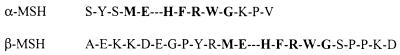Abstract
The staphylococcal exfoliative toxins (ETs) A and B (ETA and ETB) are 27-kDa exotoxins produced by certain strains of Staphylococcus aureus and are the causative agents of staphylococcal scalded-skin syndrome. The crystal structures of the ETs strongly indicate that the proteins are members of the serine protease family of enzymes, although protease activity until now has not yet been conclusively demonstrated. Here, we show that the peptide β-melanocyte-stimulating hormone (β-MSH) is cleaved by ETA and that both ETA and ETB are capable of cleaving α-MSH. Both toxins exhibit cleavage at specific glutamic acid residues in MSH peptides. Moreover, biologically inactive mutants of ETA were incapable of cleaving β-MSH.
Staphylococcal scalded-skin syndrome (SSSS) is an illness characterized most notably by the sloughing of outer epidermal layers of skin in infected patients, most of whom are neonates (12, 16). Staphylococcal exfoliative toxins (ETs) A and B (ETA and ETB) are known to be the etiologic agents of SSSS (9, 12). Both proteins are about 27 kDa in size and have approximately 40% primary sequence identity with each other (11). Prior to the solution of the three-dimensional structures of ETA (5, 18) and ETB (17), it was thought (by virtue of motifs seen in the primary sequences of the toxins) that the ETs were members of the serine protease family of proteins (2, 6, 15). The crystal structures of the toxins have shown that the ETs possess a serine protease active site and probably cleave at glutamic acid (and possibly, aspartic acid) residues, but until now, neither a substrate for the ETs nor a reliable method for assaying protease activity had been discovered. Several studies have, however, shown that the ETs possess serine esterase activity (1, 17, 18), which is an intrinsic property of serine proteases.
This observed lack of in vitro protease activity could be explained in a number of ways. First, the ETs, like many other serine proteases may require processing, by a specific agent (or agents) to become proteolytically active. Second, the protein substrate for the ETs may be very specific and not yet discovered. A combination of these two postulates may also apply.
Here, we show for the first time that ETs directly cleave melanocyte-stimulating hormones (MSH) and that this cleavage occurs after specific glutamic acid residues. Human α-MSH and β-MSH (Fig. 1) are derived from a precursor protein known as proopiomelanocortin. Proopiomelanocortin is a 31-kDa protein precursor for a variety of bioactive peptides that are known to be produced in keratinocytes, melanocytes, leukocytes, and Langerhans cells—all of which are components of the epidermis (3, 7, 8).
FIG. 1.
Amino acid sequences of α- and β-MSH. Boldfaced residues indicate a conserved region between the two peptides. Extra dashes indicate the point at which ETs cleave the MSH peptides.
Staphylococcal ETA and mutant forms of ETA were produced from RN4220 Staphylococcus aureus grown to stationary phase in pyrogen-free, dialyzable beef heart medium with 5 μg of erythromycin per ml at 37°C, and the proteins were precipitated in 4 volumes of ethanol for 48 h (4). Precipitates were resuspended in pyrogen-free water and cleared by centrifugation. Supernatants were dialyzed against deionized water for 2 days (changing water each day), and ETA and mutant forms of ETA were purified via flatbed isoelectric focusing in gradients of 3.5 to 10 and then 6 to 8. Toxin-positive fractions were collected and dialyzed against deionized water for 4 days (changing water each day). Purified toxin was quantified by use of a double immunodiffusion dilution assay (14) and lyophilized until needed. Multiple batches of toxin were used to insure reproducibility among toxin preparations. Toxin purity was assessed by reverse-phase high-pressure liquid chromatography (RHPLC) (13) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10). ETB was similarly purified from S. aureus RN4220 containing etb from strain UT0007 (grown in beef heart medium containing 5.0 μg of chloramphenicol per ml), except that during purification, ETB was resolubilized in 6.0 M urea rather than water prior to isoelectric focusing (17).
