Abstract
Exercise and diet are two essential interventions in weight control. The purpose of this study was to compare the effectiveness of two exercise training types during a ketogenic diet (KD) on appetite sensation, appetite-regulating hormones, and body composition in overweight or obese man. Thirty-six men, overweight or with obesity, voluntarily participated in this study. The participants were randomly assigned into three groups, including KD (n = 12), aerobic training during KD (AT-KD) (n = 12), and resistance training during KD (RT-KD) (n = 12) groups. The participants followed a low-carbohydrate diet for 6 weeks. Exercise training programs consisted of three sessions per week over 6 weeks. Appetite sensation was analyzed using a visual analogue scale (VAS) in fasting and postprandial states. The Enzyme-Linked Immunosorbent Assay (ELISA) method analyzed appetite-regulating hormones, including spexin, leptin, and acylated ghrelin, in a fasting state. Body composition was measured using bioelectrical impedance analysis (BIA). Furthermore, the ketosis state was monitored by measuring urinary ketones weekly. The results indicated that in both AT-KD and RT-KD groups, spexin and acylated ghrelin increased while leptin decreased without any between-group differences. Hunger and prospective food consumption (PFC) declined while satiety and fullness increased in all groups. The AT-KD group experienced a significant decrease in hunger and PFC, while fullness increased compared with the KD group. Fat mass, weight, and body mass index (BMI) decreased in all groups. Lean body mass increased in the RT-KD group (+2.66 kg) compared with both AT-KD and KD groups (−1.71 and −1.33 kg, respectively). This study demonstrated that AT-KD and RT-KD effectively altered appetite-regulating hormones and suppressed appetite sensation. In addition, both interventions had a favorable effect on weight loss and body fat reduction, with a more pronounced effect of RT-KD on maintaining lean body mass in overweight or obese men.
Keywords: Ketogenic diet, exercise training, appetite, appetite-regulating hormones, body composition
Impact Statement
To our knowledge, this is the first study conducted to compare the effectiveness of resistance and aerobic training during a ketogenic diet on appetite-regulating hormones, appetite sensation, and body composition in overweight or obese men. The efficacy of both ketogenic diet and exercise interventions in fat loss and weight control has been reported in previous studies. However, the effect of the types of training during a ketogenic diet on body composition improvement is still unclear. Our results indicate that both types of training during a ketogenic diet modified the appetite regulation hormones and suppressed the appetite sensation. Both interventions had a favorable effect on weight loss and body fat reduction, with a more pronounced effect of resistance training on maintaining lean body mass. Dietitians, trainers, and clinicians can prescribe both types of exercise training to clients with obesity during a ketogenic diet for weight control.
Introduction
Over the past 50 years, the increasing trend of obesity has become one of the most critical health challenges in the world. This problem arises not only in industrialized societies but also in developing countries. The prevalence of obesity is an important challenge that substantially increases the development of chronic diseases and diminishes quality of life and life-expectancy.1,2 Regular exercise and diet are the two essential interventions for improving body composition and weight control. A meta-analysis and systematic review study demonstrated that both interventions led to weight loss and reduced visceral adiposity. Moreover, despite a more prominent effect of the diet on total body weight loss, the role of exercise in reducing visceral adiposity is more pronounced. 3 A widely used diet for weight loss is the ketogenic diet (KD). Ketone bodies produced in the KD can be an alternative energy source to muscle, heart, and brain tissue. Most studies showed the effect of the KD on weight- and fat-loss. 4 KD focuses on low-carbohydrate (10%), medium-protein (20%) and high-fat (70%) ingestion.4,5 Athletes with long-term dietary ketosis reported extraordinarily high levels of fat oxidation (over 1.5 g/min). 6 At the same time, exercise is another essential intervention in preventing obesity and its associated risk factors. 5 Aerobic and resistance exercises are two popular types of training to control body weight and improve body composition, and are widely used in weight-loss programs.7,8 However, the questions of optimal interventions to control body weight and related mechanisms still remain. During the past decade, the role of appetite regulation hormones in obesity and weight loss has attracted considerable attention. 9 Leptin is an essential appetite-regulating hormone associated with obesity, secreted from adipose tissue, affecting glucose metabolism and insulin sensitivity. 10 Leptin regulates body weight and energy balance through its receptors in the hypothalamus and had been shown to reduce the tendency to eat in individuals with obesity. 11 Another appetite hormone associated with obesity is ghrelin, known as the “hunger hormone,” which is primarily secreted by the stomach/abdominal cells. Ghrelin regulates food intake behavior, energy homeostasis, and weight control through growth hormone-independent mechanisms. 12 Previous studies have indicated that ghrelin and leptin are involved in the short-term regulation of energy balance. 12 Furthermore, spexin is a novel neuropeptide expressed in the endocrine glands and epithelial tissues of humans that play an essential role in obesity by regulating appetite, energy metabolism, glucose/lipid metabolism, and weight loss. 13 Spexin is negatively correlated with leptin in adolescents with obesity, suggesting a potential role for spexin in regulating satiety and certain cardiovascular risk factors in children with obesity. 14 Exercise training can result in weight loss via regulating appetite sensation and appetite hormones. Several studies have been conducted to investigate the effect of exercise training on appetite sensation and appetite-regulating hormones. Shakiba et al. 15 demonstrated that 12 weeks of different types of exercise training (including endurance, resistance, and concurrent training) reduced acylated ghrelin levels, body weight, and body mass index (BMI) in men with overweight. However, the impact of resistance training was more pronounced. Most studies report decreased levels of leptin after exercise training. 16 A systematic review and meta-analysis has indicated a lower level of hungriness and higher fullness/satiety after KD, 17 leading to decreased body- and fat-mass. In addition, KD in combination with exercise can efficiently reduce body fat while maintaining lean body mass.4,18 Studies have shown the effectiveness of exercise and KD in suppressing appetite and weight loss.19,20 Regarding the widespread use of KD in disease management and weight-loss programs by dietitians, clinicians, and exercise instructors, the most effective type of exercise training during a KD on appetite, appetite-regulating hormones, and weight control has not yet been investigated. Therefore, this study aimed to compare the effectiveness of two types of exercise training during a KD on appetite, appetite-regulating hormones, and body composition in overweight or obese men.
