Abstract
The aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor thought to mediate a number of physiological roles in the body, is becoming a target of interest for the development of new therapeutics. However, previous research has demonstrated that the downstream effects of AhR ligands cannot be predicted based simply on whether a ligand acts as an agonist or antagonist and the persistence of AhR signaling is thought to be a key determining feature. The current study investigated the AhR activity of four halogenated indoles isolated from the New Zealand red alga, Rhodophyllis membranacea: 4,7-dibromo-2,3-dichloroindole (4DBDCI), 7-bromo-2,3-dichloro-6-iodoindole (BDCII), 6,7-dibromo-2,3-dichloroindole (6DBDCI) and 2,6,7-tribromo-3-chloroindole (TBCI). Their ability to activate AhR signaling, measured as CYP1A1 activity via the ethoxyresorufin O-deethylase (EROD) assay, was determined in human HepG2, mouse Hepa1c1c7 and rat H4IIE liver cancer cells. All four compounds induced CYP1A1 activity in HepG2 cells, suggesting they all acted as AhR agonizts. 4DBDCI was particularly efficacious, inducing an 11-fold increase. Hepa1c1c7 and H4IIE cells, however, were generally less responsive to the halogenated indoles. All four compounds were persistent AhR agonizts, inducing peak CYP1A1 activity after 72 h. Moreover, the 2,3,6,7-substituted BDCII, 6DBDCI and TBCI, but not 4DBDCI, competed with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) for AhR binding as observed by the inhibition of TCDD-induced CYP1A1 activity. Overall, the current study has characterized four previously untested AhR ligands, highlighting differences in species sensitivity and persistence of signaling to provide a framework for their potential future use.
Abbreviations: TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TBCI, 2,6,7-tribromo-3-chloroindole; 4DBDCI, 4,7-dibromo-2,3-dichloroindole; 6DBDCI, 6,7-dibromo-2,3-dichloroindole; FICZ, 6-formylindolo[3,2b]carbazole; BDCII, 7-bromo-2,3-dichloro-6-iodoindole; AhR, aryl hydrocarbon receptor; ARNT, AhR nuclear translocator protein; BCA, bicinchoninic acid; CYP1A1, cytochrome P4501A1; EROD, ethoxyresorufin-O-deethylase; SRB, sulforhodamine B; XRE, xenobiotic response element
Keywords: Aryl hydrocarbon receptor, Halogenated indoles, Rhodophyllis membranacea
Graphical Abstract

Highlights
-
•
Four halogenated indoles derived from algae have been found to activate the AhR.
-
•
Human, mouse and rat cell lines show different sensitivity to these AhR ligands.
-
•
EROD assays were used to highlight persistent vs transient AhR activation.
-
•
All four compounds produced persistent effects.
1. Introduction
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor of the basic helix-loop-helix-Per-ARNT-Sim family, conserved in vertebrates and expressed ubiquitously in humans [1], [2]. Though initially discovered as the mediator of polycyclic and halogenated aromatic hydrocarbon toxicity, research over the last couple of decades has demonstrated a regulatory role of the AhR in inflammatory and immune responses and cell proliferation, and its physiological significance remains to be uncovered [3], [4], [5]. Indeed, in addition to the well-studied pollutants that induce toxicity via AhR binding, an ever-increasing number of dietary-derived and endogenously-produced ligands have been reported, supporting the notion of its physiological roles [6], [7], [8].
In the canonical mechanism of AhR signaling, cytosolic ligand binding of the AhR triggers translocation of the ligand-AhR-chaperone complex into the nucleus, where the chaperone proteins, heat shock protein 90, hepatitis B virus X-associated protein and p23, dissociate to allow binding of the AhR nuclear translocator (ARNT) protein [9]. The newly-formed heterodimer then binds to specific DNA sequences, known as xenobiotic response elements (XREs), found upstream of a myriad of target genes [10], [11]. Those involved in drug metabolism, such as UDP-glucuronosyltransferase 1A6 (UGT1A6), NAD(P)H quinone oxidoreductase 1 (NQO1) and various cytochrome P450 (CYP) isoforms, are commonly associated with AhR activation [12], [13], [14], [15], [16]. In particular, CYP1A1, especially in the liver, is so tightly regulated by the AhR it often serves as the basis of environmental contaminant detection [17], [18], [19].
Outside of xenobiotic-metabolizing enzymes, however, the link between AhR activation and gene expression is unclear, with different ligands inducing different downstream transcriptional responses. A study comparing two potent AhR agonizts, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and 6-formylindolo[3,2-b]carbazole (FICZ), found that TCDD and FICZ had opposing effects on Th1 and Tfh cell frequency in influenza A virus-infected mice [20]. Similarly, Wheeler et al. [21] found that TCDD modulated immune responses to influenza A virus infection, while FICZ had no effect, which was attributed to the persistence of AhR stimulation and cell-specific responses. Another study in which total AhR activation over time was matched between ligands confirmed the idea that the downstream immune responses were dependent on both the amount and duration of AhR stimulation [22].
