Highlights
-
•
Systematic analysis of 25 studies on biofilm prevalence of nosocomial infections.
-
•
Central intravenous catheters promote colonization by biofilm-forming microorganisms.
-
•
Several in vitro and in vivo infection models are used to evaluate biofilm formation.
-
•
Among 59 and 100% of microorganisms from intravenous catheters established biofilms.
-
•
Strategies for antibiofilm agents depends of antimicrobial resistance mechanisms.
Keywords: Biofilms, Nosocomial infections, Central intravenous catheter, In vitro assays, In vivo assays
Abbreviations: CVCs, Central Venous Catheters; ICU, Intensive Care Unit; NICU, Neonatal Intensive Care Unit; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; MDR, Multidrug-resistant; XDR, Extensively drug-resistant; MDS, Multidrug-susceptible; MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, Meticillin-sensitive Staphylococcus aureus; CoNS, Coagulase-negative staphylococci; CFU, Colony-forming unit; QD, Qualitative detection; QBA, Quantitative biofilm analysis; BFI, Biofilm formation index; CV, Crystal violet; CLSM, Confocal laser scanning microscopy; SEM, Scanning electron microscopy; PGA, Poly-γ-DL-glutamic acid; EDTA, Ethylenediamine tetraacetic acid; oPDM-plus-PS, N,N′-(1,2-phenylene)dimaleimide plus protamine sulfate; CHX-M/R, Chlorhexidine, minocycline, and rifampin; PDMS, Polydimethylsiloxane; HGT, Horizontal gene transfer
Abstract
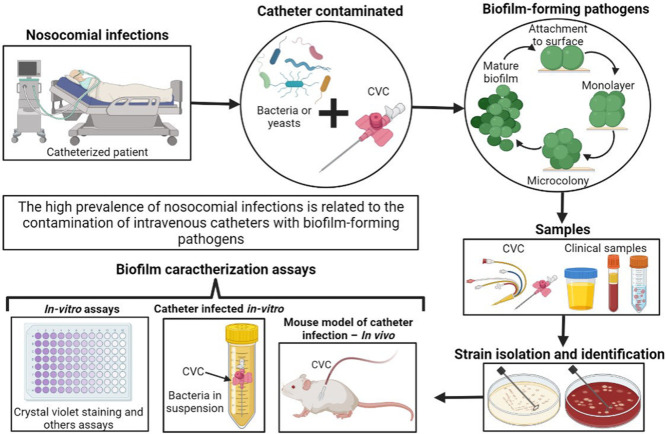
The high prevalence of nosocomial infections is related to the use of medical insertion devices such as central venous catheters (CVCs). Most of the microorganisms causing nosocomial infections are biofilm producers, this characteristic allows them to adhere to abiotic surfaces and cause initial catheter infections that can lead to bloodstream infections. Our main goal in this systematic review was to evaluate the prevalence of biofilm among CVC-related infections, particularly among Intensive Care Unit (ICU) patients, in the studies applying different in vitro and in vivo methodologies.
All studies reporting clinical isolates from patients with catheter-related nosocomial infections and biofilm evaluation published up to 24 June 2022 in the PubMed and Scopus databases were included. Twenty-five studies met the eligibility criteria and were included in this systematic review for analysis. Different methodologies were applied in the assessment of biofilm-forming microorganisms including in vitro assays, catheter-infected in vitro, and in vivo mouse models. The present study showed that between 59 and 100% of clinical isolates were able to form biofilms, and the prevalence rate of biofilm formation varied significantly between studies from different countries and regions. Among the clinical isolates collected in our study set, a wide variety of microorganisms including Gram-positive strains, Gram-negative strains, and Candida albicans were found. Many authors studied resistance mechanisms and genes related to biofilm development and surface adherence properties. In some cases, the studies also evaluated biofilm inhibition assays using various kinds of catheter coatings.
Graphical abstract
Introduction
The prevalence of hospital-acquired or nosocomial infections exceeds 25% in developing countries and up to 15% in developed countries, resulting in the death of approximately 40,000 hospitalized patients worldwide (Lemiech et al., 2021). The insertion of medical devices in the hospital setting for various patient treatment purposes has increased the incidence of nosocomial infections, including the various types of central venous catheters (CVCs) used for patient therapy and even for outpatients receiving various types of treatment such as hemodialysis, minor surgeries, some cancer treatments, among others 1(Baier et al., 2020).
CVCs are used to provide nutritional support to hospitalized patients, administer fluids and medications, and monitor hemodynamics, so their contamination with various microorganisms can lead to bloodstream infections that are a major cause of mortality, increasing the length of stay of hospitalized patients and Intensive Care Unit (ICU) stays and leading to additional health care costs (Brunelli et al., 2018). Nosocomial infections caused by catheterization procedures are frequently associated with antimicrobial-resistant microorganisms including various enterobacterial species, Enterococcus spp., Staphylococcus spp., and fungi such as Candida spp. (Liao et al., 2021).
Between 50 and 70% of nosocomial infections are caused by biofilm formation on implanted medical devices such as CVCs (Asker et al., 2021). Biofilms are made up of colonies of a wide variety of microorganisms bound by an extracellular matrix and can adhere to different surfaces such as CVCs (Muhsin Jamal et al., 2018). It is known that almost all bacteria and some fungal species have the inherent ability to form biofilms that allow them to evade the host immune response and tolerate treatment with a wide range of antibiotics and antifungals becoming a serious public health threat (Roy et al., 2018).
Therefore, this systematic review aimed to analyze studies comprising a variety of in vitro and in vivo methodologies used to study the biofilm formation of different microorganisms from nosocomial infections related to the use of central intravenous catheters.
Materials and methods
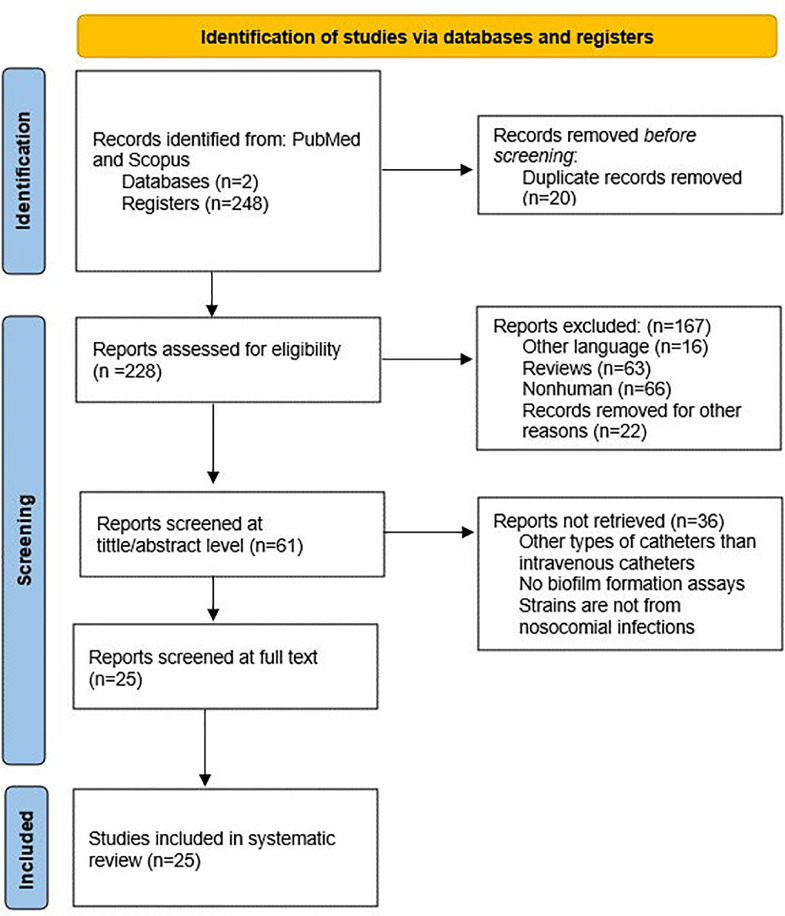
For a better understanding of the involvement of biofilm within nosocomial infections caused using CVCs, the relevant literature was reviewed. This study was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines by three independent reviewers (SPC-P, ALÑ-T, and AM). In each electronic database, a combination of MESH terms was used to conduct the search applying the following strategy: “((biofilms) AND ((microbial resistance) OR (drug resistance)) AND ((cross infection) OR (nosocomial infection)) AND ((intravenous) OR (catheters)))”. To start with the article collection each reviewer included articles related to the main topic, involving catheters and biofilms as keywords. All studies published on Scopus and PubMed databases until 24th June 2022 were retrieved. During the identification step in the databases, 248 results were found. When screening the studies set, 20 duplicate studies were removed.
The data selection for eligibility criteria from the remaining 228 articles was limited to human clinical isolates and studies in English, excluding articles in other languages (n=16), reviews (n=63), nonhuman studies (n=66), and records with irrelevant or unquantified information, editorials, congress, and meeting abstracts were also removed (n=22). In total, 167 articles were excluded from the study set. At each level, the reviewers independently screened the articles and finally merged their conclusions. An additional examination of the selected articles was realized by the remaining reviewers (AM, JR, and DG-C) focused on the homogeneity of the eligibility criteria of previous reviewers in the initial data set. Discrepancies were resolved by discussion before finalizing the records for the evaluation of eligibility criteria. In case of disagreements, the third assessor (AM) was assigned to make a final decision.
