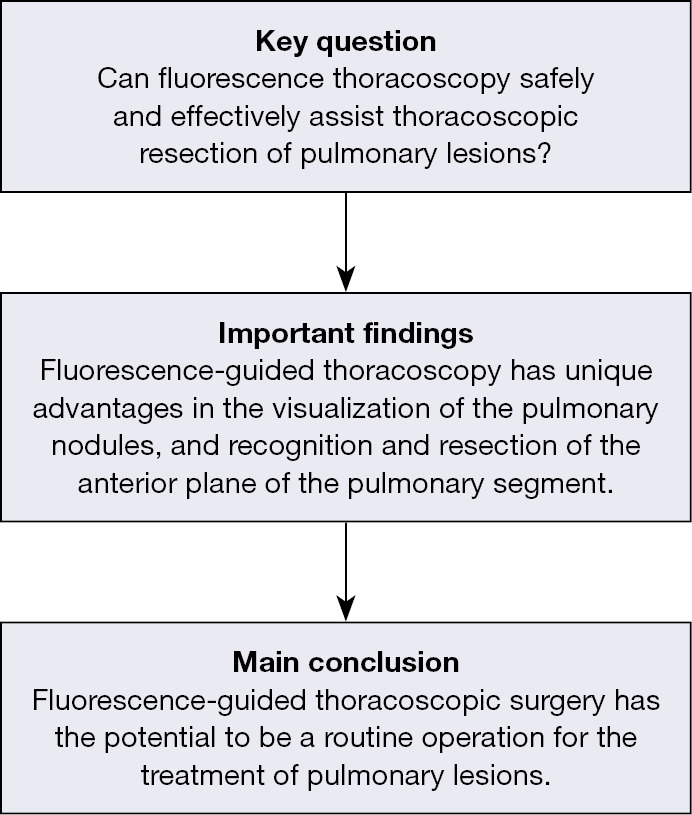
Abstract
The use of the white-light thoracoscopy is hampered by the low contrast between oncologic margins and surrounding normal parenchyma. As a result, many patients with in situ or micro-infiltrating adenocarcinoma have to undergo lobectomy due to a lack of tactile and visual feedback in the resection of solitary pulmonary nodules. Near-infrared (NIR) guided indocyanine green (ICG) fluorescence imaging technique has been widely investigated due to its unique capability in addressing the current challenges; however, there is no special consensus on the evidence and recommendations for its preoperative and intraoperative applications. This manuscript will describe the development process of a consensus on ICG fluorescence-guided thoracoscopic resection of pulmonary lesions and make recommendations that can be applied in a greater number of centers. Specifically, an expert panel of thoracic surgeons and radiographers was formed. Based on the quality of evidence and strength of recommendations, the consensus was developed in conjunction with the Chinese Guidelines on Video-assisted Thoracoscopy, and the National Comprehensive Cancer Network (NCCN) guidelines on the management of pulmonary lesions. Each of the statements was discussed and agreed upon with a unanimous consensus amongst the panel. A total of 6 consensus statements were developed. Fluorescence-guided thoracoscopy has unique advantages in the visualization of pulmonary nodules, and recognition and resection of the anterior plane of the pulmonary segment. The expert panel agrees that fluorescence-guided thoracoscopic surgery has the potential to become a routine operation for the treatment of pulmonary lesions.
Keywords: Indocyanine green (ICG), near-infrared fluorescence, thoracoscopy, pulmonary lesions
Highlight box.
Key recommendations
• Evidence for the safety and effectiveness of fluorescent imaging in thoracic surgery appears convincing, representing an excellent approach with minimal complications and contraindications.
What was recommended and what is new?
• Fluorescence-guide thoracoscopy is a new surgical approach widely used for its multiple applications. However, its role in radical resection of lung lesions remains controversial and there is no consensus on this.
• This consensus reports recent advances in fluorescence-navigated thoracoscopy, summarizing the indications and its related complications. In particular, it could be an excellent approach for identifying pulmonary nodules, intersegmental planes during thoracoscopic segmentectomy, and for evaluating the surgical margin after excision. Conversely, considering the low-quality evidence achieved for the detection of sentinel lymph nodes, further studies are needed to evaluate its role on this topic.
What is the implication, and what should change now?
• The expert panel agrees that fluorescence-guided thoracoscopic surgery has the potential to become a routine operation for the treatment of pulmonary lesions.
Introduction
Although conventional white-light video-assisted thoracic surgery (VATS) has been widely accepted and implemented over the past decades, more sophisticated surgical techniques are increasingly available in thoracic surgery. The application of white-light thoracoscopy is restricted by the low contrast between important anatomical or pathological regions and surrounding normal parenchyma. Both a randomized clinical trial in Japan and the Surveillance, Epidemiology, and End Results (SEER) program of the U.S. National Cancer Institute have demonstrated similar survival rates for sublobar resections and lobectomy for patients with peripheral-type early-stage non-small cell lung cancer (NSCLC) (1,2). Precise anatomical lung segmentectomy preserves more lung function (3) and offers a better survival prognosis, especially in elderly patients, those who have undergone a previous pulmonary resection (4,5), and those with poor cardiopulmonary reserve (6,7). However, some patients exhibit poor or absent pulmonary fissure development, which leads to difficulty in the accurate identification of lung segments during surgery and subsequently excessive removal of pulmonary parenchyma, which has been associated with unfavorable prognosis and life status. These outcomes are contrary to the enhanced recovery after surgery (ERAS) concept (8,9).
Near-infrared (NIR) guided indocyanine green (ICG) fluorescence imaging has long been applied in procedures like abdominal surgery and has demonstrated oncologic advantages over conventional operative techniques. The technology can be utilized for pulmonary nodule localization, visualization of the intersegmental plane, reverse staining, positive staining, and watershed analysis of the target pulmonary artery (10-15). The use of ICG fluorochromes has not compromised patient safety and has been associated with reduced intraoperative complications and the risk of blood clots, with fewer side effects (16). Li et al. have demonstrated that percutaneously injected ICG can stay in the lung parenchyma for up to 6 days without compromising in surgical quality afforded to the patient (16). Conversely, NIR-guided segmentectomy, after endobronchial injection (11) or intravenous injection (17) of ICG solution, allows visualization separation of targeted lung segments from normal lung parenchyma under NIR fluorescence imaging and is not likely to be affected by anthracosis secondary to deposition of light-absorbing carbons (18,19).
