Abstract
The pathophysiology underlying the loss of dopaminergic neurons in Parkinson’s disease (PD) is unclear. A gap of knowledge in the molecular and cellular events leading to degeneration of the nigrostriatal DA system is a major barrier to the development of effective therapies for PD. 1-methyl-4-phenylpyridinium (MPP+) is used as a reliable in vitro model of PD in dopaminergic neurons; however, the molecular mechanisms that lead to cell death with this model are not fully understood. Additionally, there is a lack of translational in vitro models to fully understand progressive dopaminergic neurotoxicity. Here, we propose cultures of primary human dopaminergic neuronal precursor cells (HDNPCs) as a model to study progressive dopaminergic toxicity and neuronal damage in PD. We evaluated the concentration-response of MPP+ (0–10 mM) at 24 h, using cell viability and mitochondrial activity assays (LDH, XTT, Live/Dead staining, and MitoTracker). Based on concentration-response data, we chose two concentrations (1.0 and 2.5 mM) of MPP+ to evaluate markers of autophagy and dopaminergic status [tyrosine hydroxylase (TH)] after a 24-h exposure. Exposure to MPP+ induced cytotoxicity, reduced cell viability, and decreased mitochondrial activity. MPP+ at 1.0 and 2.5 mM also induced expression of lysosome-associated membrane protein 1 (LAMP-1) and increased the ratio of light chain 3 (LC3), LC3BII/LC3BI. The expression of TH also decreased. Furthermore, α-synuclein (α-SYN) and parkin were evaluated by immunofluorescence (IF) at 1.0 and 2.5 mM MPP+ after 24 h. A qualitative analysis revealed decreased parkin expression while α-SYN aggregation was observed in the cytoplasm and the nucleus. These data suggest that in HDNPCs MPP+ can cause cytotoxicity and neuronal damage. This damage may be mediated by autophagy, dopamine synthesis, and protein aggregation. The combination of HDNPCs and MPP+ may serve as valuable in vitro model of progressive dopaminergic neurotoxicity for research into potential treatments for PD.
Keywords: MPP+, Parkinson's disease, In vitro model, Autophagy, Protein aggregation
Graphical Abstract
Highlights
-
•
High concentrations of MPP+ induced necrosis on HDNPCs, associated to reduction of metabolic and mitochondrial activity of HDNPCs.
-
•
MPP+ effects may involve dopaminergic dysfunction, autophagy initiation, aggregation of α-SYN, and decreased Parkin protein expression.
-
•
Characterization of the mechanism of action of MPP+ is relevant to understanding PD as well as for evaluating potential biomarkers and therapeutic agents.
1. Introduction
Parkinson's disease (PD) is the second-most common neurodegenerative disorder affecting 2–3% of the population older than 65 [37]. PD is a progressive central nervous system disease of unknown etiology characterized by motor impairments that arise from selective degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc) [2]. The mechanisms implicated in PD progression include mitochondrial dysfunction, oxidative stress, and protein aggregation [10]. However, what initiates these mechanisms is poorly understood. 1-methyl-4-phenylpyridinium (MPP+) is the toxic metabolite of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) and has a high-affinity for dopamine transporters (DATs), which allow it to enter dopaminergic neurons [27]. Once in the neuron, MPP+ is thought to accumulate in the mitochondria, inhibiting complex 1 of the electron transporter chain [29], causing oxidative stress and triggering cellular pathways that mimic PD pathophysiology [12].
Autophagy is one process responsible for the removal of dysfunctional proteins from the brain. Dysregulation of autophagy may result from the accumulation of abnormal proteins in the course of PD [26]. Autophagy is critical for the regulation of α-synuclein (α-SYN) protein levels and is protective against neuronal death [11]. Disrupted, autophagy has been suggested as a mechanism for MPTP/MPP+-induced toxicity [18], [41] [44]. The aggregation of α-SYN monomers, presumably caused by disrupted autophagy, has been suggested to play a crucial role in PD pathology, with α-SYN toxicity depending on the oligomerization/aggregation status [7]. Exposing dopaminergic cells to MPP+ may be an effective model of α-SYN aggregation-related neurotoxicity. However, little is known about the underlying mechanism of MPTP/MPP+-induced autophagy, but it may be related to microtubule-associated protein function. The conversion of light chain 3 (LC3) BI to LC3BII occurs as part of the normal autophagy process. Evidence for autophagy in PD-related neurodegeneration is supported by an in vitro MPP+ model of PD, where an increase in LC3BII was shown to be the result of a pro-death mechanism and may be dependent on the aggregation of α-SYN in the dopaminergic system [14].
Aggregation of α-SYN and recruitment of Parkin may be caused by mitochondrial dysfunction [15], [39]. α-SYN is an abundant neuronal protein that when misfolded, accumulates and is deposited into Lewy bodies in PD. α-SYN aggregation may induce early synaptic loss and axonal damage, both being prominent signs of nigrostriatal degeneration in PD [35], [36], [10]. Lewy bodies can contain aggregated Parkin, ubiquitin, and other proteins [8], [1]. Parkin is an E3 ubiquitin-protein ligase, which facilitates proteasomal degradation of misfolded proteins, like α-SYN in Lewy bodies [17]. The observation of parkin-α-SYN complexes in PD brains suggests these proteins interact and are co-localized in pathological structures [5]. Parkin has been implicated in the regulation of proteasomal degradation pathways as well as mitophagy [8], [9]. The colocalization of these proteins further supports disrupted autophagy as a pathological mechanism in PD.
