Short abstract
Content available: Audio Recording
Listen to an audio presentation of this article.
INTRODUCTION
The earliest known mention of bile, for use in enemas and other treatments, is found in Egypt in the Ebers Papyrus of the 16th century BCE. 1 Yet references to the liver and bile, respectively, were far from isolated in the ancient world, understandably so considering the impressive sight of the largest internal organ in the body filled with bright red blood—the life force—as depicted in the Upper Paleolithic wall painting of an eviscerated bison in the caves at Lascaux (near Montignac in the Dordogne region of southwestern France) and the unique body fluid with its distinctive yellow‐green color, odor, and bitter taste, which dictated its etymological designation and metaphors. In old Hebrew, the label used poison (ראש; see Deuteronomy 32:33 2 and Matthew 27:34 3 ) 2 , 3 , 4 focused on the taste of bile, whereas the Proto‐Indo‐European epithet *ǵʰelh₃‐ (“green, yellow”) dwells on its color and gives cognates in Old Latin fel, holus, helvus; in Ancient Greek choli χολή and chloros χλωρός that meant “yellow” and metaphorically “anger and indignation” in Latin (cholera from the Greek); in English gold and Old English ġeolu (“yellow”); and a slew of other languages.* * The alternative term in Latin, bilis, † † also denotes a yellow bitter liquid secreted by the liver that aids in digestion. 5 The Latin appellation cholera that was used for bile until the 18th century could, like bilis, also signify a touchy irascible disposition because of an excess of black bile (see later); occasionally bile and liver were metaphors for courage, anger, arrogance, daring, and amorous tendencies, ‡ ‡ even lust, 6 in English 7 and other languages. 8 Biliousness was a common symptom in Britain in the 18th century, probably related to being liverish over the next 100 to 200 years or so. 9 , 10 Yet liverish was considered a functional rather than an organic disorder of the liver, even though the general malaise and dyspepsia and indeed bad temper that the term liverish suggested had actually been recognized by army surgeons at the time of the British Raj as premonitory warnings of a liver abscess. 9 The French, of course, have their crise de foie and colique hépatique, but when a Frenchman was bad tempered, he was said to be un bilieux.
It was therefore logical to the physician of old that bile should play an important role in physical health, as well as in mood and temperament—that is, a “proportioned mixture of elements,” from Latin temperare to control or blend together the four qualities: hot, cold, moist, and dry. A mainstay of the disciples or school of Hippocrates (c.460–c.377 BCE; Figure 1) was the humoral theory of diseases, a concept that originated with the Pythagoreans two centuries earlier. Health and disease were thought to be dependent on the balance between the four main humors or body fluids, that is, the choleric warm dry yellow bile, the melancholic cold dry black bile, the sanguine warm moist blood, and the phlegmatic cold moist phlegm (Figure 2A), corresponding, respectively, to the four qualities and the four basic elements, namely, fire, earth, air, and water (Figure 2B). The Hippocratic composition that describes in detail the formation of bile and the way bile increases in the body is in Diseases IV. 11 In contrast with earlier work in which the Greek physician distinguished blood, phlegm, yellow bile, and black bile, in Diseases IV, Hippocrates mentions blood, phlegm, bile, and water. The balance (physis φύσις) between these four humors, an extension of the harmony of opposites, guarantees good health (Figure 2B). Hippocrates clearly writes that the “source of bile is the part of the liver” (probably the gallbladder). 12 , 13
FIGURE 1.

The Greek physician Hippocrates of Cos (c.460–c.377 BCE) was the first to propose that the “source of bile was part of the liver.” (A) Line drawing portrayal of Hippocrates; this drawing was copied from the first edition of the works of Hippocrates by Francisco Asulanus, edited by the Publishing House of Aldo Manuzio (Aldus Manutius), Venice in 1526. (B) Side view of a sculptured head of an elderly man, which was discovered in 1940 lying on the ground in Antica in Northern Ostia at the end of the Via Flavia, in front of the tomb of K. Markios Demetrios, a distinguished Greco‐Roman physician of the 1st century CE. The bust, which was a Roman copy made of Parian—Greek white marble—of an earlier 3rd century BCE Greek sculpture, was identified as Hippocrates by Prof. Giovanni Becatti (1912–1973), an Italian Classical Art Historian and Archaeologist of the University of Pisa, who directed excavations at Ostia Antica, as reported by D.W. Richards. 12 (A) Reproduced with permission from British Medical Journal. 9 Copyright 1938, British Medical Association.
FIGURE 2.

(A) Image of woodcut from Physiognomische Fragmente, zur Beförderung der Menschenkenntnis und Menschenliebe (1775–1778) by Johann Caspar Lavater, depicting the four humors: phlegmatic (upper left; i.e., mucus phlegm, phlegma φλέγμα), choleric (upper right, i.e., yellow bile, xanthe chole ξανθη χολή), sanguine (lower left, i.e., blood, haima αἷμα), and melancholic (lower right, i.e., black bile, melaina chole μέλαινα χολή). (B) The association between the four elements—fire, earth, water, and air (depicted on the outside of the figure)—and the four Hippocratic humors (yellow bile, black bile, blood, and phlegm), enveloped in the inside by the four qualities: dry, moist, hot, and cold. The natural state (physis, φύσις), that is, good health (eucrasis/eucrasia, εὐκρᾶσις/ία) is achieved when there is a proper blending and balance of the elements, qualities, and humors in amount and strength. When these are disproportionate, disease dyscrasia (δυσκρασία) ensues. Reproduced with permission from History of Physiology. 96 Copyright 1973, RE Karger Publishers Co.
