Abstract

The heat shock protein 90 (Hsp90) family of molecular chaperones mediates the folding and activation of client proteins associated with all 10 hallmarks of cancer. Herein, the design, synthesis, and biological validation of Hsp90α-selective inhibitors that contain a tertiary alcohol are reported. Forty-one analogues were synthesized to modulate hydrogen-bonding interactions and to probe for steric and hydrophobic interactions within the Hsp90α binding site. Cocrystal structures of lead compound 23d (IC50 = 0.25 μM, 15-fold selective vs Hsp90β) and a 5-fluoroisoindoline derivative (KUNA-111) revealed a novel binding mode that induced conformational changes within Hsp90α’s N-terminal domain. The lead Hsp90α-selective inhibitors did not manifest significant antiproliferative activity, but they did result in selective and dose-dependent degradation of Hsp90α clients in the cellular environment. Additional studies will be sought to determine the effects of the novel conformational change induced by 23d.
Keywords: Hsp90α, isoform selectivity, drug discovery, structure-based drug design, cancer
Heat shock proteins are highly conserved and ubiquitous molecular chaperones that play a variety of roles to maintain cellular proteostasis through the folding, activation, and degradation of client proteins necessary for cellular growth, survival, and differentiation.1 In response to cellular stress, members of the 90 kDa heat shock protein (Hsp90) family become highly induced and contribute to oncogenesis via the maturation of client proteins associated with all 10 hallmarks of cancer.2,3 As a result, cancer cells become dependent upon the upregulated Hsp90 heteroprotein complex, which exhibits ∼200-fold higher affinity for ATP than the Hsp90 homodimer found in normal tissue. In addition, Hsp90 binds ATP in a unique and bent conformation, which allows for the development of selective small-molecule ATP-competitive inhibitors. To date, 18 small-molecule Hsp90 pan-inhibitors have entered clinical trials for the treatment of cancer. Unfortunately, the inhibition of all four Hsp90 isoforms has led to dosing issues and toxicities, resulting in the clinical failure of most candidates.
As an alternative strategy, recent work has aimed to develop Hsp90-isoform-selective inhibitors to avoid complications associated with pan-inhibition. The four Hsp90 isoforms are highly conserved despite their separate cellular localization, function, and client proteins: Hsp90α and Hsp90β reside in the cytosol, whereas glucose-regulated protein 94 (Grp94) is localized in the endoplasmic reticulum and tumor necrosis factor receptor-associated protein 1 (Trap1) resides in the mitochondria. In contrast to the constitutively expressed cytosolic isoform Hsp90β, Hsp90α expression is inducible and exhibits differential expression, function, and regulation.4,5 As a stress-induced chaperone, Hsp90α-dependent client proteins are primarily involved in cell signaling pathways that are responsive to environmental stimuli, including cell receptors, protein kinases, and transcription factors.
In addition to its cytosolic localization, Hsp90α is secreted extracellularly (eHsp90α) to modulate wound healing and inflammation.6 eHsp90α’s interaction with cell-surface receptors such as human epidermal growth factor receptor (HER2) and extracellular clients such as matrix metalloproteinase-2 (MMP-2) are responsible for eHsp90α-regulated cell signaling, activation of the epithelial to mesenchymal transition (EMT), and induction of an aggressive and invasive phenotype.6−9 Increased expression of Hsp90α is directly correlated with cancer invasiveness and severity in several cancers, implicating Hsp90α as a biomarker for cancer diagnosis.10,11 Furthermore, high plasma levels of Hsp90α have been recognized as a clinically relevant cancer biomarker.12,13 Genetic knockdown of Hsp90α results in the degradation of oncogenic client proteins, suggesting that the administration of Hsp90α-selective inhibitors could disrupt the progression of cancers that are driven by Hsp90α-dependent substrates.14
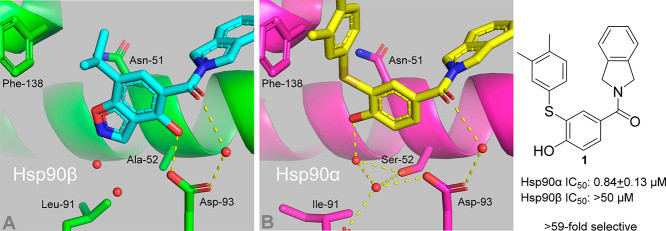
The development of inhibitors that exhibit isoform selectivity between the two cytosolic isoforms Hsp90α and Hsp90β has been difficult since they exhibit >95% identity within the N-terminal ATP binding sites, differing by only two amino acids: Ala52/Leu91 in Hsp90β (Figure 1A) and Ser52/Ile91 in Hsp90α (Figure 1B). Although there is only a two amino acid difference between the Hsp90α and Hsp90β nucleotide-binding sites, previous work led to the identification of the first Hsp90α-selective inhibitor and subsequent characterization of its binding mode, as can be seen in Figure 1B.15
Figure 1.
