter was the leading bacterial enteric pathogen causing gastroenteritis in Slovenia. The reported incidence of gastroenteritis caused by other bacterial pathogens including STEC, L. monocytogenes, Yersinia, and Salmonella varied between 1.02 and 1.4 in 2020 (European Food Safety Authority, 2021b).
3.1.2.28 Spain
Although the reported incidence for campylobacteriosis has not been available in Spain since 2016, there has been a continuous increase in the number of cases notified during the past decade, from 11,481 cases in 2014, to 13,227 cases in 2015 and to 18,411 cases in 2018 ( Table 1 ) (European Centre for Disease Prevention and Control, 2018a). In 2019 and 2020, 9,723 and 6,891 cases were reported, respectively. However, Spain did not receive data from all regions due to COVID-19 in 2019, therefore cases reported were lower than expected (European Food Safety Authority, 2021a). A recent study reported that in Madrid, Spain, the cases of Campylobacter infections reduced from 1,308 in 2019 to 391 in 2020, and Salmonella infections decreased from 462 in 2019 to 111 in 2020 due to social distancing and reduced tourism during the COVID-19 pandemic (De Miguel Buckley et al., 2020).
Ena et al. examined the epidemiology of patients with severe acute diarrhea between November 2016 and October 2018 (Ena et al., 2019). A total of 132 patients with acute diarrhea who required hospital admission were studied and Campylobacter was found to be the most frequently identified enteric pathogen (24/132), followed by Clostridioides difficile(20/132), Salmonella(20/132) and rotavirus (12/132) (Ena et al., 2019).
3.1.2.29 Sweden
Sweden showed a conspicuous decreasing trend of campylobacteriosis during the period of 2016 to 2020, with the reported incidence dropping from 111.9 per 100,000 population in 2016, to 65.4 in 2019 and 33.3 in 2020 ( Table 1 ) (European Food Safety Authority, 2021b). In 2019, a total of 6,693 cases were reported, among which 44% (2,865) cases were domestically acquired (National Veterinary Institute, 2020). A total of 137 isolates were collected from domestic cases, among which all but one were C. jejuni. The domestic incidence was higher among adults than children, and males (56%) had a higher incidence rate reported than females (National Veterinary Institute, 2020).
Publications on Campylobacter outbreaks in Sweden were rare. One Campylobacter outbreak was reported in 2014 in the South Western part of Sweden where 11 cases were identified (Lahti et al., 2017). C. jejuni was isolated from eight of the 11 cases examined and genomic analysis confirmed that human and cattle isolates of C. jejuni belonged to the same cluster, indicating cattle were the source of infection. The most likely route of transmission was found to be consumption of unpasteurised milk from the farm.
Gastroenteritis caused by other enteric pathogens including Salmonella, STEC, Yersinia and L. monocytogenes were less common, the reported incidence rates were 8.0, 4.8, 2.1 and 0.85 in 2020, respectively (European Food Safety Authority, 2021b).
3.1.2.30 Switzerland
The trend of campylobacteriosis in Switzerland fluctuated in the years between 2014 and 2019, with the reported incidence ranging between 84.2 and 95.4 per 100,000 population ( Table 1 ) (European Food Safety Authority, 2019a; European Food Safety Authority, 2021b). The reported incidence dropped to 71.7 in 2020. Campylobacter was the leading bacterial enteric pathogen causing gastroenteritis in Switzerland. The reported incidence of bacterial gastroenteritis caused by less prevalent pathogens including Salmonella, STEC and L. monocytogenes were 14.7, 8.4 and 0.67 per 100,000 population in 2020, respectively (European Food Safety Authority, 2021b). A study by Gosert et al. examined the detection of viral and bacterial pathogens from 677 stool samples collected from 504 patients with acute gastroenteritis between 2013 and 2015, and found that rotavirus (126/677), norovirus (82/677) and enterovirus (36/677) were the most prevalent viral pathogens, whilst Clostridium difficile(39/677) and Campylobacter(16/677) were the most prevalent bacterial pathogens (Gosert et al., 2018).
3.1.2.31 United Kingdom
In 2019, a total of 58,718 cases of campylobacteriosis were reported in United Kingdom, with the reported incidence being 88.1 per 100,000 population, which was the lowest level in this country since 2014 ( Table 1 ) (European Centre for Disease Prevention and Control, 2016; European Food Safety Authority, 2021a). Data for 2020 were not available in the European Union One Health 2020 Zoonoses Report due to the withdrawal of the United Kingdom from the European Union. Campylobacter was the leading bacterial enteric pathogen causing gastroenteritis in the United Kingdom, followed by Salmonella, STEC, Yersinia and L. monocytogenes whereby the reported incidence rates were 14.6, 2.4, 0.24 and 0.23 in 2019, respectively (European Food Safety Authority, 2021b).
Campylobacteriosis was the most commonly reported gastrointestinal infection in England and Wales with 56,729 cases reported in 2017 (Public Health England, 2018). The highest number of confirmed cases were found in the age group of 50-59 years old. Summer months including July and August were consistently shown to have the highest rate of infection (Public Health England, 2018). Hotels, schools, pubs and farms were settings where foodborne outbreaks frequently occur, and chicken liver containing dishes and raw drinking milks were causes for most of the foodborne outbreaks (Public Health England, 2018). During the Christmas period in 2016, a foodborne outbreak was reported in a hotel in North Yorkshire, England, of which 19 cases were identified, seven of them being positive for Campylobacter(Wensley et al., 2020). Chicken liver pâté was the food item that was most strongly associated with the disease, possibly due to inadequate cooking during the busy holiday period. In December 2016, an outbreak of campylobacteriosis was reported in North West England which affected 69 individuals. Further investigations found that the outbreak was associated with consumption of unpasteurised milk from a farm which was predominantly sold from a vending machine (Kenyon et al., 2020).
3.1.3 Oceania
National surveillance of campylobacteriosis is available in Australia and New Zealand in Oceania.
3.1.3.1 Australia
Data of campylobacteriosis in Australia were available for years 2014, 2015 and 2016, monitored by the National Notifiable Diseases Surveillance System and OzFoodNet.
There were 29,931, 22,573 and 24.164 cases of gastroenteritis caused by Campylobacters in Australia in 2014, 2015 and 2016, respectively (NNDSS Annual Report Working Group, 2019; Bell et al., 2021; NNDSS Annual Report Working Group, 2021). The reported incidence increased from 124.1 per 100,000 population in 2014 to 146.8 in 2016 ( Table 1 ).
Although foodborne outbreaks were reported, Campylobacter infections in Australia were mainly sporadic. A total of 84 Campylobacter outbreaks were identified between 2001 and 2016 consisting of 1042 cases, among which 51 outbreaks were due to the consumption of contaminated food (Moffatt et al., 2020). Among foodborne outbreaks that had specific food vehicles identified (33/51), 85% (28/33) cases were due to chicken or chicken-containing dishes, and a significant higher proportion was due to poultry-liver containing dishes (11/28) (Moffatt et al., 2020). In addition to foodborne outbreaks, the remaining cases were caused via contaminated water (6%), transmission from animal to person (2.4%), person to person (3.6%), or unknown sources (27.4%) (Moffatt et al., 2020). Similar conclusions were drawn from a recent meta-analysis on sporadic campylobacteriosis in Australia and New Zealand between 1990 and 2017, in which consuming undercooked poultry and poultry cooked outside home, having pet chickens and overseas travel were considered relevant risk factors for sporadic campylobacteriosis (Varrone et al., 2020). Approximately 28% of Campylobacter infections in Australia were detected in summer, although they often occurred in other seasons as well. Males were more commonly affected than females with a male to female ratio of 1.2:1 (NNDSS Annual Report Working Group, 2019; Bell et al., 2021; NNDSS Annual Report Working Group, 2021)
The data of campylobacteriosis notification in New South Wales (NSW), a state of Australia, were available for the year 2018, with the reported incidence being 113.54 per 100,000 population ( Table 1 ) (Communicable Diseases Branch, 2019).
Campylobacter was the leading bacterial enteric pathogen in Australia. In 2016, 49,885 notifications of gastrointestinal disease were reported, among which 24,164 were campylobacteriosis, followed by salmonellosis (18,088), shigellosis (1,406) and STEC (340) (NNDSS Annual Report Working Group, 2021). A recent study reported the isolation of enteric pathogens during 2015 and 2019 in a large diagnostic laboratory in NSW, reporting that the total positive isolates of Campylobacters from stool samples of patients with gastroenteritis during 2015 and 2019 was 11,597, followed by non-Typhi Salmonella and Aeromonas species, being 5,190 and 2,132, respectively (Yuwono et al., 2021).
3.1.3.2 New Zealand
There has been a decreasing trend of Campylobacter infection in New Zealand between 2014 and 2019. The reported incidence decreased from 150.3 per 100,000 population in 2014, to 135.2 in 2017 and further dropped to 126.1 in 2019; the case numbers decreased from 6,776 in 2014, to 6,482 in 2017 and 6,202 in 2019 ( Table 1 ) (The Institute of Environmental Science and Research Ltd, 2020; The Institute of Environmental Science and Research Ltd, 2021).
Only a minority of Campylobacter infections were due to outbreaks. In 2019, a total of 20 Campylobacter outbreaks were reported, amounting to 156 cases. The sources that caused Campylobacter outbreaks were not available, however, major risk factors causing campylobacteriosis were the consumption of contaminated food from retail premises, contacts with farm animals and consumption of contaminated water (Jeffs et al., 2019a). Similar risk factors were also identified in preceding years (The Institute of Environmental Science and Research Ltd, 2015; The Institute of Environmental Science and Research Ltd, 2016; The Institute of Environmental Science and Research Ltd, 2017; The Institute of Environmental Science and Research Ltd, 2019; The Institute of Environmental Science and Research Ltd, 2020).
Individuals in the age group of 0-4 years were found to have the highest reported incidence of Campylobacter infection compared to other age groups, being 242.7 per 100,000 individuals ( Table 1 ). Furthermore, males (reported incidence of 146.7 per 100,000) were found to have a higher incidence rate than females (reported incidence of 106.1 per 100,000). There was a distinct seasonal pattern where the highest notification rate was found during early summer. This age, gender and seasonal distribution of campylobacteriosis was consistent with previous years except for 2016 where a large outbreak involving 964 cases occurred in Hawke’s Bay, contrary to the established summer peak pattern (The Institute of Environmental Science and Research Ltd, 2017). The outbreak was found to be due to the consumption of contaminated drinking water supplied by two bores on the outskirts of Havelock North (Institute of Environmental Science and Research Ltd, 2018).
Campylobacter was the leading bacterial enteric pathogen in New Zealand. The reported incidence of other enteric diseases including salmonellosis, yersiniosis, STEC infection and shigellosis in 2019 were 24.2, 24.1, 22.4 and 4.5 per 100,000 population, respectively, much lower than that of campylobacteriosis (The Institute of Environmental Science and Research Ltd, 2021).
Campylobacter is a major pathogen causing paediatric gastroenteritis in New Zealand. An observational study from 1997 to 2015 examining non-viral gastroenteritis in the paediatric population less than 15 years of age showed that Campylobacter was the most frequently notified pathogen, contributing to 51.7% of notified cases and 43.4% of hospitalisations (Jeffs et al., 2019b). Other major notified enteric pathogens included Giardia, Cryptosporidium, non-Typhi Salmonella and Yersinia. Most of the Campylobacter infections in New Zealand were caused by C. jejuni. Nohra et al. reported that between 2005 and 2014, among the 1,601 Campylobacter clinical isolates examined, 96% (1,552/1,601) were C jejuni, and only 2.9% (47/1,601) were C. coli(Nohra et al., 2016).