Materials and methods
Participants
Following a public announcement, 36 inactive male students who were overweight or obese (age = 20.70 ± 1.42 years old; height = 182.79 ± 4.75 cm; weight = 103.83 ± 3.03 kg; BMI = 31.09 ± 3.98 kg/m2) volunteered for this study. Participants were randomly assigned to KD, aerobic training during KD (AT-KD), and resistance training during KD (RT-KD) groups. The randomization was performed in a 1:1:1 allocation ratio via random number generation. Before starting the study intervention, the participants were familiarized with the research process, training and diet programs, and testing protocols. They were then advised regarding potential risks and benefits. After the familiarization session, the participants signed written consent to be included in the study. The inclusion criteria were: a BMI > 25, not engaging in regular exercise training or KD programs over the past 6 months, and no history of smoking, drug use, or sports supplements. The exclusion criteria in this study included participation in exercise training of less than 15 sessions and the inability to adhere to the diet plan. The regional ethics committee approved the present study (ID number: IR.URMIA.REC.1399.006). Only 24 participants (eight participants in each study group) completed all the stages of the research protocol, and finally, their data were collected for further analyses. The main reasons for the withdrawal of participants were the inability to comply with exercise and diet plans, or other personal reasons.
Exercise intervention
In this study, we used two types of exercise training, namely, moderate-intensity continuous aerobic training and circuit resistance training. The effectiveness of these training programs in weight reduction has been revealed in previous research.7,8
Aerobic training
The AT-KD group performed moderate-intensity continuous aerobic training by following the KD plan over six weeks and three sessions per week with at least 1 day of rest between sessions. The intensity of training was in the range of 60–70% of the maximum heart rate (MHR = 220 minus ages). The aerobic exercise program started with running at 60% of MHR over the first 2 weeks, after which, training intensity was increased by 5% of MHR biweekly. Training intensity was controlled using a chest strap heart-rate monitor (Polar, Finland). All training sessions consisted of three parts, including standard warming up (10 min), aerobic training (40–60 min), and standard cooling down (10 min). Warm-up and cool-down included light stretching and jogging. Training duration started at 40 min for the first 2 weeks, with an additional 10 min added biweekly.
Resistance training
The RT-KD group performed circuit strength training in three sessions per week for 6 weeks with at least 1 day of rest between sessions by following the KD plan. Two short circuits of strength exercises were performed in each session. The first circuit exercises included knee flexion, bench press, and ankle extension, while the second circuit included the lat pull-down, squat, and elbow flexions. Both circuits were done for 3–5 series with 35 s of rest between exercises. A 5-min rest period between the circuits was determined for active rest and warm-up by doing similar exercises in the following circuit. The resistance training program started with three series in the first 2 weeks, with the addition of one series biweekly. Participants performed all exercises with 100% of 6RM (i.e. the participant selected the heaviest weight that could be lifted for only six consecutive repetitions). A resistance training expert supervised the participants to ensure that the exercises were performed safely and with the correct technique.
Dietary intervention
Nutritional counseling and resources to assist in adhering to the KD plan were provided by a dietitian. The diet plan was prescribed for all the participants based on carbohydrate intake with the ad libitum total calorie intake. Carbohydrate intake was restricted to approximately 50 g or 10% of daily calorie intake. An electronic and printed list of daily nutrient resources was provided. This list categorized nutrients into three groups: (1) encouraged to eat, (2) eat in moderation, and (3) foods to avoid. The list of the encouraged foods included non-processed food such as green vegetables, raw seeds and nuts, animal meat, dairy products, eggs, fish, vegetable oils, and oils from olive, coconut, and avocado. The research team online monitored the participant’s dietary intake via social media self-reporting. Sufficient fluid intake and vitamin supplementation were recommended following the KD. This study consulted a cyclical KD plan consisting of the KD for 6 days and following an ordinary diet for 1 day per week. We calculated macronutrient and calorie intake at each meal using smartphone apps such as Samsung Health and Nutrition Fact. The ketosis state was monitored weekly by urine reagent stripes (DIA strip, Diaplus Inc., Canada). A participant would be excluded from the study if urinary ketone levels were less than 4 mmol/dL. Diet composition is presented in Table 1. There were no significant differences among the groups regarding macronutrients and calorie intake.21,22
Table 1.
Diet composition.
| Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | ||
|---|---|---|---|---|---|---|---|---|
| AT-KD | Protein (g/day) | 112.1 ± 5.1 | 126.0 ± 7.0 | 130.9 ± 3.0 | 132.1 ± 4.4 | 126.4 ± 5.3 | 124.9 ± 3.9 | 123.8 ± 5.7 |
| Carbohydrate (g/day) | 255.3 ± 4.4 | 53.6 ± 1.8 | 54.1 ± 1.0 | 51.3 ± 1.4 | 50.8 ± 1.5 | 51.6 ± 0.9 | 50.8 ± 1.2 | |
| Fat (g/day) | 71.1 ± 3.1 | 150.5 ± 4.3 | 149.8 ± 7.3 | 147.3 ± 2.4 | 142.4 ± 1.0 | 140.3 ± 1.5 | 141.2 ± 4.2 | |
| Calorie intake (kcal/day) | 2109.5 ± 3.6 | 2073.1 ± 5.3 | 2088.7 ± 6.7 | 2059.4 ± 3.5 | 1991.0 ± 1.0 | 1969.8 ± 1.0 | 1964.2 ± 6.5 | |
| RT-KD | Protein (g/day) | 109.9 ± 3.9 | 129.9 ± 5.3 | 127.4 ± 2.4 | 127.2 ± 5.2 | 121.8 ± 1.5 | 123.8 ± 1.1 | 116.7 ± 8.9 |
| Carbohydrate (g/day) | 248.2 ± 6.5 | 57.0 ± 2.8 | 52.3 ± 1.5 | 53.2 ± 3.0 | 51.8 ± 1.2 | 51.6 ± 0.7 | 50.0 ± 0.8 | |
| Fat (g/day) | 73.2 ± 4.8 | 152.4 ± 6.1 | 145.4 ± 3.4 | 141.2 ± 7.9 | 142.6 ± 2.3 | 141.3 ± 3.5 | 137.4 ± 2.2 | |
| Calorie intake (kcal/day) | 2090.8 ± 5.2 | 2012.0 ± 7.1 | 2028.6 ± 3. | 1992.6 ± 8.6 | 1978.7 ± 2.0 | 1974.0 ± 6.5 | 1903.3 ± 4.2 | |
| KD | Protein (g/day) | 114.3 ± 3.7 | 127.7 ± 5.0 | 130.93 ± 1.6 | 122.7 ± 4.4 | 119.1 ± 3.3 | 121.5 ± 2.0 | 116.6 ± 4.3 |
| Carbohydrate (g/day) | 250.1 ± 7.1 | 54.6 ± 2.0 | 52.7 ± 1.2 | 52.8 ± 0.7 | 51.8 ± 1.5 | 50.7 ± 0.8 | 50.2 ± 0.8 | |
| Fat (g/day) | 70.7 ± 3.9 | 144.5 ± 9.6 | 143.9 ± 1.0 | 140.9 ± 8.0 | 143.5 ± 4.7 | 140.0 ± 3.4 | 139.6 ± 1.8 | |
| Calorie intake (kcal/day) | 2093.1 ± 6.1 | 2030.8 ± 9.7 | 2027.8 ± 9.8 | 1970.5 ± 8.1 | 1975.9 ± 4.9 | 1949.5 ± 3.6 | 1923.6 ± 1.5 | |
| Between-group p-value | Protein (g/day) | 0.45 | 0.65 | 0.15 | 0.06 | 0.06 | 0.80 | 0.47 |
| Carbohydrate (g/day) | 0.09 | 0.15 | 0.19 | 0.39 | 0.60 | 0.28 | 0.51 | |
| Fat (g/day) | 0.23 | 0.70 | 0.54 | 0.35 | 0.86 | 0.81 | 0.78 | |
| Calorie intake (kcal/day) | 0.18 | 0.30 | 0.43 | 0.24 | 0.77 | 0.70 | 0.54 |
Values are expressed as mean ± SD. AT-KD: aerobic training during a ketogenic diet group; RT-KD: resistance training during a ketogenic diet group; KD: ketogenic diet alone group.
Body composition
Total body composition was measured using the bioelectrical impedance analysis (BIA) method on the Inbody device (Inbody 3, South Korea). Using this method, the alternative hand–foot impedance small current passed through the whole body, providing values of strength and reactivity from body tissues. These impedance values were used to predict several variables, including body-fat percentage, lean body mass, and total body water. Before carrying out the test, each participant’s height, age, and gender were entered into the BIA device’s software. Participants then cleaned their hands and feet with alcohol pads and stood on the electrodes while simultaneously holding the electrode embedded handles. Bodyweight and height were measured using a standardized scale and a stadiometer (Seca, Germany).
Biochemical analysis
The Enzyme-Linked Immunosorbent Assay (ELISA) method with commercial human kits were used for analyzing serum levels of spexin (Elabscience, China, Sensitivity: 46.88 pg/mL), leptin (Elabscience, China, Sensitivity: 9.38 pg/mL), and acylated ghrelin (Elabscience, China, Sensitivity: 9.38 pg/mL). Blood samples were obtained between 08:00 and 09:00. Fasting blood samples were taken 48 h before the exercise and dietary intervention and 48 h after the last training session. The blood samples were spun in a centrifuge for 10 min at 3000 r/min, and the extracted serum was stored at −24°C until analysis.
Appetite sensation
Appetite sensation was measured using the visual analogue scale (VAS) in fasting and postprandial states. VAS is a questionnaire with eight questions on hunger; satiety; fullness; prospective food consumption (PFC); and desire to eat something fatty, salty, sweet, or savory. Each question item included a 100 mm line in length with words anchored at each end, expressing the most positive and negative rating. Subjects marked their mental sensation on line. 23 Assessments of appetite sensation included an assessment of pre-test appetite in fasting state (Pre-F), an assessment of pre-test appetite after eating breakfast (Pre-BF), an assessment of post-test appetite in fasting state (Post-F), and an assessment of post-test appetite after eating breakfast (Post-BF). The pre-test measured appetite sensation during fasting and 30 min after eating standard breakfast (30 g of cheese, two dates, half of the thin bread, and 200 mL of low-fat milk). In the post-test, the appetite sensation was measured similarly to the pre-test.