Two amino acid difference between Hsp90β and Hsp90α. (A) Cocrystal structure of the Hsp90β-selective inhibitor KUNB-31 bound to the N-terminal domain of Hsp90β (PDB code 5UCJ). (B) Cocrystal structure of Hsp90α-selective inhibitor 1 bound to the N-terminal domain of Hsp90α (PDB code 6PJS).
The subtle differences between Ser52/Ala52 in Hsp90α/Hsp90β were exploited via a phenol that retains the moiety needed to interact directly with Asp93 and Ser52 via a water-mediated hydrogen-bonding network (Figure 1B).14 As previously described, removal of the 2-phenol resulted in ∼11-fold selectivity for Hsp90α versus Hsp90β at the expense of decreased affinity, which served as the starting point for Hsp90α-selective inhibitors.14 Analysis of the cocrystal structure of 1 bound to Hsp90α suggested that selectivity for Hsp90α is also dependent upon steric bulk, which cannot be easily accommodated by Hsp90β. Therefore, this strategy was applied to the previously identified small molecule 2 that contained a tertiary alcohol in lieu of the thioether and ultimately displayed comparable selectivity and greater affinity for Hsp90α compared to 1.14
Overlay of compound 1 and compound 2 revealed the potential for hydrogen-bonding interactions between the tertiary alcohol of 2 and Asn51 (Figure 2). Therefore, functionalities were incorporated to determine the role played by the alcohol during Hsp90α binding. Modifications included the introduction of a nitrile or azide group, as shown in compounds 3 and 4, respectively, which were proposed to interact with Asn51 via a hydrogen-bond acceptor. In contrast, an amine was introduced as shown in 5 to determine whether ionic interactions with Hsp90 could be established. Phenolic ether 6 was proposed to interact with Phe138 via π–π interactions in a manner similar to 1. Molecular docking of alcohol 2 to the binding site suggested that methyl ether 7 could be accommodated in lieu of the alcohol, which differentiates the role played by this moiety as a hydrogen-bond acceptor or donor.
Figure 2.

Proposed binding mode of compound 2, highlighting the possible 2.1 Å hydrogen bond between Asn-51 and the tertiary alcohol.
The synthesis of nitrile 3 was achieved by treating alcohol 2 with trimethylsilyl cyanide in the presence of boron trifluoride diethyl etherate (Scheme 1). Similarly, 4 was produced upon exposure of 2 to sodium azide. Upon hydrogenation, 4 was reduced to amine 5. In contrast, phenol readily reacted with 2 in the presence of trifluoroacetic acid to produce ether 6, while 7 required the treatment of 2 with ceric ammonium nitrate.
Scheme 1. Synthesis of 3–7.
Conditions: (a) TMSCN, BF3·Et2O, 55%; (b) NaN3, BF3·Et2O, 65%; (c) Pd/C, HCOONH4, ethanol, 80 °C, 68%; (d) phenol, TFA/CH2Cl2 (1:9), 40 °C, 25%; (e) methanol, ceric ammonium nitrate, 80 °C, 50%.
All of the newly synthesized compounds were evaluated by a fluorescence polarization (FP) assay to determine the Hsp90α affinity and selectivity over Hsp90β. The FP assay results (Table 1) revealed that the nitrile can effectively replace the alcohol present in 2, as the binding affinities manifested by 3 and 2 were comparable. These data suggest that the tertiary alcohol of 2 interacts with Asn51 via a hydrogen-bond acceptor. In line with the proposed hypothesis, the azide-containing compound 4 exhibited an IC50 of ∼2.74 μM for Hsp90α, which indicated that the azide may also interact with Asn51, although the size and linearity of the azido group may produce a steric clash. Amine 5 exhibited a complete loss of affinity. Surprisingly, the methoxy-containing analogue 7 did not bind well (IC50 ≈ 36 μM). The reduced affinity exhibited by 7 may result from increased steric interactions between the pocket and the methoxy group. Similarly, the phenoxy-containing compound 6 also manifested a loss in Hsp90α binding affinity. Therefore, the alcohol was determined to be optimal for Hsp90α binding since the other functionalities did not improve the affinity or selectivity over 2.
Table 1. Binding Affinities and Selectivities for Hsp90α > Hsp90β Obtained by FP Assay.
| IC50 (μM)a |
|||
|---|---|---|---|
| compd | Hsp90α | Hsp90β | fold selectivity |
| 3 | 0.51 ± 0.01 | 5.10 ± 0.11 | ∼10 |
| 4 | 2.74 ± 0.20 | 14.91 ± 0.11 | ∼5 |
| 5 | >50 | >50 | NSb |
| 6 | >50 | >50 | NS |
| 7 | 36.00 ± 1.56 | >50 | >1 |
IC50 values are reported as the mean of triplicates ± SD.
NS = nonselective.