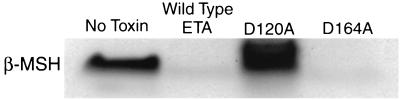
Each ET and MSH (Sigma Chemical Co., St. Louis, Mo.) were incubated in a 0.1 M NaPO4 protease assay buffer. Conditions such as temperature, pH, presence of ions, and ratio of ET to MSH were varied to optimize cleavage time. Reactions were halted by freezing and lyophilizing the proteins. Cleavage was detected by polyacrylamide gel electrophoresis in 16.5% Tris-Tricine gels (Bio-Rad, Hercules, Calif.). An example of ETA cleavage of β-MSH (50 μg ETA, 40 μg β-MSH; molar ratio, 1:8) is shown in Fig. 2. This was the highest amount of ETA relative to β-MSH tested. ETA cleaved β-MSH most efficiently at a pH of approximately 8.0, a temperature of 50°C, and at a 1 ETA:8 β-MSH molar ratio; less ETA relative to MSH resulted in less cleavage. Neither the presence of ions (a 5.0 or 50 mM concentration of Ca2+, Mg2+, Mn2+, or Zn2+) nor glycine affected cleavage of β-MSH. Under these optimized conditions, 40 μg of β-MSH was completely cleaved by 50 μg of ETA in approximately 18 h. As controls, a biologically active (i.e., epidermolytically and esterolytically active) mutant form of ETA (D164A) and a biologically inactive mutant of ETA (D120A) (17, 18) were incubated with β-MSH. After incubation, it was shown that D164A, like wild-type ETA, cleaved β-MSH, whereas the D120A mutant form of ETA did not (Fig. 2). Both of these Asp-to-Ala mutants were prepared by QuikChange site-directed mutagenesis (Stratagene, La Jolla, Calif.). Their genes were sequenced across the site of mutation to ensure that only the desired changes were made. Asp 164 is thought to be important in maintaining the structure of the active site (18). Asp 120 is part of the serine protease catalytic triad which includes Ser 195 and His 72 in addition to Asp 120. The Ser 195 residue initiates nucleophilic attack on the substrate, His 72 accepts a proton from Ser 195, and Asp 120 orients His 72 to accept the proton from Ser 195. Both wild-type ETA and the D164A mutant functioned as esterases against substrate N-t-BOC-l-glutamic acid α-phenyl ester (Sigma), whereas D120A lacked activity. Finally, wild-type ETA and the D164A mutant were able to induce skin peeling in newborn mice (12), whereas D120A also lacked that activity. Cleavage of α-MSH by either ETA or ETB could not be detected using Tris-Tricine gels due to the small size of α-MSH.
FIG. 2.
Cleavage of β-MSH by ETA as determined by polyacrylamide gel electrophoresis. Lane 1, β-MSH alone; lane 2, β-MSH treated with wild-type ETA; lane 3, β-MSH treated with a biologically inactive mutant of ETA (D120A); lane 4, β-MSH treated with a biologically active mutant of ETA (D164A). Gels were stained with Coomassie brilliant blue R250.