MPP+-induced cytotoxicity has been tested in different primary neuronal cells such as N2a mouse neuroblastoma and embryonic mouse and rat mesencephalic cells. In these assays, low micro-molar concentrations of MPP+ substantially reduce cell viability as measured by MTT and LDH [25], [43], [30]. However, the steep concentration-response to MPP+ in these models limit their utility in modelling progressive dopaminergic damage. An in vitro model better suited to study underlying mechanisms of progressive dopaminergic damage that also allows drug target identification using a broad range of concentrations may improve the drug discovery process.
Primary human dopaminergic neuronal precursor cells (HDNPCs; ABMGood T4034), in conjunction with MPP+ may serve as an appropriate in vitro model to study progressive damage to the dopaminergic system. In Parkinson’s disease (PD), two hallmarks are necessary to mimic the pathology, the degeneration of DA neurons and protein aggregates consisting mainly of α-SYN. Currently, models of PD only partially reproduce the pathophysiology of the disease, mainly because the etiology of sporadic PD is still unknown.
The use of primary human neuronal-based models to study pathophysiology of PD disease is limited, because the technical and ethical challenges. However, using primary cell-based models from human origin brings translational basis to disease modeling and will facilitate optimal high-throughput pre-clinical validation of therapeutics. We used a commercially available primary human dopaminergic neuronal precursor (HDNP) cell to evaluate dopaminergic toxicity and we ecpect that this model reproduces the two main hallmarks of PD [32]. Generation of a human cellular model would improve understanding of the development and/or progression of dopaminergic neurotoxic damage, disrupted autophagy and α-SYN accumulation. This could allow for high throughput approaches for screening biomarkers and therapeutic agents for PD. The well-known mitochondrial pathways of dopaminergic neurodegeneration have been heavily investigated in various in vitro and in vivo models of PD. However, the extensive role of protein aggregation and autophagy is poorly studied. Here, we evaluated concentration-response effects of MPP+ on α-SYN aggregation and autophagy in HDNPCs. This approach may provide a better model to understand the role of protein aggregation and autophagy in PD, improving the search for novel therapeutics.
2. Methodology
2.1. Primary cell culture
HDNPCs were purchased from ABMGood (T4034, Richmond, BC, Canada). As per vendor, cells are human-derived precursor cells [P2 fetal tissue (14–16weeks gestation)] obtained from the brain, and the isolation method used included a tyrosine free medium supplemented with a mixture of growth factors. These cells were cultured in plates coated with poly-L-lysine (PLL) at a density of 1 × 104 cells/cm2 using proprietary PriGrow IV culture medium (TM004) supplemented with 5% fetal bovine serum (TM999). At confluence, 10 ng/mL basic fibroblast growth factor (R&D Systems 4114TC), 10 ng/mL epidermal growth factor (R&D Systems 236-EG-01 M) and 100 µM dibutyryl-cyclic adenosine monophosphate (Sigma D0627) were added to PriGrow IV. Cells were maintained in this media for 7 days to achieve full differentiation to neurons prior to assay performance.
2.2. Parkinson’s disease in vitro model and treatments
MPP+ (Santa Cruz, sc-206178, Dallas, TX) was dissolved in differentiation media and then filtered (0.2 μ). Upon differentiation, HDNPCs were treated with MPP+ (0, 0.1, 0.5, 1, 5, and 10 mM), for 24 h and evaluated by lactate dehydrogenase (LDH), 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]− 2 H-tetrazolium-5-carboxanilide (XTT), MitoTracker, and live/dead assays with n = 6 per experiment. Each assay was replicated in triplicate.
Based on cytotoxicity data, two concentrations of MPP+ were selected to test for autophagy markers [lysosome-associated membrane protein 1 (LAMP-1) and (LC3BII) and dopaminergic status [tyrosine hydroxylase (TH)] by western blot (WB) after a 24-h exposure. The localization of parkin and α-SYN by immunofluorescence (IF) was also evaluated.
2.3. Cell viability assays
2.3.1. LDH assay
LDH was measured using a commercially available kit (Roche, Basel, Switzerland) 24 h after MPP+ exposure. It is a well-established method to evaluate cell viability that measures the release of intracellular enzyme LDH [3], [20]. LDH was quantified by measuring the absorbance at 490 nm with a reference wavelength of 650 nm (Synergy MX, BioTek, Winooski, VT).
2.3.2. XTT assay
The metabolic activity of HDNPCs, a functional representation of reduced cell viability, was determined using previously described methods [21]. Briefly, cells were treated with MPP+ for 24 h. Mitochondrial dehydrogenase-induced cleavage of XTT was then measured [31]. Fresh XTT reagent (30 µL at 0.2 mg/mL) in the presence of 25 µM phenazine methosulfate (PMS) was added and incubated for 2 h at 37 °C. Absorbance was measured at 450 nm with a reference wavelength of 650 nm (Synergy MX, BioTek, Winooski, VT).
2.3.3. Live/dead assay
The viability of cells was analyzed using a commercially available kit (Thermo Fisher, Waltham, MA). Briefly, after a 24-h exposure to MPP+, the medium was removed from the cultures and the cells were washed with Dulbecco’s phosphate-buffered saline (DPBS). A solution of 2 μM calcein and 4 μM ethidiumhomodimer-1 in DPBS was added for 30 min at room temperature. Micrographs were taken at 4X and live or dead cells were detected using a fluorescein isothiocyanate (FITC) or tetramethylrhodamine (TRITC) filters, respectively. Images were analyzed using ImageJ (National Institutes of Health) and the data are reported as percentage of live cells.
2.4. Mitochondrial function assay
To assess changes in mitochondrial function, MitoTracker® Orange CMTMRos (Invitrogen, Waltham, MA) staining was used. After a 24-h exposure to MPP+, media was removed, and cells were labeled with 5 μg/mL Hoechst 33342 and 100 nM MitoTracker® Orange CMTMRos for 30 min at 32 °C. Cells were then fixed in 2% paraformaldehyde. Images were then acquired at 20X for qualitative analysis. The dye is well-retained after aldehyde fixation, allowing it to be tracked under microscopy for qualitative analysis.