The gem-dimethyl group in 2 was modified to investigate the pocket surrounding the alcohol. The extension of both methyl groups to form diethyl compound 11 was pursued to explore the mutual exclusivity of the pocket. Diol 14 was prepared to investigate whether hydrogen-bonding interactions within the binding site could be enhanced. In addition, a sec-butyl group was introduced (16) to probe chain elongation and branching, while the trifluoromethyl moiety of 18 explored the potential for additional interactions.
Commercially available acid 8 was protected as benzyl ether 9 upon hydrolysis of the ester (Scheme 2). Lithium–halogen exchange of 9 with n-butyllithium followed by the addition of 3-pentanone led to 10. An isoindoline side chain was installed onto the acid moiety of 10, followed by benzyl deprotection to provide 11. Preparation of 14 began with an EDC coupling between 9 and isoindoline to produce intermediate 12. A palladium-catalyzed cross-coupling reaction between bromophenol 12 and potassium (E,Z)-2-butenyl trifluoroborate followed by benzyl removal produced 13. Dihydroxylation of intermediate 13 was performed to give 14. Monophenol 15 was treated with ethylmagnesium bromide to produce tertiary alcohol 16 (Scheme 3). Installation of the trifluoromethyl group required benzyl protection of 15 to yield 17, which was then treated with trifluoromethyltrimethylsilane and tetrabutylammonium fluoride to give 18.
Scheme 2. Synthesis of 11 and 14.
Conditions: (a) BnBr, K2CO3; (b) aq. NaOH, 95%; (c) n-BuLi, 3-pentanone, 15%; (d) EDCI·HCl, HOBt, DIPEA, 45%; (e) BCl3, 70%; (f) potassium isopentyltrifluoroborate, Pd(PPh3)4, Cs2CO3; (g) OsO4, N-methylmorpholine-N-oxide, 67%.
Scheme 3. Synthesis of 16 and 18.
Conditions: (a) ethylmagnesium bromide, 74%; (b) BnBr, K2CO3, 84%; (c) TMSCF3, TBAF, (d) BCl3, 45%.
Replacement of the dimethyl group in 2 with a diethyl moiety (11) led to decreased binding affinity (Table 2). Diol 14 did not manifest an improvement in binding affinity but instead exhibited a 10-fold decrease in IC50 value. Introduction of an ethyl group (16) resulted in increased binding affinity, which suggests that substitutions can be tolerated at one location but not both. The trifluoromethyl group (18) manifested a decrease in affinity, suggesting that fluorines adjacent to the tertiary alcohol were not well-tolerated. As a result of these studies, it was determined that hydrophobic groups proximal to the alcohol were tolerated but steric bulk and additional hydrogen-bonding interactions were not favored.
Table 2. Binding Affinities and Selectivities for Hsp90α > Hsp90β Obtained by FP Assay.
| IC50 (μM)a |
|||
|---|---|---|---|
| compd | Hsp90α | Hsp90β | fold selectivity |
| 11 | 37.87 ± 1.24 | >50 | >1 |
| 14 | 6.15 ± 0.76 | >50 | >8 |
| 16 | 0.478 ± 0.010 | 5.23 ± 0.02 | ∼11 |
| 18 | 36.00 ± 1.56 | >50 | >1 |
IC50 values are reported as the mean of triplicates ± SD.
The cocrystal structures revealed that 1 induced a conformational change in the Hsp90α N-terminal ATP binding site that resulted in a large open Site 1 (Figure 3B) compared to the closed Site 1 that results upon occupation with the pan-inhibitor AT13387 (Figure 3A). The binding mode of 1 suggests that the alkyl side chain projects toward a solvent-accessible channel to displace water molecules, resulting in a reduced dielectric constant within the binding site. The increased binding affinity observed with 16 led to the hypothesis that extension of the alkyl chain would further induce the opening of Site 1 and consequently improve binding.
Figure 3.

(A) Site 1 closed in the cocrystal structure of Hsp90 pan-inhibitor onalespib (AT13387) bound to the N-terminal domain of Hsp90α (PDB code 2XAB). (B) Site 1 open in the cocrystal structure of Hsp90α-selective inhibitor 1 bound to the N-terminus of Hsp90α (PDB code 6PJS).
Linear and branched aliphatic appendages were installed to determine the subtle differences between Hsp90α and Hsp90β. The construction of 20a–h was achieved by treating 15 with readily available Grignard reagents 19a–h (Scheme 4).15 Similarly, 20i was constructed with allylmagnesium bromide. 19j was constrained via a propargyl ether to produce 20j, which orients the ether toward the solvent channel for interactions with Asn51 and Phe138. Compound 21 was produced upon treatment of 20h with osmium tetroxide and sodium metaperiodate followed by reduction with sodium cyanoborohydride.
Scheme 4. Synthesis of 20a–k and 21.
Conditions: (a) RMgBr, THF, 80 °C, 55–77%; (b) allylmagnesium bromide, −20 °C, 50%; (c) ethymagnesium bromide, methyl propargyl ether, 58%; (d) OsO4, NMO; (e) NaBH3CN, 62%.