The involvement of the AhR in numerous physiological processes makes it an interesting therapeutic target. However, the categorization of AhR ligands into agonist or antagonist is clearly insufficient to describe the effect these ligands will have on downstream pathways and the persistence of AhR stimulation needs to be considered. In this study, the AhR activity of four halogenated marine indoles (Fig. 1) isolated from the endemic New Zealand red alga, Rhodophyllis membranacea [23], was assessed. The aim was not just to identify whether these compounds would act as AhR agonizts or antagonists, but to also identify any differences in persistence of AhR stimulation and competition with TCDD for AhR binding that could provide a more useful profile of these ligands for future experiments.
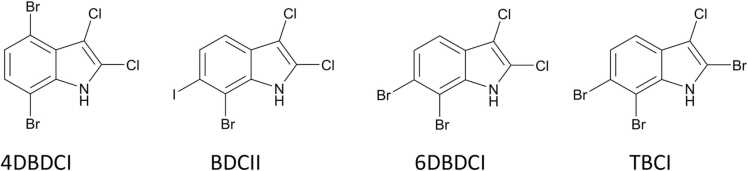
Fig. 1.
Structures of halogenated indoles. 4,7-dibromo-2,3-dichloroindole (4DBDCI), 7-bromo-2,3-dichloro-6-iodoindole (BDCII), 6,7-dibromo-2,3-dichloroindole (6DBDCI), and 2,6,7-tribromo-3-chloroindole (TBCI).
2. Materials and methods
2.1. Materials
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD, 50 μg/mL in DMSO, >99.9%) was purchased from Cambridge Isotope Laboratories (Tewksbury, MA) and GNF-351 was purchased from Santa Cruz Biotechnology (Dallas, TX). Trichloroacetic acid was purchased from Merck (Darmstadt, Germany). Resorufin ethyl ether, 3,3′-methylene-bis(4-hydroxycoumarin), sulforhodamine B and DMEM/F12 powder were purchased from Sigma (St Louis, MO). 100x L-glutamine and penicillin-streptomycin were purchased form Gibco (Dublin, Ireland) while fetal bovine serum was purchased from Cytiva Life Sciences (Marlborough, MA). HepG2, Hepa1c1c7 and H4IIE cell lines were purchased from ATCC (Manassas, VA).
2.2. Indole extraction
Indoles, 4,7-dibromo-2,3-dichloroindole (4DBDCI), 7-bromo-2,3-dichloro-6-iodoindole (BDCII), 6,7-dibromo-2,3-dichloroindole (6DBDCI) and 2,6,7-tribromo-3-chloroindole (TBCI) were extracted and purified from Rhodophyllis membranacea as previously described [23].
2.3. Cell culture
Human hepatocellular carcinoma (HepG2) and rat hepatoma (H4IIE) cells were grown in DMEM/F12 supplemented with 5% fetal bovine serum, 1% penicillin streptomycin and 1% L-glutamine. Mouse hepatoma (Hepa1c1c7) cells were grown in MEMα supplemented with 5% FBS and 1% penicillin streptomycin. All cells were incubated at 37 °C, 5% CO2 and passaged at ~80% confluence.
2.4. Cell viability
HepG2, Hepa1c1c7 and H4IIE cells were plated in 96-well plates at 1 × 104, 7.5 × 103 and 7.5 × 103 cells per well, respectively. Cells were treated the following day with the specified concentration of indole or vehicle control (0.5% DMSO) and incubated for 72 h. The sulforhodamine B (SRB) assay was used to determine the effect of the individual indoles on cell viability as previously described [24]. The resulting absorbance was measured at 510 nm using the Bio-Rad Benchmark Plus microplate spectrophotometer. Absorbances were compared to a cell density standard curve and the results are expressed as a percent of the vehicle control.
2.5. CYP1A1 catalytic activity
CYP1A1 activity was determined via the ethoxyresorufin O-deethylase (EROD) assay, as previously described [25]. For assessing EROD activity after 6 or 24 h, HepG2 cells were plated in black, clear-bottom 96-well plates at 7 × 104 cells per well, while Hepa1c1c7 and H4IIE cells were plated at 5 × 104 cells per well. For EROD assays conducted over 72 h of treatment, HepG2 cells were plated at 1.5 × 104 cells per well. In all cases, cells were allowed to attach to the wells for 24 h and were then treated with the indicated concentration of indole or vehicle control and incubated at 37 °C. EROD assays were then conducted by incubating the cells with ethoxyresorufin (5 µM) and dicoumarol (5 µM) in PBS for 30 min at 37 °C. The reaction was terminated by the addition of ice-cold methanol and the resulting fluorescence was measured on the SpectraMax i3x Multi-Mode microplate reader with an excitation/emission wavelength of 550/585 nm. Fluorescence output was expressed as a function of assay duration (30 min) and protein content, as determined by the bicinchoninic acid assay (P. K. [26]).