Statistical analysis
The Shapiro–Wilk test was used to evaluate the normality of the data. A 2 × 3 two-way mixed analysis of variance (ANOVA) was used to investigate the effectiveness of exercise training types during a KD on appetite-regulating hormones and body composition. Time (pre- vs post-test) and group (AT-KD, RT-KD, KD) were the within-subject, and between-subject factors, respectively. In addition, a 4 × 3 two-way mixed ANOVA was used to investigate the effectiveness of the two exercise training types during a KD on appetite sensation (4 measurements in time × 3 groups). A significant Time × Group interaction in the multivariate test of ANOVA indicated an effect of exercise training during a KD between pre- to post-test of relevant independent variables. The Bonferroni post hoc test was used to analyze the simple main effects of between-group changes. A paired sample t-test was used for analyzing within-group differences. Finally, effect sizes were reported by partial eta squared (ηp2). The significance level in all these analyses was set at p ⩽ 0.05. Data were analyzed using SPSS software (version 24, IBM Corporation, Armonk, NY, USA).
Results
The two-way mixed ANOVA analyses indicated no significant Time × Group interaction between spexin serum levels (P = 0.80, ηp2 = 0.02), while differences were observed in spexin levels over time (P = 0.001, ηp2 = 0.83). Paired sample t-test results revealed a significant increase in the spexin levels in all groups compared to the pre-test (P < 0.01). No significant Time × Group interaction was found in leptin levels (P = 0.55, ηp2 = 0.07), while significant differences were observed over time (P = 0.001, ηp2 = 0.68). Leptin levels significantly decreased in all groups compared to the pre-test (P < 0.05). Acylated ghrelin levels showed no significant differences in Time × Group interaction (P = 0.26, ηp2 = 0.15), but they were significantly different over time (P = 0.001, ηp2 = 0.81). Acylated ghrelin levels significantly increased in all groups compared to the pre-test (P < 0.01) (Table 2).
Table 2.
The effect of exercise training types during a ketogenic diet on appetite-regulating hormones.
| Variable | Groups | Pre-test (mean ± SD) | Post-test (mean ± SD) | Interaction | P-value | ηp2 |
|---|---|---|---|---|---|---|
| Spexin (pg/mL) | AT-KD | 210.75 ± 40.61 | 362.80 ± 46.14* | Time | 0.001 | 0.83 |
| RT-KD | 193.02 ± 28.69 | 371.69 ± 53.20* | Time × Group | 0.80 | 0.02 | |
| KD | 197.02 ± 37.92 | 374.27 ± 40.17* | Group | 0.98 | 0.002 | |
| Leptin (pg/mL) | AT-KD | 147.79 ± 35.24 | 97.96 ± 09.53* | Time | 0.001 | 0.66 |
| RT-KD | 131.67 ± 12.13 | 99.80 ± 10.99* | Time × Group | 0.55 | 0.07 | |
| KD | 139.66 ± 28.49 | 100.34 ± 12.43* | Group | 0.69 | 0.004 | |
| Acylated ghrelin (pg/mL) | AT-KD | 112.66 ± 8.88 | 165.32 ± 24.92* | Time | 0.001 | 0.81 |
| RT-KD | 111.80 ± 10.48 | 161.13 ± 9.41* | Time × Group | 0.26 | 0.15 | |
| KD | 110.66 ± 13.56 | 188.01 ± 35.92* | Group | 0.18 | 0.19 |
AT-KD: aerobic training during a ketogenic diet group; RT-KD: resistance training during a ketogenic diet group; KD: ketogenic diet alone group; SD: standard deviation.
Significant difference compared with pre-test. Values are expressed as mean ± SD.
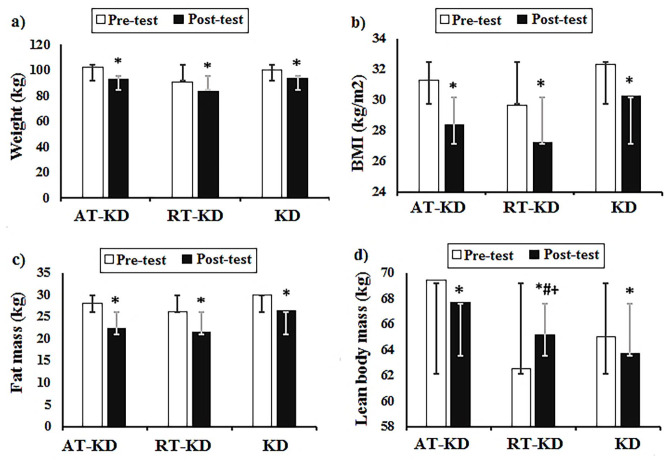
The body composition analysis indicated no significant Time × Group interaction in body fat mass (P = 0.15, ηp2 = 0.20). However, significant differences were observed in body fat mass over time (P = 0.001, ηp2 = 0.85). The mean body fat loss change was observed to be significant in the AT-KD group (−8.14 kg), the RT-KD group (−5.66 kg), and the KD group (−5.16 kg) compared with pre-test (P < 0.01). The time × group interaction results indicated a significant difference between lean body mass (P = 0.001, ηp2 = 0.85). Significant increases in lean body mass in the RT-KD group were observed compared with both AT-KD and KD groups (P = 0.001, ηp2 = 0.72). No significant differences were found in lean body mass over time (P = 0.68, Eta2 = 0.01). However, the mean lean body mass change significantly increased in the RT-KD group compared with the pre-test (+2.66 kg), but in both AT-KD and KD groups mean lean body mass change significantly decreased compared with the pre-test (−1.71 and −1.33 kg, respectively) (Figure 1).
Figure 1.
The effects of exercise training types during a ketogenic diet on body composition, including (a) weight, (b) BMI, (c) fat mass, and (d) lean body mass. Values are expressed as mean ± SD. (A color version of this figure is available in the online journal.)
AT-KD: Aerobic training during a ketogenic diet group; RT-KD: Resistance training during a ketogenic diet group; KD: ketogenic diet alone group.