The n-propyl derivative 20a maintained similar affinity as 16, which led to evaluation of the related n-butyl analogue 20b (Table 3). Surprisingly, 20b manifested an ∼3-fold improvement in binding affinity, but the selectivity against Hsp90β remained unchanged (∼13-fold). Extension to an n-pentyl moiety (20c) resulted in a minor loss of affinity. Collectively, the data obtained from isopropyl compounds 20d–f suggest that branching adjacent to the tertiary alcohol is not well-tolerated. Therefore, the pocket was further explored via a methoxy group (20g) and a terminal alcohol (21) to mimic water that is present in the channel. Methoxy-containing compound 20g manifested an ∼3-fold loss in affinity compared to n-butyl analogue 20b, whereas alcohol 21 manifested an ∼24-fold loss in affinity. These results suggest that the unprotected alcohol in 21 interacts with solvent molecules via enthalpic interactions, whereas the methoxy analogue 20 appears to displace solvent from the binding site, which leads to an increase in entropy. Ether 20j exhibited an improved affinity (IC50 ≈ 45 nM), but the selectivity for Hsp90α remained unaffected. Introduction of a terminal alkene in 20h and 20i produced compounds that manifested decreased affinity, suggesting that the alkene is disfavored because of its rigidity. Compounds 20a–j and 21 revealed that alkylation of the tertiary alcohol produces compounds that manifest improved affinity. Except for allyl derivative 20i, the selectivity for Hsp90α versus Hsp90β ranged between ∼5- and 10-fold, suggesting that modification to this region drives affinity but not selectivity.
Table 3. Binding Affinities and Selectivities for Hsp90α > Hsp90β Obtained by FP Assay.
| IC50 (μM)a |
|||
|---|---|---|---|
| compd | Hsp90α | Hsp90β | fold selectivity |
| 20a | 0.480 ± 0.05 | 4.57 ± 0.07 | ∼10 |
| 20b | 0.184 ± 0.02 | 2.40 ± 0.39 | ∼13 |
| 20c | 0.229 ± 0.01 | 2.07 ± 0.08 | ∼8 |
| 20d | 11.81 ± 0.87 | >50 | >5 |
| 20e | 4.60 ± 0.87 | >50 | ∼10 |
| 20f | 0.596 ± 0.03 | 5.16 | ∼10 |
| 20g | 0.447 ± 0.02 | 4.98 ± 0.29 | ∼10 |
| 20h | 2.49 ± 0.25 | 30.5 ± 2.3 | ∼12 |
| 20i | 2.22 ± 0.21 | >50 | ∼21 |
| 20j | 0.045 ± 0.02 | 0.426 ± 0.07 | ∼10 |
| 21 | 3.71 ± 0.25 | 17.94 ± 2.80 | ∼5 |
IC50 values are reported as the mean of triplicates ± SD.
Several Hsp90 inhibitors have been reported to exhibit a moderate preference for binding Hsp90α versus Hsp90β, but the rationale for this selectivity remains unclear.17,18 Although Site 1 is conserved among the cytosolic isoforms, conformational changes in the protein tertiary structure have been observed upon ligand binding.16 As previously discussed, Hsp90α undergoes a conformational change upon ligand binding, resulting in a large open Site 1. The observed Hsp90α selectivity may be due to differences in the bound-state energies for the ligand–Hsp90α complex. Therefore, compounds 23a–e were prepared from the respective Grignard reagents 22a–e (Scheme 5) to probe for π-stacking and hydrophobic interactions within Site 1.
Scheme 5. Synthesis of 23a–e with Linker Lengths n = 0–4.
Conditions: (a) RMgBr, THF, 80°C, 65–72%.
23a–e were evaluated for affinity in both Hsp90α and Hsp90β FP assays. In comparison to 2, 23a–c exhibited a loss of binding affinity and selectivity (∼10–13-fold vs ∼20-fold for 2). This observation led to the hypothesis that compounds with linker lengths ranging from 0 to 2 carbons were undesired or unable to open Site 1. However, 23d with a three-carbon linker exhibited improved affinity (IC50 ≈ 0.25 μM) and ∼15-fold selectivity. 23e with a four-carbon linker exhibited a similar binding profile, but a minor loss in affinity was observed.
A 5-fluoroisoindoline derivative of 23d (KUNA-111) was synthesized, and the cocrystal structure was solved (Figure 4). The fluorine substitution in the solvent-exposed region did not affect binding (Table 4). As proposed, the tertiary alcohol in KUNA-111 interacts with Asn51 via a hydrogen bond as outlined in Figure 2. Surprisingly, KUNA-111 overcame the combined entropic penalty by rotation of the propylene linker to induce a conformational change in Hsp90α’s N-terminal domain (NTD), which can be observed in the juxtaposition of the cocrystal structures of compound 1 and KUNA-111 (Figure 5). Rather than projecting directly into Site 1 as proposed, the alkyl side chain of KUNA-111 binds orthogonally to 1, resulting in a modified Site 1. A significant reorientation of multiple residues was observed upon further examination. Notably, Phe138 and Leu107 rotate, leading to the opening of a novel solvent-closed Site 1 that can accommodate the hydrophobic and sterically bulky side chain of KUNA-111.