2.6. Statistical analysis
Data were analyzed using a either a t-test, or one- or two-way ANOVA coupled with a Bonferroni post-hoc test following a significant ANOVA, as indicated in the figure legends. Statistical significance was set at p < 0.05. Data were normalized to an appropriate control, and variability in the control group was accounted for in the statistical analysis.
3. Results
3.1. Cell viability in response to halogenated indoles
To ensure that changes in enzymatic activity were not caused by cell death, the SRB assay was used to assess cell viability in response to the indoles. The results showed that indoles BDCII, 6DBDCI and TBCI were not significantly cytotoxic to any of the cell lines at concentrations up to 20 µM (Supplementary Table 1). At concentrations of 20 µM, BDCII and TBCI did decrease Hepa1c1c7 cell viability to 52% and 64% of control, respectively, but neither was statistically significant (Supplementary Table 1). 4DBDCI, however, was significantly more cytotoxic to each cell line, reducing cell viability after 72 h to 47.8 ± 6.2% (HepG2), 12.5 ± 1.3% (Hepa1c1c7) and 33.1 ± 13.5% (H4IIE) of control at 20 µM (p < 0.05, one-way ANOVA with Bonferroni post-hoc test) (Supplementary Table 1). Therefore, experiments that examined structure-activity relationships between the four indoles used the same range of concentrations at a maximum concentration of 10 µM.
3.2. Short-term induction of EROD activity
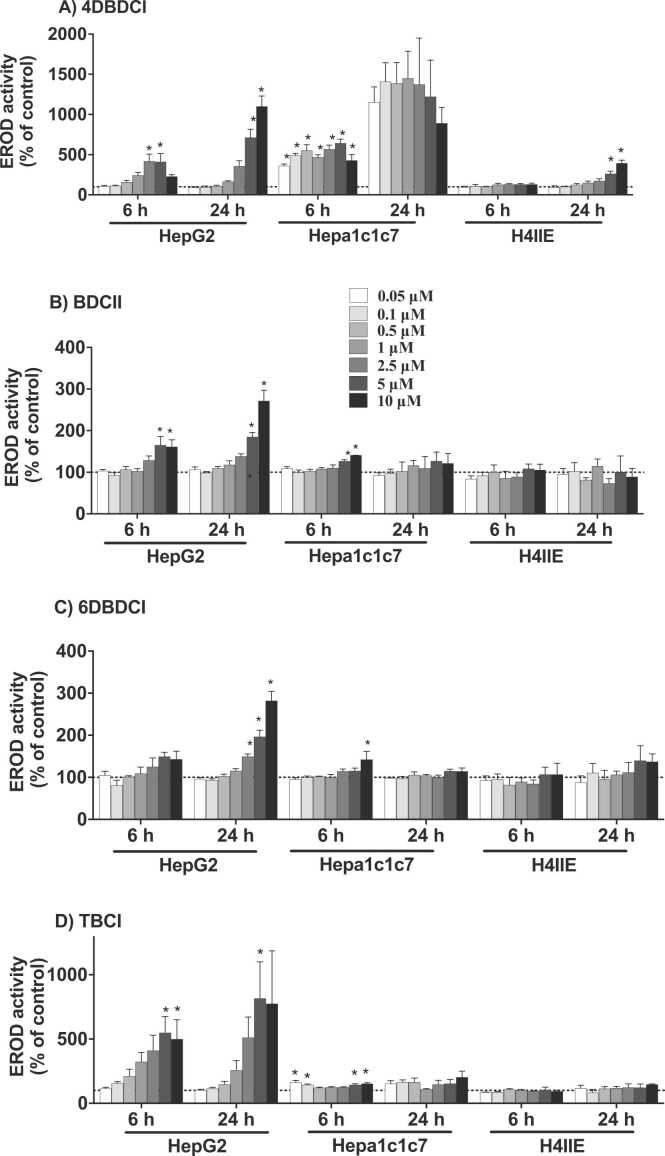
To determine whether these compounds act as AhR agonizts, their ability to induce CYP1A1 catalytic activity was determined after at 6 and 24 h. In human HepG2 cells, all four indoles caused a significant increase in EROD activity, albeit to varying extents (Figs. 2). At its peak, the most potent, 4DBDCI, increased EROD activity 11-fold relative to control. BDCII and 6DBDCI increased EROD activity 2–3-fold at 24 h, while TBCI elicited a 7-fold increase, though with considerable variability. The trends observed in HepG2 cells did not translate to either mouse hepatoma (Hepa1c1c7) or rat hepatoma (H4IIE) cells. For example, in Hepa1c1c7 cells, 4DBDCI was particularly potent with near maximal EROD induction at 0.05 μM, while BDCII, 6DBDCI and TBCI were relatively ineffective. Furthermore, H4IIE cells were the least sensitive to the halogenated indoles with only 4DBDCI increasing EROD activity ~4-fold after 24 h. Given the relative insensitivity of the mouse and rat cells to these indoles, no further investigation was conducted with these cell lines.