*Significant difference compared with the pre-test.
#Significant difference compared with the KD group.
+Significant difference compared with the RT-KD group.
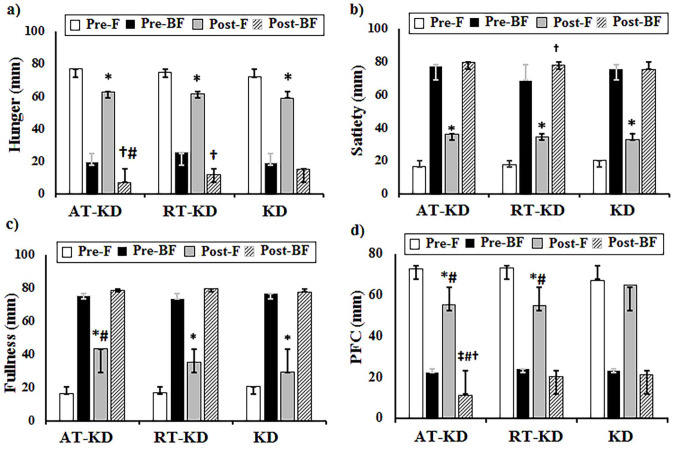
The mixed ANOVA results indicated a significant Time × Group interaction for hunger sensation (P = 0.04, ηp2 = 0.18). Hunger sensation significantly decreased in the AT-KD group in the postprandial state compared to the KD alone group (P < 0.05). Significant differences were found in hunger over time (P = 0.001, ηp2 = 0.96). Hunger sensation significantly decreased in AT-KD and RT-KD groups during fasting and postprandial states compared with the pre-test (P < 0.01). The KD alone group had a significant decrease in fasting state compared with the pre-test (P < 0.01). The Time × Group interaction analyses indicated no significant differences in satiety sensation (P = 0.33, ηp2 = 0.10). However, significant differences were observed in satiety sensation over time (P = 0.001, ηp2 = 0.94). Furthermore, satiety sensation significantly increased in all groups in the fasting state compared with the pre-test (P < 0.01). Only the RT-KD group had increased satiety sensation in the postprandial state (P < 0.05). The Time × Group interaction analyses showed a significant difference between fullness sensation (P = 0.006, ηp2 = 0.24). A significant increase was observed in fullness sensation during the postprandial state in the AT-KD group than the KD alone group (P < 0.05). Moreover, significant differences were found in fullness sensation over time (P = 0.001, ηp2 = 0.96). Fullness sensation significantly increased in all groups in the fasting state compared with the pre-test (P < 0.01). Furthermore, mixed ANOVA results revealed significant differences in Time × Group interaction between PFC (P = 0.005, ηp2 = 0.24). In the fasting state, both AT-KD and RT-KD groups had a significant decrease in PFC compared to the KD alone group (P < 0.05). The AT-KD group significantly decreased PFC in the postprandial state compared to both RT-KD and KD alone groups (P < 0.05). Significant differences were found in PFC over time (P = 0.001, ηp2 = 0.94). PFC decreased in both AT-KD and RT-KD groups in the fasting state compared to the pre-test (P < 0.05). Moreover, it was significantly decreased only in the AT-KD group in the postprandial state compared to the pre-test (P < 0.05) (Figure 2).
Figure 2.
The effects of exercise training types during a ketogenic diet on appetite sensation, including (a) hunger, (b) satiety, (c) fullness, and (d) prospective food consumption (PFC). Values are expressed as mean ± SD. (A color version of this figure is available in the online journal.)
Assessment of pre-test appetite in a fasted state (Pre-F), assessment of pre-test appetite after eating breakfast (Pre-BF), assessment of post-test appetite in a fasted state (Post-F), assessment of post-test appetite after eating breakfast (Post-BF). AT-KD: aerobic training during a ketogenic diet group; RT-KD: resistance training during a ketogenic diet group; KD: ketogenic diet alone group.
*A significant difference compared with the Pre-F.
†A significant difference compared with the Pre-BF.
#A significant difference compared with the KD group.
‡A significant difference compared with the RT-KD group.
Discussion
The efficacy of KD and exercise interventions in fat loss and weight control has been previously reported in various studies. However, the most effective exercise training types during a KD in improving body composition have not yet been determined. Moreover, the effects of exercise training types and KD on appetite and appetite-regulating hormones, and thus their effects on body composition, have not been adequately explored. This study is the first to compare the impact of resistance and aerobic training during a low-carbohydrate KD on appetite-regulating hormones, appetite sensation, and body composition in overweight or obese men.
Our findings showed significantly increased resting levels of spexin and acylated ghrelin, while resting levels of leptin decreased during 6 weeks of performing both AT-KD and RT-KD. However, between-group differences were not found in this study. To the best of our knowledge, only research conducted by Cipryan et al. has been examined the effect of exercise training along with a KD on leptin levels. They reported that engaging in 12 weeks of very low–carbohydrate KD and high-intensity interval aerobic training decreased leptin levels in healthy individuals compared to a habitual diet and exercise. 24 The effects of both exercise training and KD on leptin, ghrelin, and spexin levels separately have been investigated in other studies. Cipryan et al. 25 indicated that leptin resting levels after 4 weeks of very low-carbohydrate KD decreased compared with a habitual diet in healthy individuals. Sumithran et al. 26 observed significant reductions in leptin levels and small non-significant increases in ghrelin levels after 8 weeks of the KD.