It has been found that ICG is safe for use in patients, with a very low rate of anaphylactic complications and high tissue absorption. The use of ICG imaging reduces postoperative complications, the risk of embolism, and side effects. In addition, because the use of dilatation and collapse method is difficult to operate in the narrow space of a single lung segment, and not ideal in chronic obstructive pulmonary disease (COPD) and non-intubation surgery, the application of ICG imaging can be more convenient and reduce the operation time.
Other advantages of ICG include long duration (as mentioned above) and that ICG fluorescence is capable of deep tissue penetration and can assist in lesion localization.
However, NIR fluorescence imaging-guided resection of pulmonary lesions remains a novel technique and is not routinely performed outside of certain major academic medical centers. Therefore, the role of NIR fluorescence imaging in radical resection of lung lesions and postoperative rehabilitation remains controversial and there is no consensus on this. Here, expert consensus recommendations on NIR fluorescence-guided resection of pulmonary lesions were developed based on the evidence garnered from clinical trials, meta-analyses, case reports, and real-world surgical practice.
Methods
Literature review
An expert panel consisting entirely of thoracic surgeons was established. The medical subject terms, including “VATS”, “lung cancer”, “fluorescence”, “lung cancer”, “ICG”, “Intraoperative Molecular Imaging”, and “NIR”, were searched in the Embase, PubMed, and Cochrane Central Register of Controlled Trials databases. Reference lists of the identified eligible articles were further checked for relevance. The development process of the expert consensus is shown in Figure 1. The main references included were the Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery (2020 edition) (8), Expert Consensus on Diagnosis and Treatment Using Medical Thoracoscopy in China (2021 edition) (20), and Expert Consensus Document on Pulmonary Metastasectomy (21). A few of the general consensuses of VATS diagnosis and treatment will not be elaborated on in this document.
Figure 1.
The algorithm of consensus decision-making.
Quality assessment and data analysis
The quality of evidence and strength of recommendations were rated according to the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) approach (22).
Recommendations were based on the quality of evidence (high, moderate, low, and very low) but also on the balance between desirable and undesirable effects. Thus, strong recommendations were achievable from low-quality data and vice versa. All panel members voted for all the generated recommendations, and the consensus was defined as >75% agreement among all participants. Recommendations with a <75% agreement were revised and voted on again.
Results
The evidence base was provided for each item including expert recommendations, evidence quality, and evidence level grades were developed through group discussions (Table 1).
Table 1. The 6 consensus opinions reached by the panel.
| Recommendations | Evidence level | Recommendation grade |
|---|---|---|
| Route 1: Percutaneous ICG injection | ||
| ICG percutaneous injection under CT guidance | High | Strong |
| Route 2: ICG injection via the vessels of the target lung segment | ||
| Blocking the vessels of the target lung segment and injecting ICG via a peripheral/central vein can reveal the intersegmental plane by reverse staining | Middle | Strong |
| In patients with bronchial stenosis or poor lung dilation/collapse, the vascular reverse staining method is preferred to display the intersegmental plane compared with the dilation and collapse method | High | Strong |
| Because of the short development time when using the vascular reverse staining method, the intersegmental plane boundary can be marked quickly or repeatedly staining with repeated injections of ICG; the pulmonary veins can also be clamped to extend the staining duration appropriately |
Middle | Strong |
| Route 3: ICG injection via airway approach | ||
| Bronchoscopic guidance of ICG injection into the target lung segment via the bronchus to stain the target lung segment | Middle | Strong |
| ENB allows ICG to be injected and positioned around the target lesion through an ultra-thin bronchoscope | Middle | Strong |
ICG, indocyanine green; CT, computed tomography; ENB, electromagnetic navigation bronchoscopy.
Localization of pulmonary lesions: ICG percutaneous injection under computed tomography guidance
Preoperative ICG injection was performed under computed tomography (CT)-guided puncture to locate pulmonary nodules. Precise preoperative localization of pulmonary nodules is a key prerequisite to the identification of lesions. Localization of small pulmonary nodules is challenging. Notably, when ground-glass nodules (GGNs) do not alter the surface of the visceral pleura, the elevation of tumors cannot be perceived sin the deflated lung during VATS, making localization difficult. Suzuki et al. observed a 54% overall conversion rate from VATS to thoracotomy which increased to 63% for nodules ≤10 mm in size and >5 mm from the pleural surface (23). A NIR thoracoscopy can detect ICG injected into the lungs as a marker (24). ICG can be topically administered into the nodules 24 hours before surgery (25). Both percutaneous and bronchoscopic injection techniques have been shown to be safe and reliable methods for lung nodule localization (26-28). As percutaneous marking is not dependent on bronchial branching, it can easily be performed by interventional radiologists, using the same technique as CT-guided biopsy. The success rate of a single marking using the CT-guided percutaneous approach is high, and there is minimal risk for retained foreign bodies or vascular embolism (16).
However, there are some details to heed when applying this technique. After ICG injection, negative pressure needle extraction is required to avoid ICG overflow resulting in extensive development of the pleura. The depth of the lung lesion also affects the result of ICG imaging. According to the experience of different institutions, the ICG imaging effect is best when the depth of the lung lesion is less than 3 cm or pulmonary nodules are located outside 1/3 of the lung tissue. For some patients with extensive chest adhesion, severe emphysema, or poor pulmonary perfusion, the time of ICG-enhanced staining is shortened, and ICG may not play a full role. ICG staining should be used with caution in these patients (29). Furthermore, complications may also occur during CT-guided percutaneous injection puncture due to improper operation, it may damage the visceral pleura and lead to pneumothorax.
Recommendation
Percutaneous marking is not dependent on bronchial branching, it can easily be performed by interventional radiologists, using the same technique as CT-guided biopsy. The success rate of a single marking using the CT-guided percutaneous approach was high. In terms of cost, the CT-guided percutaneous approach costs less than other methods because it involves only the expense of using the CT scanner unless complications, such as severe pneumothorax, occur.
ICG percutaneous injection under CT guidance.
Evidence level: high.
Recommendation grade: strong.
