Abstract
Background:
SARS-CoV-2 transmission has an impact on education. In this study, we assessed the performance of rapid antigen detection tests (RADTs) versus polymerase chain reaction (PCR) for the diagnosis of SARS-CoV-2 infection in school settings, and RADT use for monitoring exposed contacts.
Methods:
In this real-world, prospective observational cohort study, high-school students and staff were recruited from 2 high schools in Montréal, Canada, and followed from Jan. 25 to June 10, 2021. Twenty-five percent of asymptomatic participants were tested weekly by RADT (nasal) and PCR (gargle). Class contacts of cases were tested. Symptomatic participants were tested by RADT (nasal) and PCR (nasal and gargle). The number of cases and outbreaks were compared with those of other high schools in the same area.
Results:
Overall, 2099 students and 286 school staff members consented to participate. The overall specificity of RADTs varied from 99.8% to 100%, with a lower sensitivity, varying from 28.6% in asymptomatic to 83.3% in symptomatic participants. Secondary cases were identified in 10 of 35 classes. Returning students to school after a 7-day quarantine, with a negative PCR result on days 6–7 after exposure, did not lead to subsequent outbreaks. Of cases for whom the source was known, 37 of 51 (72.5%) were secondary to household transmission, 13 (25.5%) to intraschool transmission, and 1 to community contacts between students in the same school.
Interpretation:
Rapid antigen detection tests did not perform well compared with PCR in asymptomatic individuals. Reinforcing policies for symptom screening when entering schools and testing symptomatic individuals with RADTs on the spot may avoid subsequent substantial exposures in class. Preprint: medRxiv — doi.org/10.1101/2021.10.13.21264960
Timely diagnosis of infection enables outbreak control through rapid isolation of index cases and subsequent contact tracing.1,2 Diagnosis of SARS-CoV-2 infection is predominantly based on polymerase chain reaction (PCR), which has a turnaround time of 24–48 hours. Rapid antigen detection tests (RADTs) are inexpensive and can be used at the point of care. They usually have high specificity and moderate sensitivity compared with PCR.3–6 Given their rapid turnaround time, RADTs allow for efficient triage and management of exposed individuals.7 The potential use of RADTs is especially relevant in schools, where outbreaks of SARS-CoV-2 infection can interrupt in-person teaching and negatively affect learning.8–11
Rapid antigen detection tests perform best in the early stages of infection, when viral load is generally high.12–15 Reported RADT sensitivity ranges from 28.9% to 98.3%, with improved sensitivity in samples with high viral loads and in symptomatic individuals.16,17 The usual limits of detection for PCR is 600–1000 viral RNA copies/mL, whereas RADTs usually have limits of detection 2–3 logs higher (105 to 106).18 Many studies have indicated the importance of high viral load dynamics with infectiousness. 19,20 For each unit increase in cycle threshold (Ct) value, the odds of recovering infectious virus decreased by 0.67, being under 10% when Ct values were greater than 35. Cycle threshold values of 17 to 32 corresponded to 105 and 101 SARS-CoV-2 RNA copies/μL, respectively.21
We aimed to determine the performance characteristics of RADTs for SARS-CoV-2 compared with PCR in high-school students and staff, and to determine whether serial testing of COVID-19 contacts would allow for safe faster return to school.
Methods
The study was conducted in 2 high schools in Montréal, Canada. Pensionnat du Saint-Nom-de-Marie (PSNM) is a private school, and École secondaire Calixa-Lavallée (ESCL) is a public school (age range 11–16 yr for both schools). Both schools followed the ministry of education recommendations by forming “classroom bubbles,” which lasted from the onset of the pandemic to the end of the 2021 school year. Masks were mandatory as of Oct. 8, 2020. Students were about 30 per class and seated 3 feet apart. School staff were invited to participate in the study. Vaccination began Apr. 9, 2021, for adults and May 25, 2021, for children aged 12 years and older.