2.5. Western Blot analysis
Protein expression of TH was evaluated. After 24 h of MPP+ exposure, HDNPCs were lysed with RIPA buffer (Cell Signaling, Danvers, MA) containing protease inhibitors, phosphatase inhibitors (Sigma-Aldrich Inc., St. Louis, MO), and phenylmethylsulfonyl fluoride (Cell Signaling, Danvers, MA). Protein concentration was determined using the bicinchoninic acid (BCA) method (Thermo Fisher Scientific, Waltham, MA). For each sample, 15 µg protein was loaded in 4–20% Tris-HCl gradient gels (Bio-Rad, Hercules, CA) and electrophoresis performed at 200 V for 60 min at 4 °C. After electrophoresis, proteins were transferred to polyvinylidene fluoride (PVDF) membranes at 100 V for 60 min at 4 °C. Membranes were blocked with PBS blocking buffer (LiCor, Lincoln, NE) for 1 h and then incubated 48 h at 4 °C with LAMP-1 (, Phosphosolutions, Aurora, CO, 1:1000), LC3BII (Cell Signaling, 1:500), TH (Millipore, Burlington, MA, 1:1000), and β-actin (Sigma-Aldrich Inc., 1:5000). Membranes were then washed and incubated with IRDye 800CW or 680 RD secondary antibodies (LiCor, 1:20,000) for 1 h at room temperature and protected from light. Band intensities were determined with the Odyssey CLx Infrared system (LiCor) and quantified with Image Studio version 5.0 (LiCor). Data were normalized to β-actin and the integrated density of each marker was expressed as ratio of LAMP-1/ βA, LC3B/ βA, TH/ βA. The most representative band for each marker or condition was chosen for the final figure.
2.6. Morphological analysis
To assess the localization of parkin and α-SYN, cells were grown on 8-well chambered slides. After a 24-h exposure to MPP+ (1.0 and 2.5 mM), cells were fixed in 4% paraformaldehyde in PBS (pH 7.4), blocked with 5% albumin (Sigma-Aldrich Inc.) and incubated with anti-Parkin, rabbit (Cell Signaling, 1:200) and anti-α-SYN, mouse (1:200, Cell Signaling,) overnight at 4 °C. Secondary antibodies [anti-rabbit- FITC or anti-mouse- CY3, Jackson ImmunoResearch, West Grove, PA, 1:500] were incubated for 2 h at room temperature. Photomicrographs were taken at 20X for qualitative analysis.
2.7. Statistical analysis (Supplementary Table 1)
Data were analyzed using one-way ANOVA followed by Tukey’s post-hoc test. Analyses were performed using GraphPad Prism 6 (GraphPad Scientific, San Diego, CA). All data are expressed as means ± SEMs.
3. Results
3.1. High concentrations of MPP+ decrease cell viability
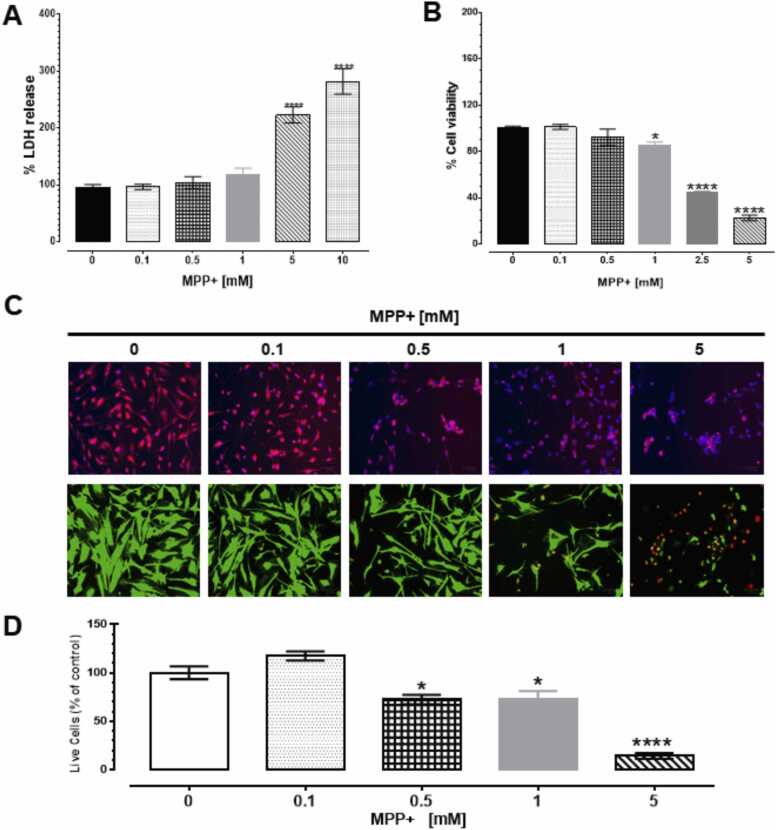
The release of LDH was quantified in the culture media after a 24-h exposure to MPP+. MPP+ at 0.1, 0.5 and 1 mM did not modify LDH release; however, 5 and 10 mM significantly increased LDH release by 128% and 186%, respectively, compared to control [F (5,30) = 38.31, p < 0.001, Fig. 1A, Supplementary table 1 (ST1)].
Fig. 1.
