Abstract
Introduction
Lung cancer screening (LCS) using low-dose CT has been demonstrated to reduce lung cancer-related mortality in large randomised controlled trials. Moving from trials to practice requires answering practical questions about the level of expertise of CT readers, the need for double reading as in trials and the potential role of artificial intelligence (AI). In addition, most LCS studies have predominantly included male participants with women being under-represented, even though the benefit of screening is greater for them. Thus, this study aims to compare the performance of a single CT reading by general radiologists trained in LCS using AI as a second reader to that of a double reading by expert thoracic radiologists, in a campaign for low-dose CT screening in high-risk women.
Methods and analysis
This observational cohort study will recruit 2400 asymptomatic women aged between 50 and 74 years, current or former smokers with at least a 20 pack-year smoking history, in 4 different French district areas. Assistance with smoking cessation will be offered to current smokers. An initial low-dose CT scan will be performed, with subsequent follow-ups at 1 year and 2 years. The primary objective is to compare CT scan readings by a single LCS-trained, AI-assisted radiologist to that of an expert double reading. The secondary objectives are: to evaluate the performance of AI as a stand-alone reader; the adherence to screening of female participants; the influence on smoking cessation; the psychological consequences of screening; the detection of chronic obstructive pulmonary disease (COPD), coronary artery disease and osteoporosis on low-dose CT scans and the costs incurred by screening.
Ethics and dissemination
Ethics approval was obtained from the Comité de Protection des Personnes Sud-Est 1 (ethics approval number: 2021-A02265-36 with an amendment on 15 July 2022). Trial results will be disseminated at conferences, through relevant patient groups and published in peer-reviewed journals.
Trial registration number
Keywords: Adult oncology, Respiratory tract tumours, Chest imaging, Computed tomography, Diagnostic radiology, Clinical trials
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The CASCADE study will answer important preliminary questions by exploring practical methods for CT readings before an organised large-scale lung cancer screening is implemented.
The study will validate the single reading of low-dose CT scans by non-expert radiologists trained in lung cancer screening.
The study will provide a prospective evaluation of artificial intelligence in lung cancer screening based on current low-dose CT technology.
The results of this study regarding adherence to screening, its psychological consequences and its effect on smoking cessation will be based only on French participants, with the limitation that the results may not be generalisable to other countries.
Due to the nature of the study design, missing data are expected in some patients.
Introduction
Background and rationale
Lung cancer is the leading cause of cancer death worldwide.1 Less common than breast cancer, it has been the main cause of cancer death in women in the USA since 1987. This was not observed in France, because the incidence of smoking started later in the female population. However, the epidemiology of female lung cancer is extremely worrying in France as is also the case in Spain.2 Lung cancer incidence and mortality in French women showed an average increase of 5% and 3% per year, respectively, during the period from 2010 to 2018.3 With an equivalent smoking history, the risk of developing lung cancer is 1.2–1.7 times higher in women than in men.4 The results of the French KBP 2020 study conducted in 82 general hospitals which included 8999 patients, were presented in early 2022. The proportion of women among lung cancer patients increased from 16% in 2000 to 34.6% in 2020, and in patients younger than 50 years, it increased to 41%.5 When diagnosed based on symptoms, 80% of patients have advanced lung cancer and are not eligible for surgical treatment, resulting in poor long-term survival.6 Screening with low-dose CT can detect lung cancer at earlier stages, thereby reducing lung cancer-related mortality in the screened population. In 2011, the National Lung Cancer Screening Trial (NLST) reported a 20% reduction in lung cancer-related mortality in the screened arm, at the cost of a high false positive rate.7 In 2020, the NELSON study reported a 26% and 33% reduction in lung cancer deaths at 10 years in male and female participants, respectively, as compared with controls.8 The overall referral rate for suspicious nodules was only 2.1% in this study, which adopted an efficient nodule management strategy based on volumetry and volumetric growth estimation for indeterminate nodules. The Multicentric Italian Lung Detection study also reported a reduction in lung cancer-related mortality of 39% in the screened arm.9 The UKLS and LUSI trials also demonstrated a reduction in lung cancer mortality through screening, despite this being significant only in women in the LUSI trial.10 11