Later, Aelius/Claudius Galenus (Κλαύδιος Γαληνός), anglicized as Galen of Pergamon (129–c.216 CE) (Figure 3), one‐time physician to the gladiators of the Temple of Pergamon's High Priest and physician to several emperors in Rome, also proposed that yellow bile comes from the liver. It was not that Galen was concerned especially with bile formation per se. Rather, he attempted to unify those ancient notions of human physiology—albeit only those with which he agreed—dating from the Babylonians, the Egyptians, the Etruscans, and especially the Greeks, who were particularly preoccupied with the location and nature of the soul, as well as the mechanisms responsible for health and disease. Galen fancied a dominant role for the liver as the “seat of sanguification—that is, the manufacture of blood § §—and the source of the veins.” In his schema for the process of digestion (Figure 4), 14 Galen incorporated the concept that the stomach could separate the useful from the useless constituents of food as chyle and provide for the former an absorption site into the portal vein for transport to the liver. Using innate heat or the heat of vegetative pneuma (i.e., the spirit) derived from inspired breath, the baser nutritive liver component of the tripartite soul (in contrast with the rational and noble, and the spirited, emotional, and affective thirds of the soul that are located in the brain and heart, respectively) completes the concoction by which blood is elaborated from chyle. More than a millennium later, Jean François Fernel (Latinized as Ioannes Fernelius), the eminent 16th‐century French physician (1497–1558), mathematician, and astronomer, ¶ ¶ who coined the neologism Physiology, concurred with this second concoction. Incidentally, chyle was also cleansed of impure residues that become fecal matter. Galen thought that blood made by the liver could contain phlegm, air, and black bile, 15 but he opposed the view of the distinguished Alexandrian anatomist and physiologist Erasistratus of Chios (310–250 BCE) that arteries contained air. 16 Incidentally, Galen mocked Erasistratus's prescient hypothesis that tiny channels exist in the liver, connecting the portal to the hepatic veins.
FIGURE 3.

Eighteenth‐century engraving of Claudius Galenus by the Prussian engraver Georg P. Busch (1707–1756) (Berlin). Reproduced with permission from The Wellcome Collection, London.
FIGURE 4.

Physiology of digestion and sanguification (manufacture of blood) according to Galen. Chyle from food partially digested in the stomach and to some extent from the small intestine passes in the portal vein to the liver where the process of digestion (coction) is completed. Partially concocted food material (chyme) also passes from the stomach into the gastrointestinal tract. Excrement from concoction in the liver is excreted as yellow bile into the intestine by way of the gallbladder and bile duct, and as black bile after passage in a retrograde manner through the portal vein, splenic vein, and spleen, by way of a hypothetical splenic duct into the stomach. The heavy earthy material that remains within the gut after digestion is excreted as feces mixed with black and yellow bile. Reproduced with permission from figure 11 in Circulation of the Blood: Men and Ideas. 14 Copyright 1964, Oxford University Press.
However, the proof that bile is really produced by the liver had to wait until the 17th century and the work of Marcello Malpighi (1628–1694) 17 (Figure 5), who is considered to be the founder of microscopical anatomy and histology, the father of physiology and embryology, and arguably the patriarch of plant anatomy as well. 18 Even though Malpighi has some 50 structures named after him, at least in Italian anatomy, arguably his greatest discovery was that of the hair‐like vascular structures in the frog's lung, 19 for which he coined the term capillaries, after capillari—from the Latin capillus/capillum for hair. Malpighi first communicated his discovery of the structure of the lung in two letters to his long‐time friend from his days in Pisa, 20 Giovanni Borelli, but later published it formally after an invitation to present his work at The Royal Society in London. 21 Notwithstanding, biliophiles should value highly Malpighi's appreciation of the acinar organization of the liver** ** and his recognition that bile originated from the liver lobule and not the gallbladder, 17 as was popularly imagined and remained so until the mid‐18th century. Malpighi's decisive experiment‐based view of hepatic bile formation was not without its deluded antagonists but was soon echoed enthusiastically by Francis Glisson 23 (1598/9–1677; Figure 6) in London. Glisson envisioned bile secretion as a process of filtration from the blood, although he could not visualize the necessary connections between the vascular and biliary systems. Yet despite this insight, Glisson actually proposed that the flow of bile results from the successful effort by the ducts to expel the irritating contents. Glisson further theorized that “irritability” was a vital property of all tissues, but this once‐popular doctrine of physiology was abandoned in the 18th century. 24 Although not necessarily an adherent to the Irritability Doctrine of his close contemporary, William Harvey (Figure 7) endorsed Glisson's vision of the excretory/secretory nature of bile formation †† †† and foresaw its detergent and cathartic properties in the intestine. 25 Harvey's intuition about the digestive role of bile in intestinal function was richly reciprocated over the succeeding centuries in studies on digestion by a luminary roster of lumenary 16th‐ to 19th‐century physiologists. ‡‡ ‡‡ But we digress.
FIGURE 5.

(A) Late 17th‐century line engraving of Marcello Malpighi by Jan Kip, after an unknown artist. Source: National Portrait Gallery, London. (B) Front cover of a rare first edition of Malpighi's De Viscerum Structura Exercitatio Anatomica, published by Giacomo Monti in Bologna in 1666, from the collection of Irwin J. Pinkus, MD. Auction at Christie's closed on December 6, 2004.
FIGURE 6.

Portrait of Francis Glisson located in the Museum of the Royal College of Physicians London. © Royal College of Physicians. It is arguable that this portrait was painted by William Faithorne (c.1620–c.1691), who was the artist responsible for the line drawing engraving that is found in the National Portrait Gallery and the Wellcome Institute, because Faithorne was not accustomed to painting in oils.
FIGURE 7.

(A) Portrait of William Harvey in the Museum of the Royal College of Physicians London. © Royal College of Physicians. This likeness that was painted around 1650 shortly before Harvey's death has been attributed to the Dutch artist Cornelius Johnson, but this is disputed. This portrait was one of only two paintings rescued from the Royal College of Physicians during the great fire of London in 1666; the heavy restoration that was necessary is evident in Harvey's distorted right hand. (B) Terracotta statue of William Harvey. Charles Bell Birch (1832–1893) and the Torquay Terracotta Company (1886). Reproduced by kind permission of The Royal Society of Medicine, London, where the statue is located in the Heritage Centre on the second floor of the library.
Criticisms by Glisson's detractors verged on mockery, 27 but their scepticism was bolstered by Glisson's own inconvenient observation that blood flow in the portal system was slow and nonpulsatile. The inescapable objection to blood flow–based bile formation was resolved when an alternative explanation to a hydrostatic mechanism for bile flow was demonstrated, 28 and the intricacies of portal hemodynamics were fully elucidated as the mysteries of portal hypertension were revealed. 29 Yet by the end of the 18th century, the impression that bile formation was the only recognized function of an organ as large as the liver was met with incredulity by the anatomic physiologists of the day. 30
It was also during the 17th century that the concept of an enterohepatic cycle emerged, which during the 18th century was seen as a mechanism to conserve the constituents of bile. The term “bile acids” was proposed by von Liebig in 1842, 31 and it was only in 1870 that the remarkable physiologist Moritz Schiff unequivocally described the enterohepatic circulation. 32 The cellular and molecular mechanisms that underlie this cycle were discovered much later. Surely the history of bile acids deserves its own essay in the current series? Meanwhile the reader is directed to the tour de force four‐score‐year history of key discoveries in bile acid chemistry and biology authored by Hofmann and Hagey 33 and the equally masterful review of cholesterol gallstone pathogenesis by Frank Lammert 34 that will appear soon in the current series.