In the eligibility step, the main inclusion criteria were the evaluation of biofilm formation and the prevalence of biofilm-related infections, including observational studies (more exactly, cohort, retrospective, and case-control studies). Furthermore, data regarding the geographical location of the study set, and the use of antimicrobial agents in clinical isolates were also extracted from the studies. All studies without information about biofilm formation or clinical isolates were consequently excluded. The method to quantify biofilm biomass was not a criterion to include or exclude any paper in this systematic review. Therefore, 25 of 61 articles were chosen. Finally, the information from the final 25 articles was analyzed and extracted in the present study.
The extracted information included the first authors’ names, time of the study, year of publication, location, sample size, microorganism identification (species), biofilm formation rate, type of study, and the type of biofilm. The initial three authors (SPC-P, ALÑ-T, and LJE-M) extracted all data, and further confirmation and final evaluation were realized by the lead authors (AM, JR, and DG-C).
Results
Study inclusion criteria and characteristics of the eligible studies
A total of 248 articles were retrieved from PubMed and Scopus based on the established search terms. Following the article selection process, 25 articles that met the inclusion criteria were included for full-text analysis. The PRISMA flow chart summarizing the search strategy is illustrated in Fig. 1. This systematic review does not include time or location delimitations, so the data collected includes studies covering different regions of the world at different times. All articles were carefully reviewed for the extraction of relevant information related to biofilm formation on intravenous catheters in patients with hospital-acquired or nosocomial infections.
Fig. 1.
Prisma flow chart of included and excluded studies of the selection process.
General effects of biofilms in nosocomial infections
The overall data from the selected studies are shown in Table 1 comprising studies conducted between 2001 and 2020 in several countries around the world from Europe (7/25), Asia (6/25), South America (6/25), North America (5/25), and Africa (1/25). In addition, several methodologies such as in vitro assays (14/25), in vitro catheter-infected assays (10/25), and in vivo mouse catheter infection models (2/25) were used to assess biofilm formation. The tested strains of different bacterial and fungal species were isolated from intravenous catheters, medical devices, and clinical sites, where 59 and 100% of the clinical isolates were able to form biofilms.
Table 1.
General information extracted from the data set selected for the present systematic review.
| Study | Region | Country | Study type |
Biofilm rate, n (%) |
Species | Type of isolated |
| (Souza et al., 2019) | South America | Brazil | CI - In vitro | 4/4 (100) |
Corynebacterium striatum MDR and MDS | CS |
| (Burton et al., 2006) | North America | Canada | CI - In vitro | 6/6 (100) |
Escherichia coli P18, Pseudomonas aeruginosa PA01, Staphylococcus epidermidis 1457, Klebsiella pneumoniae P30, Proteus mirabilis 6285, and Enterococcus faecalis 36171 | C |
| (Sohail & Latif, 2018) | Asia | Pakistan | In vitro | 203/344 (59) |
MRSA | CVC |
| (Sobczak et al., 2019) | Europe | Poland | CI - In vitro | 15/15 (100) |
Staphylococcus haemolyticus, S. capitis, S. epidermidis, S. cohnii, and Klebsiella pneumoniae | UAC, UVC |
| (Hoque et al., 2016) | Asia | India | CI - In vitro, MMCI - In vivo | 2/2 (100) |
Staphylococcus aureus y E. coli | N/A |
| (Jain et al., 2016) | North America | USA | In vitro | 18/24 (75) |
Acinetobacter baumannii | CVC |
| (Sharma et al., 2011) | Asia | India | In vitro | 61/100 (61) |
Coagulase-negative staphylococci (CoNS) | CVC, CS |
| (Ramos et al., 2019) | South America | Brazil | CI - In vitro | 2/2 (100) |
C. striatum MDR | CVC, CS |
| (Sued et al., 2017) | South America | Brazil | CI - In vitro | 5/6 (83) |
Oxacillin-resistant S. haemolyticus | MD |
| (Cerezales et al., 2018) | South America | Bolivia | In vitro | 3/3 (100) |
A. baumannii | CVC, CS |
| (Fux et al., 2005) | Europe | Switzerland | In vitro | 19/29 (66) |
CoNS | CVC, CS |
| (Mohamed Jamal et al., 2014) | North America | USA | CI - In vitro | 5/5 (100) |
MRSA, S. epidermidis, E. coli, P. aeruginosa, and Candida albicans | CS |
| (Souza et al., 2015) | South America | Brazil | CI - In vitro | 4/4 (100) |
C. striatum 1987/I, 2369/II, 1961/III and 1954/IV | CS |
| (Cherifi et al., 2014) | Europe | Belgium | In vitro | 19/20 (95) |
S. epidermidis | CVC |
| (Conlan et al., 2012) | North America | USA | In vitro | 28/28 (100) |
S. epidermidis | CVC, CS |
| (Percival et al., 2005) | Europe | UK | CI - In vitro | 6/6 (100) |
(MRSA, S. epidermidis, E. coli, P. aeruginosa, C. albicans, and K. pneumoniae | CS |
| (Petrelli et al., 2008) | Europe | Italy | In vitro | 36/39 (92) |
S. aureus and S. epidermidis | CVC |
| (Soumya et al., 2016) | Asia | India | In vitro | 40/40 (100) |
S. epidermidis, S. haemolyticus, S. saprophyticus, and S. hominis | CS |
| (Pereira et al., 2014). | South America | Brazil | In vitro | 37/40 (93) |
S. haemolyticus | CS |
| (S. Zhou et al., 2013) | Asia | China | In vitro | 15/22 (68) |
S. epidermidis | CS |
| (Mekni et al., 2012) | Africa | Tunisia | In vitro | 97/97 (100) |
S. epidermidis | CVC, CS |
| (Donelli et al., 2001) | Europe | Italy | CI - In vitro | 74/97 (76) |
S. epidermidis and S. aureus | CVC |
| (Tekeli, 2021) | Asia | Turkey | In vitro | 35/35 (100) |
S. epidermidis RP62A, S. haemolyticus, S. hominis, and S. capitis | CVC |
| (Olejnickova et al., 2014) | Europe | Czech Republic | In vitro | 149/175 (85) |
P. aeruginosa | CVC |
| (Kocianova et al., 2005) | North America | USA | MMCI - In vivo | N/A | S. epidermidis and S. aureus | CS |
CI - In vitro: Catheter-infected in vitro; MMCI - In vivo: Mouse model of catheter infection – In vivo; MDR: Multidrug-resistant; MDS: Multidrug-susceptible; MRSA: Methicillin-resistant Staphylococcus aureus; CoNS: Coagulase-negative staphylococci; CS: Clinical sites; C: Catheter; CVC: Central venous catheters; UAC: Umbilical arterial catheter; UVC: Umbilical venous catheter; MD: Medical devices; N/A: Not Available.
Characteristics of catheterized patients with nosocomial infections
From the 25 articles analyzed in this review, all samples came from patients with nosocomial infections, but some of them reported extra information about the patients. The data collected on catheterized patients are summarized in Table 2. The most common aspect of the population set is the provenience of clinical isolates from the ICU. Moreover, studies in Bolivia and the Czech Republic showed the highest rates of nosocomial infection (Cerezales et al., 2018; Olejnickova et al., 2014). However, only studies in Bolivia and Poland evaluated the correlation between the nosocomial infection rate and the use of antibiotics in all ICU patients (Cerezales et al., 2018; Sobczak et al., 2019).
Table 2.
General information on catheterized patients with nosocomial infections.
| Hospital | Unit | Country |
Nosocomial infection rate, n (%) |
Male: Female | Age range | Main reasons for catheterization | Antibiotics during catheterization | Study |
| University Children's Hospital | NICU | Poland | 15/40 (37.5) |
27:13 | 23 to 41 weeks | Prematurity, therapeutic hypothermia monitoring, congenital disorders, and meconium aspiration syndrome | Yes | (Sobczak et al., 2019) |
| N/A | N/A | Pakistan | 203/344 (59.0) |
129:74 | 10 to 60 years | N/A | N/A | (Sohail & Latif, 2018) |
| Nationwide Children's Hospital | ICU | USA | 16/22 (72.7) |
14:8 | 1 to 14 years | N/A | Yes | (Jain et al., 2016) |
| Hospital Materno-Infantil Cochapamba | ICU | Bolivia | 29/32 (90.6) |
15:14 | 1 month to 5 years | Pneumonia, septicemia, and meningitis | Yes | (Cerezales et al., 2018) |
| University Hospital of Vern | Nephrology | Switzerland | 17/26 (65.3) |
18:8 | 29 to 83 years | Dialysis | Yes | (Fux et al., 2005) |
| Erasme University Hospital | ICU, nephrology, gastroenterology, neurology | Belgium | 20/128 (15.6) |
N/A | N/A | N/A | N/A | (Cherifi et al., 2014) |
| A teaching hospital in Rio de Janeiro | NICU | Brazil | 21/40 (53.0) |
N/A | 0 to 28 days | N/A | Yes | (Pereira et al., 2014) |
| St. Anne's University Hospital | N/A | Czech Republic | 149/172 (85.0) |
104:71 | 0 to 100 years | N/A | N/A | (Olejnickova et al., 2014) |
NICU: Neonatal Intensive Care Unit; ICU: Intensive Care Unit; N/A: Not Available.
Although all samples were collected from nosocomial infections, some strains were directly isolated from catheters (Mekni et al., 2012) and other isolates came from clinical samples of nosocomial infections and subsequently were cultivated and analyzed into infected catheters (Ramos et al., 2019). Concerning gender, the recollected data showed a higher rate of nosocomial infections in men. Finally, as expected, the age range of most patients with nosocomial infections was neonates/infants (below one year) children, adults, and geriatric patients (up to 100 years of age).