While the medical benefit of screening is well established, the practicalities of its implementation still need to be evaluated, hence the need for implementation research programmes.12 13
Most lung cancer screening (LCS) studies are based on double reading,8 11 13–18 with the exception of the NLST which involved only one expert for the reading. It is estimated that the number of individuals eligible forLCS in France varies between 2.5 and 3.7 million, depending on the inclusion criteria. Training radiographers is not an option as their performance is lower than that of experienced radiologists.19 There are not enough expert thoracic radiologists for this task, especially if double reading is required, thus making it necessary to train general radiologists in LCS. Moreover, none of the LCS studies mentioned above, evaluated the role of artificial intelligence (AI) in screening. An ancillary study of 400 randomly selected CT exams in the NELSON trial reported a superior performance of computer-assisted lung nodule detection compared with double reading by radiologists, at the cost of 3.7 false positives per exam.20 The development of modern algorithms based on deep learning could solve this problem.21–24 Google engineers claimed to have developed a programme capable of diagnosing lung cancer with a performance superior to that of human doctors.21 However, their algorithm was trained on NLST data, not on current CT technology, which uses iterative image reconstruction or deep learning. Finally, most LCS studies have primarily included male participants, with women being under-represented, leading the authors of the NELSON trial to conclude that further research is needed in this subgroup.8
Objectives
Main objective: The main objective of the CASCADE study is to compare the performance of a single general radiologist trained in LCS using AI as a second reader with that of the reference standard (a double reading by expert thoracic radiologists), in a campaign for low-dose CT screening in high-risk women.
Hypothesis: a single reading of the CT scans by a general radiologist, trained in screening and assisted by an AI algorithm, which plays the role of a second reader, should have a performance comparable to that of a double reading by experts.
Secondary objectives: to evaluate:
The performance of AI as a stand-alone reader.
The screening adherence according to the different modes of invitation.
The influence of screening on smoking cessation.
The detection of three comorbidities with smoking as the causative or additional risk factor: COPD, coronary artery disease and osteoporosis.
The psychological consequences of screening.
The costs incurred by screening.
Methods
Participants, interventions and outcomes
Trial design: prospective cohort study.The study protocol is consistent with the recommendations of the European position statement on LCS, which states that individuals participating in screening programmes should be informed about the benefits and harms of screening, smoking cessation should be offered to all current smokers, and the management of solid nodules should involve semiautomatically measured volume and volume doubling time.25
We followed the recommendations of the STROBE (Strengthening The Reporting of OBservational Studies in Epidemiology) checklist.26
Study setting
The study will be conducted in four French cities, namely Paris, Rennes, Béthune and Grenoble, which represent different socioeconomic profiles. It will then be disseminated in neighbouring areas. The recruitment centres will be a university hospital in Paris and community clinics for the other three cities.
Inclusion and exclusion criteria for participants.
Inclusion criteria
Women aged 50–74 years.
Having at least 20 pack-year smoking history.
Current or former smokers who have no quit for more than 15 years.
Having given their consent and understood the need for a 2-year follow-up.
Affiliated to social security.
Exclusion criteria
Presence of clinical symptoms suggestive of malignancy (weight loss, haemoptysis) or ongoing infection (febrile cough, expectoration).
Cancer within the previous 2 years.
History of lung cancer.
Follow-up at 2 years is impossible.
Chest CT scan in the previous 2 years.
Eligibility criteria for individuals/study centres who will perform the interventions
Pulmonologists: trained in the ‘5 As’ strategy for smoking cessation.
Onsite general radiologists (first readers): trained in LCS according to the European Society of Thoracic Imaging (ESTI) LCS certification programme, available at https://www.myesti.org/lungcancerscreeningcertificationproject/https://www.myesti.org/lungcancerscreeningcertificationproject/.
Study centres: equipped with an artificial solution for lung nodule detection (Veye Lung Nodules, V.3.9.2, Aidence, Amsterdam, the Netherlands) and fulfilling the technical requirements by performing a test CT scan on a phantom.