Figure 4.

Cocrystal structure of KUNA-111, the 5-fluoroisoindoline derivative of 23d, bound to the NTD of Hsp90α (PDB code 7UR3). The structure confirmed that a hydrogen bond is present between Asn-51 and the tertiary alcohol of 23d.
Table 4. Binding Affinities and Selectivities for Hsp90α > Hsp90β Obtained by FP Assaya.
| IC50 (μM) |
||||
|---|---|---|---|---|
| compd | n | Hsp90α | Hsp90β | fold selectivity |
| 23a | 0 | 3.87 ± 0.32 | 39.32 ± 2.64 | ∼10 |
| 23b | 1 | 3.84 ± 0.31 | >50 | >13 |
| 23c | 2 | 3.03 ± 0.19 | >50 | >13 |
| 23d | 3 | 0.25 ± 0.01 | 3.67 ± 0.14 | ∼15 |
| 23e | 4 | 0.33 ± 0.87 | 4.28 ± 0.07 | ∼13 |
| KUNA-111 | 3 | 0.25 ± 0.01 | 3.79 ± 0.18 | ∼15 |
IC50 values are reported as the mean of triplicates ± SD.
Figure 5.

Cocrystal structures of phenyl thioether scaffold 1 (PDB code 6PJS) and tertiary alcohol scaffold KUNA-111 (PDB code 7UR3) bound to the NTD of Hsp90α.
The binding profile of KUNA-111 led to the investigation of phenyl substituents to improve the affinity and selectivity. Substituted phenyl derivatives 25a–j were produced by treating 17 with allylmagnesium bromide to yield tertiary allylic alcohol 24, which was coupled with various aromatic bromides via Heck coupling (Scheme 6). Subsequent removal of the benzyl group and reduction of the alkene produced the desired products (25a–j). The binding affinity and selectivity of the chlorine-substituted compounds (25a–c) remained unaffected via FP assay compared to 23d (Table 5). This suggests that a chlorine can be accommodated on the phenyl ring without loss of affinity. Therefore, a fluorine was installed at the 4-position (25d) to mitigate potential phase I metabolism and led to a moderate increase in selectivity (∼17-fold).
Scheme 6. Synthesis of 25a–j.
Conditions: (a) allylmagnesium bromide, 73%; (b) respective aromatic bromide, Pd(OAc)2, triethanolamine, 25–35%; (c) 1,4-cyclohexadiene, Pd/C, ethanol/ethyl acetate, 100 °C, 95%.
Table 5. Binding Affinity and Selectivity for Hsp90α > Hsp90β Obtained by FP Assaya.
| IC50 (μM) |
|||
|---|---|---|---|
| compd | Hsp90α | Hsp90β | fold selectivity |
| 25a | 0.279 ± 0.048 | 2.74 ± 0.34 | ∼10 |
| 25b | 0.256 ± 0.31 | 2.55 ± 0.19 | ∼10 |
| 25c | 0.381 ± 0.001 | 4.84 ± 0.19 | ∼12 |
| 25d | 0.255 ± 0.052 | 4.40 ± 0.46 | ∼17 |
| 25e | 0.726 ± 0.030 | 3.98 ± 0.04 | ∼6 |
| 25f | 0.243 ± 0.006 | 4.15 ± 0.65 | ∼17 |
| 25g | 0.278 ± 0.064 | 2.12 ± 0.08 | ∼8 |
| 25h | 0.056 ± 0.004 | 0.328 ± 0.059 | ∼6 |
| 25i | 0.086 ± 0.009 | 0.563 ± 0.026 | ∼7 |
| 25j | 0.057 ± 0.004 | 0.639 ± 0.119 | ∼11 |
IC50 values are reported as the mean of triplicates ± SD.
Methyl groups were installed on the phenyl ring to probe for additional hydrophobic interactions with the pocket. The 2-methyl analogue 25e lost affinity and selectivity compared to 23d. In contrast, the 3-methyl analogue 25f exhibited improved selectivity (∼17-fold), whereas the 4-methyl analogue 25g retained affinity but lost selectivity. Finally, a nitrogen scan was performed to probe for potential hydrogen-bonding interactions with Tyr139 and/or improved π-stacking interactions. The binding affinity was improved for all of the pyridyl analogues (25h–j), but no improvement in selectivity was observed. Unfortunately, the 2- and 4-pyridyl analogues 25h and 25j manifested selectivities of only ∼6-fold and ∼11-fold, respectively.
Flexibility associated with the three-carbon linker was investigated by the introduction of an alkyne or alkene. It was hypothesized that restriction of the alkyl chain would project the phenyl ring directly into Site 1 and result in a lower entropic penalty upon binding. Therefore, an alkyne was introduced within the linker chain (26 and 27). In addition, cis (28) and trans (29) alkene analogues of 23d were prepared.