The mechanisms by which the liver produces bile were progressively elucidated during the second half of the 20th century. Ralph W. Brauer and colleagues, 28 at the US Naval Radiological Defence Laboratory in San Francisco, California, §§ §§ were the first to address the question of the source of energy for bile formation. At that time, it was well established that the energy for urine secretion was hydrostatic pressure provided by the heart contractions and transmitted to renal arteries and capillaries. To test the hydrostatic pressure hypothesis for bile secretion, Brauer used the isolated perfused rat liver with a cannula inserted into the common bile duct and placed in a vertical position, to gauge bile secretory pressure. He could monitor the arterial perfusion pressure simultaneously and, perhaps to his surprise, he saw that the bile column always rose to a higher level than the arterial perfusion pressure, whatever latter pressure he used (above a threshold). This showed conclusively that hydrostatic pressure could not be the source of energy for secretion of bile by the liver. Brauer and colleagues 28 concluded that bile formation required metabolic energy and a process of “active transport.” The hypothesis at this stage was that the initial process of bile formation was not hydrostatic filtration but rather osmotic filtration in response to the active transport of one or several solutes into the canalicular lumen.
Five years later, in a landmark paper, Ivar Sperber, 35 from Uppsala (Sweden), proposed that “the primary event in bile formation would be the active transfer (from the cells or through the cells) of bile acids (and possibly other, less quantitatively important compounds) into the bile capillaries that nowadays are called bile canaliculi. 22 The osmotic effect of these solutes would result in a flow of water and dissolved molecules and ions into bile capillaries. In support of this theory, Sperber 35 showed experimentally that bile flow was positively related to bile salt excretion rates in bile. His theory was directly derived from his observations on renal physiology and the relationship between organic anion secretion and the flow of urine.
The hypothesis proposed by Sperber 35 was strongly supported a few years later by Henry Wheeler and his coworkers at Columbia University in the laboratory of Stanley Bradley. Wheeler et al. 36 used inulin and mannitol as markers of canalicular bile formation in bile fistula dogs. They proposed that mannitol enters canalicular bile by diffusion and is neither reabsorbed nor secreted by the biliary channels, so that its secretion rate is an estimate of canalicular bile flow. They showed that when sodium taurocholate was infused at increasing rates, both bile flow and mannitol excretion increased, but when the hormone secretin was given, bile flow increased but mannitol excretion remained stable. 36 The observation that secretin does not increase mannitol excretion is consistent with the idea that secretin stimulates secretion by the biliary epithelium (in the same way that it stimulates secretion by pancreatic ducts). These observations in four dogs were similar to the findings in guinea pigs by Lee Forker 37 and earlier by Chenderovitch et al. 38
The interpretation of these observations was complicated by the fact that mannitol is a marker of extracellular space and as such, it should not enter the hepatocyte. This apparent contradiction was resolved with the discovery of aquaporins. 39 Aquaporin 9 (also known as aquaglyceroporin, because it allows the movement of glycerol) is found on the basolateral membrane of hepatocytes 40 and allows the transport of mannitol into the hepatocyte. Because aquaporin 9 is not found on the canalicular membrane, it is likely that mannitol enters canalicular bile by a vesicular mechanism related to bile acids.
At this stage, it was reasonably well established that there are two sites of bile formation: the canaliculus and the bile ductules or ducts. Canalicular bile flow is stimulated by bile acids, while ductular/ductal bile flow is stimulated by secretin. Water movement in the bile ducts or ductules occurs through aquaporin 1, 41 which does not transport mannitol. This explains why secretin does not increase mannitol clearance.
In their original description of the linear relationship between canalicular bile flow and bile salt secretion, Wheeler et al. 36 noted that this relationship, when “extrapolated” to zero bile salt secretion, yielded a positive value. They postulated that this corresponded to a bile salt–independent fraction of canalicular bile flow. With Micheline Dumont and others in our laboratory, 42 , 43 , 44 we showed that this component of canalicular bile flow was much greater in rabbits and rats than in the dog. Jim Boyer 45 at Yale University confirmed this independently in the isolated perfused rat liver. This concept was challenged because it was argued that the “osmotic activity” of bile salts increases at low bile salt concentrations, 46 , 47 and hence at low bile salt concentrations and excretion rates, a given amount of bile salts could drive more water than at higher concentrations. However, several other observations support the view of a canalicular bile salt–independent flow in all species studied 48 , 49 , 50 (Figure 8), including humans. 51 , 52 We initially proposed that canalicular bile acid–independent bile flow was driven by Na+ transport into canaliculi mediated by the enzyme Na+, K+‐ATPase. 42 This hypothesis was refuted when it was shown that Na+, K+‐ATPase was not located on the canalicular membrane but on the basolateral membrane of hepatocytes. There is now evidence that canalicular bile salt–independent flow is driven by secretion into the canaliculi of glutathione and other thiols 53 , 54 and bicarbonate. 55 , 56 Bicarbonate secretion by the hepatocyte is mediated by a canalicular Cl−/HCO3 − exchanger. 57 , 58
FIGURE 8.

Relationship between bile flow and bile acid secretion rate in five species (rabbit, rat, dog, monkey, and human). The relation extrapolates to a positive value for a zero bile salt secretion, an estimate of the bile acid–independent bile flow. Débit biliaire = bile flow; débit des acides biliaires = bile acid secretion rate. Reproduced with permission from La Revue du praticien. 52 Copyright 1991, J.‐B. Baillière.