3.1.4 North America
United States and Canada are the only two countries with national surveillance available for campylobacteriosis.
3.1.4.1 Canada
Campylobacteriosis is monitored by The Public Health Agency of Canada’s FoodNet Canada surveillance system. The reported incidence of campylobacteriosis remained stable in Canada, ranging between 25.4 and 28.6 per 100,000 population in the years between 2014 and 2019 ( Table 1 ) (Public Health Agency of Canada, 2018b; Public Health Agency of Canada, 2018a; Public Health Agency of Canada, 2019; Public Health Agency of Canada, 2021).
Species information was available for a subset of Campylobacter cases between 2017 and 2018. C. jejuni accounted for 88-91% of all cases examined, C. coli accounted for 5-7%, and the remaining 2-5% were caused by C. upsaliensis, C. lari, C. fetus, C. rectus, C. curvus, C. hyointestinals, or C. ureolyticus(Public Health Agency of Canada, 2018b; Public Health Agency of Canada, 2018a; Public Health Agency of Canada, 2019). Children aged between 1-5 years or late adolescents aged between 20-24 were found to have a high reported incidence rate, being more than 35 per 100,000 population. Males were consistently shown to be more easily infected than females. Higher proportions of Campylobacter cases were reported during summer months from June to August, with a marked decrease at the end of the summer season.
Although a higher number of cases were reported during summer months, proportions of retail chicken samples positive for Campylobacter continued to rise after summer. It was suggested that the summer peak might be due to improved survival and replication of bacteria under warm temperatures and seasonal changes in eating behaviours such as summer barbeques (Fleury et al., 2006; Public Health Agency of Canada, 2018b; Public Health Agency of Canada, 2018a; Public Health Agency of Canada, 2019).
A recent study examining potential bacterial enteric pathogens in children with acute gastroenteritis enrolled between December 2014 and February 2018 reported Campylobacter(18/2391) to be the third most prevalent bacterial pathogen, following Salmonella(54/2391) and Aeromonas(26/2391) (Tarr et al., 2019).
3.1.4.2 United States
Campylobacteriosis in United States is monitored by the Foodborne Diseases Active Surveillance Network (Tack et al., 2020). The reported incidence of campylobacteriosis demonstrated an increasing trend for the period between 2014 and 2019, elevating from 13.3 per 100,000 population in 2014, to 19.2 in 2017, and 19.5 in 2019 ( Table 1 ) (Centers for Disease Control and Prevention, 2014; Centers for Disease Control and Prevention, 2017; Marder et al., 2017; Marder et al., 2018; Tack et al., 2019; Tack et al., 2020). There was a decrease in the rate of Campylobacter infection in 2020, in which the reported incidence dropped to 14.4 per 100,000 population (Ray et al., 2021).
According to the FoodNet Surveillance reports for 2014 and 2015 where species information was available for subsets of Campylobacter infections, 88% of cases were caused by C. jejuni, 7-8% of cases were caused by C. coli and the rest by other Campylobacter species (Centers for Disease Control and Prevention, 2014; Centers for Disease Control and Prevention, 2017). Children less than five years old had the highest reported incidence (22.2 per 100,000 population for 2014 and 20.8 for 2015) compared to other age groups. Males were found to be more frequently infected than females. The summer months of June, July and August were found to have the highest number of infections.
Although several Campylobacter outbreaks associated with a variety of sources have been reported in United States in recent years, Campylobacter infections in United States were mainly sporadic. A C. jejuni outbreak linked to puppy exposure affecting 118 individuals was reported in Ohio between 2016-2018 (Montgomery et al., 2018). A cluster of C. jejuni infection involving 39 cases in Nebraska was reported in 2017 which was found to be associated with contaminated municipal water supply (Pedati et al., 2019). C. jejuni outbreaks due to the consumption of raw milk were reported in Colorado in 2016, and Utah in 2014, which affected 99 and 17 persons, respectively (Davis et al., 2016; Burakoff et al., 2018). Lanier et al. have reviewed 28 chicken liver-associated outbreaks of campylobacteriosis and salmonellosis in United States between 2000 and 2016. Among the 28 outbreaks, 23 were campylobacteriosis, three were salmonellosis and two were caused by both bacterial pathogens (Lanier et al., 2018). Common features of these outbreaks included chicken liver dishes, inadequate cooking and food preparation settings (Lanier et al., 2018). Overall, contacts with animals, drinking raw milk, and the consumption of contaminated food, especially chicken dishes, were the major causes of Campylobacter outbreaks in United States.
Campylobacter was the leading foodborne bacterial pathogen causing gastroenteritis in United States. In 2019, a total of 25,866 foodborne infections were reported, among which 9,731 cases were caused by Campylobacter, followed by Salmonella(8,556 cases), STEC (3,127), Shigella(2,416), Yersinia(681), Vibrio(466) and Listeria(134) (Tack et al., 2020). Among the 9,731 Campylobacter infections, 85% were acquired domestically (Tack et al., 2020).
3.2 Campylobacteriosis in countries without national surveillance data
3.2.1 Africa
Currently there are no national surveillance data available in Africa. However, several studies have examined the enteric pathogens in this region.
3.2.1.1 Madagascar, Malawi and South Sudan
In Africa, Campylobacter was not the leading bacterial enteric pathogen causing gastroenteritis ( Table 2 ). In Madagascar, EPEC (3%, 6/199) was found to be the leading bacterial enteric pathogen causing diarrhea in children less than five years old, followed by Shigella(1.5%, 3/199) and Campylobacter(1%, 2/199) (Randremanana et al., 2016). Similarly, in Malawi, enteroaggregative E. coli, heat-stable enterotoxin-producing E. coli and EPEC were the leading bacterial enteric pathogens among children hospitalised with diarrhea, accounting for 51.8% (354/684), 21.2% (145/684) and 18% (123/684) of all cases, respectively. Consistently, Biswas et al. in 2017 reported diarrheagenic E. coli(68.3%) to be the major pathogen of diarrhea in the United Kingdom military personnel in South Sudan, followed by Salmonella(50.1%), and Campylobacter(0.8%, 10/127) (Biswas et al., 2019).
3.2.1.2 Ethiopia and Ghana
The presence of Campylobacter in HIV patients with diarrhea was reported by Ayele1 et al. in Ethiopia during 2019, in which Campylobacter, although being the most predominant enteric bacterial pathogen, had a low isolation rate of 4.4% (8/180), followed by Salmonella(2.8%, 5/180) and Shigella(1.1%, 2/180) (Ayele et al., 2020). Forson et al. assessed the incidence of Campylobacter in HIV patients with gastroenteritis in Ghana between 2015 and 2016, and reported that 50.7% (71/140) were positive for Campylobacter; surprisingly all isolates were C. coli(Forson et al., 2020).
3.2.1.3 Egypt
Studies from Egypt reported Campylobacter detection rates from 4.1-48% in patients with gastroenteritis ( Table 2 ).
A study by Zeinhom et al. in 2019 reported a C. jejuni isolation rate of 48% (12/25) from individuals with diarrhea across Beni-Suef Governorate; however, the detection rate was only slightly higher than that from non-diarrheic individuals (32.1%, 9/28) (Zeinhom et al., 2021). Barakat et al. reported that 38.1% (40/105) of diarrheic human stools collected in 2018 from five different governorates were PCR positive for Campylobacter, among which a majority of them were C. jejuni(92.5%, 37/40) (Barakat et al., 2020). A similar detection rate of C. jejuni was reported in Sharkia (30%, 30/100) and Ismailia (31.2%, 25/80) Governorates between 2017-2018 (Ammar et al., 2020; Abdelmageed et al., 2021). However, a much lower detection rate of C. jejuni was reported in multiple Governorates between 2015 and 2016 (17.33%, 13/75), as well as between 2013-2014 (21.5%, 20/93) (Elgabry et al., 2016; Ghoneim et al., 2020).
Among all the Egyptian regions that have Campylobacter infection reported, Zagazig city had the lowest isolation rate of C. jejuni. The study by Ahmed et al. examining the presence of C. jejuni from 270 stool samples collected from gastroenteritis patients between 2015-2018 showed that only 4.1% (11/270) were positive for C. jejuni(Abd El-Hamid et al., 2019). A very similar detection rate of C. jejuni was reported between 2013-2014 (4.1%, 10/246) (Ahmed et al., 2015).
Taken together, there seems to be an increasing trend of Campylobacter infection in Egypt, however the lack of national surveillance makes this difficult to conclude.
3.2.2 Asia
3.2.2.1 Bangladesh
Two recent studies examining the incidence of Campylobacter in diarrheal patients between 2019-2020 in Dhaka and Mymensingh reported very different results, possibly due to the geographical differences where the studies were conducted ( Table 2 ). It was found that in Dhaka, Campylobacter was the third leading bacterial pathogen being isolated from diarrheal stool samples (3.6%, 76/2135), following V. cholera(23.8%, 509/2135) and Aeromonas(17%, 363/2135) (Garbern et al., 2021). On the other hand, a much higher isolation rate was reported in Mymensingh in which 31.5% (104/330) of clinical samples had Campylobacter. Of these, 21.8% (72/330) were C. jejuni and 9.6% (32/330) were C. coli(Rahman et al., 2021). Additionally, a higher detection rate of Campylobacter was found in children less than five years old, contributing to more than half of all positive cases (Rahman et al., 2021).
3.2.2.2 China
Campylobacter infections have been reported in multiple regions of China ( Table 2 ). The most recent study examining the presence of Campylobacter in diarrheal patients between 2017-2019 in Wenzhou showed that 10.5% of patient stool samples had Campylobacter species isolated; this being the highest detection rate reported in China in the past 15 years (Zhang et al., 2020a). Li et al. examined the presence of Campylobacter in 370 diarrhea cases between 2016-2017 in Beijing. Campylobacter was the third leading enteric bacterial pathogen found in 7% (26/370) of total cases, following diarrheagenic E. coli(7.3%, 27/370) and V. parahaemolyticus(10.3%, 38/370); other enteric bacterial pathogens included Salmonella(6.2%, 23/370) and Shigella(0.3%, 1/370) (Li et al., 2018). A similar Campylobacter detection rate of 7.8% between 2017-2018 was reported by Zhang et al. (Zhang et al., 2020b).
A study on the aetiology of acute diarrhea among children under five years of age in Wuhan between 2014-2015 reported that rotavirus (25.7%, 98/381) was the leading viral enteric pathogen, whilst Salmonella(8.4%, 32/381), diarrheagenic E. coli(4.7%, 18/381) and Campylobacter(2.9%, 11/381) were the most prevalent bacterial pathogens (Zhu et al., 2016).
Another study by Wang et al. reported a very low isolation rate of Campylobacter(0.13%, 1/755) in children less than five years old in a developing region (Henan Province) between 2011 and 2014, while none of the patients (0/1422) from the developed region (Beijing) were found to be positive for Campylobacter(Wang et al., 2015). Other pathogens detected in the developing region of Henan included Shigella(16.95%, 128/755), Salmonella(5.3%, 40/755), diarrheagenic E. coli(7.15%, 54/755), Aeromonas hydrophila(3.58%, 27/755) and Yersinia(0.26%, 2/755) (Wang et al., 2015).
A large scale national-based prospective surveillance study on more than 90,000 patients with acute diarrhea across 31 provinces in China was conducted between 2009-2018. It was found that rotavirus A and norovirus were the two leading viral enteric pathogens, while C. jejuni was the sixth leading bacterial enteric pathogen detected (0.87%) following diarrheagenic E. coli(6.71%), non-Typhi Salmonella(4.41%), Shigella(2.44%), V. parahaemolyticus(2.08%) and A. hydrophila(0.99%) (Wang et al., 2021).