An alkynyl anion was generated via deprotonation of 3-phenyl-1-propyne with lithium diisopropylamide, which was reacted with 15 to yield analogue 26 (Scheme 7). The synthesis of 27 was achieved by treating 15 with 3-chloro-1-phenyl-1-propyne. The cis-alkene analogue 28 was accessed by reduction of alkyne 27 via Lindlar’s catalyst. The trans-alkene analogue 29 was achieved via Heck coupling of 24 with iodobenzene followed by removal of the benzyl ether.
Scheme 7. Synthesis of 26–29.
Conditions: (a) 3-phenyl-1-propyne, LDA, −78 °C to rt, 51%; (b) 3-chloro-1-phenyl-1-propyne, Mg, ZnCl2, 0 °C to rt, 31%; (c) Lindlar’s catalyst, 46%; (d) Pd(dba)2, P(o-tolyl)3, N,N-dicyclohexylmethylamine, dioxane, iodobenzene, 90 °C; (e) BCl3, 38%.
The binding profiles of 26–29 were revealed upon evaluation in the FP assay (Table 6). Compound 26 manifested improved binding affinity (∼40 nM IC50) but a complete loss of selectivity. In contrast, compound 27 exhibited a minor loss of affinity (∼3-fold), but an ∼11-fold improvement in selectivity was observed compared to parent compound 23d. The cis-alkene analogue 28 exhibited a slight loss of affinity and selectivity, whereas the trans-alkene analogue 29 exhibited affinity and selectivity comparable to those of 23d. These results suggest that rigidity adjacent to the tertiary alcohol is unfavorable for selectivity, but restriction of the phenyl ring in a linear orientation may favor the conformational change induced by 23d to increase the selectivity, as exemplified by 27. In addition, molecular docking of compounds 26–29 into the KUNA-111 Hsp90α NTD cocrystal structure (PDB code 7UR3) revealed a binding mode similar to that of KUNA-111 and 23d (see the Supporting Information).
Table 6. Binding Affinities and Selectivities for Hsp90α > Hsp90β Obtained by FP Assaya.
| IC50 (μM) |
|||
|---|---|---|---|
| compd | Hsp90α | Hsp90β | fold selectivity |
| 26 | 0.034 ± 0.001 | 0.048 ± 0.011 | NSb |
| 27 | 0.861 ± 0.011 | 22.35 ± 0.64 | ∼26 |
| 28 | 0.343 ± 0.004 | 4.55 ± 0.08 | ∼13 |
| 29 | 0.260 ± 0.09 | 3.97 ± 0.15 | ∼15 |
IC50 values are reported as the mean of triplicates ± SD.
NS = nonselective.
Hit compound 2 and lead compounds 23d, 25d, 27, and 29 were evaluated for selectivity versus the ER-localized Hsp90 isoform (Grp94) and the mitochondria-localized Hsp90 isoform (Trap1) via FP assay (Table 7). All of the evaluated compounds were significantly selective versus Trap1 (>100-fold), and most were >100-fold selective versus Grp94, except constrained analogues 27 and 29, which were ∼26-fold and ∼33-fold selective, respectively.
Table 7. Lead Compound Affinity for Grp94 and Trap1 Obtained by FP Assay.
| compd | Grp94 IC50 (μM)a | selectivity > Grp94 | Trap1 IC50 (μM)a | selectivity > Trap1 |
|---|---|---|---|---|
| 2 | 95.40 ± 4.61 | ∼159-fold | >100 | >166-fold |
| 23d | 6.60 ± 0.63 | ∼26-fold | >100 | >400-fold |
| 25d | 8.71 ± 0.08 | ∼33-fold | >100 | >384-fold |
| 27 | >100 | >116-fold | >100 | >116-fold |
| 29 | >100 | >384-fold | >100 | >384-fold |
IC50 values are reported as the mean of triplicates ± SD.
A single-dose NCI-60 cancer cell screen was performed with 10 μM 23d and identified nonsmall cell lung cancer cell line NCI-H522, ovarian cancer cell line SK-OV-3, and renal cell carcinoma cell line UO-31 as susceptible to Hsp90α inhibition (see the Supporting Information). Growth inhibition manifested by the lead compounds was determined against all three cell lines after 72 h via MTS cell proliferation assays (see the Supporting Information). Compared to Hsp90 pan-inhibitor AUY922, the lead Hsp90α-selective inhibitors did not manifest significant antiproliferative effects, yielding IC50 values greater than 10 μM for nearly all compounds tested (Table 8). Previous studies demonstrated that silencing of Hsp90α did not result in significant antiproliferative or cytotoxic effects.19 Therefore, selective inhibition of Hsp90α may provide a means of garnering synergistic effects or synthetic lethality in combination treatments.20
Table 8. IC50 Values for the NCI-H522, SK-OV-3, and UO-31 Cell Lines Obtained by MTS Assaya.