The next major step in understanding the mechanisms of bile secretion was to identify the membrane and cellular transporters mediating these different functions. The protein responsible for bile acid transport across the canalicular membrane was identified by photoaffinity labeling in 1991 by Müller in the Dietrich Keppler laboratory 59 and by Nishida working with Win Arias. 60 Then, Emmanuel Jacquemin, working in the late Peter Meier's laboratory in Zurich, made an important advance. He cloned the first organic anion transporter, called organic anion transporting polypeptide (OATP), 61 , 62 located on the basolateral membrane of hepatocytes and responsible for the hepatocellular uptake of the dye sulfobromophthalein (bromsulfthalein), which was once used extensively in humans (both clinically and experimentally) and animals in testing liver function 63 and of the sodium‐independent uptake of conjugated bile acids. Since then the group of OATPs (now named solute carriers [SLCs]) has been considerably extended: it includes more than 300 members organized into more than 60 families. 64 Most members of the SLC group are located in the cell membrane. The molecular cloning of OATP (SLC) was followed by that of the sodium taurocholate cotransporting polypeptide (the sodium‐dependent transporter of bile acids, also known as SLC10A1), which is expressed on the basolateral membrane, by Bruno Hagenbuch 65 in the same laboratory in Zurich. This pioneering work opened a new chapter in the understanding of biliary physiology, namely, the molecular cloning of the membrane transporters responsible for the secretion of all biliary constituents. The list of these transporters is quite long and has been the subject of several reviews, 66 , 67 regarding both hepatocytes and cholangiocytes. 68 , 69 Soon the three‐dimensional structure of these transporters was established, along with identification of mutant forms. 70 An example is given in Figure 9. The identification of the transporters implicated in bile formation led to an understanding of several genetic diseases caused by mutations of the carrier proteins, 71 mirroring remarkable progress in the knowledge of the mechanisms of hereditary hyperbilirubinemia and cholestatic syndromes. 72
FIGURE 9.
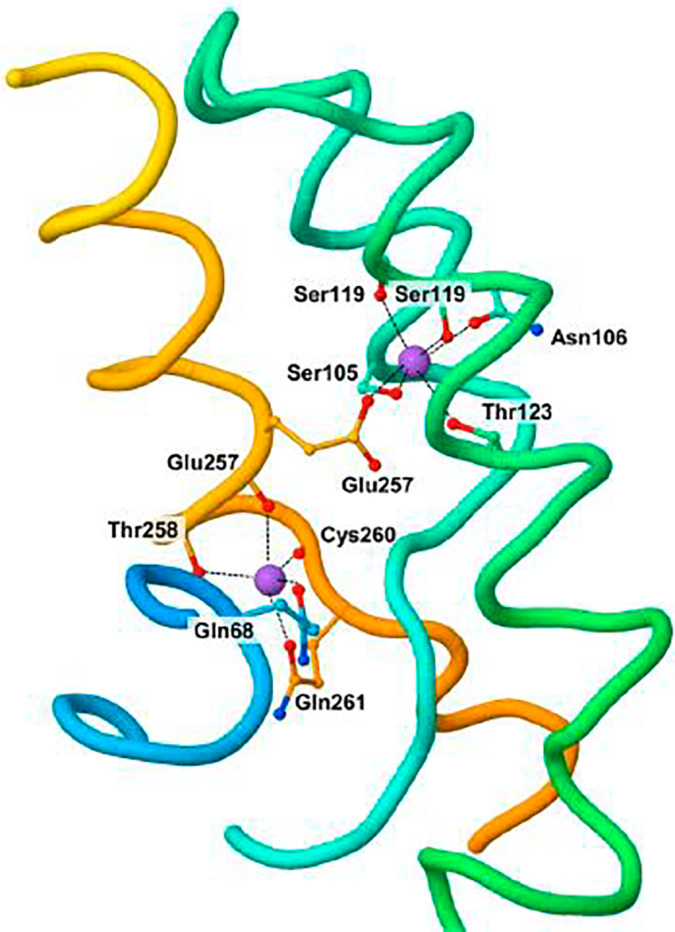
Three‐dimensional structure of sodium taurocholate cotransporting polypeptide (SLC10A1), the sodium taurocholate basolateral transporter of hepatocytes. Reproduced with permission from Current Topics in Membranes. 72 Copyright 2012, Elsevier.
As for the mechanism for bile, once formed, to flow downstream out of the canaliculi, it stands to reason that it must be hydrostatic because the primary force for secretion by hepatocytes is the osmotic pressure generated by concentrative bile acid translocation across the canalicular membranes. In the absence of bile secretory failure or biliary obstruction, bile flows from the canaliculi via the canals of Hering 22 into the biliary tree. Although not subscribing to Glisson's irritability hypothesis, bile flow from central regions of the lobule to the bile ducts is enhanced by canalicular “peristalsis” because of coordinated calcium‐stimulated contractions of pericanicular actin‐myosin microfilaments. 73 , 74 Perhaps more surprising than the finding of canalicular peristalsis upward of 40 years ago comes the reborn hypothesis that hydrostatic pressure (because of paracellular water movement) may indeed play a role in bile formation in the human liver. This and other developments in the field have recently been comprehensively reviewed. 67
Another major step in our knowledge of biliary physiology was the discovery in 1999 that bile acids serve as ligands for the nuclear receptor FXR (the farnesoid X receptor). 76 , 77 , 78 This opened the way to characterize their actions as selective signaling molecules 79 for, as we shall see, their therapeutic potential.
Medical research has a major goal, which is to improve health. Research on biliary physiology has fully reached this goal. The first medical application of bile acids in medicine was found in Asia. Chinese traditional physicians have used bear bile to treat various digestive disorders for centuries 79 a tradition that was extended to Japan and Korea. Bear bile contains ursodeoxycholic acid—from Ursa, the Latin for bear—the 7β epimer of chenodeoxycholic acid from chena, χήνα, Greek for goose. In 1972, Alan Hofmann and colleagues were the first to demonstrate that chenodeoxycholic acid was able to dissolve radiolucent (cholesterol) gallbladder gallstones, 80 the first medical treatment of this disease. However, chenodeoxycholic acid had a few side effects, including diarrhea and elevations of aminotransferases, and was superseded by ursodeoxycholic acid, after Makino et al., 81 in 1975, showed that cholesterol gallstone dissolution could be obtained, with very few side effects. This initiated a considerable number of clinical studies all over the world. 82 The multiple physiological, pathophysiological and therapeutic aspects of bile acids were discussed with great enthusiasm at the regular bile acid meetings organized generously by Herbert Falk (1924–2008) alternately in Freiburg and Basel. These scientifically and socially lavish meetings undoubtedly contributed to progress in the discipline.