Study design and interventions
This was a real-world, prospective observational cohort study comparing RADTs with PCR, from Jan. 25 to June 10, 2021. The study began during a recrudescence of SARS-CoV-2 infection in Quebec, with more than 2800 cases per day, including 1200 in Montréal alone,22,23 where the predominant circulating strains were ancestral SARS-CoV-2 and the Alpha variant as of April 2021.24
The lateral flow immunoassay (Panbio COVID-19 Ag test, Abbott Laboratories), authorized by Health Canada,25 was used. Nasal swabs were self-collected under the supervision of a research assistant, to avoid sampling bias, who then performed an RADT on site. Spring-water gargle specimens were collected for PCR testing.26 Laboratory-developed PCR was performed at Centre Hospitalier Universitaire Sainte-Justine, with a limit of detection of 400 copies/mL.27 Extraction and purification of genetic material was done with Roche’s MagNA Pure 96 System. The laboratory testing protocol and the water gargle validation have been described elsewhere.28–31 A PCR test was considered positive if the Ct was under 33, weakly positive for Ct values of 33.0–36.9, equivocal for Ct values of 37.0–39.9 and negative if the Ct value was over 40. The PCR results were not available to the performers or readers of RADTs, and the RADT results were not available to the laboratory technicians performing PCR. Participants with equivocal PCR results were usually retested. As per protocol, any test performed on a symptomatic participant that would get lost would be repeated to avoid missing data in a possible infected case. The full study protocol is available on request.
Decisions about management of cases and contacts were made by 2 members of the research team (A.C.B. and C.Q.), in collaboration with local public health (C.T.N. and O.S.). The school principals (Y.P. and D.B.) were actively involved in the study deployment, and oversaw the identification of exposed contacts and reporting them to public health, as was the case before the study.
Testing protocol in the absence of a known exposure
Surveillance screening in asymptomatic participants: Nasal swabs and gargle specimens were collected weekly for 19 weeks for RADT (nasal) and PCR (gargle) on a random sample of 25% of participants, stratified by class.
Symptomatic participant testing32: Gargle specimens for PCR and a nasal swab for RADT and PCR were performed on site. Results from RADT and PCR were reported to public health; an individual was considered infected if the PCR result was positive or weakly positive. If symptoms occurred in school, the research team proceeded with testing. If symptoms developed at home, participants were invited to get tested at school in a private room.
Management of exposed contacts of a positive individual in a class
Contacts of a confirmed positive individual were instructed to isolate at home. Students were allocated to a 7- or 14-day quarantine and staff members to a 7-day quarantine, with tests (both nasal RADT and gargle PCR) 3 days after last contact with the known positive case and up to 2 days before the end of quarantine. Rapid antigen detection testing alone was performed on days 14, 21 and 28 if the initial PCR was negative. If symptoms developed, both the RADT and PCR were performed. Students who did not consent to the study were quarantined for 14 days. Students and staff with substantial off-campus exposures (defined as per public health definitions, which usually defined substantial exposures as being less than 2 m away from an infected individual for at least 15 min) were offered on-site testing.
Outcomes
The primary outcome was to assess the performance characteristics of RADTs in asymptomatic participants randomly screened, asymptomatic close contacts of a confirmed positive case and symptomatic participants.
Secondary outcomes included the number of RADT-positive students in groups exposed to a confirmed positive index case, allocated to early (on day 8) or standard (on day 15) return to school, and number of case clusters in schools. The latter was compared with clusters in all other high schools associated with Direction de la santé publique de Montréal during the same time frame, using data from the local public health electronic platform (Akinox).
Statistical analysis
We assessed the sensitivity, specificity, and positive and negative predictive values of RADTs, compared with PCR, and we determined the 95% confidence intervals (CIs) using the Clopper–Pearson method. To determine the precision with which we could estimate our primary outcome, we implemented an agent-based model33 (Appendix 1, available at www.cmajopen.ca/content/10/4/E1027/suppl/DC1). Based on this simulation, we expected that the number of infections and tests in 1 school would be sufficient, but we added a second school to support generalizability of the findings and explore secondary objectives.
Ethics approval
This project was approved by the Centre Hospitalier Universitaire Sainte-Justine Research Ethics Board (no. MP-21-2021-3271). Informed parental consent or assent were required for all students. Parents who preferred to keep their children home for 14 days in the case of a contact could do so. Tests results were communicated to parents and students by the school. This study was funded by the Ministère de la Santé et des Services sociaux.
Results
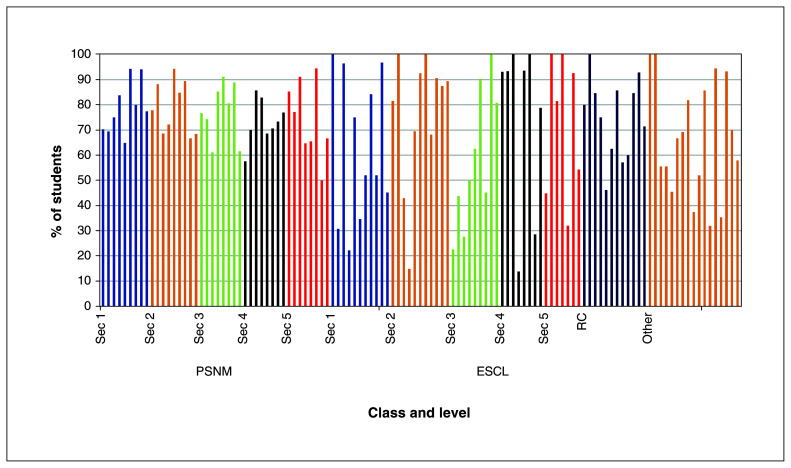
During the study period, 2099 students and 286 school staff members consented to participate. The participation rate was 78.5% and 63.5% for students (Figure 1), and 94.4% and 89.5% for staff at the 2 schools. There were no adverse events caused by performing RADTs.