Fig. 2.
CYP1A1 catalytic activity induced by halogenated indoles. HepG2, Hepa1c1c7 and H4IIE cells were treated with the various compounds (0.05 – 10 µM) or vehicle control (0.5% DMSO) for 6 or 24 h. CYP1A1 activity was determined via the EROD assay and is expressed as a percentage of the vehicle control. Bars represent the mean ± SEM of three independent experiments performed in triplicate. Data for each time-point and cell line were analyzed via a one-way ANOVA coupled with the Bonferroni post-hoc test. *significantly increased relative to the vehicle control, p < 0.05.
3.3. EROD activity in competition with GNF
While the EROD assay is generally considered adequate for determining AhR activation given the tight regulation of CYP1A1 by the AhR, the possibility remains that CYP1A1 could have been induced independent of the AhR [27], [28]. Thus, to ensure the observed effects were mediated by the AhR, EROD assays were conducted in combination with the AhR antagonist, GNF-351 (0.25 µM) [29]. The results showed that GNF-351 completely abrogated the indole-induced CYP1A1 activity, suggesting that all four compounds induced CYP1A1 via AhR binding (Fig. 3).
Fig. 3.
Antagonism of CYP1A1 catalytic activity induced by halogenated indoles. HepG2 cells were treated with 10 µM of the halogenated indoles for 24 h both alone and in combination with the AhR antagonist, GNF-351 (0.25 µM). CYP1A1 activity was determined via the EROD assay and is expressed as a percentage of the vehicle control (0.5% DMSO). Bars represent the mean ± SEM of three independent experiments performed in duplicate. Data were analyzed using a one-tailed t-test. *significantly decreased compared to the respective indole-only response, p < 0.05.
3.4. Long-term induction of EROD activity
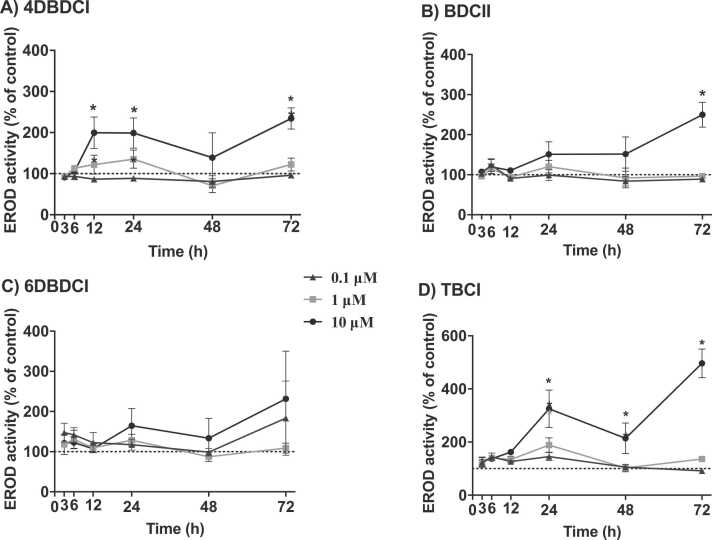
Having observed differences in short-term (6–24 h) CYP1A1 activity between the indoles, their effect on CYP1A1 activity over a 72-h period was determined in HepG2 cells. Although 1 μM or less of the halogenated indoles generally did not induce significant CYP1A1 activity at 24 h, 0.1, 1 and 10 μM of each indole was examined to see if the lower concentrations could persist and induce CYP1A1 activity. Within 12 h, the CYP1A1 activity induced by 4DBDCI (10 μM) was elevated 2-fold over control and remained elevated at 72 h (Fig. 4). Both BDCII and TBCI (10 μM) induced peak CYP1A1 activity at 72 h, reaching approximately 2.5- and 5-fold, respectively, above control. In contrast, 6DBDCI did not induce significant changes in CYP1A1 activity throughout the 72-h period, though it followed a similar trend to that observed for BDCII and TBCI (Fig. 4).
Fig. 4.
Effect of halogenated indoles on CYP1A1 activity over a time-course. HepG2 cells were treated with 0.1, 1 or 10 µM of 4DBDCI, BDCII, 6DBDCI or TBCI or vehicle control (0.5% DMSO) for 3–72 h. CYP1A1 activity was determined via the EROD assay and is expressed as a percentage of the time-matched vehicle control. Points represent the mean ± SEM of three independent experiments performed in triplicate. Data were analyzed via a two-way ANOVA followed by a Bonferroni post-hoc test. *significantly increased compared to vehicle control, p < 0.05.