MPP+ induced cytotoxicity in HDNPCs. Differentiated HDNPCs were exposed to MPP+ [0–10 mM] for 24 h. After treatment LDH release (A), metabolic activity (B), mitochondrial function (C) and the number of live/dead cells (D) were analyzed. Each value represents the mean ± SEM of three independent experiments. * p < 0.05 and **** p < 0.0001 vs. control. In the micrographs of differentiated HDNPCs exposed to MPP+, mitochondria were stained in red for MitoTracker panel. FITC-green represents live cells and TRITC-red represents dead cells in Live/Dead panel. Blue stain represents nuclei in both panels.(For interpretation of the references to colour in this figure, the reader is referred to the web version of this article.)
3.2. MPP+ reduces metabolic and mitochondrial activity
At low exposure levels of MPP+, (0.1 and 0.5 mM), cell viability was unchanged compared to control. However, 1, 2.5 and 5 mM of MPP+, significantly reduced cell viability to 15%, 55% and 77%, respectively, compared to control [F (5, 21) = 245.1, p < 0.0001, Fig. 1B, ST1]. Likewise, a MitoTracker® Orange assay that measures oxidation activity of mitochondrial respiration suggested that MPP+ affected mitochondrial function [Fig. 1C, ST1] while significantly reducing the number of live cells [F (4,50) = 34.87, p < 0.0001, Fig. 1D, ST1] by 34%, 33% and 88% compared to control in response to 0.5, 1 and 5 mM of MPP+, respectively.
3.3. MPP+ increased levels of autophagy markers and decreased levels of TH
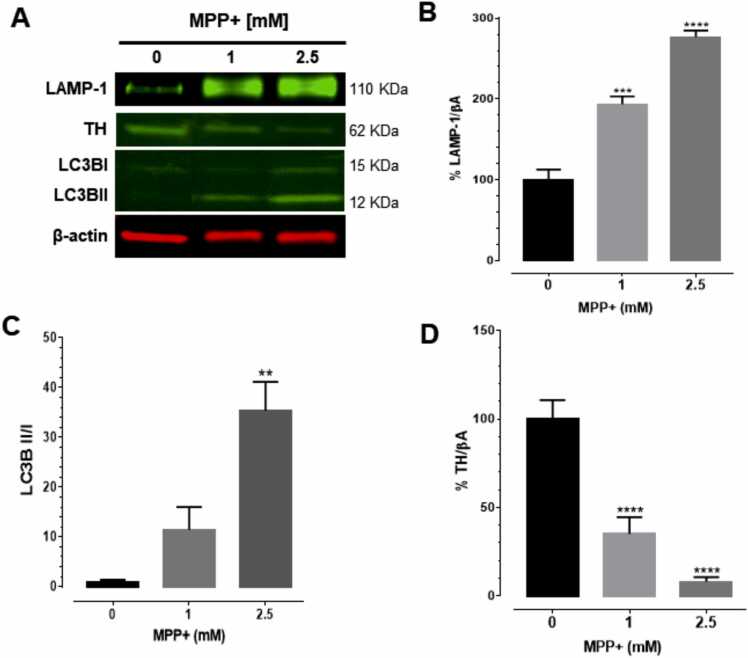
To evaluate the influence of MPP+ on autophagy response, the expression of LC3BI, LC3BII and LAMP-1 were analyzed by WB. MPP+ significantly increased LAMP-1 levels at 1 and 2.5 mM [93% and 176% compared to control] after a 24-h exposure [F (2,6) = 72.04, p < 0.0001, Fig. 2A and B, ST1]. A significant increase of 34.37-fold was observed in the ratio of LC3BII/LC3BI after exposure to MPP+ at 2.5 mM [F (2,6) = 17.16, p = 0.0033, Fig. 2C, ST1] compared to control group.
Fig. 2.
MPP+ increased levels of autophagy markers and decreased levels of tyrosine hydroxylase (TH). Differentiated HDNPCs were exposed to 1 and 2.5 mM MPP+ for 24 h. LAMP-1, TH, LC3BI and LC3BII expression were evaluated using western blot. The presence of LAMP-1, TH, LC3BI and LC3BII were detected at 110, 62, 15 and 12 kDa, respectively. Protein expression was normalized to β-actin protein. Densitometric analyses were performed using the Li-COR Image Studio software. Each value represents the mean ± SEM of three independent experiments. ** p < 0.01, *** p < 0.001 and **** p < 0.0001 vs control.
To evaluate the influence of MPP+ upon dopamine (DA) synthesis, TH expression levels were evaluated by WB. MPP+ induced a significant decrease in TH expression after 24 h of exposure. A reduction of − 65% and − 92% in response to MPP+ at 1 and 2.5 mM, respectively, compared to control group was observed [F (2,6) = 32.42, p = 0.006, Fig. 2D, ST1]. This observation suggests that MPP+ may cause a reduction of DA synthesis.
3.4. MPP+ induced nuclear localization and aggregation of α-SYN, as well as decreased Parkin expression
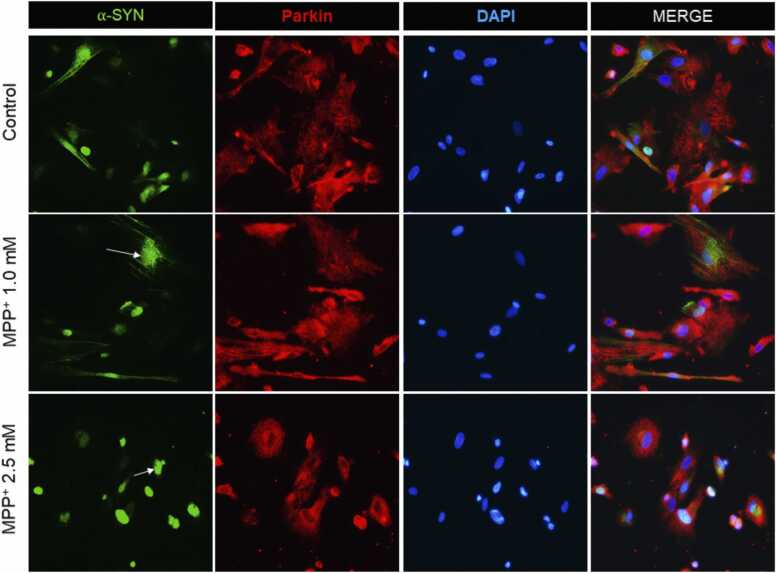
We evaluated parkin and α-SYN expression and localization using fluorescent microscopy. MPP+ promoted co-localization of parkin-α-SYN. Also, MPP+ decreased the labeling of Parkin [Fig. 3] suggesting that MPP+ may be promoting α-SYN accumulation through the inhibition of parkin. We evaluated the effects of MPP+ at 1 mM and 2.5 mM, 24 h postexposure on α-SYN and Parkin by IF. The qualitative analysis indicated that MPP+ produced nuclear localization and aggregation of α-SYN (Supplementary Fig. 3a). Additionally, a decrease in Parkin labeling was observed [Fig. 3].