Localization of pulmonary lesions: ICG injection via targeted vascular occlusion
After occlusion of target arteries and veins, ICG was injected through the peripheral/central vein to image the unresected lung segment. The intravenous injection method should proceed as follows: based on the results of preoperative CT or three-dimensional (3D) reconstruction, the vessels in the diseased lung segment(s)/subsegment(s) are transected or temporarily occluded to dissect the diseased lung tissue. Next, a prepared sterile solution of ICG is intravenously administered. Ideally, by the ICG reverse staining, a visual boundary will appear between the preserved segment and the target segment (the target lung segment will be dark on negative contrast, while the other lung segments will be green because the fluorescent contrast agent cannot enter the segmental vessel after dissection) (17). The intersegmental planes (ISPs) between the target and preserved segments can be visualized by infrared thoracoscopy (IRT). After the target lung segment can be visually separated from the other segments, it is removed with a linear cutter/stapler (30). The ICG solution diffuses within 3–5 minutes. With the help of infrared light, the lines among lung segments can be seen clearly. The target lung segments can be removed along the boundaries (31). The lung tissue is endoscopically placed in a sterile bag, removed from the chest cavity, and immediately sent for pathological evaluation. The extent of surgical resection (lobectomy vs. segmentectomy) will depend on the histopathological findings of the frozen sectional analysis and institutional practices (15).
The intravenous injection method is simple and easy to perform but has the disadvantage of the ICG being rapidly metabolized by the liver through the bronchial circulation, which leads to a short visualization time. However, repeated angiographies can be performed and may be helpful; otherwise, intraoperative clamping of the pulmonary lobar veins may enable prolongation of washout time (32). In addition, previous literature reports have demonstrated that ICG-guided watershed analysis of the target pulmonary artery for nodule localization is safe and effective, especially in selected patients undergoing thoracoscopic wedge resection, which may be a feasible and attractive alternative method for localizing non-palpable pulmonary nodules (33).
In addition, when a patient has a combination of emphysema and underdeveloped lung fissures, the alveolar inflation-deflation method will make it difficult to perform lung ISP identification. The use of NIR fluorescence thoracoscopy is a potential solution to this challenge. The combination of NIR fluorescence imaging with the intravenous ICG method to generate ISPs has gained considerable momentum recently due to its simplicity and rapidity. Misaki et al. were among the early pioneers and described their experience with ICG for the first time in pulmonary segmentectomy in 8 patients (17). In a clinical trial performed by Kasai et al., a total of 30 consecutive patients who underwent NIR-guided segmentectomy were evaluated by 2- and 1-wavelength IRT; the rate of successful identification of ISPs was 93%; the 1-wavelength group (0.5 mg/kg) had a lower ICG dose than did the 2-wavelength group (3.0 mg/kg) and longer staining duration, although the image quality was relatively poorer (34). Additional studies have focused on the evaluation of the true success rate of IRT with ICG during VATS, with multiple studies reporting high rates of ISP identification. In particular, Tarumi et al. demonstrated a success rate of 84.6% (11/13) (35), Mun et al. 95% (19/20) (36), and Bédat et al. 88% (59/67 patients) (37). In a prospective trial by Motono et al., the ISP identification efficiency was 90% (18/20) (38). Pischik et al. successfully identified ISPs in 86 of 90 consecutive patients, with a success rate of 95.6% (29). The ISP identification efficiency even reached 100% in research conducted by Guigard et al. (22/22) (39) and Chen et al. (19/19) (40). In a prospective trial, Iizuka et al. successfully identified ISPs in 98.6% of patients and found that smoking index (SI) (SI >800) and low attenuation areas >1.0% on CT were strong predictors of unfavorable ICG visibility (41). Recent studies have demonstrated that NIR fluorescence thoracoscopy is non-inferior to the inflation-deflation method in ISP identification. Notably, a retrospective study by Sun et al. found that ISPs in the fluorescence group were consistent with those in the inflation-deflation group, with the former having significantly shorter in-plane generation time and operative time than the latter (42,43). In addition, Soo Chang et al. applied the combination of NIR fluorescence imaging and intraoperative CT for ISP identification, and complete resection was achieved in all 12 consecutive patients using this approach (44).
However, some details need to be taken into account when applying the technique. The use of an ICG diluent is recommended to reduce the chance of adverse effects (38,45). The optimal cutoff volume of diluted ICG (0.0125 mg/mL) for insufflation was 8.91% of the calculated targeted pulmonary segment volume (46). However, in some cases, no fluorescent imaging can be seen after ICG localization or no pulmonary nodules can be found after excision. In this case, either an expanded excision or an anatomic excision with 3D reconstruction should be performed. Furthermore, due to the shorter staining duration of transvascular ICG injection, it may cause some difficulty in separating intersegmental planes during the prolonged use of energy instruments. In addition, intravascular ICG reverse staining is not recommended for patients who are allergic to iodine.
Recommendation
In patients with bronchial stenosis or poor lung dilation/collapse, the vascular reverse staining method is preferred to display the intersegmental plane compared with the dilation and collapse method. During intravenous injection of ICG or other ICG-based tracers, the pulmonary lobar veins can be clamped after the visual boundaries appear, and the boundaries can be marked with electrocautery, which helps to avoid a second injection due to the short visualization time. The use of an ICG diluent is recommended to ensure the duration and effectiveness of the imaging while reducing the chance of adverse effects.
ICG injection via targeted vascular occlusion.
Evidence level: middle.
Recommendation grade: strong.
Localization of pulmonary lesions: ICG injection via airway approach
By marking the target bronchus under the bronchoscope, ICG was directly injected into the corresponding target bronchus to image the lung segment to be resected. Bronchoscopic marking enables the injection of ICG-fluorescence (ICG-FL) markers into multiple lung sections without injuring the visceral pleura. Additionally, the bronchoscopic approach is more feasible for regions of the lung that are difficult to reach using a percutaneous approach, such as the subscapular area (26). Several bronchoscopic localization techniques have been advocated and validated in clinical trials, which overcome these adverse events associated with percutaneous needle marking (47-50). The endobronchial injection method offers relatively adequate visualization time for resecting the lung segment (31). In addition, describing the ISP of the lung with ICG is feasible despite the type of lung segmental anatomy, and is commonly utilized during lung segmentectomy (51).
The electromagnetic navigation bronchoscopy (ENB) approach was used to locate pulmonary nodules near the visceral pleura, based on thin-section CT reconstructed images. In this way, the ENB system, using an ultrathin bronchoscope (outer diameter: 2.8–3.5 mm), enabled deeper visualization into the tracheobronchial tree to reach the right position around the target lesion. Subsequently, dye marking was performed to inject ICG under the guidance of ENB, which can mark the position of the target lesion (52). In addition, for patients with multiple pulmonary nodules and inconvenient percutaneous puncture localization, ICG fluorescence navigation under ENB guidance is considered, which has certain advantages (53).