3.5. EROD activity in competition with TCDD
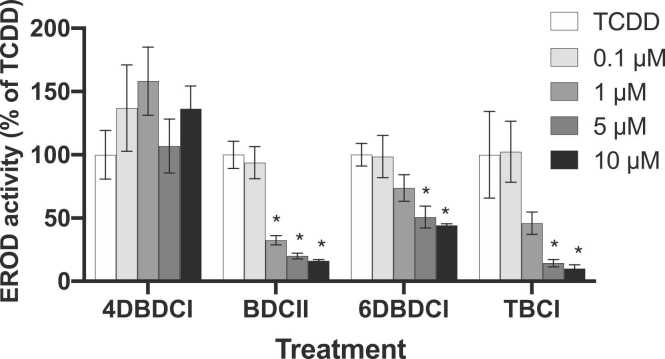
To assess the potential for these halogenated indoles to antagonize AhR binding of potent halogenated aromatic hydrocarbons, EROD assays were conducted in combination with TCDD (1 nM). The results showed that at concentrations of 1 µM BDCII, 6DBDCI and TBCI began to antagonize the TCDD-induced response (Fig. 5). At the highest concentration (10 µM) each compound effectively blocked TCDD binding to the AhR, as evidenced by the reduced EROD activity following co-treatment with the compounds and TCDD. 4DBDCI, however, was unable to antagonize the action of TCDD and appeared to increase TCDD-induced EROD activity, though this was not statistically significant.
Fig. 5.
Antagonism of TCDD-induced CYP1A1 activity by halogenated indoles. HepG2 cells were treated with TCDD (1 nM) ± 4DBDCI, BDCII, 6DBDCI or TBCI (0.1–10 µM) for 24 h. CYP1A1 activity was determined via the EROD assay and results are expressed as a percentage of the TCDD-only response. Bars represent the mean ± SEM of three independent experiments performed in duplicate. Results for each indole were analyzed via one-way ANOVA followed by the Bonferroni post-hoc test. *significantly decreased from TCDD, p < 0.05.
4. Discussion
The current study has identified four seaweed-derived indoles as AhR ligands, with some key differences in their AhR activity. The EROD activity induced by each compound in human HepG2 cells was abrogated when tested in combination with the AhR antagonist, GNF-351, confirming the AhR-dependency of the response. Moreover, though their potencies varied, these ligands appeared to act persistently, inducing peak (2–5-fold increase over control) EROD activity at 72 h. This is in contrast to the findings of DeGroot et al. [30], who investigated eight marine-derived halogenated indoles and found them to act quite transiently, with greater CYP1A1 mRNA expressed at 6 h compared to 24 h. The distinction between persistent and transient AhR ligands is considered an important determinant of the overall downstream response [31] and transient AhR ligands are thought to have a lower risk of toxicity. Although these indoles were tested for cytotoxicity in liver cell lines, potential toxicity towards other organs is an important consideration. The type and pattern of halogen substitution on an indole core likely determines the persistent or transient nature of these compounds and further investigation into this could be useful in the rational design of AhR ligands. For example, BDCII, 6DBDCI and TBCI all follow the pattern of halogen substitution at the C-2, 3, 6 and 7 positions, differing only by the type of halogens substituted and, as such, had very similar responses across all experiments in this study. Notably these three ligands were effective at inhibiting TCDD-induced EROD activity. This suggests that they can compete with TCDD for AhR binding, effectively antagonizing the effect of TCDD, while still exhibiting partial agonism. Therapeutically, this could be explored further to reduce the body burden of dioxins and dioxin-like compounds. Moreover, this is a therapeutic use in which a slightly persistent effect (to match that induced by dioxins) may be more beneficial than a transient one.
An interesting comparison can be made between 4DBDCI and 6DBDCI, differing only by the position of a single bromine (C-4 vs. C-6). The EROD activity induced by 4BDDCI appeared to be relatively stable over the course of 72 h, while that induced by 6DBDCI seemed to increase over time. A particularly interesting finding was that, unlike the other indoles which reduced TCDD-induced EROD activity, 4DBDCI appeared to increase the effect of TCDD. It is fair to assume that the mechanism through which 4DBDCI induces CYP1A1 activity is mediated by the AhR, given that GNF-351 antagonized CYP1A1 induction by 4DBDCI. This leaves two possible conclusions: 1) 4DBDCI exhibits weak affinity for the AhR that cannot outcompete TCDD, but still maintains reasonable efficacy and acts relatively persistently, or 2) 4DBDCI and TCDD are capable of binding simultaneously to the AhR to potentiate the response. Although the average EROD activities obtained for the combination treatment of TCDD and 4DBDCI were greater than that observed for TCDD alone, the large variability in the data makes it difficult to draw conclusions regarding the significance of this effect.