Few studies have reported Campylobacter outbreaks in China. In August 2019, a gastroenteritis outbreak consisting of 14 patients working at the same factory was reported in Shunyi District Beijing. The diarrhea happened after lunch which was supplied from a meal delivery company. Further examinations showed that 12 of the 14 patients, as well as two food workers were PCR positive for C. jejuni(Li et al., 2020b). Further investigations by a later study on the same outbreak did not identify the original source of infection, however duck blood curd was considered as a possible risk factor (Chen et al., 2021). Two studies conducted in 2018 reported a Campylobacter outbreak in students and teachers in Beijing following a school trip and in Hangzhou due to unknown sources (Qu et al., 2019; Yu et al., 2020). C. jejuni was found to be the causative agent of both outbreaks.
3.2.2.3 India
Limited data are available on Campylobacter infection in India ( Table 2 ). A recent study examining 400 human dysenteric stool samples between 2019 and 2020 in Vellore, South India using PCR analysis showed that EPEC (17%, 68/400) was the most dominant bacterial enteric pathogen being detected, followed by Campylobacter(12%, 48/400) and EIEC/Shigella(6.5%, 26/400) (Lakshmi Ss et al., 2022). By using genus specific PCR methods, a study by Mohakud et al. detected Campylobacter in 16.77% (52/310) of faecal samples collected from children with acute diarrhea in the peri-urban Bhubaneswar city between 2016-2017 (Mohakud et al., 2019). Infants between two and five years old were shown to have the highest rate of Campylobacter positivity (23.3%, 14/60). Co-infection with other enteric pathogens such as rotavirus, E. coli, Shigella, Cryptosporidium and adenovirus was also observed (Mohakud et al., 2019). Another study examining the presence of Campylobacter enteritis in children under five years in Northeast India between 2014-2015 reported a detection rate of 10.1% (41/407), of which 8.1% (33/407) was C. jejuni(Borkakoty et al., 2020). The highest detection rate was reported during summer (June and July, 20-18.8%) (Borkakoty et al., 2020).
3.2.2.4 Iran
A recent study by Alsalmi et al. showed that in 2019, C. jejuni(0.6%, 5/790) was the second most dominant enteric bacterial pathogen causing gastrointestinal manifestations in Oman, following Salmonella(2.5%, 20/790) ( Table 2 ) (Alsalmi et al., 2021). The detection rates of C. jejuni in diarrheal patients from Mazandaran and East Azerbaijan in 2017 and 2016 were reported to be 27% (20/74) and 27.8% (62/223), respectively (Ranjbar et al., 2017; Divsalar et al., 2019).
Mazaheri et al. reported that in Semnan between 2014 and 2015, Campylobacter was isolated from stools of 8.6% (36/419) of children (1-12 years old) with diarrhea; children in age group 6-12 years were found to have the highest frequency of positive cultures (Mazaheri et al., 2016). Around the same time between 2013-2014 in Hamadan, 10% (12/120) of the diarrheal stool samples from children less than 10 years old had Campylobacter isolated. In Central Iran, 33% (76/230) diarrheal stools from children were PCR positive for C. jejuni in 2015, among which most of the infections occurred in the age group of 1-3 years (Rastyani et al., 2015; Abbasi et al., 2019). Campylobacter infection was found to be more prevalent in girls in Semnan, contrary to that of Central Iran (Mazaheri et al., 2016; Abbasi et al., 2019).
In summary, by reviewing the incidence studies on Campylobacter infection in Iran, the positive detection rates in different regions ranged from 3.6% to 35.4%; campylobacteriosis was frequently reported in children with diarrhea ( Table 2 ).
3.2.2.5 Iraq
In Iraq, during 2017, adenovirus (34.2%, 53/155) was the leading enteric pathogen causing acute diarrhea in children less than five years old, followed by Salmonella(14.8%, 23/155), Entamoeba(13.5%, 21/155) and Campylobacter(10.9%, 17/155) (Harb et al., 2019).
3.2.2.6 Lebanon
The isolation rate of Campylobacter was reported to be 12% (35/291) in Lebanon in 2018 in children under 12 years with acute gastroenteritis (Ghssein et al., 2021). A large-scale incidence study including 1000 patients with diarrhea performed between 2016-2017 in Lebanon reported Campylobacter as the leading bacterial enteric pathogen with a detection rate of 21.5%. Other pathogens being detected were rotavirus (6.7%), Entamoeba histolytica(6%), Salmonella Typhi (5.8%), and enterohemorrhagic E. coli O157:H7 (5.6%) (Ibrahim et al., 2019). Males were found to have a slightly higher Campylobacter isolation rate as compared to females; there was a clear seasonal distribution, with summer having the highest rate of infection (Ibrahim et al., 2019).
3.2.2.7 Nepal
Campylobacter infection in children less than five years old with acute gastroenteritis in Kathmandu during 2017-2018 was reported ( Table 2 ) (Bhattarai et al., 2020). Among the 303 children participating, more than half (56.8%, 172/303) were infected with Campylobacter, of which 26.7% were Campylobacter mono-infection, and 30% were co-infected with rotavirus. Children less than six months were found to have an increased risk of Campylobacter mono-infection as compared to other age groups (Bhattarai et al., 2020).
A case-control study of traveller’s diarrhea was conducted in Kathmandu between 2012-2014 in adults from North America, Western Europe, Australia, New Zealand, Japan, Taiwan or Korea residing in Nepal for less than one year (Murphy et al., 2019). Campylobacter was the second leading bacterial enteric pathogen (20%, 88/433), following enteroaggregative E. coli(26%, 110/420) (Murphy et al., 2019). Another epidemiological surveillance study of traveller’s diarrhea conducted between 2011 and 2014 for residents of aforementioned countries visiting Nepal reported a significantly higher presence of C. concisus in traveller’s diarrhea cases (28.9%, 24/83) as compared with asymptomatic controls (4%, 3/75), suggesting emerging Campylobacter species also contributed to the traveller’s diarrhea in this area (Serichantalergs et al., 2017).
3.2.2.8 Pakistan
Noreen et al. reported that in 150 diarrheal stool samples collected from hospitalised paediatric patients between December 2014 and September 2015 in major cities of Pakistan, C. jejuni was isolated in 54.6% (82/150) of them ( Table 2 ) (Noreen et al., 2020). This isolation rate was similar to that reported in Rawalpindi and Islamabad in 2014 in which 52% (260/500) of paediatric diarrheal stool samples were culture positive for Campylobacter. C. jejuni accounted for 48.2% (241/500) of all cases, and the positive rate was much higher than that of rotavirus (26.4%) (Sadiq et al., 2019b).
3.2.2.9 Thailand
Analogous to Nepal, traveller’s diarrhea was often reported in Thailand, which was also a prevalent illness encountered by deployed military personnel ( Table 2 ). A prospective study of acute diarrhea in United States military personnel on deployment in Thailand between 2013-2017 reported Campylobacter as the leading bacterial enteric pathogen, causing 43.8% (21/48) of all cases, followed by diarrheagenic E. coli(42%, 20/48), and Salmonella(23%, 11/48) (Lurchachaiwong et al., 2020). Another study by Lertsethtakarn et al. also found that Campylobacter(31.2% 48/154) was the most prevalent enteric pathogen causing traveller’s diarrhea for individuals visiting Thailand from North America, Europe, Australia, New Zealand, Japan, Taiwan, and South Korea between 2012-2014 (Lertsethtakarn et al., 2018). Additionally, non-jejuni/coli Campylobacter species such as C. concisus and C. ureolyticus were found to be causative agents for 11.5% (3/26) and 7.7% (2/26) of traveller’s diarrhea, respectively between 2011-2014 (Serichantalergs et al., 2017).
3.2.2.10 United Arab Emirates
In the United Arab Emirates between 2017-2019, Campylobacter only contributed to a minor portion (1.9%, 4/203) of diarrheal cases in children less than five years of age, with the leading viral enteric pathogens being rotavirus (21.2%, 43/203) and norovirus (19.2%, 39/202), and leading bacterial species being EPEC(17.7%, 36/203) and enteroaggregative E. coli(10.3%, 21/203) (Alsuwaidi et al., 2021).
3.2.3 Europe
3.2.3.1 North Macedonia
Trajkovska-Dokic et al. reported that the presence of Campylobacter infection in patients with acute enteritis between 2012-2017 was 2.5% (97/3820), among which 98.63% were C. jejuni(85/97) and 12.37% (12/97) were C. coli( Table 2 ) (Trajkovska-Dokic et al., 2019). There was an increase in the Campylobacter isolation rate in 2016 (3.8%) and 2017 (6.4%) as compared with previous years, where the rates were 1.4% or below (Trajkovska-Dokic et al., 2019). This study also found a significantly higher isolation rate in individuals under 15 years old as compared those from 15-30, 31- 50 or above, but no statistical difference between genders was noted
3.2.4 Oceania
3.2.4.1 Papua New Guinea
Between 2013 and 2014, a study on acute diarrhea was conducted in four provinces in Papua New Guinea including Hela, Eastern Highlands, Madang, and Central Provinces ( Table 2 ). The common enteric pathogens detected were Shigella(38.1% 45/118), Campylobacter(33.1%) and rotavirus (20.3%) (Abdad et al., 2020).
3.3 Human hosted Campylobacter species
Some of the Campylobacter species utilise humans as their natural host and have not been isolated from other animals, which were referred to as human hosted Campylobacter species (Liu et al., 2018). Among the human hosted Campylobacter species, C. concisus was most studied. C. concisus normally colonises the human oral cavity as a commensal oral bacterium (Zhang, 2015). The presence of C. concisus in the lower parts of the human gastrointestinal tract is associated with gastrointestinal diseases such as Barrett’s oesophagus, gastroenteritis and inflammatory bowel disease (Macfarlane et al., 2007; Yang et al., 2009; Zhang et al., 2009; Man et al., 2010; Mahendran et al., 2011; Mukhopadhya et al., 2011; Zhang, 2015; Kirk et al., 2016; Liu et al., 2018). C. concisus has two genomospecies (Chung et al., 2016). Strains of genomospecies 2 C. concisus are more likely to colonise the human gastrointestinal tract (Engberg et al., 2005; Kalischuk and Inglis, 2011; Ismail et al., 2012; Wang et al., 2017). C. concisus is able to induce gut barrier defects and intestinal inflammation (Ismail et al., 2012; Mahendran et al., 2016). Additionally, a recent study showed that C. concisus has a immunomodulatory role by upregulating programmed death-ligand 1 in interferon-γ sensitised intestinal epithelial cells (Lee et al., 2021). Details on disease associations and pathogenic mechanisms of human hosted Campylobacter species were reviewed recently (Liu et al., 2018).
3.4 The impact of COVID-19 on Campylobacter infection
The epidemiological data of campylobacteriosis for the year 2020 were available in 27 countries, including 26 European countries and the United States, providing the opportunity for assessing the impact of COVID-19 on campylobacteriosis. The average incidence of campylobacteriosis reported during the years 2014-2019 was compared to that in 2020, the first pandemic year of COVID-19. There has been a drop in the reported incidence of campylobacteriosis in 22 of the 26 European countries, except France, Latvia, Luxembourg and Lithuania ( Table 1 ; Supplementary Figure 1 ).
In the United States, the decreased incidence reported was observed not only for campylobacteriosis but also enteric infections caused by other bacterial pathogens (Ray et al., 2021). A study from the United States showed that in 2020, the reported incidence of infections caused by enteric pathogens dropped by 26% as compared with 2017-2019, with only 18,462 cases reported. Furthermore, only 5% (958) of infections were associated with international travel as compared to 14% during 2017-2019, and most of these (83%, 798/958) cases occurred during January-March.