Interventions
Low-dose CT scans performed at inclusion then at 1-year and 2-year follow-ups.
An additional CT scan will be needed if one of the three previously listed CT scan results is indeterminate. All CT examinations will be performed according to the technical recommendations of the ESTI, available at https://www.myesti.org/content-esti/uploads/ESTI-LCS-technical-standards_2019-06-14.pdf.
CT scan reading modalities: general radiologist first without the use of AI, then with the use of AI as well as two independent expert thoracic radiologists.
Consultation with a pulmonologist at the inclusion visit and then at the end of the study participation, as well as in the event of an indeterminate CT scan result, after the additional CT scan.
The inclusion visit will be carried out by a pulmonologist who will:
Provide information on the methods, risks and benefits of screening presented in an information leaflet.
Check eligibility.
Offer help with smoking cessation via a tobacco dependence questionnaire (CDS, Cigarette Dependence Scale) followed by a discussion on the benefits of cessation and its methods. A prescription for nicotine substitutes will be offered. The follow-up of this care will be conducted by telephone interviews with a nurse specialised in smoking cessation. Participants who request this will be referred to a specialised smoking cessation consultation.
Look for signs suggestive of COPD according to the six-question COPD test available on the French national social health insurance (CNAM) website (https://www.ameli.fr/assure/sante/themes/bpco/symptomes-diagnosticcomplications). In the event of a positive score, the result will be communicated to the participant and her attending physician, who will consider performing spirometry.
Explain that a visual quantification of the coronary artery calcium score and a search for thoracic vertebral fractures related to osteoporosis will be performed during the CT reading. The results will be communicated to the participant and her attending physician for management.
Questionnaires: The Hospital Anxiety and Depression Scale (HADS) questionnaire will be completed after each CT scan. The Cancer worry scale and Satisfaction with Decision scale questionnaires will be completed at the inclusion and end of study visits. The CDS questionnaire for current smokers will be completed at the inclusion visit.
Management of study participants
The management of study participants will be based on the consensus of the double expert reading. The criteria for positive, negative and indeterminate screen results can be found in online supplemental appendix 1. In summary, solid nodules with a volume of less than 100 mm3 at baseline are considered a negative screen result, according to Horeweg et al.27 For a positive screen result, the CASCADE scientific committee considered and adopted the initial threshold volume of 500 mm3, which was used in the NELSON trial in order to avoid increasing the recall rate.
bmjopen-2022-067263supp001.pdf (1.6MB, pdf)
Outcomes
Main outcome
To demonstrate that the reading of CT scans by a general radiologist trained in screening, assisted by detection software, has a similar performance to that of expert double reading, using the NELSON study as a reference.
Main outcome measure
Diagnostic performance (sensitivity, specificity, predictive values and likelihood ratios) of initial readings aided by detection software. The reference standard will be the pathological report for the positive screen results and for the negative screen results, a 2-year follow-up demonstrating stability or absence of nodules on CT.
Secondary outcomes
Effectiveness of screening.
Diagnostic performance of reading without AI as the second reader, in order to assess its additional value.
Diagnostic performance of AI as a stand-alone reader.
Agreement of the different readings.
Adherence to screening.
Impact of screening on smoking cessation.
Psychological impact of screening.
Number of comorbidities (COPD, coronary heart disease) diagnosed.
Evaluation of the costs incurred by screening.
Prevalence of osteoporosis in opportunistic screening.
Secondary outcome measures
Proportion of participants with a positive screen result and the proportion of cancers confirmed.
Sensitivity, specificity, predictive values and likelihood ratios of reading without AI.
Sensitivity, specificity, predictive values and likelihood ratios of AI as stand-alone reader.
Kappa coefficient between the different readings.
Number of participants compared with the number of eligible women, having all three CT scans, time needed to include the target number of participants.
Proportion who quit smoking at the end of the study.
Cancer worry scale, Satisfaction with Decision scale, HADS questionnaires translated into French.
Number of participants in relation to the number of women included, in whom treatment is started.