Previous studies have also reported synergistic AhR activity from TCDD and other phytochemicals. Van der Heiden et al. [32] found that genistein concentration-dependently increased the effect of TCDD on the reporter luciferase response in stably transfected H4IIE, HepG2 and T47D cells. Jin et al. [33] also found that quercetin (10 μM) and TCDD (10 nM) together increased CYP1A1 mRNA more than each individual compound alone in Caco2 cells. Another study investigating a range of methyl- and methoxy-indoles identified 4-methylindole and 7-methoxyindole as two of the most efficacious AhR ligands in human cells, both of which slightly increased CYP1A1 mRNA in combination with TCDD [34]. Interestingly, a synergistic response was obtained by combination treatment of both ligands to AZ-AHR (stable HepG2 luciferase reporter) cells, compared to each indole alone, but the response to an indole combining both groups, 7-methoxy-4-methylindole, was reduced ~10-fold compared to each individual compound. A more thorough investigation into the binding dynamics of AhR ligands, particularly those possessing C-4 and C-7 substitution, both alone and in combination with TCDD, is clearly necessary to determine the specific mechanism behind these instances of enhanced CYP1A1 induction.
Importantly, EROD induction in response to these seaweed-derived indoles varied considerably among human, mouse and rat cell lines. AhR ligands are often tested in animal models of disease, despite the growing body of literature emphasizing differences between human and other mammalian AhR [35], [36], [37]. Hepa1c1c7 cells were particularly sensitive to 4DBDCI, with 0.05 µM inducing an 11-fold increase in EROD activity after 24 h. In contrast, Hepa1c1c7 cells were relatively insensitive to the indoles BDCII, 6DBDCII and TBCI, as were the H4IIE cells. Similarly, H4IIE cells were relatively insensitive to the effects of the halogenated indoles. β-naphthoflavone, a positive control for AhR signaling, induced CYP1A1 activity 9 – 19-fold in the three cell lines (Supplementary Fig. 1), suggesting that the lack of CYP1A1 induction in response to the halogenated indoles in Hepa1c1c7 and H4IIE cells was due to varying sensitivities of the cell lines to AhR ligands. Humans are thought to have evolved an AhR with greater sensitivity towards indoles, likely due to their presence in the diet, both as phytochemicals and tryptophan metabolites [38]. Similarly, DeGroot et al. [30], found that cells from various species, including fish, mouse, rat, guinea pig and human, differed in their sensitivities to a range of brominated indoles. Future studies investigating the therapeutic effects of an AhR ligand need to consider the relative sensitivity of different species in order to improve their translatability. Furthermore, it should be noted that the effect of these indoles may differ between tissues. A range of phytochemicals examined in reporter gene assays differed in responses induced not just between species (mouse vs. human) but also tissue (human breast MCF7 cells vs. human liver HepG2 cells) [39]. The tissue-specific effect of AhR ligands has led to the concept of selective AhR modulators (SAhRMs), which have enormous therapeutic potential, but require thorough testing to best understand their effect in the human body as a whole [40], [41].
A limitation of this study is that AhR activation was only assessed via EROD activity, which can be variable between experimental runs. This could potentially be improved by expressing EROD activity values as a ratio of a known inducer, such as TCDD, or normalizing the EROD activity to metabolic activity rather than protein content. However, the emphasis of this study as an initial test of these halogenated indoles was on the qualitative findings. Further validation of these results can be carried out to probe these findings further. Additionally, the pharmacokinetics of these indoles and their direct binding to the AhR have not been determined, so whether the observed CYP1A1 induction was induced by the ligand itself or a metabolite is unconfirmed. However, given that other halogenated indoles have been reported to act as AhR agonizts [30] it would be fair to assume that the observed effects are mediated by the halogenated indoles themselves.
Overall, the four previously untested AhR ligands presented here further the understanding of the potential structure-activity relationships governing AhR ligand binding and CYP1A1 induction. Given the differences observed in TCDD-competition binding and timing of CYP1A1 induction, these ligands may differ both in their AhR binding and in their induced downstream effects and thus could serve different therapeutic purposes. There is particular interest in the use of AhR ligands to modulate immune and inflammatory diseases [42], as well as cancer [43] and metabolic diseases [44]. However, species- and tissue-specific AhR effects are common and thus careful investigation into how these ligands are likely to act in humans as a whole is still required.
Funding
This work was supported by a Victoria University of Wellington Faculty Strategic Research Grant, New Zealand (UW36) and a University of Otago Deans Bequest Grant, New Zealand (119734).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Pete Northcote for his original identification of the halogenated indoles.
Author statement
All authors agree to the revisions made in this manuscript and have read and approved the final version.