However, there are some disadvantages of the bronchoscopic-assisted localization technique: Localization accuracy has some limitations, the locator procedure is complicated, and the localization costs are higher.
Recommendation
For patients with inconvenient percutaneous puncture localization and multiple pulmonary nodules, ENB-guided ICG injection is recommended. In addition, in patients with pulmonary lesions with secondary bronchial signs, lesion localization with ENB alone may be superior to CT-guided lung puncture localization because of similar accuracy but lower complication rates in the former (54).
ICG injection via airway approach.
Evidence level: middle.
Recommendation grade: strong.
Discussion
Detection of subcentimeter pulmonary nodules using conventional techniques such as tactile and visual feedback is often difficult. These challenges are further exacerbated when minimally invasive techniques are utilized. Therefore, various techniques such as the inflation-deflation method have been proposed to assess the oncologic feasibility of sub-global resections. During segmentectomy, the inflation-deflation method is challenging to perform, particularly in patients with coexisting emphysema and/or poorly developed lobar fissures, as well as being highly dependent on the skills of the operator, which can be limited in small-volume centers. IMI-guided resections have increasingly been recognized due to their ability to detect small pulmonary nodules that are difficult to find during conventional surgery as well as allow adequate visualization of resected lung segments. Several clinical trials using ICG-FL imaging have described novel findings (55,56).
According to previous reports’ findings, we summarized the indications of fluorescence imaging for thoracoscopic lung surgery and its related complications. It can be used for pulmonary nodule localization, visualization of the intersegmental plane, reverse staining, positive staining, watershed analysis of the target pulmonary artery.
The ICG-guided thoracic surgery is very safe with minimal complications and contraindications. The reported incidence of adverse reactions after ICG use is quite low (approximately 0.05%) (57). The vast majority of complications are mild, including pneumonia and air leaks, which are similar to those following white-light thoracoscopy, and require no special intervention (11,29,32,35,40,58-60). In addition, allergic reactions to ICG may be mediated by a dose-dependent pseudoallergic mechanism (61). Intravenous ICG doses for segmentectomy range from 0.25 to 2 mg/kg (and perhaps lower at 2 mg/kg), and no ICG-induced complications have been reported (17,34-36,38,41,43,62-64). In addition, low-dose ICG may achieve a clear ISP while avoiding anaphylactic shock and pharmacological toxicity; thus, ICG is generally considered safe.
Yang et al. preoperatively marked pulmonary nodules using CT-guided percutaneous or electromagnetic navigation bronchoscopic injection techniques; after the marking, none of the patients experienced a risk of bleeding or required chest tube drainage (28). Additionally, Wang et al. localized pulmonary nodules by injecting 0.1 mL of ICG under the guidance of a magnetic navigation bronchoscope. A total of 20 nodules were successfully removed in all 16 patients, and no bleeding or other complications occurred after the localization (65). Finally, Zhong et al. performed wedge resection of pulmonary nodules in 30 patients. The surgical margins were all negative on final pathology in all included cases, and none of the patients needed additional resection (59).
Matsuura et al. performed ICG (0.25 mg/kg)-based navigation for NIR thoracoscopic segmentectomy in 149 patients. Neither ICG-related adverse events nor procedure-related major complications occurred (66). The 5-year overall survival (OS) and recurrence-free survival (RFS) rates were 91.8% and 98%, respectively. Localized recurrence at the resected site did not occur in any patient (67). Similarly, a prospective study reported a good safety profile for intravenous low-dose ICG solution, with no adverse events occurring after surgery in any of the study patients, and complications occurring in 5 (25%) of the 20 patients. Another study compared the intraoperative and postoperative results in patients undergoing segmentectomy under the guidance of IRT with ICG versus the lung inflation-deflation cohort; it was found that the two groups showed no significant difference in operative time, intraoperative bleeding, or complications (35). Similarly, a propensity-score matching study found that there was no difference between NIR thoracoscopy and conventional thoracoscopy in terms of operative time and postoperative complications, with shorter hospital stays observed in the NIR thoracoscopy group (11). Liu et al. found that, compared with the inflation-deflation method, segmentectomy using NIF imaging is feasible for patients with chronic lung diseases, conferring a better ISP, shorter operation time, fewer complications, and faster recovery (67). In a retrospective analysis, Sun et al. found that the postoperative duration of air leaks was shorter in the ICG group than in the inflation-deflation group, whereas there were no significant differences in bleeding volume, chest tube duration, postoperative hospital stays, surgical margin width, or other postoperative complications (42). Geraci et al. successfully performed robotic segmentectomy under the guidance of NIR in 245 consecutive patients (68). An R0 resection was achieved when using ICG either bronchoscopically or intravenously. No postoperative 30- or 90-day death was noted; the average length of stay was 3.1 days (69). Similarly, Okumura et al. (60) demonstrated the feasibility of ICG-guided assessment in their cohort of 92 patients undergoing segmentectomy.
Wada et al. demonstrated successful completion of segmentectomy following bronchoscopic injection of ICG in 87% (13/15) of patients, and no postoperative adverse events were reported (30). Elkhouly et al. explored segmental resection after endobronchial injection of ICG diluted to 10% in 3 patients. There were no reported side effects, and no recurrence occurred during the 12 and 15 months of follow-up, respectively (31). In a propensity-matched analysis, Sekine et al. found that the short- and long-term outcomes of segmentectomy were similar between an ICG bronchoscopic injection group and an inflation-deflation method group (69). Therefore, evidence for the safety and effectiveness of fluorescent imaging for thoracoscopic sublobectomy appears convincing.
Conclusions
This consensus document summarizes recent advances in fluorescence-navigated thoracoscopy, with an attempt to further promote its application of radical resection of pulmonary lesions under the guidance of fluorescence.
Questions to be further discussed and considered
Which method do you think is better for the identification of ISPs: the inflation-deflation method or fluorescence imaging?
Chang Young Lee: I think that fluorescence imaging is a better method for the identification of ISPs during thoracoscopic segmentectomy. But there are some disadvantages or limitations to the fluorescence technique, so surgeons should know how to use the inflation-deflation technique correctly.
Eugenio Pompeo: In our view, fluorescence imaging provides a more clear view of the ISP during lung segmentectomy.
Feredun Azari: Clearly fluorescence imaging is superior in visualizing the ISPs. Currently, intuitive technologies have employed ICG NIR cameras in their robotic surgical devices which allows intersegmental anatomic delineation. This is done in realtime and can be performed in situ in the thoracic cavity.