Total cost, average cost per woman, cost per case detected.
Presence of at least one thoracic vertebral fracture or an attenuation value for the T8 vertebral body measuring less than 100 Hounsfield units.
Participant timeline
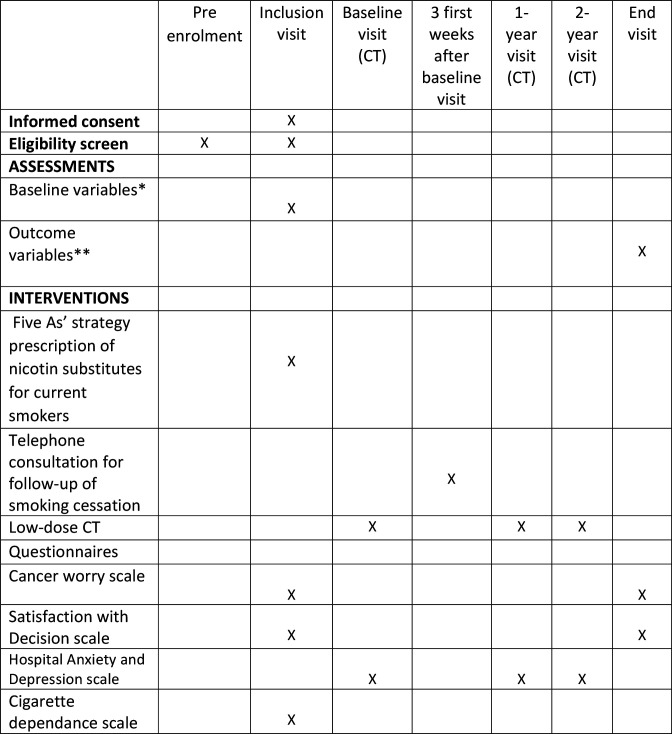
A timeline of the enrolment process, study visits, interventions and assessments performed on participants is presented in figure 1.
Figure 1.
Participant timeline. * List of collected baseline variables: Age of smoking onset, date of cessation, number of cigarettes per day, study level, family history of lung cancer, previously diagnosed coronary artery disease or osteoporosis, status in relation to other cancer screenings: breast, cervix, colon, How information about the study reached them. **List of collected outcome variables: Duration of smoking cessation, COPD confirmed by spirometry, coronary artery disease confirmed and treatment initiated (medical treatment or revascularisation), osteoporosis confirmed by additional densitometry, initiation of antiosteoporosis treatment, completion of the other recommended screenings. COPD, chronic obstructive pulmonary disease.
Sample size
The objective is to confirm a diagnostic performance comparable to that of the Nelson study after three CT scans.8 The recruitment of 2400 women over 2 years will allow us to estimate a positive predictive value of 43.5% with a 95% CI of (29.5% to 56.7%) as well as a rate of positive scans (true and false positives) of 2.1% (51/2400 women) with a 95% CI of (1.6% to 2.7%). The expected cancer rate at 2 years (0.9%, ie, 22/2400 women) can be estimated with a 95% CI of (0.5% to 1.3%).
Recruitment
The participants will be recruited through social networks (facebook, twitter …), as well as through communications via town halls, regional print and television media, with the following announcement approved by the ethics committee:
‘You are a female smoker or ex-smoker between 50 and 74 years old. You can participate in a lung cancer screening study in women by calling the following number: 06 15 06 58 35 Monday to Friday between 9 a.m. and 5 p.m. You can also contact us by email: cascade.cch@aphp.fr. Your eligibility criteria will be checked during the first telephone contact. If you are eligible, you will then be offered a consultation appointment with a pulmonologist to screen for the various tobacco-related pathologies’.
The same leaflet will be included in the invitation letter to breast cancer screening in the four participating French regions, which will be sent by the Regional Cancer Screening Coordination Centres (Centres Régionaux de Coordination du Dépistage des Cancers).