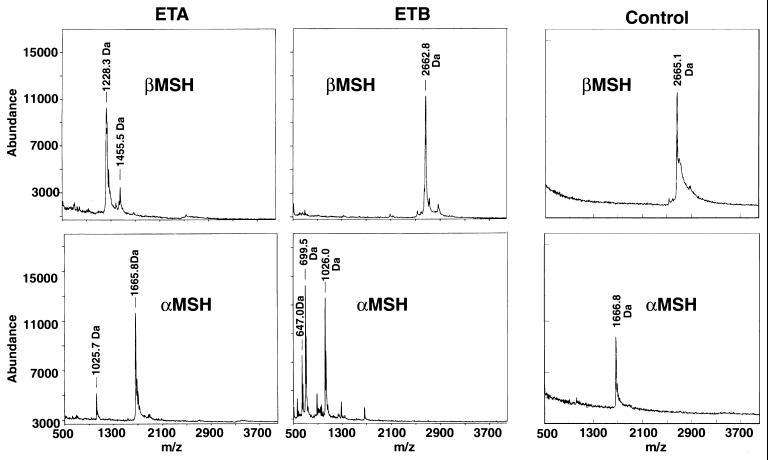
Once conditions for cleavage by ETA had been established, the ability of both of the ETs to cleave both α-MSH and β-MSH was determined by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectroscopy combined with RHPLC and sequencing of cleavage fragments. Individually, α-MSH and β-MSH (20 to 30 μg) were incubated with 20 μg of ETA or ETB in a Tris-phosphate buffer at pH = 8.0. A negative control of substrate with no toxin was incubated simultaneously. MALDI-TOF mass spectroscopy detected cleavage of β-MSH by ETA at the third glutamic acid residue (Fig. 1), based on the expected fragment sizes obtained (Fig. 3). ETB did not cleave β-MSH (Fig. 3). In addition, both ETs cleaved α-MSH after the glutamic acid at position 5, again, based on the fragment sizes obtained (Fig. 3). An additional minor peak was seen on mass spectroscopy when α-MSH was cleaved with ETB. The identity of this fragment is unknown. After RHPLC and N-terminal sequencing of the peptide fragments obtained, it was confirmed that ETA cleaved β-MSH after only the third glutamic acid residue of the peptide, not after the first or second glutamic acid residues (Fig. 1). N-terminal sequencing of peptide fragments obtained also confirmed that both ETs cleaved α-MSH after the single glutamic acid residue in the peptide. The extra peptide seen by mass spectroscopy after cleavage of α-MSH with ETB was not seen on RHPLC. Thus, as indicated above, the identity of this peak on mass spectroscopy remains unknown. These data indicate that ETs are specific for glutamic acid residues, but there was additional specificity, since ETA only cleaved after the third of the three glutamic acid residues in β-MSH. Interestingly, both peptides contain a similar motif in the region of cleavage, namely, ME-HFRWG, where “–” is the point of cleavage. This motif appears to be important for recognition of the MSH peptides by the ETs. In a database search (FASTA module, GCG version 9.0; Wisconsin Supercomputer Group), MSH peptides and their precursors from many species (not limited to mammals) share this MEHFRWG sequence. Interestingly, no other proteins or peptides were identified in the databases that share this motif. This may explain the exquisite sensitivity of the skin to ETs and why it has been so difficult to find a protein substrate for the toxins.
FIG. 3.
MALDI-TOF mass spectroscopy of α- and β-MSH pretreated with ETA or ETB. The molecular weights of intact α-MSH and β-MSH are 1,667 and 2,665, respectively, as indicated in the undigested controls.
The exact mechanism(s) by which the ETs cause SSSS remains unknown. However, the discovery that the ETs are capable of cleaving very specific substrates normally found in the epidermis (i.e., the MSH hormones) at a very specific residue may be the first step in elucidating the mechanism which causes SSSS. Until now, a reliable system for assaying the ETs for proteolytic activity had not been devised. These findings will provide researchers with a means to study the proteolytic properties of the ETs and will also contribute to our understanding of the development of SSSS.
Acknowledgments
This work was supported by PHS grants HL36611 (P.M.S.) and GM54384 (D.H.O.) from NIH. G.M.V. was supported by Biophysics Training grant GM08277 from NIH.
Timothy Leonard is gratefully acknowledged for artwork. Protein sequencing was performed by Ben Madden, Mayo Protein Core Facility, Mayo Clinic, Rochester, Minn.