Ursodeoxycholic acid was largely used in selected patients with gallstones until the advent of laparoscopic cholecystectomy in the early to mid‐1980s, chronologically in Russia, Germany, and France. For a while, the invention and innovation of laparoscopic cholecystectomy were enigmatic, 83 and its pioneers were not immediately recognized or applauded. Philippe Mouret, a French surgeon in Lyon, did not submit his work for publication because he “did not see any chance for publishing in a surgical journal,” as he stated in 1994 in an interview with Litynski. 84 But, with the help of his colleagues François Dubois in Paris and Jacques Périssat in Bordeaux, laparoscopic cholecystectomy rapidly gained widespread acceptance among surgeons and the public. It also developed very rapidly in other European countries and in the United States. In a similar hostile environment, Erich Mühe (1938–2005), an Erlangen‐trained surgeon (Figure 10) working in Böblingen, Germany, performed laparoscopic cholecystectomies a few years before Mouret, but Mühe's work was rejected by the German surgical community and remained largely ignored. 85 The colorful, often controversial history of gallstone surgery and its eventual acceptance is told with panache by Frank Lammert 34 in another forthcoming essay in this series, in which the pathogenesis of cholelithiasis is also described, lyrically. Since the advent of laparoscopic cholecystectomy, the medical dissolution of gallstones by ursodeoxycholic acid rapidly lost its indications and the favor of physicians.
FIGURE 10.

Erich Mühe, innovative surgeon and 1985 national and 1987 international cycling champion. Reproduced with permission from Journal of Minimal Access Surgery. 83 Copyright 2011, Indian Association of Gastrointestinal Endo Surgeons.
However, ursodeoxycholic acid rapidly knew an exciting rebirth. With Raoul Poupon, working with me, we reasoned that ursodeoxycholic acid was a “very special bile acid” with properties quite different from those of physiological bile acids. We had discovered, with Micheline Dumont, that it was “hypercholeretic,” with a marked increase in bicarbonate output into bile 86 (Figure 11). In addition, in contrast with physiological bile acids, it was hydrophilic, not detergent, and was not toxic to cell membranes. Because it was already approved as a drug by health agencies, Poupon 87 decided to try it in patients with primary biliary cholangitis (PBC) (termed primary biliary cirrhosis at that time). The first results were spectacular in terms of serum liver biochemical tests, 87 and a controlled trial showed a highly significant prolongation of transplant‐free survival in treated patients. 88 This result was confirmed by a combined analysis of several international trials, 89 and now ursodeoxycholic acid is universally recommended as a first‐line treatment of PBC 90 and of a number of cholestatic diseases.
FIGURE 11.

Hypercholeresis induced by ursodeoxycholate in the rat. At secretion rates greater than 500 μmol/min/100 g of body weight, ursodeoxycholate has a much greater choleretic effect than taurocholate. Débit biliaire = bile flow; débit des sels biliaires = bile salt secretion rate. Reproduced with permission from Gastroenterology. 86 Copyright 1980, American Gastroenterological Association.
The discovery that bile acids are ligands of the nuclear receptor FXR also opened a new therapeutic avenue. When they bind to FXR, physiological bile acids repress the synthesis of new bile acids through inhibition of 7α‐hydroxylase, a rate‐limiting enzyme in bile acids synthesis. 9 This is a “protective” mechanism against the accumulation of bile acids in liver cells during cholestasis. Obeticholic acid, or 6‐ethylchenodeoxycholic acid, is a much more potent agonist of FXR than physiological bile acids. When administered to patients with cholestasis, it blocks efficiently the synthesis of new bile acids, decreases bile acids in the liver, and improves markers of cholestasis. It has been successful in decreasing alkaline phosphatase and other biochemical tests in patients with PBC 91 , 92 and, more surprisingly, in patients with nonalcoholic fatty liver disease (NAFLD ¶¶ ¶¶). The interim results of an ongoing multicenter randomized trial 94 in this latter disease have been reported, 95 in which obeticholic acid 25 mg significantly improved fibrosis and key components of disease activity among patients with nonalcoholic steatohepatitis, and this is reasonably likely to predict clinical benefit.
Physiological advances in the knowledge of bile formation led to therapeutic advances that have already helped many patients and, it is hoped, will continue to do so. The history of research on bilirubin, the pigment responsible for jaundice, is also a fascinating area and will be the subject of another essay in this History of Hepatology series by Toni Herta and Ulrich Beuers.
SERIES EDITOR'S POSTSCRIPT
The gradual development of our understanding of how bile is formed exemplifies the changes in the study of the liver that have taken place over the millennia, and especially during my own investigative professional career that began in earnest enquiring into biliary lipid secretion in obese individuals in late 1976. Given the profusion of functions of the liver, the investigation of which is ably reviewed by Mousa and Kamath 64 in the current series, it is curious to reflect that at the end of the 18th century bile secretion was deemed the sole justification for hepatic existence, to which the liver was relegated once the soul was relocated elsewhere. In this context, there is no one better than Serge Erlinger to relate the “History of Research Into the Physiology of Bile,” because he was a key contributor to our understanding of bile formation, for which he was duly recognized and honored by the scientific hepatology community, including being awarded the coveted quadrennial prize by the Falk Foundation in 1980.
As with other developments and discoveries in the history of hepatology, serendipity can be relied on to play its role here too, as long as prepared minds are on hand to be favored by chance.*** *** How else can one explain that a dihydroxy‐5beta‐cholanic acid derivative of chenodeoxycholic acid, obeticholic acid, a FXR agonist that is a useful adjunct in the treatment of the cholestatic liver disease PBC, also shows promise in the treatment of NAFLD.
Prof. Erlinger is a native Parisien, whose medical studies were at the University of Paris (1957–1963), his residency (1962–1967) was fulfilled at Paris hospitals, and a research fellowship was undertaken at New York‐Presbyterian/Columbia Hospital in Stanley E. Bradley's laboratory. Under the direction of Prof. René Fauvert, he was awarded his Doctorate in Medicine in 1967 for his thesis on the mechanisms of bile secretion—what else? His glowing career thereafter included time (1973–1976) at the Institut National de la Santé et de la Recherche Médicale (INSERM) and a rise within the ranks at l'Hôpital Beaujon in Clichy to succeed (1993–2000) the renowned founder and Chief of the Liver Department, Prof. Jean‐Pierre Benhamou (1927–2008). By the bye, Serge was also Director of the Liver Pathophysiology Research Laboratory of INSERM in the same hospital (1986–1998) and also Secretary General of the European Association for the Study of the Liver (1974–1975).
It would be remiss of me not to mention other captivating facts from his biography. His childhood in Paris was interrupted in the early 1940s by the Nazi German occupation. As a 3‐year‐old he was separated from his parents (who had previously fled antisemitism in Poland) and his brother, and he was evacuated by the Assistance Publique to a small hamlet, Les Mardelles, near Châtillon‐sur‐Cher, near the Loire Valley. For 4 years, he was hidden by a compassionate local couple on their small farm in Loir‐et‐Cher. Erlinger published this poignant period in his young life until his miraculous reunion with his family at war's end, in his graphically documented autobiography Parcours d'un enfant caché (1941–1945): Une enfance aux Mardelles. ††† †††
After retirement from the University of Paris in 2000, he and his wife relocated to Rognes, a village in Provence, where he augmented his bucolic existence by pursuing studies. in history on the vexed topic of the Israeli‐Palestinian conflict (“La représentation du conflit israélo‐arabe dans les éditoriaux du Monde de 1987 à 2002”), at the university in Aix‐en‐Provence.