A web page is accessible for participants, containing a summary of the study, the information leaflet, as well as a short video presentation of the study(https://www.aphp.fr/actualite/depistage-du-cancer-du-poumon-par-scanner-faible-dose-lap-hp-lance-letude-pilote-cascade)
The total number of eligible women in the 4 participating French regions is 39 094. The inclusion target of 2400 women corresponds to 6% of the eligible population.
Patient and public involvement
The project is motivated by previous experiences with patients and discussions with patient associations. Lung Cancer Europe a lung cancer patient advocacy group expressed its support for this study, estimating that the study will evaluate essential preliminary questions before large-scale lung screening is considered. The project places the patient at the centre of the research process, by evaluating the patient’s satisfaction with their decision and the psychological impact of the screening at different study time points.
Methods: data collection, management and analysis
Data collection methods
Clinical data will be collected in each centre during the inclusion and end visits by the investigator or by a clinical research technician, supervised by the investigator. Deidentified data will be collected on an electronic form, using the cleanweb software.
Reminders by telephone, post and email will be used to schedule appointments in order to collect the data from all participants. If the participant is lost to follow-up, the contact details of the participants’ general practitioner will be used in order to collect the information of a cancer diagnosis at 2 years.
Anonymised CT images and AI reports will be transferred via secure connections to a dedicated Picture Archiving and Communicating System (SPHERE CASCADE), developed for the study. Expert readers will access CT images, but not AI reports via a secure encrypted connection, using a CE marked DICOM viewer allowing nodule segmentation and volume doubling time measurement (Veolity Lung Screening 1.7, MeVis Medical Solutions AG, Bremen, Germany).
Data management
The coordinating centre (URC Cochin) will be responsible for the development of the electronic file, and they will ensure that the data are well collected.
Statistical analysis
The statistical analysis will be carried out at Cochin Hospital Clinical Research Unit using R and/or SAS software V.9.3. A statistical analysis plan will be produced and validated by the study steering committee before freezing and analysing the data. Data analysis and reporting will follow the STARD statement recommendations (http://www.equator-network.org).
The analysis will be carried out on all the participants included in the protocol.
Quantitative variables will be described as mean and SD or median and IQRs depending on the data distribution. Qualitative variables will be described as numbers and percentages.
Diagnostic performance (sensitivity, specificity, negative and positive predictive values, positive and negative likelihood ratios) will be calculated as usual. The proportion of women with a positive CT scan and the 2 year cancer rate for the entire screened population will be estimated with their 95% CIs using the exact binomial law.
The definition used for the presence or absence of cancer is as follows:
Lung cancer: positive histology result.
Absence of cancer: absence of nodule, or stability at 2 years, or negative histology result.
In cases of persistent missing data regarding the main outcome (the information of cancer diagnosis at 2 years), multiple imputations with chained equations will be applied using the MICE package of the R statistical software.
Agreement between the different readings will be analysed using the kappa coefficient, provided with its 95% CI.
The false positives and false negatives for each reading will be calculated using the above definition of lung cancer. The analysis of other endpoints will be mainly based on descriptive statistical methods.
Cost analysis
The cost analysis is based on a non-comparative study undertaken from a health system and payer perspective over a 2-year time time frame. One expected outcome of the cost analysis is to advise at the national level on the need for the use of AI in LCS. The other reported cost data include the average screening costs with scenario analyses on screening uptake, the costs per cancer detected and the costs associated with the workup of thoracic lesions detected by screening. These will be collected prospectively at the participant level only via the study case report form, administrative data will not be queried, partly due to regulatory difficulties but mainly because it cannot differentiate workup/cancer costs from other costs. Screening programme costs include:
The fixed costs of screening invitation such as those involved if the programme is implemented (printing invitation letters and additional postage costs), retrieved from the billing systems of the regional cancer screening organisations.
The costs of the CT scan: we will use the social health insurance tariffs for the price of the most recent type of equipment, to which the radiologist fees will be added.
The cost of the AI solution is the purchase price, annual volume estimates are subjected to scenario analyses.
In the event of a positive or indeterminate result, or an incidental finding, we will estimate the healthcare costs for the following 2 years. Consultations and examinations (additional CT scan, biopsies, coronary angiography, bone densitometry and generally any assessment directly attributable to the results of the initial scan) will be valued by taking into account the social health insurance tariffs, hospital admissions (inpatient and outpatient) from the most recent national cost study.