Geographical distribution of biofilm-forming
It is well-known that biofilms are the dominant form of growth in almost all bacteria species across the world being associated with the development of nosocomial infections through catheter insertions (Olejnickova et al., 2014). The prevalence rate of biofilm-related infections significantly varied among studies of different regions and countries. As shown in Table 3, the higher prevalence rate of biofilm was reported in Africa, followed by South and North America. On the other hand, Asia reported a lower prevalence of biofilm. Nonetheless, it is important to mention that the number of studies per region significantly varied, which could easily lead to erroneous conclusions, and further studies are essential to confirm these results.
Table 3.
Analysis of biofilm-forming for different geographical regions.
| Region | Number of studies | Biofilm rate | Prevalence of biofilms (%) |
| Europe | 7 | 318/381 | 83.5 |
| Asia | 6 | 356/543 | 65.6 |
| South America | 6 | 55/59 | 93.2 |
| North America | 5 | 57/63 | 90.5 |
| Africa | 1 | 97/97 | 100 |
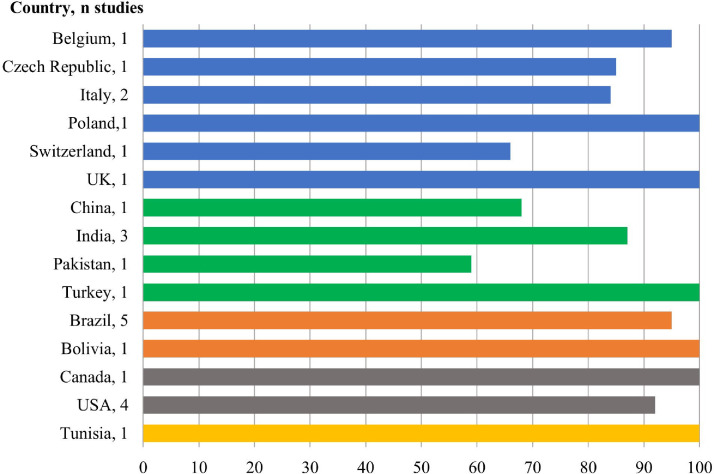
In Fig. 2, the study set is shown by country and most countries only reported one study, except for Italy (2 studies), India (3 studies), USA (4 studies), and Brazil (5 studies).
Fig. 2.
Prevalence of biofilm formation in different countries.
It is also remarkable how the prevalence of biofilm rate decreased in comparison to other countries of the same region with similar features, suggesting that more studies are needed to evaluate the real biofilm prevalence worldwide among nosocomial infections.
In vitro studies
Due to their ability to form biofilms, crystal violet methodology is usually the gold standard procedure to measure biofilm biomass. This methodology consists of the adjustment of bacterial growth cultures to a specific optical density or colony-forming unit (CFU)/mL value incubating the initial bacterial concentration into 96-well polystyrene plates containing a certain medium with some complements (Olejnickova et al., 2014). After 24h or 48h of incubating at 37°C, the media culture and bacterial planktonic cells are removed through multiple washes. Subsequently, the absorbance is measured at a certain wavelength. Although other methodologies could be applied as shown in Table 4, these techniques are usually a qualitative evaluation (such as Qualitative Detection, QD) or a quantitative evaluation less applied in biofilm research, such as Quantitative Biofilm Analysis (QBA), test for slime production, and safranin staining. The most trustful evaluation of the ability to form biofilm is its classification as weak, moderate, and strong biofilm former by the Biofilm Formation Index (BFI). However, the parameters for this classification varied between studies being a pitfall that must be solved to allow comparing data in the literature.
Table 4.
Biofilm-forming in vitro tests.
| Methodology to measure biofilm | Biofilm classification | Country | Study |
| CV (595nm) | Weak (0.1> BFI ≤ 0.5), moderate (0.5> BFI ≤ 1), and strong (BFI > 1) | Pakistan | (Sohail & Latif, 2018) |
| CV (595nm) | N/A | USA | (Jain et al., 2016) |
| Test for slime production | N/A | India | (Sharma et al., 2011) |
| CV (620nm) | Weak (0.1> BFI ≤ 0.5), moderate (0.5> BFI ≤ 1), and strong (BFI > 1) | Bolivia | (Cerezales et al., 2018) |
| CV (540 nm) | Weak (BFI ≤ 1.5), moderate (1.5> BFI ≤ 3), and strong (BFI > 3) | Belgium | (Cherifi et al., 2014) |
| Safranin (490nm) | N/A | USA | (Conlan et al., 2012) |
| CV | Weak (BFI ≤ 0.120), moderate (0.120> BFI ≤ 0.240), and strong (BFI > 0.240) | Italy | (Petrelli et al., 2008) |
| QBA | N/A | India | (Soumya et al., 2016) |
| QD | N/A | Brazil | (Pereira et al., 2014) |
| QD | Color reference scale | China | (S. Zhou et al., 2013) |
| CV (490 nm) | The BU of negative control was used to classify biofilm production | Tunisia | (Mekni et al., 2012) |
| SEM | N/A | Italy | (Donelli et al., 2001) |
| N/A | N/A | Turkey | (Tekeli, 2021) |
| CV (595nm) | N/A | Czech Republic | (Olejnickova et al., 2014) |
CV: Crystal violet; BFI: Biofilm formation index; QD: Qualitive Detection; QBA: Quantitative Biofilm Analysis; BU: Biofilm Unit; N/A: Not Available.
As shown in Table 4, all studies confirmed the ability of the clinical isolates to form biofilms and four of the studies tried to classify these biofilms. In addition, Sohail and Latif were able to classify 208 MRSA strains, from which 50% were weak, 27% were moderate, and 23% were strong biofilm formers (Sohail & Latif, 2018). Cherifi and colleagues demonstrated that 90% of Staphylococcus epidermidis isolates (18/20) were strong biofilm formers (Cherifi et al., 2014), while Petrelli and colleagues evidenced that 92% of Staphylococcus aureus, MRSA, and Staphylococcus epidermidis isolates were strong biofilm formers (36/39) (Petrelli et al., 2008).
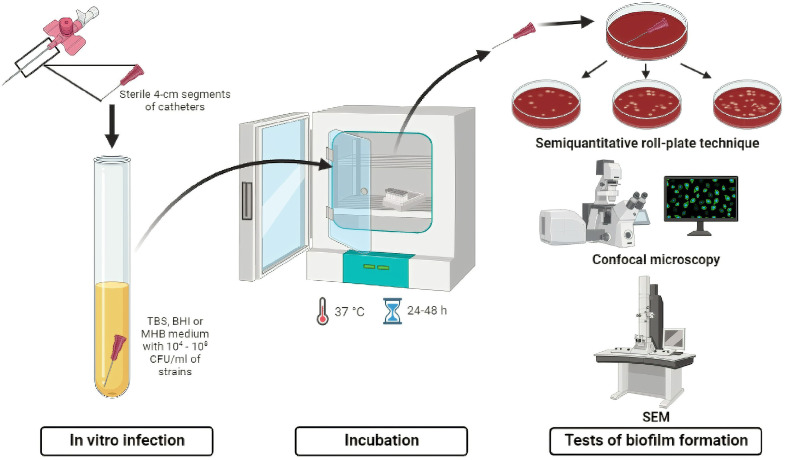
Catheter-infected in vitro
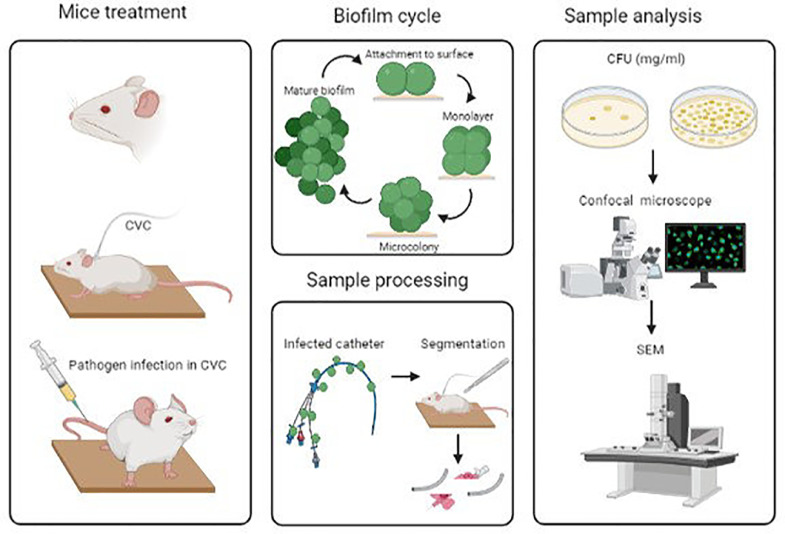
An alternative approach to evaluating the ability to form biofilms is through catheter-infected in vitro assays (Fig. 3). In this approach, clinical isolates were previously collected from different biological samples or medical devices and then cultured into well containing medium and a catheter to analyze the biofilm production (Souza et al., 2015).
Fig. 3.
Illustration of the procedure by catheter in vitro infection model.
As shown in Table 5, most studies evaluated the biofilm production on polyurethane 16-gauge percutaneous nephrostomy catheters, whereas Brazil was the country with more studies of catheter-infected in vitro.
Table 5.