REFERENCES
- 1.Bailey C J, Redpath M B. The esterolytic activity of epidermolytic toxins. Biochem J. 1992;284:177–180. doi: 10.1042/bj2840177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey C J, Smith T P. The reactive serine residue of epidermolytic toxin A. Biochem J. 1990;269:535–537. doi: 10.1042/bj2690535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhardwaj R S, Luger T A. Proopiomelanocortin production by epidermal cells: evidence for an immune neuroendocrine network in the epidermis. Arch Dermatol Res. 1994;287:85–90. doi: 10.1007/BF00370724. [DOI] [PubMed] [Google Scholar]
- 4.Blomster-Hautamaa D A, Schlievert P M. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 5.Cavarelli J, Prevost G, Bourguet W, Moulinier L, Chevrier B, Delagoutte B, Bilwes A, Mourey L, Rifai S, Piemont Y, Moras D. The structure of Staphylococcus aureus epidermolytic toxin A, an atypic serine protease, at 1.7A resolution. Structure. 1997;5:813–824. doi: 10.1016/s0969-2126(97)00235-9. [DOI] [PubMed] [Google Scholar]
- 6.Dancer S J, Garratt R, Saldanha J, Jhoti H, Evans R. The epidermolytic toxins are serine proteases. FEBS Lett. 1990;268:129–132. doi: 10.1016/0014-5793(90)80990-z. [DOI] [PubMed] [Google Scholar]
- 7.Dores R M. The proopiomelanocortin family. Prog Clin Biol Res. 1990;342:22–27. [PubMed] [Google Scholar]
- 8.Hunt G. Melanocyte stimulating hormone: a regulator of human melanocyte physiology. Pathobiology. 1995;63:12–21. doi: 10.1159/000163930. [DOI] [PubMed] [Google Scholar]
- 9.Kapral F A, Miller M M. Product of Staphylococcus aureus responsible for the scalded-skin syndrome. Infect Immun. 1971;4:541–545. doi: 10.1128/iai.4.5.541-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Lee C Y, Schmidt J J, Johnson-Winegar A D, Spero L, Iandolo J J. Sequence determination and comparison of the exfoliative toxin A and toxin B genes from Staphylococcus aureus. J Bacteriol. 1987;169:3904–3909. doi: 10.1128/jb.169.9.3904-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melish M E, Glasgow L A. The staphylococcal scalded-skin syndrome. The development of an experimental model. N Engl J Med. 1970;282:1114–1119. doi: 10.1056/NEJM197005142822002. [DOI] [PubMed] [Google Scholar]
- 13.Monday S R, Vath G M, Ferens W A, Deobald C, Rago J V, Gahr P J, Monie D D, Iandolo J J, Chapes S K, Davis W C, Ohlendorf D H, Schlievert P M, Bohach G A. Unique superantigen activity of staphylococcal exfoliative toxins. J Immunol. 1999;162:4550–4559. [PubMed] [Google Scholar]
- 14.Ouchterlony O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1962;6:30–54. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- 15.Prevost G, Rifai S, Chaix M L, Piemont Y. Functional evidence that the Ser-195 residue of staphylococcal exfoliative toxin A is essential for biological activity. Infect Immun. 1991;59:3337–3339. doi: 10.1128/iai.59.9.3337-3339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rago J V, Schlievert P M. Scalded skin syndrome. Rev Med Microbiol. 1998;9:1–7. [Google Scholar]
- 17.Vath G M, Earhart C A, Monie D D, Iandolo J J, Schlievert P M, Ohlendorf D H. The crystal structure of exfoliative toxin B: a superantigen with enzymatic activity. Biochemistry. 1999;38:10239–10246. doi: 10.1021/bi990721e. [DOI] [PubMed] [Google Scholar]
- 18.Vath G M, Earhart C A, Rago J V, Kim M H, Bohach G A, Schlievert P M, Ohlendorf D H. The structure of the superantigen exfoliative toxin A suggests a novel regulation as a serine protease. Biochemistry. 1997;36:1559–1566. doi: 10.1021/bi962614f. [DOI] [PubMed] [Google Scholar]