The total fixed and variable cost of the 2-year screening programme will be estimated with and without AI, including all downstream healthcare costs. We will calculate the average cost per participating woman, the average cost per lung cancer detected and the average cost per any relevant finding.
Methods: monitoring
Steering committee
The CASCADE study steering committee will have the overall responsibility for trial oversight, monitoring trial progress and protocol adherence.
Data monitoring
Data monitoring will be performed by research technicians who will alert the investigators by email in cases of missing data on the electronic report file.
A data monitoring committee comprising a statistician and two methodologists will perform an interim analysis halfway through the inclusions. They will review the initial statistical assumptions, regarding the prevalence of lung cancer and the performance of initial readings, especially the rates of positive and indeterminate CT scans, in order to have low confidence intervals when calculating positive predictive values.
Harms
Screening can be anxiety-provoking, especially since the participants will not have immediate results, due to a double reading being necessary. Anxiety will be evaluated at each CT scan using the HADS questionnaire. Performing an additional CT scan in the event of an indeterminate result is also a potential source of stress, and the participants will be forewarned of this possibility, as this concerned 9% of the NELSON trial participants.8
Auditing
An audit may be carried out at any time by persons appointed by the sponsor and it is independent of the investigators. Its objective is to ensure the quality of research, the validity of its results and compliance with the law and regulations in force.
Ethics and dissemination
Research ethics approval
The study protocol and the informed consent form template contained in the appendices have been approved by the Comité de Protection des Personnes Sud-Est 1. Any modifications to the protocol which may impact on the conduct of the study will be submitted to this committee for its approval and subsequently communicated to the relevant parties.
Consent
Informed consent will be obtained from the trial participants during the inclusion visit with the pulmonologist. The sponsor will ensure that each person who takes part in the research has given their written consent for access to their individual data.
Confidentiality
During the research and at its end, the data collected on the participants will be deidentified/anonymised. Only the initials of the family name and first name will be recorded, accompanied by a coded number specific to the research indicating the order of subject inclusion.
Declaration of interests
The investigators have no financial and other competing interests
Access to data
The data will be kept within the clinical research unit Unité de Recherche Clinique (URC) of Cochin Hospital.
Data access requests must be approved by the ethics committee, the CASCADE scientific committee and the sponsor APHP.
Dissemination
The study results will be disseminated at relevant conferences and societies, published in peer-reviewed journals without intervention of professional writers. It will also be disseminated through relevant patient groups. Authorship will be according to the International Committee of Medical Journal Editors guidelines.
Trial status
Recruitment started on 8 April 2022 and is expected to end in April 2024.
Supplementary Material
Acknowledgments
We would like to thank the Regional Cancer Screening Coordination Centers for their collaboration (Dr J Nicolet CRCDC-IDF, Dr Forzy CRCDC-HDF, Dr Exbrayat CRCDC-AURA, Dr E Robert CRCDC-BRETAGNE)
Footnotes
Twitter: @revelmp
Contributors: M-PR, HA, MW and ID-Z constructed the protocol and design. M-PR made the first draft of this manuscript. HA contributed with statistical advice and study design. M-PR, HA, MW, GC, EC and ID-Z contributed with a thorough evaluation of the design, method and manuscript. All authors accepted the final manuscript version.