CONFLICT OF INTEREST
Nothing to report.
Erlinger S. A history of research into the physiology of bile, from Hippocrates to molecular medicine. Clin Liver Dis. 2022;20:33–44. 10.1002/cld.1266
Footnotes
https://en.wiktionary.org/wiki/Reconstruction:Proto‐Indo‐European/ǵʰelh₃‐, last accessed December 30, 2021.
https://www.wordsense.eu/bilis/, last accessed December 30, 2021.
https://www.etymonline.com/word/bile, last accessed December 30, 2021.
To which idea the rabbis of the Talmudic period (70–640 CE) and even William Harvey (1578–1657) subscribed.
After whom the Fernelius lunar crater is named.
Resurrected later by Aron Rappaport. 23
From his years of tenure as the renowned Lumleian lecturer, 27 which began in April 1616. Harvey's manuscript notes of these lectures “Praelectiones anatomicae Universal For me Gulielmum Harveium, medicum Londinensem, anatomist. and surgeon. Professor, Year Sun. 1616, aetatis 37: praelect. April, 1617,” were rare documents that were rescued from the Great Fire, which engulfed the library that Harvey helped establish at the Royal College of Physicians, and are now in the British Museum.
Sixteenth century: Jean François Fernel and Philip Aureolus Theophrastus Bombastus von Hohenheim (i.e. Paracelsus); 17th century: Jan Baptist van Helmont and Isbrand van Diemerbroeck; 18th century: Herman Boerhaave and Albrecht von Haller; 19th century: Theodore Schwann, Reinhold Schellbach, Johannes Peter Müller, William Saunders, and William Beaumont.
This was an early military laboratory created in 1946 to study the effects of radiation and nuclear weapons. The facility was based at the Hunter's Point Naval Shipyard in San Francisco, California, to manage testing, decontamination, and disposition of US Navy ships contaminated by the pair of Operation Crossroads nuclear tests at the Bikini Atoll in the Pacific.
Recently suggested to be renamed metabolic‐associated fatty liver disease (MAFLD 94 ).
According to Pasteur's famous aphorism.
Journey of a Hidden Child (1941–1945): A Childhood in the Mardelles. Published by Le Manuscrit, Paris, 2012.
REFERENCES
- 1. Bryan CP, trans. The papyrus ebers. Ancient Egyptian medicine. London: Geoffrey Bles; 1930. p. 58, 143, 159. [Google Scholar]
- 2. Alter R. The five books of moses. A translation with commentary. New York: WW Norton and Company; 2004. [Google Scholar]
- 3. King James Holy Bible 1611 Edition. Peabody, MA: Hendrickson Publishers; 2005. [Google Scholar]
- 4. Easton MG. Illustrated bible dictionary. 3rd ed. Nashville, TN: Thomas Nelson; 1897. [Google Scholar]
- 5. de Vaan M. Etymological dictionaries of Italic and other languages. Leiden: Brill; 2008. [Google Scholar]
- 6. Cordell EF. The medicine and doctors of Horace. Bull Johns Hopkins Hosp. 1901;12:233–40. [Google Scholar]
- 7. Garrison FH. History of gastro‐enterology (special reference to American developments). Bull NY Acad Med. 1934;10:629–42. [PMC free article] [PubMed] [Google Scholar]
- 8. Mellinkoff SM. Some meanings of the liver. Gastroenterology. 1979;76:636–8. [PubMed] [Google Scholar]
- 9. Hurst A. The physical basis of “biliousness” and “wind round the heart”. Br Med J. 1938;1:661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bynum B. Biliousness. Lancet. 2002;359:176. [Google Scholar]
- 11. Richards DW. Hippocrates of Ostia. JAMA. 1968;204:1049–56. [PubMed] [Google Scholar]
- 12. Sun D. How does bile rise? Physiology of bile from Homer to Hippocrates. Bull Assoc Guillaume Budé. 2011;1:119–36. [Google Scholar]
- 13. Joly R. Hippocrates. Complete works (volume XI). Paris: Les Belles Lettres; 2003. [Google Scholar]
- 14. Bradley SE. The splanchnic circulation. In: Fishman AP, Richards DW, editors. Circulation of the blood: men and ideas. New York: Oxford University Press; 1964. p. 607–701. [Google Scholar]
- 15. Balalykin DA. Understanding of the digestive system in the context of the commensurability of medical knowledge in different periods. History Med. 2019;6:98–110. [Google Scholar]
- 16. Karamanou M, Stefanadis C, Tsoucalas G, Laios K, Androutsos G. Historical perspective: Galen's (130–201 AD) conceptions of the heart. Hellenic J Cardiol. 2015;56:197–200. [PubMed] [Google Scholar]
- 17. Malpighi M. De viscerum structura exercitatio anatomica. Bologna: Giacomo Monti; 1666. [Google Scholar]
- 18. Ghosh SK, Kumar A. Marcello Malpighi (1628–1694): pioneer of microscopic anatomy and exponent of the scientific revolution of the 17th Century. Eur J Anat. 2018;22:433–9. [Google Scholar]
- 19. West JB. Marcello Malpighi and the discovery of the pulmonary capillaries and alveoli. Am J Physiol Lung Cell Mol Physiol. 2013;304:L383–90. [DOI] [PubMed] [Google Scholar]
- 20. Wilson LG. The transformation of ancient concepts of respiration in the seventeenth century. Isis. 1960;51:161–72. [DOI] [PubMed] [Google Scholar]
- 21. Malpighi M. De pulmonibus. London: Philosophical Transactions of the Royal Society; 1661. [Google Scholar]
- 22. Gil RM, Theise ND. Rappaport, Glisson, Hering, and Mall—champions of liver microanatomy: microscopic and ultramicroscopic anatomy of the liver into the modern age. Clin Liver Dis. 2021;18:76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown MT. Physiology and the mechanical philosophy in the mid‐17th century England. Bull Hist Med. 1977;51:25–54. [PubMed] [Google Scholar]
- 24. Giglioni G. What ever happened to Francis Glisson? Albrecht Haller and the fate of eighteenth‐century irritability. Sci Context. 2008;21:465–93. [DOI] [PubMed] [Google Scholar]
- 25. O'Malley CD, Poynter FNL, Russell KF. An annotated translation of the Prelectiones Anatomiae Universalis . William Harvey, trans. Berkeley, CA: University of California Press; 1962. [Google Scholar]
- 26. Keele KD. William Harvey as morbid anatomist. Proc R Soc Med. 1962;55:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Diemerbroeck I. The anatomy of human bodies: comprehending the most modern discoveries and curiosities in that art to which is added a particular treatise of the small‐pox & measles, together with several practical observations and experienced cures. London: William Whitewood; 1694. [Google Scholar]
- 28. Brauer RW, Leong GF, Holloway RJ. Mechanics of bile secretion; effect of perfusion pressure and temperature on bile flow and bile secretion pressure. Am J Physiol. 1954;177:103–12. [DOI] [PubMed] [Google Scholar]