Fig. 3.
MPP+ induced nuclear-located α-SYN aggregation and decreased Parkin labeling. Micrographs of differentiated HDNPCs exposed to MPP+ [1 and 2.5 mM] for 24 h. Controls show normal cells with few α-SYN aggregates. The positive control, MPP+ [1 and 2.5 mM], induced aggregation α-SYN [white arrows], and decreased Parkin labeling. Axonal retraction and a decreased number of cells were observed. Parkin was stained with Alexa Fluor [red] and FITC was used to track α-SYN [green].(For interpretation of the references to colour in this figure, the reader is referred to the web version of this article.)
4. Discussion and conclusion
PD results from the death of dopaminergic neurons in the SNc. The number of studies examining the effects of neurotoxins in human dopaminergic neurons are limited. Here, we evaluated the effects of MPP+ on HDNPCs and the role of α-SYN aggregation and autophagy as possible pathways of neuronal damage in PD.
We found that MPP+ reduced cell viability at low concentrations [starting at 0.5 mM] as evidenced by decreased metabolic and mitochondrial activity. We also found that decrease in cell viability only occurred at high concentrations [> 5 mM] of MPP+, as evidenced LDH release. This finding is in line with observation of cellular death in human embryonic stem cells (hESCs)-derived dopaminergic neurons [42] and human SH-SY5Y cells [34] in response to a 24-h exposure of 5 mM MPP+. Previous studies demonstrated that MPP+ produced mitochondrial dysfunction at 1 mM in PC12 cells [22], [13] and in SH-SY5Y cells [4], giving additional support for the use of HDNPCs to evaluate mechanistic pathways involved in MPP+ toxicity. Our results are consistent with data obtained from other MPTP-induced rodent models and MPP+-induced human cell line models, in which reduced cell viability was observed.
The pathways activated by MPP+ are not limited to mitochondrial dysfunction. Evidence suggests that autophagy may be an additional mechanism of MPTP-induced toxicity [18], [41]. Experimentally, the conversion of LC3 from LC3-I to LC3-II reflects the progression of autophagy. We found the LC3I/LC3II ratio significantly increased, suggesting MPP+ induced autophagy. A dysregulation of autophagy may result from the accumulation of abnormal proteins during neurodegenerative disorders including PD [26]. An increase in LC3II was suggested in an in vitro MPP+ model of PD, indicating an autophagy-mediated death mechanism in the dopaminergic system [14]. Furthermore, lysosomal LAMP-1 is a major protein component of the lysosomal membrane required for fusion of lysosomes with phagosomes and reflects the completion of autophagy. We found that only high concentrations of MPP+ increased the conversion of LC3I to LC3II and the expression of LAMP-1, suggesting that autophagic degradation may play an alternative or simultaneous pathway activated in the toxicity of this metabolite on DA neurons. Supporting this hypothesis, other reports found that MPP+ increased autophagic vacuoles and recruitment of LC3II at 2.5 mM (LD50) in SH-SY5Y cells [45]. Likewise, there is an increase in conversion of LC3I to LC3II in response to 0.2 mM MPP+ in MN9D cells, a mouse dopaminergic cell line [23]. These data suggest that MPP+ and HDNPCs can be used to model multiple aspects of PD in a concentration-dependent manner. This agrees with the results presented here as 2.5 mM MPP+, the maximum utilized in the autophagy evaluation, induced the higher ratio of LC3II/LC3I.
Autophagy may regulate the clearance of α-SYN, aggrieving DA neurons to develop the pathogenesis of PD [38]. An upregulation of α-SYN after exposure to 0.5 mM MPP+ in SH-SY5Y cells transfected with UCA1 that over-overexpress α-SYN has been reported followed by oligomer formation, decreased cell viability and increased apoptosis [24]. Similarly, 5 mM MPP+ in SH-SY5Y cells also resulted in upregulation of α-SYN [16]. We found that MPP+ induced the accumulation and aggregation of α-SYN in the nucleus. Additionally, the distribution of α-SYN is different, with higher positive labeling in the soma rather than in the axons (at 2.5 mM MPP+), suggesting potential dysfunction in the autophagy response and axonal degeneration. Additional evaluation is needed to confirm this finding. We detected the presence of Parkin in dopaminergic neurons and its co-localization with α-SYN. MPP+ (1.0 and 2.5 mM) increased the aggregation and accumulation of α-SYN in the cells while decreasing the Parkin signal after a 24-h exposure. This suggests that MPP+ may stimulate α-SYN accumulation and aggregation through the inhibition of Parkin, a possible path to the development of Lewy bodies. It is unclear if α-SYN aggregation correlates with PD pathology, and if this aggregation precedes the loss of dopaminergic neurons [19]. However, a growing body of evidence suggests this is likely to be a key event in PD-related neurodegeneration [33].