Funding: This work was supported by Institut National du Cancer grant number INCA_14771 and by the French Ministry of Health financement dérogatoire SERI 2020
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol 2015;1:505. 10.1001/jamaoncol.2015.0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi F, Bosetti C, Fernandez E, et al. Trends in lung cancer among young European women: the rising epidemic in France and Spain. Int J Cancer 2007;121:462–5. 10.1002/ijc.22694 [DOI] [PubMed] [Google Scholar]
- 3.Pujol J-L, Thomas P-A, Giraud P, et al. Lung cancer in France. J Thorac Oncol 2021;16:21–9. 10.1016/j.jtho.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 4.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996;88:183–92. 10.1093/jnci/88.3-4.183 [DOI] [PubMed] [Google Scholar]
- 5.Debieuvre D, Molinier O, Falchero L, et al. Lung cancer trends and tumor characteristic changes over 20 years (2000-2020): results of three French consecutive nationwide prospective cohorts' studies. Lancet Reg Health Eur 2022;22:100492. 10.1016/j.lanepe.2022.100492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar J, Urban D, Amit U, et al. Long-term survival of patients with metastatic non-small-cell lung cancer over five decades. J Oncol 2021;2021:1–10. 10.1155/2021/7836264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–13. 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 9.Pastorino U, Silva M, Sestini S, et al. Prolonged lung cancer screening reduced 10-year mortality in the mild trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019;30:1672. 10.1093/annonc/mdz169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field JK, Vulkan D, Davies MPA, et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg Health Eur 2021;10:100179. 10.1016/j.lanepe.2021.100179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening-Results from the randomized German LUSI trial. Int J Cancer 2020;146:1503–13. 10.1002/ijc.32486 [DOI] [PubMed] [Google Scholar]
- 12.Martini K, Chassagnon G, Frauenfelder T, et al. Ongoing challenges in implementation of lung cancer screening. Transl Lung Cancer Res 2021;10:2347–55. 10.21037/tlcr-2021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field JK, deKoning H, Oudkerk M, et al. Implementation of lung cancer screening in Europe: challenges and potential solutions: summary of a multidisciplinary roundtable discussion. ESMO Open 2019;4:e000577. 10.1136/esmoopen-2019-000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field JK, Duffy SW, Baldwin DR, et al. The UK lung cancer screening trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess 2016;20:1–146. 10.3310/hta20400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes Pegna A, Picozzi G, Mascalchi M, et al. Design, recruitment and baseline results of the Italung trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34–40. 10.1016/j.lungcan.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 16.Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial--overall design and results of the prevalence round. J Thorac Oncol 2009;4:608–14. 10.1097/JTO.0b013e3181a0d98f [DOI] [PubMed] [Google Scholar]
- 17.Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT. Lung Cancer 2008;59:355–63. 10.1016/j.lungcan.2007.08.040 [DOI] [PubMed] [Google Scholar]
- 18.Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the mild trial. Eur J Cancer Prev 2012;21:308–15. 10.1097/CEJ.0b013e328351e1b6 [DOI] [PubMed] [Google Scholar]
- 19.Nair A, Gartland N, Barton B, et al. Comparing the performance of trained radiographers against experienced radiologists in the UK lung cancer screening (UKLS) trial. Br J Radiol 2016;89:20160301. 10.1259/bjr.20160301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, de Bock GH, Vliegenthart R, et al. Performance of computer-aided detection of pulmonary nodules in low-dose CT: comparison with double reading by nodule volume. Eur Radiol 2012;22:2076–84. 10.1007/s00330-012-2437-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med 2019. 10.1038/s41591-019-0447-x [DOI] [PubMed] [Google Scholar]
- 22.Nasrullah N, Sang J, Alam MS, et al. Automated lung nodule detection and classification using deep learning combined with multiple strategies. Sensors 2019;19:3722. 10.3390/s19173722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trajanovski S, Mavroeidis D, Swisher CL, et al. Towards radiologist-level cancer risk assessment in CT lung screening using deep learning. Comput Med Imaging Graph 2021;90:101883. 10.1016/j.compmedimag.2021.101883 [DOI] [PubMed] [Google Scholar]
- 24.Mastouri R, Khlifa N, Neji H, et al. Deep learning-based CAD schemes for the detection and classification of lung nodules from CT images: a survey. J Xray Sci Technol 2020;28:591–617. 10.3233/XST-200660 [DOI] [PubMed] [Google Scholar]
- 25.Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754–66. 10.1016/S1470-2045(17)30861-6 [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 27.Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the Nelson trial of low-dose CT screening. Lancet Oncol 2014;15:1332–41. 10.1016/S1470-2045(14)70389-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067263supp001.pdf (1.6MB, pdf)