Biofilm-forming in catheter-infected in vitro.
| Catheters type | Catheter-infected in vitro | Tests of biofilm formation | Country | Study |
| Polyurethane 16-gauge percutaneous nephrostomy catheters | In TSB medium containing 108 CFU/mL of strains | Viability of sessile forms of biofilm | Brazil | (Souza et al., 2019) |
| Coated silicon catheters | In BHI medium containing 107 CFU/mL of strains | Confocal microscopy | Canada | (Burton et al., 2006) |
| 16-gauge percutaneous nephrostomy polyurethane and silicone catheters | In TSB medium containing 106 CFU/mL of strains | Semi-quantitative roll plate method and SEM | Brazil | (Ramos et al., 2019) |
| Polyurethane 16-gauge percutaneous nephrostomy catheters | In TSB medium containing 106 CFU/mL of strains | Semi-quantitative roll plate method and SEM | Brazil | (Sued et al., 2017) |
| Polyurethane CVC coated with CHX-M/R and uncoated catheter | In MHB medium containing 5×105 CFU/mL of strains | Counts for colony growth | USA | (Muhsin Jamal et al., 2018) |
| Polyurethane 16-gauge percutaneous nephrostomy catheters | In TSB medium containing 5×106 CFU/mL of strains | Quantitative and semi-quantitative roll plate methods and SEM | Brazil | (Souza et al., 2015) |
| Triple-lumen catheter | In TSB medium containing 104 CFU/mL of strains | Counts for colony growth and SEM | UK | (Percival et al., 2005) |
SEM: Scanning Electron Microscopy; CFU: Colony-Forming Unit.
One of the most relevant results from infecting catheters in vitro was the influence of temperature on biofilm formation in abiotic surfaces (such as medical devices) evidencing that most strains showed lower biofilm production at 20°C when compared with the human body temperature at 37°C (Souza et al., 2015). When applying microscopic methodologies, the confocal analysis showed uniform colonization on uncoated catheters with S. epidermidis biofilms (Burton et al., 2006), while Scanning Electron Microscopy (SEM) showed microcolony formation of Corynebacterium striatum (Ramos et al., 2019) evidencing a large amount of mature biofilm and microcolonies of C. striatum in the surface of CVCs (Souza et al., 2015). To prevent the biofilm formation on medical devices, Percival and colleagues applied treatment with tetrasodium EDTA on catheters reducing C. albicans and MRSA biofilms after 21h, but with an additional 4h treatment, it was possible to eradicate these resistant microorganisms (Percival et al., 2005).
In vivo studies
The development of new strategies by coating CVCs to mitigate the production of biofilms has been considered a novel approach to combat infections (Hoque et al., 2016). Also, the application of animal models to reach the impact of coating CVCs allowed us to evaluate the mechanisms between the microorganism and the CVC in a host (Fig. 4) (Hoque et al., 2016; Kocianova et al., 2005). Catheter coated with polymer 7.5μg/mm2 implanted in mice with ∼1.7 × 107 CFU of MRSA showed a ∼5 log reduction and revealed no cell clusters after 96h on the surface by SEM analysis (Hoque et al., 2016). Regarding bacterial pathogenesis, S. epidermidis secretes poly-γ-DL-glutamic acid (PGA) allowing the colonization in a host, as approved by Kocianova and colleagues using a mice model infected with a PGA mutant S. epidermidis (Kocianova et al., 2005).
Fig. 4.
Illustration of the procedure by catheter in vivo infection model.
Biofilm-forming capacity according to different microorganisms
Gram-positive and negative bacteria and yeasts are well-known biofilm formers, being Staphylococcus spp., E. coli, P. aeruginosa, K. pneumoniae, and S. epidermidis the microorganisms more frequently reported in the literature (Muhsin Jamal et al., 2018). These microorganisms represent approximately 60% of all catheter biofilm-related infections. Moreover, P. aeruginosa has been used during the last decades as an in vitro model due to its ability to form biofilms (Cerezales et al., 2018; Khatoon et al., 2018). In this review, we summarized the biofilm-forming capacity of the microorganisms evaluated in our study set, as shown in Table 6.
Table 6.
Prevalence of biofilms in the different microorganisms.
| Species | Number of studies | Biofilm rate | Prevalence of biofilms (%) |
| Gram-positive bacteria | |||
| Staphylococcus aureus | 3 | 54/54 | 100 |
| Staphylococcus haemolyticus | 3 | 111/112 | 99.1 |
| Staphylococcus epidermidis | 12 | 416/416 | 100 |
| Staphylococcus capitis | 1 | 2/2 | 100 |
| Staphylococcus cohnii | 1 | 15/15 | 100 |
| Staphylococcus saprophyticus | 1 | 5/5 | 100 |
| Staphylococcus hominis | 2 | 48/48 | 100 |
| MRSA | 4 | 219/360 | 60.8 |
| Coagulase-negative staphylococci | 4 | 81/120 | 67.5 |
| Corynebacterium striatum | 3 | 10/10 | 100 |
| Gram-negative bacteria | |||
| Escherichia coli | 4 | 4/4 | 100 |
| Enterococcus faecalis | 1 | 1/1 | 100 |
| Acinetobacter baumannii | 2 | 21/24 | 87.5 |
| Klebsiella pneumoniae | 3 | 3/3 | 100 |
| Proteus mirabilis | 1 | 1/1 | 100 |
| Pseudomonas aeruginosa | 4 | 178/178 | 100 |
| Yeast | |||
| Candida albicans | 2 | 2/2 | 100 |
MRSA: Methicillin-resistant Staphylococcus aureus
Regarding the results, most of the strains in our study set showed that 13 of 18 microorganisms are 100% biofilm-formers, including several Gram-positive and negative bacteria as well as Candida albicans. Although the studies evaluated the ability to form biofilms under different laboratory-controlled conditions, all isolates are related to acquired-hospital infections, where S. epidermidis was isolated in 13 studies due to its high prevalence among patients with biofilm-associated infections (Cherifi et al., 2014). In summary, almost all staphylococcal species (such as S. epidermidis, S. saprophyticus, S. capitis, S. cohnii, S. hominis, S. aureus, and S. haemolyticus) showed a prevalence of biofilm-associated infections more than 90% in our study set. Meanwhile, MRSA evidenced a 60.8% of biofilm prevalence through four studies compiling a total of 360 isolates. Furthermore, all Gram-negative bacteria (E. coli, P. aeruginosa, K. pneumoniae, and P. mirabilis) reached 100% of biofilm prevalence except for A. baumannii evidencing 77.8% biofilm prevalence obtained in only one study. Finally, C. albicans and C. striatum also demonstrated a 100% of biofilm prevalence, being the only yeast and Gram-positive bacillus in the present review.
Biofilm and adhesion-related genes
The most prevalent biofilm-related infections are with S. aureus, S. epidermidis, and E. coli species in our study set. In staphylococcal species, several genes are involved in biofilm production being the ica gene locus extensively studied. The ica gene locus regulates the biosynthesis of polysaccharide intercellular adhesion, which plays an important role in biofilm production. In addition, mecA genes are involved in methicillin resistance and are allegedly involved in the resistance patterns as well as biofilm induction (Mekni et al., 2012; Pereira et al., 2014). However, the relationship between these genes is not well-known. It is important to mentation that not all ica-positive isolates are biofilm producers, where Mekni and colleagues demonstrated in a previous study that only 43.9% of S. epidermidis with this cluster were able to produce biofilms (Mekni et al., 2012). Usually, icaA and mecA genes are present in most of the S. aureus strains, resulting in MRSA evolution, but the presence of both genes does not indicate that a certain strain can form biofilm (Conlan et al., 2012; Donelli et al., 2001; Sharma et al., 2011). Therefore, further studies should be conducted to understand the multifactorial pathways leading to the establishment of biofilm among staphylococcal species.
On the other hand, P. aeruginosa biofilms have also been studied in the last two decades and it is an excellent biofilm producer, showing 100% of biofilm prevalence and being at the top of bacteria Gram-negative in the review. It is well-known the resilience of the mature P. aeruginosa biofilm when compared to the planktonic cells (Olejnickova et al., 2014). Nonetheless, the remaining Gram-negative bacteria are also important in nosocomial infections, in particular, Acinetobacter baumannii can be a serial problem in hospitals, because isolates are multidrug-resistant (MDR) and/or extensively drug-resistant (XDR), resulting in a high rate of mortality for patients. A. baumannii biofilm demonstrates an excellent evasion mechanism for infections and its huge antibacterial spectrum increases the complications of the patient infections and outcomes (Cerezales et al., 2018; Muhsin Jamal et al., 2018).
Prevention of biofilms on catheters
Several antibiofilm agents, such as antimicrobial CVCs, antimicrobial locks, enzymes, and polymers, have been tested with novel effects on different MDR pathogens and isolates from nosocomial infections, demonstrating significant inhibition and eradication rates against biofilms, where CVC coated with chitin derivates inhibited the biofilm formation of MRSA and CVC coated with chlorhexidine, minocycline, and rifampin showed a 100% of biofilm inhibition (Burton et al., 2006; Hoque et al., 2016; Mohamed Jamal et al., 2014; Percival et al., 2005). To evaluate the effects of different treatments after coating CVC, SEM analysis usually represents an elementary tool. As shown in Table 7, Percival and colleagues evaluated CVC coated with tetrasodium ethylenediamine tetraacetic acid (EDTA) at 40mg/mL reporting an eradication of 4 from 6 pathogens after 21h (Percival et al., 2005). On the other hand, MRSA and C. albicans biofilms were eradicated on CVCs coated with a certain polymer derivate from chitin at 7.5 μg/mm2 as reported by Hoque and colleagues through SEM analysis (Hoque et al., 2016).
Table 7.