DA release has been reported to increase in a concentration-dependent (0.001–1 mM) manner immediately (1–3 min) after MPP+ exposure in striatal synaptosomes [6]. Also, in MES23.5 cells, 100 μM MPP+ induced reduction of TH and was associated with the downregulation of Bcl-2, with cellular damage observed starting at 50 μM, in a concentration-dependent manner (10–400 μM) [40]. Likewise, abnormal dopamine metabolism, may also promote misfolded protein conformations and neurodegeneration [28]. Here, we evaluated a phenotypic marker of dopaminergic neuron loss using TH, the rate-limiting enzyme in catecholamine synthesis. We found that a 24-h exposure of 1.0 and 2.5 mM MPP+ decreased TH expression, suggesting a potential dysfunction of DA synthesis.
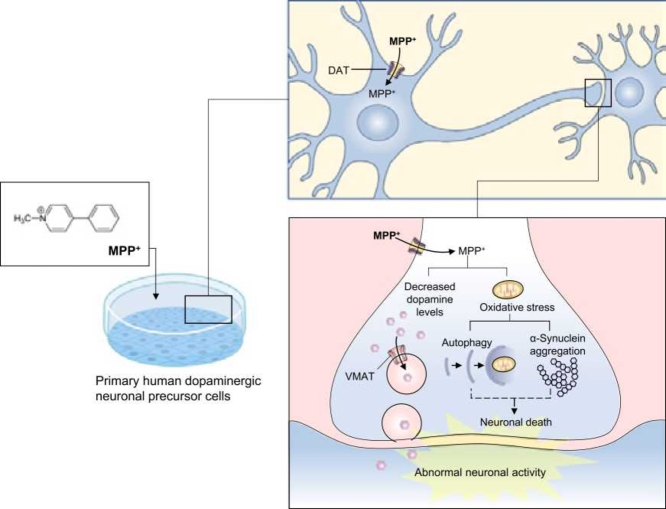
In these initial studies using HDNPCs, MPP+ led to an altered energy metabolism. We also found evidence of an additional role for autophagy, aggregation, and accumulation of α-SYN as a mechanism of possible neurodegeneration. This model, being less aggressive, may prove more translationally relevant to evaluate progressive mechanisms of dopaminergic neurodegeneration during PD [Fig. 4]. Nevertheless, additional studies are needed to clarify the molecular pathways at lower MPP+ concentrations. The response of these cells to MPP+ exposure may serve as a valuable model for evaluating potential PD biomarkers and therapeutic agents targeting autophagy.
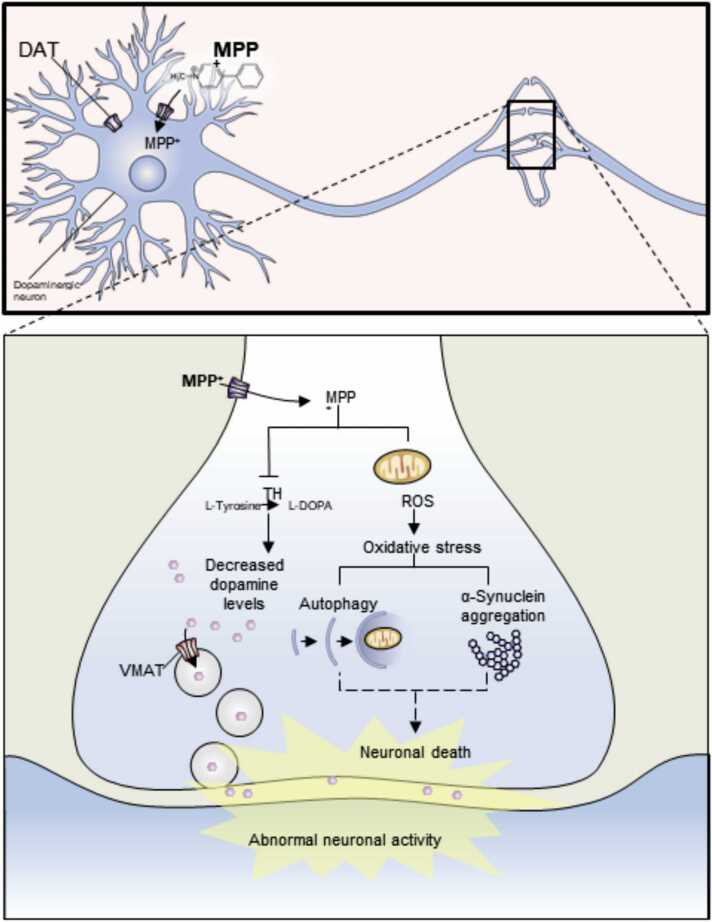
Fig. 4.
Proposed mechanism of action for MPP+ cytotoxicity in HDNPCs. HDNPCs uptake the MPP+ through dopamine transporter (DAT). Once inside the cell, MPP+ may follow two routes: (1) interact/inhibit the activity or expression of TH, decreasing the levels of DA; or (2) concentrate in the mitochondria, potentially promoting the development of oxidative stress by reactive oxygen species (ROS) generation, which in turn could activate the autophagy response and protein aggregation leading to neuronal death. Illustration was done using the biomedical PowerPoint toolkit, biology bundle, Motifolio software (Motifolio Inc. Ellicott City, MD).
5. Disclaimer
This reflects the views of the author(s) and does not necessarily reflect those of the U.S. Food and Drug Administration. Any mention of commercial products is for clarification only and is not intended as approval, endorsement, or recommendation.