- 29. Groszmann RJ, Abraldes JG. Portal hypertension: from bedside to bench. J Clin Gastroenterol. 2005;39:S125–30. [DOI] [PubMed] [Google Scholar]
- 30. Bichat MFX. General Anatomy: Applied to Physiology and the Practice of Medicine (translated by Constant Coffyn, revised and corrected by George Calvert). London: Shackell and Arrowsmith; 1824. [Google Scholar]
- 31. Liebig J. Animal chemistry, or organic chemistry in its applications to physiology and pathology. London: Taylor and Watton; 1842. [PMC free article] [PubMed] [Google Scholar]
- 32. Reuben A. The biliary cycle of Moritz Schiff. Hepatology. 2005;42:500–5. [DOI] [PubMed] [Google Scholar]
- 33. Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55:1553–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lammert F. Gallstones: the thing itself. Clin Liver Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sperber I. Secretion of organic anions in the formation of urine and bile. Pharmacol Rev. 1959;11:109–34. [PubMed] [Google Scholar]
- 36. Wheeler HO, Ross ED, Bradley SE. Canalicular bile production in dogs. Am J Physiol. 1968;214:866–74. [DOI] [PubMed] [Google Scholar]
- 37. Forker EL. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967;46:1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chenderovitch J, Phocas E, Rautureau M. Effects of hypertonic solutions on bile formation. Am J Physiol. 1963;205:863–7. [DOI] [PubMed] [Google Scholar]
- 39. Agre P, Sasaki S, Chrispeels MJ. Aquaporins: a family of water channel proteins. Am J Physiol. 1993;265:F461. [DOI] [PubMed] [Google Scholar]
- 40. Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry‐Moghaddam M, et al. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun. 2000;276:1118–28. [DOI] [PubMed] [Google Scholar]
- 41. Marinelli RA, Tietz PS, Pham LD, Rueckert L, Agre P, LaRusso NF. Secretin induces the apical insertion of aquaporin‐1 water channels in rat cholangiocytes. Am J Physiol. 1999;276:G280–6. [DOI] [PubMed] [Google Scholar]
- 42. Erlinger S, Dhumeaux D, Benhamou JP. Effect on bile formation of inhibitors of sodium transport. Nature. 1969;223:1276–7. [DOI] [PubMed] [Google Scholar]
- 43. Erlinger S, Dhumeaux D, Benhamou JP, Fauvert R. Biliary secretion of rabbit: evidence in favor of important fraction independent of bile salts. Rev Fr Etud Clin Biol. 1969;14:144–50. [PubMed] [Google Scholar]
- 44. Erlinger S, Dhumeaux D, Berthelot P, Dumont M. Effect of inhibitors of sodium transport on bile formation in the rabbit. Am J Physiol. 1970;219:416–22. [DOI] [PubMed] [Google Scholar]
- 45. Boyer JL, Klatskin G. Canalicular bile flow and bile secretory pressure. Evidence for a non‐bile salt dependent fraction in the isolated perfused rat liver. Gastroenterology. 1970;59:853–9. [PubMed] [Google Scholar]
- 46. Carpenter P, Lindenbaum S. Osmotic and activity coefficients of aqueous bile salt solutions at 25, 37, and 45°C. J Solution Chem. 1979;8:347–57. [Google Scholar]
- 47. Balabaud C, Kron KA, Gumucio JJ. The assessment of the bile salt‐nondependent fraction of canalicular bile water in the rat. J Lab Clin Med. 1977;89:393–9. [PubMed] [Google Scholar]
- 48. Berthelot P, Erlinger S, Dhumeaux D, Preaux AM. Mechanism of phenobarbital‐induced hypercholeresis in the rat. Am J Physiol. 1970;219:809–13. [DOI] [PubMed] [Google Scholar]
- 49. Ballatori N, Truong AT. Relation between biliary glutathione excretion and bile acid‐independent bile flow. Am J Physiol. 1989;256:G22–30. [DOI] [PubMed] [Google Scholar]
- 50. Graf J. Canalicular bile salt‐independent bile formation: concepts and clues from electrolyte transport in rat liver. Am J Physiol. 1983;244:G233–46. [DOI] [PubMed] [Google Scholar]
- 51. Erlinger S. The formation of bile. Rev Prat (Paris). 1991;41:2341–6. [PubMed] [Google Scholar]
- 52. Lindblad L, Schersten T. Influence of cholic and chenodeoxycholic acid on canalicular bile flow in man. Gastroenterology. 1976;70:1121–4. [PubMed] [Google Scholar]
- 53. Prandi D, Erlinger S, Glasinovic JC, Dumont M. Canalicular bile production in man. Eur J Clin Invest. 1975;5:1–6. [DOI] [PubMed] [Google Scholar]
- 54. Lauterburg BH, Smith CV, Hughes H, Mitchell JR. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest. 1984;73:124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paulusma CC, van Geer MA, Evers R, Heijn M, Ottenhoff R, Borst P, et al. Canalicular multispecific organic anion transporter/multidrug resistance protein 2 mediates low‐affinity transport of reduced glutathione. Biochem J. 1999;338(Pt 2):393–401. [PMC free article] [PubMed] [Google Scholar]
- 56. Hardison WG, Wood CA. Importance of bicarbonate in bile salt independent fraction of bile flow. Am J Physiol. 1978;235:E158–64. [DOI] [PubMed] [Google Scholar]
- 57. Van Dyke RW, Stephens JE, Scharschmidt BF. Effects of ion substitution on bile acid‐dependent and ‐independent bile formation by rat liver. J Clin Invest. 1982;70:505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strazzabosco M, Boyer JL. Regulation of intracellular pH in the hepatocyte. Mechanisms and physiological implications. J Hepatol. 1996;24:631–44. [DOI] [PubMed] [Google Scholar]
- 59. Müller M, Ishikawa T, Berger U, Klunemann C, Lucka L, Schreyer A, et al. ATP‐dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110‐kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991;266:18920–6. [PubMed] [Google Scholar]
- 60. Nishida T, Gatmaitan Z, Che M, Arias IM. Rat liver canalicular membrane vesicles contain an ATP‐dependent bile acid transport system. Proc Natl Acad Sci U S A. 1991;88:6590–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression cloning of a rat liver Na(+)‐independent organic anion transporter. Proc Natl Acad Sci U S A. 1994;91:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jacquemin E, Hagenbuch B, Wolkoff AW, Meier PJ, Boyer JL. Expression of sodium‐independent organic anion uptake systems of skate liver in Xenopus laevis oocytes. Am J Physiol. 1995;268:G18–23. [DOI] [PubMed] [Google Scholar]
- 63. Mousa OY, Kamath PS. A history of the assessment of liver performance. Clin Liver Dis. 2021;18:28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. HGNC . Gene group: solute carriers (SLC). Available from: http://www.genenames.org/cgi‐bin/genefamilies/set/752. Accessed 30 Jun 2022.