Antibiofilm studies on catheters.
| Antibiofilm on catheters assays | Antibiofilm agents | Reducing the biofilm formation | Country | Study |
| Confocal microscopy | oPDM-plus-PS | 59.1% inhibition to P. aeruginosa and 64.4% to S. epidermidis | Canada | (Burton et al., 2006) |
| SEM | Polymer derivate from chitin | Polymer-coated catheter revealed a lesser number of bacteria, thus indicating no biofilm formation on the surface | India | (Hoque et al., 2016) |
| CFU of the segments | Chlorhexidine, minocycline, and rifampin | A significant difference was reported for MRSA, P. aeruginosa, and C. albicans, while a biofilm inhibition of 100% was found against S. epidermidis | USA | (Muhsin Jamal et al., 2018) |
| SEM | Tetrasodium EDTA | After treatment eradicated the biofilm of S. epidermidis, P. aeruginosa, K. pneumoniae, and E. coli, while also reducing MRSA biofilm by 3.5 logs and C. albicans biofilm by 2.2 logs CFU |
UK | (Percival et al., 2005) |
SEM: Scanning Electron Microscopy; CFU: Colony-Forming Unit.
Likewise, when Jamal and colleagues coated CVC with chlorhexidine, minocycline, and rifampin (CHX-M/R), this antibiofilm combination proved to be effective against MRSA, P. aeruginosa, C. albicans, and S. epidermidis (Muhsin Jamal et al., 2018). Finally, N,N′-(1,2-phenylene)dimaleimide plus protamine sulfate (oPDM-plus-PS) at 50 mg/mL also inhibited the biofilm formation in 2 of the 6 isolates until 70% in P. aeruginosa and S. epidermidis (Burton et al., 2006). However, few studies were realized until now on CVCs with antibiofilm agents and further evaluation is needed.
Discussion
The importance of this systematic review lies in the fact that the high prevalence of biofilm formation on insertion medical devices, such as CVCs, dramatically increases the incidence of nosocomial infections in catheterized patients. Biofilms can usually be found on CVCs and most of them invaded the inside of the catheter depending on the duration of catheterization and length of hospital stay, which eventually lead to bloodstream infections in patients (Lewis, 2001).
The care units with a higher prevalence of nosocomial infections are NICU (Pereira et al., 2014; Sobczak et al., 2019) and ICU (Cerezales et al., 2018; Cherifi et al., 2014; Jain et al., 2016). It is well-known that ICU and NICU patients are at serious risk of contracting healthcare-associated infections or any bloodstream infection (Atiencia et al., 2022; Johnson & Quach, 2017). All evaluated clinical isolates in this systematic review were collected from patients with nosocomial infections, where most of the patients received several antibiotic treatments without success when applied against biofilms extracted from them. This issue occurred because a routine systemic treatment with antibiotics is not effective due to resistance and tolerance of biofilm organisms present on these medical devices (Donlan, 2008; Galié et al., 2018; Rodríguez-Cerdeira et al., 2020; Roy et al., 2018). When comparing gender, all studies reported differences in the rate of nosocomial infections in catheterized patients between men and women, evidencing a higher susceptibility in men to develop this type of biofilm-associated infection, as recently postulated by Tomczyk-Warunek and colleagues(Tomczyk-Warunek et al., 2021).
All clinical isolates evaluated in the present review were directly isolated from catheters and/or biological samples/biopsies from catheterized patients. The procedure of the in vitro catheter infection model was originally formulated through intravenous catheter-associated infections in patients, which showed extreme microbial colonization and related to some particular diagnoses like severe sepsis, suppurative thrombophlebitis, endocarditis, bloodstream infection, and biofilm-related strains collected from the blood or even skin (Atiencia-Carrera et al., 2022a; Mermel et al., 2009; Pinto et al., 2021).
Concerning the geographical distribution of our study set, it is remarkable that Europe showed the highest number of published studies, but America and Africa demonstrated a higher prevalence of biofilm-forming strains. However, it is important to increase the number of studies on Asia and Africa. Only one study was published in Africa, while Asian studies reported a significantly lower prevalence of biofilm infections when compared with the remaining regions. Moreover, European countries have developed several problems related to microbial infections. In particular, Italy was reported as one of the worldwide regions with an increase in deaths due to antibiotic resistance and biofilm-related infections (Atiencia-Carrera et al., 2022a; Cesta et al., 2020; Nolan et al., 2020). Other regions also described the high prevalence of nosocomial infections caused by catheterization such as Bolivia, where there has been a steady increase in the mortality rate related to nosocomial infections since 2001 (Cerezales et al., 2018; Maury Fernández et al., 2003), and Turkey, where the mortality rate associated with these infections reached values of 69% (Cevik et al., 2005; Dagi et al., 2016).
Several in vitro methods that have been developed to characterize the ability of clinical isolates in biofilm formation are usually based on colorimetric or microscopic techniques. Currently, crystal violet (CV) staining technique, due to its simplicity and sensitivity, is the preferred method for in vitro biofilm quantification and classification (Atiencia-Carrera et al., 2022b; Di Domenico et al., 2016; Stepanović et al., 2000). Another advantage of the CV staining technique, as a basic stain, is the ability to stain all living and dead cells by binding to the negative charge of surface molecules and extracellular matrix polysaccharides (Extremina et al., 2011). Although some studies found at least one biofilm-forming strain, most of the studies reported multispecies biofilms, where CV staining could be applied to either type of biofilm and classified them as strong, moderate, and weak biofilm formers. This classical classification is usually done through the BFI. The BFI is obtained through a mathematical algorithm and measures the adhesion capacity of each strain by comparing the initial and the final absorbance measurements during a certain incubation growth period (Atiencia-Carrera et al., 2022a; Castro et al., 2022; Olivares et al., 2016). This systematic review also describes studies using the procedure by catheter in vitro infection model. These studies allow predicting thebehavior of biofilm in medical devices, from initial adhesion until biofilm dispersion and even antimicrobial biofilm treatments in catheters with different and relevant clinical isolates (Asker et al., 2021; Buhmann et al., 2016; Didehdar et al., 2022). Due to the phenotypic shift associated with biofilms and the environmental and medical settings, this in vitro model allowed us to characterize altered gene expression, virulence factors, antimicrobial resistance mechanisms, and biofilm life cycle (Atiencia-Carrera et al., 2022b; Dötsch et al., 2012; Hall & Mah, 2017). Thus, most studies using CV staining technique also employ other methodologies to measure biofilm formation on catheters, such as Confocal Laser Scanning Microscopy (CLSM) and SEM. These techniques provide a high-resolution insight into the in vitro process of biofilm first attachment to the catheters’ surface. Overall, SEM analysis showed microbial colonization on catheters in all studies where this in vitro infection model was applied in our study set. While CLSM analysis could evidence the three-dimensional colonization on catheter surfaces (Burton et al., 2006; Rosenberg et al., 2019).
The catheter in vivo infection model plays a relevant role in monitoring biofilm infections, giving a pathway to characterize omics, quorum sensing molecules, immune responses, treatment outcomes, and infection evolution (Chauhan et al., 2016; Su et al., 2020). In our study set, several researchers used in vivo model to assess the antibiofilm activity of various alternative and standard therapies against several MDR strains (like MRSA and vancomycin-resistant enterococci), such as coating CVCs with polydimethylsiloxane (PDMS) to reduce biofilm development. Hoque and colleagues achieved more than 84% of biofilm reduction in all MDR strains analyzed (Hoque et al., 2016). Likewise, Zhou and colleagues demonstrated similar results when employing polymers at 7.5 μg/mm2 by coating CVCs. This type of coating showed novel antibiofilm and antimicrobial properties against MRSA and MDR S. epidermidis strains (C. Zhou et al., 2017). By SEM analysis, it was possible to confirm the conformation of the polymer agent on the CVC surface and the inhibition and eradication of biofilms. While dead/alive staining and CLSM evaluation allowed us to validate the mortality rate and biofilm composition (Chauhan et al., 2016; C. Zhou et al., 2017). The preliminary animal-model results lead to the initial evaluation to characterize further the interaction host-CVC-infection (Su et al., 2020).
Among the results obtained in our study set, most of the clinical isolates belong to S. epidermidis, regarding its importance in nosocomial infections and biofilm formation capacity. Although S. epidermidis was an underrated opportunistic pathogen, studies in the last decades became essential to clarify the importance of this species and understand its mechanisms, genes, antibiotic resistance, and biofilm ability (Le et al., 2019). S. epidermidis is nowadays characterized by its resistant intercellular network of amyloid fibers, which became an important factor during the biofilm cycle, increasing the resistance to environments, chemical factors, standard treatments, and several antibiotics (Yarawsky et al., 2020). On the other hand, MRSA is one of the major nosocomial pathogens known worldwide, in particular by the mechanisms of antibiotic resistance and biofilm formation, reaching a biofilm rate almost of 99%, being strong biofilm formers in more than 30% and 50% of them are isolated from catheters infection (Piechota et al., 2018; Suresh et al., 2019). Meanwhile, Gram-negative bacteria represent a significant percentage of nosocomial infections and potential to establish biofilm. Regarding the results obtained in the study set, more than 87% of Gram-negative isolates are biofilm producers, in agreement with the literature, being detected around 84% in CVC-related infections with E. coli, K. pneumoniae, P. aeruginosa, and Acinetobacter spp. Consequently, urge for new therapies are urgently needed to inhibit and eradicated biofilms in catheter-associated infections, as postulated by several authors (Gunardi et al., 2021; Maharjan et al., 2018; Oleksy-Wawrzyniak et al., 2021). Finally, Candida isolates equally demonstrated to be strong biofilm-formers, through their production of extracellular polysaccharides and hyphae morphology shift, showing a biofilm prevalence near 100% and so becoming an important threat in hospital-acquired infections (Bekkal brikci benhabib et al., 2021).