4 Discussion and conclusion
Globally, national surveillance data of campylobacteriosis were available from 38 countries, mostly from countries in Europe (30 countries) ( Table 1 ). The other countries with a national surveillance program for campylobacteriosis were United States, Canada, United Kingdom, Australia, New Zealand, Japan, Singapore and Korea.
The reported incidence of campylobacteriosis in countries with a national surveillance program varied greatly ( Figure 1 ; Table 1 ). Czech Republic in eastern Europe had the highest reported incidence of campylobacteriosis worldwide followed by Australia and New Zealand. Some other European countries such as Bulgaria, Poland and Romania had a low reported incidence rate of campylobacteriosis. Countries in western and northern Europe in general had a high reported incidence of campylobacteriosis ( Figure 1 ; Table 1 ). Limited data were available from countries in southern Europe. The reported incidence of campylobacteriosis in the United States and Canada was relatively low amongst developed countries ( Figure 1 ; Table 1 ). Campylobacter was the leading bacterial enteric pathogen in these countries.
Figure 1.
Reported global incidence of campylobacteriosis. Reported incidence rates of campylobacteriosis worldwide (A) and in Europe (B) are presented as per 100,000 population. Data presented in the figure were mainly from 2019. In countries where the 2019 data were not available, data from a previously available year were used. Countries with no reported incidence of campylobacteriosis available were in grey colour. Map was created with Datawrapper.
Campylobacteriosis surveillance programs provide an excellent opportunity in obtaining national data. However, the data accuracy depends on the implementation of such programs in individual countries. Due to the differences in medical and reporting systems, it is not surprising that cases of campylobacteriosis may be underreported in some countries. This, at least in part, would have contributed to their low reported campylobacteriosis incidence. Campylobacteriosis incidence may vary in different regions of a given country, particularly in large countries comprising of different climate, economic and cultural zones. The regional differences were not revealed in national data presented in this review. Despite these limitations, data obtained from national surveillance programs still provide very useful information in assessing epidemiology trend of campylobacteriosis. Individual studies reporting Campylobacter infections in humans during 2014-2021 in each country, if available, were also included in this review, which provided further information on global Campylobacter infection. However, in countries that lacked campylobacteriosis surveillance programs, these individual studies do not represent the overall epidemiology of their nations, due to the restricted subset of population that these studies were performed on.
Culture-independent molecular method is more sensitive than bacterial isolation in detecting Campylobacter species in clinical samples. In European countries, the diagnosis was generally based on cultures from human faecal samples and confirmed by both culture and PCR methods (European Food Safety Authority, 2021b). In the United States, culture-independent diagnostic test was used (Ray et al., 2021). In Canada, confirmed cases were defined as positive isolation of Campylobacter from an appropriate clinical specimen (Government of Canada, 2018). Furthermore, different detection methods were used in individual studies. The diagnostic methods used for Campylobacter detection could also contribute to variations in Campylobacter detection rates between different countries and studies.
Consumption of contaminated chicken containing food seems to be the most common driver of Campylobacter infection in developed countries. For example, in Europe, occurrence of Campylobacter in poultry meat is an important vehicle for Campylobacter infection (Guirin et al., 2020). In Australia and New Zealand, consumption of undercooked chicken or chicken-containing dishes, consumption of contaminated water, owning pet chicken and travelling overseas are major risk factors of Campylobacter infection (Moffatt et al., 2020; Varrone et al., 2020). In Canada, consumption and handling of contaminated poultry meat appeared to be the most common drivers of human campylobacteriosis (Huang et al., 2015). In United States, Campylobacter outbreaks were frequently attributed to dairy products, chicken, and vegetables (Sher et al., 2021). While outbreaks were more frequently reported in some countries such as Japan, Campylobacter infections were mainly sporadic cases in most of the developed countries. This difference may reflect cultural differences in lifestyle.
Although three Asian countries had national surveillance data of campylobacteriosis, the reported incidence was only available in Singapore ( Figure 1 ; Table 1 ). Data in other Asian countries included in this review were mainly from individual studies on isolation and detection of enteric pathogens. In some Asian countries such as Lebanon, Japan and Thailand, Campylobacter was the leading bacterial enteric pathogen. In other Asian countries such as Korea, Singapore, Iran and Iraq, Campylobacter was the second or third leading bacterial enteric pathogen (Ministry of Health Singapore, 2017; Harb et al., 2019; Alsalmi et al., 2021; Korea Disease Control and Prevention Agency, 2021); E. coli, Salmonella or Vibrio species was the leading bacterial enteric pathogens in these countries. Although national data are not available from China, a large scale national-based prospective surveillance study showed that C. jejuni was the sixth leading bacterial enteric pathogen (Wang et al., 2021). Campylobacter was reported to be the third leading bacterial enteric pathogen in several countries in Africa, following E. coli and Salmonella species. These data show that Campylobacter infection is also an important health concern in Asian countries.
C. jejuni is the major causative species of campylobacteriosis, responsible for about 90% of cases. C. coli accounted for less than 10% of Campylobacter caused gastrointestinal infections (Ministry of Health Singapore, 2019; Public Health Agency of Canada, 2019; European Food Safety Authority, 2021b). Other Campylobacter species such as C. upsaliensis, C. lari, C. fetus, C. rectus, C. curvus, C. hyointestinals, C. ureolyticus and C. concisus were also reported, although they contributed to a small portion of Campylobacter infections. Campylobacter may also co-infect humans with other bacterial enteric pathogens (Yuwono et al., 2021)
The seasonality of campylobacteriosis varied in different countries. Campylobacter infection was more common in the summer period in some countries such as European countries, Japan, Korea, Canada and United States ( Table 1 ). A winter peak was also observed during the Christmas festive season in some countries in Europe (Bless et al., 2017). In a recent study from New South Wales of Australia, Campylobacter infection did not display seasonality (Yuwono et al., 2021). Campylobacter infection was more common in males than females. These findings show that multiple factors contribute to the risk of developing Campylobacter infections.
The reported incidence and case numbers of campylobacteriosis have remained steadily high in most developed countries prior to the COVID-19 pandemic, although with fluctuations between years ( Table 1 ). It showed an increasing trend in some countries such as France and Japan. The COVID-19 pandemic has reduced the reported incidence of campylobacteriosis in most of the countries where 2020 epidemiology data were available ( Table 1 ; Supplementary Figure 1 ). In response to the COVID-19 pandemic, many countries have implemented specific policies such as restricting social gathering, restricting travel, closing restaurants and schools. These interventions have reduced the exposure of humans to foodborne pathogens, consequently reducing the infections caused by Campylobacter species and other enteric pathogens (European Food Safety Authority, 2021b; Ray et al., 2021). Furthermore, during the COVID-19 pandemic, reduced access to medical diagnostics was also likely to contribute to the reduction of campylobacteriosis. It is expected that Campylobacter infections will increase post COVID-19 pandemic as normal ways of life resume.
In conclusion, Campylobacter species is one of the most common human enteric pathogens in both developed and developing countries, with campylobacteriosis remaining a global health concern. Increased research and improved strategies are needed for the prevention and reduction of Campylobacter infection.
Author contributions
LZ conceived the project. FL and LZ wrote the first version of the manuscript. SL, JX, and SR provided critical feedback and helped with reviewing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by a Faculty Research Grant awarded to LZ from the University of New South Wales (grant number PS46772).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.979055/full#supplementary-material
Impact of COVID-19 on the reported incidence of campylobacteriosis. Reported incidence are presented as per 100,000 population. The average incidence between 2014-2019 was lower than that in 2020 due to COVID-19 pandemic in most of the countries, except France, Lithuania and Malta.
References
- Abbasi E., Van Belkum A., Ghaznavi-Rad E. (2019). Quinolone and macrolide-resistant Campylobacter jejuni in pediatric gastroenteritis patients from central Iran. Microb. Drug Resist. 25, 1080–1086. doi: 10.1089/mdr.2018.0455 [DOI] [PubMed] [Google Scholar]
- Abdad M. Y., Soli K. W., Pham B., Bande G., Maure T., Jonduo M., et al. (2020). Diarrhoeal disease surveillance in Papua new Guinea: findings and challenges. West Pac. Surveill. Response 11, 7. doi: 10.5365/wpsar.2018.9.2.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hamid M. I., Abd El-Aziz N. K., Samir M., El-Naenaeey E. Y., Abo Remela E. M., Mosbah R. A., et al. (2019). Genetic diversity of Campylobacter jejuni isolated from avian and human sources in Egypt. Front. Microbiol. 10, 2353. doi: 10.3389/fmicb.2019.02353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmageed H. A., Mandour A. S., El Gedawy A. A., Fawzy M., Furuya T., Ezzat M. (2021). Characterization of Campylobacter jejuni isolated from dogs and humans using flaA-SVR fragment sequencing in ismailia, Egypt. Comp. Immunol. Microbiol. Infect. Dis. 77, 101675. doi: 10.1016/j.cimid.2021.101675 [DOI] [PubMed] [Google Scholar]
- Ahmed H. A., El Hofy F. I., Ammar A. M., Abd El Tawab A. A., Hefny A. A. (2015). ERIC-PCR genotyping of some Campylobacter jejuni isolates of chicken and human origin in Egypt. Vector Borne Zoonot. Dis. 15, 713–717. doi: 10.1089/vbz.2015.1836 [DOI] [PubMed] [Google Scholar]
- Alsalmi A. A. S., Al Busafi S. A., Al Lamki R. N. A., Mabruk M. (2021). The ecology and antibiotic resistance patterns of gastrointestinal tract infections in a tertiary care hospital in Oman. J. Pure Appl. Microbiol. 15, 1634–1642. doi: 10.22207/JPAM.15.3.60 [DOI] [Google Scholar]
- Alsuwaidi A. R., Al Dhaheri K., Al Hamad S., George J., Ibrahim J., Ghatasheh G., et al. (2021). Etiology of diarrhea by multiplex polymerase chain reaction among young children in the united Arab Emirates: a case-control study. BMC Infect. Dis. 21, 7. doi: 10.1186/s12879-020-05693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar A. M., El-Naenaeey E. Y., El-Malt R. M. S., El-Gedawy A. A., Khalifa E., Elnahriry S. S., et al. (2020). Prevalence, antimicrobial susceptibility, virulence and genotyping of Campylobacter jejuni with a special reference to the anti-virulence potential of eugenol and beta-resorcylic acid on some multi-drug resistant isolates in Egypt. Anim. (Basel) 11, 3. doi: 10.3390/ani11010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H., Takahashi N., Yamamoto S., Maruyama H. (2017). Draft genome sequence of Campylobacter jejuni CAM970 and C. coli CAM962, associated with a large outbreak of foodborne illness in Fukuoka, Japan, in 2016. Genome Announc. 5, e00508–e00517. doi: 10.1128/genomeA.00508-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian Agency for Health and Food Safety (2020) Campylobacter. Available at: https://www.ages.at/en/(Accessed 31 Aug 2021).