- 65. Hagenbuch B, Lubbert H, Stieger B, Meier PJ. Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J Biol Chem. 1990;265:5357–60. [PubMed] [Google Scholar]
- 66. Keppler D. Progress in the molecular characterization of hepatobiliary transporters. Dig Dis. 2017;35:197–202. [DOI] [PubMed] [Google Scholar]
- 67. Boyer JL, Soroka CJ. Bile formation and secretion. J Hepatol. 2021;75:190–201. [DOI] [PubMed] [Google Scholar]
- 68. Alpini G, McGill JM, Larusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–68. [DOI] [PubMed] [Google Scholar]
- 69. Bogert PT, LaRusso NF. Cholangiocyte biology. Curr Opin Gastroenterol. 2007;23:299–305. [DOI] [PubMed] [Google Scholar]
- 70. Lazaridis KN, LaRusso NF. The cholangiopathies. Mayo Clin Proc. 2015;90:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dðring B, Lutteke T, Geyer J, Petzinger E. The SLC10 carrier family: transport functions and molecular structure. Curr Top Membr. 2012;70:105–68. [DOI] [PubMed] [Google Scholar]
- 72. Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009;51:565–80. [DOI] [PubMed] [Google Scholar]
- 73. Oshio C, Phillips MJ. Contractility of the bile canaliculi: implications for liver function. Science. 1981;212:1041–2. [DOI] [PubMed] [Google Scholar]
- 74. Watanabe S, Smith CR, Phillips MJ. Coordination of the contractile activity of bile canaliculi. Evidence from calcium microinjection of triplet hepatocytes. Lab Invest. 1985;53:275–9. [PubMed] [Google Scholar]
- 75. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5. [DOI] [PubMed] [Google Scholar]
- 76. Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. [DOI] [PubMed] [Google Scholar]
- 77. Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–53. [DOI] [PubMed] [Google Scholar]
- 78. Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: the FXR‐FGF15/19 pathway. Dig Dis. 2015;33:327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Makino I, Takebe K. Bear bile and ursodeoxycholic acid. Sogo Rinsho. 1982;31:2385–7. [Google Scholar]
- 80. Danzinger RG, Hofmann AF, Schoenfield LJ, Thistle JL. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med. 1972;286:1–8. [DOI] [PubMed] [Google Scholar]
- 81. Makino I, Shinozaki K, Yoshino K, Nakagawa S. Dissolution of cholesterol gallstones by long‐term administration of ursodeoxycholic acid. Nihon Shokakibyo Gakkai Zasshi. 1975;72:690–702. [PubMed] [Google Scholar]
- 82. Hofmann AF. Medical dissolution of gallstones by oral bile acid therapy. Am J Surg. 1989;158:198–204. [DOI] [PubMed] [Google Scholar]
- 83. Blum CA, Adams DB. Who did the first laparoscopic cholecystectomy? J Minim Access Surg. 2011;7:165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Litynski GS. Profiles in laparoscopy: Mouret, Dubois, and Périssat: the laparoscopic breakthrough in Europe (1987–1988). JSLS. 1999;3:163–7. [PMC free article] [PubMed] [Google Scholar]
- 85. Lytinski GS. Erich Mühe and the rejection of laparoscopic cholecystectomy (1985): a surgeon ahead of his time. JSLS. 1998;2:341–6. [PMC free article] [PubMed] [Google Scholar]
- 86. Dumont M, Erlinger S, Uchman S. Hypercholeresis induced by ursodeoxycholic acid and 7‐ketolithocholic acid in the rat: possible role of bicarbonate transport. Gastroenterology. 1980;79:82–9. [PubMed] [Google Scholar]
- 87. Poupon R, Chretien Y, Poupon RE, Ballet F, Calmus Y, Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet. 1987;1:834–6. [DOI] [PubMed] [Google Scholar]
- 88. Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA‐PBC Study Group. N Engl J Med. 1991;324:1548–54. [DOI] [PubMed] [Google Scholar]
- 89. Poupon RE, Lindor KD, Cauch‐Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–90. [DOI] [PubMed] [Google Scholar]
- 90. European Association for the Study of the Liver . EASL clinical practice guidelines in primary biliary cholangitis. J Hepatol. 2017;67:145–72. [DOI] [PubMed] [Google Scholar]
- 91. Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C, et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67:1890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Trauner M, Nevens F, Shiffman ML, Drenth JPH, Bowlus CL, Vargas V, et al. Long‐term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3‐year results of an international open‐label extension study. Lancet Gastroenterol Hepatol. 2019;4:445–53. [DOI] [PubMed] [Google Scholar]
- 93. Eslam M, Sanyal AJ, George J, International Consensus Panel . MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014. [DOI] [PubMed] [Google Scholar]
- 94. Ratziu V, Sanyal AJ, Loomba R, Rinella M, Harrison S, Anstee QM, et al. Regenerate: design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp Clin Trials. 2019;84:105803. [DOI] [PubMed] [Google Scholar]
- 95. Zobair M, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non‐alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet. 2019;394:2184–96. [DOI] [PubMed] [Google Scholar]
- 96. Rothschuh KE. History of physiology. Risse GB, trans. Huntington, NY: RE Karger Publishers Co.; 1973. [Google Scholar]