Nonetheless, controversies in the literature can be found about the relationship between biofilm formation and antibiotic resistance (Carcione et al., 2022; Ruhal & Kataria, 2021). Since not all S. epidermidis strains with a resistance spectrum are biofilm producers, it is believed that horizontal gene transfer (HGT) mechanisms also play a vital role in antimicrobial resistance in hospital-acquired infections (Abe et al., 2020). As postulated by Abe and colleagues, biofilms constitute a hot spot of HGT of antibiotic resistance genes, through conjugation, transformation, and transduction, in any environment like health care facilities (Abe et al., 2020). The same scenario was found in several studies with S. aureus isolates, where the correlation between biofilm production and antibiotic resistance is inconclusive. Indeed, the studies reported a higher percentage of biofilm formers, but not all of them are MRSA or meticillin-sensitive Staphylococcus aureus (MSSA), whereas sensible isolates also are biofilm positive. Moreover, ica ABCD operon is associated with biofilm production, where a polysaccharide intercellular adhesin is encoded, being an essential factor for the biofilm of Staphylococcus spp. However, the presence of the entire cluster did not always correlate with biofilm production, but there is evidence to suggest a correlation when at least two genes (icaAD) need to be co-transcribed. Therefore, the presence of ica ABCD operon does not necessarily determine the biofilm production among the strains (Abdel-Shafi et al., 2022; Kıvanç, 2018; Sharma et al., 2011). Finally, among Gram-negative isolates, the studies reported the same paradigm, appointing other potential mechanisms such as secretion of different polysaccharides, amyloid-type proteins, flagella, and virulence factors (Conlan et al., 2012; Senobar Tahaei et al., 2021; Wang et al., 2020). Although some studies evaluated novel compounds against in vitro biofilm formation by coating catheters with antimicrobials, polymers, and lock solutions (Chandra et al., 2018; Percival et al., 2005), further in vitro and in vivo studies are strictly necessary to discover and implement alternative treatments for biofilm inhibition and eradication.
Conclusions
In summary, this systematic review evaluated published studies on biofilm-forming microorganisms isolated from CVCs and analyzed the prevalence of biofilm among catheterized patients and their association with nosocomial infections. Although the reviewed studies employed different methodologies for the assessment of biofilm formation, an accurate analysis was realized describing the most frequently isolated biofilm-forming species, their biofilm classification, and geographical distribution. The information included in this systematic review uses data published worldwide and without time delimitation. Finally, our systematic analysis showed that the high prevalence rate of biofilm-forming microorganisms is related to the high occurrence of nosocomial infections in catheterized patients. Further studies are needed to determine the real incidence of biofilms and their microbial diversity in countries for which results have not yet been reported, and a full report of the clinical background of the patients in published studies is essential to better understand the interaction between biofilm-associated infections and host immune response.
Funding
This work was supported by the COCIBA research budget and Collaboration Grants 2021 of the Research Office from Universidad San Francisco de Quito (USFQ), under Project ID: 17579 entitled “Antibiotic resistance and biofilm formation among clinical isolates from intravenous catheter tips at a public hospital of Quito” and Project ID: 17577 entitled “The antibiofilm potential of lactobacilli biosurfactants against multi-drug-resistant pathogens”. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Data availability statement
All data presented in this study are available upon request by contacting the corresponding author A.M.
CRediT authorship contribution statement
Sandra Pamela Cangui-Panchi: Conceptualization, Formal analysis, Investigation, Data curation, Writing – original draft. Anahí Lizbeth Ñacato-Toapanta: Conceptualization, Formal analysis, Investigation, Data curation, Writing – original draft. Leonardo Joshué Enríquez-Martínez: Conceptualization, Formal analysis, Investigation, Writing – original draft. Jorge Reyes: Conceptualization, Writing – review & editing. Daniel Garzon-Chavez: Conceptualization, Resources, Writing – review & editing, Funding acquisition. António Machado: Conceptualization, Methodology, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
A special recognition deserves all colleagues of the Microbiology Institute of USFQ, COCIBA, and Research Office of Universidad San Francisco de Quito for their support in this study.
Footnotes
Some references seem to have sometimes a space after the first "(". Please check this typo. Thank you.
Data availability
Data will be made available on request.
References
- Abdel-Shafi S., El-Serwy H., El-Zawahry Y., Zaki M., Sitohy B., Sitohy M. The association between icaA and icaB Genes, antibiotic resistance and biofilm formation in clinical isolates of staphylococci spp. Antibiotics. 2022;11(3):389. doi: 10.3390/antibiotics11030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K., Nomura N., Suzuki S. Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020;96(5):fiaa031. doi: 10.1093/femsec/fiaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asker D., Awad T.S., Raju D., Sanchez H., Lacdao I., Gilbert S., Sivarajah P., Andes D.R., Sheppard D.C., Howell P.L., Hatton B.D. Preventing Pseudomonas aeruginosa Biofilms on Indwelling Catheters by Surface-Bound Enzymes. ACS Appl. Bio Mater. 2021;4(12):8248–8258. doi: 10.1021/acsabm.1c00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiencia-Carrera M.B., Cabezas-Mera F.S., Tejera E., Machado A. Prevalence of biofilms in Candida spp. bloodstream infections: a meta-analysis. PLoS ONE. 2022;17(2) doi: 10.1371/journal.pone.0263522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiencia-Carrera M.B., Cabezas-Mera F.S., Vizuete K., Debut A., Tejera E., Machado A. Evaluation of the biofilm life cycle between Candida albicans and Candida tropicalis. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.953168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier C., Linke L., Eder M., Schwab F., Chaberny I.F., Vonberg R.-P., Ebadi E. Incidence, risk factors and healthcare costs of central line-associated nosocomial bloodstream infections in hematologic and oncologic patients. PLOS ONE. 2020;15(1) doi: 10.1371/journal.pone.0227772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkal brikci benhabib, O., Boucherit-otmani, Z., Boucherit, K., & Djediat, C. (2021). Interaction in a dual-species biofilm of Candida albicans and Candida glabrata co-isolated from intravascular catheter. Microbial Pathogenesis, 152, 104613. https://doi.org/10.1016/j.micpath.2020.104613. [DOI] [PubMed]
- Brunelli S.M., Van Wyck D.B., Njord L., Ziebol R.J., Lynch L.E., Killion D.P. Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J. Am. Soc. Nephrol. 2018;29(4):1336–1343. doi: 10.1681/ASN.2017080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhmann, M. T., Stiefel, P., Maniura-Weber, K., & Ren, Q. (2016). In Vitro biofilm models for device-related infections. Trends in Biotechnol., 34(12), 945–948. https://doi.org/10.1016/j.tibtech.2016.05.016. [DOI] [PubMed]
- Burton E., Gawande P.V., Yakandawala N., LoVetri K., Zhanel G.G., Romeo T., Friesen A.D., Madhyastha S. Antibiofilm activity of glmu enzyme inhibitors against catheter-associated uropathogens. Antimicrobial Agents and Chemotherapy. 2006;50(5):1835–1840. doi: 10.1128/AAC.50.5.1835-1840.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcione, D., Leccese, G., Conte, G., Rossi, E., Intra, J., Bonomi, A., Sabella, S., Moreo, M., Landini, P., Brilli, M., & Paroni, M. (2022). Lack of direct correlation between biofilm formation and antimicrobial resistance in clinical staphylococcus epidermidis isolates from an Italian hospital. Microorganisms, 10(6), 1163. https://doi.org/10.3390/microorganisms10061163. [DOI] [PMC free article] [PubMed]
- Castro J., Lima Â., Sousa L.G.V., Rosca A.S., Muzny C.A., Cerca N. Crystal violet staining alone is not adequate to assess synergism or antagonism in multi-species bio fi lms of bacteria associated with bacterial vaginosis. Front. Cell. Infect. Microbiol. 2022;11(795797):1–7. doi: 10.3389/fcimb.2021.795797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezales M., Ocampo-Sosa A.A., Álvarez Montes L., Díaz Ríos C., Bustamante Z., Santos J., Martínez-Martínez L., Higgins P.G., Gallego L. High prevalence of extensively drug-resistant acinetobacter baumannii at a children hospital in Bolivia. Pediatric Infectious Disease J. 2018;37(11):1118–1123. doi: 10.1097/INF.0000000000001962. [DOI] [PubMed] [Google Scholar]
- Cesta N., Di Luca M., Corbellino M., Tavio M., Galli M., Andreoni M. Bacteriophage therapy: an overview and the position of Italian society of infectious and tropical diseases. Infezioni in Medicina. 2020;28(3):322–331. [PubMed] [Google Scholar]