Figure 1:
Proportion of participating students per class and level. Overall, there were 117 participating classes during the study period. Note: ESCL = École secondaire Calixa-Lavallée, Other = special education classes for students with learning disorders, PSNM = Pensionnat du Saint-Nom-de-Marie, RC = reception class (for students newly arrived to Canada), Sec = secondary school level.
RADT results and PCR validation (from gargle specimens only)
Asymptomatic students and staff
Of 5583 RADTs done on asymptomatic students (Table 1), 7 had an invalid PCR result on the gargle sample, 7 were equivocal and 3 were weak positive, of which 1 was negative when repeated the next day (and was excluded). Two students with equivocal or weak-positive PCR results had had a positive PCR result in the previous 90 days. The prevalence of SARS-CoV-2-positive PCR results in asymptomatic participants was 0.30% (95% CI 0.18%–0.49%). Therefore, the sensitivity of RADTs in that population was 41.20% (95% CI 21.6%–64.0%), with a specificity of 100%.
Table 1:
Performance of rapid antigen detection tests in the participant groups
| RADT (nasal) | Positive | Results, no. of participants | Invalid | Clinical performance of RADTs | Specificity, % (95% CI) | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| PCR (gargle) | Sensitivity, % (95% CI) | ||||||
|
|
|
||||||
| Negative | Equivocal or weak positive | Excluding equivocal or weak positive | Including equivocal or weak positive | ||||
| Asymptomatic students * | |||||||
|
| |||||||
| Positive | 7 | 1 | 0 | 0 |
n = 17 41.2 (21.6–64.0) |
n = 26 26.9 (13.7–46.1) |
100§ (99.9–100) |
|
| |||||||
| Negative | 10 | 5549 | 9 | 7 | |||
|
| |||||||
| Invalid | 0 | 0 | 0 | 0 | |||
|
| |||||||
| Asymptomatic students considered exposed contacts of positive index cases † | |||||||
|
| |||||||
| Positive | 4 | 6 | 0 | 0 |
n = 14 28.6 (8.4–58.1) |
n = 15 26.7 (7.8–55.1) |
99.6 (99.1–99.9) |
|
| |||||||
| Negative | 10 | 1470 | 1 | 0 | |||
|
| |||||||
| Invalid | 0 | 0 | 0 | 0 | |||
|
| |||||||
| Symptomatic students ‡ | |||||||
|
| |||||||
| Positive | 10 | 0 | 0 | 0 | 83.3 (51.6–97.9) | NA | 100.0 (98.4–100.0) |
|
| |||||||
| Negative | 2 | 223 | 0 | 0 | |||
|
| |||||||
| Invalid | 0 | 0 | 0 | 0 | |||
|
| |||||||
| Asymptomatic staff members | |||||||
|
| |||||||
| Positive | 0 | 1 | 0 | 0 | NA | NA | 99.9 (99.3–100.0) |
|
| |||||||
| Negative | 0 | 775 | 0 | 2 | |||
|
| |||||||
| Invalid | 0 | 0 | 0 | 0 | |||
|
| |||||||
| Asymptomatic staff members considered exposed contacts of positive index cases | |||||||
|
| |||||||
| Positive | 0 | 0 | 0 | 0 | NA | NA | 100.0 (94.1–100.0) |
|
| |||||||
| Negative | 0 | 61 | 0 | 0 | |||
|
| |||||||
| Invalid | 0 | 0 | 0 | 0 | |||
|
| |||||||
| Symptomatic staff members | |||||||
|
| |||||||
| Positive | 1 | 0 | 0 | 0 | 50.0 (1.3–98.7) | NA | 100.0 (94.3–100.0) |
|
| |||||||
| Negative | 1 | 62 | 0 | 0 | |||
|
| |||||||
| Invalid | 0 | 0 | 0 | 0 | |||
Note: CI = confidence interval, NA = not applicable, PCR = polymerase chain reaction, RADT = rapid antigen detection test.