Hitoshi Igai: I consider that fluorescence imaging is more useful and convenient especially in thoracoscopic surgery because inflated lung sometimes hinders surgeons from getting a good operative view. In addition, the inflation-deflation method cannot work appropriately in the emphysematous lung.
Hyun Koo Kim: Fluorescent imaging is better.
Marco Andolfi: With the spread of the VATS approach, I think fluorescence imaging represents the best method for the identification of ISPs.
Indeed, although to date the most common maneuver used for delineation of the ISP consists of the inflation-deflation method, some shortcomings, such as the limited and narrow surgical view due to the overinflated lungs, and the intra-parenchymatous diffusion of air, resulting in an imprecise marking of the ISP, in particular in patients with emphysema, were evidenced. On the other hand, fluorescence imaging allows overcoming these criticisms, guaranteeing an accurate detection of ISP without reducing surgical space in VATS and not requiring skilled surgical staff.
Masatsugu Hamaji: Both methods have advantages and disadvantages, whereas fluorescence imaging using ICG appears more straightforward and objective, without interfering with the thoracoscopic view by lung ventilation
Massimiliano Bassi: Intravenous ICG injection after suture of the segmentary pulmonary artery provides, in my opinion, the best view of the ISP. However, it requires dedicated tools that are not available worldwide.
Wolfram Karenovics: Both are good, but NIR imaging is much easier, quicker, and more convenient for VATS. This makes ICG to me superior (and I stopped using any insufflation method many years ago).
Yojiro Yutaka: I prefer fluorescence imaging to the inflation-deflation method because an inflated lung sometimes hinders the thoracoscopic view.
Yoshihisa Shimada: I used to perform the inflation-deflation method on segmentectomy, but currently do it with NIR because the latter technique is simple and convenient. When it comes to the best method for the identification of ISPs, the exposure of intersegmental pulmonary veins seems to be the best. For a lung nodule with emphysema, NIR is better. When NIR fails to identify the plane due to unknown reasons (some anatomical issues?), the inflation-deflation method is conducted. It depends on the case.
Yukinori Sakao: I think the fluorescence imaging method is much better than the inflation-deflation method.
Which is the most commonly used method for segmentectomy in your institution? Why?
Chang Young Lee: In our institution, ICG methods have been recently used.
Eugenio Pompeo: We use both the inflation-deflation method, which is simpler and easier to choose, and the ICG fluorescence imaging, which we consider more accurate but more complicated to perform.
Feredun Azari: This is a biased statement from my point of view. We are one of the largest IMI thoracic research groups in the world. We conduct very large NIR clinical trials and therefore have significant expertise in ICG-guided visualization. However, some of the surgeons at our center use the inflation-deflation method because they are comfortable with that technique, it does not require additional camera systems and adds cost to the operation
Hitoshi Igai: Our team usually uses fluorescence imaging to identify an ISP except for the patients with iodine allergies.
Hyun Koo Kim: Fluorescent imaging is a routine procedure in my hospital, which is easier to identify an ISP without an obstacle in the operative field.
Marco Andolfi: In our institution, we usually used the inflation-deflation method for typical segmentectomies. Indeed, in these cases, where only one ISP has to be identified, the demarcation is quick, easy, inexpensive, and no need for additional materials or skilled staff. Conversely, during complex/atypical segmentectomies, where the identification of 2 or 3 ISPs is often unreliable, we use systemic ICG injection that allows easy and accurate detection of ISPs based only on differential blood flow in the lung without the need for inflation.
Masatsugu Hamaji: Fluorescence imaging is now the standard method to identify ISPs because the methodology appears more straightforward and objective, without interfering with the thoracoscopic view by lung ventilation.
Massimiliano Bassi: In our institution, we routinely perform the inflation-deflation method to detect the ISP. Even if not accurate as the intravenous ICG florescence, this method is feasible, quick, intuitive, and can be performed even if an infrared light optics is not available.
Wolfram Karenovics: We do almost 100% of segmentectomies by VATS (uniportal VATS) for the last 3 years, 3 portals before. We have routinely used NIR-angiography in all segmentectomies since 2013 or 2014 to identify the ISP and in some cases to locate the lesion or to identify a sentinel node (in this case we inject the lesion with ICG at the beginning of anesthesia with ENB). We divide ISPs by stapler. Reasons: habitude, little complications, short hospital stay, few air leaks, ICG helps to delineate the segment and to make sure we cut the correct vessels (improves anatomical resection, assures good margins, quality control, etc.).
Yojiro Yutaka: As I mentioned above, fluorescence imaging is the most used in our institution, Kyoto University. Because inflation-deflation methods depend on the airway pressure or respiratory volume that the anesthesiologist applies. That is, the inflation-deflation line is dependent on the technique of inflation maneuver. The demarcation line by intravenous ICG injection can be clearly identified (sometimes unclearly) after the division of the correct vessels. We usually do that after the inflation of the lobe because intralobar circulation can be improved by ventilation. Additionally, an inflated lung sometimes hinders thoracoscopic view because the remaining lung expands after the division of the target bronchus. If I apply inflation-deflation methods, I ask the anesthesiologist to inflate only the target segment, which can check the anatomy of the target bronchus via a bronchoscope. Under ICG mode, surgeons can follow the bronchial anatomy and identify the target bronchus as a fluorescent color even in the clean surgical field. It is a tip to identify the correct bronchus to be resected.
Yoshihisa Shimada: NIR is simple and convenient.
Yukinori Sakao: We routinely use the fluorescence imaging method. I think that the method is simple, accurate, and safe.
Please describe your expectations concerning fluorescence thoracoscopy
Chang Young Lee: I think that lung cancer-specific agents will be available in the near future so small-sized lung tumors will be detected without any localization methods preoperatively using the infrared camera.
Eugenio Pompeo: I believe it will be increasingly employed in lung segmentectomy provided dedicated cameras will be easily available and widespread.
Feredun Azari: As a researcher in this field, the enthusiasm in this area will grow. IMI and fluorescence-guided resections will become commonplace over the coming decade. The FDA recently approved cytalux for fluorescence-guided surgery and will potentially evaluate it in lung cancer surgery. There has been an increasing number of tracers and technological advances in this field. I am excited about what the future holds for fluorescence-guided thoracoscopy.
Hitoshi Igai: Fluorescence imaging technique is already widespread worldwide, especially to identify an ISP in thoracoscopic segmentectomy.