- Ayele A. A., Tadesse D., Manilal A., Yohanes T., Seid M., Shewangizaw Mekuria M. (2020). Prevalence of enteric bacteria and enteroparasites in human immunodeficiency virus-infected individuals with diarrhoea attending antiretroviral treatment clinic, arba minch general hospital, southern Ethiopia. New Microbes New Infect. 38, 100789. doi: 10.1016/j.nmni.2020.100789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat A. M., Abd El-Razik K. A., Elfadaly H. A., Rabie N. S., Sadek S. A., Almuzaini A. M. (2020). Prevalence, molecular detection, and virulence gene profiles of Campylobacter species in humans and foods of animal origin. Vet. World 13, 1430. doi: 10.14202/vetworld.2020.1430-1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R., Draper A., Fearnley E., Franklin N., Glasgow K., Gregory J., et al. (2021). Monitoring the incidence and causes of disease potentially transmitted by food in Australia: Annual report of the OzFoodNet network 2016. Commun. Dis. Intell. 45. doi: 10.33321/cdi.2021.45.52 [DOI] [PubMed] [Google Scholar]
- Bhattarai V., Sharma S., Rijal K. R., Banjara M. R. (2020). Co-Infection with Campylobacter and rotavirus in less than 5 year old children with acute gastroenteritis in Nepal during 2017-2018. BMC Pediatr. 20, 68. doi: 10.1186/s12887-020-1966-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas J. S., Lentaigne J., Hill N. E., Harrison J. J., Mackenzie H., Akorli E., et al. (2019). Epidemiology and etiology of diarrhea in UK military personnel serving on the united nations mission in south Sudan in 2017: A prospective cohort study. Travel Med. Infect. Dis. 28, 34–40. doi: 10.1016/j.tmaid.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Bless P. J., Schmutz C., Mäusezahl D. (2017). The recurrent campylobacteriosis epidemic over Christmas and new year in European countries 2006–2014. BMC Res. Notes 10, 1–7. doi: 10.1186/s13104-017-2587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkakoty B., Jakharia A., Sarmah M. D., Hazarika R., Baruah P. J., Bora C. J., et al. (2020). Prevalence of Campylobacter enteritis in children under 5 years hospitalised for diarrhoea in two cities of northeast India. Indian J. Med. Microbiol. 38, 32–36. doi: 10.4103/ijmm.IJMM_19_498 [DOI] [PubMed] [Google Scholar]
- Brehony C., Lanigan D., Carroll A., Mcnamara E. (2021). Establishment of sentinel surveillance of human clinical campylobacteriosis in Ireland. Zoonoses Public Health 68, 121–130. doi: 10.1111/zph.12802 [DOI] [PubMed] [Google Scholar]
- Burakoff A., Brown K., Knutsen J., Hopewell C., Rowe S., Bennett C., et al. (2018). Outbreak of fluoroquinolone-resistant Campylobacter jejuni infections associated with raw milk consumption from a herdshare dairy — Colorado 2016. Morbid. Mortal. Weekly Rep. 67, 146. doi: 10.15585/mmwr.mm6705a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2014). Foodborne diseases active surveillance network (FoodNet): FoodNet surveillance report for 2014 (Final report)(Atlanta, Georgia: U.S. Department of Health and Human Services, CDC; ). [Google Scholar]
- Centers for Disease Control and Prevention (2017). Foodborne diseases active surveillance network (FoodNet): FoodNet 2015 surveillance report (Final data)(Atlanta, Georgia: U.S. Department of Health and Human Services, CDC; ). [Google Scholar]
- Centers for Disease Control and Prevention (2022) Guillain-Barré Syndrome. Available at: cdc.gov/campylobacter/guillain-barre.html(Accessed 4 Oct 2022).
- Chau M. L., Hartantyo S. H. P., Yap M., Kang J. S. L., Aung K. T., Gutiérrez R. A., et al. (2015). Diarrheagenic pathogens in adults attending a hospital in Singapore. BMC Infect. Dis. 16, 32. doi: 10.1186/s12879-016-1354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. W., He C., Lyu J. C., Liu X. F., Gao P., Zhen G. X., et al. (2021). Campylobacter outbreak associated with duck blood curd in 2019 in shunyi district, Beijing, China. BioMed. Environ. Sci. 34, 489–492. doi: 10.3967/bes2021.067 [DOI] [PubMed] [Google Scholar]
- Chen C., Wang L. P., Yu J. X., Chen X., Wang R. N., Yang X. Z., et al. (2019). Prevalence of enteropathogens in outpatients with acute diarrhea from urban and rural areas, southeast China 2010-2014. Am. J. Trop. Med. Hyg. 101, 310–318. doi: 10.4269/ajtmh.19-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriac P. C., Duceac L. D., Manole A., Poroch V., Delcea F., Stafie L., et al. (2017). Acute bacterial gastroenteritis as a healthcare-associated infection in pediatrics. Curr. Health Sci. J. 43, 275–281. doi: 10.12865/CHSJ.43.03.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebicz A., Śliżewska ,. K. (2018). Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: a review. Int. J. Environ. Res. Public Health 15, 863. doi: 10.3390/ijerph15050863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H. K., Tay A., Octavia S., Chen J., Liu F., Ma R., et al. (2016). Genome analysis of Campylobacter concisus strains from patients with inflammatory bowel disease and gastroenteritis provides new insights into pathogenicity. Sci. Rep. 6, 38442. doi: 10.1038/srep38442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communicable Diseases Branch (2019). NSW OzFoodNet annual surveillance report: 2018(Sydney: Health Protection NSW; ). [Google Scholar]
- Davis K. R., Dunn A. C., Burnett C., Mccullough L., Dimond M., Wagner J., et al. (2016). Campylobacter jejuni infections associated with raw milk consumption — Utah 2014. Morbid. Mortal. Weekly Rep. 65, 301–305. doi: 10.15585/mmwr.mm6512a1 [DOI] [PubMed] [Google Scholar]
- De Conto F., Di Stefano S., Buttrini M., Maccari C., Arcangeletti M. C., Chezzi C., et al. (2022). Detection of potential enteric pathogens in children with severe acute gastroenteritis using the filmarray: Results from a three - years hospital-based survey in northern Italy. Diagn. Microbiol. Infect. Dis. 102, 115611. doi: 10.1016/j.diagmicrobio.2021.115611 [DOI] [PubMed] [Google Scholar]
- De Miguel Buckley R., Trigo E., de la Calle-Prieto F., Arsuaga M., Díaz-Menéndez M. (2020). Social distancing to combat COVID-19 led to a marked decrease in food-borne infections and sexually transmitted diseases in Spain. J. Travel Med. 27, taaa134. doi: 10.1093/jtm/taaa134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divsalar G., Kaboosi H., Khoshbakht R., Shirzad-Aski H., Ghadikolaii F. P. (2019). Antimicrobial resistances, and molecular typing of Campylobacter jejuni isolates, separated from food-producing animals and diarrhea patients in Iran. Comp. Immunol. Microbiol. Infect. Dis. 65, 194–200. doi: 10.1016/j.cimid.2019.06.001 [DOI] [PubMed] [Google Scholar]
- Elgabry E. A., Barakat A. M., Abd El-Razik K. A., Abuelnaga A. S., El Fadaly H. A. (2016). Incidence of zoonotic Campylobacter jejuni in fast meal meat, grill chickens and symptomatic egyptians. Afr J. Microbiol. Res. 10, 608–615. doi: 10.5897/AJMR2016.8014 [DOI] [Google Scholar]
- Ena J., Afonso-Carrillo R. G., Bou-Collado M., Galian-Nicolas V., Reyes-Jara M. D., Martínez-Peinado C., et al. (2019). Epidemiology of severe acute diarrhea in patients requiring hospital admission. J. Emerg. Med. 57, 290–298. doi: 10.1016/j.jemermed.2019.06.009 [DOI] [PubMed] [Google Scholar]
- Engberg J., Bang D. D., Aabenhus R., Aarestrup F. M., Fussing V., Gerner-Smidt P. (2005). Campylobacter concisus: an evaluation of certain phenotypic and genotypic characteristics. Clin. Microbiol. Infect. 11, 288–295. doi: 10.1111/j.1469-0691.2005.01111.x [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (2016). Annual epidemiological report 2016 - campylobacteriosis(Stockholm: ECDC; ). [Google Scholar]
- European Centre for Disease Prevention and Control (2018. a). Campylobacteriosis. annual epidemiological report for 2015(Stockholm: ECDC; ). [Google Scholar]
- European Centre for Disease Prevention and Control (2018. b). Campylobacteriosis. annual epidemiological report for 2016(Stockholm: ECDC; ). [Google Scholar]
- European Centre for Disease Prevention and Control (2019). Campylobacteriosis. annual epidemiological report for 2017(Stockholm: ECDC; ). [Google Scholar]
- European Food Safety Authority (2019. a). The European union one health 2018 zoonoses report. EFSA J. 17, e05926. doi: 10.2903/j.efsa.2019.5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (2019. b). Trends and sources of zoonoses and zoonotic agents in foodstuffs, animals and feeding stuffs in 2018. [Google Scholar]
- European Food Safety Authority (2021. a). The European union one health 2019 zoonoses report. EFSA J. (Parma, Italy: EFSA; ) 19, e06406. doi: 10.2903/j.efsa.2021.6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (2021. b). The European union one health 2020 zoonoses report. Finland's Annual Zoonoses Reports (EFSA, Parma, Italy: ) 19, e06971. doi: 10.2903/j.efsa.2021.6971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (2021. c). Trends and sources of zoonoses and zoonotic agents in foodstuffs, animals and feedingstuffs in 2019. Finland's Annual Zoonoses Reports (Parma, Italy: EFSA; ). [Google Scholar]
- Farfán-García A. E., Imdad A., Zhang C., Arias-Guerrero M. Y., Sánchez-Álvarez N. T., Iqbal J., et al. (2020). Etiology of acute gastroenteritis among children less than 5 years of age in bucaramanga, Colombia: A case-control study. PloS Negl. Trop. Dis. 14, e0008375. doi: 10.1371/journal.pntd.0008375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury M., Charron D. F., Holt J. D., Allen O. B., Maarouf A. R. (2006). A time series analysis of the relationship of ambient temperature and common bacterial enteric infections in two Canadian provinces. Int. J. Biometeorol. 50, 385–391. doi: 10.1007/s00484-006-0028-9 [DOI] [PubMed] [Google Scholar]
- Forson A. O., Adjei D. N., Olu-Taiwo M., Quarchie M. N., Asmah H. R. (2020). Characterization of Campylobacter associated gastric enteritis among patients with human immunodeficiency virus (HIV) in a hospital in Accra, Ghana. PloS One 15, e0240242. doi: 10.1371/journal.pone.0240242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis E. (2007). Campylobacter and bacterial gastroenteritis. CMAJ 177, 570–571. doi: 10.1503/cmaj.070660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern S. C., Chu T. C., Gainey M., Kanekar S. S., Nasrin S., Qu K., et al. (2021). Multidrug-resistant enteric pathogens in older children and adults with diarrhea in Bangladesh: epidemiology and risk factors. Trop. Med. Health 49, 34. doi: 10.1186/s41182-021-00327-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoneim N. H., Abdel-Moein K. A.-A., Barakat A., Hegazi A. G., El-Razik K. A. A. E., Sadek S. A. S. (2020). Isolation and molecular characterization of campylobacter jejuni from chicken and human stool samples in Egypt. Food Sci. Technol. 41, 195–202. doi: 10.1590/fst.01620 [DOI] [Google Scholar]
- Ghssein G., Awada R., Salami A., Bahmad H. F., Awad A., Joumaa W. H., et al. (2021). Prevalence, laboratory findings and clinical characteristics of campylobacteriosis agents among hospitalized children with acute gastroenteritis in Lebanon. Pediatr. Gastroenterol. Hepatol. Nutr. 24, 346–356. doi: 10.5223/pghn.2021.24.4.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X. H., Wu H. Y., Li J., Xiao W. J., Zhang X., Chen M., et al. (2018). Epidemiology, aetiology and seasonality of infectious diarrhoea in adult outpatients through active surveillance in shanghai, China 2012-2016: a cross-sectional study. BMJ Open 8, e019699. doi: 10.1136/bmjopen-2017-019699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R., Heininger U., Hirsch H. H. (2018). Enterovirus detection in patients with acute gastroenteritis in Switzerland. J. Med. Virol. 90, 685–691. doi: 10.1002/jmv.25005 [DOI] [PubMed] [Google Scholar]
- Government of Canada (2018) Surveillance of campylobacteriosis (Campylobacter). Available at: www.canada.ca(Accessed 3 Oct 2022).