- Cevik M.A., Yilmaz G.R., Erdinc F.S., Ucler S., Tulek N.E. Relationship between nosocomial infection and mortality in a neurology intensive care unit in Turkey. The J. Hospital Infect. 2005;59(4):324–330. doi: 10.1016/J.JHIN.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Chandra J., Long L., Isham N., Mukherjee P.K., DiSciullo G., Appelt K., Ghannoum M.A. In vitro and in vivo activity of a novel catheter lock solution against bacterial and fungal biofilms. Antimicrobial Agents and Chemotherapy. 2018;62(8) doi: 10.1128/AAC.00722-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A., Ghigo J.-M., Beloin C. Study of in vivo catheter biofilm infections using pediatric central venous catheter implanted in rat. Nat. Protocols. 2016;11(3):525–541. doi: 10.1038/nprot.2016.033. [DOI] [PubMed] [Google Scholar]
- Cherifi S., Byl B., Deplano A., Nagant C., Nonhoff C., Denis O., Hallin M. Genetic characteristics and antimicrobial resistance of Staphylococcus epidermidis isolates from patients with catheter-related bloodstream infections and from colonized healthcare workers in a Belgian hospital. Ann. Clin. Microbiol. Antimicrobials. 2014;13(1):20. doi: 10.1186/1476-0711-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan S., Mijares L.A., Becker J., Blakesley R.W., Bouffard G.G., Brooks S., Coleman H., Gupta J., Gurson N., Park M., Schmidt B., Thomas P.J., Otto M., Kong H.H., Murray P.R., Segre J.A. Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates. Genome Biol. 2012;13(7) doi: 10.1186/gb-2012-13-7-r64. R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagi H.T., Findik D., Senkeles C., Arslan U. Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Ann. Clin. Microbiol. Antimicrobials. 2016;15:1–5. doi: 10.1186/s12941-016-0153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico E.G., Toma L., Provot C., Ascenzioni F., Sperduti I., Prignano G., Gallo M.T., Pimpinelli F., Bordignon V., Bernardi T., Ensoli F. Development of an in vitro assay, based on the BioFilm ring test®, for rapid profiling of biofilm-growing bacteria. Front. Microbiol. 2016;7(SEP):1429. doi: 10.3389/fmicb.2016.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didehdar M., Chegini Z., Tabaeian S.P., Razavi S., Shariati A. Cinnamomum: the new therapeutic agents for inhibition of bacterial and fungal biofilm-associated infection. Front. Cellular and Infect. Microbiol. 2022;12(July):1–19. doi: 10.3389/fcimb.2022.930624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelli G., De Paoli P., Fadda G., Marone P., Nicoletti G., Varaldo P.E., Group A.T.C.S. A multicenter study on central venous catheter-associated infections in Italy. J. Chemotherapy. 2001;13(sup4):251–262. doi: 10.1179/joc.2001.13.Supplement-2.251. [DOI] [PubMed] [Google Scholar]
- Donlan R.M. Current Topics in Microbiology and Immunology. Springer; Berlin, Heidelberg: 2008. Biofilms on central venous catheters: is eradication possible? pp. 133–161. Vol. 322. [DOI] [PubMed] [Google Scholar]
- Dötsch A., Eckweiler D., Schniederjans M., Zimmermann A., Jensen V., Scharfe M., Geffers R., Häussler S. The pseudomonas aeruginosa transcriptome in planktonic cultures and static biofilms using RNA sequencing. PLoS ONE. 2012;7(2):e31092. doi: 10.1371/journal.pone.0031092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extremina C.I., Costa L., Aguiar A.I., Peixe L., Fonseca A.P. Optimization of processing conditions for the quantification of enterococci biofilms using microtitre-plates. J. Microbiol. Methods. 2011;84(2):167–173. doi: 10.1016/j.mimet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Fux C.A., Uehlinger D., Bodmer T., Droz S., Zellweger C., Mühlemann K. Dynamics of hemodialysis catheter colonization by coagulase-negative staphylococci. Infect. Control & Hospital Epidemiol. 2005;26(6):567–574. doi: 10.1086/502586. [DOI] [PubMed] [Google Scholar]
- Galié S., García-Gutiérrez C., Miguélez E.M., Villar C.J., Lombó F. Biofilms in the food industry: health aspects and control methods. Front. Microbiol. 2018;9:898. doi: 10.3389/fmicb.2018.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunardi W.D., Karuniawati A., Umbas R., Bardosono S., Lydia A., Soebandrio A., Safari D. Biofilm-producing bacteria and risk factors (gender and duration of catheterization) characterized as catheter-associated biofilm formation. Int. J. Microbiol. 2021;2021:1–10. doi: 10.1155/2021/8869275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.W., Mah T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017;41(3):276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- Hoque J., Akkapeddi P., Ghosh C., Uppu D.S.S.M., Haldar J. A biodegradable polycationic paint that kills bacteria in vitro and in vivo. ACS Appl. Mater. Interfaces. 2016;8(43):29298–29309. doi: 10.1021/acsami.6b09804. [DOI] [PubMed] [Google Scholar]
- Jain A.L., Harding C.M., Assani K., Shrestha C.L., Haga M., Leber A., Munson R.S., Kopp B.T. Characteristics of invasive Acinetobacter species isolates recovered in a pediatric academic center. BMC Infect. Dis. 2016;16(1):346. doi: 10.1186/s12879-016-1678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal Mohamed, Rosenblatt J., Jiang Y., Hachem R., Chaftari A.-M., Raad I.I. Prevention of transmission of multidrug-resistant organisms during catheter exchange using antimicrobial catheters. Antimicrobial Agents and Chemotherapy. 2014;58(9):5291–5296. doi: 10.1128/AAC.02886-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal Muhsin, Ahmad W., Andleeb S., Jalil F., Imran M., Nawaz M.A., Hussain T., Ali M., Rafiq M., Kamil M.A. Bacterial biofilm and associated infections. J. Chinese Med. Assoc. 2018;81(1):7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Johnson, J., & Quach, C. (2017). Outbreaks in the neonatal ICU. Current Opinion in Infect. Dis. 30(4), 395–403. https://doi.org/10.1097/QCO.0000000000000383. [DOI] [PMC free article] [PubMed]
- Khatoon, Z., McTiernan, C. D., Suuronen, E. J., Mah, T.-F., Alarcon, E. I., & Alarcon Bacterial, E. I. (2018). Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon, 4, e01067. https://doi.org/10.1016/j.heliyon.2018. [DOI] [PMC free article] [PubMed]
- Kıvanç S.A. Biofilm forming capacity and antibiotic susceptibility of Staphylococcus spp. with the icaA/icaD/bap genotype isolated from ocular surface of patients with diabetes. Malawi Med. J. 2018;30(4):243. doi: 10.4314/mmj.v30i4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocianova S., Vuong C., Yao Y., Voyich J.M., Fischer E.R., DeLeo F.R., Otto M. Key role of poly-γ-dl-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J. Clin. Investigation. 2005;115(3):688–694. doi: 10.1172/JCI23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K.Y., Villaruz A.E., Zheng Y., He L., Fisher E.L., Nguyen T.H., Ho T.V., Yeh A.J., Joo H.-S., Cheung G.Y.C., Otto M. Role of phenol-soluble modulins in staphylococcus epidermidis biofilm formation and infection of indwelling medical devices. J. Mol. Biol. 2019;431(16):3015–3027. doi: 10.1016/j.jmb.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemiech E., Kiersnowska Z., Michałkiewicz M., Depta A., Marczak M. Nosocomial infections as one of the most important problems of healthcare system. Ann. Agricultural and Environ. Med. 2021;28(3):361–366. doi: 10.26444/aaem/122629. [DOI] [PubMed] [Google Scholar]
- Lewis K. Riddle of biofilm resistance. Antimicrobial Agents and Chemotherapy. 2001;45(4):999. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W.-C., Chung W.-S., Lo Y.-C., Shih W.-H., Chou C.-H., Chen C.-Y., Tu C.-Y., Ho M.-W. Changing epidemiology and prognosis of nosocomial bloodstream infection: A single-center retrospective study in Taiwan. J. Microbiol. Immunol. Infect. 2021 doi: 10.1016/j.jmii.2021.09.015. [DOI] [PubMed] [Google Scholar]
- Maharjan G., Khadka P., Siddhi Shilpakar G., Chapagain G., Dhungana G.R. Catheter-associated urinary tract infection and obstinate biofilm producers. Canadian J. Infect. Diseases and Med. Microbiol. 2018;2018:1–7. doi: 10.1155/2018/7624857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury Fernández S., Mejía Salas H., Velasco V.H. Estudio de las infecciones nosocomiales en el Hospital del Niño “Dr. Ovidio Aliaga Uria. Revista de La Sociedad Boliviana de Pediatría. 2003;42(2):93–96. [Google Scholar]
- Mekni M.A., Bouchami O., Achour W., Ben Hassen A. Strong biofilm production but not adhesion virulence factors can discriminate between invasive and commensal Staphylococcus epidermidis strains. APMIS. 2012;120(8):605–611. doi: 10.1111/j.1600-0463.2012.02877.x. [DOI] [PubMed] [Google Scholar]
- Mermel L.A., Allon M., Bouza E., Craven D.E., Flynn P., O'Grady N.P., Raad I.I., Rijnders B.J.A., Sherertz R.J., Warren D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin. Infect. Diseases. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan L.M., Turnbull L., Katrib M., Osvath S.R., Losa D., Lazenby J.J., Whitchurch C.B. Pseudomonas aeruginosa is capable of natural transformation in biofilms. Microbiology. 2020;166(10):995–1003. doi: 10.1099/mic.0.000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnickova, K., Hola, V., & Ruzicka, F. (2014). Catheter-related infections caused by Pseudomonas aeruginosa : virulence factors involved and their relationships. Pathogens and Disease, 72(2), n/a-n/a. https://doi.org/10.1111/2049-632X.12188. [DOI] [PubMed]