However, the procedure to locate the tumor itself using fluorescence imaging has not been established. More and more unpalpable tumors have been recently detected in line with the advancement of computed tomography. In such a case, the procedure to locate the tumor itself can help surgeons resect the tumor with sufficient margin.
Hyun Koo Kim: Localization, identification of ISP, SLN, and the tumor itself imaging could be applied in near future.
Marco Andolfi: I think a fluorescence thoracoscopy is an excellent approach, very useful for thoracic surgeons, especially now that hybrid ORs are starting to spread worldwide. Based on published data and the extension of the surgical indication to elderly patients with co-morbidities and younger patients with early-stage NSCLC or GGO, I see a bright future for this new method in particular concerning its application for identifying small pulmonary nodules, ISPs during thoracoscopic segmentectomy, and for evaluating the surgical margin after excision. Conversely, as previously reported, considering the low-quality evidence achieved for the detection of sentinel lymph nodes, I think that further studies are needed to evaluate its role in the context of lymph node sampling in early-stage lung cancer.
Masatsugu Hamaji: I consider the role of fluorescence thoracoscopy will expand. We previously performed prospective clinical trials utilizing fluorescence thoracoscopy: virtual-assisted lung mapping by ICG and NIR imaging using intravenous ICG to locate pulmonary metastases.
Massimiliano Bassi: I believe that the advancement of knowledge and available tools will lead ICG-guided thoracoscopy to become a routine practice worldwide in the future. The advantages of the ICG in the localization of small pulmonary nodules and the detection of the ISPs during segmentectomy are already evident. I believe that in the future selective lymph node sampling in early-stage lung cancer through the ICG-guided detection of sentinel lymph nodes will become an effective option. However, before employing technical solutions, a definitive role of the sentinel lymph nodes in lung cancer must be achieved.
Wolfram Karenovics: Many of them have been mentioned already: Identification of segment limits and ISPs, identification of small lesions, identification of sentinel nodes, quality control, the possibility of repeating the injection, check perfusion of muscle flaps, possibly check perfusion of bronchial anastomosis, real-time analysis of lung perfusion.
Yojiro Yutaka: Now I prefer uniportal VATS than robot-assisted thoracic surgery (RATS). ICG mode in RATS is not satisfactory in terms of image sharpness, so the improvement of imaging in RATS seems to be necessary.
Yoshihisa Shimada: NIR techniques are highly useful for VATS. We also use this to check capillary blood flows after bronchoplasty.
Yukinori Sakao: I expect it to be applied as a method for distinguishing tumors from inflammatory lesions and for sentinel lymph node navigation.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-810/coif). Dr. WZ serves as an unpaid associate Editor-in-Chief of Translational Lung Cancer Research from February 2021 to January 2023. FA has been named in a provisional IMI guided optical biopsy patent outside the scope of this manuscript. ADLS reports consulting fees from Medela, speaker’s honoraria from Astra Zeneca, Nestle, and Medtronic. ADLS serves as an International Director in Society of Thoracic Surgeons, as a Council Member of Asian Society for Cardio-Vascular & Thoracic Surgery, and as a Board Member of the Asia Thoracoscopic Education Platform. The other authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. 10.1016/j.jtcvs.2019.03.090 [DOI] [PubMed] [Google Scholar]
- 2.Moon MH, Moon YK, Moon SW. Segmentectomy versus lobectomy in early non-small cell lung cancer of 2 cm or less in size: A population-based study. Respirology 2018;23:695-703. 10.1111/resp.13277 [DOI] [PubMed] [Google Scholar]
- 3.Nomori H, Shiraishi A, Cong Y, et al. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2018;53:640-7. 10.1093/ejcts/ezx357 [DOI] [PubMed] [Google Scholar]
- 4.Vecchiato M, Martino A, Sponza M, et al. Thoracic duct identification with indocyanine green fluorescence during minimally invasive esophagectomy with patient in prone position. Dis Esophagus 2020;33:doaa030. 10.1093/dote/doaa030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibas BJ, Costa-de-Carvalho RL, Pola-Dos-Reis F, et al. Video-assisted thoracoscopic thoracic duct ligation with near-infrared fluorescence imaging with indocyanine green. J Bras Pneumol 2019;45:e20180401. 10.1590/1806-3713/e20180401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yutaka Y, Sonobe M, Kawaguchi A, et al. Prognostic impact of preoperative comorbidities in geriatric patients with early-stage lung cancer: Significance of sublobar resection as a compromise procedure. Lung Cancer 2018;125:192-7. 10.1016/j.lungcan.2018.09.023 [DOI] [PubMed] [Google Scholar]
- 7.Razi SS, John MM, Sainathan S, et al. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res 2016;200:683-9. 10.1016/j.jss.2015.08.045 [DOI] [PubMed] [Google Scholar]
- 8.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. 10.1093/ejcts/ezy301 [DOI] [PubMed] [Google Scholar]
- 9.Gao S, Barello S, Chen L, et al. Clinical guidelines on perioperative management strategies for enhanced recovery after lung surgery. Transl Lung Cancer Res 2019;8:1174-87. 10.21037/tlcr.2019.12.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Teh CSC, Ishizawa T, et al. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg 2021;274:97-106. 10.1097/SLA.0000000000004718 [DOI] [PubMed] [Google Scholar]
- 11.Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. 10.1016/j.jtcvs.2012.01.079 [DOI] [PubMed] [Google Scholar]
- 12.Mangano A, Masrur MA, Bustos R, et al. Near-Infrared Indocyanine Green-Enhanced Fluorescence and Minimally Invasive Colorectal Surgery: Review of the Literature. Surg Technol Int 2018;33:77-83. [PubMed] [Google Scholar]