According to previous studies, the reasons for the reduced leptin levels and increased ghrelin levels after the KD may be related to compensation manner to changes in energy homeostasis, reduction in fat, and body mass.24,26 Moreover, decreased leptin serum levels during ketosis increased adiponectin serum levels and free fatty acid in adipose tissues. 27 Previous exercise training intervention studies on serum levels of leptin and acylated ghrelin are similar to those in the current study.28,29 In a systematic review, Ouerghi et al. 30 claimed that acute exercise suppressed serum levels of acylated ghrelin and long-term exercise training increased ghrelin levels regardless of the participants’ conditioning and the exercise characteristics. In a meta-analysis study, Rostas et al. reported that long-term exercise training decreased leptin levels in middle-aged or older overweight or obese individuals. They also concluded that resistance training had a more pronounced effect on reducing serum levels of leptin than aerobic training. 31 Possible reasons for these changes in leptin and ghrelin serum levels after exercise training include compensation for changes in energy homeostasis, improved leptin sensitivity, prolonged fasting, β-adrenergic signaling, and reductions in fat mass and body weight.16,30 –32 In addition, the exogenous leptin administration increased ghrelin mRNA in the stomach. 33 Changes in ghrelin levels in response to weight loss may be secondary to reducing leptin levels. Increased spexin concentrations in this study are consistent with those in previous studies. Al-Daghri et al. 34 reported increased spexin levels in women with prediabetes after 6 months of diet and exercise interventions. In addition, Khadir et al. 35 have indicated that exercise training increased spexin levels in obese and diabetic cases. Overall, the observation of no significant between-group differences in appetite-regulating hormones in this study could be due to a similar reduction in body fat mass and body weight between the study groups. Because exercise training and KD have similar mechanisms in altering appetite-regulating hormones, it seems that the lack of a significant difference existing between training types along with KD and KD alone could be due to the adequate stimulus provided by KD.
Analysis of appetite sensation revealed that hunger and PFC decreased in both AT-KD and RT-KD groups in fasting and postprandial states after 6 weeks. The KD group only had a reduced hunger sensation in the fasting state. Furthermore, the AT-KD group experienced significant declines in hunger and PFC relative to the KD group. All three groups had an increased satiety and fullness in the fasting state. Moreover, the AT-KD group had a significant increase in fullness compared with the KD group. To our knowledge, there has been no research on the impact of exercise training types during a KD on appetite. However, similar to our findings, previous studies have observed appetite suppression after 4 and 12 weeks of KD in obese individuals.17,36,37 By contrast, Struik et al. 38 have observed the increased PFC after 4 weeks of the KD in overweight or obese individuals with type 2 diabetes. It is noteworthy that several mechanisms, including direct effects of ketone bodies on reducing central orexigenic signaling, changes in appetite-regulating hormones, changes in the gut microbiota, increased fatty acids, and elevated postprandial blood glucose concentrations, may contribute to the appetite-suppression effects of KD.20,26 However, contradictory results have been reported for changes in appetite after long-term exercise. 16 In a systematic review and meta-analysis study, Beaulieu et al. 39 have demonstrated that exercise training leads to increased hunger and dietary restraint in the fasting state, a decreased disinhibition, and no significant changes in satiety and fullness feeling. Evidence suggests that exercise training typically results in partial compensation for energy consumption, linked to beneficial changes in appetite-regulating hormones after exercise. 40 Our findings showed that both AT-KD and RT-KD could similarly suppress appetite sensation, and the AT-KD had a more pronounced effect than the KD alone. Favorable changes in leptin and spexin levels through decreases in hypothalamic inflammation could be possible mechanisms for the suppression of appetite feelings following exercise training and KD in individuals with obesity.32,41 According to the results of most previous studies, chronic exercise training and KD increased ghrelin and decreased leptin levels related to body weight and fat mass reduction. However, after acute exercise, because of the temporary and transitory effects of these hormones, circulating levels of ghrelin decreased and circulating levels of leptin increased.16,30 –32 As regards this, incompatible findings between appetite-regulating hormones and appetite sensation in this study may be related to assessing chronic changes of appetite sensation after exercise training and KD. In contrast, effects of leptin and acylated ghrelin in appetite control are acute and transitory. As mentioned above, probably the antiinflammatory effects of these hormones suppressed appetite sensation after regular exercise training and KD. Moreover, contradictory results reported in previous studies may be related to appetite sensation analyzing methods, duration of exercise training and KD plan, the intensity of training, and subjects’ condition (healthy subjects vs disease subjects).
In this study, fat mass, body weight, and BMI decreased following all three interventions, with no significant differences being observed between the interventions. These findings are consistent with most studies conducted on KD along with exercise training regardless of the type of exercise training.15,18,21 Lean body mass significantly increased in the RT-KD compared to AT-KD and KD alone. Furthermore, lean body mass decreased in AT-KD and KD alone. Most previous studies indicated the positive effects of the RT-KD on increasing and maintaining lean body mass.18,21 Similar to our results, Clark’s meta-analysis has indicated that KD and exercise training alone or in combination (endurance or resistance training with KD) could improve body composition in overweight and obese individuals. Moreover, Clark claimed that RT-KD was more beneficial in body fat reduction with lean body mass retention than AT-KD and other exercise methods. 42 However, several reviews have reported negative or non-significant effects of the RT-KD on lean body mass changes.19,43 Inconsistent findings may be due to differences in body composition measurement methods, diet composition, exercise training design, and subject characteristics. However, activating AMP-activated protein kinase may be a potential mechanism to decrease lean body mass after performing AT-KD and KD alone. This is due to AMPK phosphorylation blunting Akt/mTOR pathways. 44 No significant differences observed in body weight and fat mass reduction between two exercise training types during a KD and KD alone interventions may reflect this hypothesis that KD alone intervention has a ceiling effect and exercise training did not provide an additional benefit. This finding can be related to similar mechanisms to changes in appetite feeling and appetite-regulating hormones through the exercise program and KD. Another reason why there are no differences between the interventions may be the small sample size and the short training period in this study.