CRediT authorship contribution statement
Elvis Cuevas: Writing – original draft, Conceptualization, Data curation. Aida Guzman: Experiment design and execution. Susan M. Burks: Writing – review & editing. Alejandro Ramirez-Lee: Writing – review & editing. Syed F. Ali: Writing – review & editing. Syed Z. Imam: Funding acquisition, Conceptualization, Writing – review & editing, Data Analysis, interpretation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by FDA/NCTR protocol E07616.01, and postdoctoral fellowships from the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the FDA (to A. Guzman-Lopez, A. Ramirez-Lee).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.03.047.
Appendix A. Supplementary material
Supplementary material
.
(ST1). Summary of the data. Data were analyzed using One-way ANOVA with post Tukey’s multiple comparisons test. Each value represents the mean ± SEM of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 vs control.
.
References
- 1.Abeliovich A., Flint Beal M. Parkinsonism genes: Culprits and clues. J. Neurochem. 2006;99:1062–1072. doi: 10.1111/j.1471-4159.2006.04102.x. [DOI] [PubMed] [Google Scholar]
- 2.Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 3.Chan F.K., Moriwaki K., De Rosa M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013;979:65–70. doi: 10.1007/978-1-62703-290-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y.F., Zhu G.Q., Wang M., Cheng H., Zhou A., Wang N., Fang N., Wang X.C., Xiao X.Q., Chen Z.W., Li Q.L. Involvement of ubiquitin proteasome system in protective mechanisms of Puerarin to MPP(+)-elicited apoptosis. Neurosci. Res. 2009;63:52–58. doi: 10.1016/j.neures.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Choi P., Golts N., Snyder H., Chong M., Petrucelli L., Hardy J., Sparkman D., Cochran E., Lee J.M., Wolozin B. Co-association of parkin and alpha-synuclein. Neuroreport. 2001;12:2839–2843. doi: 10.1097/00001756-200109170-00017. [DOI] [PubMed] [Google Scholar]
- 6.Clarke P.B., Reuben M. Inhibition by dizocilpine (MK-801) of striatal dopamine release induced by MPTP and MPP+: Possible action at the dopamine transporter. Br. J. Pharm. 1995;114:315–322. doi: 10.1111/j.1476-5381.1995.tb13229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway K.A., Harper J.D., Lansbury P.T. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 8.Cookson M.R. The biochemistry of Parkinson’s disease. Annu Rev. Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 9.Dagda R.K., Chu C.T. Mitochondrial quality control: insights on how Parkinson’s disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. J. Bioenerg. Biomembr. 2009;41:473–479. doi: 10.1007/s10863-009-9255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dauer W., Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 11.Friedman L.G., Lachenmayer M.L., Wang J., He L., Poulose S.M., Komatsu M., Holstein G.R., Yue Z. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J. Neurosci. 2012;32:7585–7593. doi: 10.1523/JNEUROSCI.5809-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa E., Takeshige K., Oishi T., Murai Y., Minakami S. 1-Methyl-4-Phenylpyridinium (Mpp+) induces nadh-dependent superoxide formation and enhances nadh-dependent lipid-peroxidation in bovine heart submitochondrial particles. Biochem. Biophys. Res. Commun. 1990;170:1049–1055. doi: 10.1016/0006-291x(90)90498-c. [DOI] [PubMed] [Google Scholar]
- 13.Huang J.Z., Chen Y.Z., Su M., Zheng H.F., Yang Y.P., Chen J., Liu C.F. dl-3-n-Butylphthalide prevents oxidative damage and reduces mitochondrial dysfunction in an MPP(+)-induced cellular model of Parkinson’s disease. Neurosci. Lett. 2010;475:89–94. doi: 10.1016/j.neulet.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Hung K.C., Huang H.J., Lin M.W., Lei Y.P., Lin A.M.Y. Roles of autophagy in MPP+-induced neurotoxicity in vivo: The involvement of mitochondria and alpha-synuclein aggregation. Plos One. 2014;9 doi: 10.1371/journal.pone.0091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesko H., Lenkiewicz A.M., Wilkaniec A., Adamczyk A. The interplay between parkin and alpha-synuclein; possible implications for the pathogenesis of Parkinson’s disease. Acta Neurobiol. Exp. (Wars. ) 2019;79:276–289. [PubMed] [Google Scholar]
- 16.Kalivendi S.V., Cunningham S., Kotamraju S., Joseph J., Hillard C.J., Kalyanaraman B. Alpha-synuclein up-regulation and aggregation during MPP+-induced apoptosis in neuroblastoma cells: intermediacy of transferrin receptor iron and hydrogen peroxide. J. Biol. Chem. 2004;279:15240–15247. doi: 10.1074/jbc.M312497200. [DOI] [PubMed] [Google Scholar]
- 17.Khandelwal P.J., Moussa C. The relationship between Parkin and protein aggregation in neurodegenerative diseases. Front. Psychiatry. 2010;1:15. doi: 10.3389/fpsyt.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo J.H., Cho J.Y. Treadmill exercise attenuates alpha-synuclein levels by promoting mitochondrial function and autophagy possibly via SIRT1 in the chronic MPTP/P-induced mouse model of Parkinson’s disease. Neurotox. Res. 2017 doi: 10.1007/s12640-017-9770-5. [DOI] [PubMed] [Google Scholar]
- 19.Kramer M.L., Schulz-Schaeffer W.J. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J. Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P., Nagarajan A., Uchil P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018;2018 doi: 10.1101/pdb.prot095497. [DOI] [PubMed] [Google Scholar]
- 21.Lantz S.M., Cuevas E., Robinson B.L., Paule M.G., Ali S.F., Imam S.Z. The role of harmane and norharmane in in vitro dopaminergic function. J. Drug Alcohol Res. 2015;4:1–8. [Google Scholar]