Handling Editor: Lawrence Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.05.016.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Hahn M.E. Aryl hydrocarbon receptors: diversity and evolution. Chem. Biol. Interact. 2002;141(1–2):131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Hahn M.E., Karchner S.I., Shapiro M.A., Perera S.A. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc. Natl. Acad. Sci. USA. 1997;94(25):13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao N., Whitelaw M.L. The emerging roles of AhR in physiology and immunity. Biochem. Pharmacol. 2013;86(5):561–570. doi: 10.1016/j.bcp.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Kou Z., Dai W. Aryl hydrocarbon receptor: its roles in physiology. Biochem. Pharmacol. 2021;185 doi: 10.1016/j.bcp.2021.114428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong F., Perdew G.H. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes. 2020;12(1):1859812. doi: 10.1080/19490976.2020.1859812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubbard T.D., Murray I.A., Perdew G.H. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos. 2015;43(10):1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamas B., Natividad J.M., Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11(4):1024–1038. doi: 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- 8.Manzella C., Singhal M., Alrefai W.A., Saksena S., Dudeja P.K., Gill R.K. Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci. Rep. 2018;8(1):6103. doi: 10.1038/s41598-018-24213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright Eric J., Pereira De Castro Karen, Joshi, Aditya D., Elferink Cornelis J. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr. Opin. Toxicol. 2017;2:87–92. doi: 10.1016/j.cotox.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo R., Matthews J. High-resolution genome-wide mapping of AHR and ARNT binding sites by ChIP-Seq. Toxicol. Sci. 2012;130(2):349–361. doi: 10.1093/toxsci/kfs253. [DOI] [PubMed] [Google Scholar]
- 11.Pansoy A., Ahmed S., Valen E., Sandelin A., Matthews J. 3-Methylcholanthrene induces differential recruitment of aryl hydrocarbon receptor to human promoters. Toxicol. Sci. 2010;117(1):90–100. doi: 10.1093/toxsci/kfq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bock K.W., Bock-Hennig B.S. UDP-glucuronosyltransferases (UGTs): from purification of Ah-receptor-inducible UGT1A6 to coordinate regulation of subsets of CYPs, UGTs, and ABC transporters by nuclear receptors. Drug Metab. Rev. 2010;42(1):6–13. doi: 10.3109/03602530903205492. [DOI] [PubMed] [Google Scholar]
- 13.Münzel Peter A., Schmohl Stephan, Heel H., Kälberer K., Bock-Hennig, Barbara S., Bock Karl Walter. Induction of human UDP glucuronosyltransferases (UGT1A6, UGT1A9, and UGT2B7) by t-butylhydroquinone and 2,3,7,8-tetrachlorodibenzo-p-dioxin in Caco-2 cells. Drug Metab. Dispos. 1999;27(5):569–573. [PubMed] [Google Scholar]
- 14.Ramadoss P., Marcus C., Perdew G.H. Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2005;1(1):9–21. doi: 10.1517/17425255.1.1.9. [DOI] [PubMed] [Google Scholar]
- 15.Yeager R.L., Reisman S.A., Aleksunes L.M., Klaassen C.D. Introducing the “TCDD-inducible AhR-Nrf2 gene battery”. Toxicol. Sci. 2009;111(2):238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yueh M.F., Bonzo J.A., Tukey R.H. The role of Ah receptor in induction of human UDP-glucuronosyltransferase 1A1. Methods Enzymol. 2005;400:75–91. doi: 10.1016/s0076-6879(05)00005-4. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin W.S. Phase 0 of the xenobiotic response: nuclear receptors and other transcription factors as a first step in protection from xenobiotics. Nucl. Recept. Res. 2019:6. doi: 10.32527/2019/101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godard-Codding C.A., Clark R., Fossi M.C., Marsili L., Maltese S., West A.G., Stegeman J.J. Pacific Ocean-wide profile of CYP1A1 expression, stable carbon and nitrogen isotope ratios, and organic contaminant burden in sperm whale skin biopsies. Environ. Health Perspect. 2011;119(3):337–343. doi: 10.1289/ehp.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung J.H., Yim B., Jeong S., Yoon M.S., Kim B.M., Ha S.Y., Lee Y.M. Development and evaluation of olive flounder cyp1a1-luciferase assay for effective detection of CYP1A-inducing contaminants in coastal sediments. Environ. Sci. Technol. 2020;54(23):15170–15179. doi: 10.1021/acs.est.0c06921. [DOI] [PubMed] [Google Scholar]
- 20.Boule Lisbeth A., Burke, Catherine G., Jin Guang-Bi, Lawrence B.Paige. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci. Rep. 2018;8(1):1826. doi: 10.1038/s41598-018-20197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler J.L., Martin K.C., Resseguie E., Lawrence B.P. Differential consequences of two distinct AhR ligands on innate and adaptive immune responses to influenza A virus. Toxicol. Sci. 2014;137(2):324–334. doi: 10.1093/toxsci/kft255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrlich A.K., Pennington J.M., Bisson W.H., Kolluri S.K., Kerkvliet N.I. TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol. Sci. 2018;161(2):310–320. doi: 10.1093/toxsci/kfx215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolner Victoria H., Jones Cori M., Field Jessica J., Fadzilah Nazmi H., Munkacsi Andrew B., Miller John H., Northcote Peter T. Polyhalogenated indoles from the red alga Rhodophyllis membranacea: the first isolation of bromo-chloro-iodo secondary metabolites. J. Nat. Prod. 2016;79(3):463–469. doi: 10.1021/acs.jnatprod.5b00831. [DOI] [PubMed] [Google Scholar]