Prevalence of SARS-CoV-2 infection, based on PCR results (including equivocal and weakly positive results): 0.30% (95% CI 0.18%–0.49%).
Prevalence of SARS-CoV-2 infection, based on PCR results (including equivocal and weakly positive results): 0.7% (95% CI 0.5%–1.6%).
Prevalence of SARS-CoV-2 infection, based on PCR results (including equivocal and weakly positive results): 5.1% (95% CI 2.65%–8.71%).
The specificity of RADT in asymptomatic students was 99.98% when adjusted to 2 decimal places.
Of 784 asymptomatic RADT screening tests done on asymptomatic randomly screened staff members, 2 had invalid PCR results and 6 of the tests were lost (the latter is not shown in Table 1). Only 1 case had a positive RADT, but a negative PCR, giving a specificity of 99.9% (95% CI 99.3%– 100%) in that group (Table 1).
Asymptomatic exposed contacts at school
A total of 1491 RADTs and 1491 PCR tests were done on asymptomatic students exposed to a positive classmate index case at day 3 and 2 days before returning to class. After exclusion of 1 equivocal PCR result, SARS-CoV-2 prevalence in this exposed group was 0.7% (95% CI 0.5%–1.6%). The sensitivity of RADTs was 28.6% (95% CI 8.4%–58.1%), with a specificity of 99.6% (95% CI 99.1%–99.9%) (Table 1). Of 627 RADTs done for asymptomatic exposed contacts on days 14, 21 and 28, only 1 was positive (also positive by PCR when tested on day 12 — see “Assessment of SARS-CoV-2 transmission after return to school of exposed contacts”). A total of 61 RADTs and PCR tests were done for staff members on day 3 and day 7 after a contact with a positive index case in school (Table 1). All were negative.
Symptomatic students and staff
Overall, 235 students developed symptoms and were tested on site for SARS-CoV-2. As shown in Table 1, 10 had a positive RADT and 12 had a positive PCR test (prevalence 5.1%, 95% CI 2.7%–8.7%). The sensitivity of RADTs in that population was 83.3% (95% CI 51.6%–97.9%), with a specificity of 100% (95% CI 98.4%–100%). Sixty-four staff members were tested on site for symptoms compatible with COVID-19. One had a positive RADT and PCR test. One positive case was identified by PCR after a negative RADT (sensitivity of 50.0%, 95% CI 1.3%–98.7%; specificity of 100%, 95% CI 94.3%–100%).
Of 235 symptomatic children, 225 had recorded their onset of symptoms, with a median time of 1 (range 0–33) day. Overall, 46.7% (n = 105/225) were tested with RADT and PCR on the day of symptom onset.
Assessment of SARS-CoV-2 transmission after return to school of exposed contacts
We identified 76 PCR-positive cases (gargle or nasal), including 3 cases in staff. Of the 35 classes included in the study where there was a positive case, 20 returned on day 8 after contact, if the gargle PCR test was negative on days 6 or 7.
Secondary cases were identified in 10 classes. The number of secondary cases in each class were 1 (n = 8 classes), 3 (n = 1 class) and 4 (n = 1 class). Four secondary cases had a positive RADT, including 3 asymptomatic students and 1 symptomatic student who tested positive by RADT and PCR on day 12, with symptoms starting on day 9 after last contact with the positive classmate — a community exposure was also suspected. No tertiary case occurred. Outbreaks were limited to the classroom bubble and to school friends seen outside of school. Of cases for whom the source was known, 37 of 51 (72.5%) were secondary to household transmission, 13 (25.5%) to intraschool transmission and 1 to community contacts between students in the same school.
During the same period, outbreaks declared in other Montréal schools had a lower proportion of asymptomatic cases (31.8%) compared with ESCL (55.6%) and PSNM (85.7%) (Appendix 2, available at www.cmajopen.ca/content/10/4/E1027/suppl/DC1).
Interpretation
Rapid antigen detection tests were purchased worldwide as a tool to prevent outbreaks. However, their use is limited by the paucity of evidence regarding their performance in children. In this study, we prospectively compared the performance of RADTs and PCR tests for the purpose of limiting transmission of SARS-CoV-2 in schools. In a context of lower SARS-CoV-2 prevalence in school than in the community,23 we observed only 7 false-positive RADTs during the 5-month study (all in asymptomatic individuals), and the specificity of RADTs remained excellent overall. However, the sensitivity was much lower, varying between 28.6% in asymptomatic and 83.3% in symptomatic students.