| IC50 (μM)b |
|||
|---|---|---|---|
| compd | NCI-H522 | SK-OV-3 | UO-31 |
| 2 | 10.98 ± 1.21 | 24.55 ± 2.71 | 30.07 ± 1.08 |
| 23d | 14.81 ± 4.30 | 38.00 ± 0.28 | 77.41 ± 20.75 |
| 25d | 17.37 ± 0.94 | 49.20 ± 4.31 | 44.49 ± 6.30 |
| 27 | 9.34 ± 1.79 | 36.71 ± 2.40 | 53.54 ± 11.66 |
| 29 | 16.66 ± 0.95 | 58.18 ± 4.92 | 85.38 ± 13.69 |
| AUY922 | 0.00536 ± 0.00072 | 0.00974 ± 0.00045 | 0.00935 ± 0.00175 |
Cells were treated with Hsp90α-selective compound 2, 23d, 25d, 27, 29, or AUY922 (Hsp90 pan-inhibitor) or DMSO (vehicle) for 72 h.
IC50 values are reported as averages ± SEM (N = 3 independent replicates).
The selective degradation of Hsp90-dependent client proteins was determined by treating the NCI-H522 and SK-OV-3 cell lines with 23d and 27, followed by Western blot analysis. The Hsp90 client proteins Her2, Raf-1, and Akt as well as the Hsp90α-dependent clients survivin and c-Src were observed to decrease in a dose-dependent manner in response to 23d and 27 (Figure 6). Likewise, p-Akt and p-Src decreased, suggesting that inhibition of Hsp90α restricted Hsp90α-dependent kinase signaling cascades (Figure 6A). CDK4, an Hsp90β-dependent client, was degraded at higher concentrations, supporting the selective inhibition of Hsp90α over Hsp90β in the cellular context. During the heat shock response, the expression of heat shock proteins Hsp90 and Hsp70 is induced to refold proteins that have become denatured. In a manner similar to Hsp90 pan-inhibitors (AUY922), Hsp70 and Hsp90 levels increased in response to Hsp90α-selective inhibitors 23d and 27, indicating an induction of the heat shock response.
Figure 6.
Western blot analysis for the degradation of Hsp90α-dependent client proteins in NCI-H522 and SK-OV-3 cells after 24 h treatment with (A) 23d or (B) 27. 0.2% DMSO (vehicle) and 30 nM AUY922 (Hsp90 pan-inhibitor) were used as controls.
In summary, 41 Hsp90α-selective analogues were synthesized to probe for hydrogen-bonding interactions with Asn51 and steric and hydrophobic interactions within the Hsp90α binding site. A tertiary alcohol was found to be optimal, while adjacent steric bulk was not tolerated. Therefore, linear and branched aliphatic appendages were introduced to probe for structural differences in Site 1 between Hsp90α and Hsp90β. The results yielded 23d with a three-carbon phenyl linker, which exhibited improved affinity (IC50 ≈ 0.25 μM) and ∼15-fold selectivity. The cocrystal structure of the 5-fluoroisoindoline derivative of 23d (KUNA-111) bound to the Hsp90α NTD was solved. KUNA-111 induced conformational changes within Hsp90α’s NTD, which revealed a novel binding mode. The lead compounds were found to exhibit >100-fold selectivity versus the ER (Grp94) and mitochondrial (Trap1) Hsp90 isoforms. These new Hsp90α-selective inhibitors did not manifest significant antiproliferative activity, in agreement with prior findings. However, they did induce selective and dose-dependent degradation of Hsp90α clients in the cellular environment. Current studies are underway to investigate the effects of the novel binding conformation induced by 23d.
Acknowledgments
The authors gratefully acknowledge financial support of this work from the National Institutes of Health (CA167079 to B.S.J.B. and CA219907 to R.L.M. and J.D.) and the Oklahoma Agricultural Experiment Station (OKL03159 to R.L.M. and OKL03060 to J.D.). This research was also supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (Award P20GM103640). We thank the staff of beamline 14 at the Stanford Synchrotron Radiation Lightsource for their support.
Glossary
Abbreviations
- Hsp90
heat shock protein 90
- Grp94
glucose-regulated protein 94
- Trap1
tumor necrosis factor receptor-associated protein 1
- eHsp90α
extracellular heat shock protein 90 α
- HER2
human epidermal growth factor receptor
- MMP-2
matrix metalloproteinase-2
- EMT
epithelial to mesenchymal transition
- FP
fluorescence polarization
- NTD
N-terminal domain
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00327.
The authors declare no competing financial interest.