- 24.Vichai V., Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006;1(3):1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 25.Schiwy A., Brinkmann M., Thiem I., Guder G., Winkens K., Eichbaum K., Hollert H. Determination of the CYP1A-inducing potential of single substances, mixtures and extracts of samples in the micro-EROD assay with H4IIE cells. Nat. Protoc. 2015;10(11):1728–1741. doi: 10.1038/nprot.2015.108. [DOI] [PubMed] [Google Scholar]
- 26.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Klenk D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 27.Guigal N., Seree E., Bourgarel-Rey V., Barra Y. Induction of CYP1A1 by serum independent of AhR pathway. Biochem. Biophys. Res. Commun. 2000;267(2):572–576. doi: 10.1006/bbrc.1999.1959. [DOI] [PubMed] [Google Scholar]
- 28.Villard P.H., Barlesi F., Armand M., Dao T.M., Pascussi J.M., Fouchier F., Seree E. CYP1A1 induction in the colon by serum: involvement of the PPARα pathway and evidence for a new specific human PPREα site. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0014629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith K.J., Murray I.A., Tanos R., Tellew J., Boitano A.E., Bisson W.H., Perdew G.H. Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. J. Pharmacol. Exp. Ther. 2011;338(1):318–327. doi: 10.1124/jpet.110.178392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeGroot D.E., Franks D.G., Higa T., Tanaka J., Hahn M.E., Denison M.S. Naturally occurring marine brominated indoles are aryl hydrocarbon receptor ligands/agonists. Chem. Res. Toxicol. 2015;28(6):1176–1185. doi: 10.1021/acs.chemrestox.5b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell K.A., Elferink C.J. Timing is everything: consequences of transient and sustained AhR activity. Biochem. Pharmacol. 2009;77(6):947–956. doi: 10.1016/j.bcp.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Heiden E., Bechoux N., Muller M., Sergent T., Schneider Y.J., Larondelle Y., Scippo M.L. Food flavonoid aryl hydrocarbon receptor-mediated agonistic/antagonistic/synergic activities in human and rat reporter gene assays. Anal. Chim. Acta. 2009;637(1–2):337–345. doi: 10.1016/j.aca.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 33.Jin U.H., Park H., Li X., Davidson L.A., Allred C., Patil B., Safe S. Structure-dependent modulation of aryl hydrocarbon receptor-mediated activities by flavonoids. Toxicol. Sci. 2018;164(1):205–217. doi: 10.1093/toxsci/kfy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stepankova M., Bartonkova I., Jiskrova E., Vrzal R., Mani S., Kortagere S., Dvorak Z. Methylindoles and methoxyindoles are agonists and antagonists of human aryl hydrocarbon receptor. Mol. Pharmacol. 2018;93(6):631–644. doi: 10.1124/mol.118.112151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flaveny C.A., Perdew G.H. Transgenic humanized AHR mouse reveals differences between human and mouse AHR ligand selectivity. Mol. Cell. Pharmacol. 2009;1(3):119–123. doi: 10.4255/mcpharmacol.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriguchi Takashi, Motohashi Hozumi, Hosoya Tomonori, Nakajima Osamu, Takahashi Satoru, Ohsako Seiichiroh, Yamamoto Masayuki. Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse. Proc. Natl. Acad. Sci. USA. 2003;100(10):5652–5657. doi: 10.1073/pnas.1037886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flaveny C.A., Murray I.A., Perdew G.H. Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicol. Sci. 2010;114(2):217–225. doi: 10.1093/toxsci/kfp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray I.A., Perdew G.H. How Ah receptor ligand specificity became important in understanding its physiological function. Int. J. Mol. Sci. 2020;21(24) doi: 10.3390/ijms21249614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S., Qin C., Safe S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ. Health Perspect. 2003;111(16):1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safe S., Jin U.H., Park H., Chapkin R.S., Jayaraman A. Aryl hydrocarbon receptor (AHR) ligands as selective AHR modulators (SAhRMs) Int. J. Mol. Sci. 2020;21(18) doi: 10.3390/ijms21186654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolciami D., Ballarotto M., Gargaro M., López-Cara L.C., Fallarino F., Macchiarulo A. Targeting Aryl hydrocarbon receptor for next-generation immunotherapies: selective modulators (SAhRMs) versus rapidly metabolized ligands (RMAhRLs) Eur. J. Med. Chem. 2020;185 doi: 10.1016/j.ejmech.2019.111842. [DOI] [PubMed] [Google Scholar]
- 42.Busbee P.B., Rouse M., Nagarkatti M., Nagarkatti P.S. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 2013;71(6):353–369. doi: 10.1111/nure.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paris A., Tardif N., Galibert M.D., Corre S. AhR and cancer: from gene profiling to targeted therapy. Int. J. Mol. Sci. 2021;22(2) doi: 10.3390/ijms22020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girer N.G., Tomlinson C.R., Elferink C.J. The aryl hydrocarbon receptor in energy balance: the road from dioxin-induced wasting syndrome to combating obesity with Ahr ligands. Int. J. Mol. Sci. 2020;22(1) doi: 10.3390/ijms22010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.