A recent large observational study described the use of RADTs in asymptomatic individuals as beneficial, reporting a sensitivity of 64.4% (95% CI 58.3%–70.2%).34 However, this could be overestimated as not all asymptomatic individuals had a confirmatory PCR test. In our study, only a few positive cases were detected by RADTs (overall 7/6358, 0.1%) in asymptomatic individuals who were randomly tested. Ten additional cases were detected by PCR from gargle specimens. Two full-time research assistants were in each school, in addition to local school staff who were supporting the study rollout. This level of resources may not be available in most schools for random screening of asymptomatic individuals, given low sensitivity in that setting.
Rapid antigen detection tests identified SARS-CoV-2-positive symptomatic cases in 15 minutes, allowing for prompt isolation, contact tracing and testing. The overall sensitivity of RADTs in symptomatic staff and students was 78.6% (95% CI 49.2%–95.3%). This finding is in agreement with results of other published studies.14,15,35–37 Sood and colleagues recently described that the positive concordance of RADTs was higher among symptomatic children (64.4%) than asymptomatic children (51.1%) presenting at a walk-in testing site.36 L’Huillier and colleagues described a sensitivity of 73.0% in symptomatic versus 43.3% in asymptomatic children.37 The authors described the peak of sensitivity on day 2 after onset of symptoms, with a subsequent decrease to 56% by day 5. In our study, about half of symptomatic students (with a recorded date of symptom onset) were tested with RADT and PCR on the day their symptoms started. Our reported RADT sensitivity may have been higher had students been tested on subsequent days. However, the usefulness of RADTs is to control outbreaks; therefore, delaying testing to enhance sensitivity would be counterproductive. This trade-off may not apply to the Delta variant, for which the kinetic of infection may differ.38,39
Rapid antigen detection tests identified 28.6% of positive asymptomatic exposed school contacts, which was similar to findings recently described by Torres and colleagues for non-household contacts (sensitivity 35.7%).40 Although this percentage is low, the rapid diagnosis of SARS-CoV-2 infection in exposed individuals allowed local public health to quickly manage these students’ household contacts who, at the time, were required to isolate until the result of the day 3 testing. Most positive cases in students were assumed to be from household SARS-CoV-2 transmission. Students were often sent to school despite having a known positive contact. Active screening of symptoms and history of noteworthy exposures should be reinforced to prevent school outbreaks. Thirteen of 51 cases were acquired from school, with 15 cases in the same class bubble (in 5 classes overall). Therefore, the asymptomatic nature of this infection makes screening for school contacts essential. Our results show that using a more sensitive method, such as PCR, may be more reliable for that purpose.
The strengths of this study include its prospective design and the real-world use of RADTs versus PCR tests. We assigned participants to earlier versus standard return to school with serial RADTs, showing that there were no secondary outbreaks with shorter quarantine. Although the study was not powered to rule out secondary outbreaks, our finding aligns with other recently published data47 and may allow policy-makers to consider reducing the duration of quarantine for exposed contacts, provided a PCR test is negative on days 6 or 7.
Limitations
This study had several limitations. We did not collect data regarding adherence to public health measures, nor did we systematically document exposures occurring outside of school. However, for the most part, we were able to identify when evident household transmission occurred and relied on the transparency of participants. We cannot infer whether PCR-positive individuals were contagious; however, we used PCR as the gold standard test to consider individuals infected, as was being done by the public health jurisdiction during the time that the study took place. Since then, there has been some evidence suggesting that RADT results may correlate well with live viral culture.41 The study was performed before the advent of the Delta variant in our region. Because the RADT detects the nucleocapsid protein, we expect that its sensitivity and specificity would not be affected negatively, as viral loads of Delta variant infections are reported to be higher.38 In addition, recent data show that infectious viral loads are not lower in double-dose vaccinated individuals than in unvaccinated Omicron-infected individuals, but are reduced in boosted individuals.42 Therefore, the findings of this study may not be as generalizable to the latter group. As of November 2022, 21% of youth aged 12–17 years had received a third vaccine dose in Quebec. 43 In addition, recently published studies indicate that the performance of the Panbio RADT is adequate for detection of the Omicron variant in cohorts largely vaccinated with 2-dose and 3-dose vaccine regimens, respectively.44,45 Some participants may have received their first dose of vaccine during the last few weeks of the study; we did not collect data regarding vaccination. However, infection and transmission can still occur despite vaccination,46 and vaccination acceptance rates may vary in time; therefore, the findings of this study related to the use of RADTs are relevant. Finally, the sensitivity of RADTs in symptomatic individuals was based on a relatively small number of people with PCR-confirmed SARS-CoV-2 infection.