- 13.Nykänen AI, Mariscal A, Ali A, et al. Near-infrared fluorescence imaging during ex vivo lung perfusion: Noninvasive real-time evaluation of regional lung perfusion and edema. J Thorac Cardiovasc Surg 2022;164:e185-203. 10.1016/j.jtcvs.2022.02.048 [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Zhang Q, Wang Z, et al. Feasibility Investigation of Fluorescence Method in Uniport Thoracoscopic Anatomical Segmentectomy for Identifying the Intersegmental Boundary Line. Zhongguo Fei Ai Za Zhi 2021;24:756-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy GT, Azari FS, Bernstein E, et al. Targeted Intraoperative Molecular Imaging for Localizing Nonpalpable Tumors and Quantifying Resection Margin Distances. JAMA Surg 2021;156:1043-50. 10.1001/jamasurg.2021.3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Zeng Y, Liu J, et al. Indocyanine Green Remains in the Lung for up to 6 Days. Ann Thorac Surg 2020;110:e385-6. 10.1016/j.athoracsur.2020.03.076 [DOI] [PubMed] [Google Scholar]
- 17.Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. 10.1016/j.jtcvs.2010.07.020 [DOI] [PubMed] [Google Scholar]
- 18.Rho J, Lee JW, Quan YH, et al. Fluorescent and Iodized Emulsion for Preoperative Localization of Pulmonary Nodules. Ann Surg 2021;273:989-96. 10.1097/SLA.0000000000003300 [DOI] [PubMed] [Google Scholar]
- 19.Azari F, Kennedy G, Zhang K, et al. Effects of Light-absorbing Carbons in Intraoperative Molecular Imaging-Guided Lung Cancer Resections. Mol Imaging Biol 2022. [Epub ahead of print]. doi: . 10.1007/s11307-021-01699-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin F, Wang H, Li Q, et al. Expert consensus for diagnosis and treatment using medical thoracoscopy in China. J Thorac Dis 2020;12:1799-810. 10.21037/jtd-19-2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handy JR, Bremner RM, Crocenzi TS, et al. Expert Consensus Document on Pulmonary Metastasectomy. Ann Thorac Surg 2019;107:631-49. 10.1016/j.athoracsur.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. 10.1378/chest.115.2.563 [DOI] [PubMed] [Google Scholar]
- 24.Anayama T, Qiu J, Chan H, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg 2015;99:224-30. 10.1016/j.athoracsur.2014.07.050 [DOI] [PubMed] [Google Scholar]
- 25.Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. 10.1016/j.athoracsur.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anayama T, Hirohashi K, Miyazaki R, et al. Near-infrared dye marking for thoracoscopic resection of small-sized pulmonary nodules: comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg 2018;13:5. 10.1186/s13019-018-0697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chino S, Kuriyama K, Isohashi K, et al. Percutaneous localization of pulmonary nodules with CT guidance for lung resection: use of dyes. Nihon Igaku Hoshasen Gakkai Zasshi 2003;63:308-10. [PubMed] [Google Scholar]
- 28.Yang YL, Li ZZ, Huang WC, et al. Electromagnetic navigation bronchoscopic localization versus percutaneous CT-guided localization for thoracoscopic resection of small pulmonary nodules. Thorac Cancer 2021;12:468-74. 10.1111/1759-7714.13775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pischik VG, Kovalenko A. The role of indocyanine green fluorescence for intersegmental plane identification during video-assisted thoracoscopic surgery segmentectomies. J Thorac Dis 2018;10:S3704-11. 10.21037/jtd.2018.04.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wada H, Yamamoto T, Morimoto J, et al. Near-Infrared-Guided Pulmonary Segmentectomy After Endobronchial Indocyanine Green Injection. Ann Thorac Surg 2020;109:396-403. 10.1016/j.athoracsur.2019.08.083 [DOI] [PubMed] [Google Scholar]
- 31.Elkhouly AG, Cristino B, Pompeo E. A new method of infrared-fluorescence-enhanced thoracoscopic segmentectomy. Asian Cardiovasc Thorac Ann 2018;26:247-9. 10.1177/0218492317751827 [DOI] [PubMed] [Google Scholar]
- 32.Ito A, Takao M, Shimamoto A, et al. Prolonged intravenous indocyanine green visualization by temporary pulmonary vein clamping: real-time intraoperative fluorescence image guide for thoracoscopic anatomical segmentectomy. Eur J Cardiothorac Surg 2017;52:1225-6. 10.1093/ejcts/ezx233 [DOI] [PubMed] [Google Scholar]
- 33.Chu XP, Chen ZH, Lin SM, et al. Watershed analysis of the target pulmonary artery for real-time localization of non-palpable pulmonary nodules. Transl Lung Cancer Res 2021;10:1711-9. 10.21037/tlcr-20-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013;44:1103-7. 10.1093/ejcts/ezt168 [DOI] [PubMed] [Google Scholar]
- 35.Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. 10.1093/ejcts/ezt565 [DOI] [PubMed] [Google Scholar]
- 36.Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. 10.21037/jovs.2017.05.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bédat B, Triponez F, Sadowski SM, et al. Impact of near-infrared angiography on the quality of anatomical resection during video-assisted thoracic surgery segmentectomy. J Thorac Dis 2018;10:S1229-34. 10.21037/jtd.2018.01.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motono N, Iwai S, Funasaki A, et al. Low-dose indocyanine green fluorescence-navigated segmentectomy: prospective analysis of 20 cases and review of previous reports. J Thorac Dis 2019;11:702-7. 10.21037/jtd.2019.02.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guigard S, Triponez F, Bédat B, et al. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg 2017;25:703-9. 10.1093/icvts/ivx225 [DOI] [PubMed] [Google Scholar]
- 40.Chen R, Ma Y, Li C, et al. A Pilot Study of Pulmonary Segmentectomy With Indocyanine Green Near-Infrared Angiography. Surg Innov 2019;26:337-43. 10.1177/1553350618824914 [DOI] [PubMed] [Google Scholar]
- 41.Iizuka S, Kuroda H, Yoshimura K, et al. Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis 2016;8:985-91. 10.21037/jtd.2016.03.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Zhang Q, Wang Z, et al. Feasibility investigation of near-infrared fluorescence imaging with intravenous indocyanine green method in uniport video-assisted thoracoscopic anatomical segmentectomy for identifying the intersegmental boundary line. Thorac Cancer 2021;12:1407-14. 10.1111/1759-7714.13923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y, Zhang Q, Wang Z, et al. Is the near-infrared fluorescence imaging with intravenous indocyanine green method for identifying the intersegmental plane concordant with the modified inflation-deflation method in lung segmentectomy? Thorac Cancer 2019;10:2013-21. 10.1111/1759-7714.13192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soo Chang S, Go T, Matsuura N, et al. Intraoperative Navigation System during Thoracoscopic Segmentectomy for Non-palpable Pulmonary Tumors; Infrared Thoracoscopy (IRT)-Indocyanine Green (ICG) and Intraoperative Computed Tomography(CT)-assisted Method. Kyobu Geka 2019;72:488-93. [PubMed] [Google Scholar]