The strength of this study was to compare the effectiveness of two types of training during a KD on appetite-regulating hormones in obesity, especially spexin, a novel appetite-regulating neuropeptide. The limitations of this research include the measurement of appetite-regulating hormones only in a fasting state, the small sample size, and the short period of training and dietary intervention. Therefore, it is recommended that hormones should be measured in both fasting and postprandial states, use of large sample size, and the duration of the training and diet intervention should be increased up to 12 weeks. Moreover, it is better to examine the cellular and molecular pathways involved in appetite control and appetite-regulating hormones in weight loss by conducting KD and exercise training interventions in future animal studies.
Our results showed that the two types of training during a KD and a KD alone similarly modified the appetite-regulating hormones in overweight and obese men. Both AT-KD and RT-KD led to a reduction in hunger and PFC feeling and an increase in satiety and fullness feeling. AT-KD had pronounced effectiveness in suppressing appetite over KD alone. Fat mass, body weight, and BMI were reduced after AT-KD and RT-KD, with a slightly more reduction in AT-KD. However, lean body mass increased after the RT-KD and decreased after performing AT-KD and KD alone. Appetite and appetite regulation hormones seem to have a double-sided effect on body composition. Changes in appetite and appetite-regulating hormones have led to changes in body composition and vice versa. However, both types of exercise during the KD positively affected weight loss and body fat reduction in men. However, the effectiveness of RT-KD in maintaining and increase lean body mass is more notable than AT-KD and KD alone.
Footnotes
Authors’ Contributions: All the authors were involved in designing, interpreting the studies, analyzing the data and revising the manuscript. AV conducted the experiments. KKh wrote the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This research obtained the ethical approval with ID number: IR.URMIA.REC.1399.006 from University/Regional Research Ethics Committee of Urmia University.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kazem Khodaei  https://orcid.org/0000-0003-1566-2851
https://orcid.org/0000-0003-1566-2851
References
- 1. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics 2015;33:673–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288–98 [DOI] [PubMed] [Google Scholar]
- 3. Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev 2016;17:664–90 [DOI] [PubMed] [Google Scholar]
- 4. Paoli A. Ketogenic diet for obesity: friend or foe? Int J Environ Res Public Health 2014;11:2092–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volek JS, Vanheest JL, Forsythe CE. Diet and exercise for weight loss: a review of current issues. Sports Med 2005;35:1–9 [DOI] [PubMed] [Google Scholar]
- 6. Volek JS, Freidenreich DJ, Saenz C, Kunces LJ, Creighton BC, Bartley JM, Davitt PM, Munoz CX, Anderson JM, Maresh CM, Lee EC, Schuenke MD, Aerni G, Kraemer WJ, Phinney SD. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016;65:100–10 [DOI] [PubMed] [Google Scholar]
- 7. Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev 2017;18:635–46 [DOI] [PubMed] [Google Scholar]
- 8. Alcaraz PE, Perez-Gomez J, Chavarrias M, Blazevich AJ. Similarity in adaptations to high-resistance circuit vs. traditional strength training in resistance-trained men. J Strength Cond Res 2011;25:2519–27 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki K, Jayasena CN, Bloom SR. Obesity and appetite control. Exp Diabetes Res 2012;2012:824305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 2007;8:21–34 [DOI] [PubMed] [Google Scholar]
- 11. Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010;21:643–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patterson M, Bloom SR, Gardiner JV. Ghrelin and appetite control in humans–potential application in the treatment of obesity. Peptides 2011;32:2290–4 [DOI] [PubMed] [Google Scholar]
- 13. Walewski JL, Ge F, Lobdell H, 4th, Levin N, Schwartz GJ, Vasselli JR, Pomp A, Dakin G, Berk PD. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity (Silver Spring) 2014; 22:1643–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar S, Hossain MJ, Javed A, Kullo IJ, Balagopal PB. Relationship of circulating spexin with markers of cardiovascular disease: a pilot study in adolescents with obesity. Pediatr Obes 2018;13:374–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shakiba E, Sheikholeslami-Vatani D, Rostamzadeh N, Karim H. The type of training program affects appetite-regulating hormones and body weight in overweight sedentary men. Appl Physiol Nutr Metab 2019;44:282–7 [DOI] [PubMed] [Google Scholar]
- 16. Dorling J, Broom DR, Burns SF, Clayton DJ, Deighton K, James LJ, King JA, Miyashita M, Thackray AE, Batterham RL, Stensel DJ. Acute and chronic effects of exercise on appetite, energy intake, and appetite-related hormones: the modulating effect of adiposity, sex, and habitual physical activity. Nutrients 2018;10:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gibson AA, Seimon RV, Lee CM, Ayre J, Franklin J, Markovic TP, Caterson ID, Sainsbury A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes Rev 2015;16:64–76 [DOI] [PubMed] [Google Scholar]
- 18. Jabekk PT, Moe IA, Meen HD, Tomten SE, Høstmark AT. Resistance training in overweight women on a ketogenic diet conserved lean body mass while reducing body fat. Nutr Metab (Lond) 2010;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashtary-Larky D, Bagheri R, Asbaghi O, Tinsley GM, Kooti W, Abbasnezhad A, Afrisham R, Wong A. Effects of resistance training combined with a ketogenic diet on body composition: a systematic review and meta-analysis. Crit Rev Food Sci Nutr 2021:1–16 [DOI] [PubMed] [Google Scholar]