Conclusion
Our findings contribute to the growing evidence that the use of RADTs leads to rapid diagnosis of SARS-CoV-2 infection in symptomatic individuals. However, RADTs did not perform well compared with PCR in asymptomatic individuals. In our study, teenagers were able to proceed to self-collection of swabs, while supervised by a research assistant. It may be helpful to reinforce policies for symptom screening when entering schools, where symptomatic individuals could be tested with RADTs to avoid substantial in-class exposures. A negative RADT could still mean that symptoms are due to SARS-CoV-2, but with a viral load too low to be detected and therefore less likely to transmit at that point. In such instances, a subsequent sample tested by PCR would be useful.
Supplementary Material
Footnotes
Competing interests: Ana Blanchard reports project grants from Réseau de recherche en Santé Respiratoire du Québec and Cystic Fibrosis Foundation (payments to Centre Hospitalier Universitaire Sainte-Justine). Annie-Claude Labbé reports honoraria for a lecture from Hologic, speakers honoraria from Merck and Pfizer, and provision of reagents for SARS-CoV-2 testing from Seegene, DiaSorin and Roche Diagnostics, as part of another study. Kate Zinszer reports grant funding from the Public Health Agency of Canada, Canadian Institutes of Health Research and Fonds de Recherche du Québec — Santé. Caroline Quach reports a project grant from Ministère de la Santé et des Services sociaux — Québec (payments to Centre Hospitalier Universitaire Sainte-Justine). No other competing interests were declared.
This article has been peer reviewed.
Contributors: Caroline Quach conceptualized and designed the study, acquired funding, carried out the data analysis and drafted the initial manuscript. Marc Desforges, Annie-Claude Labbé, Cat Tuong Nguyen, Kate Zinszer, Jean Longtin, Jiannis Ragoussis and David L. Buckeridge conceptualized and designed the study. Kelsey Adams and Marie-Ève Benoit collected data, and managed and coordinated the research project on site. Yves Petit, Dominic Besner, Zineb Laghdir and Geneviève Leduc managed and coordinated the research project on site. Ana Blanchard carried out the data analysis and drafted the initial manuscript. Olivier Séguin carried out the data analysis. All authors critically reviewed and revised the manuscript, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by the Quebec Ministry of Health and Social Services. The study sponsor did not have a role in study design, interpretation of data, writing of the report or decision to submit the paper for publication.
Data sharing: Anonymized data may be accessed on request, by contacting the corresponding author.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/4/E1027/suppl/DC1.
References
- 1.Hellewell J, Abbott S, Gimma A, et al. Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–96. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kretzschmar ME, Rozhnova G, Bootsma MCJ, et al. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020;5:e452–9. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azar MM, Landry ML. Detection of influenza A and B viruses and respiratory syncytial virus by use of Clinical Laboratory Improvement Amendments of 1988 (CLIA): waived point-of-care assays — a paradigm shift to molecular tests. J Clin Microbiol. 2018;56:e00367–18. doi: 10.1128/JCM.00367-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan CA, Caya C, Papenburg J. Rapid and simple molecular tests for the detection of respiratory syncytial virus: a review. Expert Rev Mol Diagn. 2018;18:617–29. doi: 10.1080/14737159.2018.1487293. [DOI] [PubMed] [Google Scholar]
- 5.Chartrand C, Tremblay N, Renaud C, et al. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol. 2015;53:3738–49. doi: 10.1128/JCM.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papenburg J, Buckeridge DL, De Serres G, et al. Host and viral factors affecting clinical performance of a rapid diagnostic test for respiratory syncytial virus in hospitalized children. J Pediatr. 2013;163:911–3. doi: 10.1016/j.jpeds.2013.03.067. [DOI] [PubMed] [Google Scholar]
- 7.Peeling RW, Olliaro PL, Boeras DI, et al. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis. 2021;21:e290–5. doi: 10.1016/S1473-3099(21)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Z, Idris S, Zhang Y, et al. The impact of COVID-19 pandemic outbreak on education and mental health of Chinese children aged 7–15 years: an online survey. BMC Pediatr. 2021;21:95. doi: 10.1186/s12887-021-02550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koirala A, Wood N, Macartney K. Testing for SARS-CoV-2 infection: a key strategy to keeping schools and universities open. Lancet Child Adolesc Health. 2021;5:387–9. doi: 10.1016/S2352-4642(21)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engzell P, Frey A, Verhagen MD. Learning loss due to school closures during the COVID-19 pandemic. Proc Natl Acad Sci U S A. 2021;118:e2022376118. doi: 10.1073/pnas.2022376118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stringer N, Keys E. Learning during the pandemic: review of international research. London (UK): Public Health England; 2021. [accessed 2021 Sept. 7]. Available: https://www.gov.uk/government/publications/learning-during-the-pandemic/learning-during-the-pandemic-review-of-international-research#authors. [Google Scholar]