Supplementary Material
References
- Hartl F. U.; Bracher A.; Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475 (7356), 324–332. 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Rutherford S. L.; Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature 1998, 396 (6709), 336–342. 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144 (5), 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Zuehlke A. D.; Beebe K.; Neckers L.; Prince T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570 (1), 8–16. 10.1016/j.gene.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Sahu D.; Tsen F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim. Biophys. Acta: Mol. Cell Res. 2012, 1823 (3), 730–741. 10.1016/j.bbamcr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y.; Wang C. Y.; Chen S. H.; Liu J.; Fu Y.; Luo Y. Z. Extracellular HSP90α and clusterin synergistically promote breast cancer epithelial-to-mesenchymal transition and metastasis via LRP1. J. Cell Sci. 2019, 132 (15), jcs228213. 10.1242/jcs.228213. [DOI] [PubMed] [Google Scholar]
- Eustace B. K.; Sakurai T.; Stewart J. K.; Yimlamai D.; Unger C.; Zehetmeier C.; Lain B.; Torella C.; Henning S. W.; Beste G.; Scroggins B. T.; Neckers L.; Ilag L. L.; Jay D. G. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat. Cell Biol. 2004, 6 (6), 507–514. 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Bohonowych J. E.; Hance M. W.; Nolan K. D.; Defee M.; Parsons C. H.; Isaacs J. S. Extracellular Hsp90 Mediates an NF- kBDependent Inflammatory Stromal Program: Implications for the Prostate Tumor Microenvironment. Prostate 2014, 74 (4), 395–407. 10.1002/pros.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.; Chen S.; Han H.; Li H.; Huang Z.; Zhang J.; Yin Q.; Wang X.; Ma X.; Dai P.; Duan D.; Zou F.; Chen X. Expression of Hsp90α and cyclin B1 were related to prognosis of esophageal squamous cell carcinoma and keratin pearl formation. Int. J. Clin. Exp. Pathol. 2014, 7 (4), 1544–1552. [PMC free article] [PubMed] [Google Scholar]
- Tian W. L.; He F.; Fu X.; Lin J. T.; Tang P.; Huang Y. M.; Guo R.; Sun L. High expression of heat shock protein 90 alpha and its significance in human acute leukemia cells. Gene 2014, 542 (2), 122–128. 10.1016/j.gene.2014.03.046. [DOI] [PubMed] [Google Scholar]
- Zhou W. W.; Yang Y. H.; Wang Z. Z.; Liu Y.; Najafi M. L. Impact of HSP90α, CEA, NSE, SCC, and CYFRA21-1 on Lung Cancer Patients. J. Healthcare Eng. 2021, 2021, 6929971. 10.1155/2021/6929971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sourbier C. Plasma HSP90α and liver cancer: a potential biomarker?. eBioMedicine 2017, 25, 7–8. 10.1016/j.ebiom.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Li J.; Zhang P.; Hou Q. Y.; Feng S.; Liu L. S.; Cui D. W.; Shi H. B.; Fu Y.; Luo Y. Z. A novel pan-cancer biomarker plasma heat shock protein 90alpha and its diagnosis determinants in clinic. Cancer Sci. 2019, 110 (9), 2941–2959. 10.1111/cas.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S. J.; Khandelwal A.; Banerjee M.; Balch M.; Peng S. X.; Davis R. E.; Merfeld T.; Munthali V.; Deng J. P.; Matts R. L.; Blagg B. SJ. Selective Inhibition of the Hsp90 alpha Isoform. Angew. Chem., Int. Ed. 2021, 60 (19), 10547–10551. 10.1002/anie.202015422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarinov D. A.; Kuznetsov D. M.; Voloshina A. D.; Lyubina A. P.; Strobykina A. S.; Mukhitova F. K.; Polyancev F. M.; Mironov V. F. Synthesis of 2-(2-hydroxyaryl)alkenylphosphonium salts from phosphine oxides via ring-closing ring-opening approach and their antimicrobial evaluation. Tetrahedron 2016, 72 (51), 8493–8501. 10.1016/j.tet.2016.11.023. [DOI] [Google Scholar]
- Li J.; Soroka J.; Buchner J. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochim. Biophys. Acta: Mol. Cell Res. 2012, 1823 (3), 624–635. 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Patel P. D.; Yan P. R.; Seidler P. M.; Patel H. J.; Sun W. L.; Yang C. H.; Que N. S.; Taldone T.; Finotti P.; Stephani R. A.; Gewirth D. T.; Chiosis G. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol. 2013, 9 (11), 677–684. 10.1038/nchembio.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Lin C.; Qin X. C.; Dong X. P.; Tu Z. C.; Tang F.; Chen C. N.; Zhang J. C. Synthesis and biological evaluation of 3,5-disubstituted-4-alkynylisoxozales as a novel class of HSP90 inhibitors. Bioorg. Med. Chem. Lett. 2015, 25 (16), 3129–3134. 10.1016/j.bmcl.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Cruickshanks N.; Shervington L.; Patel R.; Munje C.; Thakkar D.; Shervington A. Can hsp90α-Targeted siRNA Combined with TMZ Be a Future Therapy for Glioma?. Cancer Invest. 2010, 28 (6), 608–614. 10.3109/07357901003630967. [DOI] [PubMed] [Google Scholar]
- Mehta A.; Shervington A.; Howl J.; Jones S.; Shervington L. Can RNAi-mediated hsp90α knockdown in combination with 17-AAG be a therapy for glioma?. FEBS Open Biol. 2013, 3, 271–278. 10.1016/j.fob.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.