- Oleksy-Wawrzyniak M., Junka A., Brożyna M., Paweł M., Kwiek B., Nowak M., Mączyńska B., Bartoszewicz M. The in vitro ability of klebsiella pneumoniae to form biofilm and the potential of various compounds to eradicate it from urinary catheters. Pathogens. 2021;11(1):42. doi: 10.3390/pathogens11010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares E., Badel-Berchoux S., Provot C., Jaulhac B., Prévost G., Bernardi T., Jehl F. The BioFilm ring test: a rapid method for routine analysis of pseudomonas aeruginosa biofilm formation kinetics. J. Clin. Microbiol. 2016;54(3):657–661. doi: 10.1128/JCM.02938-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival S.L., Kite P., Eastwood K., Murga R., Carr J., Arduino M.J., Donlan R.M. Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect. Control & Hospital Epidemiol. 2005;26(6):515–519. doi: 10.1086/502577. [DOI] [PubMed] [Google Scholar]
- Pereira, P. M. A., Binatti, V. B., Sued, B. P. R., Ramos, J. N., Peixoto, R. S., Simões, C., de Castro, E. A., Duarte, J. L. M. B., Vieira, V. V., Hirata, R., Santos, K. R. N., Mattos-Guaraldi, A. L., & Pereira, J. A. A. (2014). Staphylococcus haemolyticus disseminated among neonates with bacteremia in a neonatal intensive care unit in Rio de Janeiro, Brazil. Diagnostic Microbiol. Infect. Disease, 78(1), 85–92. https://doi.org/10.1016/j.diagmicrobio.2013.06.026. [DOI] [PubMed]
- Petrelli D., Repetto A., D'Ercole S., Rombini S., Ripa S., Prenna M., Vitali L.A. Analysis of meticillin-susceptible and meticillin-resistant biofilm-forming Staphylococcus aureus from catheter infections isolated in a large Italian hospital. J. Med. Microbiol. 2008;57(3):364–372. doi: 10.1099/jmm.0.47621-0. [DOI] [PubMed] [Google Scholar]
- Piechota M., Kot B., Frankowska-Maciejewska A., Grużewska A., Woźniak-Kosek A. Biofilm formation by methicillin-resistant and methicillin-sensitive staphylococcus aureus strains from hospitalized patients in Poland. BioMed Res. Int. 2018;2018:1–7. doi: 10.1155/2018/4657396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto H., Simões M., Borges A. Prevalence and impact of biofilms on bloodstream and urinary tract infections: a systematic review and meta-analysis. Antibiotics. 2021;10(7):1–24. doi: 10.3390/antibiotics10070825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J.N., Souza C., Faria Y.V., da Silva E.C., Veras J.F.C., Baio P.V.P., Seabra S.H., de Oliveira Moreira L., Hirata Júnior R., Mattos-Guaraldi A.L., Vieira V.V. Bloodstream and catheter-related infections due to different clones of multidrug-resistant and biofilm producer Corynebacterium striatum. BMC Infect. Diseases. 2019;19(1):672. doi: 10.1186/s12879-019-4294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Cerdeira C., Martínez-Herrera E., Carnero-Gregorio M., López-Barcenas A., Fabbrocini G., Fida M., El-Samahy M., González-Cespón J.L. Pathogenesis and clinical relevance of candida biofilms in vulvovaginal candidiasis. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.544480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Azevedo N.F., Ivask A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Reports. 2019;9(1):6483. doi: 10.1038/s41598-019-42906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R., Tiwari M., Donelli G., Tiwari V. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9(1):522–554. doi: 10.1080/21505594.2017.1313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhal R., Kataria R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol. Res. 2021;251 doi: 10.1016/j.micres.2021.126829. [DOI] [PubMed] [Google Scholar]
- Senobar Tahaei, S. A., Stájer, A., Barrak, I., Ostorházi, E., Szabó, D., & Gajdács, M. (2021). Correlation between biofilm-formation and the antibiotic resistant phenotype in staphylococcus aureus isolates: a laboratory-based study in hungary and a review of the literature. Infect. Drug Resistance, 14, 1155–1168. https://doi.org/10.2147/IDR.S303992. [DOI] [PMC free article] [PubMed]
- Sharma P., Lahiri K., Kapila K. Conventional and molecular characterization of coagulase-negative staphylococcus in hospital isolates. Indian J. Pathology and Microbiol. 2011;54(1):85. doi: 10.4103/0377-4929.77331. [DOI] [PubMed] [Google Scholar]
- Sobczak A., Klepacka J., Amrom D., Żak I., Kruczek P., Kwinta P. Umbilical catheters as vectors for generalized bacterial infection in premature infants regardless of antibiotic use. J. Med. Microbiol. 2019;68(9):1306–1313. doi: 10.1099/jmm.0.001034. [DOI] [PubMed] [Google Scholar]
- Sohail M., Latif Z. Molecular analysis, biofilm formation, and susceptibility of methicillin-resistant Staphylococcus aureus strains causing community- and health care-associated infections in central venous catheters. Revista Da Sociedade Brasileira de Medicina Tropical. 2018;51(5):603–609. doi: 10.1590/0037-8682-0373-2017. [DOI] [PubMed] [Google Scholar]
- Soumya K.R., Mathew S., Sugathan S., Mathew J., Radhakrishnan E.K. Studies on prevalence of biofilm associated genes and primary observation on sas X gene in clinical isolates of coagulase negative staphylococci (CoNS) APMIS. 2016;124(4):319–326. doi: 10.1111/apm.12510. [DOI] [PubMed] [Google Scholar]
- Souza, C. de, Faria, Y. V., Sant'Anna, L. de O., Viana, V. G., Seabra, S. H., Souza, M. C. de, Vieira, V. V., Hirata JúniorR., MoreiraL. deO., & Mattos-Guaraldi, A. L. de. (2015). Biofilm production by multiresistant Corynebacterium striatumassociated with nosocomial outbreak. Memórias Do Instituto Oswaldo Cruz, 110(2), 242–248. https://doi.org/10.1590/0074-02760140373. [DOI] [PMC free article] [PubMed]
- Souza C.de, Simpson-Louredo L., Mota H.F., Faria Y.V., Cabral F.de O., Colodette S.dos S., Canellas M.E.F.C., Cucinelli A.do E.S., Luna M.das G.de, Santos C.da S., Moreira L.de O., Mattos-Guaraldi A.L. Virulence potential of Corynebacterium striatum towards Caenorhabditis elegans. Antonie van Leeuwenhoek. 2019;112(9):1331–1340. doi: 10.1007/s10482-019-01265-9. [DOI] [PubMed] [Google Scholar]
- Stepanović S., Vuković D., Dakić I., Savić B., Švabić-Vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods. 2000;40(2):175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- Su S., Yin P., Li J., Chen G., Wang Y., Qu D., Li Z., Xue X., Luo X., Li M. In vitro and in vivo anti-biofilm activity of pyran derivative against Staphylococcus aureus and Pseudomonas aeruginosa. J. Infect. Public Health. 2020;13(5):791–799. doi: 10.1016/j.jiph.2019.10.010. [DOI] [PubMed] [Google Scholar]
- Sued, B. P. R., Pereira, P. M. A., Faria, Y. V., Ramos, J. N., Binatti, V. B., Santos, K. R. N. dos, Seabra, S. H., Hirata Júnior, R., Vieira, V. V., Mattos-Guaraldi, A. L., & Pereira, J. A. A. (2017). Sphygmomanometers and thermometers as potential fomites of Staphylococcus haemolyticus: biofilm formation in the presence of antibiotics. Memórias Do Instituto Oswaldo Cruz, 112(3), 188–195. https://doi.org/10.1590/0074-02760160381. [DOI] [PMC free article] [PubMed]
- Suresh M.K., Biswas R., Biswas L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2019;309(1):1–12. doi: 10.1016/j.ijmm.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Tekeli A. Detection of sasX gene and distribution of SCCmec types in invasive and non-invasive coagulase-negative staphylococci. Balkan Med. J. 2021;37(4):215–221. doi: 10.4274/balkanmedj.galenos.2020.2019.8.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczyk-Warunek A., Blicharski T., Blicharski R., Pluta R., Dobrowolski P., Muszyński S., Tomaszewska E., Jabłoński M. Retrospective study of nosocomial infections in the orthopaedic and rehabilitation clinic of the medical university of lublin in the years 2018–2020. J. Clin. Med. 2021;10(14):3179. doi: 10.3390/jcm10143179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhao G., Chao X., Xie L., Wang H. The characteristic of virulence, biofilm and antibiotic resistance of klebsiella pneumoniae. Int. J. Environ. Res. Public Health. 2020;17(17):6278. doi: 10.3390/ijerph17176278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarawsky A.E., Johns S.L., Schuck P., Herr A.B. The biofilm adhesion protein Aap from Staphylococcus epidermidis forms zinc-dependent amyloid fibers. J. Biol. Chem. 2020;295(14):4411–4427. doi: 10.1074/jbc.RA119.010874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C., Wu, Y., Thappeta, K. R. V., Subramanian, J. T. L., Pranantyo, D., Kang, E.-T., Duan, H., Kline, K., & Chan-Park, M. B. (2017). In Vivo anti-biofilm and anti-bacterial non-leachable coating thermally polymerized on cylindrical catheter. ACS Appl. Mater. Interfaces, 9(41), 36269–36280. https://doi.org/10.1021/acsami.7b07053. [DOI] [PubMed]
- Zhou S., Chao X., Fei M., Dai Y., Liu B. Analysis of S. Epidermidis icaA and icaD genes by polymerase chain reaction and slime production: a case control study. BMC Infect. Diseases. 2013;13(1):242. doi: 10.1186/1471-2334-13-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this study are available upon request by contacting the corresponding author A.M.
Data will be made available on request.