- 12.Guidance for antigen testing for SARS-CoV-2 for healthcare providers testing individuals in the community. Atlanta: Centers for Disease Control and Prevention; [accessed 2021 Aug. 7]. updated 2022 Apr 4. Available https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html. [Google Scholar]
- 13.Interim guidance on the use of rapid antigen detection tests for the identification of SARS-CoV-2 infection. Ottawa: Public Health Agency of Canada; [accessed 2021 Aug. 7]. modified 2021 Feb. 23. Available: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/use-rapid-antigen-detection-tests.html. [Google Scholar]
- 14.Pickering S, Batra R, Merrick B, et al. Comparative performance of SARS-CoV-2 lateral flow antigen tests and association with detection of infectious virus in clinical specimens: a single-centre laboratory evaluation study. Lancet Microbe. 2021;2:e461–71. doi: 10.1016/S2666-5247(21)00143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagenhäuser I, Knies K, Rauschenberger V, et al. Clinical performance evaluation of SARS-CoV-2 rapid antigen testing in point of care usage in comparison to RT-qPCR. EBioMedicine. 2021;69:103455. doi: 10.1016/j.ebiom.2021.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinnes J, Deeks JJ, Berhane S, et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayer J, Kasapic D, Zemmrich C. Real-world clinical performance of commercial SARS-CoV-2 rapid antigen tests in suspected COVID-19: a systematic meta-analysis of available data as of November 20, 2020. Int J Infect Dis. 2021;108:592–602. doi: 10.1016/j.ijid.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mertens P, De Vos N, Martiny D, et al. LHUB-ULB SARS-CoV-2 Working Diagnostic Group. Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Front Med (Lausanne) 2020;7:225. doi: 10.3389/fmed.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawasuji H, Takegoshi Y, Kaneda M, et al. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS One. 2020;15:e0243597. doi: 10.1371/journal.pone.0243597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenoy S. SARS-CoV-2 (COVID-19), viral load and clinical outcomes; lessons learned one year into the pandemic: a systematic review. World J Crit Care Med. 2021;10:132–50. doi: 10.5492/wjccm.v10.i4.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandolini M, Taddei F, Marino MM, et al. Correlating qRT-PCR, dPCR and viral titration for the identification and quantification of SARS-CoV-2: a new approach for infection management. Viruses. 2021;13:1022. doi: 10.3390/v13061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Données COVID-19 au Québec. Québec: Institut national de sant publique du Québec; [accessed 2022 Mar. 30]. updated 2022 June 22. Available: https://www.inspq.qc.ca/covid-19/donnees. [Google Scholar]
- 23.Situation of the coronavirus (COVID-19) in Montréal. Montréal: Santé Montréal; [accessed 2021 Oct. 4]. updated 2022 June 28. Available: https://santemontreal.qc.ca/en/public/coronavirus-covid-19/situation-of-the-coronavirus-covid-19-in-montreal/ [Google Scholar]
- 24.Données sur les variants du SRAS-CoV-2 au Québec. Québec: Institut national de sant publique du Québec; [accessed 2022 Apr. 11]. updated 2022 June 22. Available: https://www.inspq.qc.ca/covid-19/donnees/variants. [Google Scholar]
- 25.Interim guidance for the detection of SARS-CoV-2 with the Abbott Panbio COVID-19 antigen rapid test. Ottawa: Public Health Agency of Canada; [accessed 2021 Aug. 7]. modified 2021 Mar. 8. Available: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2021-47/issue-1-january-2021/interim-guidance-detection-sars-cov-2-abbott-panbio-antigen-rapid-test.html. [Google Scholar]