- 22.Lee E.S., Chen H., Charlton C.G., Soliman K.F. The role of phospholipid methylation in 1-methyl-4-phenyl-pyridinium ion (MPP+)-induced neurotoxicity in PC12 cells. Neurotoxicology. 2005;26:945–957. doi: 10.1016/j.neuro.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Li R., Chen J. Salidroside protects dopaminergic neurons by enhancing pink1/parkin-mediated mitophagy. Oxid. Med Cell Longev. 2019;2019 doi: 10.1155/2019/9341018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu M., Sun W.L., Shen J., Wei M., Chen B., Qi Y.J., Xu C.S. LncRNA-UCA1 promotes PD development by upregulating SNCA. Eur. Rev. Med Pharm. Sci. 2018;22:7908–7915. doi: 10.26355/eurrev_201811_16417. [DOI] [PubMed] [Google Scholar]
- 25.Lundius E.G., Stroth N., Vukojevic V., Terenius L., Svenningsson P. Functional GPR37 trafficking protects against toxicity induced by 6-OHDA, MPP+ or rotenone in a catecholaminergic cell line. J. Neurochem. 2013;124:410–417. doi: 10.1111/jnc.12081. [DOI] [PubMed] [Google Scholar]
- 26.Lynch-Day M.A., Mao K., Wang K., Zhao M., Klionsky D.J. The role of autophagy in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer R.A., Kindt M.V., Heikkila R.E. Prevention of the nigrostriatal toxicity of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine by inhibitors of 3,4-Dihydroxyphenylethylamine transport. J. Neurochem. 1986;47:1073–1079. doi: 10.1111/j.1471-4159.1986.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 28.Mor D.E., Daniels M.J., Ischiropoulos H. The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Mov. Disord. 2019;34:167–179. doi: 10.1002/mds.27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicklas W.J., Vyas I., Heikkila R.E. Inhibition of nadh-linked oxidation in brain mitochondria by 1-Methyl-4-Phenyl-Pyridine, a metabolite of the neurotoxin, 1-Methyl-4-Phenyl-1,2,5,6-Tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 30.Radad K.S., Al-Shraim M.M., Moustafa M.F., Rausch W.D. Neuroprotective role of thymoquinone against 1-methyl-4-phenylpyridinium-induced dopaminergic cell death in primary mesencephalic cell culture. Neurosci. (Riyadh) 2015;20:10–16. [PMC free article] [PubMed] [Google Scholar]
- 31.Roehm N.W., Rodgers G.H., Hatfield S.M., Glasebrook A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 32.Schommer J., Marwarha G., Schommer T., Flick T., Lund J., Ghribi O. 27-Hydroxycholesterol increases alpha-synuclein protein levels through proteasomal inhibition in human dopaminergic neurons. BMC Neurosci. 2018;19:17. doi: 10.1186/s12868-018-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Schulz-Schaeffer W.J. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Q., Geng Y., Li Y., Wang L., Qin J. Long noncoding RNA NORAD regulates MPP+-induced Parkinson’s disease model cells. J. Chem. Neuroanat. 2019;101 doi: 10.1016/j.jchemneu.2019.101668. [DOI] [PubMed] [Google Scholar]
- 35.Spillantini M.G., Crowther R.A., Jakes R., Cairns N.J., Lantos P.L., Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 36.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tysnes O.B., Storstein A. Epidemiology of Parkinson’s disease. J. Neural Transm. (Vienna) 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 38.Vogiatzi T., Xilouri M., Vekrellis K., Stefanis L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J. Biol. Chem. 2008;283:23542–23556. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkaniec A., Lenkiewicz A.M., Czapski G.A., Jesko H.M., Hilgier W., Brodzik R., Gassowska-Dobrowolska M., Culmsee C., Adamczyk A. Extracellular alpha-synuclein oligomers induce parkin S-Nitrosylation: Relevance to sporadic Parkinson’s disease etiopathology. Mol. Neurobiol. 2019;56:125–140. doi: 10.1007/s12035-018-1082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu A.L., Jiang M.C., Chen X.H., Chen W.F. Icariin protects against MPP(+)-induced neurotoxicity in MES23.5 cells. Sheng Li Xue Bao. 2016;68:585–591. [PubMed] [Google Scholar]
- 41.Xu Y.D., Cui C., Sun M.F., Zhu Y.L., Chu M., Shi Y.W., Lin S.L., Yang X.S., Shen Y.Q. Neuroprotective effects of loganin on MPTP-induced Parkinson’s disease mice: Neurochemistry, glial reaction and autophagy studies. J. Cell Biochem. 2017 doi: 10.1002/jcb.26010. [DOI] [PubMed] [Google Scholar]
- 42.Zeng X., Chen J., Deng X., Liu Y., Rao M.S., Cadet J.L., Freed W.J. An in vitro model of human dopaminergic neurons derived from embryonic stem cells: MPP+ toxicity and GDNF neuroprotection. Neuropsychopharmacology. 2006;31:2708–2715. doi: 10.1038/sj.npp.1301125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng X., Pan Z.G., Shao Y., Wu X.N., Liu S.X., Li N.L., Wang W.M. SKF-96365 attenuates toxin-induced neuronal injury through opposite regulatory effects on Homer1a and Homer1b/c in cultured rat mesencephalic cells. Neurosci. Lett. 2013;543:183–188. doi: 10.1016/j.neulet.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Wu J.Y., Weng L.H., Li X.X., Yu L.J., Xu Y. Valproic acid protects against MPP+-mediated neurotoxicity in SH-SY5Y cells through autophagy. Neurosci. Lett. 2017;638:60–68. doi: 10.1016/j.neulet.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J.H., Horbinski C., Guo F., Watkins S., Uchiyama Y., Chu C.T. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am. J. Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
(ST1). Summary of the data. Data were analyzed using One-way ANOVA with post Tukey’s multiple comparisons test. Each value represents the mean ± SEM of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 vs control.