- Guirin G. F., Brusa V., Adriani C. D., Leotta G. A. (2020). Prevalence of campylobacter jejuni and campylobacter coli from broilers at conventional and kosher abattoirs and retail stores. Rev. Argent. microbiol. 52, 217–220. doi: 10.1016/j.ram.2019.07.002 [DOI] [PubMed] [Google Scholar]
- Harb A., Abraham S., Rusdi B., Laird T., O'dea M., Habib I. (2019). Molecular detection and epidemiological features of selected bacterial, viral, and parasitic enteropathogens in stool specimens from children with acute diarrhea in thi-qar governorate, Iraq. Int. J. Environ. Res. Public Health 16, 1573. doi: 10.3390/ijerph16091573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka N., Shimizu A., Somroop S., Li Y., Asakura M., Nagita A., et al. (2017). High prevalence of campylobacter ureolyticus in stool specimens of children with diarrhea in Japan. Jpn J. Infect. Dis. 70, 455–457. doi: 10.7883/yoken.JJID.2016.428 [DOI] [PubMed] [Google Scholar]
- Health Protection Surveillance Centre (2016). Annual epidemiology report 2015. (Dublin, Ireland: HSE HPSC; ) [Google Scholar]
- Health Protection Surveillance Centre (2018). Campylobacteriosis in Ireland 2017 (Dublin, Ireland: HSE HPSC; ). [Google Scholar]
- Health Protection Surveillance Centre (2019). Campylobacteriosis in Ireland 2018 (Dublin, Ireland: HSE HPSC; ). [Google Scholar]
- Health Protection Surveillance Centre (2021). Infectious disease notifications in Ireland 2015 - 2020. (Dublin, Ireland: HSE HPSC; ). [Google Scholar]
- Health Protection Surveillance Centre (2022). Infectious disease notifications in Ireland 2019 - 2021. (Dublin, Ireland: HSE HPSC; ) [Google Scholar]
- Herrador B. G., Lund V., Fonahn W., Hisdal H., Hygen H. O., Hyllestad S., et al. (2021). Heavy weather events, water quality and gastroenteritis in Norway. One Health 13, 100297. doi: 10.1016/j.onehlt.2021.100297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hse Health Protection Surveillance Centre (2018). Annual epidemiology report 2016. [Google Scholar]
- Huang H., Brooks B. W., Lowman R., Carrillo C. D. (2015). Campylobacter species in animal, food, and environmental sources, and relevant testing programs in Canada. Can. J. Microbiol. 61, 701–721. doi: 10.1139/cjm-2014-0770 [DOI] [PubMed] [Google Scholar]
- Hyllestad S., Iversen A., Macdonald E., Amato E., Borge Bå S., Bøe A., et al. (2020). Large Waterborne Campylobacter outbreak: use of multiple approaches to investigate contamination of the drinking water supply system, Norway, June 2019. Euro Surveill. 25, 2000011. doi: 10.2807/1560-7917.ES.2020.25.35.2000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim J. N., Eghnatios E., El Roz A., Fardoun T., Ghssein G. (2019). Prevalence, antimicrobial resistance and risk factors for campylobacteriosis in Lebanon. J. Infect. Dev. Ctries 13, 11–20. doi: 10.3855/jidc.10729 [DOI] [PubMed] [Google Scholar]
- Ismail Y., Mahendran V., Octavia S., Day A. S., Riordan S. M., Grimm M. C., et al. (2012). Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease. PloS One 7, e38217. doi: 10.1371/journal.pone.0038217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gómara M., Jere K. C., Hungerford D., Bar-Zeev N., Shioda K., Kanjerwa O., et al. (2019). Etiology of diarrhea among hospitalized children in Blantyre, Malawi, following rotavirus vaccine introduction: A case-control study. J. Infect. Dis. 220, 213–218. doi: 10.1093/infdis/jiz084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs E., Williman J., Martin N., Brunton C., Walls T. (2019. a). Epidemiology of Campylobacter gastroenteritis in new Zealand children and the effect of the Campylobacter strategy: A 20-year observational study. Pediatr. Infect. Dis. J. 38, 569–576. doi: 10.1097/INF.0000000000002228 [DOI] [PubMed] [Google Scholar]
- Jeffs E., Williman J., Martin N., Brunton C., Walls T. (2019. b). The epidemiology of non-viral gastroenteritis in new Zealand children from 1997 to 2015: an observational study. BMC Public Health 19, 18. doi: 10.1186/s12889-018-6229-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensen K. G., Schjørring S., Gantzhorn M. R., Vester C. T., Nielsen H. L., Engberg J. H., et al. (2021). Whole genome sequencing data used for surveillance of Campylobacter infections: detection of a large continuous outbreak, Denmark 2019. Euro Surveill. 26, 2001396. doi: 10.2807/1560-7917.ES.2021.26.22.2001396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalischuk L., Inglis G. (2011). Comparative genotypic and pathogenic examination of Campylobacter concisus isolates from diarrheic and non-diarrheic humans. BMC Microbiol. 11, 53. doi: 10.1186/1471-2180-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J., Inns T., Aird H., Swift C., Astbury J., Forester E., et al. (2020). Campylobacter outbreak associated with raw drinking milk, north West England 2016. Epidemiol. Infect. 148, e13. doi: 10.1017/S0950268820000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K. F., Nielsen H. L., Thorlacius-Ussing O., Nielsen H. (2016). Optimized cultivation of Campylobacter concisus from gut mucosal biopsies in inflammatory bowel disease. Gut Pathog. 8, 27. doi: 10.1186/s13099-016-0111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S. H., Heng Y. X., Jiang B., Ng L. S. Y., Sim D. M. F., Tan T. Y. (2022). Evaluation of the clinical sensitivity and specificity of the BD max™ enteric bacterial panel for molecular detection of pathogens for acute gastroenteritis in the Singaporean population. J. Microbiol. Methods 197, 106478. doi: 10.1016/j.mimet.2022.106478 [DOI] [PubMed] [Google Scholar]
- Korea Disease Control and Prevention Agency (2015). Epidemiological investigation of infectious diseases in Korea annual report 2014. (Cheongju, Korea: KDCA; ). [Google Scholar]
- Korea Disease Control and Prevention Agency (2016). Epidemiological investigation of infectious diseases in Korea annual report 2015. (Cheongju, Korea: KDCA; ). [Google Scholar]
- Korea Disease Control and Prevention Agency (2017). Epidemiological investigation of infectious diseases in Korea annual report 2016. (Cheongju, Korea: KDCA; ). [Google Scholar]
- Korea Disease Control and Prevention Agency (2018). Epidemiological investigation of infectious diseases in Korea annual report 2017. (Cheongju, Korea: KDCA; ). [Google Scholar]
- Korea Disease Control and Prevention Agency (2019). Epidemiological investigation of infectious diseases in Korea annual report 2018. (Cheongju, Korea: KDCA; ). [Google Scholar]
- Korea Disease Control and Prevention Agency (2021). Epidemiological investigation of infectious diseases in Korea annual report 2019. (Cheongju, Korea: KDCA; ). [Google Scholar]
- Kuhn K. G., Nielsen E. M., Mølbak K., Ethelberg S. (2018). Determinants of sporadic Campylobacter infections in Denmark: a nationwide case-control study among children and young adults. Clin. Epidemiol. 10, 1695. doi: 10.2147/CLEP.S177141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti E., Rehn M., Ockborn G., Hansson I., Ågren J., Engvall E. O., et al. (2017). Outbreak of campylobacteriosis following a dairy farm visit: confirmation by genotyping. Foodborne Pathog. Dis. 14, 326–332. doi: 10.1089/fpd.2016.2257 [DOI] [PubMed] [Google Scholar]
- Lakshmi Ss J., Prabaa Ms D., Murugan D., Anandan S., Veeraraghavan B. (2022). Real-time multiplex PCR assay reveals the increased prevalence of Campylobacter spp and diarrhoeagenic Escherichia coli in humans from vellore, south India. J. Med. Microbiol. 71, 001478. doi: 10.1099/jmm.0.001478 [DOI] [PubMed] [Google Scholar]
- Lanier W. A., Hale K. R., Geissler A. L., Dewey-Mattia D. (2018). Chicken liver-associated outbreaks of campylobacteriosis and salmonellosis, united states 2000-2016: Identifying opportunities for prevention. Foodborne Pathog. Dis. 15, 726–733. doi: 10.1089/fpd.2018.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastovica A. J., On S. L., Zhang L. (2014). The family campylobacteraceae. Prokary., (Berlin Heidelberg: Springer-Verlag; ) 307–335. doi: 10.1007/978-3-642-39044-9_274 [DOI] [Google Scholar]
- Lee S. A., Liu F., Yun D. Y., Riordan S. M., Tay A. C. Y., Liu L., et al. (2021). Campylobacter concisus upregulates PD-L1 mRNA expression in IFN-γ sensitized intestinal epithelial cells and induces cell death in esophageal epithelial cells. J. Oral. Microbiol. 13, 1978732. doi: 10.1080/20002297.2021.1978732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertsethtakarn P., Silapong S., Sakpaisal P., Serichantalergs O., Ruamsap N., Lurchachaiwong W., et al. (2018). Travelers' diarrhea in Thailand: A quantitative analysis using TaqMan® array card. Clin. Infect. Dis. 67, 120–127. doi: 10.1093/cid/ciy040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Ma R., Wang Y., Zhang L. (2018). The clinical importance of Campylobacter concisus and other human hosted Campylobacter species. Front. Cell Infect. Microbiol. 8, 243. doi: 10.3389/fcimb.2018.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Wolfert M. A., Wei N., Huizinga R., Jacobs B. C., Boons G.-J. (2020. a). Chemoenzymatic synthesis of Campylobacter jejuni lipo-oligosaccharide core domains to examine Guillain–Barré syndrome serum antibody specificities. J. Am. Chem. Soc. 142, 19611–19621. doi: 10.1021/jacs.0c08583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang S., He M., Zhang Y., Fu Y., Liang H., et al. (2018). Prevalence and molecular characterization of Campylobacter spp. isolated from patients with diarrhea in shunyi, Beijing. Front. Microbiol. 9, 52. doi: 10.3389/fmicb.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhou G., Gao P., Gu Y., Wang H., Zhang S., et al. (2020. b). Gastroenteritis outbreak caused by campylobacter jejuni - Beijing, China, august 2019. China CDC Wkly 2, 422–425. doi: 10.46234/ccdcw2020.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurchachaiwong W., Serichantalergs O., Lertsethtakarn P., Ruamsap N., Srijan A., Oransathid W., et al. (2020). Enteric etiological surveillance in acute diarrhea stool of united states military personnel on deployment in Thailand 2013-2017. Gut Pathog. 12, 17. doi: 10.1186/s13099-020-00356-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S., Furrie E., Macfarlane G. T., Dillon J. F. (2007). Microbial colonization of the upper gastrointestinal tract in patients with barrett's esophagus. Clin. Infect. Dis. 45, 29–38. doi: 10.1086/518578 [DOI] [PubMed] [Google Scholar]