- 26.Goldfarb DM, Tilley P, Al-Rawahi GN, et al. Self-collected saline gargle samples as an alternative to health care worker-collected nasopharyngeal swabs for COVID-19 diagnosis in outpatients. J Clin Microbiol. 2021;59:e02427–20. doi: 10.1128/JCM.02427-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benoit P, Labbé A-C, Lalancette L, et al. G-SPIT group. Comparison of SARS-CoV-2 detection with the Cobas(R) 6800/8800 system on gargle samples using two sample processing methods with combined oropharyngeal/nasopharyngeal swab. J Med Virol. 2021;93:6837–40. doi: 10.1002/jmv.27245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumaresq J, Coutlée F, Dufresne PJ, et al. Natural spring water gargle and direct RT-PCR for the diagnosis of COVID-19 (COVID-SPRING study) J Clin Virol. 2021;144:104995. doi: 10.1016/j.jcv.2021.104995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gobeille Paré S, Bestman-Smith J, Fafard J, et al. G-SPIT study group. Natural spring water gargle samples as an alternative to nasopharyngeal swabs for SARS-CoV-2 detection using a laboratory-developed test. J Med Virol. 2022;94:985–93. doi: 10.1002/jmv.27407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boutin C-A, Grandjean-Lapierre S, Gagnon S, et al. Comparison of SARS-CoV-2 detection from combined nasopharyngeal/oropharyngeal swab samples by a laboratory-developed real-time RT-PCR test and the Roche SARS-CoV-2 assay on a cobas 8800 instrument. J Clin Virol. 2020;132:104615. doi: 10.1016/j.jcv.2020.104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Symptoms, transmission and treatment. Québec: Gouvernement du Québec; [accessed 2022 Apr. 13]. updated 2022 May 9. Available: https://www.quebec.ca/en/health/health-issues/a-z/2019-coronavirus/symptoms-transmission-treatment. [Google Scholar]
- 33.Phillips B, Browne DT, Anand M, et al. Model-based projections for COVID-19 outbreak size and student-days lost to closure in Ontario childcare centres and primary schools. Sci Rep. 2021;11:6402. doi: 10.1038/s41598-021-85302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wachinger J, Olaru ID, Horner S, et al. The potential of SARS-CoV-2 antigen-detection tests in the screening of asymptomatic persons. Clin Microbiol Infect. 2021;27:1700e1–3. doi: 10.1016/j.cmi.2021.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Möckel M, Corman VM, Stegemann MS, et al. SARS-CoV-2 antigen rapid immunoassay for diagnosis of COVID-19 in the emergency department. Biomarkers. 2021;26:213–20. doi: 10.1080/1354750X.2021.1876769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sood N, Shetgiri R, Rodriguez A, et al. Evaluation of the Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection in children: implications for screening in a school setting. PLoS One. 2021;16:e0249710. doi: 10.1371/journal.pone.0249710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.L’Huillier AG, Lacour M, Sadiku D, et al. Diagnostic accuracy of SARS-CoV-2 rapid antigen detection testing in symptomatic and asymptomatic children in the clinical setting. J Clin Microbiol. 2021;59:e0099121. doi: 10.1128/JCM.00991-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolze A, Cirulli ET, Luo S, et al. SARS-CoV-2 variant Delta rapidly displaced variant Alpha in the United States and led to higher viral loads. Cell Rep Med. 2022;3:100564. doi: 10.1016/j.xcrm.2022.100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li B, Deng A, Li K, et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Nat Commun. 2022;13:460. doi: 10.1038/s41467-022-28089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres I, Poujois S, Albert E, et al. Evaluation of a rapid antigen test (Panbio™ COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clin Microbiol Infect. 2021;27:636e1–4. doi: 10.1016/j.cmi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pekosz A, Parvu V, Li M, et al. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis. 2021;73:e2861–6. doi: 10.1093/cid/ciaa1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puhach O, Adea K, Hulo N, et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. 2022;28:1491–1500. doi: 10.1038/s41591-022-01816-0. [DOI] [PubMed] [Google Scholar]
- 43.Données de vaccination contre la COVID-19 au Québec. Québec: Institut national de santé publique du Québec; [accessed 2022 Nov. 9]. modified 2022 June 29. Available: https://mobile.inspq.qc.ca/covid-19/donnees/vaccination. [Google Scholar]
- 44.de Michelena P, Torres I, Ramos-García Á, et al. Real-life performance of a COVID-19 rapid antigen detection test targeting the SARS-CoV-2 nucleoprotein for diagnosis of COVID-19 due to the Omicron variant. J Infect. 2022;84:e64–6. doi: 10.1016/j.jinf.2022.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galliez RM, Bomfim L, Mariani D, et al. Evaluation of the Panbio COVID-19 antigen rapid diagnostic test in subjects infected with Omicron using different specimens. Microbiol Spectr. 2022:e0125022. doi: 10.1128/spectrum.01250-22. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Science brief: COVID-19 vaccines and vaccination. Atlanta: Centers for Disease Control and Prevention; [accessed 2022 Apr. 23]. updated 2021 Sept 15. Available https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html. [Google Scholar]
- 47.Nelson EJ, McKune SL, Ryan KA, et al. SARS-CoV-2 positivity on or after 9 days among quarantined student contacts of confirmed cases. JAMA. 2021;325:1561–2. doi: 10.1001/jama.2021.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.