- 45.Jackson TL, Hillenkamp J, Knight BC, et al. Safety testing of indocyanine green and trypan blue using retinal pigment epithelium and glial cell cultures. Invest Ophthalmol Vis Sci 2004;45:2778-85. 10.1167/iovs.04-0320 [DOI] [PubMed] [Google Scholar]
- 46.Anayama T, Hirohashi K, Miyazaki R, et al. Fluorescence visualization of the intersegmental plane by bronchoscopic instillation of indocyanine green into the targeted segmental bronchus: determination of the optimal settings. J Int Med Res 2021;49:300060521990202. 10.1177/0300060521990202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. 10.1016/j.chest.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 48.Yi JH, Choi PJ, Bang JH, et al. Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review. J Thorac Dis 2018;10:E59-64. 10.21037/jtd.2017.12.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ueda K, Uemura Y, Sato M. Protocol for the VAL-MAP 2.0 trial: a multicentre, single-arm, phase III trial to evaluate the effectiveness of virtual-assisted lung mapping by bronchoscopic dye injection and microcoil implementation in patients with small pulmonary nodules in Japan. BMJ Open 2019;9:e028018. 10.1136/bmjopen-2018-028018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshiyasu N, Sato M, Yamaguchi H, et al. Risk Factors for Invisible Intraoperative Markings After Virtual-Assisted Lung Mapping. Ann Thorac Surg 2022;114:1903-10. 10.1016/j.athoracsur.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 51.Yotsukura M, Okubo Y, Yoshida Y, et al. Indocyanine green imaging for pulmonary segmentectomy. JTCVS Tech 2021;6:151-8. 10.1016/j.xjtc.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saito Y. Recent advances in electromagnetic navigation bronchoscopy for localization of peripheral pulmonary nodules. J Thorac Dis 2022;14:802-4. 10.21037/jtd-22-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng C, Fu X, Yuan Z, et al. Application of electromagnetic navigation bronchoscopy-guided microwave ablation in multiple pulmonary nodules: a single-centre study. Eur J Cardiothorac Surg 2022;62:ezac071. 10.1093/ejcts/ezac071 [DOI] [PubMed] [Google Scholar]
- 54.Kim YW, Kim HJ, Song MJ, et al. Utility and safety of sole electromagnetic navigation bronchoscopy under moderate sedation for lung cancer diagnosis. Transl Lung Cancer Res 2022;11:462-71. 10.21037/tlcr-21-846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tokuno J, Chen-Yoshikawa TF, Nakajima D, et al. Improved visualization of virtual-assisted lung mapping by indocyanine green. JTCVS Tech 2021;10:542-9. 10.1016/j.xjtc.2021.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamaji M, Chen-Yoshikawa TF, Minami M, et al. Near-Infrared Imaging Using Intravenous Indocyanine Green at a Conventional Dose to Locate Pulmonary Metastases: A Pilot Study. Thorac Cardiovasc Surg 2019;67:688-91. 10.1055/s-0038-1675346 [DOI] [PubMed] [Google Scholar]
- 57.Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529-33. 10.1016/S0161-6420(94)31303-0 [DOI] [PubMed] [Google Scholar]
- 58.Wang C, Liu Y, Yang L, et al. Effectiveness and safety of CT-guided percutaneous intrapulmonary injection of indocyanine green for localization of pulmonary nodules and ground glass opacity. Zhonghua Yi Xue Za Zhi 2020;100:538-40. [DOI] [PubMed] [Google Scholar]
- 59.Zhong L, Hu W, Li S, et al. Clinical study of video-assisted thoracoscopic surgery wedge resection in early-stage lung cancer by tumor mapping with indocyanine green. Wideochir Inne Tech Maloinwazyjne 2019;14:545-50. 10.5114/wiitm.2019.89986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okumura N. Thoracoscopic Stapler-based Segmentectomy Using Indocyanine Green(ICG)-infrared Thoracoscopy. Kyobu Geka 2019;72:496-500. [PubMed] [Google Scholar]
- 61.Lam S, MacAulay C, leRiche JC, et al. Detection and localization of early lung cancer by fluorescence bronchoscopy. Cancer 2000;89:2468-73. [DOI] [PubMed] [Google Scholar]
- 62.Kim Y, Rho J, Quan YH, et al. Simultaneous visualization of pulmonary nodules and intersegmental planes on fluorescent images in pulmonary segmentectomy. Eur J Cardiothorac Surg 2020;58:i77-84. 10.1093/ejcts/ezaa064 [DOI] [PubMed] [Google Scholar]
- 63.Tarumi S, Yokomise H. Video-assisted Thoracoscopic Segmentectomy Using Infrared Thoracoscopy with Indocyanine Green. Kyobu Geka 2016;69:671-5. [PubMed] [Google Scholar]
- 64.Wen CT, Liu YY, Fang HY, et al. Image-guided video-assisted thoracoscopic small lung tumor resection using near-infrared marking. Surg Endosc 2018;32:4673-80. 10.1007/s00464-018-6252-7 [DOI] [PubMed] [Google Scholar]
- 65.Wang G, Lin Y, Luo K, et al. Feasibility of injecting Fluorescent Agent under the Guidance of Electromagnetic Navigation Bronchoscopy in Pulmonary Nodule Resection. Zhongguo Fei Ai Za Zhi 2020;23:503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuura Y, Mun M, Ichinose J, et al. Recent fluorescence-based optical imaging for video-assisted thoracoscopic surgery segmentectomy. Ann Transl Med 2019;7:32. 10.21037/atm.2019.01.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z, Yang R, Cao H. Near-infrared intraoperative imaging with indocyanine green is beneficial in video-assisted thoracoscopic segmentectomy for patients with chronic lung diseases: a retrospective single-center propensity-score matched analysis. J Cardiothorac Surg 2020;15:303. 10.1186/s13019-020-01310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geraci TC, Ferrari-Light D, Kent A, et al. Technique, Outcomes With Navigational Bronchoscopy Using Indocyanine Green for Robotic Segmentectomy. Ann Thorac Surg. 2019;108:363-369. 10.1016/j.athoracsur.2019.03.032 [DOI] [PubMed] [Google Scholar]
- 69.Sekine Y, Itoh T, Toyoda T, et al. Precise Anatomical Sublobar Resection Using a 3D Medical Image Analyzer and Fluorescence-Guided Surgery With Transbronchial Instillation of Indocyanine Green. Semin Thorac Cardiovasc Surg 2019;31:595-602. 10.1053/j.semtcvs.2019.01.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