- Mahendran V., Liu F., Riordan S., Grimm M., Tanaka M., Zhang L. (2016). Examination of the effects of Campylobacter concisus zonula occludens toxin on intestinal epithelial cells and macrophages. Gut Pathog. 8, 18. doi: 10.1186/s13099-016-0101-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran V., Riordan S. M., Grimm M. C., Tran T. A., Major J., Kaakoush N. O., et al. (2011). Prevalence of Campylobacter species in adult crohn's disease and the preferential colonization sites of Campylobacter species in the human intestine. PloS One 6, e25417. doi: 10.1371/journal.pone.0025417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S. M., Zhang L., Day A. S., Leach S. T., Lemberg D. A., Mitchell H. (2010). Campylobacter concisus and other Campylobacter species in children with newly diagnosed crohn's disease. Inflammation Bowel Dis. 16, 1008–1016. doi: 10.1002/ibd.21157 [DOI] [PubMed] [Google Scholar]
- Marder E. P., Cieslak P. R., Cronquist A. B., Dunn J., Lathrop S., Rabatsky-Ehr T., et al. (2017). Incidence and trends of infections with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance — foodborne diseases active surveillance network, 10 U.S. sites 2013–2016. Morbid. mortal. weekly Rep. 66, 397. doi: 10.15585/mmwr.mm6615a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. P., Griffin P. M., Cieslak P. R., Dunn J., Hurd S., Jervis R., et al. (2018). Preliminary incidence and trends of infections with pathogens transmitted commonly through food — foodborne diseases active surveillance network, 10 U.S. sites 2006–2017. Morbid. Mortal. Weekly Rep. 67, 324. doi: 10.15585/mmwr.mm6711a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri M., Haji Rezaei M., Aalinezhad M., Sharif M. R., Akhavan T. (2016). Clinical and laboratory characteristics of pediatric Campylobacter spp. acute gastroenteritis. Arch. Pediatr. Infect. Dis., 4, 1–6. doi: 10.4414/smw.2022.w30113 [DOI] [Google Scholar]
- Meistere I., Ķibilds J., Eglīte L., Alksne L., Avsejenko J., Cibrovska A., et al. (2019). Campylobacter species prevalence, characterisation of antimicrobial resistance and analysis of whole-genome sequence of isolates from livestock and humans, Latvia 2008 to 2016. Euro Surveill. 24, 1800357. doi: 10.2807/1560-7917.ES.2019.24.31.1800357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health Singapore (2015). Communicable diseases surveillance Singapore 2014. (College of Medicine Building: Singapore, MOH; ). [Google Scholar]
- Ministry of Health Singapore (2016). Communicable diseases surveillance Singapore 2015. (College of Medicine Building, Singapore: MOH; ). [Google Scholar]
- Ministry of Health Singapore (2017). Communicable diseases surveillance Singapore 2016. (College of Medicine Building, Singapore: MOH; ). [Google Scholar]
- Ministry of Health Singapore (2018). Communicable diseases surveillance Singapore 2017. (College of Medicine Building, Singapore: MOH; ). [Google Scholar]
- Ministry of Health Singapore (2019). Communicable diseases surveillance Singapore 2018. (College of Medicine Building, Singapore: MOH; ). [Google Scholar]
- Moffatt C. R., Fearnley E., Bell R., Wright R., Gregory J., Sloan-Gardner T., et al. (2020). Characteristics of Campylobacter gastroenteritis outbreaks in Australia 2001 to 2016. Foodborne Pathog. Dis. 17, 308–315. doi: 10.1089/fpd.2019.2731 [DOI] [PubMed] [Google Scholar]
- Mohakud N. K., Patra S. D., Kumar S., Sahu P. S., Misra N., Shrivastava A. K. (2019). Detection and molecular typing of campylobacter isolates from human and animal faeces in coastal belt of odisha, India. Indian J. Med. Microbiol. 37, 345. doi: 10.4103/ijmm.IJMM_19_394 [DOI] [PubMed] [Google Scholar]
- Montgomery M. P., Robertson S., Koski L., Salehi E., Stevenson L. M., Silver R., et al. (2018). Multidrug-resistant Campylobacter jejuni outbreak linked to puppy exposure–united states 2016–2018. Morbid. Mortal. Weekly Rep. 67, 1032. doi: 10.15585/mmwr.mm6737a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen N., Jonasson S. A., Lavesson I. V., Emberland K. E., Litleskare S., Wensaas K.-A., et al. (2021). Characteristics of hospitalized patients during a large waterborne outbreak of Campylobacter jejuni in Norway. PloS One 16, e0248464. doi: 10.1371/journal.pone.0248464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhya I., Thomson J. M., Hansen R., Berry S. H., El-Omar E. M., Hold G. L. (2011). Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. PloS One 6, e21490–e21490. doi: 10.1371/journal.pone.0021490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H., Bodhidatta L., Sornsakrin S., Khadka B., Pokhrel A., Shakya S., et al. (2019). Traveler's diarrhea in Nepal-changes in etiology and antimicrobial resistance. J. Travel Med. 26, taz054. doi: 10.1093/jtm/taz054 [DOI] [PubMed] [Google Scholar]
- National Food Institute (2021). Annual report on zoonoses in Denmark 2020. (Kemitorvet, Denmark: DTU Food; ) [Google Scholar]
- National Veterinary Institute (2019). Surveillance of infectous diseases in animals and humans in Sweden 2018(Uppsala, Sweden: SVA; ). [Google Scholar]
- National Veterinary Institute (2020). Surveillance of infectous diseases in animals and humans in Sweden 2019(Uppsala, Sweden: SVA; ). [Google Scholar]
- NNDSS Annual Report Working Group (2016). Australia’s notifiable disease status 2014: Annual report of the national notifiable diseases surveillance system, (Canberra, Australia: CDI; ) Vol. 40. 1–98. [DOI] [PubMed] [Google Scholar]
- NNDSS Annual Report Working Group (2019). Australia's notifiable disease status 2015: Annual report of the national notifiable diseases surveillance system, (Canberra, Australia: CDI; ) Vol. 43. 1–192. [PubMed] [Google Scholar]
- NNDSS Annual Report Working Group (2021). Australia's notifiable disease status 2016: Annual report of the national notifiable diseases surveillance system, (Canberra, Australia: CDI; ) Vol. 45. 1–196. [DOI] [PubMed] [Google Scholar]
- Nohra A., Grinberg A., Midwinter A. C., Marshall J. C., Collins-Emerson J. M., French N. P. (2016). Molecular epidemiology of Campylobacter coli strains isolated from different sources in new Zealand between 2005 and 2014. Appl. Environ. Microbiol. 82, 4363–4370. doi: 10.1128/AEM.00934-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreen Z., Siddiqui F., Javed S., Wren B. W., Bokhari H. (2020). Transmission of multidrug-resistant Campylobacter jejuni to children from different sources in Pakistan. J. Glob. Antimicrob. Resist. 20, 219–224. doi: 10.1016/j.jgar.2019.07.018 [DOI] [PubMed] [Google Scholar]
- Okada K., Wongboot W., Kamjumphol W., Suebwongsa N., Wangroongsarb P., Kluabwang P., et al. (2020). Etiologic features of diarrheagenic microbes in stool specimens from patients with acute diarrhea in Thailand. Sci. Rep. 10, 4009. doi: 10.1038/s41598-020-60711-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova M., Alexandrova E., Donkov G., Mitova-Mineva Y., Kantardjiev T., Velev V. (2020). Campylobacter infections among Bulgarian children: molecular characterization and antimicrobial susceptibility. Biotechnol. Biotechnol. Equip. 34, 1038–1042. doi: 10.1080/13102818.2020.1817783 [DOI] [Google Scholar]
- Pavlova M. R., Dobreva E. G., Ivanova K. I., Asseva G. D., Ivanov I. N., Petrov P. K., et al. (2016). Multiplex PCR assay for identification and differentiation of Campylobacter jejuni and Campylobacter coli isolates. Folia Med. (Plovdiv) 58, 95–100. doi: 10.1515/folmed-2016-0016 [DOI] [PubMed] [Google Scholar]
- Pavlova M., Velev V., Karageorgiev M., Alexandrova E., Kamenov E., Kantardjiev T. (2019). Surveillance data on bacterial enterocolitis in Bulgaria for 2014-2018. Probl. Infect. Parasit. Dis. 47, 28–33. [Google Scholar]
- Pedati C., Koirala S., Safranek T., Buss B. F., Carlson A. V. (2019). Campylobacteriosis outbreak associated with contaminated municipal water supply — Nebraska 2017. Morbid. Mortal. Weekly Rep. 68, 169. doi: 10.15585/mmwr.mm6807a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada (2018. a). FoodNet Canada annual report 2016. (Ottawa, Canada: PHAC; ). [Google Scholar]
- Public Health Agency of Canada (2018. b). FoodNet Canada annual report 2017. (Ottawa, Canada: PHAC; ). [Google Scholar]
- Public Health Agency of Canada (2019). FoodNet Canada annual report 2018. (Ottawa, Canada: PHAC; ). [Google Scholar]
- Public Health Agency of Canada (2021) Reported cases from 1924 to 2019 in Canada - notifiable diseases on-line. Available at: https://dsol-smed.phac-aspc.gc.ca(Accessed 13 Sep 2021).
- Public Health England (2018) Campylobacter data 2008 to 2017. Available at: https://www.gov.uk/(Accessed 31 Jan 2022).