- 20. Roekenes J, Martins C. Ketogenic diets and appetite regulation. Curr Opin Clin Nutr Metab Care 2021;24:359–63 [DOI] [PubMed] [Google Scholar]
- 21. Vargas S, Romance R, Petro JL, Bonilla DA, Galancho I, Espinar S, Kreider RB, Benitez-Porres J. Efficacy of ketogenic diet on body composition during resistance training in trained men: a randomized controlled trial. J Int Soc Sports Nutr 2018;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greene DA, Varley BJ, Hartwig TB, Chapman P, Rigney M. A low-carbohydrate ketogenic diet reduces body mass without compromising performance in powerlifting and olympic weightlifting athletes. J Strength Cond Res 2018;32:3373–82 [DOI] [PubMed] [Google Scholar]
- 23. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000;24:38–48 [DOI] [PubMed] [Google Scholar]
- 24. Cipryan L, Dostal T, Plews DJ, Hofmann P, Laursen PB. Adiponectin/leptin ratio increases after a 12-week very low-carbohydrate, high-fat diet, and exercise training in healthy individuals: a non-randomized, parallel design study. Nutr Res 2021;87:22–30 [DOI] [PubMed] [Google Scholar]
- 25. Cipryan L, Maffetone PB, Plews DJ, Laursen PB. Effects of a four-week very low-carbohydrate high-fat diet on biomarkers of inflammation: non-randomised parallel-group study. Nutr Health 2020;26:35–42 [DOI] [PubMed] [Google Scholar]
- 26. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur J Clin Nutr 2013;67:759–64 [DOI] [PubMed] [Google Scholar]
- 27. Deemer SE, Plaisance EP, Martins C. Impact of ketosis on appetite regulation-a review. Nutr Res 2020;77:1–11 [DOI] [PubMed] [Google Scholar]
- 28. Dundar A, Kocahan S, Sahin L. Associations of apelin, leptin, irisin, ghrelin, insulin, glucose levels, and lipid parameters with physical activity during eight weeks of regular exercise training. Arch Physiol Biochem 2021;127:291–5 [DOI] [PubMed] [Google Scholar]
- 29. Gueugnon C, Mougin F, Nguyen NU, Bouhaddi M, Nicolet-Guénat M, Dumoulin G. Ghrelin and PYY levels in adolescents with severe obesity: effects of weight loss induced by long-term exercise training and modified food habits. Eur J Appl Physiol 2012;112:1797–805 [DOI] [PubMed] [Google Scholar]
- 30. Ouerghi N, Feki M, Bragazzi NL, Knechtle B, Hill L, Nikolaidis PT, Bouassida A. Ghrelin response to acute and chronic exercise: insights and implications from a systematic review of the literature. Sports Med 2021;51:2389–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rostás I, Pótó L, Mátrai P, Hegyi P, Tenk J, Garami A, Illés A, Solymár M, Pétervári E, Szűcs Á, Párniczky A, Pécsi D, Rumbus Z, Zsiborás C, Füredi N, Balaskó M. In middle-aged and old obese patients, training intervention reduces leptin level: a meta-analysis. PLoS ONE 2017;12: e0182801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzalez-Gil AM, Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients 2020;12:1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept 2004;119:77–81 [DOI] [PubMed] [Google Scholar]
- 34. Al-Daghri NM, Wani K, Yakout SM, Al-Hazmi H, Amer OE, Hussain SD, Sabico S, Ansari MGA, Al-Musharaf S, Alenad AM, Alokail MS, Clerici M. Favorable changes in fasting glucose in a 6-month self-monitored lifestyle modification programme inversely affects spexin levels in females with prediabetes. Sci Rep 2019;9:9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khadir A, Kavalakatt S, Madhu D, Devarajan S, Abubaker J, Al-Mulla F, Tiss A. Spexin as an indicator of beneficial effects of exercise in human obesity and diabetes. Sci Rep 2020;10:10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008;87:44–55 [DOI] [PubMed] [Google Scholar]
- 37. Mohorko N, Cernelic-Bizjak M, Poklar-Vatovec T, Grom G, Kenig S, Petelin A, Jenko-Praznikar Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr Res 2019;62:64–77 [DOI] [PubMed] [Google Scholar]
- 38. Struik NA, Brinkworth GD, Thompson CH, Buckley JD, Wittert G, Luscombe-Marsh ND. Very low and higher carbohydrate diets promote differential appetite responses in adults with type 2 diabetes: a randomized trial. J Nutr 2020;150:800–5 [DOI] [PubMed] [Google Scholar]
- 39. Beaulieu K, Blundell JE, van Baak MA, Battista F, Busetto L, Carraca EV, Dicker D, Encantado J, Ermolao A, Farpour-Lambert N, Pramono A, Woodward E, Bellicha A, Oppert JM. Effect of exercise training interventions on energy intake and appetite control in adults with overweight or obesity: a systematic review and meta-analysis. Obes Rev 2021;22:e13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab 2010;57:36–42 [DOI] [PubMed] [Google Scholar]
- 41. Tran A, He W, Chen JTC, Belsham DD. Spexin: its role, regulation, and therapeutic potential in the hypothalamus. Pharmacol Ther 2021;8: 108033. [DOI] [PubMed] [Google Scholar]
- 42. Clark JE. Diet, exercise or diet with exercise: comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18-65 years old) who are overfat, or obese; systematic review and meta-analysis. J Diabetes Metab Disord 2015;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coleman JL, Carrigan CT, Margolis LM. Body composition changes in physically active individuals consuming ketogenic diets: a systematic review. J Int Soc Sports Nutr 2021;18:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paoli A, Cancellara P, Pompei P, Moro T. Ketogenic diet and skeletal muscle hypertrophy: a frenemy relationship? J Hum Kinet 2019;68:233–47 [DOI] [PMC free article] [PubMed] [Google Scholar]