- Qu M., Zhang M., Zhang X., Jia L., Xu J., Chu Y., et al. (2019). Molecular and epidemiologyical analysis of a Campylobacter jejuni outbreak in China 2018. J. Infect. Dev. Ctries 13, 1086–1094. doi: 10.3855/jidc.11408 [DOI] [PubMed] [Google Scholar]
- Radziszewski F., Kucharczyk B., Sadkowska-Todys M. (2018). Campylobacteriosis in Poland in 2015 and 2016. 100th years Natl. Inst. Public Health Natl. Inst. Hyg. 72, 399–405. doi: 10.32394/pe.72.4.15 [DOI] [PubMed] [Google Scholar]
- Radziszewski F., Kucharczyk B., Sadkowska-Todys M. (2019). Campylobacteriosis in Poland in 2017. Przeglad Epidemiol. 73, 437–443. doi: 10.32394/pe.73.41 [DOI] [PubMed] [Google Scholar]
- Rahman M. A., Paul P. R., Hoque N., Islam S. S., Haque A. K. M. Z., Sikder M. H., et al. (2021). Prevalence and antimicrobial resistance of Campylobacter species in diarrheal patients in mymensingh, Bangladesh. BioMed. Res. Int. 2021, 9229485. doi: 10.1155/2021/9229485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan H., Jackson C., Hinton J. A. (2015). Screening and rapid identification of campylobacter spp. DNA by flaA PCR based method on chicken and human fecal samples in Egypt. Int. J. Poult. Sci. 14, 252. doi: 10.3923/ijps.2015.252.256 [DOI] [Google Scholar]
- Randremanana R. V., Razafindratsimandresy R., Andriatahina T., Randriamanantena A., Ravelomanana L., Randrianirina F., et al. (2016). Etiologies, risk factors and impact of severe diarrhea in the under-fives in moramanga and Antananarivo, Madagascar. PloS One 11, e0158862. doi: 10.1371/journal.pone.0158862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar R., Babazadeh D., Jonaidi-Jafari N. (2017). Prevalence of Campylobacter jejuni in adult patients with inflammatory bacterial diarrhea, East Azerbaijan, Iran. Acta Med. Mediterr. 33, 901–908. doi: 10.19193/0393-6384_2017_1s_134 [DOI] [Google Scholar]
- Rastyani S., Alikhani M. Y., Sedighi I., Kazemi S., Kohan H. F., Arabestani M. R. (2015). Campylobacter jejuni and Campylobacter coli in children with acute diarrhea in health centers of hamadan, Iran. Avicenna J. Clin. Microbiol. Infect. 2, 29791–29791. doi: 10.17795/ajcmi-29791 [DOI] [Google Scholar]
- Ray L. C., Collins J. P., Griffin P. M., Shah H. J., Boyle M. M., Cieslak P. R., et al. (2021). Decreased incidence of infections caused by pathogens transmitted commonly through food during the COVID-19 pandemic–foodborne diseases active surveillance network, 10 U.S. sites 2017–2020. Morbid. Mortal. Weekly Rep. 70, 1332. doi: 10.15585/mmwr.mm7038a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq A., Bokhari H., Noreen Z., Asghar R. M., Bostan N. (2019. a). Magnitude of rotavirus a and Campylobacter jejuni infections in children with diarrhea in twin cities of rawalpindi and Islamabad, Pakistan. BMC Infect. Dis. 19, 978. doi: 10.1186/s12879-019-4575-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq A., Bokhari H., Noreen Z., Asghar R. M., Bostan N. (2019. b). Magnitude of rotavirus a and Campylobacter jejuni infections in children with diarrhea in twin cities of rawalpindi and Islamabad, Pakistan. BMC Infect. Dis. 19, 978. doi: 10.1186/s12879-019-4575-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadkowska-Todys M., Kucharczyk B. (2016). Campylobacteriosis in Poland in 2013 and 2014. Przeglad Epidemiol. 70, 209–215. [PubMed] [Google Scholar]
- Serichantalergs O., Ruekit S., Pandey P., Anuras S., Mason C., Bodhidatta L., et al. (2017). Incidence of Campylobacter concisus and C. ureolyticus in traveler’s diarrhea cases and asymptomatic controls in Nepal and Thailand. Gut Pathog. 9, 47. doi: 10.1186/s13099-017-0197-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams S., Ghorbanalizadgan M., Haj Mahmmodi S., Piccirillo A. (2017). Evaluation of a multiplex PCR assay for the identification of Campylobacter jejuni and Campylobacter coli . Infect. Epidemiol. Microbiol. 3, 6–8. doi: 10.18869/modares.iem.3.1.6 [DOI] [Google Scholar]
- Shen H., Zhang J., Li Y., Xie S., Jiang Y., Wu Y., et al. (2016). The 12 gastrointestinal pathogens spectrum of acute infectious diarrhea in a sentinel hospital, shenzhen, China. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A. A., Ashraf M. A., Mustafa B. E., Raza M. M. (2021). Epidemiological trends of foodborne campylobacter outbreaks in the united states of America 1998–2016. Food Microbiol. 97, 103751. doi: 10.1016/j.fm.2021.103751 [DOI] [PubMed] [Google Scholar]
- Sorgentone S., Busani L., Calistri P., Robuffo G., Bellino S., Acciari V., et al. (2021). A large food-borne outbreak of campylobacteriosis in kindergartens and primary schools in pescara, Italy, may-June 2018. J. Med. Microbiol. 70, 001262. doi: 10.1099/jmm.0.001262 [DOI] [PubMed] [Google Scholar]
- Stanyevic B., Sepich M., Biondi S., Baroncelli G. I., Peroni D., Di Cicco M. (2021). The evolving epidemiology of acute gastroenteritis in hospitalized children in Italy. Eur. J. Pediatr. 181, 1–10. doi: 10.1007/s00431-021-04210-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statens Serum Institut (2018) Campylobacter infections 2016-2017. Available at: https://en.ssi.dk(Accessed 31 Aug 2021).
- Statens Serum Institut (2019) Campylobacter infections 2018-2019. Available at: https://en.ssi.dk(Accessed 31 Aug 2021).
- Tack D. M., Marder E. P., Griffin P. M., Cieslak P. R., Dunn J., Hurd S., et al. (2019). Preliminary incidence and trends of infections with pathogens transmitted commonly through food — foodborne diseases active surveillance network, 10 U.S. sites 2015–2018. Morbid. Mortal. Weekly Rep. 68, 369. doi: 10.15585/mmwr.mm6816a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack D. M., Ray L., Griffin P. M., Cieslak P. R., Dunn J., Rissman T., et al. (2020). Preliminary incidence and trends of infections with pathogens transmitted commonly through food — foodborne diseases active surveillance network, 10 U.S. sites 2016–2019. Morbid. Mortal. Weekly Rep. 69, 509. doi: 10.15585/mmwr.mm6917a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. A. M., Chui L., Lee B. E., Pang X. L., Ali S., Nettel-Aguirre A., et al. (2019). Performance of stool-testing recommendations for acute gastroenteritis when used to identify children with 9 potential bacterial enteropathogens. Clin. Infect. Dis. 69, 1173–1182. doi: 10.1093/cid/ciy1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Institute of Environmental Science and Research Ltd (2015). Notifiable diseases in new Zealand: Annual report 2014(Porirua, New Zealand: ESR; ). [Google Scholar]
- The Institute of Environmental Science and Research Ltd (2016). Notifiable diseases in new Zealand: Annual report 2015(Porirua, New Zealand: ESR; ). [Google Scholar]
- The Institute of Environmental Science and Research Ltd (2017). Notifiable diseases in new Zealand: Annual report 2016(Porirua, New Zealand: ESR; ). [Google Scholar]
- The Institute of Environmental Science and Research Ltd (2018). Annual summary of outbreaks in new Zealand 2016. (Porirua, New Zealand: ESR; ) [Google Scholar]
- The Institute of Environmental Science and Research Ltd (2019). Notifiable diseases in new Zealand: Annual report 2017(Porirua, New Zealand: ESR; ). [Google Scholar]
- The Institute of Environmental Science and Research Ltd (2020). Notifiable diseases in new Zealand: Annual report 2018(Porirua, New Zealand: ESR; ). [Google Scholar]
- The Institute of Environmental Science and Research Ltd (2021). Notifiable diseases in new Zealand: Annual report 2019(Porirua, New Zealand: ESR; ). [Google Scholar]
- Trajkovska-Dokic E., Mihajlov K., Mirchevska G., Kostovski M., Blazevska A., Stojkovska S. (2019). Antimicrobial susceptibility of Campylobacter isolates in the capital of north Macedonia. prilozi 40, 73–80. doi: 10.2478/prilozi-2019-0017 [DOI] [PubMed] [Google Scholar]
- Tzani M., Mellou K., Kyritsi M., Kolokythopoulou F., Vontas A., Sideroglou T., et al. (2020). Evidence for waterborne origin of an extended mixed gastroenteritis outbreak in a town in northern Greece 2019. Epidemiol. Infect. 149, e83. doi: 10.1017/S0950268820002976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrone L., Glass K., Stafford R. J., Kirk M. D., Selvey L., Team C. P. (2020). A meta-analysis of case-control studies examining sporadic campylobacteriosis in Australia and new Zealand from 1990 to 2016. Aust. N Z J. Public Health 44, 313–319. doi: 10.1111/1753-6405.12998 [DOI] [PubMed] [Google Scholar]
- Vetchapitak T., Misawa N. (2019). Current status of Campylobacter food poisoning in Japan. Food Saf., D–19-00001. doi: 10.14252/foodsafetyfscj.D-19-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu F., Zhang X., Chung H. K., Riordan S. M., Grimm M. C., et al. (2017). Campylobacter concisus genomospecies 2 is better adapted to the human gastrointestinal tract as compared with Campylobacter concisus genomospecies 1. Front. Physiol. 8, 543. doi: 10.3389/fphys.2017.00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang J., Sun H., Xia S., Duan R., Liang J., et al. (2015). Etiology of childhood infectious diarrhea in a developed region of China: Compared to childhood diarrhea in a developing region and adult diarrhea in a developed region. PloS One 10, e0142136. doi: 10.1371/journal.pone.0142136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. P., Zhou S. X., Wang X., Lu Q. B., Shi L. S., Ren X., et al. (2021). Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat. Commun. 12, 2464. doi: 10.1038/s41467-021-22551-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensley A., Padfield S., Hughes G. J. (2020). An outbreak of campylobacteriosis at a hotel in England: the ongoing risk due to consumption of chicken liver dishes. Epidemiol. Infect. 148, e32. doi: 10.1017/S095026882000028X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Lu X., Nossa C. W., Francois F., Peek R. M., Pei Z. (2009). Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137, 588–597. doi: 10.1053/j.gastro.2009.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H. (2020). Declining Vibrio parahaemolyticus and Salmonella, increasing Campylobacter and persisting norovirus food poisonings: inference derived from food poisoning statistics of Japan. Jpn J. Infect. Dis. 73, 102–110. doi: 10.7883/yoken.JJID.2019.247 [DOI] [PubMed] [Google Scholar]
- Yu H., Elbediwi M., Zhou X., Shuai H., Lou X., Wang H., et al. (2020). Epidemiological and genomic characterization of Campylobacter jejuni isolates from a foodborne outbreak at hangzhou, China. Int. J. Mol. Sci. 21, 3001. doi: 10.3390/ijms21083001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuwono C., Wehrhahn M. C., Liu F., Riordan S. M., Zhang L. (2021). The isolation of Aeromonas species and other common enteric bacterial pathogens from patients with gastroenteritis in an Australian population. Microorganisms 9, 1440. doi: 10.3390/microorganisms9071440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinhom M. M. A., Abdel-Latef G. K., Corke H. (2021). Prevalence, characterization, and control of Campylobacter jejuni isolated from raw milk, cheese, and human stool samples in beni-suef governorate, Egypt. Foodborne Pathog. Dis. 18, 322–330. doi: 10.1089/fpd.2020.2895 [DOI] [PubMed] [Google Scholar]
- Zhang L. (2015). Oral Campylobacter species: Initiators of a subgroup of inflammatory bowel disease? World J. Gastroenterol. 21, 9239–9244. doi: 10.3748/wjg.v21.i31.9239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lai S., Yu J., Geng Q., Yang W., Chen Y., et al. (2017). Etiology of acute diarrhea in the elderly in China: A six-year observational study. PloS One 12, e0173881. doi: 10.1371/journal.pone.0173881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li Y., Shao Y., Hu Y., Lou H., Chen X., et al. (2020. a). Molecular characterization and antibiotic resistant profiles of Campylobacter species isolated from poultry and diarrheal patients in southeastern China 2017–2019. Front. Microbiol. 11, 1244. doi: 10.3389/fmicb.2020.01244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Man S. M., Day A. S., Leach S. T., Lemberg D. A., Dutt S., et al. (2009). Detection and isolation of Campylobacter species other than C. jejuni from children with crohn's disease. J. Clin. Microbiol. 47, 453–455. doi: 10.1128/JCM.01949-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhang X., Liu Y., Jiang J., Shen Z., Chen Q., et al. (2020. b). Multilocus sequence types and antimicrobial resistance of Campylobacter jejuni and C. coli isolates of human patients from Beijing, China 2017–2018. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.554784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Yu F., Chen X., Cui D., Cheng Y., Xie G., et al. (2016). Enteropathogens in children less than 5 years of age with acute diarrhea: a 5-year surveillance study in the southeast coast of China. BMC Infect. Dis. 16, 434. doi: 10.1186/s12879-016-1760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.-H., Tian L., Cheng Z.-J., Liu W.-Y., Li S., Yu W.-T., et al. (2016). Viral and bacterial etiology of acute diarrhea among children under 5 years of age in wuhan, China. Chin. Med. J. 129, 1939. doi: 10.4103/0366-6999.187852 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Impact of COVID-19 on the reported incidence of campylobacteriosis. Reported incidence are presented as per 100,000 population. The average incidence between 2014-2019 was lower than that in 2020 due to COVID-19 pandemic in most of the countries, except France, Lithuania and Malta.